Bone Morphogenetic Protein Compositions
a technology of morphogenetic proteins and compositions, applied in the field of bone morphogenetic proteins, can solve the problems of difficult formulation of certain therapeutically significant proteins, and achieve the effects of high recovery rate, enhanced stability, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
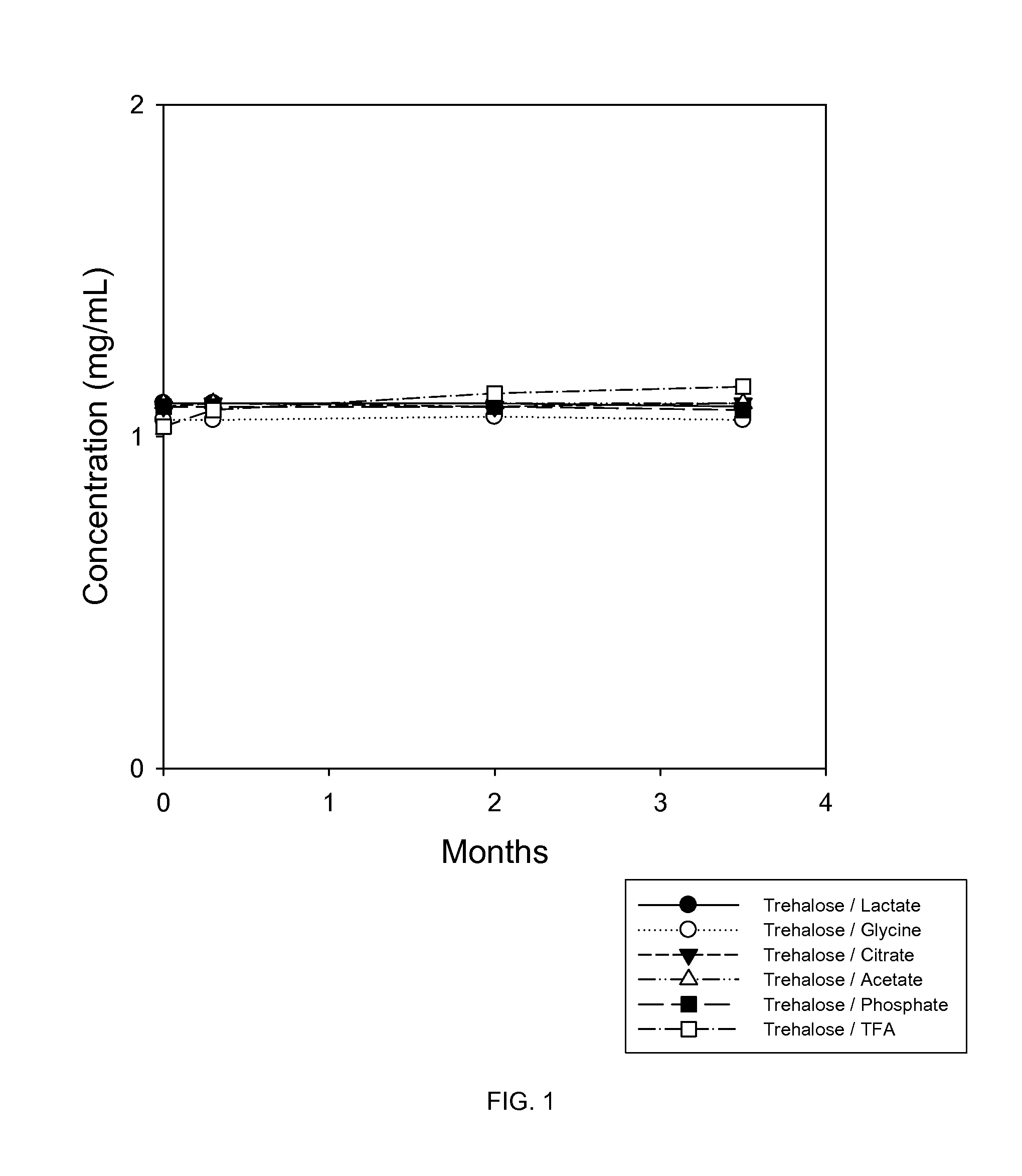
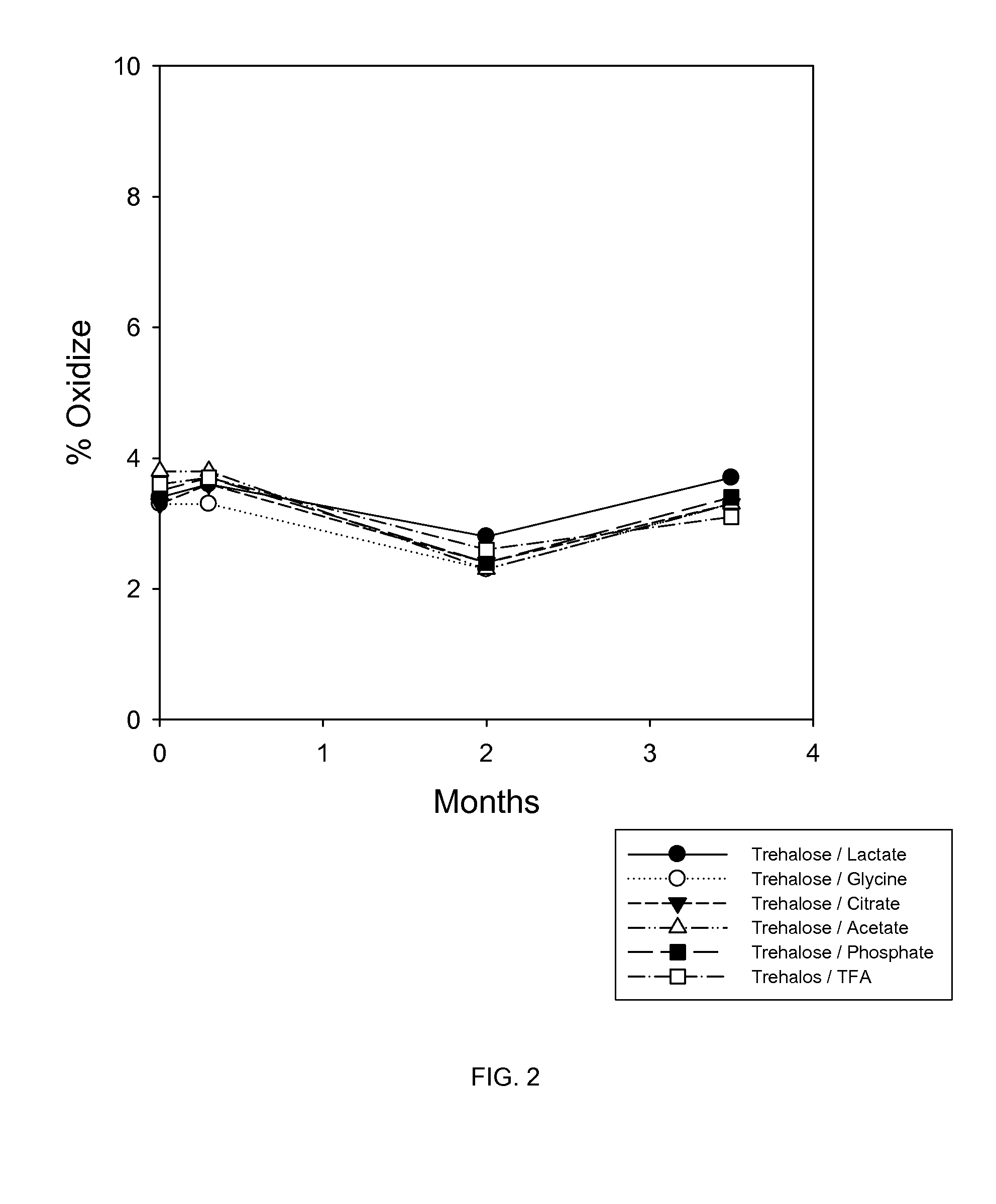
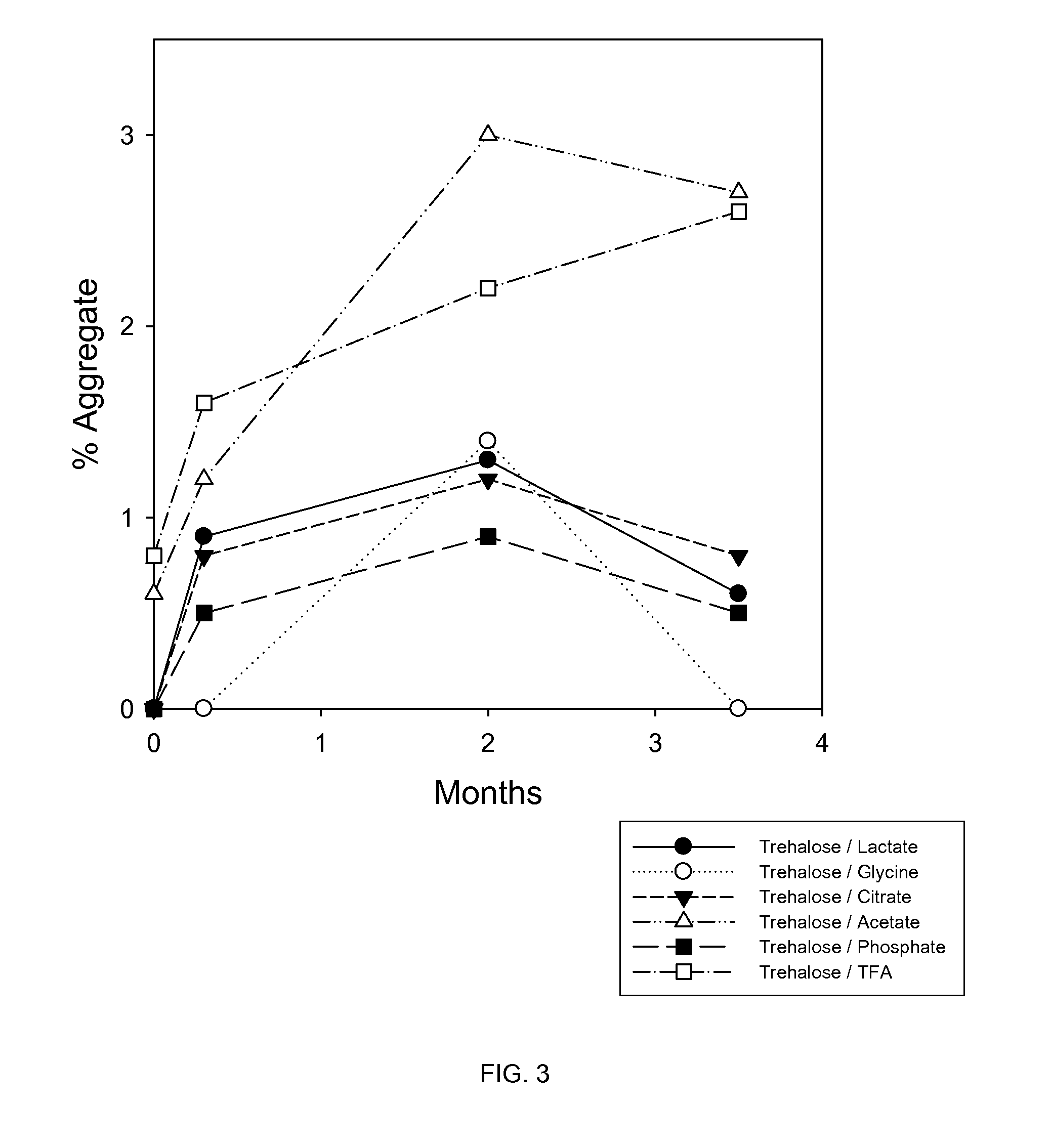
A Study of the Stability of BMP-7 at 40° C. in Various Buffers with 5% Trehalose
[0105]In this study, trehalose and various buffers were tested for their effects on protecting 1 mg / mL BMP-7 solution during lyophilization and storage at 40° C. The buffers tested were 5 mM glycine, 5 mM citrate, 10 mM lactate, 5 mM phosphate, 0.05% TFA and 100 mM acetic acid.
[0106]The buffers were prepared as follows:
5 mM Glycine, pH 3 Buffered Solution
[0107]Two liters of 5 mM glycine solution were prepared by weighing 0.7507 g of glycine (MW=75.07) into a 2000 mL beaker. The glycine was dissolved in Milli-Q® water with stirring, the pH was measured and adjusted to pH 3 with 5 N HCl, and the final volume adjusted to 2000 mL.
5 mM Citrate, pH 3 Buffered Solution
[0108]Two liters of 5 mM citrate solution were prepared by weighing 2.1014 g of citric acid monohydrate (MW=210.14) into a 2000 mL beaker. The citric acid was dissolved in Milli-Q® water with stirring, the pH was measured and adjusted to pH 3 with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com