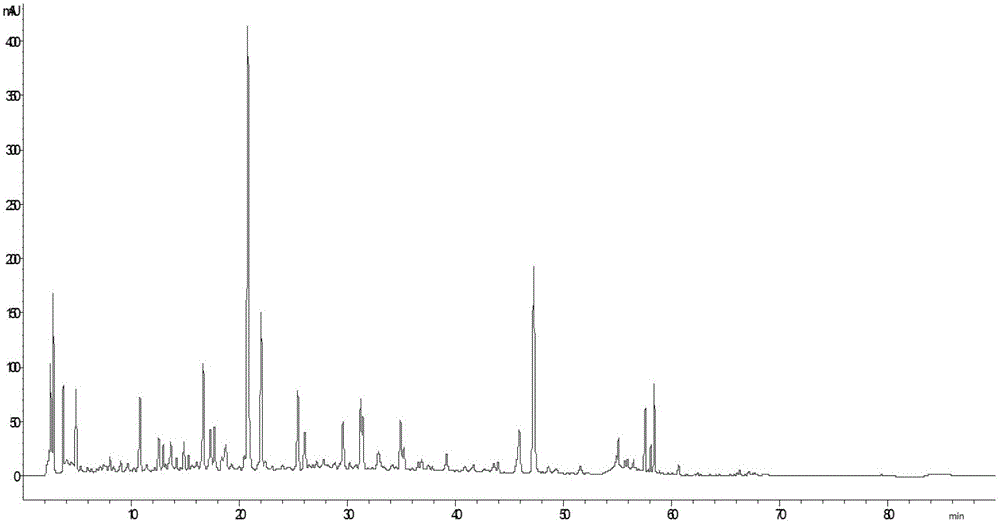
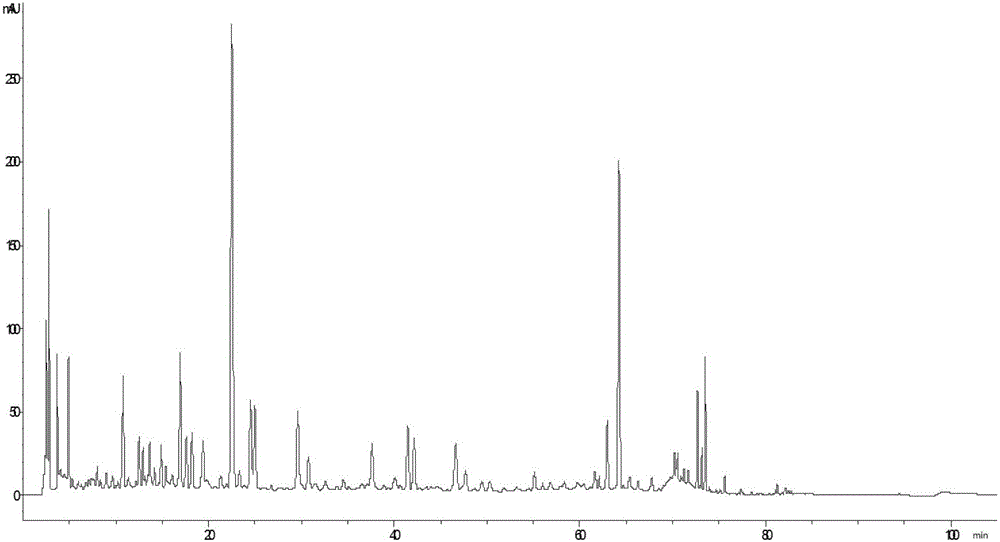
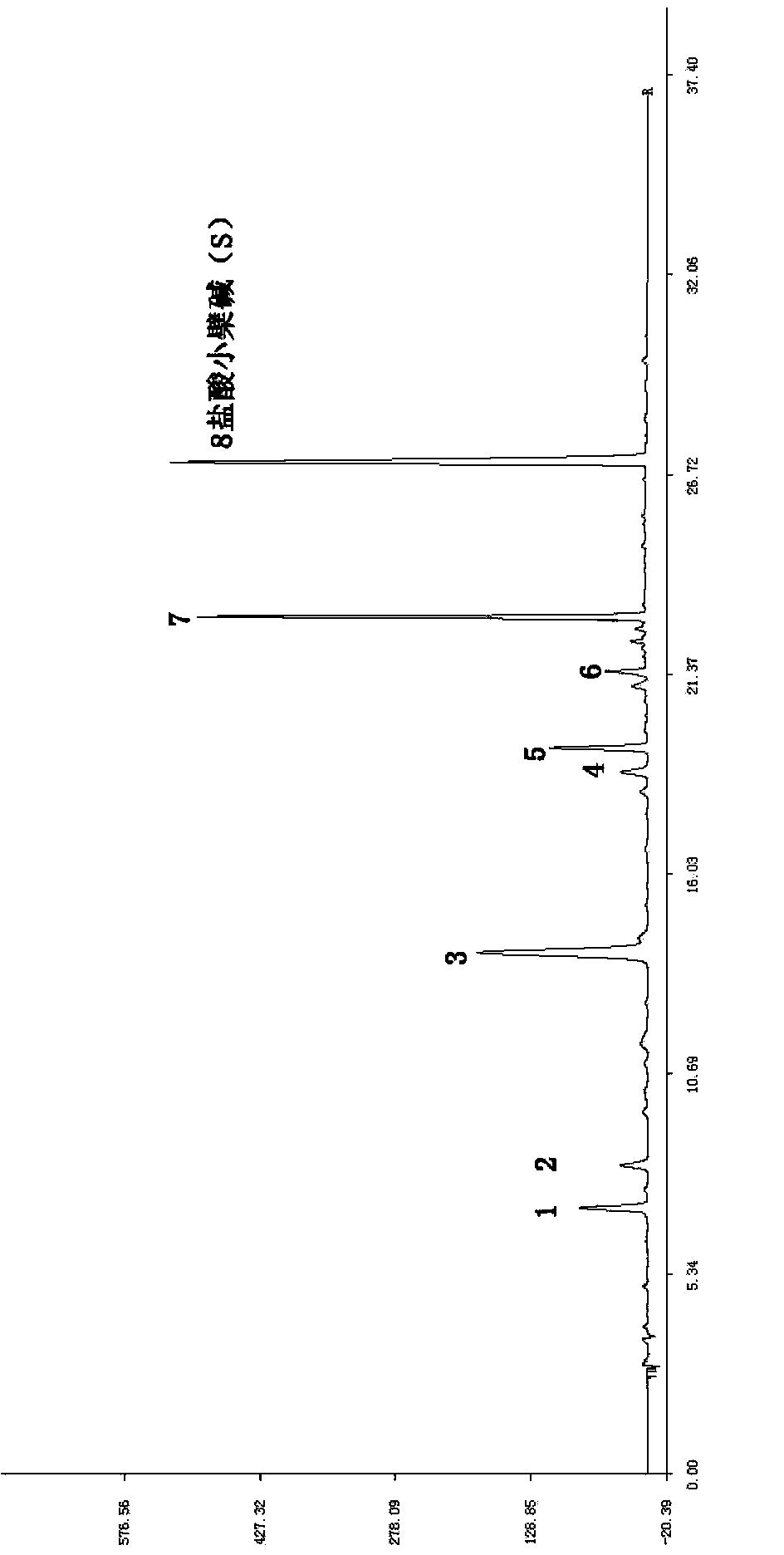
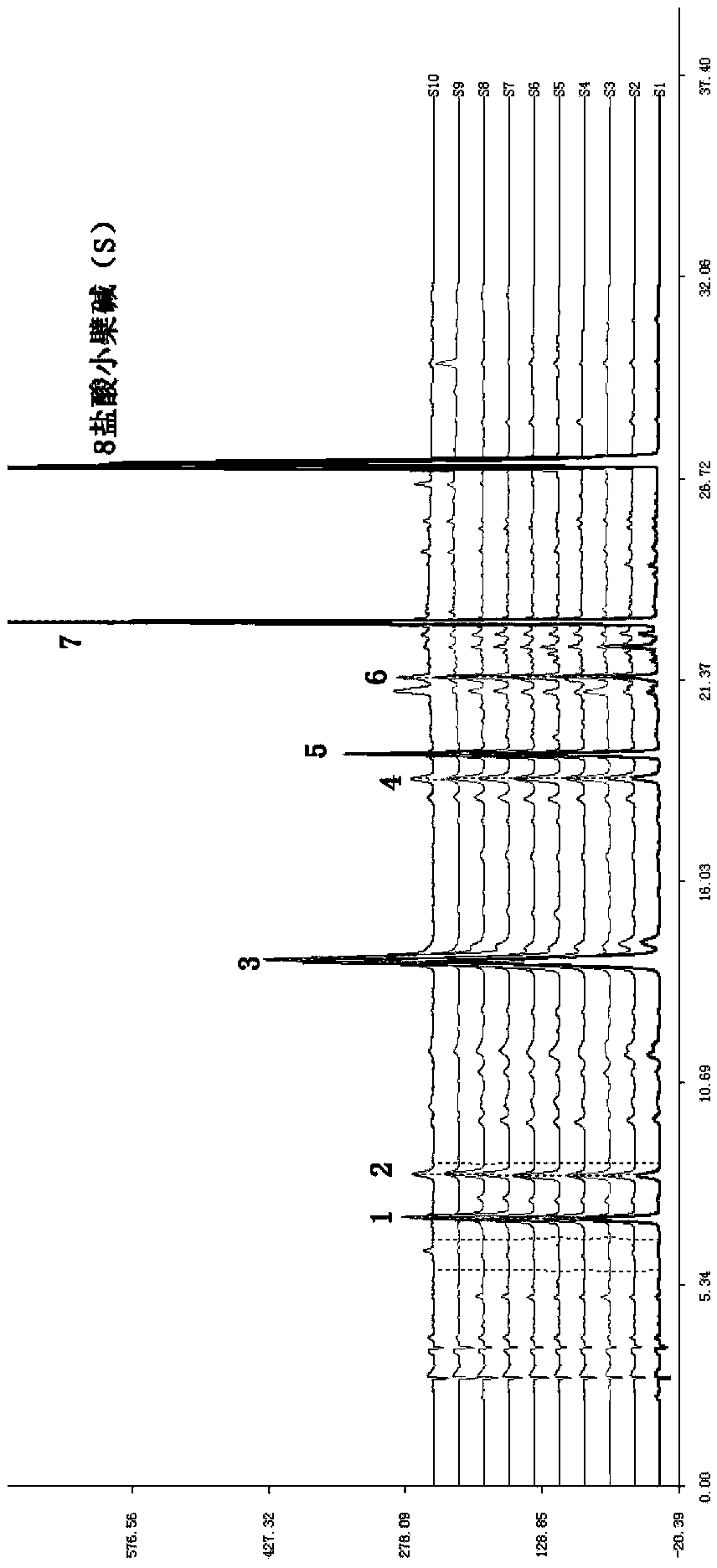
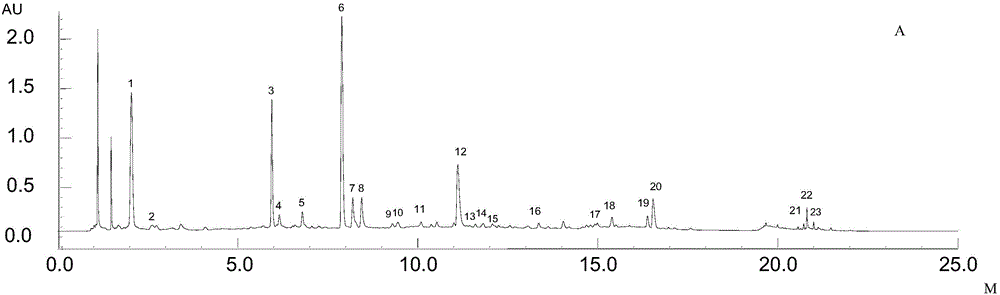
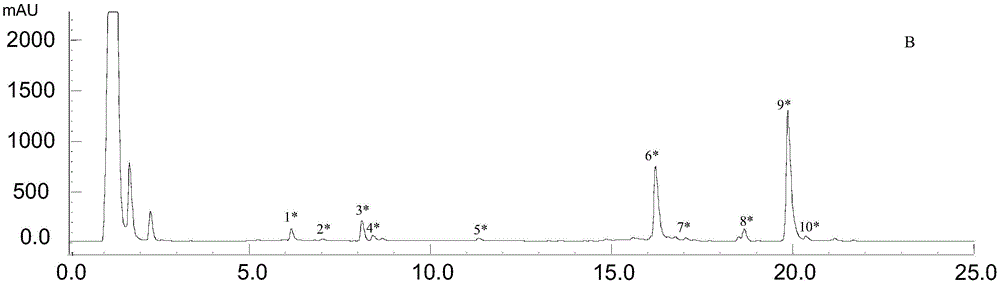
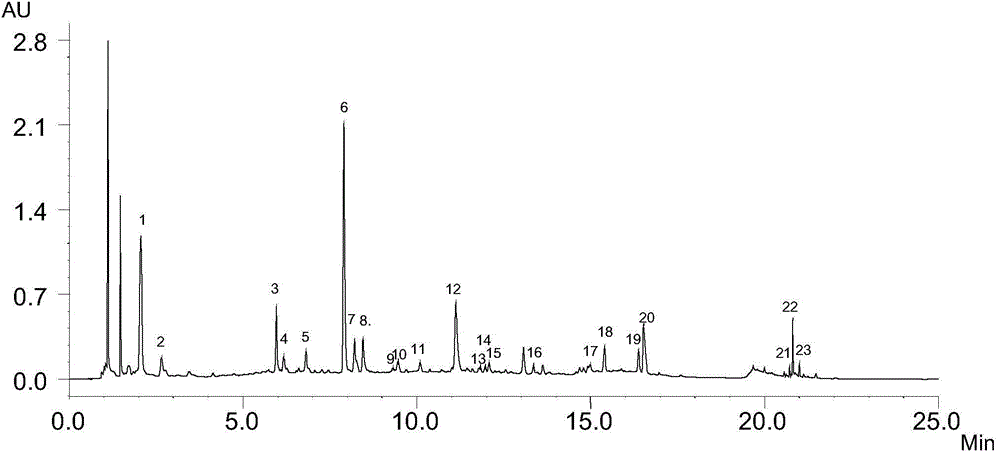
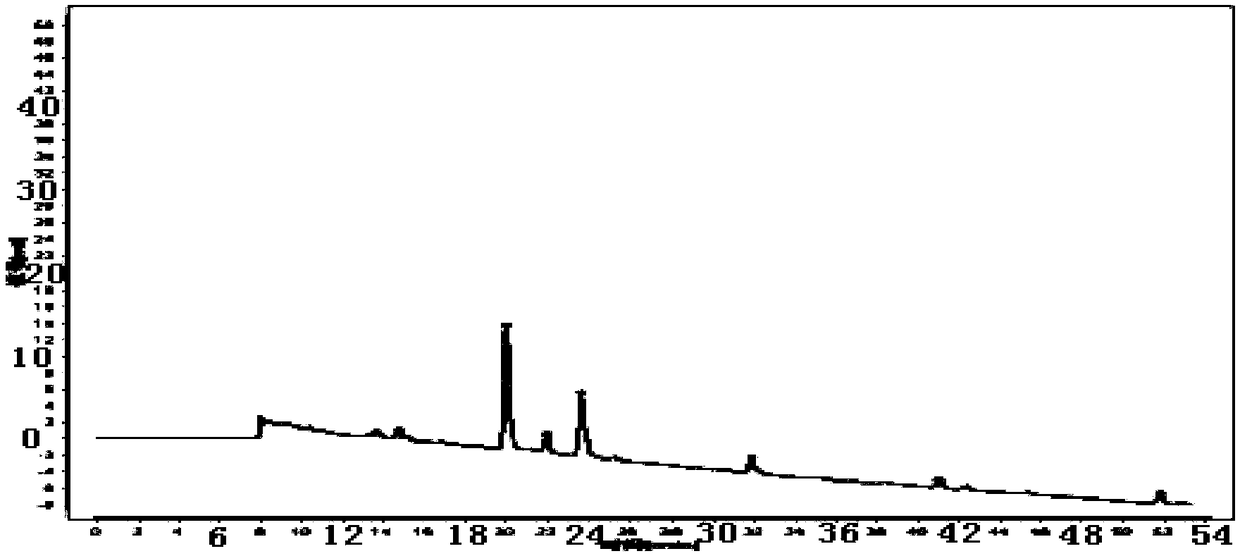
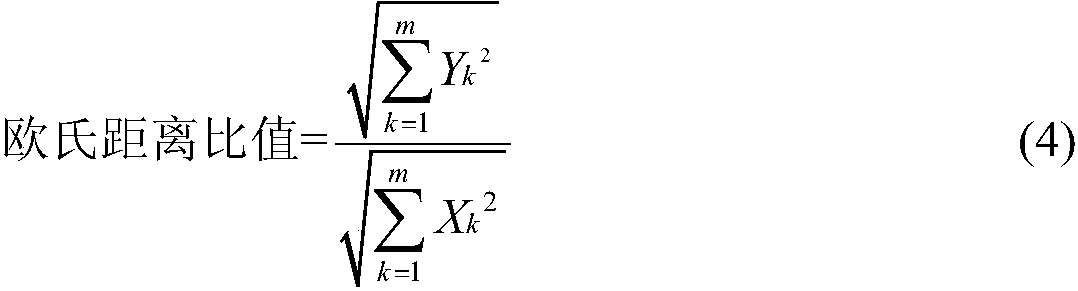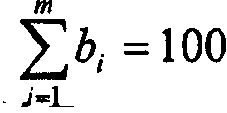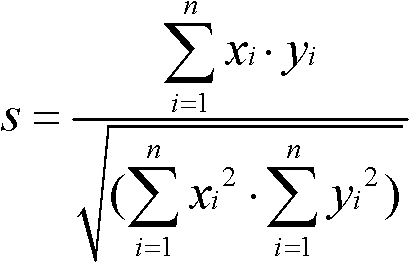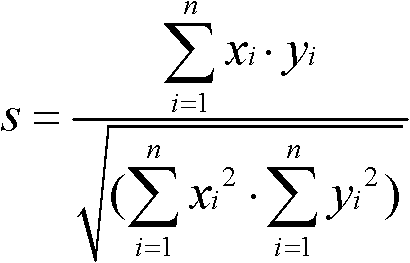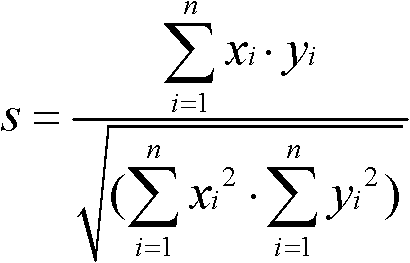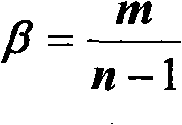Patents
Literature
226 results about "Chromatographic fingerprint" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HPLC fingerprint spectrum measurement method for standard double-harmonizing decoction
ActiveCN106501434AGuaranteed stabilityGuarantee the safety of useComponent separationHplc fingerprintData matching
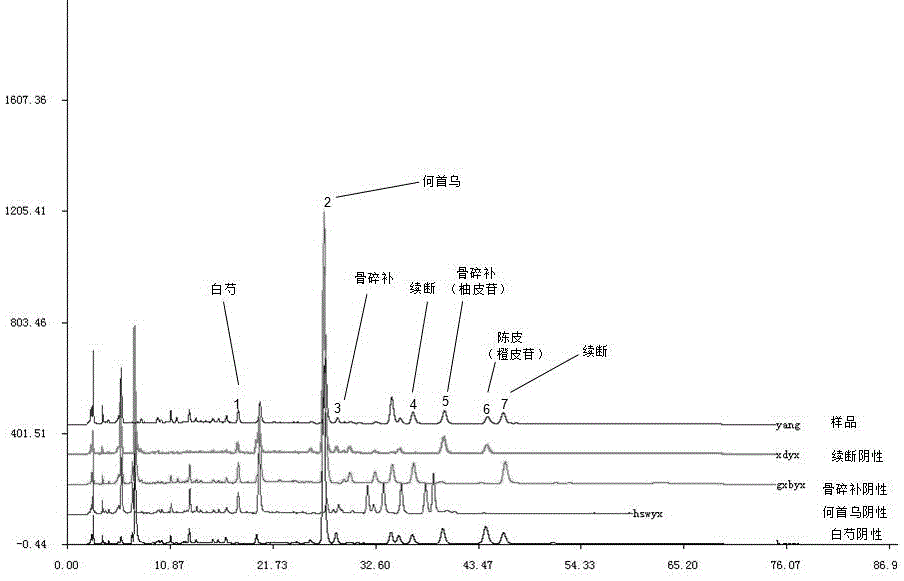
The invention provides a HPLC fingerprint spectrum measurement method for standard double-harmonizing decoction, belonging to the technical field of traditional Chinese medicine. The method comprises the following steps: (1) preparing a test sample solution; (2) preparing a mixed reference solution; (3) preparing a single medicine negative reference solution; (4) precisely absorbing the test sample solution, the mixed reference solution and the single medicine negative reference solution respectively and injecting into a high performance liquid chromatograph and measuring to obtain the liquid chromatogram of the test sample solution, the mixed reference solution and the single medicine negative reference solution; and (5) performing data matching on the liquid chromatogram of the test sample solution, the mixed reference solution and the single medicine negative reference solution by use of the traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system formulated by the Chinese committee of pharmacopeia to obtain a standard fingerprint spectrum. According to the invention, the quality of standard granules of double-harmonizing decoction is effectively controlled, the curative effect of the drug is guaranteed, and more formal quality control is realized on the classical famous prescription.
Owner:天津同仁堂集团股份有限公司
Gas-chromatography fingerprinting for rapidly identifying edible oil
InactiveCN101398412AHigh sensitivityGood precisionComponent separationTesting foodFood safetyGas liquid chromatographic
The invention provides a gas chromatographic fingerprint method for fast distinguishing edible oil, comprising the following steps of: A. establishing a fingerprinting for the volatile constituents of the edible oil: the edible oil with the same origin and brand is used as a proof sample and the gas chromatographic fingerprinting of the volatile constituents thereof is established by gas chromatographic analysis; and B. detecting samples: an edible oil sample to be detected is taken and treated with sampling with the proof sample under the same condition to obtain the fingerprinting of the detected sample, the fingerprinting of the two is taken as the qualitative base by the analysis of a direct observational method and fingerprinting software. By the observation of the methodology, the fingerprinting made by the volatile constituents of the edible oil proves that the precision, the stability and the reproducibility thereof all have better application prospect, thus being suitable for generalization in the technical field of food safety detection.
Owner:HEBEI UNIVERSITY
Fingerprint map detection method for ganmaoling granules
ActiveCN104181248AFully reflect the quality informationControl internal qualityComponent separationTraditional medicineChromatographic fingerprint
The invention belongs to the field of traditional Chinese medicinal preparation detection, and in particular relates to a fingerprint map detection method for ganmaoling granules. According to the method disclosed by the invention, traditional Chinese medicinal components, including linarin, in the ganmaoling granules are detected by utilizing a high-performance liquid chromatography; quality information of the ganmaoling granules can be reflected comprehensively; the intrinsic quality of the ganmaoling granules can be effectively controlled; moreover, the established method is relatively high in stability, precision and reproducibility; a traditional Chinese medicine chromatographic fingerprint map similarity evaluation system specified by the Pharmacopoeia Committee of China is adopted, so that the operation is quick, and results are objective and accurate.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA
Intelligent fragrance blending and simulating method of cigarette flavor
ActiveCN103436361AImprove scienceEfficientComponent separationEssential-oils/perfumesFlavorOrganoleptic
