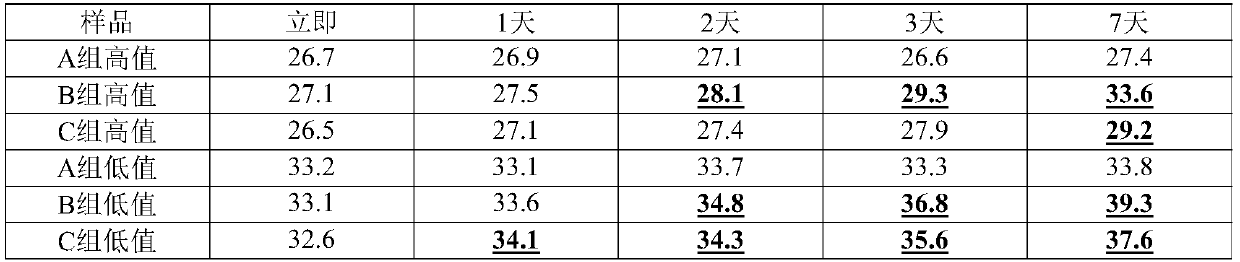
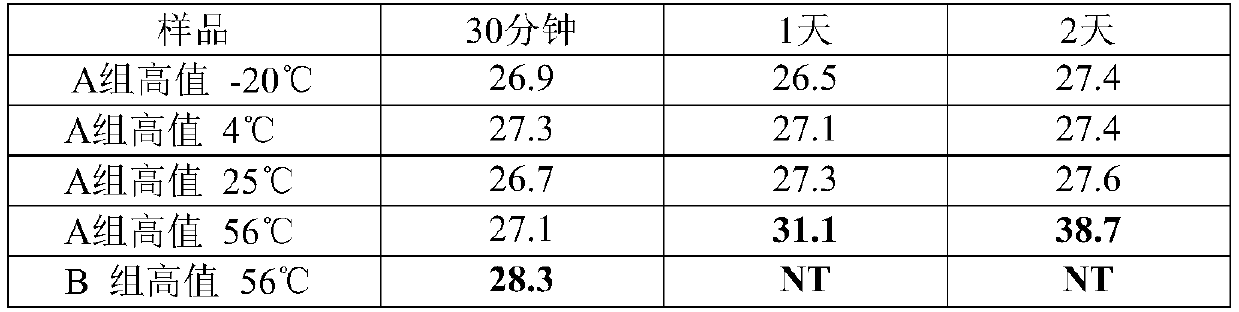
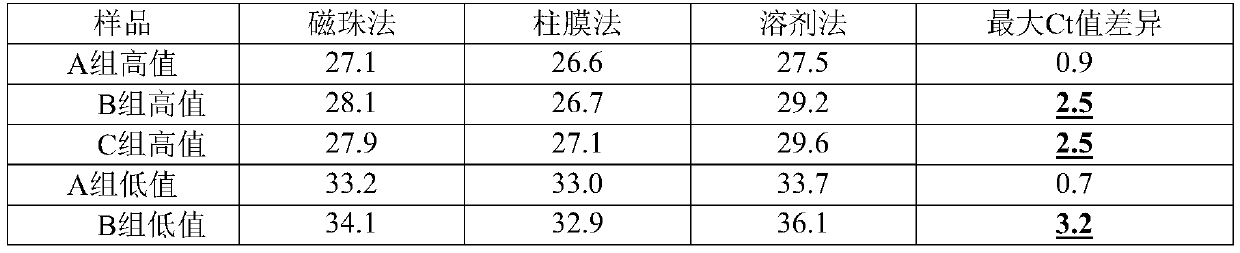
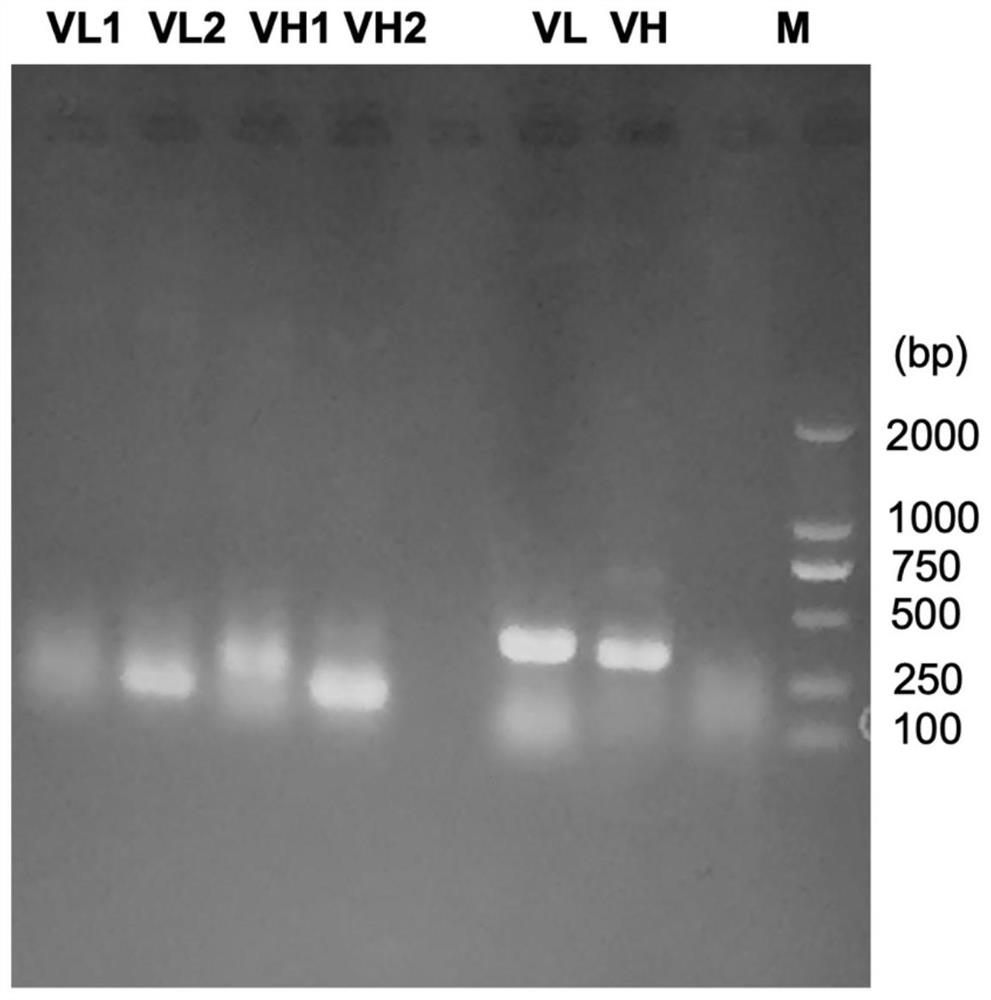
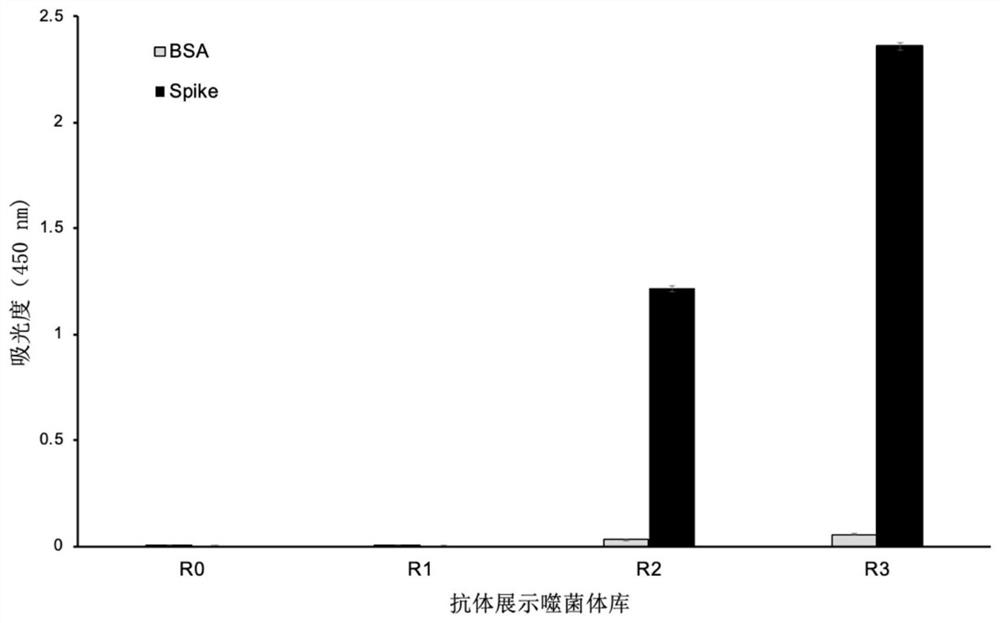
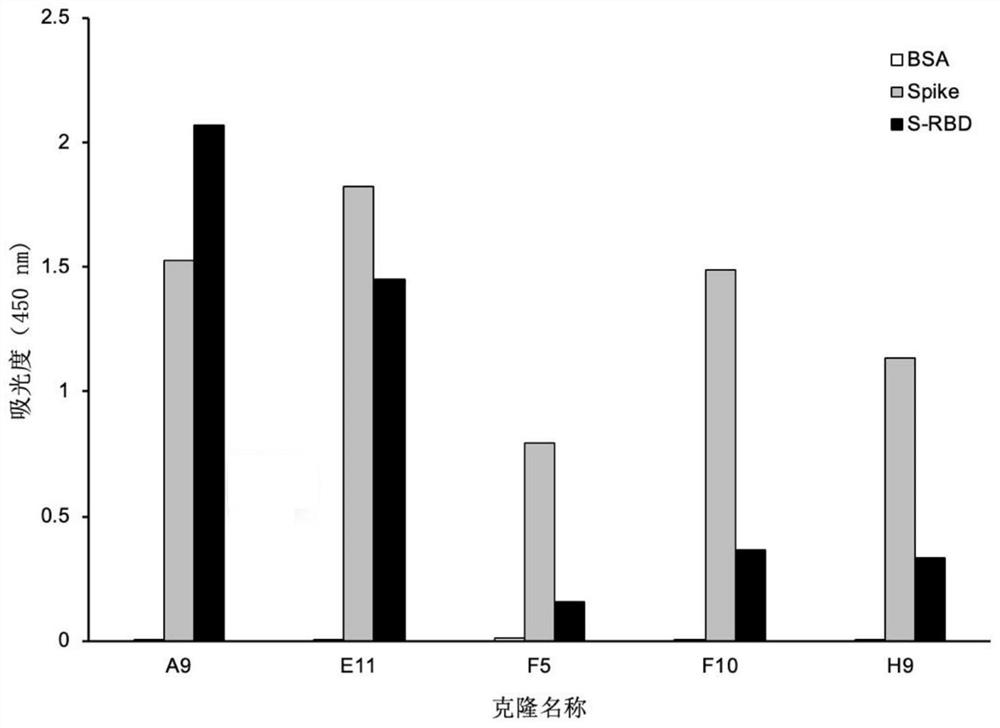
Patents
Literature
711 results about "Viral test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral Tests. Test Overview. A viral test is done to find infection-causing viruses. Viruses grow only in living cells. Viruses cause disease by destroying or damaging the cells they infect, damaging the body's immune system, changing the genetic material (DNA) of the cells they infect, or causing inflammation that can damage an organ.
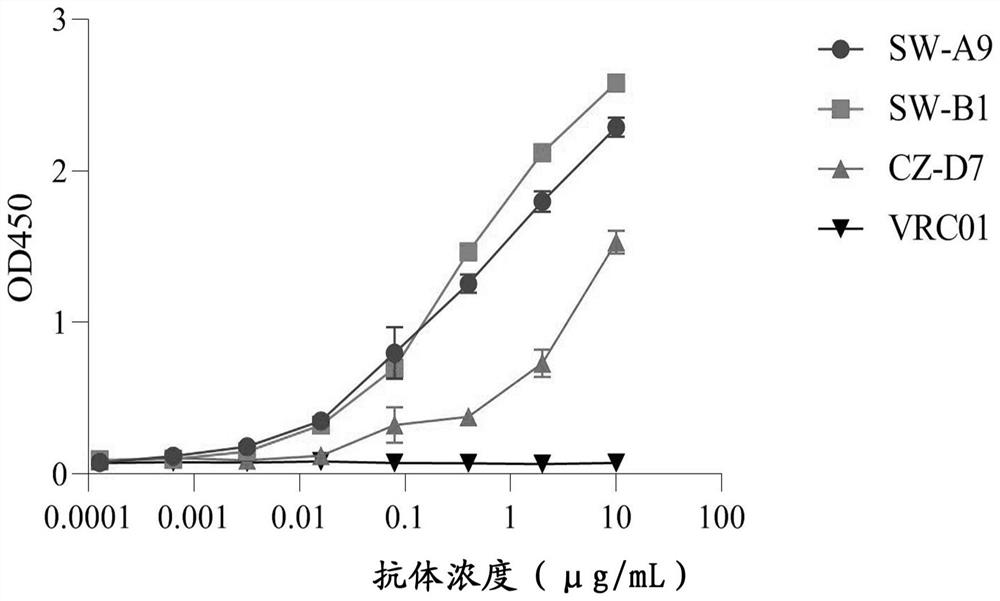
Human SARS-CoV-2 monoclonal antibody and preparation method and application thereof
PendingCN111153991AHigh affinityStrong specificitySsRNA viruses positive-senseVirus peptidesBALB/cMonoclonal antibody agent
The invention discloses a human SARS-CoV-2 monoclonal antibody. The preparation method of the human SARS-CoV-2 monoclonal antibody comprises the steps: adopting SARS-CoV Nucleocapsid recombinant protein as immunogen, immunizing BALB / c mice, performing fusion and subcloning on spleen cells and myeloma cells of mice, then performing a large amount of repeated screening and domestication of cell lines through commercialized products SARS-CoV-2 Nucleocapsid and MERS Nucleocapsid so as to obtain a hybridoma cell line capable of secreting the SARS-CoV-2-resistant N monoclonal antibody with high affinity and high specificity finally and successfully, and finally performing ascites preparation and purification so as to obtain the monoclonal antibody, wherein the amino acid sequence of the SARS-CoVNucleocapsid recombinant protein is shown in SEQ ID No. 1. The invention also discloses application of the monoclonal antibody in preparation of SARS-CoV-2 virus detection products and preparation ofdrugs for inhibiting the SARS-CoV-2 viruses. The monoclonal antibody can be used for detecting the SARS-CoV-2 in human throat swabs / pulmonary secretions and other samples by using a double-antibody sandwich method, and can be applied to diagnosis and prevention and control of SARS-CoV-2 virus infection and scientific researches of viruses and other study.
