Patents
Literature
62results about How to "Characterizing quality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
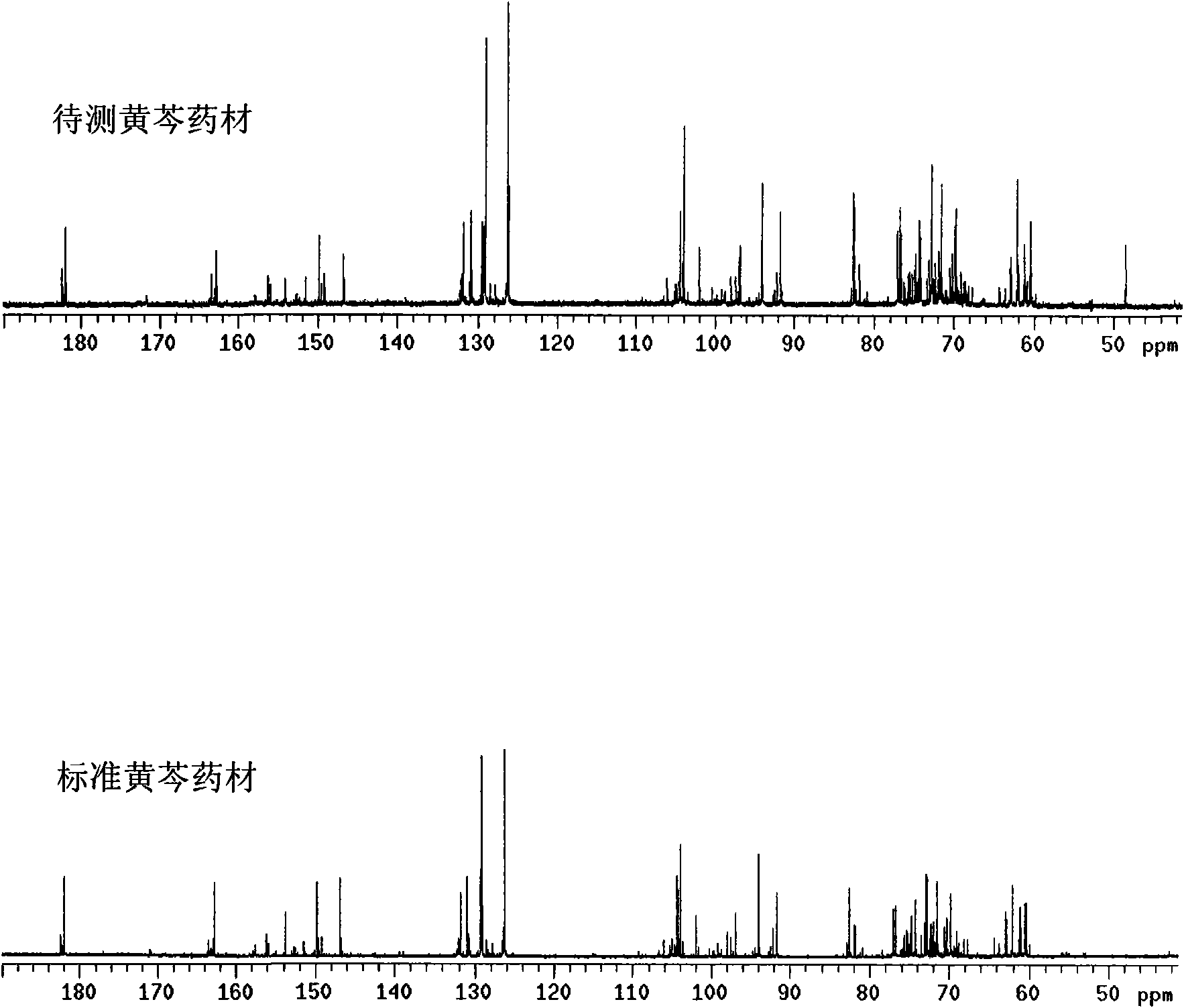
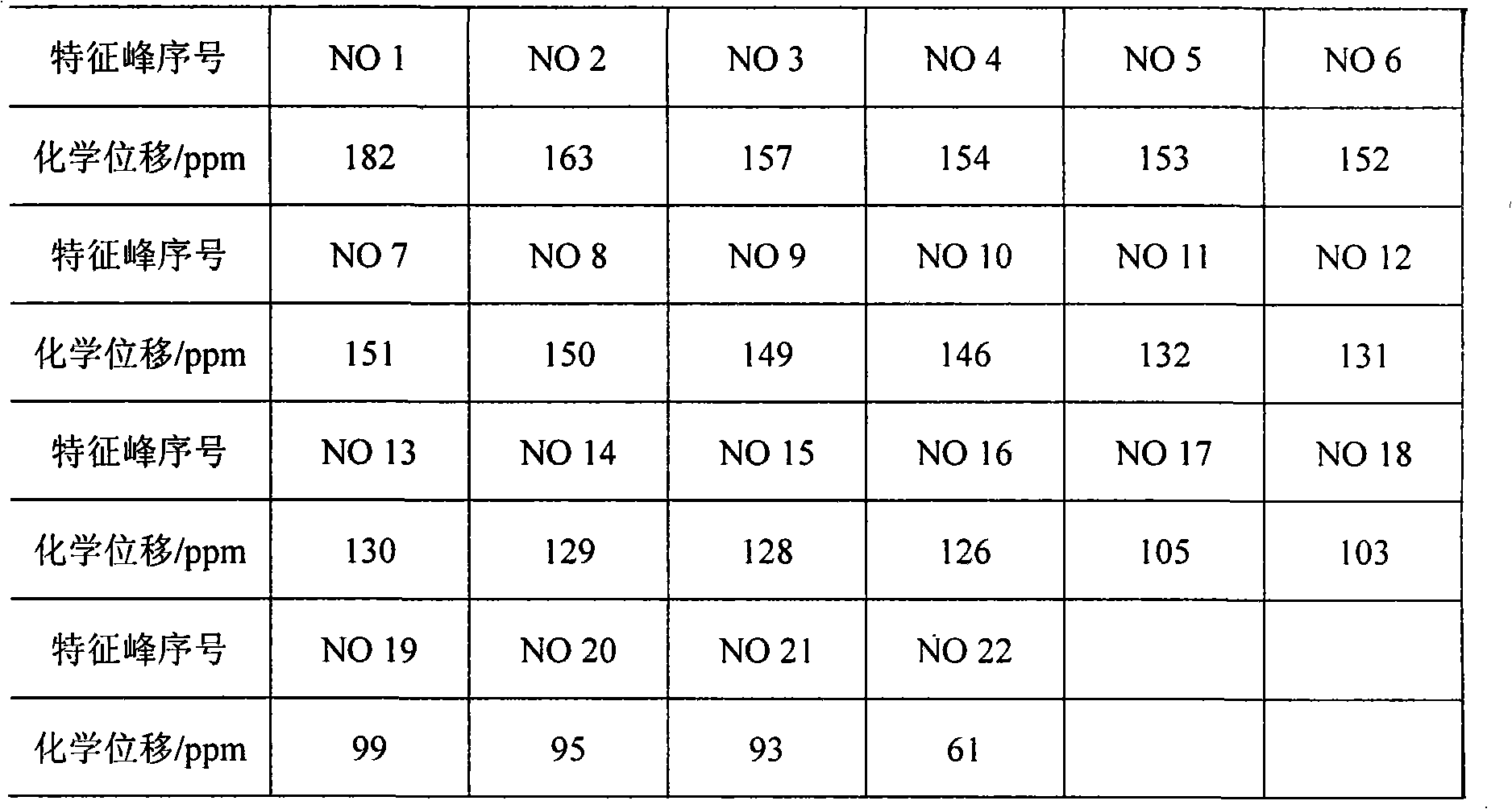
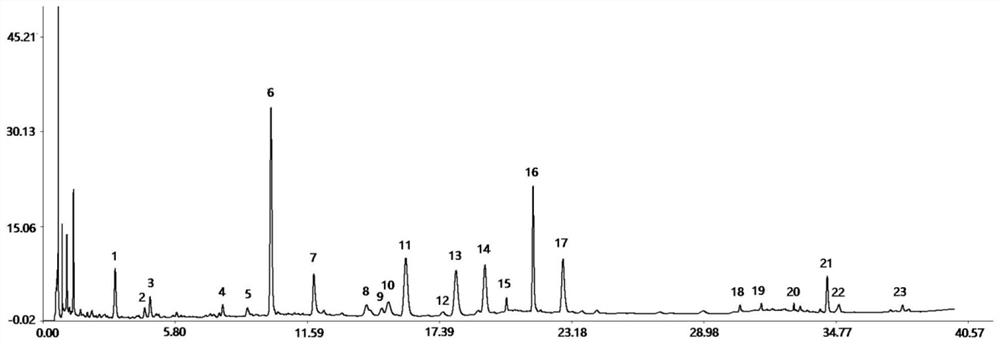
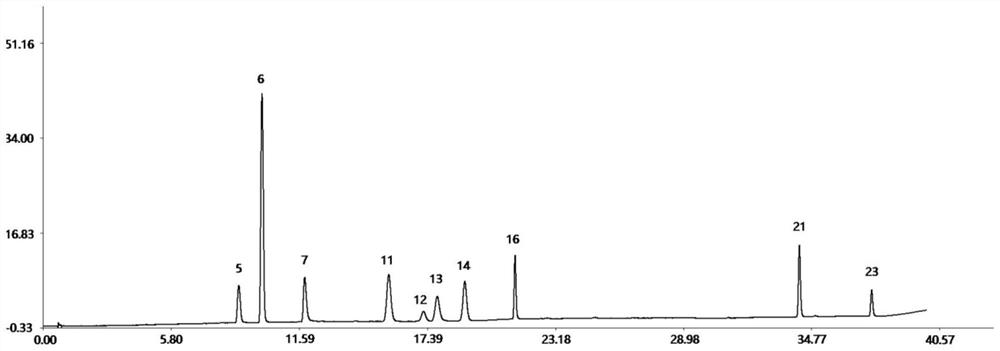
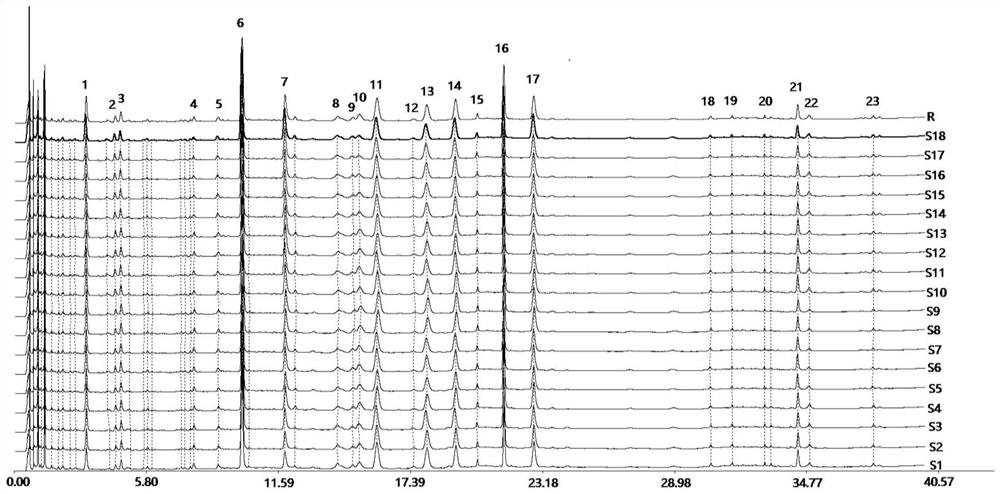
Method for controlling quality of radix scutellariae medicinal materials
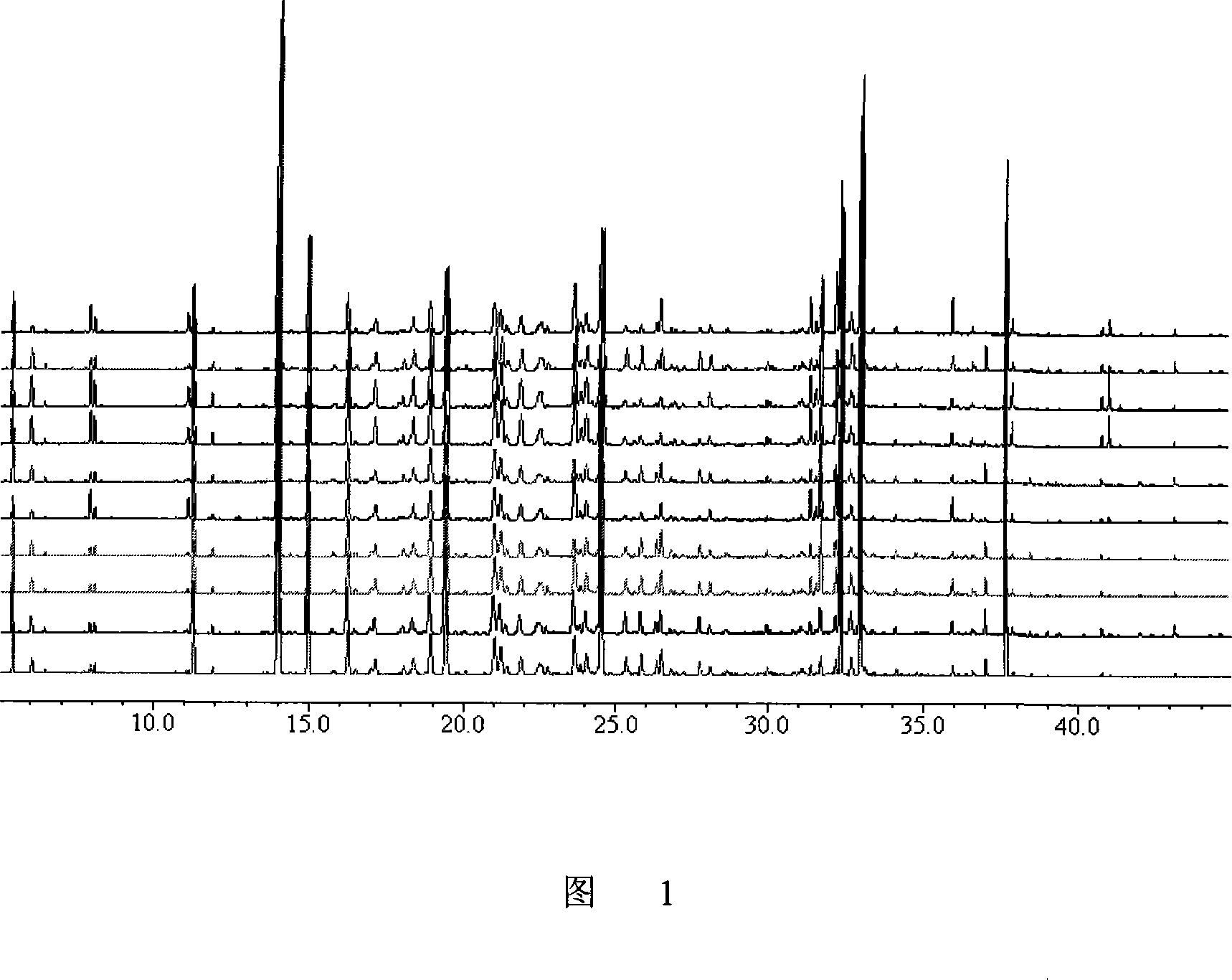
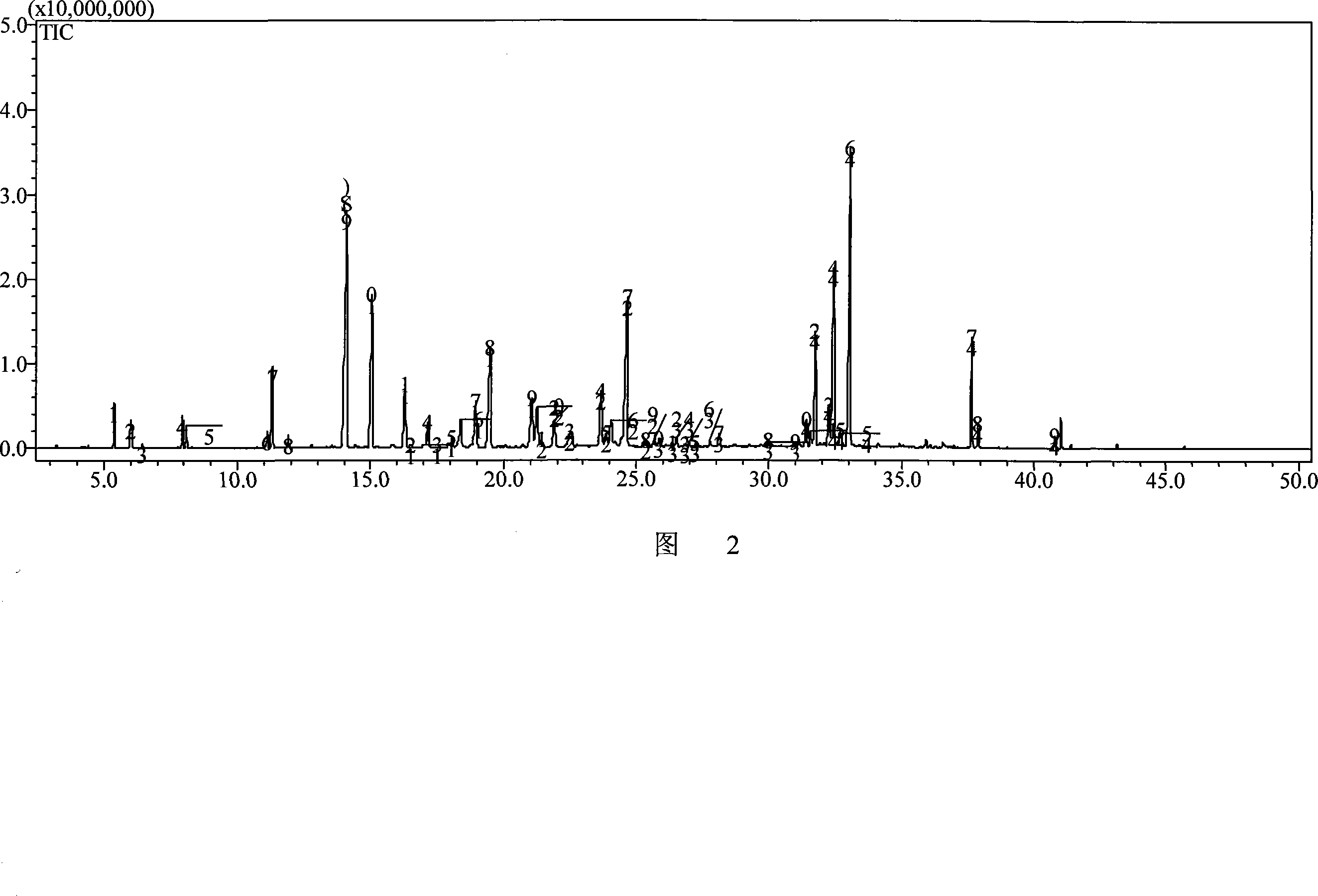
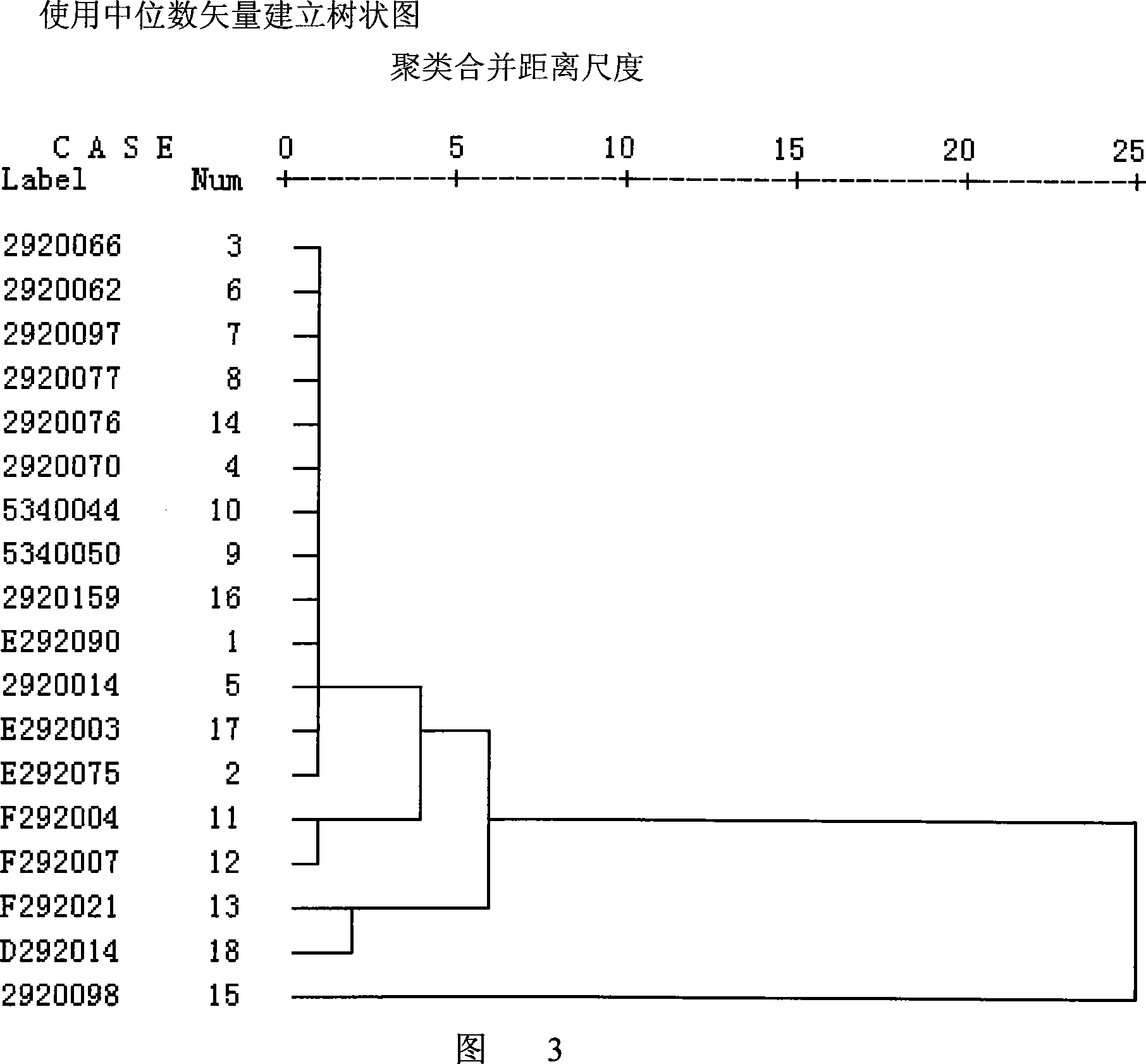
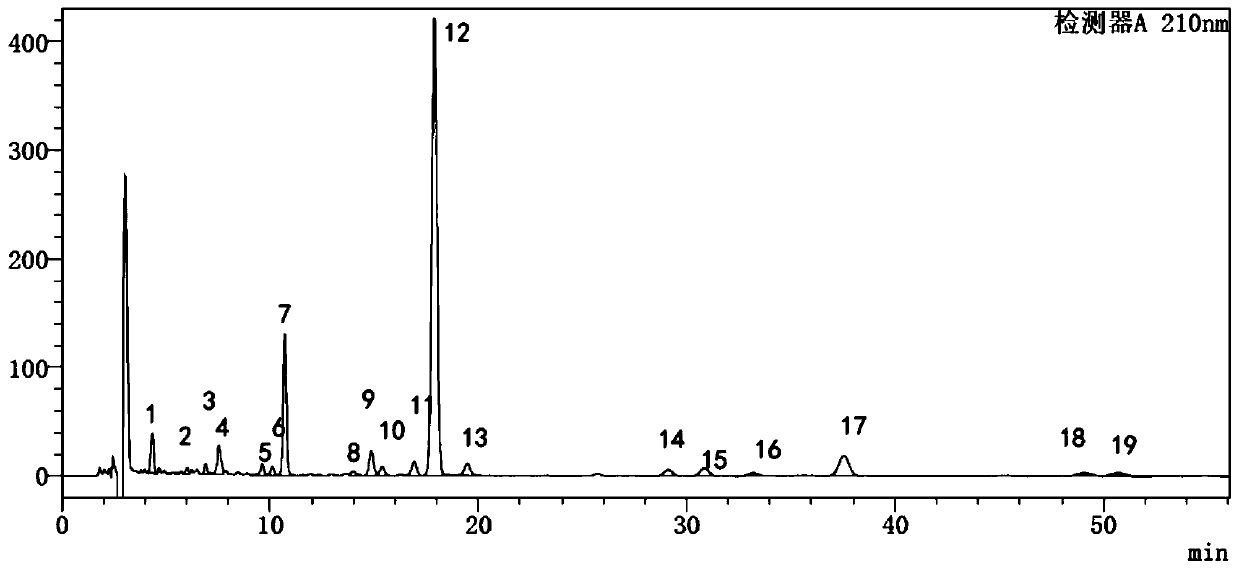
InactiveCN101606970ACharacterizing qualityAvoid one-sidednessAnalysis using nuclear magnetic resonancePlant ingredientsMedicinal herbsFingerprint
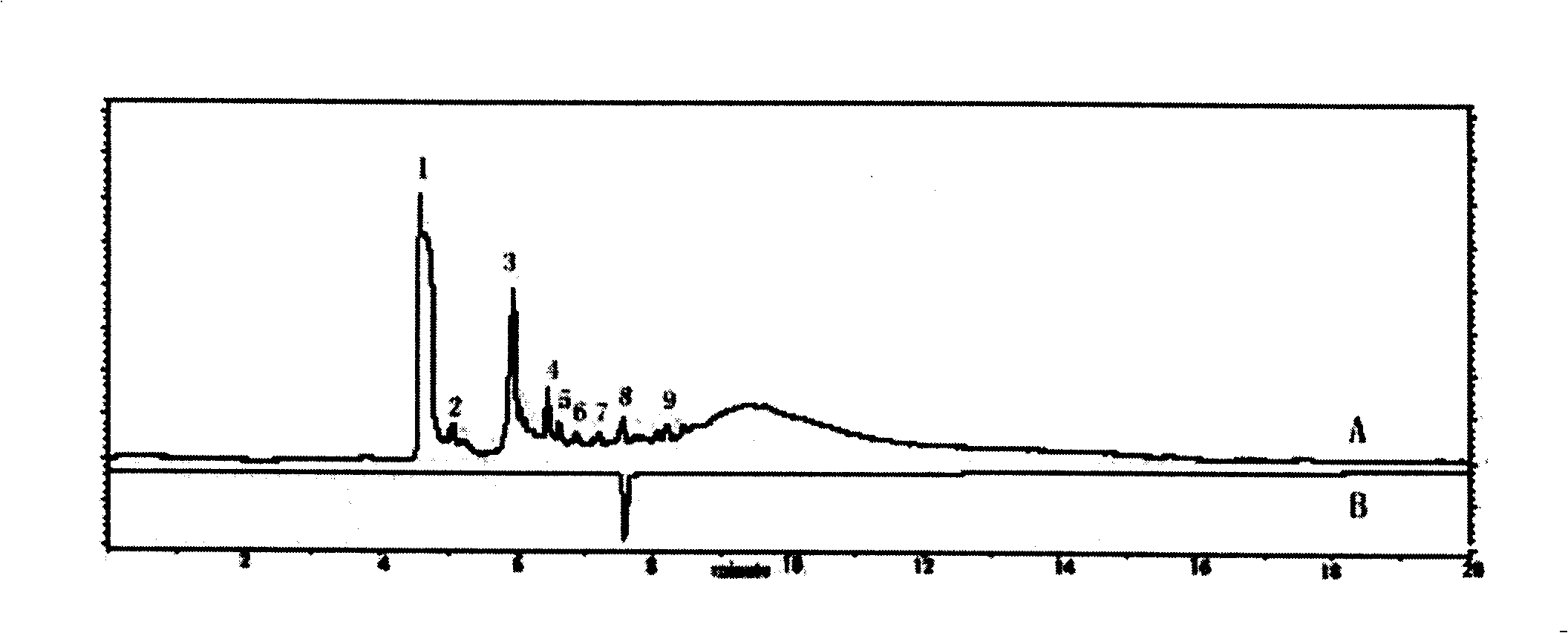
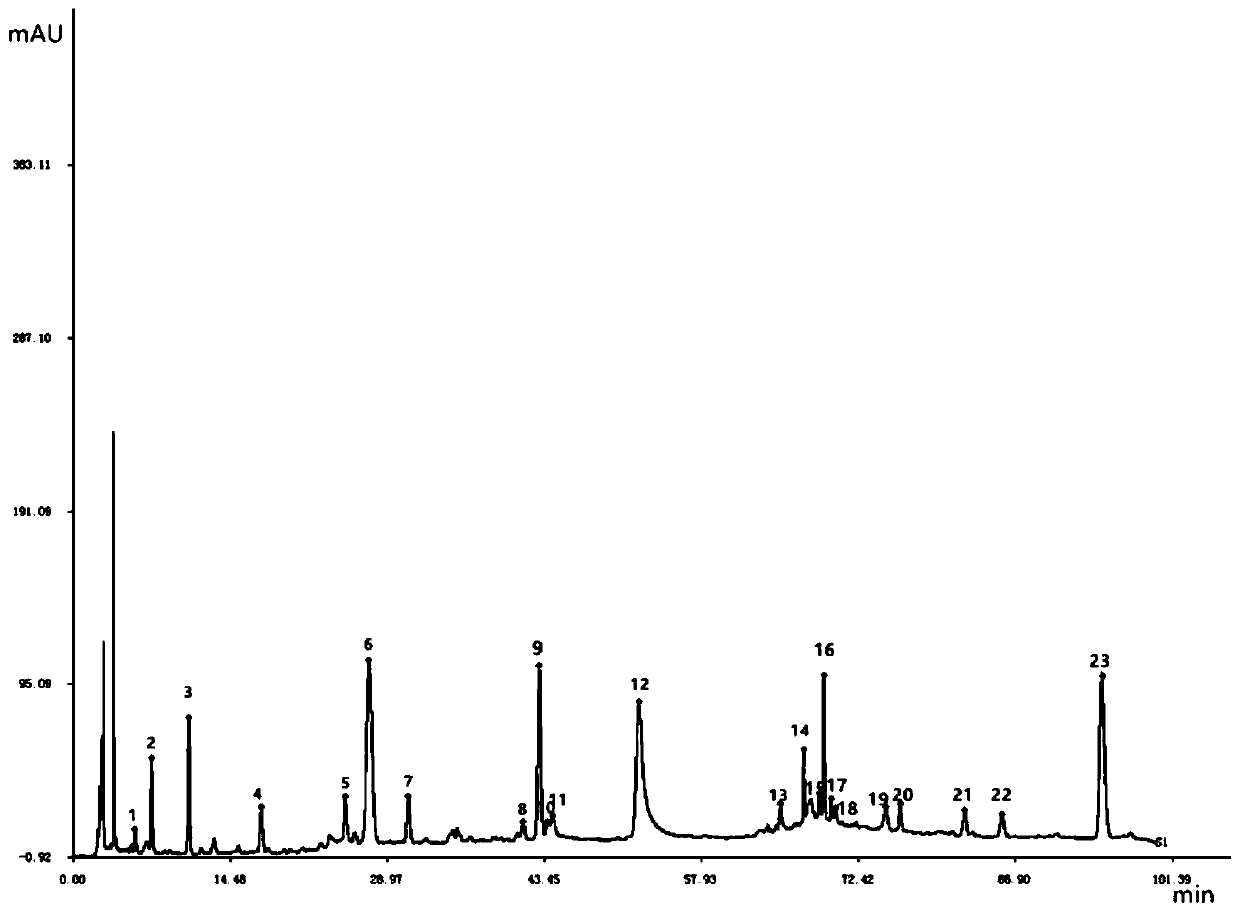
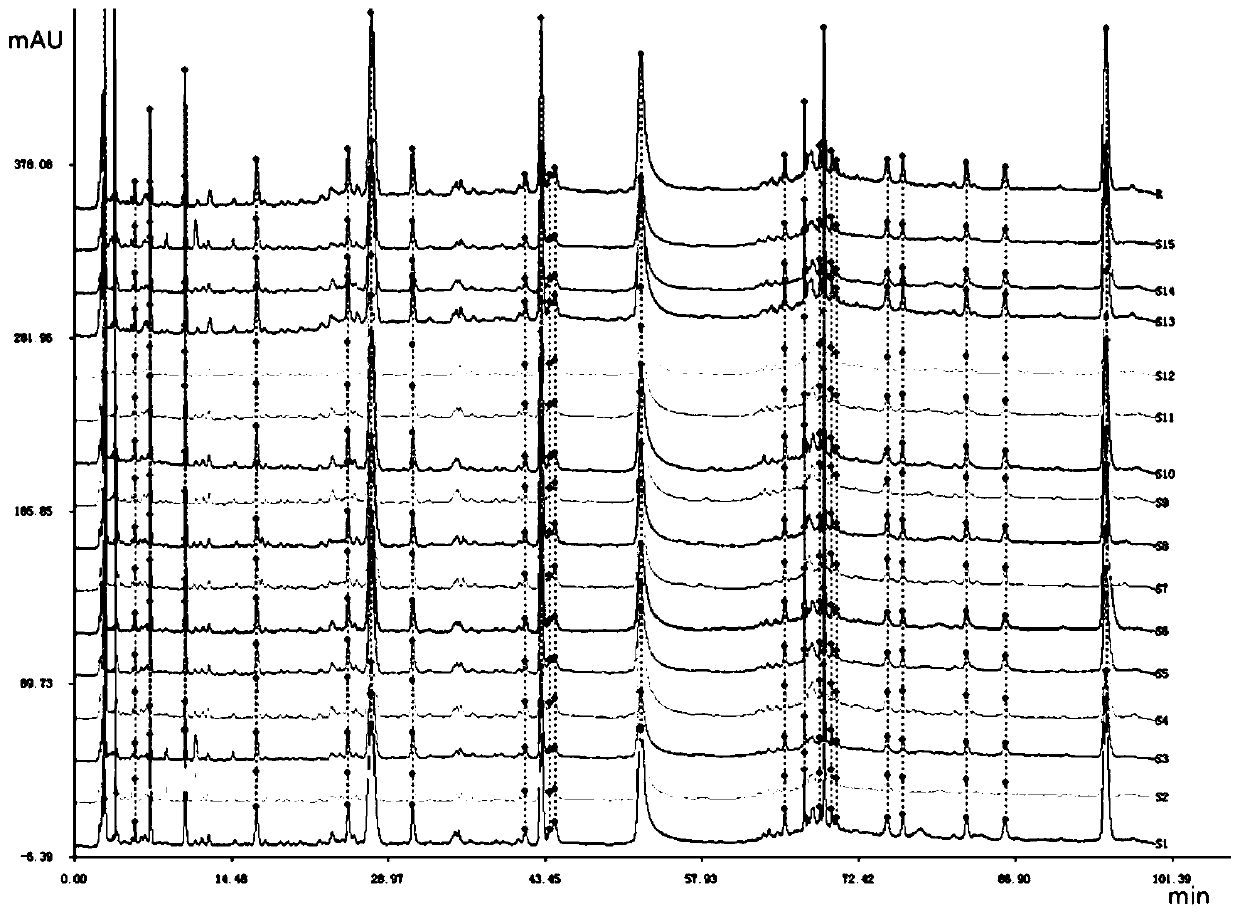
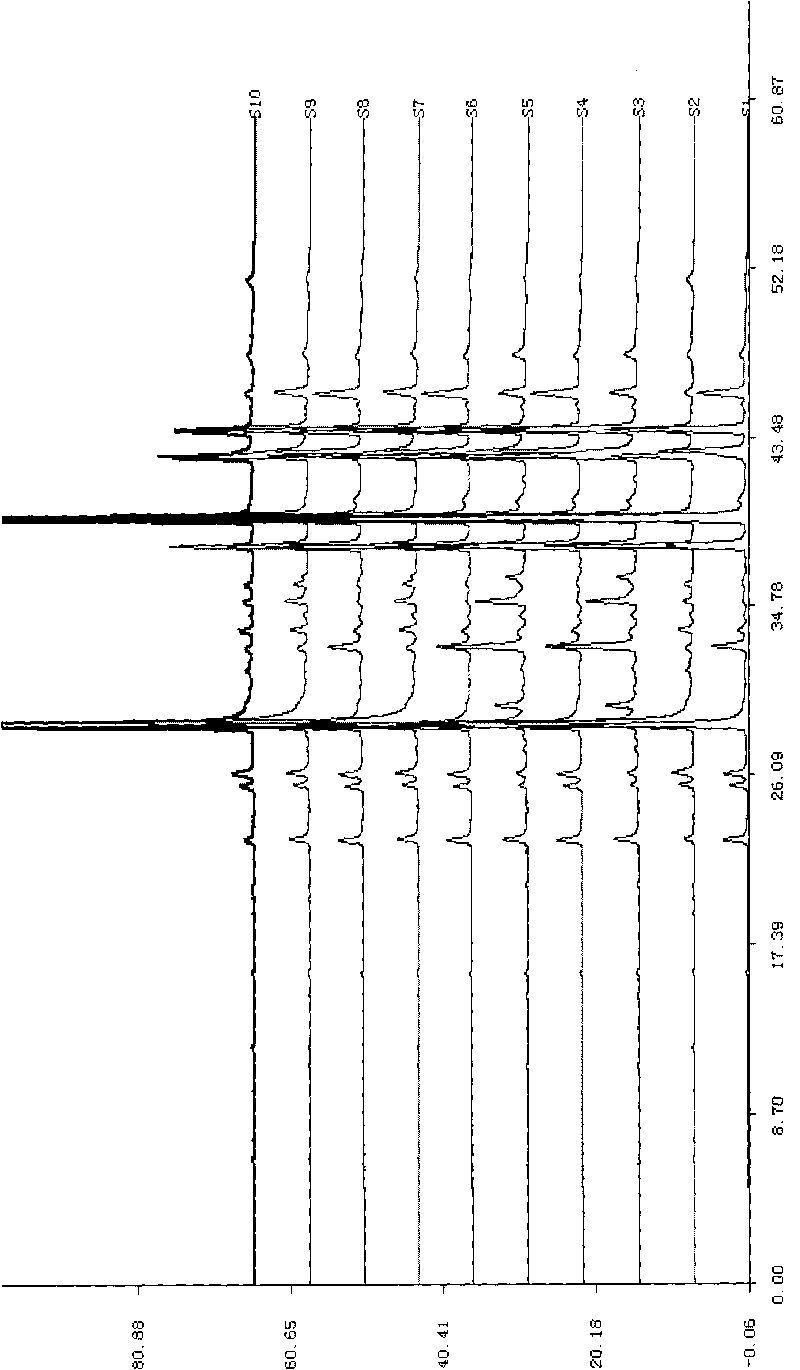
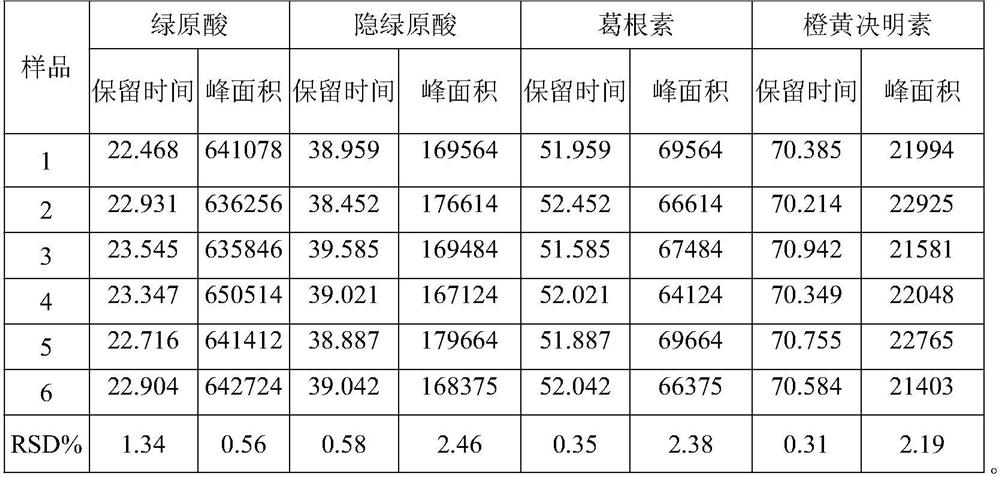
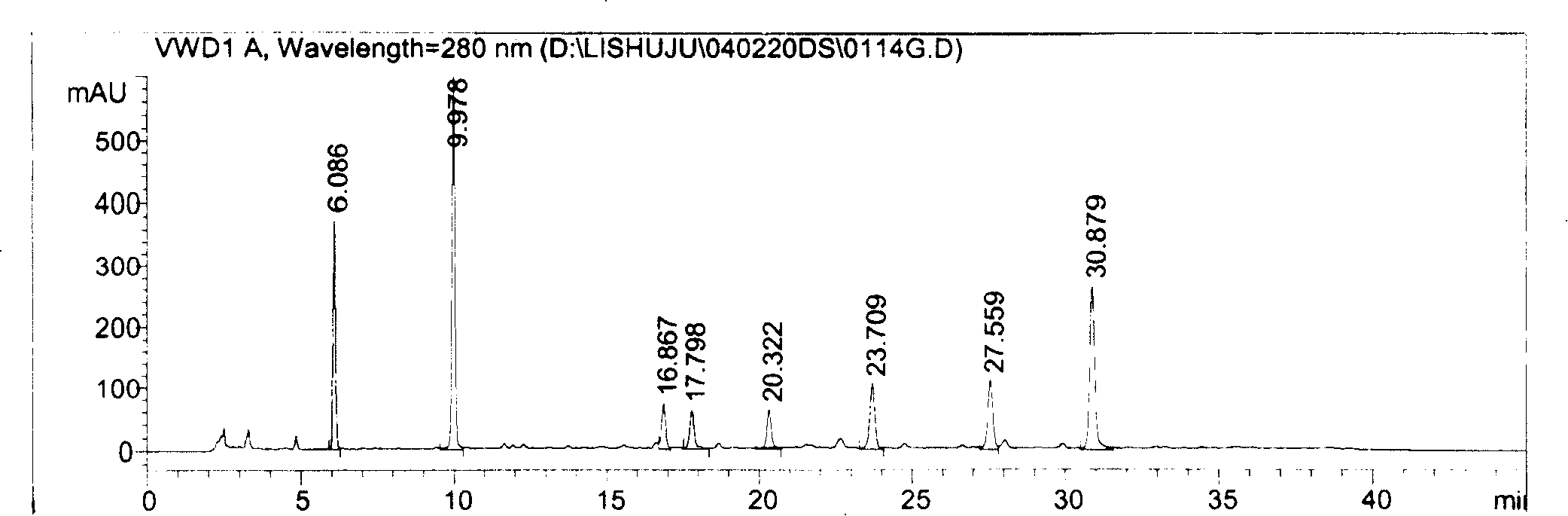
The invention relates to a method for controlling the quality of radix scutellariae medicinal materials, comprising the following steps: taking standard radix scutellariae medical materials, obtaining a nuclear magnetic resonance spectroscopy thereof, and using characteristic ingredients of the radix scutellariae to identify the characteristic peak of medicinal effective ingredients as the characteristic peak