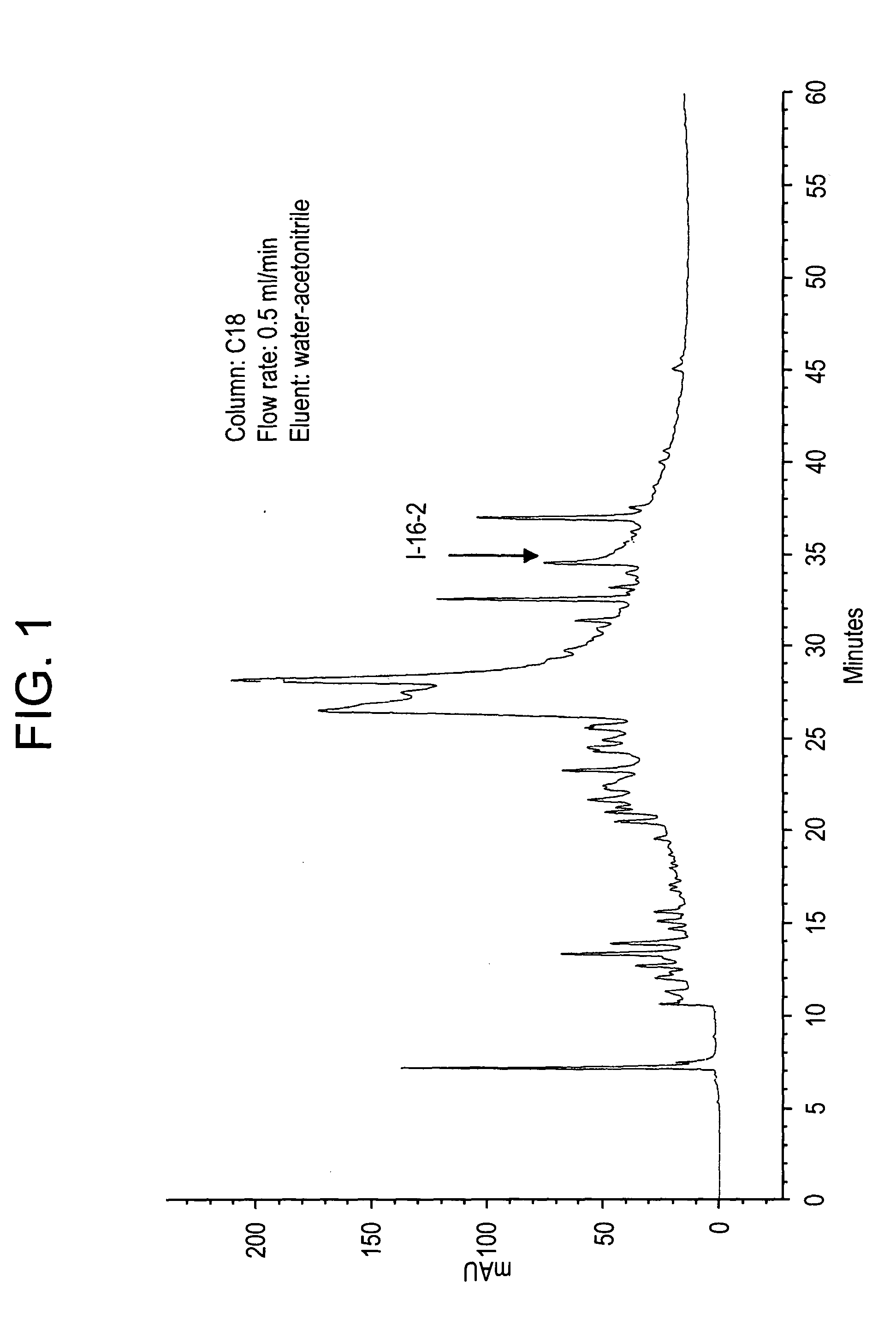
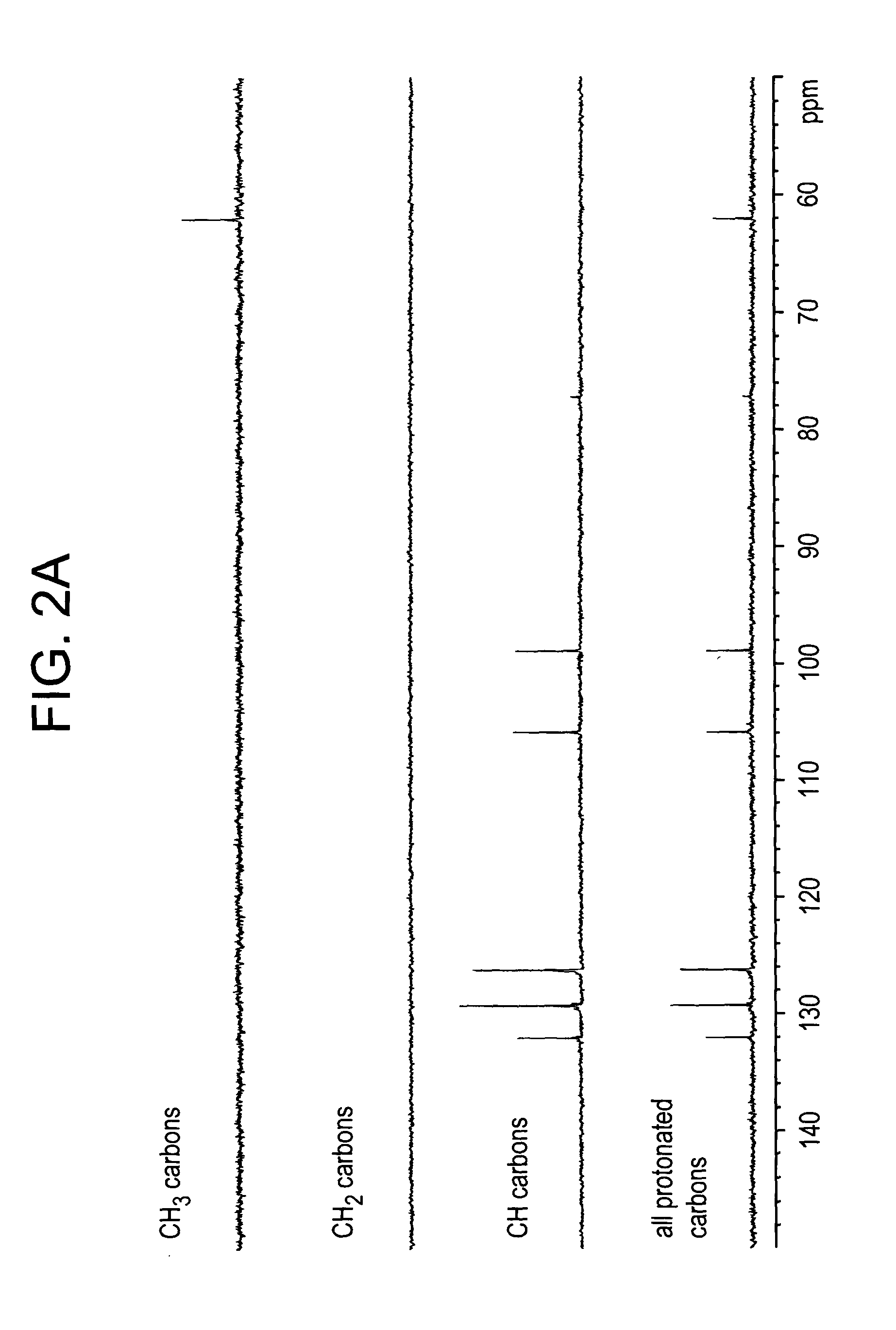
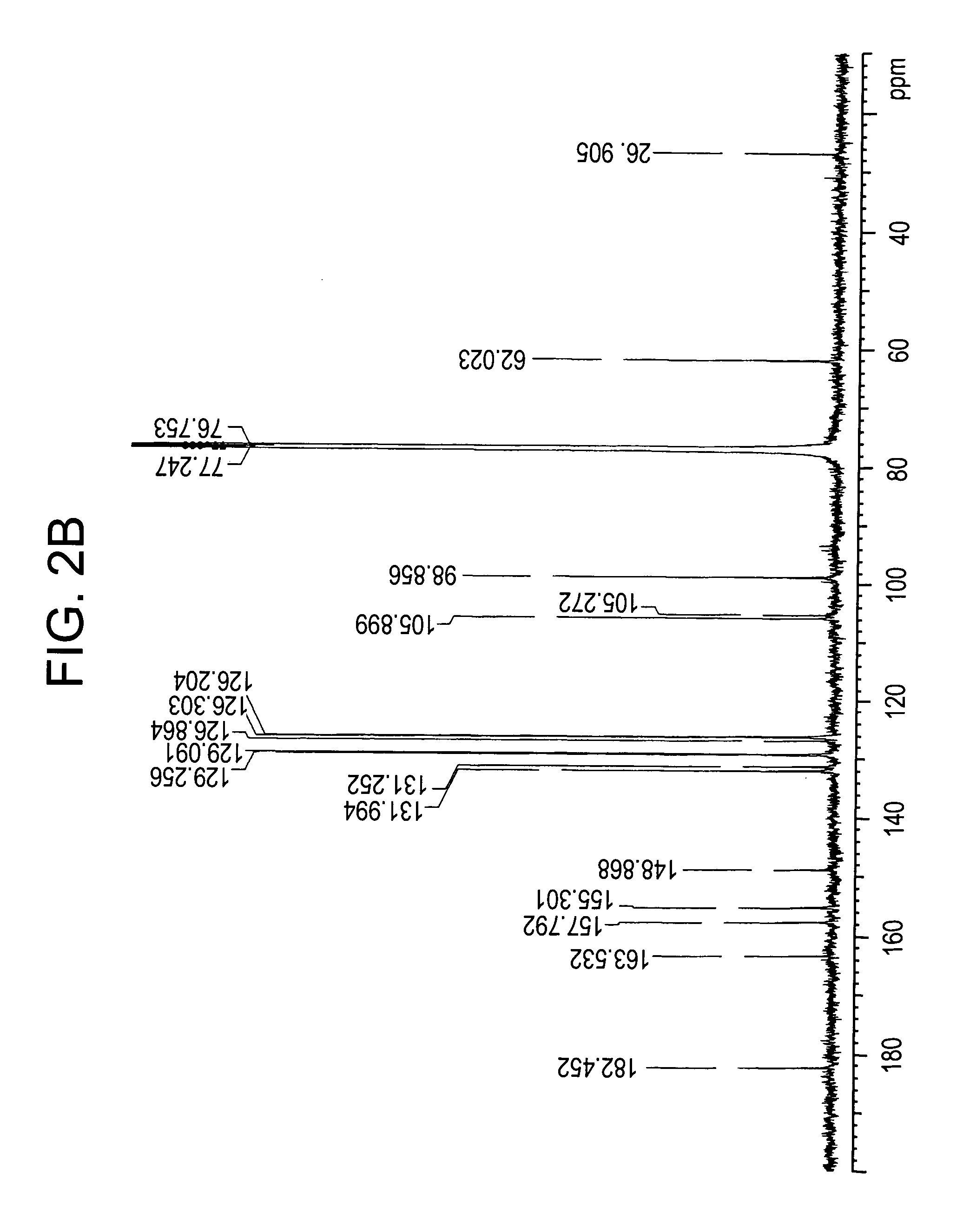
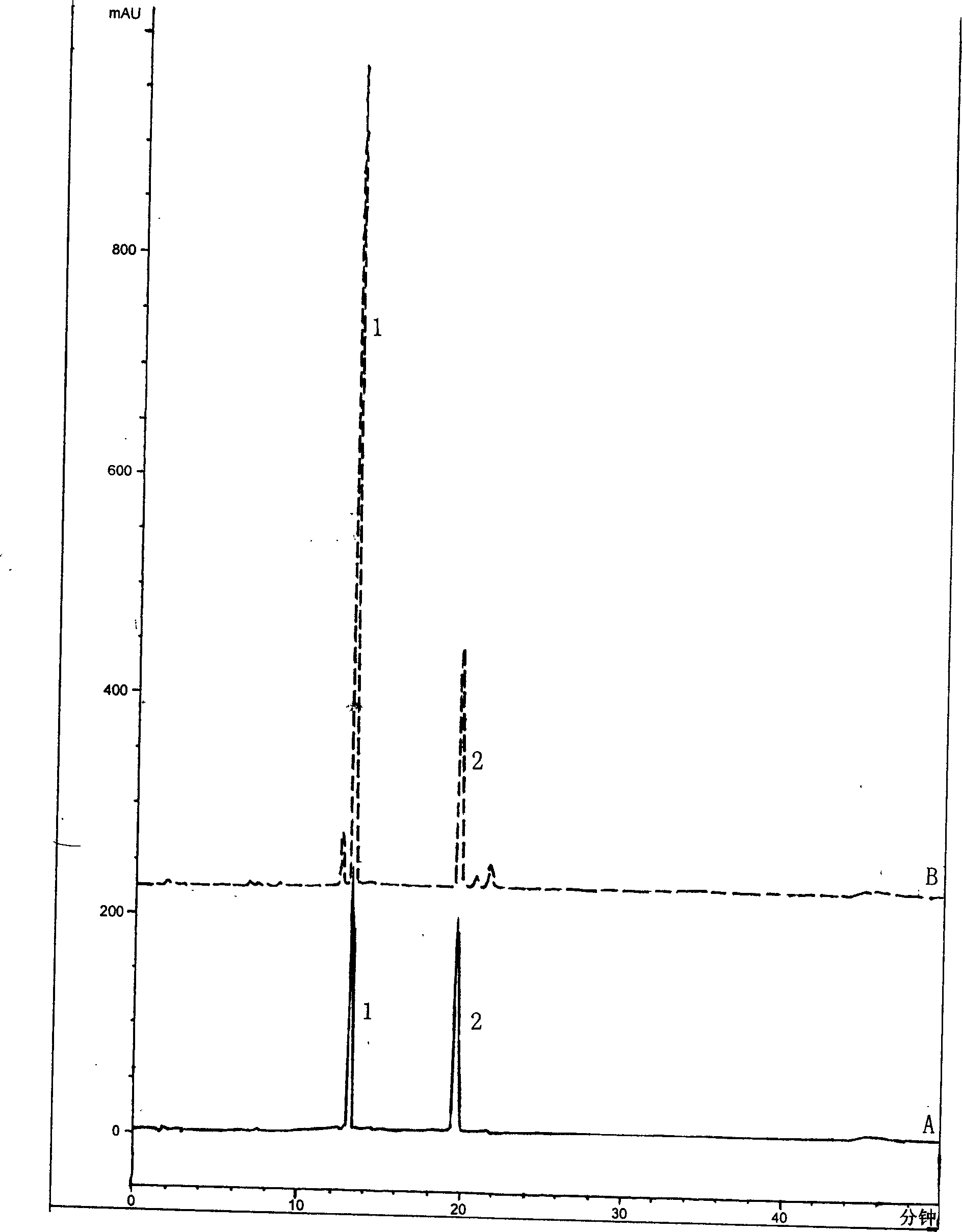
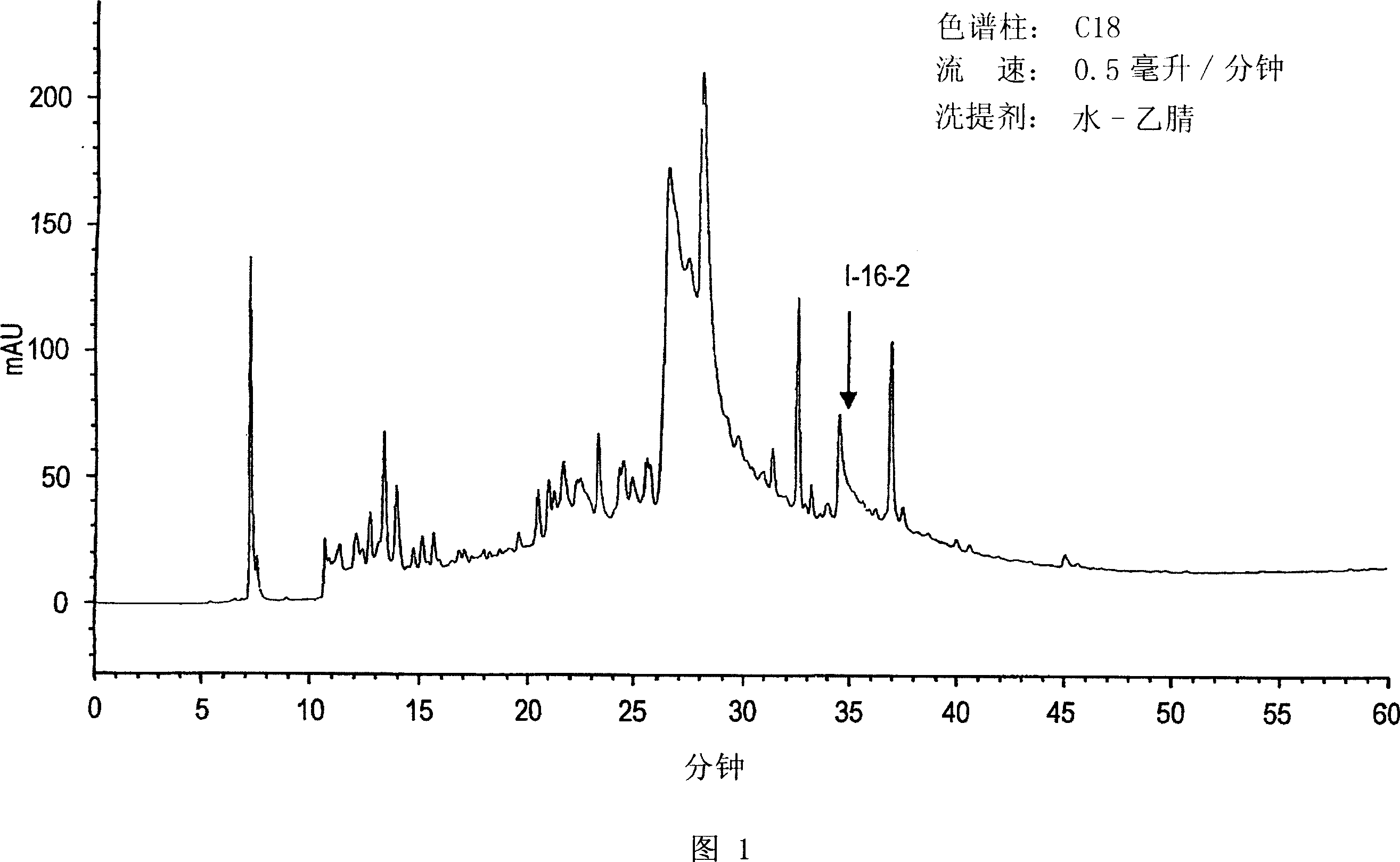
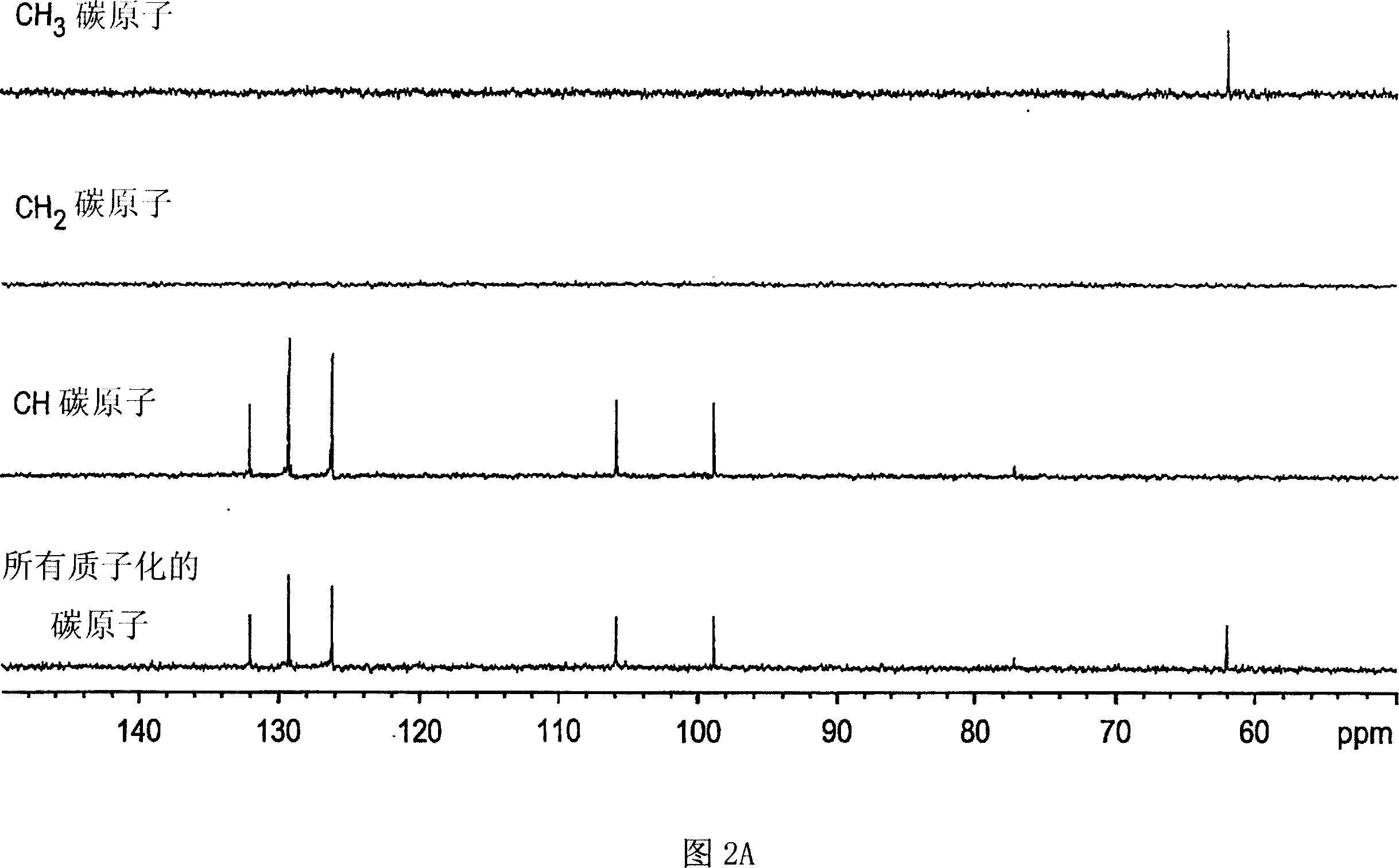
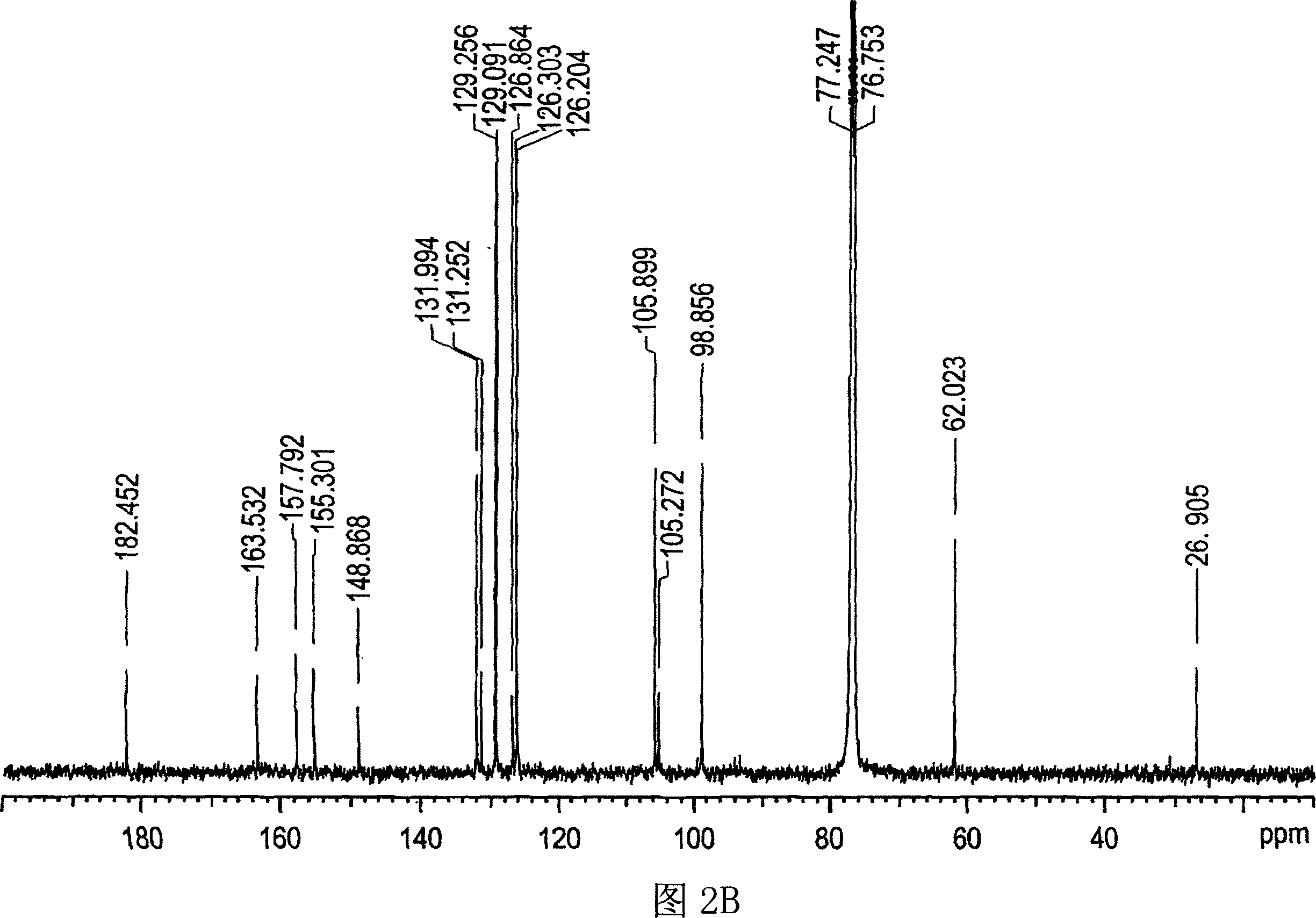
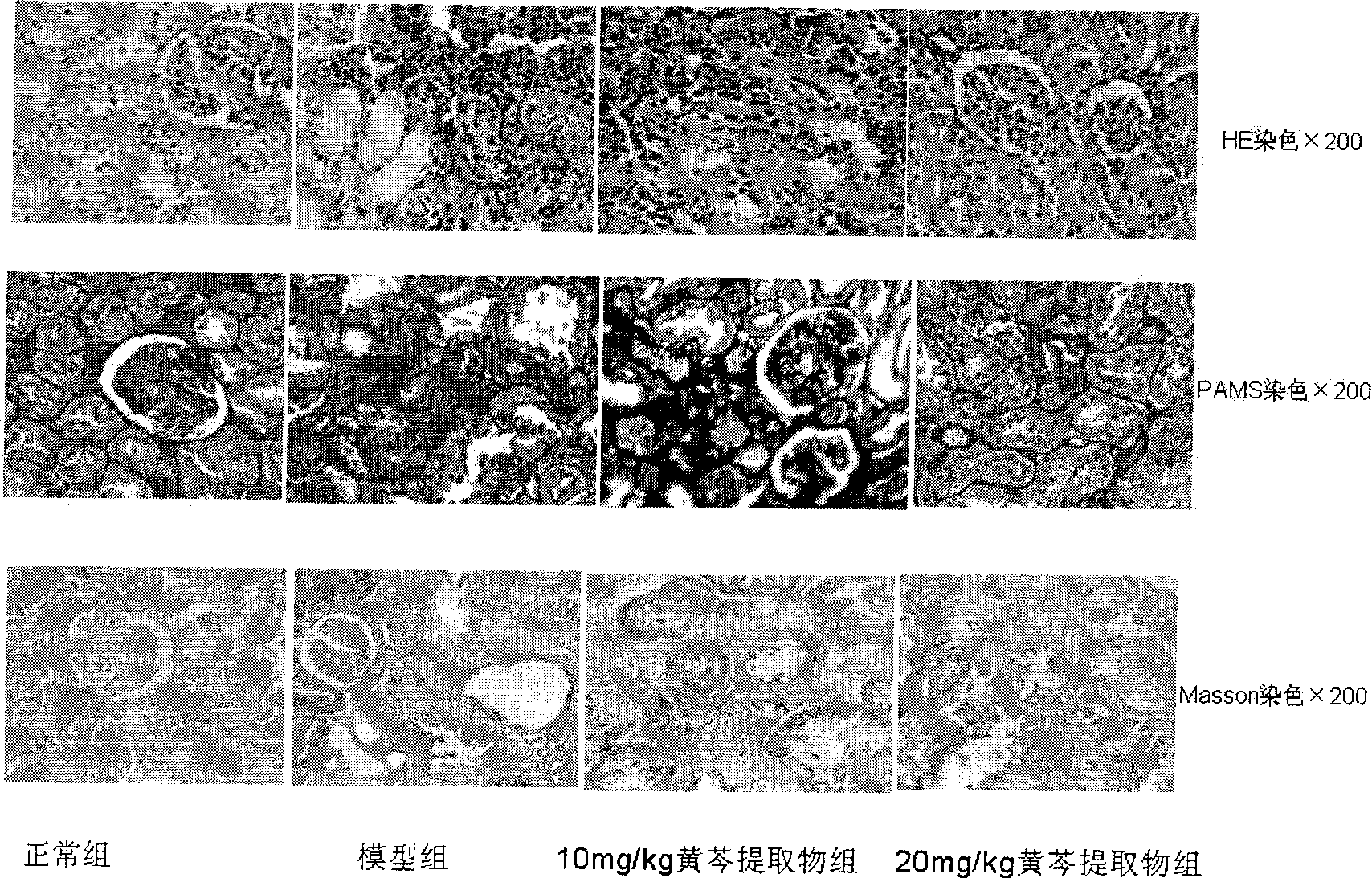
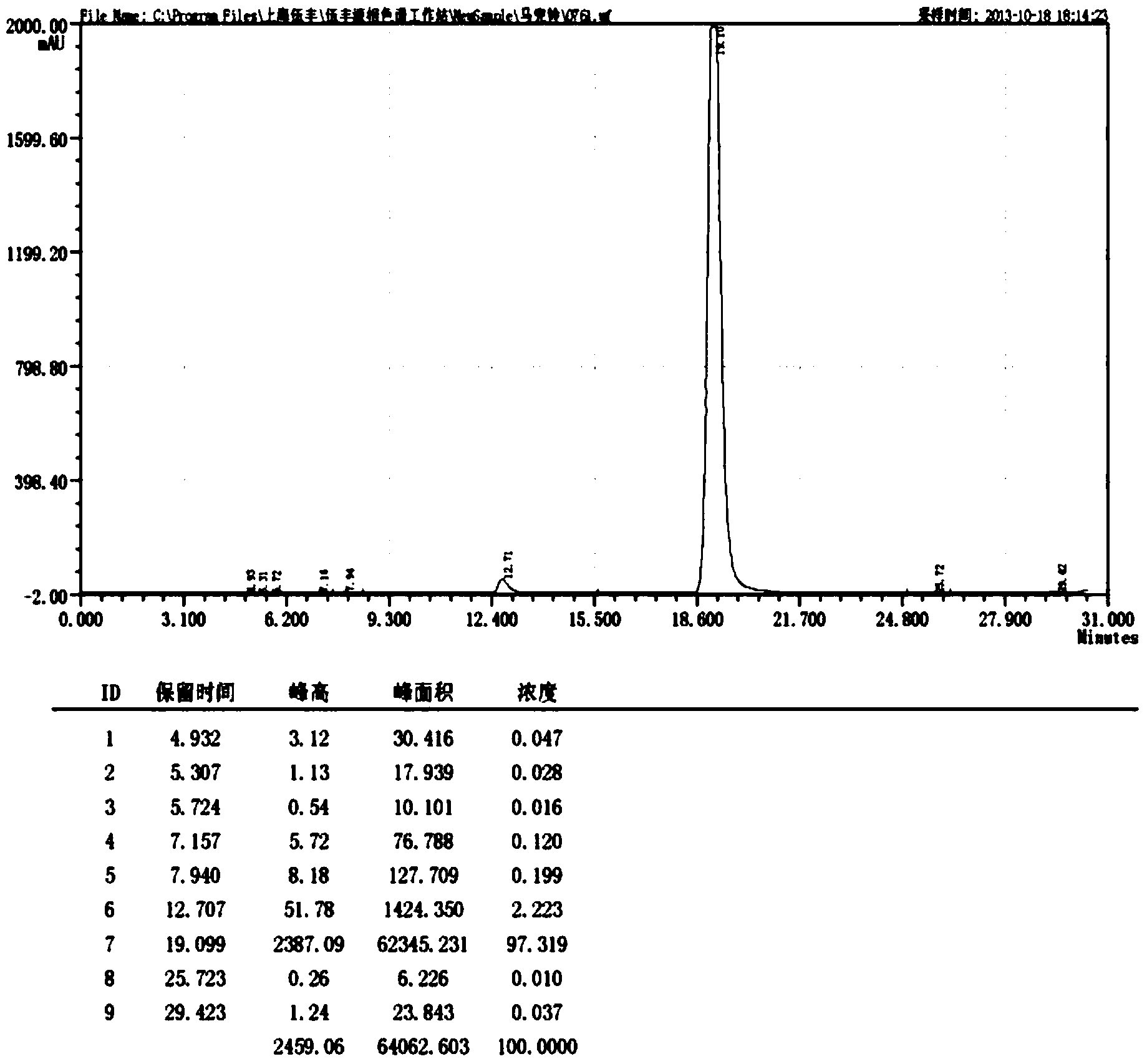
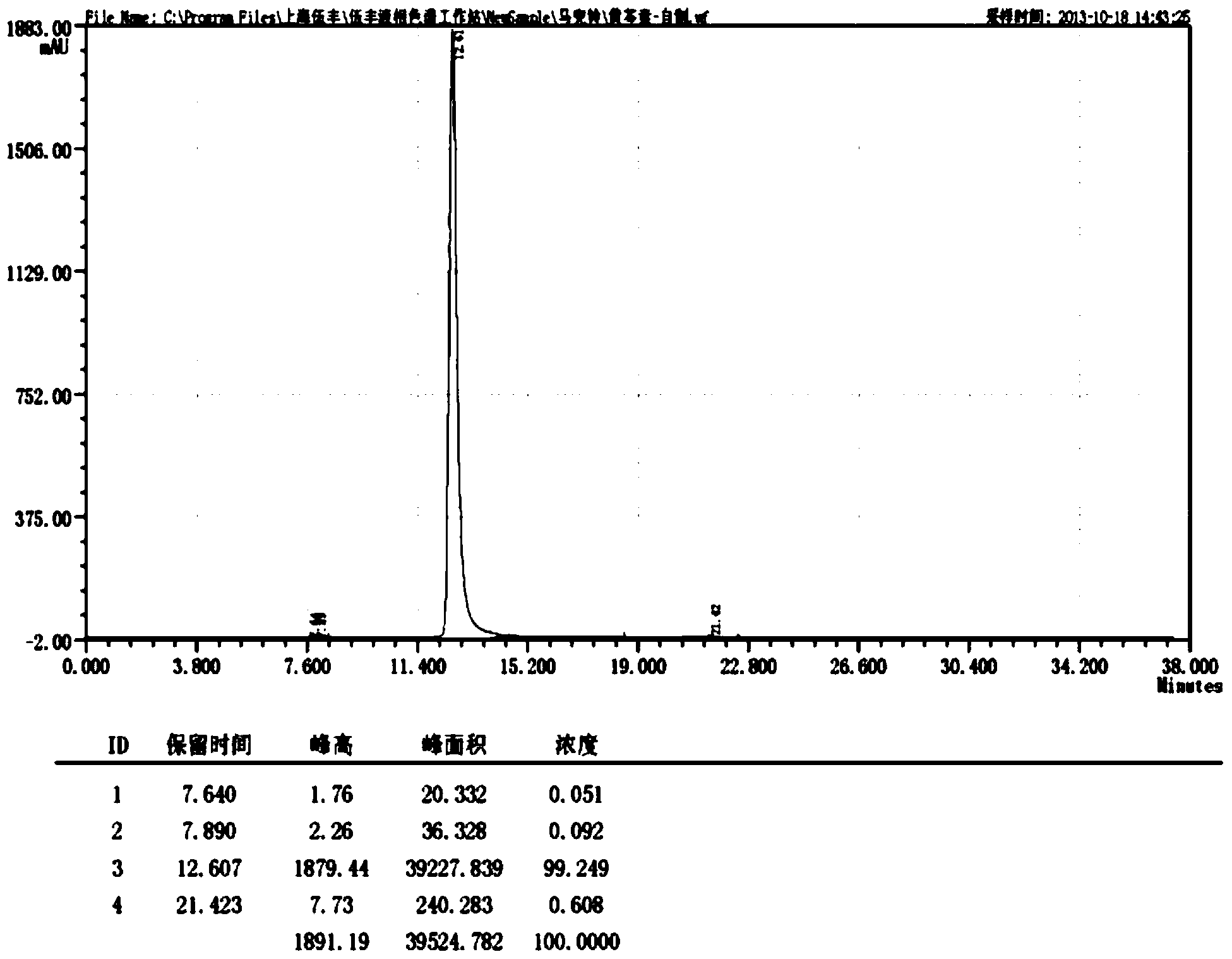
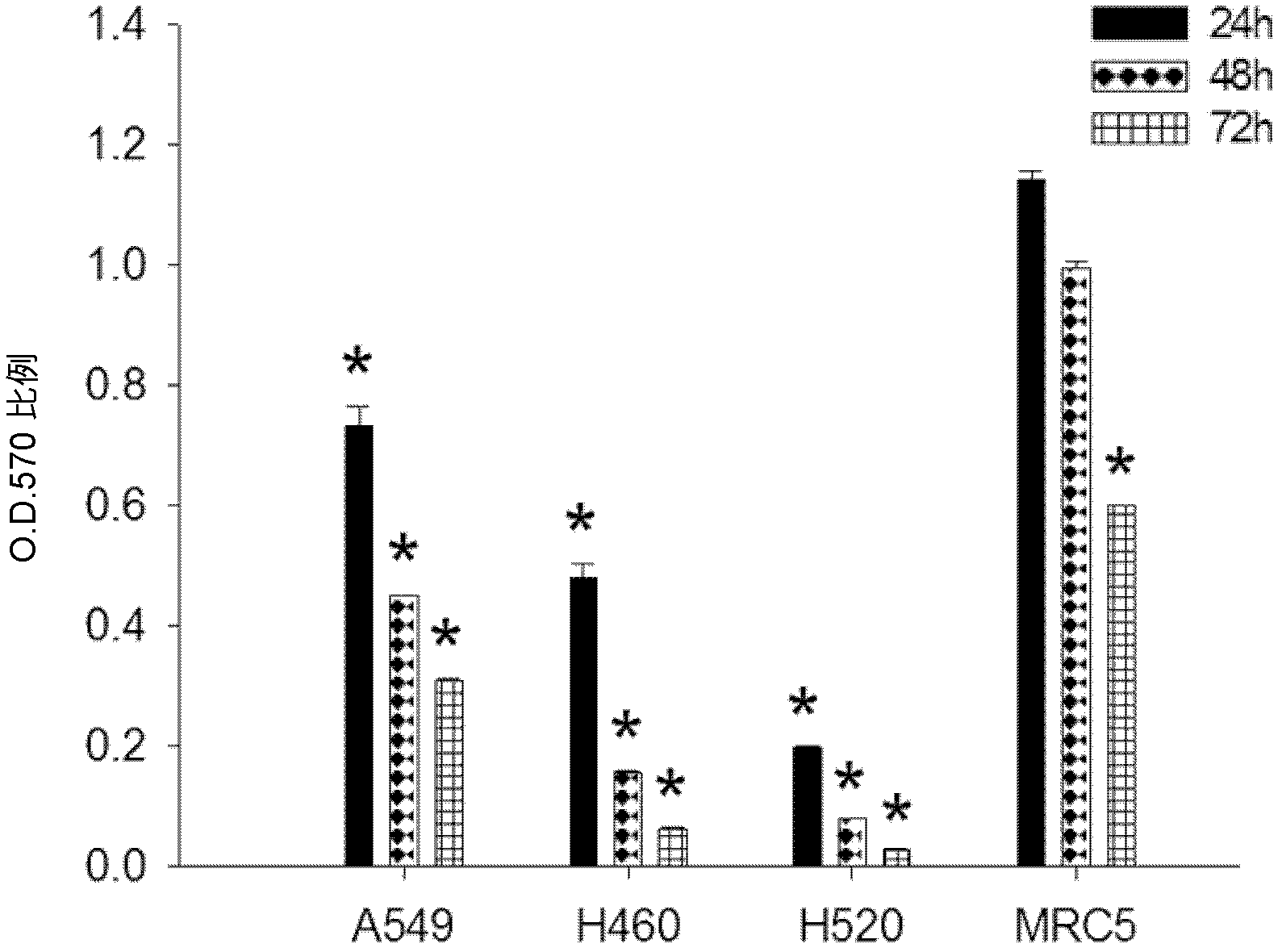
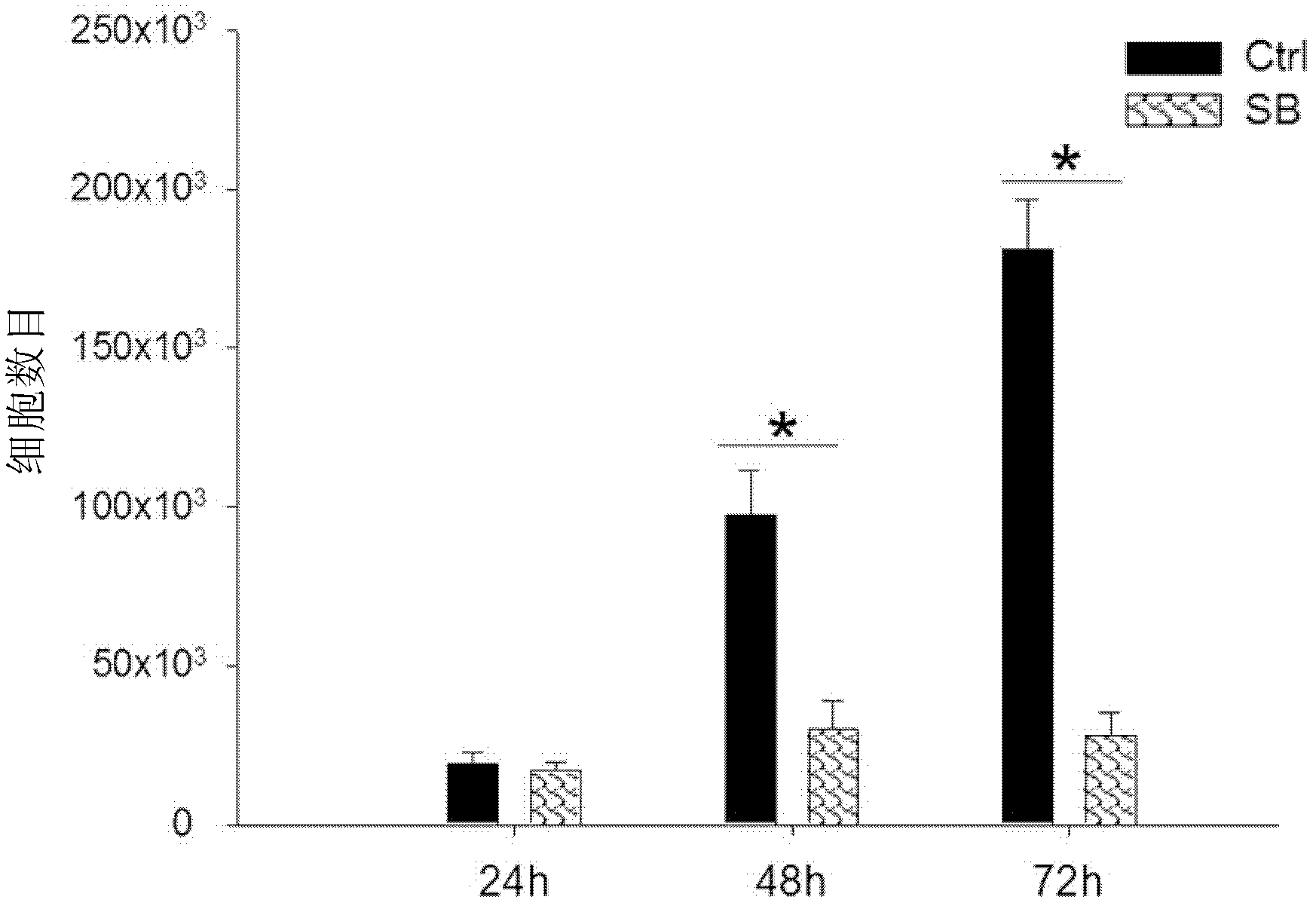
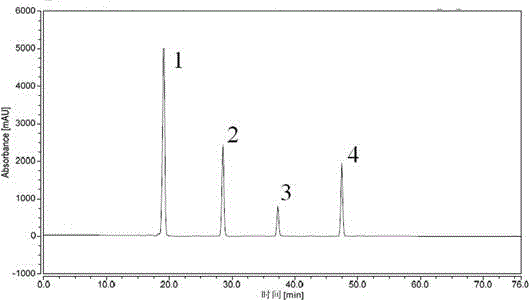
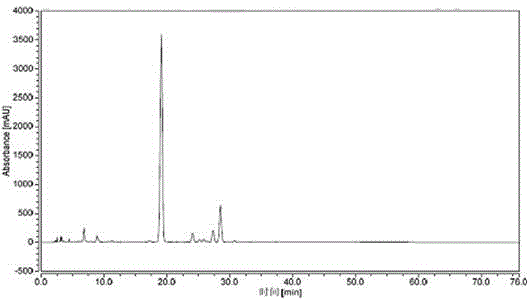
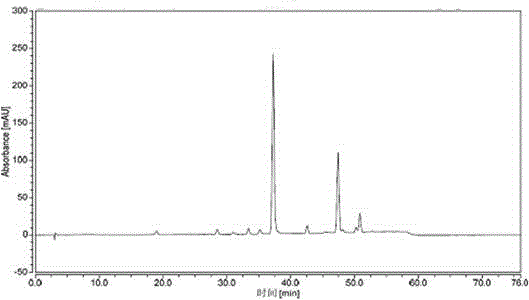
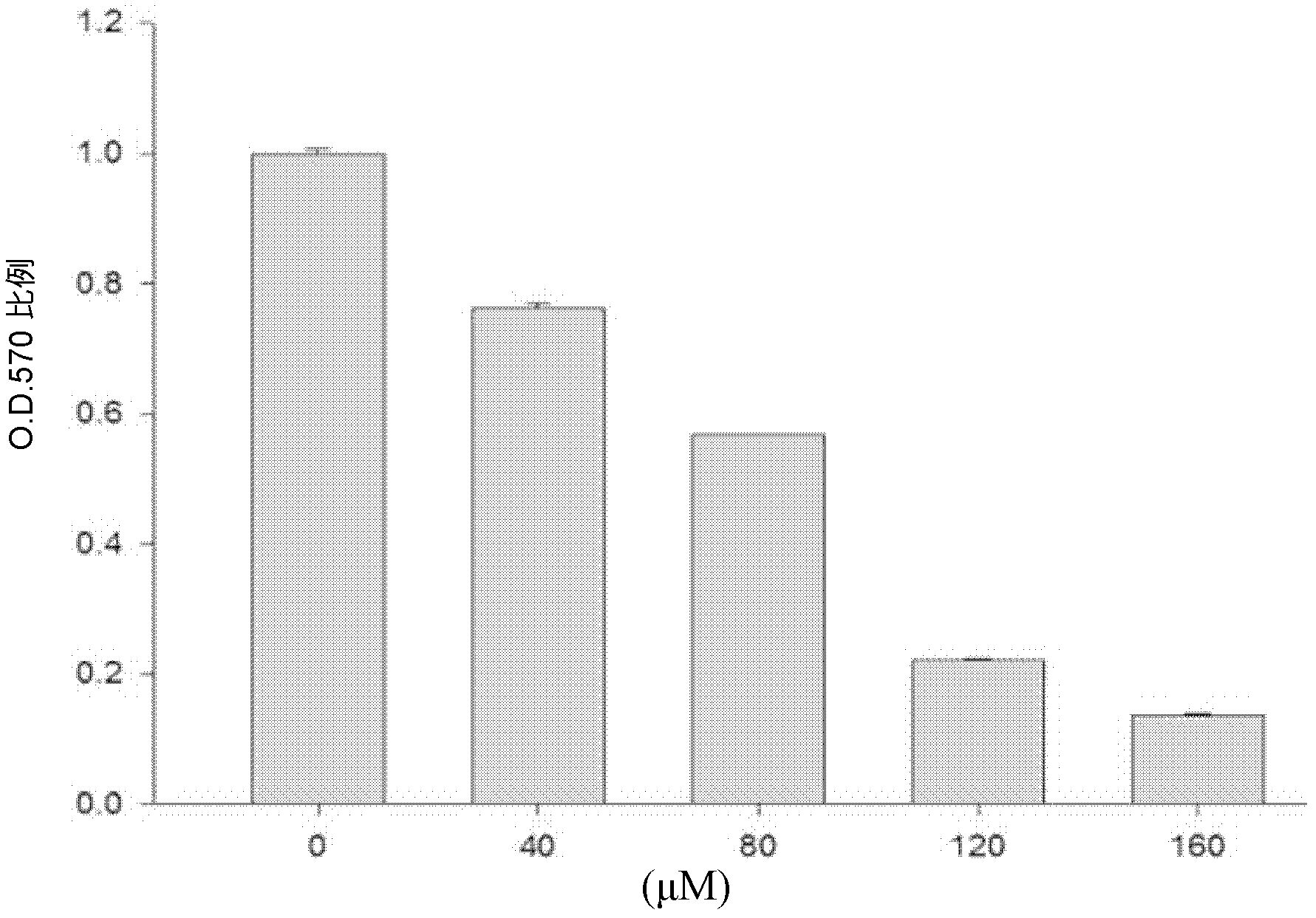Patents
Literature
110 results about "Wogonin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
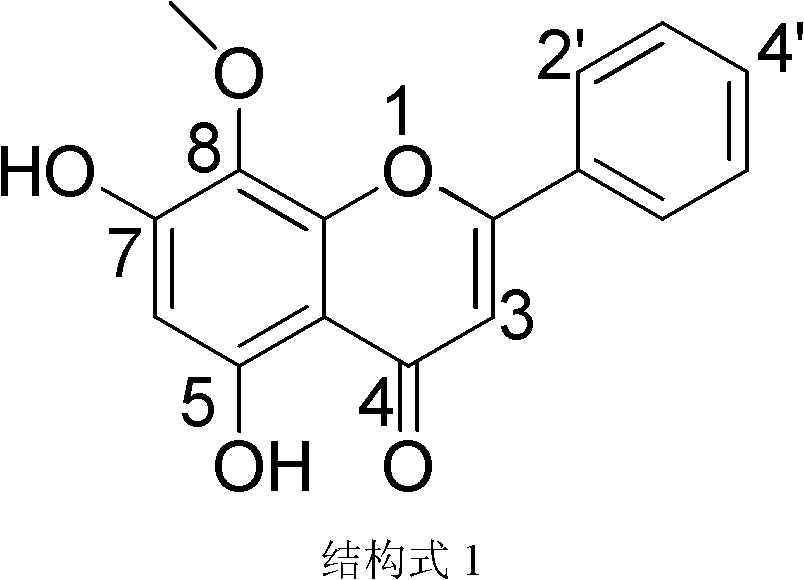
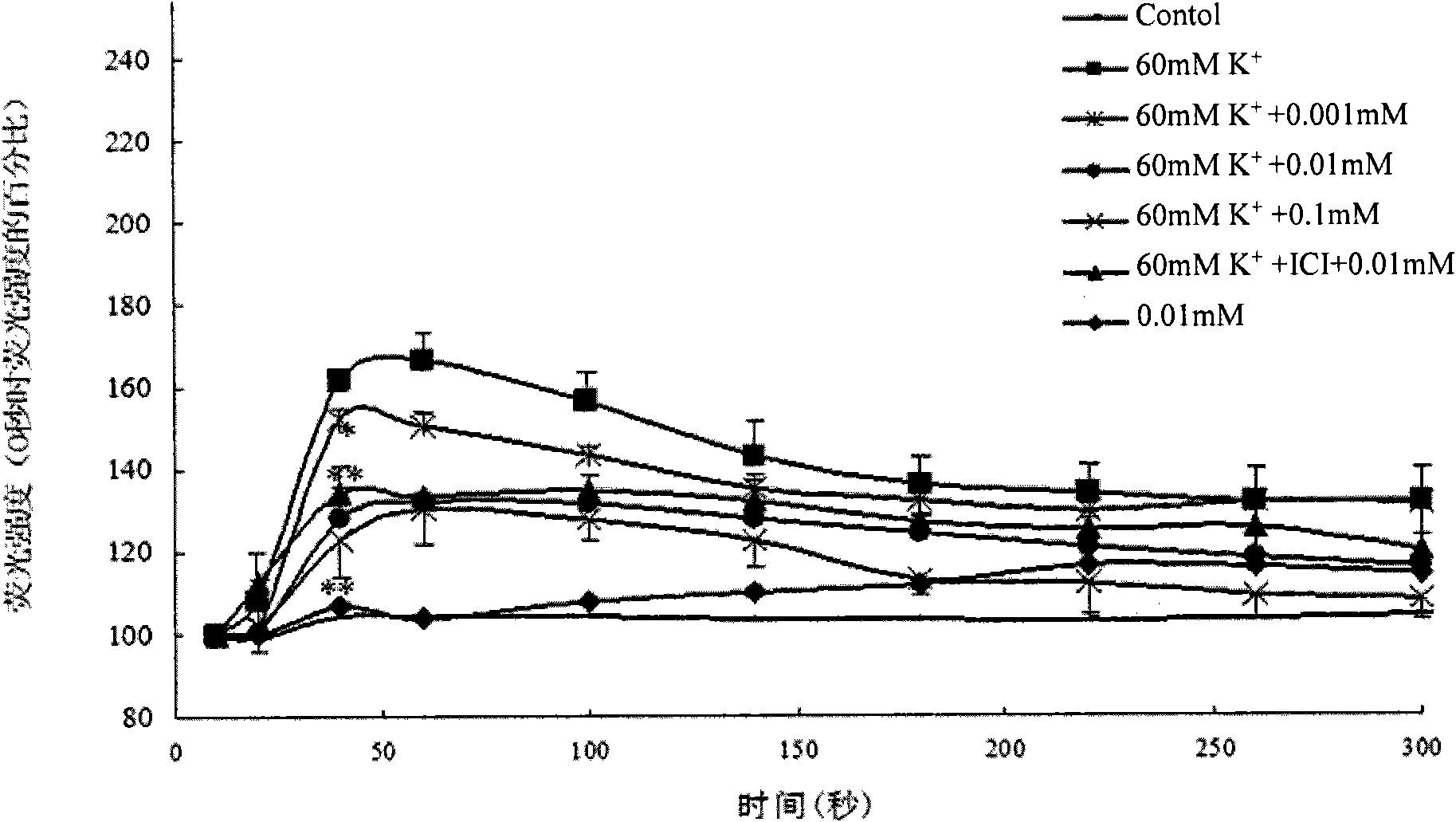
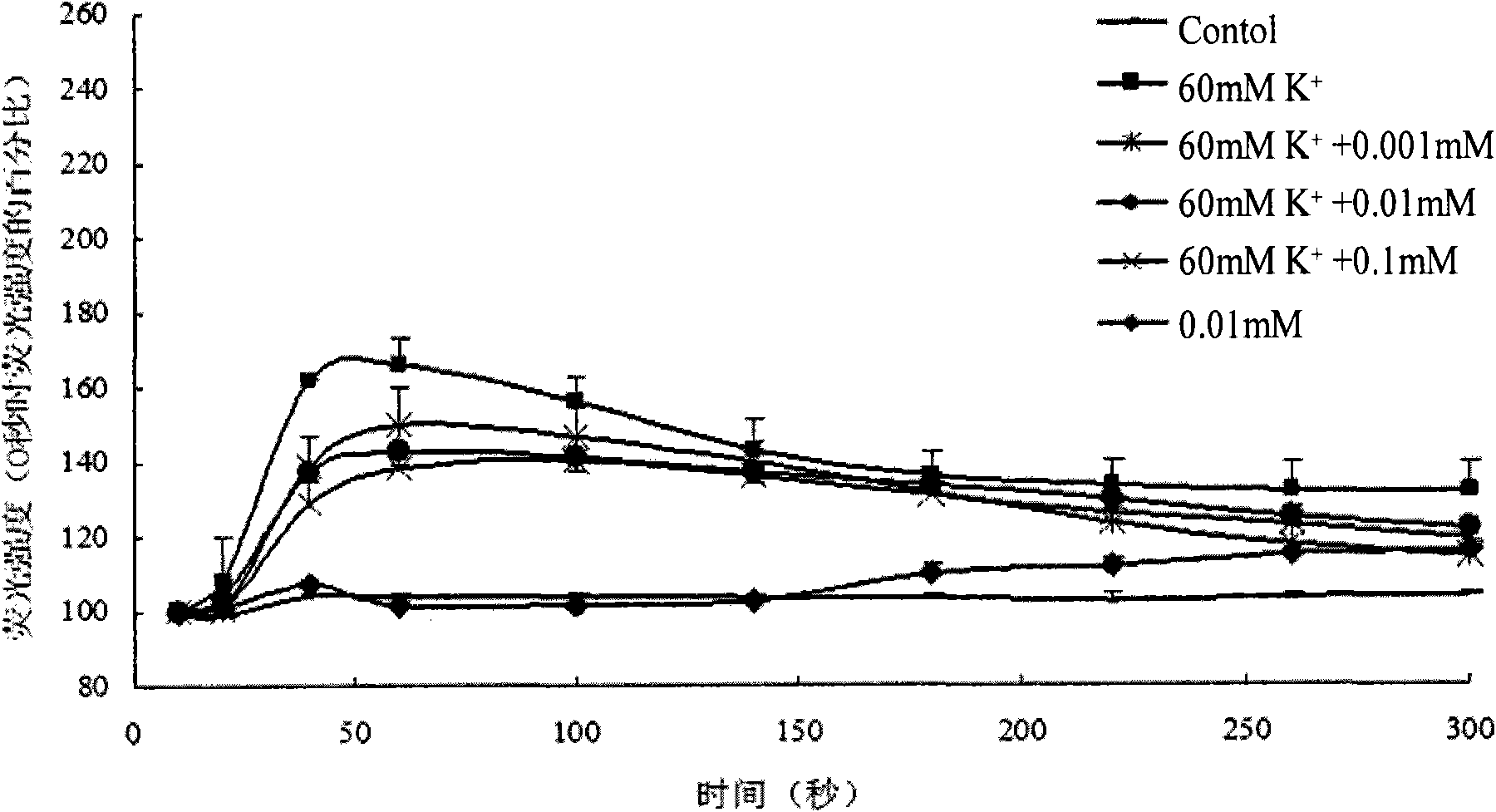
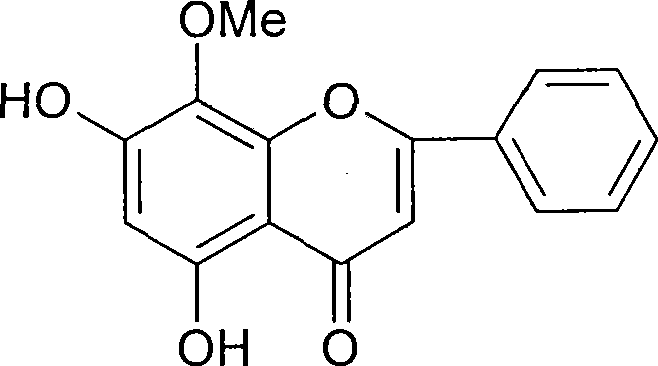
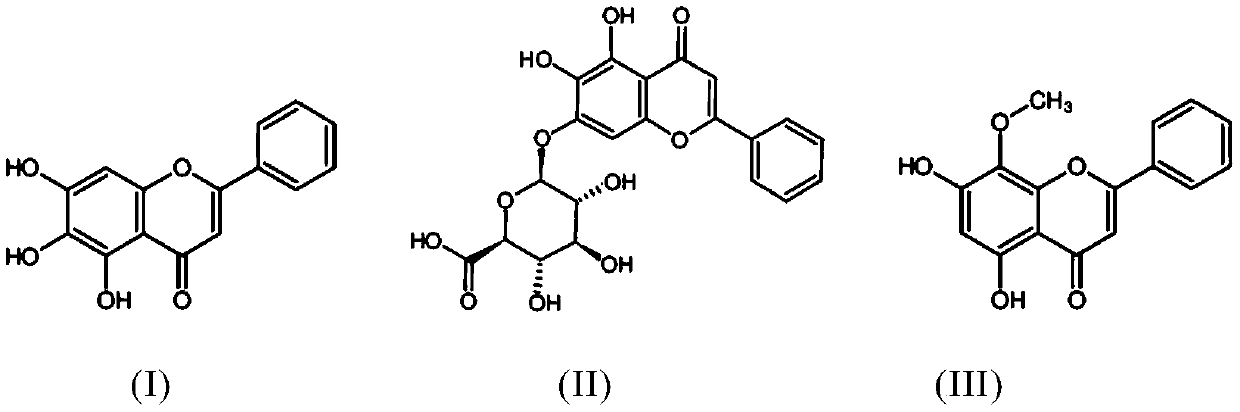
Wogonin is an O-methylated flavone, a flavonoid-like chemical compound which was found in Scutellaria baicalensis. The glycosides of wogonin are known as wogonosides. For example, oroxindin is a wogonin glucuronide isolated from Oroxylum indicum. It is one of the active ingredients of Sho-Saiko-To, a Japanese herbal supplement.
Botanical extract compositions and methods of use
A composition having phytoestrogenic and anti-cancer activity is described. The composition comprises wogonin, isoliquiritigenin, coumestrol, their pharmaceutically acceptable salts or esters, their selectively substituted analogs, or combinations thereof. The compositions may also include an