Owner:BEIJING BIOSYNTHESIS BIOTECH
Method for Detection of Cancer Cells Using Virus
InactiveUS20090311664A1Microbiological testing/measurementMammal material medical ingredientsNon cancerCancer cell
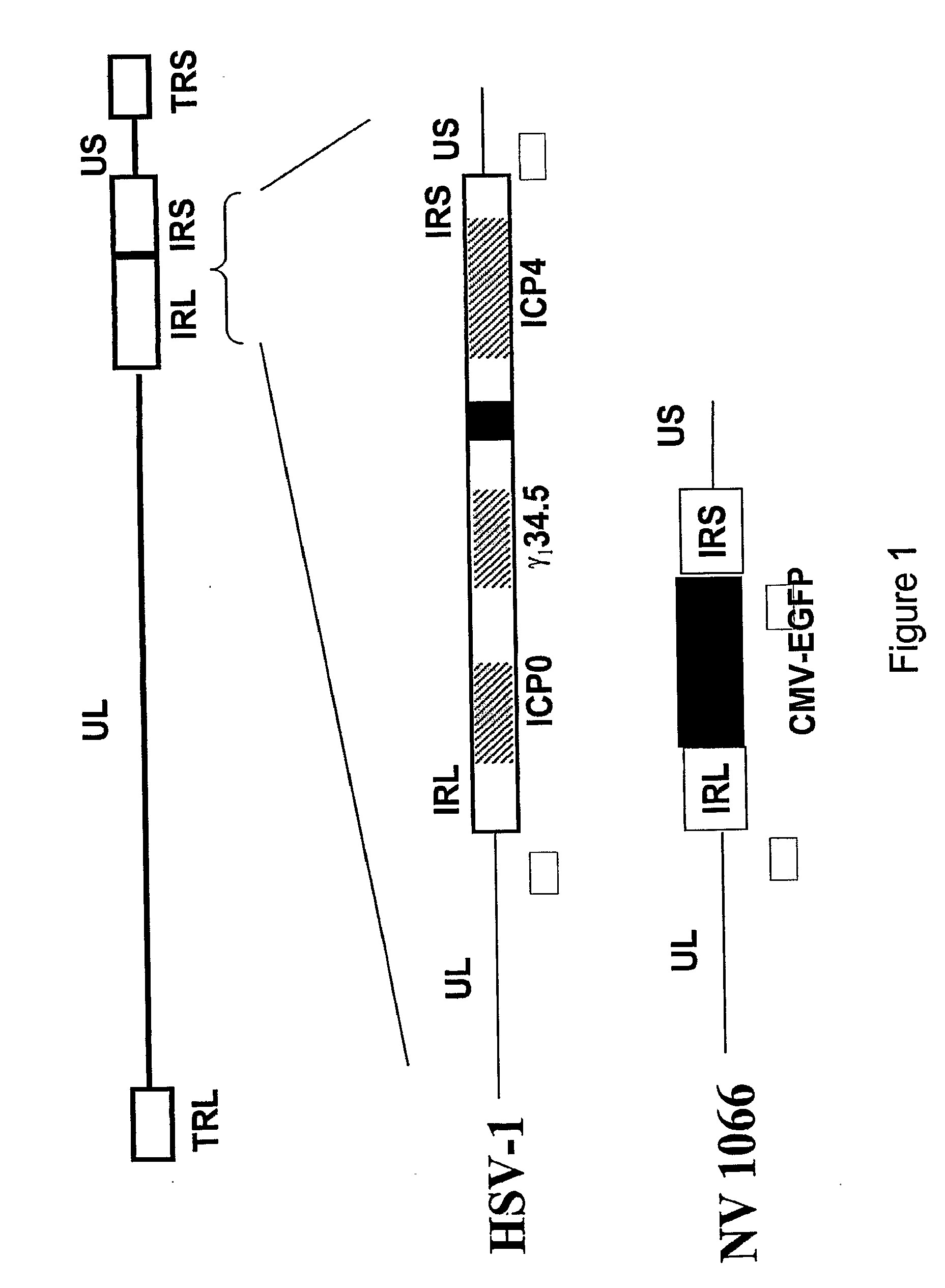
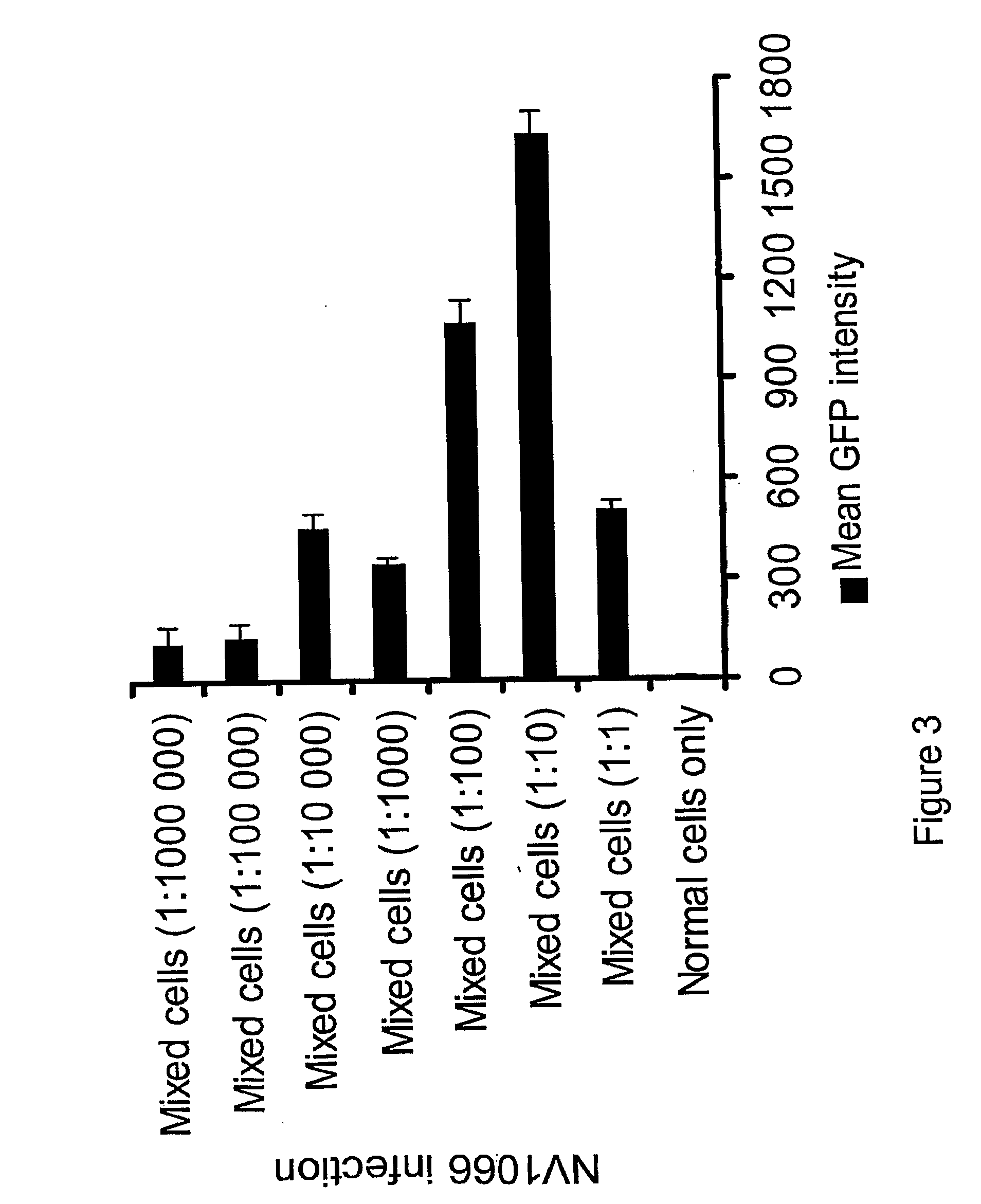
The invention relates to compositions and methods for cancer cell detection in bodily samples wherein a cancer cell can be detected within a mixed population of cancer cells and non-cancer cells. The invention also relates to compositions and methods that may be used in cancer cell detection, specifically viruses that are replication-competent conditional to a cancer cell, in particular an oncolytic herpes virus, such as NV 1066 and a vaccinia virus, such as GLV-1h68. Provided are methods and kits for using these viruses that preferentially replicate in cancer cells and may also preferentially infect cancer cells for specific identification of such cancer cells, even when a cancer cell is present, for example, at a ratio of one infected cancer cell in a background often thousand non-cancer cells, thus further providing a reproducible and sensitive screening method for cancer detection, monitoring and prognosis.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Selective Reaction Monitoring (SRM) Derived Protein Profiles for Cancer and other Pathologic Entities
InactiveUS20130288233A1Microbiological testing/measurementBiological material analysisProtein proteinSpecific protein
The invention relates to a method of detecting and quantifying small peptides derived from proteins from a range of different clinical samples using the Selective Reaction Monitoring (SRM) profiling technique. By targeting these unique peptides which specifically identify particular proteins, the present invention enables multiple samples to be run in a multiplexed fashion in order to identify, diagnose, quantitate and profile a full range of benign and pathologic entities, including but not limited to, the complete range of cancers and the spectrum of inflammatory diseases, including inflammatory cell typing and bone marrow cell typing. The SRM assay is capable of performing clinical blood typing and it can also act as a diagnostic test to identify women at highest risk for cervical cancer base on Human Papillomavirus (HPV) testing.
Owner:MAP IP HLDG
Nanobody against SARS-COV-2 virus S protein RBD structure domain and use thereof
InactiveCN111825762AAvoid infectionStrong specificityImmunoglobulins against virusesAntiviralsViral ReceptorPharmaceutical drug
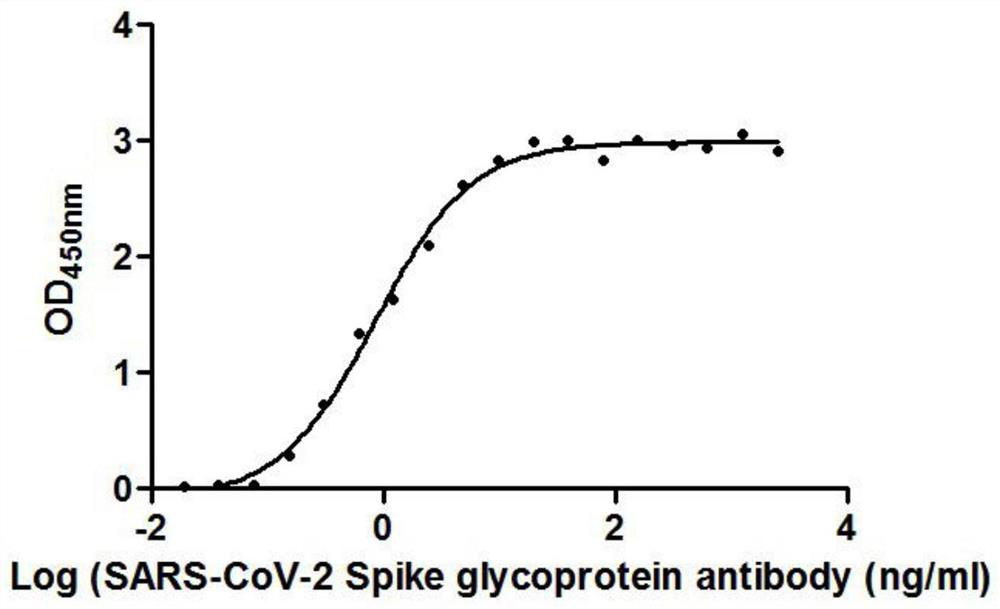
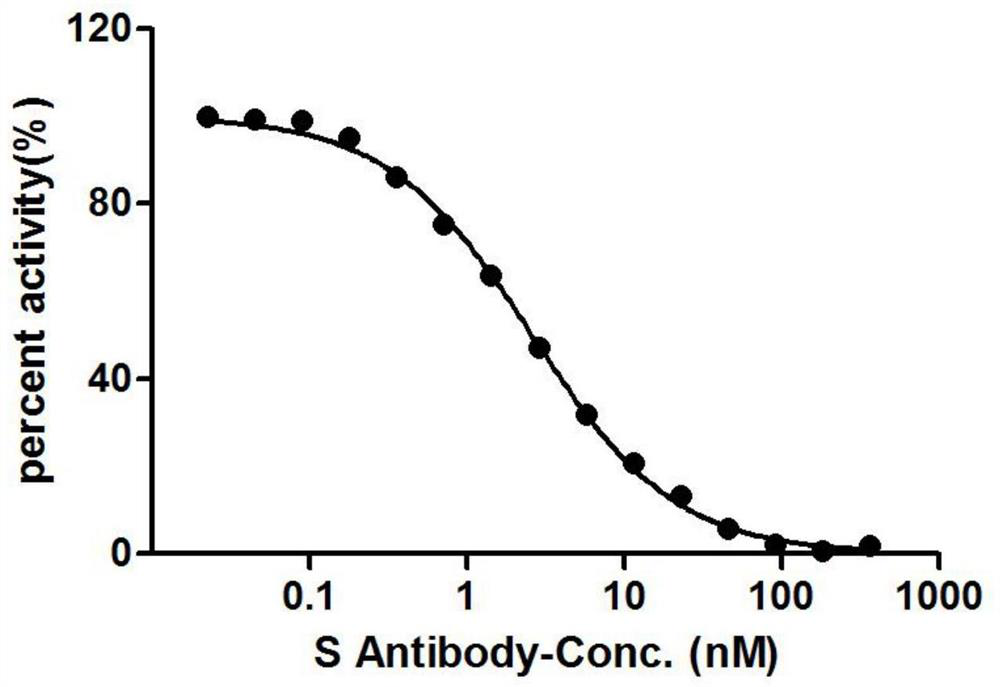
The invention provides a nanobody against a SARS-COV-2 virus S protein RBD structure domain. The nanobody can recognize the RBD structure domain of SARS-COV-2 virus S protein, and includes one or moreof nanobody1-nanobody19. The nanobody can specifically bind to an S protein RBD region of SARS-COV-2 and block the binding of the virus to a cell receptor ACE2, and EC50 of the binding of the antibody to RBD is 0.4212ng / ml-325.24ng / ml. The nanobody competes with viral receptor ACE2 protein, and the IC50 is 2.433nm-100.02nm. The invention further provides use of the nanobody and recombinant antibody thereof in preparing drugs for inhibiting SARS-COV-2 virus infection, and preparing a SARS-COV-2 virus detection reagent or kit.