The invention discloses an intelligent fragrance blending and simulating method of a cigarette flavor, which comprises the steps that a chromatographic fingerprint map characteristic database of a raw cigarette fragrance material is established; a targeted flavor is subjected to chromatographic fingerprint map analysis; components of the raw fragrance material, which compose the targeted flavor are obtained; a compound flavor is compounded preliminarily; a professional fragrance blending group evaluates a fragrance characteristic of the compound flavor, adjusts a proportion, compares the similarity of a chromatographic fingerprint map, and supplements the raw fragrance material insufficient in fragrance; evaluation, proportion adjustment, comparison of the similarity of the chromatographic fingerprint map, and supplement of the raw fragrance material are conducted repeatedly till the similarity between the chromatographic fingerprint map of the finally obtained compound flavor and that of the targeted flavor is not less than 90%; the finally obtained compound flavor and the targeted flavor are subjected to a cigarette flavoring test; results of smoke panel tests after the compound flavor and the targeted flavor are subjected to cigarette flavoring are compared by a cigarette style and sensory quality evaluation method; and if the results are the same, a fragrance simulating flavor of the targeted flavor is formed. The method is simple and direct in procedure, high in accuracy and wide in application scope.
Owner:CHINA TOBACCO HUNAN INDAL CORP
Quality testing method for fingerprint of herbal medicine musk
The invention discloses a quality testing method for fingerprint of herbal medicine musk. The method includes establishing a gas chromatographic fingerprint of the musk by gas chromatography, and analyzing chemical components of different types of musk by gas chromatography and mass spectrometry. A test sample is processed by specific process, chromatographic detection is optimized, and tests show that the method is stable, precise and repeatable. Three established reference musk fingerprints are well specific. Similarity of the three types of musk is researched under integration of non-integration conditions of muscone, and more accurate basis is provided for testing quality of the three different types of musk. In addition, common compounds of the three types of musk are obtained by identification by GC-MS (gas chromatography and mass spectrometry) and can be used as basis for further identification of synthetic musk, domestic musk and natural musk.
Owner:ZHANGZHOU PIEN TZE HUANG PHARM
Sophora fungus mycoplasma extract identification and detection method
ActiveCN102621260AControl contentRaise quality standardsComponent separationMonosaccharide compositionAnion-exchange chromatography
The invention relates to a sophora fungus mycoplasma extract identification and detection method. The identification method is characterized in that an anion exchange chromatography method is used for establishing chromatographic fingerprint composed by monosaccharide of proteoglycan protein in the sophora fungus mycoplasma extract, and the quality is controlled by comparing the similarity of a sample fingerprint spectrum and a fingerprint spectrum of a standard extract. The detection method is characterized in that the anion exchange chromatography method is used for detecting the monosaccharide composition and content of proteoglycan protein of the sophora fungus mycoplasma extract, thereby the content limit can be made. The fingerprint spectrum of the anion exchange chromatography method combines the content determination method to realize the effective control of quality of the sophora fungus mycoplasma extract and the relative products as well as distinguish with other products. The method of the invention has the advantages of rapidity, convenience and strong specificity.
Owner:QIDONG GAITIANLI MEDICINES
Detection method of compound danshen dripping pills
ActiveCN102119961AQuality improvementImprove product qualityHydroxy compound active ingredientsComponent separationSalvia miltiorrhizaChromatographic fingerprint
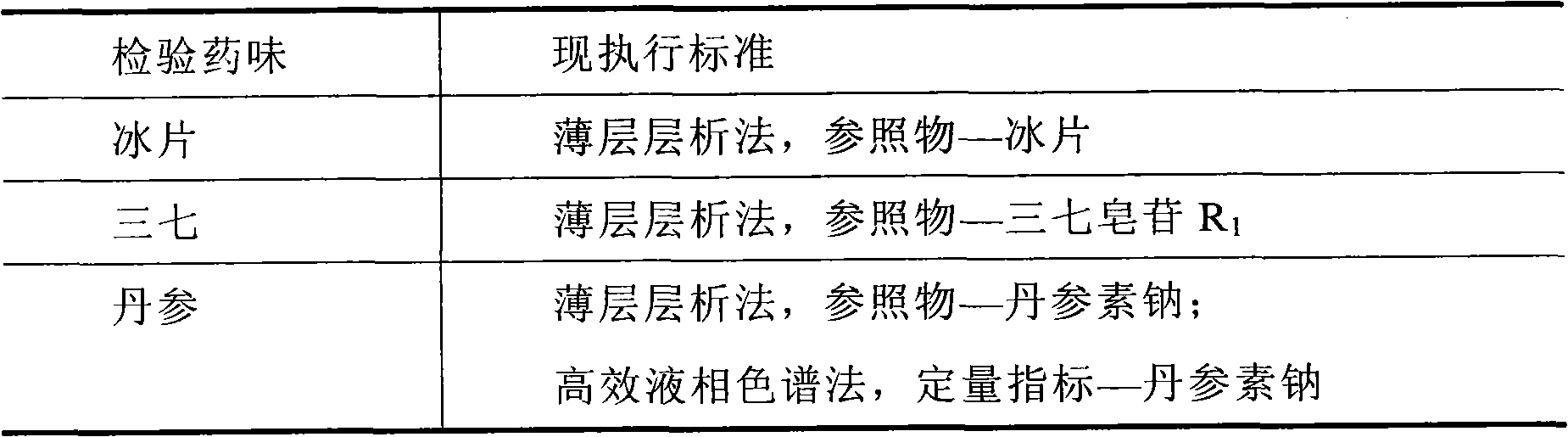
The invention relates to a detection method of compound danshen dripping pills, comprising the following contents of: observation of characters, discrimination of contents, inspection of contents, comparison of finger prints and assaying of contained components. The detection method provided by the invention comprises a discriminating method of the medicinal component panax notoginseng, the discriminating method adopts thin-layer chromatography, the reference materials adopt a panax notoginseng reference medicinal material, a ginsenoside Rg1 reference substance, a ginsenoside Re reference substance, a ginsenoside Rb1 reference substance and a panax notoginseng saponin R1 reference substance. The detection method provided by the invention also comprises a discriminating method and an assaying method of the medicinal component danshen, wherein the discriminating method adopts a sub-2 mu m liquid chromatography technology to perform chromatographic fingerprint discrimination, and adopts sodium danshensu as a reference material; and the assaying method adopts a sub-2 mu m liquid chromatography technology to perform assaying on the components such as danshensu, panax notoginseng saponin R1, ginsenoside Rg1 and ginsenoside Rb1 in the danshen.
