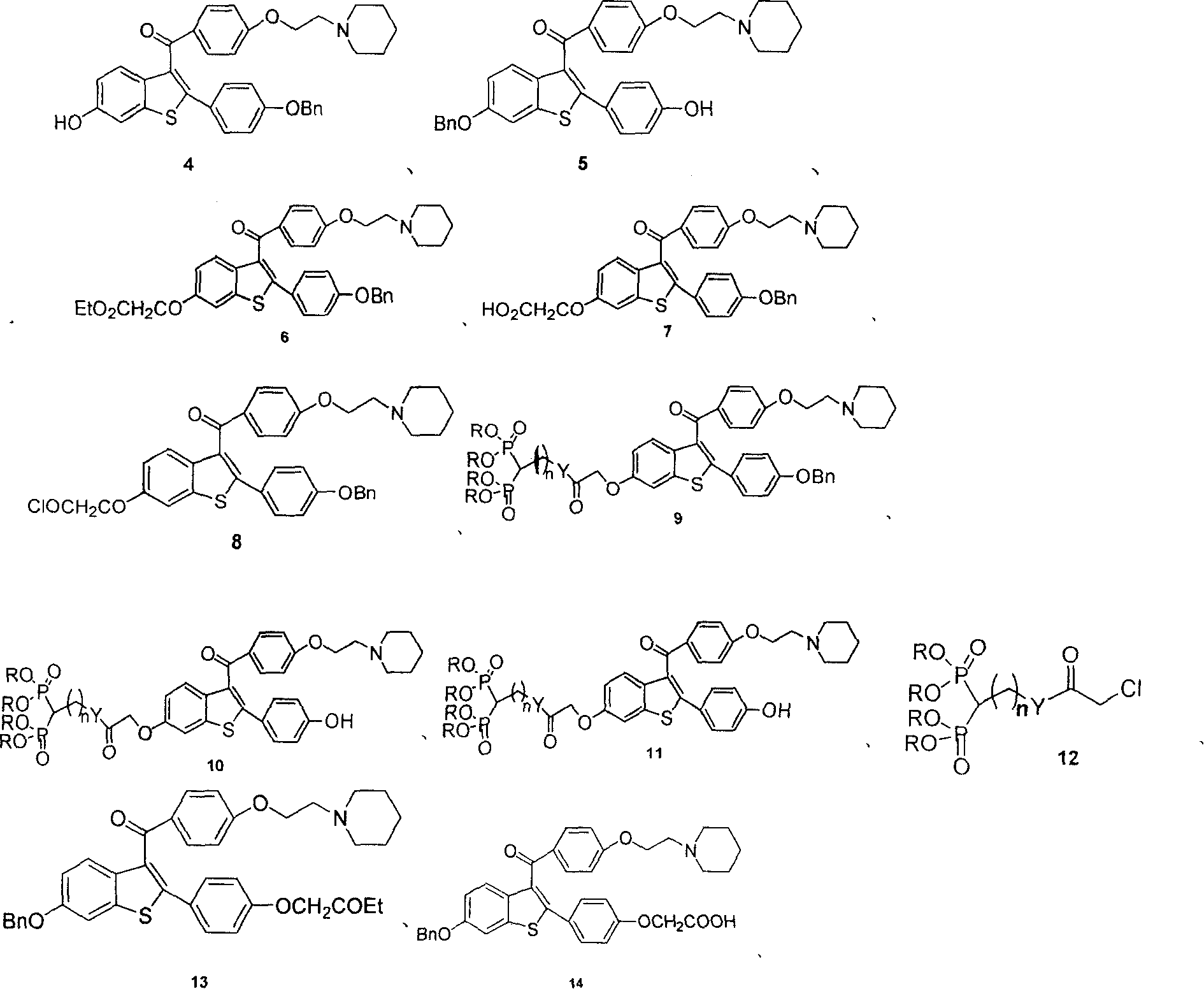Patents
Literature
58 results about "Raloxifene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
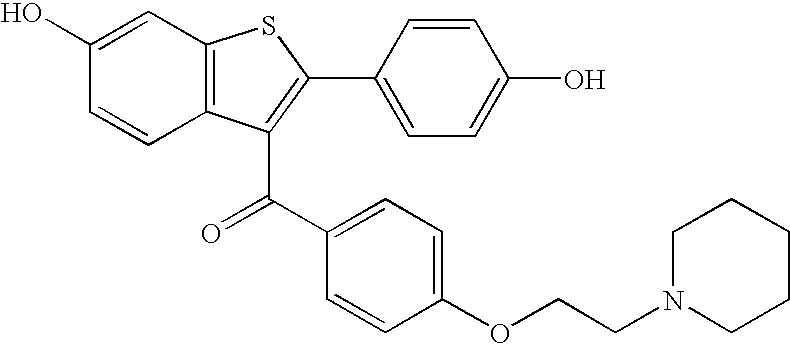
Raloxifene is used by women to prevent and treat bone loss (osteoporosis) after menopause.
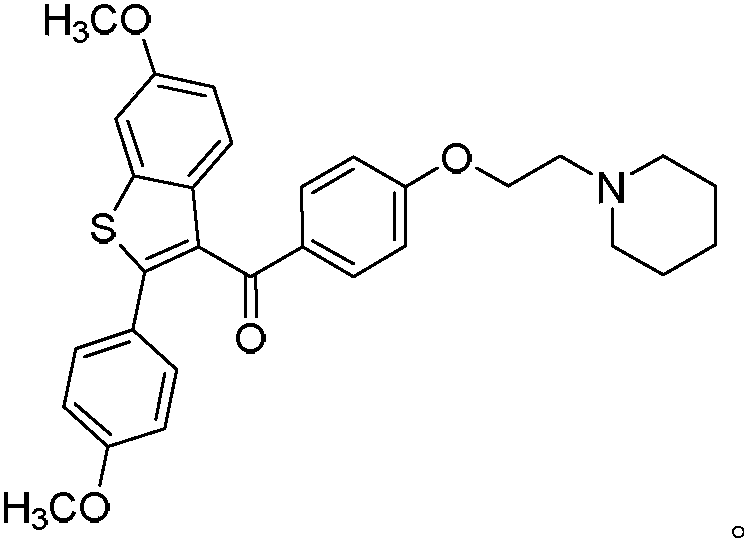
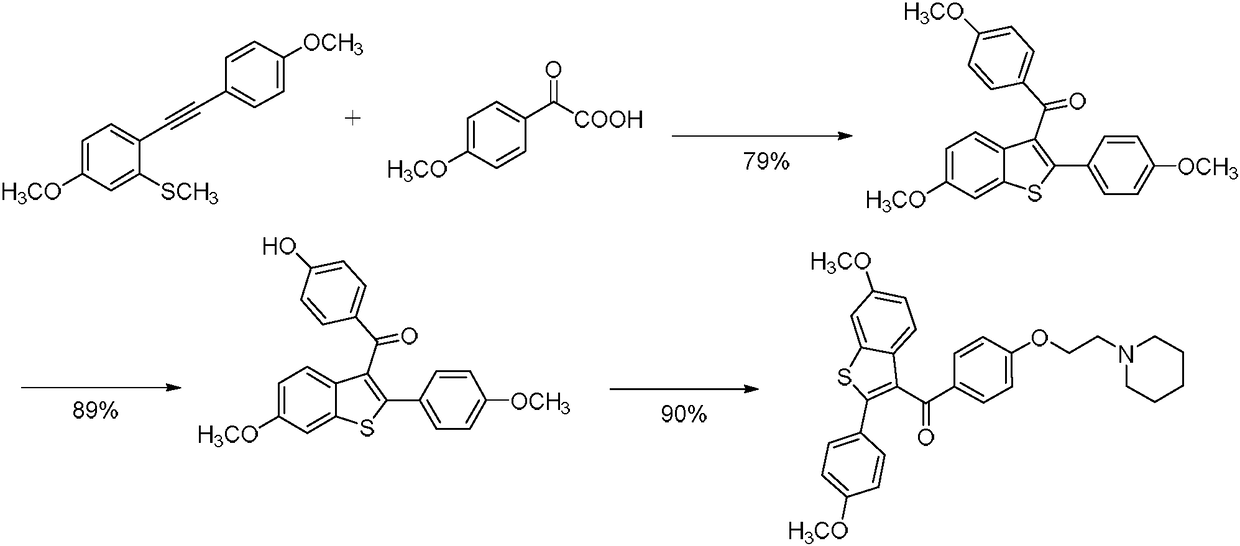
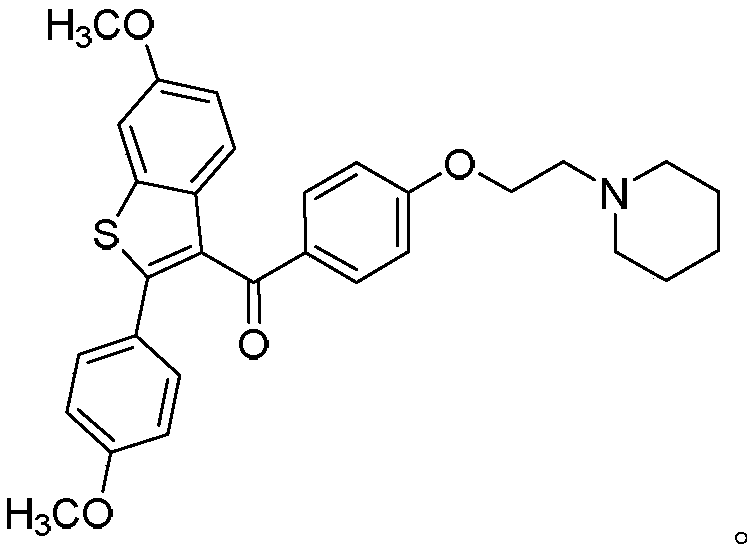
Nanoparticulate benzothiophene formulations
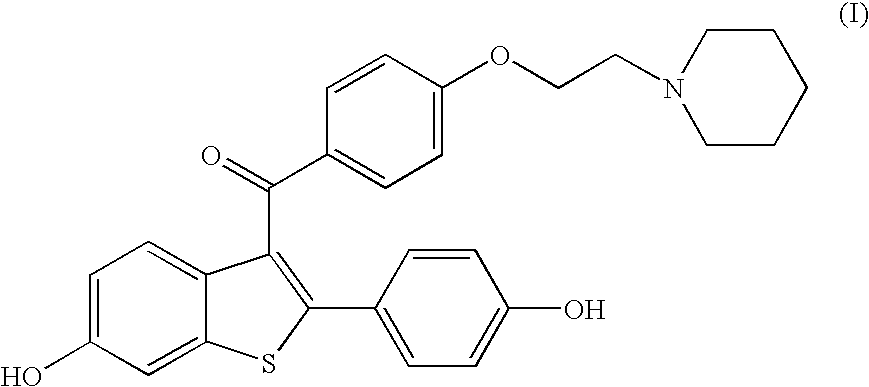
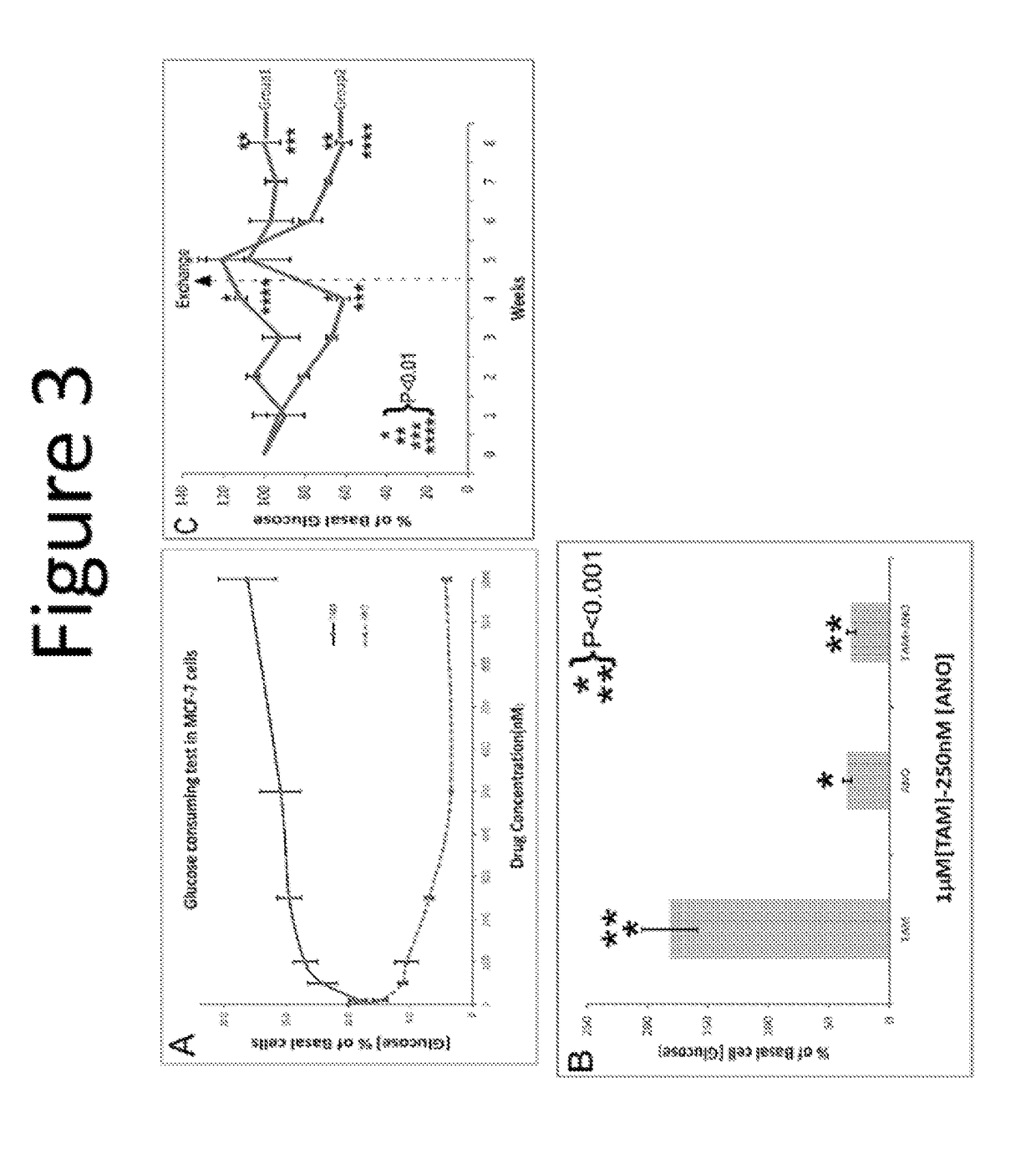
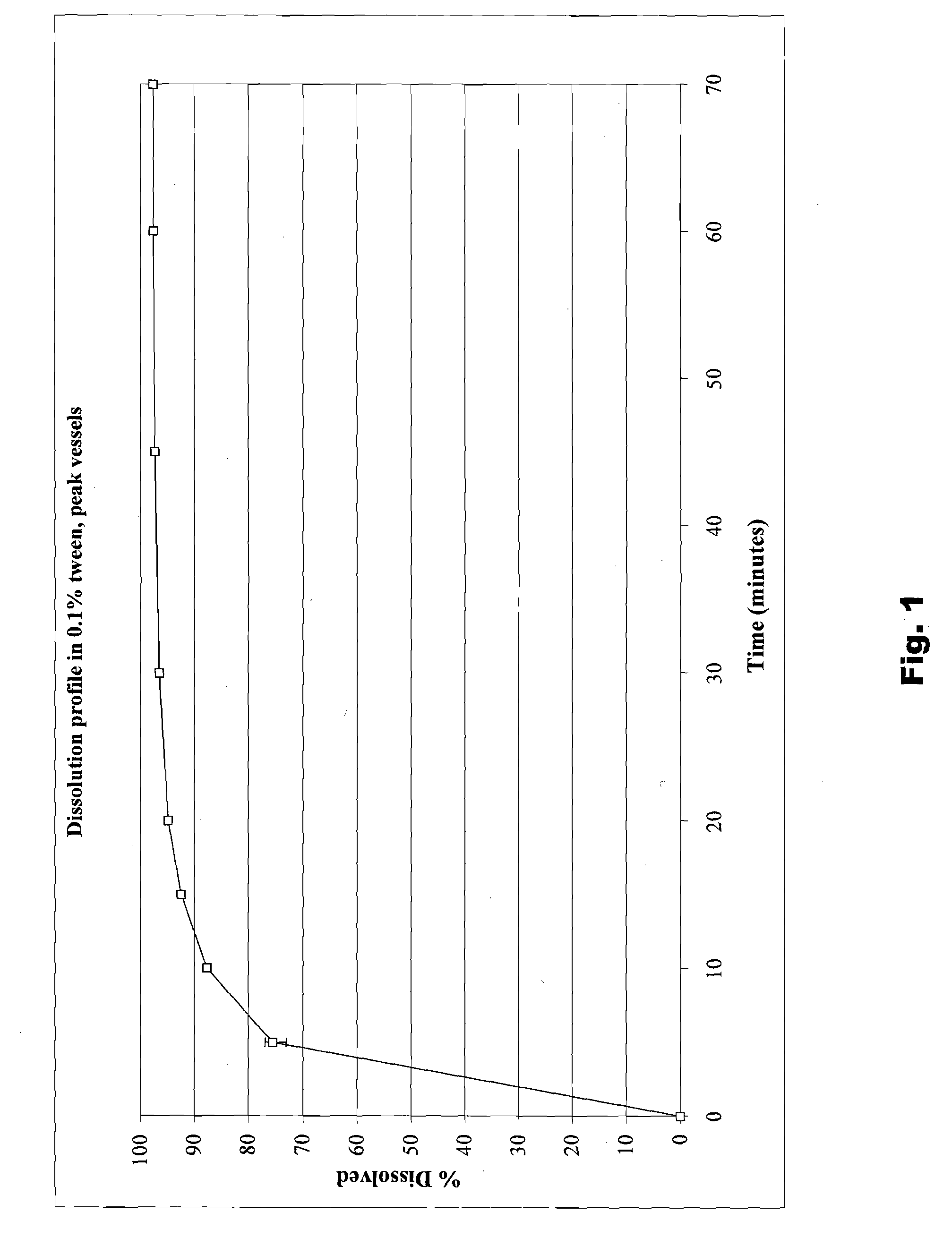
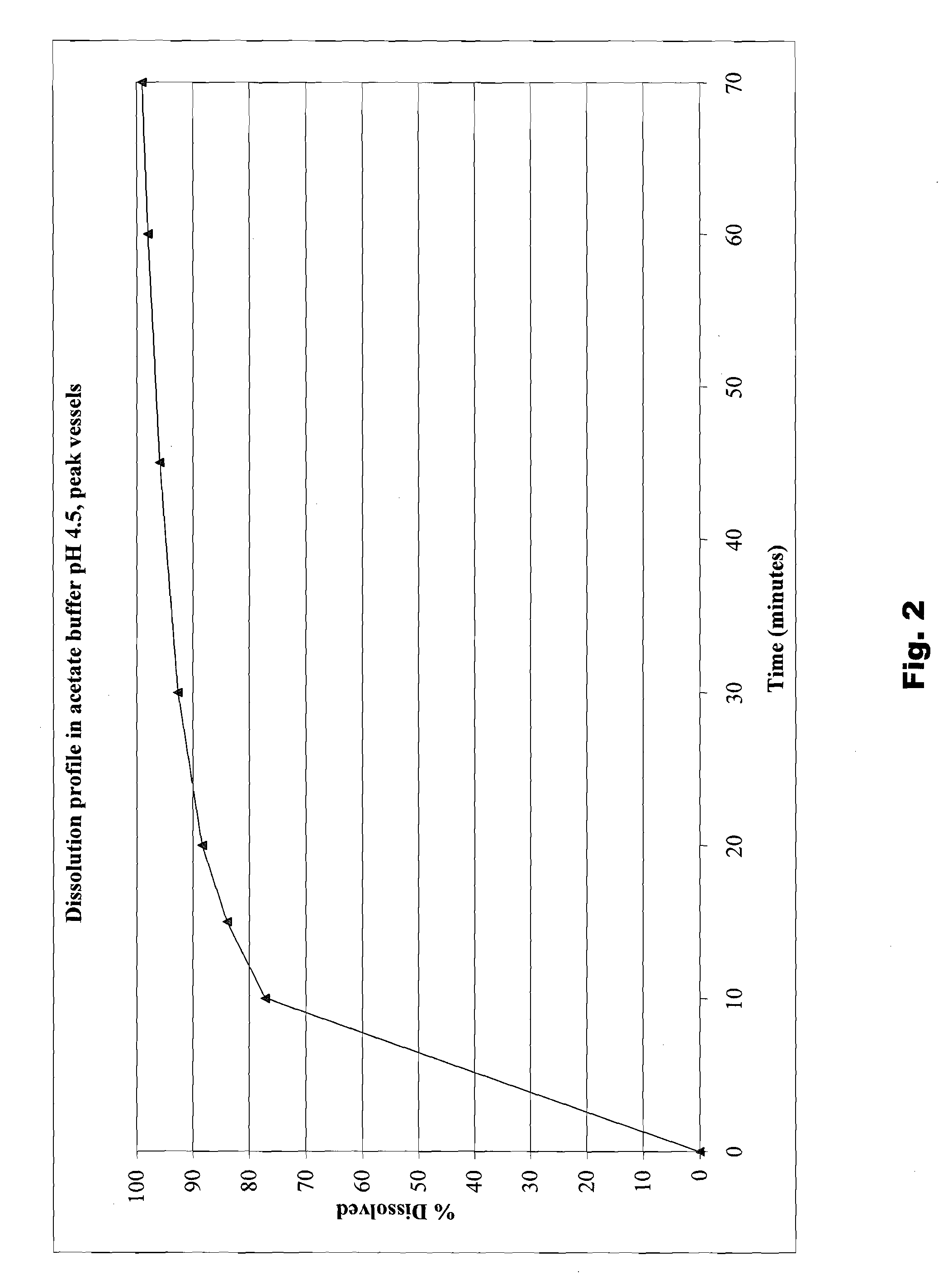
The present invention is directed to benzothiophene compositions, preferably nanoparticulate raloxifene hydrochloride compositions having improved pharmacokinetic profiles, improved bioavailability, dissolution rates and efficacy. In one embodiment, the raloxifene hydrochloride nanoparticulate composition have an effective average particle size of less than about 2000 nm.
Owner:ELAN PHRMA INT LTD
Selective androgen receptor modulators
This invention provides pharmaceutical compositions comprising combination of SARM compounds and antiresorptive agents such as SERM compounds, including, inter-alia, Raloxifene, and uses thereof for treating osteoporosis and associated diseases.
Owner:UNIV OF TENNESSEE RES FOUND
Implantable drug delivery compositions and methods of treatment thereof
Owner:BRAEBURN PHARMA INC
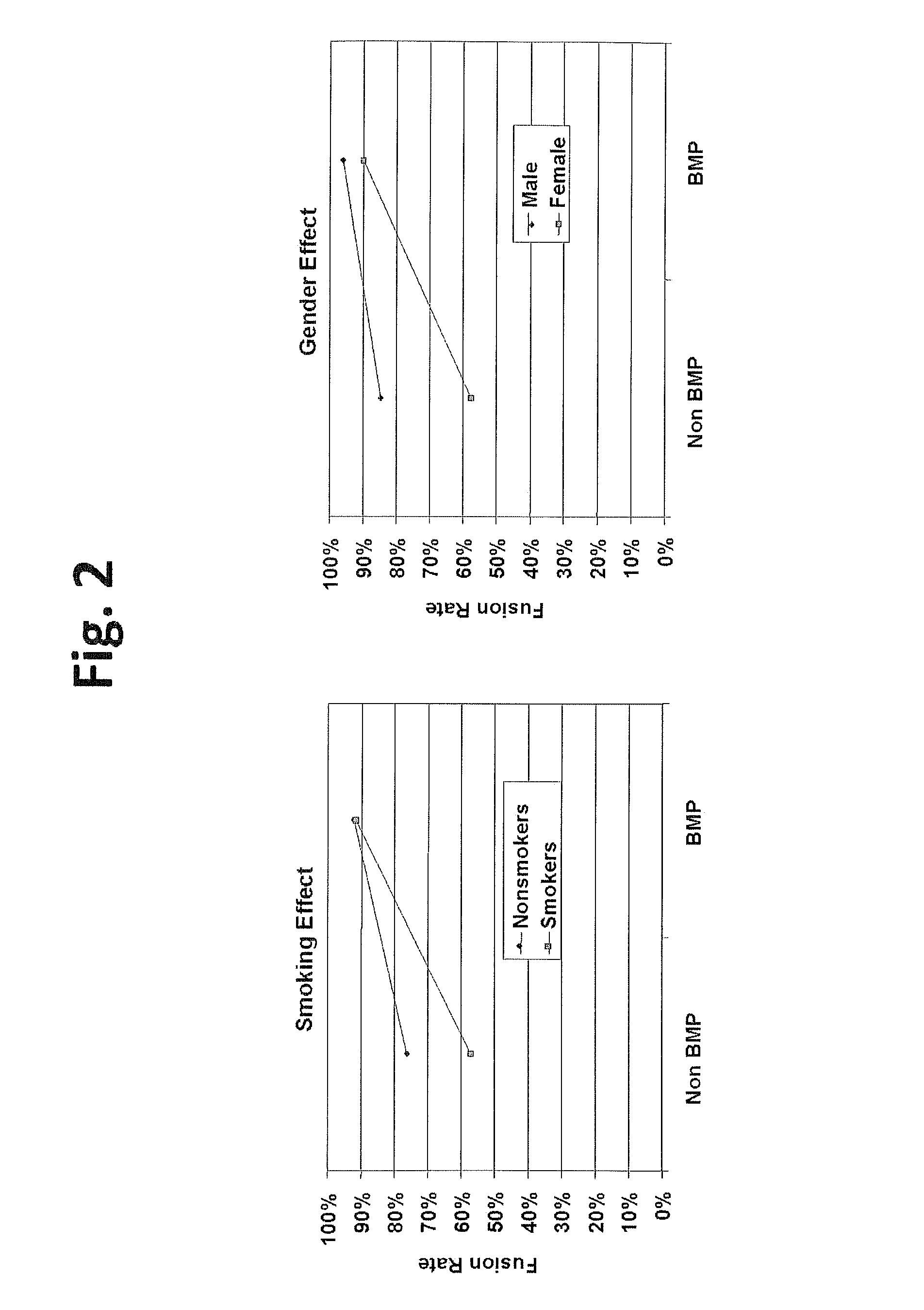
Use of selective estrogen receptor modulator for joint fusion and other repair or healing of connective tissue
Methods for facilitating joint immobilization or fusion using selective estrogen receptor modulator (SERM) such as raloxifene are disclosed. The SERM may be administered systemically or locally. In conjunction with SERM, other therapeutic agents such as calcium, vitamin D, bone morphogenetic protein may be administered simultaneously. The method can similarly be applied to facilitate bone repair, bone healing, and connective tissue healing processes in a patient.
