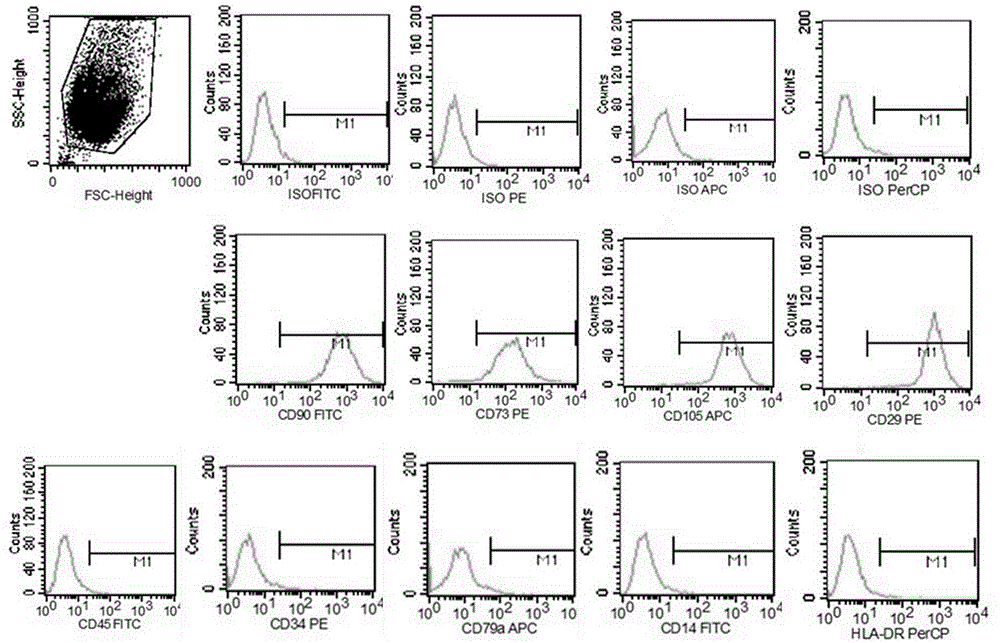
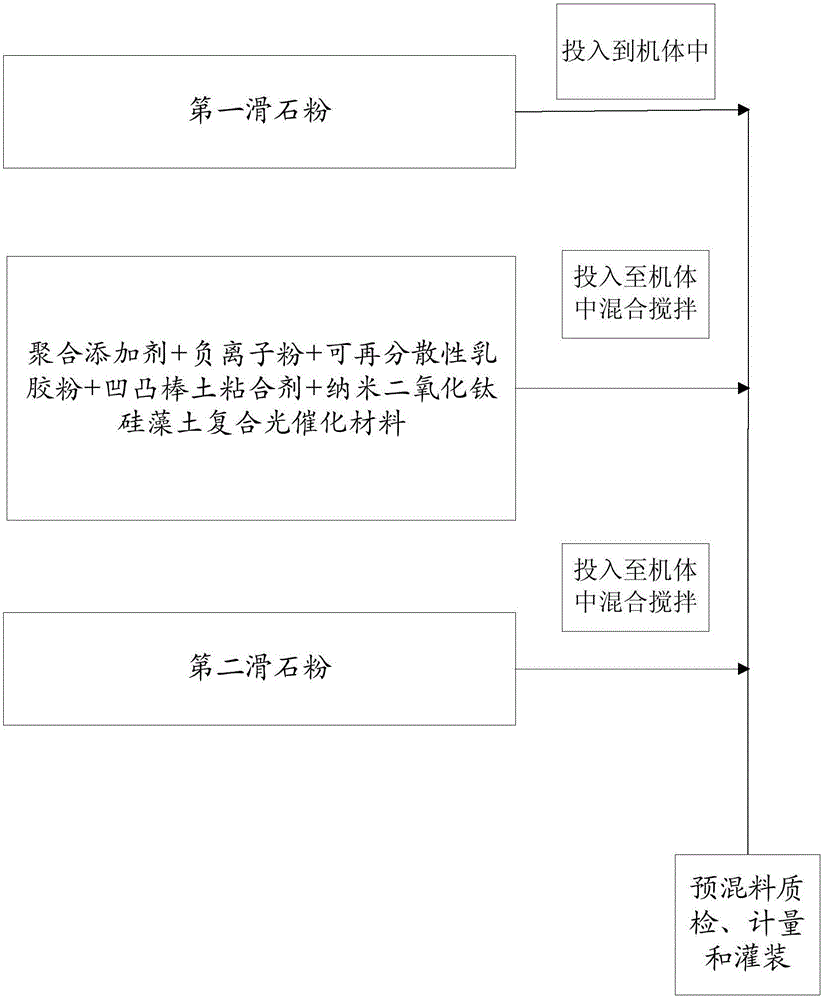
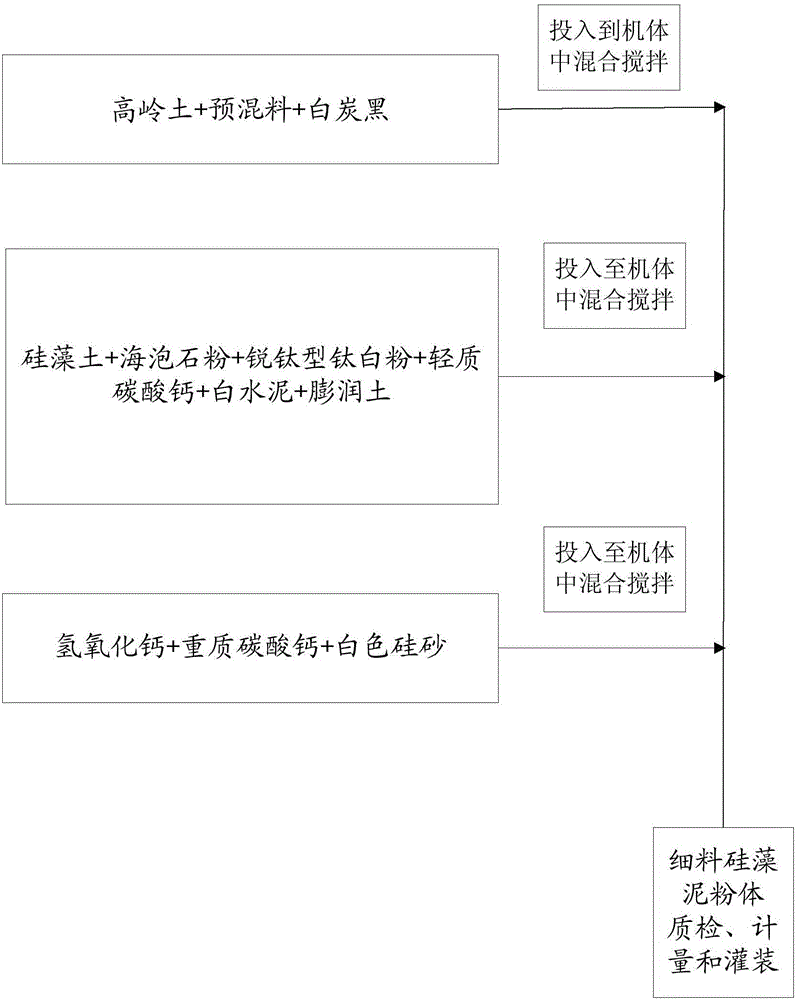
Patents
Literature
116 results about "Fusion rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
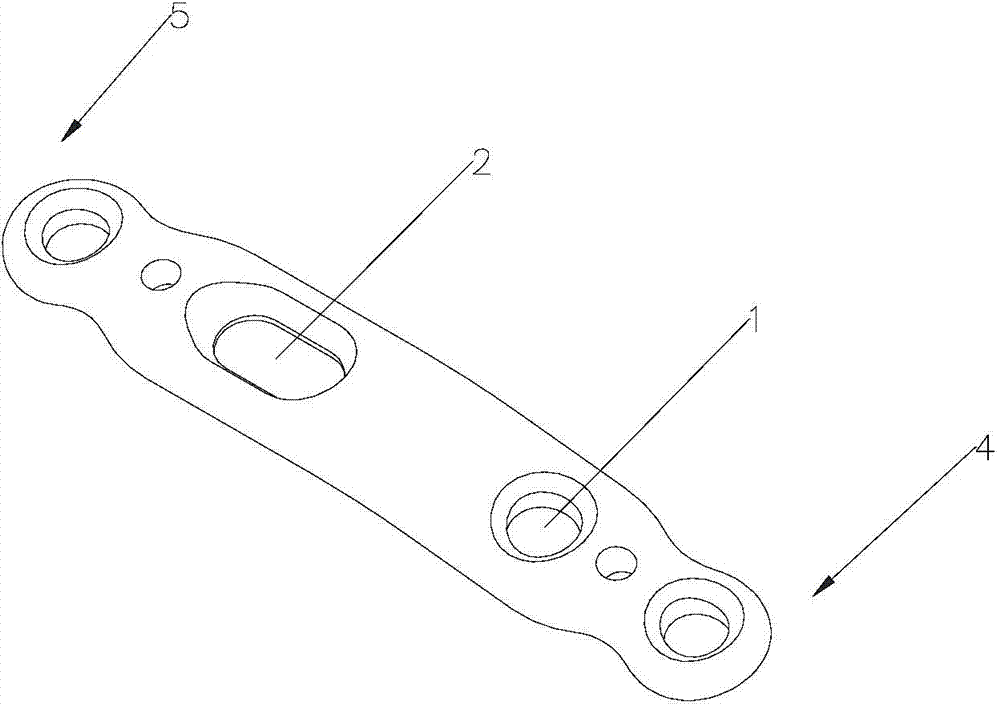
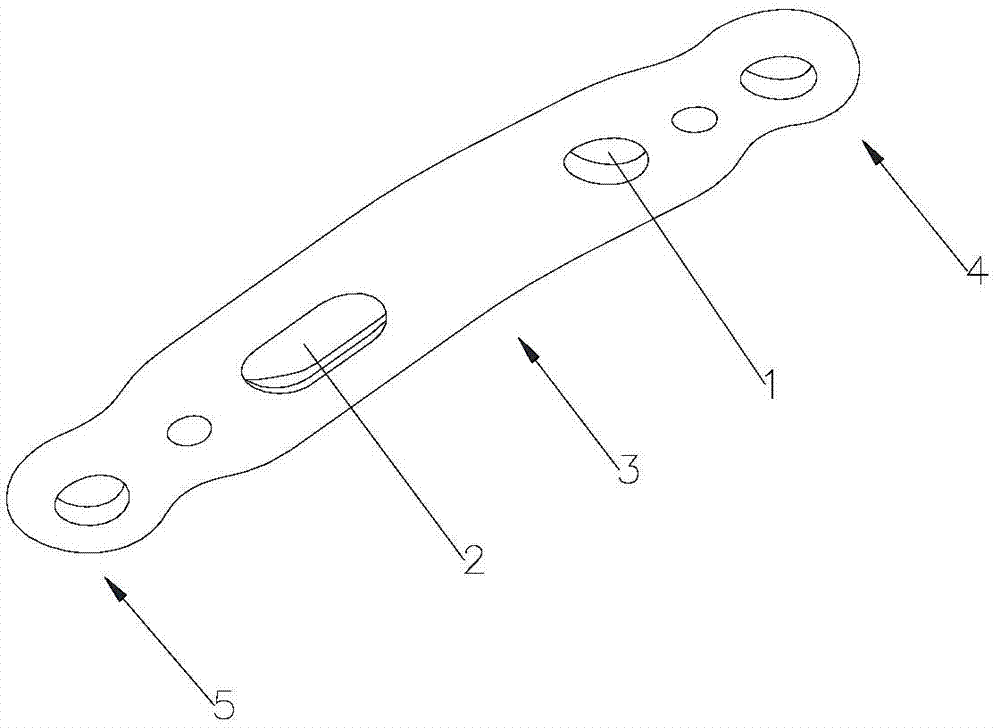
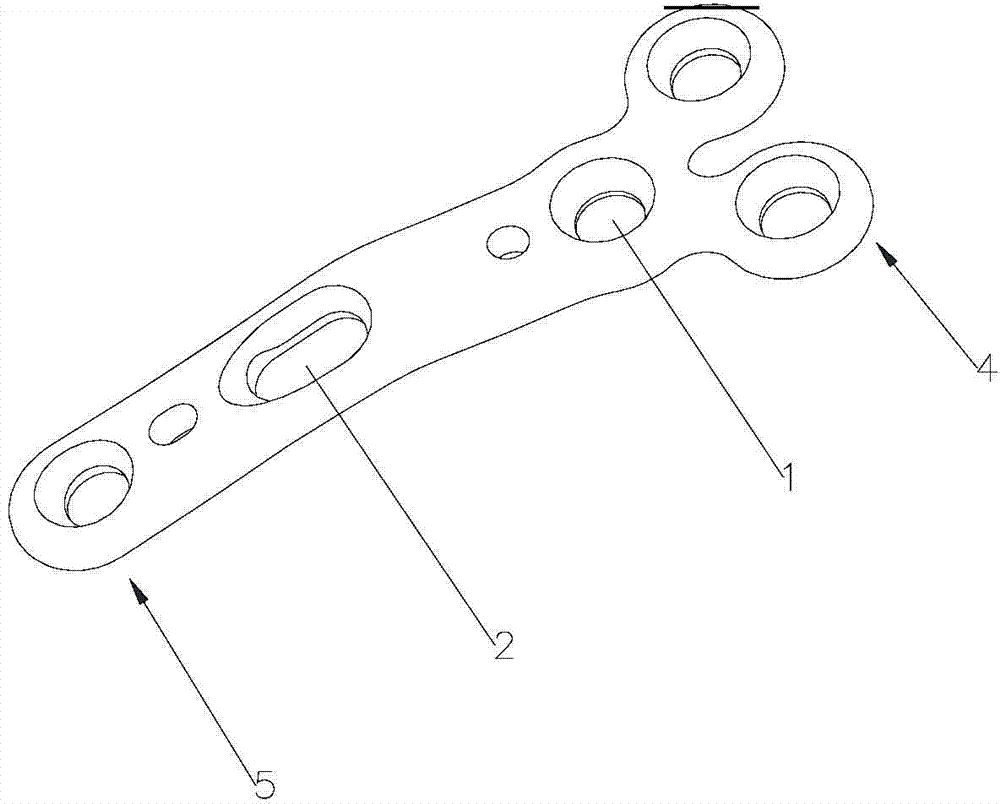
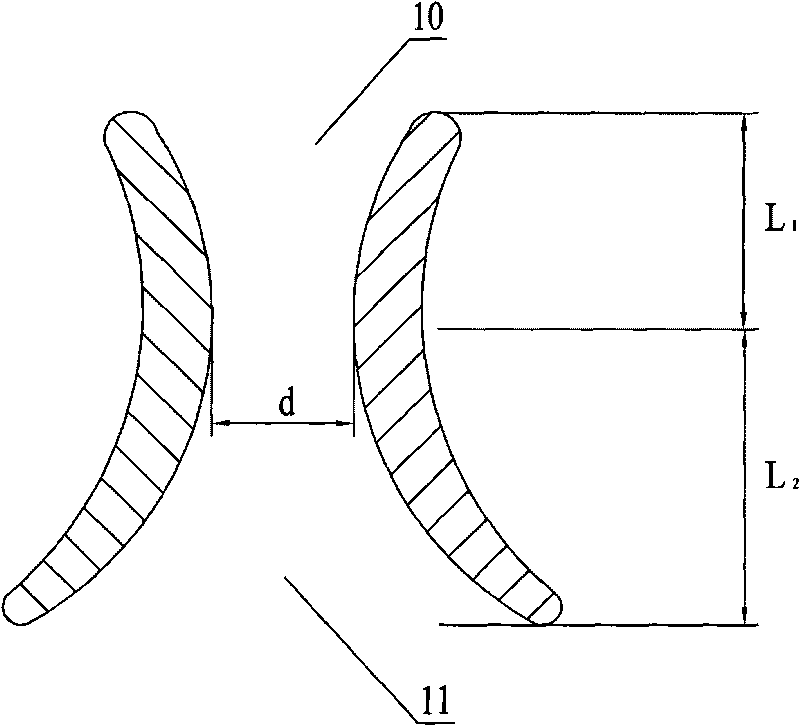
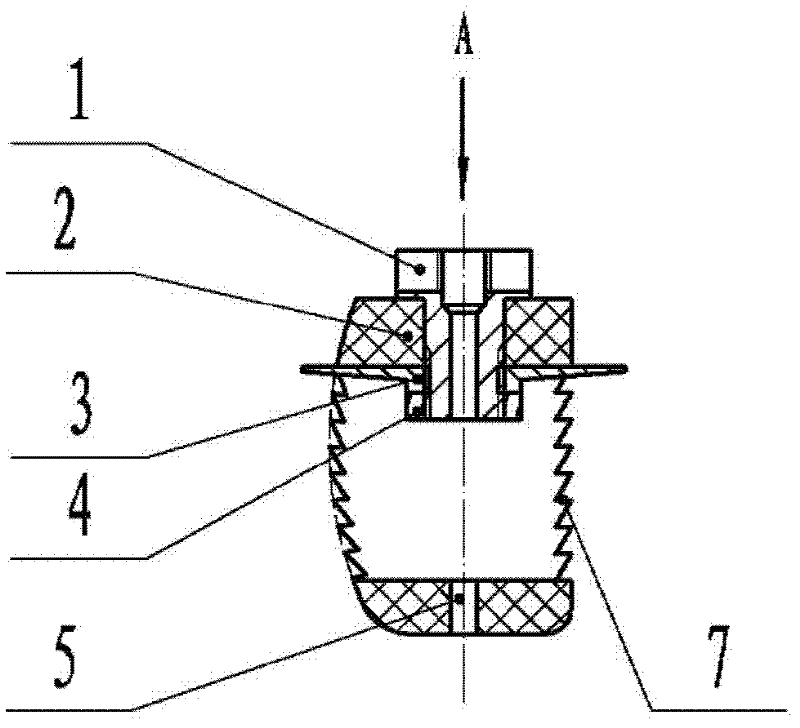
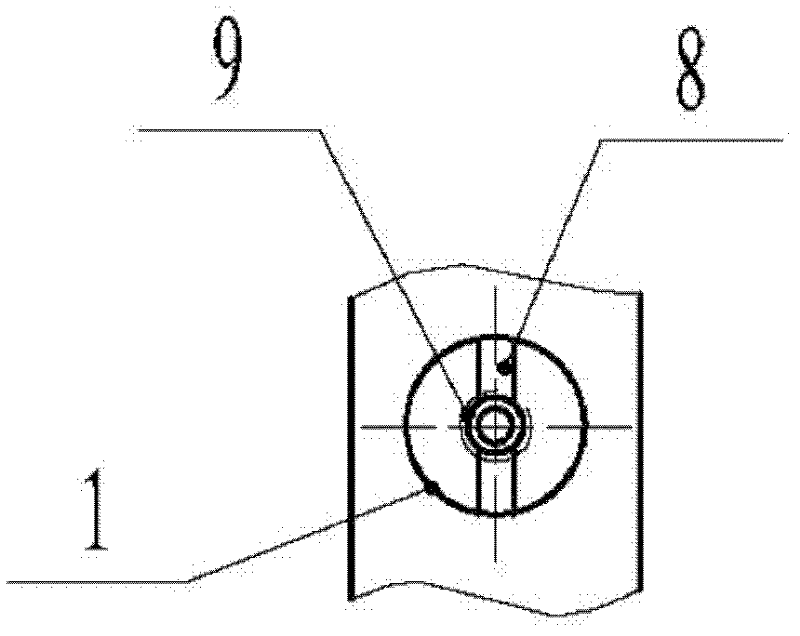
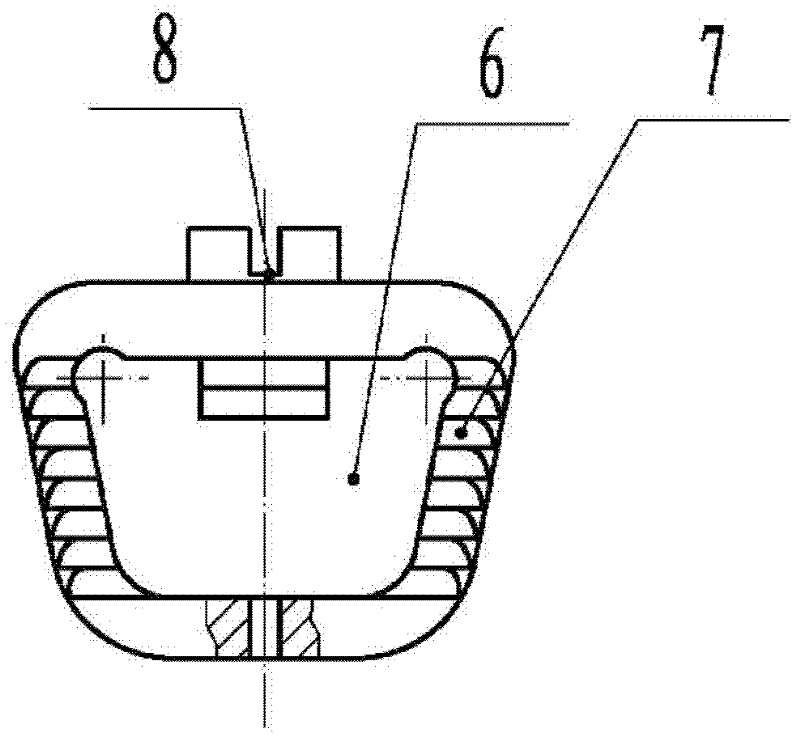
First tread wedge joint plate
The invention relates to the technical field of medical machinery, in particular to a first tread wedge joint plate. The first tread wedge joint plate comprises a plurality of fixing holes which are used for fixing the first tread wedge joint plate on a first metatarsal bone and an internal side wedge bone; a sliding hole which enables pressing between bones to be convenient during fusion of the first tread wedge joint is formed in the first tread wedge joint plate. According to the first tread wedge joint plate, tender moving of the first tread wedge joint surface is allowed when the bridge first tread wedge joint plate is fixed, namely, the first tread wedge joint surface gristle is kept and the joint is limited from moving; when the first tread wedge joint fuses the first tread wedge joint plate to be fixed, bone grafting after joint surface gristle is removed, pressing of the first tread wedge joint surface is achieved through a sliding hole at a far end of the first tread wedge joint plate, and accordingly the fusion rate is improved; the first tread wedge joint plate is in an anatomy type design, anatomical reduction is convenient, pre-bending of the first tread wedge joint plate during operation is not required, and the intensity of the first tread wedge joint plate is avoided from being influenced by bending during the operation.
Owner:上海斯地德商务咨询中心
Mixed porous structure interbody fusion cage and preparation method thereof
InactiveCN102440852AGood mechanical compatibilityGood bone conductionSpinal implantsFreeze-dryingReticular formation
Disclosed are a mixed porous structure interbody fusion cage and a preparation method thereof. The interbody fusion cage comprises a porous metal support and porous structure filling bodies, the porous metal support is a three-dimensional net-shaped structure, a plurality of holes are arranged in the porous metal support, and the porous structure filling bodies are fully filled in the holes. The preparation method includes steps that the metal rapid forming technology is directly combined with the freeze drying technology, the porous metal support is manufactured via a structural design and the direct metal rapid forming technology, then uniformly mixed polymer gel or polymer / biological ceramic compound gel is poured in the porous metal support to realize freeze treatment, so that the porous structure filling bodies with the micropore feature are formed after freeze drying, and the mixed porous structure interbody fusion cage is obtained. Mechanical compatibility is good, contact area between the mixed porous structure interbody fusion cage and natural centrum is further increased, instant stability is good, fusion rate is improved, and the mixed porous structure interbody fusion cage and the preparation method thereof can be used for treating clinical degenerative disc diseases.
Owner:SHANGHAI JIAO TONG UNIV
Fabric including low-melting fiber
InactiveUS20100317248A1Satisfactory flexibilityUse in some applicationFlame-proof filament manufactureWoven fabricsYarnFiber
Fabrics including a low-melting fiber are provided. In an embodiment, the fabric includes a regular fiber and a low-melting fiber. The low-melting fiber is directly included in either warps or wefts or both. Alternatively, a blended or plied fiber of the regular fiber and the low-melting fiber is included in either warps or wefts or both. The low-melting fiber has a fusion rate of 30 to 100%. The fabric has a yarn slip length of 0.1 to 2.5 mm.
Owner:WOONGJIN CHEM
Method for separating and culturing umbilical cord blood mesenchymal stem cells
ActiveCN104630144AIncrease growth rateHigh recovery rateSkeletal/connective tissue cellsPrimary cellBottle
The invention provides a method for separating and culturing umbilical cord blood mesenchymal stem cells. The method comprises the steps of carrying out secondary separation on umbilical cord blood mononuclear cells by using a separating medium, and then inoculating the umbilical cord blood mononuclear cells into a culture bottle in which fibronectin and CD90 monoclonal antibodies are coated; and culturing for 4-5 days by using a serum-free culture system and simulating the in-vivo low-oxygen growth environment of the mesenchymal stem cells, then, removing suspension cells, further culturing adherent cells, and subculturing after the fusion rate of primary cells is up to 60%. By using the method for separating and culturing umbilical cord blood mesenchymal stem cells, provided by the invention, the problems of adherence infirmness, low culture success rate, low purity and the like caused in the extraction and culture processes of the umbilical cord blood mesenchymal stem cells are effectively solved, and the safety in clinical application is improved. In addition, the application ranges of the umbilical cord blood mesenchymal stem cells are widened, the utilization values of the umbilical cord blood mesenchymal stem cells are developed, and the clinical application prospects of the umbilical cord blood mesenchymal stem cells are widened.