Owner:TIANJIN TASLY PHARMA CO LTD
Method for culture of alpinia galanga and its quality-control
InactiveCN1947488AComponent separationColor/spectral properties measurementsAdditive ingredientGas phase
A method for culturing the rhizome of alpinia galanga includes such steps as tissue culture, transplanting, field management, applying fertilizer, controlling diseases and pests and harvesting. Its processing, transportation and storage are also disclosed. Its quality control method includes UV spectrophotometry, thin-layer chromatography, gas-phase chromatographic fingerprint, and efficient liquid-phase chromatography for measuring the content of 1'-acetoxychavicol acetate.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Evaluation method for chromatographic fingerprint similarity
ActiveCN103278591AReliable identificationQuality improvementComponent separationPeak valueComputer science
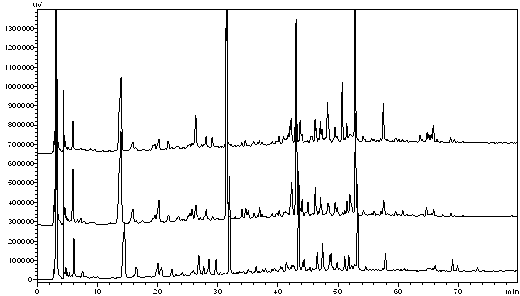
The invention discloses an evaluation method for chromatographic fingerprint similarity. The method comprises the following steps of: creating a contrast fingerprint through a standard sample; creating a sample fingerprint of a sample to be evaluated under chromatographic conditions same as those for creating the contrast fingerprint; comparing each peak value in the sample fingerprint with the corresponding peak value in the contrast fingerprint to obtain the ratio fingerprint vector and contrast ratio fingerprint vector of the sample; and calculating the similarity S of the two ratio vectors. The evaluation method disclosed by the invention not only is capable of reflecting the similarity of shared peaks of the fingerprint of the sample to be evaluated and the standard fingerprint, but also considers the effects of the number and the sizes of non-shared peaks on product quality, thus being capable of integrally reflecting the similarity of fingerprints of samples. The similarity can be used for sensitively and quantitatively expressing the qualitative and quantitative difference between the sample fingerprint and the standard fingerprint, reliably verifying medicines and food and better controlling the quality of medicines and food from quality stability and safety.
Owner:XUZHOU NORMAL UNIVERSITY
Quality control method of ginkgolide injection
ActiveCN101647829AStrong specificityGood reproducibilityComponent separationGinkgophyta medical ingredientsGinkgolideQuality control
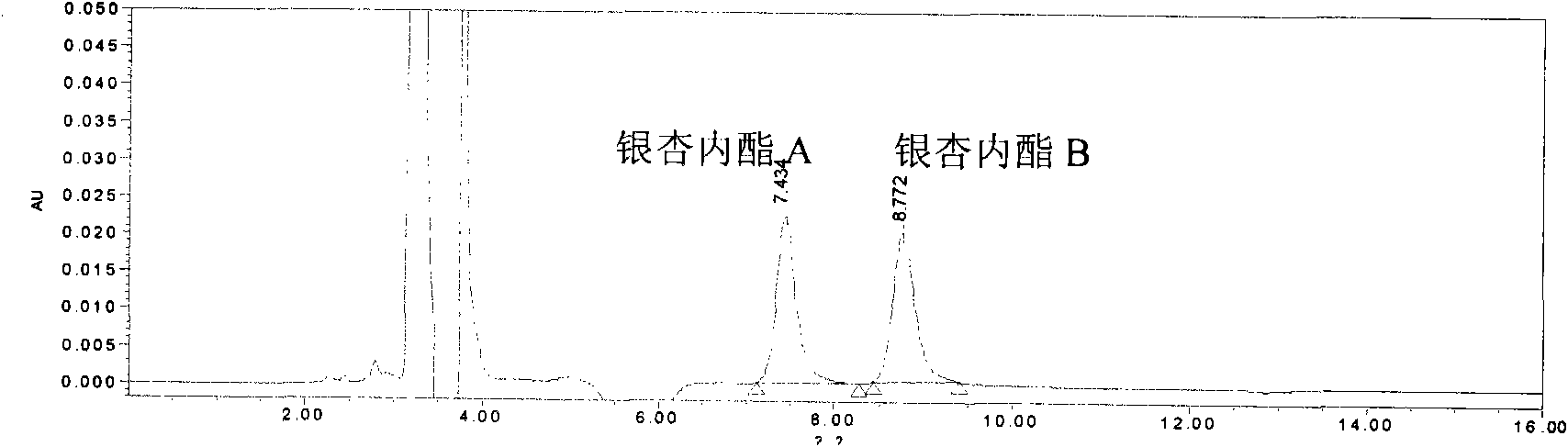
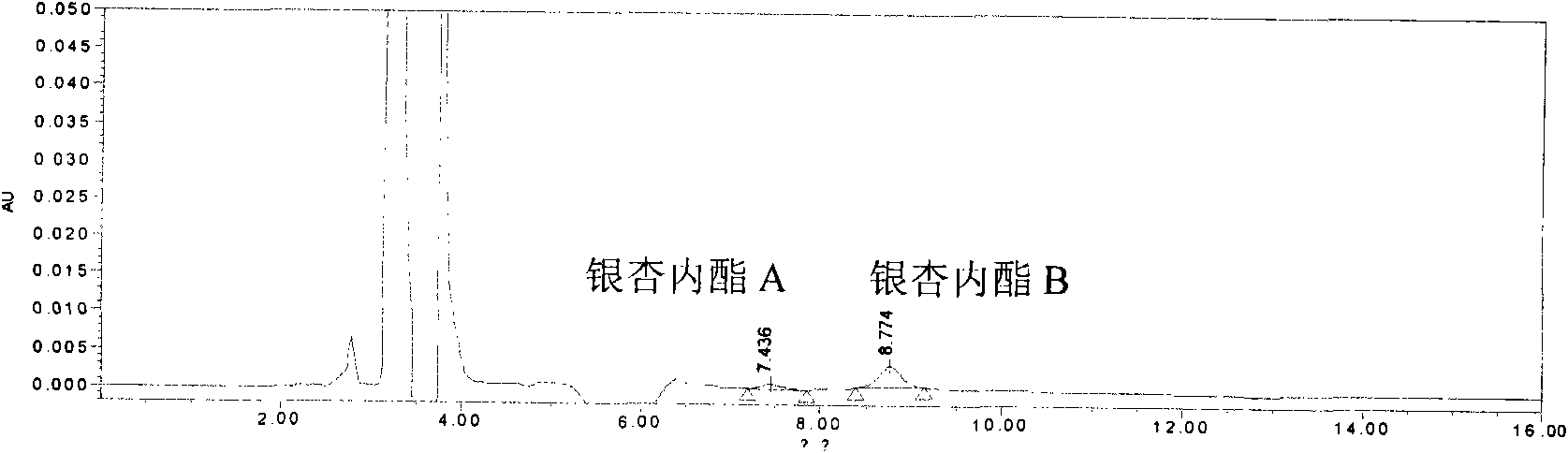
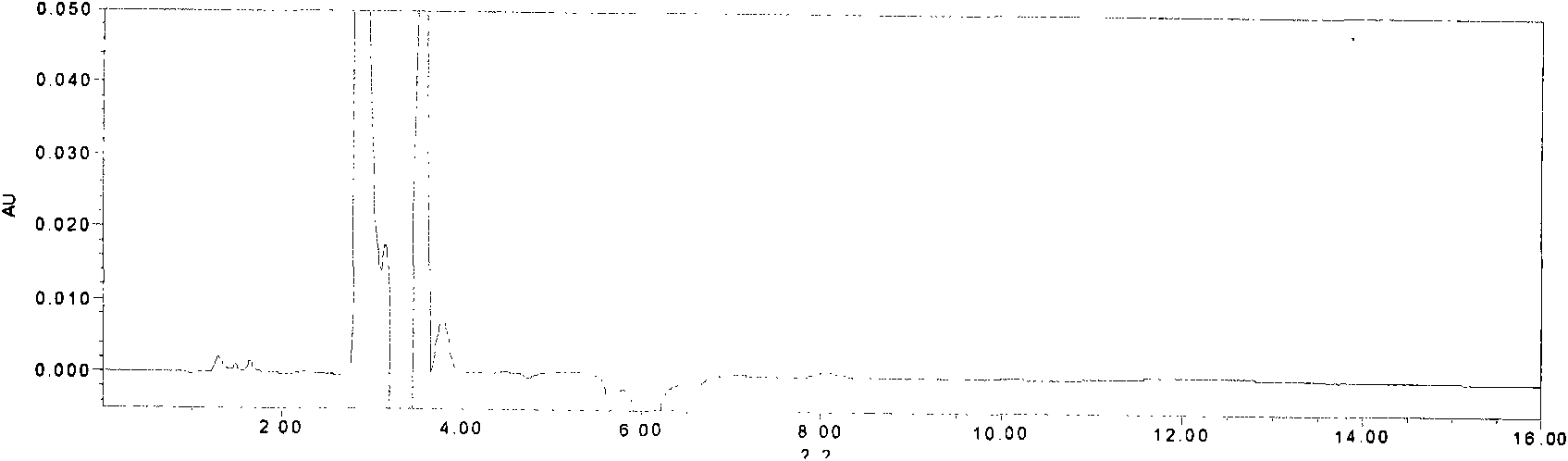
The invention relates to a quality control method of injection, in particular to a quality control method of ginkgolide injection. The invention provides a method for controlling the quality of the ginkgolide injection by adopting high performance liquid chromatography. The method can control the quality of the ginkgolide injection by adopting one or more of a content determination method, a limittest method of dissociation ginkgolide and a chromatographic fingerprint method. The quality control method provided by the invention has the advantages of having high precision, stability and repeatability, and achieving the aim of effectively controlling the quality of the ginkgolide injection in an all-round way.
Owner:JIANGSU KANION PHARMA CO LTD
Method for quickly and accurately evaluating quality stability of cigarette product
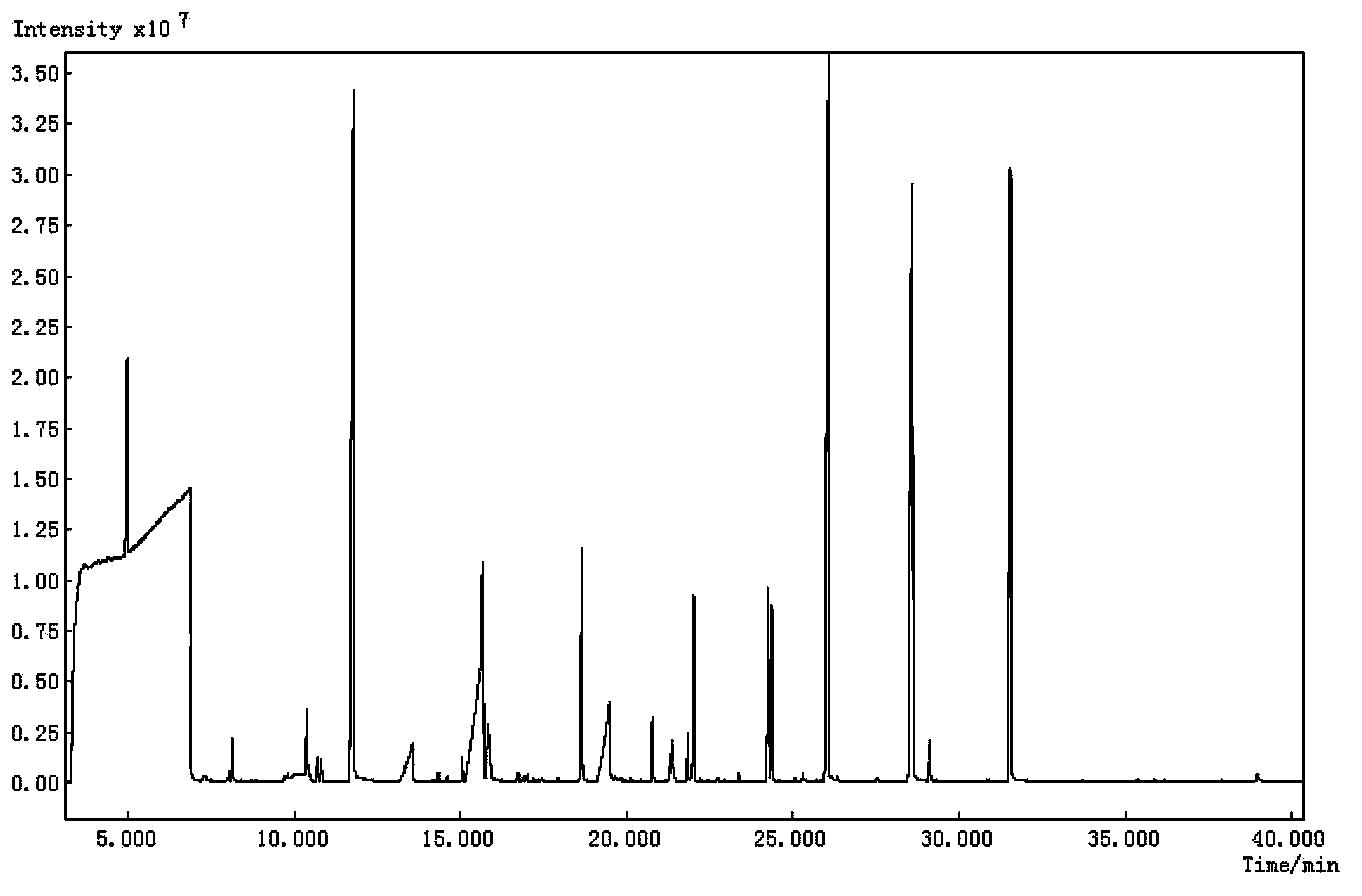
ActiveCN104569263AEfficiently evaluate quality stabilityEfficiently evaluate quality consistencyComponent separationVapor phase chromatographyMass spectrometry
The invention discloses a method for quickly and accurately evaluating the quality stability of a cigarette product. The method comprises the steps of collecting GC-MS chromatograms of more than 10 batches of cigarette products which have the same brand specification as that of the cigarette product to be detected and are purchased in the proper way by a purge trap-gas chromatography-mass spectrometer combination method, and averagely acquiring a chromatograph fingerprint of the cigarette of the brand specification by stacking; collecting the GC-MS chromatograms of a batch of cigarette samples to be detected, and analyzing the similarity between the GC-MS chromatograms of a batch of cigarette samples to be detected and the chromatograph fingerprint of the cigarette of the brand specification by a relevant coefficient method, and judging the cigarette products with the similarity being greater than or equal to 90 percent to be cigarette products with stable quality and the cigarette products with the similarity being smaller than 90 percent to be cigarette products with unstable quality. The method has the advantages of simplicity in operation treatment, high spectrum specificity, quickness and accuracy in conclusion making, and the like.
