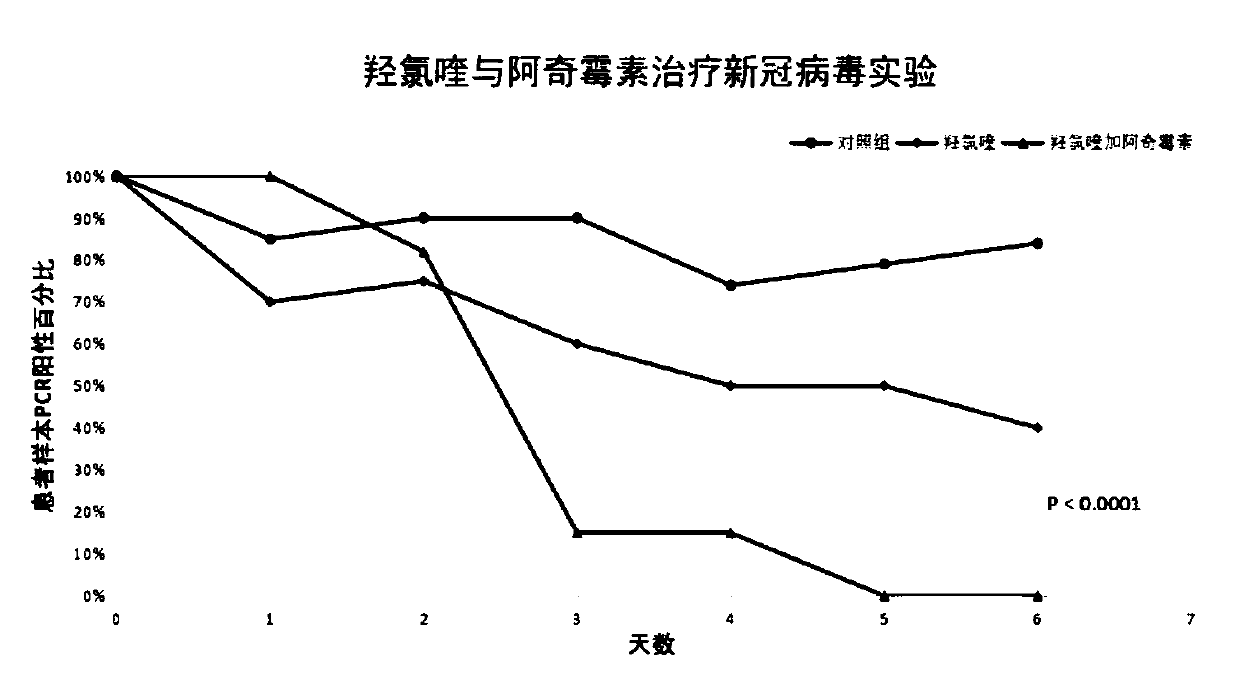
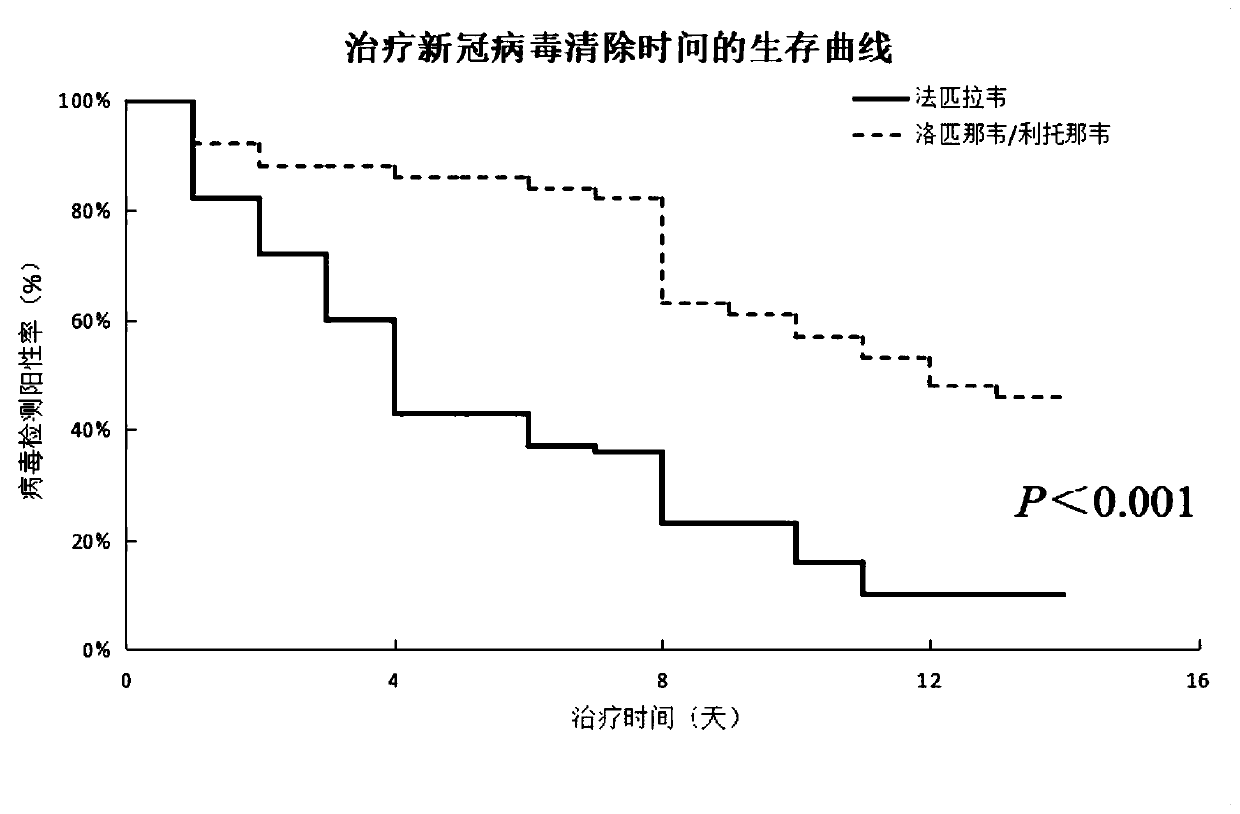
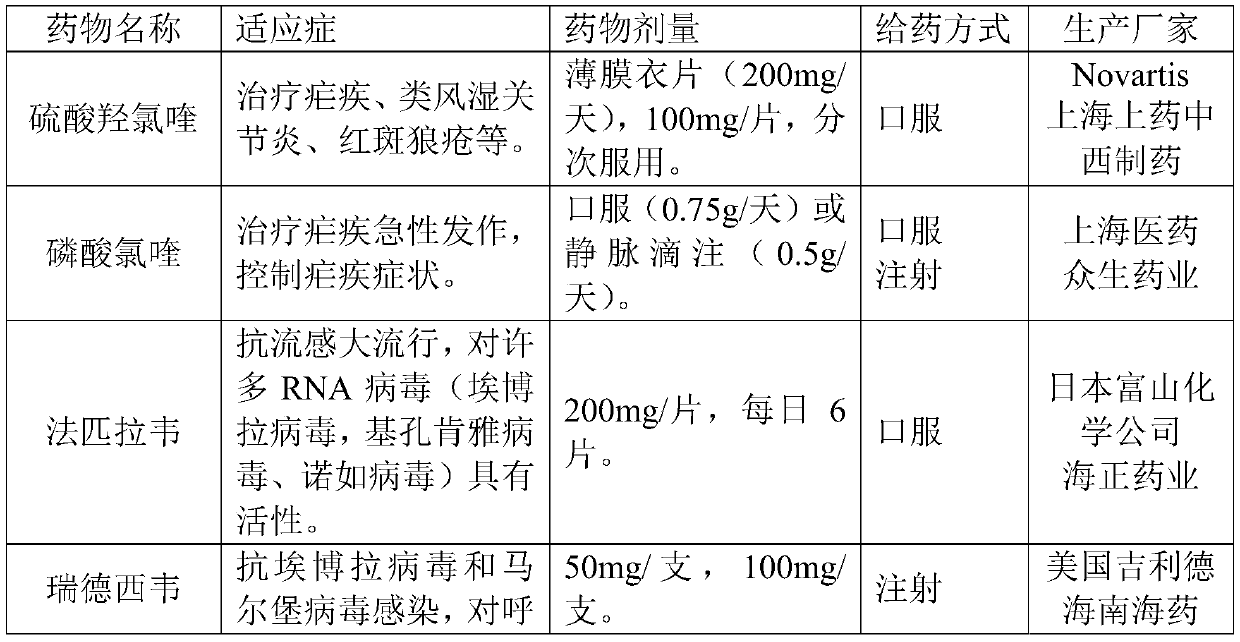
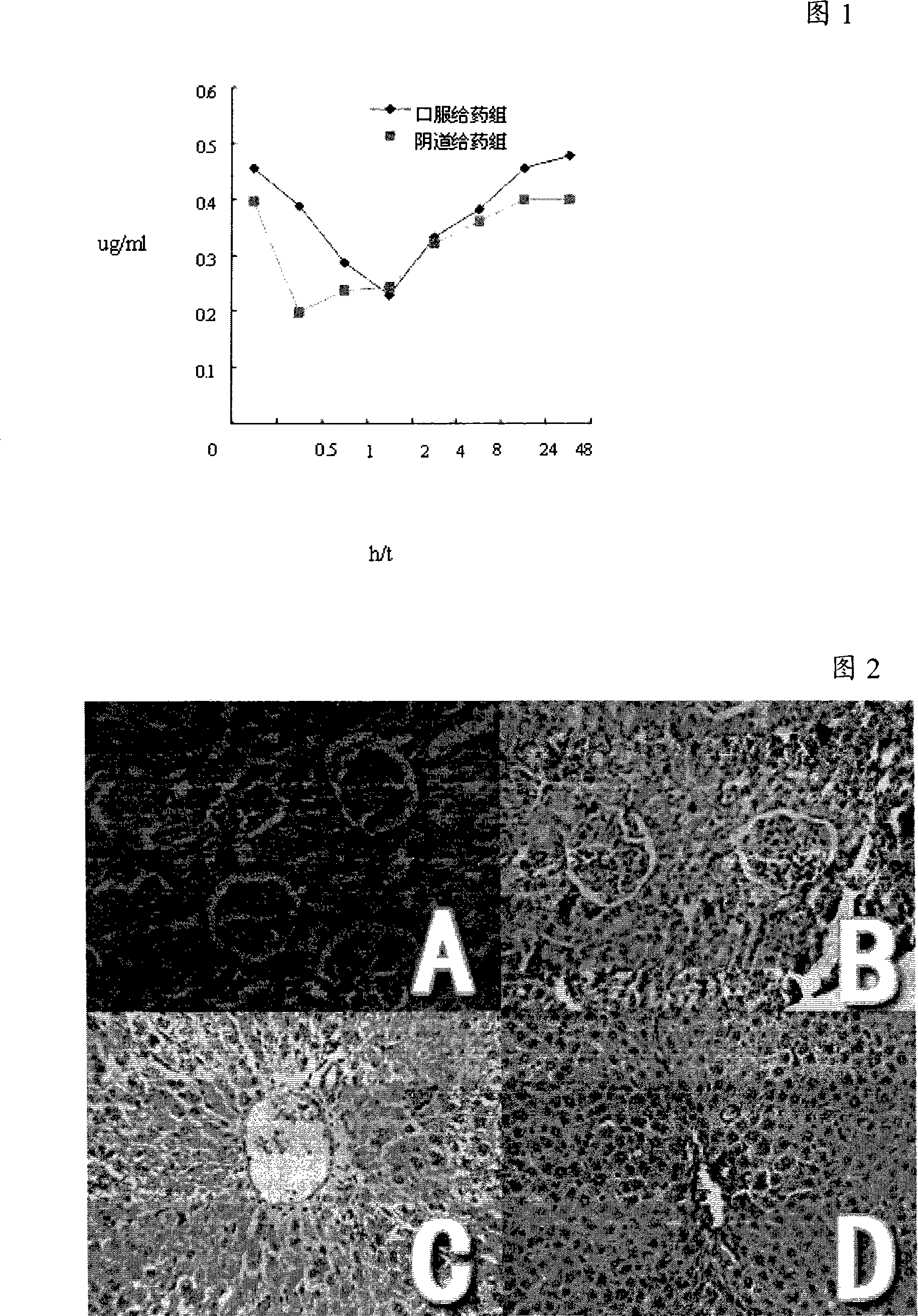
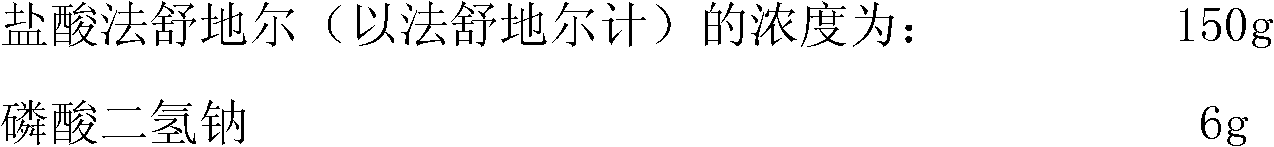Patents
Literature
47results about How to "Avoid the first pass effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inhalation spray of antivirus medicines
PendingCN111297838ASmall doseSmall toxicityOrganic active ingredientsDispersion deliveryPulmonary infectionActive agent
The invention relates to an inhalation spray of antivirus medicines. The inhalation spray comprises the following components in percentage by mass of 0%-30% of antivirus activity agents, 0%-30% of auxiliary agents, 0%-30% of taste masking agents and the balance solvents, wherein the content of the antivirus activity agents and the content of the auxiliary agents are not 0% at the same time. Compared with the prior art, the inhalation spray disclosed by the application has the purpose that during outbreak period of epidemic corona viruses and other viruses, people do not need to occupy medicalresources in short supply and only need to inhale the antivirus medicines into respiratory tracts, medicine administration is accurately targeted, and the objective of preventing virus infection and propagation can be achieved; in addition, through united medication of the antivirus activity agents and the auxiliary agents, besides, various medicines are inhaled into the respiratory tract of a patient, synergistic treatment effects are generated, the viruses are eliminated, and serious respiratory tract and infection and serious pulmonary infection caused by the viruses can be treated; and chloroquine type antivirus medicines and macrocyclic antibiotics are inhaled through spraying for united medication, and the dosage of the medicines can be notably reduced, so that side effects of prolonged QT intervals, Tdp and sudden cardiac death caused by the medicines can be reduced.
