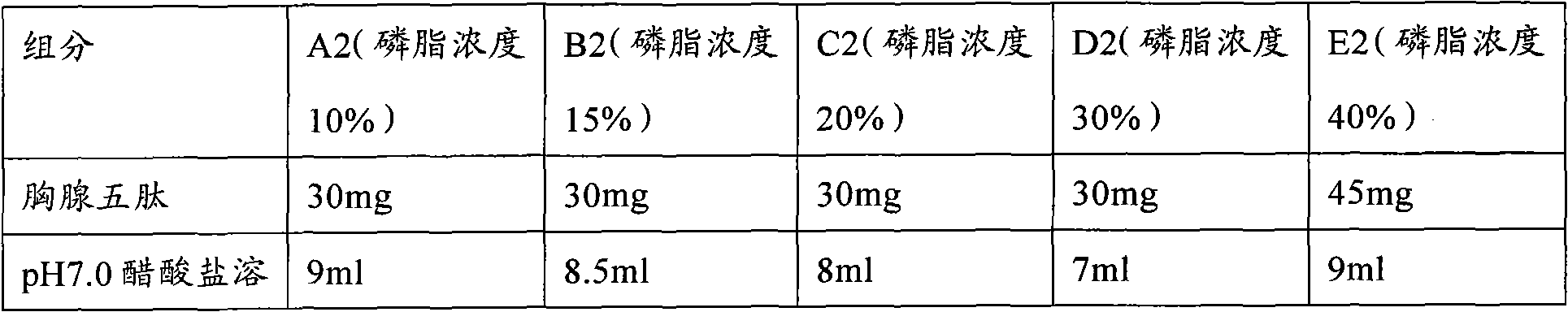
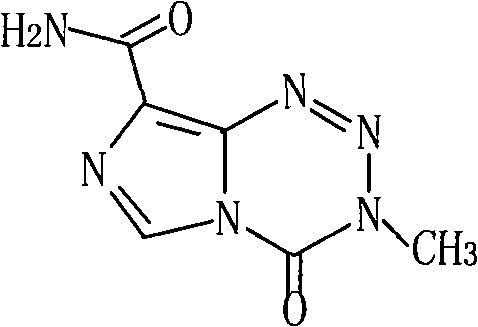
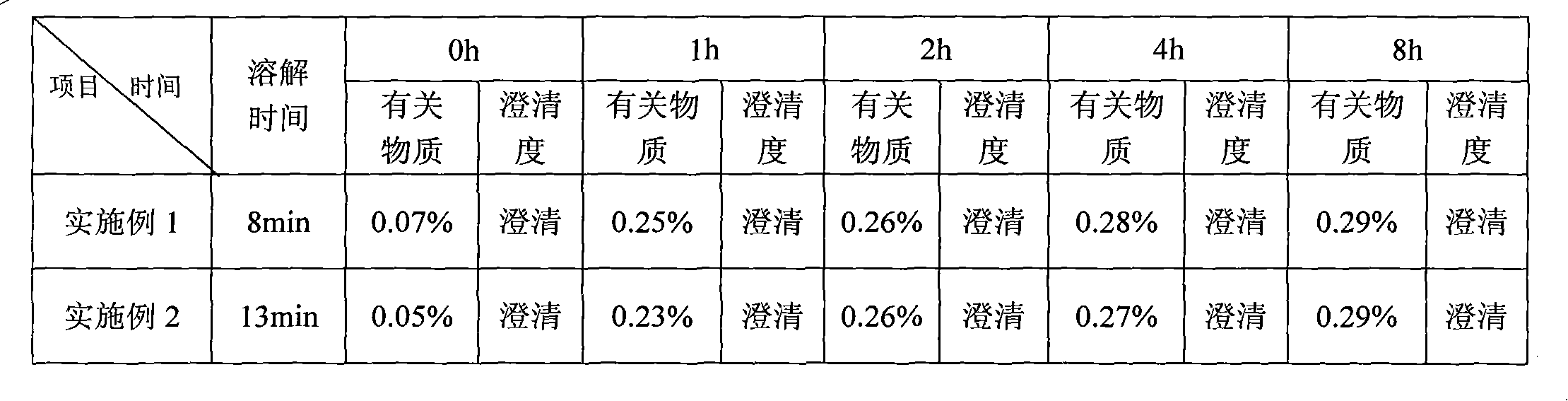
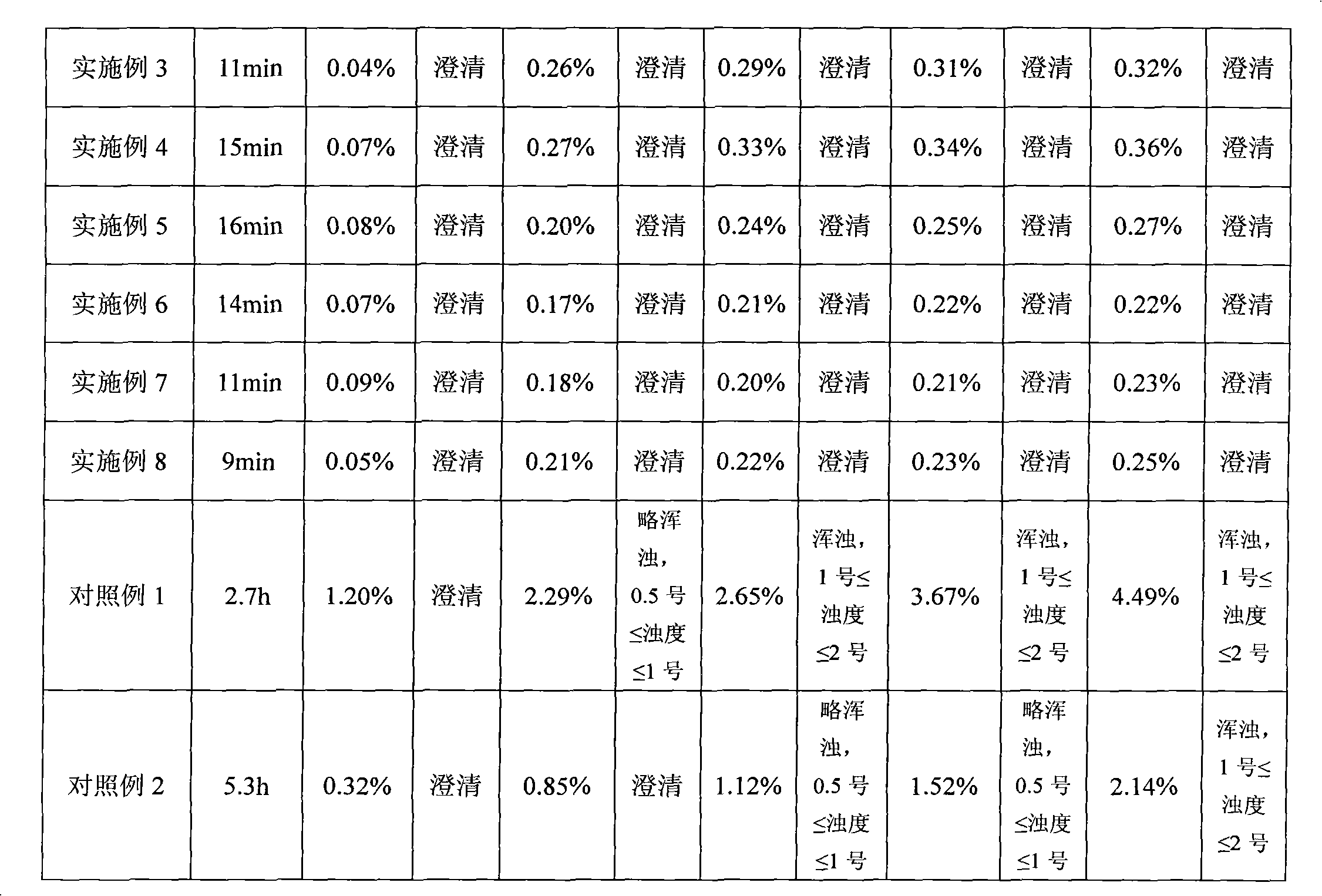
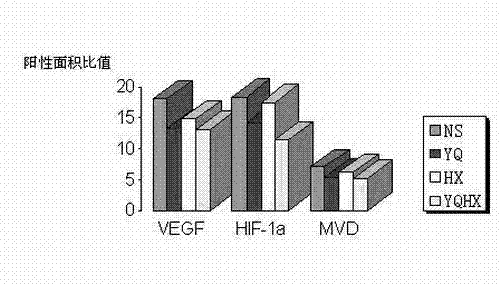
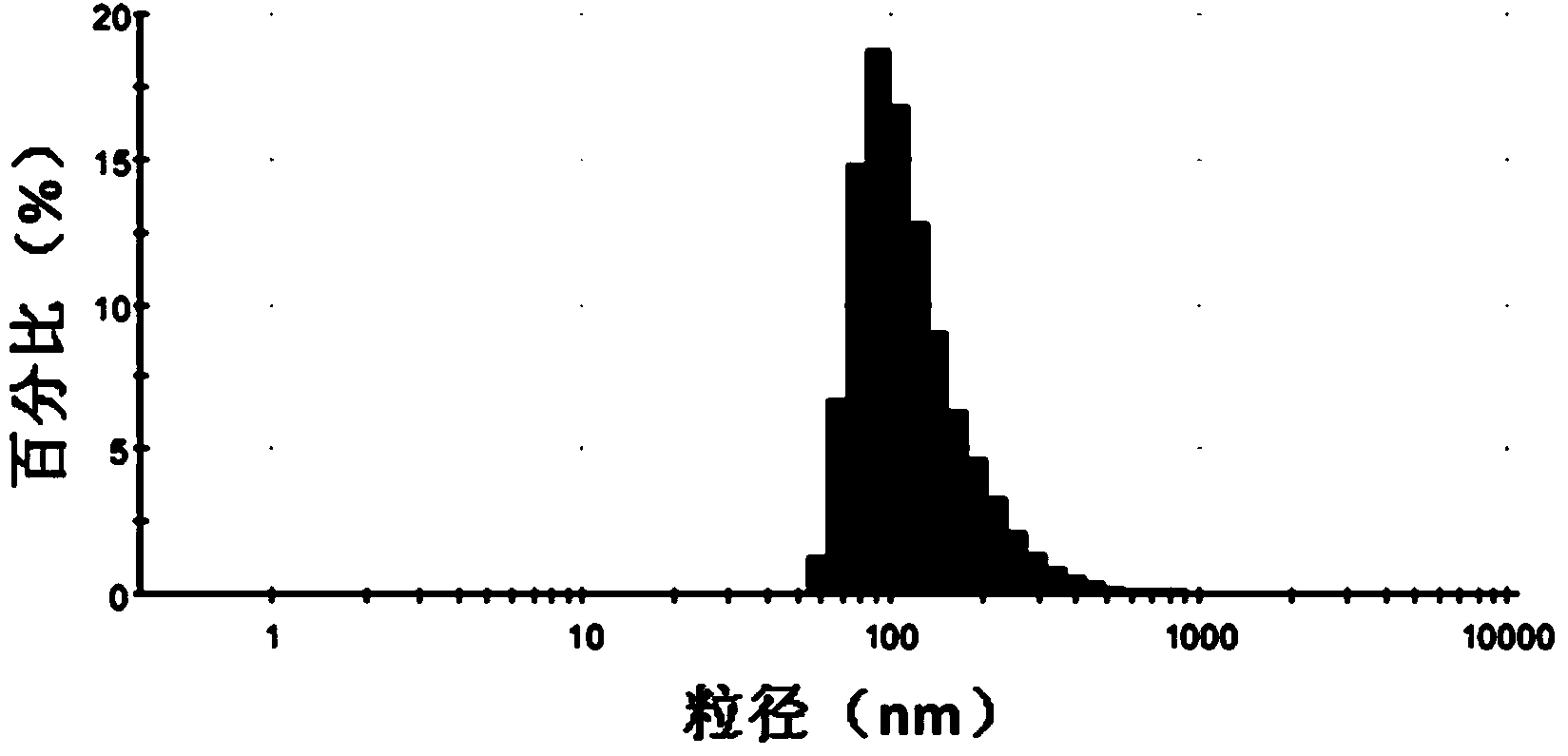
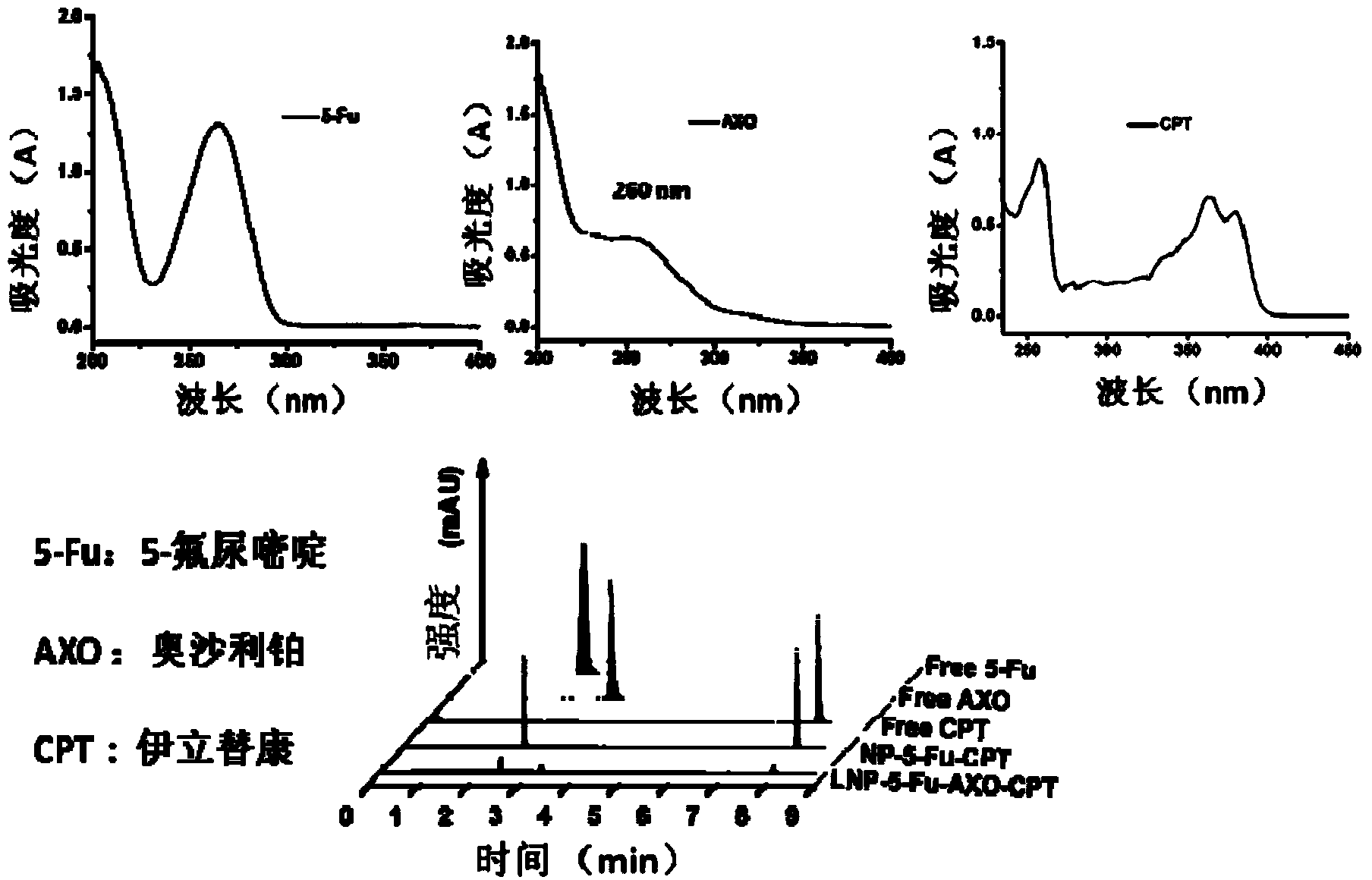
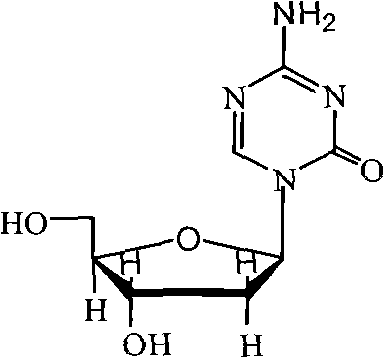
Patents
Literature
353results about How to "Reduce toxic and side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
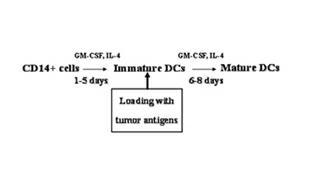
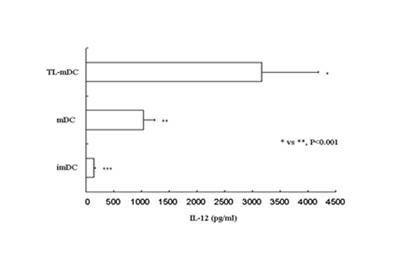
Preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen
ActiveCN102091327ASignificant technological progressConvenient for clinical operationBlood/immune system cellsAntibody medical ingredientsCytotoxicityT lymphocyte
