New monoterpene compounds and preparation method thereof
A technology of compound and monoterpene, which is applied in the field of new monoterpene compound and its preparation, to achieve good analgesic effect, less environmental pollution, and a single conversion product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
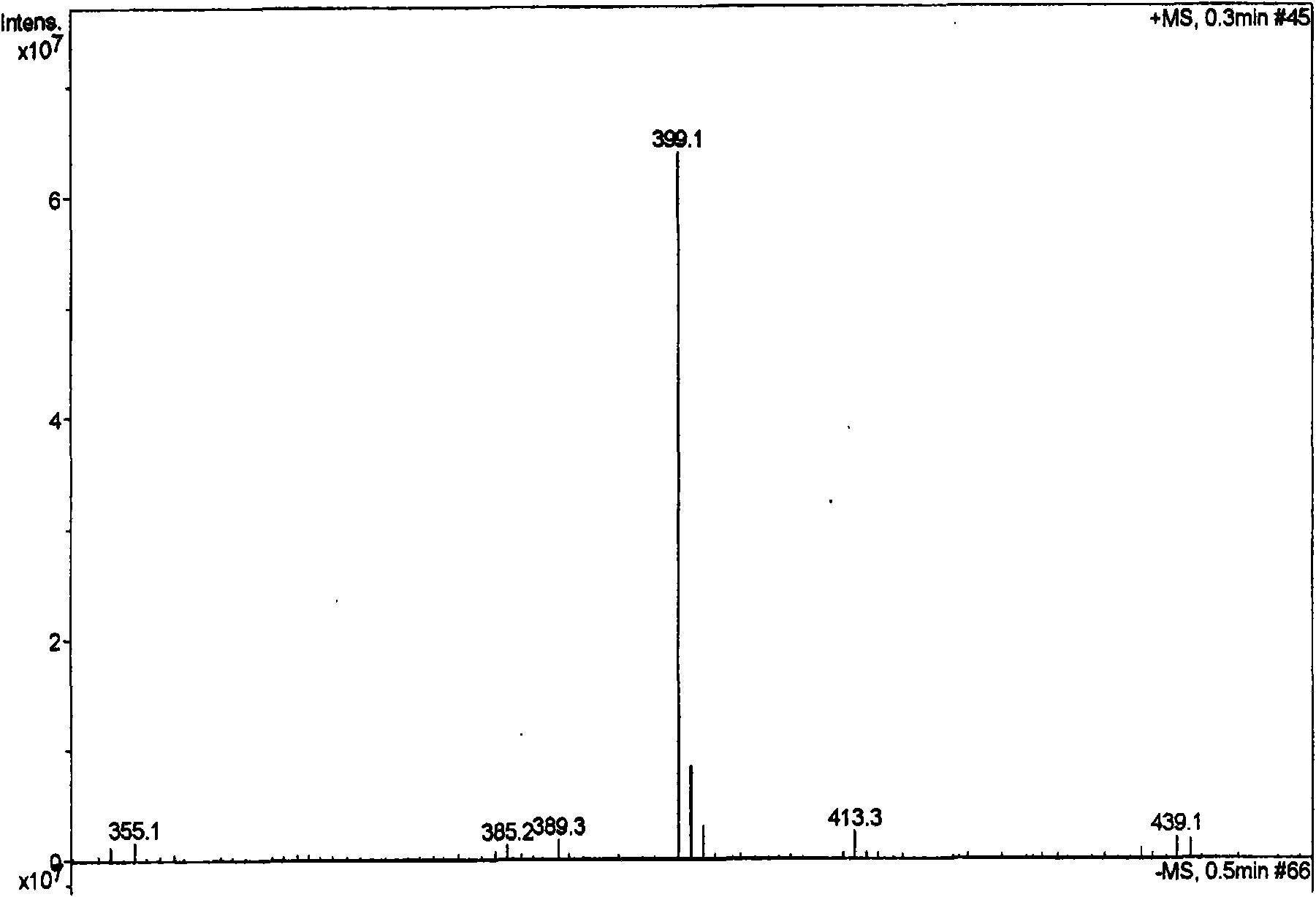
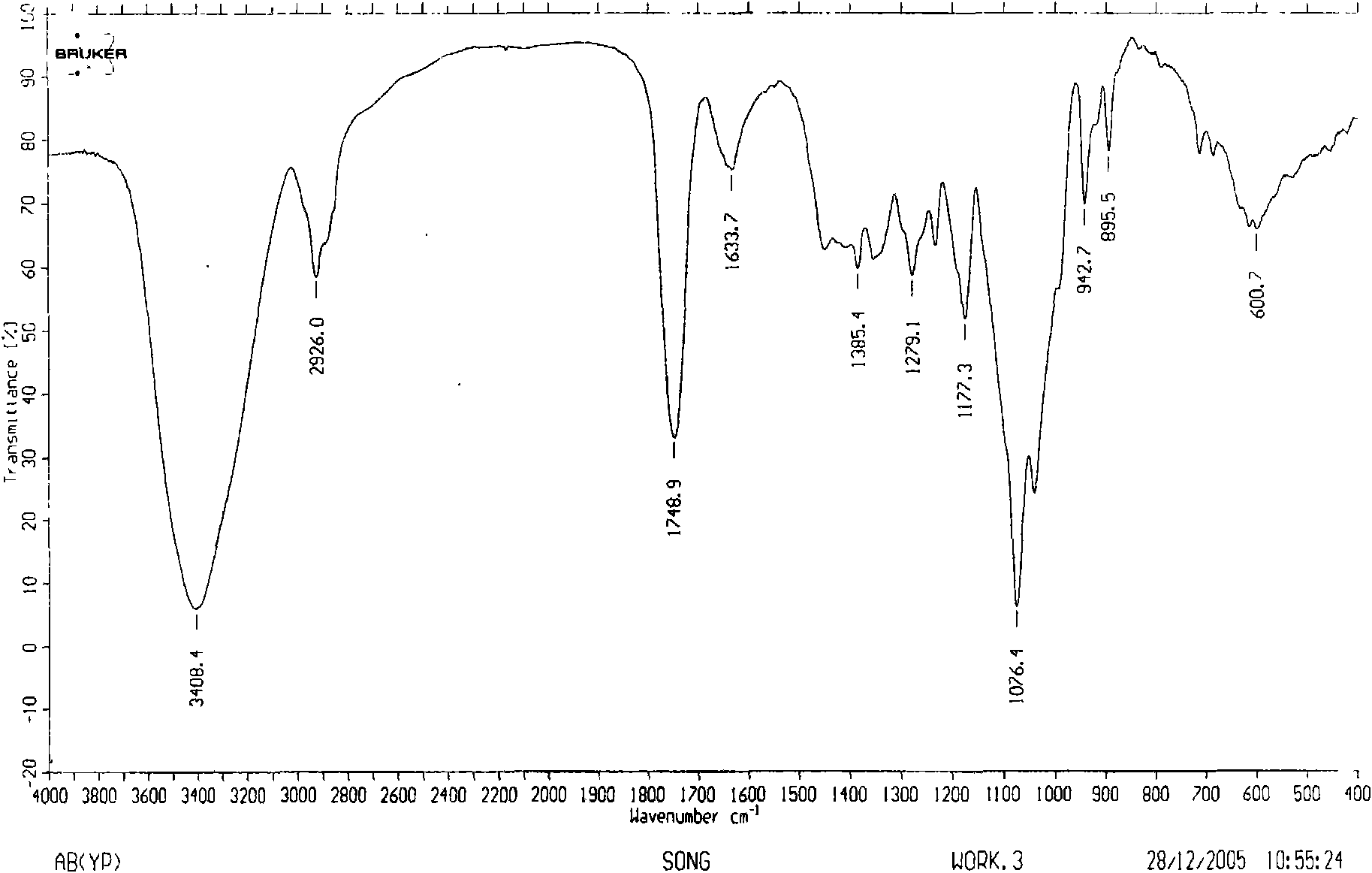
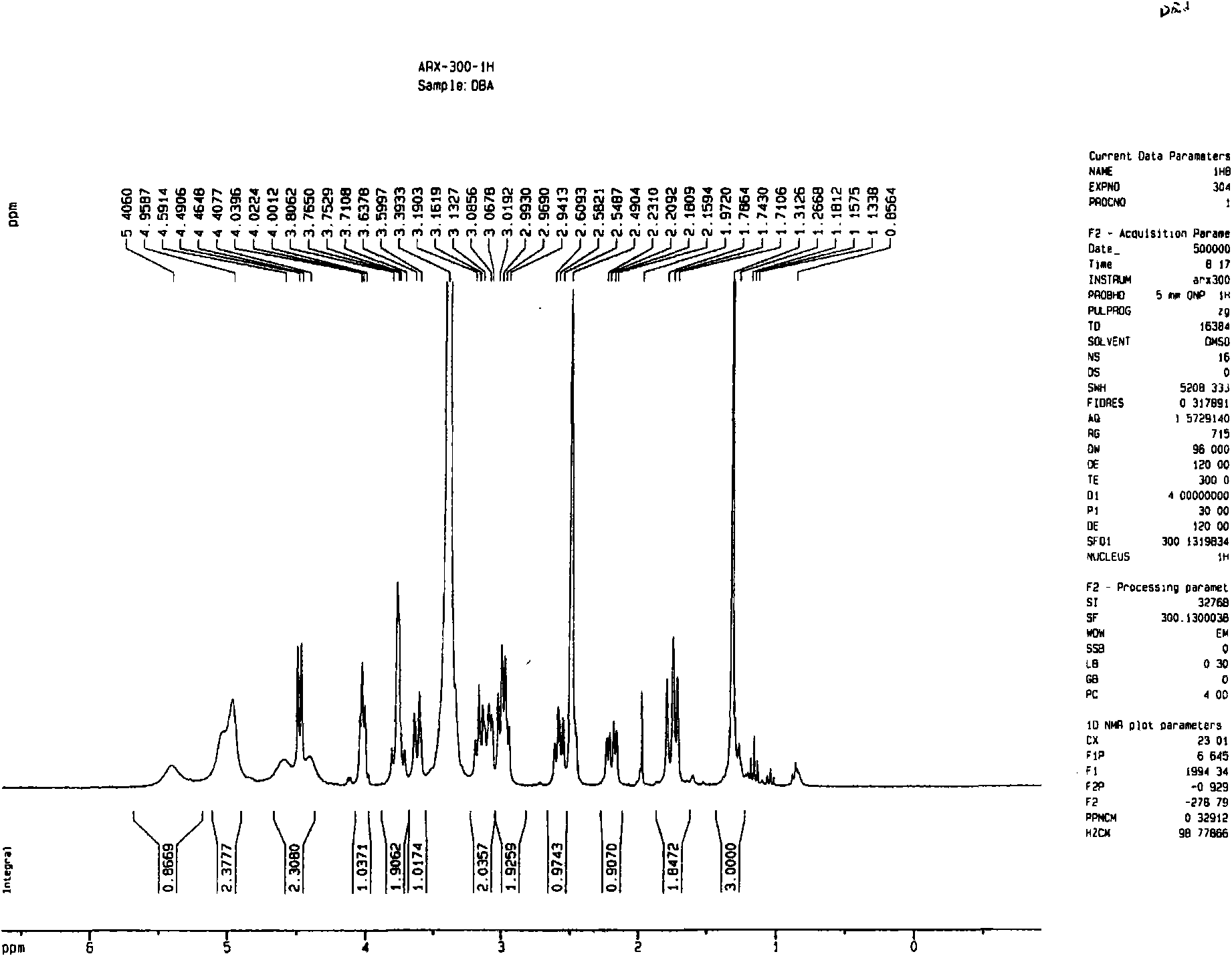
Image
Examples
experiment example 1
[0042] 1. Analgesic effect of compounds I and II--rat writhing experiment
[0043] Experimental method: 50 male rats were taken and randomly divided into blank control group, positive control group, paeony glucoside injection group (100mg / kg), compound I group (100mg / kg) and compound II group (100mg / kg ), 10 animals in each group. Respectively tail vein injection of physiological saline, positive drug (Kanglaite injection), paeony glucoside injection, compound I salt solution and compound II salt solution, after 30 minutes of administration, intraperitoneal injection of 1% acetic acid (0.5ml / only), observe and record the number of writhing times in the two time periods of 0-15 minutes