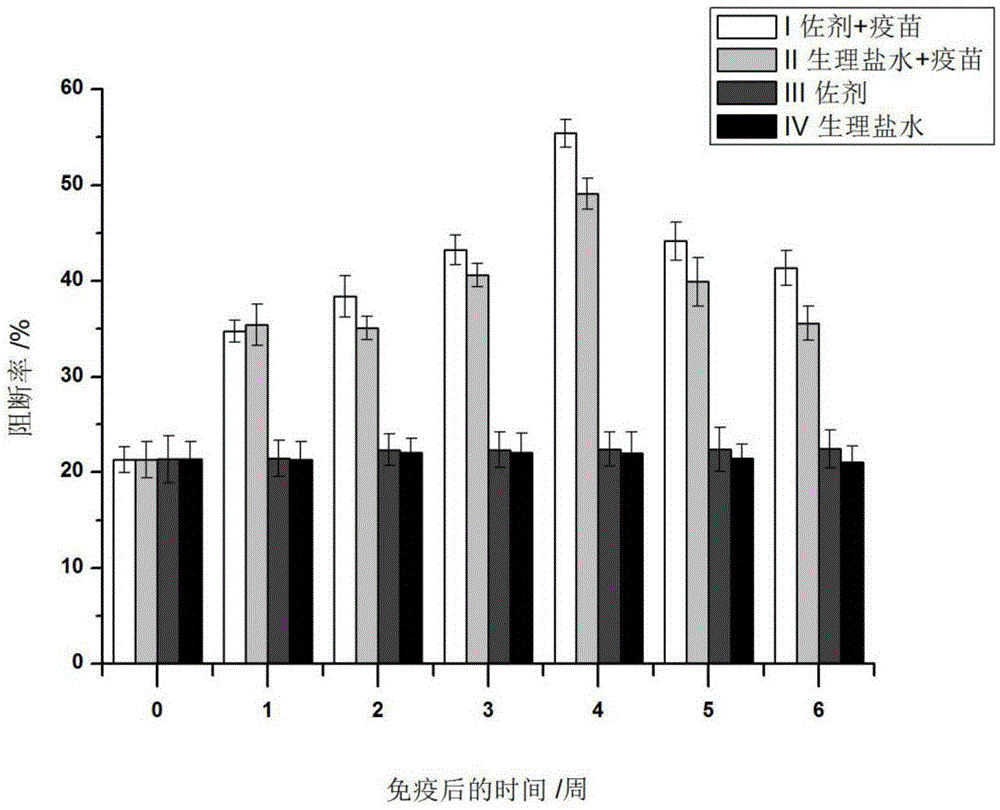
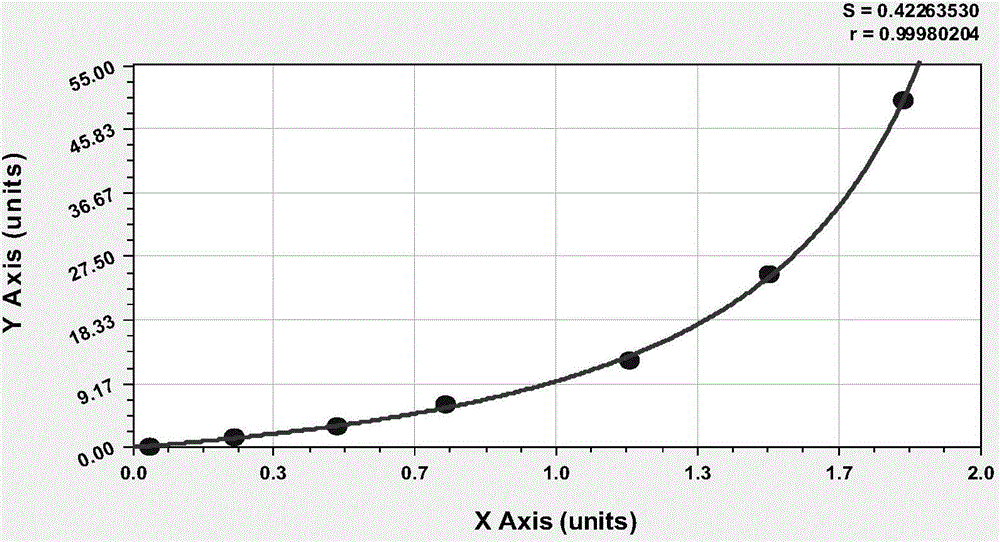
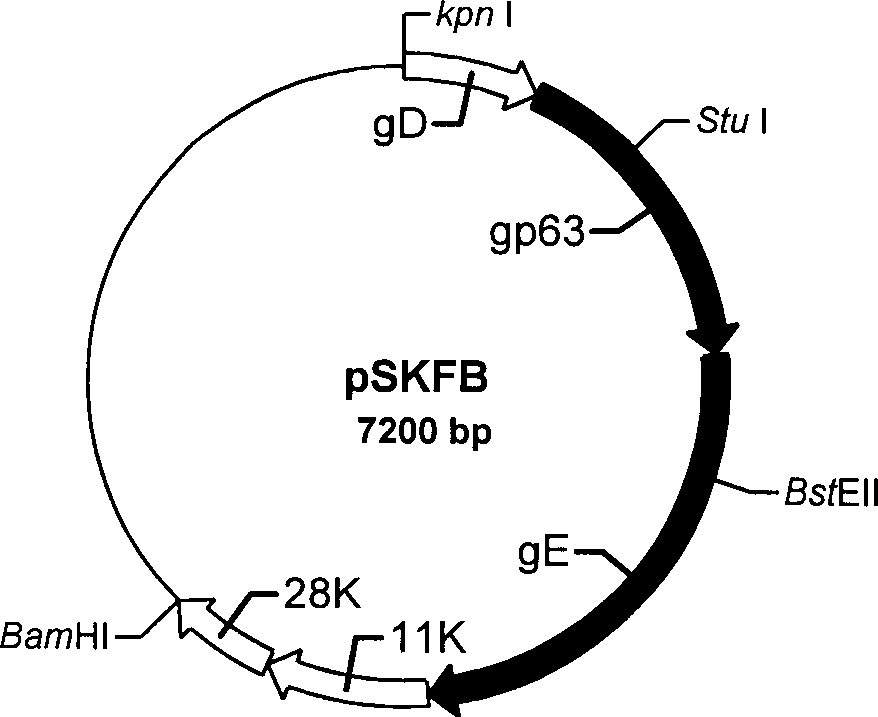
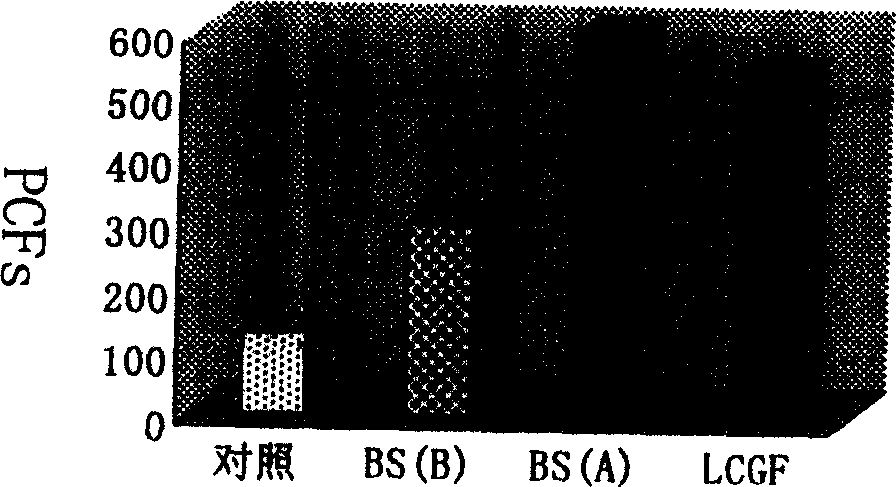
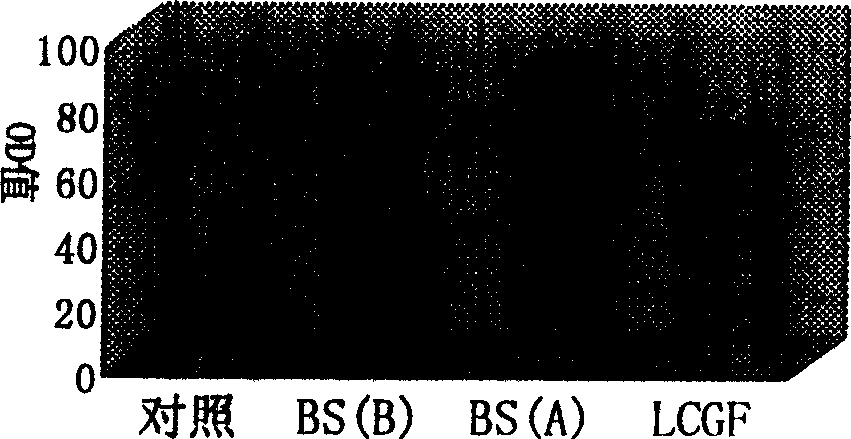
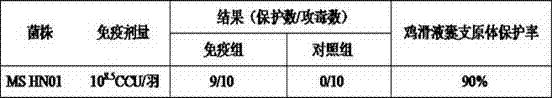
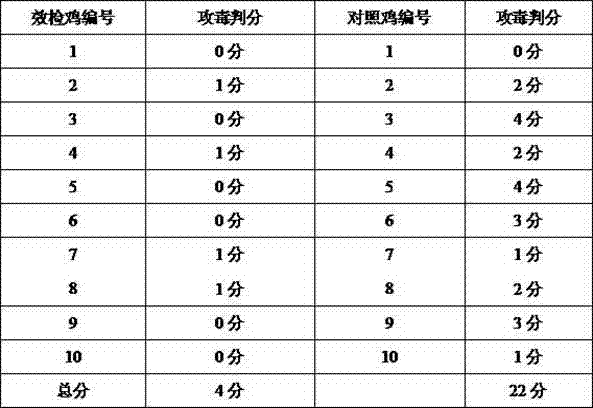
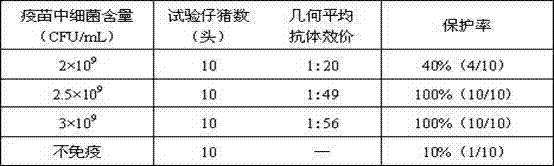
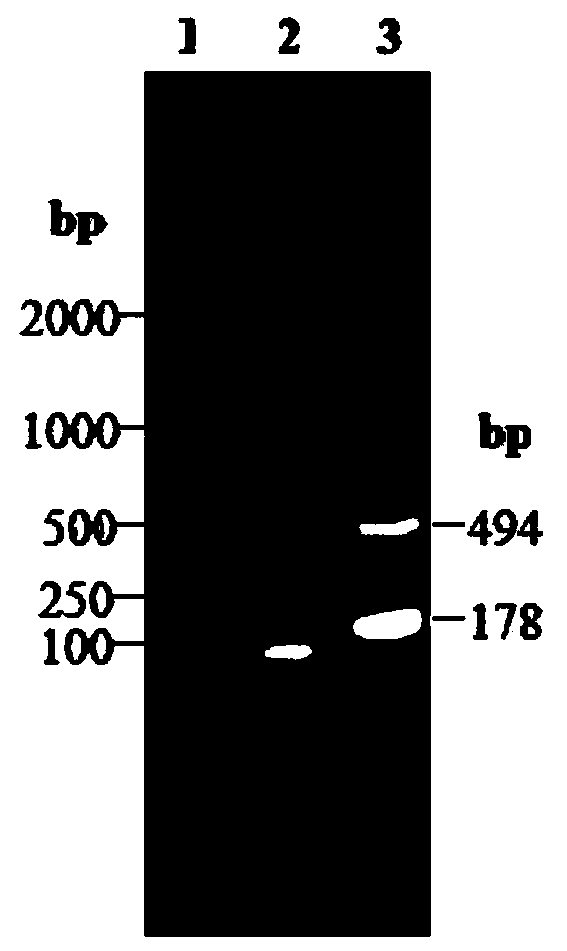
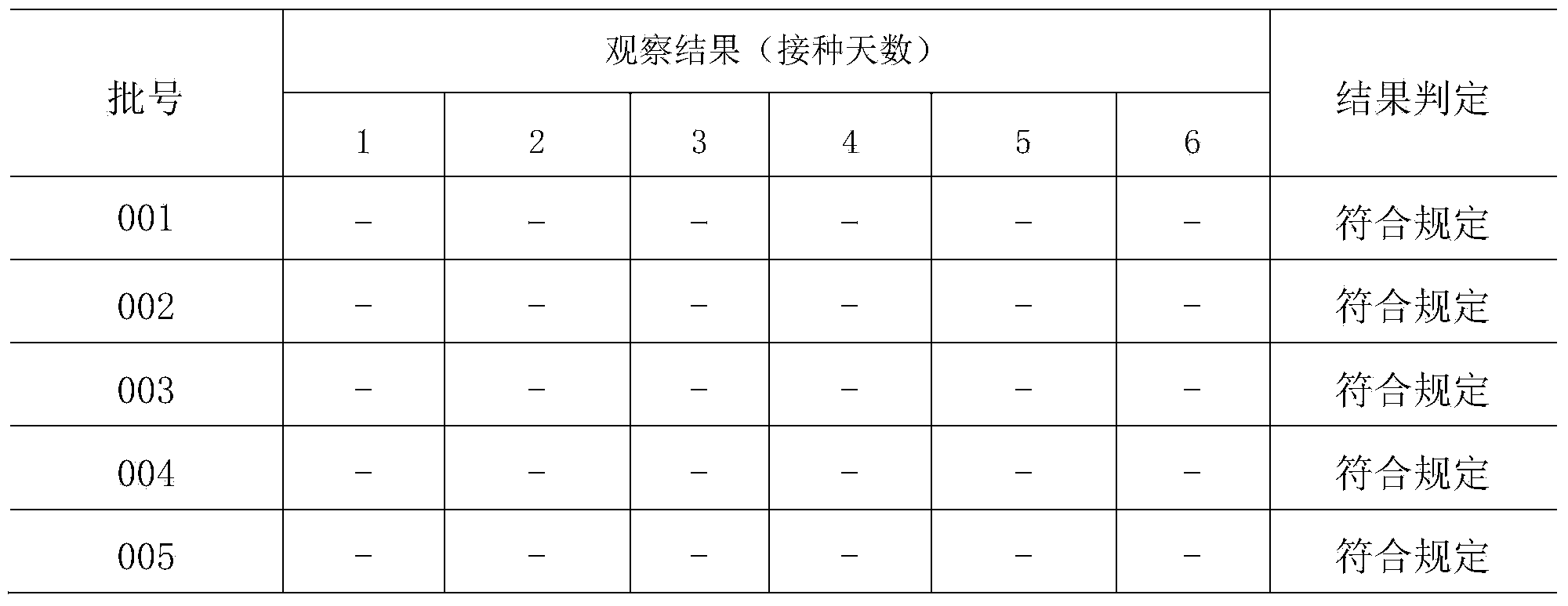
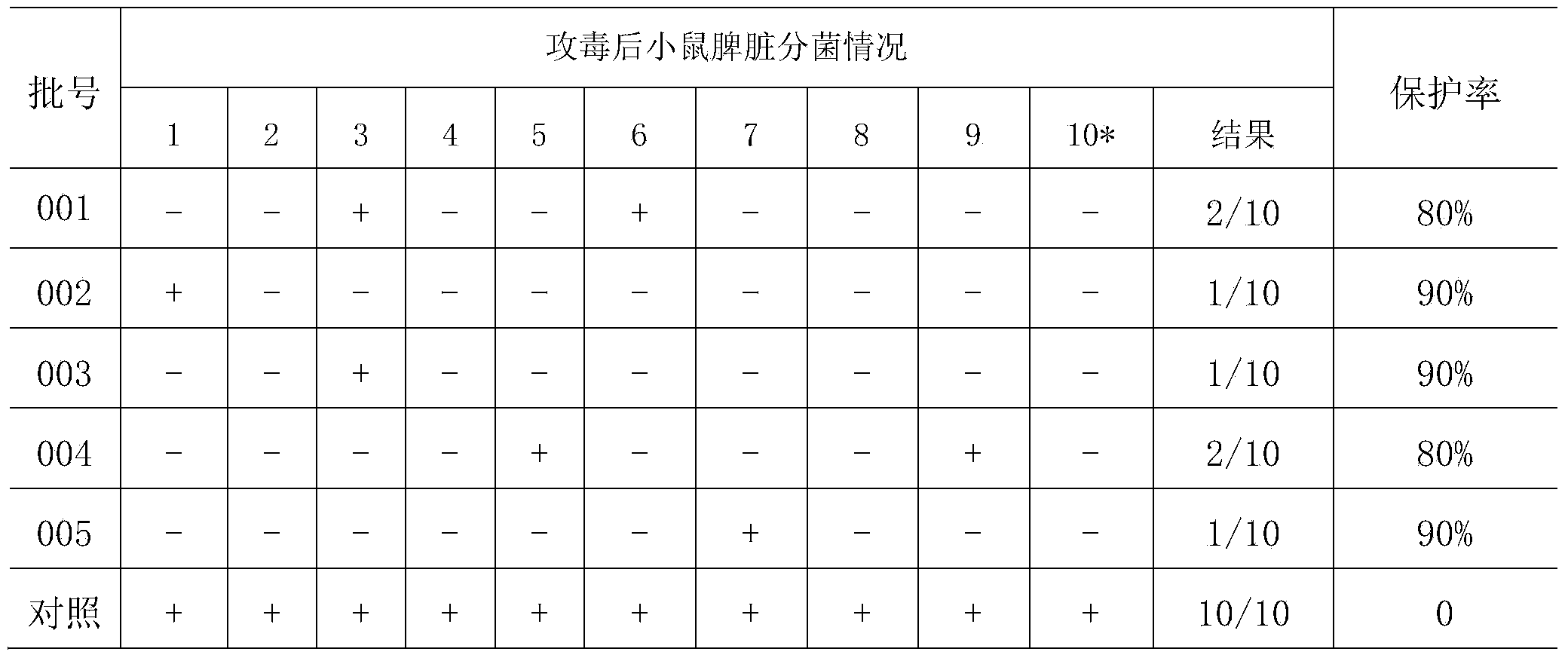
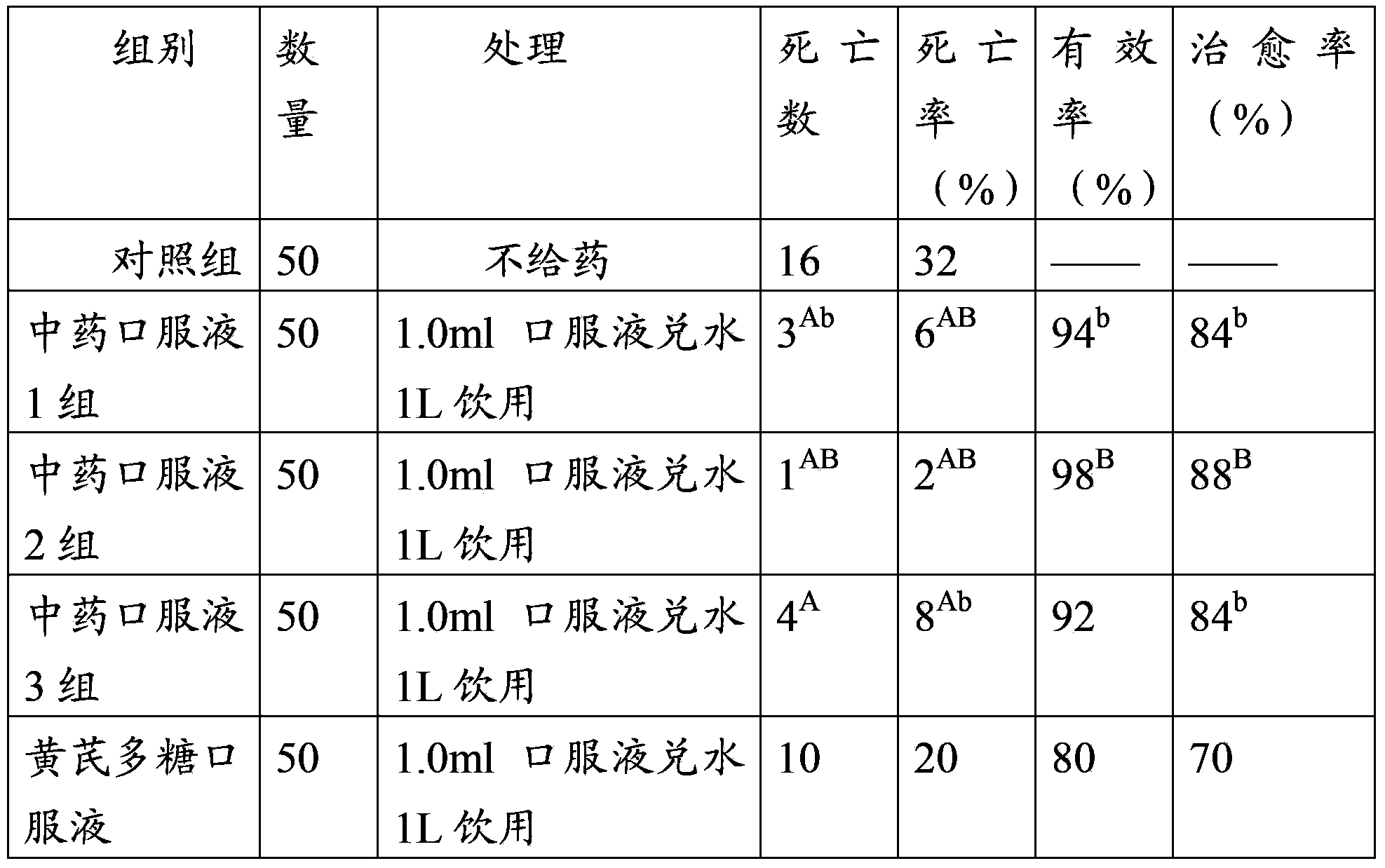
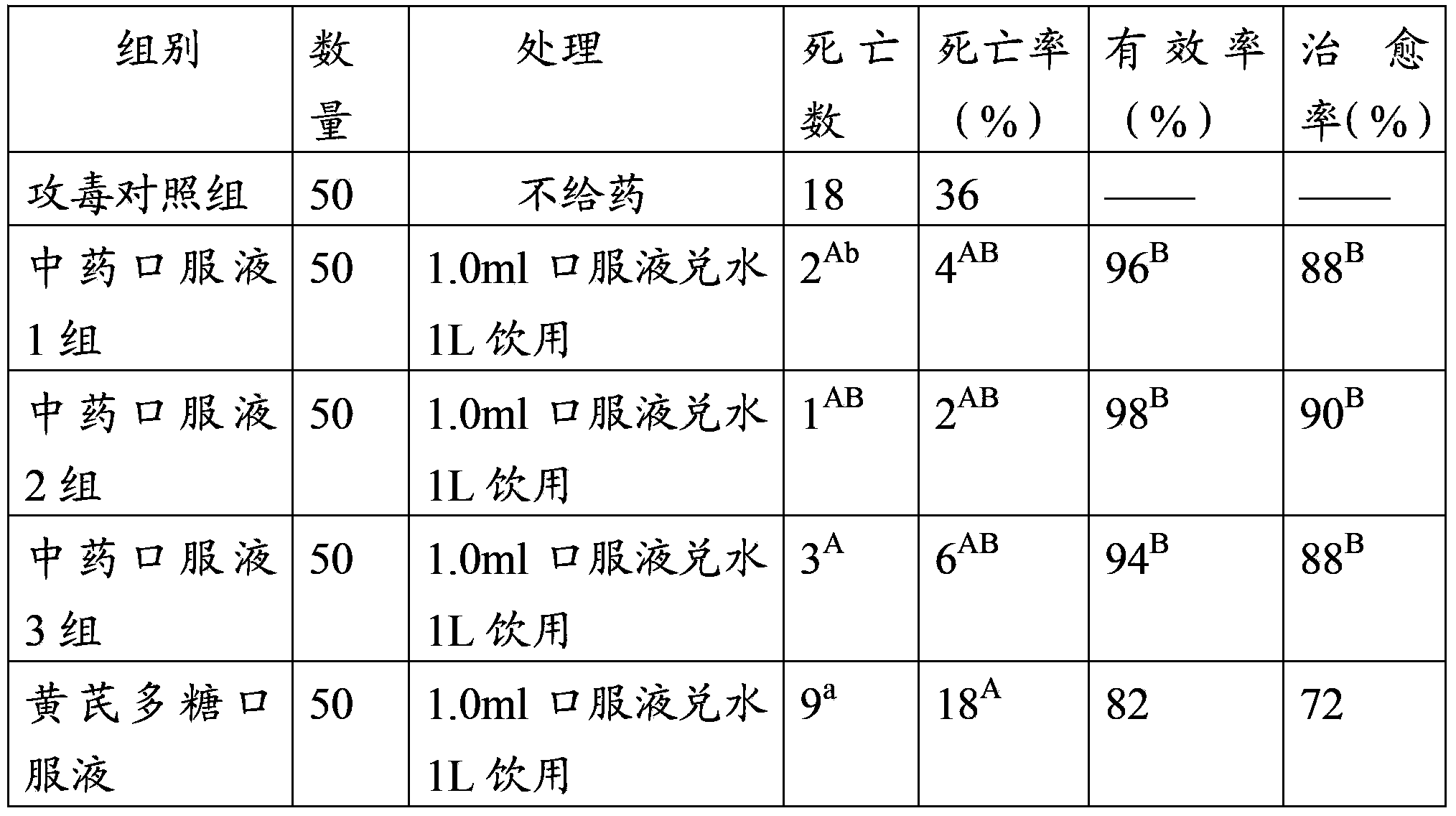
