Patents
Literature
237 results about "Epidemic strain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
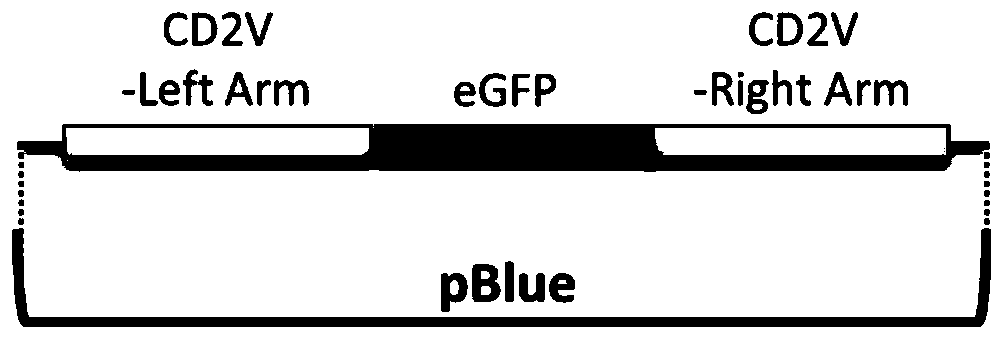
Gene deletion attenuated African swine fever virus and application thereof as vaccine
ActiveCN110093324AGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
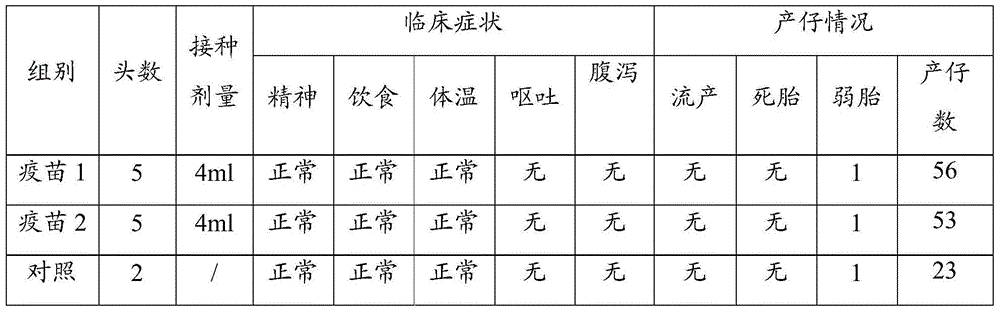
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Porcine epidemic diarrhea recombinant baculovirus gene engineering subunit vaccine, preparation method and application thereof
InactiveCN103585625AImprove abilitiesTargetedMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of biological vaccine preparation, and particularly relates to a porcine epidemic diarrhea (PED) recombinant baculovirus gene engineering subunit vaccine, a preparation method and an application thereof. According to the present invention, S1 gene and M gene of the current new PEDV epidemic strain are selected as reference sequences, a baculovirus expression system is adopted to express S1 protein or partial S1 protein and M protein, and the obtained recombinant protein is prepared into a subunit vaccine for effectively controlling PED occurrence; with the PED recombinant baculovirus gene engineering subunit vaccine produced by using the method, the defect of the current PEDV traditional vaccine is solved; and the PED recombinant baculovirus gene engineering subunit vaccine can be used for prevention and treatment of PEDV infections and related diseases caused by PEDV, and can further be used for preparation of coating antigen of PEDV detection antibody ELISA kits.
Owner:SOUTH CHINA AGRI UNIV
Porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as preparation method and application thereof
InactiveCN103122353AImprove expression levelHigh expressionGenetic material ingredientsAntiviralsEscherichia coliShuttle vector
The invention discloses porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as a preparation method and application thereof. Sequences of VP0, VP1 and VP3 genes are artificially synthesized by referring to an FMDV (Foot And Mouth Disease Virus) O-type epidemic strain gene sequence; the VP0, VP1 and VP3 genes are connected to pFBDPHmHNM1P10eGFP plasmid by adopting the plasmid as a framework vector, so that a baculovirus transfer vector pFBDPHmVP013 is obtained. The baculovirus transfer vector pFBDPHmVP013 is mixed with DH10Bac escherichia coli competent cells, and the positive bacterial colony is selected to obtain a recombinant shuttle vector Bacmid; the shuttle vetcor Bacmid is transferred with a sf9 cell, and the recombinant baculovirus QP-Ac-FVLP is obtained by collecting the cell supernatant. The recombinant baculovirus can be used for efficiently expressing FMDVVP0, Vp1 and Vp3 proteins and forming virus-like particles. And the virus-like particles are used for preparing subunit vaccine, so that the organism is induced to generate specific immunity response after the mouse is immunized.
Owner:HUAZHONG AGRI UNIV
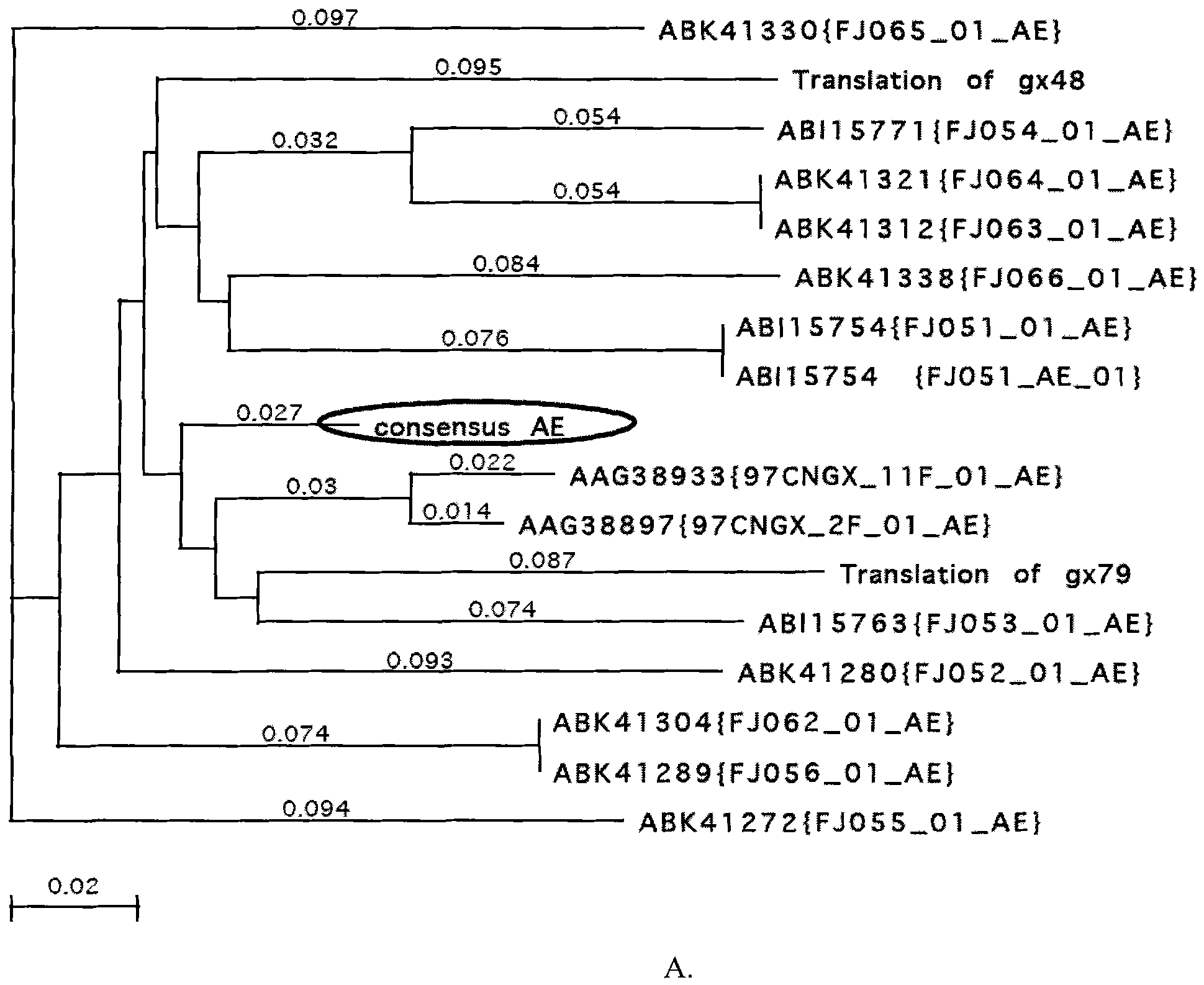
HIV-1gp120 gene consensus sequence optimized by codon and gp120 nucleic acid vaccine
InactiveCN101885760AEfficient expressionEffective stimulationGenetic material ingredientsVirus peptidesEscherichia coliFhit gene
The invention belongs to the technical field of biological medicine, relating to an HIV-1gp120 gene consensus sequence optimized by codons and a gp120 nucleic acid vaccine. In the invention, a Chinese HIV-1 epidemic strain (A / E recombinant subtype, B / C recombinant subtype and ThB subtype) envelope protein gp120 consensus amino acid sequence is obtained by applying multi-sequence comparative analysis; a gp120 gene segment encoding the consensus amino acid sequence is prepared by using an artificially synthesized method, and the gene is optimized by the codons and combines preferences of mammalian cells and escherichia coli codons; and on the basis, HIV-1gp120 nucleic acid vaccine consisting of the gp120 gene and eukaryotic expression vector pJW4303 is constructed. The nucleic acid vaccine can be applied to animal and human immunization and expression and production of the gp120 protein.
