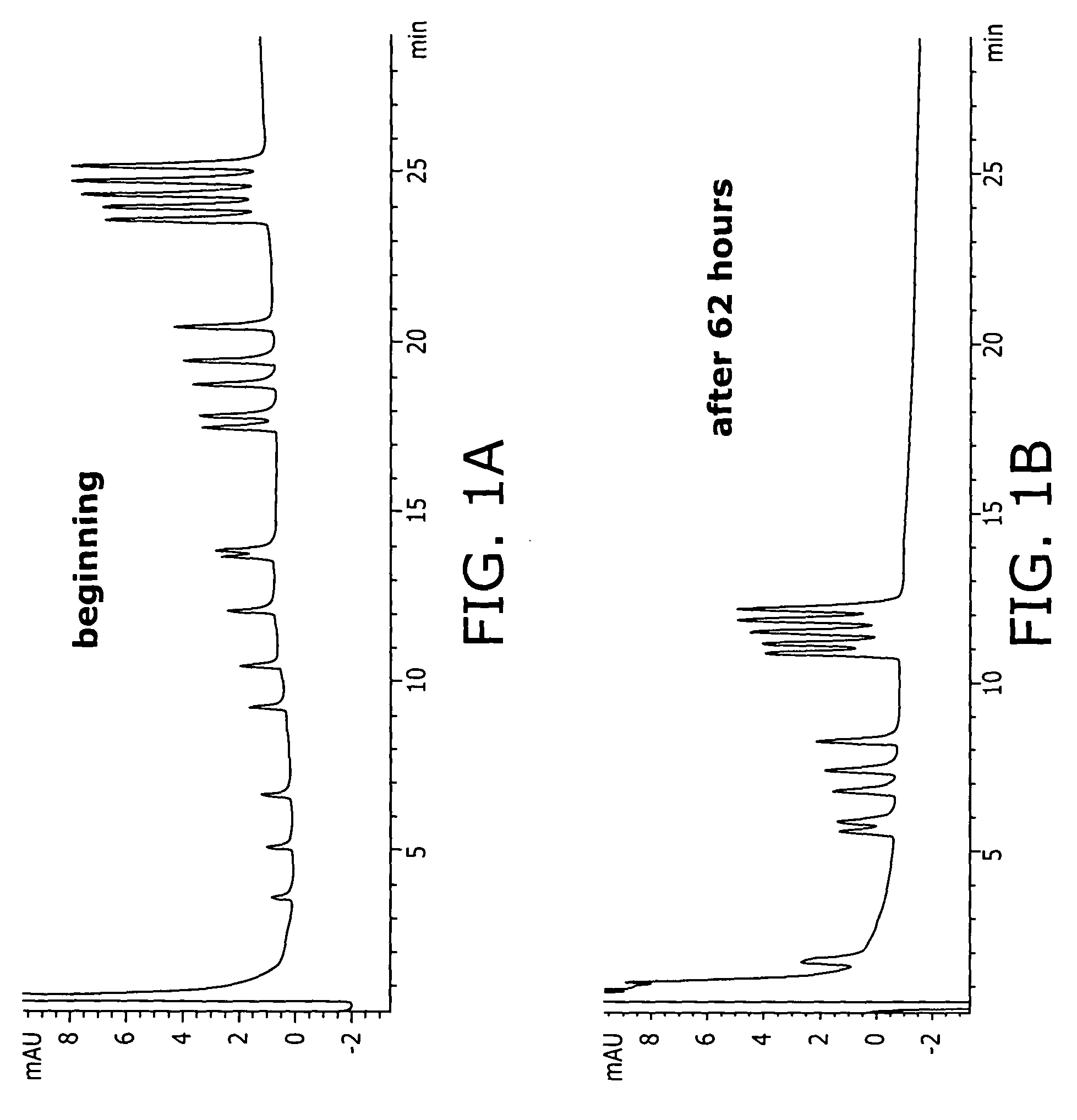
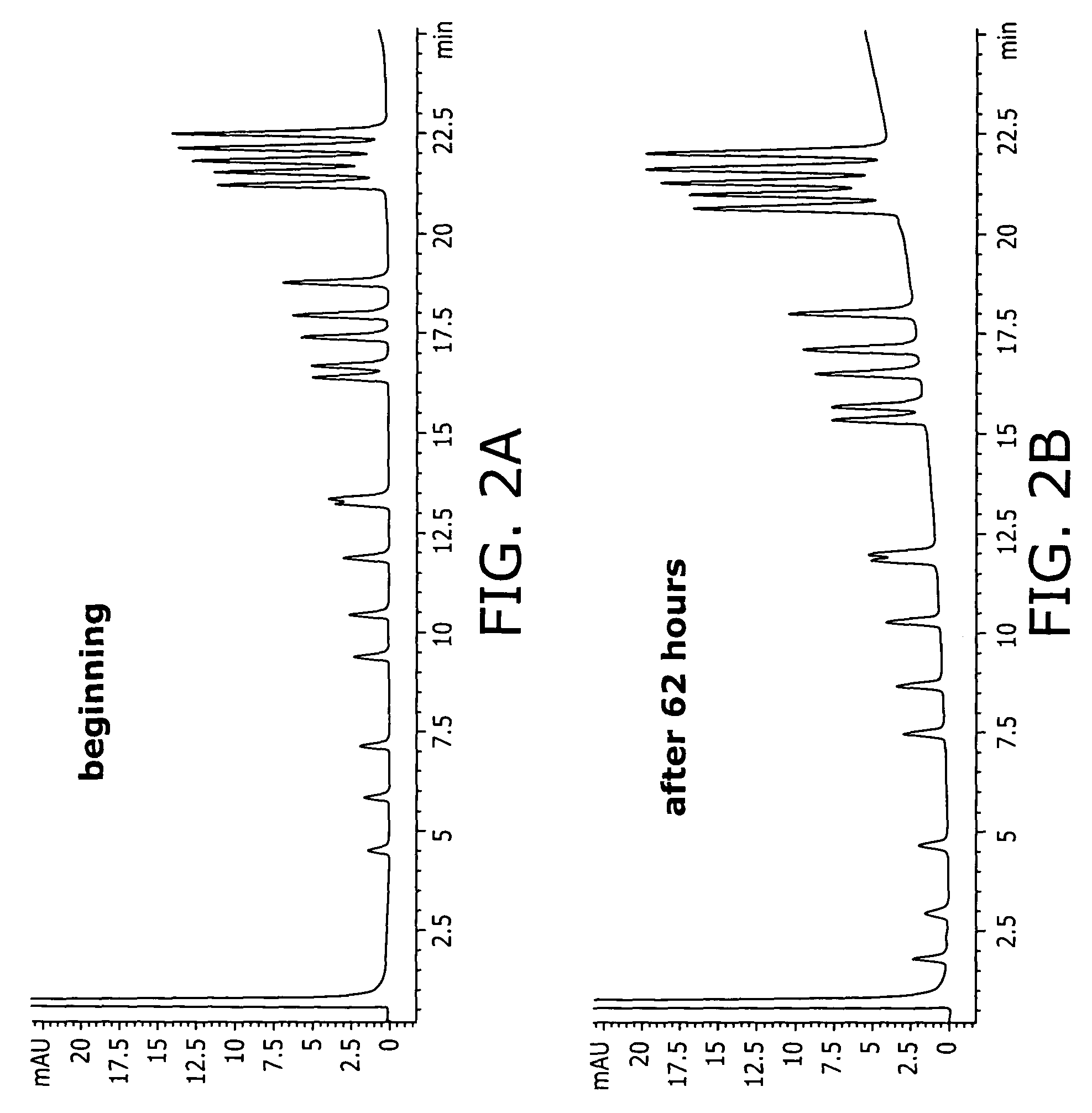
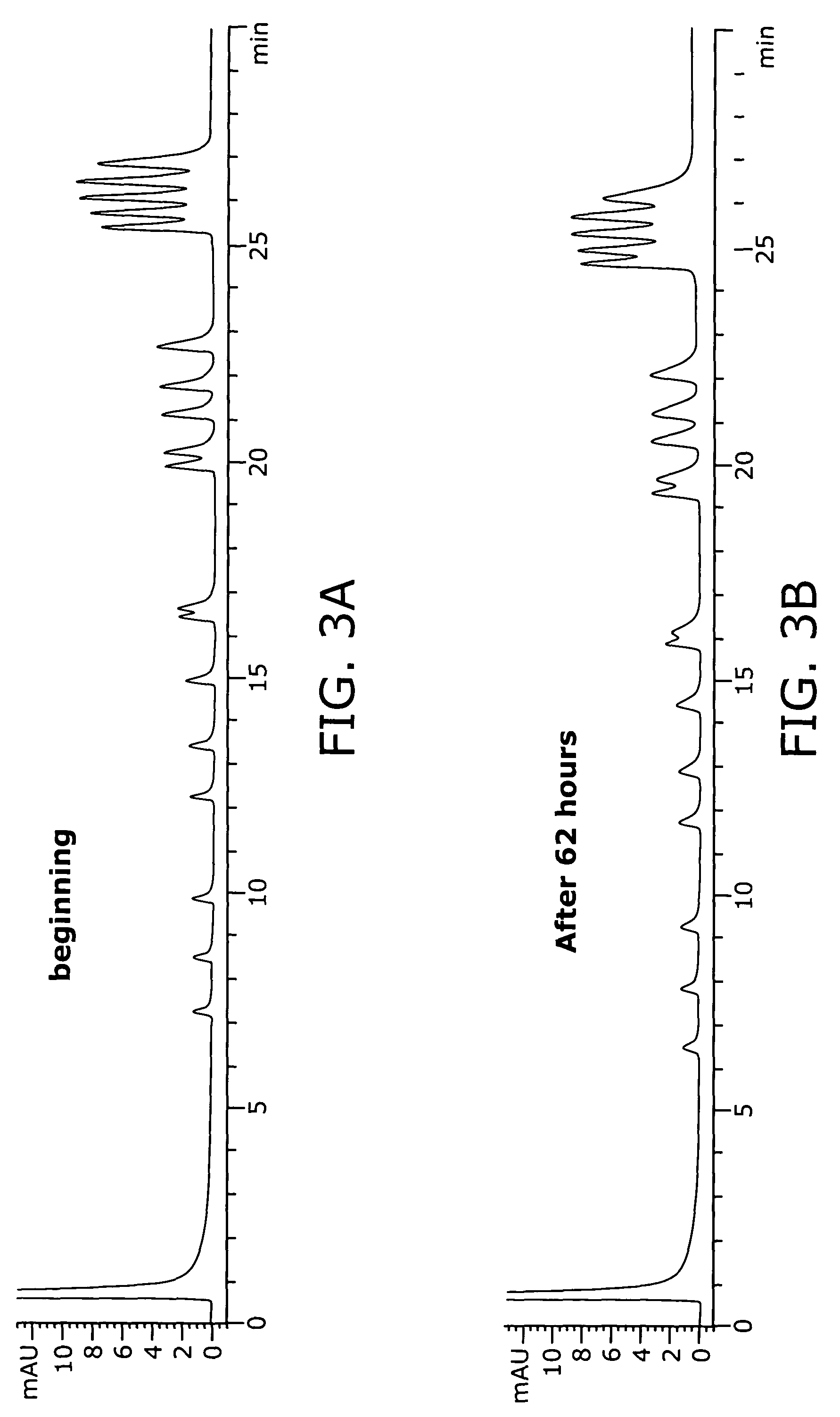
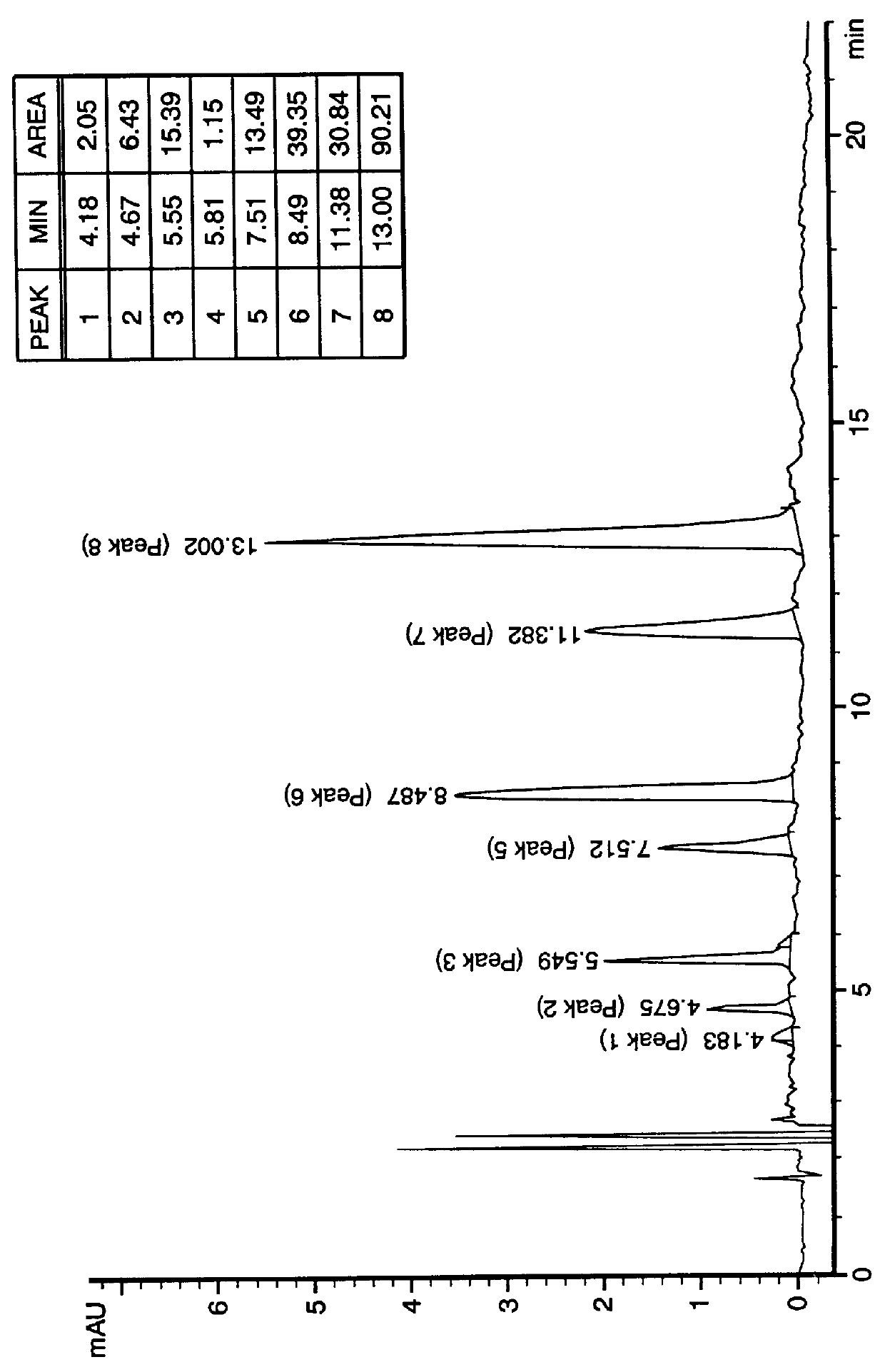
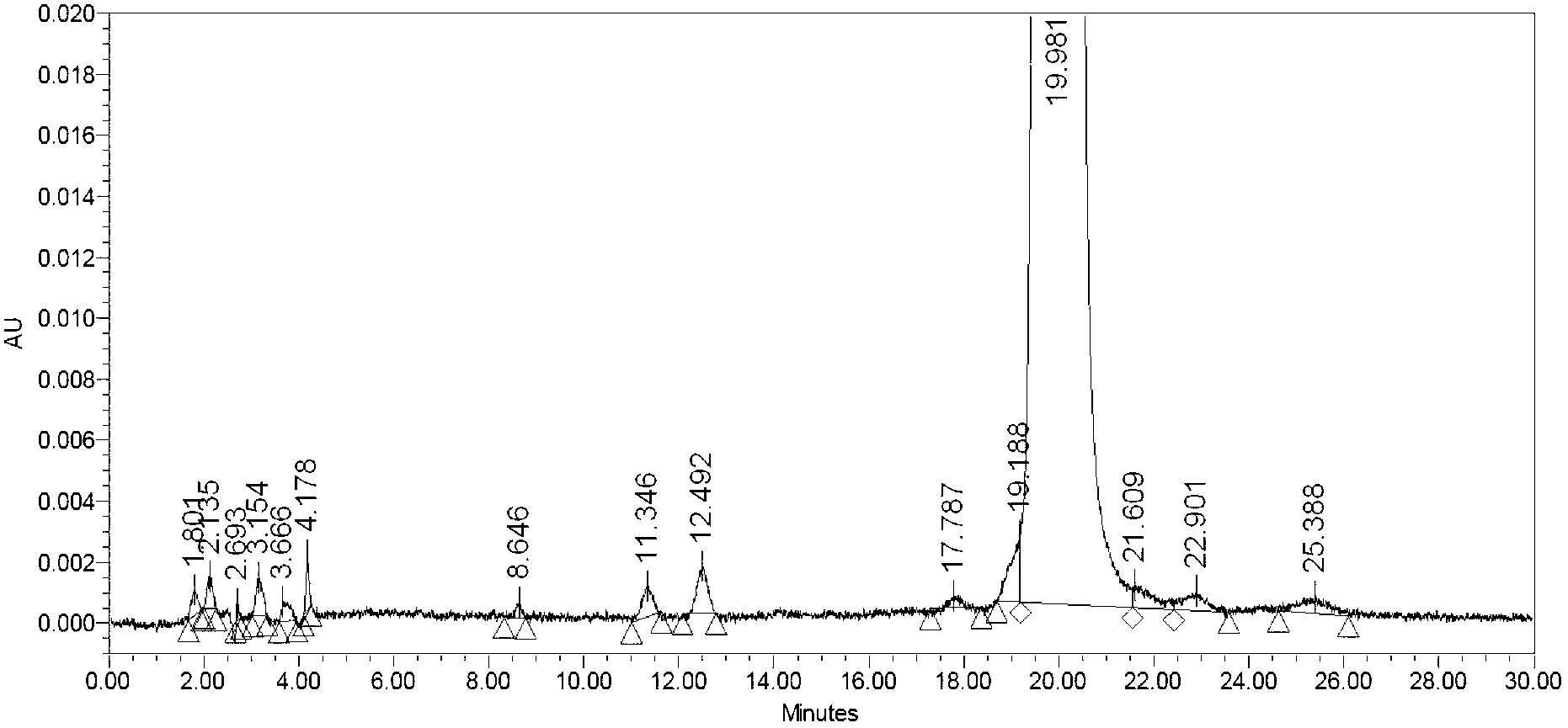
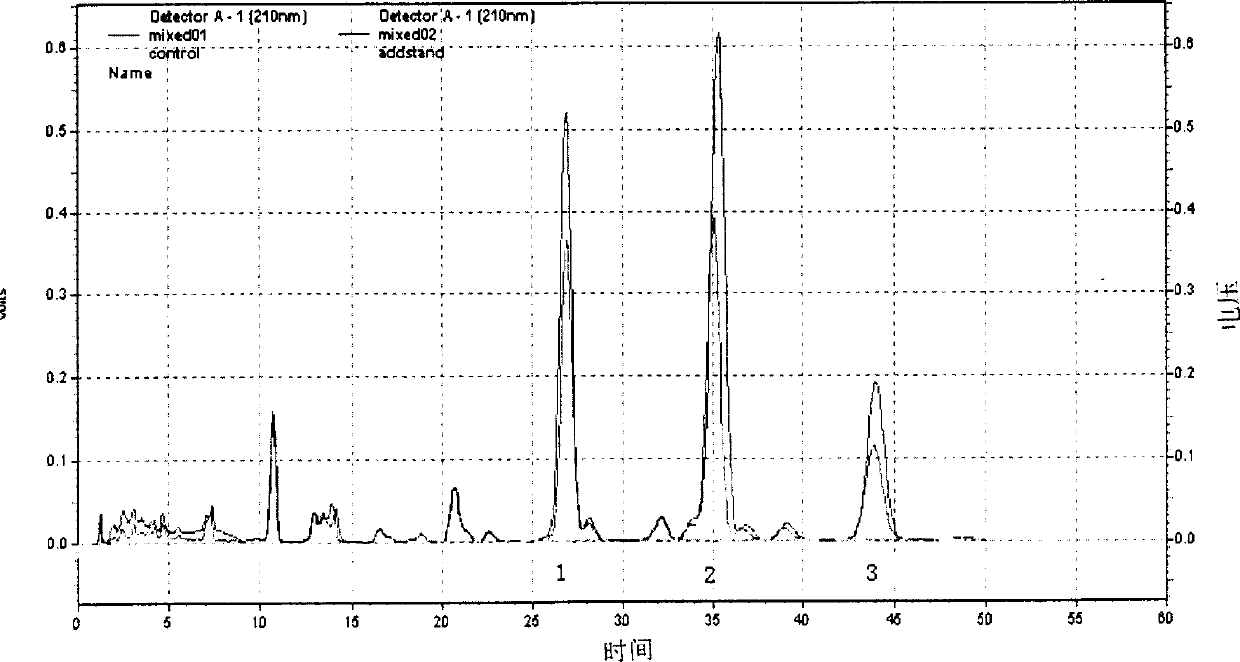
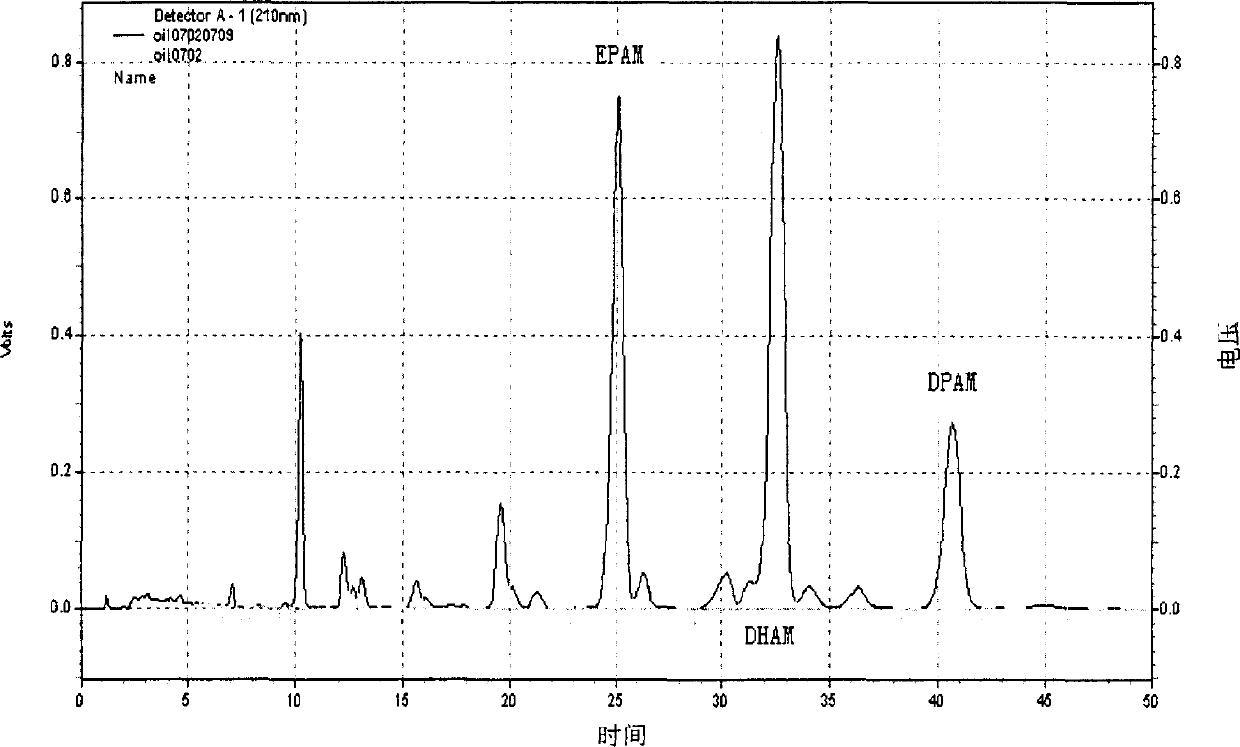
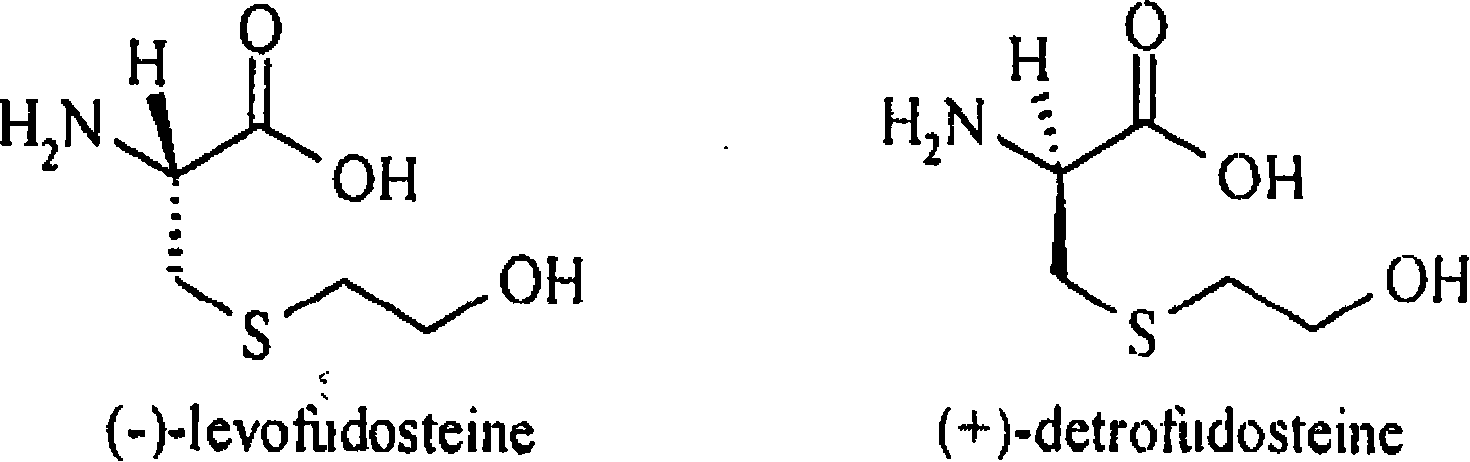Patents
Literature
639 results about "Hplc method" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
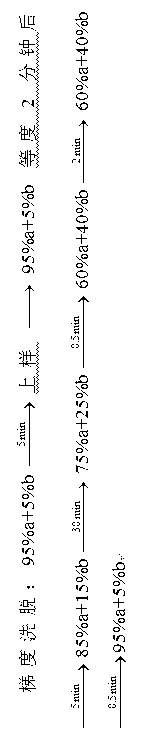
The process is influenced by the nature of the analytes and generally follows the following steps: step 1 - selection of the HPLC method and initial system. step 2 - selection of initial conditions. step 3 - selectivity optimization. step 4 - system optimization.
Additives for reversed-phase HPLC mobile phases
InactiveUS20050011836A1Efficient separationExtended column lifetimeIon-exchange process apparatusComponent separationNatural productAnalyte
The present invention provides silica-based reversed-phase HPLC methods that lead to higher retention of the analytes in the column and longer column lifetimes than usually observed under medium to high pH aqueous mobile phase conditions. The inventive methods comprise eluting the HPLC column using an aqueous mobile phase comprising at least one fluorinated additive. Preferred