Owner:CUSABIO TECH LLC
Anti-rabies virus monoclonal antibody and preparation method and application
InactiveCN101560255AExperimental costs are highEasy to operateImmunoglobulins against virusesTissue cultureImmunoblot AnalysisHybridoma cell
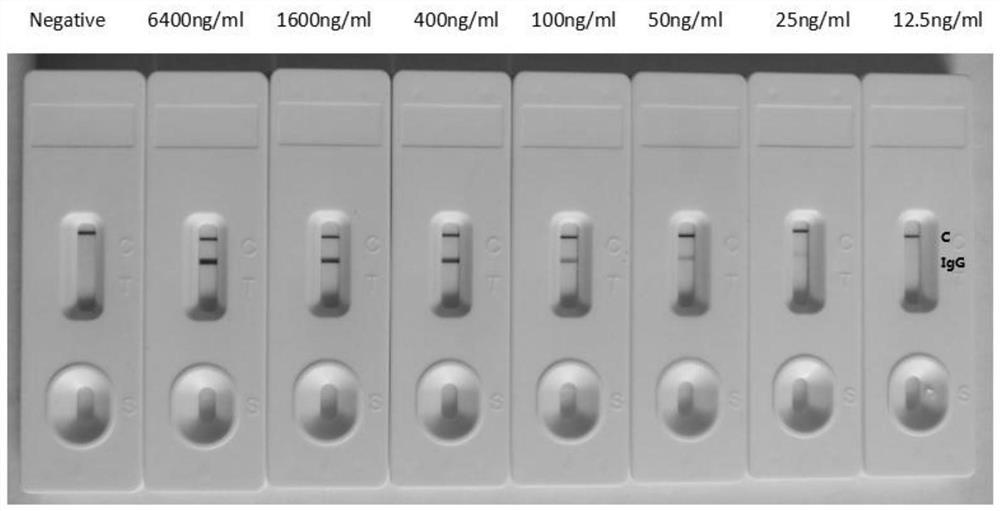
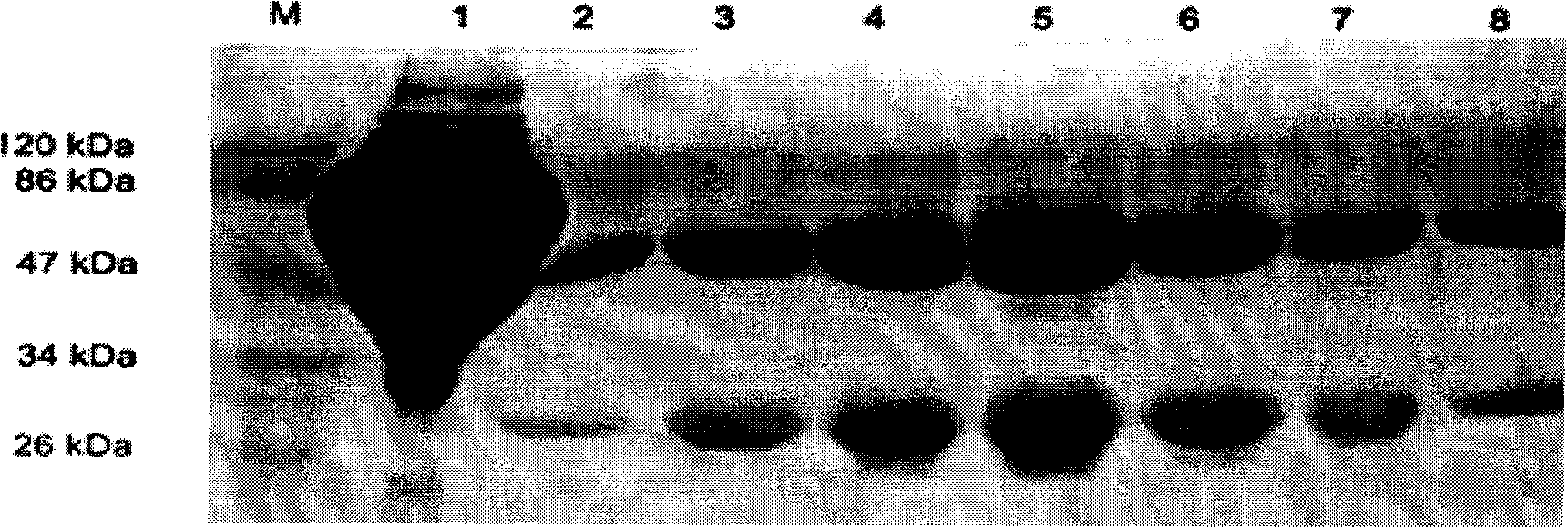
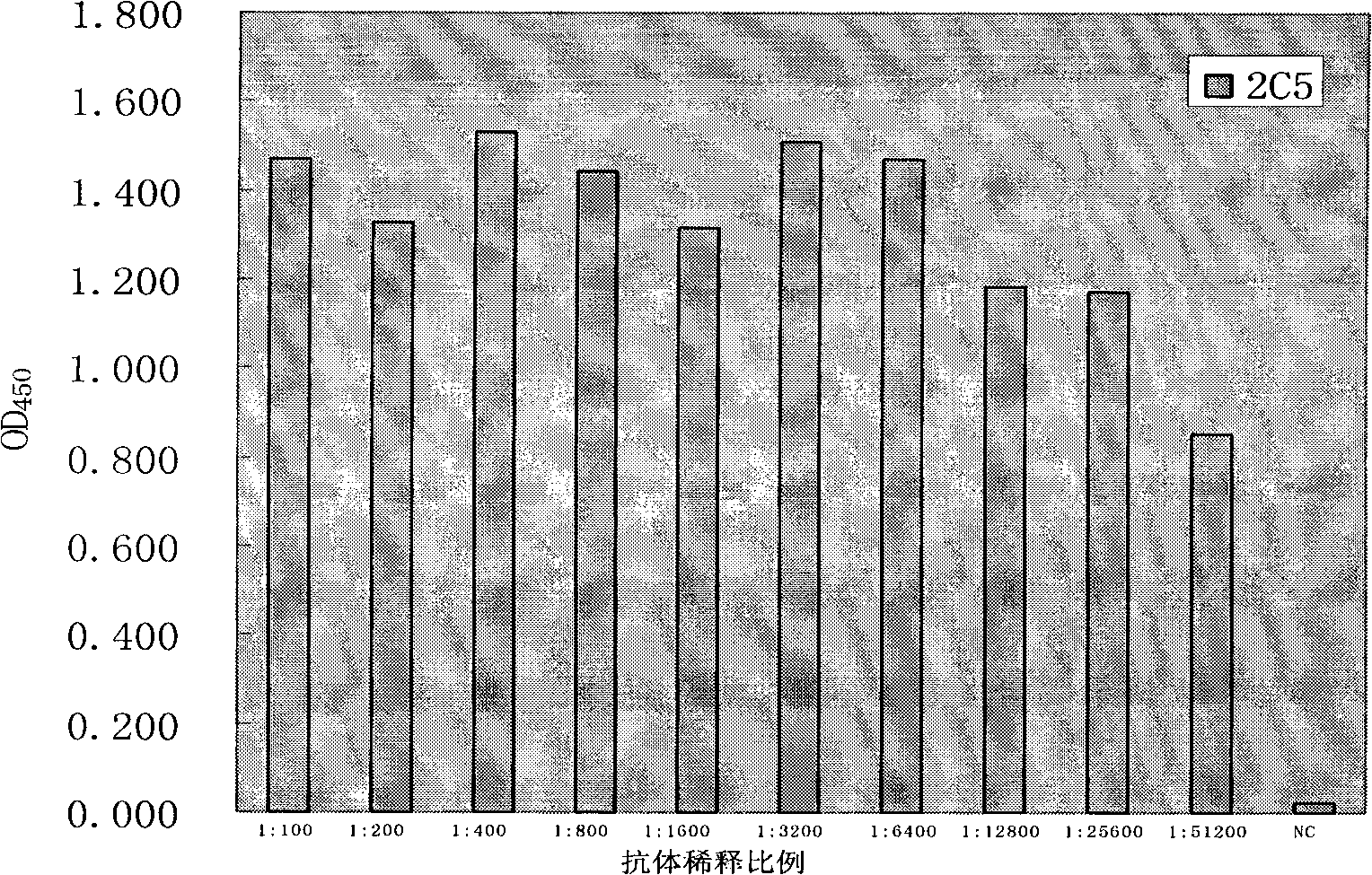
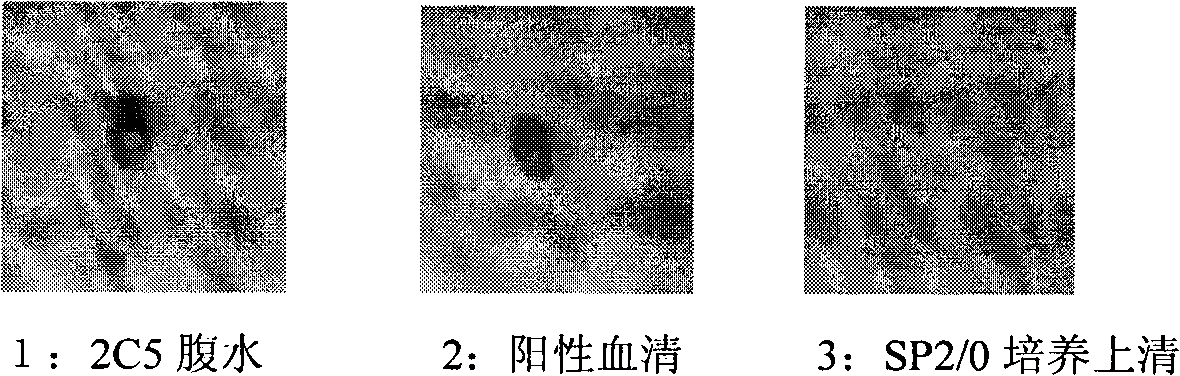
The invention discloses an anti-rabies virus monoclonal antibody and a preparation method and an application, belonging to the field of biomedicine and particularly relating to the preparation of a monoclonal antibody capable of identifying rabies virus and the application. The monoclonal antibody of the invention is screened by indirect Enzyme-linked immunosorbent assay (ELISA), and specificity and affinity thereof combined with antigen are identified by methods such as polyacrylamide gel electrophoresis analysis, speckle ELISA, immunoblot analysis and the like. The anti-rabies virus monoclonal antibody of the invention can be applied in multiple testing methods of antigen of rabies virus and can be also applied in the preparation of rabies virus detecting kit. The anti-rabies virus monoclonal antibody is secreted by anti-rabies virus monoclonal antibody hybridoma cell strain 2C5 with the preservation number being CGMCC No.3014.
Owner:NANJING MEDICAL UNIV +1
Primer system and method for detecting and analyzing avian influenza virus
InactiveCN101058833AQuick checkAccurate detectionMicrobiological testing/measurementDNA preparationFowlHypotype
The invention provides a method for detecting fowl influenza virus and parting primer system and augmenting fowl influenza virus RNA asymmetrically. The invention designs and screens 25 pairs multiple asymmetrical RT-PCR primers, a pair general primer, 52 specific probes and 3 quality control probes according to the report fowl influenza virus total hypotype genome sequence in Genebank, wherein every pair in 25 pairs multiple asymmetrical RT-PCR primers is fit for a hypotype of fowl influenza virus, the primer system comprising 25 pairs multiple asymmetrical RT-PCR primers and a pair general primer can be used for detecting and parting fowl influenza virus, the primer system builds the method of asymmetrically augmenting fowl influenza virus RNA and detecting and parting fowl influenza virus. The invention provides strong specificity, high sensibility, which also proceeds the first step application.
Owner:中国检验检疫科学研究院动植物检疫研究所
RNA virus inactivation preservation solution and application thereof
ActiveCN111363729AAchieve immediate lysis and inactivationHigh sensitivityMicrobiological testing/measurementInactivation/attenuationLithium chlorideActive agent
The present invention discloses an RNA virus inactivation preservation solution. The inactivation preservation solution comprises ammonium salt, 1-ethyl-3-methylimidazolium bisulfate, lithium chloride, ethylenediaminetetraacetic acid, an RNase inhibitor, a surfactant, a reducing agent, a buffer agent and water. The present invention also provides an RNA virus sample inactivation and preservation method and an RNA virus detection method, and the method is conducted by using the inactivation preservation solution. The preservation solution has low cost, inhibits RNase activity, preserves RNA integrity, realizes preservation and transportation of samples at room temperature, can also quickly inactivate pathogens at room temperature, reduces infection risks of medical personnel, and reduces false negatives in detection. At the same time, the inactivation preservation solution can realize a lysis function, does not contain chaotropic reagents and alcohols, is compatible with mainstream nucleic acid extraction kits on the market, greatly saves time of an entire process, and is particularly suitable for 2019 new coronavirus (COVID-19) inactivation and lysis steps after swab sampling.
Owner:苏州白垩纪生物科技有限公司
Phage display antibody library and monoclonal antibodies aiming at novel coronavirus SARS-CoV-2 and obtained by panning based on same
ActiveCN111778218ANeutralizes contagionMicroorganism based processesImmunoglobulins against virusesEscherichia coliMonoclonal antibody agent