anti-cancer agent and / or an immune stimulant. A method for treating or preventing cancer or an estrogen-related disorder includes administering a therapeutically effective amount of the compositions is described. The compositions are particularly useful in the treatment of hormone-related cancers.
Owner:ACTIVEPHYTO TECH LTD
Total flavone glycoside extract of Radix scutellariae, Rodix scutellariae monomer flavone glycoside, its preparation and use
This is a kind of baicalin total flavones glucoside distillation, baicalin monomer flavones glucoside and its preparation method and application, belonging to the Chinese native medicine drugs manufacturing technology field. The invention obtains the baical in total flavones glucoside distillation mainly containing biacalein, or baical in flavones glucoside monomer compound mainly containing biacalein or wogonoside or oroxylin; comminute baixcal, add water, have alcoholysis reaction at 41 -46 Deg. C, the baialinase and baicalin flavones glucoside naturally existing in baixcal directly have alcoholysis reaction without distillation and separation. The pharmacy preparation includes various pharmacy preparations comprised of officinal auxiliary materials with baicalin total flavones glucoside distillation or monomer flavones glucoside as pharmacy active components, and are used to prepare drugs for curing liver diseases and AIDS. The reaction condition of the invention is mild, and it has high distilling rate, good product quality, low energy waste simple craft, no pollution and low production cost.
Owner:SHANDONG UNIV
