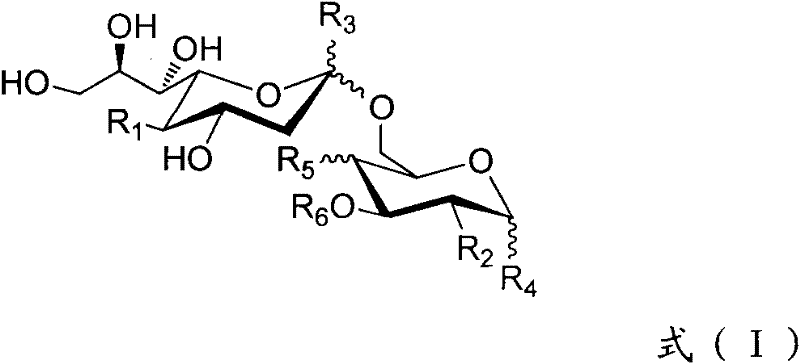
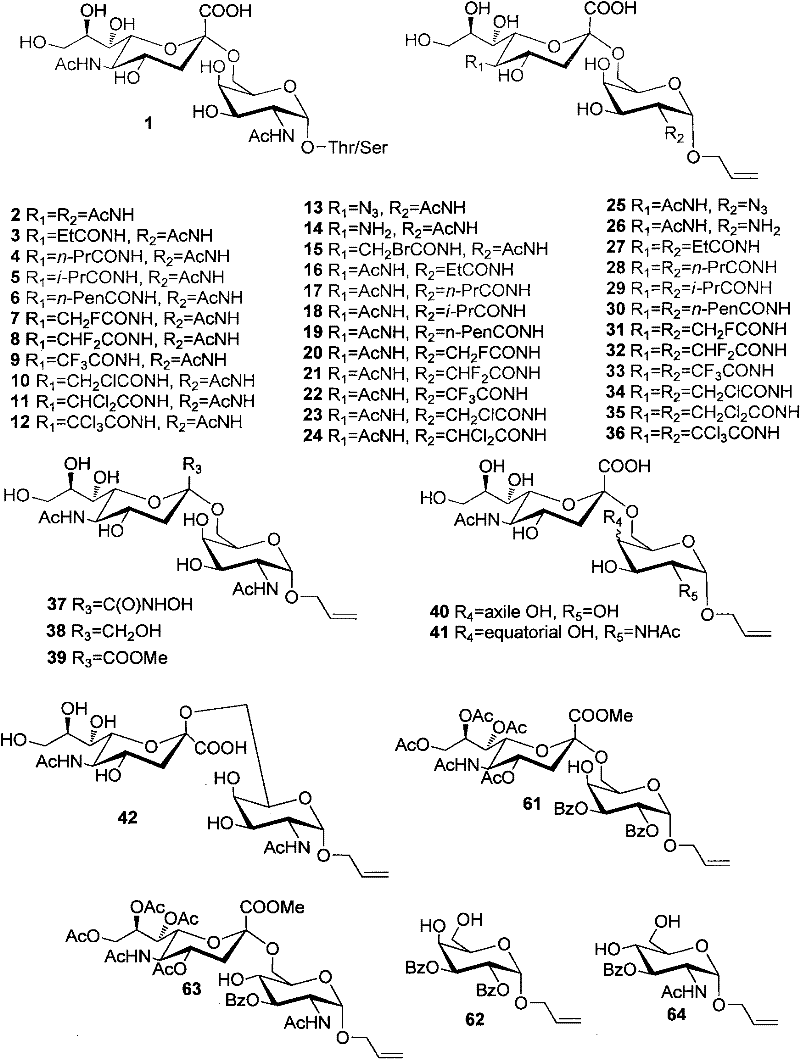
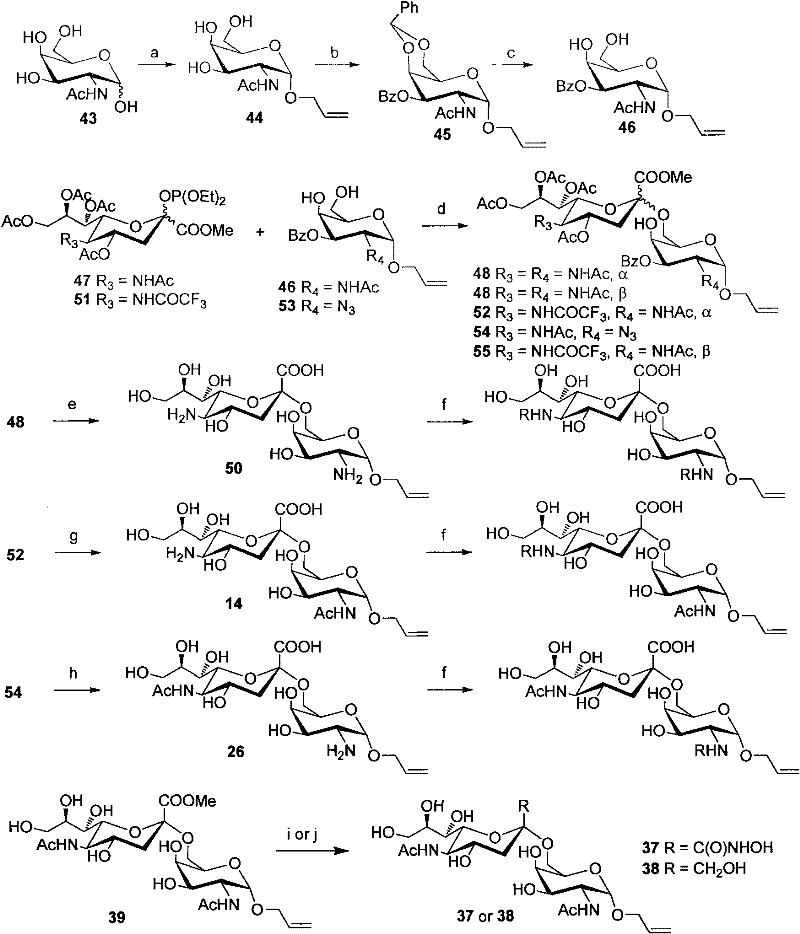
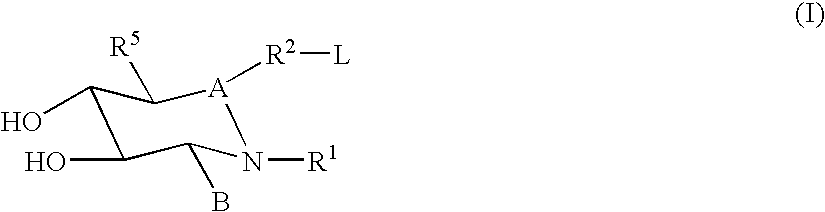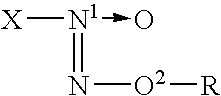Patents
Literature
152 results about "Pyranose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
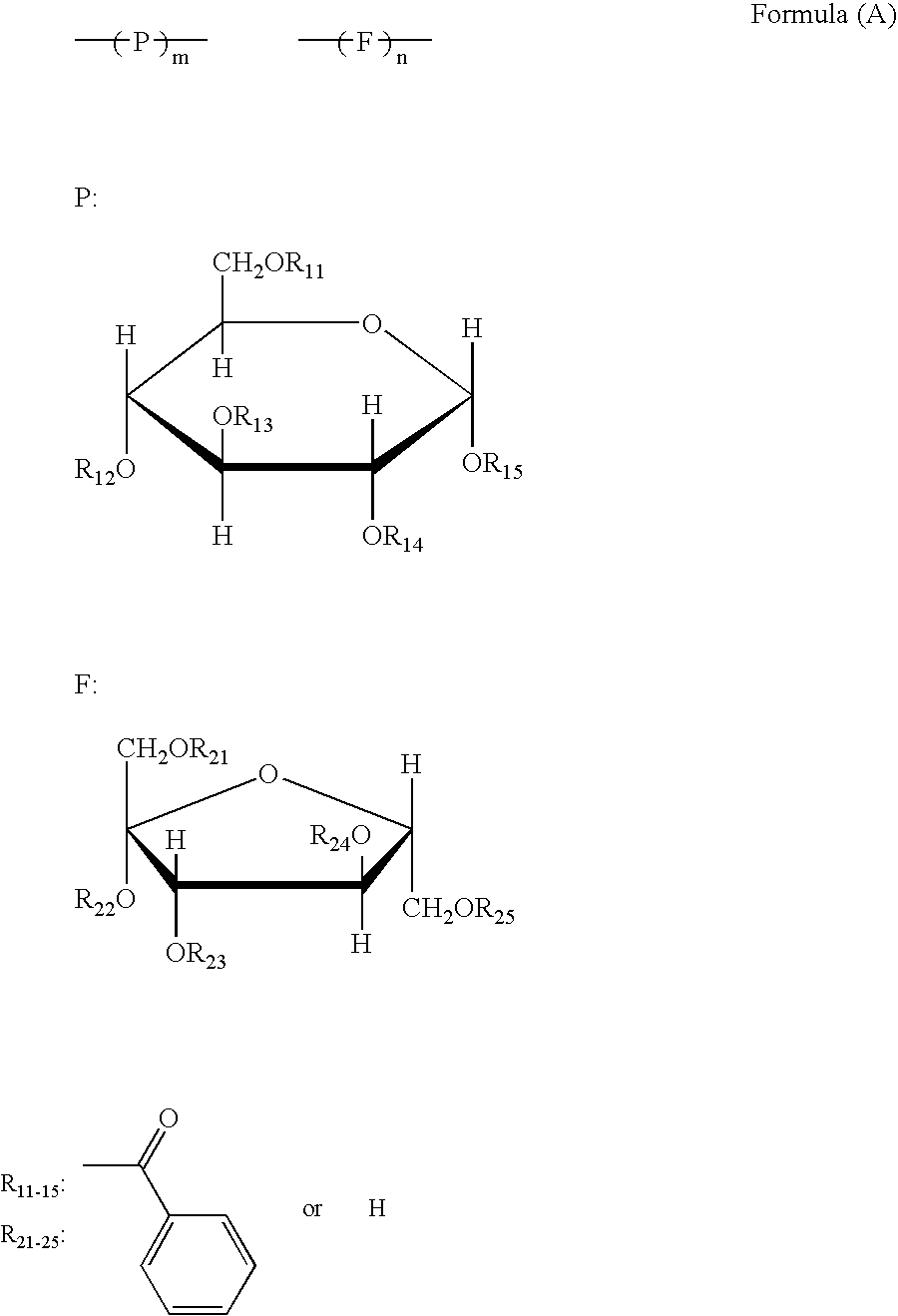
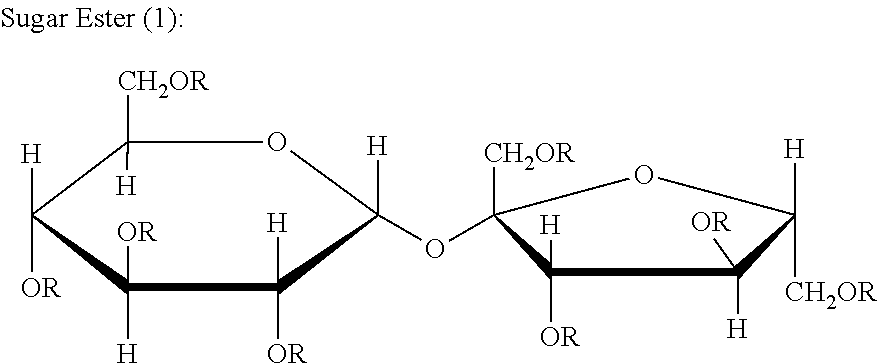
Pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds. A pyranose in which the anomeric OH at C(l) has been converted into an OR group is called a pyranoside.
