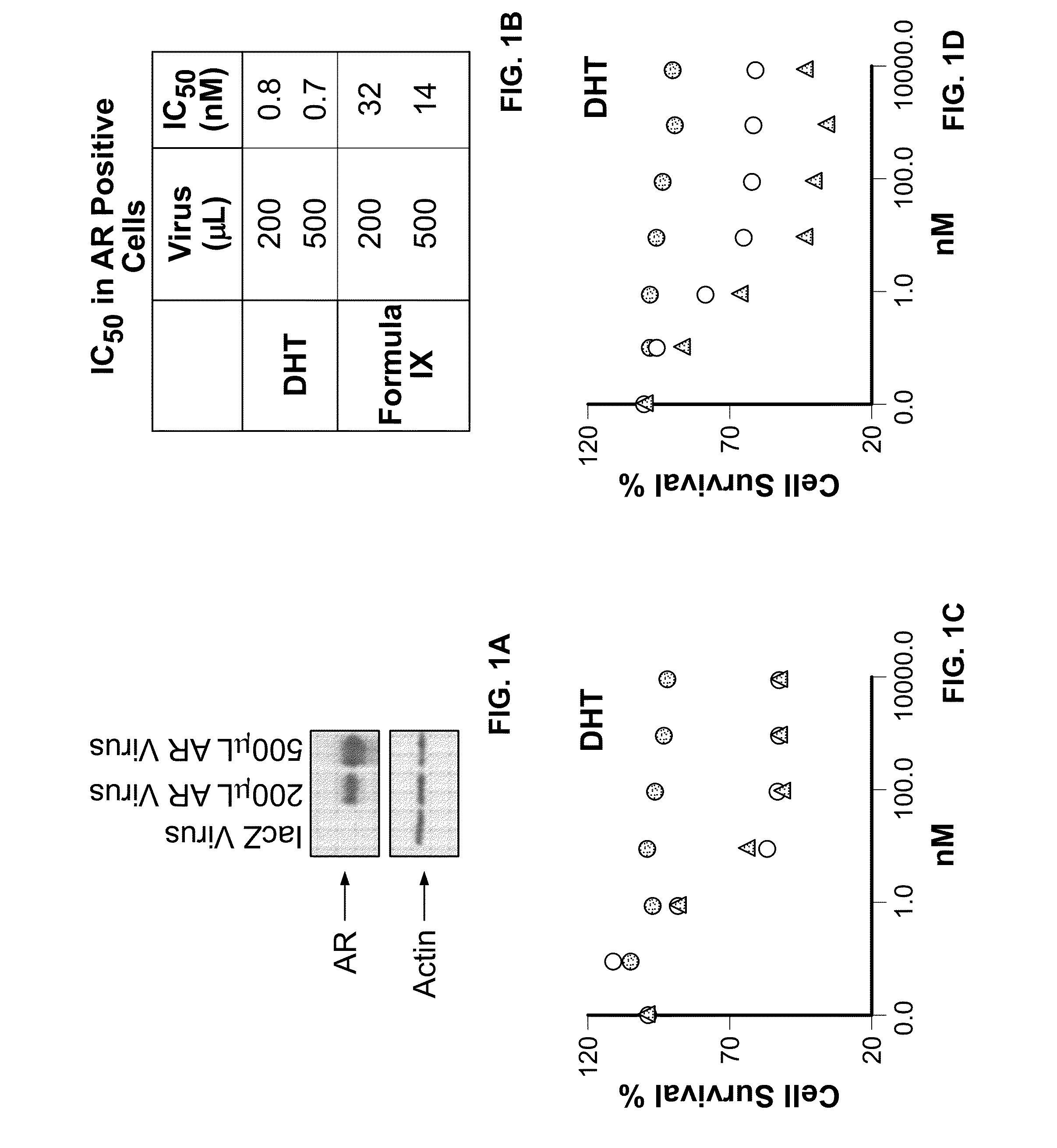
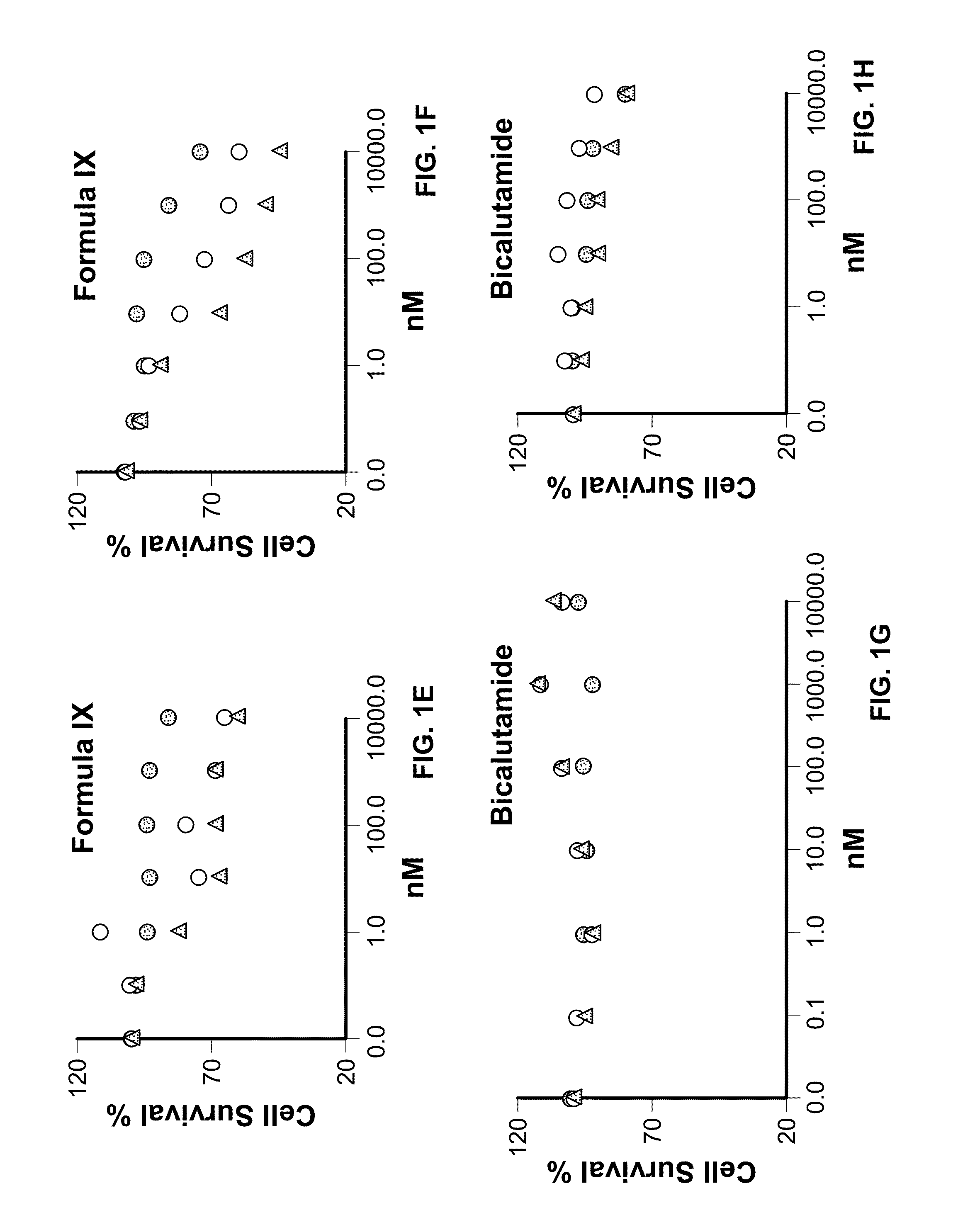
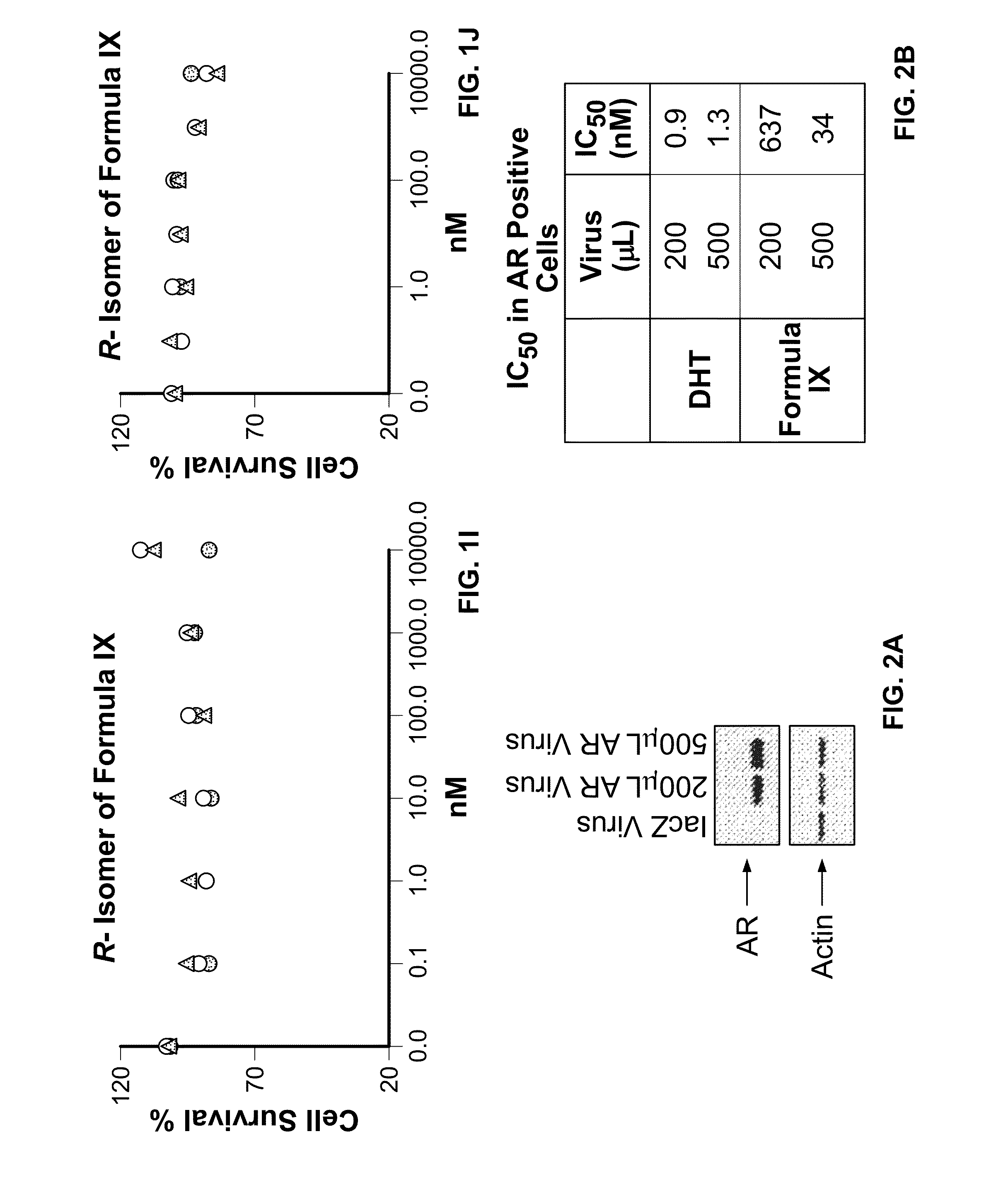
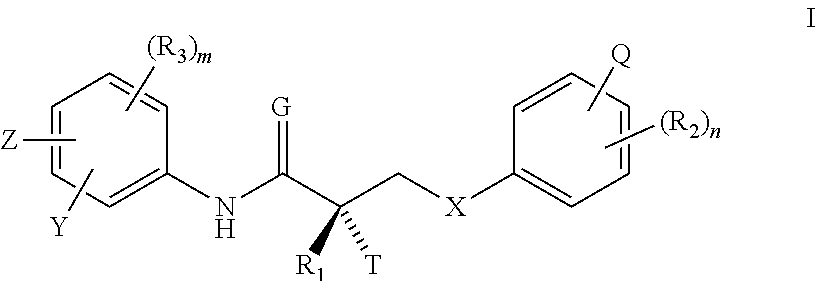
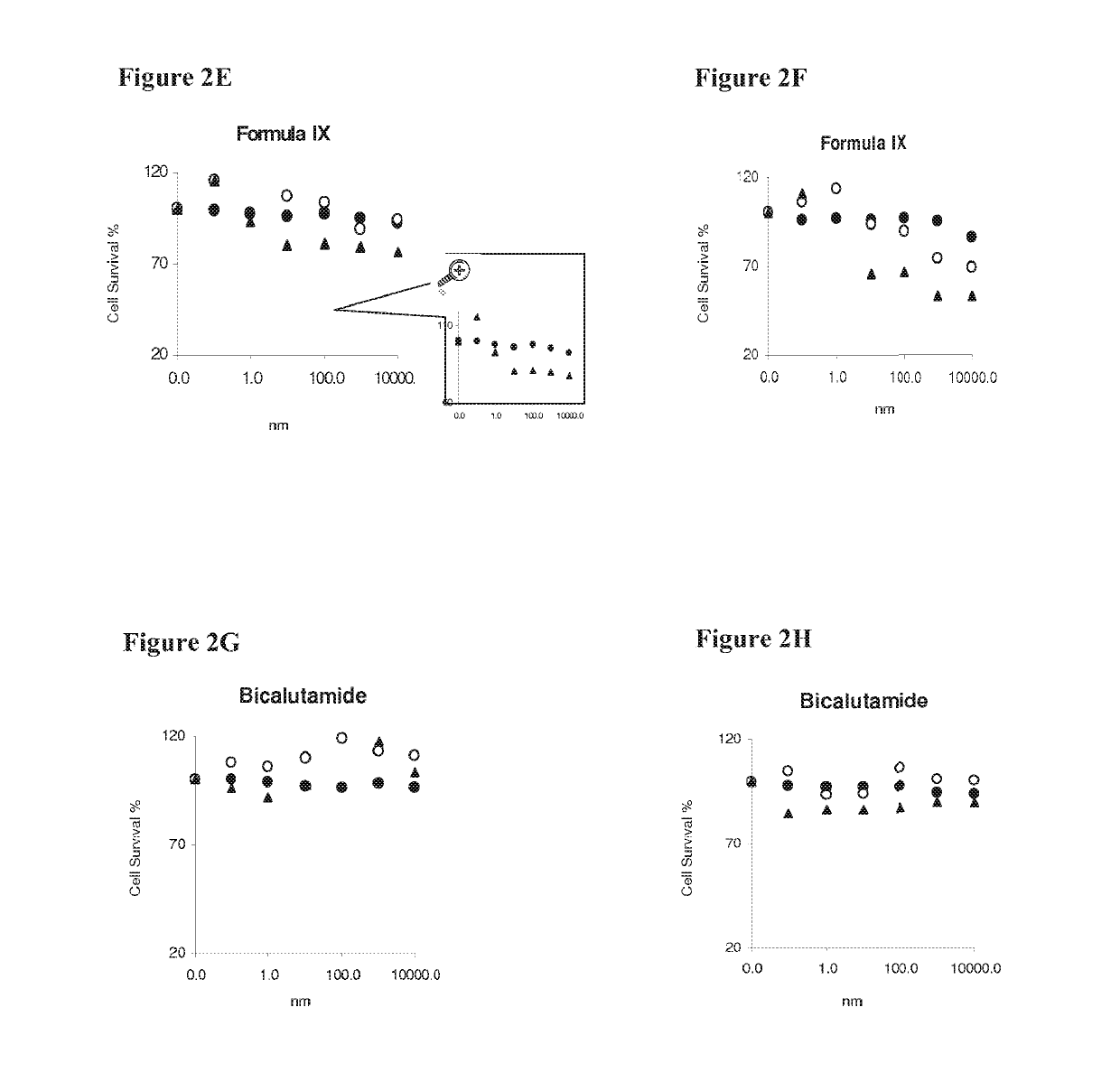
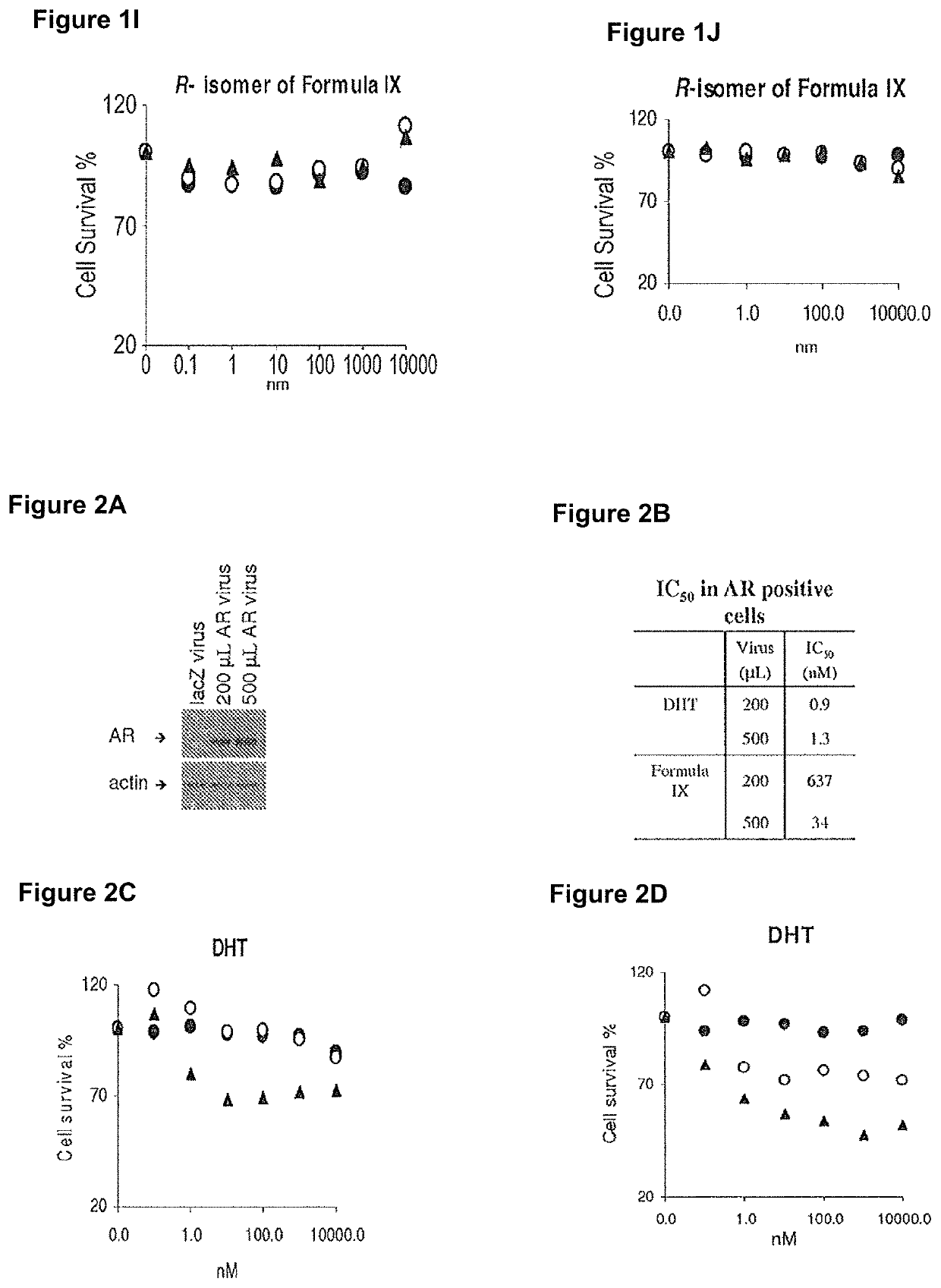
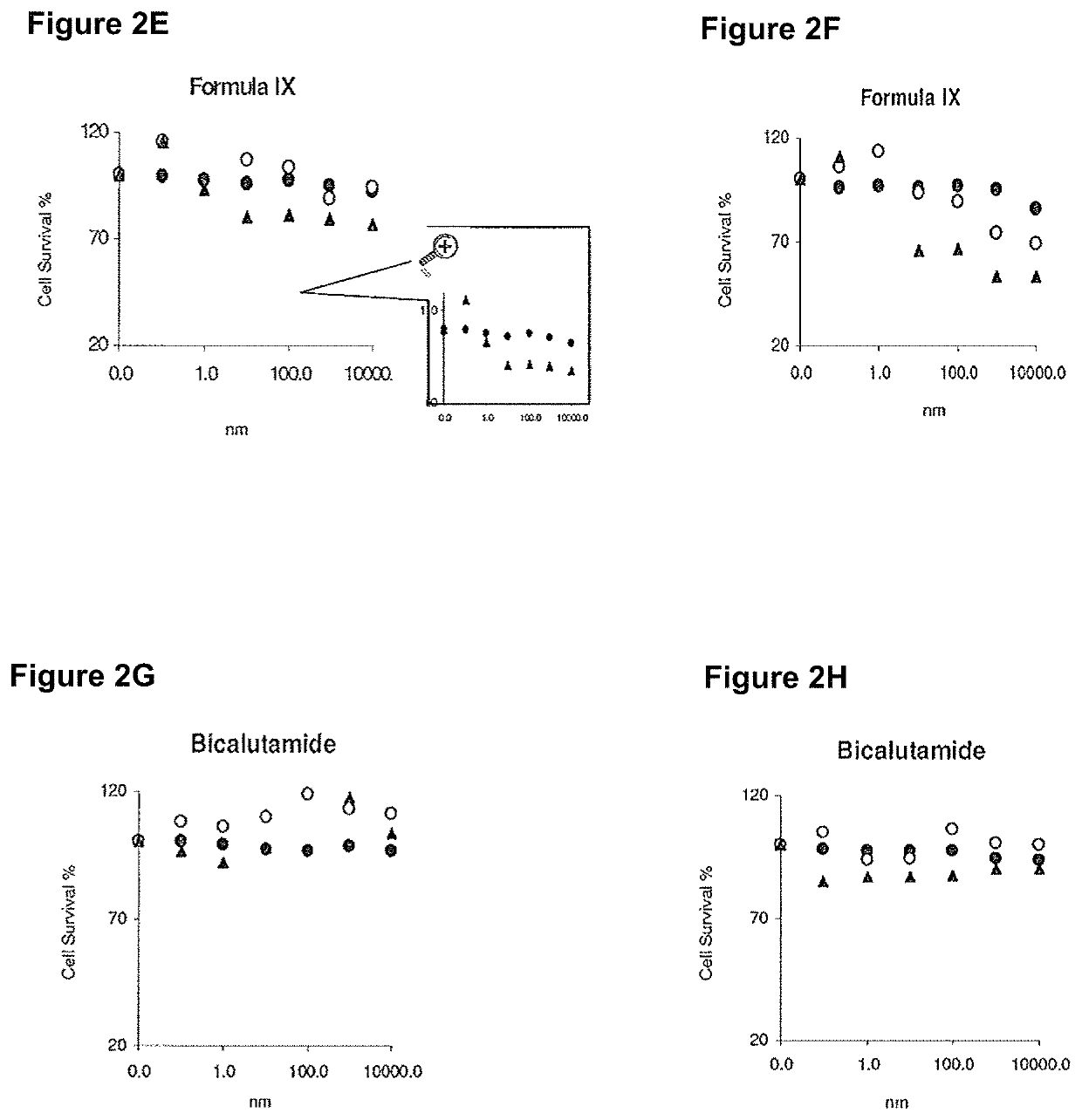
Patents
Literature
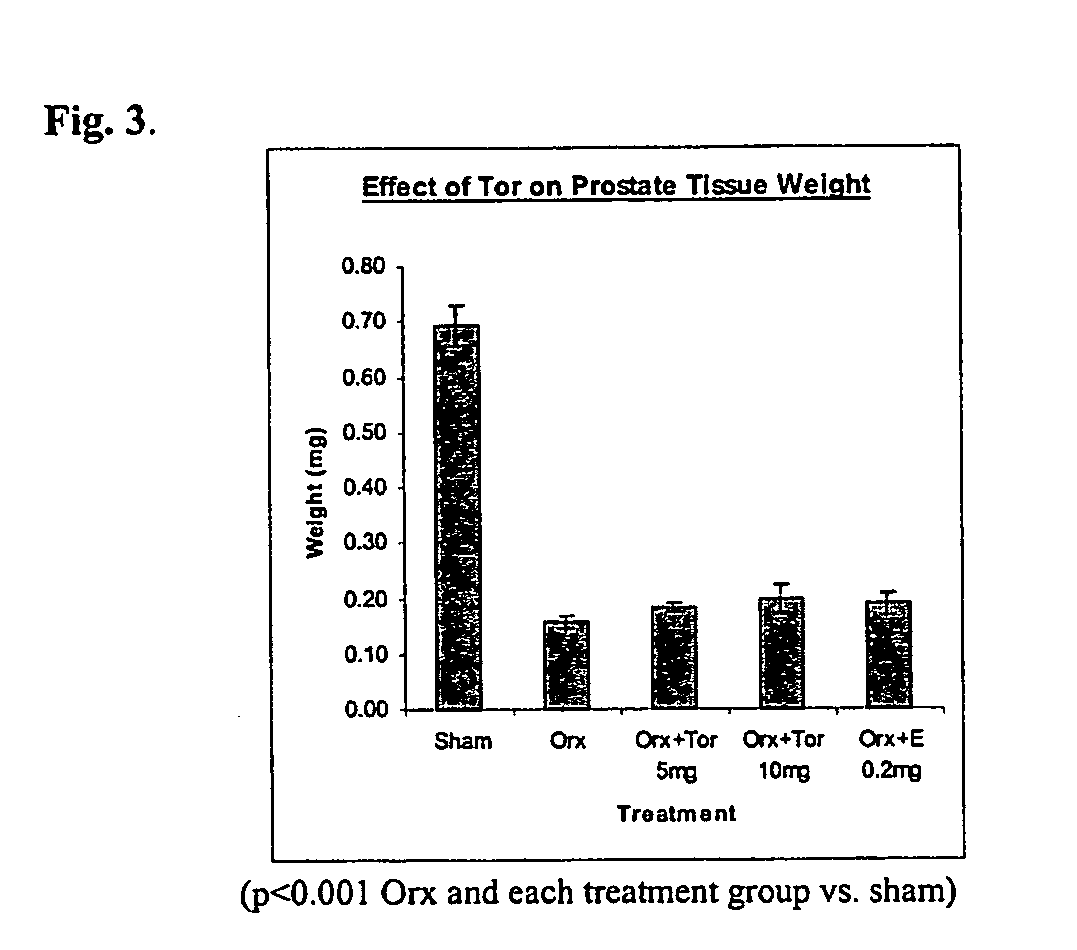
37 results about "Toremifene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
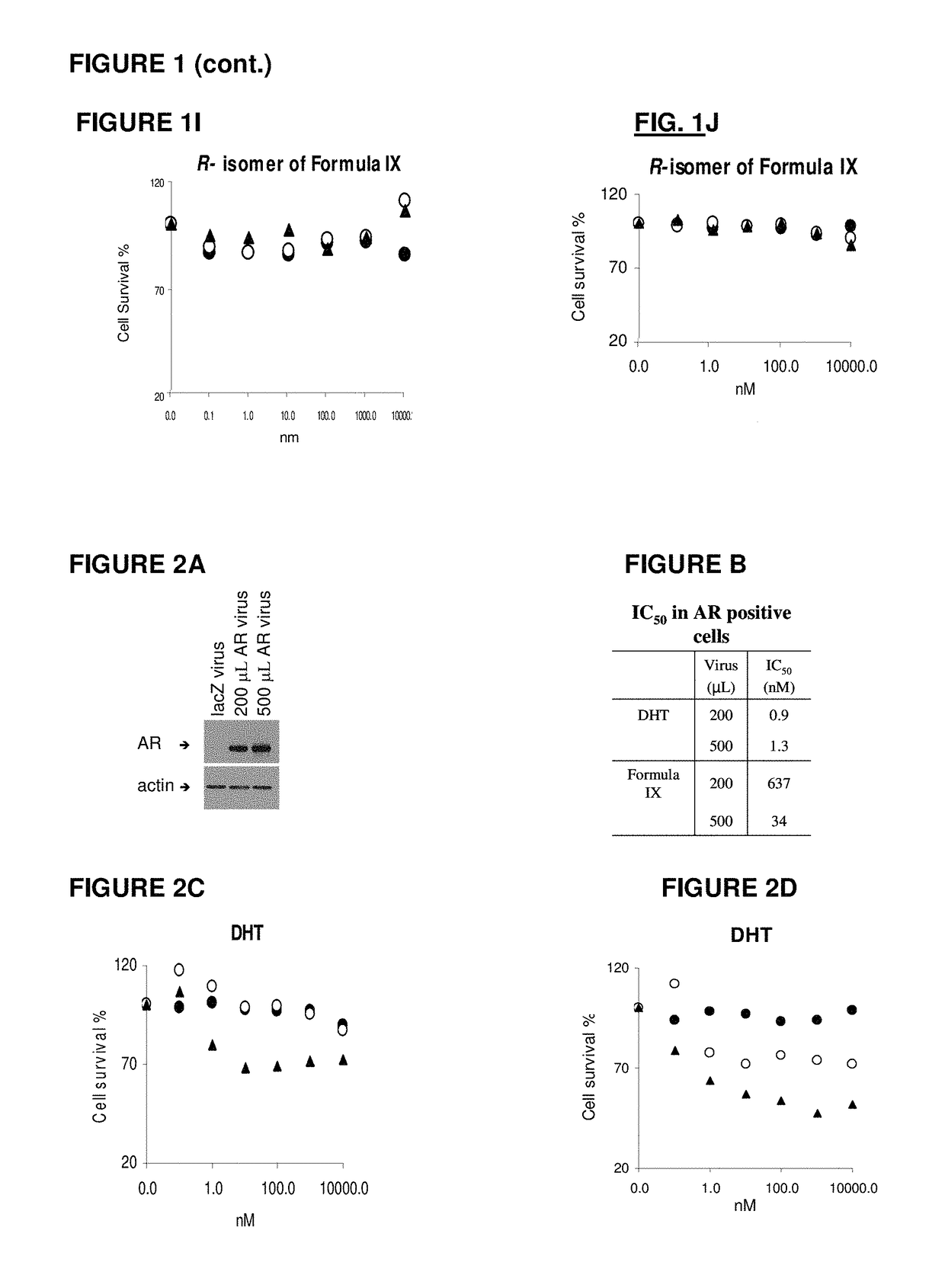
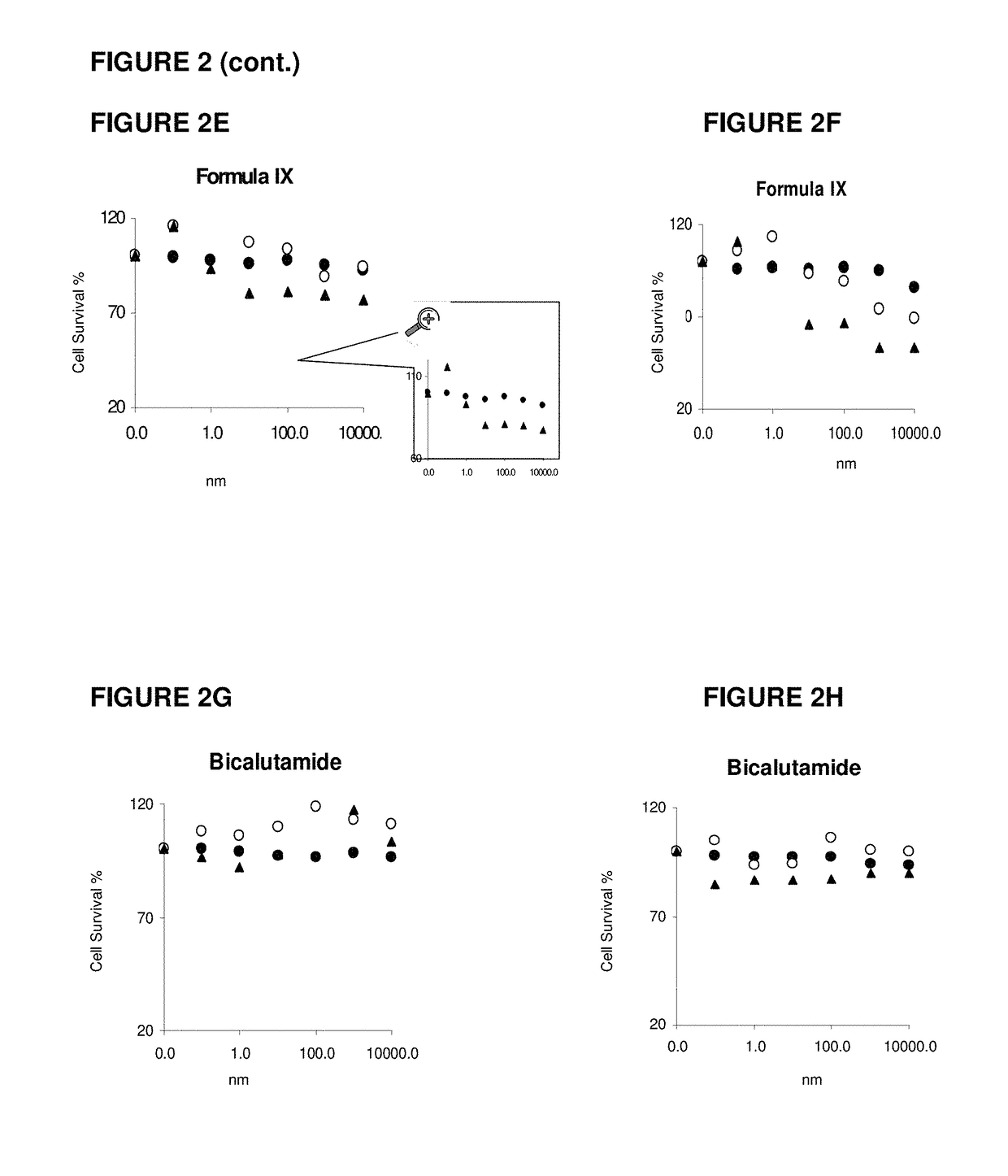
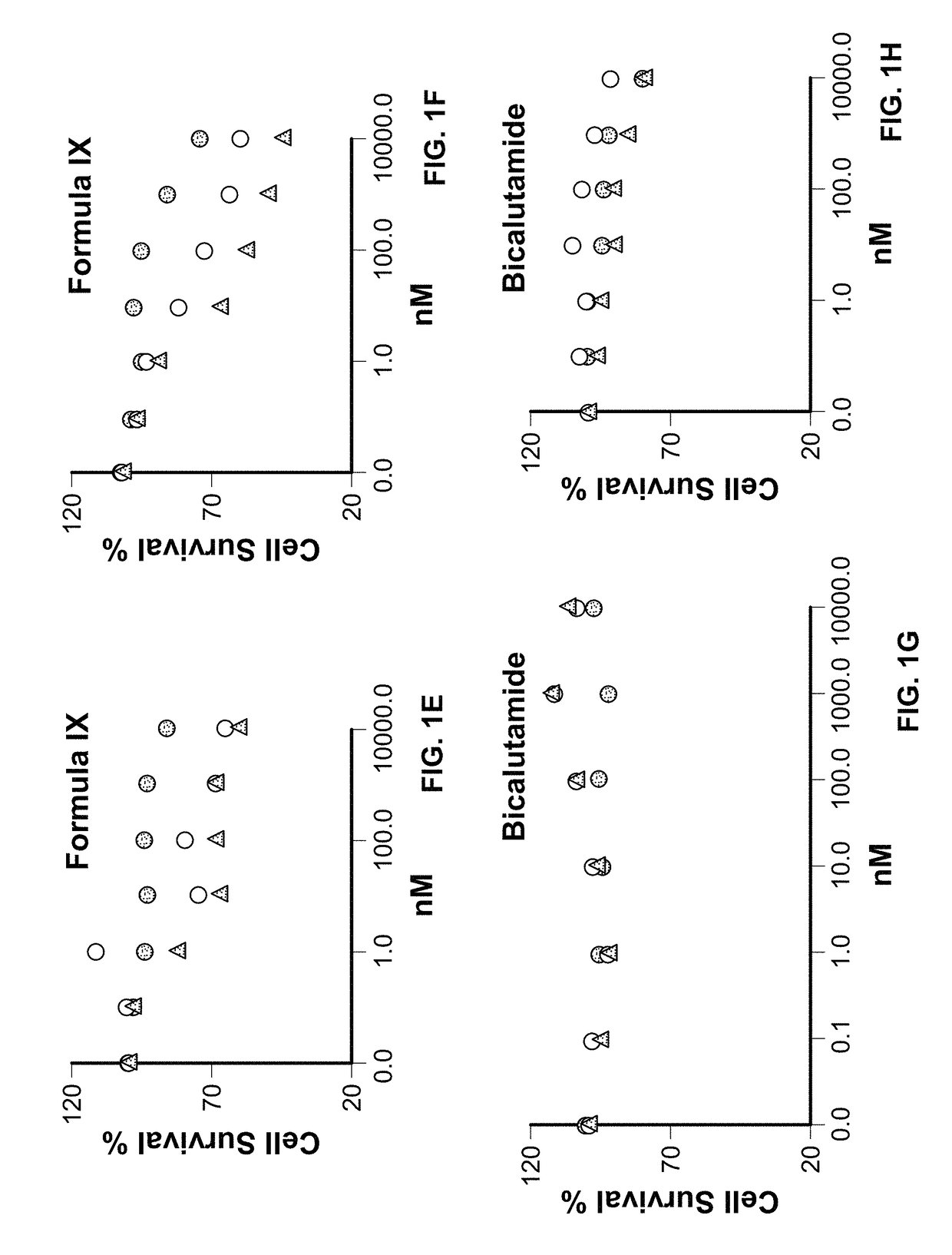
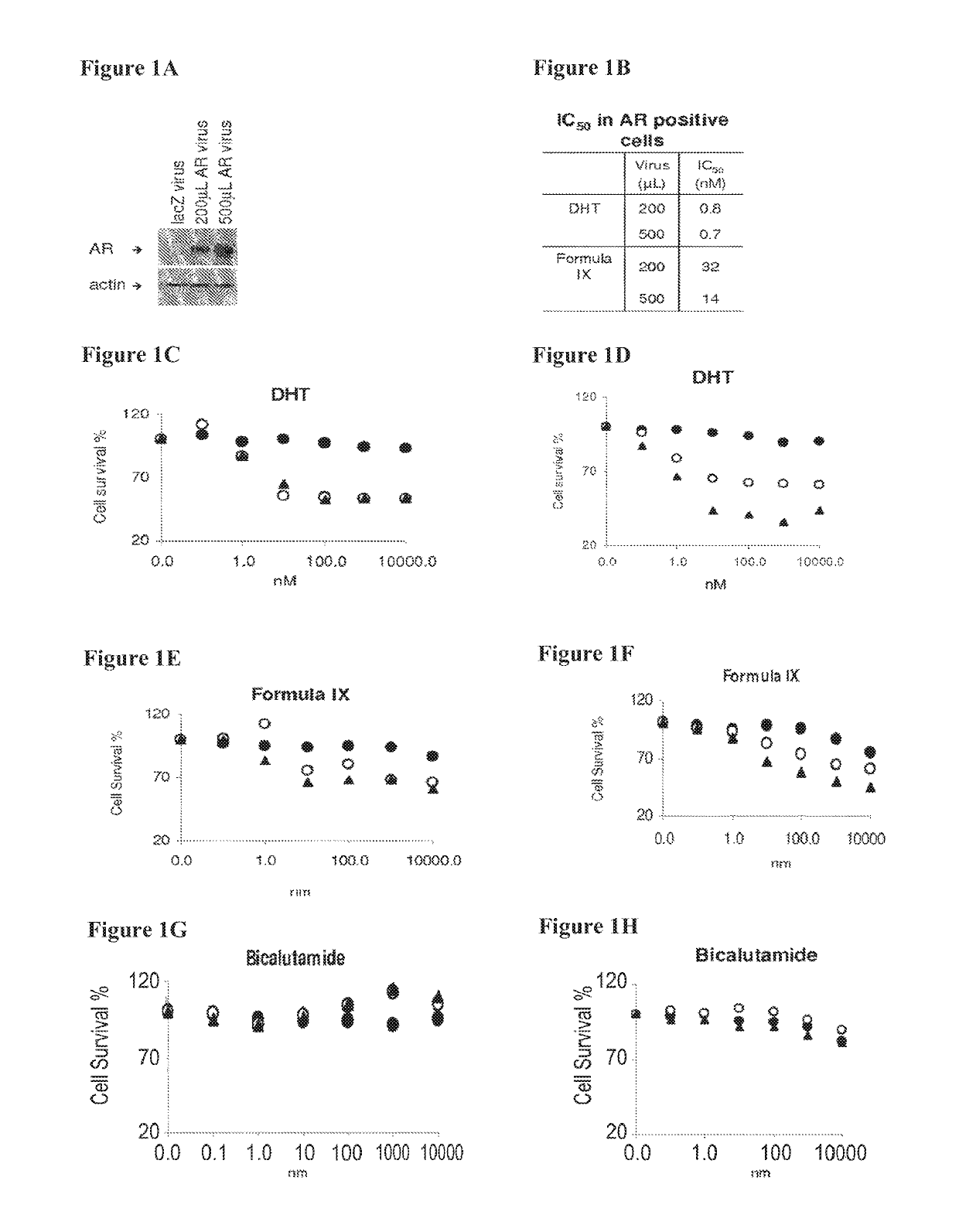
Toremifene is used in postmenopausal women to treat breast cancer that has spread to other parts of the body (metastatic breast cancer). It is usually used to treat cancer that needs estrogen, a female hormone, in order to grow (estrogen-receptor positive).
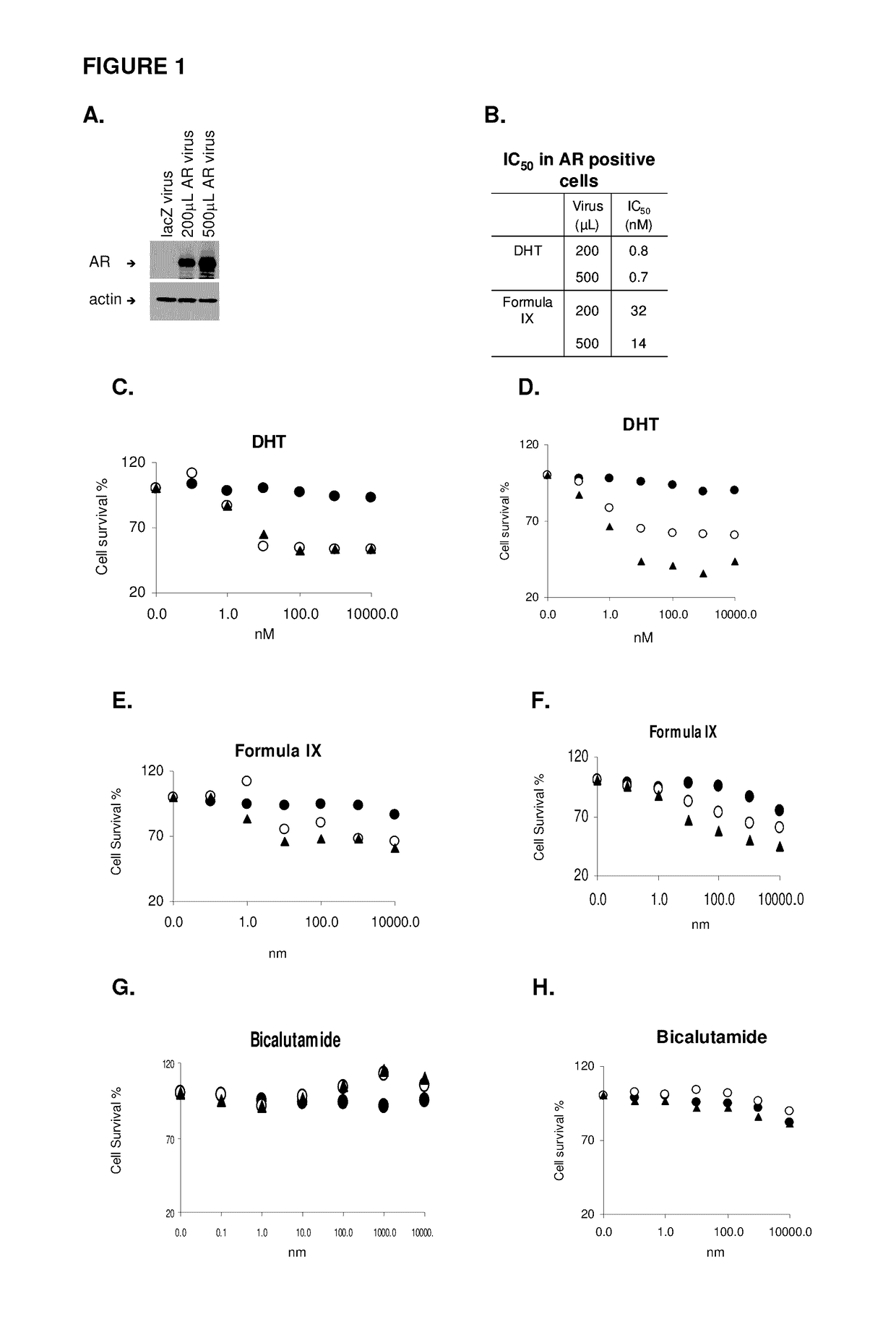
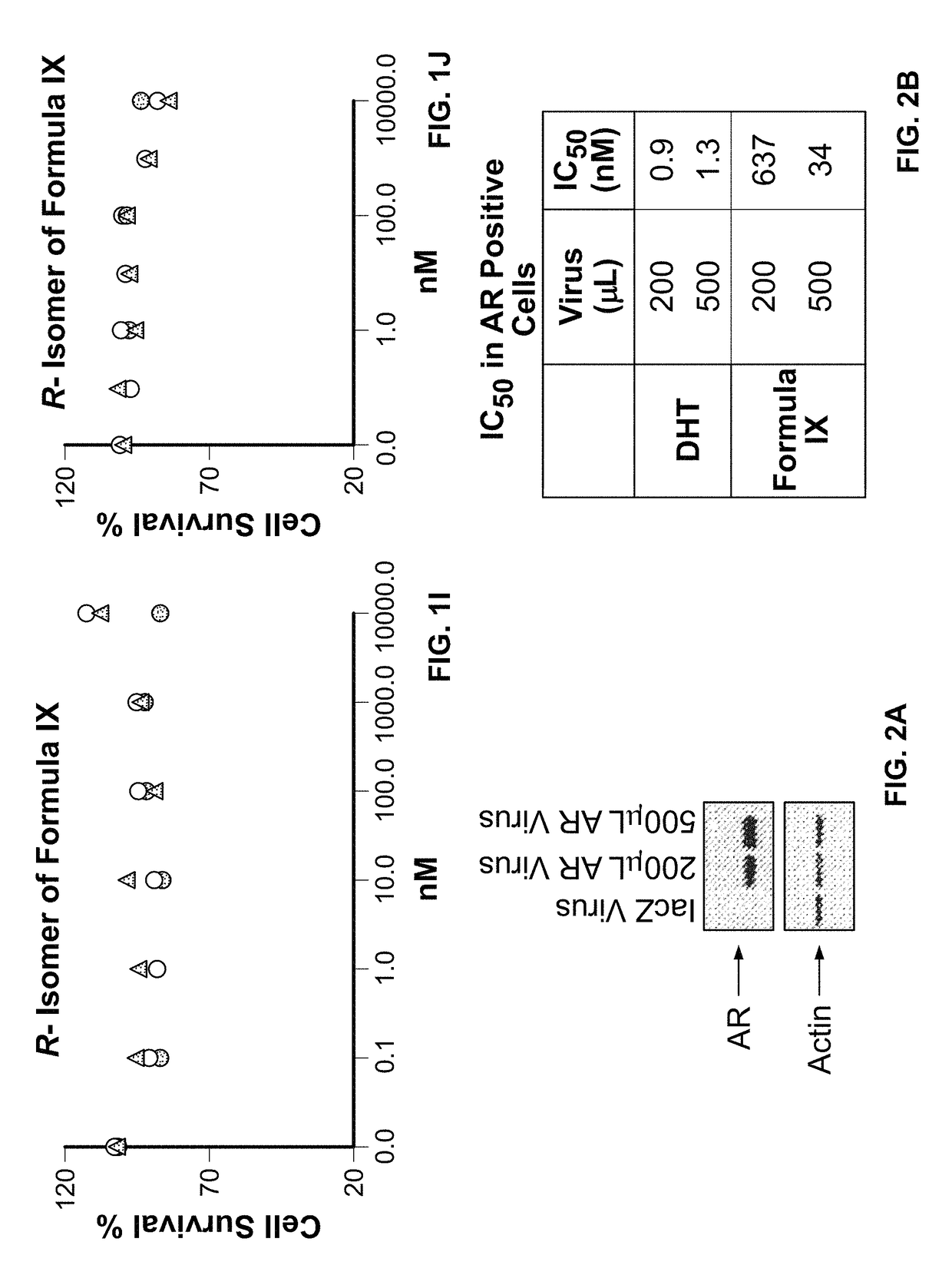
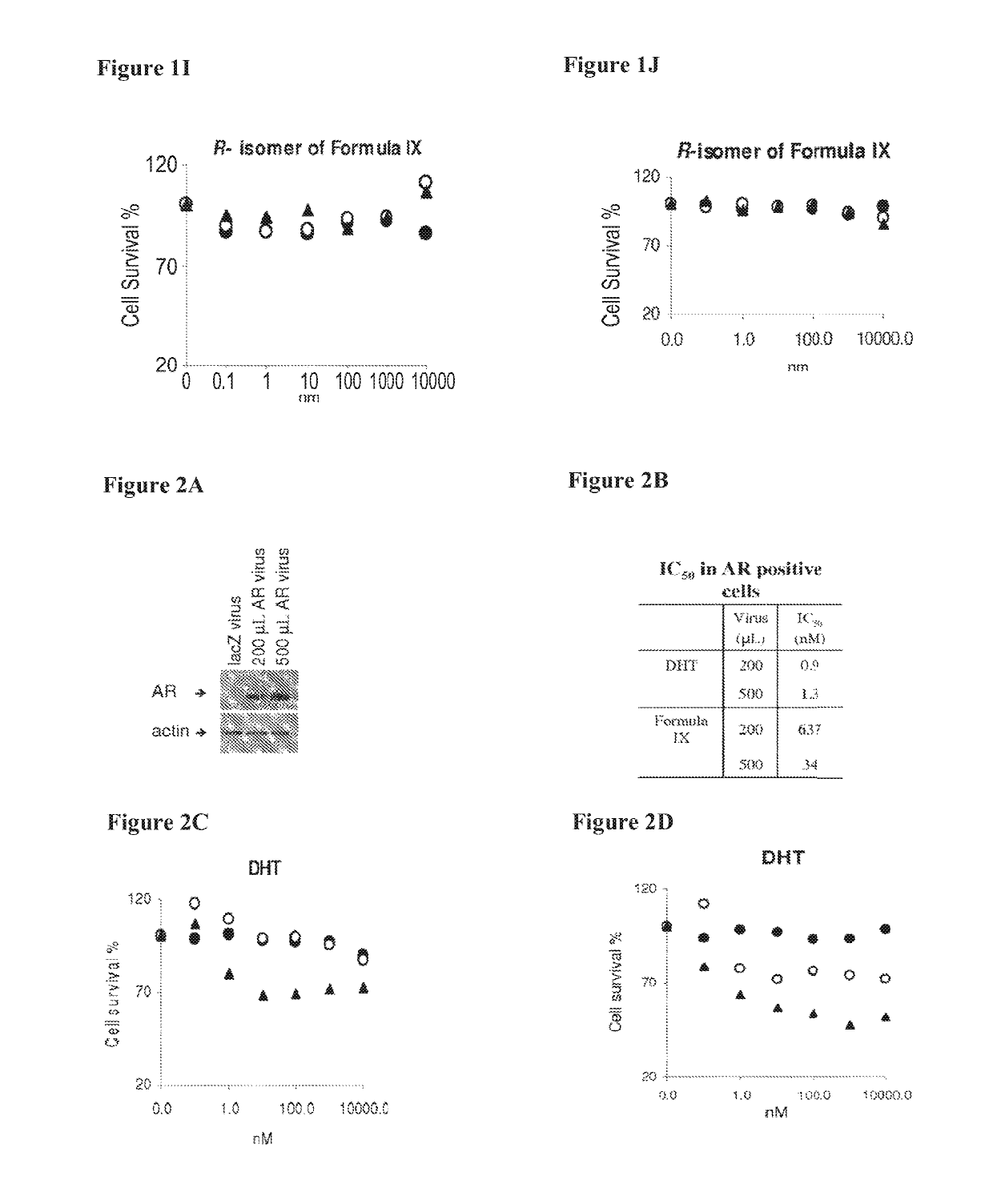
Method of treating estrogen receptor (ER) -positive breast cancers with selective androgen receptor modulator (SARMS)
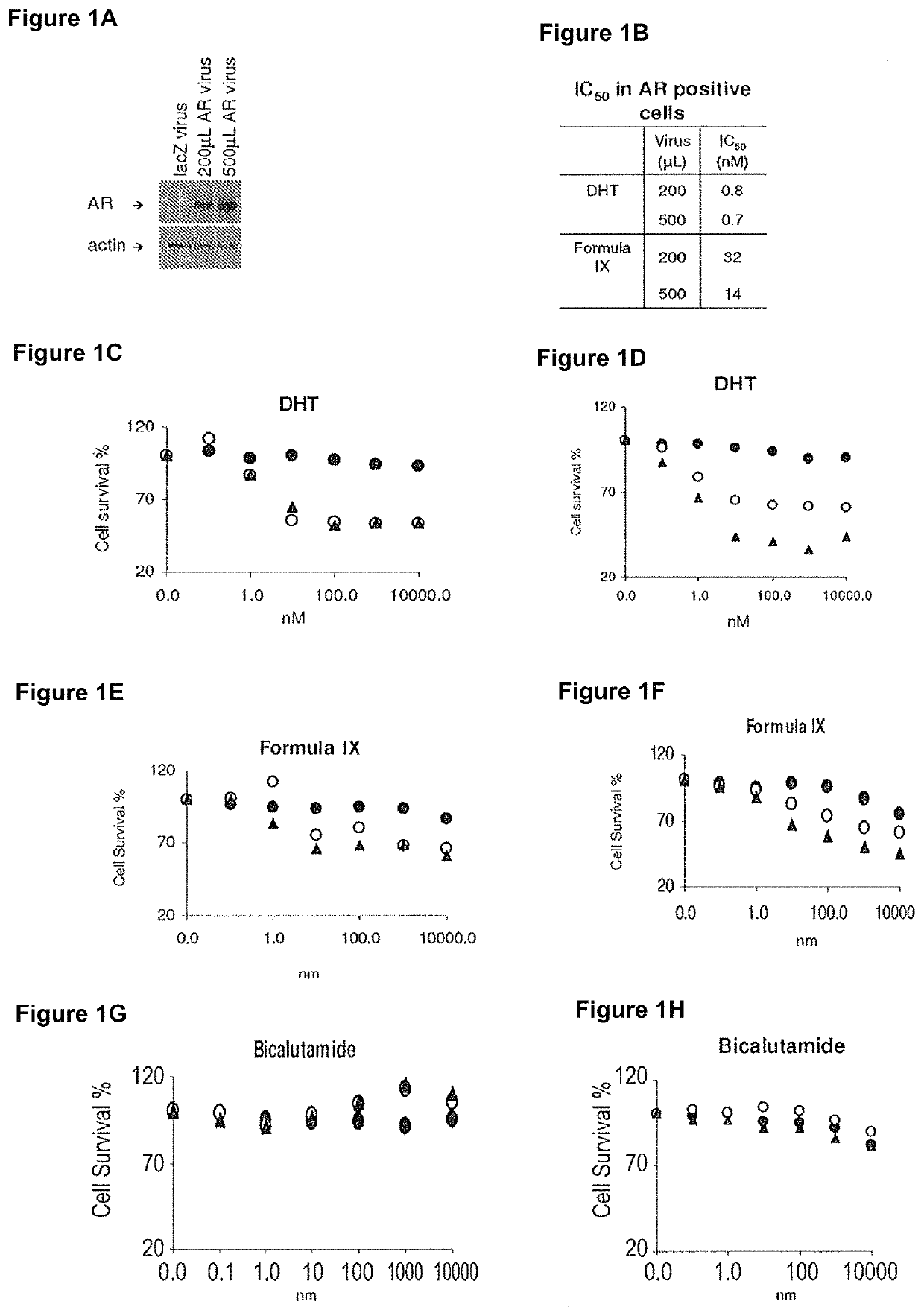