additives are polyfluorinated alcohols such as 2,2,2-trifluoroethanol and 1,1,1,3,3,3-hexafluoroisopropanol. The methods of the present invention may be used for analyzing, separating, purifying, and / or isolating small organic molecules, natural products, as well as biomolecules such as polypeptides, oligonucleotides and polynucleotides (e.g., DNA fragments).
Owner:AGILENT TECH INC
In vivo stain composition, process of manufacture, and methods of use to identify dysplastic tissue
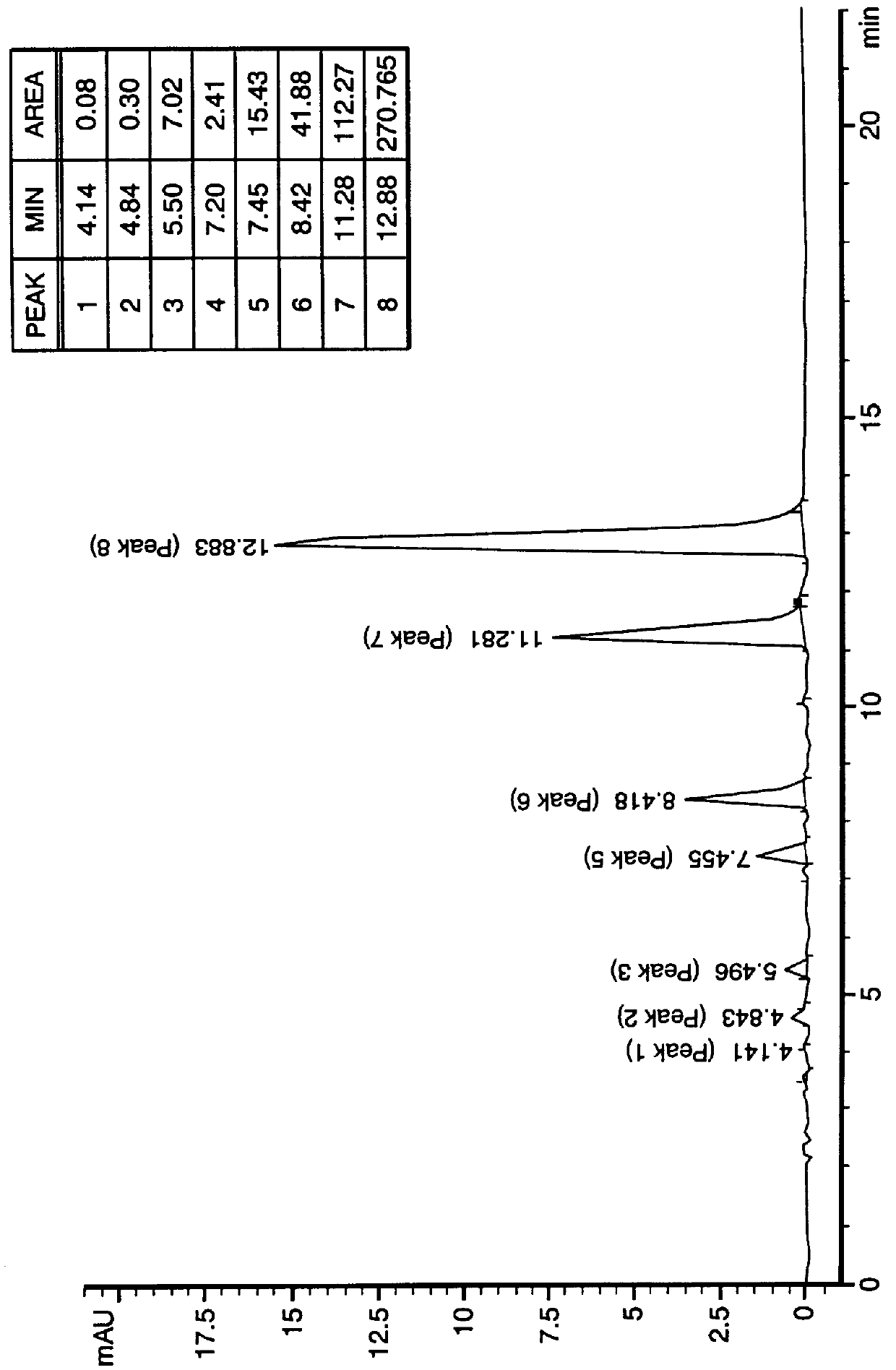
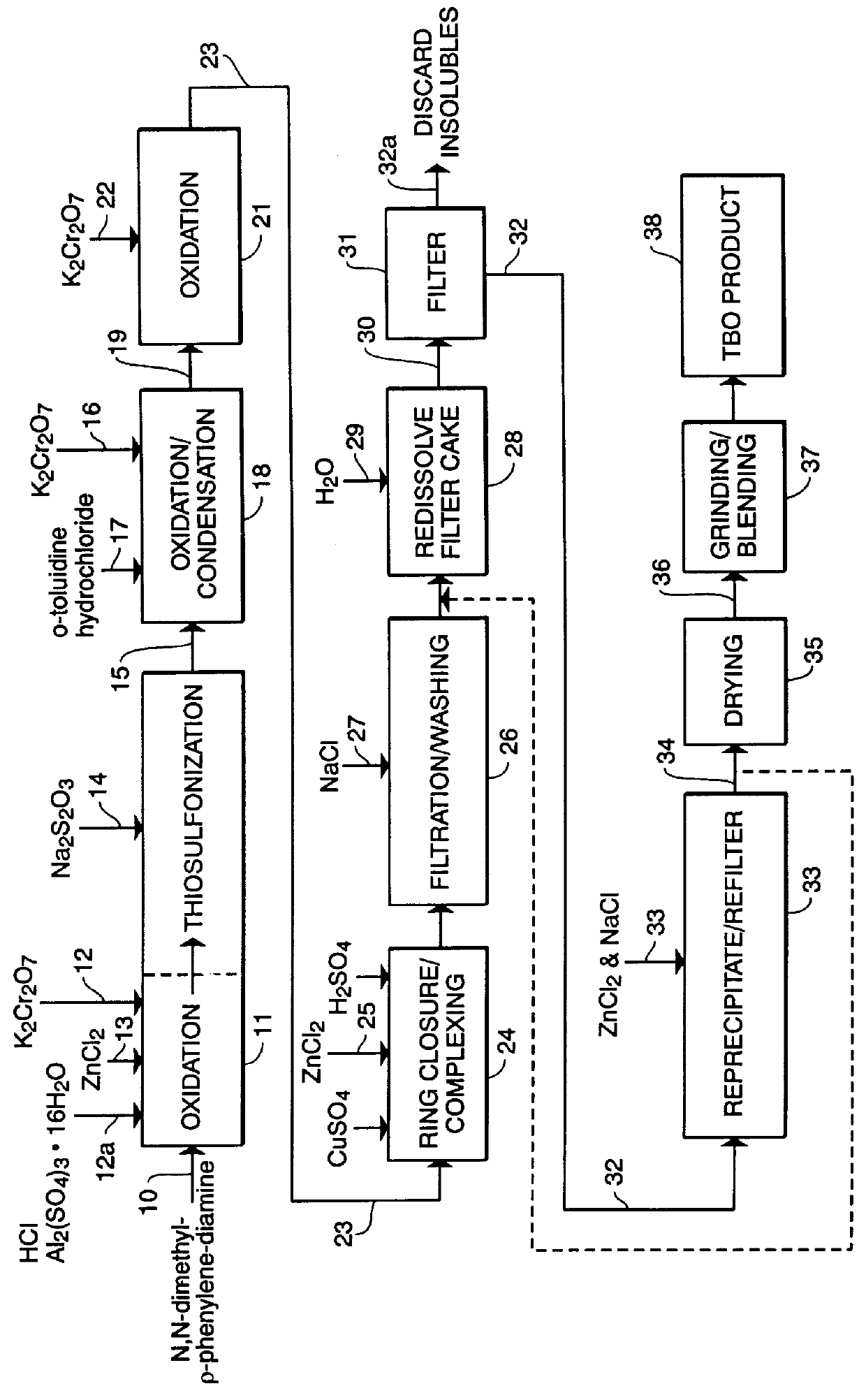
PCT No. PCT / US97 / 20981 Sec. 371 Date May 20, 1999 Sec. 102(e) Date May 20, 1999 PCT Filed Nov. 13, 1997 PCT Pub. No. WO99 / 25388 PCT Pub. Date May 27, 1999N-demethylated and N,N-demethylated derivatives of toluidine blue O and compositions which include these derivatives and the conformational isomers of toluidine blue O. Improved methods for the detection of dysplastic oral tissue using such compositions. Processes for synthesis of toluidine blue O products, in which a complexing agent is introduced prior to the last stage of oxidation of a three-step synthesis from N,N-dimethyl- rho -phenylenediamine. An HPLC method for characterizing toluidine blue O products in which the mobile phase is an aqueous solution of an organic acid.