Owner:中国烟草总公司山东省公司
Novel method for chromatographic finger printing and standardization of single medicines and formulations
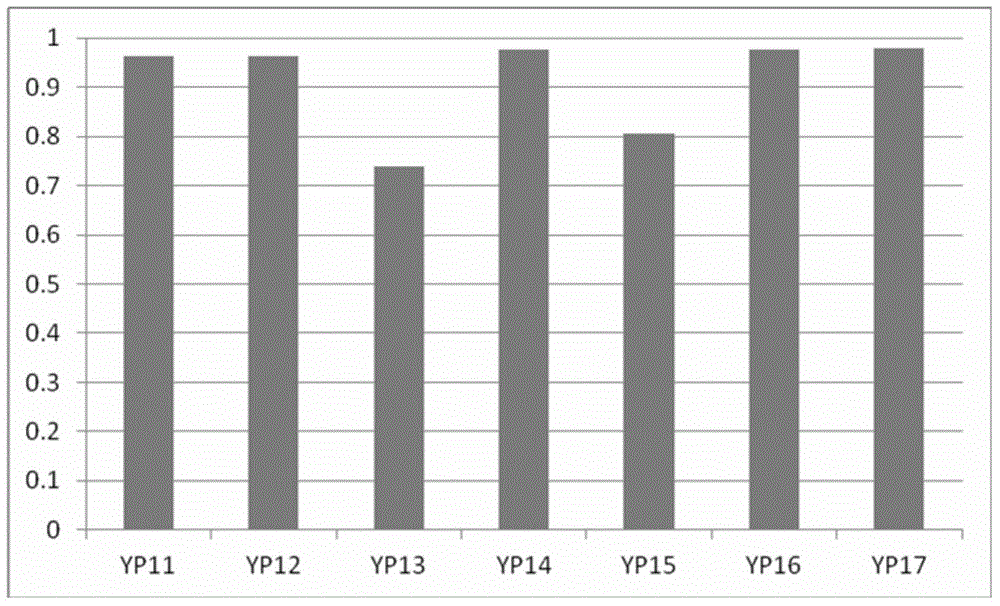
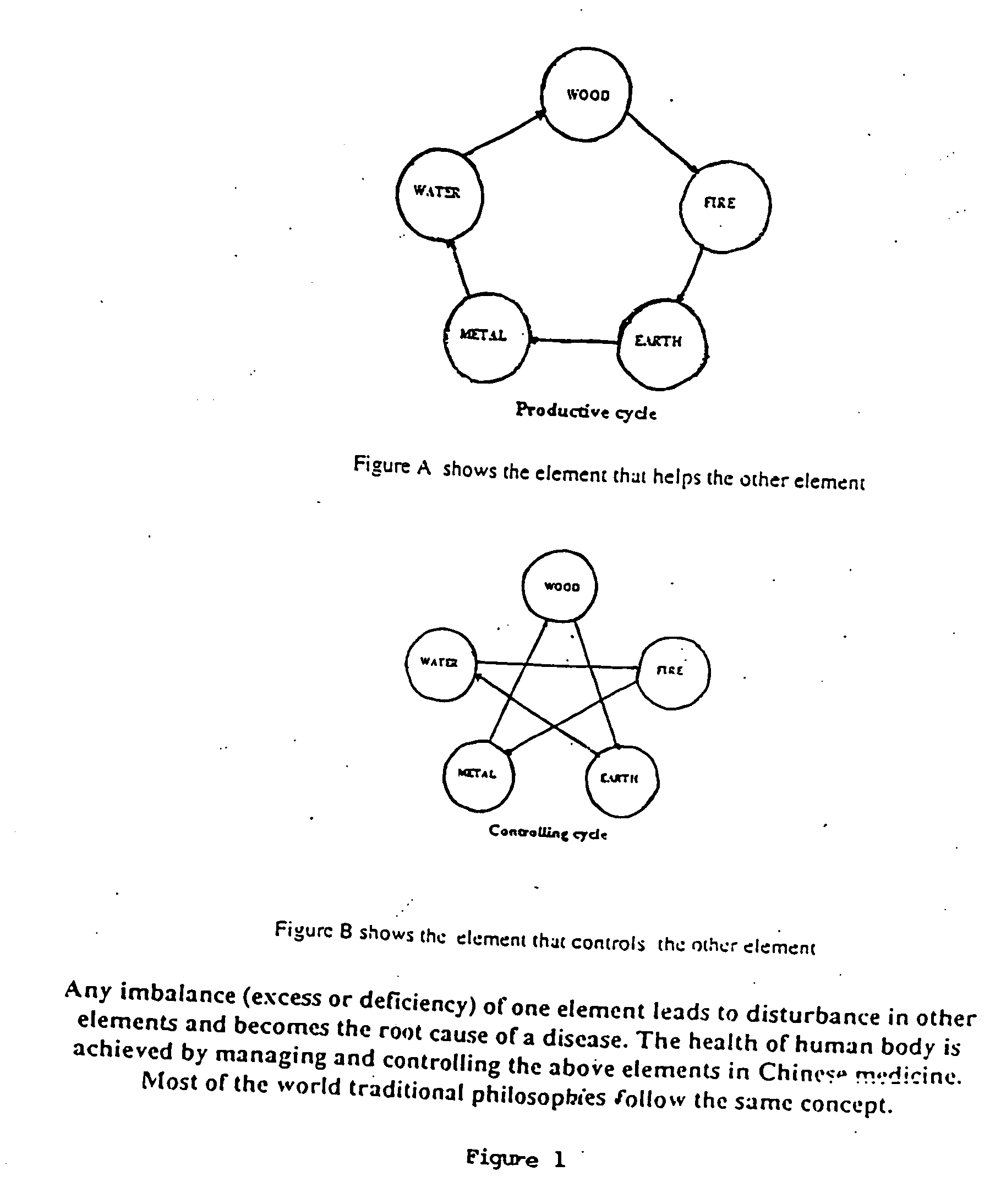
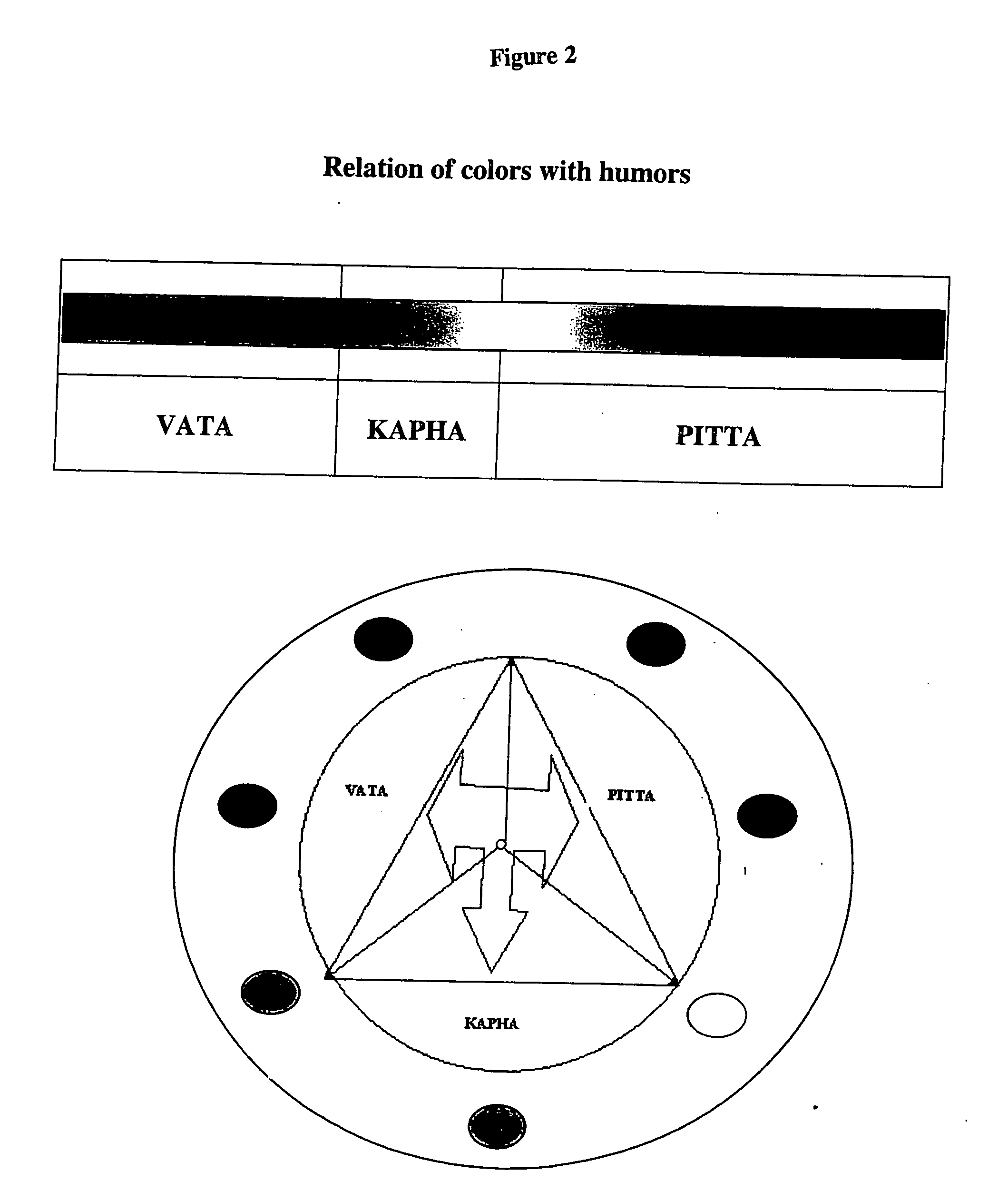
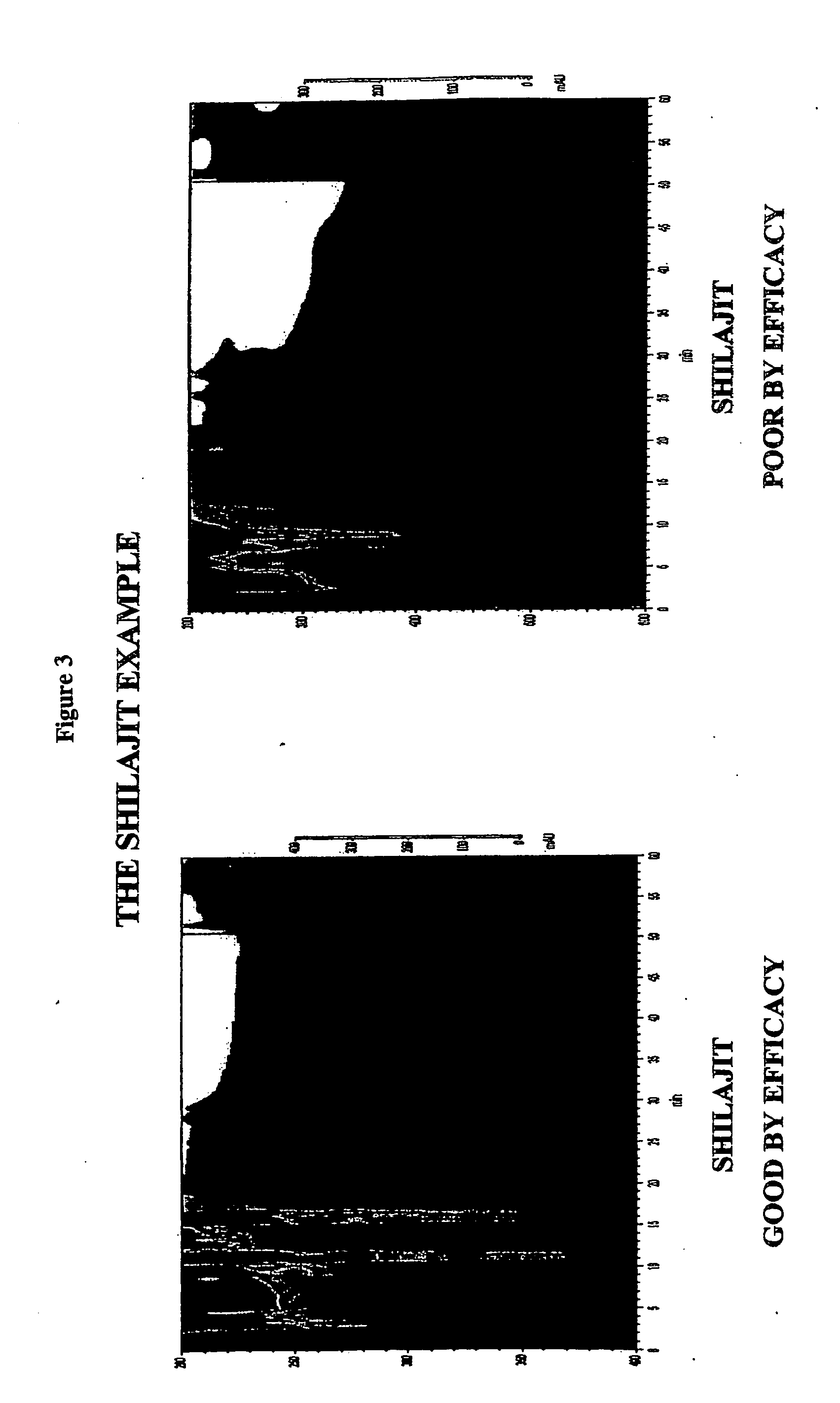
The present invention provides a method for the chromatographic fingerprinting, chemical and therapeutic standardization, bar-coding of the fingerprints and preparation of a data base for Enterprise Resource Planning (ERP) and Customer Relationship Management (CRM) machines and applications of medicines in general and traditional medicines in particular; this invention includes a software based instrumental method and a novel method of fingerprinting and standardization is proposed for the above purpose and the said method for the chromatographic finger printing which facilitates to correlate the traditional therapeutic standardization methods with the chemical properties of the medicines and humors and provides a rational basis to understand the methods used for the said purpose.