Owner:王世霞 +3
Porcine epizootic diarrhea virus strain and vaccine composition, preparation method and application thereof
ActiveCN106148287AImprove immune efficiencyImprove securityDigestive systemAntiviralsBiologyDiarrhea
The invention discloses a porcine epizootic diarrhea virus strain with good immunogenicity and an inactivated vaccine prepared through the porcine epizootic diarrhea virus strain. The porcine epizootic diarrhea virus strain is a current epidemic strain, and good immunogenicity and stability are achieved; compared with a commercially available vaccine strain, the vaccine prepared through the strain has the advantages of being good in safety, high in immune protective capability, high in immune efficacy and the like, and porcine epizootic diarrhea can be comprehensively and effectively prevented and treated.
Owner:PU LIKE BIO ENG
Asia1 type foot-and-mouth disease recombinant virus and preparation method and application thereof
The invention relates to an Asia1 type foot-and-mouth disease recombinant virus without pathogenicity for a host and a preparation method and application thereof. A saving system is efficient eukaryotic plasmids which are constructed by gene engineering and can express exact foot-and-mouth disease virus genome RNA (Ribonucleic Acid), and therefore the foot-and-mouth disease recombinant virus can be constructed and prepared; vaccine strains with high titer and good antigen matching property can be prepared by using the plasmids, can be prepared into live vaccines or inactivated vaccines and can effectively stimulate bodies to produce immune response after being used for immunizing pigs and cattle, provide an immune protective effect on the pigs and the cattle and effectively protect GV and GII prevalent strains, the immune protection rate can reach 100 percent, and the median protective dose (PD50) is 6.34 to 13.59; and the recombinant virus has the advantages of high titer, high antigen matching property with the prevalent strains, wide antigen spectrum and high immune protection rate, does not have pathogenicity for pig and cattle hosts, does not form toxemia or expel toxin, and can be applied to prevention and control of Asia1 type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
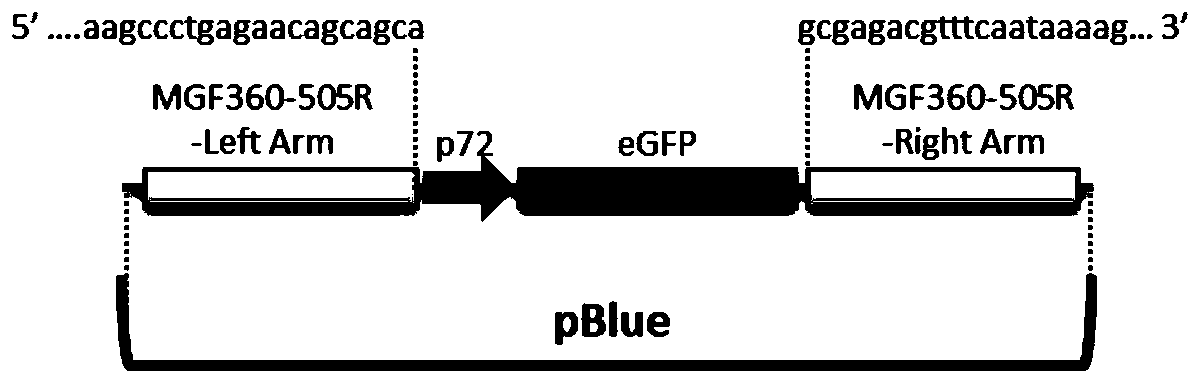
Attenuated African swine fever virus with gene deletion and its application as a vaccine
ActiveCN110093324BGood immune protectionFull Poison Attack ProtectionViral antigen ingredientsVirus peptidesAfrican swine feverGenetic engineering
The invention relates to a gene deletion attenuated African swine fever virus as a vaccine and the vaccine, and a construction method thereof. An African swine fever Chinese epidemic strain Pig / CN / HLJ / 2018 is adopted, a virulence gene of the African swine fever virus is deleted by a genetic engineering technology, and the gene deletion virus of MGF360-505R deletion and joint deletion of CD2V and MGF360-505R is obtained. Experiments show that the two virus strains can provide 100% immune protection against the African swine fever Chinese epidemic virulent strains, can be used as vaccines for safe and effective prevention and control of African swine fever in China, and have great social value.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
O type foot-and-mouth disease virus variant as well as coding gene and application thereof
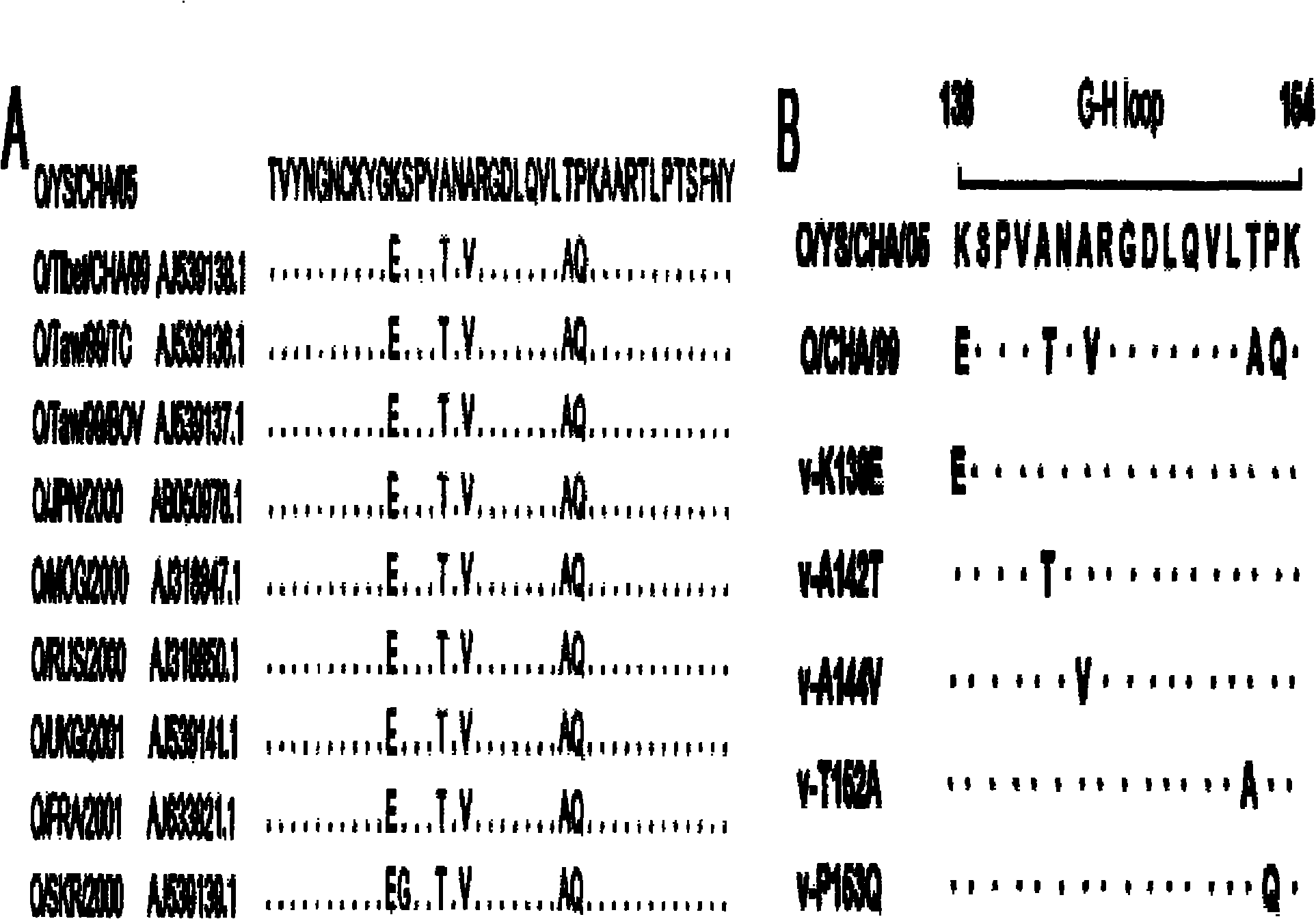
The invention discloses an O type foot-and-mouth disease virus variant as well as a coding gene and application thereof. In the invention, an O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 is firstly separated out, the nucleotide sequence of the O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 SEQ ID NO: 1, and the amino acid sequence is SEQ ID NO: 2. In comparison with a VP1 amino acid sequence, the virus strain has 7 variable sites, five of which are centralized in a G-H ring. Mutation of the sites ensures that the virus variant has the capability of escaping from host immunity so as to have the superiority for becoming a popular virus strain. Therefore, the variant can be employed to prepare an inactivated vaccine for prevention and treatment of the variant and relevant strains, dominant antigen epitope of the variant can be employed to prepare a synthetic peptide vaccine, and the variant can be employed to develop novel O type foot-and-mouth disease virus vaccines such as VLP vaccine and the like. Therefore, the invention has important value in controlling the popularity of O / YS / CHA / 05 and relevant variable strains.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