Application of extracting agent and deep-eutectic solvent in determining of effective ingredients in traditional Chinese medicine
InactiveCN108088943AImprove extraction efficiencyReduce lossComponent separationAdditive ingredientRadix Astragali seu Hedysari
The invention relates to the technical field of preparation of extracting agents and deep-eutectic solvents, and relates to application of an extracting agent and a deep-eutectic solvent in determining of effective ingredients in traditional Chinese medicine, in particular to application in determining of flavonoid ingredient in radix scutellariae, and application of an extracting agent and a deep-eutectic solvent in determining of effective ingredients of baicalin, baicalein, wogonoside, wogonin and scutellarin in radix scutellariae. The application is characterized in that the radix scutellariae is used as the raw material, the deep-eutectic solvent, especially choline deep-eutectic solvent, is used as the extracting agent, the water is used as a thinner for reducing the system viscosity, and the flavonoid compound is forcibly extracted under the ultrasonic condition. An extracting method has the advantages that the extracting efficiency of the flavonoid ingredient is higher, the extracting conditions are moderate, and the operation is simple and convenient. The extracting agent has the characteristics of no burning explosion and volatizing, small loss, low energy consumption andthe like. The extracting method is also suitable for extracting the flavonoid-containing traditional Chinese medicines, such as radix astragali seu hedysari, liquorice root, radix puerariae and radixsophorae flavescentis.
