Patents
Literature
92 results about "Degenerative disc disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition where one or more discs in the spine lose their strength.
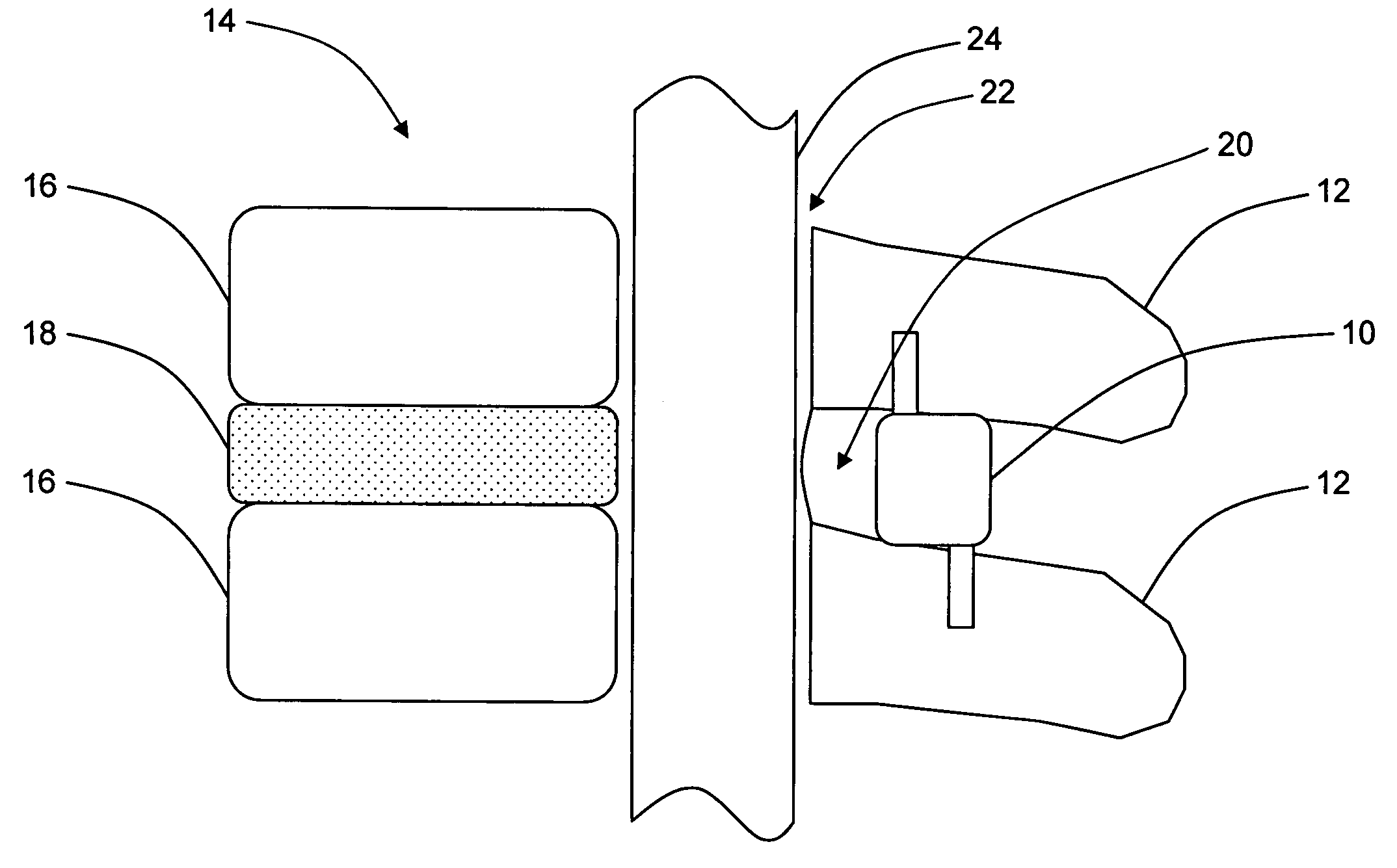
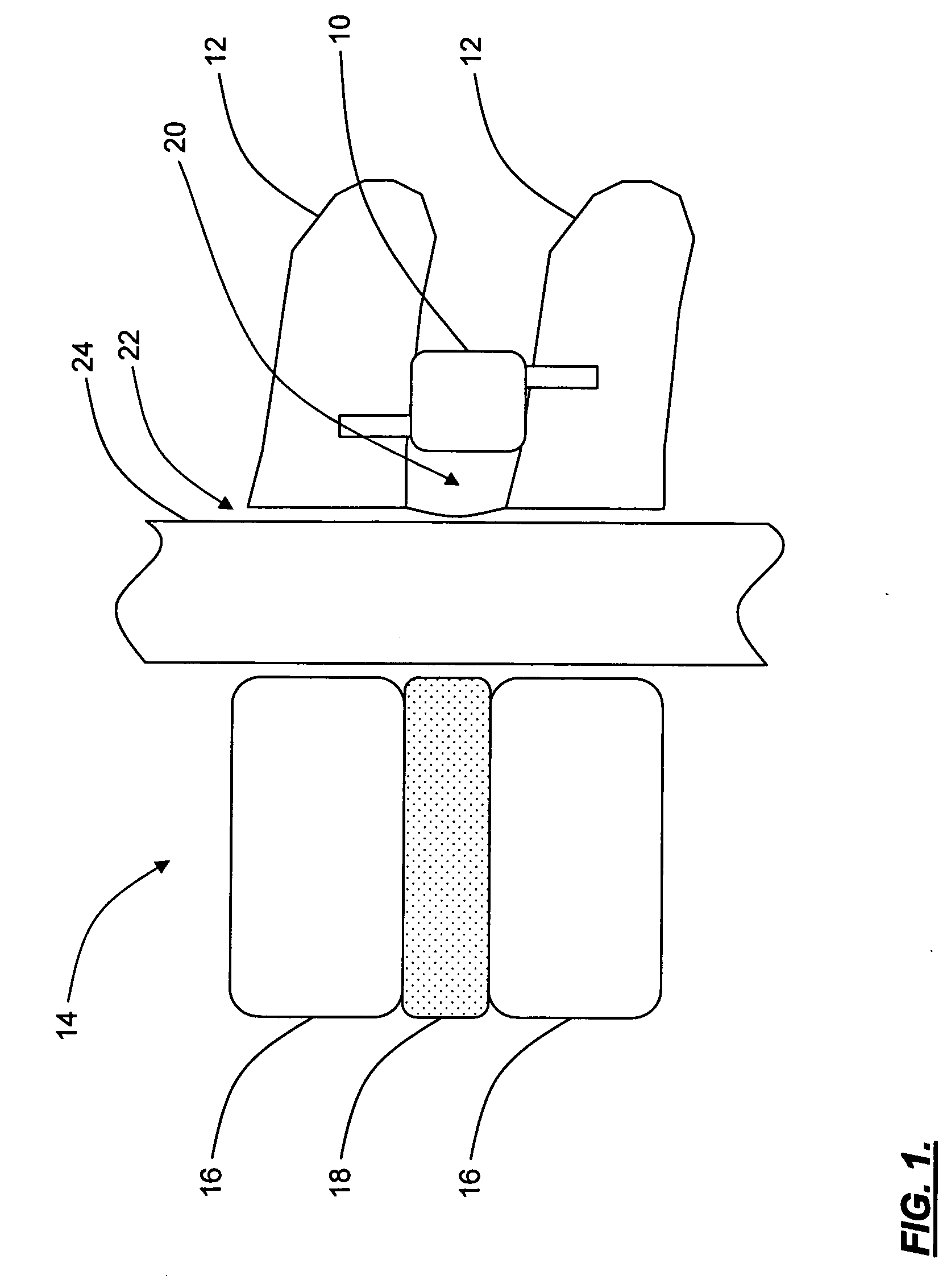
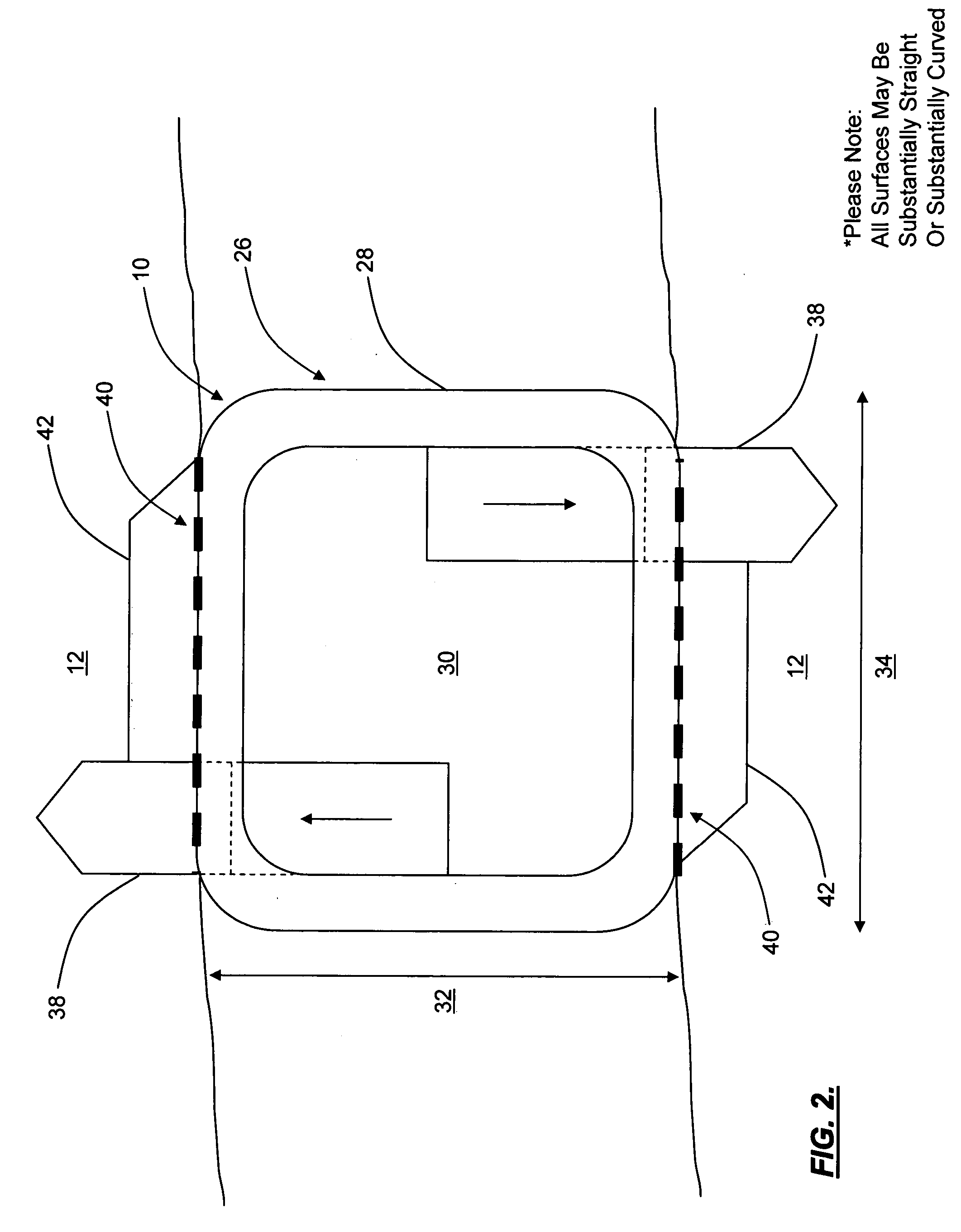
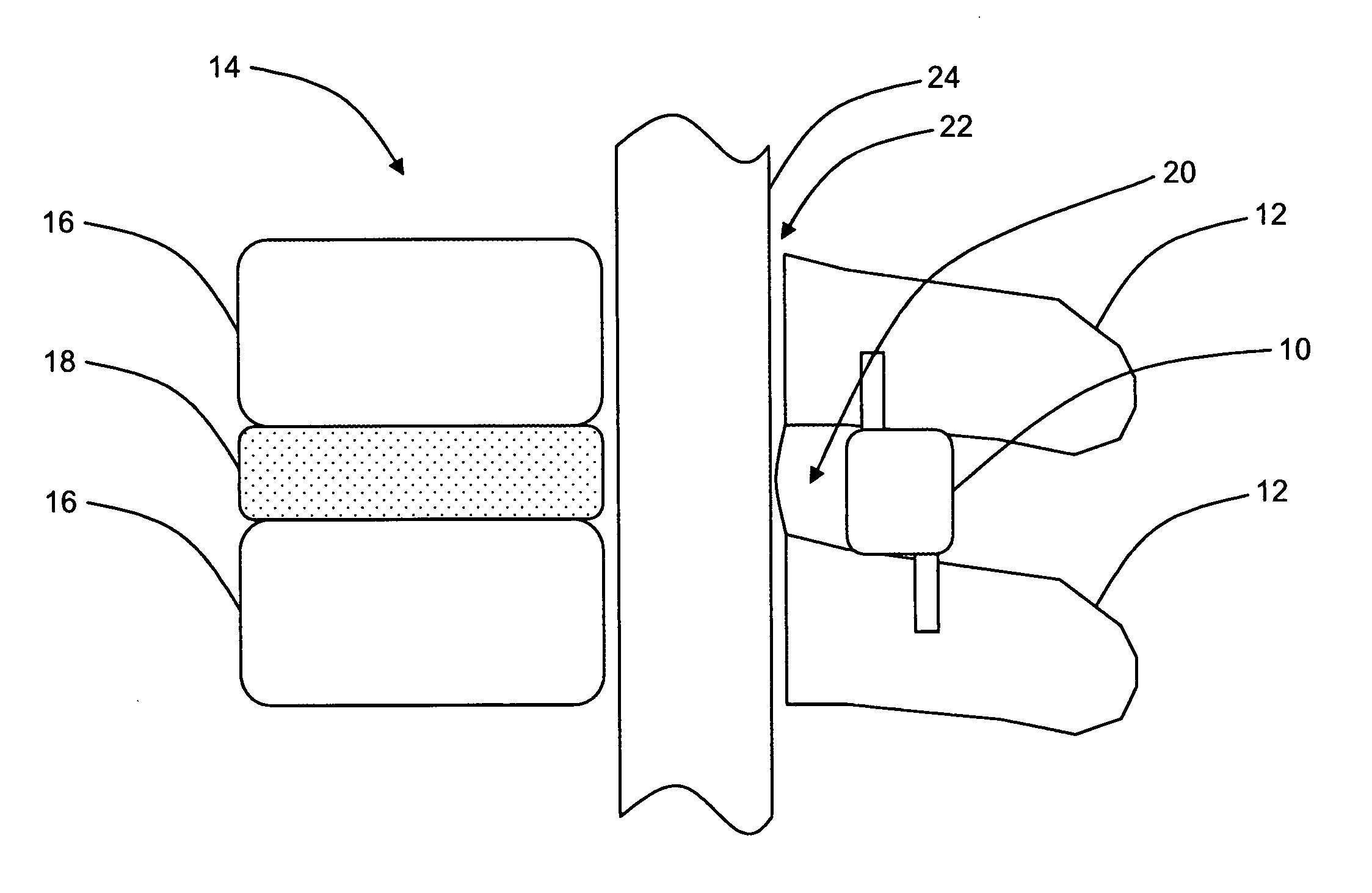
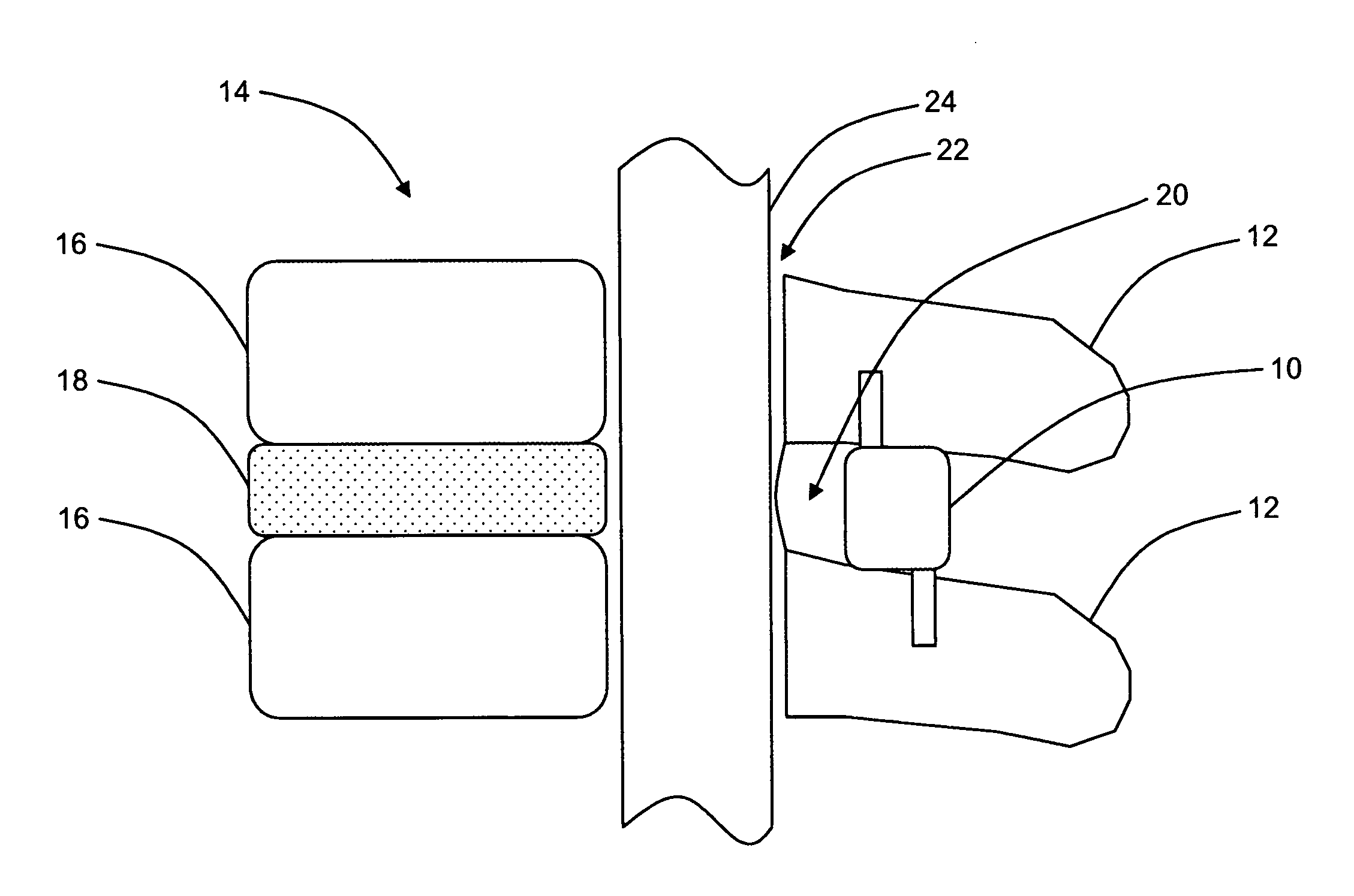
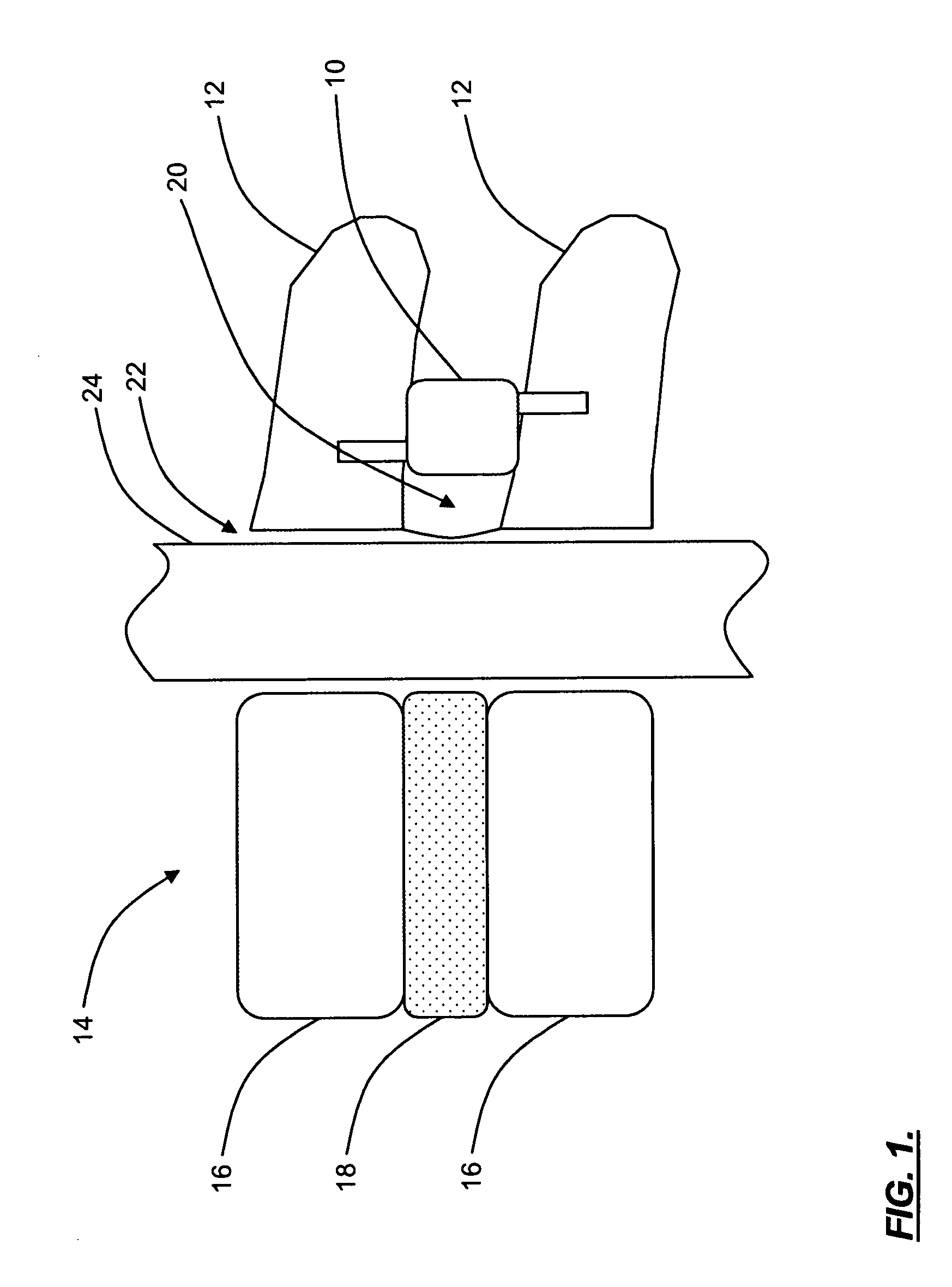
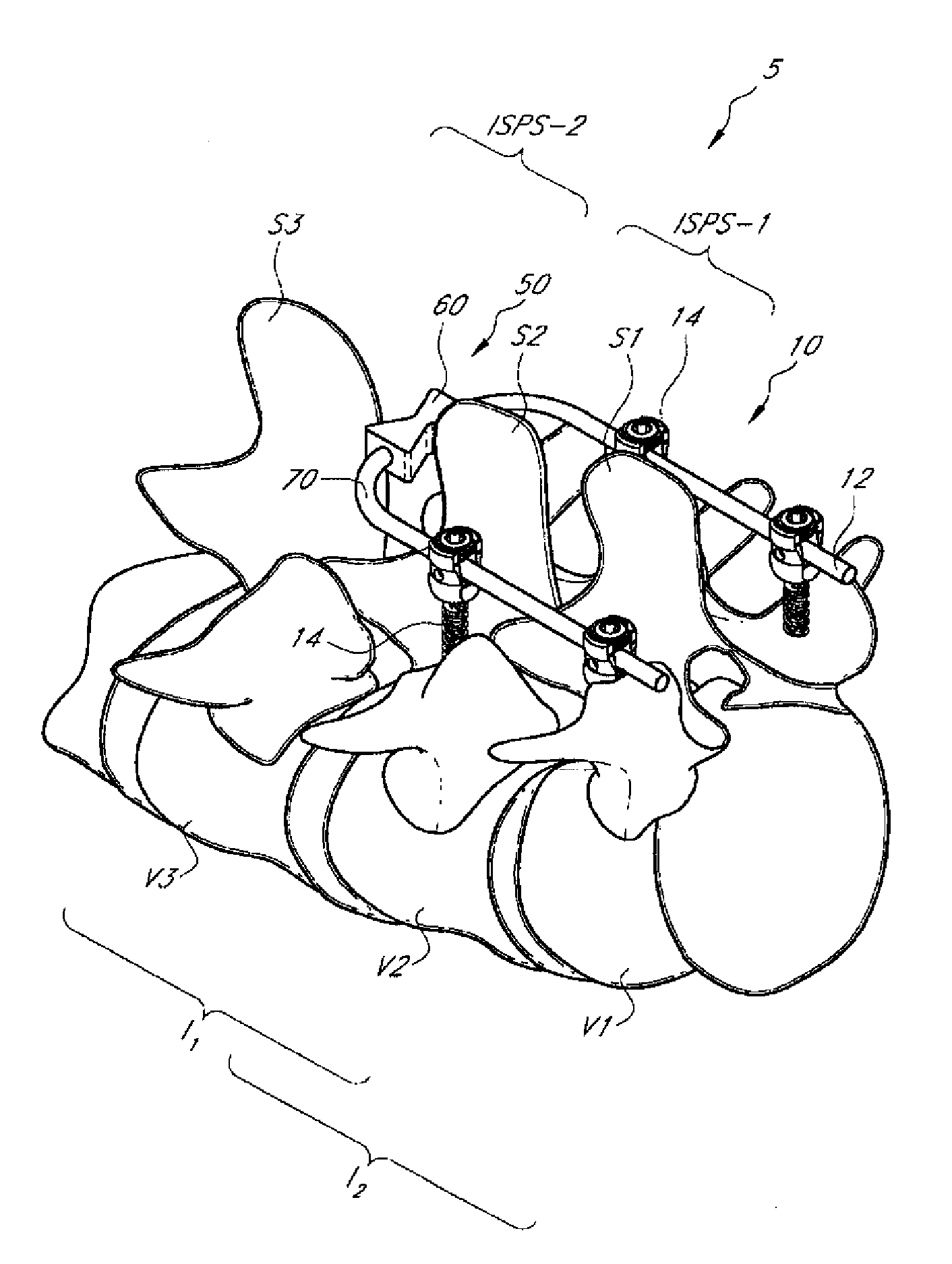
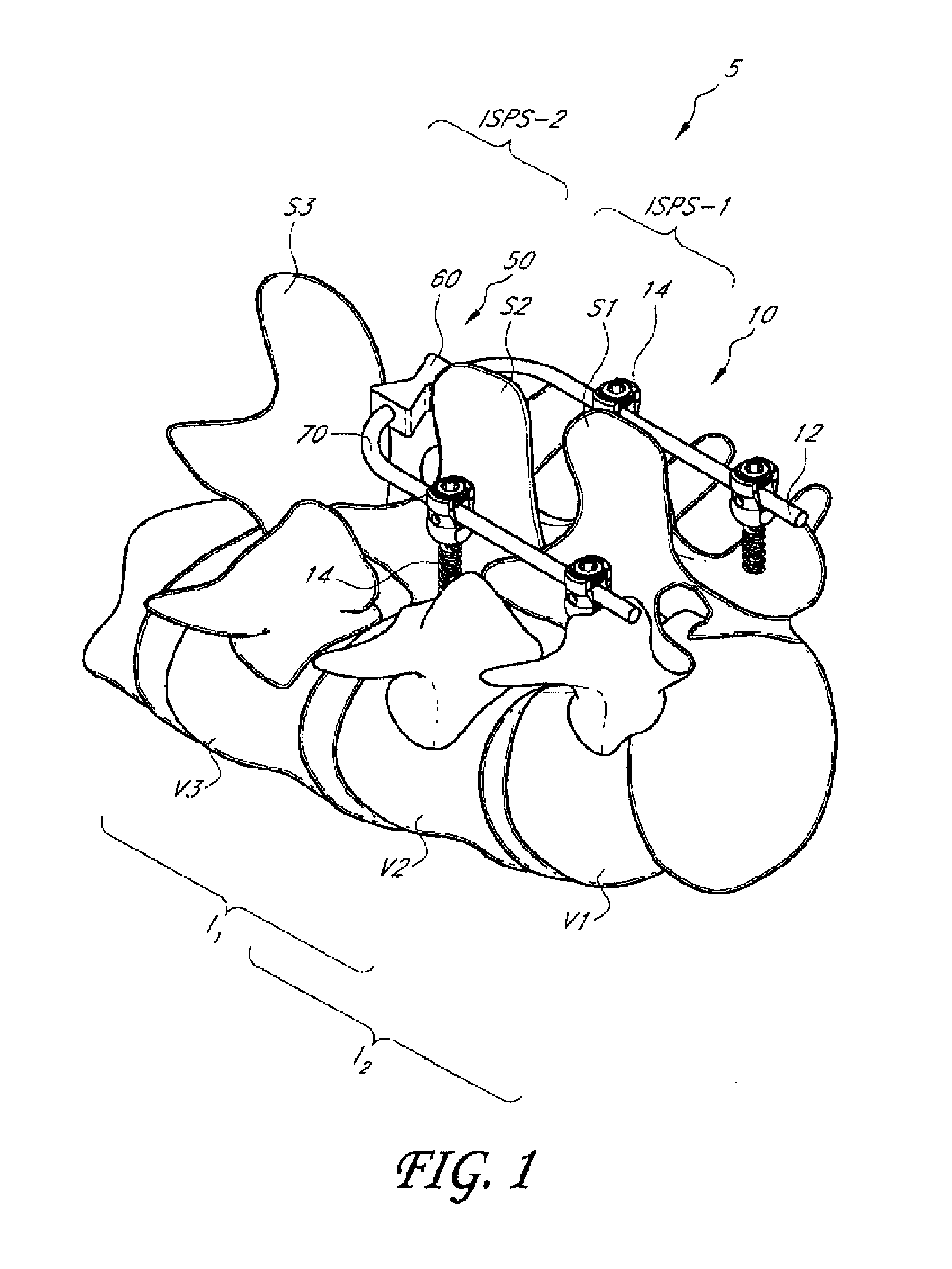
Interspinous distraction devices and associated methods of insertion
InactiveUS20060106397A1Securely holdInternal osteosythesisJoint implantsDistractionMinimally invasive procedures
In various embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
Interspinous distraction devices and associated methods of insertion
In various exemplary embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
Method of treating degenerative spinal disorders
InactiveUS20080045949A1Reduce loadPromotes extracellular matrix productionInternal osteosythesisAntipyreticDevice implantDisease
The present invention describes methods for treating a degenerative intervertebral disc disorder comprising implanting a disc stabilization device into a subject and administering at least one therapeutic agent which promotes healing of the disc to the subject. The invention also includes methods of promoting healing of damaged or degenerated intervertebral discs comprising decreasing the load of the disc through a disc stabilization device and inhibiting the inflammatory process. Also described are hydrogels for use in combination with extradiscal stabilization devices, as intradiscal stabilization devices, as drug carriers, and as combinations thereof.
Owner:ABBOTT LAB INC +1
Spine replacement system for the treatment of spine instability and degenerative disc disease
InactiveUS20070093903A1Avoids prosthetic movementInhibit migrationInternal osteosythesisBone implantDiseaseInstability
Apparatuses and methods for single disc arthroplasty and multi-segmental spine replacement. The implant assembly includes a first cage (20) adapted to be rigidly attached to a first vertebra, a second cage (20) adapted to be rigidly attached to a second vertebrae, and a spinal disc replacement prosthesis (10) positioned between the first and second vertebrae. The spinal disc replacement prosthesis preferably includes a resilient body having two or more adjustable fluid-filled compartments.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Microstructured implant surfaces
An implantable device for treating disc degenerative disease and arthritis of the spine. The implant is sized for placement into an intravertebral disc space. The implant has a body with a predetermined, defined, repeating, three-dimensional pattern at least partially on at least one of its surfaces. The pattern is adapted to create a surface area of bone-contacting features that enhance in-growth and biological attachment to a biocompatible material. Also disclosed are process steps for making the implant.
