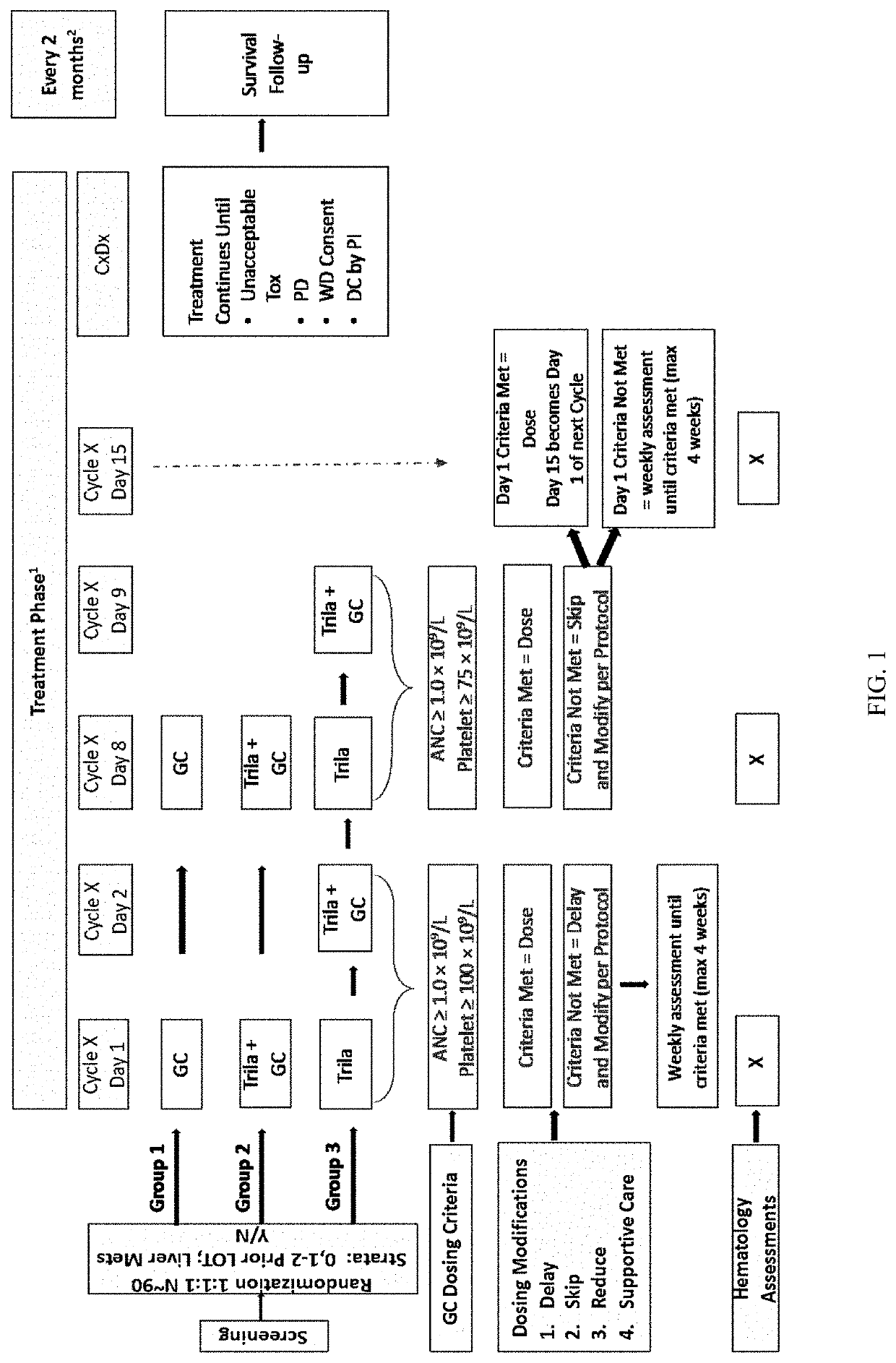
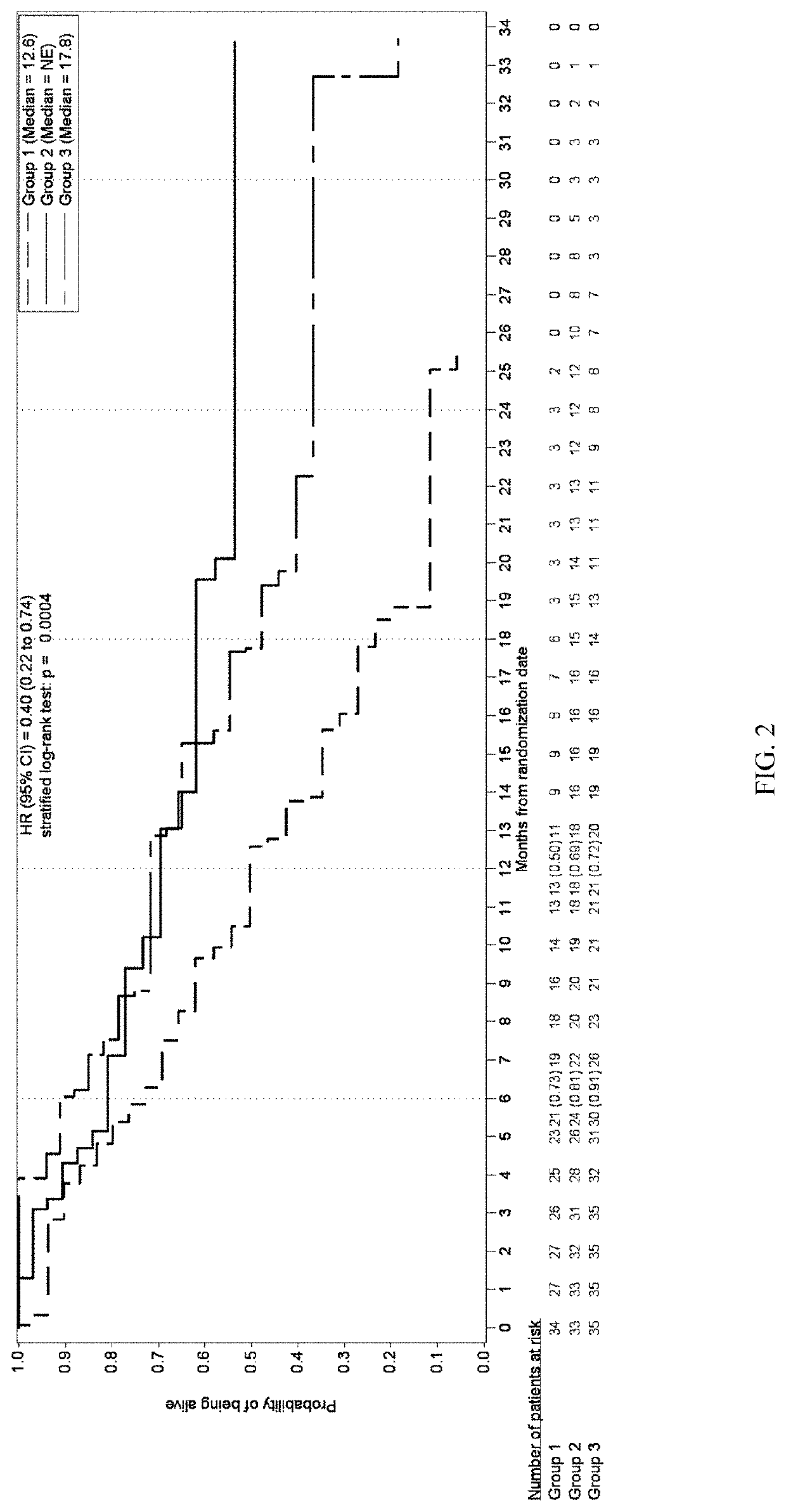
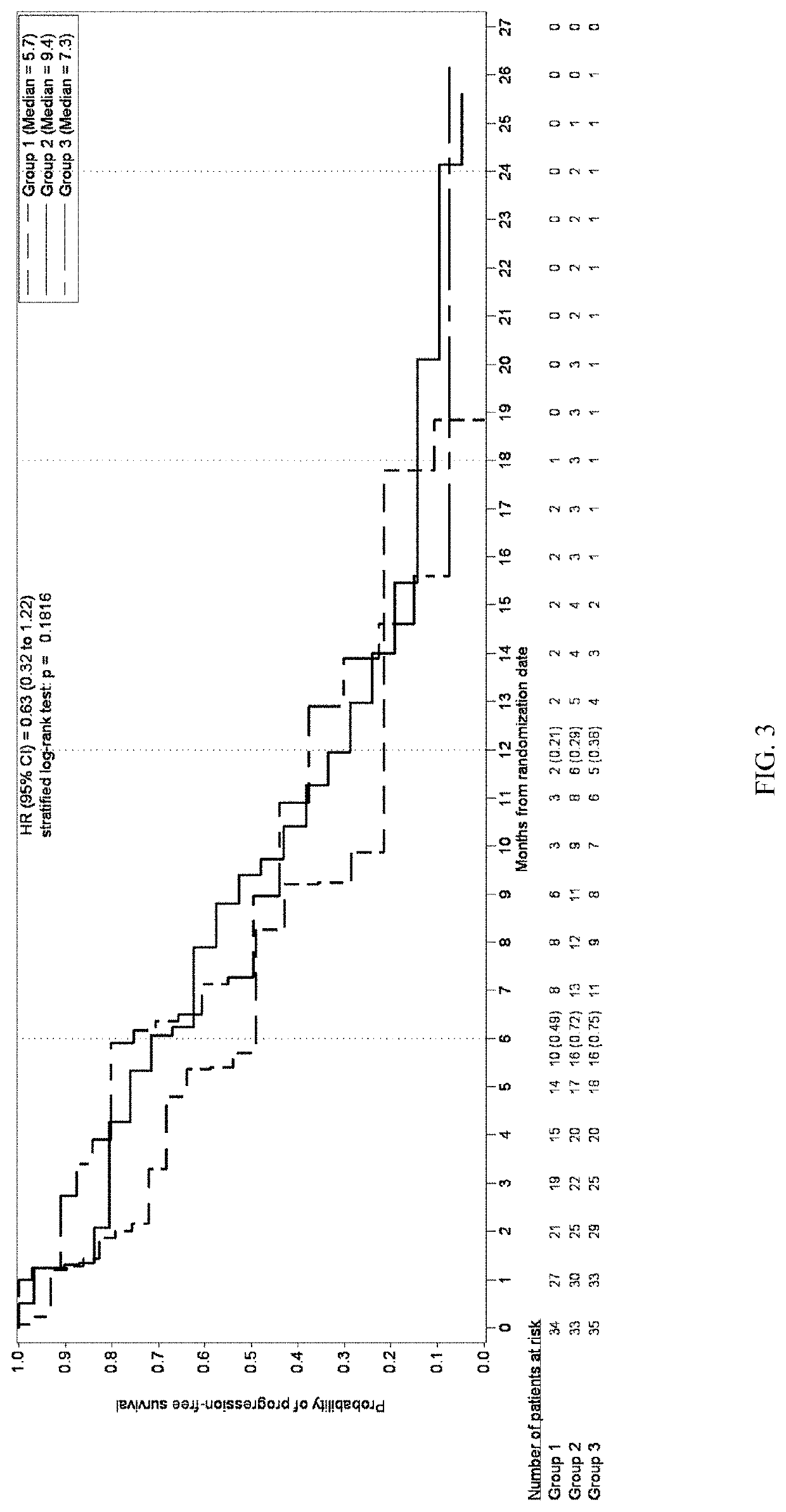
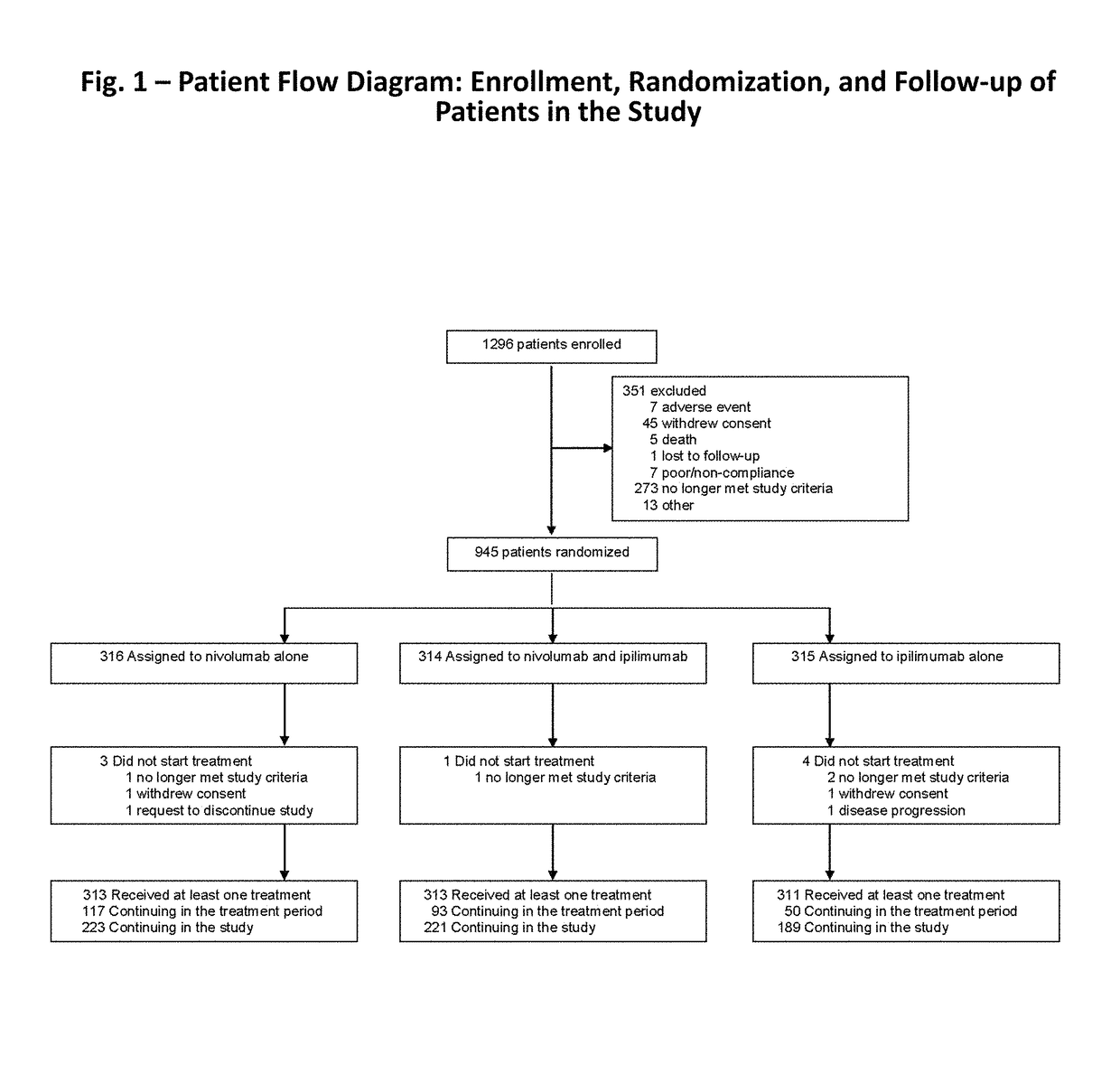
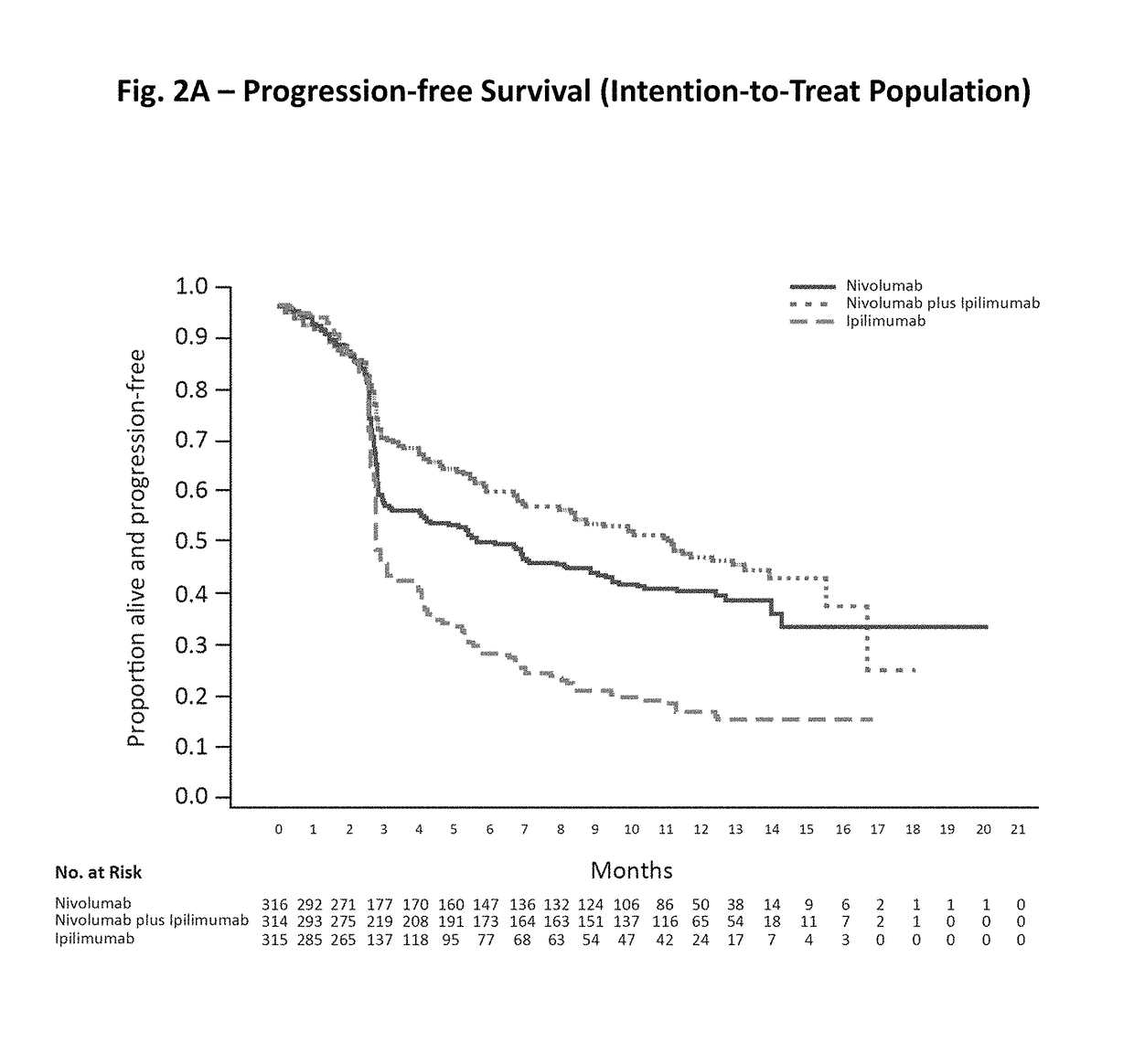
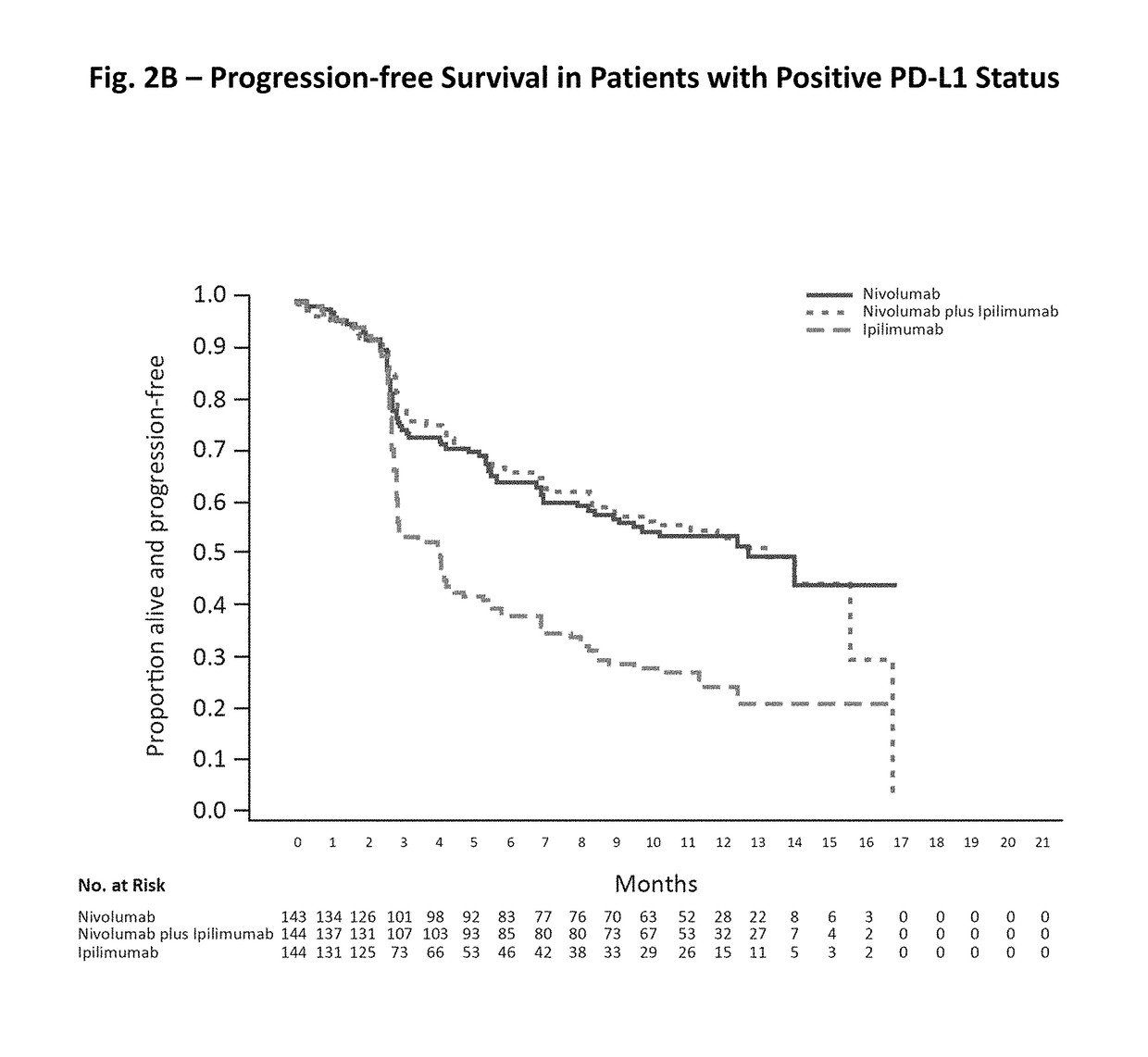
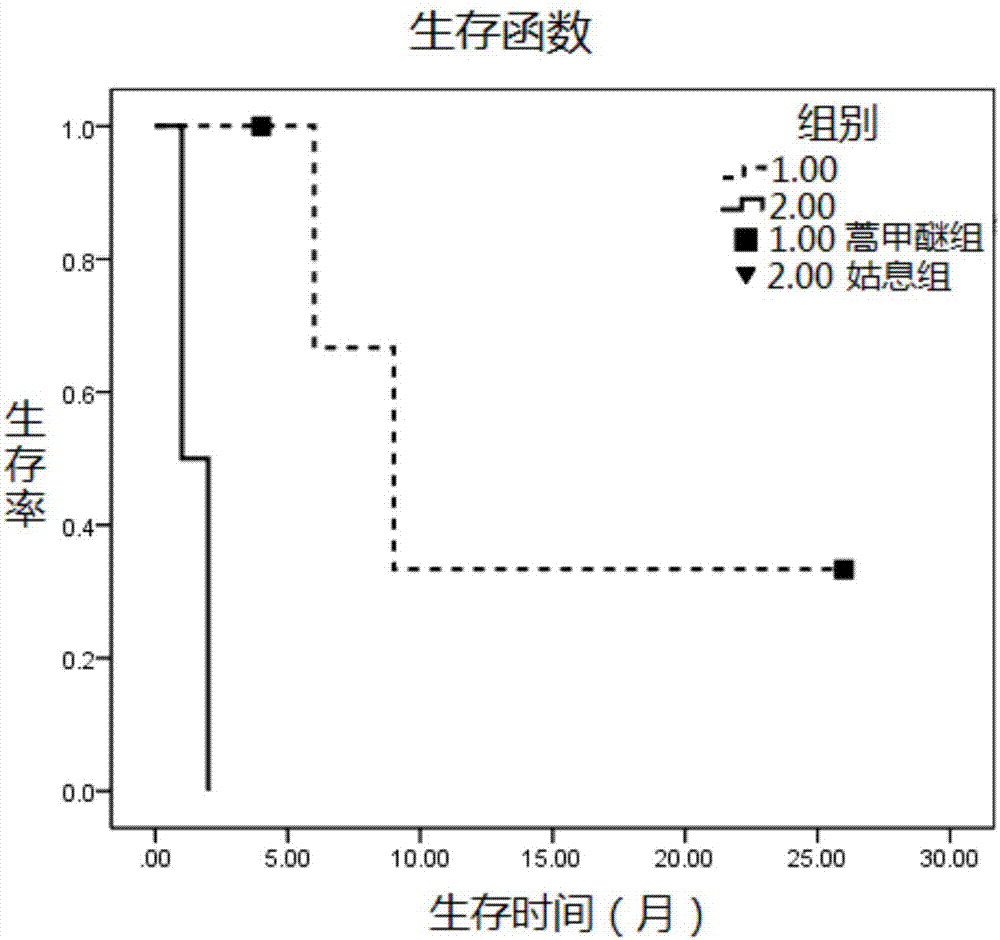
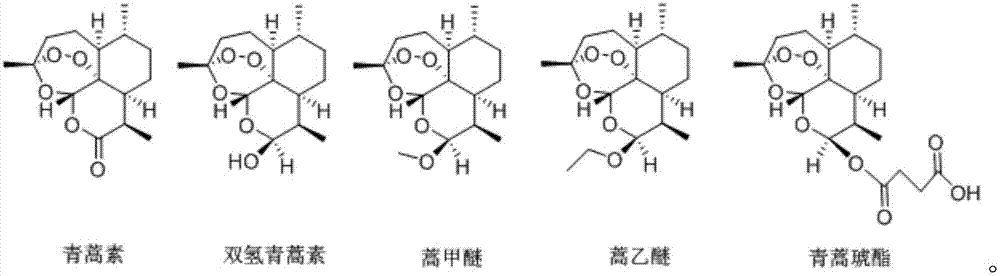
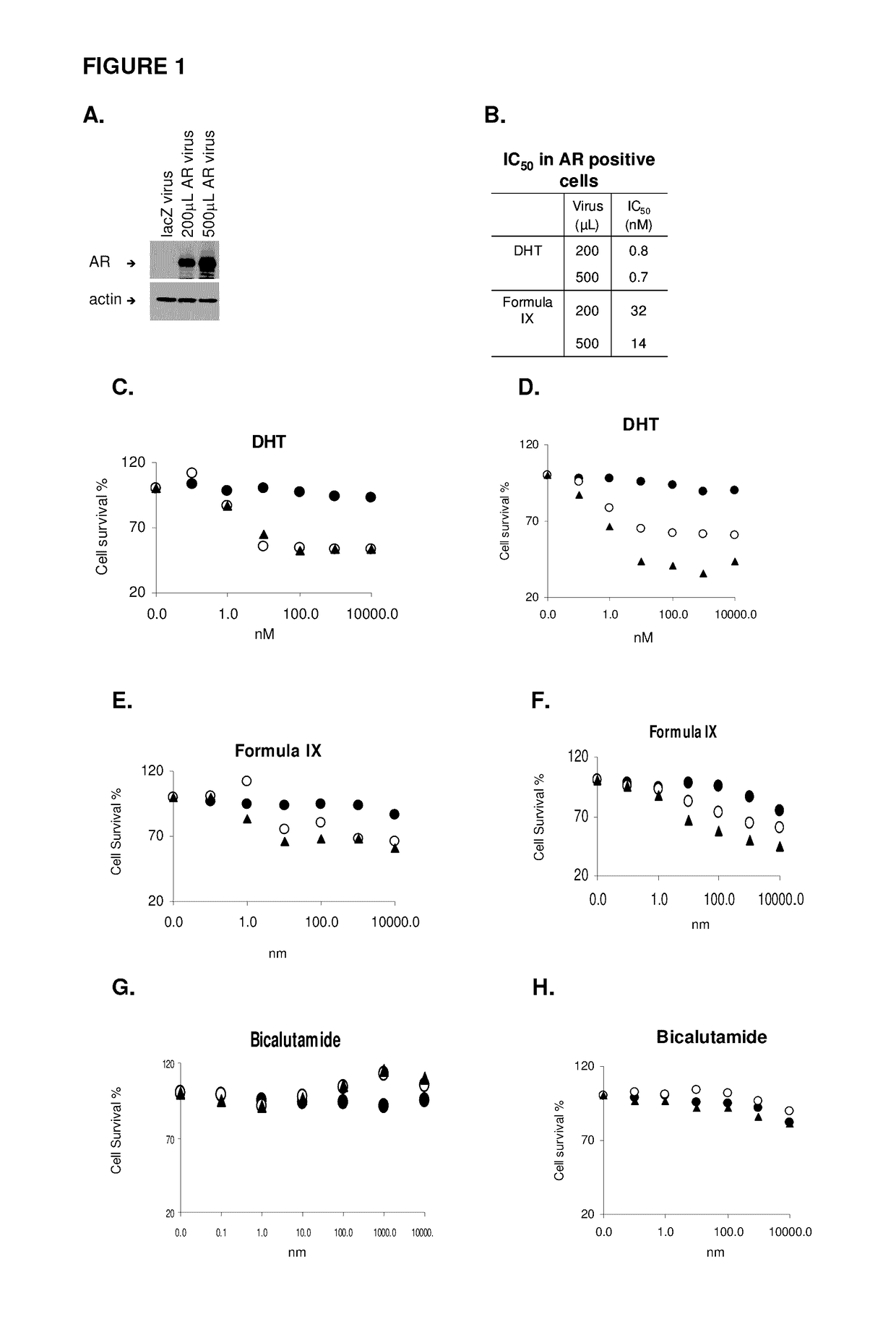
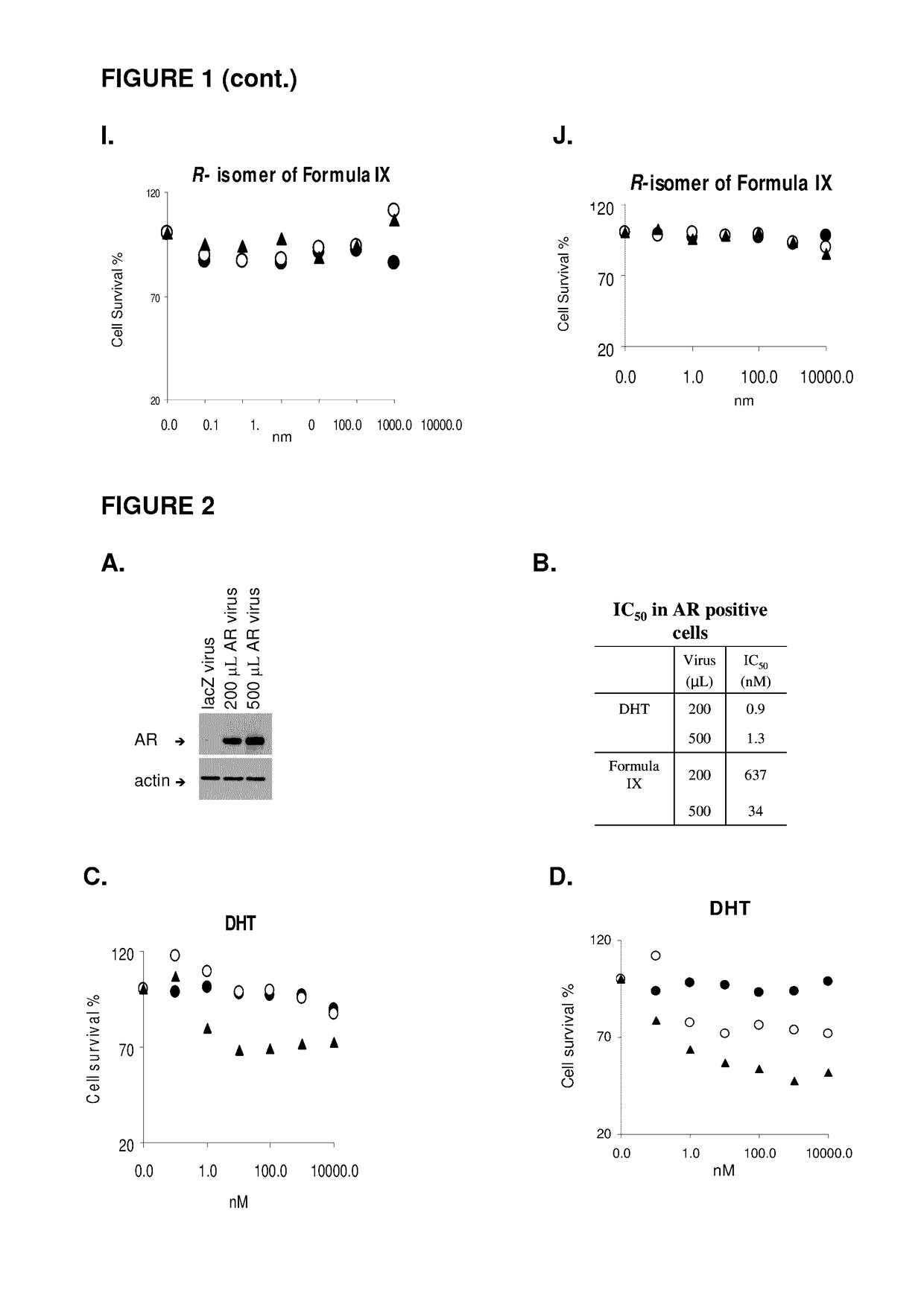
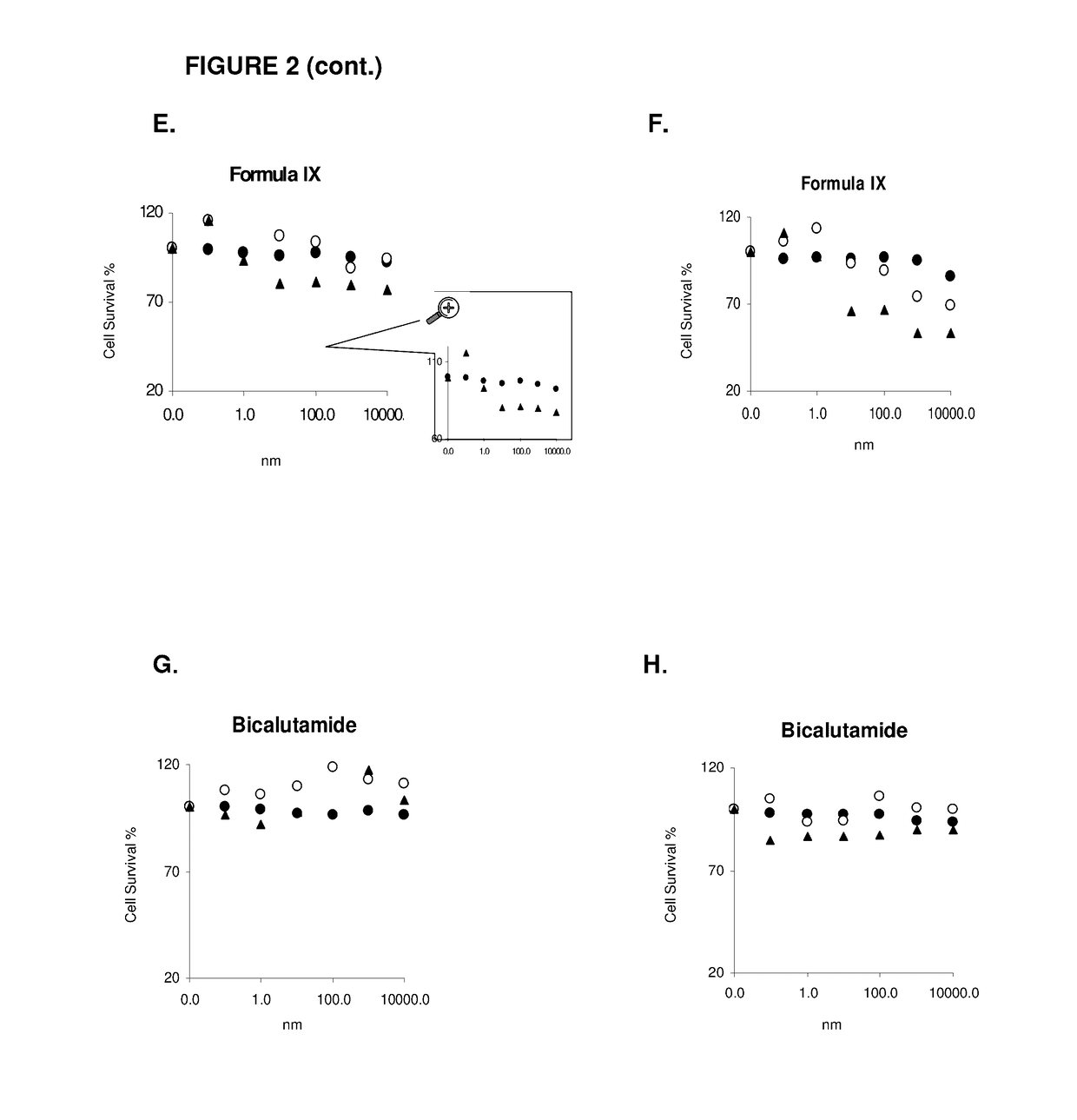
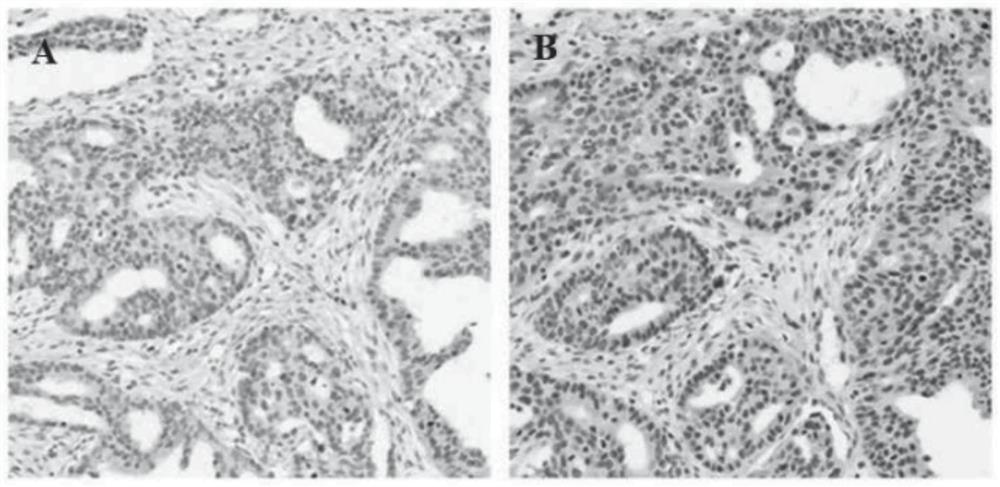
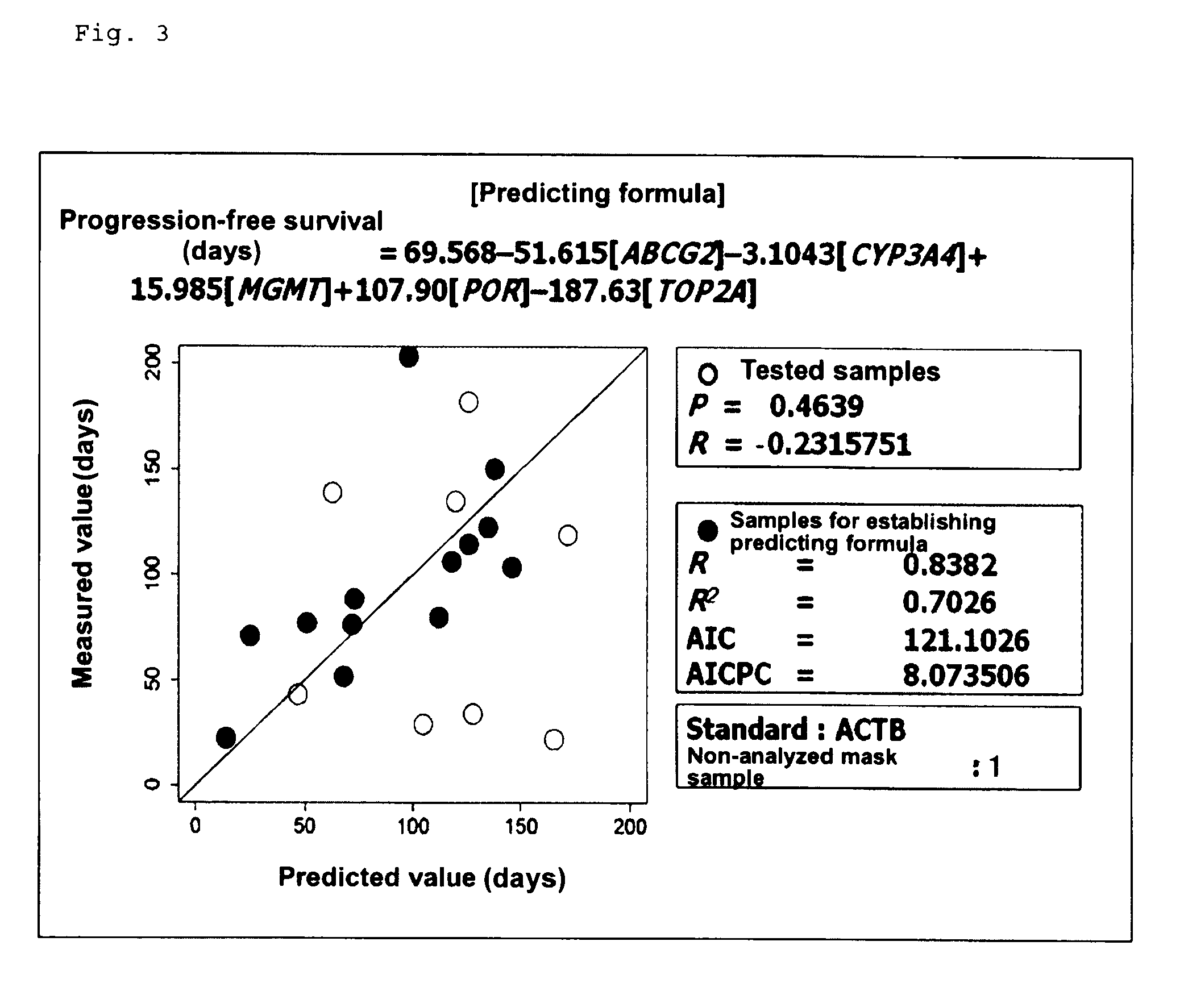
Patents
Literature
79 results about "Progression-free survival" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
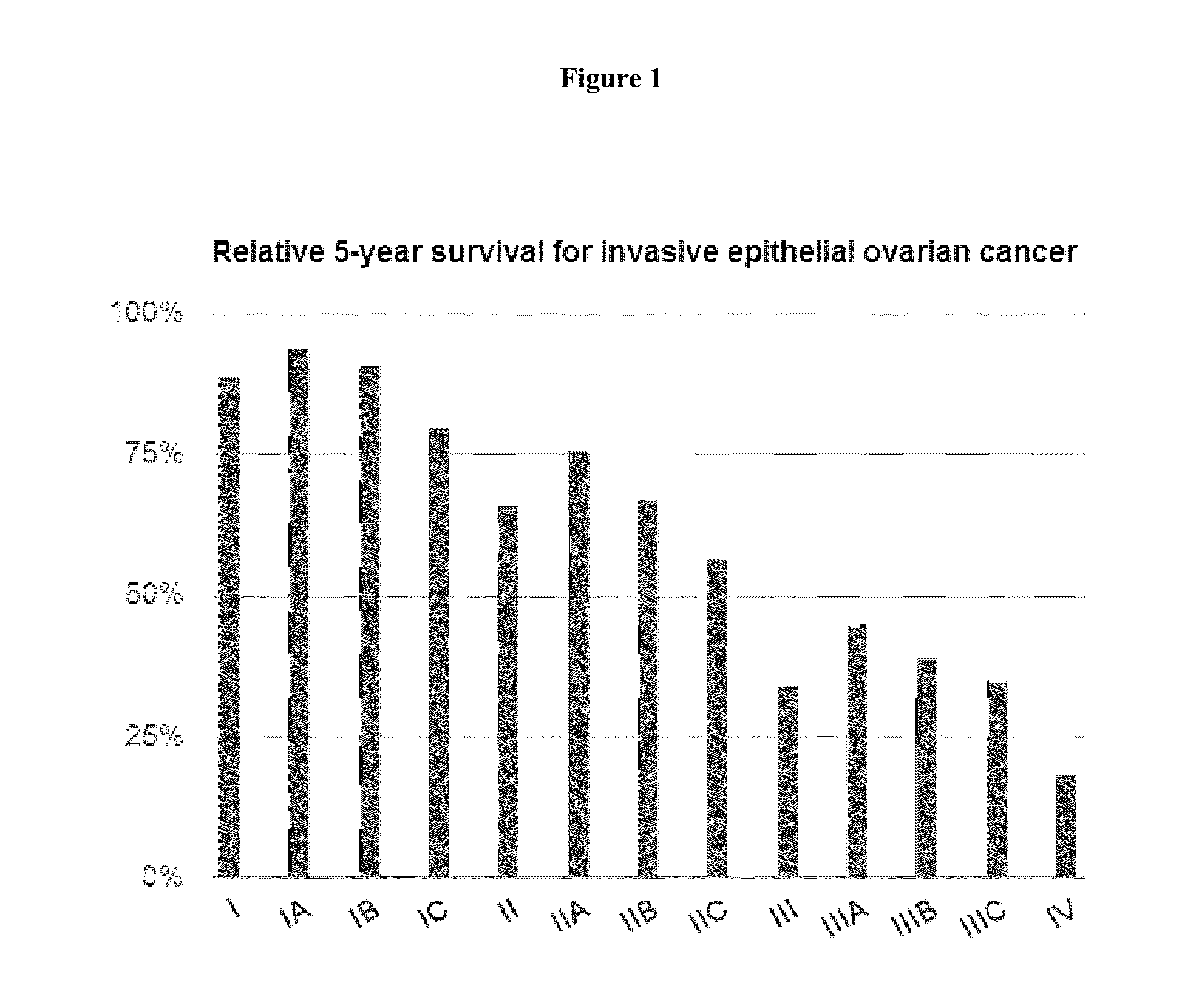
Progression-free survival (PFS) is "the length of time during and after the treatment of a disease, such as cancer, that a patient lives with the disease but it does not get worse". In oncology, PFS usually refers to situations in which a tumor is present, as demonstrated