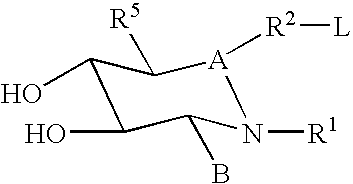
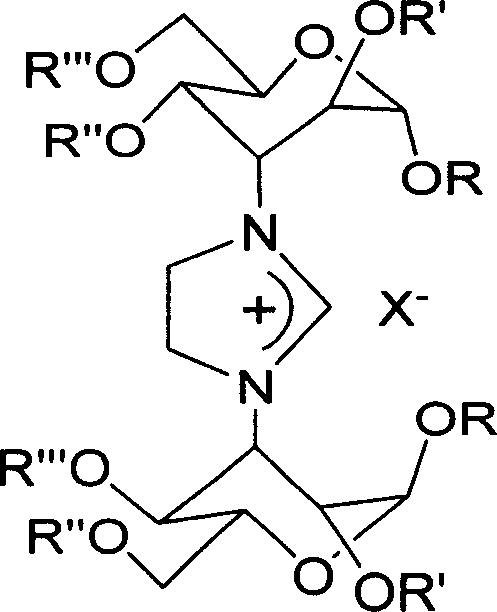
Treatment or prophylaxis of diseases caused by pilus-forming bacteria
InactiveUS6962791B2Reduce capacityReduce pathogenicityBiocideCompound screeningBacteroidesBinding free energy
Novel methods for the treatment and / or prophylaxis of diseases caused by tissue-adhering bacteria are disclosed. By interacting with periplasmic molecular chaperones it is achieved that the assembly of pili is prevented or inhibited and thereby the infectivity of the bacteria is diminished. Also disclosed are methods for screening for drugs as well as methods for the de novo design of such drugs, methods which rely on novel computer drug modelling methods involving an approximative calculation of binding free energy between macromolecules. Finally, novel pyranosides which are believed to be capable of interacting with periplasmic molecular chaperones are also disclosed.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
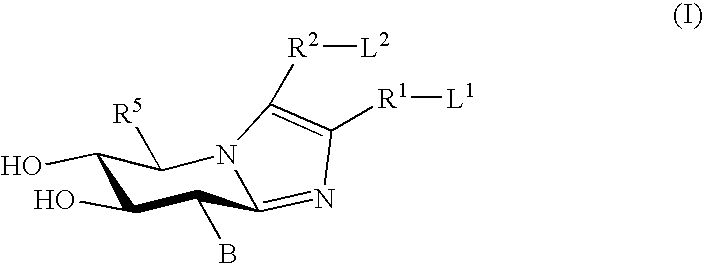
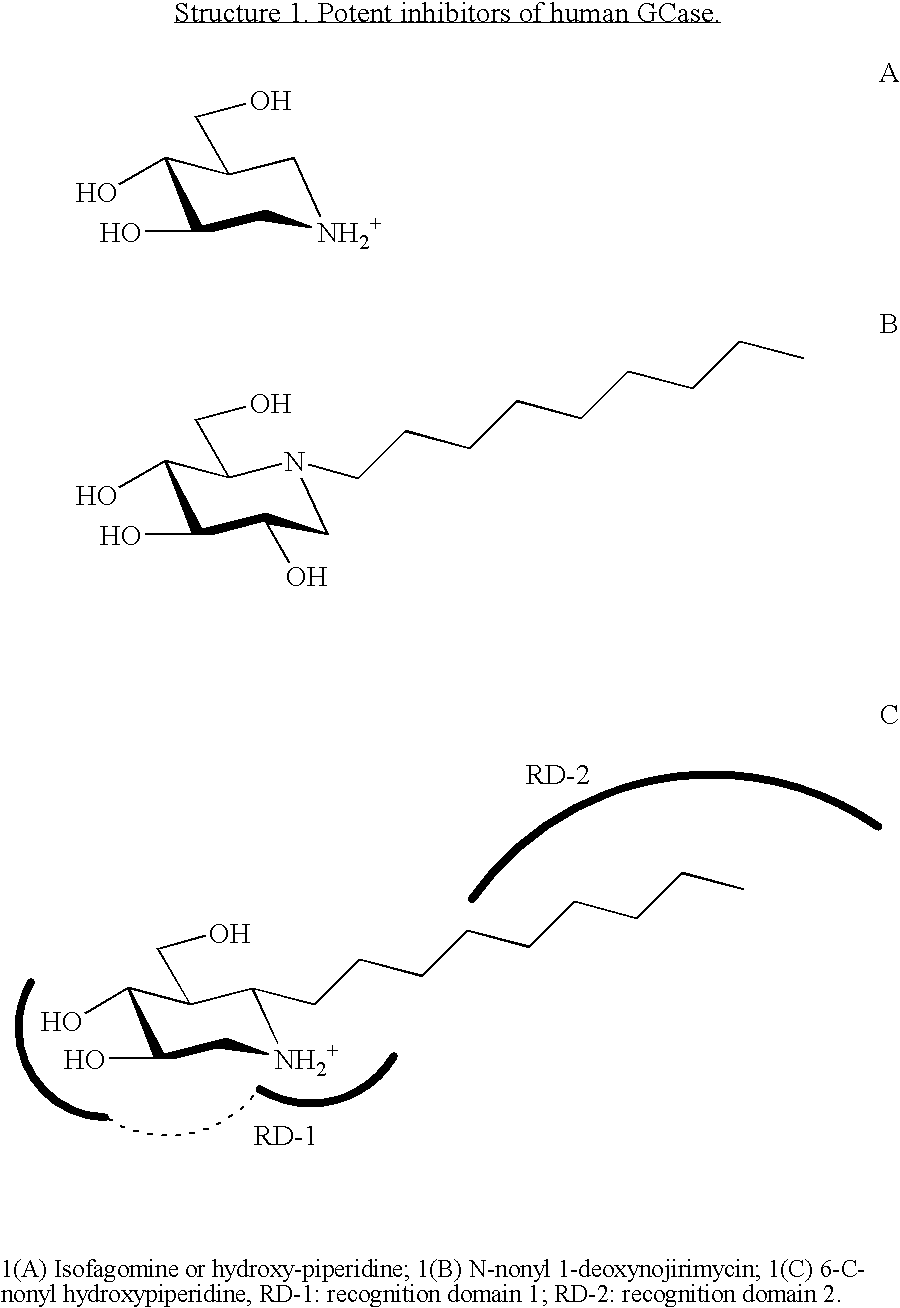
Glucoimidazole and polyhydroxycyclohexenyl amine derivatives to treat gaucher disease
InactiveUS20050137223A1Stabilize GCaseEffective and stableBiocideNervous disorderPyranoseActive site
Owner:AMICUS THERAPEUTICS INC
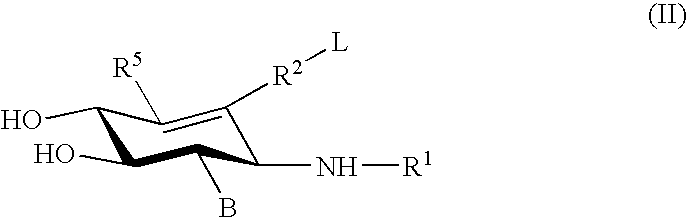
Hydroxy piperidine derivatives to treat gaucher disease
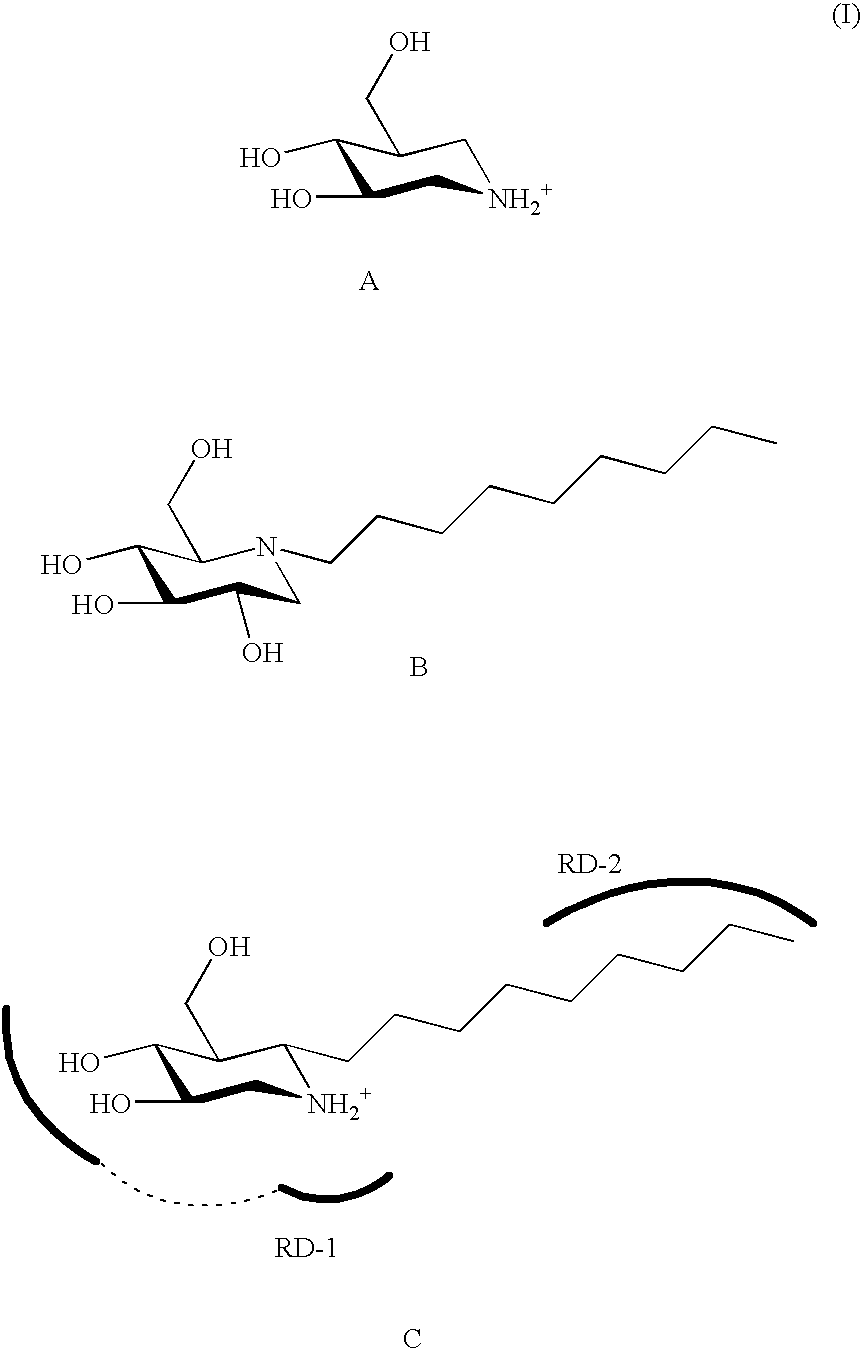
The present invention provides novel hydroxy piperidine (HP) derivatives having (i) a positive charge at the position corresponding to the anomeric position of a pyranose ring; (ii) a short, flexible linker emanating from the corresponding position of the ring oxygen in a pyranose; and (iii) a lipophilic moiety connected to the linker and pharmaceutically acceptable salts thereof. The linker can be absent if the lipophilic moiety corresponds to a hydrocarbon chain with a linear length of 6 or more carbons. The present invention further provides a method for treating individuals having Gaucher disease by administering the novel HP derivative as “active-site specific chaperones” for the mutant glucocerebrosidase associated with the disease.
Owner:AMICUS THERAPEUTICS INC
Optical film, polarizing plate and liquid crystal display apparatus
InactiveUS20090068377A1Improve flatnessMaintain good propertiesLiquid crystal compositionsEsterified saccharide compoundsCelluloseFuran
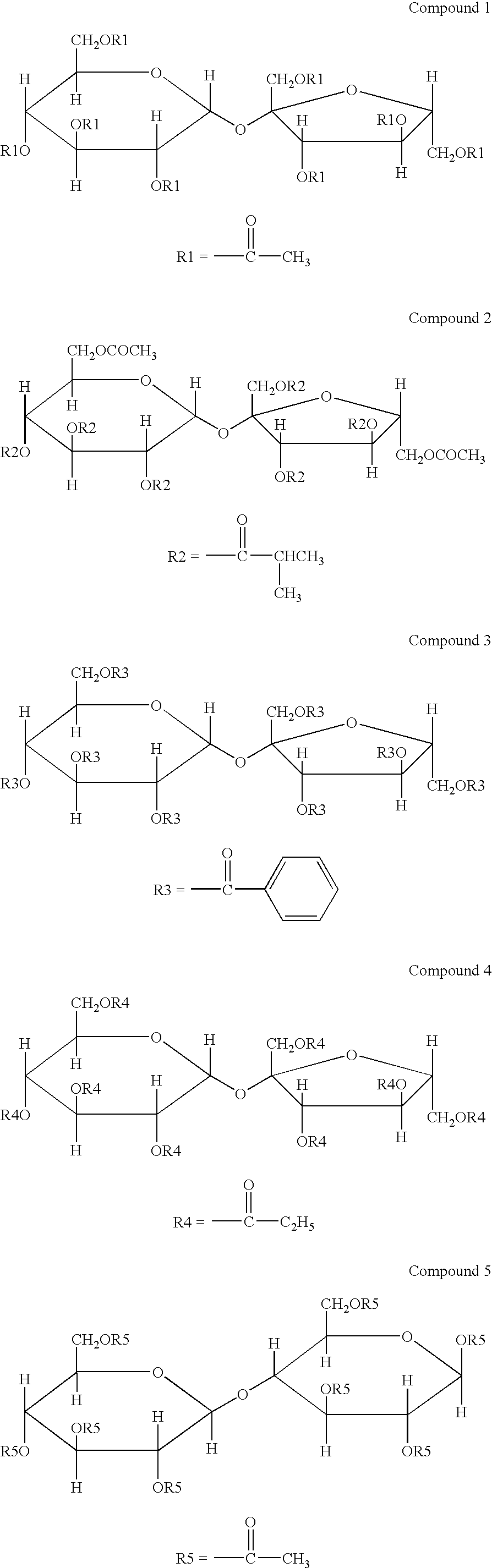
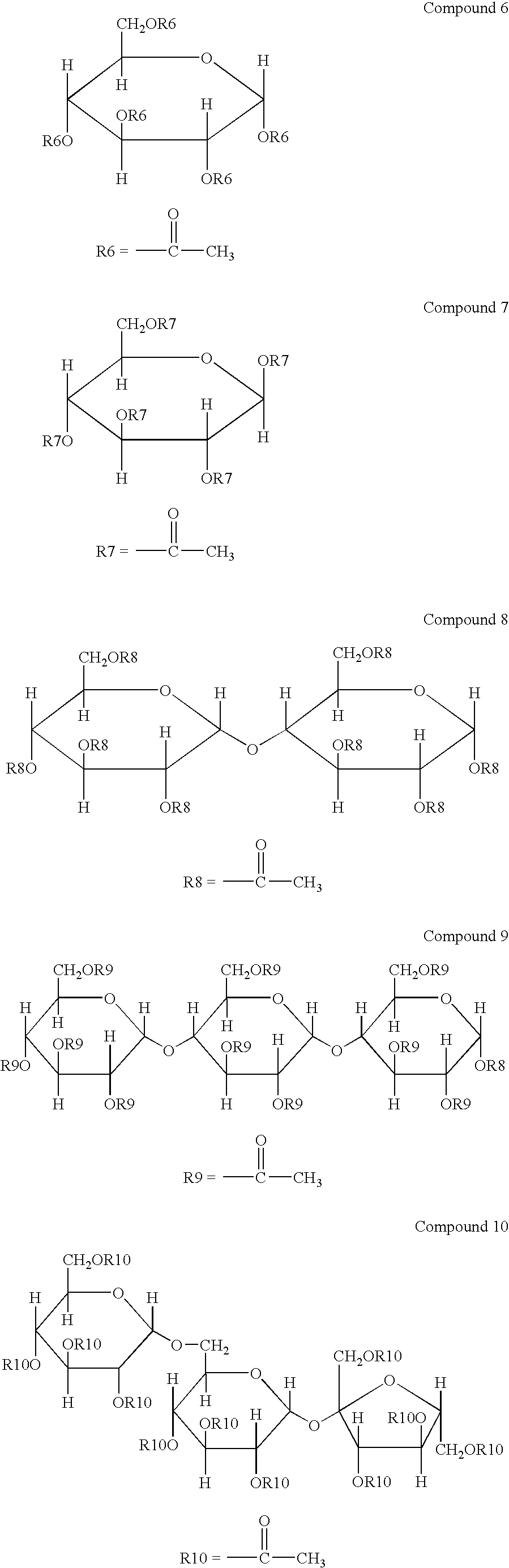
Disclosed is an optical film having a hard coat layer on a cellulose ester film, which contains an ester compound composed of a furanose structure or a pyranose structures and a sum of a number of the furanose structures or the pyranose structure is 1 to 12, provided that a part of hydroxy groups of the furanose structure or the pyranose structure has been esterified and a part of hydroxy groups of the furanose structure or the pyranose structure remains.
