Anti-inflammatory tablet HPLC fingerprint construction method
A technology of fingerprint spectrum and construction method, which is applied in the quality control of anti-inflammatory tablets and the field of high-performance liquid phase fingerprint spectrum, and achieves the effects of good absorption value, good peak shape and easy identification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 of the present invention: detection of fingerprints of different batches of Xiaoyan Tablets
[0034] 1 Instruments and reagents
[0035] 1.1 Agilent 1220 high performance liquid chromatography (Agilent, USA); KQ-100 ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd.); RE-98 rotary evaporator (Shanghai Yarong Biochemical Instrument Factory); 124S electronic balance ( Beijing Sartorius Balance Co., Ltd.).
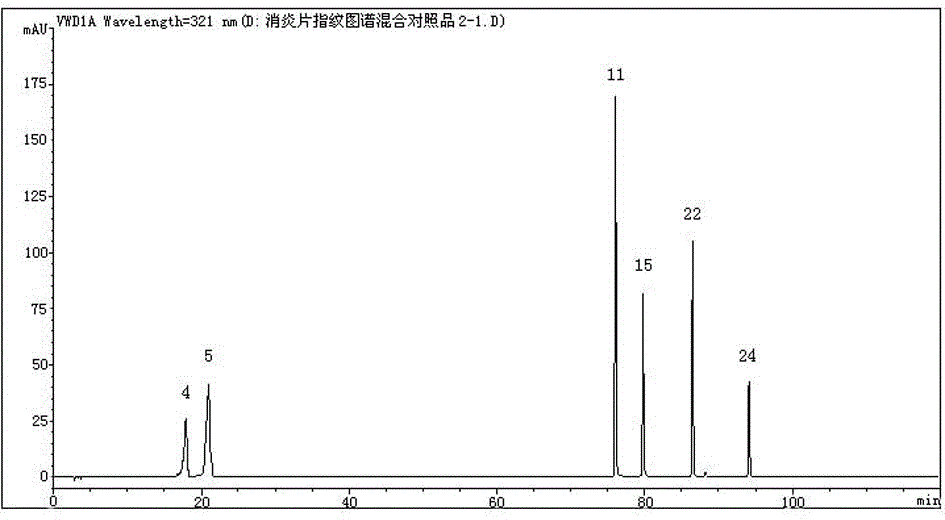
[0036] 1.2 Purified water (Wahaha), acetonitrile (chromatographically pure), and other reagents are analytically pure. Chlorogenic acid, teretin B, baicalin, baicalin, baicalein, wogonin reference substances (lot numbers 110753-200413, 110741-200506, 110715-201016, 111528-200606, 111595-200905, 111514-200403, China Institute for Food and Drug Control). Anti-inflammatory tablets (batch numbers: 150301, 150302, 150303, 150304, 150305, 150601, 150801, 150802, 150803, 150804, 150805, revised Pharmaceutical Group Co., Ltd.).
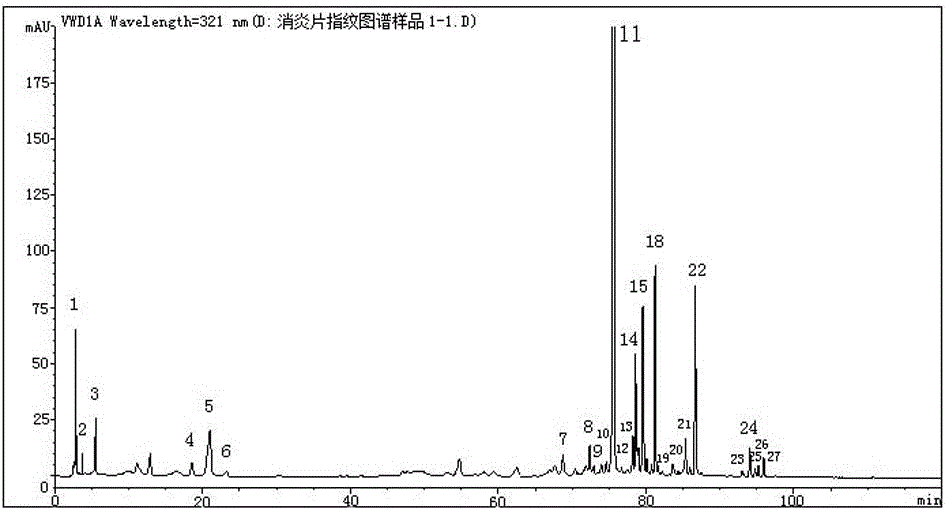
[0037] 2 Determinatio...
Embodiment 2
[0050] Example 2: Determination and data analysis of fingerprints of 11 batches of Xiaoyan Tablets
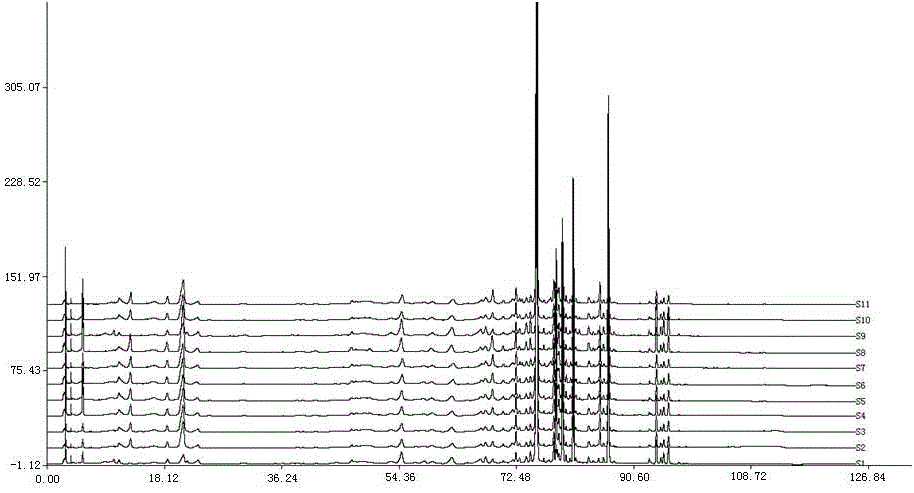
[0051] 11 batches of anti-inflammatory tablets were taken and tested according to the conditions of Example 1 to obtain the HPLC spectra of 11 batches of samples. Figure 3.
[0052] Through the comparison of 11 batches of PHLC chromatograms, the similarity evaluation is carried out to determine its characteristic common peaks: import the chromatograms of 11 batches of Xiaoyan Tablets into the "Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System" 2004A Edition (National Pharmacopoeia Commission), and use the common peaks mode, set the time window width to 0.1min, and generated standard fingerprints by the average method, and determined 27 common peaks. The similarities of the 11 batches of Xiaoyan Tablets are all greater than 0.9, as shown in Table 3. The relative retention times of the 27 common peaks are basically the same, but there are large difference...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com