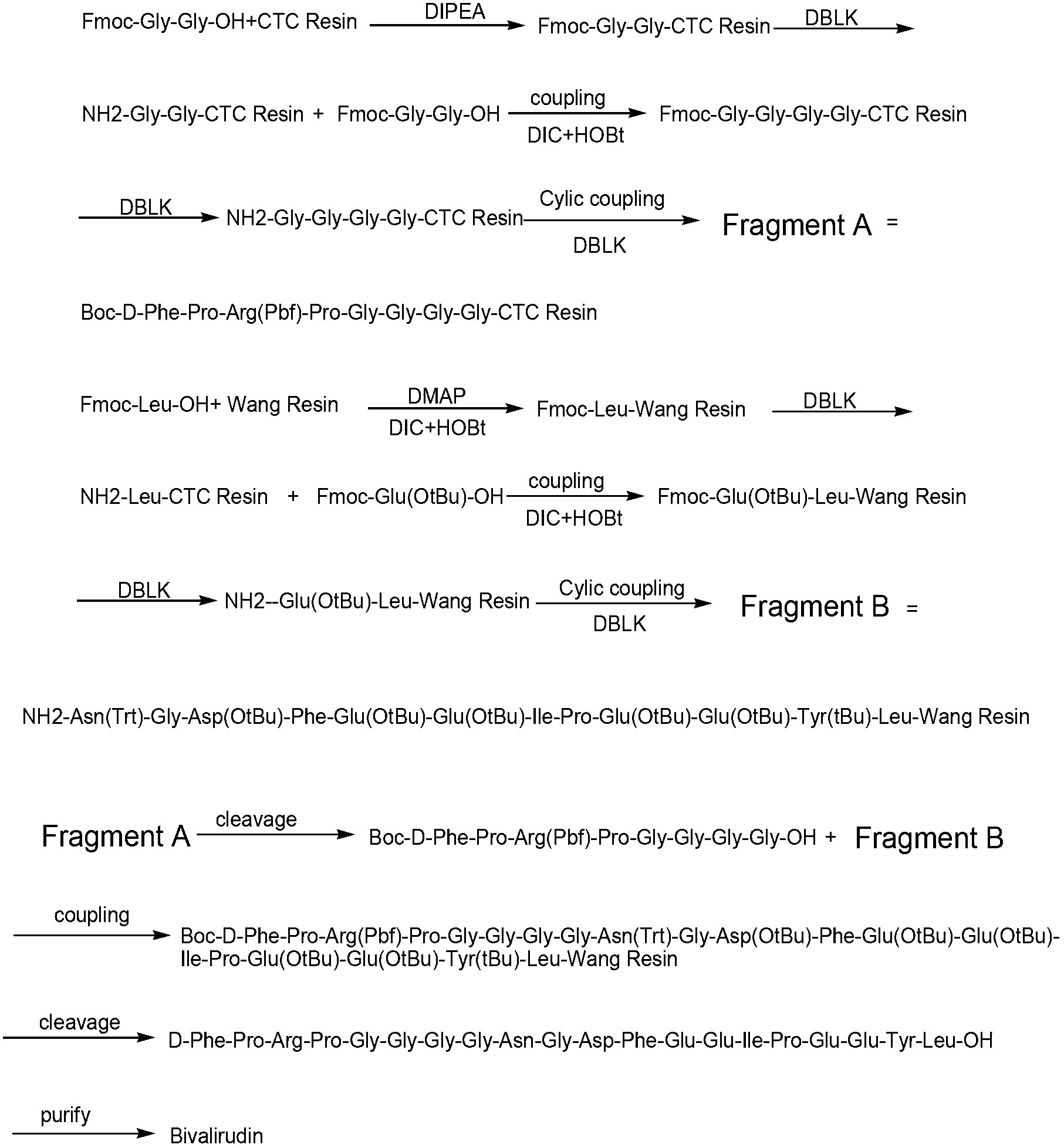
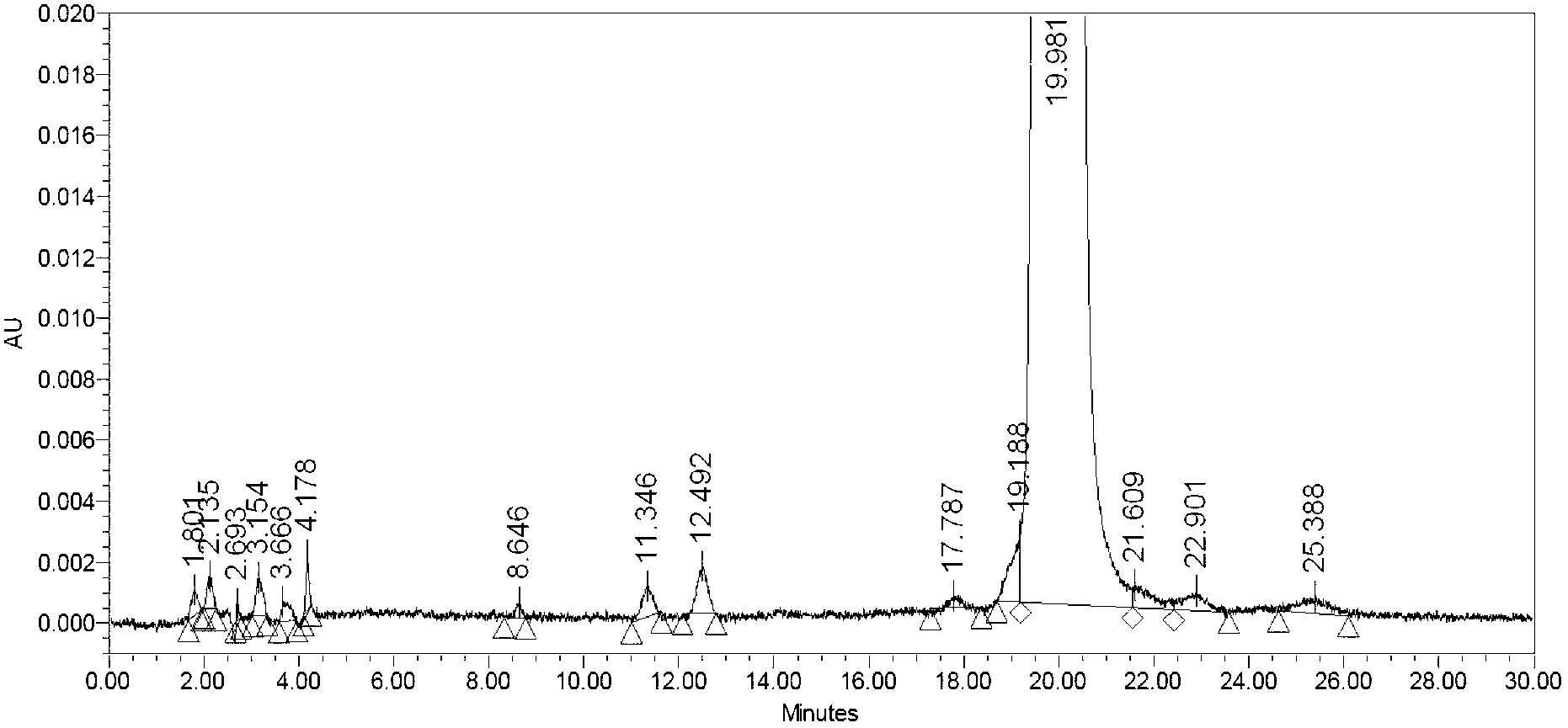
Preparation method of anticoagulant polypeptide
A crude peptide and peptide resin technology, applied in the field of medicinal chemistry, can solve the problems of high cost, many purification steps, and unsatisfactory results, and achieve the effects of low cost, simple operation, and considerable economic and practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of Fmoc-Gly-Gly-CTC Resin with a degree of substitution of 0.6mmol / g
[0042] Weigh 300g of 2-CTC resin with a substitution degree of 1.0mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, take 79.65g of Fmoc-Gly-Gly-OH and wash it with DMF Dissolved, activated by adding 97.8mL DIPEA in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, reacted for 2 hours, added 243mL of anhydrous methanol to block for 30min. Wash with DMF for 3 times, DCM for 3 times, shrink and dry with methanol to obtain Fmoc-Gly-Gly-CTC resin, and the detected substitution degree is 0.628mmol / g.
Embodiment 2
[0043] Example 2: Preparation of Fmoc-Gly-Gly-CTC Resin with a degree of substitution of 1.0mmol / g
[0044] Weigh 300g of 2-CTC resin with a substitution degree of 1.2mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then take 159.3g of Fmoc-Gly-Gly-OH and wash it with DMF Dissolved, activated by adding 195.6mL DIPEA in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, and reacted for 2 hours, then added 243mL of anhydrous methanol to block for 30min. Wash with DMF for 3 times, DCM for 3 times, shrink and dry with methanol to obtain Fmoc-Gly-Gly-CTC resin, and the detected substitution degree is 0.988mmol / g.
Embodiment 3
[0045] Example 3: Preparation of Fmoc-Gly-Gly-CTC Resin with a degree of substitution of 0.8mmol / g
[0046] Weigh 300g of 2-CTC resin with a substitution degree of 1.0mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, take 106.2g of Fmoc-Gly-Gly-OH and wash it with DMF Dissolved, activated by adding 130.4mL DIPEA in an ice-water bath, then added to the above-mentioned reaction column filled with resin, reacted for 2 hours, added 243mL of anhydrous methanol to block for 30min. Wash with DMF for 3 times, DCM for 3 times, shrink and dry with methanol to obtain Fmoc-Gly-Gly-CTC resin, the detection degree of substitution is 0.806mmol / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com