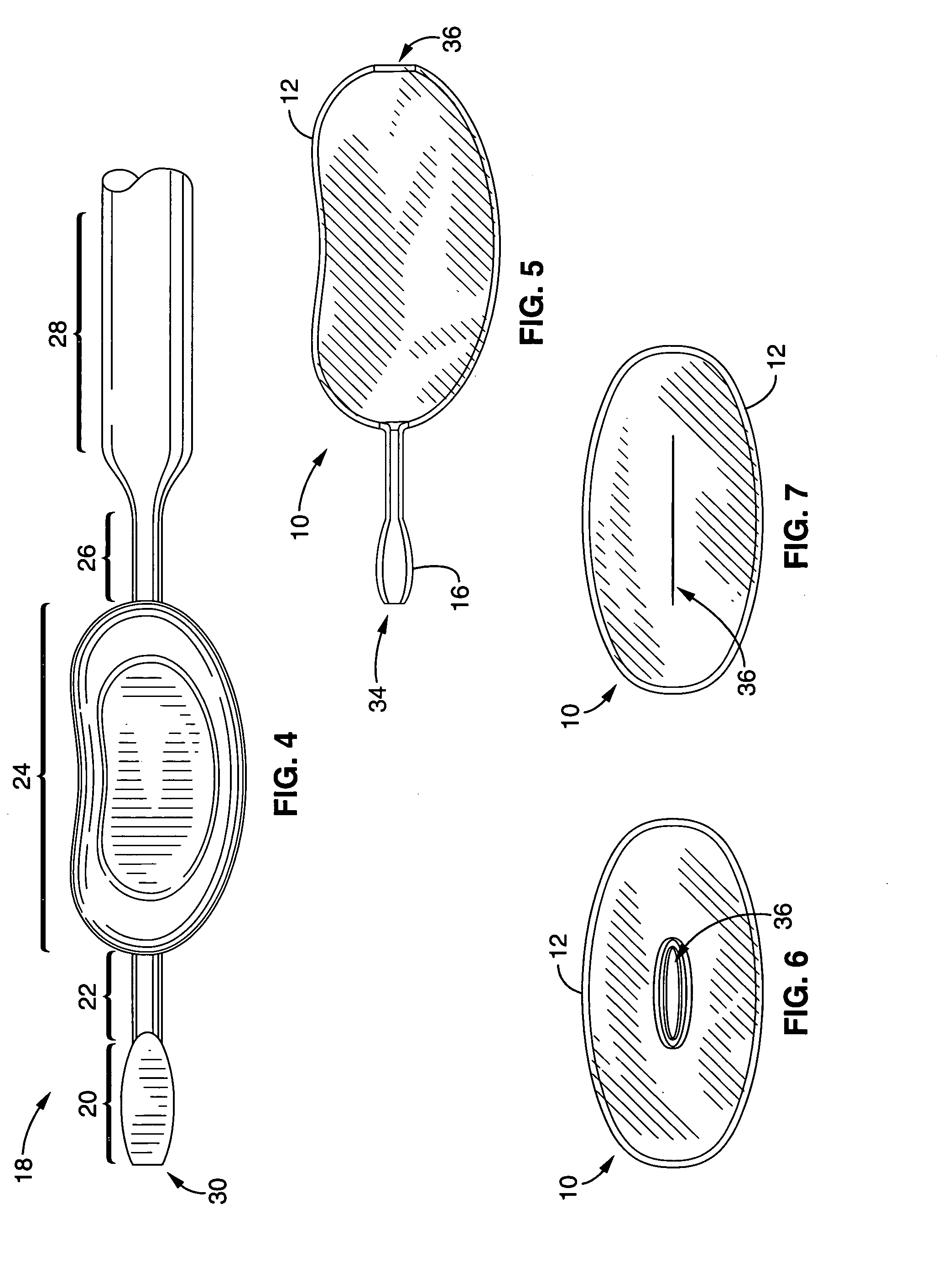
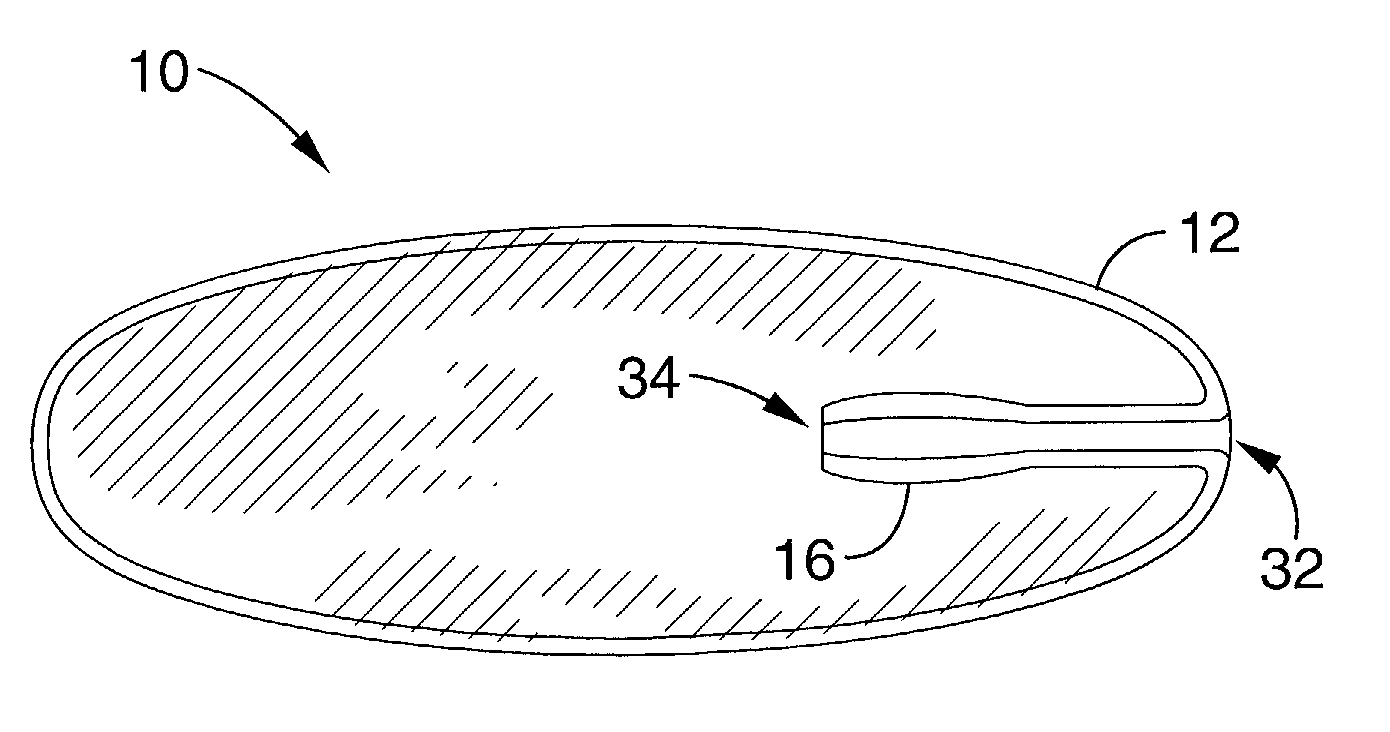
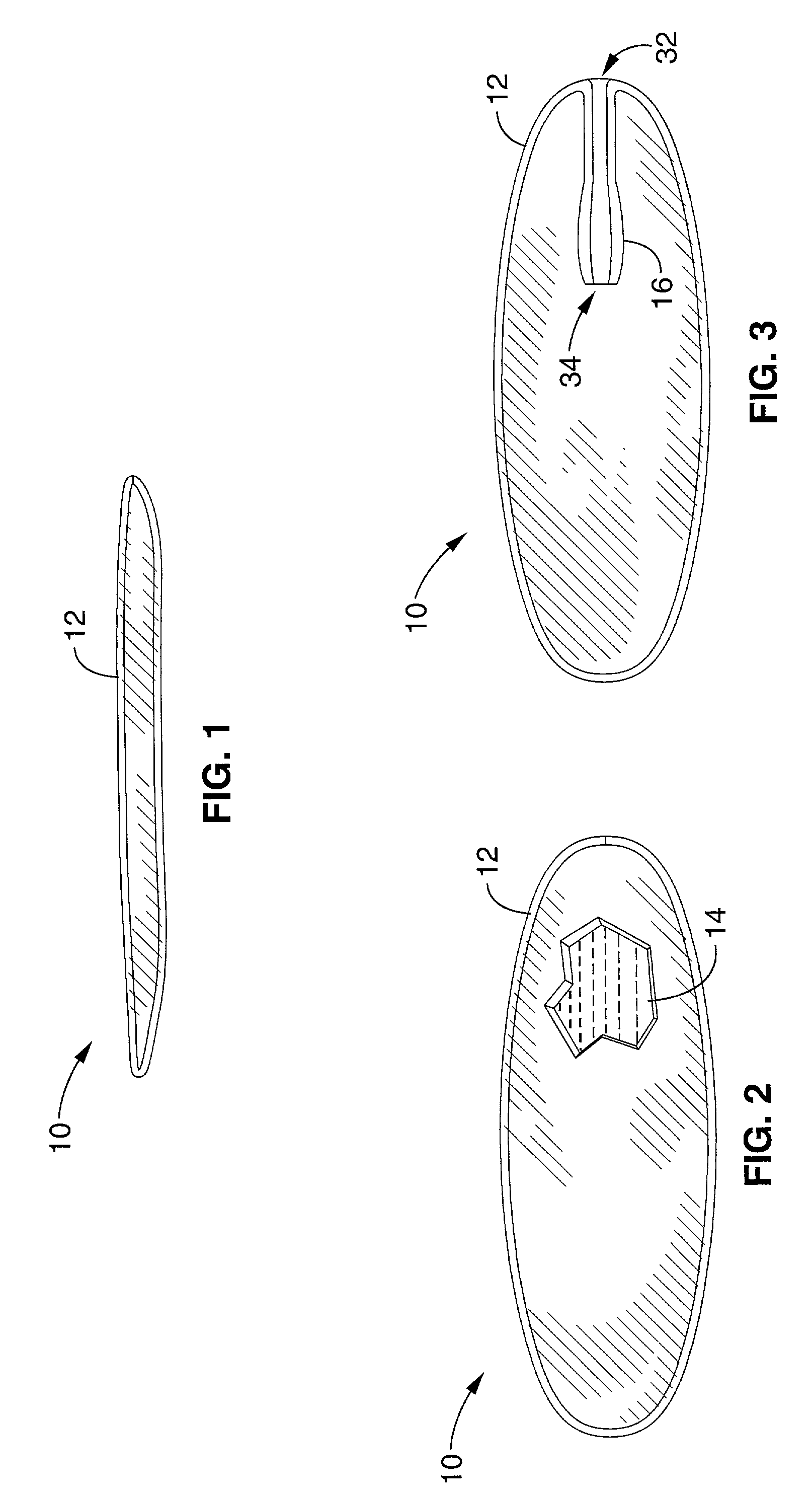
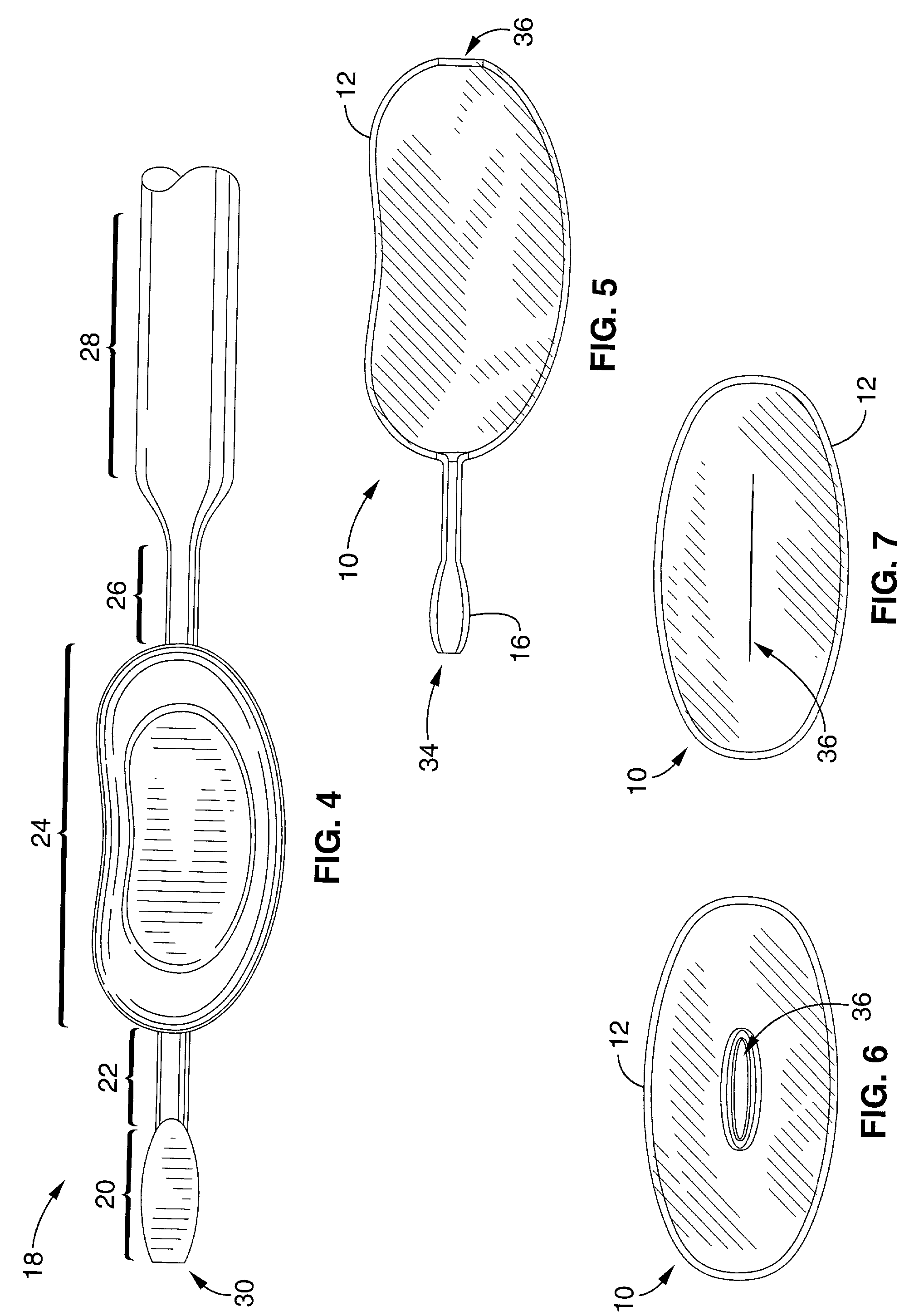
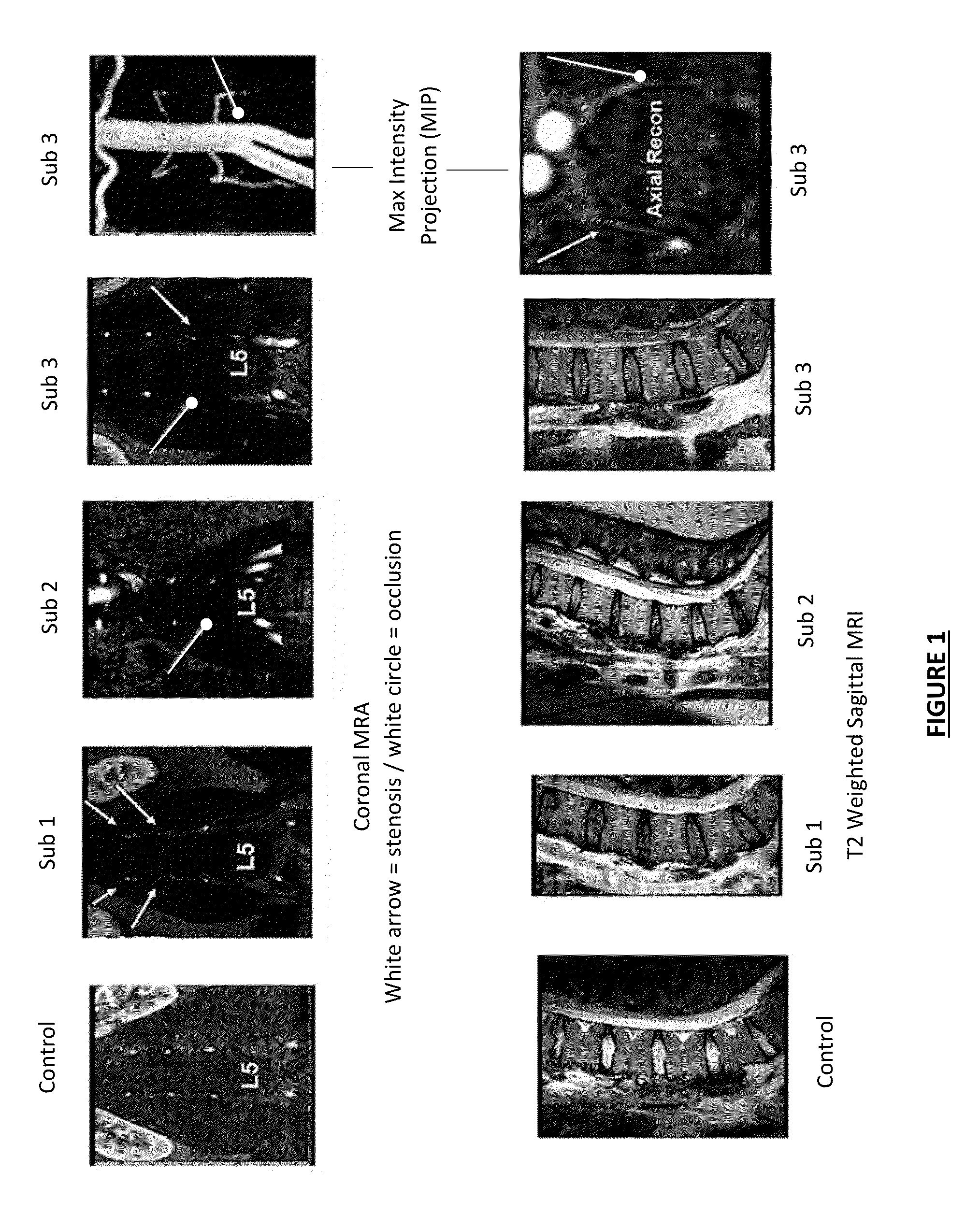
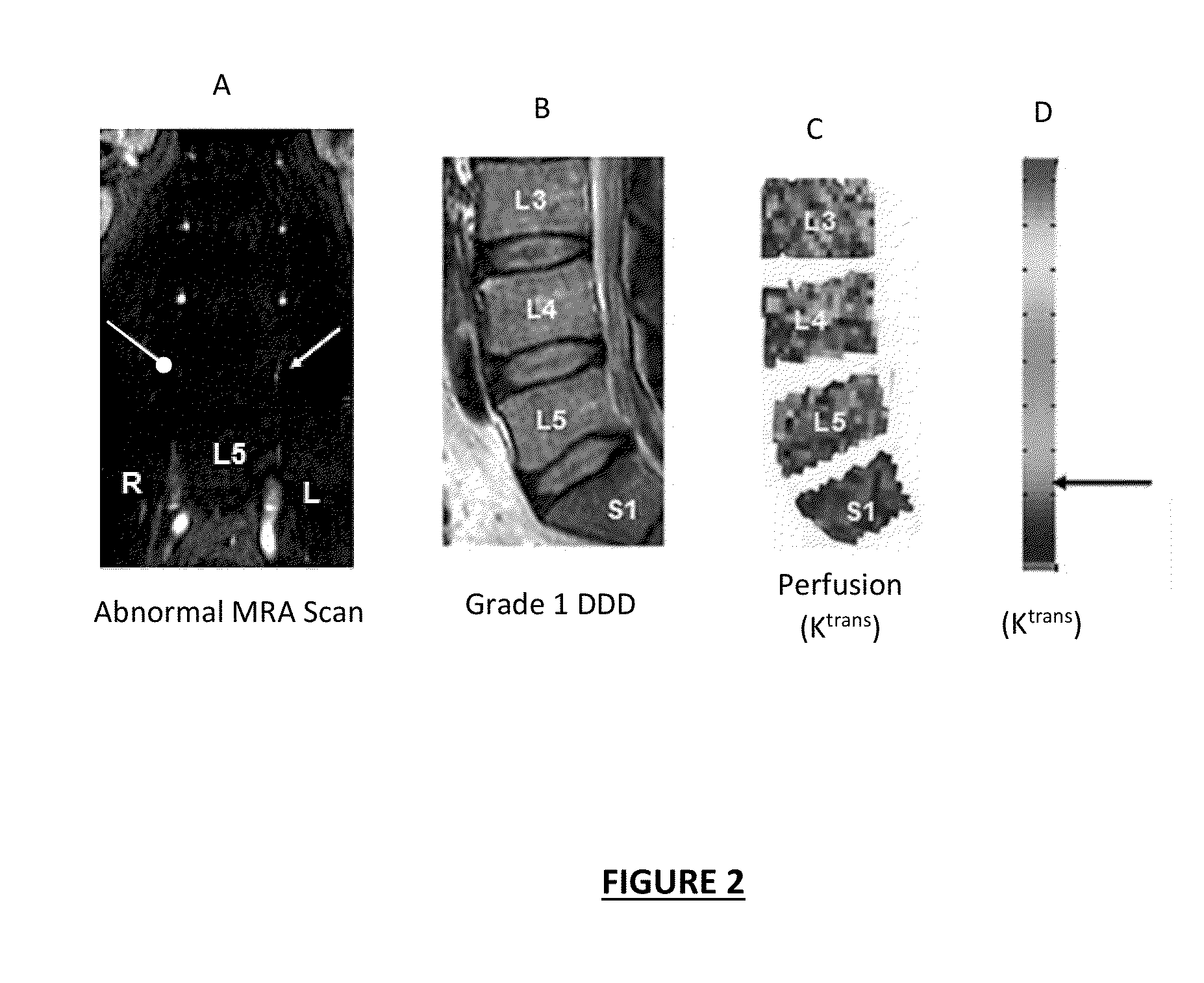
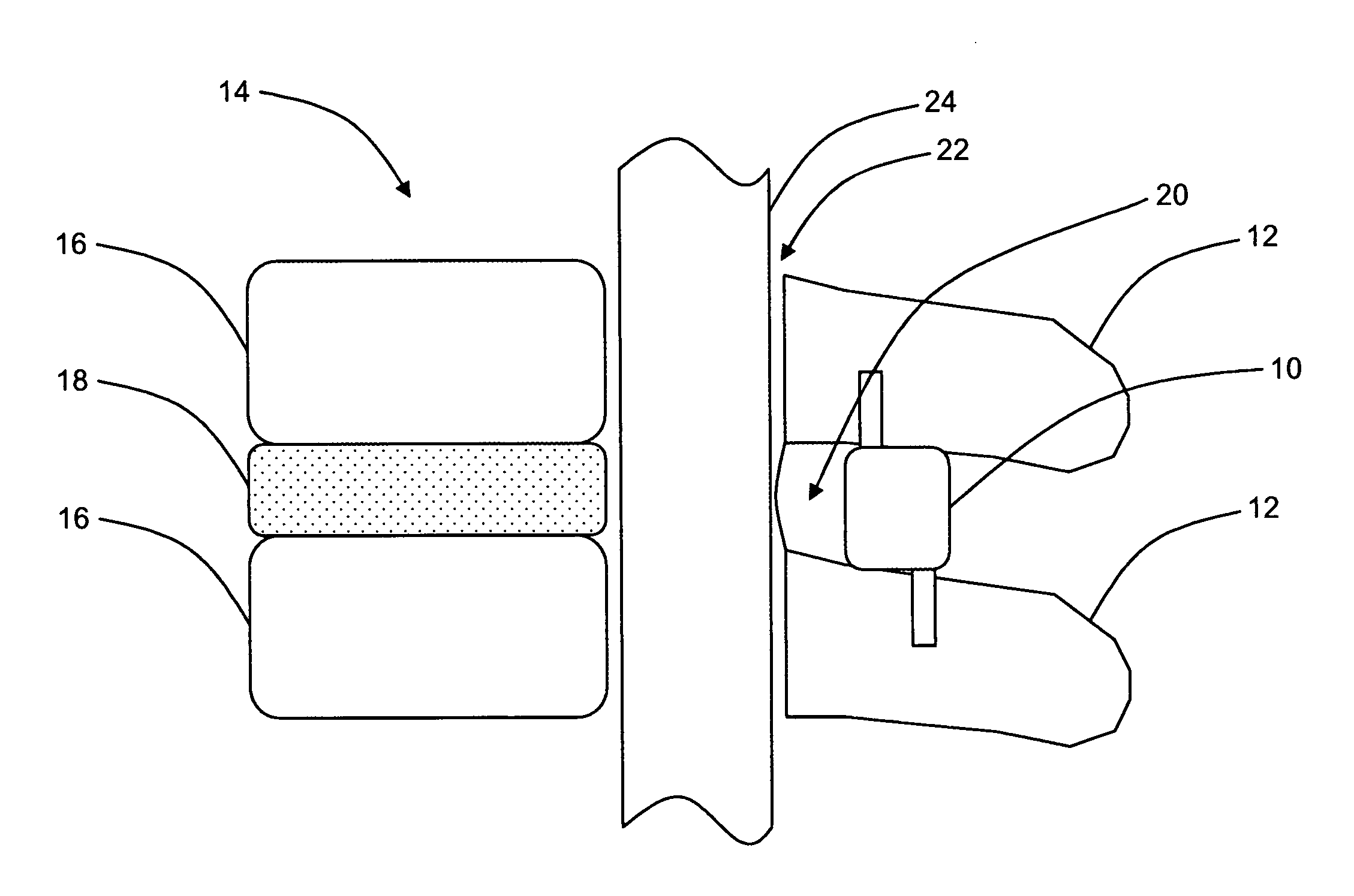
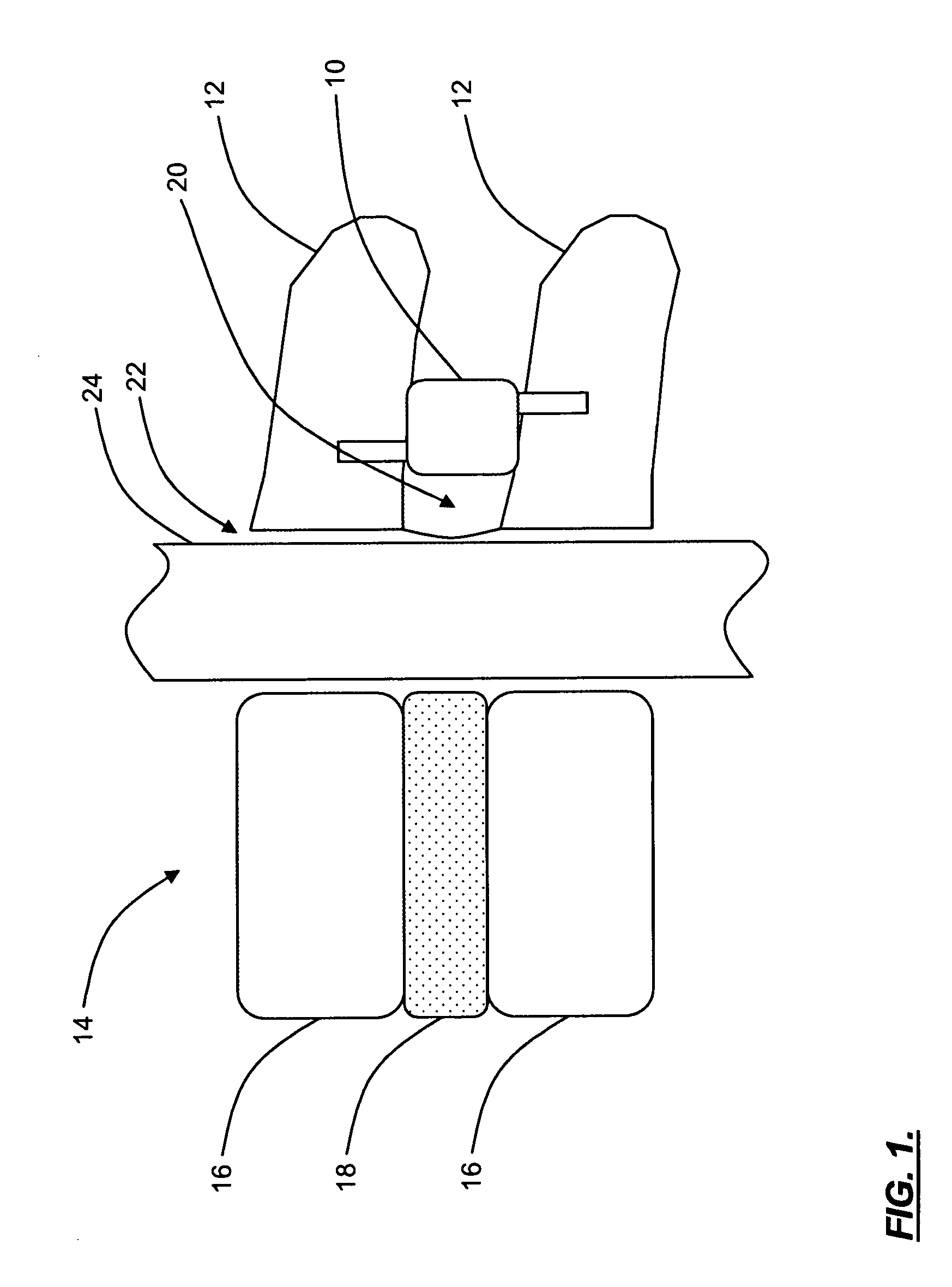
Patents
Literature
97 results about "Degenerated intervertebral disc" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intervertebral disc arthroplasty: also called Artificial Disc Replacement (ADR), or Total Disc Replacement (TDR), is a type of arthroplasty. It is a surgical procedure in which degenerated intervertebral discs in the spinal column are replaced with artificial ones in the lumbar (lower) or cervical (upper) spine.
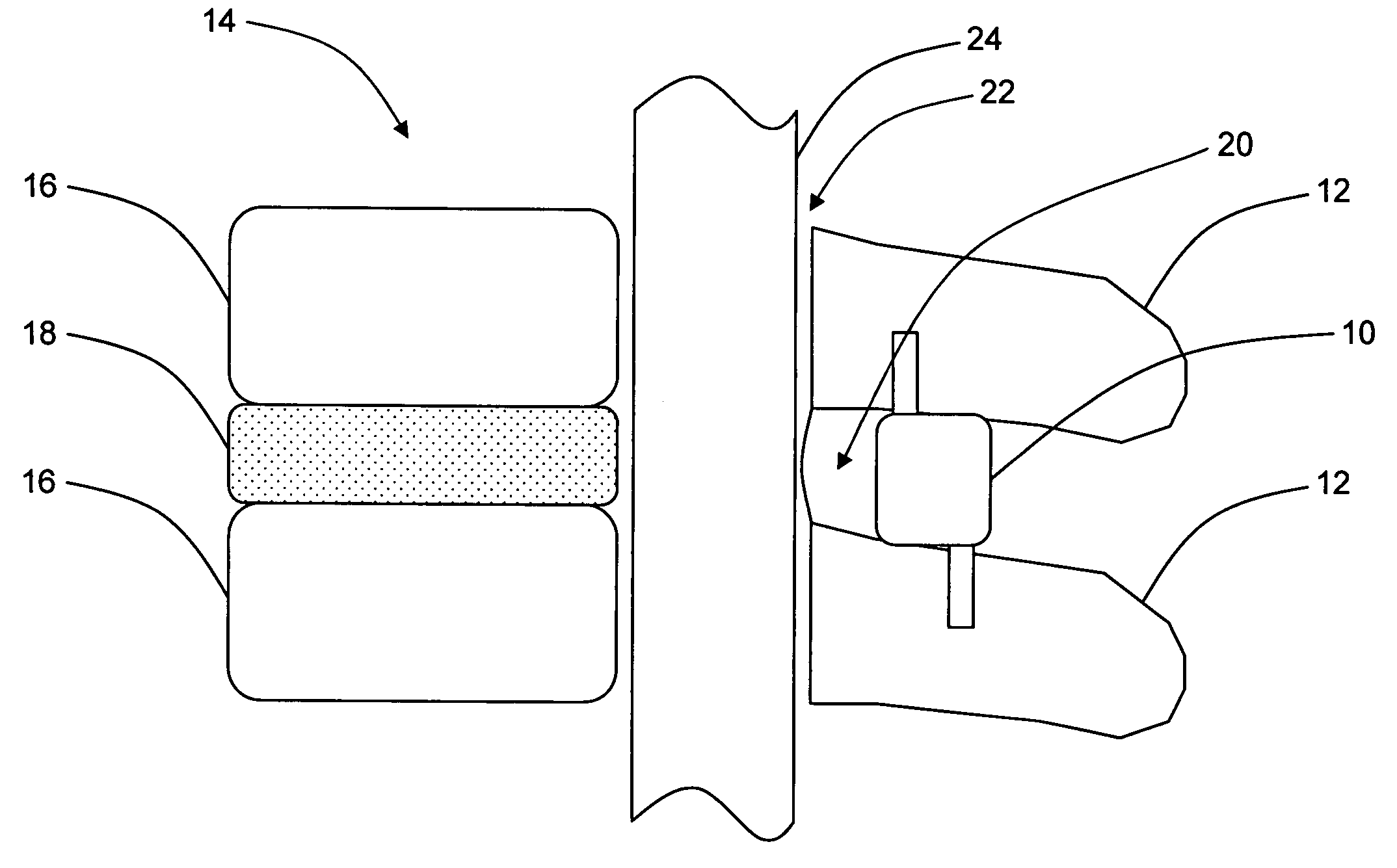
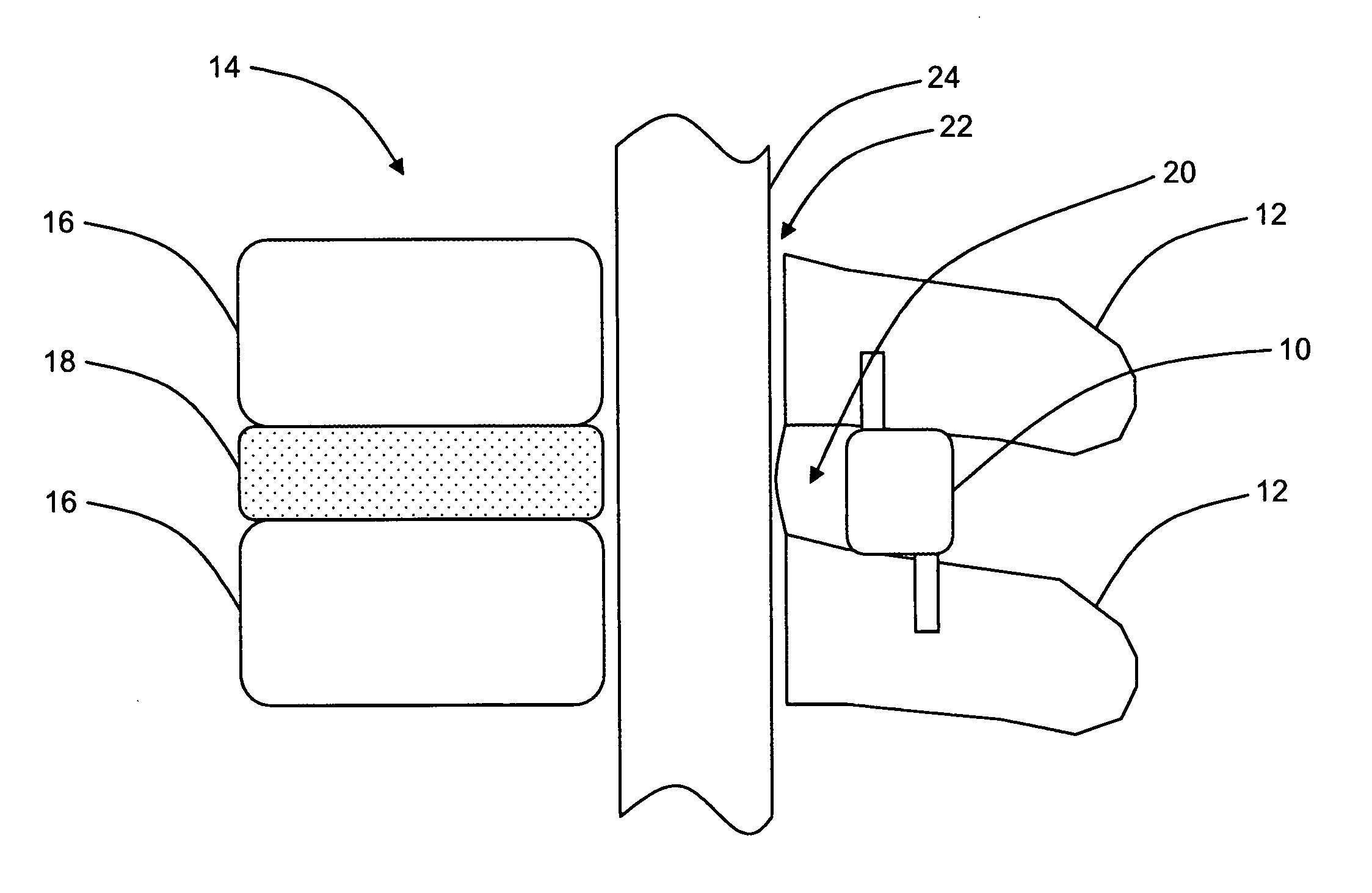
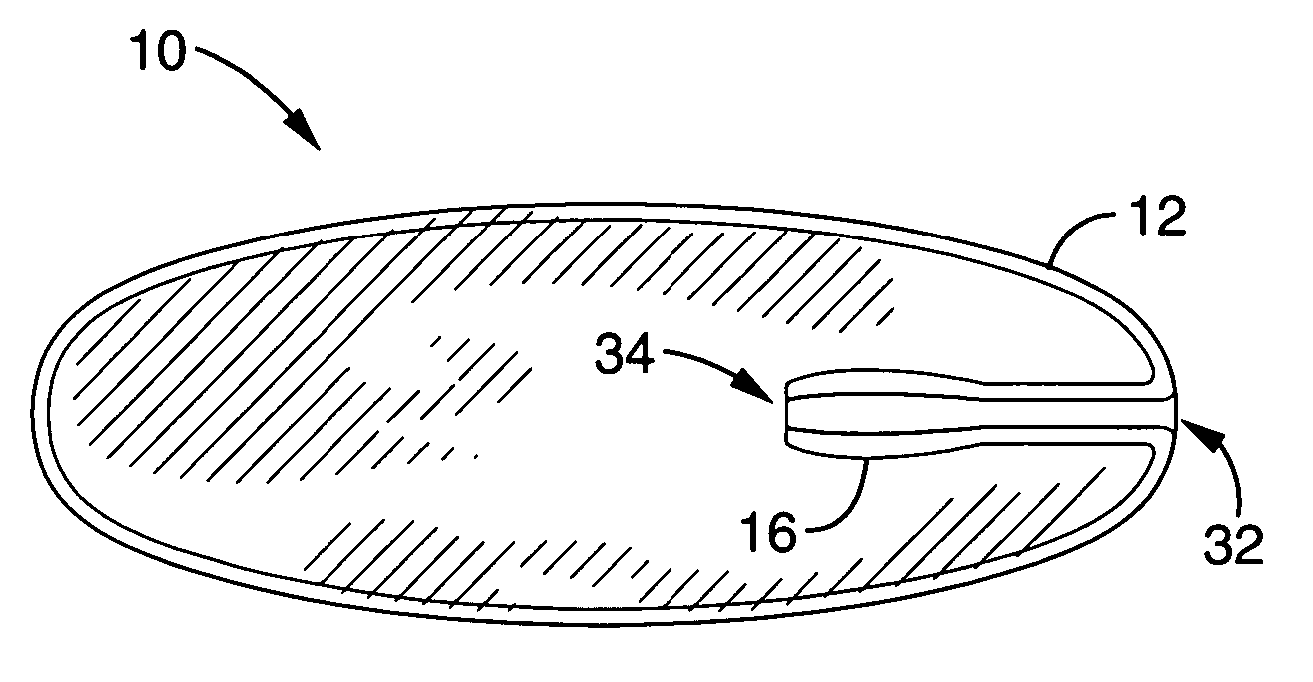
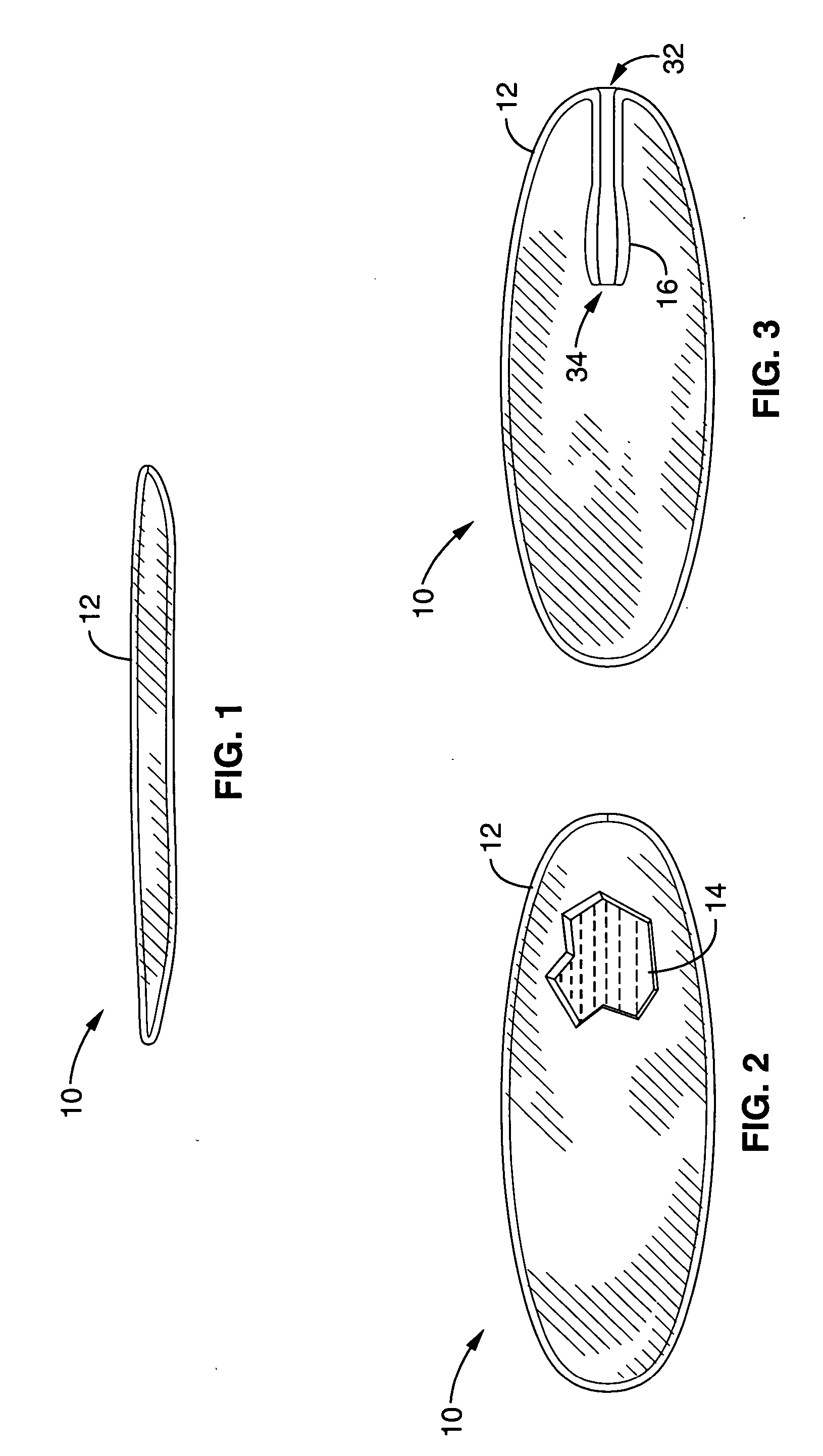
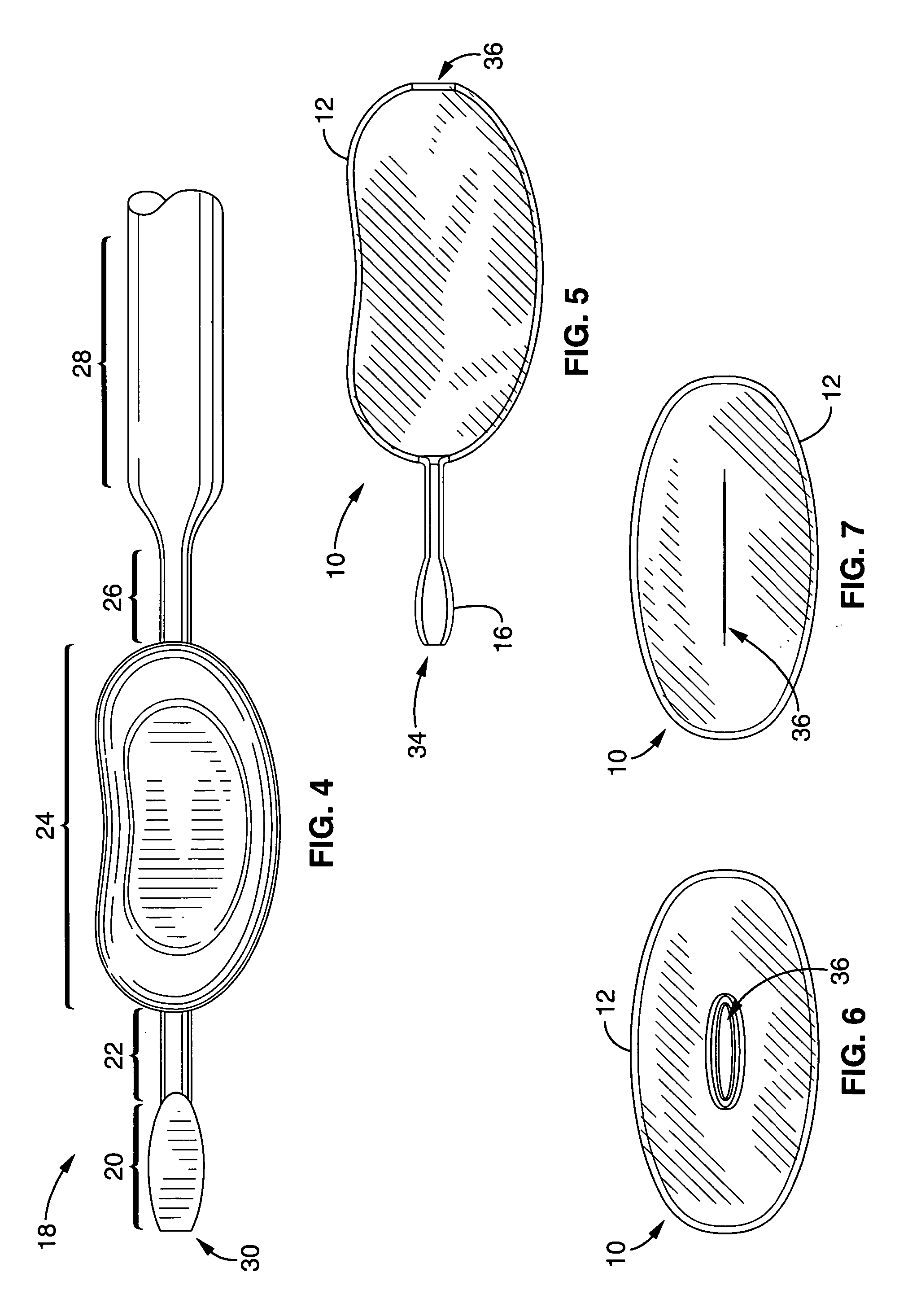
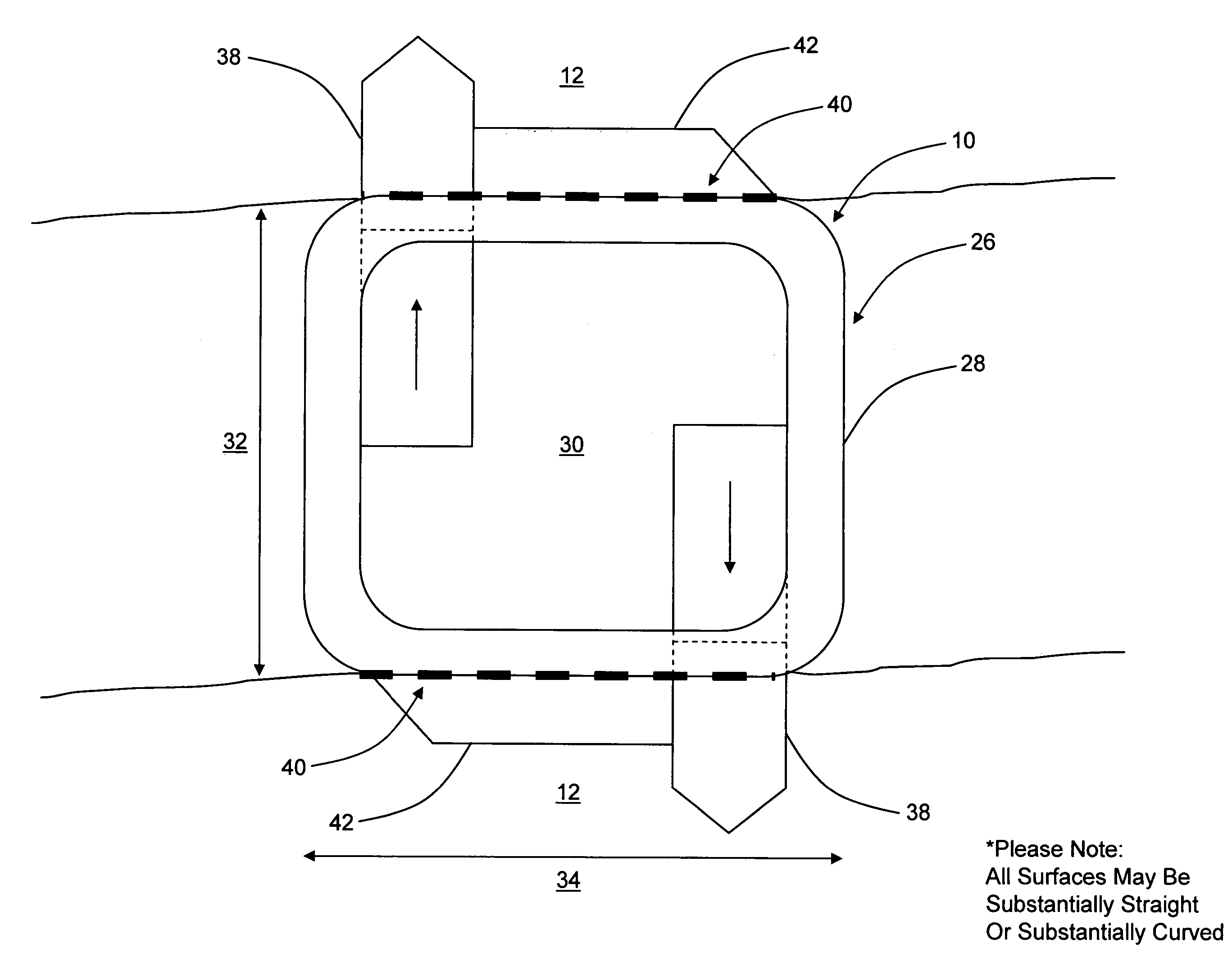
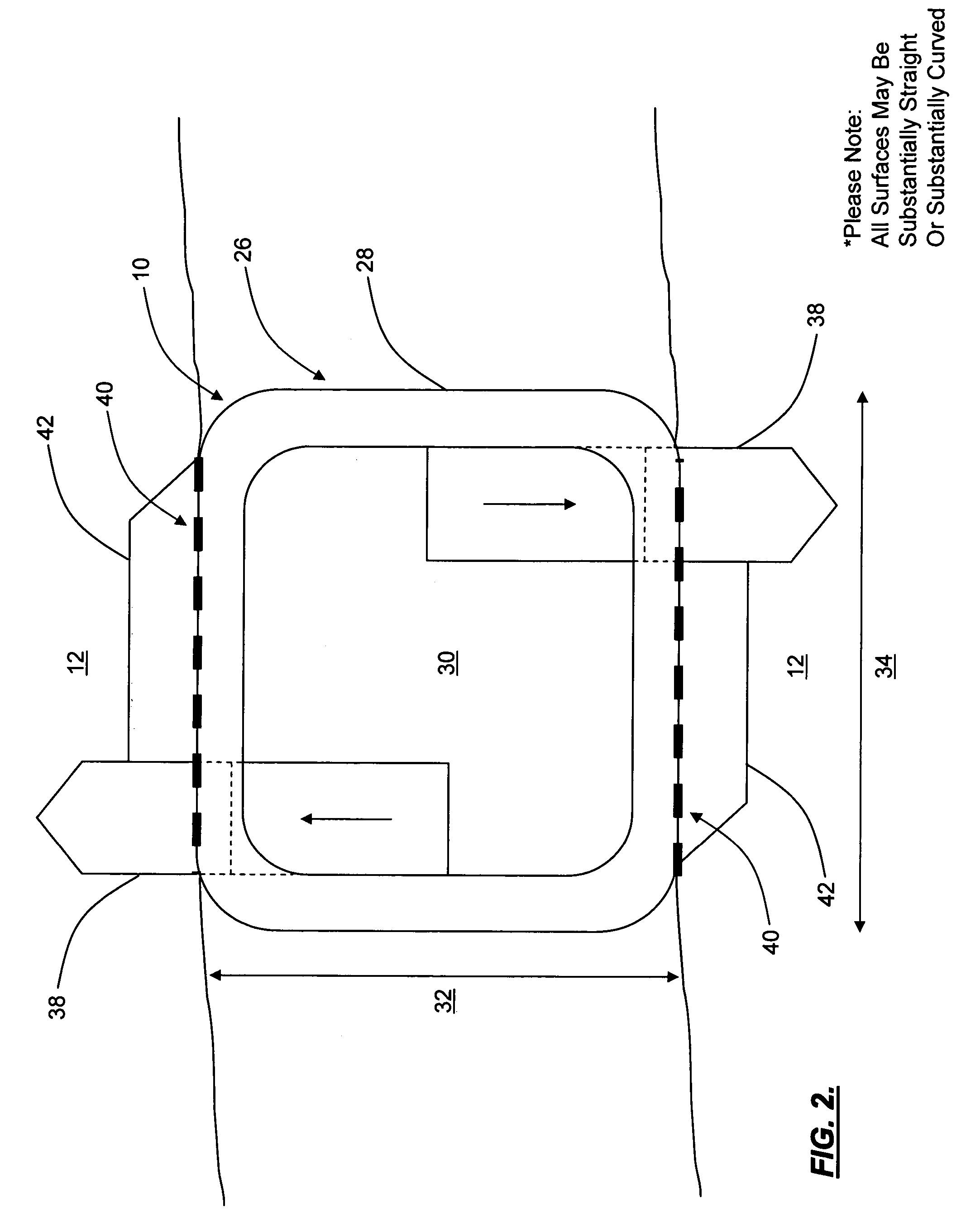
Interspinous distraction devices and associated methods of insertion
InactiveUS20060106397A1Securely holdInternal osteosythesisJoint implantsDistractionMinimally invasive procedures
In various embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
Interspinous distraction devices and associated methods of insertion