Owner:STARK JOHN G
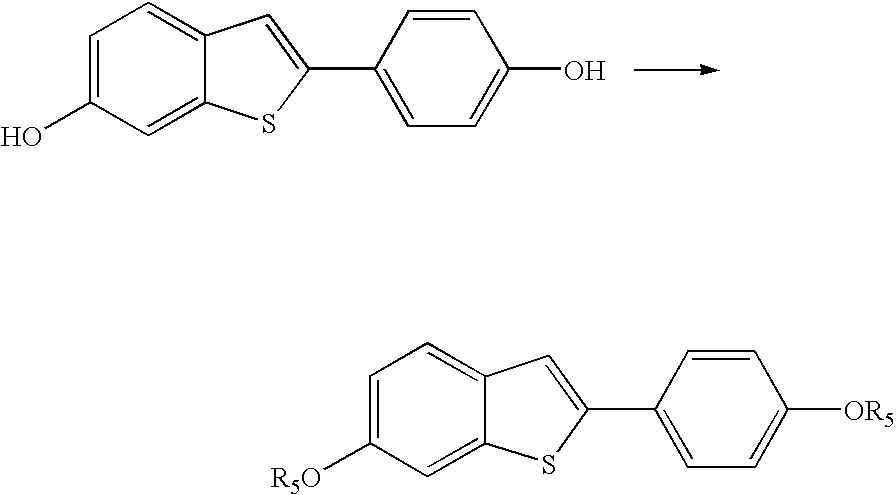
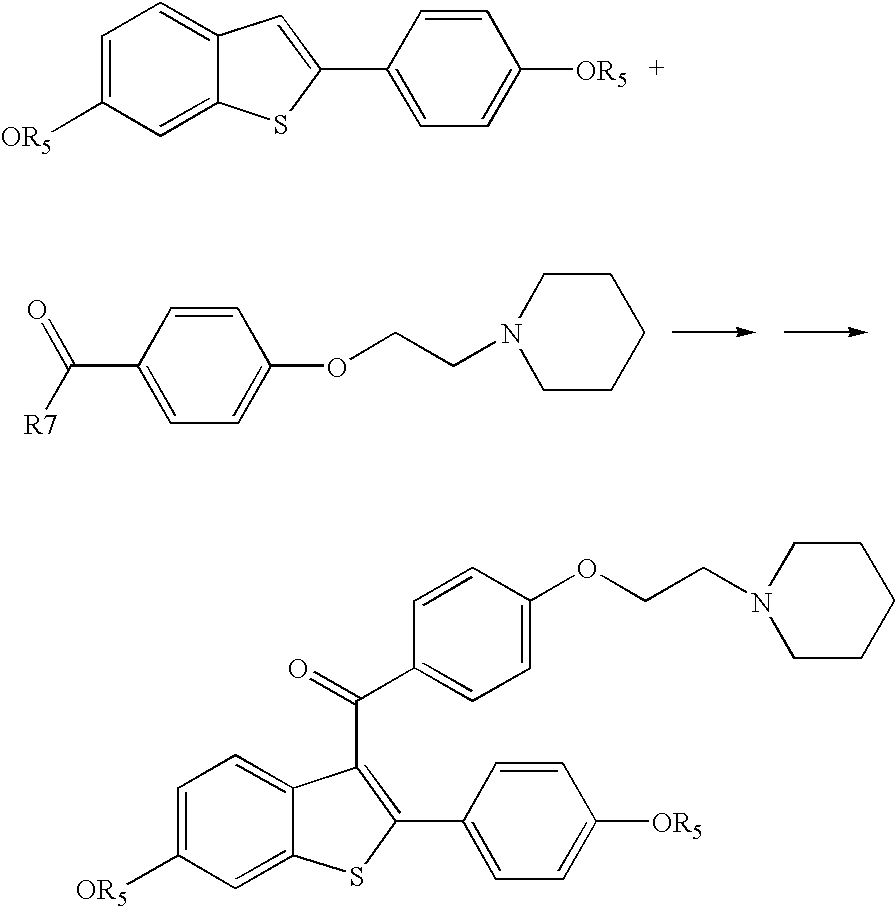
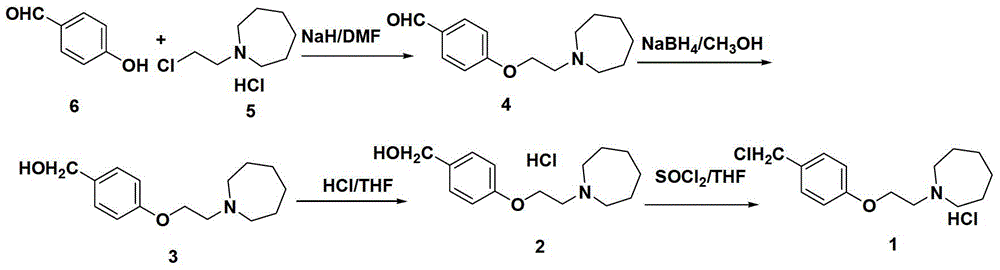
Process for preparing raloxifene hydrochloride
InactiveUS20070100147A1High purityHigh yieldOrganic active ingredientsOrganic chemistryStrong acidsSolvent
Process for preparing raloxifene hydrochloride with a purity greater than 98% and low aluminium content comprising the following stages a) demethylation of 6-methoxy-2-(4-methoxyphenyl)benzo[b]thiophene in pyridine and hydrochloric acid to obtain 6-hydroxy2-(4-hydroxyphenyl)benzo[b]thiophene in pyridine hydrochloride, b) acetylation of 6-hydroxy-2-(4hydroxyphonyl)benzo[b]thiophene with an acetylating agent to obtain the corresponding 6-acetoxy-2-(4 acetoxyphenyl)benzo[b]thiophene, c) acylation of 6-acetoxy-2-(4-acetoxyphonyl)benzo[b]thiophene with 4-(2 piperidinoethoxy)benzoylchloride hydrochloride with aluminium trichloride in halogenated solvent to obtain 6-acetoxy-2-(4acetoxyphenyl)-3-[4-(2 piperidinoethoxy)benzoyl]-benzo[b]thiophene, d) hydrolysis of 6-acetoxy-2-(4-acetoxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyll benzo[b]thiophene according to the following operating conditions: d1) treatment of 6-acetoxy-2-(4-acetoxyphonyl)-3-[4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene with alkaline hydroxide in alcohol solvent, d2) acidification of the product obtained in the preceding stage (d1) with a strong acid, to obtain the corresponding raloxifene salt with the strong acid, characterised in that the strong acid used in stage (d2) is concentrated hydrochloric acid.