of the radix scutellariae; taking a radix scutellariae sample to be tested, adopting the same method as a preparation method, a test condition and a test method of the standard radix scutellariae medical materials to test, obtaining a fingerprint electropherogram, and comparing the nuclear magnetic resonance spectroscopy of the radix scutellariae medicinal materials to be tested with the fingerprint electropherogram of the standard radix scutellariae medical materials. According to the differences of characteristic resonance peaks of the fingerprint electropherogram between the radix scutellariae medicinal materials to be tested and the standard radix scutellariae medical materials and number of common characteristic resonance peaks, the radix scutellariae medicinal materials can be classified as the following grades: high-class products: N is greater than 20, first-class products: N is greater than and equal to 18 and is less than or equal to 20, second-class products: N is greater than or equal to 16 and is less than 18, qualified products: N is greater than or equal to 14 and is less than 16, and unqualified products: N is less than 14.
Owner:SHANDONG ANALYSIS & TEST CENT
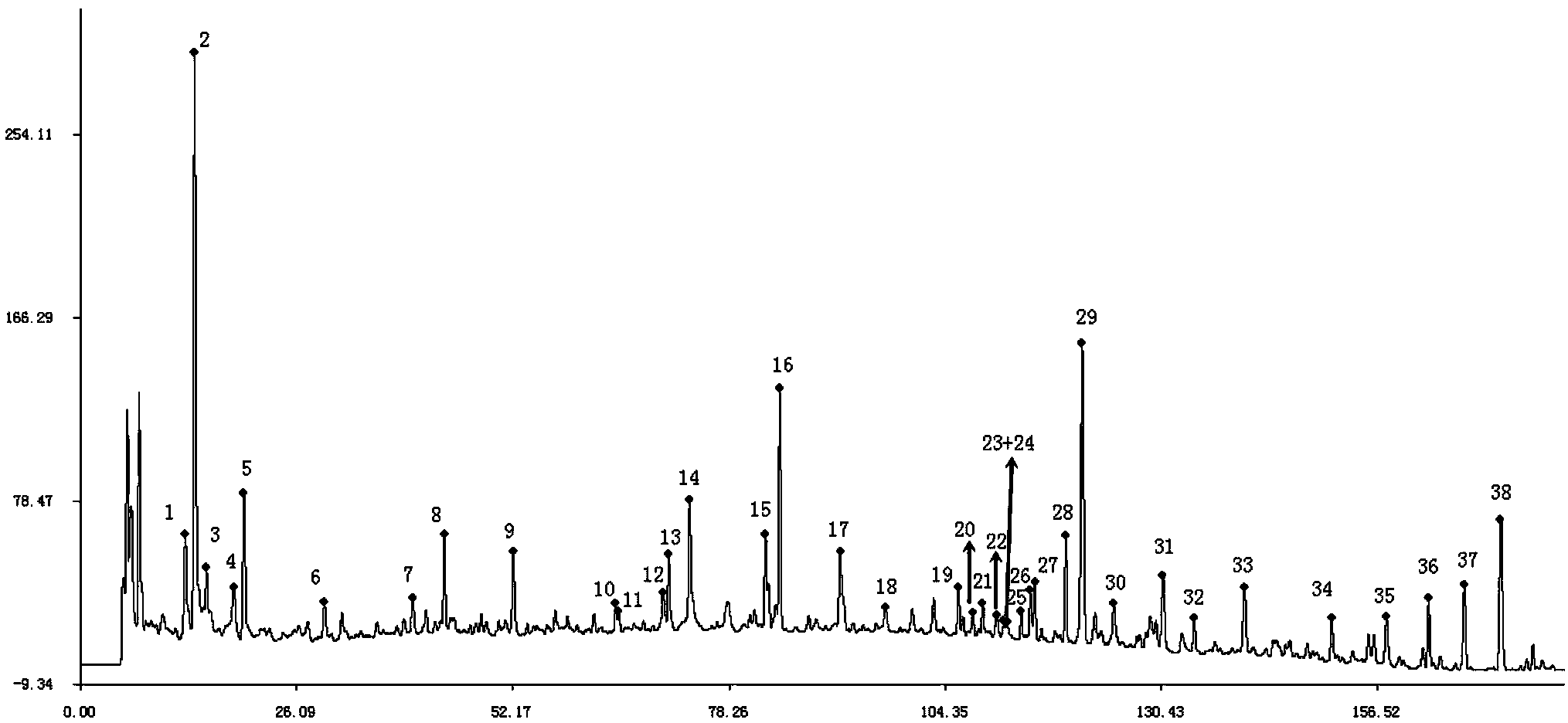
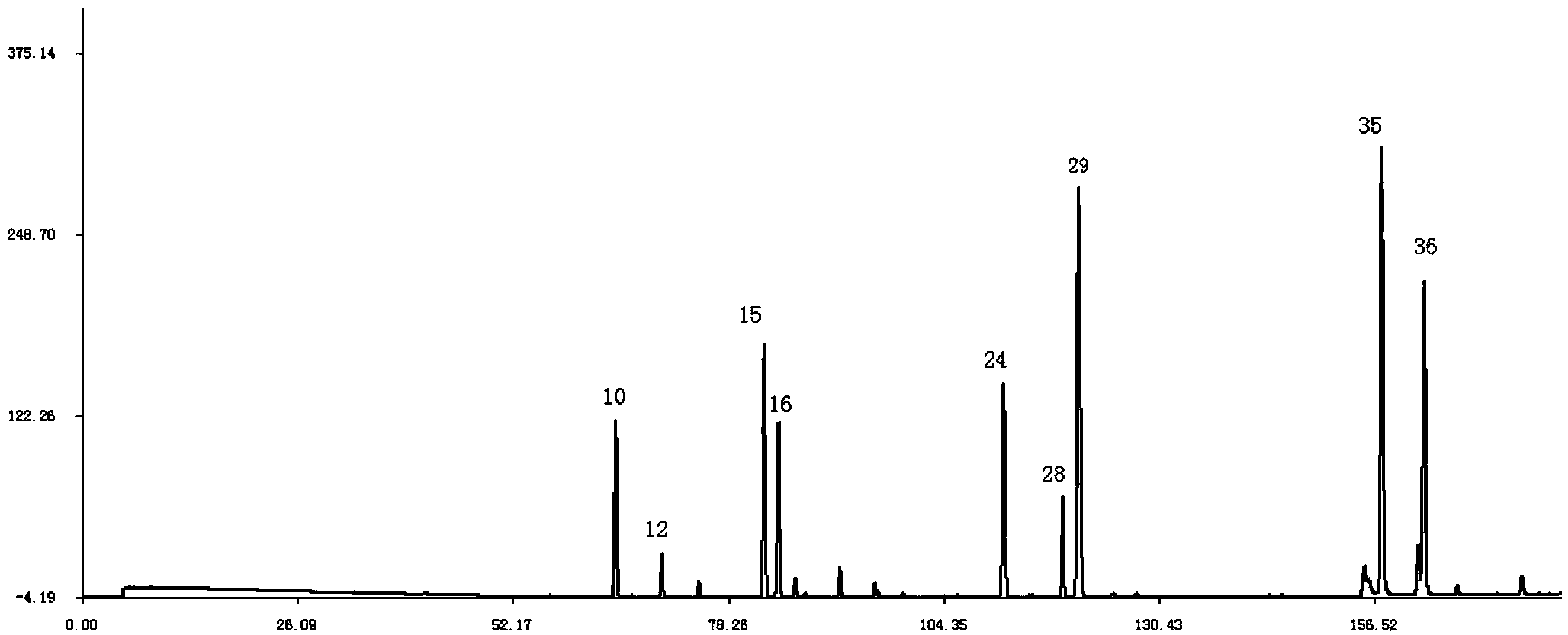
Shenqi hypoglycemic preparation HPLC standard finger print and construction method thereof
The present invention discloses a Shenqi hypoglycemic preparation HPLC standard finger print and a construction method thereof. The construction method comprises the following steps: preparing test sample solutions and a reference solution, measuring the test sample solutions and the reference solution by using the HPLC method and the linear gradient elution process, so as to obtain the Shenqi hypoglycemic preparation finger prints, wherein the chromatographic condition is that a mobile phase A is acetonitrile, a mobile phase B is 0.1% (v / v) phosphoric acid solution, the detection wavelength is 203nm, the column temperature is 25-35 DEG C and the flow rate is 0.8-1.2mL / min. The common characteristic peaks of at least ten finger prints are used as the characteristic peaks of the standard finger print; the standard finger print comprises 38 characteristic peaks, and the numbers of the characteristic peaks of saponin of ginseng stem and leaf, astragalus, Chinese magnoliaving, rehmannia root and raspberry are 13, 4, 7, 3 and 4 respectively. The Shenqi hypoglycemic preparation HPLC standard finger print and the construction method thereof can comprehensively and accurately evaluate the whole quality of the Shenqi hypoglycemic preparation, and facilitate to ensure the quality and the clinical curative effect of the Shenqi hypoglycemic preparation.