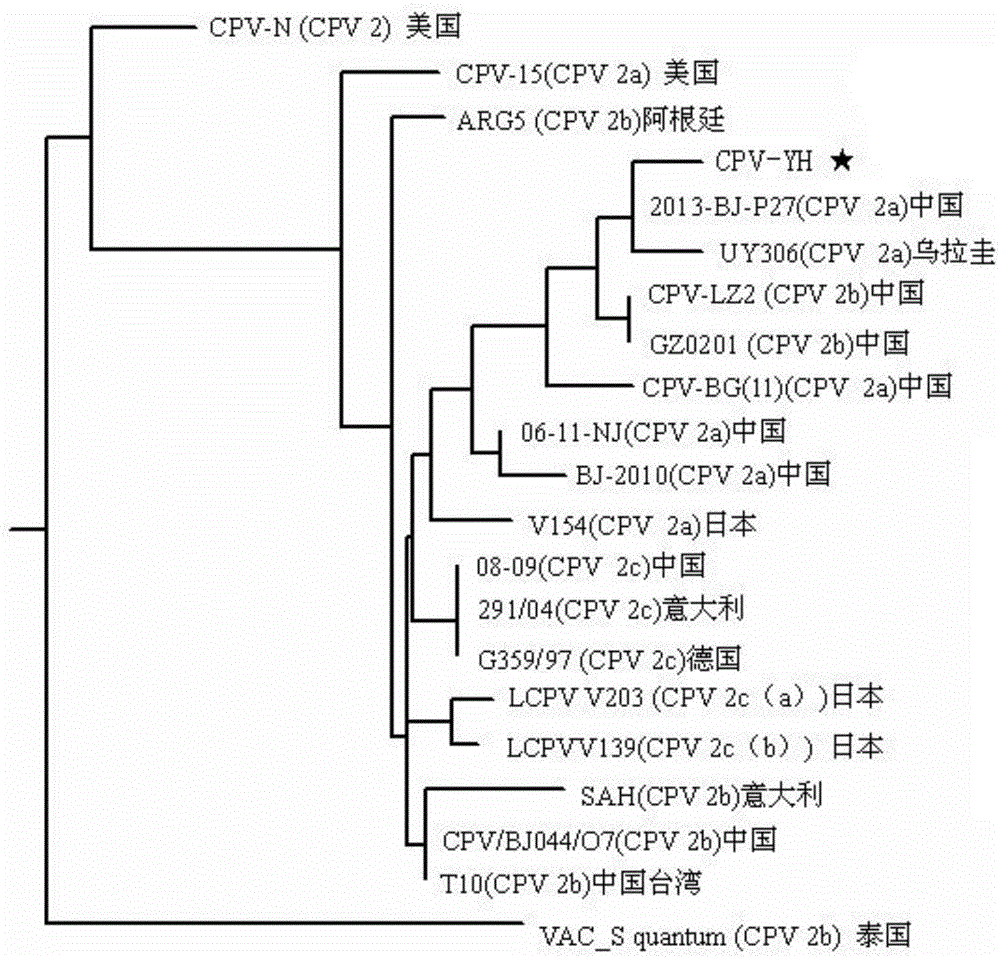
Canine parvovirus strain CPV-YH and applications thereof
ActiveCN106591242AProliferate fastHigh titerMicrobiological testing/measurementInactivation/attenuationCanine parvovirusRaw material
The invention provides a canine parvovirus strain CPV-YH and applications thereof, and belongs to the technical field of microbes. The preservation number of the provided canine parvovirus strain CPV-YH is CGMCC No.12990. The invention also provides applications of the canine parvovirus strain CPV-YH in the preparation of canine parvovirus vaccines and canine parvovirus diagnostic reagents. Based on the canine parvovirus strain CPV-YH, the invention also provides a vaccine composition for preventing and / or treating diseases caused by canine parvovirus and a reagent for detecting a canine parvovirus strain and / or diagnosing diseases caused by canine parvovirus. A novel content and direction are provided for the research on the variation of epidemic virus; a novel strain is provided for the canine parvovirus vaccine development; and a high quality raw material and choice are provided for the vaccine development.
Owner:北京世纪元亨动物防疫技术有限公司
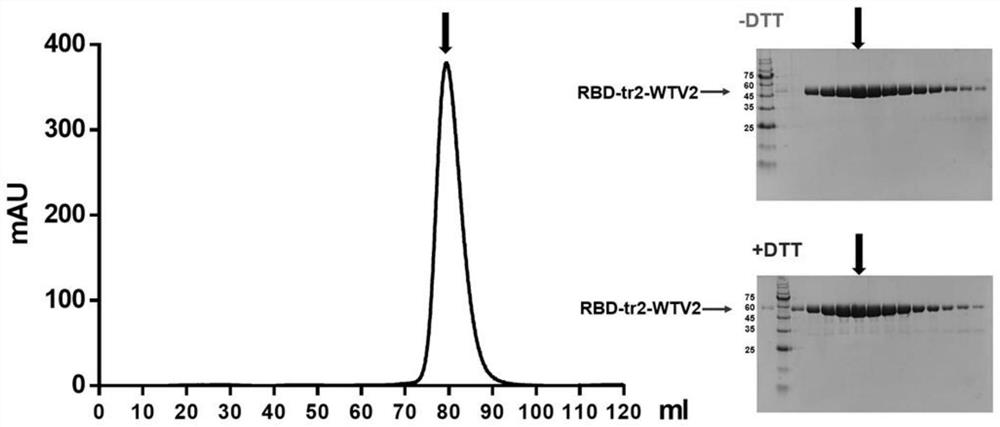
Recombinant novel coronavirus RBD tripolymer protein vaccine capable of generating broad-spectrum cross-neutralization activity as well as preparation method and application of recombinant novel coronavirus RBD tripolymer protein vaccine
ActiveCN113292640AAvoid infectionSsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigenDisease
The invention discloses a recombinant RBD trimer protein capable of simultaneously generating cross neutralization activity aiming at multiple novel coronavirus (SARS-CoV-2) epidemic strains, the trimer protein is composed of subunits of three novel coronavirus S protein RBD regions, and the amino acid sequences of the three novel coronavirus RBD regions are the same or at least one is different; and when the amino acid sequences of the three novel coronavirus RBD regions are the same, the amino acid sequences are the amino acid sequences shown as SEQ ID No.2 or SEQ ID No.3, or sequences with more than 95% of homology with the amino acid sequences shown as SEQ ID No.2 or SEQ ID No.3. The RBD trimer protein is taken as an antigen and supplemented with an adjuvant to immunize an organism, so that a high-titer neutralizing antibody aiming at various novel coronavirus epidemic strains can be generated at the same time, and the antibody has certain broad spectrum and can be used for treating and / or preventing novel coronavirus infection and / or novel coronavirus diseases.
Owner:NAT VACCINE & SERUM INST
HIV-1 genotype and drug resistant mutation site detection kit and application thereof
InactiveCN104946794AWide coverageStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationVirus strainDetection rate
The invention discloses an HIV-1 genotype and drug resistant mutation site detection kit and application thereof. The kit comprises set of primers detecting to-be-detected HIV-1 genome's genotype and drug resistant mutation site. The set of primers include amplification primer pairs and sequencing primer sets. Experiments prove that the detection kit provided by the invention ensures the detection capability of low-frequency mutant strains, greatly makes up the disadvantages of poor amplification efficiency, low detection rate and low low-frequency mutation detection rate of similar kits imported from abroad to China HIV epidemic strains, has the characteristics of simple and fast operation, high efficiency, economical efficiency and high throughput, etc., not only lays a solid foundation for domestic production of HIV drug resistant detection reagents, but also opens up broad prospect for domestic large-scale detection of HIV virus strain drug resistant situation.