The invention discloses a phage display antibody library and five strains of screened antibodies capable of being combined with S protein of novel coronavirus SARS-CoV-2. Mutation is introduced into an ultra-variable region of an antibody variable region based on synthetic biology and a phage display technology, and a gene is transferred into escherichia coli, so that a synthetic antibody librarycontaining 108 kinds of antibodies is constructed; the phage display antibody library provided by the invention can perform screening to obtain the antibody with the specificity and the detection function, so that powerful resources of biological research and medical diagnosis are expanded; and the five strains of antibodies capable of being combined with the S protein of the novel coronavirus arefurther screened out and can be used for detecting the virus, part of the antibodies can block combination of the virus and cells, and the antibodies have the capacity of neutralizing novel coronavirus infectivity, can be used for preparing a novel coronavirus detection product, preparing a drug for inhibiting the novel coronavirus and preparing a pharmaceutical preparation for preventing or treating diseases caused by the novel coronavirus, and have a wide application prospect.
Owner:山东宽和正生物医药有限公司
Novel coronavirus antigen epitope and application thereof
ActiveCN111848753AImproving immunogenicityHigh titerSsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigen epitopeCoronavirus vaccination
The invention provides a novel coronavirus antigen epitope and application thereof. A super computer is used for simulating S, E, M and N protein structures of the novel coronavirus, and novel coronavirus epitopes SEQ ID NO 1-38 are obtained through calculation. The epitopes have very good immunogenicity; an antibody generated by inducing a part of epitope polypeptide has the effect of neutralizing the novel coronavirus; the antigen epitope can be combined with antibodies in serum of novel coronavirus patients at home and abroad, has the potential of resisting various novel coronavirus strains, and also has the potential of identifying and applying different strains. The epitope provided by the invention can be used for (1) research and development of novel coronavirus vaccines and universal coronavirus vaccines such as MERS viruses and SARS viruses; and (2) preparing a novel coronavirus antibody, and further detecting and typing viruses and treating diseases caused by the viruses. Thenovel coronavirus antigen epitope has a wide application prospect in the aspects of prevention of coronavirus and propagation and outbreak thereof, and detection and diagnosis of virus strains.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Methods for detection or measurement of viruses
InactiveUS20110262892A1Improve automationMicrobiological testing/measurementBiological material analysisViral antibodyActive agent
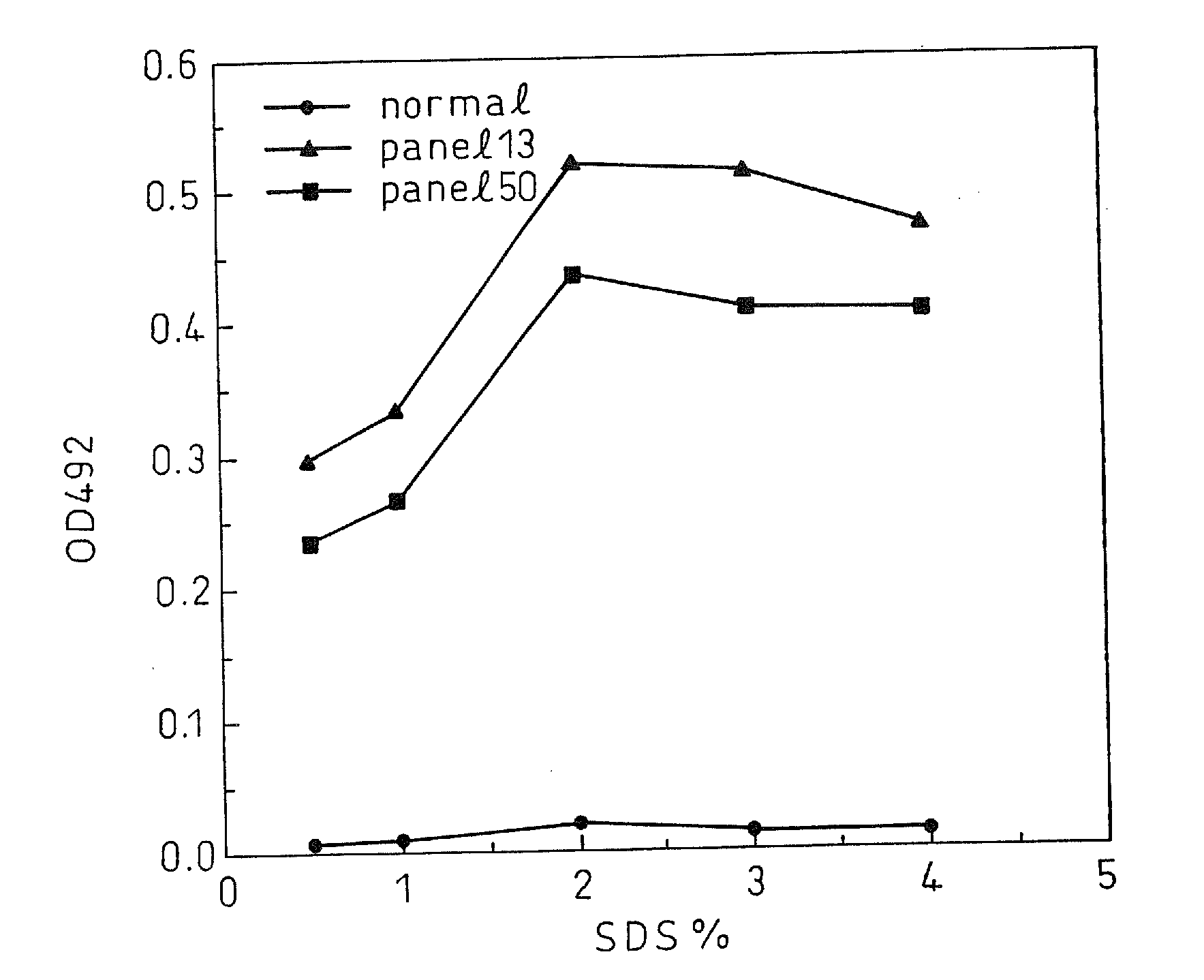
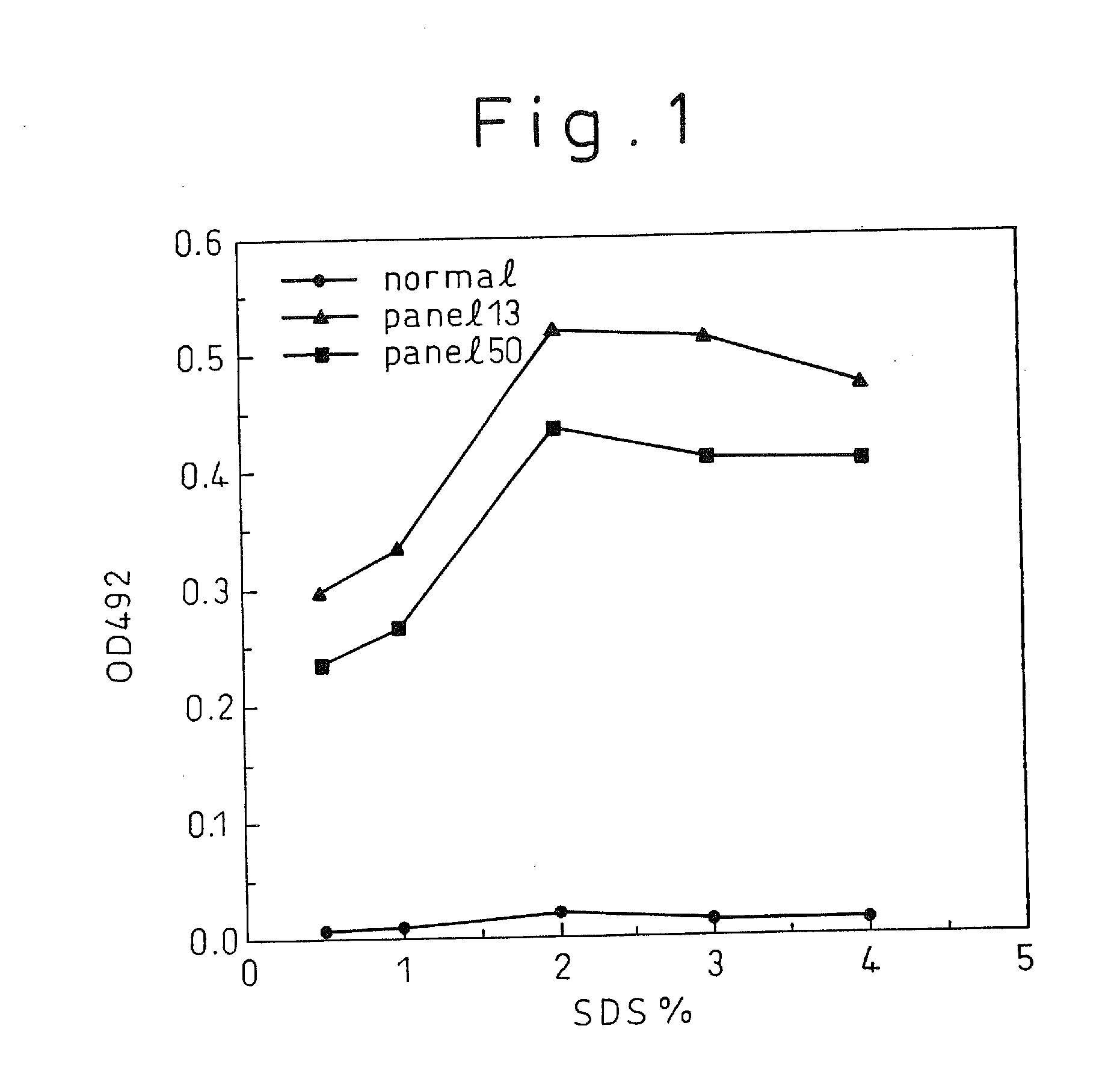
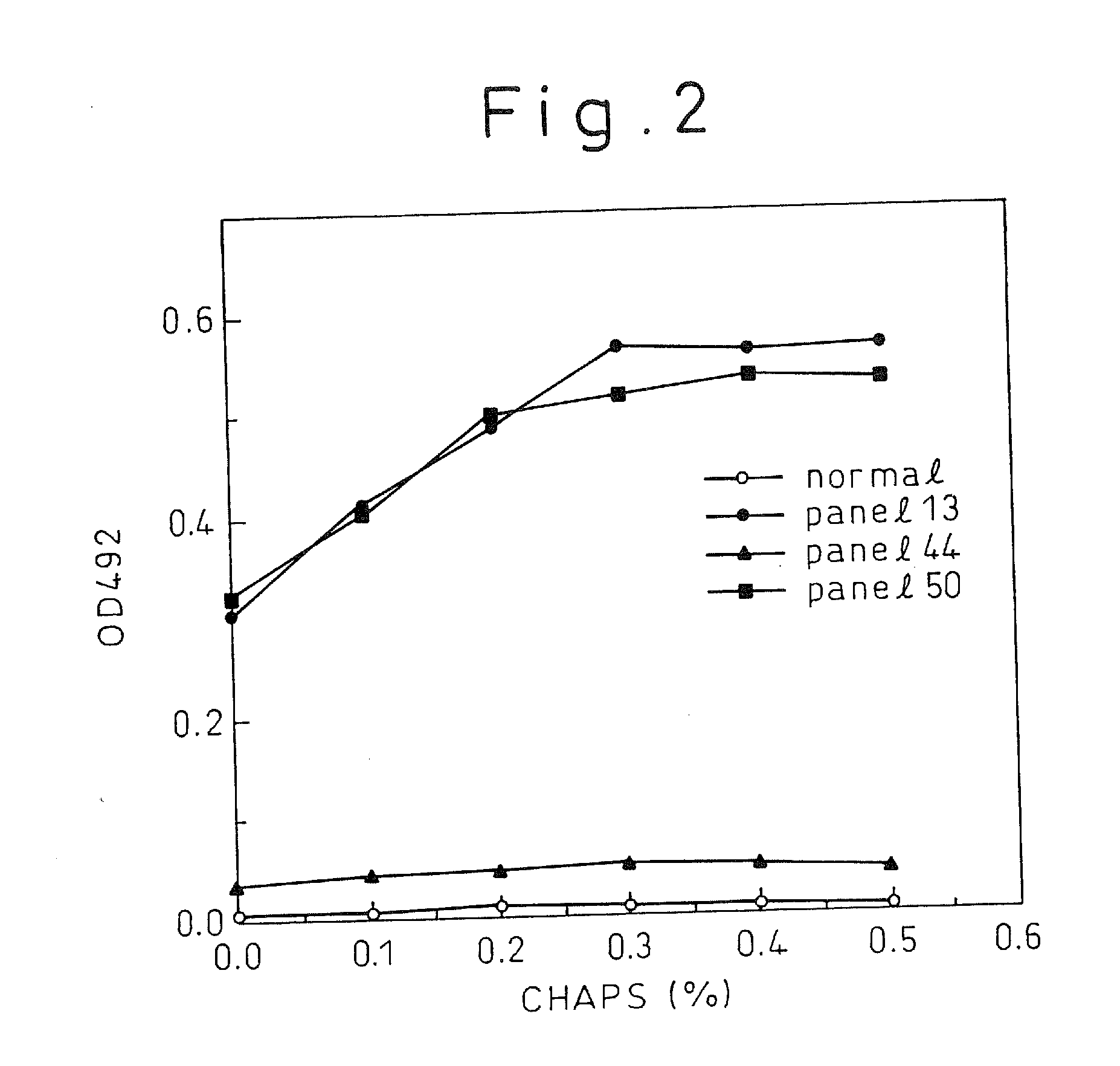
A method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) an anionic surfactant and (2) an amphoteric surfactant, nonionic surfactant or protein denaturant; a virus assay method using said treating method; a method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) a chaotropic ion and (2) an acidifying agent; a virus assay method using said treating method; a virus assay method, characterized in that a virus antigen and a virus antibody are measured based on their binding to their probe in the presence of a surfactant with an alkyl group of 10 or more carbon atoms and a secondary, tertiary or quaternary amine, or a nonionic surfactant, or of both of them; and a monoclonal antibody and a hybridoma producing the same for carrying out said method.
Owner:AOYAGI KATSUMI +4
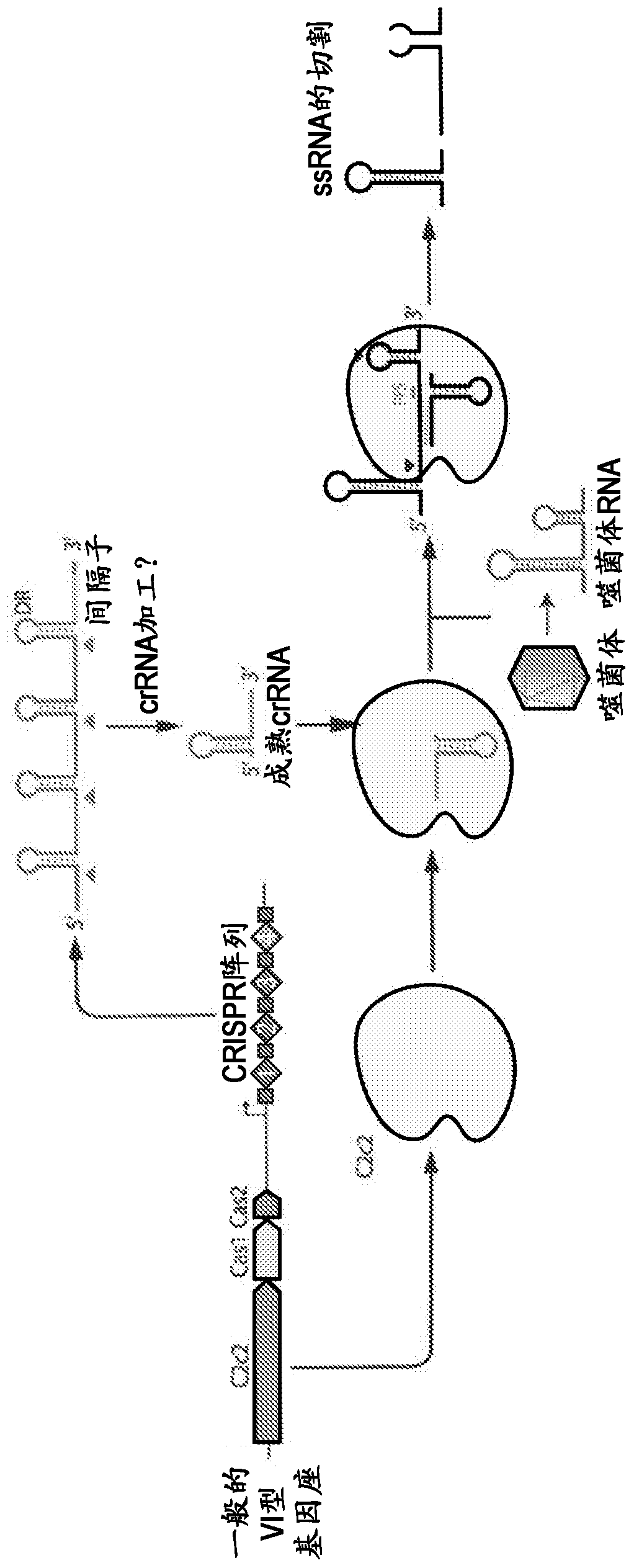
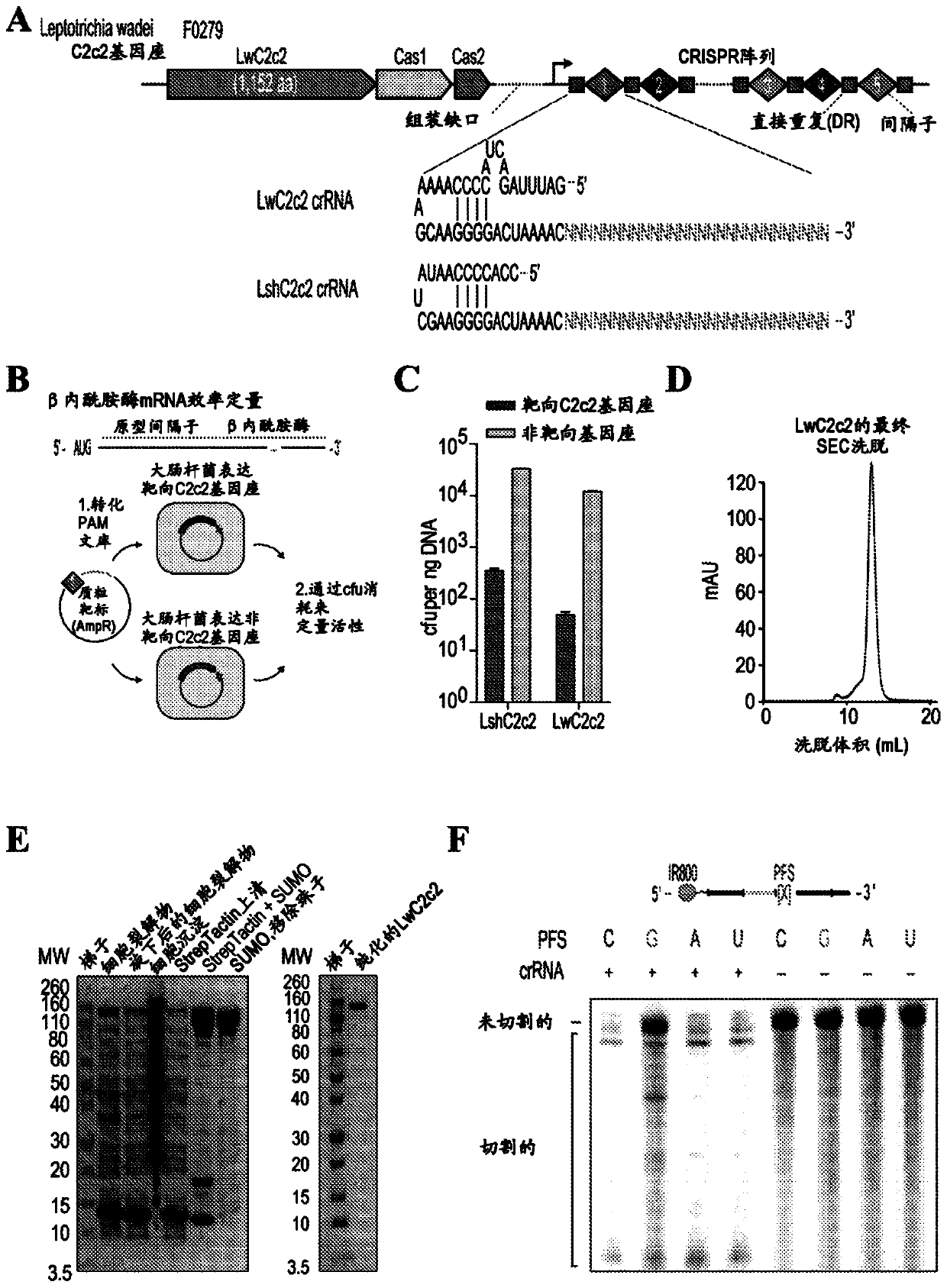
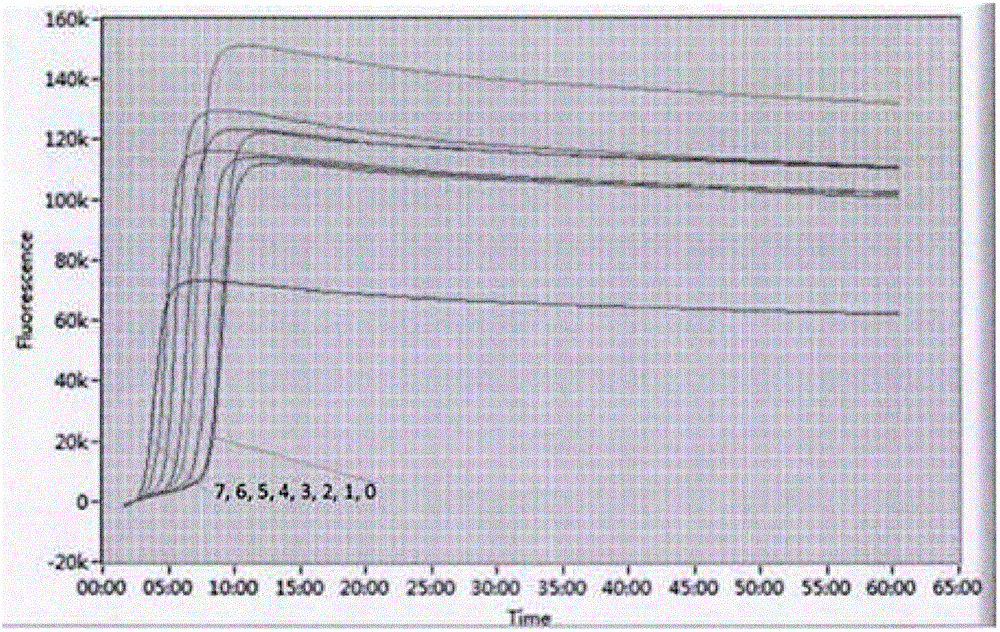
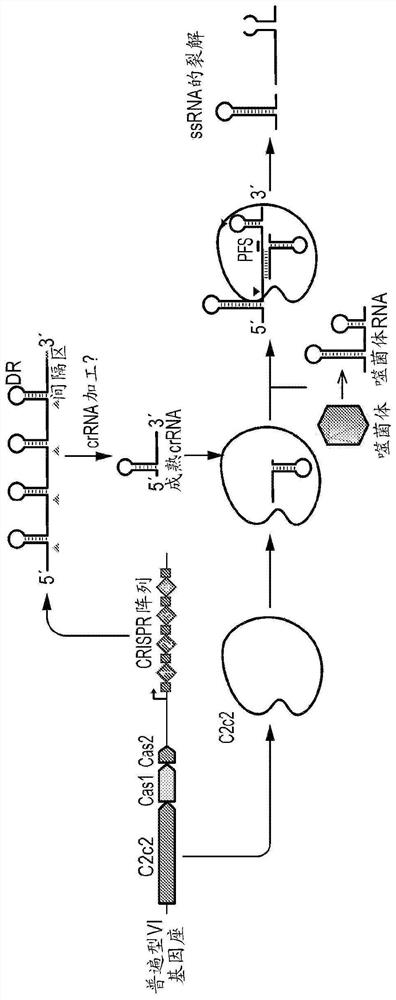
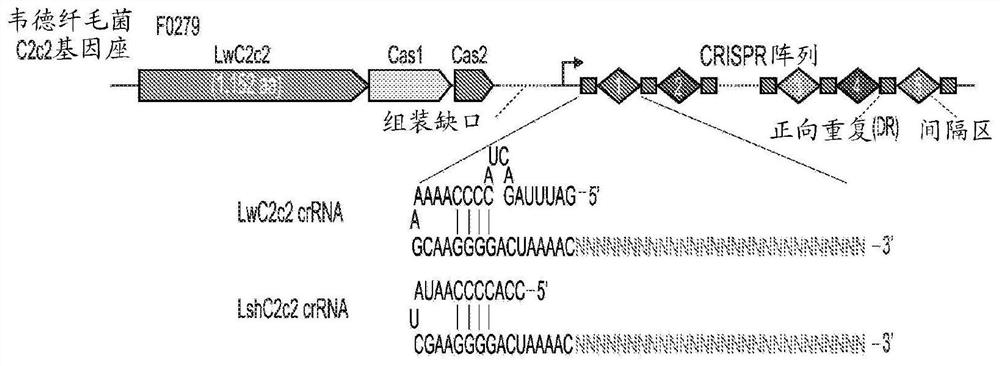
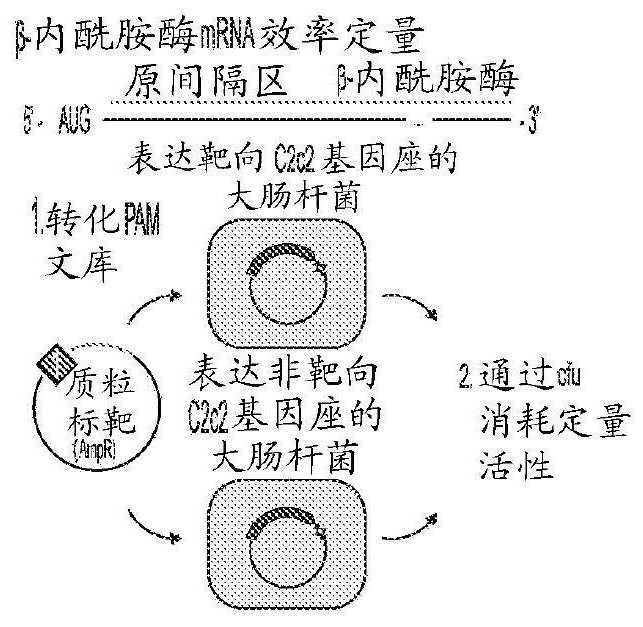
Crispr effector system based diagnostics
The embodiments disclosed herein utilized RNA targeting effectors to provide a robust CRISPR-based diagnostic with attomolar sensitivity. Embodiments disclosed herein can detect both DNA and RNA withcomparable levels of sensitivity and can differentiate targets from non-targets based on single base pair differences. Moreover, the embodiments disclosed herein can be prepared in freeze-dried formatfor convenient distribution and point-of-care (POC) applications. Such embodiments are useful in multiple scenarios in human health including, for example, viral detection, bacterial strain typing, sensitive genotyping, and detection of disease-associated cell free DNA.