Owner:TITAN SPINE
Degenerative disc regeneration techniques
InactiveUS20070093905A1Reducing TNF-a productionLowering IL-1 productionSpinal implantsTissue regenerationIntervertebral diskDegenerative disc disease
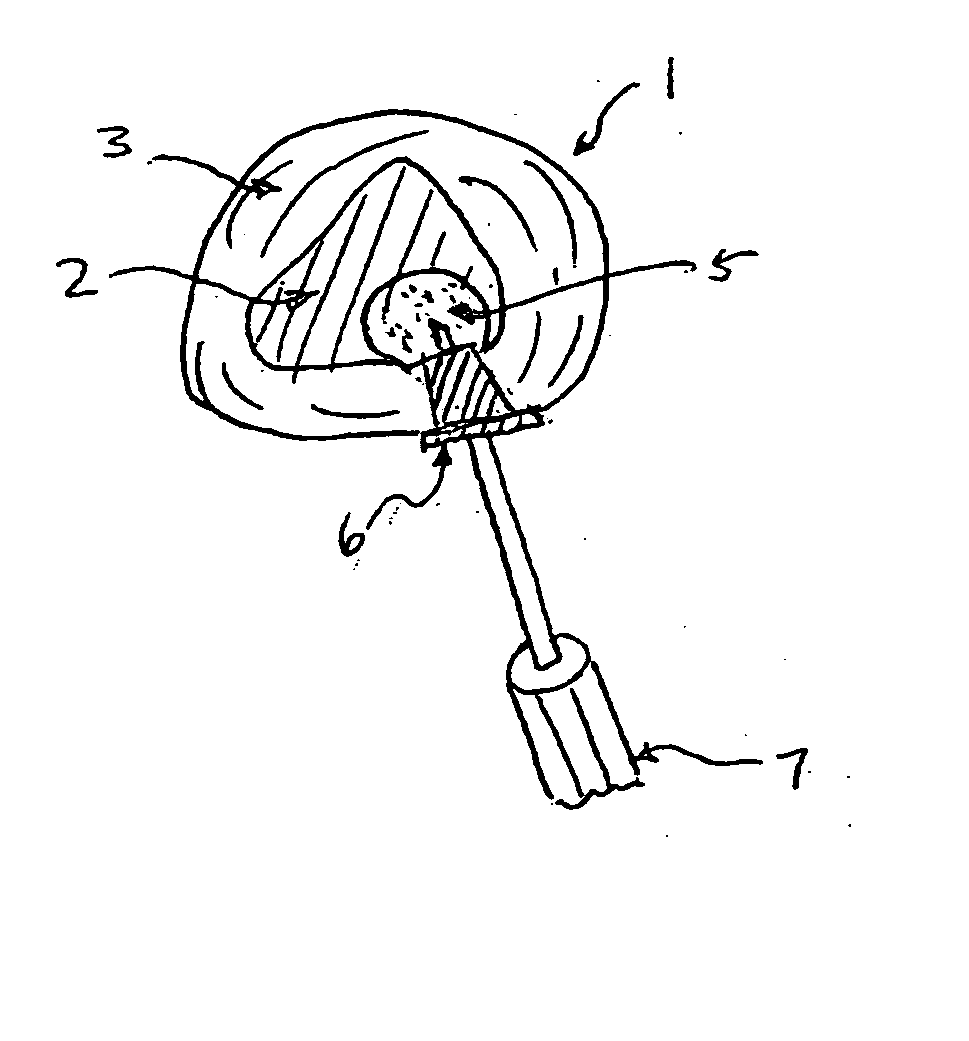
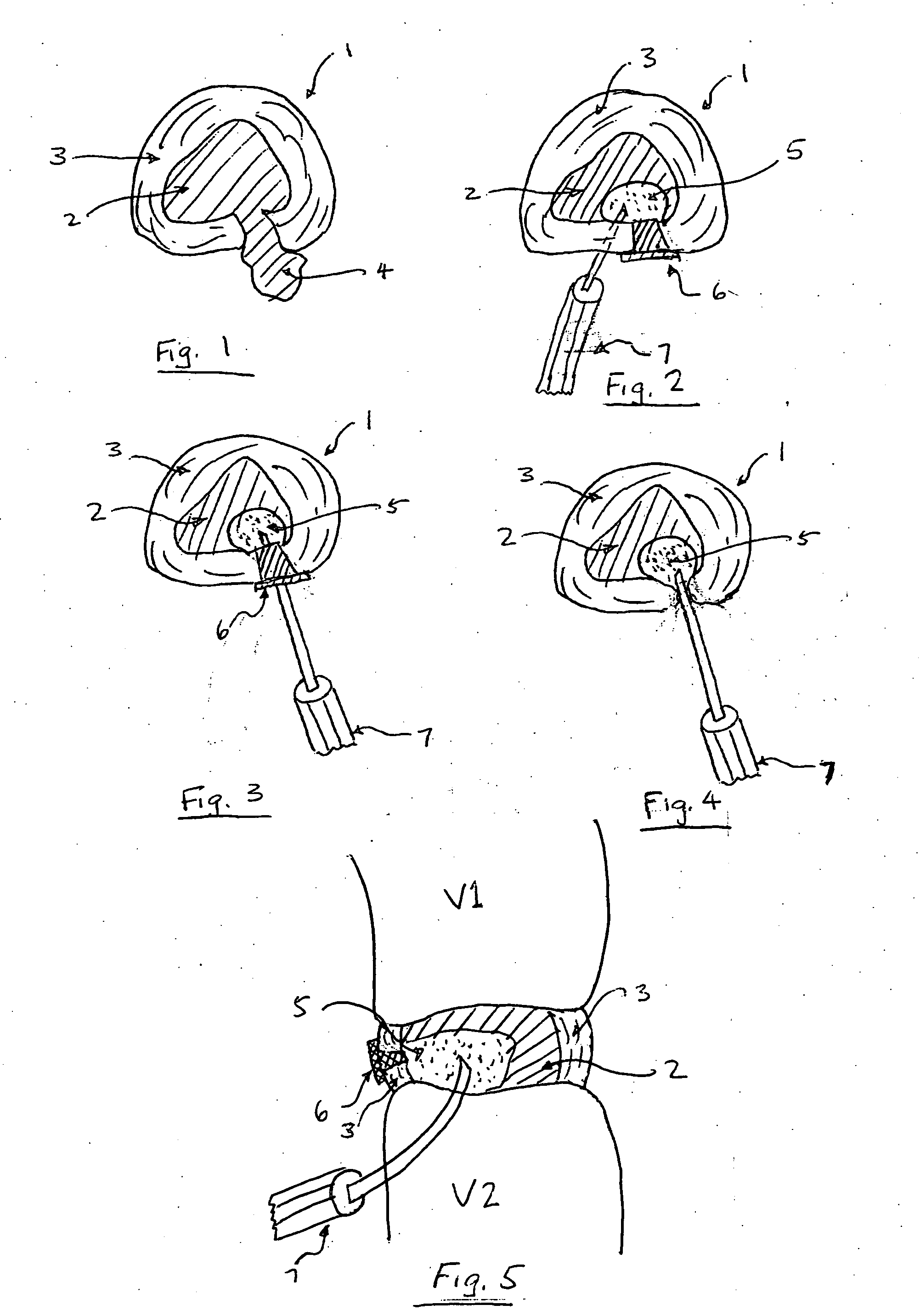
In the repair / regenerate of the intervertebral disc, all or a portion of the nucleus pulposus or annulus fibrosus is excised and treated for reinsertion into the disc or adjacent or alternate level disc. Alternatively certain bioactive agents may be injected into the degenerative disc without excising disc material.
Owner:DEPUY SPINE INC (US) +1
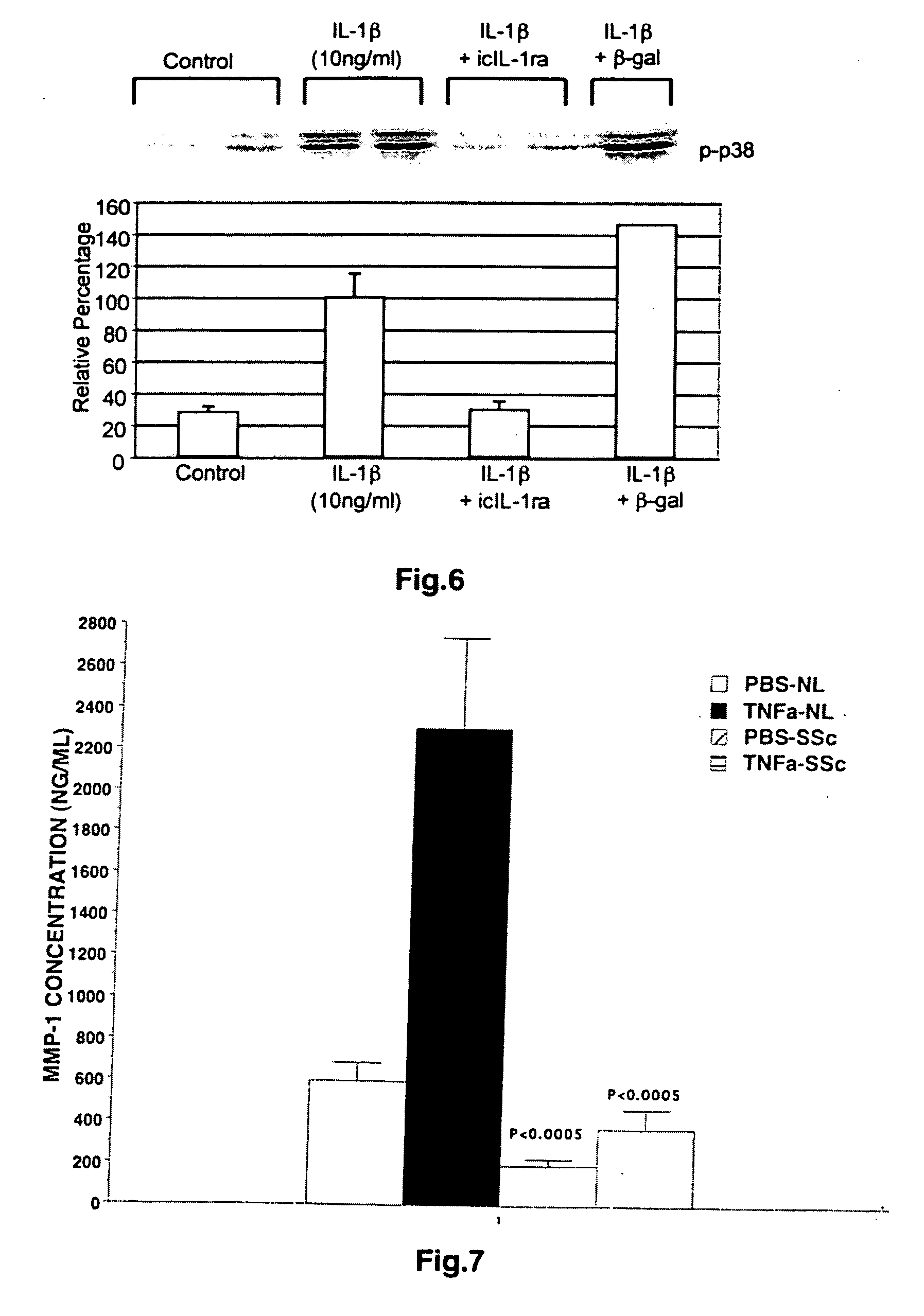
Intracellular interleukin-1 receptor antagonist and uses thereof
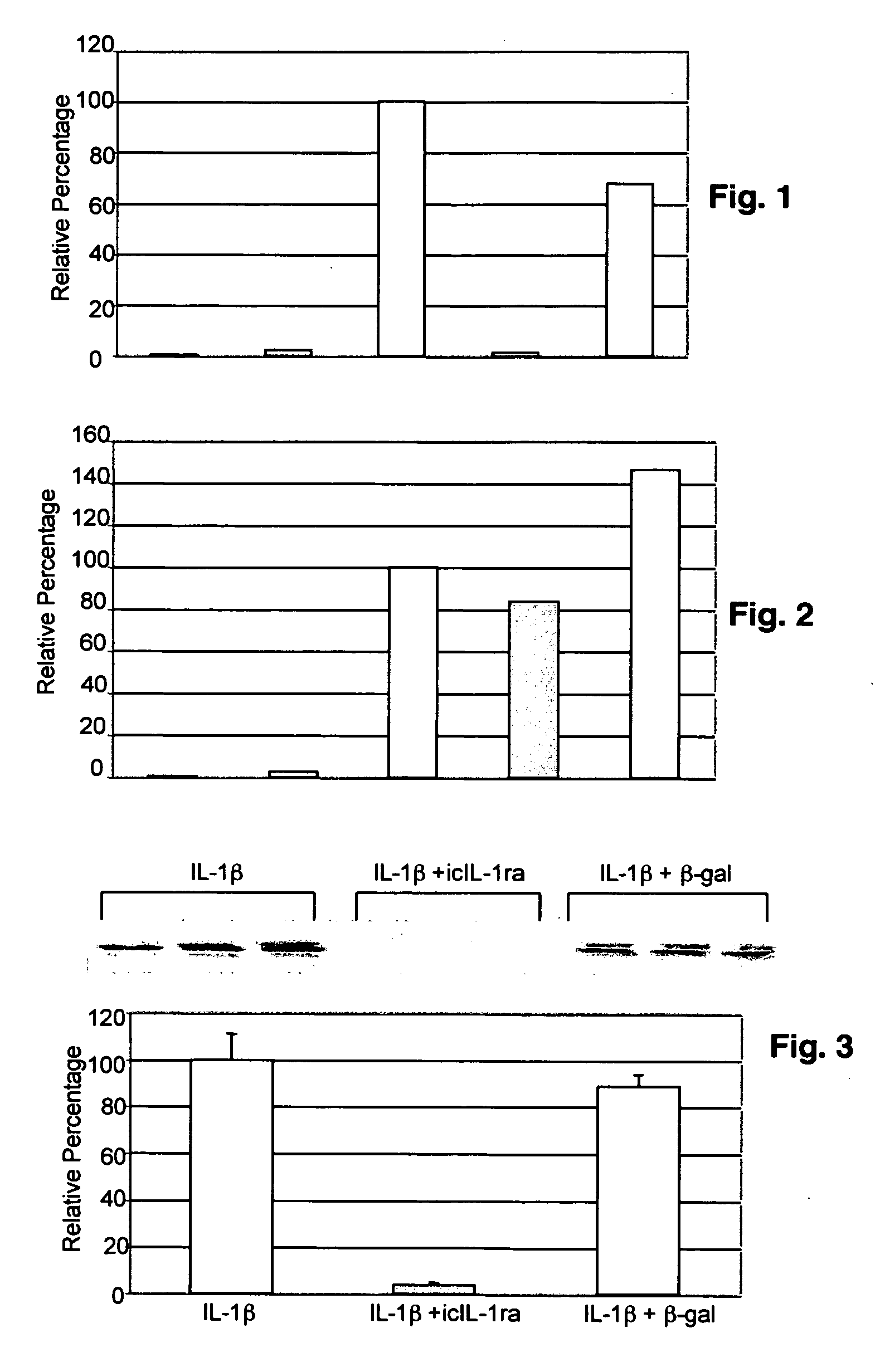
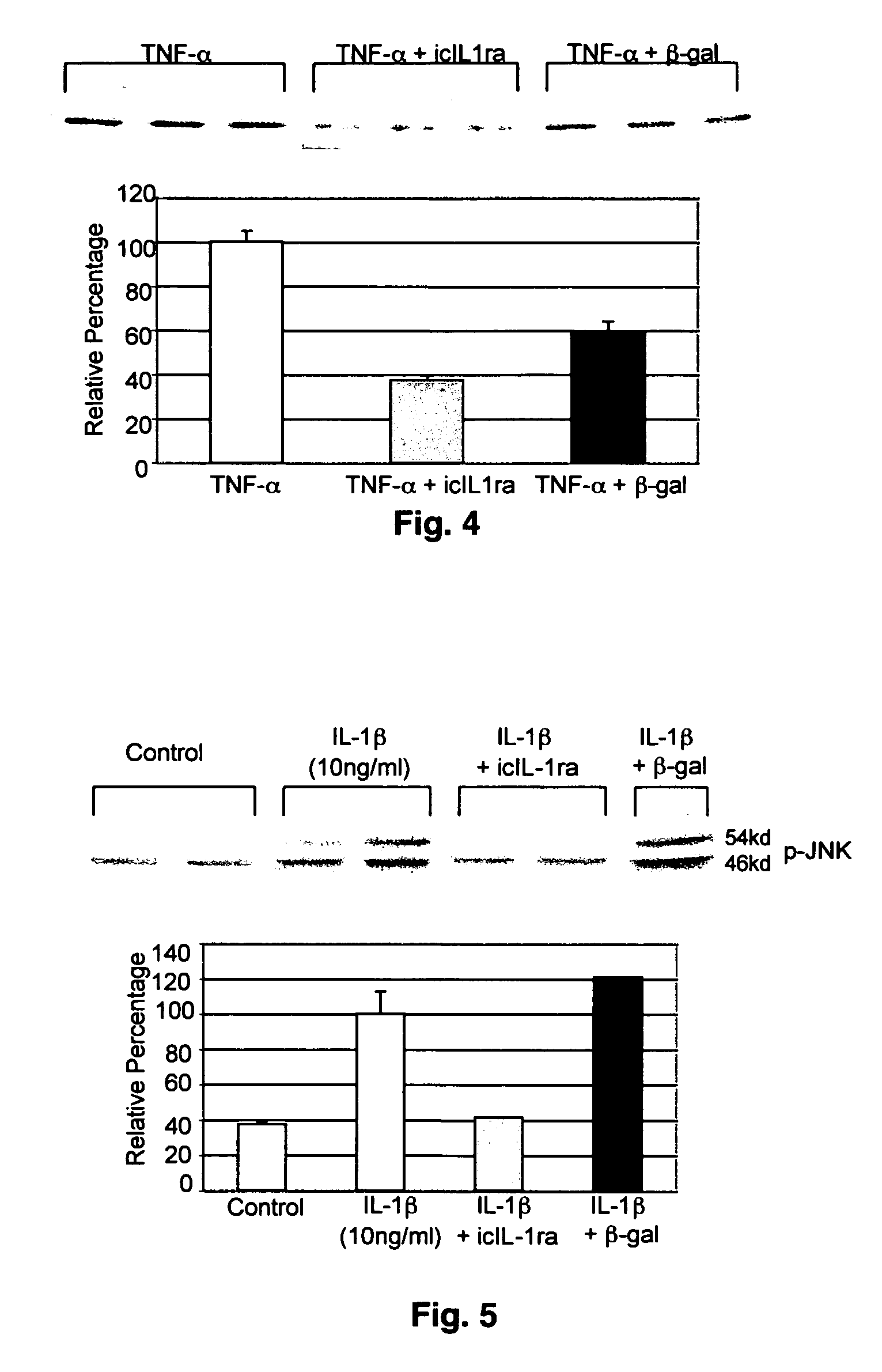
Matrix metalloproteinases are major mediators of tissue destruction in various chronic inflammatory disorders. The present invention demonstrates that over- expression of intracellular isoform of IL-1 receptor antagonist confers to recipient cells resistance to signaling pathways of proinflammatory cytokines (such as tumor necrosis factor alpha and IL-1 beta) that induce matrix metalloproteinase and subsequent tissue degradation. Hence, over-expression of intracellular IL-1 receptor antagonist may inhibit tissue destruction in various inflammatory disorders such as rheumatoid arthritis, other arthritides, degenerative intervertebral disc disease and chronic skin ulcers that occurs in diabetes mellitus and bed-ridden patients.
