Patents
Literature
52 results about "Magnoflorine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
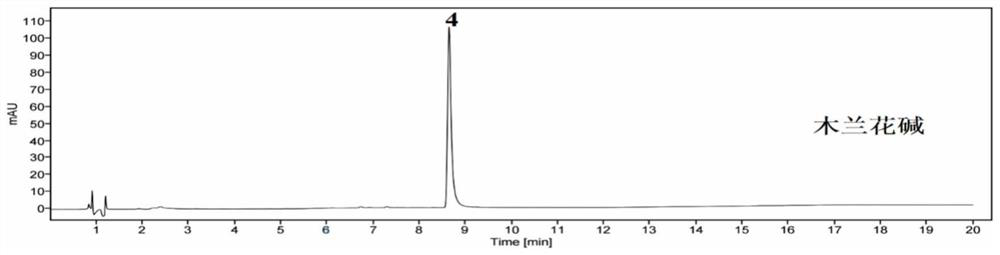
(S)-Magnoflorine is a quaternary benzylisoquinoline alkaloid (BIA) of the aporphine structural subgroup which has been isolated from various species of the Menispermaceae family, such as Pachygone ovata, Sinomenium acutum, and Cissampelos pareira.
Compositions and methods of use for extracts of magnoliaceae plants
InactiveUS6582735B2Reduce and preventWithout reduction and loss of motor functionBiocideNervous disorderHonokiolMagnolol
The invention relates to compositions and methods for preventing, treating, or managing sleeplessness, restlessness, weight gain including weight gain due to stress or lack of sleep, or all three comprising the administration of a prophylactically and therapeutically effective amount of Magnoliaceae plant or extracts thereof to a mammal in need of such therapy. Preferably the mammal is human and the compositions have comprise at least two compounds selected from magnolol, honokiol, and magnoflorine. Alternatively, the compositions may also comprise about 2% honokiol by weight of the composition.
Owner:INTERHEALTH NUTRACEUTICALS
Method for separating and purifying coptis alkaloids by utilizing polyamide resin
The invention relates to the natural medicament field, in particular to a method for separating and purifying coptis alkaloids by utilizing polyamide resin. The method is characterized in that polyamide is used to deal with coptis coarse extract, adding the mixture in a polyamide column, dichloromethane is used for elution or the mixed solvent of dichloromethane and methanol is used for gradient elution, the fractions are inspected through thin layer chromatography, the same fractions are combined to separate out seven alkaloids, namely berberine, palmatine, coptisine, columbamine, jatrorrhizine, magnoflorine and groenlandcine. The method has the advantages of simple process operation, low sample loss, higher separation efficiency and lower production cost.
Owner:CHINA PHARM UNIV
Pharmaceutical composition for preventing and treating rheumatoid arthritis
InactiveCN101700249AInhibition of clinical onsetOrganic active ingredientsAntipyreticChlorogenic acidEarly rheumatoid arthritis
The invention discloses a pharmaceutical composition for preventing and treating rheumatoid arthritis. The traditional Chinese medicine raw materials for constituting active ingredients of the pharmaceutical composition by weight are as follows: 5.5-6.0mg of berberine, 0.3-0.4mg of palmatine, 1.5-2.0mg of jatrorrhizine, 1.5-1.8mg of magnoflorine, 0.1-0.2mg of phellodendrine, 0.2-0.3mg of coptisine, 55-60mg of baicalin, 1.5-2.0mg of baicalein, 5.0-7.0mg of wogonoside, 0.03-0.05mg of wogonin, 20-25mg of geniposide, 0.1-0.2mg of crocin and 0.25-0.3mg of chlorogenic acid. The results of pharmacological tests show that the pharmaceutical composition can significantly reduce the disease severity of CIA mice with the rheumatoid arthritis, significantly improve the inflammation and the immune injury situation at lesion sites in comparison with a control group and be used for preparing drugs for preventing and treating the rheumatoid arthritis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Mangnolia officinalis alkaloid, extraction method and applications thereof
InactiveCN101966228ASimple extraction and preparation processEasy to operateMetabolism disorderAntiviralsStrong acidsOfficinalis
The invention belongs to the organic chemistry technical field, relating to an extraction method of mangnolia officinalis active constituent alkaloid and applications thereof used as alpha-glucuroide inhibitor. The mangnolia officinalis alkaloid of the invention comprises magnoflorine, liriodenine, anonaine, roemerine and total alkaloids of lysicamine, and mangnolia officinalis bark is taken as a raw material and then is extracted with acid aqueous solution at normal temperature or by heating; the extracting solution passes through strong-acid-type cation exchange resin or weak-acid-type cation exchange resin; and the active component is adsorbed on the resin; the resin column is washed by water, is eluted with ammonia water or alcoholic solution, and the obtained product is concentrated and dried to obtain total mangnolia officinalis alkaloids. The invention has the advantages of simple extraction and preparation process, easy operation and low cost and the like.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Dicranostigma leptopodum extract and extraction method and application thereof
ActiveCN106565724AReduce development costsHigh antibacterial activityBiocideOrganic chemistryDicranostigma leptopodumMagnoflorine
The invention relates to dicranostigma leptopodum extract and an extraction method and application thereof. Based on researches on the antimicrobial activity and active ingredients of dicranostigma leptopodum, it is found that the dicranostigma leptopodum extract has remarkable inhibitory activity for various plant pathogens and has the potential to prepare a plant antibacterial agent as an effective ingredient. Meanwhile, it is also found that the dicranostigma leptopodum extract contains multiple effective ingredients with magnoflorine as a majority. The invention further provides a method and process for quickly gathering the effective ingredients in the dicranostigma leptopodum.