Owner:广东万年青制药股份有限公司
Establishment method of curcuma aromatica medicine fingerprint map and the fingerprint map thereof
The invention discloses an establishment method of a curcuma aromatica medicine fingerprint map and the fingerprint map thereof, and particularly, provides establishment of a HPLC fingerprint map for controlling the quality of curcuma aromatica medicine. The method includes the steps of: 1) preparation of a reference substance solution: preparing the reference substance solutions of curdione, curcumadiol, procurcumenol; 2) preparation of a sample solution: extracting the curcuma aromatica medicine and filtering an extract liquid; 3) HPLC detection to obtain the fingerprint map: performing HPLC detection with octadecylsilane chemically bonded silica as a filler and an acetonitrile-tetrahydrofuran-0.1% phosphoric acid water system as a mobile phase in a chromatographic column in a gradient elution manner, wherein column temperature is 30 DEG C, ultraviolet detection wavelength is 230-250 nm and time is 60-65 min; and 4) similarity evaluation: introducing the obtained fingerprint map into "fingerprint similarity evaluation system of traditional Chinese medicine chromatography" (2004A version) similarity software for performing similarity evaluation. The method is simple, quick and accurate, has good repeatability, and provides powerful guarantee for comprehensive and effective control on quality of the curcuma aromatica medicine.
Owner:GUILIN EIGHT PLUS ONE PHARMA CO LTD
Measuring method of agastache for dispelling turbidity soft capsules fingerprint
ActiveCN101183091ACharacterizing qualityAccurate evaluation of qualityComponent separationTesting medicinal preparationsTurbidityPeak area
The present invention relates to a determining method for the fingerprint map of a pogostemi herba healthy energy soft capsule, which belongs to the field of Chinese medicine modernization. The determining method for the fingerprint map of the pogostemi herba healthy energy soft capsule comprises: the preparation of a sample; the preparation of reference object solution; chromatostrip condition: the chromatographic column is a plastic quartz capillary column; polysiloxane is stationary phase; procedure temperature: initial temperature is between 40 DEG C to 100 DEG C and raised to 230 DEG C to 280 DEG C at the speed of 2 DEG C to 6 DEG C per minute; the temperature in a vaporizer is between 230 DEG C to 280 DEG C; an interface temperature is between 230 DEG C to 280 DEG C; the range of mass charge ratio is 10 to 1000. The determining method: chromatogram maps in 1 hour are recorded by using GC-MS; taking the acreage of the patchouli alcohol as 1 to calculate the relative ratio of the peak area. The advantages of the present invention are that the fingerprint technique and the GC-MS equipment are combined to carry out nature and quantified determinations; the present invention can be taken as the standard fingerprint of the pogostemi herba healthy energy soft capsule content determination to discriminate active principles; the method is of stable, high accuracy and good reproducibility.
Owner:津药达仁堂集团股份有限公司达仁堂制药厂
Method for detecting quality of medicinal water extracts of Qipi oral liquid based on HPLC (High Performance Liquid Chromatography) fingerprint spectrum
InactiveCN102435692AStable qualityThe method is simpleComponent separationHplc fingerprintFingerprint
The invention provides a method for detecting quality of medicinal water extracts of Qipi oral liquid based on an HPLC (High Performance Liquid Chromatography) fingerprint spectrum. The method comprises the following steps of: preparing a comparison product solution and a tested sample solution; performing HPLC measurement; establishing a standard fingerprint spectrum; and detecting the sample. According to the method, a freezing technology and quality of the medicinal water extracts of the Qipi oral liquid can be effectively represented, energy source is saved and labor hour is reduced; the method is beneficial to monitoring the quality of products; and the method has the advantages of simple method, excellent stability, high precision, excellent repeatability, and the like.
Owner:长春澜江医药科技有限公司
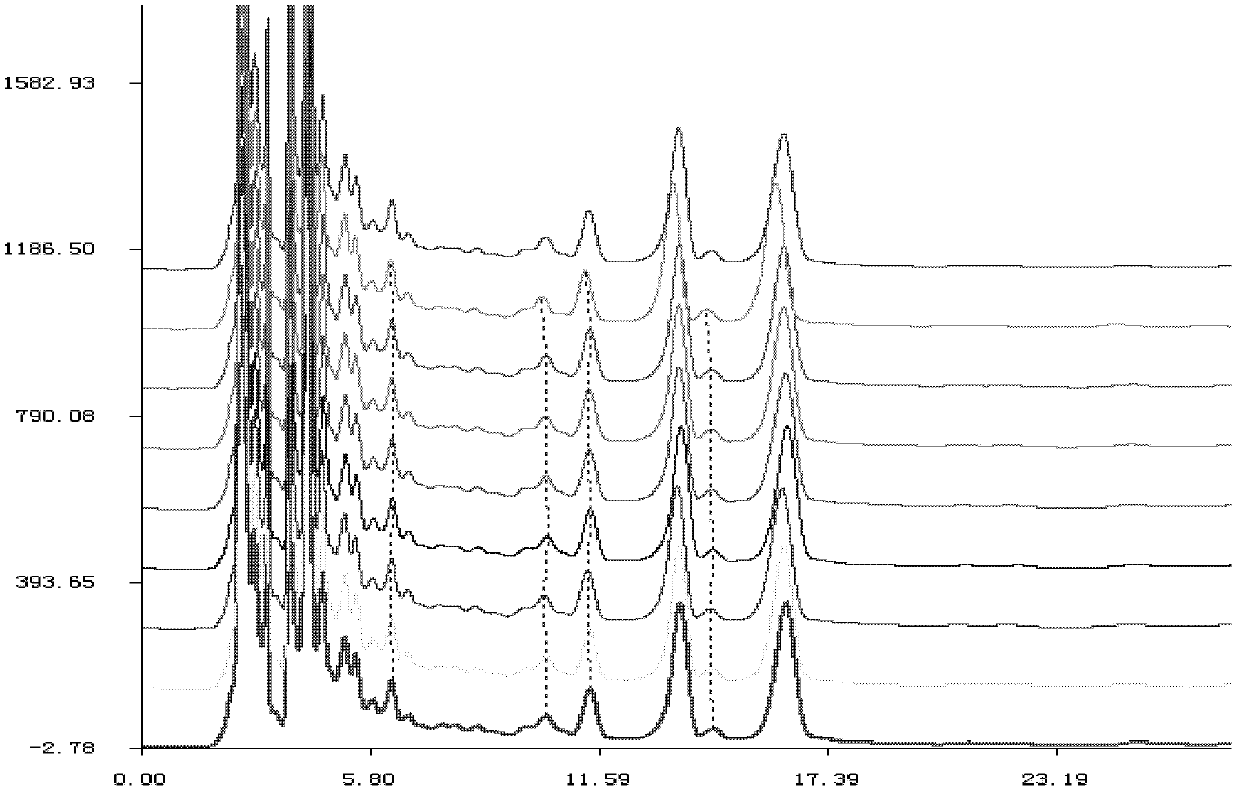
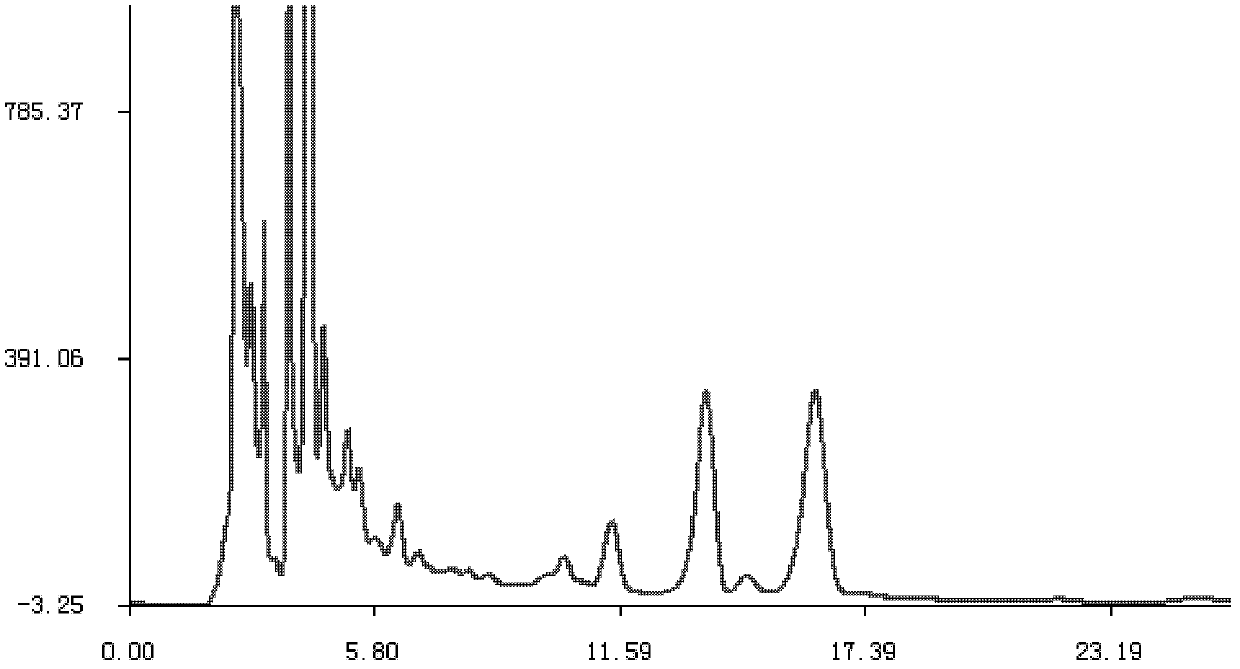
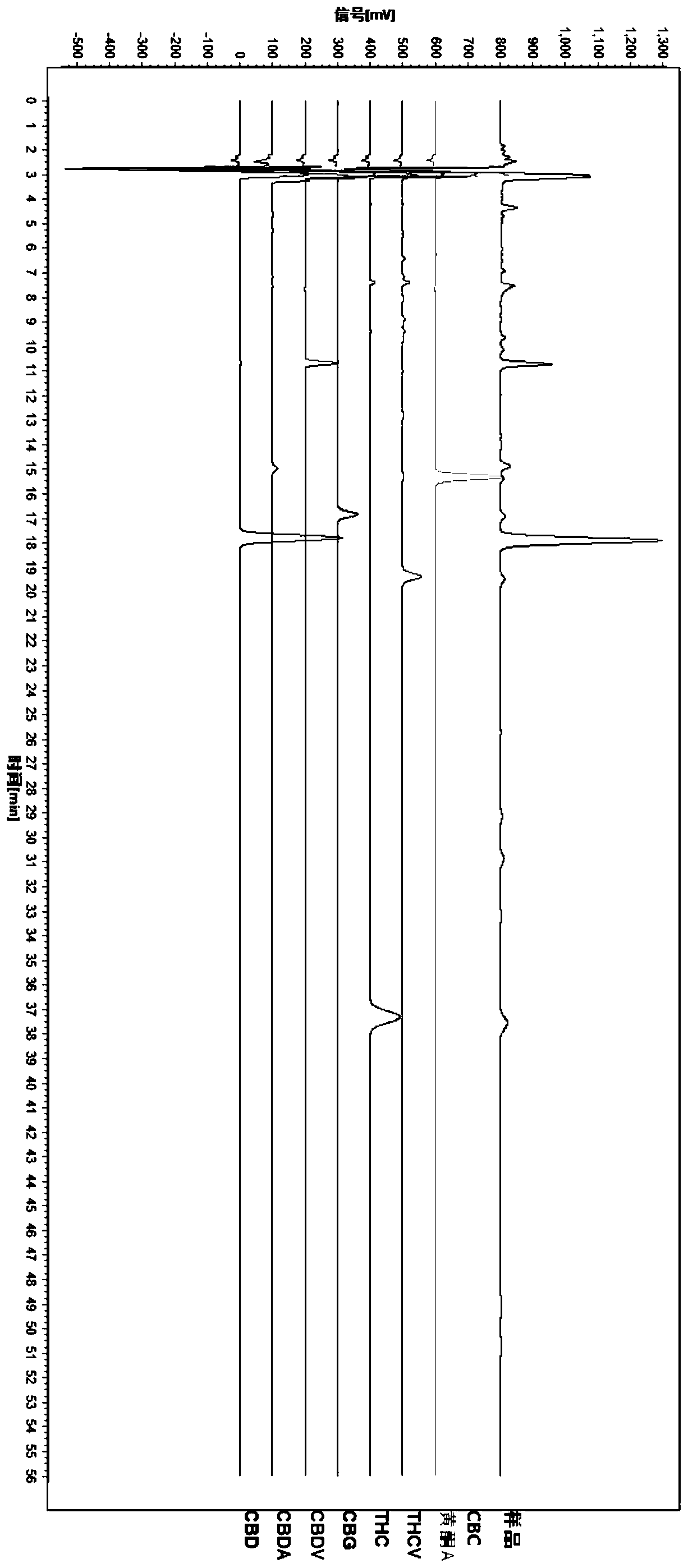
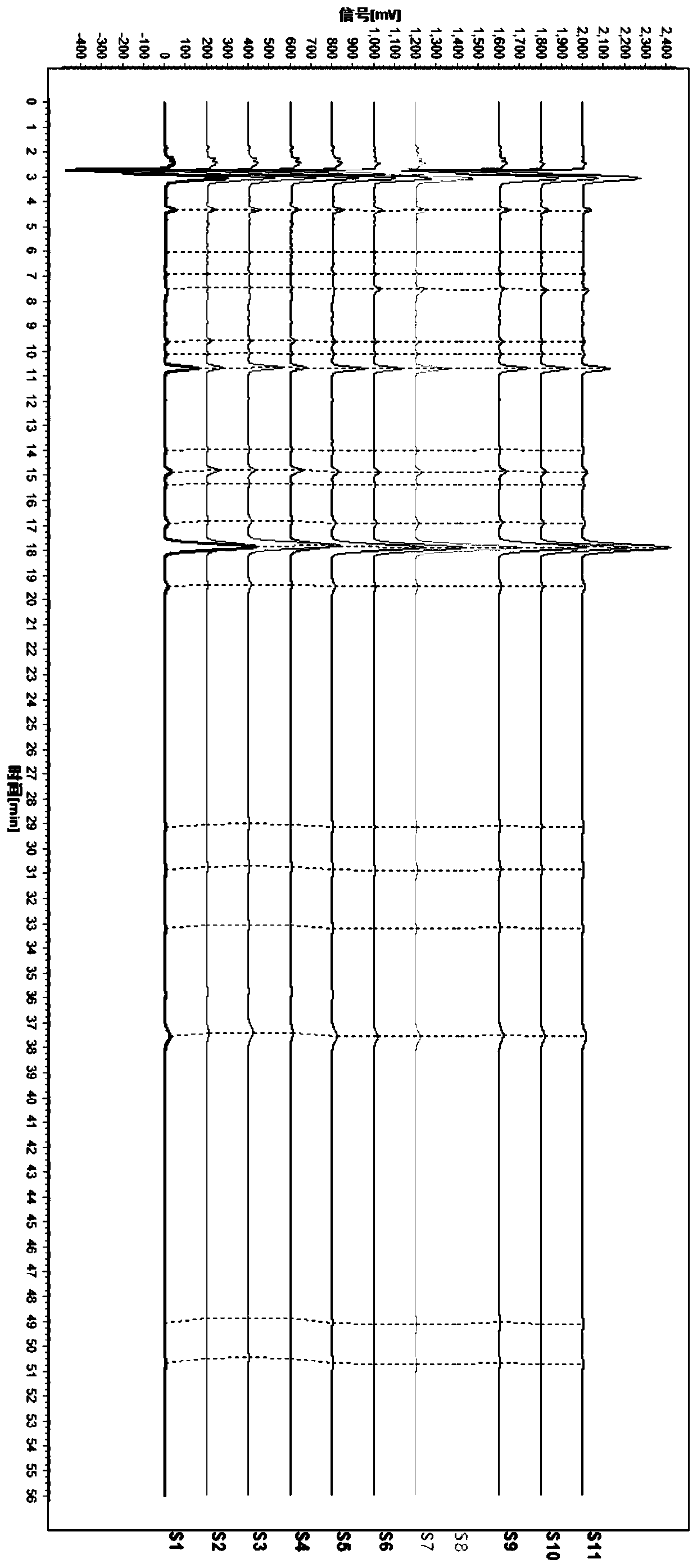
HPLC fingerprint spectrum determination method for cannabis sativa
InactiveCN110133148AAvoid one-sidednessReduce the possibilityComponent separationCannabidiolic acidFingerprint
The invention provides an HPLC fingerprint spectrum determination method for cannabis sativa. The method is characterized by comprising the steps of (1) preparing a test solution: taking the cannabissativa medicinal material, crushing, sieving and baking the cannabis sativa medicinal material, precisely weighing baked cannabis sativa medicinal material powder, adding an extracting agent, carryingout ultrasonic extraction, carrying out standing until the room temperature is reached, passing through a microporous filter membrane, and taking subsequent filtrate; (2) preparing a reference solution: taking cannabidiolic acid, cannabidivarin, cannabigerol, cannabidiol, delta 9-tetrahydrocannabinol, cannabichromene, tetrahydrocannabinol and geranylflavone A reference substances, and adding methanol to dissolve the reference substances so as to prepare the reference substance solution; and (3) performing determination: precisely sucking the reference solution and the test solution into an HPLC, performing determination, and recording a chromatogram.