Owner:CAPITALBIO CORP +2
Porcine epidemic diarrhea virus attenuated strain, vaccine composition prepared therefrom, and application
The invention relates to a porcine epidemic diarrhea virus attenuated strain. The porcine epidemic diarrhea virus attenuated strain is a nucleotide fragment or termination translator in a porcine epidemic diarrhea virus S gene encoding sequence, for encoding last nine amino acids EAFEKVHVQ or a homologous fragment thereof. The porcine epidemic diarrhea virus attenuated strain is a porcine epidemic diarrhea virus epidemic strain attenuated strain, and has the advantages of substantially reduced pig pathogenicity, no return after pig immunization, good immunogenicity and realization of effective resistance of immunized pigs to virulent attack. The invention also relates to a vaccine composition obtained by adopting the porcine epidemic diarrhea virus attenuated strain as a live virus antigen, a porcine epidemic diarrhea virus attenuated strain mutation S protein, and a preparation method of the porcine epidemic diarrhea virus attenuated strain.
Owner:PU LIKE BIO ENG
Degenerate primer for detecting virus belonging to virus family Arenaviruses and RT-PCR detection method
InactiveCN103224999AReduce degeneracyRaise the annealing temperatureMicrobiological testing/measurementDNA/RNA fragmentationLymphocytic choriomeningitis virus infectionFamily Arenaviridae
The invention discloses a degenerate primer for detecting virus belonging to the virus family Arenaviruses and a RT-PCR detection method. The degenerate primer can sensitively detect the virus belonging to the virus family Arenaviridae, and is a universal primer which is specific for the virus belonging to the virus family Arenaviridae, and can be used for detecting lymphocytic choriomeningitis virus which is widely distributed, and also can be used for detecting epidemic strains of other Arenaviruses, thereby preventing foreign epidemic strains from affecting our country, and the primer also can be used for detecting unknown viruses which is homologous to the expansion gene, thereby discovering new virus. Because a codehop primer design method is used, the degeneracy of the primer is reduced and the primer annealing temperature is increased simultaneously, thereby improving the singularity of PCR reaction of the designed degenerate primer.
Owner:中华人民共和国大榭出入境检验检疫局
Canine influenza virus monoclonal antibody hybridoma cell strain F112 and application thereof
ActiveCN107034197AStrong antiviral activityRapid test diagnosisImmunoglobulins against virusesAntiviralsLymphocyteTiter
The invention relates to canine influenza virus monoclonal antibody hybridoma cell strain F112 and belongs to the technical field of biology. Immune antigens are prepared by using canine influenza virus epidemic strain A / Canine / Nanjing / 11 / 2012 (H3N2) through lymphocyte hybridoma technique, the efficient hybridoma cell strain F112 is screened out from the antigens, and the canine influenza virus monoclonal antibody hybridoma cell strain F112 has hemagglutination inhibition titer of 212 and neutralizing titer of 105 for canine influenza virus. A canine influenza preventive therapeutic agent prepared by using F112 monoclonal antibody is used for preventing and treating canine influenza, under the effective rate of 100%. The efficient F112 monoclonal antibody is applicable to the detection and diagnosis of canine influenza and the preventive therapy, and has a promising application prospect.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
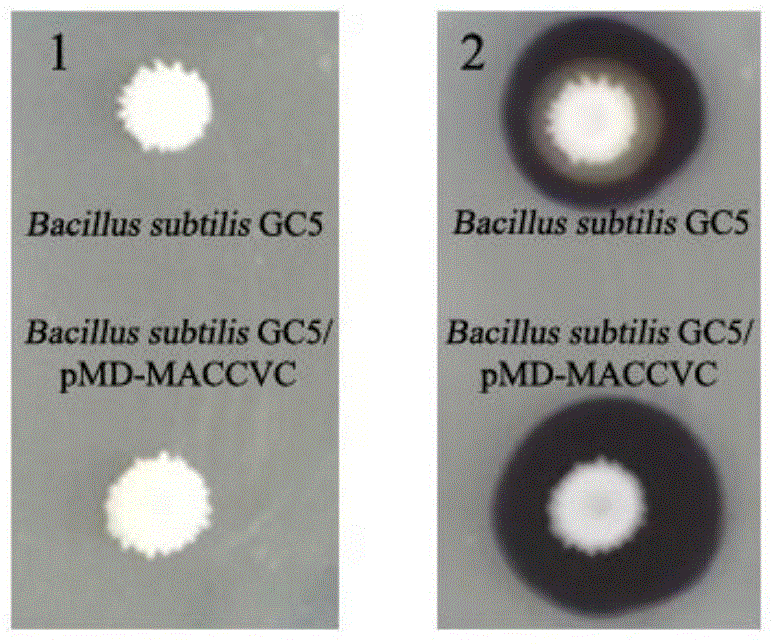
Recombinant bacillus displaying GCRV VP7 proteins on surface of bacillus subtilis GC5 and preparation method
ActiveCN104560860AAvoid immunogenicityAvoid security issuesBacteriaVirus peptidesBiotechnologyAntigen
The invention discloses a recombinant bacillus displaying GCRV VP7 proteins on the surface of bacillus subtilis GC5 and a preparation method. The preparation method comprises the following steps: (1) obtaining a present epidemic strain type-II GCRV VP7 nucleotide sequence; (2) separating wide bacillus from the body of a grass carp, determining the wide bacillus to be bacillus subtilis, and naming the wide bacillus as Bacillus subtilis GC5, CCTCC NO: M2014654; (3) constructing a fusion expression recombinant integrated carrier, wherein a vp7 sequence in recombinant plasmids is the nucleotide sequence shown in SEQ ID No. 1; (4) preparing and authenticating recombinant bacillus subtilis, CCTCC NO: M2014655; (5) inducing and authenticating the recombinant bacillus displaying VP7 on the surface. The generation rate of spores is up to 100%, and the recombinant bacillus is simple and convenient to produce, low in cost, and capable of being used as a feed additive; the recombinant bacillus has an intestinal customization capacity, as well as is beneficial to regulating the intestinal bacterial colony balances of animals, improving the body immunocompetence of the animals, and enhancing the nutrition metabolism functions of the animals. The spores are high in stress resistance and easy for large-scale production, as well as solve the problem of instability of GCRV VP7 proteins used as immunizing antigens in extreme environments.