Owner:NORTHWEST A & F UNIV
Lanqin oral solution fingerprint spectrum establishment method, fingerprint spectrum of Lanqin oral solution and application of fingerprint spectrum
ActiveCN110108825ACharacterizing qualityAvoid one-sidednessComponent separationBenzoic acidChlorogenic acid
The invention discloses a Lanqin oral solution fingerprint spectrum establishment method, a fingerprint spectrum of a Lanqin oral solution and application of the fingerprint spectrum. A test solutionis prepared from the Lanqin oral solution, a reference substance solution is a solution which dissolves adenosine, epigoitrin, phellodendrine chloride, chlorogenic acid, magnoflorine, geniposide, benzoic acid, berberine hydrochloride, baicalin, wogonoside, baicalein and wogonin, and the liquid chromatography is measured by using a high-performance liquid chromatograph; the obtained liquid chromatography is introduced into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system for analysis, and the Lanqin oral solution fingerprint spectrum is obtained after multi-point correction, data matching and analysis. According to the Lanqin oral solution fingerprint spectrum establishment method and the fingerprint spectrum of the Lanqin oral solution, the quality information of the Lanqin oral solution can be comprehensively reflected, and uniform and stable product quality is ensured.
Owner:YANGTZE RIVER PHARMA GRP JIA NGSU LONGFENGTANG TRADITIONAL CHINESE MEDICINE CO LTD +2
HPLC method for simultaneously determining contents of four alkaloid components in cortex berberidis
InactiveCN105675761AImprove quality controlPromote development and utilizationComponent separationMagnoflorineChemistry
The invention discloses an HPLC method for simultaneously determining the contents of four alkaloid components in berberis bark. The four alkaloid components are magnolialine, jatrorrhizine, palmatine and berberine, comprising the following steps: (1) prepare need testing solution; (2) prepare reference substance solution; (3) need test solution and reference substance solution are injected into high-performance liquid chromatograph detection respectively; of magnolialine, jatrorrhizine, palmatine and berberine. The method of the invention is accurate, reliable, simple and quick, and lays a foundation for improving the quality control level of barberry bark and promoting the development and utilization of medicinal materials. Simultaneously, the method of the present invention also accurately detects four alkaloid components in different parts of different gene source varieties of barberry bark, which provides an effective reference for the clinical application of barberry bark bark.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Application of preservative in preservative paper of cakes and preparation method thereof
InactiveCN106172676AHas bactericidal and antibacterial effectsWith detoxificationBakery product preservationActivated carbonPolyvinyl alcohol
The present invention relates to a preservative, discloses an application of the preservative in preservative paper of cakes and a preparation method thereof, and solves the problems that relatively complicated devices needed in a special technology in the prior art cause the increase of costs and a direct addition of preservatives in the cakes is harmful to human body. The preservative includes the following raw materials in parts by weight: 0.1-0.3 part of a lemon extract, 0.5-1 part of a garlic extract, 0.2-0.3 part of a fresh herba portulacae extract, 0.3-0.5 part of an eupatorium adenophora leaf extract, 0.5-1 part of L-ascorbyl palmitate, 0.03-0.1 part of butyl hydroxy anisd, 1-3 parts of licorice powder, 0.3-0.8 part of activated carbon powder, 5-10 parts of polyvinyl alcohol, 4-8 parts of cationic polyacrylamide, 0.3-0.5 part of chitosan, 0.3-0.5 part of magnoflorine and 50-70 parts of deionized water.
Owner:厦门陈纪乐肴居食品有限公司
Compositions and methods of use for extracts of magnoliaceae plants
InactiveUS20030206975A1Reduce and preventWithout reduction and loss of motor functionBiocideNervous disorderHonokiolMagnolol
Owner:INTERHEALTH NUTRACEUTICALS
Method for preparing magnoflorine from Chinese medicinal Chelidonium majus L.
InactiveCN102464616AReduce usageThe method is simple and feasibleOrganic chemistryMagnoflorineChromatographic column
The invention belongs to the field of Chinese medicines, and provides a method for preparing magnoflorine from Chinese medicinal Chelidonium majus L.. The method comprises the following steps of: (1) extracting: extracting Chinese medicinal Chelidonium majus L. with 70 percent ethanol, filtering, concentrating a filtrate, adding ethanol till the ethanol content is 80 percent, filtering, recovering a filtrate till ethanol smell disappears, adding water for diluting, filtering, removing undissolved substances, and adjusting the pH of a filtrate to 1-3 to obtain a standby liquid I; (2) performing primary resin purification: making the standby liquid I flow through a macroporous resin chromatographic column, eluting with water, eluting with 10 percent ethanol, collecting an eluent, filtering, and adjusting the pH of a filtrate to 8-10 to obtain a standby liquid II; (3) performing secondary resin purification: making the standby liquid II flow through a macroporous resin chromatographic column, eluting with water till a color becomes light, eluting with 40 percent ethanol, collecting an eluent, concentrating and drying to obtain a crude product; and (4) refining: dissolving the crude product with water, making the dissolved crude product flow through a C-18 filler chromatographic column, eluting with water, eliminating a fluid, eluting with 5 percent ethanol, collecting an eluent, concentrating and drying to obtain magnoflorine.