Owner:HANYI BIO TECH CO LTD
Lanqin oral solution fingerprint spectrum establishment method, fingerprint spectrum of Lanqin oral solution and application of fingerprint spectrum
ActiveCN110108825ACharacterizing qualityAvoid one-sidednessComponent separationBenzoic acidChlorogenic acid
The invention discloses a Lanqin oral solution fingerprint spectrum establishment method, a fingerprint spectrum of a Lanqin oral solution and application of the fingerprint spectrum. A test solutionis prepared from the Lanqin oral solution, a reference substance solution is a solution which dissolves adenosine, epigoitrin, phellodendrine chloride, chlorogenic acid, magnoflorine, geniposide, benzoic acid, berberine hydrochloride, baicalin, wogonoside, baicalein and wogonin, and the liquid chromatography is measured by using a high-performance liquid chromatograph; the obtained liquid chromatography is introduced into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system for analysis, and the Lanqin oral solution fingerprint spectrum is obtained after multi-point correction, data matching and analysis. According to the Lanqin oral solution fingerprint spectrum establishment method and the fingerprint spectrum of the Lanqin oral solution, the quality information of the Lanqin oral solution can be comprehensively reflected, and uniform and stable product quality is ensured.
Owner:YANGTZE RIVER PHARMA GRP JIA NGSU LONGFENGTANG TRADITIONAL CHINESE MEDICINE CO LTD +2
Propolis supercritical CO2 extract GC-MS fingerprint and construction method thereof
InactiveCN102043031AReduce lossesThe amount of sample analyzed is smallComponent separationBiotechnologyPropolis
The invention relates to the field of detection methods of active ingredients of functional foods, and discloses a propolis supercritical CO2 extract gas chromatography-mass spectrometer (GC-MS) fingerprint and a construction method thereof. The propolis supercritical CO2 extract GC-MS fingerprint has 8 common peaks, wherein the total length of the fingerprint is 60 minutes. The construction method comprises the following steps of: placing a propolis sample in a supercritical CO2 extraction kettle to extract so as to obtain a supercritical CO2 extract of the propolis; weighing the propolis sample and placing in a sample bottle to perform solid-phase micro-extraction; performing GC-MS analysis; and finally comparing the GC-MS fingerprints of propolis supercritical CO2 extracts in different regions, and extracting the common peaks to form the GC-MS fingerprint of the propolis supercritical CO2 extract. The invention has the advantages that: the fingerprint is convenient and stable, has high precision and repeatability, is easy to master and the like. The time for detecting the sample is short; and the obtained propolis supercritical CO2 extract contains more than 120 volatile components.
Owner:JIANGSU UNIV
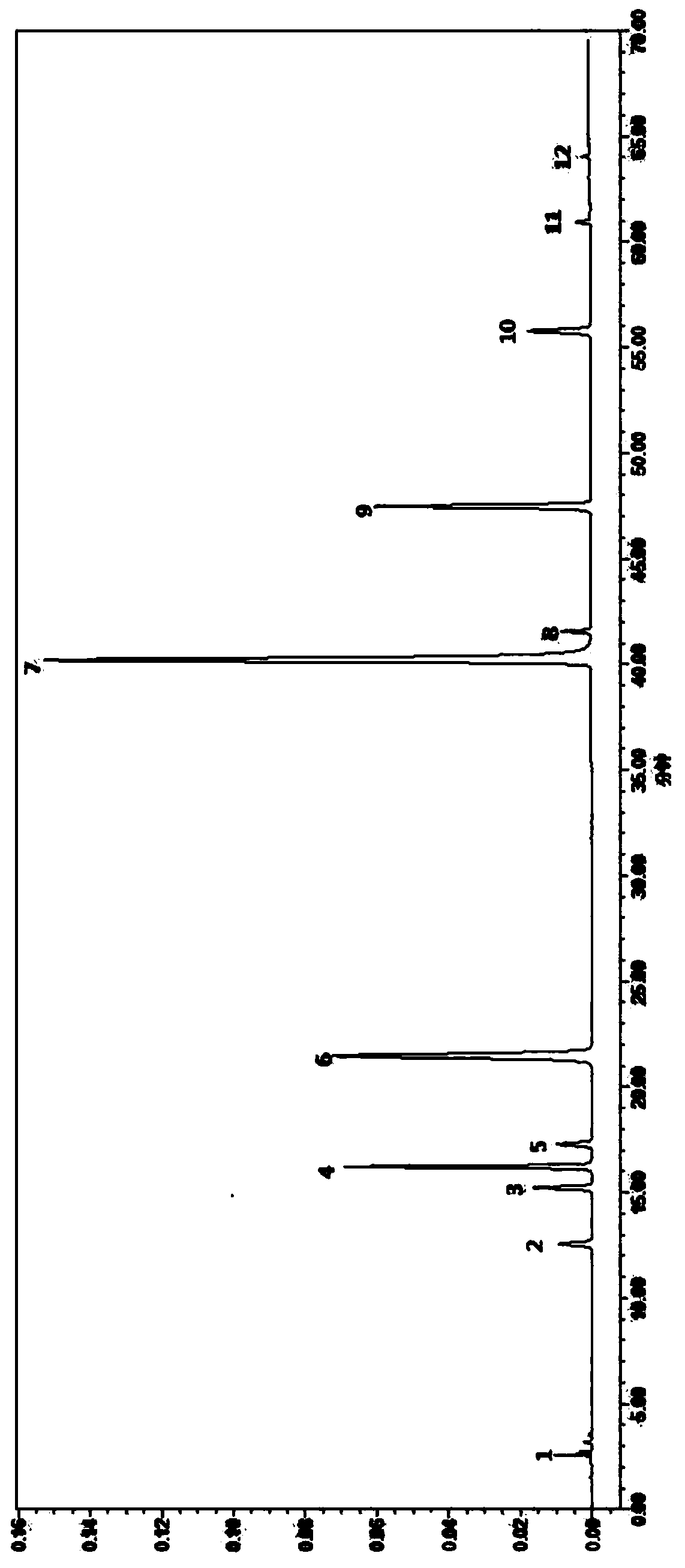
High performance liquid chromatography (HPLC) standard fingerprint spectrum of lucid ganoderma capsule preparation and construction method and application of standard fingerprint spectrum
ActiveCN104007198AEffectively Characterize QualityCharacterizing qualityComponent separationPhosphoric acidColumn temperature
The invention discloses a high performance liquid chromatography (HPLC) standard fingerprint spectrum of a lucid ganoderma capsule preparation and a construction method and application of the standard fingerprint spectrum. The construction method comprises the following steps: preparing a test solution and a reference substance solution, and carrying out linear gradient elution, wherein a mobile phase A is acetonitrile, a mobile phase B is 0.05-0.15 percent (v / v) phosphoric acid aqueous solution, the detection wavelength is 252nm, the column temperature is 25-35 DEG C, the flow velocity is 1.0mL / min; measuring through HPLC, obtaining a lucid ganoderma capsule fingerprint spectrum, and taking a common characteristic peak of at least 14 batches of fingerprint spectrums as the characteristic peak of the standard fingerprint spectrum, wherein the standard fingerprint spectrum totally has 40 characteristic peaks, and the numbers of characteristic peaks belonging to lucid ganoderma, bighead atractylodes rhizome, rhizoma polygonati, the seed of Chinese dodder, schisandra chinensis, liquorice and the fruit of Chinese wolfberry are respectively 20, 3, 3, 5, 1, 5 and 2. According to the method and the standard fingerprint spectrum, the overall quality of the lucid ganoderma preparation can be comprehensively and accurately evaluated, and the quality and drug effect of the lucid ganoderma preparation are guaranteed.
Owner:INFINITUS (CHINA) CO LTD
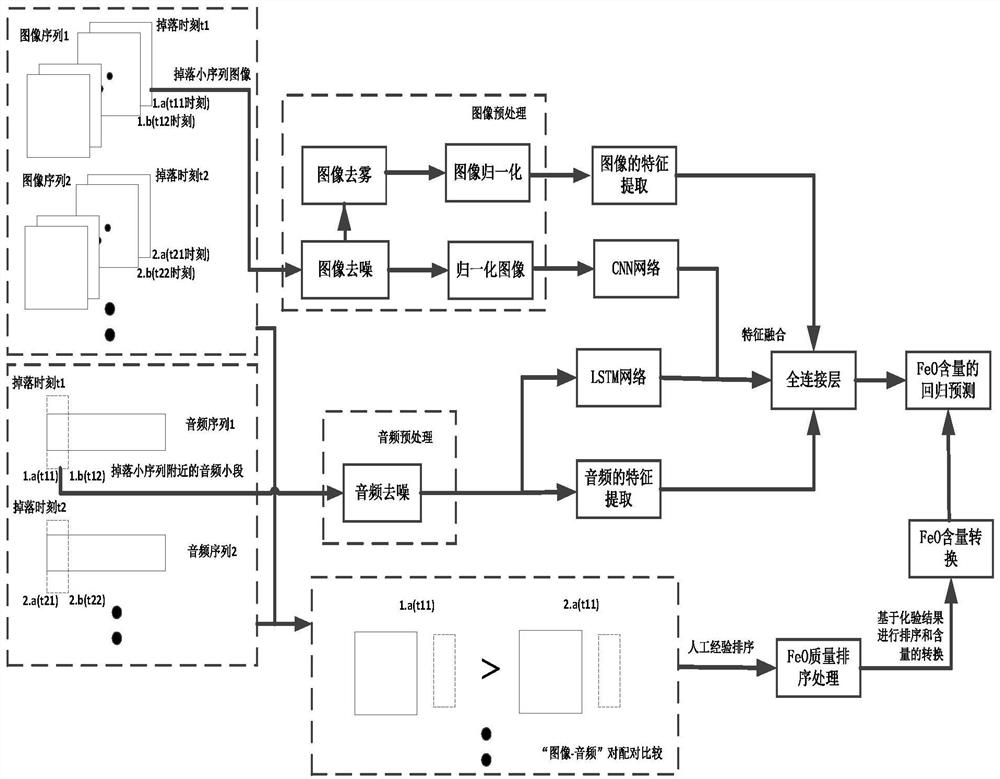
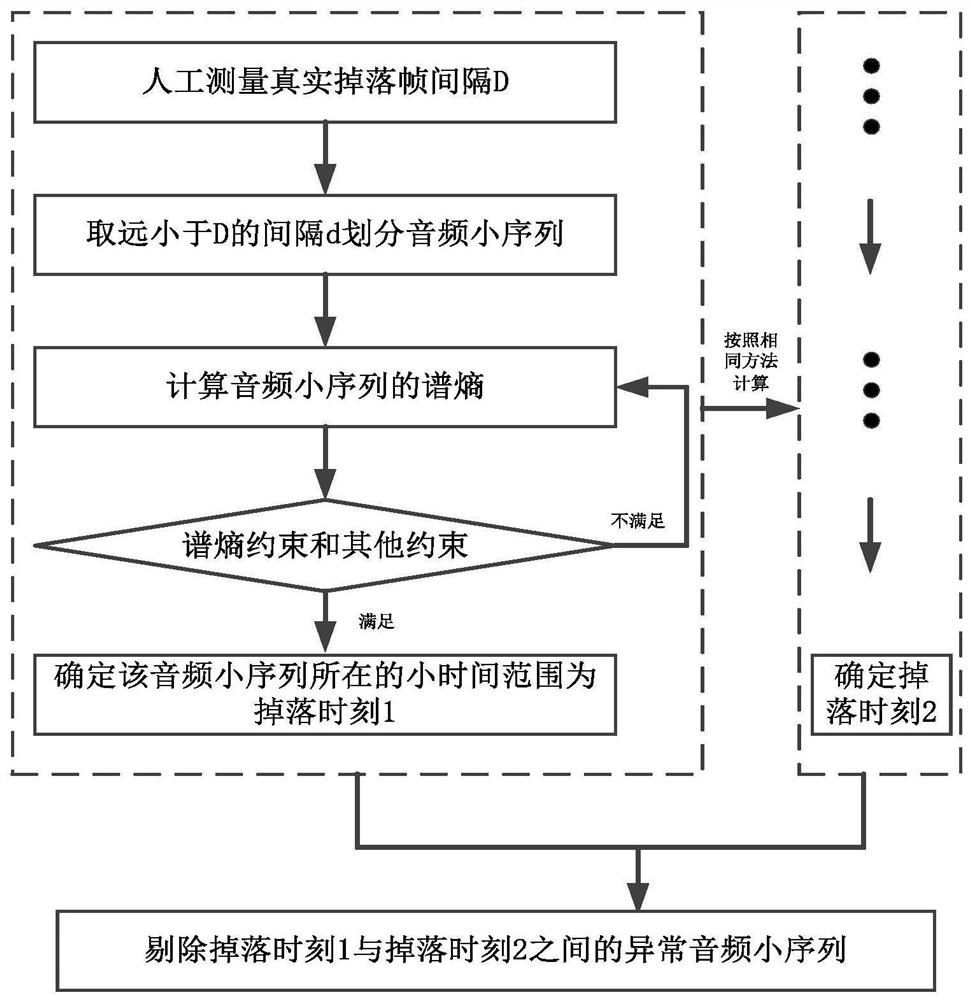
FeO content prediction method based on sintering machine tail section video
ActiveCN112329558AReduce the impact of quality judgmentsAccurate extractionImage enhancementImage analysisMachine learningImaging Feature
The invention discloses a FeO content prediction method based on a sintering machine tail section video, and relates to the field of steel production. The method mainly comprises the following steps:determining a falling moment, removing an abnormal audio sequence, dividing an image sequence and preprocessing, carrying out audio preprocessing, extracting image features, extracting audio features,constructing a video quality database, and constructing, training and testing a quality prediction model. Compared with the prior art, the method has the advantages that the severe conditions of thesintering machine tail are considered, the characteristics of the video information are represented more comprehensively from the aspects of images and audios, and quantitative prediction of the FeO content can be achieved.
Owner:SHANGHAI JIAO TONG UNIV
Method for evaluating quality of loquat leaf medicinal materials
InactiveCN101354381AEffectively Characterize QualityCharacterizing qualityComponent separationPreparing sample for investigationCapillary electrophoresisTest sample
The invention relates to a method for building a fingerprint of loquat leaf medicinal materials and a fingerprint thereof. The method comprises the following steps of: firstly collecting the loquat leaf medicinal materials for over 10 batches, preparing test sample solution and a reference product solution, implementing measurement by referring to and applying a high-efficiency liquid chromatography method and a high-efficiency capillary electrophoresis method, and finally implementing analysis by applying a computation software of the similarity between the fingerprint of traditional Chinese medicines (which is recommended by the State Pharmacopoeia Committee). The method of the invention has the advantages of simplicity, stability, high precision, good reproducibility and easy grasp, can grasp the variety and the quality condition of the loquat leaves from the integral characteristic face and the high profile of chromatogram, and can be used as one of the methods for evaluating the quality of the loquat leaves and distinguishing the loquat leaves between true and false.
Owner:PUTIAN INST OF TRADITIONAL CHINESE MEDICINE
Method for establishing fingerprint spectrum of rhizoma alismatis decoction and fingerprint spectrum
InactiveCN111398437ACharacterizing qualityFocus on integrityComponent separationAgainst vector-borne diseasesMedicinal herbsLactone II
The invention discloses a method for establishing a fingerprint spectrum of rhizoma alismatis decoction and the fingerprint spectrum. The method comprises the following steps: (1) preparing rhizoma alismatis decoction by adopting rhizoma alismatis and bighead atractylodes rhizomes with different producing areas and different batch numbers, and extracting rhizoma alismatis water decoction by utilizing a methanol solution to obtain a test solution; dissolving atractylodes macrocephala lactone I, atractylodes macrocephala lactone II, atractylodes macrocephala lactone III, alisol A, alisol B and 23-acetyl alisol B by using a methanol solution to prepare a reference substance solution; and (2), carrying out ultra-high liquid chromatography analysis on the test solution and the reference solution, and recording the chromatogram. The rhizoma alismatis decoction fingerprint spectrum established by the method provided by the invention can effectively characterize the quality of the rhizoma alismatis decoction, and provides a basis for monitoring the quality of medicinal materials. Meanwhile, the method has the advantages of being simple, rapid, stable, good in reproducibility, high in precision and the like.