In various exemplary embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
Systems, devices and methods for treatment of intervertebral disorders
InactiveUS20060149380A1Increase heightMinimally invasiveSuture equipmentsSpinal implantsFiberElastomer
A bioactive / biodegradable nucleus implant for repairing degenerated intervertebral discs that is inflated inside the nucleus space after the degenerated nucleus has been removed to re-pressurize the nuclear space within the intervertebral disc. The implant is inflated with a high molecular weight fluid, gel or combination of fluid and elastomer, preferably an under-hydrated HA hydrogel / growth factor mixture with or without host cells. The implant includes an internal, integral, self-sealing valve that allows one-way filling of the implant after it is placed within the disc, and is made from a material that allows fibrous in growth thereby stabilizing the implant. A variety of substances can be incorporated into the implant to promote healing, prevent infection, or arrest pain.
Owner:RGT UNIV OF CALIFORNIA
Biodegradable/bioactive nucleus pulposus implant and method for treating degenerated intervertebral discs
A bioactive / biodegradable nucleus implant for repairing degenerated intervertebral discs that is inflated inside the nucleus space after the degenerated nucleus has been removed to re-pressurize the nuclear space within the intervertebral disc. The implant is inflated with a high molecular weight fluid, gel or combination of fluid and elastomer, preferably an under-hydrated HA hydrogel / growth factor mixture with or without host cells. The implant includes an internal, integral, self-sealing valve that allows one-way filling of the implant after it is placed within the disc, and is made from a material that allows fibrous in growth thereby stabilizing the implant. A variety of substances can be incorporated into the implant to promote healing, prevent infection, or arrest pain.
Owner:RGT UNIV OF CALIFORNIA
Unit for treatment of the degeneration of an intervertebral disc
InactiveUS20060235532A1Limit mechanical stressInternal osteosythesisJoint implantsMedicineIntervertebral disk
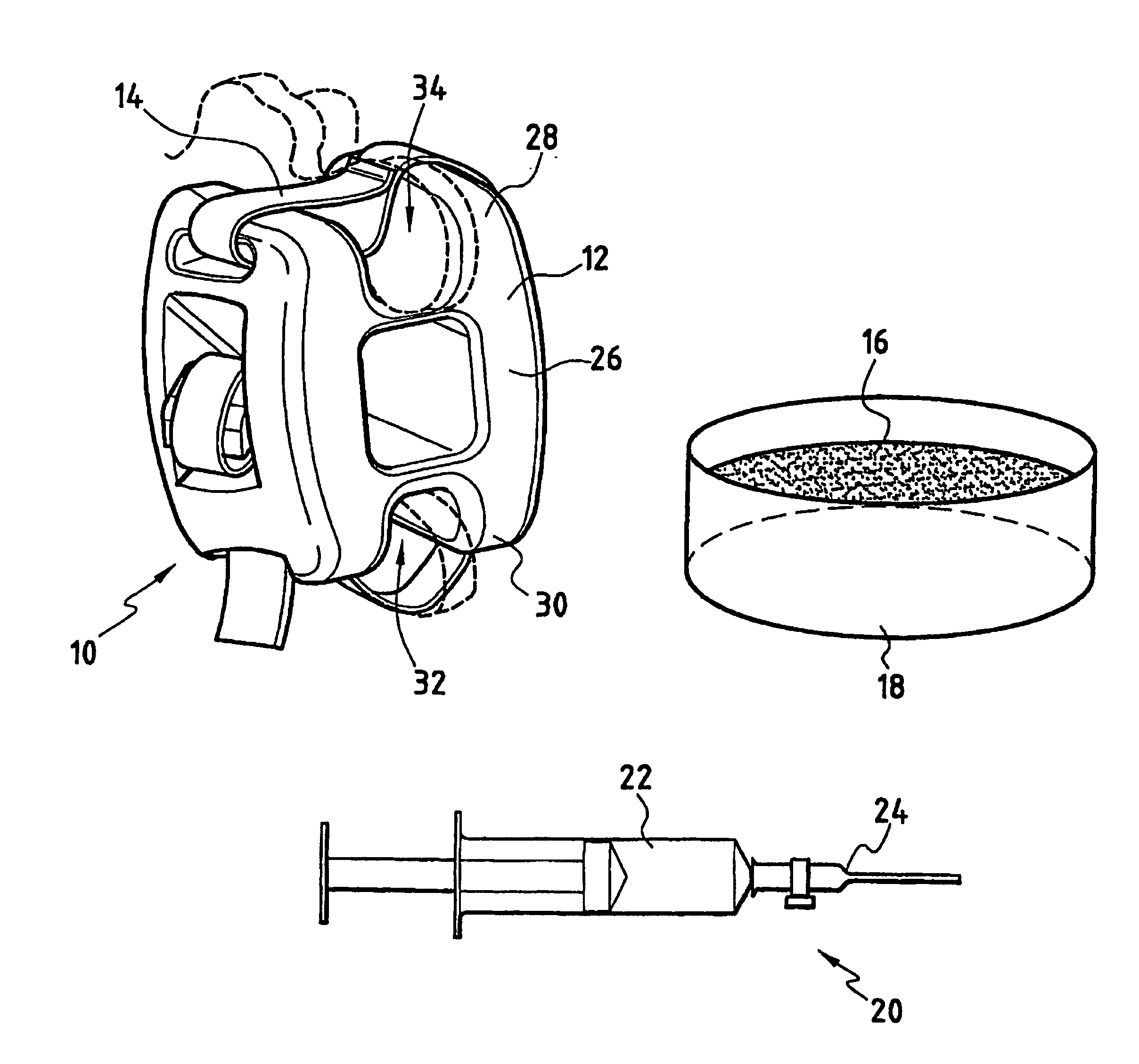
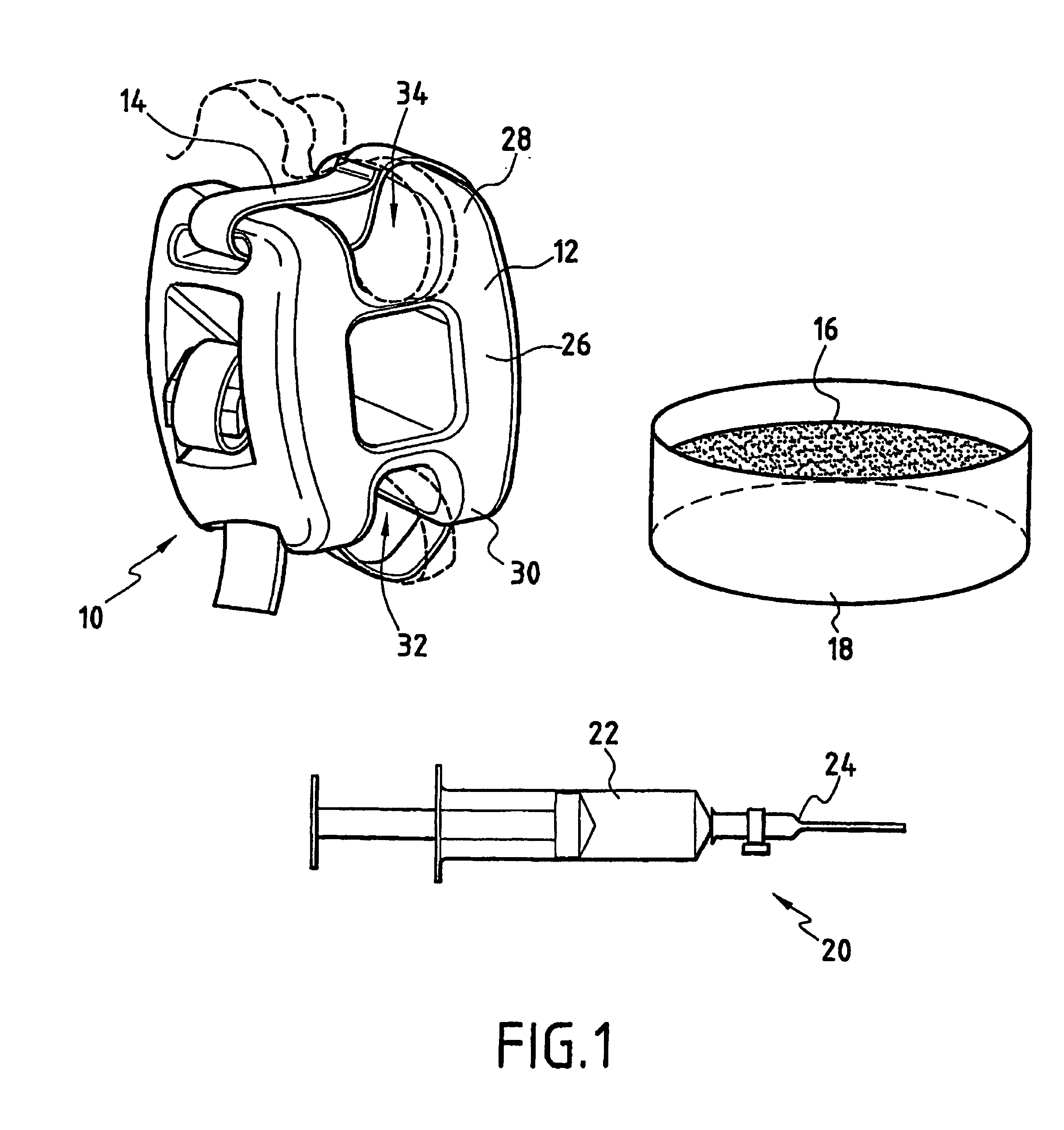
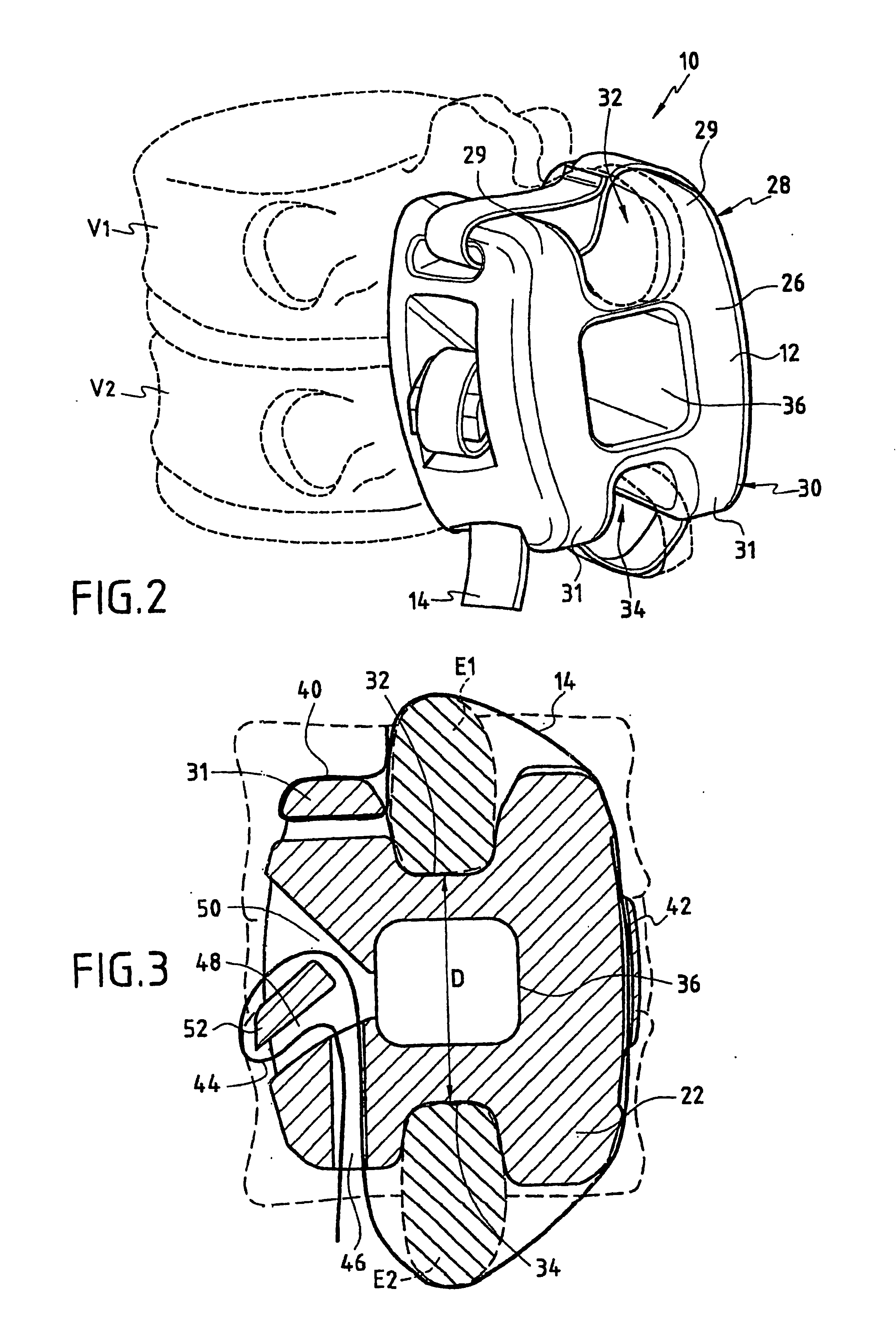
The invention relates to an assembly for treating degeneration of a damaged intervertebral disk disposed between two vertebrae. The treatment assembly includes cells that may be analogous to those of the intervertebral disk, and that are suitable for implanting in said disk; and an intervertebral implant. The intervertebral implant includes an intervertebral spacer for placing between said vertebrae, and a fastener device for fastening said spacer on said vertebrae.