Owner:UNIV OF TENNESSEE RES FOUND
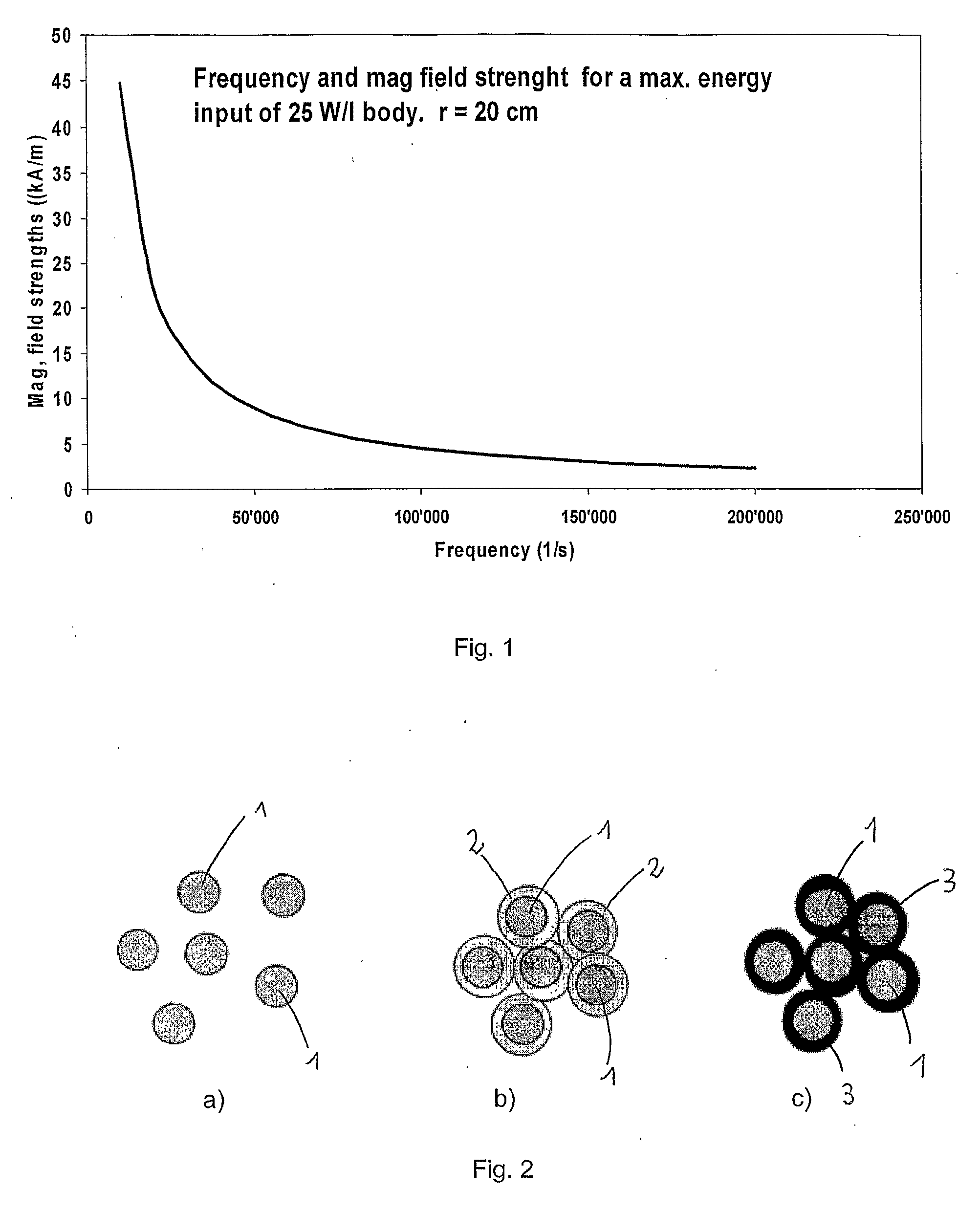
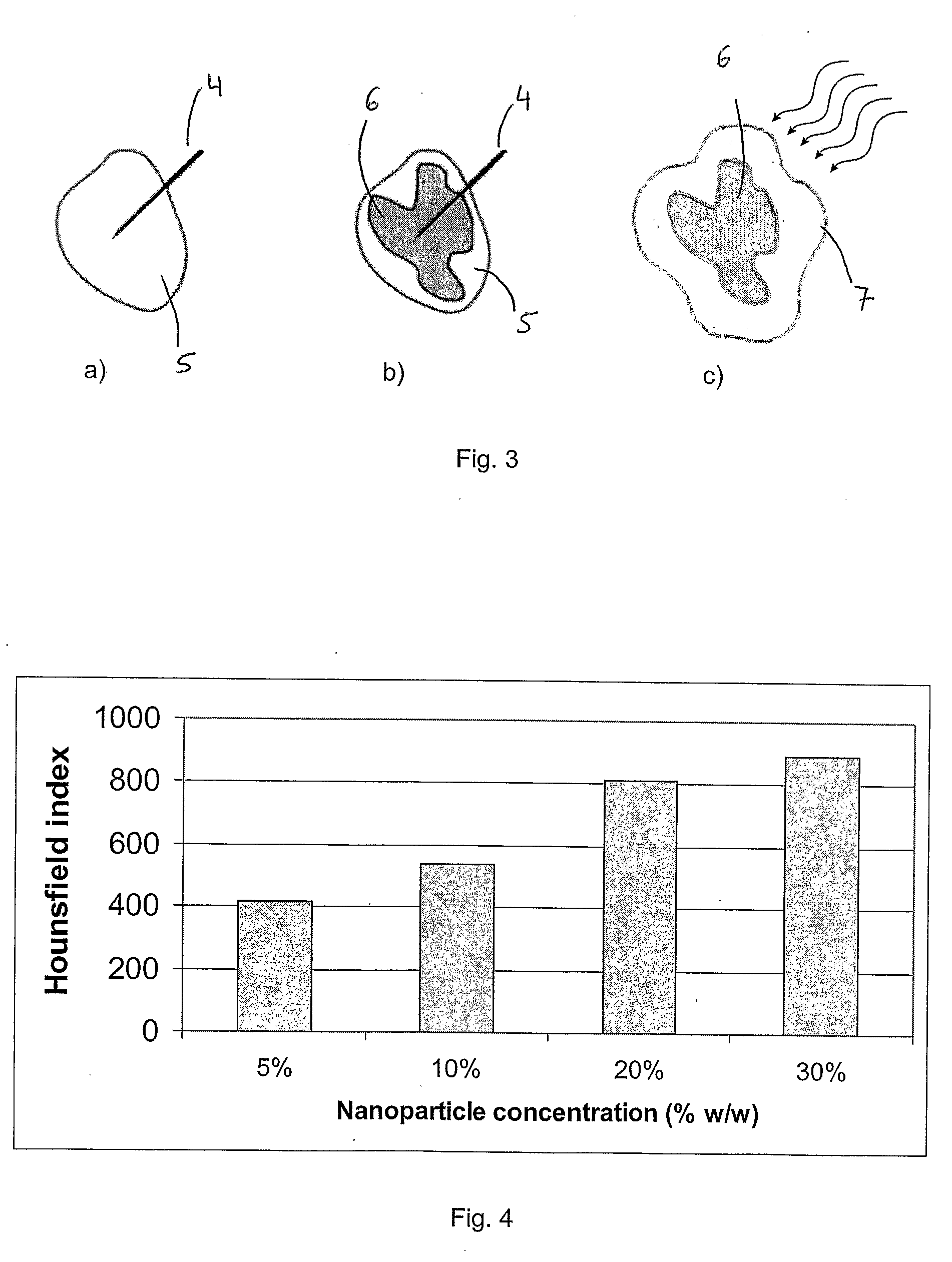
Injectable superparamagnetic nanoparticles for treatment by hyperthermia and use for forming an hyperthermic implant
The injectable formulation for treatment by hyperthermia comprises a liquid carrier and heat-generating superparamagnetic iron oxide nanoparticles having a mean diameter not greater than 20 nm. Said injectable formulation is able to form in-situ a hyperthermic solid or semi-solid implant upon contact with a body fluid or tissue. Said hyperthermic solid or semi-solid implant may be useful for treating a tumor or a degenerative disc disease by hyperthermia.
Owner:UNIVERSITY OF GENEVA +2
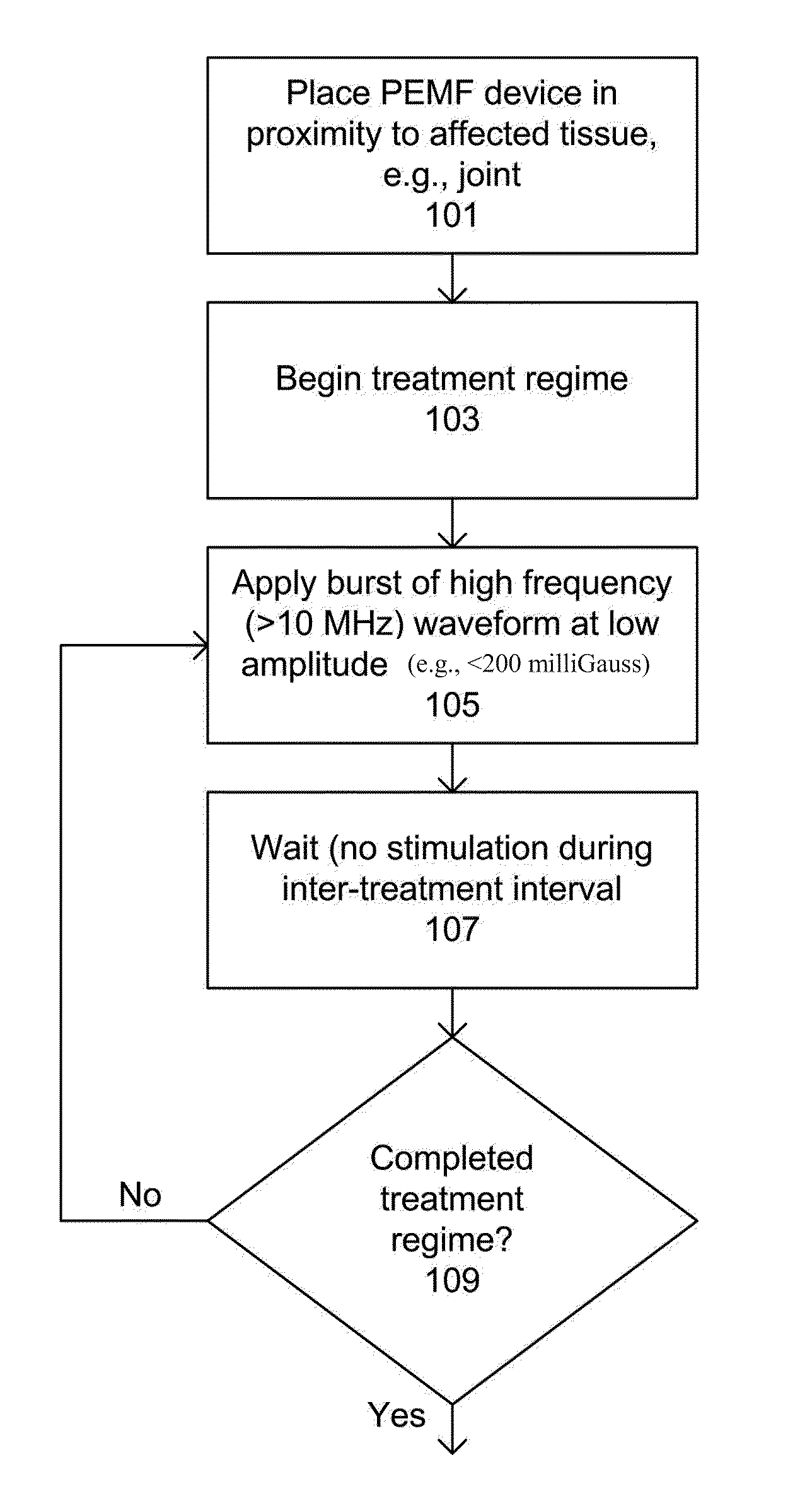
Devices and method for treatment of degenerative joint diseases with electromagnetic fields
InactiveUS20110207989A1Reduce pro-inflammatory cytokine pro-inflammatoryReduce pro-inflammatory other pro-inflammatory pathwayElectrotherapyMagnetotherapy using coils/electromagnetsDiseaseElectromagnetic field
Described herein are devices and methods for treating degenerative joint diseases with electromagnetic fields using one or more waveforms that are configured to modulate Ca2+ binding to calmodulin and thereby modulate calmodulin-dependent nitric oxide signaling within joint and other affected tissue for the purpose of reducing pain and inflammation, as well as enhancing the healing and regeneration of such tissue.
Owner:ENDONOVO THERAPEUTICS INC
Microstructured implant surfaces
An implantable device for treating disc degenerative disease and arthritis of the spine. The implant is sized for placement into an intravertebral disc space. The implant has a body with a predetermined, defined, repeating, three-dimensional pattern at least partially on at least one of its surfaces. The pattern is adapted to create a surface area of bone-contacting features that enhance in-growth and biological attachment to a biocompatible material. Also disclosed are process steps for making the implant.
Owner:TITAN SPINE
Devices and method for treatment of degenerative joint diseases with electromagnetic fields
InactiveUS8961385B2Enhance immune responseEnhanced signalElectrotherapyMagnetotherapy using coils/electromagnetsDiseaseElectromagnetic field
Described herein are devices and methods for treating degenerative joint diseases with electromagnetic fields using one or more waveforms that are configured to modulate Ca2+ binding to calmodulin and thereby modulate calmodulin-dependent nitric oxide signaling within joint and other affected tissue for the purpose of reducing pain and inflammation, as well as enhancing the healing and regeneration of such tissue.
Owner:ENDONOVO THERAPEUTICS INC
Treatment of degenerative joint disease
ActiveUS20130090292A1Improve throughputLimit deliverySkeletal disorderPharmaceutical delivery mechanismAmino acid side chainDiketopiperazines
The invention provides a method of treating a degenerative joint disease. The method comprises administering an effective amount of a pharmaceutical composition comprising a diketopiperazine with amino acid side chains of aspartic acid and alanine (DA-DKP). The invention also provides a pharmaceutical product as well as a kit comprising DA-DKP.
Owner:AMPIO PHARMA
Method of treating a subject suffering from degenerative disc disease using a matrix metalloprotease inhibitor
ActiveUS20070190149A1Effective treatmentRelieve symptomsPowder deliveryOrganic active ingredientsDegenerated intervertebral discMatrix metalloproteinase inhibitor
The present invention provides a method for treating a vertebrate subject suffering from a degenerative disc disease by administering an inhibitor of a matrix metalloprotease (MMP) to the subject in an amount effective to treat the subject.
Owner:DISCOGEN
Concentrated Protein Preparations of Bone Morphogenetic Proteins and Methods of Use Thereof
InactiveUS20100144631A1Efficient preparationHigh recovery rateSenses disorderNervous disorderDiseaseWhole body
Disclosed herein are heretofore undescribed preparations of highly concentrated, solubilized proteins, such as but not limited to, Bone Morphogenetic Proteins. Such protein preparations can be formulated in an aqueous carrier at protein concentrations in excess of 10 mg / ml when using the methods of manufacture taught herein. Such methods yield stable protein preparations in either solubilized or lyophilized form. The protein preparations of the present invention are particularly beneficial when administered either locally or systemically, in part, because low administration volumes can be accomplished. This is especially important for local treatment of certain anatomic locations such as, for example, the synovial fluid of a joint when treating osteoarthritis with BMP-7 or the intradiscal space when treating degenerative disc disease with BMP-7.
Owner:MARIEL THERAPEUTICS
Mixed porous structure interbody fusion cage and preparation method thereof
InactiveCN102440852AGood mechanical compatibilityGood bone conductionSpinal implantsFreeze-dryingReticular formation
Disclosed are a mixed porous structure interbody fusion cage and a preparation method thereof. The interbody fusion cage comprises a porous metal support and porous structure filling bodies, the porous metal support is a three-dimensional net-shaped structure, a plurality of holes are arranged in the porous metal support, and the porous structure filling bodies are fully filled in the holes. The preparation method includes steps that the metal rapid forming technology is directly combined with the freeze drying technology, the porous metal support is manufactured via a structural design and the direct metal rapid forming technology, then uniformly mixed polymer gel or polymer / biological ceramic compound gel is poured in the porous metal support to realize freeze treatment, so that the porous structure filling bodies with the micropore feature are formed after freeze drying, and the mixed porous structure interbody fusion cage is obtained. Mechanical compatibility is good, contact area between the mixed porous structure interbody fusion cage and natural centrum is further increased, instant stability is good, fusion rate is improved, and the mixed porous structure interbody fusion cage and the preparation method thereof can be used for treating clinical degenerative disc diseases.