Owner:THE BROAD INST INC +2
Human papillomavirus HPV DNA fragment, specific primer and application thereof
InactiveCN101851630ASimple methodMature technologyMicrobiological testing/measurementMicroorganism based processesDiseaseHuman papillomavirus DNA
The invention provides a human papillomavirus HPV DNA fragment, a mix primer for human papillomavirus HPV DNA detection and / or parting and application thereof. The mixed primer can specifically amplify to obtain one or a plurality of DNA sequences from HPV6, HPV 11, HPV 16, HPV 18, HPV 26, HPV 31, HPV 33, HPV 35, HPV 39, HPV 40, HPV 42, HPV 43, HPV 44, HPV45, HPV 51, HPV 52, HPV 53, HPV 54, HPV 55, HPV 56, HPV 58, HPV 59, HPV 61, HPV 66, HPV 68, HPV 70, HPV 72, HPV 73, HPV 81, HPV 82 and HPV 83, can be used for the human papillomavirus HPV DNA detection and / or parting and prepared into a PCR kit. The invention can detect common types of HPV at a time, greatly improve the HPV detection efficiency, overcome the defect of missed detection of latent infection by a serology and IHC detection method, realize early diagnosis of HPV diseases, and is suitable for clinical HPV gene detection and parting and guides prevention and cure of cervical carcinoma.
Owner:白向阳 +1
Zika virus loop-mediated isothermal amplification detection kit and using method
InactiveCN106244726AMicrobiological testing/measurementMicroorganism based processesZika virusFluorescence
The invention discloses a loop-mediated isothermal amplification kit for detecting Zika viruses and a using method of the kit. The kit is characterized by consisting of a Zika virus envelop protein (E) gene loop-mediated isothermal amplification primer mixed solution, a loop-mediated isothermal amplification reaction pre-mixed solution, a Zika virus E gene positive quality control and a Zika virus E gene negative quality control, and the kit is applicable to the rapid detection of the Zika viruses. The Zika virus E gene loop-mediated isothermal amplification primer group comprises a pair of outer primers (5'-3' sequences are shown as: AAGCACTGGCTGGTTCAC and TCCAGAGCTCCAGCAAGG), a pair of inner primers (5'-3' sequences are shown as: GTGGAGTTCCGGTGTCTGCCAAGGAGTGGTTCCACGACAT and AGAGTTCAAGGACGCACATGCCTGCTCCTTCTTGACTCCCTA) and a pair of loop primers (5'-3' sequences are shown as: CAGCGTGCCAAGGTAATGGA and AAAAGGCAAACTGTCGTGGT). The using method of the kit is characterized in that the real-time rapid diagnosis of the Zika viruses can be achieved by virtue of an isothermal amplification fluorescent detection system. The method is strong in specificity and high in sensitivity; therefore, a convenient and rapid way is provided for the prevention and control of the Zika viruses and for conducing trend investigation and analysis.
Owner:CHINA INSPECTION LAB TECH CO LTD
Crispr effector system based multiplex diagnostics
The embodiments disclosed herein utilized RNA targeting effectors to provide a robust CRISPR-based diagnostic with attomolar sensitivity. Embodiments disclosed herein can detect broth DNA and RNA withcomparable levels of sensitivity and can differentiate targets from non-targets based on single base pair differences. Moreover, the embodiments disclosed herein can be prepared in freeze-dried format for convenient distribution and point-of-care (POC) applications. Such embodiments are useful in multiple scenarios in human health including, for example, viral detection, bacterial strain typing,sensitive genotyping, and detection of disease-associated cell free DNA.
Owner:THE BROAD INST INC +2
Probe and primer for detecting novel coronavirus SARS-CoV-2, kit, detection method and application
ActiveCN111560472AOutbreak controlGuaranteed reliabilityMicrobiological testing/measurementBiological testingNucleic acid detectionViral nucleic acid
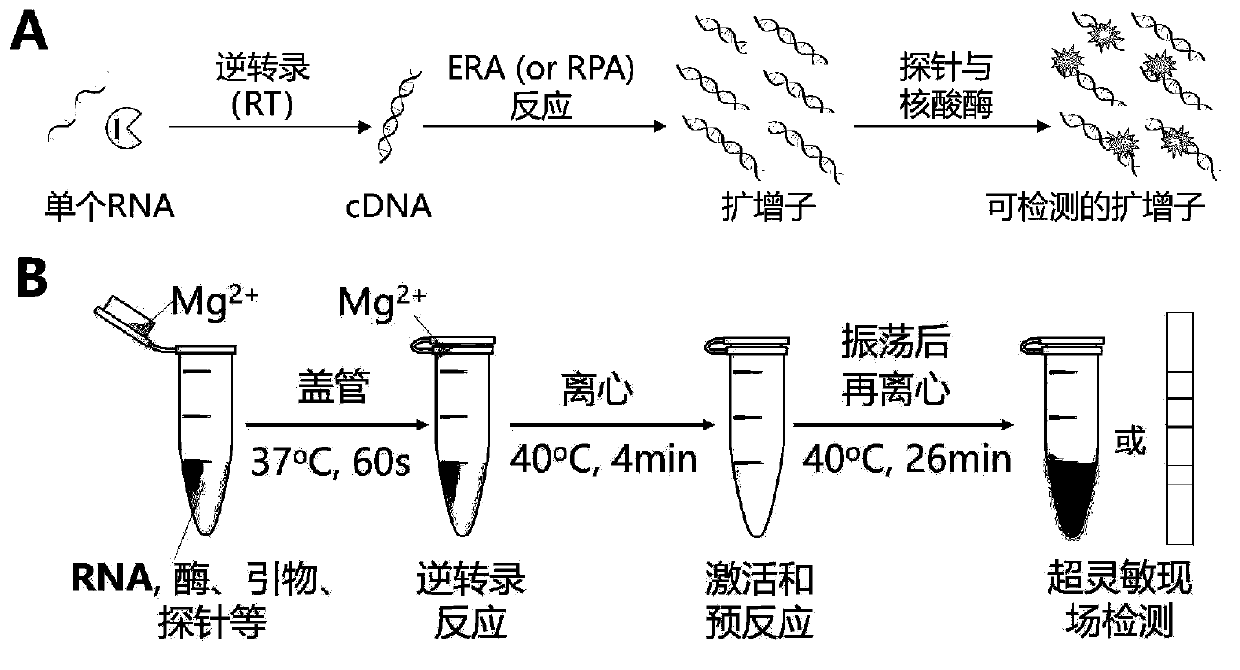
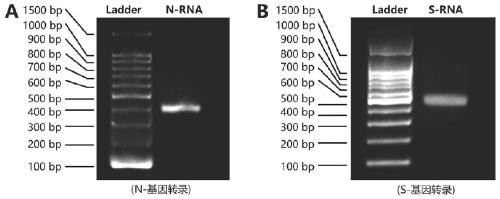
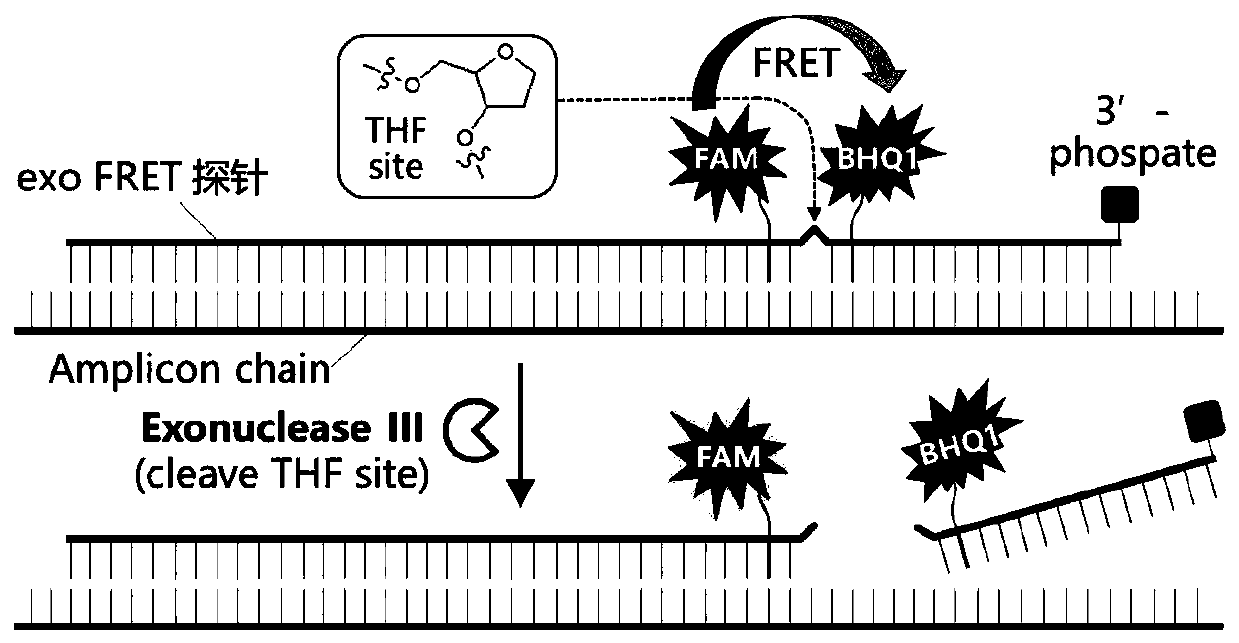
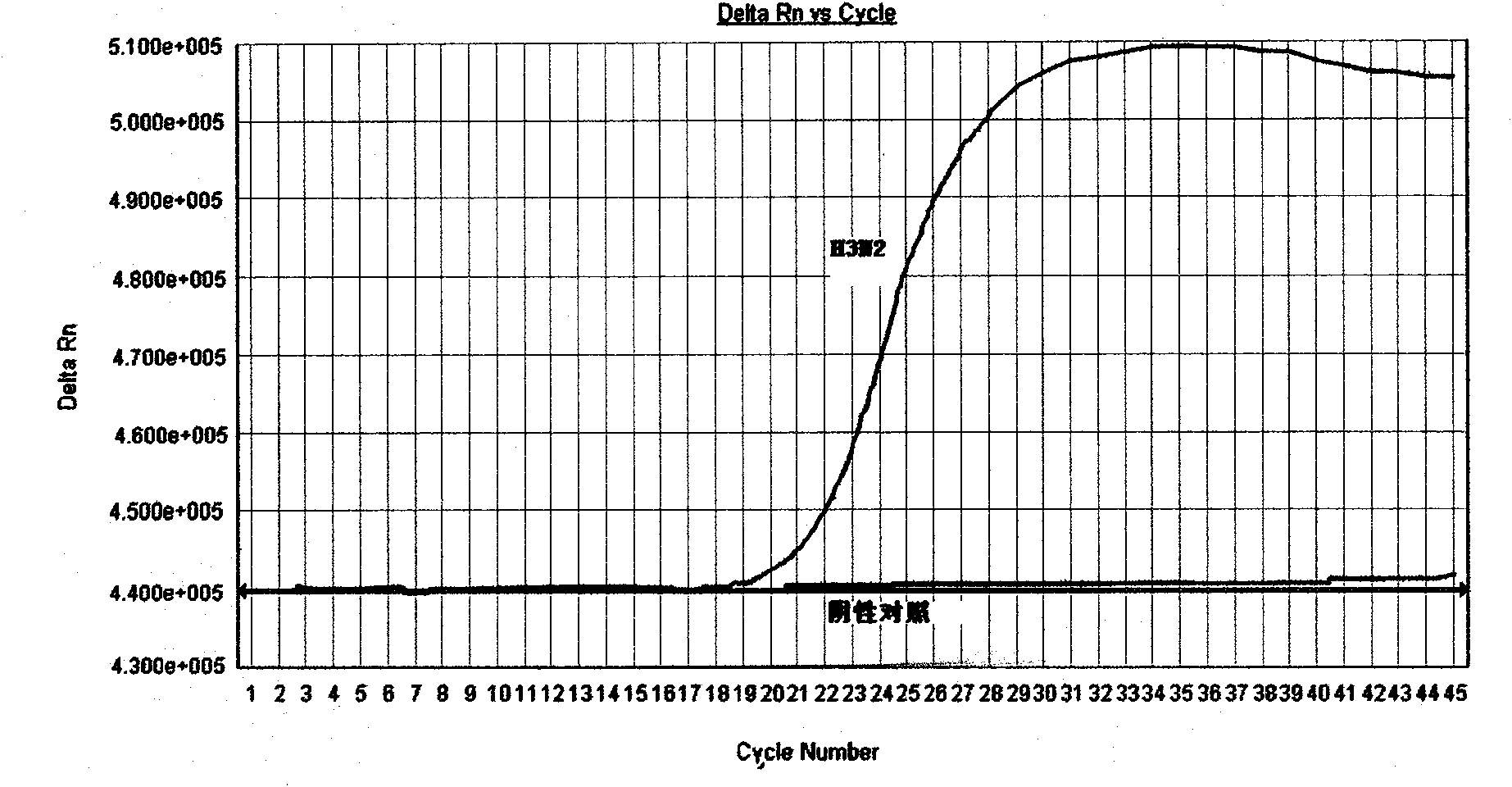
The invention discloses a probe, a primer, a kit, and a detection method for detecting novel coronavirus SARS-CoV-2, and application, belongs to the technical field of molecular biology nucleic acid detection technology, and aims to solve problems in field detection, reduce pollution caused in detection process, increase virus RNA detection sensitivity and specificity. The invention provides the probe and the primer for detecting novel coronavirus SARS-CoV-2, and the kit containing the probe and the primer. The invention further provides a whole-course encapsulated procedure for exponential amplification from RNA (WEPEAR method). According to the invention, the detection sensitivity on N gene as low as 1ag (about 4 copies) is realized; the detection sensitivity on S gene as low as 1 copy is achieved, meanwhile, double genes of virus nucleic acid RNA based on RT-RPA (or RT-ERA) are detected at the same time so as to further improve the reliability of the detection result, and the kit can be used for field detection or household detection.
