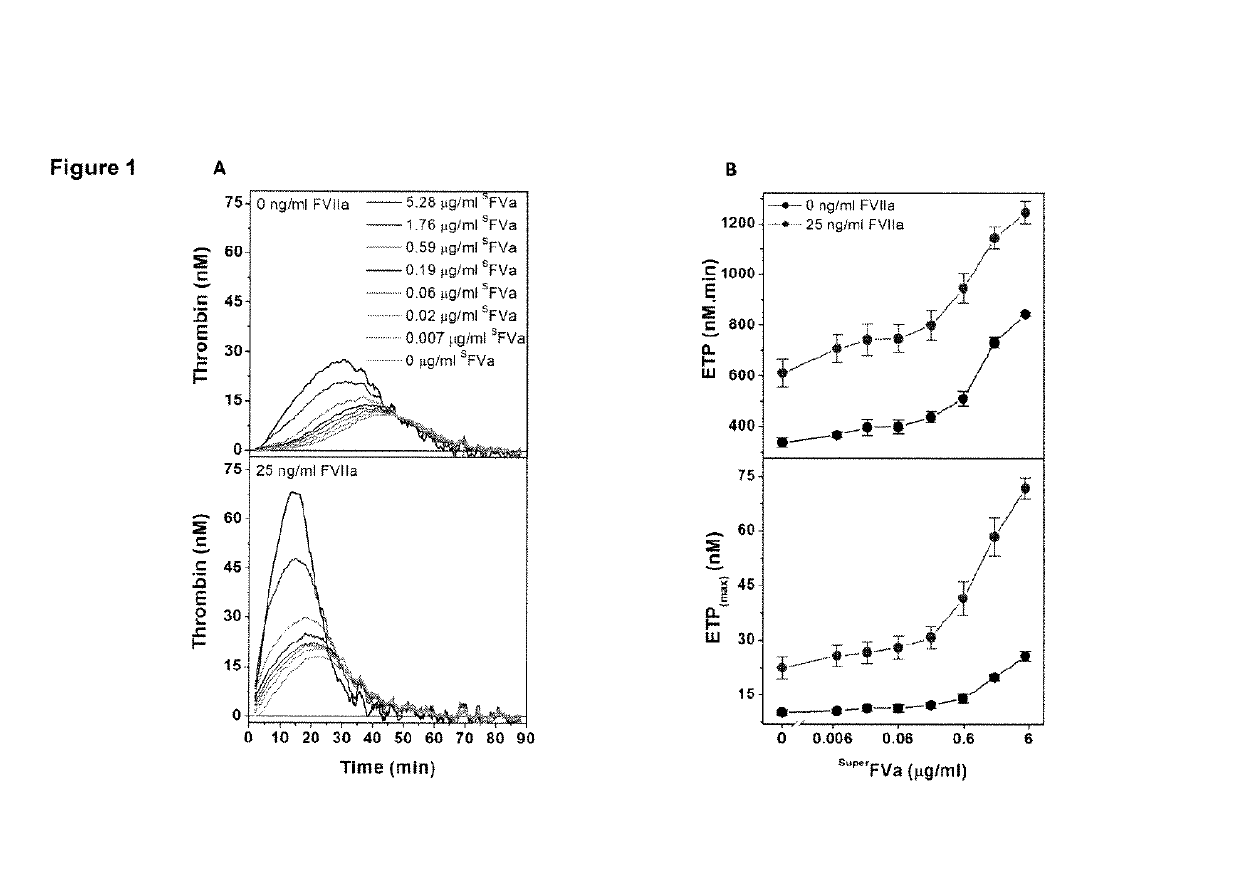
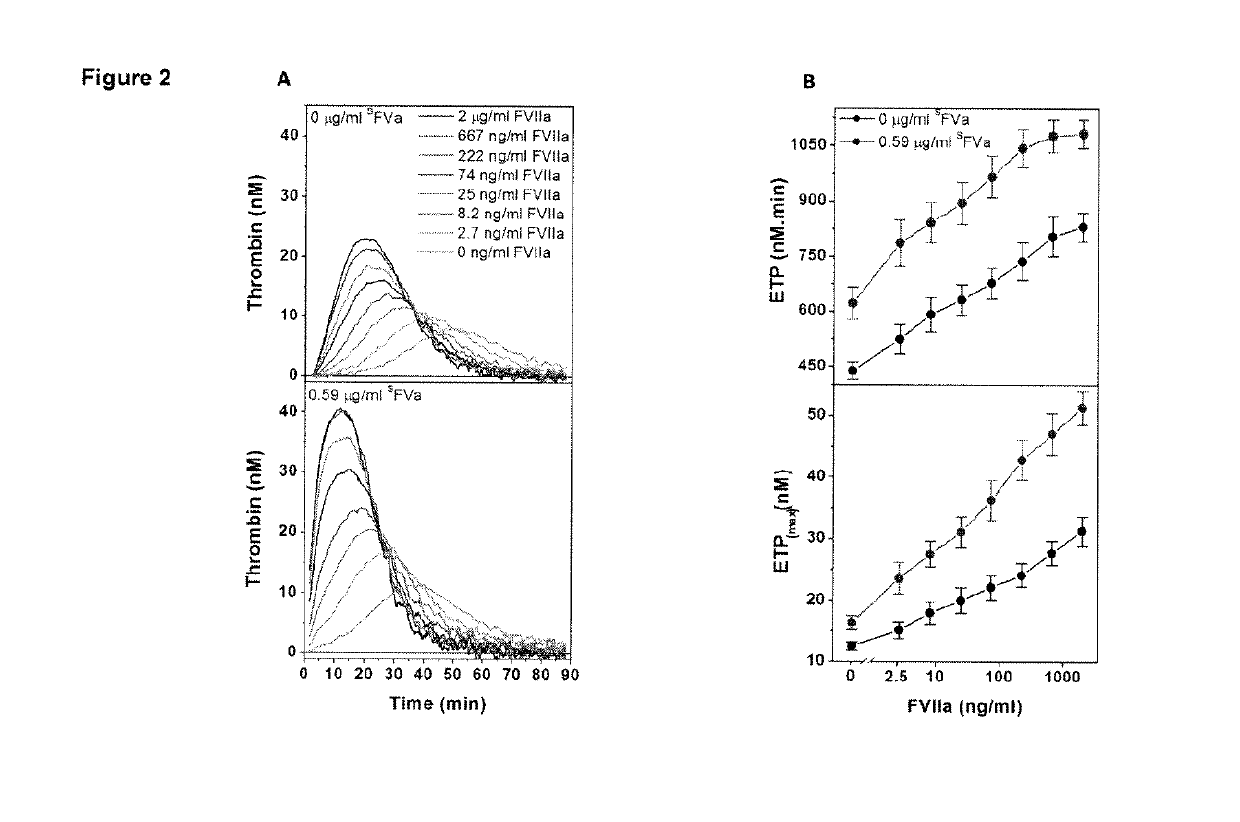
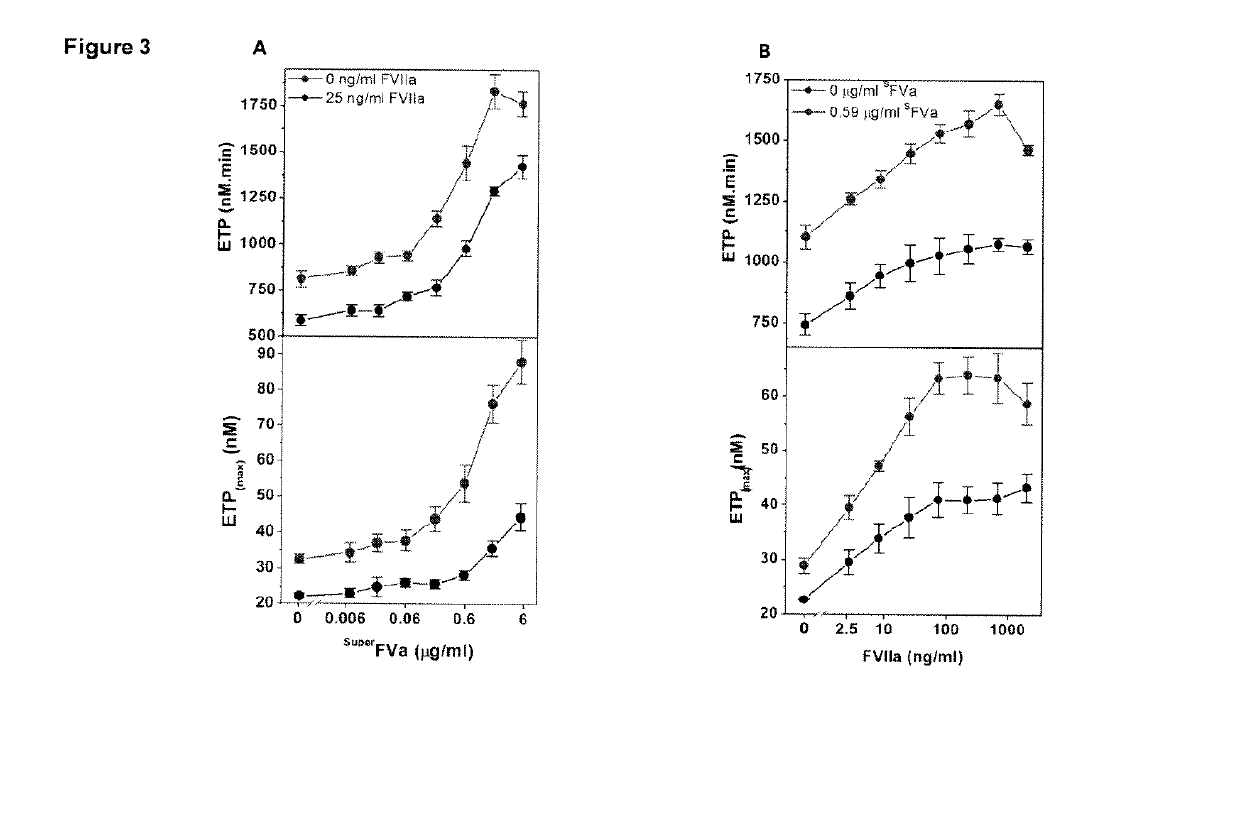
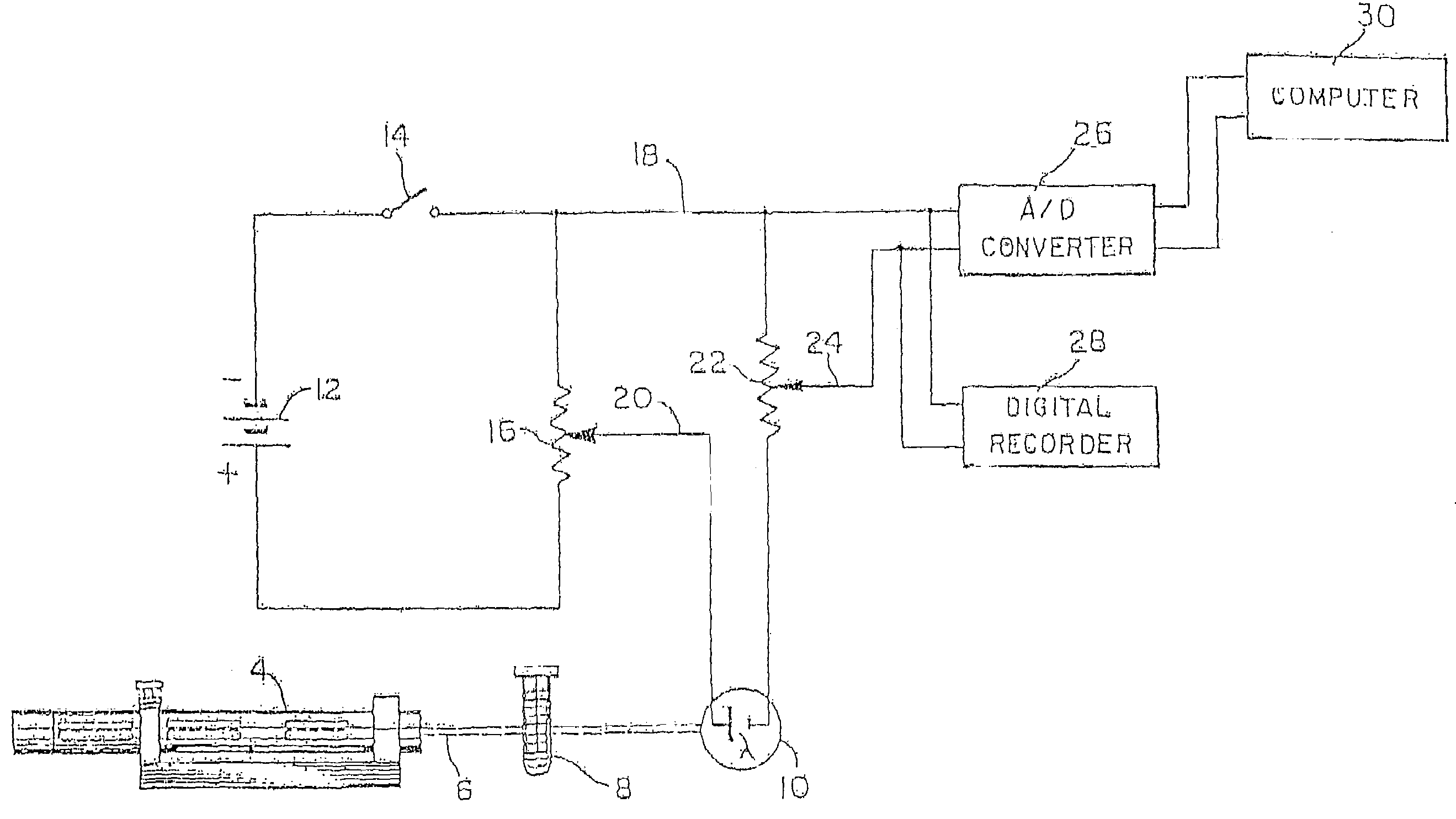
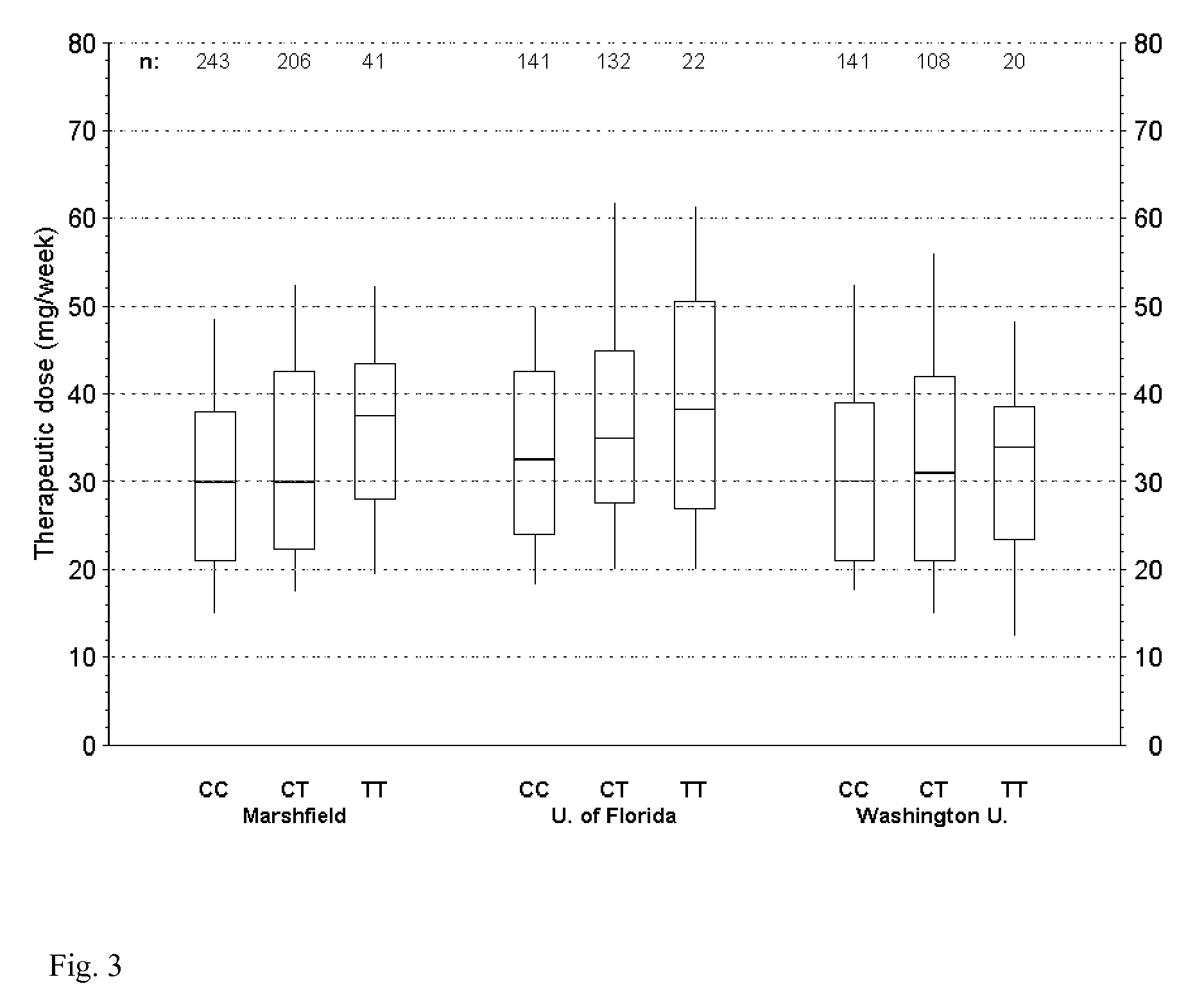Patents
Literature
68 results about "Anticoagulant therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anticoagulant therapy is a course of drug therapy in which anticoagulant medications are administered to a patient to slow the rate at which the patient's blood clots. There are a number of reasons for a patient to be put on anticoagulant therapy, ranging from deep vein thrombosis to atrial fibrillation.
