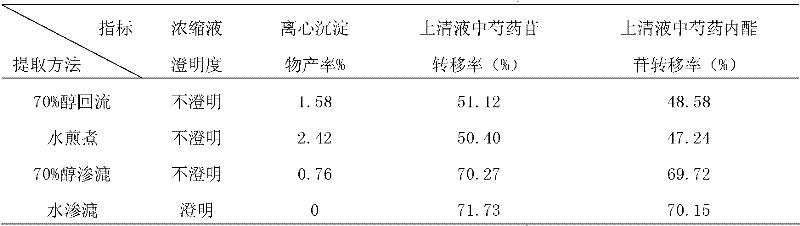
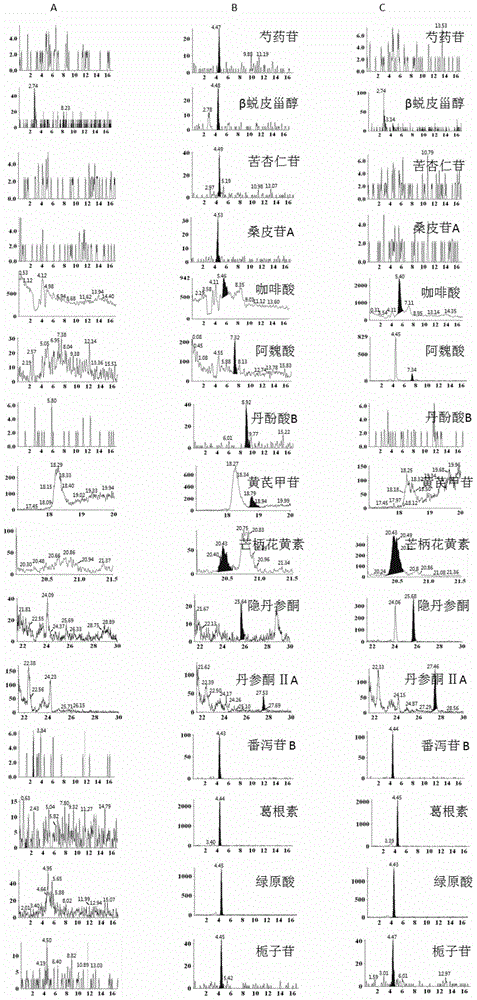
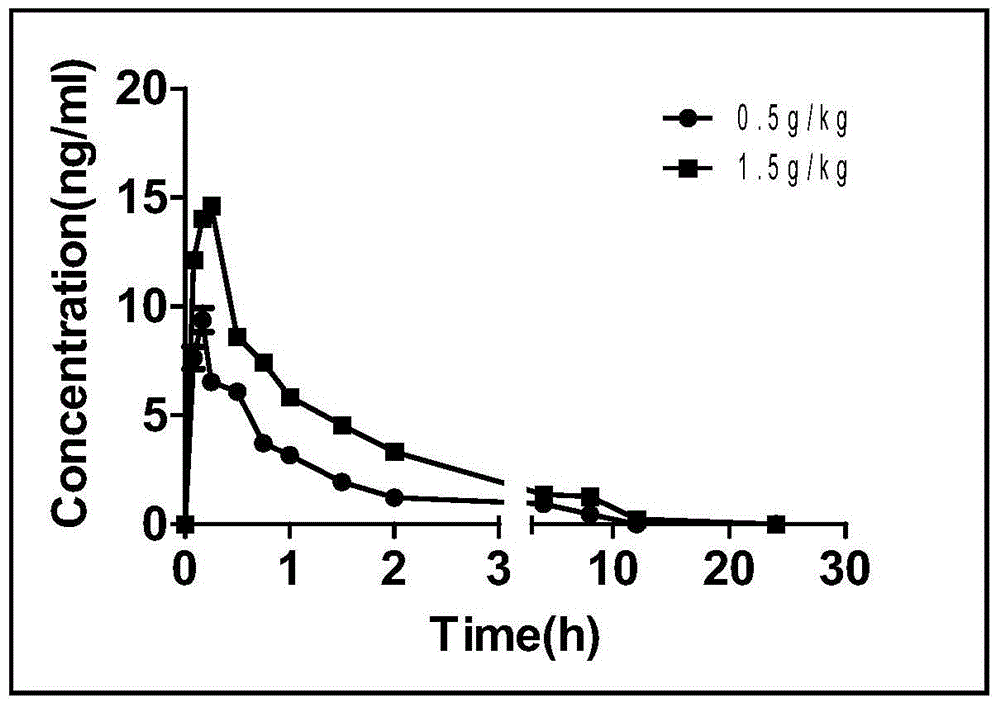
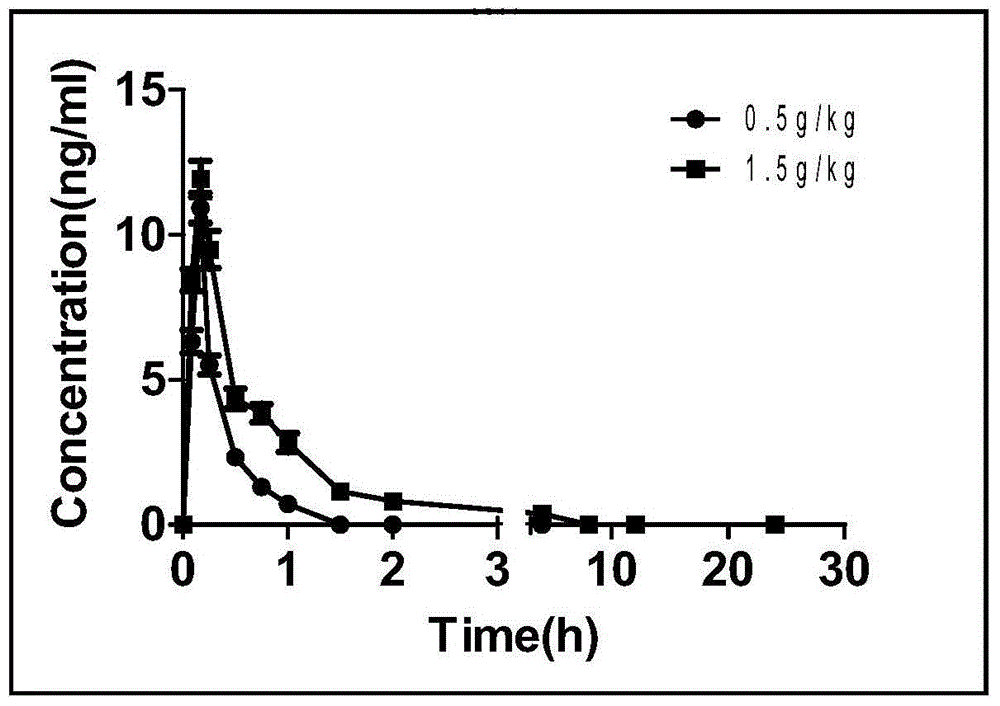
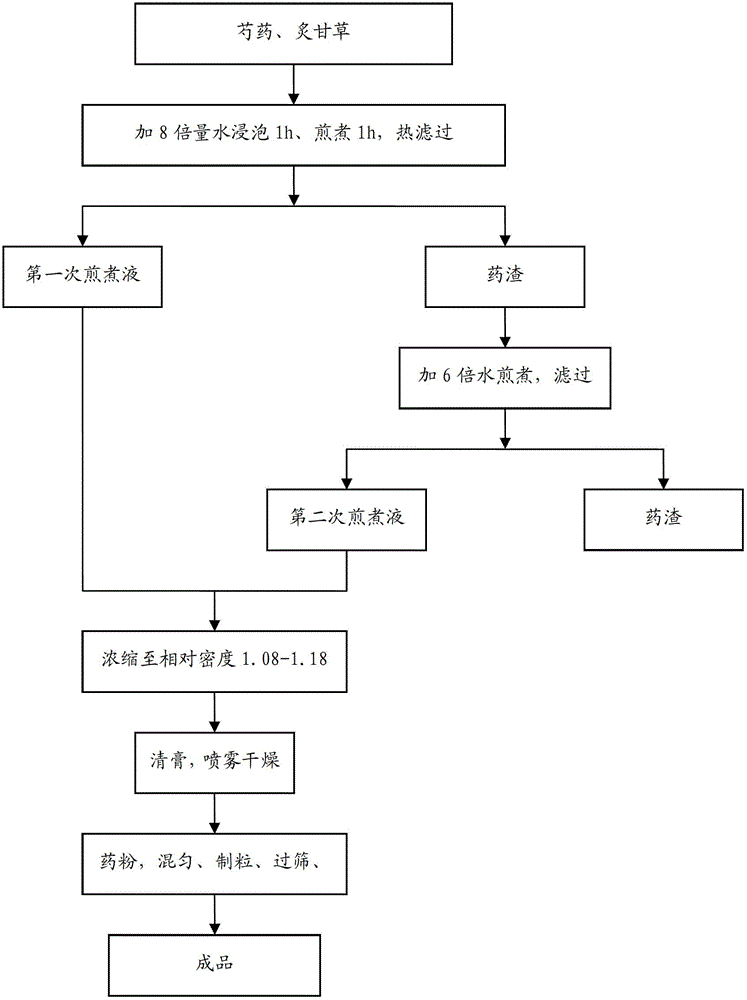
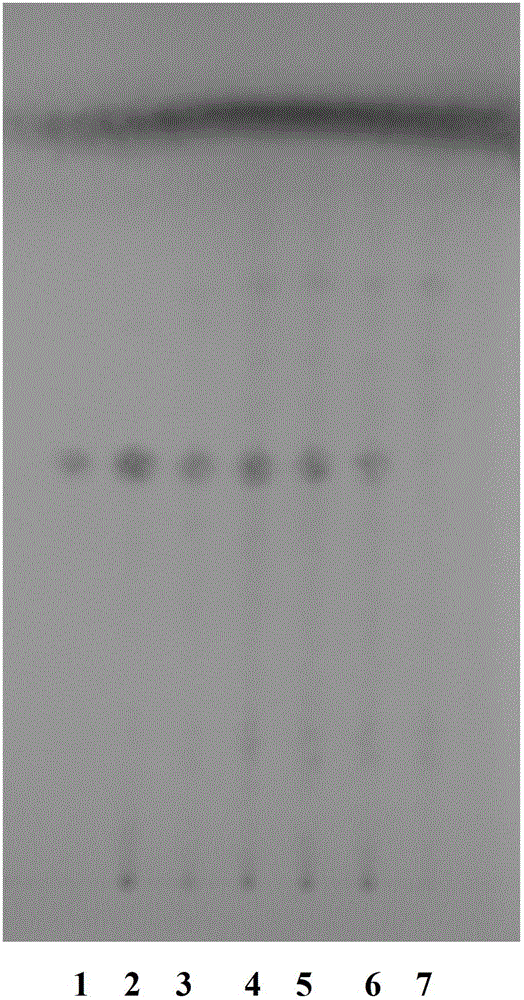
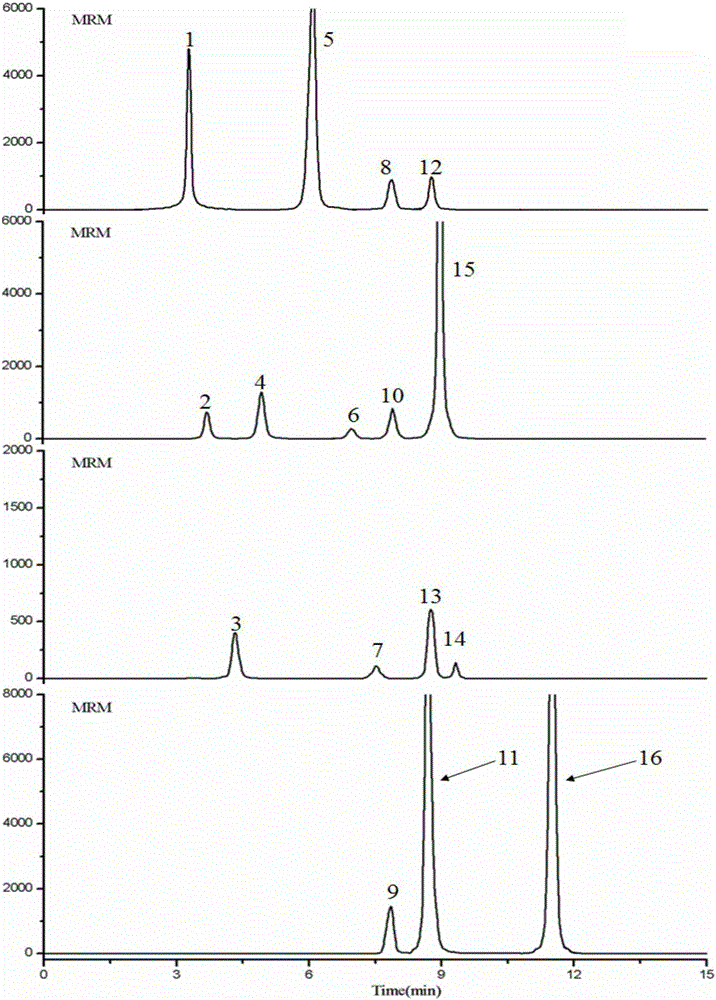
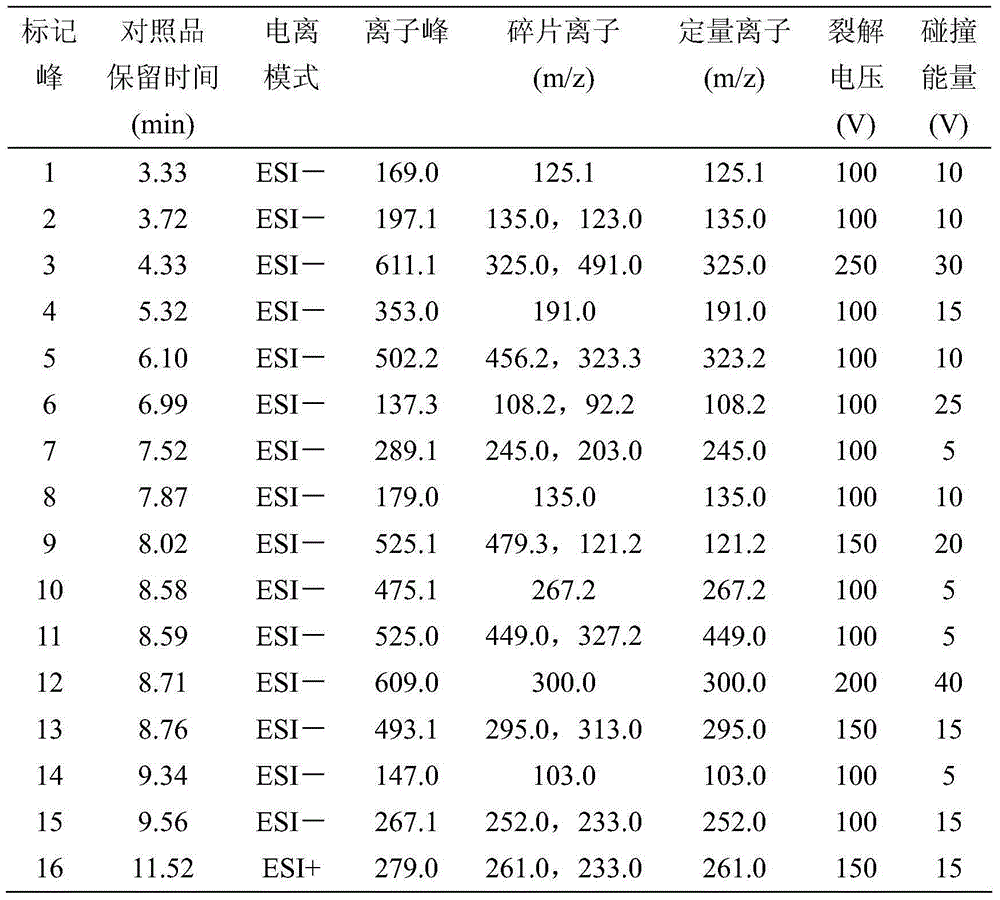
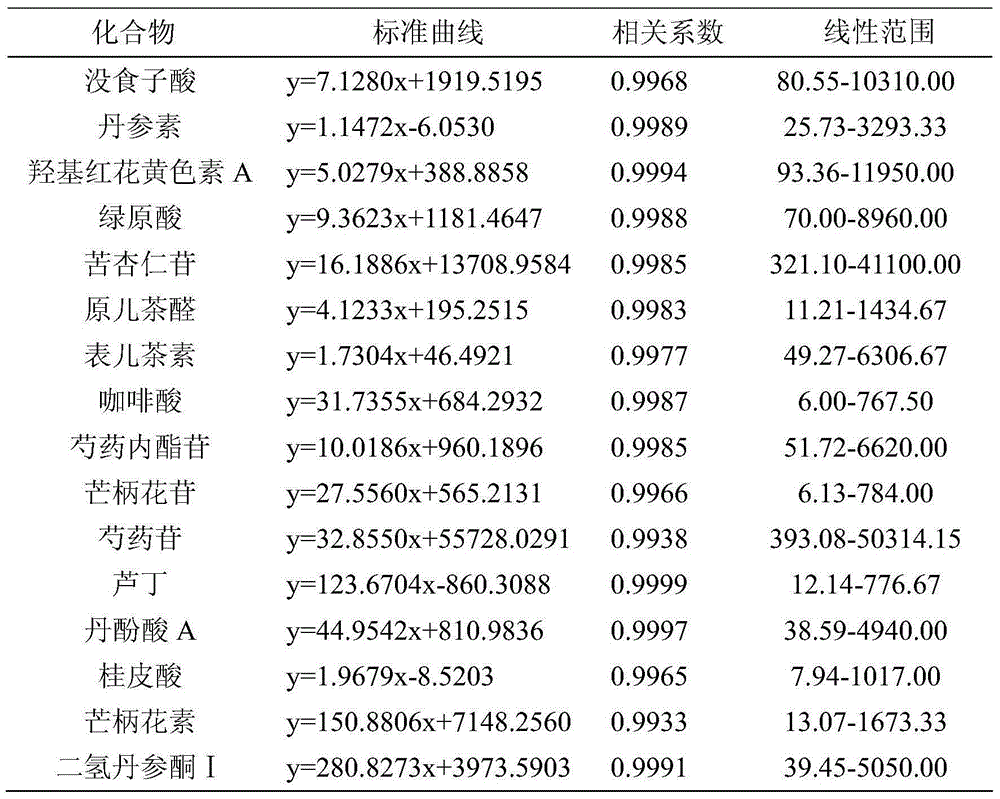
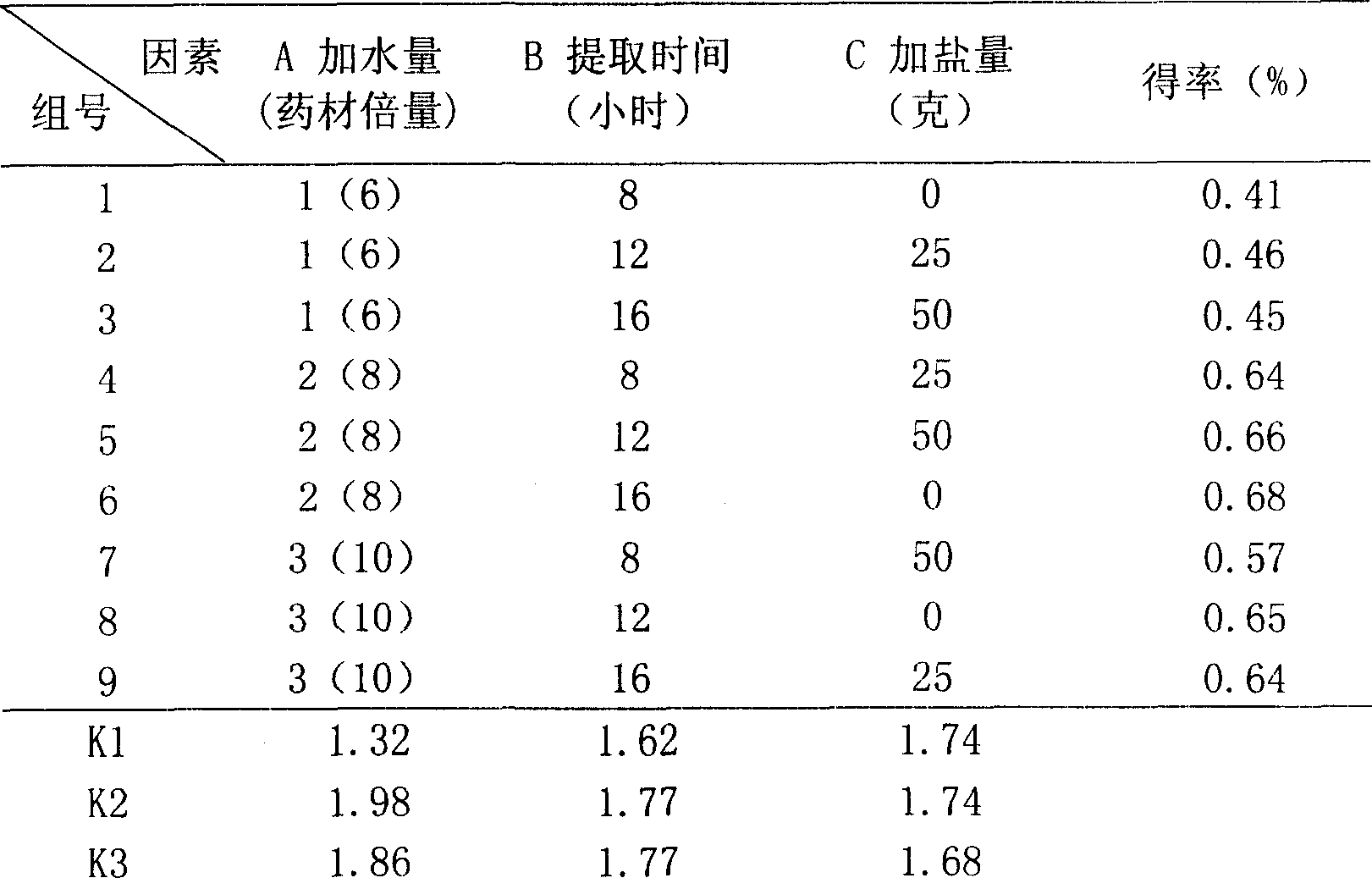
Patents
Literature
642 results about "Paeoniflorin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Paeoniflorin is a chemical compound which is one of the major constituents of an herbal medicine derived from Paeonia lactiflora. It can also be isolated from the fresh water fern Salvinia molesta. In Paeonia, it can form new compounds with addition of phenolic substituents. In a study in female rats, paeoniflorin was found to inhibit the production of testosterone within the ovaries by promoting the activity of aromatase. In mice, paeoniflorin was shown to protect against neuroinflammation and depression-like behavior induced by IFN alpha.
Extract of total glucosides of paeony and the preparing method thereof
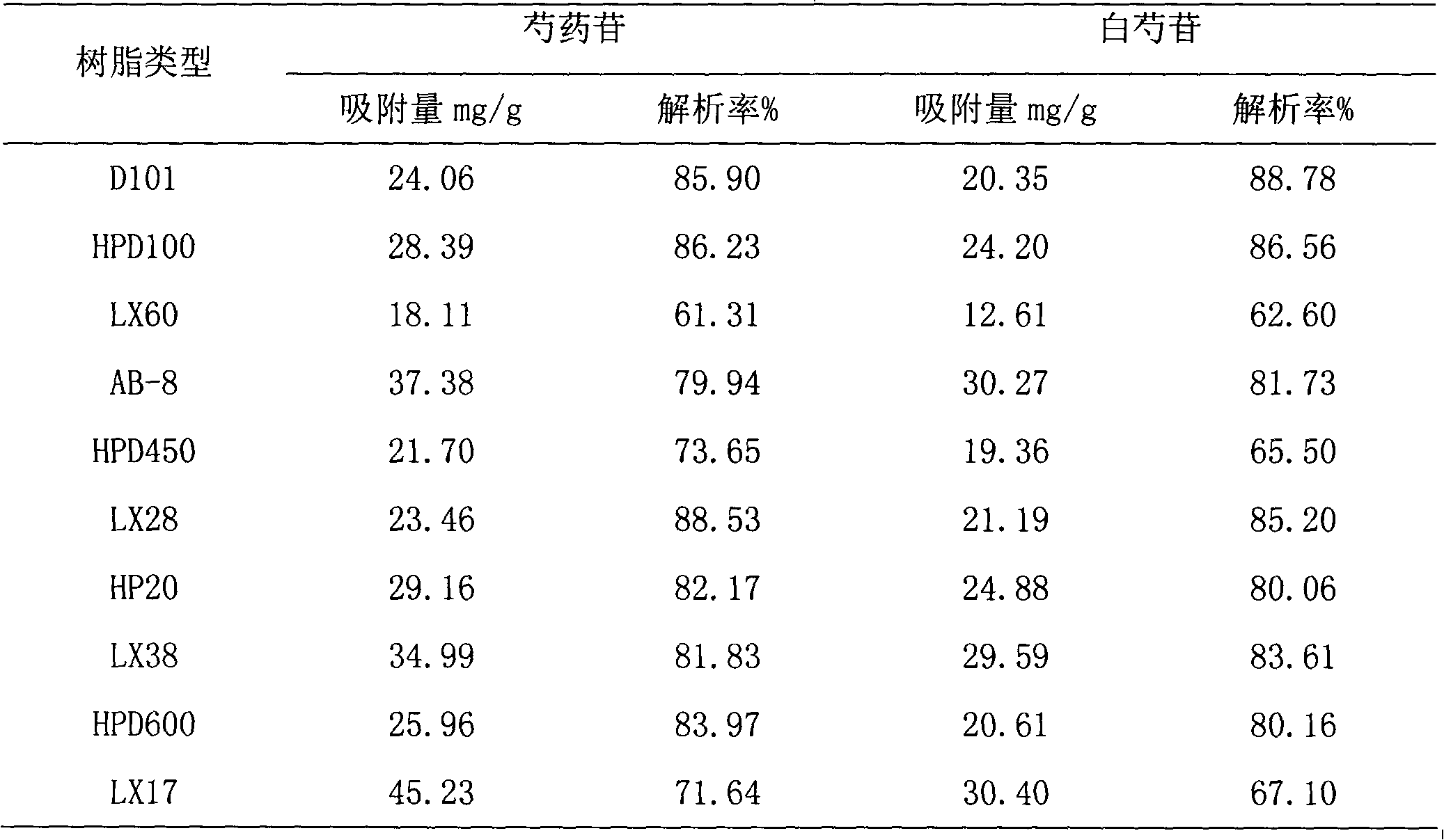