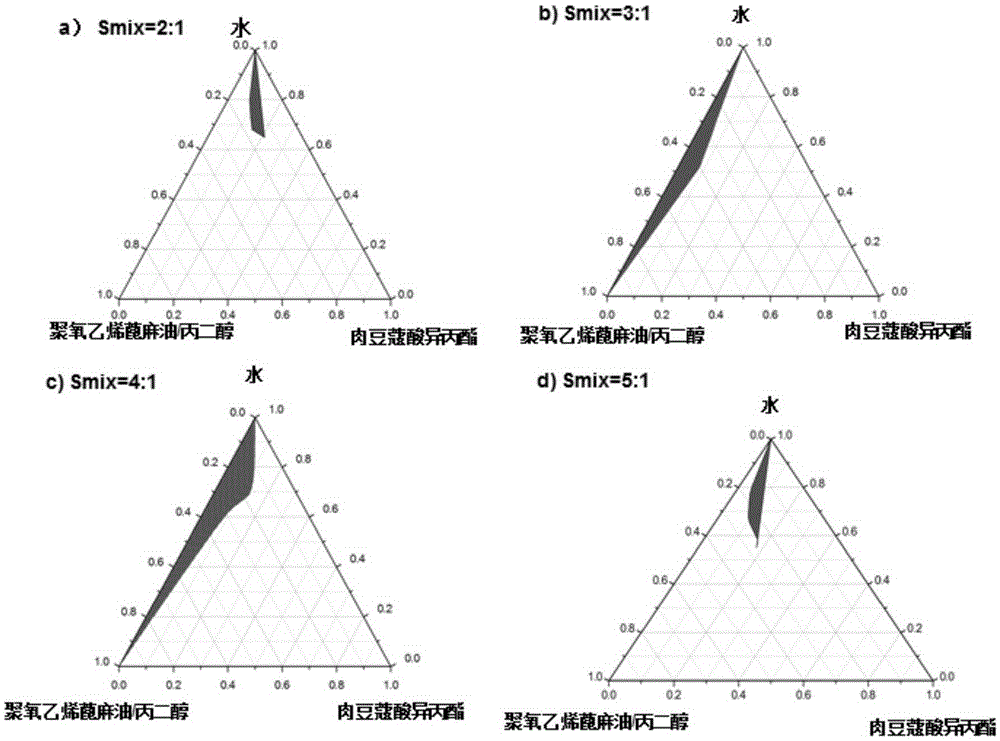
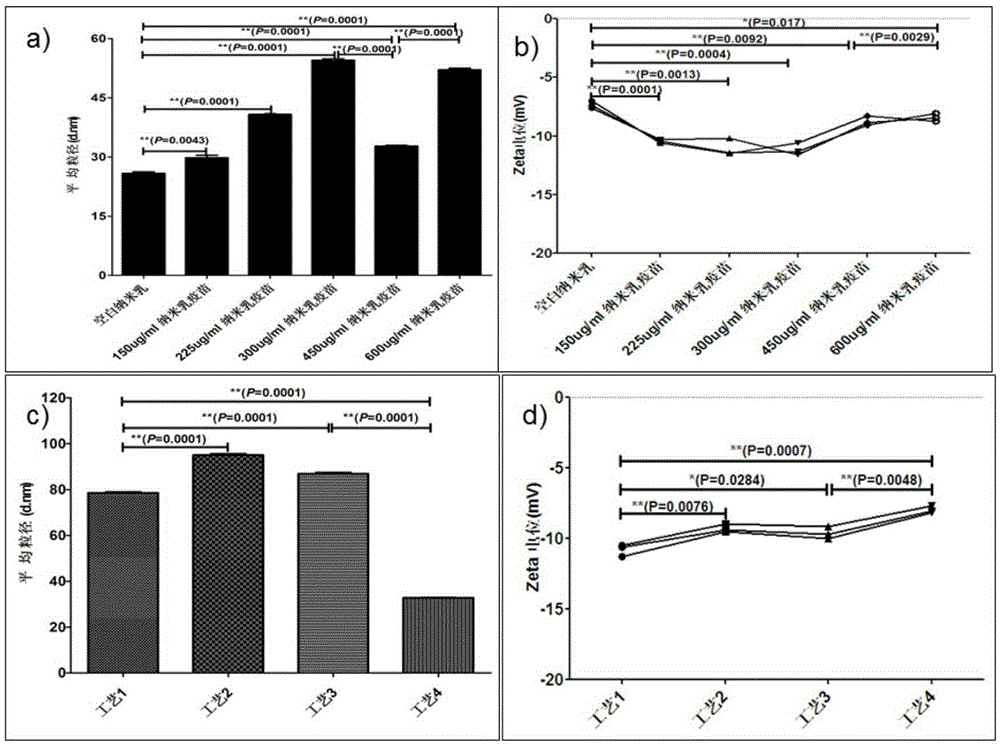
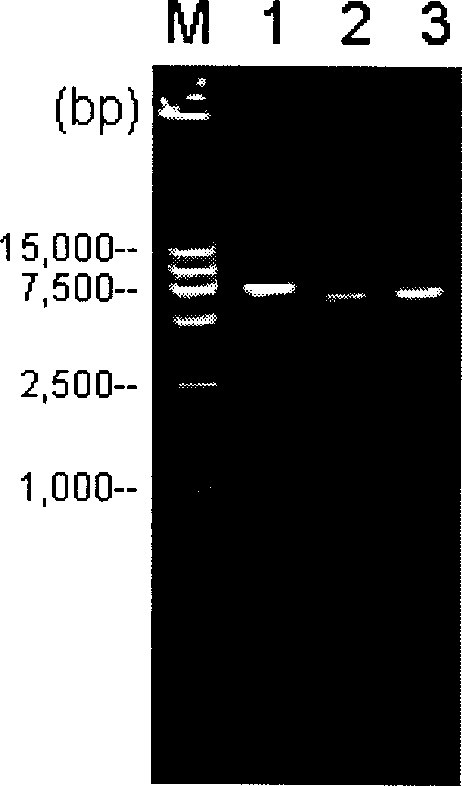Patents
Literature
1203 results about "Immune effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
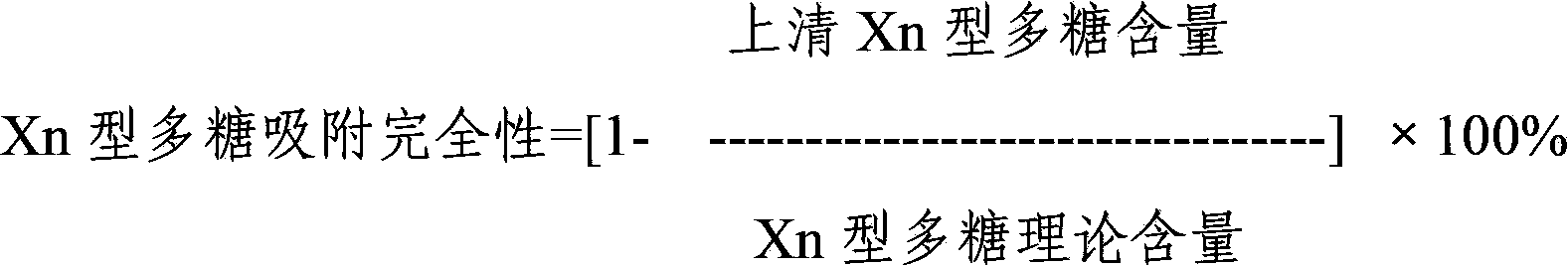
Immune effects are always temporary, and only offer immunity for the stated period or while the source is active: after this, the effect is removed. Immune effects on a hero belong to that specific hero, not to the controlling player.