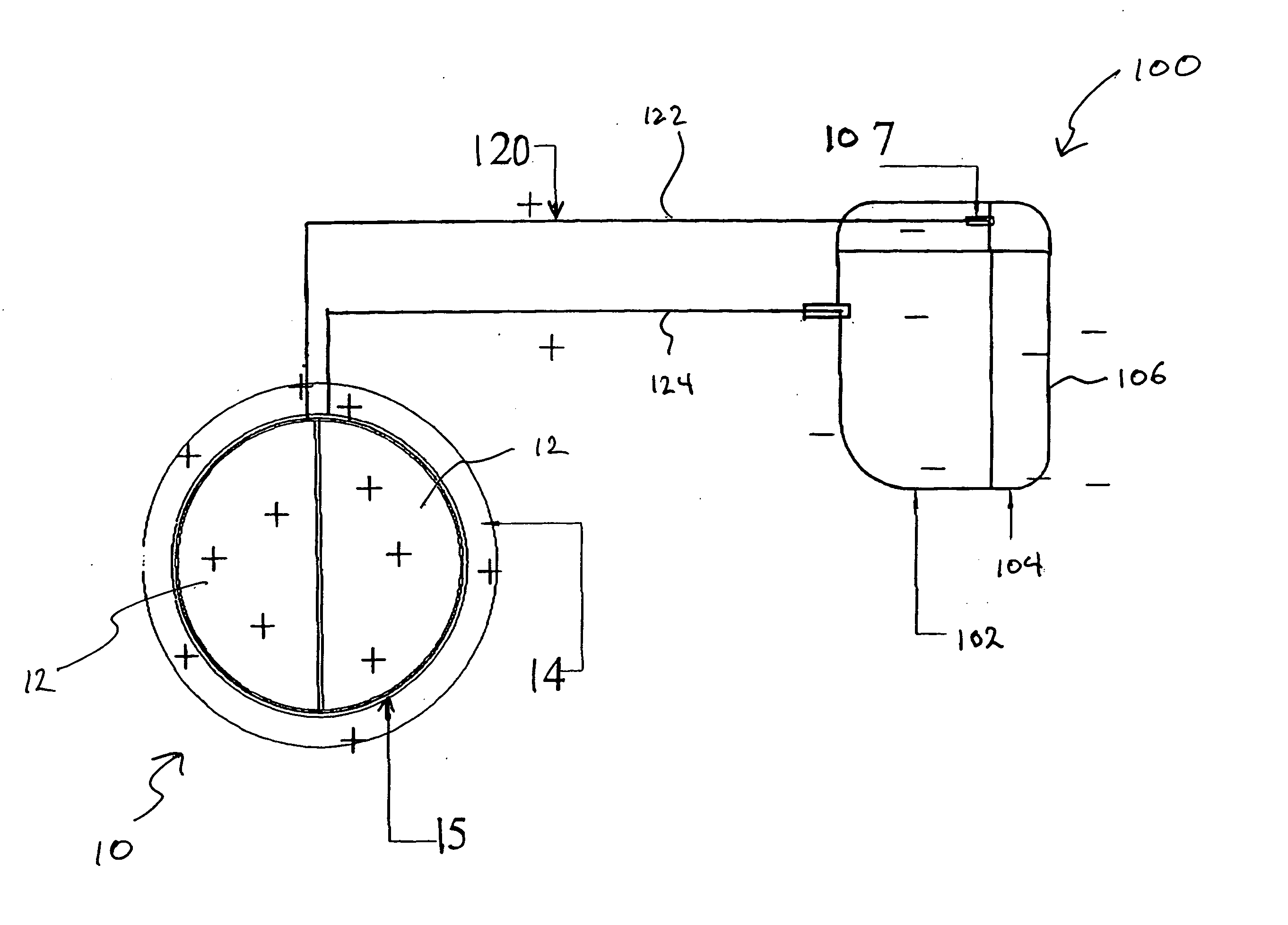
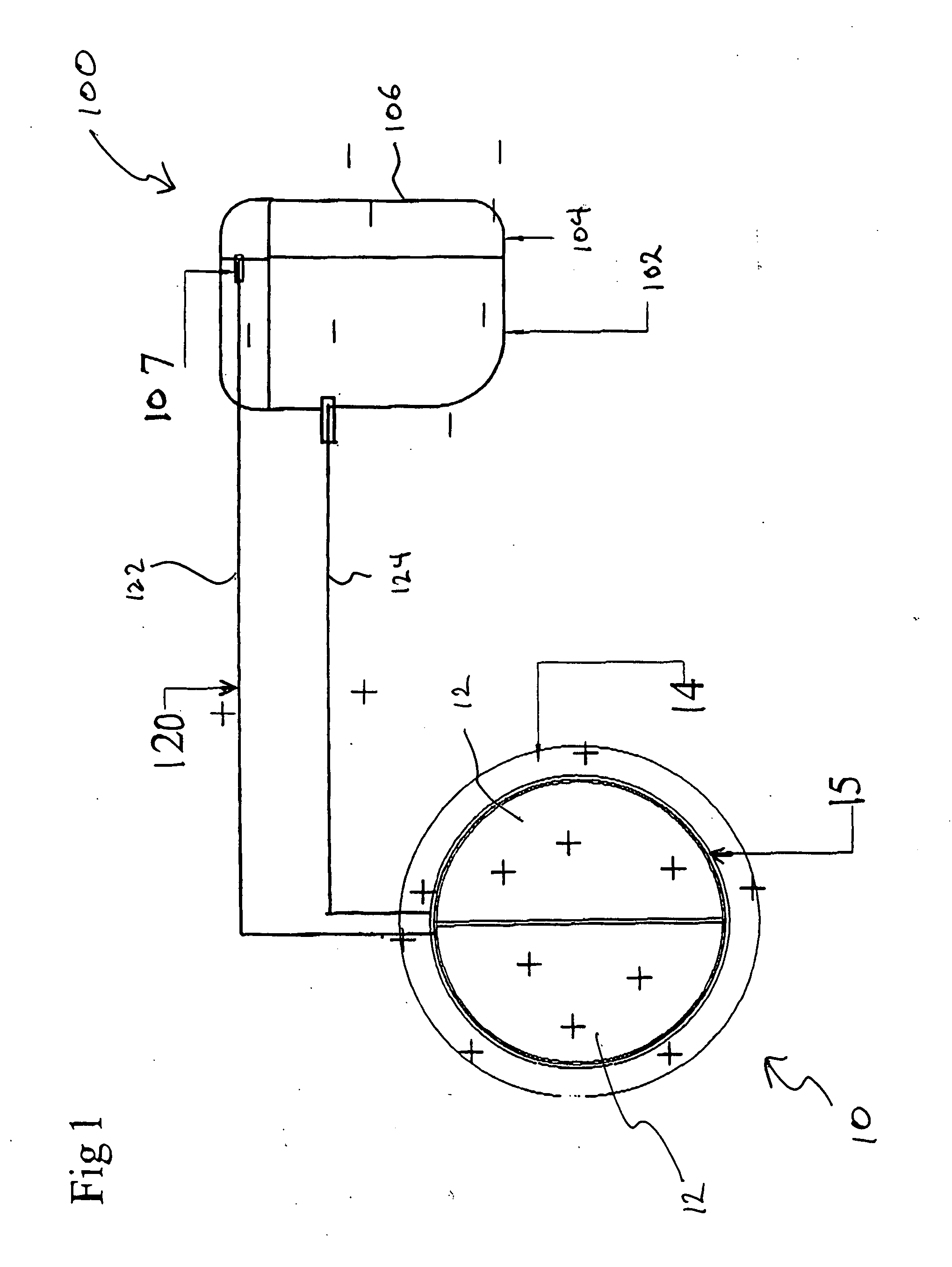
Method of rendering a mechanical heart valve non-thrombogenic with an electrical device
InactiveUS20050021134A1Reduce and eliminate clottingEasy accessElectrotherapyHeart valvesThrombusElectrical devices
A mechanical device for implantation into a patient's body is designed or modified to be electrically charged to prevent coagulation on the device, thereby extending the life of the device and alleviating the need for the patient to utilize anticoagulant therapy. The device may be a heart valve and is electrically charged by being connected to a power source. The power source is preferably a battery pack implanted in the body and is connected to the device by connector wires. The charge applied to the device may be negative or positive, as long as it helps to repel platelets and / or red blood cells from the device in order to help prevent coagulation on one or more surfaces of the device.
Owner:JS VASCULAR
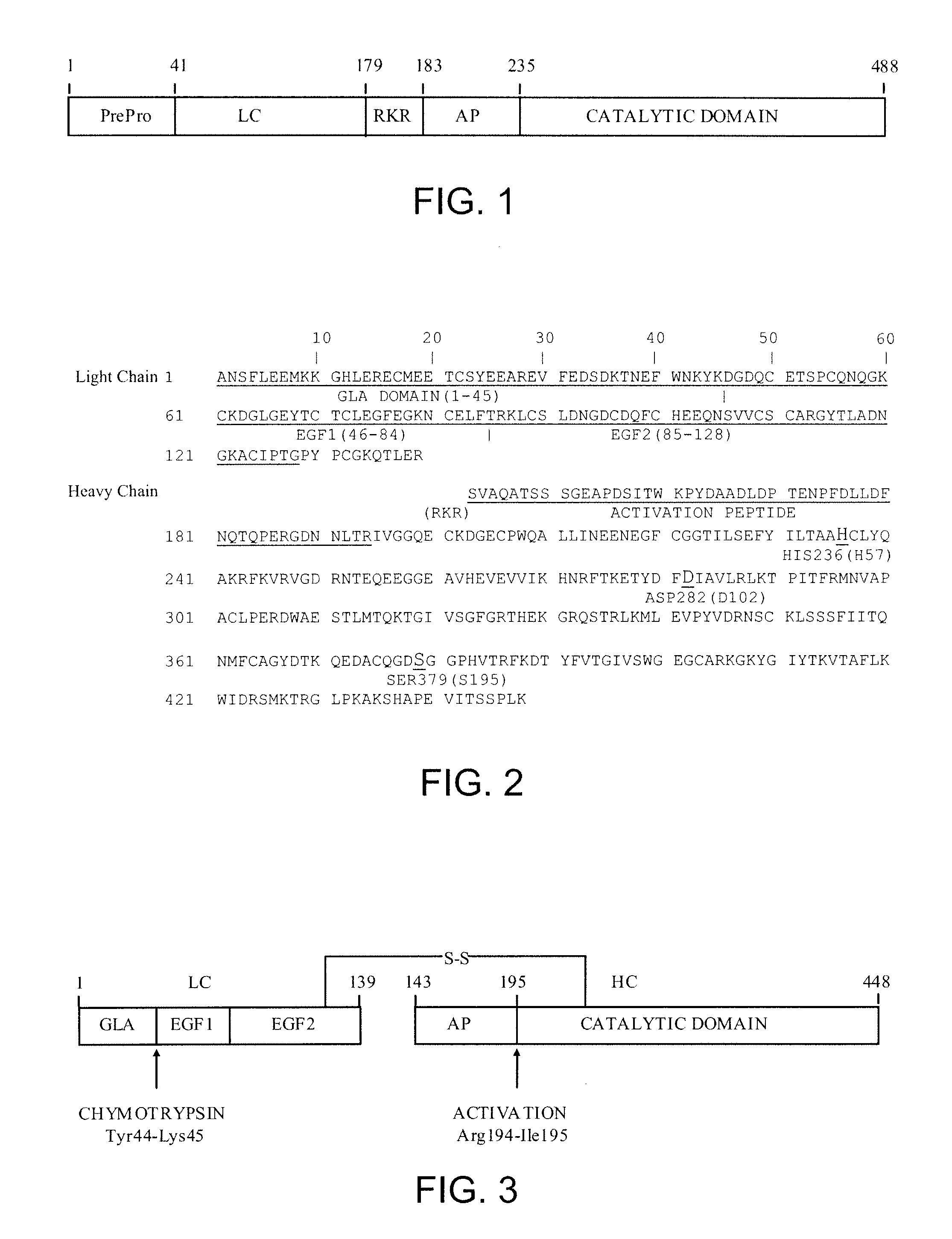
