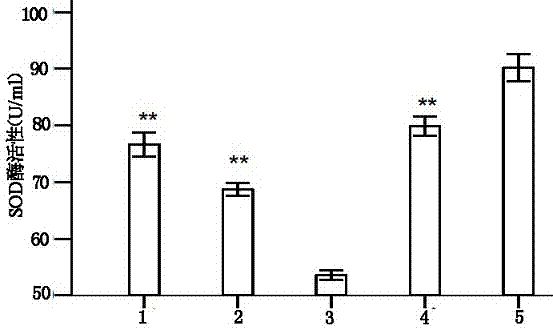
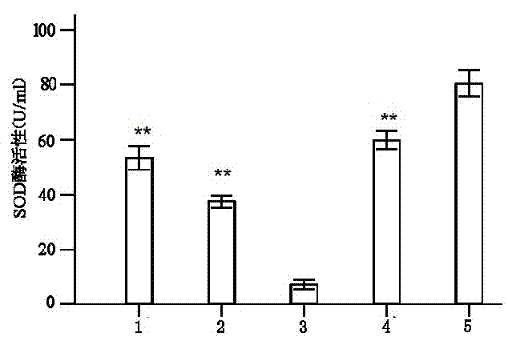
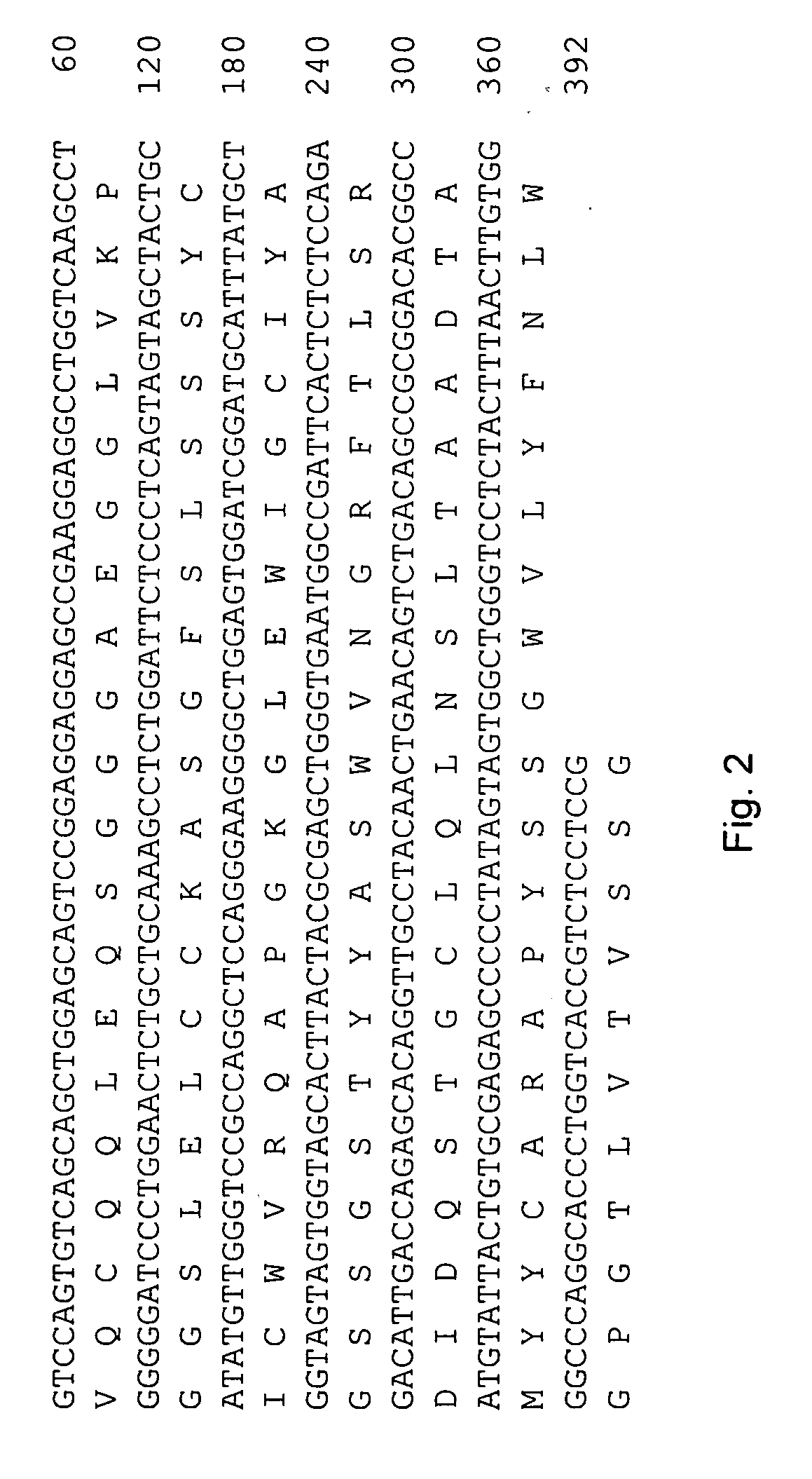
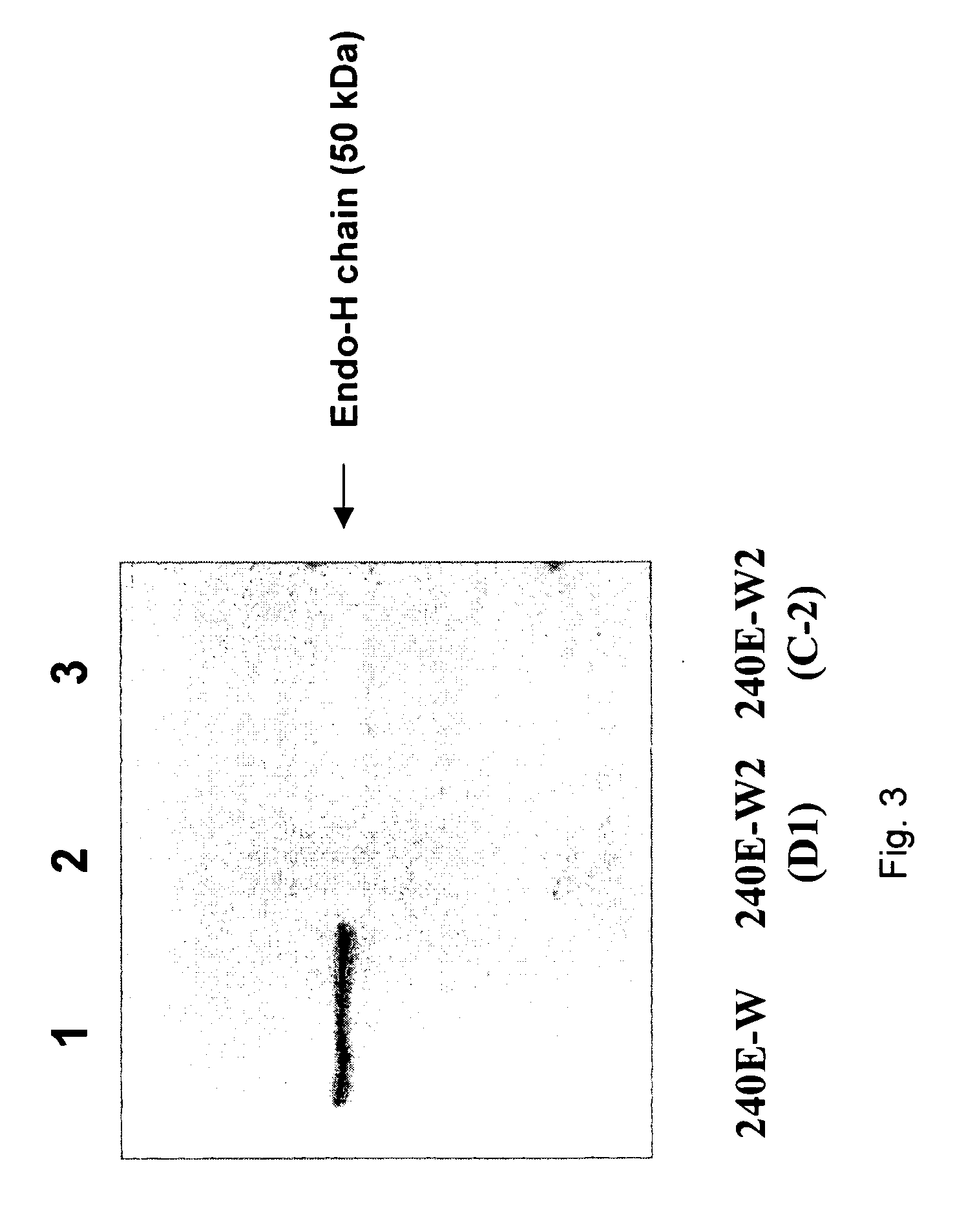
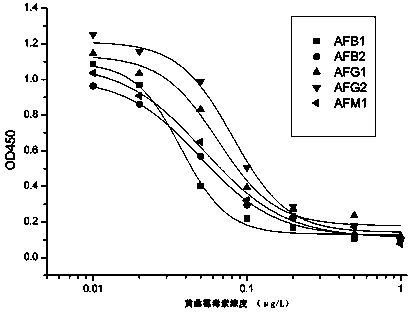
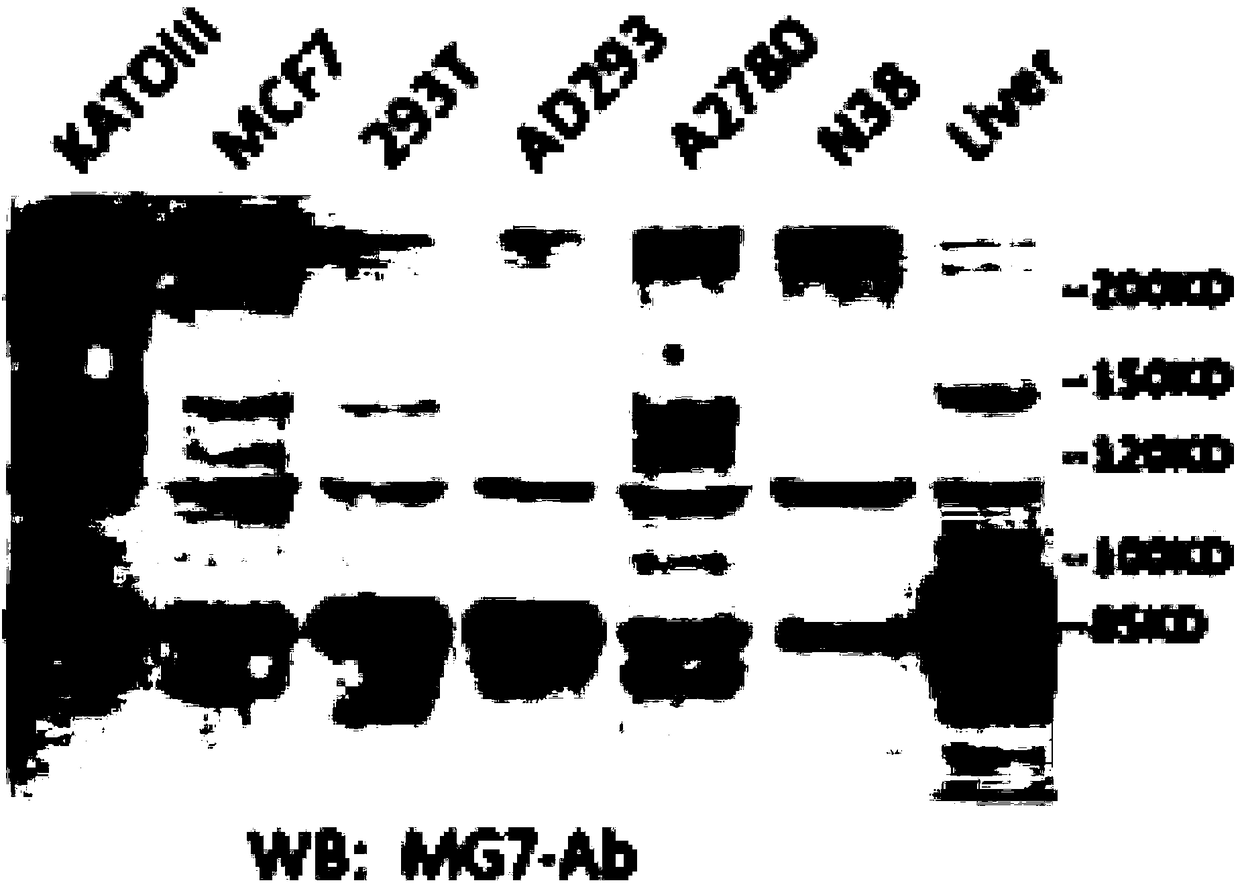
Patents
Literature
286 results about "Cell fusion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cell fusion is an important cellular process in which several uninuclear cells (cells with a single nucleus) combine to form a multinuclear cell, known as a syncytium. Cell fusion occurs during differentiation of muscle, bone and trophoblast cells, during embryogenesis, and during morphogenesis. Cell fusion is a necessary event in the maturation of cells so that they maintain their specific functions throughout growth.
Human SARS-CoV-2 monoclonal antibody and preparation method and application thereof
PendingCN111153991AHigh affinityStrong specificitySsRNA viruses positive-senseVirus peptidesBALB/cMonoclonal antibody agent
The invention discloses a human SARS-CoV-2 monoclonal antibody. The preparation method of the human SARS-CoV-2 monoclonal antibody comprises the steps: adopting SARS-CoV Nucleocapsid recombinant protein as immunogen, immunizing BALB / c mice, performing fusion and subcloning on spleen cells and myeloma cells of mice, then performing a large amount of repeated screening and domestication of cell lines through commercialized products SARS-CoV-2 Nucleocapsid and MERS Nucleocapsid so as to obtain a hybridoma cell line capable of secreting the SARS-CoV-2-resistant N monoclonal antibody with high affinity and high specificity finally and successfully, and finally performing ascites preparation and purification so as to obtain the monoclonal antibody, wherein the amino acid sequence of the SARS-CoVNucleocapsid recombinant protein is shown in SEQ ID No. 1. The invention also discloses application of the monoclonal antibody in preparation of SARS-CoV-2 virus detection products and preparation ofdrugs for inhibiting the SARS-CoV-2 viruses. The monoclonal antibody can be used for detecting the SARS-CoV-2 in human throat swabs / pulmonary secretions and other samples by using a double-antibody sandwich method, and can be applied to diagnosis and prevention and control of SARS-CoV-2 virus infection and scientific researches of viruses and other study.
Owner:BEIJING BIOSYNTHESIS BIOTECH
Human fat mesenchymal stem cell extract and lyophilized powder and application thereof
ActiveCN103784474ABiologically activeEasy to prepareCosmetic preparationsToilet preparationsBiotechnologyChemical products
The invention discloses a human fat mesenchymal stem cell extract and lyophilized powder and applications thereof. The human fat mesenchymal stem cell extract is prepared by the steps: collecting subcultured P3-P30 generations of human fat mesenchymal stem cells, culturing with cell growth liquid until 80% of cells are fused, removing culture liquid, washing the cells with a phosphate buffer solution for three times, adding into a serum-free culture solution, continuously culturing for 72 hours, collecting the serum-free culture solution, digesting uncollected cells with a digestion solution, crushing the cells with an ultrasonic crushing device, centrifuging, and then collecting supernatant; and mixing the collected serum-free culture solution and the collected supernatant, filtering, collecting filtrate, concentrating, and sterilizing. The human fat mesenchymal stem cell extract and the lyophilized powder thereof have multiple biological activities, can be used for preparing wound healing or cell repair medicaments and other biopharmaceutical products, and can also be used for preparing whitening products or skin anti-wrinkle products and other fine chemical products.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Fusion proteins
InactiveUS20110091437A1Peptide/protein ingredientsAntibody mimetics/scaffoldsBinding siteCytotoxicity
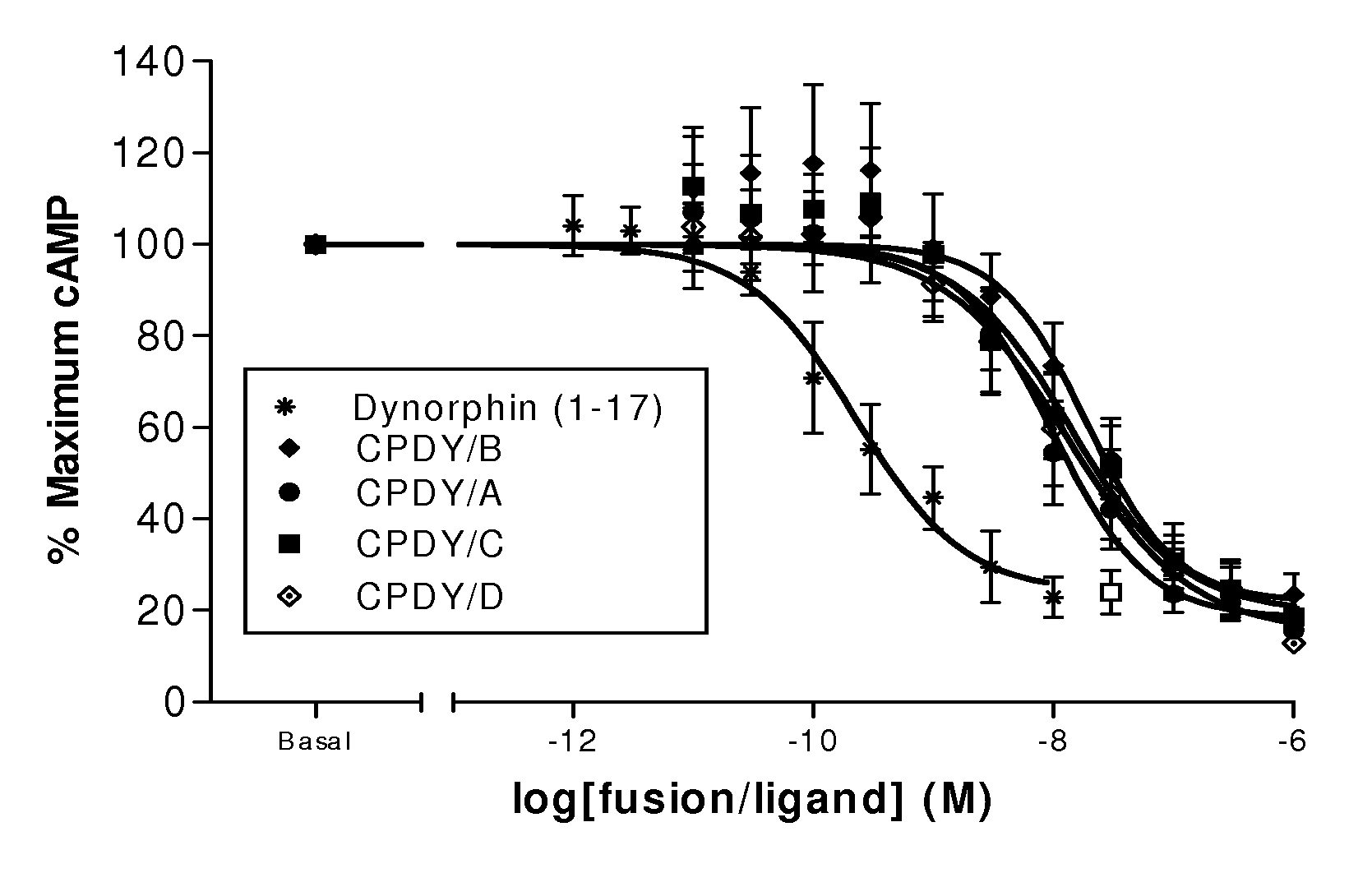
A single chain, polypeptide fusion protein, comprising: a non-cytotoxic protease, or a fragment thereof, which protease or protease fragment is capable of cleaving a protein of the exocytic fusion apparatus of a nociceptive sensory afferent; a dynorphin Targeting Moiety that is capable of binding to a Binding Site on the nociceptive sensory afferent, which Binding Site is capable of undergoing endocytosis to be incorporated into an endosome within the nociceptive sensory afferent; a protease cleavage site at which site the fusion protein is cleavable by a protease, wherein the protease cleavage site is located between the non-cytotoxic protease or fragment thereof and the dynorphin Targeting Moiety; and a translocation domain that is capable of translocating the protease or protease fragment from within an endosome, across the endosomal membrane and into the cytosol of the nociceptive sensory afferent. Nucleic acid sequences encoding the polypeptide fusion proteins, methods of preparing same and uses thereof are also described.