and 15-30 minutes after injection of acetic acid. As shown in Table 1, compounds I and II can significantly reduce the number of writhing reactions in rats, and have slightly stronger analgesic effects than paeony glucoside injection. There was also a significant difference between the positiv...
Embodiment 2
[0057] Dried herba peony slices 1Kg, boiled with water to extract twice, each time for 2 hours, the extract was filtered and passed through a reverse adsorption resin column, and then eluted with alcohol-water solution (50%), and the eluent was concentrated and then layered with alumina column Analysis was carried out to obtain 12.5 g of extract rich in pinane monoterpene glycosides (the content of paeonifloride glycosides detected by liquid phase was 42.2%). 5 g of the pinane-like monoterpene glycoside extract was dissolved and added to the fermentation broth of the bacteria [Penicillium melini 3.4474 (xxl)] that had been cultured for 48 hours, and the shake flask was removed after continuing to cultivate for 72 hours. The fermentation broth was suction filtered to remove the mycelium, the filtrate was concentrated under reduced pressure to a small volume, extracted three times with an equal amount of ethyl acetate, the extracts were combined, concentrated under reduced pressu...
Embodiment 3
[0059] 500 mg of compound I was dissolved and added to the fermentation broth of the thallus [Cocephala racemosa 3.264 (xxl)] that had been cultured for 48 hours, and the shake flask was removed after continuing to cultivate for 72 hours. The fermentation broth was suction filtered to remove the mycelium, the filtrate was concentrated under reduced pressure to a small volume, extracted three times with an equal amount of ethyl acetate, the extracts were combined, concentrated under reduced pressure, and dried in vacuo to obtain 600 mg of coarse powder. Dissolve the coarse powder with methanol, stir in 1.8g of 100-140 mesh silica gel, evaporate the solvent until dry, and wait for sample loading. Take 24g of 200-300 mesh silica gel, wet-pack the column, and then add the mixed sample silica gel for column chromatography. Using dichloromethane:methanol (30:1) as the eluent, collected in sections, detected by TLC, and combined single spots to obtain 200 mg of white powder (yield 70...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com