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; l) prolonging survival of a subject with breast cancer, and / or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Owner:UNIV OF TENNESSEE RES FOUND
Method of treating androgen receptor (AR) -positive breast cancers with selective androgen receptor modulator (SARMS)
ActiveUS20140350102A1Treating and preventing and suppressing and inhibiting metastasisProlonged progression-free survivalBiocideOrganic chemistryToremifeneLymphatic Spread
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Prevention and treatment of androgen-deprivation induced osteoporosis
InactiveUS20060269611A1Suppressing inhibiting reducing riskAvoid lostPowder deliveryOrganic active ingredientsToremifeneMetabolite
This invention provides a method of treating androgen-deprivation induced osteoporosis, bone fractures or loss of bone mineral density (BMD) in a male human subject suffering from prostate cancer by administering a pharmaceutical composition comprising Toremifene and / or its analog, derivative, isomer, metabolite, pharmaceutically acceptable salt, pharmaceutical product, hydrate, N-oxide, or any combination thereof, to the subject.
Owner:GTX INCORPORATED
Treatment of androgen-deprivation induced osteoporosis
The present invention provides methods for reducing the incidence of, inhibiting, suppressing, reducing the incidence of, and treating androgen-deprivation induced osteoporosis, bone fractures and / or loss of bone mineral density (BMD) in men having prostate cancer, comprising administering to a male human subject having prostate cancer a toremifene and / or its analog, derivative, isomer, metabolite, pharmaceutically acceptable salt, pharmaceutical product, hydrate, N-oxide, or any combination thereof. The present invention also provides methods of treating, preventing, suppressing, inhibiting, or reducing the incidence of hot flashes, gynecomastia, and / or hair loss in male humans having prostate cancer, comprising same.
Owner:GTX INCORPORATED
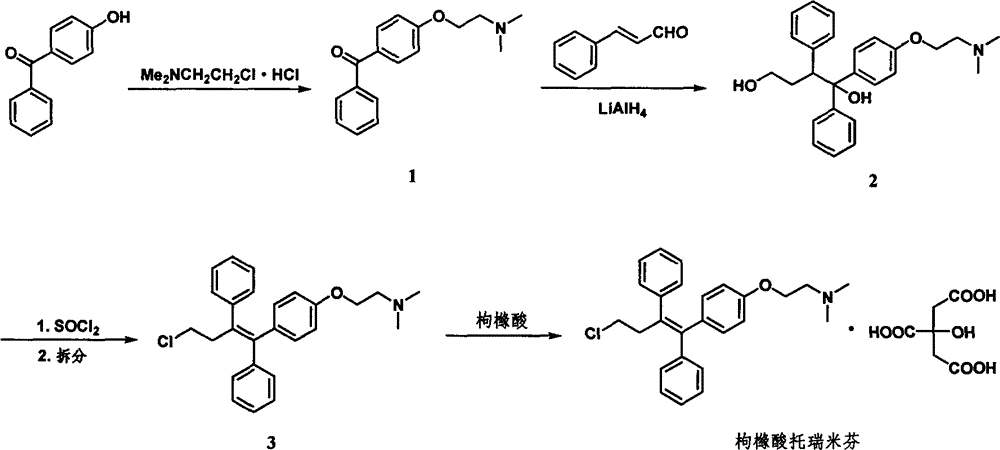
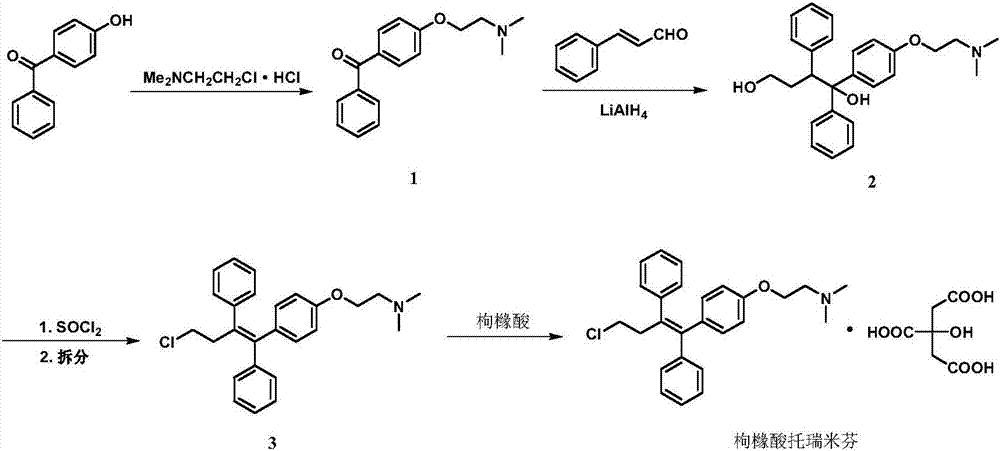
Synthesis method of toremifene
ActiveCN104230723AHigh stereoselectivityEasy to separateOrganic compound preparationAmino-hyroxy compound preparationToremifeneSynthesis methods
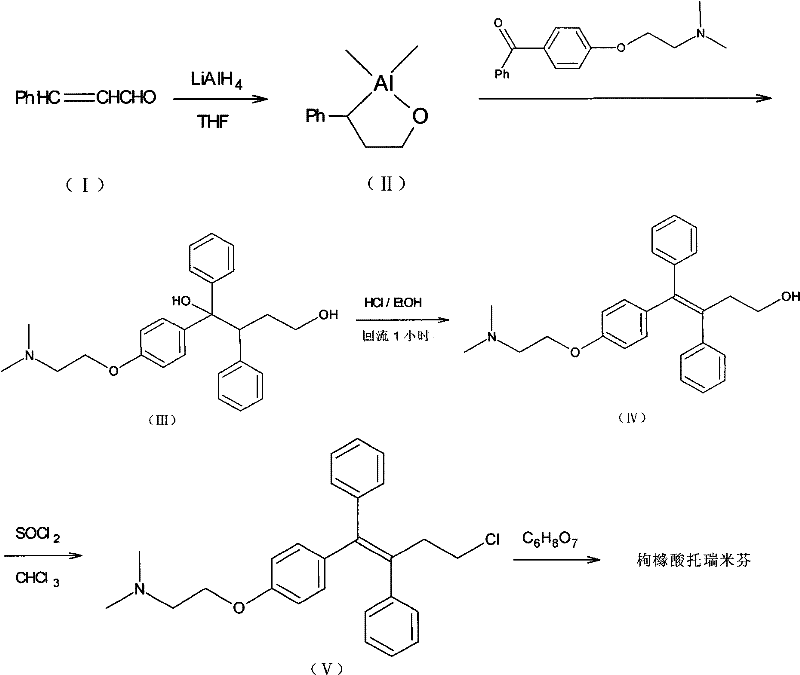
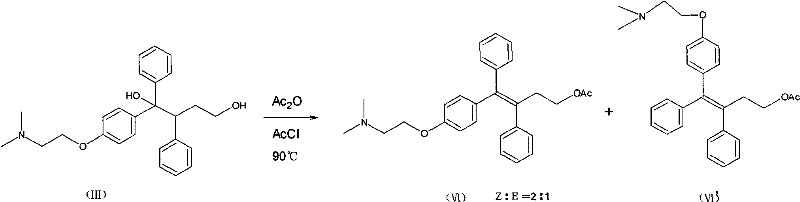
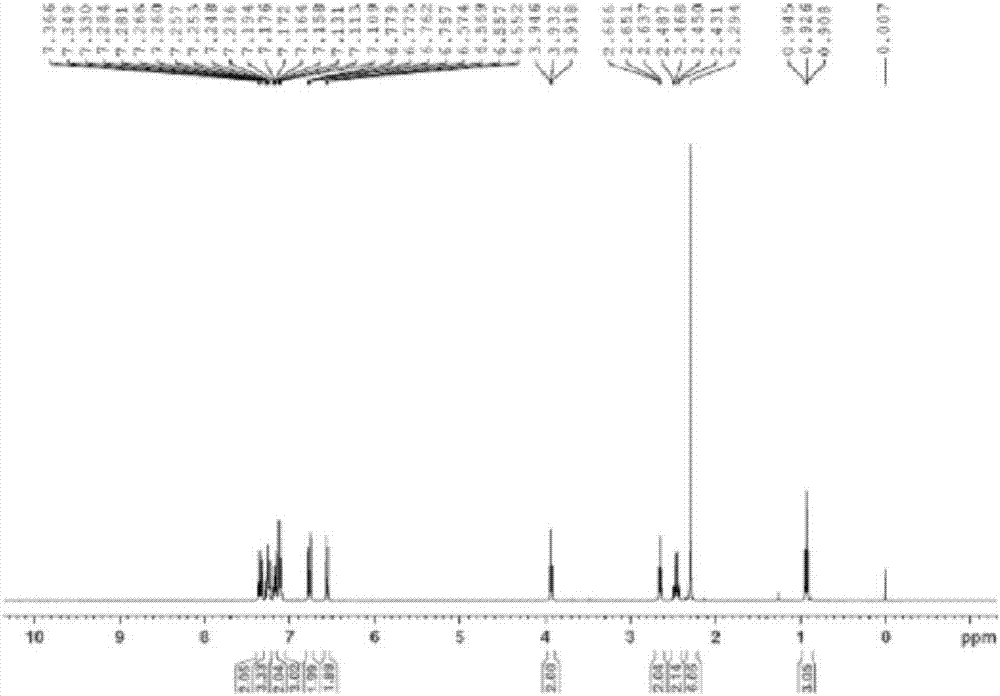
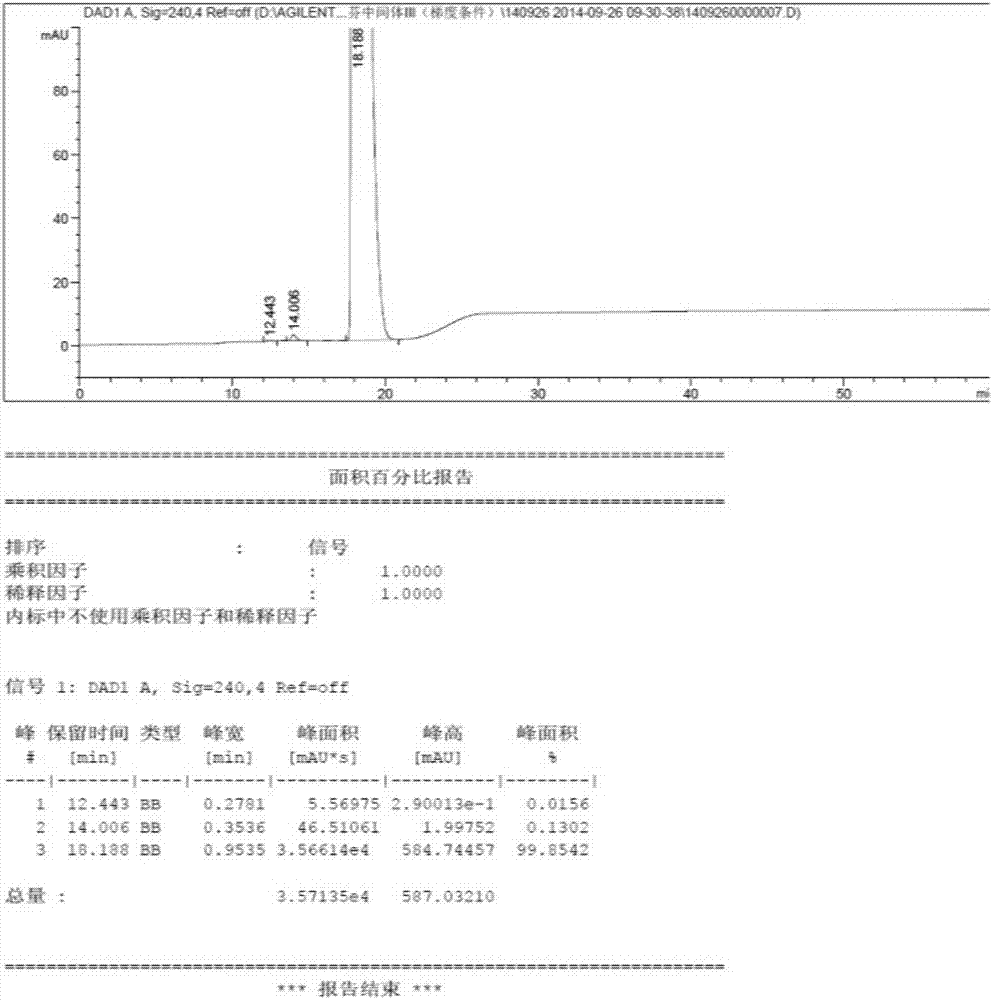
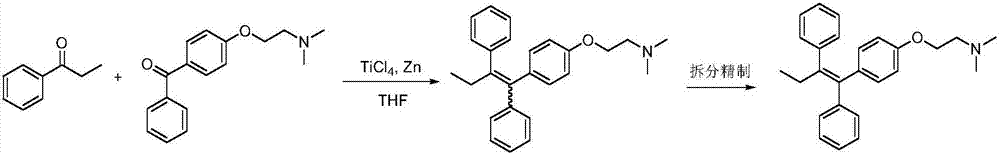
The invention provides a synthesis method of toremifene. The synthesis method comprises the following steps: S1. performing McMurry reaction to a compound B as shown in a structural formula II and a compound C as shown in a structural formula III, to obtain a compound D as shown in a structural formula IV; S2. performing selective alkylation reaction of phenolic hydroxyl to the compound D and a compound E as shown in a structural formula V or hydrochloride of the compound E, to obtain a compound F as shown in a structural formula VI; and S3. reacting the compound F with thionyl chloride, to obtain the toremifene, wherein the structural formula II, the structural formula III, the structural formula IV, the structural formula V and the structural formula VI are respectively shown in the specification. The obtained compound D has higher stereoselectivity, therefore, the reaction yield of the toremifene with a Z configuration can be increased when the compound D serves as an intermediate to synthesize the toremifene, and the purity of the toremifene with the Z configuration can be improved; and the synthesis method is stable in technology, mild in reaction conditions, the intermediate is easily separated, and the synthesis method can be used for mass production.