Owner:宁波合康生物医药科技有限公司
Broad-spectrum cancer-treating Chinese medicine prepn and its prepn process
InactiveCN1772262ASignificant effectImprove general conditionCapsule deliveryAntineoplastic agentsSide effectTreatment effect
The present invention relates to one kind of broad-spectrum cancer treating Chinese medicine capsule and its preparation process. It is prepared with trichosanthes root, edible tulip bulb, zedoary, pinellia tuber, grisaema tuber and other Chinese medicinal materials. It has the functions of nourishing Yin, promoting blood circulation, warming Yang, promoting urination, dredging meridian and dissipating mass. The animal experiment and the clinical application show that the Chinese medicine has obvious curative effect, no negative reaction and no toxic side effect.
Owner:吴军杰
Female surface coating contraceptive
InactiveCN101181638AImprove permeabilityAvoid damageOrganic active ingredientsSexual disorderAnti-ProgestinOral medicine
The invention relates to a surface coating adopting contraceptive for women, which consists of one or more than one active components such as compound, infiltrating accelerant, and humectant in anti-progestin medicine or progestational hormone medicine and estrogen medicine as well as normal raw materials such as oily component, aqueous component, macromolecule material, surface active agent, bacteriostat, chemical inhibitor and spice and so on. The invention can be made into electuary, emulsion, cream, jellies, spray and liquid cream so as to be used in contraception, urgent contraception, lactation contraception and reproductive health. The contraceptive adopts a method of being coated on the skin or the mucosa surface to reach the aim so as to prevent the first-pass effect and the gastrointestinal tract effect of the oral medicine, thereby reducing the injure to the liver and the kidney, reducing toxic and side effect, providing new preparation, method and route of administration for enlarging the range of the medicine-user, and making the contraception become more simple, convenient, easier and more willing to be accepted.
Owner:程定超
Nebulizer for treating respiratory system diseases
ActiveCN103893165AQuick effectAvoid the first pass effectAntibacterial agentsAntimycoticsDiseaseNebulizer
The invention provides a nebulizer for treating respiratory system diseases. The nebulizer contains (A) 0.1-20 g / L of terpenoid, (B) 0.1-20 g / L of a non-ionic surfactant, and (C) 0.1-20 g / L of an osmotic pressure regulator. The nebulizer can form medicinal steam, mist or aerosol through a sprayer or an atomizer and the like, and is inhaled by the respiratory tract or locally sprayed. The nebulizer has the following advantages that 1, the target is directly arrived, the response is quick, and local action or general action can be exerted; 2, the first-pass effect of the liver and the destroying and degradation of gastrointestinal tracts can be avoided, and the bioavailability is high; 3, the nebulizer has good compliance.
Owner:BEIJING GRAND JOHAUM PHARMA CO LTD
Combination antihypertensive wafer
InactiveCN101472557AEfficient use ofPrecise coordinationOrganic active ingredientsPharmaceutical delivery mechanismDepressantTherapeutic treatment
The present invention relates to a planar-shaped drug preparation that quickly dissolves or decomposes in an aqueous environment, for the application of active ingredient combinations for the treatment of hypertension, wherein the drug preparations contain at least two active ingredients that are suitable for the treatment of hypertension, and wherein the antihypertensive drug is selected from the group that encompasses beta receptor blockers, alpha receptor blockers, calcium antagonists, ACE inhibitors, AT1 antagonists, centrally acting antihypertensive drugs, direct vasodilators, and diuretics. The present invention also relates to the use of active ingredient combinations according to the invention for the production of an oral drug preparation for the treatment of high blood pressure, to a method for the therapeutic treatment of hypertension, and to a method for the production of a planar-shaped drug preparation.