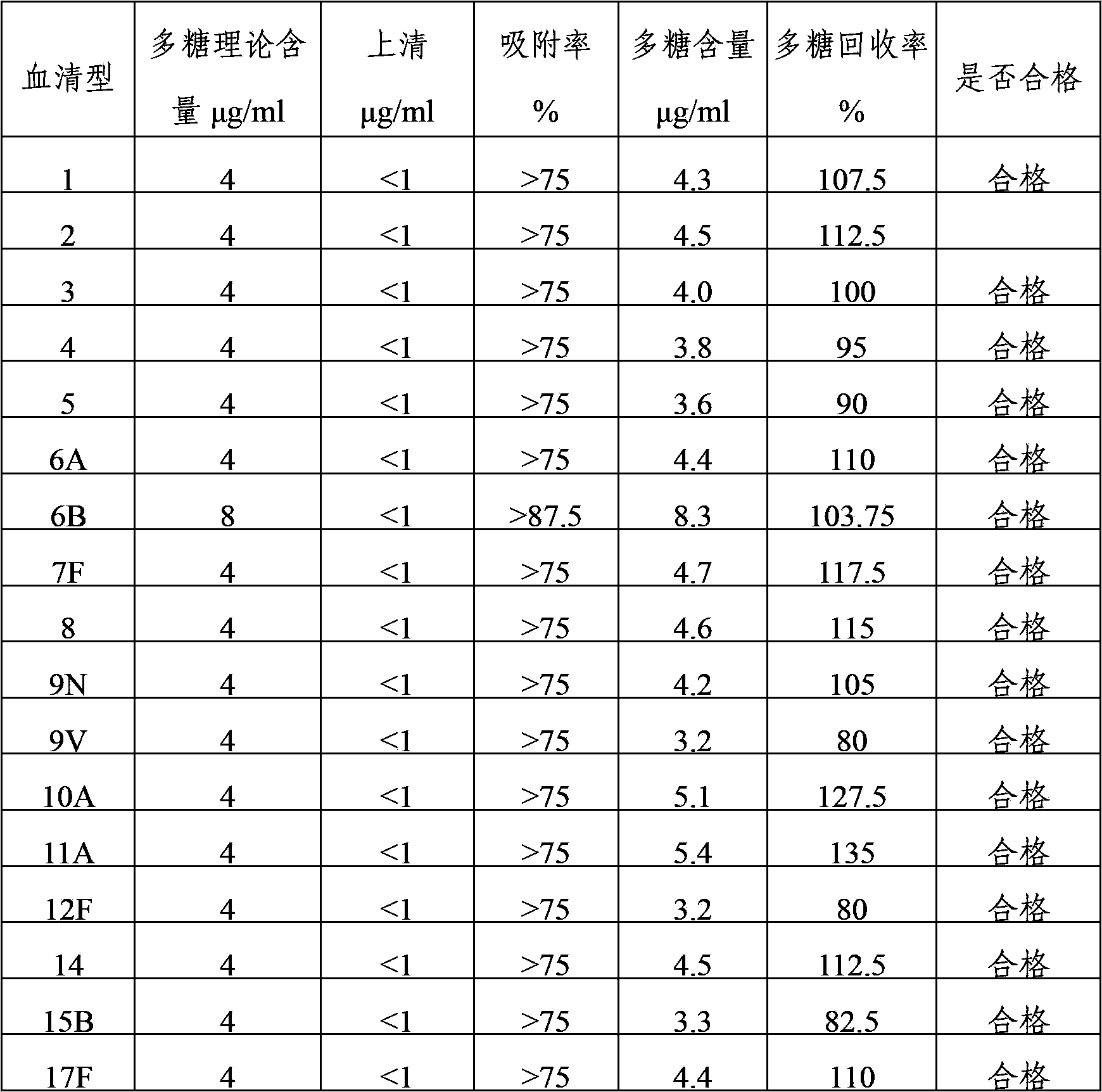
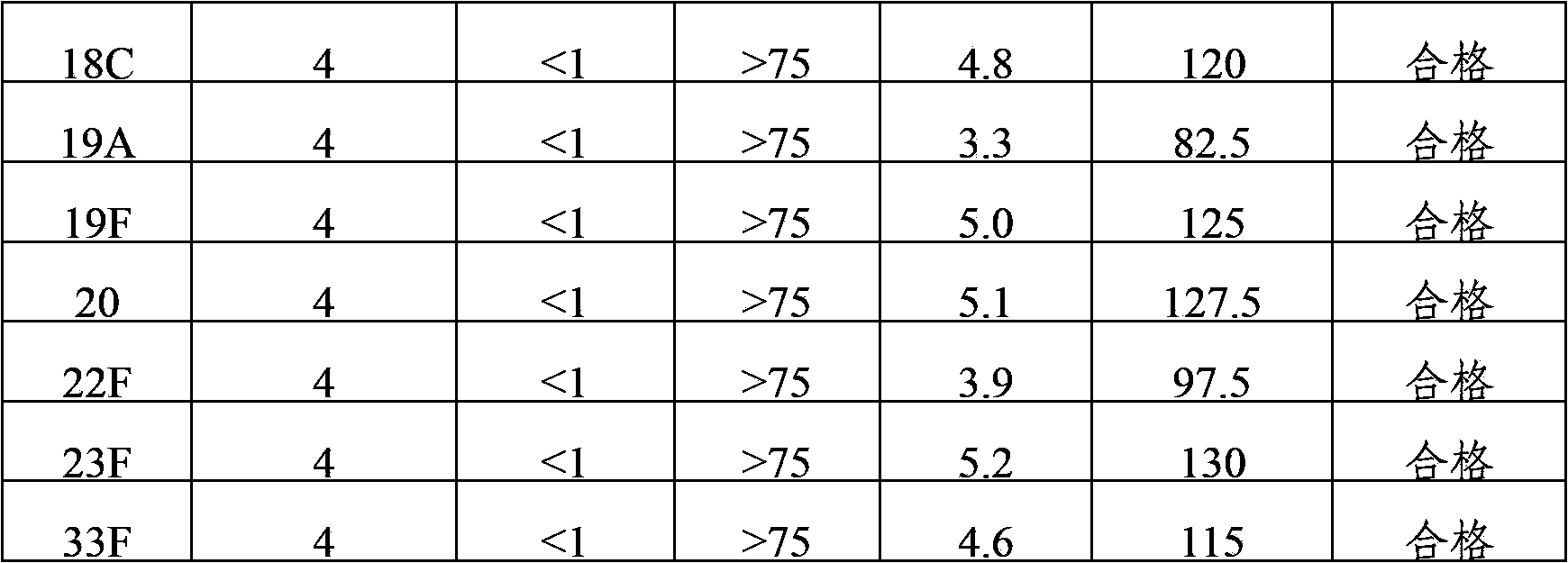
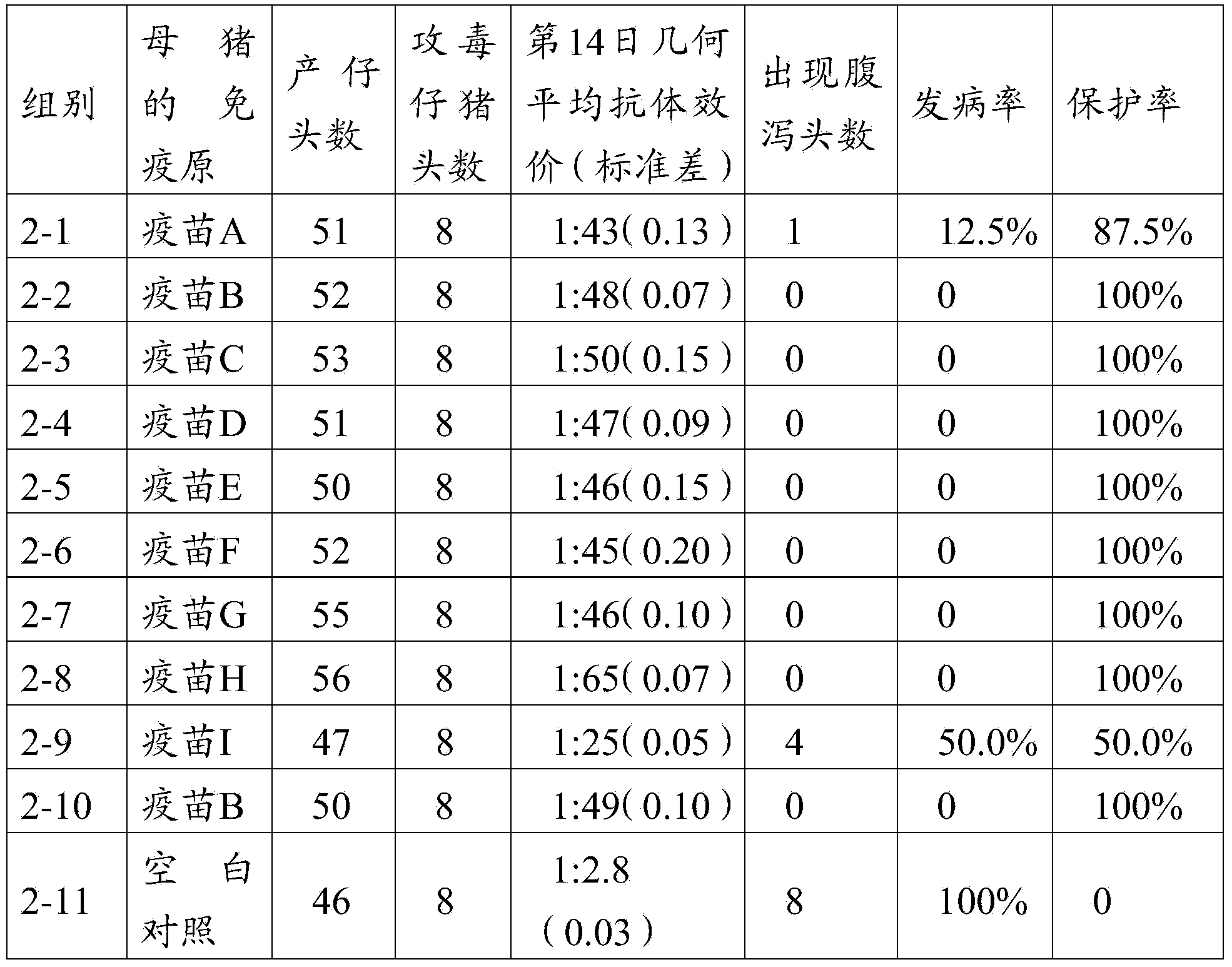
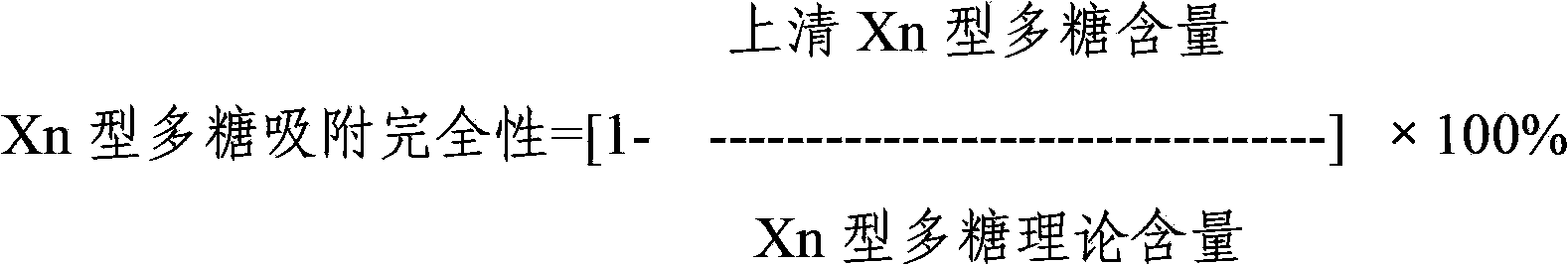
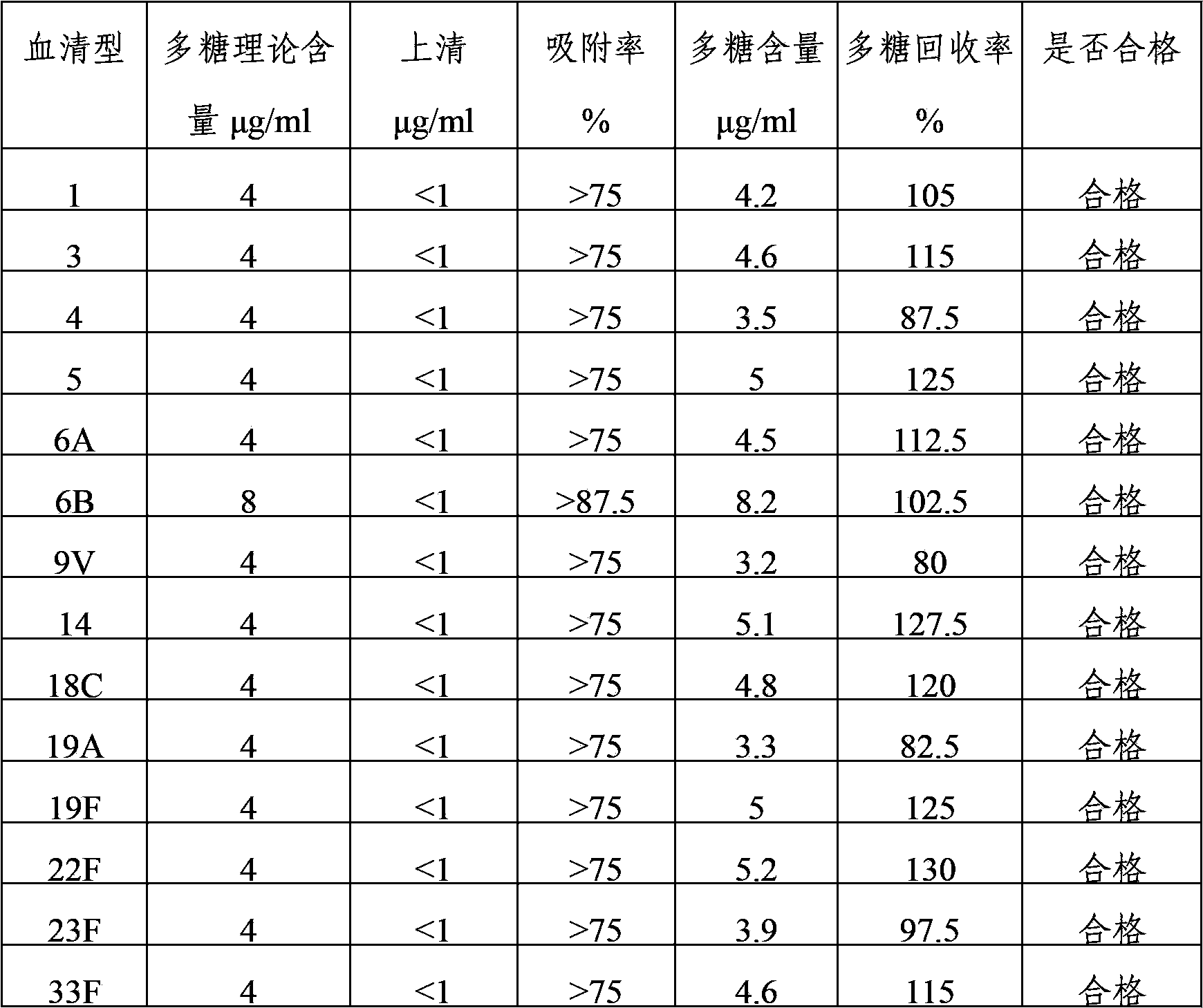
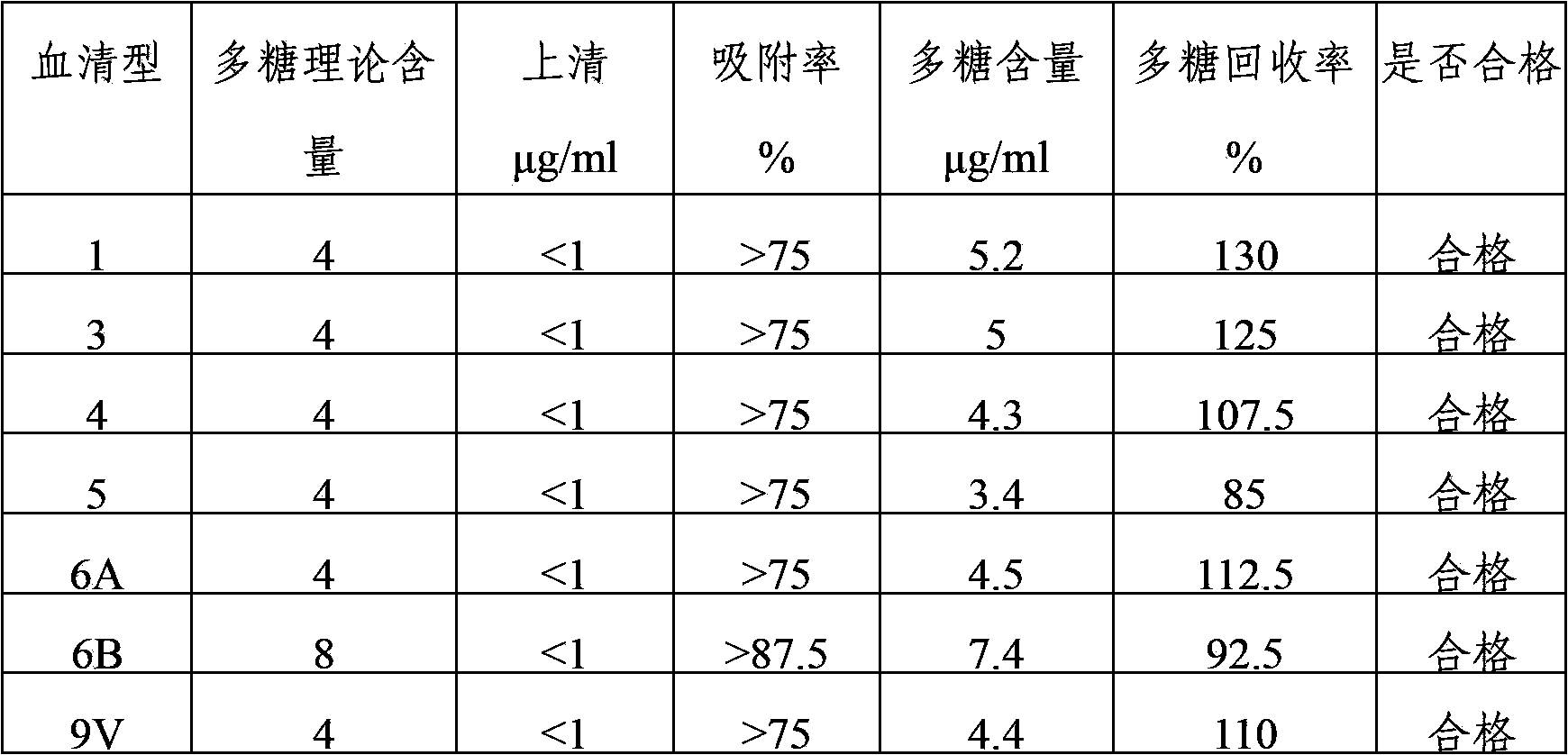
Multivalence pneumococcus capsular polysaccharide-protein conjugate composition and preparation method thereof
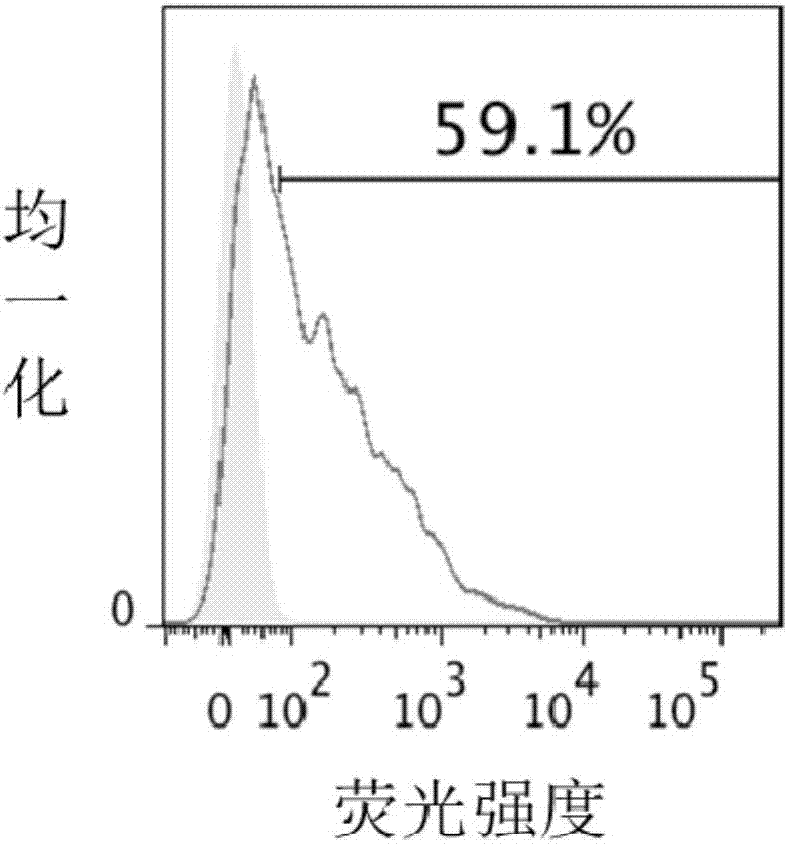
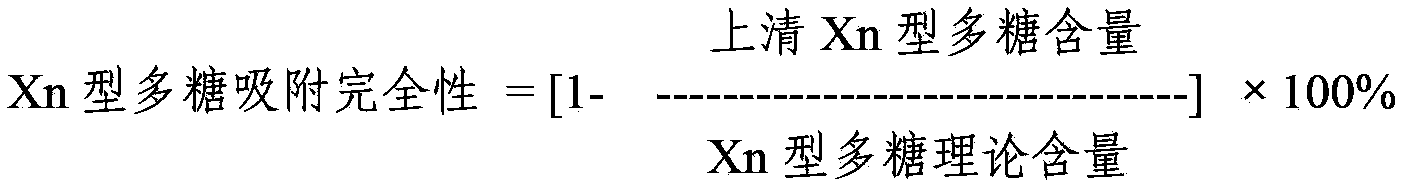
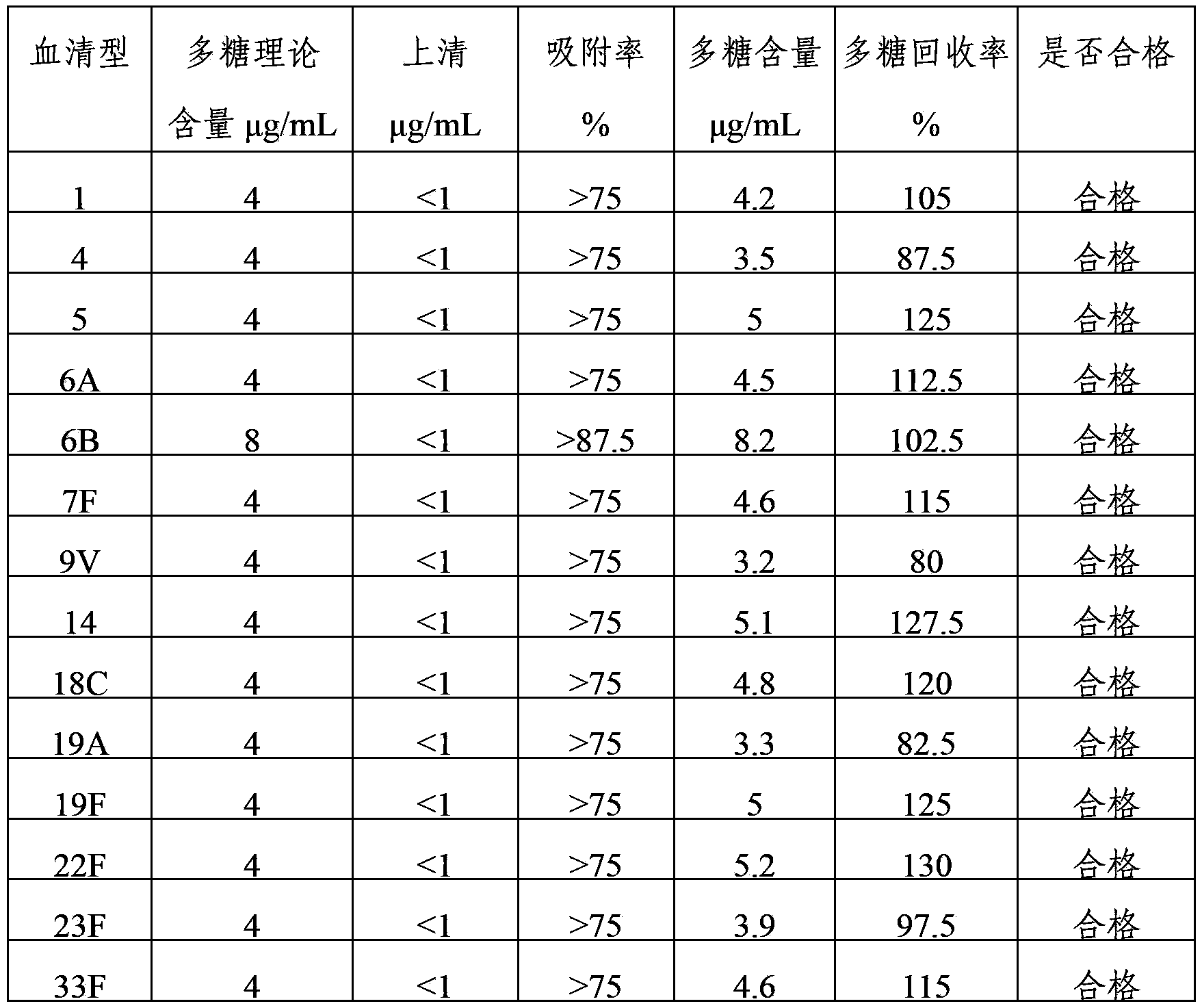
ActiveCN103656631AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsConjugate vaccineDisease
The invention provides a multivalence pneumococcus capsular polysaccharide-protein conjugate composition and a preparation method thereof. The conjugate composition is prepared from capsular polysaccharides of pneumococcus of 24 different serotypes and a carrier protein in a covalence connection manner, wherein the 24 different serotypes are 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The conjugate composition is good in adsorption effect and stability, has multiple immunogenicity and protection properties aiming at invasion of pneumococcus of the 24 serotypes, and is superior to the low-valence pneumonia composition in the market, and the immune response is higher than that of uncombined compositions. By inoculating a multivalence pneumococcus capsular polysaccharide conjugate vaccine prepared from the conjugate composition, the inoculation injection times can be reduced, the immunization procedure can be simplified, and meanwhile human beings and animals can be effectively prevented from diseases resulted from the pneumococcus of the 24 serotypes, and the conjugate composition is wide in coverage range and good in immune effect.
Owner:SINOVAC RES & DEV
Vaccine adjuvant as well as preparation method and application thereof
ActiveCN104147599AHelp with immunityImprove the effectiveness of anti-virus protectionAntiviralsEmulsion deliveryImmune effectsMedicine
The invention discloses an oil-in-water type vaccine adjuvant as well as a preparation method and application thereof. The oil-in-water type nano-emulsion vaccine adjuvant comprises the following components in percentage by mass: 0.1-10 percent of oil phase, 0.1-10 percent of emulsifier, 0.1-3 percent of stabilizer, 0.1-3 percent of complexing agent and 0.01-10 percent of immunopotentiator. The preparation method comprises the following steps: (1) uniformly dispersing the immunopotentiator, the stabilizer and the complexing agent in water, thereby obtaining an aqueous phase; (2) mixing an oil phase and an emulsifier, thereby obtaining an oil phase; (3) slowly adding the oil phase into the aqueous phase, and continuously stirring, thereby forming a stable emulsion; (4) regulating the pH value of the emulsion, and fixing the volume to obtain a primary emulsion; and (5) performing high-speed shearing and high-pressure homogenizing on the primary emulsion. The vaccine adjuvant provided by the invention is simple in preparation, convenient to use and small in side reactions, is used for diluting vaccines, particularly swine fever live vaccines and is high in stability and good in immune effect.
Owner:HUAZHONG UNIV OF SCI & TECH +1
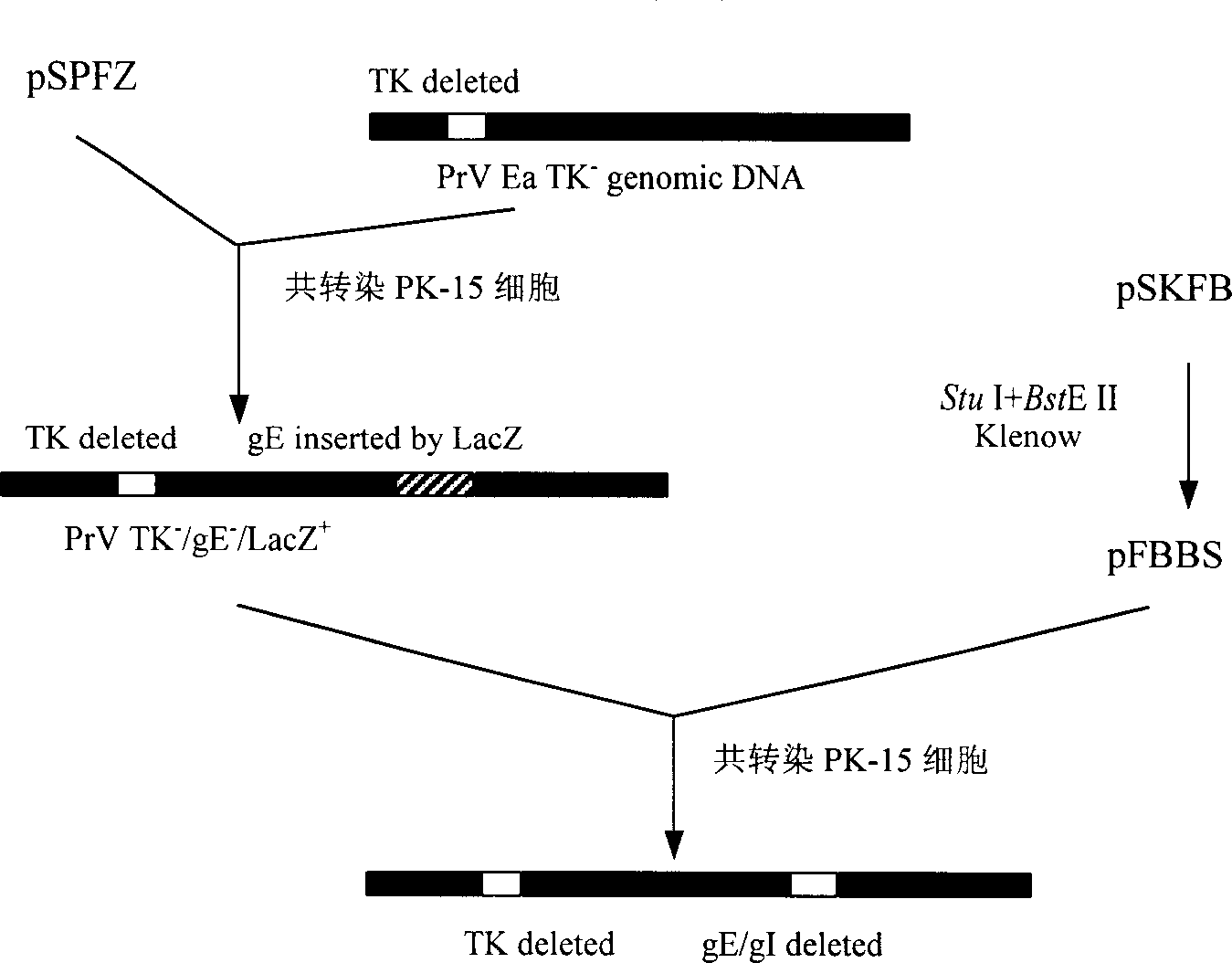
Pseudorabies TK*/gE*/gI* gene dificiency mark live vaccine and preparation method thereof