Owner:SHANGHAI JIAO TONG UNIV
Treatment of degenerative joint disease
ActiveUS20140256642A1Improve throughputLimit deliveryPharmaceutical delivery mechanismSkeletal disorderAmino acid side chainDiketopiperazines
The invention provides a method of treating a degenerative joint disease. The method comprises administering an effective amount of a pharmaceutical composition comprising a diketopiperazine with amino acid side chains of aspartic acid and alanine (DA-DKP). The invention also provides a pharmaceutical product as well as a kit comprising DA-DKP.
Owner:AMPIO PHARMA INC
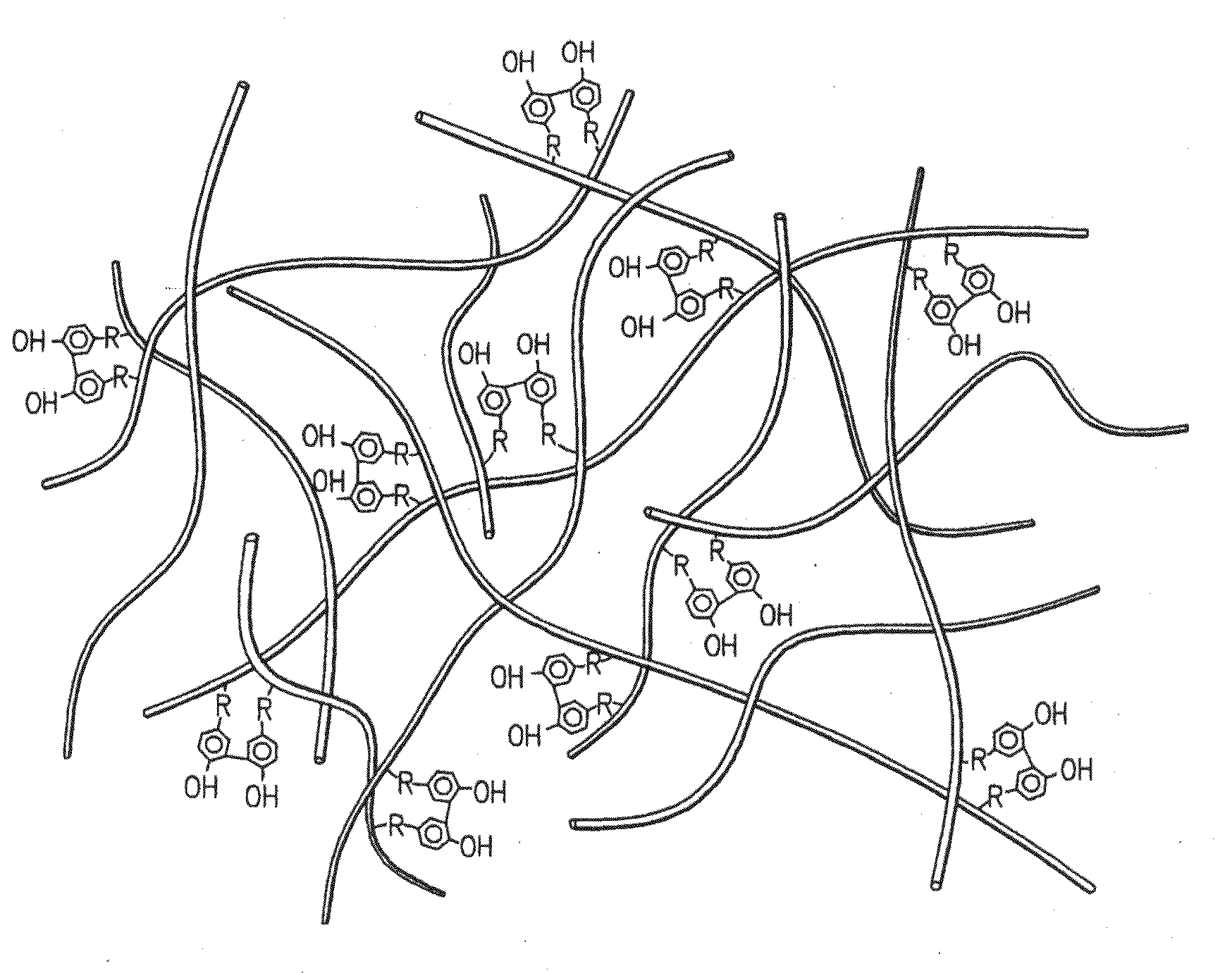
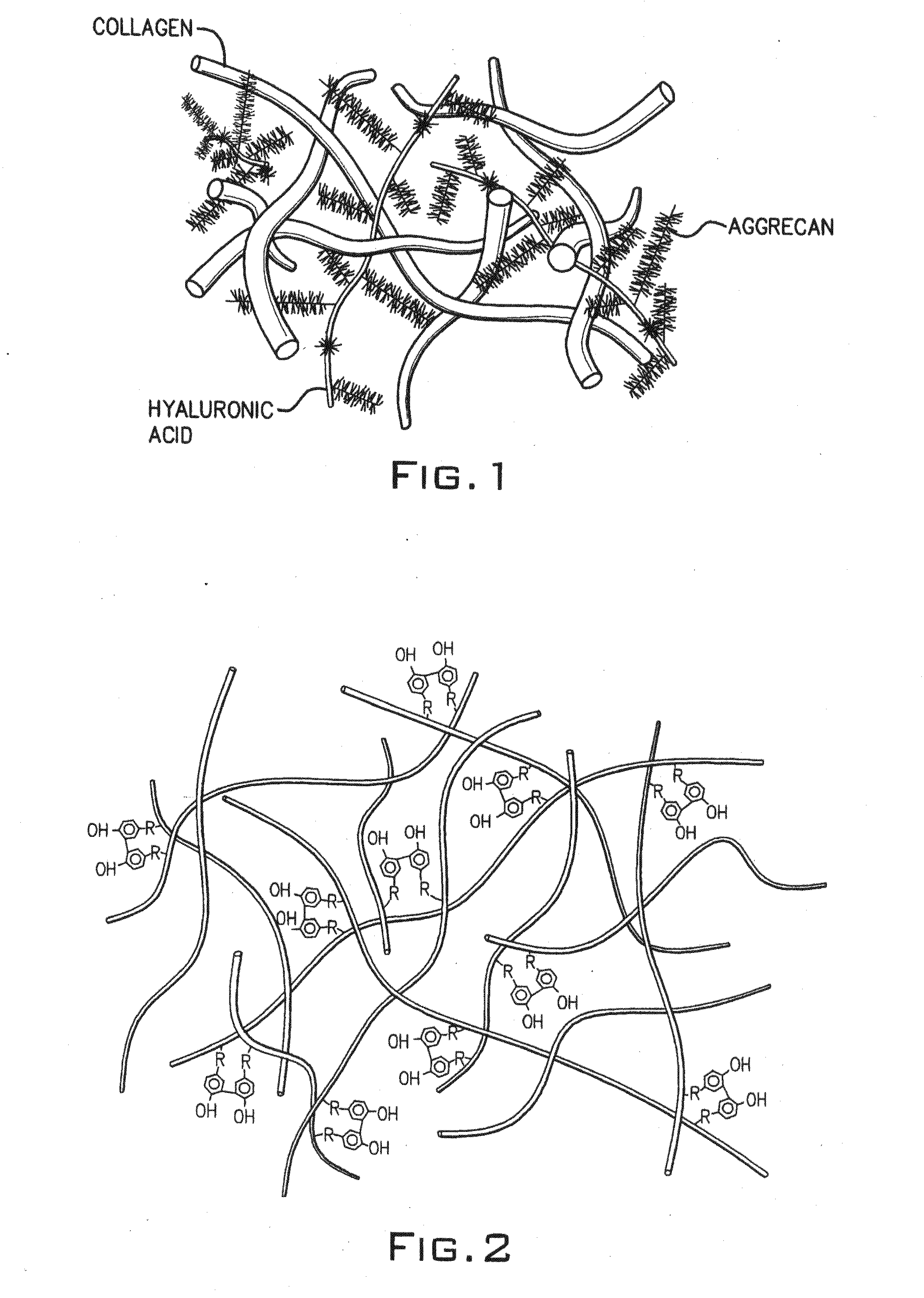
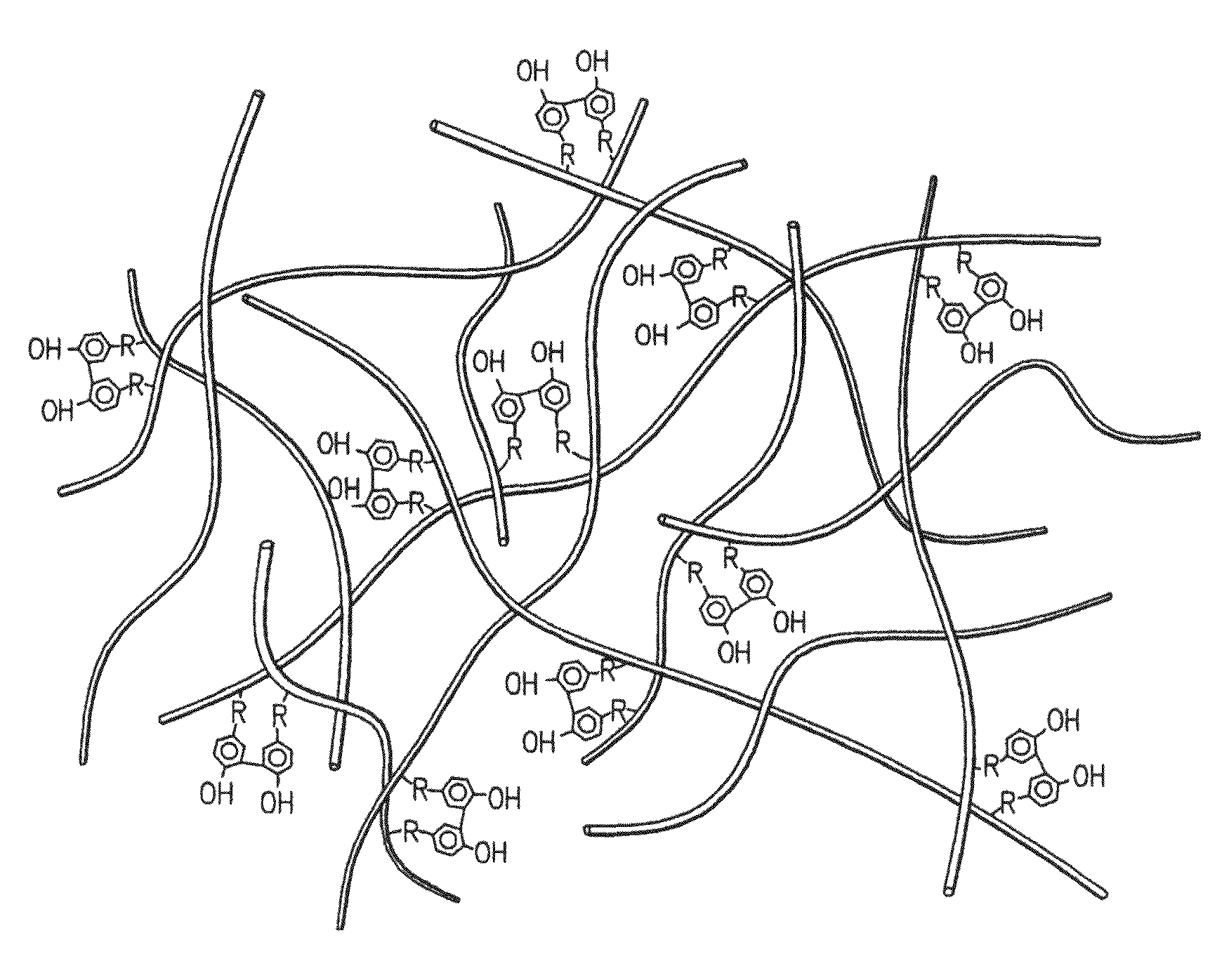
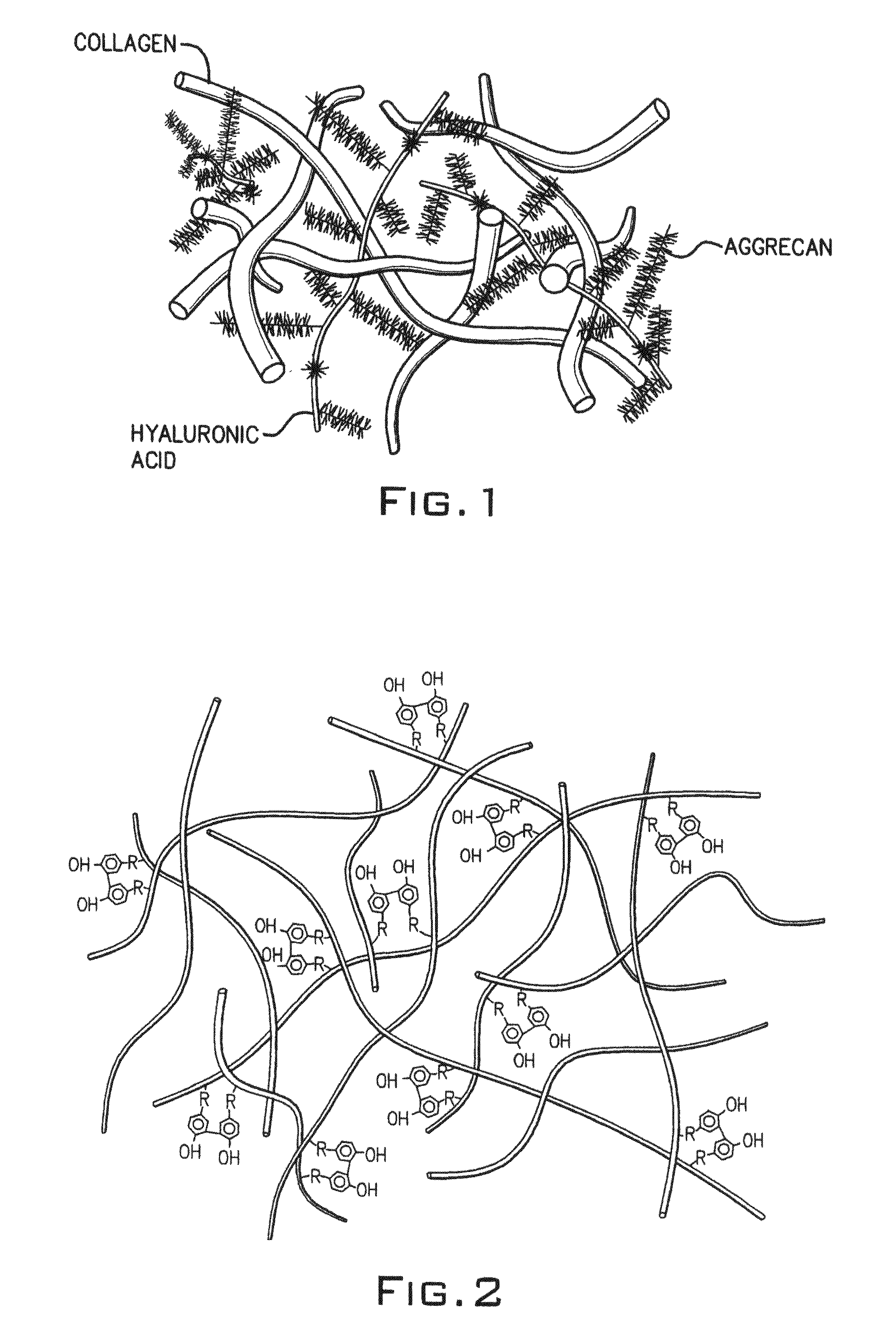
Hydroxyphenyl cross-linked macromolecular network and applications thereof
A synthetic nucleus pulposus is provided that is useful in treatment of degenerative disc disease, augmentation of a degenerate disc, and alleviation of back pain. In an embodiment the synthetic nucleus pulposus comprises hyaluronan macromolecules that have been cross-linked via dihydroxyphenyl linkages. The synthetic nucleus pulposus restores or improves the water-retention capability of the disc. A method of treating an intervertebral disc with the cross-linked hyaluronan macromolecules is also provided. A method of regenerative therapy to address loss of cells of nucleus pulposus of an intervertebral disc based on treatment with the cross-linked hyaluronan macromolecules and mesenchymal stem cells is also provided.
Owner:THE CLEVELAND CLINIC FOUND
Therapeutic Angiogenesis for Treatment of the Spine and Other Tissues
ActiveUS20130230454A1Lower pHDecreased blood flowUltrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsDiseaseSpine disorder
Methods for the diagnosis and treatment of ischemic spinal conditions, degenerative disc disease, back pain and / or other tissue pathologies. Patients with ischemic spine disease can be categorized into subsets that are deemed to have potential to respond to therapy. In particular, therapies are disclosed which involve stimulation of neovascularization so as to increase perfusion of spinal and other anatomies.