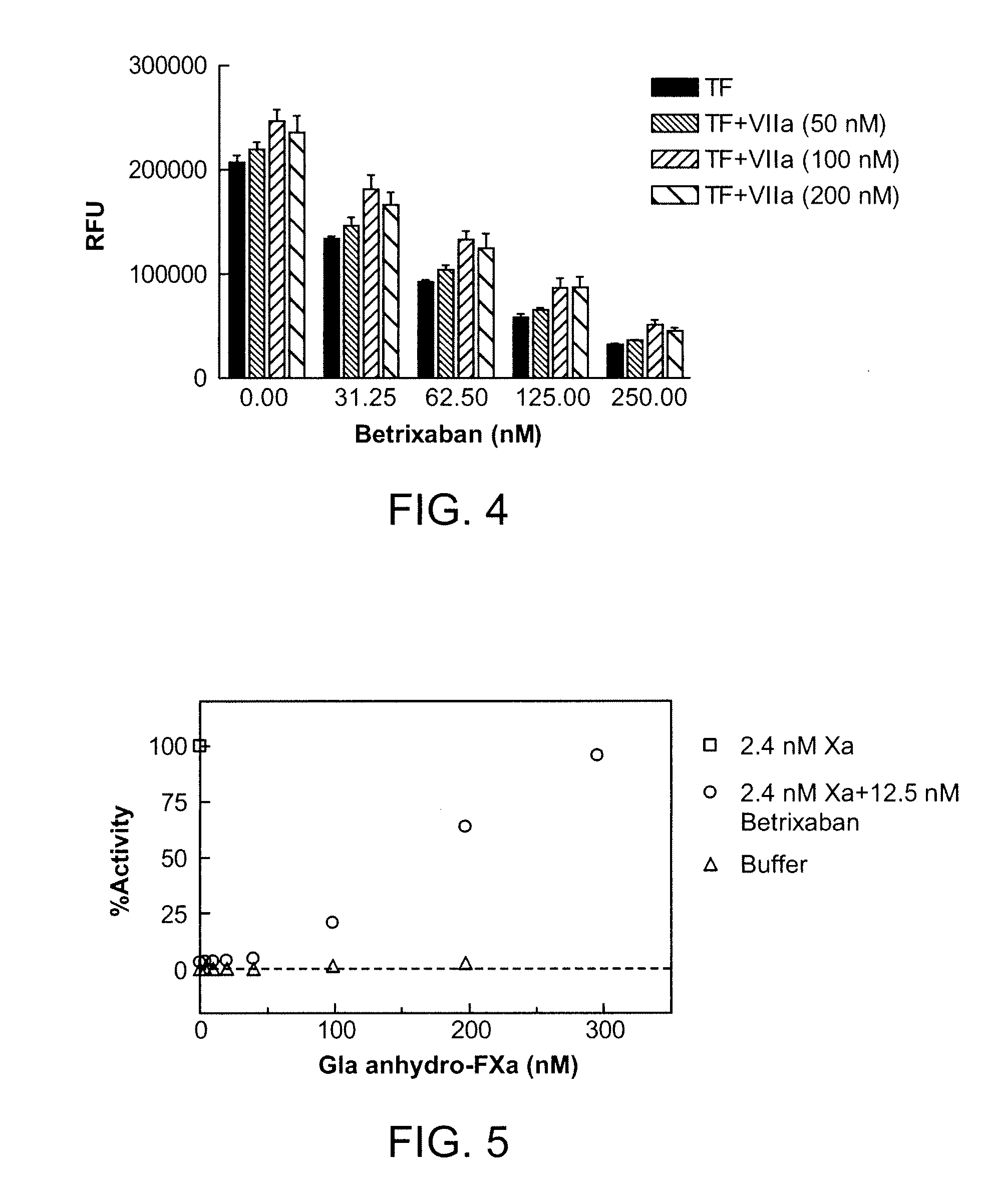
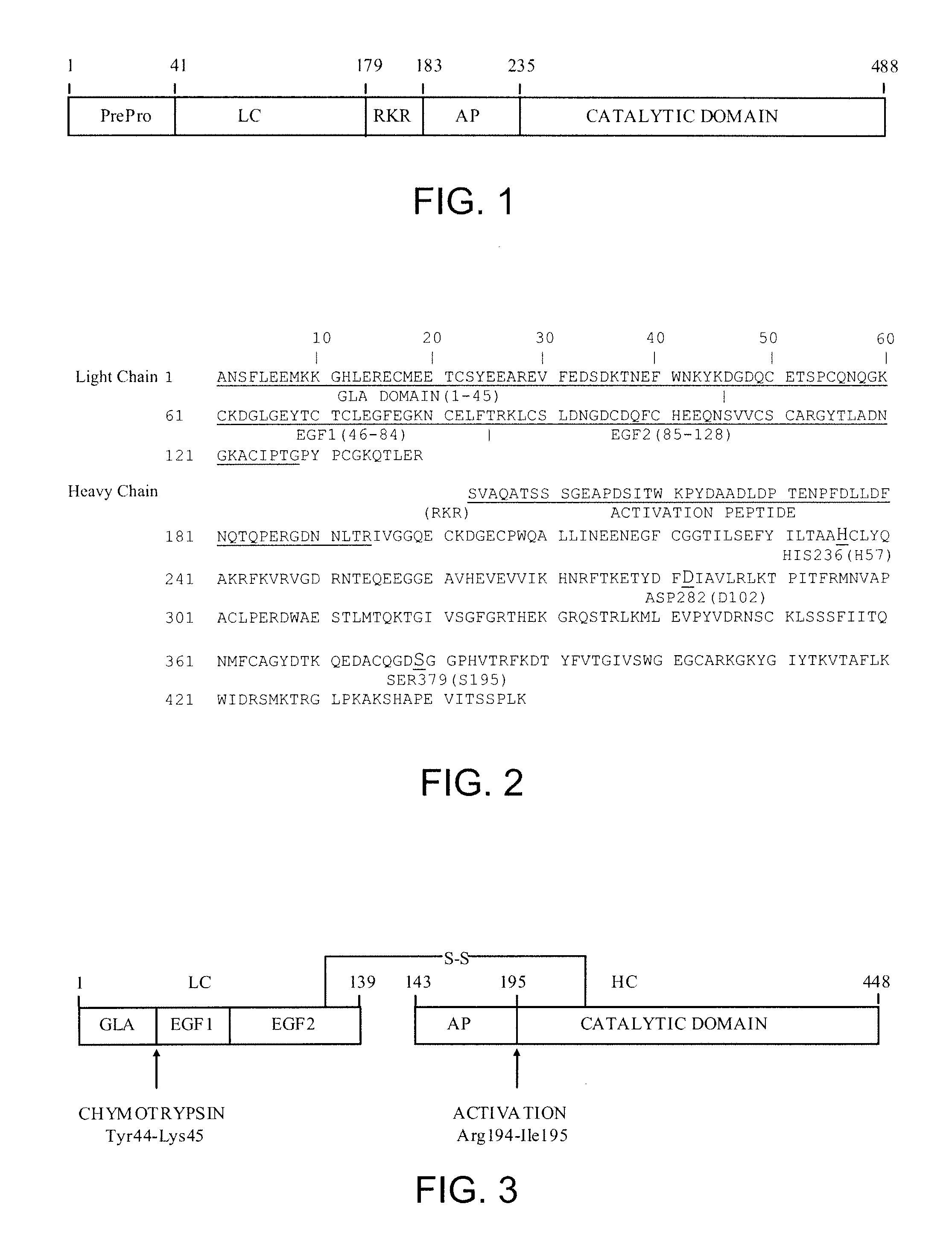
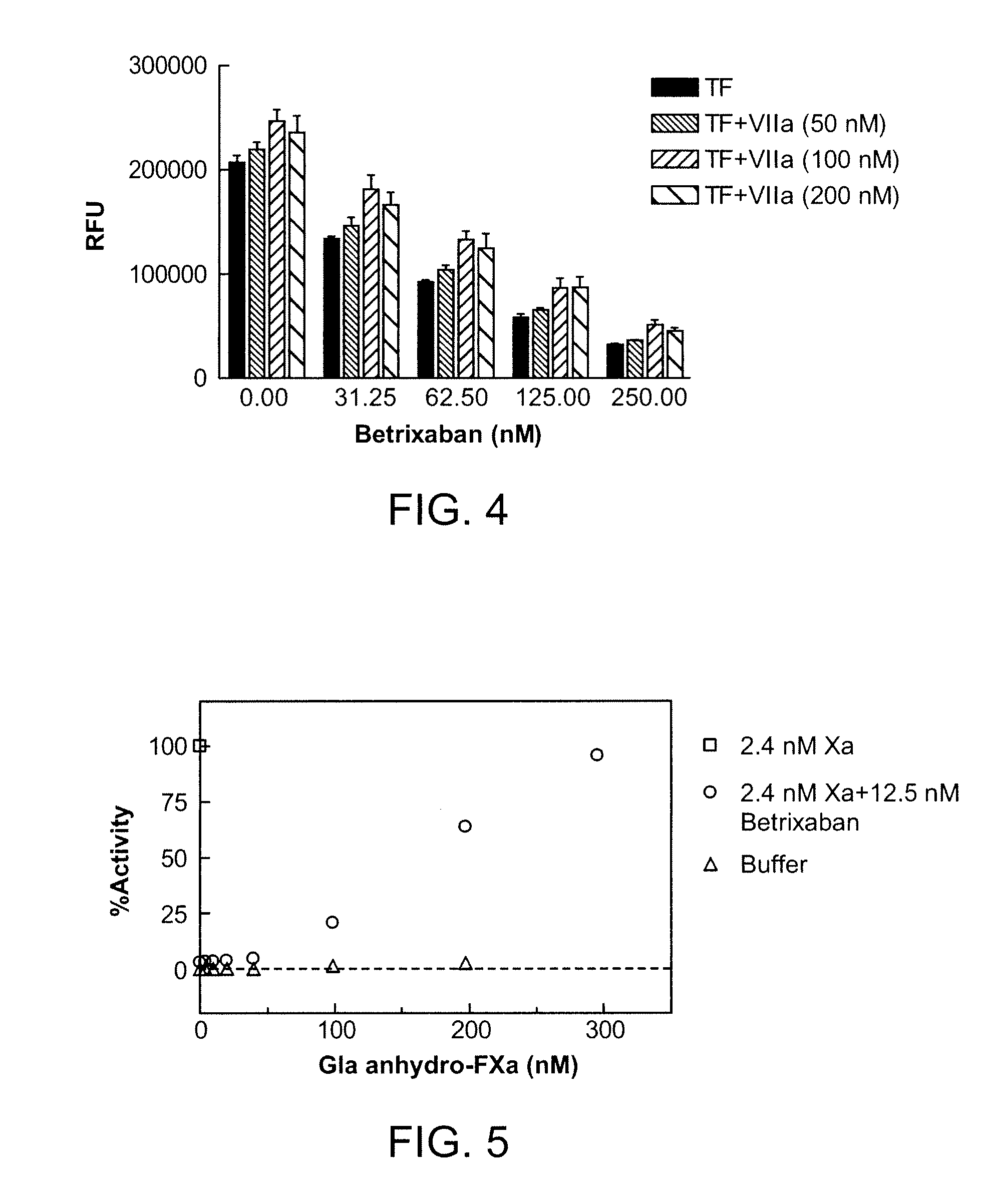
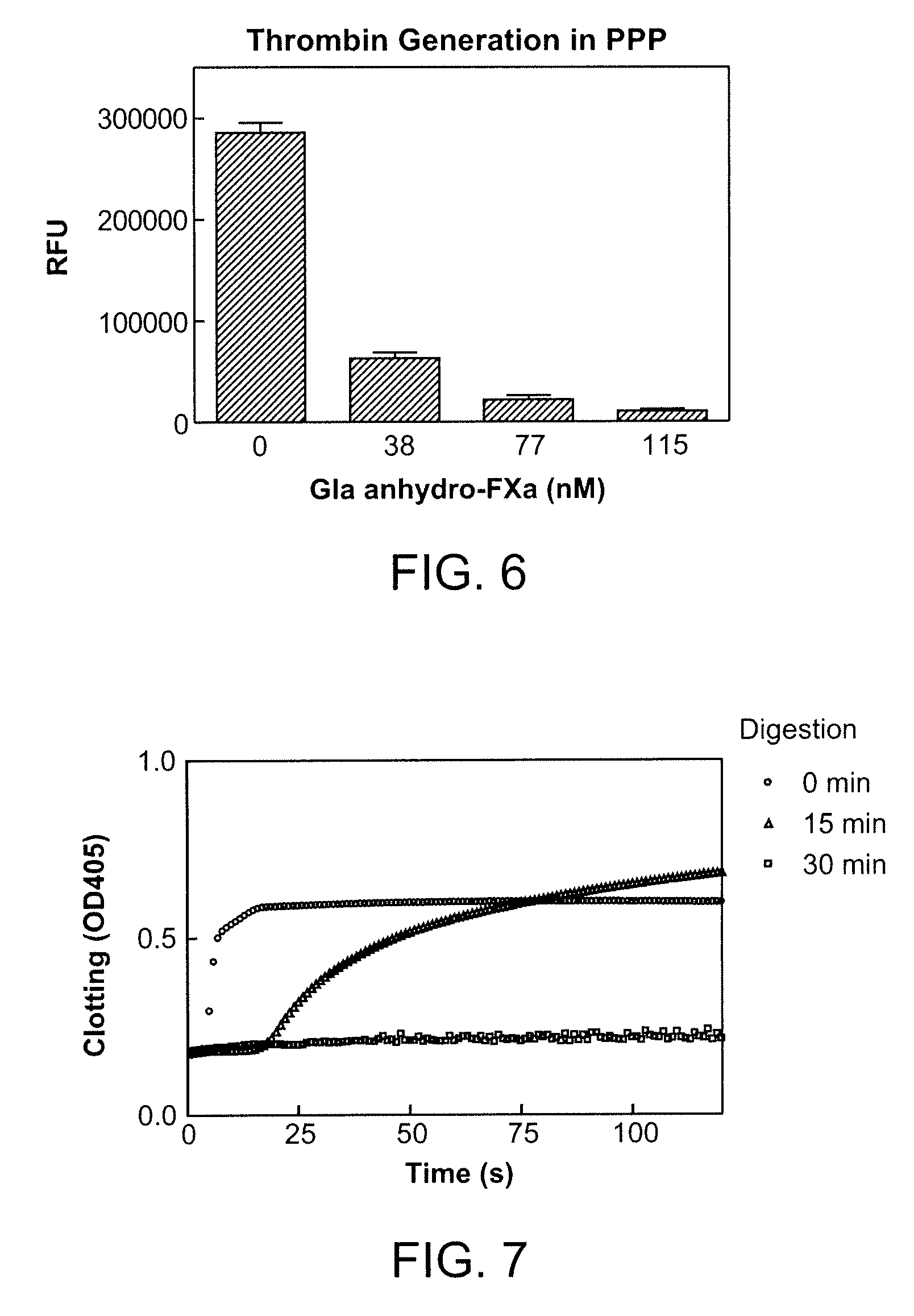
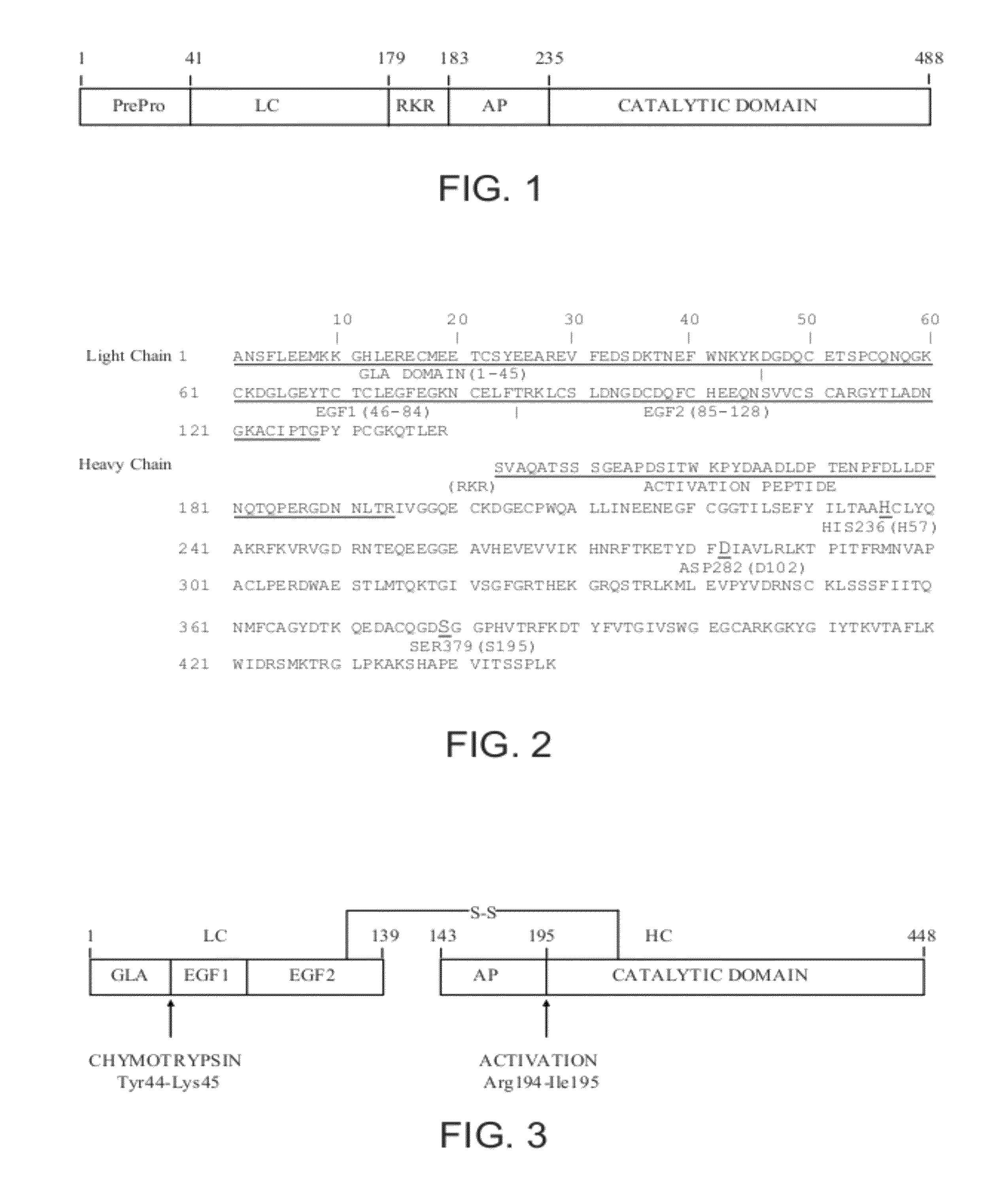
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20090098119A1Reduce and remove intrinsic procoagulantReduce and remove and anticoagulant activityOrganic active ingredientsHydrolasesMedicineAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Method for monitoring coagulability and hypercoagulable states
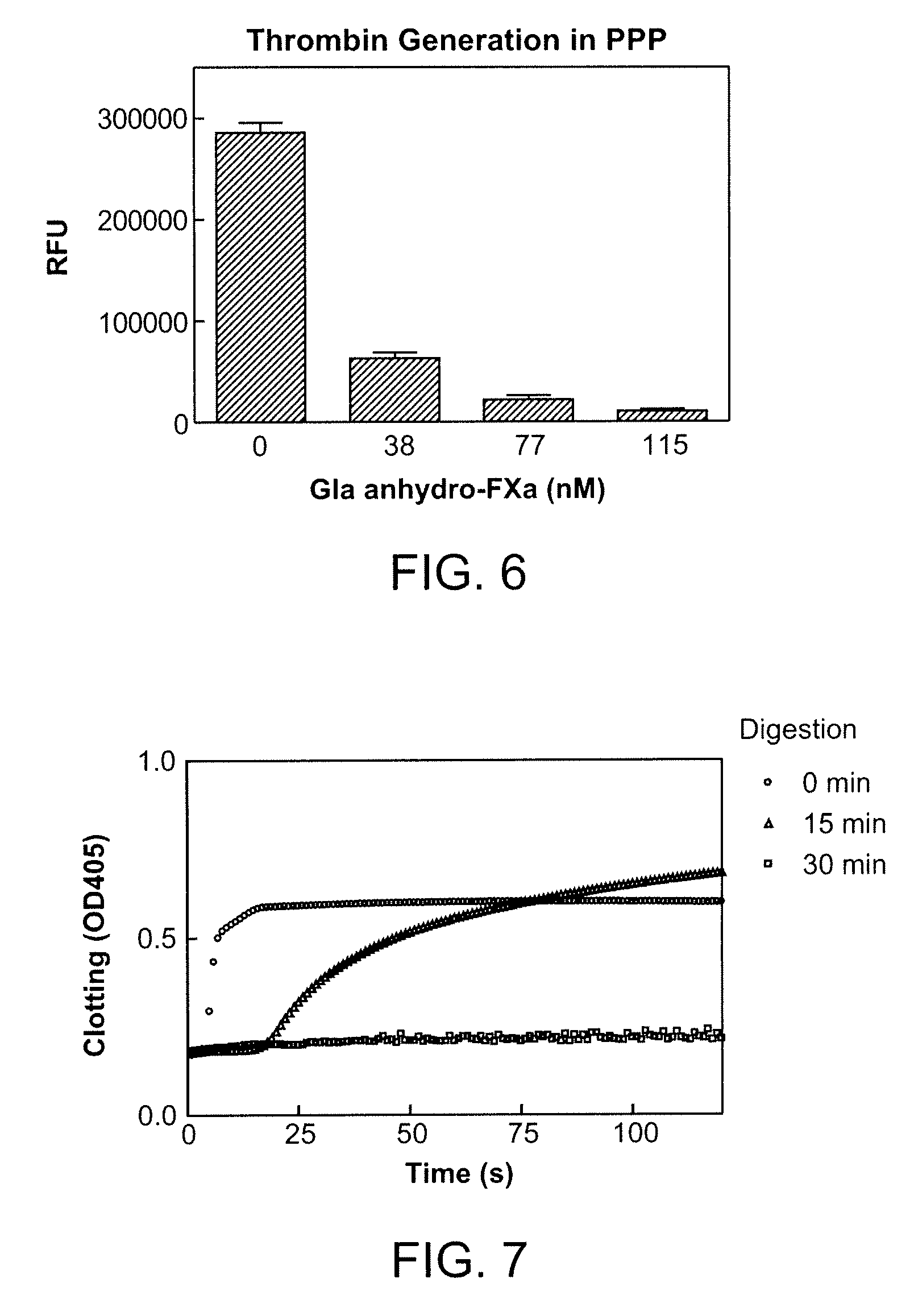
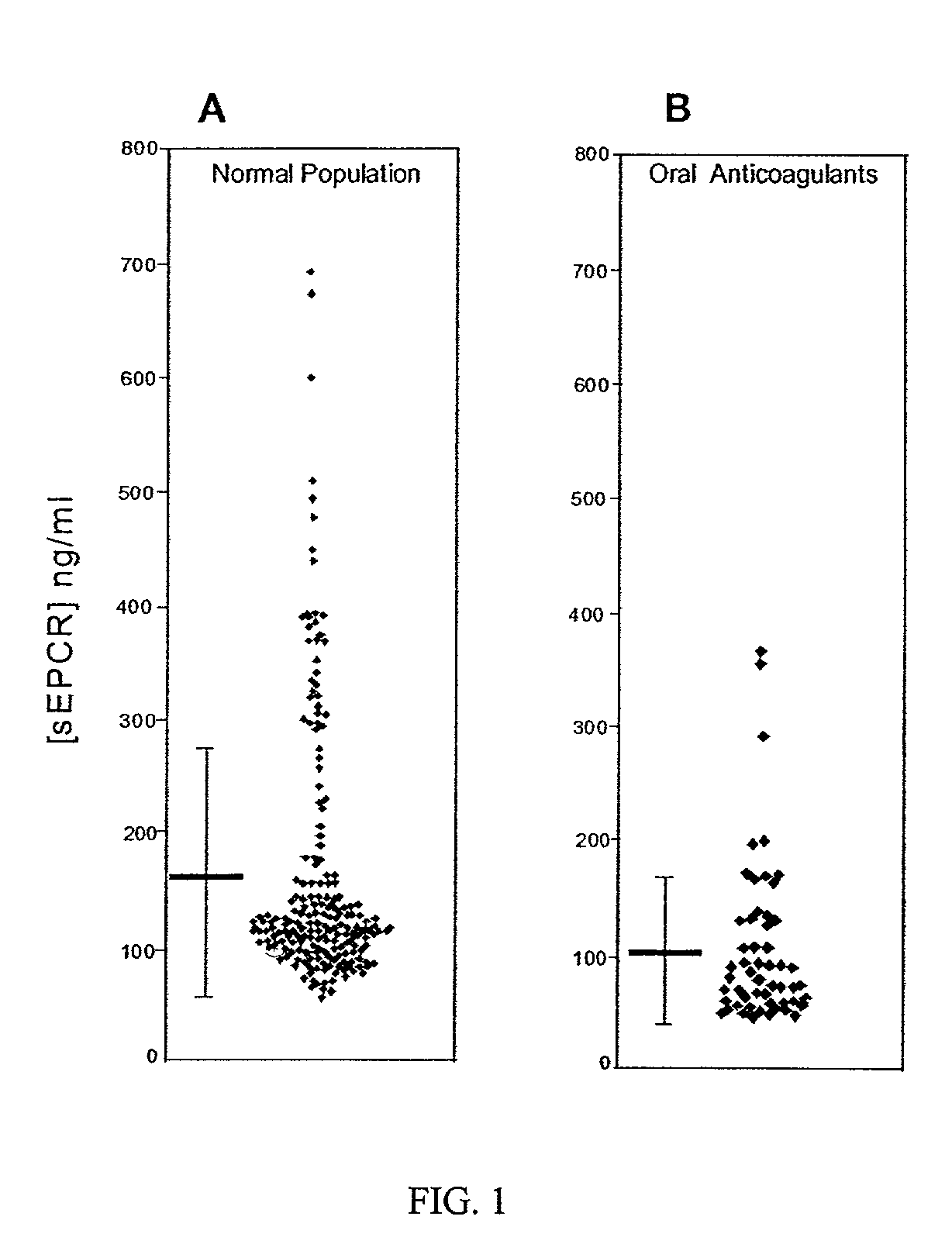
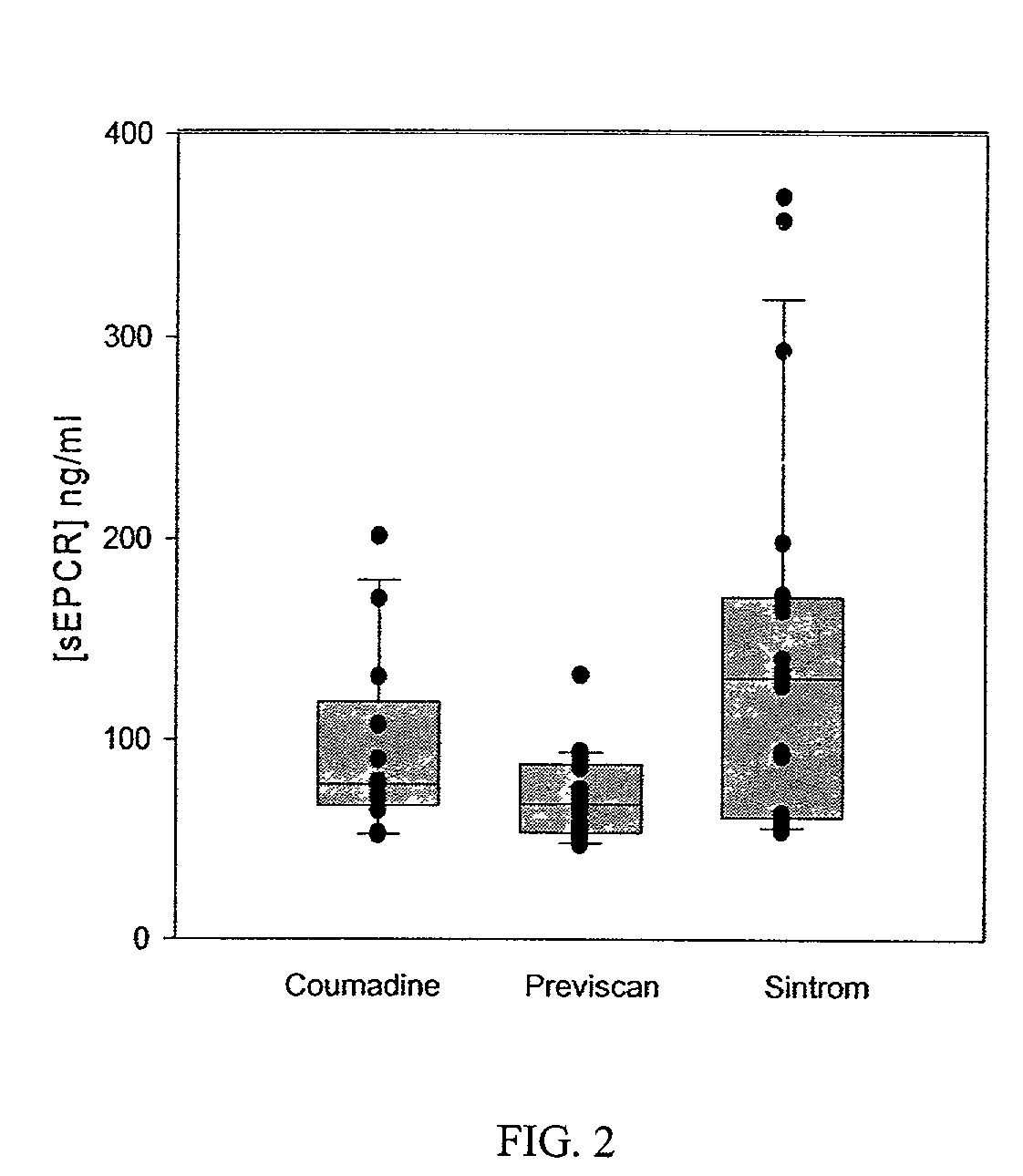
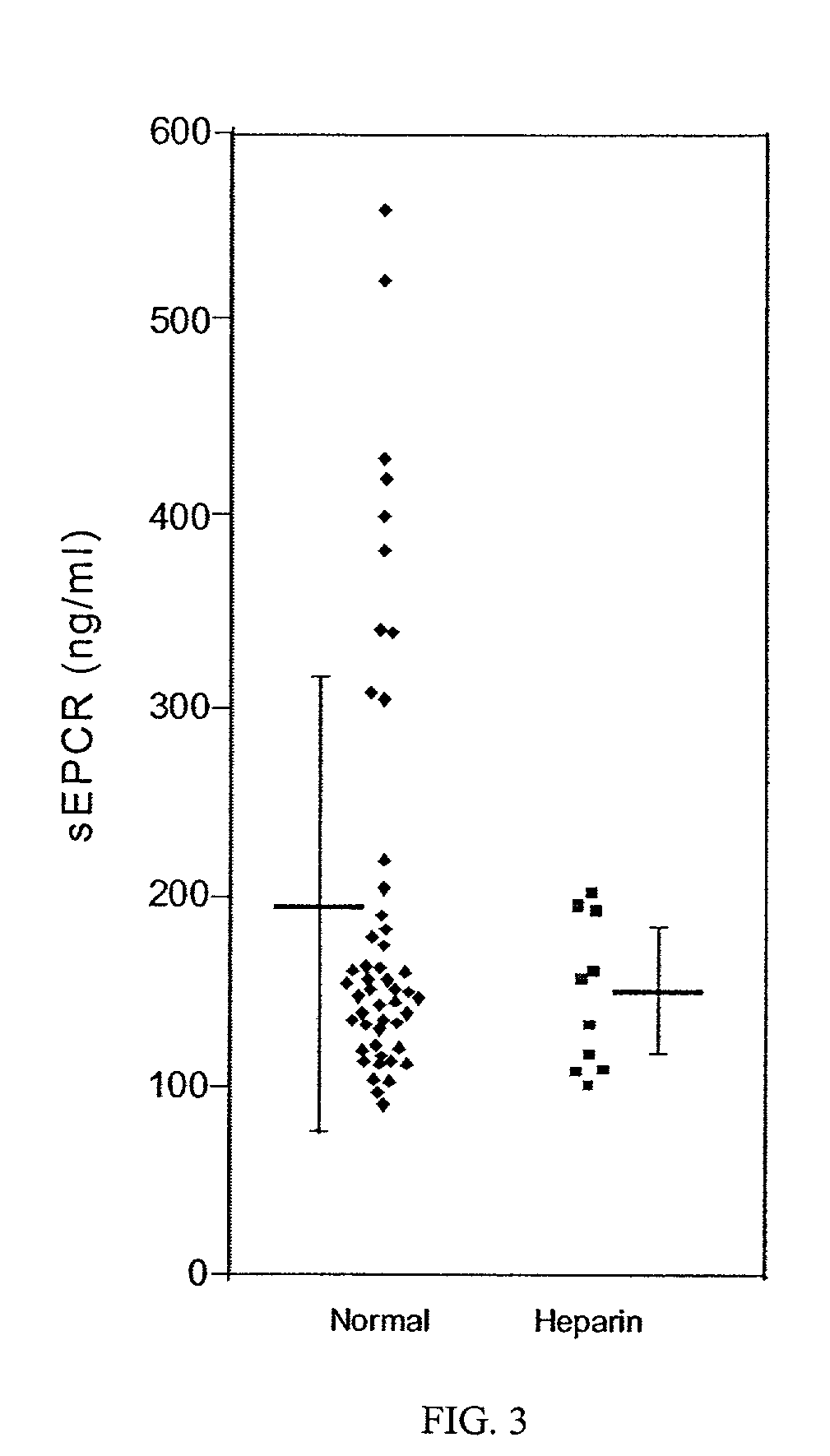
The assay of soluble endothelial protein C receptor (sEPCR) is useful to monitor effective thrombin levels and a hypercoagulable state. An assay for sEPCR is therefore useful to monitor ongoing effectiveness of anticoagulant therapy. A sEPCR ELISA assay is particularly useful for this purpose. A state of hypercoagulability in patients or normal individuals can also be identified by such an assay.