Owner:ASYMCHEM LAB TIANJIN +4
Method of treating her2-positive breast cancers with selective androgen receptor modulators (SARMS)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, palbociclib (Ibrance), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; and / or HER2-positive; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Method for realizing high-stereoselectivity synthesis of Toremifene by configuration conversion method
InactiveCN102126969AHigh stereoselectivityHigh reaction yieldOrganic compound preparationAmino-hyroxy compound preparationSolubilityToremifene
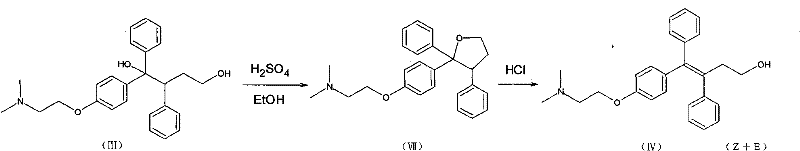
The invention discloses a method for realizing high-stereoselectivity synthesis of Toremifene (Z-type). In the method, configuration conversion is performed on an intermediate triaryl butenol under acid catalysis; and the required Z-type triaryl butenol is separated out continuously according to the difference of solubility, so that balance is damaged and E-type triaryl butenol is converted into the Z-type triaryl butenol continuously. The obtained Z-type triaryl butenol is chloridized by thionyl chloride to obtain an antitumor medicament, namely the Toremifene.
Owner:NINGBO TEAM PHARMA
Sex steroid precursors alone or in combination with selective estrogen receptor modulators for the prevention and treatment of dyspareunia in postmenopausal women
ActiveUS20160058774A1Efficient treatment methodReduced activityBiocideOrganic active ingredientsToremifeneDisease
Owner:MYRIEL PHARM LLC
The synthetic method of toremifene
ActiveCN104230723BHigh stereoselectivityEasy to separateOrganic compound preparationAmino-hyroxy compound preparationToremifeneStructural formula
The invention provides a synthetic method of toremifene. The synthesis method comprises: step S1, making a MacMurray reaction between compound B having structural formula II and compound C having structural formula III to obtain compound D having structural formula IV; step S2, making compound D react with compound E having structural formula V or The hydrochloride of compound E undergoes a selective alkylation reaction on the phenolic hydroxyl group to obtain compound F with structural formula VI; step S3, reacting compound F with thionyl chloride to obtain toremifene, wherein structural formula II is : Structural formula III is: Structural formula IV is Structural formula Ⅴ is ClCH 2 CH 2 N(CH 3 ) 2 ; Structural formula Ⅵ is The stereoselectivity of the obtained compound D is higher, so when using it as an intermediate to synthesize toremifene, the reaction yield of the toremifene of the Z configuration can be increased, and the toremifene of the Z configuration can be improved. purity; and the process is stable, the reaction conditions are mild, the intermediates are easy to separate, and can be used for large-scale production.
Owner:ASYMCHEM LAB TIANJIN +4
Method of treating HER2-positive breast cancers with selective androgen receptor modulators (SARMS)
ActiveUS10258596B2MicrocapsulesNitrile/isonitrile active ingredientsToremifeneHER2 Positive Breast Cancer
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, palbociclib (Ibrance), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; and / or HER2-positive; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Method of treating androgen receptor (AR)-positive breast cancers with selective androgen receptor modulator (SARMs)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
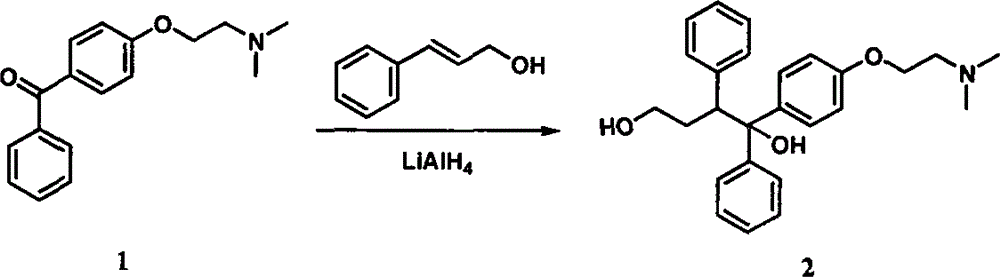
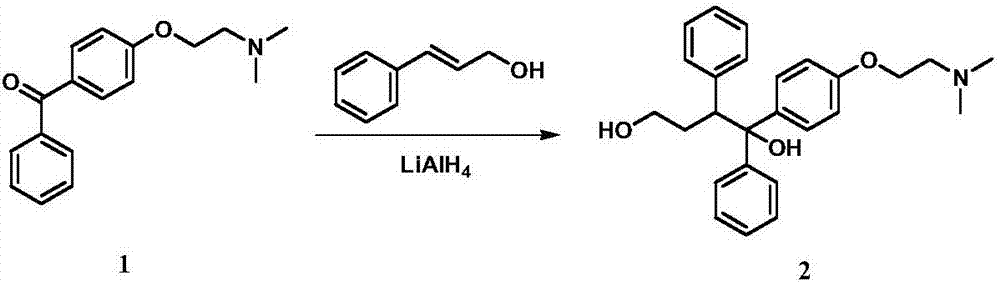
New preparation method of citric acid toremifene intermediate
InactiveCN106187791ARaise the reaction temperatureShort reaction timeOrganic compound preparationAmino-hyroxy compound preparationToremifeneALUMINUM HYDRIDE
The invention discloses new methods for producing and preparing a citric acid toremifene key intermediate 4-[2-(N,N-dimethylamino)ethoxy]benzophenone(hereinafter referred to as an intermediate 1) and 1,2-diphenyl-1-[4-[2- ( N,N-dimethylamino)ethoxy]phenyl group]-1,4-butanediol (hereinafter referred to as an intermediate 2). The intermediate 1 is prepared by using a non-water-soluble resolvent as a main solvent, a water-soluble reagent as an auxiliary solvent, and an inorganic base as an acid acceptor, and is formed by an alkylation reaction of 4-hydroxybenzophenone and N,N-dimethylaminoethyl chloride hydrochloride. The reaction speed is high, the post-treatment is simple, convenient and safe, and few three wastes are generated; and the yield and the purity of the intermediate 1 are high, the new method is especially suitable for industrialized production. The intermediate 2 is prepared from cinnamic aldehyde (or cinnamyl alcohol), lithium aluminum hydride and the intermediate 1 in an ether solvent, the post-treatment of the intermediate 2 is performed in a manner of performing destroying with alkali liquor, and drying with a drying agent, and the intermediate 2 is separated in a direct filtering manner. The new method has the characteristics of being quick, environmental-friendly, high in yield and the like.
Owner:NINGBO TEAM PHARMA
Preparation method of citric acid toremifene intermediates
ActiveCN106966911AAvoid it happening againEasy to operateOrganic compound preparationAmino-hyroxy compound preparationToremifeneOil phase
The invention relates to a preparation method of citric acid toremifene intermediates 4-[2-(N,N-dimethylamino)ethyoxyl diphenylketone and 1,2-diphenyl-1-[4[2-[2-N,N- dimethylamino]ethoxyl]-1,4-butanediol. The preparation method of the 4-[2-(N,N-dimethylamino)ethyoxyl diphenylketone comprises the steps that 4-hydroxy diphenyl ketone, inorganic alkali, a first solvent and a second solvent are mixed and are stirred at the temperature of 53-58 DEG C for 0.8-2 hours, then cooling is performed to 40 DEG C or below, N,N-dimethylamino chloroethane hydrochloride is added, heating is performed to reach 80-85 DEG C, and stirring reaction is performed for 2-5 hours; cooling is performed to 40 DEG C or below, drinking water is added, stirring is performed to make the mixture layered, and an upper-layer oil phase is taken; the oil phase is washed with 4% sodium hydroxide solution till it is detected through TLC that the 4-hydroxy diphenyl ketone disappears, then washing is performed with drinking water till pH is 8-9; the obtained product is subjected to decompression at the temperature of 55-60 DEG C to evaporate out the solvent, and the citric acid toremifene intermediates 4-[2-(N,N-dimethylamino)ethyoxyl diphenylketone is obtained after cooling.
Owner:NINGBO TEAM PHARMA
Method of treating androgen receptor (AR)-positive breast cancers with selective androgen receptor modulator (SARMs)
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; l) prolonging survival of a subject with breast cancer, and / or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Owner:UNIV OF TENNESSEE RES FOUND
Method of treating er mutant expressing breast cancers with selective androgen receptor modulators (SARMS)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Sex steroid precursors alone or in combination with a selective estrogen receptor modulator and/or with estrogens and/or a type 5 cGMP phosphodiesterase inhibitor for the prevention and treatment of vaginal dryness and sexual dysfunction in postmenopausal women
ActiveUS8835413B2Efficient treatment methodReduced activityBiocideSuppositories deliveryDiseaseToremifene
Owner:MYRIEL PHARM LLC
Method of treating androgen receptor (AR)-positive breast cancers with selective androgen receptor modulator (SARMs)
Owner:UNIV OF TENNESSEE RES FOUND
Method of treating estrogen receptor (ER)-positive breast cancers with selective androgen receptor modulator (SARMS)
Owner:UNIV OF TENNESSEE RES FOUND
Method of treating HER2-positive breast cancers with selective androgen receptor modulators (SARMS)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, palbociclib (Ibrance), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; and / or HER2-positive; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Application of toremifene citrate in preparation of medicines for inhibiting tumor cell metastasis and diffusion
InactiveCN104666284AAbility to inhibit metastasisReduce the number of deathsOrganic active ingredientsAntineoplastic agentsToremifenePharmaceutical drug
The invention discloses an application of toremifene citrate in preparation of medicines for inhibiting tumor cell metastasis and diffusion. The metastasis ability of tumor cells is inhibited by the medicines prepared from the toremifene citrate, wherein the toremifene citrate is a low-toxicity medicine, and can play a relatively large blocking role in a tumor metastasis process; and meanwhile, treatment is carried out by virtue of other related tumor medicines, so that the mortality amount of normal cells can be greatly reduced; most of the existing tumor medicines are cytotoxic medicines; and a large number of normal cells die when the tumor cells are killed.
Owner:NANJING ZHONG HONG HUA FEI INFORMATION TECH CO LTD
Toremifene citrate nano suspension, toremifene citrate tablet for treating ebola and preparation methods thereof
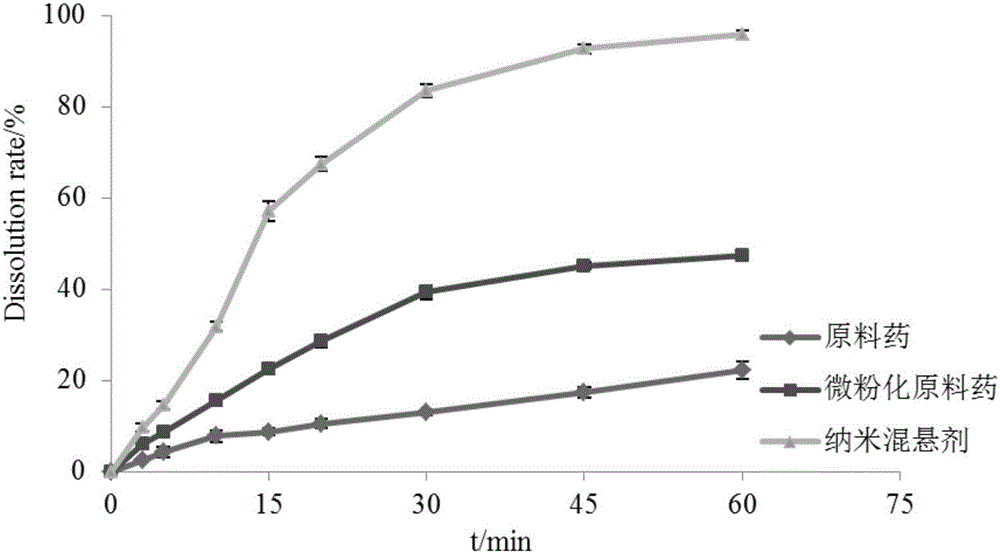
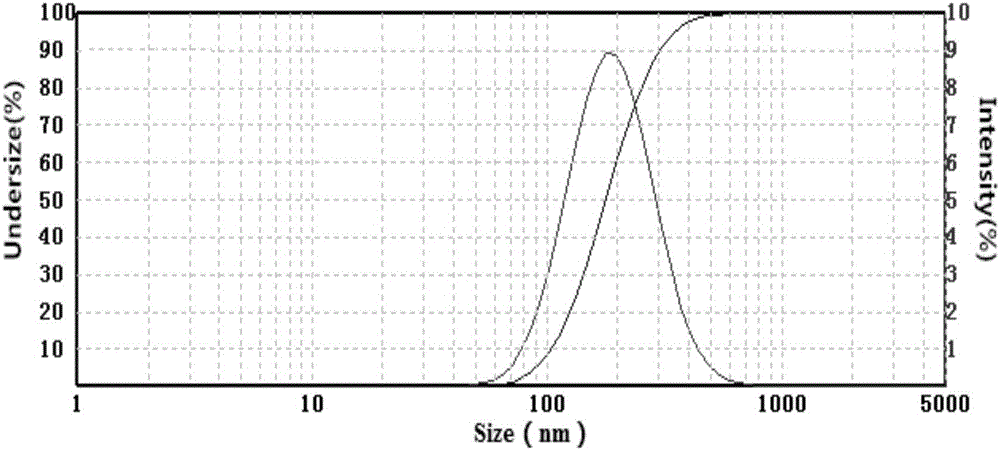
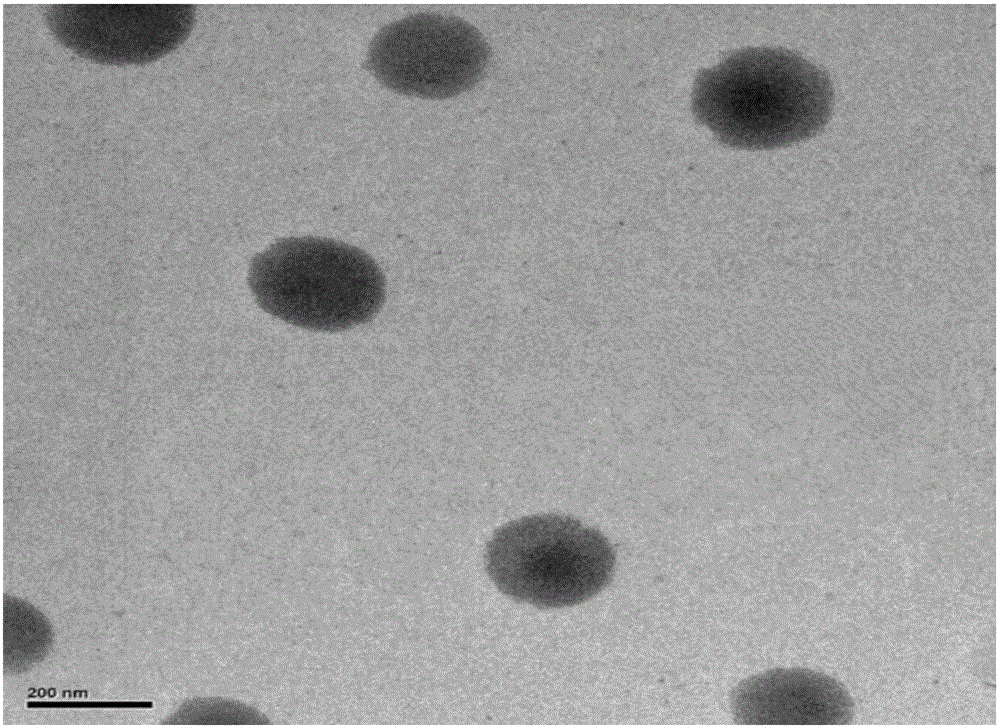
InactiveCN106821976AImprove solubilityHigh dissolution rateOrganic active ingredientsAntiviralsSolubilityFluidized bed drying
The invention discloses a toremifene citrate nano suspension, a toremifene citrate tablet for treating ebola and preparation methods thereof, and belongs to the technical field of medicines. The toremifene citrate nano suspension is prepared from the following raw materials in parts by weight: 1 part of toremifene citrate, 0.1 to 2 parts of stabilizer and 10 to 500 parts of redistilled water. The toremifene citrate tablet is prepared from an effective component and a pharmaceutically acceptable carrier, wherein the effective component is the toremifene citrate nano suspension. The medicine toremifene citrate tablet provided by the invention has the following advantages that the solubility and the dissolution rate of the toremifene citrate are ameliorated by utilizing a nano suspending technique, the adhesiveness of the toremifene citrate to a gastrointestinal mucous membrane is increased, the first pass effect is avoided, the oral bioavailability is improved, and further, the drug loading capacity is high, and the like; meanwhile, a fluidized bed drying technique is adopted; the process is simple; the industrial mass production is suitable.