Owner:LTS LOHMANN THERAPIE-SYST AG
Lappaconitine transdermal patch and preparation method thereof
InactiveCN101574331AEasy to useImprove bioavailabilityOrganic active ingredientsAntipyreticTransdermal patchSide effect
The invention discloses a lappaconitine transdermal patch and preparation method thereof. The patch comprises a back sheet, a drug storing layer and a protection layer, wherein, the drug storing layer is composed of the raw materials of lappaconitine and pressure sensitive adhesive, or a right amount of transdermal accelerant is added to finally prepare lappaconitine transdermal patch. The lappaconitine transdermal patch of the invention has the advantages of convenient use, high bioavailability and lasting action, avoids first-pass effect of liver as well as damage to gastrointestinal tract, reduces drug toxicity and side effect, improves bioavailability of drug and safety of treatment, and has obvious effect on anti-inflammatory and analgesia.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
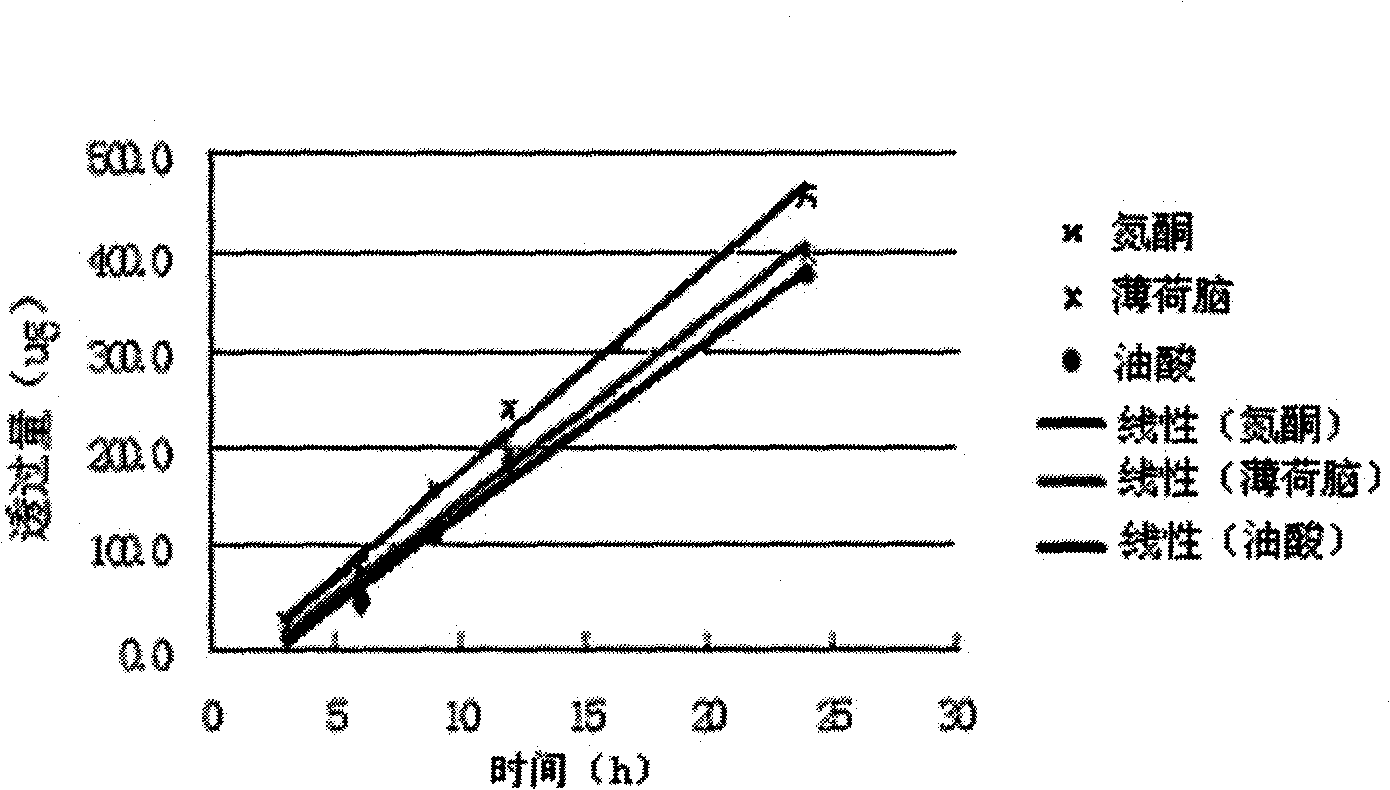
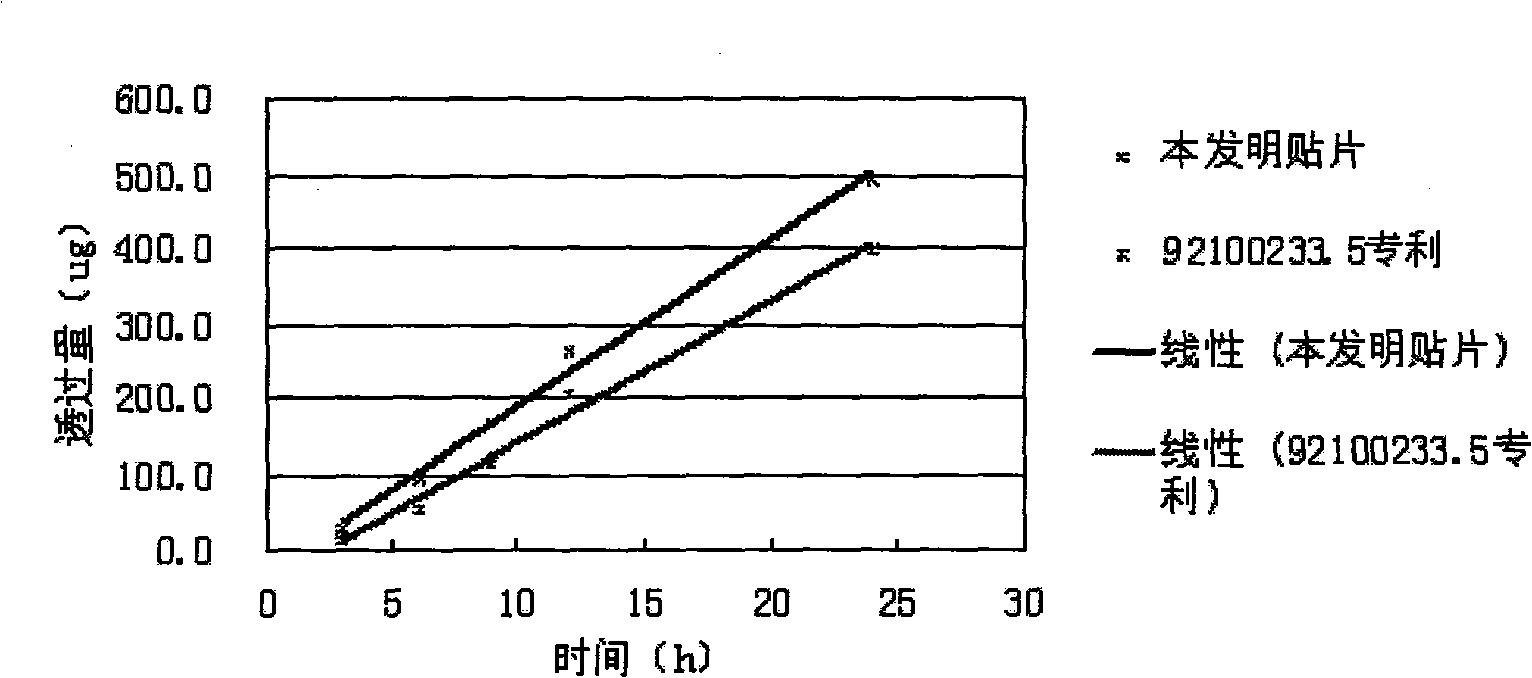
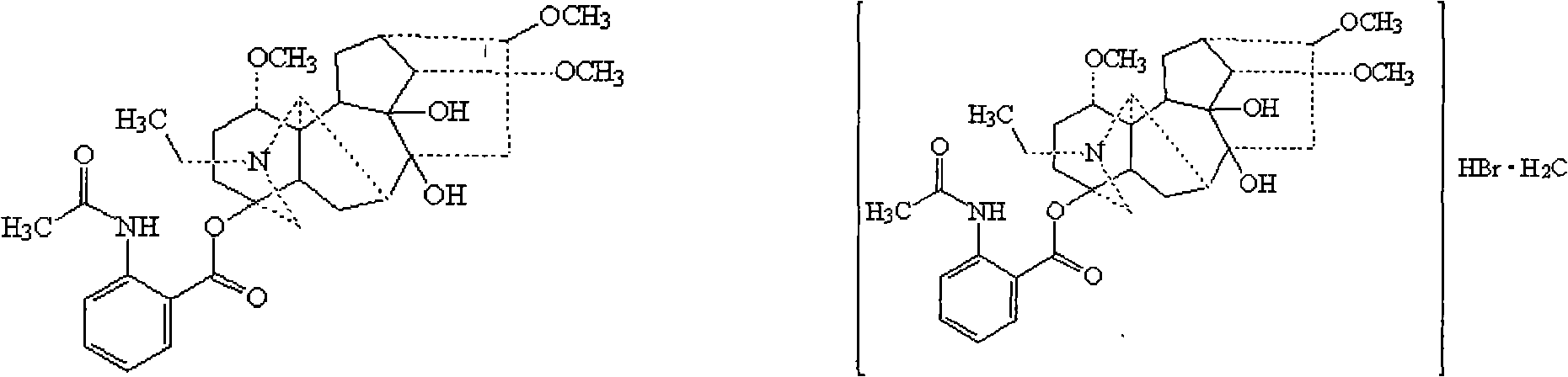
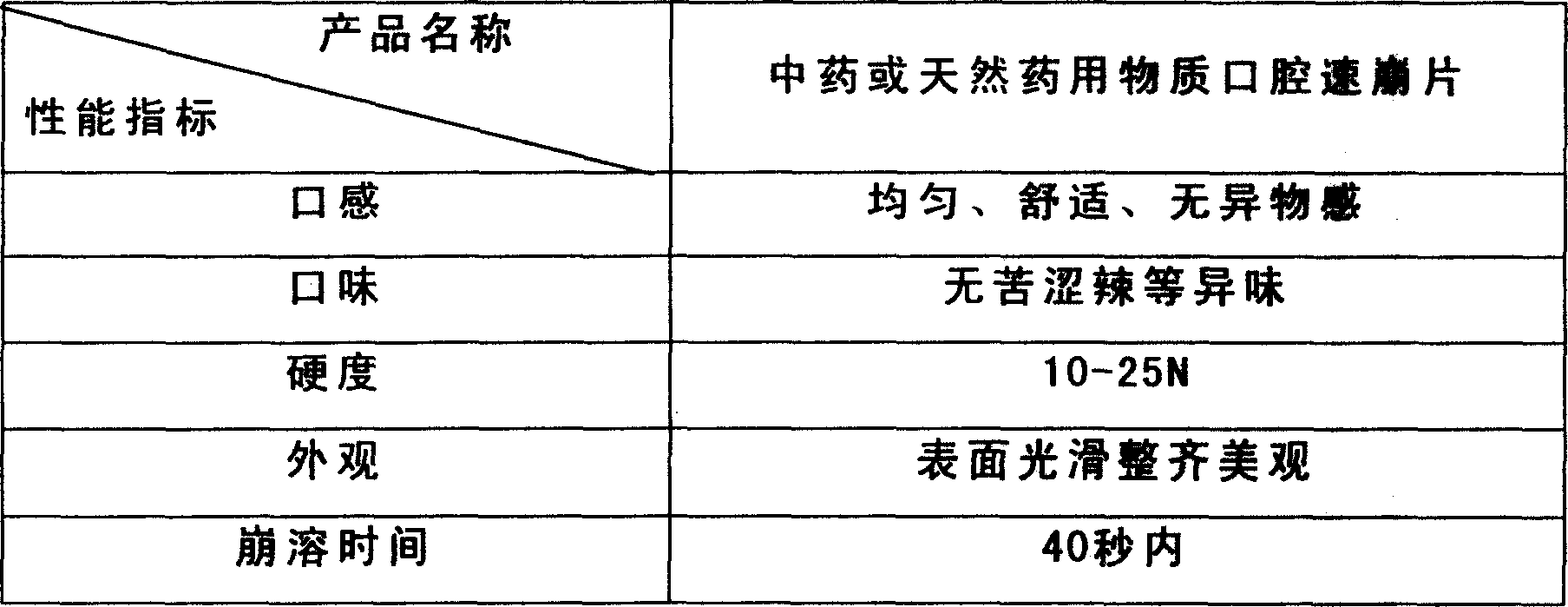
Mouth cavity fast disintegrating tablet of Chinese medicine or natural medical matters and its preparing method
InactiveCN1473560AAvoid the "first pass effect"Promote dissolutionOrganic active ingredientsUnknown materialsForeign matterHigh volume manufacturing
The present invention relates to a kind of oral cavity fast disintegrating tablet of Chinese medicine or natural medicine, and the cavity fast disintegrating tablet comprises active component of Chinese medicine or natural medicine and medicinal applicable supplementary material. The active component of Chinese medicine or natural medicine is compound separated from Chinese medicine or natural medicine plant. The oral cavity fast disintegrating tablet can dissolve in oral cavity fast, in 40 sec, without needing water drunk, has no foreign matter feeling and may be leached in 15 min. The oral cavity fast disintegrating tablet may be produced with common tablet press.
Owner:NINGBO LIWAH PHARM CO LTD
Traditional Chinese medicine composition with bacteriostatic, anti-inflammatory, hemostatic and analgesic effects and preparation method thereof
InactiveCN111000891AImprove anti-inflammatory and antibacterial effectAntibacterial and anti-inflammatory fastAntipyreticAerosol deliveryMedicinal herbsPolyethylene glycol
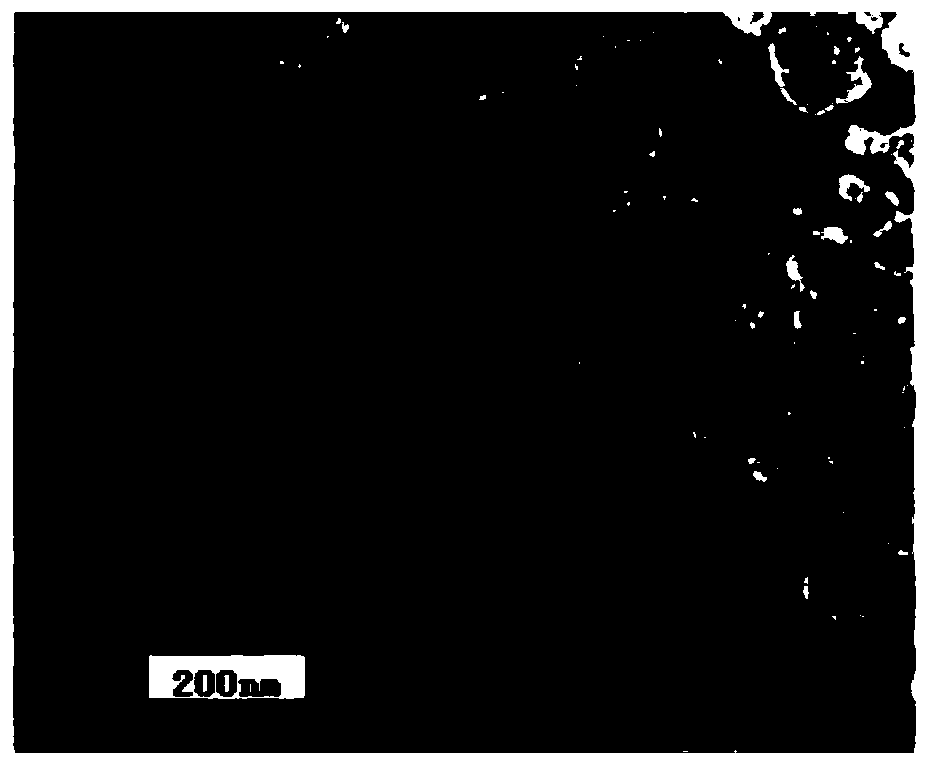
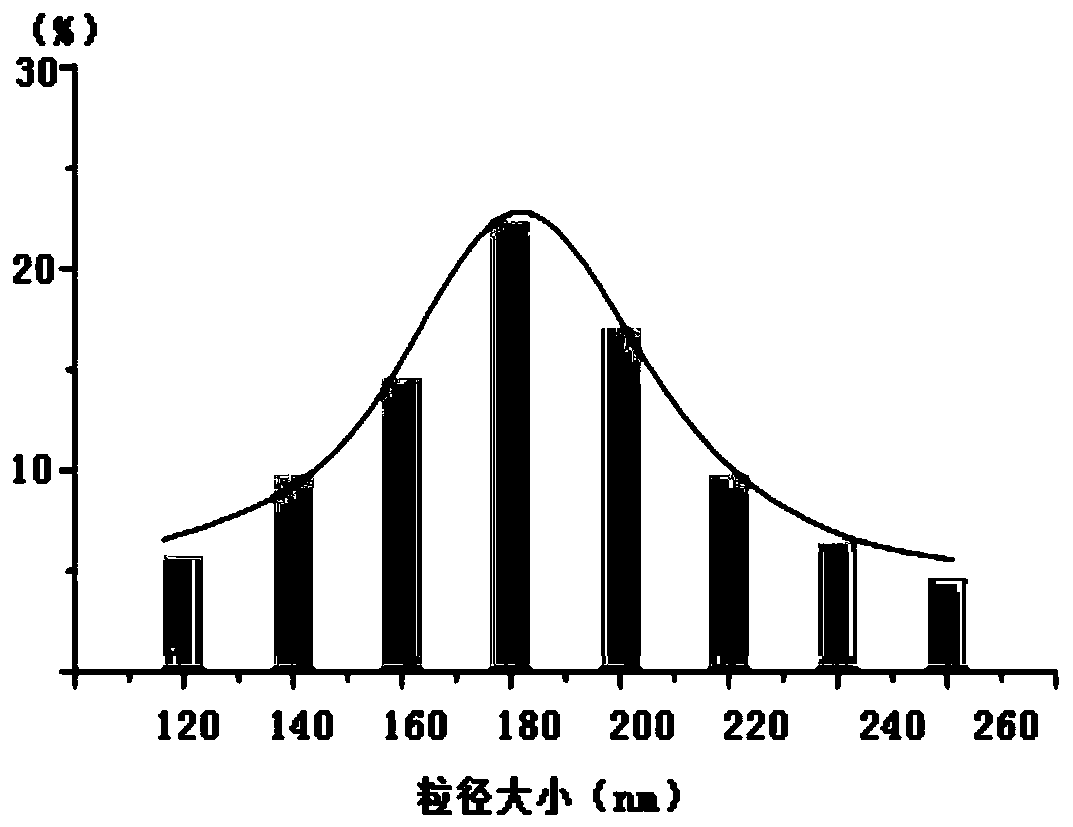
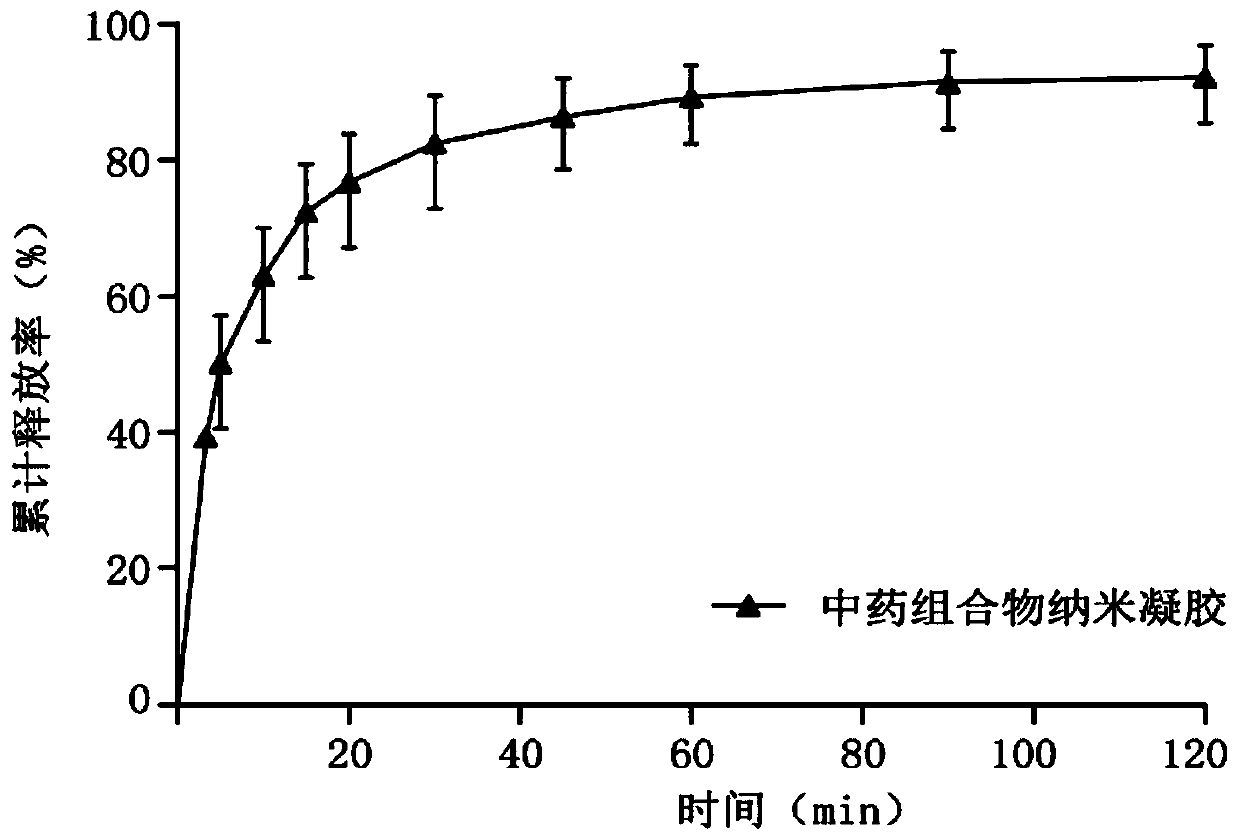
The invention discloses a traditional Chinese medicine composition with bacteriostatic, anti-inflammatory, hemostatic and analgesic effects and a preparation method thereof. According to the method, apithecellobium clypearia extract and a lamiophlomis rotata extract are used as raw materials; poly(lactic-co-glycolic acid) PLGA, vitamin E polyethylene glycol succinate TPGS and poloxamer are used as a drug-loaded matrix. The preparation method comprises the following steps: mixing raw medicinal materials with the drug-loaded matrix to form an oil phase and an oil-in-water phase, performing probe ultrasonic emulsification to obtain a nano-suspension, and adding carbomer, collagen tripeptide, oat beta-glucan and propylene glycol as a gel matrix to obtain traditional Chinese medicine composition nanogel. The prepared traditional Chinese medicine composition nanogel has the particle size of 120 nm-260 nm, has good drug loading capacity, obviously improves the solubility and bioavailabilityof hydrophobic drugs, inhibits bacteria, diminishes inflammation, stops bleeding and relieves pain, and is definite in curative effect.