Owner:SHENYANG PHARMA UNIVERSITY
Inhibitors and Enhancers of Uridine Diphosphate-Glucuronosyltransferase 2B (UGT2B)
ActiveUS20090074708A1Increase heightReduced activityBiocideHydroxy compound active ingredientsPolyethylene glycolEriodictyol
A UGT2B inhibitor capable of increasing the bio-availability of a drug, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: capillarisin, isorhamnetin, β-naphthoflavone, α-naphthoflavone, hesperetin, terpineol, (+)-limonene, β-myrcene, swertiamarin, eriodictyol, cineole, apigenin, baicalin, ursolic acid, isovitexin, lauryl alcohol, puerarin, trans-cinnamaldehyde, 3-phenylpropyl acetate, isoliquritigenin, paeoniflorin, gallic acid, genistein, glycyrrhizin, protocatechuic acid, ethyl myristate, umbelliferone, PEG (Polyethylene glycol) 400, PEG 2000, PEG 4000, Tween 20, Tween 60, Tween 80, BRIJ® 58, BRIJ® 76, Pluronic® F68, Pluronic® F127, and a combination thereof. A UGT2B enhancer capable of enhancing a clearance rate of morphine-like analgesic agents, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: nordihydroguaiaretic acid, wogonin, trans-cinnamic acid, baicalein, quercetin, daidzein, oleanolic acid, homoorientin, hesperetin, narigin, neohesperidin, (+)-epicatechin, hesperidin, liquiritin, eriodictyol, formononetin, quercitrin, genkwanin, kaempferol, isoquercitrin, (+)-catechin, naringenin, daidzin, (−)-epicatechin, luteolin-7-glucoside, ergosterol, rutin, luteolin, ethyl myristate, apigenin, 3-phenylpropyl acetate, umbelliferone, glycyrrhizin, protocatechuic acid, poncirin, isovitexin, 6-gingerol, cineole, genistein, trans-cinnamaldehyde, and a combination thereof.