Owner:DEN MAT HLDG
Triterpene saponin in camellia seeds, preparation method and medical use thereof
InactiveCN101392015AHigh yieldHigh purityOrganic active ingredientsMetabolism disorderHplc methodAdemetionine
The invention pertains to the field of medical technology and provides triterpenoid saponin in camellia seeds, and a preparation method and medical application thereof. The triterpenoid saponin is shown in a general formula (1), wherein, R1 is hydrogen or oxhydryl or acyloxy; R2 is the hydrogen or the acyloxy; R3 is the hydrogen or the acyloxy; R4 is the hydrogen or the acyloxy; R5 is methyl or methylol or aldehyde group or carboxyl or metheyl carboxyl; R6 is the hydrogen or orglycosyl; the preparation method comprises the following steps: macroporous resins are degreased, extracted by ethanol, decocted in water, desugared and decolored to obtain crude total saponins and then go though an opened ODS chromatographic column and repeated HPLC method to obtain the total saponins of camellia seeds and monomer theasaponin. The triterpenoid saponin compounds in the camellia seeds have the functions of protecting gastric mucosa, being antineoplastic, reducing blood sugar and blood fat and thelike and are used for preparing medicaments or health-care foods having the functions of protecting gastric mucosa, being antineoplastic and reducing blood sugar and blood fat.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of bamboo leaf flavone
InactiveCN101391060AReduce pollutionAchieve purificationFood preparationPlant ingredientsAlcoholHplc method
The invention discloses a preparing method for a bamboo leaves flavone extractive, which includes the following steps: breeds with high flavone content which are selected form 30 kinds of bamboo leaves can be taken as the production materials trough HPLC method; the selected bamboo leaves are dried at low temperature and lucifuge and smashed; and the C1-C3 is used for extracting; the lixivium is filtered and condensed in vacuum so as to obtain bamboo leaves gross extractive; and the gross extractive is purified through centrifugalizing, ceramic membrane, ultrafiltration membrane, nanofiltration membrane so as to obtain the bamboo leaves processed liquid; and the bamboo leaves processed liquid is separated and purified by certain size polymeric adsorbent resin, and is cooled to dry in vacuum or to dry with sprayer, so as to obtain the bamboo leaves flavone extractive with 40% to 65% of the flavone content.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
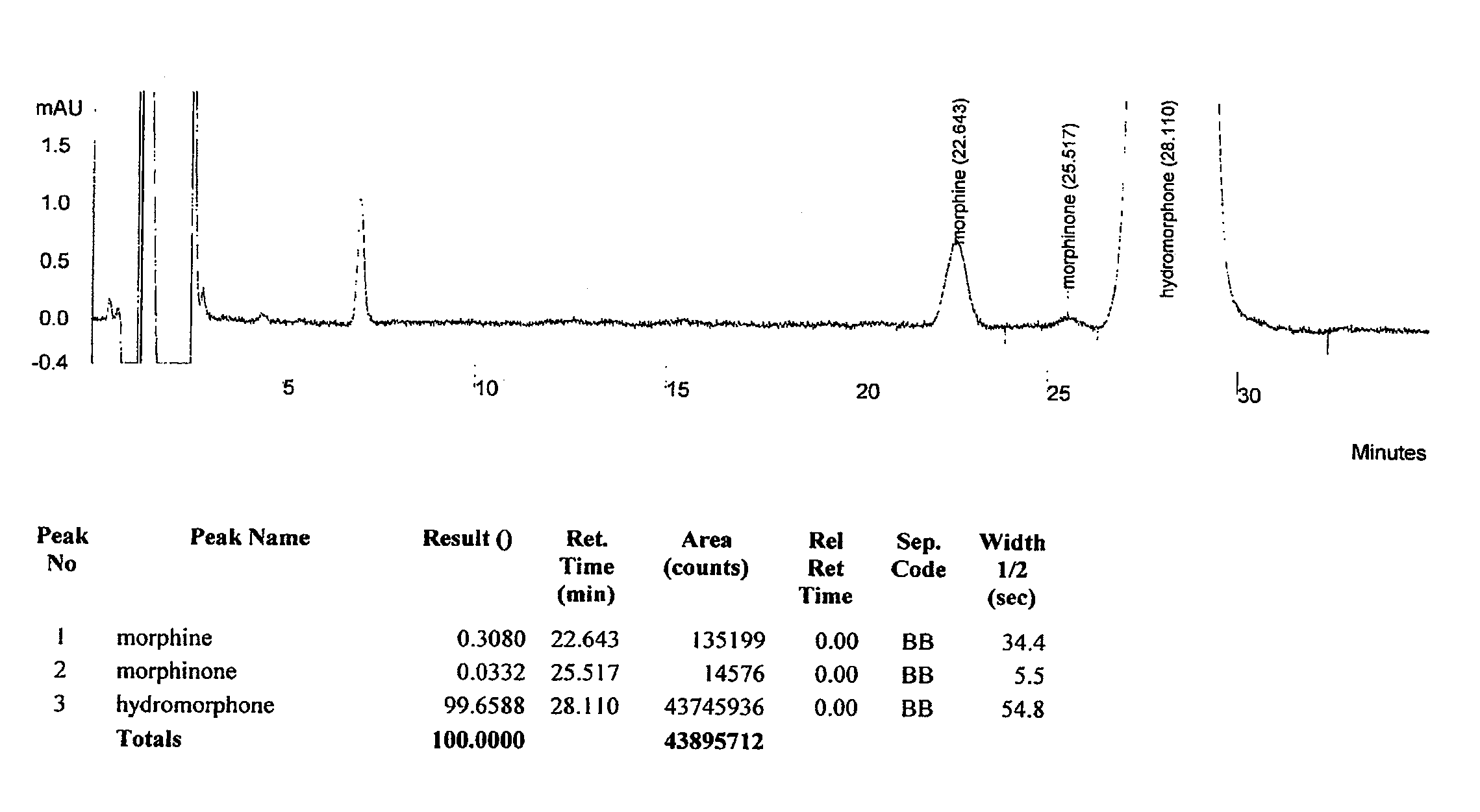
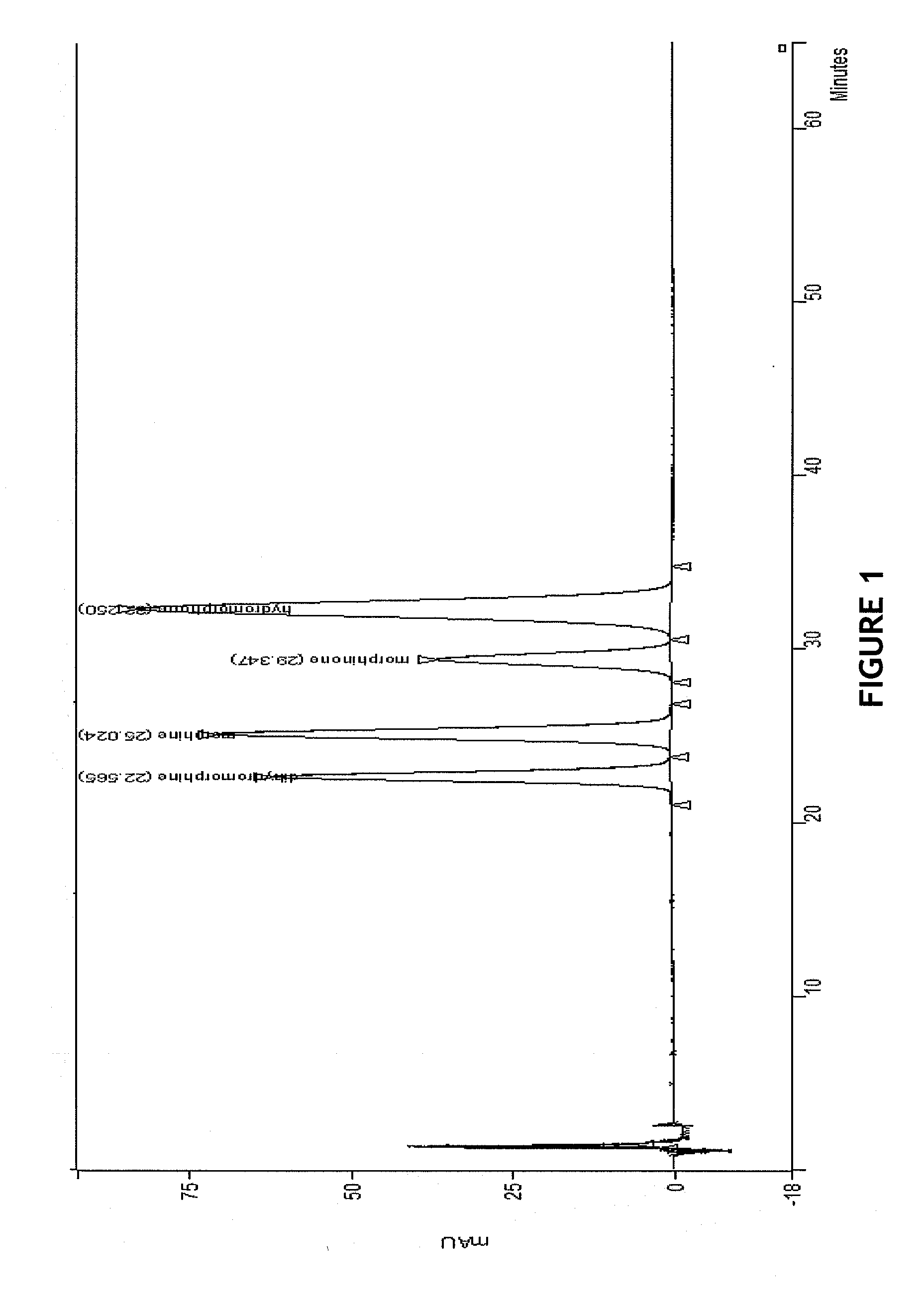
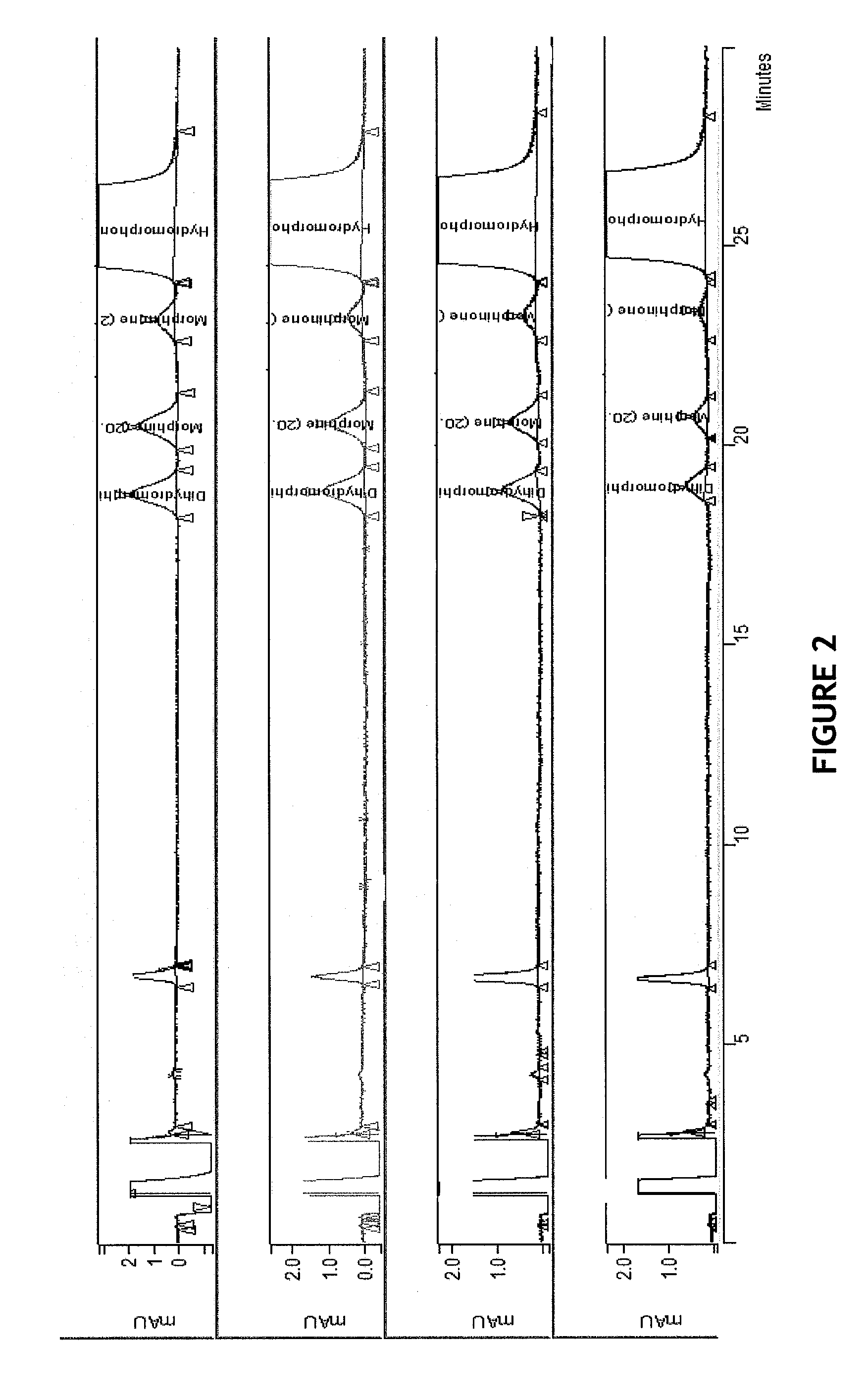
HPLC method for separation and detection of hydromorphone and related opioid pharmacophores
InactiveUS20080206883A1Component separationColor/spectral properties measurementsHplc methodPharmacophore
HPLC methods are provided to separate and detect morphinone, morphine, and dihydromorphine in the presence of hydromorphone. The isocratic HPLC methods employ ion-pair solute-solute ion-exchange mobile phase techniques in reversed phase chromatography. Method conditions in the disclosure provide separation and quantification of opioid pharmacophores in accordance with federal guidelines for obtaining resolution between analytes R≧2.0; tailing factor T≦2.0, capacity factor 2<k′≦50, and theoretical plate number N≧2000 for each opioid analyte peak.
Owner:CODY LAB
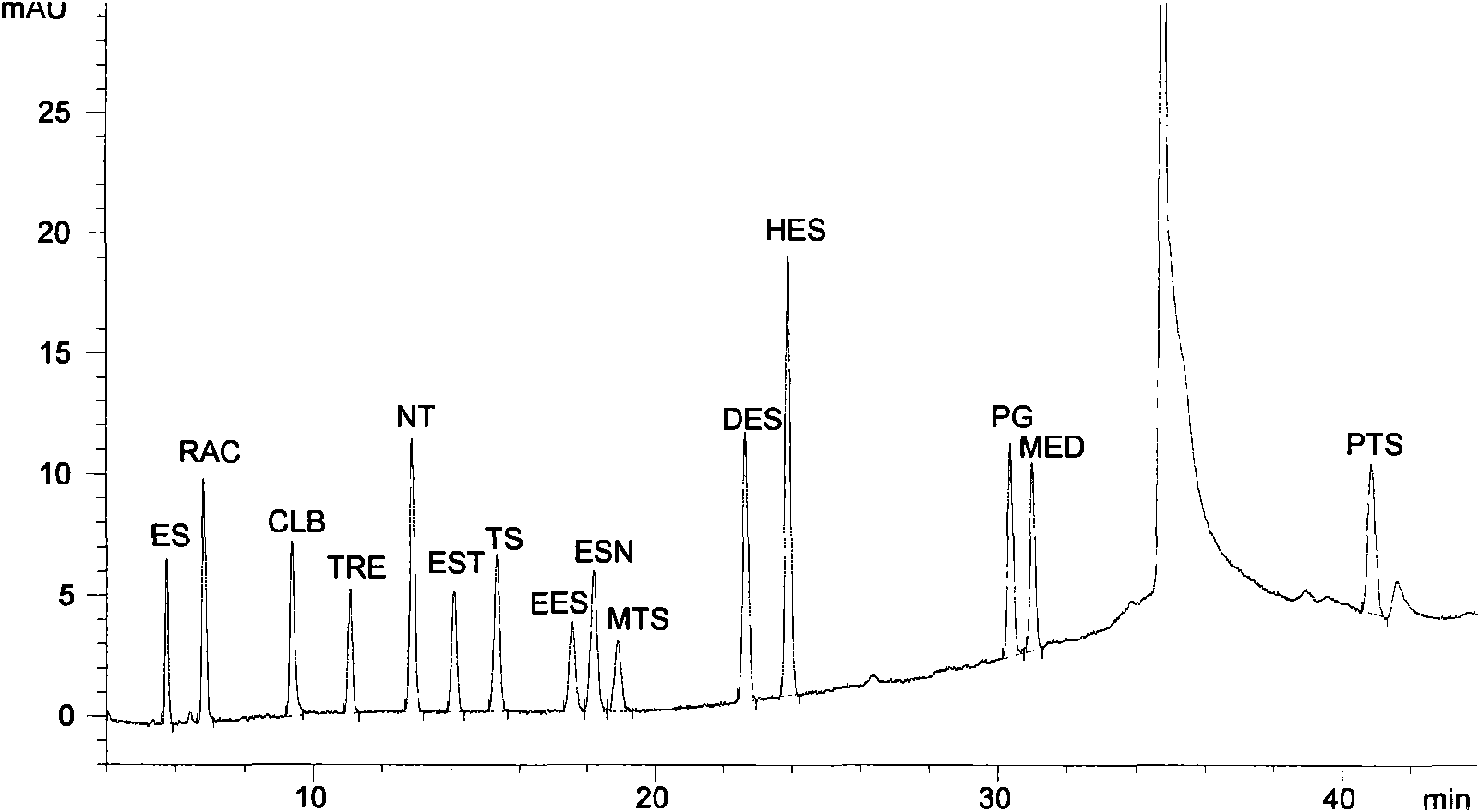
Liquid chromatography for synchronously detecting 15 anabolic hormone residues in food
ActiveCN101551362ALow toxicityLow costComponent separationPreparing sample for investigationWater bathsHplc method
The invention relates to a liquid chromatography for detecting anabolic hormone drug residue in animal-derived food, which is characterized by first sampling: animal musculature is taken off the fat and connective tissue, then minced and evenly ground, and the sample is weighed; extracting: anabolic hormone extract is extracted from the weighed sample by methanol ultrasonic extraction, the extract is evaporated to dryness in water bath through a rotary evaporator, and the residue is dissolved by methanol aqueous solution; purifying: C18 Solidoid extraction column on the extract is carried out solid phase extraction; and testing by devices: the filtrate is tested by opposite phase high efficiency liquid chromatography, quantified by external reference method-peak area, and then carried out with binary gradient elution. In the invention, the detected sample is complex biological sample; the established method can complete one detection in about one hour and is simple and fast, with reliable sensitivity and low cost; the method can analyze more veterinary hormone drug species than the synchronous usage of the existing GC or HPLC methods with easier operation, and has lower cost than the existing GC / MS and LC / MS or LC / MS / MS methods with low solvent toxicity.