Owner:江西杏林白马药业股份有限公司
Traditional Chinese medicine foot bath preparation prepared by utilizing microbial fermentation and preparation method thereof
InactiveCN105125666AImprove permeabilityImprove efficiencyHeavy metal active ingredientsAntimycoticsDiseaseCuticle
The invention discloses a traditional Chinese medicine foot bath preparation prepared by utilizing microbial fermentation and a preparation method thereof and belongs to the field of parenteral administration preparations. The preparation method includes: placing brewer's yeast, candida utilis and lactobacillus reuteri in a water solution of ginkgo leaf extract, algae powder, menthol, baking soda, edible salt and glucose at 37-40 DEG C for anaerobic cultivation to obtain microbial fermentation foot bath preparation engineering bacterium liquid. The bacterium liquid is adopted for fermenting compound traditional Chinese medicine. The ginkgo leaf extract has promoting effect on expanding pores and blood vessels and increasing administration speed, menthol has transdermal efficacy and can improve permeability of drug in a plantar dermis layer and a cuticle layer, density of foot bath liquid prepared by baking soda, edible salt and glucose is greater than that of body fluid, so that osmotic pressure of the foot bath liquid is increased. Toxic and harmful substances in the compound traditional Chinese medicine are degraded after fermentation, and absorbing effect of traditional Chinese medicine activity agent is improved by utilizing synergistic effect of beneficial bacteria and active ingredients of the traditional Chinese medicine. The traditional Chinese medicine foot bath preparation is effective in disease dispelling, healthcare and scientific administration.
Owner:朱民生
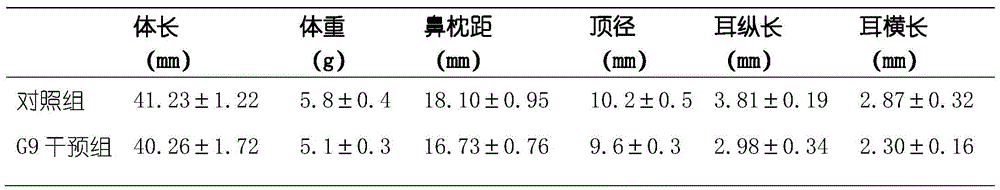
Method for preparing congenital microtia animal model
InactiveCN105616395AAvoid the first pass effectAvoid decompositionHydroxy compound active ingredientsPharmaceutical delivery mechanismPregnancyAnimal science
The invention belongs to the technical field of animal model preparation, and in particular relates to a method for preparing a congenital microtia animal model. The method for preparing the congenital microtia animal model comprises the following operation steps: (1) feeding rats enabling to mate; (2) selecting pregnant female rats, and feeding in different cages randomly; (3) performing hypodermic injection of 30-60 mg / kg of retinoic acid to the female rats after 8-13.5 days of pregnancy; (4) normally feeding till 20 days of pregnancy, taking off the necks of the pregnant rats, and taking off embryos from deciduae, thereby obtaining the ongenital microtia animal model. The preparation method of the ongenital microtia animal model is simple to operate, low in cost, convenient to copy, good in repeatability and high in success rate, and compared with a transgenic congenital microtia animal model or a congenital microtia animal model prepared through gene knockout, the congenital microtia animal model is relatively close to the natural process of disease occurrence.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
Percutaneous drug administration cream ,ointment of letrozole and preparation method thereof
InactiveCN101172107AGood curative effectLittle side effectsOrganic active ingredientsAerosol deliverySide effectSkin penetration
The invention relates to the cream and the ointment of Letrozole tablet percutaneous administration and the preparation method of the cream and the ointment thereof, and relates to the cream and the ointment of the percutaneous administration and the preparation method of the cream and the ointment thereof. The invention solves the problems that the prior Letrozole tablet oral preparation cannot avoid the first pass effect of liver and the bad reaction is numerous. The cream and the ointment of the percutaneous administration are characterized in that the cream and the ointment of the percutaneous administration comprise raw materials and penetrating agent with the following percentages: 0.01 to 20 percent of Letrozole tablet and 0.1 to 20 percent of percutaneous penetrating agent. The invention has the method that through vitro and a skin penetration test, the Letrozole tablet only with oral preparation is filtered out a formula with good non-steady state / steady state distribution by a skin release curve, and then the cream and the ointment of the percutaneous administration are prepared, so as to lead principal medicine to avoid the liver first pass effect of oral administration to directly reach mammary gland target tissue, and to lead the concentration of target tissue internal medicine to be improved. Compared with the prior preparation at home and at abroad, the invention technically has the advantages that the prepared external preparation can reduce the side effect of Letrozole tablet and improve the curative effect of the Letrozole tablet, thereby the invention has good promotion and application value.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
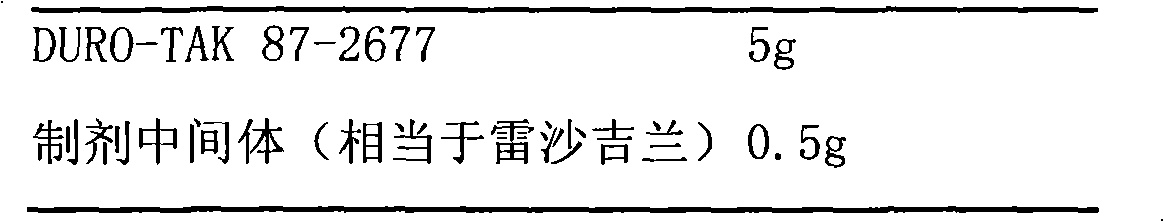
Slow release long-acting rasagiline transdermal patch with high bioavailability and preparation method thereof
InactiveCN102100682AStable absorptionAvoid the first pass effectOrganic active ingredientsNervous disorderTransdermal patchAcrylic polymer
The invention relates to a slow release long-acting rasagiline transdermal patch with high bioavailability and a preparation method thereof. The patch is characterized in that effective amount of rasagiline and a substrate are contained, wherein the concentration of rasagiline is in the range of 0.1 mg / cm2 - 2 mg / cm2, every patch contains 1 mg - 10 mg of rasagiline, and the substrate contains one or more than two kinds of the following substances: acrylic polymers containing no carboxylic group and silicone polymers with silanol groups capped by alkyls. The time of maximum plasma drug concentration of the patch is prolonged to be 5 times or more that of oral tablets, and the bioavailability of the patch is 130 percent or more of that of oral tablets. The patch in the invention can be applied to effectively inhibit monoamine oxidase B (MAOB) for at least 3 days, in addition, the preparation method is simple and suitable for industrial production.
Owner:CHONGQING PHARMA RES INST
Transdermal drug delivery system for treating infantile diarrhea and preparation method thereof
InactiveCN101972276ASmall side effectsConvenient treatmentAnthropod material medical ingredientsDigestive systemDiseaseAdhesive
The invention discloses a transdermal drug delivery system for treating infantile diarrhea. The transdermal drug delivery system consists of a substrate layer, a backing layer and a protective film; and the substrate layer comprises the following components in percentage by weight: 0.5 to 55 percent of gallnut extract, 5 to 60 percent of adhesive, 3 to 20 percent of organic solvent, 1 to 20 percent of plasticizer, 2 to 30 percent of thickening agent, and 1 to 10 percent of penetration enhancer. The substrate layer of the traditional Chinese medicine gallnut transdermal drug delivery system has high compatibility and adhesion with skin; the substrate layer has high stability; and the transdermal drug delivery system can be adhered to infantile Shenque acupoint (that is, navel), has the advantages of treating an inside disease outside the body of a patient, treating and preventing, avoiding injection and administration, being accepted by sick children and parents easily, along with simplicity, convenience and high operability.
Owner:SOUTH CHINA UNIV OF TECH
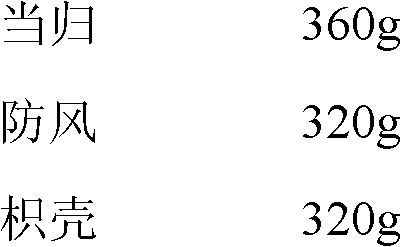
Cataplasm for curing acute injury of soft tissues and preparation method thereof
InactiveCN102579783AGood followabilitySymptoms improvedAntipyreticAnalgesicsPatient complianceIrritation
The invention relates to a cataplasm for curing acute injury of soft tissues and a preparation method thereof. The cataplasm is prepared by raw materials of angelica extract, saposhnikoviae radix extract, bitter orange extract, anemarrhena asphodeloides extract, rhizoma arisaematis (processed) extract, fructus trichosanthis extract, angelica dahurica extract, carthamus tinctorius extract, thunberg fritillary bulb extract and suitable accessories. The cataplasm consists of rear lining layers, ointments containing drugs, and cover lining layers. The cataplasm takes effect quickly, performances of moisture and air permeability are good, usage is comfortable and convenient, irritation on skins is small, and compliance of patients is high.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
Frovatriptan inhalation aerosol powder, preparation method thereof and application of powder
InactiveCN110251492AGood lung deposition rateGood for storagePowder deliveryOrganic active ingredientsMicrometerFrovatriptan
The invention discloses frovatriptan inhalation aerosol powder which comprises frovatriptan micro-powder and carrier micro-powder. The particle sizes of the frovatriptan micro-powder are d10=0.8-1.8 micrometers, d50=1.5-4.5 micrometers and d90=3.0-9.0 micrometers, the particle sizes of more than 95% of the frovatriptan micro-powder are smaller than 10 micrometers, the particle sizes of the carrier micro-powder are d10=20-45 micrometers, d50=80-110 micrometers and d90=120-180 micrometers, and the weight ratio of the frovatriptan micro-powder to the carrier micro-powder is 1:(1-1000). A preparation method of the frovatriptan inhalation aerosol powder includes the steps of frovatriptan micronizing, carrier micronizing, carrier granulation, mixing and sub-packaging. Capsules are filled with the blended powder by a micro-capsule filling machine, and the powder is sub-packaged to prepare the capsules containing the frovatriptan inhalation aerosol powder, and the filling amount of each capsule is 1-200mg. The frovatriptan inhalation aerosol powder includes a nasal inhalation mode and an oral inhalation mode, and frovatriptan can exist in a physiological salt form. The frovatriptan inhalation aerosol powder is high in bioavailability and rapid in effect.
