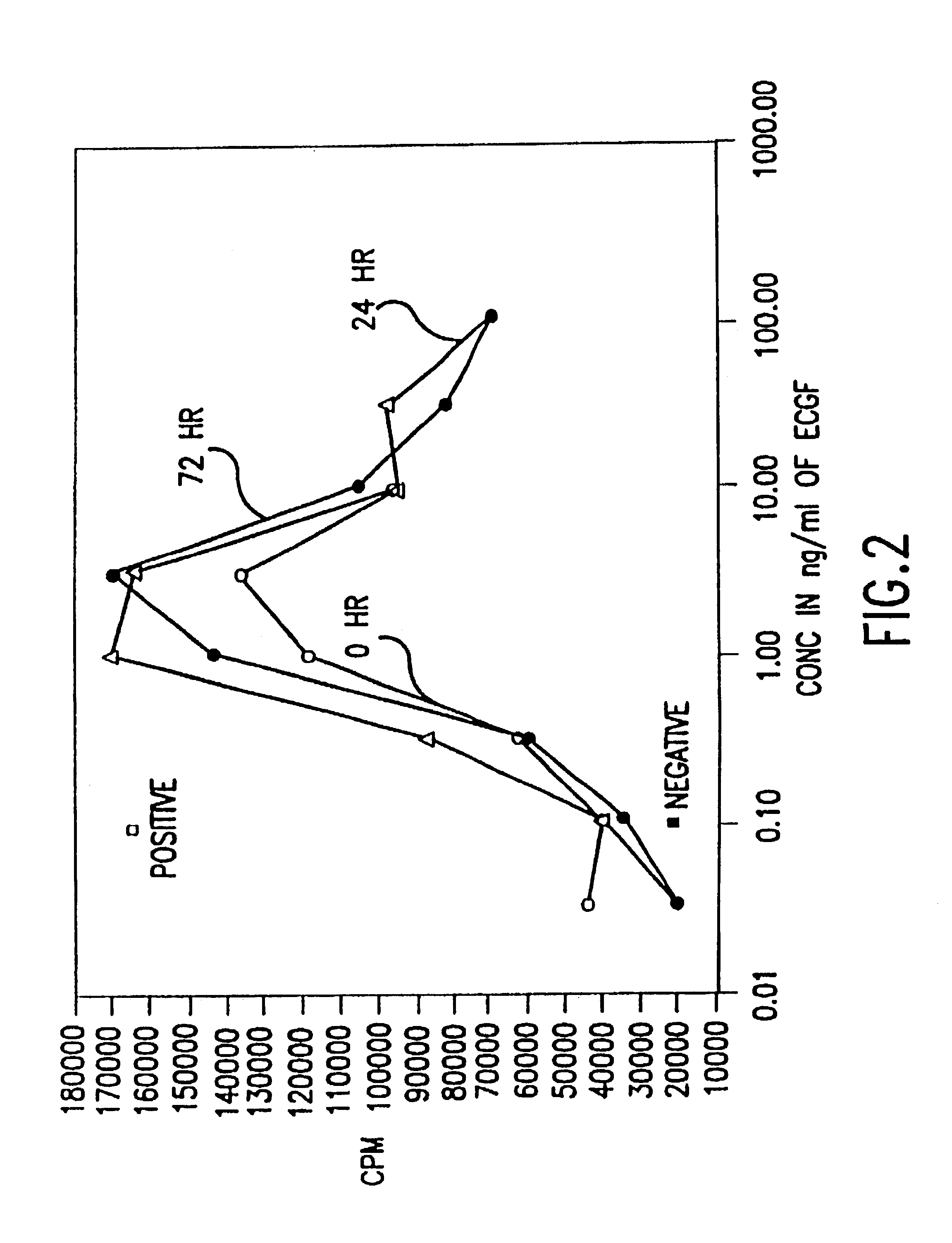
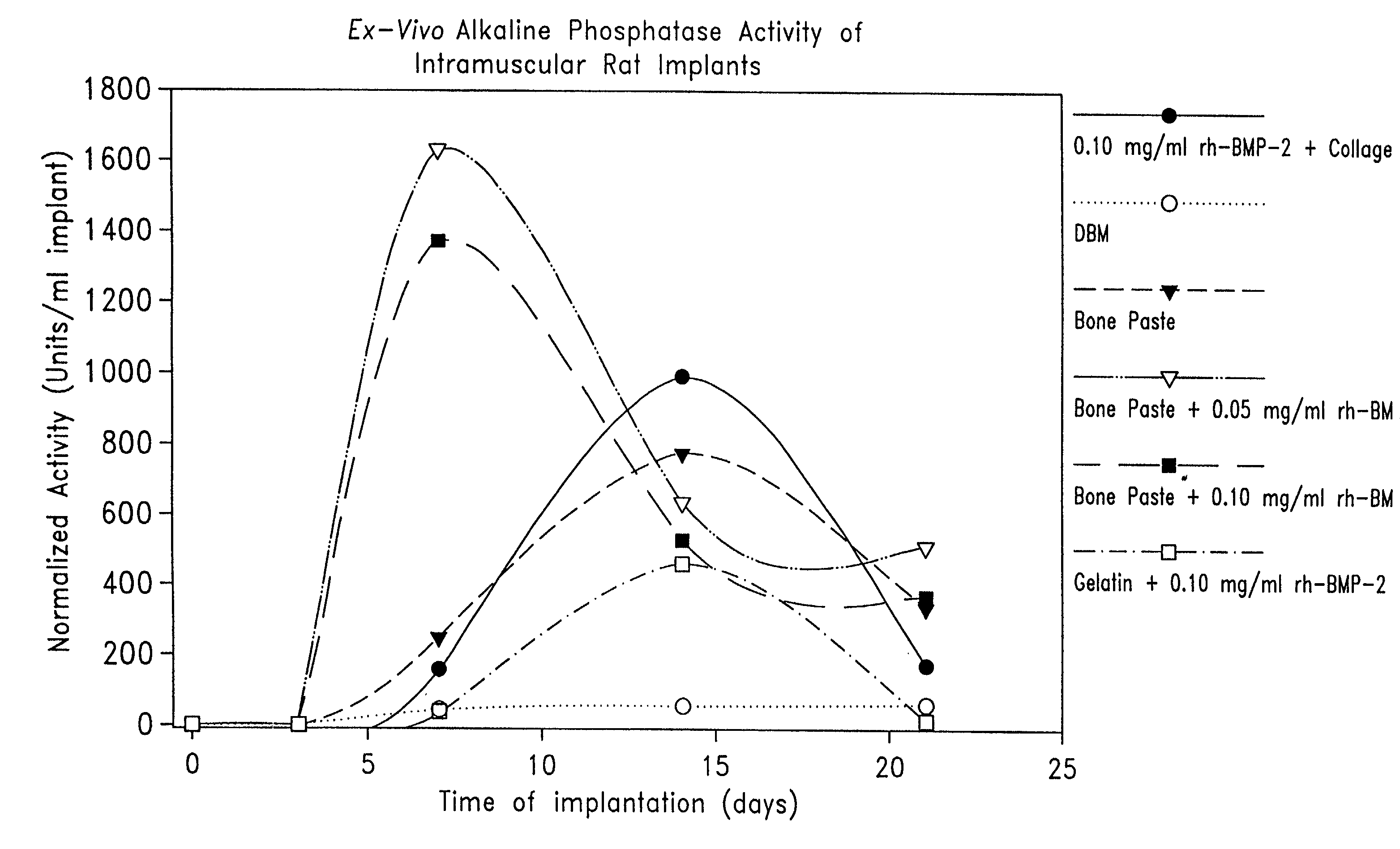
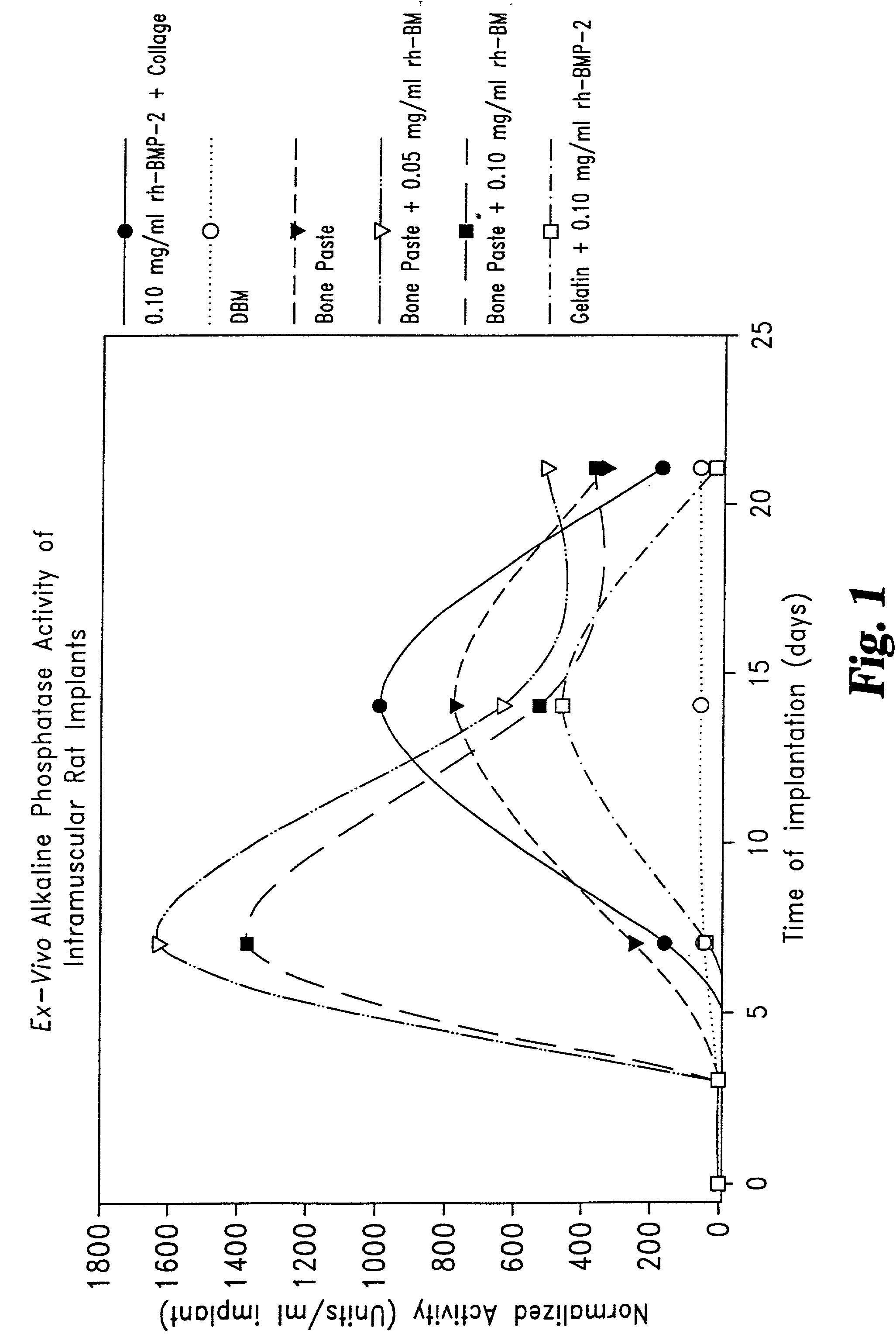
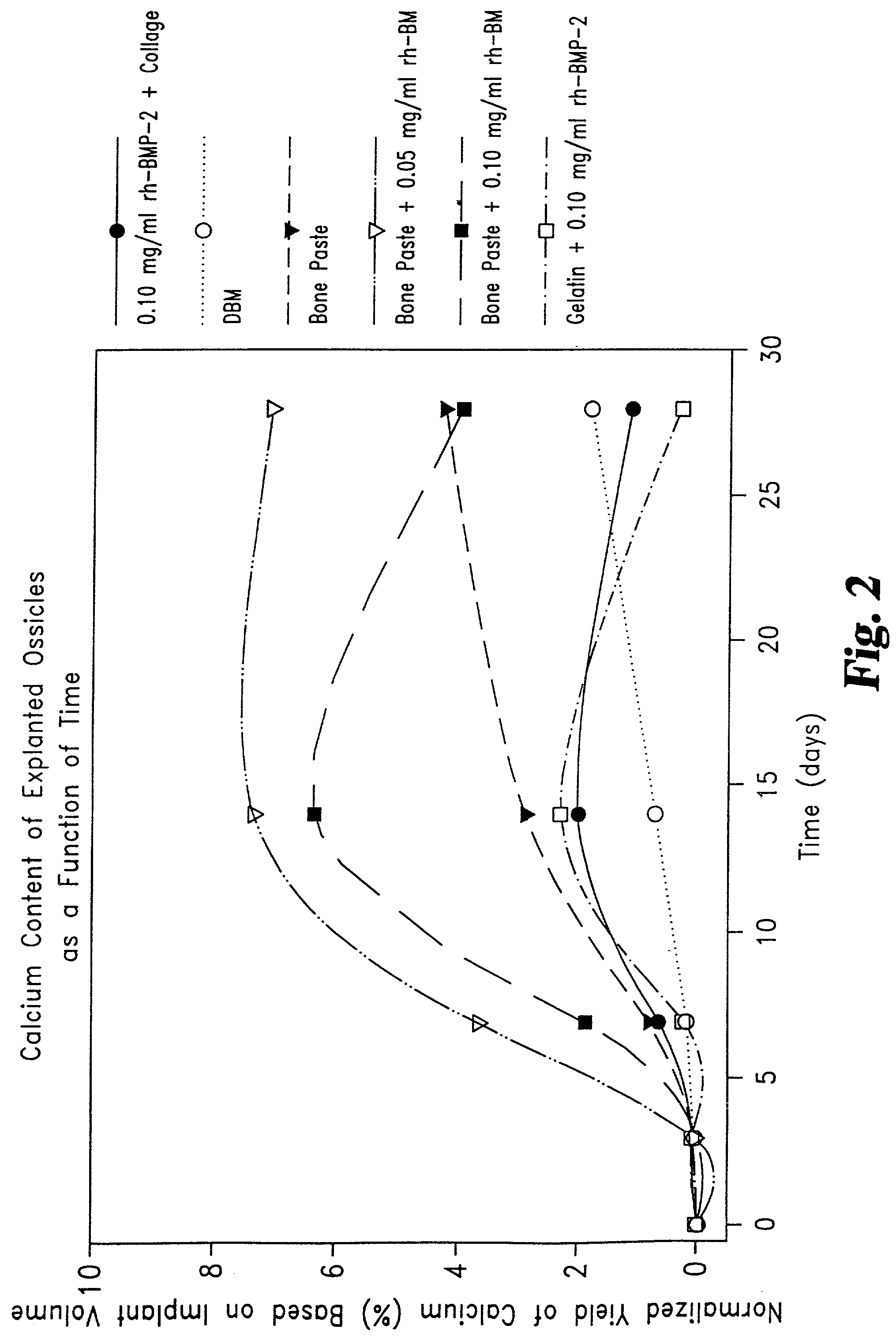
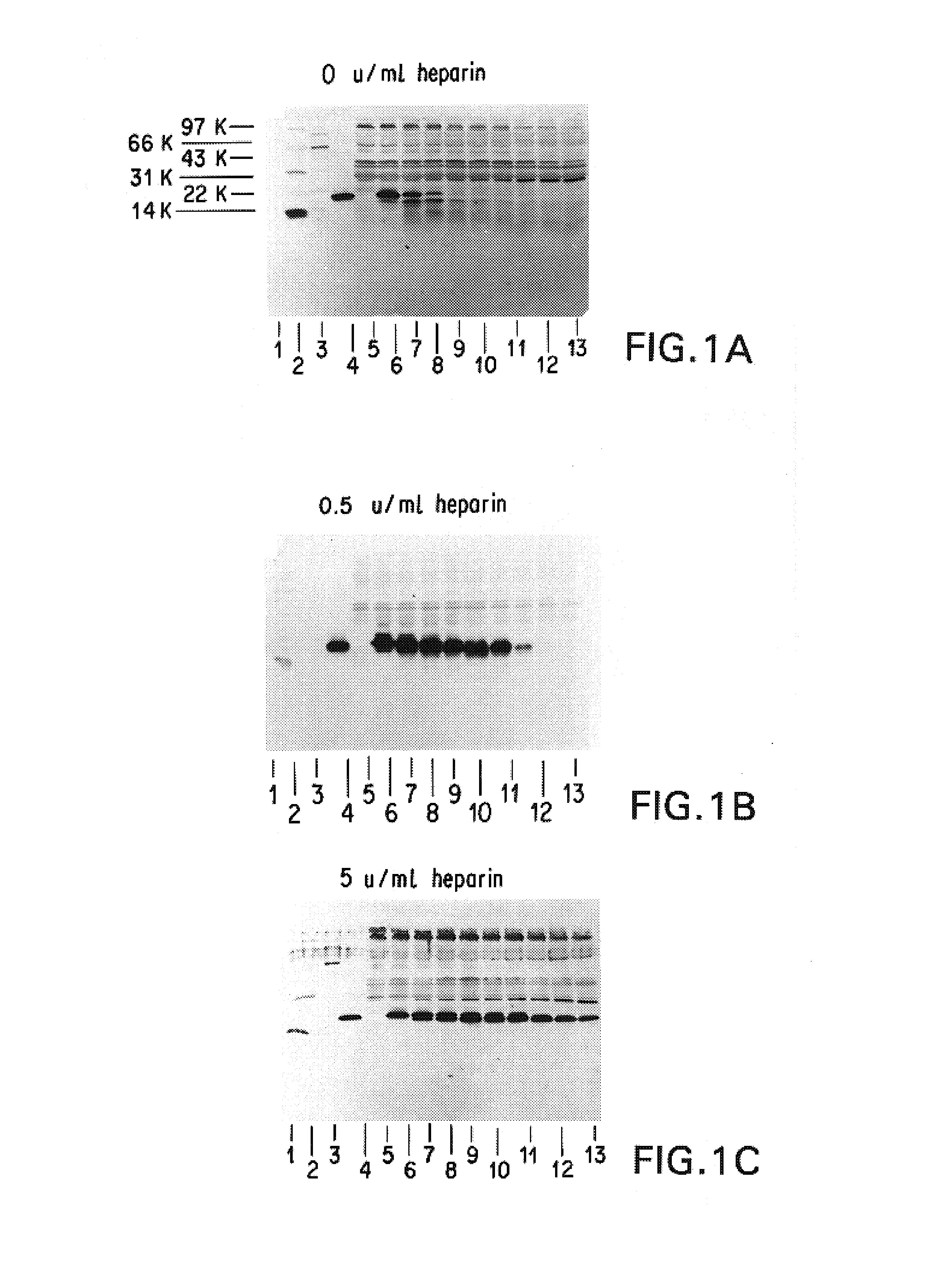
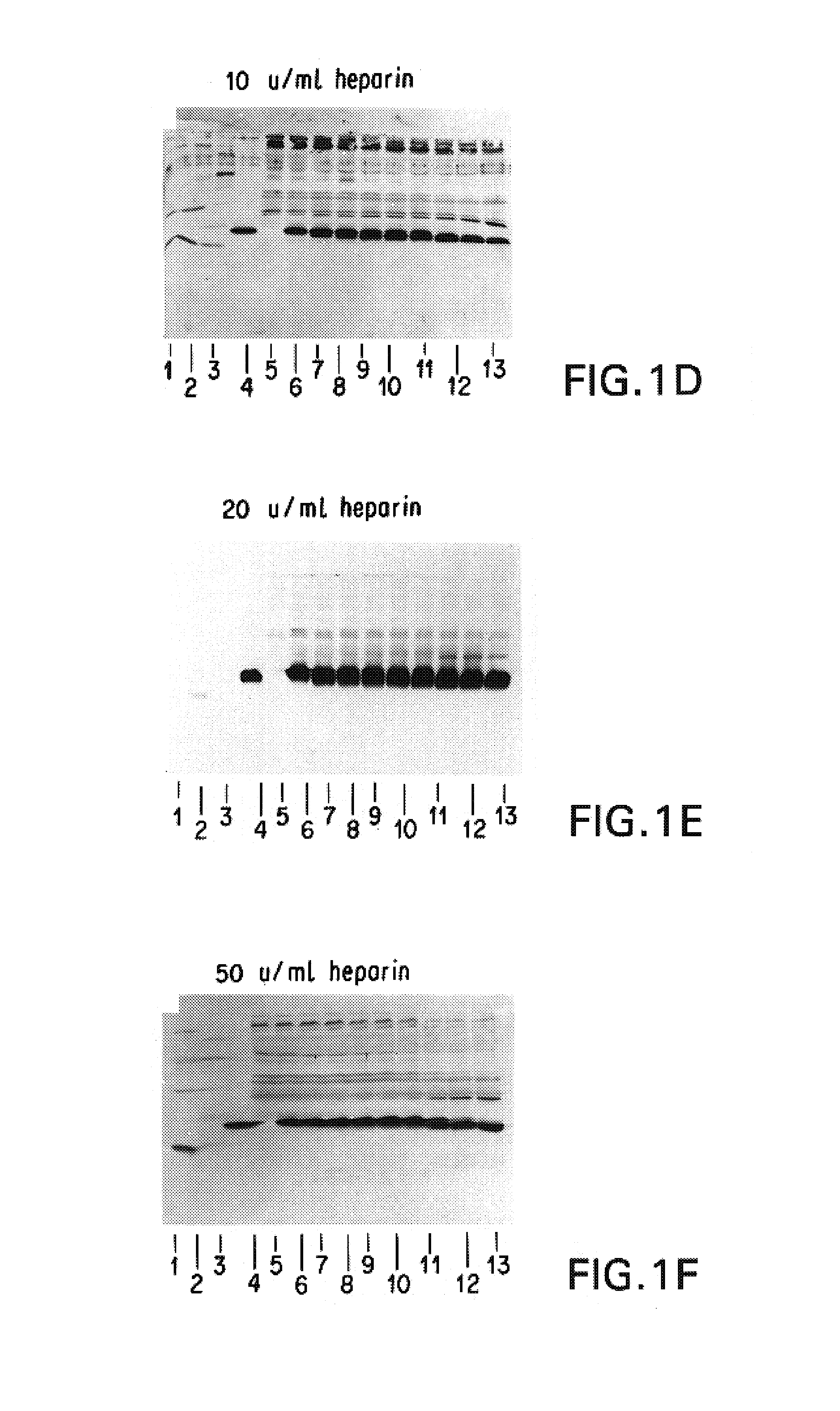
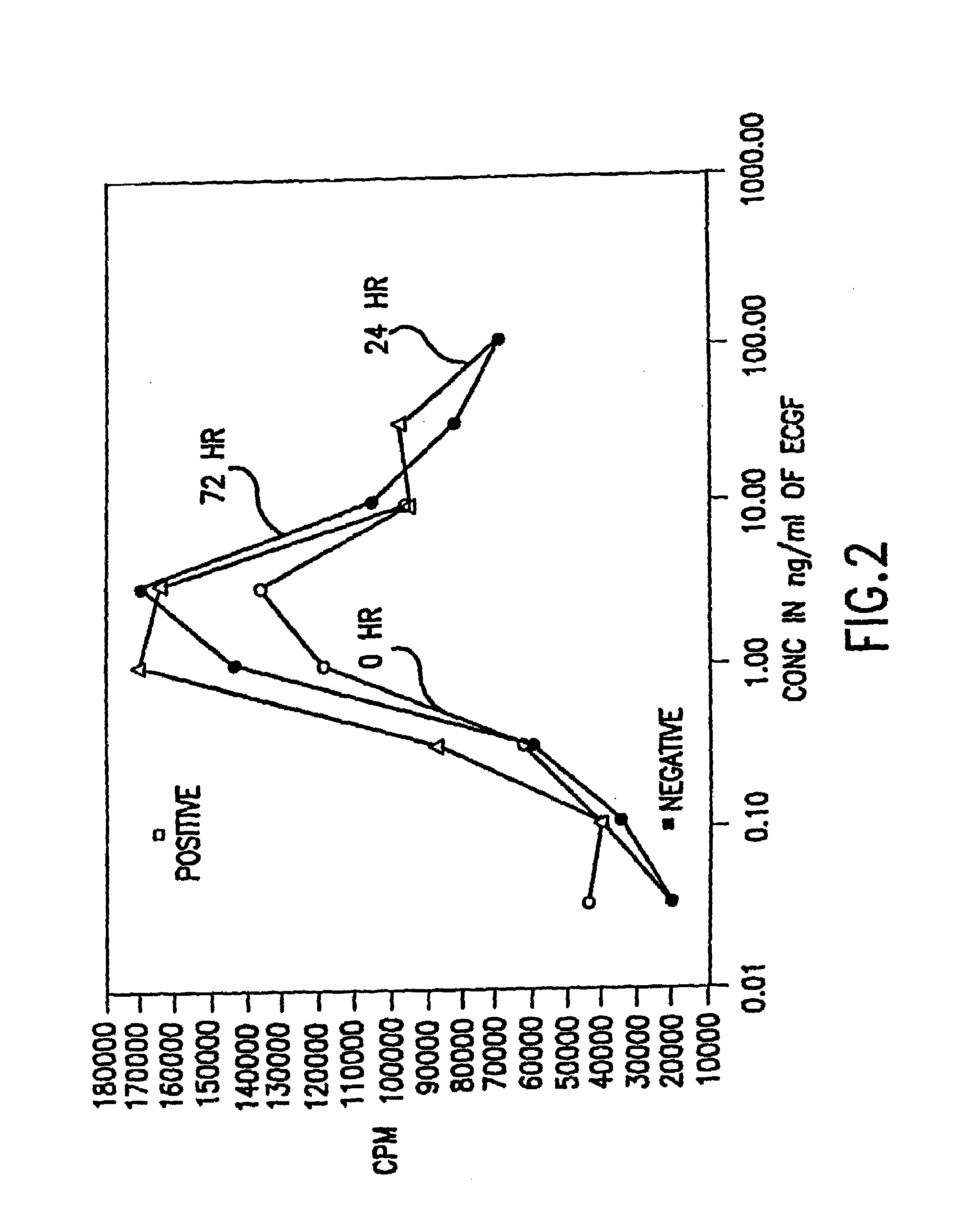
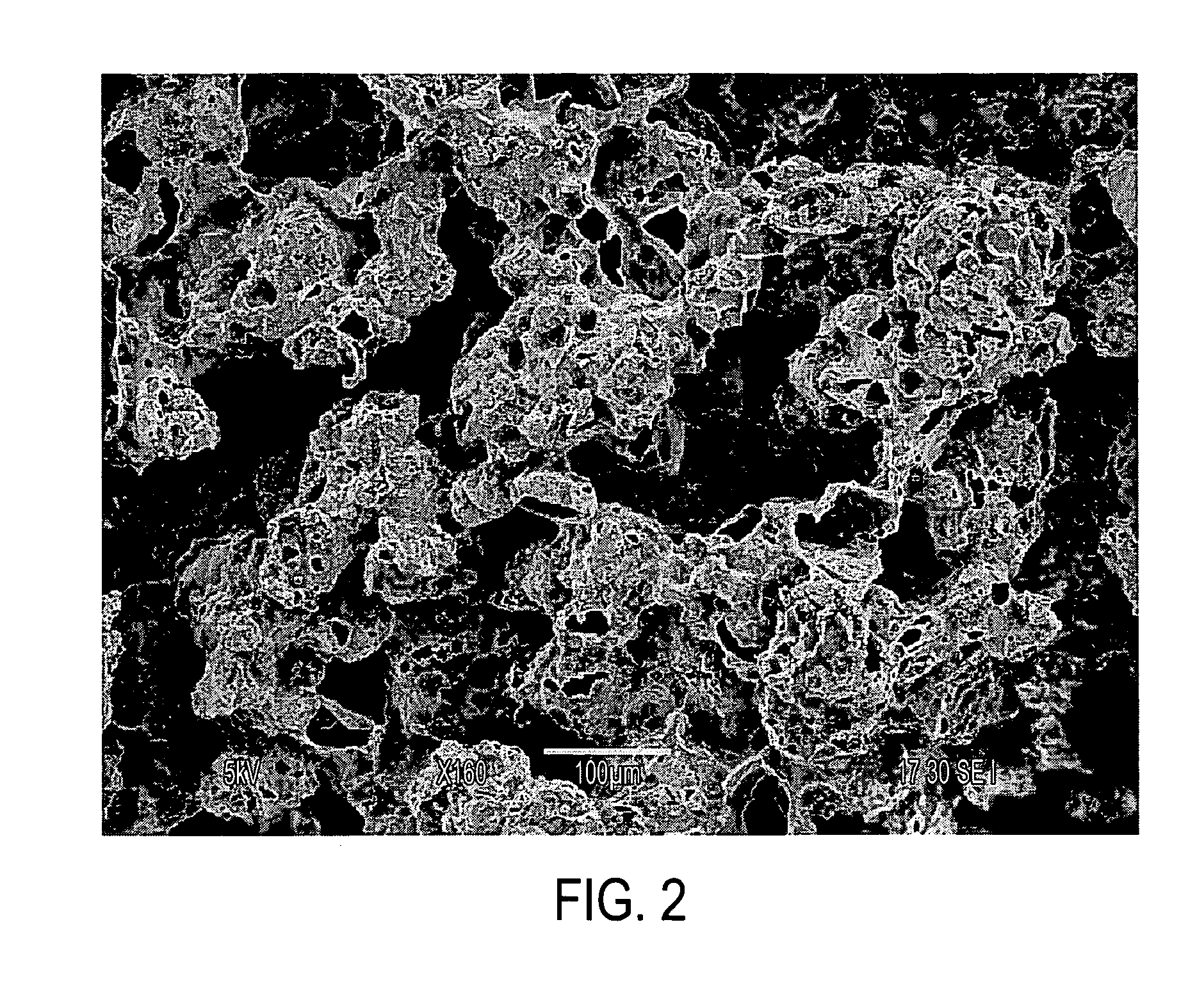
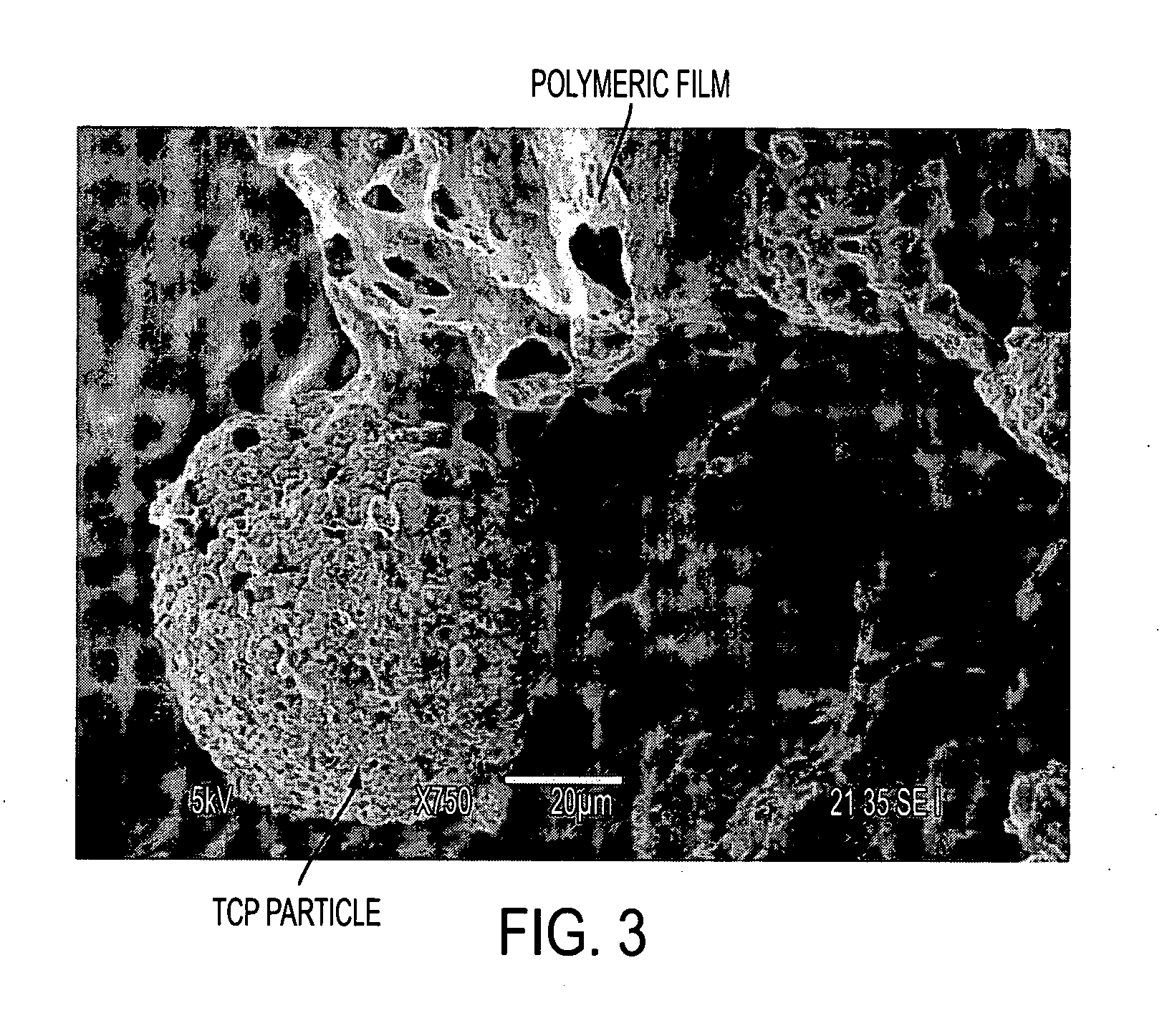
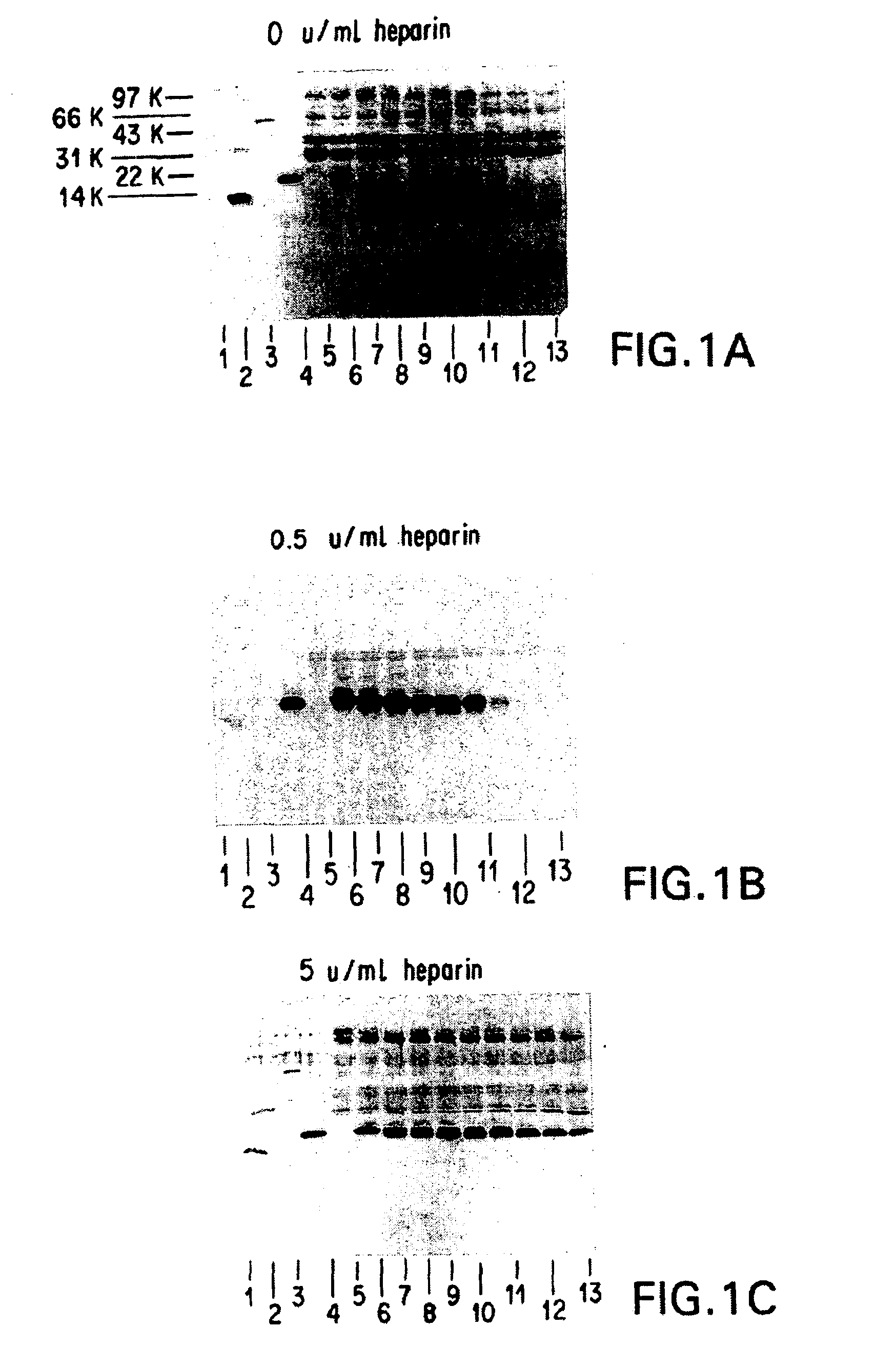
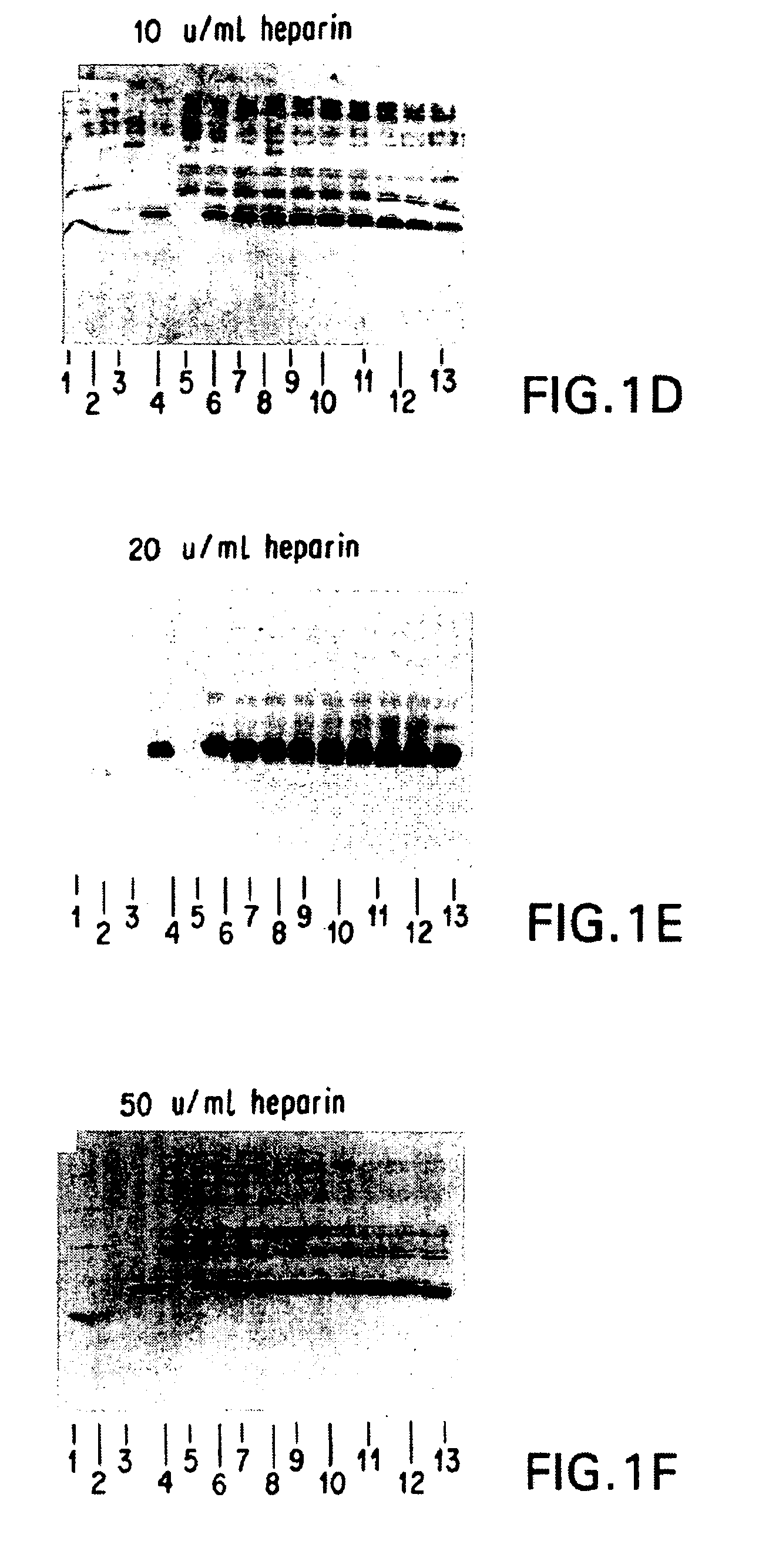
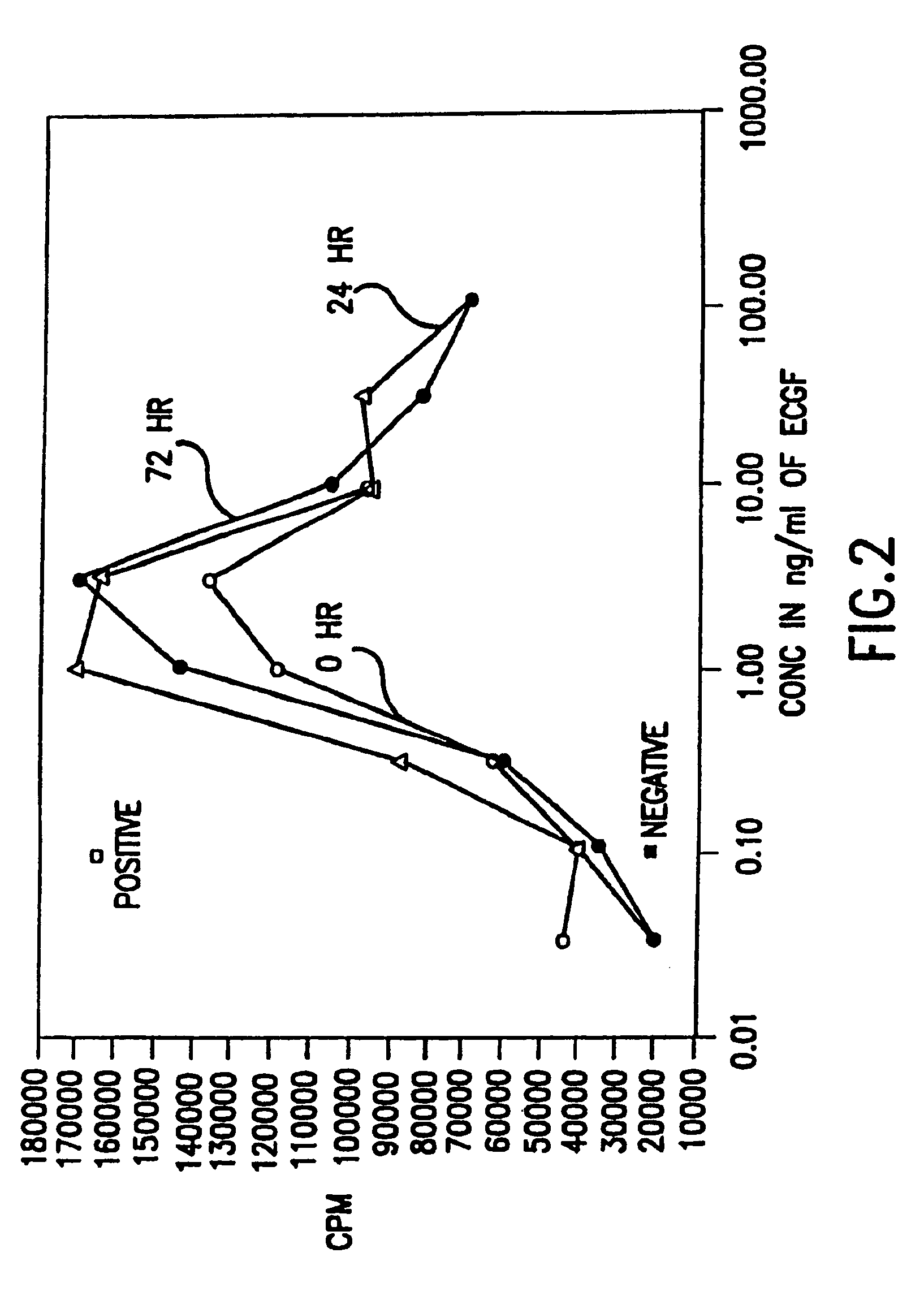
Patents
Literature
440 results about "Bone morphogenetic protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bone morphogenetic proteins (BMPs) are a group of growth factors also known as cytokines and as metabologens. Originally discovered by their ability to induce the formation of bone and cartilage, BMPs are now considered to constitute a group of pivotal morphogenetic signals, orchestrating tissue architecture throughout the body. The important functioning of BMP signals in physiology is emphasized by the multitude of roles for dysregulated BMP signalling in pathological processes. Cancerous disease often involves misregulation of the BMP signalling system. Absence of BMP signalling is, for instance, an important factor in the progression of colon cancer, and conversely, overactivation of BMP signalling following reflux-induced esophagitis provokes Barrett's esophagus and is thus instrumental in the development of adenocarcinoma in the proximal portion of the gastrointestinal tract.