The invention discloses a peony total glycosides extract from traditional medicine white peony root or red peony root and preparing method, which is characterized by the following: comprising peony glycosides, white peony glycosides, peony new glycosides, peony inner ester A, B, C, oxidation peony glycosides, benzoyl peony glycosides, derivant and so on; choosing one or several methods from solvent extraction, solvent extraction process, macroreticular absorption resin method, column chromatography, supercritical fluid chromatography, liquid-liquid counter-current partition chromatography and so on; producing extract; setting sum of various terpene glycosides percentage content at 5-100%(w / w); setting content of peony glycosides at 5-100%(w / w).
Owner:石任兵 +1
Quality standard of Moluo Dan.and detecting method thereof
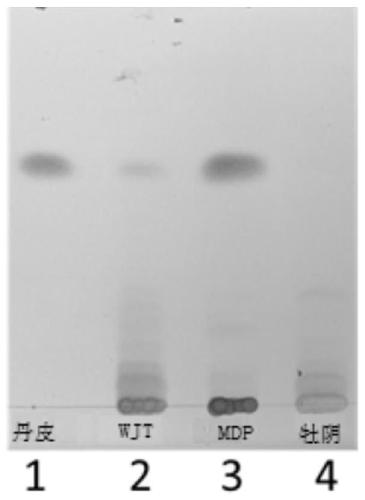
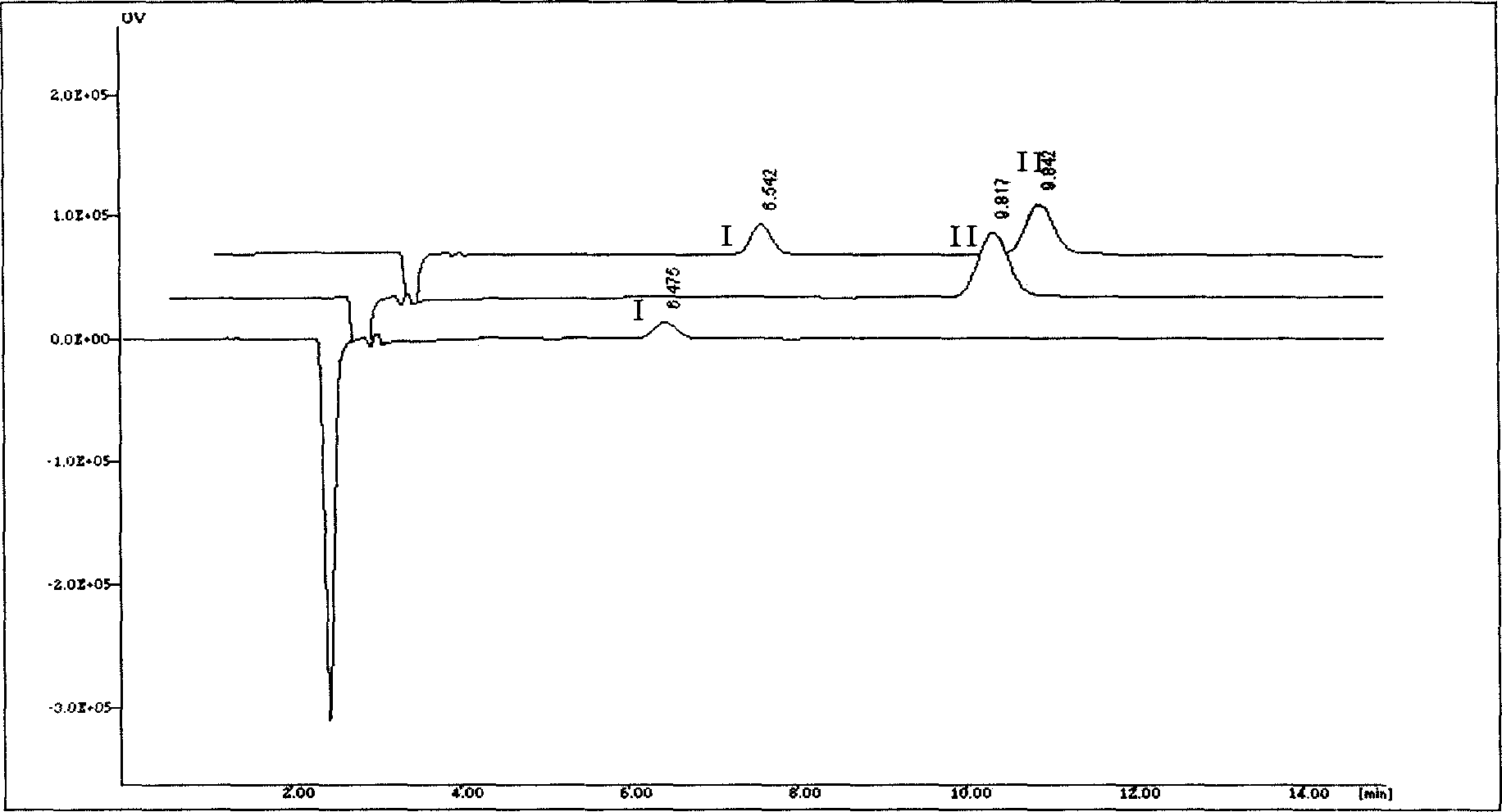
ActiveCN101109733AGood linear relationshipImprove accuracyMaterial analysis by observing effect on chemical indicatorComponent separationOphiopogon japonicusThin layer
The invention discloses a method for controlling the quality of Moluo Dan, which comprises the discrimination method for thin layer of Ophiopogon japonicus, pseudo-ginseng, Sanguisorba officinalis, angelica sinensis, Ligusticum wallichii and corydalis tuber, as well as the content determining of paeoniflorin (C23H28O11) in white Chinese herbaceous peony. The white Chinese herbaceous peony contained in each 1g of Moluo Dan is expressed by C23H28O11, which shall not be less than 0.8 mg.