Owner:VENTURIS THERAPEUTICS INC
Interspinous distraction devices and associated methods of insertion
In various exemplary embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
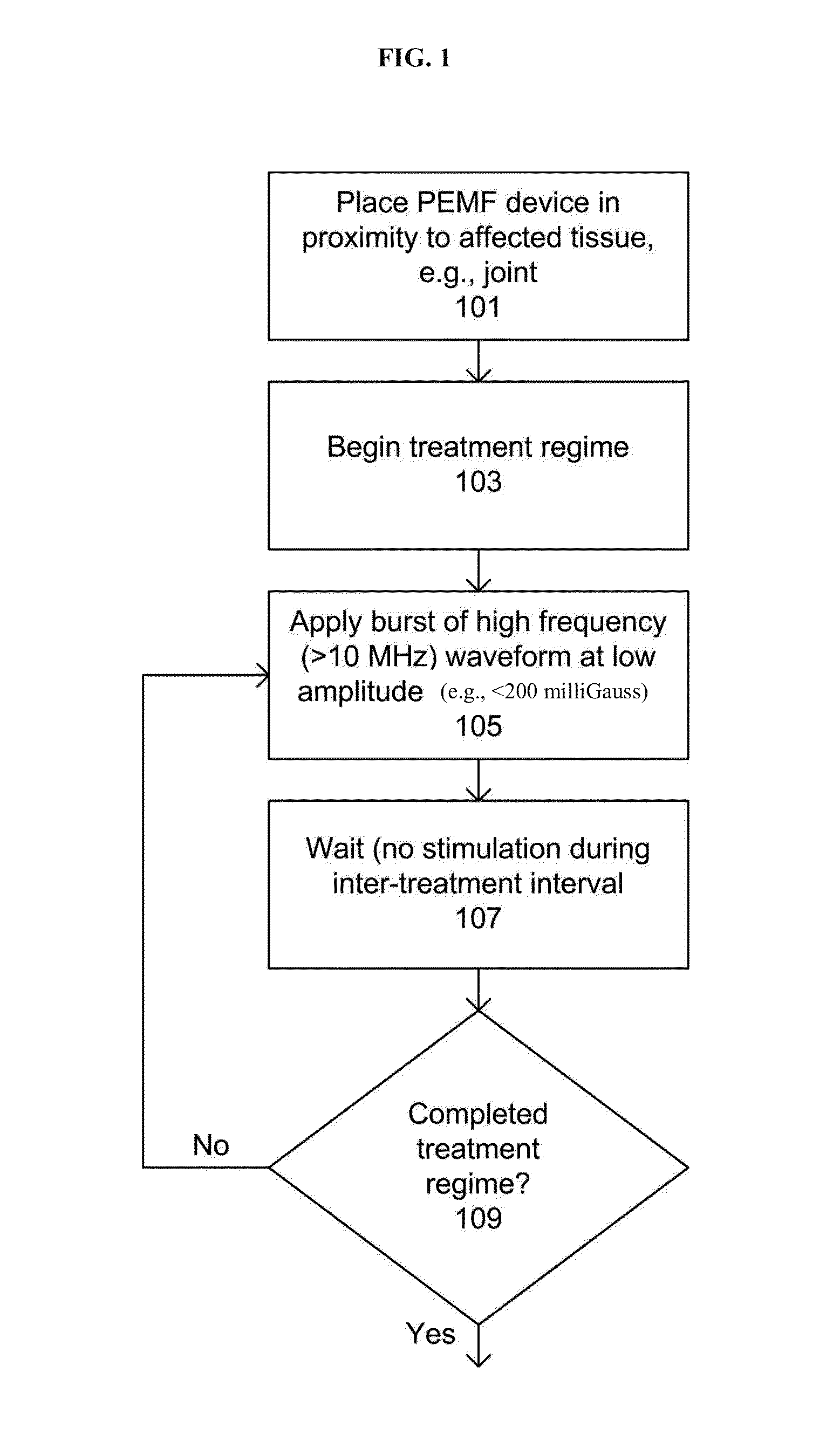
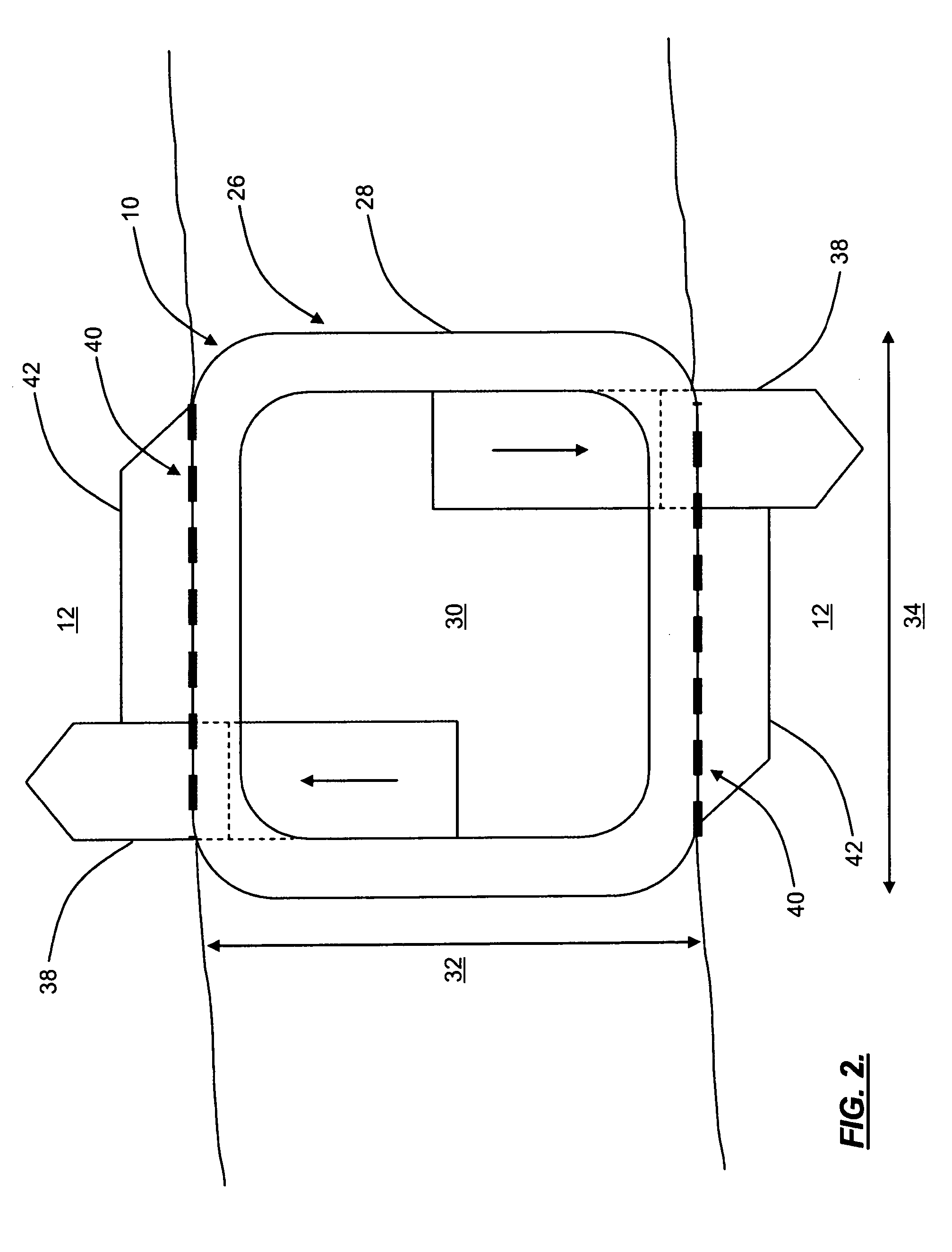
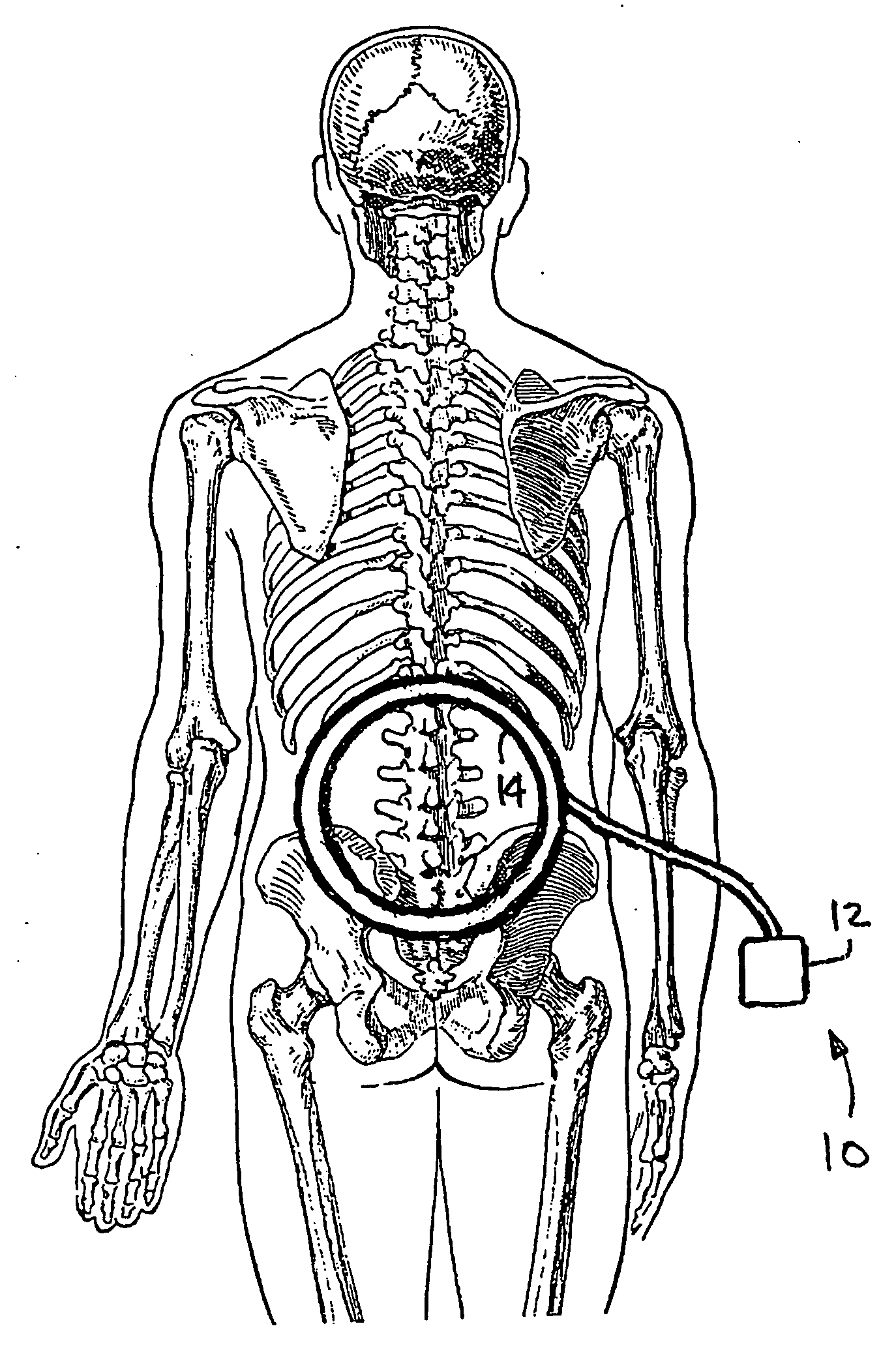
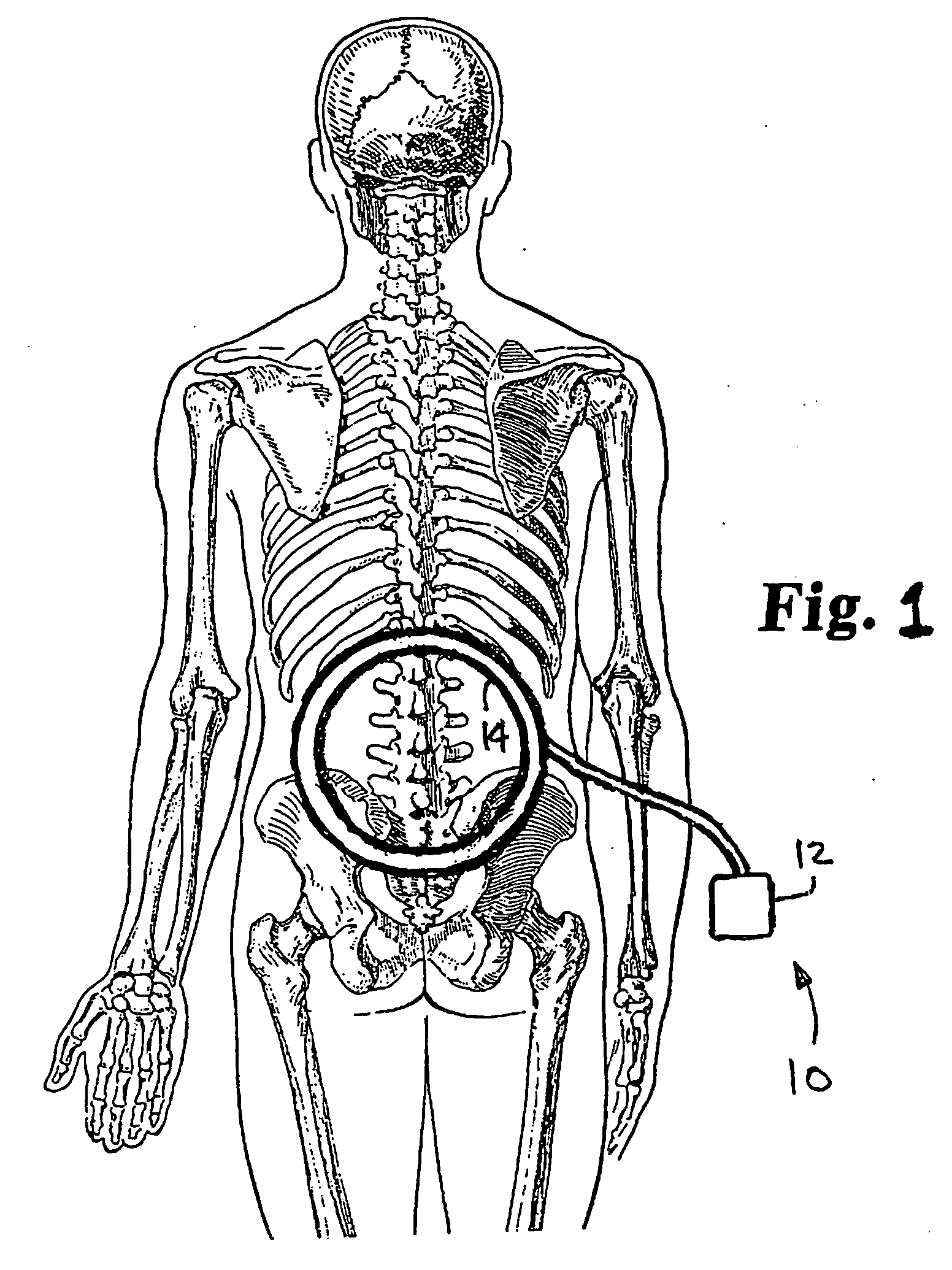
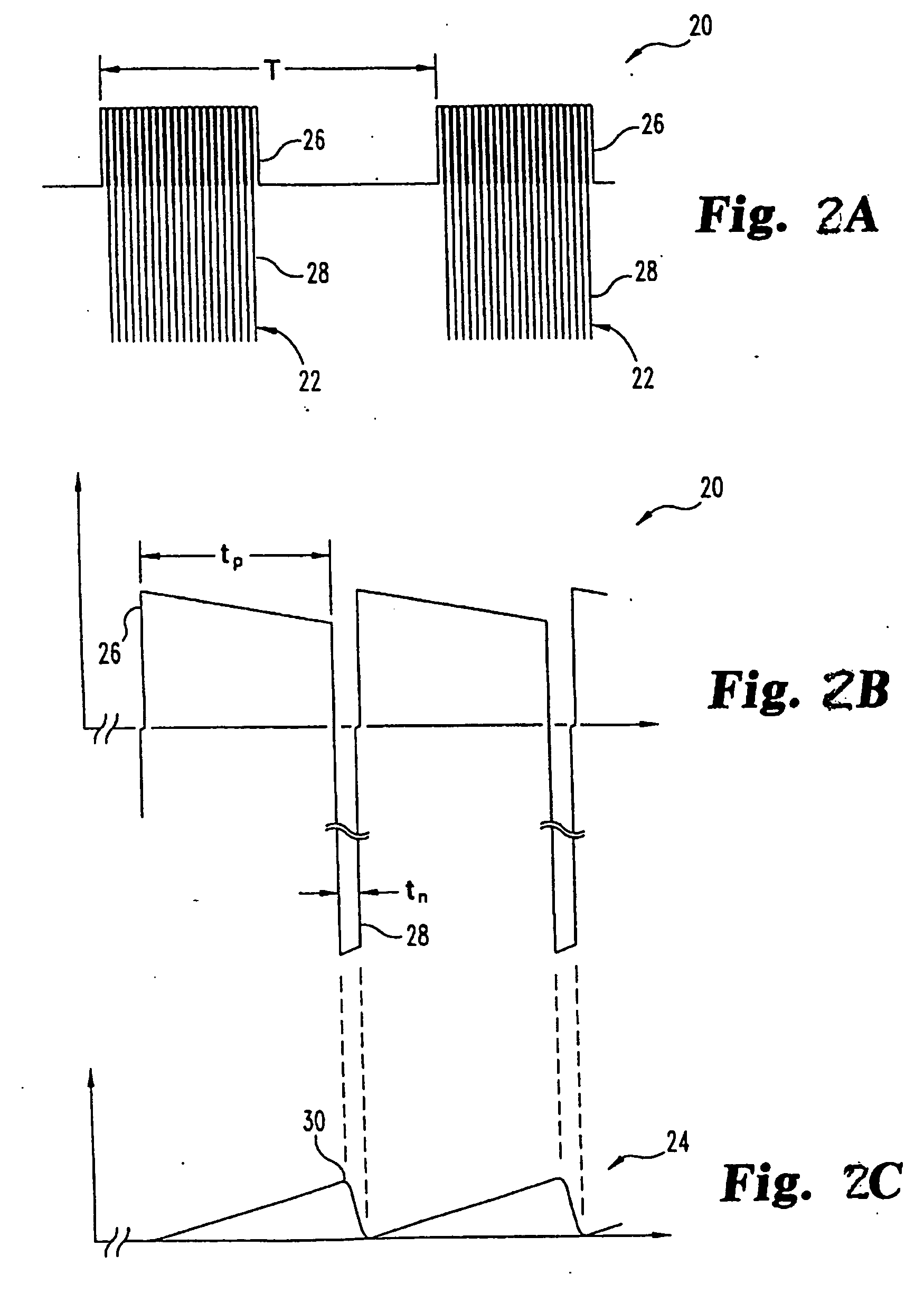
Pulsed electromagnetic field method of treatment of degenerative disc disease
A pulsed electromagnetic field method of treating degenerative disc disease, wherein, in one embodiment, a patient in need of treatment for degenerative disc disease is administered a pulsed electromagnetic field (PEMF) having repetitive pulse bursts approximately 5 ms in duration, with a pulse burst repetition rate of approximately 15 Hz.