Owner:KONICA MINOLTA OPTO
Polarizing plate protective film, polarizing plate and liquid crystal display device
ActiveUS20080049324A1High retardation stabilityLiquid crystal compositionsPolarising elementsTectorial membraneCellulose
Owner:KONICA MINOLTA OPTO
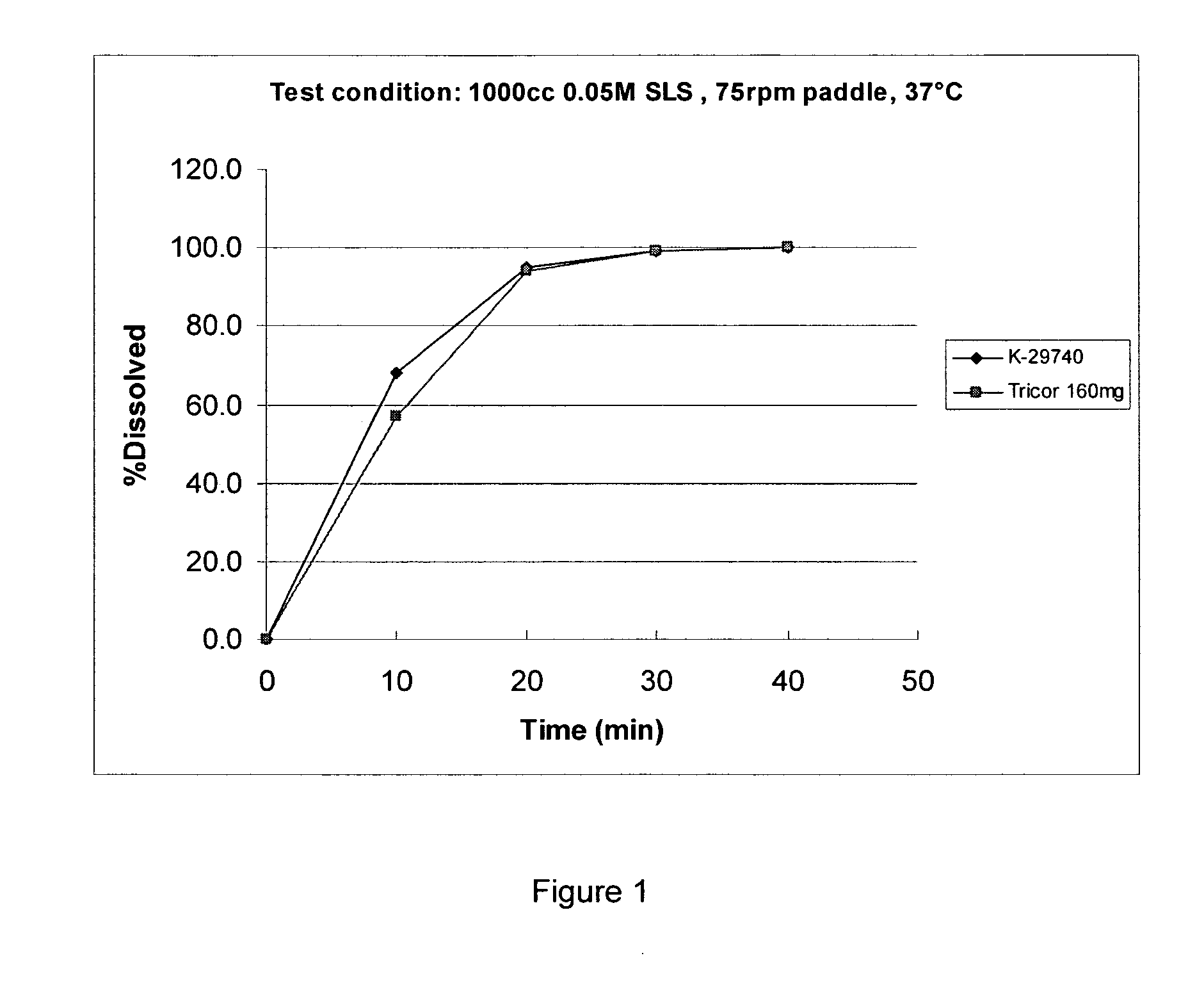
Novel granulation process
One of the objects of the invention relates to a pharmaceutical composition in the form of a granulate, wherein the granulates comprises an active pharmaceutical ingredient (API) having a poor water solubility intimately associated with at least one pharmaceutically acceptable sugar, and optionally or preferably at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar, wherein the active pharmaceutically ingredient has a water solubility less than about 20 mg / ml. The at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar is selected from the group consisting of disintegrants, wetting agents, diluents, binders, lubricants, glidants, coloring agents and flavoring agents. The at least one pharmaceutically acceptable sugar is preferably selected from pyranosyl pyranoses, such as lactose. Another object of the invention relates to a process for preparing a pharmaceutical granulate, comprising (a) combining an API having poor water solubility with a solution comprising at least one pharmaceutically acceptable sugar, for example a pyranosyl pyranose such as lactose, and a solvent, and optionally at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar to form a combined mixture; (b) drying the combined mixture of step (a); and (c) comminuting the product of step (b) to obtain the granulate.
Owner:TEVA PHARM USA INC
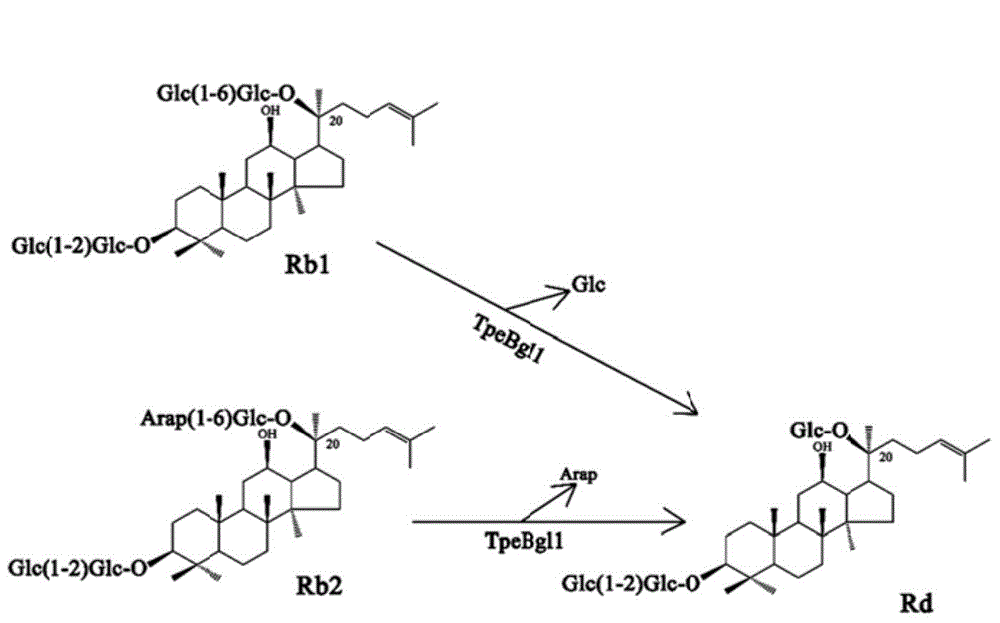
Beta-glucosidase as well as preparation method and application thereof
ActiveCN104611313AGood thermal stabilityStrong conversion abilityFermentationVector-based foreign material introductionAlgluceraseGinsenoside Rb1
The invention provides a beta-glucosidase as well as a preparation method and an application thereof. The amino acid sequence of beta-glucosidase is shown as SEQ ID NO.1. The beta-glucosidase has excellent thermal stability, can resist high temperature, has higher beta-1,6-glucosidic bond hydrolysis capacity as well as higher alpha-1,6-arabinopyranoside bond hydrolysis capacity and has higher transformation capacity for ginsenoside Rb1 and Rb2. After the beta-glucosidase is incubated with the ginsenoside Rb1 and Rb2 for certain period, ginsenoside Rb1 and Rb2 are almost transformed into ginsenoside Rd completely. According to a preparation method of TPEBGL1, high-efficiency expression can be realized without an inducer IPTG (isopropyl beta-D-1-thiogalactopyranoside).
Owner:NANJING FORESTRY UNIV
Novel pharmaceutical granulate
One of the objects of the invention relates to a pharmaceutical composition in the form of a granulate, wherein the granulates comprises an active pharmaceutical ingredient (API) having a poor water solubility intimately associated with at least one pharmaceutically acceptable sugar, and optionally or preferably at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar, wherein the active pharmaceutically ingredient has a water solubility less than about 20 mg / ml. The at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar is selected from the group consisting of disintegrants, wetting agents, diluents, binders, lubricants, glidants, coloring agents and flavoring agents. The at least one pharmaceutically acceptable sugar is preferably selected from pyranosyl pyranoses, such as lactose. Another object of the invention relates to a process for preparing a pharmaceutical granulate, comprising (a) combining an API having poor water solubility with a solution comprising at least one pharmaceutically acceptable sugar, for example a pyranosyl pyranose such as lactose, and a solvent, and optionally at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar to form a combined mixture; (b) drying the combined mixture of step (a); and (c) comminuting the product of step (b) to obtain the granulate.
Owner:TEVA PHARM USA INC
Novel coloring compound and recording material using the same
InactiveUS20060183046A1Bright in hueGood weather resistanceOrganic chemistryMeasurement apparatus componentsPyranoseHydrogen
Disclosed herein are a coloring compound represented by the general formula: A-L1-X-L2-B (1) wherein A is a coloring moiety, B is a stabilizing moiety having fading-preventing ability, L1 and L2 are linkers for linking A, X and B by covalent bonding and denote, independently of each other, any group of —O—, —CO—, —OCO—, —NR1— (in which R1 is a group selected from hydrogen, alkyl, aryl and aralkyl groups), —NHCO—, —NHCOO—, —NHCONH—, —NHCSNH—, —SO—, —SO2—, —SO2NH—, —S—, —SS— and —CH2-, with the proviso that L1 and L2 are not —CH2— at the same time, and X is a spacer moiety and denotes any group of an alkylene group having 1 to 10 carbon atoms, an alkenylene group having 2 to 10 carbon atoms, an alkynylene group having 2 to 10 carbon atoms, an alkoxyalkylene group having 1 to 10 carbon atoms, a cycloalkylene group having 5 to 7 carbon atoms, an arylene group having 6 to 10 carbon atoms and a pyranose type saccharide having I to 7 saccharide units, and a recording material using the coloring compound as a coloring material.
