Ready-to-use adjuvant of livestock vaccines, preparation and applications thereof
A vaccine and adjuvant technology, applied in the field of compound adjuvant preparation, can solve the problems of inability to meet the needs of epidemic disease prevention and control in aquaculture industry, inability to continuously stimulate the immune response of the body, and uncommon application of vaccines, so as to prolong the protection period and improve the retention. and presentation ability, the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Adjuvant preparation
[0032] Recipe 1:
[0033] White oil 10 g,
[0034] Tween 80 0.5g,
[0035] Sibon 85 0.5g,
[0036] Chitosan 2.0g,
[0037] Carbomer 971P 0.1g,
[0038] Water 83.9g.
[0039] Recipe 2:
[0040] Squalane 10 g,
[0041] Pluronic 121 0.5g,
[0042] Sibon 80 0.5g,
[0043] Carbomer 971P 3.0g,
[0044] Water 83.0g.
[0045] Recipe 3:
[0046] Squalane 10g, Squalene 10g
[0047] Pluronic 121 5g, Tween 80 0.3g,
[0048] Siben 80 5g, Siben 85 0.3g,
[0049] Hydroxymethyl Cellulose 1.0g, Dimethyl Dioctadecyl Ammonium Bromide 1.0g,
[0050] Water 67.4g.
[0051] Recipe 4:
[0052] Squalane 15g, white oil 15g,
[0053] Pluronic 121 0.2g, Tween 80 8.8g,
[0054] Siben 80 0.2g, Siben 85 8.8g,
[0055] Hydroxymethyl Cellulose 2.0g, Carbomer 971P 3.0g,
[0056] Water 47.0g.
[0057] Recipe 5:
[0058] Squalane 5 g,
[0059] Pluronic 121 0.1g,
[0060] Siben 80 0.1g,
[0061] Carbomer 971P 1.0g, Dimethyl Dioctadecyl Ammonium Br...
Embodiment 2
[0071] Example 2 Preparation and properties of oil-in-water vaccine against porcine foot-and-mouth disease
[0072] (1) Preparation of ready-to-use adjuvants
[0073] The ready-to-use adjuvants 1-6 were prepared according to the method in Example 1. ISA206 adjuvant was purchased from SEPPIC Company.
[0074] (2) Prepare porcine foot-and-mouth disease inactivated virus liquid
[0075]Inactivated porcine foot-and-mouth disease virus liquid (type O, II) was provided by Jinyu Baoling Biological Pharmaceutical Co., Ltd., and the virus content in every 0.2 ml of virus liquid before inactivation was 10 7.0 LD 50 / 0.2ml. The porcine foot-and-mouth disease inactivated virus liquid is prepared in accordance with the relevant requirements of "Compilation of Quality Standards for Veterinary Biological Quality" (2006-2008). Pig foot-and-mouth disease inactivated virus liquid (type O, II) is referred to as pig foot-and-mouth disease inactivated virus liquid.
[0076] (3) Preparati...
Embodiment 3
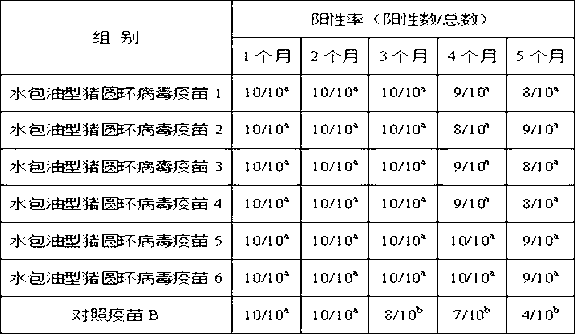
[0089] Example 3 Application of oil-in-water porcine circovirus vaccine
[0090] (1) Preparation of porcine circovirus type 2 inactivated virus solution
[0091] Porcine circovirus type 2 inactivated virus liquid (DBN-SX07 strain) was provided by Chengdu Tianbang Biotechnology Co., Ltd., and the virus content per ml of virus liquid before inactivation was 10 5.5 TCID 50 . The inactivated virus liquid complies with the relevant requirements of the "Compilation of Quality Standards for Veterinary Biological Quality" (2006-2008).
[0092] (2) Preparation of oil-in-water porcine circovirus vaccine
[0093] The ready-to-use adjuvants 1-6 were mixed with the porcine circovirus type 2 inactivated virus solution respectively, and the oil-in-water porcine circovirus vaccines 1-6 were prepared evenly by hand shaking. The mass ratio of the ready-to-use adjuvant 1 in the oil-in-water porcine circovirus vaccine 1 to the porcine circovirus type 2 inactivated virus liquid is 1:0.5. In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com