The invention belongs to preparation of biological cell formulations, and in particular relates to a preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen. The preparation method comprises the following steps: preparing the autologous tumor associated holoantigen, collecting and separately culturing DCs, impacting the DCs by the autologous tumor associated holoantigen, maturing the DCs and preparing autologous tumor antigen specific DC vaccine. The invention solves the problems in the prior art that the immunogenicity of tumor antigen is not strong enough, the antigen target spots are incomplete, the tumor antigens of most tumor patients are difficult to acquire, and the like. The DC vaccine provided by the invention has the advantages of effectively inducing tumor antigen specific cytotoxic T lymphocyte (CTL) in vitro and in vivo, efficiently generating specific cytotoxicity on tumors, having high overall effective rate and no obvious toxic side effects in clinical application, and the like.
Owner:玥特农生物科技河北有限责任公司
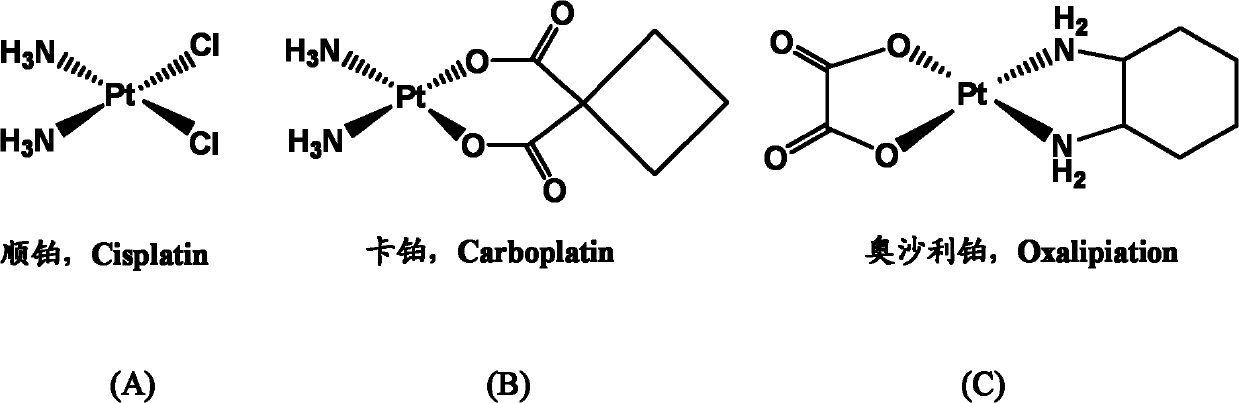
Nano micelle of biodegradable macromolecular-bonding Pt(IV) anti-cancer medicament and preparation method thereof
ActiveCN102120036AReduce toxic and side effectsNo toxicityHeavy metal active ingredientsPharmaceutical non-active ingredientsCarboplatinPolyester
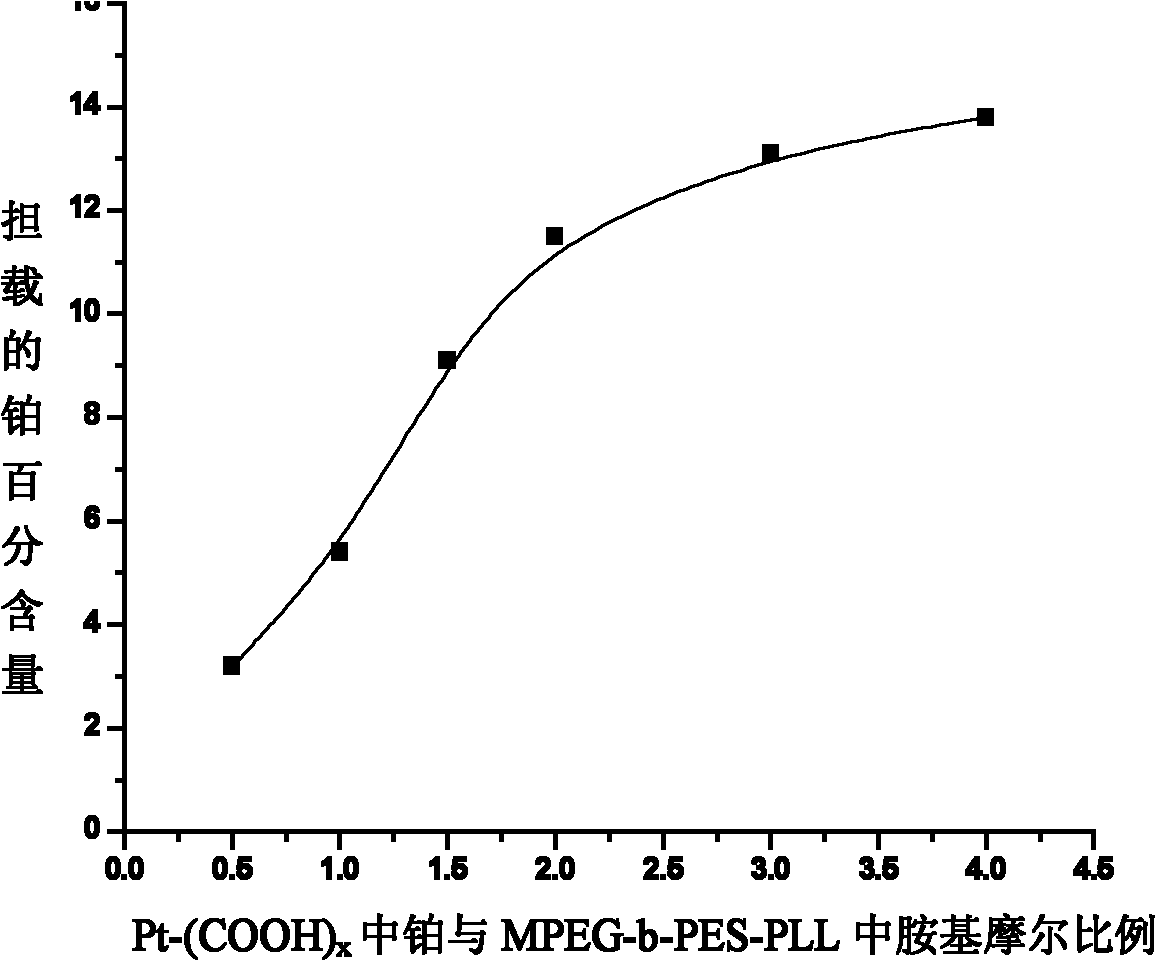
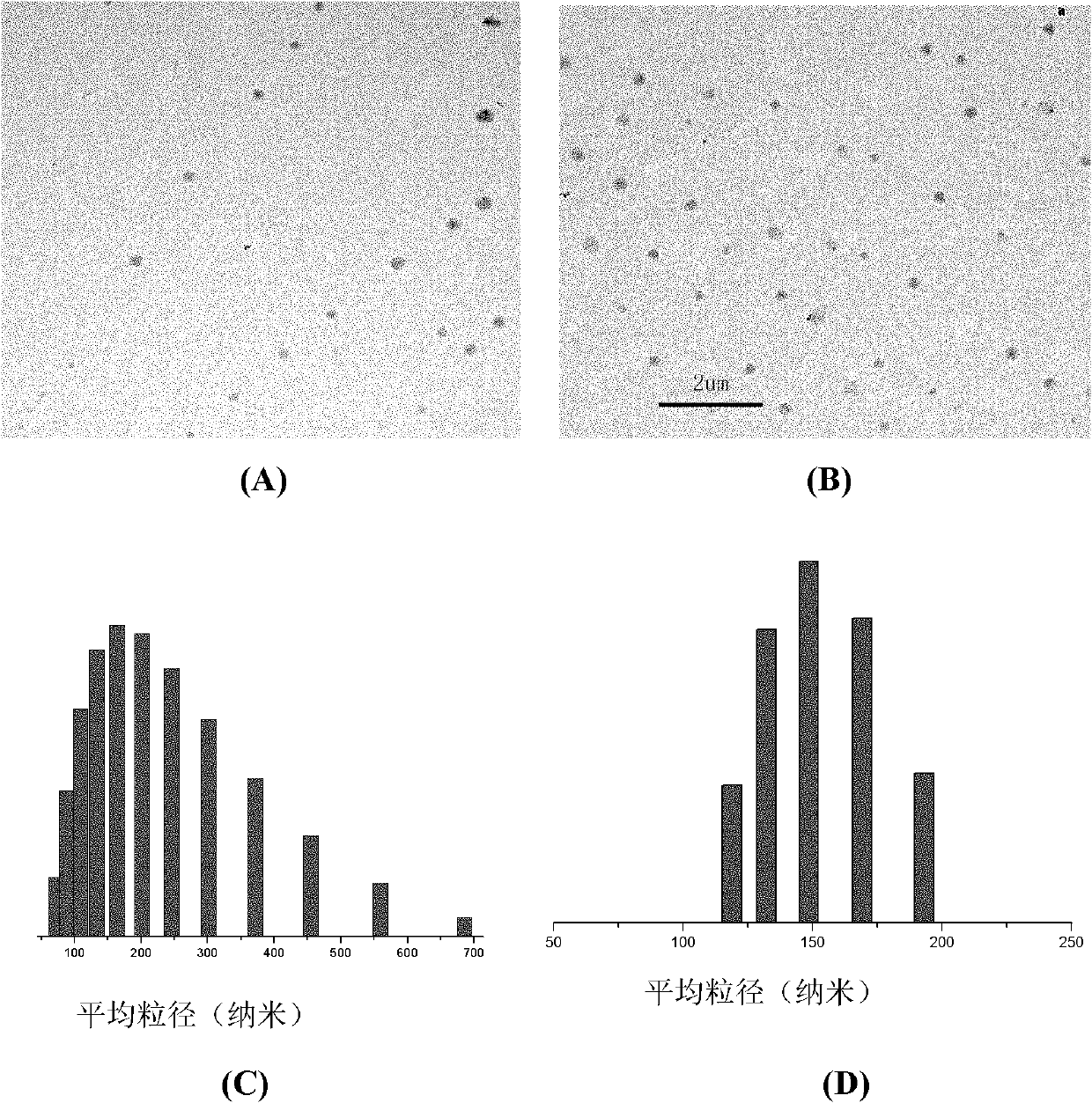
The invention relates to a nano micelle of a biodegradable macromolecular-bonding Pt(IV) anti-cancer medicament and a preparation method thereof. The structural formula of the anti-cancer medicament is defined in the specification, wherein the biodegradable macromolecule is tri-block copolymer, namely polyethylene glycol-b-polyester-b-polylysine; and tetravalency platinum coordination compound Pt(IV) is connected with a side amino on the block of polylysine on a macromolecular carrier through alpha, omega-polyethylene glycol. The carrier is non-toxic and is water-soluble, thus being convenient for reacting with the platinum(IV) coordination compound in water phase; a nano micelle form can be formed through self assembly; a platinum(IV) coordination compound is reduced to platinum(II), namely, cis-platinum, Carboplatin or Oxaliplatin, and the anti-cancer effect is known; the synthesis of the nano micelle is easy; the reduction potential of platinum(IV) is low, so that the platinum(IV) can be rapidly reduced to platinum(II) in cancer cells to take treatment effect; and the platinum(IV) is connected to the side chain of the macromolecule rather than the end of a chain, a macromolecular chain can be connected with multiple platinum(IV)s, and the content of platinum can be as high as 10-20%.