Owner:HANDAN PHARMA
Plant source bacteriostasis and detoxification deodorant and preparation method and application of plant source bacteriostasis and detoxification deodorant
InactiveCN103168803ASpeed up conversionGood antibacterial effectBiocideDispersed particle separationHordeum vulgareCapsaicin
The invention provides a plant source bacteriostasis and detoxification deodorant. The plant source bacteriostasis and detoxification deodorant is prepared by the following plant materials in parts by weight: 10-15 parts of tobacco stems, 12-20 parts of Ilex latifolia Thunb stems, 2-8 parts of Helianthus tuberosus Linn leaves, 5-15 parts of ginkgo biloba, 8-16 parts of capsaicin, 1-5 parts of tanshinone, 15-25 parts of orange peel, 2-8 parts of paeoniflorin, 1-5 parts of tea seed, 8-15 parts of ginger stems, 15-35 parts of wild chrysanthemum flower, 5-20 parts of garlic, 5-15 parts of ginger, 20-35 parts of sophora alopecuroides, 5-10 parts of pine needle oil, 15-30 parts of artemisia annua, 20-50 parts of green beans, 30-60 parts of barley and 20-35 parts of corn. The plant source bacteriostasis and detoxification deodorant provided by the invention has the advantages of high biological activity, strong broad-spectrum antibacterial property, fast insect and egg killing, good environment-friendly property, safety, high efficiency and the like. The invention also provides a preparation method and application of the plant source bacteriostasis and detoxification deodorant.
Owner:陈士进 +2
Composition of paeoniflorin and albiflorin and its preparation method
A composition of paeoniflorin and albiflorin is prepared from peony through extracting in water to obtain aqueous solution, resin adsorption, alcohol eluting, concentrating, and chromatography by alumina column and silica gel column. It can be used to prepare to medicines in different forms.
Owner:SHENYANG PHARMA UNIVERSITY
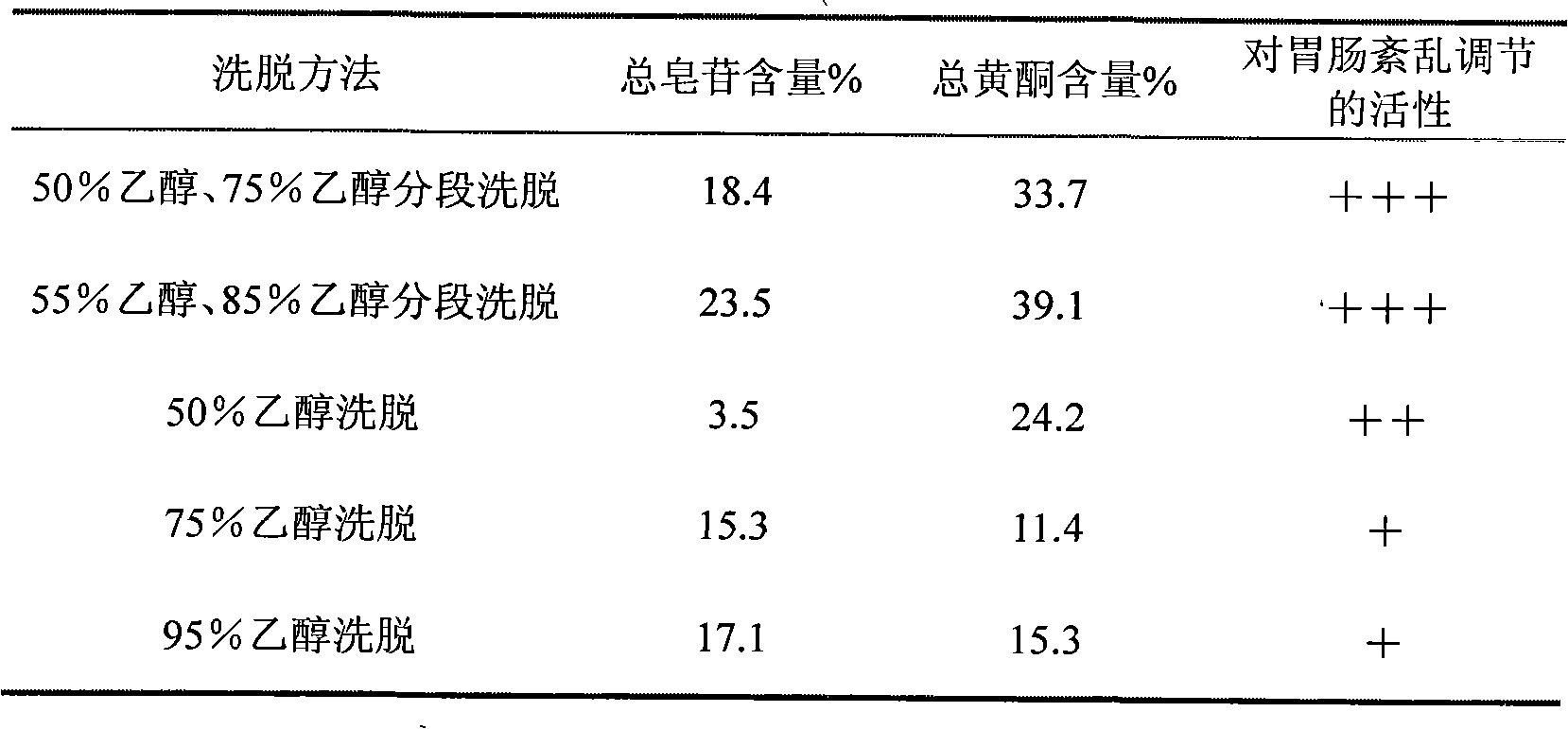
Composition for curing gastrointestinal functional disorders, preparation method thereof and application thereof in preparing drugs for curing gastrointestinal functional disorders
The invention relates to a composition for curing gastrointestinal functional disorders, a preparation method thereof and an application thereof in preparing drugs for curing the gastrointestinal functional disorders. The bulk drugs of the composition comprise radix bupleuri, radix glycyrrhizae preparata, fruit of immature citron and white paeony root, which follow a weight proportion of 6:6:6:6-9. The composition comprises the following components by weight percent: 24.5-46.5% of total flavonoids, 10.5-31.6% of total saporins, 17.0-35.4% of naringin, 1.1-8.5% of glycyrrhizic acid and 3.2-13.7% of paeoniflorin. The preparation method is as follows: condensing water solution of the bulk drugs; using macroporous absorption resin for absorption, continuing to wash till the state of colorlessness after the water solution completely passes through a resin column; using 50-85% by weight of ethanol for elution, and collecting eluent; reducing the pressure of solvent, recovering the solvent, drying the solvent, and finally obtaining the composition. Compared with decoction and western medicines, the extract of the composition achieves better and more obvious effect in curing the gastrointestinal functional disorders.
Owner:山东花王粮油集团有限公司
Glycosides compound monomer in Chinese herbaceous peony, and method for extracting and separating same
InactiveCN1420122AMeet purity requirementsSimple methodSugar derivativesSugar derivatives preparationAlcoholPaeoniflorin
A paeoniflorin monomer as a novel medicine is prepared from white (or red) peony through swelling in aqueous solution of alcohol, thermal reflux, concentrating, ultrasonic extracting in acetone solution, concentrating, mixing with gum powder, concentrating, vacuum chromatography, eluting, concentrating, separating by silica gel column, eluting and distribution chromatography. Its advantages are simple process, low cost and high yield.
Owner:SHANGHAI INNOVATIVE RESEARCH CENTER OF TRADITIONAL CHINESE MEDICINE
Method for establishing fingerprint spectrum of liver-enhancing medicine
ActiveCN102539553AEfficient separationEffective characterizationComponent separationColumn temperatureGradient elution
The invention provides a method for establishing a fingerprint spectrum of a liver-enhancing medicine. The liver-enhancing medicine is composed of oriental wormwood, isatis root, angelica, white paeony root, red-rooted salvia root, Radix curcumae, Astragalus mongholicus, Codonopsis pilosula, rhizoma alismatis, sealwort, rehmannia, yam, hawthorn, large-leaved gentian, liquorice and medicated leaven. The establishing method comprises the step of detecting paeoniflorin in the liver-enhancing medicine by using a high efficiency liquid chromatography method, wherein conditions are as follows: a chromatographic column takes octadecylsilane chemically bonded silica as a filling material; mobile phases comprise a mobile phase A which is acetonitrile and a mobile phase B which is an acidic water solution, and the mobile phases are subjected to gradient elution; the flow velocity is 1.0mL / min; the column temperature is 30 DEG C; the detection wavelength is 210nm to 400nm; and the number of theoretical plates is calculated according to the paeoniflorin peak and should not be less than 6000. The obtained fingerprint spectrum is a chromatographic peak which mainly takes active ingredients of the large-leaved gentian, the white paeony root, the liquorice, the red-rooted salvia root, the oriental wormwood and the astragalus mongholicus in raw materials of the liver-enhancing medicine as main parts. According to the establishing method provided by the invention, the fingerprint spectrum capable of comprehensively representing the active ingredients of the liver-enhancing medicine can be effectively obtained and has the characteristics of high precision, stability and repeatability. The obtained fingerprint spectrum can be used for guaranteeing the stability and consistency of the product quality, so that the safety and effectiveness of a product are guaranteed.