Owner:ERREGIERRE
Method for chemoprevention of prostate cancer
InactiveUS20060270641A1Safe and effectivePreventing prostate carcinogenesisBiocideAnimal repellantsRaloxifeneMetabolite
This invention relates to the chemoprevention of prostate cancer and, more particularly, to a method of suppressing or inhibiting latent prostate cancer comprising administering to a mammalian subject a chemopreventive agent, for example raloxifene, or its analog, derivative, isomer, metabolite, pharmaceutically acceptable salt, ester, or N-oxide, or mixtures thereof. The chemopreventive agent prevents, prevents recurrence of, suppresses or inhibits prostate carcinogenesis and treats prostate cancer.
Owner:UNIV OF TENNESSEE RES FOUND
Fatty acid raloxifene derivatives and their uses
The invention relates to fatty acid raloxifene derivatives; compositions comprising an effective amount of a fatty acid raloxifene derivative; and methods for treating osteoporosis or preventing invasive breast cancer in postmenopausal women comprising the administration of an effective amount of a fatty acid raloxifene derivative.
Owner:CATABASIS PHARMA
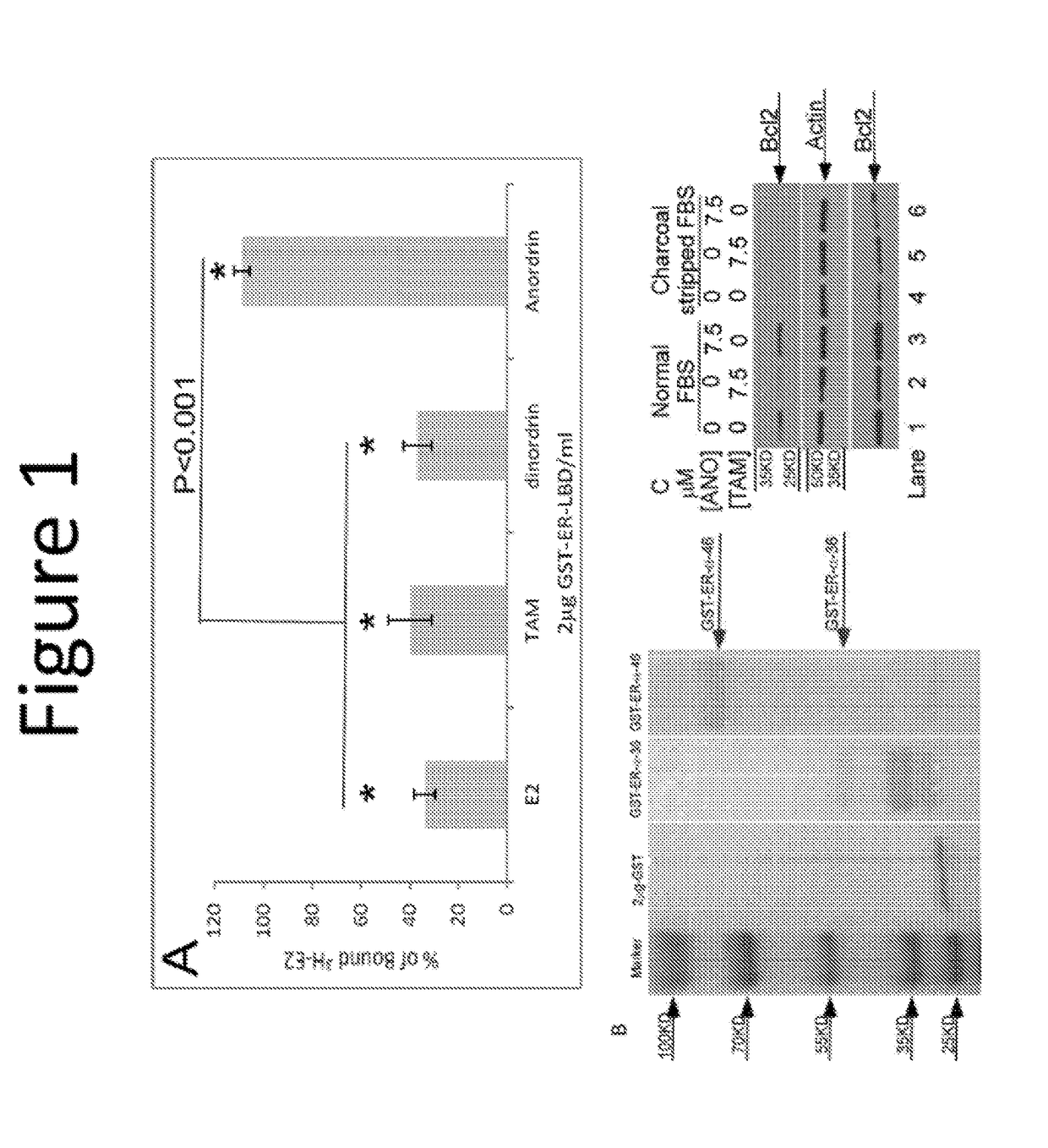
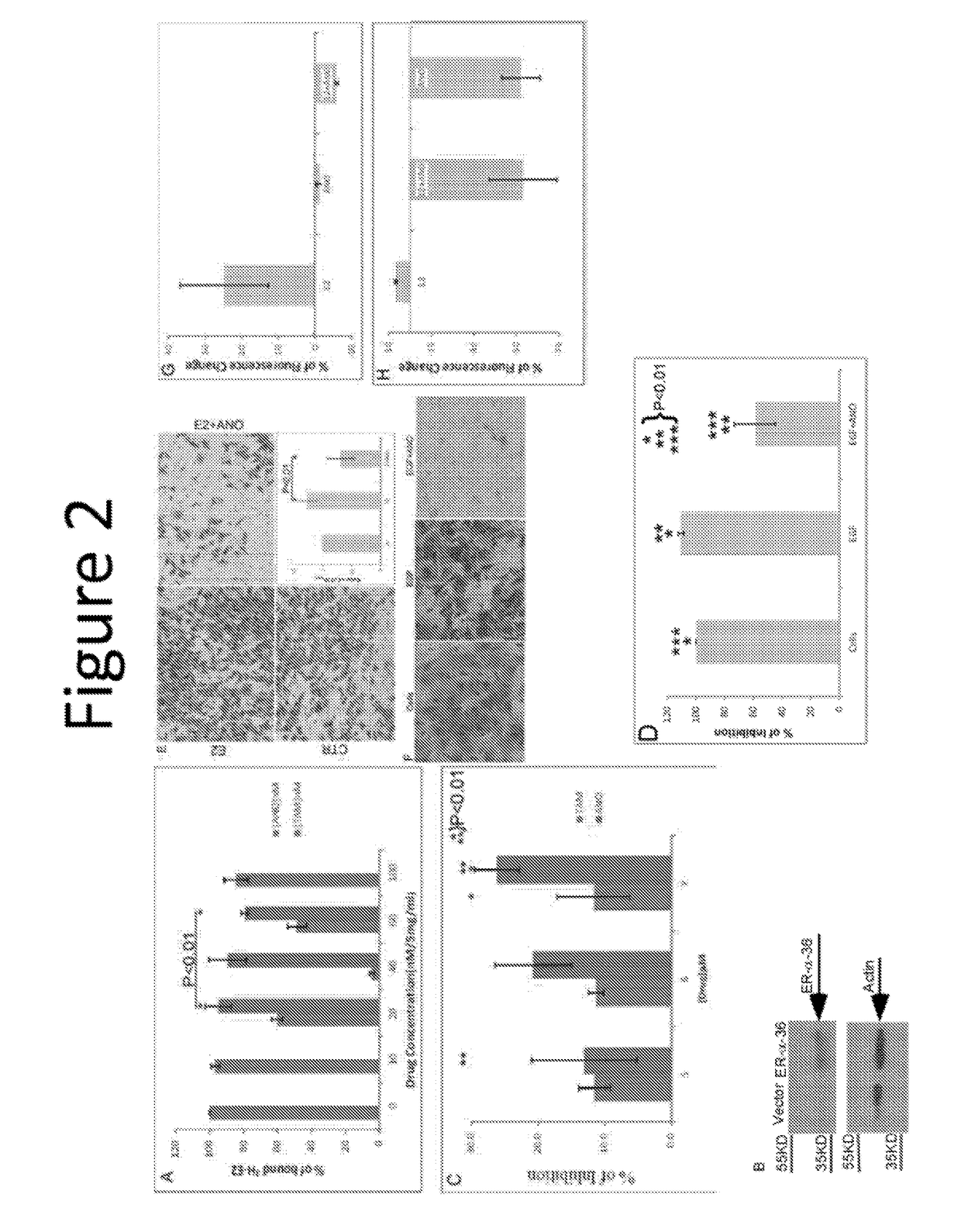
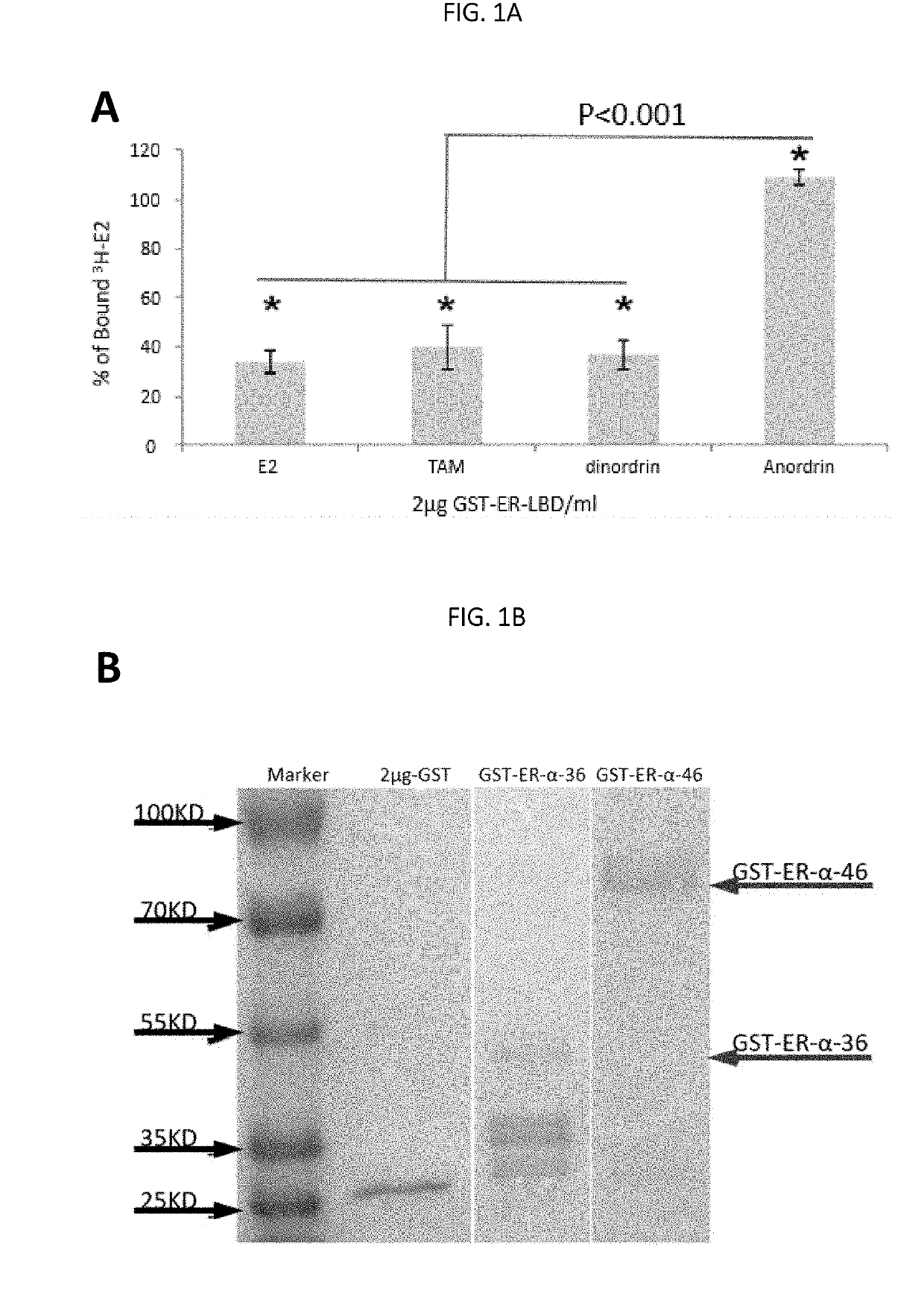
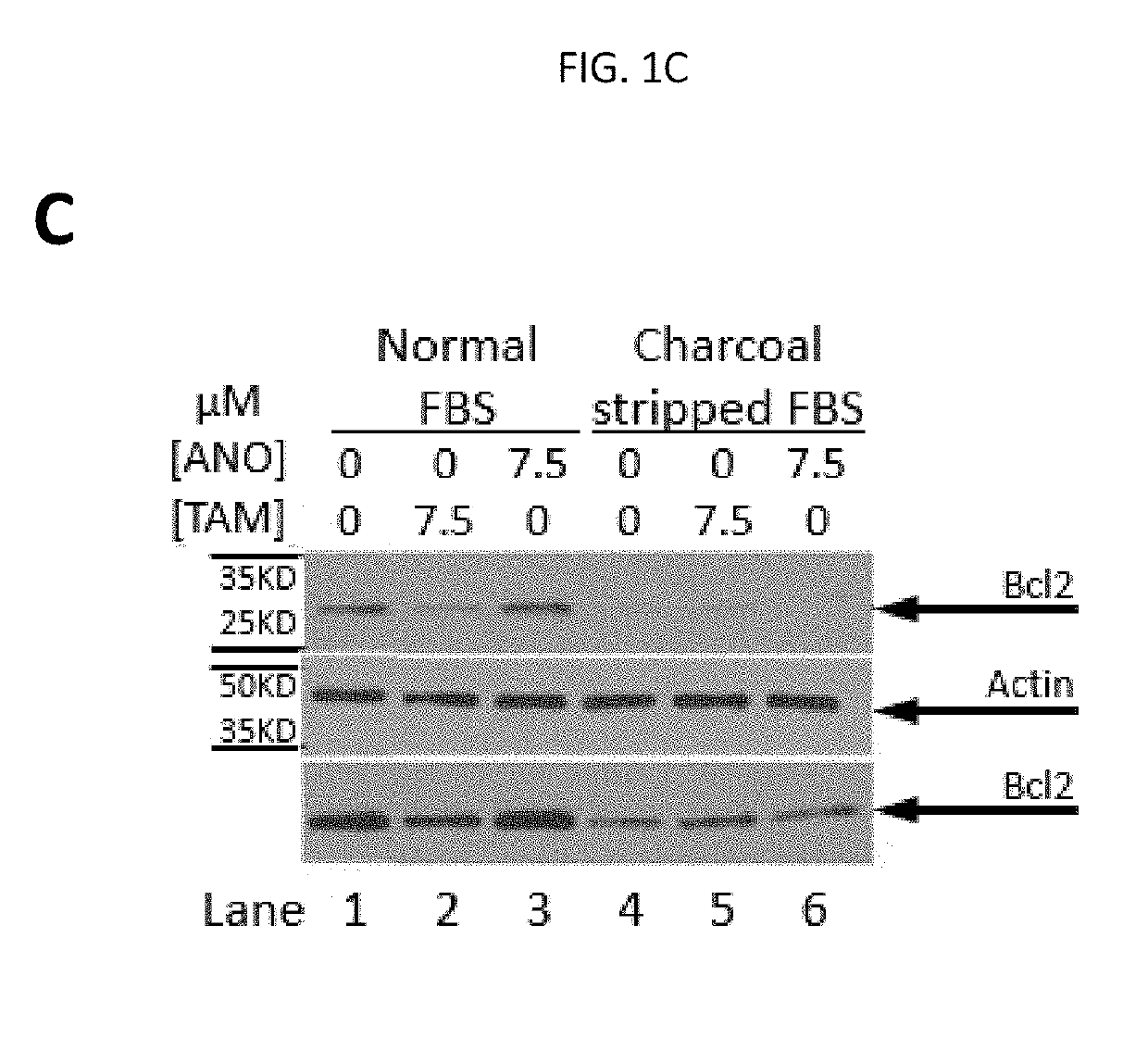
Anordrin compositions and methods for treating diseases
ActiveUS20170151263A1Eliminate side effectsReduce concentrationOrganic active ingredientsMetabolism disorderDiseaseRaloxifene
The present invention provides methods and compositions for treating cancer, reducing side effects, and reducing postmenopausal symptoms comprising anordrin or analog thereof (such as anordrin) alone or in combination with at least one other agent selected from the group consisting of tamoxifen, raloxifene or functional equivalent thereof, and an aromatase inhibitor.
Owner:ZHEJIANG JIACHI DEV PHARMA LTD +1
Raloxifene composition
A pharmaceutical composition comprising raloxifene or a pharmaceutically acceptable salt thereof, a mixed cellulose excipient, and a disintegrant can be conveniently made.