Owner:HEALTH PROTECTION AGENCY +1
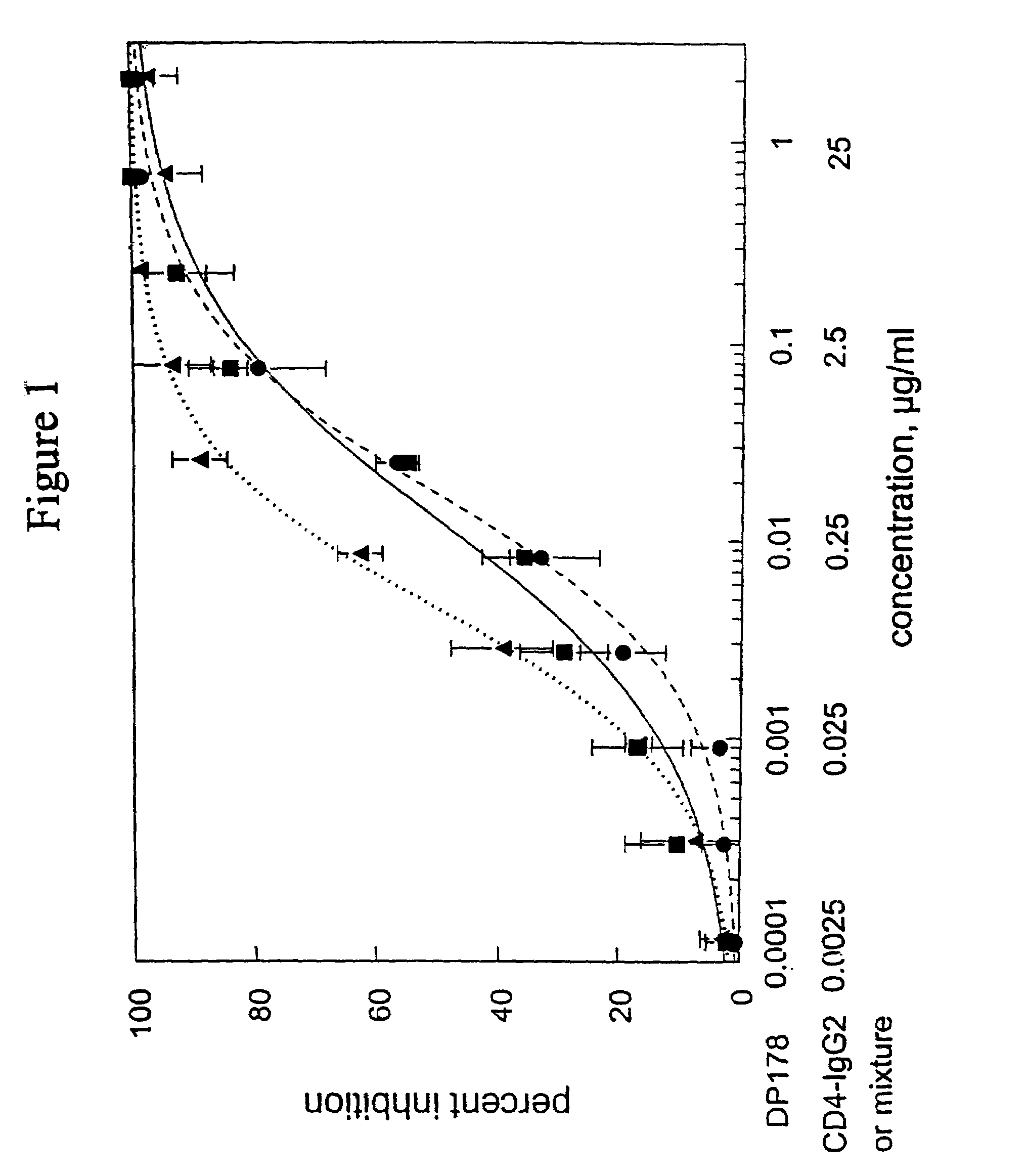
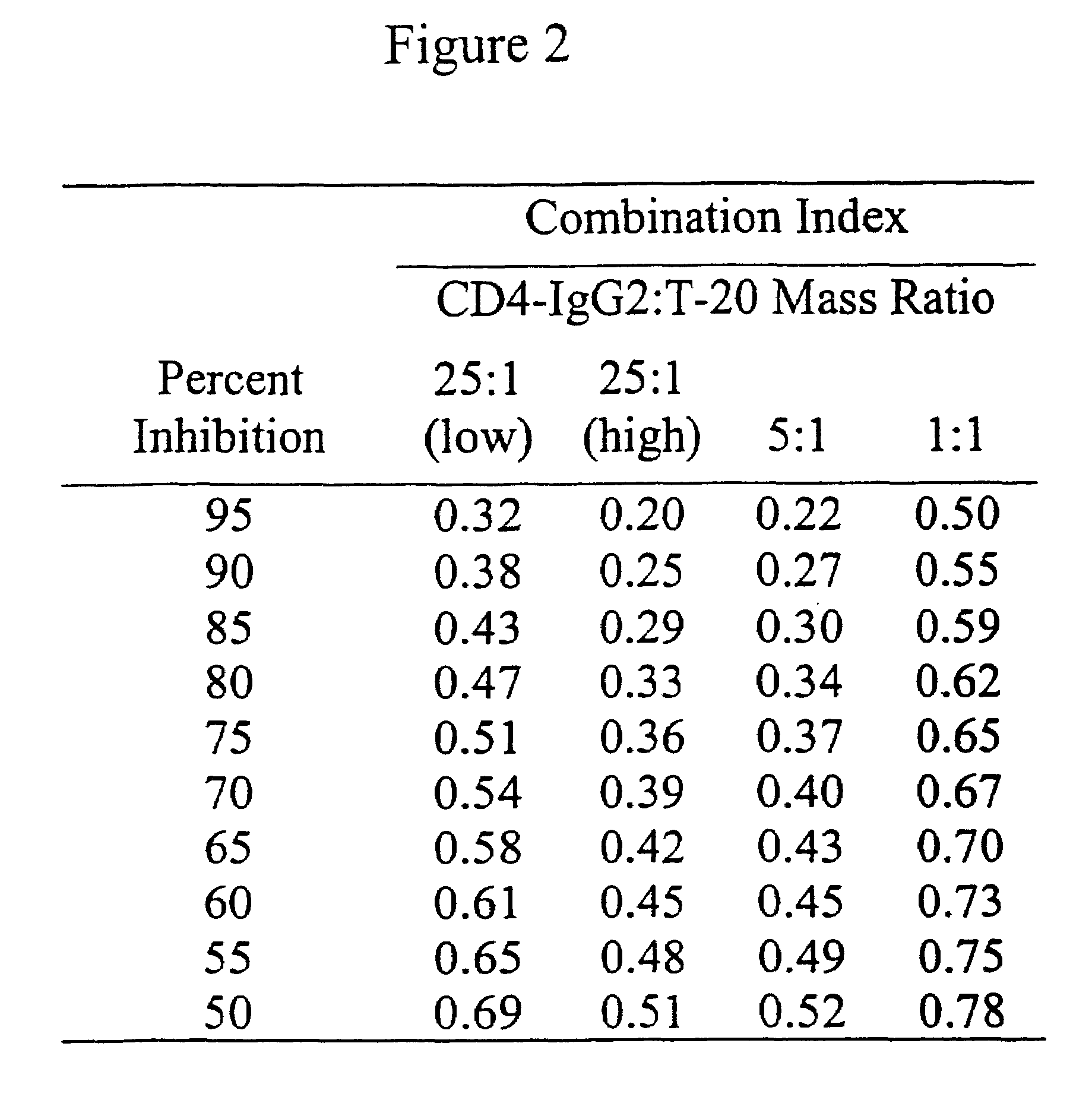
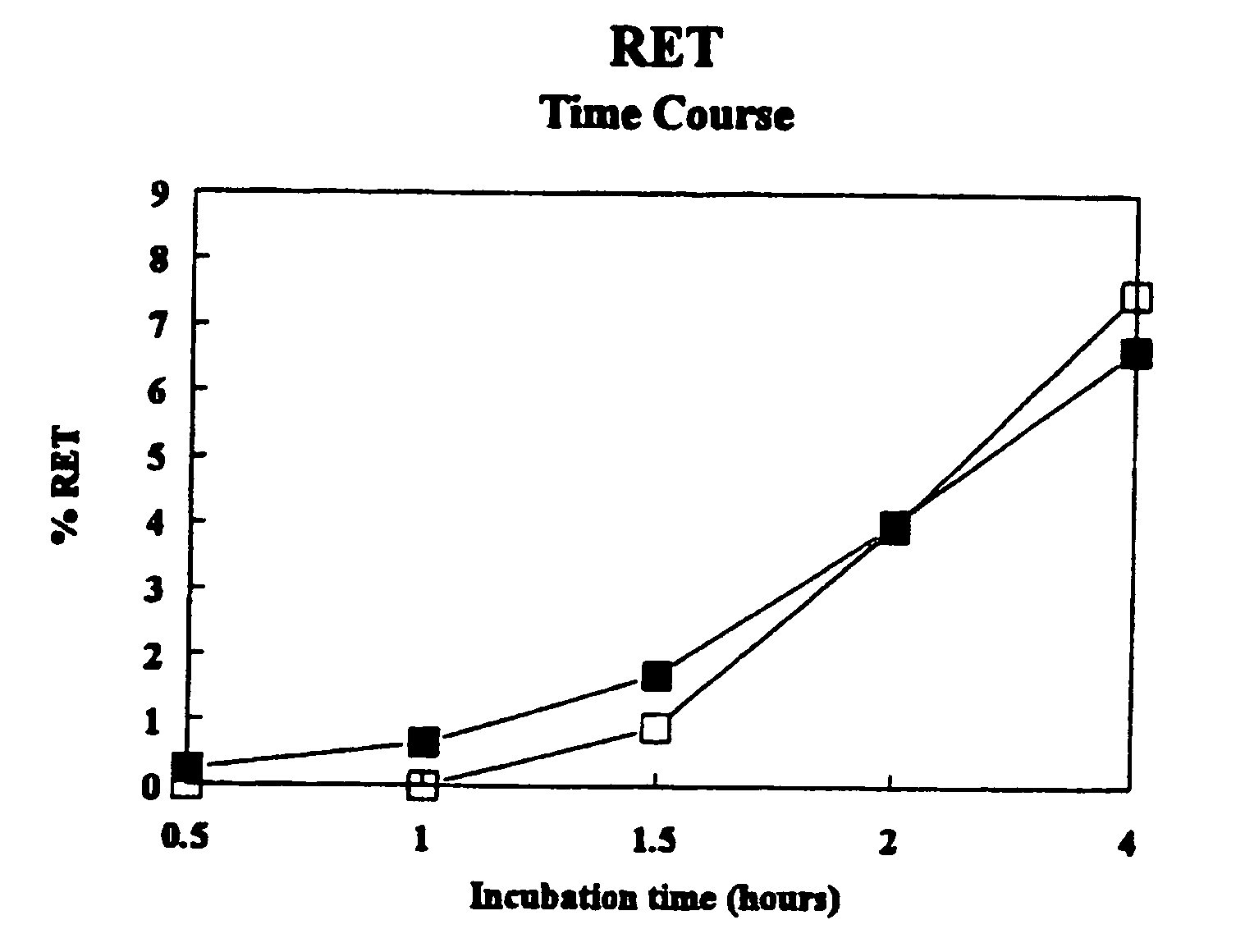
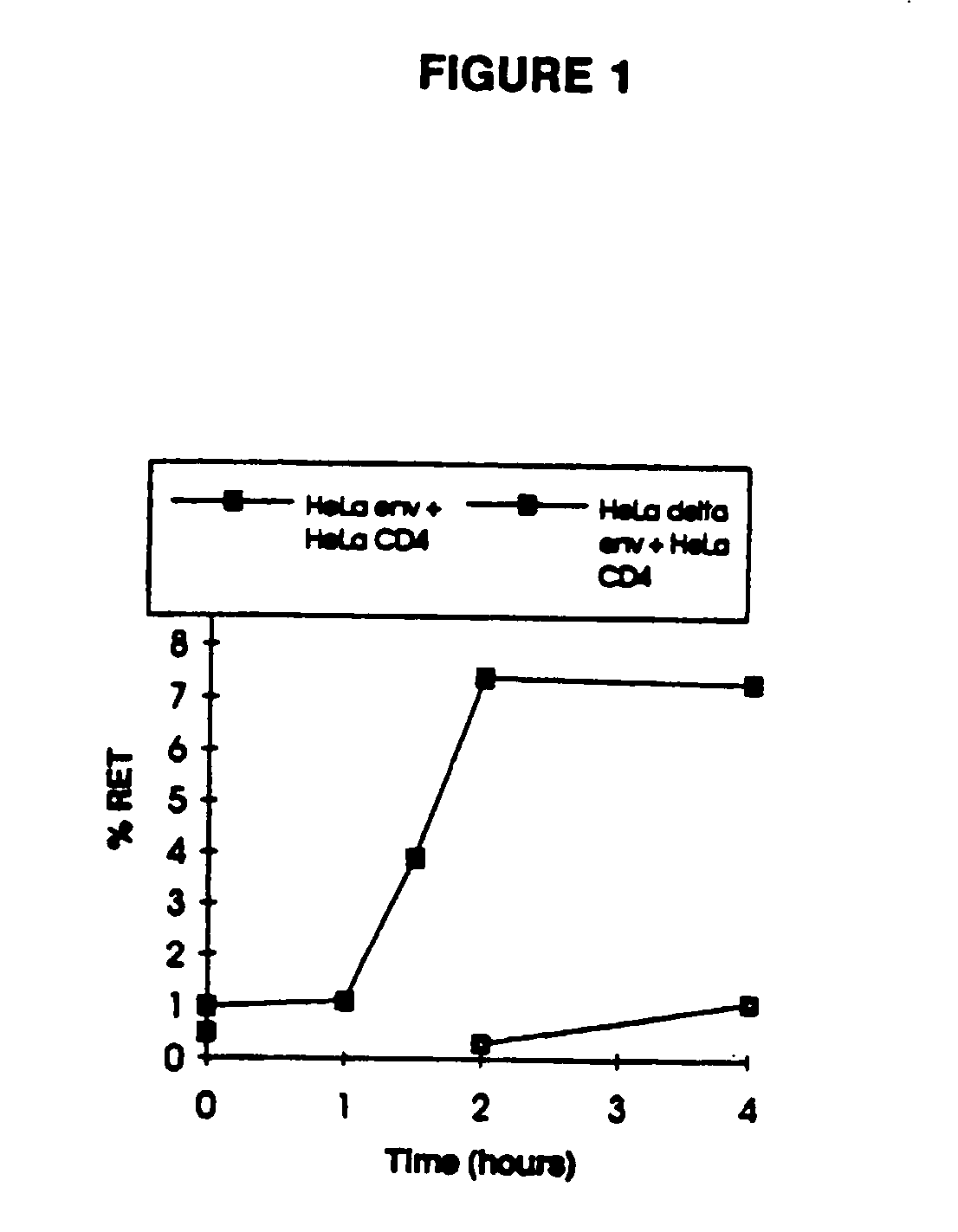
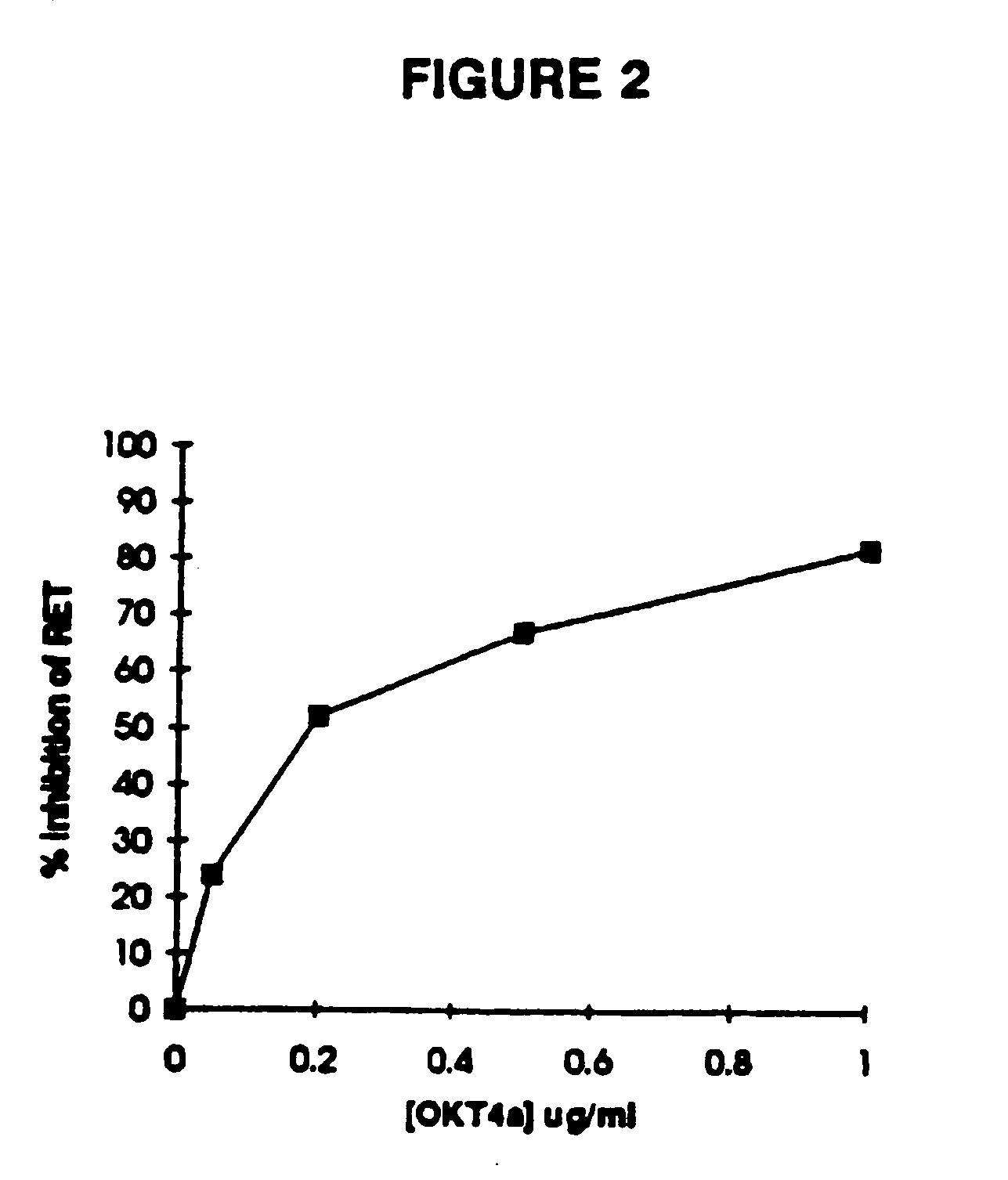
Methods for using resonance energy transfer-based assay of HIV-1 envelope glycoprotein-mediated membrane fusion, and kits for practicing same
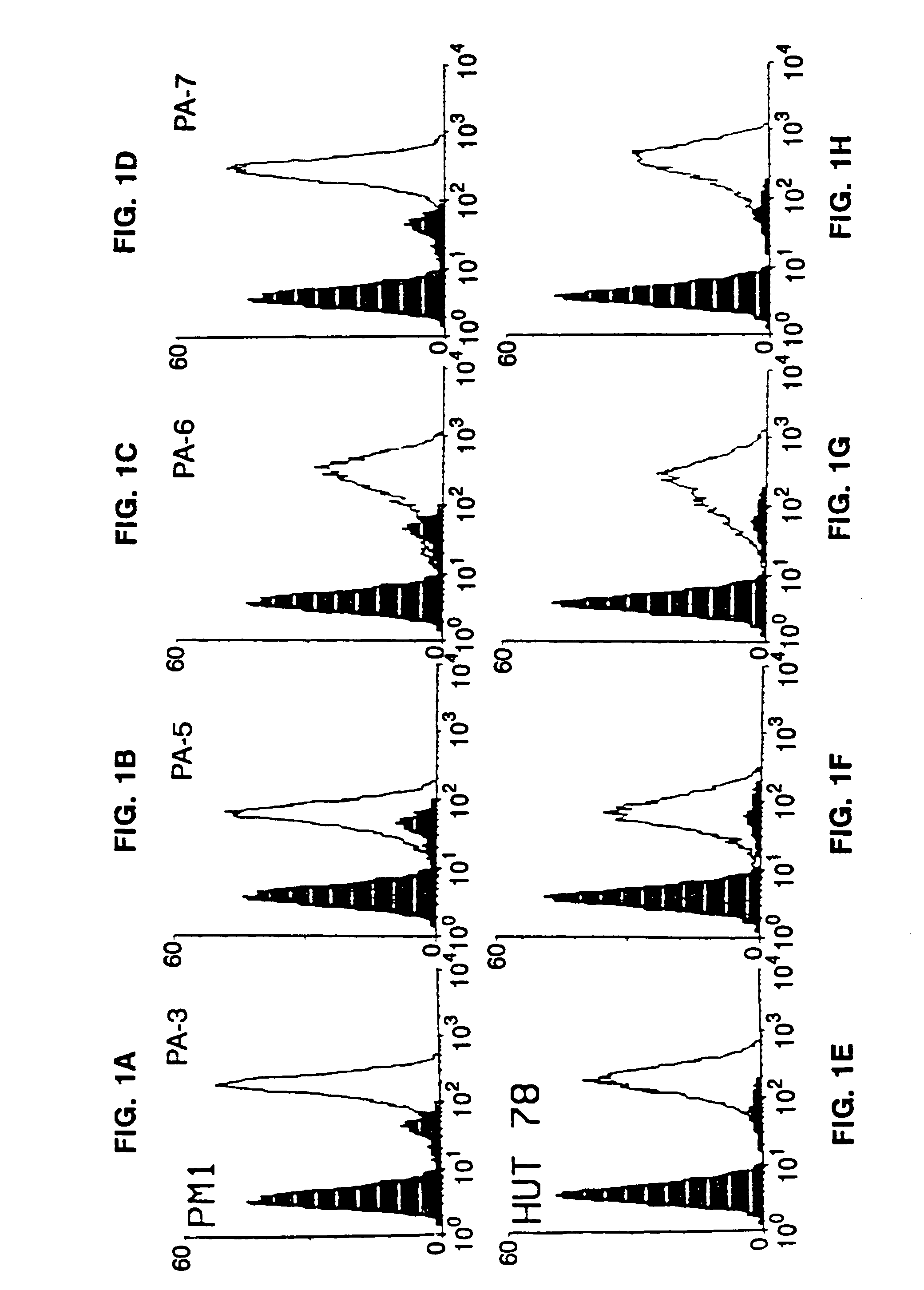
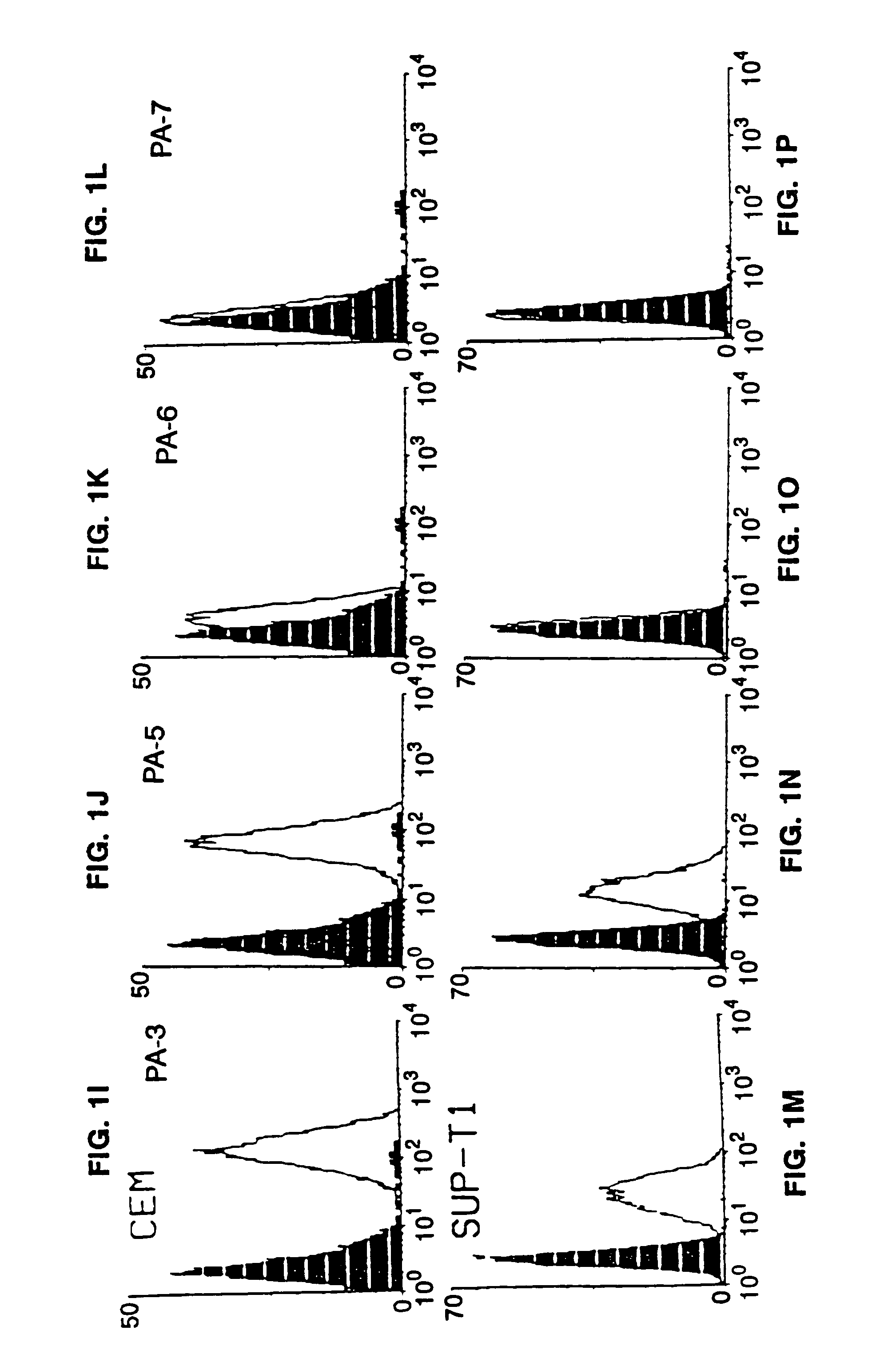
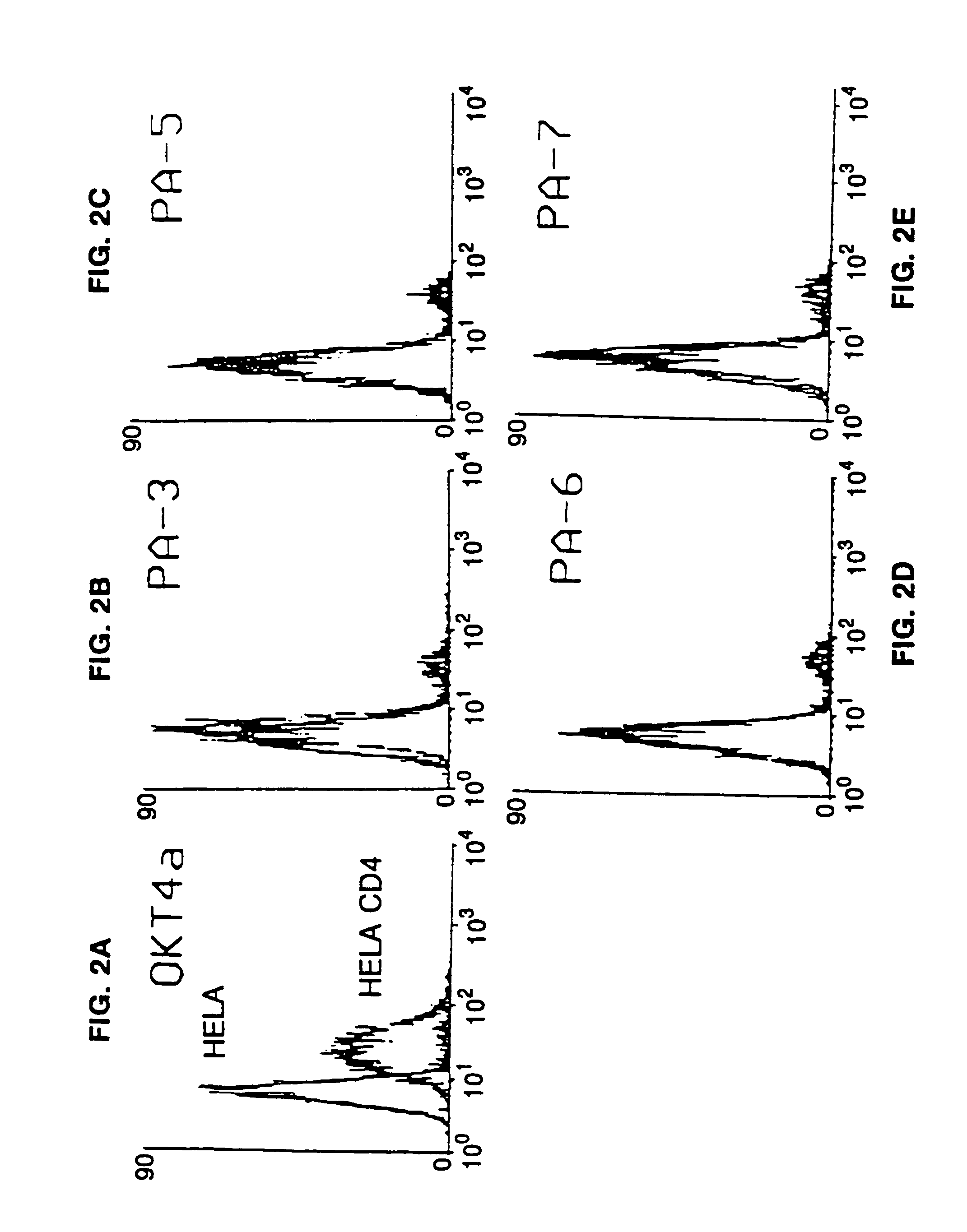
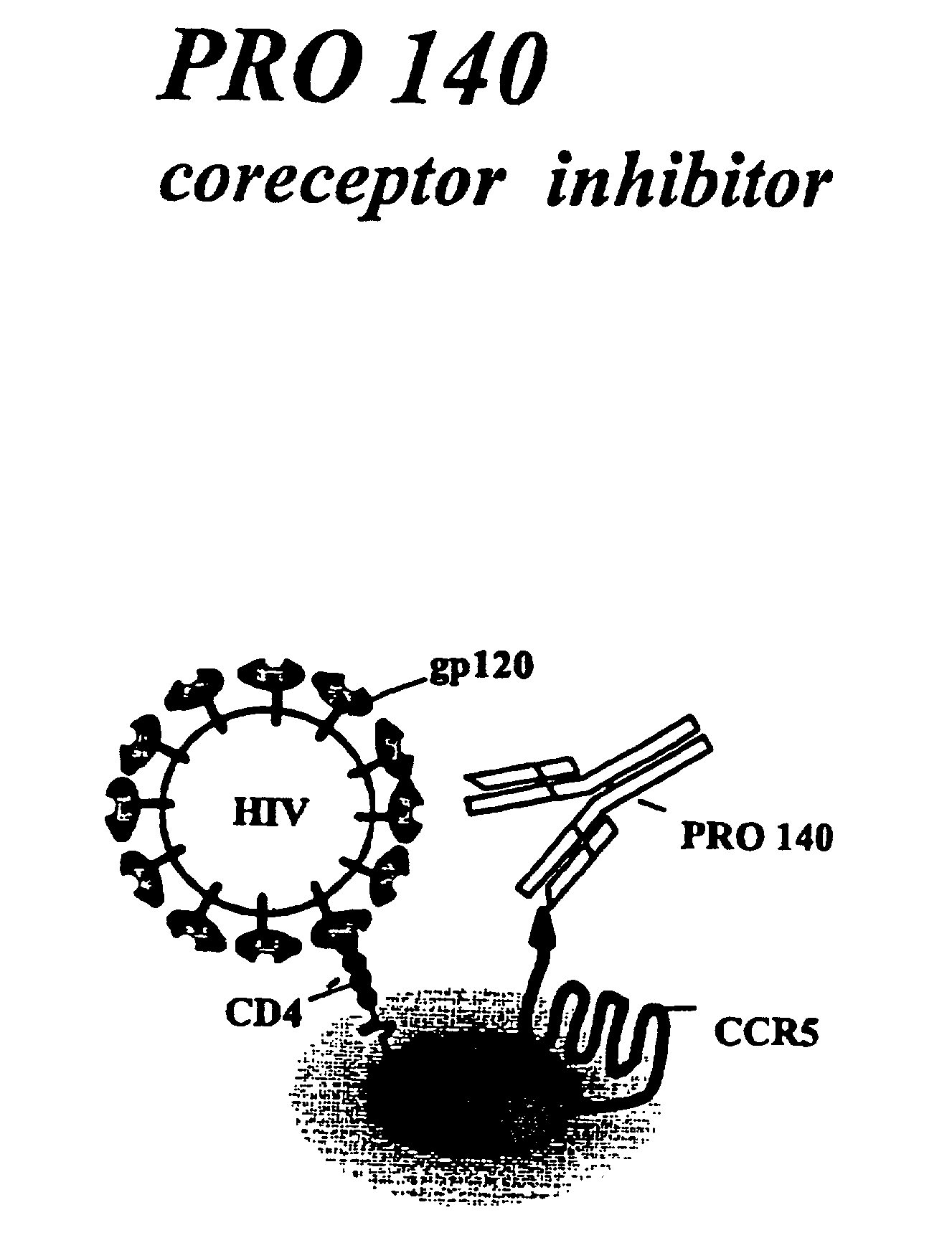
This invention provides: agents determined to be capable of specifically inhibiting the fusion of a macrophage-tropic primary isolate of HIV-1 to a CD4+ cell, but not a T cell-tropic isolate of HIV-1 to a CD4+ cell; and agents determined to be capable of specifically inhibiting the fusion of a T cell-tropic isolate of HIV-1 to a CD4+ cell, but not a macrophage-tropic primary isolate of HIV-1 to a CD4+ cell. This invention also provides: agents capable of specifically inhibiting the fusion of a macrophage tropic primary isolate of HIV-1 with a CD+ cell susceptible to infection by a macrophage-tropic primary isolate of HIV-1; and agents capable of specifically inhibiting the fusion of a T cell-tropic isolate of HIV-1 with a CD4+ cell susceptible to infection by a T cell-tropic isolate of HIV-1. The agents include but are not limited to antibodies. This invention further provides: methods of inhibiting fusion of a macrophage-tropic primary isolate of HIV-1 with a CD+ cell susceptible to infection by a macrophage-tropic primary isolate of HIV-1 which comprises contacting the CD4+ cell with an amount of an agent capable of specifically inhibiting such fusion so as to thereby inhibit such fusion; and methods of inhibiting fusion of a T cell-tropic isolate of HIV-1 with a CD4+ cell susceptible to infection by a T cell-tropic isolate of HIV-1 which comprises contacting the CD4+ cell with an amount of an agent capable of specifically inhibiting such fusion so as to thereby inhibit such fusion.
Owner:CYTODYN
Methods for inhibiting HIV-1 infection
This invention provides an antibody capable of specifically inhibiting the fusion of an HIV-1 envelope glycoprotein+ cell with an appropriate CD4+ cell without cross reacting with the HIV-1 envelope glycoprotein or CD4 and capable of inhibiting infection by one or more strains of HIV-1. This antibody is then used to identify a molecule which is important for HIV infection. Different uses of the antibody and the molecule are described.