by laboratory testing, radiologic testing, or clinically. Similarly, "disease-free survival" is when patients have had operations and are left with no detectable disease.
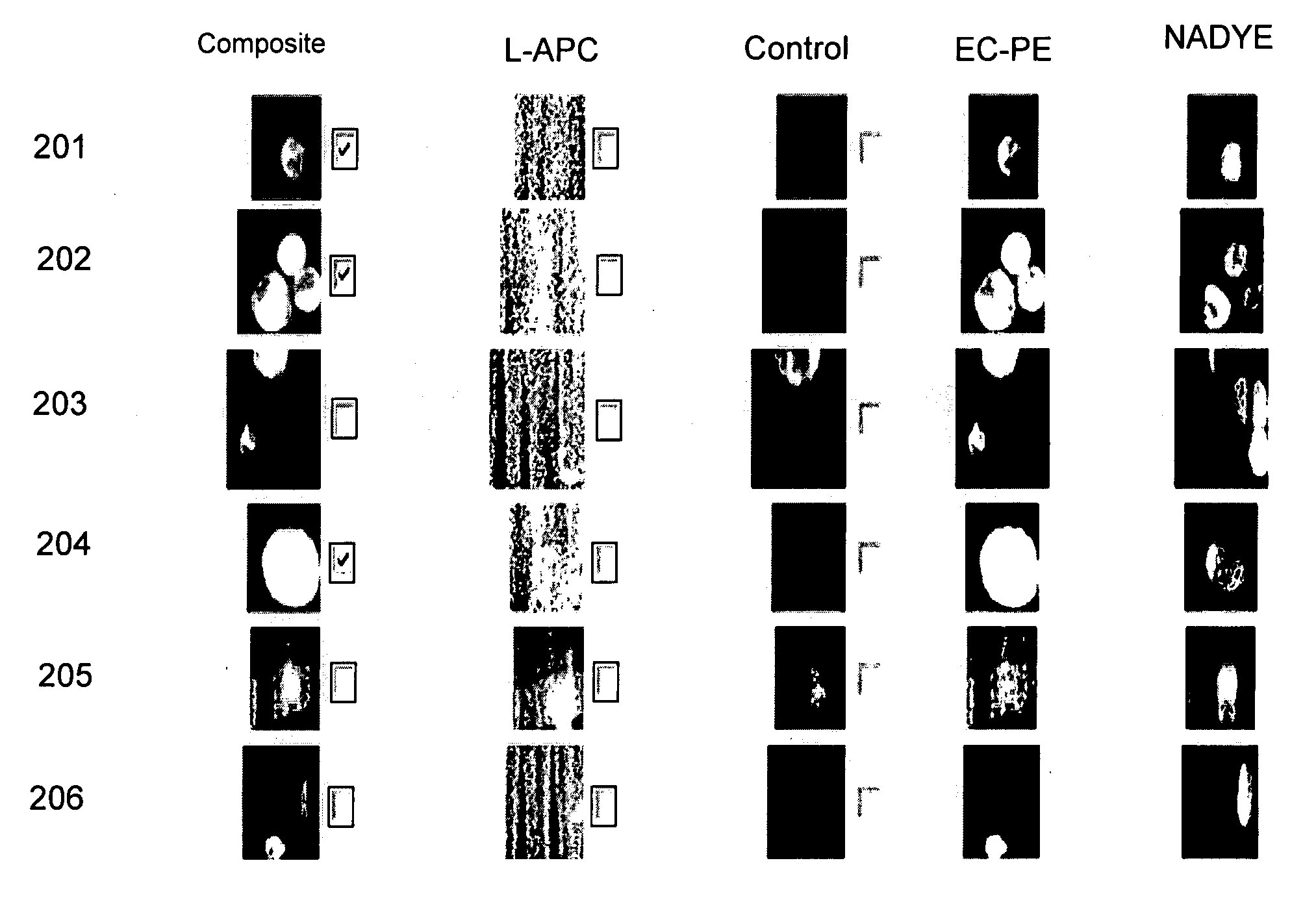
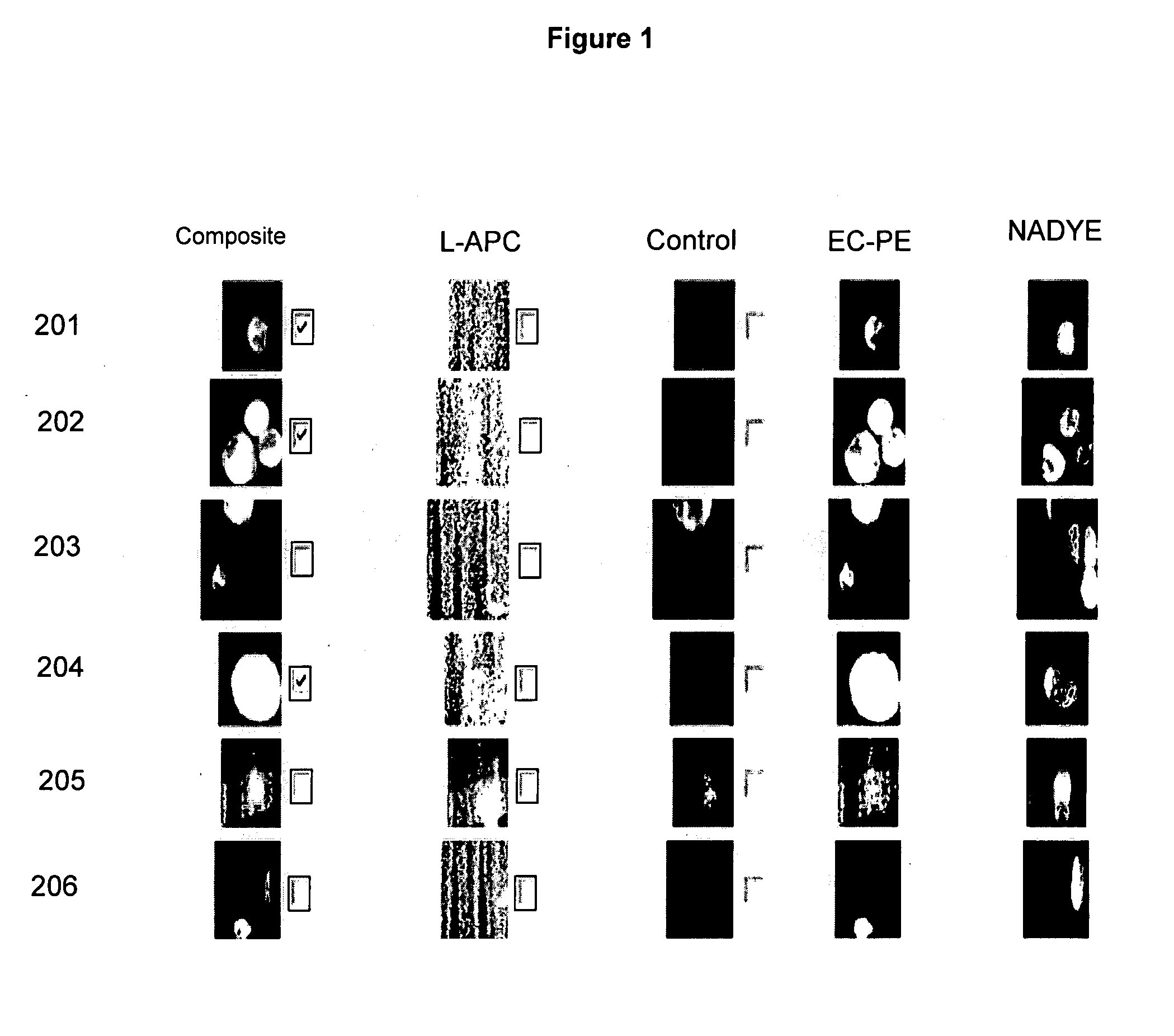
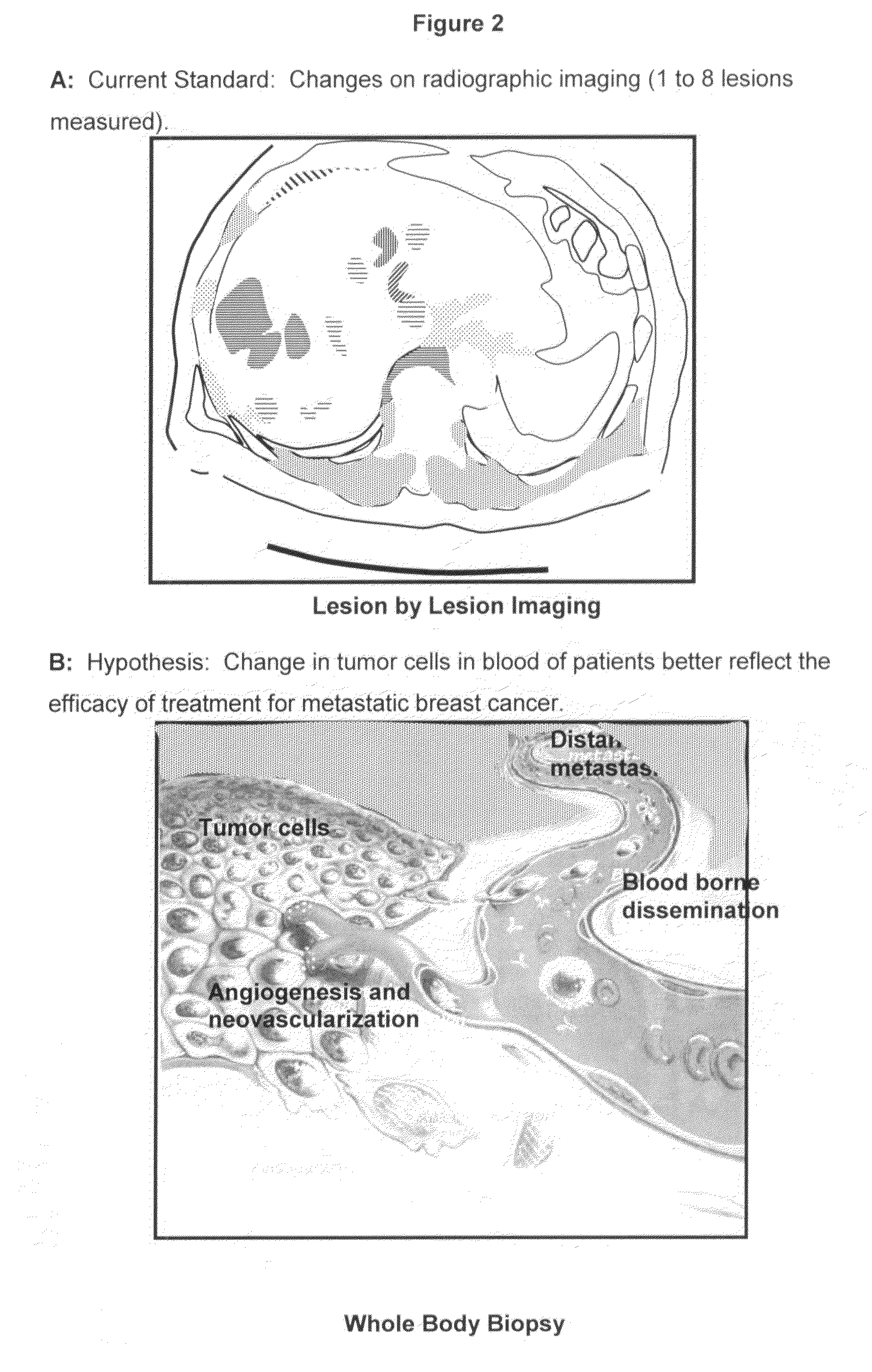
Method for predicting progression free and overall survival at each follow-up time point during therapy of metastatic breast cancer patients using circulating tumor cells
InactiveUS20090061456A1Less side effectsImprove the quality of lifeDisease diagnosisBiological testingOncologyDisease progression
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:VERIDEX LCC
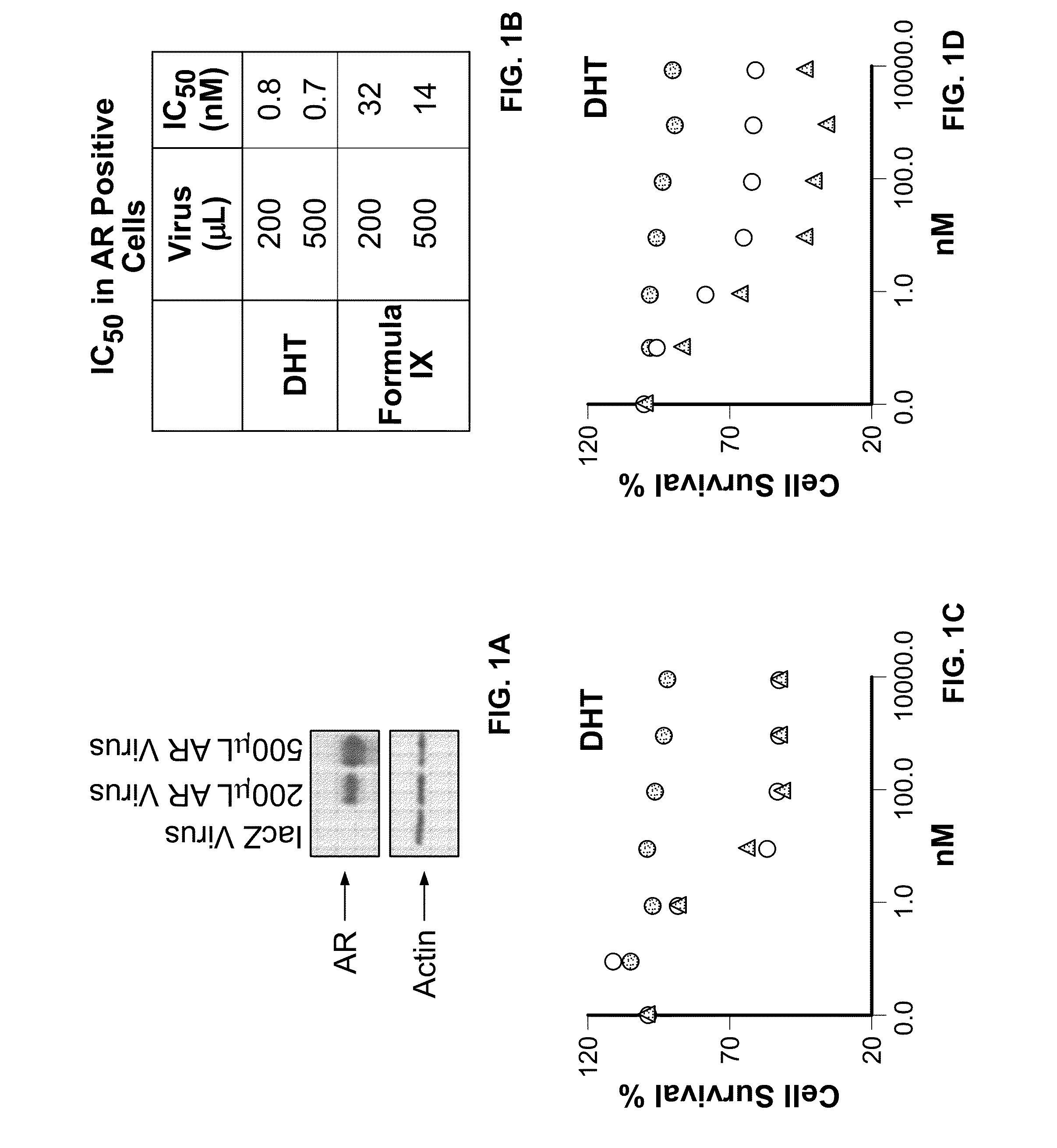
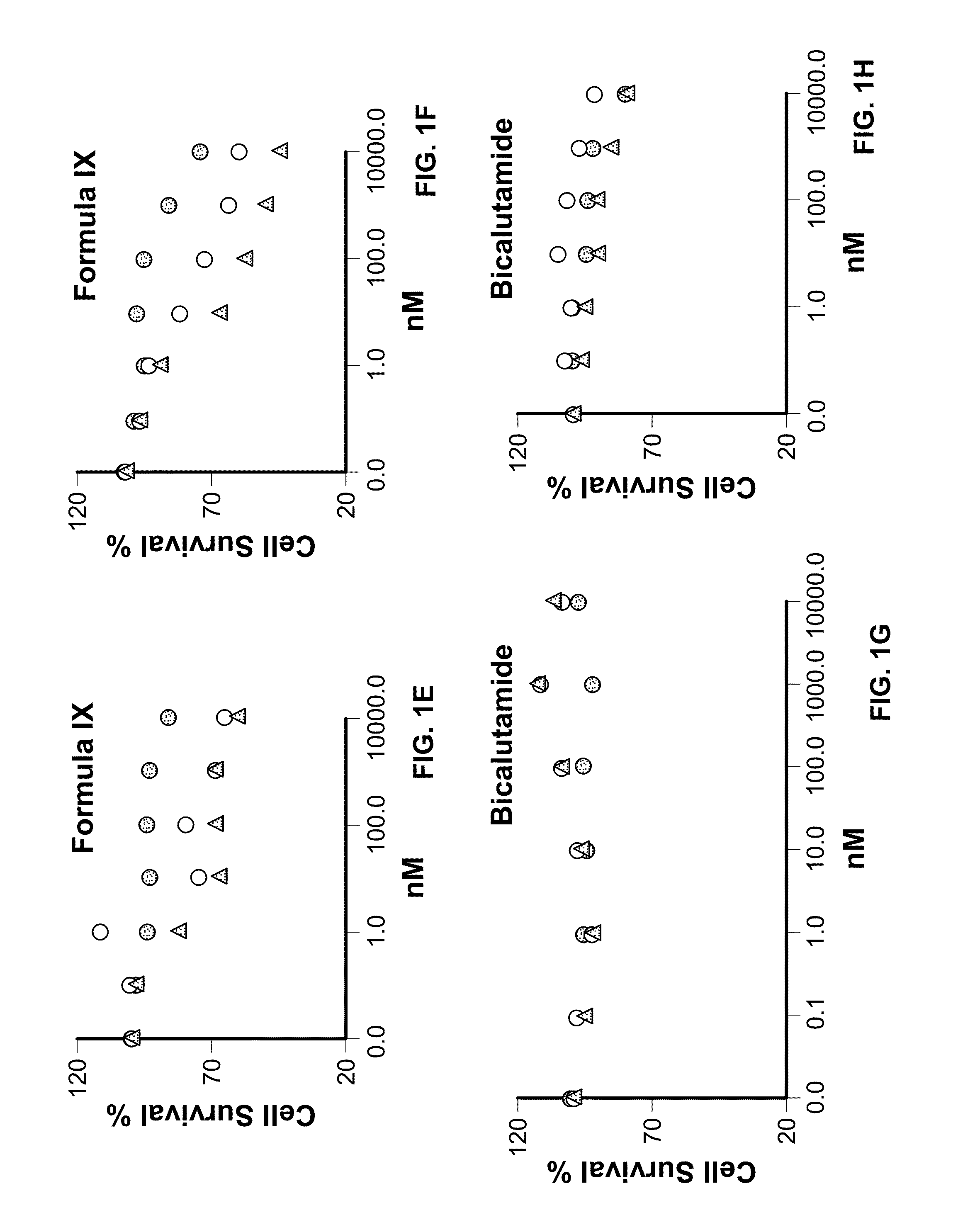
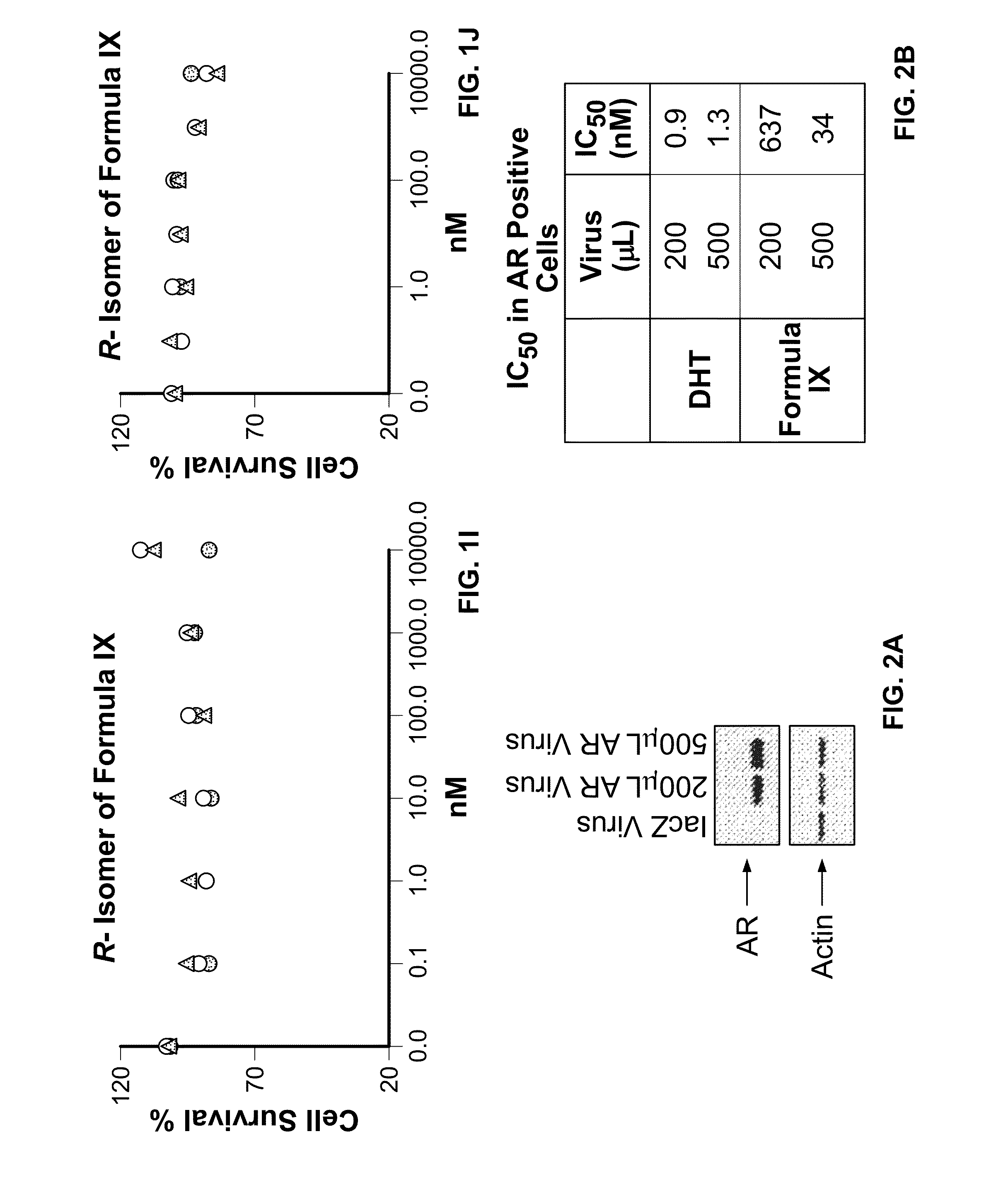
Method of treating estrogen receptor (ER) -positive breast cancers with selective androgen receptor modulator (SARMS)