Owner:HARBIN INST OF TECH
Detection method and detection kit of influenza A virus, H1N1 and H3N2 subtype influenza virus
InactiveCN101818207AIncreased sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesReaction conditionsTaqMan
The invention discloses a detection method and a detection kit of influenza A virus, H1N1 and H3N2 subtype influenza virus, which has the advantages of specificity, sensitivity, good repeatability, fastness and low cost. A specific primer and a TaqMan probe are designed through sequence alignment for searching a highly conserved area according to a North American variant strain M gene of Influenza A Viruse(H1N1)virus in 2009, an HA gene sequence and the HA gene sequence of Influenza A Viruse(H3N2) epidemic strain, which are published in GeneBank; key reagents such as the probe, the primer and positive control (to structure the influenza A virus, the H1N1 and the H3N2 subtype influenza virus gene recombination clone plasmid) in the detection method are developed; and the influenza A virus, H1N1 and H3N2 subtype influenza virus real-time fluorescence RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection method is established through the optimization of various reaction conditions and the tests on the specificity, the sensitivity and the repeatability. The detection method can be used for simultaneously and specifically detecting the influenza A virus, the H1N1 subtype influenza virus and the H3N2 subtype influenza virus in one test.
Owner:中华人民共和国珠海出入境检验检疫局
Humanized broad-spectrum high-neutralizing-activity monoclonal antibody against novel coronavirus and application
PendingCN113512113AHigh sourceImprove stabilityGenetically modified cellsImmunoglobulins against virusesAntigenTGE VACCINE
The invention discloses a group of humanized broad-spectrum monoclonal neutralizing antibodies for resisting SARS-COV-2 virus, and the neutralizing antibodies are obtained by screening through a single B cell flow sorting-antibody gene amplification pairing expression technology and have a unique CDR region; theneutralizing antibodies can be specifically combined with SARS-COV-2 and can effectively neutralize a plurality of international epidemic virus strains (a novel coronavirus mutant strain A, a novel coronavirus mutant strain B.1. 1.7, a novel coronavirus mutant strain B.1.351, a novel coronavirus mutant strain P.1, a novel coronavirus mutant strain B.1.617.1 and a novel coronavirus mutant strain B.1.617.2) at present, wherein the IC50 is about 0.1 [mu]g / mL. The present invention also relates to methods of preparation and uses of the set of neutralizing antibodies. The three antibodies have the effect of synergistically neutralizing viruses when being used in a pairwise combined manner, so that the combination of the three antibodies can be used for emergency prevention and / or treatment of COVID-19, has the characteristics of full humanization, high expression and good stability, and is suitable for industrialization. In addition, the antibody can also be used for preparing an SARS-COV-2 virus detection reagent, finding effective neutralizing epitopes and developing SARS-COV-2 recombinant protein and subunit vaccines.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
Enzyme-linked immunologic diagnosis kit for core antigen of C type hepatitis virus and method for preparing same
Disclosed are a hepatitis C virus antigen enzyme-linked immunoassay reagent box and a method for making the same. The invention obtains the cell strain of excretive anti HCV core antigen by analyzing the core antigen array of the hepatitis C virus different type and cloning the core antigen gene of HCV, purifying out the high activity monoclonal antibody with four core aa expression sites of HCV, wherein the Cab1 and Cabs are used as coating antibodies, the Cab3 and Cab4 are used as enzyme labeled antibodies; employing double antibodies sandwich technology to prepare HCV-cAg ELISA diagnosing reagent box.
Owner:湖南景达基因有限公司
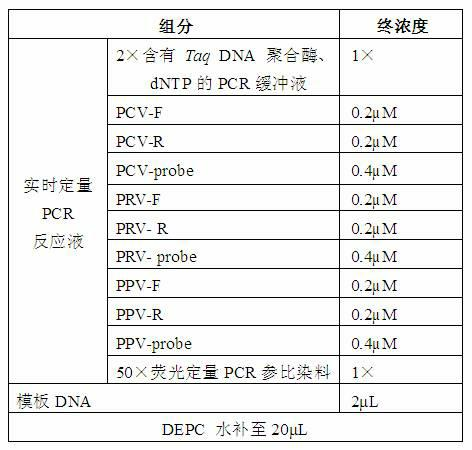
Primers, probes and detection kits for detection of porcine circovirus, porcine pseudorabies virus and porcine parvovirus
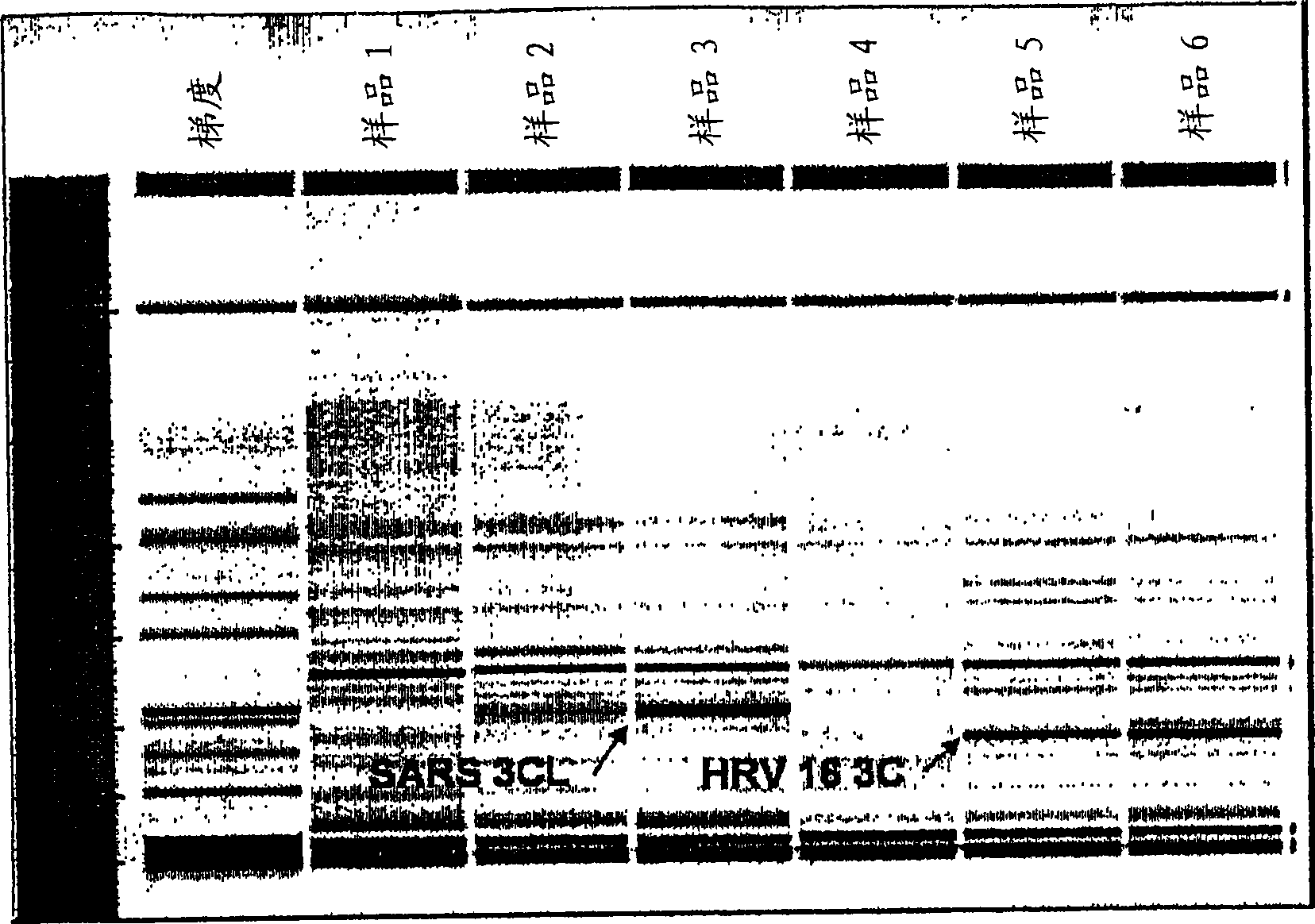
ActiveCN102277455AImprove detection efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceAssayViral test
The invention discloses a primer, probe and assay kit used for detecting porcine circovirus, porcine pseudorabies virus and porcine parvovirus. The primer and probe used for detecting are shown as sequences from SEQIDNO:1 to SEQIDNO:9 in a sequence table. The invention also provides an assay kit used for detecting the porcine circovirus, the porcine pseudorabies virus and porcine parvovirus. The primer and the probe which are adopted by the invention have strong specificity, and three viruses can be detected in one step, thus cost is saved, steps are reduced, and efficiency is improved. The method provided by the invention has the characteristics of high specificity, high sensitivity, high efficiency and low cost and is applicable to analysis of a larger number of samples.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Rapid prion-detection assay
Assays are provided for rapid detection, with high specificity of the pathogenic form of prion protein responsible for neurodegenerative diseases affecting humans and animals, such as transmissible spongiform encephalopathy in bovine, sheep, and cats. Also provided are assays for testing animal feedstock, such as animal feed, for the presence or concentration of pathogenic prion protein. Results are available in from about 0.5 to about 20 minutes and preferably within from about 5 to about 10 minutes. The assays employ proteinase-K to remove normal prion protein from a biological sample, so that the sample may be analyzed by immunochromatography to determine the presence and concentration of pathogenic prion protein. Because the proteinase-K is immobilized on a solid support for in situ removal of interfering components, the present invention obviates the need for subsequent extraction of the desired analyte. All aspects of the present invention are suitable for quantifying the minimal detectable amount of pathogenic prion protein in a biological sample. Moreover, the simplicity of sample preparation makes the present invention suitable for use in the field.
Owner:PRION DEVAL LAB
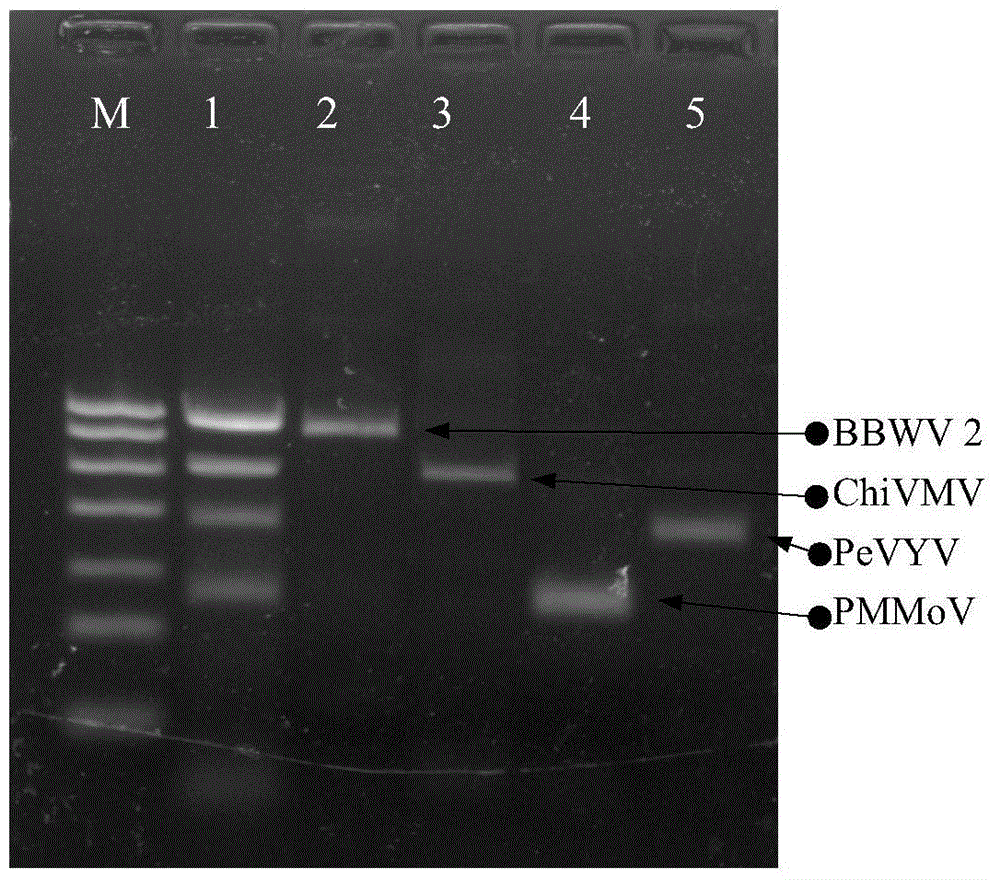
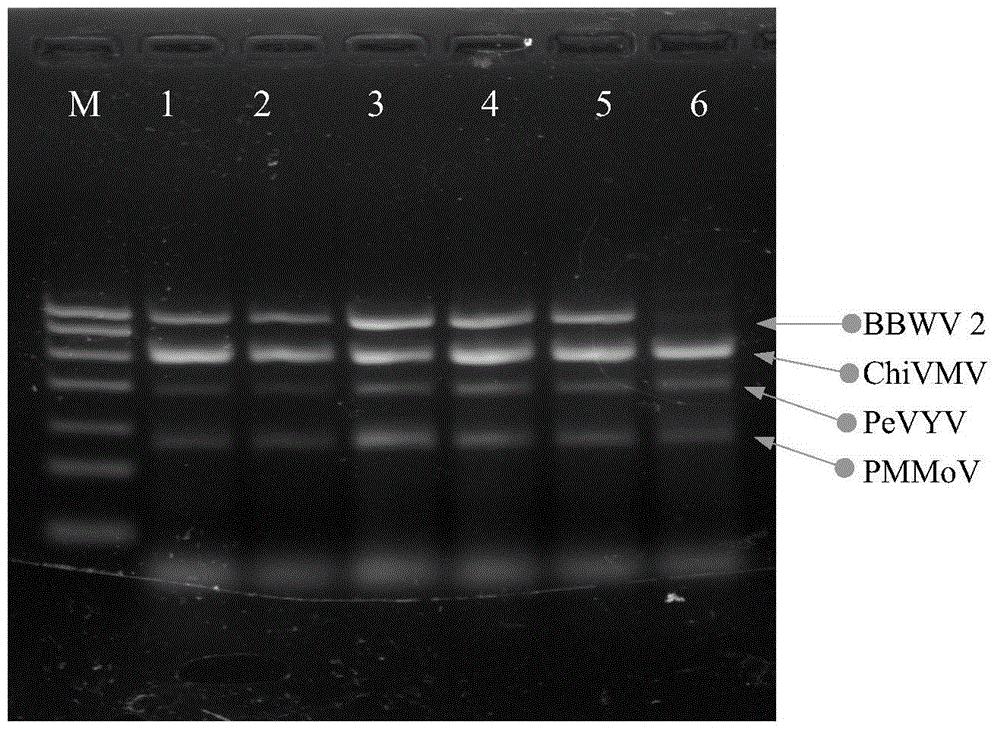
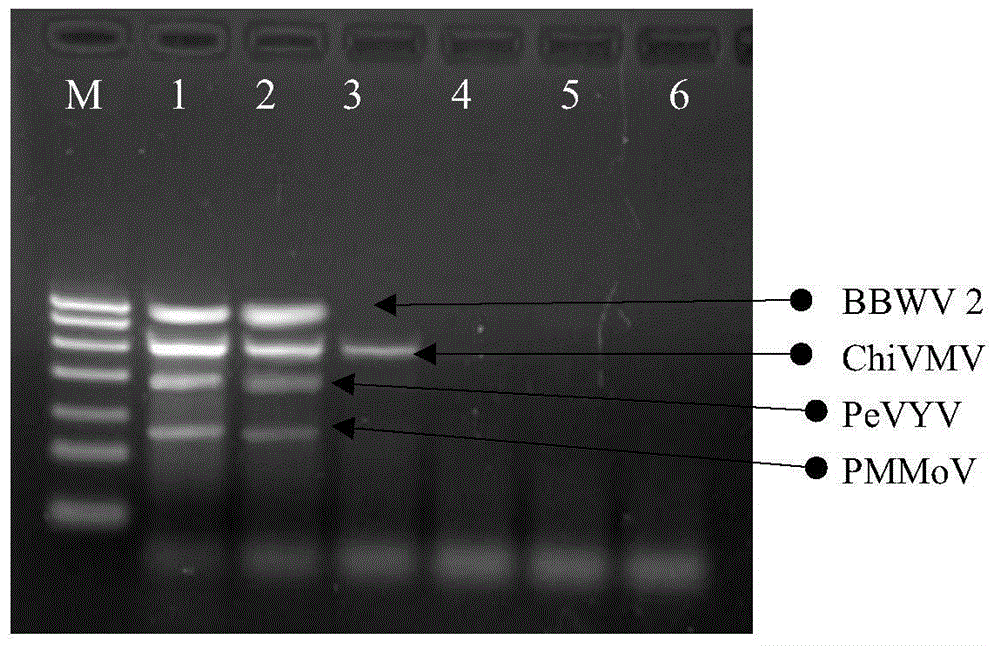
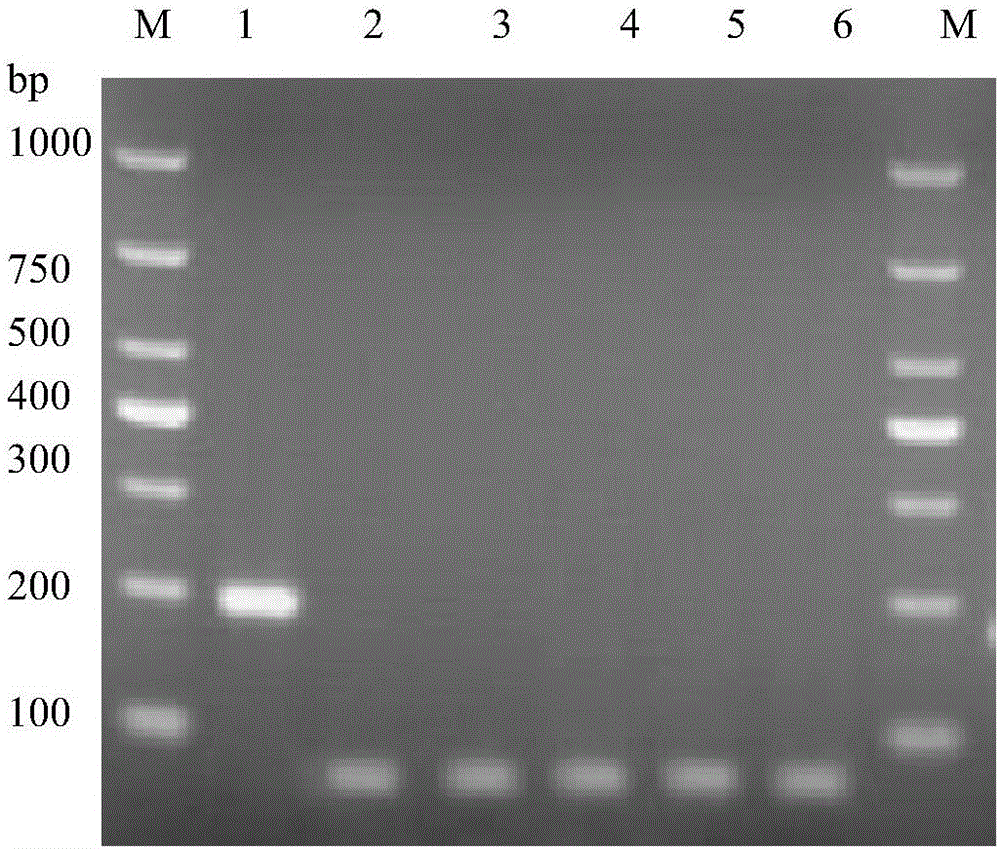
Quadruple RT-PCR (reverse transcription-polymerase chain reaction) detection primers and method capable of simultaneously detecting multiple chili viruses
InactiveCN105177188AIncrease varietyImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseBroad bean wilt virus
The invention discloses quadruple RT-PCR (reverse transcription-polymerase chain reaction) detection primers and a method capable of simultaneously detecting multiple chili viruses. The method adopts four pairs of primers disclosed as SEQ ID NO:1-SEQ ID NO:8; and one RT-PCR process can simultaneously detect four chili viruses: Broad bean wilt virus 2 (BBWV 2), Pepper vein yellows virus (PeVYV), Chilli veinal mottle virus (ChiVMV) and Pepper mildmottle virus (PMMoV). Compared with the existing single RT-PCR and duplex RT-PCR detection methods, the method disclosed by the invention can simultaneously detect the four chili viruses by one RT-PCR process, and is capable of detecting more virus varieties, greatly enhancing the detection efficiency and obviously lowering the detection cost.