Owner:NAT DEFENSE MEDICAL CENT
Botanical extract compositions and methods of use
InactiveCN1984648AUnknown materialsHeterocyclic compound active ingredientsCancer preventionIMMUNE STIMULANTS
A composition having phytoestrogenic and anti-cancer activity is described. The composition comprises wogonin, isoliquiritigenin, coumestrol, their pharmaceutically acceptable salts or esters, their selectively substituted analogs, or combinations thereof. The compositions may also include an anti-cancer agent and / or an immune stimulant. A method for treating or preventing cancer or an estrogen related disorder includes administering a therapeutically effective amount of the compositions is described. The compositions are particularly useful in the treatment of hormone-related cancers.
Owner:MEDICAL RES & EDUCATION ASSOCS
Scullcap total-flavonoid aglycone extract, preparation method and use thereof
ActiveCN101455718AImprove weight ratioReduce collagen depositionUrinary disorderPlant ingredientsScutellareinMouse Stomach
The invention discloses a radix scutellariae total flavonoids aglycon extract, also a preparation method of radix scutellariae total flavonoids aglycon extract and functions of treating and resisting renal fibrosis. Content of total flavonoids aglycons in the radix scutellariae total flavonoids aglycon extract is 45-70%. The extract is mainly composed of scutellarein, wogonin, oroxylin A and other aglycons. The preparation method of the extract is as follows: crushing radix scutellariae roots, screening by a No. 1 screen; adding 1-5 times amount of water, uniformly stirring, keeping temperature for enzymolysis at 32-40 DEG C for 5-9 hours, baking under vacuum at 60 DEG C, adding 2-8 times amount of ethyl acetate, continuously extracting for 6-10 hours, depressurizing, recycling solvent, thus obtaining total flavonoids aglycon extract. The extract can obviously relieve renal fibrosis symptoms when irrigated into a mouse stomach with renal fibrosis caused by mercuric chloride moulding and when a group of mice having the extract is compared with group of mice without having the extract. Results suggest that the total flavonoids aglycon extract has a dose dependence in preventing renal fibrosis.
Owner:SHANGHAI UNIV OF T C M
Gene sequence related to flavone composite in radix scutellariae and application thereof
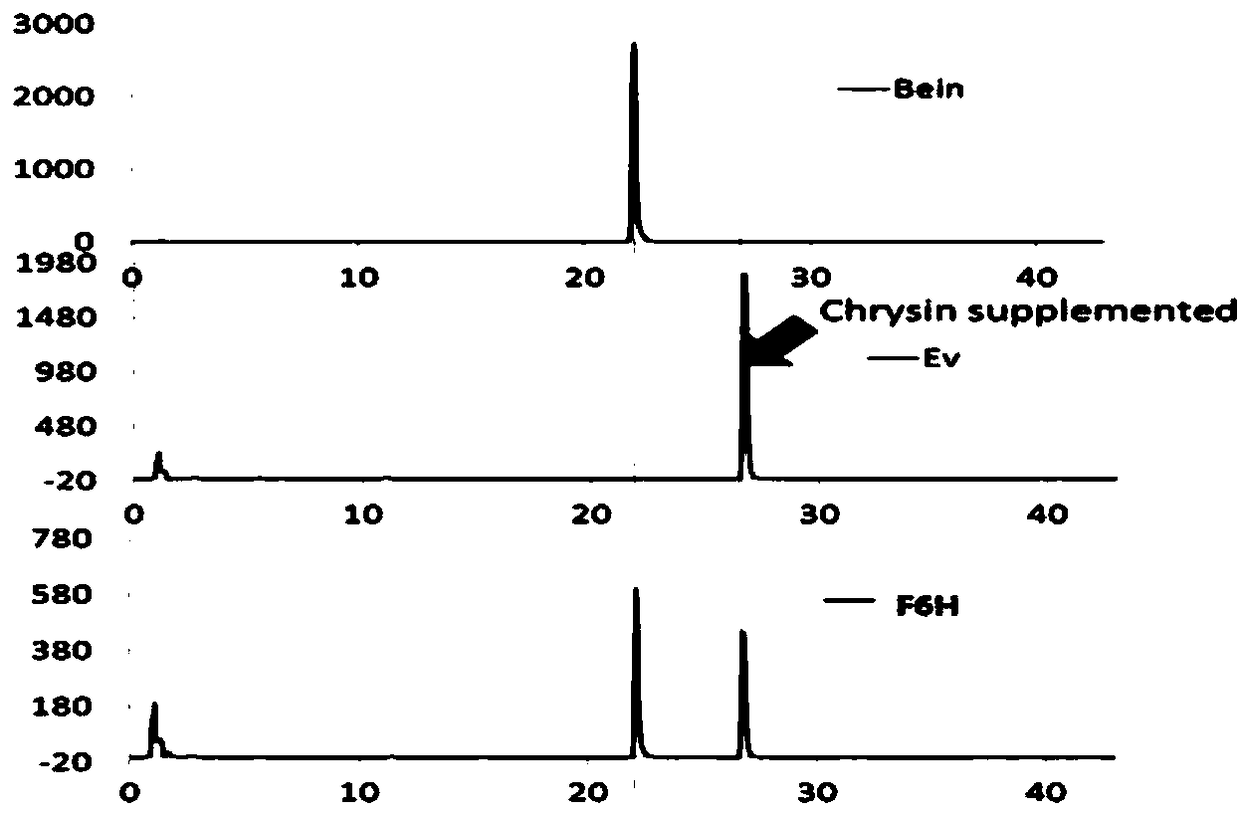
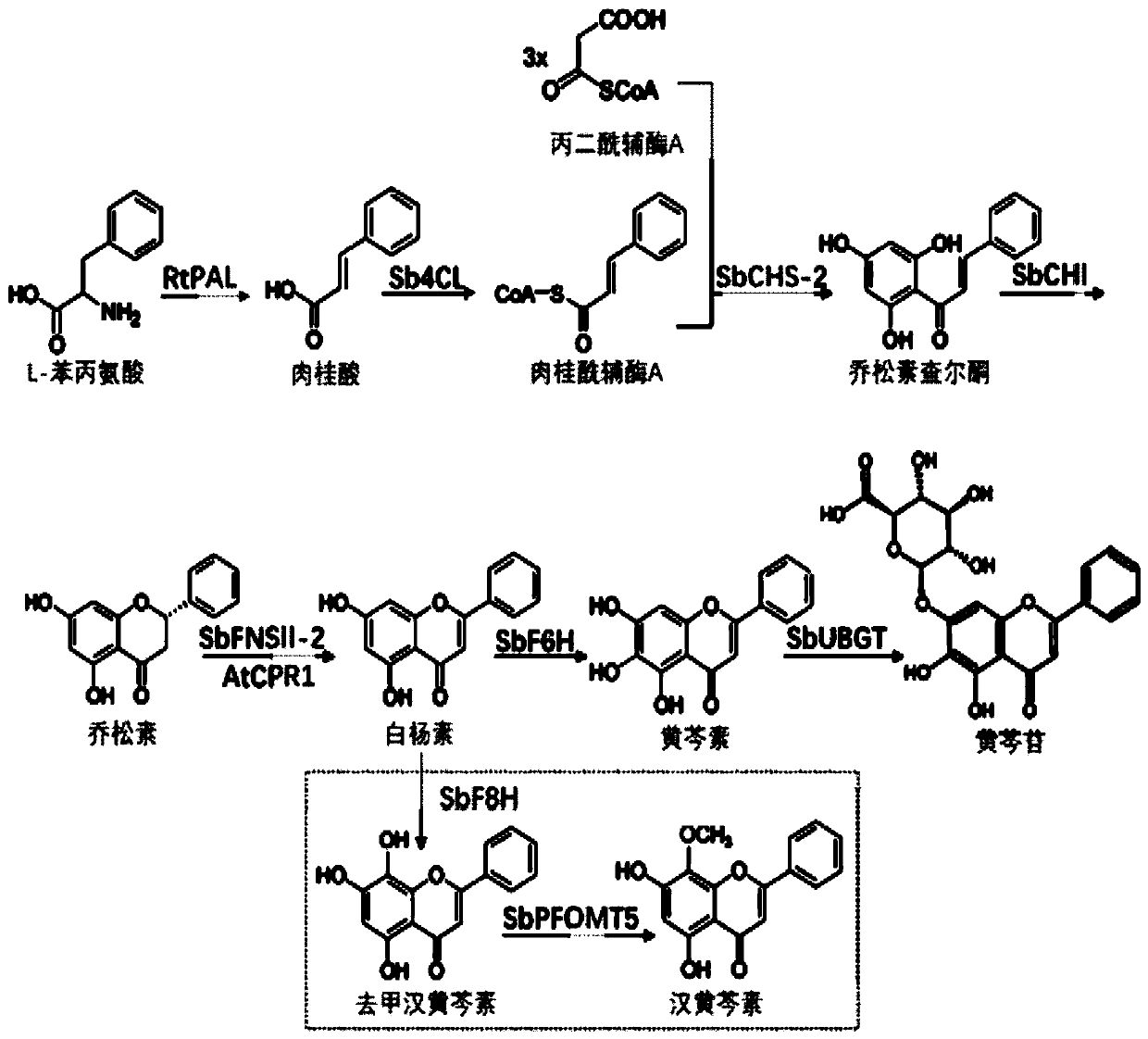
The invention discloses a gene related to flavone composite in radix scutellariae. The gene includes one or several in flavone synthetase gene with a sequence shown in SEQ ID No.1, flavone 6-site hydroxylase gene with a sequence shown in SEQ ID No.3 and flavone 8-site hydroxylase gene with a sequence shown in SEQ ID No.5. The invention further provides primer composite for amplifying the gene, protein encoded by the gene, a recombinant vector, host cell or transgenic cell line containing the gene and application thereof. The protein encoded by the gene disclosed by the invention can participate in synthesizing baicalein and wogonin and catalyzing synthesis and hydroxylation reaction of flavone matters of the similar structures, can provide very good basis for producing effective active matters, provides theoretical basis for producing baicalein, wogonin and aglycone of the baicalein and the wogonin in a large scale and establishes solid basis for industrially producing other related flavonoid compound.
Owner:SHANGHAI CHENSHAN BOTANICAL GARDEN
New application of chemical component of eucommia bark used as plant estrogen
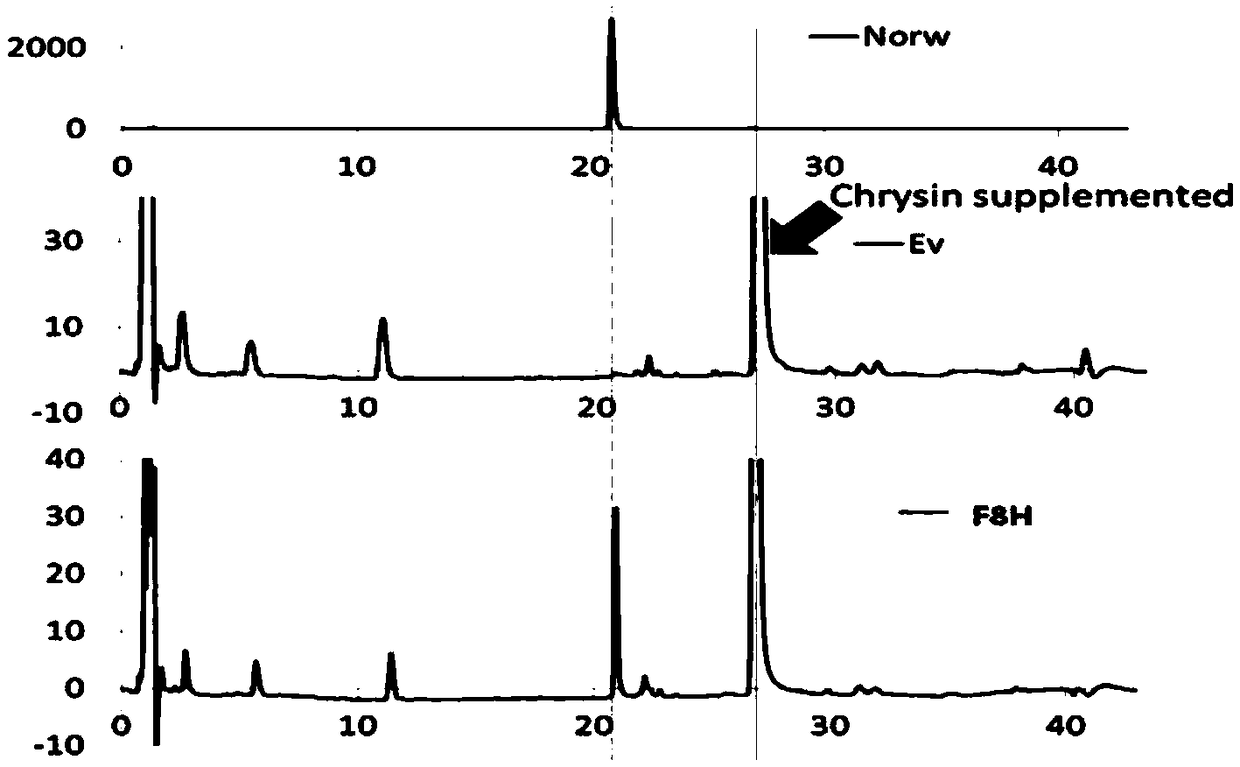
The invention discloses a new application of a chemical component of chemical component used as plant estrogen, in particular relates to an application of an eucommia bark extract in preparing a medicine for treating and / or preventing a disease relevant to the estrogen hyposecretion of a mammal (particularly a human). The invention relates to an application of a composition in preparing a medicine for treating and / or preventing a disease relevant to the estrogen hyposecretion of a mammal (particularly a human), which is selected from one or more of the following components: betulinic acid, genipin, aucubin, pinoresinol diglucoside, syringaresinol diglucoside, abietinol monoglucoside, syringaresinol monoglucoside, wogonin, oroxylin A, baicalein and dihydrochalcone 3-O-beta-D-glucoside and a medicine composition containing the eucommia bark extract or an active component in the eucommia bark extract. The eucommia bark extract and a monomer chemical component in the eucommia bark extracthave the effect of effective plant estrogen.
Owner:JIANGXI POZIN PHARMA
Inhibitor or promoter of uridinediphosphate glucuronosyltransferase 2B (UGT2B)
The present invention provides one kind of UGT2B inhibitor capable of raising the bioavailability of medicine. The UGT2B inhibitor is one or the composition of capillarisin, isorhamnetin, beta-naphthoflavone and other compounds and in the form of alkali or pharmaceutically acceptable salt. The present invention also provides one kind of UGT2B promoter capable of promoting the detoxication function of liver. The UGT2B promoter is one or the composition of nordihydroguaiaretic acid, wogonin, trans-cinnamicacid and other compounds and in the form of alkali or pharmaceutically acceptable salt.
Owner:INT EDUCATION FOUND
Method for simultaneously separating wogonin and baicalein monomers from scutellaria baicalensis
InactiveCN103555784AGood clinical valueHigh yieldFermentationBulk chemical productionBaicaleinEnzyme
The invention provides a method for simultaneously separating wogonin and baicalein monomers from scutellaria baicalensis. The method comprises the steps of crushing a scutellaria baicalensis medicinal material, adding a proper amount of water and edible enzyme to carry out enzymolysis, supercritically extracting the medicinal material subjected to the enzymolysis, fractionally collecting the medicinal material by using an entrainer, and re-crystallizing to respectively obtain baicalein and wogonin. By using the method, baicalein compound monomers can be simultaneously extracted from the scutellaria baicalensis medicinal material, the yield is increased by over 3 times than the yield of wogonin and baicalein monomers obtained by using a direct separation method, and the purities of the prepared wogonin and baicalein monomers are both over 98%; moreover, the method is simple and convenient in flow, environment-friendly, very important in production practical significance, and suitable for large-scale industrial production.
