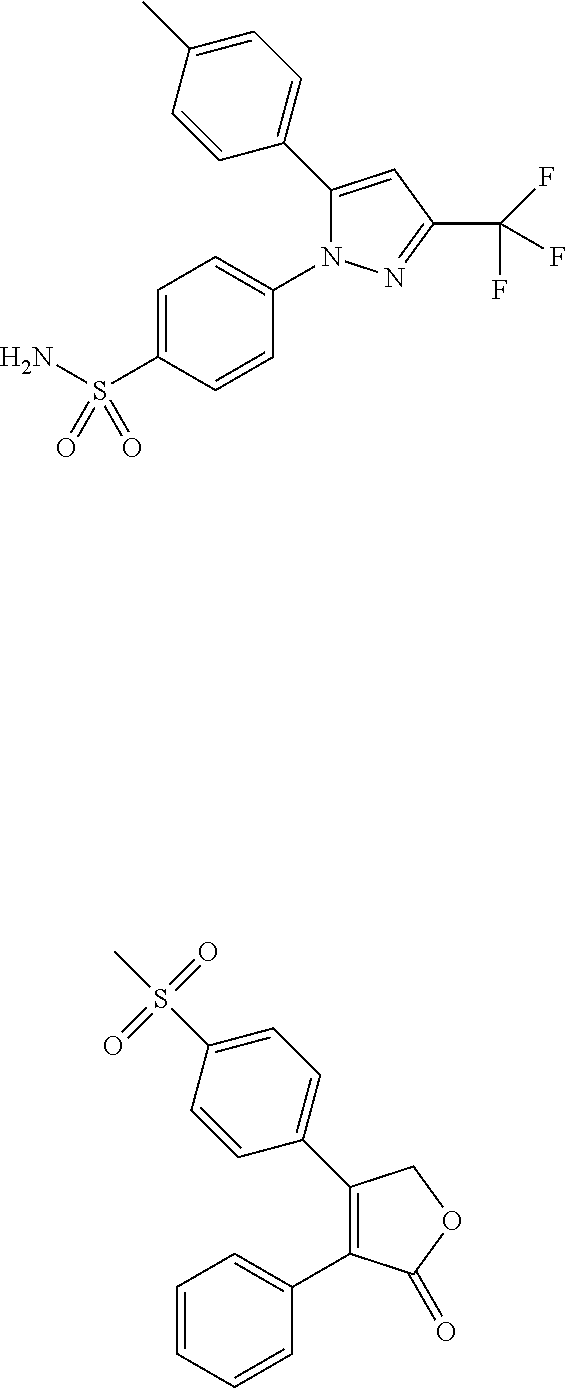
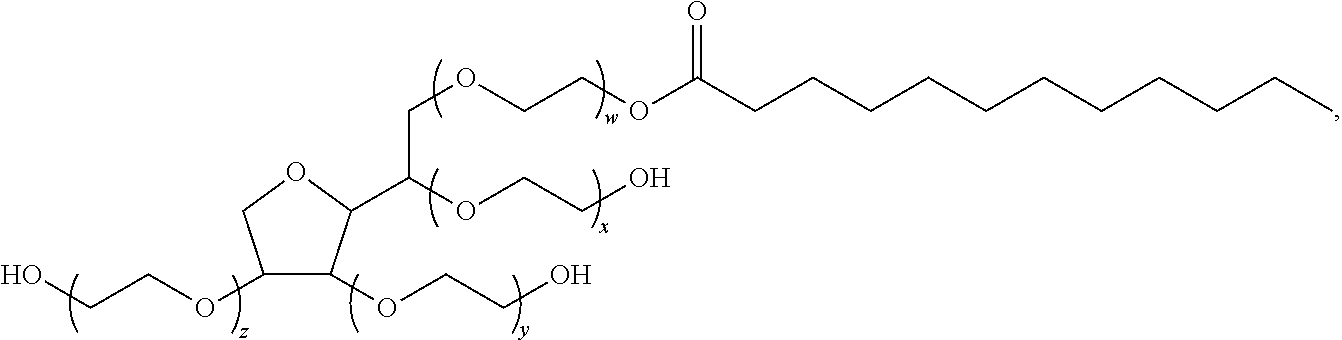
Patents
Literature
49results about How to "Reduce potential side-effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
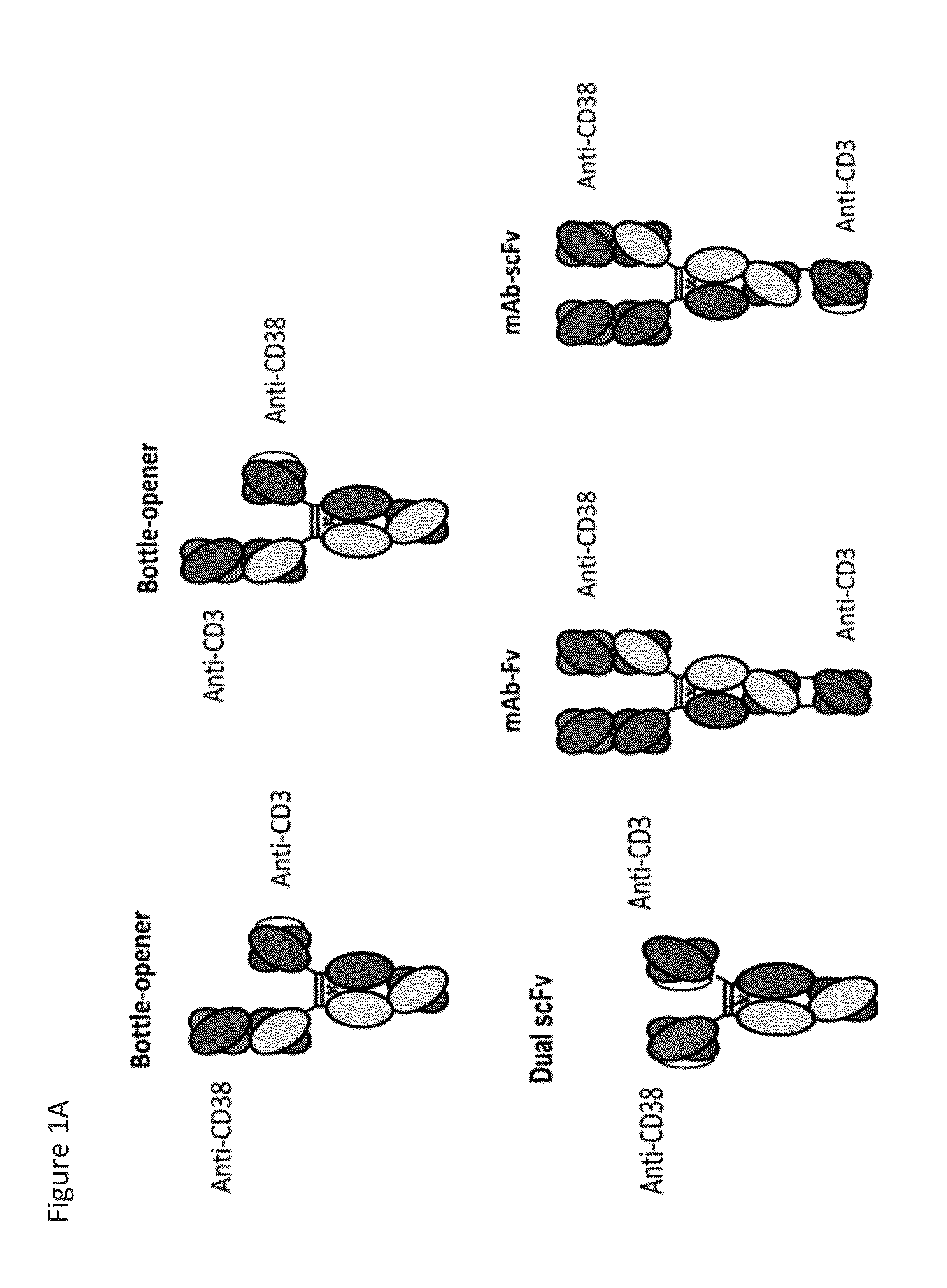
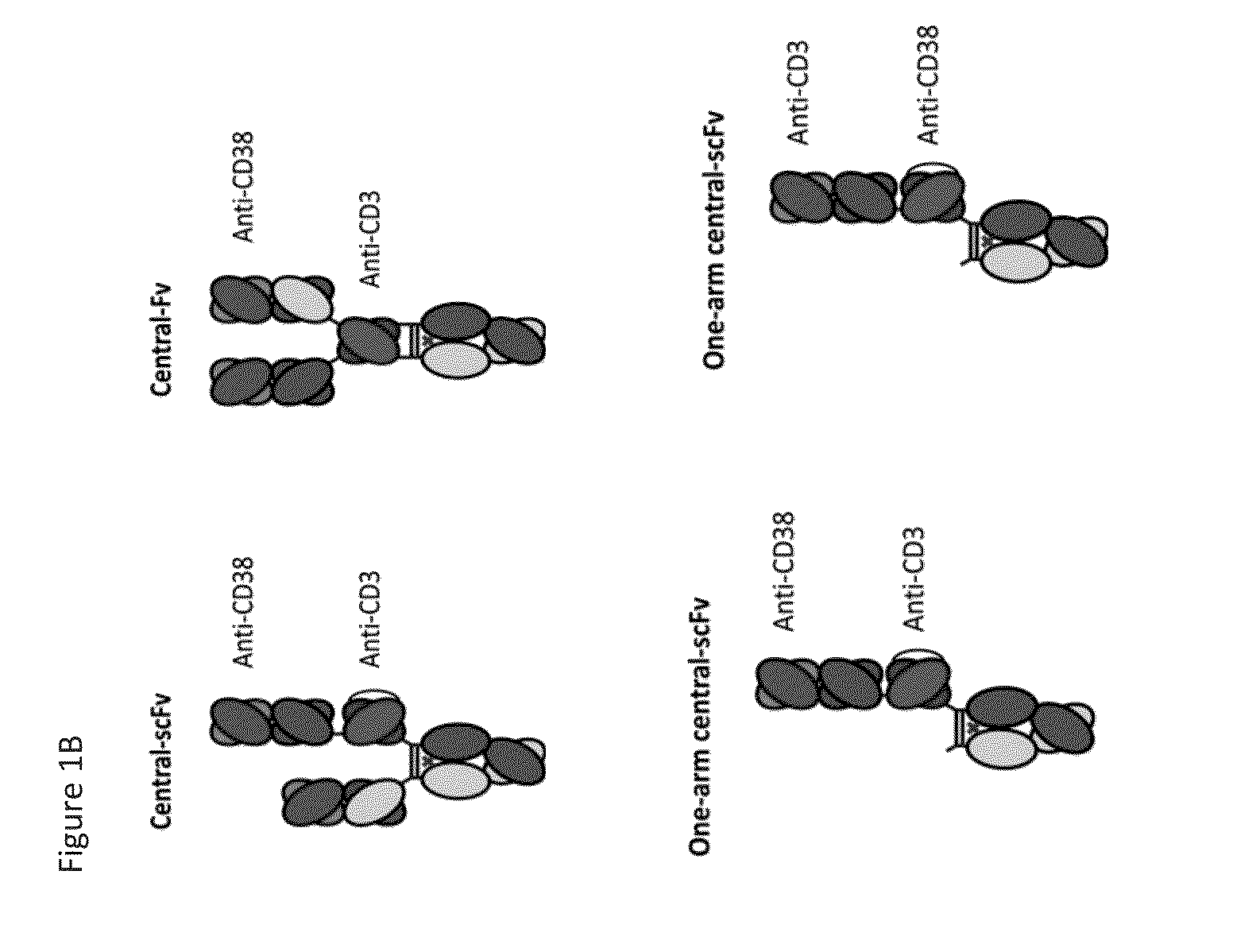
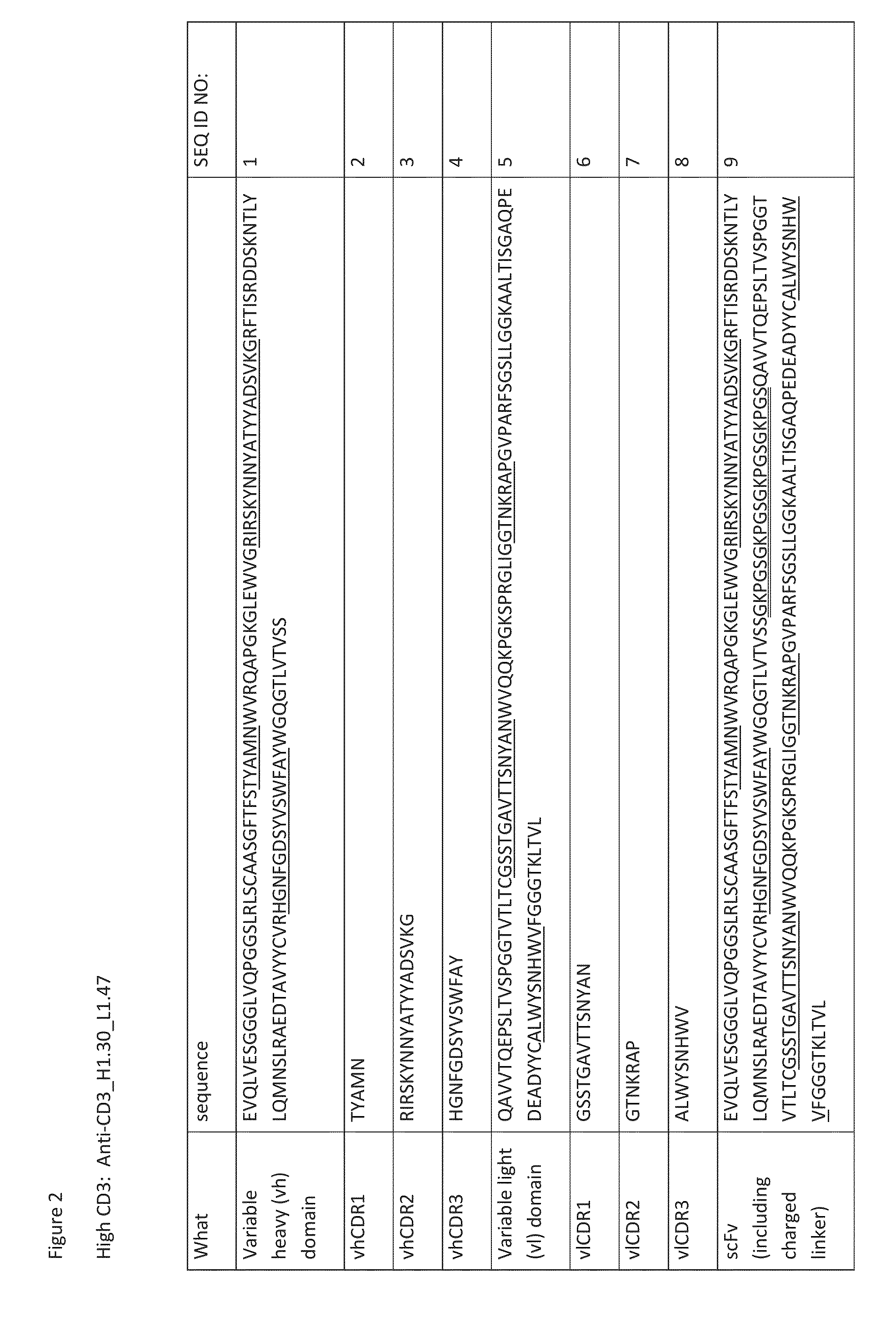
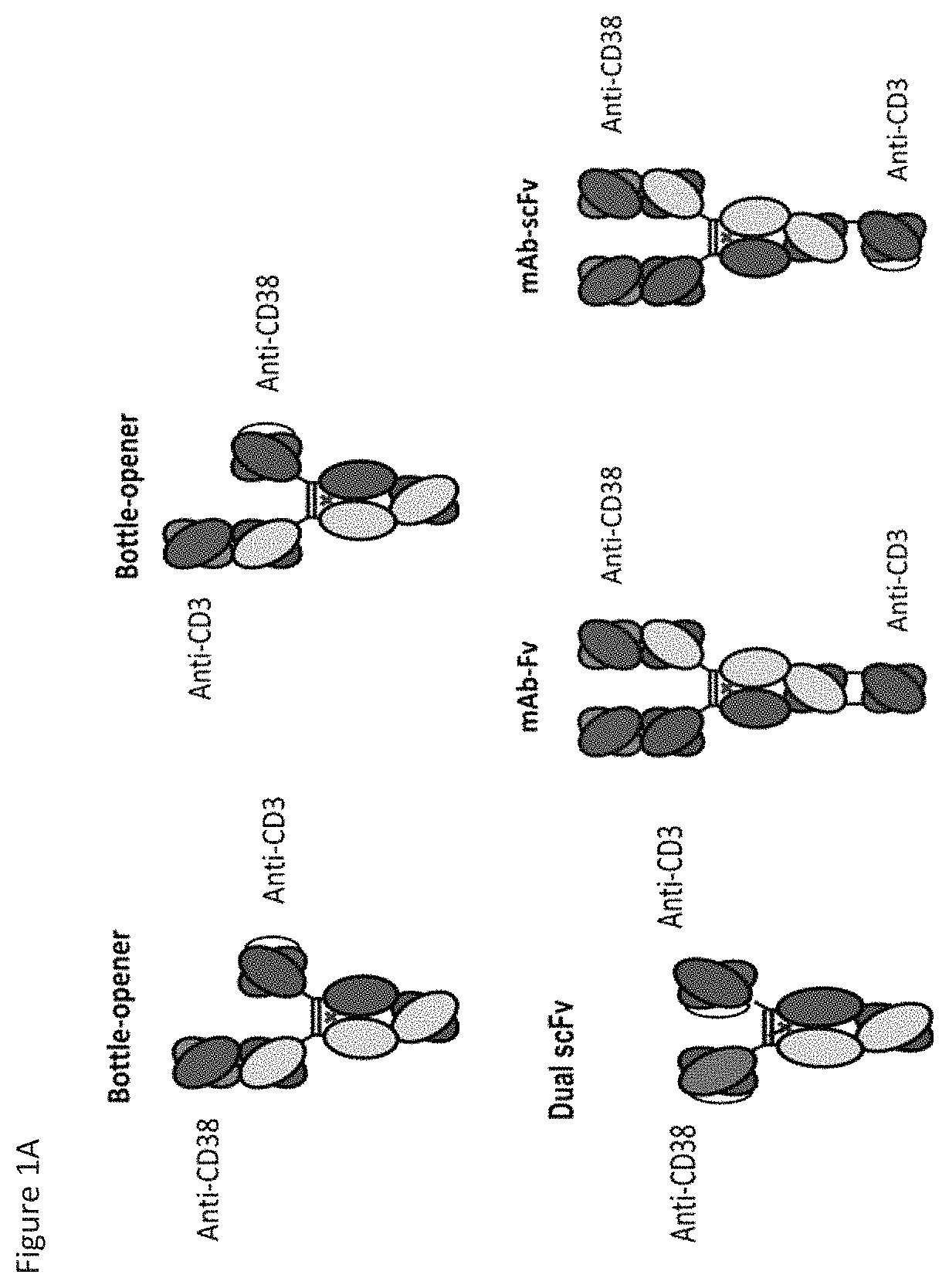
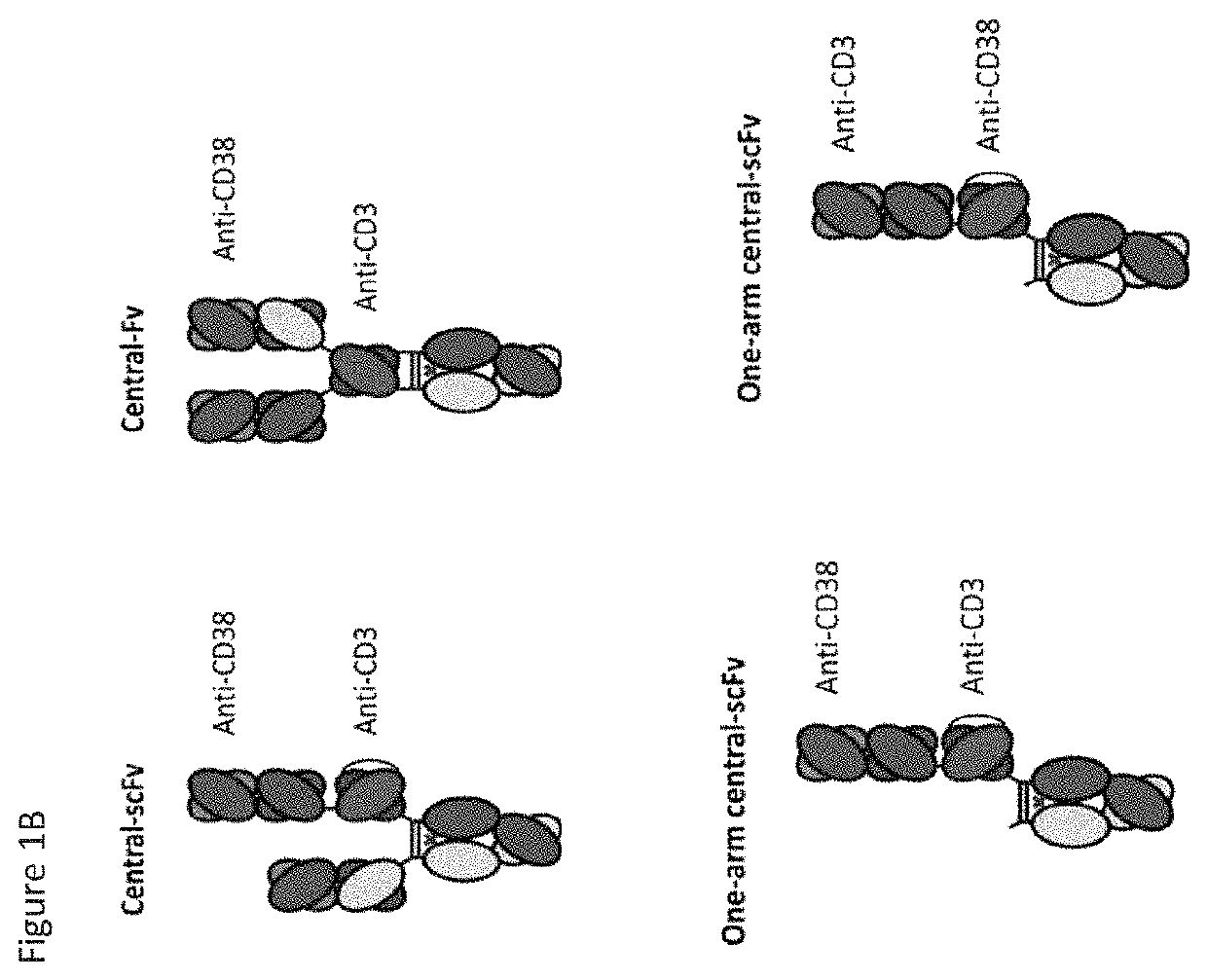
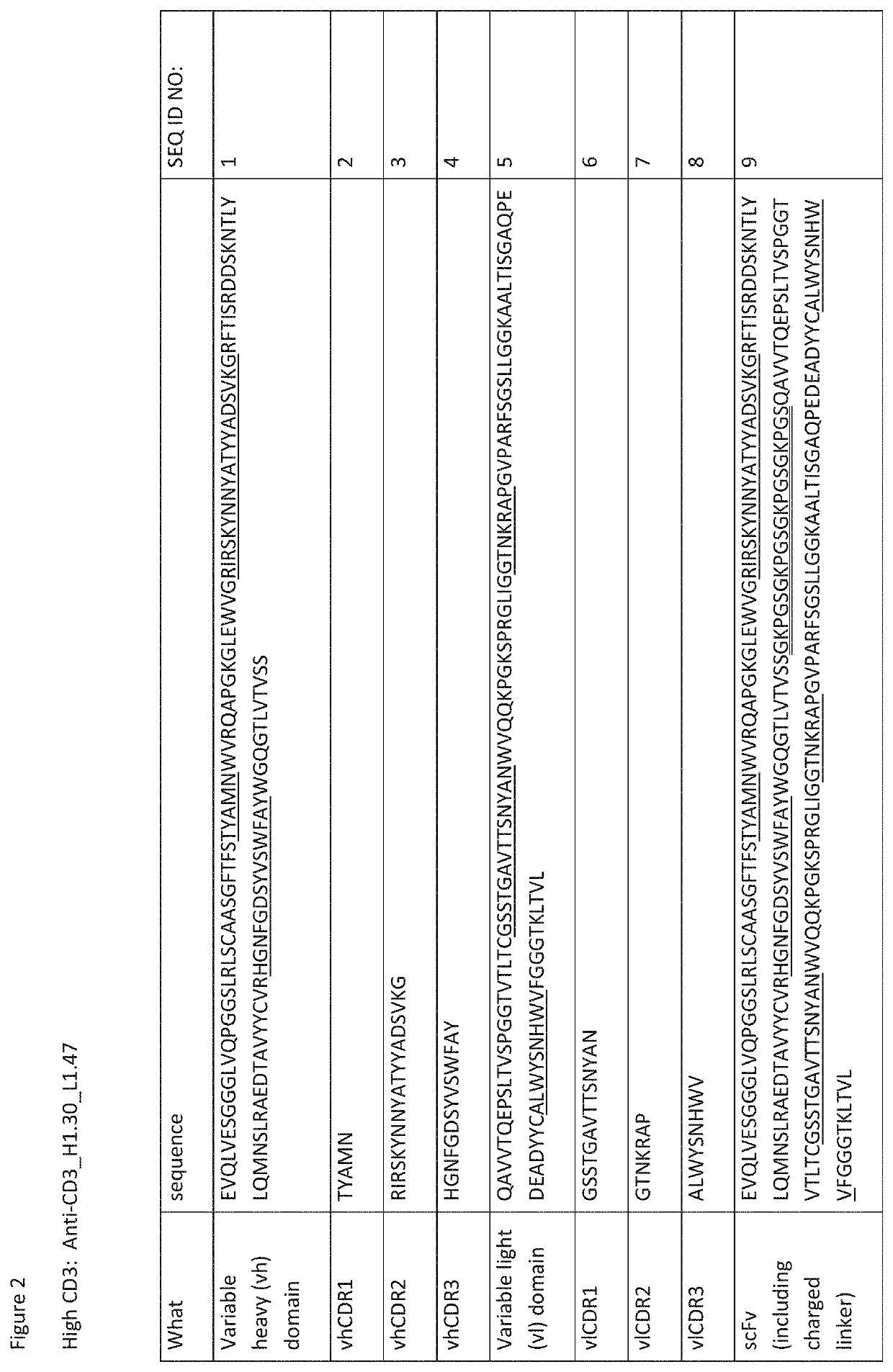
Heterodimeric antibodies that bind cd3 and cd38
ActiveUS20160215063A1Reduce potential side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntibodyCD38
Owner:XENCOR