Owner:HUNAN AGRICULTURAL UNIV
Method for detecting bean pod mottle virus based on RPA technology, RPA primer and kit
InactiveCN106755592ASave complex designShort detection timeMicrobiological testing/measurementMicroorganism based processesBean pod mottle virusNucleotide
The invention provides a primer pair, wherein one primer comprises the nucleotide sequence shown as the SEQ ID NO: 1, the other primer comprises the nucleotide sequence shown as the SEQ ID NO: 2. The invention also provides the application of the primer pair to detection or aided detection on bean pod mottle virus, or to detection or aided detection on bean pod mottle virus products and a method for detecting the bean pod mottle virus based on a RPA technology. Experiments show that the method for detecting the bean pod mottle virus based on the RPA technology is good in specificity and high in sensitivity, the detection time is short, a special instrument is not needed, the applicable range is wide, and guiding significance is realized in import and export plant and product testing.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
African swine fever virus ASFV-LAMP detection primer set and reagent kit
PendingCN110093457AGuaranteed accuracyAvoid missing detectionMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionAfrican swine fever
The invention relates to an African swine fever virus ASFV-LAMP detection primer set, reagent kit and detection method. In order to cope with the epidemic situation of African swine fever in Chin at 2018, an African swine fever virus P72 gene is used as an LAMP detection primer set. The primer set specially detects the African swine fever virus gene P72, ASFV can be effectively detected, a new technique means is provided for prevention and control of the African swine fever, and toxin strain detection and entry and exit quick screening are facilitated. The LAMP detection method can realize quick detection of the African swine fever viruses, detection results can be directly tested visually, and the entire reaction can be realized only needing heating. The relying of a traditional nucleic acid detection technique on a PCR instrument can be shaken off. The method is high in detection sensitivity, high in specificity, good in repeatability and high in detection speed, and can be used as an effectively on-field detection means for African swine fever.
Owner:陕西诺威利华生物科技有限公司
Primer and method for carrying out specific detection and absolute quantification on tomato spotted wilt virus
InactiveCN104120193AMicrobiological testing/measurementDNA/RNA fragmentationSpecific detectionFluorescence
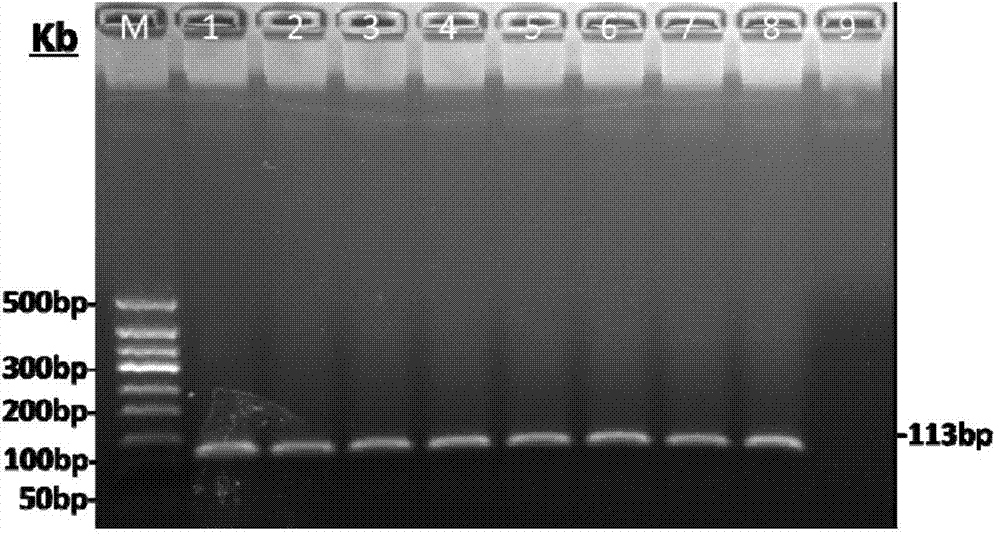
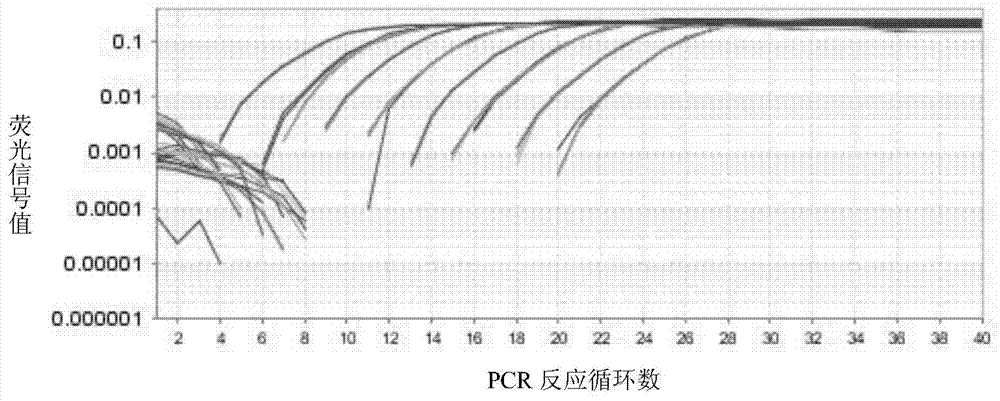
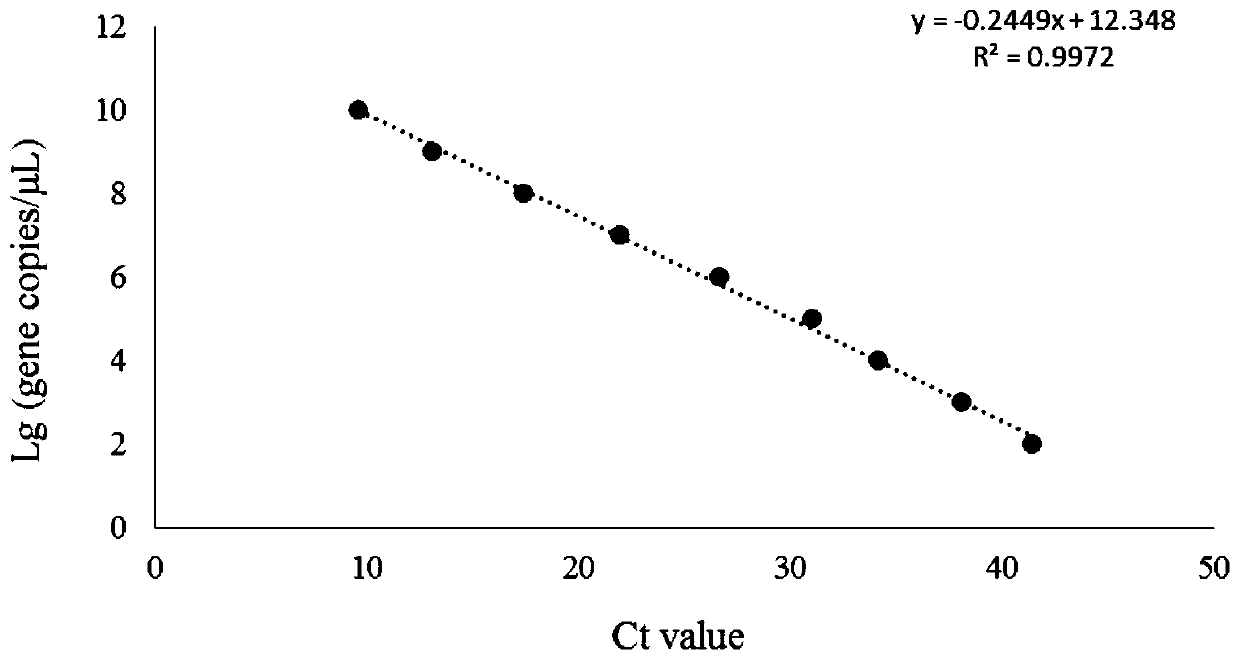
The invention relates to a primer and a method for carrying out specific detection and absolute quantification on a tomato spotted wilt virus and belongs to the field of plant virus detection. According to the primer and method disclosed by the invention, the tomato spotted wilt virus is taken as an object of study, a more rapid, simple, accurate and sensitive method for quantitatively detecting a plant virus is established, the method comprises the following steps of (1) establishing a standard curve according to the standard plasmid; (2) obtaining the cDNA of a sample to be detected; synthesizing cDNA by virtue of reverse transcription; and carrying out fluorescence quantitative PCR by virtue of using cDNA as a template and TSWV-113F / TSWV-113R as a primer to obtain the Ct value of the sample to be detected; and (3) calculating the copy number of the tomato spotted wilt virus in the sample to be detected by virtue of the standard curve. By virtue of the method, tomato spotted wilt virus within the sample can be accurately quantified in 4-5 hours, the time is saved and the experimental steps are simplified, the method is contributed to taking corresponding remedial measures before the onset of plant diseases to prevent huge economic losses brought by the widespread of the virus among crops.
Owner:INST OF VEGETABLE & FLOWERS CHINESE ACAD OF AGRI SCI
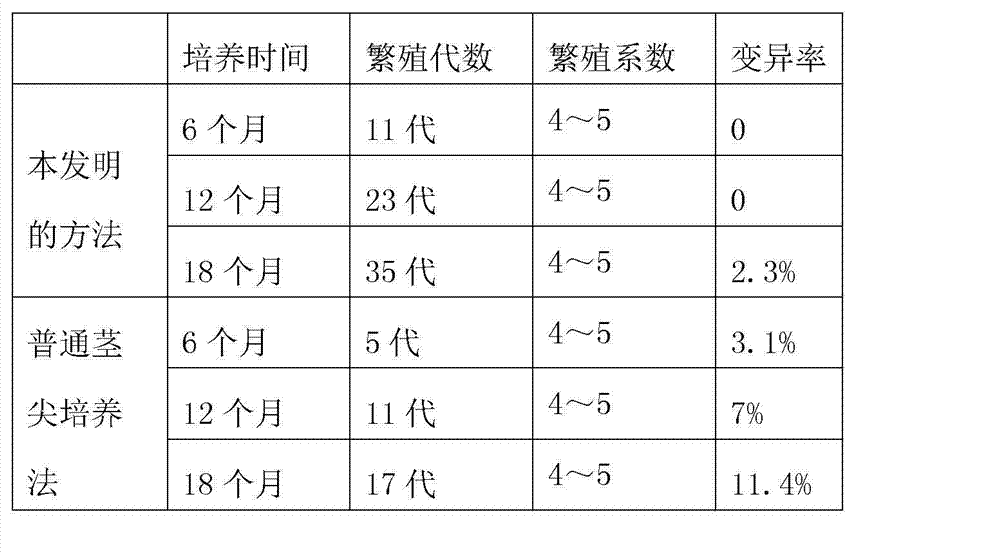
Method for breeding Pseudostellaria heterophylla by virus-free tissue culture
InactiveCN102524067AImprove stress resistanceIncrease productionPlant tissue cultureHorticulture methodsBiotechnologyPseudostellaria
The invention relates to a method for breeding Pseudostellaria heterophylla by virus-free tissue culture. The method comprises the following steps of: selecting explants, performing induction culture, detecting viruses, performing enrichment culture, performing rooting culture, propagating virus-free tissue-culture seedlings, acclimating, transplanting, and producing virus-free Pseudostellaria heterophylla stock seeds, first generation seeds and second-generation propagation seeds (production seeds). The method is easy to operate and has a low inoculation contamination rate and a high survival rate. The virus elimination operation is simple, rapid and complete, the propagation coefficient is increased by 10-15 times, and the propagation speed is high. The virus-free tissue-culture seedlings are suitable for industrialized production. The root tubers produced from the virus-free tissue-culture seedlings have resistance, are better in yield and quality than virus-infected seedlings, and can be produced commercially.
Owner:贵州昌昊中药发展有限公司 +1
Method for efficiently and industrially producing sweet potato detoxification tissue culture seedlings
InactiveCN103202232ALow variabilityFast growthPlant tissue cultureHorticulture methodsCell buddingSeedling
The invention discloses a method for efficiently and industrially producing sweet potato detoxification tissue culture seedlings. The method comprises the following steps of: (1) material culture, (2) material disinfection, (3) explant bud induction, (4) subculture multiplication and culture, (5) virus detection, (6) rooting culture, and (7) seedling hardening and transplantation. According to the principle that the quantity of the viruses at the growing point at the top of a plant is far less than the quantity at the lower part of the plant, the generation number of taking the growing point is enlarged, so that the aim of detoxification is achieved. According to the method provided by the invention, low hormone concentration is adopted, the MS basic culture medium is improved and a three-day dark culture method is used, so that the aberration rate of the detoxification tissue culture seedlings is greatly reduced, the growth rate of the tissue culture seedlings is accelerated, the reproductive cycle is shortened, the expanding propagation speed is high and the production cost is low.