Owner:中国人民解放军第三O二医院
Non-invasive method of evaluating breast cancers for selective androgen receptor modulator (SARM) therapy
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; treating a subject suffering from ER mutant expressing breast cancer and / or treating breast cancer in a subject, by first determining the 18F-16β-fluoro-5α-dihydrotestosterone (18F-DHT) tumor uptake and identifying said subject as having AR-positive breast cancer based on 18F-DHT tumor uptake, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
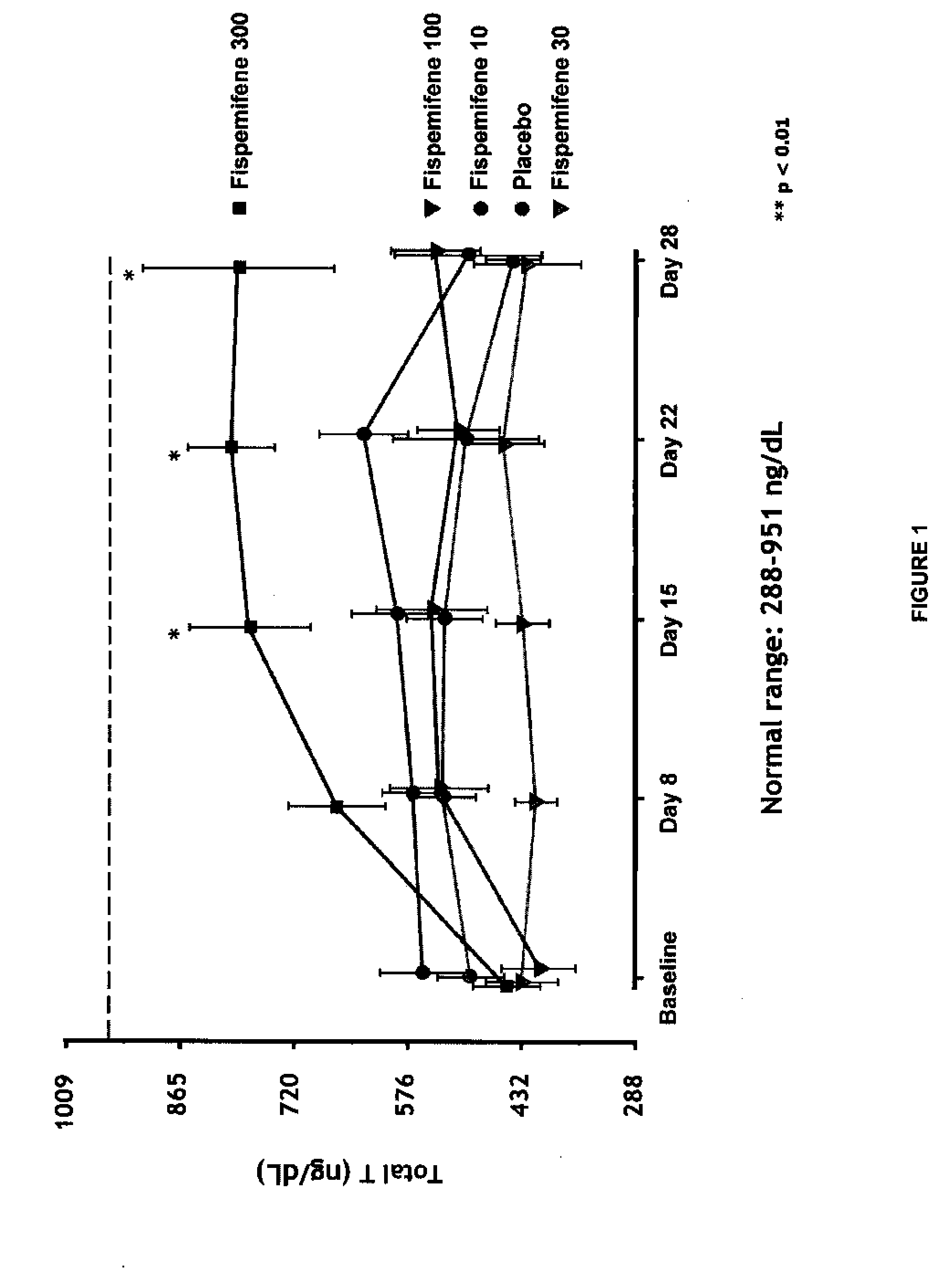
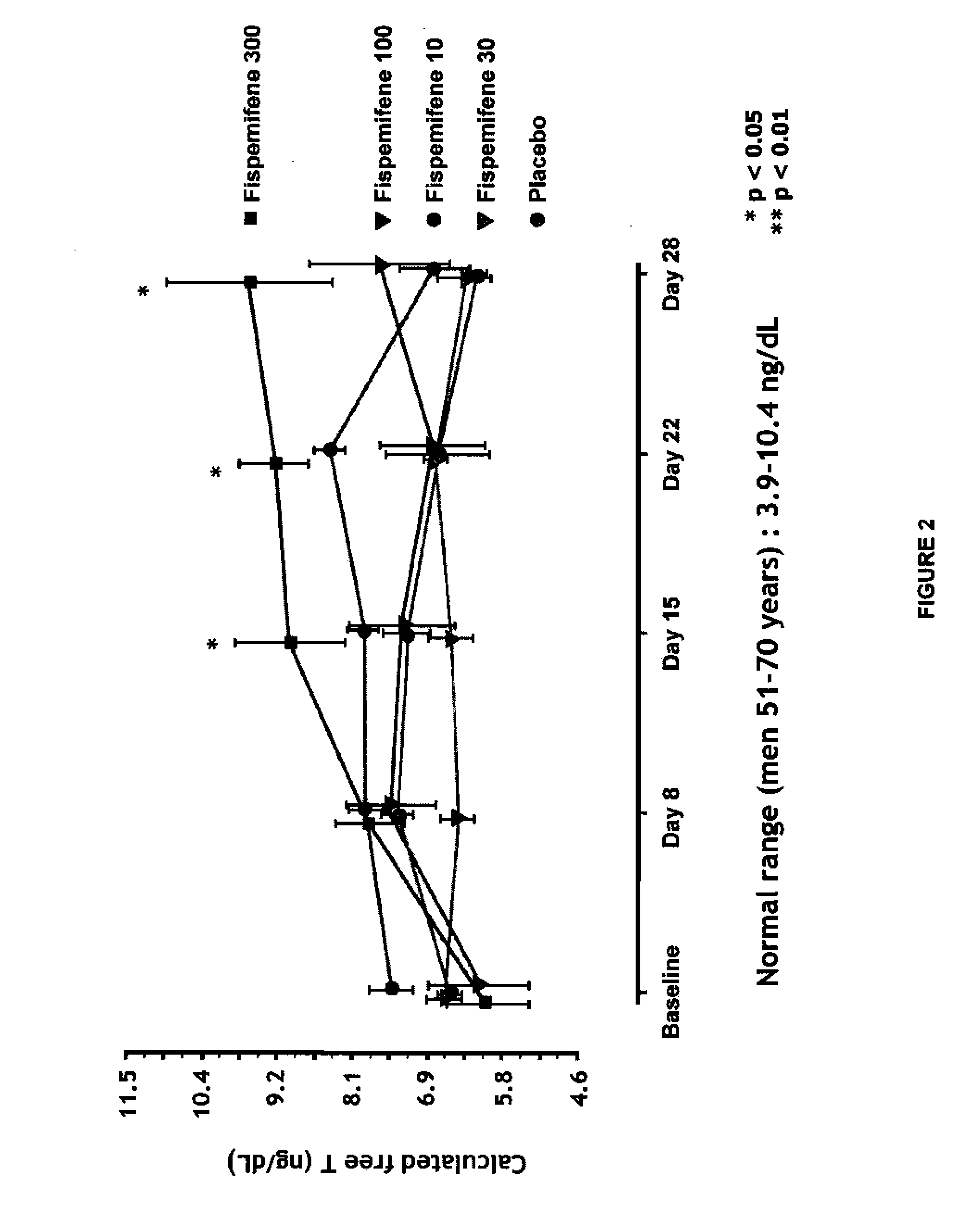
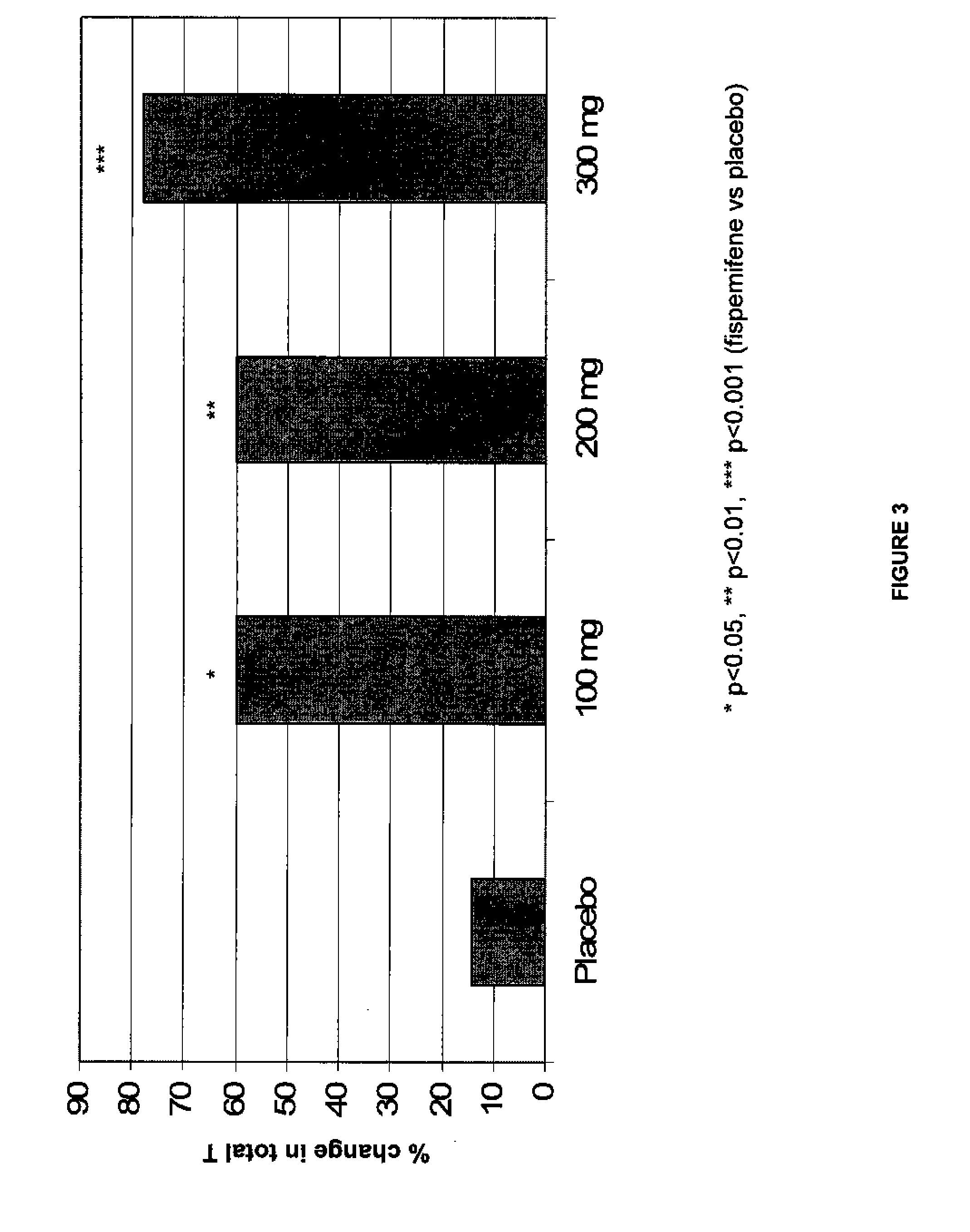
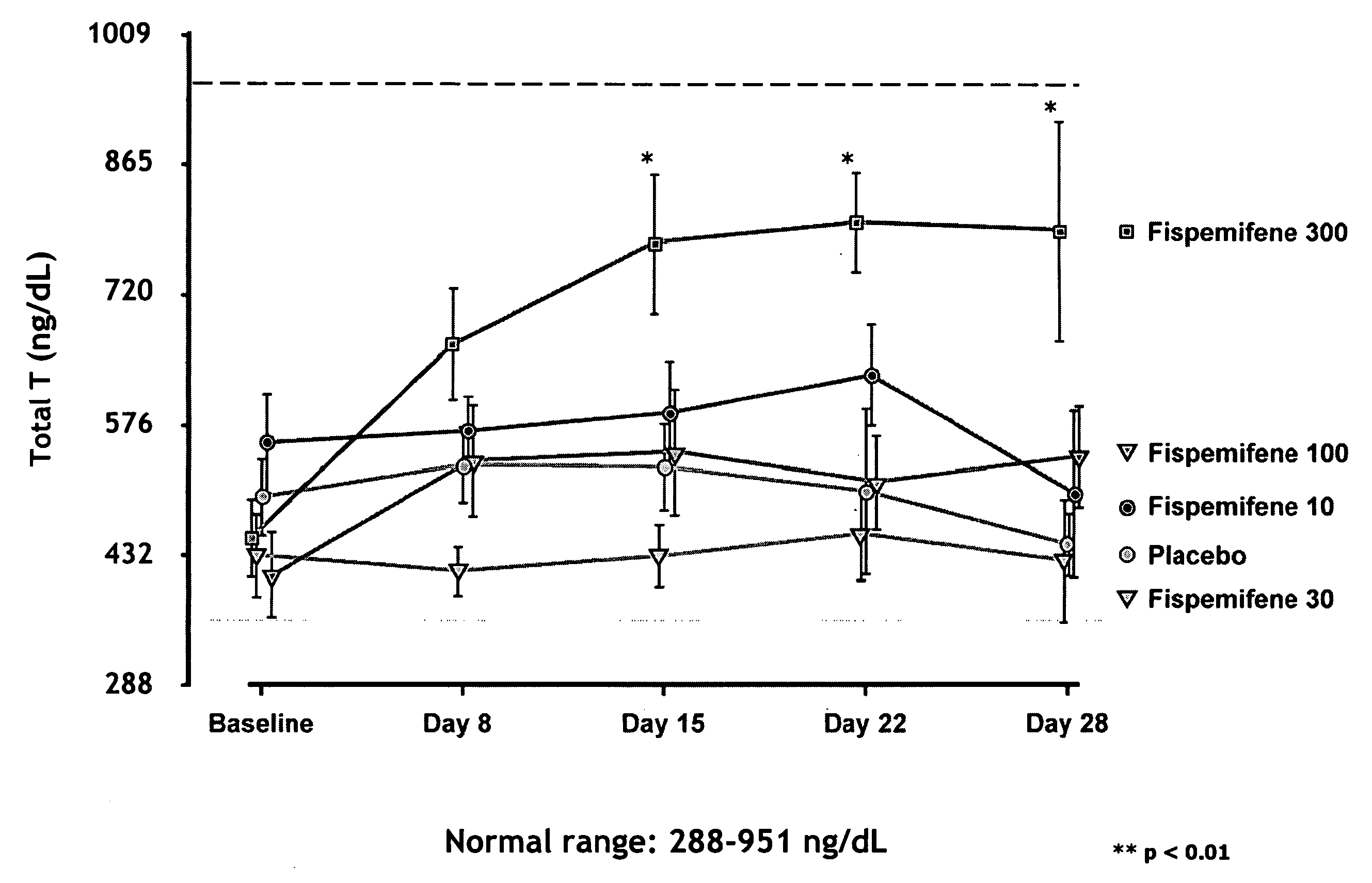
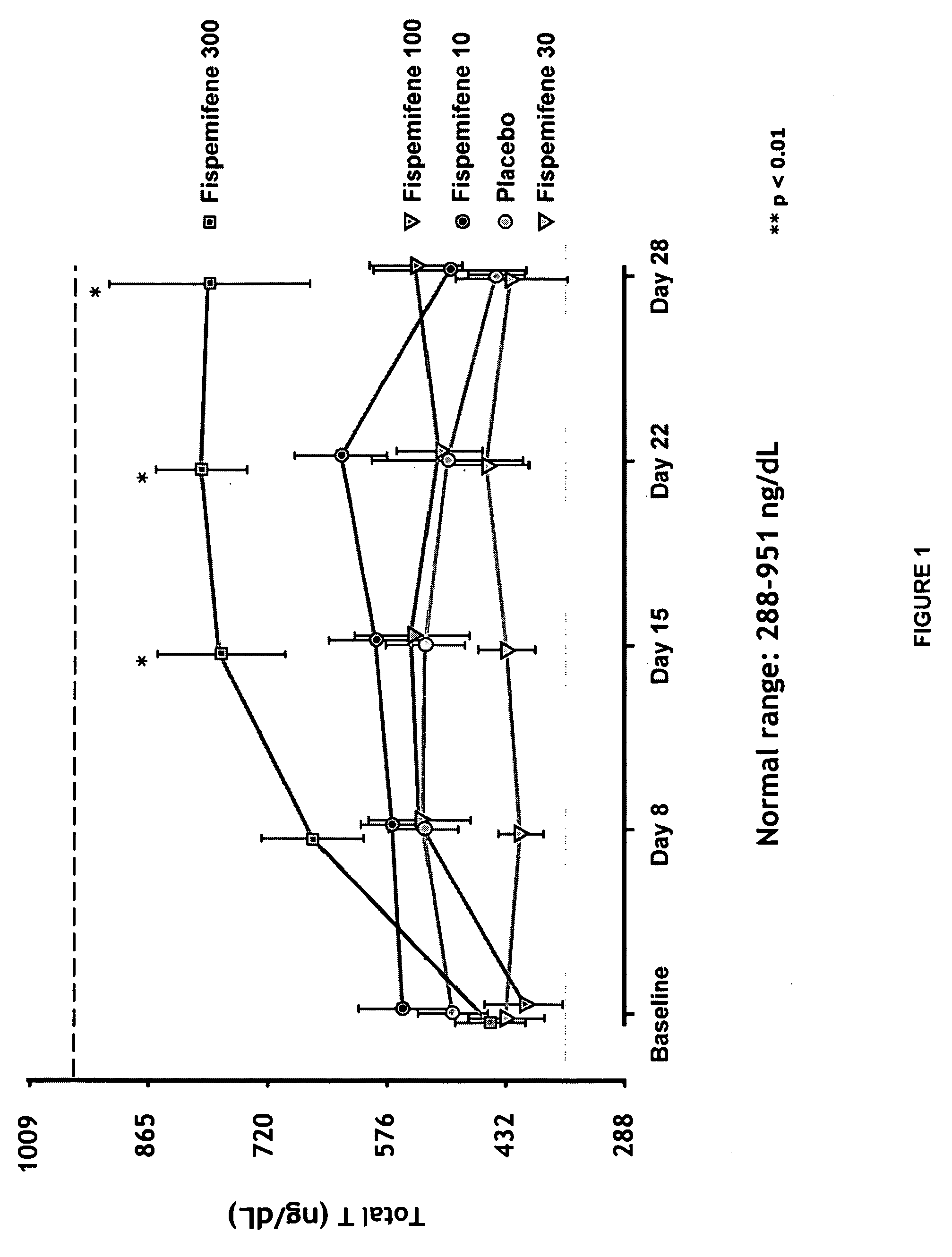
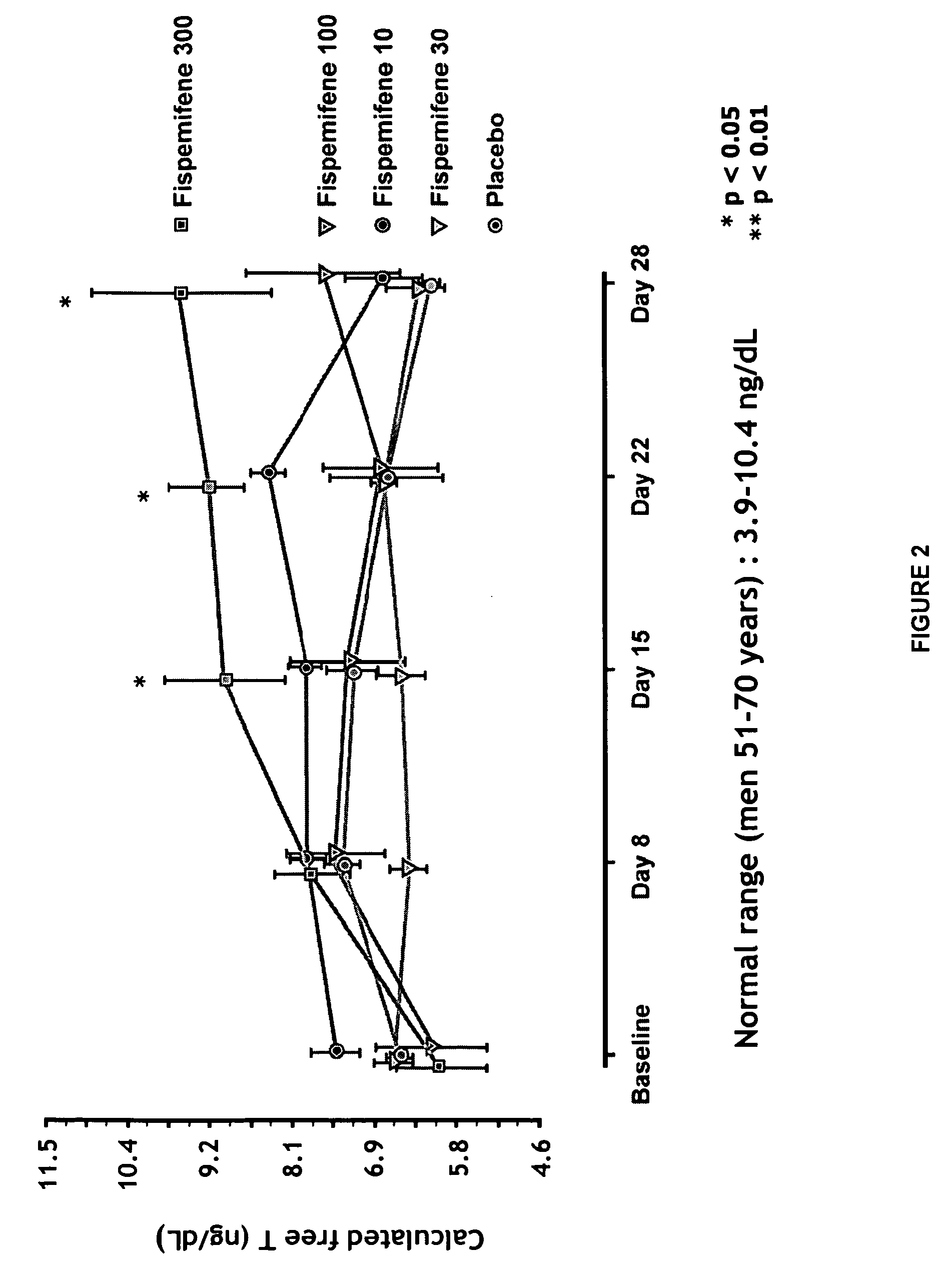
Methods for the treatment of erectile dysfunction using fispemifene
InactiveUS20100261729A1Improve erectile functionImprove the level ofPharmaceutical delivery mechanismEther/acetal active ingredientsToremifeneEnclomifene
A method of treatment of erectile dysfunction (ED) comprises the step of administering fispemifene to a subject in need thereof in an amount effective to raise the subject's testosterone level. Fispemifene may be used in combination with a PDE-5 inhibitor in individuals who have failed to respond sufficiently to conventional ED treatment. Methods are also disclosed of treating ED by administering clomifene, enclomifene, ospemifene, toremifene and mixtures thereof in combination with a PDE-5 inhibitor.
Owner:QUATRX PHARMA
Methods for the treatment of erectile dysfunction using fispemifene
InactiveUS20080312239A1Improve erectile functionImprove the level ofBiocideEther/acetal active ingredientsToremifeneEnclomifene
A method of treatment of erectile dysfunction (ED) comprises the step of administering fispemifene to a subject in need thereof in an amount effective to raise the subject's testosterone level. Fispemifene may be used in combination with a PDE-5 inhibitor in individuals who have failed to respond sufficiently to conventional ED treatment. Methods are also disclosed of treating ED by administering clomifene, enclomifene, ospemifene, toremifene and mixtures thereof in combination with a PDE-5 inhibitor.
Owner:QUATRX PHARMA
Treatment of androgen-deprivation induced osteoporosis
InactiveUS20080249183A1Preventing ADT-induced osteoporosisIncrease bone densityOrganic active ingredientsBiocideToremifeneMetabolite