Owner:OKLAHOMA MEDICAL RES FOUND
Antidotes for factor Xa inhibitors and methods of using the same
ActiveUS8153590B2Reduces and removes anticoagulant effectReduced activityPeptide/protein ingredientsMammal material medical ingredientsAntidoteFactor Xa Inhibitor
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Antidotes for factor Xa inhibitors and methods of using the same
ActiveUS8268783B2Reduces and removes anticoagulant effectReduced activityBiocidePeptide/protein ingredientsFactor XAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor X and factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of reversing anticoagulation, stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Combination anticoagulant therapy with a compound that acts as a factor xa inhibitor
InactiveUS20080254036A1Predictable levelImprove efficacyBiocideAntipyreticFactor iiFactor Xa Inhibitor
The present invention is directed to methods of using combination therapies containing [2-({4-[(dimethylamino)iminomethyl]phenyl}carbonylamino)-5-methoxyphenyl]-N-(5-chloro(2-pyridyl))carboxamide for the treatment of thrombotic disease(s) and pharmaceutical compositions thereof.
Owner:MILLENNIUM PHARMA INC
Method of providing hemostasis in Anti-coagulated blood
InactiveUS20080254147A1Promote formationBiocideInanimate material medical ingredientsMedicineB hemophilia
In a method of clotting blood in which the blood exhibits a reduced tendency to clot and may be from a person undergoing an anticoagulant therapy or having type A or B hemophilia or von Willebrand disease, a therapeutically effective amount of a composition comprising clay as the active ingredient is administered to a wound from which the blood emanates. Upon contacting the blood, this clay, which may be kaolin, bentonite, or any type of layered clay, causes the blood to clot. In a method of arresting blood flowing from a wound, a therapeutically effective amount of a composition comprising clay as the active ingredient is administered to the bleeding wound. In this method, the blood has a reduced tendency to clot and may be from a person undergoing an anticoagulant therapy or having at least one of hemophilia A or B or von Willebrand disease.
Owner:TELEFLEX LIFE SCI LTD
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20100255000A1Preventing and reducing bleedingReduces and removes anticoagulant effectPeptide/protein ingredientsHydrolasesMedicineAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor X and factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of reversing anticoagulation, stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Liquid, aqueous, pharmaceutical compositions of factor VII polypeptides
InactiveUS20060063714A1Good storage stabilityPeptide/protein ingredientsInorganic non-active ingredientsFactor VIIaClotting factor deficiency
The invention relates to a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (e.g. human Factor VIIa) and a buffering agent; wherein the molar ratio of non-complexed calcium ions (Ca2+) to the Factor VII polypeptide is lower than 0.5. The composition may further comprise a stabilizing agent (e.g. copper or magnesium ions, benzamidine, or guanidine), a non-ionic surfactant, a tonicity modifying agent, an antioxidant and a preservative. The composition is useful for treating a Factor VII-responsive syndrome, such as bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor XI deficiency, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy.
Owner:NOVO NORDISK AS
Antidotes for factor Xa inhibitors and methods of using the same in combination with blood coagulating agents
ActiveUS8455439B2Low effective doseReduce potential side effectsPeptide/protein ingredientsMammal material medical ingredientsAntidoteFactor Xa Inhibitor
The present invention relates to antidotes of anticoagulants targeting factor Xa which antidotes are used in combination with blood coagulating agents or other heparin antidotes to prevent or reduce bleeding in a subject. The antidotes described herein have reduced or no intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is or will be undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Antidotes for factor xa inhibitors and methods of using the same in combination with blood coagulating agents
ActiveUS20100125052A1Reduce potential side effectsLow effective dosePeptide/protein ingredientsMammal material medical ingredientsMedicineAntidote
The present invention relates to antidotes of anticoagulants targeting factor Xa which antidotes are used in combination with blood coagulating agents or other heparin antidotes to prevent or reduce bleeding in a subject. The antidotes described herein have reduced or no intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is or will be undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
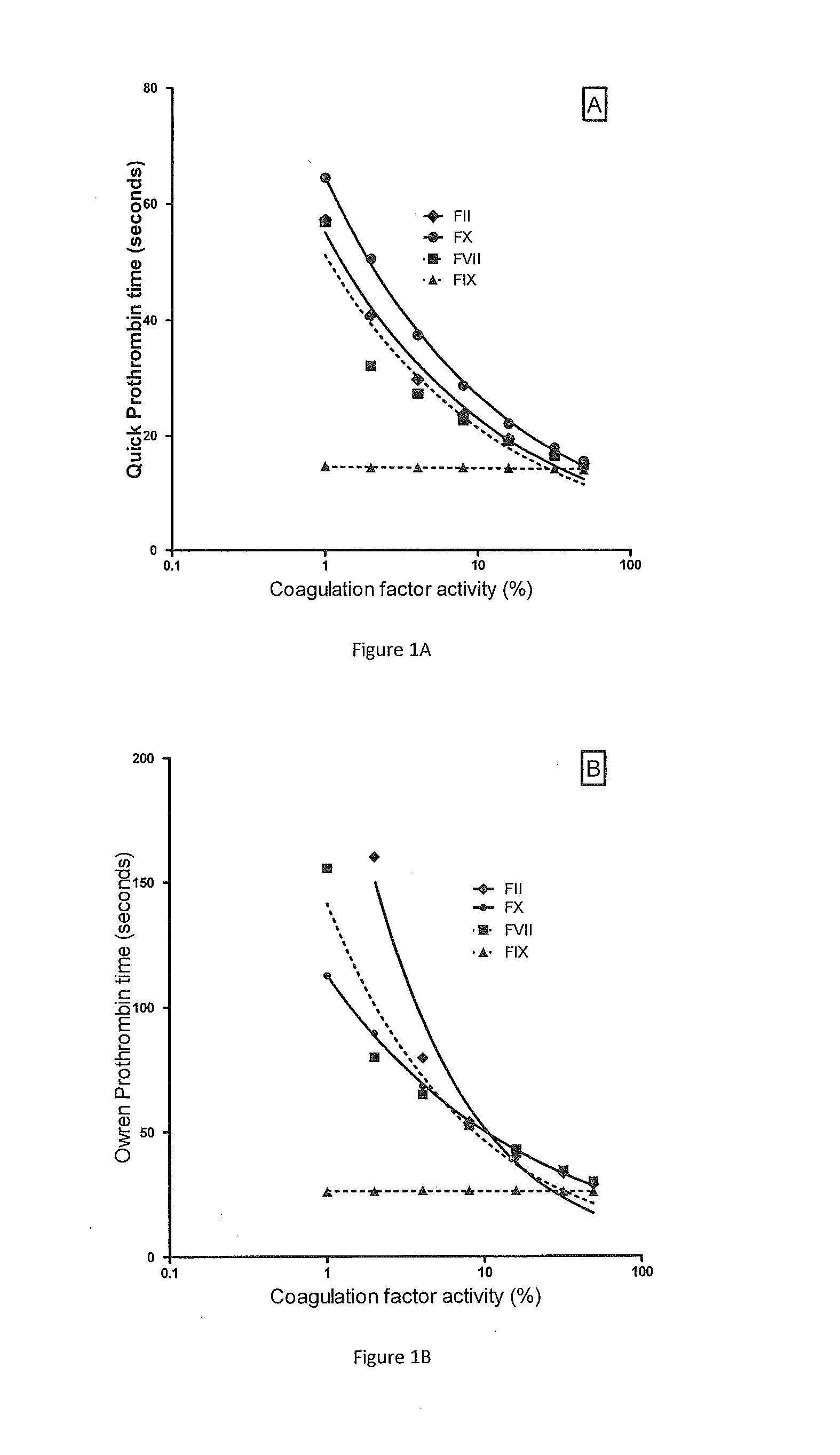
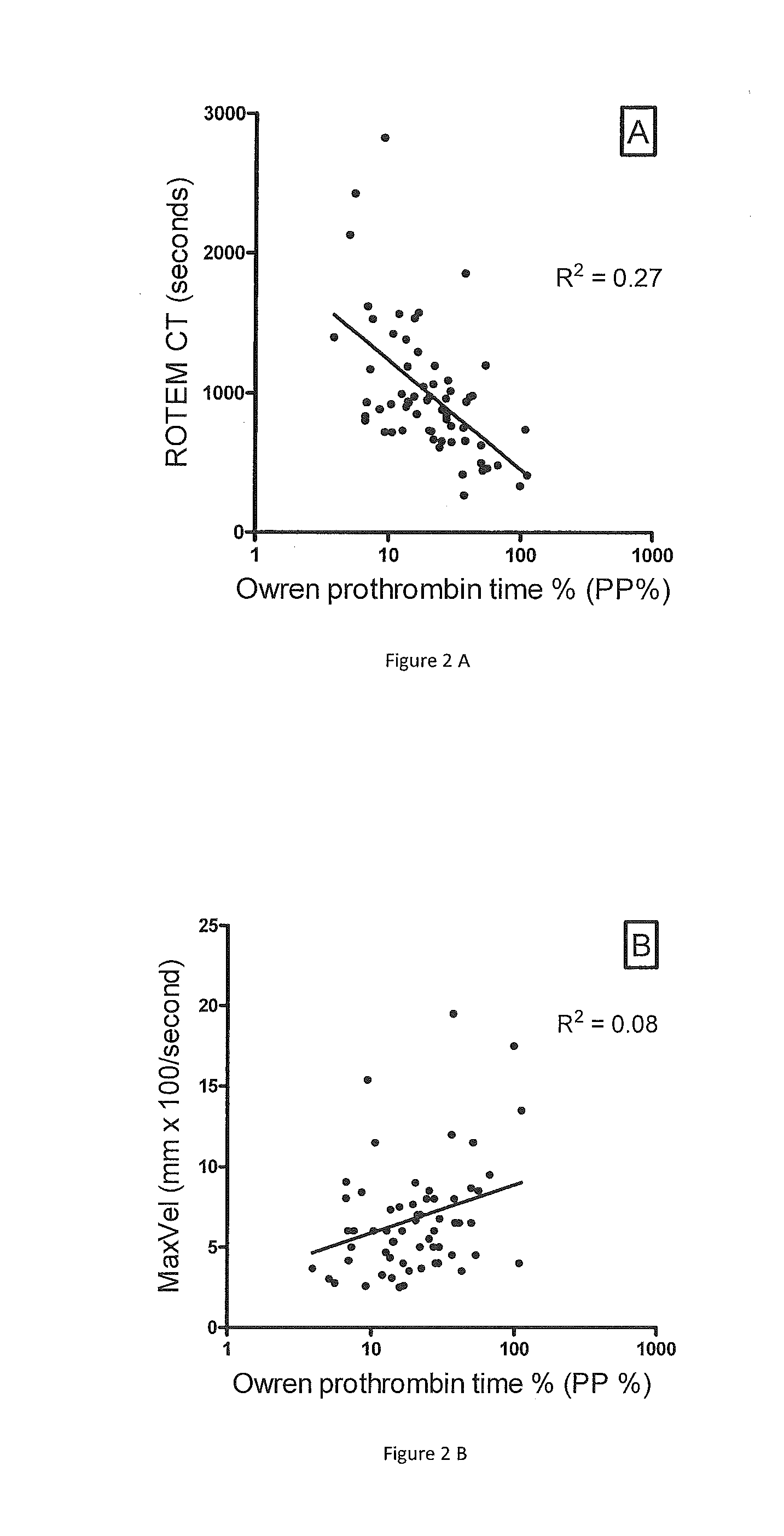
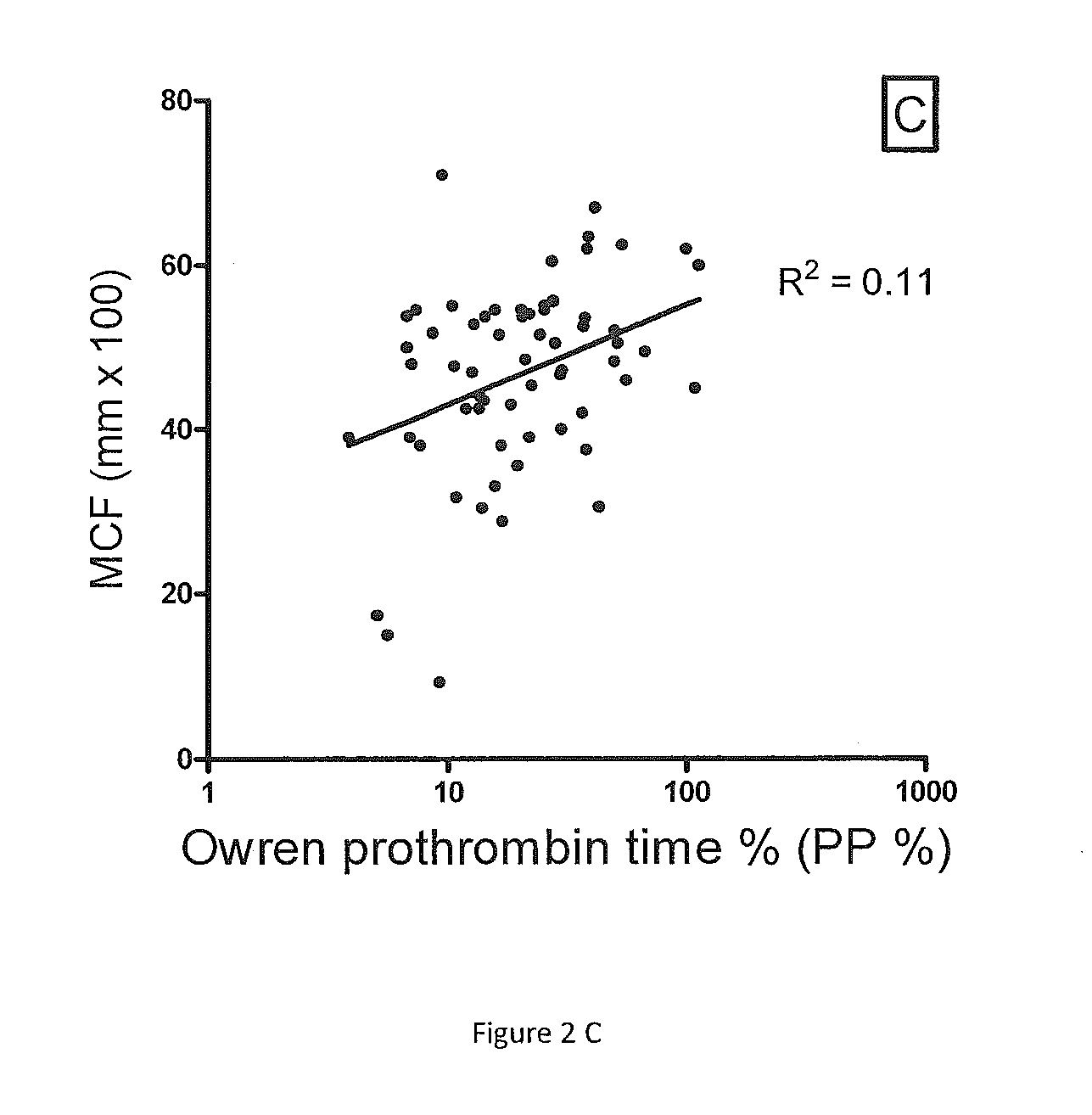
Method for monitoring anticoagulant therapy
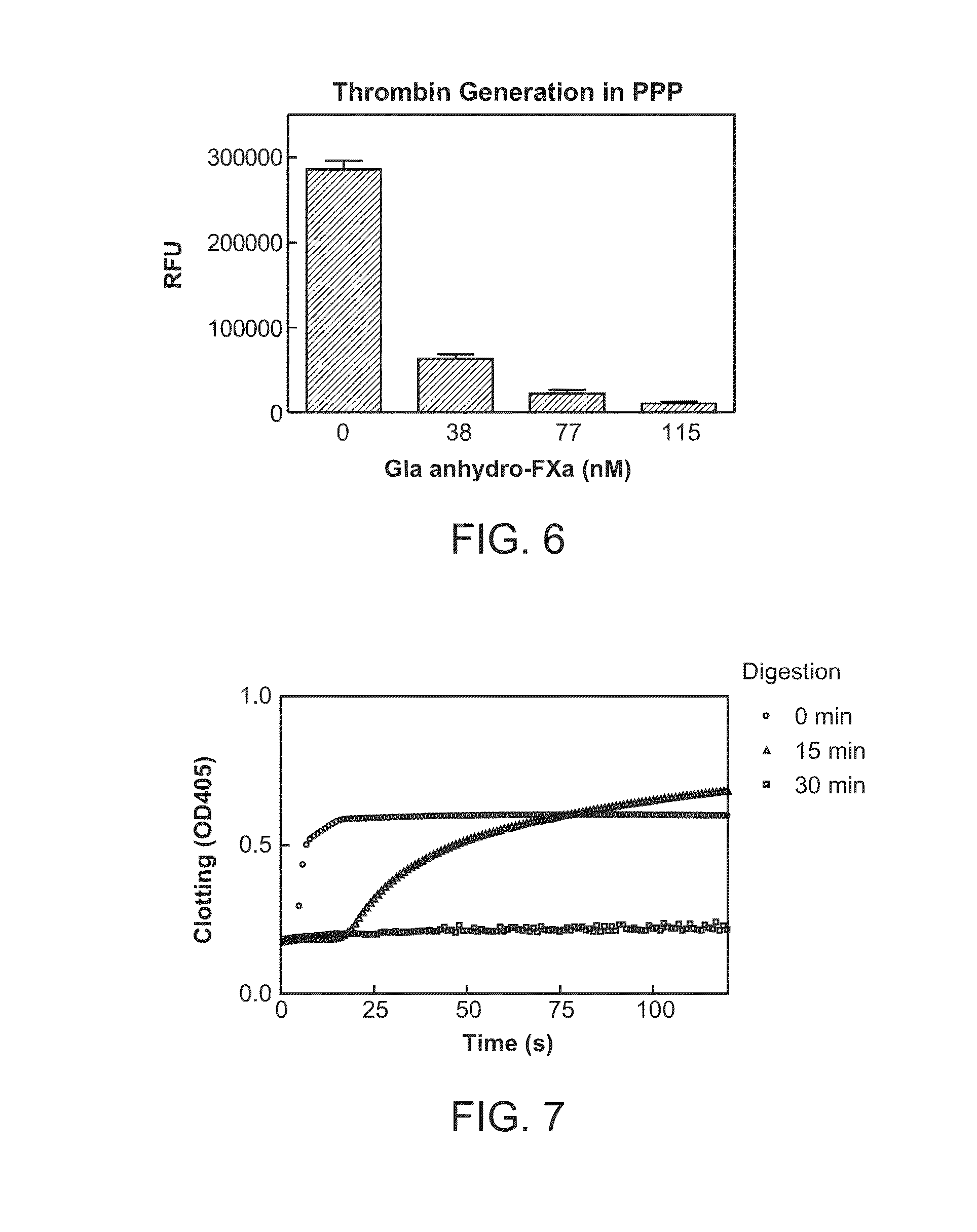
ActiveUS20120231485A1Antithrombotic effectAccurate assessmentMicrobiological testing/measurementChemiluminescene/bioluminescenceTest sampleFactor ii
A method of measuring the combined activity of both and only coagulation factors II and X for the purpose of monitoring anticoagulant therapy, and kits for using the method. The method involves mixing of test plasma from a human to be tested with specially prepared plasma deficient in both and only coagulation factors II and X but with normal levels of other factors (referred to herein as Fiix-deficient plasma or Fiix plasma), in order to correct for any possible deficiency in other coagulation factors than FII and FX in the test sample. By adding a coagulation reagent and calcium, the generation of thrombin or fibrin can be measured. Kits of the invention comprise a coagulation reagent, calcium and specially made plasma that is deficient in both and only factor II and factor FX.