Owner:DADALA VIJAYA KUMAR +1
System for evaluation of Chinese medicine numeralization color spectrum dactylogram similarity
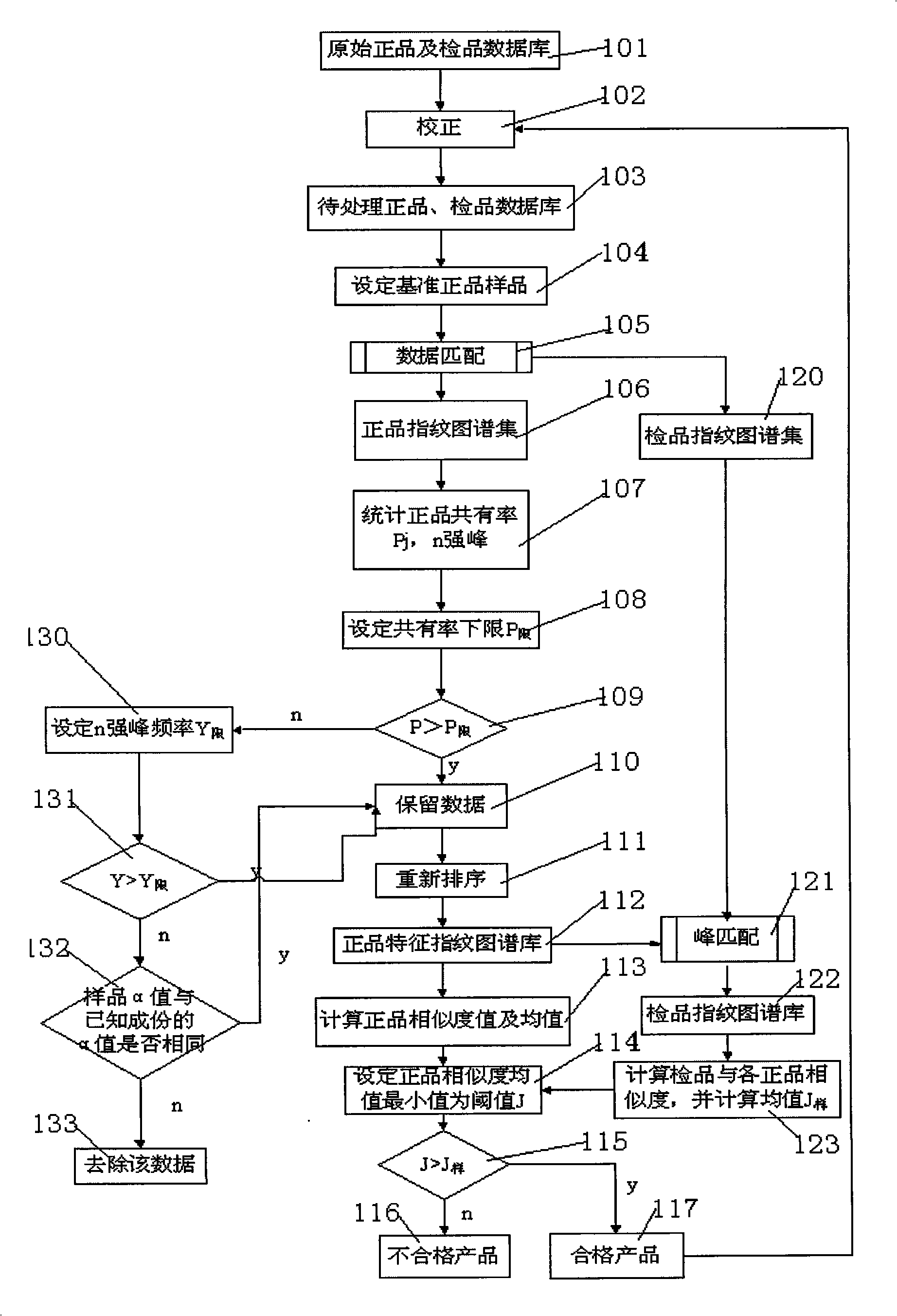
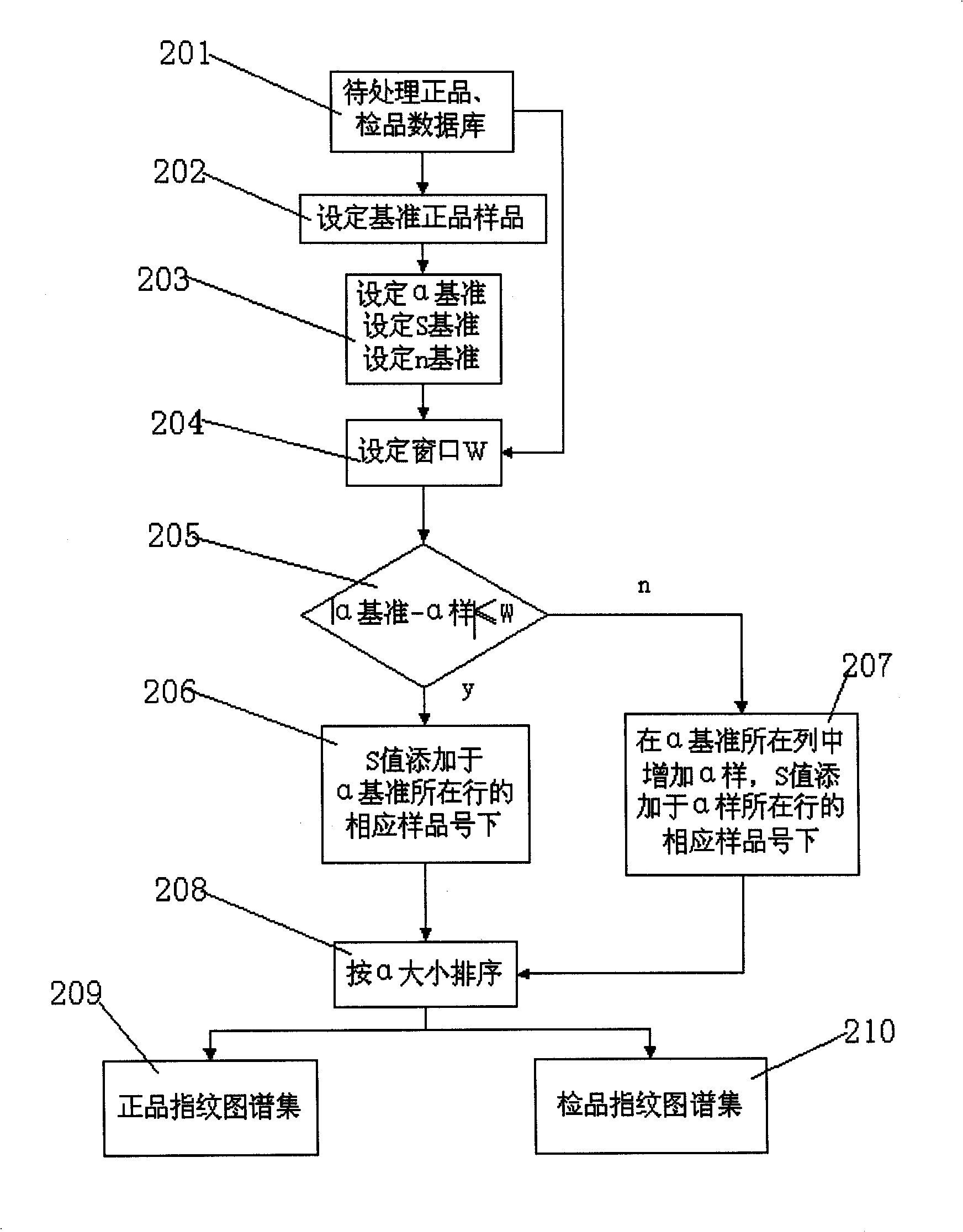
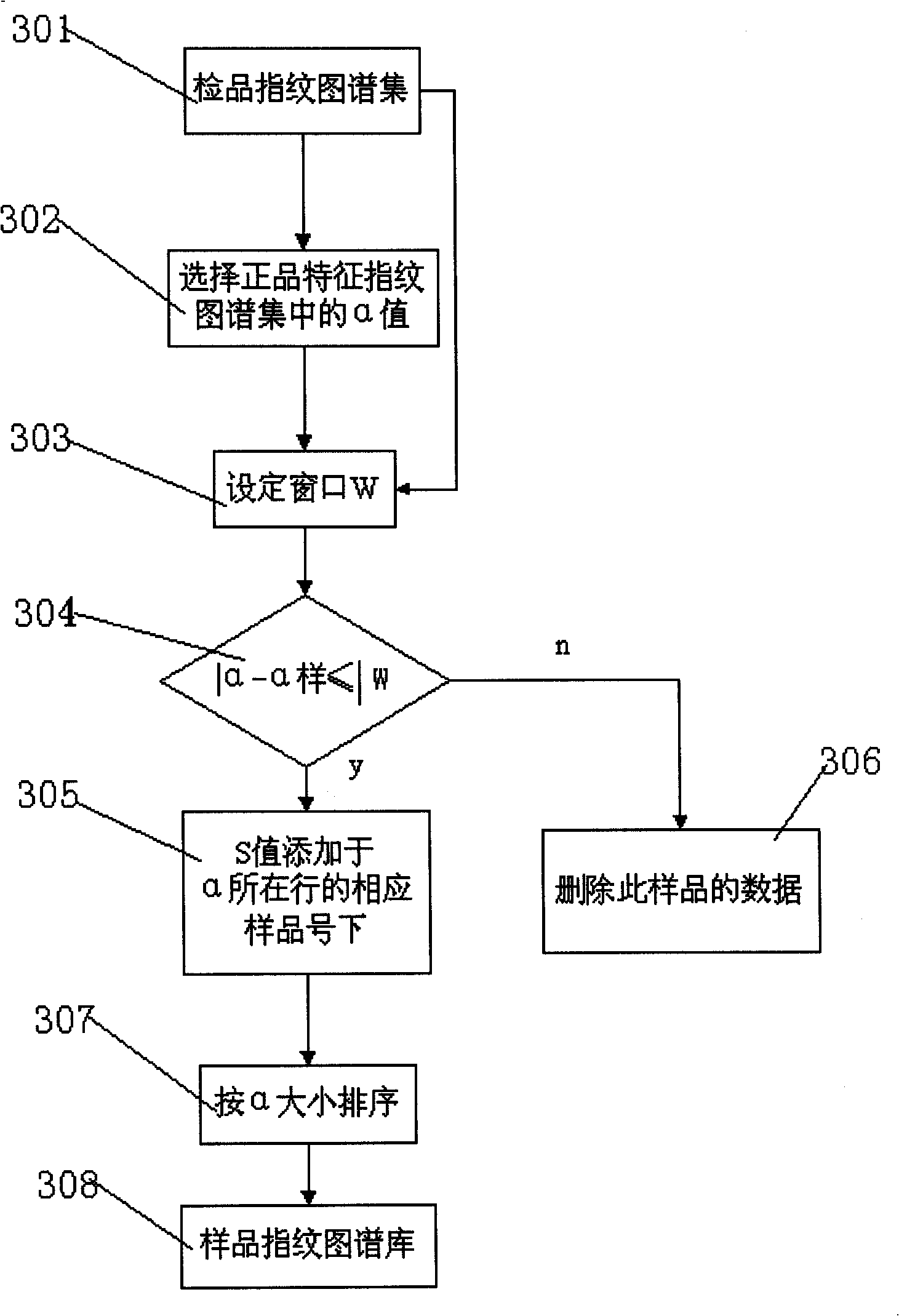
InactiveCN101256177AEfficient removalDigitizationComponent separationSpecial data processing applicationsPattern recognitionTest sample
The invention discloses a system for evaluating the similarity of digital chromatographic fingerprint of Chinese medicine, which includes the test of the substances by HPLC, input of the test result into the digital computer which saves programs, the digital computer which saves programs executes the following step: inputting data of quality goods and tested samples being tested by the HPLC into the original database, matching the data of quality goods and tested samples with the standard data, forming a quality goods fingerprint collection, a quality goods characteristic fingerprint database and a tested sample fingerprint collection; processing peak matches between the data in the tested sample fingerprint collection with the data in the quality goods characteristic fingerprint database, forming a tested sample fingerprint database, calculating the similarity between the quality goods and the similarity between the tested samples and every quality good, if the similarity mean of the tested samples is larger than the similarity mean J of the quality goods characteristic fingerprint database, the product is a qualified product, compared with the prior art, the peak-to-peak comparability is improved, the scientific processing is combined with integrity and fuzzyness, the datamation of the map is realized.
Owner:WANNAN MEDICAL COLLEGE
Method for building quality control chromatography fingerprint maps of traditional Chinese medicine herbal tea and herbal medicine beverage products
The invention discloses a method for building quality control chromatography fingerprint maps of traditional Chinese medicine herbal tea and herbal medicine beverage products. The method comprises the steps of: (1) preprocessing a sample and processing the sample by one method selected from following methods: (1) a freeing, drying-extracting method, (2) a thermal evaporating, drying-extracting method, and (3) a solid-phase extraction method; (2) conducting HPLC (high performance liquid chromatography) fingerprint map analysis. According to the method, the quality control chromatography fingerprint maps of the traditional Chinese medicine herbal tea and herbal medicine beverage products built by the method disclosed by the invention can be used for carrying out macroscopic and fine constituent quality control on the product, and the blank that the Chinese herbal medicine beverage such as the traditional Chinese medicine herbal tea and herbal medicine herbal tea only has requirements on raw material and auxiliary material, sensory indexes and health indexes in quality control, but has no macroscopic quality control indexes of related phytochemical constituents at present is filled.