Owner:ZIMMER SPINE INC
Compositions and methods for intervertebral disc reformation
Methods of reforming degenerated intervertebral discs. Hybrid materials useful in methods of reforming degenerated intervertebral discs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and methods for intervertebral disc reformation
Methods of reforming degenerated intervertebral discs are provided in accordance with methods of the invention. Hybrid materials useful in methods of the present invention are also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method of treating degenerative spinal disorders
InactiveUS20080045949A1Reduce loadPromotes extracellular matrix productionInternal osteosythesisAntipyreticDevice implantDisease
The present invention describes methods for treating a degenerative intervertebral disc disorder comprising implanting a disc stabilization device into a subject and administering at least one therapeutic agent which promotes healing of the disc to the subject. The invention also includes methods of promoting healing of damaged or degenerated intervertebral discs comprising decreasing the load of the disc through a disc stabilization device and inhibiting the inflammatory process. Also described are hydrogels for use in combination with extradiscal stabilization devices, as intradiscal stabilization devices, as drug carriers, and as combinations thereof.
Owner:ABBOTT LAB INC +1
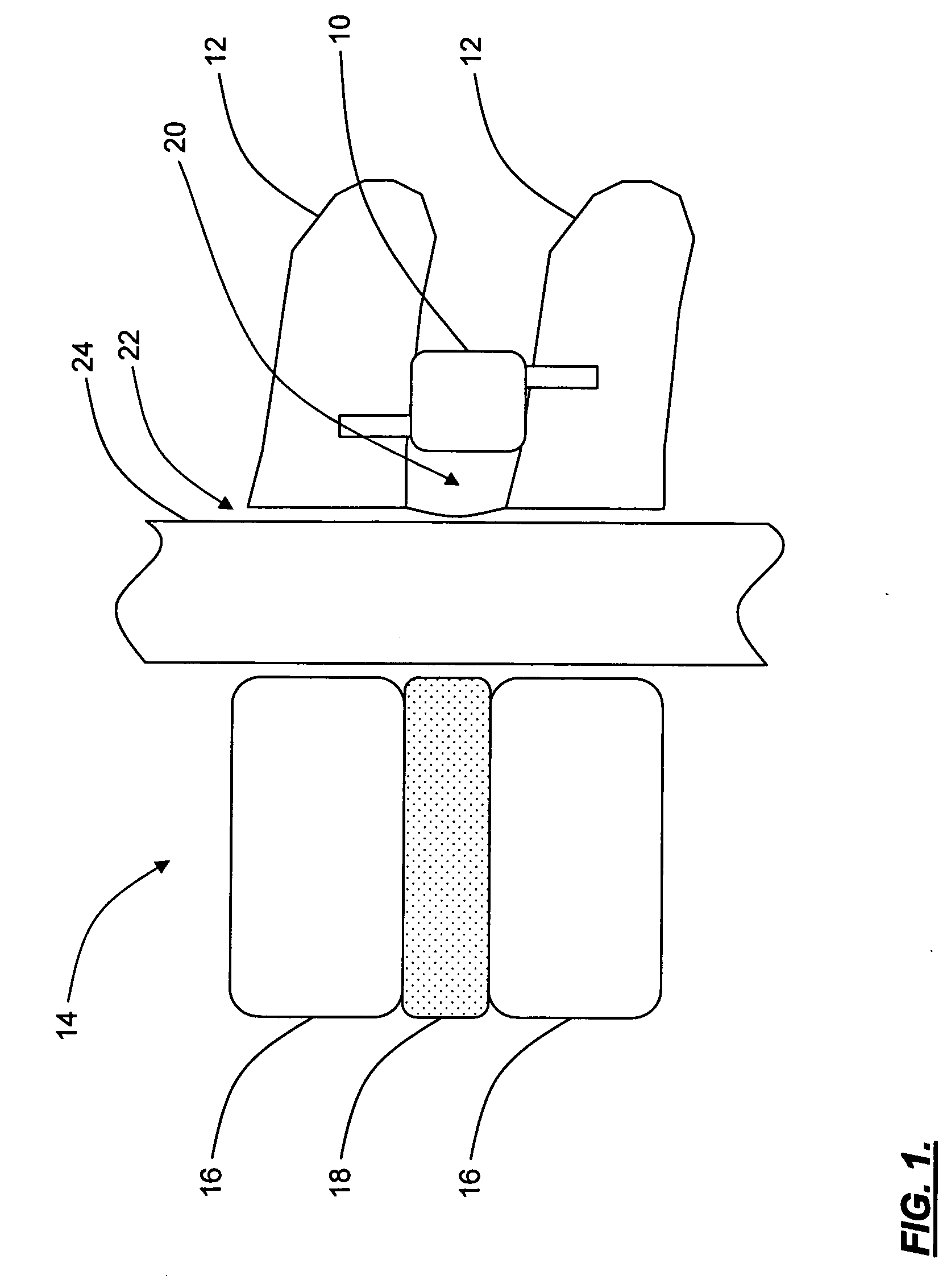
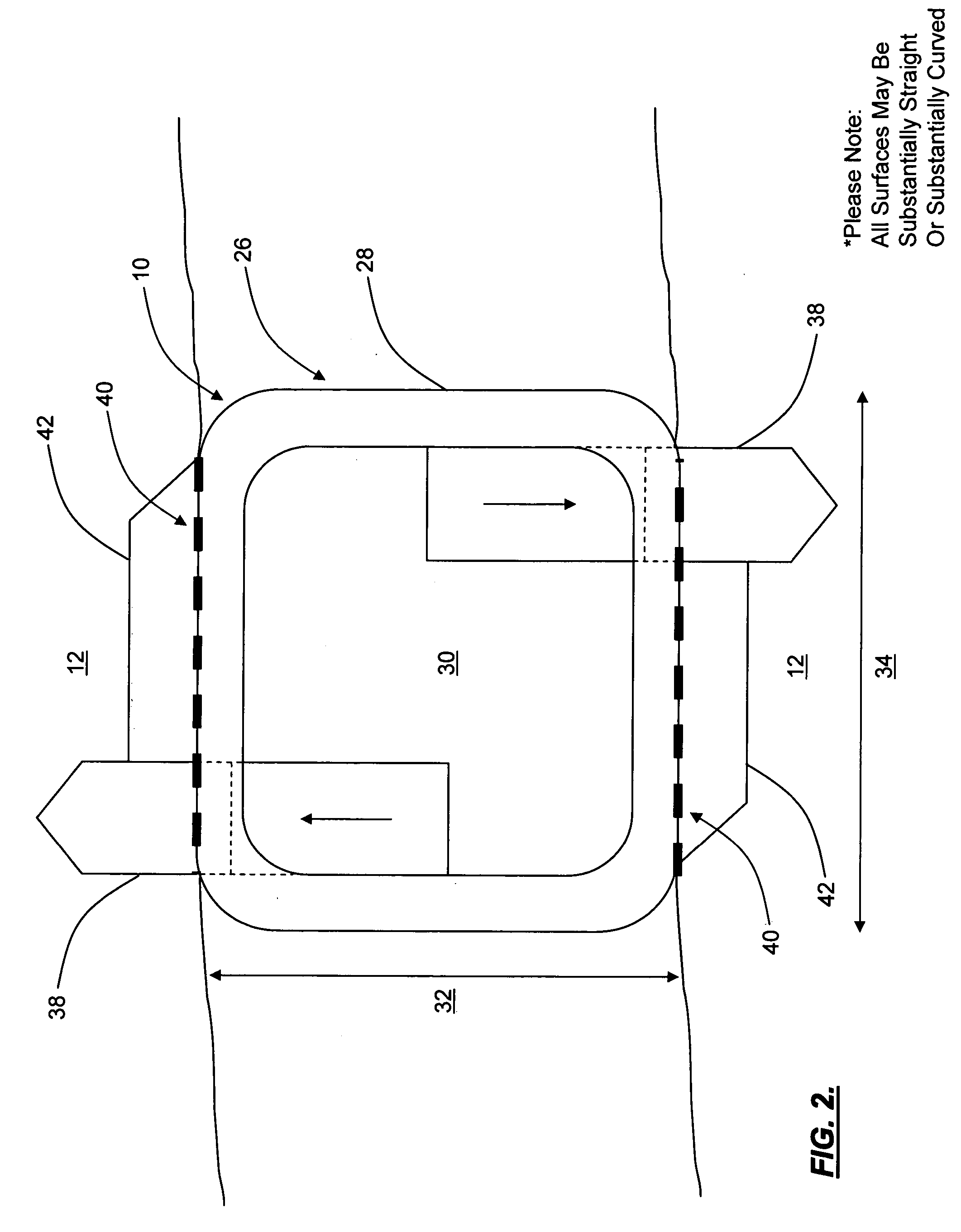
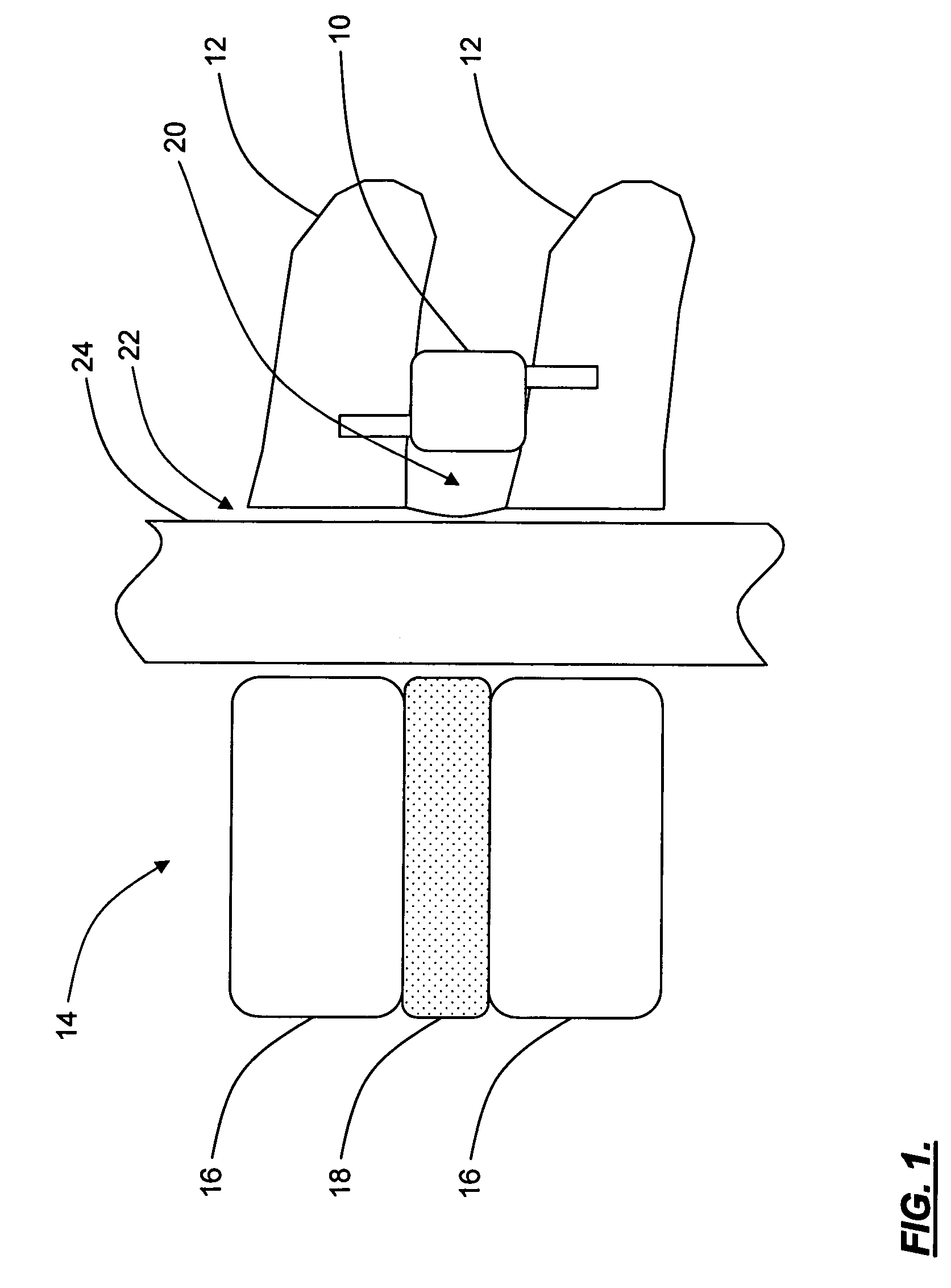
Spine replacement system for the treatment of spine instability and degenerative disc disease
InactiveUS20070093903A1Avoids prosthetic movementInhibit migrationInternal osteosythesisBone implantDiseaseInstability
Apparatuses and methods for single disc arthroplasty and multi-segmental spine replacement. The implant assembly includes a first cage (20) adapted to be rigidly attached to a first vertebra, a second cage (20) adapted to be rigidly attached to a second vertebrae, and a spinal disc replacement prosthesis (10) positioned between the first and second vertebrae. The spinal disc replacement prosthesis preferably includes a resilient body having two or more adjustable fluid-filled compartments.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Device for treating back pain by re-establishing the exchage of nutrient & waste
InactiveUS20060247600A1Minimize twistingFriction minimizationStentsInternal osteosythesisBACK DISCOMFORTBack pain
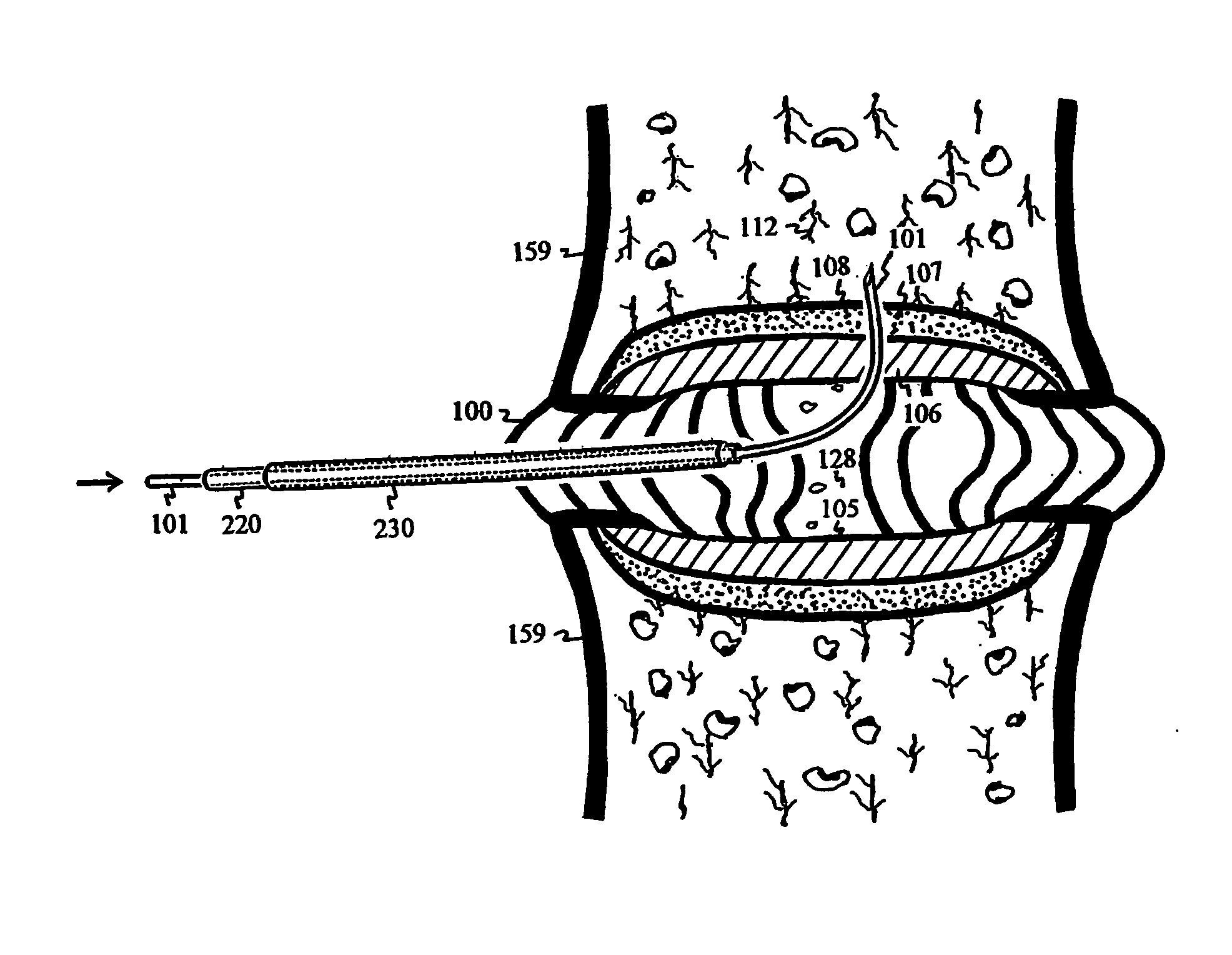
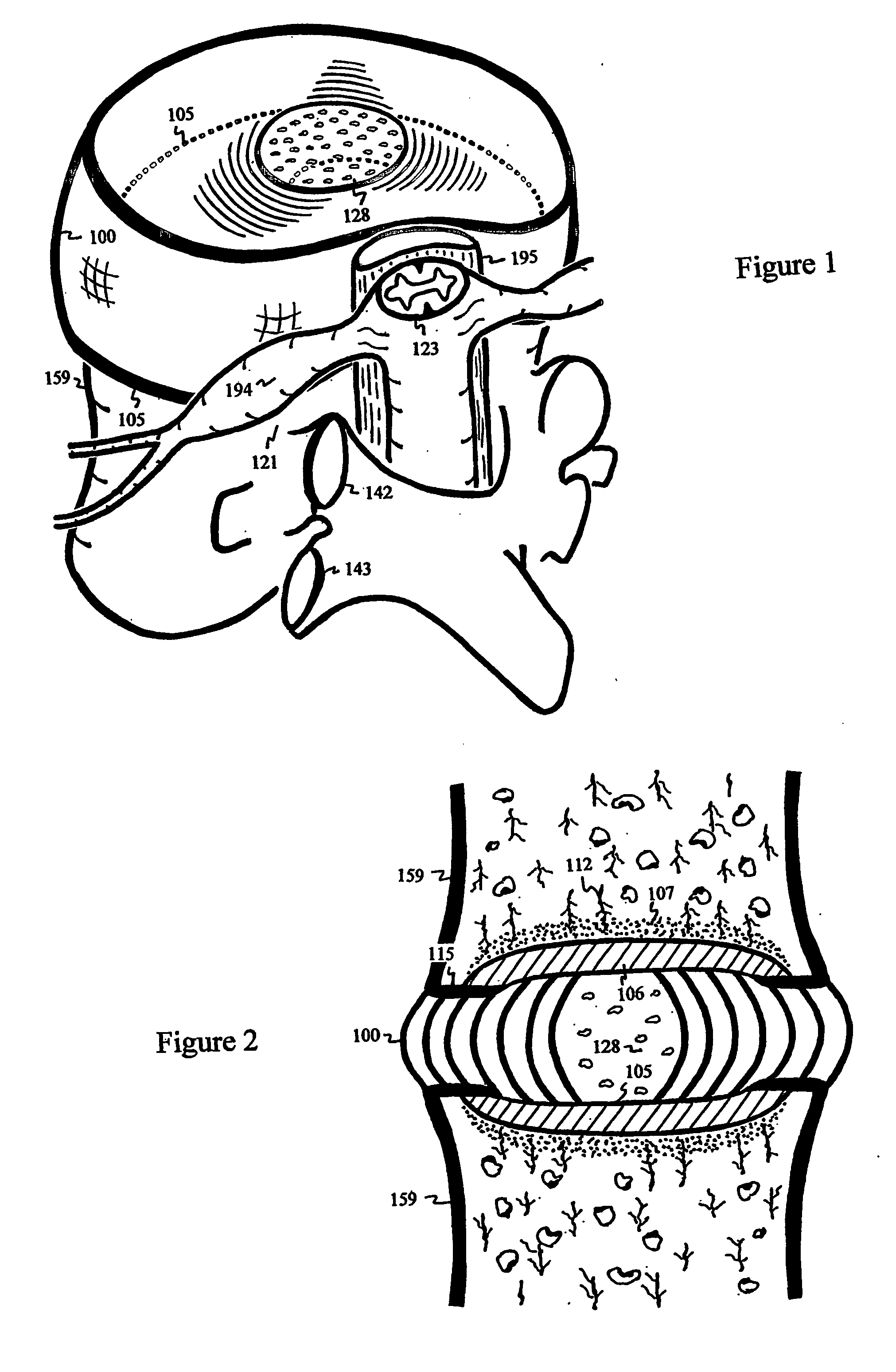
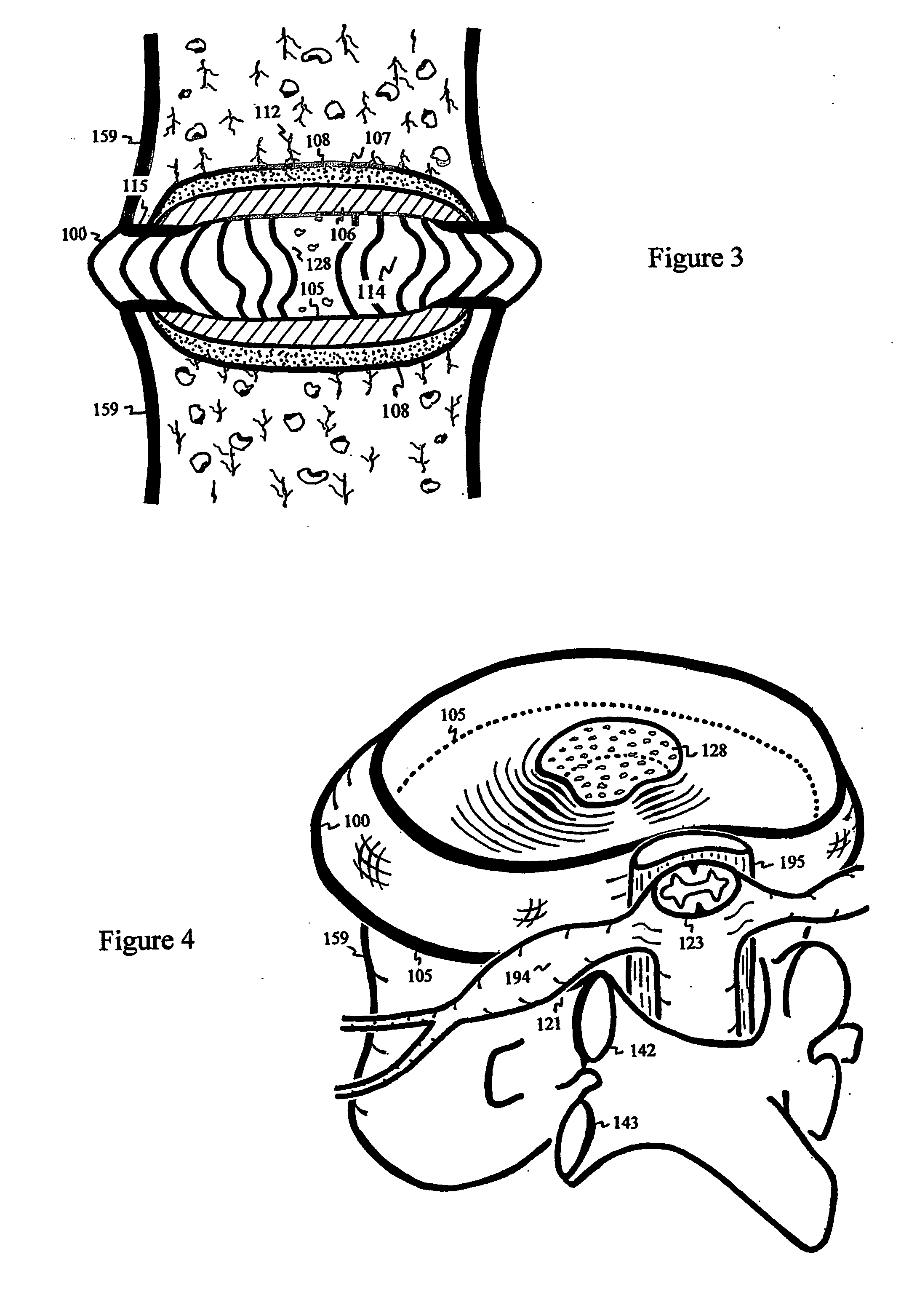
The intervertebral disc is avascular. With aging, endplates become occluded by calcified layers, and diffusion of nutrients and oxygen into the disc diminishes. The disc degenerates, and pain ensues. Conduits are delivered and deployed into the intervertebral disc to reestablish the exchange of nutrients and waste between the disc and bodily circulation to stop or reverse disc degeneration and relieve pain. The intervertebral disc installed with semi-permeable conduits may be used as an immuno-isolated capsule to encapsulate donor cells capable of biosynthesizing therapeutic molecules. The semi-permeable conduits establish the exchange of nutrients and therapeutic molecules between disc and bodily circulation to treat a disease without using immunosuppressive drugs.