Bispecific anti-VEGF/anti-ANG-2 antibodies and their use in the treatment of ocular vascular diseases
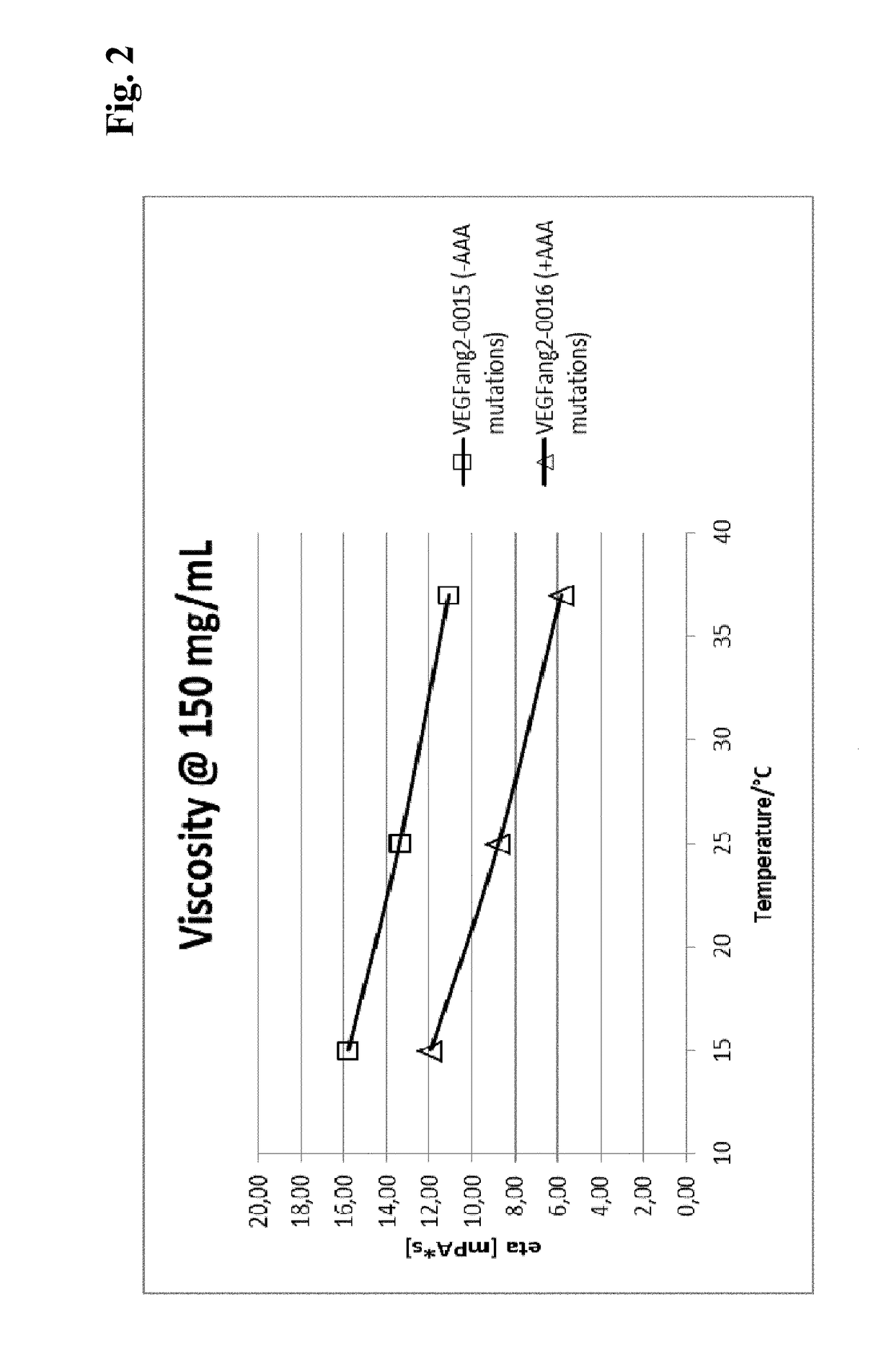
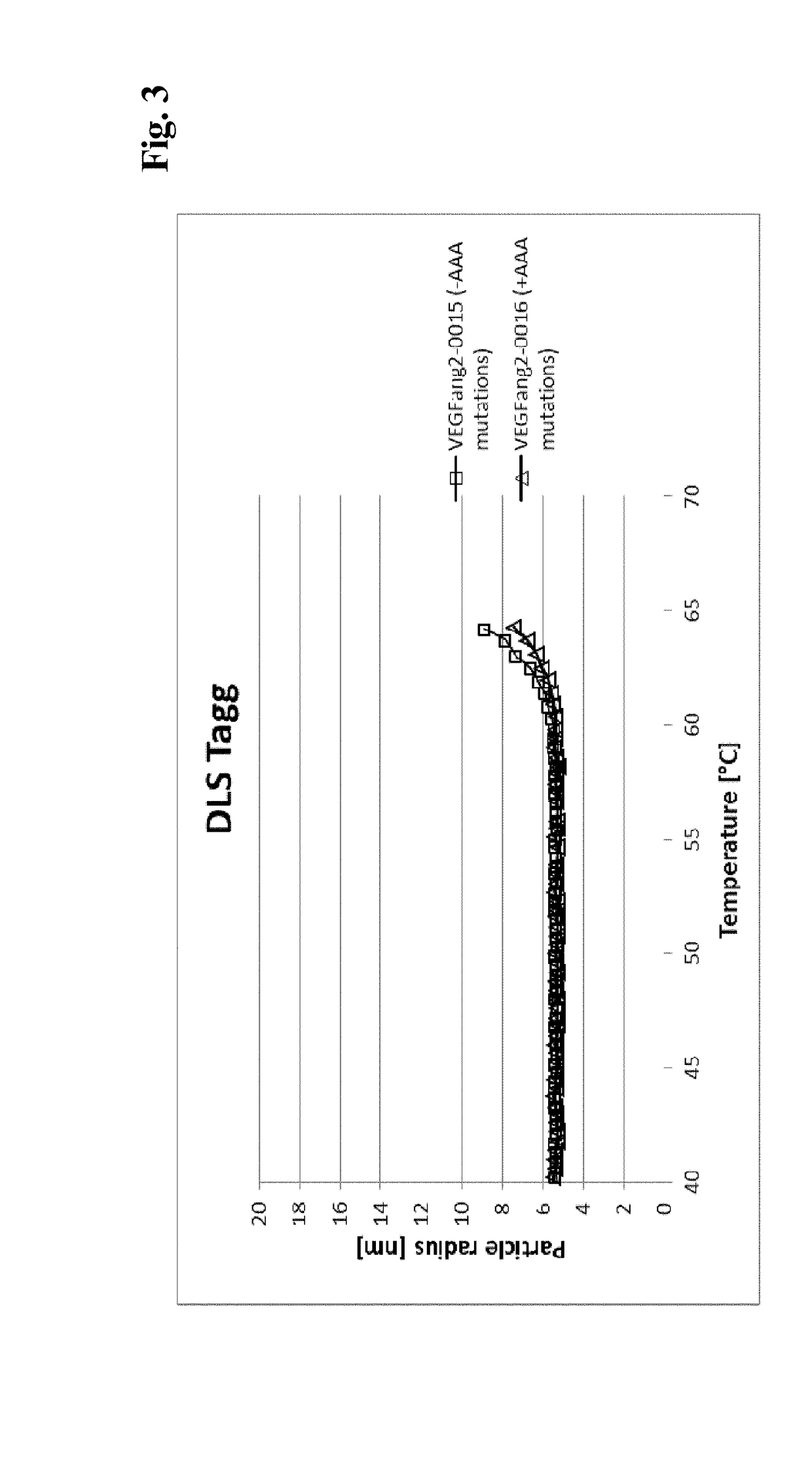
ActiveUS9695233B2Low viscosityHighly valuable propertySenses disorderImmunoglobulins against growth factorsVascular diseaseBispecific antibody
The present invention relates to bispecific antibody against human vascular endothelial growth factor (VEGF / VEGF-A) and against human angiopoietin-2 (ANG-2) of human IgG1 or IgG4 subclass with mutations I253A, H310A, and H435A, methods for their production, pharmaceutical compositions containing said antibodies, and uses thereof.