Owner:CYTODYN
Formation of Hybrid Cells by Fusion of Lineage Committed Cells with Stem Cells
InactiveUS20060084167A1Limit reductive divisionImprove stabilityFused cellsOrgan functionNormal tissue
The potential of a stem cell to differentiate into specialized cell types for restoring normal tissue / organ function has stimulated interest in stem cell research. The methods used to coax stem cells differentiate into specialized cells still remain in their infancy stages. The disclosed invention is the generation of mammalian or avian cell hybrids formed from fusing lineage committed somatic cells with nucleated stem cells or nucleated transit amplifying cells. The fusion of lineage committed somatic cells with nucleated stem cells, or nucleated transit amplifying cells as described herein facilitates stem cell differentiation and lineage commitment of hybrid cells and can be aided by inclusion of an encapsulation step. By the fusion of cells in this invention, this invention also provides for methods to restore damaged tissue or the expression of defective, dysfunctional, decreased, lost or not previously expressed bio-pharmaceutical products.
Owner:COHENFORD MENASHI A +1
Compositions and methods for inhibition of HIV-1 infection
InactiveUS7138119B2Inhibit infectionAvoid infectionAnimal cellsPeptide/protein ingredientsMolecular biologyGlycophorin
Owner:CYTODYN
Fusion proteins
A single chain, polypeptide fusion protein, comprising: a non-cytotoxic protease, or a fragment thereof, which protease or protease fragment is capable of cleaving a protein of the exocytic fusion apparatus of a nociceptive sensory afferent; a dynorphin Targeting Moiety that is capable of binding to a Binding Site on the nociceptive sensory afferent, which Binding Site is capable of undergoing endocytosis to be incorporated into an endosome within the nociceptive sensory afferent; a protease cleavage site at which site the fusion protein is cleavable by a protease, wherein the protease cleavage site is located between the non-cytotoxic protease or fragment thereof and the dynorphin Targeting Moiety; and a translocation domain that is capable of translocating the protease or protease fragment from within an endosome, across the endosomal membrane and into the cytosol of the nociceptive sensory afferent. Nucleic acid sequences encoding the polypeptide fusion proteins, methods of preparing same and uses thereof are also described.
Owner:ALLERGAN INC
Methods for promoting fusion and reprogramming of somatic cells
InactiveUS20110136145A1Microbiological testing/measurementGenetically modified cellsDemethylaseMethyltransferase Gene
The invention features methods for reprogramming somatic cells by treating the cells with one or more agents to induce de-differentiation, in particular by targeting demethylase and methyltransferase genes. The invention also features methods of monitoring somatic cell fusion and reprogramming and methods of identifying agents that alter somatic cell fusion and reprogramming. The invention also features reprogrammed cells and kits.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods for assaying inhibition of HIV-1 envelope glycoprotein-mediated membrane fusion
Owner:PROGENICS PHARMA INC
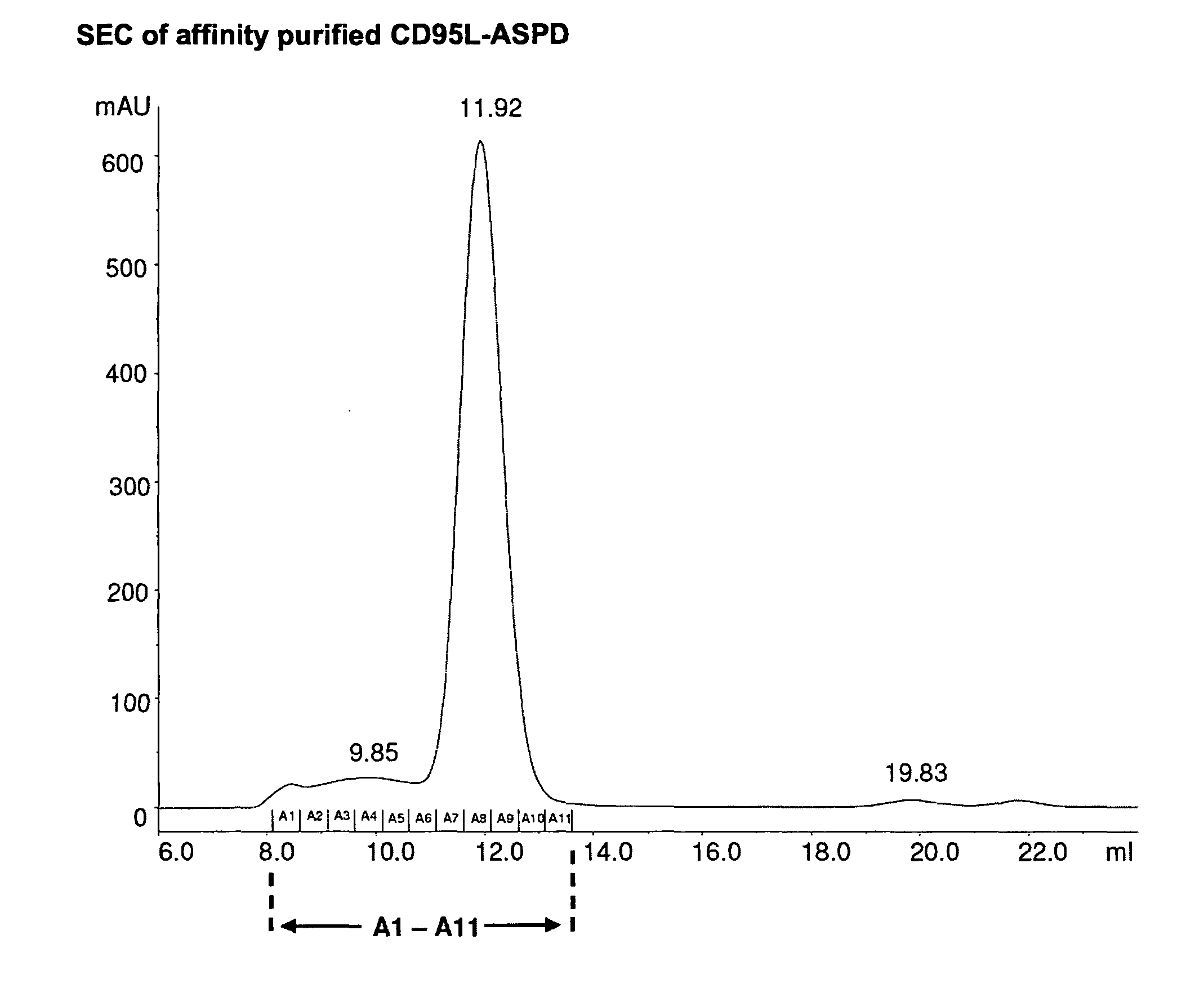
TNF superfamily collectin fusion proteins
The present invention refers to a fusion protein comprising a TNF-superfamily (TNFSF) cytokine or a receptor binding domain thereof fused to a collectin trimerization domain, to a nucleic acid molecule encoding the fusion protein, and to a cell comprising the nucleic acid molecule. The fusion protein is present as a trimeric complex or as an oligomer thereof. The fusion protein, the nucleic acid, and the cell is suitable as pharmaceutical composition or for therapeutic, diagnostic and / or research applications.
Owner:APOGENIX AG
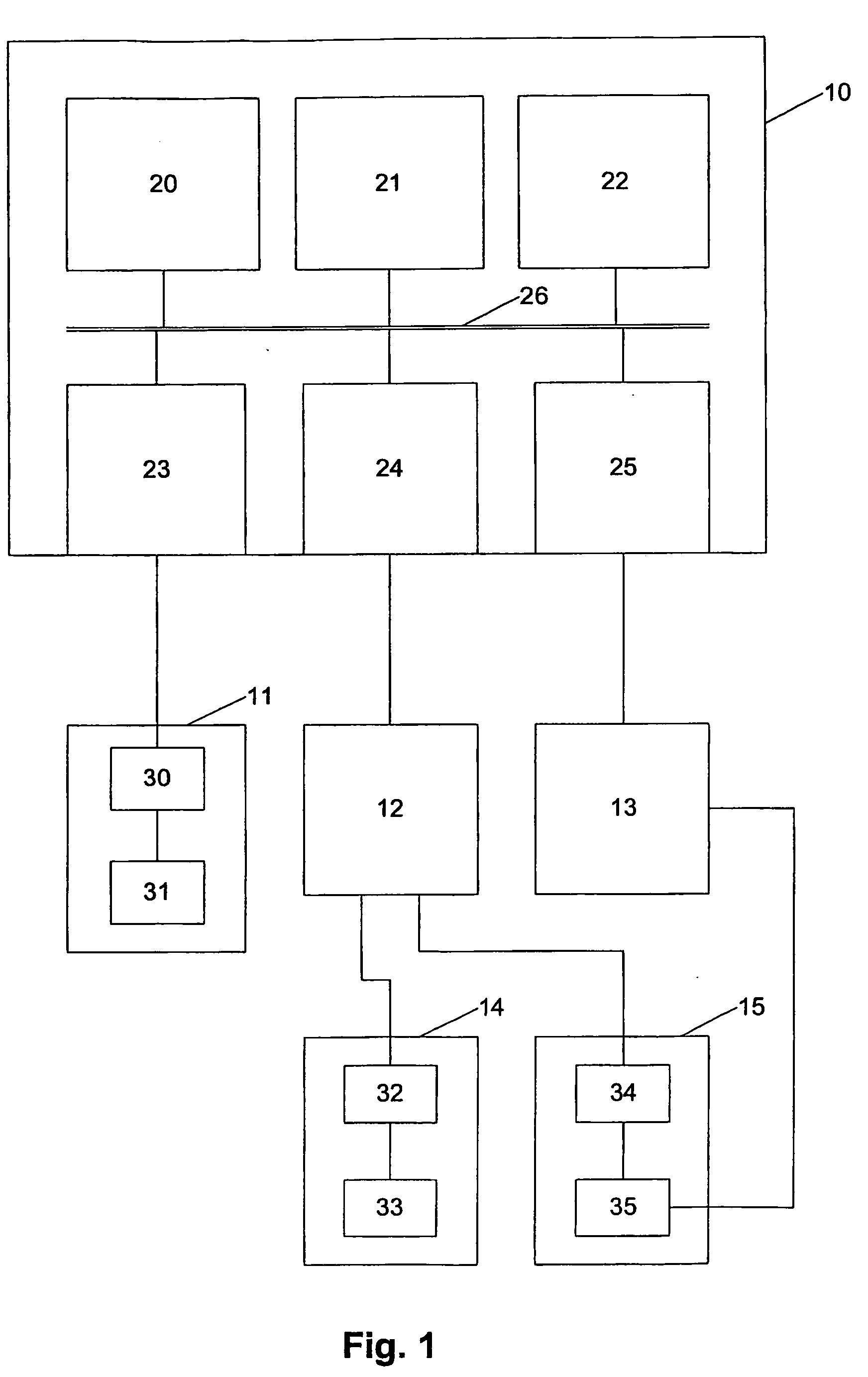
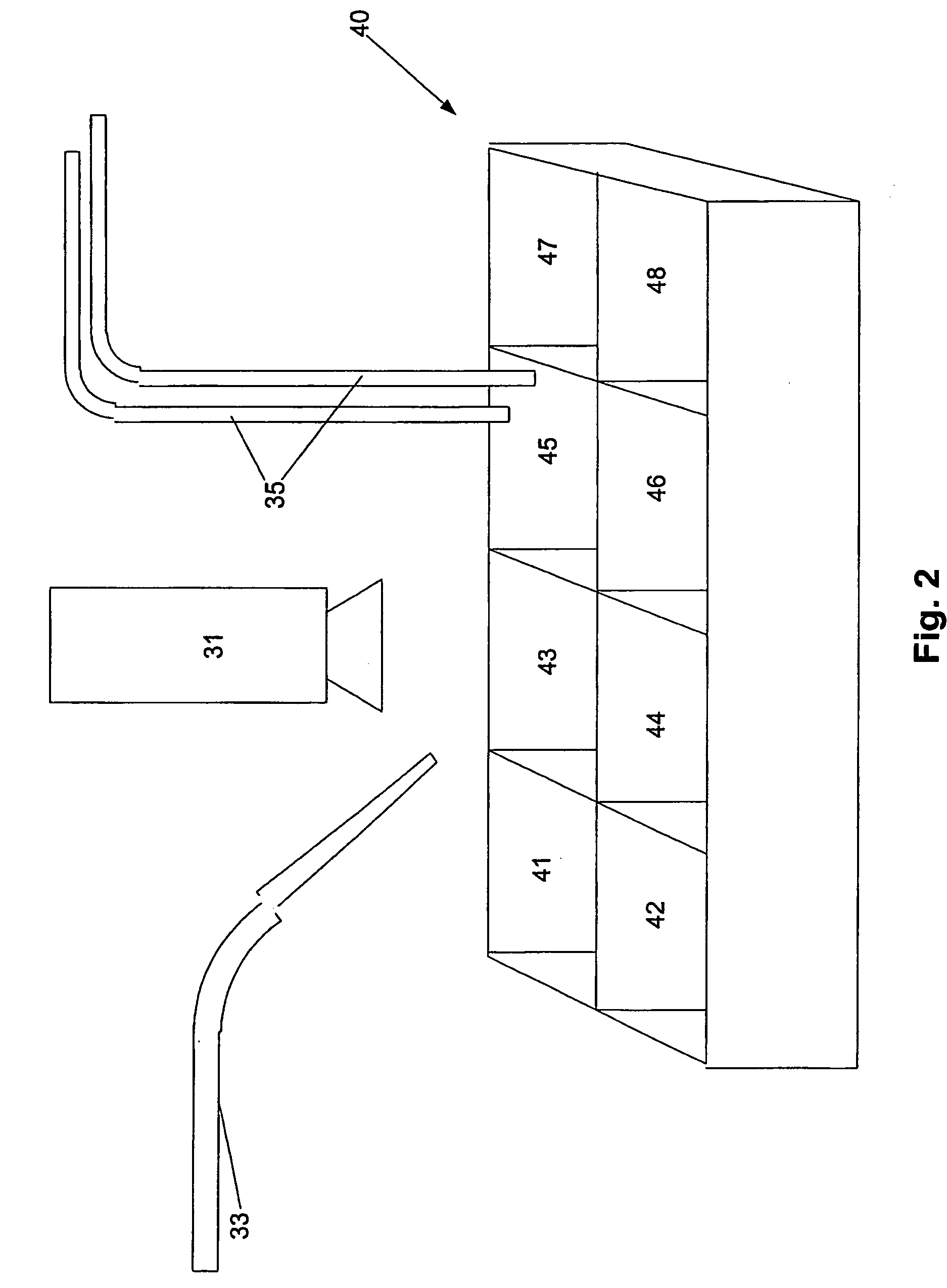
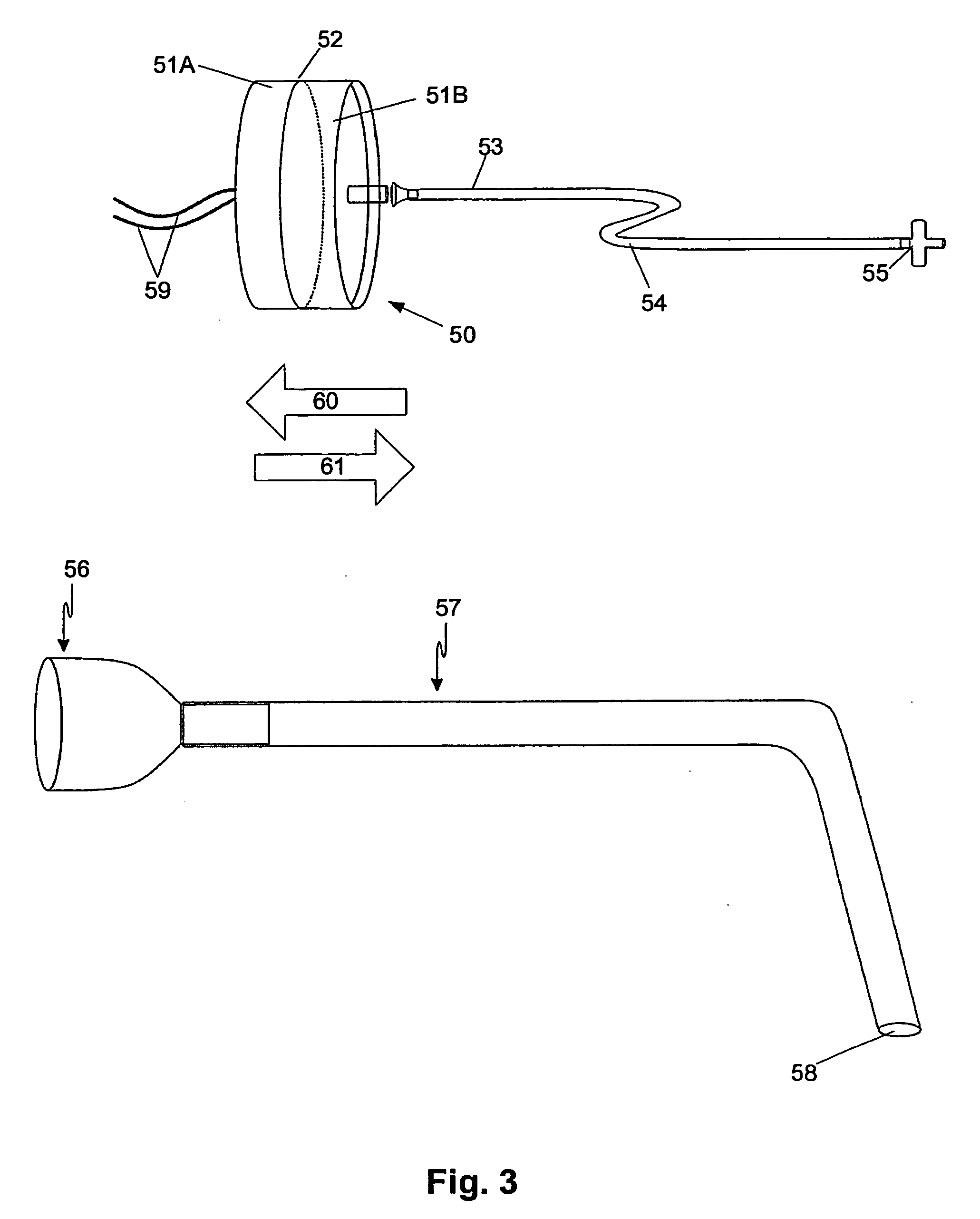
Electrofusionof cells and apparatus therefore
The present invention relates to a method and apparatus for fusing first and second cells. In particular, the method includes positioning the first and second cells between two electrodes (35) in a fluid filled container (40) using a pipette system (33), with the first and second cells being held separated from each electrode. Once this has been achieved a current having a predetermined waveform is applied to the electrodes (35) to generate a predetermined electric field thereby causing the cells to fuse.