Owner:FIIX DIAGNOSTICS
Combination therapy for anticoagulation
A combination anticoagulation medicament including vitamin K with warfarin in an oral form is described. Between 50 and 5000 micrograms of vitamin K are combined in a single oral medication with 0.5 to 15 milligrams of warfarin for administration. The combination of vitamin K with warfarin in a single orally dosed form is a novel approach to improving the effectiveness of anticoagulation. The combination allows for broader application of warfarin in medical anticoagulation and reduces the variability of anticoagulation due to the influences of diet, additional medications, nutritional status, changes in physical condition, and potentially other factors. Use of the combination therapy improves the safety of warfarin as an appropriate anticoagulant for many medical conditions.
Owner:SCIENTIA MEDICA DONA A DEO
Method and apparatus for determining anticoagulant therapy factors
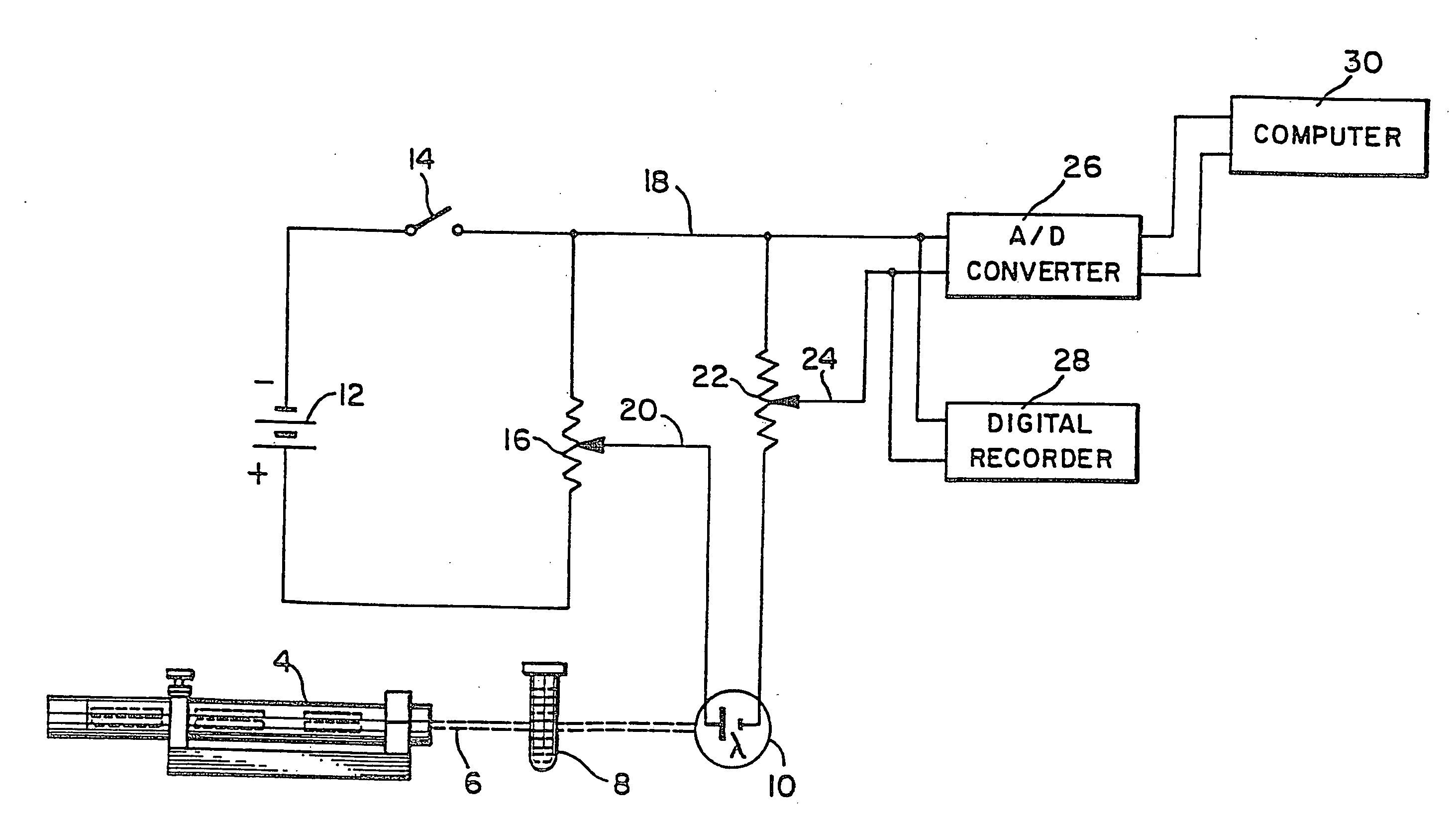
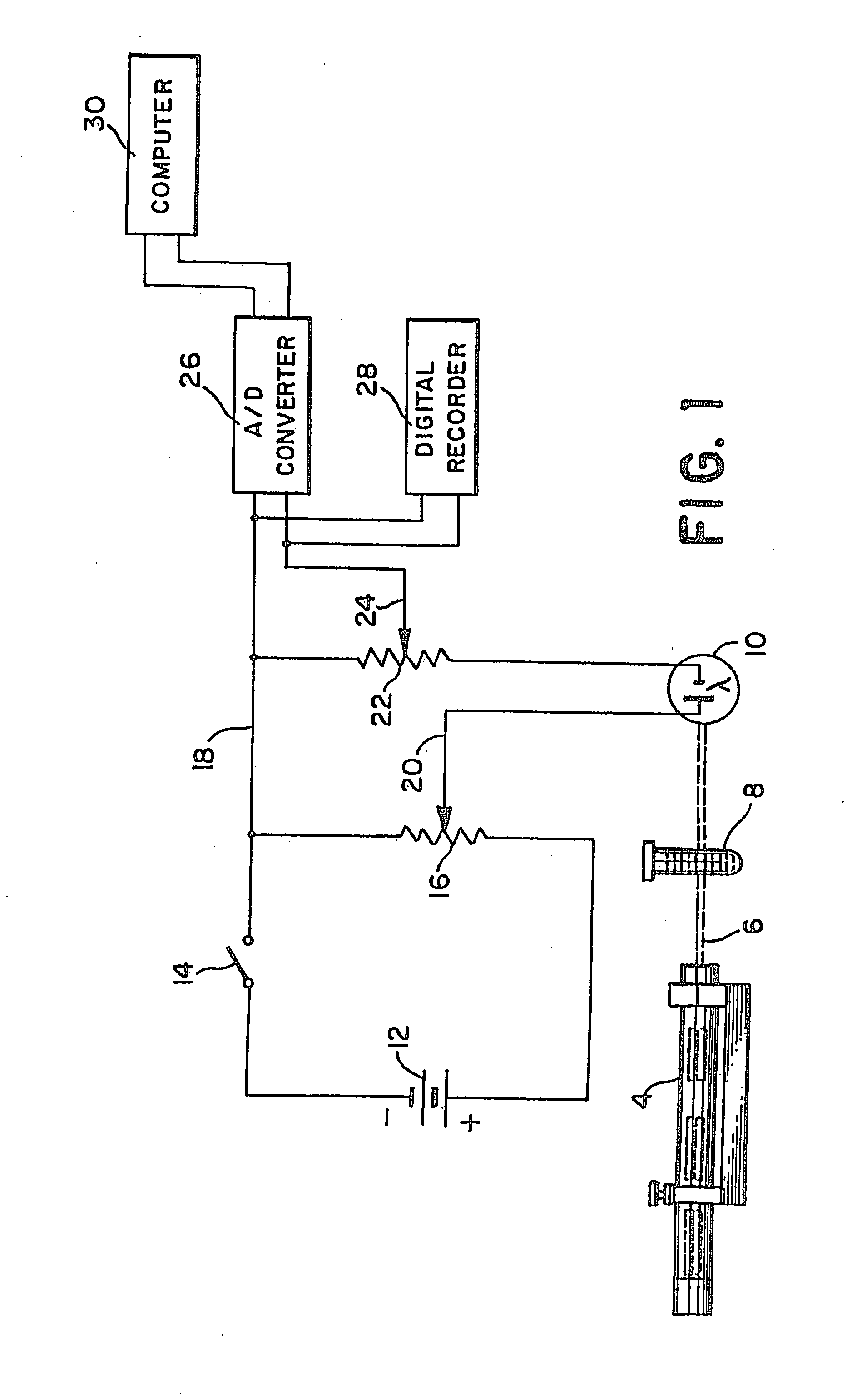
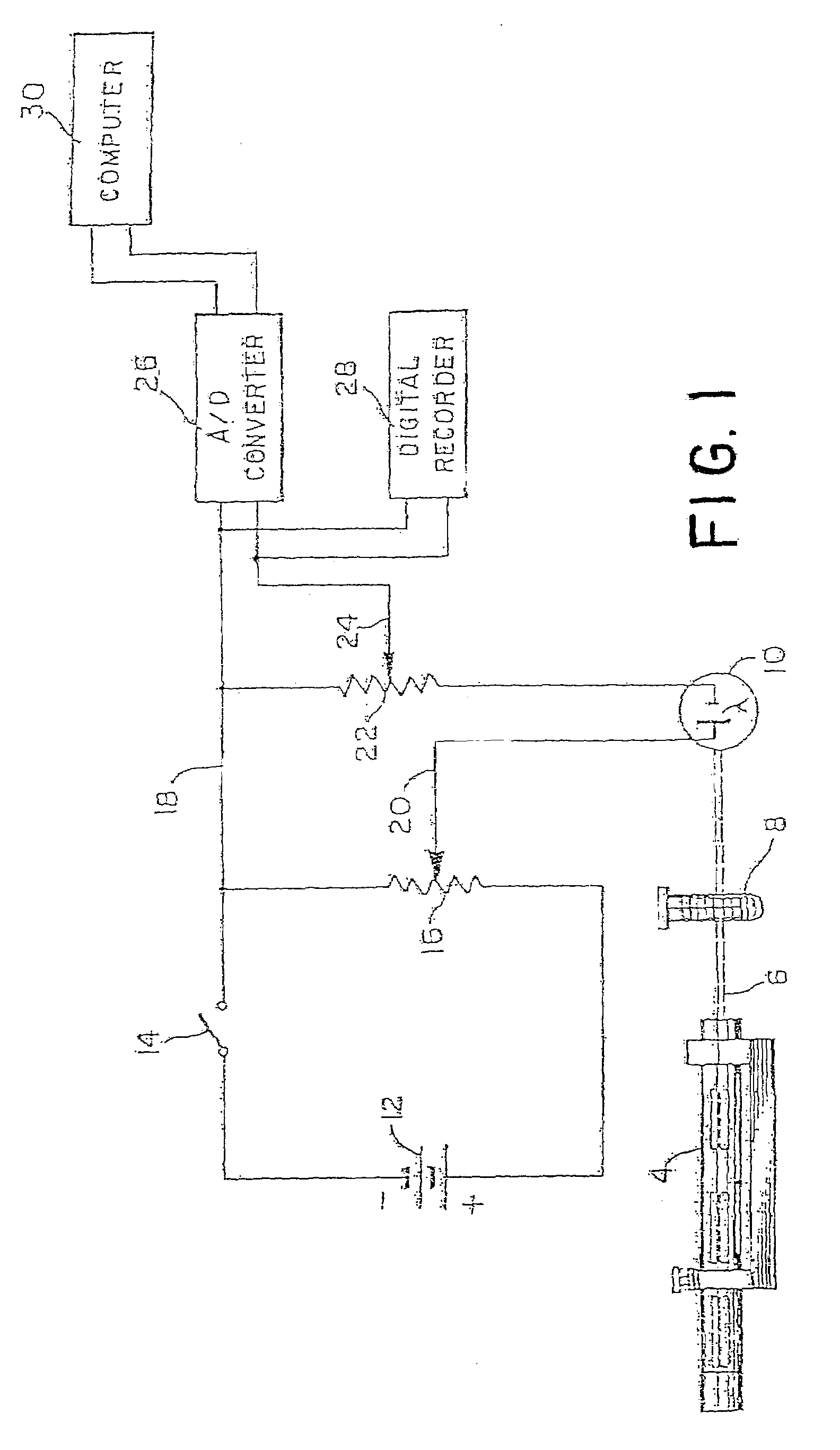
InactiveUS20110224292A1BiocideAnalysis by subjecting material to chemical reactionAnti coagulationCoagulation reagent
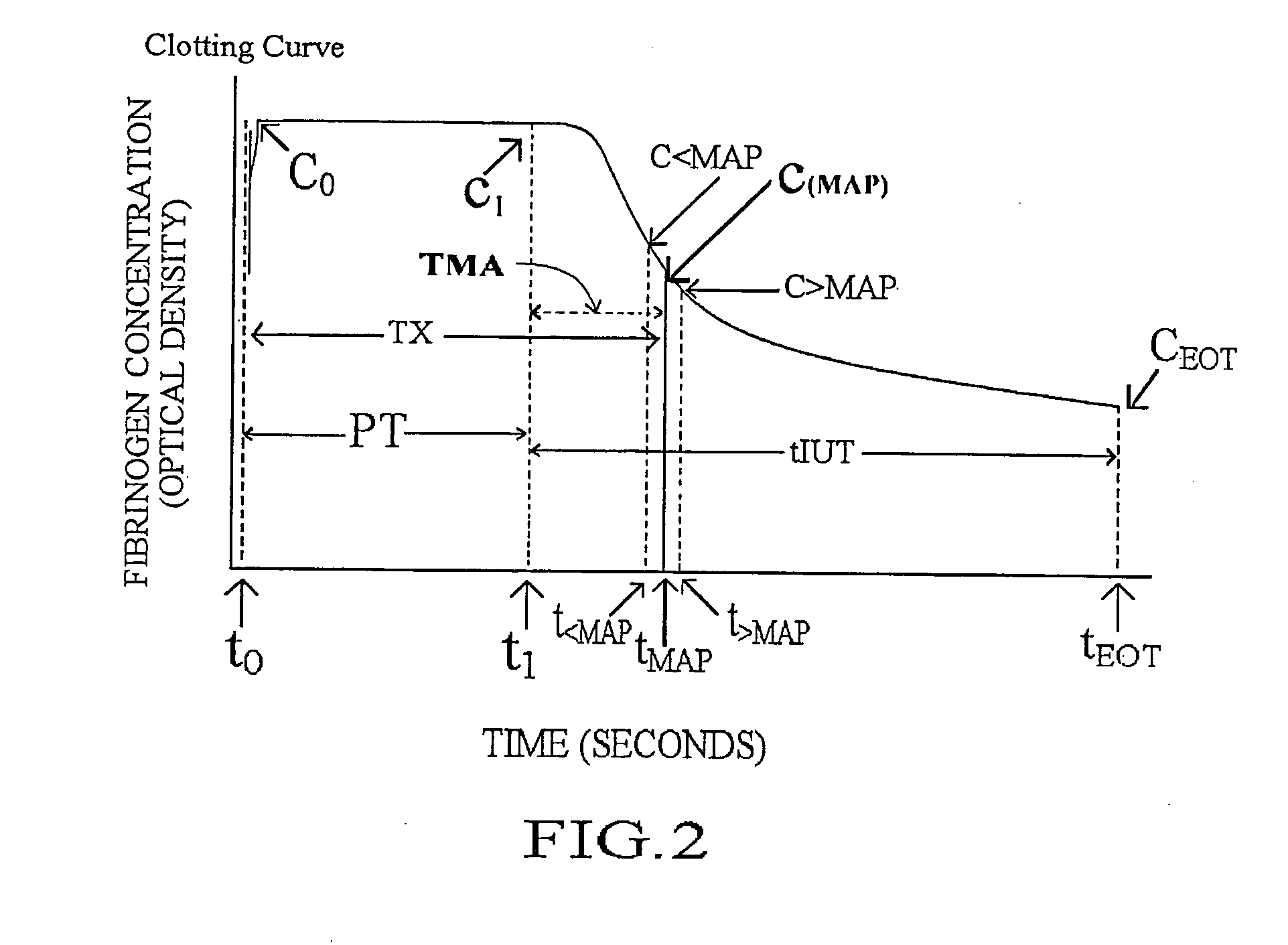
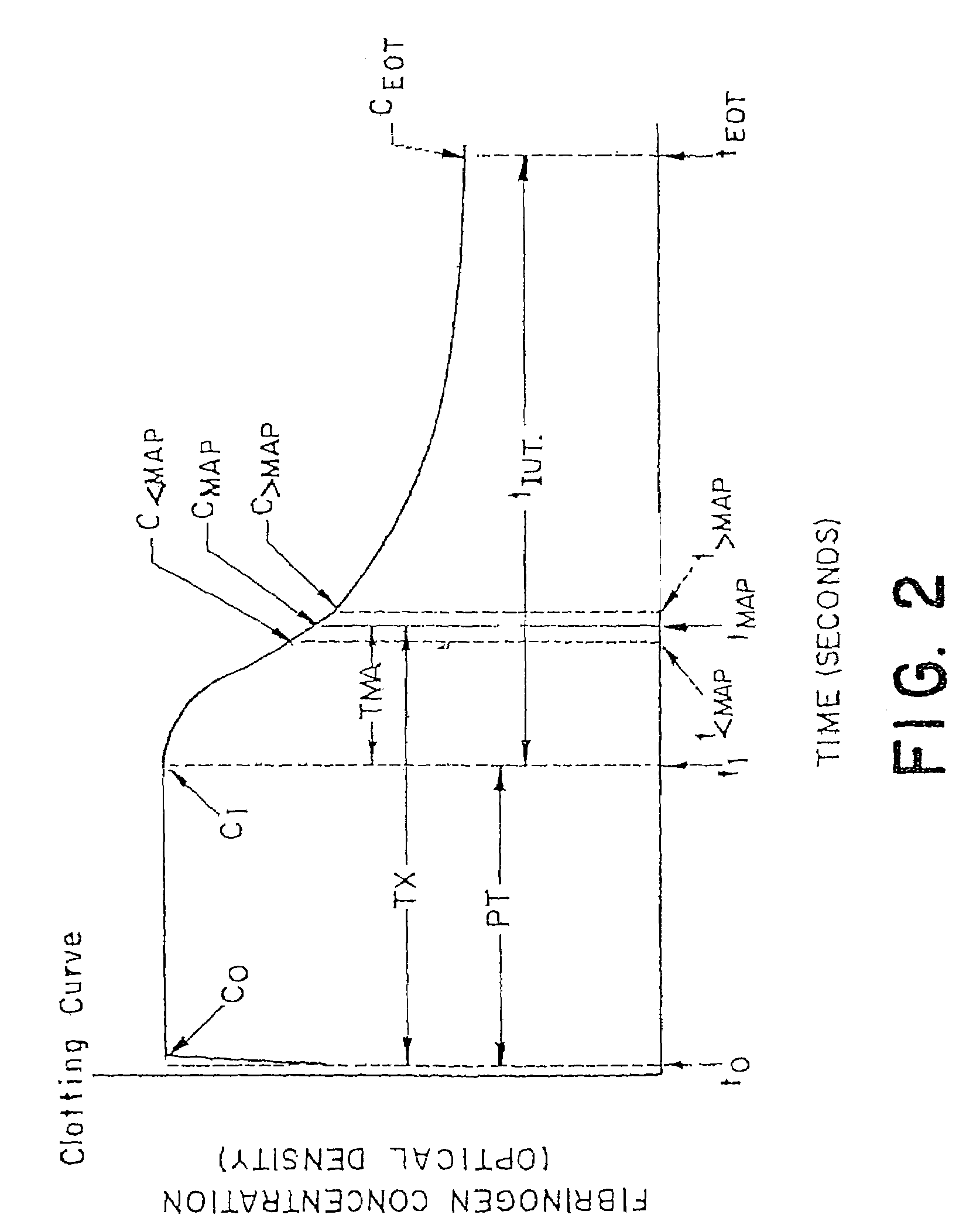
Methods and apparatus are disclosed for determining new anticoagulant therapy factors for monitoring oral anticoagulant therapy to help prevent excessive bleeding or deleterious blood clots that might otherwise occur before, during or after surgery. The inventive methods and apparatus provide an International Normalization Ratio (INR) based on a coagulation reaction with a blood sample of a living being. Embodiments include methods and apparatus for determining an anticoagulant therapy factor without requiring use of a mean normal prothrombin time determination or an ISI, and may be carried out with the patient sample and a coagulation reagent, where the coagulation reagent may be selected from a number of coagulation reagents. One embodiment provides an INRs value which is determined from a prothrombin time (PT or T1) of a patient blood sample and a