Owner:SHIJIAZHUANG DONGFANG PHARMA
Quality detection method of traditional Chinese medicine preparation
ActiveCN111044624AReasonable quality inspectionEasy to separateComponent separationBiotechnologyGallic acid ester
The invention relates to a quality detection method of a traditional Chinese medicine preparation. The method comprises the following steps: respectively taking gallic acid, albiflorin, paeoniflorin,ferulic acid, liquiritin, beta-ecdysterone, senkyunolide I, glycyrrhizin, cinnamic acid, cinnamyl aldehyde, paeonol, ammonium glycyrrhizinate and ligustilide as reference substances, and establishinga fingerprint spectrum of the traditional Chinese medicine preparation by adopting high performance liquid chromatography; identifying Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba,moutan bark, ginseng, cassia bark, licorice root and the root of bidentate achyranthes of the traditional Chinese medicine preparation by adopting thin-layer chromatography. The traditional Chinese medicine preparation is prepared by using the following raw materials: Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba, cassia bark, moutan bark, zedoray rhizome, ginseng, licorice root and the root of bidentate achyranthes. With the two detection ways, comprehensive quality evaluation can be conducted on the meridian-warming decoction traditional Chinese medicine preparation. Themethod is simple, convenient, high in accuracy and high in reproducibility; a scientific basis can be provided for quality detection and evaluation of the meridian-warming decoction traditional Chinese medicine preparation, and the product quality is effectively controlled.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
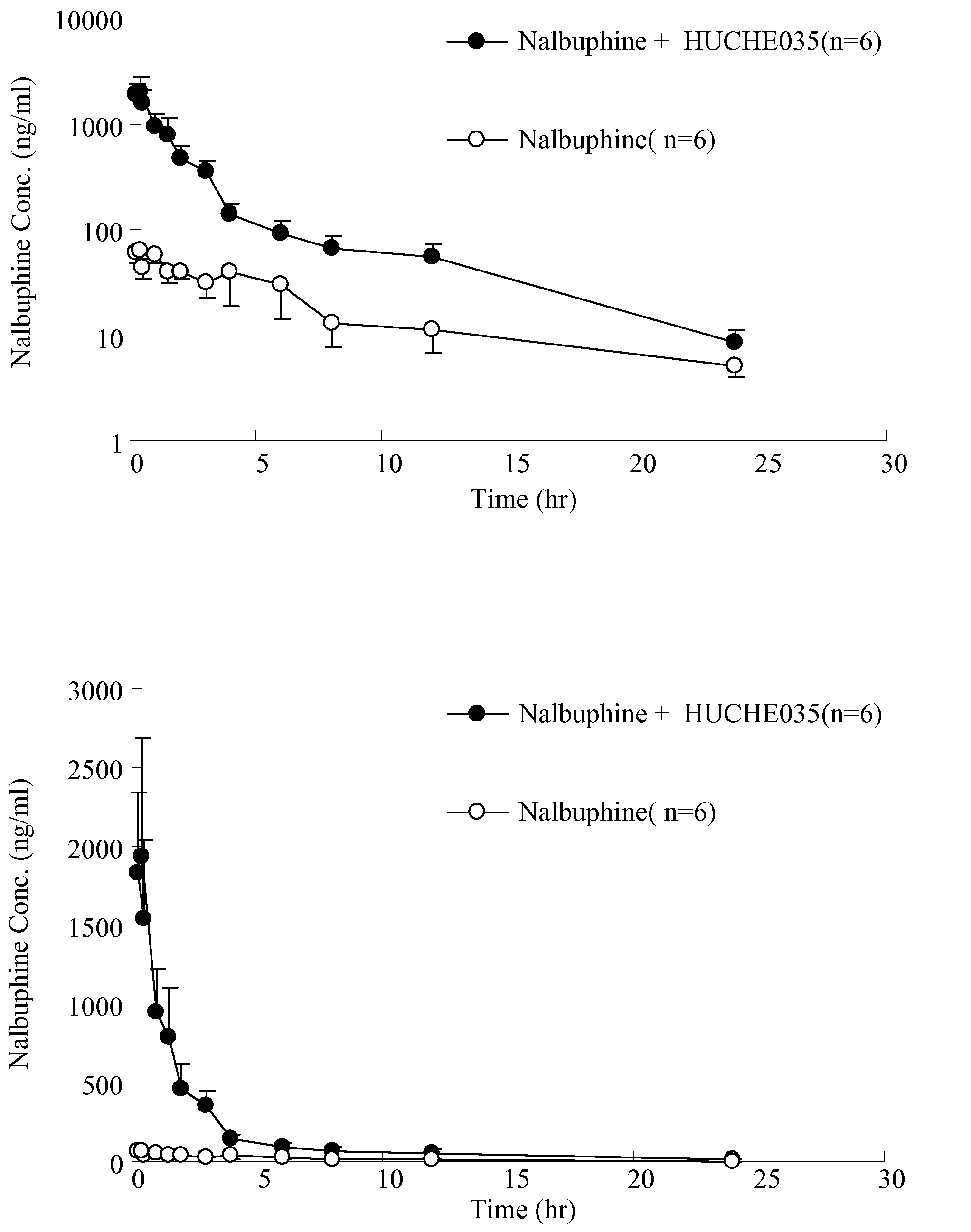
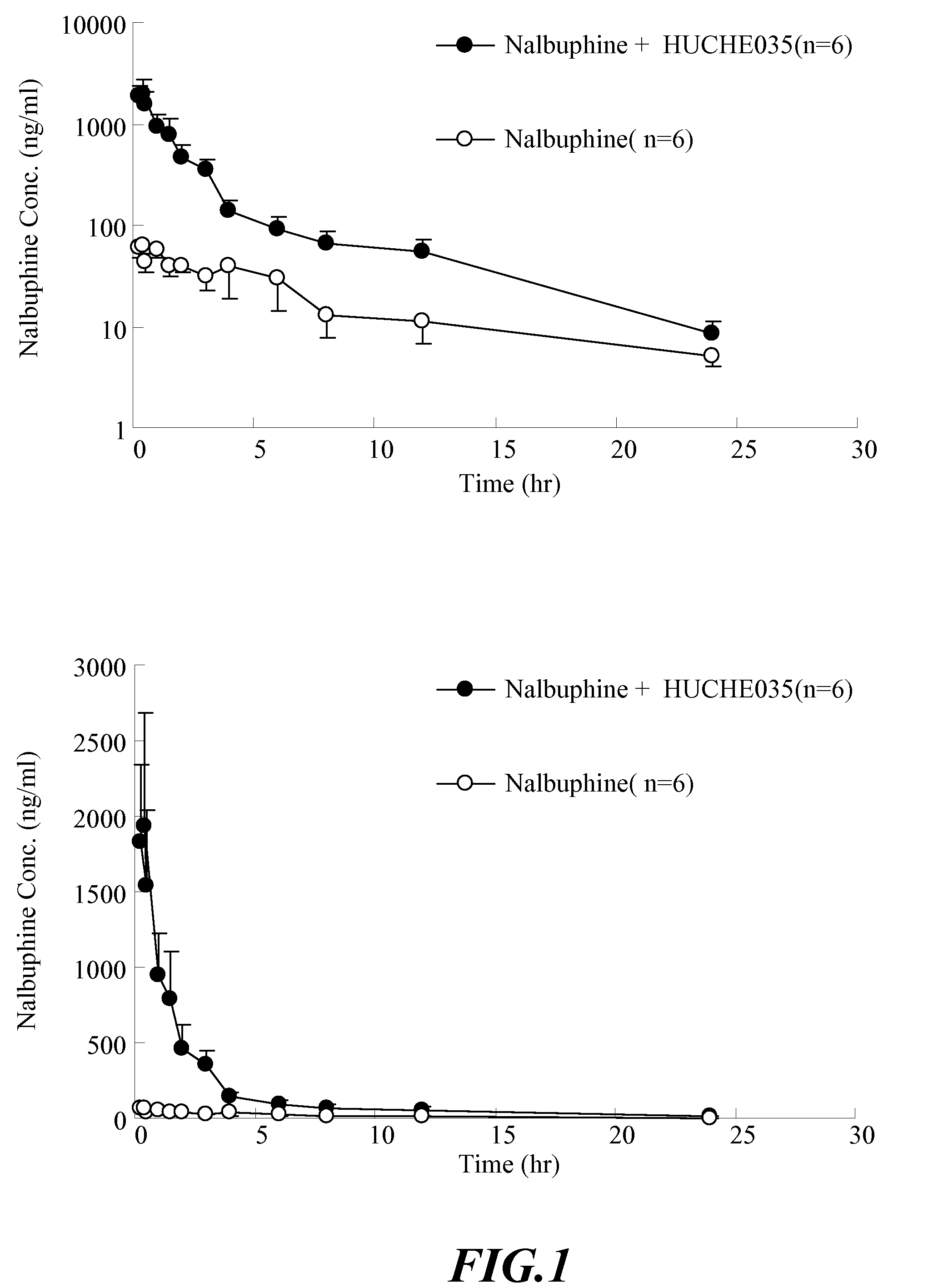
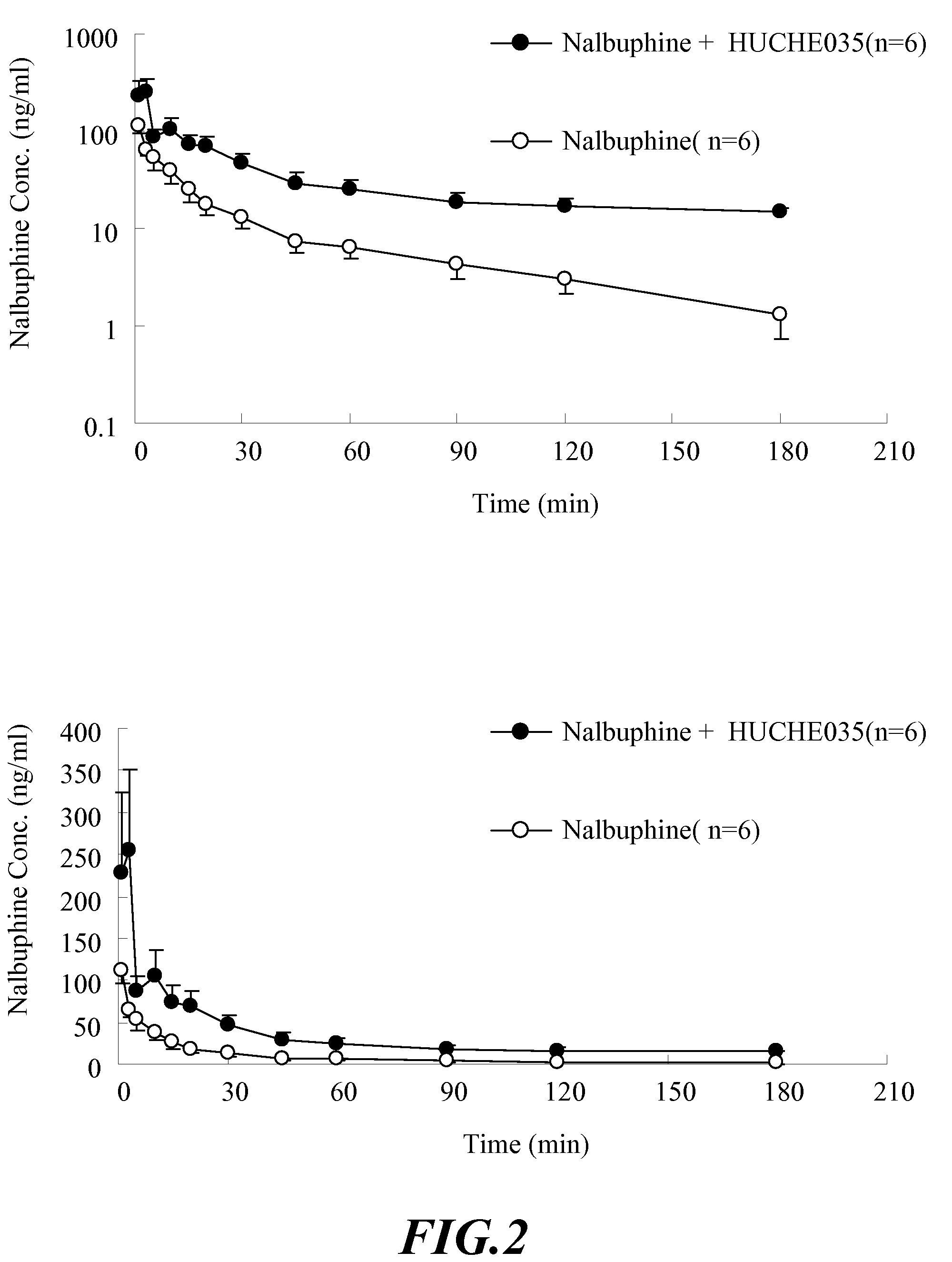
Inhibitors and Enhancers of Uridine Diphosphate-Glucuronosyltransferase 2B (UGT2B)
ActiveUS20090074708A1Increase heightReduced activityBiocideHydroxy compound active ingredientsPolyethylene glycolEriodictyol
A UGT2B inhibitor capable of increasing the bio-availability of a drug, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: capillarisin, isorhamnetin, β-naphthoflavone, α-naphthoflavone, hesperetin, terpineol, (+)-limonene, β-myrcene, swertiamarin, eriodictyol, cineole, apigenin, baicalin, ursolic acid, isovitexin, lauryl alcohol, puerarin, trans-cinnamaldehyde, 3-phenylpropyl acetate, isoliquritigenin, paeoniflorin, gallic acid, genistein, glycyrrhizin, protocatechuic acid, ethyl myristate, umbelliferone, PEG (Polyethylene glycol) 400, PEG 2000, PEG 4000, Tween 20, Tween 60, Tween 80, BRIJ® 58, BRIJ® 76, Pluronic® F68, Pluronic® F127, and a combination thereof. A UGT2B enhancer capable of enhancing a clearance rate of morphine-like analgesic agents, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: nordihydroguaiaretic acid, wogonin, trans-cinnamic acid, baicalein, quercetin, daidzein, oleanolic acid, homoorientin, hesperetin, narigin, neohesperidin, (+)-epicatechin, hesperidin, liquiritin, eriodictyol, formononetin, quercitrin, genkwanin, kaempferol, isoquercitrin, (+)-catechin, naringenin, daidzin, (−)-epicatechin, luteolin-7-glucoside, ergosterol, rutin, luteolin, ethyl myristate, apigenin, 3-phenylpropyl acetate, umbelliferone, glycyrrhizin, protocatechuic acid, poncirin, isovitexin, 6-gingerol, cineole, genistein, trans-cinnamaldehyde, and a combination thereof.
Owner:NAT DEFENSE MEDICAL CENT
Face cosmetic
InactiveCN101496775AUnique formulaFormulation InnovationCosmetic preparationsToilet preparationsIrritationGluconic acid
The invention provides a facial make-up with excellent effect on moisturizing, whitening and nourishing and small irritation to skin. The facial make-up consists of make-up substrate and make-up active substances, wherein the make-up active substances comprise glycolic acid, kojic acid, arbutin, aloin, baicalin, paeoniflorin, glycyrrhizic acid, squalane, hyaluronic acid and chitin to be prepared into toner, facial cream, facial cleanser, facial mask, gel and external soft capsule preparations.
Owner:徐伟玲
Method for extracting paeoniflorin from peony seed meal
The invention relates to the plant extraction field and concretely relates to a new plant source for paeoniflorin extraction, and a paeoniflorin extraction method. The method for extracting paeoniflorin from peony seed meal is characterized in that the method comprises the steps of crushing, enzymatic hydrolysis, ultrasonic extraction with ethanol, microwave extraction with ethanol, macro-porous resin adsorption, alumina column adsorption, titanium rod filtration, membrane ultrafiltration, nanofiltration, concentration, and drying to obtain a paeoniflorin extract. According to the invention, waste peony seed meal which is treated as a raw material undergoes enzymatic hydrolysis, is added with the organic solvent ethanol for paeoniflorin extraction, and is extracted through a macro-porous adsorption resin, an alumina column, a titanium rod and membrane filtration, so the paeoniflorin extraction rate reaches 61.6%.
Owner:HEZE YAO & SHUN PEONY BIOTECH
The preparation method of total paeoniflorin
ActiveCN102258588ALow costHigh extraction rateNervous disorderAntipyreticPropanolGlycoside formation
The invention provides a preparation method of peony general glycoside, which comprises the following steps: soaking radix paeoniae alba or red paeonia in 2-10 times (weight) of hydrophilic solvent, wherein the hydrophilic solvent is water or a water solution containing less than 30% of methanol, ethanol, propanol and isopropanol; after soaking, percolating at low temperature of 5-40 DEG C; and concentrating the percolate, filtering to obtain the extracting solution, and passing the extracting solution through macroporous resin. The transfer rate of penoniflorin and peony Albiflorin is 85-90%, and the purity of the peony general glycoside extract is high.
Owner:北京采瑞医药科技研究院有限公司
Method for simultaneously detecting main components of Naoxintong capsule in plasma
ActiveCN104614456AInhibit aggregationImprove neurological deficitsComponent separationAstragalosideSalvianolic acid B
The invention provides a method for simultaneously detecting main components of paeoniflorin, beta ecdysterone, laetrile, mulberroside A, caffeic acid, ferulic acid, salvianolic acid B, astragaloside, formononetin, cryptotanshinone and tanshinone IIA of a Naoxintong capsule in a plasma sample by a liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). In a liquid chromatogram, a mobile phase consists of acetonitrile and a formic acid aqueous solution of which the volume fraction is 0.1%, and gradient elution is used. A mass spectrum uses a quick positive and negative ions switching and analyzing mode and an MRM (Multiple Reaction Monitoring) scanning manner. After the Naoxintong capsule is taken, the situations of the changes of the blood-medicine concentration of several kinds of main components in the plasma of a rat are detected at the same time. The methodological survey results indicate that the established method conforms to determination requirements on biological samples in a body; the method is good in sensitivity, high in specificity, stable, reliable, and suitable for detecting substances with lower contents.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE +1
Peony and liquorice soup formula granule, preparation method and detection method of peony and liquorice soup formula granule
InactiveCN103330758AEasy to storeEasy to carry and useComponent separationAntipyreticLiquoricesPaeoniflorin
The invention provides a peony and liquorice soup formula granule, and a preparation method and a detection method of the peony and liquorice soup formula granule. The preparation method comprises the steps of weighing peony and liquorice at a mass ratio of 1:1, adding water for decocting and extracting, merging filtrate, decompressing and condensing the filtrate to obtain a concentrated solution, drying the concentrated spray, adding water for granulating and sieving, and obtaining the peony and liquorice soup formula granule. The detection method comprises the steps of identifying the peony and liquorice soup formula granule by a thin-layer chromatography, and measuring the heavy metal content, the organo-chlorine pesticide residual amount, the fingerprint spectrum, the extract content and the paeoniflorin content of the peony and liquorice soup formula granule. The peony and liquorice soup formula granule inherits advantages of a traditional Chinese medicine single-formula granule, fully considers interaction during decocting (or other processing) of a decoction piece, and has a higher value, and the detection method allows the quality of the peony and liquorice soup formula granule to be controlled more effectively, so that the peony and liquorice soup formula granule is safer to use.