The present invention discloses a construction of three-gene defected recombinant pseudorabies virus (PrV) strain, vaccine prepared by said strain, method for constructing said strain and method for preparing said vaccine. The described recombinant pseudorabies virus strain defects genes of TK.gE ang gI, and contains no exogenous gene. The described vacine belongs to the freeze-dried live vaccine made up by virus liquid containing said invention and gelatin. Said invented live vaccine can be inoculated on the piglet, slaughter pig and sow with farrow, and can obtain obious immune effect. Said vaccine has good biological safety, and can be used for preventing and curing pseudorabies.
Owner:HUAZHONG AGRI UNIV
Targeted ligand-PEG (polyethylene glycol)-cholesterol/tocopherol derivative, and preparation method and application of derivative
InactiveCN103623416AGenetic material ingredientsPharmaceutical non-active ingredientsTumor targetCholesterol
The invention belongs to the field of pharmaceutic preparations, and relates to a targeted ligand-PEG (polyethylene glycol)-cholesterol / tocopherol derivative, and a preparation method and an application of the derivative, in particular to an application of the derivative in a composite mechanism mediate tumor targeted drug delivery system. According to the derivative, the preparation method and the application, a drug delivery system consisting of the targeted ligand-PEG-cholesterol / tocopherol derivative with a targeting function, a long circulating function and a pH (power of hydrogen) sensitive function as a functional material, a cation liposome and a drug can simultaneously carry anticancer polypeptide and gene / chemotherapy drugs. According to the system, different drugs are jointly entrapped into the lipid nano drug delivery system and delivered into a targeted cell by utilizing a physicochemical property difference between components or by in-vivo specific targeted recognition, signal conduction blocking and pH triggering PEG chain breaking charge reversion methods, so that a targeted tumor therapeutic effect is improved. According to the derivative, the preparation method and the application, the advantages of the system in directional delivery, co-delivery and the like are proved by in-vivo and in-vitro activity evaluation, the anticancer activity is improved significantly, and definite synergic therapeutic and immunity effects are provided.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV) and its application
InactiveCN102321639ANeutralizing activityViral antigen ingredientsInactivation/attenuationImmune effectsVirus-like particle
The invention relates to a preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV). The method comprises the steps of: modifying genetic elements of a structural protein encoding gene C-E3-E2-6K-E1 of CHIKV, cloning the modified genetic elements into the expression vector of an insect cell, then transfecting the obtained recombined expression vector and baculovirus linear DNA respectively to an SF9 insect cell and making the cell secrete and express CHIKV VLPs. Additionally, the invention also makes preliminary studies on the immune effects of CHIKV VLPs and applicationof CHIKV VLPs in virus specific antibody detection, thus laying a foundation for research and preparation of immunological detection reagents and even vaccines based on CHIKV VLPs.