Owner:中国医科大学 +1
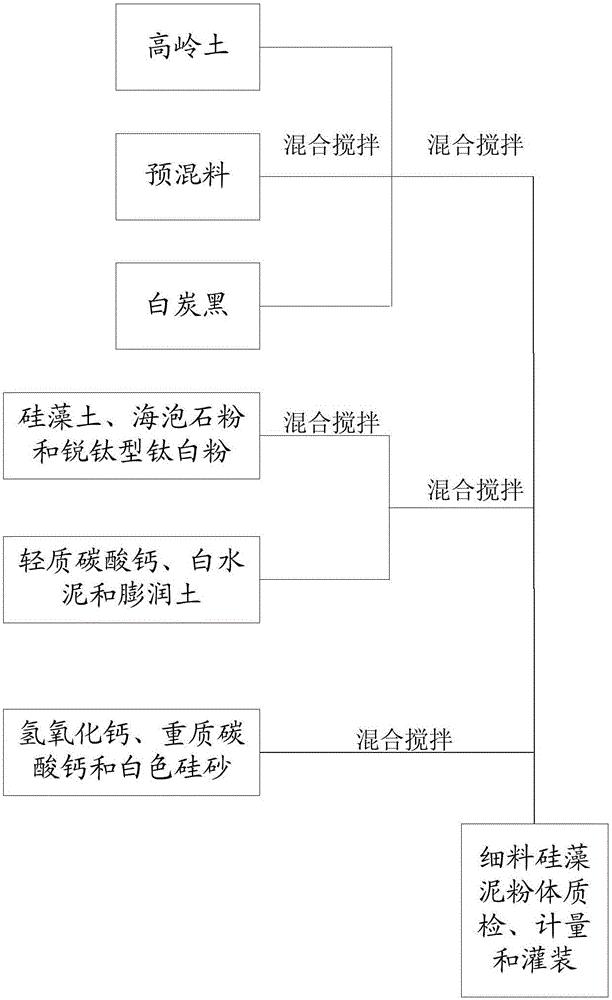
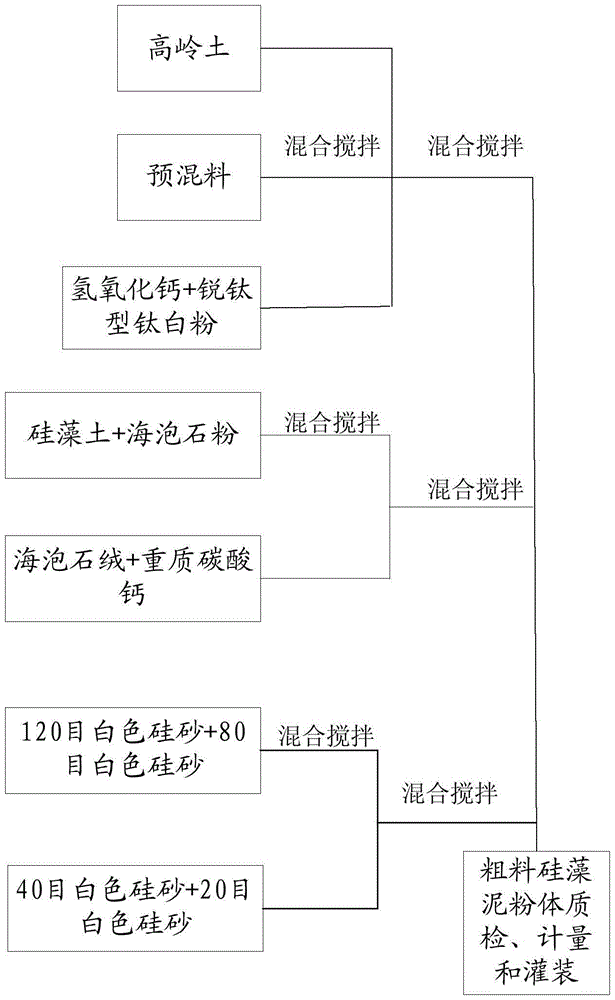
Fine diatom ooze powder and production technology thereof
The invention provides a production technology of fine diatom ooze powder. The technology is simple. The water solubility of the diatom ooze powder is improved through specific mixing steps, the fusion rate of the diatom ooze powder and water is increased, the construction window phase is prolonged, and the long-term using effect can be achieved through one-time stirring. The surface decorative effect problem caused when diatom ooze products in the current market are constructed and coated on a wall can be solved, and the construction and coating efficiency of the diatom ooze products can be improved. The stirring time is short in the using process, energy consumption is reduced, and the overall cost of the diatom ooze products is lowered.
Owner:石家庄星海高科非金属矿业材料有限责任公司
Laser repair method for worn bearing cylinder of gas turbo-expander
ActiveCN102127763AImprove mechanical propertiesAchieve the purpose of repairMetallic material coating processesHardnessEngineering
The invention discloses a laser repair method for a worn bearing cylinder of a gas turbo-expander after wear. The method comprises the following steps of: detecting the size of each part of a bearing cylinder matrix of the gas turbo-expander before repair and determining a worn part and the size of the worn part; smoothly grinding the surface of a workpiece; cleaning to remove a fatigue layer of a damaged part of the bearing cylinder; detecting hardness and components of the material of the bearing cylinder matrix; preparing nickel-based alloy powder according to a detection result, preheating the bearing cylinder to the temperature of between 80 and 150 DEG C and fixing the bearing cylinder on a laser machine tool; adjusting operating data of a laser, setting parameters of a laser cladding technology, prearranging a powder layer with thickness of 2mm, and performing laser cladding on the damaged part of the bearing cylinder; and detecting and finishing after repair. The method has the advantages that: a repair layer has a few cracks and has high fusion rate with a raw material, the performance is greatly improved after repair, and the like.
Owner:河北瑞兆激光再制造技术股份有限公司
Method for separating and amplifying mesenchymal stem cells from umbilical cord
InactiveCN102660503AShorten the timeThe time is shortened to affect the effect of tissue adheringSkeletal/connective tissue cellsUmbilical cord tissueBottle
The invention relates to a method for separating and amplifying mesenchymal stem cells from an umbilical cord. The method comprises the following steps of: disinfecting and cleaning an umbilical cord tissue; cutting the tissue into pieces, and paving in another cell culture plate to ensure that the tissue pieces are air-dried until the tissue is attached to the plate; culturing the umbilical cord tissue in a culture medium special for the mesenchymal stem cells, supplementing / replacing liquid and clearing all umbilical cord tissue pieces when the umbilical cord tissue is cultured for 11 to 13 days, and continuing to culture; completely replacing liquid every 1 to 3 days later on; separating wall attaching cells from the bottom of the plate by using digestive enzyme when the fusion rate of the wall attaching cells in the plate reaches about 50-70 percent; centrifuging and removing a supernatant, adding into the culture medium special for the mesenchymal stem cells, suspending the cells again, and inoculating in a T25 cell culture bottle for subculture and amplification culture; and replacing liquid once every 1 to 3 days later on until the fusion rate reaches 70-90 percent to obtain the mesenchymal stem cells. The method can be effectively used for separating and amplifying the mesenchymal stem cells.
Owner:BOYALIFE
Extraction and fusion method for strawberry protoplast
InactiveCN102505004AIncrease vitalityIncrease the probability of one-to-one fusionGenetic engineeringFermentationResearch ObjectPolyethylene glycol
The invention provides an optimal isolation and fusion method for strawberry protoplasts, which utilizes strawberry leaves as research objects. According to the method, the protoplasts with high activity are obtained with both the pre-treatment method and the enzymolysis method, and 30-45% polyethylene glycol (PEG) 6000 is combined with high Ca<2+> method and high pH method so as to induce one-to-one protoplast fusion with improved probability. The method disclosed by the invention has the advantages of mild action, more than 85% of the activity of the isolated protoplast, one-to-one fusion rate up to 11.7%, simple operation, and possibility of forming hybrid cell. The method is suitable for inducing and culturing the new breed by overcoming distant hybridization barrier, and has certain significance in the biological breeding aspect.