Owner:APOLLO LIFE SCI
Method for modifying chromosomes
InactiveUS20090007282A1High homologous recombination efficiencyImprove efficiencyStable introduction of DNANucleic acid vectorGeneticsMicrocell
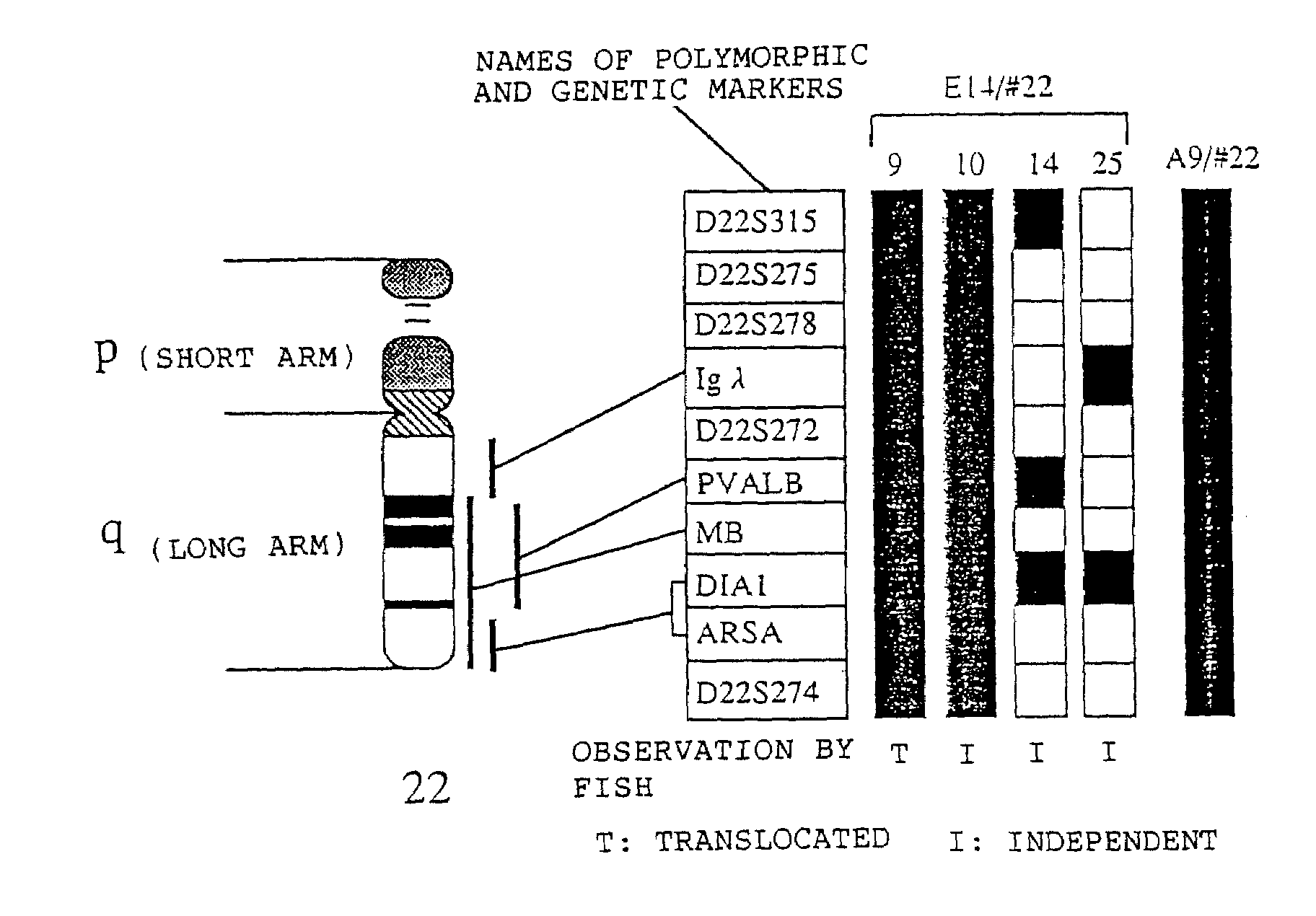
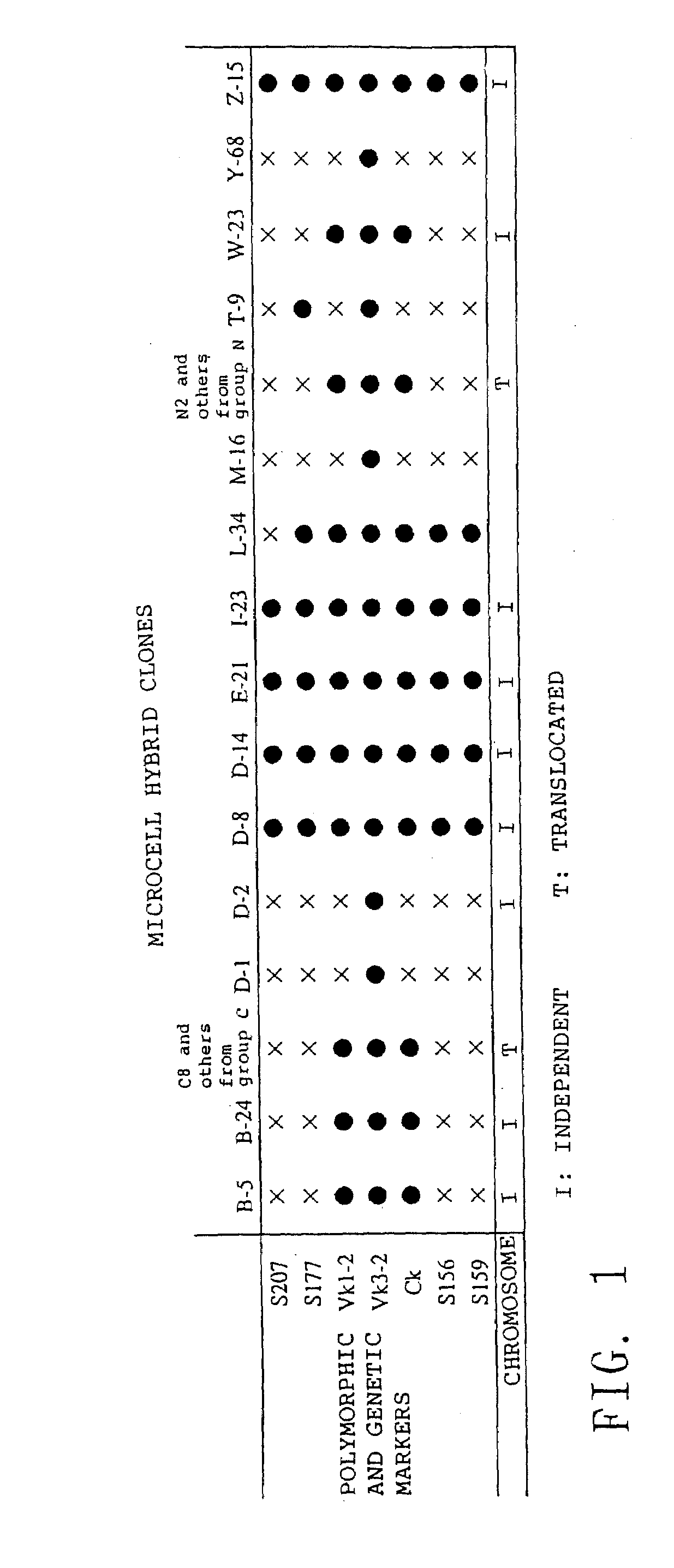
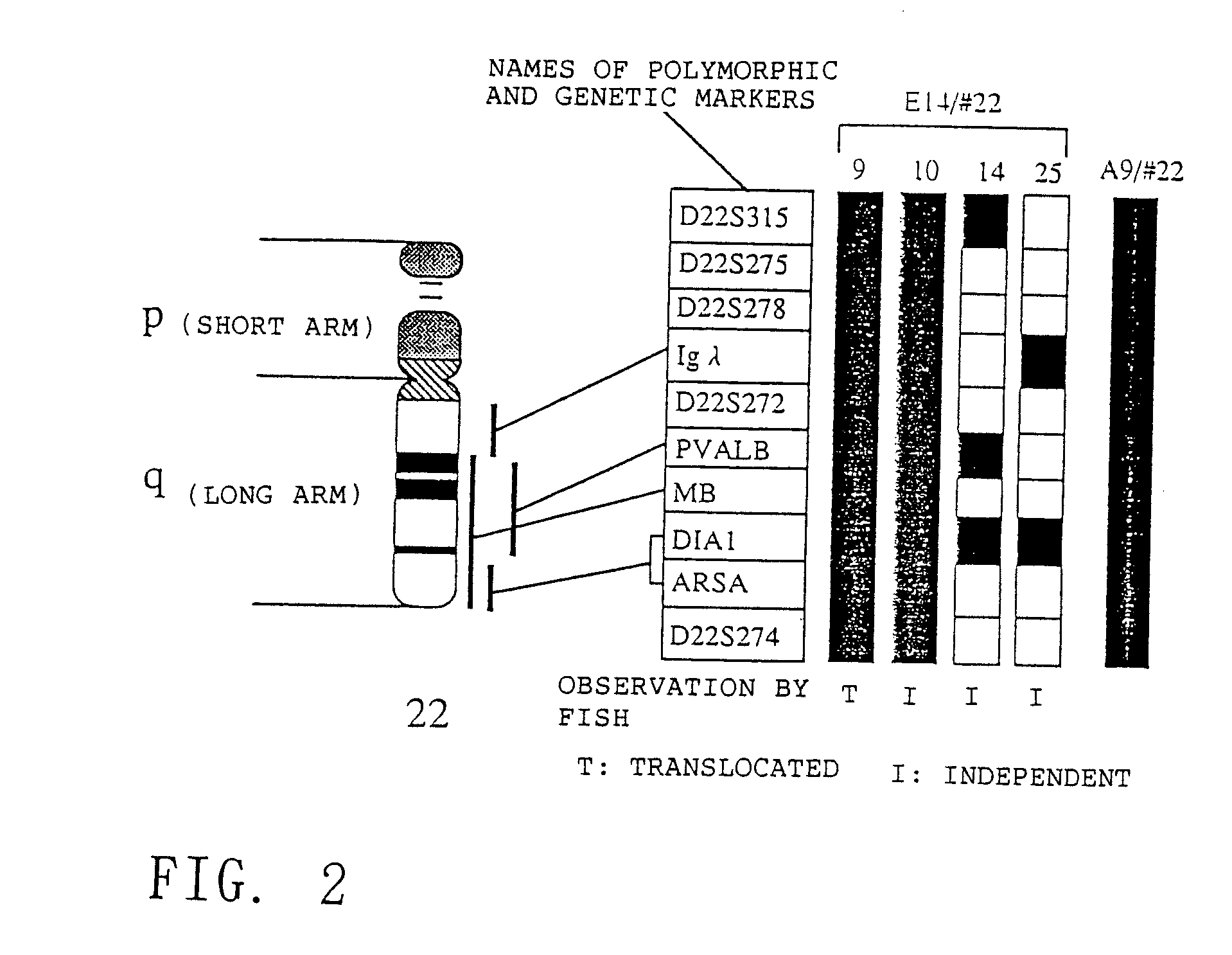
The present invention relates to a method for producing a modified foreign chromosome(s) or a fragment(s) thereof, which comprises the steps of: (a) preparing a microcell comprising a foreign chromosome(s) or a fragment(s) thereof, and transferring said foreign chromosome(s) or a fragment(s) into a cell with high homologous recombination efficiency through its fusion with said microcell; (b) in said cell with high homologous recombination efficiency, inserting a targeting vector by homologous recombination into a desired site of said foreign chromosome(s) or a fragment(s) thereof, and / or a desired site of a chromosome(s) derived from said cell with high homologous recombination efficiency, thereby marking said desired site; and (c) in said cell with high homologous recombination efficiency, causing deletion and / or translocation to occur at the marked site of said foreign chromosome(s) or a fragment(s) thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
Placental villus plate mesenchymal stem cells and extraction method thereof
InactiveCN106085952APrevent intrusionReduce usageCell dissociation methodsSkeletal/connective tissue cellsFiltrationAntibiotic Y
The invention discloses placental villus plate mesenchymal stem cells. An extraction method of the placental villus plate mesenchymal stem cells includes the following steps that 1, a placental villus plate is obtained; 2, blood vessels and villus tissue are removed; 3, the villus plate is cleaned till the blood color disappears; 4, the villus plate is sheared into 0.5-5 mm<2> tissue microblocks, and the tissue microblocks are cleaned; 5, tissue digestive juice containing pancreatin, collagenase II, collagenase IV and hyaluronidase is added for digestion; 6, filtration and centrifugation are carried out; 7, culture is carried out; 8, cell digestion is carried out with 0.25% pancreatin when the cell confluence reaches 70-90%; 9, subculturing is carried out; 10, detection is carried out; 11, cryopreservation is carried out; 12, database building is carried out. According to the method, the steps are simple, and a large number of mesenchymal stem cells can be rapidly obtained from the placental tissue; the separation process does not need too much cleaning operation; the use of antibiotics is omitted, so that the purity of the placental villus plate mesenchymal stem cells obtained after separation is high.
Owner:四川华皓生物科技有限公司
Preparation method of HRPII protein monoclonal antibody of plasmodium falciparum
ActiveCN101659975AGood repeatabilityAchieve serial expressionMicroorganism based processesFermentationChemical synthesisEscherichia coli
The invention relates to a preparation method of HRPII protein monoclonal antibody of plasmodium falciparum. The preparation method comprises the following steps of: adopting HRPII protein of plasmodium falciparum as target antigen and respectively analyzing and selecting two dominant antigen epitopes of A and B; respectively repeating the two dominant antigen epitopes of A and B, then continuously connecting four glycine and forming recombinant protein C; adopting most securest code of escherichia coli and converting the amino acid sequence of the recombinant protein C into corresponding nucleotide sequence; carrying out chemical synthesis to the former step to obtain the nucleotide sequence, and respectively adding enzyme cutting sites BamHI and EcoRI at the upstream and downstream thereof; inserting nucleotide fragment obtained by the former step into expression carrier PET-28a(+), constructing recombinant protein C expression carrier and inducing to express the recombinant proteinC in the escherichia coli BL21 (DE3); carrying out ultrasonic bacteria breaking and low-temperature centrifugation, then taking supernatant of the solution, affining a chromatographic column by nickel-agarose, eluting and obtaining purified recombinant protein C; after immunizing Balb / c mouse with the recombinant protein C for a plurality of times, taking and fusing spleen cells with sp2 / 0 myelomacells, and obtaining six hybridoma cell lines by multiple rounds of screening; and purifying monoclonal antibody, respectively marking horse radish peroxidase and prorating matching and combination of optimum monoclonal antibody by ELISA orthogonal experiment.