Owner:ROCHE GLYCART AG
Antidotes for factor Xa inhibitors and methods of using the same in combination with blood coagulating agents
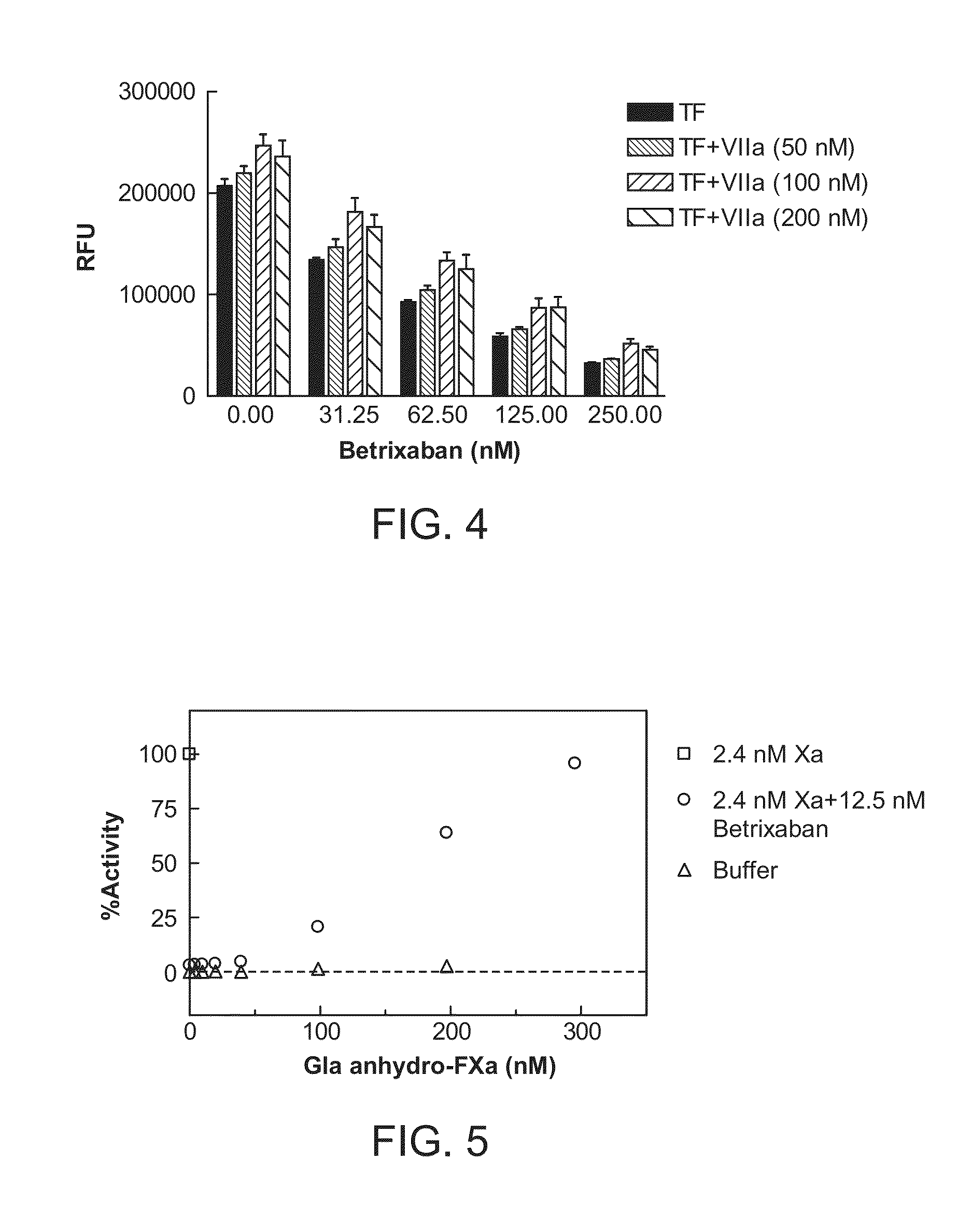
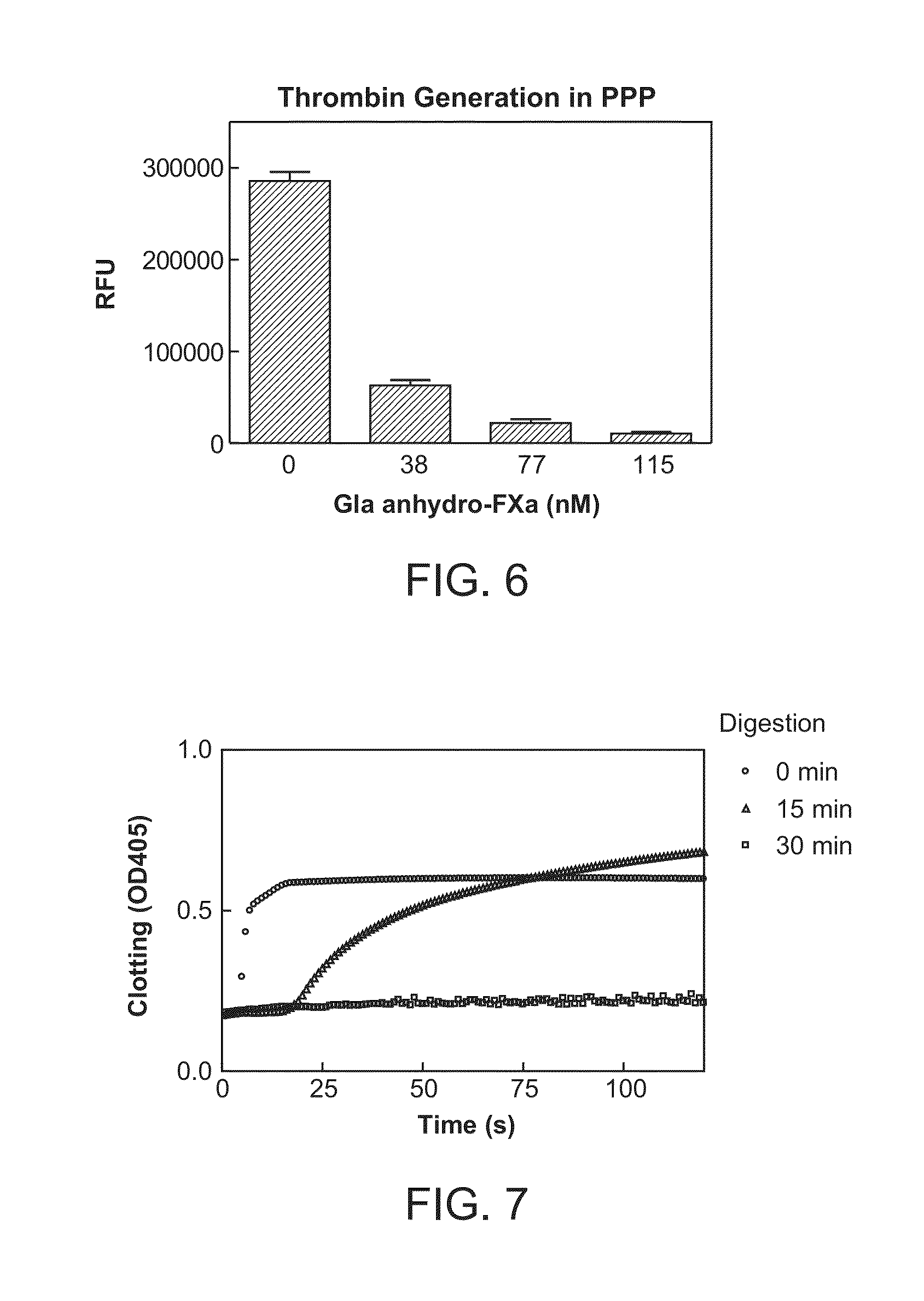
ActiveUS8455439B2Low effective doseReduce potential side effectsPeptide/protein ingredientsMammal material medical ingredientsAntidoteFactor Xa Inhibitor
The present invention relates to antidotes of anticoagulants targeting factor Xa which antidotes are used in combination with blood coagulating agents or other heparin antidotes to prevent or reduce bleeding in a subject. The antidotes described herein have reduced or no intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is or will be undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Cox-2 inhibitors and related compounds, and systems and methods for delivery thereof
InactiveUS20140004177A1Improve efficiencyReduce riskBiocideNervous disorderMedicineCompound (substance)
The present invention generally relates to the transdermal delivery of various compounds. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising a COX-2 inhibitor and / or a salt thereof, and optionally, a hostile biophysical environment and / or a nitric oxide donor. In some cases, the composition may be stabilized using a combination of a stabilization polymer (such as xanthan gum, KELTROL® BT and / or KELTROL® RD), propylene glycol, and a polysorbate surfactant such as Polysorbate 20, which combination unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
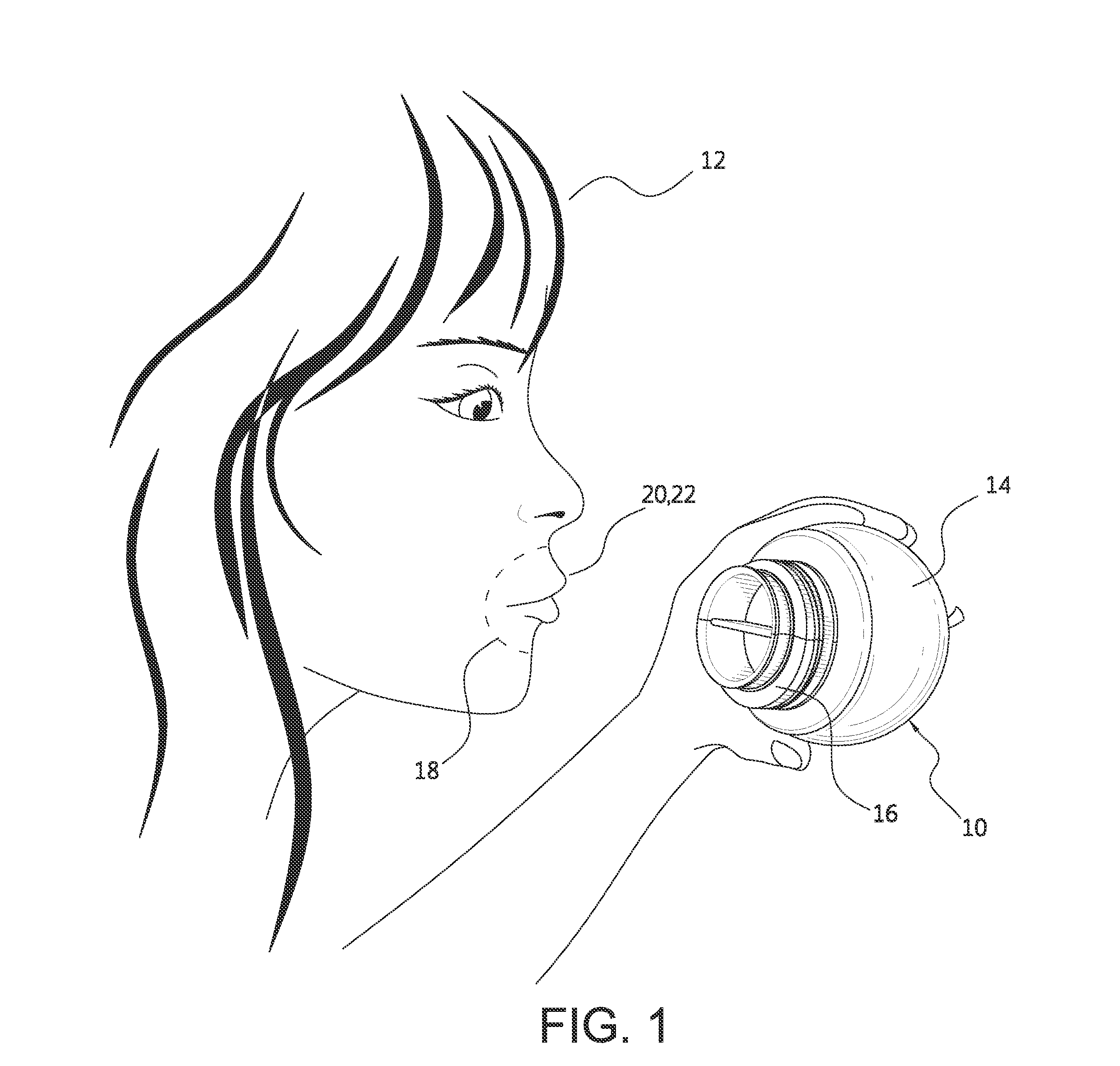
Lip enhancement and enlargement device
ActiveUS20130046211A1Reduce potential side effectsReduce stressPneumatic massageDiagnosticsUpper lipEngineering
A lip enhancement and enlargement device includes a suction element and a lip shaper coupled to the suction element. Each type of lip shaper includes one or more features that allow the device to shape and contour lips. A lip shaper may include one or more contouring elements that change the shape of an upper lip, for example. A lip shaper may also include shaping and contouring elements that form a fuller, unitary lip lobe or two fuller, lip lobes. Lip enhancement and enlargement kits and methods are also disclosed.
Owner:HO THIENNA
Treatment of erectile dysfunction and other indications
ActiveUS20140086980A1Improve efficiencyFast actionBiocideInorganic non-active ingredientsNitric oxidePhosphodiesterase Type 5 Inhibitors
The present invention generally relates to the transdermal delivery of various compounds. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising a phosphodiesterase type 5 inhibitor and / or a salt thereof, and optionally, a hostile biophysical environment and / or a nitric oxide donor. In some cases, the composition may be stabilized using a combination of a stabilization polymer (such as xanthan gum, KELTROL® BT and / or KELTROL® RD), propylene glycol, and a polysorbate surfactant such as Polysorbate 20, which combination unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Treatment of erectile dysfunction and other indications
InactiveUS20140051707A1Enhancing local deliveryReduce systemic amount of compoundBiocideInorganic non-active ingredientsNitric oxidePhosphodiesterase Type 5 Inhibitors
The present invention generally relates to the transdermal delivery of various compounds. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising a phosphodiesterase type 5 inhibitor and / or a salt thereof, and optionally, a hostile biophysical environment and / or a nitric oxide donor. In some cases, the composition may be stabilized using a combination of a stabilization polymer (such as xanthan gum, KELTROL® BT and / or KELTROL® RD), propylene glycol, and a polysorbate surfactant such as Polysorbate 20, which combination unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Systems and methods for treatment of allergies and other indications
ActiveUS20140044774A1Lower systemic exposureIncrease efficiencyBiocideInorganic non-active ingredientsNitric oxidePropylene glycol
The present invention generally relates to the transdermal delivery of various compounds. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising an H1 antihistamine and / or a salt thereof, and optionally, a hostile biophysical environment and / or a nitric oxide donor. In some cases, the composition may be stabilized using a combination of a stabilization polymer (such as xanthan gum, KELTROL® BT and / or KELTROL® RD), propylene glycol, and a polysorbate surfactant such as Polysorbate 20, which combination unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
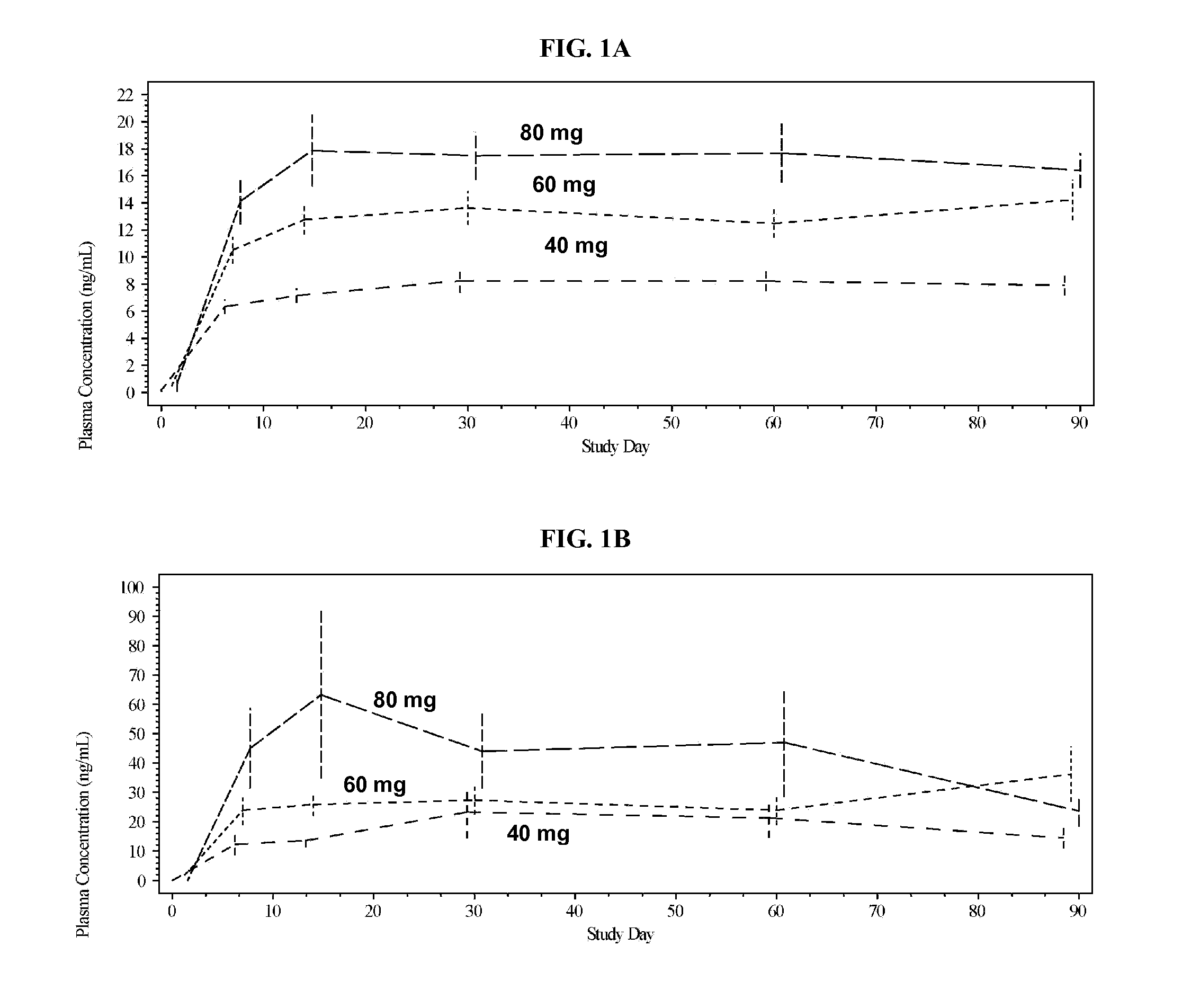
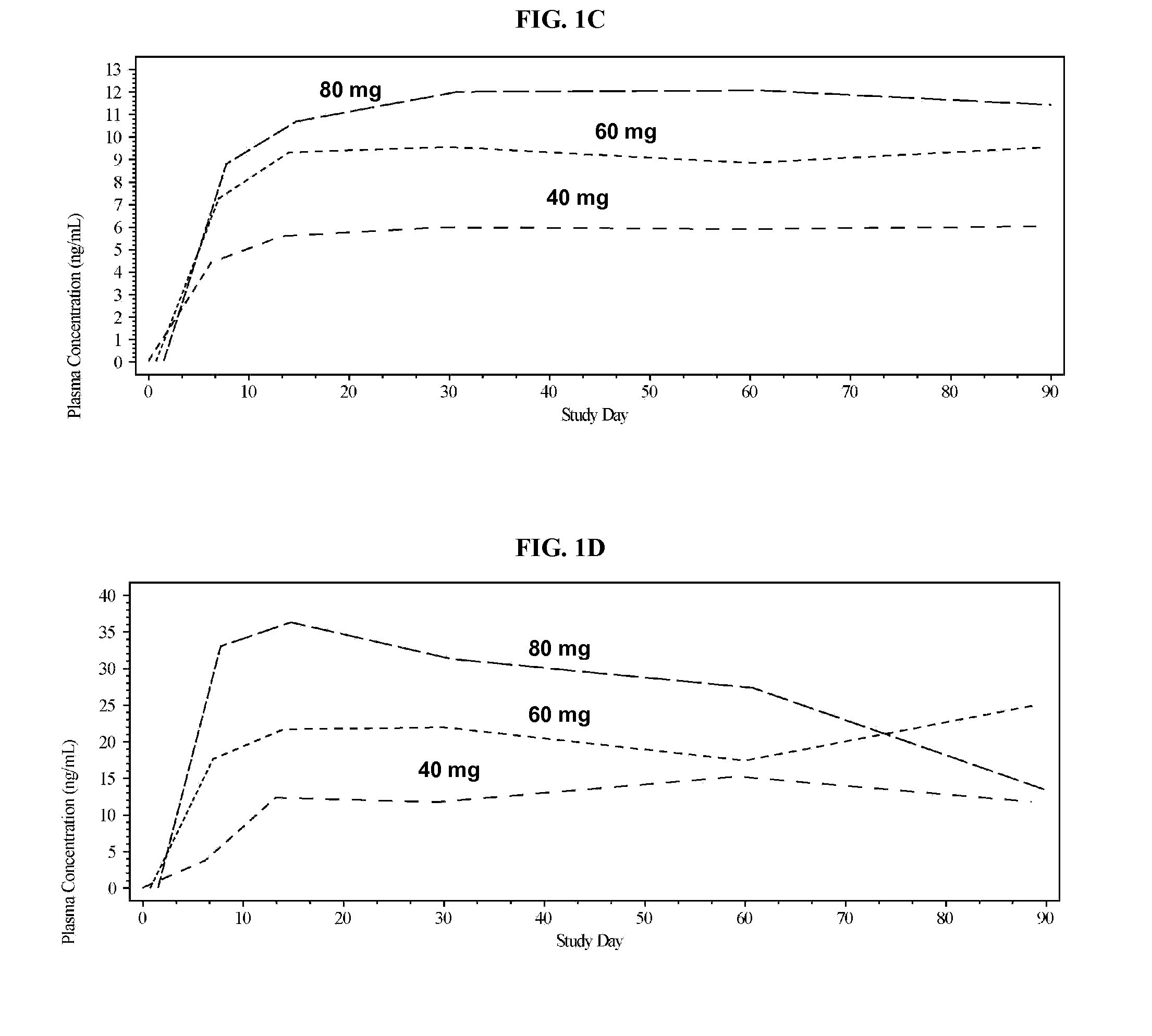
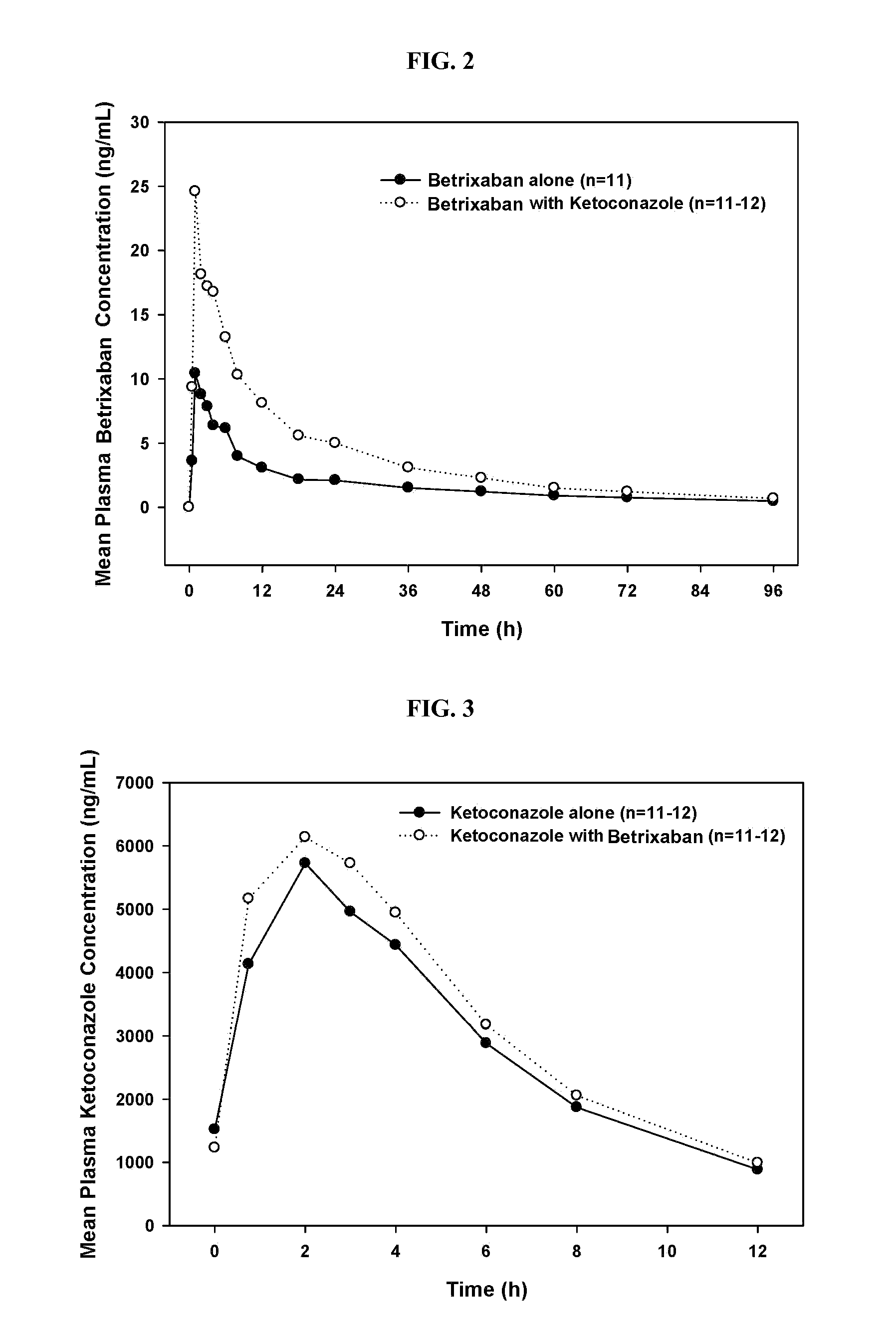
Methods and formulations of treating thrombosis with betrixaban and a p-glycoprotein inhibitor
InactiveUS20120095019A1Increase exposureReduce the amount requiredBiocideAnimal repellantsBetrixabanFactor Xa Inhibitor
This invention is directed to methods of inhibiting coagulation or treating thrombosis using a factor Xa inhibitor and a P-glycoprotein (Pgp) inhibitor. The invention is also directed to formulations used in the methods.