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; l) prolonging survival of a subject with breast cancer, and / or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Owner:UNIV OF TENNESSEE RES FOUND
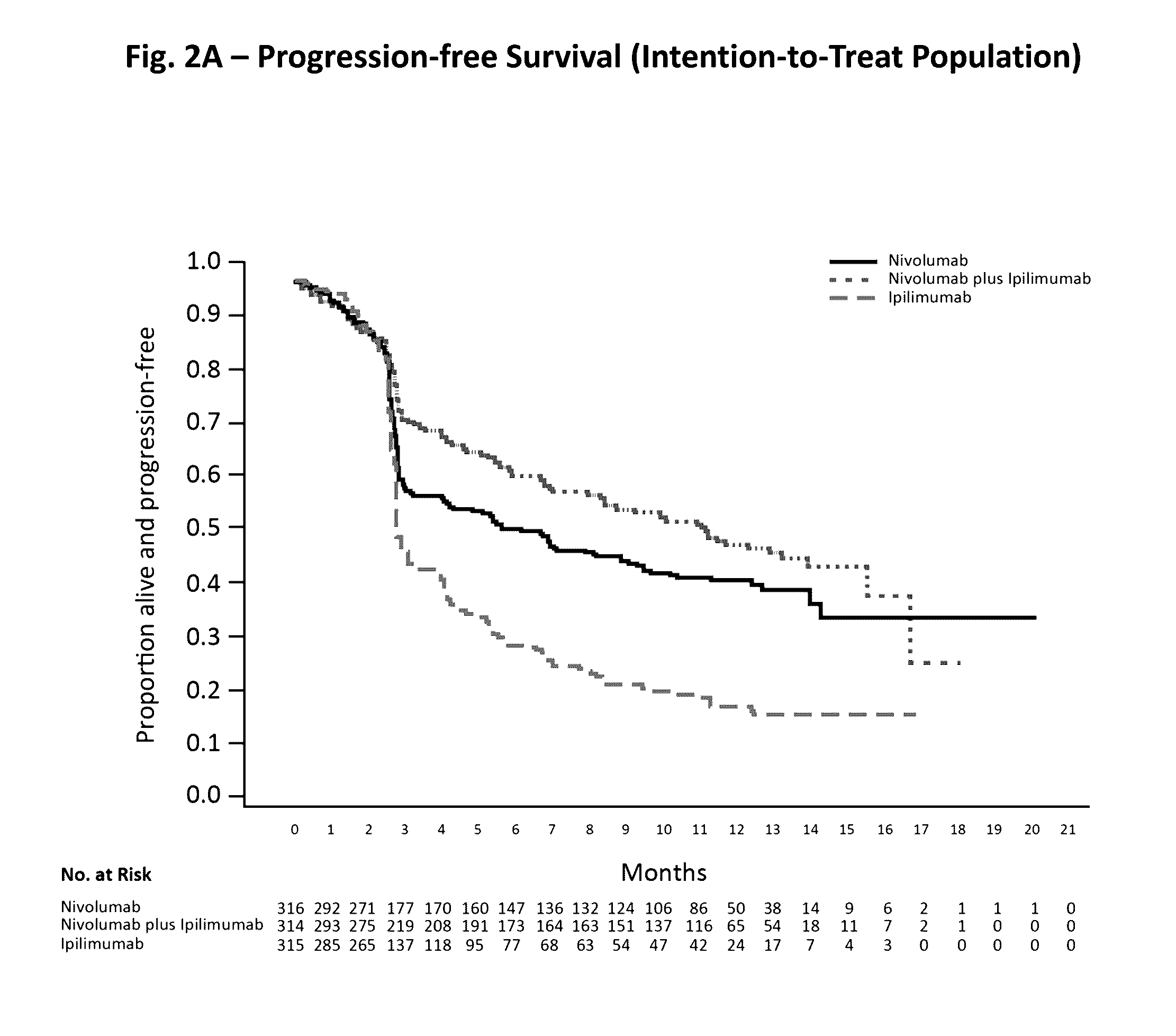
Treatment of PD-L1-Positive Melanoma Using an Anti-PD-1 Antibody
InactiveUS20160362489A1Shrink tumorBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsProgression-free survivalMelanoma
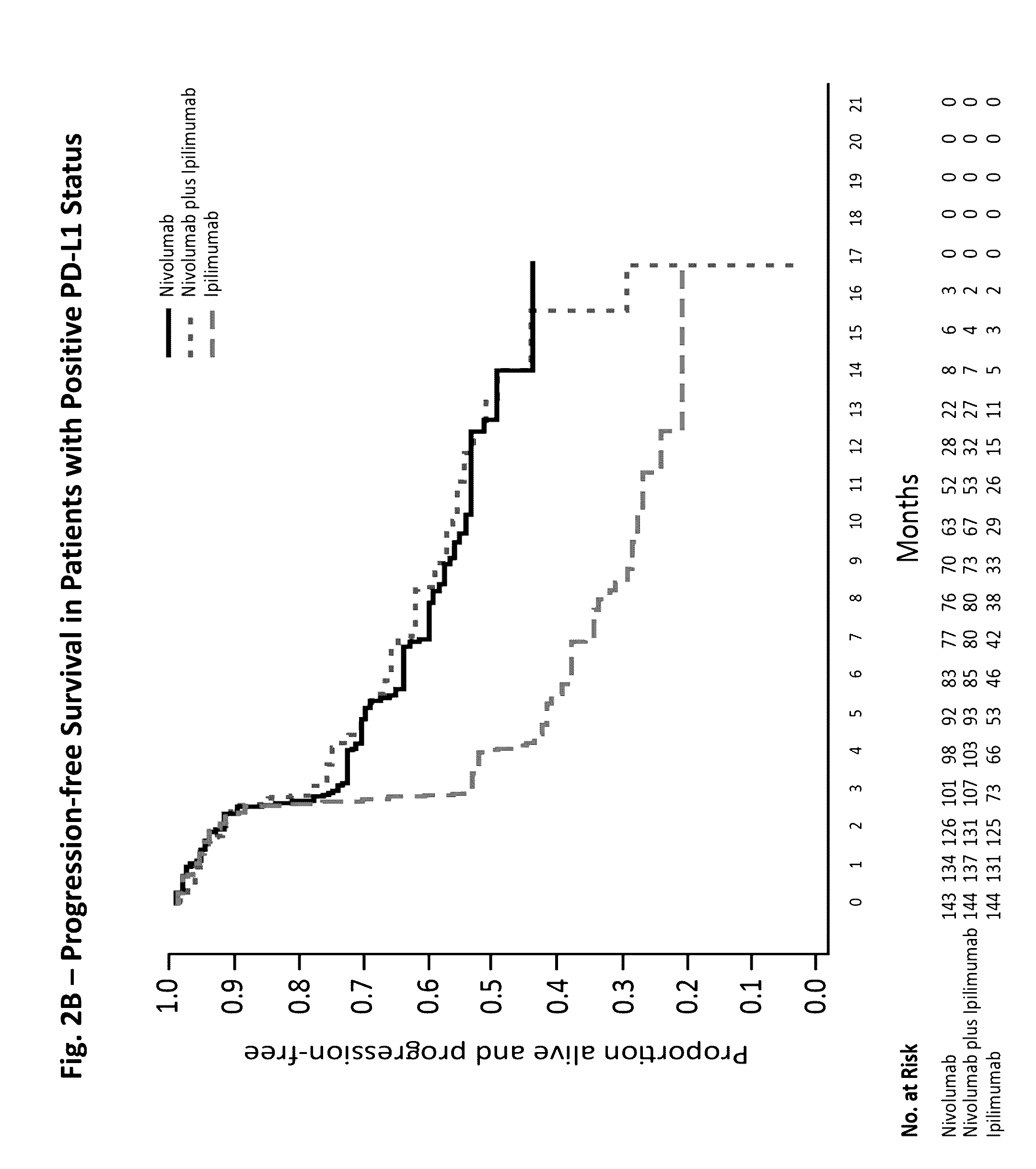
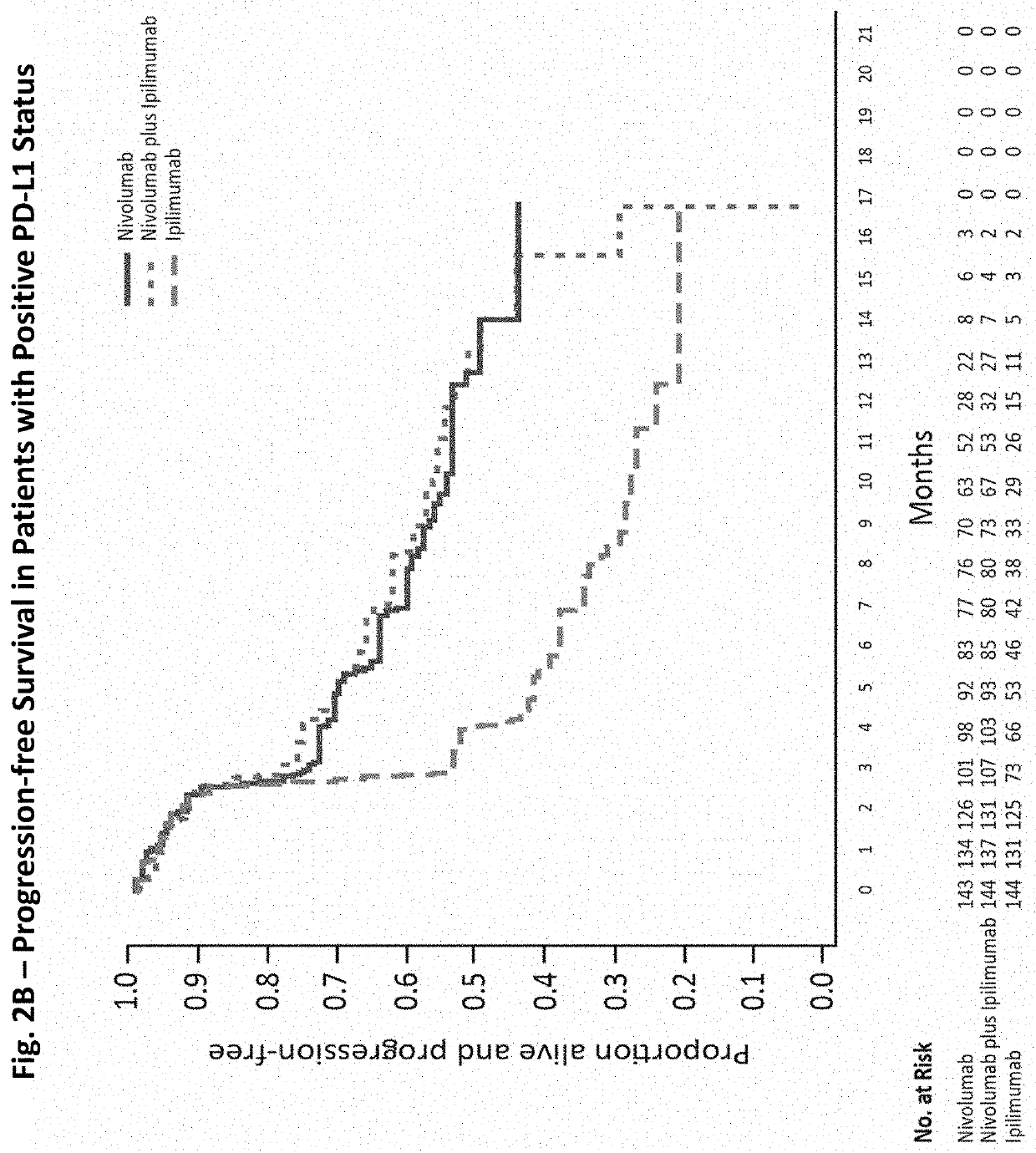
The invention provides a method of treating a melanoma comprising (i) identifying a patient having a PD-L1 positive melanoma and (ii) administering to the patient an anti-PD-1 antibody or an antigen-binding portion thereof (“an anti-PD-1 antibody monotherapy”). The methods of the invention can extend progression-free survival for over 12 months and / or reduces the tumor size at least about 10%, about 20%, about 30%, about 40%, or about 50% compared to the tumor size prior to the administration.