Owner:INST OF AQUATIC LIFE ACAD SINICA
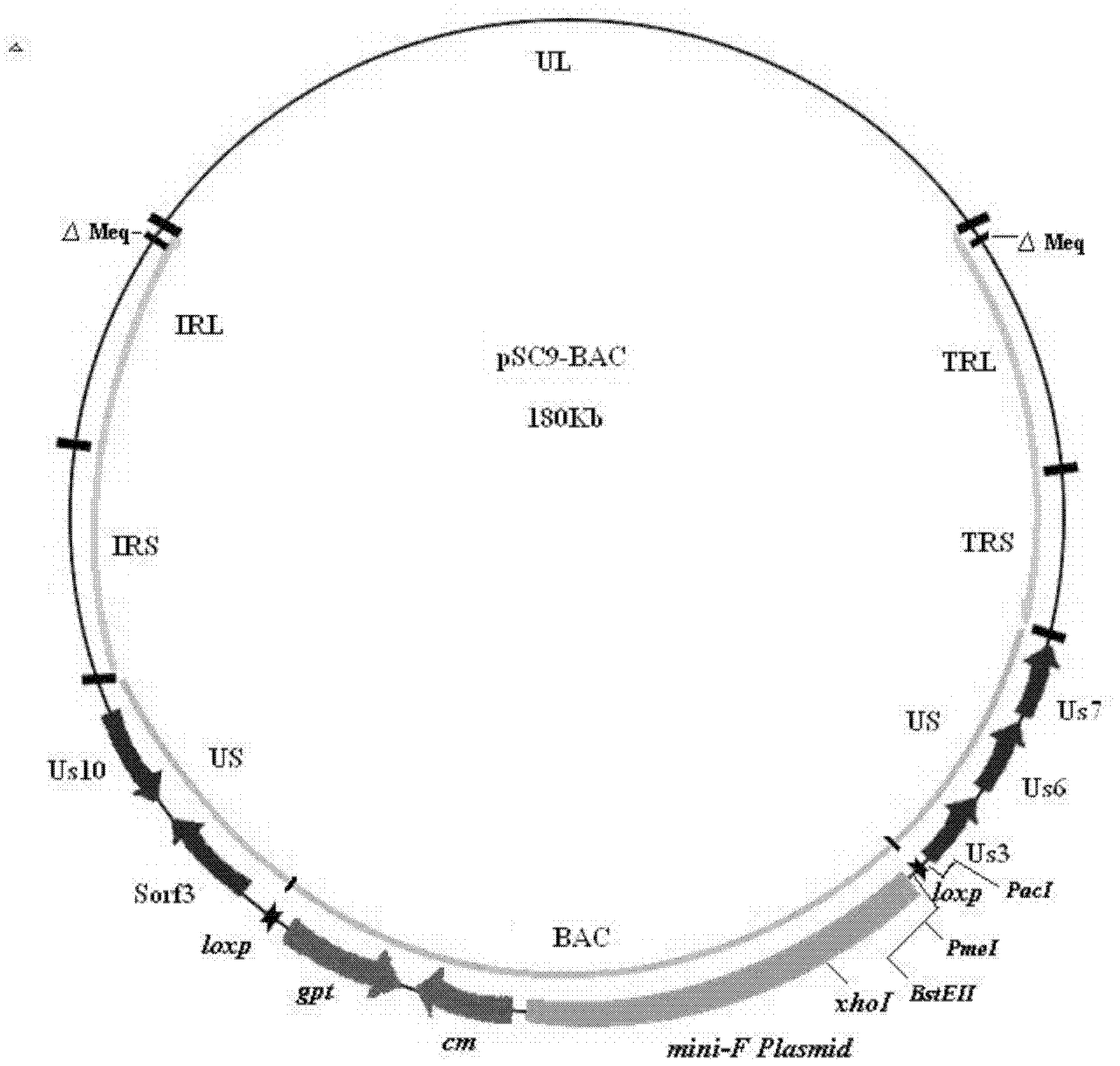
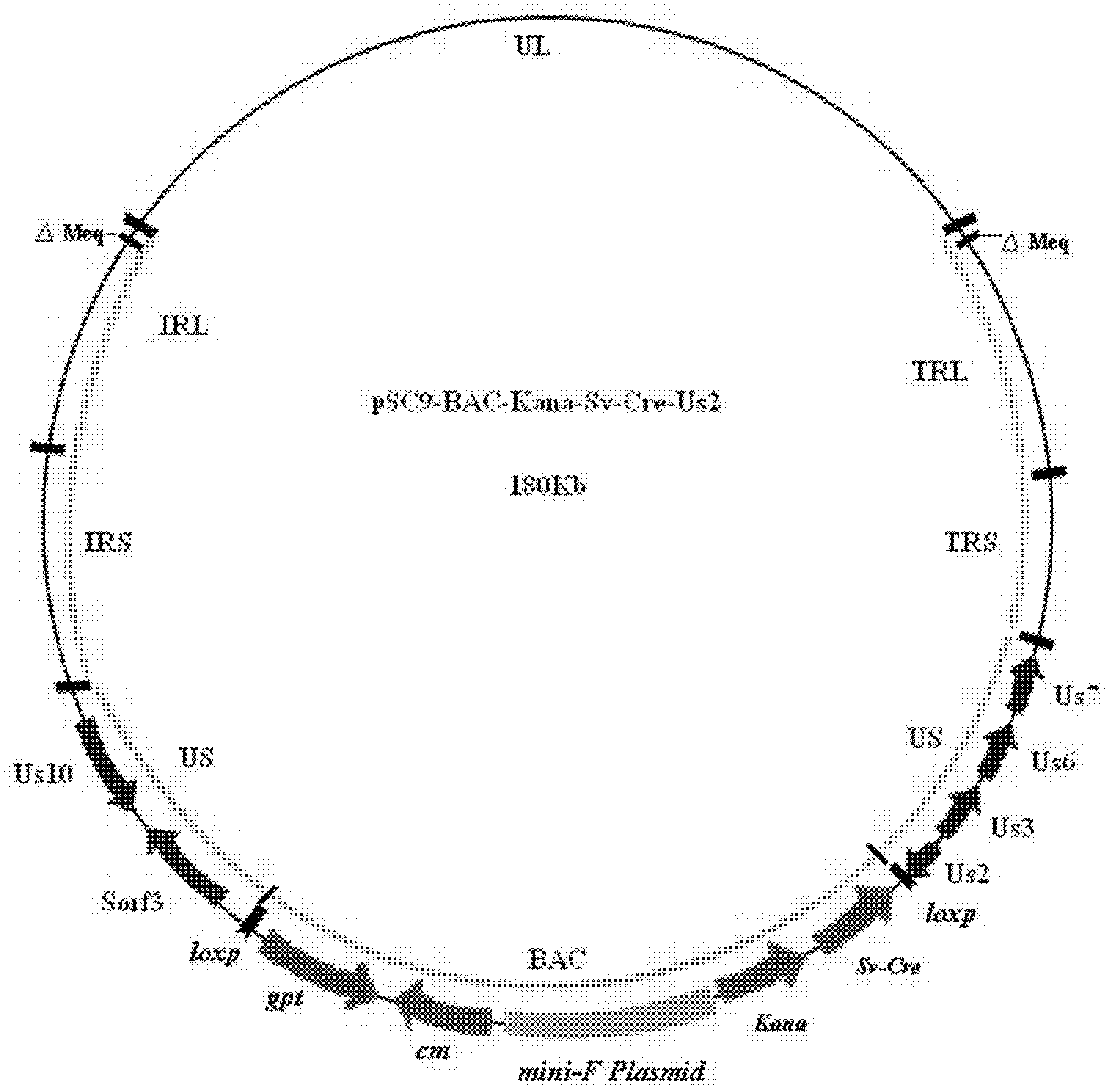
Construction and application of recombinant Chicken Marek's Disease Virus SC9-1 strain and SC9-2 strain
The invention relates to a construction and an application of recombinant Chicken Marek's Disease Virus SC9-1 strain and SC9-2 strain. The recombinant virus construction method solves the technical problem that kanr gene cannot be knocked out again by a present method after kanr gene containing flp recognition sites at two ends is continuously used twice to knock out two specific functional genes on the same virus genome. The obtained recombinant virus MDV SC 9-1 strain and MDV SC 9-2 strain are used as production strains of Marek's Disease live vaccine. The prepared vaccine prevents the very virulent or emerging very virulent plus MDV induced chicken Marek's disease. The protective immunity effect of the vaccine is superior to CVI988 / Rispens strain vaccine which is mostly widely used in foreign and domestic markets at present. The antigenicity of the recombinant virus is more similar to that of Chinese epidemic strain than the antigenicity of the similar virus rMd5deltameq which has been published in the United State. The strains provided by the invention will not induce tumor and has no immune suppression effect. Therefore, the strains are more applicable to China.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Porcine epidemic diarrhea virus strain MY01 and application thereof
InactiveCN105462936AEpidemic preventionAvoid spreadingSsRNA viruses positive-senseViral antigen ingredientsDiseaseMicroorganism
The invention relates to a porcine epidemic diarrhea virus strain MY01 and application thereof and belongs to the field of porcine epidemic diarrhea virus vaccine reagents. The porcine epidemic diarrhea virus strain MY01 is collected in China General Microbiological Culture Collection Center under CGMCC No. 11495. The porcine epidemic diarrhea virus strain MY01 is higher in pathogenicity for pigs and good in immunogenicity, a deactivated oil emulsion vaccine prepared using the strain is safe and reliable, homologous attacking protection can be provided, protection is also provided for PEDV (porcine epidemic diarrhea virus) epidemic strains, higher immunity can be generated after immunization, inoculated pigs are significantly lower in morbidity and mortality, the immunization effect is superior than that of existing commercial vaccines in the market, and the strain has the advantage of competing with like products home and abroad, can effectively prevent the prevalence and spread of porcine epidemic diarrhea virus and reduce economic cost caused by the disease and has a promising application prospect.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
O-type aftosa synthetic peptide vaccine
ActiveCN101659695AImprove protectionFree from attackAntiviralsPeptide preparation methodsChemical synthesisPeptide vaccine
The invention provides O-type aftosa synthetic peptide vaccine, and in particular relates to polypeptide or polypeptide polymer thereof used in the vaccine as well as the vaccine containing the polypeptide or the polypeptide polymer thereof and a preparation method thereof. The polypeptide has amino acid sequences shown in SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3. The O-type aftosa synthetic peptide vaccine carries out chemical synthesis of potential antigen site peptide segments by carrying out sequencing of domestic aftosa epidemic strains to study the variation of the main antigen sites ofaftosa and combining with computer assistant to carry out antigen site analytical prediction. Candidate polypeptide antigens are screened out by carrying out large numbers of animal experiments and aftosa virus antigen sites are optimized according to the screening result; and T cell epitope and B cell epitope are effectively combined to improve the immune effects of the polypeptide antigens. TheO-type aftosa synthetic peptide vaccine can effectively cope with the antigen variation of aftosa virus and has ideal biosafety and easy large-scale synthesis, thereby having a good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Fusion protein of porcine pseudorabies virus and preparation method, application and vaccine of fusion protein
ActiveCN109134669AImprove antigen broad spectrumGood broad-spectrum antigenAntibody mimetics/scaffoldsVirus peptidesAntigenResearch Object
The invention relates to the technical field of biology, in particular to a fusion protein of porcine pseudorabies virus and a preparation method, application and vaccine of the fusion protein. The fusion protein of porcine pseudorabies virus comprises a gB section and a gD section, wherein the gB section is expressed by the nucleotide sequence shown in SEQ ID NO.1; and the gD section is expressedby the nucleotide sequence shown in SEQ ID NO.2. The sequences shown in SEQ ID NO.1 and SEQ ID NO.2 are the sequences obtained through contrast and analysis by selecting genes of classical strains and current epidemic strains as research objects, and codon optimization and modification are preformed on the sequences, so that the broad spectrum of fusion protein antigen is further improved and theantigen expression amount is increased. The invention further provides a preparation method and application of the fusion protein, and the vaccine for preparation.