Owner:ALEXION PHARMA INC
Systems and methods for treatment of allergies and other indications
ActiveUS9289495B2Improve efficiencyFast actionInorganic non-active ingredientsPharmaceutical delivery mechanismMedicineAllergy
The present invention generally relates to the transdermal delivery of various compounds. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising an H1 antihistamine and / or a salt thereof, and optionally, a hostile biophysical environment and / or a nitric oxide donor. In some cases, the composition may be stabilized using a combination of a stabilization polymer (such as xanthan gum, KELTROL® BT and / or KELTROL® RD), propylene glycol, and a polysorbate surfactant such as Polysorbate 20, which combination unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Fatty acid-pharmaceutical agent conjugates
InactiveUS8314077B2Reduce potential side effectsReduced activityBiocideCarbohydrate active ingredientsNervous systemFatty acid
The invention provides conjugates of fatty acids and pharmaceutical agents useful in treating noncentral nervous system conditions. Methods for selectively targeting pharmaceutical agents to desired tissues are provided.
Owner:LUITPOLD PHARMA INC
Propulsive drug delivery from a swallowable device into a patients intestinal tract
PendingUS20200276426A1Improve pharmacokineticsQuick releaseBalloon catheterMedical devicesSmall intestinePharmaceutical drug
Embodiments of the invention provide swallowable devices, preparations, and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs or other therapeutic agents (TA) into a wall of the GI tract such as the stomach or small intestine. The swallowable device comprises a sensor, a combustible propellant (CP) and a therapeutic agent preparation (TAP) comprising at least one TA. The sensor triggers the CP to ignite and propel the TAP into the wall of the GI tract in response to an external condition or change in external condition. Embodiments of the invention are particularly useful for orally delivering drugs or other TAs which are degraded within the GI tract and require parenteral injection.
Owner:RANI THERAPEUTICS
Heterodimeric antibodies that bind CD3 and CD38
ActiveUS10526417B2Reduce potential side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesCell biology
Owner:XENCOR INC
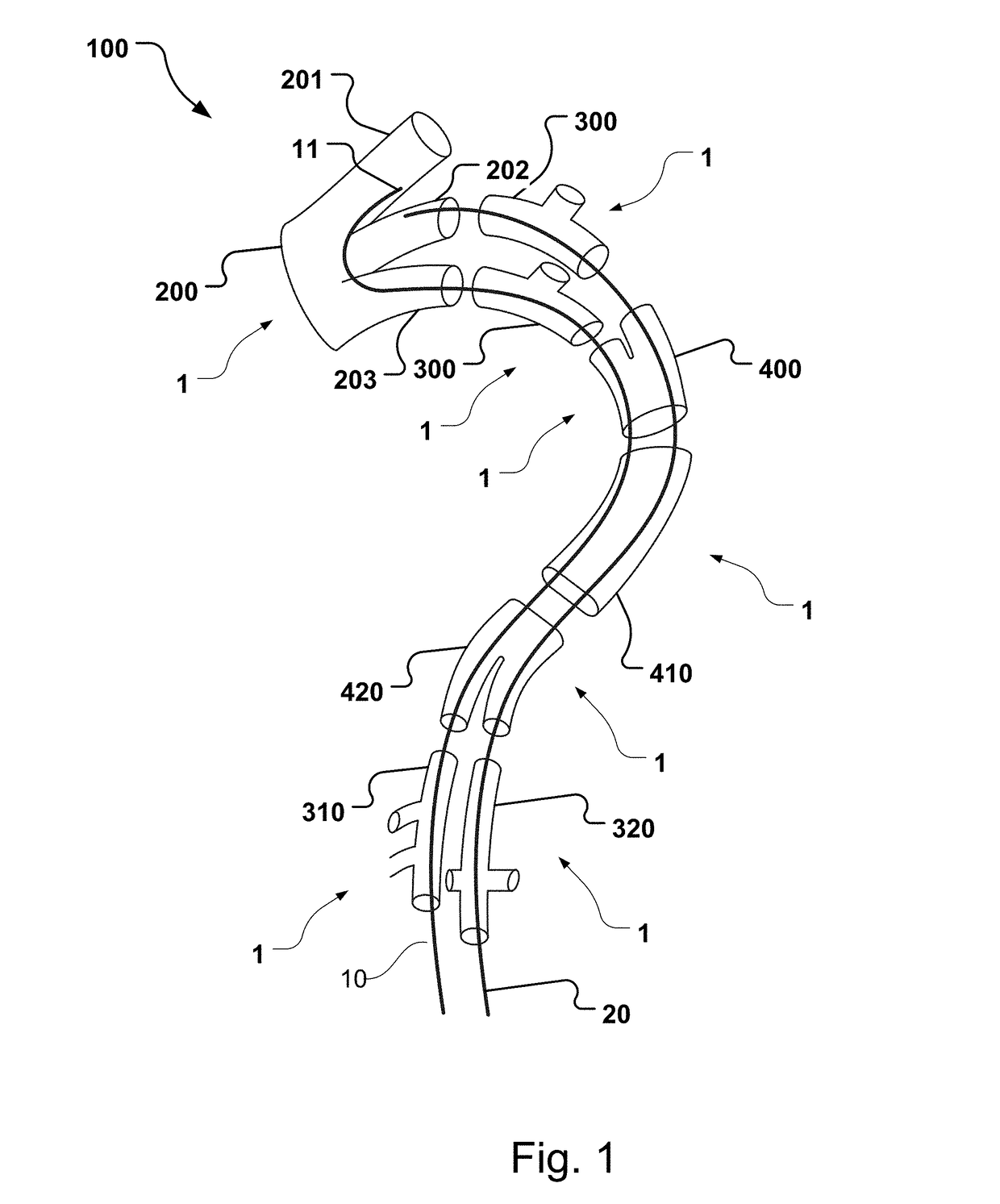
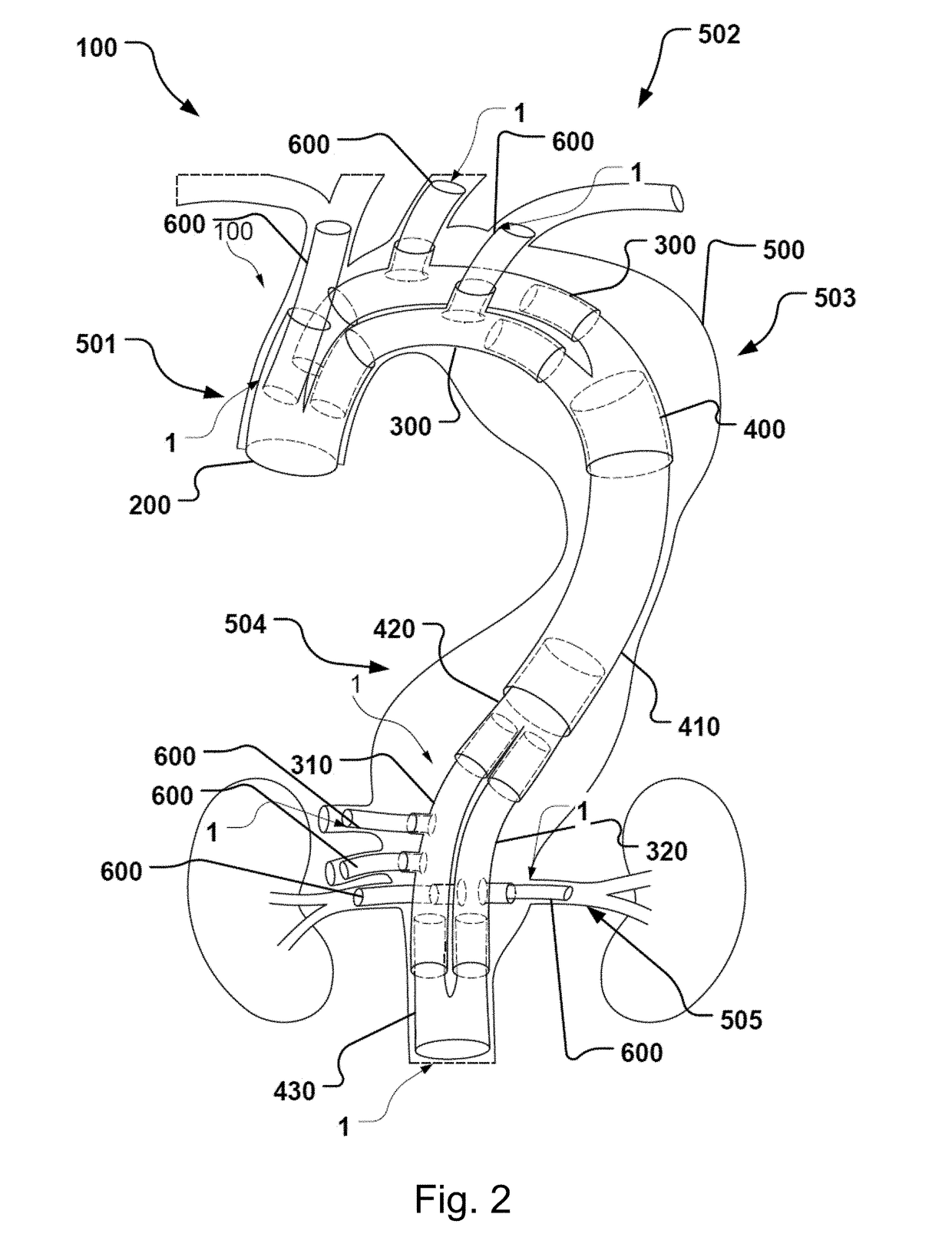
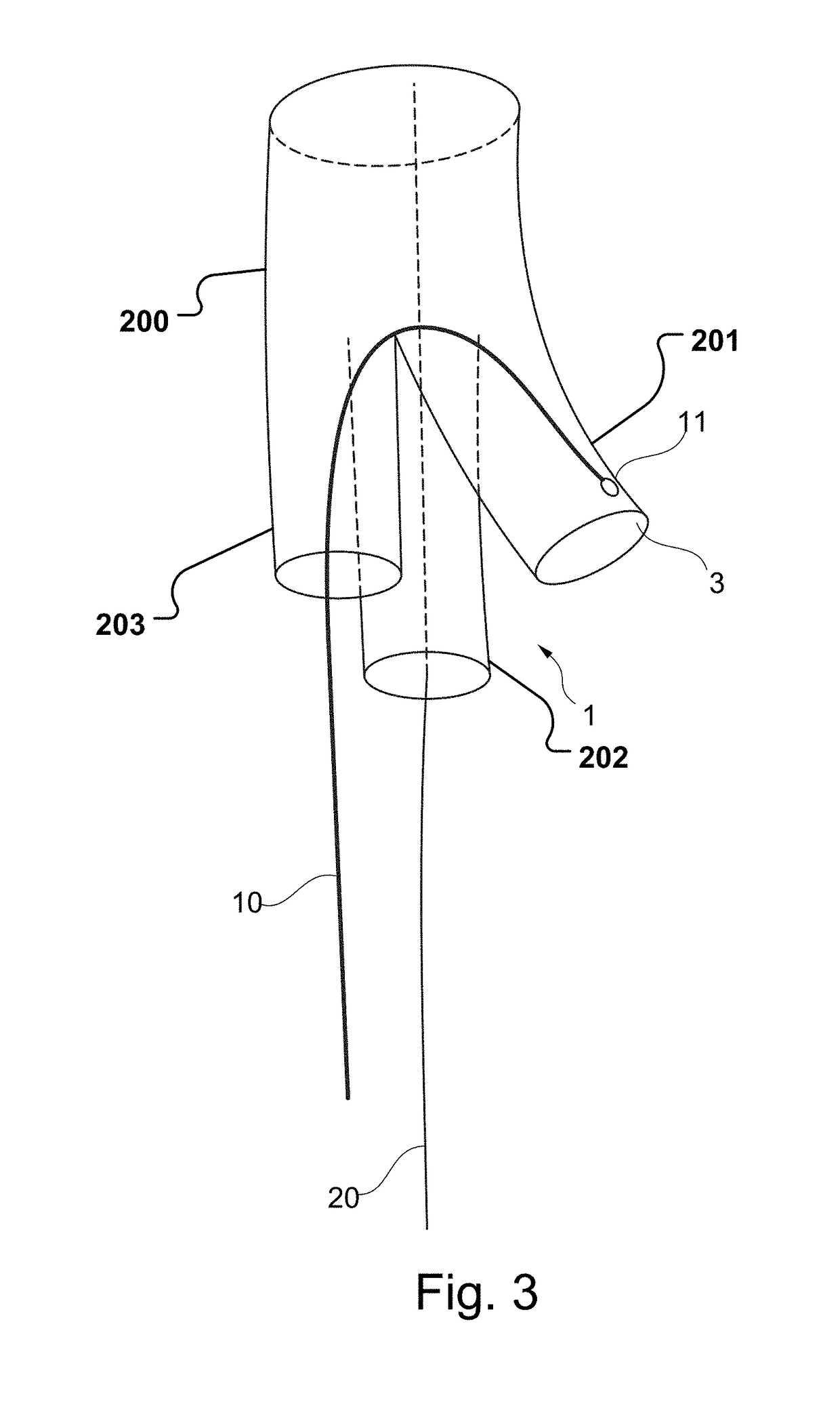
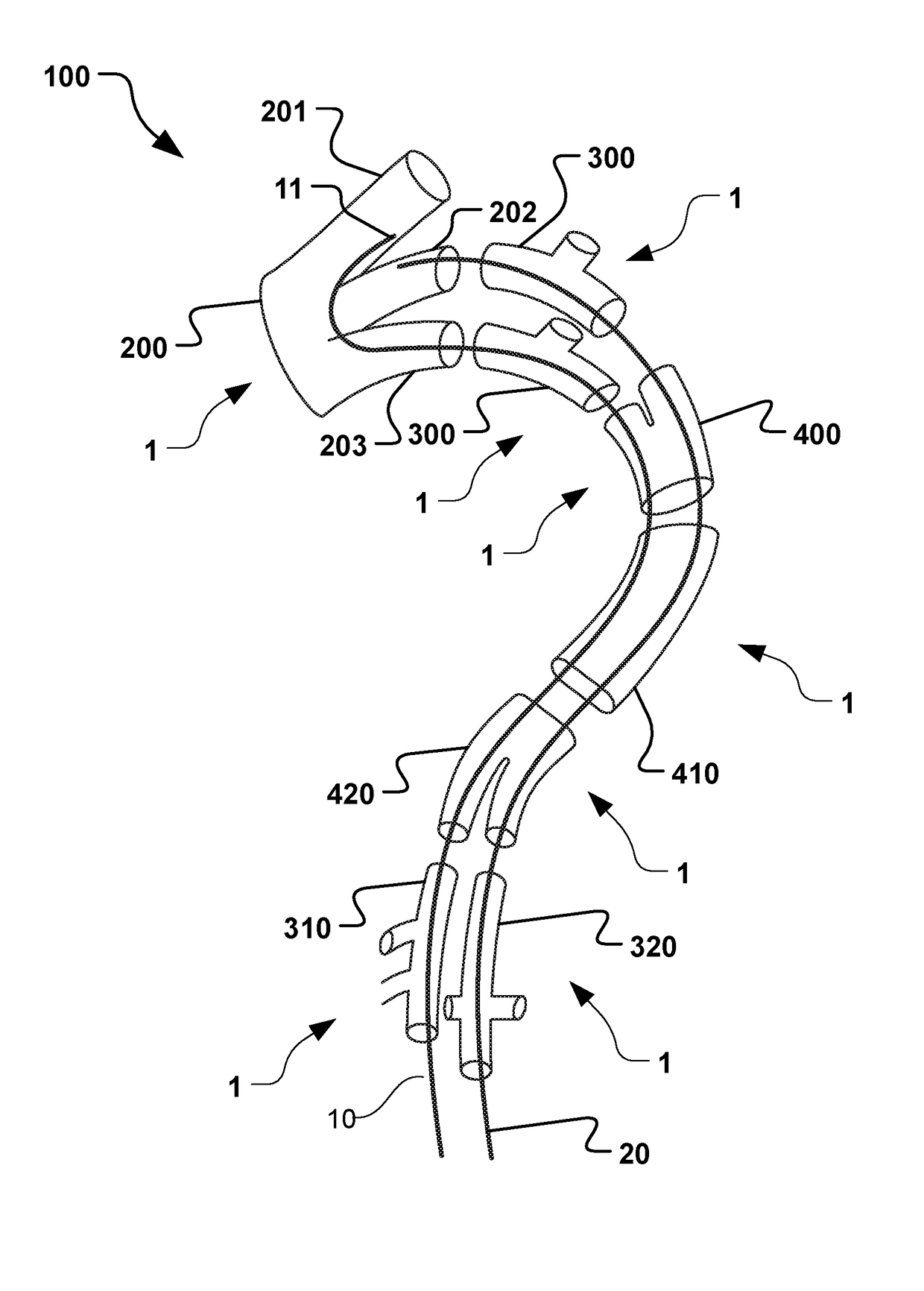
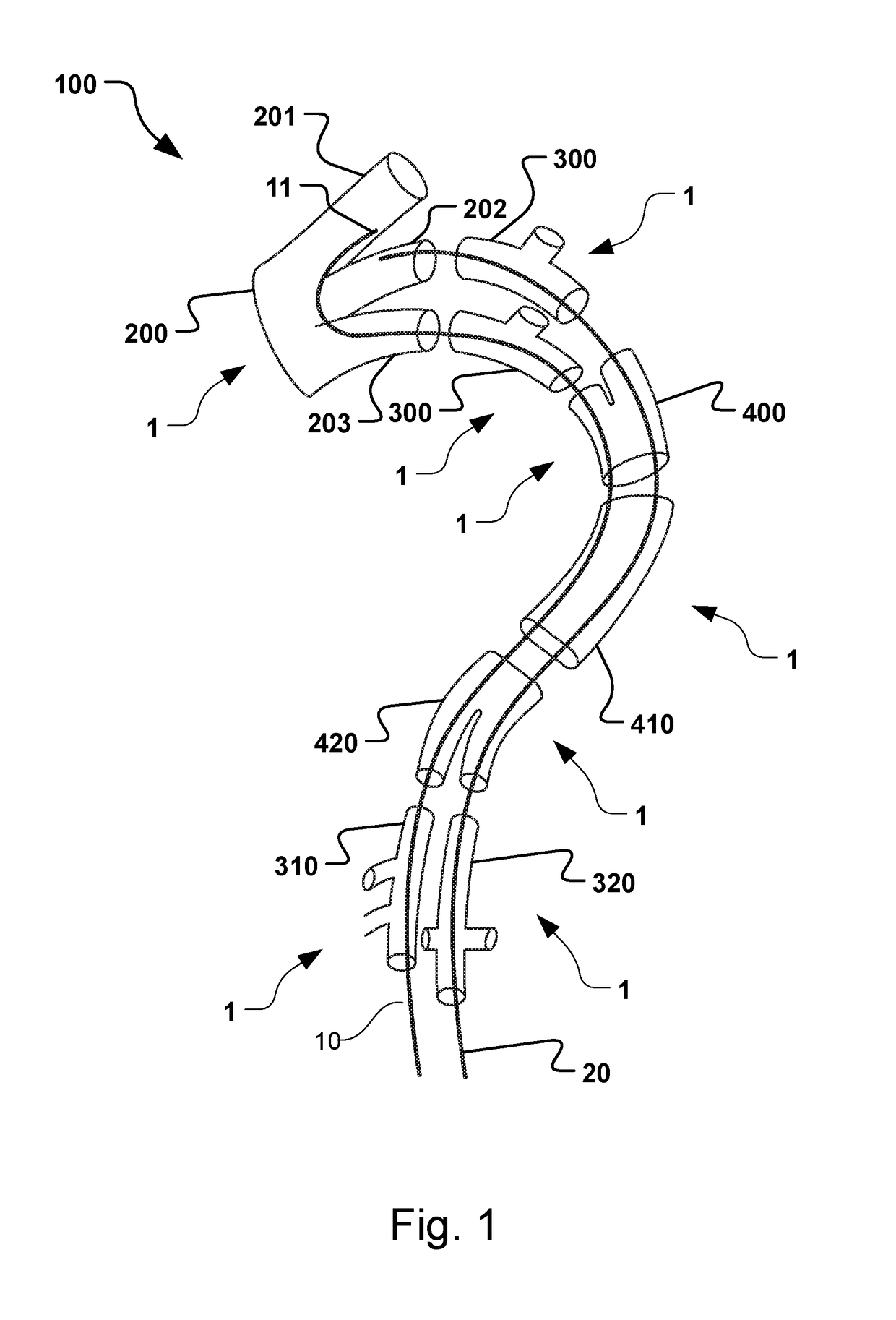
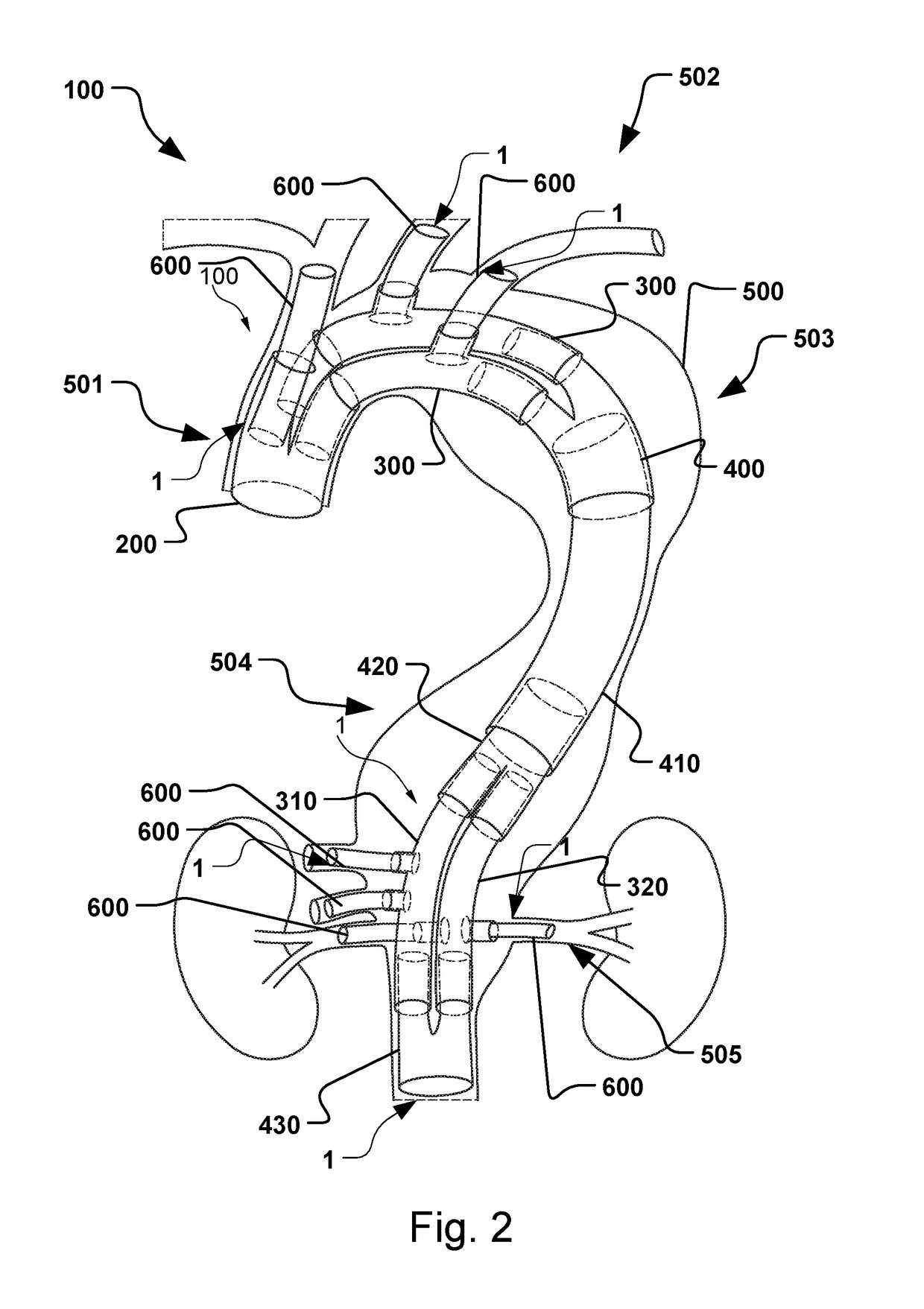
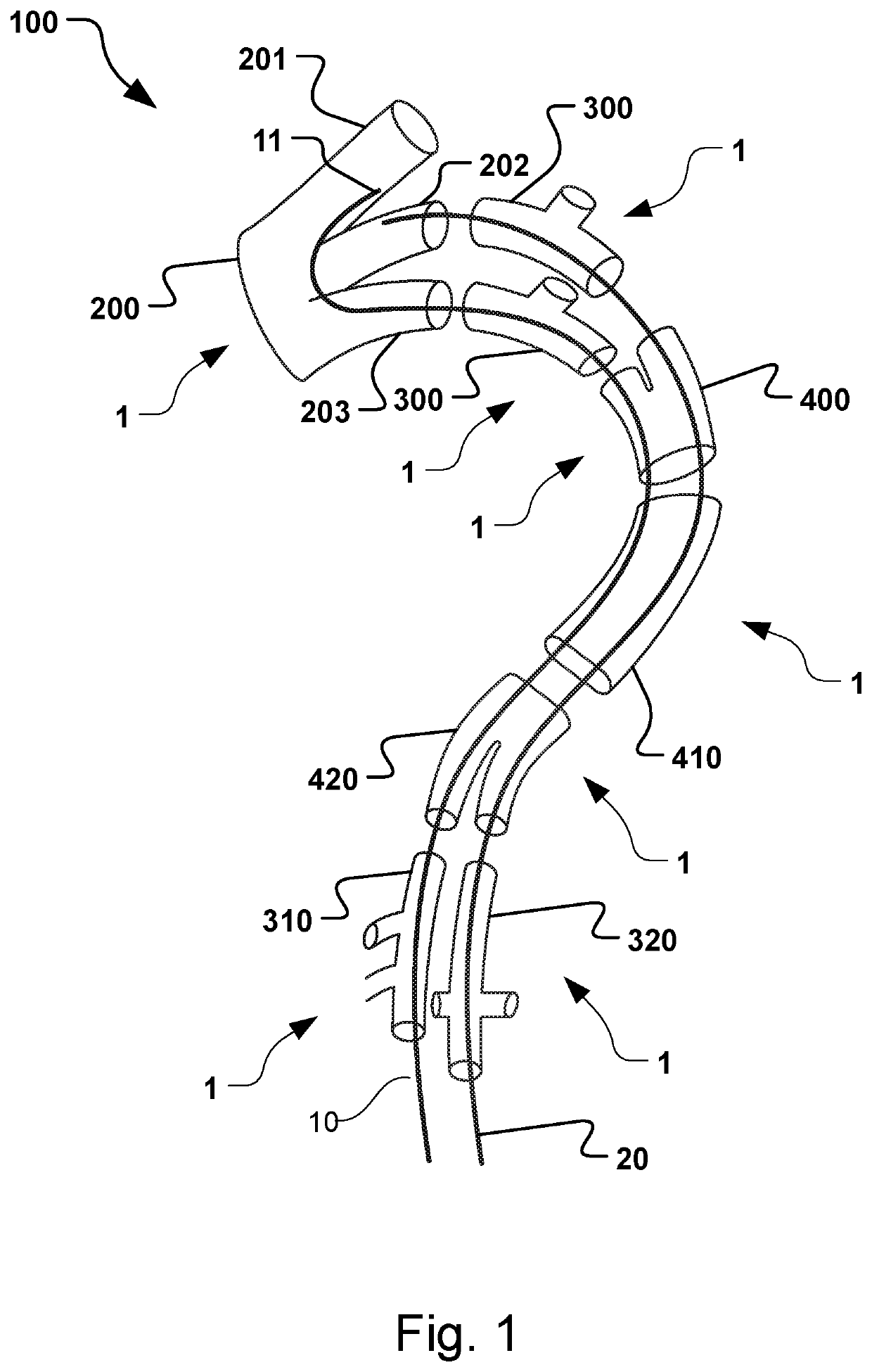
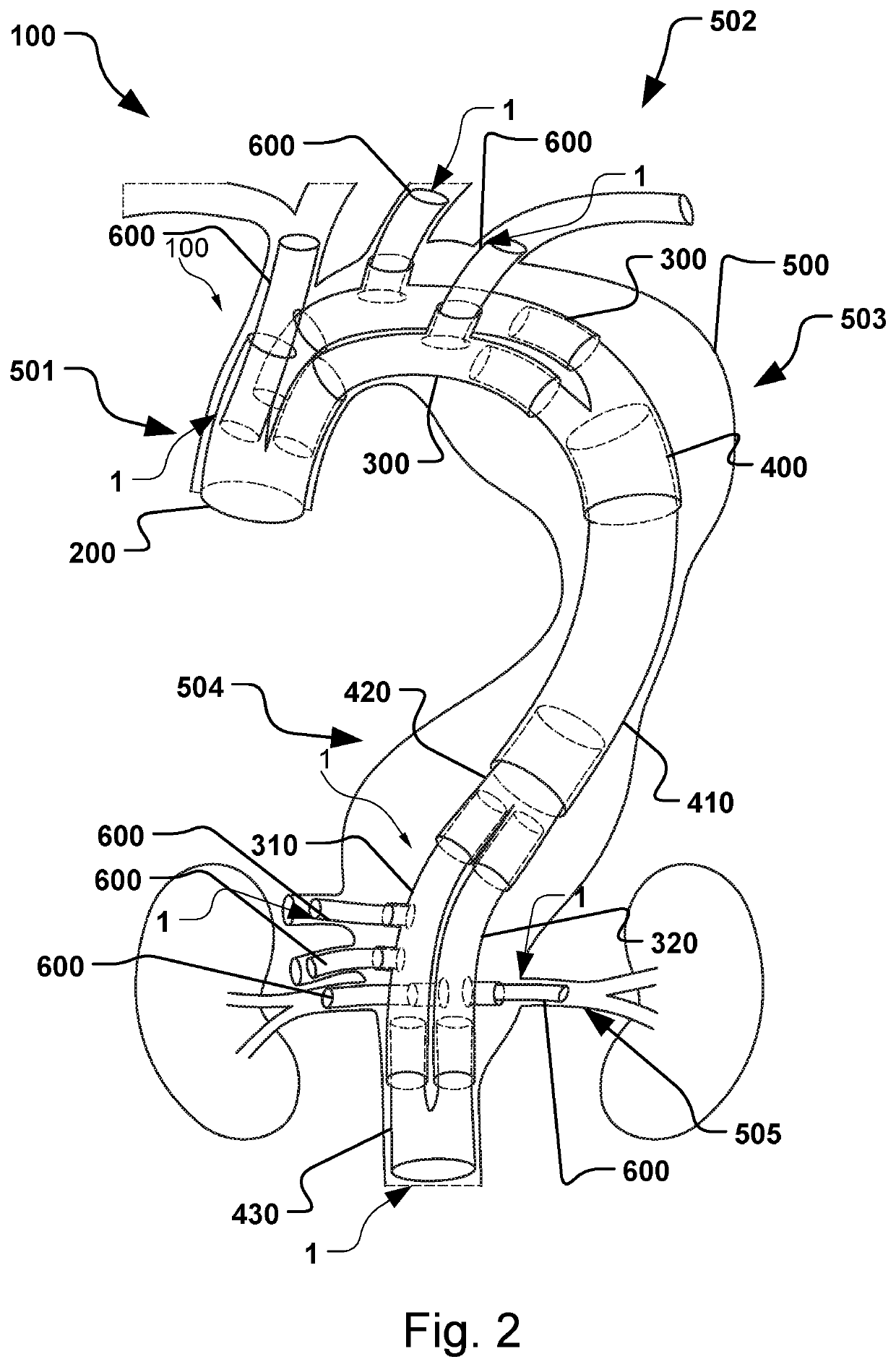
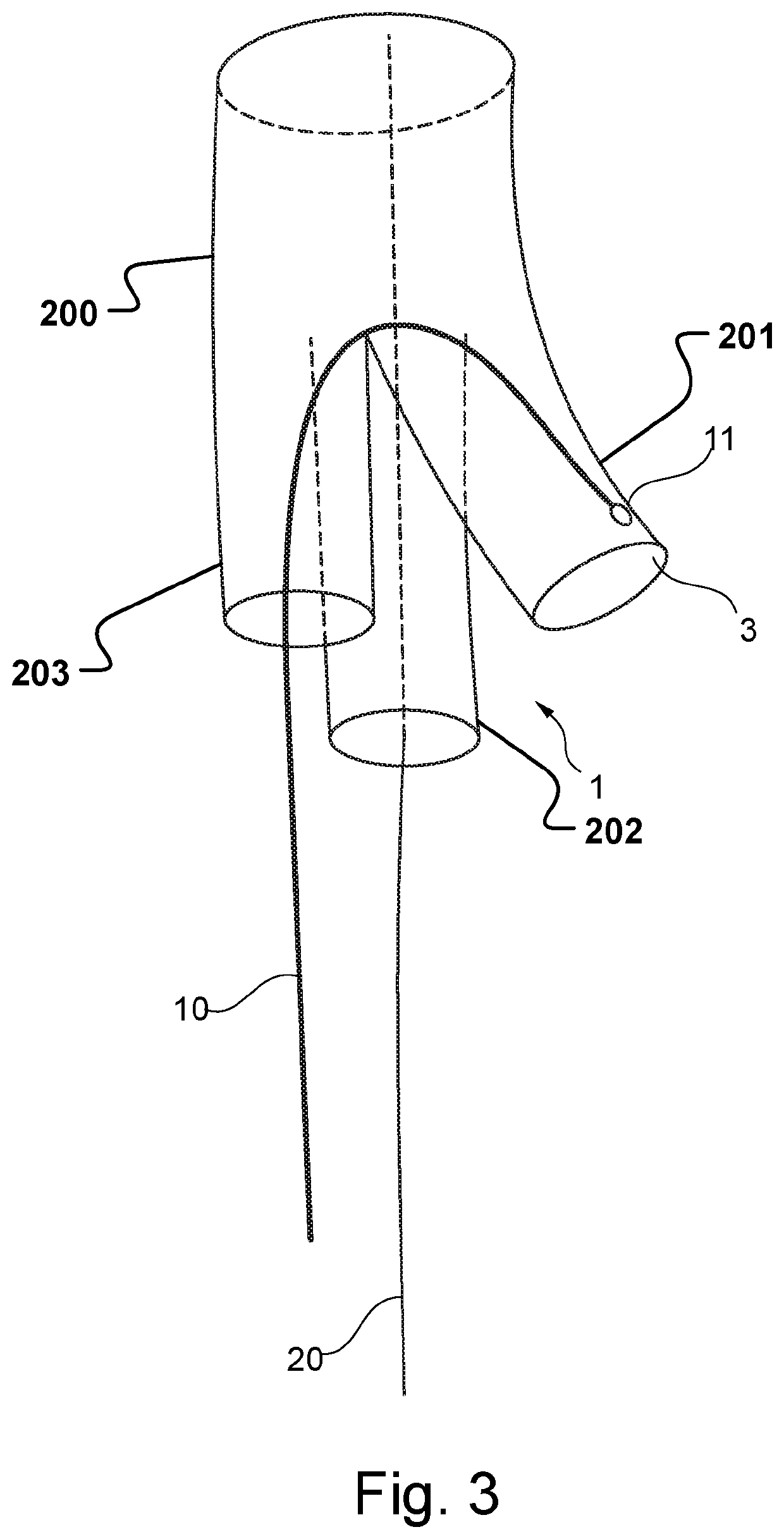
Vascular Medical Device, System And Method
ActiveUS20190083229A1Shorten operation timeImprove securityStentsBlood vesselsInsertion stentMedical device
The present application discloses a covered stent and a method for navigating the covered stent to a branch vessel, the covered stent including a main body and at least one lateral side branch connected to the main body. A system of covered stents and a method for implanting, including interconnecting the covered stents is also disclosed.