Owner:CANON KK
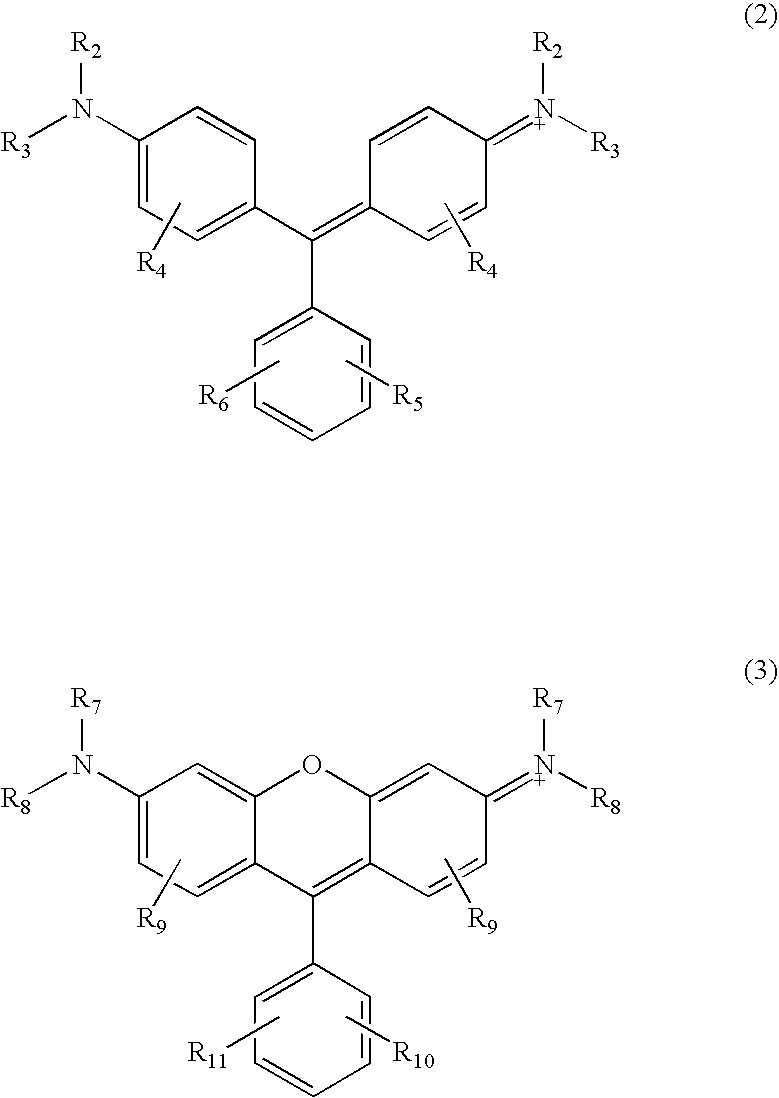
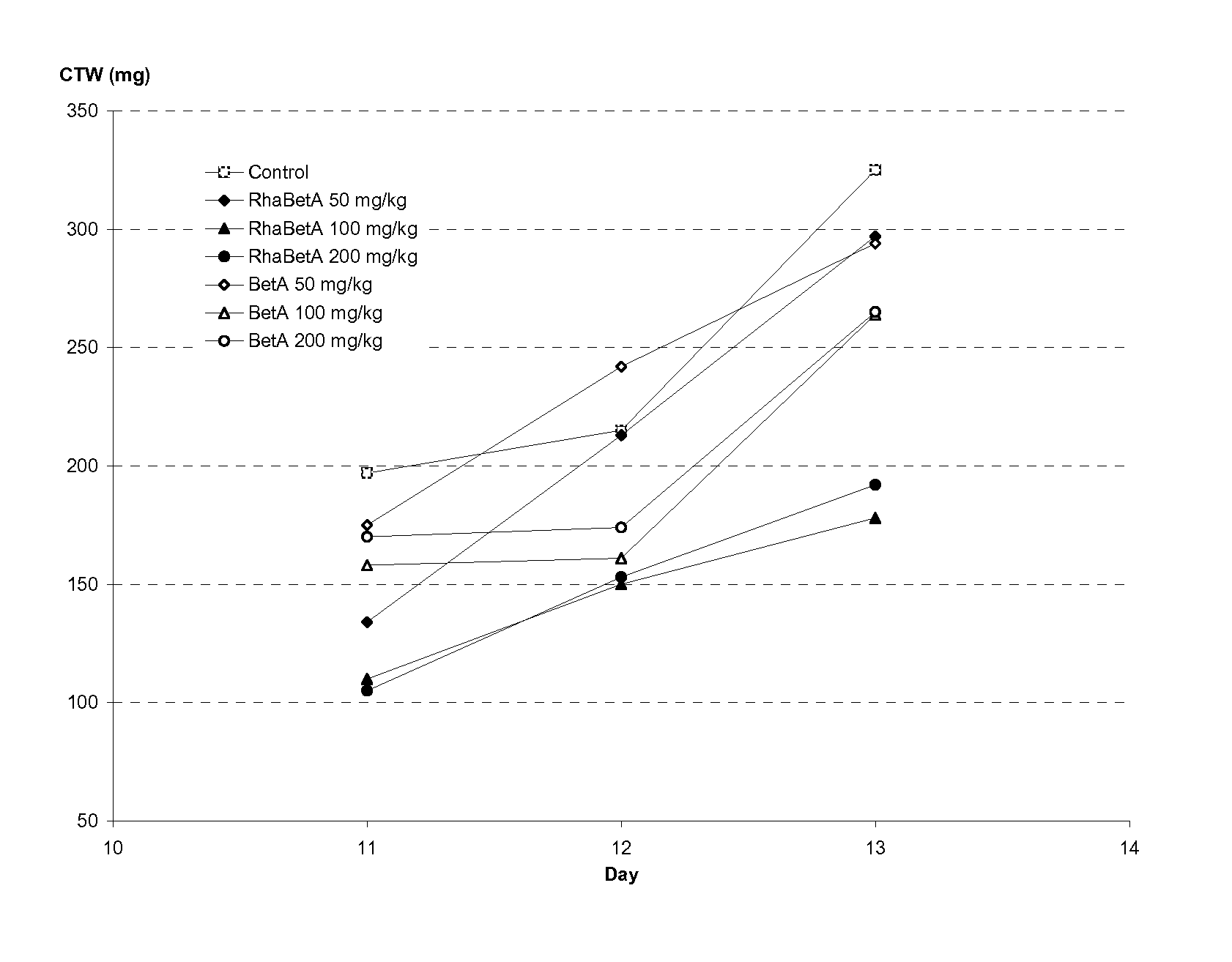
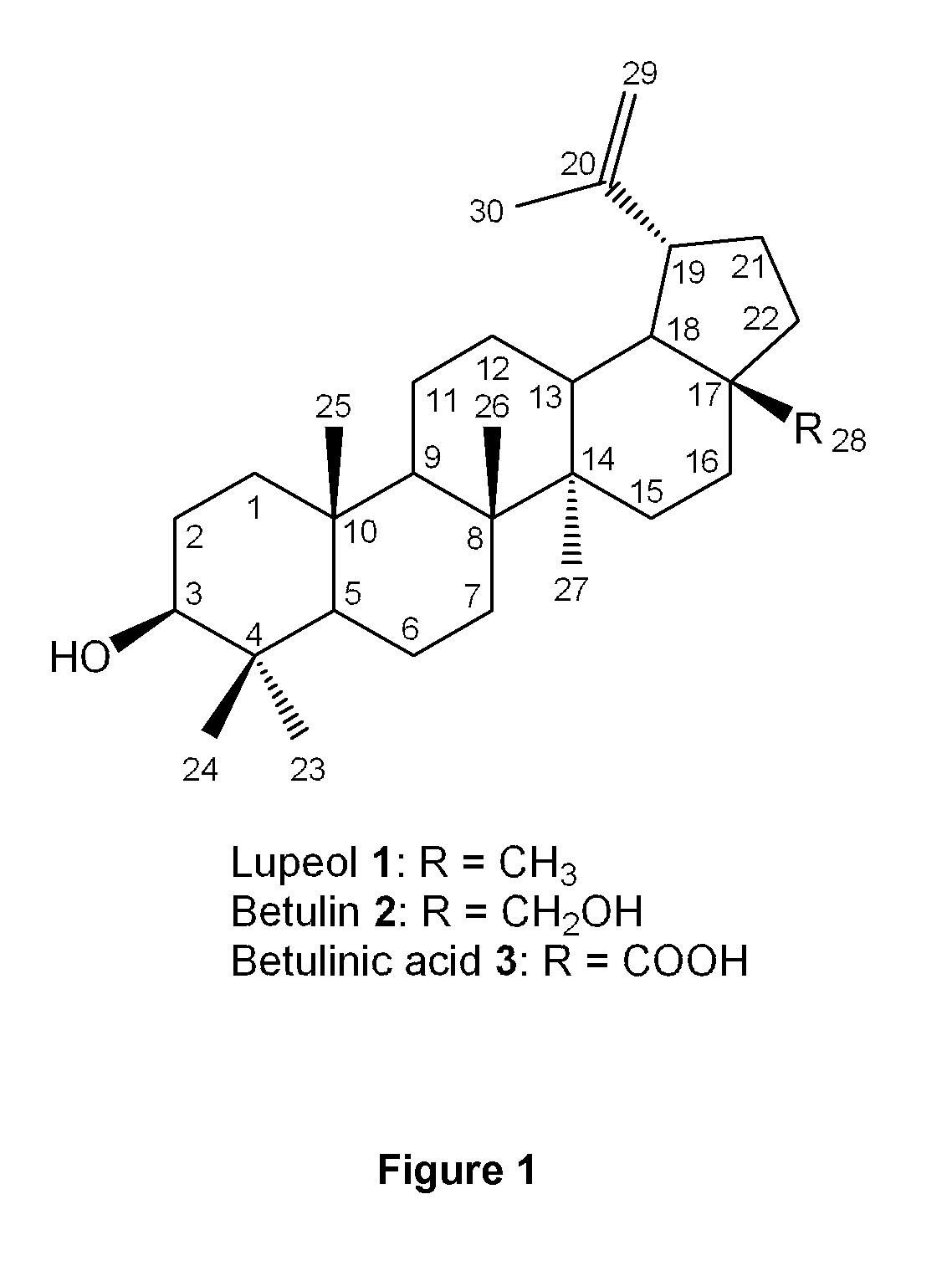
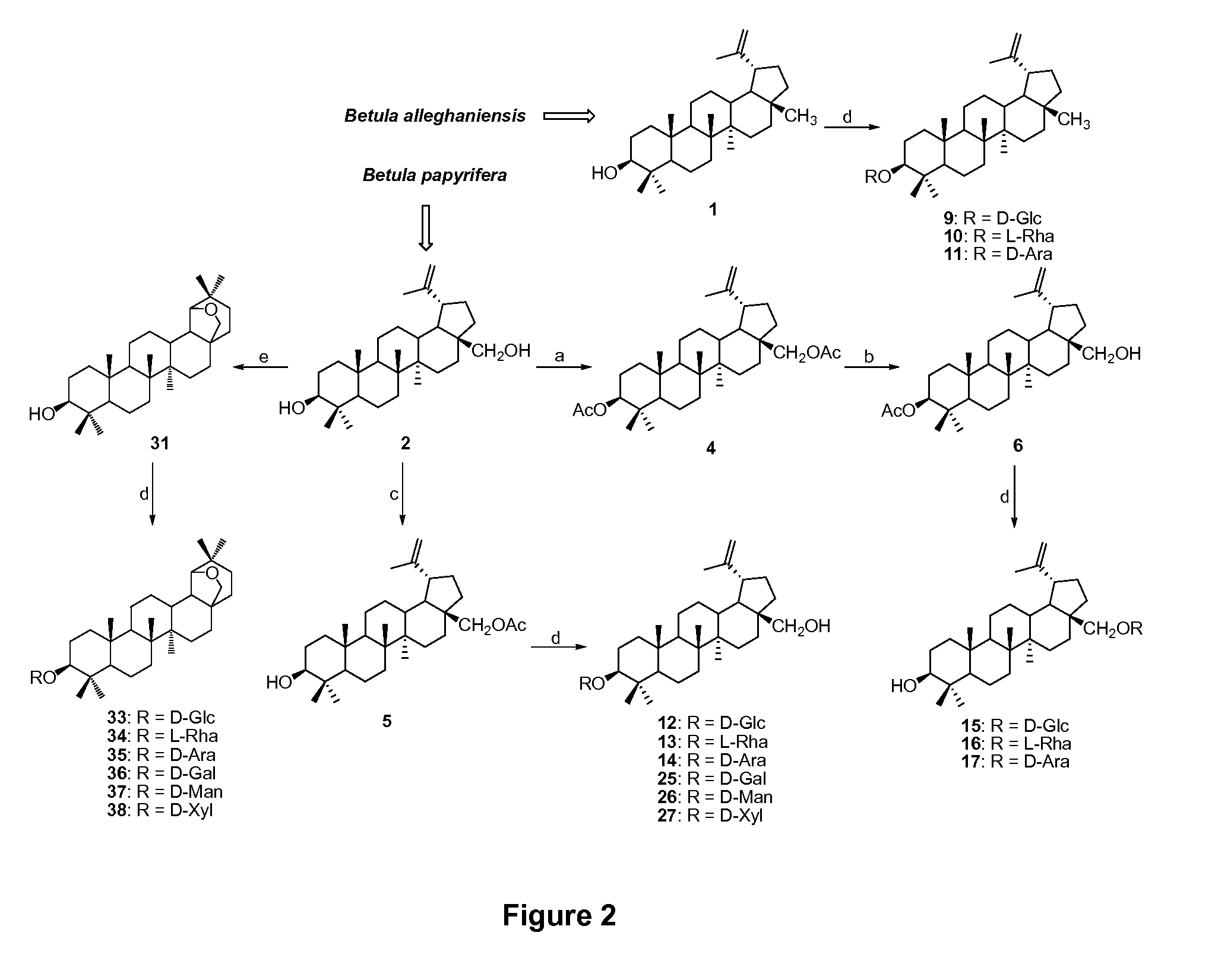
Triterpenes derivatives and uses thereof as antitumor agents or Anti-inflammatory agents
Owner:UNIV DU QUEBEC CHICOUTIMI
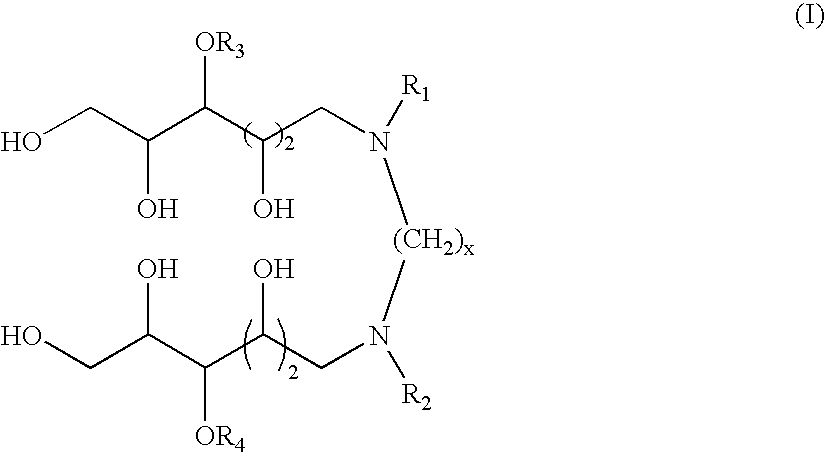
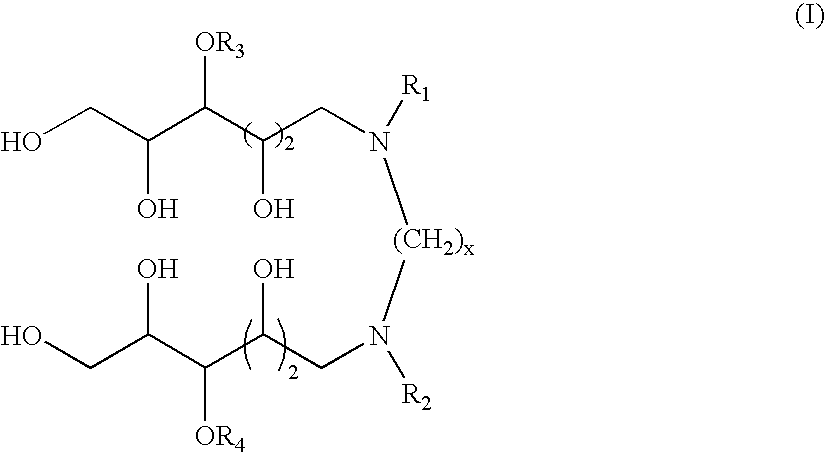
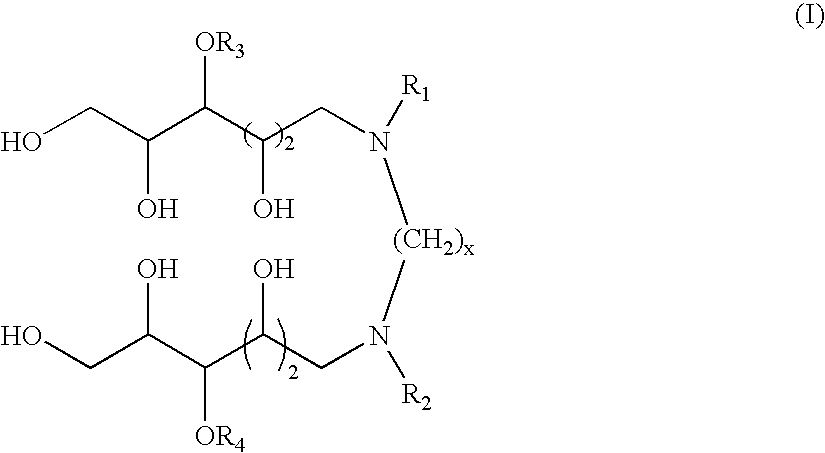
N,N'-dialkyl derivatives of polyhydroxyalkyl alkylenediamines
Surfactant compositions containing compounds according to structure (I), and methods of making them, are disclosed. The compounds provide reduced dynamic and equilibrium surface tension, good solubility, moderate foaming, and good cleaning performance. The methods for making them involve reaction of N-(polyhydroxyalkyl)-alkylamines with dinitriles, dialdehydes, or acetals or hemiacetals of dialdehydes in the presence of hydrogen and a transition metal catalyst. In structure (I), x is an integer from about 1 to 12, R1 and R2 are independently C3-C30 linear alkyl, cyclic alkyl, branched alkyl, alkenyl, aryl, alkylaryl, alkoxyalkyl, and dialkylaminoalkyl; and R3 and R4 are independently hydrogen or a pyranosyl group such as α-D-glucopyranosyl, β-D-glucopyranosyl, or β-D-galactopyranosyl.