Owner:天康生物制药有限公司
Meningococcal Outer Membarne Vesicles
OMVs targeted against specific epidemic strains can be highly effective in controlling localised outbreaks of disease. In combination with large-scale and reproducible manufacturing techniques, a vaccine can be rapidly produced after an outbreak. The invention provides a method for preparing a meningococcal outer membrane vesicle (OMV) vaccine, comprising the steps of: (i) identifying the serosubtype of a meningococcal strain associated with an outbreak of meningococcal meningitis; (ii) preparing OMVs from a meningococcal strain having the serosubtype identified in step (i) for use in vaccine manufacture. The method may comprise one or both of the further steps of (iii) formulating said OMVs as a vaccine; and (iv) distributing said vaccine in a geographical area affected by or likely to be affected by said outbreak. The meningococcal strain will typically be in serogroup B, but may be instead by in serogroup A, C, W135, Y, etc.
Owner:NOVARTIS AG
Influenza B virus Vero cell productive adaptive strain and preparation and application thereof
ActiveCN101619306AImprove protectionOvercome deficienciesInactivation/attenuationMicroorganism based processesTiterInfluenza vaccine
The invention provides an influenza B virus Vero cell productive adaptive strain and a preparation method and application thereof. The influenza B virus Vero cell productive adaptive strain is named as B / Yunnan / 2 / 2005va(B) with a preserved number of CGMCC No.2931. The virus strain can be continuously subcultured on the Vero cell, and a clotting titer can be kept over 1: 512. Through detecting, the virus strain is the influenza B virus, belonging to a Yamagata pedigree. The virus strain includes eight genetic fragments of the influenza B virus and has the characteristic of continuously producing the Vero cells. On the one hand, the virus strain is inoculated in the Vero cell, the supernate of a culture is collected to obtain a influenza B virus vaccine which has favorable immunogenicity and proactive effects through an experiment; on the other hand, the virus strain also can be used as a donor virus strain which is reassorted through a natural selective pressure or a reverse genetics to obtain the adaptive strain of an epidemic strain on the Vero cell, thereby an epidemic strain Vero cell influenza vaccine is prepared.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Recombinant porcine pseudorabies virus for expressing GP protein of porcine reproductive and respiratory syndrome virus, and application
ActiveCN110628730AInviolableStrong targetingSsRNA viruses positive-senseViral antigen ingredientsAntigenVirulent characteristics
The invention provides a recombinant porcine pseudorabies virus for expressing GP protein of a porcine reproductive and respiratory syndrome virus, and an application. A PRV virus strain genome is quickly edited through a Crispr / Cas9 gene editing technique and a Cre / lox recombination system, virulence genes gE, gI and TK of the PRV virus strain genome are subjected to fixedpoint deletion, and an antigenic gene of a NADC30-like strain is subjected to fixedpoint insertion at a gG position. According to the recombinant porcine pseudorabies virus for expressing GP protein of a porcine reproductiveand respiratory syndrome virus disclosed by the invention, non-transmembrane regional coding sequences of GP3 protein, GP4 protein, GP5 protein and GP6 protein of an epidemic PRRSV strain PRRSV NADC30-like are selected as antigenic genes for the first time, the kinds of antigens are more comprehensive, and the antigenic genes have higher applicability on current PRRSV epidemic situations, and arebetter in immunoprotection effects. A live vaccine provided by the invention can protect target animals from being invaded by PRV and PRRSV in a more pointed manner, and a powerful tool is provided for preventing and controlling epidemic situations of porcine pseudorabies and porcine reproductive and respiratory syndromes in China.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Recombinant PRRSV virus-like particles having immunogenicity and preparation thereof
ActiveCN109385435ABroad-spectrum cross-immunogenicityImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsSpecific immunityTransfer vector
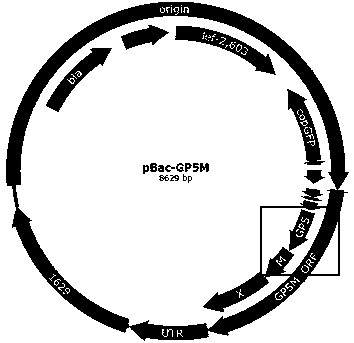
