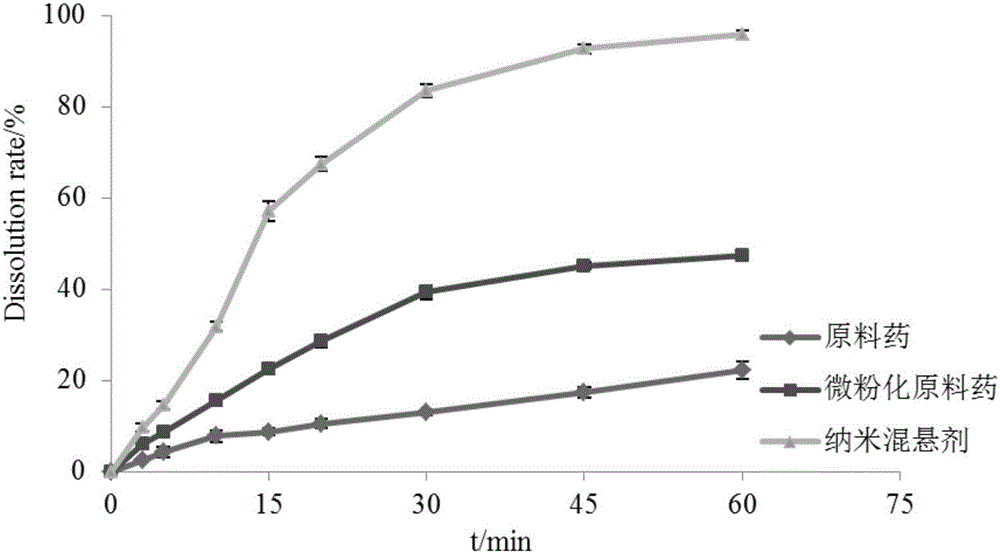
Toremifene citrate nano suspension, toremifene citrate tablet for treating ebola and preparation methods thereof
A technology of toremifene citrate and nanosuspension, which is applied in the field of medicine, can solve the problems of low oral bioavailability, large dose of anti-Ebola treatment, and low solubility of toremifene citrate. Achieve the effect of improving oral bioavailability, improving solubility and dissolution rate, and increasing adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: A preparation of toremifene citrate nanosuspension for the treatment of Ebola:
[0051]Weigh the raw materials containing the following weights in every 1000g toremifene citrate nanosuspension:
[0052]
[0053] Preparation method: Disperse toremifene citrate, PVP K30 and SDS in water, stir well and mix well, cut with high-speed shearing machine at 12000rmp for 3min, then use high-pressure homogenization method at 100Mpa, cycle 15 homogenization cycles .
Embodiment 2
[0054] Example 2: A preparation of toremifene citrate nanosuspension for the treatment of Ebola:
[0055] Weigh the raw materials containing the following weights in every 1000g toremifene citrate nanosuspension:
[0056]
[0057] Preparation method: disperse PVP K30 and SDS in redistilled water as a dispersion medium, mix well to form suspension A, disperse toremifene citrate in suspension A, mix well to form suspension B, Then suspensoid B was cut with a high-speed shearing machine at 12000rmp for 5 minutes to obtain suspension C, and then high-pressure homogenization method was used at 100Mpa to circulate 15 homogenization cycles to obtain toremifene citrate suspension agent.
Embodiment 3
[0058] Example 3: A preparation of toremifene citrate nanosuspension for the treatment of Ebola:
[0059] Weigh the raw materials containing the following weights in every 1000g toremifene citrate nanosuspension:
[0060]
[0061] Preparation method: Disperse toremifene citrate, PVP K30 and SDS in water, stir well and mix well, cut with high-speed shearing machine at 12000rmp for 3min, then use high-pressure homogenization method at 100Mpa, cycle 15 homogenization cycles .
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com