Owner:ALEEVE MEDICAL INC
Biodegradable/bioactive nucleus pulposus implant and method for treating degenerated intervertebral discs
InactiveUS20060293751A1Minimally invasive disk repairNo loss of mobilityJoint implantsSpinal implantsElastomerFiber
A bioactive / biodegradable nucleus implant for repairing degenerated intervertebral discs that is inflated inside the nucleus space after the degenerated nucleus has been removed to re-pressurize the nuclear space within the intervertebral disc. The implant is inflated with a high molecular weight fluid, gel or combination of fluid and elastomer, preferably an under-hydrated HA hydrogel / growth factor mixture with or without host cells. The implant includes an internal, integral, self-sealing valve that allows one-way filling of the implant after it is placed within the disc, and is made from a material that allows fibrous in growth thereby stabilizing the implant. A variety of substances can be incorporated into the implant to promote healing, prevent infection, or arrest pain.
Owner:RGT UNIV OF CALIFORNIA
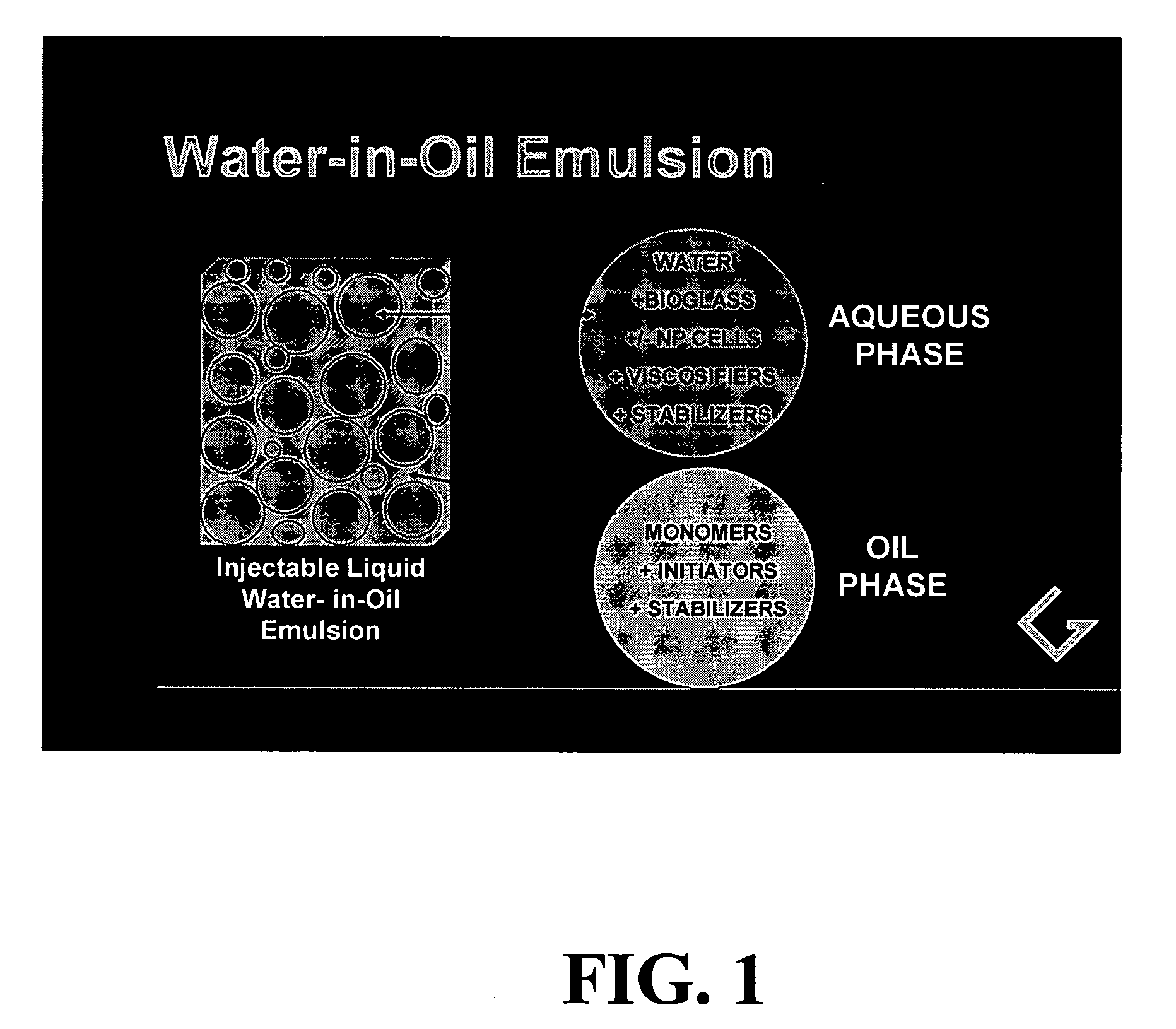
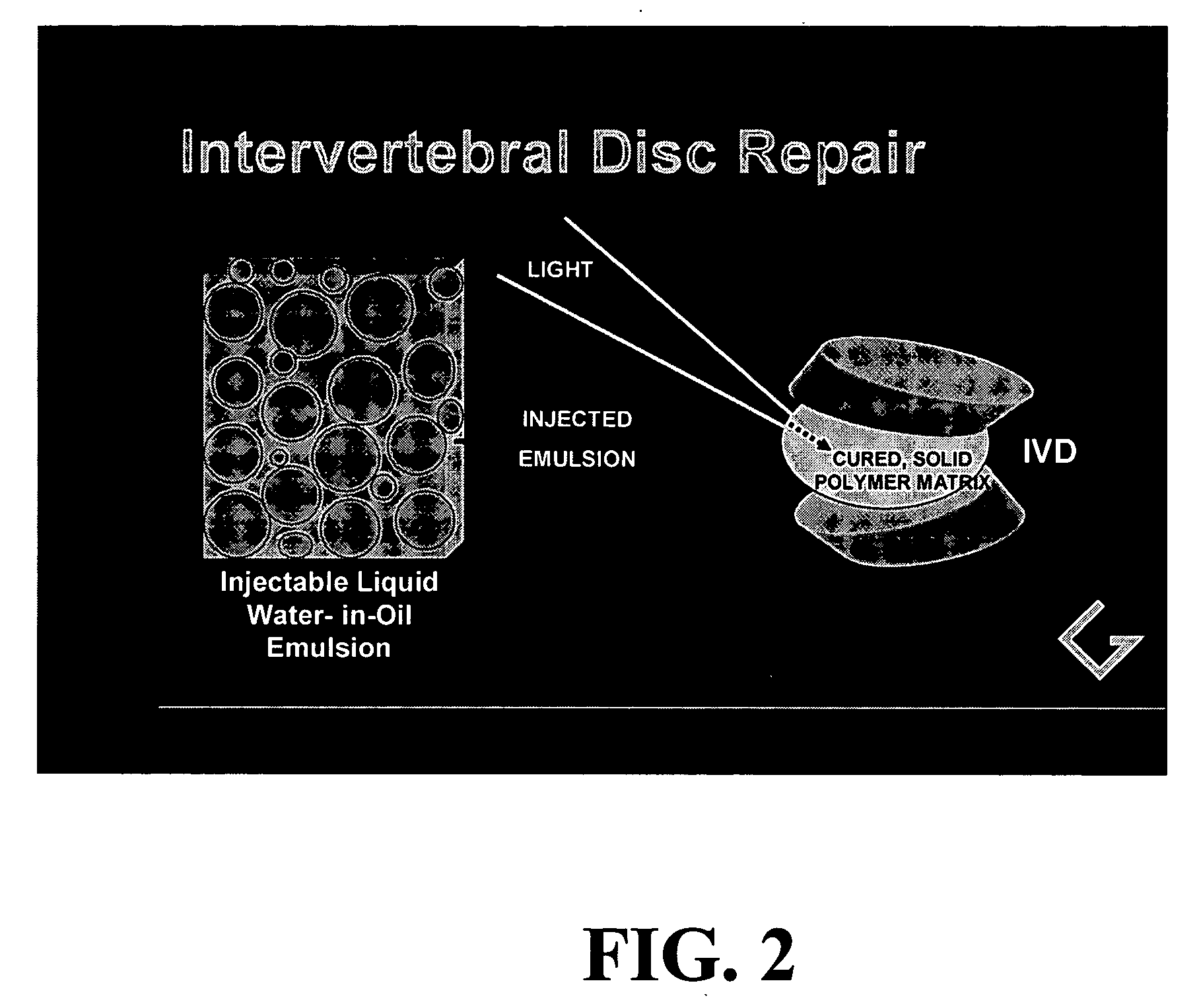
Polymerizable emulsions for tissue engineering
InactiveUS20050112186A1Not provoke autoimmune and inflammatory responseImprove performance and stability and durabilityCosmetic preparationsPowder deliveryWater in oil emulsionNon invasive
Provided are biocompatible viscoelastic solid materials derived from polymerization of fluid water-in-oil emulsions, along with methods of their preparation and methods for their use for tissue engineering applications, including for reforming diseased, damaged or degenerated intervertebral discs by acceptably non-invasive means.
Owner:GENTIS
Disk Replacement Endoprosthesis
A disk replacement endoprosthesis and a procedure for implantation of the endoprosthesis to treat pain, nerve root compression, and neural injury caused by degeneration or injury to vertebral disks. The endoprosthesis may include an intervertebral wedge or other body having a lead-in distractor. A fastening plate may be attached to the intervertebral wedge by a joint such that the first fastening plate may move from a first configuration in which the first fastening plate is in-line with the intervertebral wedge to facilitate insertion of the endoprosthesis to a second configuration in which the first fastening plate is in contact with an anterior surface of the inferior vertebral body for attachment thereto.
Owner:KYPHON
Interspinous distraction devices and associated methods of insertion
InactiveUS7918875B2Securely holdInternal osteosythesisJoint implantsDistractionLess invasive surgery
In various embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
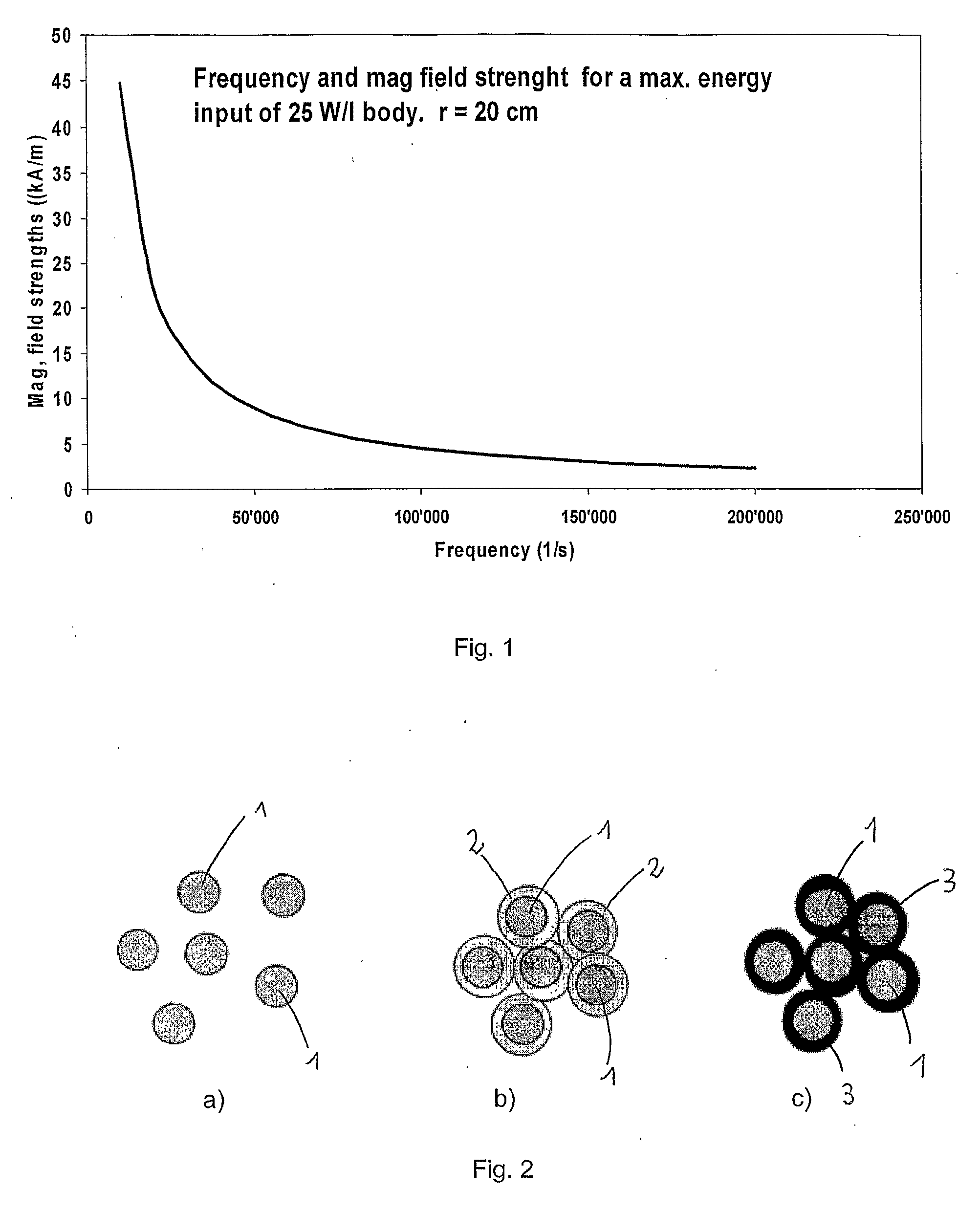
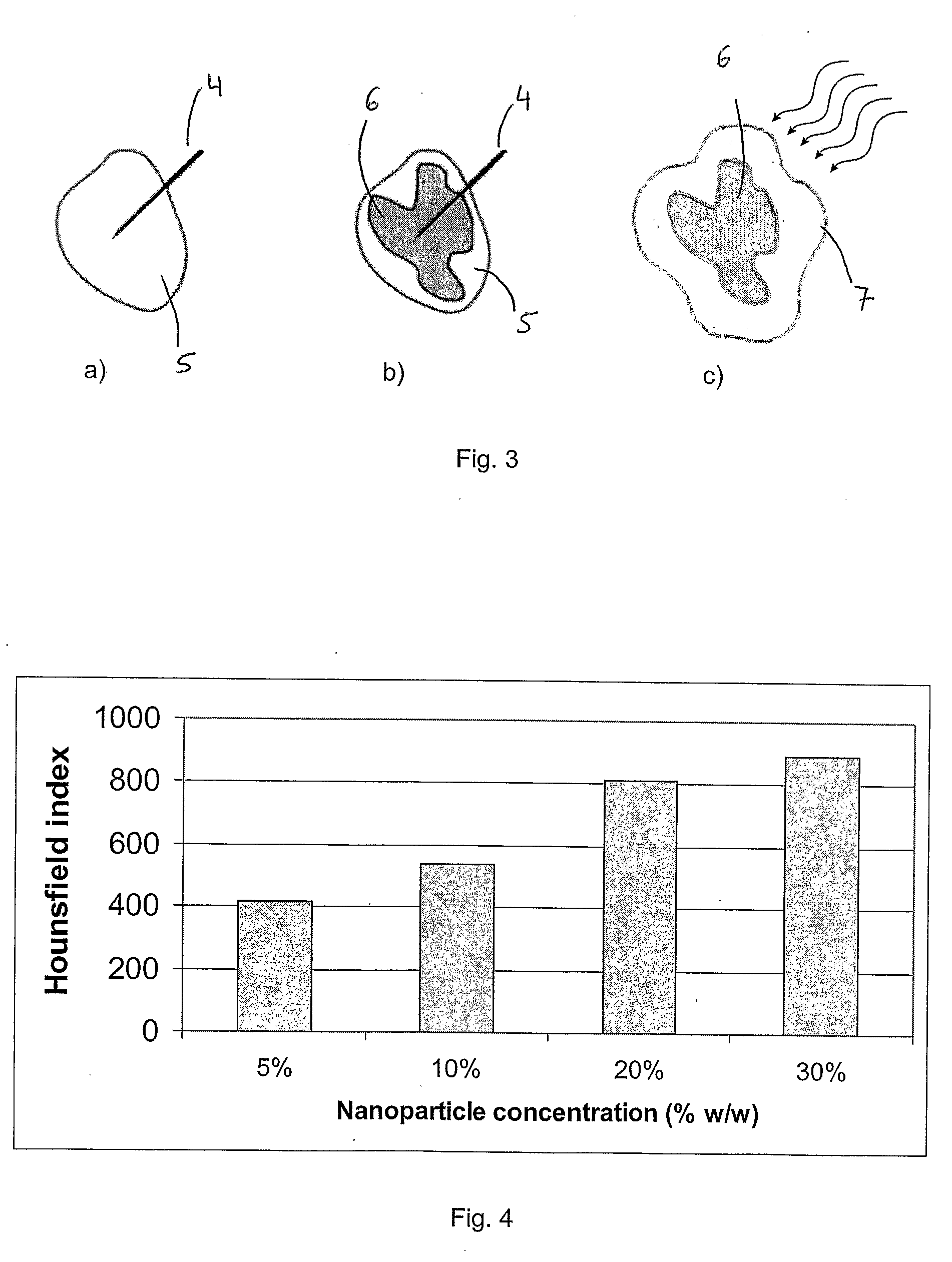
Injectable superparamagnetic nanoparticles for treatment by hyperthermia and use for forming an hyperthermic implant
The injectable formulation for treatment by hyperthermia comprises a liquid carrier and heat-generating superparamagnetic iron oxide nanoparticles having a mean diameter not greater than 20 nm. Said injectable formulation is able to form in-situ a hyperthermic solid or semi-solid implant upon contact with a body fluid or tissue. Said hyperthermic solid or semi-solid implant may be useful for treating a tumor or a degenerative disc disease by hyperthermia.