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
Naphtholactams and lactones as bone morphogenetic protein active agents
InactiveUS6030967AHigh toxicological thresholdReduce riskBiocideOrganic chemistryActive agentCarbon chain
PCT No. PCT / JP97 / 02858 Sec. 371 Date Oct. 30, 1997 Sec. 102(e) Date Oct. 30, 1997 PCT Filed Aug. 19, 1997 PCT Pub. No. WO98 / 07705 PCT Pub. Date Feb. 26, 1998A compound of the formula: wherein Q is an optionally substituted carbon atom or N(O)p wherein p is 0 or 1; Y is an optionally substituted methylene group, S(O)q wherein q is an integer of 0 to 2, or an optionally substituted imino group; Z1 is a C1-3 alkylene group which may have an oxo group or a thioxo group and may contain etherified oxygen or sulfur within the carbon chain; Z2 is an optionally substituted C1-3 alkylene group; Ar is an optionally substituted carbocyclic group or an optionally substituted heterocyclic group; one of R1 and R2 is a hydrogen atom, a halogen atom, a hydroxyl group, an optionally substituted lower alkyl group, or an optionally substituted lower alkoxy group; the other is a halogen atom, a hydroxyl group, an optionally substituted lower alkyl group, or an optionally substituted lower alkoxy group; or R1 and R2 taken together with adjacent -c=c- form a ring; and ring A is a benzene ring which may be substituted in addition to R1 and R2; or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
Osteogenic fusion device
InactiveUS7179293B2Support of loadMinimize radiopacityBone implantJoint implantsDevice formBone growth
An interbody osteogenic fusion device is provided that includes opposite end pieces with an integral central element. The end pieces are sized to maintain the height of an intervertebral disc space. The central element has a much smaller diameter so that the osteogenic fusion device forms an annular pocke around the central element. An osteogenic material is disposed within the annular pocket between the opposite end pieces. In one embodiment,the osteogenic material constitutes a collagen sheet soaked in asolution containing a bone morphogenetic protein. The osteogenic fusion device is configured so that the osteogenic material is in direct contact with the adjacent vertebral bone. In addition to the enhanced area of contact between the vertebral bone and the fusion material, the inventive osteogenic fusion device reduces stress-shielding and minimizes the radiopacity of the implant so that growth of the fusion mass can be continuously assessed. In yet another embodiment, the osteogenic fusion device includes at least one end piece with a truncated surface. The osteogenic fusion devices of the present invention may be combined with other fusion devices to form an implant system. The implant system includes at least one load bearing member having a truncated surface configured to nest within another load bearing member, preferably the load bearing, osteogenic fusion device of the present invention. The invention also provides implant systems comprising adjacent load bearing members connected to one another to resist lateral separation. Methods of promoting fusion bone growth in the space between adjacent vertebrae utilizing devices and systems of the invention are also described.
Owner:WARSAW ORTHOPEDIC INC
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39321E1Decreasing thrombogenicityLow antigenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant dressing, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, antiinflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant dressing.
Owner:AMERICAN NAT RED CROSS
Osteogenic paste compositions and uses thereof
InactiveUS7172629B2Rapid and premature resorptionDiminish and eliminate capacityImpression capsSurgical adhesivesMineral matrixImplantation Site
Described are osteogenic paste compositions with enhanced osteoinductive properties for use in bone repair. Compositions comprising a quickly resorbable paste carrier, a more slowly resorbed mineral matrix, and Bone Morphogenetic Protein (BMP) or other osteogenic factor are described which enable increased osteoinductive activity while retaining a reliable scaffold for the formation of new bone at the implant site. Methods for making and methods for therapeutic use of the compositions are also disclosed.
Owner:WARSAW ORTHOPEDIC INC
Methods for treating wound tissue and forming a supplemented fibrin matrix
InactiveUS7196054B1Low antigenicityDecreasing thrombogenicityOrganic active ingredientsSurgical adhesivesTissue sealantVascular dilatation
Owner:AMERICAN NAT RED CROSS
Deposition of calcium-phosphate (CaP) and calcium-phosphate with bone morphogenic protein (CaP+BMP) coatings on metallic and polymeric surfaces
InactiveUS20080097618A1Reduce the amount of solutionHigh densityImpression capsBone implantPolymeric surfaceSolubility