Owner:INST OF FOOD CROPS HAINAN ACAD OF AGRI SCI
Porcine reproductive and respiratory syndrome virus RT-LAMP detection kit and detection method thereof
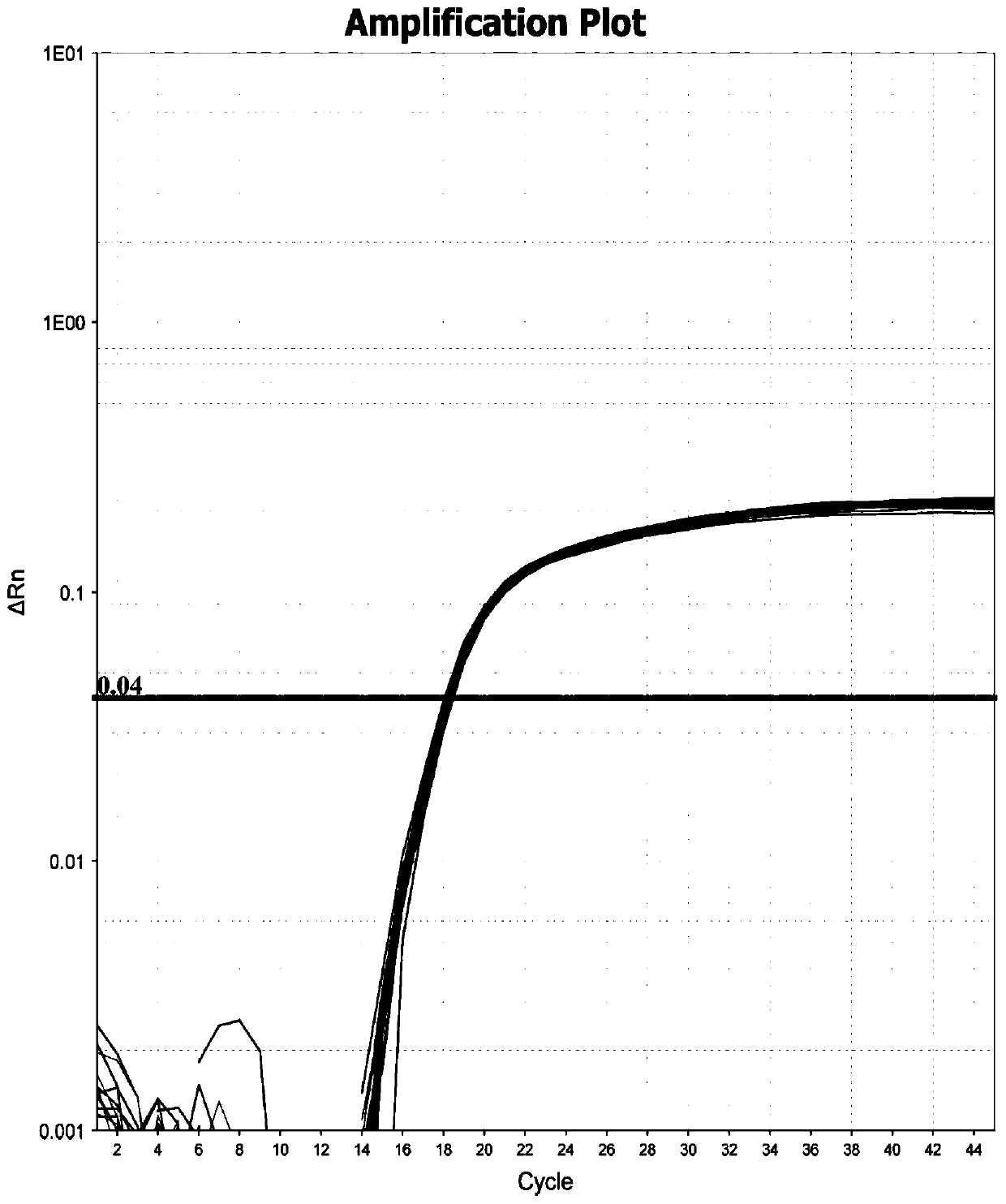
ActiveCN101575650AEfficient and specific amplificationThe detection process is fastMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementRNA extractionHighly pathogenic
The invention relates to a porcine reproductive and respiratory syndrome virus RT-LAMP detection kit and a detection method thereof. Primers required by PRSSV RT-LAMP reaction system are designed according to the sequence of porcine reproductive and respiratory syndrome virus (PRSSV) published by GenBank; PRSSV virus RNA is extracted with the virus RNA extraction reagent (LBBII-RNA) designed and prepared by the inventor, the PRSSV RT-LAMP reaction system established in the invention is utilized for detection, and color developing agent is added after the reaction to judge the result; the result shows that the PRSSV virus RNA obtains efficient specific amplification after the reaction is conducted for 45 minutes at the temperature of 63 DEG C; and then, quick detection of porcine reproductive and respiratory syndrome virus American classical strain and NSP2 variant strain (highly pathogenic porcine reproductive and respiratory syndrome virus strain) is conducted by SpuI enzyme cutting. Compared with the prior art, the invention has quick detection, high sensitivity, low reaction cost, convenient and fast operation, which is capable of differentiating American classical strain and NSP2 variant strain (highly pathogenic porcine reproductive and respiratory syndrome virus strain) and meets the requirement of multi-level detection.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Compositions and methods using same for the detection of viruses
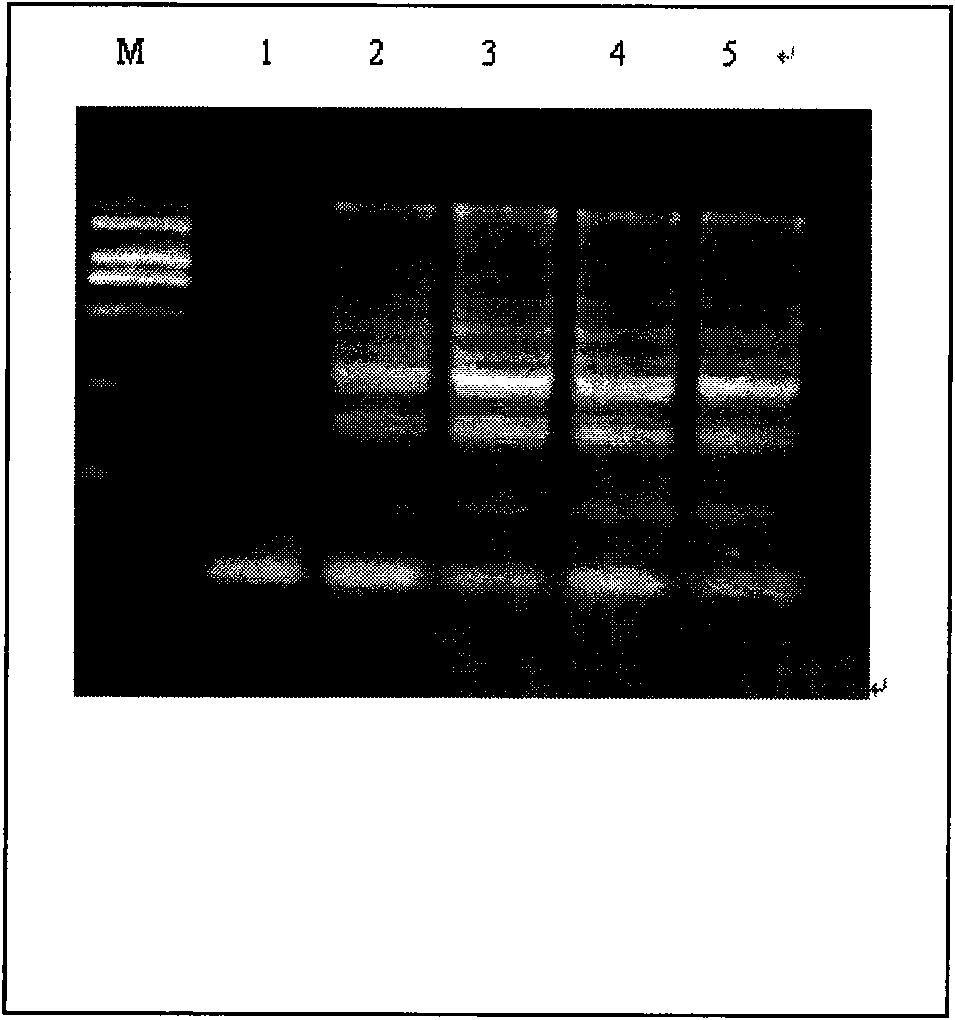
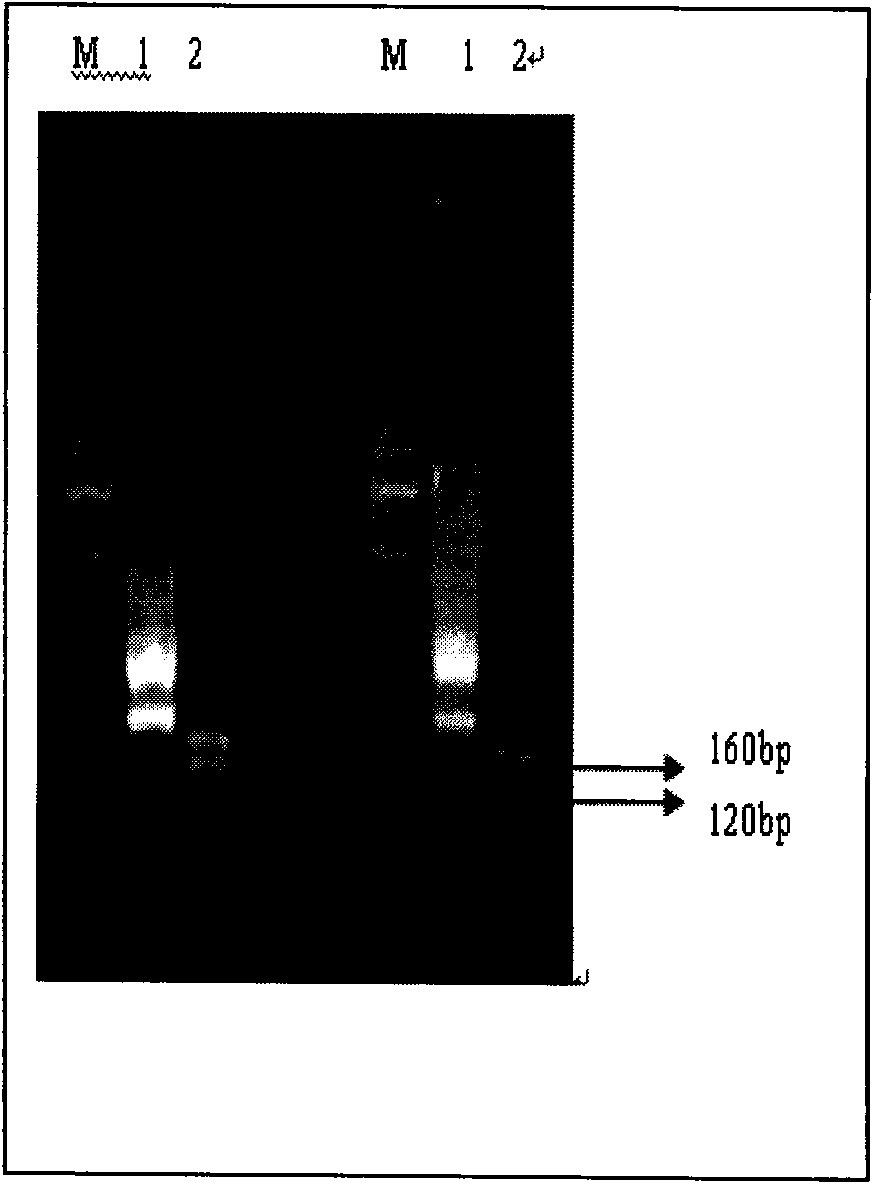
An isolated peptide is provided. The isolated peptide comprising an amino acid sequence selected from the group consisting of SEQ ID NO: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 and 47, said amino acid sequence being no more than 14 amino acids in length. Also provided are compositions which comprise the peptides and use of same in the detection of viruses.
Owner:MND DIAGNOSTICS LTD
Real-time fluorescent PCR primer probe combination and kit for detecting African swine fever virus wild strains
ActiveCN110760617AEasy to controlImprove purification effectMicrobiological testing/measurementMicroorganism based processesAnimal virusAfrican swine fever
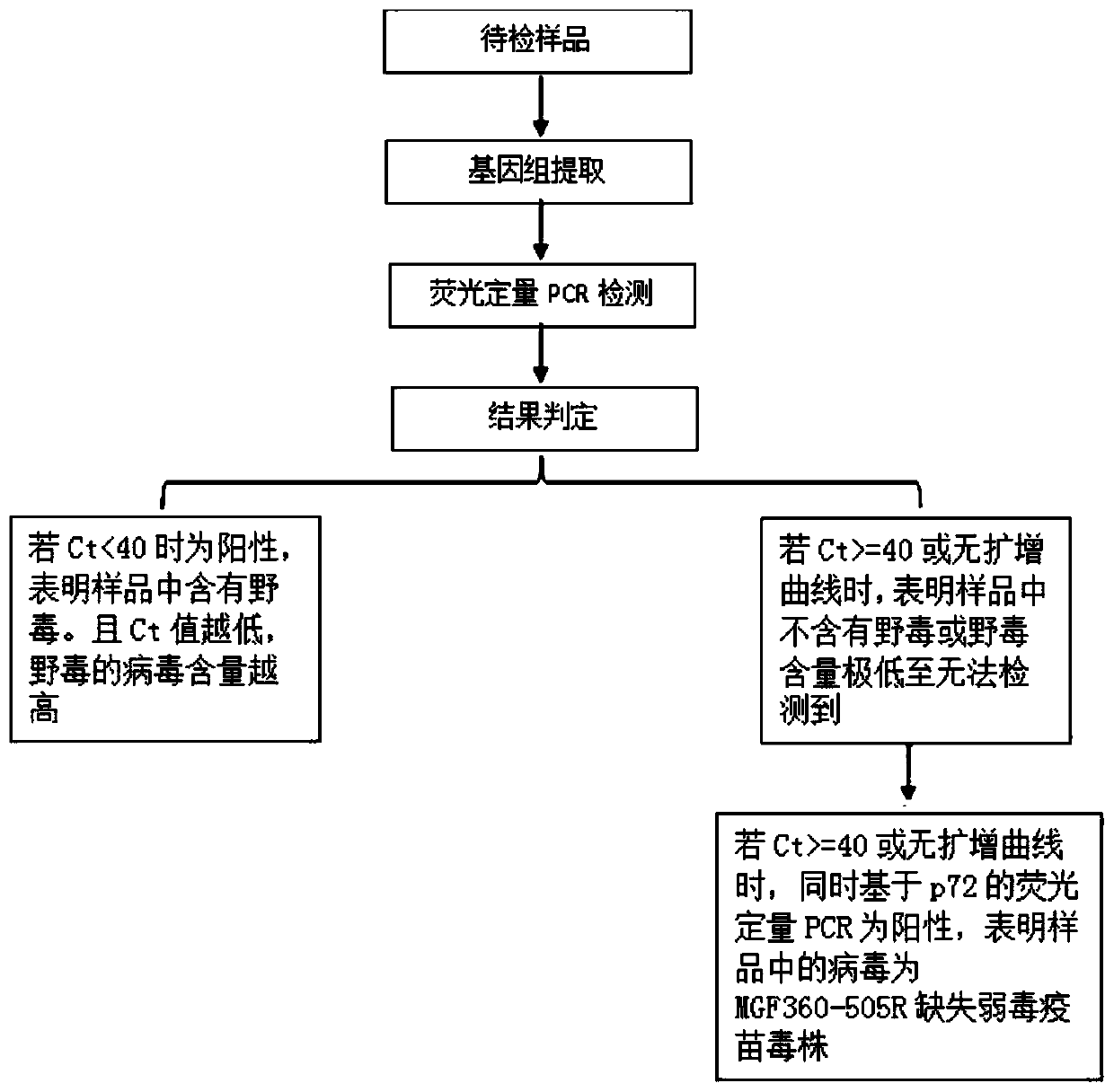
The invention relates to the technical field of animal virus detection in the field of veterinarians, and particularly discloses a real-time fluorescent PCR primer probe combination and a kit for detecting African swine fever virus wild strains. A PCR method based on the kit can not only specifically detect the African swine fever virus wild strains, but also serve as a method for distinguishing between the African swine fever virus wild strains and MGF360-505R loss attenuated vaccine virus strains when in use in combination with current p72 fluorescent PCR, therefore, important tools are provided for detecting the African swine fever virus wild strains and animals infected by the African swine fever virus wild strains, and control over African swine fevers and purification of the Africanswine fevers are promoted.
Owner:广纳达康(广州)生物科技有限公司
Oriental lily seed ball virus removing method
InactiveCN102577965AReduce cross infectionImprove survival ratePlant tissue cultureHorticulture methodsViral testCallus
The invention provides an oriental lily seed ball virus removing method, which specifically comprises the following steps of: A) stripping of explant growth points, B) heat treatment virus removal, C) cleaning and sterilization of explants, D) acquisition, induction and culture of growth points, E) virus-free culture and F) virus detection. The oriental lily seed ball virus removing method has the advantages that the defects existing in the prior art are overcome, the defects caused by a reason that a single method is used are overcome by comprehensively using a heat treatment virus removing method, a shoot tip culture virus removing method and a callus virus removing method, the probability of virus cross infection can be effectively reduced, the virus removal rate is high, the survival rate is high and the culture period of seed balls can be effectively shortened.
Owner:YUXI MINGZHU FLOWER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com