Owner:中国疾病预防控制中心病毒病预防控制所
Targeted genetic engineering vaccine for African swine fever immune system
ActiveCN110760006APrevent Dependency (ADE) EffectsGood immune effectAntibody mimetics/scaffoldsVirus peptidesImmune effectsAfrican swine fever
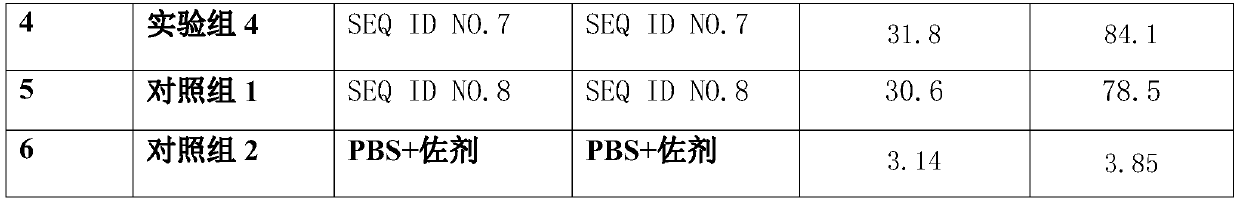
The invention belongs to the technical field of vaccines, and particularly relates to a targeted genetic engineering vaccine for an African swine fever immune system. The main raw material of the African swine fever immune system targeted genetic engineering vaccine is African swine fever fusion protein. The African swine fever fusion protein comprises a fragment selected from p72 protein, a fragment selected from p54 protein and a fragment selected from p30 protein. Wherein the fragment selected from the p72 protein at least comprises a sequence as shown in SEQ ID NO.1, the fragment selectedfrom the p54 protein at least comprises a sequence as shown in SEQ ID NO.2, and the fragment selected from the p30 protein at least comprises a sequence as shown in SEQ ID NO.3. The African swine fever fusion protein has the advantages of good immune effect and the like.
Owner:河南省生物工程技术研究中心 +1
African swine fever B and T cell tandem epitope fusion vaccine
InactiveCN111018995AGood immune effectAvoid the risk of accelerated viral infectionAntibody mimetics/scaffoldsViral antigen ingredientsAfrican swine feverTGE VACCINE
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever B and T cell tandem epitope fusion vaccine. The main component of the African swine fever B and T cell tandem epitope fusion vaccine is African swine fever tandem epitope fusion protein. The African swine fever tandem epitope fusion protein comprises a B cell neutralizing epitope peptidefragment and a T cell epitope; and the B cell neutralizing epitope peptide comprises the following fragments: at least one neutralizing epitope peptide of each of p72, p54, p30 proteins. When the African swine fever tandem epitope fusion protein is used as a vaccine, the immune effect is good; and the antibody level significantly higher than that of a control group can be detected after one immunization. Since the non-neutralizing epitope is reduced as much as possible in the fusion protein, the risk of accelerating virus infection (ADE effect and antibody dependence enhancement effect) by anon-neutralizing antibody after immunization can be avoided.
Owner:河南省生物工程技术研究中心 +1
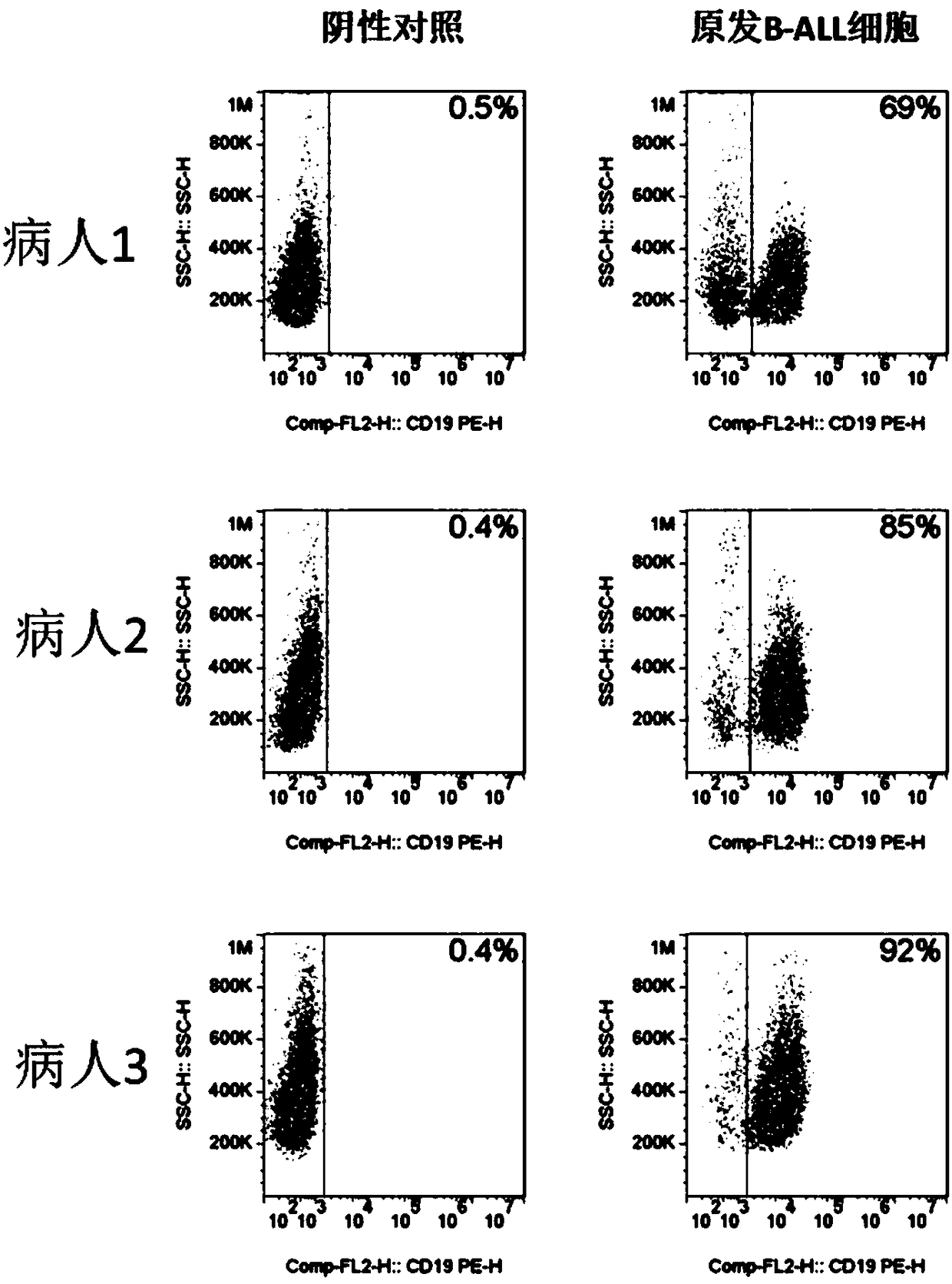
CD19-based chimeric antigen receptor and application thereof
PendingCN108383914ALess prone to stormsGood killing effectPeptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthSide effect
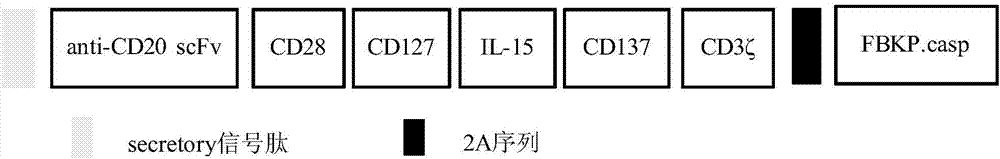
The invention relates to a CD19-based chimeric antigen receptor and application thereof, in particular to a lentivirus vector material built by a chimeric antigen receptor T (CAR-T) cell technology using a tumor specific target point CD19 as the basis, a method, and application thereof to anti-tumor treatment. The chimeric antigen receptor is formed by serially connecting an antigen combination structure domain, a membrane spaning structure domain, a costimulatory signal conduction region, a CD3 zeta signal conduction structure domain and an inducible suicide fusion structure domain, wherein the antigen combination structure domain is combined with the tumor surface antigen; the tumor surface antigen is CD19. The chimeric antigen receptor is subjected to specific gene transformation on theT cell stimulation signals. Compared with other chimeric antigen receptors, the chimeric antigen receptor provided by the invention has a better reaction effect and higher safety, so that the CAR-T cells have higher immune effects and low side effects; the treatment effect and safety of the CAR-T cells are enhanced.
Owner:BEIJING MEIKANG JIMIAN BIOTECH CO LTD
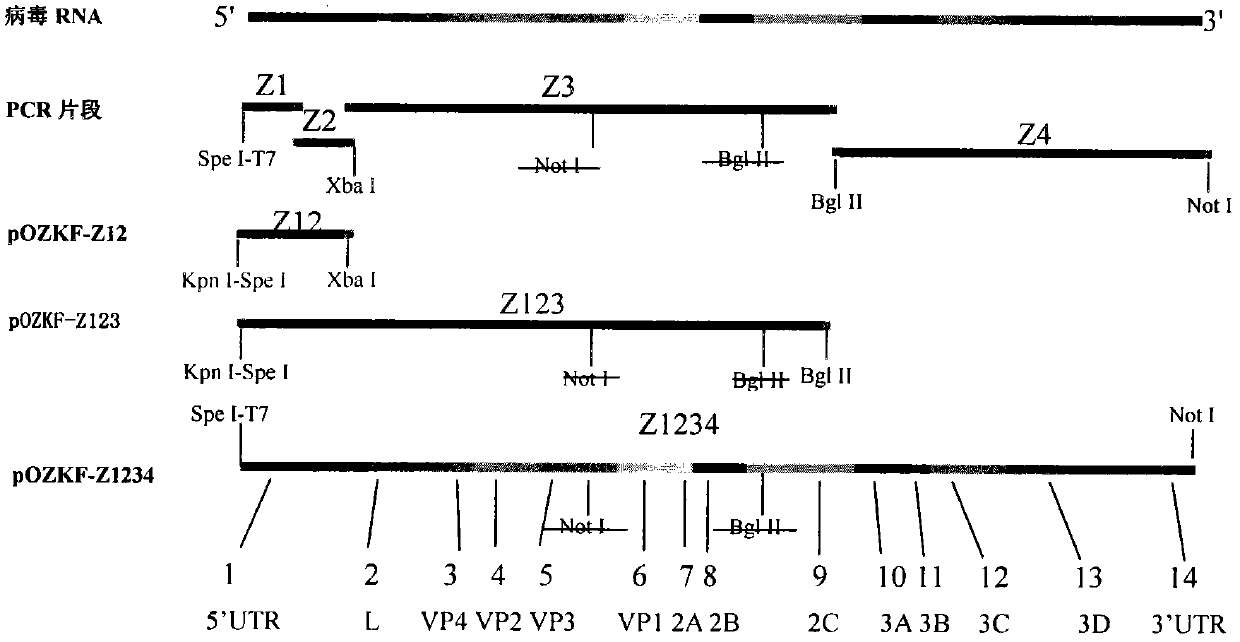
Method for expanding antigen spectrum of foot-and-mouth disease vaccine strain by reverse genetic operation and preparation method of vaccine
ActiveCN101948811AHigh protection rateBroad antigen spectrumVirus peptidesMicroorganism based processesImmune effectsSoutheast asia
The invention relates to a method for expanding the antigen spectrum of a foot-and-mouth disease vaccine strain by reverse genetic operation and a preparation method of a vaccine. The amino acid sequence of the VP3 and VP1 structural proteins of the foot-and-mouth disease virus strain of the invention is represented by the amino acid residues from a position 304 to a position 736 in SEQ ID No.4. Experiments show that the vaccine prepared from the mutant virus strain obtained by the invention can resist porcine epidemic viruses of China O / TL / Taiwan / 97 lineage, Pan-Asia O / China / 99 lineage and Southeast Asia Myanmar O / GS / 2010 / 98 lineage, has a characteristic of wide antigen spectrum, can immunize pigs and obviously improve the rate of protection against foot-and-mouth disease viruses which are of the same type and have antigenicity difference, achieves an immune effect of cross protection, and is expected to play an important role in the prevention and control of foot-and-mouth disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
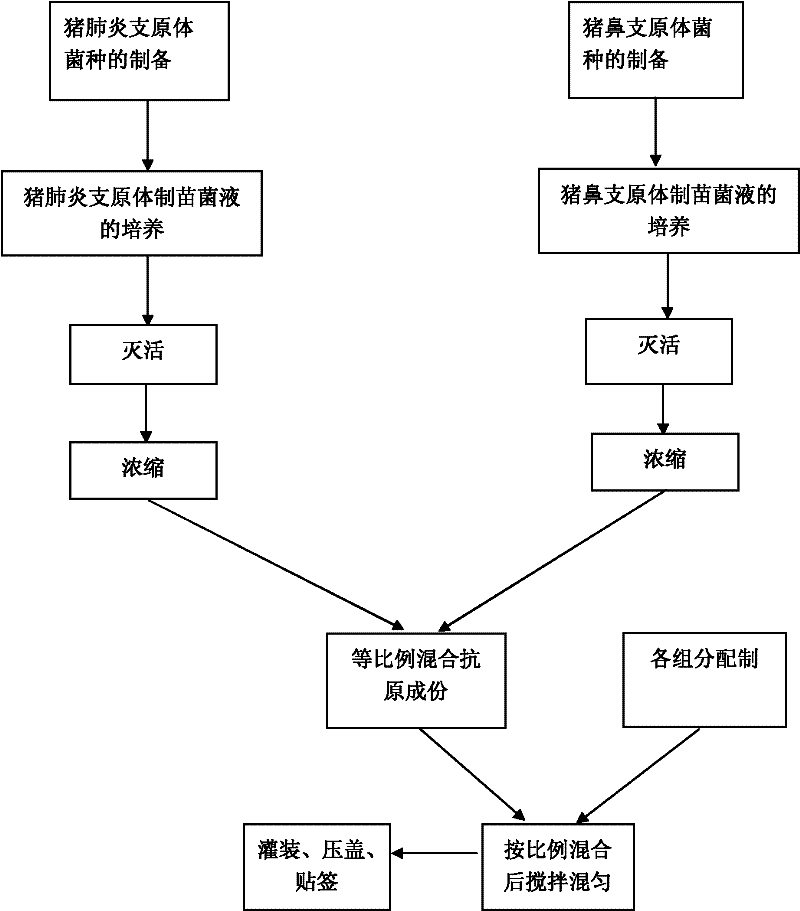
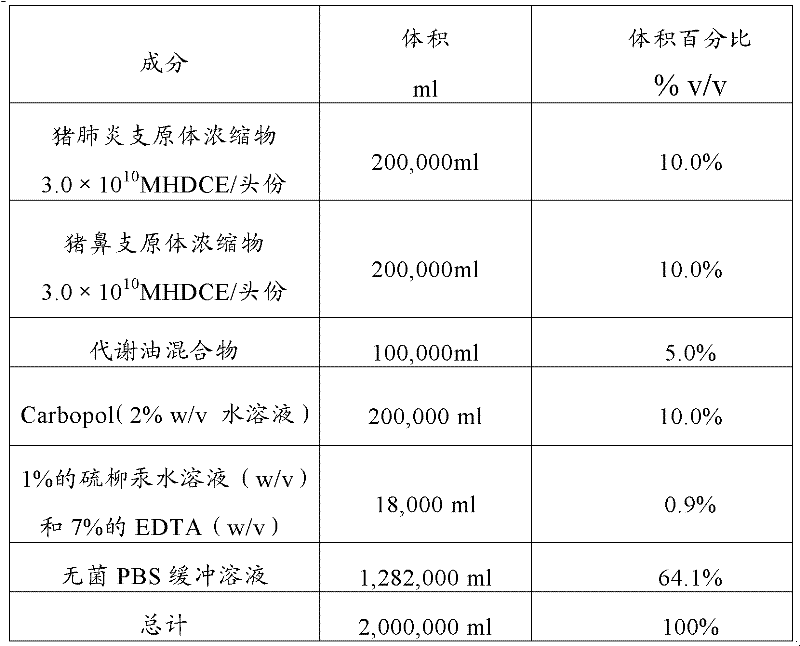
Mycoplasma hyopneumoniae and Mycoplasma hyorhinosus dual inactivated vaccine and preparation method thereof
ActiveCN102258776AStrong specificityGood immune effectAntibacterial agentsBacterial antigen ingredientsDiseaseImmune effects
The invention provides a combined inactivated vaccine against mycoplasma hyopneumoniae (MHP) and mycoplasma hyorhinis. The combined inactivated vaccine contains the MHP and the mycoplasma hyorhinis with preferred content as well as a Carbomer adjuvant with concentration of 10% (V / V). The invention further provides a preparation method of the combined inactivated vaccine against the MHP and the mycoplasma hyorhinis. The preparation method comprises the following steps: preparing production strains; preparing bacterium solution for producing seedlings; and inactivating, concentrating and blending to obtain the vaccine. The combined inactivated vaccine has the advantages of strong specificity and good immunity, thus solving the problem of specific infection caused by the MHP in the current domestic breeding farm and obtaining the mycoplasma hyorhinis vaccine under a blank state at home and abroad at present. The combined inactivated vaccine has the beneficial effects that the step of vaccine inoculation is simplified, trouble caused by a plurality of inoculation and easily produced side effects are avoided, and vaccine cost is saved, thus being especially applicable to preventing andtreating mixed infection diseases in the breeding farms at home and abroad and the like; and compared with the existing single vaccine, application range is widened and immune effect is enhanced.