This invention provides a method of treating androgen-deprivation induced osteoporosis, bone fractures or loss of bone mineral density (BMD) in a male human subject suffering from prostate cancer, wherein the subject has a precipitous decline in androgen levels, by administering a pharmaceutical composition comprising Toremifene or its analog, derivative, isomer, metabolite, pharmaceutically acceptable salt, pharmaceutical product, hydrate, N-oxide, or any combination thereof, to the subject, wherein the method increases bone density without increasing androgen and specifically testosterone levels in the subject.
Owner:GTX INCORPORATED
Method for chemoprevention of prostate cancer
The invention relates to uses of antiestrogen in the medicine preparation. The medicine is used for preventing, limiting and restraining the prostatic cancer or potential prostatic cancer, and for decreasing the danger of prostatic cancer or potential prostatic cancer, or curing the prostatic cancer or potential prostatic cancer, wherein, the antiestrogen is Toremifene, Raloxifene, Tamoxifen, Idoxifene or Droloxifene.
Owner:UNIV OF TENNESSEE RES FOUND
Treatment of androgen-deprivation induced osteoporosis
InactiveCN101056621AReduce the incidence of hair lossOrganic active ingredientsPowder deliveryToremifeneMetabolite
The present invention provides methods for reducing the incidence of, inhibiting, suppressing, and treating androgen-deprivation induced osteoporosis, bone fractures and / or loss of bone mineral density (BMD) in men having prostate cancer, comprising administering to a male human subject having prostate cancer a toremifene and / or its analog, derivative, isomer, metabolite. pharmaceutically acceptable salt, pharmaceutical product, hydrate, N-oxide. or any combination thereof. The present invention also provides methods of treating, preventing, suppressing, inhibiting, or reducing the incidence of hot flashes, gynecomastia, and / or hair loss in a subject, comprising same.
Owner:GTX INCORPORATED
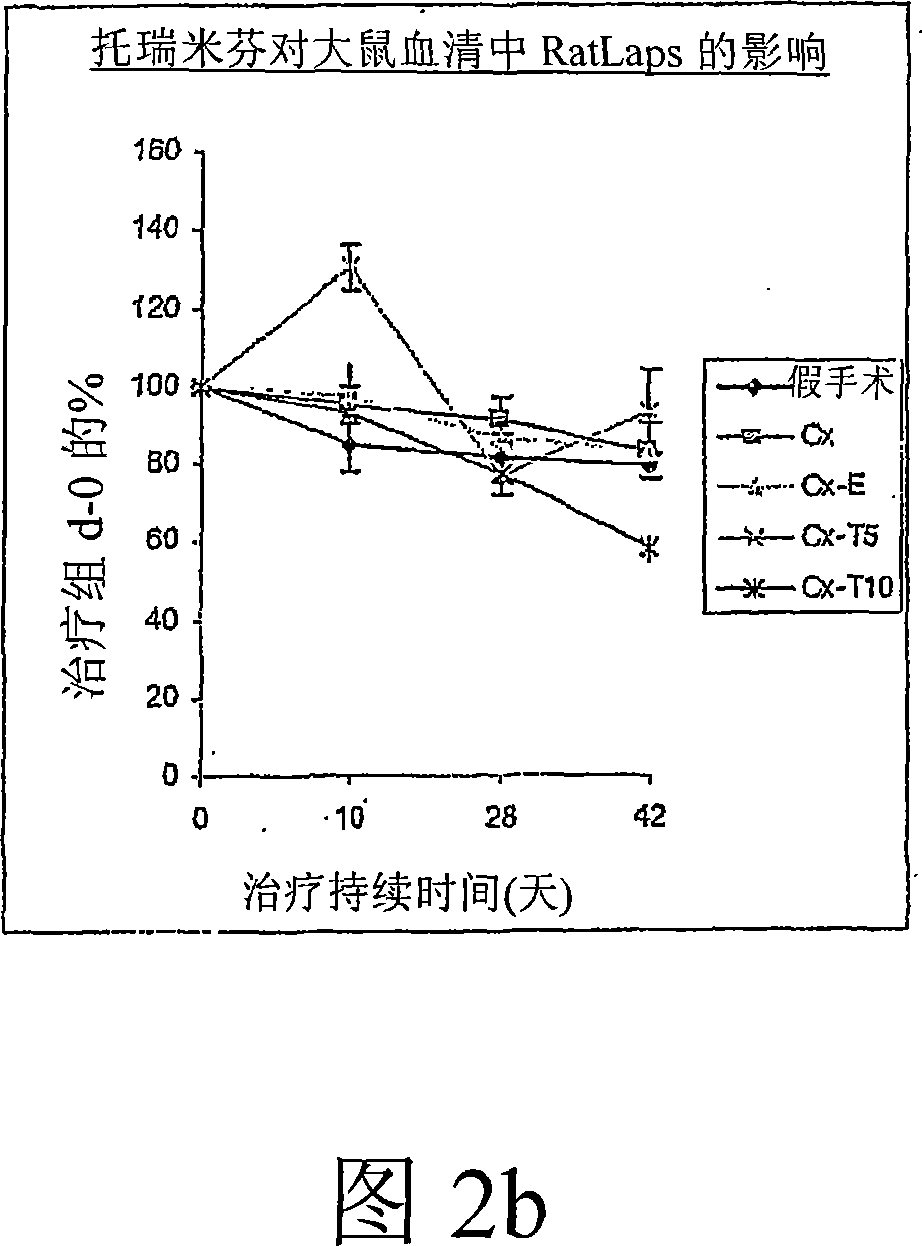
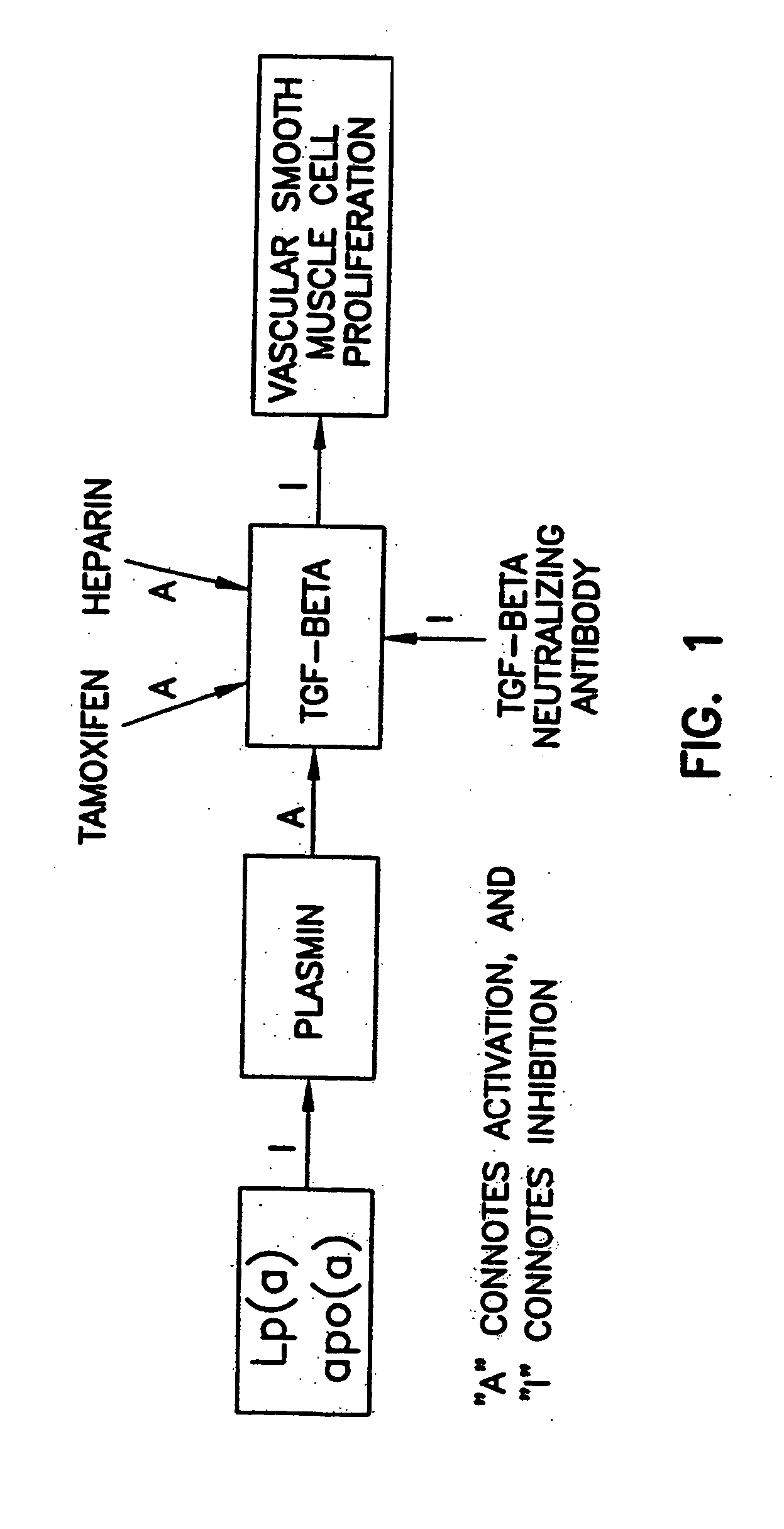
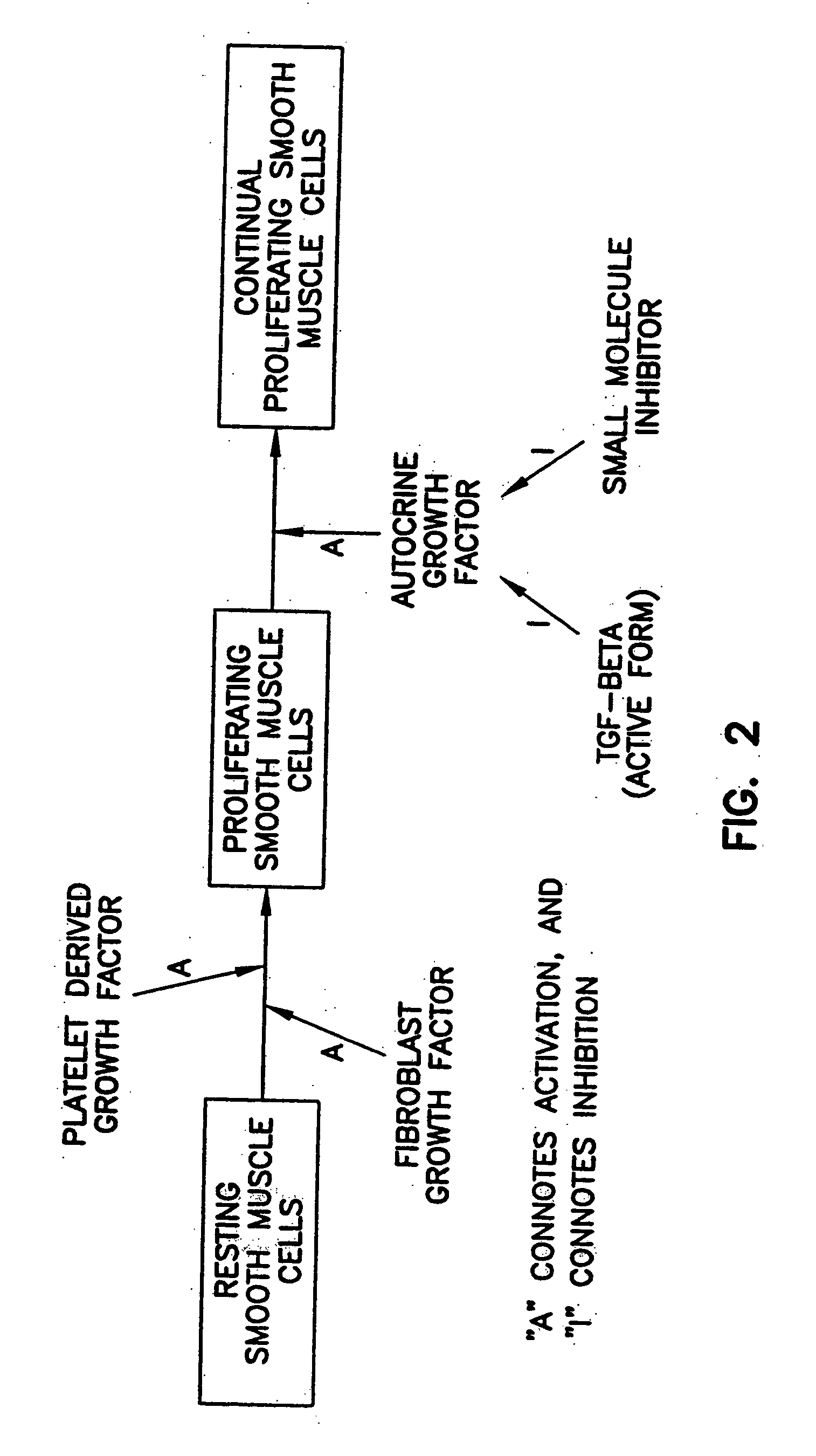
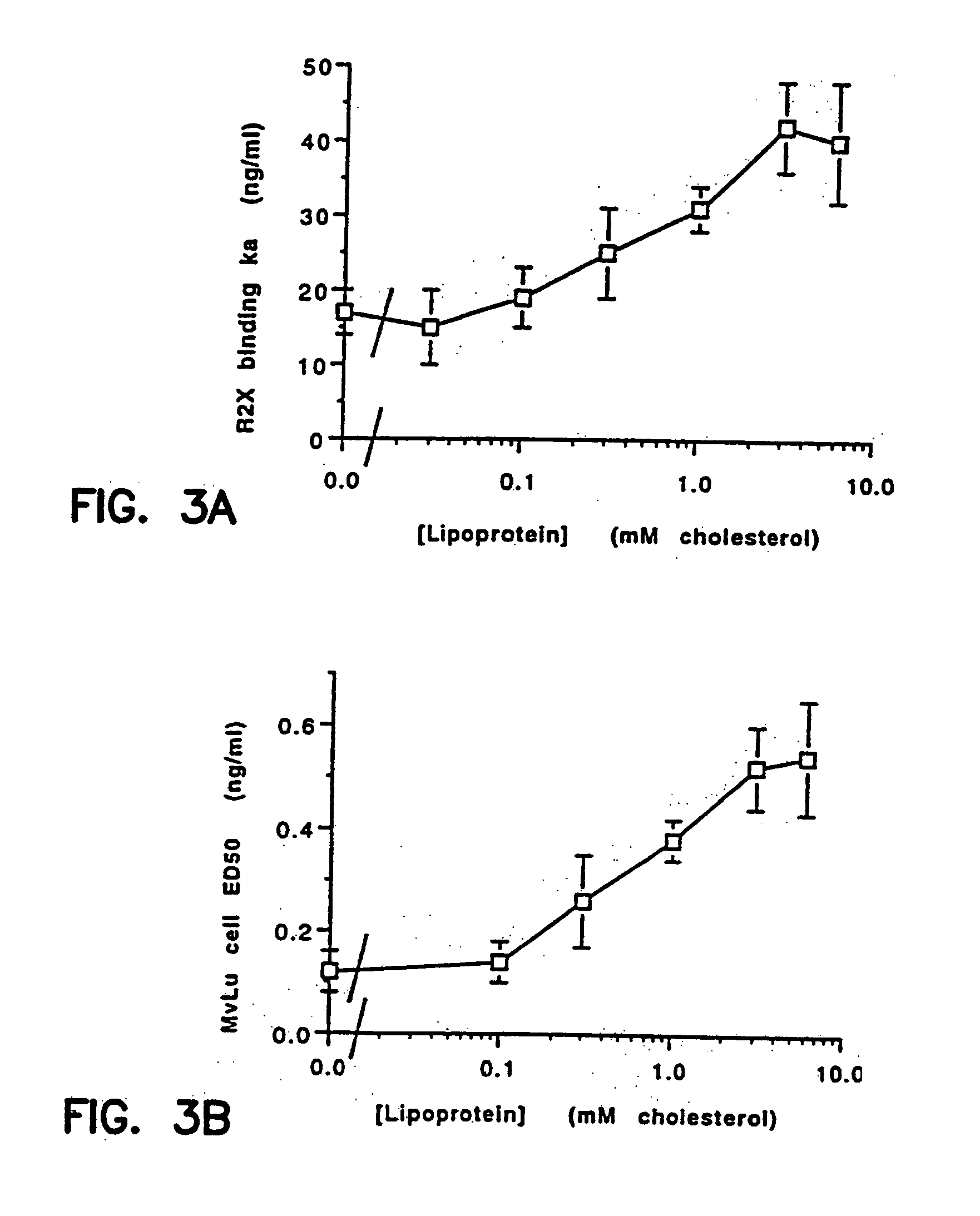
Prevention and treatment of cardiovascular pathologies with tamoxifen analogues
InactiveUS20060135560A1Improve the level ofReduce the overall diameterBiocideOrganic chemistryToremifeneRisk stroke
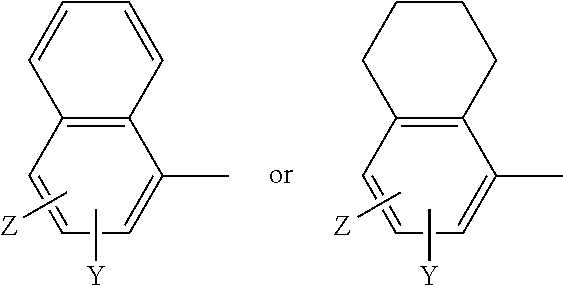
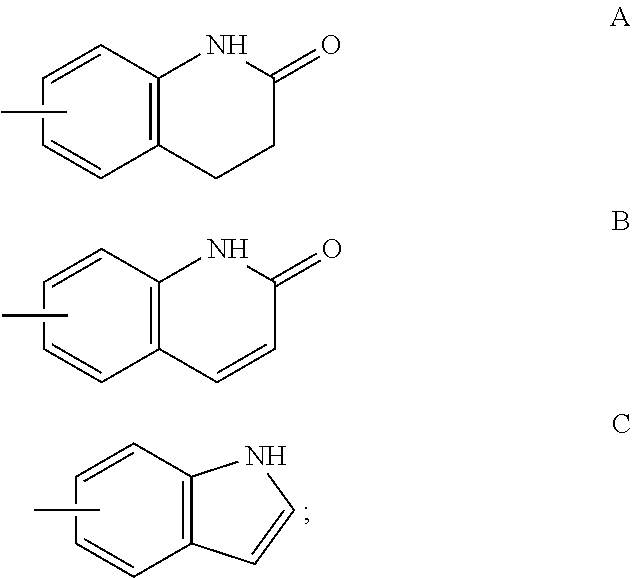
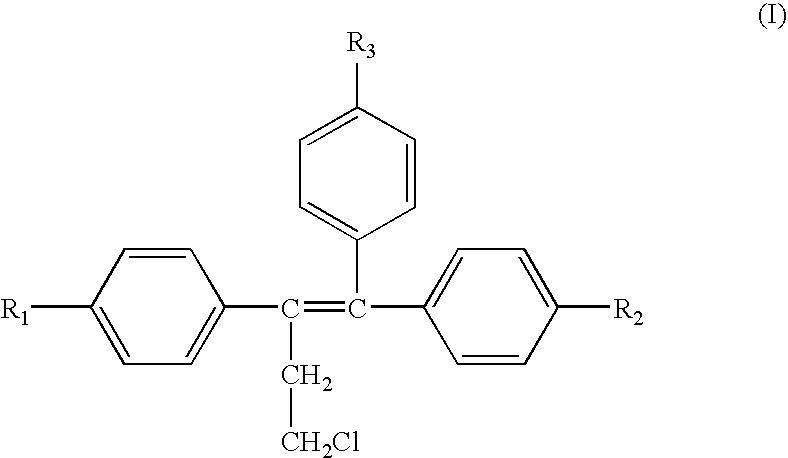
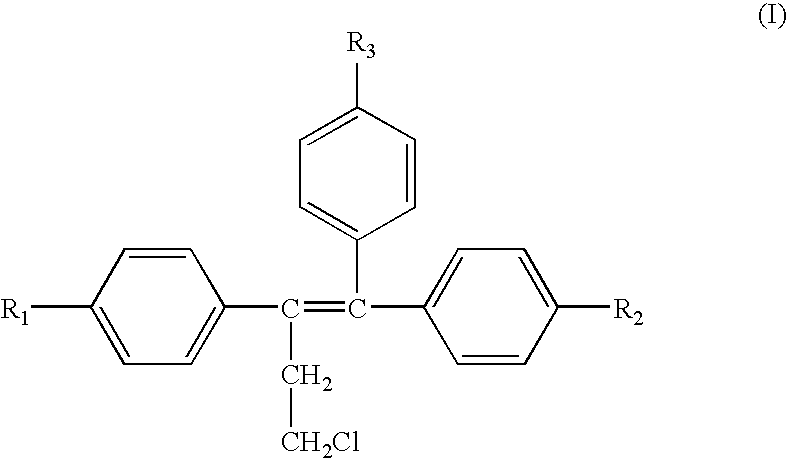
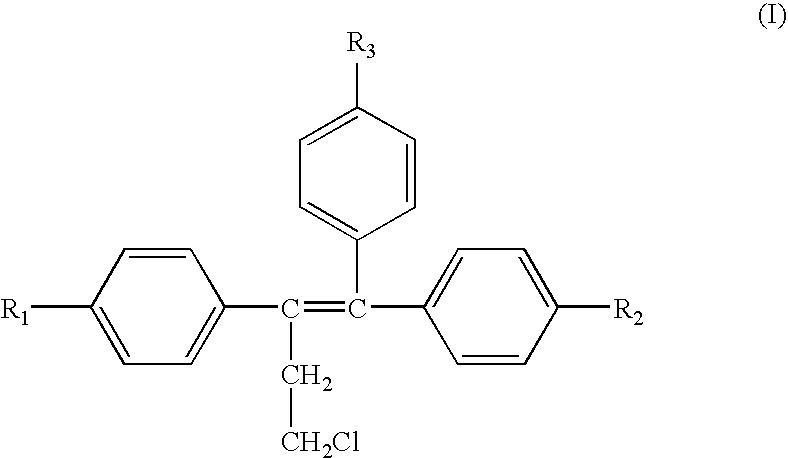