Owner:JIANGSU KANION PHARMA CO LTD
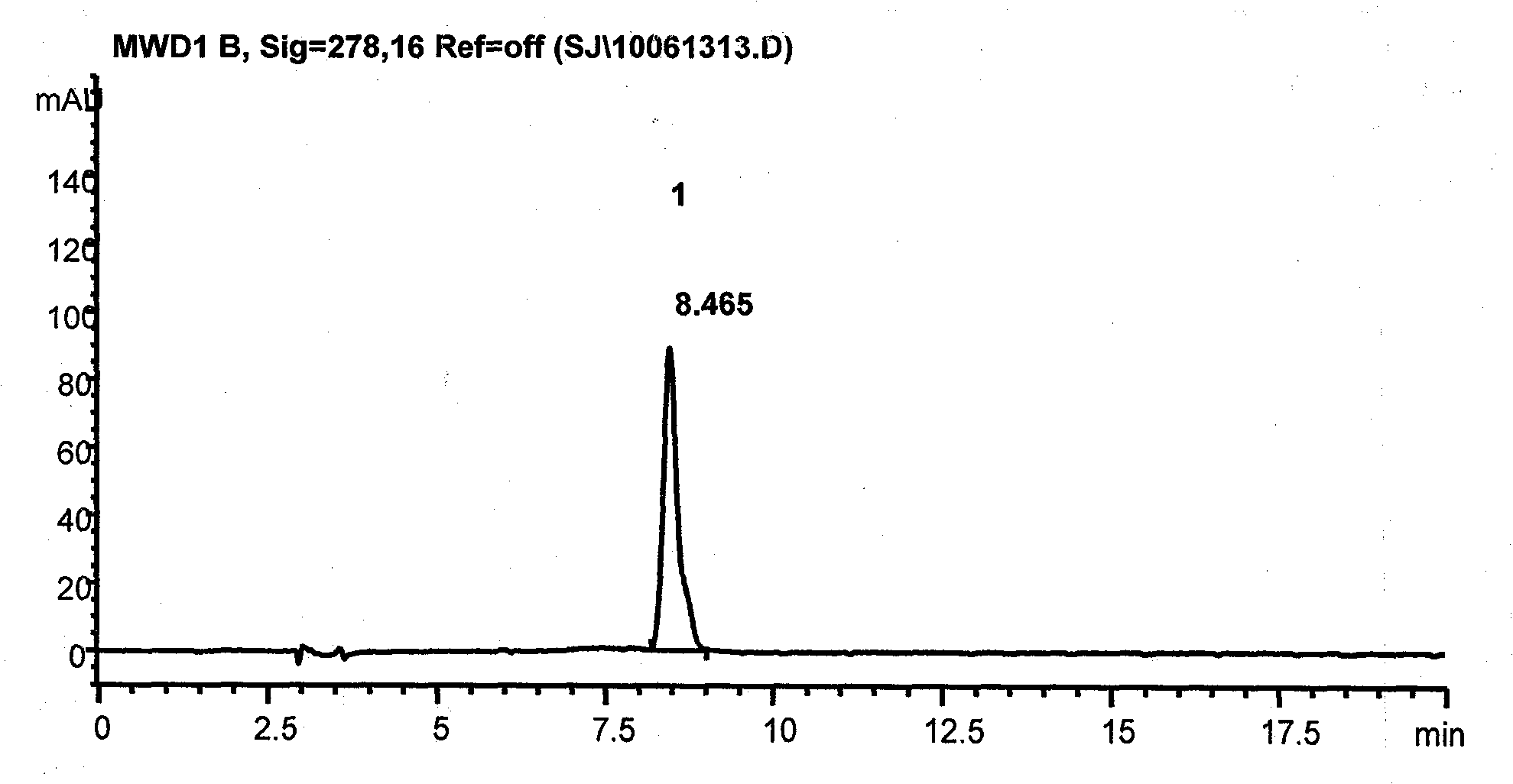
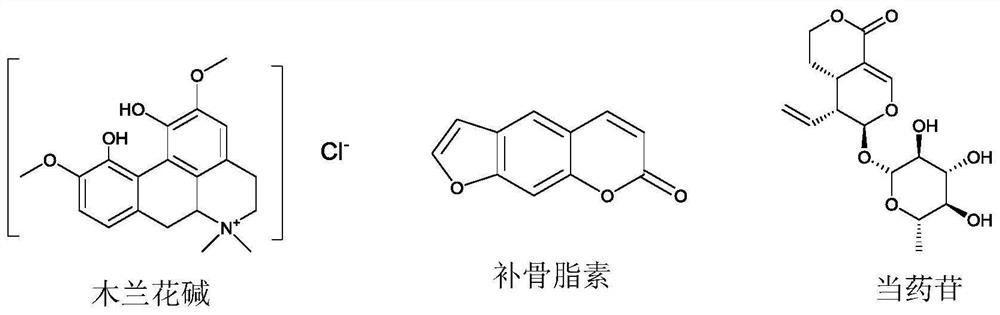
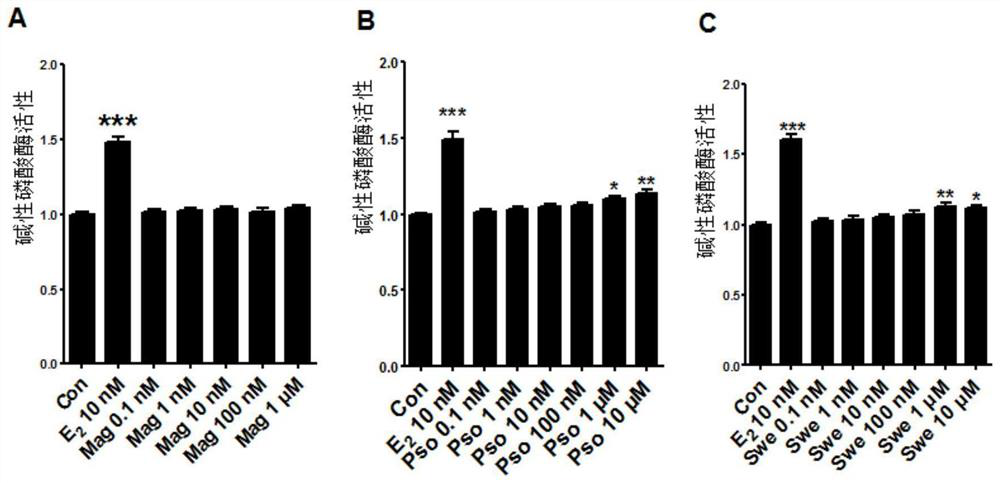
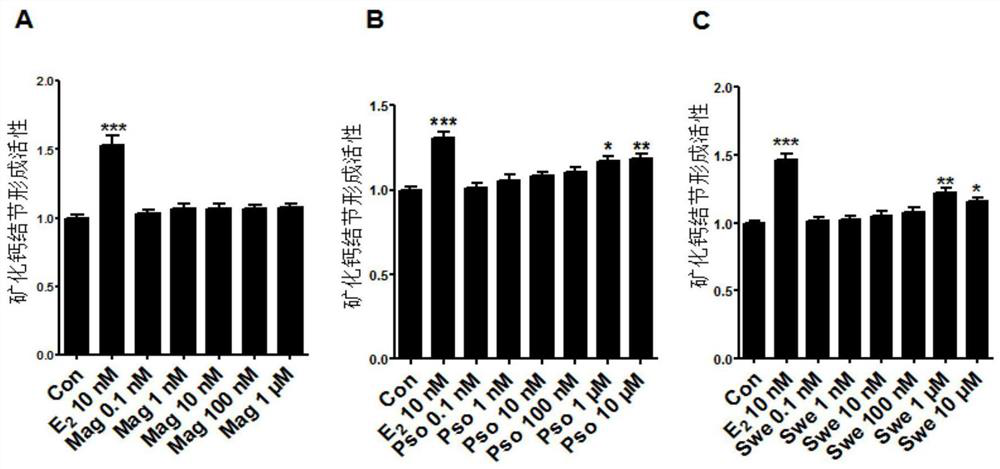
Application of magnoflorine in preparation of bone regulation drug synergist and pharmaceutical composition containing magnoflorine
ActiveCN113116897APromote differentiationHigh activitySkeletal disorderHeterocyclic compound active ingredientsPharmaceutical drugMagnoflorine
The invention discloses an application of magnoflorine in preparation of a bone regulation drug synergist and a pharmaceutical composition containing magnoflorine, and belongs to the technical field of biological medicines. The magnoflorine can be used for preparing the bone regulation drug synergist and can further be used for preparing a pharmaceutical composition with the effect of promoting osteoblast differentiation and mineralization. The pharmaceutical composition is prepared from one or two of psoralen and sweroside, and magnoflorine. The magnoflorine can be widely applied to the field of medicines, and can provide a new medicine source for the treatment of osteoporosis diseases.
Owner:JINAN UNIVERSITY
Tissue culture method for Aristolochia heterophylla
InactiveCN107494282AImprove germination rateEx vivo regenerationPlant tissue cultureHorticulture methodsBudExodermis
The invention discloses a tissue culture method for Aristolochia heterophylla. Aristolochia heterophylla is an Aristolochiaceae plant and can be harvested in the four seasons, preferably in autumn. After the root of Aristolochia heterophylla is dug out, silt and fibrous roots on the root of Aristolochia heterophylla are removed; then the root is dried to a semi-dry state in the sun; and if the root has a diameter of 2.5 cm or more, the root is halved and dried in the sun. The drug properties of Aristolochia heterophylla are that the root of Aristolochia heterophylla is cylindrical, has light brown or light brown-yellow cortex, and has a light grayish yellow color after the cortex is peeled; Aristolochia heterophylla is firm and not prone to breakage; a broken surface is uneven and spinous; a cross section is offwhite to yellow white; and Aristolochia heterophylla is mealy and has thick cortex and bitter and slightly astringent taste. Aristolochia heterophylla mainly comprises aristolochic acid, beta-sitosterol, magnoflorine, allantoin, etc. Aristolochia heterophylla is mainly used for dispelling wind, promoting urination, eliminating dampness and easing pain and applied to treatment of oedema in the head, face, arms and legs and pain in the waist and the knee joints. According to the invention, an Aristolochia heterophylla stem with a bud is used as an explant and subjected to bud induction, bud proliferation culture, rooting culture, domestication, transplanting and the like so as to realize in-vitro regeneration of Aristolochia heterophylla; so the tissue culture method provides technical support for mass breeding, rapid propagation and novel species promotion of Aristolochia heterophylla.