Owner:TONGHUA NORMAL UNIV
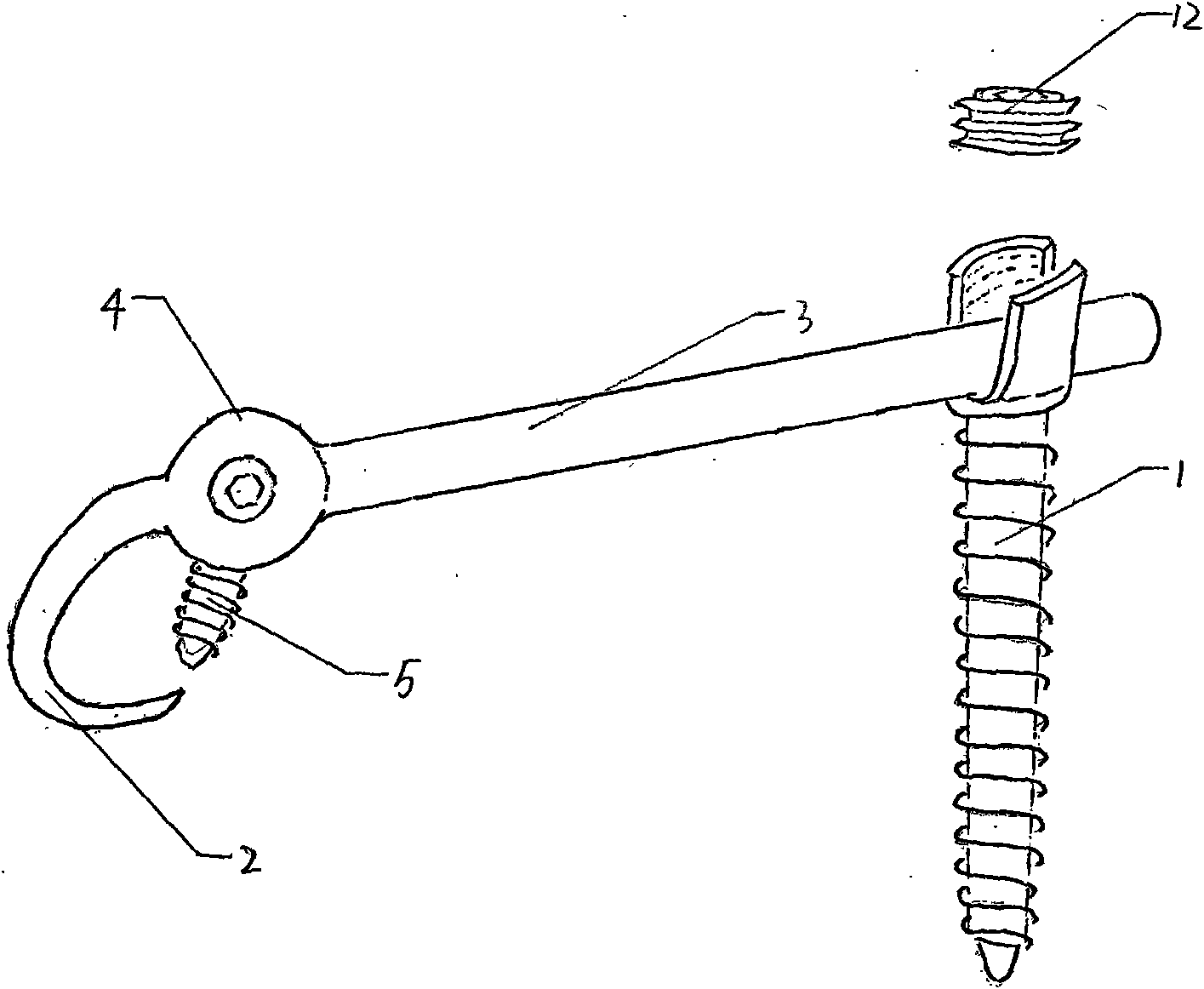
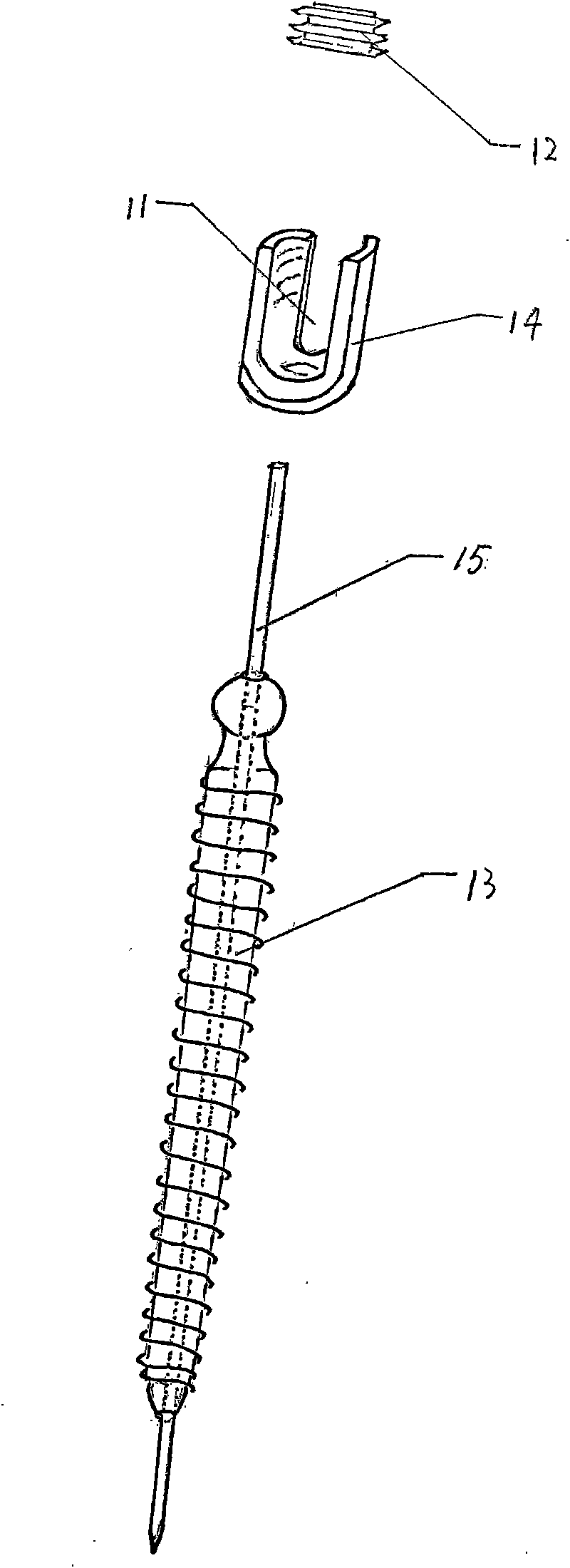
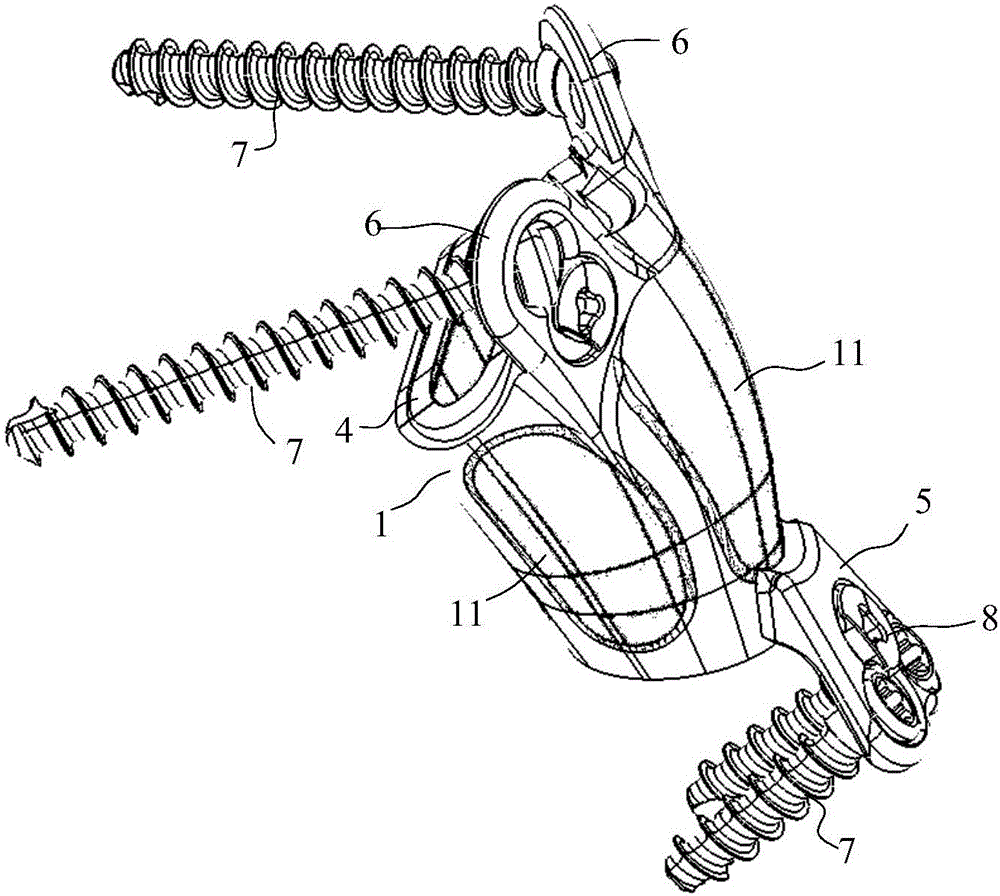
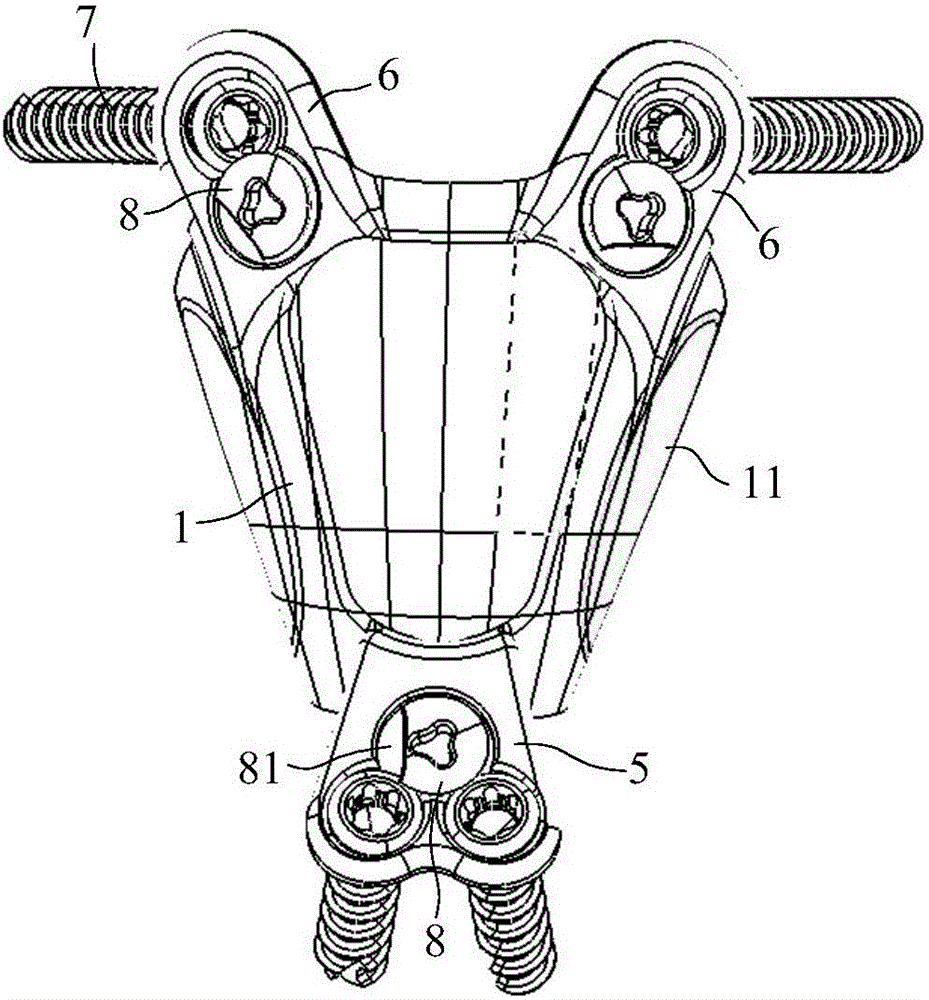
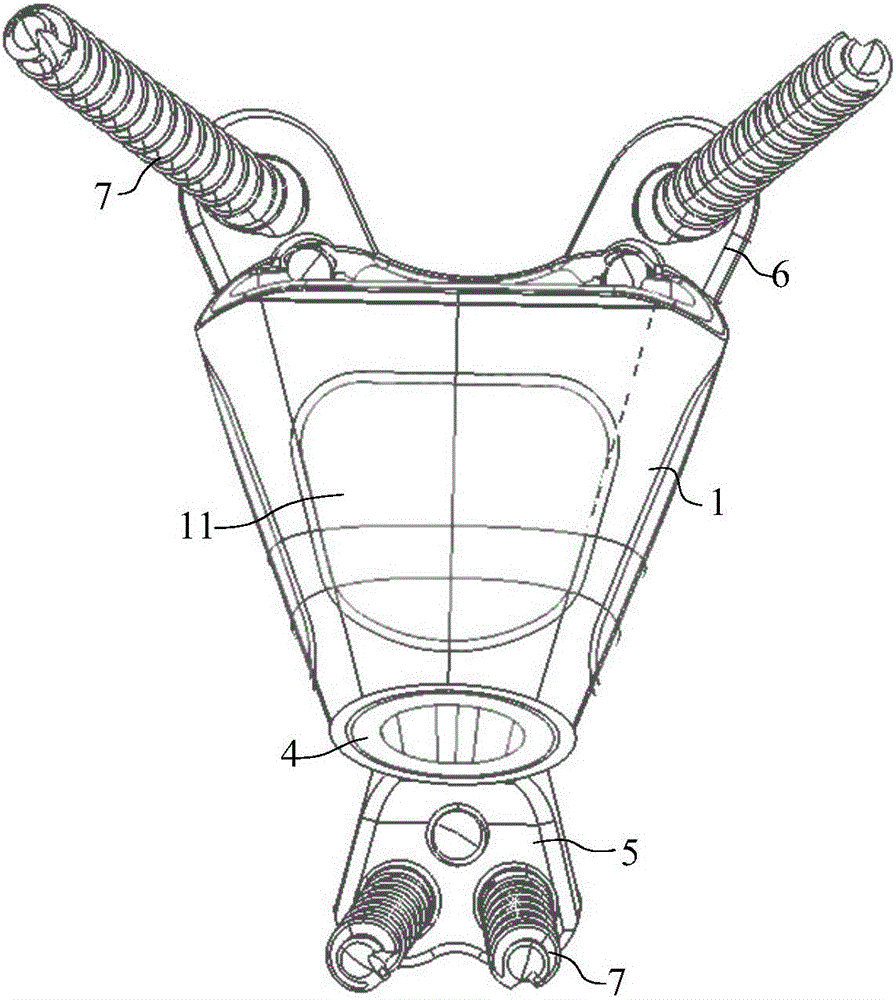
Lumbar spondylolysis restoration internal fixation system
InactiveCN101579253AEliminate frettingEliminate instabilityInternal osteosythesisEngineeringInternal fixation
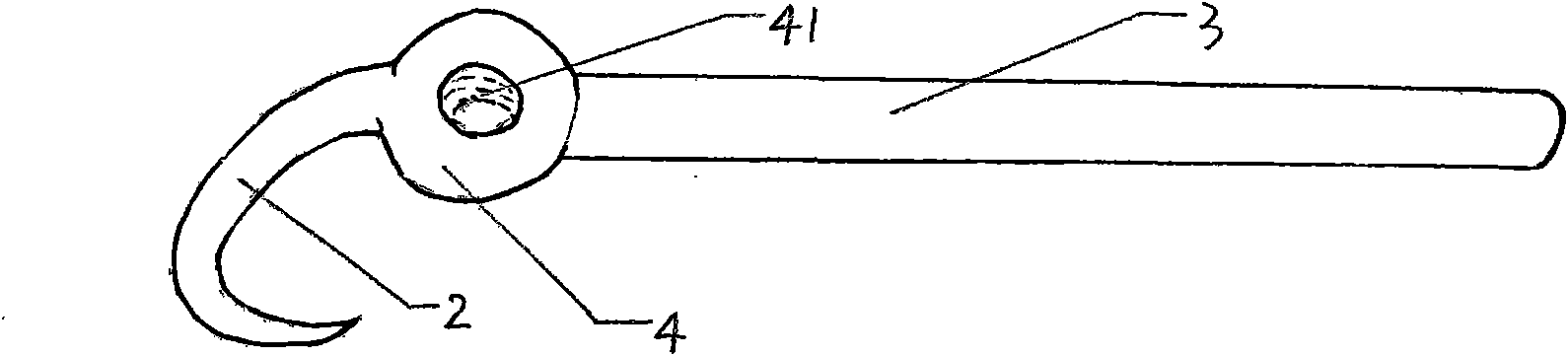
The invention relates to a lumbar spondylolysis restoration internal fixation system, comprising a pedicle screw implanted into pedicle and a vertebral plate hook hooking the lower edge of a vertebral plate; the pedicle screw is connected with the vertebral plate hook by a cylindrical connecting rod; the head part of the pedicle screw is provided with a U-type groove; the connecting rod can be arranged in the U-type groove in a sliding manner; the top part of the U-type groove is provided with internal threads; the U-type groove is connected with a plug screw which is used for fixing the connecting rod; the vertebral plate hook is fixedly connected to the connecting rod by a locking plate; the vertebral plate hook is positioned at the end, which is far from the pedicle screw, of the connecting rod; the locking plate is an expanded part at the tail end of the connecting rod; the vertebral plate hook, the locking plate and the connecting rod are integrated to form a repository; and the locking plate is fixed on the vertebral plate by locking screws and is provided with locking holes matched with the locking screws. The invention has the advantages of elimination of tender moving of the vertebral plate after operation, high positioning precision of screws, simple and convenient operation, reservation of lumbar motion segment and high fusion rate of fracture end after operation.