Owner:SWISS CAPITAL ENG AG
Lip enhancement and enlargement device
ActiveUS9549868B2Reduce potential side effectsReduce stressPneumatic massageCupping glassesUpper lipEngineering
A lip enhancement and enlargement device includes a suction element and a lip shaper coupled to the suction element. A lip shaper may include one or more features that allow the device to shape and contour lips. A lip shaper may include one or more contouring elements that change the shape of an upper lip, for example. A lip shaper may also include shaping and contouring elements that form a fuller, unitary lip lobe or two fuller, lip lobes. Lip enhancement and enlargement kits and methods are also disclosed.
Owner:HO THIENNA
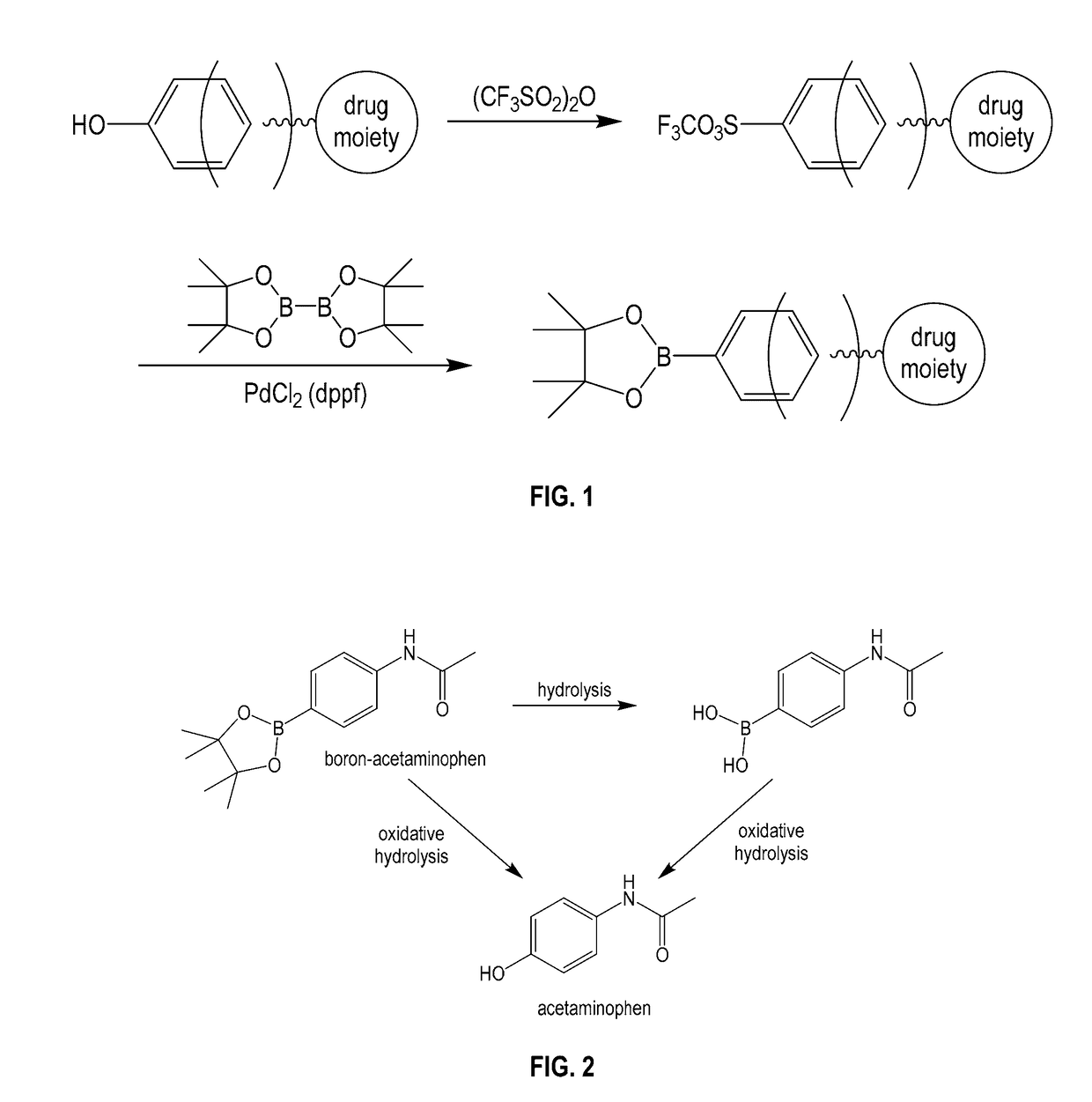
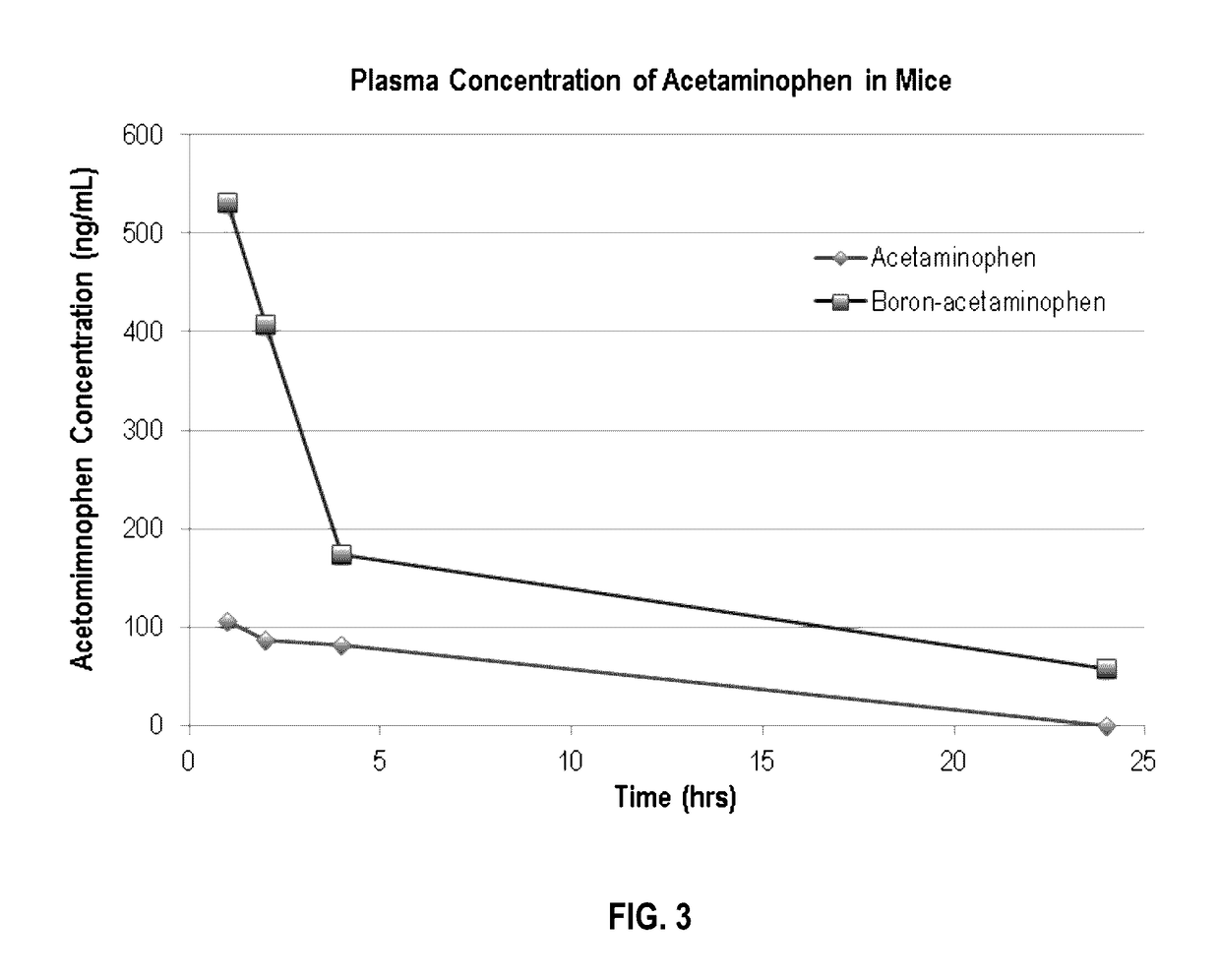
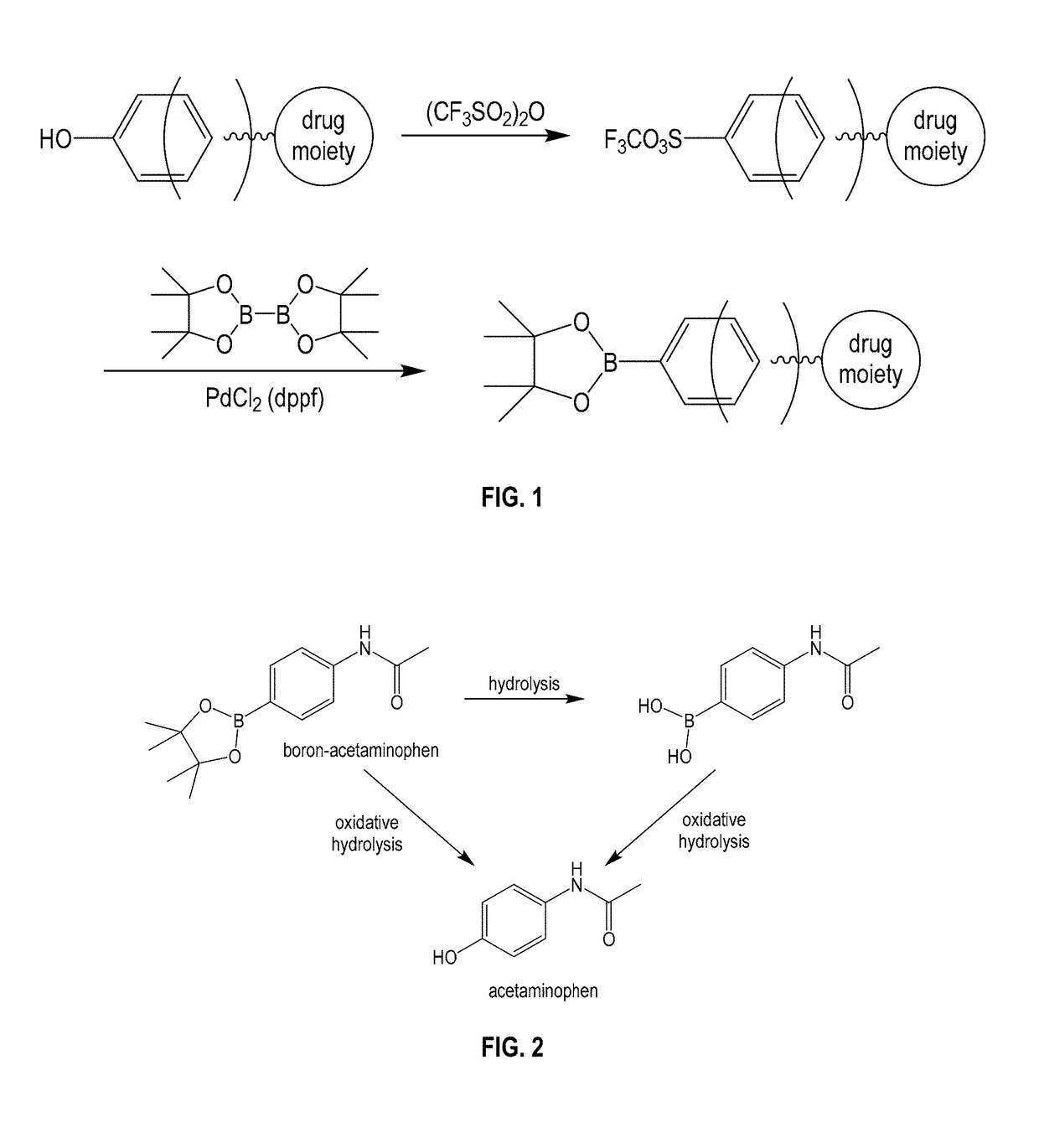
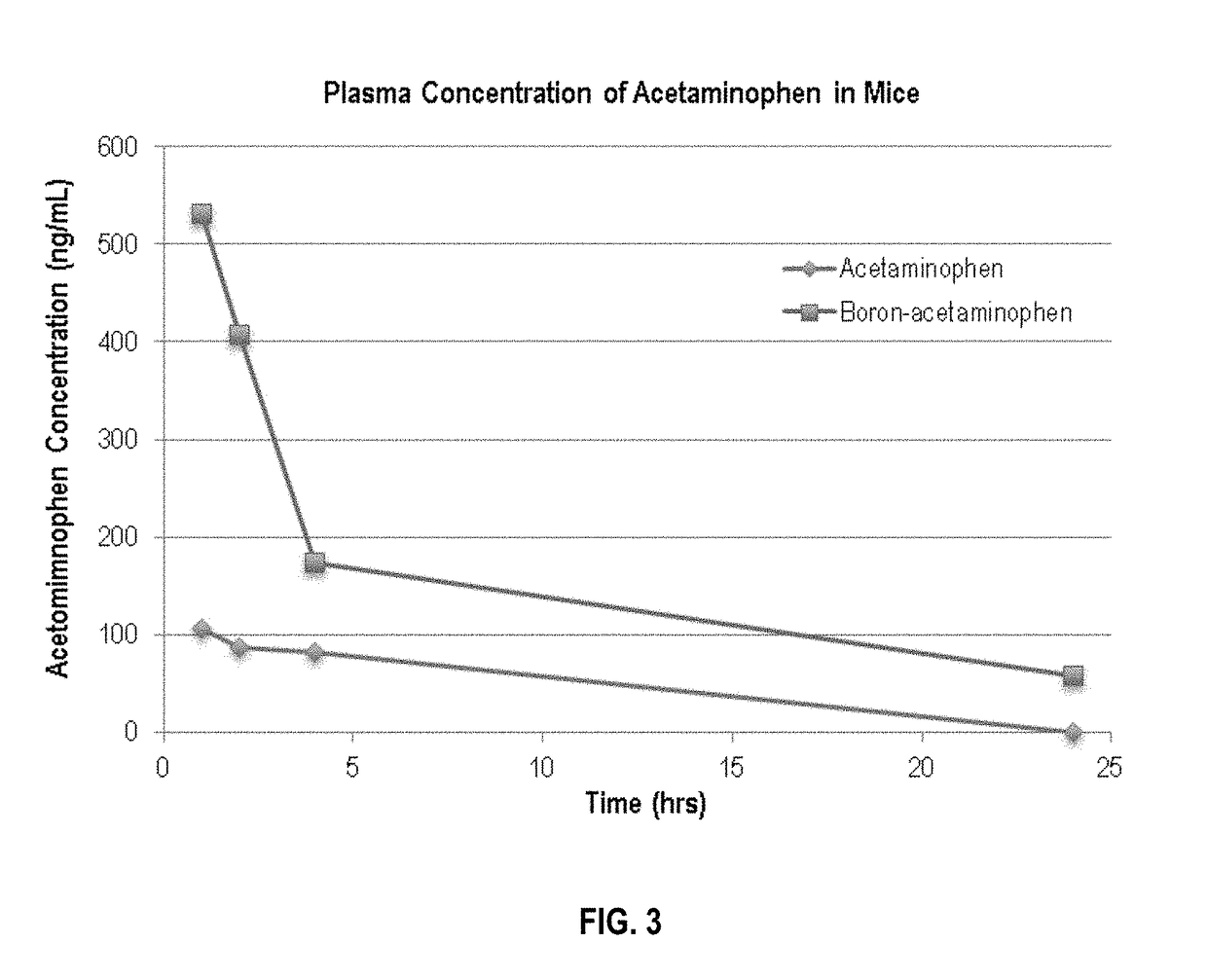
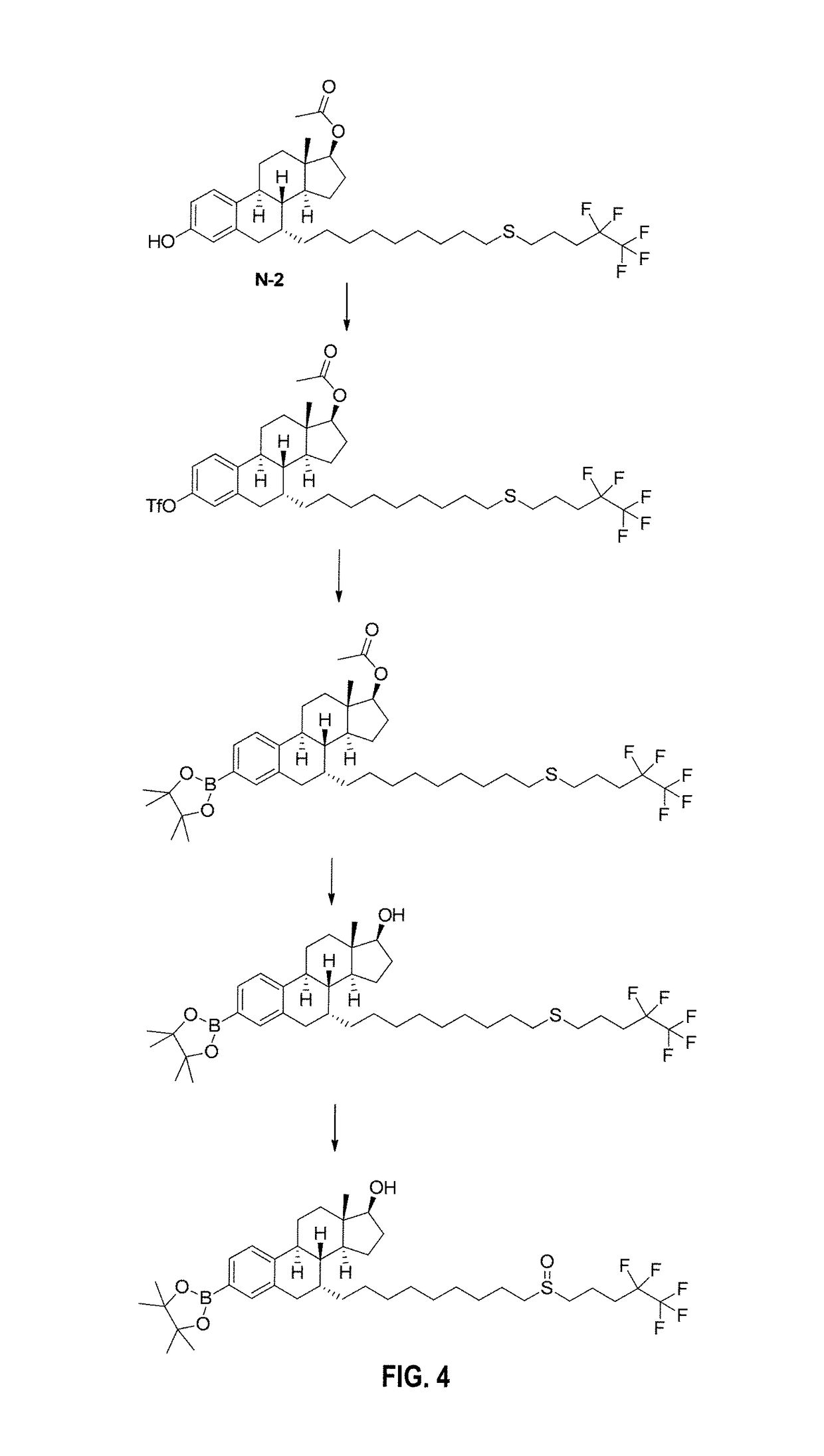
Boron-based prodrug strategy for increased bioavailability and lower-dosage requirements for drug molecules containing at least one phenol (or aromatic hydroxyl) group
ActiveUS20170137443A1Improve bioavailabilityHigh retention rateAntibacterial agentsNervous disorderTherapeutic effectBioavailability
Boron-based prodrugs of phenol- or aromatic hydroxyl group-containing therapeutic molecules (“original drugs”), uses thereof, and methods of making the same, are provided for achieving, for example, improved bioavailability, prolonged retention (e.g., in a circulatory system) and, in particular, significantly lowered therapeutically effective dosage in order to reduce adverse effects while maintaining the desired therapeutic effects of the original drugs.