Owner:李正美
Use and preparation method of magnoflorine in preparation of medicament for preventing and treating insomnia and depression
InactiveCN108635352AImprove efficiencyLarge amount of preparationOrganic active ingredientsNervous disorderDiseaseCountercurrent chromatography
The invention discloses a use and preparation method of magnoflorine in preparation of a medicament for preventing and treating insomnia and depression. The preparation method utilizes high-speed countercurrent chromatography to separate magnoflorine. Compared with the prior art, the method for separating and purifying an alkaloid monomer magnoflorine has high monomer preparation and purificationefficiency, a large preparation amount, a low loss, high product purity, simple operation, economy and environmental friendliness. The high performance liquid chromatography (HPLC) detection result shows that the alkaloid monomer magnoflorine separated and purified through one step has purity of 95% or more. The animal pharmacodynamic experimental result shows that the magnoflorine can significantly improve and treat insomnia and depression.
Owner:TIANJIN MEDICAL UNIV
Medicine composition for preventing and treating depression and insomnia diseases and preparation method and application thereof
InactiveCN108498526AImprove efficiencyHigh purityOrganic active ingredientsSaccharide with heterocyclic radicalsEnvironmental resistanceDisease
The invention discloses a medicine composition for preventing and treating the depression and insomnia diseases and a preparation method and application thereof. The medicine composition is prepared from active ingredients of magnoflorine and spinosin in proportion. The invention further provides a method for separating and preparing magnoflorine and spinosin from spina date seeds through high-speed counter-current chromatography. According to the separation conditions, in a solvent system, the ratio of n-butyl alcohol to ethyl acetate to water is (1-3):(1-5):(2-7), the upper phase is a stationary phase, and the lower phase is a mobile phase. The preparation method has the advantages of being good in separation effect, large in preparation amount, low in loss, easy and convenient to operate, economical and environmentally friendly. Experimental studies of animal pharmacodynamics show that the composition has a remarkable improving and treating function on the depression and insomnia diseases.