Owner:杭州新脉生物科技有限公司
Method for constructing hybridoma cell line of heterogeneity of seereting human monoclonal antibody for anti hepatitis B
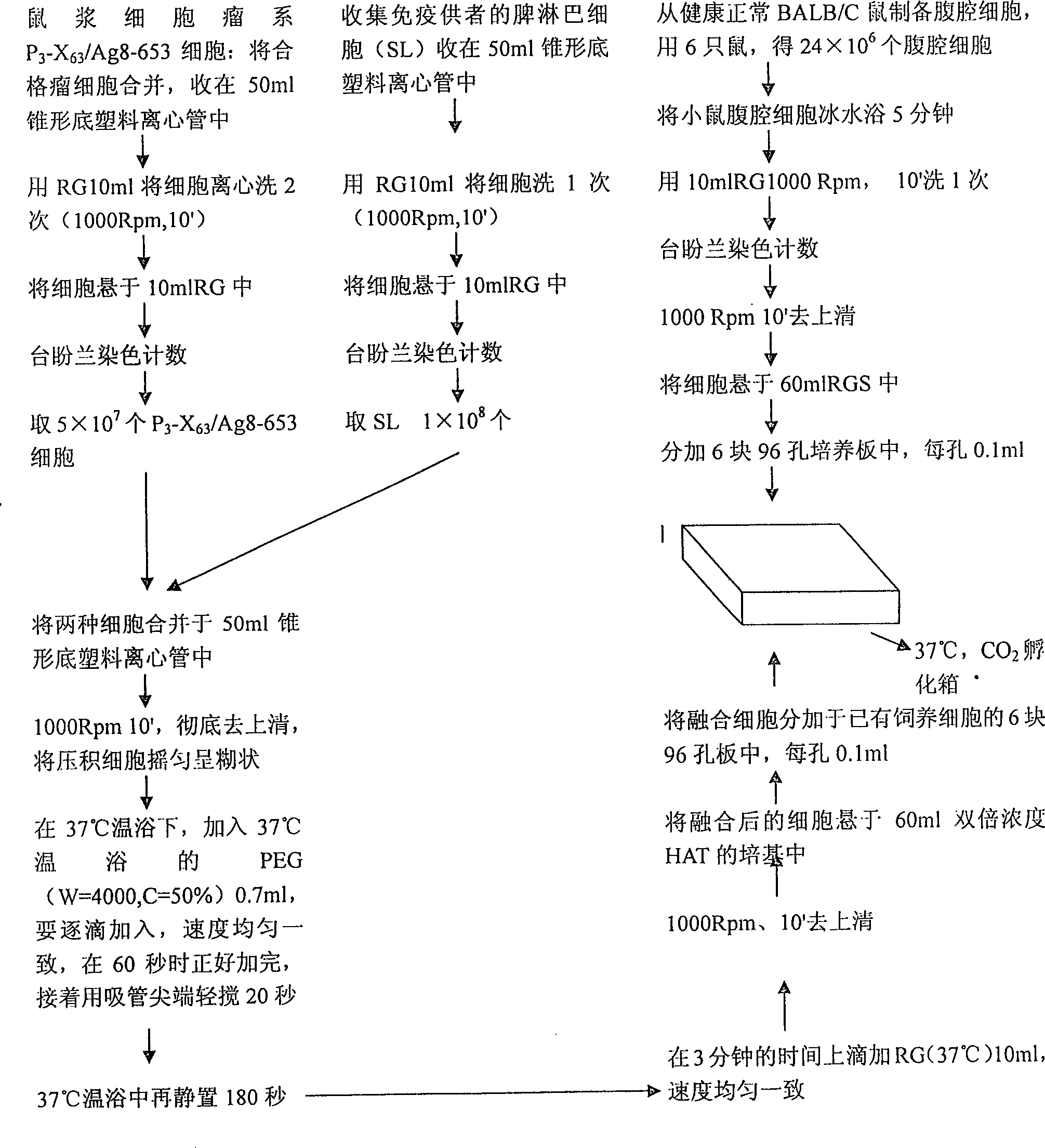
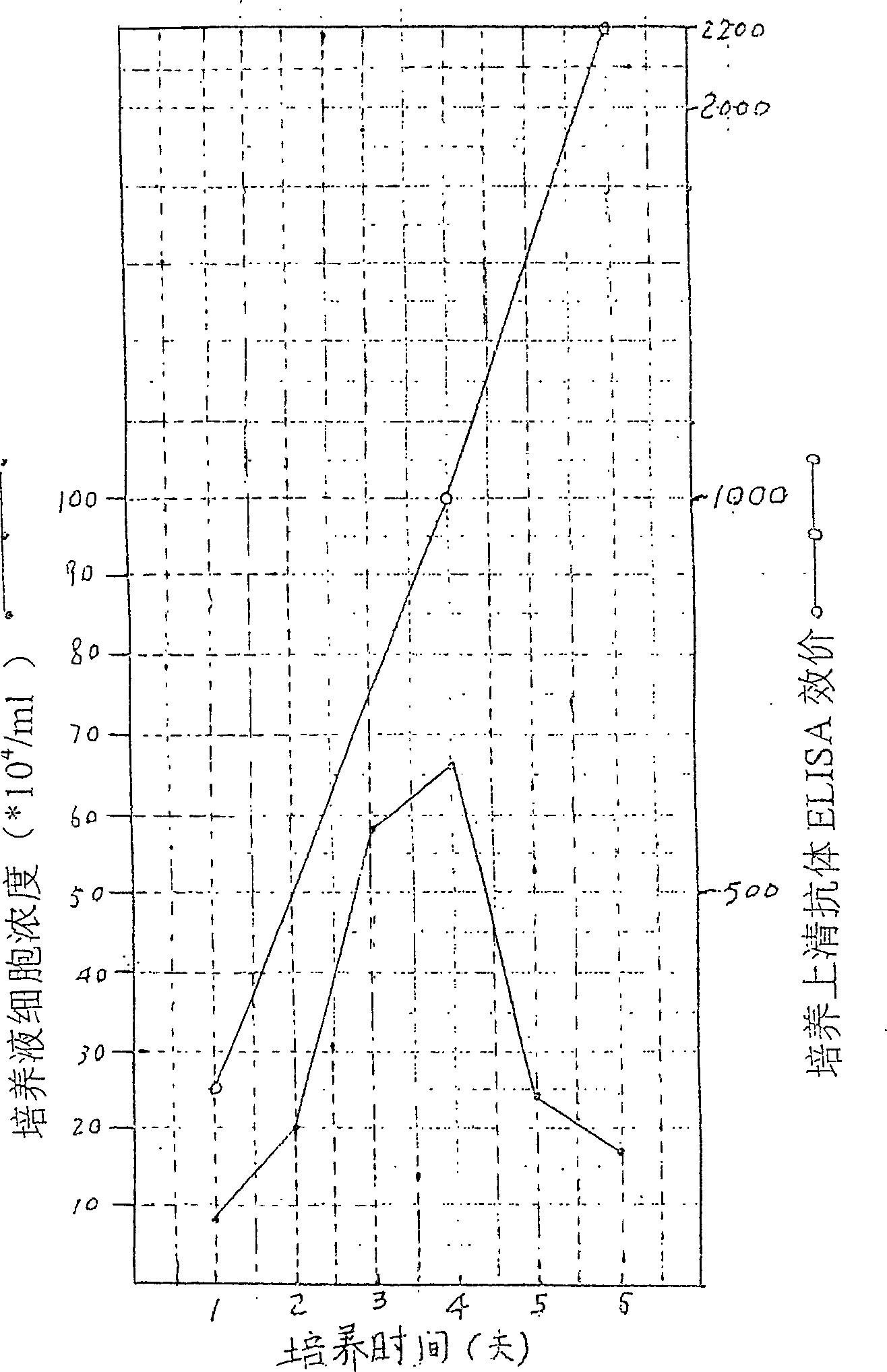
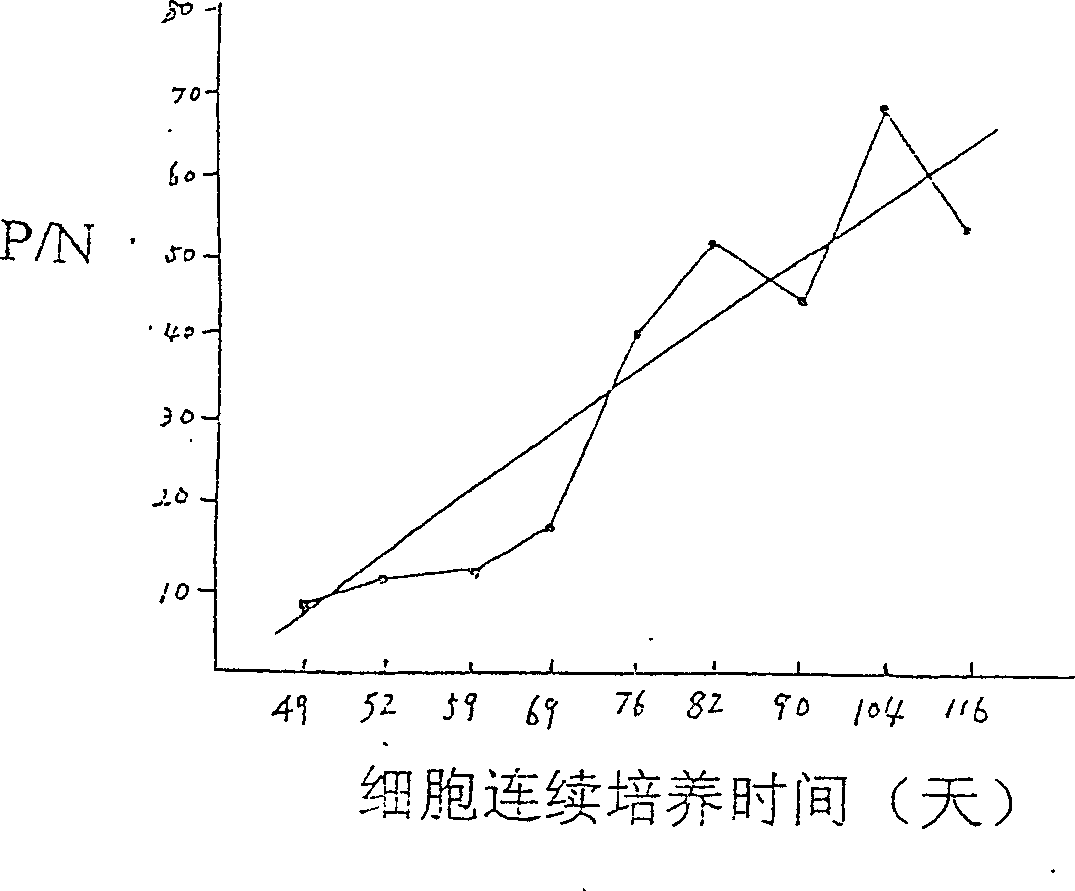
This invention provides a method for establishing hetero-hybrid tumor engineering cell sequence for producing human monoclonal antibody for anti-hepatitis B virus. The anti-HBs positive human splenic lymph cell (SL) is fused with mouse plasmacytoma sequence P3-X63 / Ag8-653 cell. Limiting dilution method is used for cloning the positive antibody poro-hybrid-tumor cell to obtain hetero hybrid tumor engineering cell sequence which produces human monoclonal antibody for anti-hepatitis B virus, with stable and high product, rate. Said monoclonal antibody is proceeded with purification and cross-linkage with interferon to produce target prepn. of anti-hepatitis B virus human monoclonal antibody crosslinking with itnerferon, and passive immune prepn. for hepatitis B, and testing agent for hepatitis B.
Owner:SHAANXI JIUZHOU BIOTECH
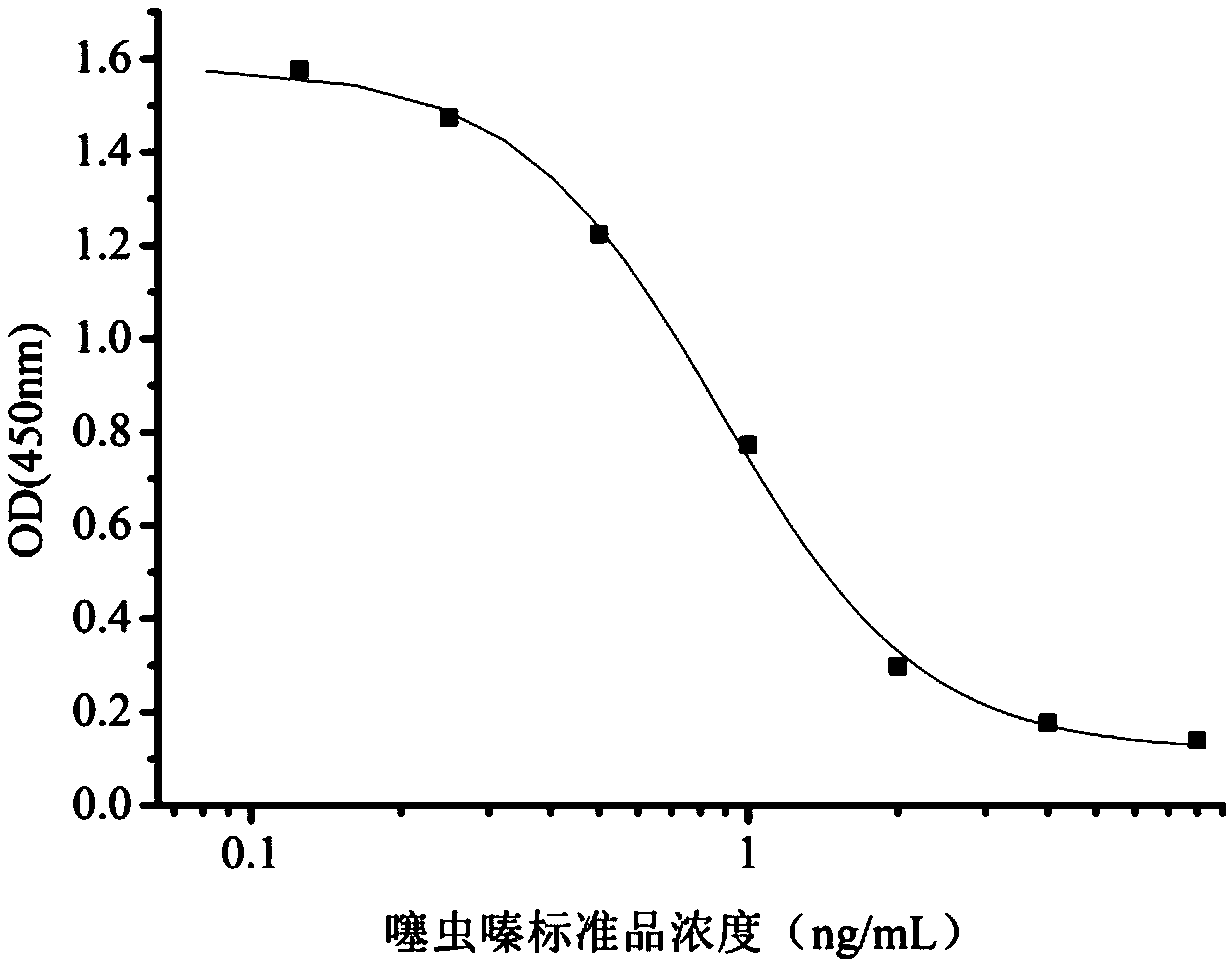
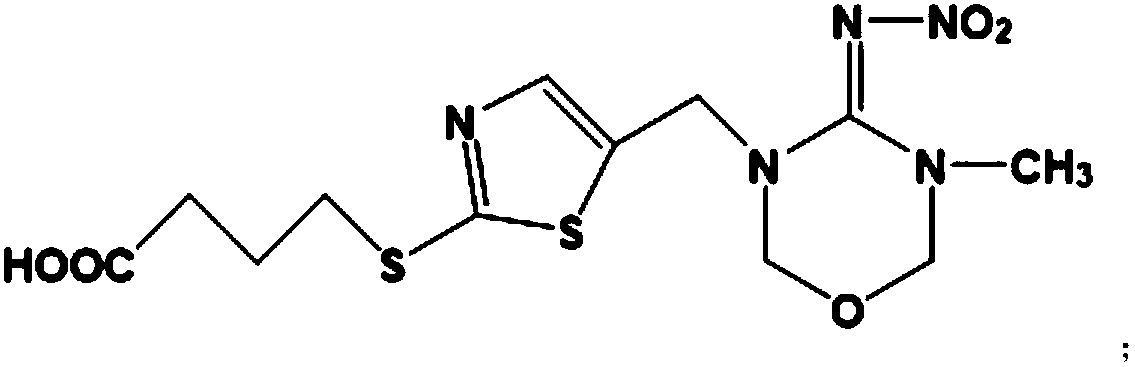
Hybridoma cell strain secreting thiamethoxam monoclonal antibody and application thereof
InactiveCN108998422AHigh detection sensitivityImprove featuresMicroorganism based processesDepsipeptidesBALB/cIc50 values
The invention relates to a hybridoma cell strain secreting thiamethoxacin monoclonal antibody and application thereof, belonging to the field of food safety immunodetection. The accession number of the hybridoma cell strain is CGMCC No. 14699. According to the invention, a complete Freund's adjuvant is used for primary immunization of a BALB / c mouse, then an incomplete Freund's adjuvant is used for booster immunization three times, and a thiamethoxam complete antigen containing no adjuvant is used for impact immunization once, so the BALB / c mouse is immunized; and then the high-titer low-IC50spleen cells of the immunized mouse are fused with mouse myeloma cells by using a PEG method, and then the cell strain is obtained through indirect competitive ELISA screening and subcloning three times. The monoclonal antibody secreted by the cell strain has good specificity and detection sensitivity (with an IC50 value of 0.81 ng / mL) to thiamethoxam and can be used for detection of thiamethoxamresidues in food.
Owner:JIANGNAN UNIV +1
Enrofloxacin monoclonal antibody and application
InactiveCN101565690AStrong specificityRapid and Sensitive DetectionTissue cultureImmunoglobulinsBALB/cIon exchange
The invention relates to an enrofloxacin monoclonal antibody and application, relates to hybridoma strains thereof, and belongs to the technical field of immunochemistry. The enrofloxacin monoclonal antibody is generated by mouse hybridoma strains 6A4 and 8E6. The preparation method comprises the following steps that: enrofloxacin and carrier proteins BSA, HAS and OVA are coupled by a carbodiimide method to synthesize artificial immunogens EnR-BSA, EnR-HSA and coatingen EnR-OVA; a Balb / c mouse is immunized by the synthesized artificial immunogens EnR-BSA and EnR-HSA; a spleen cell of the immunized mouse is extracted to be fused with a SP2 / O myeloma cell and coated by the coatingen EnR-OVA; indirect ELISA method and indirect competition ELISA method are established to screen the hybridoma strains which can stably secrete specific antibody; the obtained cell strain immunized Balb / c mouse is used to prepare ascites; the ascites is purified by a caprylic acid-ammonium method and an ion exchange method; and valences of antibodies of two purified cell strains reach over 1.024*10 and 1.28*10. The monoclonal antibody has strong specificity, can be applied to preparation of enrofloxacin residue inspection kit and aerosol test strip, and can sensibly and quickly inspect the enrofloxacin residue.