theoretical end of test time (TEOT), where a theoretical clotting area is used to determine the INRs value according to the expression, INRs=T1*TEOT*MUL, where MUL is a multiplier that takes into account pixel parity and sampling times. The INRs may be used to determine a course of treatment for a patient or other living being without regard to the specific coagulation regent used to generate the coagulation data (e.g., time and optical activity values).
Owner:WADA
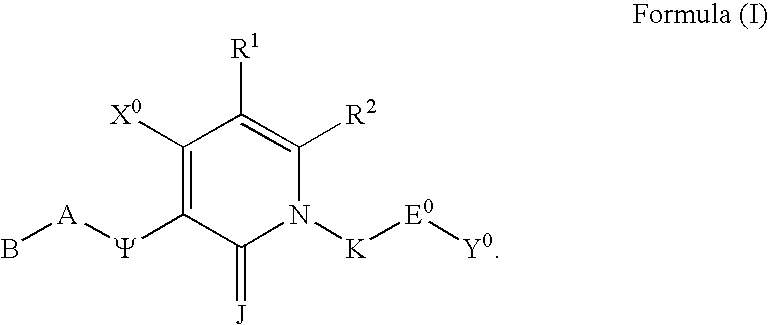
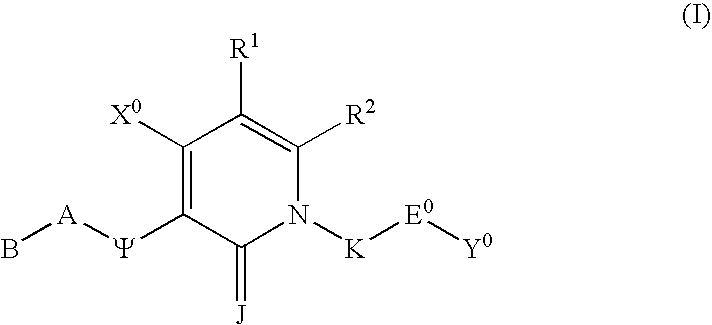
Substituted polycyclic aryl and heteroaryl pyridones useful for selective inhibition of the coagulation cascade
InactiveUS6867217B1Preventing and treating thrombotic conditionPrevent thrombosisBiocideOrganic chemistryDiseaseAryl
The invention relates to substituted polycyclic aryl and heteroaryl pyridone compounds useful as inhibitors of serine proteases of the coagulation cascade and compounds, compositions and methods for anticoagulant therapy for the treatment and prevention of a variety of thrombotic conditions including coronary artery and cerebrovascular diseases.
Owner:PHARMACIA CORP
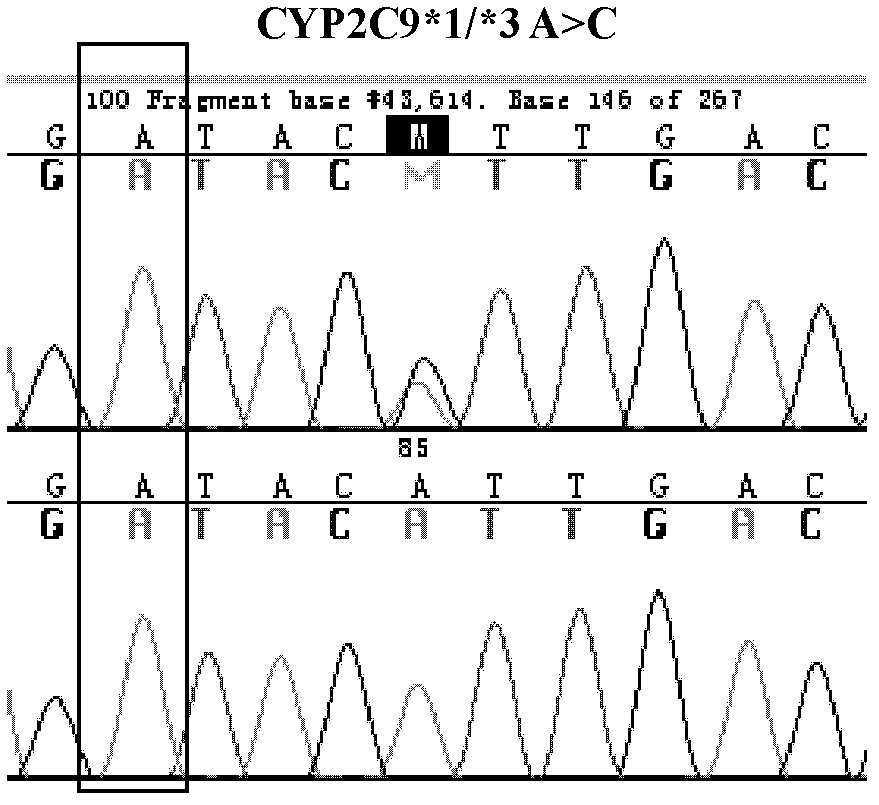
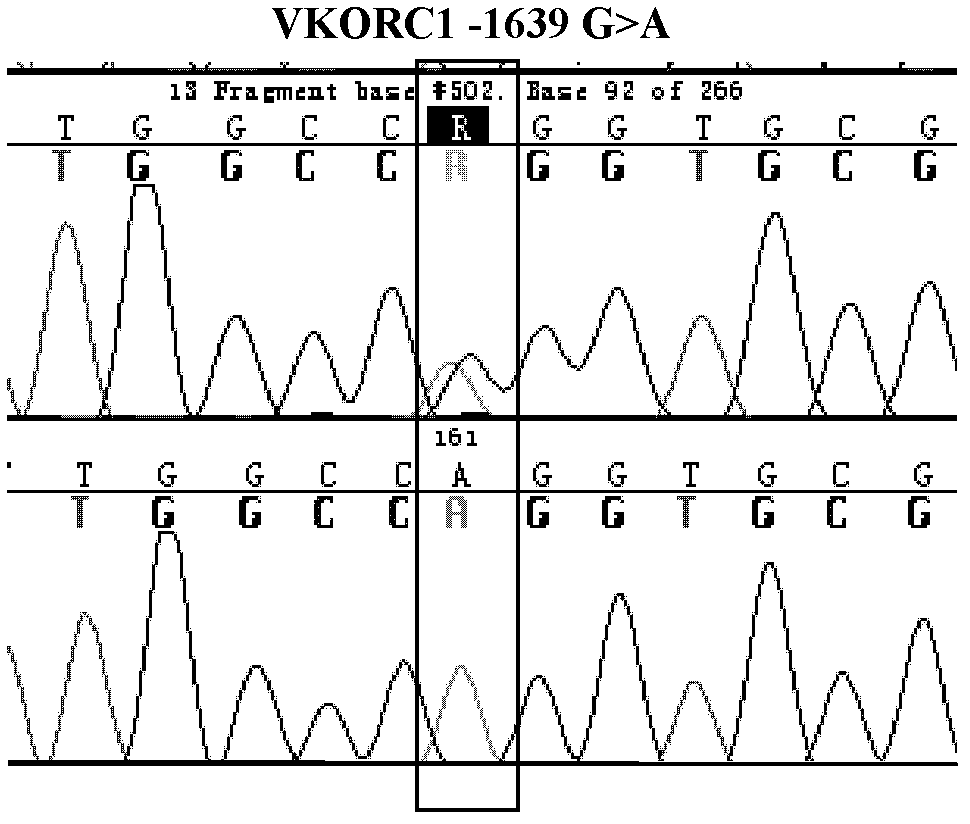
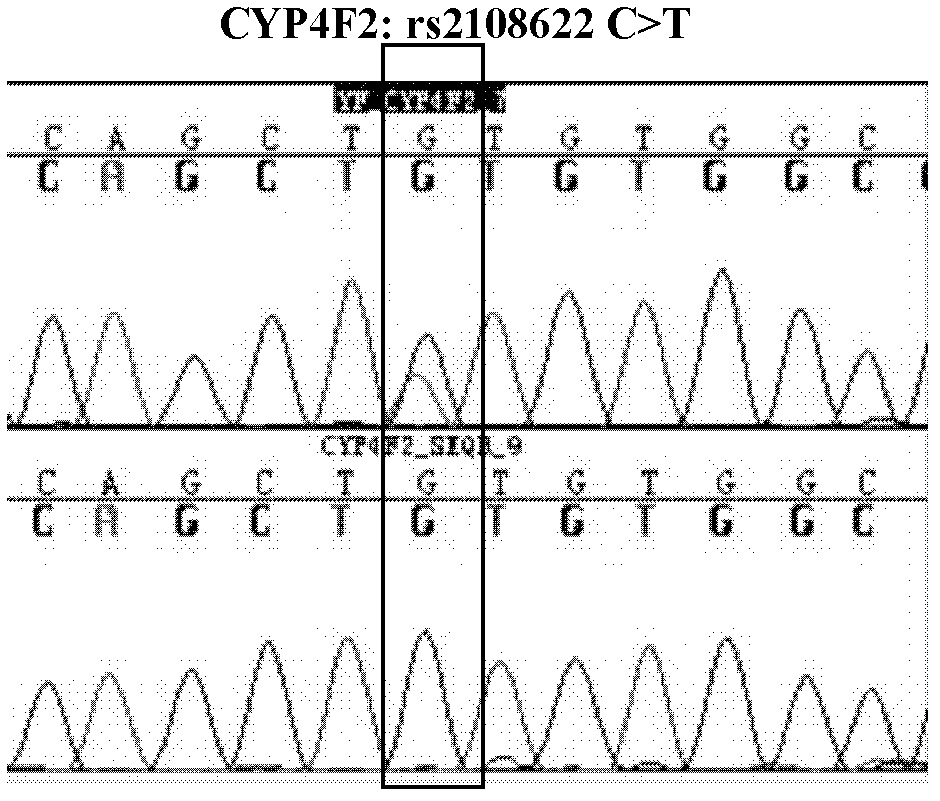
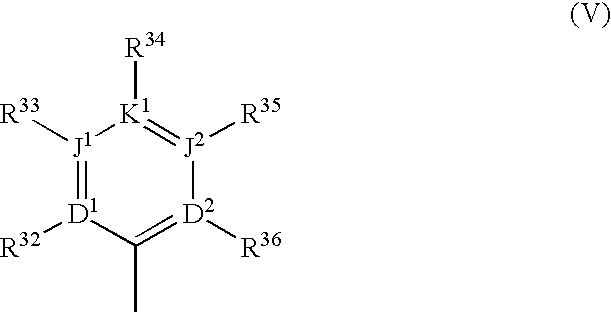
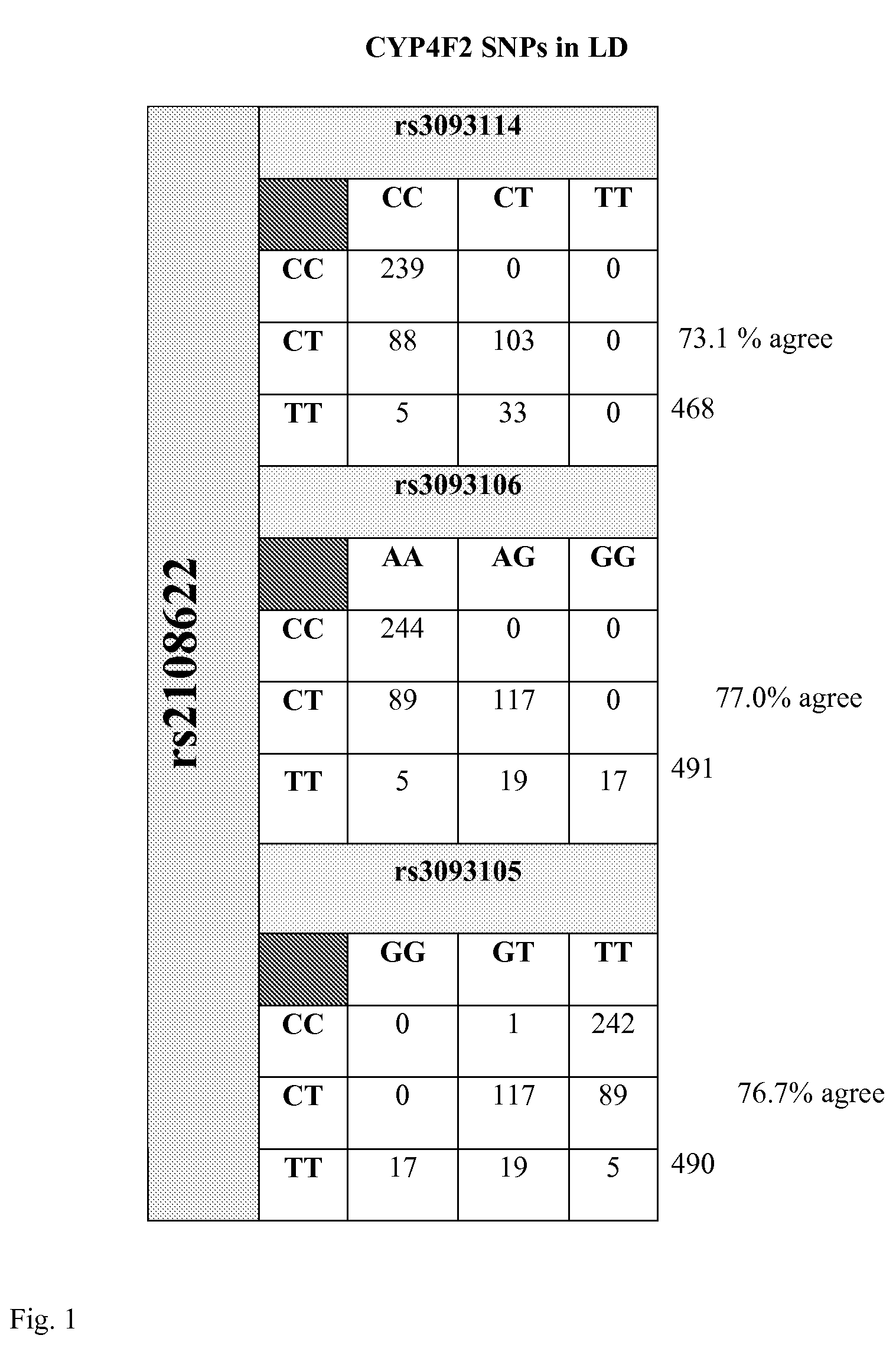
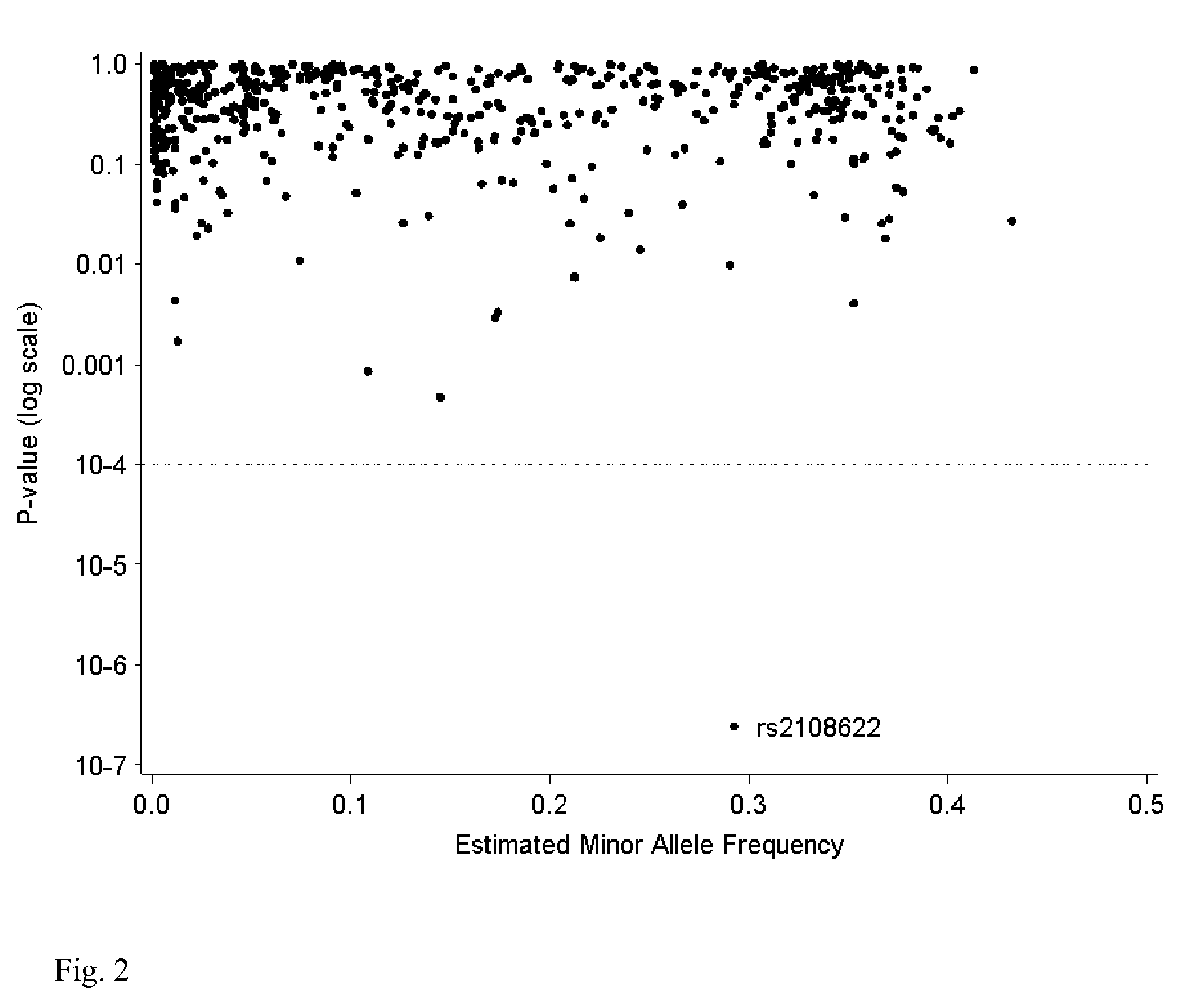
Warfarin individual anticoagulant pharmacogenomics detection kit suitable for Chinese population
The invention provides a warfarin individual anticoagulant pharmacogenomics detection kit suitable for Chinese population, which mainly comprises CYP2C9, VKORC1 and CYP4F2 related gene-type amplification primers, CYP2C9, VKORC1 and CYP4F2 related gene-type sequencing primers, a polymerase chain reaction (PCR) reagent and a sequencing reagent. The warfarin individual anticoagulant pharmacogenomicsdetection kit suitable for the Chinese population also can comprise a specification used for explaining a warfarin pharmacogenomics dose prediction model suitable for the Chinese population or / and a computer readable storage medium recording the