Owner:PU LIKE BIO ENG
Porcine epidemic diarrhea vaccine composition, and preparation method and application thereof
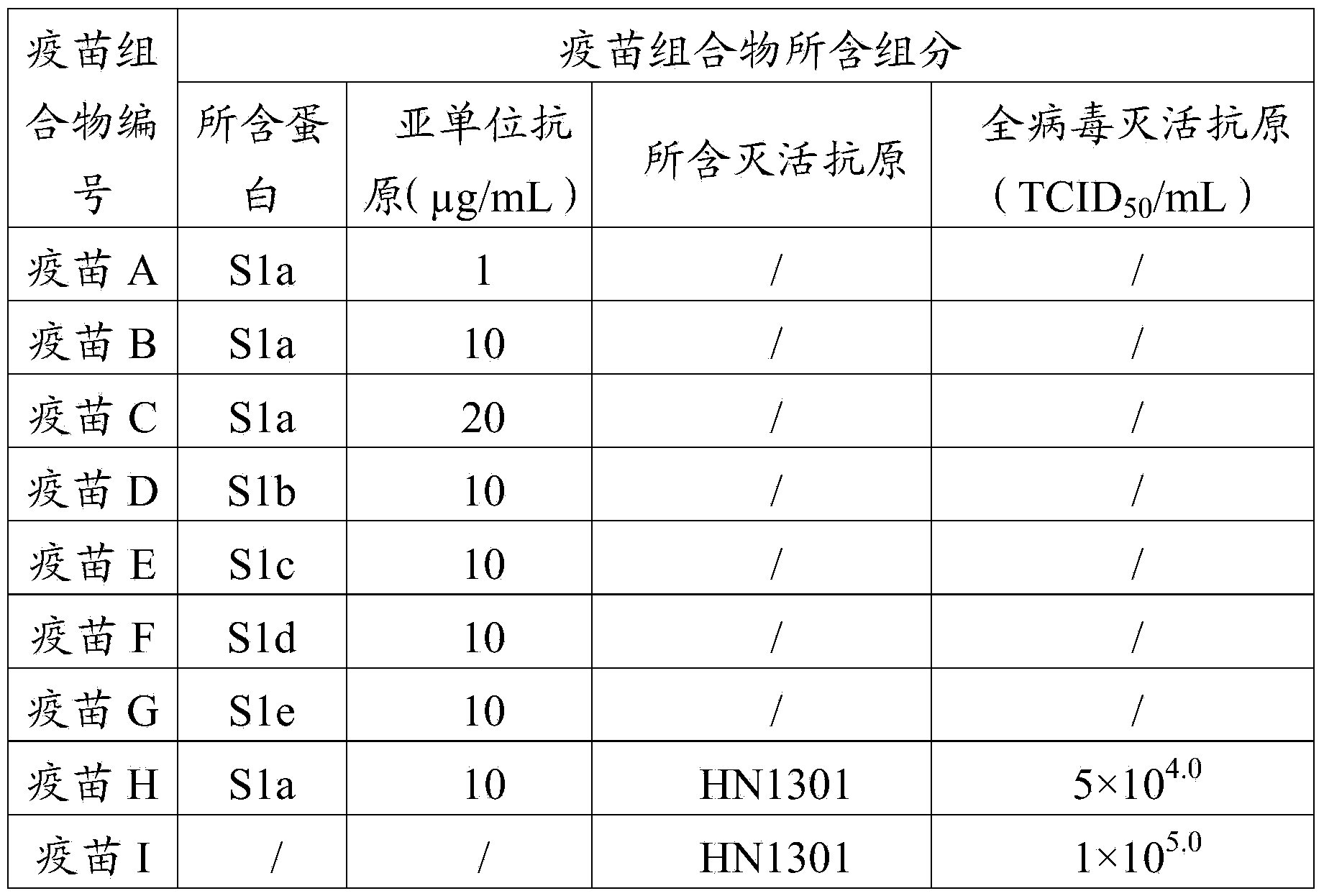
The invention provides a porcine epidemic diarrhea vaccine composition. The vaccine composition contains a porcine epidemic diarrhea virus subunit antigen and a vector. The invention also provides a porcine epidemic diarrhea vaccine composition, and the vaccine composition comprises a porcine epidemic diarrhea virus subunit antigen, a porcine epidemic diarrhea totivirus inactivated antigen and a vector. The invention also discloses a preparation method of the vaccine composition and application thereof to preparation of drugs for preventing and / or treating porcine epidemic diarrhea. An S1 protein in the vaccine composition can conduct large amount of recombinant expression of components in the vaccine composition by means of genetic engineering, so as to realize short time consumption as well as facilitate large-scale production. When the vaccine composition contains the S1 protein and porcine epidemic diarrhea totivirus inactivated antigen, the vaccine composition has better immune effect than single usage of totivirus inactivated antigen, and can effectively prevent diseases caused by variants of porcine epidemic diarrhea and common strains.
Owner:PU LIKE BIO ENG
Multivalent pneumococcus capsular polysaccharide-protein conjugated composition and preparation method thereof
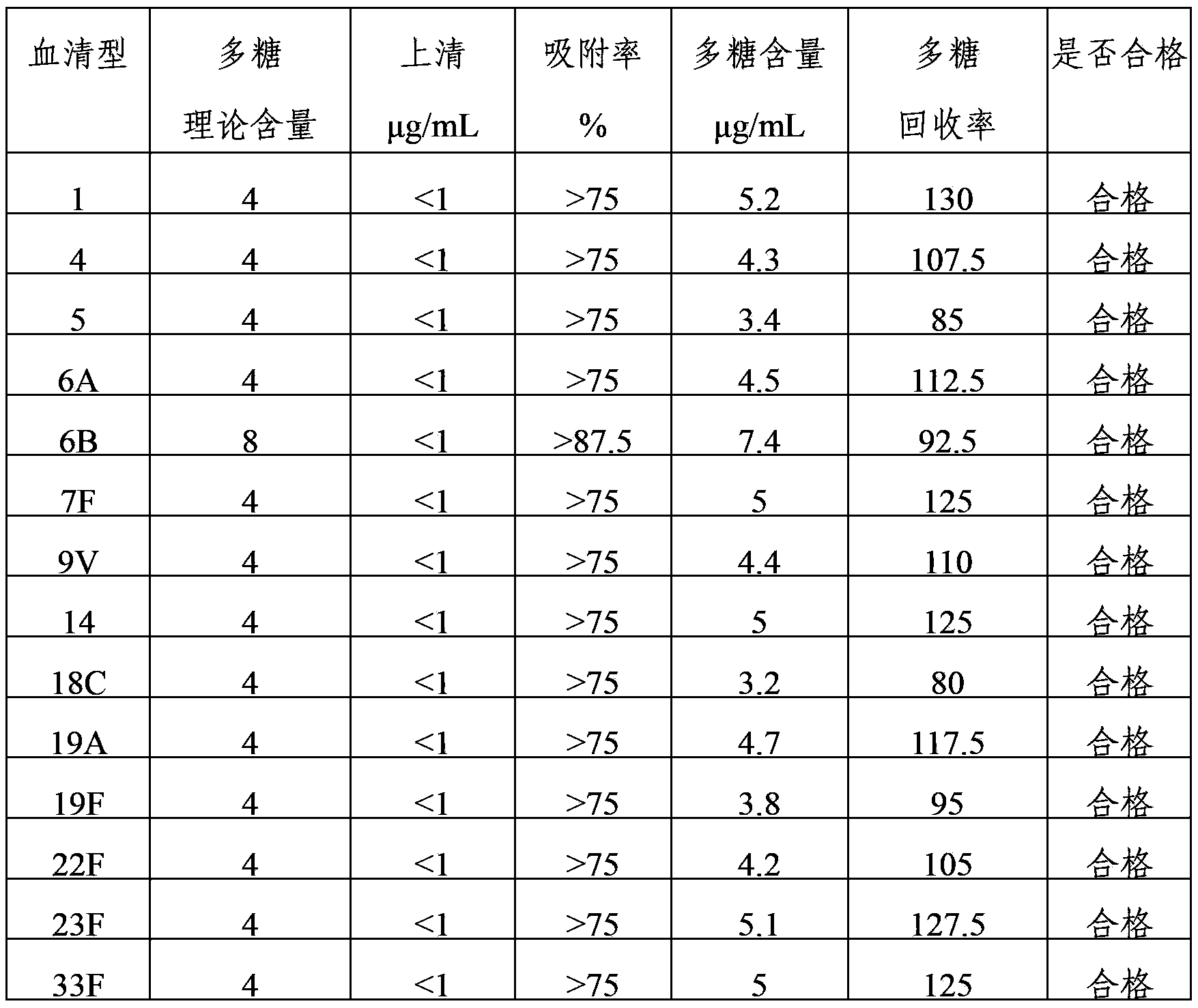
InactiveCN103623401AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsConjugate vaccineDisease
The invention provides a multivalent pneumococcus capsular polysaccharide-protein conjugated composition and a preparation method thereof. The conjugated composition is formed through covalent linkages between pneumococcus capsular polysaccharides of 14 different serotypes and a carrier protein, wherein the 14 different serotypes are: 1, 3, 4, 5, 6A, 6B, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F. The conjugated composition has a good absorption effect and stability, and has multiple immunogenicity and protective property aiming at the invasion of pneumococcus of the 14 serotypes; the effect of the conjugated composition is better than that of the low price and low quality pneumonia-treating compositions on the market, and moreover the immune response of the conjugated composition is higher than that of the composition that has not been combined. By using the multivalent pneumococcus capsular polysaccharide conjugate vaccine containing the conjugated composition, the inoculation times can be reduced, the immune procedure is simplified, diseases of humans and animals caused by the pneumococcus of the 14 serotypes can be effectively prevented, moreover the disease coverage is wider, and the immune effect is better.
Owner:SINOVAC RES & DEV
Veterinary compound acanthopanax granules with immunopotentiation and preparation method thereof
ActiveCN101559095AImprove immunityImprove disease resistanceGranular deliveryImmunological disordersDiseaseAnti stress
The invention provides veterinary compound acanthopanax granules with immunopotentiation, which is prepared from the following Chinese medicament raw materials by weight percentage: 70 to 60 percent of acanthopanax and the balance of astragalus root, wherein 100 grams of granules contain 100 grams of crud drugs. The compound acanthopanax granules produced by the method have good stability and safety, are mainly applied to strengthening the immunological effect of a vaccine and strengthening the immunity of organisms and the anti-stress efficacy clinically, and is applied to treating various immunosuppressive diseases of chickens and other animals and birds, strengthening the immunological effect, and treating symptoms of hypoimmunity, inappetence and the like caused by transportations, groups changes, abrupt climate changes and the like.
Owner:HENAN XINZHENGHAO BIO ENG
2 type subunit vaccine for porcine circovirus as well as preparation method and application thereof
InactiveCN102517331AQuick responseHigh activityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
The invention relates to a 2 type subunit vaccine for a porcine circovirus as well as a preparation method and application thereof. A recombinant bacilliform virus contains double promoters (a polyhedrin promoter and a P10 promoter), a coding gene of a Cap protein with double copying can be expressed, and the expression efficiency of the protein is obviously enhanced; moreover, the Cap protein expressed by an inserted foreign gene does not contain an excess sequence, virus-like particles (VLPs) can be effectively formed, and the immunogenicity of an expressed protein is enhanced; furthermore, a produced antigen has high content; and according to the 2 type subunit vaccine for the porcine circovirus, which is disclosed by the invention, the productivity ratio and the quality of a viral protein of the 2 type subunit vaccine for the porcine circovirus are obviously enhanced, and a prepared vaccine composition has the advantages of stable and persistent immune effect, high safety and the like.