Owner:KANGMEI PHARMA +1
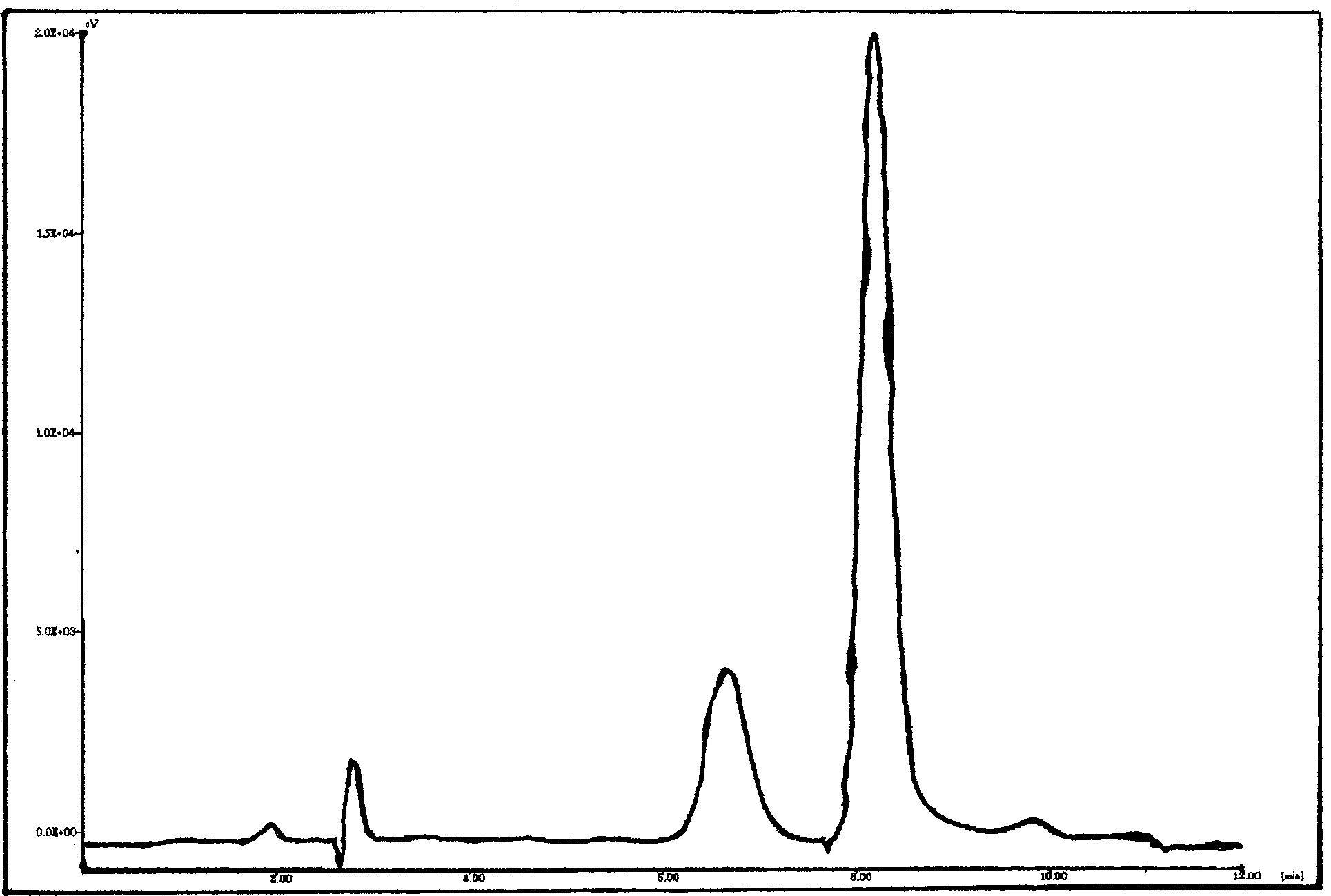
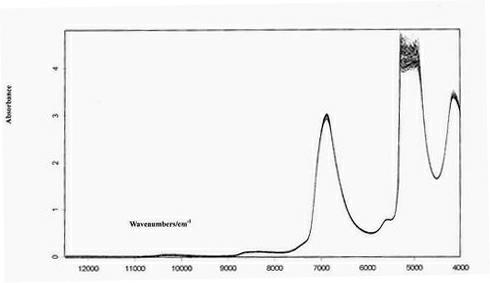
NIR online detection method for paeoniflorin content of white paeony root extract
ActiveCN102058682ARealize the whole process of real-time quality monitoringSimple and fast operationComponent separationColor/spectral properties measurementsInfraredAlcohol
The invention discloses a near infrared (NIR) online detection method for paeoniflorin content of white paeony root extract, and belongs to the technical field of Chinese medicament research. The method comprises the following steps of: a) acquiring near infrared spectrum and paeoniflorin content of white paeony root extract samples of set number; b) establishing a detection model by a partial least squares method according to the near infrared spectrum and the paeoniflorin content; and c) acquiring the near infrared spectrum of the white paeony root extract sample to be detected on line, and inputting the near infrared spectrum to the detection model to obtain the paeoniflorin content of the sample to be detected so as to realize online detection of the paeoniflorin content of the white paeony root extract. The method can be used for an alcohol extraction process of white paeony root, and can also be used for a concentration process of the white paeony root alcohol extract. The method can be used for detection and quality monitoring of Chinese medicaments.
Owner:TIANJIN TASLY MORDEN TCM RESOURCES
Method for extracting peony polysaccharide from peony seed meals
The invention relates to the field of plant extraction and in particular relates to a novel method for extracting peony polysaccharide from peony seed meals. The method for extracting peony polysaccharide from peony seed meals comprises the following steps of crushing, carrying out enzymatic hydrolysis, carrying out ultrasonic extraction with ethanol, carrying out microwave extraction with ethanol, adsorbing with macroporous resin, carrying out enzymatic hydrolysis and removing protein by membrane ultrafiltration, decolorizing, and concentrating and drying to obtain the peony polysaccharide. The method disclosed by the invention has the beneficial effects that the waste peony seed meals are taken as raw materials, peony seed meals is firstly subjected to enzymatic hydrolysis by an enzymatic method, and then an organic solvent such as ethanol is added to extract paeoniflorin, then adsorption is carried out by macroporous resin, and enzymatic hydrolysis is carried out, protein is removed by ultrafiltration membrane and concentrated and dried to obtain the peony polysaccharide of which the yield reaches 12.8%.
Owner:HEZE YAO & SHUN PEONY BIOTECH
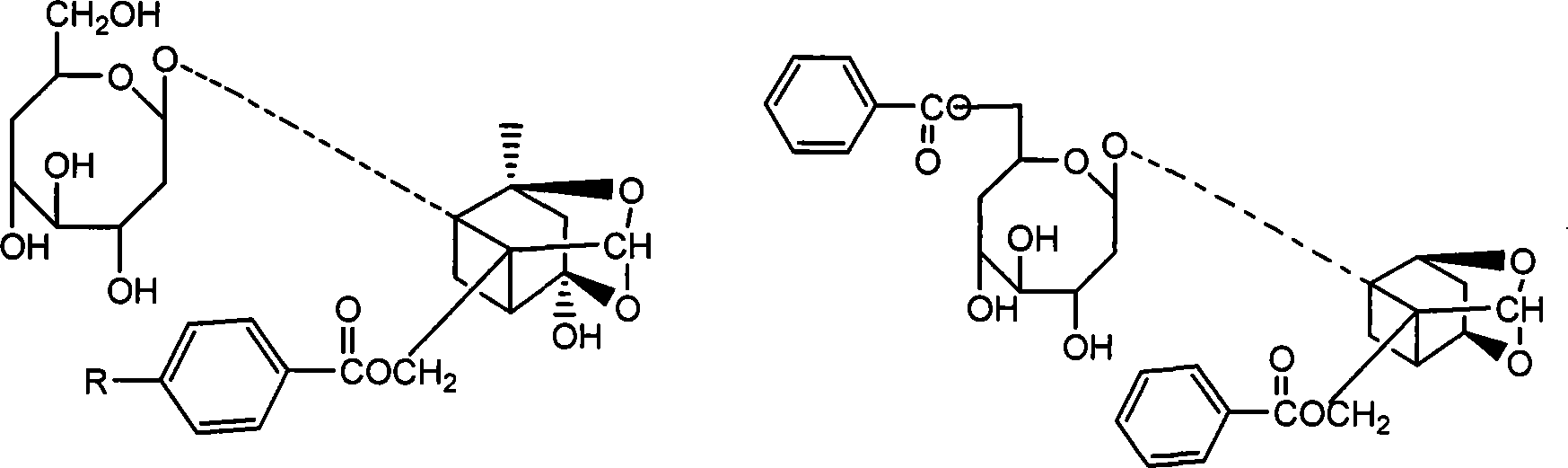
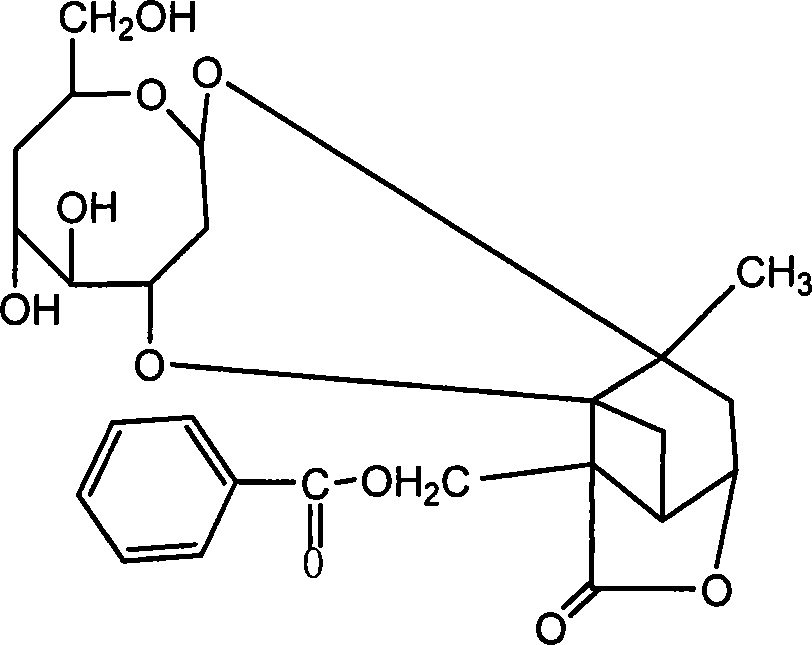
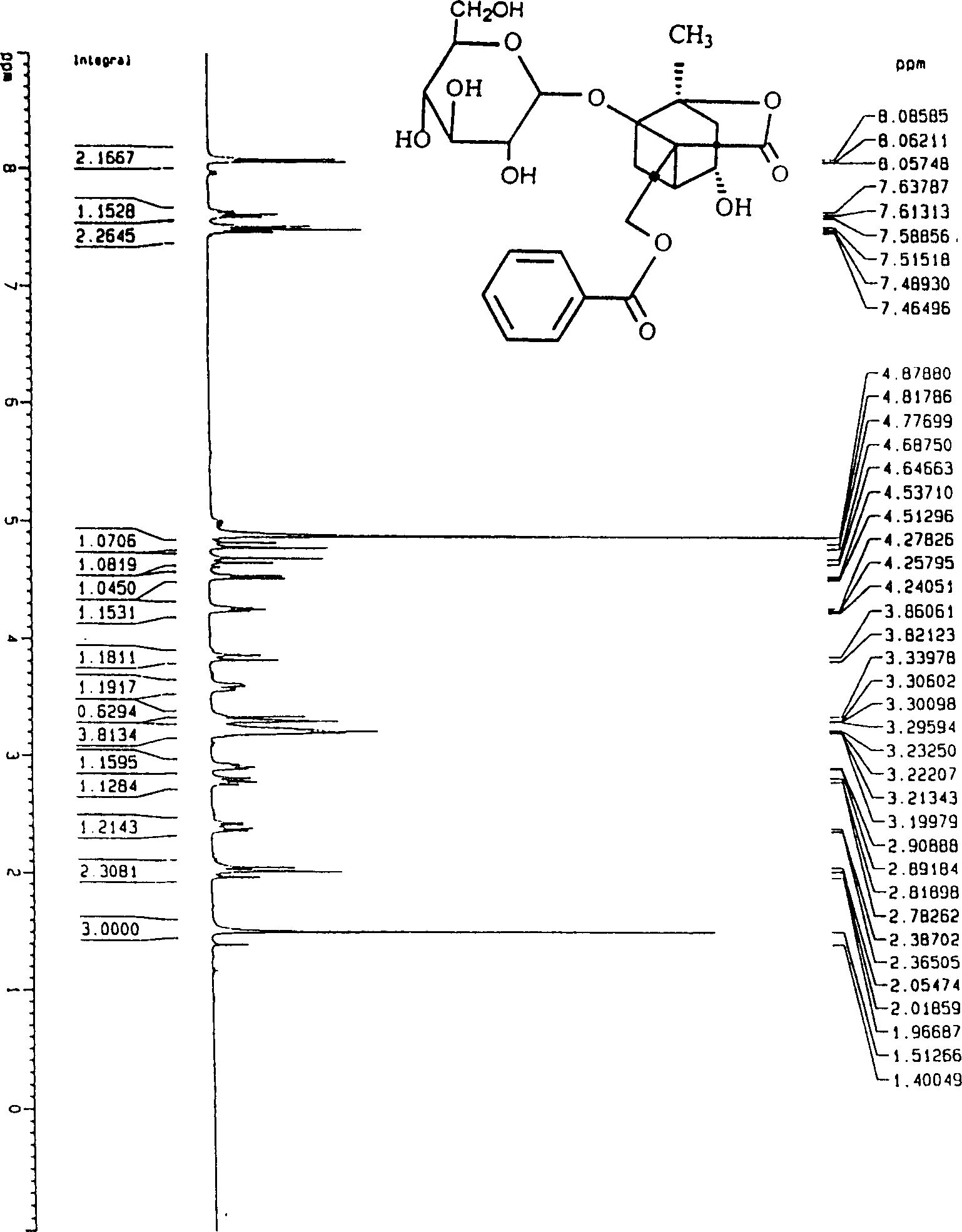
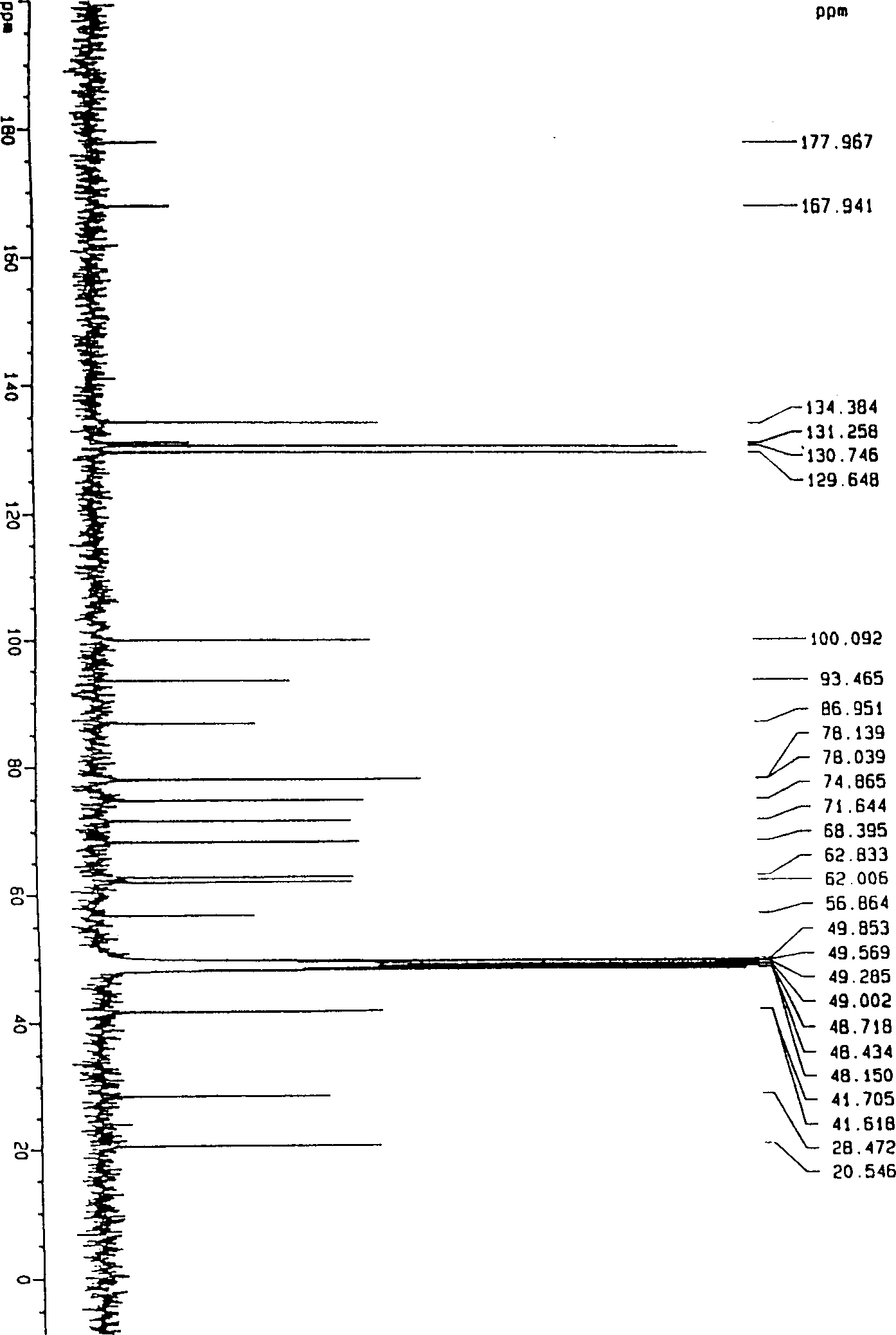
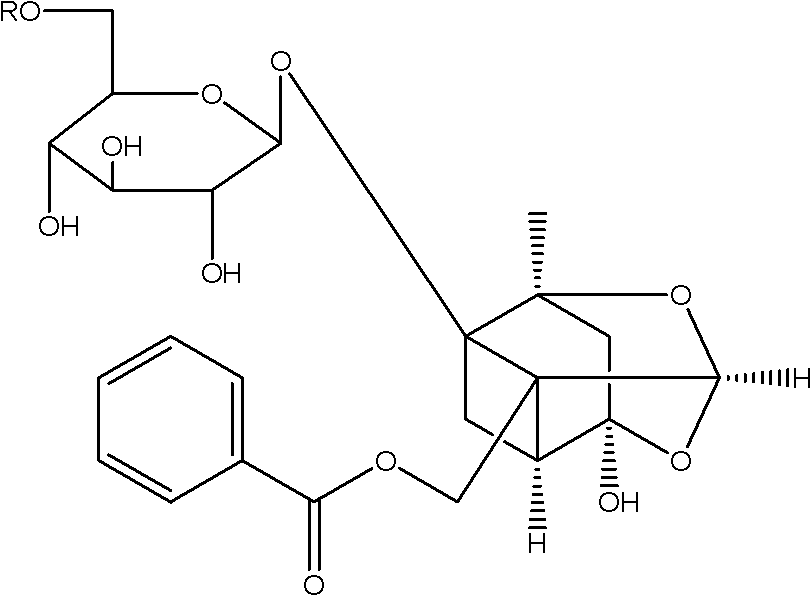
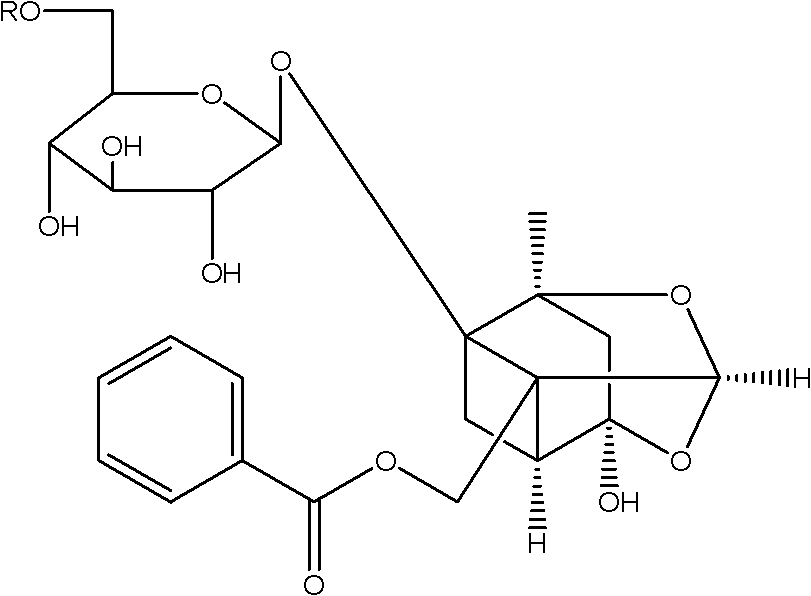
Paeoniflorin aromatic ester derivative, preparation method and applications thereof
ActiveCN102603827AImprove bioavailabilityImprove securityOrganic active ingredientsSugar derivativesSolventMethyl group
The invention discloses a paeoniflorin aromatic ester derivative, a preparation method and applications of the paeoniflorin aromatic ester derivative. The general structure formula of the paeoniflorin aromatic ester derivative is as follows: in the formula, R is selected from benzoyl, phenylsulfonyl, phenylacetyl, benzene propionyl, benzene acryloyl, p-chlorobenzoyl, 3, 5-dimethoxybenzoyl, 2-( acetoxyl group) benzoyl, 2-methyl-4-(2'-methyl propyl) phenylacetyl or 2-[(2', 6-dichlorophenyl) amino]- phenylacetyl. The preparation method comprises the steps of taking paeoniflorin as raw materials, conducting esterification on the paeoniflorin in a solvent through aromatic acyl chloride or aromatic anhydride, then conducting washing and column chromatography to obtain the paeoniflorin aromatic ester derivative. The paeoniflorin aromatic ester derivative has the advantages that the bioavailability of the paeoniflorin is improved and the anti-inflammatory action is strong, and can be made into injection, tablets, capsules, dropping pills and particles for treating chronic infectious arthritis.