The invention is a medical implantable device which is coated by the method according to the invention. The surface of the substrate used for the implantable device, in the raw condition, following a cleaning regime and physiochemical pretreatments, is coated using a biomimetic process in a supersaturated calcium phosphate solution (SCPS) to obtain the desired coating coverage and morphology maintaining a ratio of calcium to phosphorus pH, as well as solution temperature plays a major role in yielding precipitation of the proper phase of CaP so that composition, morphologies, crystal structures, and solubility characteristics are optimal for the deposition process. The biomimetic coating adds the attribute of osteoconductivity to the implant device. To maximize bone growth, the implant must also induce bone growth, or possess the attribute of osteoinductivity. This attribute is acquired by the use of therapeutic agents, i.e. bone morphogenic proteins (BMP), growth factors, stem cells, etc. The preparation of the SCPS solution is slightly altered so that during the immersion of the implant in the SCPS, the therapeutic agents are co-precipitated and bonded with the CaP directly on the underlying surface of the implant device. A final dipping process into a BMP solution provides an initial burst of cellular activity. For delivering stem and / or progenitor cell, after drying the dipped solution of BMP, the cells are cultured on the surface of the implant.
Owner:HERKOWITZ HARRY N
Muscle-derived cells (MDCs) for treating muscle- or bone-related injury or dysfunction
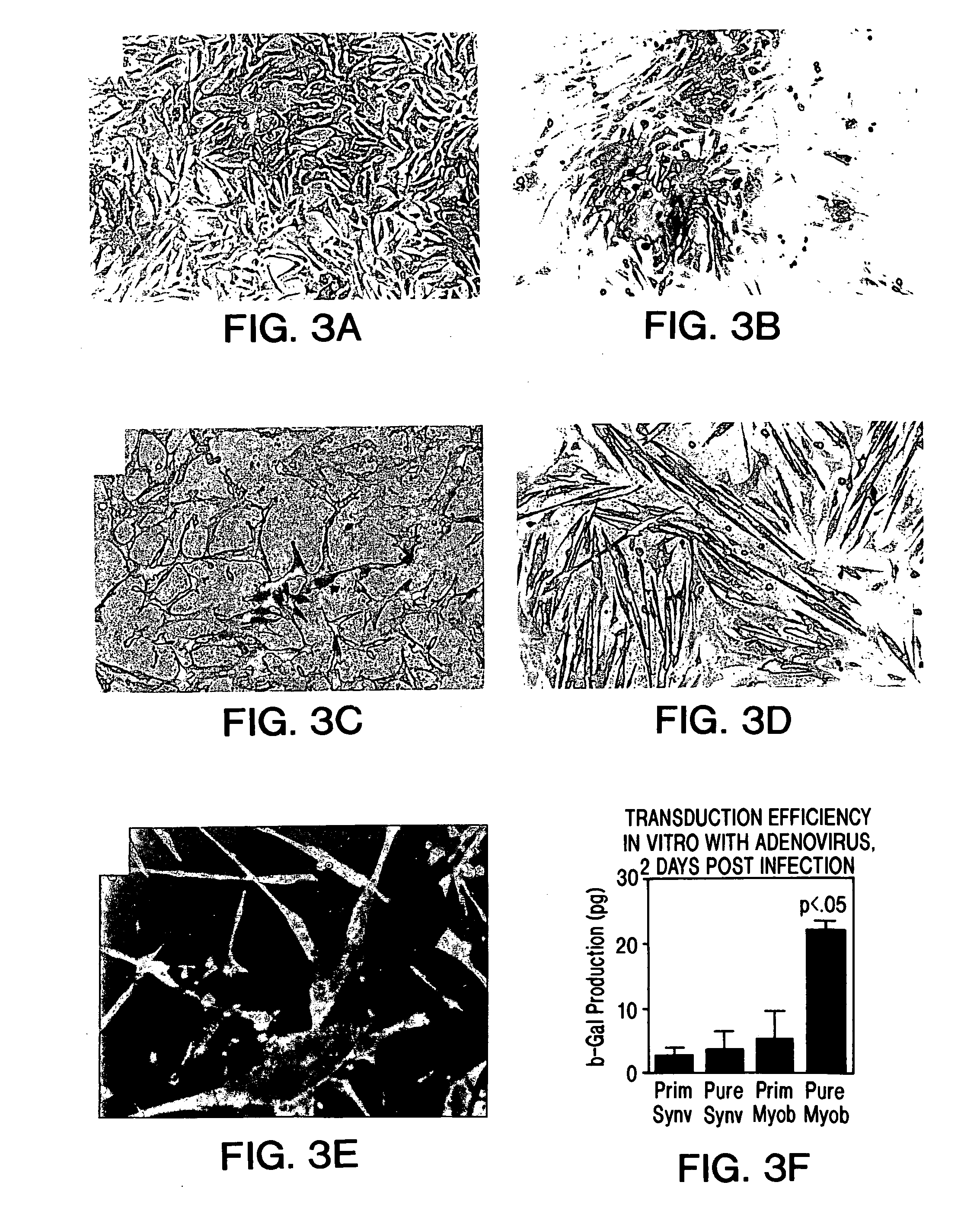
The present invention provides muscle-derived cells, preferably myoblasts and muscle-derived stem cells, genetically engineered to contain and express one or more heterologous genes or functional segments of such genes, for delivery of the encoded gene products at or near sites of musculoskeletal, bone, ligament, meniscus, cartilage or genitourinary disease, injury, defect, or dysfunction. Ex vivo myoblast mediated gene delivery of human inducible nitric oxide synthase, and the resulting production of nitric oxide at and around the site of injury, are particularly provided by the invention as a treatment for lower genitourinary tract dysfunctions. Ex vivo gene transfer for the musculoskeletal system includes genes encoding acidic fibroblast growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor, platelet derived growth factor, transforming growth factor-β, transforming growth factor-α, nerve growth factor and interleukin-1 receptor antagonist protein (IRAP), bone morphogenetic protein (BMPs), cartilage derived morphogenetic protein (CDMPs), vascular endothelial growth factor (VEGF), and sonic hedgehog proteins.
Owner:UNIVERSITY OF PITTSBURGH
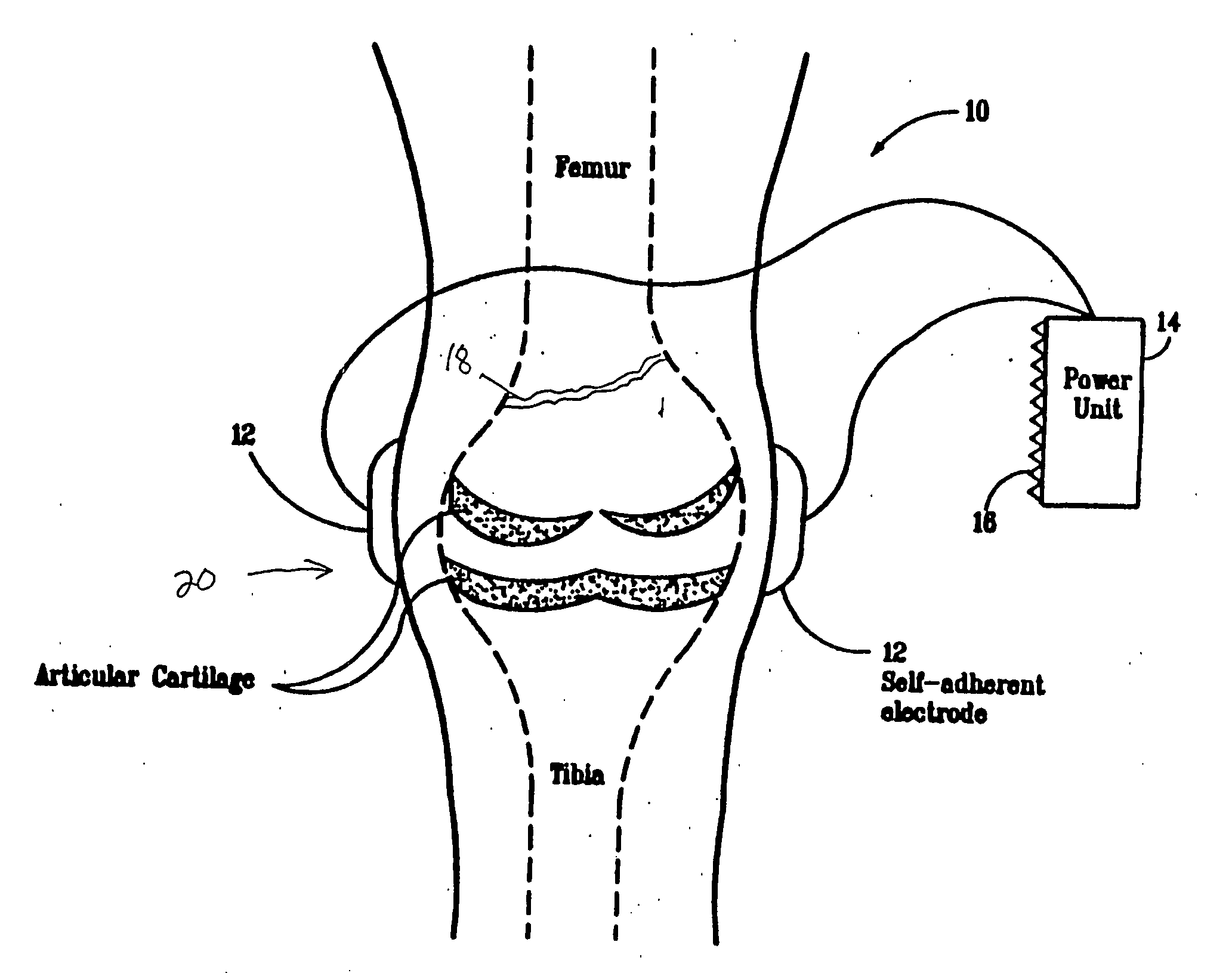
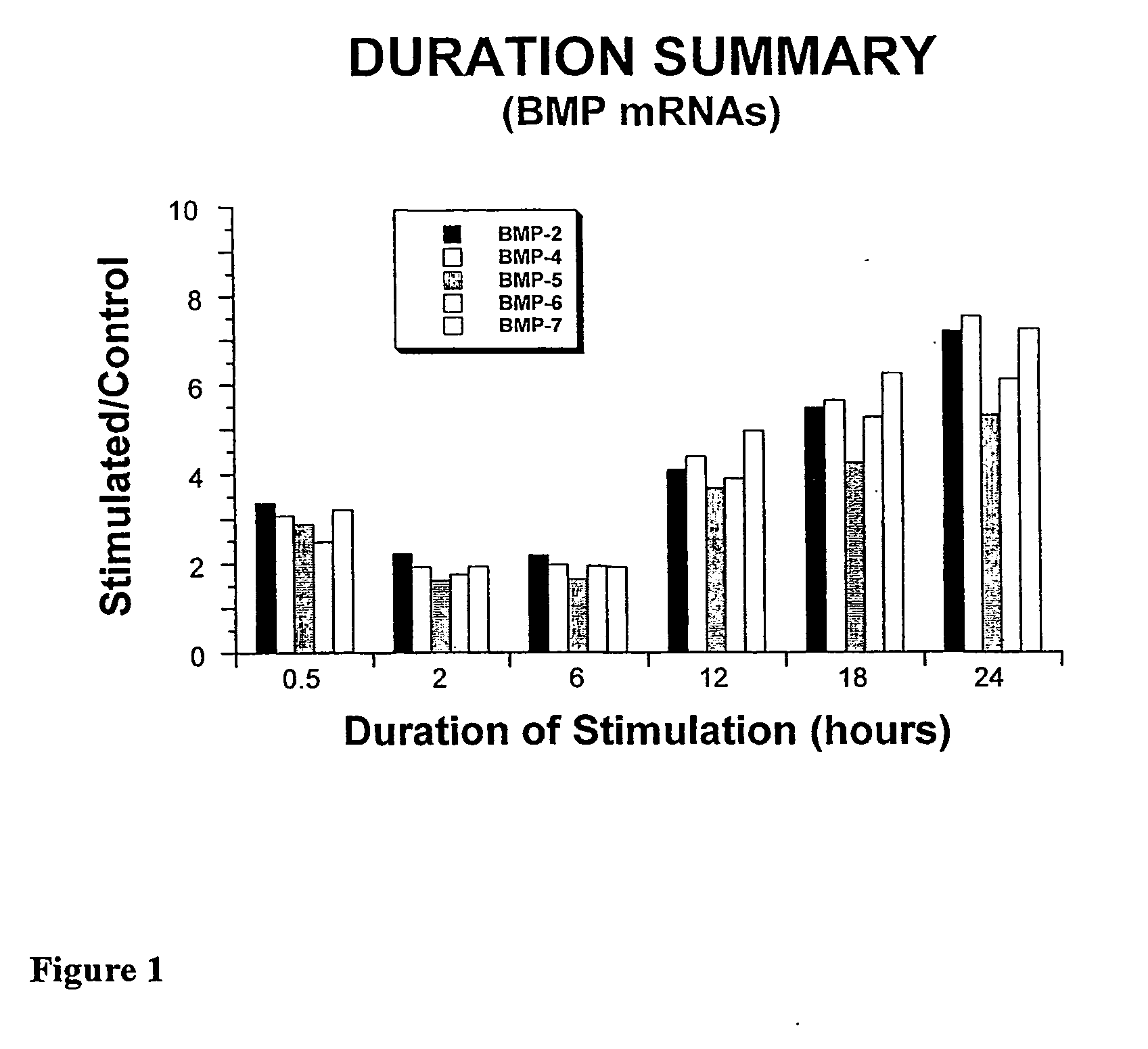
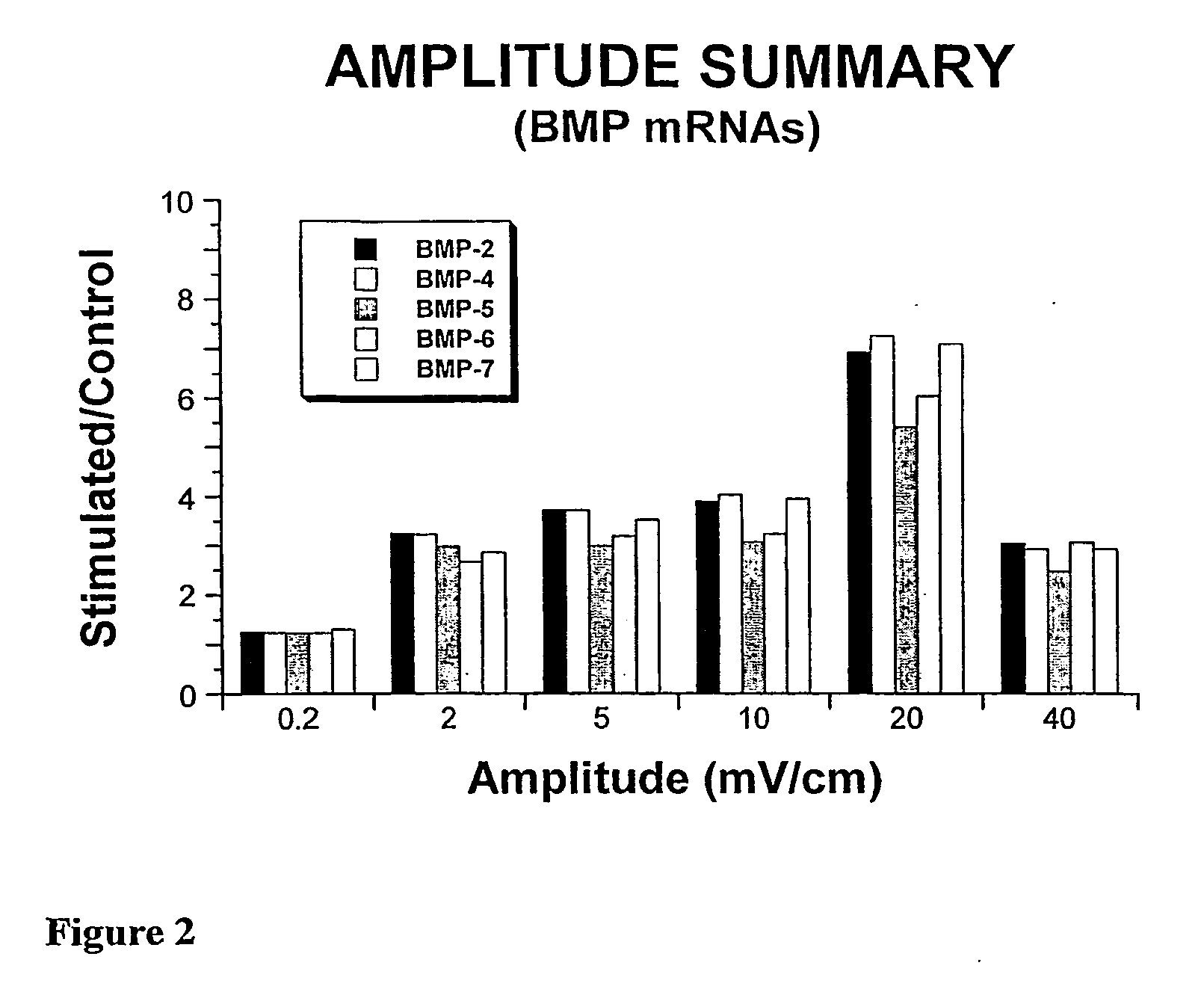
System and Method of Up-Regulating Bone Morphogenetic Proteins (Bmp) Gene Expression in Bone Cells Via the Application of Fields Generated by Specific and Selective Electric and Electromagnetic Signals
InactiveUS20070299472A1High expressionBiocideElectrotherapyHuman DNA sequencingBone Morphogenetic Protein Gene
Methods and devices are described for the regulation of bone morphogenetic protein gene expression in bone cells via the application of fields generated by specific and selective electric and electromagnetic signals in the treatment of diseased or injured bone. By gene expression is meant the up-regulation or down-regulation of the process whereby specific portions (genes) of the human genome (DNA) are transcribed into mRNA and subsequently translated into protein. Methods and devices are provided for the targeted treatment of injured or diseased bone tissue that include generating specific and selective electric and electromagnetic signals that generate fields optimized for increase of bone morphogenetic protein gene expression and exposing bone to the fields generated by specific and selective signals so as to regulate bone morphogenetic protein gene expression in such bone tissue. The resulting methods and devices are useful for the targeted treatment of bone fractures, fractures at risk, delayed unions, nonunion of fractures, bone defects, spine fusions, osteonecrosis or avascular necrosis, as an adjunct to other therapies in the treatment of one or all of the above, and in the treatment of osteoporosis.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods and devices for differentiating pluripotent stem cells into cells of the pancreatic lineage
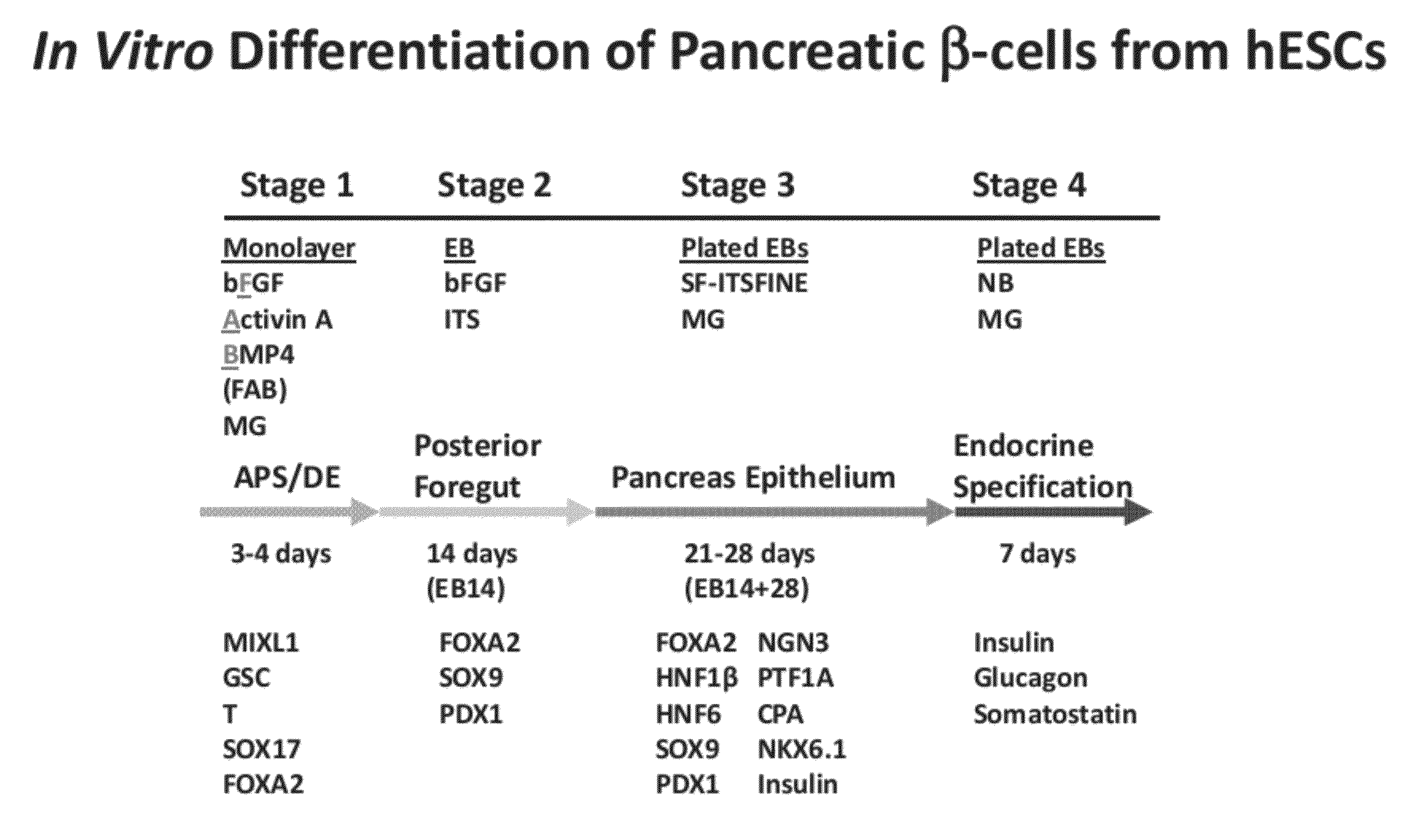
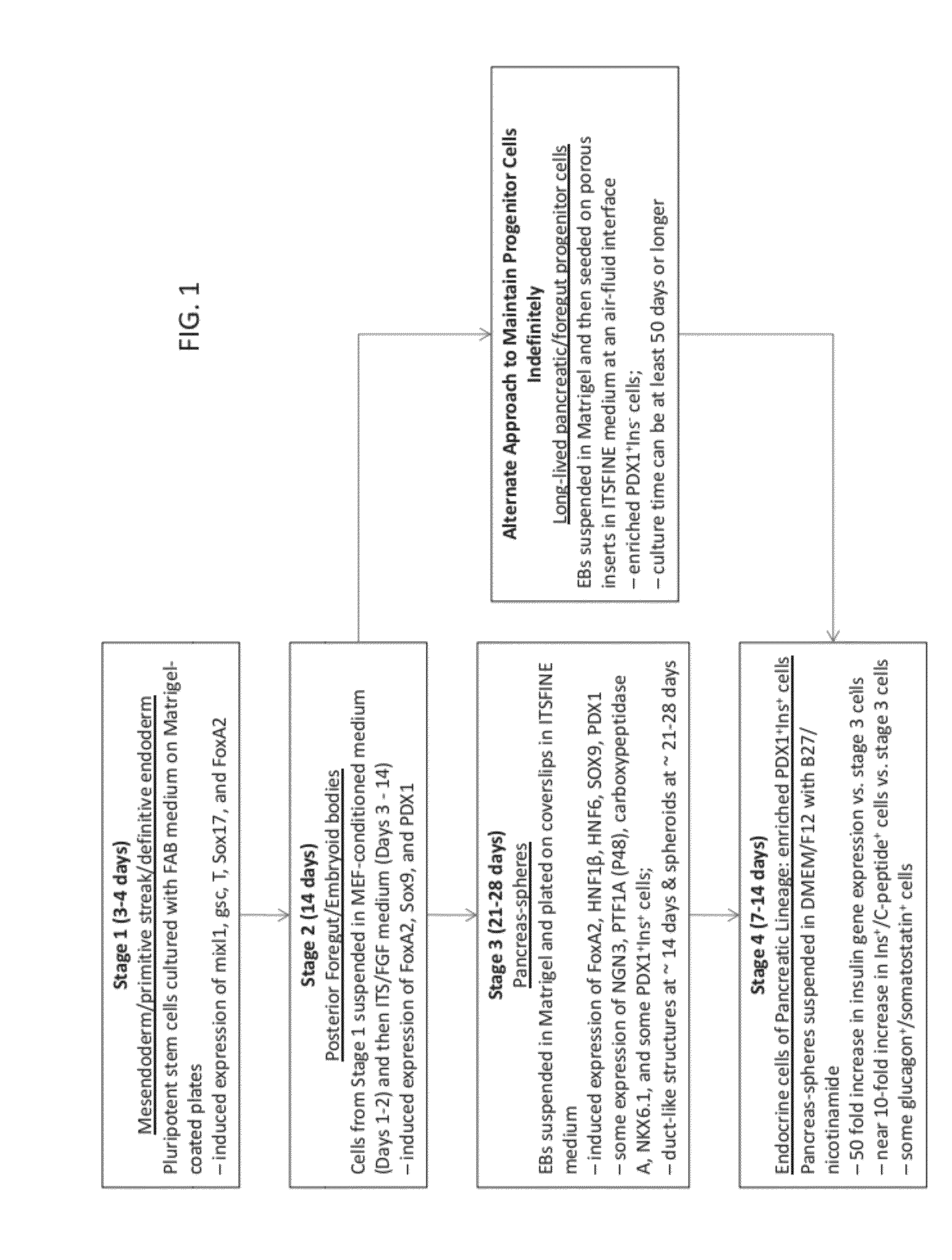
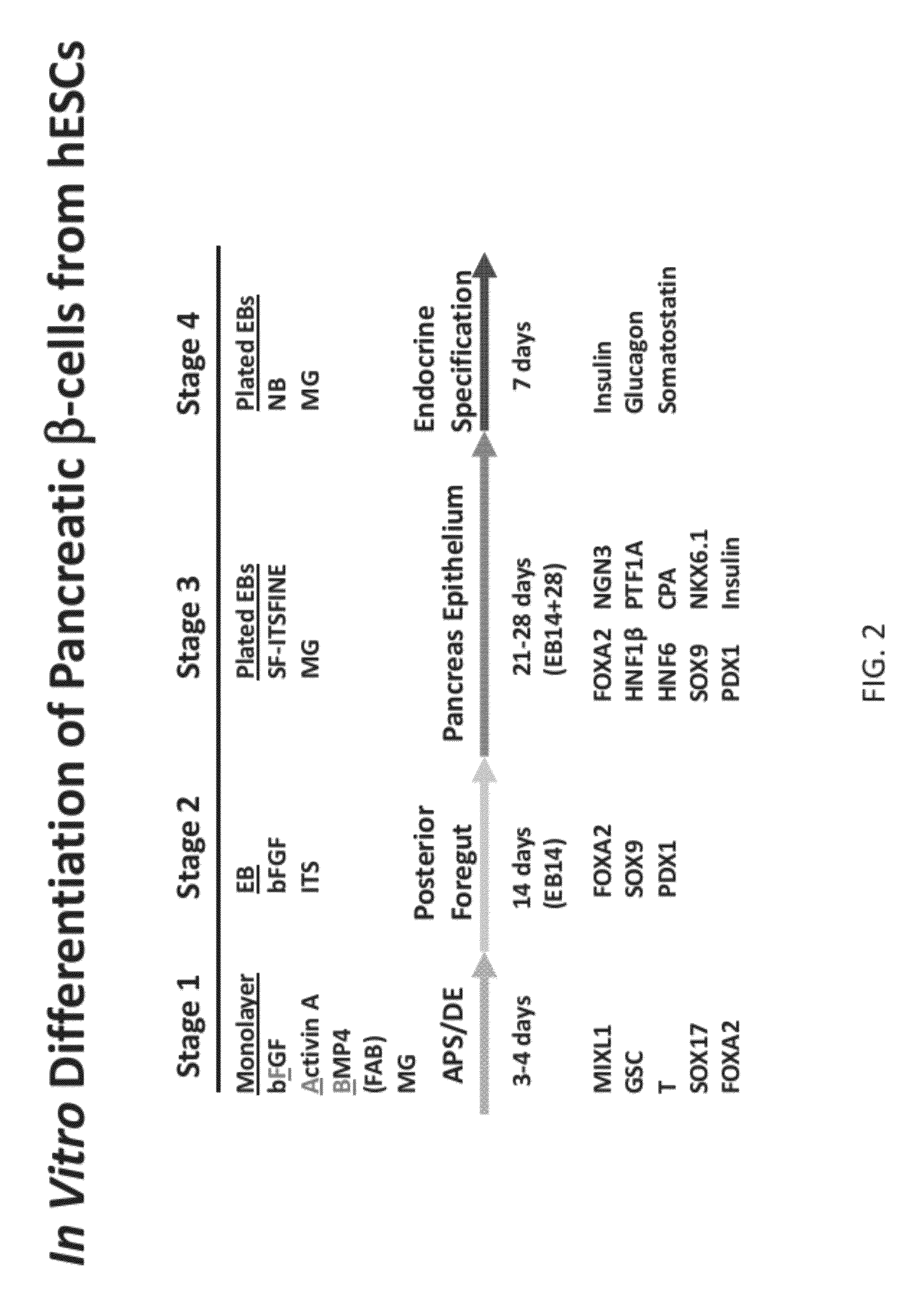
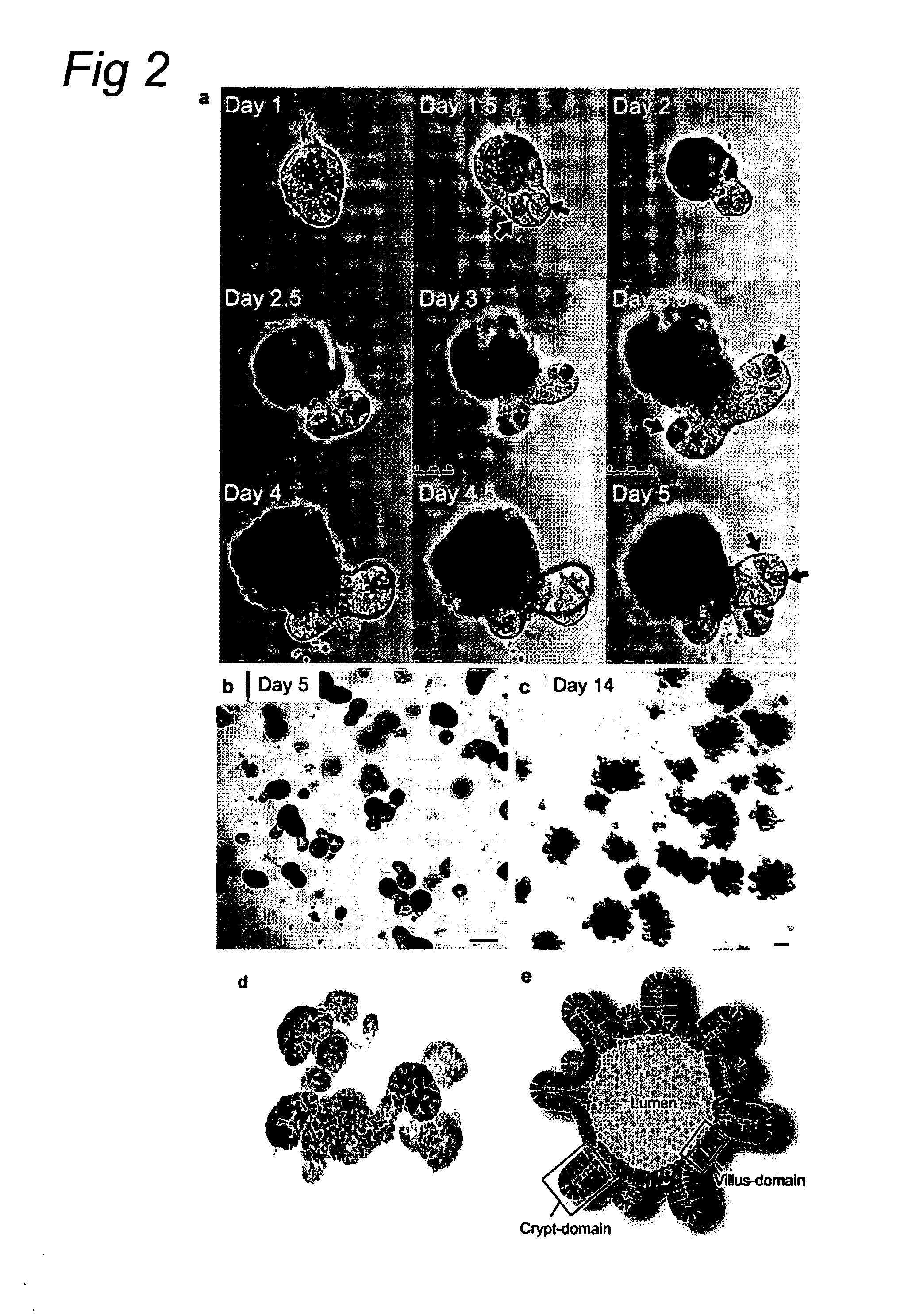
Methods and devices for culturing human pluripotent stem cells to produce cells of the pancreatic lineage are disclosed. The methods include steps of culturing the stem cells under conditions that induce the expression of mesendoderm / primitive streak and definitive endoderm markers in a chemically defined medium including an effective amount of i) fibroblast growth factor, ii) Activin A, and iii) bone morphogenetic protein. The methods further include the steps of culturing cells under conditions favoring the formation of at least one of intact embryoid bodies and pancreatic progenitor PDX1+ Ins− cells.
Owner:WISCONSIN ALUMNI RES FOUND
Methods and compositions for healing and repair of articular cartilage
InactiveUS7323445B2Improve stabilityIncrease ultimate strengthPeptide/protein ingredientsGenetic material ingredientsDiseaseMedicine
Methods and compositions are provided for the treatment of articular cartilage defects and disease involving the combination of tissue, such as osteochondral grafts, with active growth factor. The active growth factor is preferably a composition containing at least one bone morphogenetic protein and a suitable carrier. The method results in the regeneration and / or functional repair of articular cartilage tissue.