Owner:SHAANXI INST OF INT TRADE & COMMERCE
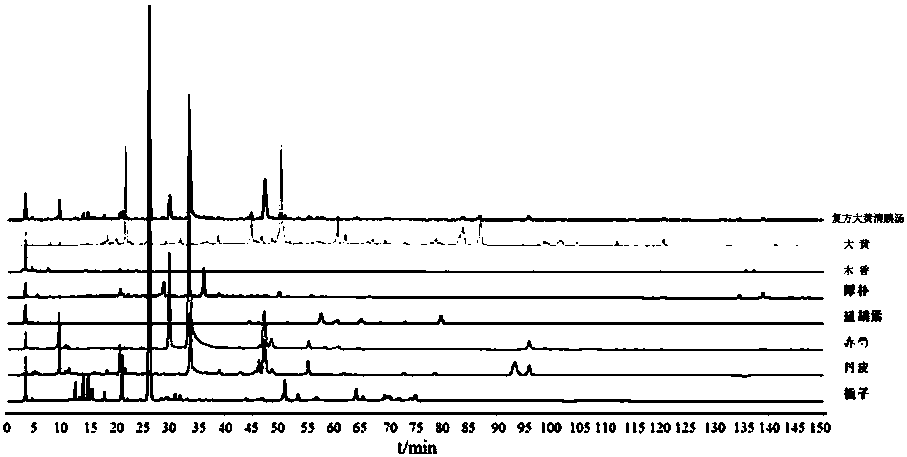
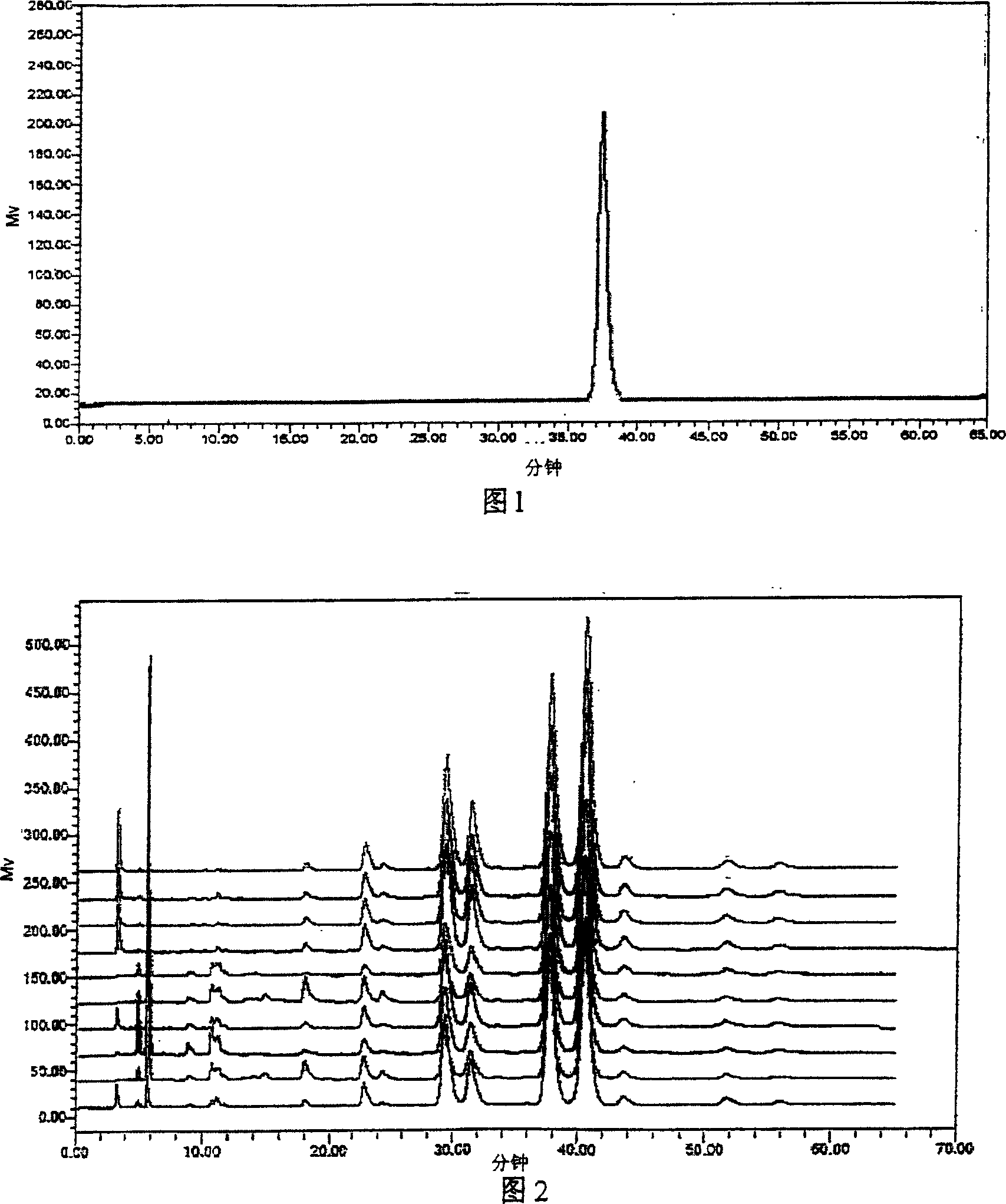
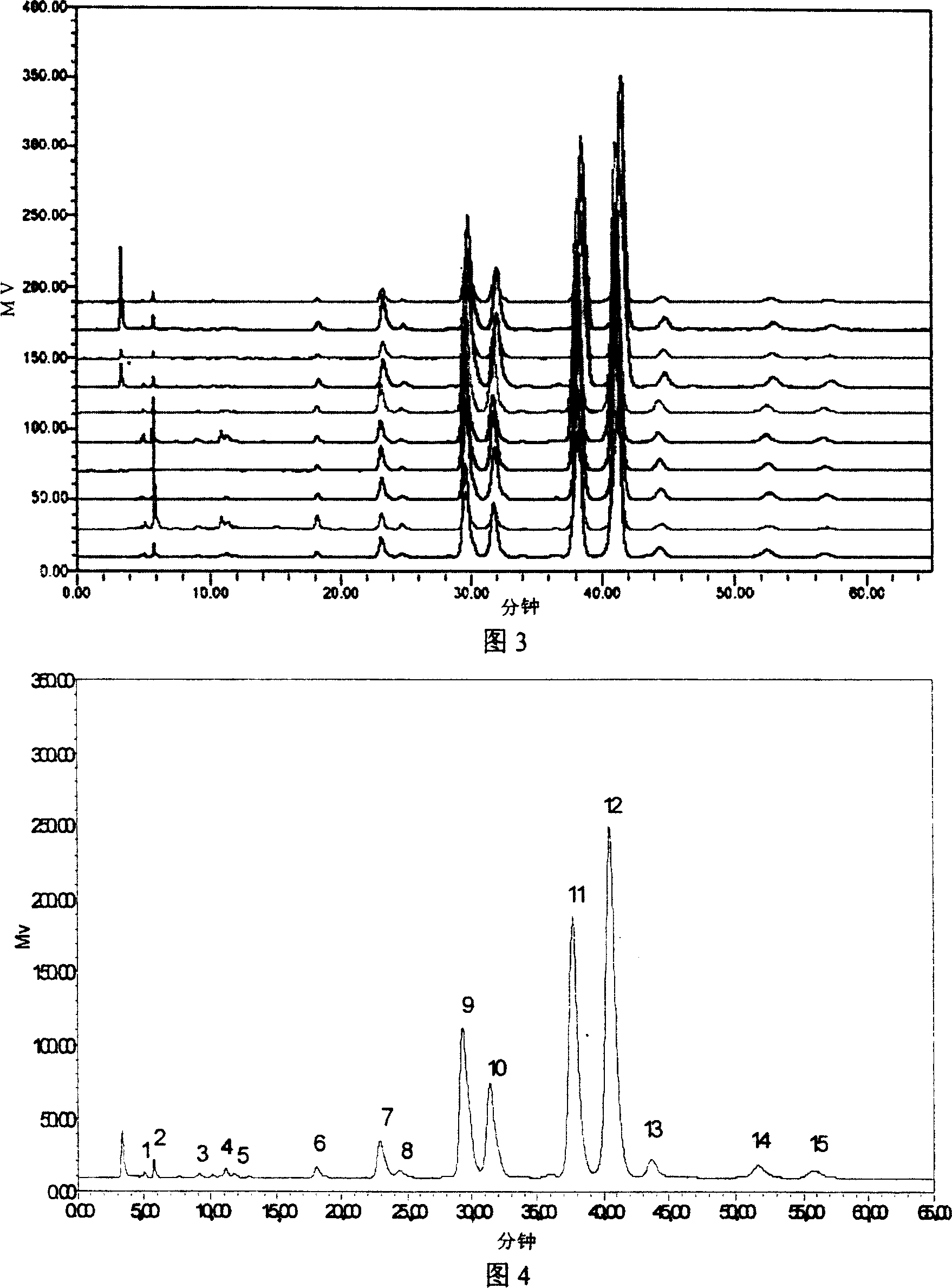
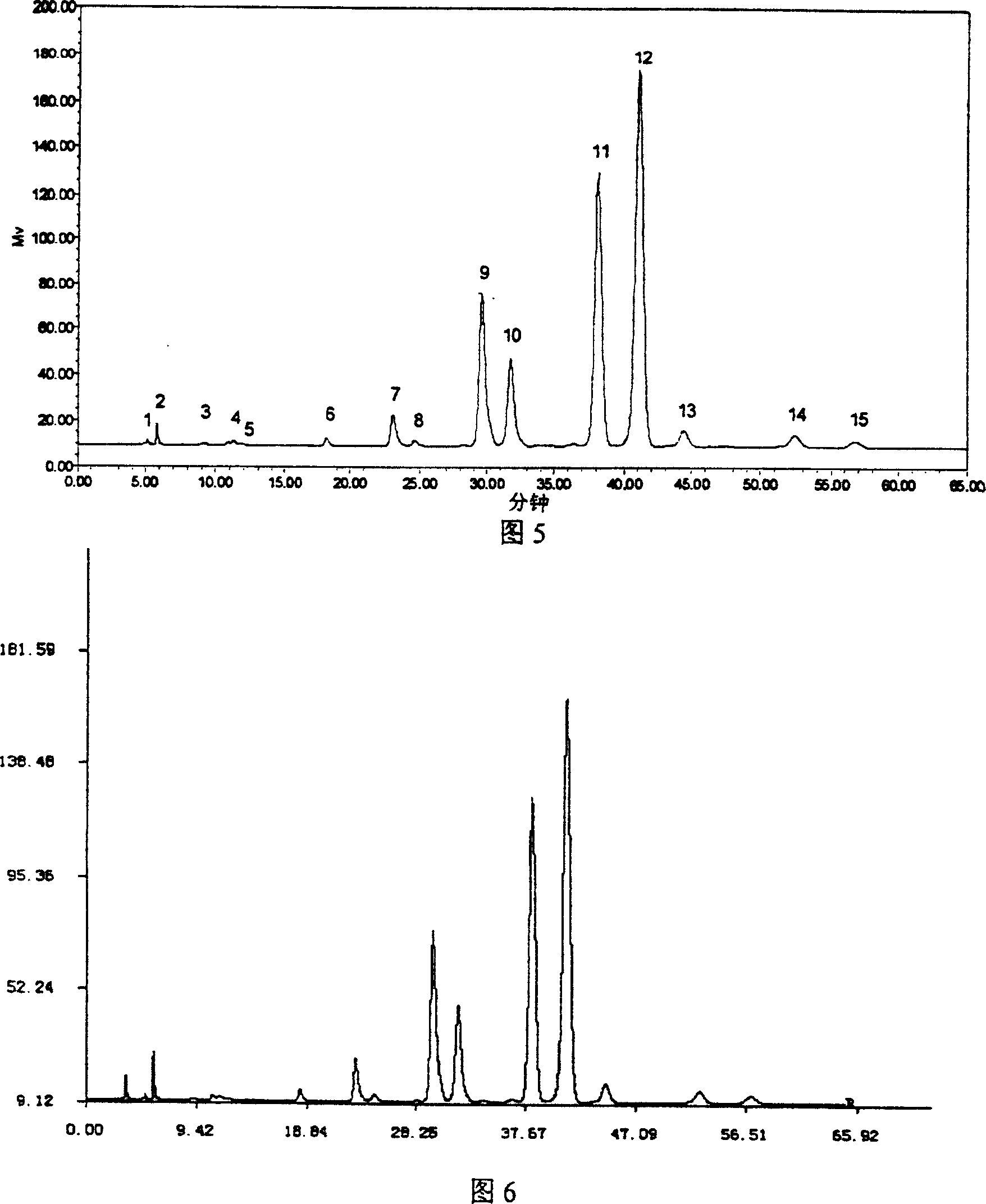
Establishment method of compound rheum officinale Qingyi decoction fingerprint
ActiveCN107860832AEffectively Characterize QualityCharacterizing qualityComponent separationAdditive ingredientRetention time
The invention provides an establishment method of a compound rheum officinale Qingyi decoction fingerprint. The establishment method comprises the following steps: preparation of a sample solution fortest, a single-flavor ingredient medicinal material sample solution for test and a reference substance solution, determination of chromatographic conditions of an HPLC (high performance liquid chromatography), and determination of a fingerprint, wherein the conditions of the HPLC are described as follows: a column is UItimate LP-C18 (250mm*4.6mm, 5mum), the flow velocity is 1.0mL / min, the columntemperature is 30 DEG C, the detection wavelength is 237nm, acetonitrile (A)-0.1% phosphoric acid solution (B) is adopted, and gradient elution is adopted. In the establishment method, the compound rheum officinale Qingyi decoction fingerprint established in 'Traditional Chinese Medicine Chromatographic Fingerprint Evaluation System 2012' is adopted, and the fingerprint is formed by chromatographyinformation of 42 common peaks; through comparison with the retention time of medicinal materials of all compositions and the reference substance and an ultraviolet spectrogram, the common peaks attributed and part chromatographic peaks are identified, so that a technical means of quality control by a single ingredient or various effective ingredients is changed.
Owner:ZUNYI MEDICAL UNIVERSITY
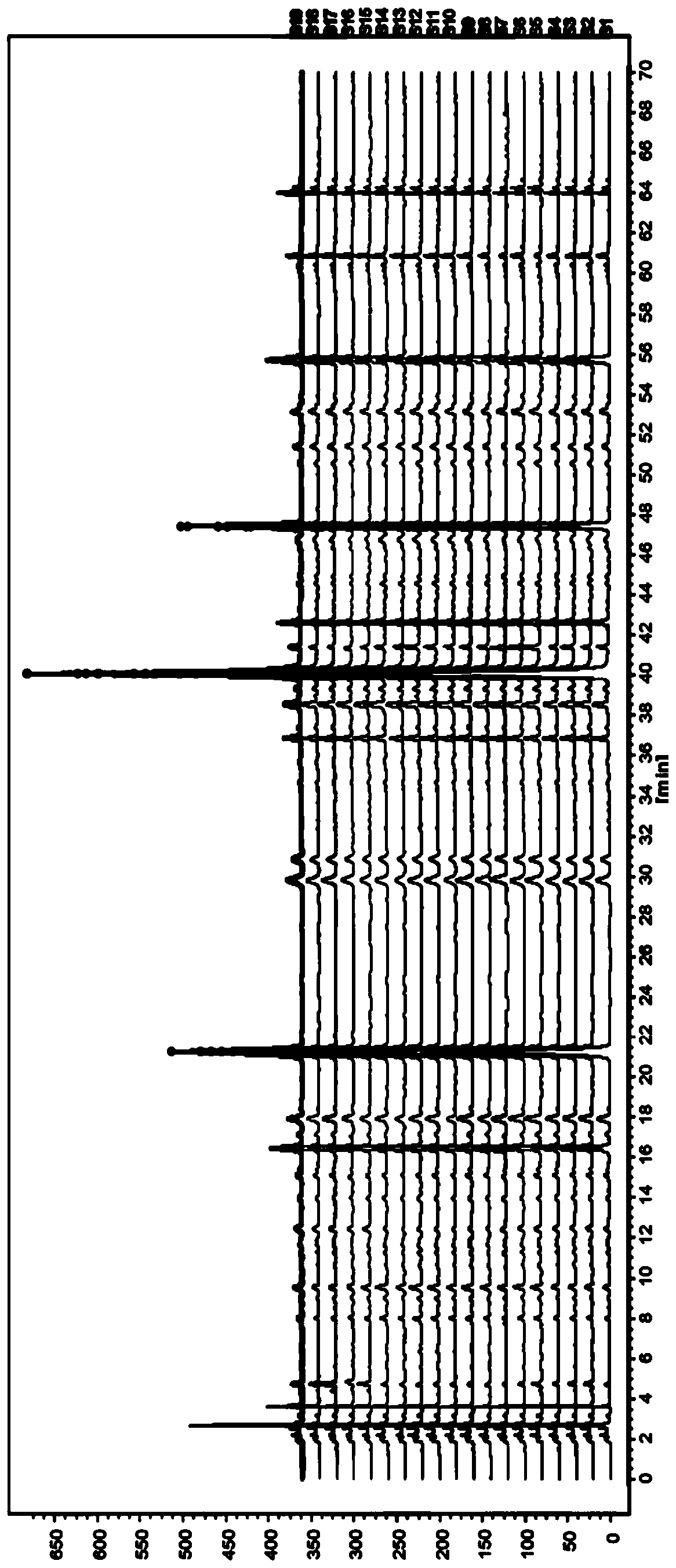
Establishment of lucid ganderma spore and lucid ganderma spore oil finger print atlas and standard finger print atlas
ActiveCN100362345CEffectively Characterize QualityCharacterizing qualityComponent separationHplc fingerprintSpore
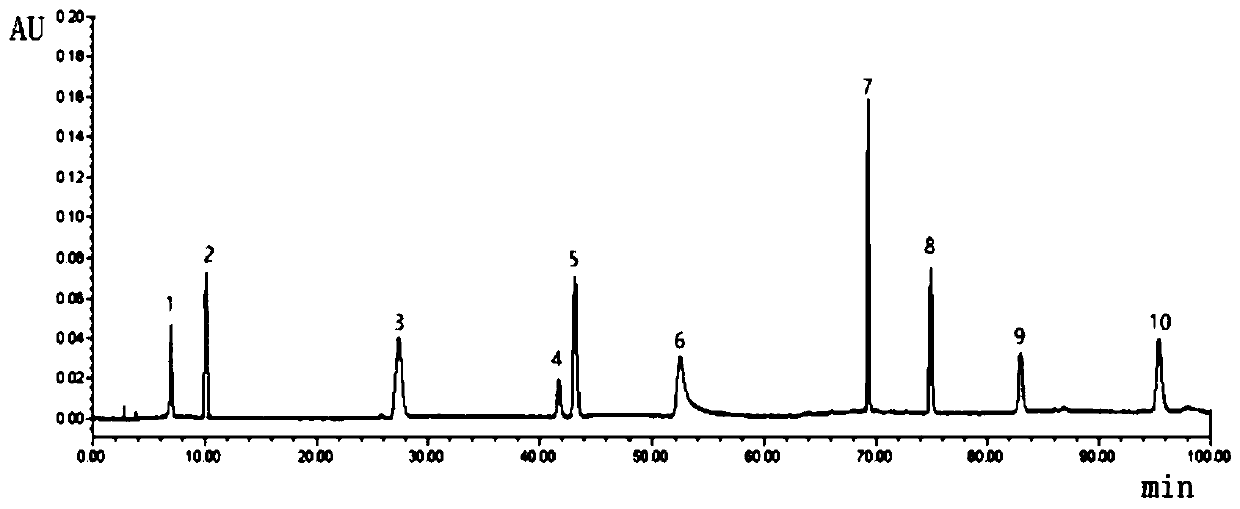
The invention is related to method for controlling quality of traditional Chinese medicinal materials and productions. Comparing fingerprints of high performance liquid chromatography HPLC for ten batches of ganoderma lucidum spore, oil of ganoderma lucidum spore determines mutual characters so as to obtain standard fingerprints. There are 15 mutual peaks in fingerprints. There are 4 peaks with its area exceeding 5% of total peak area. Features of the invention are: simple, stable, high accuracy, good reproducibility, easy of mastering. The invention discloses total new method for controlling quality of ganoderma lucidum spore, and oil of ganoderma lucidum spore, and distinguishing true or false products.