Owner:BRISTOL MYERS SQUIBB CO
Biological markers predictive of Anti-cancer response to epidermal growth factor receptor kinase inhibitors
InactiveUS20120142028A1Long survival progression free survivalEffectiveness of treatmentDisease diagnosisAntineoplastic agentsEpidermal Growth Factor Receptor KinaseErlotinib
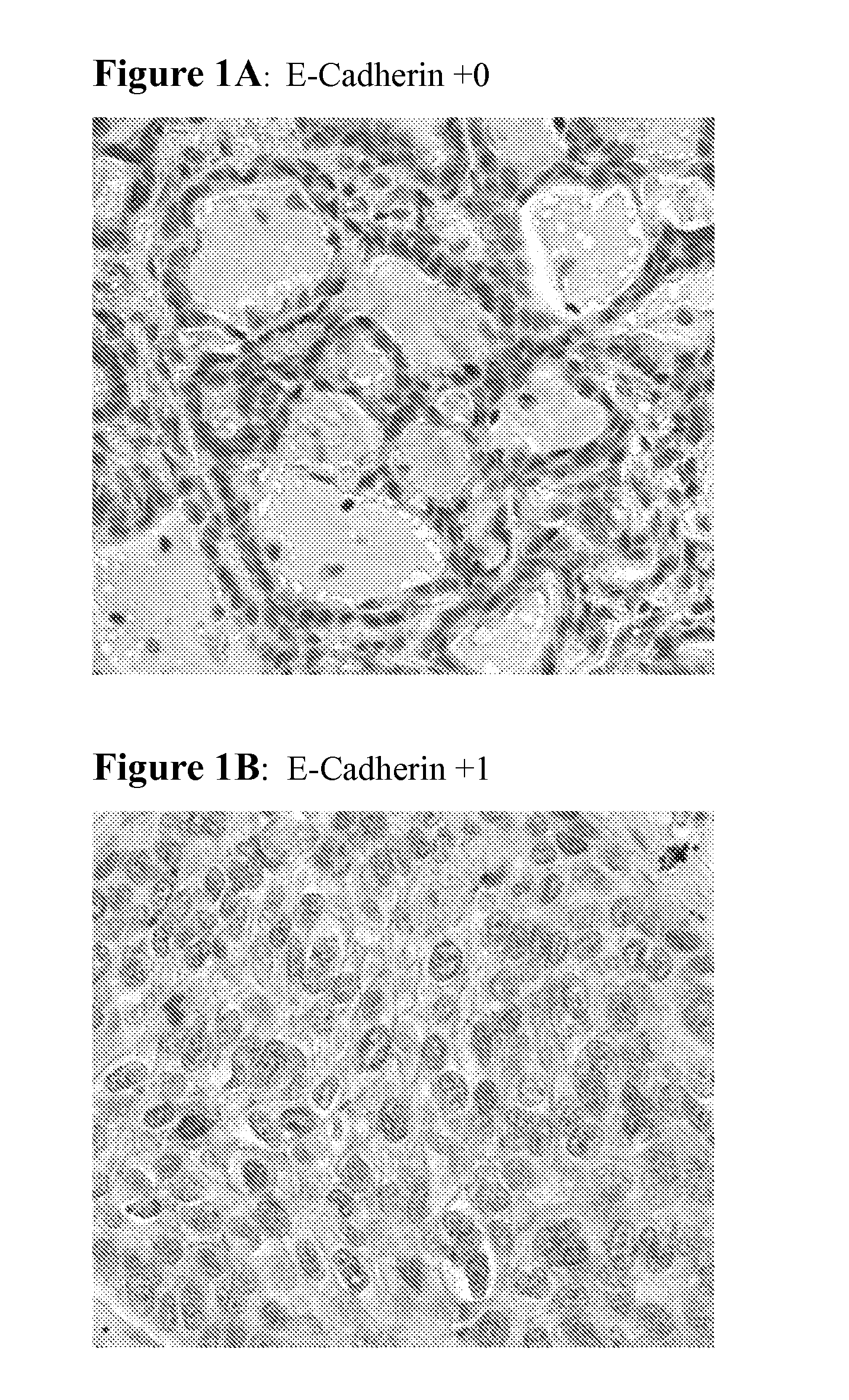
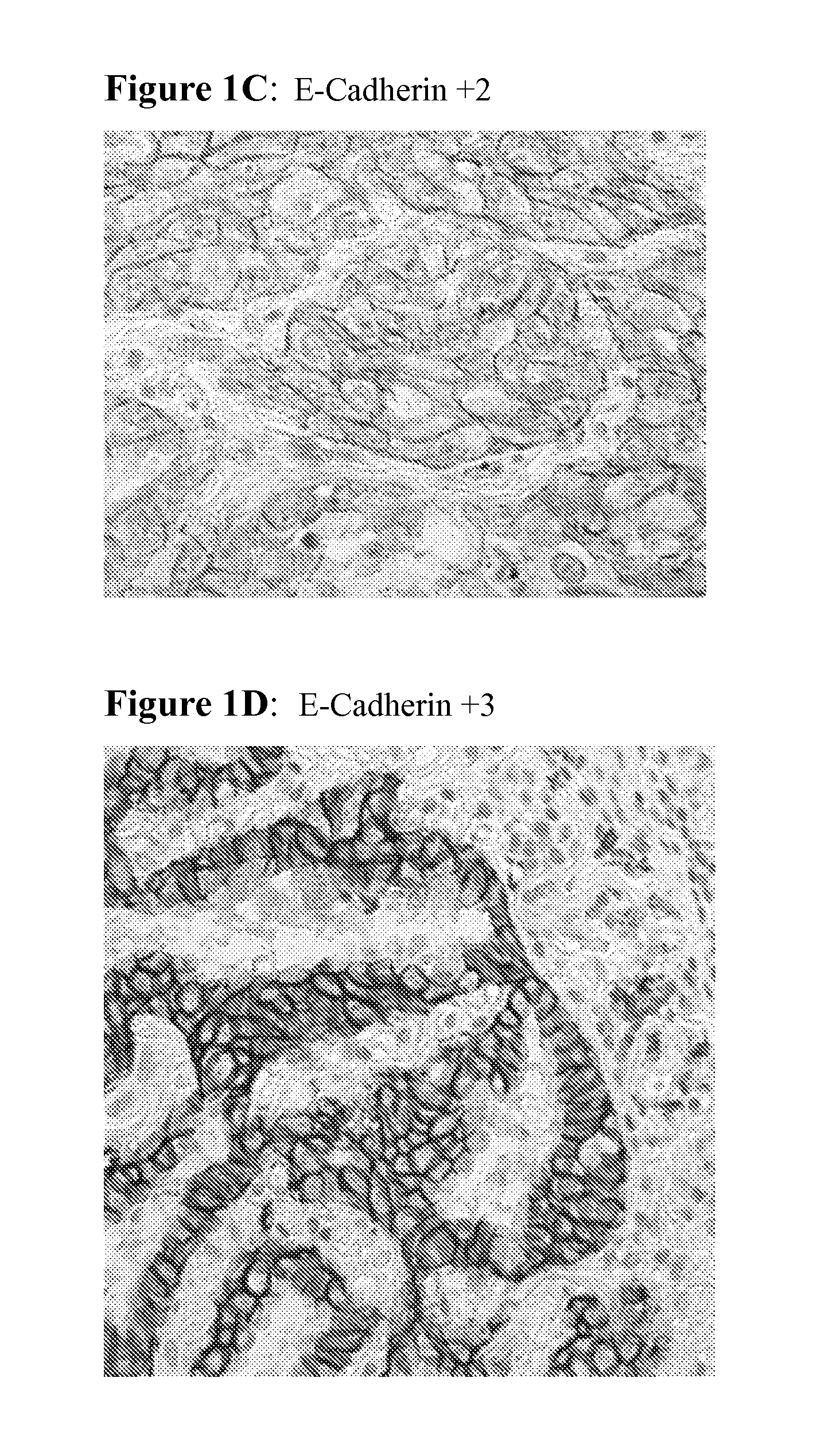
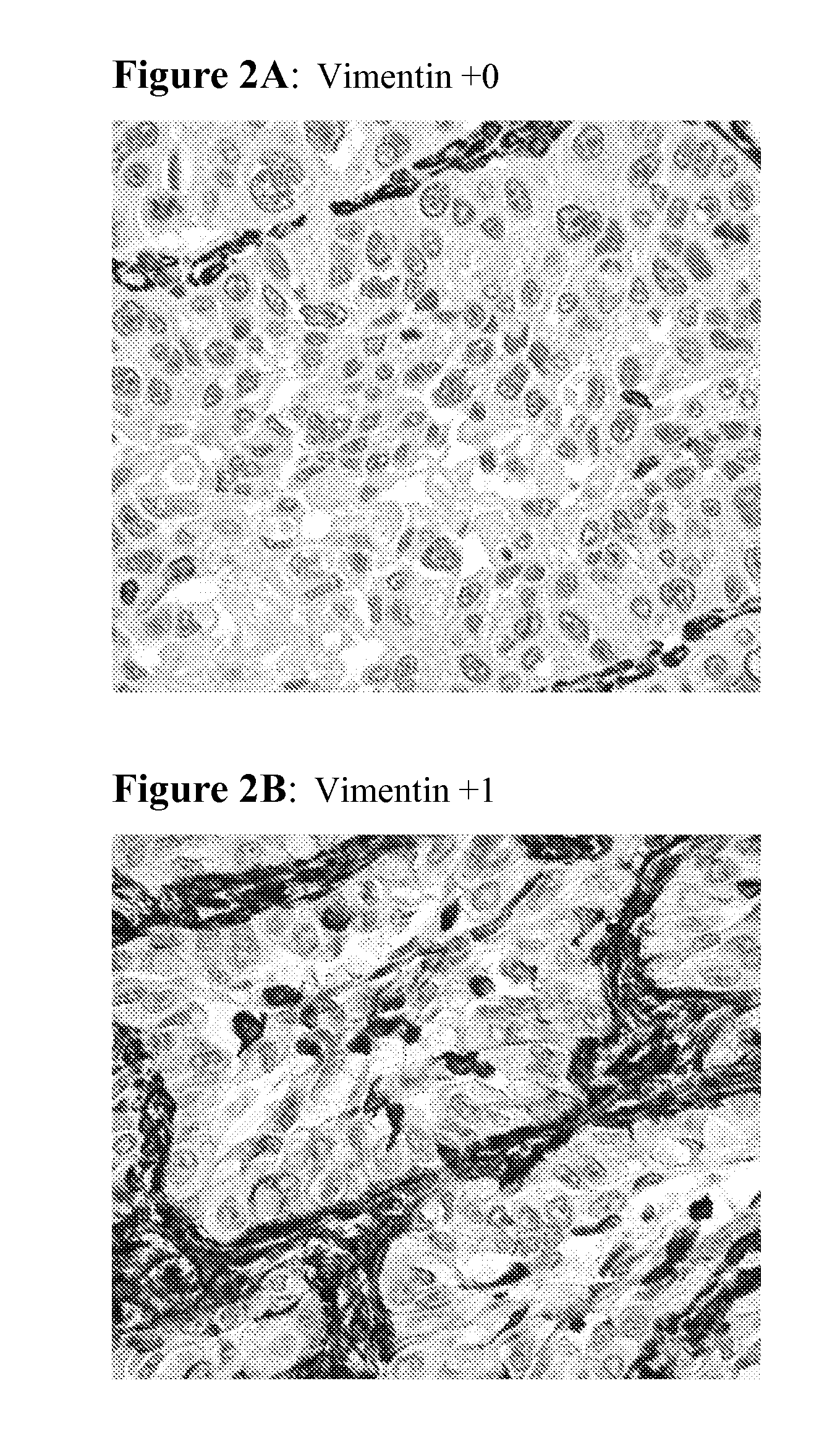
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. These methods are based on the surprising discovery that the effectiveness of treatment with an EGFR kinase inhibitor is predicted by whether a patient's tumor cells express a high or a low level of the biomarkers vimentin and E-cadherin, such that patients whose tumors express a high level of at least one of the biomarkers vimentin and E-cadherin have a longer overall survival and progression free survival than patients whose tumors express a low level of both vimentin and E-cadherin. The present invention further provides a method for treating tumors or tumor metastases in a patient, comprising the steps of diagnosing a patient's likely responsiveness to an EGFR kinase inhibitor by assessing whether tumor cells express a high level of at least one of the biomarkers vimentin and E-cadherin, and administering to said patient a therapeutically effective amount of an EGFR kinase inhibitor (e.g. erlotinib), particularly when effectiveness of the inhibitor is predicted.
Owner:OSI PHARMA LLC
Auxiliary assessment method for prognosis of nasopharynx cancer based on enhanced MRI radiomics
ActiveCN111657945ANo ionizing radiation damageClear structureDiagnostic recording/measuringSensorsIndividualized treatmentNasopharyngeal cancer
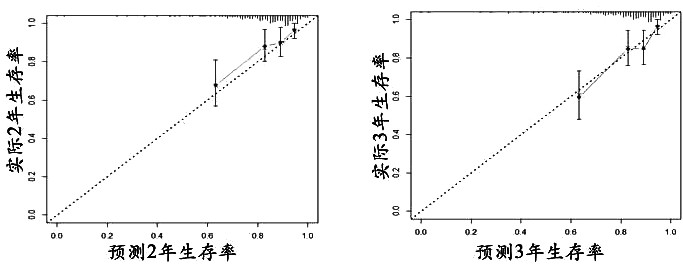
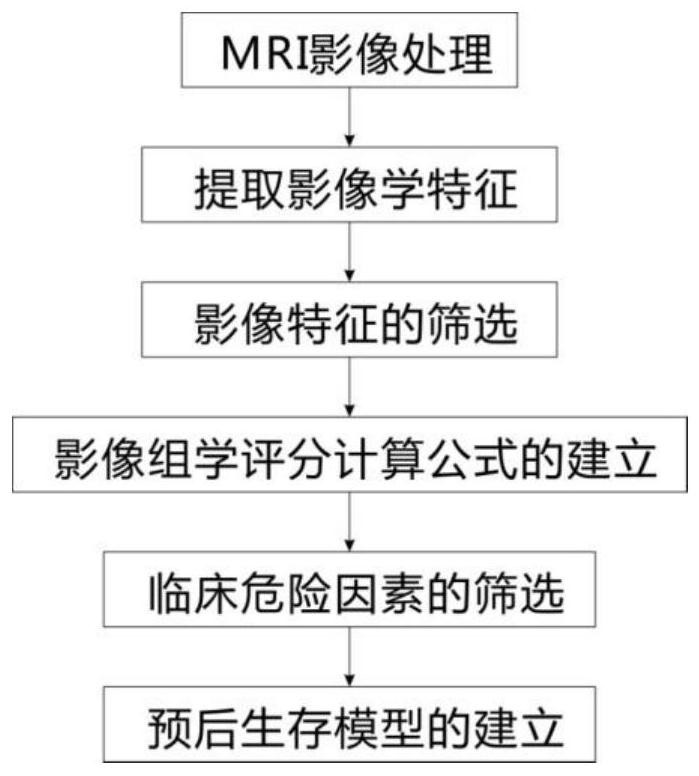
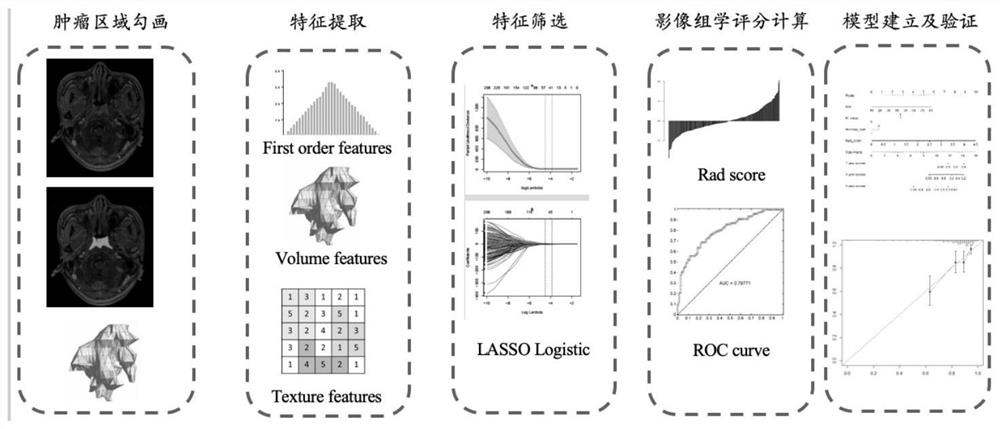
The invention relates to an auxiliary assessment method for prognosis of nasopharynx cancer based on enhanced MRI radiomics. The auxiliary assessment method comprises the steps of: (1), performing MRIimage processing; (2), extracting imaging features; (3), screening the imaging features; (4), establishing a radiomics scoring formula; (5), screening clinical risk factors; and (6), establishing a prognostic survival model: establishing a prognostic observation model through combination of a radiomics score and the clinical risk factors of a patient with nasopharynx cancer, performing qualitative and quantitative prediction on the PFS (progression free survival) of the patient, and furthermore, assessing the performance of the prognostic survival model. The auxiliary assessment method in theinvention has little harm to the image examination of the patient; qualitative and quantitative analysis on the survival time of a specific patient is carried out; therefore, a doctor is assisted tomake an individualized treatment and follow-up visit scheme; furthermore, the doctor is assisted to assess the survival and recurrence time of the patient; simultaneously, the performance of the obtained prognostic survival model is verified; and thus, the accuracy of a prognostic prediction model is ensured.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
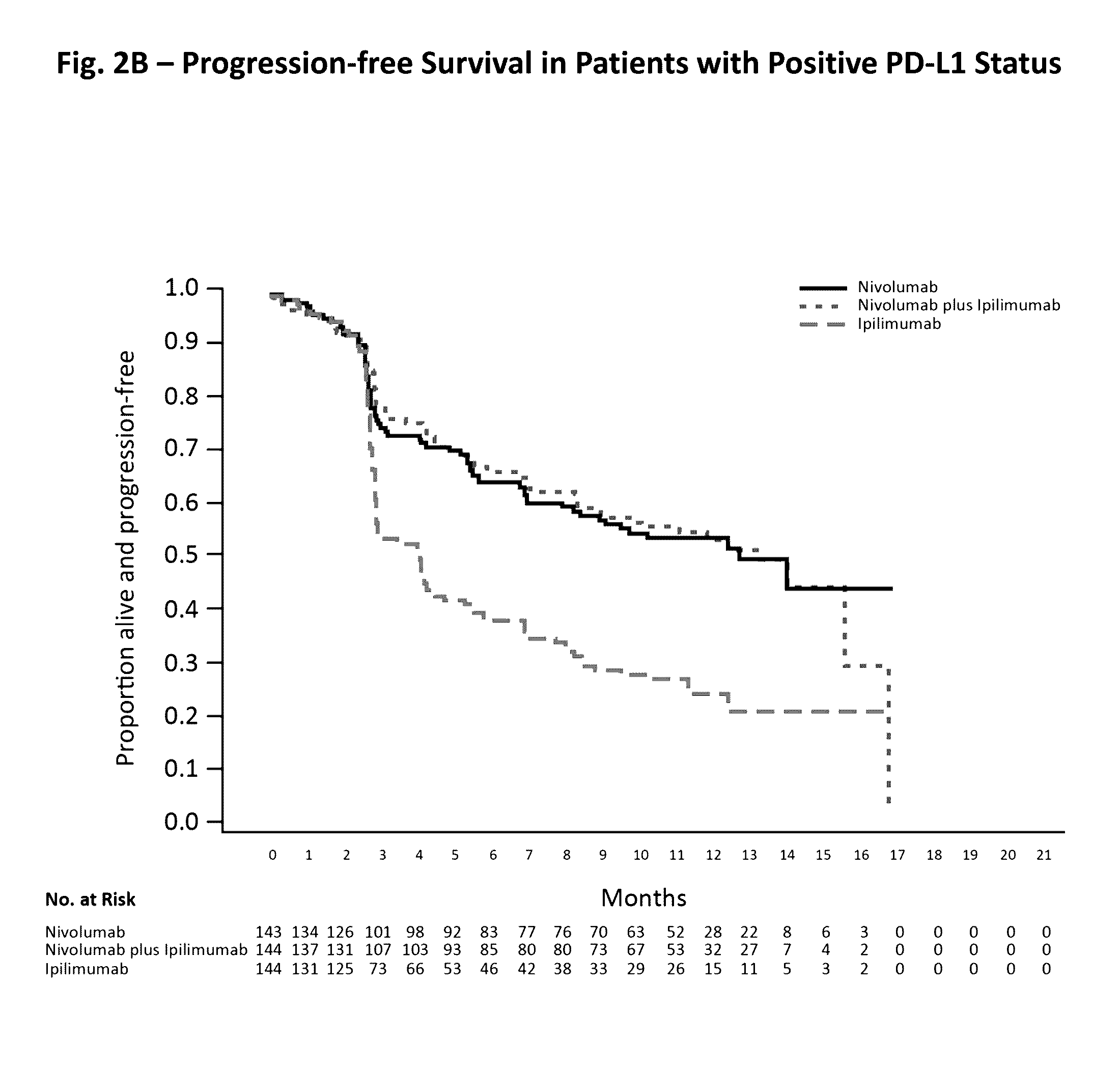
Treatment of PD-L1-Negative Melanoma Using an Anti-PD-1 Antibody and an Anti-CTLA-4 Antibody
ActiveUS20160340428A1Shrink tumorIncreasing objective response rateBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsProgression-free survivalMelanoma
The invention provides a method of treating a melanoma comprising (i) identifying a patient having a PD-L1-negative melanoma and (ii) administering to the patient a combination of an anti-PD-1 antibody or an antigen-binding portion thereof and an anti-CTLA-4 antibody or an antigen-binding portion thereof. The methods of the invention can extend progression-free survival for over 8 months and / or reduces the tumor size at least about 10%, about 20%, about 30%, about 40%, or about 50% compared to the tumor size prior to the administration.
Owner:BRISTOL MYERS SQUIBB CO
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
InactiveUS20160175438A1Prolong progression-free survivalReduce riskOrganic active ingredientsAntibody ingredientsHER2 Positive Breast CancerProgression-free survival
The present application describes uses for Pertuzumab, a first-in-class HER2 dimerization inhibitor. In particular, the application describes methods for extending progression free survival in a HER2-positive breast cancer patient population; combining two HER2 antibodies to treat HER2-positive cancer without increasing cardiac toxicity; and treating HER2-positive cancer by co-administering a mixture of Pertuzumab and Trastuzumab from the same intravenous bag.
Owner:GENENTECH INC
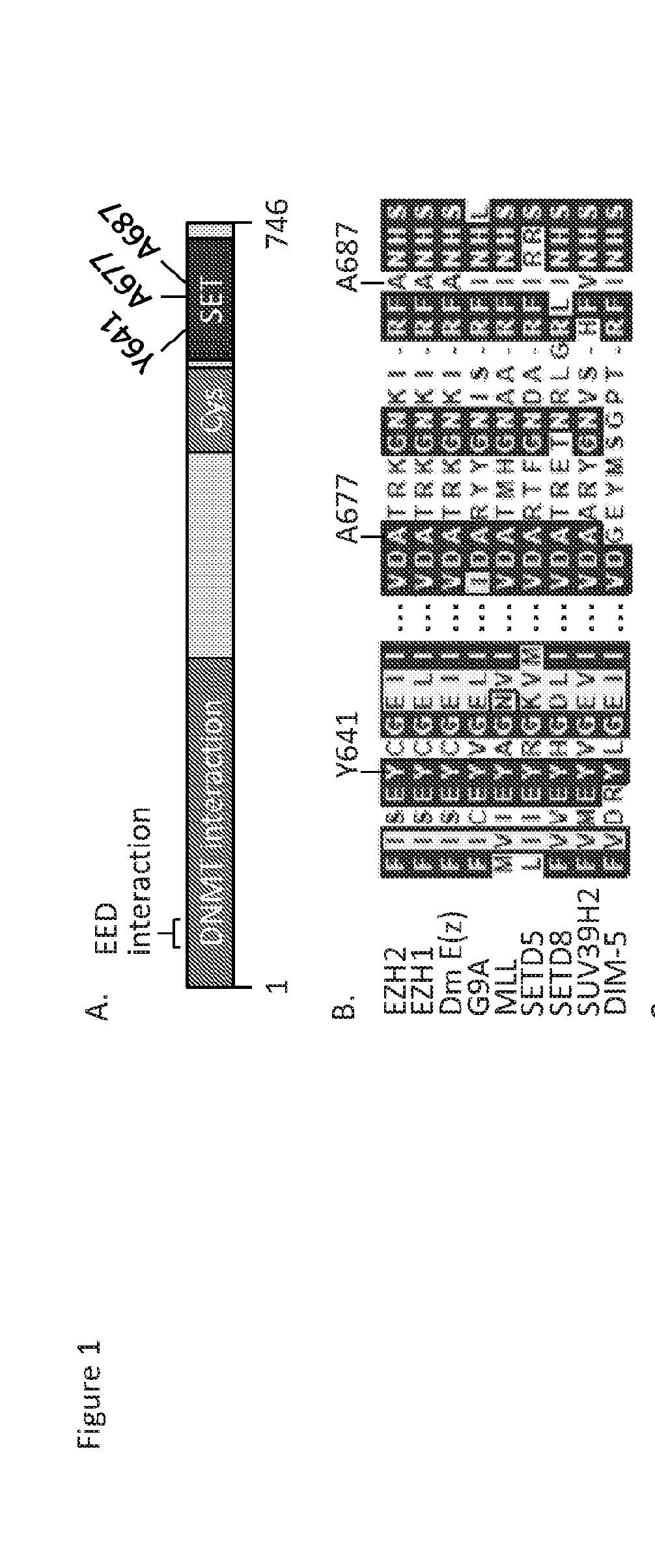
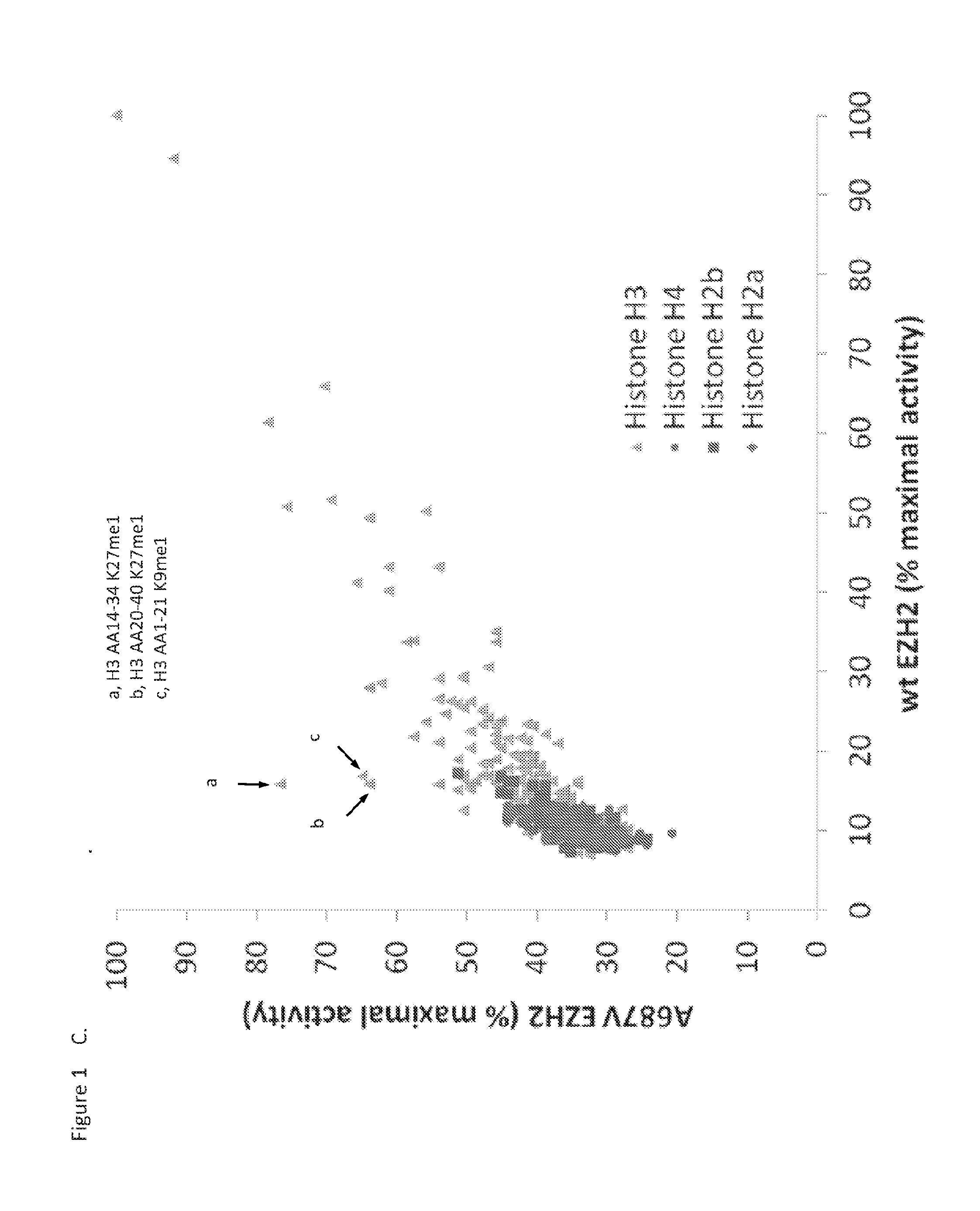
Methods of treating cancer patients responding to ezh2 inhibitor gsk126
InactiveUS20160361309A1Improve the level ofOrganic active ingredientsMicrobiological testing/measurementProgression-free survivalOncology
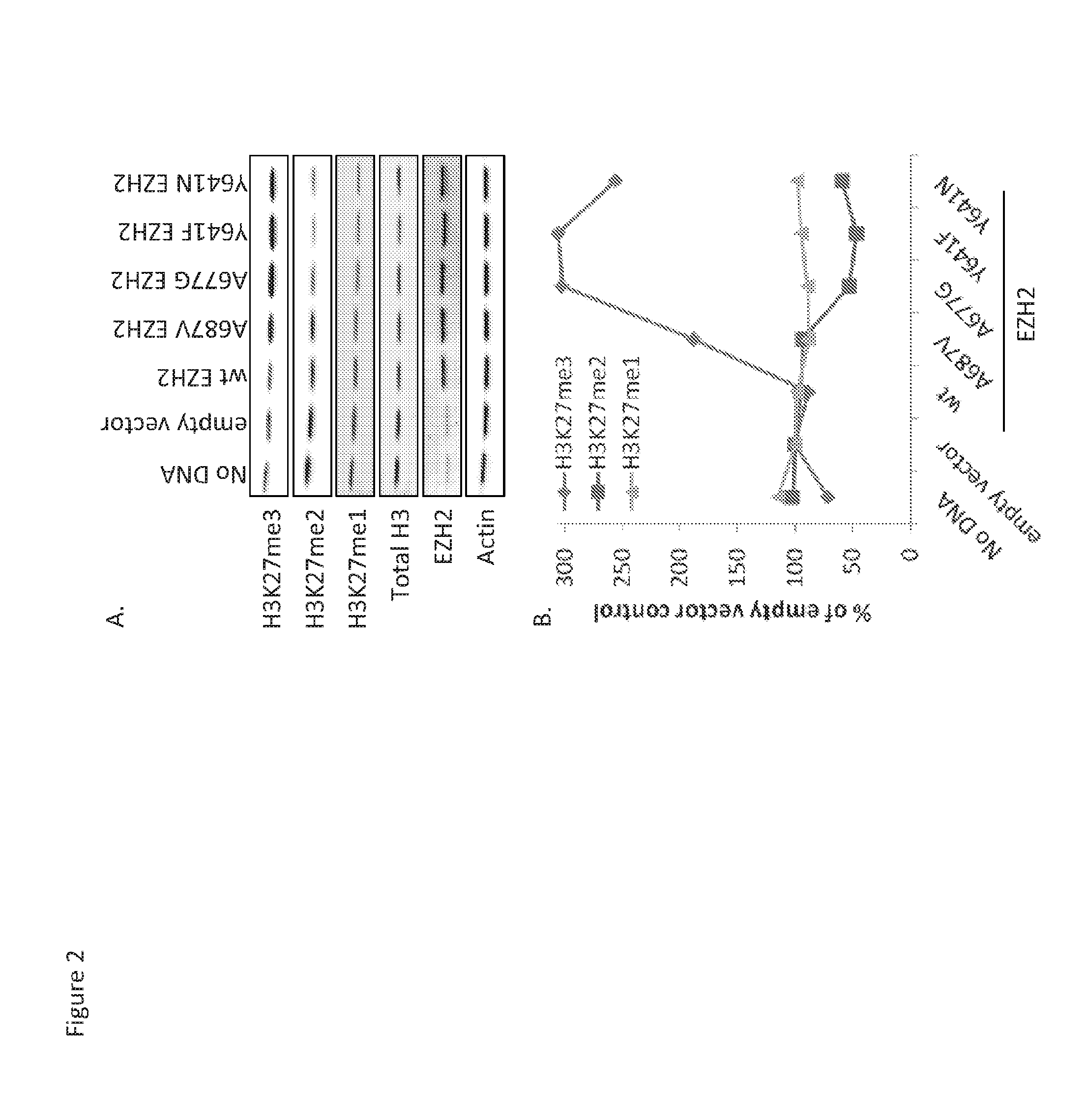
Disclosed herein are methods of treating cancer in a human, where the methods include determining at least one of the following in one or more samples from the human: the presence or absence of an alanine to valine mutation at residue 687 (A687V) in EZH2 in a sample from the human; or the presence or absence of an increased level of H3K27me2 in a sample from the human as compared to a control; and administering to the human an effective amount of the EZH2 inhibitor GSK126 or a pharmaceutically acceptable salt thereof if the A687V mutation is present, or an increased level of H3K27me2 is not present, or both, in the one or more samples, which is indicative of an increased likelihood of increased response rate and / or prolonged progression free survival.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Administration of Karenitecin for the Treatment of Platinum and/or Taxane Chemotherapy-Resistant or -Refractory Advanced Ovarian Cancer
ActiveUS20140336150A1Increase probabilityReduce adverse effectsBiocideSilicon compound active ingredientsPlatinumProgression-free survival
The present invention discloses and claims methods and compositions for the treatment of subjects having advanced ovarian cancer, including platinum and / or taxane chemotherapy resistant or refractory sub-populations, with the administration to the subject having advanced ovarian cancer of the silicon-containing highly lipophilic camptothecin derivative (HLCD), Karenitecin (also known as BNP1350; cositecan; 7-[(2′-trimethylsilyl)ethyl]-20(S) camptothecin) in an amount sufficient to provide a therapeutic benefit. The administration of Karenitecin by intravenous (i.v.) and / or oral methodologies are also disclosed and claimed. Methods for the administration of Karenitecin to increase Progression Free Survival (PFS) are also disclosed and claimed herein.