Owner:GUANGZHOU HANFANG PHARMA
Composition of paeoniflorin and peony lactone glycoside with function of increasing leukocyte
InactiveCN1706397AImprove indexClassification normalOrganic active ingredientsPavoniaWhite blood cell
The present invention relates to medicine composition extracted from Paeonia lactifora Pall and containing the active components paeoniflorin and albiflorin in the total content of 50-95 % and weight ratio of 0.1-10. The composition is suitable for being prepared into various medicine preparations, including delayed releasing preparation, controlled releasing preparation, body cavity administrated preparation, spray, transdermal preparation, oral preparation, injection and transfused fluid preparation alone or together with other medicine components. The composition is suitable for preparing medicine for treating leucopenia, throbocytopenia and achreocythemia of different causes.
Owner:SHENYANG PHARMA UNIVERSITY
Blood nourishing and brain arousing particle quality detection method
ActiveCN102998410ASimple methodGood repeatabilityComponent separationUrsolic acidRehmannia glutinosa
The invention relates to the medicinal field, and concretely relates to a blood nourishing and brain arousing particle quality detection method. The method comprises identification of ursolic acid in Spica Prunellae, identification of Rhizoma Corydalis, identification of Semen Cassiae, identification of Chinese angelica and Ligusticum wallichii, identification of rosmarinic acid in Spica Prunellae, identification of alkaloids in Ligusticum wallichii, identification of Levistilide in Ligusticum wallichii, identification of Millettia dielsiana, identification of white peony root, identification of prepared rehmannia root, identification of Gambir Plant, and determination of the content of an active ingredient paeoniflorin in the blood nourishing and brain arousing particle.
Owner:天士力东北现代中药资源有限公司
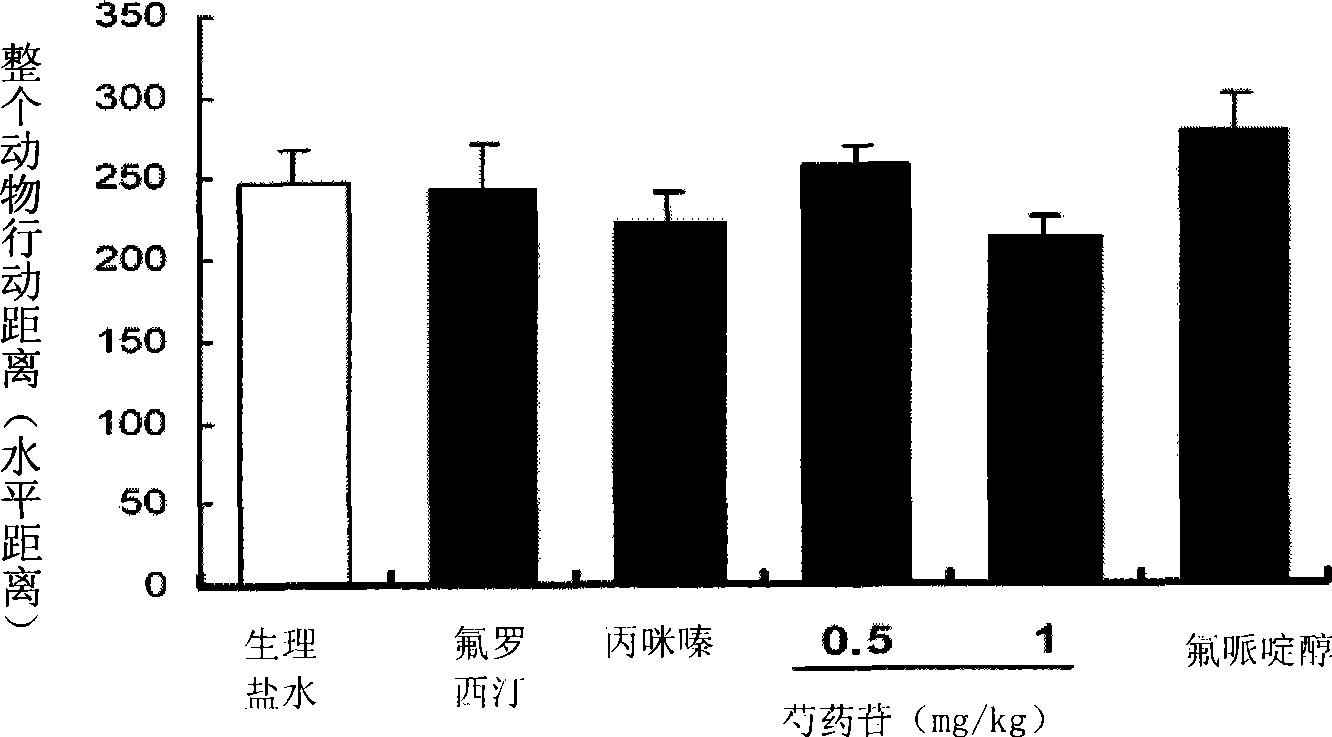
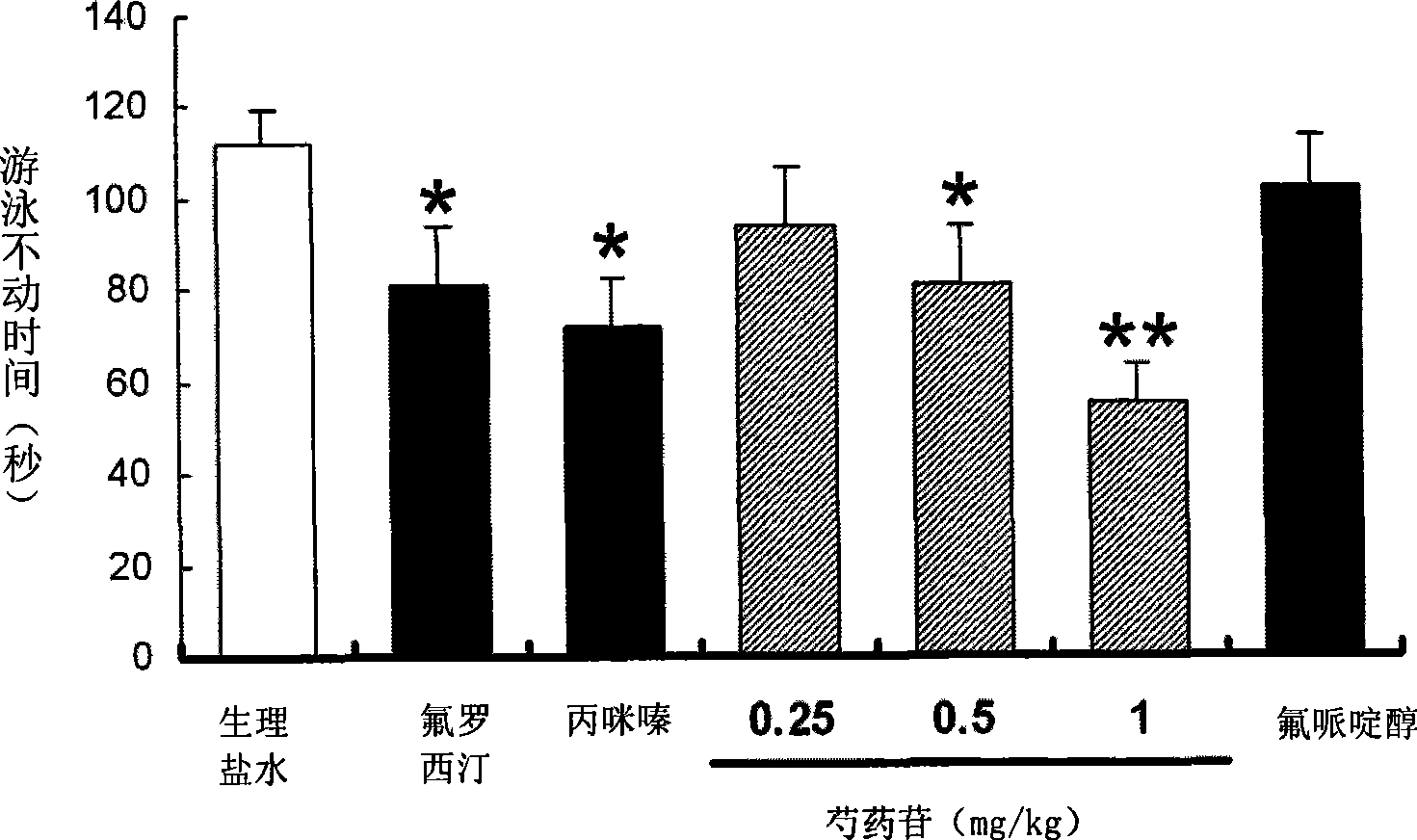
Use of penoniflorin in preparing medicine for preventing and treating depression and medicine composition thereof
InactiveCN101385736ASmall side effectsGood effectOrganic active ingredientsNervous disorderSide effectCurative effect
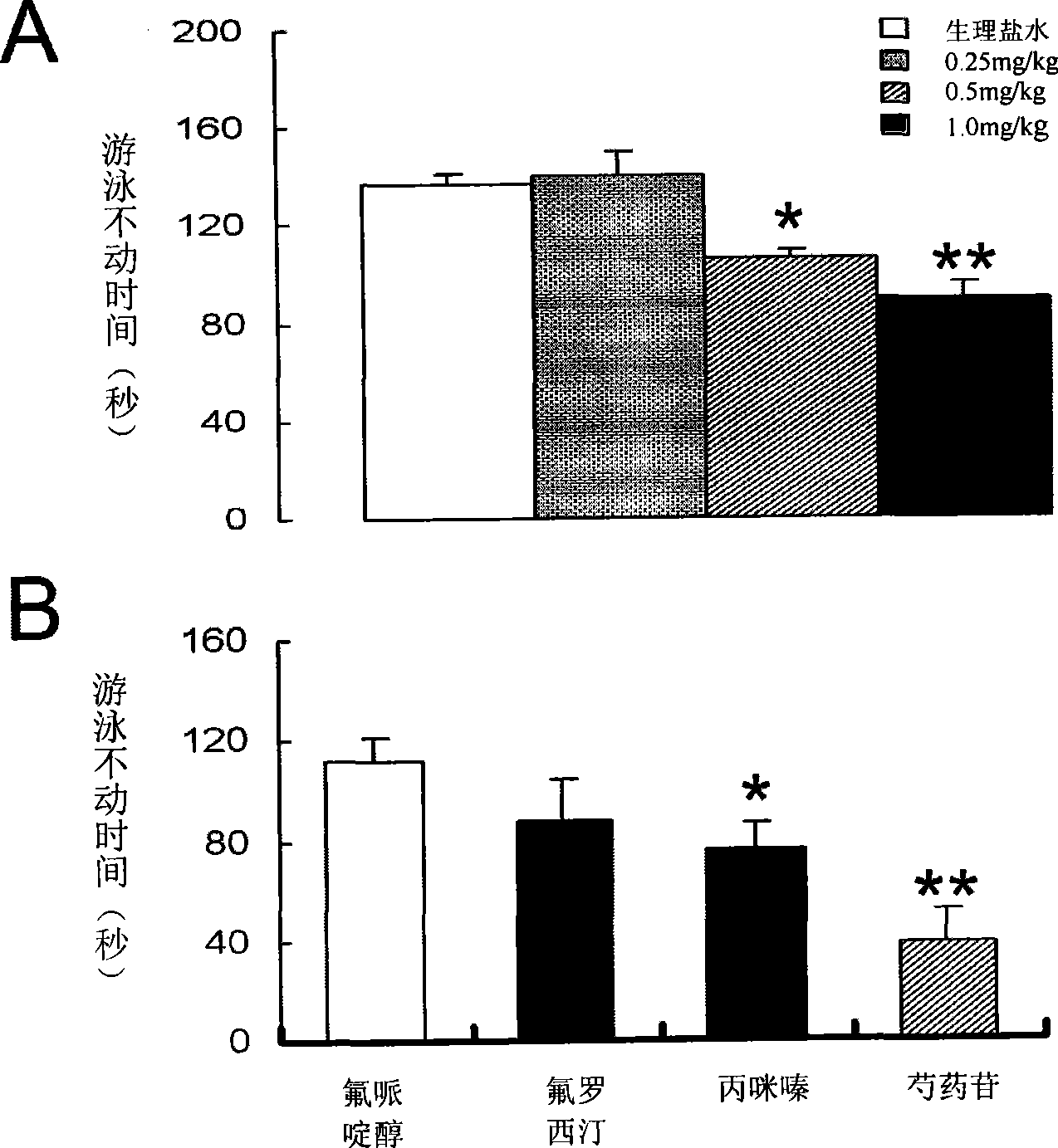
The invention relates to an application of paeoniflorin in drugs for preventing and treating depression and a pharmaceutical composition thereof, and the pharmaceutical composition contains effective dose of the paeoniflorin and a pharmaceutical acceptable carrier. The pharmaceutical composition can be prepared into various conventional liquid formulations or solid formulations. The paeoniflorin is proved to have better efficacy for the depression and long duration of the efficacy through a rat forced swimming test, a open field test and a new environment feeding inhibition test. The drugs which take the paeoniflorin as an active ingredient and are used for treating and preventing the depression have good effects, long duration, insignificant toxicity and side effect and low price.
Owner:SOUTHERN MEDICAL UNIVERSITY
Quality control method of total glycosides single preparation of white paeony roots
ActiveCN102138985AQuality assuranceGuaranteed curative effectComponent separationPlant ingredientsClinical efficacyCurative effect
The invention provides a quality control method of a preparation of white paeony roots. The method comprises the following steps of: establishing a synchronous content measuring method of white paeony root herbs, white paeony root total glycosides and paeoniflorin and albiflorin in the white paeony root preparation by adopting the same liquid-phase chromatography condition; and accurately measuring the contents of the paeoniflorin and the albiflorin. The method is simple to preprocess a sample, keeps complete characteristic components and provides a stable sample solution and has higher accuracy, favorable reproduction and a certain specificity; characteristic peaks in the obtained fingerprint map have favorable separation effect, the fingerprint maps of the white paeony root herbs, the white paeony root total glycosides and the preparation have favorable relativity; the standard fingerprint map of the white paeony root herbs is established at a new angle by utilizing the relativity research of the fingerprint maps, can be used for identifying the qualities of the white paeony root herbs and improving the controllability of the production process of the white paeony root preparation and is favorable to ensuring the quality stability and the clinical curative effect of the white paeony root preparation.