Owner:TIANJIN SHILAN TECH CO LTD
Pharmaceutical composition for inhibiting cancer stem cell growth or cancer cell metastasis by using wogonin, and application thereof
InactiveCN102973550ASuppress bottleneckOrganic active ingredientsAntineoplastic agentsCancer cellLymphatic Spread
The invention relates to a pharmaceutical composition for inhibiting cancer stem cell growth or cancer cell metastasis by using wogonin, and an application thereof. The pharmaceutical composition comprises a wogonin compound and a pharmaceutically acceptable vector. The application of the pharmaceutical composition is to prepare medicaments for inhibiting the cancer stem cell growth or the cancer cell metastasis by using the wogonin compound.
Owner:谢秀梅 +1
Method for simultaneously extracting baicalin, baicalein and wogonin from scutellaria baicalensis
ActiveCN104610401AImprove resource utilizationEfficient extractionSugar derivativesSugar derivatives preparationBaicaleinAlcohol ethyl
The invention discloses a method for simultaneously extracting baicalin, baicalein and wogonin from scutellaria baicalensis. The method comprises the following steps: extracting scutellaria baicalensis with water and concentrating to obtain a concentrated solution; rinsing the concentrated solution on a macroporous adsorption resin column till the solution is colorless, and then respectively eluting by using 60-70vol% ethyl alcohol and 75-80vol% ethyl alcohol and collecting eluants respectively; concentrating the eluant containing baicalin under reduced pressure, regulating pH, preserving the heat, standing, filtering to obtain pure baicalin; and evaporating the eluant containing baicalein and wogonin to dryness, eluting with methyl alcohol on a gel column, collecting baicalein fraction and wogonin fraction, and removing methyl alcohol out of the fractions to obtain pure baicalein and pure wogonin. The method has the advantages that the extraction rate is high, the loss of active ingredients is reduced and the resource utilization rate of scutellaria baicalensis is improved.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
Method of preparing, separating and purifying baicalein and wogonin by endogenous enzymatic hydrolysis of baicalin and wogonoside in scutellaria
InactiveCN103160549AHigh purityEfficient extractionOrganic chemistryFermentationCavitationSilica gel
The invention relates to a method of preparing, separating and purifying baicalein and wogonin by endogenous enzymatic hydrolysis of baicalin and wogonoside in scutellaria, and aims at providing a simple, safe, economical and high-efficient method of extracting, separating and purifying the high-purity baicalein and wogonin. An adopted technical scheme is that the traditional Chinese medicine scutellaria is used as a raw material, a unique endogenous enzyme induction bioconversion technology, a negative-pressure cavitation extraction technology, a liquid-liquid extraction technique, a normal-phase silica-gel medium-and-low-pressure preparation liquid chromatography technology, a low-temperature crystallization and recrystallization technology and other high-efficient conversion, extraction, separation and purification technologies are used, and finally the high-purity baicalein and wogonin are obtained, wherein conversion rates of the baicalin and the wogonoside can respectively reach 98.39 % and 98.16 %, contents of the target products: baicalein and wogonin are increased by 4.47 times and 2.85 times, and a purity can be more than 98 %. The method overcomes disadvantages of large damaging effects on the baicalin and the wogonoside, low conversion rate and serious pollution existing in prior art, has advantages of simple production process, safe and environmental protection, high conversion rate and high target compound purity, and has a very important significance for development of medicines and health care products, and industrial production and application.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Pharmaceutical composition for preventing and treating rheumatoid arthritis
InactiveCN101700249AInhibition of clinical onsetOrganic active ingredientsAntipyreticChlorogenic acidEarly rheumatoid arthritis
The invention discloses a pharmaceutical composition for preventing and treating rheumatoid arthritis. The traditional Chinese medicine raw materials for constituting active ingredients of the pharmaceutical composition by weight are as follows: 5.5-6.0mg of berberine, 0.3-0.4mg of palmatine, 1.5-2.0mg of jatrorrhizine, 1.5-1.8mg of magnoflorine, 0.1-0.2mg of phellodendrine, 0.2-0.3mg of coptisine, 55-60mg of baicalin, 1.5-2.0mg of baicalein, 5.0-7.0mg of wogonoside, 0.03-0.05mg of wogonin, 20-25mg of geniposide, 0.1-0.2mg of crocin and 0.25-0.3mg of chlorogenic acid. The results of pharmacological tests show that the pharmaceutical composition can significantly reduce the disease severity of CIA mice with the rheumatoid arthritis, significantly improve the inflammation and the immune injury situation at lesion sites in comparison with a control group and be used for preparing drugs for preventing and treating the rheumatoid arthritis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
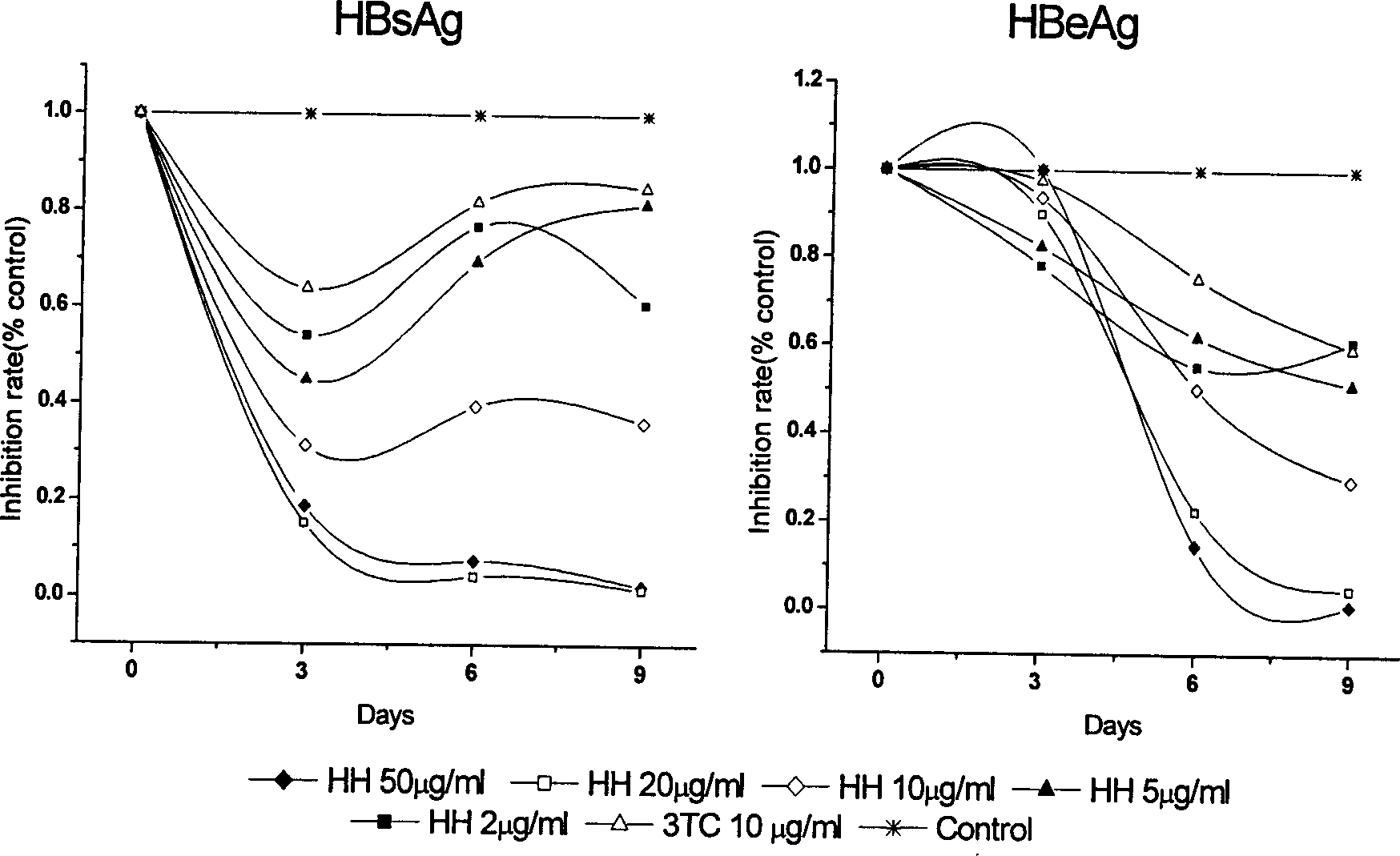
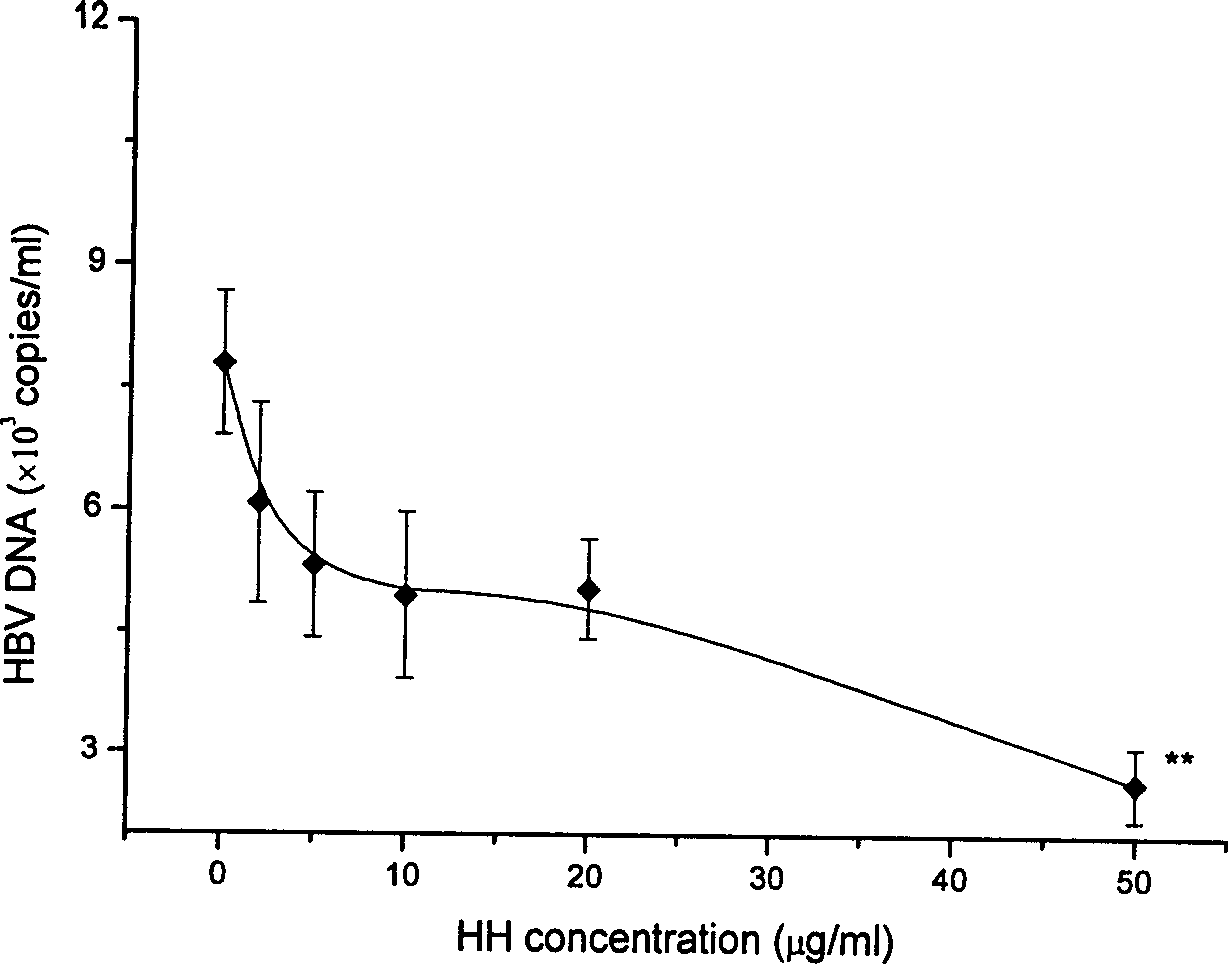
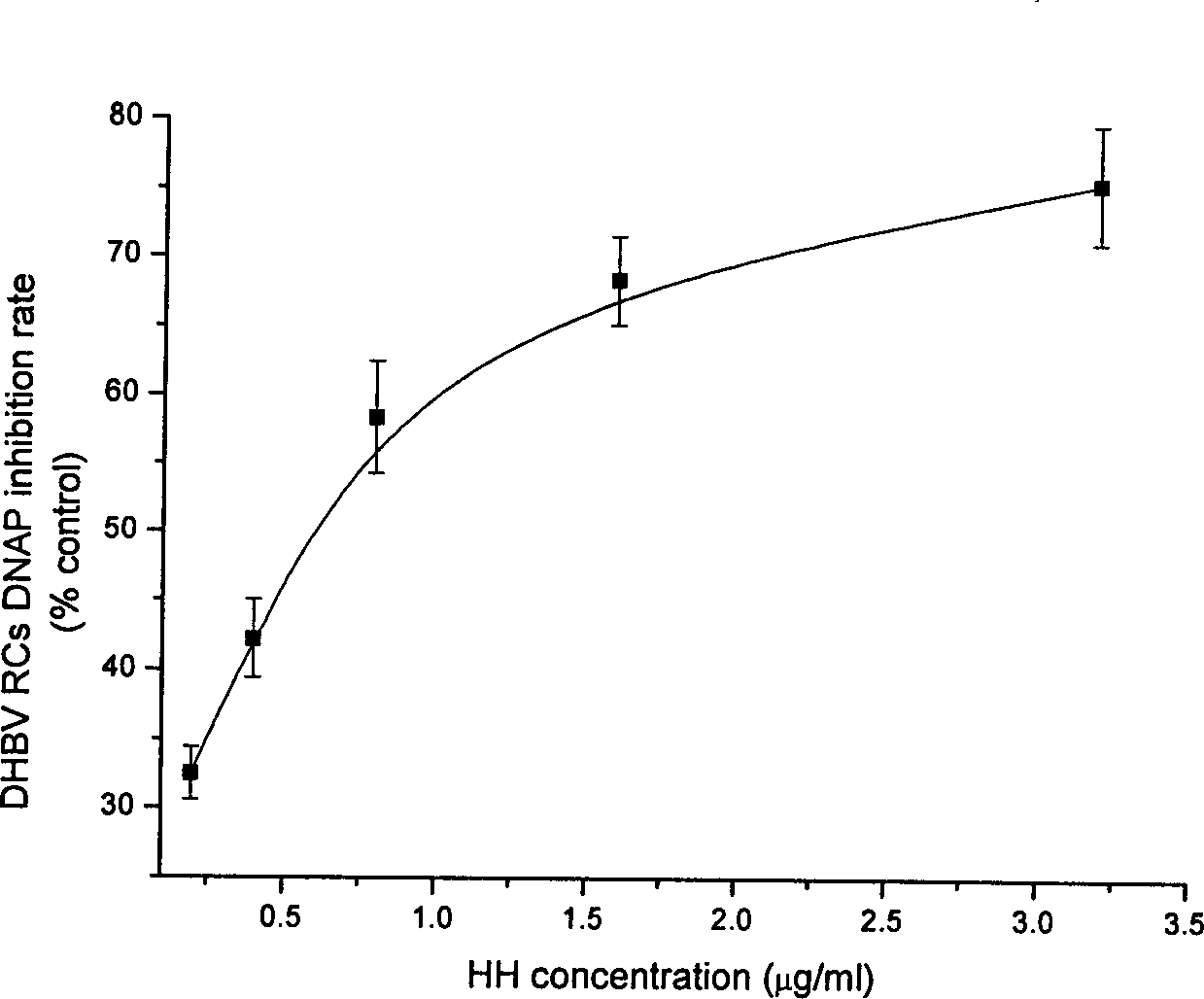
Application of wogonin for preparing medicine to treat or prevent hepatitis B
ActiveCN1785174AInhibition of replicationSignificant effectOrganic active ingredientsDigestive systemE AntigensSerum ige
Owner:CHINA PHARM UNIV +1
Novel purpose of eucommia chemical components as blood vessel protective agent
ActiveCN102552372APrevent proliferationNervous disorderMetabolism disorderDiseaseVascular proliferation
Owner:JIANGXI POZIN PHARMA
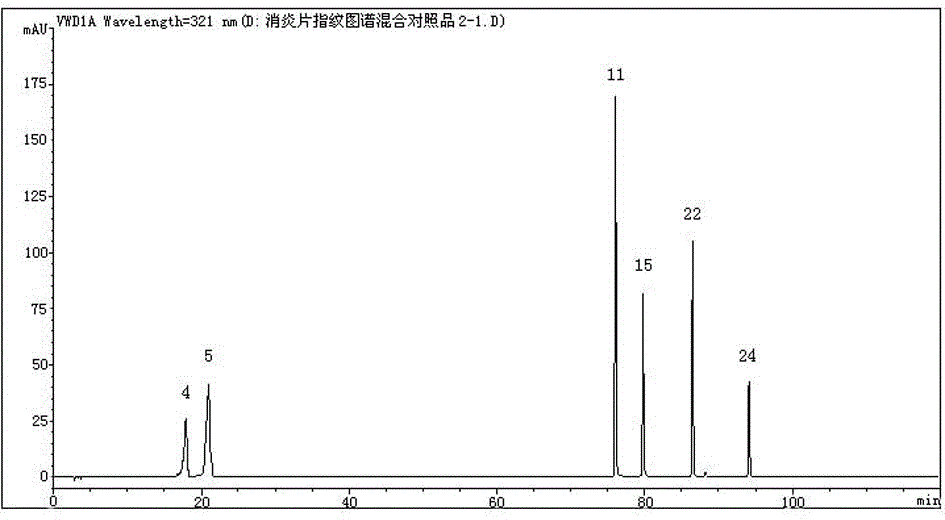
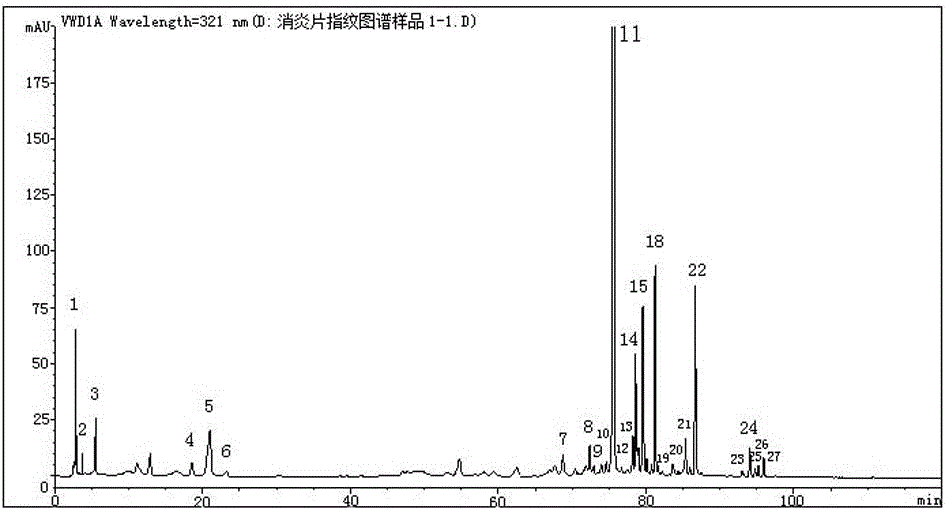