Owner:TIANJIN MEDICAL UNIV
Content determination method of magnoflorine in dichocarpum sutchuenense medicinal material
The invention discloses a content determination method of magnoflorine in a dichocarpum sutchuenense medicinal material. The content determination method of magnoflorine is as follows: chromatographiccondition: Pntulips BP-C18 column (4.6 mm*250 mm, 2.5 microns); mobile phase: mobile phase A is acetonitrile, mobile phase B is 0.1% phosphoric acid- 0.1% triethylamine aqueous solution, gradient elution; detection wavelength: 260-280 nm; column temperature: 20-30 DEG C; flow velocity: 0.8-1.2 mL.min<-1>; and sample size: 5-20 microliters. According to the invention, magnoflorine is used as the determination index, a high performance liquid chromatography method is adopted, and a content determination method of the dichocarpum sutchuenense medicinal material is established, thus providing experimental bases for quality standard research of the dichocarpum sutchuenense medicinal material. The determination method is accurate, has high sensitivity, good repeatability and reliable results, and provides basis for quality control and evaluation of the dichocarpum sutchuenense medicinal material.
Owner:贵州中医药大学
Medicinal composition for treating neuropathic pain
InactiveCN102861045ASignificant effectOrganic active ingredientsNervous disorderClinical efficacyEfficacy
The invention discloses a medicinal composition for treating neuropathic pain. The medicine provided by the invention is prepared by magnoflorine, ranunculin, piperine and eugenol as raw material medicines and can be prepared into various dosage forms according to a conventional preparation technology. With the adoption of the medicinal composition for treating neuropathic pain, the defects in the prior art can be overcome, and the invention provides the safe medicinal composition for treating neuropathic pain with significant efficacy. The medicine provided by the invention is simple in preparation method and low in cost, and clinical tests prove that the medicine for treating the neuropathic pain has significant clinical effects, including prosopalgia, ischialgia, radicular pain, postherpetic neuralgia and diabetic peripheral neuritis and the like.
Owner:TAISHAN MEDICAL UNIV
Preservation method for mangoes
InactiveCN109169867AInhibition of transpirationInhibition of steam actionFruits/vegetable preservation by coatingOXALIC ACID DIHYDRATESodium Bentonite
The invention belongs to the technical field of fruit preservation and specifically discloses a preservation method for mangoes. The preservation method for mangoes comprises the following steps: 1) preparing a mango fresh-keeping agent: weighting 3-8 parts of oxalic acid, 1-4 parts of ferulic acid, 1-2 parts of nanometer zinc oxide, 1-3 parts of methyl jasmonate, 1-5 parts of phenylalanine and 2-4 parts of magnoflorine, in parts by weight, and then mixing and dissolving the weighted raw materials; 2) weighting 5-10 parts of chitosan, 1-4 parts of bentonite, 0.5-1 part of sodium dehydroacetateand 1-5 parts of plant extract powder, in parts by weight, and then mixing and dissolving the weighted raw materials. The preservation method for mangoes provided by the invention, the fresh-keepingagent and the fresh-keeping coating are combined for performing dual treatment on mangoes, so that respiration rate and transpiration of the mangoes during a storage process can be effectively reduced, and meanwhile, the antibacterial effect is excellent, so that the preservation effect of mangoes is effectively promoted.
Owner:广西田阳县创新农业综合开发有限公司
Medicinal composition for preventing and treating neuropathic pain
InactiveCN102920726AGood anti-inflammatory and analgesic effectHas the effect of relaxing meridians and dredging collateralsOrganic active ingredientsNervous disorderClinical efficacyTrigeminal neuralgia
The invention discloses a medicinal composition for preventing and treating neuropathic pain. The medicine is prepared from magnoflorine and ranunculin which are used as bulk pharmaceuticals in a certain proportion; the medicine can be prepared into various preparations by the conventional preparation process; the defects in the prior art are overcome; the medicinal composition which is used for treating neuropathic pain and is obvious in curative effect and safe is provided; the provided medicine is easy to prepare and low in cost; and the clinical test proves that the medicine has an obvious clinical effect on neuropathic pain comprising prosopalgia, ischialgia, sciatica of nerve roots, postherpetic neuralgia and diabetic peripheral polyneuritis and the like.
Owner:TAISHAN MEDICAL UNIV
Mango preservative and preparation method thereof
InactiveCN109042848AReduce transpirationReduce steam actionFruit and vegetables preservationAdditive ingredientPreservative
The invention belongs to the technical field of fruit fresh keeping and particularly discloses a mango preservative and a preparation method thereof. The mango preservative is prepared from the following raw materials including oxalic acid, ferulic acid, difenoconazole, phenylalanine, tryptophan, magnoflorine and a plant extracting solution; and the plant extracting solution is prepared from the following raw materials including flos caryophylli, spica prunellae, cortex cinnamomi, flos inulae and sisal hemp. Through mutual effect of various components, the mango preservative effectively improves the rate of intact fruit of mango storage, lowers the weight loss ratio, prolongs the fresh keeping time, and is good in fresh keeping effect.