Owner:SYNTHON BV
Therapy using a combination of raloxifene and alendronate
Provided are methods of treating bone disease including, but not limited to, osteoporosis, metastatic bone disease, or Paget's disease, by administering a combination of raloxifene and alendronate in a manner that mitigates the formation of ulcerative adverse events.
Owner:TEVA PHARM USA INC
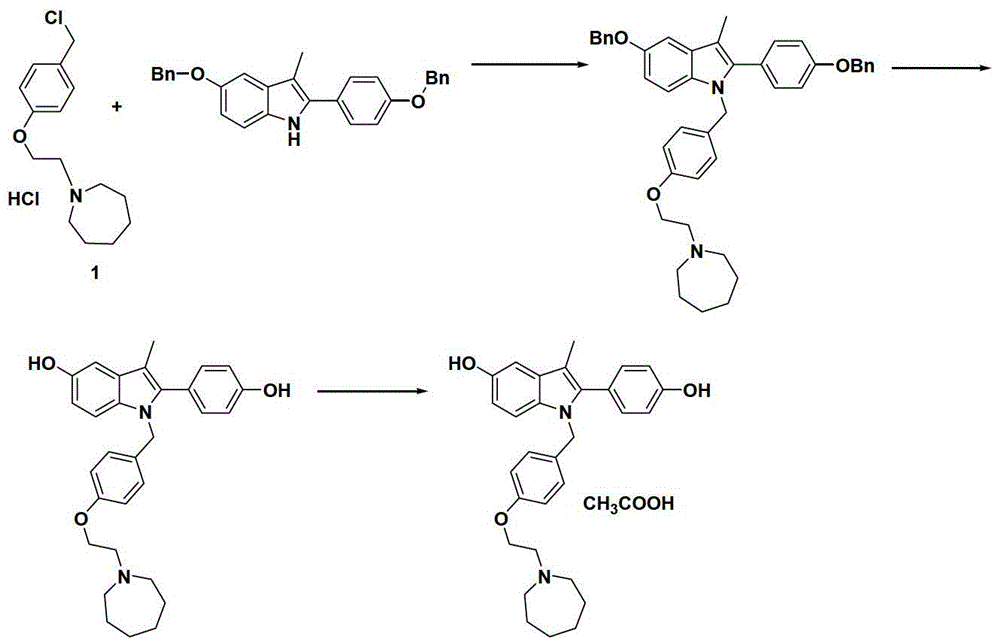
Raloxifene derivative and preparation method thereof
ActiveCN109320494ASimple ingredientsThe reaction conditions are easy to achieveOrganic chemistryRaloxifeneHazardous substance
The invention discloses a raloxifene derivative. A molecular structural formula is shown in the description. According to the raloxifene derivative prepared by the invention, raw materials are simpleand reaction conditions are easy to realize; the yield is relatively high; no harmful substances are generated in a reaction process; the raloxifene derivative is simple in raw materials and reactionconditions.
Owner:WENZHOU UNIVERSITY
Anordrin compositions and methods for treating diseases
ActiveUS10231978B2Eliminate side effectsElevated sugar uptakeOrganic active ingredientsMetabolism disorderDiseaseRaloxifene
The present invention provides methods and compositions for treating cancer, reducing side effects, and reducing postmenopausal symptoms comprising anordrin or analog thereof (such as anordrin) alone or in combination with at least one other agent selected from the group consisting of tamoxifen, raloxifene or functional equivalent thereof, and an aromatase inhibitor.
Owner:ZHEJIANG JIACHI PHARMA DEV LTD +1
Bone microenvironment modulated migraine treatments
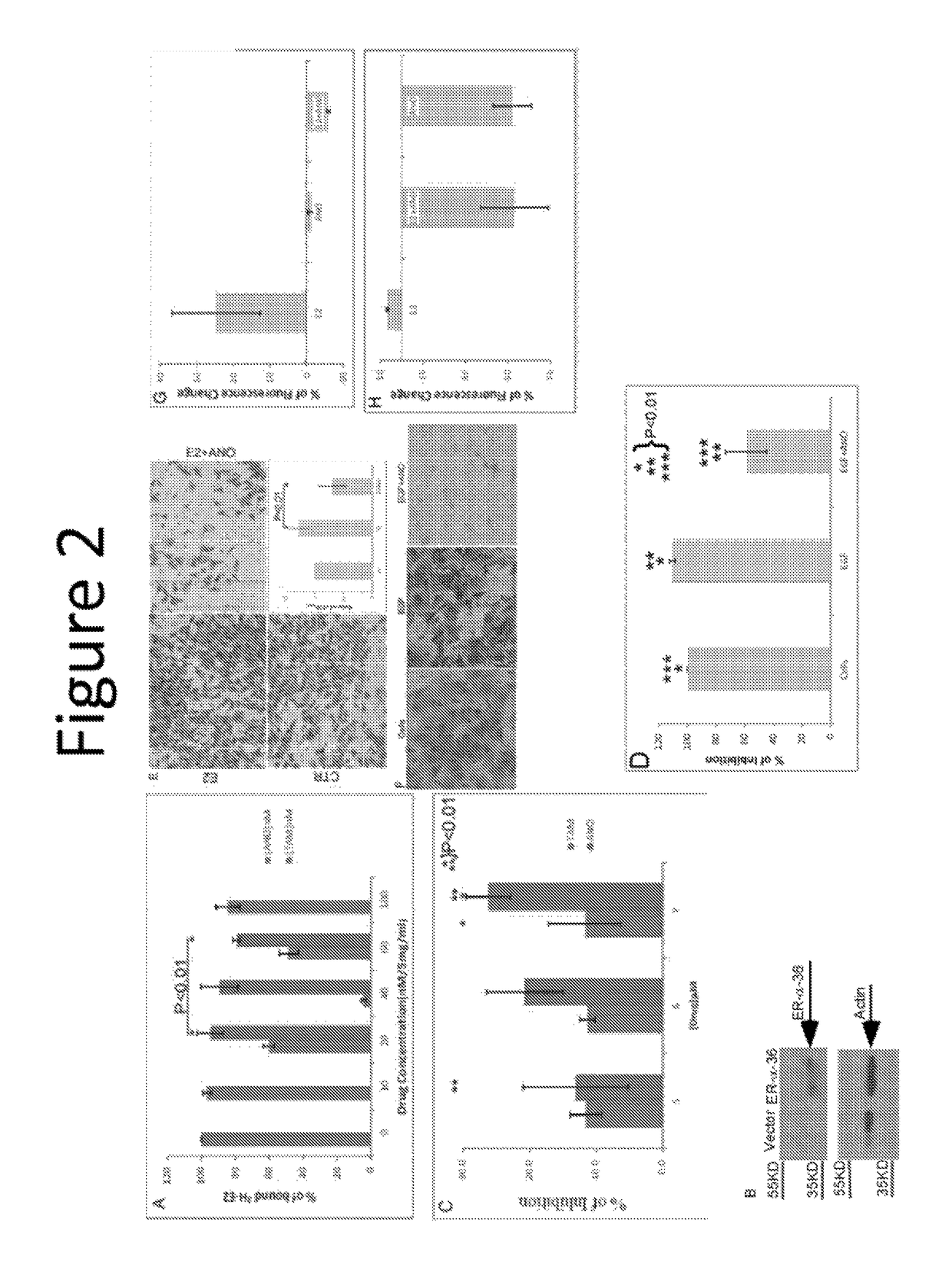
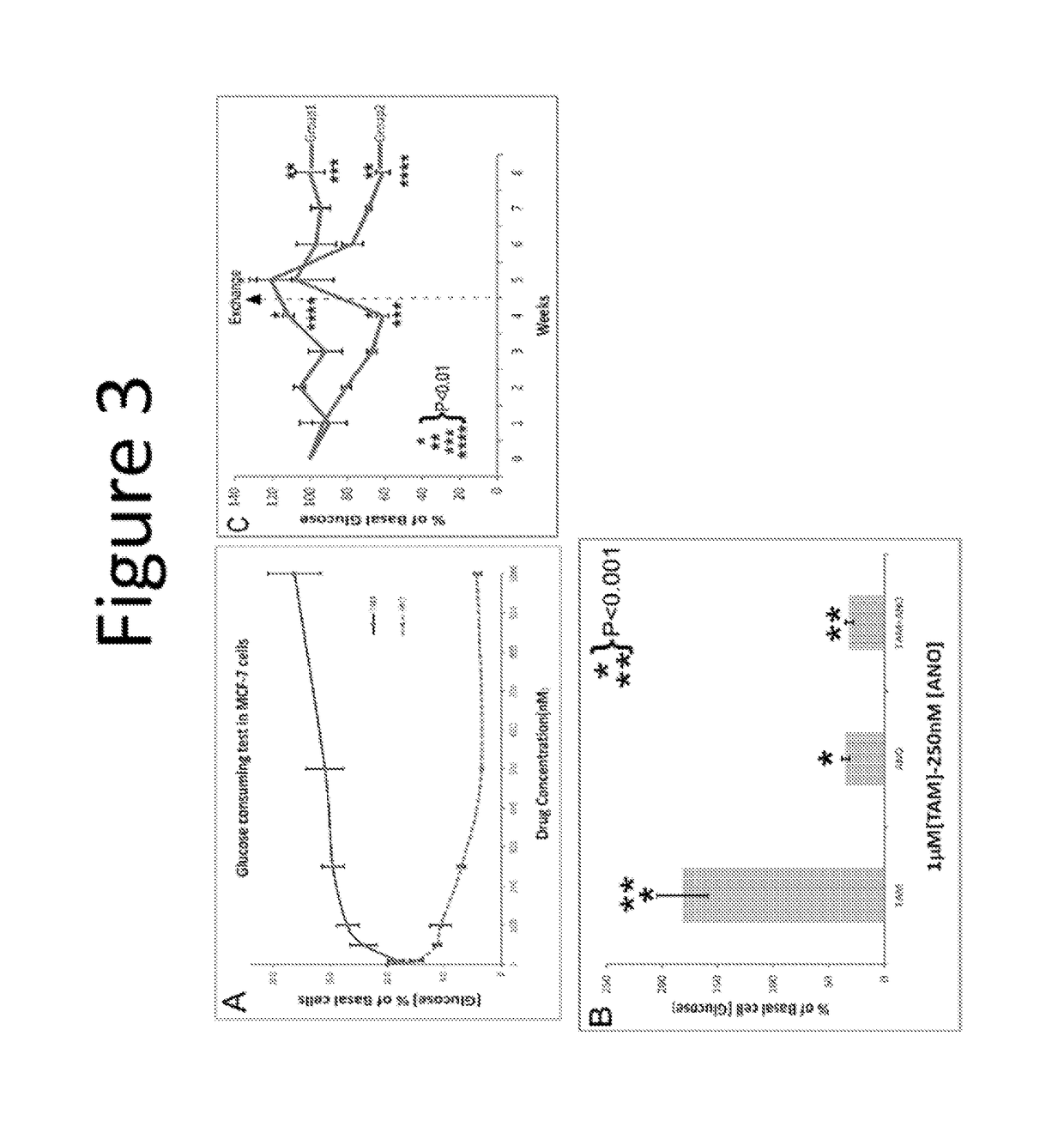
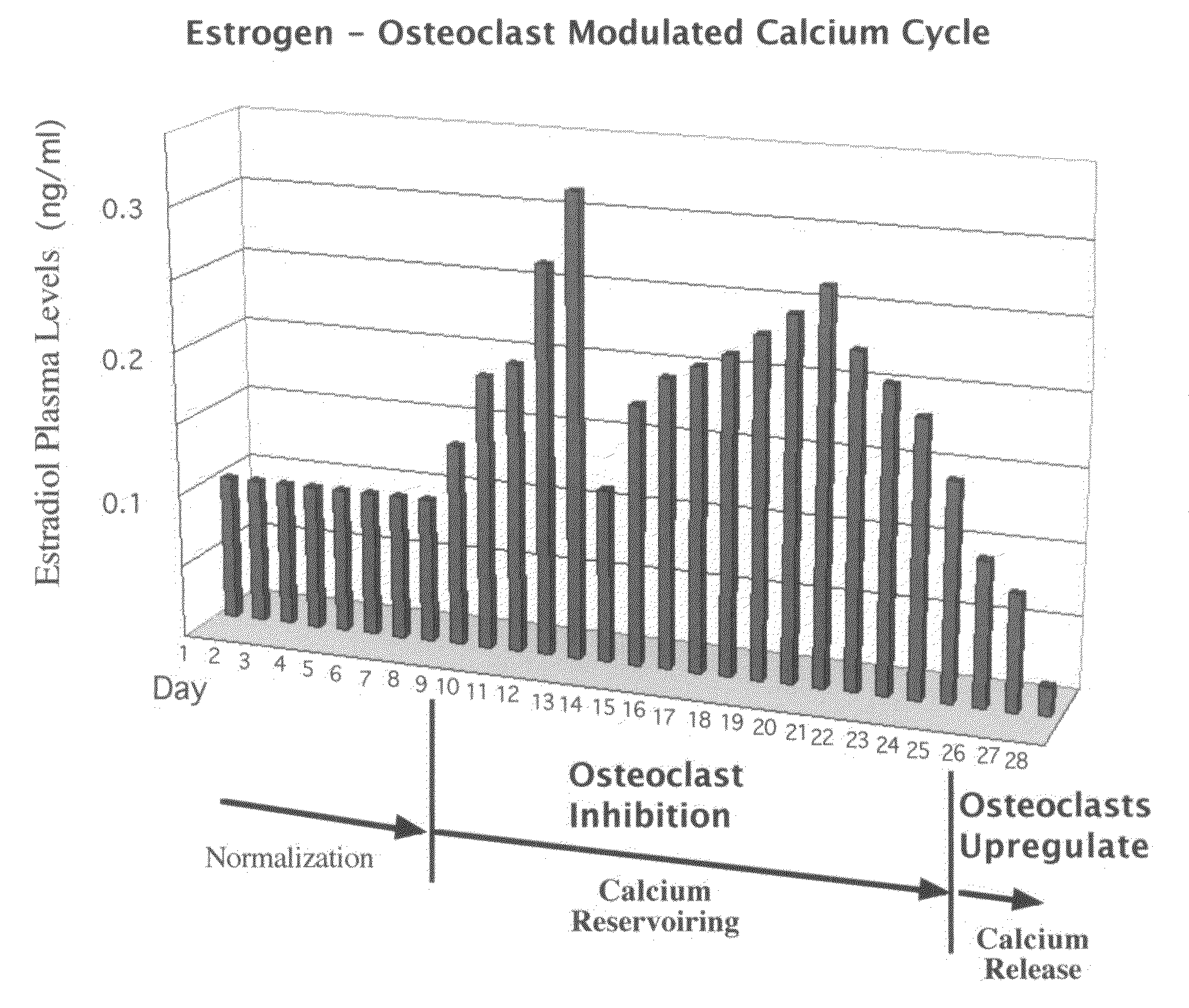
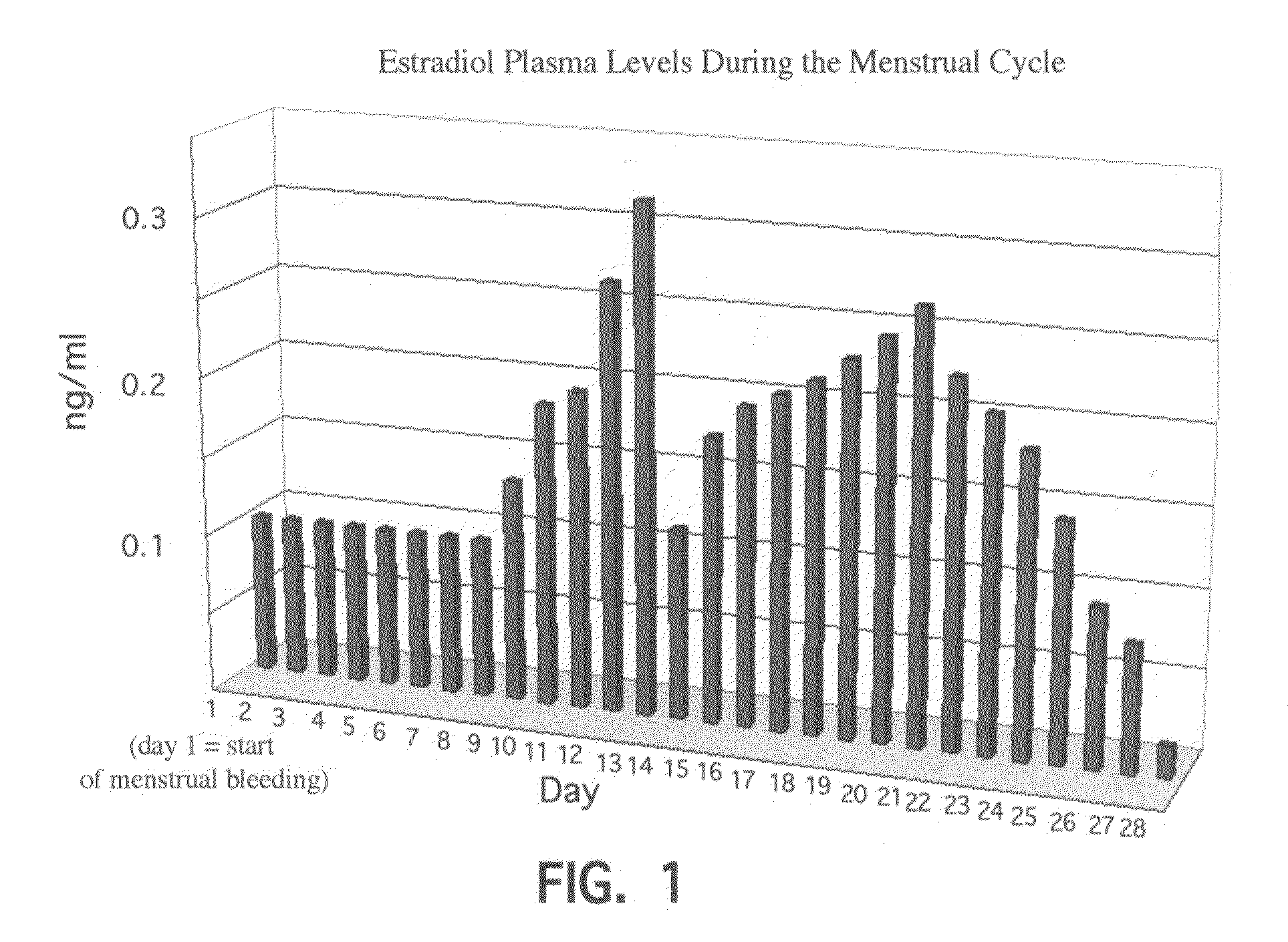
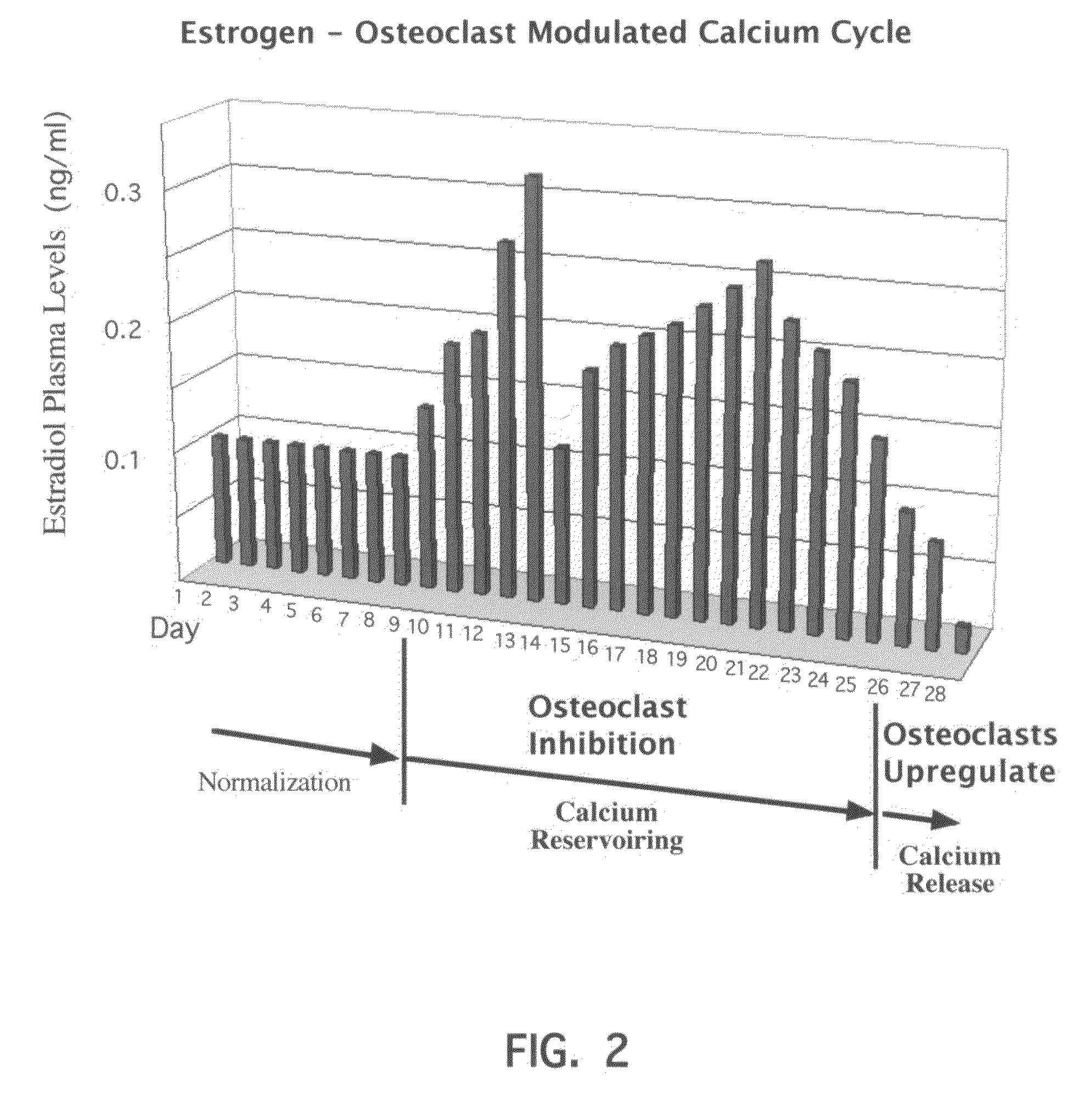
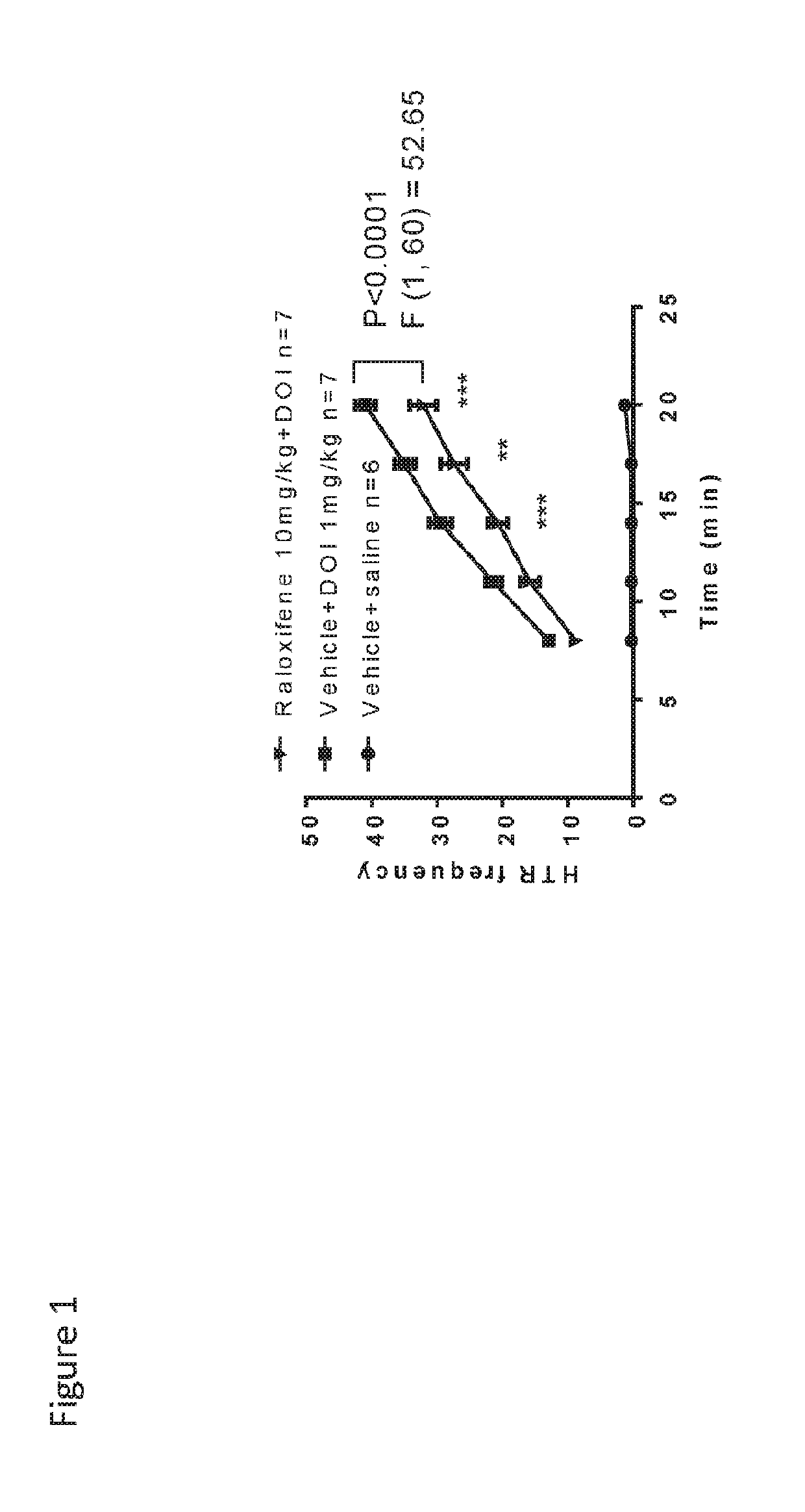
Novel etiology and pathogenesis of premenstrual headache and premenstrual migraine are presented and novel treatment methods are provided. Present invention identifies how declining estrogen results in a transient elevation in extracellular calcium concentrations via osteoclast upregulation. The elevated extracellular calcium pathogenesis is then traced, from bone to brain, and includes depolarization of nerves, hyperactive neurotransmitter release, and hyperactive muscle contractility. Treatment methods are provided that target the earliest steps of the underlying etiology, in order to provide the most efficacious treatment possible. The treatment methods presented include use of compounds such as calcitonin and SERMs such as raloxifene.