Owner:陈哲
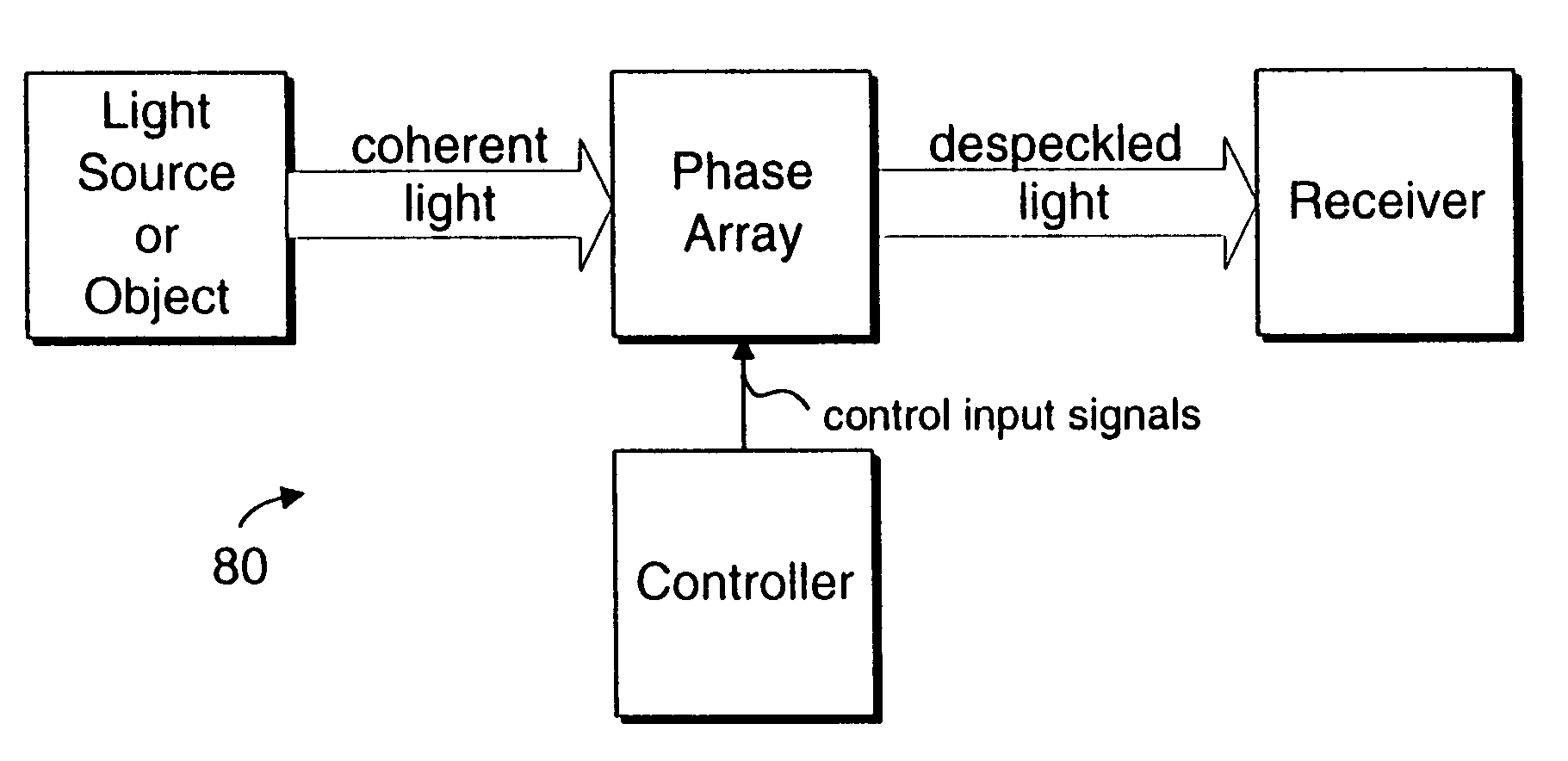
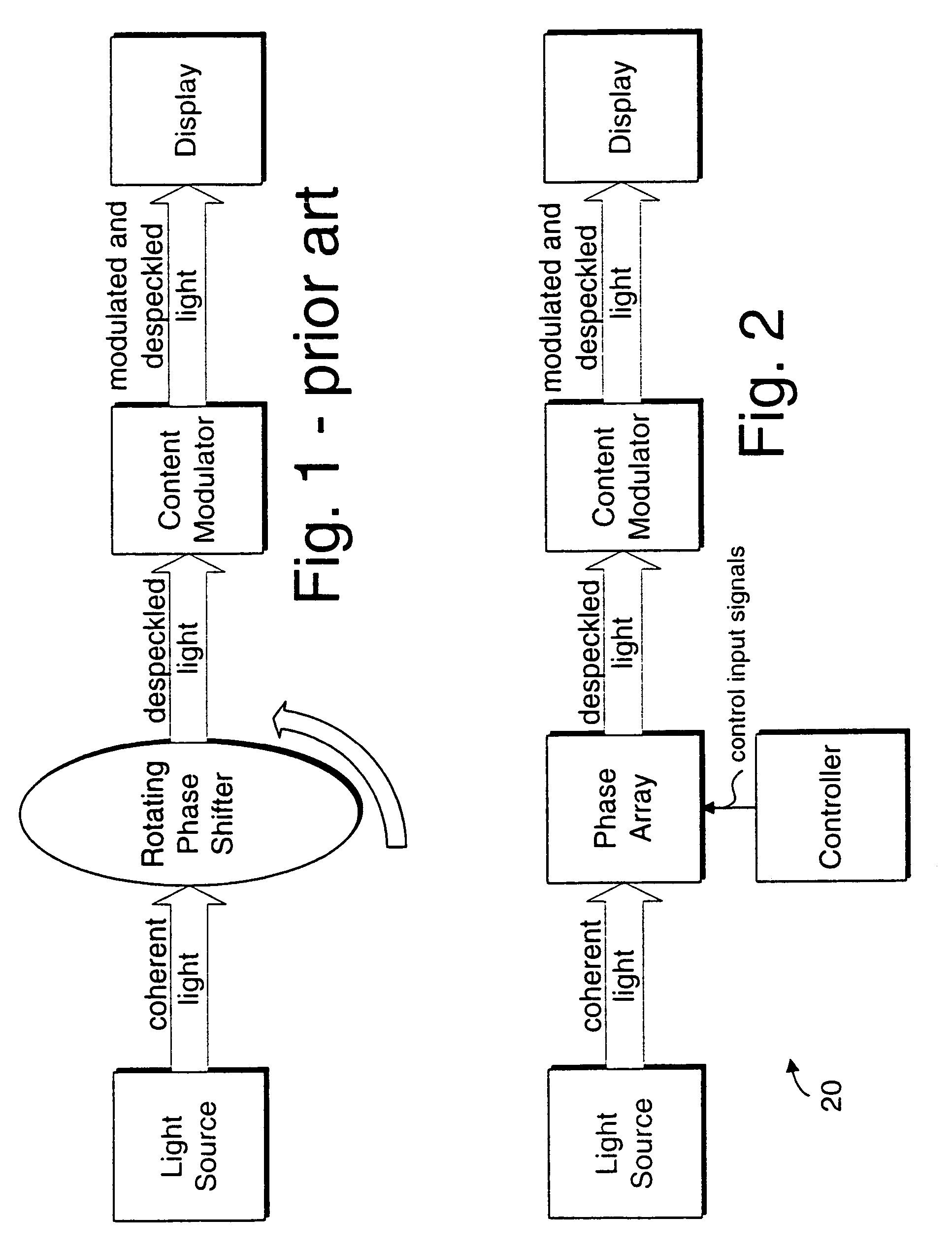
Coherent light despeckling
InactiveUS20040183954A1Television system detailsTelevision system scanning detailsPhase shiftedDisplay device
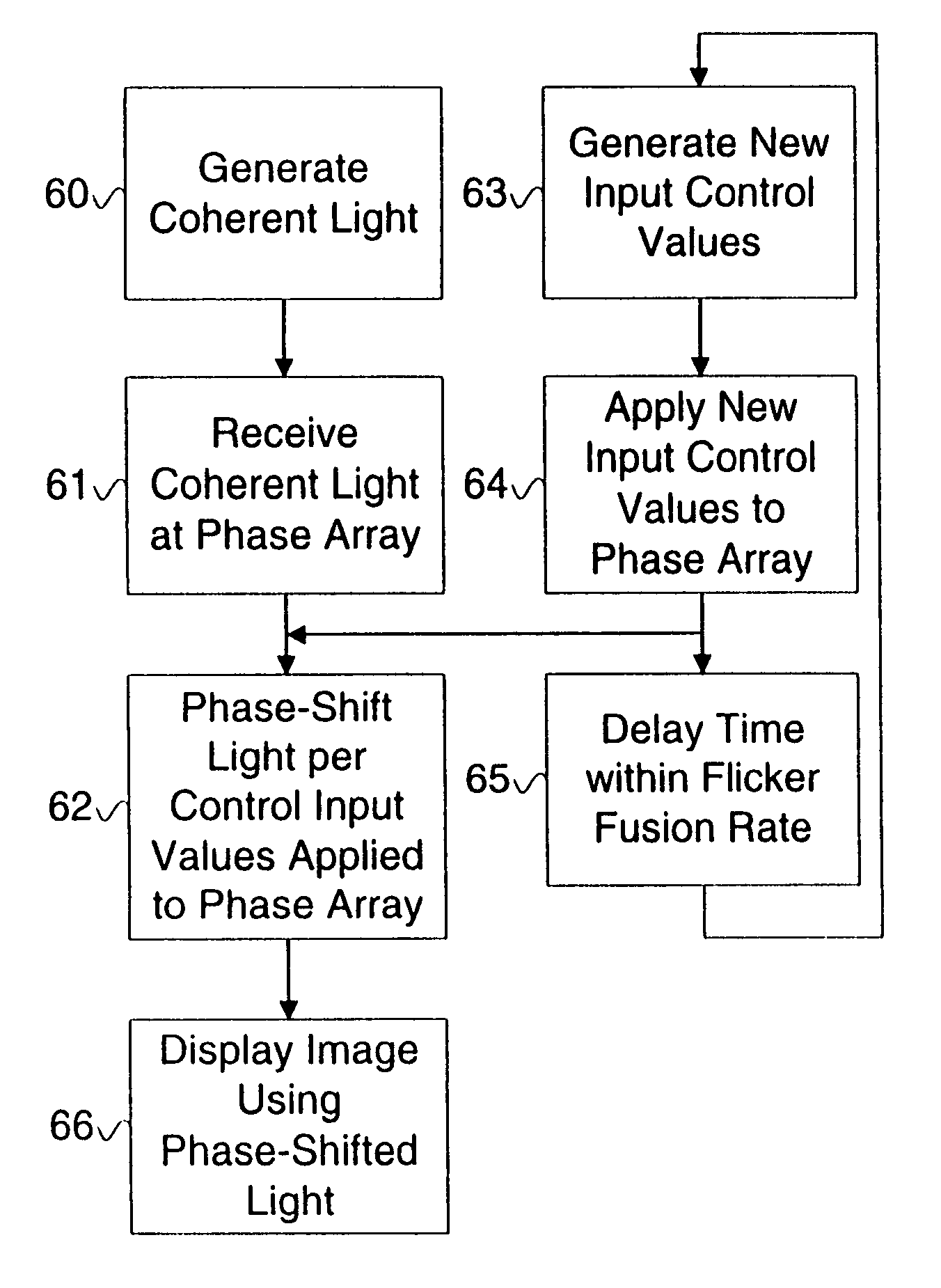
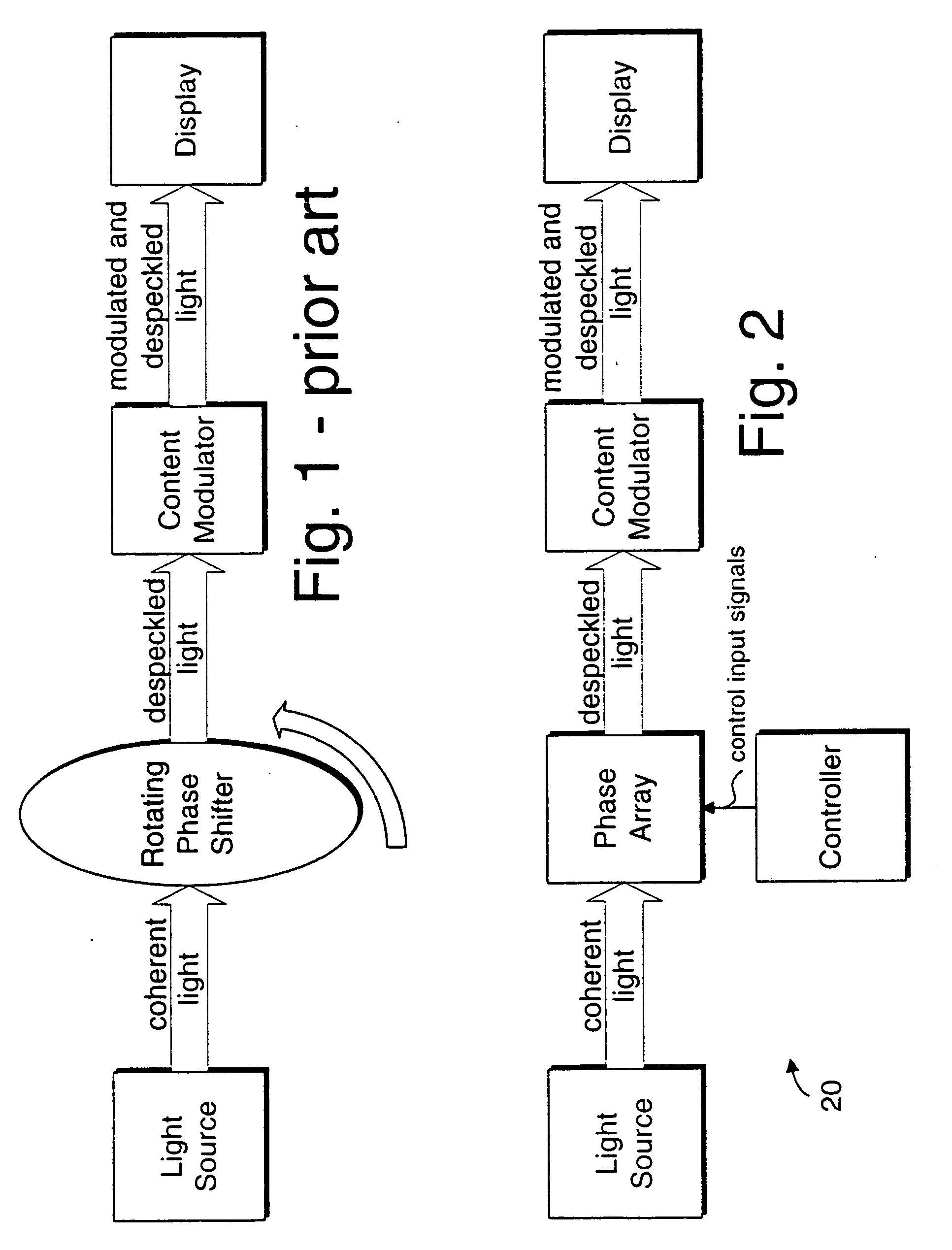
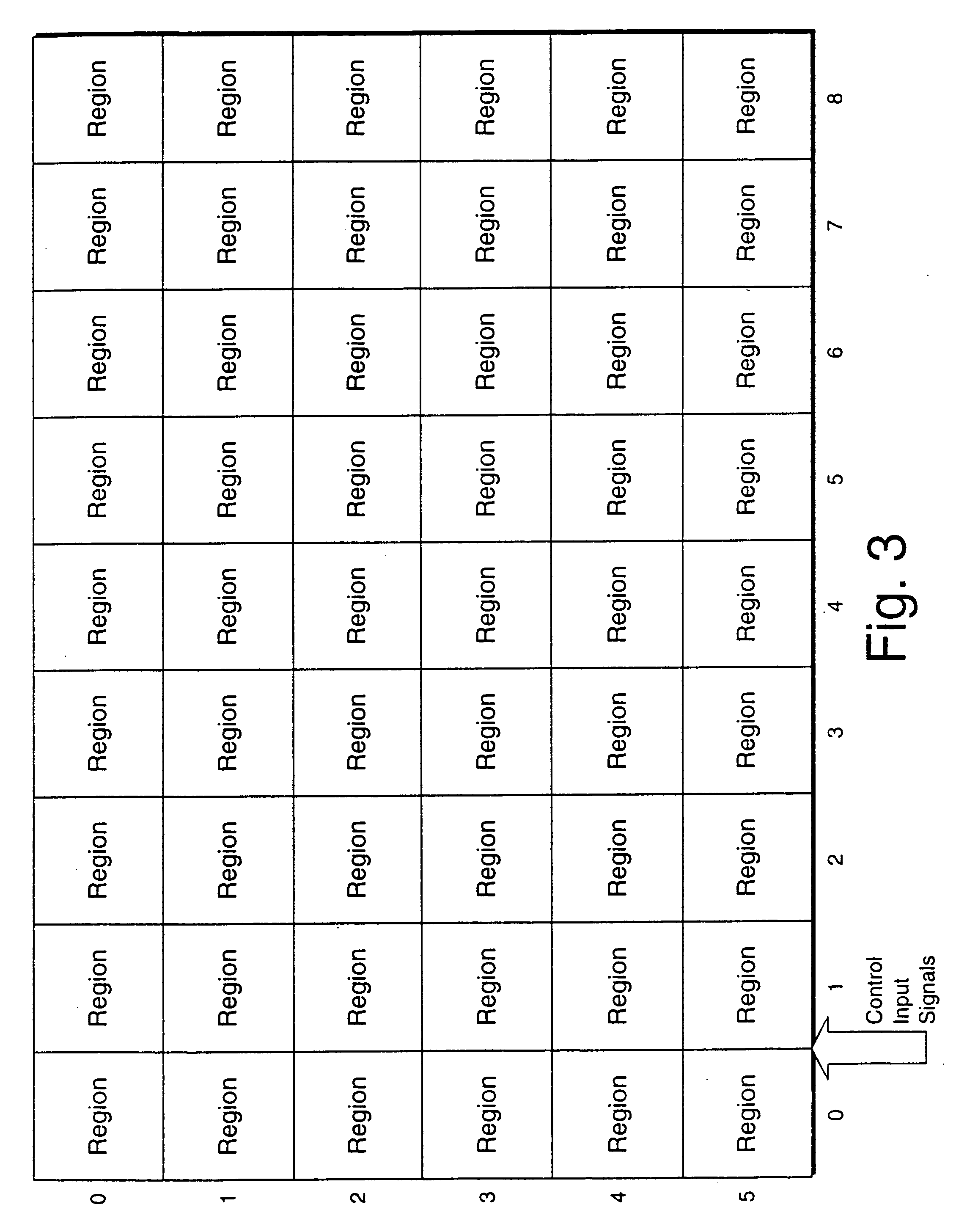
A display device or a receiver device for use with coherent light. A controller applies phase shift values to a multi-region phase array at a frequency sufficiently higher than the flicker fusion rate of the human eye or other intended receiver in order to remove the perception of speckling artifacts which would otherwise appear due to the coherency of the light.