Owner:WUHAN CHOPPER BIOLOGY
Ready-to-use adjuvant of livestock vaccines, preparation and applications thereof
ActiveCN103071153ASolve side effectsReduce dosageImmunological disordersEmulsion deliveryBiotechnologyAdjuvant
The invention provides a ready-to-use adjuvant of livestock vaccines, preparation and applications thereof, belonging to the field of the adjuvant of livestock vaccines. The ready-to-use adjuvant is prepared from the following substances: 5-30 percent of oil, 0.1-10 percent of hydrophilic surfactant, 0.1-10 percent of oleophilic surfactant, 0.1-10 percent of polymeric micelle substance and 40-90 percent of water. The invention also provides a preparation method of the ready-to-use adjuvant, and the method comprises the following steps: respectively weighing the substances, mixing and then emulsifying, and degerming to obtain the ready-to-use adjuvant of the livestock vaccines. The invention also provides an oil-in-water type vaccine with the ready-to-use adjuvant. The ready-to-use adjuvant is an oil-in-water type adjuvant and has the advantages of simple ingredients, stable dosage form, convenience in use and easiness in injection. The preparation method of the ready-to-use adjuvant has a simple process and is low in cost, and the obtained dosage form is stable. The oil-in-water type vaccine has the advantages of long stable period, easiness in storage, good immune effect, protection period prolonging, easiness in injection and small side reaction.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
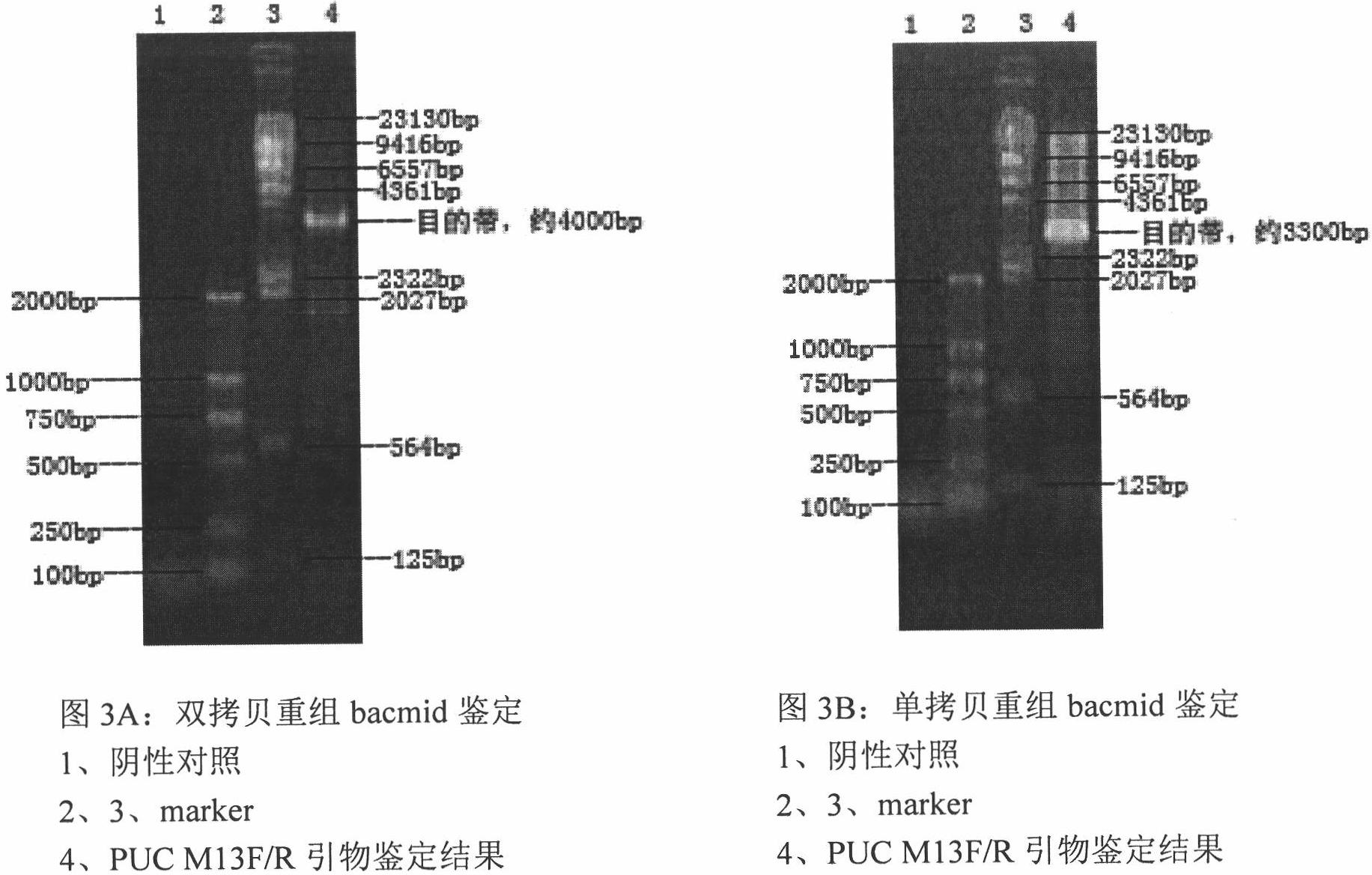
Recombinant low-virulent vaccine strain of chicken infectious bursal disease viruses (IBDV) and application thereof
ActiveCN101935637ANon-pathogenicGood spiritsViral antigen ingredientsMicroorganism based processesProtective antigenOrganism
The invention discloses a recombinant low-virulent vaccine strain of chicken infectious bursal disease viruses (IBDV) and application thereof. In the invention, a major protective antigen gene VP2 of an epidemic superhigh virulent strain is cloned, the nucleotide of the gene VP2 is modified by mutation and then used for replacing a corresponding segment of a Gt genome of a low-virulent strain of the IBDV, so that the infectious clone of a recombinant genome of the IBDV is constructed, and the recombinant low-virulent vaccine strain is saved and identified by using an IBDV reverse genetic operation system. The microbial collection number of the vaccine strain is CGMCC No.3749. The recombinant low-virulent vaccine strain of the invention has high replicability, genetic stability and safety. The immune effect of the low-virulent vaccine strain of the invention is as good as that of the medium-virulent vaccine strain, but is superior to that of the low-virulent vaccine strain. The biological safety of the low-virulent vaccine strain of the invention is superior to that of the medium-virulent vaccine strain. As the vaccine strain, the recombinant low-virulent vaccine strain of the invention has the characteristics of high efficiency and low toxicity, is a good candidate vaccine strain and can be used for controlling chicken infectious bursal disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
H9N2 avian influenza virus vaccine strain and application of H9N2 avian influenza virus vaccine strain in immune protection
The present invention relates to the field of animal virology, and provides a recombinant chicken-origin H9N2 avian influenza virus vaccine strain and a method for isolation, identification and purification of the strain. The invention further relates to a research of biological characteristics of the strain, especially to a research of characteristics of the strain adopted as the vaccine strain,and an evaluation of immune effects of the strain on SPF chickens. The preservation number of the strain is CCTCCNO:V201030. According to the present invention, the antigen variation conditions of the virus strain and other isolated virus strains are represented from the molecular level; after the virus strain is prepared into the vaccine, the prepared vaccine is adopted to immunize the 4 week old SPF chickens, with the protection effect analysis of the homologous H9 influenza wild virus strain and the heterologous H9 influenza wild virus strain, the results show that the influenza virus strain can be adopted as the spare vaccine strain of H9 subtype avian influenza. With the present invention, the spare vaccine strain is provided for prevention of the avian influenza outbreak by using the vaccine, the molecular biology technology program is provided for screen of the avian influenza virus vaccine strain, the molecular biology background is provided for study of the mechanism of animal infection by the avian influenza, and the important public health significance is provided.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Bursa of Fabricius heptapeptide with immune regulation effect
InactiveCN101830968ASynthetic technology is matureImprove efficiencyPeptide preparation methodsAntibody medical ingredientsSide effectImmunologic Competence
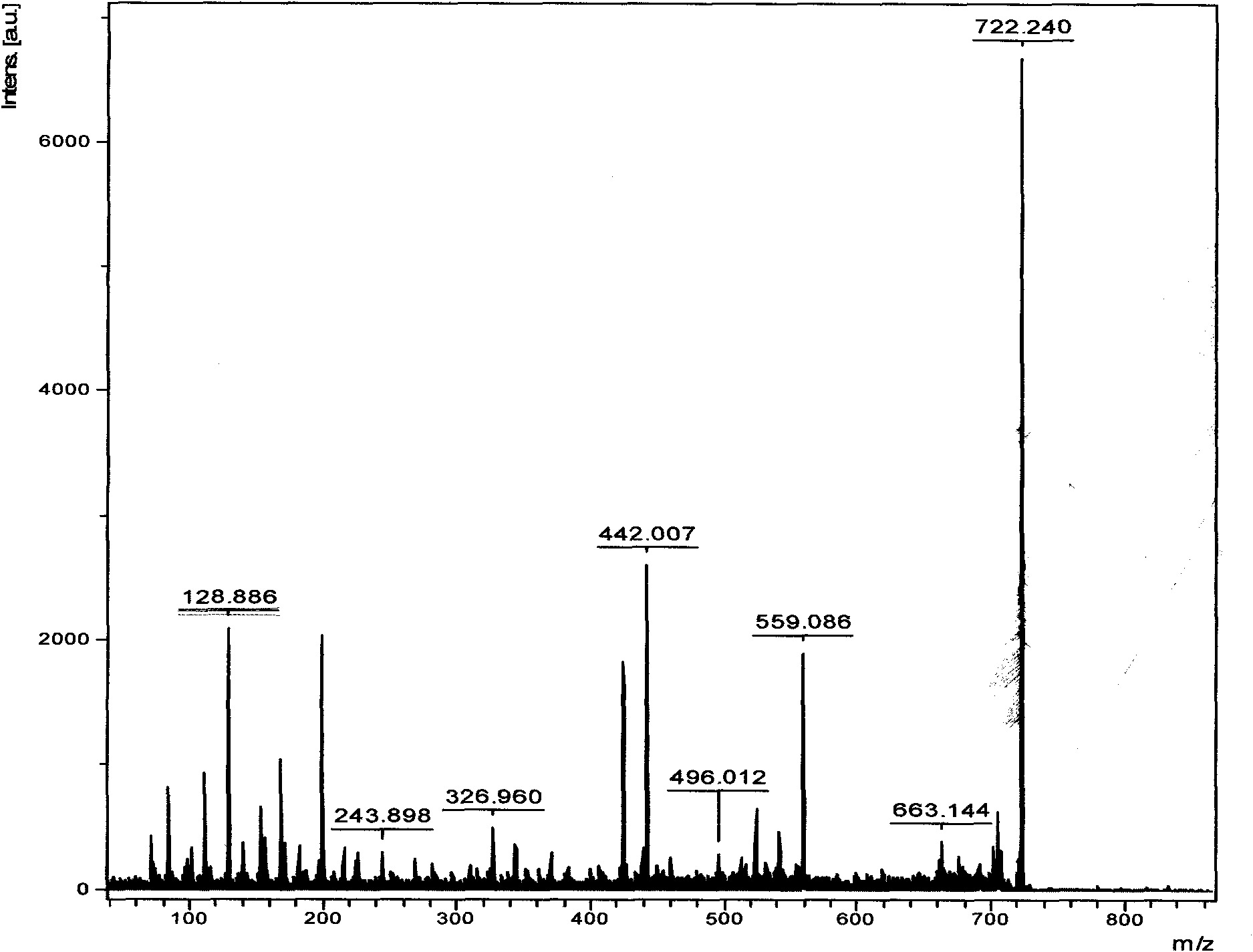
The invention relates to bursa of Fabricius heptapeptide with immune regulation effect and application thereof in immunity (in improving the immune capability of animals, improving the immune effect of vaccines and affecting the activity of tumor cells), and belongs to the field of immunology. The molecular weight of the separated heptapeptide is 722.240, the amino acid sequence is EPASGMM, and the heptapeptide has a simple structure, no toxic or side effect and extremely weak immunogenic property. The heptapeptide can be separated and extracted from bursa of Fabricius, also can be chemically synthesized, has low cost, and can be massively produced. The bursa of Fabricius heptapeptide has induction effect on the production of antibody and subtype thereof, and meanwhile can regulate the production of cell factors, partition of T lymphocytes and proliferation of spleen cells, and promote the immune reaction of the cells. The bursa of Fabricius heptapeptide is an immune regulation factor on functions, has wide application prospect on the aspects of immune regulation, immune therapy and the like, and can be applied in the fields of basic immune research, clinical application research and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
Bursopoietin extracting method and its use in disease treating and immune
InactiveCN1528783AImprove immunityIncrease body fluidsAnimal feeding stuffTripeptide ingredientsAdjuvantAntimicrobial drug
The invention relates to a bursin extracting method and its application to curing disease and immunity, having important value in application in the aspects of heightening organismal immunity and acting as immunoenhancer, heightening effect of vaccine, etc., and able to heighten body fluid and cell immune functions of mammal at the same time. It can be used to prevent and cure infectious diseases and young animal diseases singly or together with other drugs such as antivirus and antibacterial drugs or immunomodulators, also be applied to animal vaccine as adjuvant or immunoenhancer to strengthen the disease-resistant ability and immunoresponse ability to peculiar antigens, thus heightening the immune effect.
Owner:王爱华 +1
Preparation method of mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine
ActiveCN103479995AImproving immunogenicityAvoid infectionAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma synoviae
The invention relates to a preparation method of a mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine. The method comprises the steps as follows: a mycoplasma gallisepticum virulent CR strain and a mycoplasma synoviae HN01 strain which have good immunogenicity are inoculated on a proper culture medium for cultivation, so that a culture is acquired; and the culture is inactivated through a formaldehyde solution and then is mixed with an oil emulsion adjuvant and emulsified, so that the mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine is prepared. The mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine is used for preventing mycoplasma gallisepticum and mycoplasma synoviae diseases, and can realize immunization, prevent two pathogens at the same time and reduce the workload of immunization; and the prepared vaccine is stable in performance, good in immune effect, and more suitable for actual production of China.
Owner:兆丰华生物科技(南京)有限公司
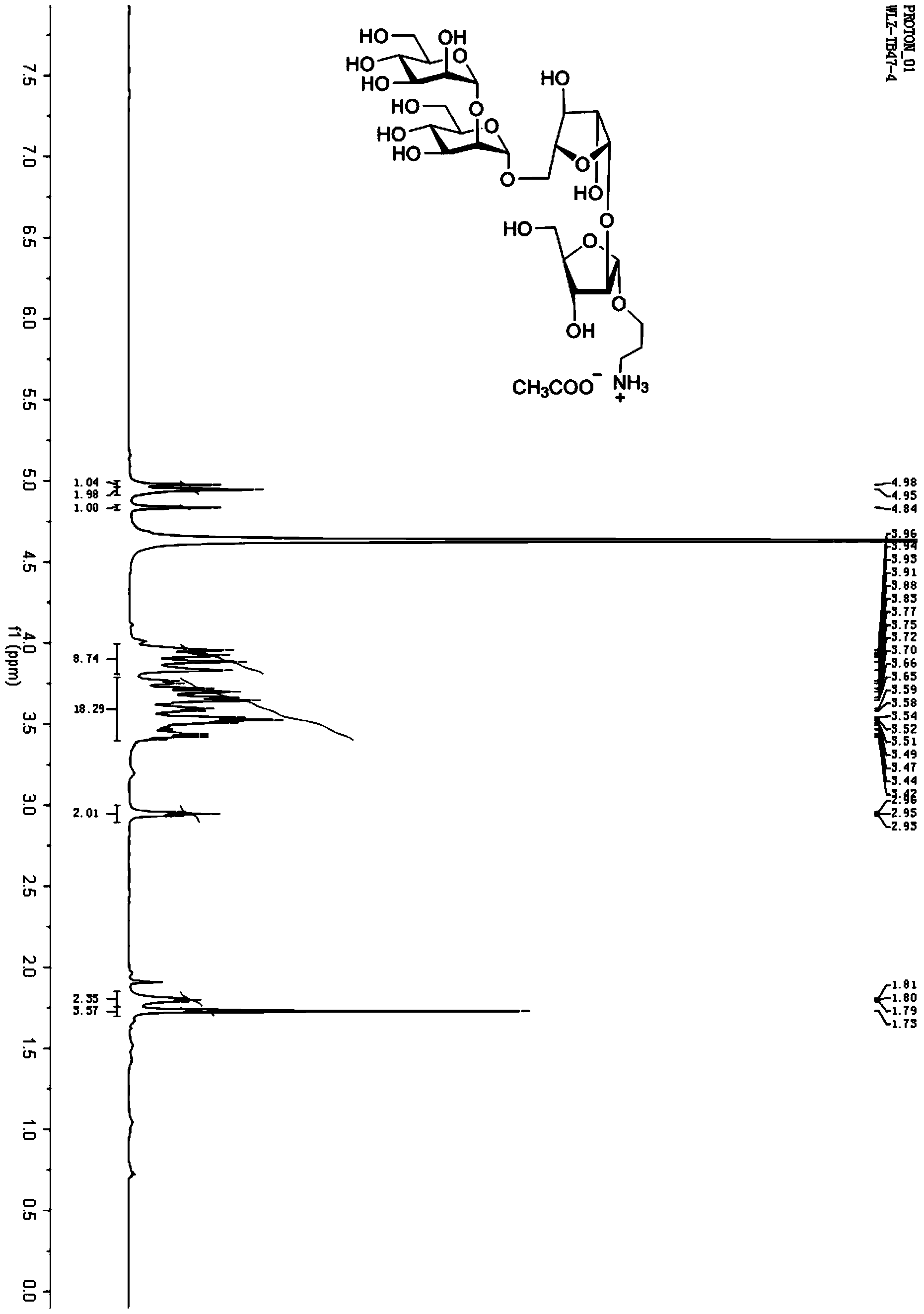
Mycobacterium tuberculosis LAM oligosaccharide conjugate as well as preparation method and application thereof
ActiveCN104004085AGood immune protectionAvoid drug resistanceAntibacterial agentsBacterial antigen ingredientsVaccination against tuberculosisChemical structure
The invention relates to a mycobacterium tuberculosis LAM oligosaccharide conjugate as well as a preparation method and application thereof. A structural general formula of the mycobacterium tuberculosis LAM oligosaccharide conjugate is described in the specification. The invention also relates to application of the mycobacterium tuberculosis LAM oligosaccharide conjugate in preparation of a tuberculosis vaccine. In the mycobacterium tuberculosis LAM oligosaccharide conjugate, a chemical structure of oligosaccharide is definite and single, is not a mixture and can be synthesized by adopting a chemical method, the problem that immune protection force of a Bacilli Calmette Guerin vaccine is small can be solved, better immune effect can be produced to the crowd with low immunity, and the problem that bacterial drug resistance is produced as antibiotics are greatly used, can be solved.
Owner:SHANDONG UNIV
Porcine pseudorabies virus vaccine composition and preparation method and application thereof
ActiveCN104248757AShort timeEase of mass productionAntiviralsAntibody medical ingredientsDiseaseImmune effects
The invention provides a porcine pseudorabies virus vaccine composition. The porcine pseudorabies virus vaccine composition contains a porcine pseudorabies virus subunit antigen, or a recombinant Newcastle disease virus, namely a porcine pseudorabies virus vector. The invention further provides the preparation method and application of the vaccine composition. The vaccine composition can effectively prevent related diseases of a porcine pseudorabies virus and related diseases infected by the porcine pseudorabies virus. The combination of immunogenicity antigens in the porcine pseudorabies virus vaccine composition can be induced to generate a synergetic immune effect, thereby having good immune effect, and further lowering the immune usage amount to lower the immunization cost.