Owner:CROWN BIOSCIENCE INC
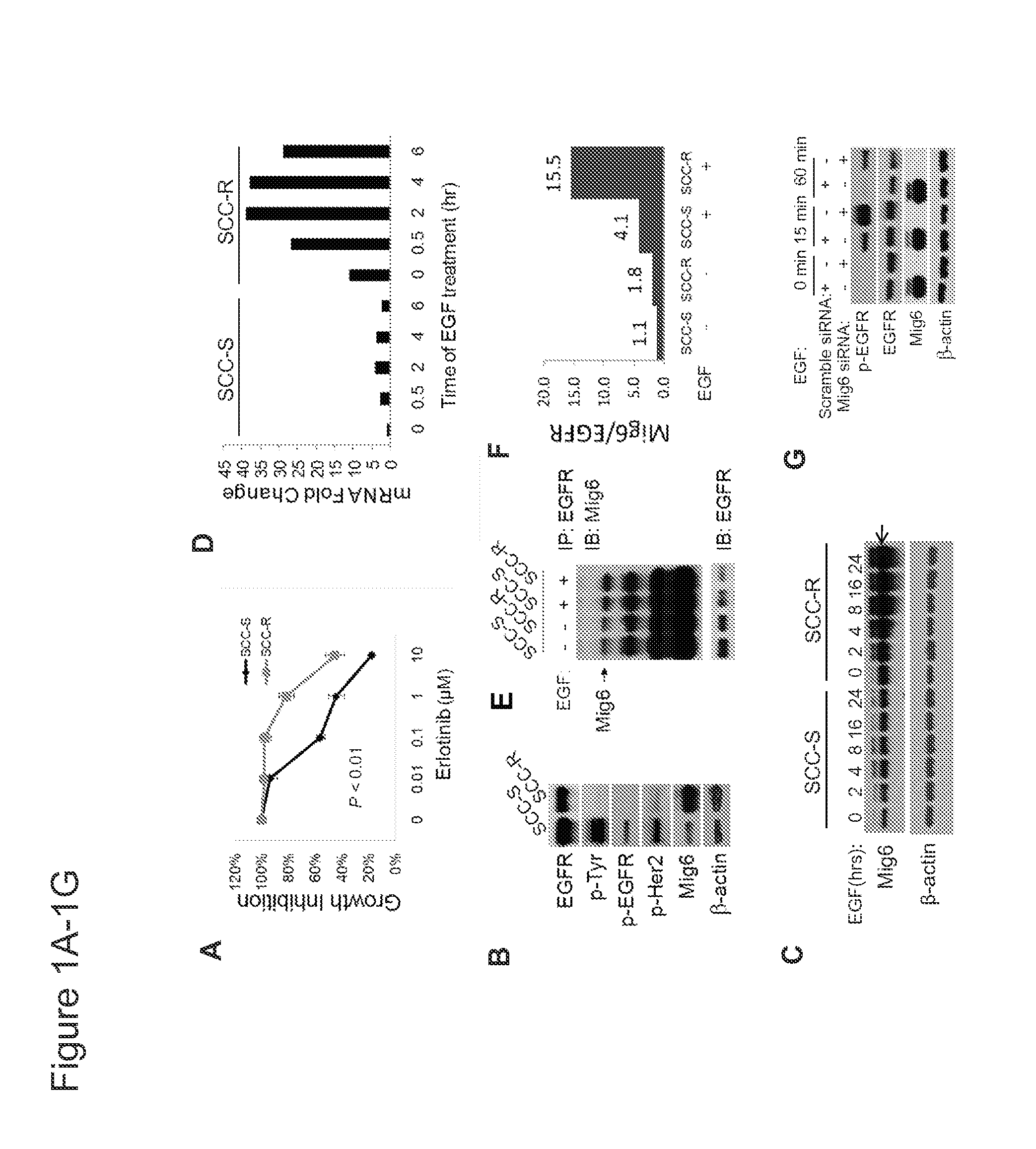
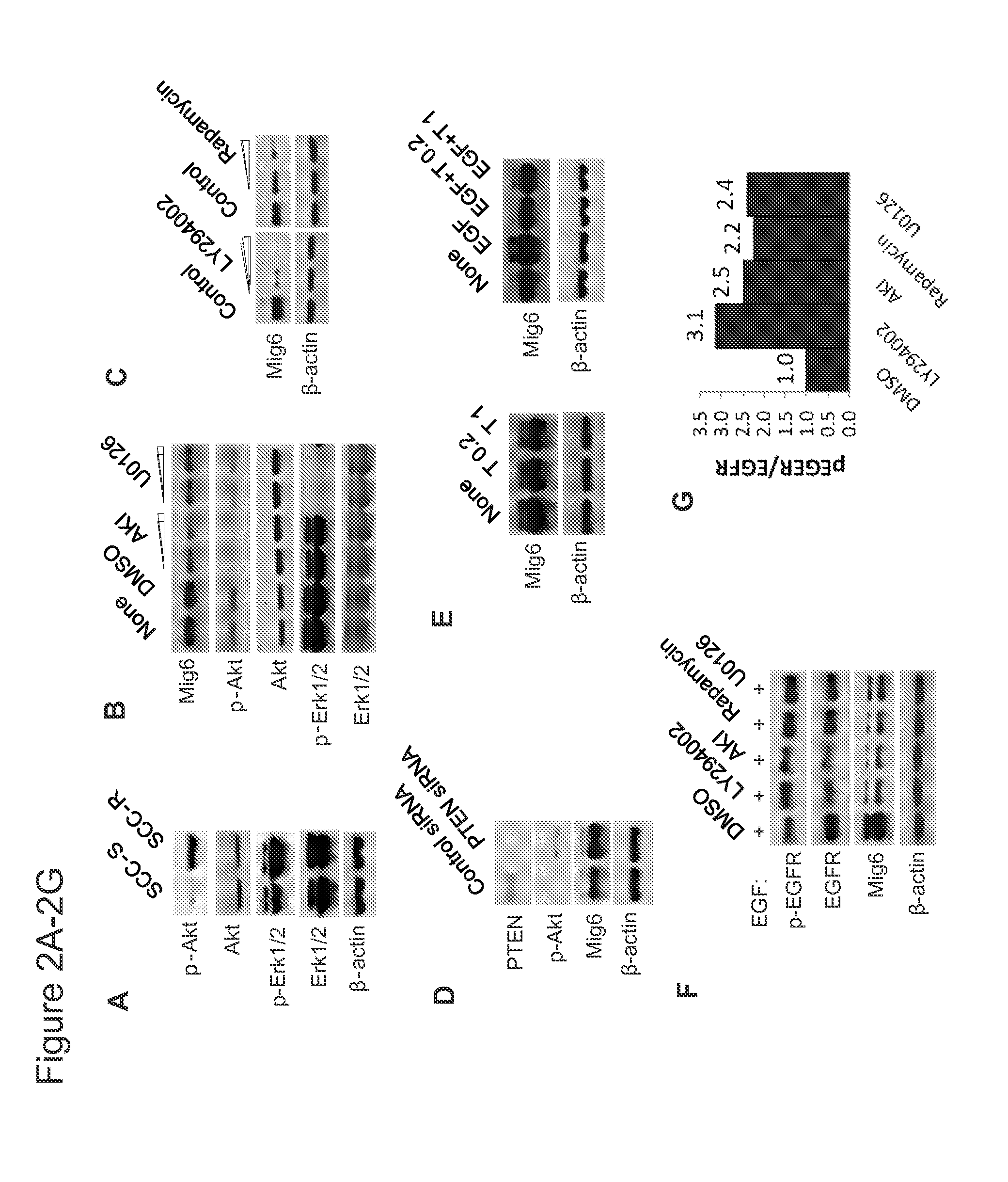
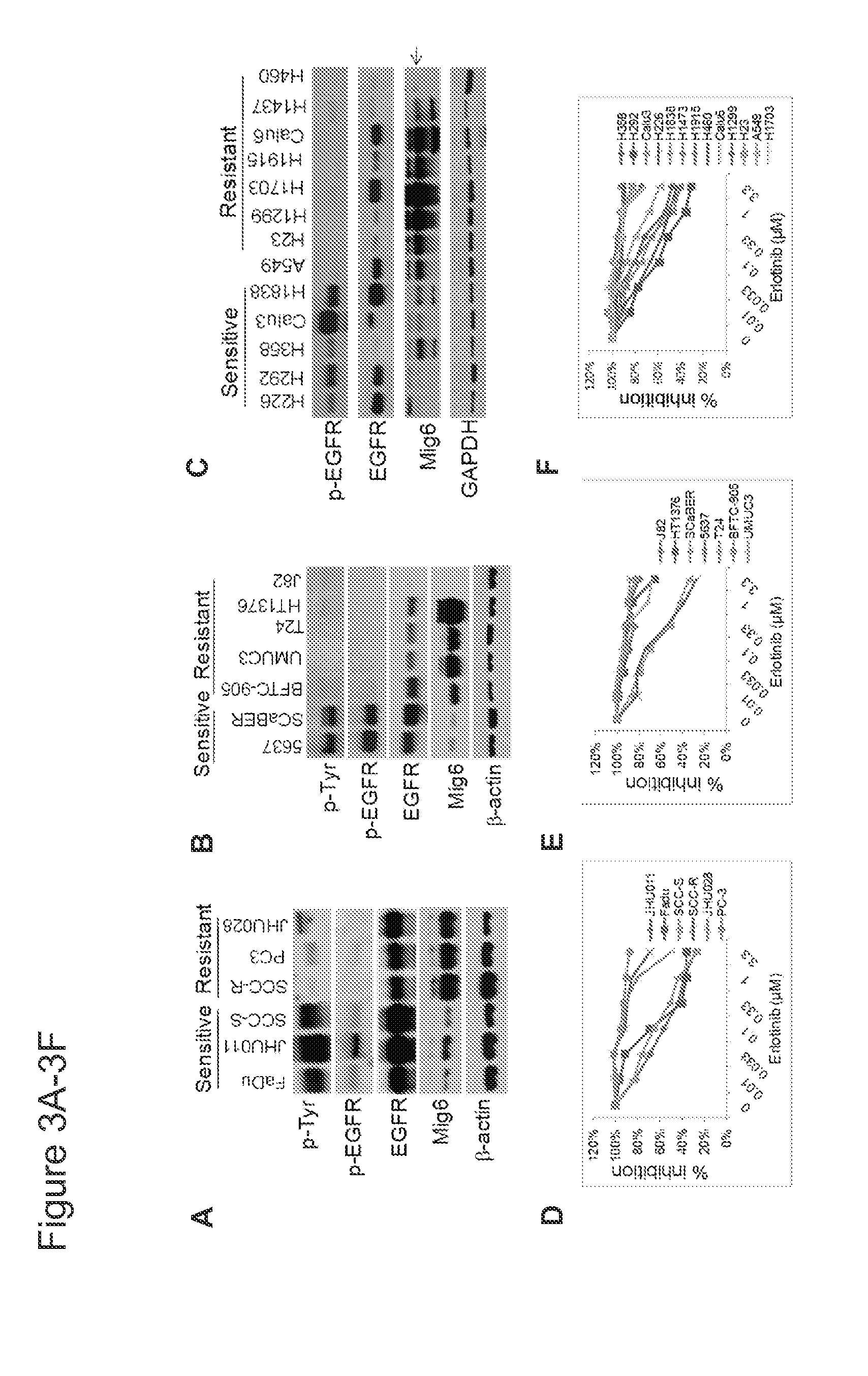
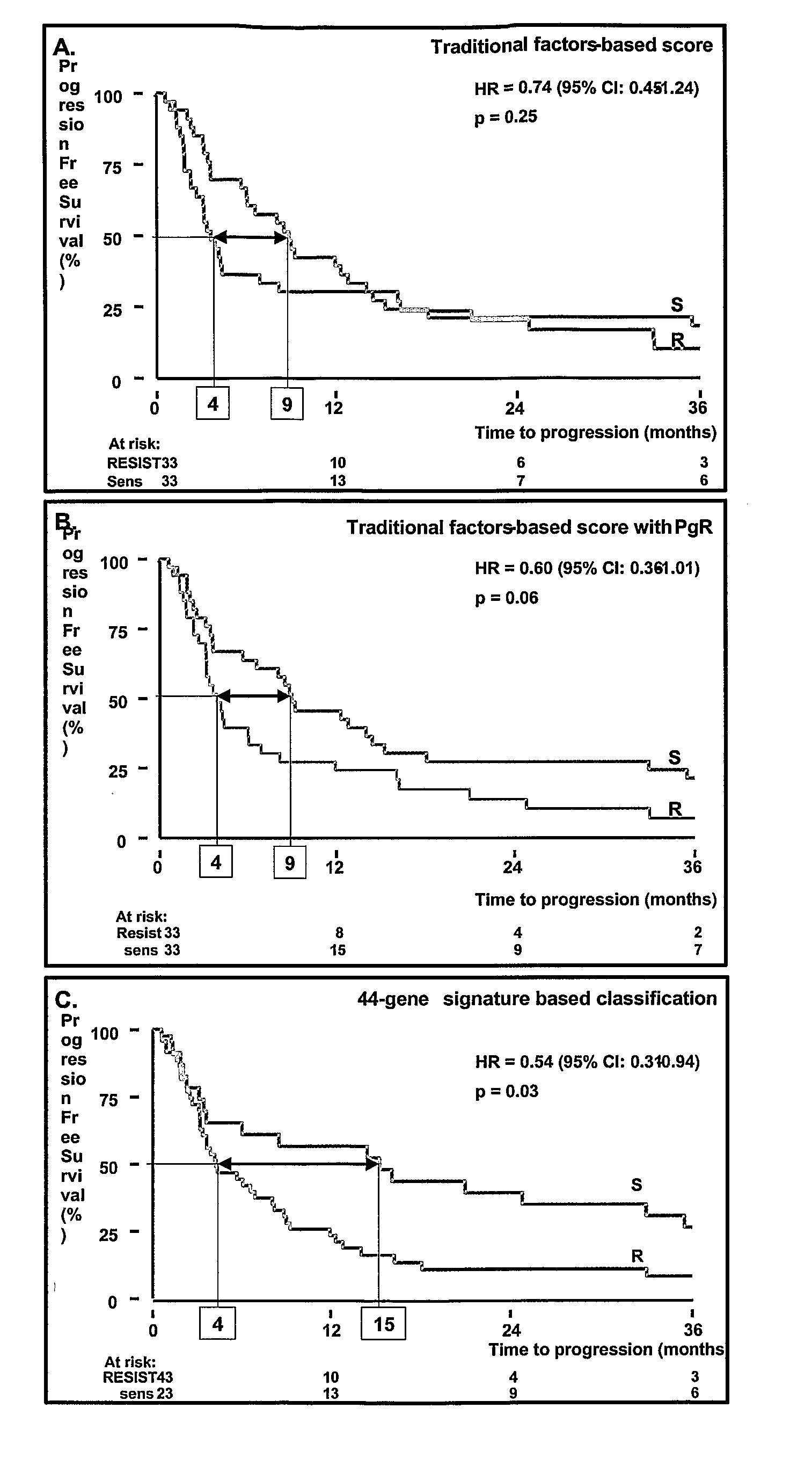
Mig6 and therapeutic efficacy
We identify markers capable of guiding the decision to incorporate epidermal growth factor receptor (EGFR) inhibitors, in particular EGFR tyrosine kinase inhibitors (TKIs), into chemotherapeutic regimens. Mitogen-inducible gene 6 (Mig6), a negative regulator of EGFR, is selectively upregulated during the development of resistance to the EGFR tyrosine kinase inhibitor (TKI) erlotinib, resulting in decreased EGFR phosphorylation. The ratio of Mig6 / EGFR expression highly correlates with erlotinib sensitivity. A low Mig6 / EGFR ratio correlates with a high response rate to gefitinib and a marked increase in progression-free survival for patients. The ratio of Mig6 to EGFR is a major predictor of biologic and clinical responses to EGFR inhibitors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Predicting Response And Outcome Of Metastatic Breast Cancer Anti-Estrogen Therapy
InactiveUS20080113345A1Useful in treatmentSugar derivativesMicrobiological testing/measurementProgression-free survivalTamoxifen treatment
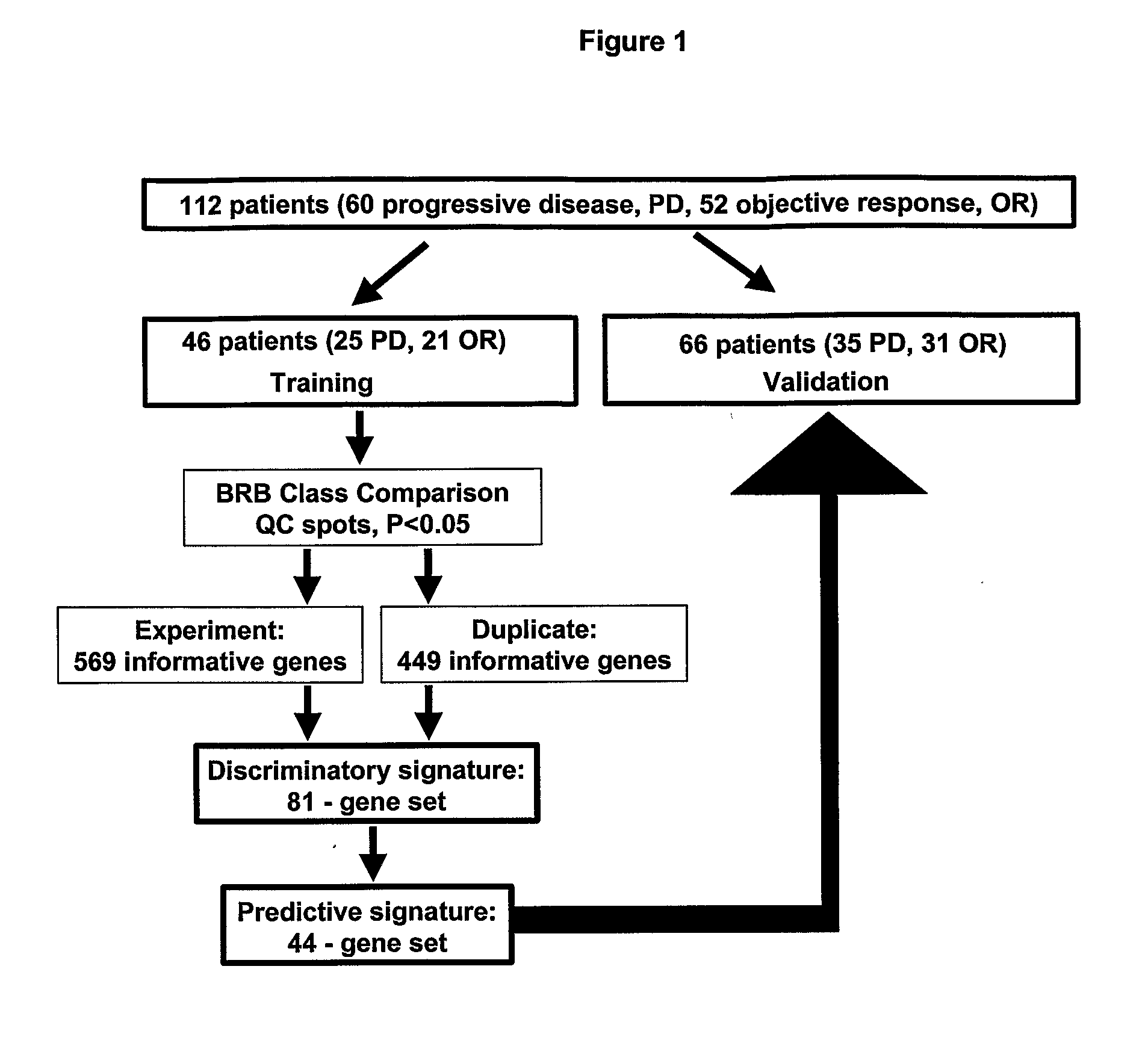
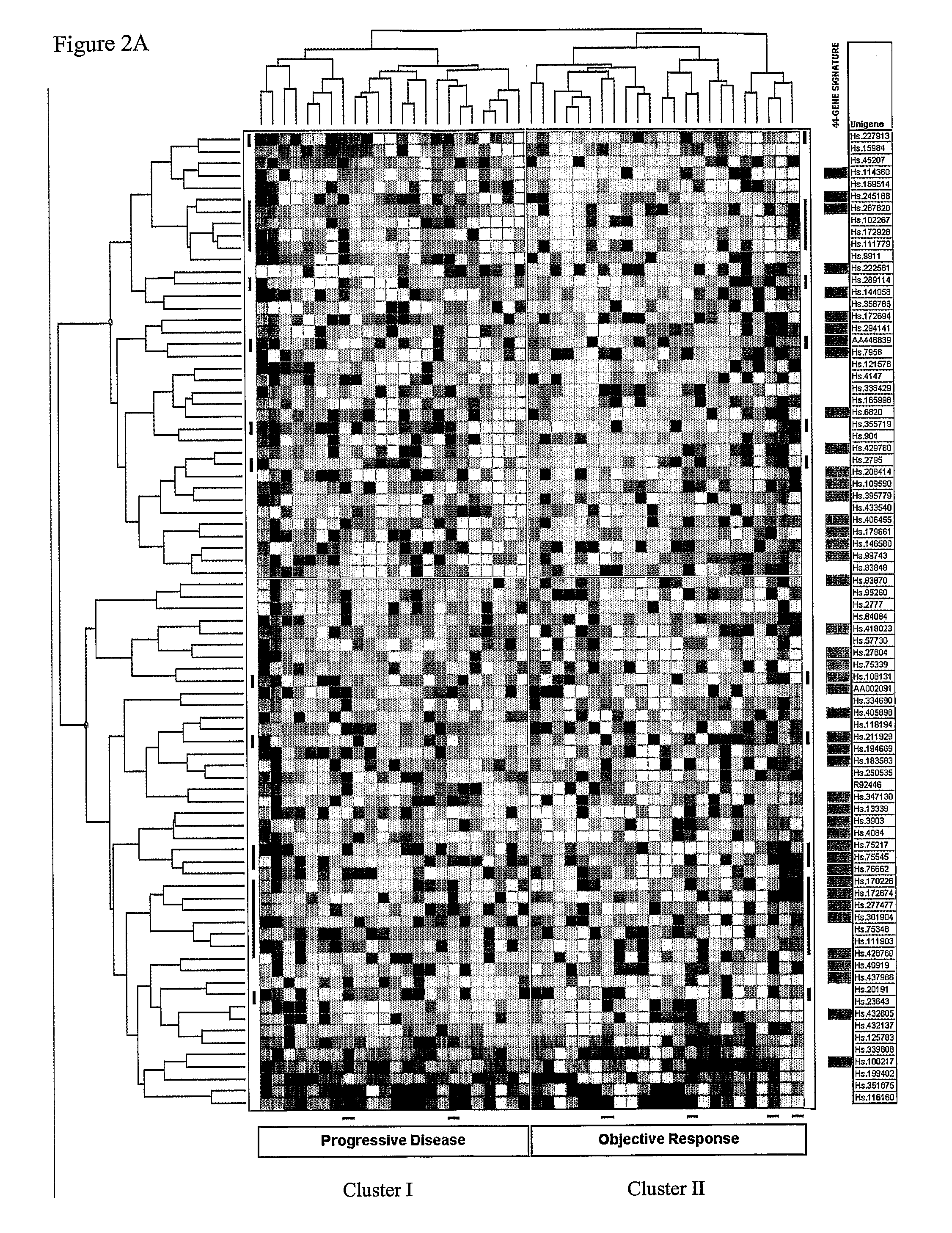
Gene signatures, specific marker genes, and diagnostic assays for predicting progression free survival and objective response to anti-estrogen, e. g., tamoxifen therapy for recurring breast cancer patients are described.