Owner:上海国矗生物科技有限公司
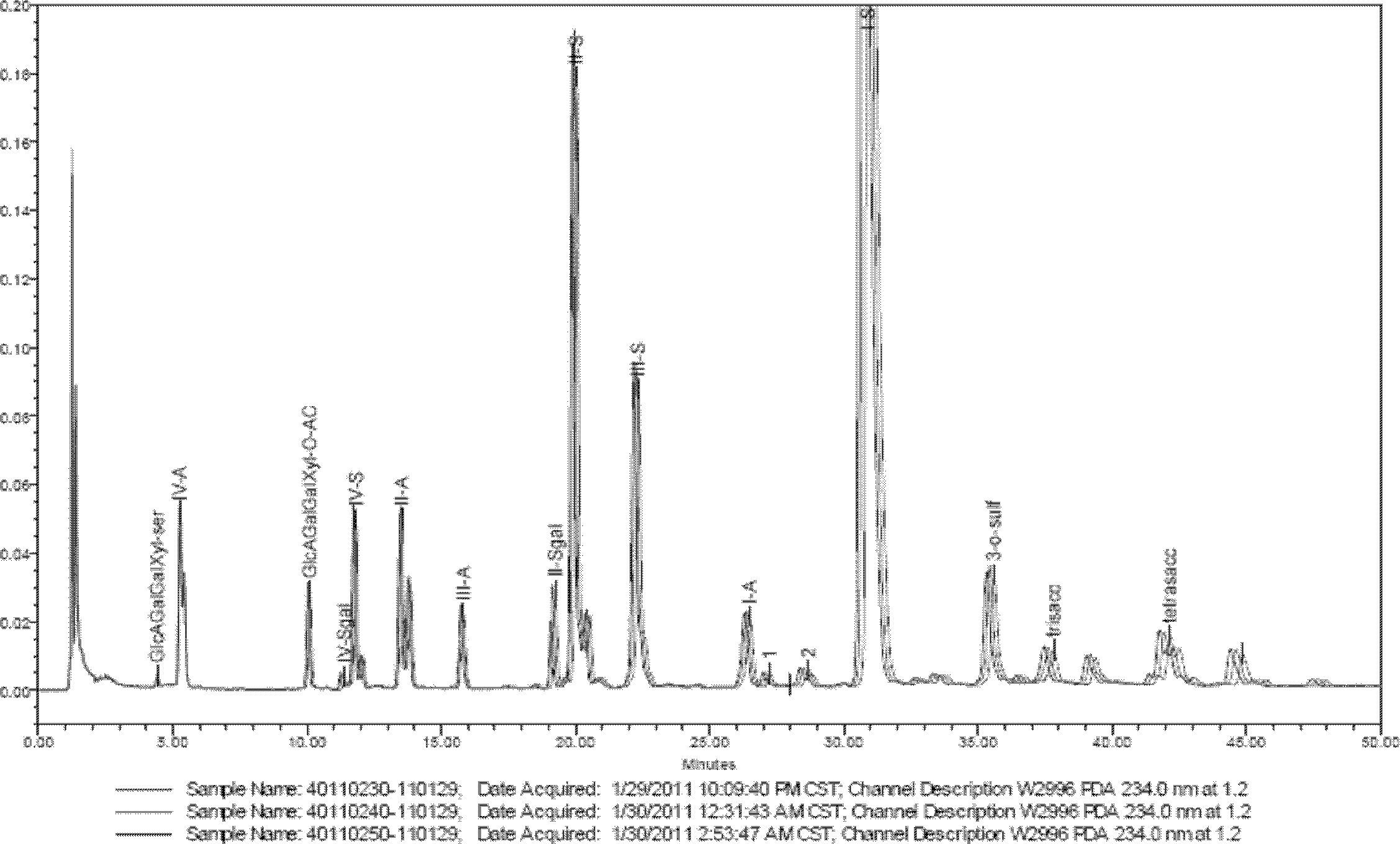
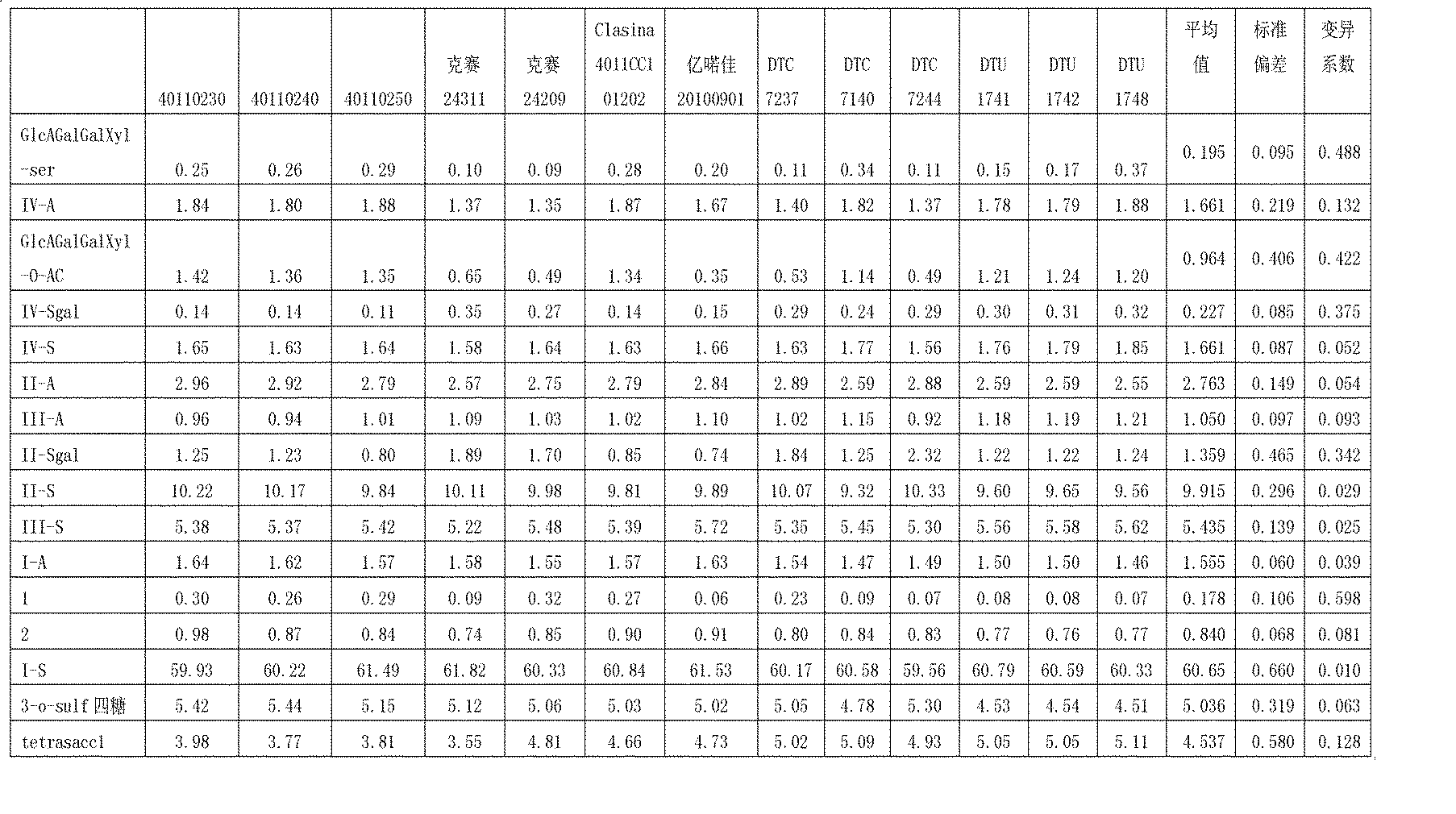
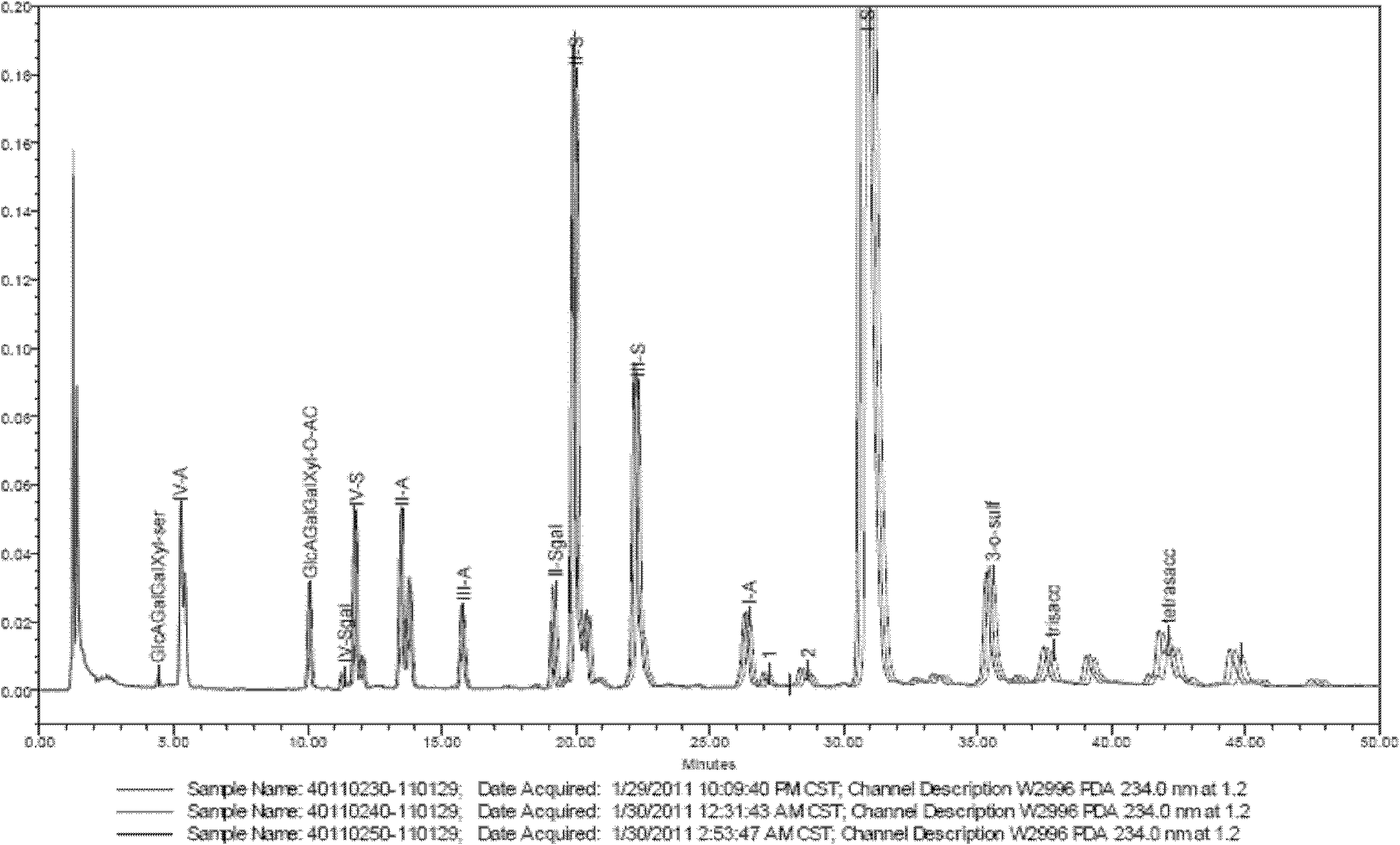
Enzymolysis-HPLC method for detecting enoxaparin
The invention discloses an enzymolysis-HPLC method for detecting enoxaparin. The method comprises steps of: a. complete enzymolysis of enoxaparin: adding a mixed enzyme solution with enzyme I, enzyme II and enzyme III in a ratio of 8:1:2 to an enoxaparin solution with a concentration of 10-200mg / ml and carrying out an enzymolysis at room temperature for 48 h. b. HPLC analysis: carrying out a SAX-HPLC analysis on the degradation products; c. calculation of variety and content of disaccharide and tetrose units: determining variety and content of disaccharide and tetrose units according to a wash out time of prior standard disaccharide and tetrose and calculating percentage content of each of the eight disaccharides and one tetrasaccharide.
Owner:SHENZHEN TECHDOW PHARM CO LTD
Asiaticoside and preparation thereof
The invention discloses an asiaticoside and a preparation method thereof. The Centella asiatica is adopted as the raw material and is extracted by water or low-carbon ethanol; the extract is separated and purified by column chromatography, the resin is decolored, and the extract is crystallized, recrystallized and dried and finally the extractive mainly composed of by the asiaticoside is obtained, wherein, the weight of the asiaticoside acounts for not less than 95 percent. The preparation method utilizes the modern biological extraction technology and adopts the technology processes of aqueous extract or ethanol extract, separation and purification by column chromatography, decoloring, crystallization and recrystallization to extract the asiaticoside from the hydrocotyle asiatica; the purity of the asiaticoside is improved by the resin column chromatography and recrystallization to lead the extractive contain more than 95 percent of the asiaticoside (determined by HPLC method); in addition, the technology process is simple, the production period is short, the consumption of organic solvent is less, and the production cost is low, thus being applicable to the commercial production.
Owner:卢照凯 +1
Method for extracting chimonin
ActiveCN1733785AAvoid lostHigh content of glycosidesSugar derivativesSugar derivatives preparationAlcoholHplc method
The invention discloses a method to extract chimonin by using mango leaves, almond leave or other plants contained chimonin as material, extracting with menstruum of water or low-carbon alcohols, condensing, separating to purify with pole chromatography, crystallizing and drying; wherein, the extract method comprises heating extract, ultrasonic extract, or microwave extract. The product had chimonin content more than 60% measured by HPLC method. This invention also avoids the loss to active composition, simplifies process, and cuts production cycle.
Owner:GUILIN NATURAL INGREDIENTS CORP
Method for quickly on-line detection of traditional Chinese medicine Kuhuang injection effective ingredient using near infra red spectrum
InactiveCN101078685AColor/spectral properties measurementsTesting medicinal preparationsReal time analysisHplc method
The invention discloses a method for measuring effective component content in kuhuang injection on line by near-infrared spectroscopy quickly. Effective components in kuhuang injection mainly comprise aloe-emodin, rhein, emodin, chrysophanol, physcion, sophocarpine and matrine so on. Near-infrared absorption spectroscopy collecting kuhuang injection make data of effective component content relate with near-infrared spectroscopy by HPLC method. Calibration model is built by partial least-squares regression method. Quick on-line measurement for effective component in injection can be accomplished. Studying result shows that near-infrared spectroscopy analyzing method can measure effective component in kuhuang injection effectively. The method is provided with simple pretreatment for sample, quick measurement and measuring multiple components at the same time. It can be used in on-line analysis and on-line quality control in tcm manufacture process.
Owner:常熟雷允上制药有限公司
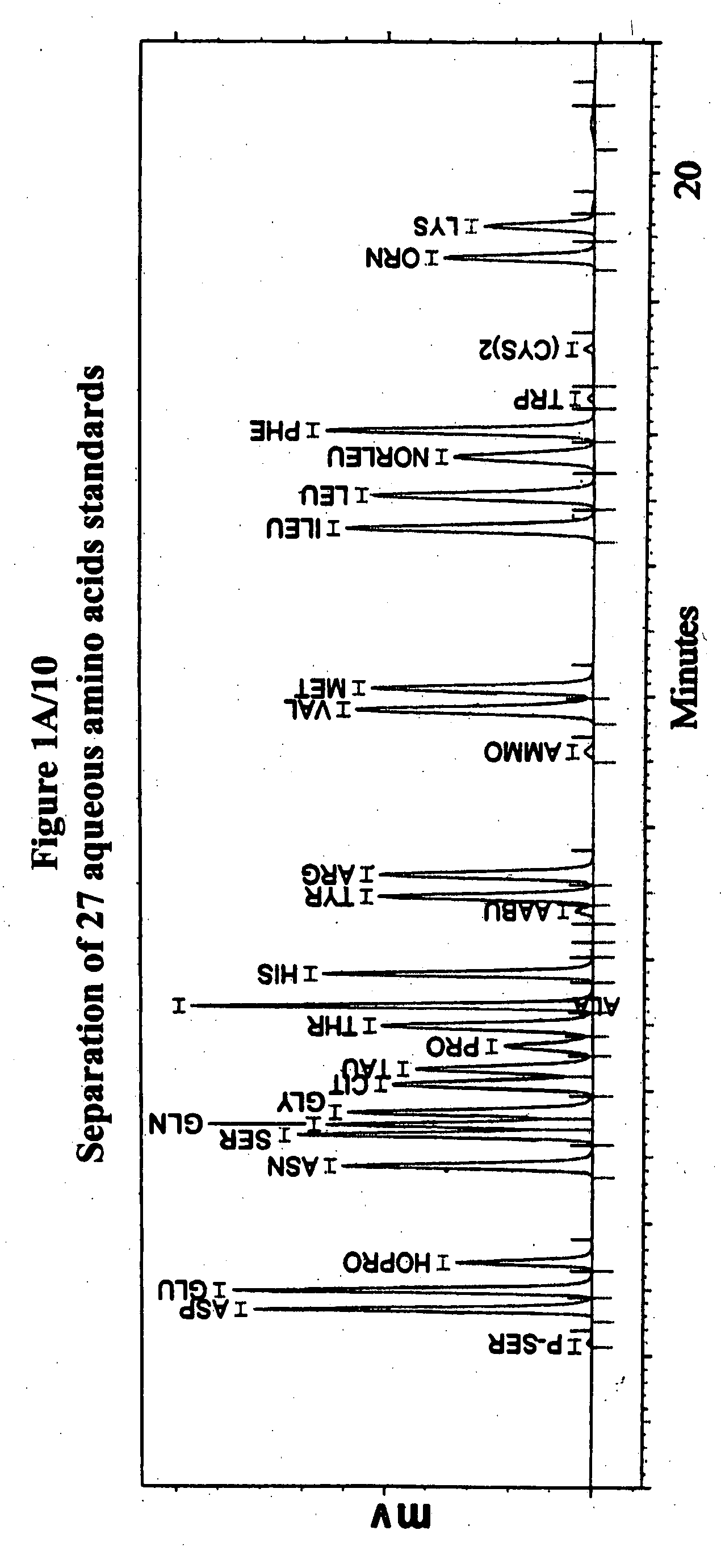
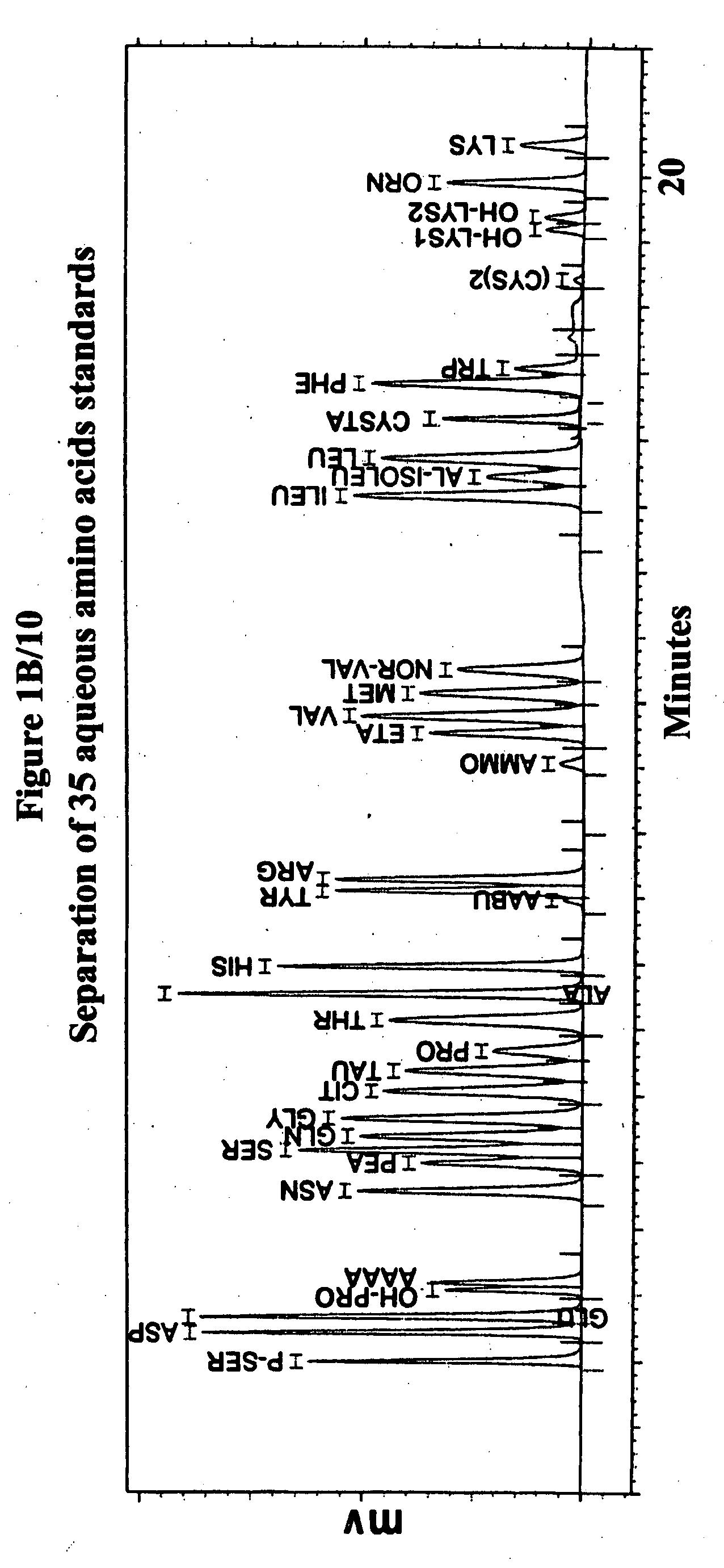
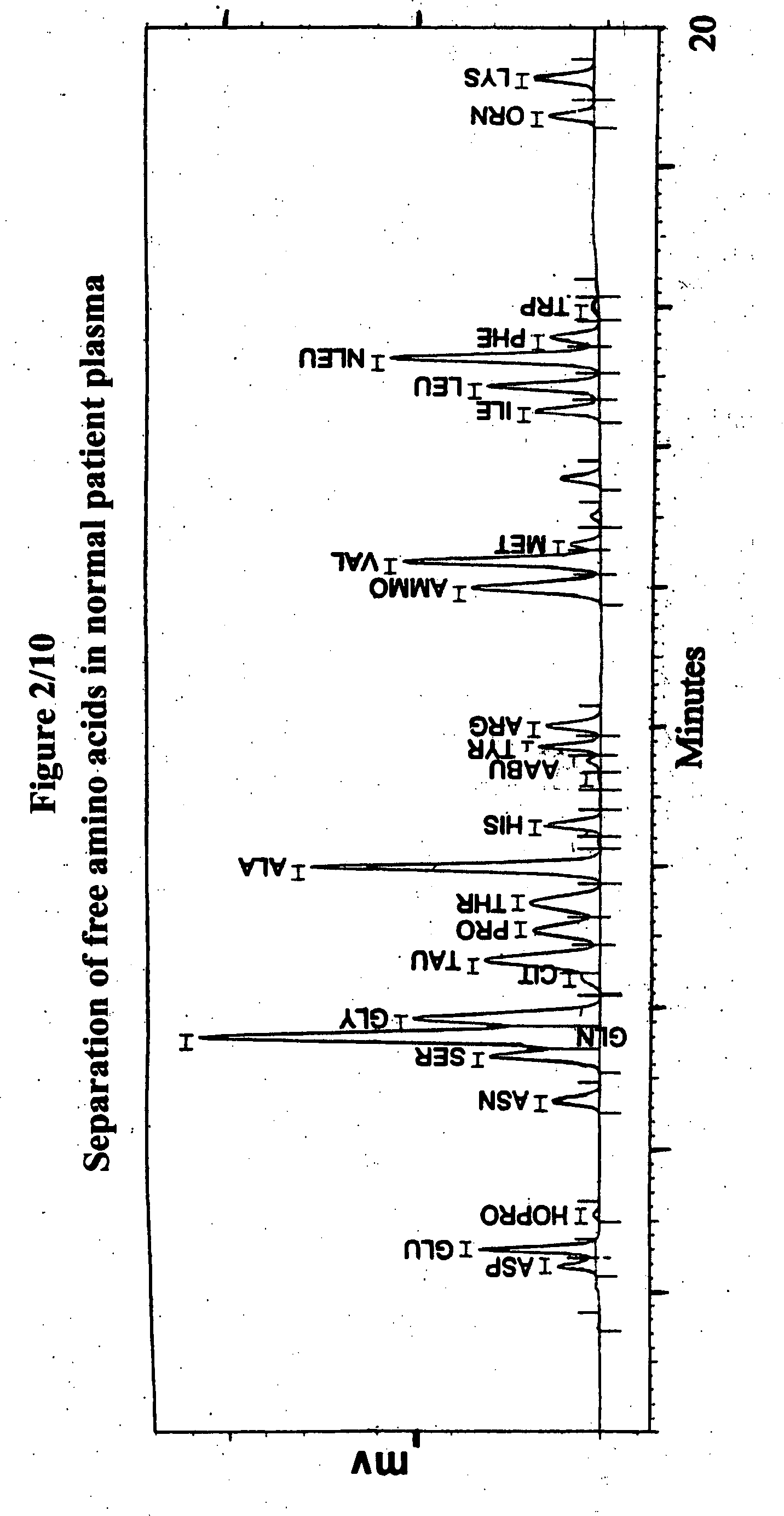
Human plasma free amino acids profile using pre-column derivatizing reagent- 1-naphthylisocyanate and high performance liquid chromatographic method
InactiveUS20070281361A1Component separationSolid sorbent liquid separationCysteine thiolateHplc method
After many decades, 1-Naphthylisocyanate (NIC) has been identified as the most ideal pre column derivatization fluorescent tag for reversed phase Liquid Chromatographic (HPLC) analysis of all free amino acids (AA) in biological samples. NIC forms very stable derivatives with all AAs in one minute. Using NIC, the first, most simple, robust, sensitive (femto mole), and economical high pressure binary gradient, HPLC method, has been developed. It estimates 35 (and 2 internal standards) AAs in human plasma in record shortest time of 20 minutes and has been validated for precision (n=16, <6%), accuracy 95 %, linearity (0 to 1200 μM / L), and analyzing normal and abnormal patients. It can provide with in 20 minutes the first and best plasma free AAs profile that includes Homocysteine, Cysteine, Alloisoleucine, and Cystathionine and a 27 AAs profile using a blood spot (3 μl plasma). A sample can be analyzed with in one hour of its arrival in the laboratory.