Owner:PU LIKE BIO ENG
Haemophilus parasuis LC strain and application thereof
ActiveCN102399724AStrong pathogenicityImproving immunogenicityAntibacterial agentsBacteriaHeterologousDisease
The invention relates to the field of haemophilus parasuis vaccines in veterinary biological products, in particular to a haemophilus parasuis LC strain. The collection number of the strain is CGMCC (China General Microbiological Culture Collection Center) No.5257. The invention also relates to application of the haemophilus parasuis LC strain to preparation of haemophilus parasuis inactivated vaccines. The haemophilus parasuis LC strain has stronger pathogenicity to pigs and has better immunogenicity; an inactivated alumina gel vaccine prepared by the strain is safe and reliable; not only a homologous attacking protection is provided, but also a better cross protection to blood serums type 4, type 5, type 10, type 12, type 14 and type 15 HPS (Hantavirus Pulmonary Syndrome) heterologous attacking can be provided; after the pigs are immunized, a stronger immunity can be generated and the morbidity and the mortality of the inoculated pigs are obviously reduced; the immune effect achieves or is better than the traditional commercialized vaccines in the market; the vaccine has the advantages to compete with like products at home and abroad and is capable of effectively preventing the epidemic and the transmission of a haemophilus parasuis disease and reducing the economic losses caused by the disease, so that the application range is wide.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Low virulent strain of Brucella and vaccine thereof
ActiveCN103981139AImprove immunityImprove securityAntibacterial agentsBacterial antigen ingredientsSerum igeImmune effects
The invention relates to a low virulent strain of Brucella and a vaccine thereof. According to a vaccine strain, a coarse low virulent strain RA343 of Brucella abortus is screened through a domestication and selection technology combining antibiotics and A-factor serum. The coarse low virulent strain is remarkably improved in safety and still remains a good immune effect on the Brucella is still maintained. A Brucella vaccine prepared by using the low virulent strain is to change the current situation that Brucella vaccine-immunized animals and wild strain-infected animals are hard to differentiate, and the safety of existing vaccine is effectively improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Broad-spectrum safe anti influenza A virus vaccine for animals
ActiveCN101643721AImprove immunityAnti-leakageMicroorganism based processesAntiviralsCell membraneMutant
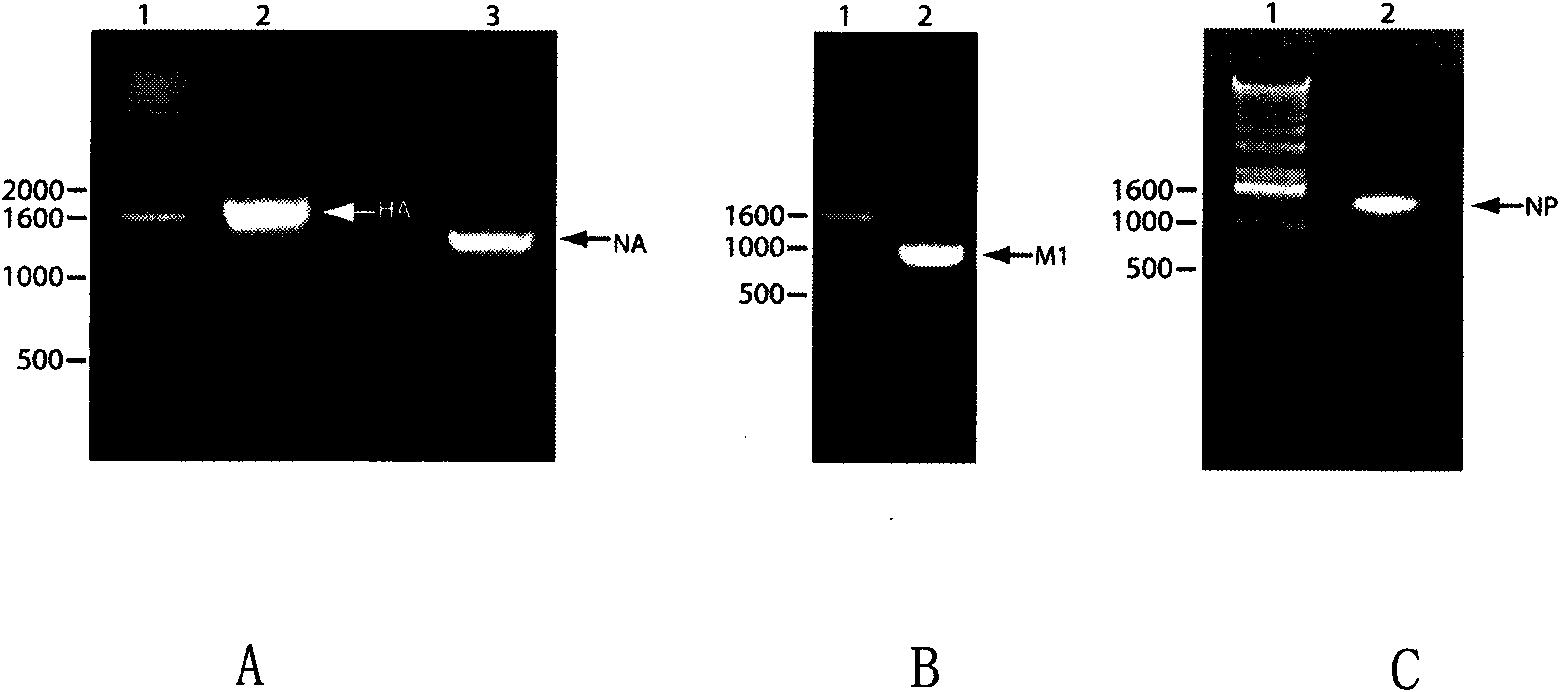
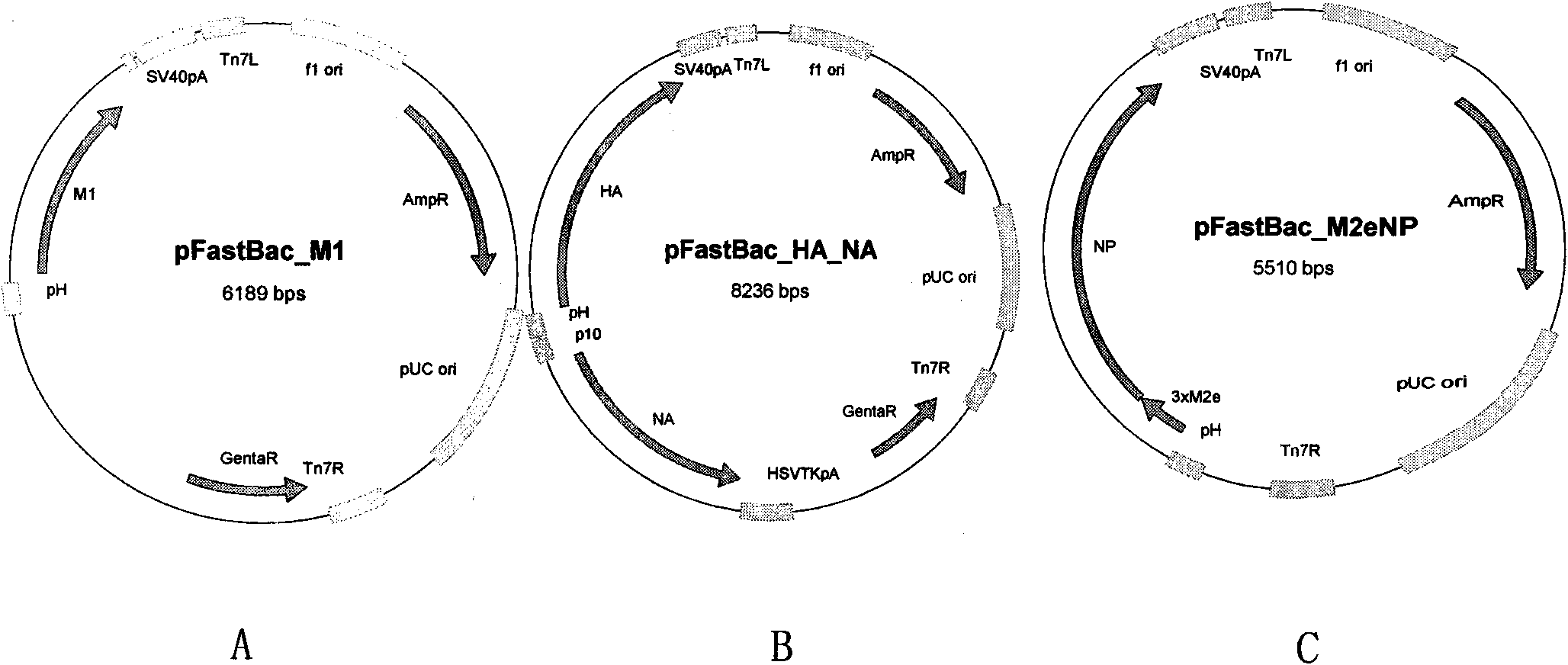
Recombinant virus-like particle contains influenza A virus matrix protein M1, surface film proteins HA and NA and M2eNP fusion protein or protein obtained by modification of mutant of at least one protein and the rest proteins, and the recombinant virus-like particle is non-replicative; wherein the M2eNP fusion protein is polymer formed by one M2e polypeptide or a plurality of M2e polypeptides atthe external end of cell membrane of matrix protein M2 or is formed by fusion of nucleoprotein NP and polymer formed by one M2e polypeptide through artificial modification or a plurality of M2e polypeptides after modification; and the nucleoprotein NP is coupled with recombinant matrix protein M1 after recombination expression and embedded in the recombinant virus-like particle. The vaccine produced by the recombinant virus-like particle can be directly applied to various animals for prevention of infection and spread of influenza A virus. The vaccine is safe in use and obvious in immune effect. Production period is short, technical operation is simple, and no purification is required, thus the vaccine is low in cost.
Owner:许雁
Chimeric antigen receptor based on CD20, and applications thereof
ActiveCN107245107AImprove bindingNot easy to mutateAntibody mimetics/scaffoldsMammal material medical ingredientsCD20Single-Chain Antibodies
The invention relates to a chimeric antigen receptor based on CD20, and applications thereof, and more specifically relates to a cell technology construction method of chimeric antigen receptor T (CAR-T) taking tumor specific target spot CD20 as a base, and applications of the chimeric antigen receptor T in treatment of tumor. The chimeric antigen receptor T is composed of an antigen binding domain, a transmembrane domain, a costimulatory signal transduction region, and a CD3 zeta signal transduction domine via series connection; wherein the antigen binding domain is used for binding tumor surface antigens, and the tumor surface antigen is CD20. According to applications, specific gene modification of single-chain antibody of tumor surface antigen CD20 is carried out, the modified antibody is capable of increasing antigen-antibody binding force, mutation is not easily caused. Compared with other chimeric antigen receptors and other tumor antigens, the chimeric antigen receptor possesses following advantages: the effect is better, target spot expression quantity is higher, immune effect on CAR-T cells is improved, and treatment effect of CAR-T cells is improved.
Owner:SHENZHEN GENO IMMUNE MEDICAL INST
Multivalent pneumococcus capsular polysaccharide-protein conjugated composition and preparation method thereof
InactiveCN104069488AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsDiseaseConjugate vaccine
The invention provides a multivalent pneumococcus capsular polysaccharide-protein conjugated composition and a preparation method thereof. The conjugated composition is formed by covalent linkage of multivalent pneumococcus capsular polysaccharides of 14 different serotypes and carrier protein, wherein the 14 serotypes include 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F. The conjugated composition has good adsorption effect and good stability, has multiple immunogenicity and protective performance against invasion of the pneumococcus of 14 serotypes, is superior to on-sale low-valent pneumonia compositions, and the immune response of the conjugated composition disclosed by the invention is higher than that of an uncombined composition. The inoculating injection frequency can be reduced by using the multivalent pneumococcus capsular polysaccharide conjugate vaccine containing the conjugated composition, the immune process can be simplified, and diseases of human and animals caused by the 14 serotypes of pneumococcal bacteria can be effectively prevented. The conjugated composition has wider coverage and better immune effect.
Owner:SINOVAC RES & DEV
Traditional Chinese medicinal composition and preparation method and application
InactiveCN104208128AImprove immunityWith preventionClimate change adaptationAntiviralsImmune effectsViral disease
A traditional Chinese medicinal composition and its preparation method and application are disclosed. The invention provides a traditional Chinese medicinal composition, raw materials of which comprise, by weight, 10-90 parts of Radix Astragali and 10-90 parts of licorice. The traditional Chinese medicinal composition has an effect of preventing and curing livestock and poultry viral diseases. In addition, the traditional Chinese medicinal composition also can be used along with a livestock and poultry vaccine so as to enhance the immune effect of the vaccine.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Oil-in-water type nanometer emulsion adjuvant and MRSA nanometer emulsion adjuvant vaccine and preparing method thereof
ActiveCN105251002ASimple ingredientsDosage stableAntibacterial agentsEmulsion deliveryIntramuscular injectionSurface-active agents
The invention discloses an oil-in-water type nanometer emulsion adjuvant and MRSA nanometer emulsion adjuvant vaccine and a preparing method thereof. The nanometer emulsion adjuvant is prepared from, by mass, 1-30% of surface active agent, 0.1-15% of cosurfactant, 0.1-15% of oil phase and 40-98.8% of water; the adjuvant is clear and transparent liquid from appearance, the particle size ranges from 1 nm to 100 nm, viscosity is low, the adjuvant belongs to a highly thermodynamics stabilizing system, and therefore high-speed centrifugation is stable without layering; when being compatible with the vaccine, the adjuvant can wrap the vaccine or be directly mixed with the vaccine physically for use, and immunity can be achieved by means of administration routes such as intramuscular injection and nasal drip. The adjuvant has the advantages of being low in cost, convenient to administrate, small in toxicity, good in liquidity and small in irritation to organism, and avoiding cross infection, the immune effect and stability of the vaccine are effectively improved, and the adjuvant has a wide application prospect.
Owner:ARMY MEDICAL UNIV +1
Preparation method and application of staphylococcus aureus isdbid-trap fusion protein
ActiveCN102276730AImprove immunityImproving immunogenicityAntibacterial agentsBacteriaAntigenStaphylococcus aureus
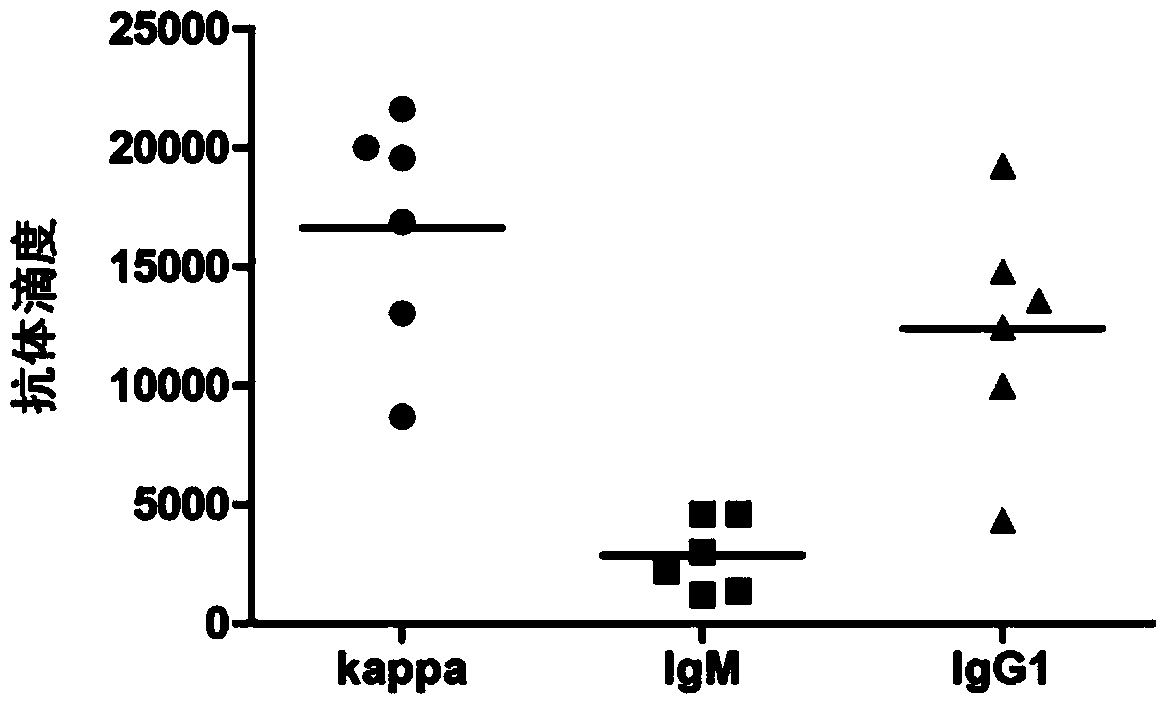
The invention provides staphylococcus aureus Iron-regulated surface determinant B immunodominant fragment (IsdBid)-target of RNAIII activating protein (TRAP) fusion protein. The amino acid sequence of the staphylococcus aureus IsdBid-TRAP fusion protein is shown by SEQ ID No. 1. A preparation method for the staphylococcus aureus IsdBid-TRAP fusion protein comprises the following steps of: selecting a staphylococcus aureus IsdB immunodominant fragment (IsdB immunodominant fragment, IsdBid), connecting the IsdBid and a trap gene by using Linker through the overlapping primer extension polymerase chain reaction (PCR) technology, performing pronucleus expression on a gene of the IsdBid-TRAP fusion protein, and purifying the fusion protein. According to the result of detection, the immune effect of a vaccine which is prepared from the IsdBid-TRAP fusion protein and is used for immunizing mice is obviously superior to that of the single immunization of the IsdB and TRAP and the mixed immunization of the IsdB and the TRAP, so the staphylococcus aureus IsdBid-TRAP fusion protein is an ideal candidate antigen for preparing staphylococcus aureus vaccines.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com