The invention discloses recombinant porcine reproductive and respiratory syndrome virus (PRRSV) virus-like particles (VLP) and a preparation method and an application thereof. Based on comparative analysis of GP5 of a PRRSV epidemic strain and an M gene sequence, a GP5 and M tandem sequence GP5M is synthesized artificially, the synthesized GP5M gene sequence is cloned into a vector with a pBAC5 plasmid as a skeleton, the baculovirus transfer vector pBAC-PRRSVGP5M is obtained, the recombinant bacmid rBacmid-GP5M is obtained, sf9 cells are transfected with the bacmid, and the recombinant baculovirus Ac-PRRSVGP5M is obtained. The PRRSV GP5 and M protein are expressed efficiently by the recombinant baculovirus, and the virus-like particles are formed. A subunit vaccine prepared by the proteinexpressed by the recombinant baculovirus can induce a body to produce a specific immune response after immunizing animals and can protect the pig body against the strong poison attacking of porcine reproductive and respiratory syndrome virus.
Owner:陕西诺威利华生物科技有限公司
Novel coronavirus multivalent antigen as well as preparation method and application thereof
PendingCN114369172AEfficient induction of immune responseEnhanced activation of the immune responseSsRNA viruses positive-senseViral antigen ingredientsAntigenReceptor
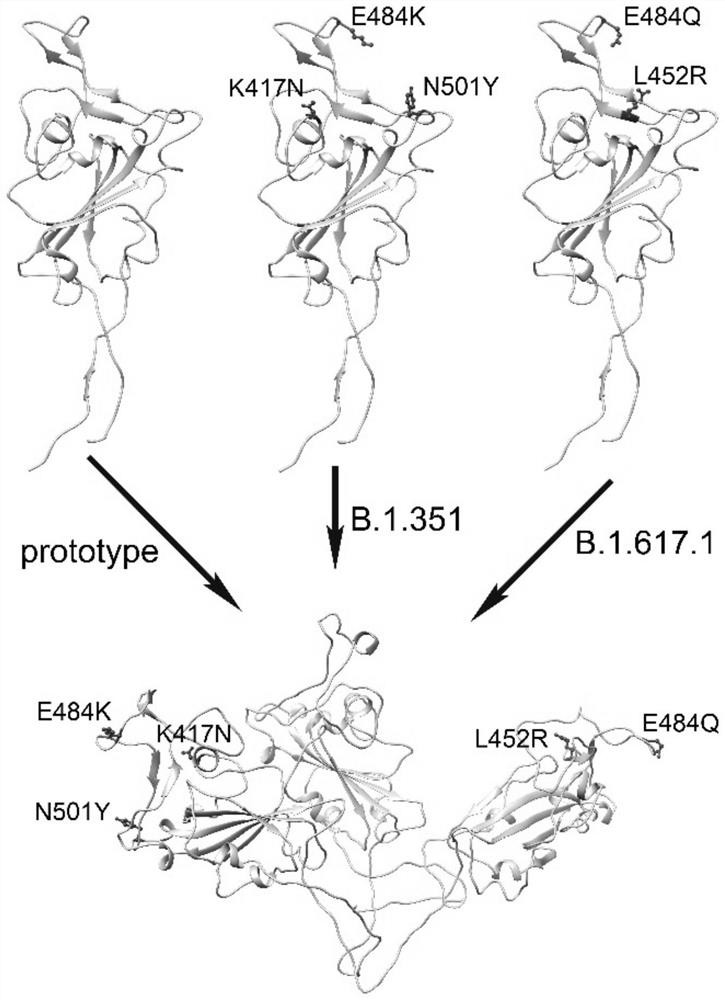
The invention relates to a novel coronavirus multivalent antigen as well as a preparation method and application thereof. The invention discloses a novel coronavirus antigen. The amino acid sequence comprises an amino acid sequence arranged according to a (A-B)-(A-B ') style or an amino acid sequence arranged according to a (A-B)-C-(A-B') style, A-B represents a partial amino acid sequence or a full-length amino acid sequence of a receptor binding region of the surface spike protein of the novel coronavirus, C represents a connecting amino acid sequence, and C represents a connecting amino acid sequence; a-B'represents an amino acid sequence obtained by substituting an amino acid sequence of A-B with one or more amino acids in K417N, E484K or N501Y. Compared with two original strain RBD protein tandem repeat dimers, the novel coronavirus multivalent antigen disclosed by the invention can activate a broad-spectrum protection antibody, and has a very good prevention effect on the original strains and current epidemic strains.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Meningococcal outer membrane vesicles
Owner:NOVARTIS AG
Influenza A virus Vero cell adapted strain and application thereof
ActiveCN101560503ATo satisfy the market's needsAvoid reduced vaccine efficacyInactivation/attenuationMicroorganism based processesHemagglutininReassortment
The invention discloses an influenza A virus Vero cell adapted strain and application thereof. The influenza A virus Vero cell adapted strain is named as A / Yunnan / 1 / 2005Va(H3N2), and the preserving number of the strain is CCTCC NO:V200514. The strain comprises an H3 subgroup hemagglutinin gene, an N2 subgroup neuraminidase gene and 6 internal genes for high yield of characteristic influenza viruses on Vero cells. The blood coagulation titer can be maintained at more than 1,024 by continuous subculturing of the strain on the Vero cells. The strain can be taken as a donor strain and subjected to genetic reassortment with an epidemic strain, or reverse genetics technology is adopted to perform genetic reassortment on the 6 internal genes and 2 surface protein genes of the epidemic strain to obtain the Vero cell adapted strain of the epidemic strain, and finally the adapted strain is used for preparing a Vero cell influenza vaccine or a Vero cell pandemic influenza vaccine.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Degenerate reverse transcription-polymerase chain reaction (RT-PCR) detection reagent and kit for hantavirus group
InactiveCN102382907AStrong specificityIncreased sensitivityMicrobiological testing/measurementOligonucleotide primersInverse polymerase chain reaction
The invention discloses a degenerate reverse transcription-polymerase chain reaction (RT-PCR) detection reagent and a kit for hantavirus group. The detection reagent comprises a pair of degenerate heterozygous oligonucleotide primers, and the sequences of the degenerate heterozygous oligonucleotide primers are respectively G1: GCAACAGCAACATGGTTTcartaytayac and G2: CTTCTTCATTCATATTTCCATGCarnccyttytc; the non-merger consensus sequence of the 5' ends in the primers plays a role in stabilizing the combination of a 3' merger core area and a template under the condition that the degeneracy of the primers is not increased, so that the specificity of the degenerate PCR reaction is improved, various viruses in hantavirus can be amplified and detected, the homologous unknown viruses of the extendedgenes can also be detected and the amplified target fragments can be sequenced by the detection reagent; and the kit are relatively high in sensitivity and good in hantavirus group specificity, can be used for detecting domestic popular Hantaan and Seoul viruses, and can also be used for detecting other oversea epidemic strains. The degenerate RT-PCR detection reagent and the kit for the hantavirus group are high in sensitivity, good in hantavirus specificity and universal.