Owner:HARIHARAN MEENAKSHISUNDARAM
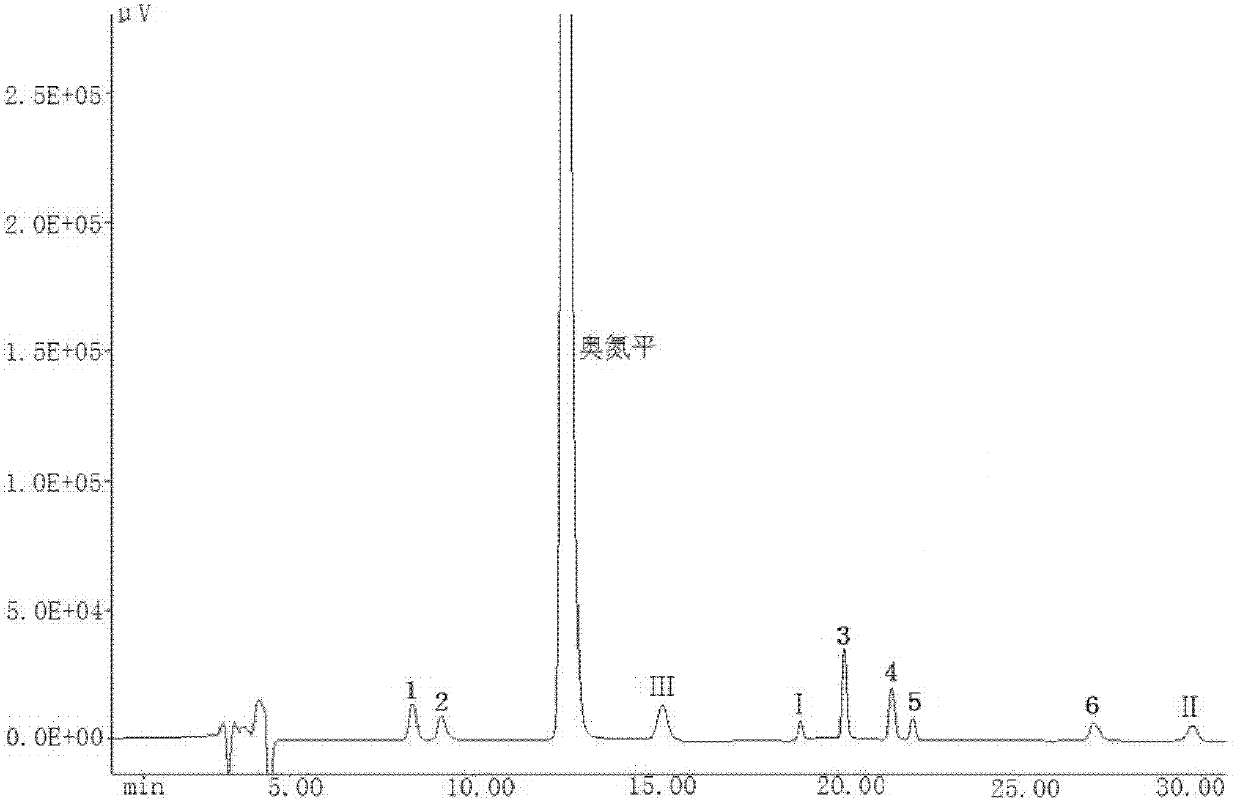
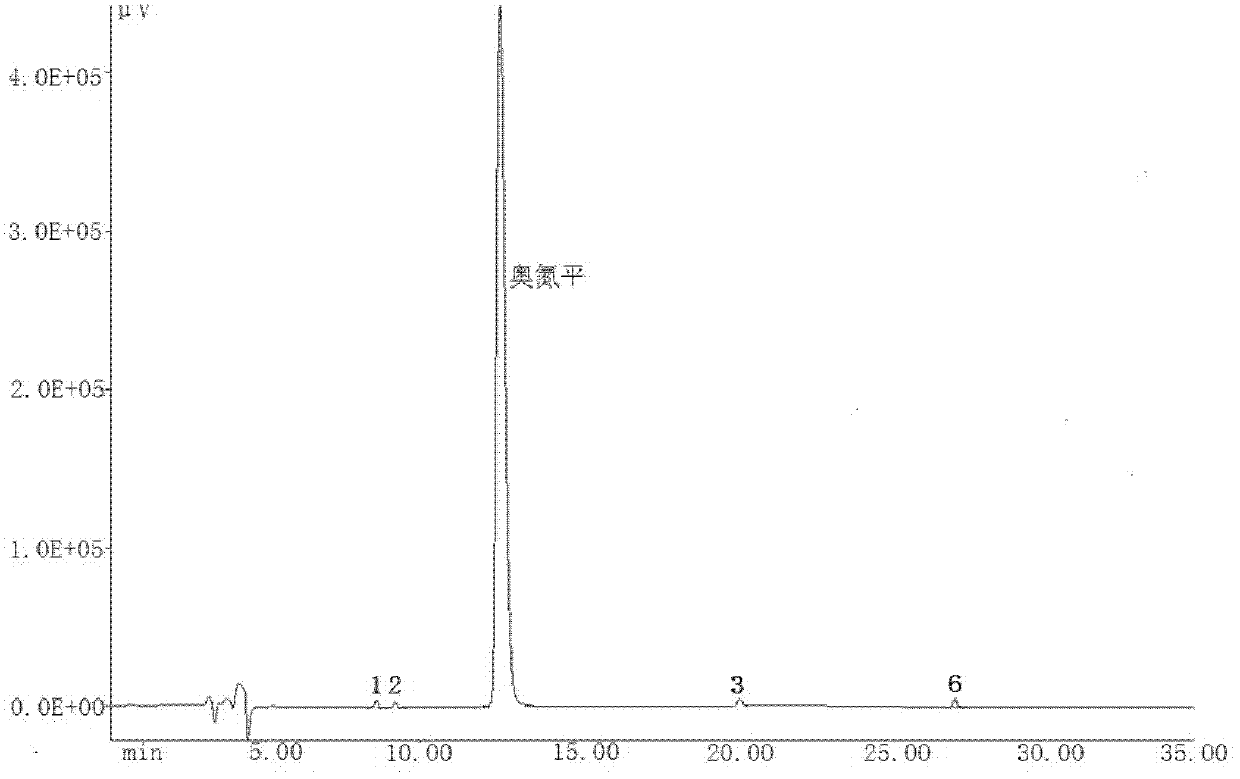
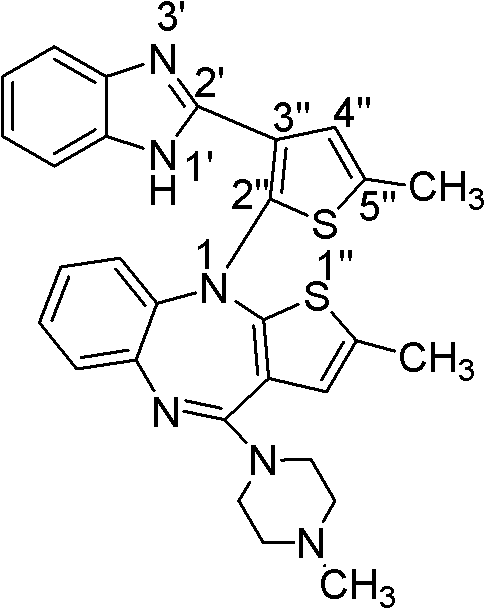
A kind of olanzapine related substance and its preparation method and high performance liquid chromatography analysis method
ActiveCN102276624ARich varietyHigh sensitivityOrganic chemistryComponent separationHplc methodGradient elution
The invention discloses an olanzapine related substance and a preparation method as well as a high-efficiency liquid-phase chromatographic analysis method thereof. The olanzapine related substance has a structural formula shown in the specification. The preparation method of the olanzapine related substance comprises the following steps of: concentrating olanzapine ethanol recrystallization mother liquor; and separating through silica gel column chromatography to prepare the olanzapine related substance. In addition, the invention provides the high-efficiency liquid-phase chromatographic analysis method of the olanzapine related substance. In the high-efficiency liquid-phase chromatographic analysis method, a reversed phase C18 chromatographic column is selected and used, the detection wavelength is 220-280nm, the flow velocity is 0.8-1.0ml / minute, the column temperature is 25-30 DEG C, acetonitrile and a 0.1-0.4-percent buffer solution of glacial acetic acid and triethylamine in equal proportion are used as a mobile phase to perform gradient elution; the olanzapine related substance and other eight related substances can be simultaneously detected; and thus, quality control of olanzapine and olanzapine-containing medicaments can be completely, scientifically, effectively and quickly realized.
Owner:DALIAN UNIV OF TECH
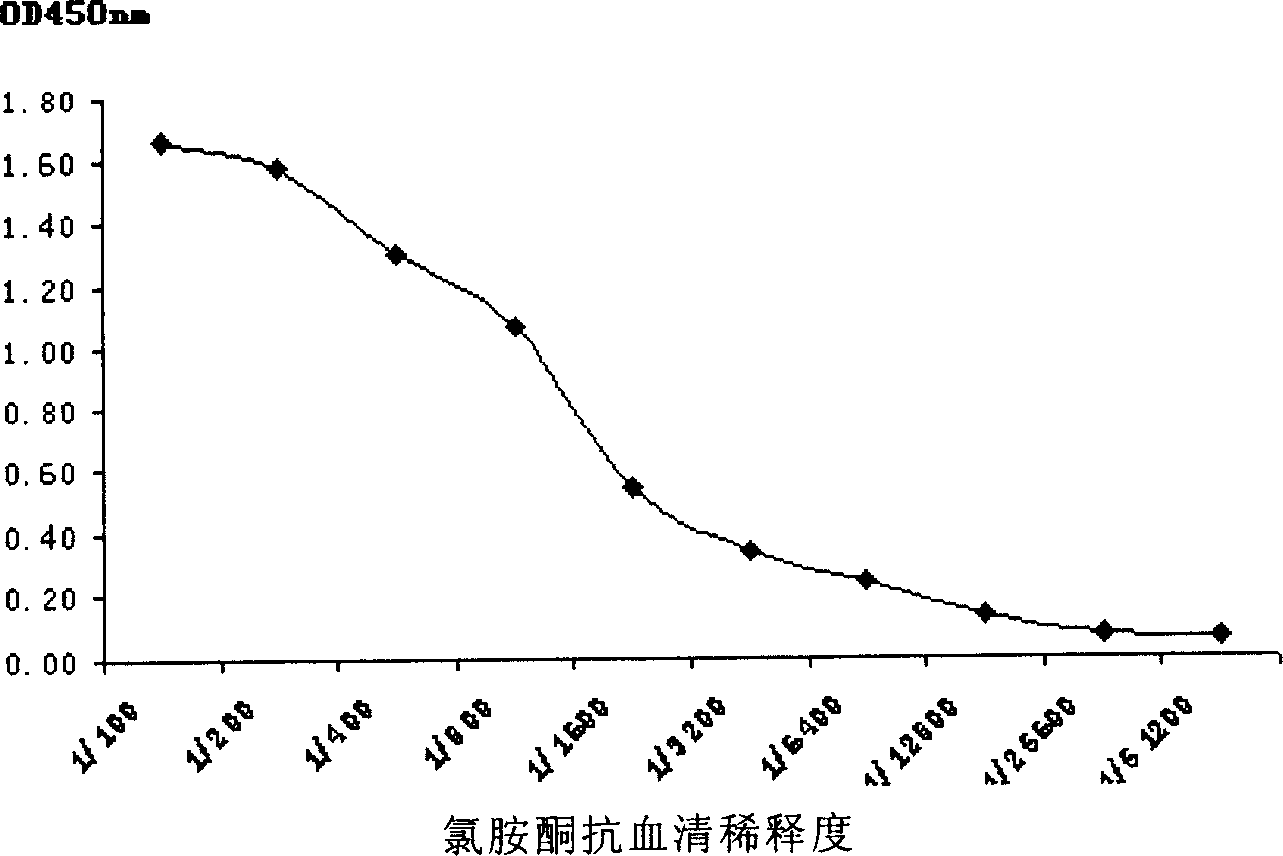
Monoclonal antibody for ketamine detection and immune detection board
ActiveCN1907953AImproving immunogenicityPreserved immunoreactivitySerum albuminBiological testingHplc methodMetabolite
The invention discloses a hapten and complete antigen used for ketamine detection and antibody preparation. The invention also discloses an anti-ketamine monoclonal antibody prepared by the complete antigen and a colloidal gold-labeled ketamine monoclonal antibody immunoassay plate used for detecting ketamine in drugs or urine, etc., human samples. Compared with HPLC method, the invented detection plate is simple, portable and easy to carry, and can be used for spot detection without need of expensive equipment. The whole detection for ketamine by using the detection plate can be completed in 10 minutes with sensitivity up to 50 ng and with no cross reaction with 39 kinds of common pharmaceuticals, drugs, and ketamine metabolites in vivo.
Owner:SHANGHAI CRIMINAL SCI TECH RES INST +1
Method for simultaneously determining contents of chlorogenic acid, caffeic acid in dandelion preparation by HPLC
InactiveCN101324546AHigh recovery rateHigh sensitivityComponent separationChlorogenic acidHplc method
The invention relates to a method for simultaneously measuring the content of chlorogenic acid and caffeic acid in a dandelion preparation by utilizing the HPLC method. The measurement method can effectively solve the problem that the chlorogenic acid and the caffeic acid are taken as the index components to simultaneously carry out the content measurement in order to lay the foundation for the quality standard of the dandelion preparation. The specific technical proposal comprises the following steps: respectively preparing a sample solution to be measured and a reference product solution, gradient-eluting with the RP-HPLC method by taking the methanol-phosphoric acid water as the mobile phase and measuring the content of the chlorogenic acid and the caffeic acid. The method has the advantages of convenience, reliability, rapidness, high sensitivity, good repeatability and high recovery rate, takes the chlorogenic acid and the caffeic acid as the index components, lays the foundation for the quality standard establishment of dandelion and the preparation thereof and provides more scientific basis.