Owner:广西田阳县创新农业综合开发有限公司
Cortex phellodendron chinese identification method and application thereof
InactiveCN109507312ACharacterizing qualityAvoid one-sidednessComponent separationPrincipal component analysisMedicine
The invention discloses a cortex phellodendron chinese identification method. According to the identification method, cortex phellodendron chinese is used for preparing a solution of a test product, solutions in which a berberine hydrochloride reference substance, a phellodendrine chloride reference substance and a magnoflorine reference substance are dissolved are used as reference substance solutions, and through high performance liquid chromatograph measurement, liquid chromatograms of the solution of the test product and the reference substance solutions are obtained respectively; the obtained liquid chromatograms are guided into a traditional Chinese medicine chromatograph fingerprint similarity evaluation system for analysis, through analysis, a cortex phellodendron chinese fingerprint is obtained, and hierarchical cluster analysis or principal component analysis or partial least square analysis is adopted for processing data of the cortex phellodendron chinese fingerprint, so that classification identification is conducted on the cortex phellodendron chinese or processed products of the cortex phellodendron chinese. According to the cortex phellodendron chinese identification method, on the basis of building the cortex phellodendron chinese fingerprint, the cortex phellodendron chinese index component content is measured, and by combining different chemical mode identification methods, the cortex phellodendron chinese, the processed products of the cortex phellodendron chinese and fake cortex phellodendri amurensis can be effectively identified and distinguished.
Owner:YANGTZE RIVER PHARM GRP CO LTD +1
UPLC specific chromatogram establishing method and detection method of radix semiaquilegiae medicinal material
ActiveCN111077245AQuality fully reflectsStrong specificityComponent separationMedicinal herbsPhosphoric acid
The invention relates to a UPLC specific chromatogram establishment method and a detection method of a radix semiaquilegiae medicinal material. The establishment method comprises the following steps:preparing a reference substance solution: taking a griffonilide reference substance, a lithospermoside reference substance and a magnoflorine reference substance, and adding a solvent for dissolutionto obtain a reference substance solution; preparing a test solution: taking a radix semiaquilegiae medicinal material powder, adding the solvent for extraction, filtering an extracting solution, and taking subsequent filtrate to obtain the test solution; and injecting the reference substance solution and the test solution into an ultra-high performance liquid chromatograph for detection, wherein amobile phase A adopted by the ultra-high performance liquid chromatograph is methanol, a mobile phase B adopted by the ultra-high performance liquid chromatograph is a phosphoric acid aqueous solution with a volume fraction of 0.03%-0.07%, and an elution mode is gradient elution. The establishing method can be used to obtain the specific chromatogram of the radix semiaquilegiae medicinal material, wherein 12 characteristic peaks are included. Besides, contents of griffonilide and magnoflorine in the radix semiaquilegiae medicinal material can be measured at the same time, the quality of the radix semiaquilegiae medicinal material can be comprehensively reflected, and the method is accurate, reliable, easy and convenient to operate and good in reproducibility.
Owner:GUANGDONG YIFANG PHARMA
GPR35 receptor novel agonist and application thereof
The invention relates to a targeting active molecule in a natural product and an application thereof, and in particular relates to a GPR35 receptor new agonist in the natural product and an application thereof. The GPR35 receptor new agonist comprises the active ingredients of one or more than two of compounds of rheinic acid, quercetin, 7-demethylated gingko biflavone, prohypericin, baicalin, tetrahydrodimethoxydibenzoquinoline diol, aloe-emodin, norbodine, daidzein, tanshinone IIA, sweet clover, magnoflorine, cryptotanshinone and isofulvic alcohol and corresponding pharmaceutically acceptable salt forming compounds. In-vitro cell experiments show that natural compounds included in the GPR35 receptor new agonist have an agonistic effect on the GPR35 receptor. At present, researches show that the GPR35 receptor is closely related to asthma, heart failure, hypertension, inflammation, coronary heart disease, metabolic syndrome, pain, cancer and other diseases. The GPR35 receptor novel agonist provides candidate compounds for drug development of the related diseases.
Owner:TAIZHOU GUOKEHUAWU BIOMEDICAL TECH CO LTD
Low-damage dyed wig rinsing technology
InactiveCN109537262AAccelerated aging rateImprove delamination effectNon-surface-active detergent compositionsDetergent mixture composition preparationOxalateUltraviolet lights
The invention discloses a low-damage dyed wig rinsing technology. The low-damage dyed wig rinsing technology comprises the following steps: (1), adding anhydrous ethanol and the like into deionized water, mixing, suspending a dyed wig into a sealed aging chamber, atomizing a mixed solution, introducing into the aging chamber for aging treatment, taking out, then irradiating by ultraviolet light toobtain an aged wig; (2), adding oxalic acid and the like into the deionized water, mixing, immersing the aged wig therein for oscillating treatment, taking out, then immersing in a magnoflorine solution to obtain an acidified and alkalized wig, and suspending in a reaction kettle, and inflating ozone for heating treatment to obtain an oxidized wig; (3), immersing the wig in gasoline for cleaningto obtained a cleaned wig; (4), immersing the cleaned wig into a plant rinsing liquid, and placing in a temperature-variable box for temperature-variable treatment to obtain a rinsed wig; (5), cleaning the rinsed wig by using an ethanol solution with the concentration of 50% to obtain a restored wig.
Owner:太和县瑞丝源发制品有限公司
Magnoflorine preparations and preparation methods and application
ActiveCN108478575AImprove permeabilityImprove bioavailabilityOrganic active ingredientsNervous disorderDiseaseOrganic solvent
The invention discloses magnoflorine preparations and preparation methods and application. One magnoflorine preparation is magnoflorine phospholipid complex, and the other magnoflorine preparation isnasal magnoflorine phospholipid complex-temperature sensitive in-situ gel. By the preparation methods, the characteristics of a good separating effect, large preparation amount, low loss and simple and convenient operation are achieved. In the related magnoflorine phospholipid complex, the mass ratio of magnoflorine to phospholipid is (1-4):(2-1), and the related preparation methods are achieved by dissolving and mixing components in an organic solvent according to a formula ratio and preparing at a proper temperature. The obtained magnoflorine phospholipid complex is then added into the temperature sensitive in-situ gel as an auxiliary material to obtain the magnoflorine phospholipid complex-temperature sensitive in-situ gel. Animal pharmacodynamic experimental studies show that the magnoflorine and two preparations thereof have significant improving and therapeutic effects on depression and insomnia.