Owner:ZAMOYSKI MARK +1
Sex steroid precursors alone or in combination with selective estrogen receptor modulators for the prevention and treatment of dyspareunia in postmenopausal women
ActiveUS20160058774A1Efficient treatment methodReduced activityBiocideOrganic active ingredientsToremifeneDisease
Owner:MYRIEL PHARM LLC
Raloxifene hydrochloride phospholipid complex solid dispersion and preparation thereof
ActiveCN109925314AImprove bioavailabilityIncrease profitOrganic active ingredientsSkeletal disorderPhospholipid complexBioavailability
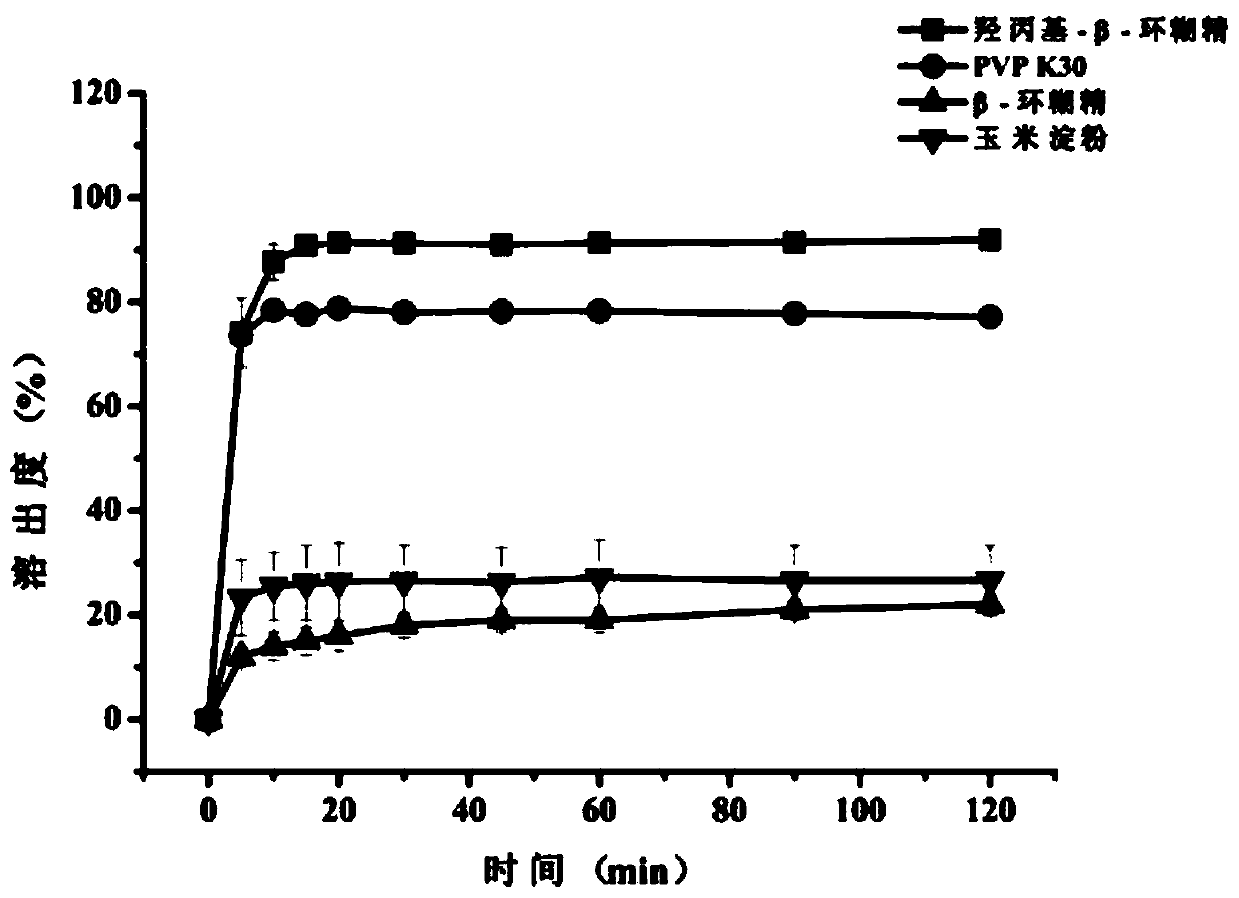
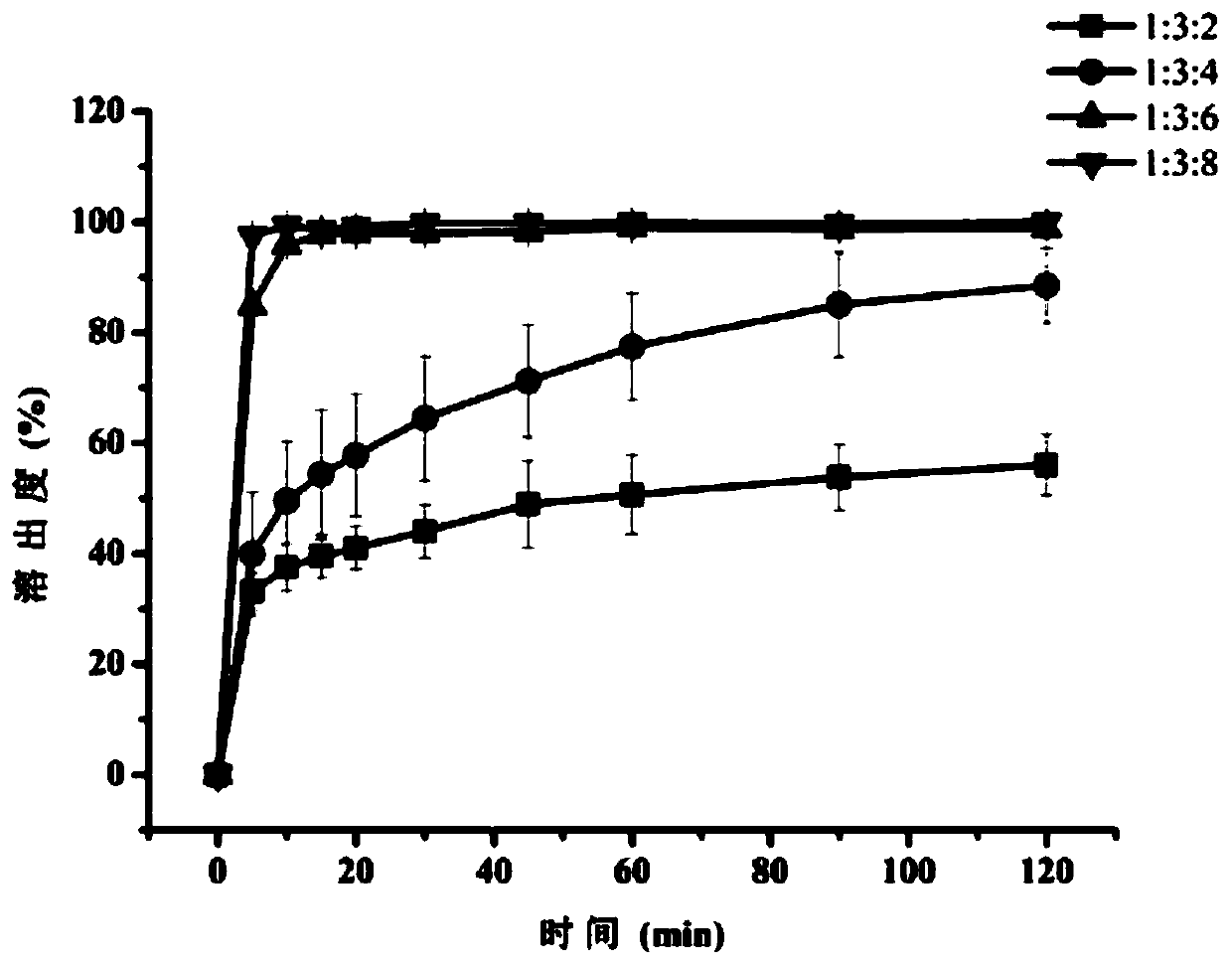
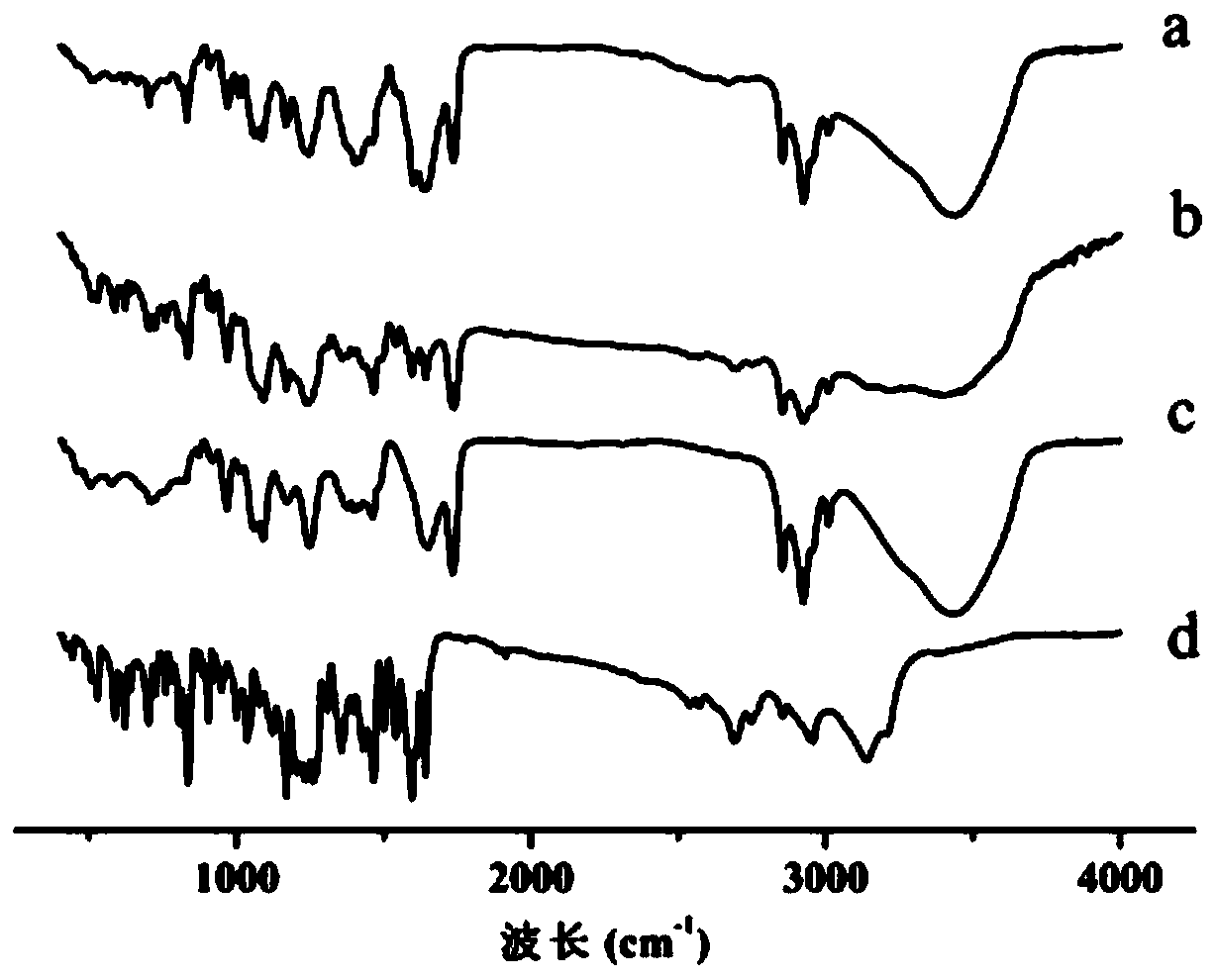
The invention belongs to the technical field of a medicine, and discloses a raloxifene hydrochloride phospholipid complex solid dispersion and a preparation method thereof. The raloxifene hydrochloride phospholipid complex solid dispersion consists of raloxifene hydrochloride, phospholipid and hydroxypropyl-beta-cyclodextrin. The raloxifene hydrochloride phospholipid complex solid dispersion is prepared by the following steps: controlling the mass ratio of raloxifene hydrochloride to phospholipid to prepare a pharmaceutically acceptable raloxifene hydrochloride phospholipid complex, and takinghydroxypropyl-beta-cyclodextrin as a carrier for preparation of the solid dispersion. The dosage form not only improves the in-vitro dissolution and solubility of raloxifene hydrochloride in the phospholipid complex, but also significantly improves the bioavailability of raloxifene hydrochloride, and has certain drug-forming properties. The prepared raloxifene hydrochloride phospholipid complex solid dispersion is mixed with the pharmaceutically acceptable carrier to prepare a clinically acceptable dosage form, and the preparation process is simple and feasible, and is advantageous for industrialized large-scale production.
Owner:SHENYANG PHARMA UNIVERSITY
Raloxifene sprinkle composition
The present invention relates to a capsule composition of raloxifene comprising multiparticulates comprising a) a core comprising raloxifene, and b) a taste-masking coating present in amount of from about 0.5% to about 50% w / w based on the core weight.