Owner:XAVIER UNIVERSITY
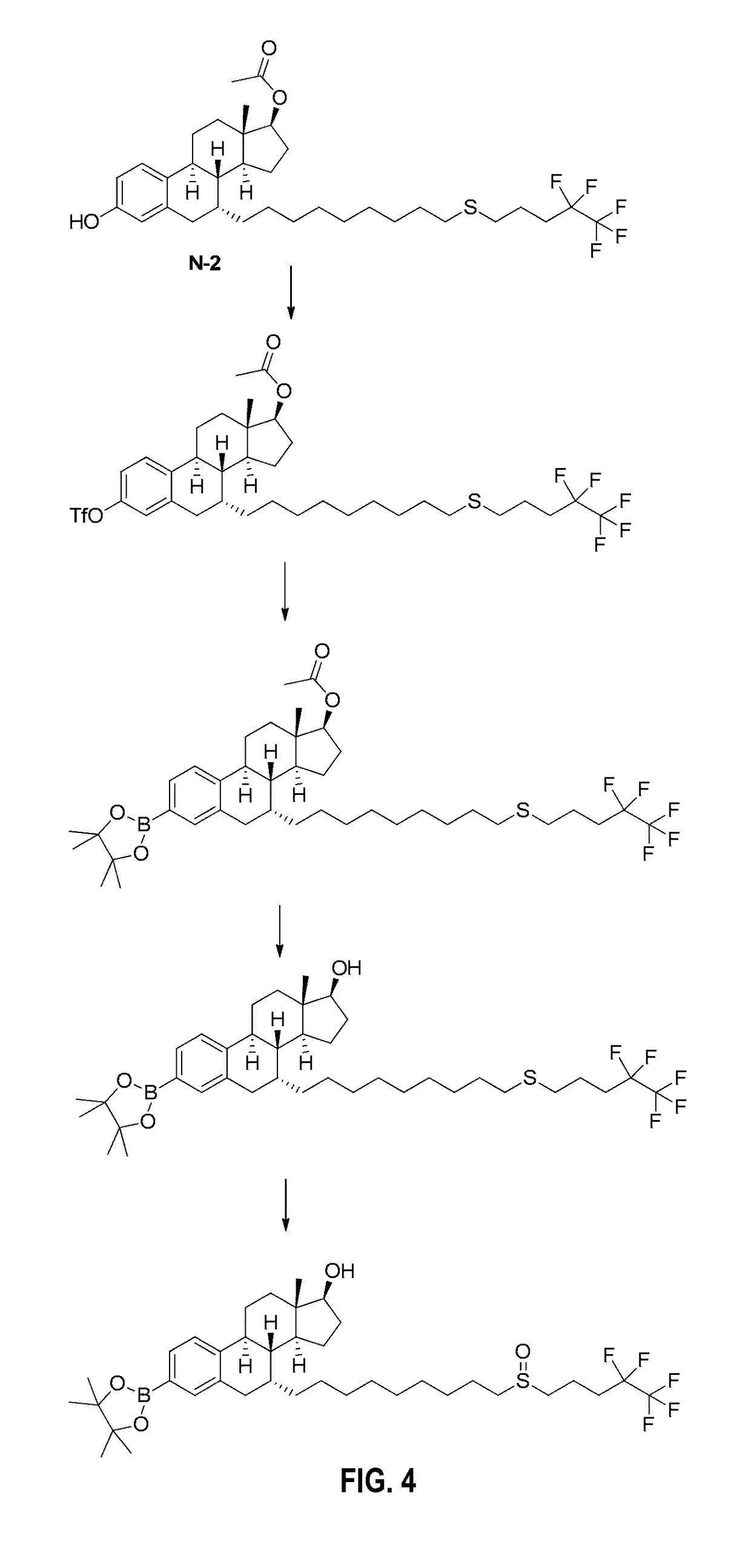
Compositions, methods and uses for the treatment of diabetes and related conditions by controlling blood glucose level
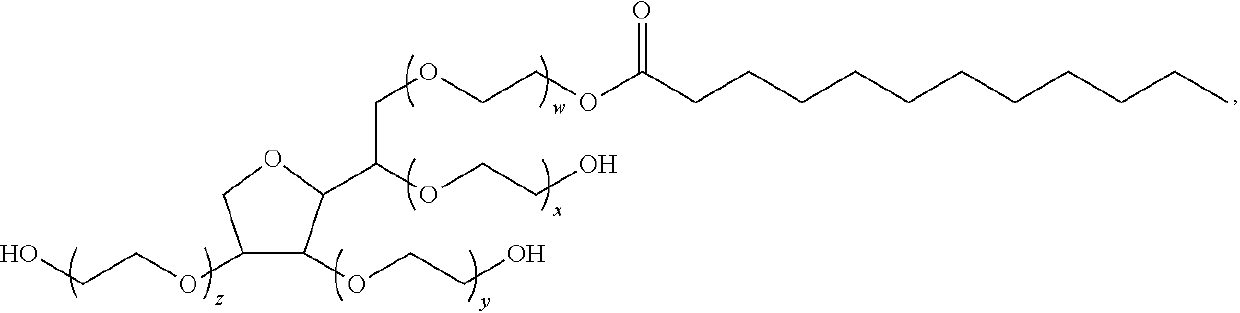
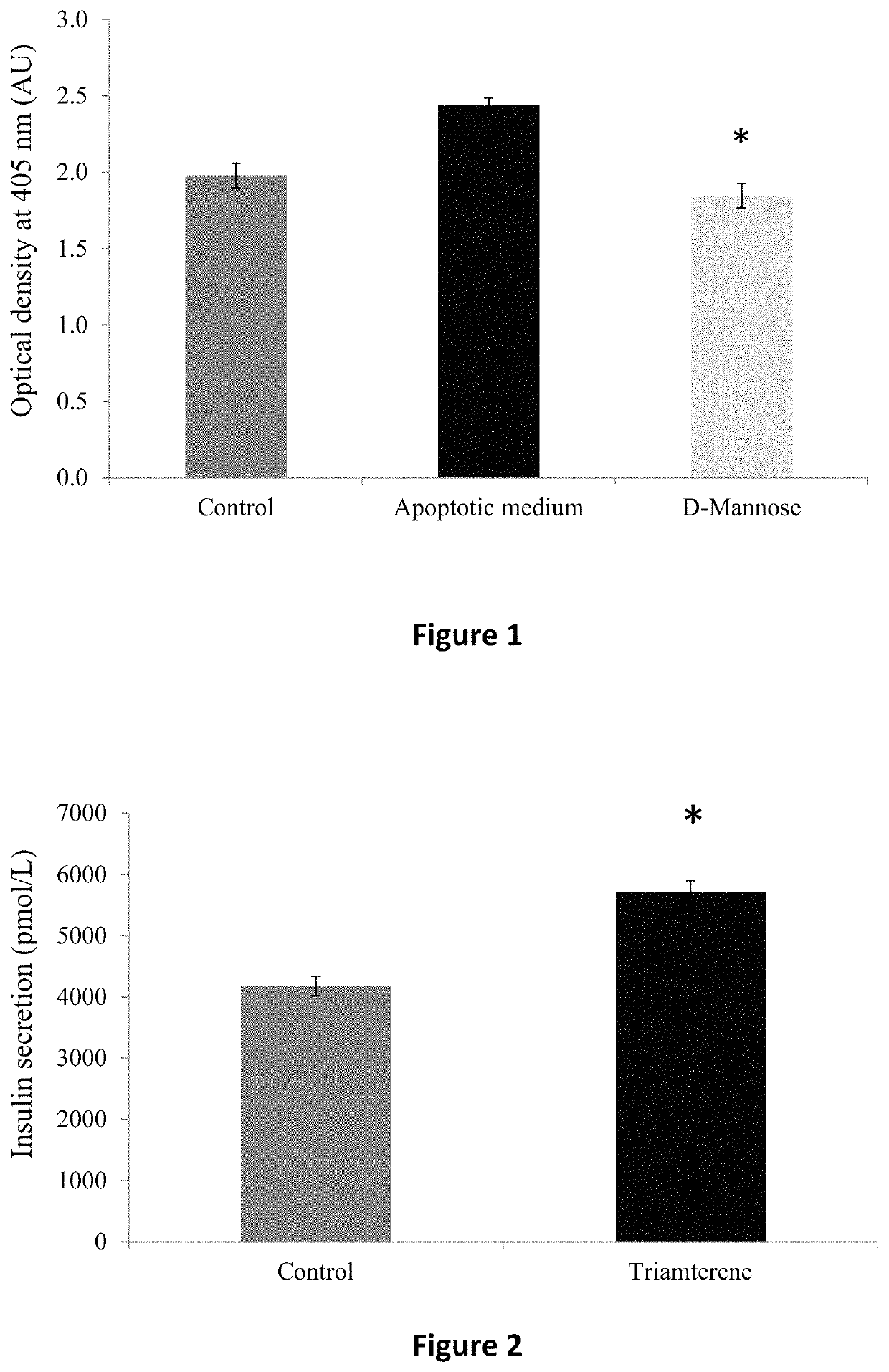
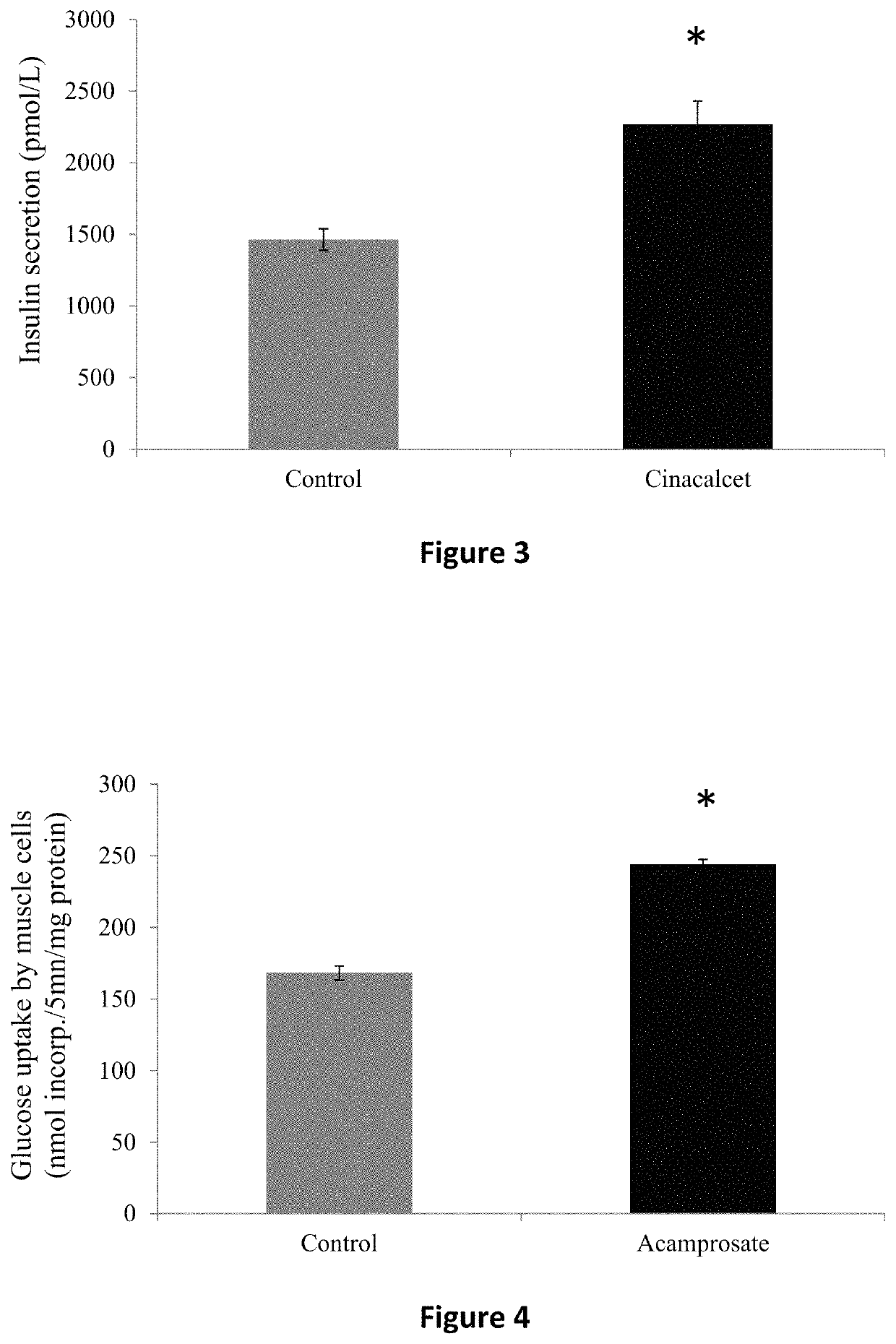
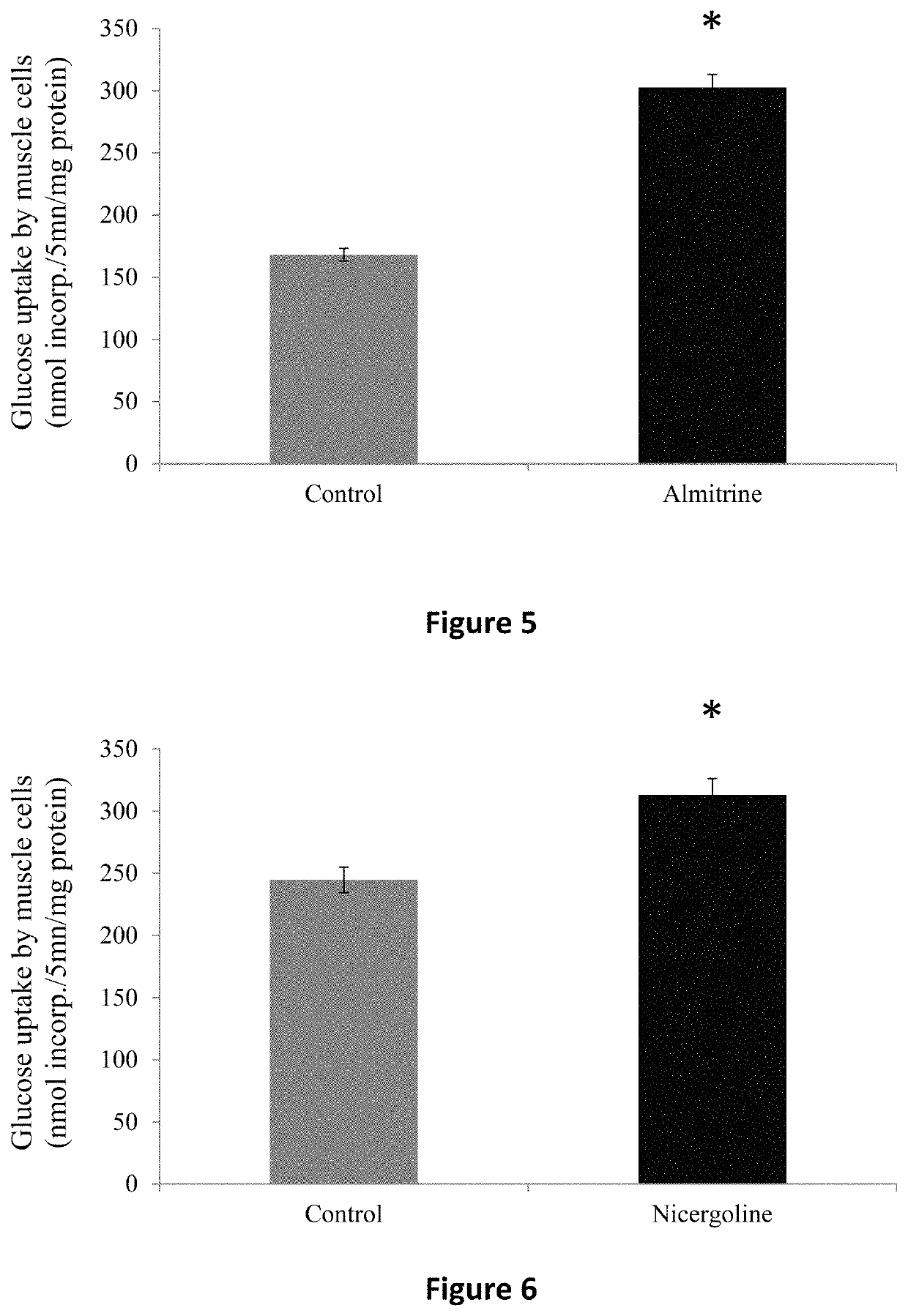
ActiveUS20160235693A1Reduce insulin resistanceEfficient clinical benefitAmide active ingredientsEster active ingredientsDiseaseBlood sugar
The present invention relates to compositions and methods for controlling glycaemia in a mammalian in need thereof. The present invention relates to compositions and methods for the treatment of diabetes disease and related disorders. More specifically, the present invention relates to novel therapies or combinatorial therapies of diabetes and related disorders, based on compositions controlling the blood glucose level.
Owner:PHARNEXT
Patient selection for enhancement of Anti-tumor immunity in cancer patients
PendingUS20220175787A1Enhance immune activationPromote antitumor immunityMicrobiological testing/measurementAntibody ingredientsChemotherapy combinationsPharmaceutical medicine
A method for increasing the progression free survival or overall survival of a patient with cancer comprising: determining if the cancer has a surrounding microenvironment that is favorable to immune modulation; determining if the chemotherapy regimen induces immunogenic cell death, and if both are yes, administering an effective amount of a CDK 4 / 6 inhibitor selected from Compounds I, II, III, IV, or V, or a pharmaceutically acceptable salt thereof, wherein the CDK4 / 6 inhibitor is administered prior to the administration of the chemotherapy or optionally prior to and concurrently with chemotherapy; and, wherein the increase in progression free survival or overall survival is in comparison to the progression free survival or overall survival based on administration of the chemotherapy alone, either based on literature or otherwise publicly available evidence, a comparative during preclinical or clinical trials, or other means accepted by persons skilled in the field.