Owner:EUROPEAN BIOINFORMATICS INSTITUTE
Hydroxyphenyl cross-linked macromolecular network and applications thereof
A synthetic nucleus pulposus is provided that is useful in treatment of degenerative disc disease, augmentation of a degenerate disc, and alleviation of back pain. In an embodiment the synthetic nucleus pulposus comprises hyaluronan macromolecules that have been cross-linked via dihydroxyphenyl linkages. The synthetic nucleus pulposus restores or improves the water-retention capability of the disc. A method of treating an intervertebral disc with the cross-linked hyaluronan macromolecules is also provided. A method of regenerative therapy to address loss of cells of nucleus pulposus of an intervertebral disc based on treatment with the cross-linked hyaluronan macromolecules and mesenchymal stem cells is also provided.
Owner:THE CLEVELAND CLINIC FOUND
Enhanced adipose tissue
InactiveUS20060018887A1Good treatment effectIncrease heightBiocidePeptide/protein ingredientsDiseaseActive agent
An enhanced adipose tissue containing native adipose tissue and a concentrated amount of an active agent derived from adipose tissue, wherein the enhanced adipose tissue is preferably injected into the intervertebral disc of a patient suffering from degenerative disc disease.
Owner:DEPUY SPINE INC (US) +1
Systems and methods for reducing adjacent level disc disease
InactiveUS20100069961A1Reduce chanceResisting compressionInternal osteosythesisJoint implantsDiseaseRange of motion
Owner:ZIMMER SPINE INC
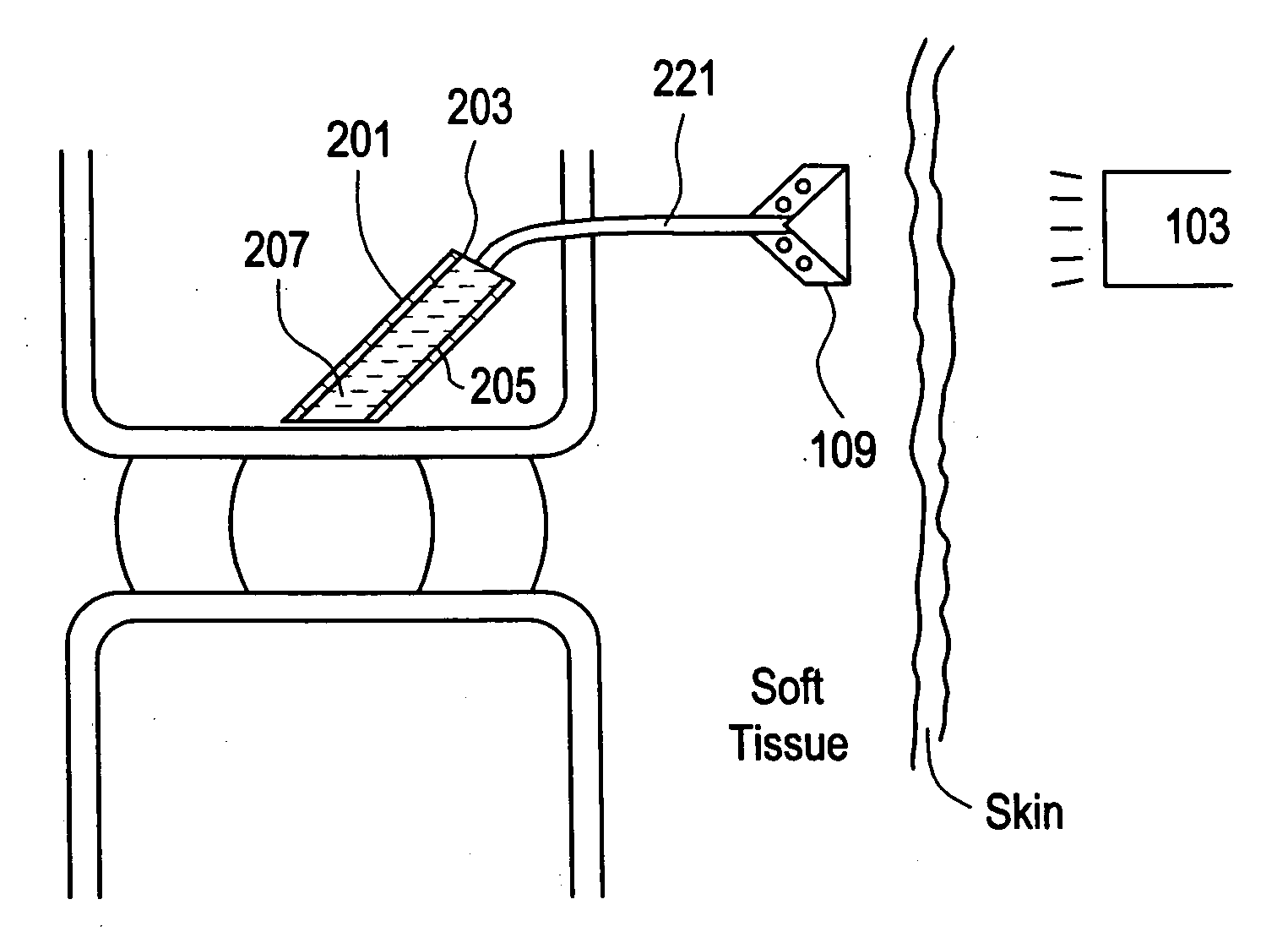
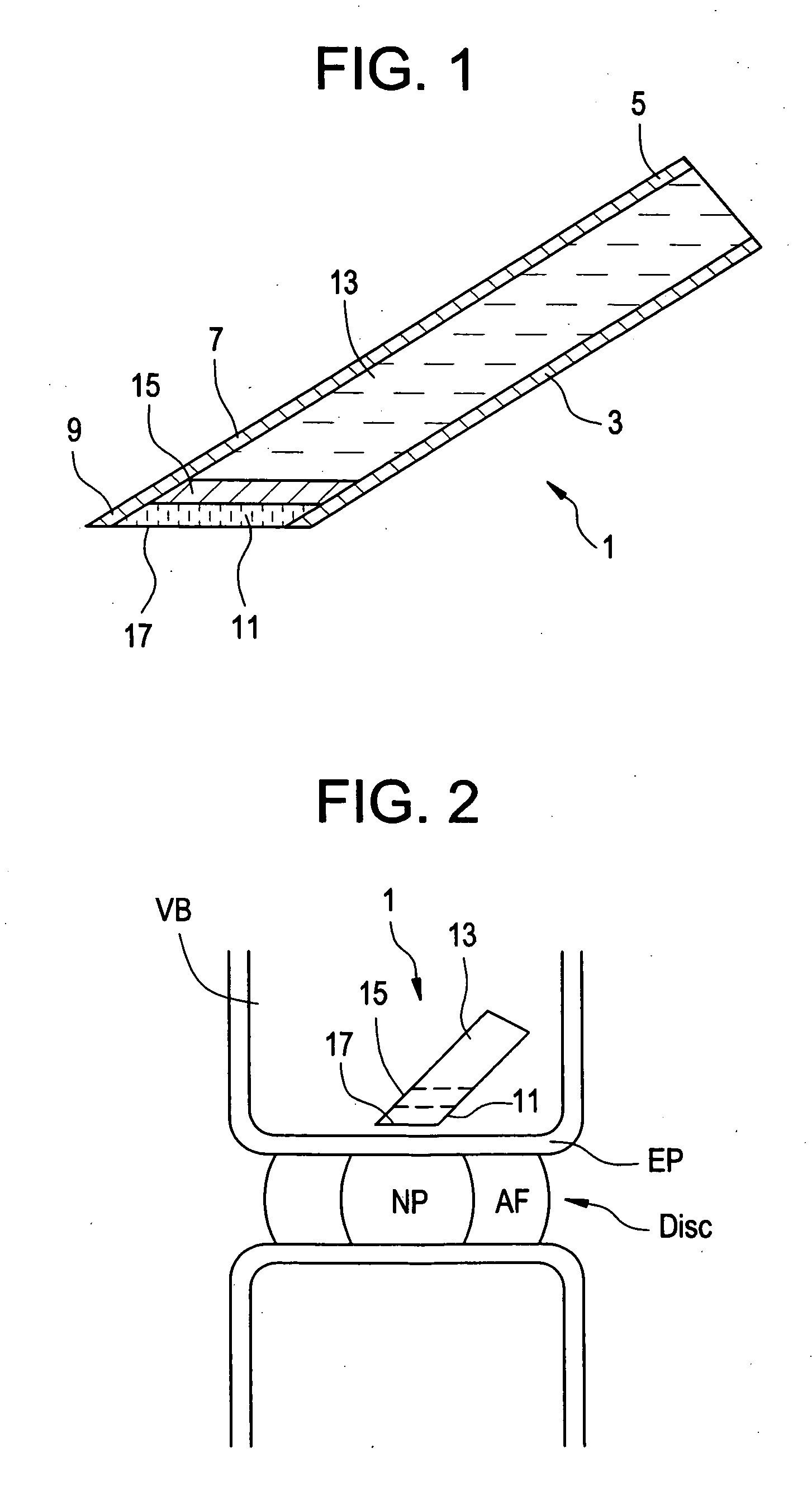
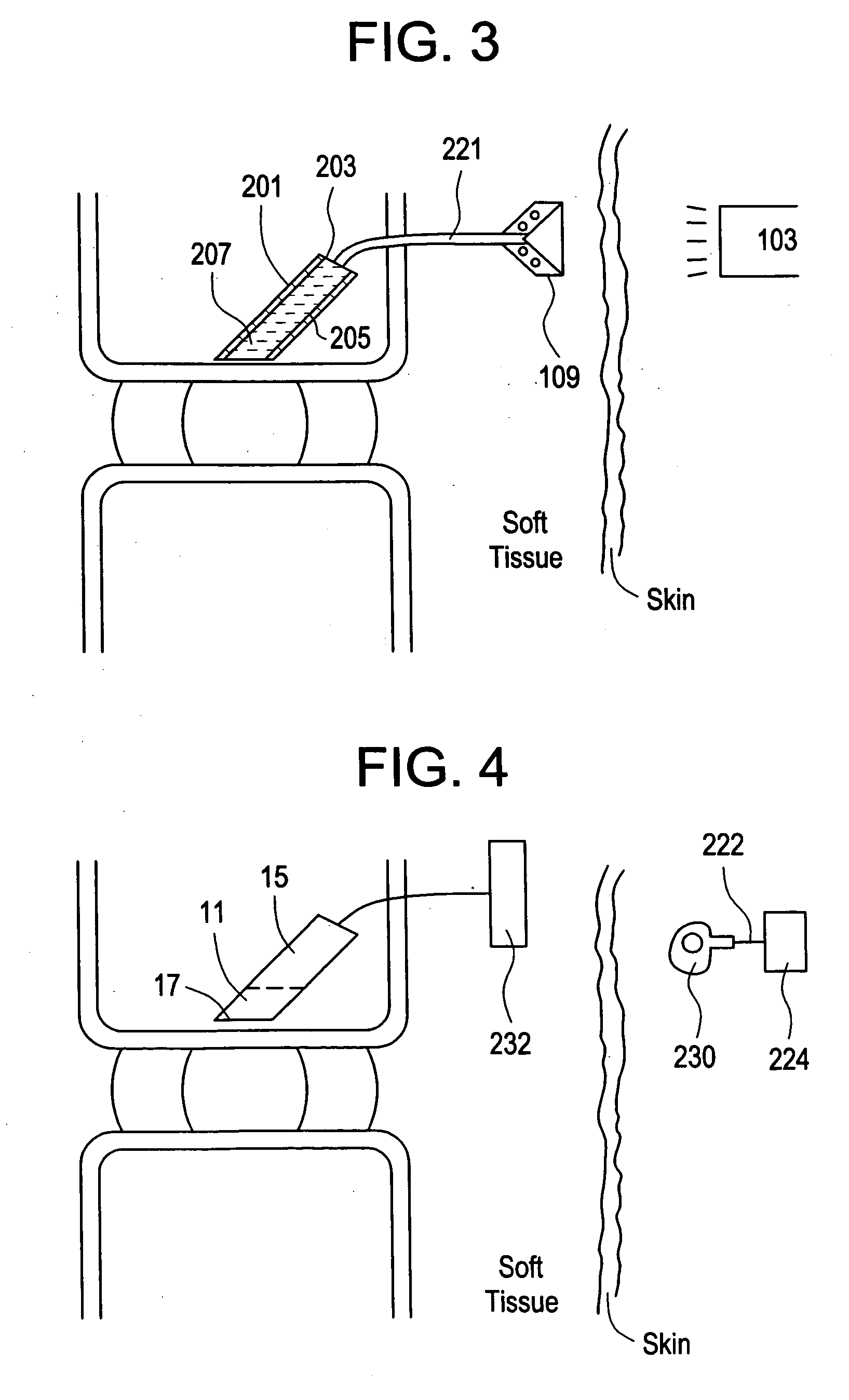
Red light implant for treating degenerative disc disease
ActiveUS20070073363A1Effective in cartilage repairUseful in therapyDiagnosticsSurgeryDiseaseDegenerated intervertebral disc
Owner:DEPUY SYNTHES PROD INC
Method for treating degenerative disc disease using noninvasive capacitively coupled electrical stimulation device
ActiveUS8082038B2Prevents further degenerationRestore mechanical propertiesElectrotherapyCapacitanceElectricity
A method for treatment of degenerative disc disease using capacitively coupled electrical stimulation. In one embodiment, a subject diagnosed as having degenerative disc disease is treated by placing first and second electrodes on the subject's body at the site of an identified disc in a state of degenerative disc disease, and applying an electric field to the identified disc via the first and second electrodes with the intent to treat the degenerative disc disease. The electric field is created with an electrical signal having a frequency within a range of 20 to 100 kHz and having a symmetrical waveform with an amplitude within a range of 0.1 to 20 volts peak-to-peak, preferably a frequency of approximately 60 kHz and an amplitude of approximately 5 volts peak-to-peak.
Owner:EUROPEAN BIOINFORMATICS INSTITUTE
Method for treatment of cartilage disorders with centella extract
The invention is a method for the treatment of mammalian articular cartilage disorders, inflammatory joint disease, trauma-related cartilage injuries, and degenerative disc disease. The method involves treating the affected area with a composition containing a therapeutically effective dose of Centella extract. The composition is delivered locally by parenteral administration to the affected site.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Enhanced adipose tissue
InactiveUS8715733B2Good treatment effectEnhanced adipose tissueBiocidePowder deliveryDiseaseActive agent
An enhanced adipose tissue containing native adipose tissue and a concentrated amount of an active agent derived from adipose tissue, wherein the enhanced adipose tissue is preferably injected into the intervertebral disc of a patient suffering from degenerative disc disease.