Owner:UNIVERSITY OF GENEVA +2
Structurally reinforced spinal nucleus implants
InactiveUS20090149958A1Reduced dimensionLamination ancillary operationsHollow inflatable ballsSpinal cordIntervertebral space
A spinal nucleus implant is provided which includes a braided three-dimensional reinforcement member having a polymeric matrix imbued therein, the implant configured to have a shape consistent with a cavity within an intervertebral disc space. The polymeric matrix may be a fluid absorbing polymer, e.g., a hydrogel or a substantially non-fluid absorbing in-situ curable elastic polymer. A method of making a spinal nucleus implant is provided which includes providing a braided three-dimensional reinforcement member configured and dimensioned to have a shape consistent with a cavity in an intervertebral space and infusing the braided three-dimensional reinforcement member with a liquid polymer capable of forming a polymeric matrix. Also provided is a spinal nucleus implant including a three-dimensional reinforcement member adapted and configured to undergo anisotropic expansion, the implant configured to have a shape consistent with a cavity within an intervertebral disc space. A method of treating a degenerating intervertebral disc includes creating an incision in an annulus; removing at least a portion of a nucleus pulposus; and inserting, through the incision, a spinal nucleus implant according to the present disclosure.
Owner:REPLICATION MEDICAL
Mesenchymal stem cell isolation and transplantation method and system to be used in a clinical setting
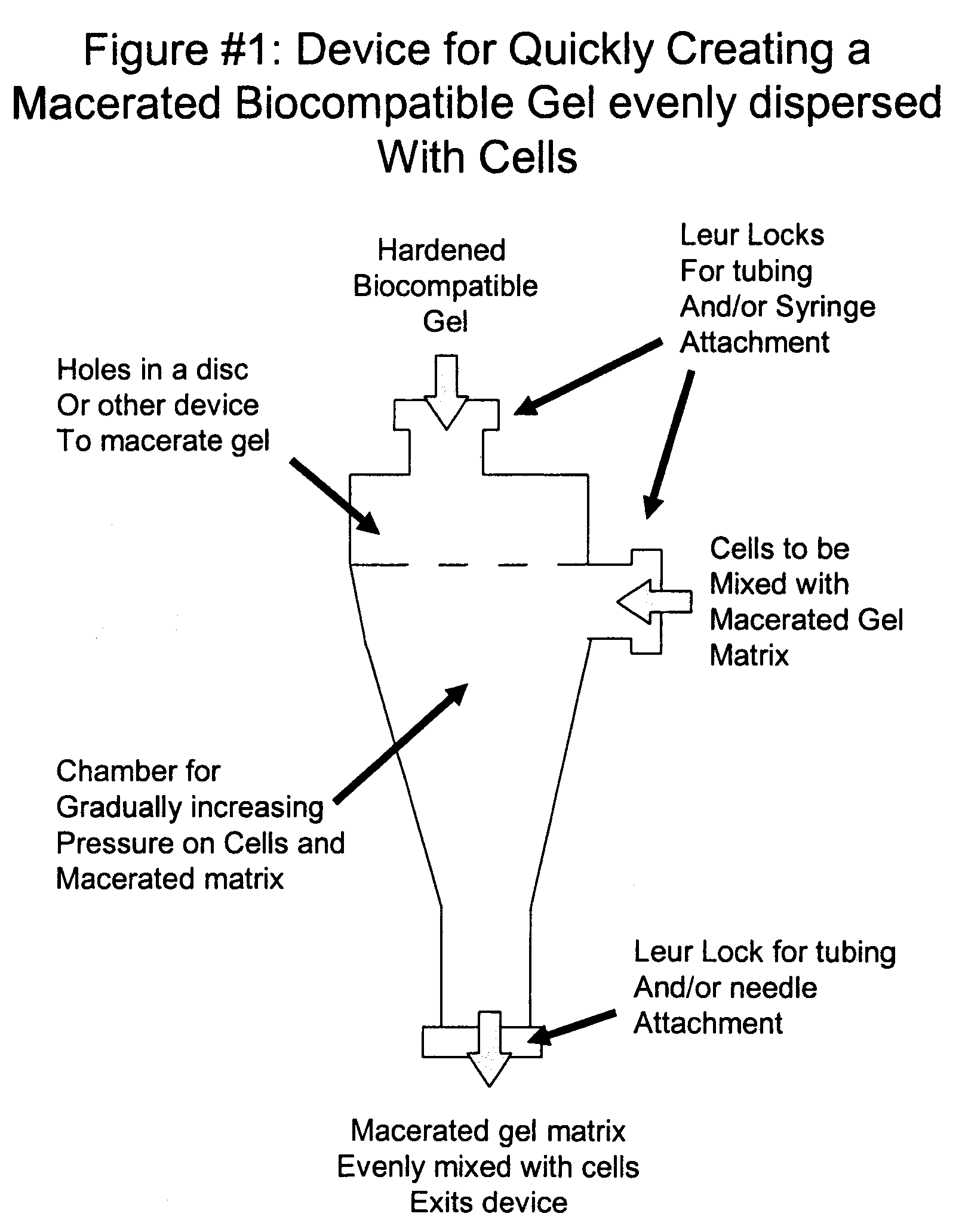
A system and method for the percutaneous, autologous transplantation of mesenchymal stem cells and progenitor helper cells (PHC) from bone marrow to degenerated intervertebral discs or joints. This method is designed to be used by operating room staff in a clinical setting to isolate a mesenchymal stem cell population and PHC during the same surgical procedure as transplantation. The method can be used as a two step procedure where cells are harvested, then isolated, then reimplanted at a later time. In addition, experimental techniques are described to determine which bone marrow cells should be removed via negative selection to generate a PHC population most likely to regenerate certain tissue types in-vitro as well as which combination of fibrinogen and hyaluronic acid and which degree of gel maceration provides the best matrix for in-vitro and in-vivo regeneration of joints and intervertebral discs.
Owner:REGENERATIVE SCI LLC
Method of treating a subject suffering from degenerative disc disease using a matrix metalloprotease inhibitor
ActiveUS20070190149A1Effective treatmentRelieve symptomsPowder deliveryOrganic active ingredientsDegenerated intervertebral discMatrix metalloproteinase inhibitor
The present invention provides a method for treating a vertebrate subject suffering from a degenerative disc disease by administering an inhibitor of a matrix metalloprotease (MMP) to the subject in an amount effective to treat the subject.
Owner:DISCOGEN
Concentrated Protein Preparations of Bone Morphogenetic Proteins and Methods of Use Thereof
InactiveUS20100144631A1Efficient preparationHigh recovery rateSenses disorderNervous disorderDiseaseWhole body
Disclosed herein are heretofore undescribed preparations of highly concentrated, solubilized proteins, such as but not limited to, Bone Morphogenetic Proteins. Such protein preparations can be formulated in an aqueous carrier at protein concentrations in excess of 10 mg / ml when using the methods of manufacture taught herein. Such methods yield stable protein preparations in either solubilized or lyophilized form. The protein preparations of the present invention are particularly beneficial when administered either locally or systemically, in part, because low administration volumes can be accomplished. This is especially important for local treatment of certain anatomic locations such as, for example, the synovial fluid of a joint when treating osteoarthritis with BMP-7 or the intradiscal space when treating degenerative disc disease with BMP-7.
Owner:MARIEL THERAPEUTICS
Mixed porous structure interbody fusion cage and preparation method thereof
InactiveCN102440852AGood mechanical compatibilityGood bone conductionSpinal implantsFreeze-dryingReticular formation