warfarin pharmacogenomics dose prediction model suitable for the Chinese population. The warfarin pharmacogenomics detection kit provided by the invention is simple in preparation and is convenient in use. By adopting the kit, the warfarin anticoagulant therapy dose of Chinese patient population can be accurately estimated through detecting warfarin pharmacogenomics indexes, integrating clinical environmental factors and utilizing warfarin pharmacogenomics dose prediction model software suitable for the Chinese population, which is established by the invention.
Owner:尹彤
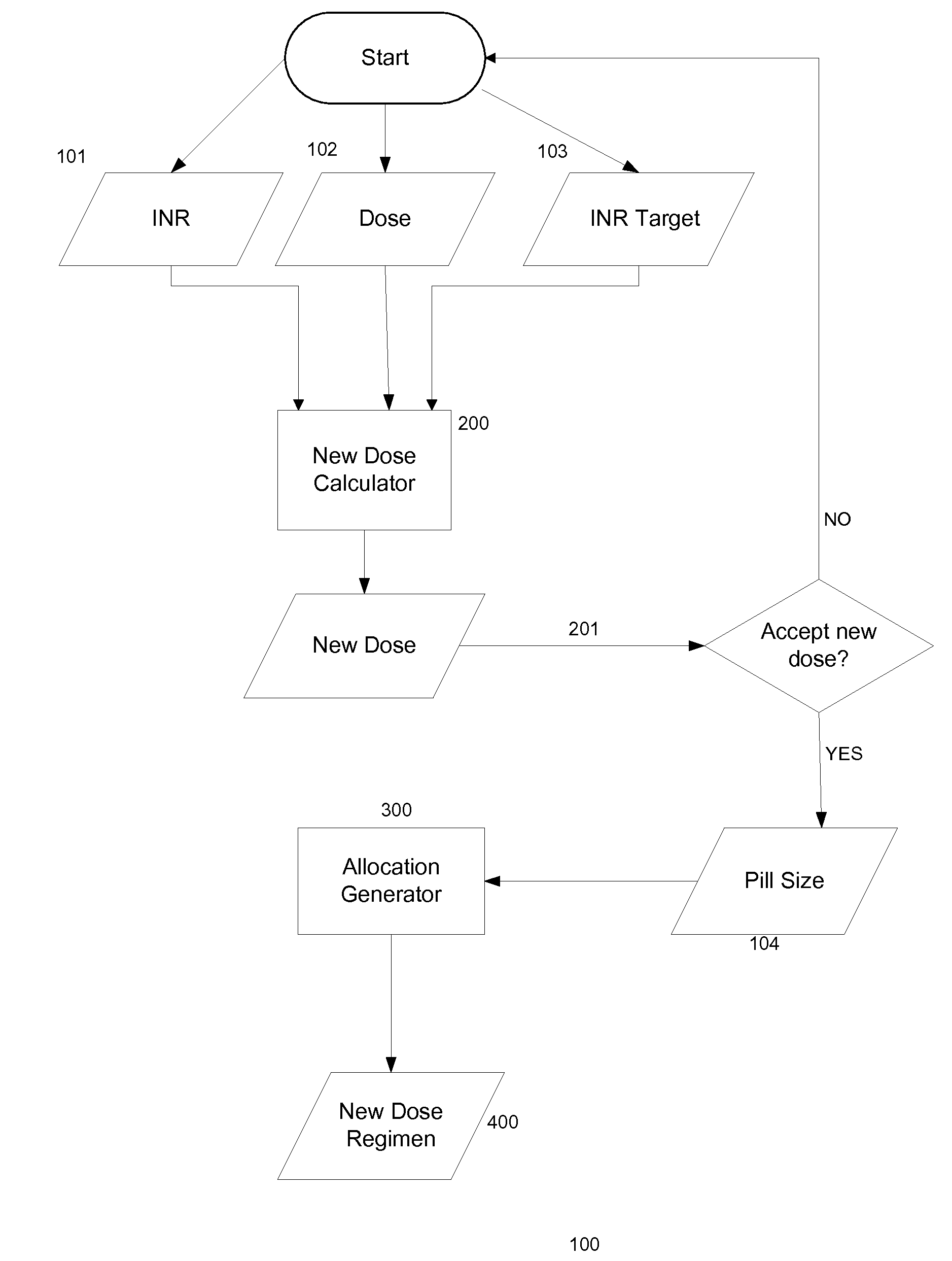
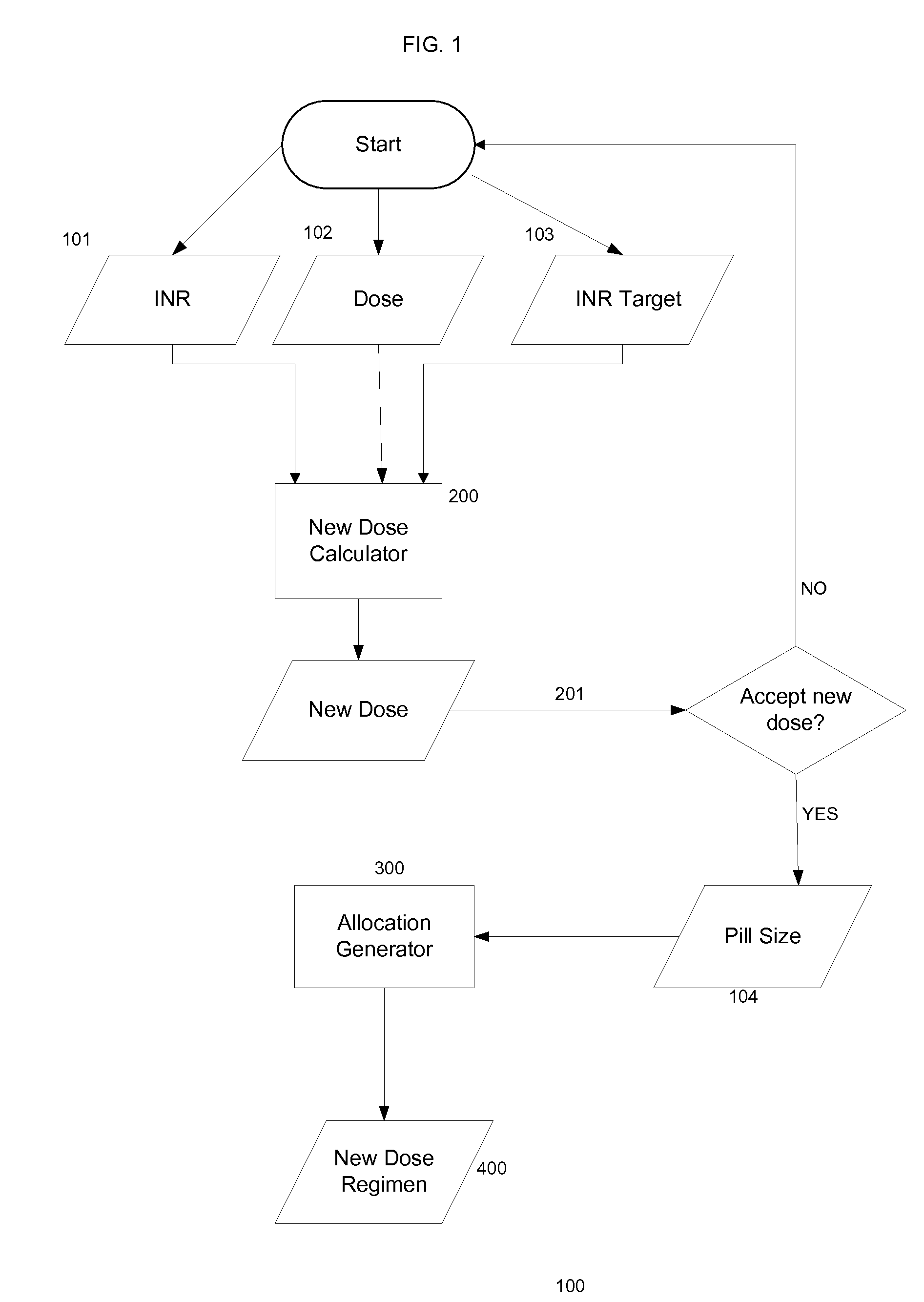
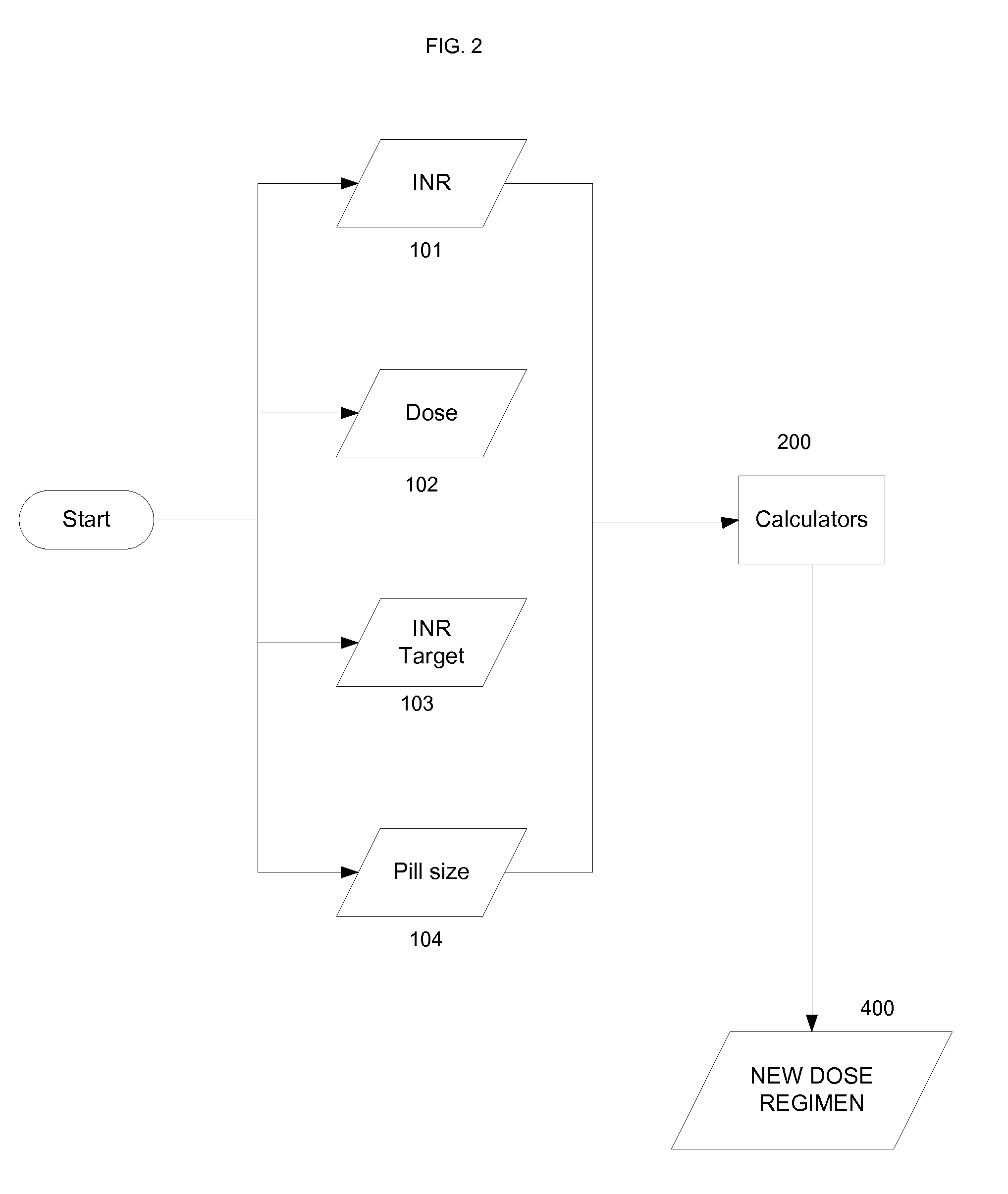
Methods for management of anticoagulation therapy
InactiveUS20090216561A1Therapeutically effectiveData processing applicationsDrug and medicationsMedical recordPoint of care
Presented herein are methods for determining dosages of anticoagulants for a patient. Also presented herein are apparatuses for determining dosages of anticoagulants for a patient. Further presented herein are electronic medical records systems comprising a program for determining dosages of anticoagulants for a patient. Further presented herein are clinical decision support programs for determining dosages of anticoagulants for a patient. Further presented herein are virtual anticoagulation clinics for determining dosages of anticoagulants for a patient. Further presented herein are point of care anticoagulation devices for determining dosages of anticoagulants for a patient.
Owner:SWEDISH HEALTH SERVICES
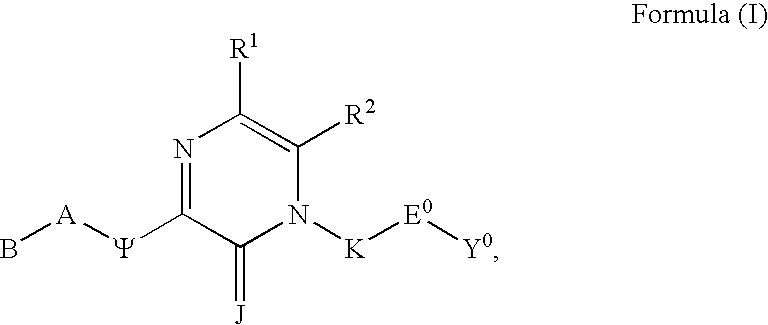
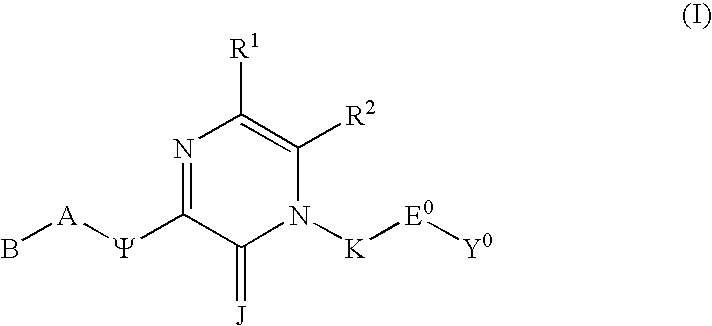
Substituted polycyclic aryl and heteroaryl 1,2,4-triazinones useful for selective inhibition of the coagulation cascade
The invention relates to substituted polycyclic aryl and heteroaryl pyrimidinone compounds useful as inhibitors of serine proteases of the coagulation cascade and compounds, compositions and methods for anticoagulant therapy for the treatment and prevention of a variety of thrombotic conditions including coronary artery and cerebrovascular diseases.