Owner:HENAN UNIV OF CHINESE MEDICINE
Method for decomposing chiral mobile phase additive RP-HPLC of fudosteine enantiomer
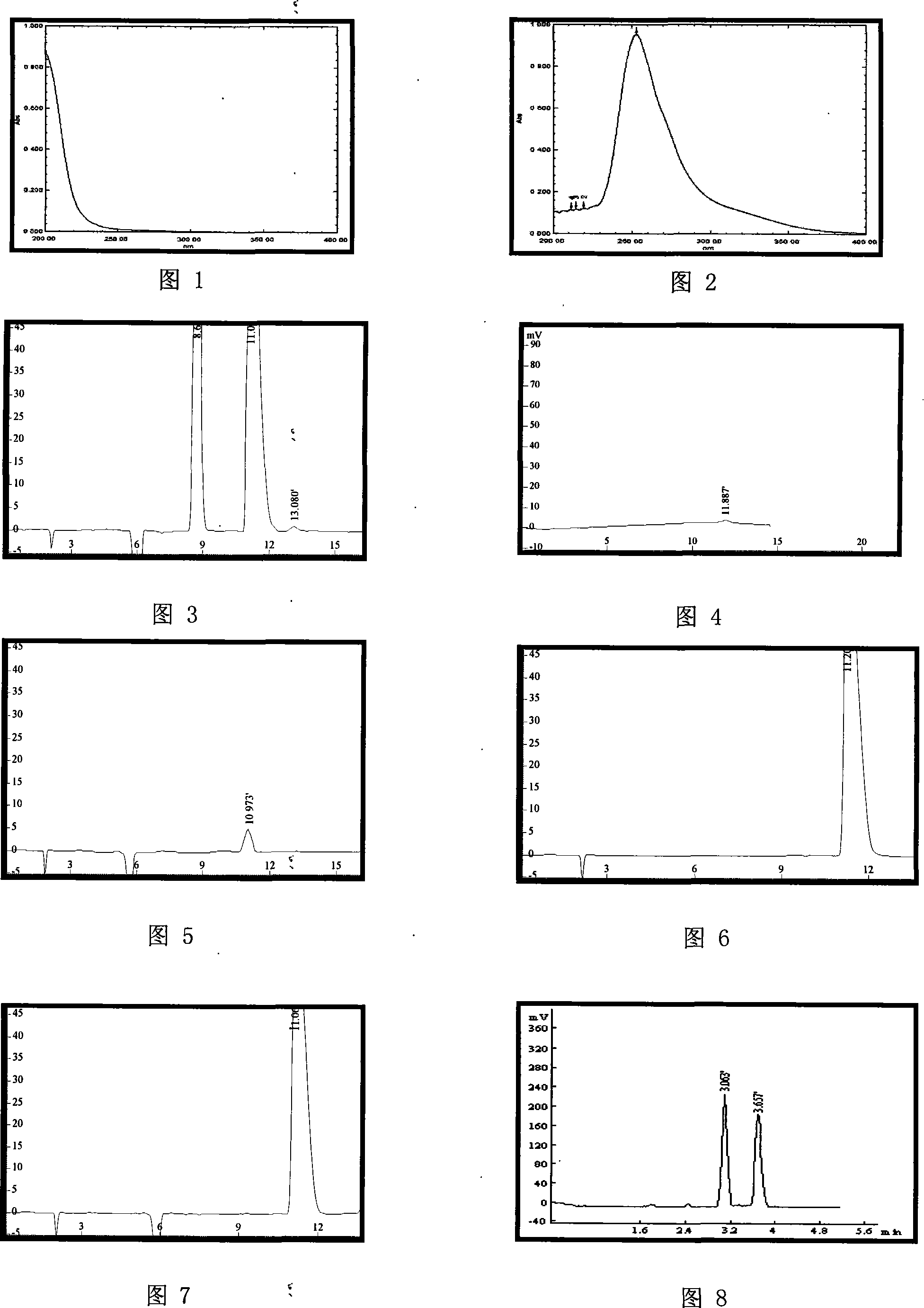
InactiveCN101161642AFast and accurate separabilityFast and Accurate DeterminationOrganic compound preparationOptically-active compound separationFUDOSTEINEHplc method
The present invention belongs to analytical chemistry field, and relates to separation and determination of Fudosteine and the enantiomer thereof (impurity). The present invention adopts RP-HPLC method, and adds a chiral metal synergist into a chromatogram flow phase system to form a tri-diastereoisomer coordination compound, because the obtained diastereoisomer coordination compound has stable structure with energy difference and capability to carry out three-dimensional selective absorption and repulsion reaction with the fixed phase, the two enantiomers can be separated from each other. The method can separate and determine Fudosteine and the enantiomer thereof (impurity), thereby making the quality of both Fudosteine and the agent containing Fudosteine controllable.
Owner:NANJING MEDICAL UNIV
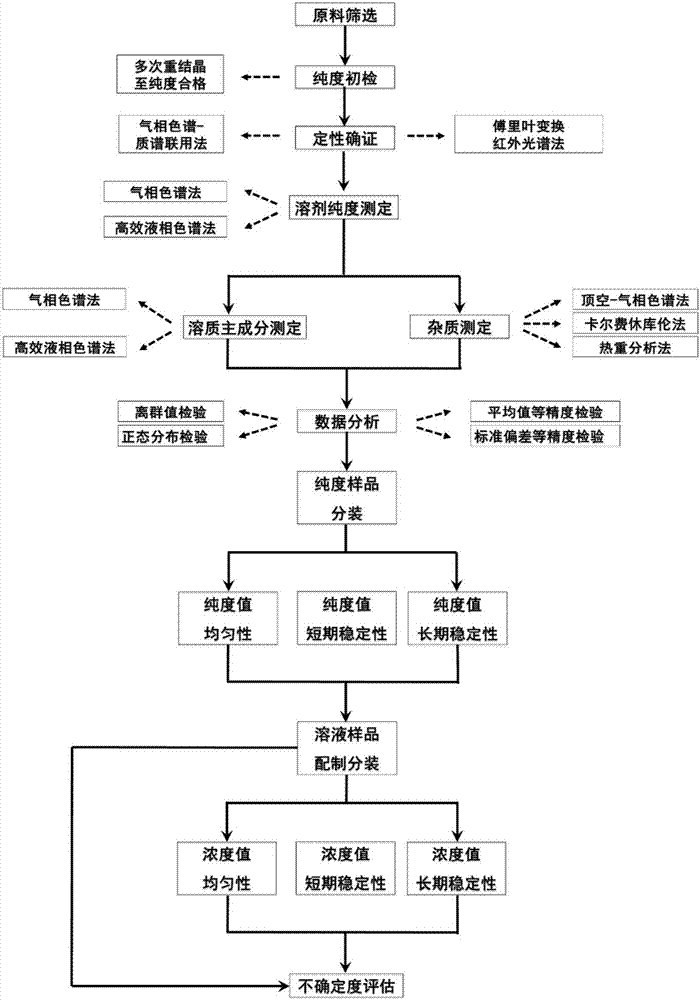
Method for measuring purity values and uncertainty degrees of standard substances of phenol and hydroquinone
ActiveCN107167529AAccurate measurementHigh purityWeighing by removing componentComponent separationHplc methodGas chromatography–mass spectrometry
The invention discloses a method for measuring purity values and uncertainty degrees of standard substances of phenol and hydroquinone. The method comprises the following steps: screening raw materials for purity qualification; performing qualitative confirmation on to-be-measured samples by combining a gas chromatography-mass spectrometry with a Fourier transform infrared spectroscopy; measuring the purity of the to-be-measured samples by combining three methods different in principle including a GC method, an HPLC method and a DSC method; sub-packaging the to-be-measured samples; checking uniformity and stability; calculating to obtain the uncertainty degree caused by purity measurement; calculating the uncertainty degree caused by the uniformity and the stability according to step four; and synthesizing all the uncertainty degrees to obtain a total uncertainty degree. The method provided by the invention has accurate qualification, reduces system error, adopts a strict statistical data processing method and a comprehensive uncertainty degree evaluation method, ensures accuracy, stability and traceability of measured values, and obtains high-purity standard substances of phenol and hydroquinone.
Owner:ZHEJIANG MEASUREMENT SCI RES INST
Method for preparing eptifibatide
InactiveCN102702320AReduces chances of exposure to alkaliReduce generationPeptide preparation methodsHplc methodCombinatorial chemistry
The invention relates to a method for preparing eptifibatide serving as a polypeptide medicament by combing solid and liquid phases, which belongs to the technical field of synthesis of polypeptides. According to the technical scheme of the invention, the method comprises the following steps of: (1) preparing a fragment A, i.e., 4peptide2-CTC resin by using a solid phase; (2) preparing a fragmentB, i.e., 3peptide-Sieber resin by using a solid phase; (3) cracking peptide resin; (4) preparing a fully-protected linear peptide from the fragments A and B by adopting a liquid phase fragment condensation method; (6) oxidizing with I2 in a liquid phase; (7) cracking to obtain crude eptifibatide; and (8) purifying the crude eptifibatide with a HPLC (High Performance Liquid Chromatography) method to obtain fine eptifibatide finally. According to the method, racemization products of Cys and Asp can be reduced greatly, and the yield and purity of a product are increased.
Owner:HYBIO PHARMA
Method for determining lignocelluloses component content of plant straw
InactiveCN104655784AScientific and reasonable designThe steps are detailed and completeWeighing by removing componentComponent separationFiberHplc method
The invention provides a method for determining the lignocelluloses component content of plant straw, and relates to the field of agronomy. The method particularly comprises the following steps: firstly, selecting the plant straw of a tested material for pretreatment; then carrying out two-step acidolysis respectively by adopting 72% concentrated sulfuric acid and 4% dilute sulfuric acid, and degrading and separating a lignocelluloses component in the plant straw; then accurately determining the content of cellulose and semi-cellulose by adopting an HPLC method, determining the content of acid-soluble lignin in a degraded liquid by adopting a UV method, and determining the content of acid-insoluble lignin by adopting a firing method. The method remedies the defects of insufficient pretreatment, extensive treatment process and inaccurate quantitative determination method caused by adopting a traditional method, gets a new breakthrough of determining the content of semi-fibers of lignin of the plant straw by adopting the HPLC method, can be used for accurately measuring the content of the lignocelluloses component in various plant straw, is accurate and reliable in result, and has wide reference and use values in the fields of agricultural crop production, biochemical analysis, biomass novel energy utilization and the like.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
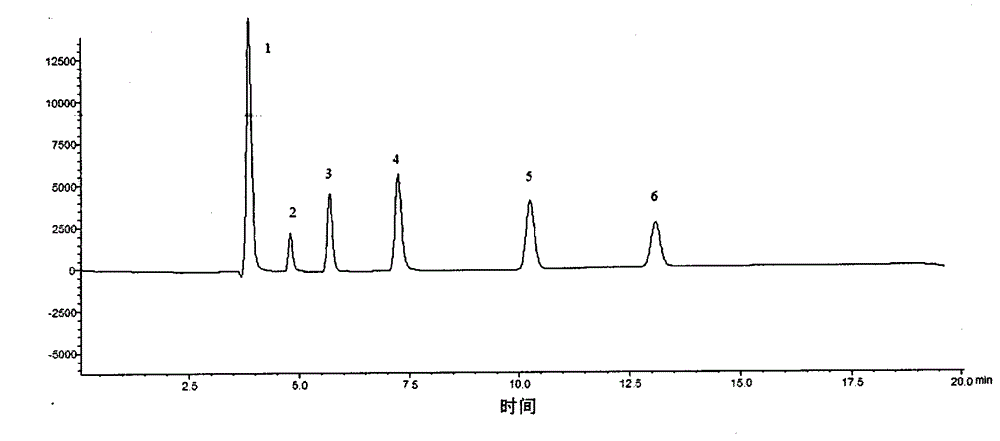
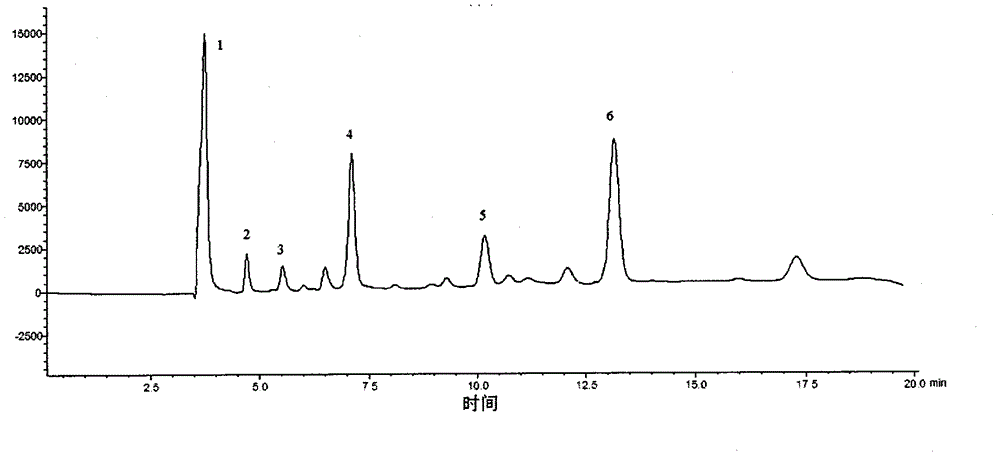
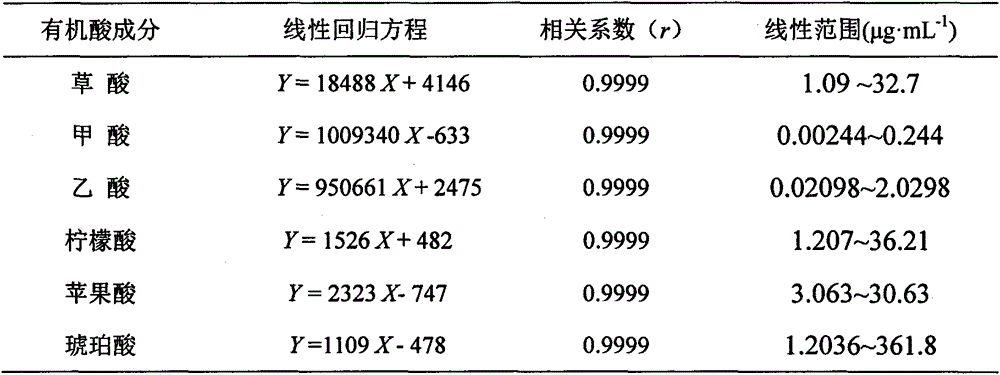
HPLC (High Performance Liquid Chromatography) method for simultaneously determining content of six organic acids in pinellia ternata
InactiveCN104597160AImprove linearityGood reproducibilityComponent separationHplc methodPhosphoric acid
The invention relates to an HPLC (High Performance Liquid Chromatography) method for simultaneously determining content of six organic acids in pinellia ternata. The method comprises the following steps: (1) preparing a sample solution; (2) preparing a comparison product solution; (3) determining by a high performance liquid chromatography: taking the comparison product solution and the sample solution and carrying out determination analysis under following chromatographic conditions: a chromatographic column is GL InterSustain-C18 (4.6mm*250mm, 5 microns), and a mobile phase is a phosphoric acid adjusted 0.03mol / L (NH4)H2PO4 buffering solution with the pH value of 2.0 and methanol; and the volume ratio is 97 to 3, the flow speed is 0.8mL / min, the column temperature is 30 DEG C and the detection wavelength is 210nm. Under the conditions of the method, oxalic acid, formic acid, malic acid, acetic acid, citric acid and succinic acid in pinellia ternata are effectively separated, and methodological results meet the analysis determination requirements. Compared with the traditional potentiometric titration method, the measurement for content of total organic acids is clear and objective by virtue of the HPLC method disclosed by the invention, and the content of the six organic acids in pinellia ternata can be effectively and rapidly determined and the quality of pinellia ternata can be comprehensively and accurately controlled.
Owner:CHONGQING MEDICAL UNIVERSITY
Tech. for extracting high purity chlorogenic acid from honeysuckle
The invention relates to a honeysuckle high purity chlorogenic acid distilling technology. The technology includes four steps: adding NaHSO3, yield promoting agent and clarifier, sheet frame filter-press, passing macro hole adsorbing resin to gain concentrated solution, spraying and drying to gain taking orally chlorogenic acid; adding alcohol and depositing, adding water taking second clarifying process, taking active carbon process, pressure decreasing and concentrating, spraying and drying to gain honeysuckle distilling material; adding beta-CD into the clarified solution to take complex reaction, adding cyclohexanone decomplexing to gain concentrated chlorogenic acid, drying in vacuum could gain the chlorogenic acid that the content could be 90%; dissolving the concentrated solution by alcohol, using silica gel chromatography column to take section analysis, testing, combining the same section, decreasing pressure and drying, crystallizing, the chlorogenic acid that has the content over 95% would be gained, which was tested by HPLC method.