Owner:ERASMUS MC
Application of scoring system based on immune gene pair in prediction of immunotherapy effect of non-small cell lung cancer patient
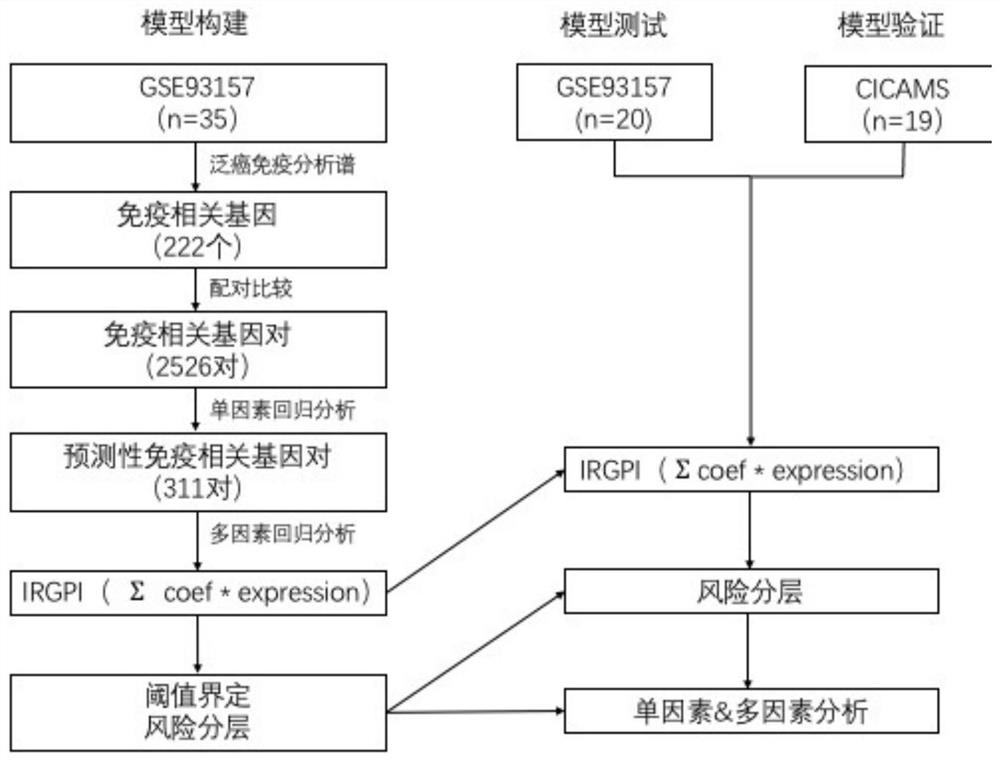
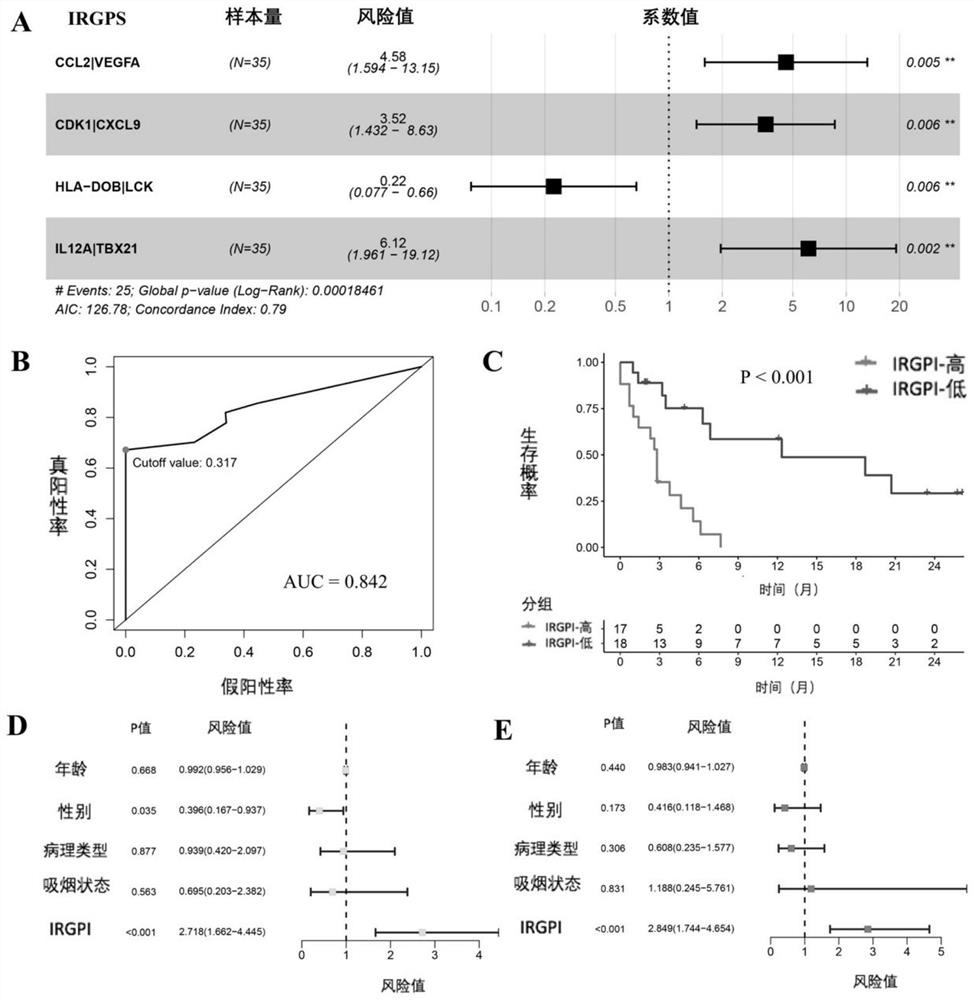
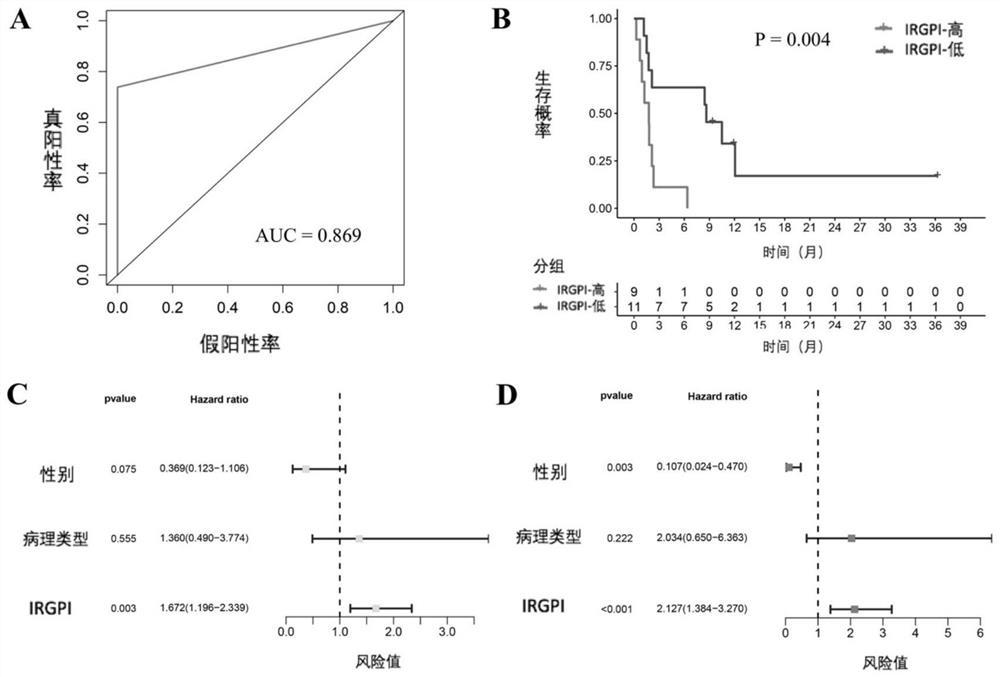
The invention discloses an application of a scoring system based on an immunogene pair in prediction of immunotherapy curative effect and prognosis of a non-small cell lung cancer patient. The scoring system disclosed by the invention is carried out based on the following four immune related gene pairs: CCL2 and VEGFA genes, CDK1 and CXCL9 genes, HLA-DOB and LCK genes, and IL-12A and TBX21 genes. The IRGP index (IRGPI) calculated based on an immune gene pair scoring system constructed by the invention is significantly related to the progression-free lifetime of a non-small cell lung cancer patient receiving anti-PD-1 immunotherapy. Therefore, the four immune-related gene pairs and the scoring system constructed according to the four immune-related gene pairs can be used for performing curative effect and prognosis prediction on the non-small cell lung cancer patient receiving anti-PD-1 immunotherapy.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
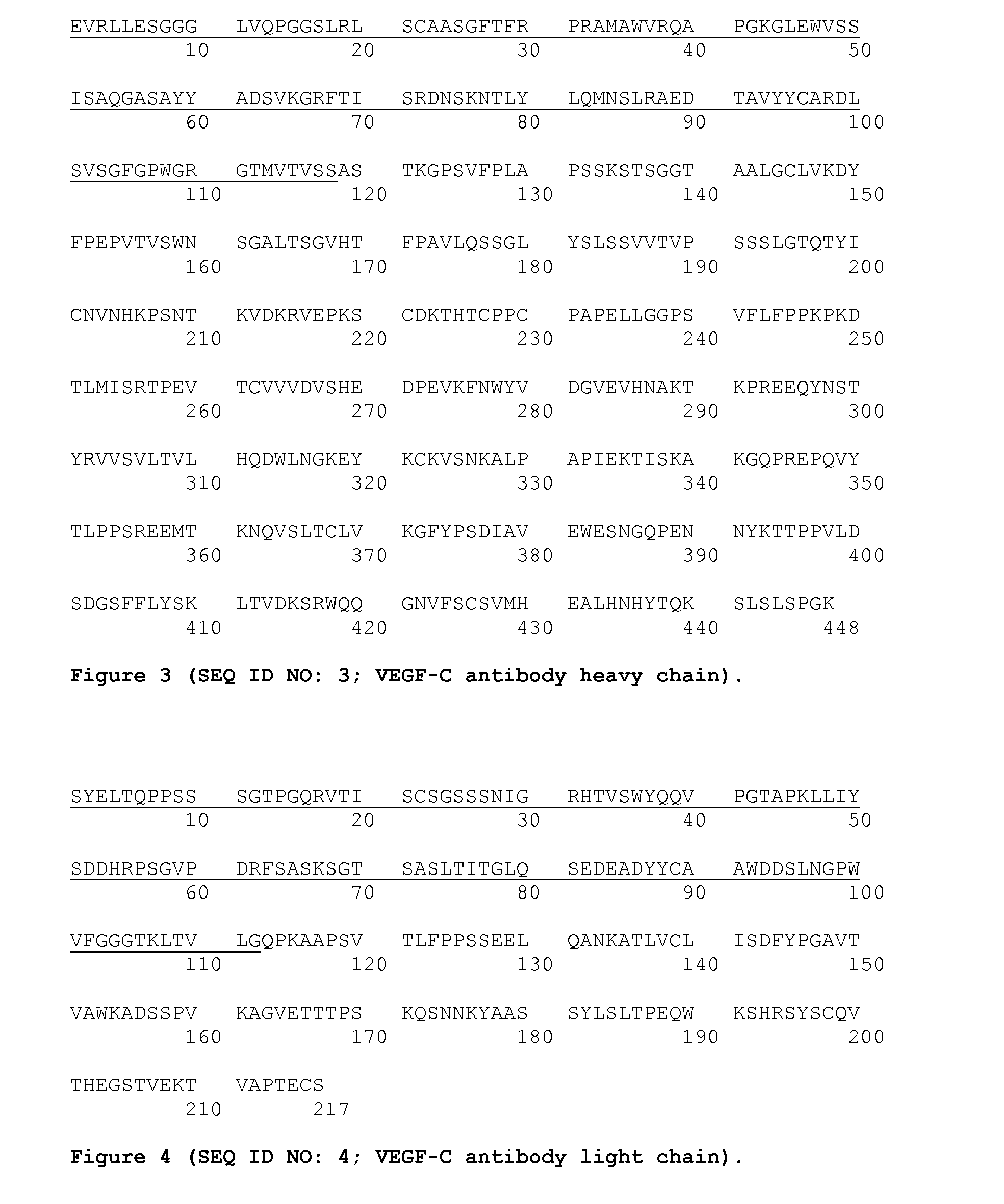
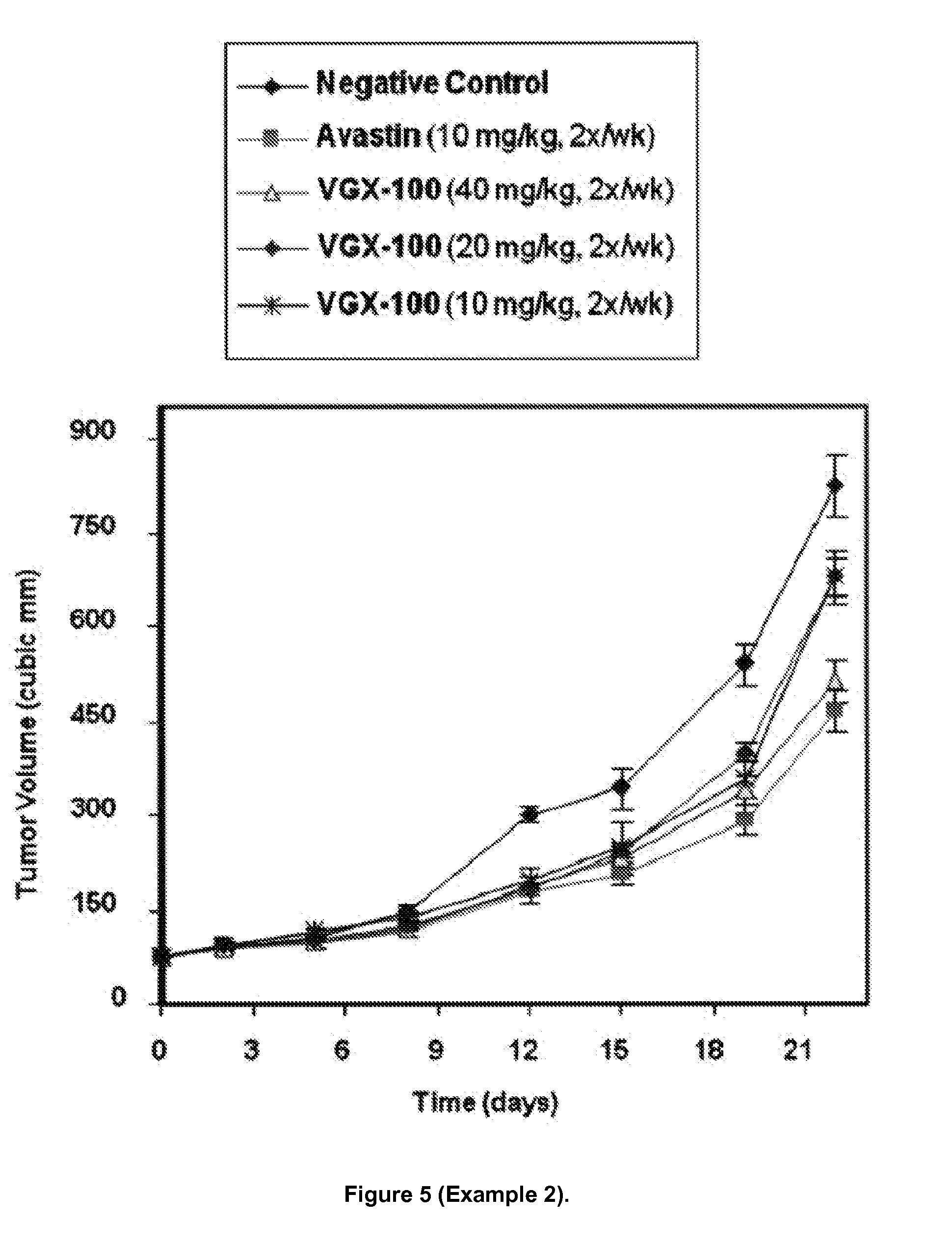
Combination treatment with vegf-c antagonists
InactiveUS20120207671A1Prevent and ameliorate metastasisNervous disorderPeptide/protein ingredientsProgression-free survivalGlioblastoma
The invention relates to a method and kit for treating cancer in a human subject, the method comprising administering to the subject in combination therapeutically effective amounts of a VEGF-C antagonist and an anti-neoplastic composition, and the kit comprising a VEGF-C antagonist for administering to the subject in combination with an anti-neoplastic composition. The invention further relates to methods for: increasing the duration of survival of, increasing the progression-free survival of, increasing the duration of response of, or treating, a subject or a group of human subjects susceptible to or diagnosed as having a cancer; or treating a human subject or a group of human subjects having metastatic colorectal cancer, prostate cancer, pancreatic cancer or glioblastoma, the methods comprising administering to the subject or subjects in the group in combination effective amounts of a VEGF-C antagonist and an anti-neoplastic composition.
Owner:VEGENICS PTY LTD
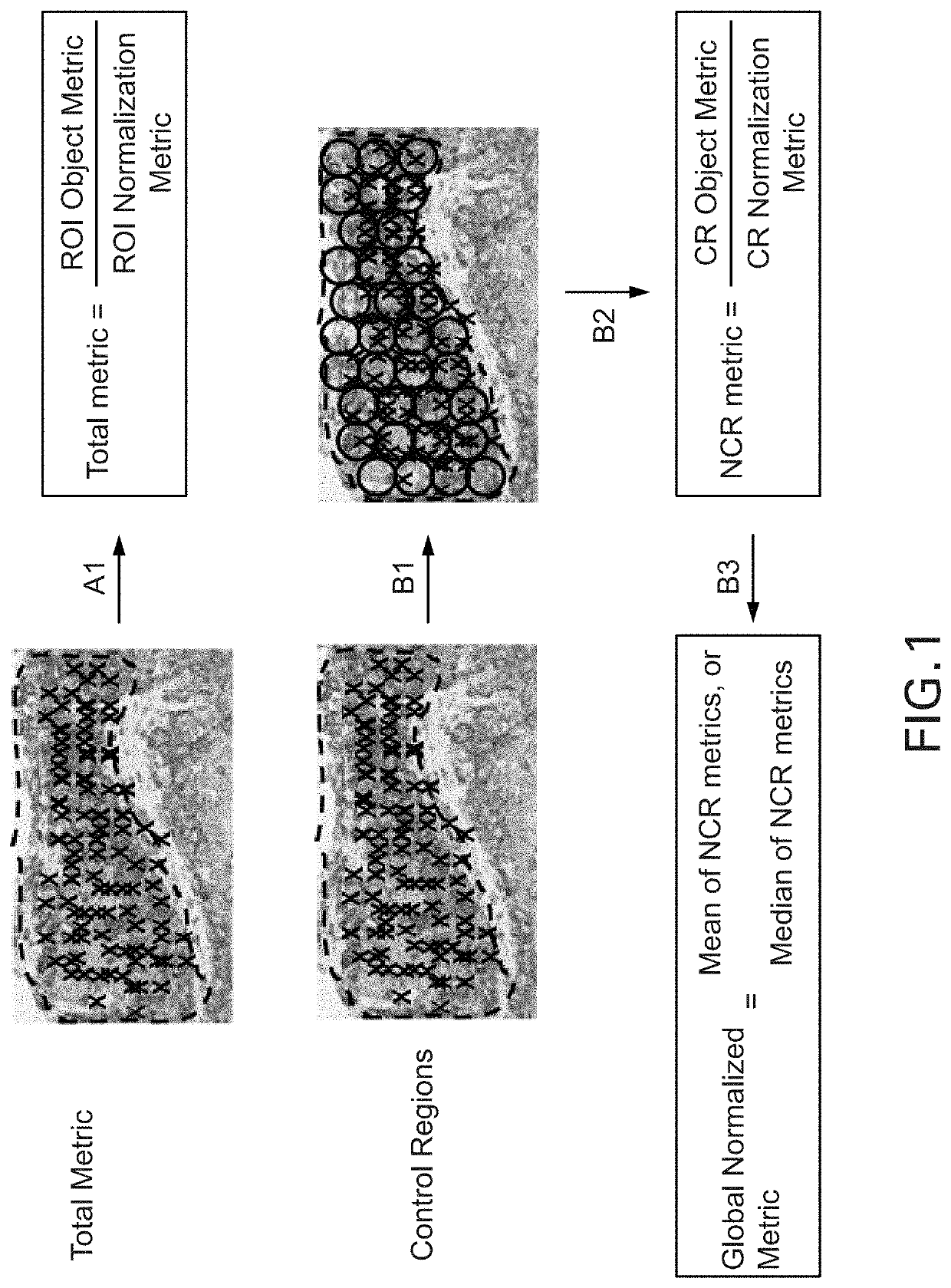
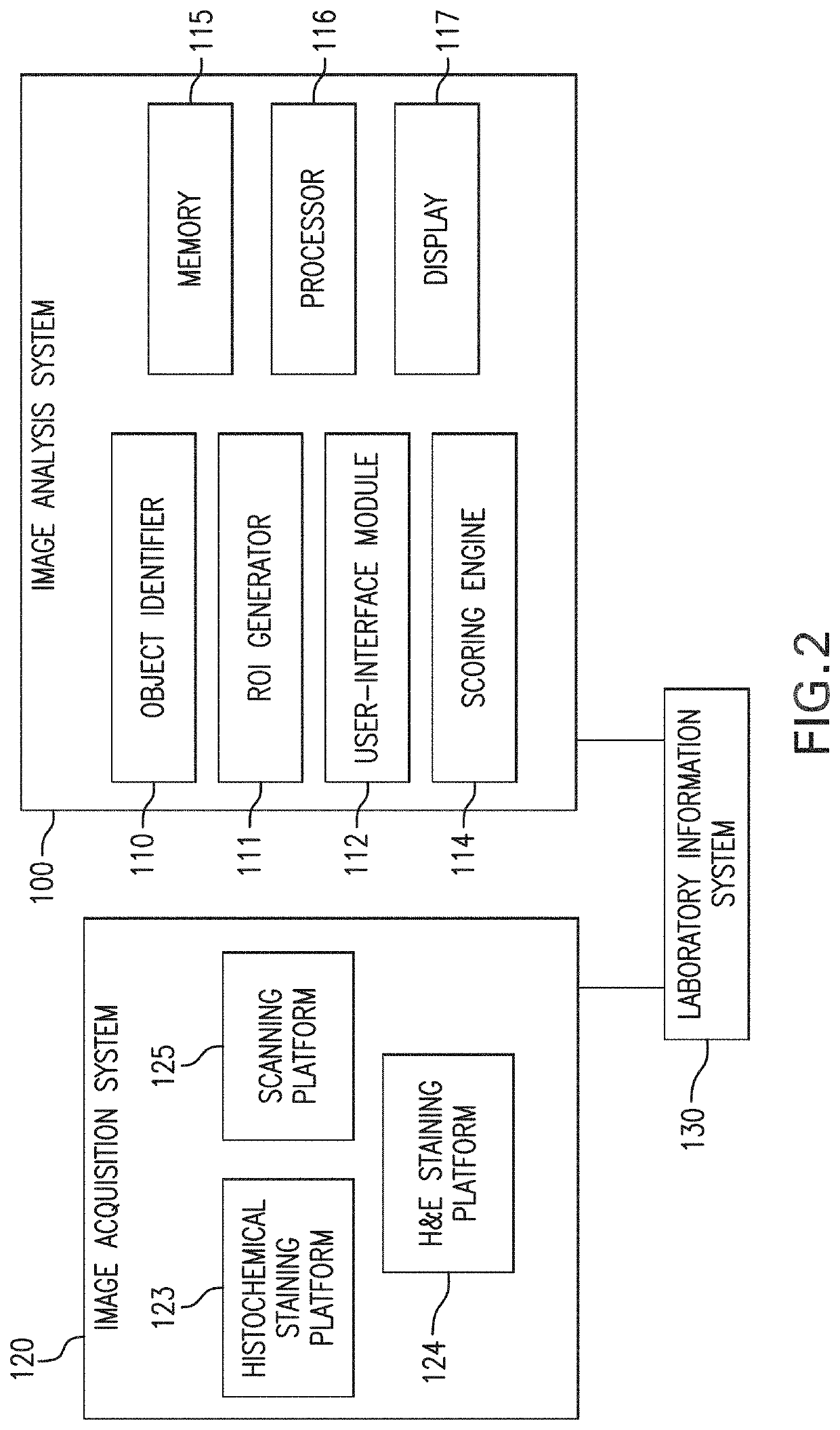
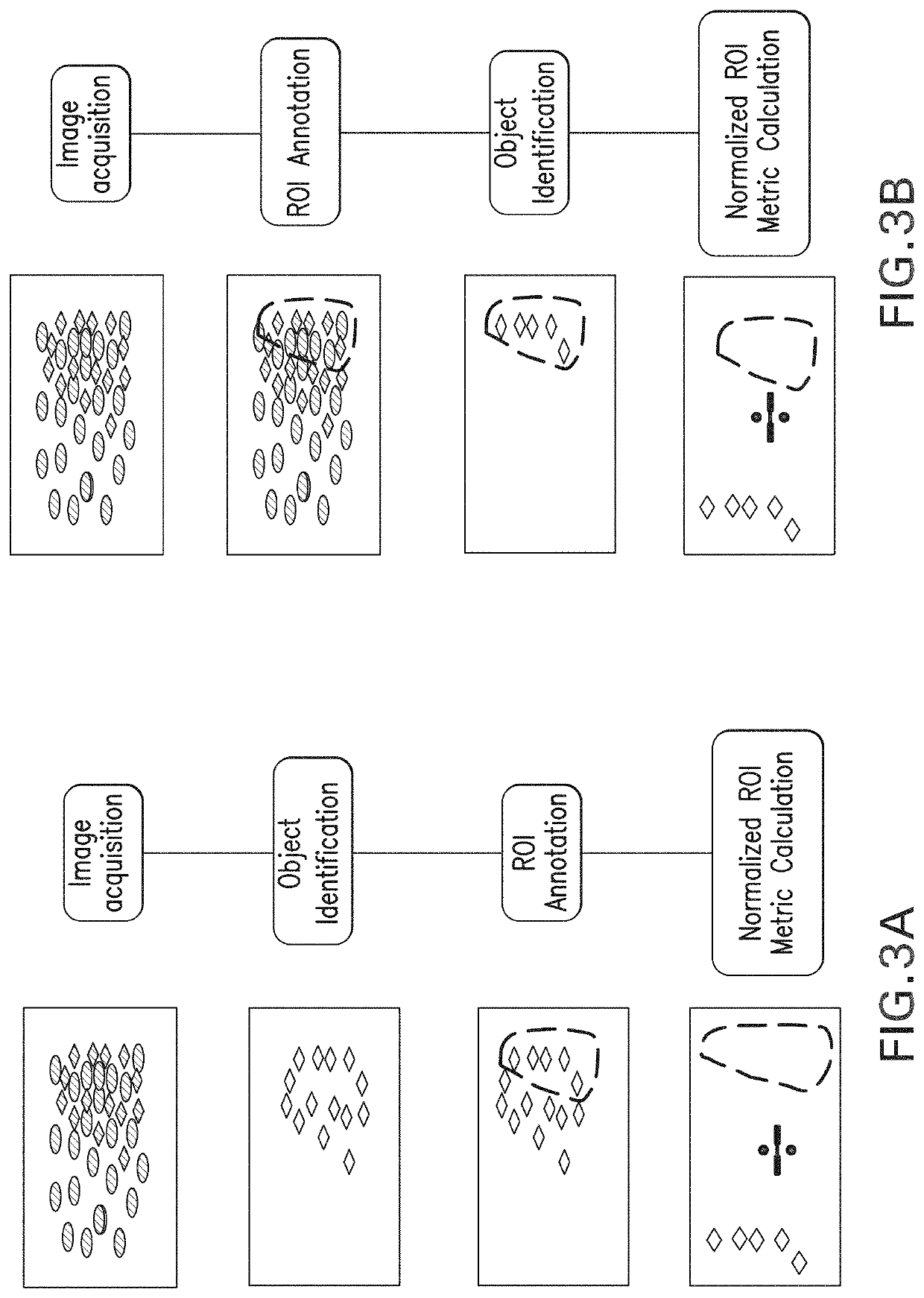
Methods and systems for evaluation of immune cell infiltrate in tumor samples
ActiveUS20200234442A1Improves prognosticGood prediction accuracyImage enhancementImage analysisProgression-free survivalCell marker
Immune context scores are calculated for tumor tissue samples using continuous scoring functions. Feature metrics for at least one immune cell marker are calculated for a region or regions of interest, the feature metrics including at least a quantitative measure of human CD3 or total lymphocyte counts. A continuous scoring function is then applied to a feature vector including the feature metric and at least one additional metric related to an immunological biomarker, the output of which is an immune context score. The immune context score may then be plotted as a function of a diagnostic or treatment metric, such as a prognostic metric (e.g. overall survival, disease-specific survival, progression-free survival) or a predictive metric (e.g. likelihood of response to a particular treatment course). The immune context score may then be incorporated into diagnostic and / or treatment decisions.