Owner:GENETICS INST INC
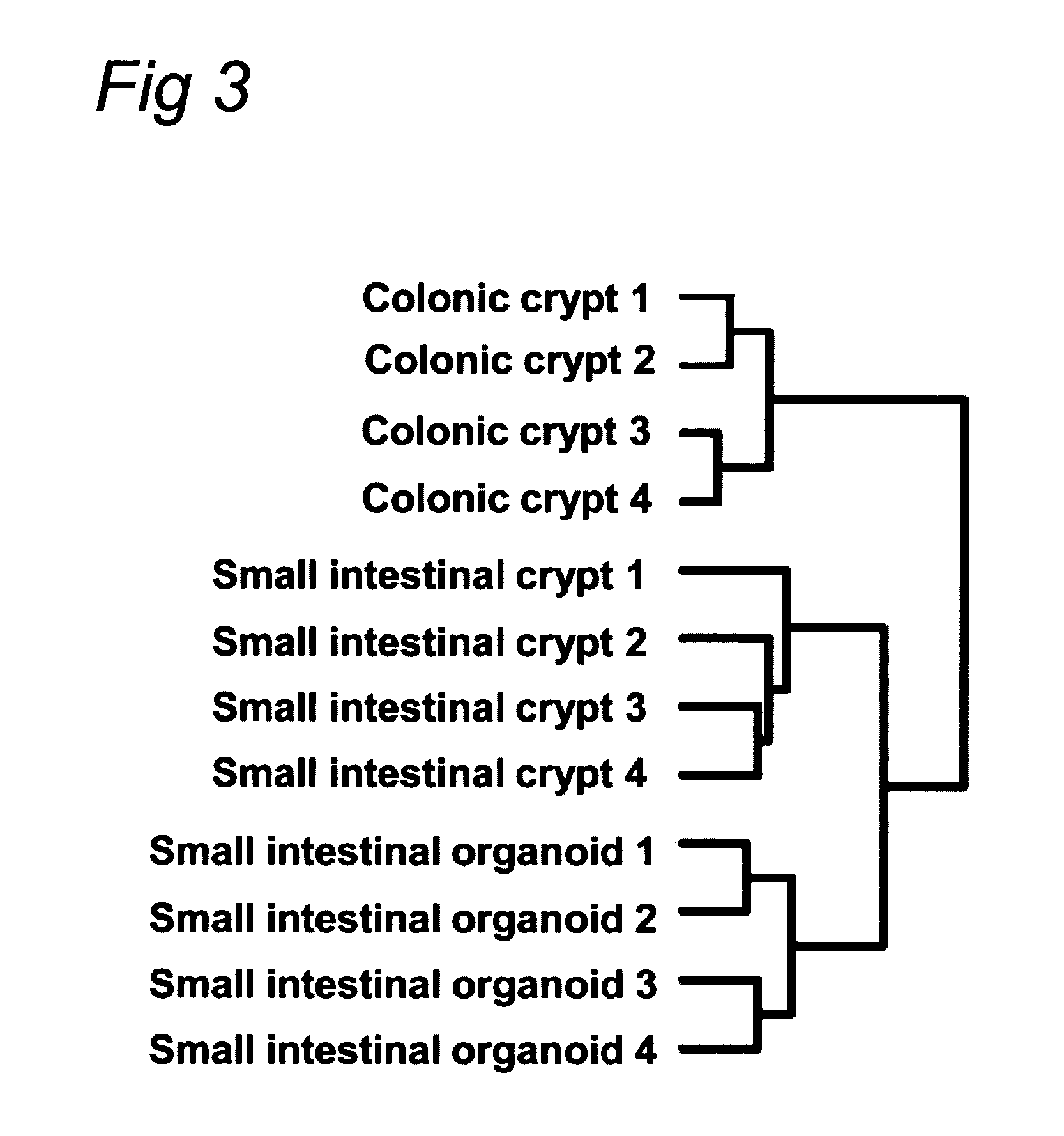
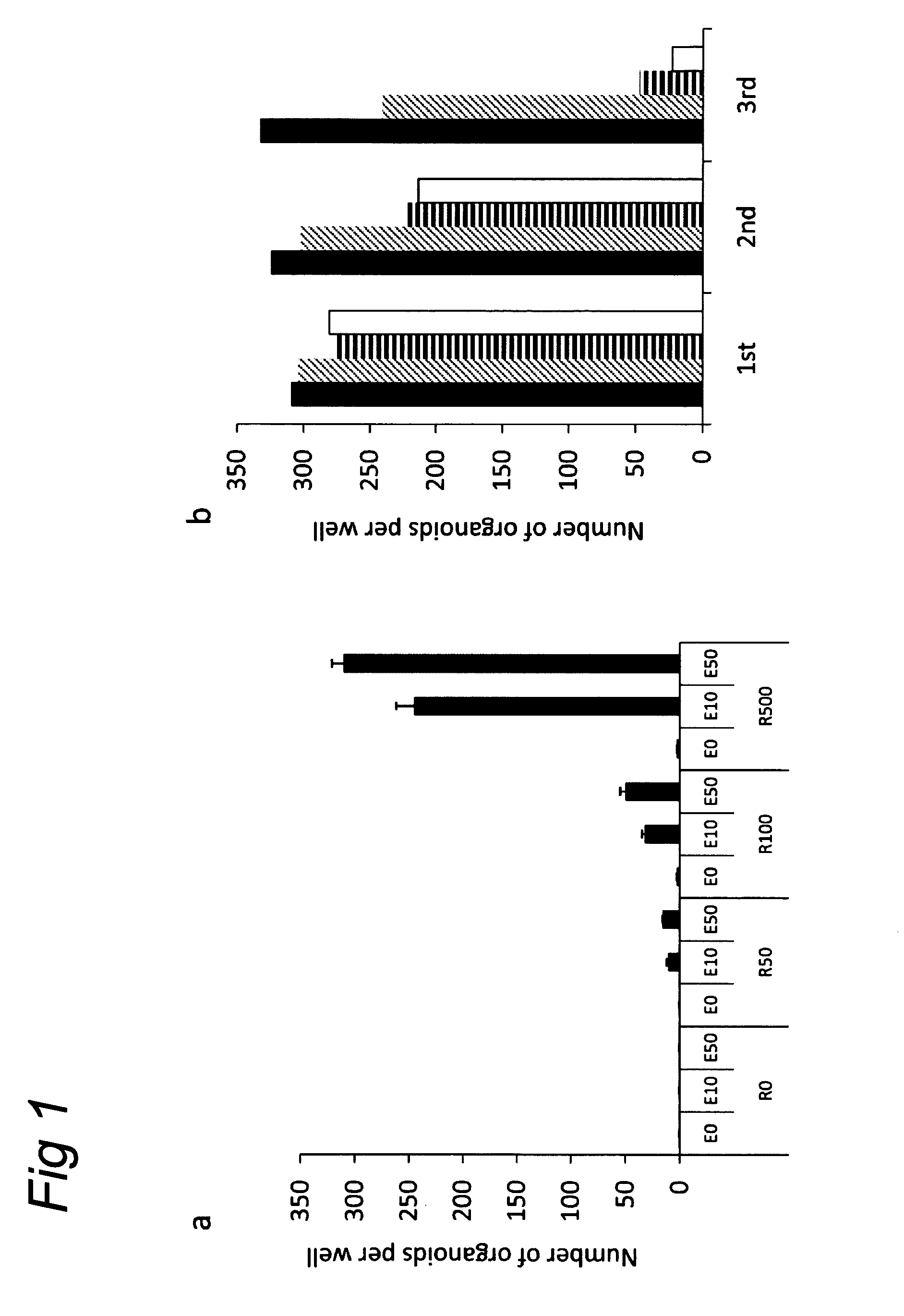
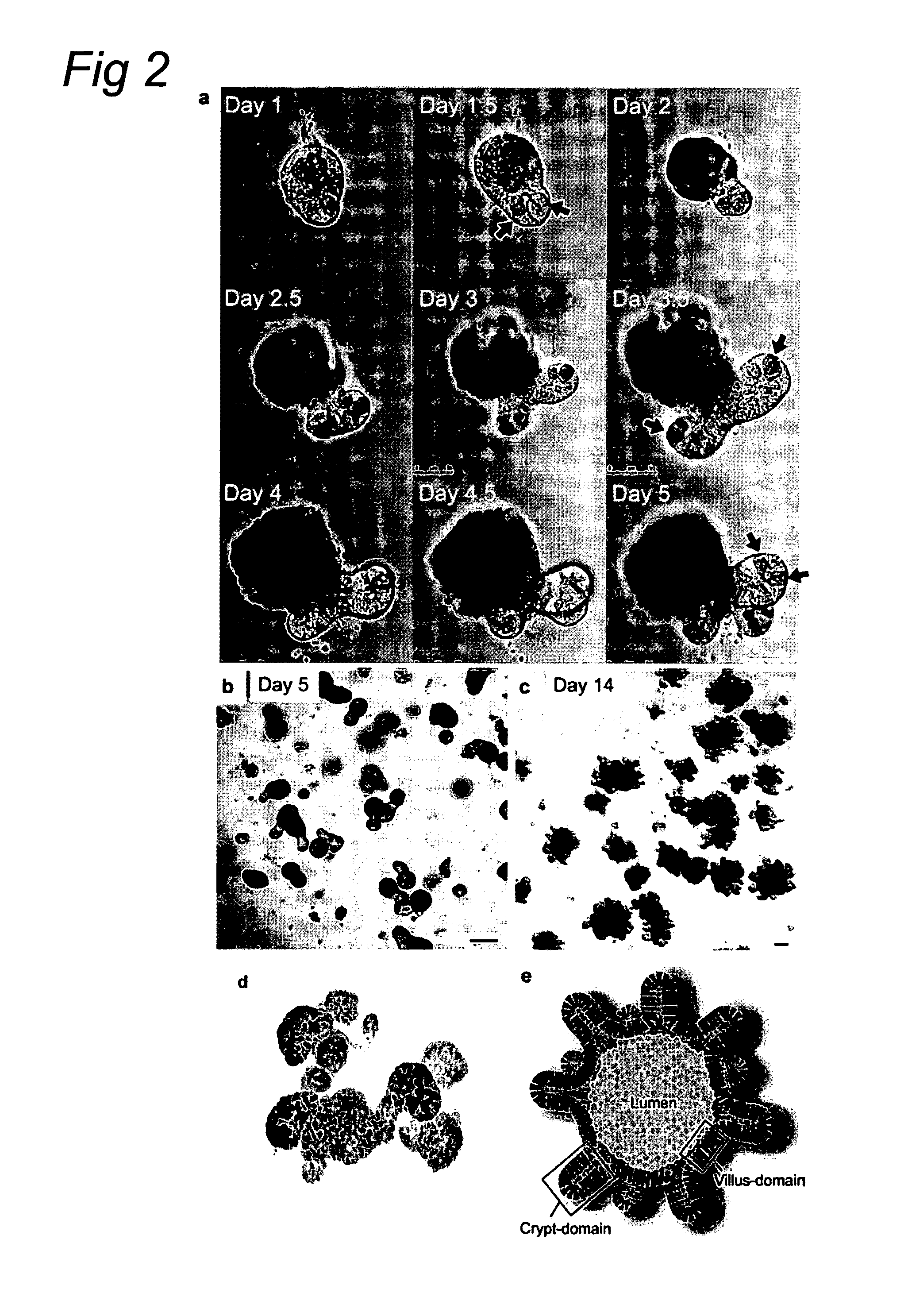
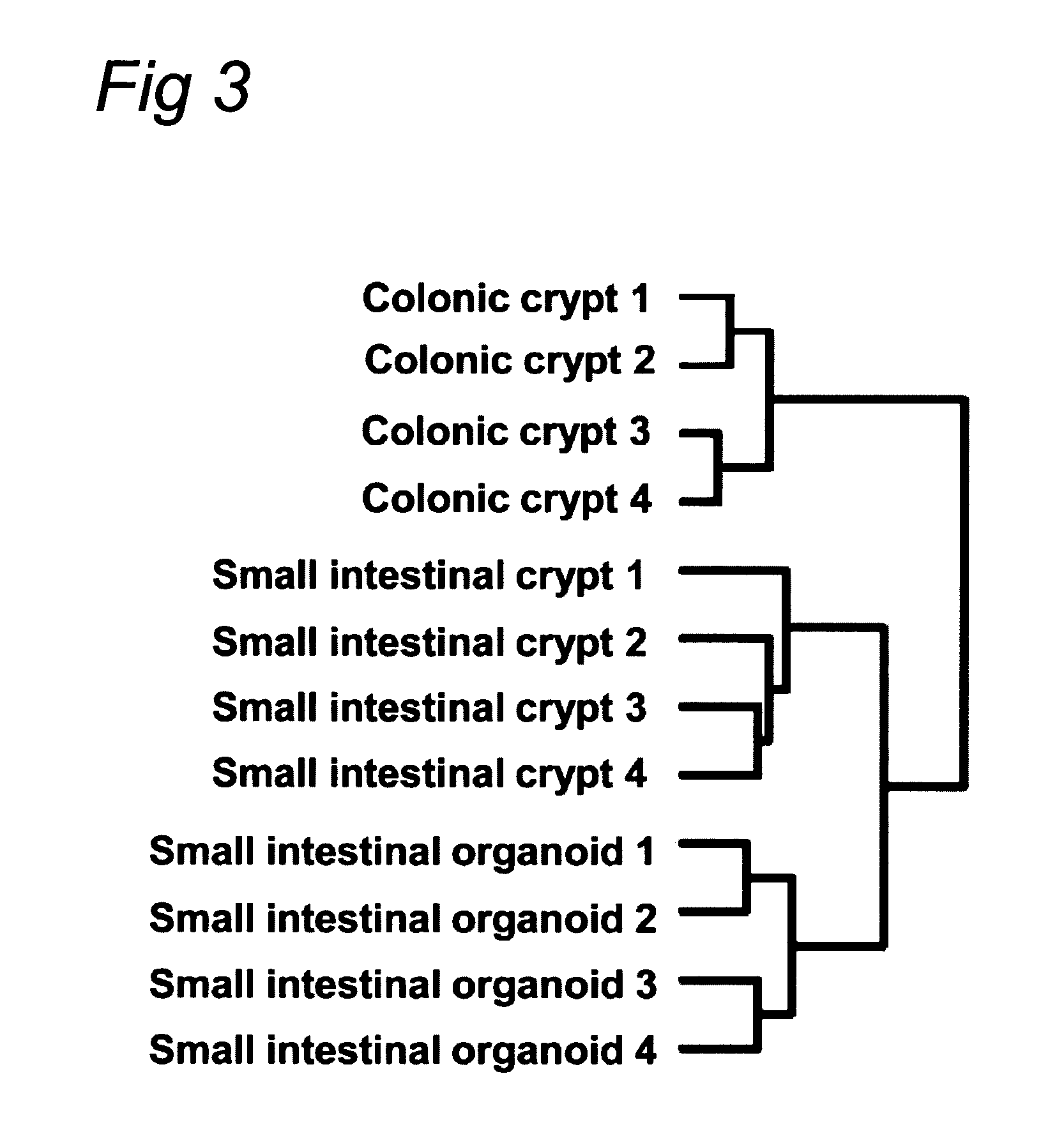
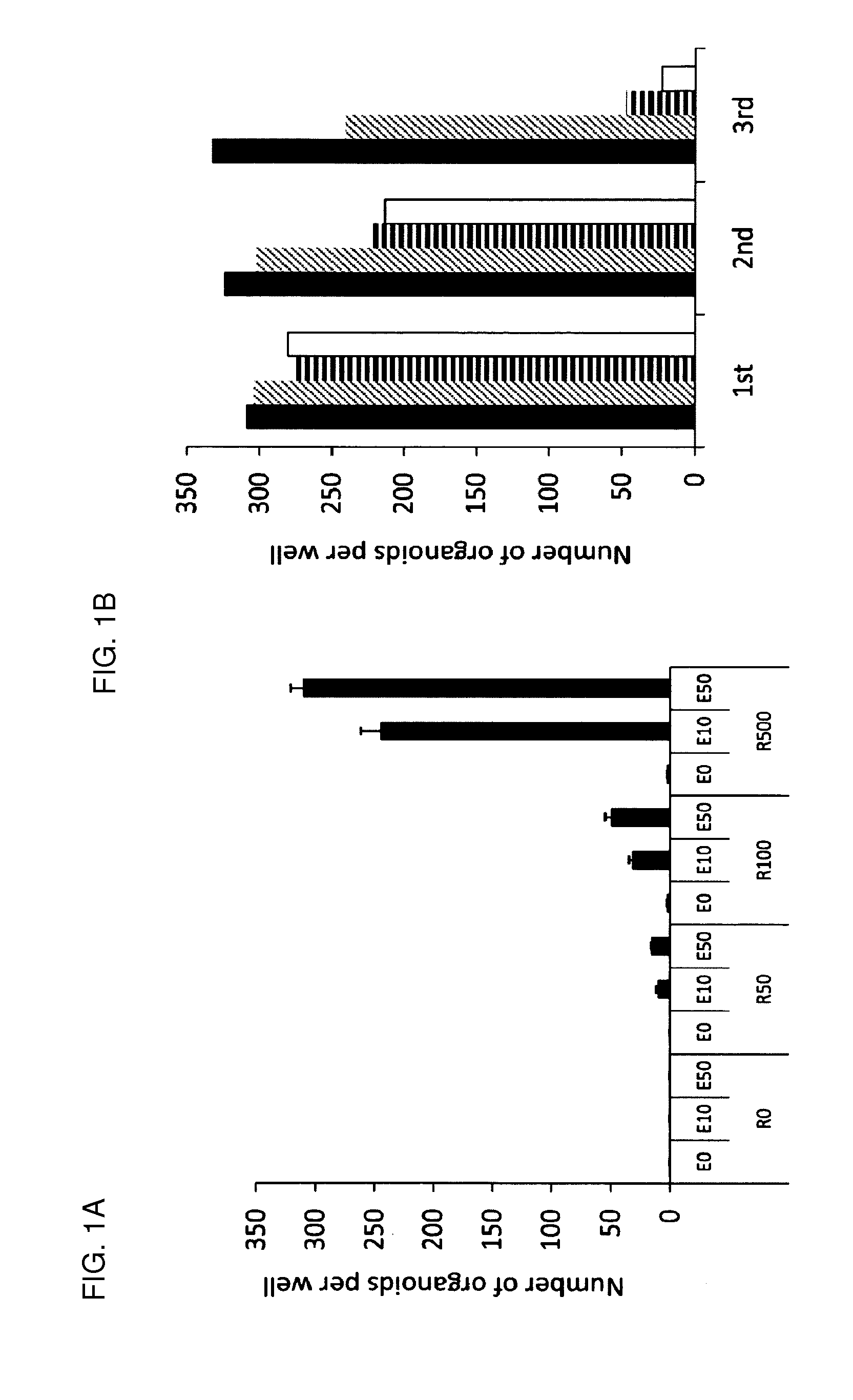
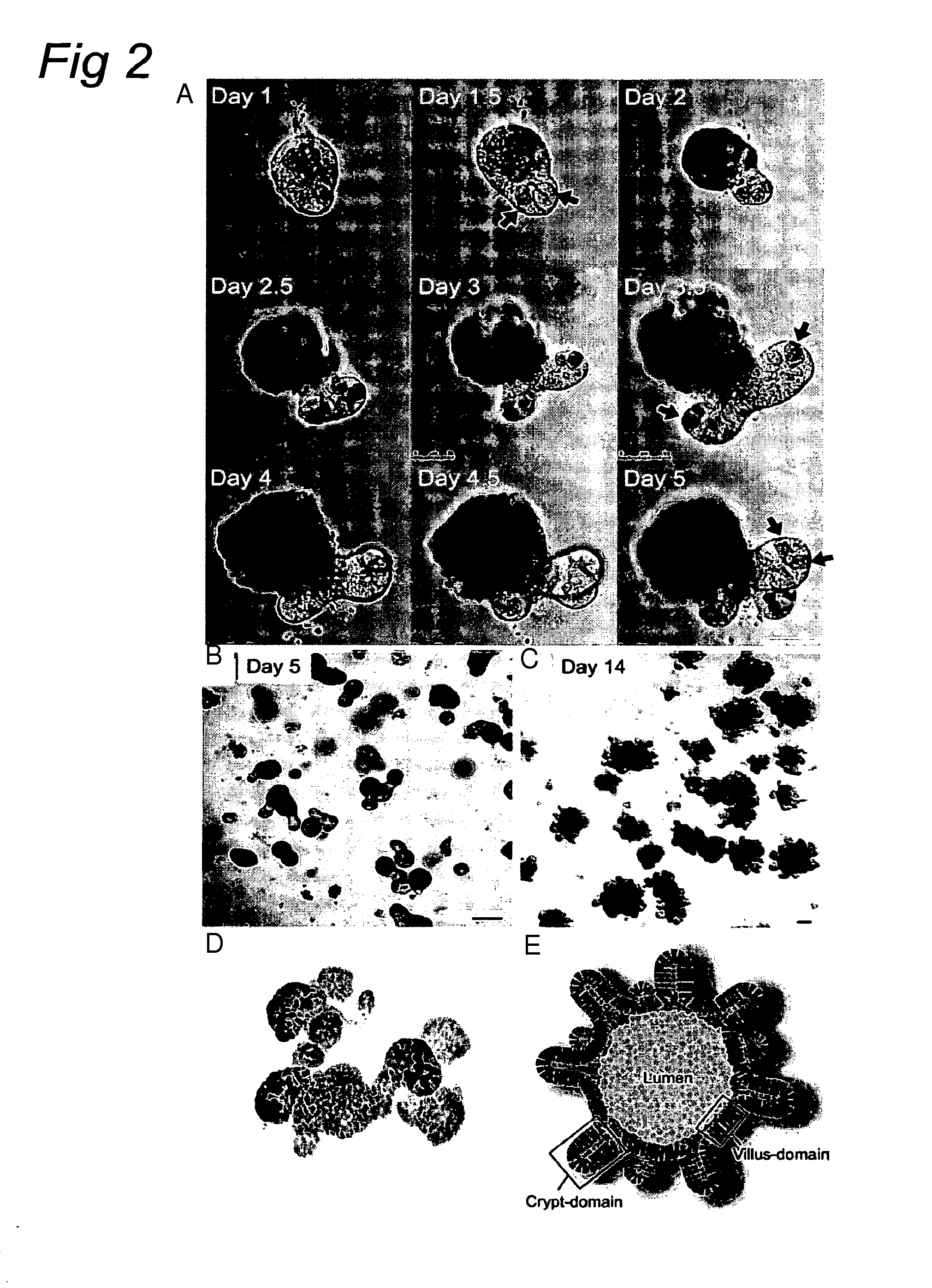
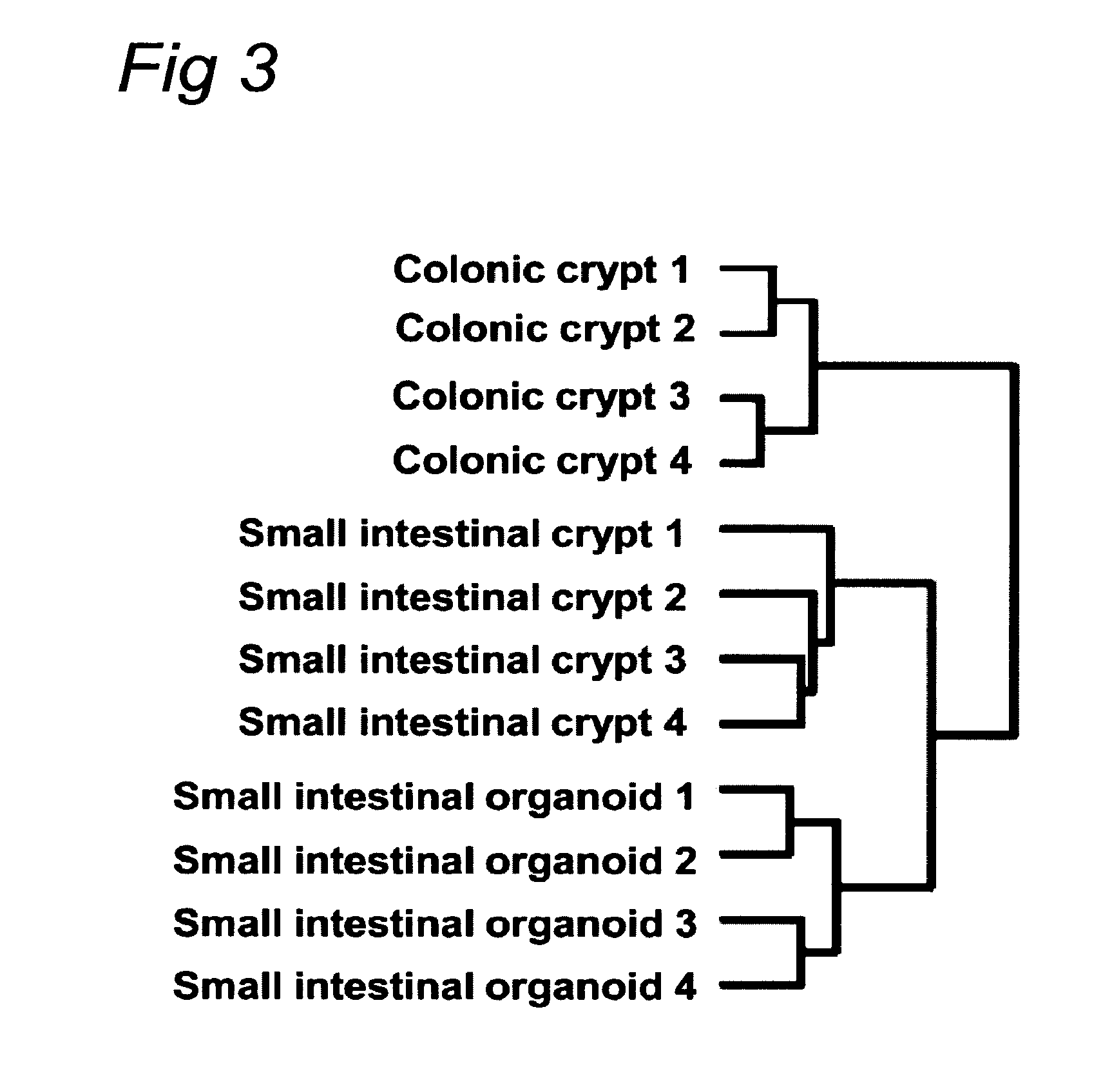
Culture medium for epithelial stem cells and organoids comprising said stem cells
ActiveUS20120028355A1Profound effectGastrointestinal cellsMetabolism disorderCell culture mediaAdenoma
The invention relates to a method for culturing epithelial stem cells, isolated tissue fragments comprising the epithelial stem cells, or adenoma cells, and culturing the cells or fragments in the presence of a Bone Morphogenetic Protein (BMP) inhibitor, a mitogenic growth factor, and a Wnt agonist when culturing epithelial stem cells and isolated tissue fragments. The invention further relates to a cell culture medium comprising a BMP inhibitor, a mitogenic growth factor, and a Wnt agonist, to the use of the culture medium, and to crypt-villus organoids, gastric organoids and pancreatic organoids that are formed in the culture medium.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
Degradable cage for bone fusion
InactiveUS20110282392A1Improve biological activityIncrease capacityInternal osteosythesisPeptide/protein ingredientsPolycaprolactoneBiomedical engineering
A cage for facilitating fusion of bones, such as vertebrae, or fusion of adjacent bone surfaces is disclosed. In one form, the cage includes a plurality of spaced apart walls comprising a biodegradable polymeric material (e.g., polycaprolactone); an osteoconductive mineral coating (e.g., a calcium compound) on at least a portion of the walls; and a bioactive agent (e.g., a bone morphogenetic protein) associated with the polymeric material and / or the coating. The bioactive agent is present in amount that induces ossification between the bones or adjacent bone surfaces. The cage may also include a fixation plate connected to at least one of the walls.
Owner:DEPUY SYNTHES PROD INC
Biodegradable osteogenic porous biomedical implant with impermeable membrane
InactiveUS20100161074A1Facilitate mechanical shaping and fillingFacilitate and enhance proliferationDental implantsSurgical adhesivesBone tissueBone growth
A biomedical implant is disclosed with osteogenic factors and a solid impermeable membrane occluding a portion of its surface for the generation of new bone growth at the target site of the implant. The implant is porous, bioresorbable, and forms a three dimensional architectural scaffold for the formation of new bone tissue. The implant is formed with a polymer or collagen, bone morphogenetic protein and ceramic particles.
Owner:WARSAW ORTHOPEDIC INC
Bone Morphogenetic Protein Compositions
InactiveUS20100015230A1Improve stabilityEfficient preparationPowder deliverySenses disorderDiseaseSkeletal tissues
The present invention relates to compositions of bone morphogenetic proteins, particularly solid and liquid formulations of such proteins comprising one or more stabilizing excipients. The invention further provides methods of producing the compositions, kits comprising the compositions and methods of using the compositions in the treatment of diseases of skeletal and non-skeletal tissues.