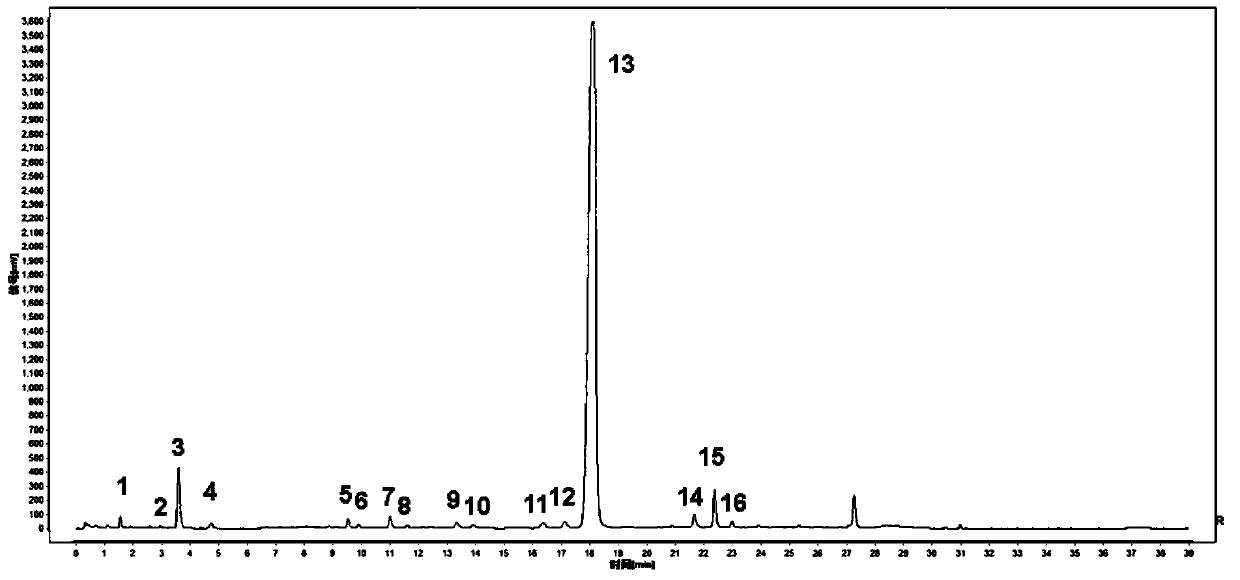
Anti-inflammatory tablet HPLC fingerprint construction method
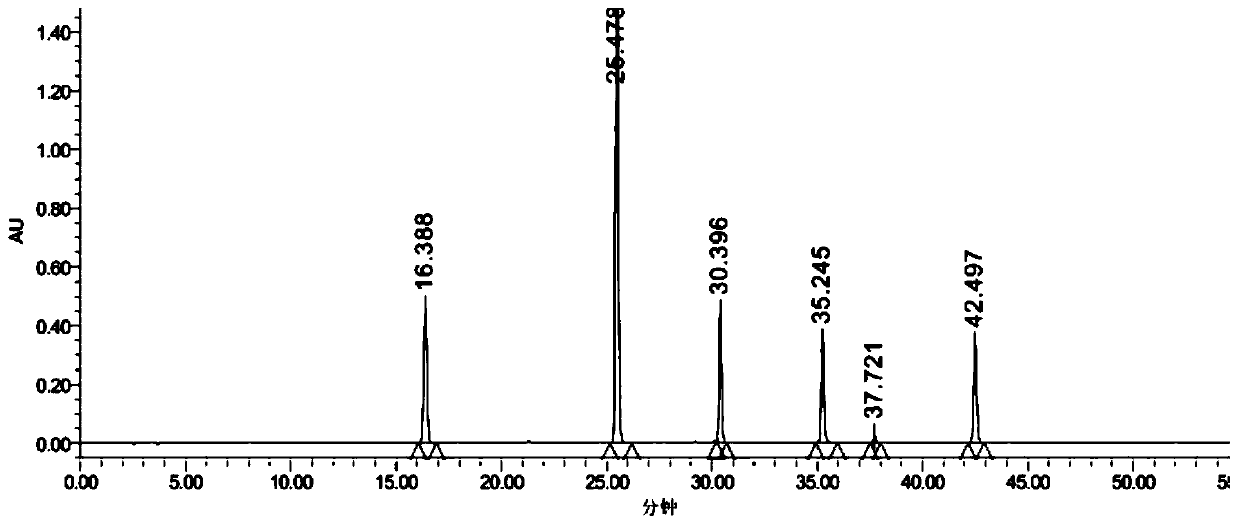
An anti-inflammatory tablet HPLC fingerprint construction method comprises the following steps: 1, preparing a test solution: grinding an anti-inflammatory tablet, weighing the ground tablet, placing the ground tablet in an extractor, adding petroleum ether, carrying out hot reflux extraction, removing the petroleum ether, adding methanol, carrying out ultrasonic treatment, supplementing methanol, and filtering the obtained solution; 2, preparing a reference solution: taking a chlorogenic acid reference substance, an aesculetin reference substance, a scutelloside reference substance, a linarin reference substance, a baicalein reference substance and a wogonin reference substance; and 3, determining: respectively taking the reference solution and the test solution, respectively injecting the reference solution and the test solution to a liquid chromatograph, recording the chromatogram in 120min, and processing the chromatogram through using fingerprint software to obtain the fingerprint of the anti-inflammatory tablet. The method has the advantages of establishing the common mode of the HPLC characteristic fingerprint of the anti-inflammatory tablet, calibration of 27 common peaks, effective characterization of the quality of the anti-inflammatory tablet, overcoming of unicity and one-sidedness of original quality control methods, and high application values.
Owner:吉林修正药业新药开发有限公司 +1
Lanqin oral solution fingerprint spectrum establishment method, fingerprint spectrum of Lanqin oral solution and application of fingerprint spectrum
ActiveCN110108825ACharacterizing qualityAvoid one-sidednessComponent separationBenzoic acidChlorogenic acid
The invention discloses a Lanqin oral solution fingerprint spectrum establishment method, a fingerprint spectrum of a Lanqin oral solution and application of the fingerprint spectrum. A test solutionis prepared from the Lanqin oral solution, a reference substance solution is a solution which dissolves adenosine, epigoitrin, phellodendrine chloride, chlorogenic acid, magnoflorine, geniposide, benzoic acid, berberine hydrochloride, baicalin, wogonoside, baicalein and wogonin, and the liquid chromatography is measured by using a high-performance liquid chromatograph; the obtained liquid chromatography is introduced into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system for analysis, and the Lanqin oral solution fingerprint spectrum is obtained after multi-point correction, data matching and analysis. According to the Lanqin oral solution fingerprint spectrum establishment method and the fingerprint spectrum of the Lanqin oral solution, the quality information of the Lanqin oral solution can be comprehensively reflected, and uniform and stable product quality is ensured.
Owner:YANGTZE RIVER PHARMA GRP JIA NGSU LONGFENGTANG TRADITIONAL CHINESE MEDICINE CO LTD +2
Application wogonin for preparing medicine to treat leukaemia
ActiveCN1785175ALow toxicityGood effectOrganic active ingredientsAntineoplastic agentsLeukemiaWogonin
An application of wogonin in preparing the medicines for treating particular-type leukemia by inducing the differentiation of leukemia cells to particle system is disclosed.
Owner:CHINA PHARM UNIV +1
Use of wogonin in preparation of drug for treating chronic kidney disease
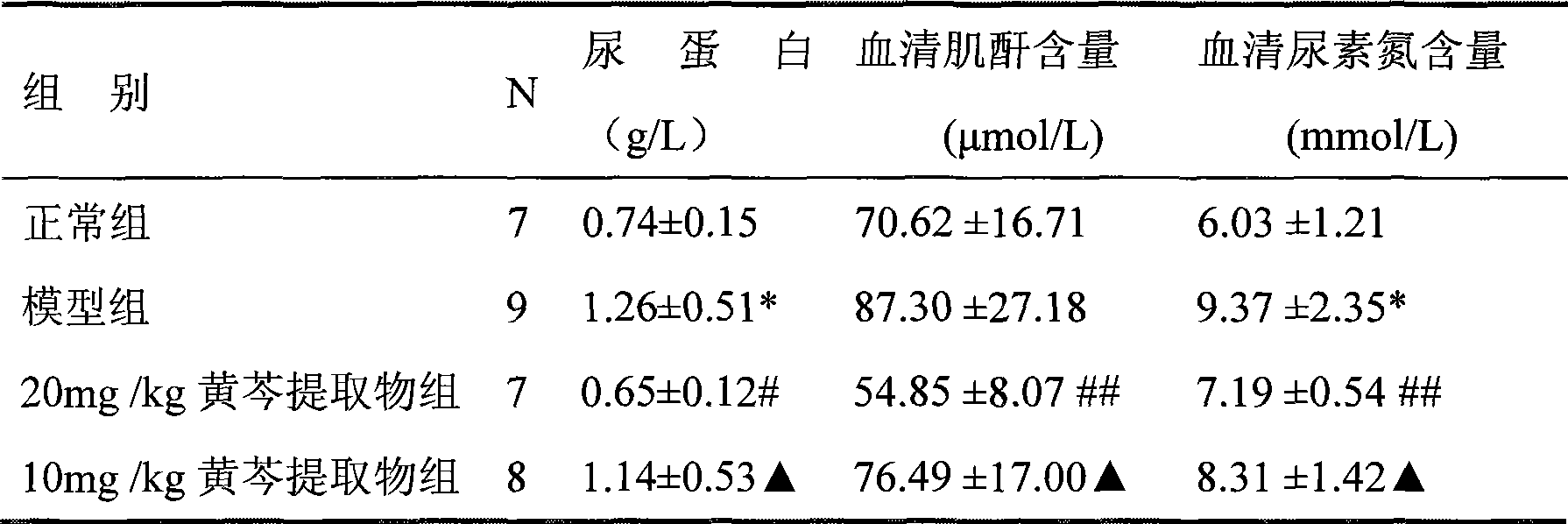
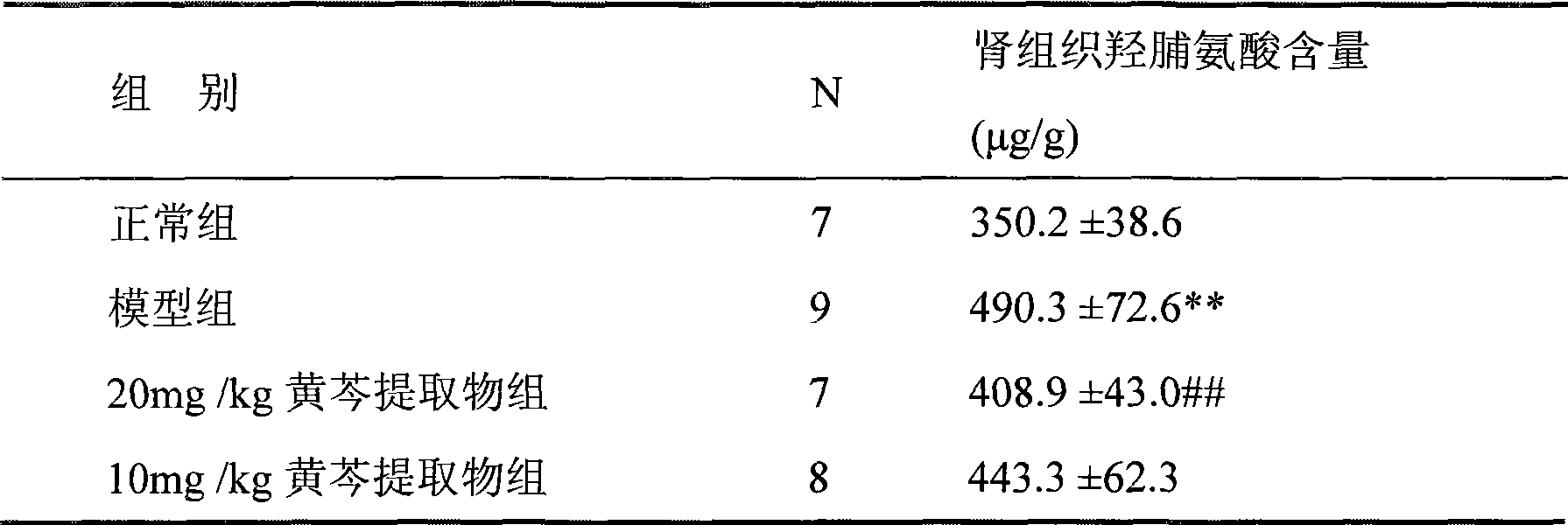
The invention relates to wogonin and derivatives thereof, a prodrug of wogonin as well as medical use of wogonin and biologically-physiologically acceptable salts of wogonin and derivatives thereof for treating a chronic kidney disease, particularly for treating renal fibrosis and renal fibrosis-induced diseases.
Owner:SHANGHAI UNIV OF T C M
Radix scutellariae total flavone extract and use of Radix scutellaride glucoside in preparing medicine for treating osteoporosis
The present invention relates to application of scutellaria total flavone extract and baicalin in preparation of medicine for curing and / or preventing osteoporosis. The baicalin is 5,6-dihydroxyflavone-7-0-glucoglycuronide, and the scutellaria total flavone extract includes baicalin, baicalein, wogonin, wogonoside and neobaicalein, etc. as its main effective components.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Phlegm-heat clearing injection fingerprint spectrum establishment method and fingerprint spectrum thereof
ActiveCN110187041AAvoid one-sidednessHigh precisionComponent separationDicaffeoylquinic acidInjection solution
The invention relates to the technical field of medicine detection, and in particular relates to a phlegm-heat clearing injection fingerprint spectrum establishment method and a fingerprint spectrum thereof; the phlegm-heat clearing injection is prepared from scutellaria baicalensis, bear gall powder, cornu gorais, honeysuckle and fructus forsythiae, and a phlegm-heat clearing injection fingerprint spectrum is established by detecting the phlegm-heat clearing injection components by adopting an ultra-high performance liquid chromatography method; the method specifically comprises the followingsteps of S1, preparing a reference substance solution: taking a proper amount of caffeic acid, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3, 4-dicaffeoylquinic acid and 3, 5-dicaffeoylquinic acid, 4, 5-dicaffeoylquinic acid, forsythiaside D, baicalin, scutellarin, chrysin-7-O-glucuronide, oroxylin-7-O-glucuronide and a wogonin reference substance, and adding methanol to prepare a solution which is 20-50 [mu]g in 1ml. according to the phlegm-heat clearing injection fingerprint spectrum establishment method provided by the invention, the method is simple and convenient to operate, stable in result, high in reproducibility and high in precision.