Owner:VENTANA MEDICAL SYST INC
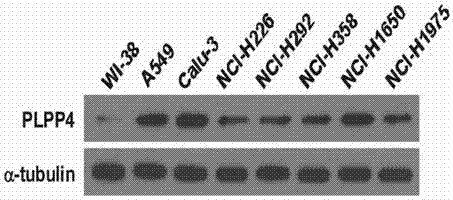
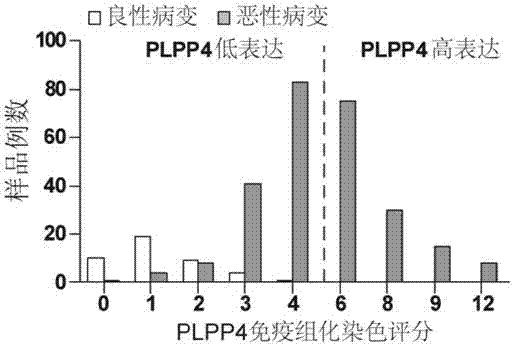
Application of PLPP4 ((phospholipid phosphatase 4) as diagnosis, treatment and prognosis target for NSCLC (non-small-cell lung carcinoma)
ActiveCN107326071AOrganic active ingredientsMicrobiological testing/measurementFluorescenceClinico pathological
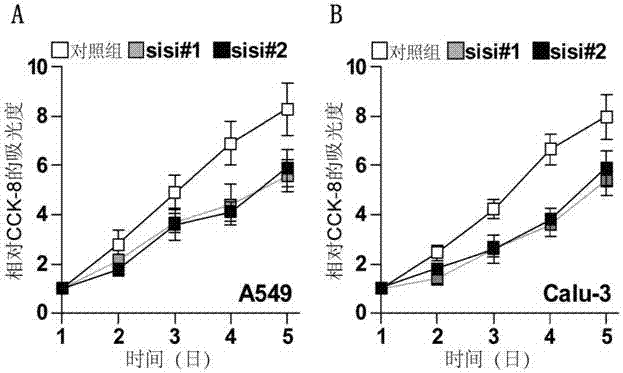
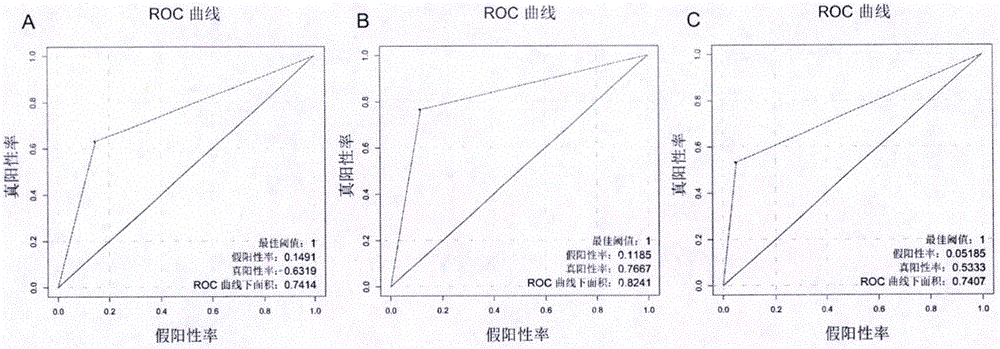
The invention discloses an application of PLPP4 ((phospholipid phosphatase 4) as a diagnosis, treatment and prognosis target for NSCLC (non-small-cell lung carcinoma). High expression of PLPP4 in tumor tissue and cell lines of NSCLC is discovered, high expression of PLPP4 in lung malignant tumor tissue is related to low clinical pathological grades as well as short overall survival time and progression-free survival time of a patient, in-vitro NSCLC cell proliferation and in-vivo tumorigenesis can be affected by silencing PLPP4, and all the information indicates that PLPP4 can serve as the diagnosis, treatment and prognosis target for NSCLC. Primers performing fluorescent quantitative detection on PLPP4 are taken as reagents for diagnosis and prognosis of NSCLC, and LNA modified sisiRNA capable of specifically silencing PLPP4 is taken as a treatment medicine for NSCLC.
Owner:江门市中心医院
IDH1-R132H and ATRX expression based glioma prognostic system
The invention relates to an IDH1-R132H and ATRX expression based glioma prognostic system which comprises (1), a tumor paraffin-embedding kit for glioma patients and an operating instruction thereof if necessary; (2), a kit for immunohistochemically testing ATRX protein expression level of tumor of glioma patients and an operating instruction thereof if necessary; and (3), a kit for immunohistochemically testing IDH1-R132H protein expression of tumor of glioma patients and an operating instruction thereof if necessary. Progression free survival (PFS) of WHO II-level and III-level of glioma is typed according to loss states of IDH1-R132H and ATRX; patients with both loss of IDH1-R132H and ATRX has longer PFS than patients with both expression of IDH1-R132H and ATRX, and PFS of patients with IDH-WT is close to PFS of patients with glioblastoma.
Owner:江涛 +2
Patient selection for enhancement of Anti-tumor immunity in cancer patients
PendingUS20220175787A1Enhance immune activationPromote antitumor immunityMicrobiological testing/measurementAntibody ingredientsChemotherapy combinationsPharmaceutical medicine
A method for increasing the progression free survival or overall survival of a patient with cancer comprising: determining if the cancer has a surrounding microenvironment that is favorable to immune modulation; determining if the chemotherapy regimen induces immunogenic cell death, and if both are yes, administering an effective amount of a CDK 4 / 6 inhibitor selected from Compounds I, II, III, IV, or V, or a pharmaceutically acceptable salt thereof, wherein the CDK4 / 6 inhibitor is administered prior to the administration of the chemotherapy or optionally prior to and concurrently with chemotherapy; and, wherein the increase in progression free survival or overall survival is in comparison to the progression free survival or overall survival based on administration of the chemotherapy alone, either based on literature or otherwise publicly available evidence, a comparative during preclinical or clinical trials, or other means accepted by persons skilled in the field.
Owner:G1 THERAPEUTICS INC
Treatment of PD-L1-negative melanoma using an anti-PD-1 antibody and an anti-CTLA-4 antibody
ActiveUS10174113B2Shrink tumorIncrease ratingsBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsProgression-free survivalMelanoma
The invention provides a method of treating a melanoma comprising (i) identifying a patient having a PD-L1-negative melanoma and (ii) administering to the patient a combination of an anti-PD-1 antibody or an antigen-binding portion thereof and an anti-CTLA-4 antibody or an antigen-binding portion thereof. The methods of the invention can extend progression-free survival for over 8 months and / or reduces the tumor size at least about 10%, about 20%, about 30%, about 40%, or about 50% compared to the tumor size prior to the administration.
Owner:BRISTOL MYERS SQUIBB CO
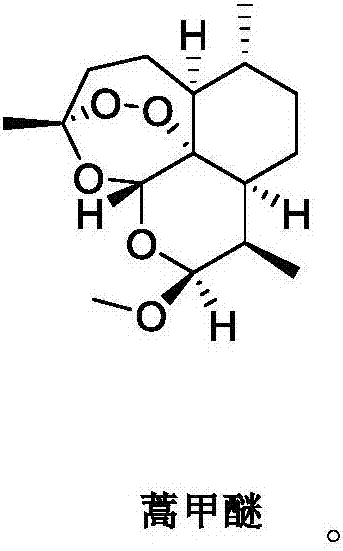
Applications of artemisinin compounds in preparing medicines for treating liposarcoma
ActiveCN107569482AOrganic active ingredientsAntineoplastic agentsProgression-free survivalLife quality
The invention relates to the field of medicines, and in particular relates to applications of artemisinin compounds in preparing medicines for treating liposarcoma. The artemisinin compounds are shownin the description. The experiment proves that the compounds can obviously prolong the progression free survival (PFS) of patients and improve the life quality of the patients, while the number of circulating tumor cells (CTCs) is obviously reduced, the ratios of T cell subsets in the peripheral blood are obviously improved, in addition, the safety is high, and the applications are disclosed forthe first time and have the outstanding substantive features. The substances can be applied to preparation of medicines for treating liposarcoma and have good market application prospects.
Owner:北京维恩派科技有限公司
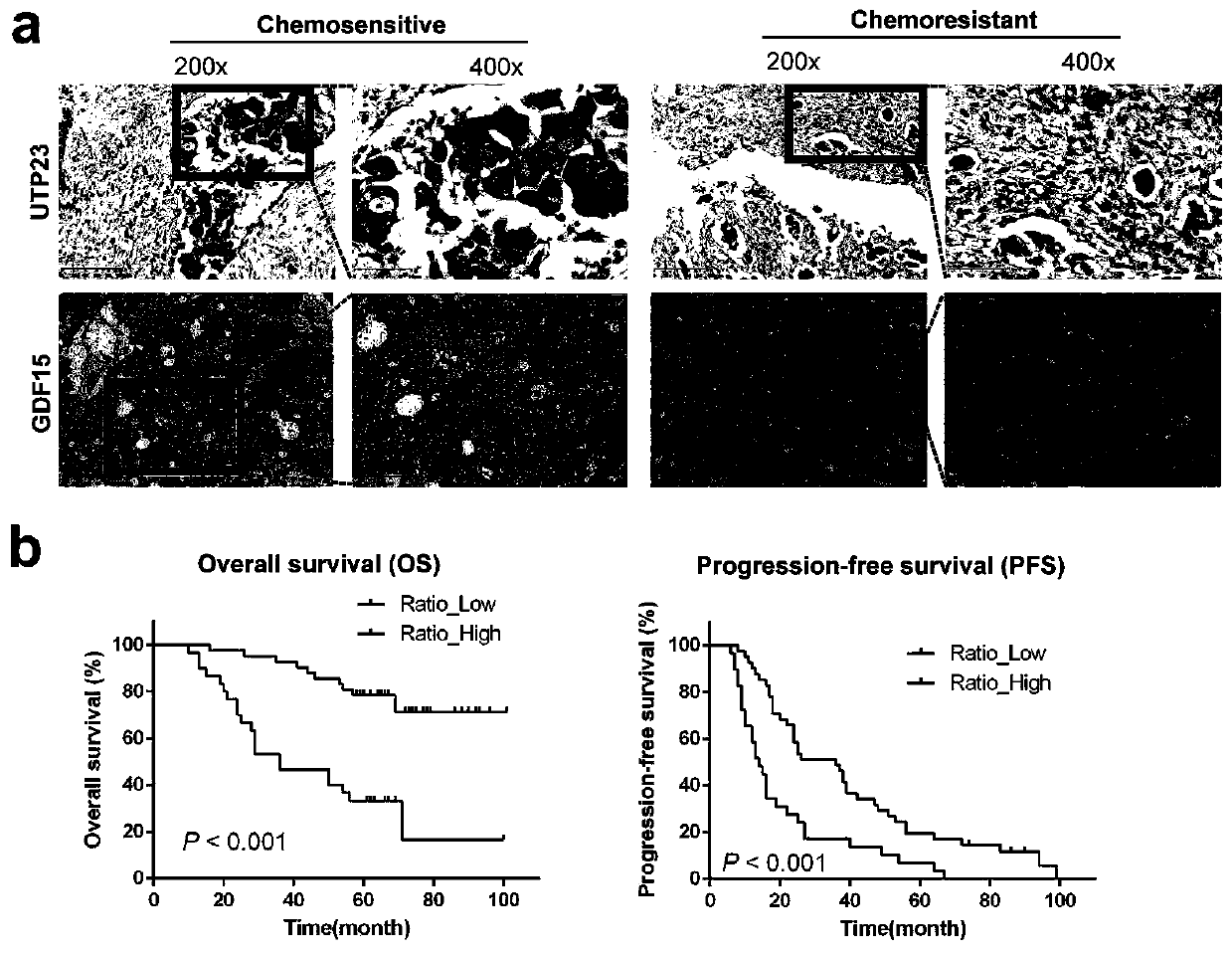
Ovarian cancer prognosis marker combination and application thereof
PendingCN110846414AImproved prognosisMicrobiological testing/measurementBiological testingGood prognosisCancer research
The invention discloses an ovarian cancer drug-resistance marker UTP23, an expression ratio of an existing gene GDF15 and related prognosis of ovarian cancers. Particularly, when the ratio is low, anovarian cancer case shows poor prognosis according to prognostic indicators such as chemotherapy sensitivity, drug resistance, satisfactory cytoreductive surgery, progression free survival and overallsurvival. When the ratio is high, an ovarian cancer patient shows good prognosis, namely, clinical therapy of the ovarian cancers includes chemotherapy and surgical therapy to provide prognosis prompt.
Owner:ZHEJIANG CANCER HOSPITAL
Method of treating androgen receptor (AR)-positive breast cancers with selective androgen receptor modulator (SARMs)
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; l) prolonging survival of a subject with breast cancer, and / or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Owner:UNIV OF TENNESSEE RES FOUND
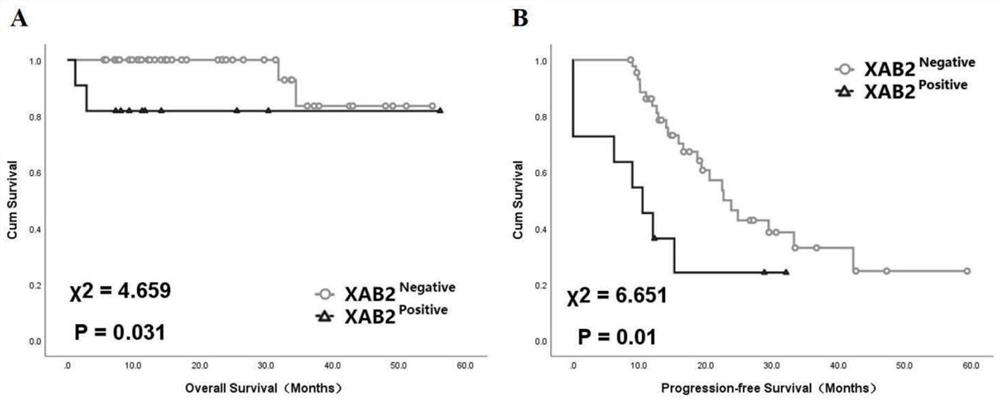
XAB2 protein as marker for prognosis and/or prediction of platinum drug resistance of ovarian cancer
PendingCN111781358AShort risk of platinum resistanceShort survival rateDisease diagnosisBiological testingCancer cellSurvival ratio
The invention belongs to the technical field of biomedicine, and particularly discloses application of XAB2 protein as a marker for prognosis and / or prediction of platinum drug resistance of ovarian cancer. The invention discloses a protein-XAB2 closely related to generation of platinum drug resistance of ovarian cancer cells; XAB2 protein expression in ovarian cancer tissues is detected through an immunohistochemical method, ovarian cancer prognosis is judged, and / or platinum drug resistance is predicted. The expression level of the XAB2 protein in ovarian cancer is related to the survival rate of patients, the prognosis of patients with high expression of the XAB2 protein is poor, and the total survival rate and the progression-free survival rate are both reduced. The expression level ofthe XAB2 protein is not only related to prognosis of a patient, but also high in expression level of the XAB2 protein is related to platinum drug resistance of an ovarian cancer patient, and the riskof platinum drug resistance of the patient with high expression level of the XAB2 protein is increased. The XAB2 protein is used as a biomarker for prognosis of ovarian cancer and / or prediction of platinum drug resistance, and has broad application prospects and huge potential social benefits.
Owner:张瑜
Treatment of pd-l1-positive melanoma using an Anti-pd-1 antibody
InactiveUS20200010549A1Shrink tumorBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsStage melanomaProgression-free survival
Owner:BRISTOL MYERS SQUIBB CO
Application of E3 ubiquitination protein ligase UBR5 in preparation of tumor diagnosis or prognosis evaluation kit
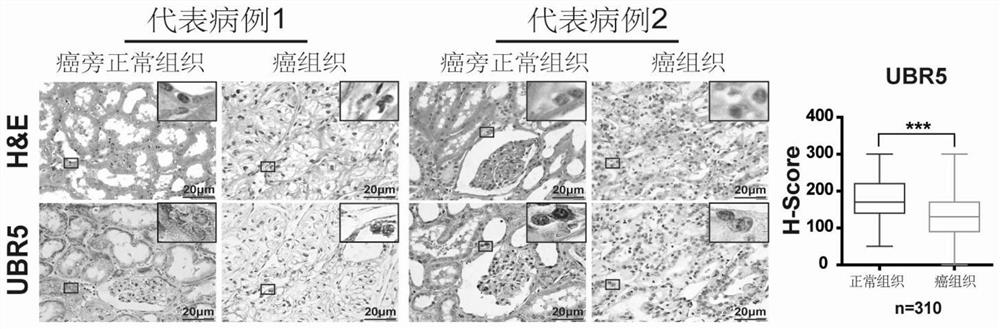
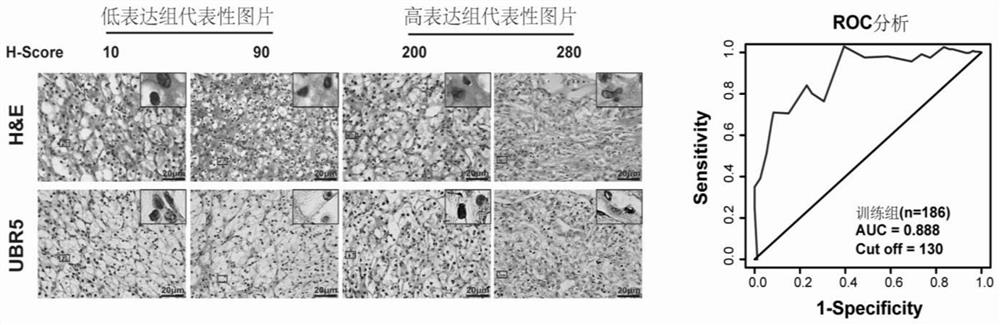
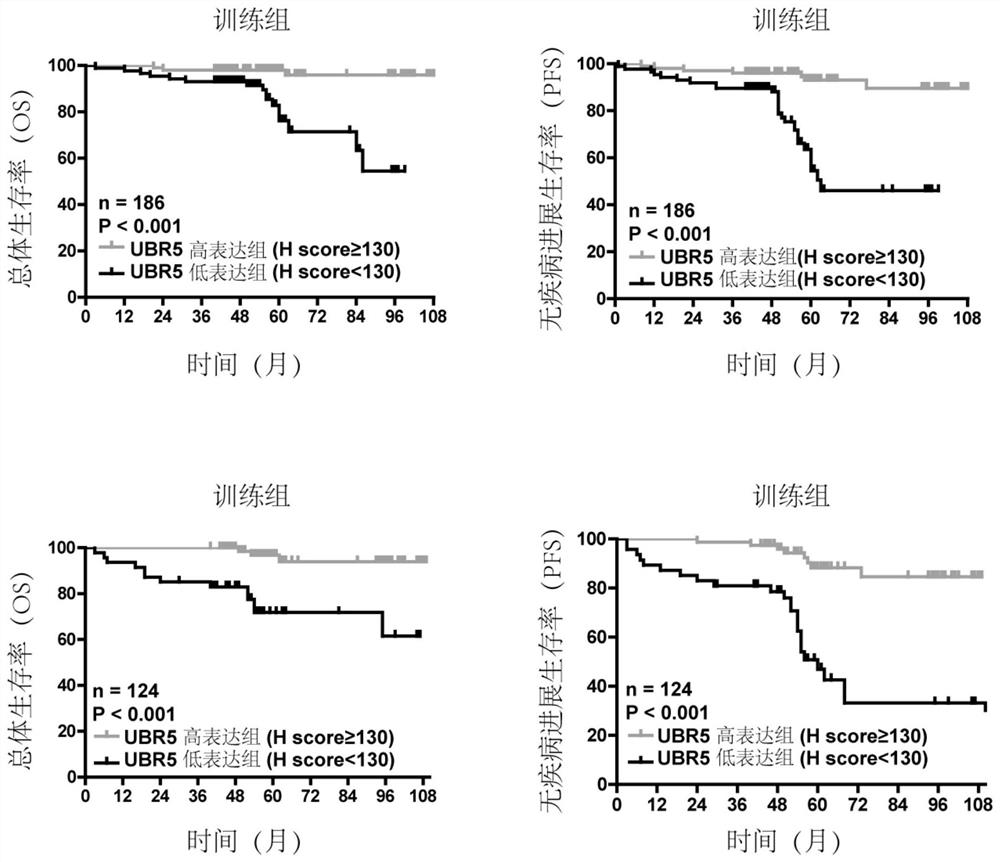
PendingCN111912985APrecise riskAccurate Prognosis PredictionMaterial analysisProgression-free survivalUbiquitinated Proteins
The invention relates to the field of biotechnology and medical diagnosis, in particular to application of E3 ubiquitination protein ligase UBR5 in preparation of a renal clear cell carcinoma diagnosis or prognosis evaluation reagent or kit and a corresponding detection kit. Researches find that the expression of E3 ubiquitination protein ligase UBR5 molecules in patients with renal clear cell carcinoma is generally reduced, the expression level of the E3 ubiquitination protein ligase UBR5 molecules is remarkably related to prognosis of the patients, and the total lifetime (OS) and progression-free lifetime (PFS) of the patients with renal clear cell carcinoma can be better predicted by combining the expression of UBR5.
Owner:SHANGHAI CITY PUDONG NEW AREA GONGLI HOSPITAL
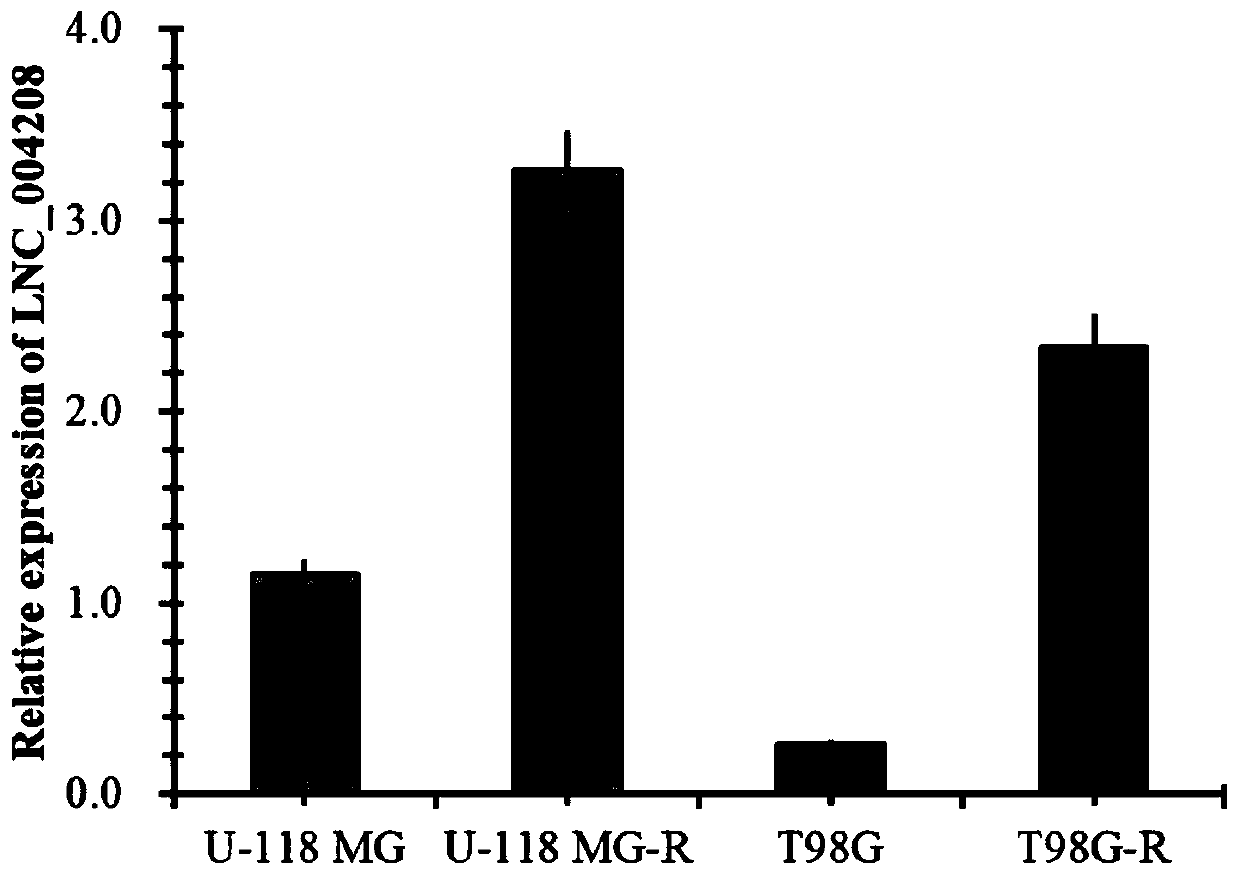
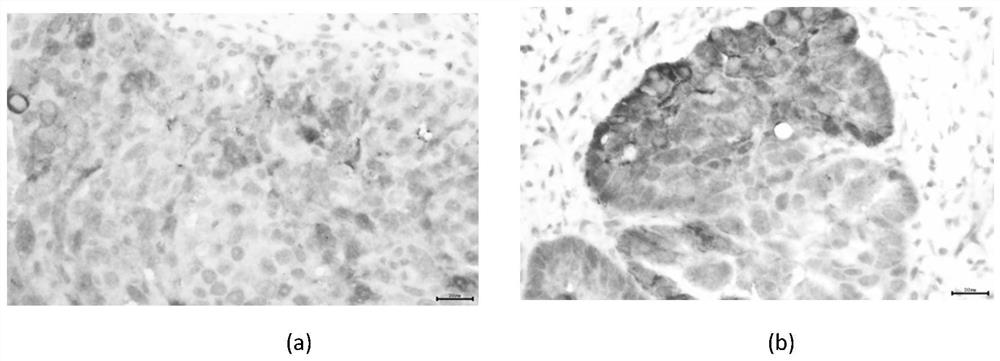
Application of incrnalnc_004208 and its detection reagents in the preparation of glioma prognosis reagents
InactiveCN109679957BMicrobiological testing/measurementDNA/RNA fragmentationDiseaseProgression-free survival
The invention discloses an application of IncRNA LNC_004208 and detection reagent thereof in preparing a glioma prognosis reagent. Through research on and utilization of IncRNA sequencing, the invention finds that IncRNA LNC_004208 has ultrahigh expression in a temozolomide-resisted glioma cell line; through telephone follow-up for 104 glioma patients, detailed inquiry for their first onset time,level, treatment condition, relapse situation, other diseases, other drugs, relapse time, death time, and the like, and registration for survival time and state, a result shows that average survival time and progression free survival of high expression patients are obviously shorter than those of low expression or no expression patients and are related to reactivity of temozolomide; thus, LNC_004208 is a molecular marker related to glioma prognosis; IncRNA LNC_004208 is high in expression; prognosis of patients is poor.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
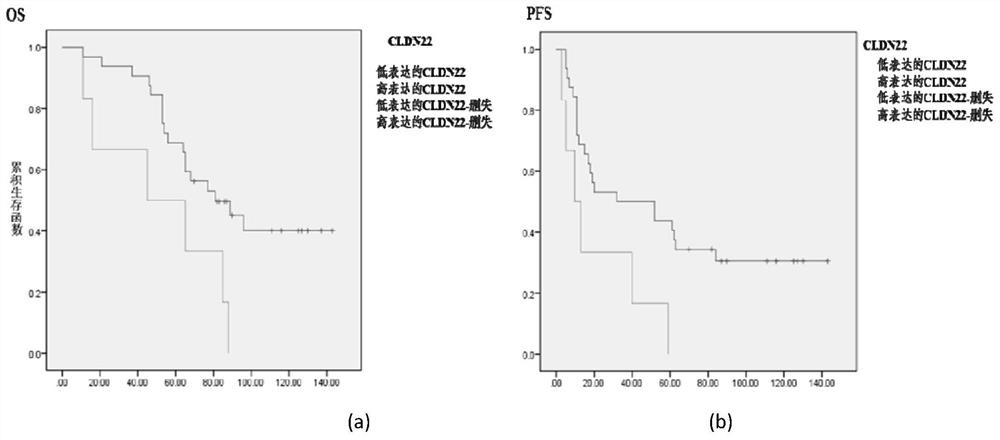
Prognostic diagnosis marker Claudin 22 of ovarian cancer and application of prognostic diagnosis marker Claudin 22
ActiveCN112229998ATumor differentiation degree predictionBiological material analysisSurgical treatmentProgression-free survival
The invention relates to a prognosis marker Claudin 22 of ovarian cancer and application thereof, and firstly finds that the expression quantity of the ovarian cancer drug-resistant marker Claudin 22is related to the prognosis of ovarian cancer, specifically, in prognosis indexes such as no progression lifetime and total lifetime when Claudin 22 is highly expressed, ovarian cancer cases show poorprognosis, and prognosis accuracy is high. The discovery of the prognosis marker Claudin 22 provides prognosis prompts for clinical treatment of ovarian cancer, including chemotherapy and surgical treatment.