Owner:中华人民共和国大榭出入境检验检疫局
Inactivated vaccine for preventing and treating paramyxovirus disease of pigeon and its prepn
InactiveCN101020054AQuality is not affectedReduce production capacityAnthropod material medical ingredientsViral antigen ingredientsDiseaseAdjuvant
The present invention proposes one kind of inactivated vaccine for preventing and treating type-I paramyxovirus disease of pigeon and its preparation process. The inactivated vaccine for preventing and treating type-I paramyxovirus disease of pigeon is prepared through screening out type-I paramyxovirus PA / PMV-I spawn GDP199902 CCTCC V200605 with powerful immunogenicity and high protecting rate against epidemic strain, inoculating un-immunized chick embryo, extracting PA / PMV-I antigen from dead chick embryo fluid, inactivating PA / PMV-I antigen, and adding adjuvant. The vaccine is used for preventing and treating PA / PMV-I disease of pigeon, and is specific, safe and effective.
Owner:广东省家禽科学研究所
Method for screening candidate bacterial strain from fish streptococcus agalactiae vaccine
ActiveCN102676683AHigh immune protection rateLarge range of protectionMicrobiological testing/measurementMicroorganism based processesGenotype AnalysisBacterial strain
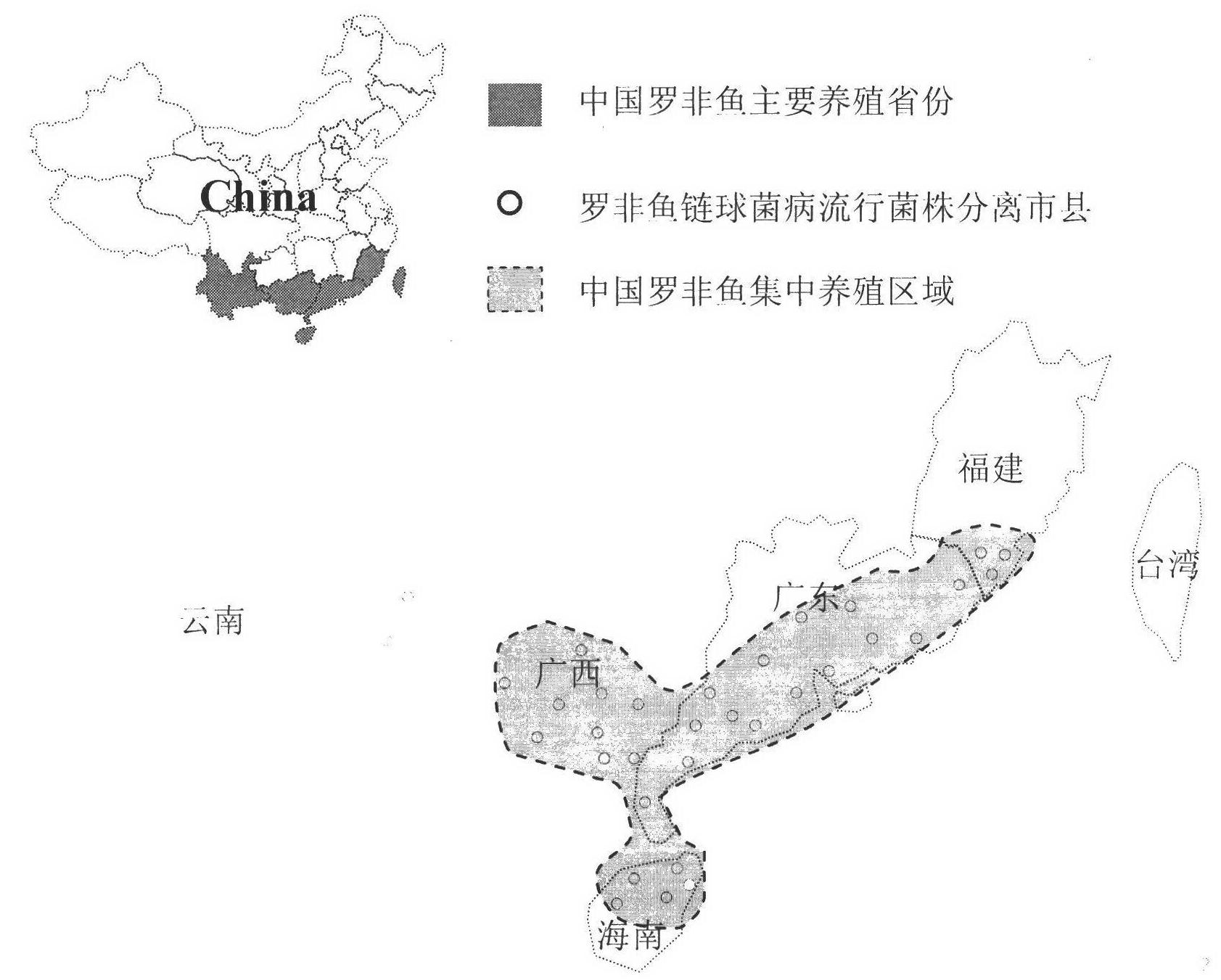
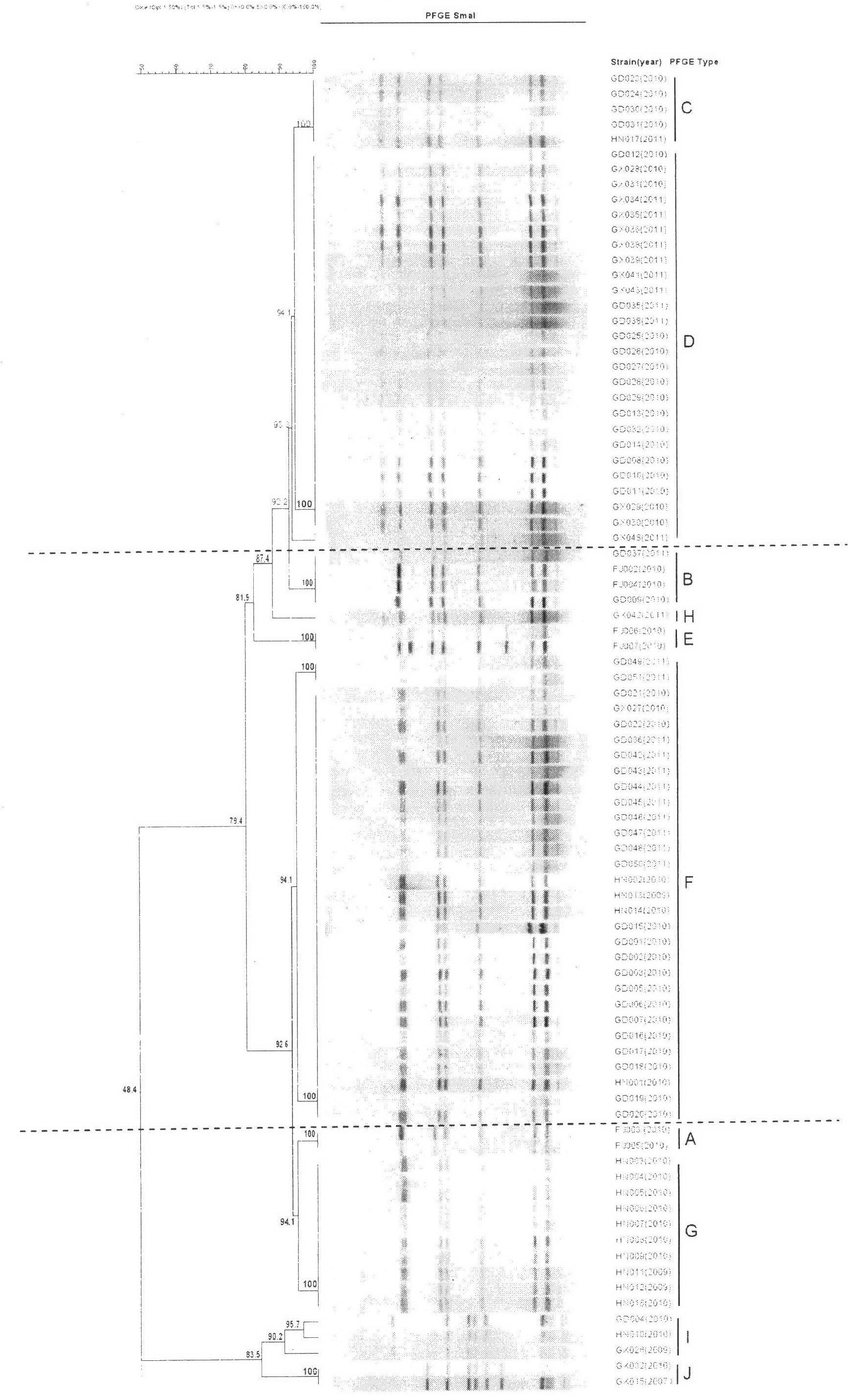
The invention discloses a method for screening candidate bacterial strains from fish streptococcus agalactiae vaccines. The method comprises the steps: separation and breed conservation of tilapia streptococcicosis agalactiae epidemic strains, identification of the epidemic strains and the establishing of a pathogenic library, PFGE genotype analysis of epidemic strains, toxicity determination of PFGE genotype representative strains, and immunogenicity and protection domain test of the PFGE genotype representative strains so as to obtain a candidate bacterial strains combination of a tilapia streptococcicosis agalactiae immunoprophylaxis vaccine in China. The technology has the characterizes of strong pertinence, high screening efficiency, remarkable effect and the like, and the screen candidate bacterial strains combination of the tilapia streptococcosis agalactiae immunoprophylaxis vaccine in China can protect 90% of genotypes and 96.47% of epidemic strains in the pathogenic library. The technology provides a new method for screening the candidate bacterial strains from the fish streptococcus agalactiae vaccines, is suitable for screening the candidate bacterial strains from the fish streptococcus agalactiae vaccines, and has significance and using value in immune prevention and control on fish streptococcicosis.
Owner:GUANGXI INST OF FISHERIES
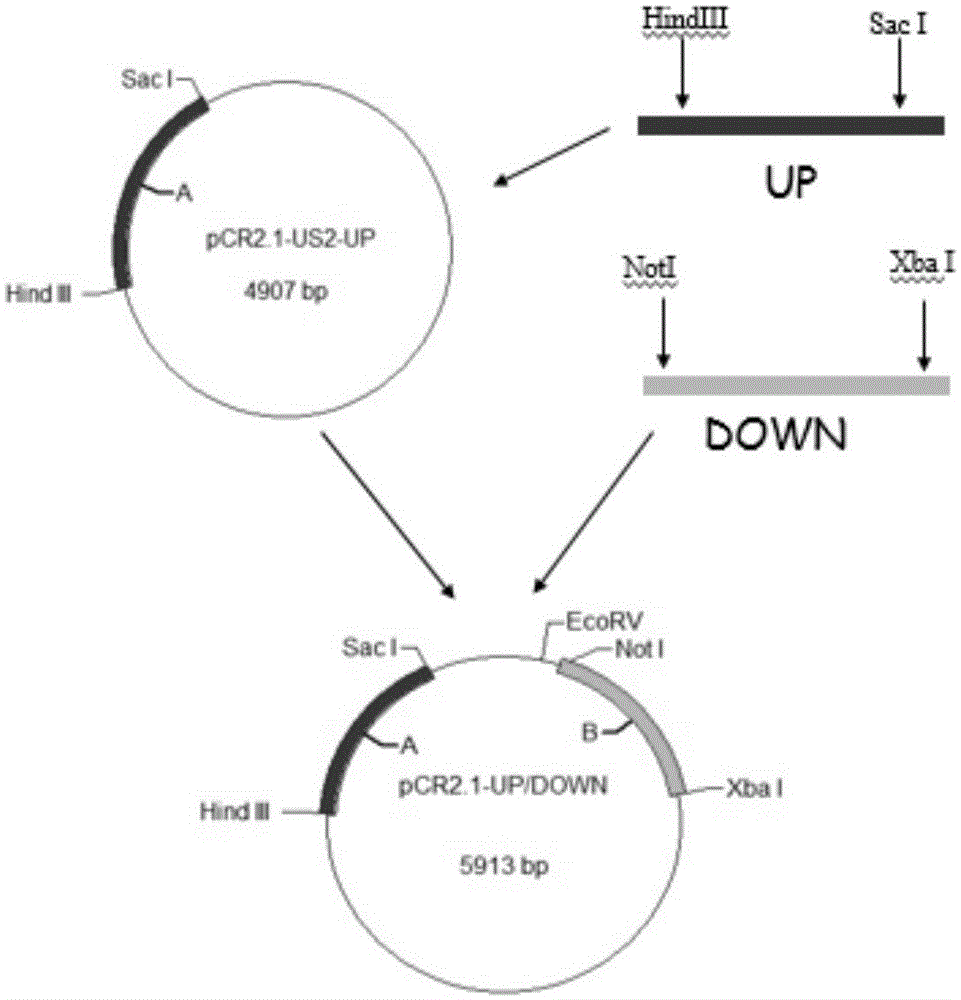
RHVT (recombinant Herpesvirus of Turkey)-H9HA (H9 hemagglutinin) and construction method thereof
InactiveCN105002146AMicroorganism based processesViruses/bacteriophagesHemagglutininAvian influenza virus
The invention relates to an rHVT (recombinant Herpesvirus of Turkey)-H9HA (H9 hemagglutinin) for expressing H9 subtype AIV (avian influenza virus) HA protein and a construction method thereof. The collection number of a vaccine strain rHVT-H9HA is CGMCC No: 10907. A GFP (green fluorescent protein) expression cassette is separated from a carrier pEGFP-C1 (plasmid enhanced green fluorescent protein) and is inserted into an HVT genome, and a recombinant virus rHVT-GFP is obtained. Through homologous recombination, a GFP gene of the rHVT-GFP is replaced with an HA gene of H9N2 subtype AIV epidemic strain A / Chicken / Jiangsu / WJ57 / 2012, a recombinant virus without fluorescence is selected, and the rHVT-H9HA for stably expressing the H9 subtype AIV HA gene is obtained. The recombinant virus strain is low in cost, good in safety, long in immunity period and suitable for large-scale production of vaccine and can be used for making the vaccine.
Owner:YANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com