Owner:吉林市博禹祥实工贸有限公司
Application of low-concentration vesicular phospholipid gel as slow release carrier for small-molecule peptide drug
ActiveCN102125517AHigh encapsulation efficiencyReduce the impact of stabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMultiple formsPeptide drug
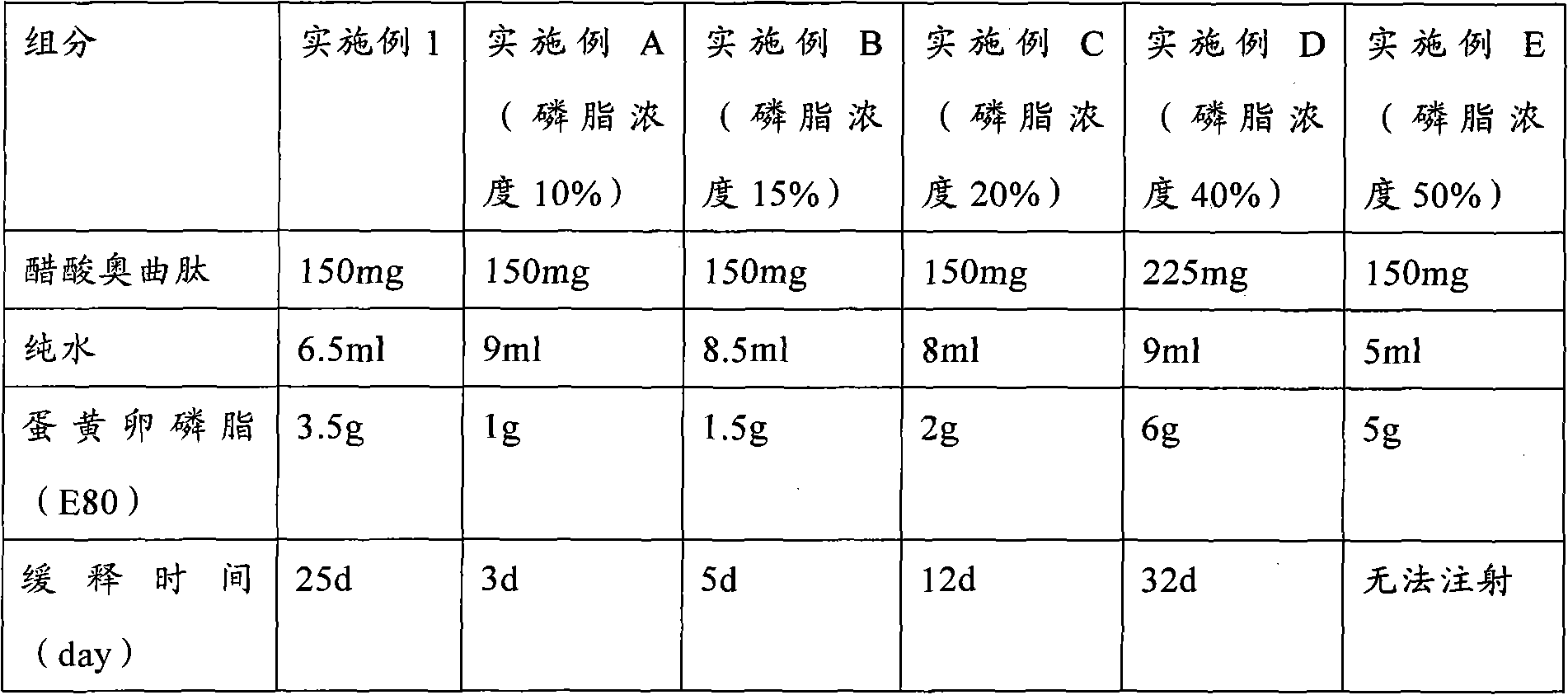
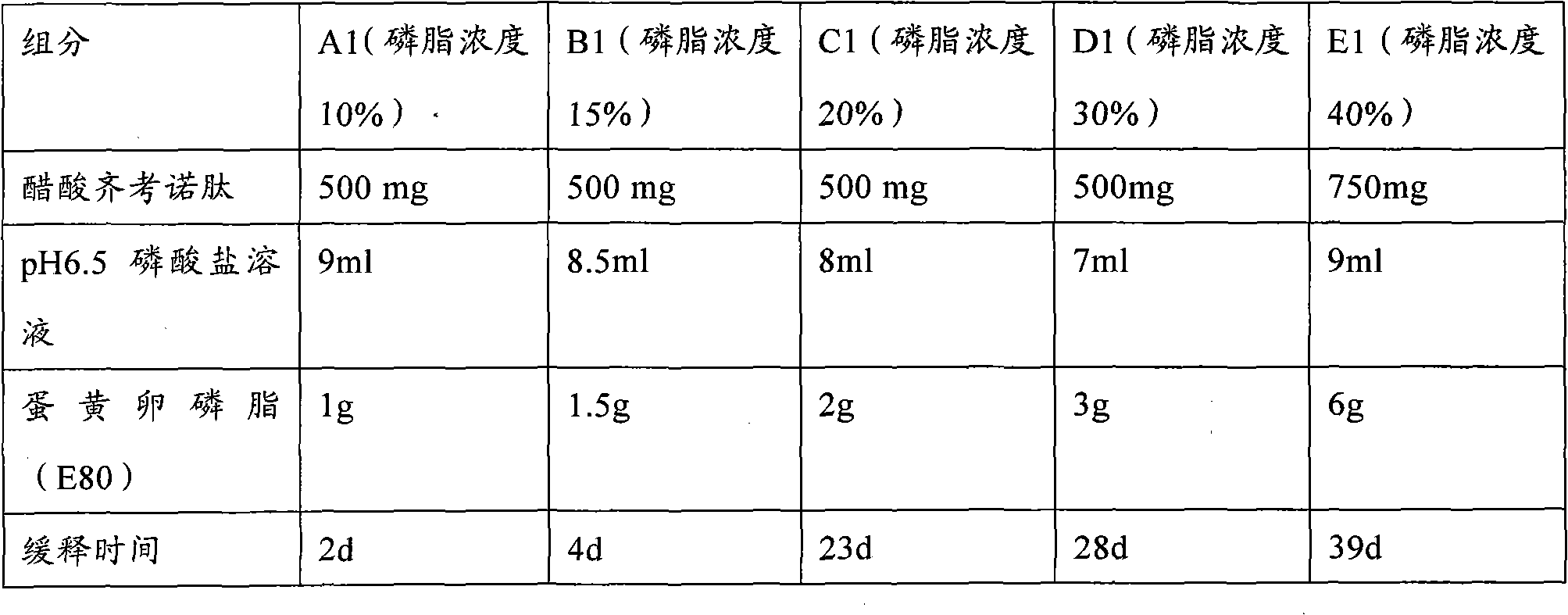
The invention provides a low-concentration vesicular phospholipid gel formulation of a small-molecule peptide drug and also provides a preparation method of the vesicular phospholipid gel formulation of the small-molecule peptide drug. In the low-concentration vesicular gel formulation, the content of phospholipids ranges from 20% to 40%, and the small-molecule peptide drug with a molecular weight ranging from about 300 D to about 3300 D is encapsulated in the phospholipids. The low-concentration vesicular phospholipid gel formulation is available in various drug administration forms suitable for injection drug administration, external drug administration and the like, has good biocompatibility, high drug-carrying capacity and good stability, and particularly has a long-acting slow release effect.