Owner:AIR PROD & CHEM INC
Method for producing optical film
InactiveUS20080122128A1Good effectImprove display qualityProtein adhesivesOptical articlesCellulosePyranose
A method for producing an optical film is disclosed. A cellulose ester, a sugar ester prepared by esterification of a sugar compound formed by 1 to 12 of structures selected by a furanose structure and a pyranose structure and an acryl type polymer having a weight average molecular weight of from 5,000 to 30,000 are subjected to melt-casting.
Owner:KONICA MINOLTA OPTO
Cellulose acylate film, its production method, polarizer and liquid crystal display device
ActiveUS20110255037A1High optical appearanceGood dispersionPolarising elementsOptical articlesPyranoseCellulose
A cellulose acylate film containing a cellulose acylate and a sugar ester compound having from 1 to 12 pyranose structures or furanose structures in which at least one hydroxyl group is esterified, the film satisfying 40 nm≦Re(550)≦60 nm and 100 nm≦Rth(550)≦140 nm, and having a dimensional change of −0.5 to 0.5% and an internal haze of at most 0.1%.
Owner:FUJIFILM CORP
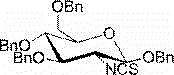
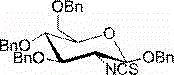
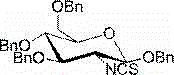
2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose, and synthetic method and application thereof
ActiveCN104961781AEasy to separate and purifySimple and fast operationSugar derivativesSugar derivatives preparationSulfonyl chloridePyranose
The invention relates to 2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose. The invention also relates to a synthetic method for 2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose. The synthetic method comprises the following concrete steps: reacting 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl-beta-D-pyranose hydrochloride and carbon disulfide with triethylamine; and then reacting a reaction product with p-toluene sulfonyl chloride so as to obtain 2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose, wherein acetonitrile is used as a solvent for the reaction, reaction temperature is 0 DEG C, and reaction time is 1 to 2 h. The synthetic method has the advantages of easy and safe operation, small environmental pollution, high yield and simple post-treatment. The prepared 2-deoxy-2-isorhodanate-1,3,4,6-tetra-O-benzyl-beta-D-glucopyranose can be used as an organic synthesis intermediate for synthesis of a glucosamine derivative.
Owner:HUAIHAI INST OF TECH
Composition for preventing or treating degenerative brain diseases comprising a hydrolysate of ginsenosides
InactiveUS20050233984A1Avoid toxicityPrevented beta-amyloid toxicityBiocideNervous disorderPyranoseHydrolysate
A pharmaceutical composition for preventing or treating a degenerative brain disease comprising a compound of formula I or a or a pharmaceutically acceptable salt thereof as an active ingredient: (I) wherein, R1 is H or Glc-Glc-; R2 is H or OH; R3 is H, glucose, Ara(p)-Glc- or Ara(f)-Glc-; Glc is Glucose; Ara(p) is arabinose in pyranose form: and Ara(f) is arabinose in furanose form.
Owner:DIGITAL BIO TECHNOLOGY CO LTD
Puerarin monocrystal and preparation method thereof
The invention relates to a puerarin monocrystal and a preparation method thereof. The puerarin monocrystal is named crystal form A, the CCDC number is 708830, the crystal structure is asymmetrical, each crystal cell contains two asymmetrical molecules, i.e. molecule a and molecule b, each of which carries a molecular crystal water, and moreover, the binding sites of the crystal waters are different. The crystal water of the molecule a and a hydroxyl group on a benzene ring carry out hydrogen bonding, the crystal water of the molecule b and a carbonyl group on a flavone mother nucleus carry out hydrogen bonding, the hydroxymethyl group of the glucose of the molecule a is at the position of an equatorial bond and perpendicular to a ring plane, the oxygen atom of the hydroxymethyl group is in a saccharide ring plane, the hydroxymethyl group of the glucose of the molecule b is at the position of an axial bond and perpendicular to a ring plane, the hydrogen on the rest pyranose carboatomic ring is an axial bond, the hydroxyl group is positioned at an equatorial bond, and the molecular formulas of the molecule a and the molecule b are the same, i.e. C21H20O9.H2O. The absolute configuration of the puerarin monocrystal is an asymmetrical isomer. The melting point of the puerarin monocrystal is increased, and the heat stability is remarkably enhanced compared with powder. The average purity of the puerarin crystal form A reaches 99.8 percent, higher than 99.1 percent of average purity of bulk pharmaceutical chemicals, and the quality is remarkably increased.
Owner:张幸国
Cell membrane imaging fluorescence probe and application thereof
ActiveCN108558967AImage stabilizationStable fluorescenceSugar derivativesColor/spectral properties measurementsFluorescenceBiocompatibility Testing
The invention relates to a cell membrane imaging fluorescence probe, in particular to glycosyl-substituted perylene bisimide derivative cell membrane imaging dye. An alkyl chain is introduced into oneend of the derivative, and pyranose is introduced into the other end. The dye can be self-assembled into nano-particles in vitro and enter cells, and then are redistributed in cell membranes, and after the dye is excited, the cell membranes can be specifically imaged. Compared with traditional cell membrane fluorescence dye, the dye is high in biocompatibility and lasting in light and acid stability, and the preparation method is simple, economical and capable of being widely used in cell membrane imaging research, especially, the research on imaging of the cell membranes of tumor cells in aweak acid environment.
Owner:HENAN UNIVERSITY
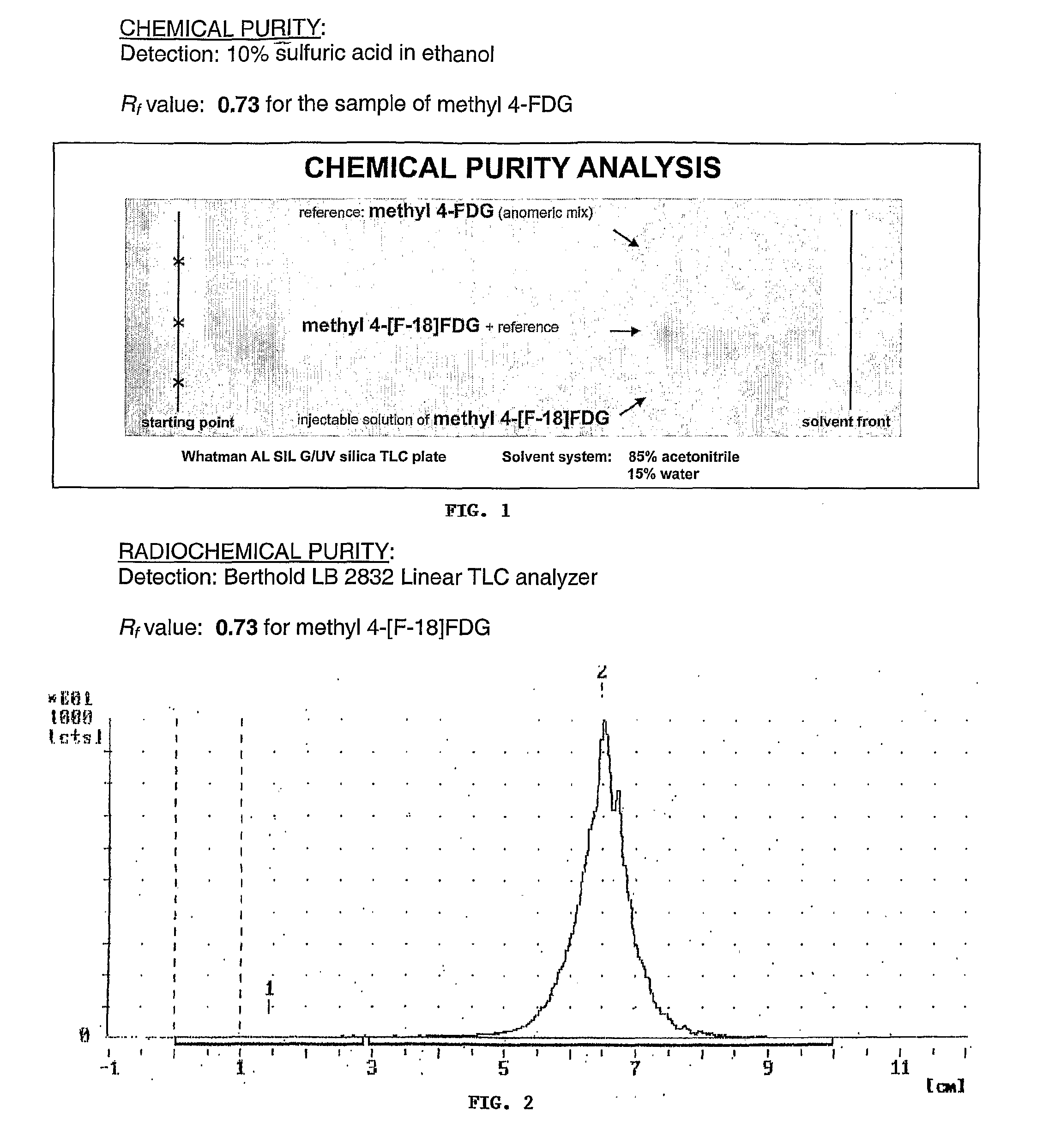
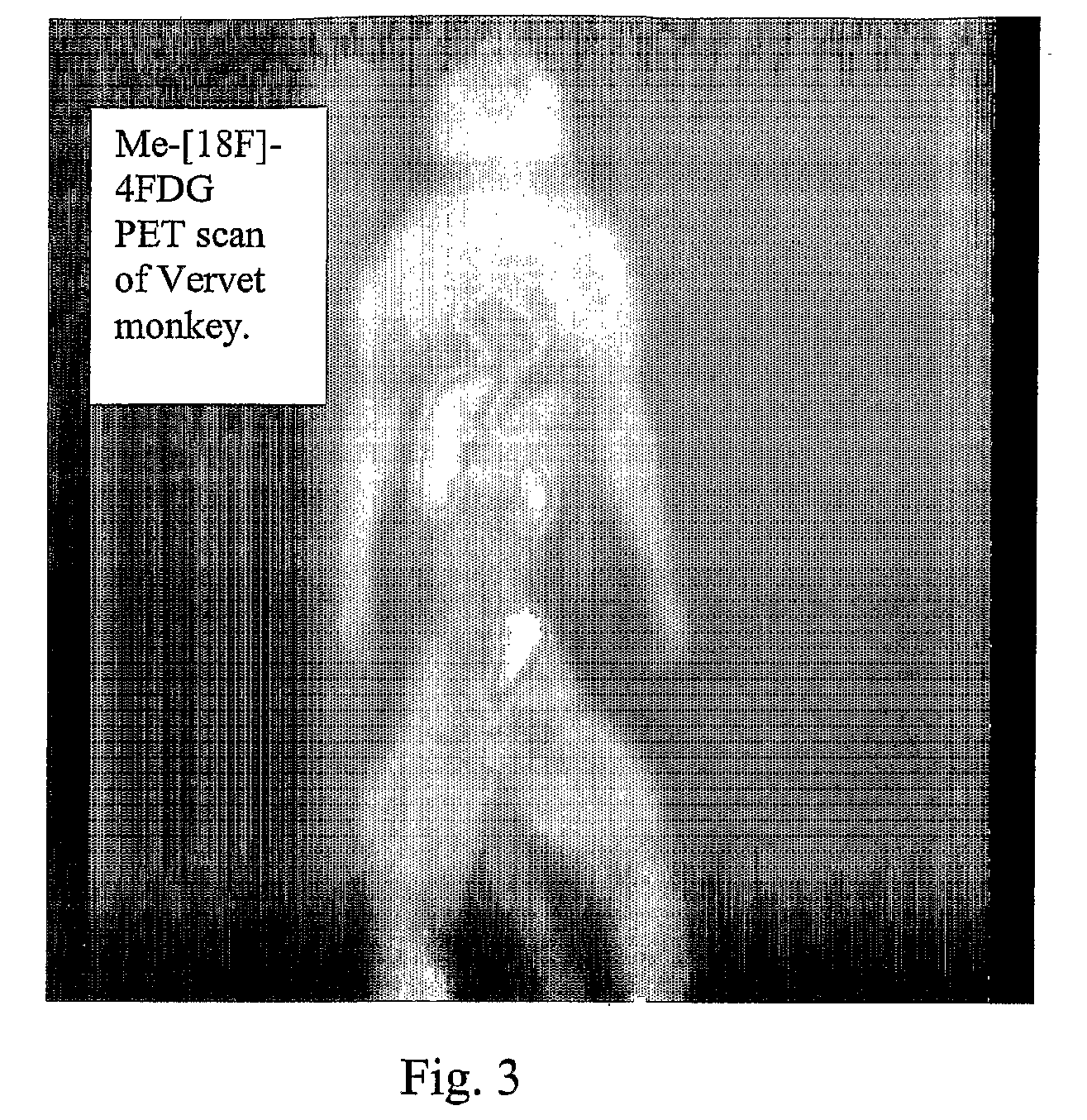
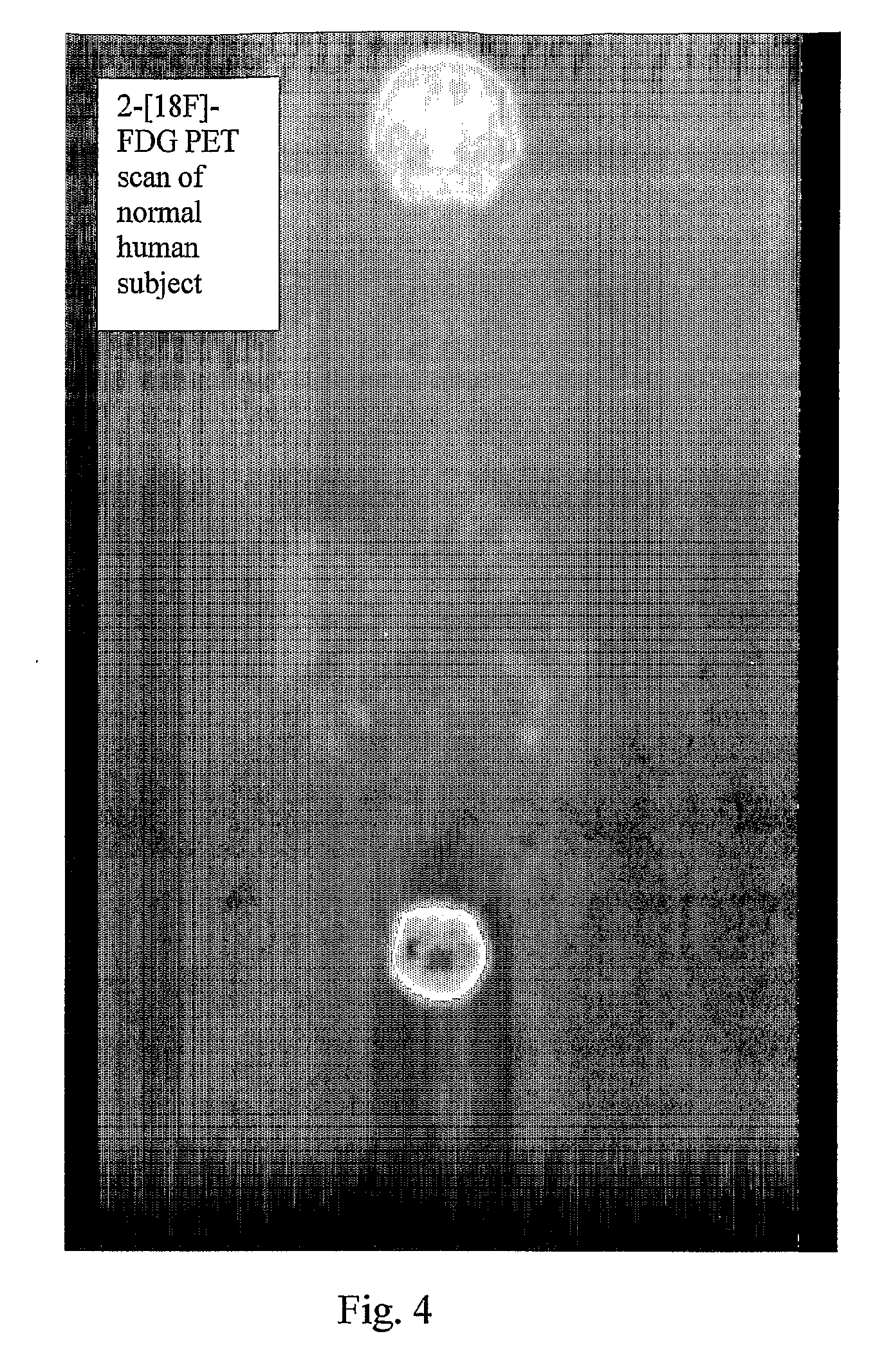
Tracers for monitoring the activity of sodium/glucose cotransporters in health and disease
ActiveUS20100008856A1Improve assessmentIn-vivo radioactive preparationsSugar derivativesMedicineIn Vitro Techniques
Radiolabeled tracers for sodium / glucose cotransporters (SGLTs), their synthesis, and their use are provided. The tracers are methyl or ethyl pyranosides having an equatorial hydroxyl group at carbon-2 and a C1 preferred conformation, radio-labeled with 18F, 123I, or 124I, or free hexoses radiolabeled with 18F, 123I, or 124I. Also provided are in vivo and in vitro techniques for using these and other tracers as analytical and diagnostic tools to study glucose transport, in health and disease, and to evaluate therapeutic interventions.