Owner:GUANGZHOU HANFANG PHARMA
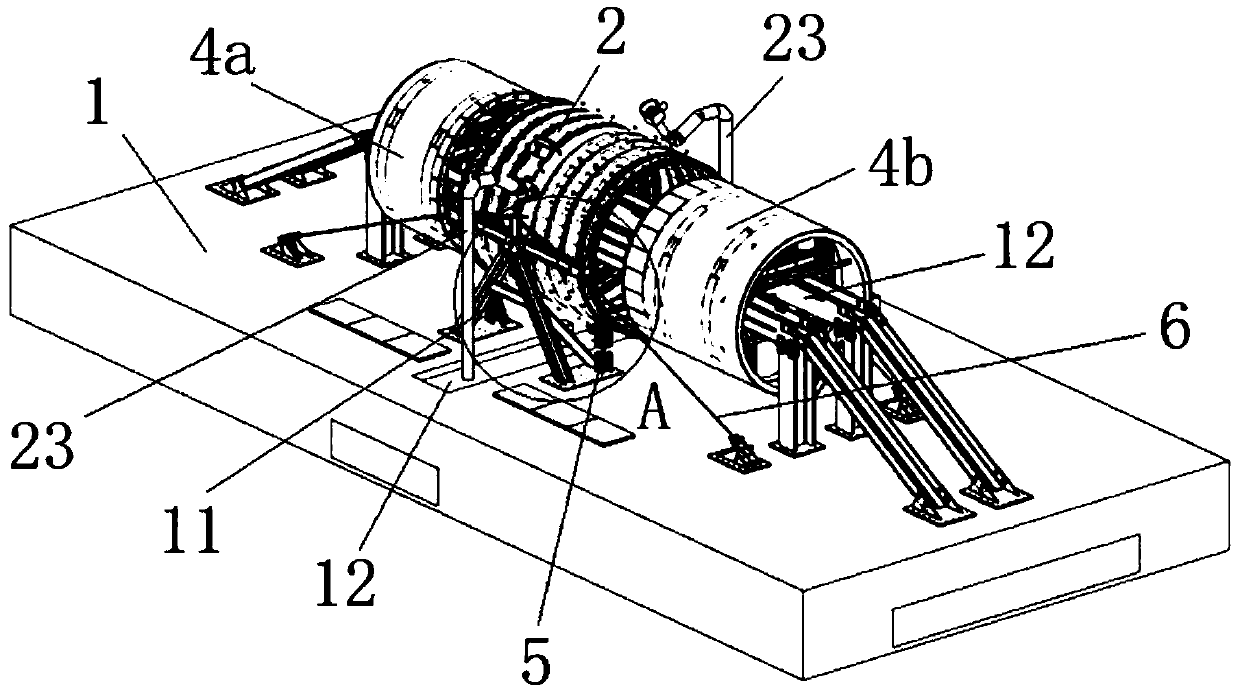
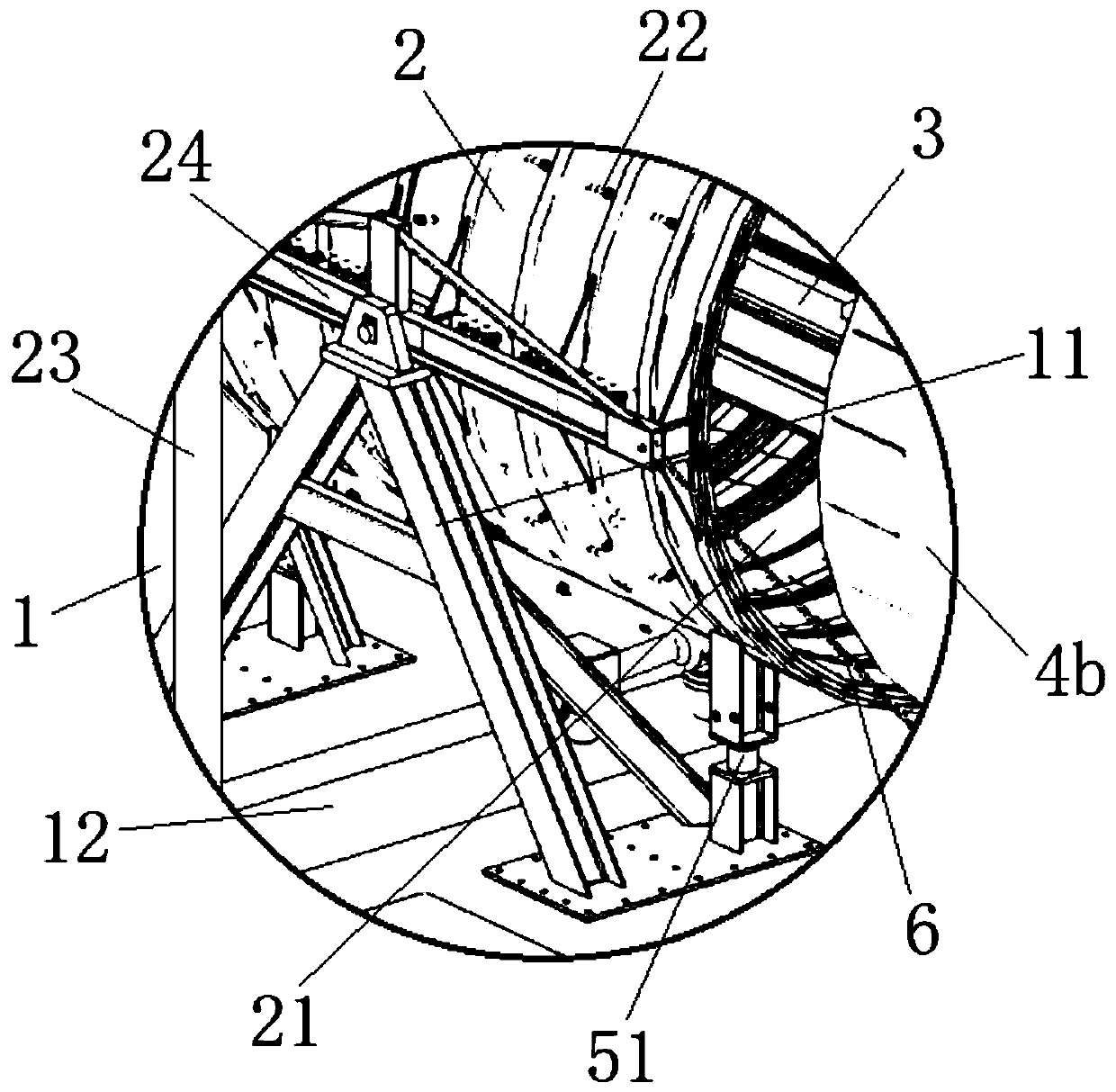
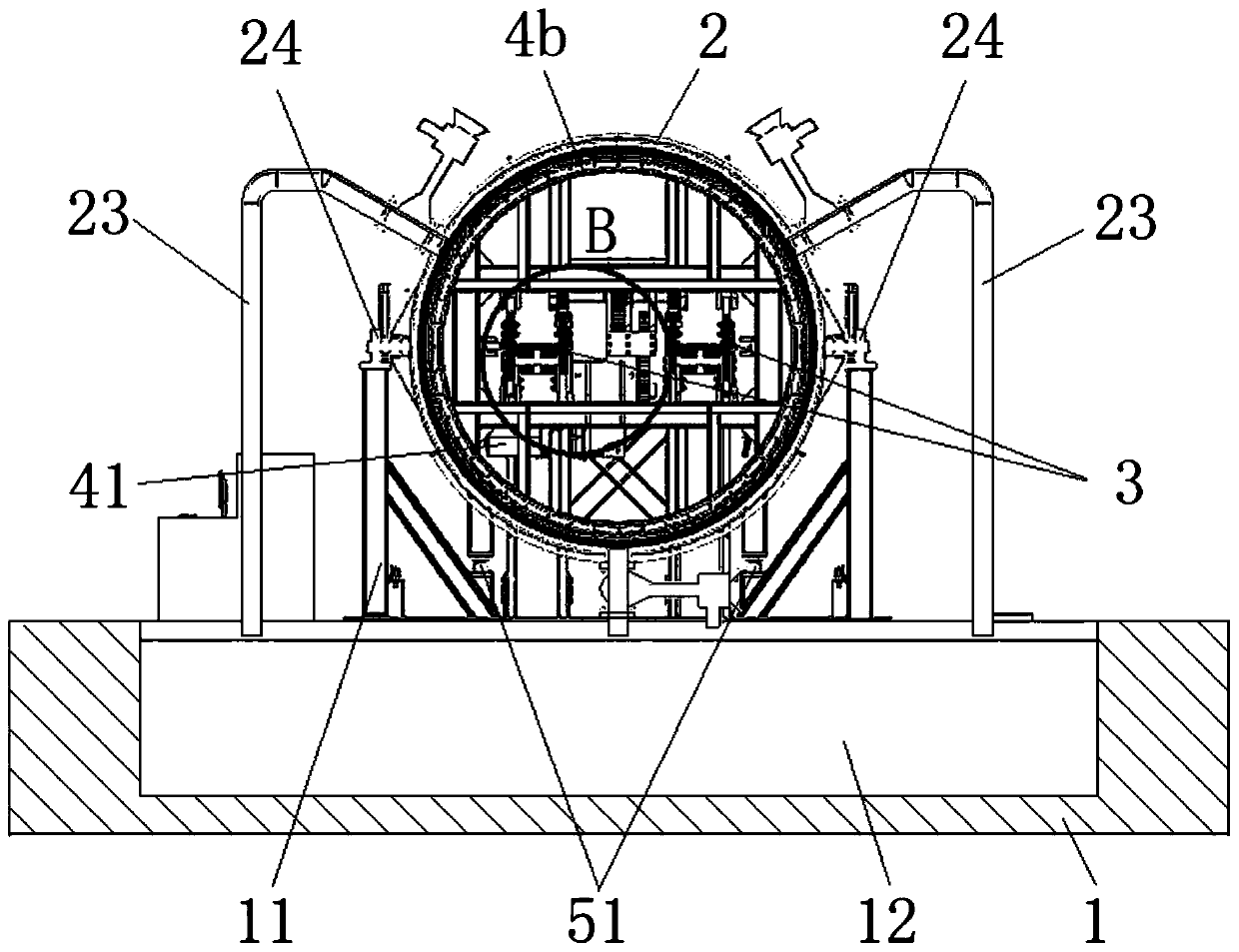
Shield tail brush dynamic sealing pressure resistance test equipment
PendingCN111426433ACharacterizing qualityMachine part testingMeasurement of fluid loss/gain rateShield machineShield tunneling
The invention belongs to the technical field of test equipment, and relates to shield tail brush dynamic sealing pressure resistance test equipment. The equipment comprises a base, a support frame spanning the middle part of the base, a simulated shield tail steel cylinder pivoted above the support frame, a transmission track horizontally penetrating through the axial hollow part of the simulatedshield tail steel cylinder, and a simulated segment and a self-sealing steel cylinder which can move along the transmission track, wherein the simulated segment and the self-sealing steel cylinder arerespectively disposed at two sides of the simulation shield tail steel cylinder. The outer diameters of the simulated segment and the self-sealing steel cylinder are smaller than the inner diameter of the simulation shield tail steel cylinder, and the axes of the simulated segment and the self-sealing steel cylinder are collinear with the horizontal axis of the simulation shield tail steel cylinder; the inner wall of the simulation shield tail steel cylinder is provided with a plurality of oil receiving ring grooves used for installing shield tail brushes in a mirror symmetry mode, the innersides of the oil receiving ring grooves are communicated with a plurality of pressure sensors, and the bottom of the simulation shield tail steel cylinder is provided with a segment gap adjusting device used for adjusting the angle. The structure can simulate the dynamic process of the shield tunneling machine, and can test the wear resistance and dynamic sealing performance of the shield tail brush at the same time.
Owner:昆山众备机械设备有限公司
Detection method for fingerprint spectrum of kidney-tonifying pregnancy-assisting granules
ActiveCN111175428AGuaranteed curative effectComprehensive detection effectComponent separationAgainst vector-borne diseasesPregnancyKidney
The invention discloses a detection method of a fingerprint spectrum of kidney-tonifying pregnancy-assisting granules. The detection method comprises the following steps: step 1, preparing a kidney-tonifying pregnancy-assisting granule test solution; step 2, preparing a mixed reference substance solution; step 3, precisely sucking the mixed reference substance solution and the test solution respectively, injecting the solutions into a liquid chromatograph, and recording chromatograms; step 4, exporting a fingerprint spectrum instrument of the kidney-tonifying pregnancy-assisting granules, importing the fingerprint spectrum instrument into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system, and selecting chromatographic peaks existing in chromatograms of different batches of kidney-tonifying pregnancy-assisting granules as common peaks; generating a reference fingerprint spectrum of the kidney-tonifying pregnancy-assisting granules by usingan average value calculation method; calculating the relative retention time and the relative peak area of each common peak; and comparing the fingerprint spectrum of the kidney-tonifying pregnancy-assisting granules with the spectrum of a mixed standard substance, and identifying the peak of main components. The fingerprint spectrum of the kidney-tonifying pregnancy-assisting granules provided bythe invention can comprehensively and objectively represent the quality of the kidney-tonifying pregnancy-assisting granules. And the detection method has the advantages of simplicity, convenience, stability, high precision, good reproducibility and the like.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
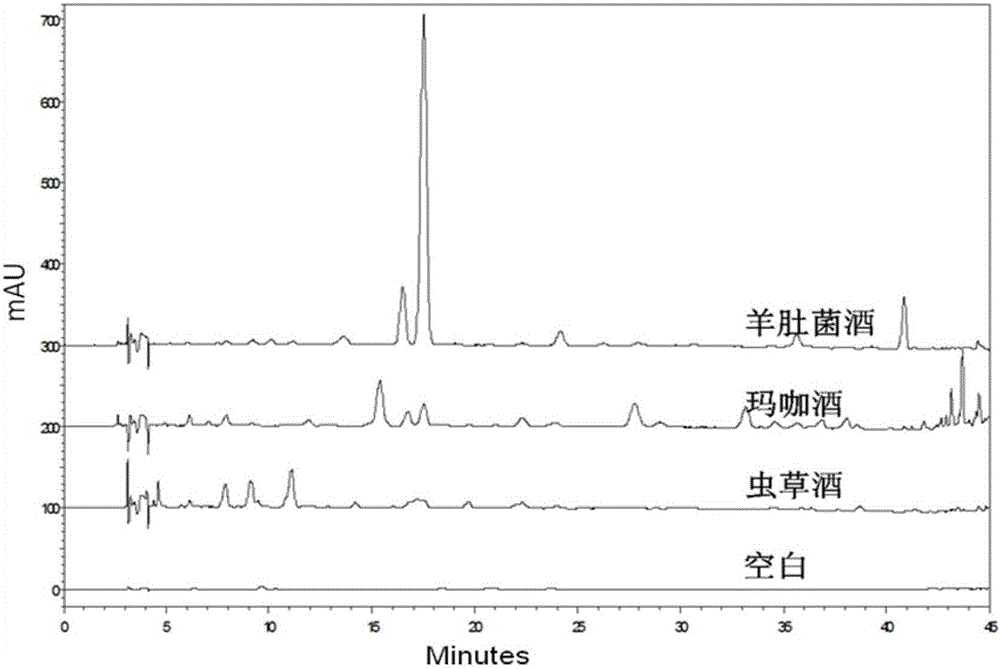
Morchella esculenta wine and method for establishing high-performance liquid phase fingerprint chromatogram thereof
ActiveCN106353432AMellow smellLong lasting fragranceComponent separationAlcoholic beverage preparationFrostCLARITY
The invention provides a preparation method of morchella esculenta wine. The method comprises the following steps: firstly, treating fresh and clean morchella esculenta at a high pressure of 80-120MPa for 8-15min, then freezing the morchella esculenta at a temperature of -30 to -25 DEG C for 1-2 days, removing frost flakes from the surface of the morchella esculenta, and then soaking the morchella esculenta in basic liquor, thereby obtaining the morchella esculenta wine with a unique flavor and an extremely good mouthfeel. Then, a fingerprint chromatogram of the morchella esculenta wine is established by adopting high-performance liquid chromatography. The preparation method of the morchella esculenta wine is simple, the product is savory and mellow and keeps the scent for a long time, and the method can significantly prolong the discoloring time of the product and effectively keep the clarity and stability of a wine body. Meanwhile, the method for establishing the fingerprint chromatogram of the morchella esculenta wine is easy to implement, and the stability, reproducibility and specificity of the method are good, and can provide a reference for quality control of the morchella esculenta wine.