Owner:成都师创生物医药科技有限公司
Temozolomide freeze-dried preparation
ActiveCN101869551ASolve the speed problemSolve poor resolubilityOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFreeze-drying
The invention discloses a temozolomide freeze-dried preparation. Every 100ml of the preparation comprises 1 to 2,000 mg of temozolomide, 1 to 2,000 mg of solubilizer, 801 to 2,000 mg of polysorbate, 5 to 5,000 mg of mannitol, 1 to 2,000 mg of buffering agent, 0.5 to 1,000 mg of hydrochloric acid and the balance of water. The preparation of the invention overcomes the shortcomings of slow dissolving speed, poor redissolution, fussy operation and unqualified solution clarity of the freeze-dried preparation, has the technical effects that: the preparation of the invention has high redissolution; solid is completely dissolved to be clear and colorless by adding water into the freeze-dried preparation and shaking slightly; purity is over 99.5 percent and single impurity content is below 0.3 percent by detecting the purity and the content by using HPLC; through stability experimental investigation, relative substances and content of the preparation are not changed remarkably; and quality is stable.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Ready-to-use adjuvant of livestock vaccines, preparation and applications thereof
ActiveCN103071153ASolve side effectsReduce dosageImmunological disordersEmulsion deliveryBiotechnologyAdjuvant
The invention provides a ready-to-use adjuvant of livestock vaccines, preparation and applications thereof, belonging to the field of the adjuvant of livestock vaccines. The ready-to-use adjuvant is prepared from the following substances: 5-30 percent of oil, 0.1-10 percent of hydrophilic surfactant, 0.1-10 percent of oleophilic surfactant, 0.1-10 percent of polymeric micelle substance and 40-90 percent of water. The invention also provides a preparation method of the ready-to-use adjuvant, and the method comprises the following steps: respectively weighing the substances, mixing and then emulsifying, and degerming to obtain the ready-to-use adjuvant of the livestock vaccines. The invention also provides an oil-in-water type vaccine with the ready-to-use adjuvant. The ready-to-use adjuvant is an oil-in-water type adjuvant and has the advantages of simple ingredients, stable dosage form, convenience in use and easiness in injection. The preparation method of the ready-to-use adjuvant has a simple process and is low in cost, and the obtained dosage form is stable. The oil-in-water type vaccine has the advantages of long stable period, easiness in storage, good immune effect, protection period prolonging, easiness in injection and small side reaction.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Tulobuterol containing pressure-sensitive adhesive, transdermal paster, and its preparing method and use
ActiveCN1640388ALow costImprove productivityOrganic active ingredientsRespiratory disorderTransdermal patchPolymer science
The present invention discloses a transcutaneous plaster containing tulobuterol, its preparation method and application. The pressure-sensitive adhesive used by said invention is a hot-melt pressure-sensitive adhesive whose melting point is 50-250 deg.C. the described hot-melt pressure-sensitive adhesive is an adhesive made up by using thermoplastic polymer as main raw material, and hs the hot-melting and pressure-sensitive double characteristics. Said product contains no organic solvent, and its coating speed is quick, and its cost is low.
Owner:YABAO PHARMA GRP CO LTD