Owner:ZHUHAI RESPROLY PHARM TECH CO LTD
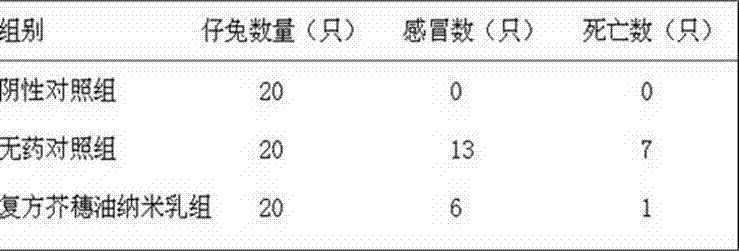
Compound oil-in-water schizonepeta spike oil nanoemulsion composition and preparation method thereof
InactiveCN102397330APrevention and treatment of cold diseasesGood water solubilityAntiviralsRespiratory disorderNasal cavitySaposhnikovia
The invention discloses a compound oil-in-water schizonepeta spike oil nanoemulsion composition, which consists of the following raw materials in percentage by mass: 0.1 to 6.0 percent of schizonepeta spike oil, 0.1 to 5.0 percent of divaricate saposhnikovia root oil, 0.1 to 5.0 percent of weeping forsythiae capsule oil, 0.1 to 2.0 percent of peppermint oil, 0.01 to 0.65 percent of sodium houttuyfonate, 19.0 to 33.0 percent of surfactant and the balance of distilled water, wherein the total percentage by massweight of the raw materials is 100 percent. The nanoemulsion has the effects of relieving exterior syndrome by diaphoresis, clearing away heat and toxic materials, removing pathogenic wind and raising the nonspecific immunity of an organism, and is used for preventing and treating inappetence, increased temperature, cold intolerance and lack of power, which are caused by cold, and sore throat, cough, rhinorrhea, mucus even purulent secretion and the like fully filled in a nasal cavity, which are caused by upper respiratory tract infection, for livestock and poultryanimals.
Owner:NORTHWEST A & F UNIV
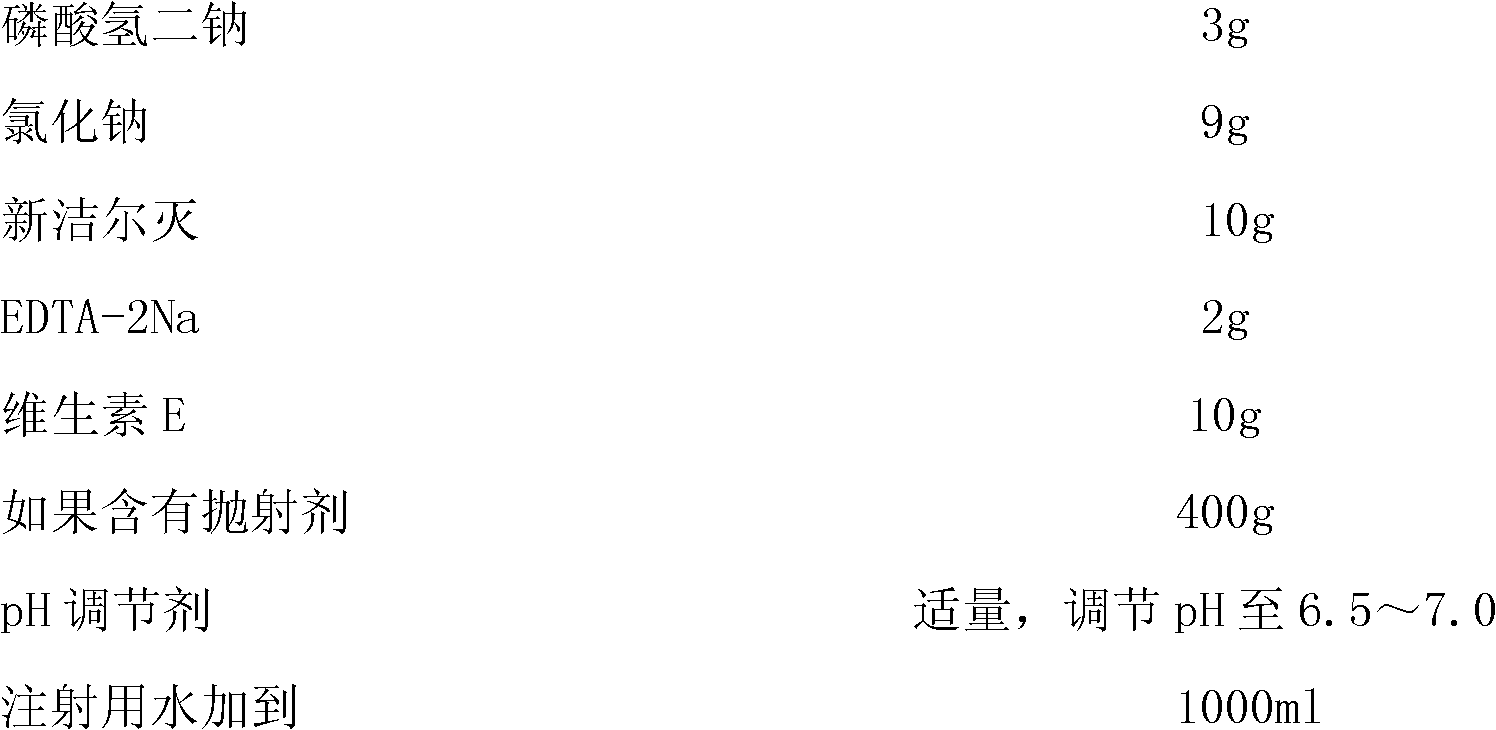
Nasal spray or aerosol containing Fasudil
ActiveCN102138901AImprove absorption rateShould not be toxicOrganic active ingredientsLiposomal deliveryActive componentNasal spray
The invention relates to a nasal spray or aerosol containing Fasudil. The active component in the preparation can be absorbed rapidly through the nasal mucosa so that the response is rapid. The nasal spray or aerosol has the advantages of high bioavailability and good compliance, and is convenient in use and carrying. The preparation provided by the invention contains Fasudil hydrochloride and pharmaceutically acceptable excipients.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Nebulized inhalers for the treatment of respiratory diseases
ActiveCN103893165BQuick effectAvoid the first pass effectAntibacterial agentsAntimycoticsDiseaseRespiratory tract disease
The invention provides a nebulizer for treating respiratory system diseases. The nebulizer contains (A) 0.1-20 g / L of terpenoid, (B) 0.1-20 g / L of a non-ionic surfactant, and (C) 0.1-20 g / L of an osmotic pressure regulator. The nebulizer can form medicinal steam, mist or aerosol through a sprayer or an atomizer and the like, and is inhaled by the respiratory tract or locally sprayed. The nebulizer has the following advantages that 1, the target is directly arrived, the response is quick, and local action or general action can be exerted; 2, the first-pass effect of the liver and the destroying and degradation of gastrointestinal tracts can be avoided, and the bioavailability is high; 3, the nebulizer has good compliance.
Owner:BEIJING GRAND JOHAUM PHARMA CO LTD
Reeombinnt human granulocyte colony stimulating factor microemulsion
InactiveCN101062406AImprove bioavailabilityAvoid the "first pass effect"Peptide/protein ingredientsEmulsion deliveryMass ratioG-csf therapy
The invention discloses a retooling human granulocyte colony stimulating factor microemulsion to medicinal technique, which comprises the following steps: allocating mass ratio of retooling human granulocyte colony stimulating factor, surface activator, cosurfactant, oil phase and water phase at 1 : (1-5) : (0. 5-3) : (1-3) : (500-2000); adding the retooling human granulocyte colony stimulating factor into oil phase; stirring evenly; dispersing in water phase; getting the product. This product is fit for treat neutrophilic granulocytopenia, which can keep bioavailability with the same effect in injection give medicine.
Owner:QINGDAO UNIV
Raloxifene emplastrum preparation and preparation method thereof
InactiveCN101700241AConvenient treatmentStabilize local blood drug concentrationOrganic active ingredientsSkeletal disorderTransdermal patchAdditive ingredient
The invention relates to a raloxifene emplastrum and a preparation method thereof. Raloxifene microemulsion is made into a transdermal emplastrum which is divided into an anti-adhesion layer, a viscose layer, a backlining layer and a medicine storage layer. The raloxifene microemulsion taken as a medical ingredient of the raloxifene emplastrum comprises the following ingredients: raloxifene taken as an active ingredient, polysorbate taken as surfactant, propanediol taken as cosurfactant, pure water or distilled water, and the like. Except for the medical ingredient, a plurality of nonpolar polymers, plasticizing agent, caking agent, transdermal enhancing agent and antioxidant are also added in the emplastrum. Compared with the prior dosage forms, such as tablets, the invention has the remarkable advantages of durable medical effect, safety, low toxicity, simple, convenient and sanitary, and accurate medical dosage.
Owner:河南省生物工程技术研究中心
Compound oil-in-water type largehead atractylodes rhizome oil nanoemulsion composition and preparation method thereof
InactiveCN102416061AImprove stabilityHigh thermodynamic stabilityDigestive systemAldehyde active ingredientsDuodenal ulcerRhizome
The invention discloses a compound oil-in-water type largehead atractylodes rhizome oil nanoemulsion composition. The nanoemulsion composition has a diameter size which ranges from 1 nm to 100 nm and consists of the following substances in percentage by mass: 0.1-7.5 percent of largehead atractylodes rhizome oil, 0.1-5.0 percent of atractylis oil, 0.1-5.0 percent of cinnamaldehyde, 0.1-3.0 percent of patchouli oil, 0.05-3.0 percent of dried tangerine peel oil, 0.05-2.0 percent of costus root oil, 0-32.0 percent of polyoxyethylene ether castor oil, 0-32.0 percent of polyoxyethylene (40) hydrogenated castor oil and the balance of distilled water, wherein the sum of the mass percentages of the components is 100 percent. The nanoemulsion composition has the effects of resisting gastrointestinal ulcer, resisting inflammation, adjusting gastrointestinal motility, and secretion of digestive juice, invigorating spleen, harmonizing stomach, eliminating dampness, tonifying spleen, stopping vomiting, sterilizing and the like. The nanoemulsion composition can be used for treatment, daily stomach health care and the like of gastric ulcer, duodenal ulcer and various kinds of acute and chronic gastritis.