Owner:RGT UNIV OF CALIFORNIA
Sialic acid (α-(2→6))-d-pyranose derivative and its synthesis method and application
ActiveCN102276662ANovel structureHigh activityEsterified saccharide compoundsOrganic active ingredientsPyranoseAntiendomysial antibodies
The invention discloses an N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative and its synthetic method and use. The sialic acid (alpha-(2-6))-D-amino pyranose derivative shown in the formula (I) is synthesized from raw materials of D-galactosamine (glucose) and sialic acid and is coupled with a carrier protein or a polypeptide to form a glycoprotein (glycopeptide) conjugate. In a structure of the N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative, a derivative acyl group replaces an acetyl group and thus the structure is novel. The N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative has good activity in anti-tumor vaccines. A result of an experiment on mice shows that through structural derivatization, a carbohydrate antigen based vaccine can produce an effective immune response and a mass of antibodies and IgG / IgM is improved obviously. The antibodies can identify specifically tumor cells expressing STn and thus anti-tumor effects are realized. STn antigens can be expressed on multiple tumors and thus the N-acyl group modified sialic acid (alpha-(2-6))-D-amino pyranose derivative has a wide application scope.
Owner:PEKING UNIV
Platycodon grandiflorum polysaccharide, and degradation product, preparation method and application thereof
ActiveCN102477103ASignificant anti-angiogenic effectOrganic active ingredientsSugar derivativesPyranoseDisease
The invention relates to platycodon grandiflorum polysaccharide extracted from rhizome of platycodon grandiflorum, a degradation product of the polysaccharide, a method for extracting the platycodon grandiflorum polysaccharide from the rhizome of the platycodon grandiflorum, a method for preparing the degradation product of the platycodon grandiflorum polysaccharide, and application of the platycodon grandiflorum polysaccharide and the degradation product thereof to preparing a medicine for treating anti-tumour cell angiogenesis diseases. The structure of the platycodon grandiflorum polysaccharide disclosed by the invention is (1->4)-alpha-D- pyranose homogalacturonan, has the polymerization degree of 15-100, the corresponding molecular weight of 2.0-18.0 kD, and the specific rotation of [alpha]D19+125 DEG (c0.05, H2O), and is represented by the following structural formula described in the specification. The molecular weight range of a degradation product obtained by partial acid hydrolysis of the platycodon grandiflorum pectin polysaccharide is 1.4-3.0 kDa. Vivo experiments prove that the platycodon grandiflorum polysaccharide can obviously inhibit lumen generation function of a human microvascular endothelial cell (HMEC-1) and is hopefully used as a novel anti-tumour medicine for inhibiting the growth of a solid tumour by inhibiting angiogenesis.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
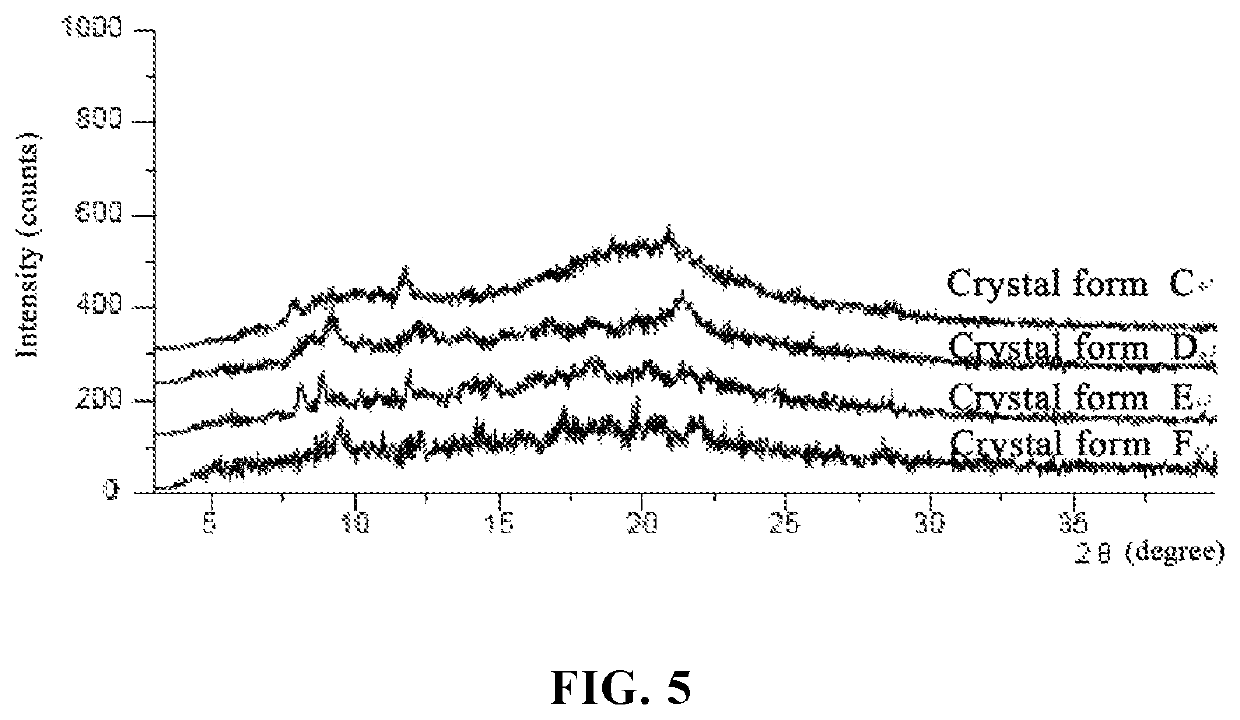
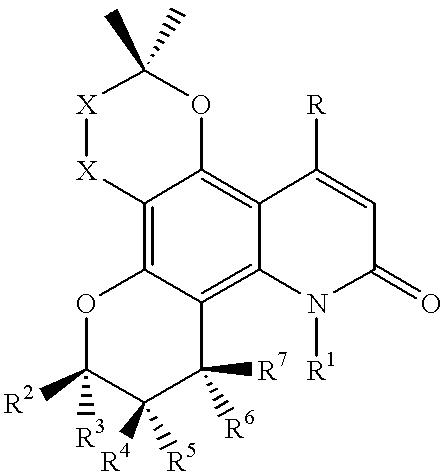
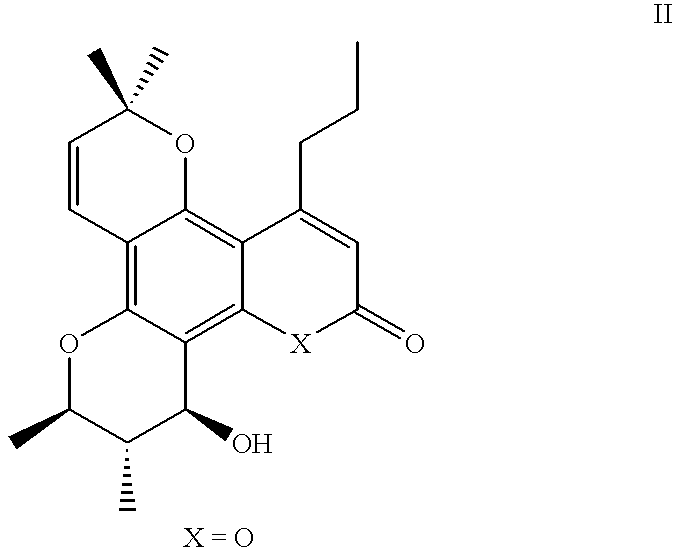
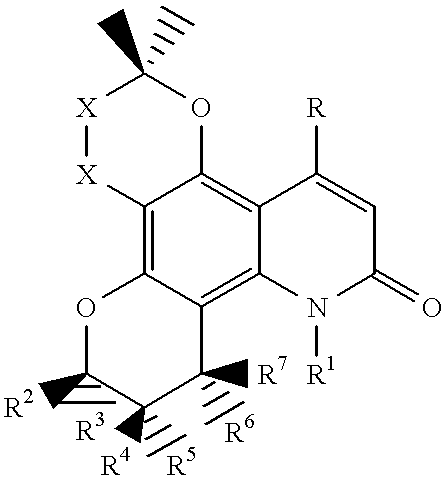
Salt of pyranose-substituted heterocyclic compound, preparation method therefor and use thereof
ActiveUS11155571B2Improve druggabilityHigh puritySugar derivativesMetabolism disorderPyranoseGlucuronate
The present application relates to a salt of a pyranose-substituted heterocyclic compound, a preparation method therefor, and use thereof, and in particular, to an acid addition salt of a compound of formula (I) or a prodrug thereof, and further relates to D-glucuronate of a crystalline compound of formula (I) or a prodrug thereof. D-glucuronate of a crystalline compound of formula (II) has particular advantages in terms of crystallizability, subsequent purification, stability, formulation medicinal properties or quality control, and is most applicable for improving the formulation pharmaceutical properties, purity and quality control, as well as large-scale process development of such drugs.