Biodegradable high-polymer bonded photoactive Pt (IV) anticancer medicament micelle and preparation method thereof
ActiveCN102416181ASignificant photoactivityFast releasePowder deliveryPharmaceutical non-active ingredientsChemical synthesisPolyester
The invention provides a biodegradable high-polymer bonded photoactive Pt (IV) anticancer medicament micelle and a preparation method thereof, which relate to the field of novel chemical synthesis medicaments and preparations thereof and are used for solving the problem that a high-polymer-platinum (IV) bonded medicament does not have optical activity in the prior art. In an anticancer medicament, ethylene glycol-b-polyester-b-polylysine serving as a biodegradable triblock copolymer or polyethylene glycol-b-poly(ester-co-carbonic ester) serving as a diblock copolymer is taken as a carrier polymer, and Pt (IV) is a photoactive tetravalent platinum composition coordinated with dihydroxyl in the axial direction. The invention further provides a preparation method of the biodegradable high-polymer bonded photoactive Pt (IV) anticancer medicament micelle. The anticancer medicament prepared with the method has remarkable optical activity; under the irradiation of UV (Ultraviolet) of 365 nanometers, Pt (IV) is reduced into Pt (II); a cis-platinum or oxaliplatin antitumor active part is contained; and the mass content of platinum can be up to 10-20 percent.
Owner:吉林市博禹祥实工贸有限公司
Transdermal plaster of rivastigmine and preparation process thereof
InactiveCN1994290AEasy to useClear curative effectOrganic non-active ingredientsEster active ingredientsTransdermal patchRivastigmine
The invention relates to a method for preparing kabalatin adhere agent for treating senile dementia, wherein said adhere agent can stably hold blood drug density and reduce feeding time, with high safety.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
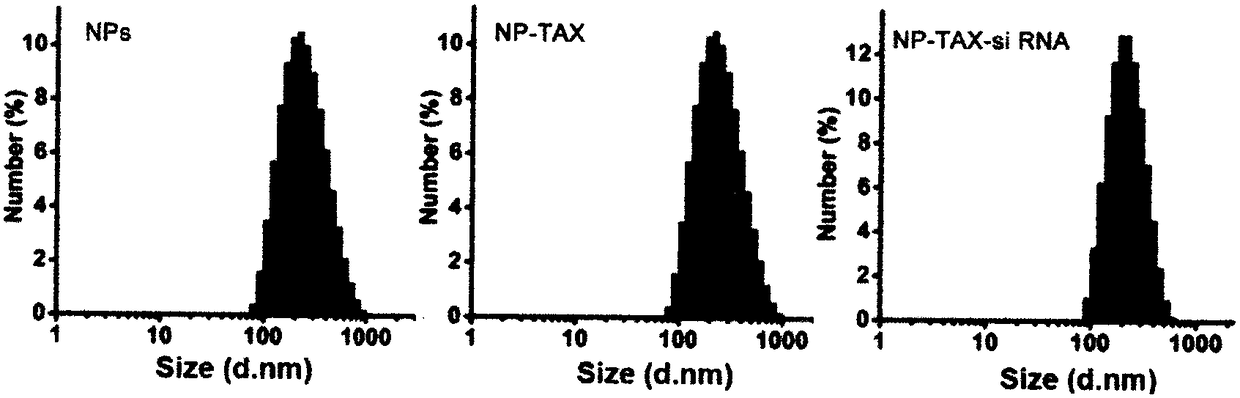
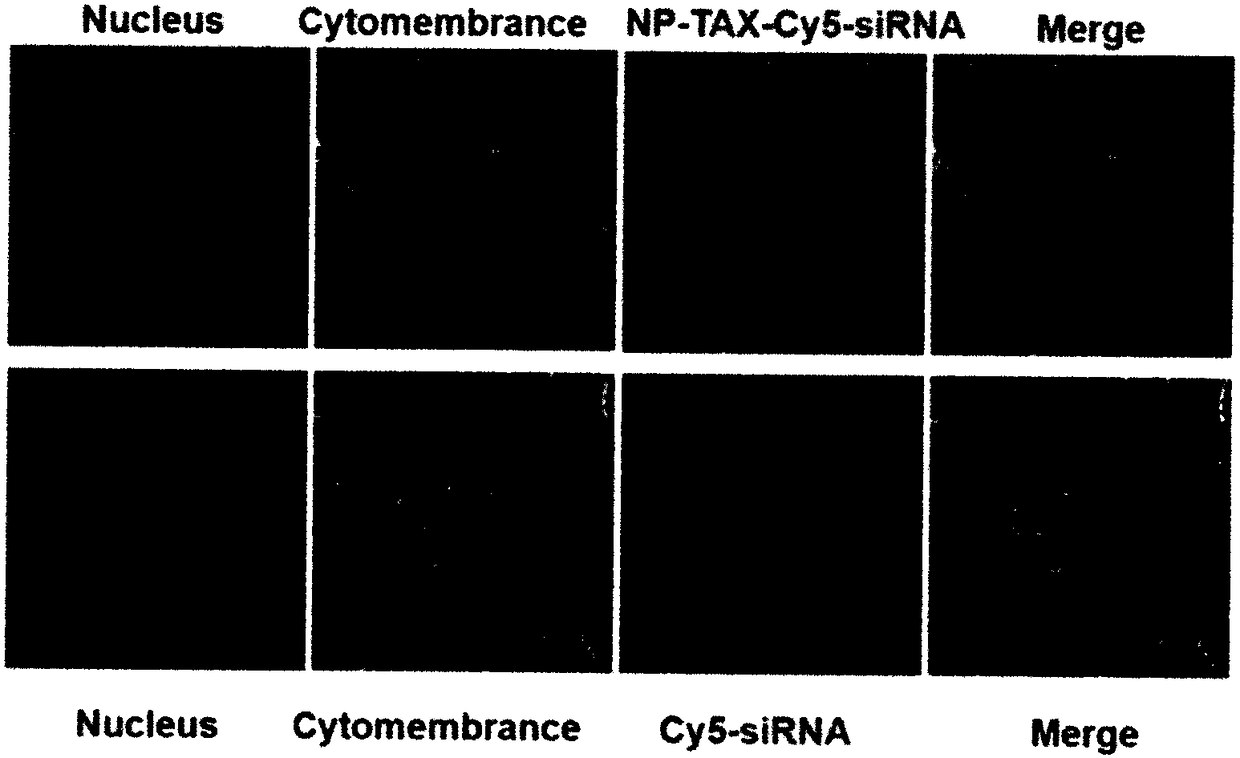
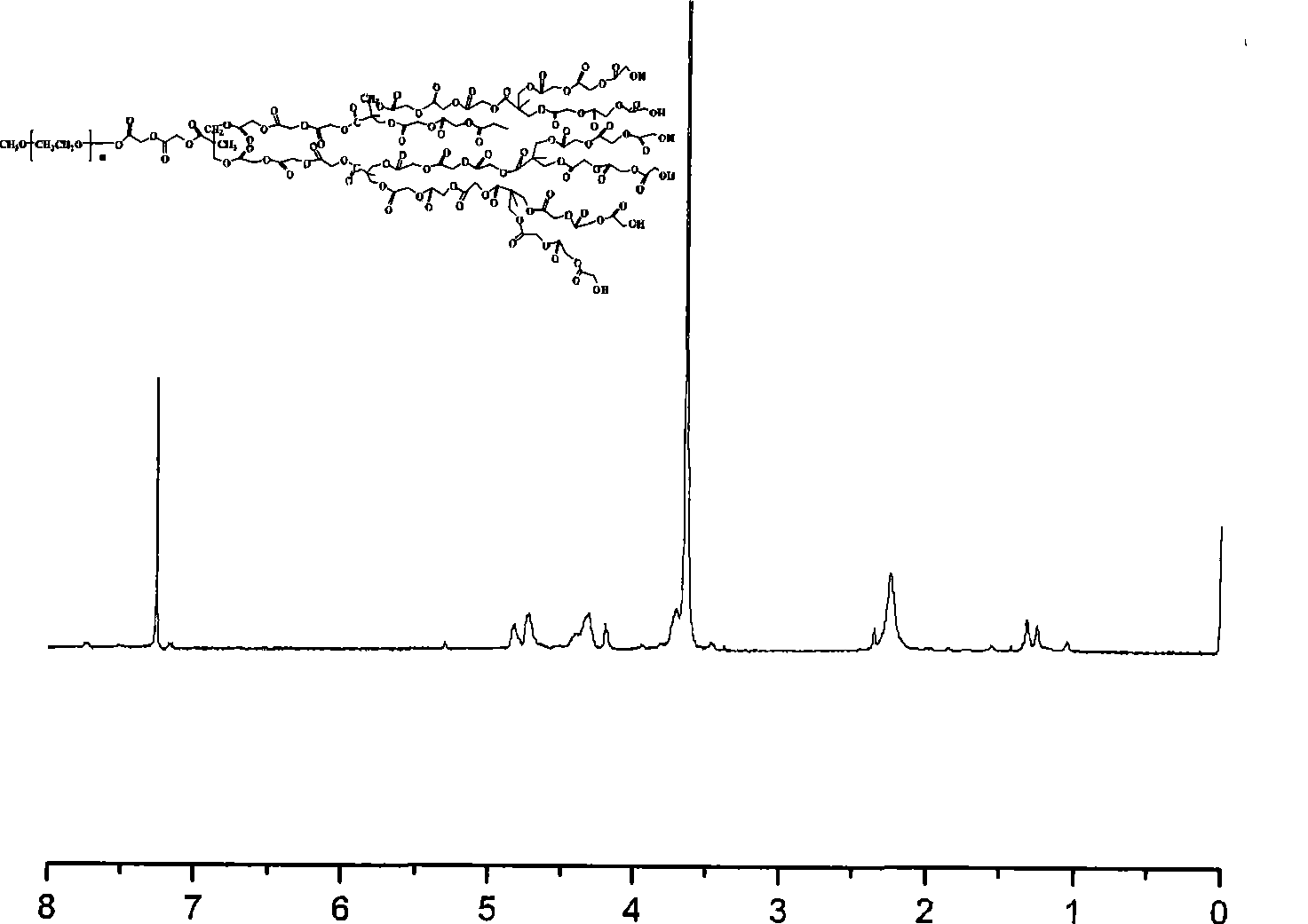
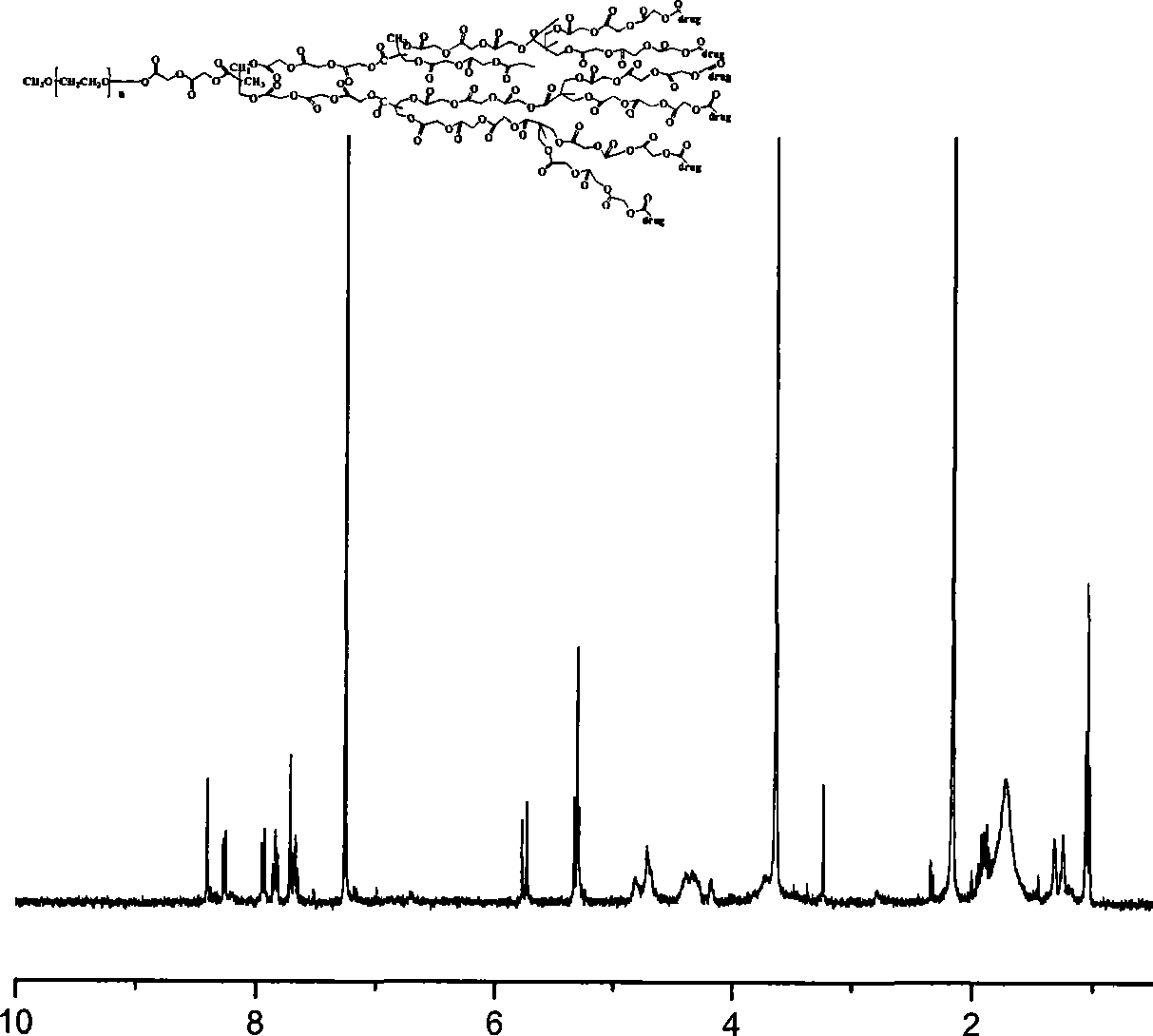
Preparation method of tumor targeted nanoparticle carrier co-loaded with breast cancer chemotherapeutic drug MTDH siRNA
ActiveCN108096583AGood curative effectReduce toxic and side effectsOrganic active ingredientsPharmaceutical non-active ingredientsBiocompatibility TestingMTDH
The invention discloses a preparation method of a tumor targeted nanoparticle carrier co-loaded with breast cancer chemotherapeutic drug MTDH siRNA. A PEI-PLGA (polyethyleneimine-poly(lactic-co-glycolic acid)) polymer is dissolved in dichloromethane, deionized water is added, the solution is subjected to ultrasonic crushing and emulsified into a homogenous emulsion, vinyl alcohol, hydrophobic taxol and dichloromethane are mixed and added to the emulsion, the mixture is subjected to ultrasonic crushing and emulsified, the emulsion is evaporated, a nanoparticle suspension is obtained, nanoparticle cores coated with taxol are prepared from the nanoparticle suspension, and then, bleaching, stirring and centrifugation are performed. The breast cancer chemotherapeutic drug and nucleic acid are carried into breast cancer tumor cells with high-expression MTDH genes to inhibit cell proliferation and have significant in-vitro and in-vivo tumor targeting. The carrier has clear anti-tumor effects,used carrier materials have high safety, and the carrier has good biocompatibility and biodegradability and has no biotoxicity or immunogenicity. The preparation process is simple, easy to operate, time-saving, energy-saving and suitable for mass production.