A method for treating or preventing cardiovascular pathologies by administering a compound of the formula (I): wherein Z is C═O or a covalent bond; Y is H or O(C1-C4)alkyl, R1 and R2 are individually (C1-C4)alkyl or together with N are a saturated heterocyclic group, R3 is ethyl or chloroethyl, R4 is H, R5 is I, O(C1-C4)alkyl or H and R6 is I, O(C1-C4)alkyl or H with the proviso that when R4, R5, and R6 are H, R3 is not ethyl; or a pharmaceutically acceptable salt thereof, effective to elevate the level of TGF-beta to treat and / or prevent conditions such as atherosclerosis, thrombosis, myocardial infarction, and stroke is provided. Useful compounds include idoxifene, toremifene or salts thereof. Further provided is a method for identifying an agent that elevates the level of TGF-beta. Another embodiment of the invention is an assay or kit to determine TGF-beta in vitro. Also provided is a therapeutic method comprising inhibiting smooth muscle cell proliferation associated with procedural vascular trauma employing the administration of tamoxifin or structural analogs thereof, including compounds of formula (I).
Owner:PONIARD PHARMA INC
Preparation method of tamoxifen
ActiveCN106957235AHigh yieldSimple processOrganic compound preparationAmino-hyroxy compound preparationToremifeneFiltration
The invention relates to a preparation method of tamoxifen. The preparation method is characterized by comprising the following steps of adding toremifene, a catalyst and an alkali compound into an alcohols solvent, and stirring for reacting for 10 to 24 hours at a hydrogen atmosphere at 0.1 to 1.0MPa and 20 to 150 DEG C; then cooling a reaction system to the room temperature, carrying out suction filtration, and vaporizing and eliminating the solvent at normal pressure or reduced pressure; adding ethyl acetate and water for washing, standing for layering, drying an upper-layer organic phase with anhydrous sodium sulfate, filtering to remove sodium sulfate, vaporizing and eliminating the ethyl acetate at reduced pressure, obtaining a pulp, and adding acetone into the pulp, wherein the ratio of the volume of the acetone to the weight of the pulp is 2 to 5ml / g; then heating to 50 to 56 DEG C for dissolving, and cooling to 0 DEG C for separating crystals out; drying the crystals to obtain the white needle-like solid tamoxifen.
Owner:NINGBO TEAM PHARMA
Method of treating ER mutant expressing breast cancers with selective androgen receptor modulators (SARMs)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com