Owner:STRYKER CORP
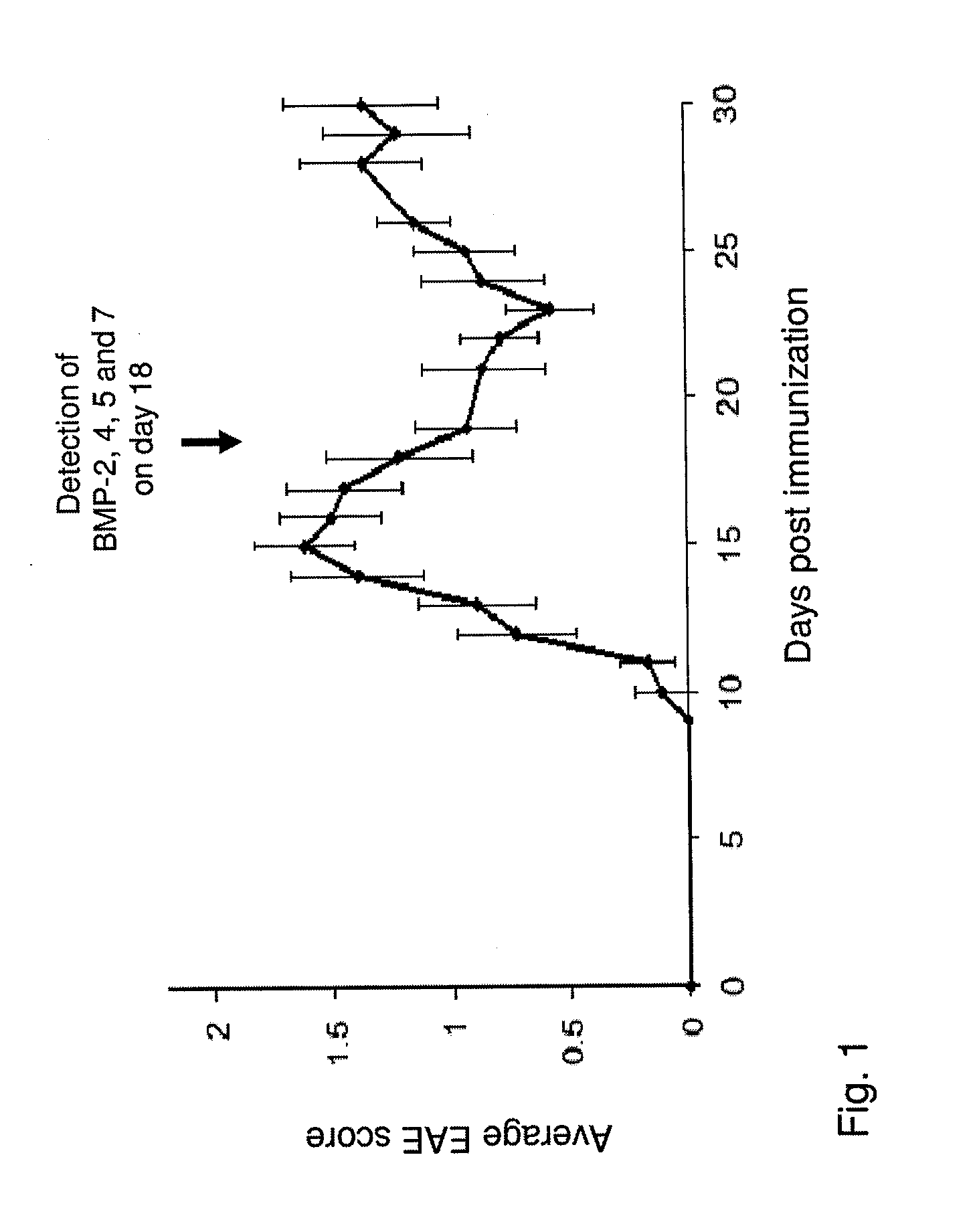
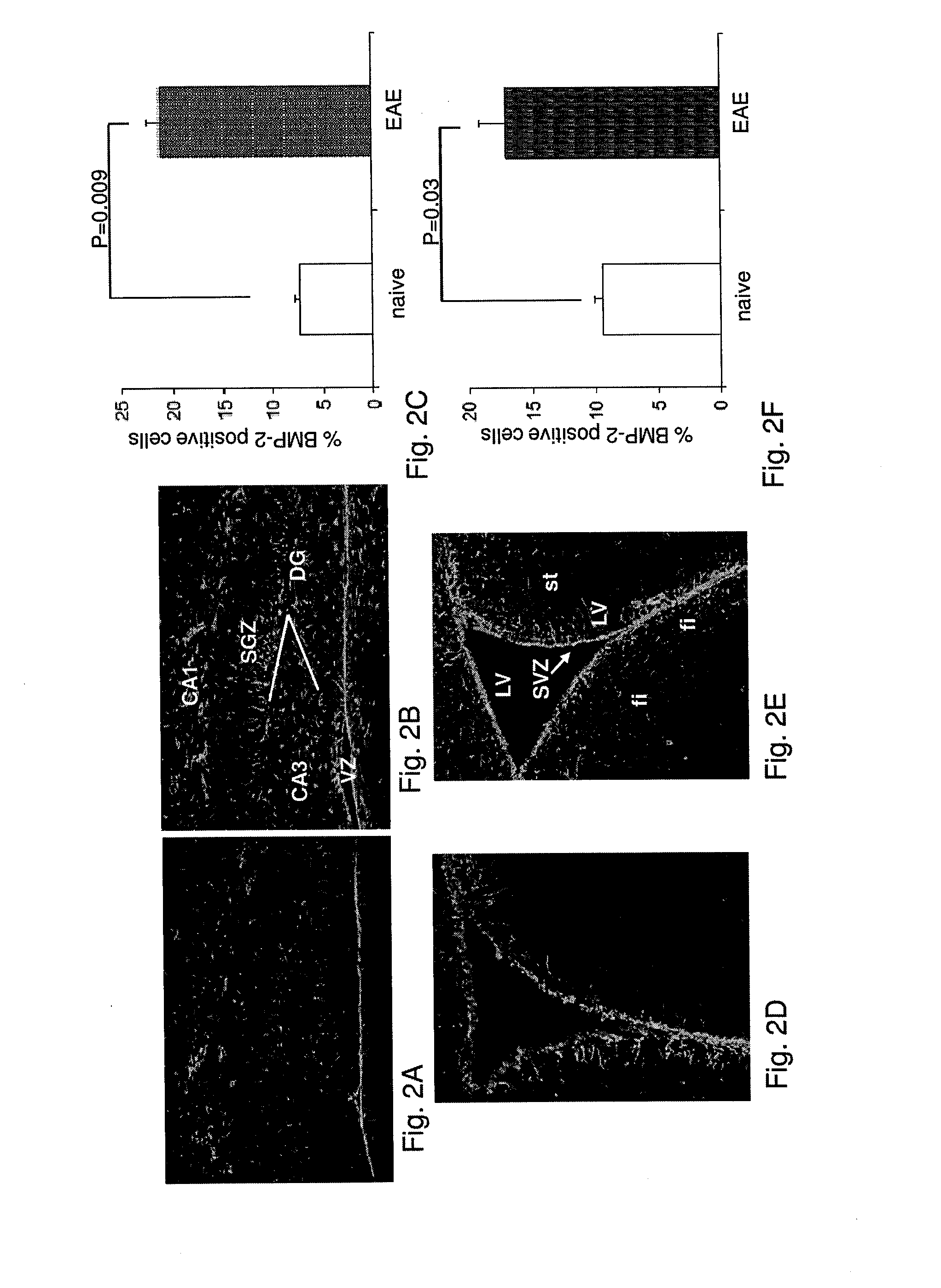
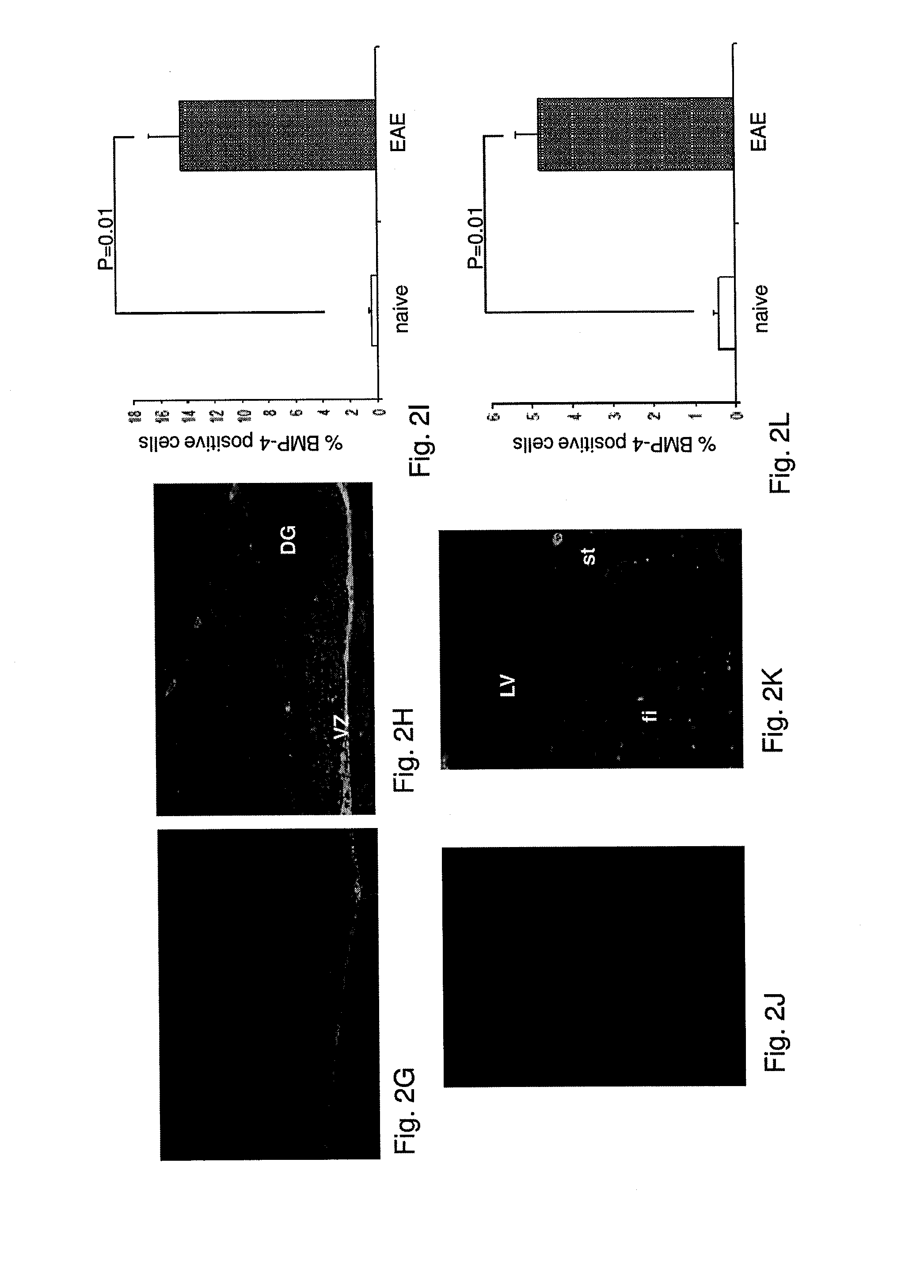
Use of blocking agents of bone morphogenic protein (BMP) signalling for the treatment of neuroinflammatory and neurodegenerative diseases
The invention provides pharmaceutical compositions for the treatment of neuroinflammatory or neurodegenerative diseases comprising a single or a combination of several blocking agent(s) of Bone Morphogenic Protein (BMP) signaling. The invention further provides methods of treatment of neuroinflammatory or neurodegenerative diseases comprising administering to a patient in need thereof the pharmaceutical compositions of the invention.
Owner:THE MEDICAL RES INFRASTRUCTURE & HEALTH SERVICES FUND OF THE TEL AVIV MEDICAL CENT
Culture medium for epithelial stem cells and organoids comprising the stem cells
The invention relates to a method for culturing epithelial stem cells, isolated tissue fragments comprising the epithelial stem cells, or adenoma cells, and culturing the cells or fragments in the presence of a Bone Morphogenetic Protein (BMP) inhibitor, a mitogenic growth factor, and a Wnt agonist when culturing epithelial stem cells and isolated tissue fragments. The invention further relates to a cell culture medium comprising a BMP inhibitor, a mitogenic growth factor, and a Wnt agonist, to the use of the culture medium, and to crypt-villus organoids, gastric organoids and pancreatic organoids that are formed in the culture medium.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
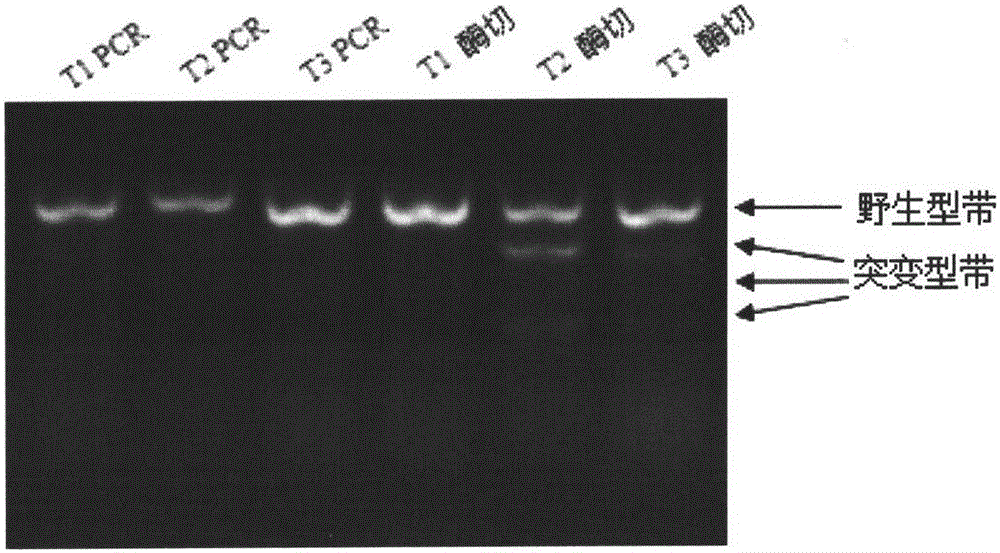
Cell model obtained after targeted knockout of rabbit bone morphogenetic protein-2 (BMP2) gene based on CRISPR/Cas9 and application thereof
InactiveCN106047803AEasy to prepareLow costBone-inducing factorOsteogenic factorMorphogenesisMesenchymal stem cell
The invention relates to a cell model obtained after targeted knockout of a rabbit BMP2 gene based on CRISPR / Cas9 and application thereof, belonging to the technical field of molecular biology and biomedicine. According to the invention corresponding oligos are synthesized for three targeting sites of the rabbit BMP2 gene on the design principles of CRISPR / Cas9 and are constructed on px458 vectors; and a CRISPR / Cas9 system is constructed directed at the three targeting sites in rabbit mesenchymal stem cells, and the CRISPR / Cas9 system can effectively knock out the rabbit BMP2 gene, is easy to operate and has high rabbit BMP2 gene knockout efficiency. The cell model disclosed in the invention can greatly promote research related to the functions and signaling pathways of the BMP2 gene.
Owner:QINGDAO JIAOZHOU CENT HOSPITAL
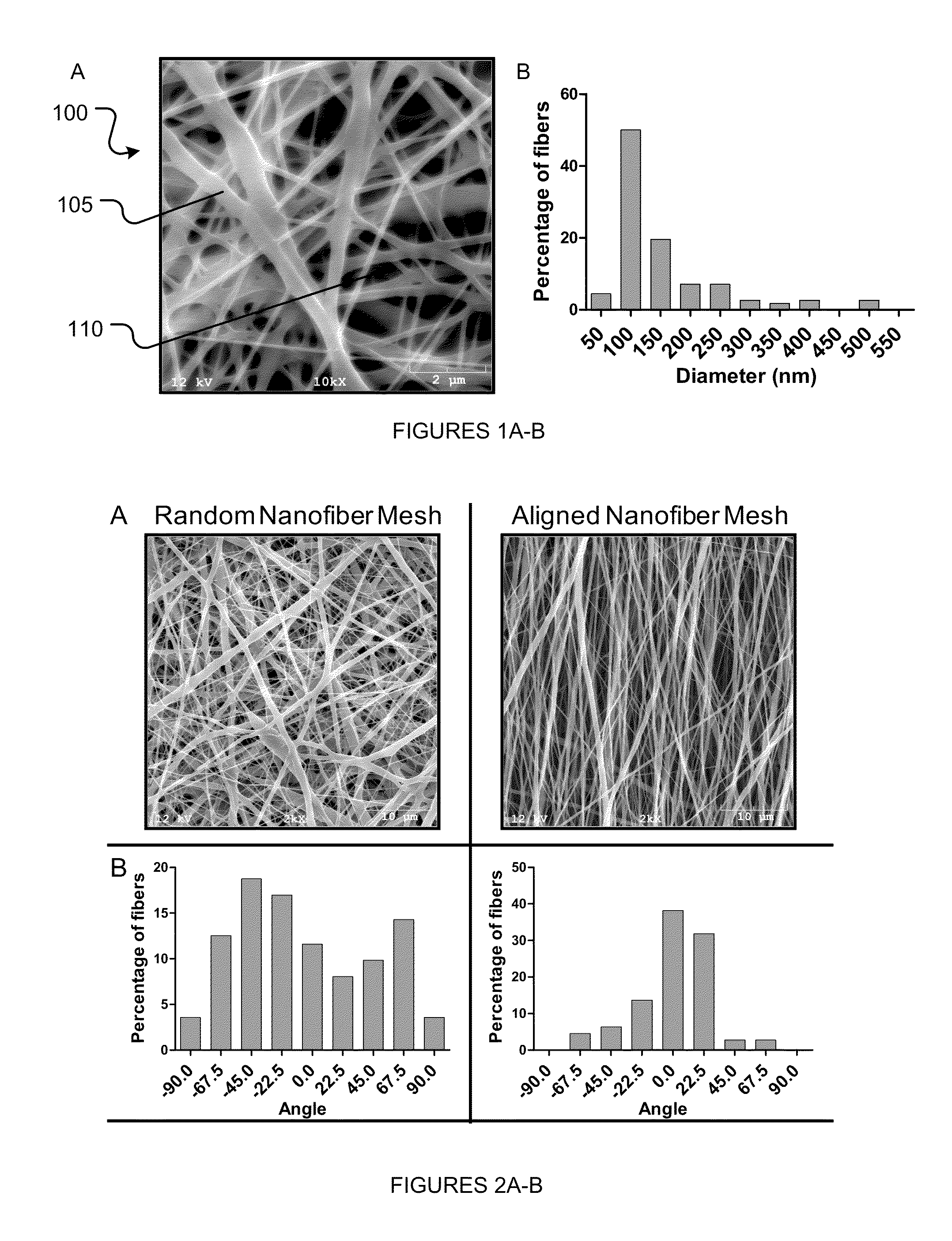
Systems and methods to affect anatomical structures
ActiveUS20100168771A1Pharmaceutical delivery mechanismTissue regenerationAnatomical structuresActive agent
The various embodiments of the present invention are generally directed to systems and methods to affect anatomical structures. More particularly, the various embodiments of the present invention are directed to systems and methods to regenerate bone. An aspect of the present invention comprises a system for affecting an anatomical structure, comprising: a nanofiber mesh configured to substantially conform to an anatomical structure, wherein at least a portion of the nanofiber mesh defines a fillable space; a carrier substance comprising an active agent, such as a bone morphogenetic protein, wherein the carrier substance is disposed within the fillable space.
Owner:GEORGIA TECH RES CORP +1
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39192E1Increased longevityImprove stabilityAntibacterial agentsPowder deliveryTissue sealantVascular dilatation
This invention provides supplemented tissue sealants, methods for their production and use thereof. Disclosed are tissue sealants supplemented with at least one cytotoxin or cell proliferation inhibiting composition. The composition may be further supplemented with, for example, one or more antibodies, analgesics, anticoagulants, anti-inflammatory compounds, antimicrobial compositions, cytokines, drugs, growth factors, interferons, hormones, lipids, deminearlized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like.
Owner:AMERICAN NAT RED CROSS
Compositions and methods for treating joints
Methods and compositions are disclosed for an intra-articular injection for the treatment of osteoarthritis. The methods and compositions comprising combinations of hyaluronic acid and a bone morphogenetic protein, like rhGDF-5, can be useful for any synovial joint, including the knee, shoulder, hip, ankle, hands, spinal facet, or temporomandibular joint, both for the relief of pain and for slowing disease progression.
Owner:DEPUY SYNTHES PROD INC
Manufacturing process, such as three dimensional printing, including binding of water-soluble material followed by softening and flowing and forming films of organic-solvent-soluble material
InactiveUS20070009606A1Effectively osteoinductiveHigh porosityAdditive manufacturing apparatusPhotosensitive materialsManufacturing technologyAdditive ingredient
The invention includes biostructures which may be characterized as having substantially all of the organic-solvent-soluble material in the form of a network of irregularly shaped perforated films. The biostructure may further include particles of a substantially-insoluble material, which may be a member of the calcium phosphate family. The biostructure may be osteoconductive. The biostructure may further contain an Active Pharmaceutical Ingredient or other bioactive substance. The API may be a substance which stimulates the production of bone morphogenetic protein, such as Lovastatin or related substances, thereby making the biostructure effectively osteoinductive. One or more of the polymers may have a resorption rate in the human body such as to control the release of the API. Methods of manufacture are also disclosed.
Owner:MASSACHUSETTS INST OF TECH +1
Degradable wound repair material and preparation method thereof
InactiveCN103830768AGood biocompatibilityGood for degradation and absorptionAbsorbent padsBandagesWhite blood cellPancreatic hormone
The invention relates to a degradable wound repair material and a preparation method thereof. The degradable wound repair material includes a matrix component and an auxiliary component, wherein the matrix component includes a protein ingredient, and the auxiliary component includes at least one of an antibacterial agent and an active factor. Specifically, the antibacterial agent is a synthetic antibacterial drug, an inorganic antibacterial agent, an organic antibacterial agent, or a natural antibacterial agent, and the active factor is at least one of the following active factors: an epidermal growth factor, an FGF vascular endothelial growth factor, a platelet-derived growth factor, a platelet activating factor, an insulin-like growth factor, a tumor necrosis factor, interleukin, colony stimulating factor-1, various bone morphogenetic proteins or transforming growth factors. By loading the antibacterial agent and active factor component on the basis of the matrix component, the degradable wound repair material provided by the invention can have biological activity on the basis of meeting degradability, can better promote wound repair, and has good anti-infection properties during use.