Owner:INTEL CORP
Laser repairing technology for aluminum foil roll crack
InactiveCN102677047AImprove tissue uniformityAvoid it happening againMetallic material coating processesLaser beam welding apparatusAlcladOptoelectronics
The invention relates to a laser repairing technology for an aluminum foil roll crack. The laser repairing technology for the aluminum foil roll crack comprises the following steps: A, cleaning up a roll; B, preheating the roll at a low temperature; C, optimizing technical parameters, and performing laser cladding on polished and cleaned pits on the roll, wherein in the process, the roll is vibrated, the temperature of the roll is kept to be 180-250 DEG C until the pits are filled, a cladding layer is higher than the roll surface, and used alloy powder comprises the following components by weight percent: 10-15% of Cr, 1-2% of B, 0.5-1.5% of Si and Fe in balancing amount; and D. detecting. The laser repairing technology for the aluminum foil roll crack can perform laser cladding on the roll crack and can effectively fill a very deep crack to making the deep crack meet a using requirement; the structural uniformity of the cladding layer is good; and the fusion rate of the cladding layer and a matrix is high.
Owner:DANYANG HONGTU LASER TECH
Preparation method and application of exosome derived from human olfactory mucosa mesenchymal stem cells
InactiveCN108004206AWon't hurtGuaranteed amountSkeletal/connective tissue cellsUnknown materialsOlfactory mucosaPolyethylene glycol
The invention provides a preparation method of an exosome derived from human olfactory mucosa mesenchymal stem cells, and belongs to the technical field of stem cells. When the fusion rate of P4-generation and / or P5-generation olfactory mucosa mesenchymal stem cells is 85% to 90%, a cultured object is subjected to first centrifugal separation, obtained first supernate is subjected to second centrifugal separation, obtained second supernate is subjected to third centrifugal separation, the obtained third supernate is filtered, the obtained filtrate is mixed with a polyethylene glycol solution,after standing and incubation for 8-10 hours, fourth centrifugal separation is conducted, the obtained fourth precipitate is washed by normal saline, and after fifth centrifugal separation, the obtained precipitate is the exosome. By adopting the method, a vesica of the exosome can not be damaged, the amount of the obtained exosome is ensured, and the obtained exosome can promote the proliferationof human-brain microvascular epithelial cells and improve the cell migration rate.
Owner:HUNAN NORMAL UNIVERSITY
Coherent light despeckling
Owner:INTEL CORP
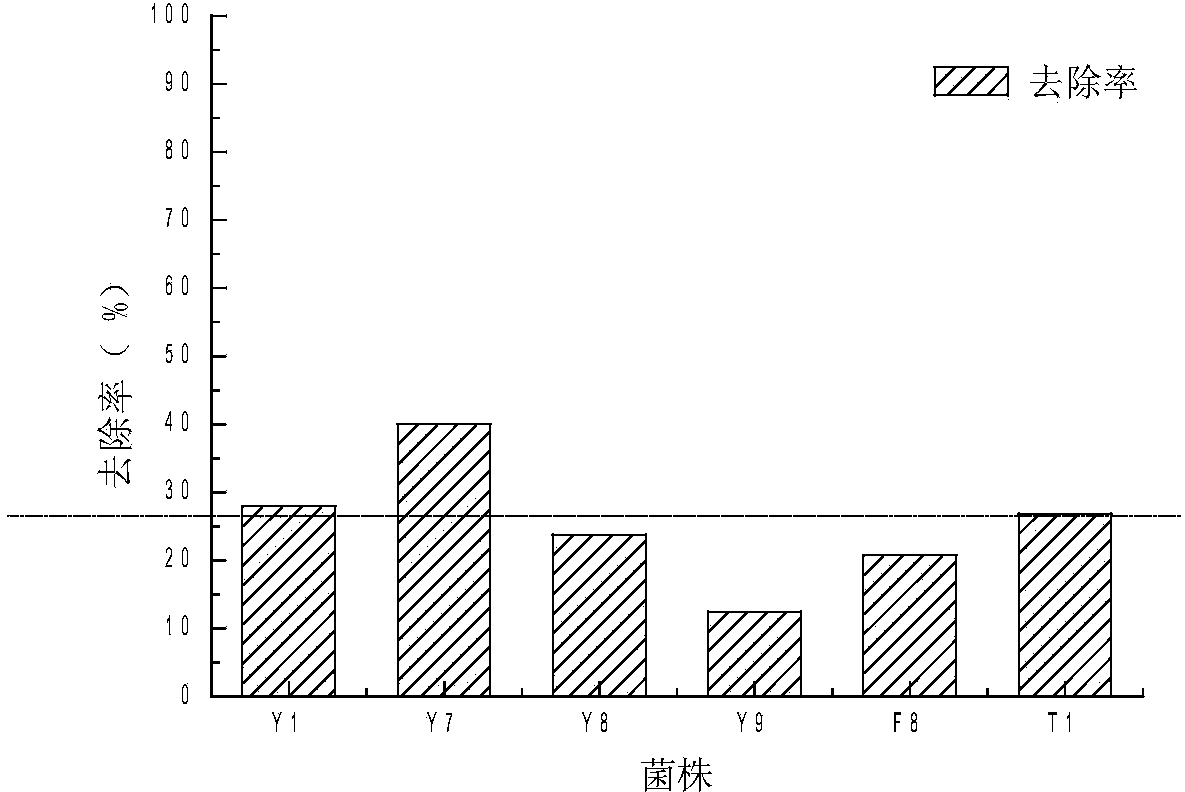
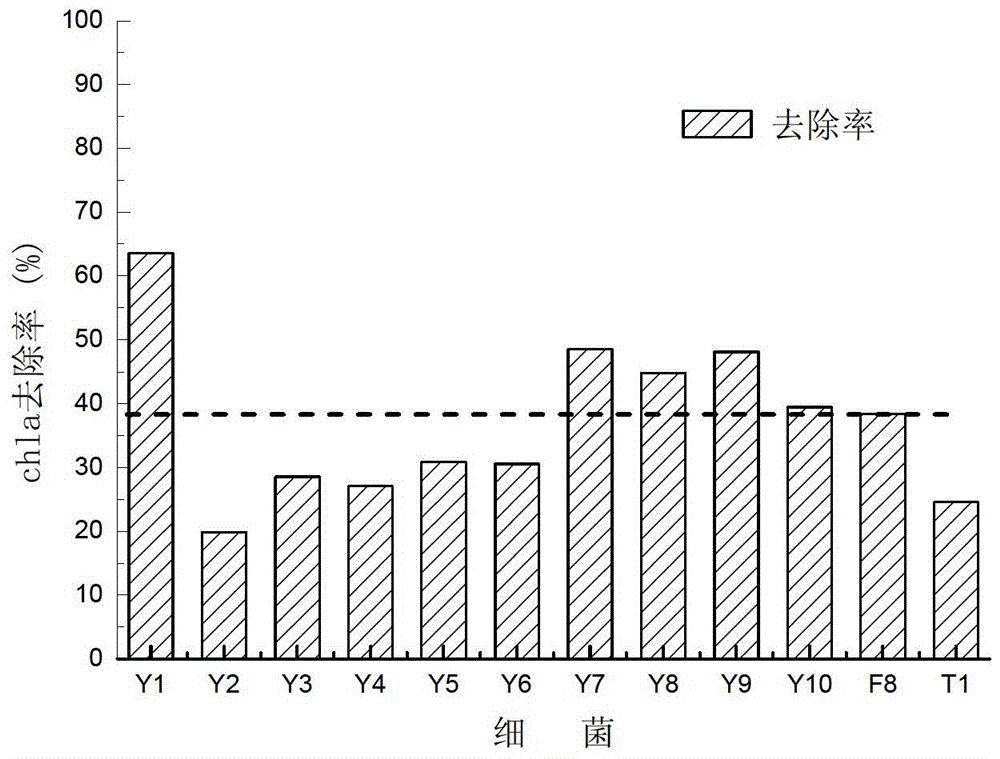
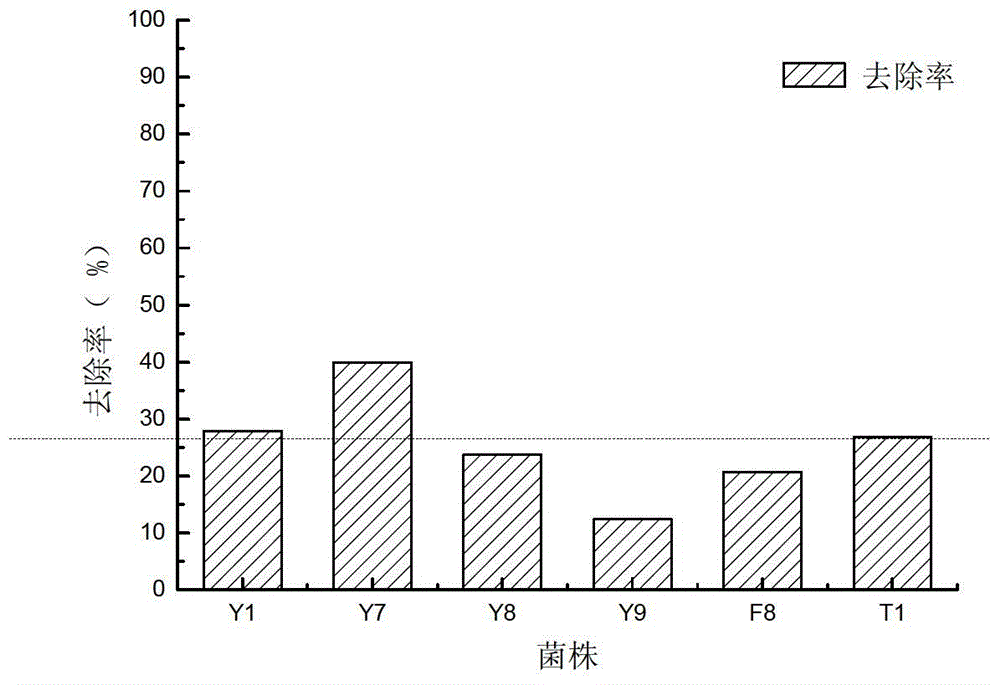
Alga lysing/algal toxin degradation double-effect engineering strain Y1 and construction method thereof
The invention discloses an alga lysing / algal toxin degradation double-effect engineering strain Y1 and a construction method thereof, belonging to the field of microbiology for treatment of blue-green algae. Fusion is performed on parent strains with alga lysing and algal toxin degradation functions by utilizing a protoplast fusion technology to construct the double-effect engineering strain with excellent traits of parents, namely Bacillussphaericus with the collection number of CGMCCNO. 7519. The alga lysing and the derived algal toxin pollution problem are synchronously solved. The protoplast fusion rate can achieve 31.97% under optimal conditions, and the double-effect engineering strain has certain guide effects on solving the problems of blue-green alga bloom and the derived MC-LR (microcystin-LR) pollution and constructing the engineering strain integrating the MC-LR degradation function and the alga lysing property into a whole by performing a protoplast fusion technology, thereby providing reference for achieving the effect on treating both the root course and the symptoms for solving the large-area blue-green alga bloom problem.
Owner:ANHUI HUANGHE WATER RESOURCE POLYTRON TECH INC
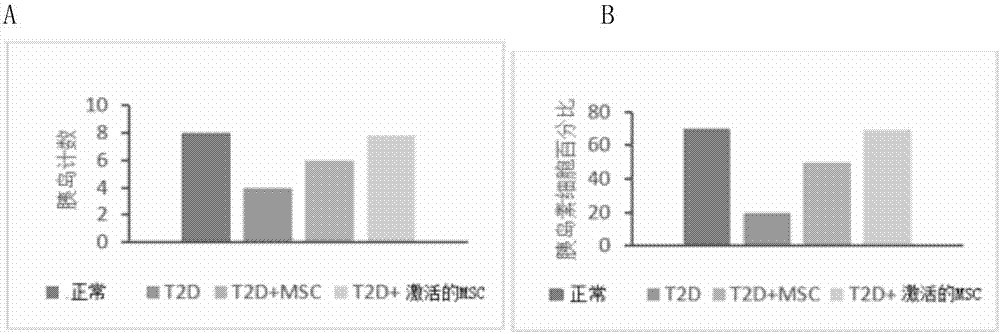
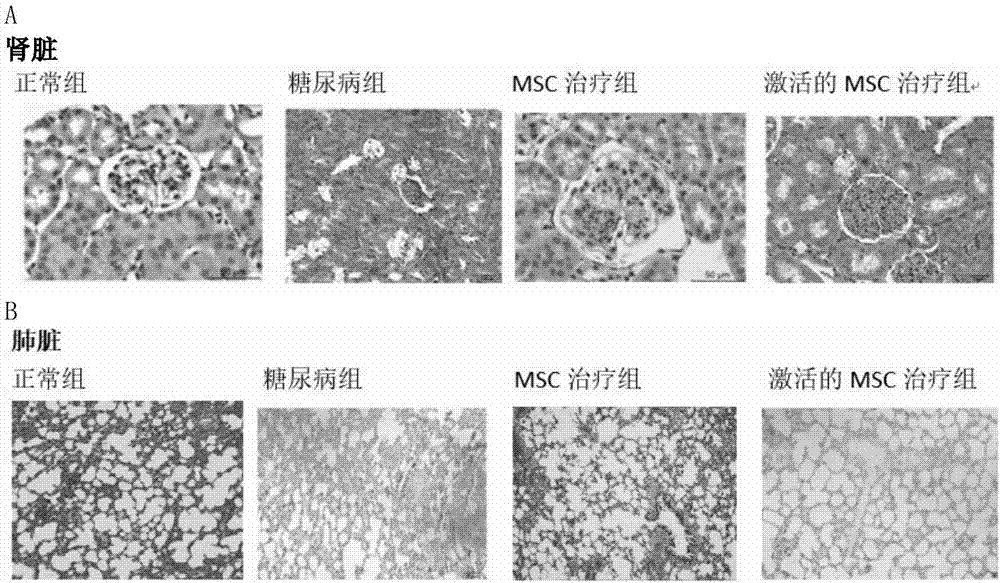
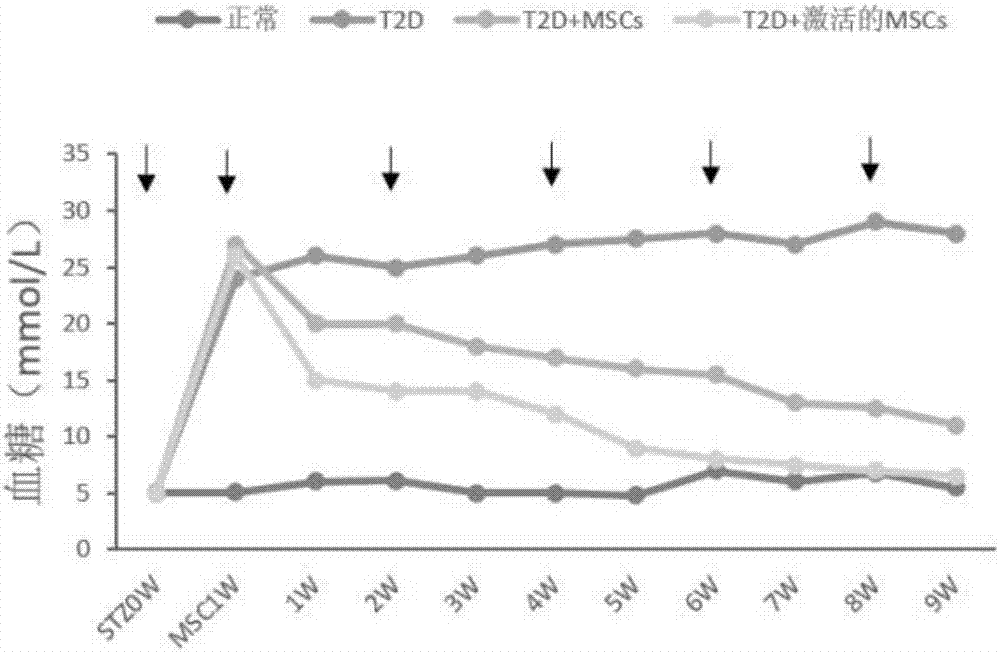
Active mesenchymal stem cell injection for diabetes mellitus infusion
InactiveCN107090431APromote secretionDecreased glucose toleranceMetabolism disorderCulture processDiabrezideStem cell culture
The invention provides a preparation method of active mesenchymal stem cells, active mesenchymal stem cell injection for diabetes mellitus infusion and application of the injection in preparing drugs for diabetes mellitus. The active mesenchymal stem cells are mesenchymal stem cells activated by diabetes mellitus (DM)-like micro-environment, and the preparation method comprises the steps that when separated and extracted umbilical-cord, bone-marrow or adipose-derived mesenchymal stem cells are cultivated until the fusion rate reaches 40-50%, a glucose solution with the final concentration of 20-50 mmol / L and palmitic acid with the final concentration of 0.2-0.4 mmol / L are added, cultivation is conducted for 46-50 hours, and when the fusion rate of the mesenchymal stem cells reaches 70-80%, the mesenchymal stem cells are digested, neutralized, washed, collected and activated. The active mesenchymal stem cell injection for the diabetes mellitus infusion comprises the mesenchymal stem cells activated by the DM-like micro-environment, human serum albumin, compound vitamins, mannitol, glucose and normal saline. The active mesenchymal stem cell injection for the diabetes mellitus infusion can be used for treating type II diabetes mellitus.