Owner:NORTHWEST A & F UNIV
Insulin sustained-release oral patch as well as preparation method and application thereof
PendingCN113893333AStable hypoglycemic effectSimple processPeptide/protein ingredientsMetabolism disorderPharmaceutical AidsPharmaceutical formulation
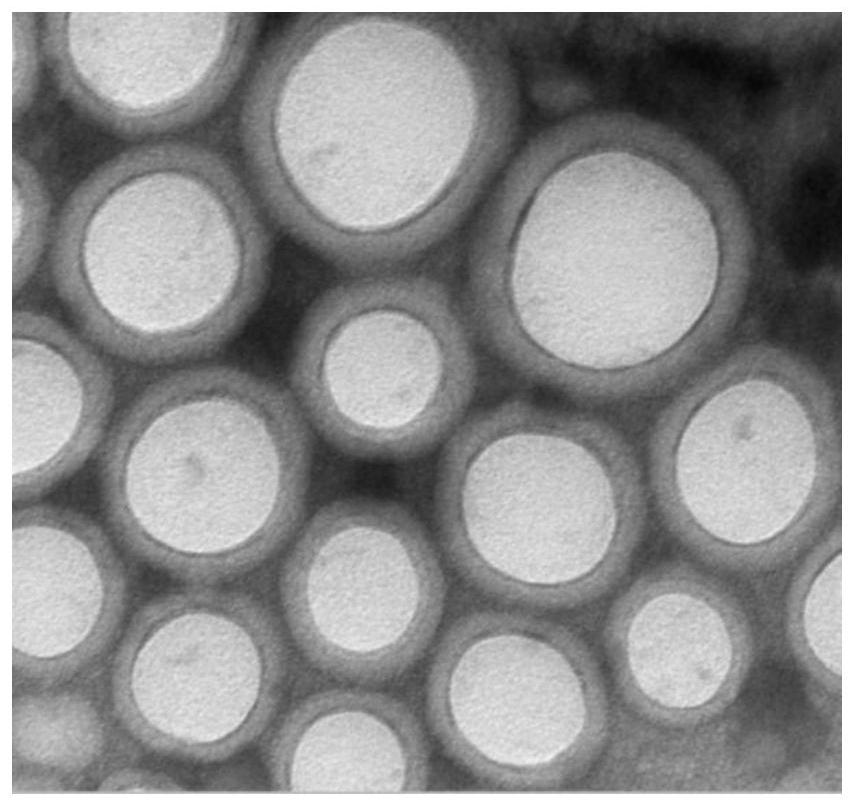
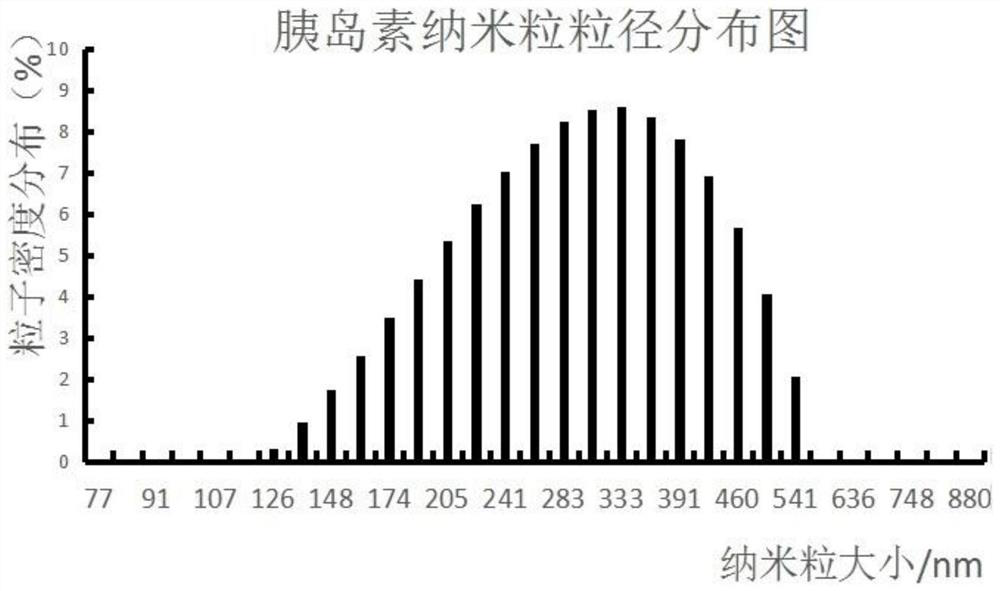
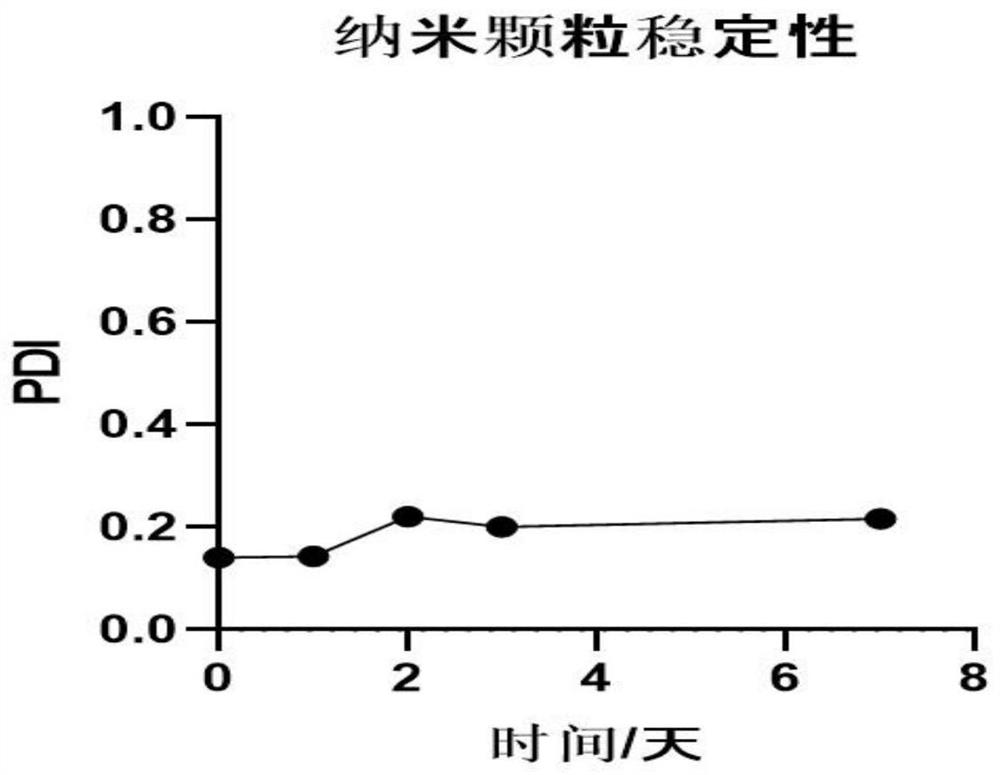
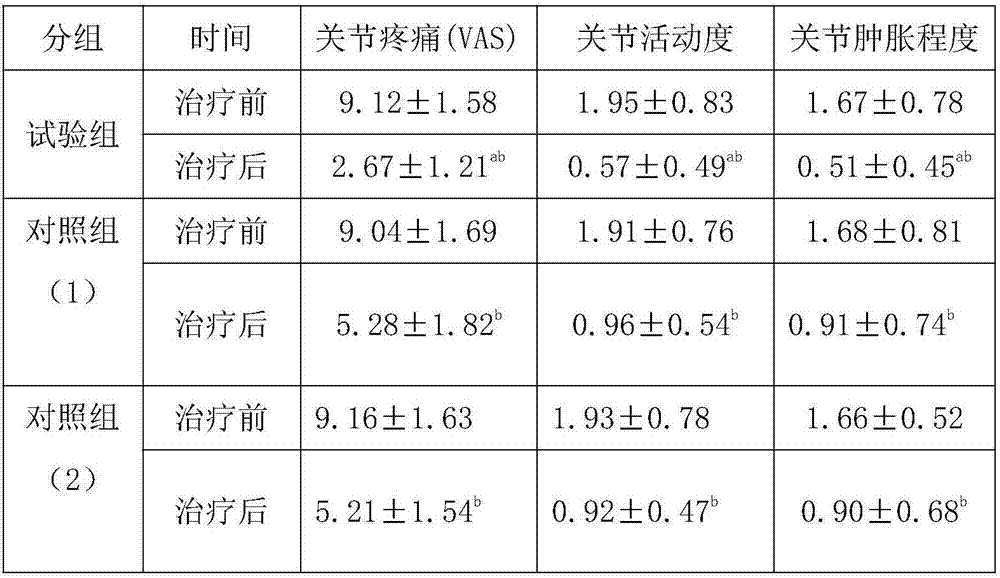
The invention relates to the field of biomedicines, and particularly relates to an insulin sustained-release oral patch as well as a preparation method and an application thereof. The pharmaceutical preparation is characterized by comprising nanoparticles and pharmaceutical excipients that comprise a filler, an adhesive and a lubricant. The dosage form of the pharmaceutical preparation is an oral patch. According to the invention, insulin is prepared into the sustained-release oral patch, thereby avoiding first-pass effect of gastrointestinal tracts, prolong action time of the medicine, improving curative effect and economic benefit of the medicine, reducing influence of enzymes on the insulin, and relieving pain of patients due to injection. The oral patch of the present invention is high in adhesive capacity, good in release and moderate in dissolution rate.
Owner:CHINA PHARM UNIV
Preparation for gout, soft tissue injury and rheumatalgia, and preparation method thereof
InactiveCN107158322AThe party is rigorousGood curative effectAntipyreticAerosol deliverySide effectRheumatism
The present invention discloses a preparation for gout, soft tissue injury and rheumatalgia, and a preparation method thereof, wherein the preparation comprises the following raw materials by weight: 20-40 parts of clematis chinensis osbeck, 20-60 parts of zanthoxylum nitidum, 10-30 parts of cremastra appendiculata, 20-40 parts of panax notoginseng, 20-60 parts of angelica sinensis, 60-100 parts of curcuma longa l, 20-40 parts of cape jasmine, 10-30 parts of safflower, and 10-30 parts of rhizoma paridis. According to the present invention, the preparation has effects of heat clearing, blood cooling, wind evil expelling, dampness removing, blood circulation activation, blood stasis removing, tendon relaxing and activating, swelling subsiding and pain stopping, can provide good treatment effects for gout, soft tissue injury and rheumatalgia, and further has characteristics of convenient use, safety, no toxicity, and no side effect; and the preparation method has characteristics of simple process, low production cost and low energy consumption, and is suitable for industrial production.