Owner:SUN PHARMA INDS
Hydrochloric acid raloxifene dispersible tablet and preparation method thereof
InactiveCN103830197AImprove bioavailabilityQuality improvementOrganic active ingredientsSkeletal disorderRaloxifeneSolubility
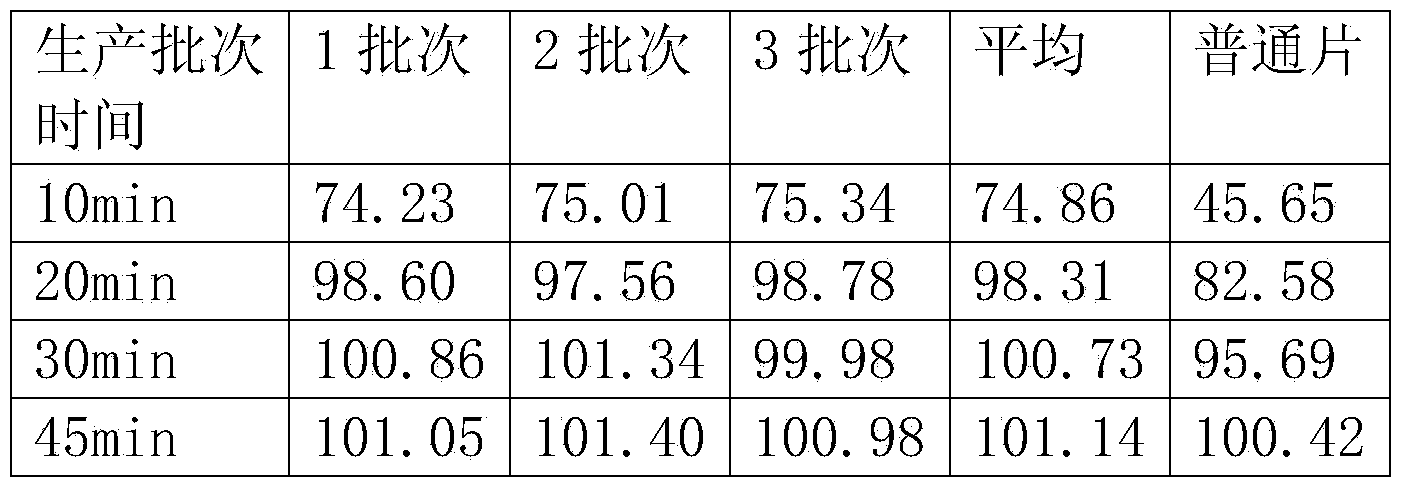
The invention discloses a hydrochloric acid raloxifene dispersible tablet and a preparation method and an application thereof. The hydrochloric acid raloxifene dispersible tablet consists of the following components by weight percent: 5-50% of hydrochloric acid raloxifene, 10-40% of a filling agent, 10-50% of a disintegrating agent, 10-50% of an acidifying agent, 0.1-15% of an adhesive and 0.1-20% of a lubricating agent and flow aid. In comparison with the ordinary tablet, the hydrochloric acid raloxifene dispersible tablet does not contain surface active agents, is favorable in solubility, dispersibility and disintegrative, and can disintegrate completely within 1min. The hadrochloric acid raloxifene dispersible tablet prepared by the method is high in dissolution rate, good in biological availability, rapid in body distribution, stable in quality and good in mouth feeling. The preparation method is simple and feasible and suitable for industrial production.
Owner:崔书豪
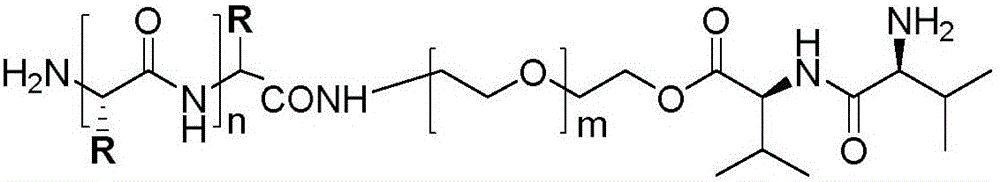
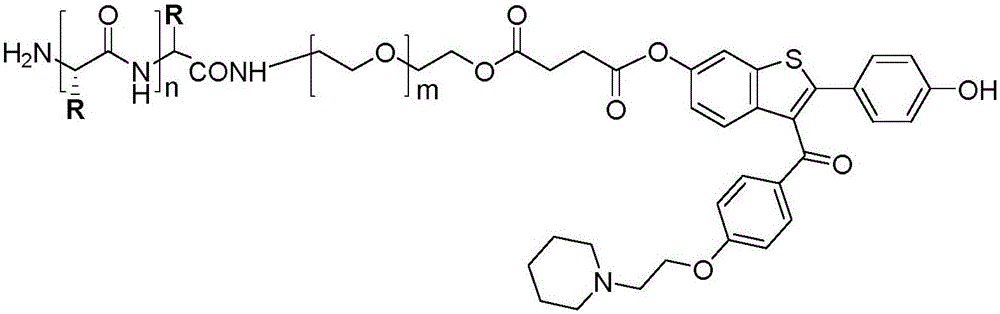
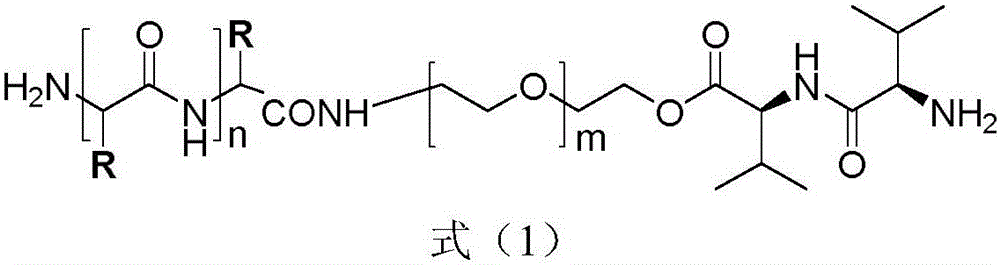
Hybrid subtype tumor targeted nano-micelle and purpose thereof
ActiveCN105687135AIncrease release rateLow toxicityOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetDipeptide
The invention discloses a hybrid subtype tumor targeted nano-micelle and a purpose thereof. The micelle is obtained through mixing and self assembly of a polymer 1 and a polymer 2, wherein the hydrophilic ends of the polymer 1 and the polymer 2 are respectively L-valine-L-valine dipeptide and raloxifene-modified polyethyleneglycol embedding sections; the hydrophobic end is polymer histidine or polymer lactamine. The micelle belongs to a kind of nano-micelle based on PepT1 and ER as the target, and can coat hydrophobic anti-tumor medicine; in-vitro study shows that the stability of the micelle is obviously superior to non-hybrid micelle; meanwhile, the type of nano-micelle medication system can be selectively taken by PepT1 and ER high-expression tumor cells; the tumor cells shows selective cell toxicity, and can be applied to preparation of tumor targeted treatment medicine.