Owner:北京恒峰铭成生物科技有限公司
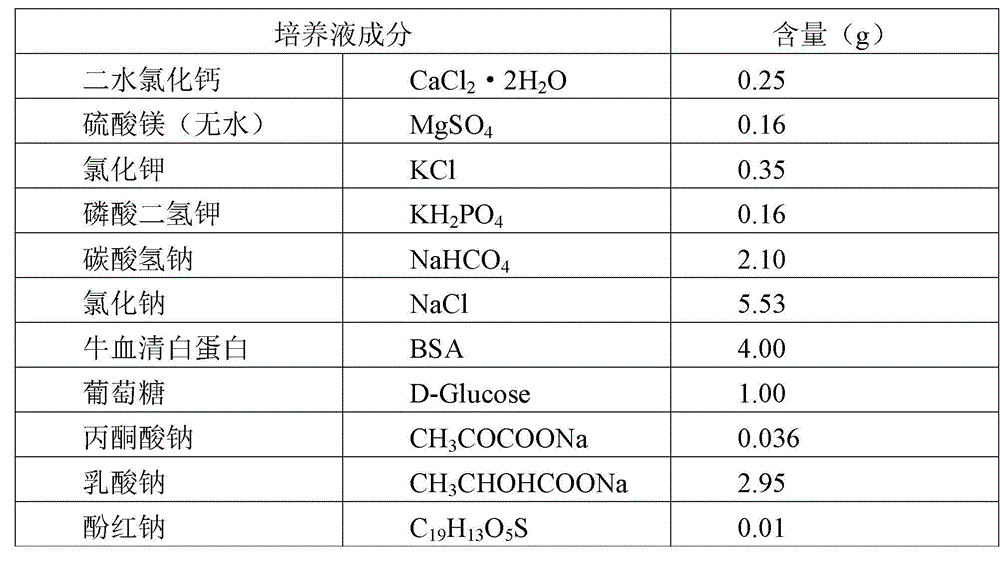
Sheep embryonic cell culture fluid
The invention provides sheep embryonic cell culture fluid. The sheep embryonic cell culture fluid comprises the following components: calcium chloride dehydrate, anhydrous magnesium sulfate, potassium chloride, monopotassium phosphate, sodium bicarbonate, sodium chloride, bovine serum albumin, glucose, sodium pyruvate, sodium lactate and phenol red sodium salt. Embryonic fluid at a volume by concentration of 10% is also added to the culture fluid so that the culture fluid has a great cell culture effect. According to the culture fluid, the components collaborate with each other, so that the irritation to an early embryo is reduced, and the embryo can grow well; and in addition, 10% of uterine embryonic fluid of a ewe is added to the culture fluid, so that the fusion rate of a reconstructed embryo can be remarkably increased. Compared with the culture fluid without the embryonic fluid, the culture with the embryonic fluid has the capability of obviously increasing the survival rate and blastocyst rate of in-vitro cultured embryos; and the culture fluid provided by the invention also can improve uterine receptivity, therefore, the lambing percentage is increased obviously.
Owner:青岛森淼生物技术有限公司
Fabric including low-melting fiber
InactiveCN101922077AEffective asMeet the flexibilityFlame-proof filament manufactureWoven fabricsYarnFiber
Owner:熊津可蜜珂耳
Pre-expanded polypropylene resin particle, and method for production thereof
A pre-expanded polypropylene resin particle can be produced in the following manner: a polypropylene resin particle produced by an under water cut method, water, a dispersing agent, and a foaming agent are charged in a pressure-resistant container, the resulting mixture is heated to a temperature equal to or higher than the softening temperature of the polypropylene resin particle to allow the polypropylene resin particle to be impregnated with the foaming agent under pressure, and the resulting product is released into the atmosphere having a pressure lower than the pressure of the inside of the pressure-resistant container. As the polypropylene resin composition, a composition is used which comprises 100 parts by weight of a polypropylene resin and 1 to 20 parts by weight of a polyethylene resin having a melt viscosity of 10 to 2000 mPa·s at 140° C., whereby it becomes possible to reduce the pressure of a heated molding vapor required for producing an in-mold expanded molding product having a fusion rate of 75% or higher.
Owner:KANEKA CORP
Cell pairing and fusion chip
ActiveCN101717717AIngenious structureImprove fusion rateBioreactor/fermenter combinationsBiological substance pretreatmentsChemical poisoningPairing
The invention belongs to the biomedicine technical field, relating to a cell pairing and fusion chip. The cell pairing and fusion chip comprises a chip body provided with a hollow and closed cell fusion chamber, wherein a cell trap consisting of two symmetrical semilunar banks is arranged at the bottom plate of the cell fusion chamber. Each bank comprises a short end and a long end; the projecting arc surfaces of the two semilunar banks face to each other, of which the short ends form a first opening, while the long ends form a second opening; the bottom part of the semilunar bank is fixed on the bottom plate; a definite interval exists between the top part of the semilunar bank and the top plate of cell fusion chamber; and the first sampling hole corresponding to the first opening direction of the cell trap and the second sampling hole corresponding to the second opening direction of the cell trap are arranged along the diagonal direction of the cell fusion chamber. The chip with simple and ingenious structure can extremely improve cell paring rate and fusion rate, reduce the chemical poisoning effect of inducer to cell, and be operated easily.
Owner:JIANGYIN STEMEASY BIOTECH LTD
Cervical spine locking and fusion device
InactiveCN102335051APrevent slipping outRelieve painInternal osteosythesisSpinal implantsEngineeringCervical spine
The invention belongs to medical appliances, and in particular relates to a cervical spine locking and fusion device, characterized in that: perforated fusion holes are formed on a main body and used as bone graft implanting spaces; and a rotatable fixing device composed of bolts, a blade and nuts is arranged at the front part of the main body of the fusion device, wherein the blade is fixed together with the main body through the bolts and nuts, and the blade can be rotated to be locked by the bolts. The main body of the fusion device presents an arc shape on the upper surface and a wedge shape on the lower surface, and both the upper and lower surfaces are provided with anti-slip serrations for enhancing stability. According to the invention, the cervical spine locking and fusion device is implanted into the intervertenral space, and the blade is rotated 90 degrees to be retained into upper and lower vertebral bodies, thereby preventing the fusion device from slipping out of the vertebral bodies, enhancing the stability at the early stage of operations, improving the fusion rate and restoring an ideal vertebral body height, and the cervical spine locking and fusion device has the characteristics of short operation time, safety and alleviation of suffering of patients.
Owner:ZHEJIANG CANWELL MEDICAL DEVICES CO LTD
Expandable polystyrene resin beads, process for the preparation of them, and foam made by using the same
Foamable modified polystyrene resin particles (E) comprising a particle of a modified polystyrene resin (C) containing a blowing agent (D), wherein the modified polystyrene resin (C) comprises conjugated diene polymer rubber particles (B) dispersed uniformly throughout a polystyrene resin (A) and when the foamable modified polystyrene resin particle (E) is expanded, there is substantially no deformation of the rubber particles (B) before and after the expansion. A foamed article of a modified polystyrene resin which has a cell membrane of the modified polystyrene resin comprising a polystyrene resin and conjugated diene polymer rubber particles dispersed uniformly throughout the polystyrene resin and has a fusion rate of not less than 50 %, wherein the rubber particles maintain substantially spherical form in the cell membrane. According to the present invention, the foamed article of the modified polystyrene resin having break resistance and fusion rate which are equal to those of a high impact polystyrene foamed article can be provided at low cost.
Owner:KANEKA CORP
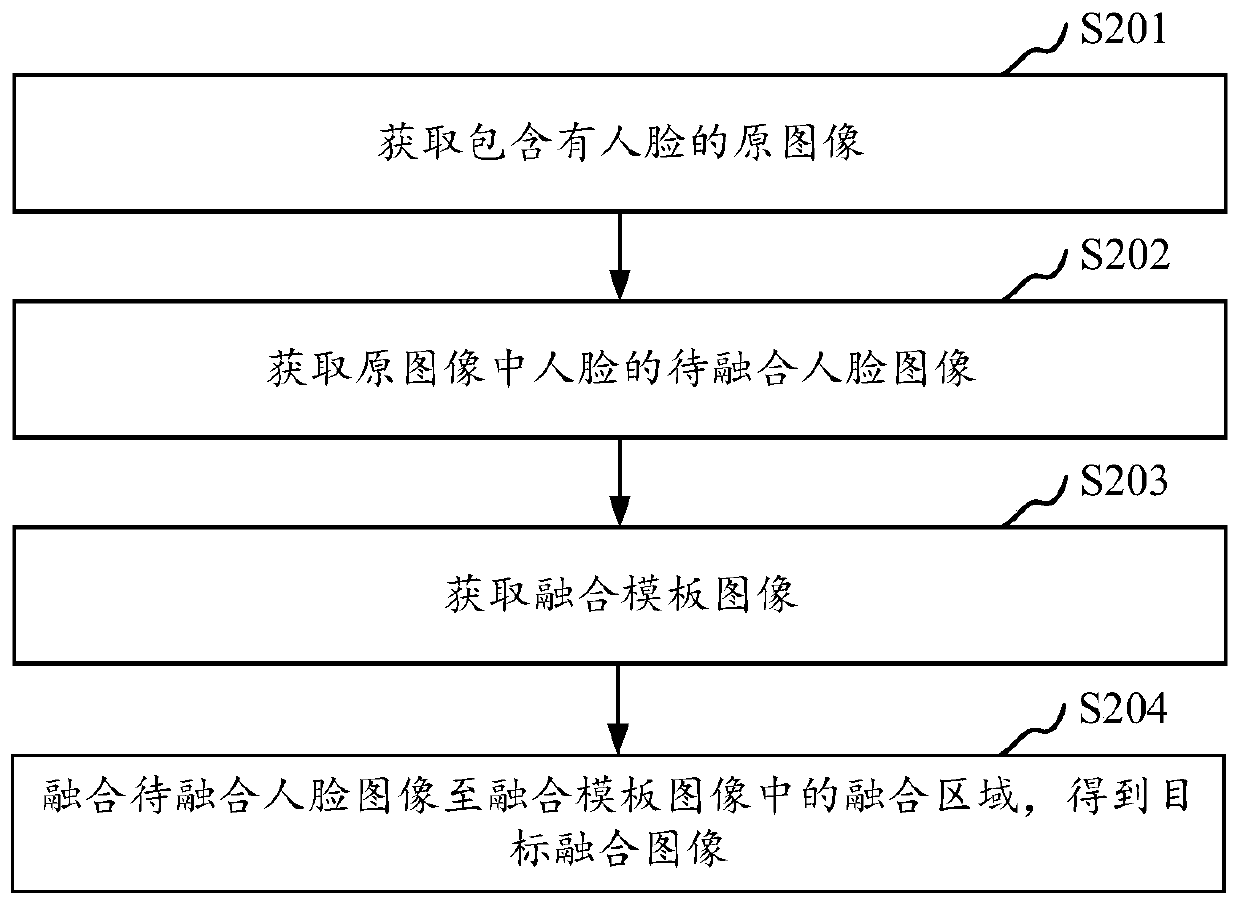
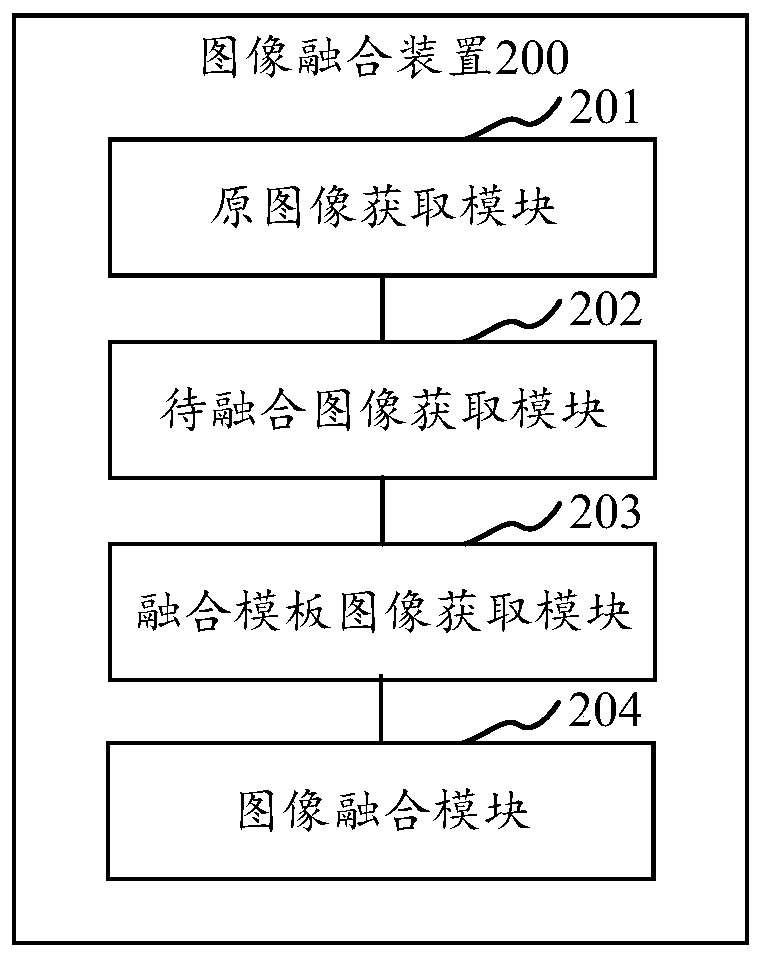
Image fusion method and device, computer equipment and storage medium
InactiveCN109801249AIncrease fusion rateImage enhancementCharacter and pattern recognitionPattern recognitionComputer equipment
The invention relates to an image fusion method. The invention discloses a device, computer equipment and a storage medium. The method comprises the following steps of: obtaining a face image to be fused of a face in an original image, obtaining a fusion template image by the face image to be fused according to an image generated by key features of the face of the original image, and fusing the face image to be fused to a fusion region in the fusion template image to obtain a target fusion image, Through the face image to be fused generated by the face in the original image, the face is represented according to the face key features in the face image to be fused, and the fusion rate of image fusion is improved.