Owner:SHANGHAI KAIBAO PHARMA
New usage of eucommia ulmoides chemical composition as vessel protective agent
InactiveCN101972294APrevent proliferationHas a blood pressure lowering effectOrganic active ingredientsNervous disorderDiseaseOxygen
The invention relates to a new usage of eucommia ulmoides chemical composition as vessel protective agent, in particular to the new application of the eucommia ulmoides or eucommia ulmoides extractive for preparing the medicines as vessel protective agent and / or antihypertensions, or the medicines for curing and / or preventing angiogenesis disease. The eucommia ulmoides extractive contains at least one component selected from the group consisting of geniposide, wogonin, chiba A, alpha-oxygen-belta-D-glucopyranosyl-4,2',4'-trihydroxy dihydrochalcone and betulinic acid. According to the invention, the five chemical components or the eucommia ulmoides extractive containing the chemical components can be used as effective vessel protective agent and / or antihypertensions for curing and / or preventing angiogenesis disease.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Application of wogonin in the preparing of medicine for treating gastric cancer
ActiveCN101062029ALow toxicityStrong growth inhibitory effectOrganic active ingredientsAntineoplastic agentsMedicineStomach cancer
The invention discloses an application of wogonin to prepare stomach cancer medicine in pharmacy domain, which is characterized by the following: possessing distinctive growth inhibitory action of the wogonin for human stomach cancer MGC803 bare mouse transplanting tumor. This invention provides better choice for stomach cancer patient.
Owner:CHINA PHARM UNIV +1
Quality detection method for traditional Chinese medicine pediatric cold-relieving granules
ActiveCN110736799ASimple and fast operationAnalytical data is accurateComponent separationBaicaleinFluid phase
The invention relates to a quality detection method for traditional Chinese medicine pediatric cold-relieving granules. According to the invention, the contents of baicalin, wogonoside, baicalein, wogonin, liquiritin and ammonium glycyrrhizinate in the traditional Chinese medicine pediatric cold-relieving granules are determined by high performance liquid chromatography. According to the invention, the separation degree between each to-be-detected component in the chromatogram of a test sample and the adjacent peak is greater than 1.5, and negative control has no interference, so that the quality safety of the granules is further ensured, evaluation is comprehensive, and the detection method has the advantages of high practicability, high operability, cost saving and the like in operation.
Owner:SHANDONG MINGREN FURUIDA PHARMA
Technique of preparing high-purity wogonin by extraction and separation method
InactiveCN101333202AOrganic chemistryPlant ingredientsHigh concentrationCountercurrent chromatography
The invention relates to a preparation technology for extracting and separating high-purity wogonin; the scutellaria material is crushed and soaked with water; then the material is joined with a proper amount of solvent and then extracted, filtered and concentrated; the concentrated liquid is separated through macroporous resin column chromatography and high-speed countercurrent chromatography successively to prepare the high-concentration wogonin. The invention has stable process and high wogonin content in the finished product; the content of wogonin reaches more than 96%, based on the measurement through high-performance liquid phase method.
Owner:孙蓉
Method for analyzing all components of angelica sinensis six-yellow decoction
PendingCN112229934AAccurate analysisEasy to analyzeComponent separationAstragalosideChlorogenic acid
The invention provides a method for analyzing all components of an angelica sinensis six-yellow decoction, and belongs to the technical field of pharmaceutical analysis. An ultra-high performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) technology is adopted to detect an angelica sinensis six-yellow decoction sample, and 15 chemical reference substances are adopted at the same time: baicalin, wogonoside, berberine hydrochloride, epiberberine hydrochloride, coptisine hydrochloride, palmatine hydrochloride, phellodendrine hydrochloride, astragaloside, calycosin-7-glucoside, ferulic acid, chlorogenic acid, ligustilide, verbascoside, baicalein and wogonin are adopted to confirm a detection result of the angelica sinensis six-yellow decoction andclearly identify 68 components; comprehensive analysis of the components of the angelica sinensis six-yellow decoction is realized, and the reliability of quality control is improved.
Owner:山东宏济堂制药集团股份有限公司
Method for measuring contents of various ingredients in cold cough syrup
InactiveCN105758978AGuaranteed separation effectFast and effective high-throughput analysisComponent separationAdditive ingredientVolumetric flask
The invention provides a method for measuring the contents of baicalin, baicalein and wogonin in cold cough syrup.The method for measuring the contents of baicalin, baicalein and wogonin in cold cough syrup aims at being accurate in measurement and easy to implement.The method comprises the steps that firstly, a standard solution is prepared; secondly, a test solution is prepared, wherein l ml of the test solution is measured and put in a 20 ml volumetric flask and dissolved with 50% methyl alcohol, ultrasonic treatment is carried out for 5 min, 50% of the solution is used for reaching the constant volume of the 20 ml scale mark, even mixing is carried out, 1 mL of the solution is precisely measured and passes through a C18-SPE column at the flow speed around 1 mL / min, elution is carried out with 2 mL of 50% methyl alcohol, the flowing-out solution and the elution solution are mixed and filtered with a 0.22-micrometer organic membrane, and a sample to be detected is obtained and put into a high performance liquid chromatograph for detection.The method belongs to the technical field of chemical detection.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
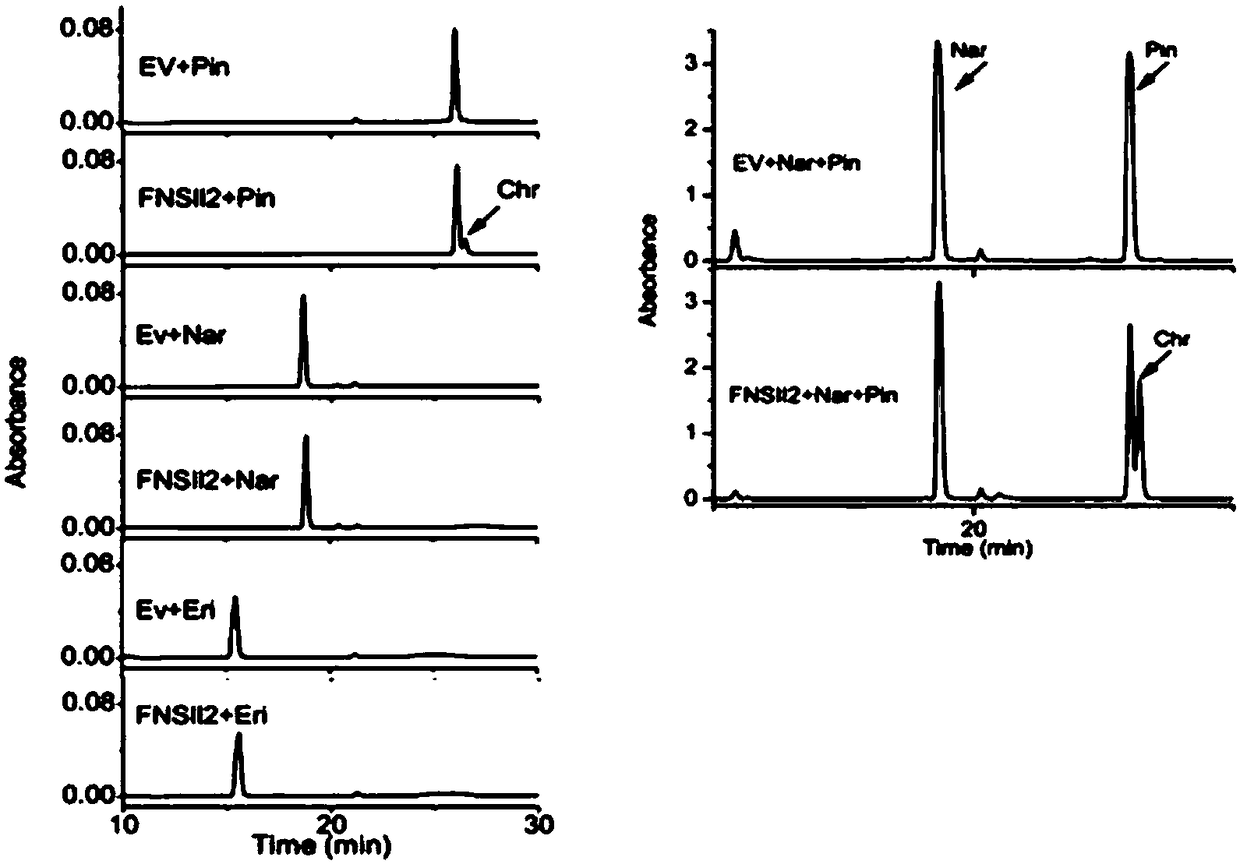
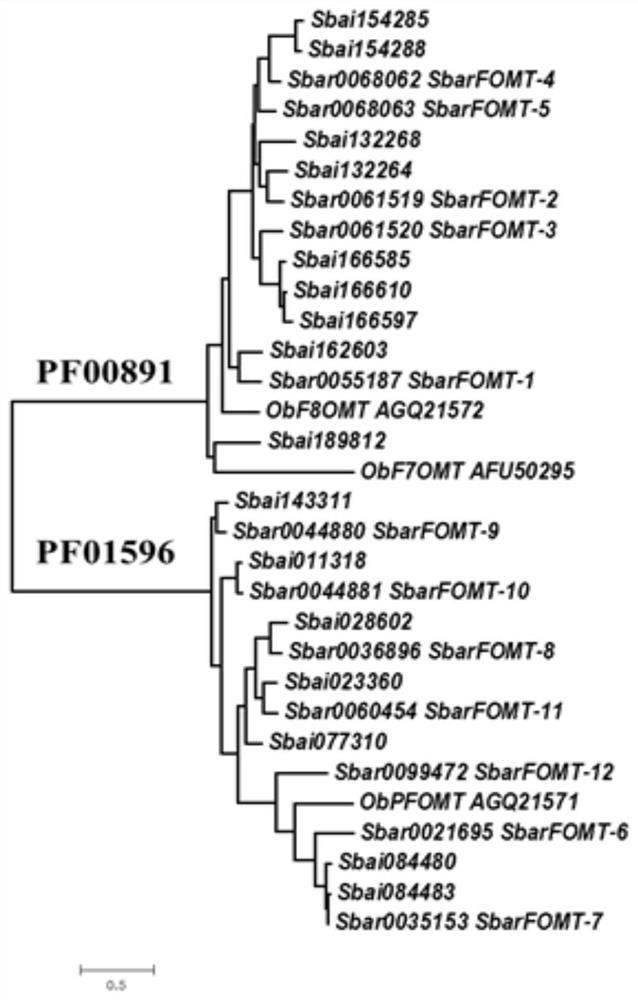
Flavone-O-methyltransferase, and application thereof in synthesis of wogonin, isowogonin and moslosooflavone
The invention provides flavone-O-methyltransferase, which comprises one or more of flavone-8-methyltransferase with the amino acid sequences as shown in SEQ ID No.1, SEQ ID No.2, SEQ ID No.3, SEQ ID No.4 and SEQ ID No.5, and flavone-7-methyltransferase with the amino acid sequences as shown in SEQ ID No.6 and SEQ ID No.7 The invention also provides a nucleotide sequence for coding the amino acid sequence, a recombinant vector containing the polynucleotide sequence for coding the above amino acid, a recombinant host cell and application. The flavone-O-methyltransferase disclosed by the invention can be used for synthesizing flavones such as t he wogonin, the isowogonin and the moslosooflavone, and can be used for catalyzing the synthesis and methylation reaction of flavonoid substances with a similar structure; and a foundation is laid for industrial large-scale fermentation production or enzymatic conversion method production of methylated flavonoid compounds.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
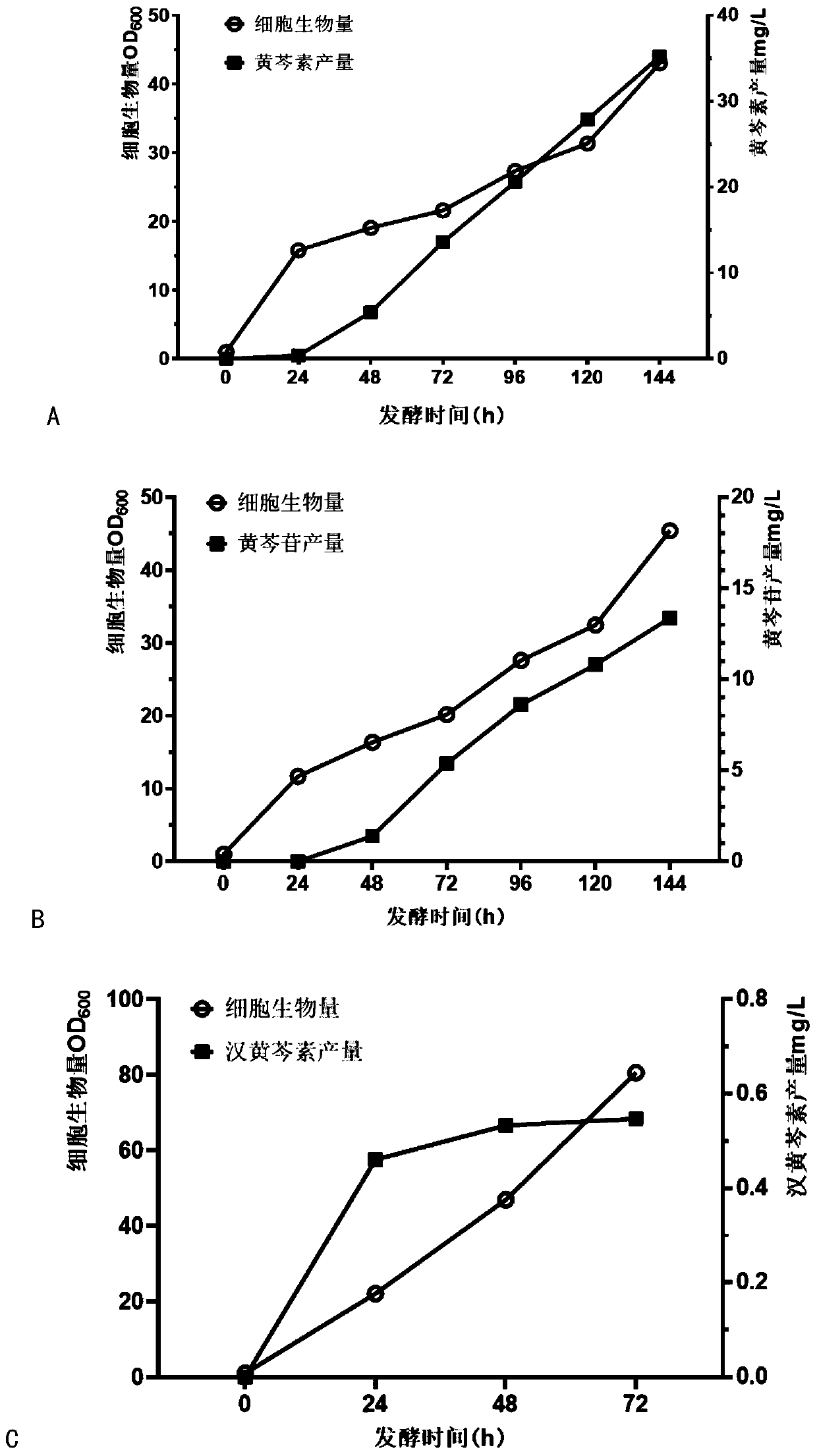
Genetically engineered saccharomycetes for producing baicalein compounds as well as construction method and application of genetically engineered saccharomycetes
The invention relates to genetically engineered saccharomycetes for producing baicalein compounds as well as a construction method and application of the genetically engineered saccharomycetes. A series of exogenous genes are introduced into yeast engineering bacteria through a gene recombination technology to obtain the yeast engineering bacteria capable of producing baicalein, baicalin or wogonin. The yeast engineering bacteria have the characteristics of a low metabolic background, strong heterologous expression ability, no need of additional addition of a precursor, capability of synthesizing a final product in a whole-cell manner, easiness in separation of the final product and the like, and a novel thought is provided for industrial production of flavonoid drugs.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com