Owner:HUNAN NORMAL UNIVERSITY
Constructing method of electrospray ionization mass spectrometry fingerprint of Guangdong herbal tea granules and standard fingerprint
InactiveCN102914587AEffective massEffective source differentiationPreparing sample for investigationMaterial analysis by electric/magnetic meansAdditive ingredientPrincipal component analysis
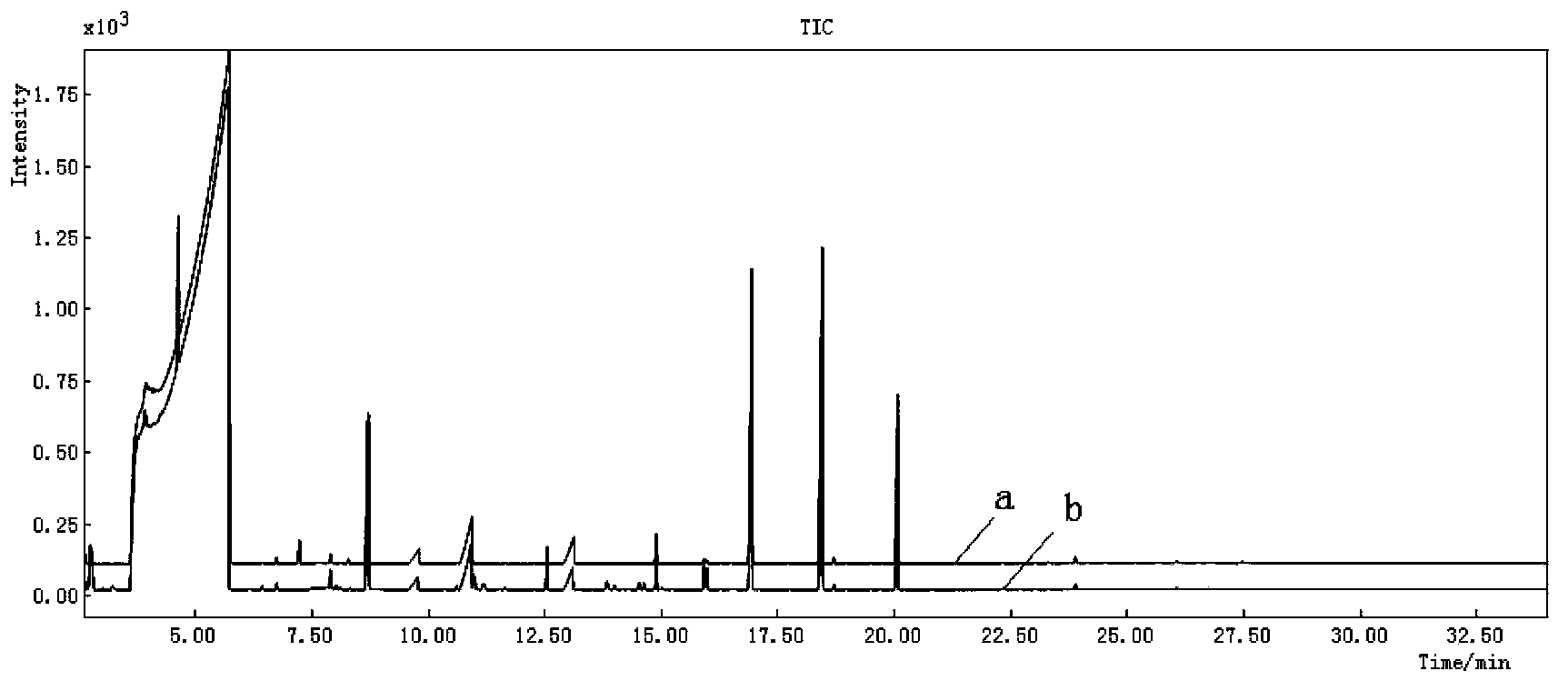
The invention provides a constructing method of an electrospray ionization mass spectrometry fingerprint of Guangdong herbal tea granules and a standard fingerprint, and relates to a quality control method of a traditional Chinese medicine preparation, in particular to a method for establishing the electrospray ionization mass spectrometry fingerprint to achieve quality evaluation and source distinguishment of the Guangdong herbal tea granules. The method comprises the following steps: (1) preparation of a test solution; (2) mass spectrometry condition: electrospray ionization mass spectrometry is directly used for detection, mass spectrometry contour map is recorded in a full-scanning mode, and scanning quality range m / z is 100-1000; (3) detection: the fingerprint is directly obtained by the electrospray ionization mass spectrometry; (4) standard fingerprint: the fingerprint of a test product uses the fingerprint according to the Similarity Evaluation System for Chromatographic Fingerprint of TCM(2004 A edition); and the standard fingerprint is generated through calculation using a mean value method; and (5) main ingredient analysis: main ingredients of the fingerprint of the test product are analyzed by using statistics software, so as to evaluate the quality and distinguish sources. The constructing method of an electrospray ionization mass spectrometry fingerprint of the Guangdong herbal tea granules, provided by the invention, is beneficial for monitoring product quality and has the advantages as follows: the quality of the Guangdong herbal tea granules is rapidly, efficiently and reliably represented; simple and convenient method, stability, high sensitivity and good repetitiveness are achieved; requirements on high throughput analysis are satisfied; and the quality of a product can be rapidly and accurately identified.
Owner:CHINA NAT ANALYTICAL CENT GUANGZHOU
Construction method for ion chromatography fingerprint spectrums of ganoderma lucidum spore powder polysaccharide
ActiveCN102645504AMethod stableHigh precisionComponent separationAnion-exchange chromatographyOligosaccharide
The invention discloses a construction method for ion chromatography fingerprint spectrums of ganoderma lucidum spore powder polysaccharide, which comprises acid-enzyme partial hydrolysis of the ganoderma lucidum spore powder polysaccharide, the ion chromatography fingerprint analysis of monosaccharide components and oligosaccharide components of hydrolysis products of the ganoderma lucidum spore powder polysaccharide and the confirmation of standard fingerprint spectrums. By means of the high performance anion-exchange chromatography (HPAEC) analysis and the comparison of the fingerprint spectrums of polysaccharide contents in ganoderma lucidum spore powder samples from 20 different production places, the common fingerprint characteristics of the polysaccharide contents in the ganoderma lucidum spore powder samples are determined, the standard fingerprint spectrums of the monosaccharide components and the oligosaccharide components are obtained respectively, and 11 monosaccharide common characteristic peaks in monosaccharide spectrums, 4 oligosaccharide common peaks in the oligosaccharide spectrum and 1 polysaccharide peak are determined. According to the construction method for the ion chromatography fingerprint spectrums of the ganoderma lucidum spore powder polysaccharide, the method is stable, the precision degree is high, the reproducibility is good, the method is easy to master, quality conditions and production places of the ganoderma lucidum spore powder polysaccharide can be grasped from two aspects of fingerprint spectrums of the monosaccharide components and the oligosaccharide components of the acid-enzyme partial hydrolysis products which can reflect the components and structural characteristics of the ganoderma lucidum spore powder polysaccharide, and a new scientific method is provided for the quality control and the authenticity identification of the ganoderma lucidum spore powders.
Owner:中食都庆(山东)生物技术有限公司
Method for evaluating intrinsic quality stability of cigarette blasting bead
ActiveCN107941978AImprove quality and safety control levelNot easy to missComponent separationRetention timeInternal standard
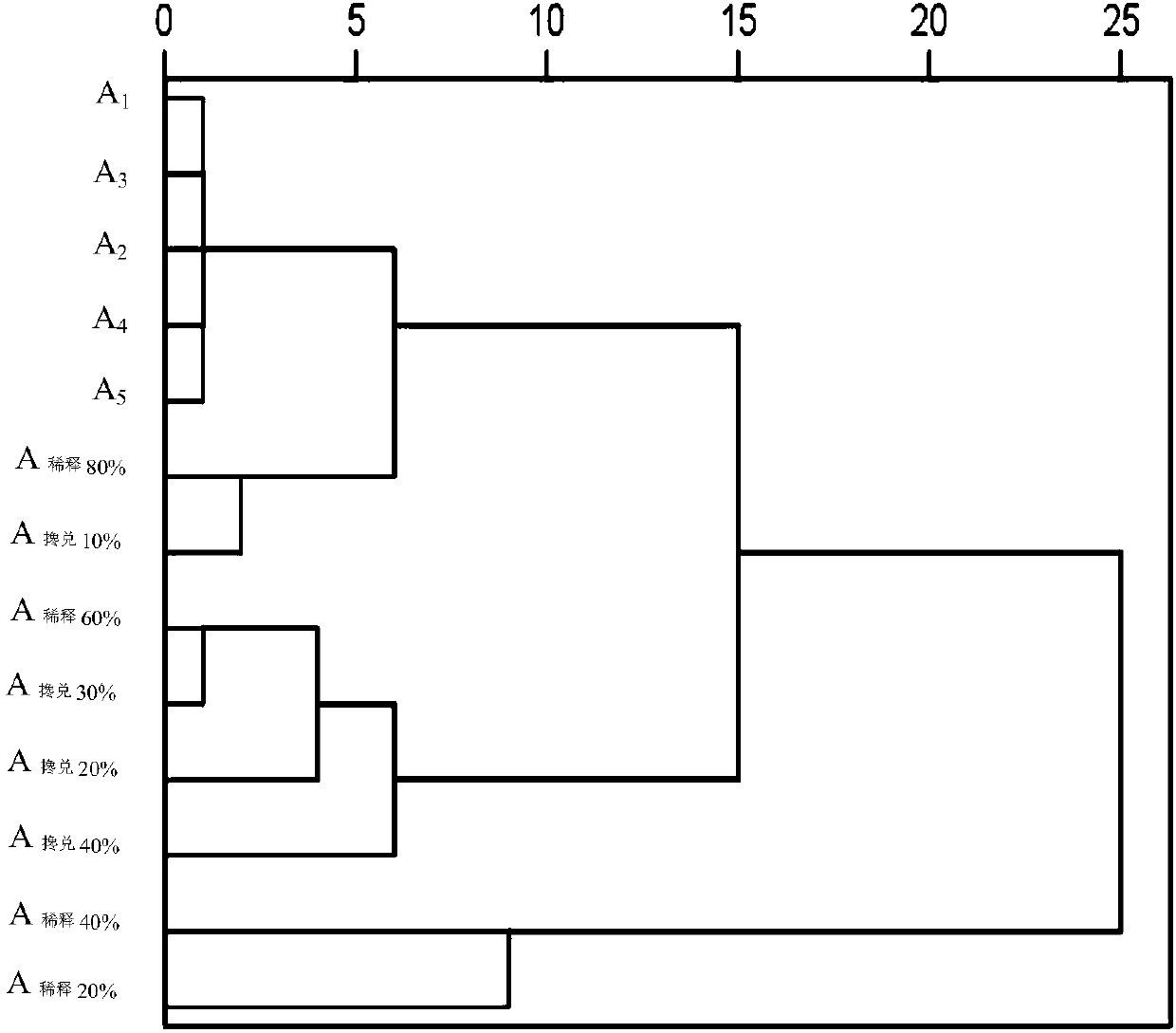
The invention relates to a method for evaluating the intrinsic quality stability of a cigarette blasting bead. The method comprises the following steps: grinding a plurality of batches of cigarette blasting bead samples separately, carrying out filtering to obtain blasting bead contents, adding an internal standard solution into the blasting bead contents, taking a supernatant after extraction, and filtering the supernatant to obtain filtrate, wherein the ratio of the blasting bead contents to internal standard substances in the internal standard solution is 1mL:(0.5-2.0)mg; carrying out gas chromatography-mass spectrometry analysis on the filtrate, taking an internal standard peak as a reference peak, determining a common peak, calculating a relative retention time alpha and a relative peak area S value of a chromatographic peak in the cigarette blasting bead sample, and establishing a chromatographic fingerprint of each blasting bead content sample; obtaining a euclidean distance, aeuclidean distance ratio and similarity by using the relative peak area S value of each component in each batch of cigarette blasting bead samples, and evaluating the intrinsic quality stability of the cigarette blasting bead. The method provided by the invention can comprehensively analyze the intrinsic quality of the cigarette blasting bead and reflect the fluctuation of the intrinsic quality.
Owner:CHINA TOBACCO SHAANXI IND
Method for guaranteeing its component content stability in Chinese medicine extract blending optimization
InactiveCN1586509AStable and uniform qualityTake advantage ofComponent separationUnknown materialsMedicineMixing ratio
The method of blending Chinese medicine extract to ensure the component content stability includes the following steps: determining the standard extract and measuring its chromatographic fingerprint and effective component content; extracting the medicine material of different batches separately to obtain the extracts and measuring the chromatographic fingerprint and effective component content of the batches; inputting the chromatographic fingerprint and effective component content of the batches and the chromatographic fingerprint and effective component content of the standard extract into the blending mold to obtain optimized mixing ratio; and mixing the extracts of the different batches based on the calculated values to obtain the extract with the same quality as the standard extract. The method of the present invention can ensure the stable Chinese medicine product quality.