Owner:SHENZHEN TCL NEW-TECH CO LTD
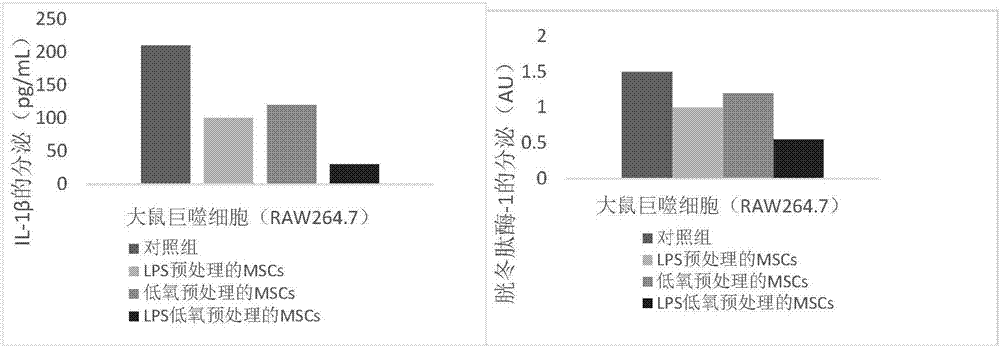
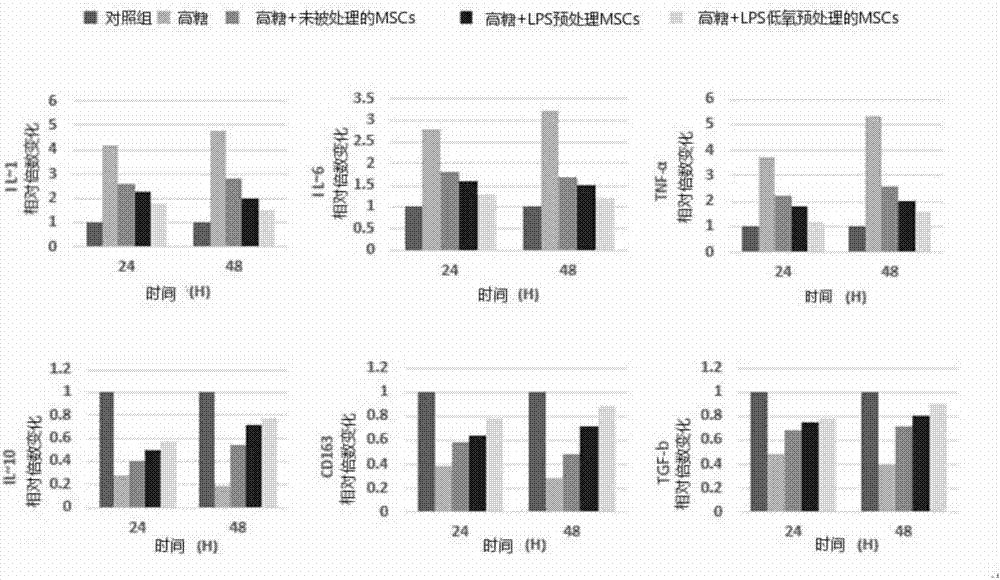
Preparing method and application of anti-chronic inflammation mesenchymal stem cells
ActiveCN106978394AGood anti-inflammatory effectGood treatment effectAntipyreticAnalgesicsSerum free mediaPhosphate
The invention provides a preparing method and application of anti-chronic inflammation mesenchymal stem cells. The preparing method comprises the steps of culturing mesenchymal stem cells obtained through separation and extraction till the fusion rate reaches 70-80%, removing a culture medium in a mesenchymal stem cell culture dish, and flushing the mesenchymal stem cells with phosphate buffer; then adding a serum-free medium containing 50-200 ng / mL lipopolysaccharide; and adjusting the hypoxia state till oxygen content becomes 5-10%, and culturing the mesenchymal stem cells for 16-18 h in the hypoxia state. The anti-chronic inflammation mesenchymal stem cells can be used for treating chronic inflammation.
Owner:恒峰铭成生物科技(上海)有限公司
Increased depth of field microscope and associated devices, systems, and methods
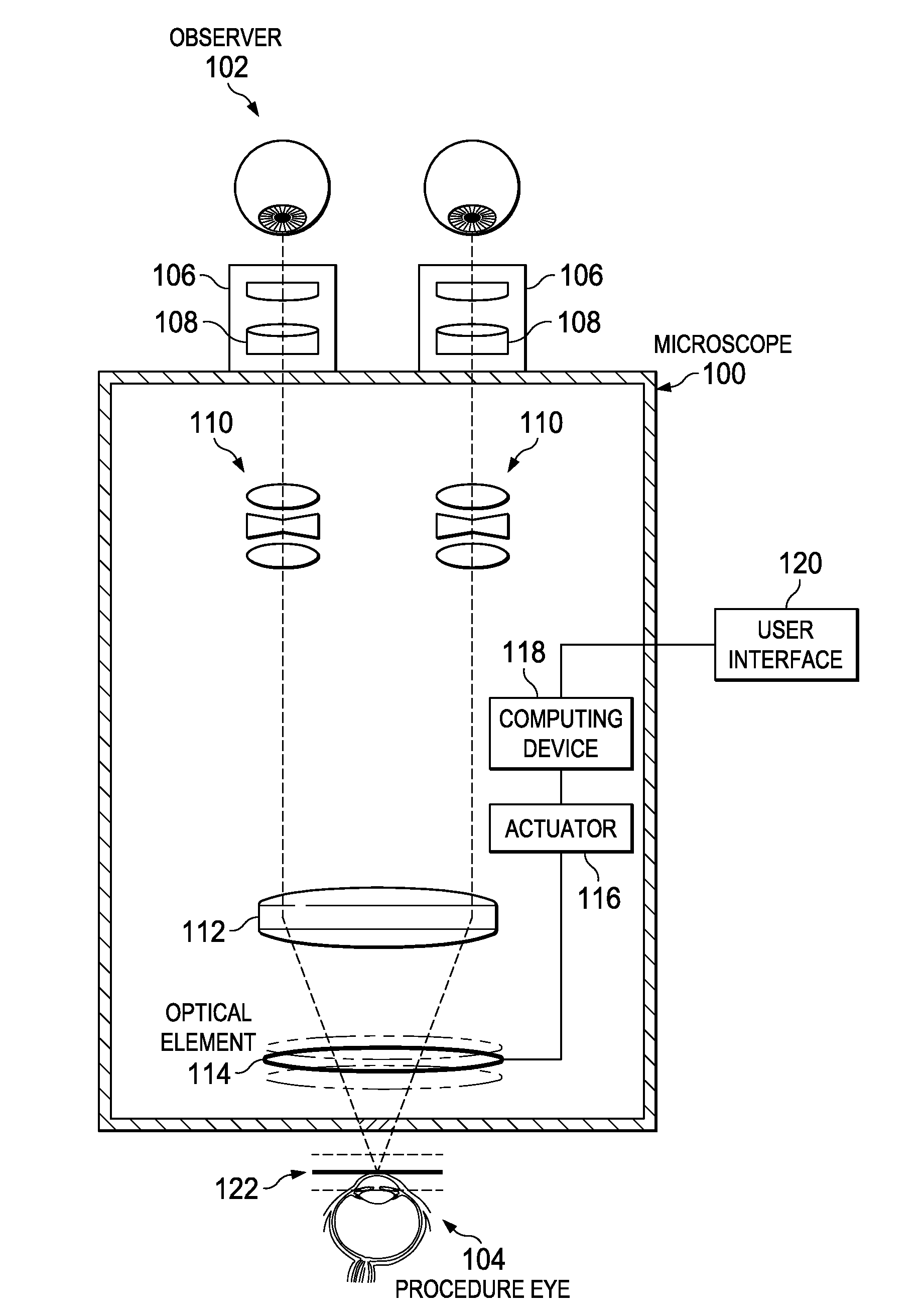
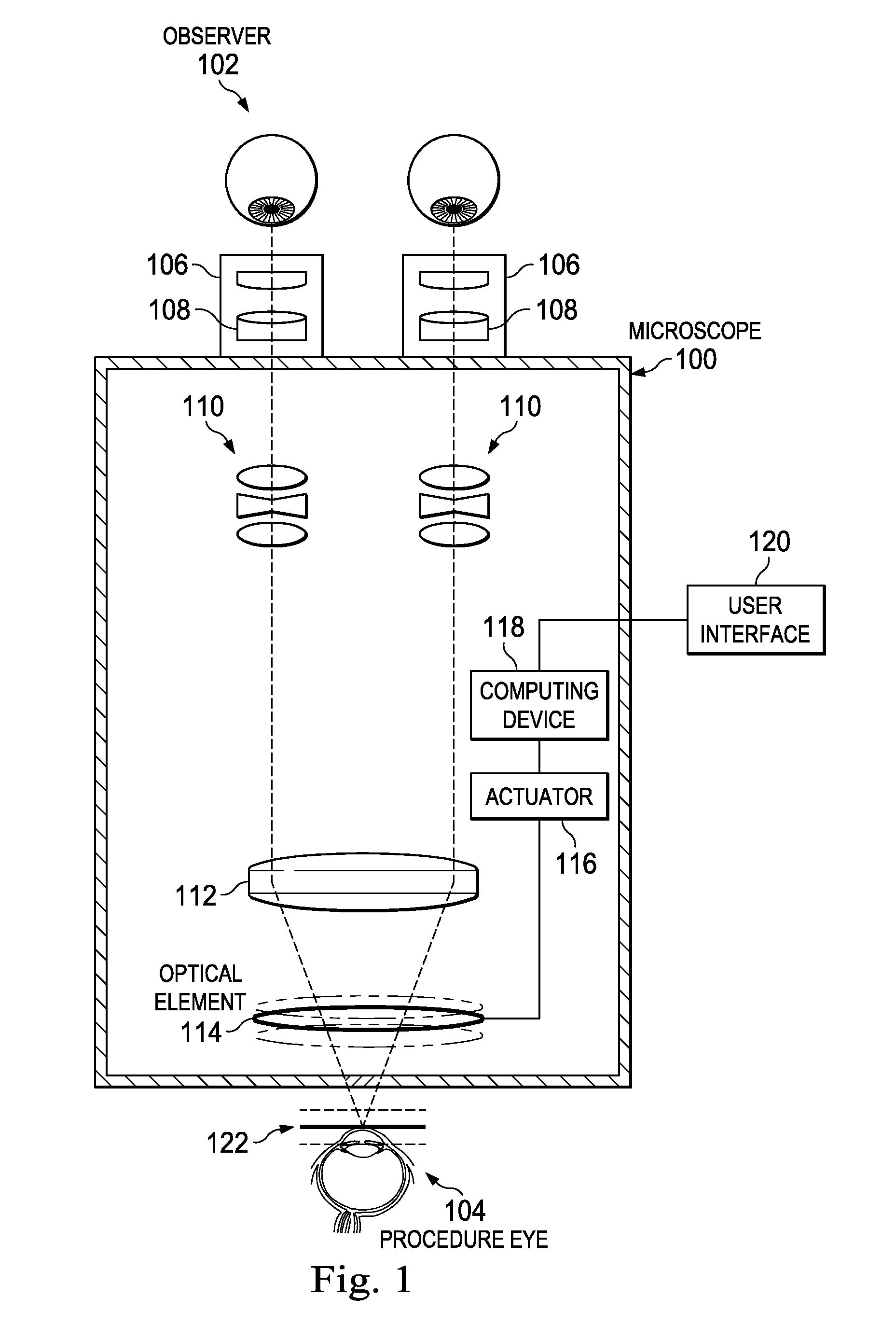
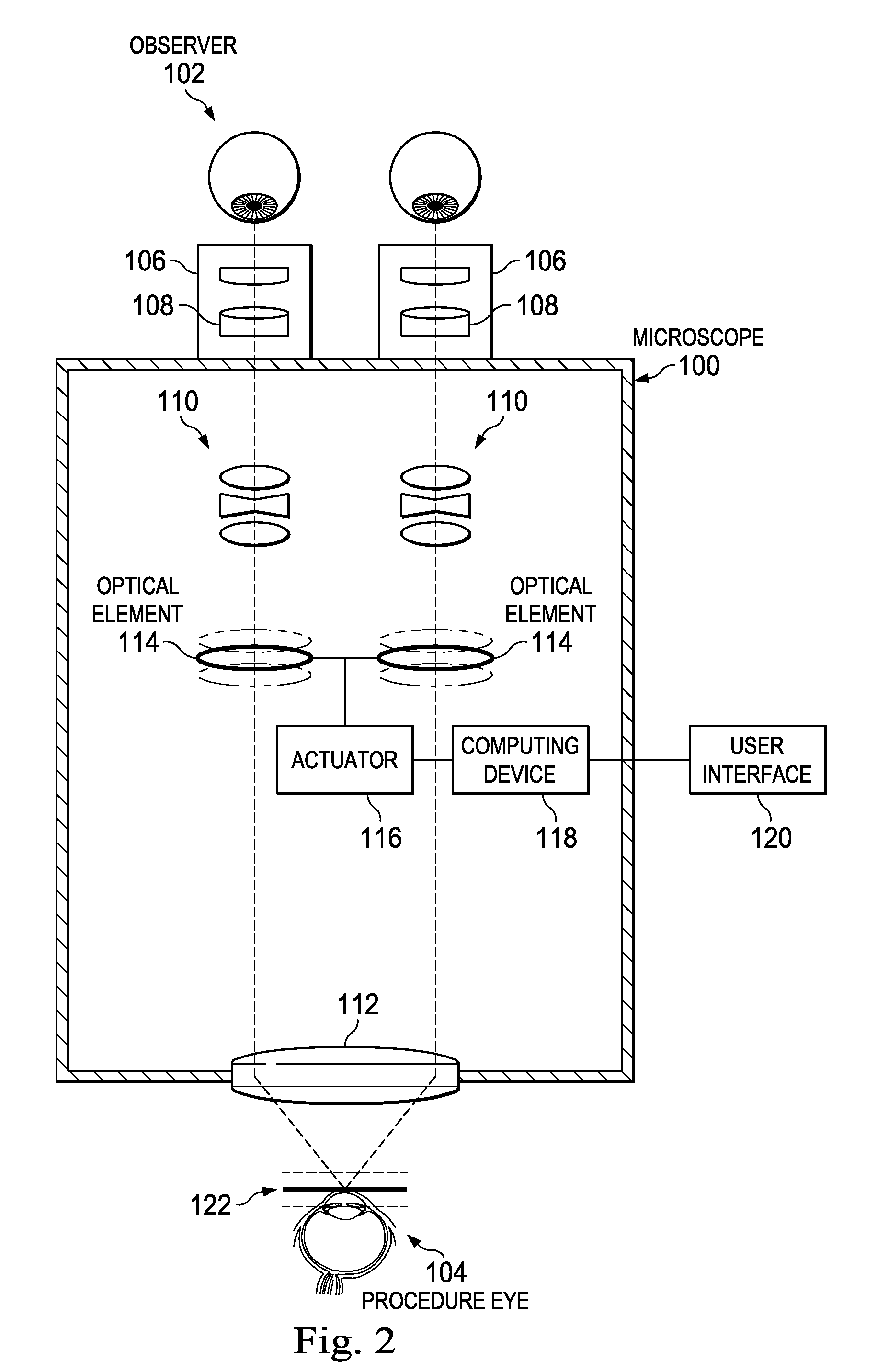
ActiveUS20160022133A1Improve doctor 's viewMicroscopesSurgical microscopesControl signalDepth of field
An ophthalmic surgical microscope can include a movable optical element positioned in an optical pathway of light reflected from a surgical field. The movable optical element can be configured to oscillate in a direction along the optical pathway. The microscope can include an actuator coupled to the movable optical element and configured to move in response to a control signal. The microscope can include a computing device in communication with the actuator and configured to generate the control signal to move the movable optical element. In some embodiments, the computing device is configured to generate the control signal to move the movable optical element with an oscillation frequency greater than the critical flicker fusion rate.
Owner:ALCON INC
A kind of algae-dissolving/algae toxin-degrading double-effect engineering bacteria y7 and its construction method
The invention provides a dual-effect engineering bacterium Y7 capable of lysing algae and degrading algal toxin and a construction method thereof, and belongs to the microbiological field of blue-green algae treatment. The dual-effect engineering bacterium Y7 capable of lysing algae and degrading microcystin-LR (MC-LR) is prepared based on a protoplast fusion technology in which the protoplast fusion rate can be up to 28.17% under optimal conditions. The dual-effect engineering bacterium Y7 provided by the invention is used for solving the problems of the outbreak of blue-green algae and the pollution of derived MC-LR, plays a guiding role in constructing a engineering bacterium integrating an MC-LR degradation function and algae-lysing property by virtue of an improved protoplast fusion technique and practical application and provides reference for solving the problem large-scale blue-green algae blooms fundamentally.
Owner:ANHUI HUANGHE WATER RESOURCE POLYTRON TECH INC
Coarse diatom ooze powder and production process thereof
The invention provides coarse diatom ooze powder and a production process thereof. The production process is simple, the water solubility of the diatom ooze powder is improved through specific mixing procedures, the fusion rate between the diatom ooze powder and water is improved, the construction window phase is prolonged, and the effect of long-time use can be achieved through one-time stirring. By means of the coarse diatom ooze powder and the production process thereof, the problem of surface decoration effect of a wall which is constructed and coated with a diatom ooze product on current market can be solved, and the construction efficiency of the diatom ooze product can be improved as well, in the using process, the stirring time is short, the energy consumption is saved, and the total cost of the diatom ooze product is reduced.