Owner:YABAO PHARMA GRP CO LTD +1
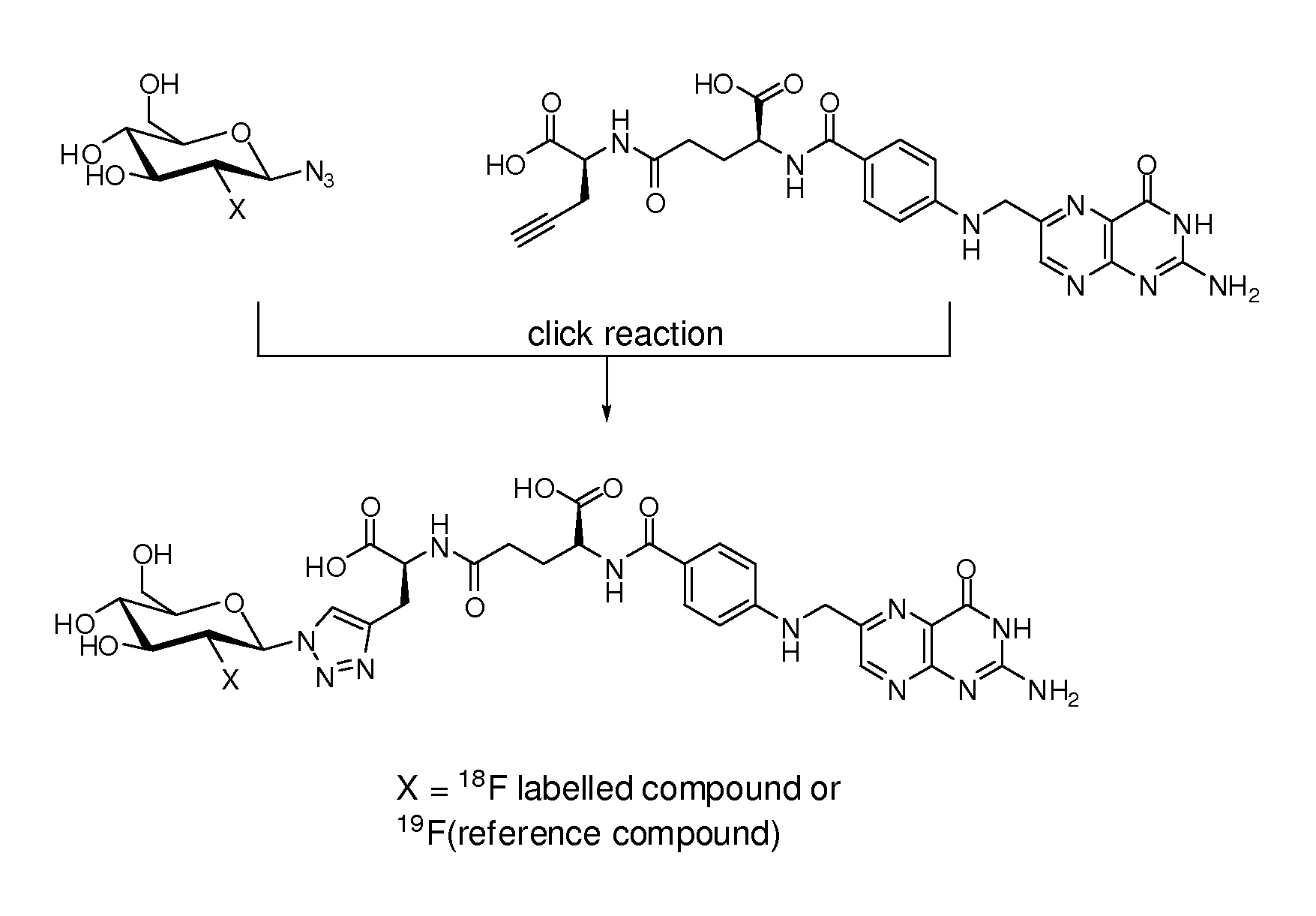
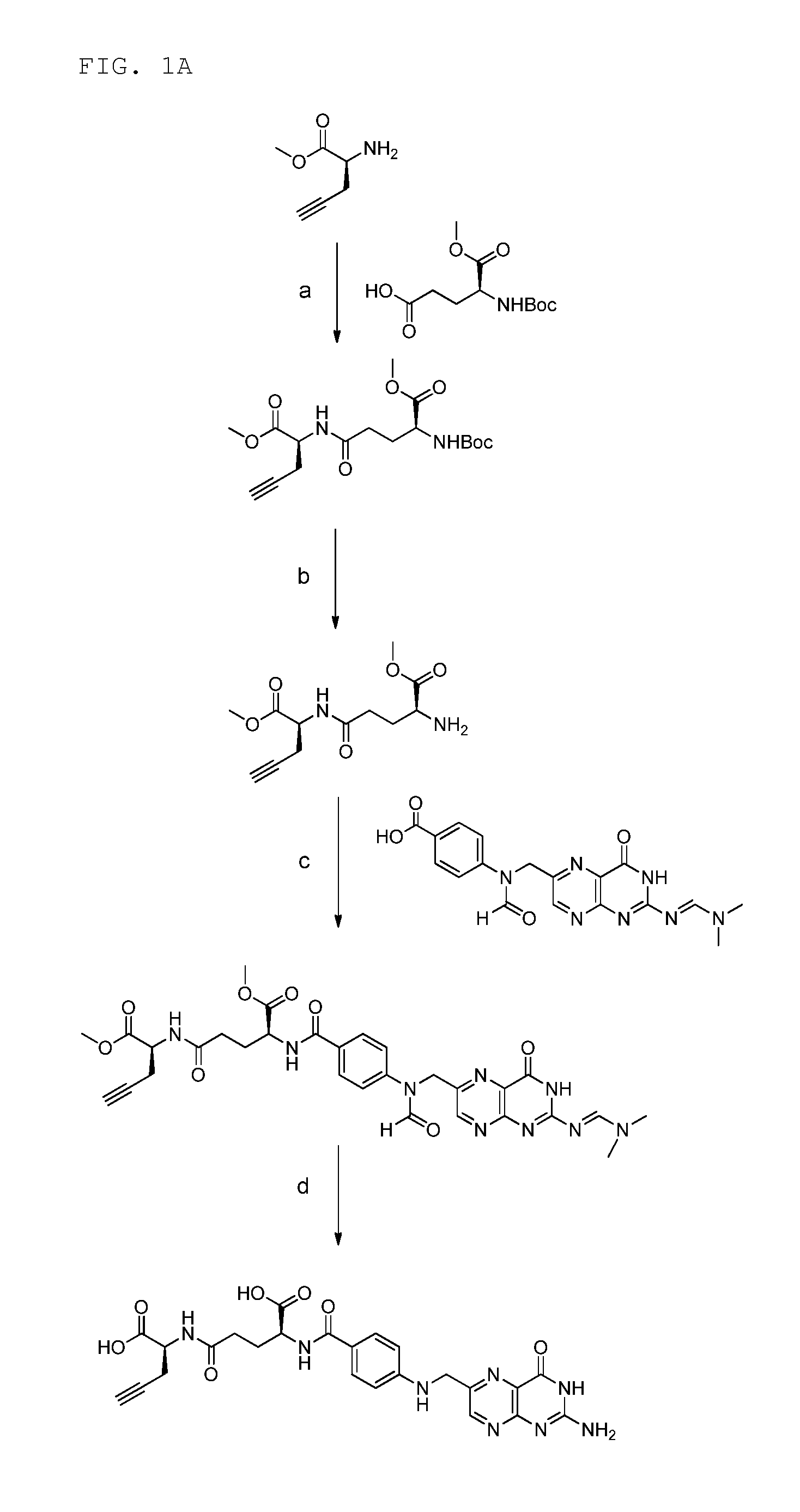
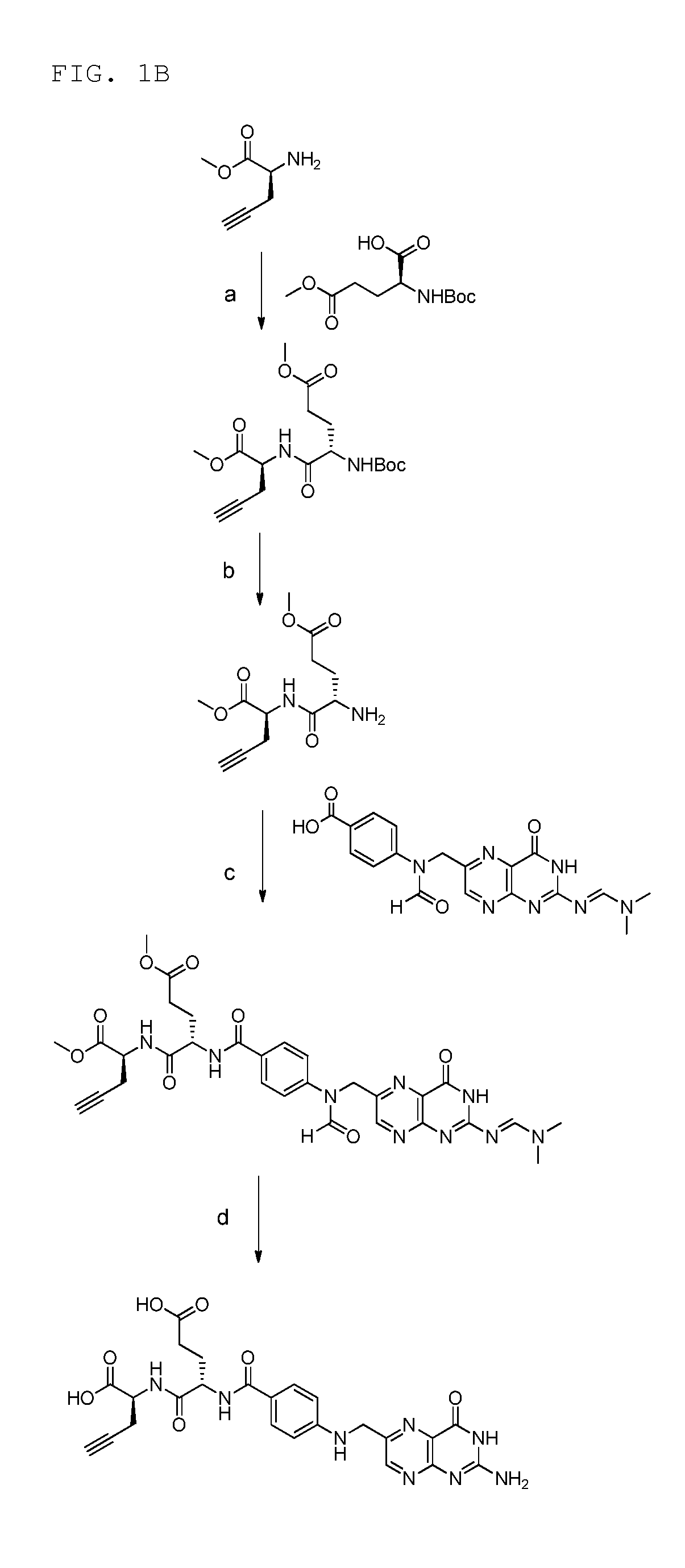
18f-saccharide-folates
ActiveUS20140193337A1Easy to manageIsotope introduction to sugar derivativesSugar derivativesDrugFolic acid
The present invention is directed towards new 18F-folate radiopharmaceuticals, wherein the 18F isotope is linked via a prosthetic group, more specifically via a prosthetic group having a saccharide group, such as a cyclic mono- or oligosaccharide, preferably based on a pyranoside or furanoside, which is covalently linked to the glutamate portion of a folate or derivative thereof, a method of their preparation, as well as their use in diagnosis and monitoring of cancer and inflammatory and autoimmune diseases and therapy thereof.
Owner:MERCK & CIE KG
New monoterpene compounds and preparation method thereof
InactiveCN101555237AGood analgesic effectReduce pollutionOrganic active ingredientsSugar derivativesNonaneMicrobial transformation
The invention pertains to the technical field of medicine, which discloses new monoterpene compounds and a preparation method thereof. A compound I is prepared by the following steps of conducting microbial transformation of carboxylesterase on an extract which is rich in pinane monoterpene and separating the transformation product by utilizing a silica gel column chromatography method. A compoundII is prepared by the following steps of conducting microbial transformation of glucosaccharase on the compound I and separating the transformation products by utilizing a silica gel column chromatography method. The chemical names of the compound I and the compound II are respectively (1R, 3R, 4R, 6S, 9S)-6-methyl-4-hydroxyl-1- (Bata-D-glucopyranoside oxyl)-9-(hydroxymethyl-7-oxotrane (4.3.0.0<3, 9>) nonane-8-ketone and (1R, 3R, 4R, 6S, 9S)-6-methyl-1, 4-dihydroxyl-9-(hydroxymethyl-7-oxotrane (4.3.0.0<3, 9>) nonane-8-ketone. Pharmacological tests show that the two monoterpene compounds disclosed by the invention have good antinociceptive effect. The preparation method is simple and has low cost and less pollution to environment.
Owner:SHENYANG PHARMA UNIVERSITY
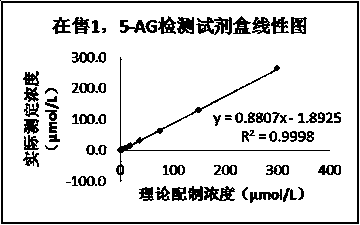
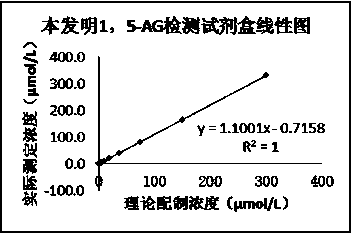
Kit for detecting 1, 5-AG with good stability and method
InactiveCN110702676AEliminate distractionsImprove accuracyMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsPyranoseConcentrations glucose
The invention discloses a kit for detecting 1, 5-AG with good stability and a method in the field of medical detection. According to the kit disclosed by the invention, a reagent R1 and a reagent R2 are kept stable within an expiry date under the interference of high glucose concentration by selecting a proper method and a proper stabilizer. According to the kit, the content of 1, 5-AG in a humanserum sample is determined by adopting a pyranose oxidase method. The test result shows that the kit for detecting 1, 5-AG is high in glucose interference resistance compared with other kits sold on the market, can eliminate 1-30 mM / L of endogenous glucose interference, can be stored for 7 days stably under the 37 DEG C acceleration condition, has better acceleration stability than other 1,5-AG measurement kits on the market, has the advantages of high accuracy, high precision, wide linear range, good stability and the like, and can be widely used in the diagnosis and treatment of diabetes.
Owner:CO HEALTH BEIJING LAB
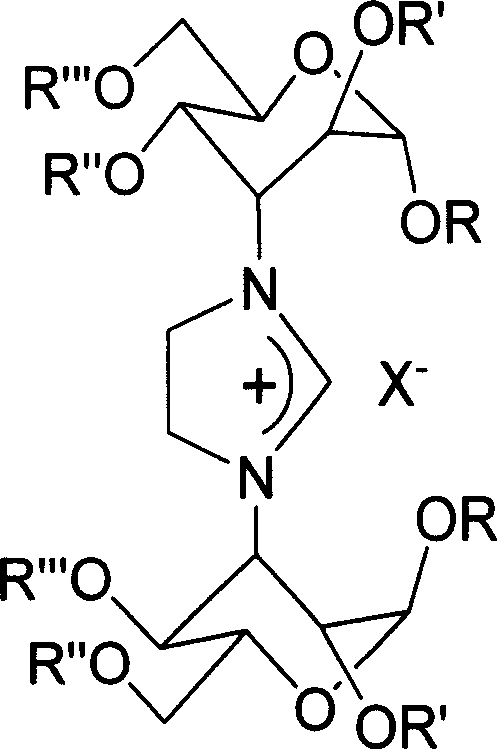
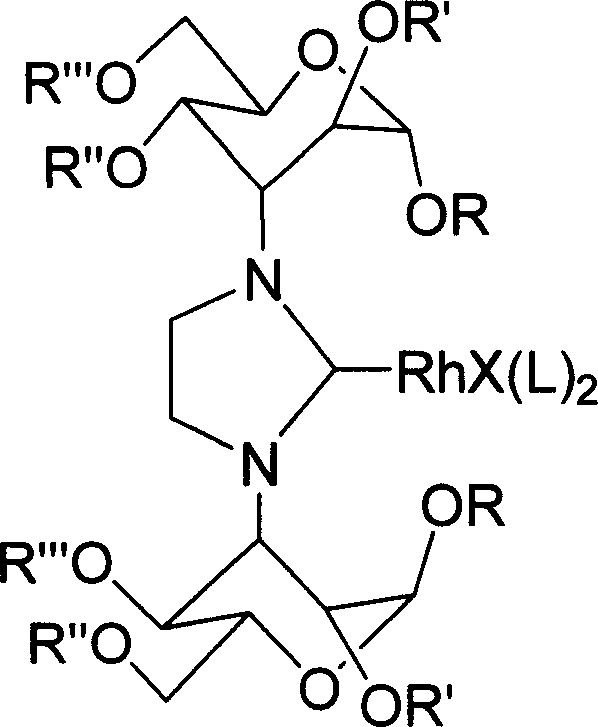
Imidazoline salt, rhodium complex of carbohydrate derivative and its preparation method
InactiveCN1995057ASugar derivativesGroup 8/9/10/18 element organic compoundsCarbohydrate derivativeDeoxygenation
The invention discloses a making method of rhodium complex compound of imidazoline and coordination of carbohydrate derivant, which comprises the following steps: reacting N,N'-di (methyl 3-deoxygenation-4, 6-O-benzyl crossing base-alpha-D-altronic pyran glycoside-3-base) ethanediamine and 40 ml ammonium chloride in the triethoxymethane for 3-4. 2h; cooling; filtering; washing through petroleum ether to obtain chiral imidazoline ligand; blending the ligand and [RhCl(COD)]2 and NaOtBu to react in the 20ml THF for 48h at 45 deg. c; filtering; purifying through column chromatography to obtain the product as high-property catalyst.