Owner:TIANJIN MEDICAL UNIV
Process for extracting and purifying magnoflorine from garden columbine family plant
InactiveCN102001998AReduce the amount of extractionAvoid damageOrganic chemistryEthyl acetateMacroporous resin
The invention relates to a process for extracting and purifying magnoflorine from a garden columbine family plant. The process comprises the steps of pulverizing a raw material, decocting in acid water, concentrating by a film, adjusting pH by alkali, extracting by ethyl acetate, carrying out macroporous resin-magnesium oxide mixed column chromatography and recrystallizing by normal hexane-acetone and drying to obtain a finished product. The method for producing the magnoflorine has the advantages of short period and low energy consumption and large preparation amount and is suitable for mass production.
Owner:NANJING ZELANG AGRI DEV
A method for constructing uplc characteristic map of magnolia formula granules and its component content determination
ActiveCN111089930BFully display the characteristics of chemical compositionRealize qualitative analysisComponent separationFormularyChemical composition
The invention relates to a method for constructing UPLC characteristic map of magnolia formula granules and determination of its component content. The method for establishing the UPLC characteristic map of magnolia formula granules comprises the following steps: (1) Precisely weighing magnolia formula granules to prepare magnolia formula granules The test solution; (2) The magnolia formula granule test solution was analyzed by ultra-high performance liquid chromatography, and the UPLC characteristic spectrum of the magnolia formula granule was obtained. The characteristic map of magnolia formula granules constructed by the present invention fully demonstrates the chemical composition characteristics of magnolia formula granules. The characteristic map constructed by the present invention comprehensively reflects the characteristic peak information of samples, and the method is stable, with high precision and good reproducibility The present invention controls the quality of magnolia formula granules from three angles of UPLC characteristic map, magnolanin content determination and magnolia alkali content determination, and can more effectively evaluate the quality of magnolia formula granules.
Owner:GUANGDONG YIFANG PHARMA
Pharmaceutical composition for preventing and treating rheumatoid arthritis
The invention discloses a pharmaceutical composition for preventing and treating rheumatoid arthritis. The traditional Chinese medicine raw materials for constituting active ingredients of the pharmaceutical composition by weight are as follows: 5.5-6.0mg of berberine, 0.3-0.4mg of palmatine, 1.5-2.0mg of jatrorrhizine, 1.5-1.8mg of magnoflorine, 0.1-0.2mg of phellodendrine, 0.2-0.3mg of coptisine, 55-60mg of baicalin, 1.5-2.0mg of baicalein, 5.0-7.0mg of wogonoside, 0.03-0.05mg of wogonin, 20-25mg of geniposide, 0.1-0.2mg of crocin and 0.25-0.3mg of chlorogenic acid. The results of pharmacological tests show that the pharmaceutical composition can significantly reduce the disease severity of CIA mice with the rheumatoid arthritis, significantly improve the inflammation and the immune injury situation at lesion sites in comparison with a control group and be used for preparing drugs for preventing and treating the rheumatoid arthritis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Pharmaceutical composition used for treating small cell carcinoma
InactiveCN103948627AHeavy metal active ingredientsOrganic active ingredientsSmall-cell carcinomaPuerarin
The invention belongs to the field of medicines, and relates to a composition for inhibiting cancer cell proliferation. The invention provides a pharmaceutical composition for treating lung cancer, especially small cell carcinoma, and particularly relates to a pharmaceutical composition consisting of cis-platinum, magnoflorine and puerarin in a weight ratio of 10:(0.5-5):(0.5-5), preferably 10:(1.5-3.5):(0.5-1.5), and more preferably 10:2:1.
Owner:QINGDAO CENT HOSPITAL
Method for constructing characteristic chromatogram of caulis sinomenii
ActiveCN114858938AFully reflectEfficient methodComponent separationAgainst vector-borne diseasesMedicinal herbsFormulary
The invention discloses a method for constructing a characteristic chromatogram of caulis sinomenii, which is characterized in that sinomenine, magnoflorine and clove resinol diglucoside are taken as characteristic components, common peaks of different original caulis sinomenii samples are confirmed by an efficient detection method, and the characteristic chromatogram is constructed. According to the method, samples with different basic sources can be distinguished, and alkaloid and lignan contained in the caulis sinomenii are taken as representative components, so that the product quality can be relatively comprehensively reflected; the method is suitable for rapid and comprehensive quality control of caulis sinomenii medicinal materials, decoction pieces, extracts, standard decoction, formula granules and standard samples, preparation intermediate products and finished products of classic famous prescriptions containing caulis sinomenii, and is efficient, convenient to operate and suitable for quality monitoring of continuous production.