Owner:浙江马益环保科技有限公司
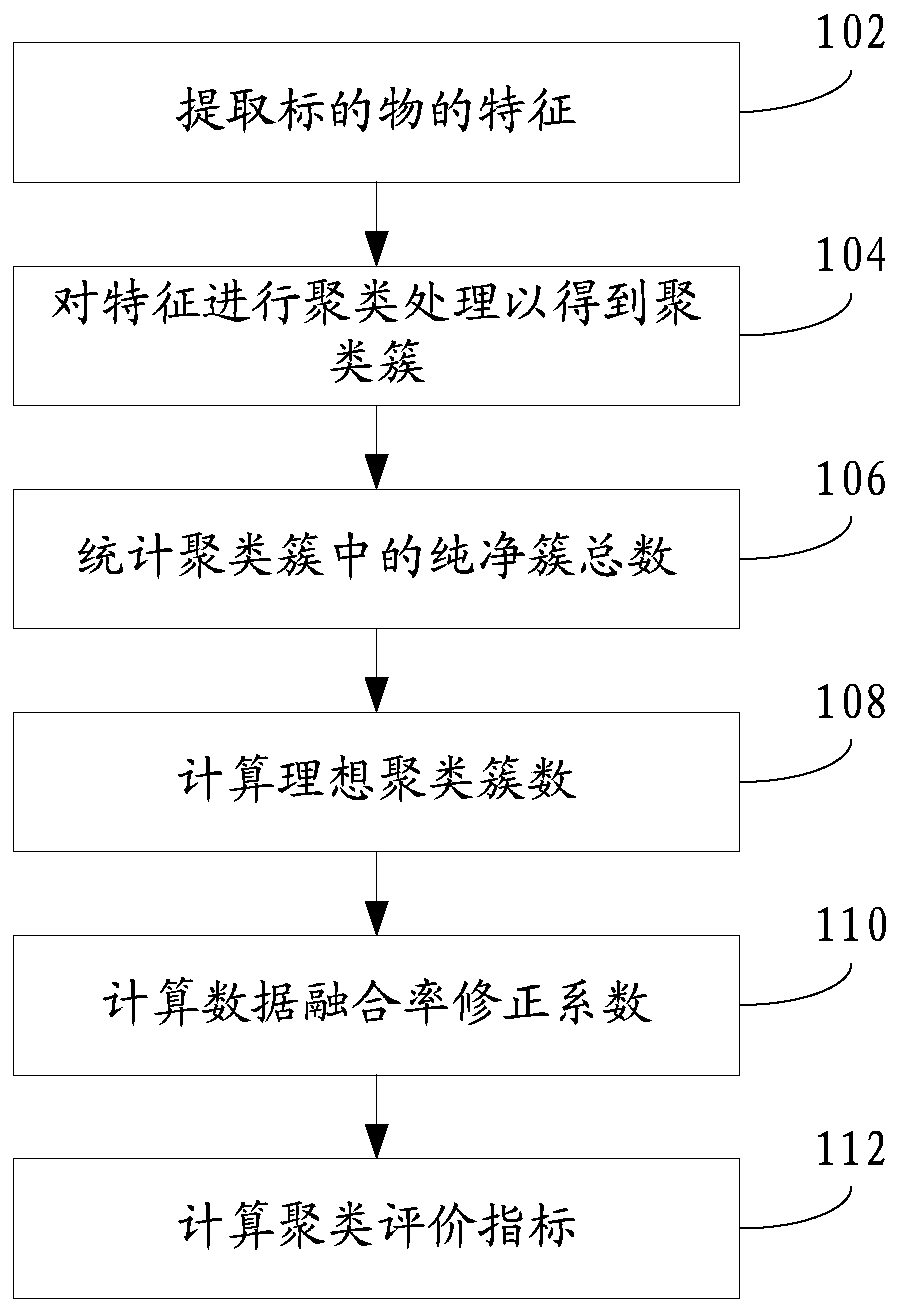
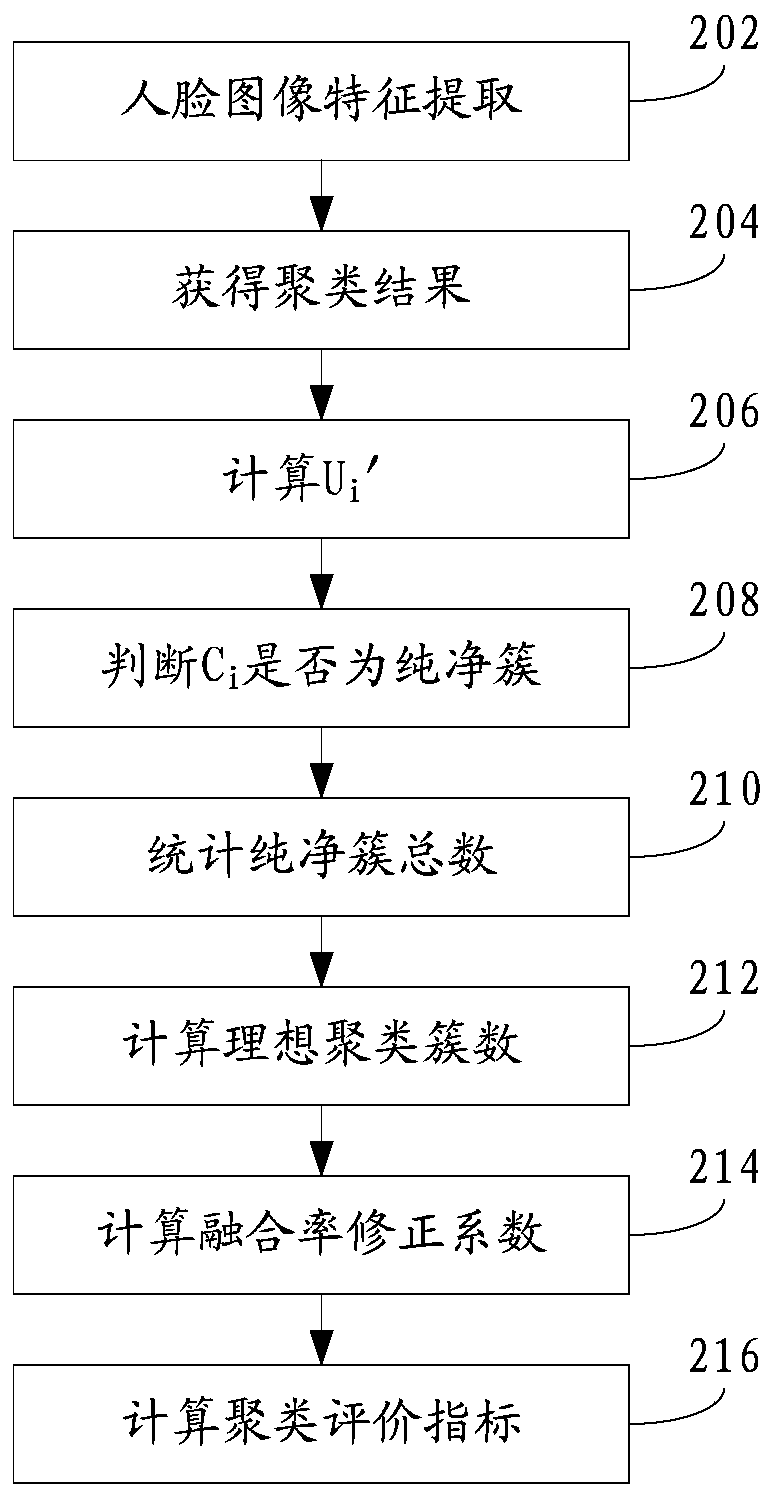
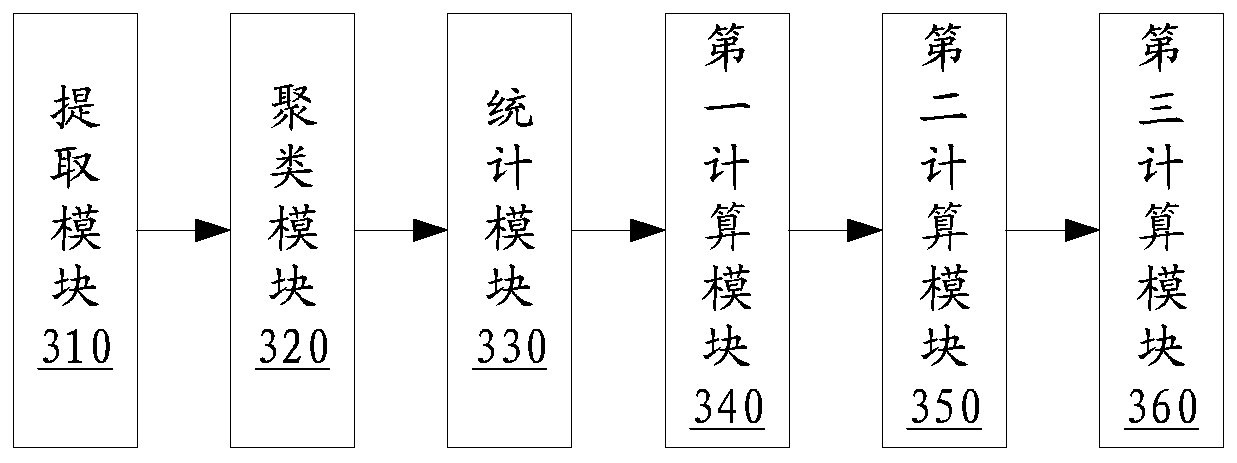
Clustering result evaluation method and system
ActiveCN110580510ACharacter and pattern recognitionEnergy efficient computingData miningEvaluation system
The invention discloses a clustering result evaluation method, which comprises the steps of extracting features of a target, the data volume of the features being M; clustering the features to obtaina clustering cluster C = {C1, C2,..., CK}, where K is a positive integer; counting the total number CP of pure clusters in the clustering cluster C, wherein P is a positive integer and is greater thanor equal to 0 and less than or equal to K; calculating an ideal clustering cluster number CI; and calculating a data fusion rate correction coefficient eta, and calculating a clustering evaluation index HI. The invention further discloses a clustering result evaluation system. According to the method and the system provided by the invention, the total number of the pure clusters and the ideal clustering cluster number are counted, so that the data fusion rate correction coefficient is calculated, the clustering evaluation index can be quickly obtained, and the index objectively and effectively reflects the accuracy of the clustering result.
Owner:SHENZHEN ZTE NETVIEW TECH +1
Exocrine body as well as preparation method and application thereof as tumor vaccine
ActiveCN105132386AHigh purityEasy to prepare in large quantitiesTissue cultureAntibody medical ingredientsPhosphateTumor antigen
The invention discloses an exocrine body as well as preparation method and application thereof as a tumor vaccine, wherein the method comprises the following steps: (1) preparing NDV-Ulster virus strain modified tumor cells; (2) preparing novel phosphate antigen or biphosphonate drug stimulated and activated antigen presenting cells gamma-delta T-APC; (3) preparing a fusion cell of the gamma-delta T-APC and the NDV-Ulster modified tumor cells; (4) purifying the fusion cell; (5) culturing the fusion cell; (6) preparing a fusion cell exocrine body; and (7) preparing a vaccine. The invention has the following beneficial effects: more novel antigen presenting cells gamma-delta T-APC are prepared by the method, and a fusion rate between the gamma-delta T-APC and the modified tumor cells is higher; and the obtained exocrine body is more stable in antigen combination, more complete in tumor antigen titer and stronger in immunogenicity, and the exocrine body is high in fusion cell and exocrine body tumor antigen loading.
Owner:BEIJING DOING TIMES BIOMEDICAL TECH
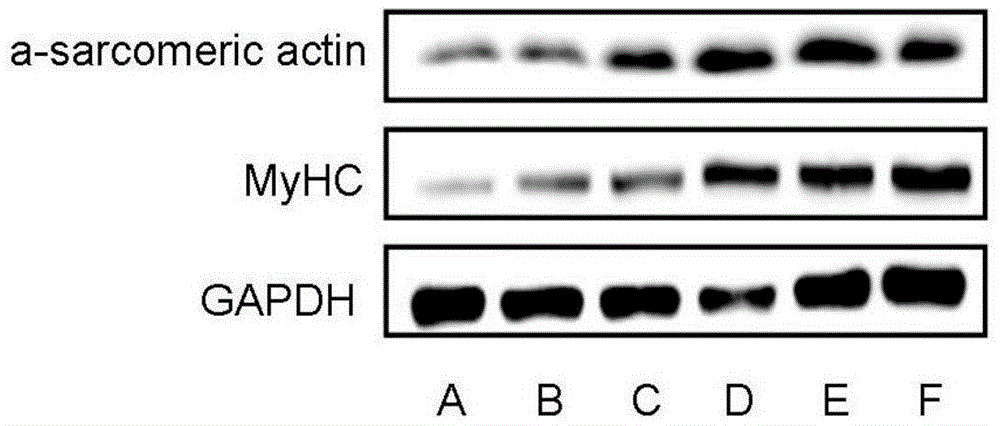
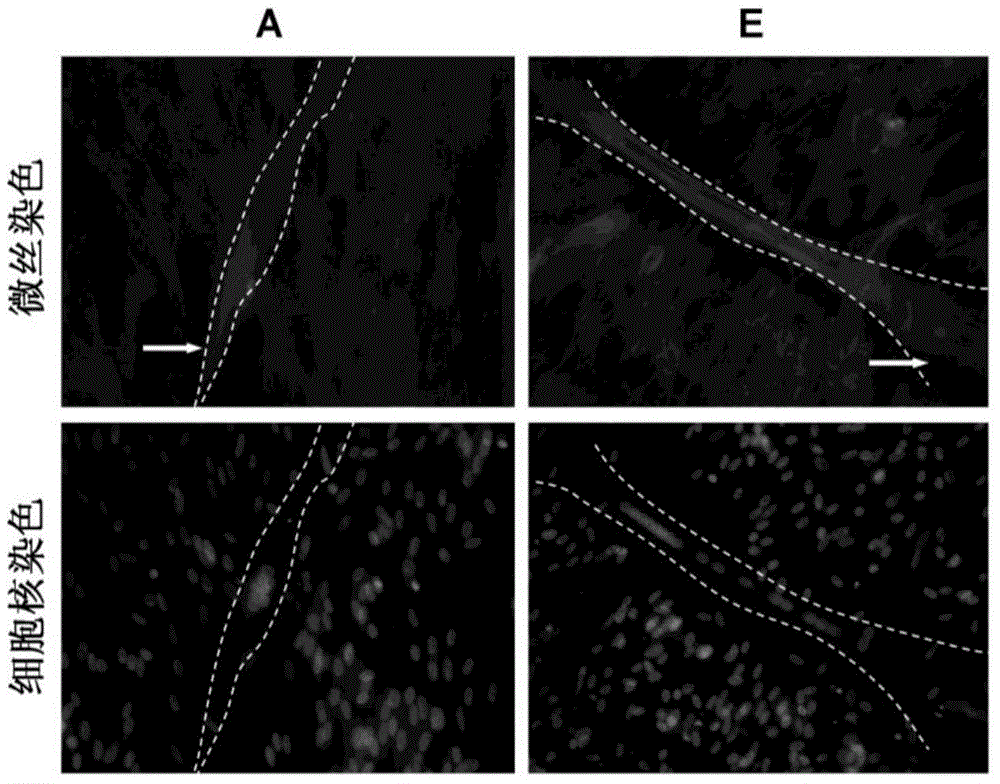
Preparation for enhancing differentiation capacity of chicken skeletal muscle myoblast and application thereof
ActiveCN104152406AInhibit apoptosisPromote migrationSkeletal/connective tissue cellsBiotechnologyCalcium pyruvate
The invention discloses a preparation for enhancing differentiation capacity of chicken skeletal muscle myoblast and application thereof, and belongs to the technical field of biological engineering. The preparation provided by the invention is composed of a muscle derived fibroblast extractive, pyruvate and calcium salt. The muscle derived fibroblast extractive is obtained from the steps of: conducting ultrasonic cracking on chicken muscle derived fibroblast, isolating to obtain a protein solution less than 10KDa, and conducting vacuum drying. Calcium salt and pyruvate can be calcium pyruvate. The preparation provided by the invention has the characteristics of simple and easily available composition and simple and effective usage, and can significantly improve the fusion rate of chicken skeletal muscle myoblast and promote the increase of skeletal muscle fiber diameter and expression of functional contractile protein. The preparation provided by the invention can be used for culturing myoblast; and the fusion rate of the myoblast is increased by 32.01%, the average number of nuclei in myotubes is increased by 91.63%, and average diameter of myotubes is increased by 45.59%.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Artificial axis support body
InactiveCN106175997APrevent looseningAvoid failureInternal osteosythesisSpinal implantsFailure rateProsthesis
The invention discloses an artificial axis support body, applicable to the technical field of medical apparatus. The artificial axis support body comprises a supporting main body, wherein the supporting main body comprises a crown part and a body part; a top profiling face is formed on the top face of the crown part and a bottom profiling face is formed on the bottom face of the body part; a cavity is formed in the supporting main body; a porous grid bracket is arranged in the cavity; on the surface of the supporting main body, a plurality of windows are formed in the cavity; a lower fixing lug is arranged at the bottom end of the body part and upper fixing lugs are arranged at the top end of the crown part; both the upper fixing lugs and the lower fixing lug are provided with fixing holes; the supporting main body can be in fastening connection to an atlas by virtue of screws; and the supporting main body can be in fastening connection to a third cervical vertebra by virtue of screws. The supporting main body, by virtue of the upper and lower profiling faces, can form dissecting fit, and meanwhile, the upper fixing lugs are connected to the atlas and the lower fixing lug is connected to the third cervical vertebra, so that the artificial axis support body is good in supporting and reconstruction stability and capable of preventing a prosthesis from becoming loosen and failed after an operation, and the artificial axis support body is easily to fix, good in shape matching performance, low in fixation failure rate after the operation and high in fusion rate; therefore, the artificial axis support body can meet complex operation demands.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com