Owner:NINGBO LIWAH PHARM CO LTD
Method for preparing paeoniflorin and albiflorin
ActiveCN102492005AExpand and enrich effective comprehensive utilizationTake advantage ofSugar derivativesSugar derivatives preparationOrganic solventAlcohol
The invention discloses a method for preparing paeoniflorin and albiflorin, which mainly includes the following steps: selecting peony plant raw materials containing the paeoniflorin and the albiflorin, adopting water or alcohol-water mixed solvent to perform extraction, mixing extract, concentrating, filtering, absorbing the obtained crude extract by adopting pre-preprocessed macroporous resin, performing gradient elution by using ethanol-water mixed solvent, collecting 30% to 75% of ethanol elution parts eluent to perform concentration and drying. The obtained refined extract adopts pressurized column chromatography, using silica gel and modified silica gel as fillers, appropriate organic solvent is separated in mobile phase, flow parts containing the paeoniflorin and the albiflorin are collected respectively, concentrated and dried to obtain paeoniflorin finished products and albiflorin finished products with the purity more than 90%. The method for preparing the paeoniflorin and the albiflorin is concise in process, strong in operability, convenient in automation, capable of achieving comprehensive utilization of plant resources, convenient in solvent recycling, low in energy consumption, high in product purity, easy to achieve and high in industrialization.
Owner:SHANGHAI HUIWEN BIO TECH
Microemulsion of total glucosides of paeony and active components
InactiveCN101601737AGood dispersionReduced bioavailabilityAntipyreticAnalgesicsSide effectActive component
The invention discloses a microemulsion of total glucosides of paeony and active components, which consists of the total glucosides of paeony and the active components, a surfactant, a cosurfactant, an oil phase and a water phase. The microemulsion has transparent appearance, a particle size of between 10 and 100 nanometers and good stability, and can remarkably improve the absorption of paeoniflorin so as to improve the bioavailability of medicaments, reduce the toxic side effects, and improve the curative effect.
Owner:魏伟 +1
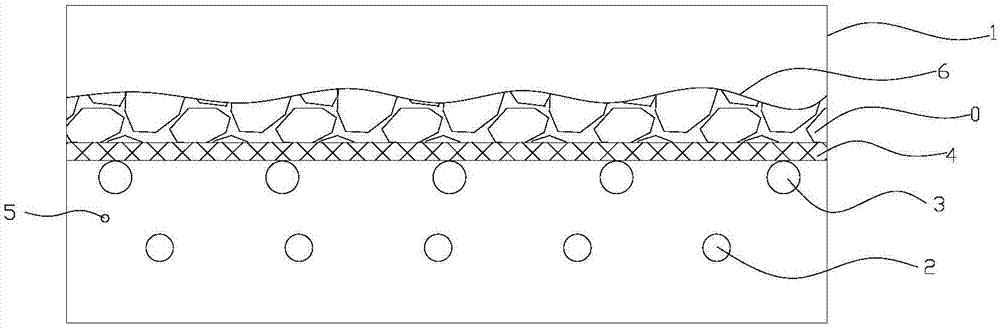
Processing method and device for white peony root decoction pieces
ActiveCN107281282ASolve the problem of many irregular-shaped pieces of materialImprove molding qualityPlant ingredientsControl systemEngineering
The invention discloses a processing method for white peony root decoction pieces. The processing method comprises the steps of cleaning; moistening: putting a white peony root material on a partition plate with meshes, adding cold water to submerge the material, heating a steam pipe until the water temperature is 40-50 DEG C and carrying out heat preservation for 6-8h; steaming and moistening: discharging water until the water level is below the partition plate, heating the steam pipe and carrying out heat preservation at 40-50 DEG C for 1-2h; slicing: cutting the material into pieces when the material is hot; and drying. Compared with a traditional processing method, the method is high in piece molding rate and small in ratio of a special-shaped piece; and the water soaking time is shortened, so that the content of paeoniflorin in the white peony root decoction pieces is greater than that through a traditional processing method. The invention further provides a processing device for the white peony root decoction pieces. The processing device comprises a drug moistening pool, wherein a steam generator, the steam pipe, a bracket, the partition plate, a temperature sensor and a control system are arranged in the drug moistening pool. According to the device, the molding quality of the white peony root decoction pieces can be effectively improved.