Owner:DEPUY SPINE INC (US) +1
Compositions of adult disc stem cells and methods for the treatment of degenerative disc disease
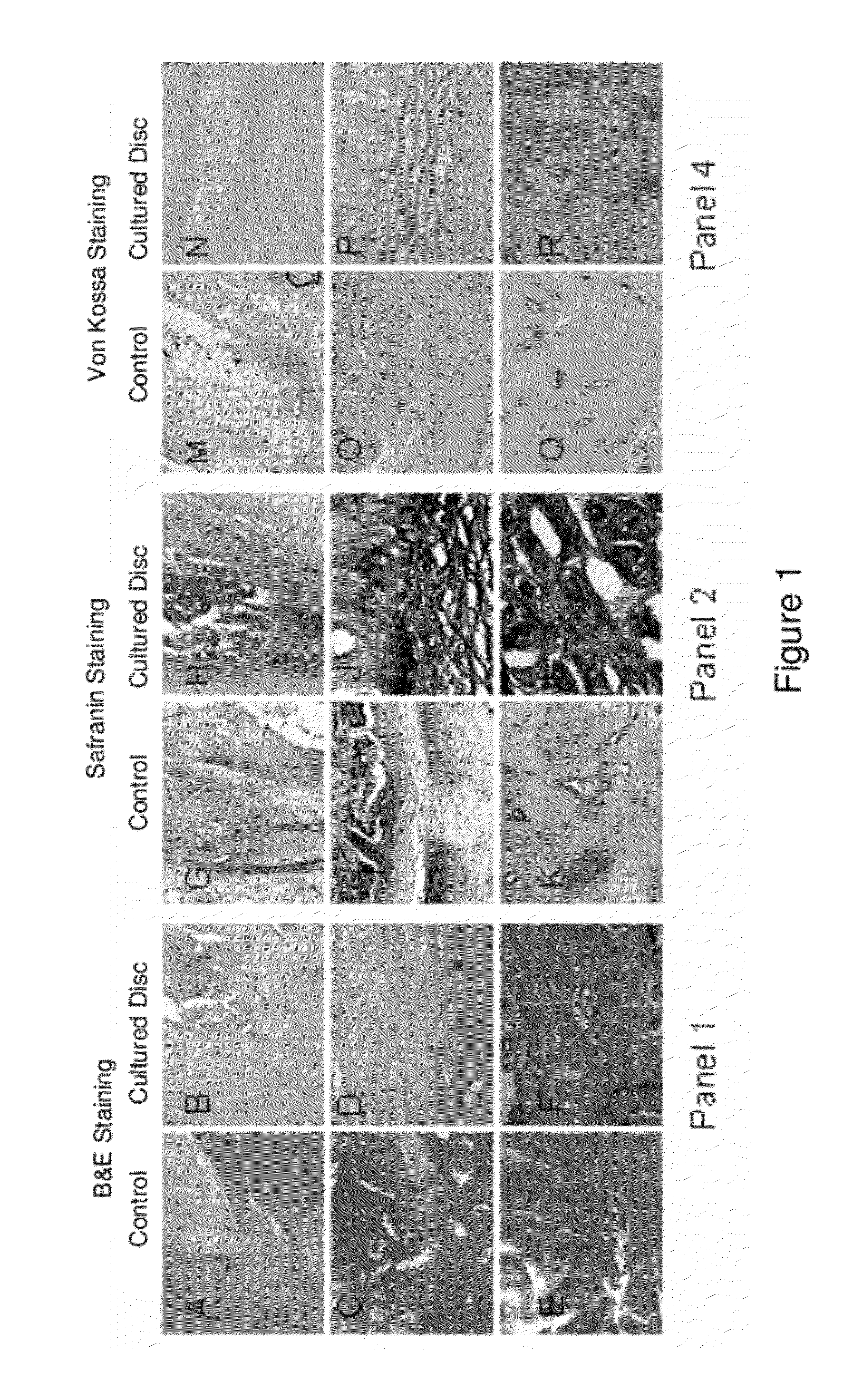
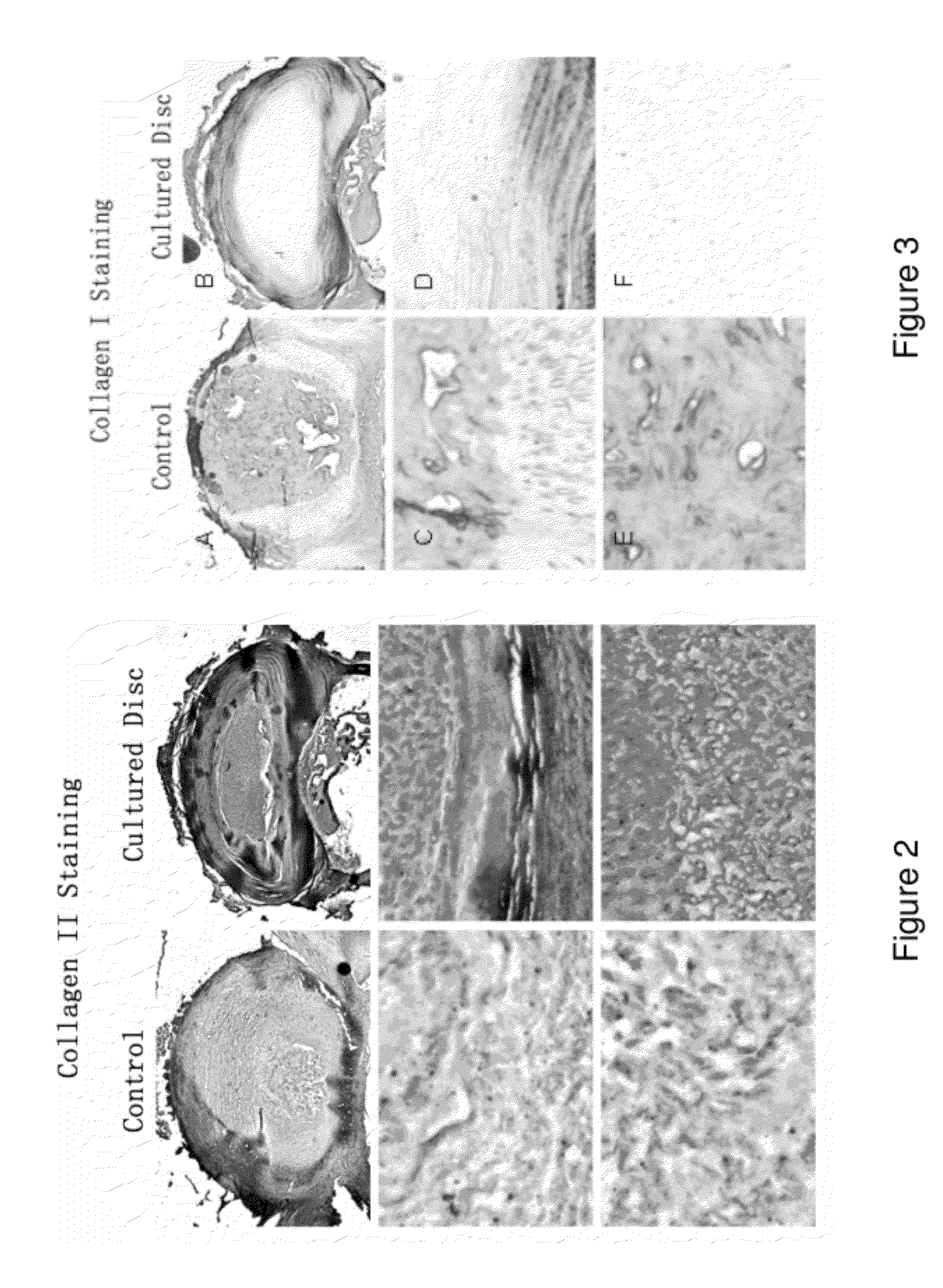
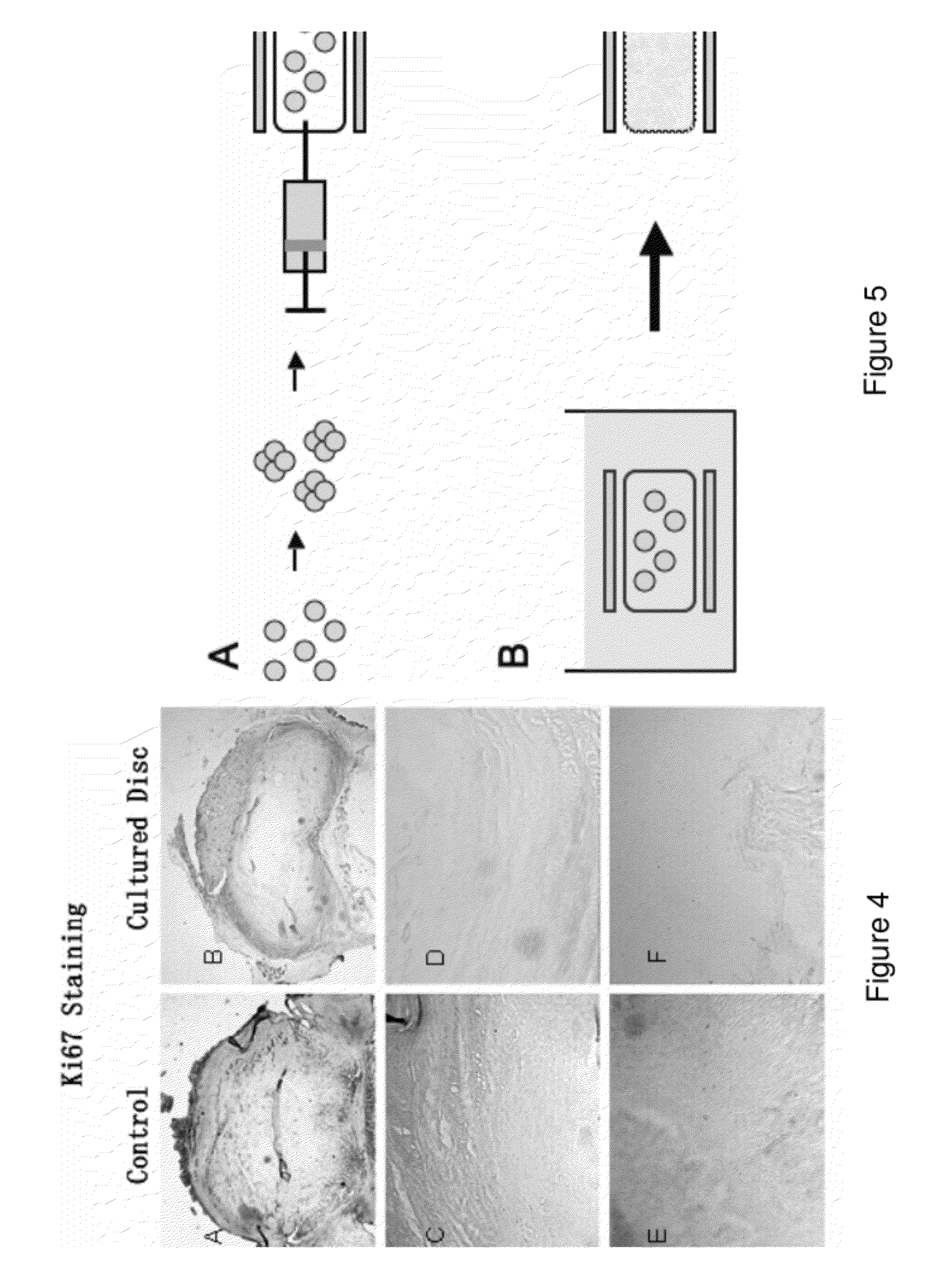
This invention provides a tissue growth apparatus comprising one or more discospheres and a method of producing nucleus pulposus cells comprising the step of growing one or more discospheres on the tissue growth apparatus. This invention also provides a neo-engineered disc comprising nucleus pulposus cells, and related methods of production and methods of use.
Owner:DISCGENICS
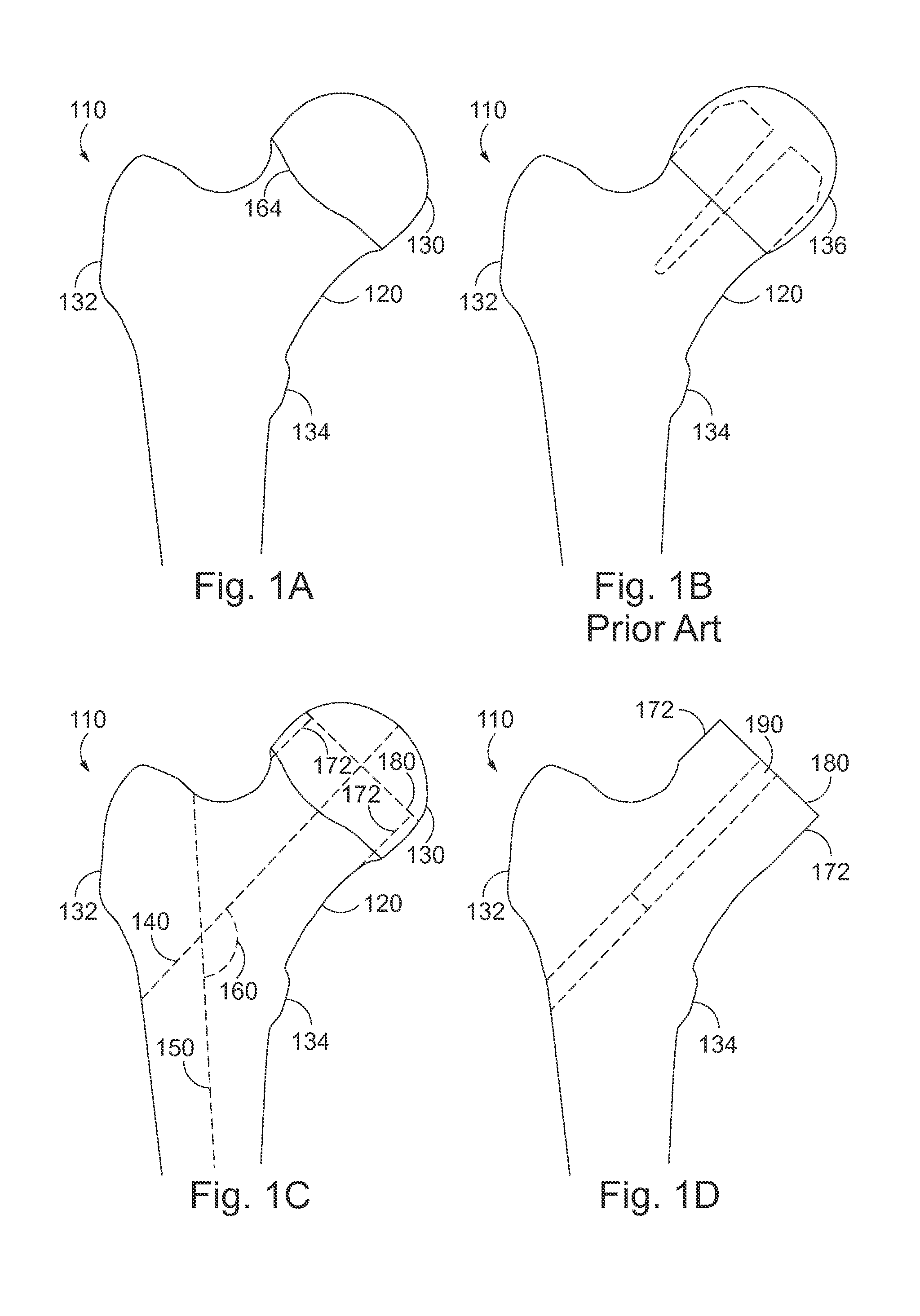
Variable hip resurfacing femoral implant
InactiveUS20160045320A1Improve structural strengthSupplement construct strengthSurgeryJoint implantsRight femoral headCoxal joint
A variable hip resurfacing implant which allows for varying configuration options to account for characteristics of underlying degenerative hip joint disease and for both un-cemented and cemented application. The variable hip resurfacing implant generally includes a femoral head portion, bearing surface, outer rim, inner planar surface, central stem, and a central stem threaded hole.
Owner:REVORTHO
Fibroblasts for treatment of degenerative disc disease
PendingUS20140314726A1Improving biomechanical functionImproving disc heightBiocideSkeletal disorderTissue repairBiomechanics
The present invention concerns methods and compositions for differentiating cells, including human fibroblasts, into chondrocyte-like cells via in vivo mechanical strain. In particular aspects, fibroblasts are delivered to a joint, such as an intervertebral disc, following which the fibroblasts differentiate into chondrocyte-like cells to treat dysfunction of cartilage therein, including to repair degenerated discs, for example. The fibroblasts that do not differentiate to chondrocytic cells because of the location of the cells, as in the fissures of annulus, or other biomechanical and biochemical micro-environment factors, may produce fibrous matrix molecule(s) in aiding tissue repair and regeneration in both nucleus pulposus and annulus fibrosus. In certain aspects, the fibroblasts prior to delivery to the individual are managed in the absence of growth factors, in vitro mechanical strain, and / or matrix molecules, for example.
Owner:SPINALCYTE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com