Owner:FUJIAN NORMAL UNIV
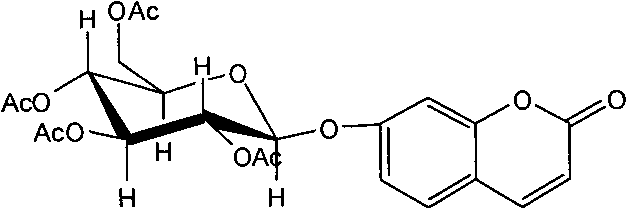
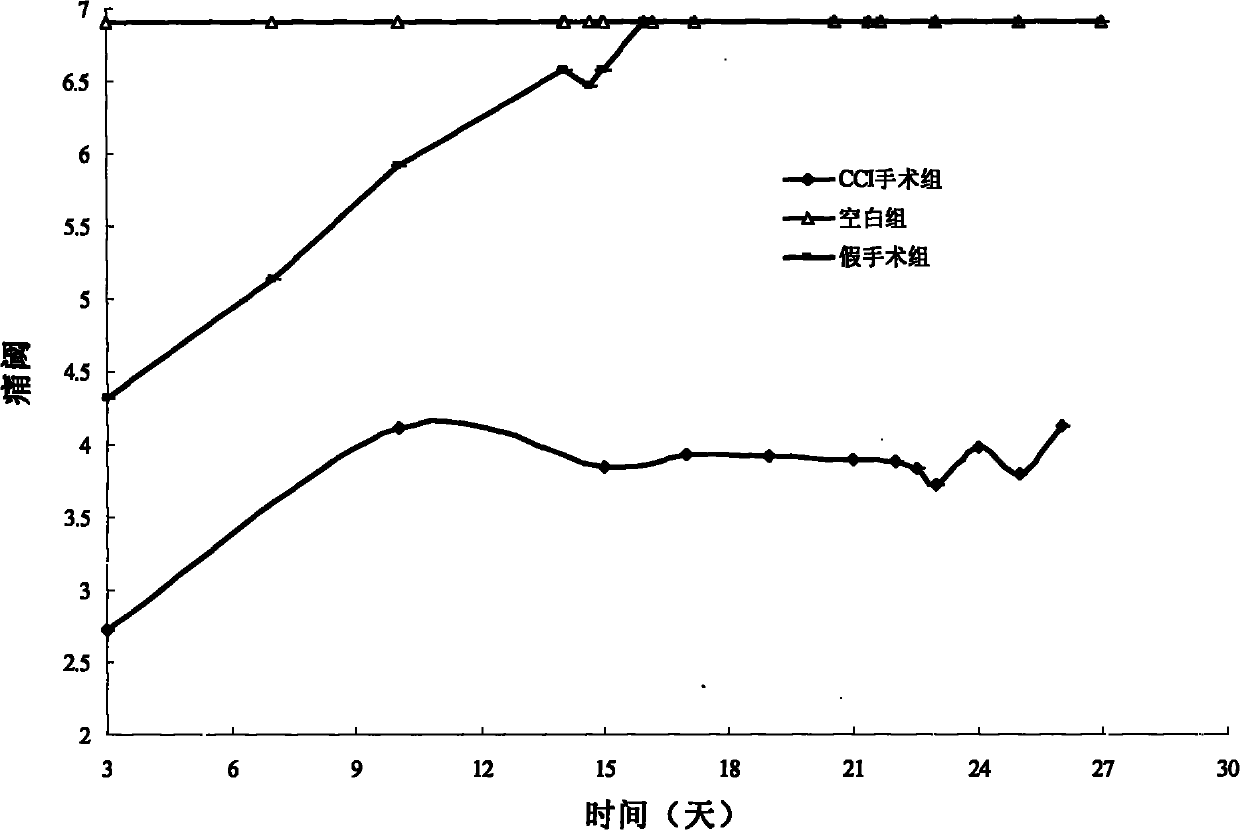
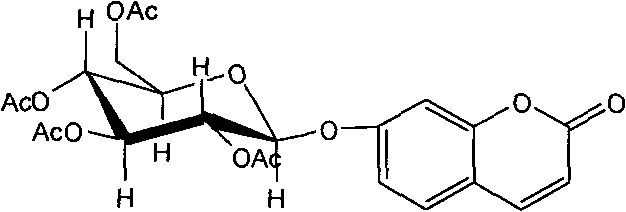
Application of 7-O-beta-D-acetylation sugar-coumarin compounds in treating chronic neuropathic pains
The invention relates to application of 7-O-beta-D-acetylation sugar-coumarin compounds in treating chronic neuropathic pains. The 7-O-beta-D-acetylation sugar-coumarin compounds comprise a pharmaceutically acceptable salt, ester or a solvate thereof, and the application relates to the application of the salt, the ester or the solvate in preparing medicaments for treating the chronic neuropathic pains, wherein a glycosyl part is arbitrary pyranose or furanose, the hydroxy of which is totally acetylated; a representative compound is 7-O-beta-D-acetylation glucose-coumarin; and the structural formula is disclosed in the specification. On a classical sciatic nerve chronic compression injury (CCI) model, through single intragastric administration and continuous intragastric administration for 7 days, the compound shows definite treatment effect of resisting nervous pains. At a dose point appointed by an experiment, the compound can obviously improve the mechanical stimulus pain threshold, the nervous pain resistant efficacy is equivalent to that of Gabapentin which is a contrast medicament, and the duration is superior to that of the Gabapentin. Proved by research results, the compound can be used for treating the chronic neuropathic pains.
Owner:YUNNAN UNIV
Dipyrano-quinolinones useful as anti viral agents and a process for preparing the same
InactiveUS6191279B1Good metabolic stabilityOrganic active ingredientsOrganic chemistryPyranoseEnantiomer
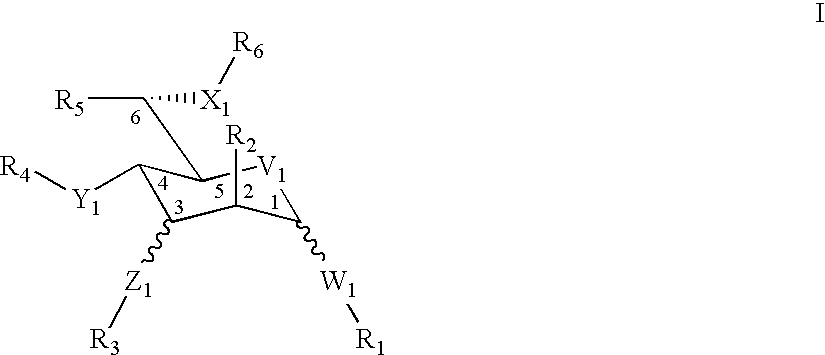
The invention relates to novel dipyrano-quinolinone class of compounds having the general formula:Wherein R is hydrogen, alkyl optionally substituted about C-1 to C-10 alkenyl optionally substituted about C-1 to C-10 with one or more double bounds, alkynyl optionally substituted about C-1 to C-10 with one or more triple bonds, aryl, hetero aryl, carbocyclic aryl, alkyl aryl, alcyclic compounds, C-1 to C-6 alkyl with terminal dialkyl amino group, thio alkyl, hydroxyl alkyl groups;R1 is H, lower dialkyl amino alkyls such as methyl, ethyl, propyl, and other alkyl groups or b-amino acid moieties, hydroxy alkyl groups having optionally substituted about C-1 to C-10 carbons, acid amides such as aliphatic acids, aromatic acids, sulphonic acids trihalo acids.x-x is either a carbon-carbon single bond or a carbon-carbon double bond;R2 and R3, R4 and R5 are each independently hydrogen and methyl there by resulting the cis and trans diastereomers as well as enantiomers;R4 and R5 are each independently hydrogen and methyl while R6 and R7 are each independently hydrogen and hydroxyl / -OR8, where R8 is independently alkyl, aryl alkyl, amino alkyl, hydroxy alkyl with C-1 to C-10 carbons, sugars which include mono saccharides both in the furanose form as well as pyranose form, amino sugars, disaccharides, amino acids, small peptides through lower alkyl spacer groups, thereby resulting the cis and trans diastereomers as well as enantiomers; and a process for producing the above novel dipyrano-quinolinone class of compounds.
Owner:COUNCIL OF SCI & IND RES
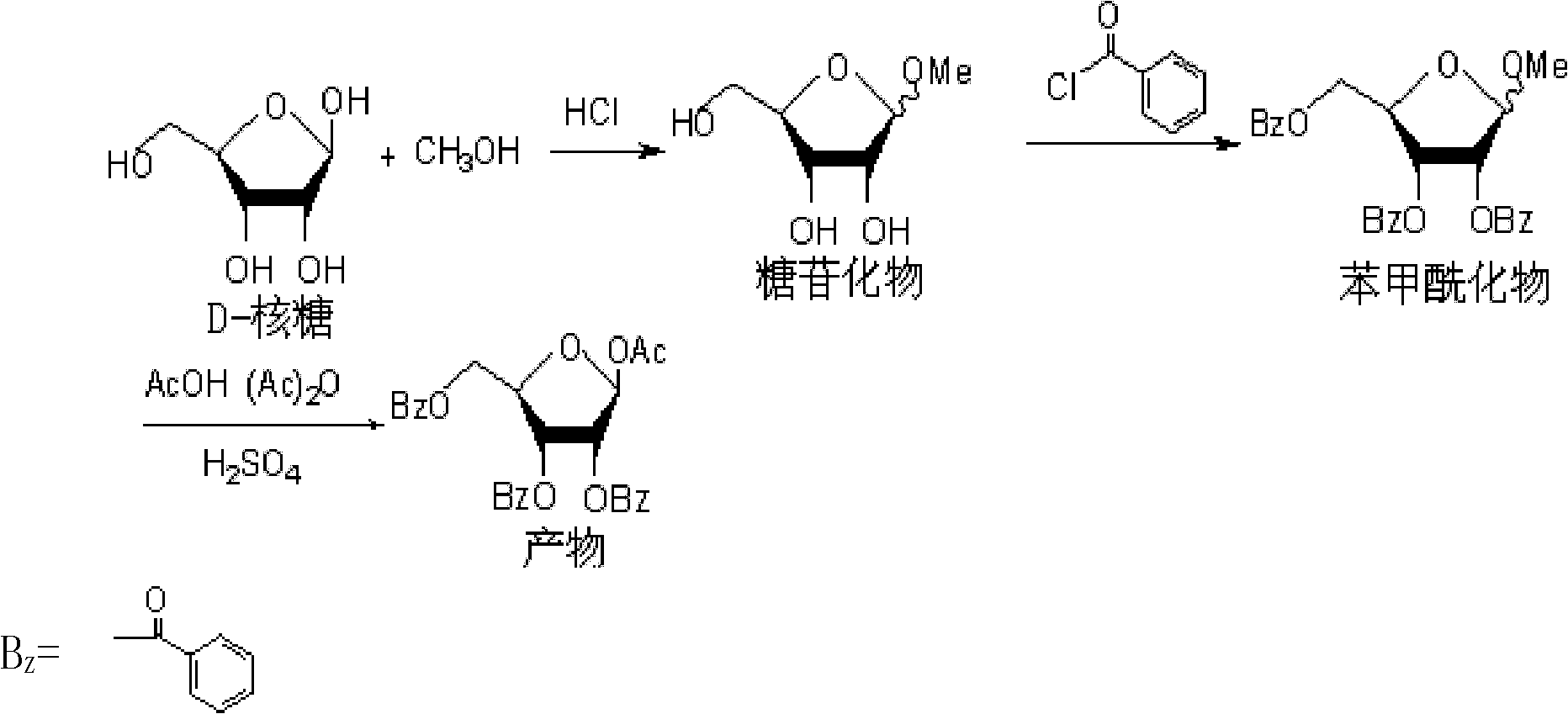
Preparation technology of 1-O-acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose
InactiveCN102659856ALow Nitrogenous Organic ContentImprove working environmentEsterified saccharide compoundsSugar derivativesPyranoseAcetic acid
The invention relates to the technical field of chemical synthesis, and especially relates to a preparation technology of 1-O-acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose, which comprises a glycosilation reaction, a benzoylation reaction and an acetylation reaction. According to the invention, the low temperature glycosilation reaction is carried out at 0-5DEG C, so the generation of a pyranose ring is effectively reduced; the benzoylation reaction is carried out through adopting an inorganic weak base, so the raw material cost, and contents of nitrogen-containing organic matters in process wastewater are reduced; a cosolvent is added to an acetylation reaction system, so the solidification of glacial acetic acid is effectively inhibited, the fluidity of the reaction system is improved, and stirring can fully perform effects, and is in favor of smooth carrying-out of the reaction. The whole preparation technology which has the advantages of simple operation, high product yield, low cost, and low contents of the nitrogen-containing organic matters in the wastewater is suitable for industrialized production.
Owner:LIVZON GROUP CHANGZHOU KONY PHARMA
Polarizer protective film, manufacturing method thereof, polarizing plate and liquid crystal display
InactiveUS20070254115A1Reduce distortionSlow changeLiquid crystal compositionsPolarising elementsPyranoseCellulose
A polarizer protective film comprising: a cellulose ester; a saccharide ester having an ester of compound (A), the compound (A) having one furanose structure or one pyranose structure in the molecule, wherein all or a part of OH groups in the compound (A) are esterified, or an ester of compound (B), the compound (B) having two to twelve of at least one of a furanose structure and a pyranose structure bonded in the molecule, wherein all or a part of OH groups in the compound (B) are esterified; and a compound represented by Formula (R) or (Ra):
Owner:KONICA MINOLTA OPTO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com