Owner:广西大海阳光药业有限公司
Fasudil-containing oral spray or aerosol
ActiveCN102144971AShould not be toxicShould not be irritatingOrganic active ingredientsAerosol deliveryAerosol drugsMouth mucosa
The invention relates to fasudil-containing oral spray or aerosol in which active constituents can be quickly absorbed through the mouth mucosa to work quickly; and the spray or the aerosol has the advantages that the spray or the aerosol is high in bioavailability, excellent in compliance, portable, convenient to use and the like. The spray or the aerosol comprises the following constituents: fasudil hydrochloride, and pharmaceutically acceptable accessories selected from buffering agent, osmotic pressure regulator, flavoring agent, preservative, absorbefacient, antioxidant, bioadhesive agent, pH modifier, dissolvent and the like.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Antitumor composition inhalation powder aerosols and preparation method thereof
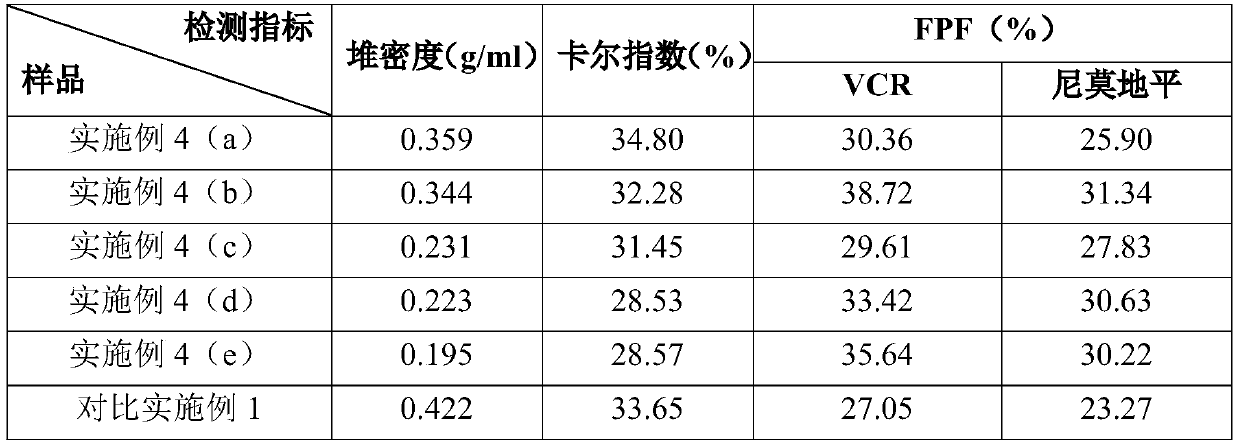
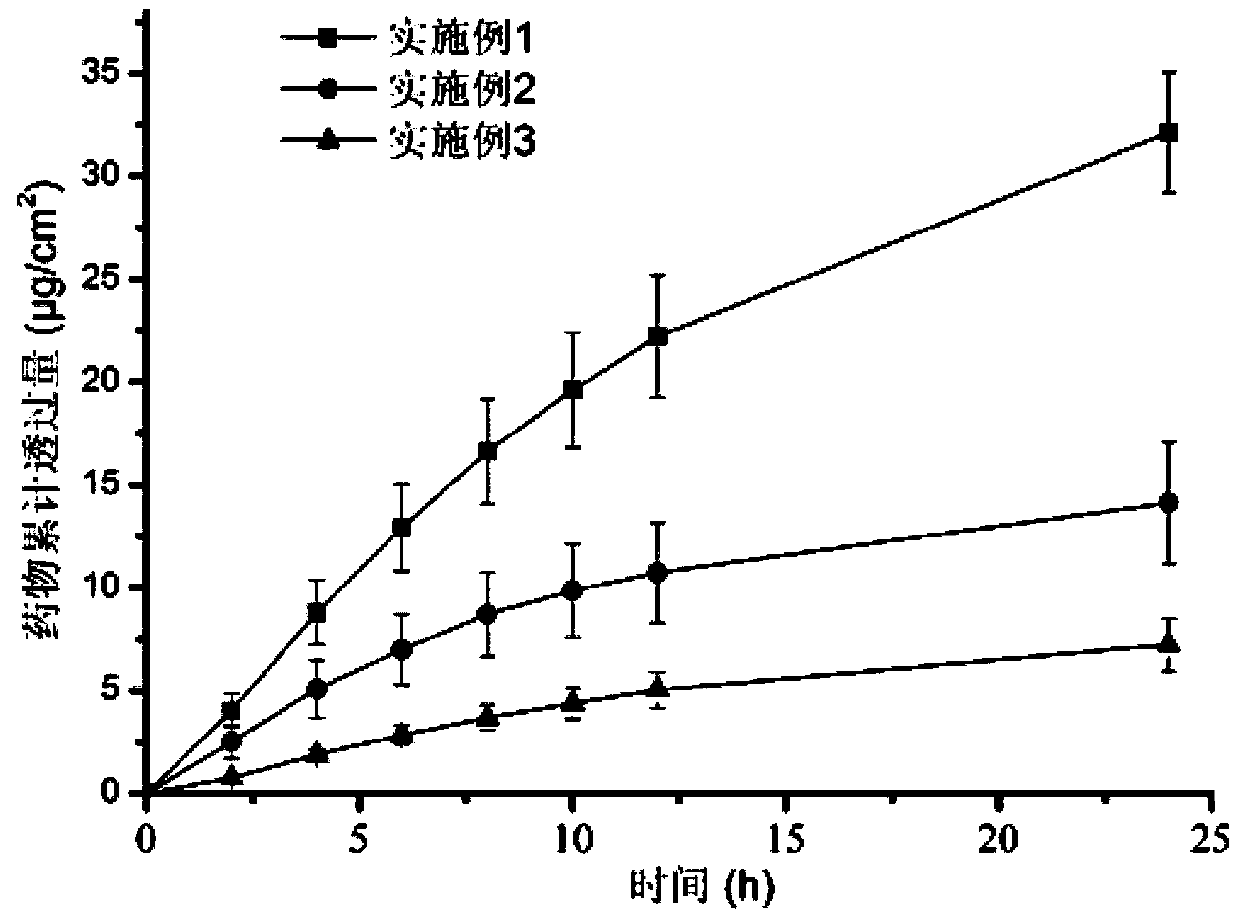
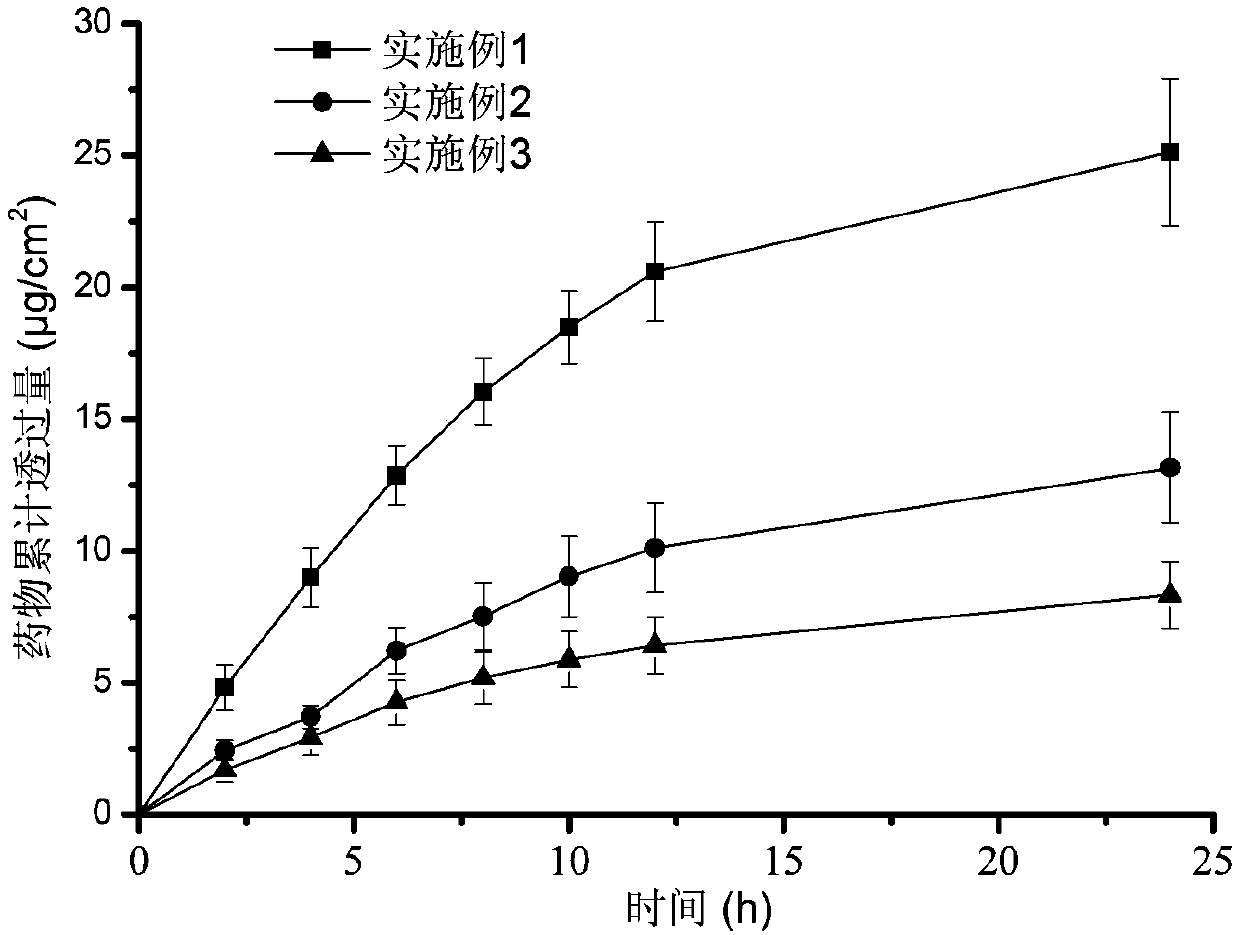
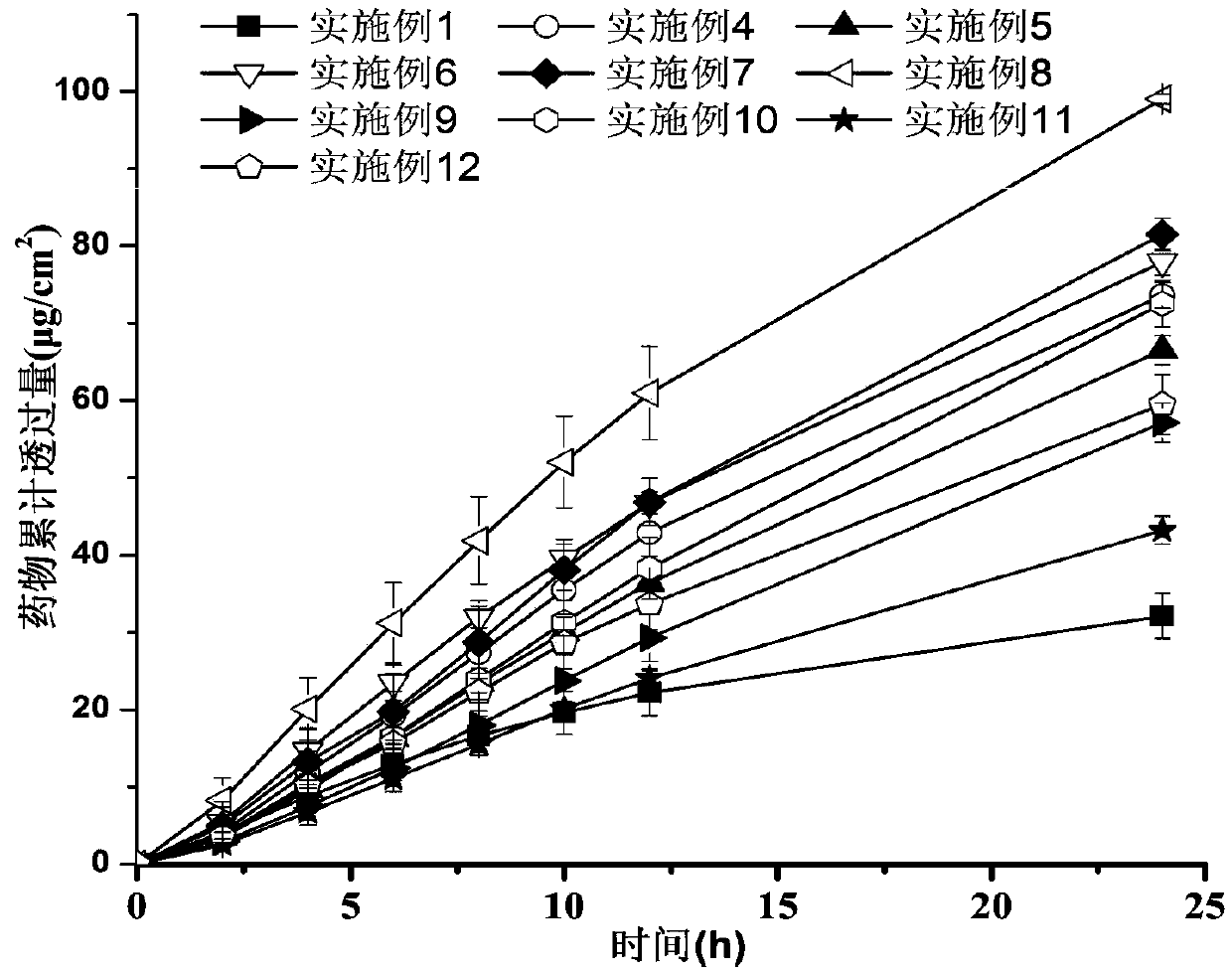
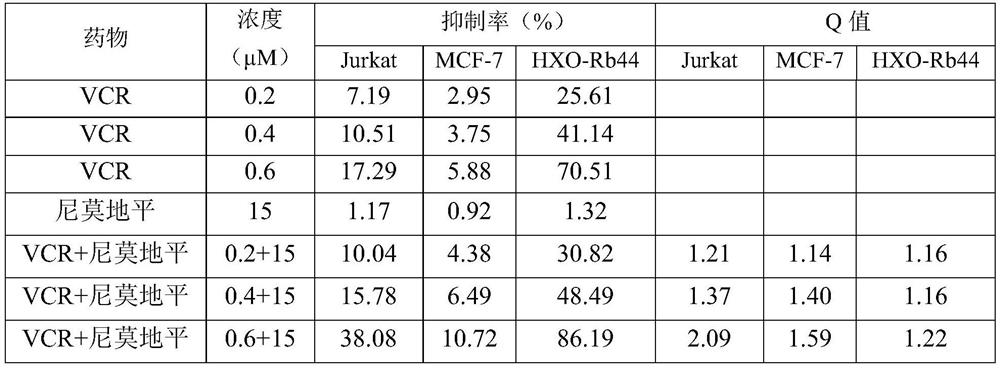
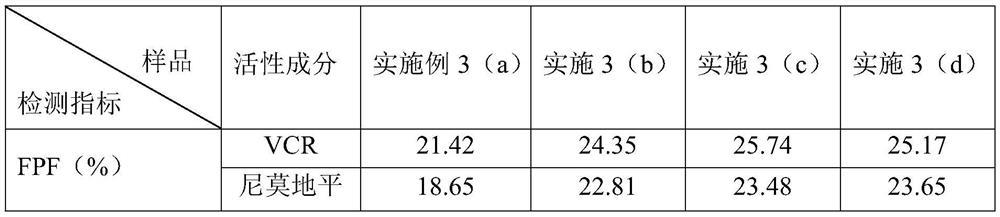
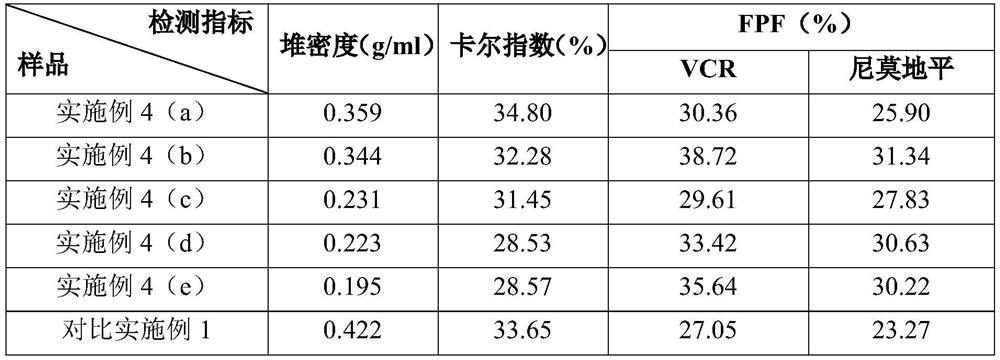
ActiveCN111067879AEfficient separationIncrease deposition ratePowder deliveryOrganic active ingredientsNimodipinePharmaceutical drug
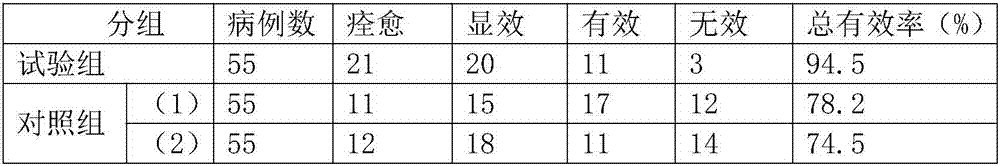
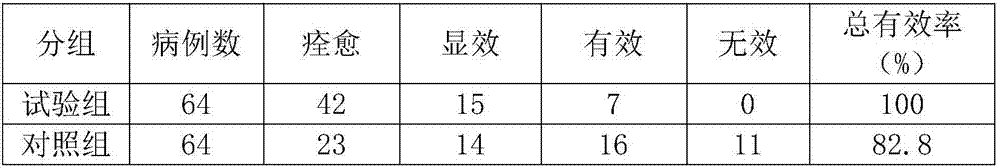
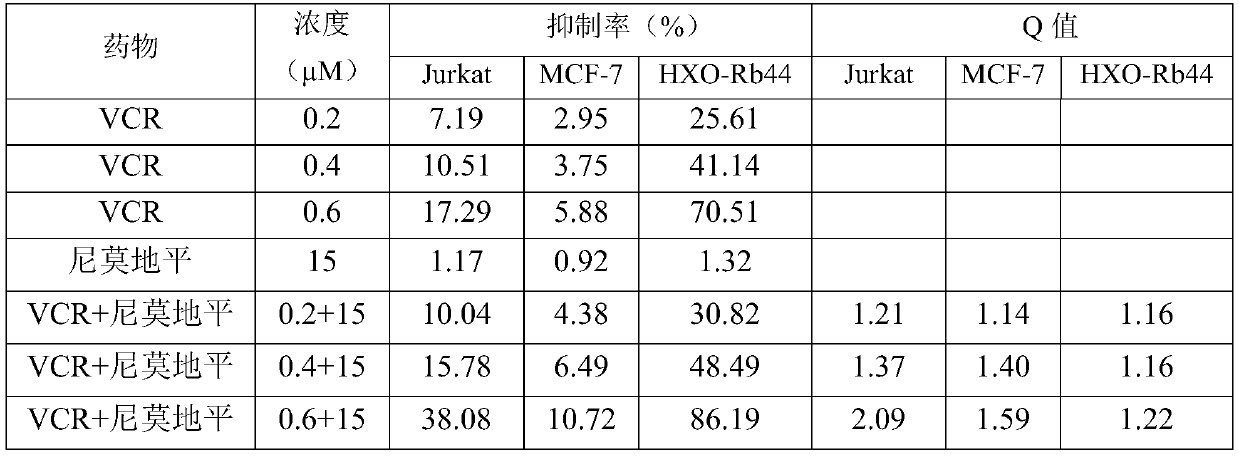
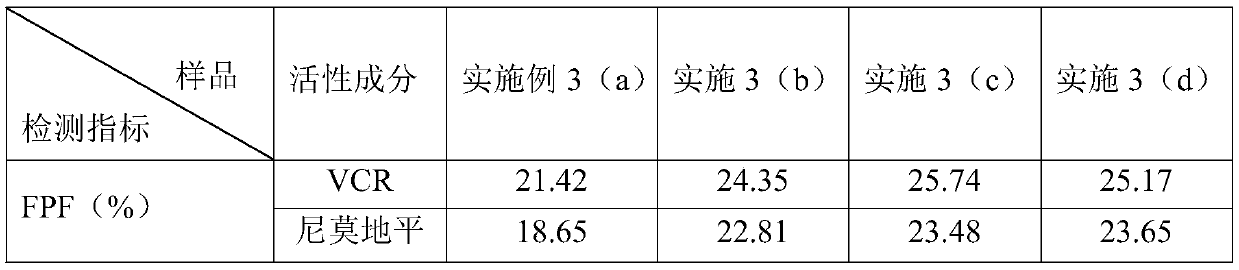
The invention discloses antitumor composition inhalation powder aerosols. The antitumor composition inhalation powder aerosols consist of the following raw materials in parts by weight of 100-500 parts of vincristine sulfate, 500-30000 parts of nimodipine, 2500-40000 parts of carriers and 2-2000 parts of additives, wherein the particle diameter of the vincristine sulfate and the nimodipine is D10=0.2-1.5 [mu]m, D50=1.0-4.0 [mu]m and D90=2.5-8.0 [mu]m, and NLT98% is smaller than 10 [mu]m; the particle diameter of the carriers is D10=2-40 [mu]m, D50=10-110 [mu]m and D90=50-180 [mu]m; and at least 98% of the particle diameter of additional particles of the additives is lower than 50 [mu]m by weight. The invention also provides a preparation method of the antitumor composition inhalation powder aerosols. The preparation method comprises the following steps of performing micronizing treatment on the vincristine sulfate, the nimodipine and the additives; performing particle diameter treatment on the carriers and the additives; performing dispersal adjustment and control on micronized medicines; mixing the micronized medicines with carrier particles and the additives to obtain mixtures; and stuffing the mixtures in No. 3 deep-color plant capsules with a minor-quantity capsule stuffing agent to obtain powder aerosol capsules. The antitumor composition inhalation powder aerosols disclosed by the invention can reinforce cancer resisting treatment effects, alleviate toxic and side effects of VCR and increase biological availability.
Owner:ZHUHAI RESPROLY PHARM TECH CO LTD
Compound oil-in-water schizonepeta spike oil nanoemulsion composition and preparation method thereof
InactiveCN102397330BPrevention and treatment of cold diseasesGood water solubilityAntiviralsRespiratory disorderNasal cavitySaposhnikovia
The invention discloses a compound oil-in-water schizonepeta spike oil nanoemulsion composition, which consists of the following raw materials in percentage by mass: 0.1 to 6.0 percent of schizonepeta spike oil, 0.1 to 5.0 percent of divaricate saposhnikovia root oil, 0.1 to 5.0 percent of weeping forsythiae capsule oil, 0.1 to 2.0 percent of peppermint oil, 0.01 to 0.65 percent of sodium houttuyfonate, 19.0 to 33.0 percent of surfactant and the balance of distilled water, wherein the total percentage by massweight of the raw materials is 100 percent. The nanoemulsion has the effects of relieving exterior syndrome by diaphoresis, clearing away heat and toxic materials, removing pathogenic wind and raising the nonspecific immunity of an organism, and is used for preventing and treating inappetence, increased temperature, cold intolerance and lack of power, which are caused by cold, and sore throat, cough, rhinorrhea, mucus even purulent secretion and the like fully filled in a nasal cavity, which are caused by upper respiratory tract infection, for livestock and poultryanimals.