Owner:泰州市蛋白质工程研究院
Obtaining method of stem cell conditioned medium, and application of stem cell conditioned medium in skin ageing resistance
Owner:河南中科干细胞基因工程有限公司
Serine protease specific monoclonal antibodies and their use
InactiveUS20010016331A1High sensitivityImprove specificityAnimal cellsHydrolasesSerine proteaseMyeloma cell
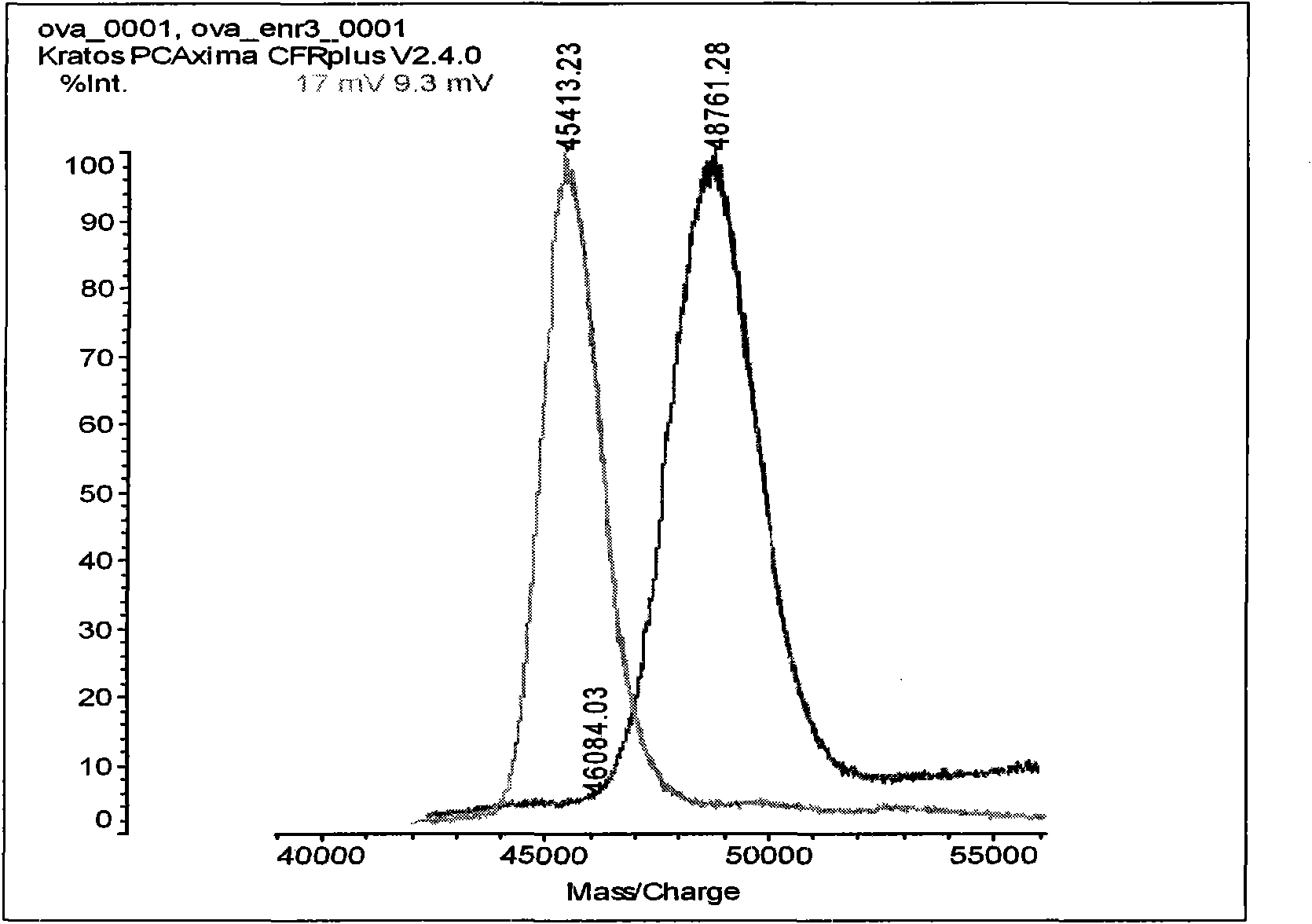
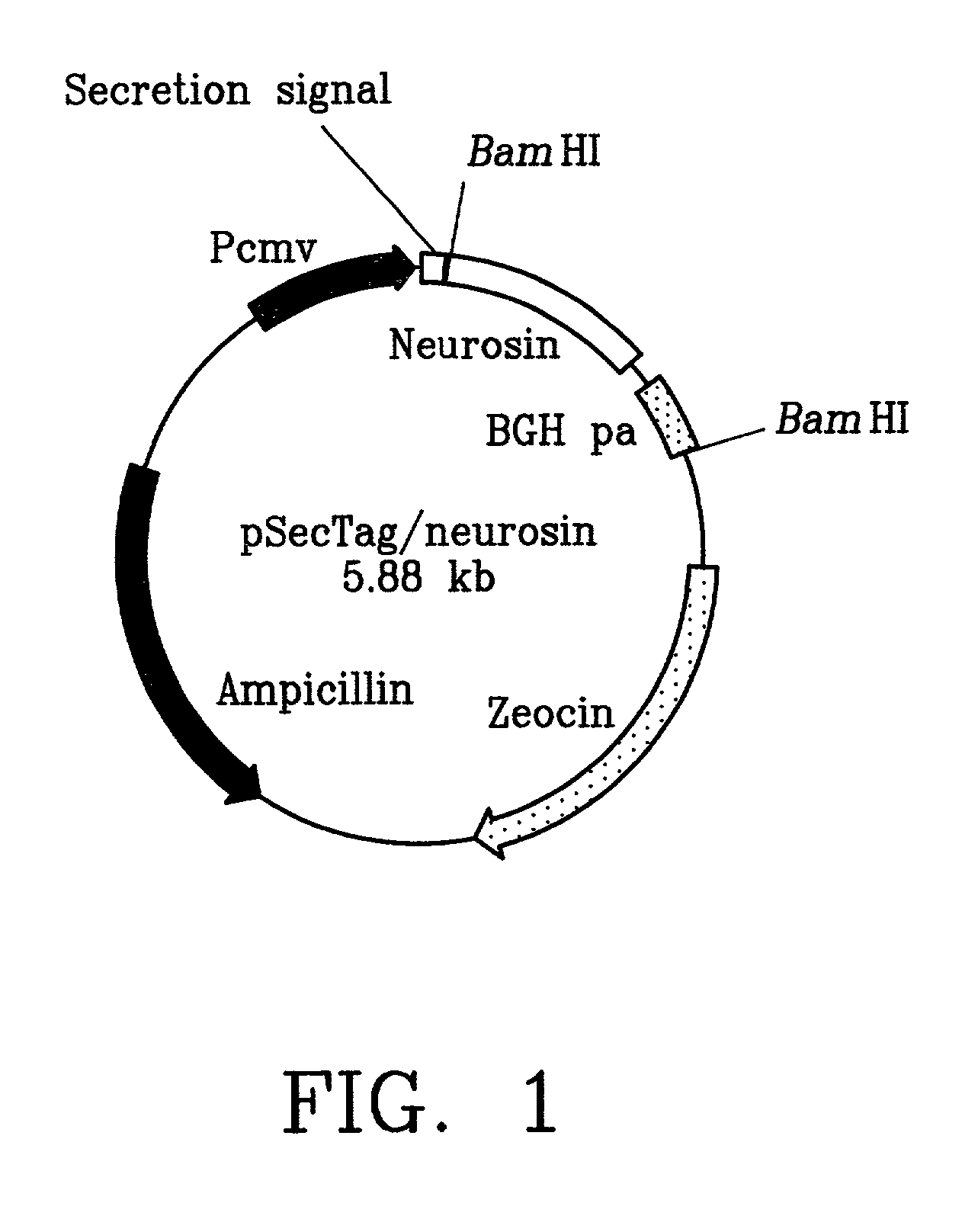
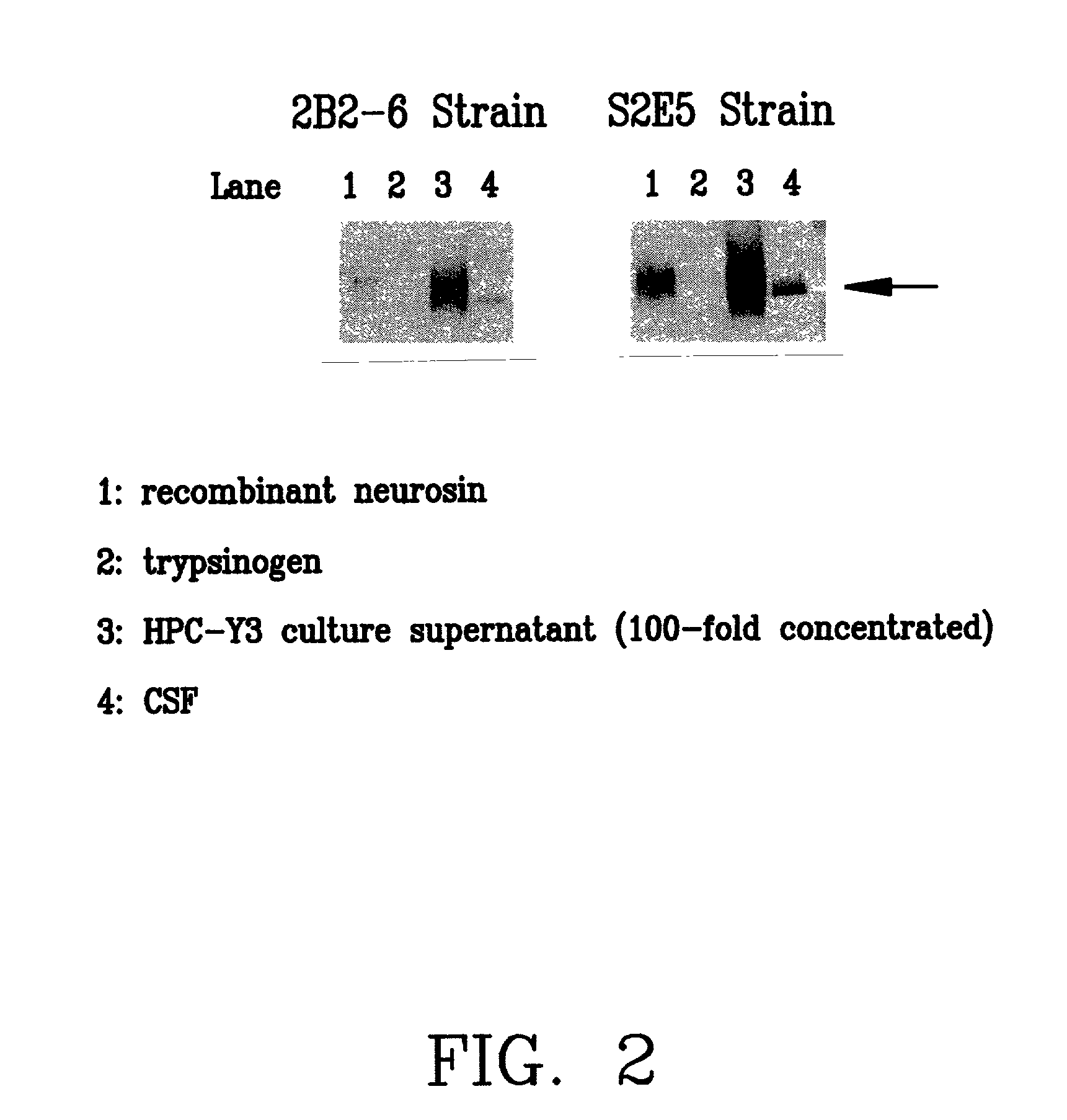
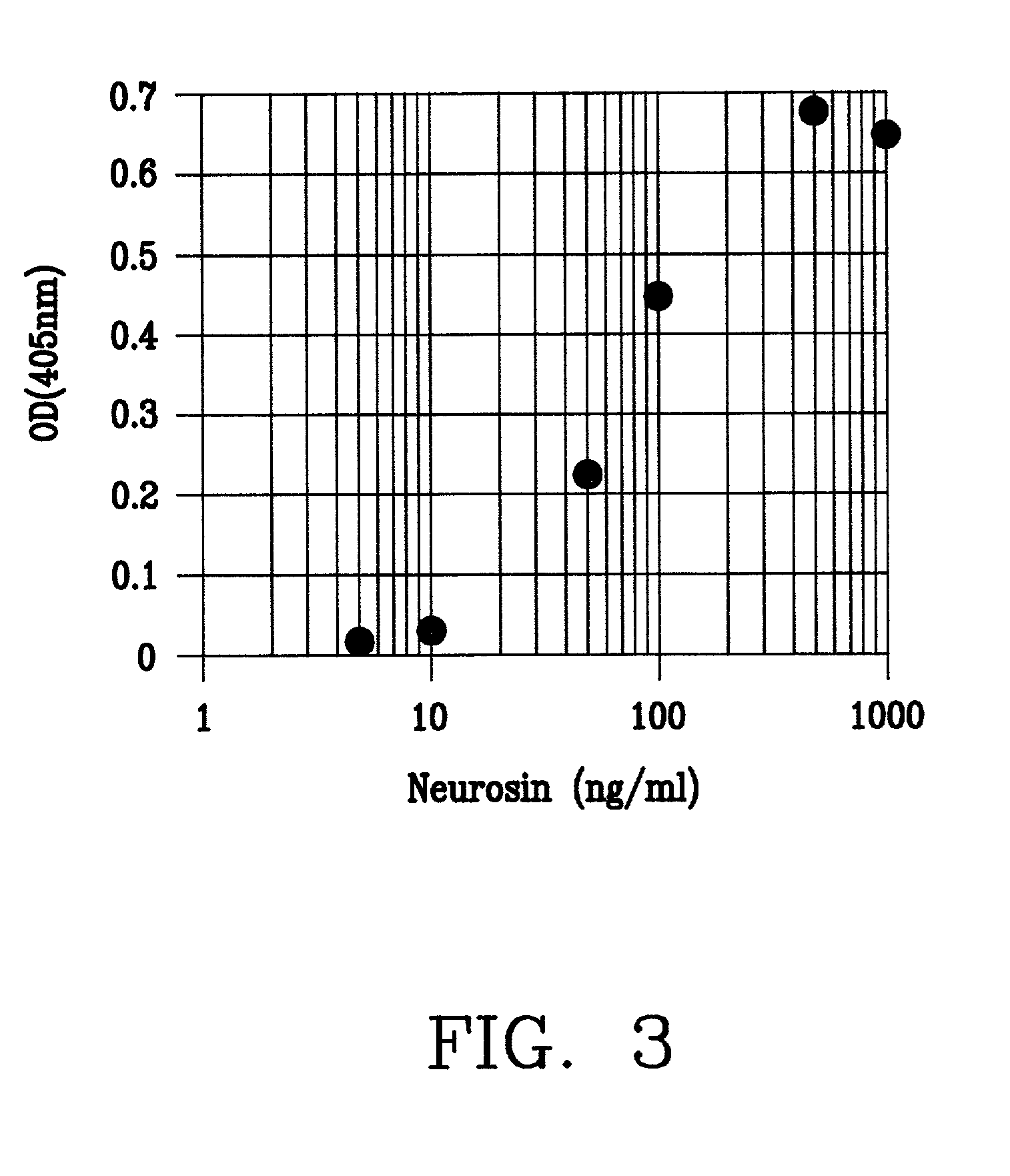
A monoclonal antibody binding selectively to neurosin obtained from hybridomas, in particular, strain 2B2-6 and strain S2E5 showing stable proliferation ability. These hydridomas are obtained by fusing mouse spleen cells having a high antibody titer against neurosin with mouse-derived myeloma cells, screening fused cells being highly reactive with neurosin, and thus producing an antibody binding specifically to neurosin. By using this antibody, various diseases in which neurosin participates can be diagnosed.
Owner:FUSO PHARMA INDS
Tissue culturing method for sugarcane seeds
InactiveCN102273409APromote growthDrought and flood toleranceHorticulture methodsPlant tissue cultureDiseaseImmunocompetence
The invention discloses a tissue culturing method for sugarcane seeds. The seed of the general sugarcane is directly the tail in the sugarcane or just the whole sugarcane. If the seeds are kept for a plurality of years, various germs and viruses intrude into the body to cause degeneration of the breed and continuous reduction of the yield. The general method for selecting high-quality seed of the sugarcane is to select variation strains for high-quality seed breeding, so the yield is not increased so greatly. The existing hybrid sugarcane is obtained by hybridizing different well-bred sugarcanes, so the hybrid sugarcane breed is generally hard to embody excellent quality. The tissue culturing method for sugarcane seeds uses cell tissues of bamboo shoots, stevia rebaudiana and sugarcane for cell fusion hybridization, so that the hybrid sugarcane seed prepared by the method disclosed by the invention is characterized by growing strongly, resisting drought, flood, falling, bad environment, diseases and insect pests, and having high immunocompetence. The hybrid sugarcane grows quickly like bamboo shoots and also has the character that the sweet taste of the stevia rebaudianum is better than the sweet taste of the sugarcane, so that the hybrid sugarcane seed prepared by the method disclosed by the invention uniquely has the character of excellent hybrid quality.
Owner:蔡明
Method for manufacturing antigen-specific antibody-producing hybridomas employing a single antigen-specific B lymphocyte and method for manufacturing monoclonal antibody
A method for producing a monoclonal antibody and antigen-specific antibody-producing hybridomas using a single antigen-specific B lymphocyte is provided. The method for producing antigen-specific antibody-producing hybridomas comprises selecting a single B lymphocyte reacting specifically with a certain antigen, an “antigen-specific B lymphocyte”, culturing the antigen-specific B lymphocyte selected, and fusing the cultured antigen-specific B lymphocyte with myeloma cells to obtain the hybridomas. The monoclonal antibody is produced with thus obtained hybridomas.
Owner:TOYAMA NEW IND ORG +4
Fusion partner for production of monoclonal rabbit antibodies
The invention provides a rabbit-derived immortal B-lymphocyte capable of fusion with a rabbit splenocyte to produce a hybrid cell that produces an antibody. The immortal B-lymphocyte does not detectably express endogenous immunoglobulin heavy chain and may contain, in certain embodiments, an altered immunoglobulin heavy chain-encoding gene. A hybridoma resulting from fusion between the subject immortal B-lymphocyte and a rabbit antibody-producing cell is provided, as is a method of using that hybridoma to produce an antibody. The subject invention finds use in a variety of different diagnostic, therapeutic and research applications.