Owner:KANGMEI PHARMA
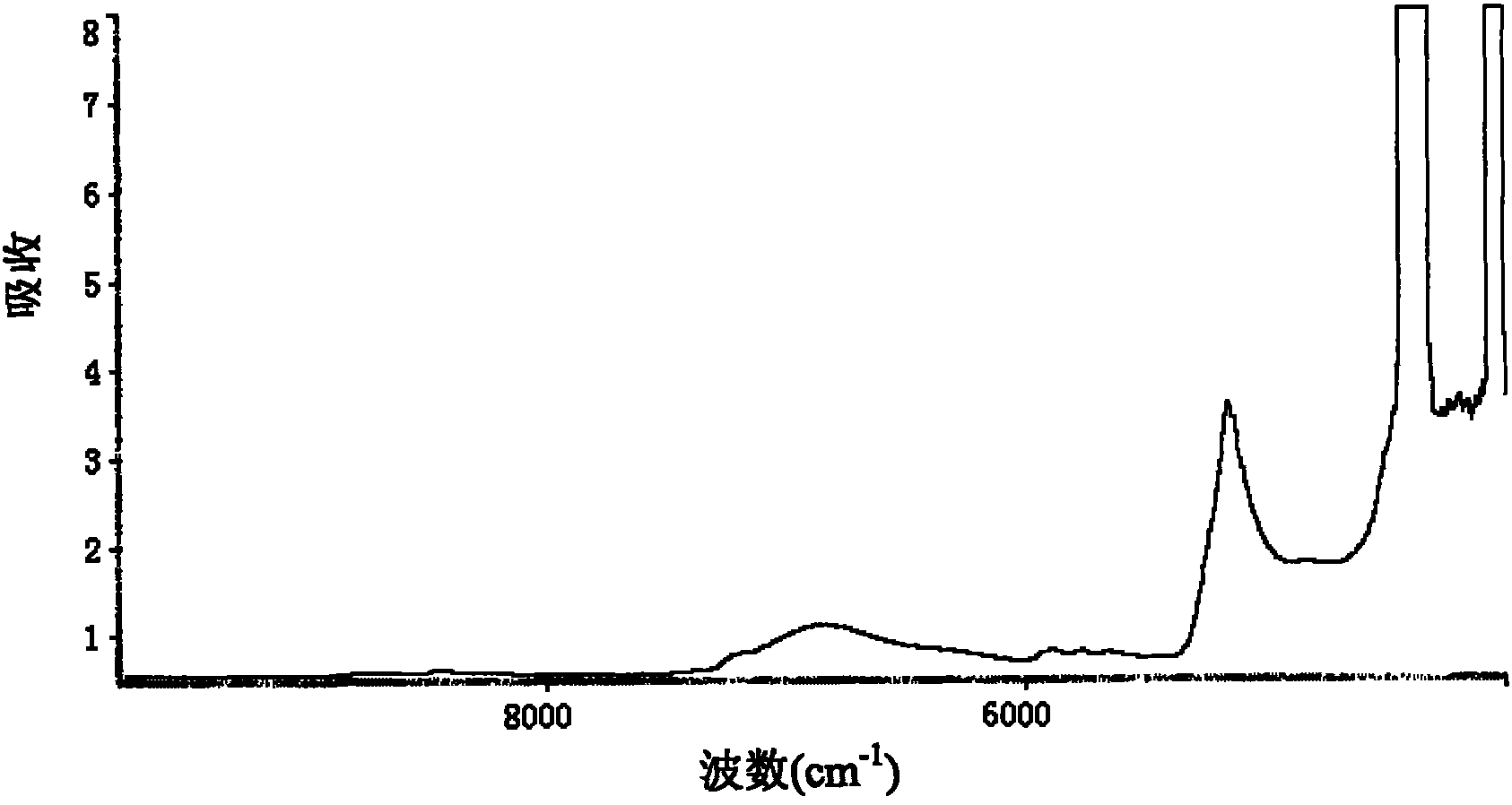
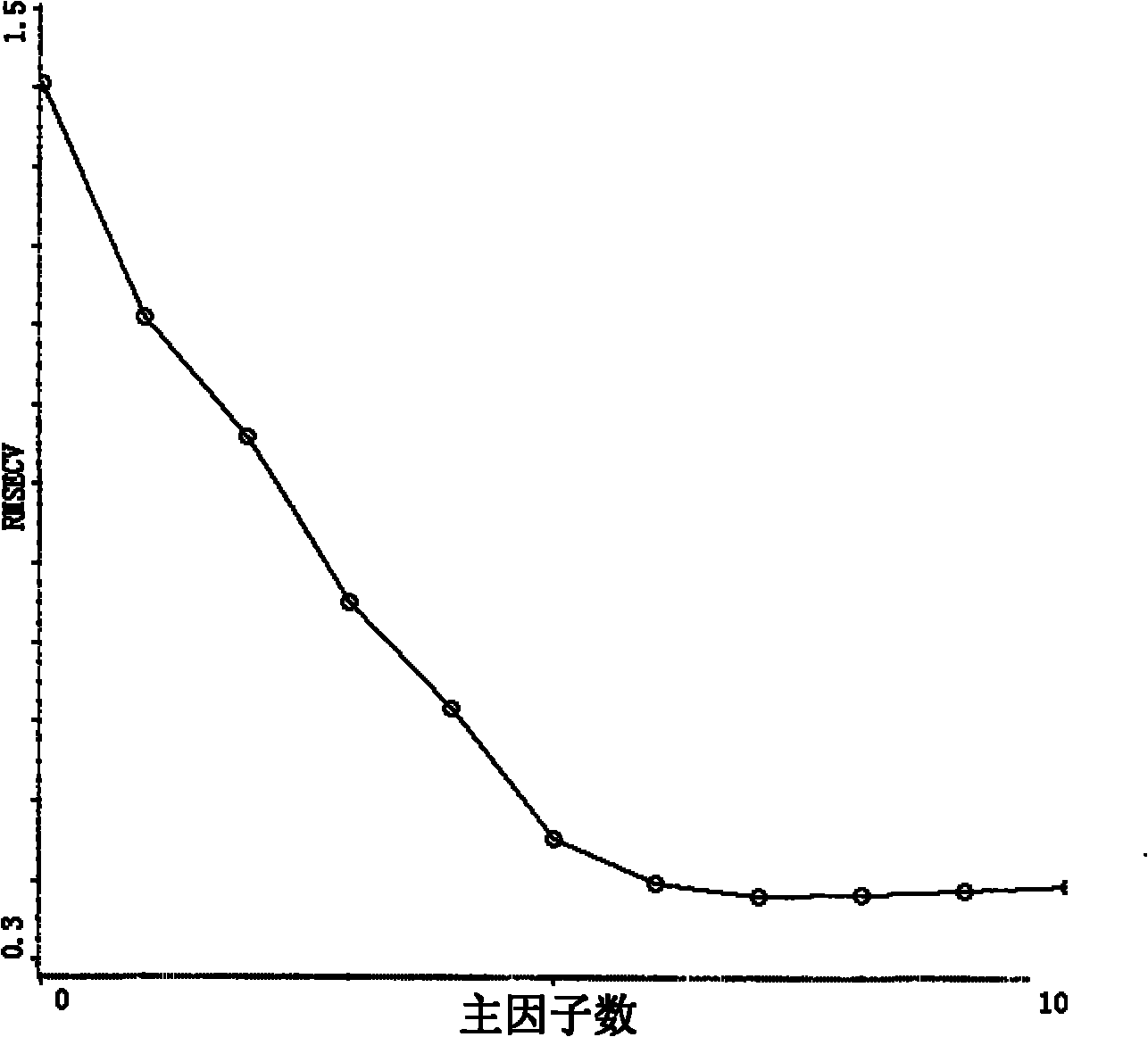
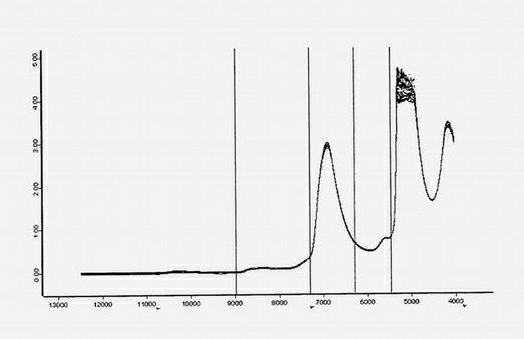
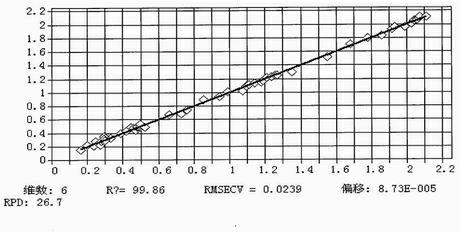
Method for measuring content of paeoniflorin in radix paeoniae alba extracting process by aid of near infrared spectrums
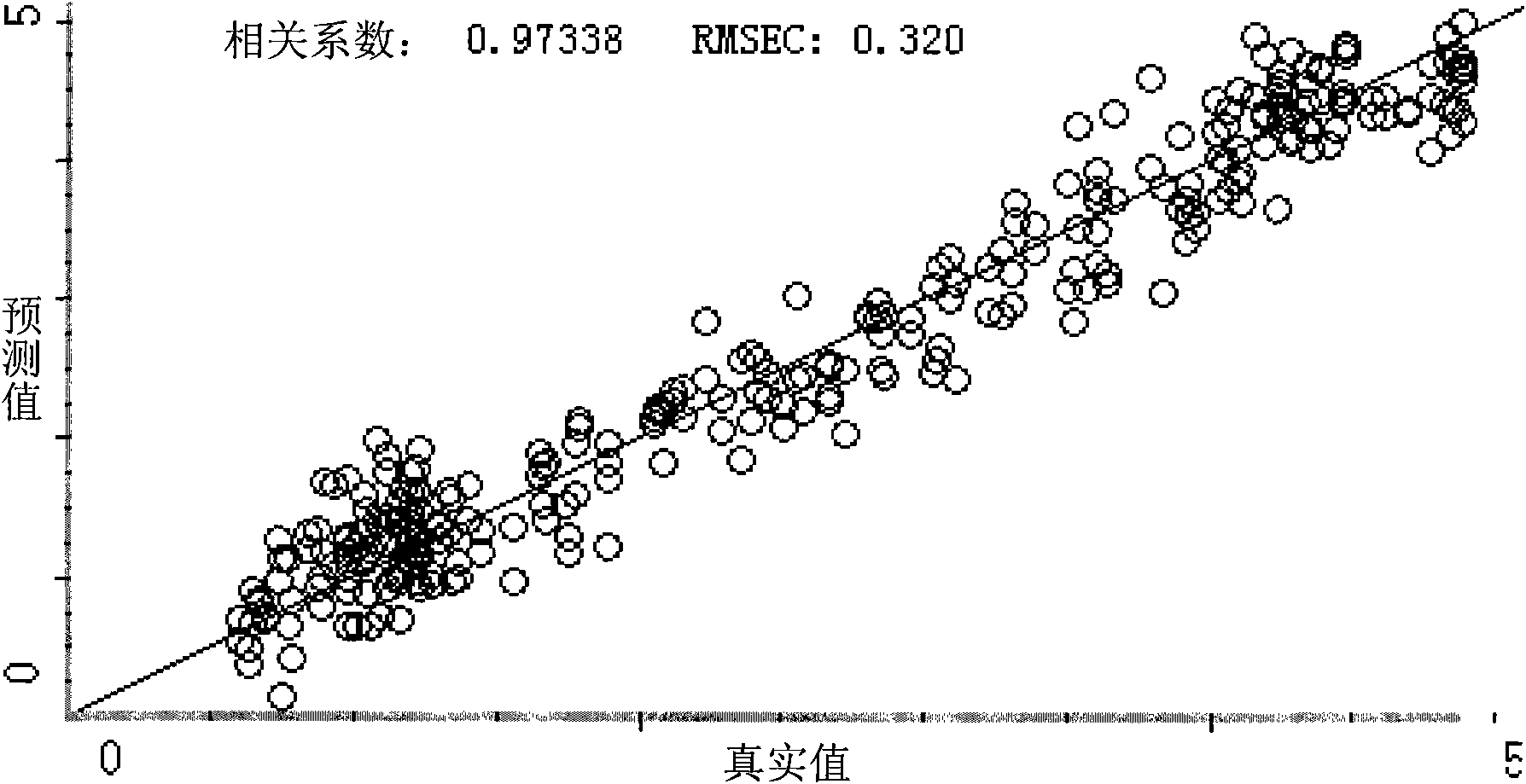
InactiveCN102636449AEliminate spectral biasImprove analysis efficiencyColor/spectral properties measurementsPaeoniflorinComputer science
The invention provides a method for measuring the content of paeoniflorin in radix paeoniae alba extracting process by the aid of near infrared spectrums, which can effectively overcome the shortcoming that in a conventional traditional Chinese medicine extracting process, the efficiency of a quality control method is low and operation is troublesome. The technical scheme includes that the method includes steps of collecting iconic radix paeoniae alba extracting process liquid as a correcting sample set at first; scanning the correcting sample set by the near infrared spectrums; selecting by means of waveband selection and spectrum pre-processing; and obtaining characteristic spectrum information which can mostly embody the characteristics of the paeoniflorin. Data, which are measured by a high performance liquid chromatography method by the aid of partial least squares, of the content of the paeoniflorin are relevant to the characteristic spectrum information of a sample, and accordingly a multi-element correcting model for the content of the paeoniflorin in the radix paeoniae alba extracting process is built. The built paeoniflorin quantitative correcting model is used for fast detecting the paeoniflorin in an unknown radix paeoniae alba extracting process. Results show that near infrared spectrum technology can be used for effectively detecting the content of the paeoniflorin in the radix paeoniae alba extracting process. The method has the advantages of simplicity, fastness, high accuracy and the like, can effectively monitor the change of the content of the paeoniflorin in the radix paeoniae alba extracting process as compared with a common detecting method by the aid of an instrument, has a high practical value, and can be popularized and applied to a traditional Chinese medicine production process.
Owner:JIANGXI HERBFINE HI TECH +1
Rapid separation liquid chromatography detection method for naoxintong capsules
ActiveCN105241980AInhibit bindingQuality assuranceComponent separationChlorogenic acidGallic acid ester
The invention relates to a rapid separation liquid chromatography detection method for naoxintong capsules. The method comprises the chromatographic conditions that octadecyl bonded silica gel columns serve as filling agents, 0.1% formic acid water serves as an A mobile phase, acetonitrile serves as a B mobile phase, the volume ratio of the A mobile phase to the B mobile phase is 10-85:15-90, and linear gradient elution is performed, the flow velocity is set at 0.20 mL.min<-1>, the column temperature is set at 25 DEG C, and the sample introduction volume is 5 microliters. The content of gallic acid, tanshinol, hydroxysafflor yellow A, chlorogenic acid, amygdalin, protocatechualdehyde, epicatechin, caffeic acid, albiflorin std, ononin, paeoniflorin, rutin, salvianolic acid A, cinnamic acid, fermlononetin, dihydrotanshinone I in the naoxintong capsules is detected. The detection method has the advantages of being rapid, stable and accurate.
Owner:SHAANXI BUCHANG PHARMA
Chinese medicine composition for treating dizziness and its preparing and quality control method
ActiveCN1616013AImprove quality controlGuaranteed uniformityComponent separationUnknown materialsPinelliaS syndrome
The Chinese medicine composition is used in treating dizziness, nausea and vomiting, especially Meeniere's syndrome, and has the functions of eliminating phlegm, preventing dizziness, regulating the middle warmer and stopping vomiting. It is prepared with 12 kinds of Chinese medicinal materials, including gastrodia tuber, hooked uncaria, oriental water plantain, pinellia tuber, etc. and contains also beta-cyclodextrin as volatile oil including agent, beta-cyclodextrin as co-solvent for liposoluble component, aspartame as corrective and dextrin as excipient. The quality control method during the preparation process includes thin layer identification of hooked uncaria, white peony root, Chuanxiong rhizome and licorice, and measurement of gastrodin in gastrodia tuber as main material and paeoniflorin in white peony root.
Owner:LUNAN PHARMA GROUP CORPORATION
Paeoniflorin and glycyrrhetinic acid composition and preparation method and application thereof
ActiveCN101926815AEasy to takeDefinite curative effectOrganic active ingredientsAntipyreticMedicineAdditive ingredient
The invention provides a paeoniflorin and glycyrrhetinic acid composition and a preparation method and application thereof for treating rheumatoid arthritis and eczema. The composition comprises paeoniflorin and glycyrrhetinic acid in a weight part ratio of 1:0.25-10. The composition can be prepared into various pharmaceutical formulations such as tablets, capsules, pills and the like; and the composition and other active ingredients form amulti-ingredient medicinal composition for providing a medicament with convenient administration and definite effect. The composition has good effect of treating rheumatoid arthritis and eczema.
Owner:NINGBO LIWAH PHARM CO LTD
Composition for treating climacteric syndrome, preparation and quality control method thereof
ActiveCN101293063AGood effectClear curative effectComponent separationSexual disorderBaical Skullcap RootAdemetionine
The invention discloses a pharmaceutical composition for treating women menopausal syndrome, a preparation method and a quality control method. The pharmaceutical composition of the invention is composed of pharmaceutical raw materials of glossy privet fruit, palmleaf raspberry fruit, south dodder seed, tuber fleeceflower root, cortex lycii radicis, ladybell root, dwarf lilyturf tuber, baical skullcap root, rehmannia root, white paeony root, red paeony, Chinese angelica, nacre and blackend swallowwort root; the preparation method is that: the pharmaceutical raw materials are selected, ground into fine powder, screened and evenly mixed; every 100g of powder is added by 42g of refining honey and appropriate amount of water, pill making and low-temperature drying are carried out, thus preparing the pharmaceutical composition. The preparation method adopts high performance liquid chromatography to carry out the content measurement on paeoniflorin. The pharmaceutical composition of the invention has good efficacy for treating women menopausal syndrome.
Owner:北京富国堂医药科技有限公司
Building method of HPLC characteristic chromatogram of radix paeoniae alba and detection method of radix paeoniae alba
InactiveCN108061768AComprehensive identificationAccurate identificationComponent separationAdditive ingredientTraditional medicine
The invention discloses a building method of an HPLC characteristic chromatogram of radix paeoniae alba and a detection method of radix paeoniae alba. For a quality control problem of radix paeoniae alba, a sulfur-containing radix paeoniae alba HPLC characteristic chromatogram and a sulfur-free radix paeoniae alba HPLC characteristic chromatogram are built, and a high performance liquid chromatography-mass spectrometry analytic method is adopted to analyze and identify ingredients of sulfur-containing radix paeoniae alba and sulfur-free radix paeoniae alba. Whether radix paeoniae alba is fumigated with sulfur or not is judged by adopting the built HPLC characteristic chromatogram of radix paeoniae alba to identify a paeoniflorin sulfite product which is produced after dry sulphitation of radix paeoniae alba. Compared with a conventional sulfur dioxide detection method, the detection method provided by the invention is simple, high in sensitivity, strong in specificity and excellent inreproducibility, can be used for detecting whether radix paeoniae alba is fumigated with sulfur or not, therefore, can identify the authenticity and quality of radix paeoniae alba more comprehensively, accurately and quickly, and is particularly suitable for detecting and identifying large-scale production of radix paeoniae alba.
Owner:GUANGDONG YIFANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com