Owner:SHENZHEN LANDO BIOMATERIALS
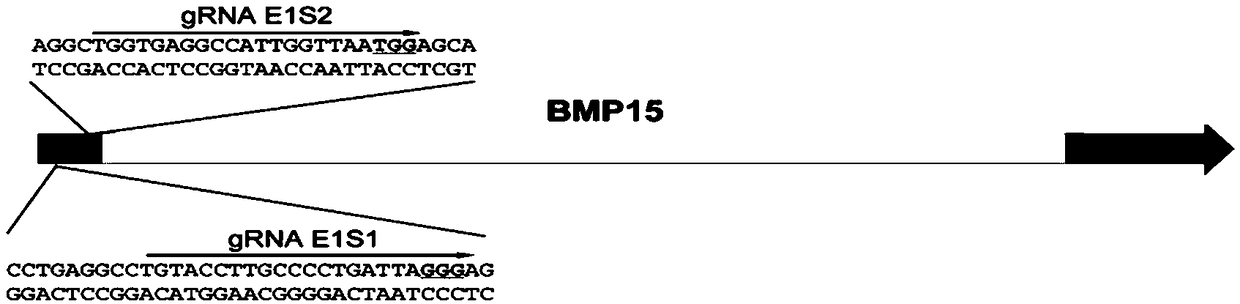
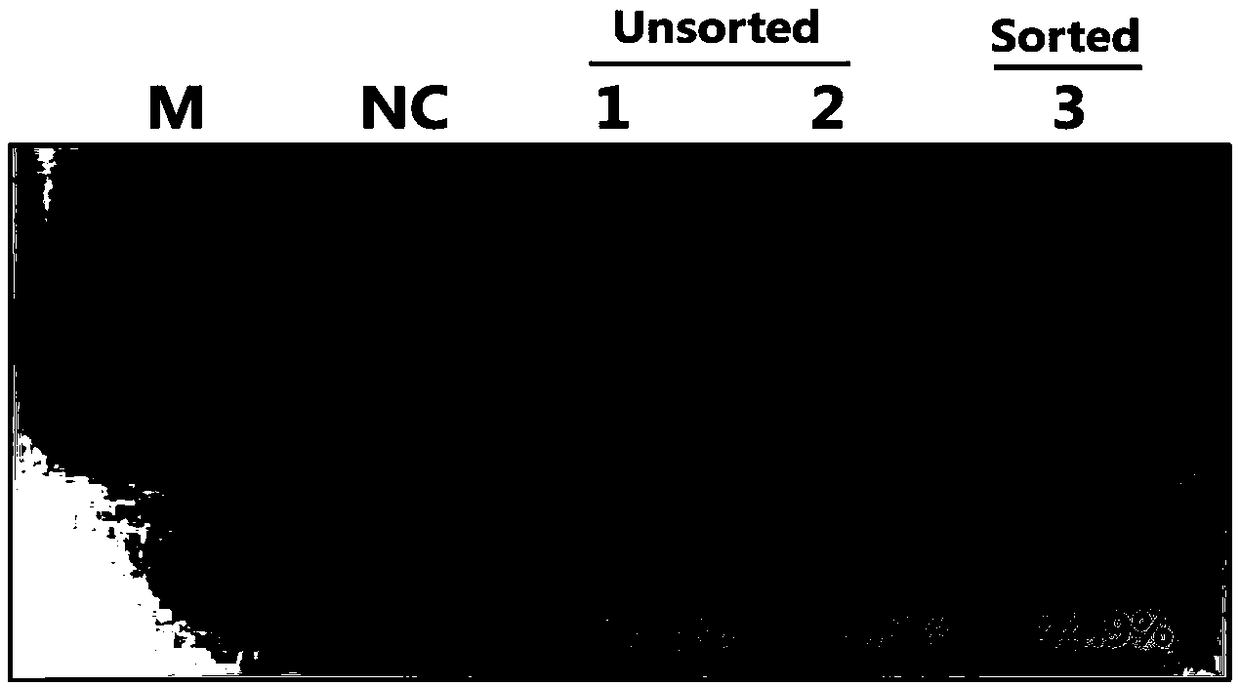
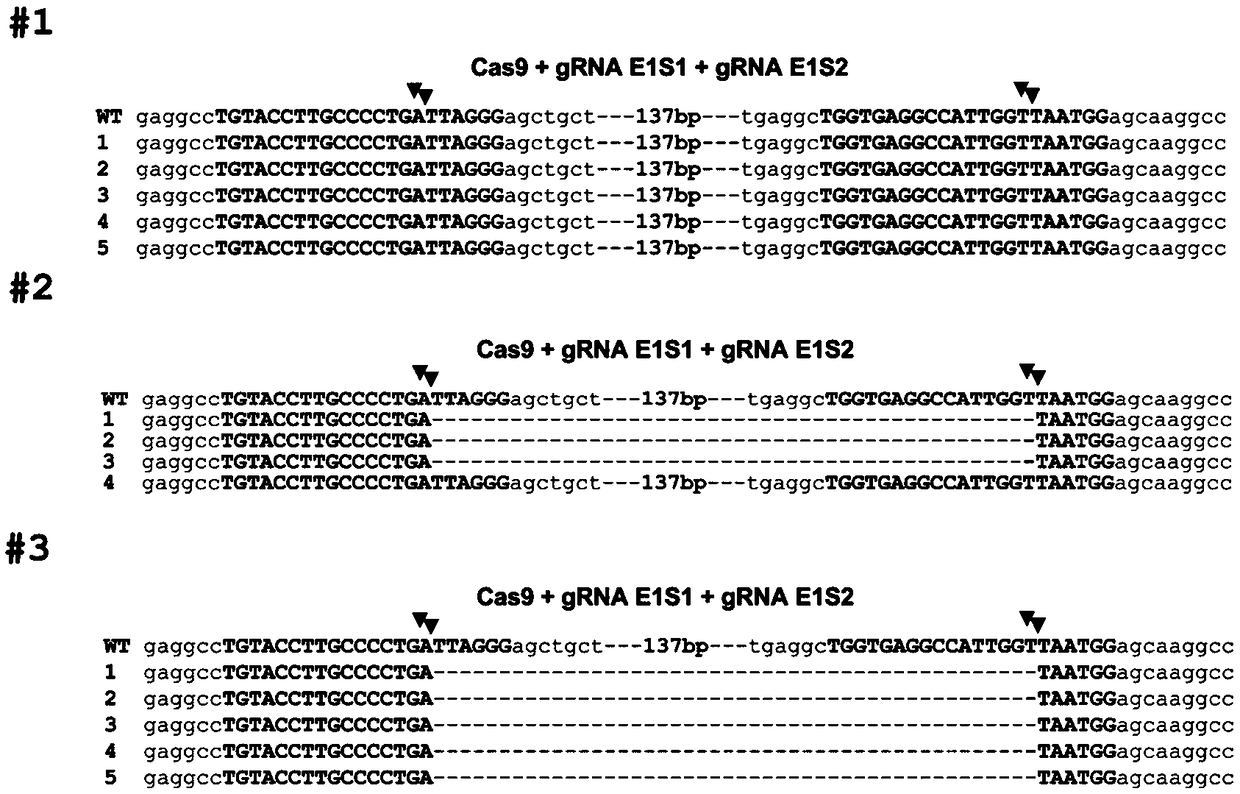
Method for editing swine BMP15 (bone morphogenetic protein 15) gene by using CRISPR/Cas9
InactiveCN108753835AEfficient and precise deletionImprove reproductive performanceBone-inducing factorStable introduction of DNADNA fragmentationExon
The invention discloses a method for editing a swine BMP15 (bone morphogenetic protein 15) gene by using CRISPR / Cas9. The method comprises the steps as follows: designing two gRNAs on an exon 1 of theBMP15 of a target swine genome, and constructing pX458 and pX459 vectors respectively, so that the BMP15 gene loses functions due to accurate deletion of DNA fragments. Compared with single gRNA medicated editing, the method has the advantage that the BMP15 gene can lose functions more effectively due to accurate deletion of DNA fragments of the exon.
Owner:SUN YAT SEN UNIV
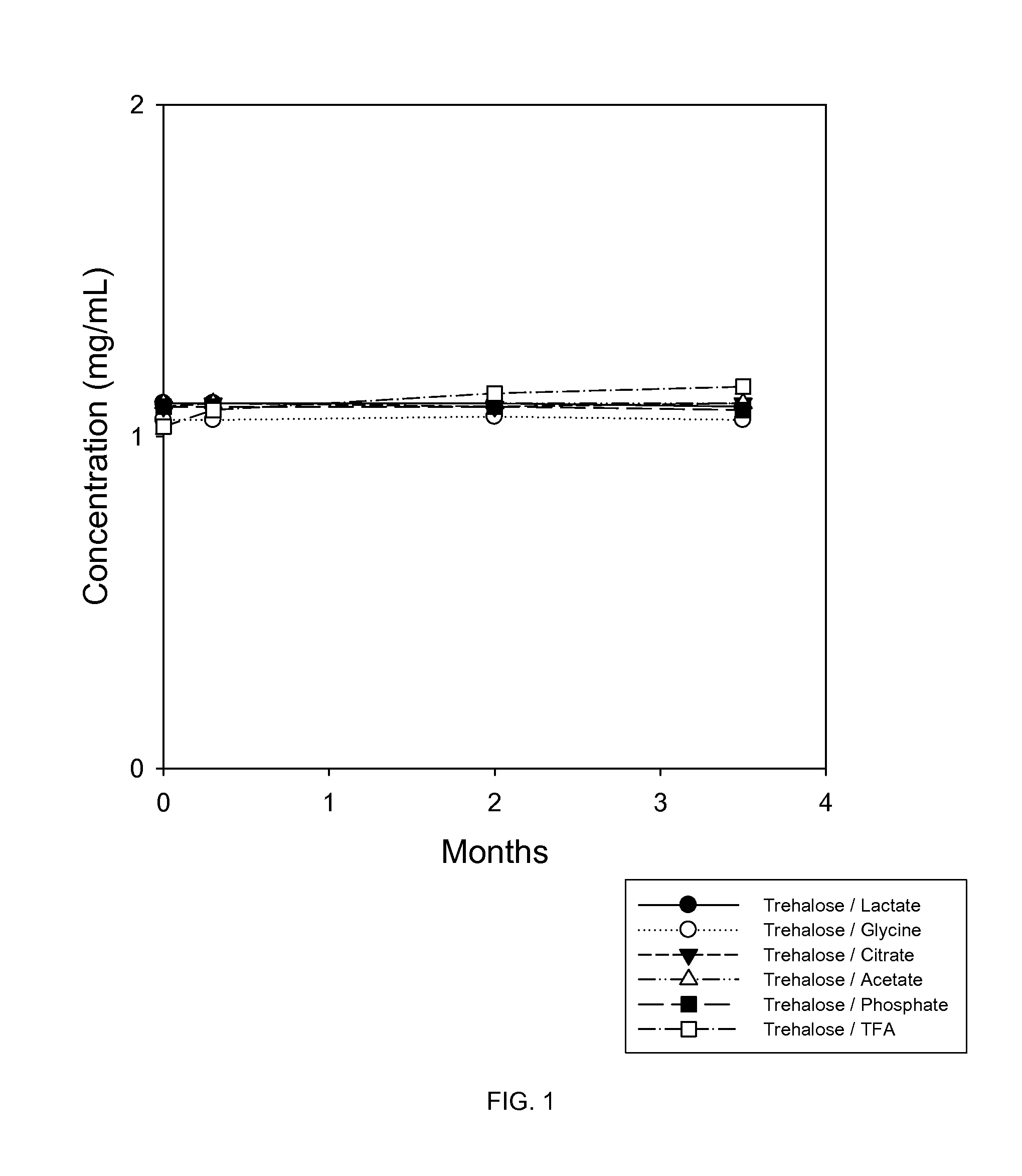
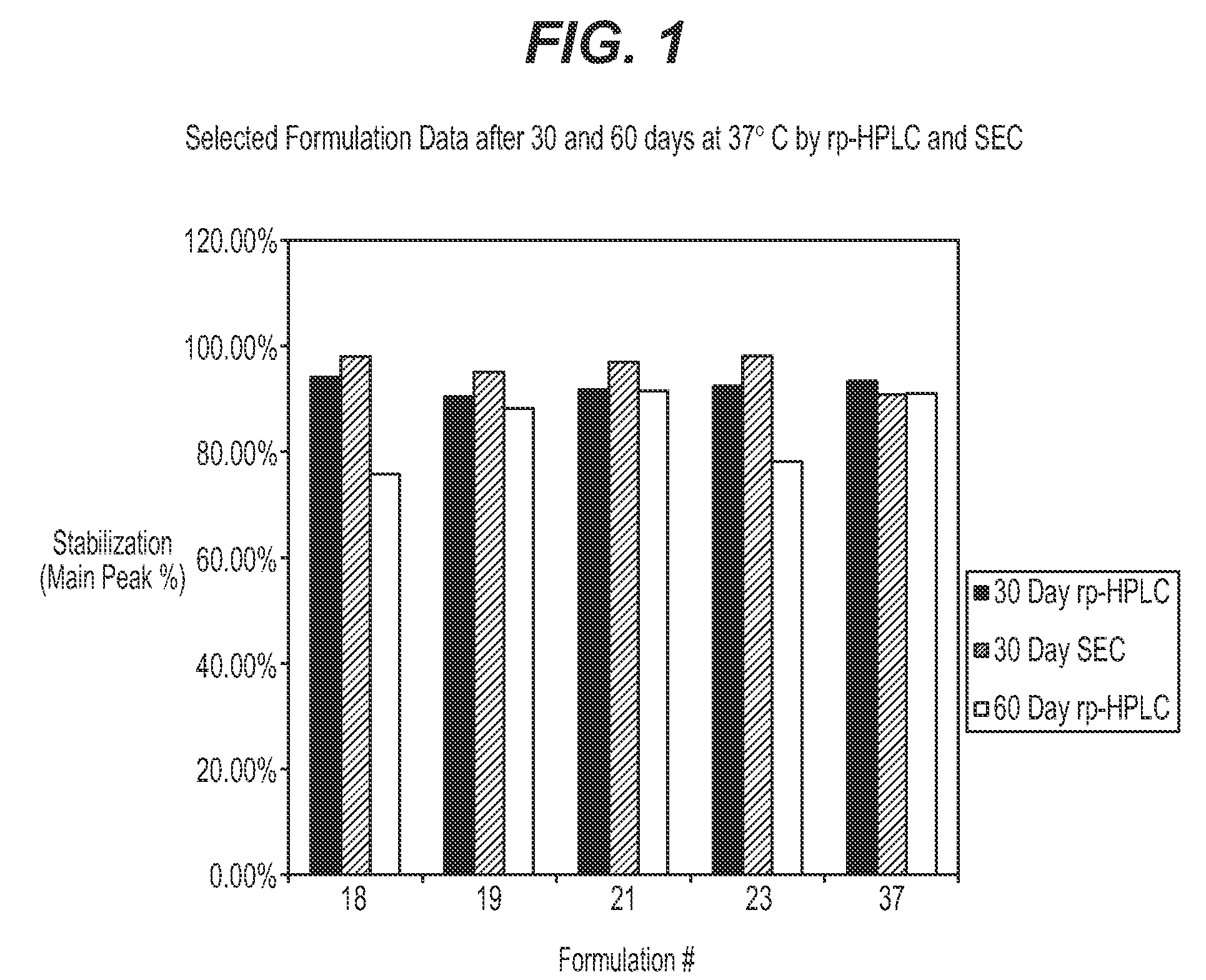
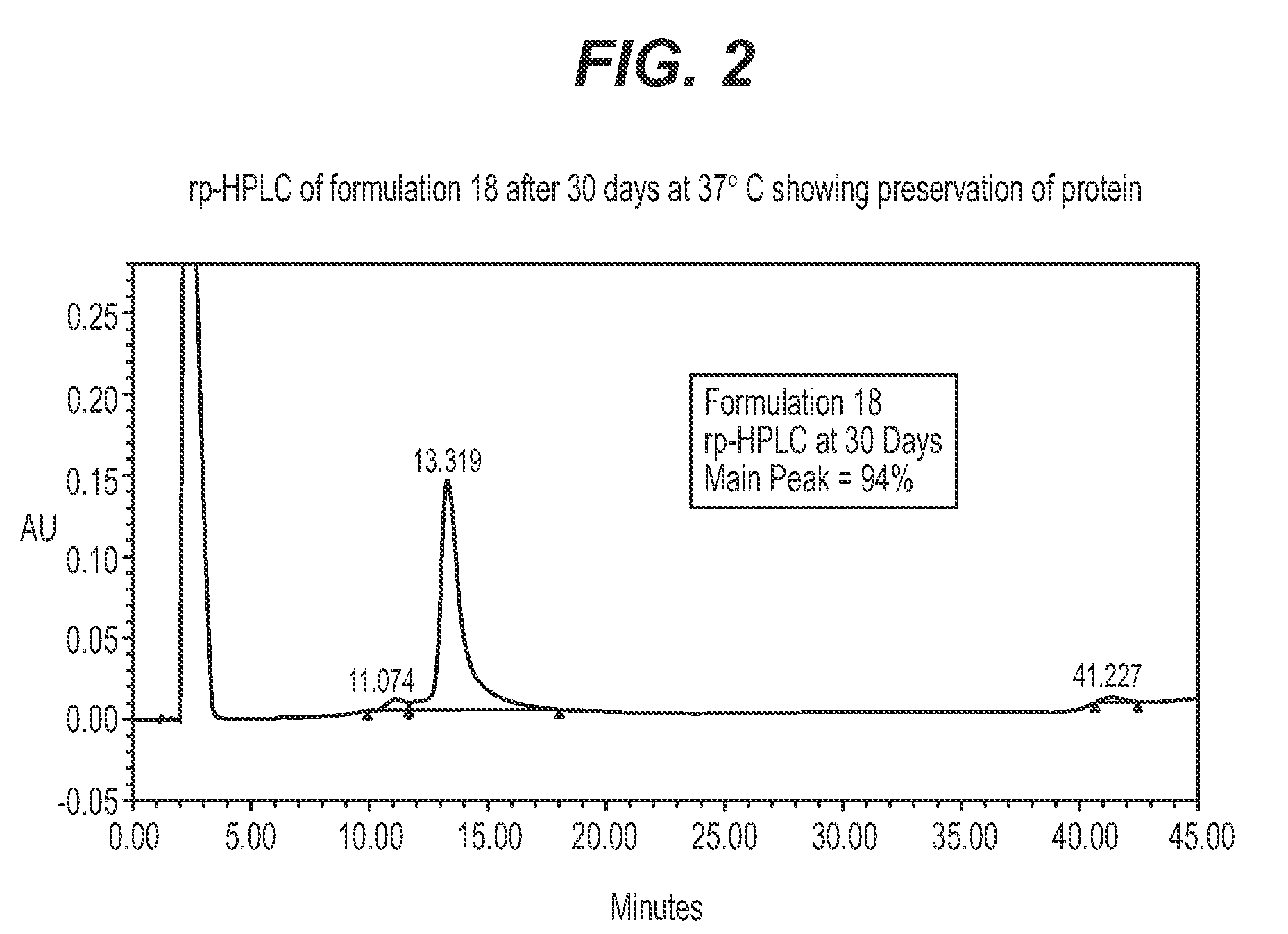
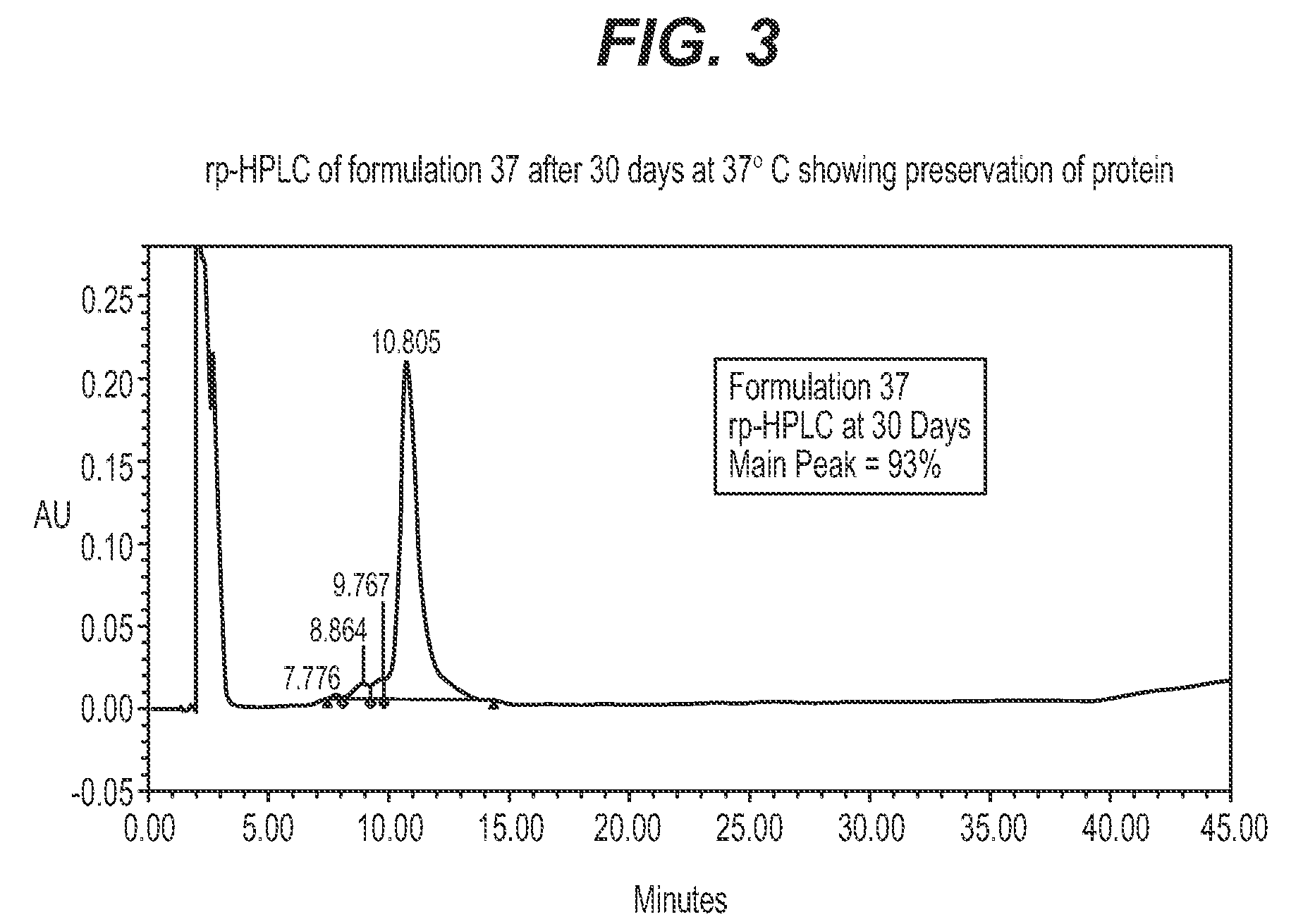
Protein formulations for use at elevated temperatures
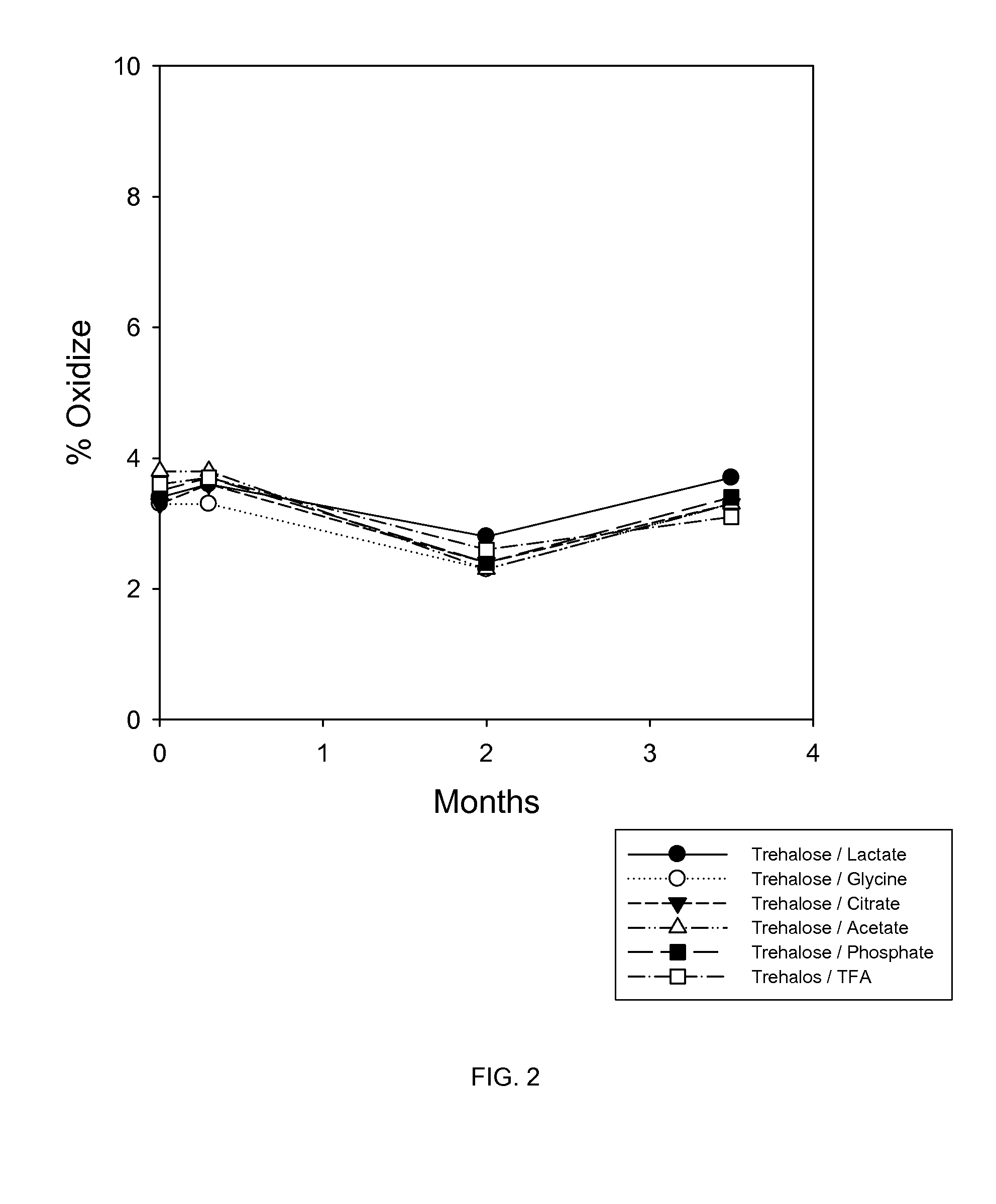
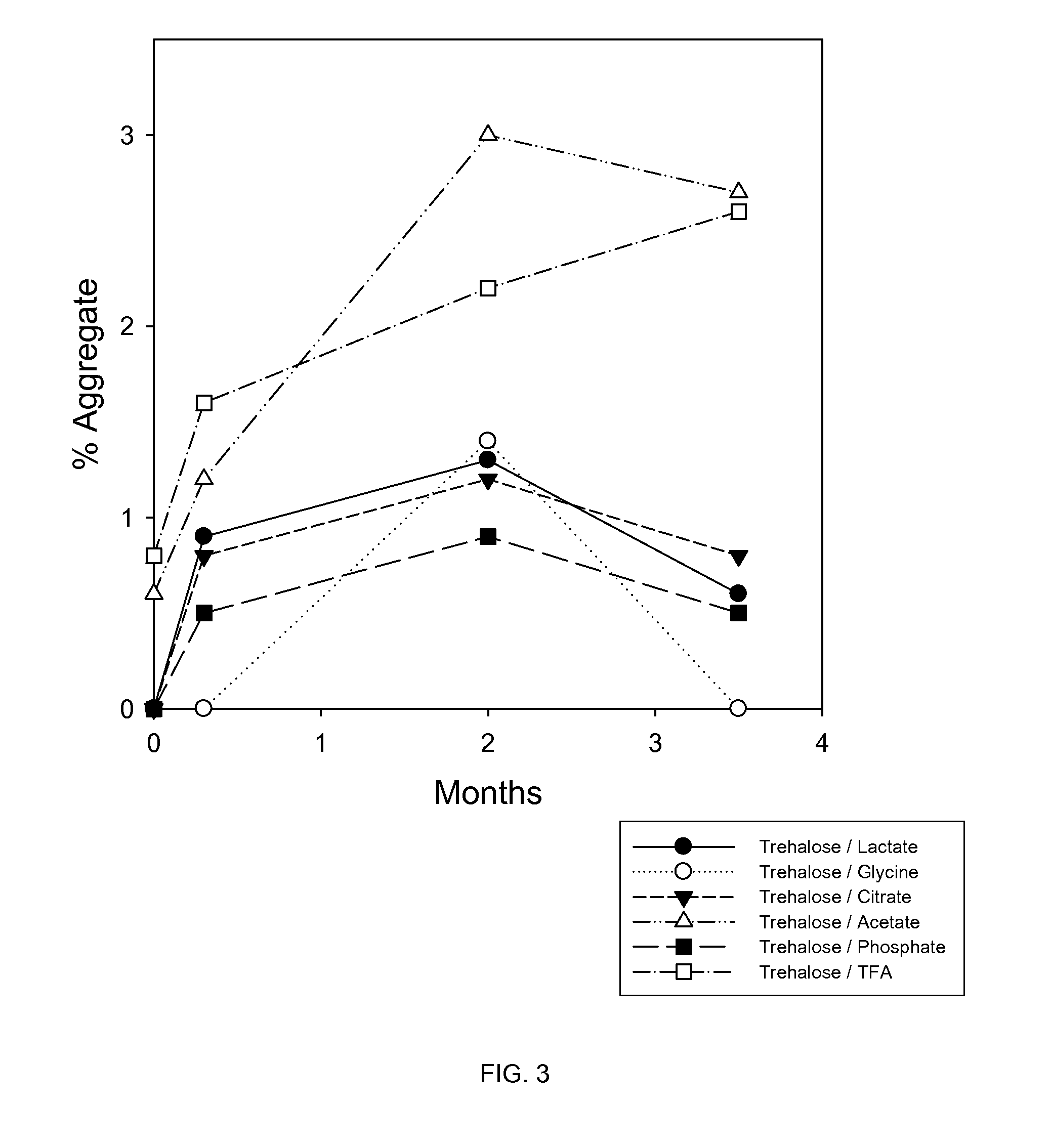
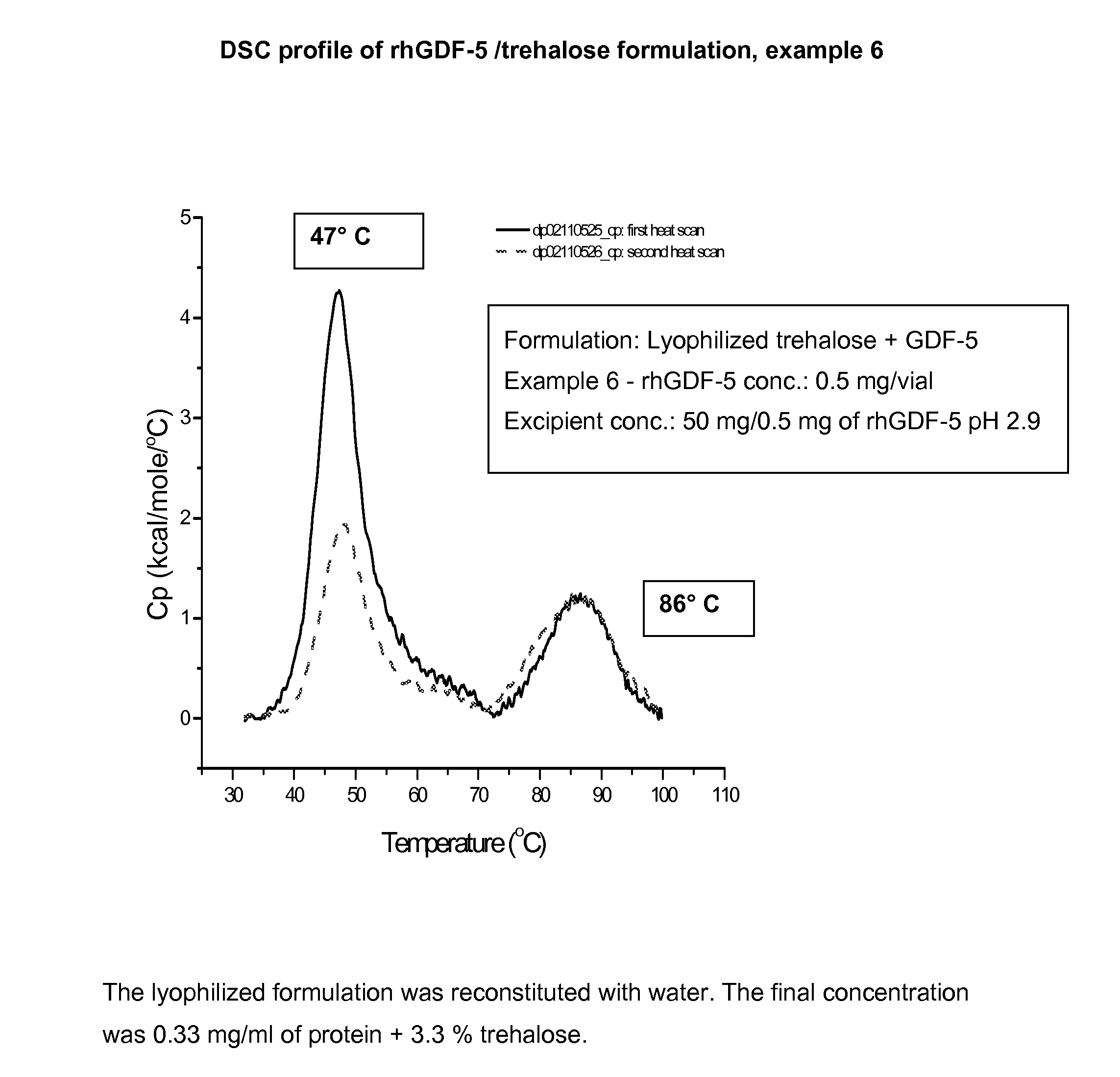
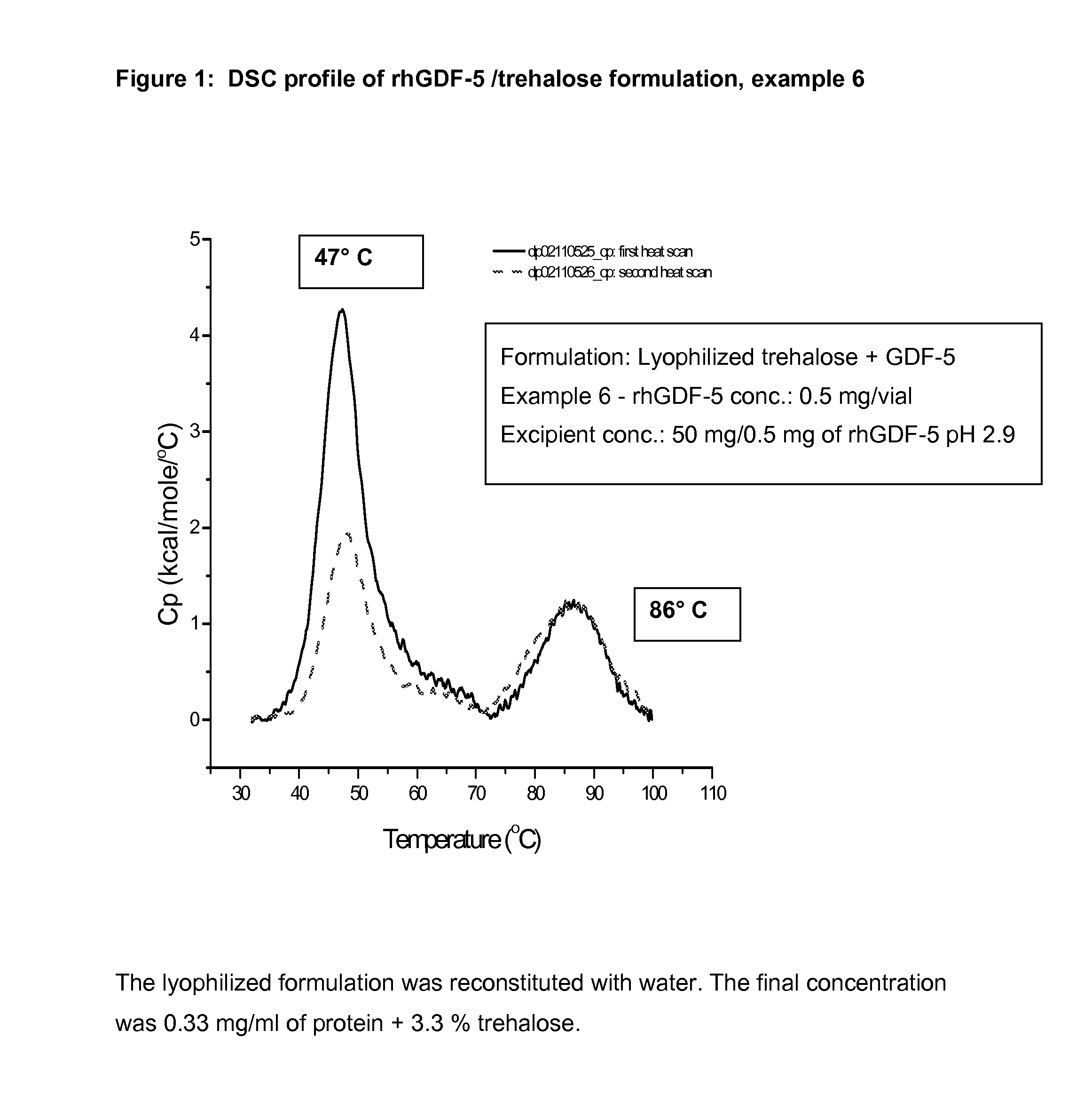
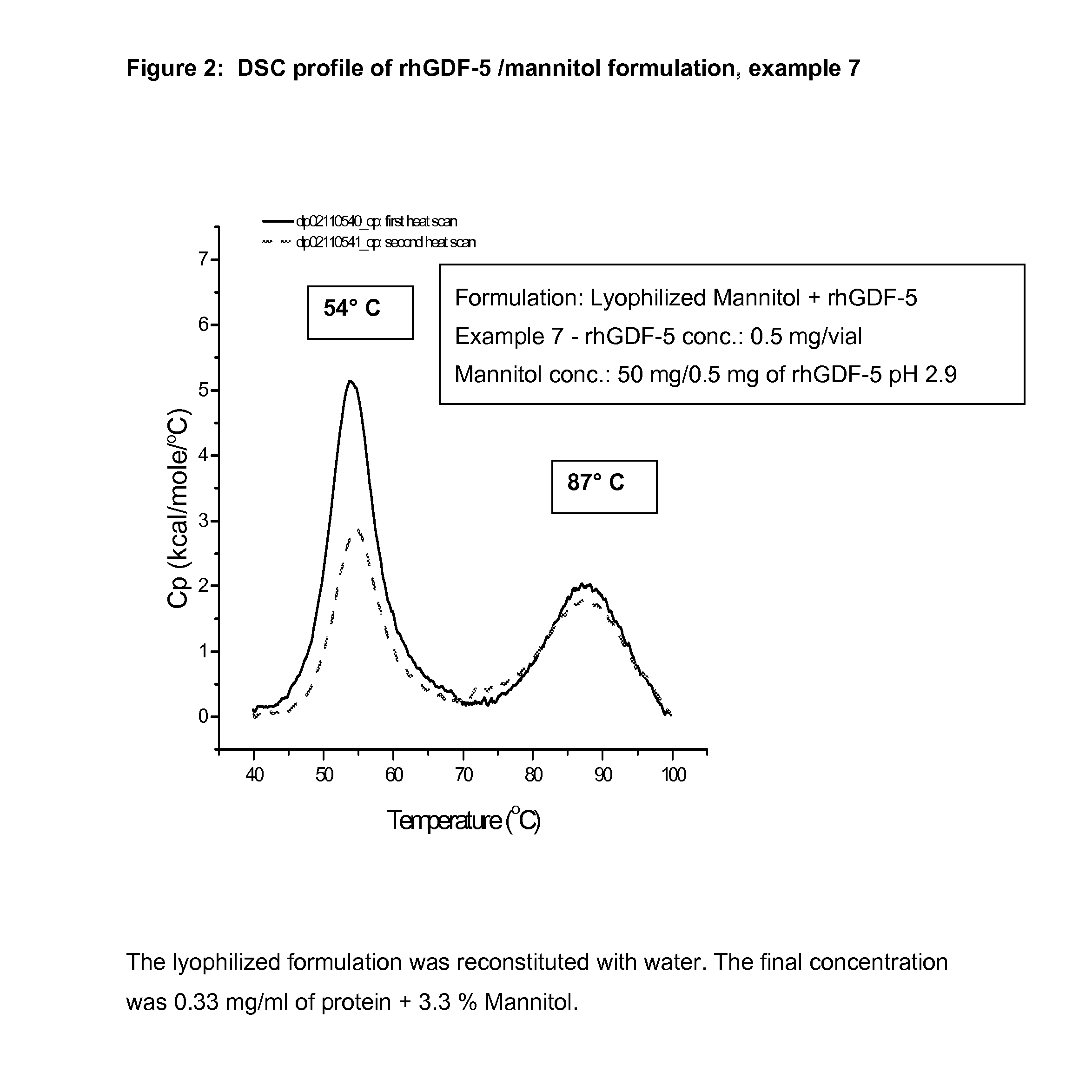
Liquid formulations of bone morphogenetic proteins are provided for prolonged use at elevated temperatures. More specifically, the invention relates to liquid formulations comprising rhGDF-5, trehalose, and one or more biocompatible excipients that provide stability to the protein for at least 30 days at temperatures up to body temperature.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Highly-mineralized osteogenic sponge compositions, and uses thereof
InactiveUS7563455B2Increased resorptionDiminish and eliminate capacityPeptide/protein ingredientsBone implantImplantation SiteProtein C
Osteogenic sponge compositions having enhanced osteoinductive properties for use in bone repair are described. The compositions include a quickly resorbable porous carrier, a more slowly resorbed mineral scaffold and an osteogenic factor, preferably a bone morphogenetic protein. The compositions enable increased osteoinductive activity while retaining a reliable scaffold for the formation of new bone at an implant site. Methods for therapeutic use of the compositions are also described.
Owner:WARSAW ORTHOPEDIC INC
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39298E1Decreasing thrombogenicityLow antigenicityOrganic active ingredientsPowder deliveryTissue sealantVascular dilatation