Owner:EPITOMICS INC
Anti-aflatoxin general type monoclonal antibody hybridoma cell line and application thereof
ActiveCN104004717AHigh detection sensitivityHigh affinityMicroorganism based processesTissue cultureBALB/cPolyethylene glycol
Owner:无锡迪腾敏生物科技有限公司 +1
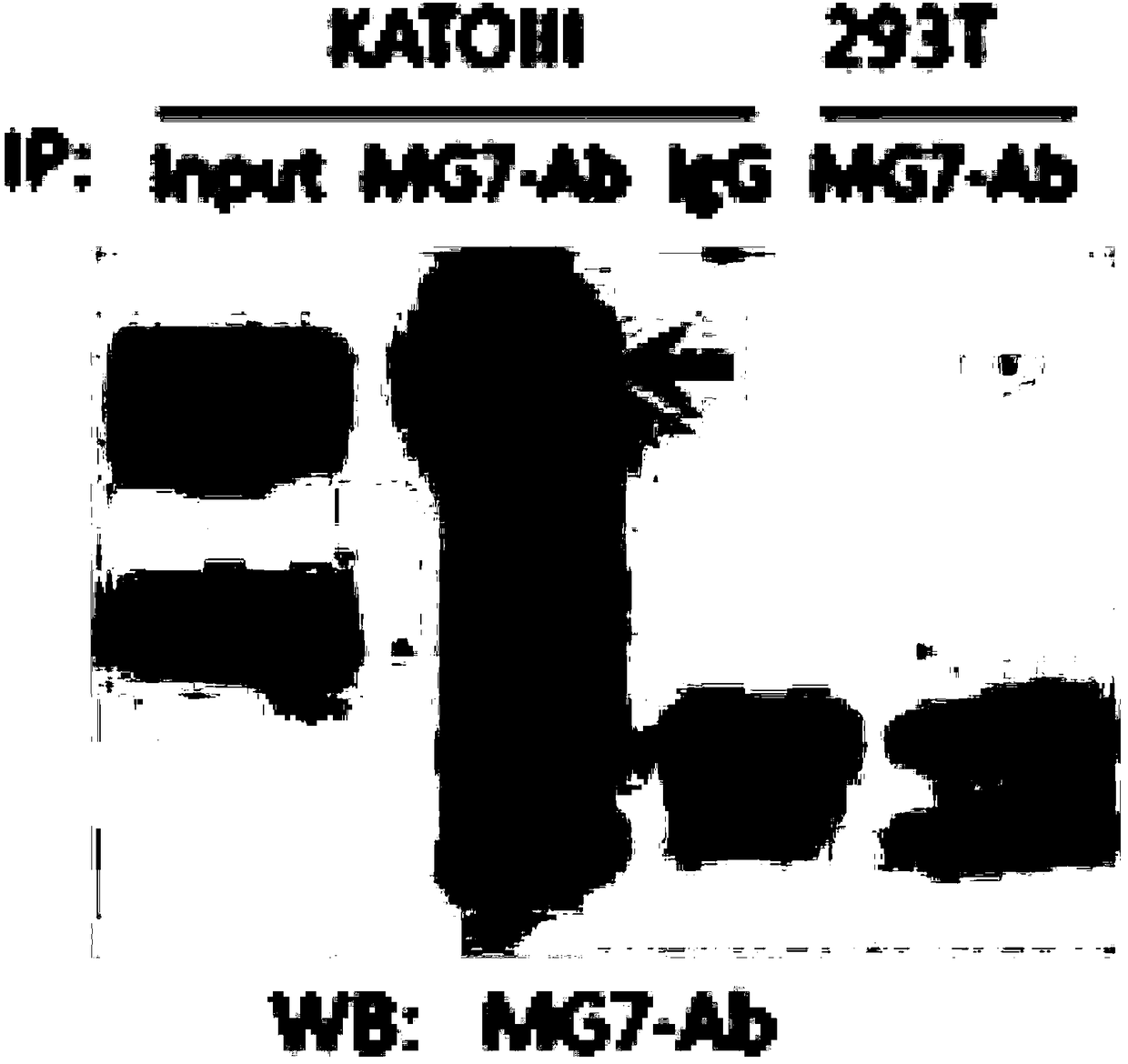
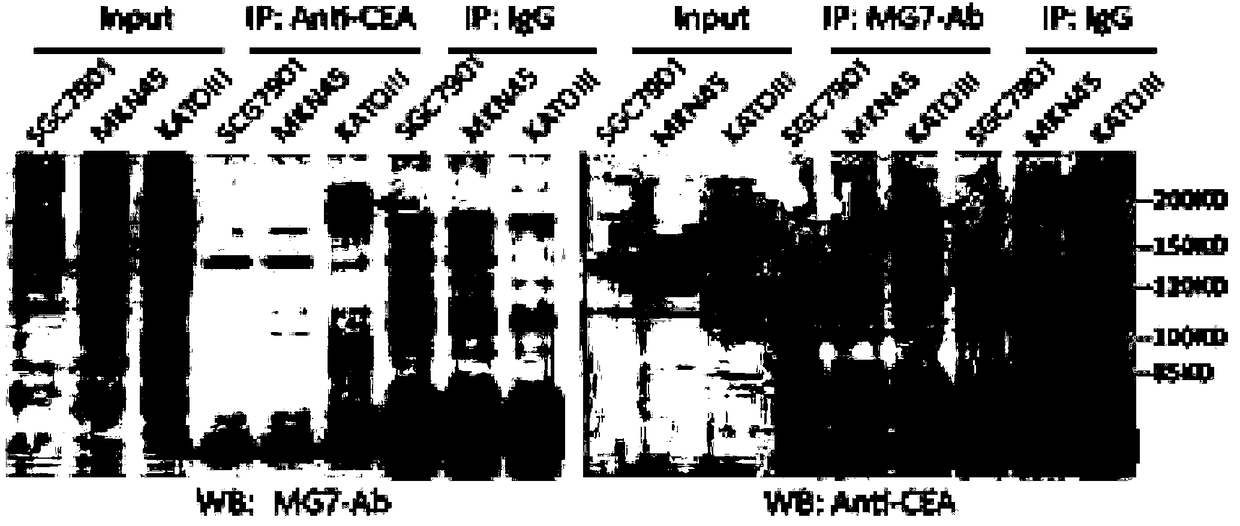
Anti-human CEACAM5 monoclonal antibody and preparation method and application thereof
ActiveCN108341876AHigh affinityImprove featuresMicroorganism based processesImmunoglobulins against cell receptors/antigens/surface-determinantsStainingImmunoprecipitation
The invention discloses an anti-human CEACAM5 monoclonal antibody and a preparation method and application thereof, and the anti-human CEACAM5 monoclonal antibody is produced by a mouse hybridoma cellline with the accession number CCTCC NO: C2016129. The preparation method comprises the following steps: S1, immunizing a mouse with whole cell protein, lysed by human gastric cancer cells, as an immunogen; S2, fusing spleen cells of the immunized mouse obtained in the step S1 with mouse myeloma cells; S3, selecting a hybridoma cell line stably secreting antibodies after cell cloning occurs during the cell fusing in the step 2; and S4, preparing the anti-human CEACAM5 monoclonal antibody from the hybridoma cell line stably secreting the antibodies obtained in the step 3. The monoclonal antibody can be used to prepare a composition for treating a malignant tumor. The anti-human CEACAM5 monoclonal antibody can be widely used in various routine operations such as flow cytometry, immunohistochemical staining and immunoprecipitation.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
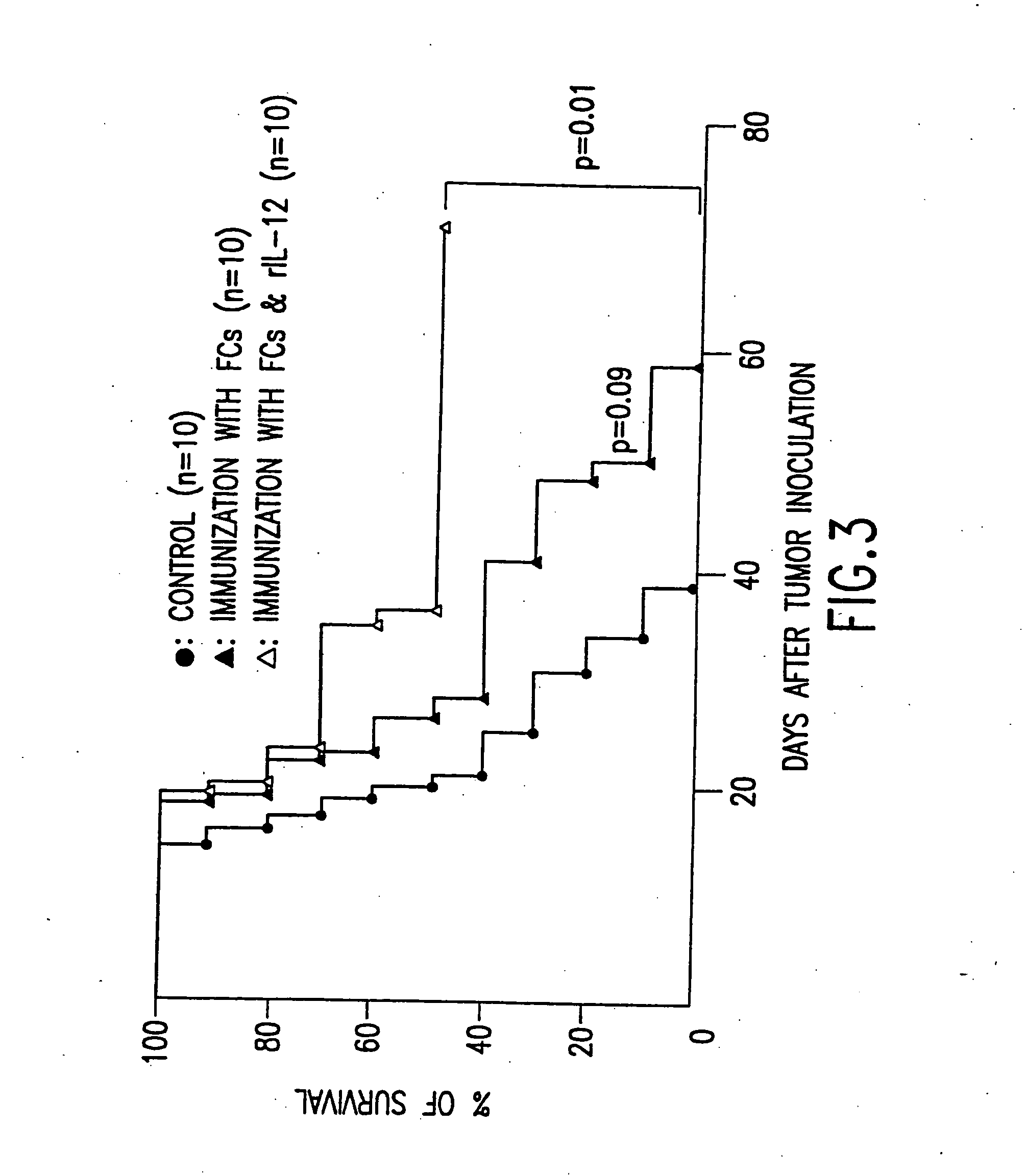
Combined immunotherapy of fusion cells and interleukin-12 for treatment of cancer
InactiveUS20050180951A1Increase stimulationBiocidePeptide/protein ingredientsWhite blood cellCancer research
The present invention relates to methods and treatment protocols for the immunotherapy of cancer by administering a therapeutically effective dose of fusion cells formed by fusion of autologous dendritic cells and autologous non-dendritic cells in combination with interleukin-12.
Owner:OHNO
Non-cytotoxic protein conjugates
ActiveUS20120207735A1Polypeptide with localisation/targeting motifSugar derivativesCytotoxicityProtein translocation
The present invention is directed to non-cytotoxic protein conjugates for inhibition or reduction of exocytic fusion in a nociceptive sensory afferent cell. The protein conjugates comprise: (i) a Targeting Moiety (TM), wherein the TM is an agonist of a receptor present on a nociceptive sensory afferent cell, and wherein the receptor undergoes endocytosis to be incorporated into an endosome within the nociceptive sensory afferent cell; (ii) a non-cytotoxic protease or a fragment thereof, wherein the protease or protease fragment is capable of cleaving a protein of the exocytic fusion to apparatus of the nociceptive sensory afferent cell; and (iii) a Translocation Domain, wherein the Translocation Domain translocates the protease or protease fragment from within the endosome, across the endosomal membrane, and into the cytosol of the nociceptive sensory afferent cell wherein the Targeting Moiety is selected from the group consisting of BAM, β-endorphin, bradykinin, substance P, dynorphin and / or nociceptin.
Owner:ALLERGAN INC
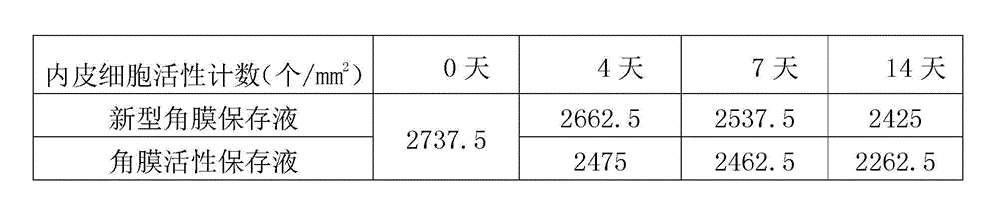
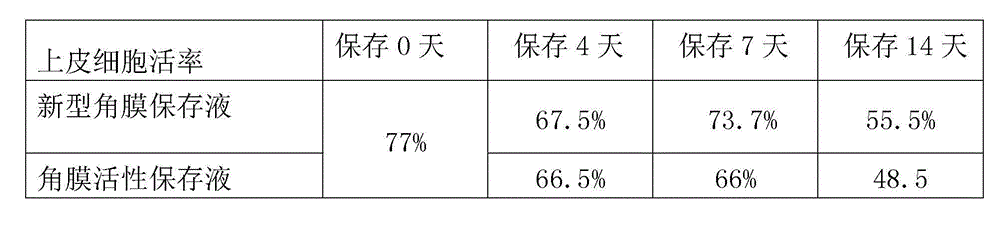
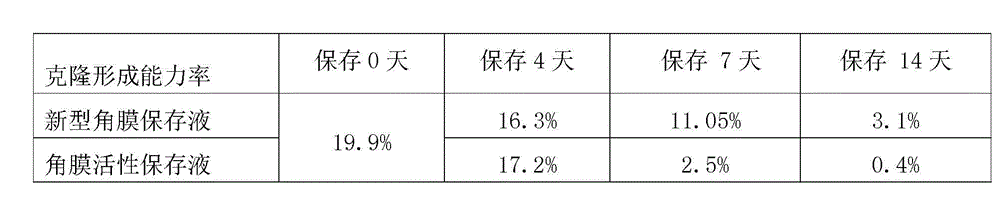
Cornea metaphase preservation solution, and preparing and using methods thereof
The invention provides a cornea metaphase preservation solution, and preparing and using methods thereof. The cornea metaphase preservation solution is a cell culture minimum essential medium (MDM) with chondroitin sulfate, low molecular dextran, L-glutamine, dexamethasone, tobramycin, 2-hydroxyethyl and Y-27632 added. The preservation solution not only can keep activity and normal morphology of cornea endothelial cells, but also can enhance viability of corneal limbus epithelial cells and improve clone ability of corneal limbus stem cells. And especially, a medium to long term preservation effect is obvious, phenomena of deformation, conjugation and the like of endothelial cells of a control group cornea do not appear in long term preservation, endothelial morphology of the control group cornea is consistent with endothelial morphology of a cornea preserved for 4 days, and the endothelial cells of the control group cornea are still regular and few in cell conjugation phenomena. The preservation solution can effectively prevent cell apoptosis phenomena during the process of preserving isolated cornea materials, increases activity and clone forming ability of the corneal limbus stem cells, and enables the cornea to maintain a transparent feature in a long preservation time.
Owner:SHANDONG EYE INST
Antibody for resisting human CD79a extracellular terminal protein, coding gene and application
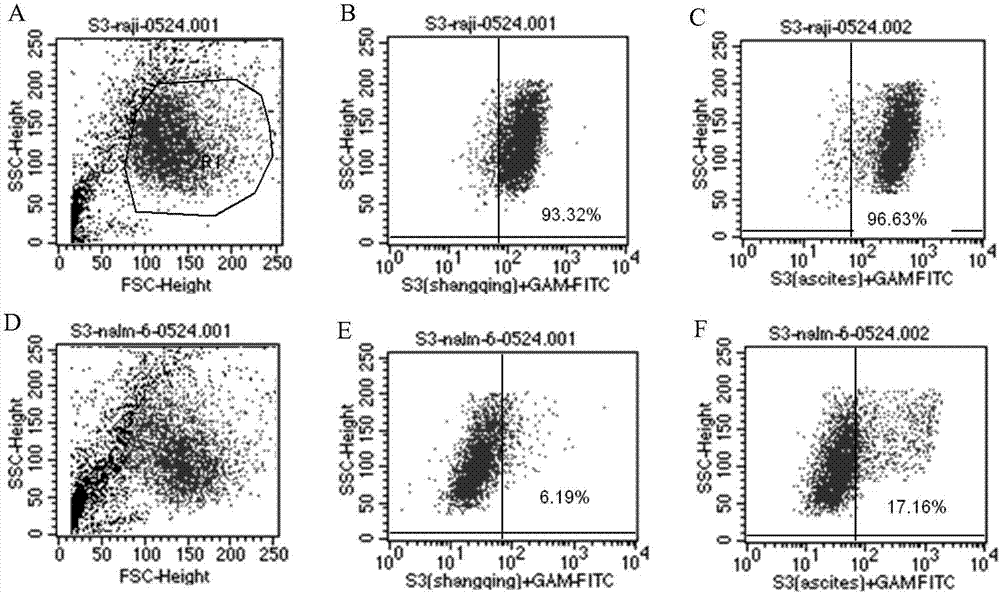
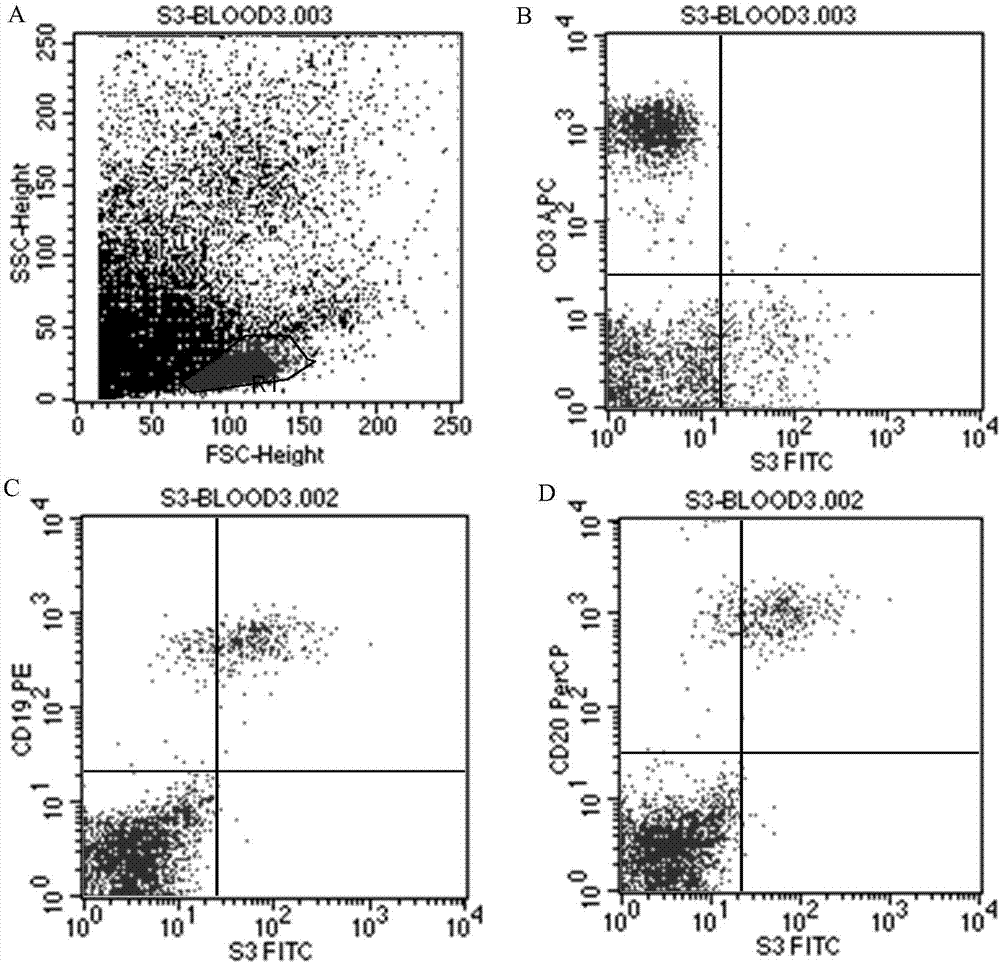
ActiveCN107488230AEfficient identificationHigh internalization rateOrganic active ingredientsTetrapeptide ingredientsDiseaseSequence analysis
The invention discloses an antibody for resisting human CD79a extracellular terminal protein, a coding gene and application. The antibody is characterized in that a human B cell lymphoma Raji cell line is used as an immunogen for immunizing mice; splenocyte is obtained and then is fused with a mouse myeloma cell to obtain a hybridoma cell; a monoclonal antibody for specifically aiming at the human CD79a extracellular terminal protein is obtained via screening; an amino acid sequence of the monoclonal antibody is obtained by sequence analysis; variable region sequence with a heavy chain and a light chain and CDR (Complementarity-Determining Region) sequences with light chains and heavy chains are obtained via analyzing; an chimeric antibody expressed via cloning can be used for effectively and specifically recognizing the human CD79a extracellular terminal protein; in addition, when the antibody acts on human B lymphocyte, the internalization rate is high; the antibody has a good prospect for preparing a targeted drug of targeted B lymphocyte and can be used for treating diseases associated with the B lymphocyte.
Owner:ZHEJIANG UNIV
Monoclonal antibody of ractopamine and preparation method and application thereof
ActiveCN101921730ASensitive detectionHigh sensitivityMicroorganism based processesTissue cultureBALB/cOctanoic Acids
The invention relates to a monoclonal antibody of ractopamine and a preparation method and application thereof, which belongs to the field of immunochemical technology. In the invention, the ractopamine and carrier proteins BSA, HSA and OVA are coupled by a mixed anhydride method to synthesize artificial immunogens RAC-BSA and RAC-HSA and coating antigen RAC-OVA; a Balb / c mouse is immunized by the synthesized artificial immunogens RAC-BSA and RAC-HSA; the spleen cell of the immune mouse is fused with the SP2 / 0 myeloma cell and the two are coated by the coating antigen RAC-OVA; AND an indirect ELISA method and an indirect competition ELISA method are established to screen out a hybridoma cell strain (5D8) capable of stably excreting specific antibody. The Balb / c mouse is immunized by the obtained cell strain to prepare ascites which is purified by an octanoic acid-ammonium sulfate method and an ion exchange method, and the titer of the purified antibody is more than 5.12*10<5>. The monoclonal antibody has strong specificity, is used for preparing a ractopamine residue detection kit and colloidal gold test paper strips and can sensitively and quickly detect the ractopamine residue.
Owner:JIANGSU HUACHUANG MEDICINE RES & DEV PLATFORM MANAGEMENT CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com