Owner:JIANGYIN TIANJIANG PHARMA
Pharmaceutical composition and application thereof in preparation of anti-osteoporosis drugs
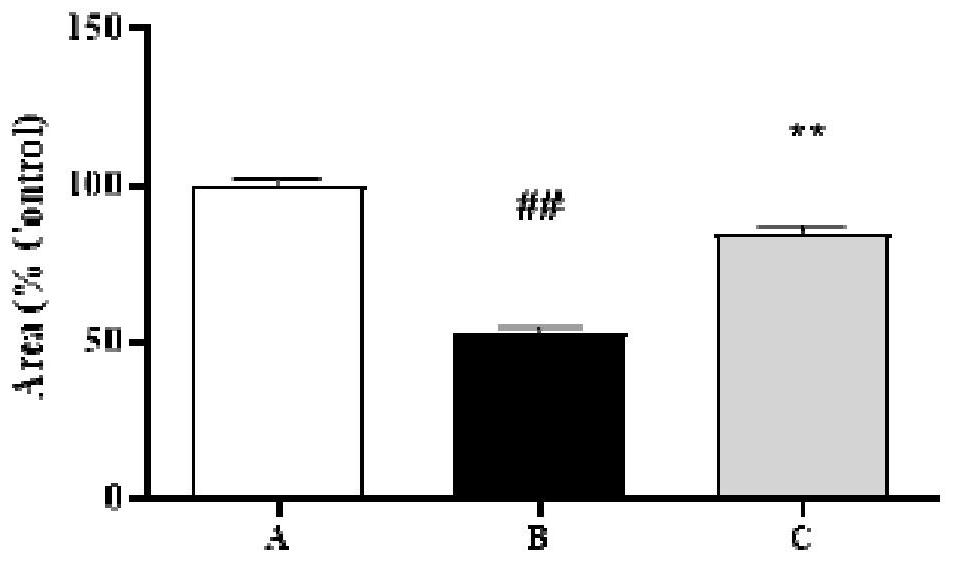
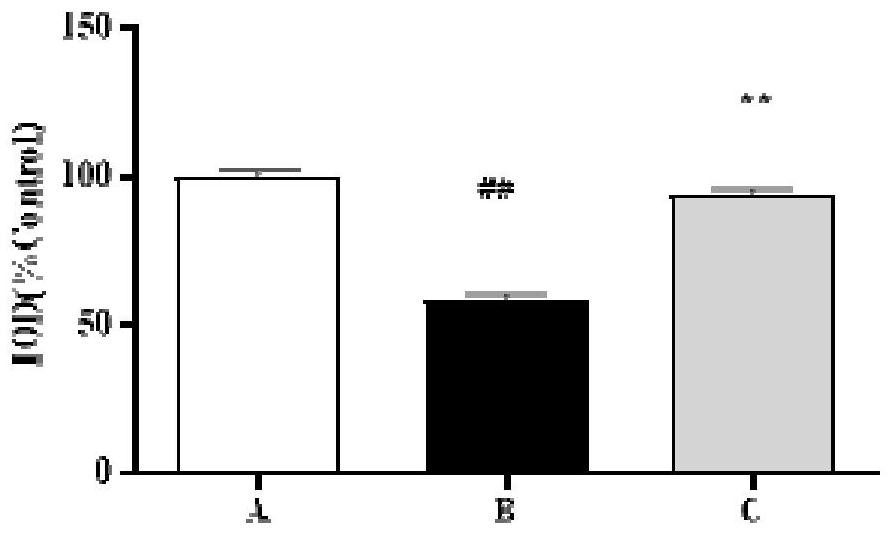
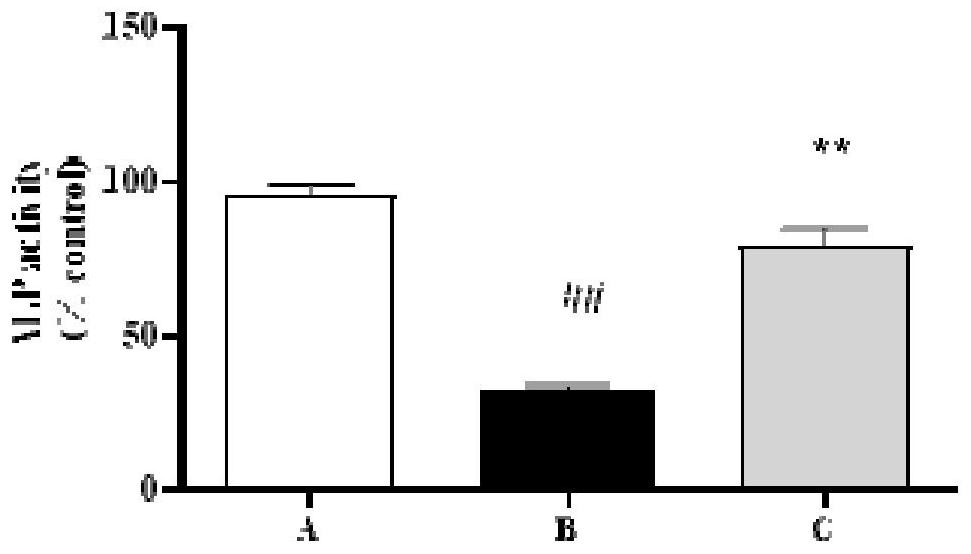
ActiveCN114288296ALower blood sugarEasy to useOrganic active ingredientsNervous disorderSide effectAnti osteoporosis
The invention discloses a pharmaceutical composition and application thereof in preparation of anti-osteoporosis drugs. The pharmaceutical composition is prepared from the following components in parts by mass: 7.64 to 40.90 parts of mangiferin, 4.26 to 7.39 parts of tetrahydroepiberberine, 24.33 to 35.31 parts of phellodendrine, 10.11 to 14.64 parts of magnoflorine, 2.30 to 4.26 parts of 13-hydroxyl oxidized berberine, 0.12 to 0.29 part of palmatine and 17.96 to 30.71 parts of berberine. The pharmaceutical composition not only can significantly reduce blood sugar, but also can significantly improve the skull area and skull optical density of the zebra fish with diabetic osteoporosis; the activity of alkaline phosphatase is improved, and bone formation related gene expression is promoted; the activity of tartrate-resistant acid phosphatase is reduced, and the expression of bone resorption related genes is reduced; the traditional Chinese medicine composition is safe and reliable to use, and has a good research and development prospect of anti-osteoporosis medicines.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com