This invention provides methods for the localized delivery of supplemented tissue sealants, wherein the supplemented tissue sealants comprise at least one composition which is selected from one or more antibodies, analgesics, anticoagulants, anti-inflammatory compounds, antimicrobial compositions, antiproliferatives, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Further provided are methods of using the site-specific supplemented tissue sealants, including preparation of a biomaterial.
Owner:AMERICAN NAT RED CROSS
Protein stabilization formulations
InactiveUS20080147077A1More riskEfficient use ofSuture equipmentsPeptide/protein ingredientsBone morphogenetic proteinProtein formation
The present invention is directed to stabilizing Bone Morphogenetic Protein in various lyophilized formulations and compositions. The present invention comprises formulations primarily including trehalose as an excipient for lyophilized compositions and their subsequent storage and reconstitution, and can also optionally include other excipients, including buffers and surfactants.
Owner:DEPUY SYNTHES PROD INC
Culture medium for epithelial stem cells and organoids comprising the stem cells
The invention relates to a method for culturing epithelial stem cells, isolated tissue fragments comprising said epithelial stem cells, or adenoma cells, and culturing the cells or fragments in the presence of a Bone Morphogenetic Protein (BMP) inhibitor, a mitogenic growth factor, and a Wnt agonist when culturing epithelial stem cells and isolated tissue fragments. The invention further relates to a cell culture medium comprising a BMP inhibitor, a mitogenic growth factor, and a Wnt agonist, to the use of said culture medium, and to crypt-villus organoids, gastric organoids and pancreatic organoids that are formed in said culture medium.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
Degradable Cage Coated With Mineral Layers For Spinal Interbody Fusion
ActiveUS20080215093A1Improve biological activityIncrease capacityInternal osteosythesisPeptide/protein ingredientsDrug biological activityPolycaprolactone
A cage for facilitating fusion of bones, such as vertebrae, or fusion of adjacent bone surfaces is disclosed. In one form, the cage includes a plurality of spaced apart walls comprising a biodegradable polymeric material (e.g., polycaprolactone); an osteoconductive mineral coating (e.g., a calcium compound) on at least a portion of the walls; and a bioactive agent (e.g., a bone morphogenetic protein) associated with the polymeric material and / or the coating. The bioactive agent is present in amount that induces ossification between the bones or adjacent bone surfaces. The cage may also include a fixation plate connected to at least one of the walls.
Owner:WISCONSIN ALUMNI RES FOUND +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com