Owner:ZHEJIANG CANCER HOSPITAL
Combination Treatment with VEGF-C Antagonists
InactiveUS20130344065A1Prevent and ameliorate metastasisNervous disorderPeptide/protein ingredientsProgression-free survivalGlioblastoma
The invention relates to a method and kit for treating cancer in a human subject, the method comprising administering to the subject in combination therapeutically effective amounts of a VEGF-C antagonist and an anti-neoplastic composition, and the kit comprising a VEGF-C antagonist for administering to the subject in combination with an anti-neoplastic composition. The invention further relates to methods for: increasing the duration of survival of, increasing the progression-free survival of, increasing the duration of response of, or treating, a subject or a group of human subjects susceptible to or diagnosed as having a cancer; or treating a human subject or a group of human subjects having metastatic colorectal cancer, prostate cancer, pancreatic cancer or glioblastoma, the methods comprising administering to the subject or subjects in the group in combination effective amounts of a VEGF-C antagonist and an anti-neoplastic composition.
Owner:VEGENICS PTY LTD
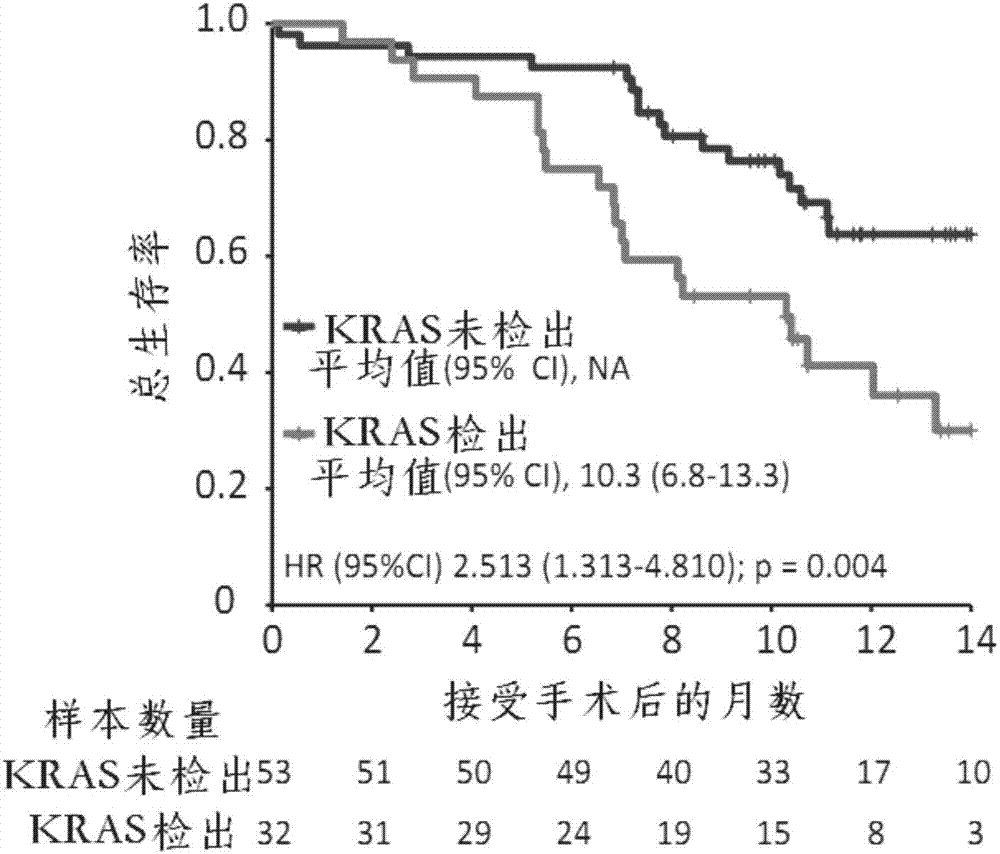
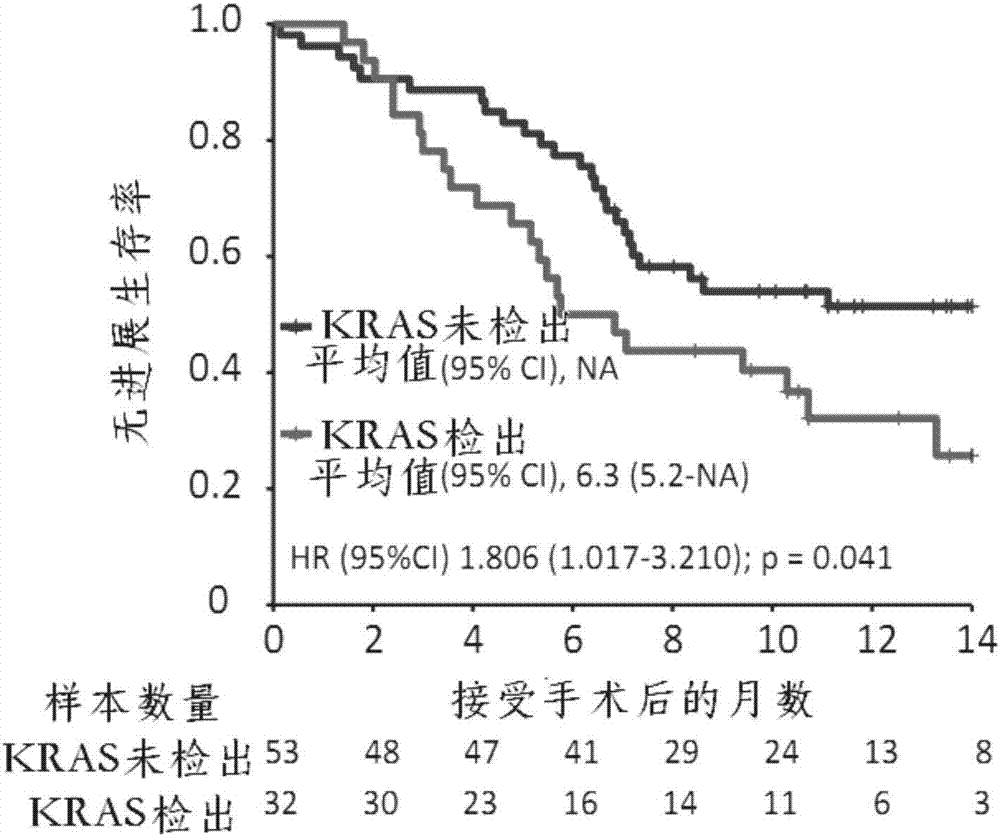
Application of KRAS (kirsten ratsarcoma viral oncogene) serving as biomarker in pancreatic cancer
The invention relates to the technical field of biology, in particular to application of KRAS (kirsten ratsarcoma viral oncogene) serving as a biomarker in pancreatic cancer and provides application of a substance for detecting G12V mutation in KRAS gene or active fragments thereof to preparation of a kit used for assessment of pancreatic cancer treatment effects and / or judgment of pancreatic cancer prognosis. According to discovery of remarkable correlation between KRAS mutation in pre-operation cfDNA and shortening of overall survival and progression-free survival, a prognosis value of KRAS cfDNA assessment is revealed, and a method of how to provide information for clinical treatment of patients suffering from the pancreatic cancer on the basis of cfDNA biomarker detection assistance is founded, and accordingly individual treatment of the pancreatic cancer can be promoted.
Owner:SHANGHAI CHANGHAI HOSPITAL
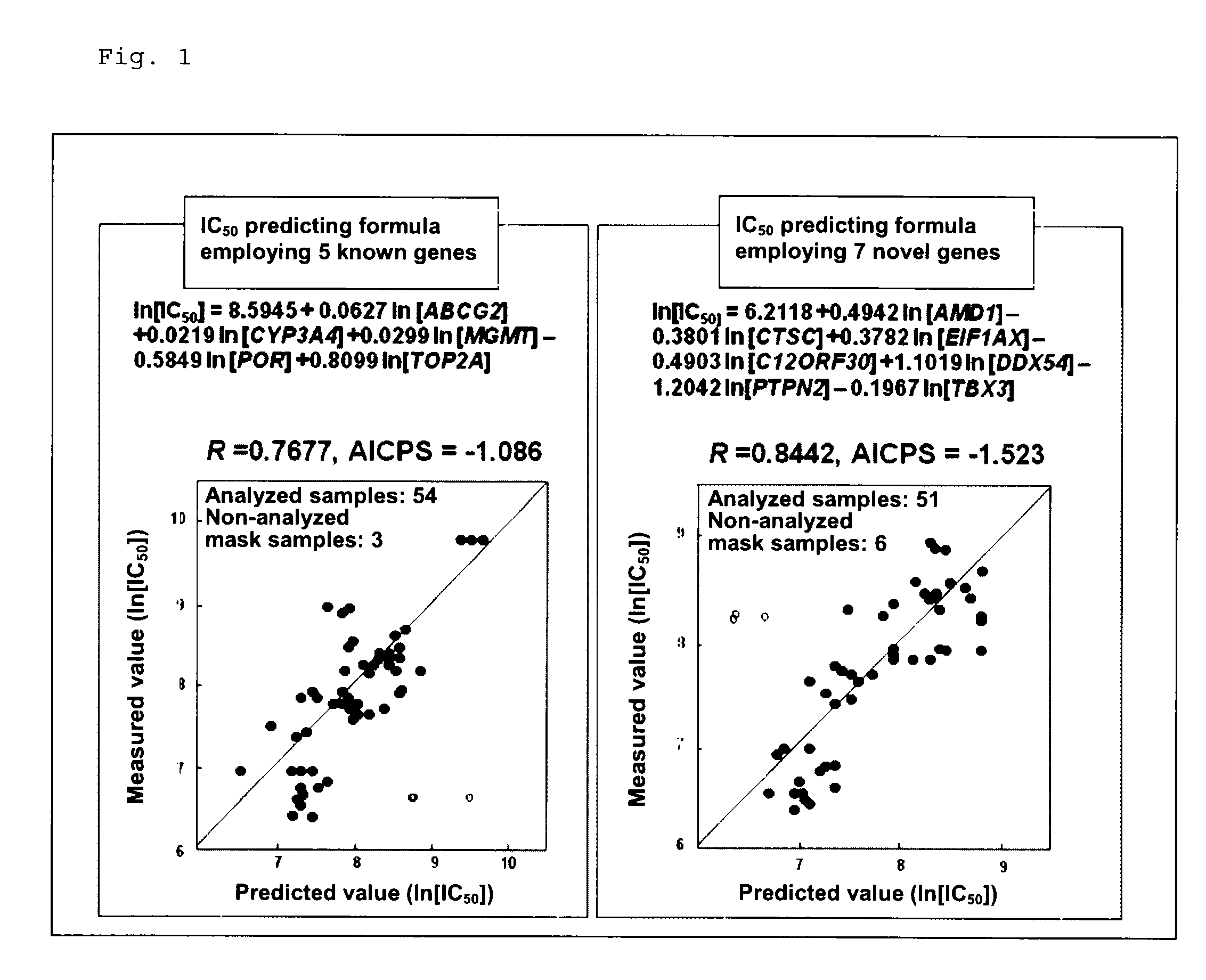
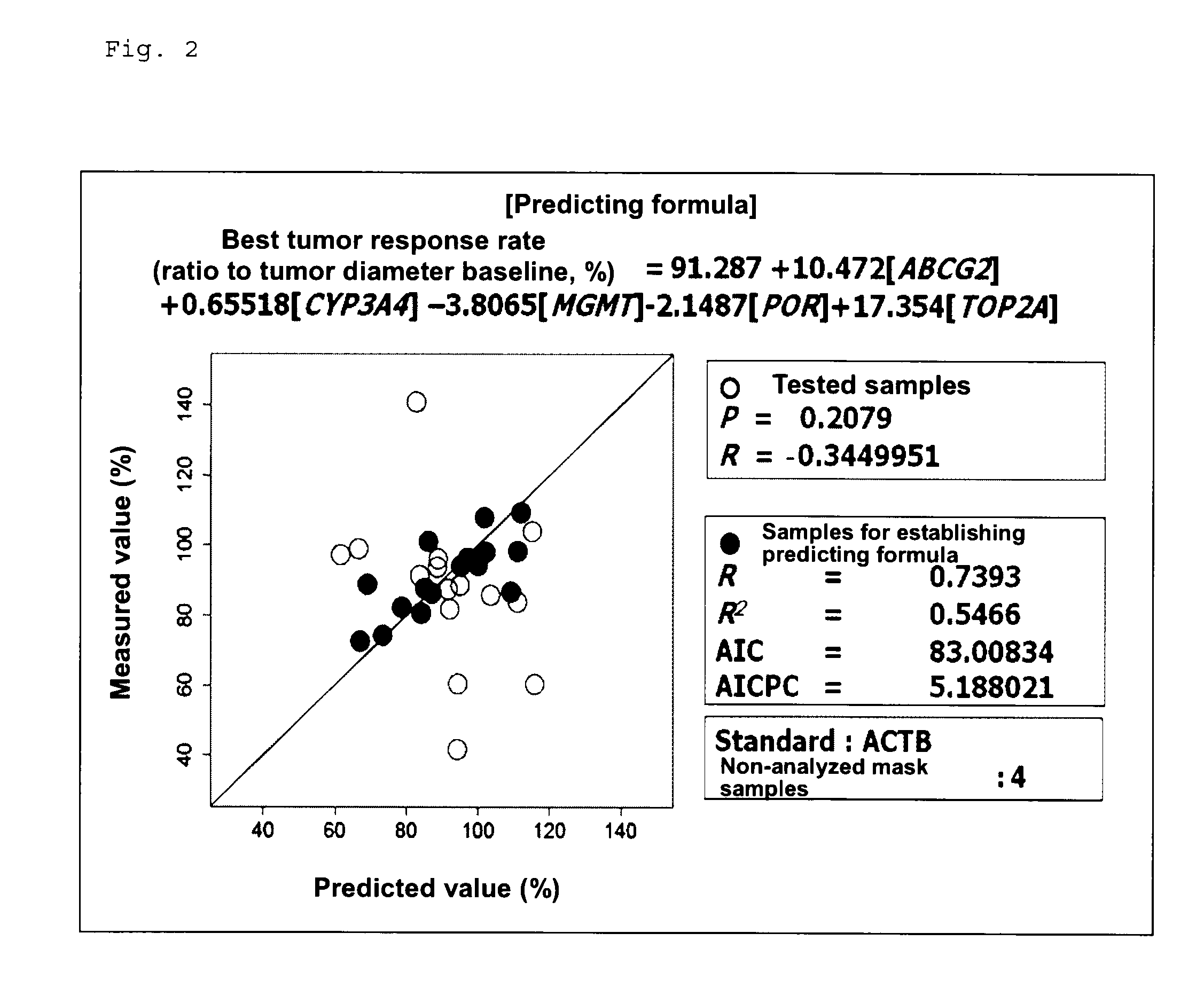
Method for determining sensitivity to irinotecan and use thereof
InactiveUS9107918B2High therapeutic effectReduce the burden onBiocideLibrary screeningProgression-free survivalTumor response
To provide a method for determining the sensitivity of a patient to irinotecan, SN-38, and / or a salt thereof, which method can determine the therapeutic response of the patient and to provide a novel cancer therapeutic means employing the method.The method for determining the sensitivity of a subject to irinotecan, SN-38, and / or a salt thereof includes measuring the expression levels of AMD1 gene, CTSC gene, EIF1AX gene, C12orf30 gene, DDX54 gene, PTPN2 gene, and TBX3 gene in a specimen, and calculating the best tumor response rate (%), overall survival (days), or progression-free survival (days) from formulas (1) to (3).
Owner:YAKULT HONSHA KK
bTMB marker, detection kit and application bTMB marker
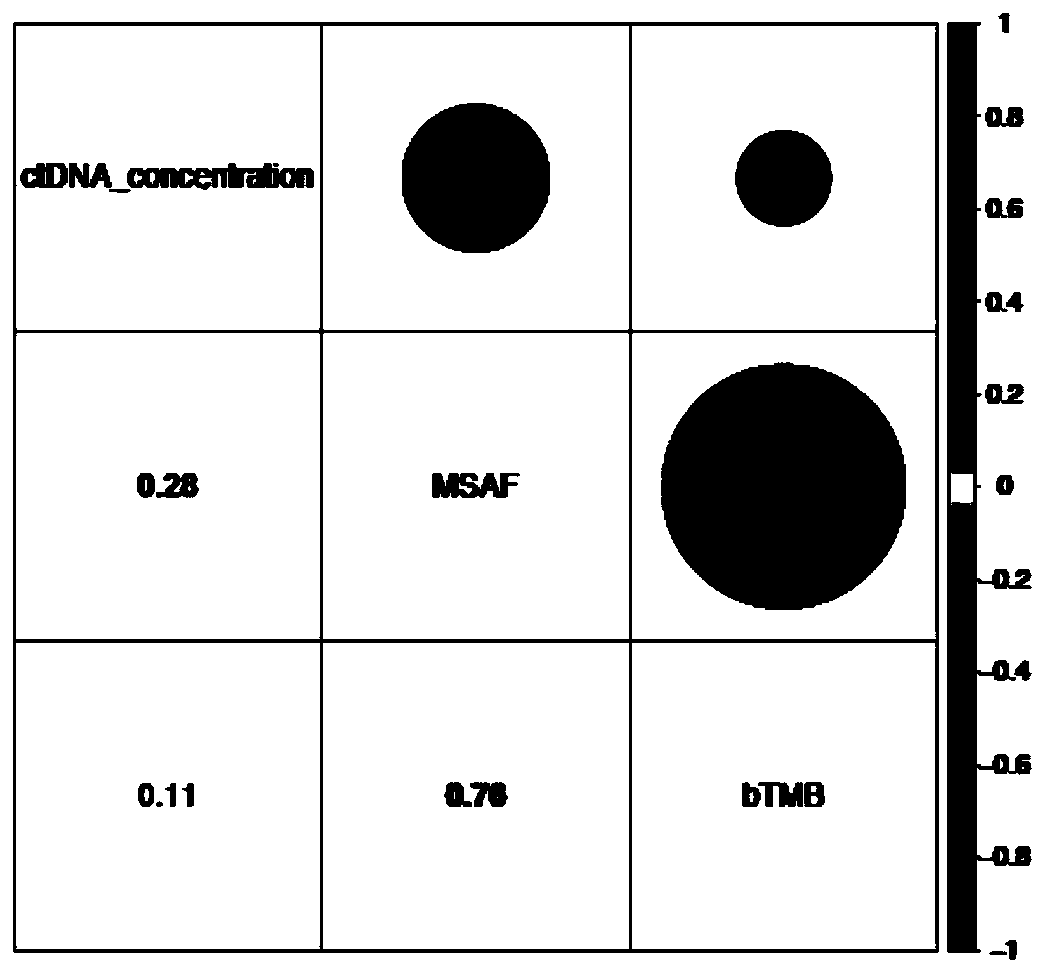
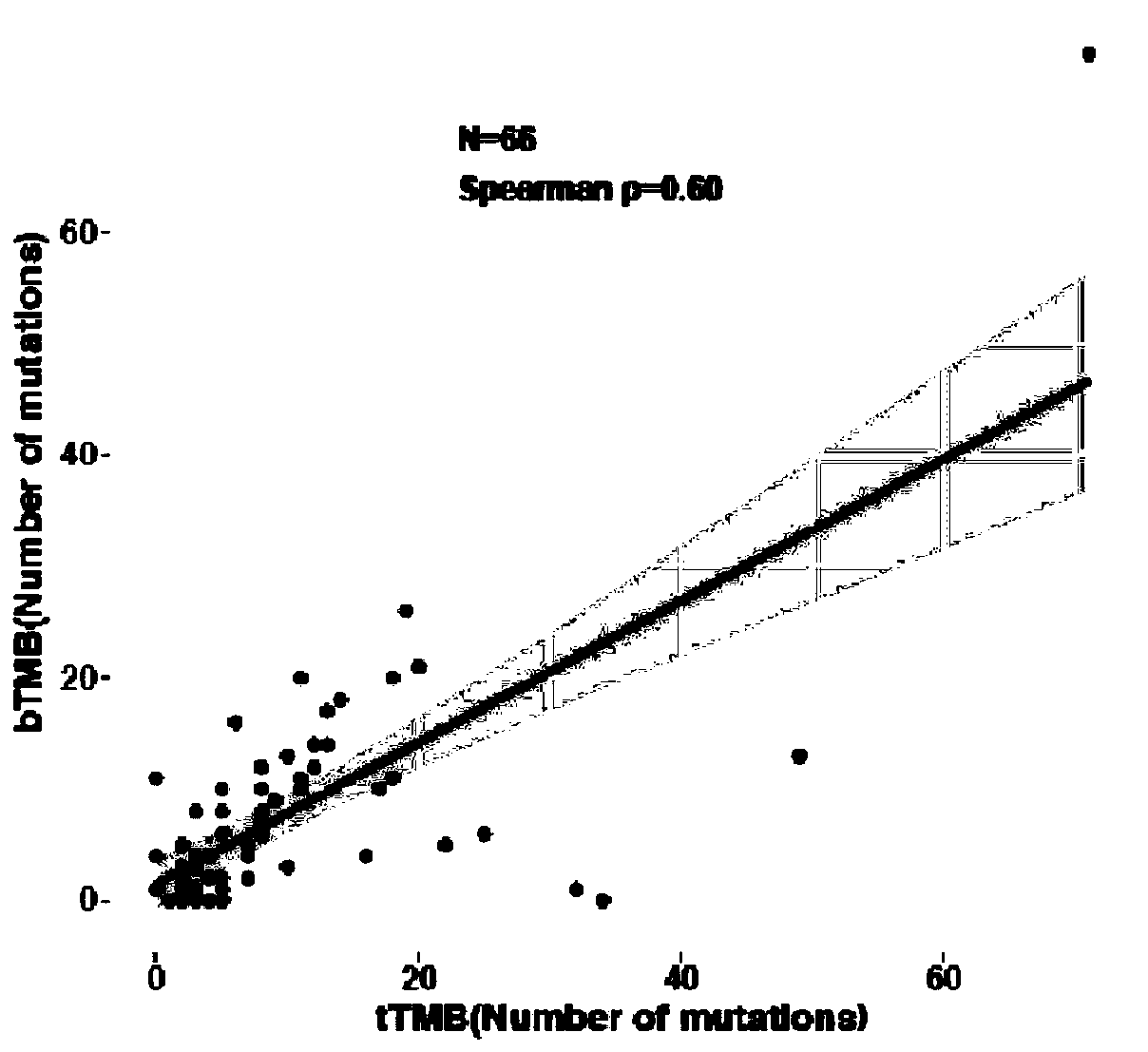
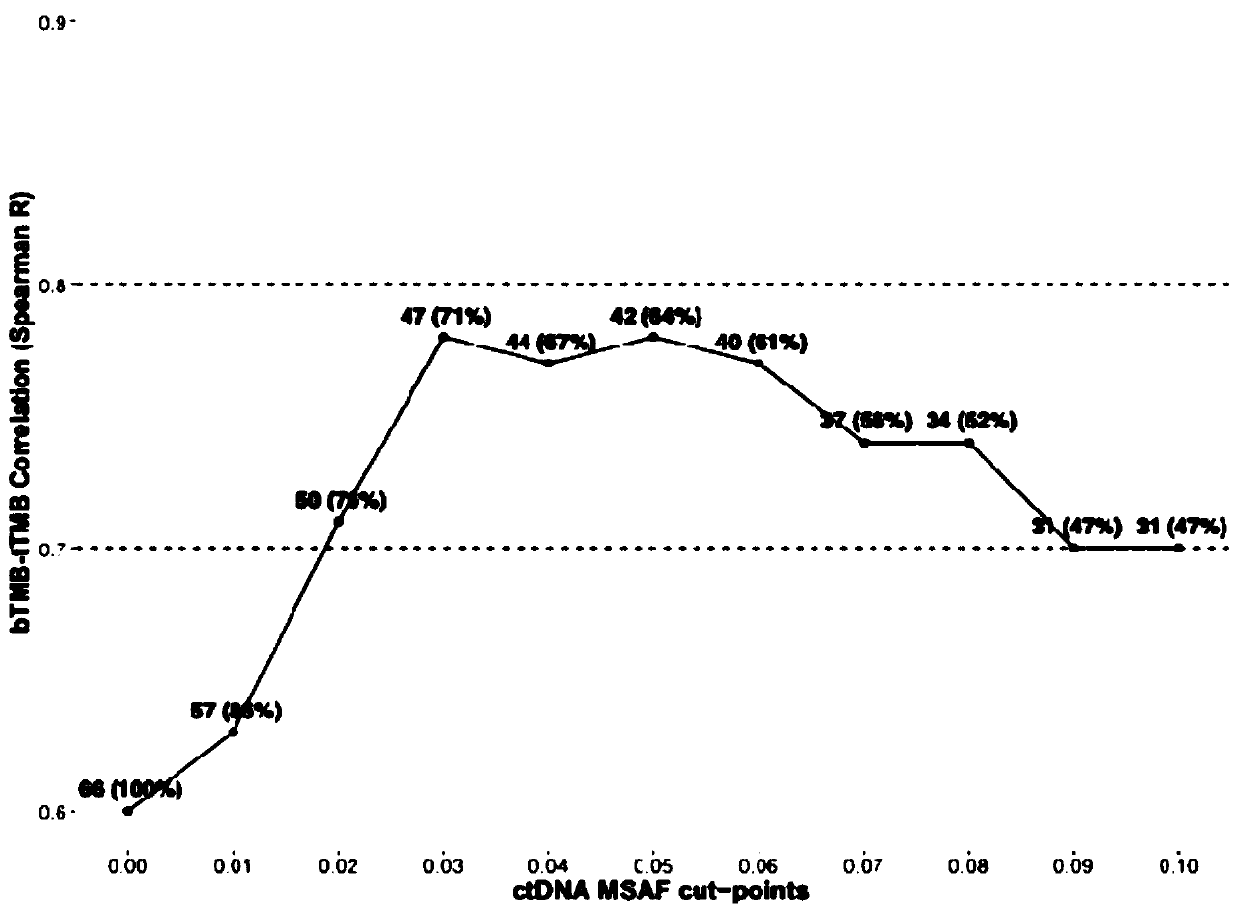
InactiveCN111424084APromote enrichmentBioreactor/fermenter combinationsBiological substance pretreatmentsTherapeutic effectCancer research
The invention relates to a blood tumor mutation burden (bTMB) marker, a detection kit and an application the bTMB marker, and belongs to the technical field of medical molecular biology. The inventionverifies clinical practicability of panel estimated bTMB as a biomarker, and the bTMB can be used for identifying patients responding to immunotherapy. On the other hand, research finds that circulating tumor DNA (ctDNA) MSAF is a new biomarker for predicting the therapeutic effect of immunotherapy. Importantly, low ctDNA release is significantly correlated with progression free survival (PFS) benefits, and comprehensive analysis of MSAF and bTMB can greatly enhance enrichment of potential responders. The research result is based on the liquid biopsy of blood, and the ctDNA has important clinical significance and practicability as a source of molecular markers of cancer immunotherapy.
Owner:GENESEEQ TECH INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com