Owner:SOUTHEAST UNIV
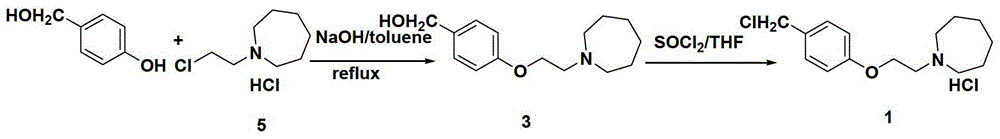
The preparation method of bazedoxifene acetate intermediate
ActiveCN104151265BFew reaction stepsSimple post-processingOrganic chemistryRaloxifeneAfter treatment
The invention discloses a preparation method of bazedoxifene acetate intermediate. The present invention provides a preparation method of bazedoxifene acetate intermediate compound 3, and the preparation method comprises the following steps: step 1, in a polar aprotic solvent in the presence of an alkali, intermediate compound 4 is obtained by condensation reaction of p-hydroxy benzaldehyde 6 and compound 5; and step 2, the reaction solution obtained in step 1, without after treatment, is directly mixed with a protic solvent, then is reduced by a reductant to obtain the bazedoxifene acetate intermediate compound 3. The preparation method has the advantages of short course, mild reaction conditions, safe operation, simple post treatment process, high conversion rate, high product yield, good purity, environmental friendliness and suitability for industrialized production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Sex steroid precursors alone or in combination with a selective estrogen receptor modulator and/or with estrogens and/or a type 5 cGMP phosphodiesterase inhibitor for the prevention and treatment of vaginal dryness and sexual dysfunction in postmenopausal women
ActiveUS8835413B2Efficient treatment methodReduced activityBiocideSuppositories deliveryDiseaseToremifene
Owner:MYRIEL PHARM LLC
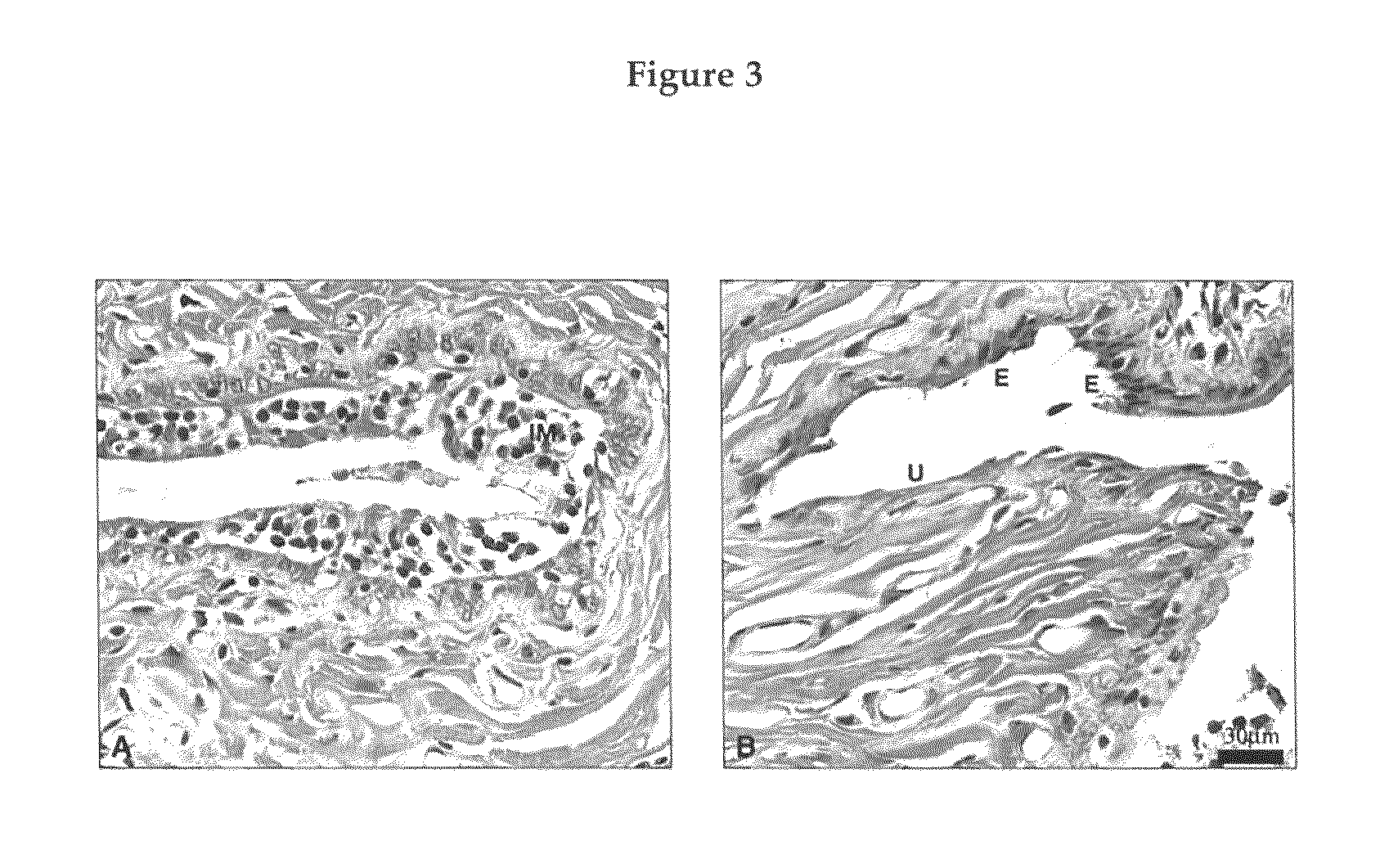
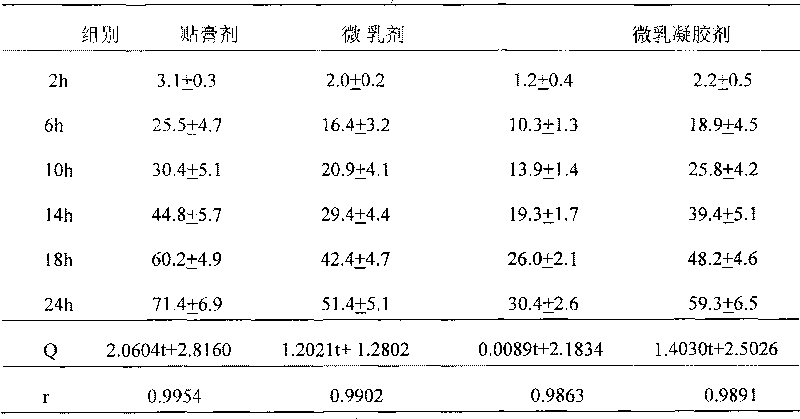
Raloxifene emplastrum preparation and preparation method thereof
InactiveCN101700241AConvenient treatmentStabilize local blood drug concentrationOrganic active ingredientsSkeletal disorderTransdermal patchAdditive ingredient
The invention relates to a raloxifene emplastrum and a preparation method thereof. Raloxifene microemulsion is made into a transdermal emplastrum which is divided into an anti-adhesion layer, a viscose layer, a backlining layer and a medicine storage layer. The raloxifene microemulsion taken as a medical ingredient of the raloxifene emplastrum comprises the following ingredients: raloxifene taken as an active ingredient, polysorbate taken as surfactant, propanediol taken as cosurfactant, pure water or distilled water, and the like. Except for the medical ingredient, a plurality of nonpolar polymers, plasticizing agent, caking agent, transdermal enhancing agent and antioxidant are also added in the emplastrum. Compared with the prior dosage forms, such as tablets, the invention has the remarkable advantages of durable medical effect, safety, low toxicity, simple, convenient and sanitary, and accurate medical dosage.
Owner:河南省生物工程技术研究中心
Anordrin compositions and methods for treating diseases
ActiveUS20190262360A1Eliminate side effectsElevated sugar uptakeOrganic active ingredientsMetabolism disorderDiseaseRaloxifene
The present invention provides methods and compositions for treating cancer, reducing side effects, and reducing postmenopausal symptoms comprising anordrin or analog thereof (such as anordrin) alone or in combination with at least one other agent selected from the group consisting of tamoxifen, raloxifene or functional equivalent thereof, and an aromatase inhibitor.
Owner:ZHEJIANG JIACHI PHARMA DEV LTD +1
Program randomization for cyber-attack resilient control in programmable logic controllers
ActiveUS20190008845A1Good treatment effectReduced psychoactive effectProgramme controlHydroxy compound active ingredientsRaloxifeneCyber-attack
The present invention provides a method of treatment of a neuropsychiatric disorder characterized by repetitive phenotype, comprising administering to a human or non-human subject in need thereof a therapeutically effective dose of a pharmaceutical composition comprising at least one active agent selected from a group of active agents comprising CB2 receptor inverse agonists and mixed CB2 / SERM ligands. The present invention further provides pharmaceutical compositions comprising 4′-0-methylhonokiol, raloxifene, and their derivatives, HU-308 and / or BCP.
Owner:SIEMENS AG
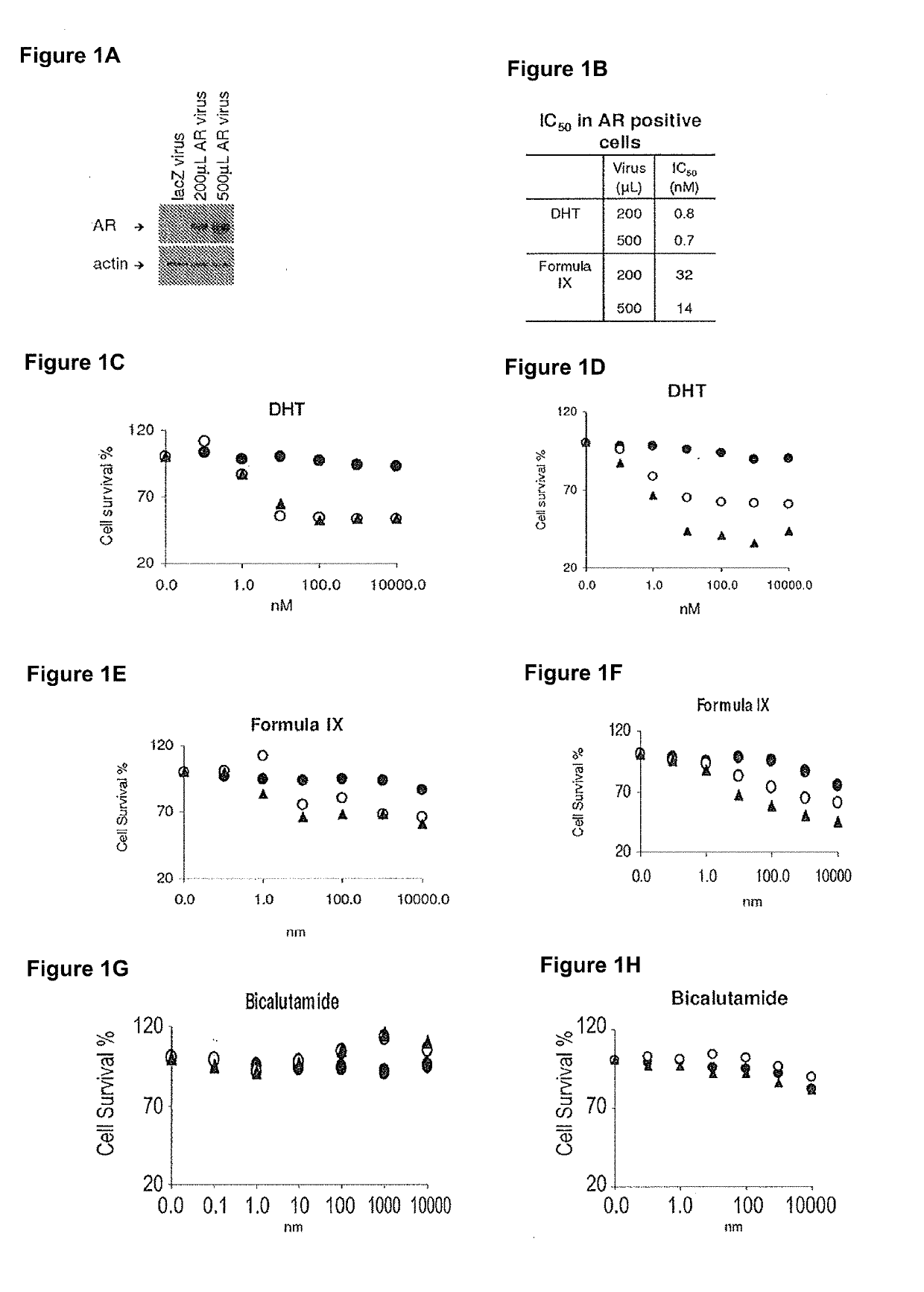
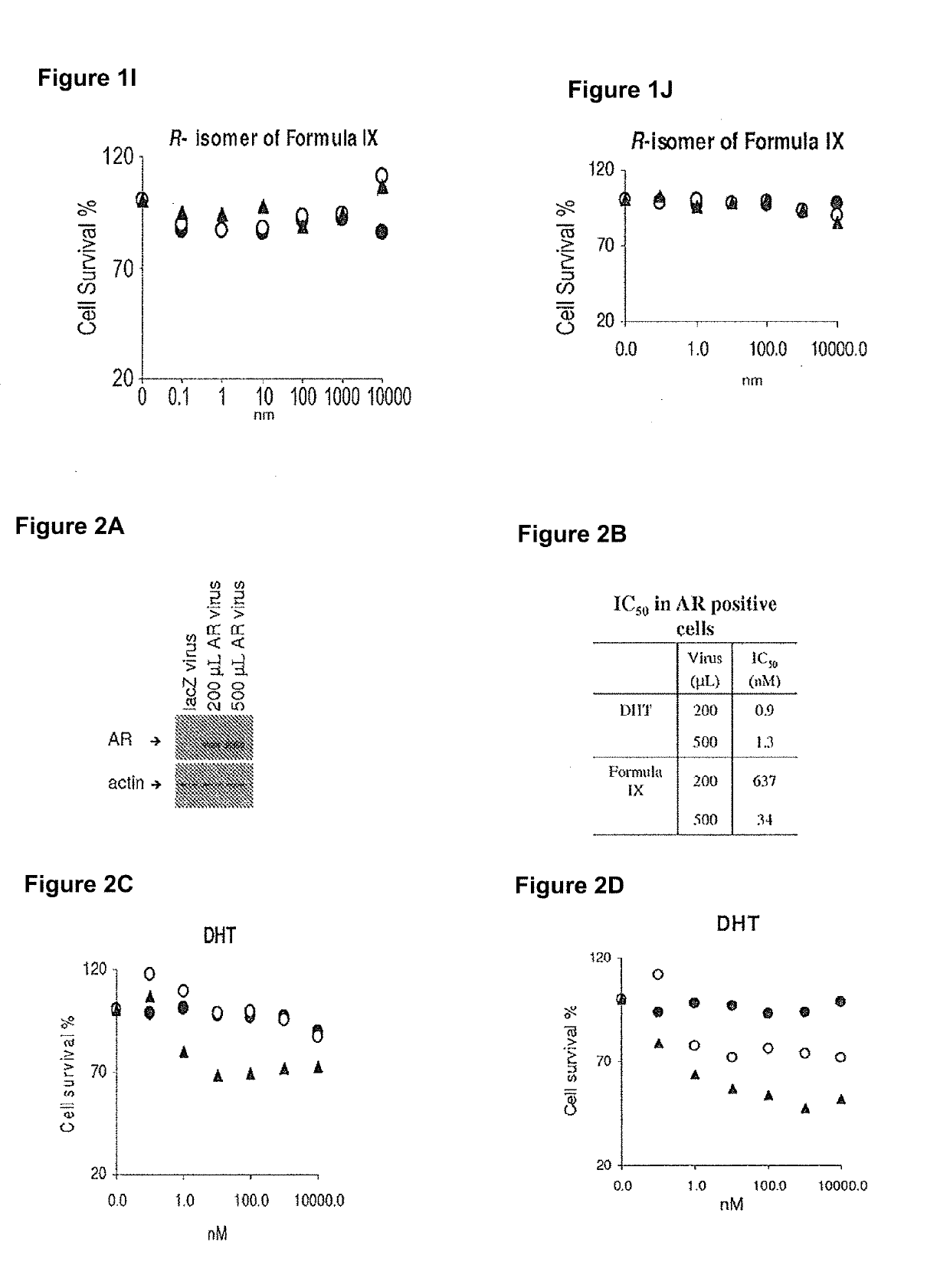
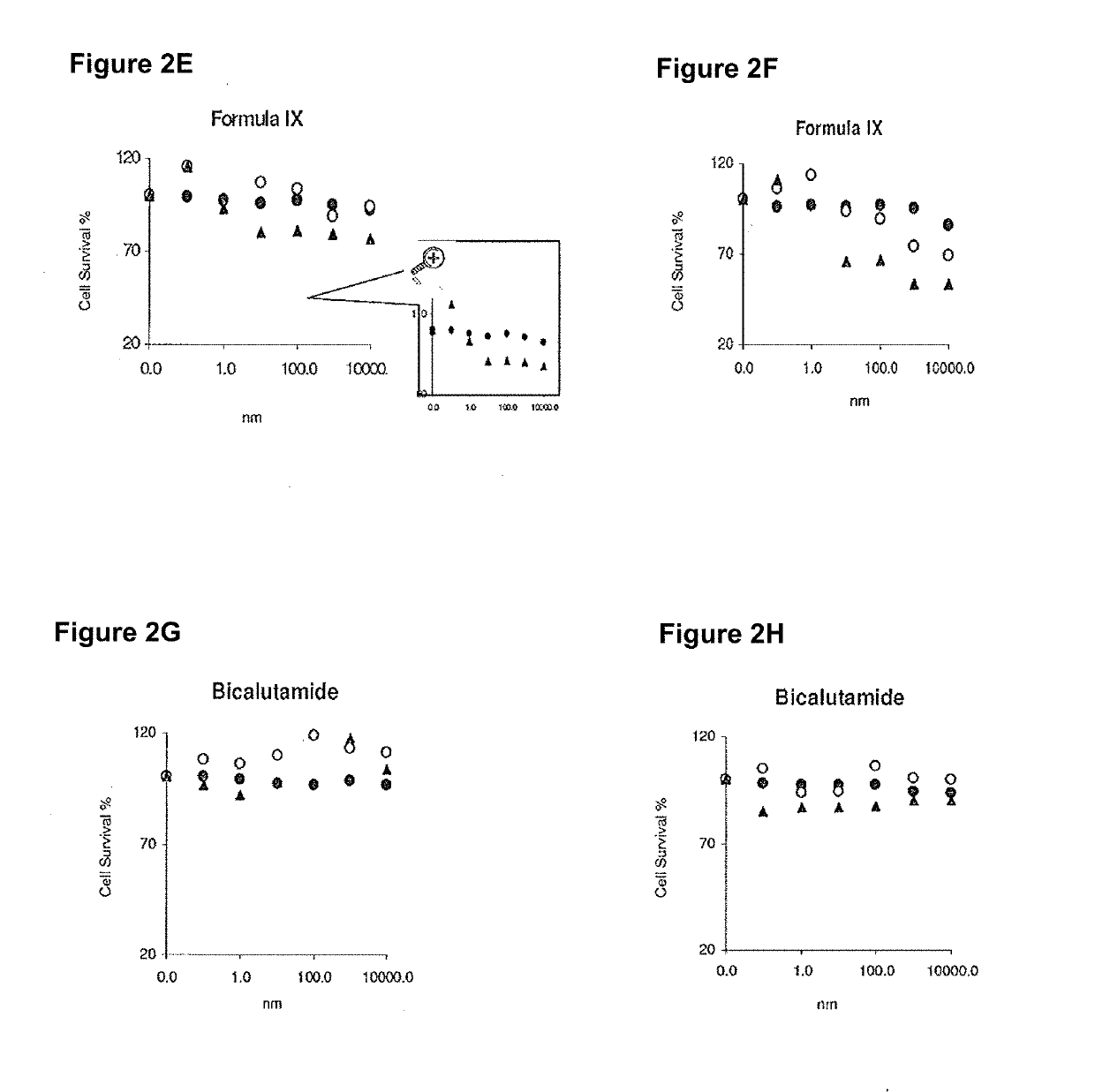
Non-invasive method of evaluating breast cancers for selective androgen receptor modulator (SARM) therapy
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; treating a subject suffering from ER mutant expressing breast cancer and / or treating breast cancer in a subject, by first determining the 18F-16β-fluoro-5α-dihydrotestosterone (18F-DHT) tumor uptake and identifying said subject as having AR-positive breast cancer based on 18F-DHT tumor uptake, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Treatment of mental, movement and behavioral disorders
InactiveUS20190321355A1Good treatment effectReduced psychoactive effectNervous disorderHydroxy compound active ingredientsRaloxifeneDisease
The present invention provides a method of treatment of a neuropsychiatric disorder characterized by repetitive pheno-type, comprising administering to a human or non-human subject in need thereof a therapeutically effective dose of a pharmaceutical composition comprising at least one active agent selected from a group of active agents comprising CB2 receptor inverse agonists and mixed CB2 / SERM ligands. The present invention further provides pharmaceutical compositions comprising 4′-0-methylhonokiol, raloxifene, and their derivatives, HU-308 and / or BCP.