Owner:CANGYUAN PHARMA DEV FUZHOU
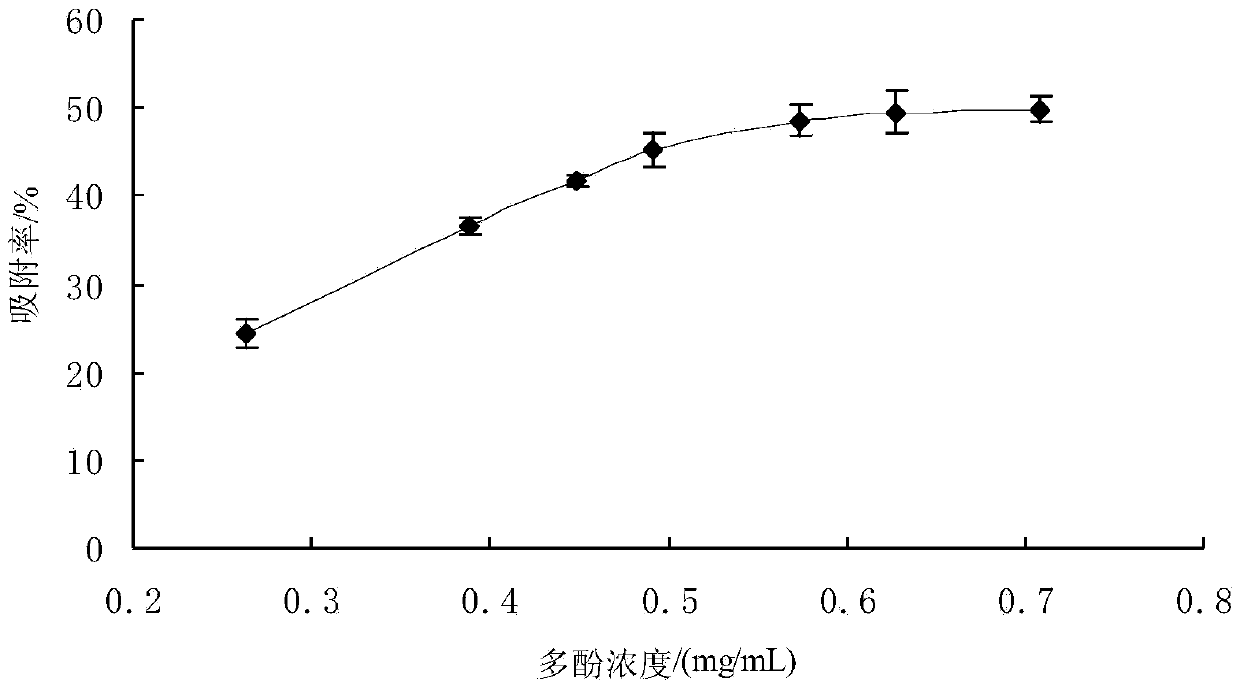
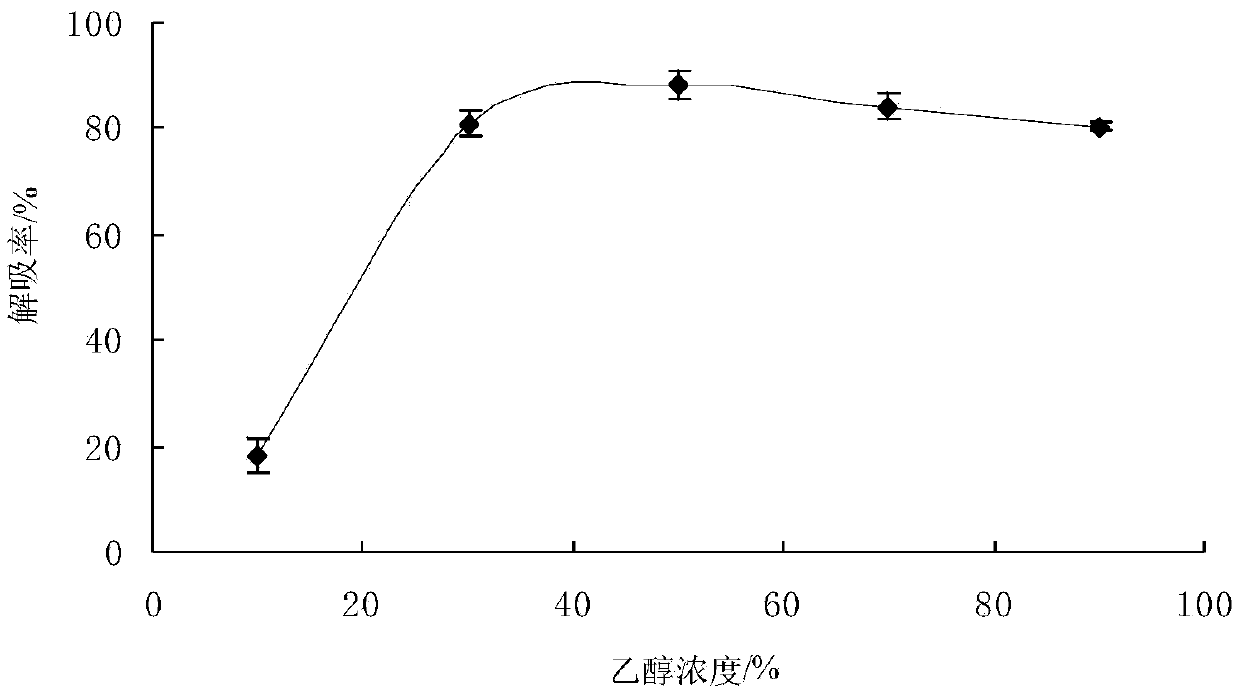
Method for separation purification of walnut green seedcase polyphenol substances by macroporous resin
The invention discloses a method for separation purification of walnut green seedcase polyphenol substances by macroporous resin. The method comprises 1, carrying out extraction on walnut green seedcase dry powder at a temperature of 35-40 DEG C through ethanol with content of 50% under ultrasonic action many times, carrying out vacuum filtration on the extract, evaporating the filtrate to obtain walnut green seedcase polyphenol crude extract, diluting the walnut green seedcase polyphenol crude extract until a desired concentration is obtained, and carrying out refrigeration for next use, 2, determining total polyphenol content of walnut green seedcase by a Folin-Ciocaileu method, 3, carrying out macroporous resin pretreatment, 4, carrying out macroporous resin static adsorption and desorption, 5, carrying out macroporous resin dynamic adsorption and desorption, and 6, through a HPLC method, determining free phenol content and composition of the walnut green seedcase polyphenol extract obtained by the macroporous resin separation purification so that the whole separation purification process is finished. The method provides the novel approach for separation purification of walnut green seedcase polyphenol substances, can be operated simply and realizes high separation purification quality and efficiency.
Owner:HENGSHAN COUNTY HONGMENGYUAN TECH IND CO LTD
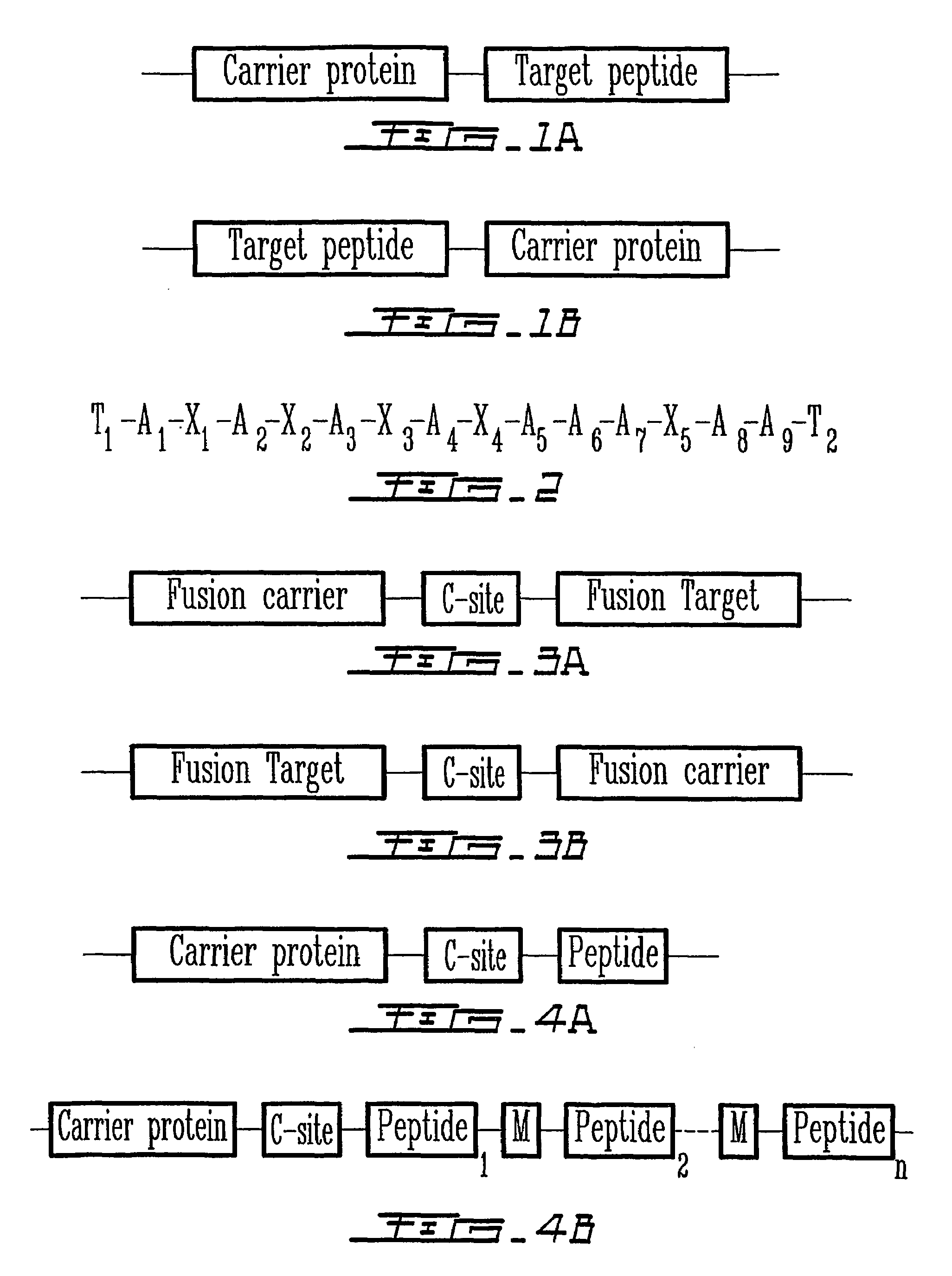
Staphylococcal nuclease fusion proteins for the production of recombinant peptides
InactiveUS7390639B2Easy to disassembleNot complicate subsequent stepSugar derivativesBacteriaInclusion bodiesHplc method
Peptides are produced as fusions with a suitable carrier protein. The carrier protein disclosed herein are adapted from the N-terminal domain of staphylococcus nuclease. This novel carrier protein acts to promote the over-expression of the peptide-protein fusion in the form of inclusion bodies, which minimizes in-cell proteolysis of desired peptides. The fusion protein is readily purified by conventional procedures or His-tag affinity chromatography when His-tag is inserted into the fusion protein. The target peptide is released from the purified fusion protein by a simple cleavage step and separated from the librated carrier protein by use of a reverse-phase HPLC process or by repeating the same affinity purification method. A particular advantage of the disclosed method, in addition to the obvious advantage of high yields, is its use for producing isotopically labeled peptides for NMR characterization of bioactive peptides and their interactions with target proteins.
Owner:NAT RES COUNCIL OF CANADA
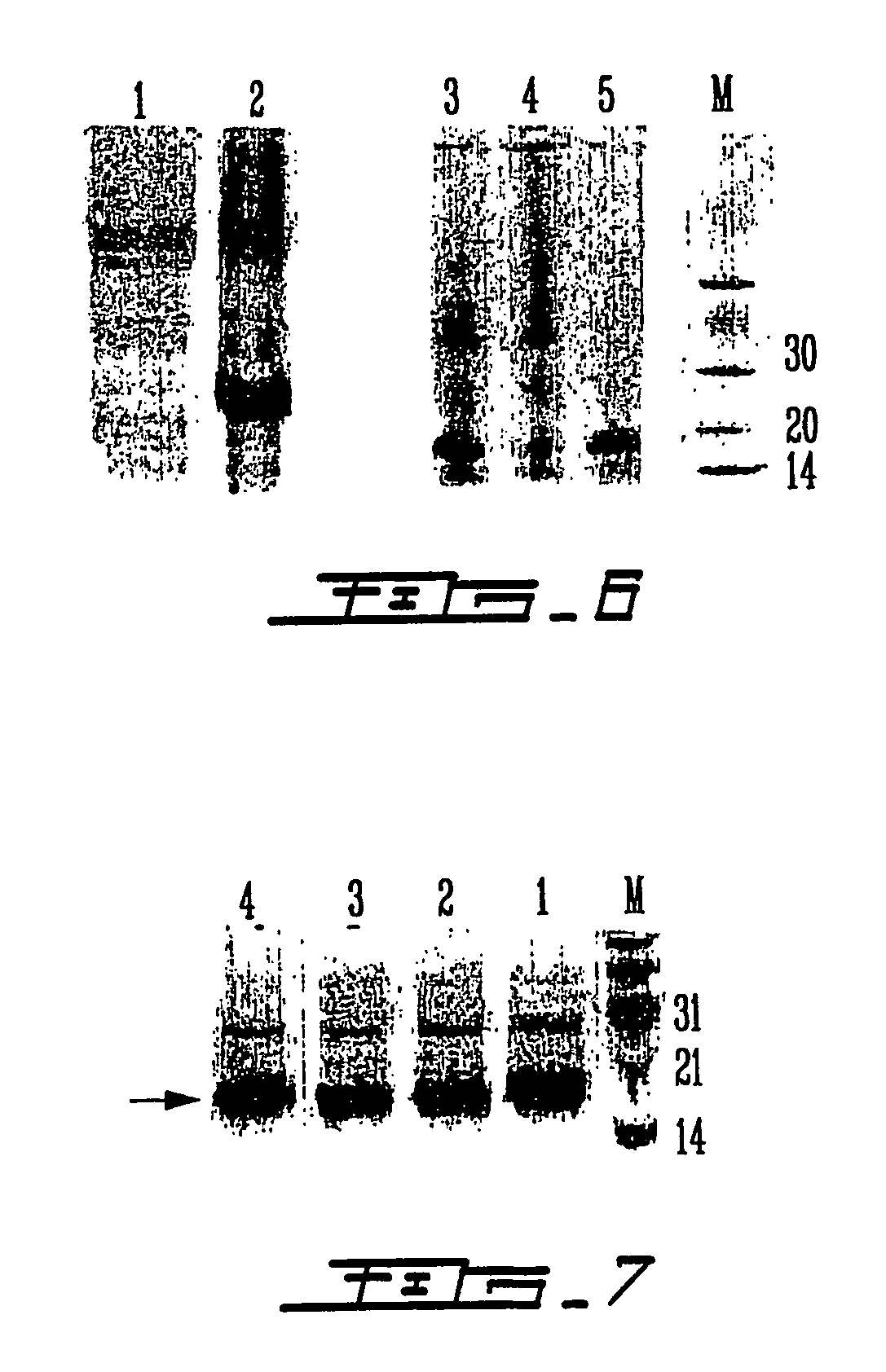
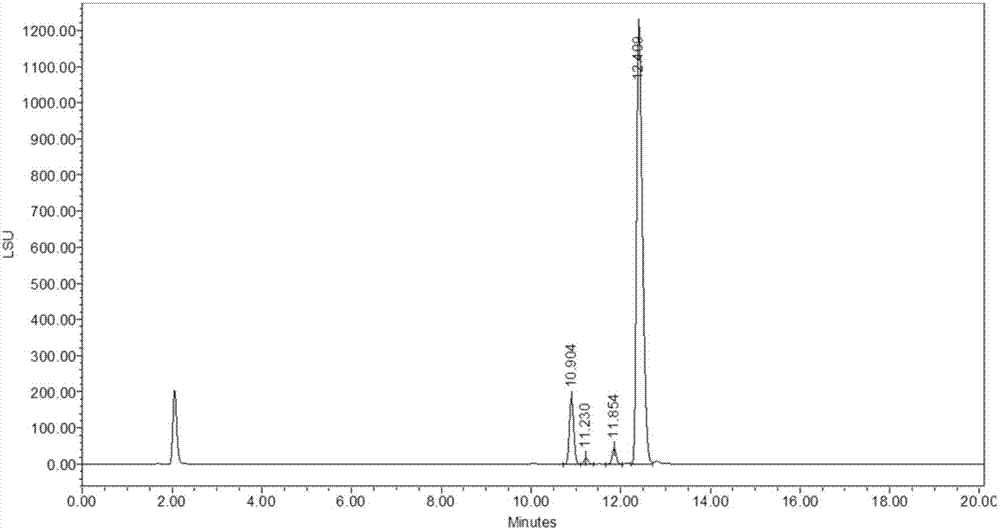
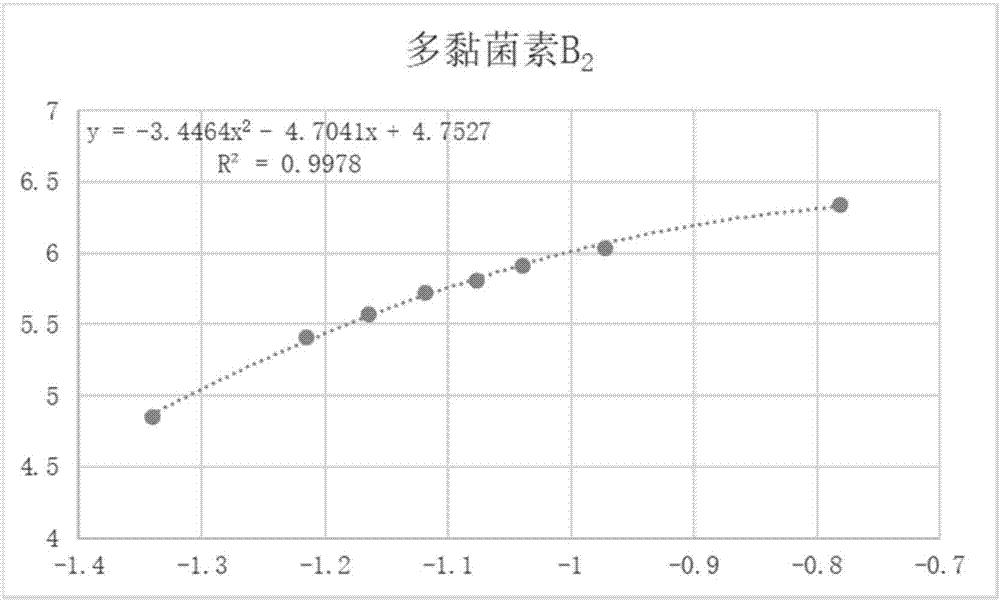
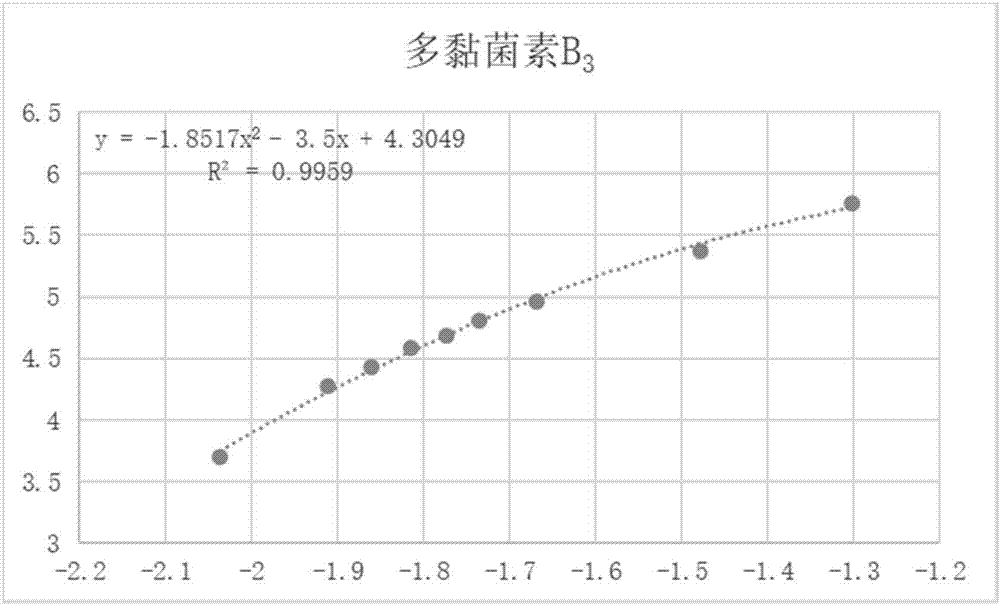
Analytical detection method and application of polymyxin sulfate B
The invention discloses an analytical detection method of polymyxin sulfate B, comprising the steps of analytically detecting polymyxin sulfate B via high-performance liquid chromatography in combination with an evaporative light scatterer. The method allows four components in polymyxin sulfate B to be fully separated, has high specificity, is simple to perform, is free of disturbance by other organic impurities, is approximately effective to HPLC (high-performance liquid chromatography) of EP 9.0 bulk pharmaceutical chemicals in terms of high sensitivity, good simplicity and high speed, and allows the contents of components in a polymyxin sulfate B preparation to be measured more accurately.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Purification method of ganirelix acetate
ActiveCN102993274AGood removal effectHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsGanirelixPurification methods
The invention provides a purification method of ganirelix acetate. The purification method comprises the steps of (1) purification of ganirelix crude peptide, wherein octadecylsilane bonded silica is adopted as a fixed phase, perchlorate / phosphoric acid solution with certain concentration is taken as an A phase and acetonitrile is taken as a B phase, the ganirelix crude peptide is purified by a gradient-elution high performance liquid chromatography (HPLC) method; (2) salt conversion and purification, wherein the alkylsilane bonded silica is taken as the fixed phase, glacial acetic acid solution with a certain concentration is taken as the A phase and the acetonitrile is taken as the B phase, salt conversion and purification are carried out by adopting the gradient elution HPLC method, and the solution collected and subjected to freeze-drying to obtain the ganirelix acetate. The invention aims at providing the purification method of the ganirelix acetate with stable and controllable process, high yield, high purity, and wide practical value and application prospect.