Disclosed are a mixed porous structure interbody fusion cage and a preparation method thereof. The interbody fusion cage comprises a porous metal support and porous structure filling bodies, the porous metal support is a three-dimensional net-shaped structure, a plurality of holes are arranged in the porous metal support, and the porous structure filling bodies are fully filled in the holes. The preparation method includes steps that the metal rapid forming technology is directly combined with the freeze drying technology, the porous metal support is manufactured via a structural design and the direct metal rapid forming technology, then uniformly mixed polymer gel or polymer / biological ceramic compound gel is poured in the porous metal support to realize freeze treatment, so that the porous structure filling bodies with the micropore feature are formed after freeze drying, and the mixed porous structure interbody fusion cage is obtained. Mechanical compatibility is good, contact area between the mixed porous structure interbody fusion cage and natural centrum is further increased, instant stability is good, fusion rate is improved, and the mixed porous structure interbody fusion cage and the preparation method thereof can be used for treating clinical degenerative disc diseases.
Owner:SHANGHAI JIAO TONG UNIV
Demineralized cortical bone implants
InactiveUS20120116515A1Easy to distinguishPromote healingInternal osteosythesisBone implantBone implantBone Cortex
Implants comprising a plurality of separate cortical bone units, which have been at least partially demineralized and are osteoinductive, are described herein. The implants can be used in methods for treating bone. Also, disclosed are methods for treating spinal conditions using these implants. The spinal conditions include but are not limited to repairing damage to or defects in the spine, such as fractures in a vertebral body or degeneration of spinal discs.
Owner:SPINEOLOGY +1
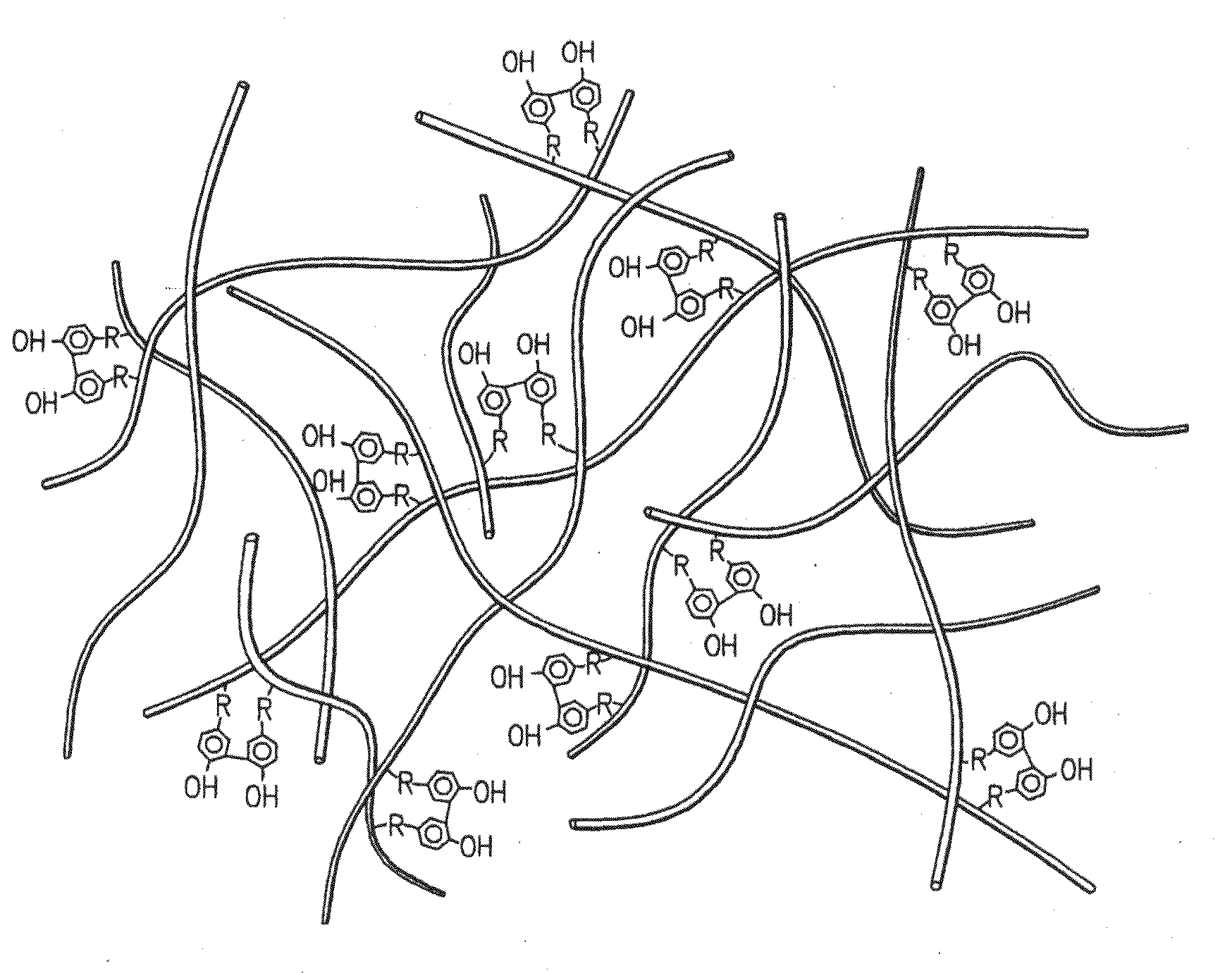
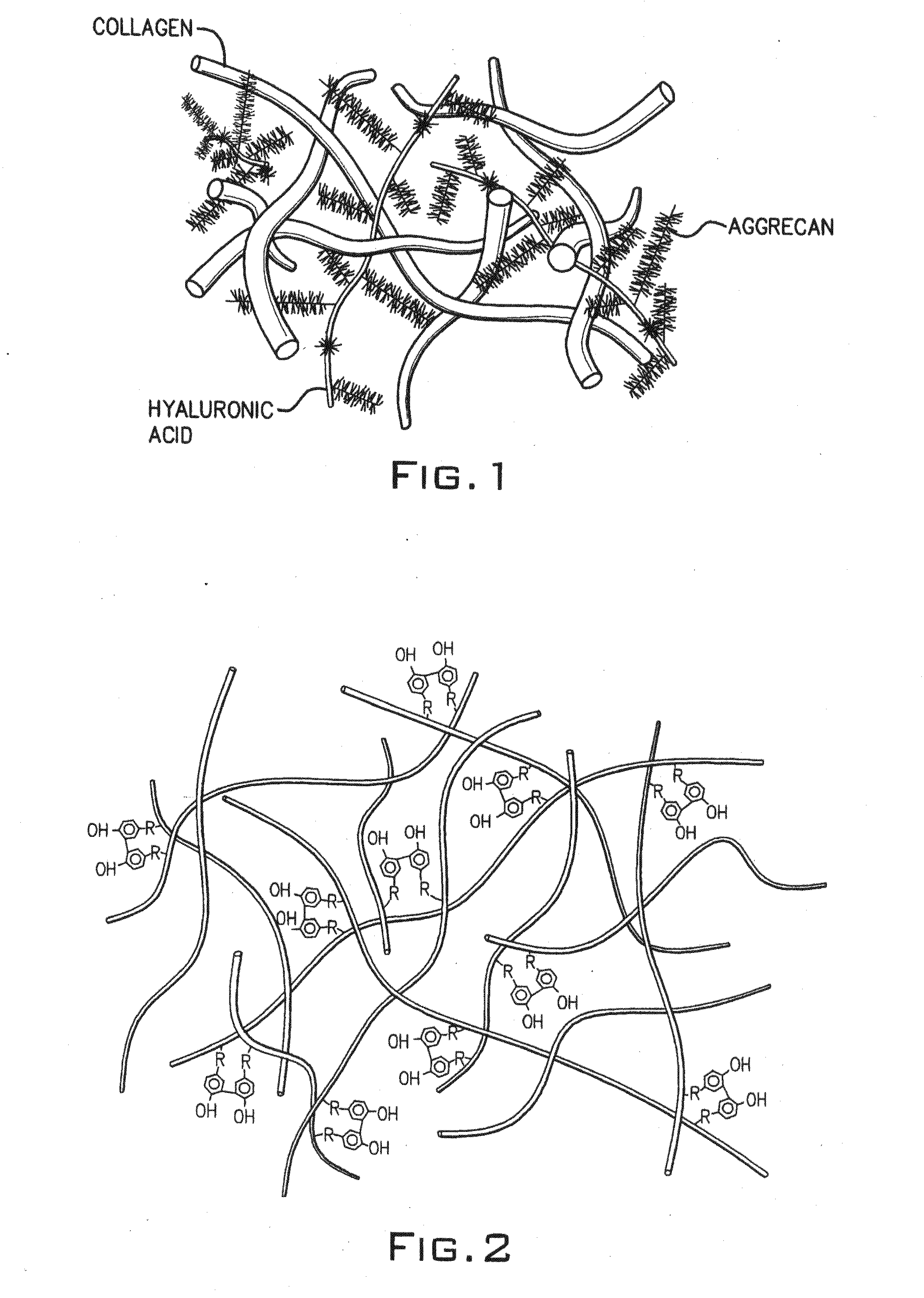
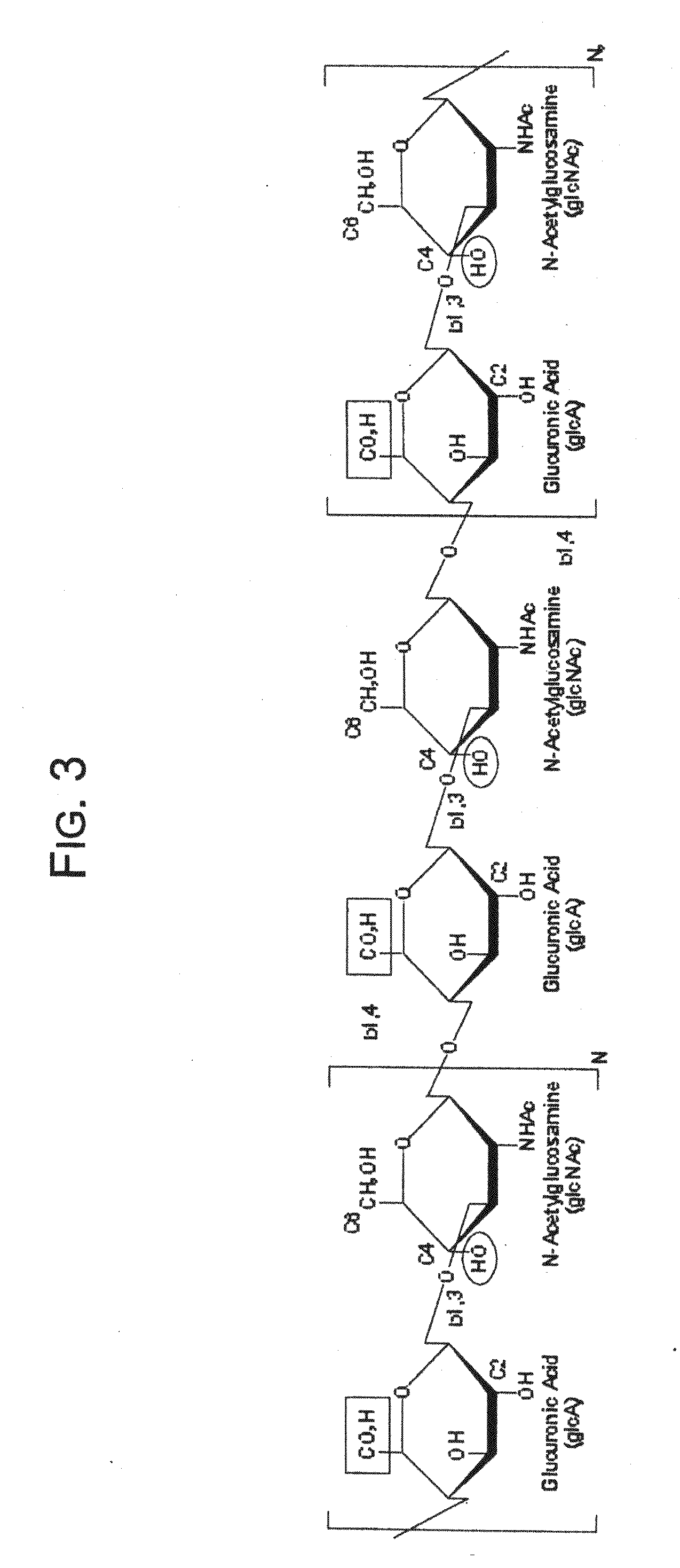
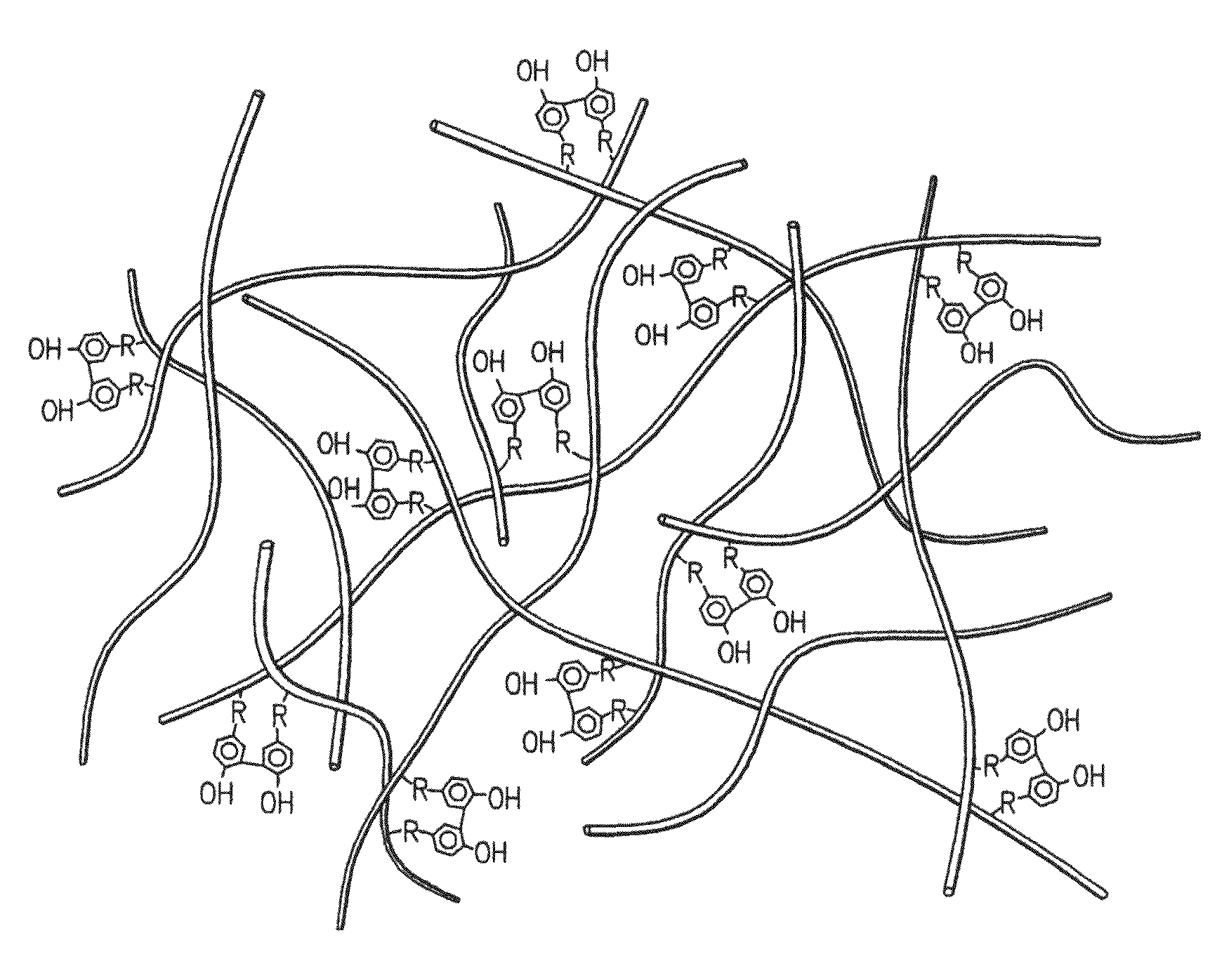
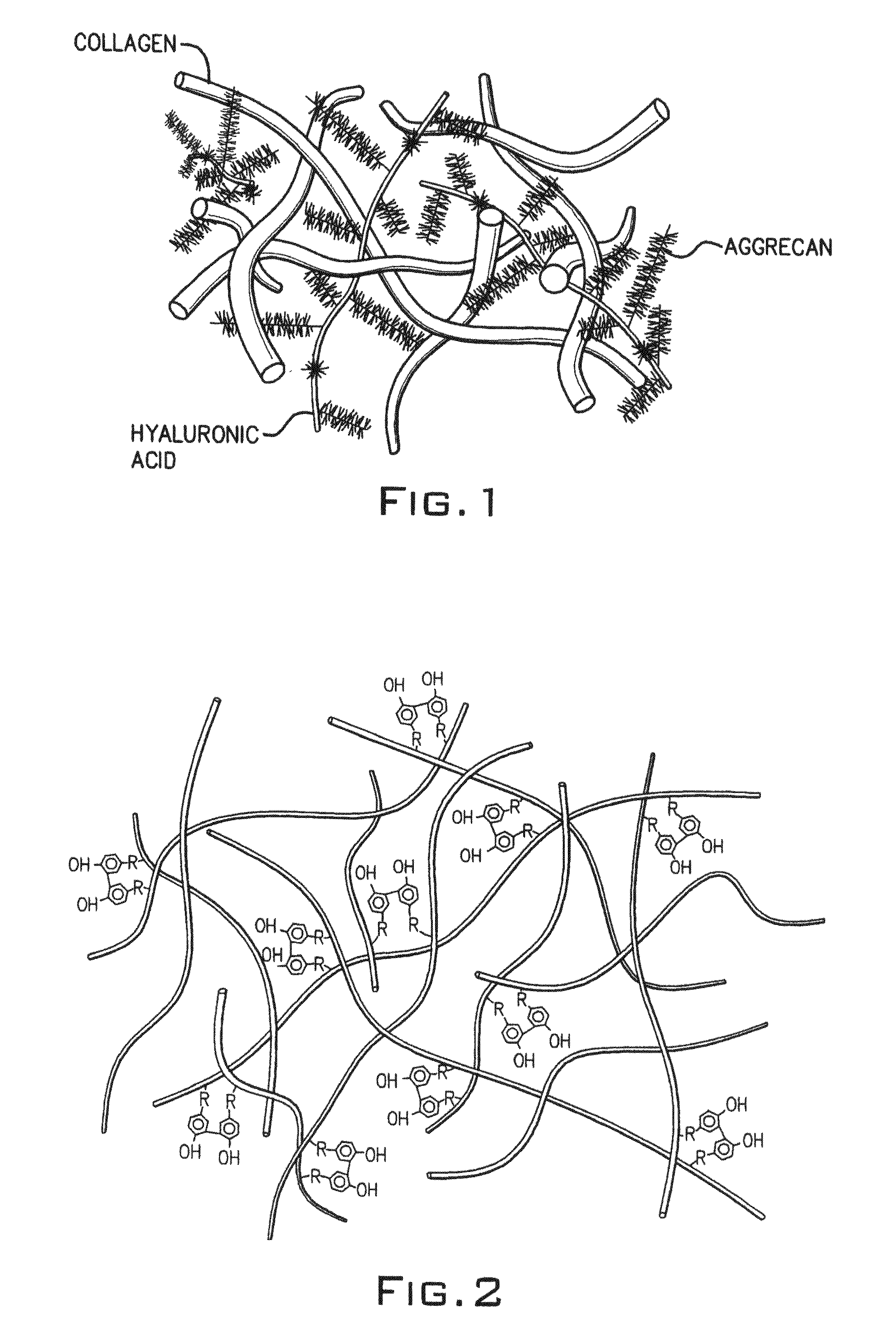
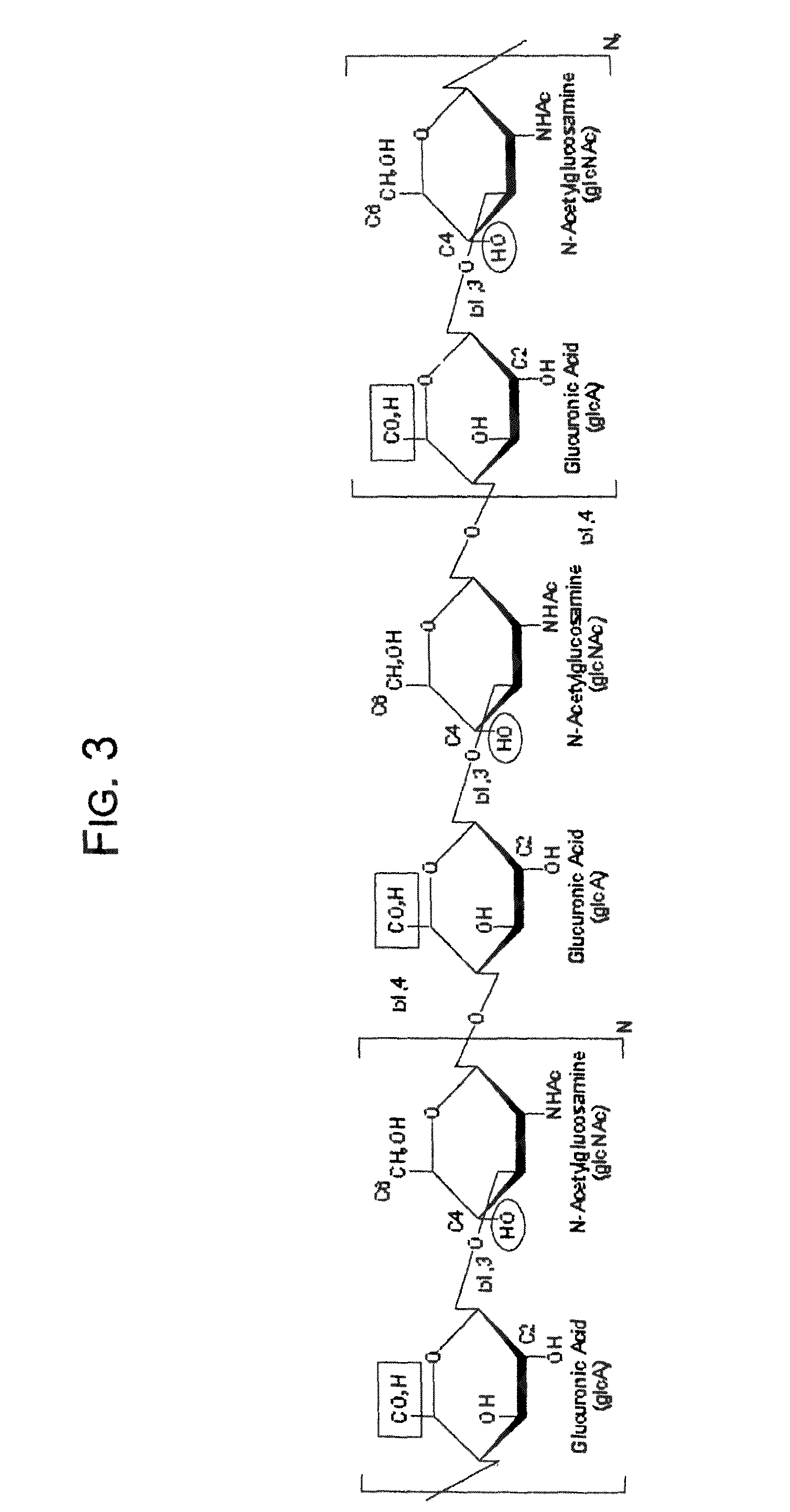
Hydroxyphenyl cross-linked macromolecular network and applications thereof
A synthetic nucleus pulposus is provided that is useful in treatment of degenerative disc disease, augmentation of a degenerate disc, and alleviation of back pain. In an embodiment the synthetic nucleus pulposus comprises hyaluronan macromolecules that have been cross-linked via dihydroxyphenyl linkages. The synthetic nucleus pulposus restores or improves the water-retention capability of the disc. A method of treating an intervertebral disc with the cross-linked hyaluronan macromolecules is also provided. A method of regenerative therapy to address loss of cells of nucleus pulposus of an intervertebral disc based on treatment with the cross-linked hyaluronan macromolecules and mesenchymal stem cells is also provided.
Owner:THE CLEVELAND CLINIC FOUND
Therapeutic Angiogenesis for Treatment of the Spine and Other Tissues
ActiveUS20130230454A1Lower pHDecreased blood flowUltrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsDiseaseSpine disorder
Methods for the diagnosis and treatment of ischemic spinal conditions, degenerative disc disease, back pain and / or other tissue pathologies. Patients with ischemic spine disease can be categorized into subsets that are deemed to have potential to respond to therapy. In particular, therapies are disclosed which involve stimulation of neovascularization so as to increase perfusion of spinal and other anatomies.
Owner:VENTURIS THERAPEUTICS INC
Interspinous distraction devices and associated methods of insertion
In various exemplary embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
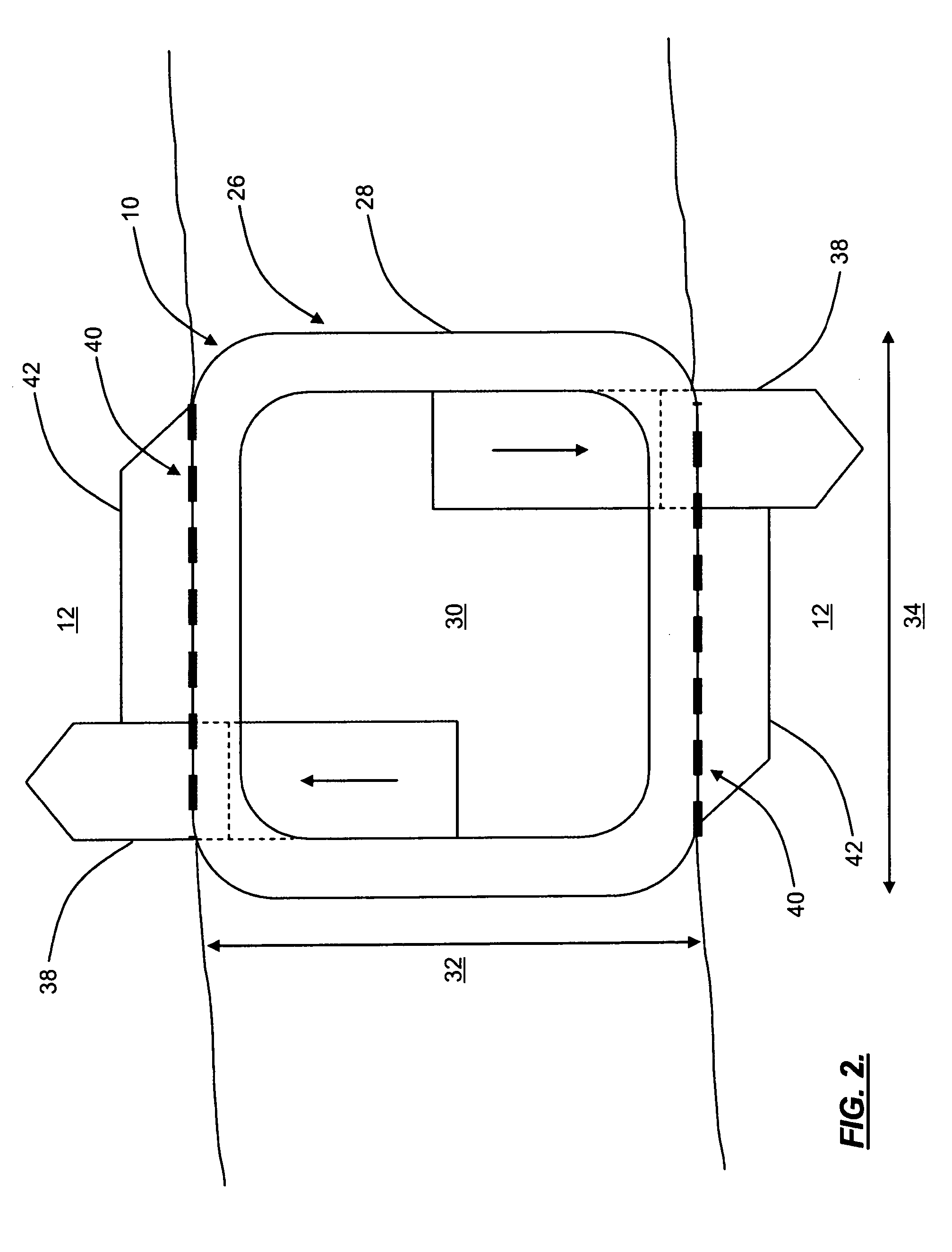
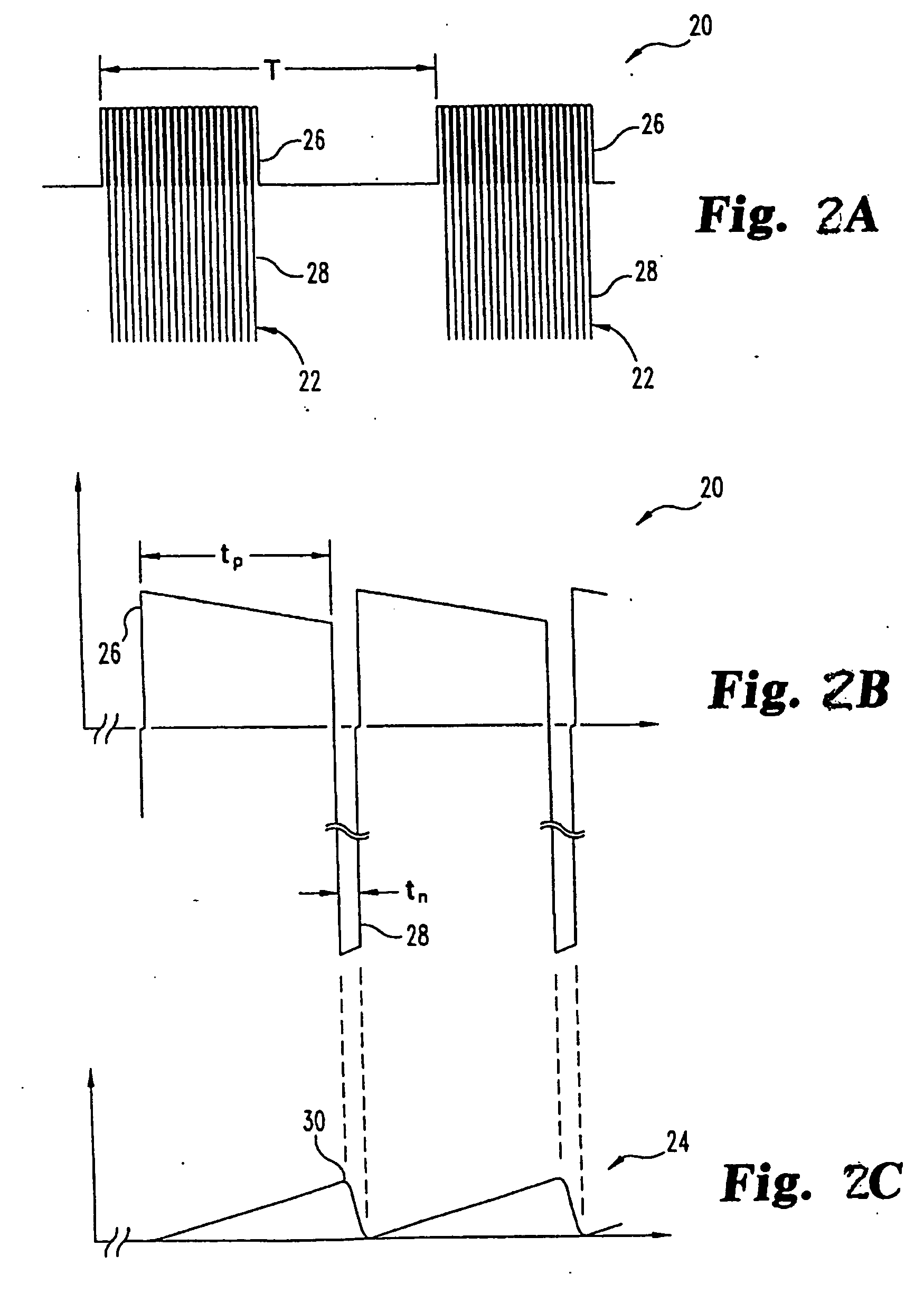
Pulsed electromagnetic field method of treatment of degenerative disc disease
A pulsed electromagnetic field method of treating degenerative disc disease, wherein, in one embodiment, a patient in need of treatment for degenerative disc disease is administered a pulsed electromagnetic field (PEMF) having repetitive pulse bursts approximately 5 ms in duration, with a pulse burst repetition rate of approximately 15 Hz.
Owner:EUROPEAN BIOINFORMATICS INSTITUTE
Hydroxyphenyl cross-linked macromolecular network and applications thereof
A synthetic nucleus pulposus is provided that is useful in treatment of degenerative disc disease, augmentation of a degenerate disc, and alleviation of back pain. In an embodiment the synthetic nucleus pulposus comprises hyaluronan macromolecules that have been cross-linked via dihydroxyphenyl linkages. The synthetic nucleus pulposus restores or improves the water-retention capability of the disc. A method of treating an intervertebral disc with the cross-linked hyaluronan macromolecules is also provided. A method of regenerative therapy to address loss of cells of nucleus pulposus of an intervertebral disc based on treatment with the cross-linked hyaluronan macromolecules and mesenchymal stem cells is also provided.