Owner:NORTHWEST A & F UNIV
Traditional Chinese medicine composition for relieving primary dysmenorrhea, preparation method and dysmenorrhea patch
PendingCN113274476AImprove cold backache, lower abdomen swelling and other symptomsTargetedOrganic non-active ingredientsUnknown materialsCyathula officinalisCassia
The invention relates to a traditional Chinese medicine composition for relieving primary dysmenorrhea, a preparation method of the traditional Chinese medicine composition and a dysmenorrhea patch, belongs to the technical field of traditional Chinese medicines, and solves the problem that in the prior art, pain of primary dysmenorrhea cannot be relieved. The traditional Chinese medicine composition for relieving primary dysmenorrhea provided by the invention is prepared from 2-3 parts of peach kernels, 2-3 parts of flos carthami, 3-5 parts of radix angelicae sinensis, 1-2 parts of olibanum, 1-2 parts of myrrh, 3-5 parts of radix cyathulae, 3-5 parts of garden balsam stems, 2-3 parts of rhizoma curcumae longae, 3-5 parts of ramulus mori, 1-2 parts of fructus viticis, 3-5 parts of cassia twigs and 1-2 parts of trogopterus dung. The invention further provides a preparation method of the traditional Chinese medicine composition and the dysmenorrhea patch containing the traditional Chinese medicine composition components. The traditional Chinese medicine composition is suitable for treating primary dysmenorrhea of common gynecological diseases.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Aconite and glycyrrhiza composition transdermal patch
The invention provides an aconite and glycyrrhiza composition transdermal patch. The transdermal patch consists of a backing layer, a medicine storage layer and an anti-sticking layer. The medicine storage layer comprises 4-15wt% of aconite effective constituent extract, 4-15wt% of glycyrrhiza effective constituent extract, 60-90wt% of adhesive and 1-15wt% of skin penetration enhancer. A preparation method of the aconite and glycyrrhiza composition transdermal patch comprises the following steps of: completely and uniformly mixing the aconite effective constituent extract, the glycyrrhiza effective constituent extract, the adhesive and the skin penetration enhancer, coating a mixture on the anti-sticking layer, drying for 10 to 30 minutes at a temperature of 50-70 DEG C, and then covering the backing layer on the medicine storage layer, and punching to obtain a finished product. The transdermal patch provided by the invention is remarkable in analgesic effect, good in skin permeability, strong in treating specificity, low in toxic and side effects and convenient to use, and is applied to treating symptoms caused by rheumatic arthritis, rheumatoid arthritis and the like, such as pain, swelling and ankylosis, so that the aconite and glycyrrhiza composition transdermal patch has an extensive market application prospect.
Owner:云南白药集团无锡药业有限公司
Composition and atomization spray capable of resisting infectious diseases and preparation method of composition
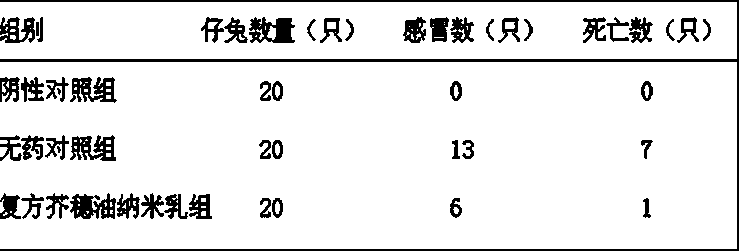
InactiveCN106860862ARich in capillariesEfficient absorptionDispersion deliveryAerosol deliveryAntibodyBlood plasma
The invention relates to a method for preparing a composition capable of resisting infectious diseases. The method comprises the following steps: S1: drawing blood plasma from infection-free recovered patients at a recovery period of the infectious diseases; S2, extracting protein from the blood plasma to obtain crude protein extract; S3: freeze-drying the crude protein extract to obtain powder, namely the composition capable of resisting the infectious diseases. The invention further relates to the composition capable of resisting the infectious diseases, which is prepared by the method, and also relates to atomization spray capable of resisting the infectious diseases, which is prepared from the composition. The composition and the atomization spray, provided by the invention, contain antibodies aiming at corresponding infectious diseases and can be used for treating and preventing the infectious diseases.
Owner:江苏安泰生物技术有限公司
A kind of antitumor composition inhalation powder spray and preparation method thereof
ActiveCN111067879BEfficient separationIncrease deposition ratePowder deliveryOrganic active ingredientsNimodipinePharmaceutical drug
Owner:ZHUHAI RESPROLY PHARM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com