Owner:东莞东阳光保健品研发有限公司
Finger print atlas determination method of Chinese medicinal material tangerine peel water extraction
ActiveCN1830472AAvoid one-sidednessQuality is easy to controlComponent separationPlant ingredientsRefluxSilica gel
A fingerprint assay method for detecting the aquatic extract of tangerine peel to evaluate the quality of tangerine peel includes such steps as adding the tangerine peel in water, reflux extracting, filtering, adsorbing by macro-reticular resin column, water washing, washing with ethanol or methanol, eluting with ethanol or methanol, collecting eluting liquid, evaporating off ethanol or methanol, loading in measuring glass, adding water, and efficient liquid-phase chromatography to obtain fingerprint.
Owner:JIANGZHONG PHARMA
Determination method for radix curcumae fingerprint and standard fingerprint thereof
ActiveCN108693275AImprove the quality control technology of medicinal materialsEffective representation qualityComponent separationFingerprintChemistry
The invention relates to a determination method for radix curcumae fingerprint and a standard fingerprint thereof. The determination method comprises the following steps of preparing of a test samplesolution, preparing of a reference sample solution, determining by gas chromatography, and determining by liquid chromatography, so as to obtain a chromatogram map; contrasting the obtained standard radix curcumae gas fingerprint and the liquid fingerprint, wherein the qualified product is obtained when the obtained standard radix curcumae gas fingerprint is consistent with the liquid fingerprint.The determination method has the advantages that the determination method is simple, rapid, accurate, stable and reliable, and is used for controlling the quality of the radix curcumae; the quality of the radix curcumae can be more comprehensively, objectively and scientifically evaluated; the validity of the preparation is guaranteed.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
Determination method for fingerprint chromatogram of HuangShiXiangShengWan preparation and standard fingerprint chromatogram thereof
ActiveCN104374838AEffective massEffective representation qualityComponent separationTest articleReference product
The invention relates to a determination method for a fingerprint chromatogram of a HuangShiXiangShengWan preparation and a standard fingerprint chromatogram thereof. The determination method comprises the steps of preparing a test article solution, preparing a reference product solution, injecting the test article solution and the reference product solution into a gas chromatograph to carry out determination, and on the basis of the standard fingerprint chromatogram, judging whether the quality of the obtained HuangShiXiangShengWan is qualified data by using the determination method. The determination method is simple, fast, accurate, stable and reliable, can be used for controlling the quality of a HuangShiXiangShengWan sample, can be used for evaluating the quality of the HuangShiXiangShengWan in a comprehensive, objective and scientific manner and can guarantee effectiveness of the preparation.
Owner:WUXI JIYU SHANHE PHARM CO LTD
Method for determining effective component of total glycoside in smilax glabra ethanol water solution extract
ActiveCN101732540ACharacterizing qualityAvoid one-sidednessAntipyreticComponent separationColumn temperatureGradient elution
The invention relates to a method for determining an effective component of total glycoside in smilax glabra ethanol water solution extract and belongs to the field of pharmaceutical analysis methods. The method adopts a high-efficiency liquid chromatography to establish a fingerprint chromatogram characterized by taking the astilbin chromatographic peak as an internal reference peak, comprising the following steps of (a) putting 1g of smilax glabra total glycoside dry powder into a 25mL volumetric flask, adding methanol for dissolution and diluting the solution to a scale to obtain sample solution; (b) putting astilbin into methanol solution to prepare reference solution with the concentration of 0.1mg of astilbin per ml; (c) including chromatographic conditions: the chromatographic column is octadecylsilyl as filling; the mobile phase: A phase is methanol, and B phase is gradient elution formed by 0.3 percent of acetic acid solution; the flow velocity is 0.8ml / min; the detection wavelength is 323-327nm; the column temperature is 25-35 DEG C; and the theoretical plate number is over 3000 based on astilbin peak; and (d) determination. The method is simple, convenient and stable, has high precision, good reproducibility and easy control and can characterize the mass of the smilax glabra total glycoside.
Owner:GUANGZHOU BAIYUNSHAN JINGXIUTANG PHARM CO LTD
Fingerprint detection method of Xiaoyao pills
PendingCN114113356ACharacterizing qualityMonitor qualityComponent separationAgainst vector-borne diseasesBiotechnologyTest sample
The invention discloses a fingerprint spectrum detection method of Xiaoyao pills. The method comprises the following steps: step 1, preparing a Xiaoyao pill test solution; 2, preparing a mixed reference substance solution; 3, respectively and precisely sucking the test sample and the reference substance solution, injecting the test sample and the reference substance solution into a liquid chromatogram instrument, and recording chromatograms; and 4, exporting the Xiaoyao pill fingerprint spectrum instrument, importing into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system, selecting chromatographic peaks existing in the chromatograms of different batches of Xiaoyao pills as common peaks, generating a contrast fingerprint spectrum of the Xiaoyao pills by using an average value calculation method, and calculating the relative retention time and the relative peak area of each common peak. The Xiaoyao pill fingerprint spectrum researched by the invention can comprehensively and objectively evaluate the quality of Xiaoyao pills. The detection method provided by the invention has the advantages of good stability, high accuracy, good repeatability, high precision and the like.
Owner:NANJING HAICHANG CHINESE MEDICINE GRPCO LTD +1
Determination method for HPLC-ELSD fingerprint spectrum of Lotrimin Sol Lotrimin
ActiveCN109239251ACharacterizing qualityGood peak shapeComponent separationQuality dataQuality control
The invention relates to a determination method for the HPLC-ELSD fingerprint spectrum of Lotrimin Sol Lotrimin. The determination method comprises the following steps of: preparing a sample solution;preparing a control sample solution; injecting the solutions into a liquid chromatograph (evaporative light scattering detector) to determine; and judging whether the quality data of the Lotrimin SolLotrimin obtained by the determination method is qualified or not on the basis of the standard fingerprint spectrum. The method is simple, rapid, accurate, stable and reliable, can be used for the quality control of a Lotrimin Sol Lotrimin sample, and can comprehensively, objectively and scientifically evaluate the quality of the Lotrimin Sol Lotrimin so as to guarantee the effectiveness of the preparation.
Owner:WUXI JIYU SHANHE PHARM CO LTD +1
Detection method for fingerprint spectrum of instant spina date seed heart-calming drink solid beverage
ActiveCN113655163AObjective qualityAccurate massComponent separationAgainst vector-borne diseasesMedicineProcess engineering
The invention discloses a detection method for the fingerprint spectrum of an instant spina date seed heart calming solid beverage. The method comprises the following steps of: step 1, preparing a test solution; step 2, preparing a mixed reference substance solution; step 3, respectively and precisely sucking the mixed reference substance solution and the test solution, injecting the mixed reference substance solution and the test solution into a liquid chromatograph, and recording chromatograms; step 4, exporting the fingerprint spectrum of the test solution from an instrument, importing the fingerprint spectrum into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system, and selecting chromatographic peaks existing in chromatograms of different batches of instant spina date seed heart-calming drink solid beverages as common peaks; generating a control fingerprint spectrum of the instant spina date heart-calming drink solid beverages by using an average value calculation method; calculating the relative retention time and the relative peak area of each common peak; and comparing with the spectrum with the spectrum of the mixed standard product, and identifying main component peaks. The fingerprint spectrum can comprehensively and objectively characterize the quality of the instant spina date seed heart-calming drink solid beverage. The detection method has the advantages of simplicity, stability, high precision, good reproducibility and the like.
Owner:NANJING HAICHANG CHINESE MEDICINE GRPCO LTD +1
Detection Method of Fingerprint of Ming Ge Shu Qing Drink
ActiveCN110850019BComprehensive detection effectComprehensive evaluationComponent separationPhysical chemistryAnalytical chemistry
The invention discloses a method for detecting fingerprints of Mingge Shuqing Drink, the method comprising the following steps: step 1, preparation of Mingge Shuqing drink test solution; step 2, preparation of a mixed reference solution; step 3, precise absorption of the mixed control The product solution and the test solution are injected into the liquid chromatograph, and the chromatograms are recorded; step 4, the Ming Ge Shu Qing drink fingerprint instrument obtained in step 3 is exported, and imported into the similarity evaluation system of traditional Chinese medicine chromatographic fingerprints, and different batches of fingerprints are selected. The chromatographic peaks that all exist in the chromatogram of Geshu Qingyin are used as common peaks; the control fingerprint of Minggeshu Qingyin is generated by the average value calculation method; the fingerprint of Minggeshu Qingyin obtained in step 5 and step 3 is compared with the mixed standard spectrum , to identify the main components. The fingerprint of Minggeshu Qingyin provided by the invention can comprehensively and objectively characterize the quality of Minggeshuqingyin; and has the advantages of simple, stable, high precision, good reproducibility and the like.
Owner:陈斌
Method for constructing HPLC (High Performance Liquid Chromatography) fingerprint spectrum of medicinal water extracts of Qipi oral liquid
InactiveCN102435692BCharacterizing qualityMonitor qualityComponent separationHplc fingerprintMedicine
The invention provides a method for detecting quality of medicinal water extracts of Qipi oral liquid based on an HPLC (High Performance Liquid Chromatography) fingerprint spectrum. The method comprises the following steps of: preparing a comparison product solution and a tested sample solution; performing HPLC measurement; establishing a standard fingerprint spectrum; and detecting the sample. According to the method, a freezing technology and quality of the medicinal water extracts of the Qipi oral liquid can be effectively represented, energy source is saved and labor hour is reduced; the method is beneficial to monitoring the quality of products; and the method has the advantages of simple method, excellent stability, high precision, excellent repeatability, and the like.
Owner:长春澜江医药科技有限公司
Fingerprint spectrum detection method for Ganshuang granules
PendingCN113533605AImprove reliabilityGood reproducibilityComponent separationAgainst vector-borne diseasesMethanolChinese herbology
The invention belongs to the technical field of spectrum detection, and relates to a fingerprint spectrum detection method for Ganshuang granules, which comprises: 1) carrying out ultrasonic extraction on Ganshuang granules by using methanol to obtain a to-be-detected test solution; (2) carrying out high performance liquid chromatography detection on the test solution by adopting ultra-high performance liquid chromatography, and recording chromatographic data within 40 minutes; and generating the fingerprint spectrum of the Ganshuang granules from the chromatographic data according to the 'Traditional Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System A'. The detection method provided by the invention has the characteristics of high accuracy, high stability and good repeatability, can comprehensively reflect the quality of the Ganshuang granules, and ensures the product quality.
Owner:BAODING BUCHANG TIANHAO PHARMA +1
Method for establishing evodia rutaecarpa liposoluble fingerprint and standard fingerprint thereof
InactiveCN100478684CImprove quality and efficacyThe method is simpleComponent separationFingerprintSoftware
The invention provides the establishment method of the lipid-soluble fingerprint diagram for the evodia and its standard fingerprint diagram which includes: making of the trying aqua, making of the comparing aqua, detecting by the HPLC, using fingerprint diagram software to treat the data and diagram and then get the lipid-soluble fingerprint diagram of the evodia. Compared with the existing technique, the invention is simple, stability, the precision is so much high and re-appear is good; The method to withdraw the lipid-soluble composition is brief and the time is short; the peak of lipid-soluble fingerprint diagram is many and the peak form is good, so it is easy to discriminate; The standard fingerprint diagram built up can be availably token by the quality of the evodia. Therefore, it provides a new standard for appraising the evodia.
Owner:GUIZHOU NORMAL UNIVERSITY
Quality control of compound Danshen root drops
ActiveCN100381817CCharacterizing qualityConducive to all aspects of monitoringComponent separationPill deliveryChemistryFingerprint
The invention relate to quality control method of compound Danshen root drops. The method comprises: measuring the fingerprint pattern of the compound Danshen root drops control sample by high efficiency liquid chromatography; measuring the fingerprint pattern of product to be measured of the compound Danshen root drops by the same method and in the same condition; comparing two results. When the absorption peak numbers of two results achieve the value described in the invention, the product to be measured is qualified.
Owner:TIANJIN TASLY PHARMA CO LTD
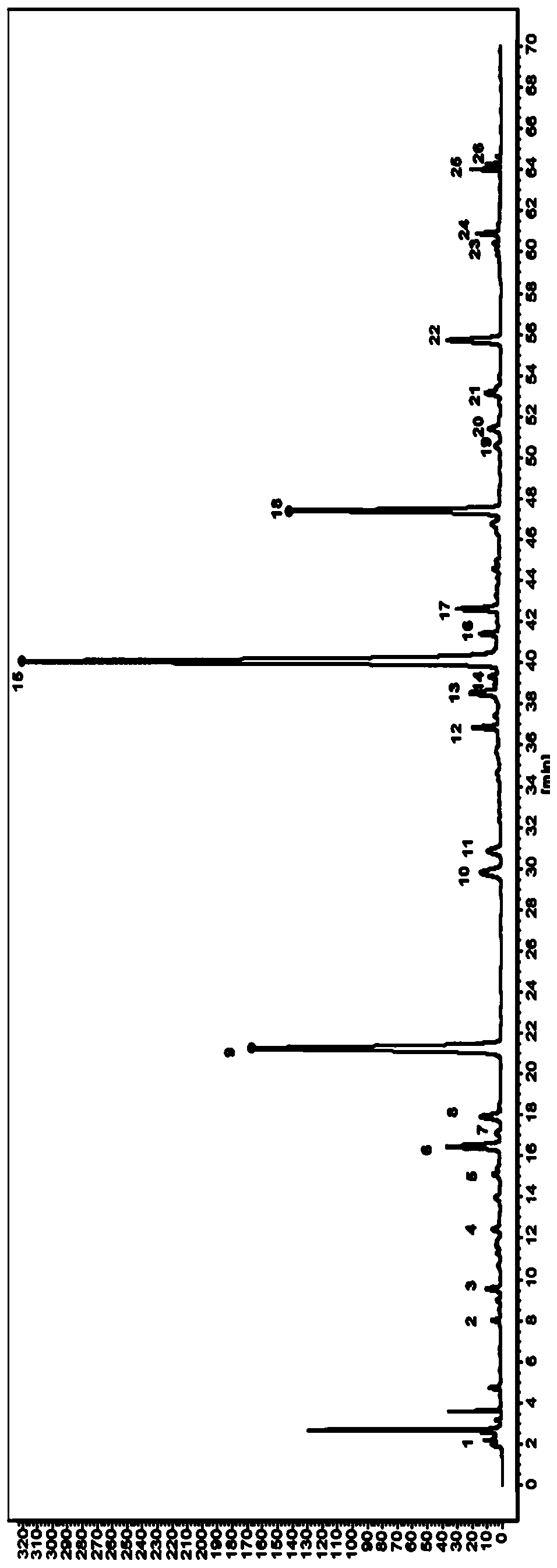
A method for constructing hplc fingerprint of Xiaoerfeike granule
ActiveCN113237974BComprehensive chemical informationCharacterizing qualityComponent separationHplc fingerprintMedicinal herbs
The invention relates to a method for constructing an HPLC fingerprint of Xiaoerfeike granules, which belongs to the field of medicine. According to the HPLC fingerprint of Xiaoerfeike granules, the invention optimizes the conditions of the mobile phase, detection wavelength, gradient elution procedure and the like, and establishes the detection conditions of the fingerprint. Based on multiple batches of samples, a standard fingerprint of Xiaoerfeike Granules was established, which identified 24 common peaks, and carried out the identification of medicinal materials and components of the related common peaks. The method of the invention can comprehensively control the quality of Xiaoer Feike Granules, so as to better ensure the quality stability, consistency and controllability of Xiaoer Feike Granules, thereby ensuring the safety and effectiveness of Xiaoer Feike Granules . The present invention comprehensively analyzes the status of each component in Xiaoer Feike Granules through systematic identification of components and the attribution of single medicinal materials with common peaks in different batches of samples, which can provide an important reference for the quality control of Xiaoer Feike Granules.
Owner:CHANGCHUN RENMIN PHARMA GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com