Owner:THE CLEVELAND CLINIC FOUND
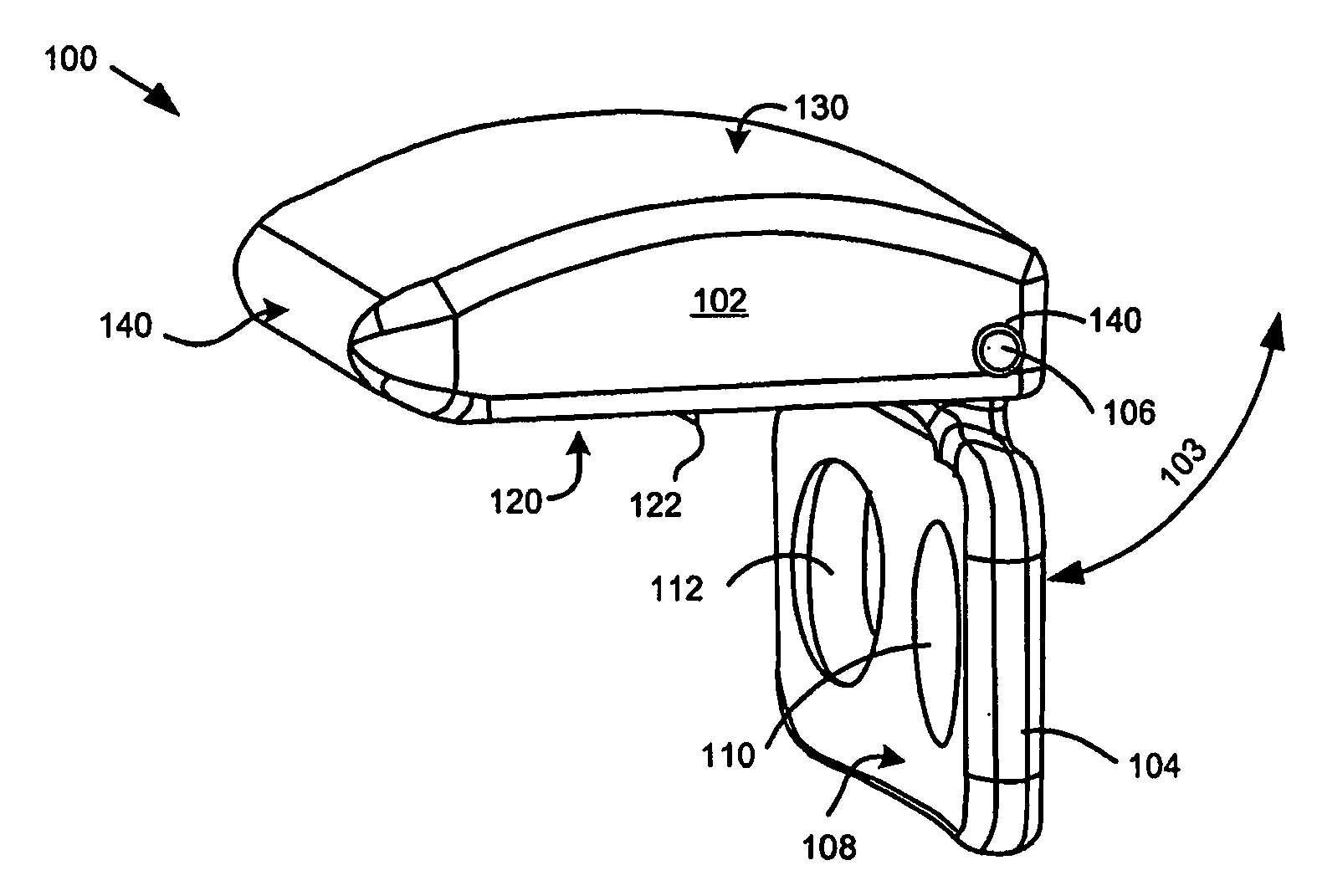
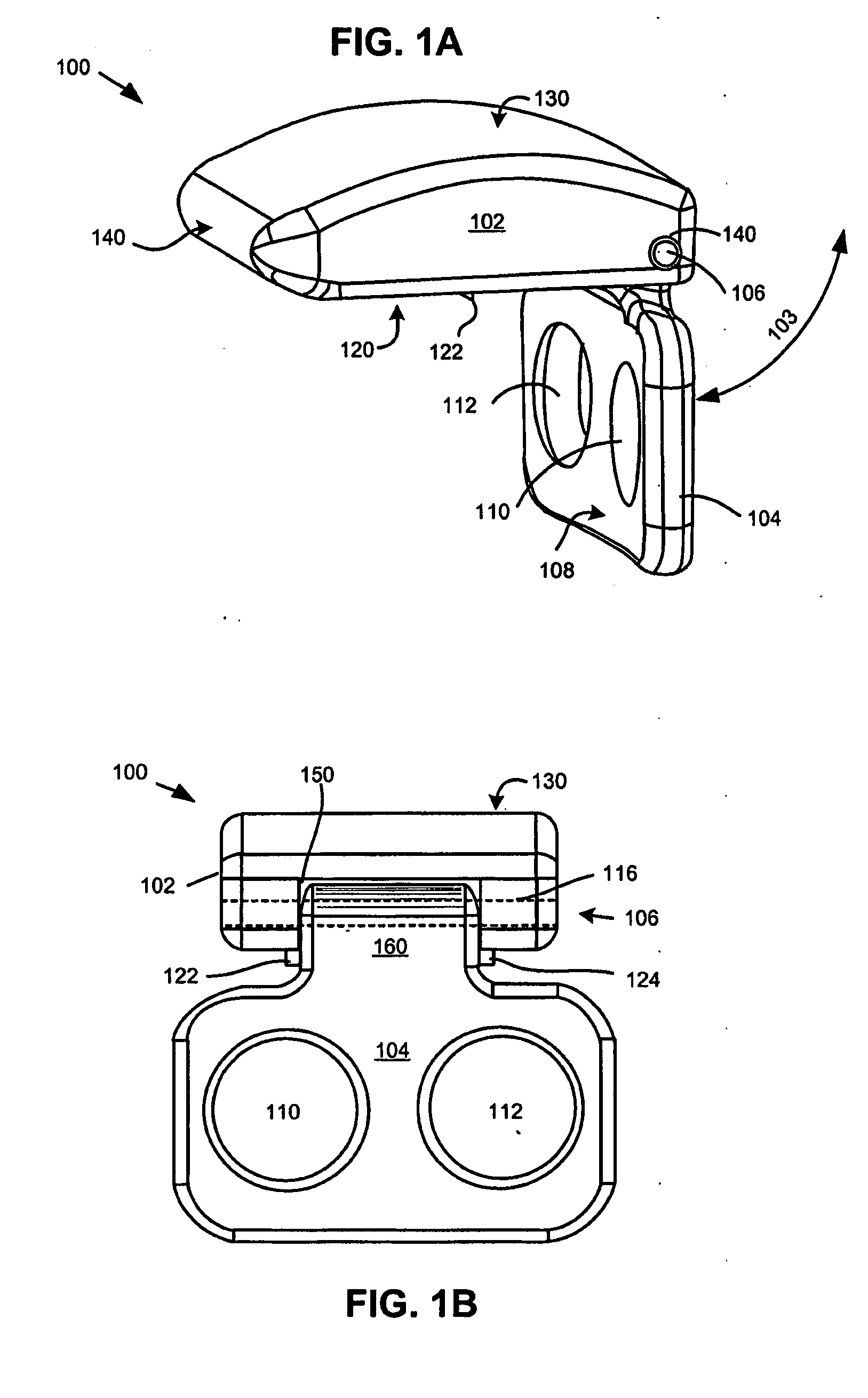
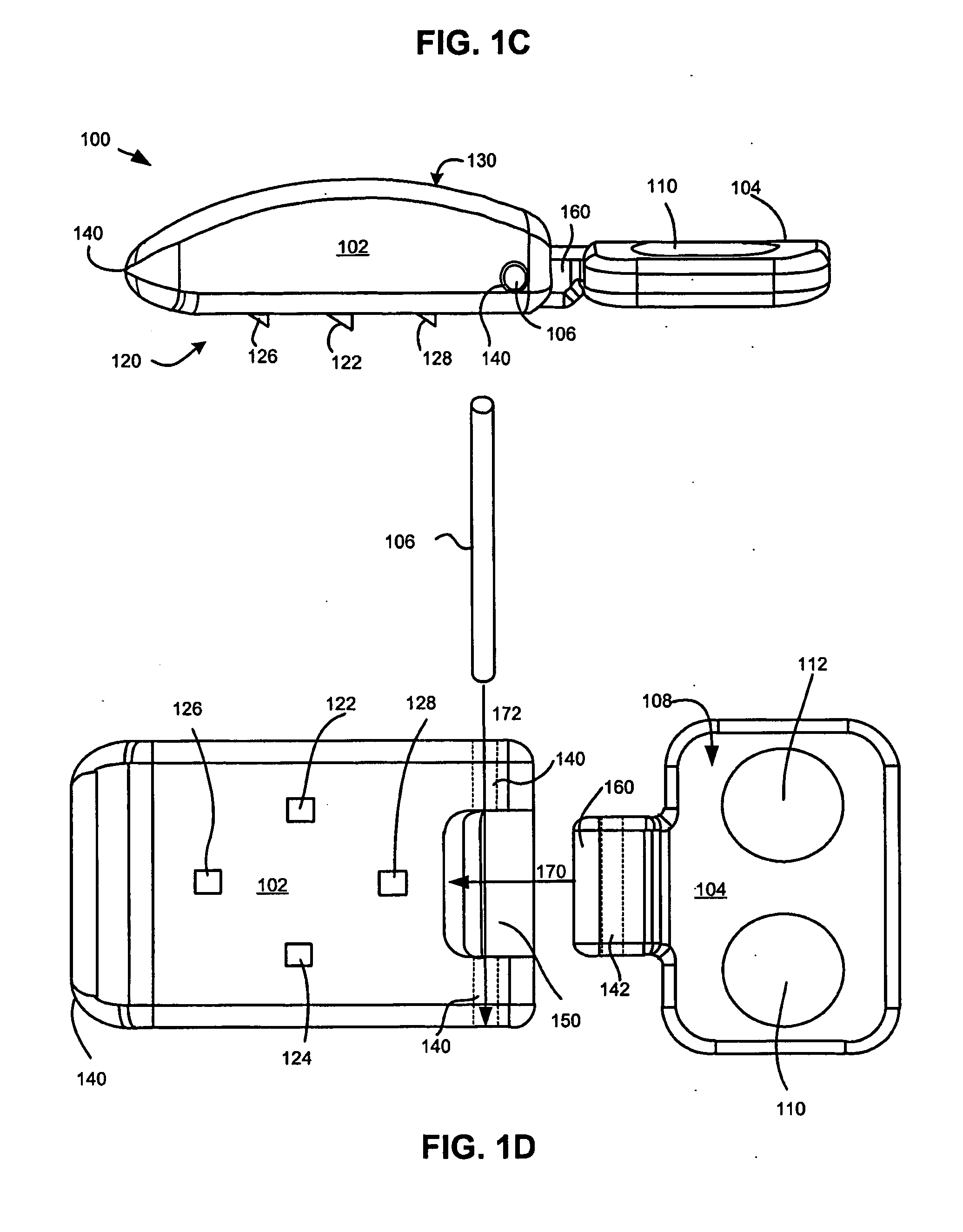
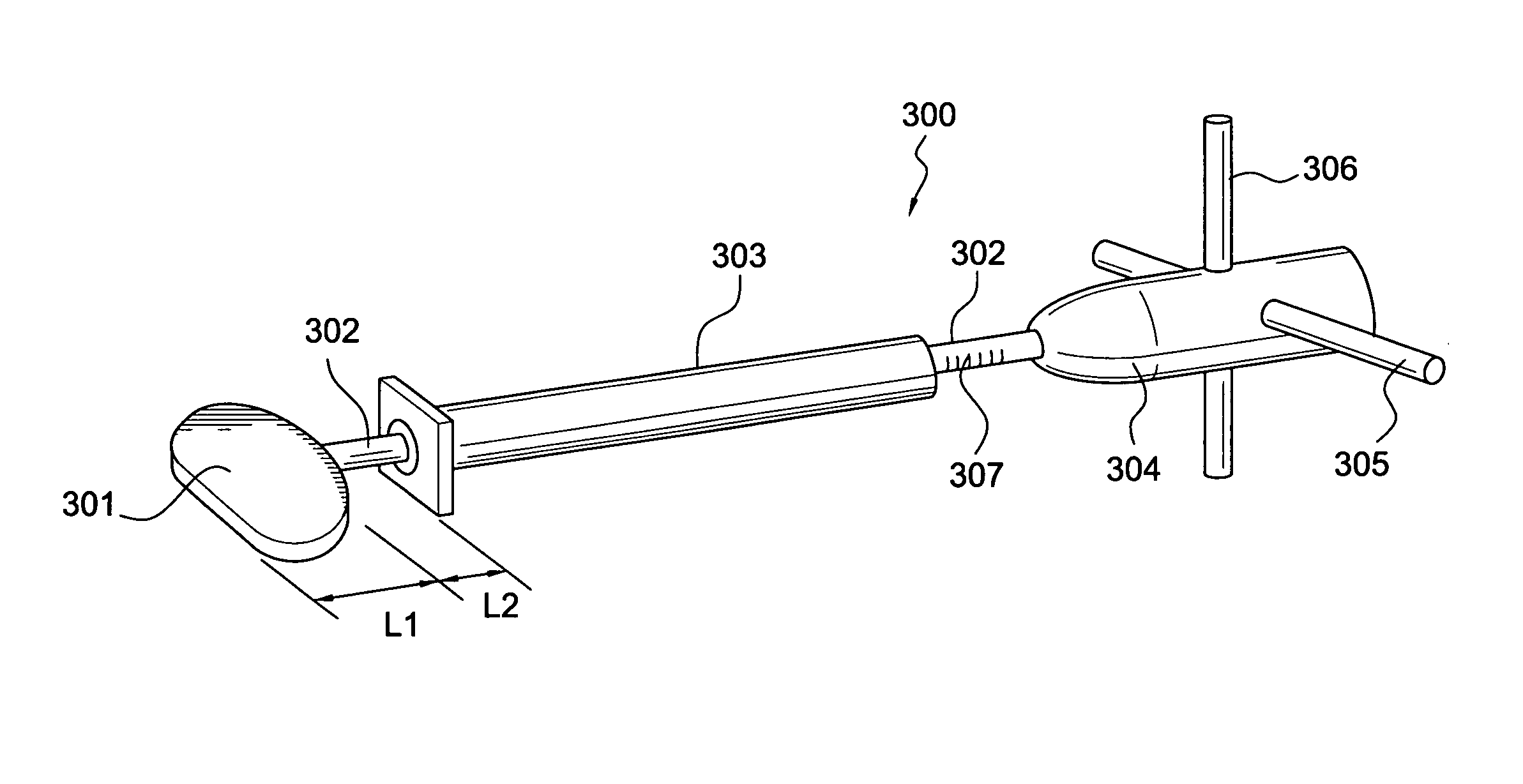
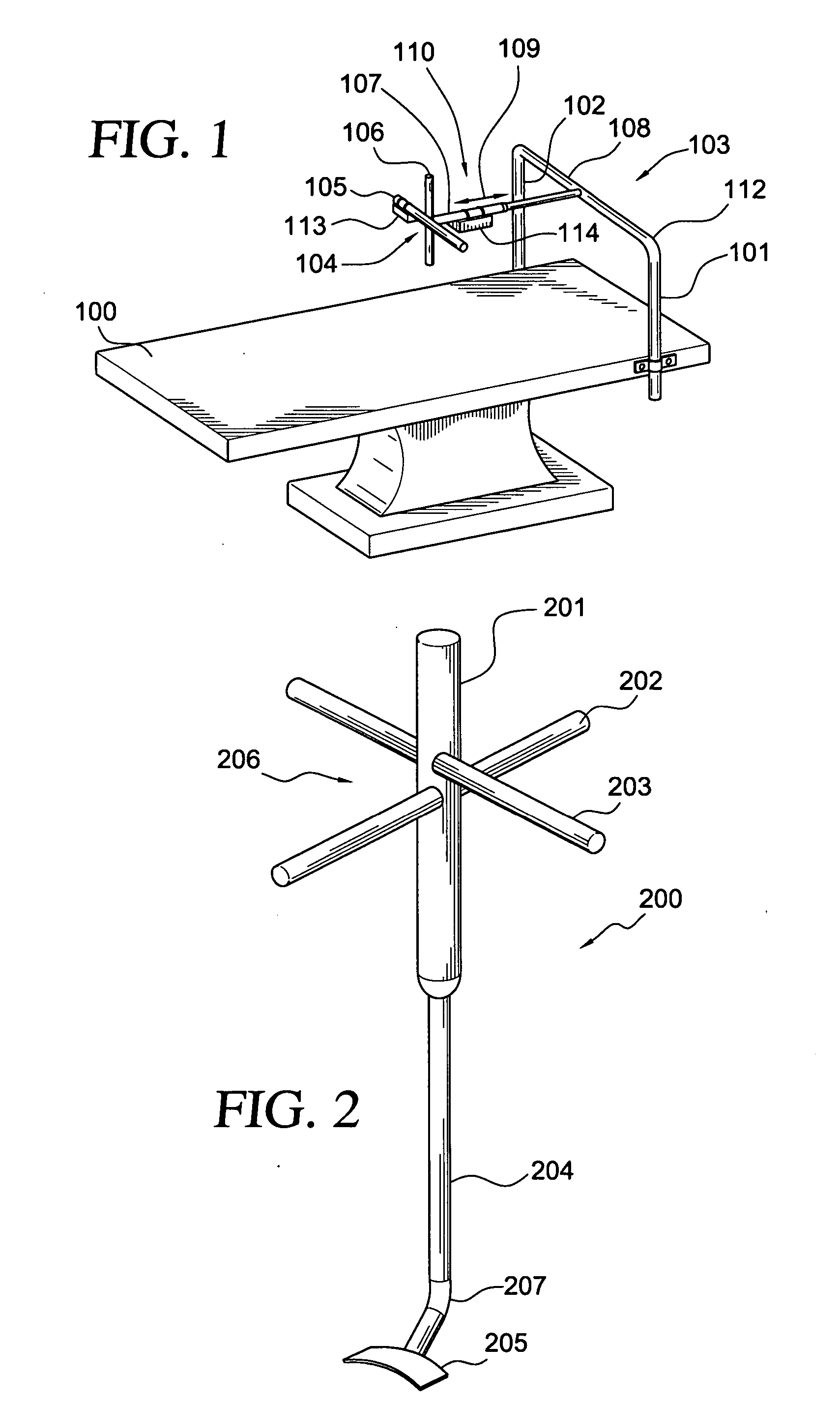
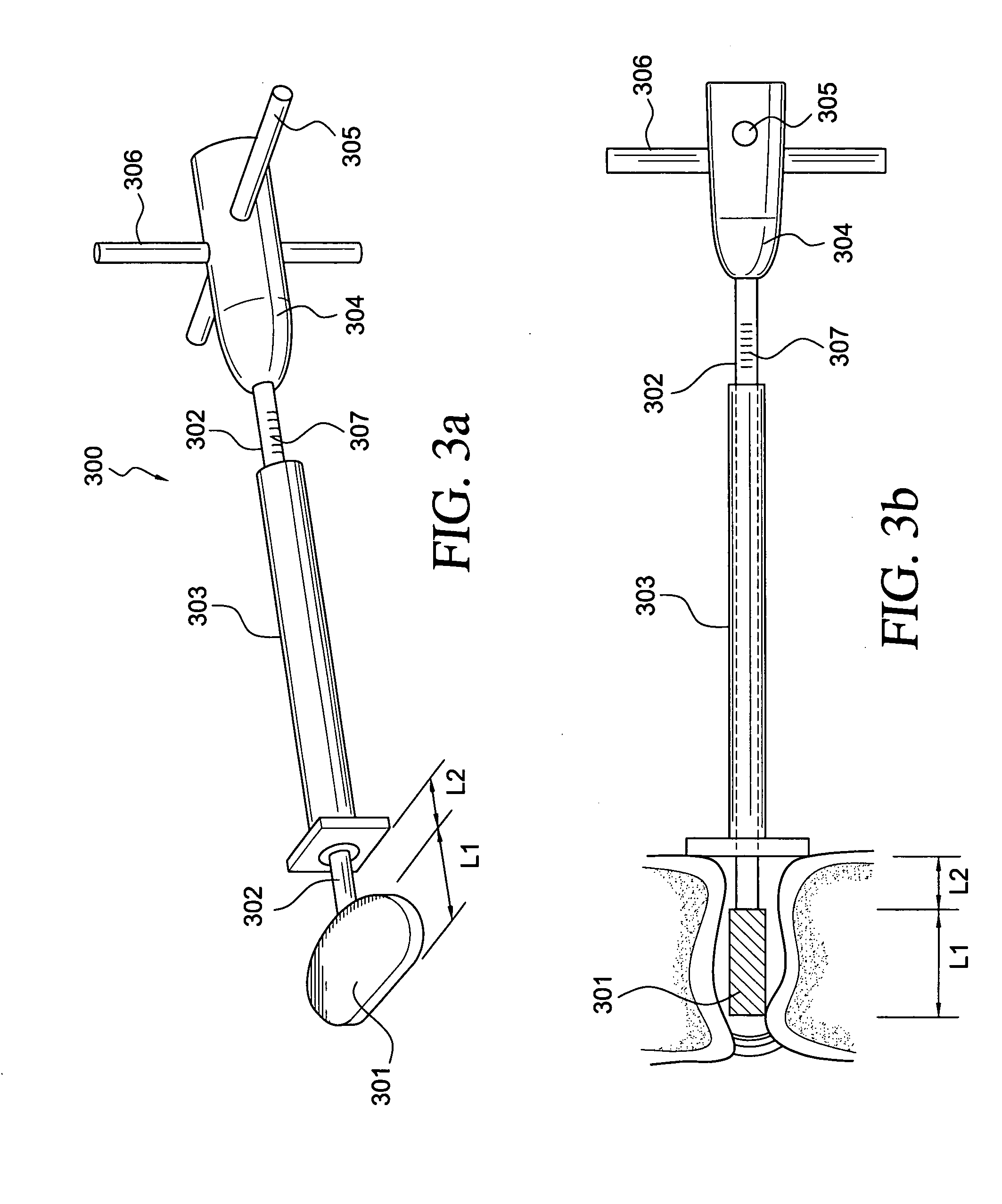
Intervertebral disc replacement and surgical instruments therefor
InactiveUS20060276800A1Precise positioningSmall sizeDiagnosticsSpinal implantsSurgical departmentDegenerated intervertebral disc
A suite of surgical tools for use in implantation of an intervertebral disc prosthesis is provided. The surgical tools include an external alignment frame (EAF), an annulus incision guide (AIG), a depth gauge, a positioning / alignment block, a disc space distractor, an endplate drill guide (EDG), and a vertebral endplate cutter (VEC), any or all of which can be used by a surgeon in a procedure for preparing an implantation site by excising all or part of a degenerated intervertebral disc and implanting an intervertebral disc prosthesis in the site so prepared.
Owner:NEXGEN SPINE
Enhanced adipose tissue
InactiveUS20060018887A1Good treatment effectIncrease heightBiocidePeptide/protein ingredientsDiseaseActive agent
An enhanced adipose tissue containing native adipose tissue and a concentrated amount of an active agent derived from adipose tissue, wherein the enhanced adipose tissue is preferably injected into the intervertebral disc of a patient suffering from degenerative disc disease.
Owner:DEPUY SPINE INC (US) +1
Systems and methods for reducing adjacent level disc disease
InactiveUS20100069961A1Reduce chanceResisting compressionInternal osteosythesisJoint implantsDiseaseRange of motion
Owner:ZIMMER SPINE INC
Red light implant for treating degenerative disc disease
ActiveUS20070073363A1Effective in cartilage repairUseful in therapyDiagnosticsSurgeryDiseaseDegenerated intervertebral disc
Owner:DEPUY SYNTHES PROD INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com