Owner:PHARMACIA CORP
Liquid, aqueous pharmaceutical composition of Factor VII polypeptides
InactiveUS20060166882A1Improve stabilityHeavy metal active ingredientsBiocideClotting factor deficiencyOxidation state
Owner:NOVO NORDISK HEALTH CARE AG
Substituted polycyclic aryl and heteroaryl uracils useful for selective inhibition of the coagulation cascade
The invention relates to substituted polycyclic aryl and heteroaryl uracil compounds useful as inhibitors of serine proteases of the coagulation cascade and compounds, compositions and methods for anticoagulant therapy for the treatment and prevention of a variety of thrombotic conditions including coronary artery and cerebrovascular diseases.
Owner:PHARMACIA CORP
Combination product comprising melagatran and factor VIIa inhibitor
There is provided a combination product comprising: (A) melagatran or a pharmaceutically-acceptable derivative thereof; and (B) a Factor VIIa inhibitor or a pharmaceutically-acceptable derivative thereof, wherein each of components (A) and (B) is formulated in admixture with a pharmaceutically-acceptable adjuvant, diluent or carrier, as well as the use of such a combination product in the treatment of a condition where anticoagulant therapy is indicated.
Owner:ASTRAZENECA AB
Methods and compositions for preventing or treating tissue calcification
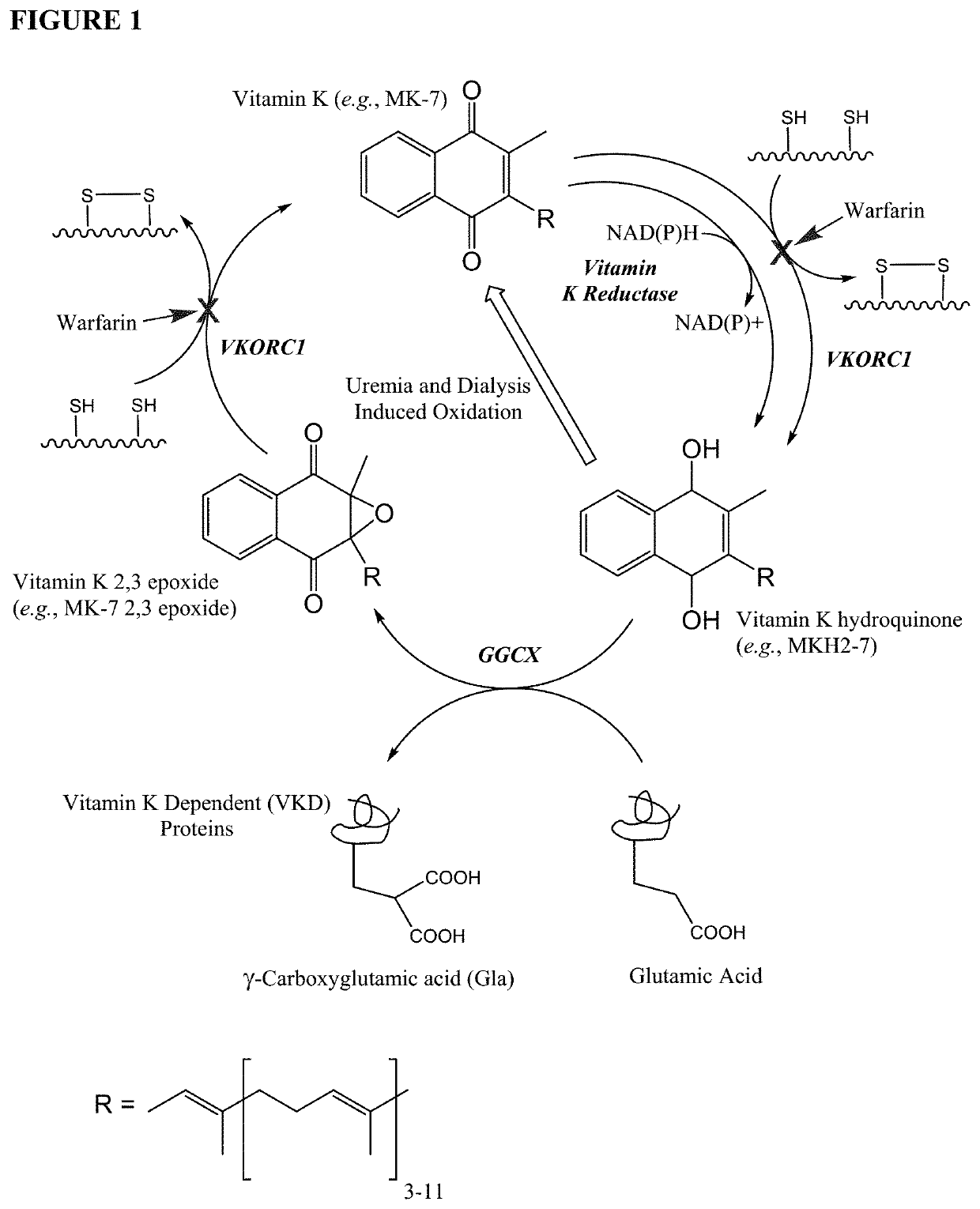
ActiveUS10688064B2Prevent and slow progression of and arrest and reverse tissue calcificationHydroxy compound active ingredientsMetabolism disorderAnti coagulationEnd stage renal failure
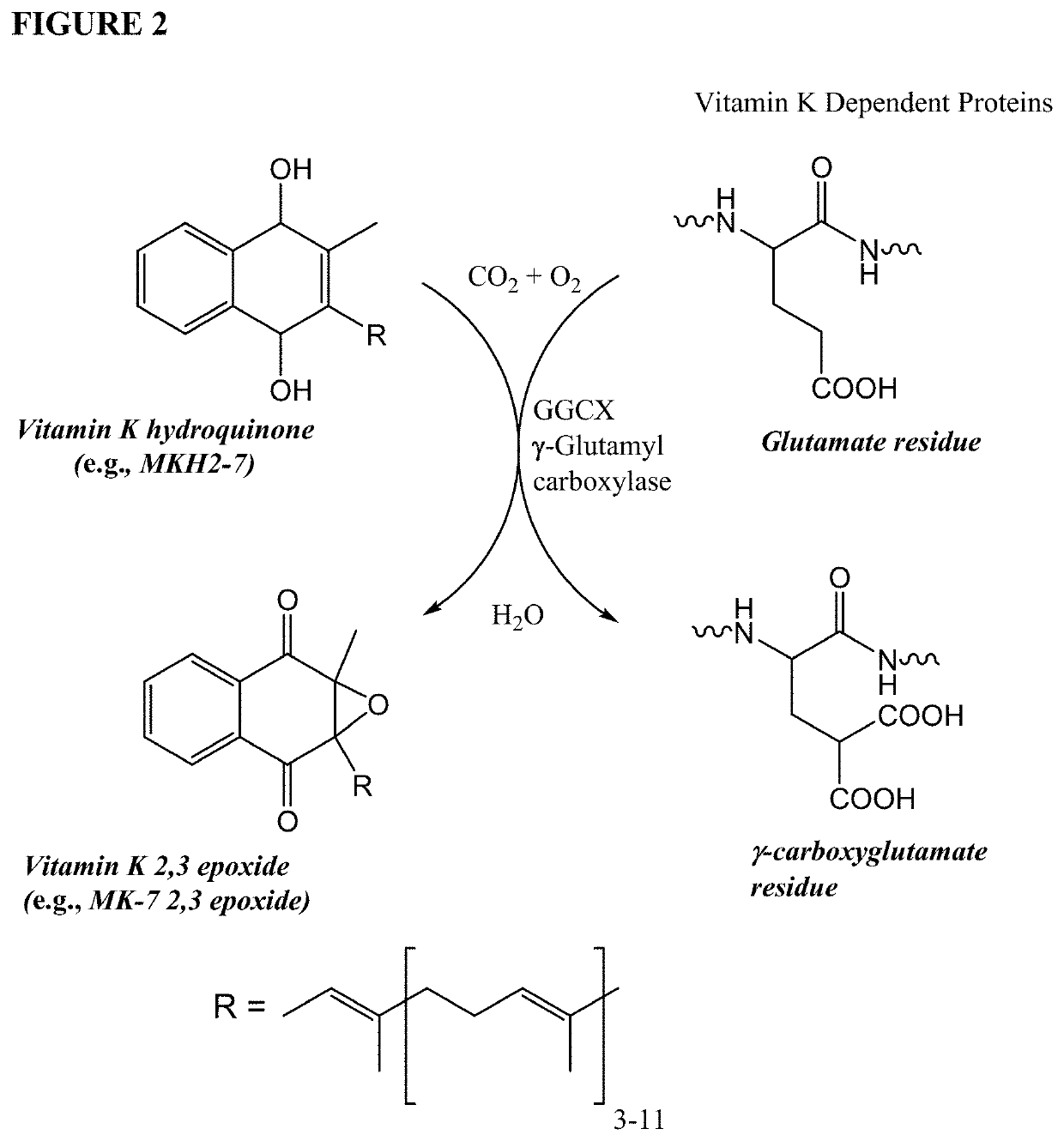
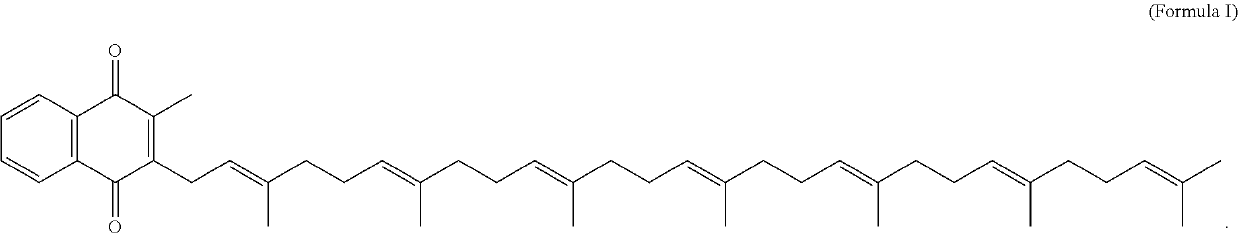
The invention provides methods and compositions for preventing or treating (e.g., slowing the progression of, arresting, and / or reversing) tissue calcification in a subject in need thereof and, more particularly, the invention relates to methods of using menaquinone-7 (MK-7) and / or menaquinol-7 (MKH2-7) for preventing or treating (e.g., slowing the progression of, arresting, and / or reversing) tissue calcification in a subject with diabetes, chronic kidney disease, end stage renal failure, or a subject undergoing hemodialysis and / or receiving anticoagulant therapy. The invention further provides methods and compositions for reducing one or more symptoms of chronic obstructive pulmonary disorder (COPD), including using menaquinone-7 (MK-7) and / or menaquinol-7 (MKH2-7), for preventing or treating (e.g., slowing the progression of, arresting, and / or reversing) one or more symptoms of COPD.
Owner:EPIZON PHARMA INC
Substituted polycyclic aryl and heteroaryl pyrazinones useful for selective inhibition of the coagulation cascade
InactiveUS6908919B2Prevent thrombosisThrombotic conditionBiocidePeptide/protein ingredientsArylCoronary arteries
The invention relates to substituted polycyclic aryl and heteroaryl pyrazinone compounds useful as inhibitors of serine proteases of the coagulation cascade and compounds, compositions and methods for anticoagulant therapy for the treatment and prevention of a variety of thrombotic conditions including coronary artery and cerebrovascular diseases.
Owner:PHARMACIA CORP
Method for administering anticoagulation therapy
The present invention provides a method for use in treating a patient with an anticoagulant to optimize drug therapy and / or to prevent an adverse drug response. More particularly, the present invention relates to a method and system for use in treating a patient with Coumadin® or a substance containing warfarin. Methods of the present invention utilize variables that include the patient's CYP4F2 genotype.
Owner:MARSHFIELD CLINIC
Therapy for treatment or prevention of conditions associated with bleeding or hypocoagulation
ActiveUS10407488B2High specific activityIncrease resistancePowder deliveryPeptide/protein ingredientsHalf-lifeOverproduction
Owner:RGT UNIV OF CALIFORNIA +1
Liquid, Aqueous Pharmaceutical Composition of Factor VII Polypeptides
InactiveUS20100166730A1Improve stabilityHeavy metal active ingredientsPeptide/protein ingredientsClotting factor deficiencyOxidation state
The present invention is directed to liquid, aqueous pharmaceutical compositions containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome, e.g., bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy. More particularly, the invention relates to liquid compositions stabilised against chemical and / or physical degradation. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; at least one metal-containing agent (iii), wherein said metal is selected from the group consisting of first transition series metals of oxidation state +II, except zinc, such as chromium, manganese, iron, cobalt, nickel, and copper; and a non-ionic surfactant (iv).
Owner:NOVO NORDISK HEALTH CARE AG
Method and apparatus for determining anticoagulant therapy factors
InactiveUS7276377B2Material thermal conductivityMaterial analysis by observing effect on chemical indicatorBlood plasmaOral anticoagulant
Methods and apparatus are disclosed for determining a new anticoagulant therapy factor (nATF) for monitoring oral anticoagulant therapy to help prevent excessive bleeding or deleterious blood clots that might otherwise occur before, during or after surgery. In one embodiment, the new anticoagulant therapy factor is based upon a determination of a new fibrinogen transformation rate (nFTR) which, in turn, is dependent on a maximum acceleration point (MAP) for fibrinogen (FBG) conversion. The new anticoagulant therapy factor quantity is also based upon the time to maximum acceleration from the time of reagent injection (TX) into a plasma sample, but does not require the difficulty of obtaining prior art International Normalized Ratio (INR) and International Sensitivity Index (ISI) parameters. Other embodiments provide methods and apparatus for determining an anticoagulant therapy factor without requiring use of a mean normal prothrombin time determination or ISI.
Owner:WADA
Liquid, aqueous, pharmaceutical compositions of factor VII polypeptides
The present invention relates to an aqueous liquid pharmaceutical composition comprising a factor VII polypeptide (eg human factor VIIa) and a buffer; wherein the molar concentration ratio of uncomplexed calcium ions (Ca2+) to factor VII polypeptide is less than 0.5. The composition may further include stabilizers (such as copper or magnesium ions, benzamidine or guanidine), nonionic surfactants, tonicity regulators, antioxidants and preservatives. The composition is useful in the treatment of factor VII-responsive syndromes, such as bleeding disorders, including: those disorders resulting from deficiencies of clotting factors (e.g., hemophilia A, hemophilia B, factor XI deficiency, factor VII deficiency), thrombocytopenia or Willebrand disease or bleeding disorders due to clotting factor inhibitors; and intracerebral hemorrhage or excessive bleeding from any cause. These formulations may also be administered to patients in conjunction with surgery or other trauma therapy or to patients receiving anticoagulant therapy.
Owner:NOVO NORDISK AS
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20130129693A1Reduces and removes anticoagulant effectReduced activityPeptide/protein ingredientsHydrolasesMedicineAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor X and factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of reversing anticoagulation, stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMACEUTICALS INC
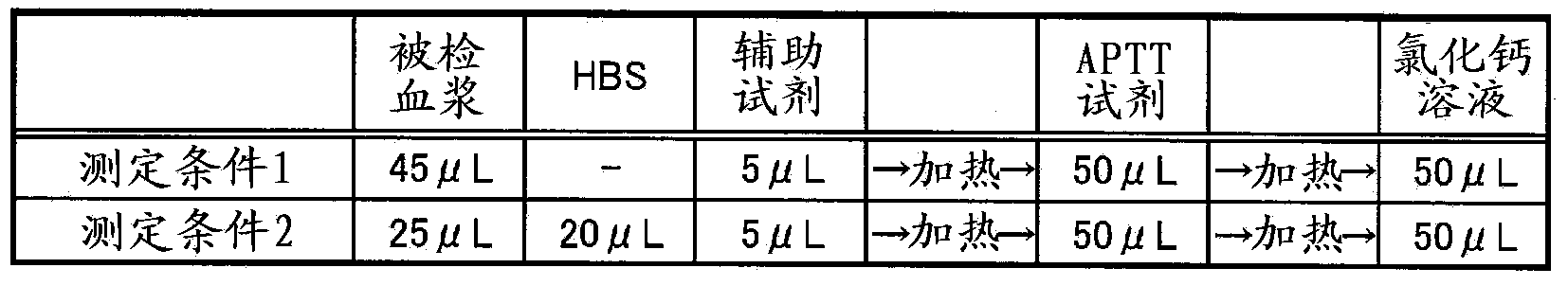
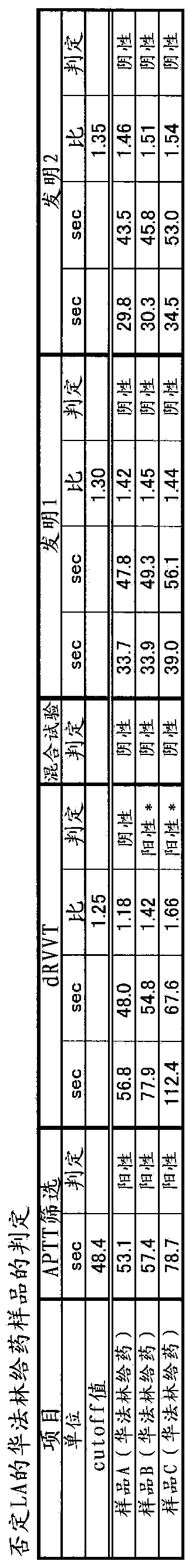
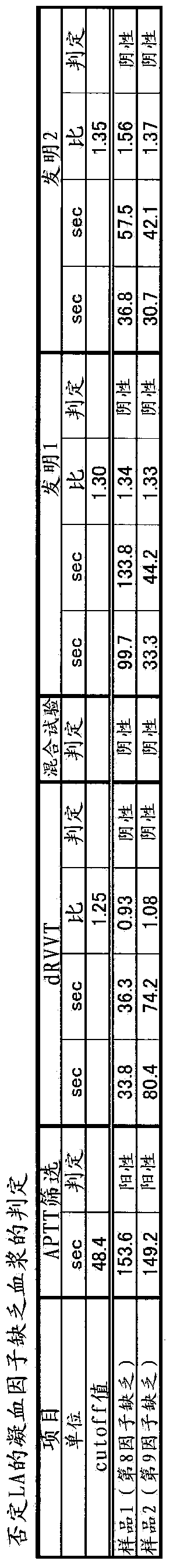
Method for detecting lupus anticoagulants
Provided is a simple method for detecting lupus anticoagulants without using blood plasma from healthy individuals, which is capable of distinguishing between blood-coagulation-factor deficiencies, even in blood samples originating from patients undergoing anticoagulant therapies using warfarin, heparin, or the like, without being affected by said anticoagulant therapies. The method for detecting the lupus anticoagulants is characterized in that it involves the following three steps, (A), (B) and (C): (A) a step in which a buffering solution composition containing blood coagulation factors is added to a blood sample and to a diluted sample of said blood sample both before and during the measurement of the blood coagulation time; (B) a step in which the blood coagulation time is measured for each of the samples from step (A); and (C) a step in which the blood coagulation times of each of the samples obtained in step (B) are compared.
Owner:SCHOOL JURIDICAL PERSON HIGASHI NIPPON GAKUEN +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com