Owner:ZHEJIANG UNIV
Quality monitoring and control method for tobacco essence perfume
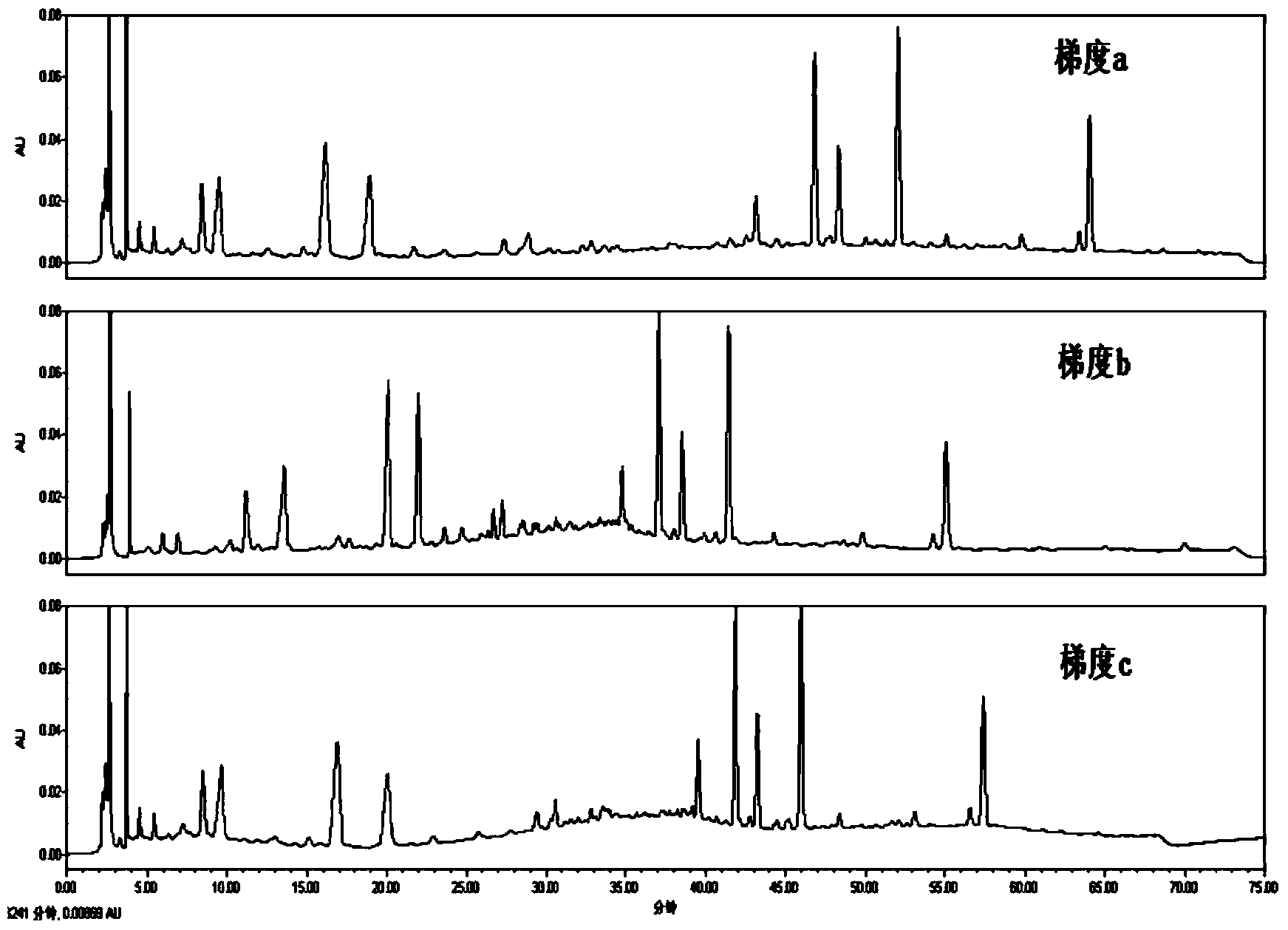
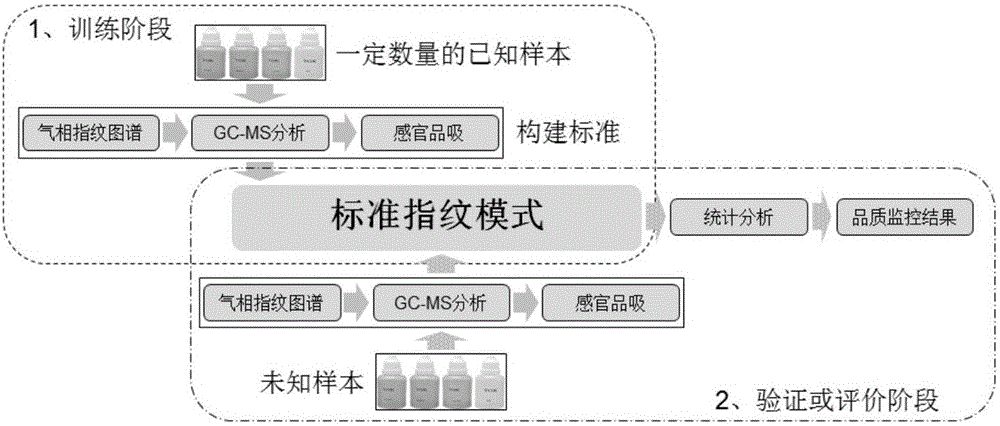
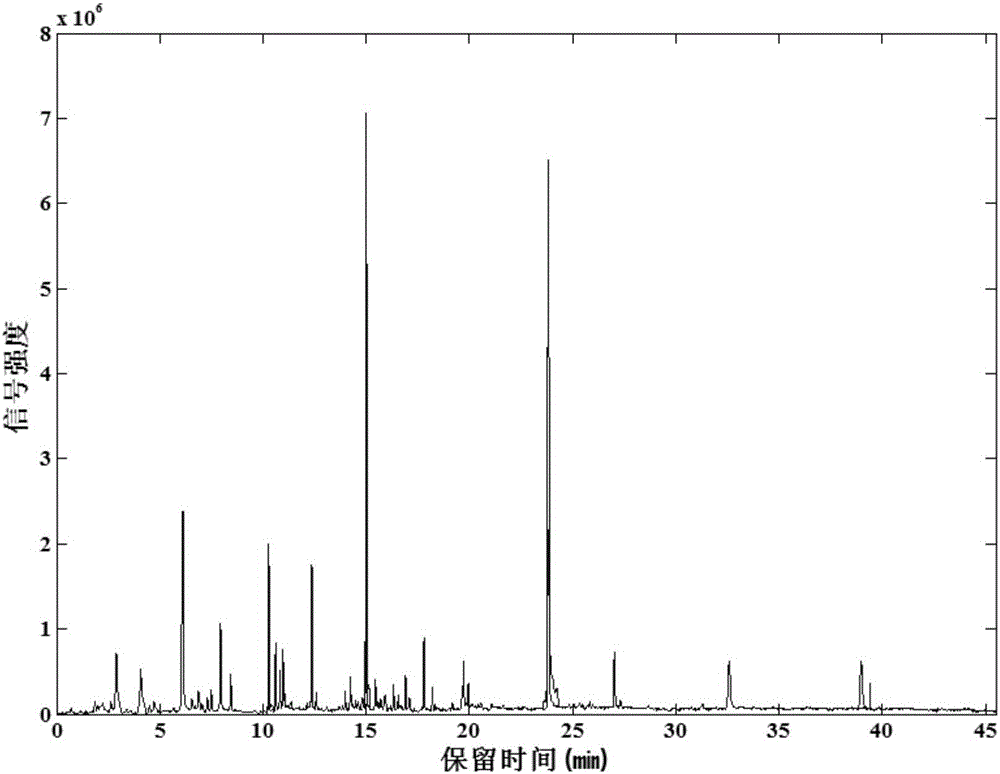
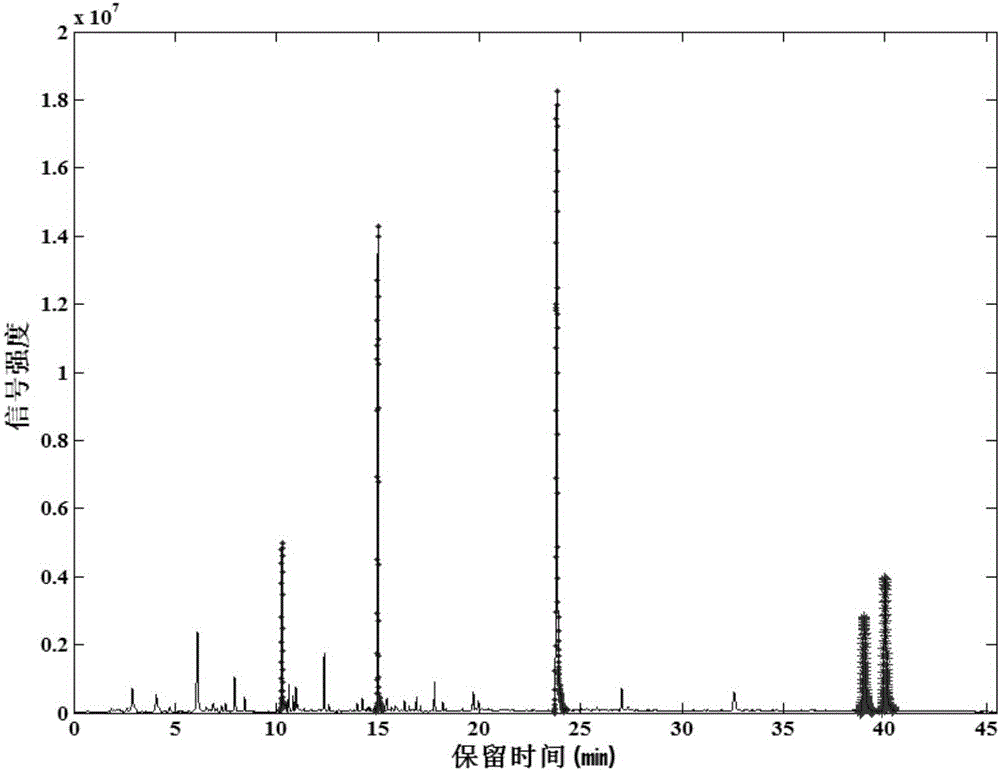
The invention relates to the field of tobacco preparation and discloses a quality monitoring and control method for tobacco essence perfume. In the method, a traditional fingerprint technology, a two-dimensional gas chromatograph-mass spectrometer (GC-MS) and sensory smoking evaluation are organically combined, so that advantages of the three methods are combined together while the defects are complemented by each other, thereby achieving the object of effectively control the quality of the essence perfume. The method particularly includes the steps of: 1) collecting gas-phase chromatograph and fingerprint spectrum of the essence perfume; 2) obtaining information of a key fragrant component through the GC-MS and quantitatively weighting the information in the fingerprint spectrum; 3) finally quantizing experience of a smoking evaluation expert, and quantitatively adding the chemical components, which cannot be effectively detected by means of analysis instruments, to the fingerprint spectrum in manners of component simulation and weighting, thereby forming a holographic fingerprint spectrum with combination of multiple characters; 4) on the basis of the holographic fingerprint spectrum, establishing a fingerprint spectrum database of the target essence perfume and establishing a standard mode; and 5) through a statistic analysis method, performing quality monitoring and control of a new essence perfume sample or effective identification of a unknown sample. Compared with a conventional quality monitoring and control method, the method provides more abundant and comprehensive information and more accurate and reliable result. The method has wide application value and market prospect.
Owner:DALIAN CHEM DATA SOLUTION TECH CO LTD
Method for verification of color spectrum fingerprint pattern peak purity
InactiveCN101256175APurity verificationEasy to handleComponent separationSpecial data processing applicationsHat matrixComputer science
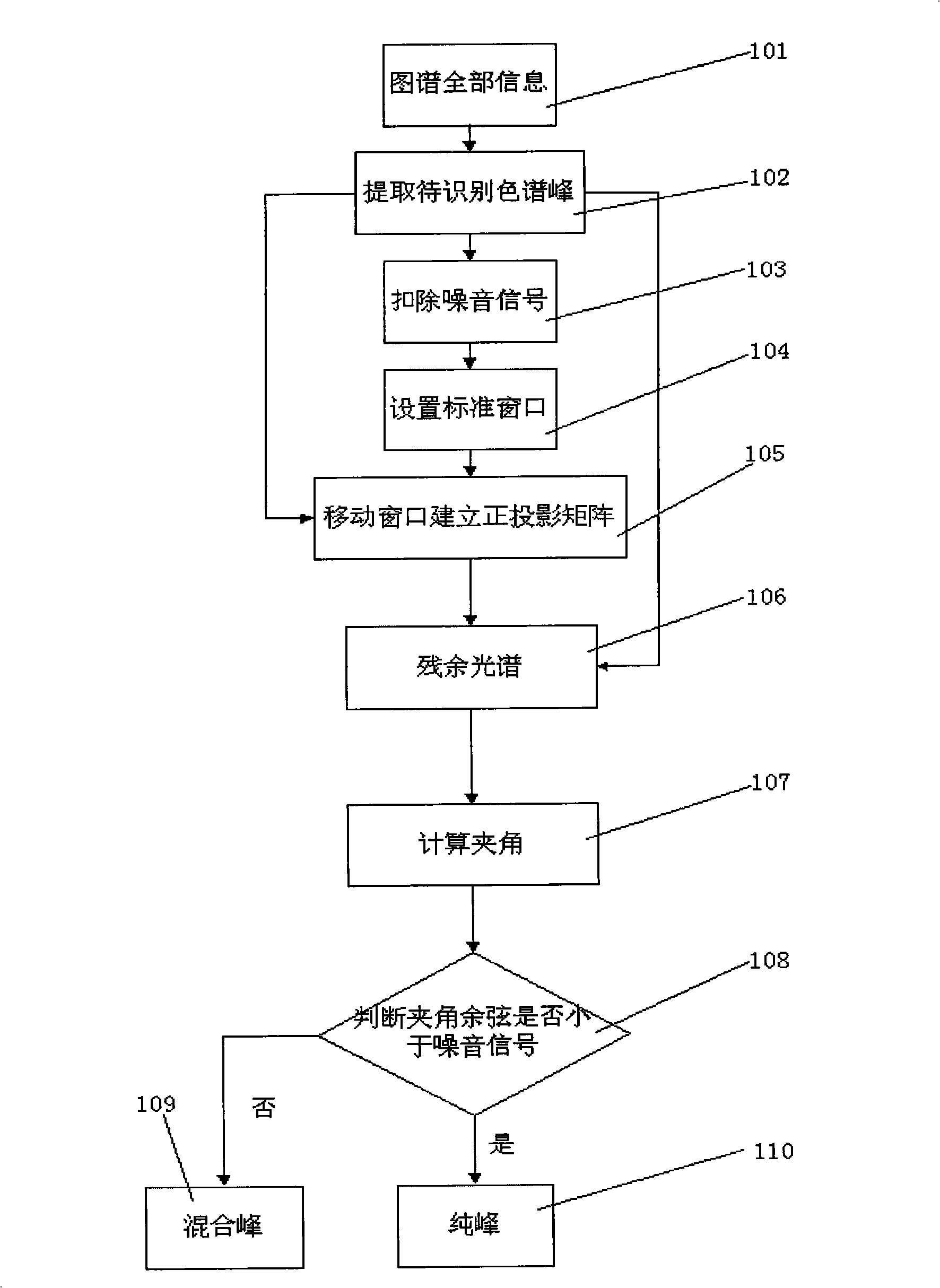
The invention discloses a method for testing purity of chromatographic fingerprint peak, which includes the following steps: processing the chromatographic fingerprint to the Chinese medicine by HPLC with DAD tester, obtaining whole information of the fingerprints; extracting chromatographic peaks to be identified in the chromatogram; deducting noise signals from the chromatographic peaks to be extracted and identified; setting standard windows; moving windows and establishing an orthogonal projection matrix; projecting chromatographic peaks to be identified to the orthogonal projection matrix, obtaining the residual spectrum; calculating the included angle between the chromatographic peak to be identified and the residual spectrum; determining whether the cosine of the included angle is less than the noise signal; if the cosine of the included angle is less than the noise signal, the chromatographic peak to be identified is a pure peak; if the cosine of the included angle is more than the noise signal, the chromatographic peak to be identified is a mixed peak. Compared with the prior art, the purity of the chromatographic peak is directly judged without standard or reference substance, the process is fast and easy, and is programmed.
Owner:WANNAN MEDICAL COLLEGE
Establishing method of fingerprint spectrum of honeysuckle-fructus forsythiae heat-clearing tablets and fingerprint spectrum
The invention discloses an establishing method of a fingerprint spectrum of honeysuckle-fructus forsythiae heat-clearing tablets and the fingerprint spectrum. The establishing method of the fingerprint spectrum of the honeysuckle-fructus forsythiae heat-clearing tablets includes the following steps: step 1, preparing a test sample solution of the honeysuckle-fructus forsythiae heat-clearing tablets; step 2, respectively precisely sucking the test sample solution and injecting into liquid chromatographs, and recording a chromatogram; step 3, exporting the honeysuckle-fructus forsythiae heat-clearing tablet fingerprint spectrum obtained in the step 2 from the instrument, and importing into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system; selecting chromatographic peaks existing in chromatograms of different batches of the honeysuckle-fructus forsythiae heat-clearing tablets as common peaks; generating a reference fingerprint spectrum of the honeysuckle-fructus forsythiae heat-clearing tablets by an average value calculation method; and calculating the relative retention time and the relative peak area of each common peak. The honeysuckle-fructus forsythiae heat-clearing tablet fingerprint spectrum established by the method provided by the invention can effectively characterize the quality of the honeysuckle-fructus forsythiae heat-clearing tablets, and is conducive to comprehensive supervisory control of the drug quality. The method has the advantages of being simple, convenient, stable, high in precision, good in reproducibility and the like.
Owner:JIANGSU KANION PHARMA CO LTD
Establishing method of double-solvent fused HPLC fingerprint of medicinal phellodendron and standard fingerprint of medicinal phellodendron
InactiveCN103869003ALarge amount of informationAchieve comprehensiveComponent separationHplc fingerprintMedicine
The invention provides an establishing method of double-solvent fused HPLC fingerprint of medicinal phellodendron. The method comprises the following steps: (1) preparing a reference solution: preparing a berberine hydrochloride reference solution; (2) preparing test solutions: weighing medicinal phellodendron or cortex phellodendri chinensis, extracting the weighed medicinal phellodendron or cortex phellodendri chinensis with two solvents, wherein the two solvents are representative and complementary, filtering the extracting solutions with a micro-porous filter membrane so as to obtain the test solutions; (3) measuring the obtained test solutions through a high performance liquid chromatography (HPLC) method so as to obtain fingerprints, wherein the chromatography conditions are as follows: chromatographic column is filled with octadecylsilane chemically-bonded silica, gradient elution is adopted, and the ultraviolet detection wavelength is 300 nm; (4) fusing the two fingerprints of parts respectively extracted by two solvents so as to establish a double-solvent fused fingerprint of medicinal phellodendron. The test fingerprint is evaluated by the "Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine" published by Chinese Pharmacopoeia Commission (Version 2004A). The establish fingerprint has rich fingerprint characteristic information and the characteristics of simple method, good repeatability, and reliability, is capable of identifying cortex phellodendri chinensis, medicinal phellodendron and counterfeit, and provides a reference for identification and overall quality control of medicinal phellodendron.