Owner:G1 THERAPEUTICS INC
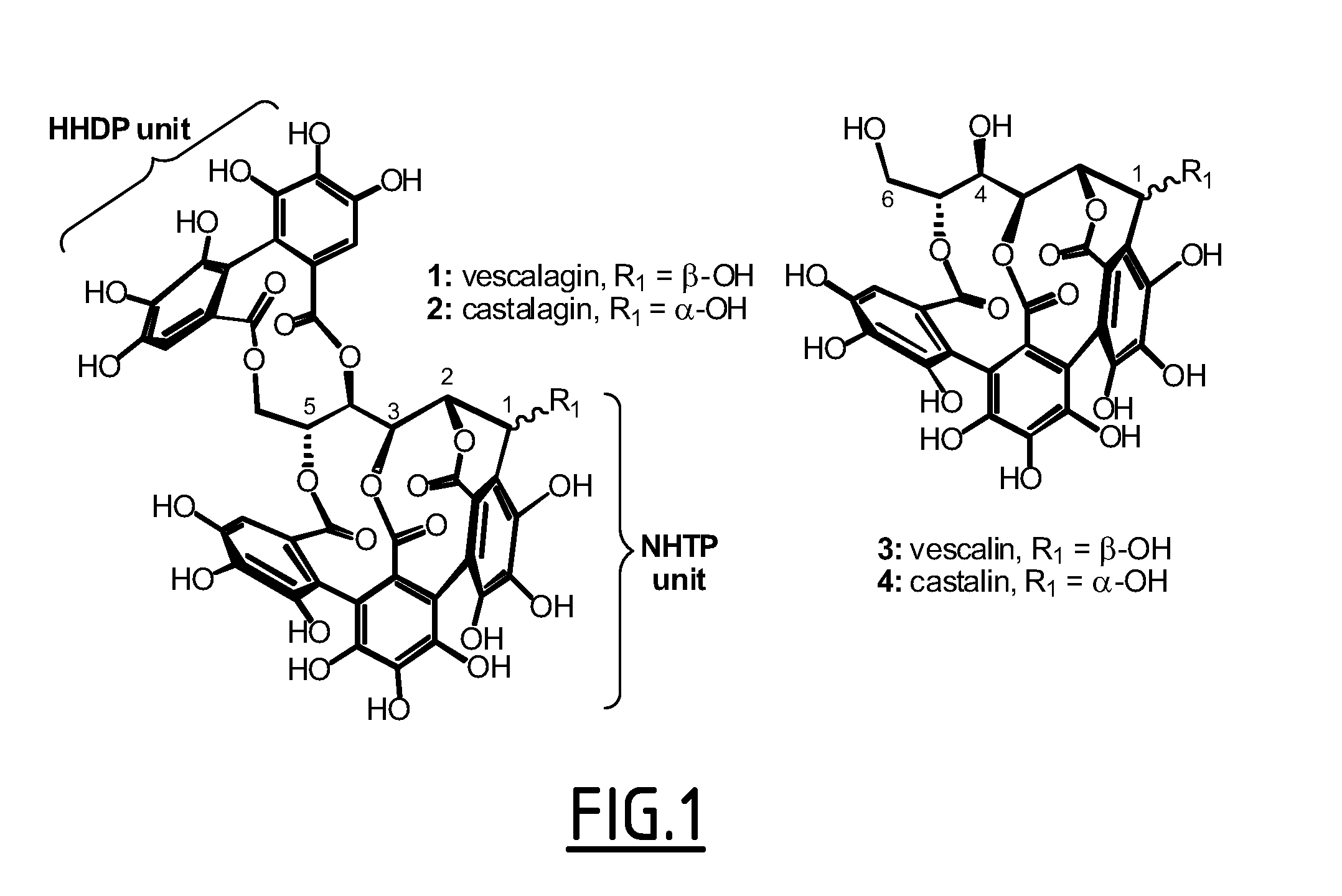
C-glucosidic ellagitannin compounds for use for altering the supramolecular arrangement of actin and for the treatment of osteoporosis, cancer, bacterial infection and viral infection
InactiveUS20130324595A1Avoid spreadingEffective absorptionBiocideOrganic active ingredientsMetaboliteCellular mechanism
The present invention concerns a C-glucosidic ellagitannin compound or a metabolite thereof for use for altering the supramolecular arrangement of actin in an individual suffering from osteoporosis, cancer, bacterial infection, or viral infection. It also pertains to pharmaceutical compositions comprising a C-glucosidic ellagitannin compound and / or metabolites thereof and one or more physiologically acceptable carriers. It finally concerns a C-glucosidic ellagitannin compound or a metabolite thereof, optionally detectably labeled, for in vitro use as a tool for studying cellular mechanisms involving actin, or for detecting F-actin in a cell.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Prophylactic treatment of migraine
ActiveUS8680081B2Reduce the risk of badOvercome problemsBiocideSalicyclic acid active ingredientsSalicylic acidCyclooxygenase
The present invention provides methods and compositions for the prophylactic or targeted prophylactic treatment of migraine. In one embodiment a patient is regularly given a therapeutically effective amount of a combination of a cyclooxygenase-2 inhibitor and acetylsalicylic acid or active derivative, for prophylactic treatment of migraine. In another embodiment, a patient, at a time prior to a determined time window, is administered a therapeutically effective amount of a cyclooxygenase-2 inhibitor, either alone or in combination with acetylsalicylic acid or active derivative, to prevent or reduce migraine symptoms during the time window. Representative compositions include a cyclooxygenase-2 inhibitor and acetylsalicylic acid or salicylate salt.
Owner:CELSPRIN
Vascular Medical Device, System And Method
ActiveUS20170340461A1Shorten operation timeImprove securityStentsBlood vesselsMedical deviceCovered stent
The present application discloses a covered stent and a method for navigating the covered stent to a branch vessel, the covered stent comprising a main body and at least one lateral side branch connected to the main body, wherein the lateral side branch is flexible and expandable. A system of covered stents and a method for interconnecting the covered stents is also disclosed.
Owner:SWISS CAPITAL ENG AG
Human tissue specific drug screening procedure
InactiveUS20040241688A1Facilitate methodReduce potential side effectsMicrobiological testing/measurementBiological testingDiseaseScreening procedures
A method of using tissue cartridges containing one or more tissues samples in configuration allowing screening of drug candidates against normal or known disease states. The inventive method generates binding information for multiple drug-human tissue sections. This binding information helps identify drug candidates having specific binding characteristics allowing for selection of potential drug candidates having specific binding characteristics allowing for selection of potential drug candidates that have the desired binding qualities. The ability to understand binding characteristics allows drug discovery methods that reduce potential side effects.
Owner:BUKUSOGLU CUNEYT
Vascular medical device, system and method
ActiveUS10821009B2Shorten operation timeImprove securityStentsBlood vesselsCovered stentMedical device
The present application discloses a covered stent and a method for navigating the covered stent to a branch vessel, the covered stent comprising a main body and at least one lateral side branch connected to the main body, wherein the lateral side branch is flexible and expandable. A system of covered stents and a method for interconnecting the covered stents is also disclosed.
Owner:SWISS CAPITAL ENG AG
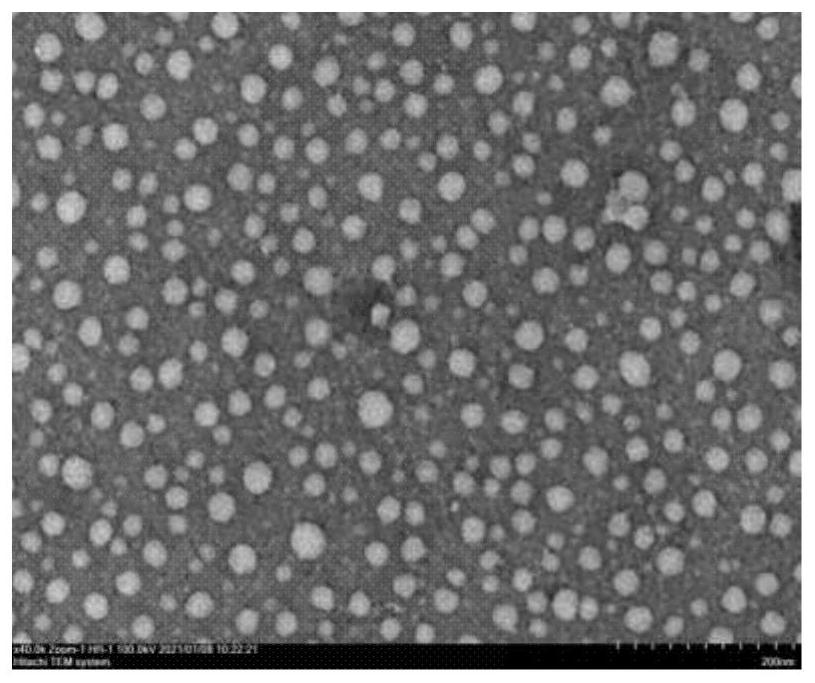
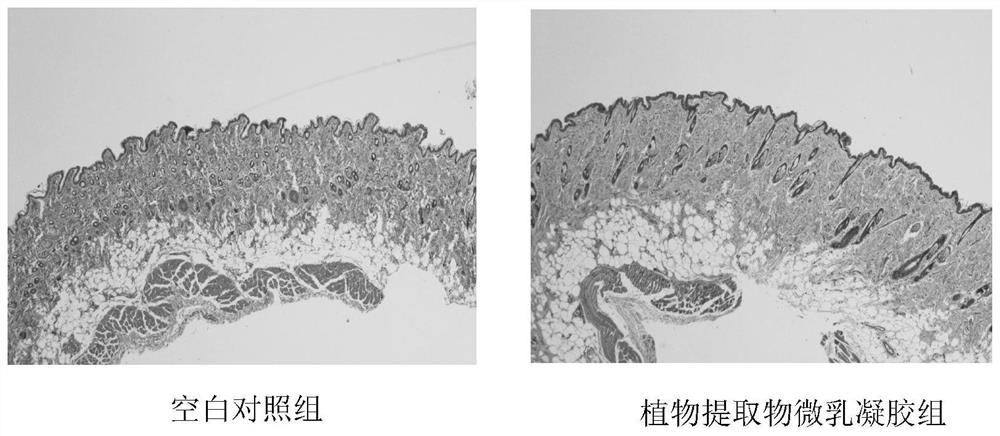
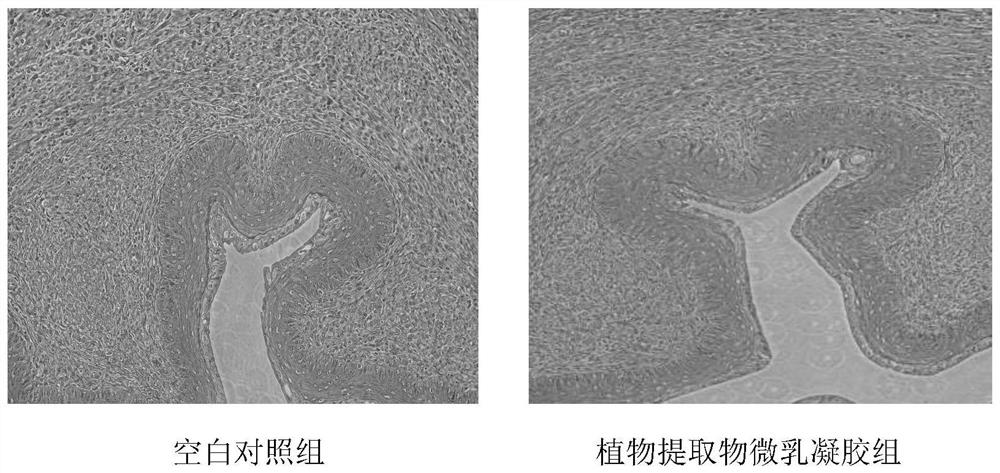
Microemulsion gel of anti-human papillomavirus(HPV) plant extract and preparation method and application of microemulsion gel
ActiveCN113244357ASolve the problem of poor infection prevention and control effectIncrease intakeAntibacterial agentsAntimycoticsBiotechnologyHuman papillomavirus
The invention discloses microemulsion gel of an anti-human papillomavirus(HPV) plant extract and a preparation method and application of the microemulsion gel. The microemulsion gel comprises a plant extract and auxiliary materials, and the plant extract includes one or more of a rhizoma curcuma extract, a folium eucalypti extract, a radix sophorae flavescentis extract, a fructus cnidii extract, a radix stemonae extract and a cortex phellodendri extract. According to the invention, the natural plant extract not only can serve as a component of a carrier but also can serve as an active ingredient to enhance the medicine efficacy; by taking carbomer as a matrix, a local topical preparation with antibacterial, anti-inflammatory and antiviral functions is prepared; and the microemulsion gel used for HPV infection and prevention, bacterial and fungal vaginitis, cervical erosion, and daily care of women's private parts especially can reduce mRNA expression levels of key oncogenes E6 and E7 in Hela cells. The microemulsion gel prepared by the invention has high safety, high stability, convenience in administration and high patient compliance; and the processing technology is simple, cost is low and large-scale production is facilitated.
Owner:CHINA PHARM UNIV
Methods and compositions for treating an alphavirus infection
ActiveUS20180134778A1Shorten the timeReduce morbiditySsRNA viruses positive-senseViral antigen ingredientsViral infectionTransforming growth factor beta
Methods and pharmaceutical compositions for preventing, treating or suppressing symptoms of a disorder associated with an alphavirus infection. In particular, the present invention relates to preventing, treating or suppressing symptoms of a disorder associated with an alphavirus infection through inhibiting the activity and / or expression of Transforming Growth Factor Beta (TGF-β) in a subject suffering from or at risk for suffering from an alphavirus infection.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
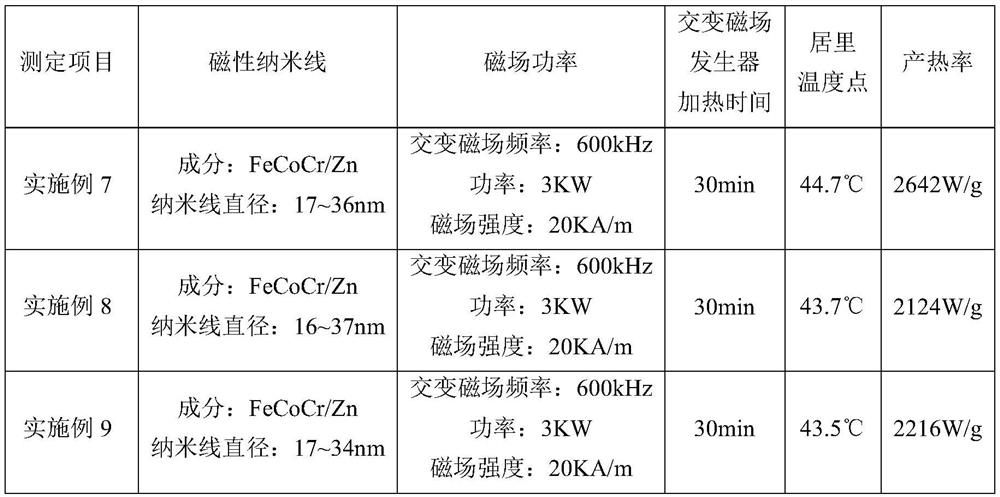
Self-temperature-control magnetic nanowire and preparation method thereof
ActiveCN112499691AIncrease heat production rateSmall doseEnergy modified materialsCobalt compoundsAluminum anodeMagnetite Nanoparticles
The invention relates to a self-temperature-control magnetic nanowire and s preparation method thereof, and belongs to the technical field of tumor magnetic induction thermotherapy. In order to solvethe problems of low heat production rate and agglomeration of magnetic nanoparticles, the invention provides a preparation method of a self-temperature-control magnetic nanowire. The preparation method comprises the following steps: anodizing an aluminum foil to prepare an aluminum anodized micro-channel template, depositing zinc-cobalt-chromium ferrite hydroxide in the micro-channel template by using a chemical coprecipitation method, performing sintering to form a zinc-cobalt-chromium ferrite magnetic nanowire, and carrying out surface hydrophobization treatment on the magnetic nanowire. Theself-temperature-control magnetic nanowire prepared by the invention has the diameter of 5-45nm, the length of 5-30mu m and the Curie temperature point of 42-45 DEG C, is stable in chemical propertyand excellent in magnetic property, has the Curie point temperature meeting the magnetic hyperthermia requirement, has the advantages of high heat yield, low possibility of agglomeration, good performance consistency and good biocompatibility, and can be used for batch production of magnetic nano media and magnetic hot seeds for magnetic hyperthermia.
Owner:重庆海士智能科技研究院有限公司 +1
Boron-based prodrug strategy for increased bioavailability and lower-dosage requirements for drug molecules containing at least one phenol (or aromatic hydroxyl) group
ActiveUS10112962B2High retention rateElevated plasma concentrationAntibacterial agentsAntimycoticsTherapeutic effectPhenol
Boron-based prodrugs of phenol- or aromatic hydroxyl group-containing therapeutic molecules (“original drugs”), uses thereof, and methods of making the same, are provided for achieving, for example, improved bioavailability, prolonged retention (e.g., in a circulatory system) and, in particular, significantly lowered therapeutically effective dosage in order to reduce adverse effects while maintaining the desired therapeutic effects of the original drugs.
Owner:XAVIER UNIVERSITY
Method of adding botanical agents/dietary supplements to pharmaceutical agents in a pharmacotherapeutic regimen
InactiveUS20140178311A1Improve actionDeleterious effectBiocideOrganic active ingredientsRegimenDietary supplement
A method for administering an active pharmaceutical agent (APA) is provided that includes the steps of: a) providing a medicine having a dose of at least one APA and at least one active botanical agent (ABA) or dietary supplement; and b) dosing the APA within the medicine such that within each subsequent dose of the medicine, the amount of the APA decreases and the amount of the ABA increases, or remains the same, or decreases relative to an earlier amount. A trace of the APA may or may not remain in the pill toward the end of medicine application.
Owner:LEVINE JOSHUA D +1
Treatment of erectile dysfunction and other indications
ActiveUS20210100807A1Improve efficiencyFast actionAerosol deliveryInorganic non-active ingredientsSexual impotenceActive agent
The present invention generally relates to the transdermal delivery of various compounds. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising a phosphodiesterase type 5 inhibitor and / or a salt thereof, and optionally, a hostile biophysical environment and / or a nitric oxide donor. In some cases, the composition may be stabilized using a combination of a stabilization polymer (such as xanthan gum, KELTROL® BT and / or KELTROL® RD), propylene glycol, and a polysorbate surfactant such as Polysorbate 20, which combination unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Compositions, methods and uses for the treatment of diabetes and related conditions by controlling blood glucose level
ActiveUS10617689B2Reduce insulin resistanceEfficient clinical benefitAmide active ingredientsEster active ingredientsDiseasePharmacology
The present invention relates to compositions and methods for controlling glycaemia in a mammalian in need thereof. The present invention relates to compositions and methods for the treatment of diabetes disease and related disorders. More specifically, the present invention relates to novel therapies or combinatorial therapies of diabetes and related disorders, based on compositions controlling the blood glucose level.
Owner:PHARNEXT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com