Owner:HYBIO PHARMA
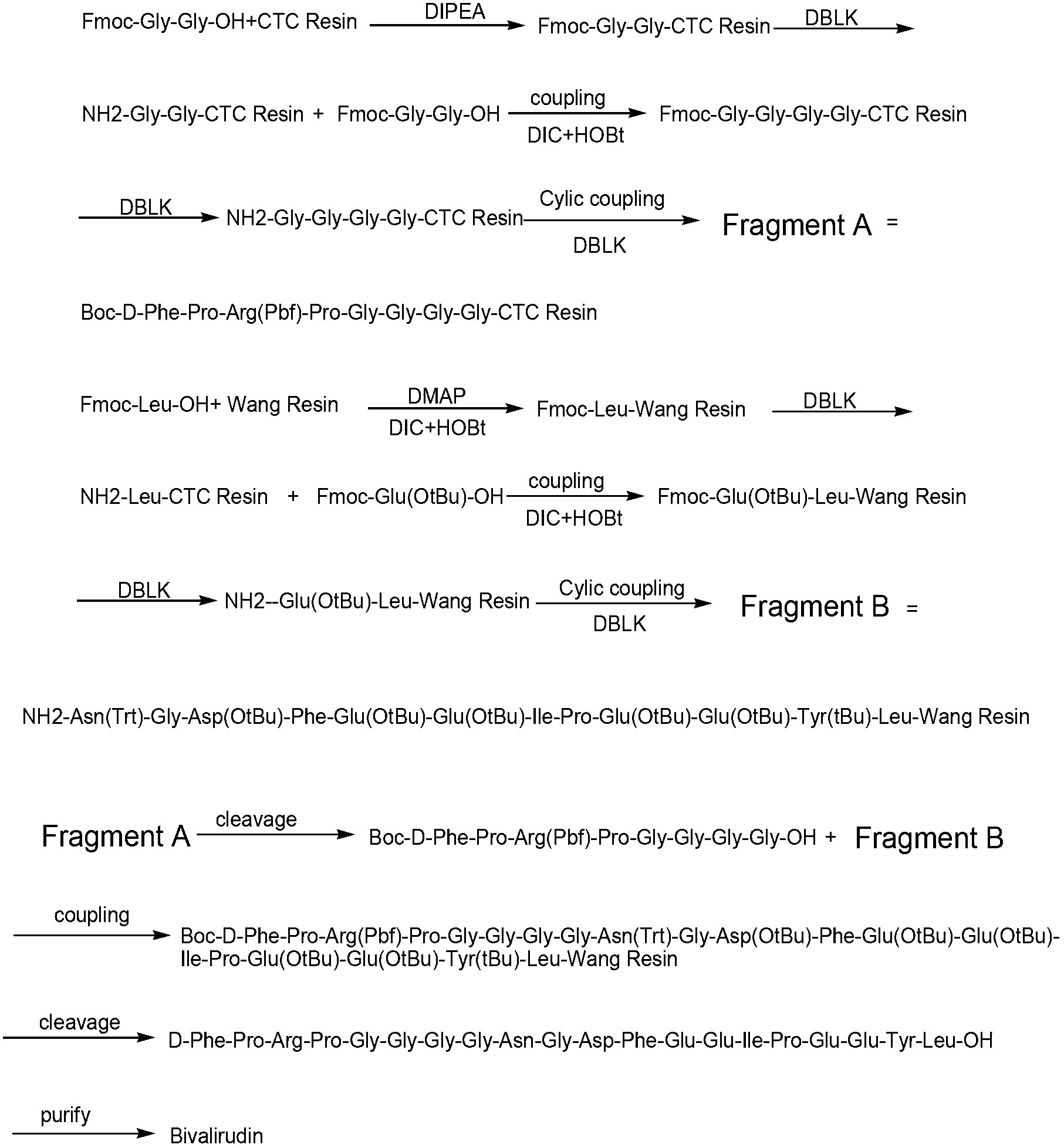
Preparation method of anticoagulant polypeptide
The invention relates to a preparation method of an anticoagulant polypeptide and discloses a preparation method of bivalirudin, and belongs to the technical field of pharmaceutical chemistry. The preparation method comprises the following steps: purifying a bivalirudin crude peptide by a reverse phase / weak cation exchange mixed-mode HPLC (high performance liquid chromatography) method; transferring a salt by a reverse phase HPLC method; and collecting and lyophilizing a solution to obtain the bivalirudin. The preparation method is easy to operate, low in cost and high in gram yield of the bivalirudin and is applicable for large-scale industrial production of the bivalirudin; by virtue of hydrophobicity and chargeability difference, impurities in the crude peptide can be separated and removed well at one time; and the prepared bivalirudin is high in purity and low in impurity content and has a considerable economic value and a wide application prospect.
Owner:HYBIO PHARMA
Determination method of effective component in aliphatic oil
InactiveCN1758060AShorten the timeSave organic solventComponent separationChromatographic separationHplc method
This invention discloses a test method for the effective components in fat oil, which applies the HPLC method to reduce the derivation operation steps of a sample and save time and reagents, not necessary to apply the step of extraction and applies suitable chromatograph conditions to separate the target components from impurities completely.
Owner:NAT INST FOR THE CONTROL OF PHARMA & BIOLOGICAL PROD
HPLC method for separating and analyzing voriconazole prodrug related substances
The method discloses an HPLC method for separating and analyzing related substances of voriconazole prodrug. High performance liquid chromatography is adopted, C18, phenyl column, and cyano column are used as chromatographic columns, buffer solution (pH value 2-9) and organic solvent The mobile phase is composed in a certain proportion, and the method can quickly, effectively and accurately separate and analyze related substances of voriconazole phosphate or its medicinal salt.
Owner:HC SYNTHETIC PHARMA CO LTD
High performance liquid chromatography (HPLC) method for analyzing and separating optical isomer of pantoprazole sodium
InactiveCN102141547AEfficient analytical separationGuaranteed stabilityComponent separationCelluloseCarbamate
The invention relates to a high performance liquid chromatography (HPLC) method for analyzing and separating an optical isomer of pantoprazole sodium. Cellulose 3,5-dimethyl phenyl carbamate chiral column OD-RH (Chiralcel, 150mm*4.6mm, 5 microns) is used, and a mixed solvent of water-acetonitrile in certain proportion is used as a mobile phase for separating and analyzing the optical isomer of the pantoprazole sodium.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Method for preparing high-content phosphatidyl ethanolamine
ActiveCN104592293AAchieve decolorizationTo achieve impurity removalPhosphatide foodstuff compositionsAlkaneHplc method
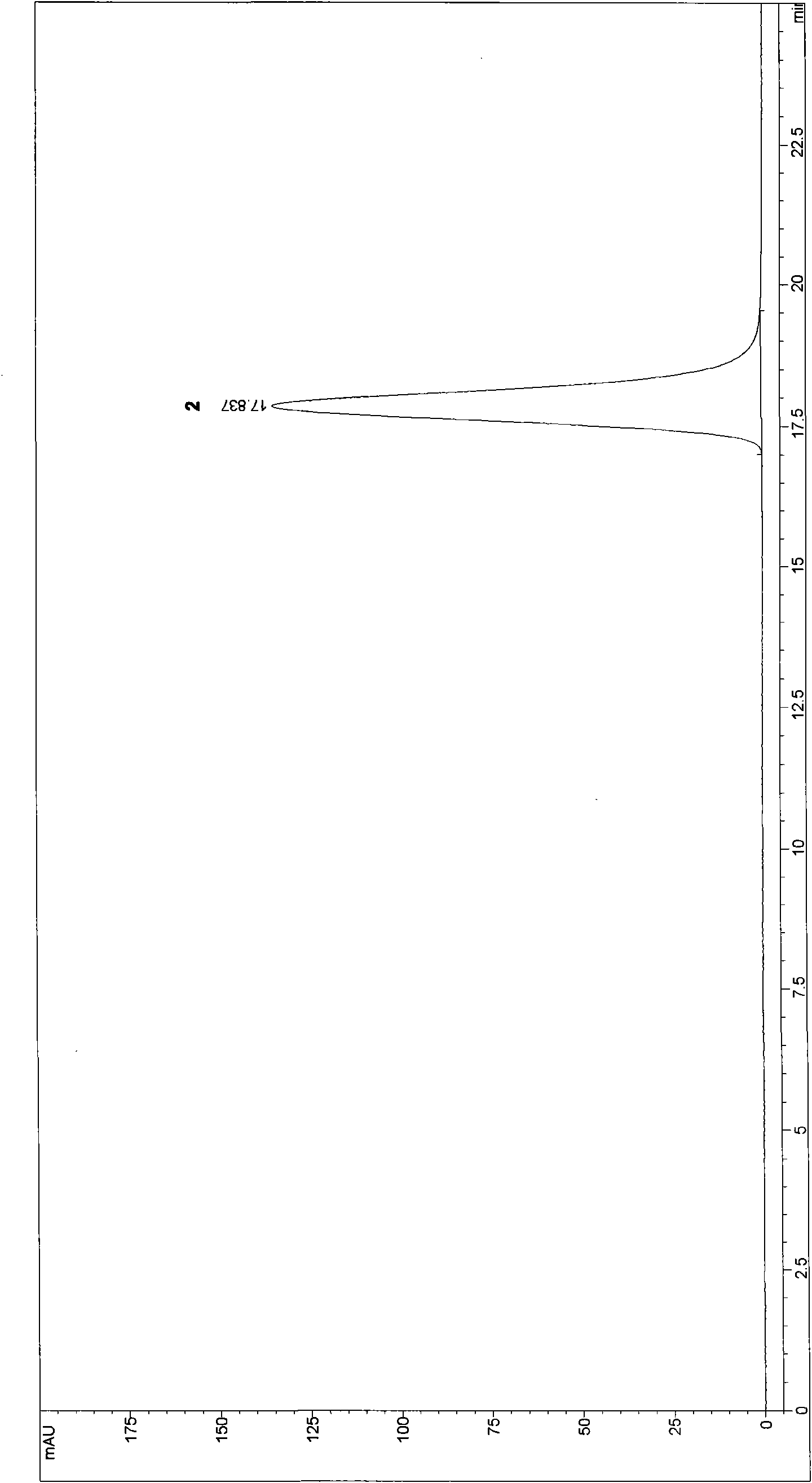
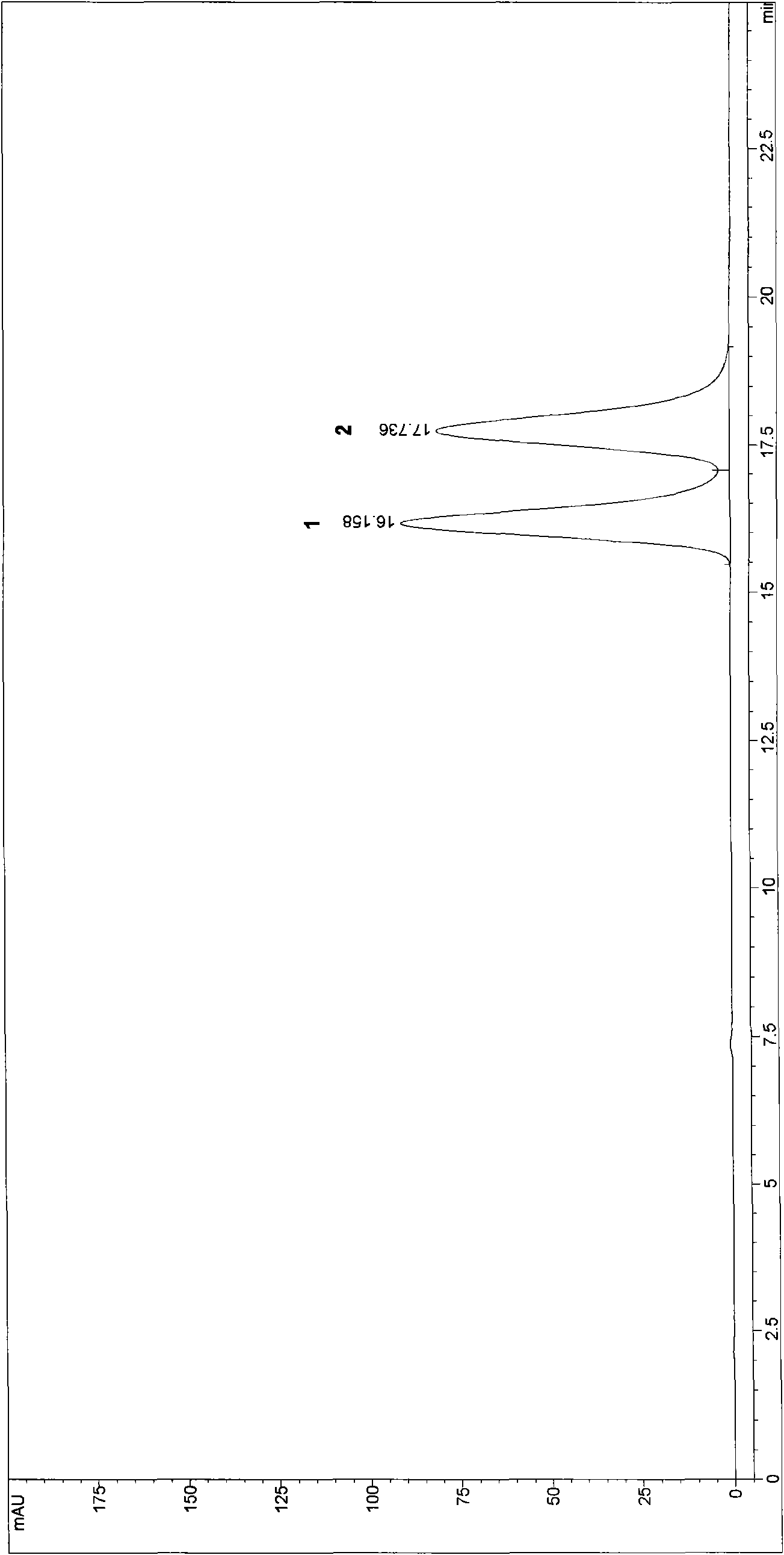
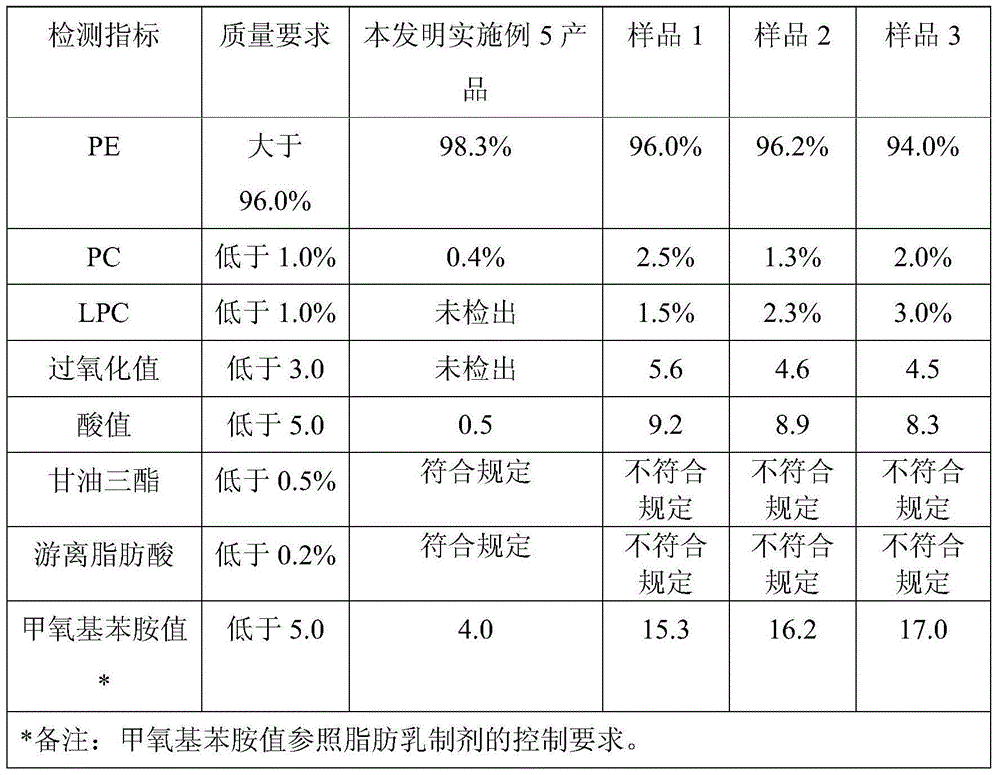
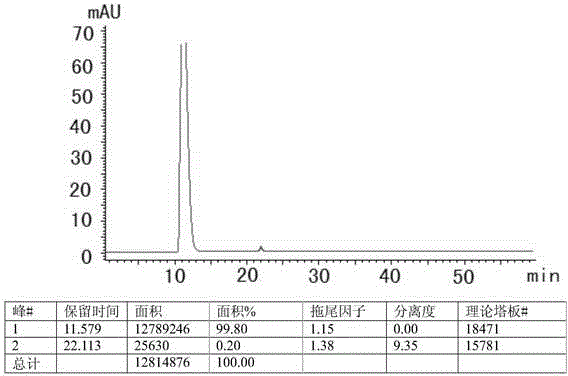
The invention discloses a method for preparing high-content phosphatidyl ethanolamine. The method comprises the following steps of dissolving a cephalin crude product as a raw material with an organic solvent, eluting twice with a ternary mixed solvent comprising polyhalogenated alkanes, lower alcohol and water by column chromatography and drying to obtain the phosphatidyl ethanolamine product, wherein the obtained phosphatidyl ethanolamine product is high in content (96.0%-99.9% obtained by an HPLC method), low in content of impurities (in which the content of phosphatidylcholine is less than 0.5%, the content of lysophosphatidylcholine is less than 0.5 %, the content of free fatty acid is less than 0.2%, and the content of triglyceride is less than 0.2%), low in oxidation indexes (of which the acid number is less than 1.0, the peroxide value is less than 1.0 and the anisidine value is less than 5.0), and the product quality meets the quality requirements of drug phospholipid for injecting.
Owner:GUANGZHOU HANFANG PHARMA
HPLC method for measuring related substances in Favipiravir
The invention discloses an HPLC method for measuring related substances in Favipiravir. According to the HPLC method for measuring related substances in Favipiravir, disclosed by the invention, specifically, a diode array detector is adopted, and acetonitrile (mobile phase A)-phosphate solution (mobile phase B) serves as a mobile phase. The method comprises the following steps: taking a proper amount of Favipiravir and related preparations containing Favipiravir, adding the substances into the mobile phase for preparing a solution of which every 1ml contains 0.2mg of Favipiravir, and taking the solution as a test solution; diluting into a solution of which every 1ml contains about 0.2mu g of Favipiravir by using the mobile phase, and taking the solution as a contrast solution; respectively performing sample introduction, wherein the sum of each impurity peak area in the chromatogram of the test solution is not more than the main peak area of the contrast solution. According to the method for detecting the related substances in Favipiravir and related preparations containing Favipiravir, disclosed by the invention, the conditions of the impurities and degradation products of Favipiravir can be rapidly and accurately detected. The operation is simple and convenient, the sensitivity is high, and the product quality can be well controlled.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com