Owner:DIHON PHARMA GROUP +1
Construction method and applications of high performance liquid chromatography (HPLC) fingerprint of alkaloid compounds of dendrobe
InactiveCN103604877AExtract completelyImprove overall lifespanComponent separationHplc fingerprintAmmonium hydroxide
The invention discloses a construction method of fingerprint of alkaloid compounds of dendrobe. According to the construction method, thermostatic waterbath reflux extraction technology is adopted; dendrobe powder is immersed in ammonium hydroxide, and is subjected to extraction with chloroform, filtration, condensation and drying so as to obtain a dendrobe total alkaloid crude product; the dendrobe total alkaloid crude product is dissolved in methanol so as to obtain a stock solution; and the stock solution is used for high performance liquid chromatographic analysis so as to obtain the dendrobe alkaloid compound fingerprint. Advantages of the construction method are that: the obtained chromatographic fingerprint is capable of comprehensively reflecting alkaloid compounds contained by dendrobium, reflecting complexity of dendrobium alkaloid compounds, and providing scientific basis for identification of different dendrobium varieties; the constructed HPLC fingerprint possesses relatively high stability and repeatability; and scientific reference is provided for quality control of dendrobium variety.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Fingerprint detection method of Xiaokeqing preparation
The invention relates to a fingerprint detection method of a Xiaokeqing preparation. The method comprises the following steps: 1, preparing the chromatogram of a sample to be detected: taking a Xiaokeqing preparation sample, adding an extract solvent which is an aqueous solution containing a proportion of methanol or ethanol, carrying out ultrasonic dissolving, adding the solvent to adjust the concentration of the obtained solution, filtering through a millipore filtration membrane, taking the obtained filtrate, and injecting the filtrate to an ultrahigh performance liquid chromatograph to obtain the chromatogram; and 2, calculating the similarity of the chromatogram of the sample to be detected and the a standard control fingerprint: introducing the chromatogram integration signal of the sample to be detected into software of Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (Version A) published by Chinese Pharmacopoeia Commission, and comparing the similarity of the chromatogram of the sample to be detected with the similarity of the standard control fingerprint.
Owner:TIANJIN TASLY PHARMA CO LTD
Black prepared lateral root of aconite extracting solution and shenfu injection obtained by adopting real-time release method in automatic black prepared lateral root of aconite extracting process
ActiveCN108459096AImprove batch-to-batch stabilityGood product quality and stabilityComponent separationPharmaceutical delivery mechanismAdditive ingredientUltrafiltration
The invention discloses a black prepared lateral root of aconite extracting solution and shenfu injection obtained by adopting a real-time release method in the automatic black prepared lateral root of aconite extracting process, and relates to the field of traditional Chinese medicine injection production testing. The real-time release method in the automatic black prepared lateral root of aconite extracting process comprises the steps of collecting and detecting the alkaloid content and a chromatographic fingerprint spectrum of a black prepared lateral root of aconite raw material sample; preparing acid volume extraction according to the alkaloid content of black prepared lateral root of aconite raw materials, and adopting an ultrafiltration membrane for removing impurities to obtain theblack prepared lateral root of aconite extracting solution; performing online collecting and detecting on the alkaloid content and the chromatographic fingerprint spectrum of the black prepared lateral root of aconite extracting solution sample; according to the alkaloid content of the black prepared lateral root of aconite extracting solution, black prepared lateral root of aconite extracting technological parameters are adjusted. Due to the fact that different raw materials correspond to different technological parameters, the stable effective ingredient is obtained, the batched stability and safety for reducing toxicity and increasing effect of the black prepared lateral root of aconite are improved, meanwhile, application of a poisonous and harmful reagents is lowered, the product quality stability of the obtained black prepared lateral root of aconite extracting solution and shenfu injection is good, and the clinic pharmaceutical safety and effectiveness are high.
Owner:YAAN THREE NINE PHARMA
Method for constructing HPLC characteristic chromatogram of Chinese patent medicine 'Yishen Bugu liquid'
The invention provides a method for constructing an HPLC characteristic chromatogram of a Chinese patent medicine 'Yishen Bugu liquid'. The method comprises the following steps: (1) preparation of a solution of a tested object; (2) preparation of a solution of a reference substance: a proper amount of the naringin reference substance is taken and weighed precisely, methanol is added for preparing a solution, wherein every 1ml of the solution contains about 0.1mg of the substance; (3) determination: 5-15[mu]l of the solution of the reference substance and 5-15[mu]l of the solution of the tested object are separately and precisely absorbed, the solutions are injected into a high performance liquid chromatograph, chromatogram from the first minute to the twentieth minute is recorded, the chromatogram data is introduced into a <similarity evaluation system for chromatographic fingerprint applied in traditional Chinese medicine> 2004A edition for analysis, and the specific chromatogram of the 'Yishen Bugu liquid' is obtained. The method has the active effects that the established characteristic chromatogram has high technical content, unicity and one-sidedness of quality control of the 'Yishen Bugu liquid' preparation are avoided, high absorption conditions of near ultraviolet impurity peaks are avoided, and the possibility of artificial treatment of product quality for reaching the standard is reduced. The method has the advantages of multiple chromatograph peaks, good peak shape, easy identification, high similarity, good specificity, good stability, and reliable accuracy.
Owner:TONGYAO PHARMA GROUP CORP
Similarity detection method for chromatographic fingerprint spectrogram
InactiveCN102507815AAutomatic measurement is simple and convenientHigh precisionComponent separationRetention timeStandard samples
A similarity detection method for a chromatographic fingerprint spectrogram includes the following steps: 1 building a polymer standard substance cracking spectrogram base: adopting a cracking gas chromatograph to detect a serial of high polymer standard samples, ruling that spectrum conditions used in each sample detection need to be identical with spectrum conditions used in detection of high polymer standard samples, and forming the polymer standard substance cracking spectrogram base by importing detected experimental data; and 2 matching with actual samples: A firstly choosing to add a current spectrogram, entering actually detected sample data for temporary storing, and leading reservation time of the entered data to be correctable; B choosing a standard spectrogram base to be compared, choosing one or several spectrogram bases to be compared with actually detected samples according to previously built spectrogram bases, and calculating similarity s through cosine of included angles. The similarity detection method for the chromatographic fingerprint spectrogram performs measurement automatically, is simple, convenient and high in accuracy.
Owner:ZHEJIANG UNIV OF TECH
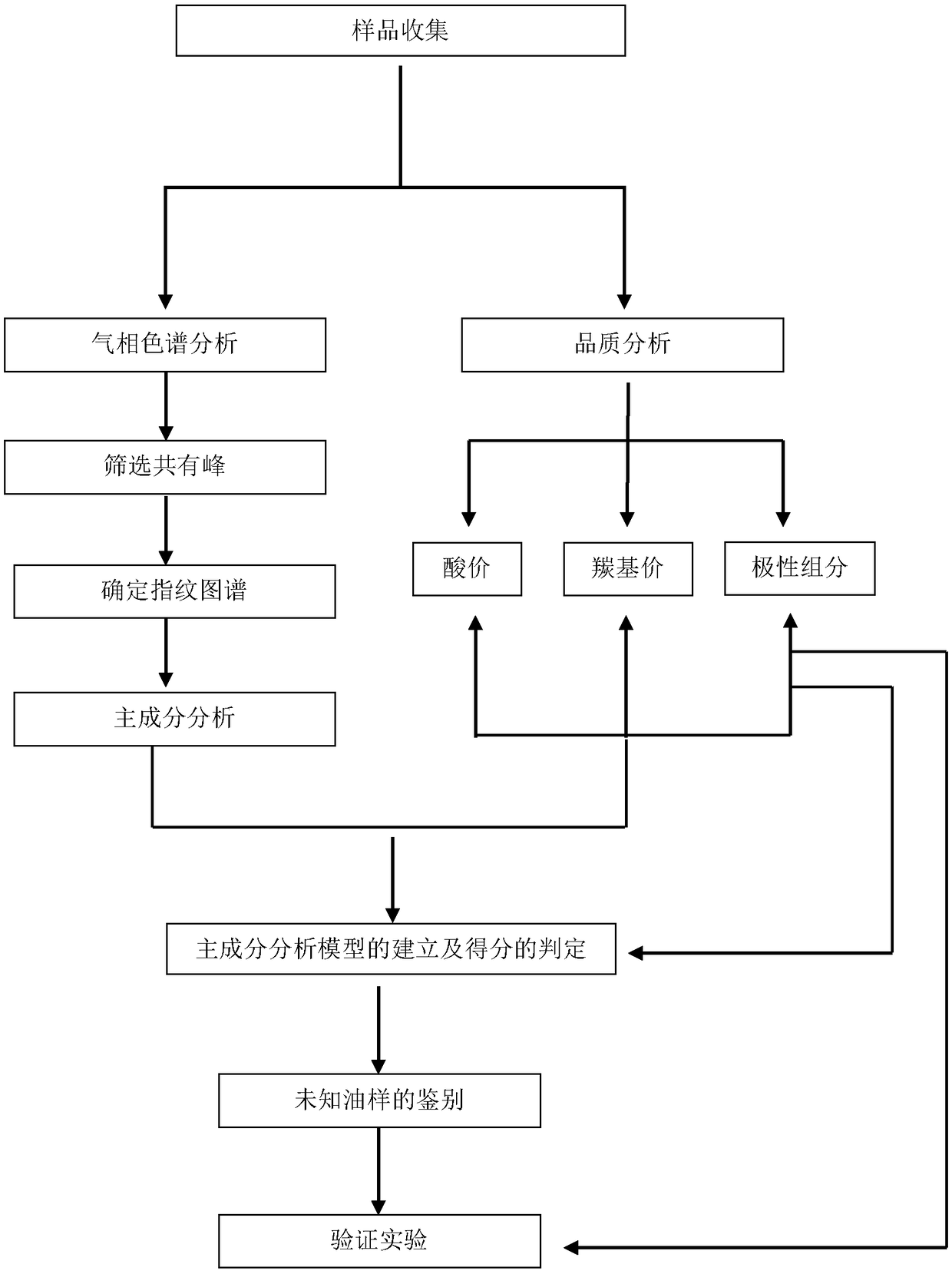
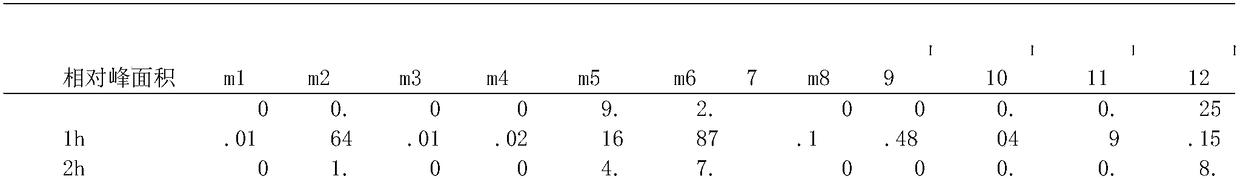
Method for distinguishing quality of frying oil through combination of gas chromatographic fingerprint and principal component analysis method
InactiveCN108845045AMeet the requirements of rapid detectionFast measurementComponent separationGas phasePrincipal component analysis
The invention belongs to the technical field of fats and oils identification, and discloses a method for distinguishing quality of frying oil through combination of gas chromatographic fingerprint anda principal component analysis method. The method is based on an established gas chromatographic fingerprint library as a sample; a common characteristic peak is extracted according to a similarity software, and characteristic peak data are analyzed by the principal component analysis method; and the quality of edible oil under different frying depths of the frying oil is effectively identified and distinguished. The quality of the edible oil under different frying depths identified by the principal component analysis method obtained by the method of the invention and by a national standard method is consistent; and the method is an effective and highly reliable method for identifying the quality of the frying oil.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Chinese medicine color spectrum fingerprint pattern characteristic digitalization and full-qualitative full-quantitative quality control method
The invention discloses a digital control method of super-information characteristics of traditional Chinese medicine chromatographic fingerprint, and 37 characteristic indicators are combined with the computer software technology to be used for the production quality control of traditional Chinese medicine materials, traditional Chinese medicine extracts and traditional Chinese medicine preparations. The invention simultaneously discloses an overall qualitative and overall quantitative quality control method of the traditional Chinese medicine chromatographic fingerprint, which uses the ratio qualitative similarity SF', Q percent of the content similarity, QF percent of the correction content similarity, C percent of the projection content similarity, P percent of the quantitative similarity, W percent of the norm length percentage, M percent of the average weight percentage, MF percent of the correction average weight percentage, d percent of the Euclidean distance percentage, Delta C percent of the projection content similarity error and other indicators and is further combined with the computer software technology to be used for the production quality control of the traditional Chinese medicine materials, the traditional Chinese medicine extracts, the traditional Chinese medicine preparations and botanical drugs. The digital control method is used for the evaluation of the fingerprint of the traditional Chinese medicine or the traditional Chinese medicine preparations and the confirmation and the evaluation of the test conditions, thus leading the test results to have quantitative reference indicators under the different conditions, which has great practicality.
Owner:SHENYANG PHARMA UNIVERSITY
Lanqin oral solution fingerprint spectrum establishment method, fingerprint spectrum of Lanqin oral solution and application of fingerprint spectrum
ActiveCN110108825ACharacterizing qualityAvoid one-sidednessComponent separationBenzoic acidChlorogenic acid
The invention discloses a Lanqin oral solution fingerprint spectrum establishment method, a fingerprint spectrum of a Lanqin oral solution and application of the fingerprint spectrum. A test solutionis prepared from the Lanqin oral solution, a reference substance solution is a solution which dissolves adenosine, epigoitrin, phellodendrine chloride, chlorogenic acid, magnoflorine, geniposide, benzoic acid, berberine hydrochloride, baicalin, wogonoside, baicalein and wogonin, and the liquid chromatography is measured by using a high-performance liquid chromatograph; the obtained liquid chromatography is introduced into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system for analysis, and the Lanqin oral solution fingerprint spectrum is obtained after multi-point correction, data matching and analysis. According to the Lanqin oral solution fingerprint spectrum establishment method and the fingerprint spectrum of the Lanqin oral solution, the quality information of the Lanqin oral solution can be comprehensively reflected, and uniform and stable product quality is ensured.
Owner:YANGTZE RIVER PHARMA GRP JIA NGSU LONGFENGTANG TRADITIONAL CHINESE MEDICINE CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com