Owner:ANAVI GOFFER SHARON
Application of Raloxifene compounds in preparing medicament for preventing and controlling benign prostatahyperplasie
InactiveCN101366716AStrong antagonistic effectReduce thicknessOrganic active ingredientsUrinary disorderRaloxifeneAndrogen
The invention belongs to the medicinal field, and in particular discloses application of a raloxifene compound in preparing medicines for preventing and treating nonmalignant prostate hyperplasia. As verified by cell and animal model experiments, the raloxifene compound has obvious inhibition effect on proliferation of prostate interstitial cells, can resist the proliferation promoting effect of estradiol on the interstitial cells, and has obvious effect of treating and improving the pathologic change that estrogen / androgen induces prostate interstitial proliferation of an emasculated mouse.
Owner:NANKAI UNIV
Method for chemoprevention of prostate cancer
The invention relates to uses of antiestrogen in the medicine preparation. The medicine is used for preventing, limiting and restraining the prostatic cancer or potential prostatic cancer, and for decreasing the danger of prostatic cancer or potential prostatic cancer, or curing the prostatic cancer or potential prostatic cancer, wherein, the antiestrogen is Toremifene, Raloxifene, Tamoxifen, Idoxifene or Droloxifene.
Owner:UNIV OF TENNESSEE RES FOUND
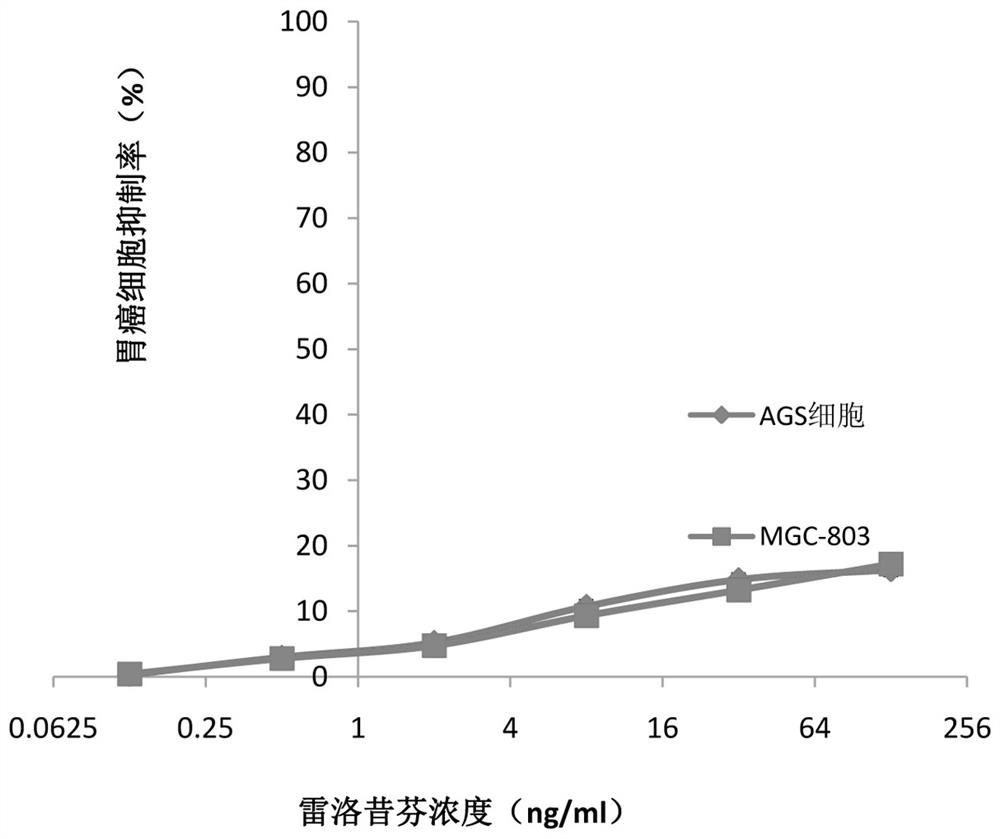
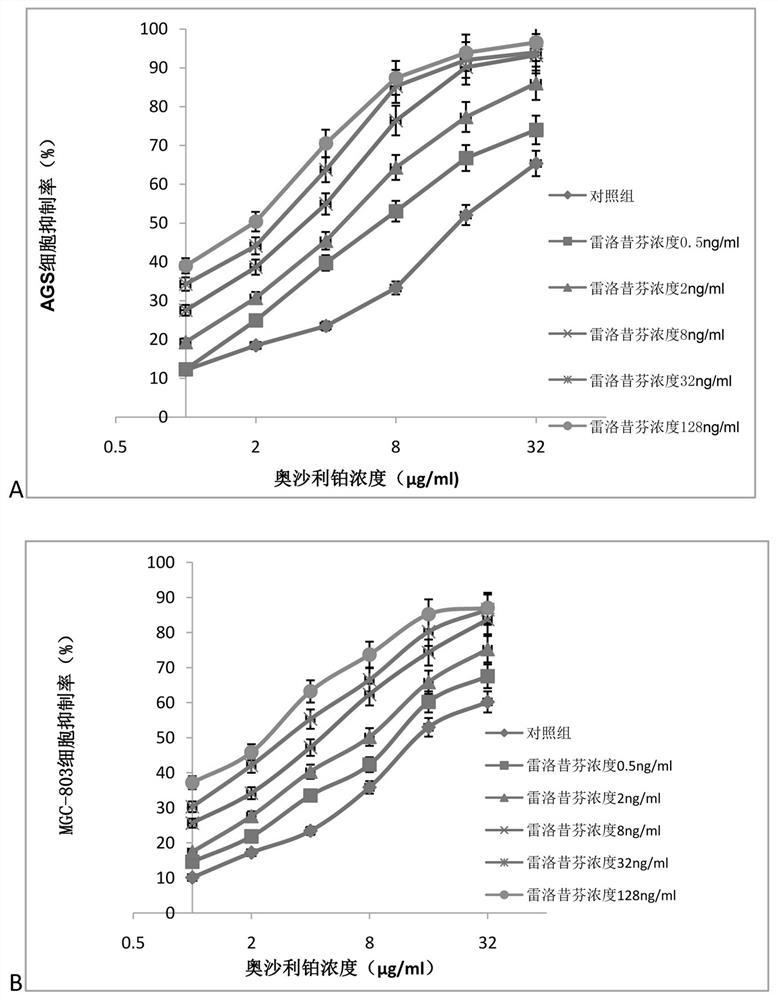
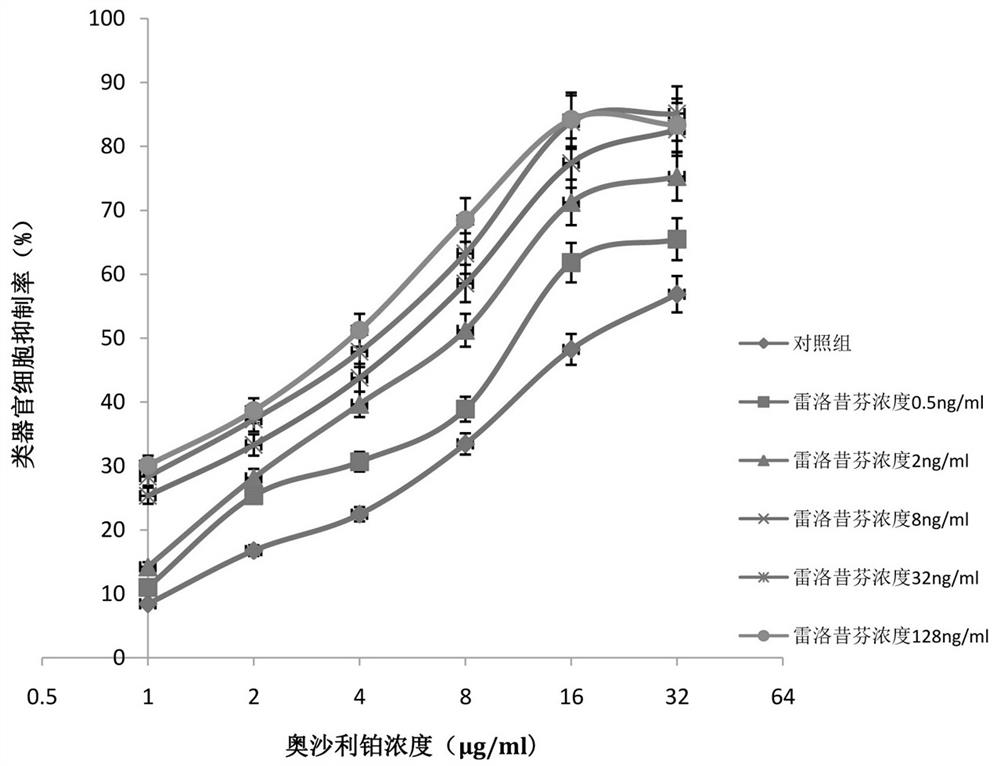
Application of raloxifene and oxaliplatin combined medicine in preparation of medicine for treating gastric cancer
InactiveCN112426428AIncreased sensitivityReduce dosageOrganic active ingredientsAntineoplastic agentsRaloxifeneSide effect
The invention belongs to the field of medicine, and particularly relates to an application of raloxifene and oxaliplatin combined medicine in preparation of a medicine for treating gastric cancer. Theapplication of the raloxifene and oxaliplatin combined medicine in preparation of the medicine for treating gastric cancer is proposed for the first time, raloxifene and oxaliplatin have an obvious synergistic effect, the curative effect is effectively improved, the curative effect is more remarkable compared with that of single components, and the lethality to tumor cells is improved; and the dosage is effectively reduced, so that the toxic and side effects are reduced. The combined use of raloxifene and oxaliplatin can save the cost, reduce the economic burden of patients, provide a new wayfor the prevention and treatment of gastric cancer, and have broad application prospects in the field of medicine and pharmacology.
Owner:阿耳法猫(杭州)人工智能生物科技有限公司
Raloxifene sprinkle composition
ActiveUS20170340573A1Patient compliance is goodLow water solubilityMicrocapsulesElectrical componentsRaloxifeneMedicine
The present invention relates to a capsule composition of raloxifene comprising multiparticulates comprising a) a core comprising raloxifene, and b) a taste-masking coating present in amount of from about 0.5% to about 50% w / w based on the core weight.
Owner:SUN PHARMA INDS
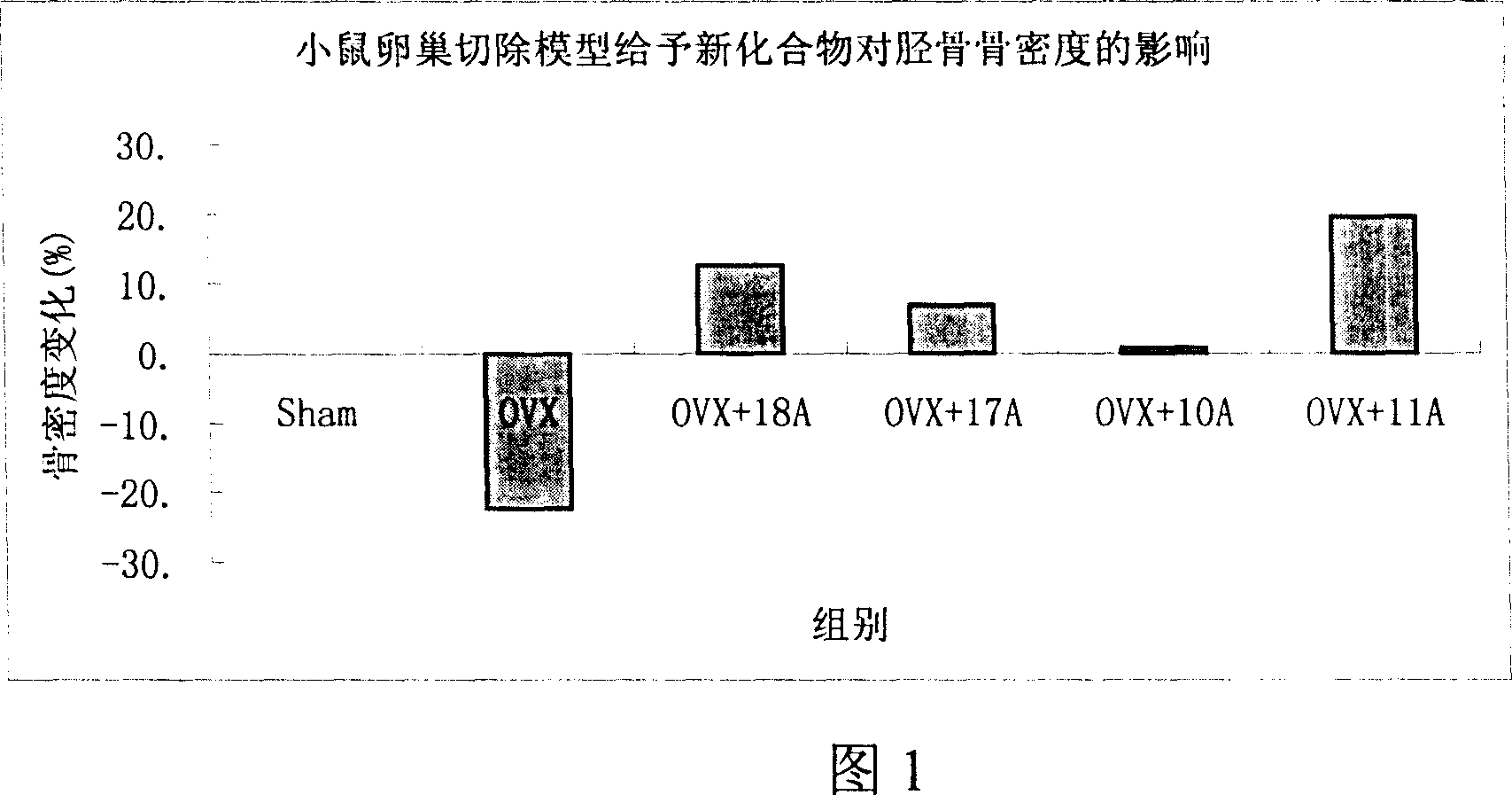
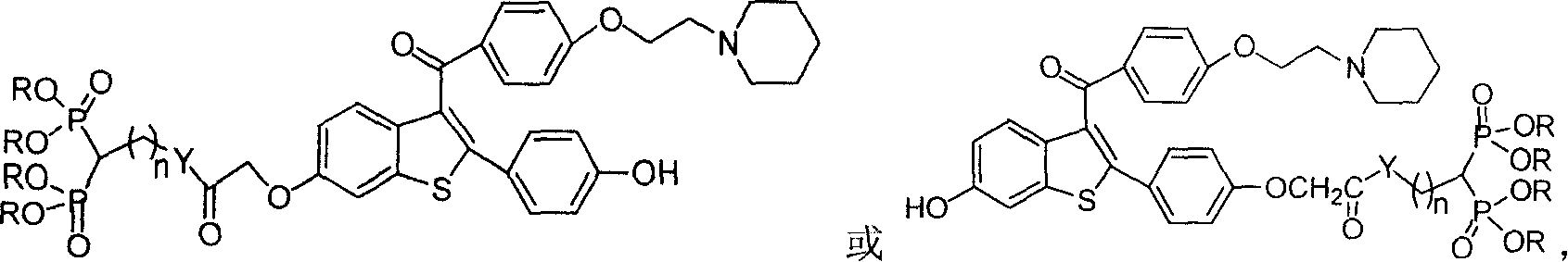
Medication towards target of bone, synthetic method and application
InactiveCN100339383CCurb churnGood anti-osteoporosis effectOrganic active ingredientsGroup 5/15 element organic compoundsDiseaseMedicine
A bone-targetable medicine and its synthesizing process are disclosed. It is a biphosphorous acid compound which has the action to suppress bone absorption, better asteotropism and better chelating function, so it can be used to treat relative diseases.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com