Owner:宋振川
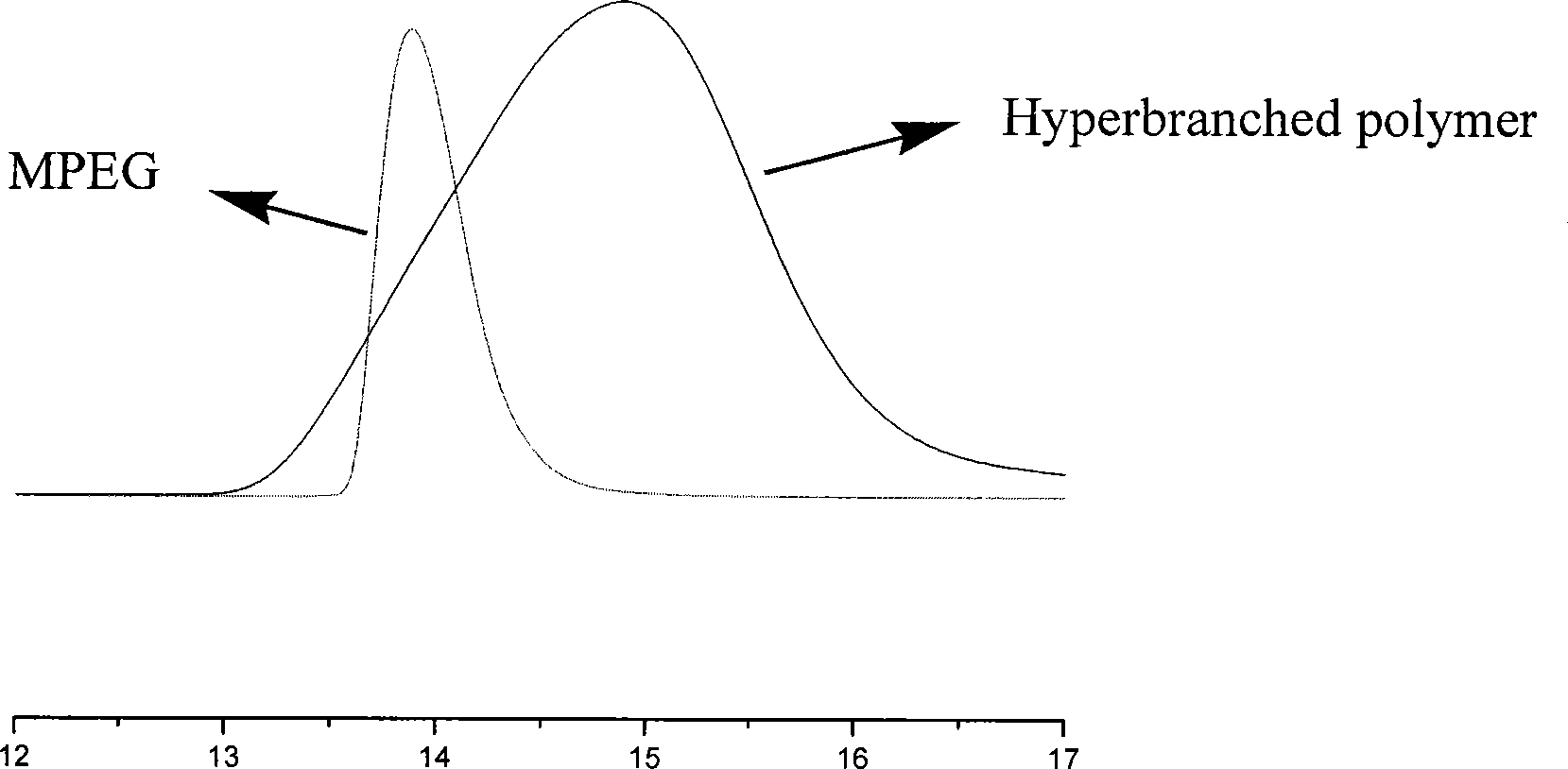
Anti-tumor prodrug using novel amphipathic hyperbranched polyesters as carrier and preparation method
InactiveCN101474411AGood curative effectReduce toxic and side effectsOrganic active ingredientsPharmaceutical non-active ingredientsWater basedPropanoic acid
The invention provides an antitumor prodrug with novel amphiphilic hyper branched polyester as a carrier and a preparation method thereof. The novel biodegradable amphiphilic hyper branched polyester is constructed as being based on dimethylol propionic acid, an oligomer of glycolic acid or lactic acid and polyethyleneglycol or polyethylene glycol monomethyl ether. A peripheral functional group of the obtained hyper branched polymer is a hydroxyl group. The hyper branched polymer is reacted with estolide to be transformed into the hydroxyl group, the hydroxyl group is esterified with antitumor drug with the existence of condensing agent to obtain a prodrug of the antitumor drug. The introduction of the oligomer of the glycolic acid or the lactic acid or an alternative oligomer thereof improves the biocompatibility of drug carrier material, and the degrading speed of the oligomer in body is controlled by regulating the polymerization degree and the chemical composition of the oligomer to realize the action of controlling the release speed of the drug, so that the invention has very good drug sustained controlled release action and avoids the toxic side effect to normal cells caused by the burst release of the antitumor drug. The prodrug has an amphiphilic property, so that the prodrug can be made into a water-based preparation and improves the hydrophilicity of the antitumor drug greatly.
Owner:XIANGTAN UNIV
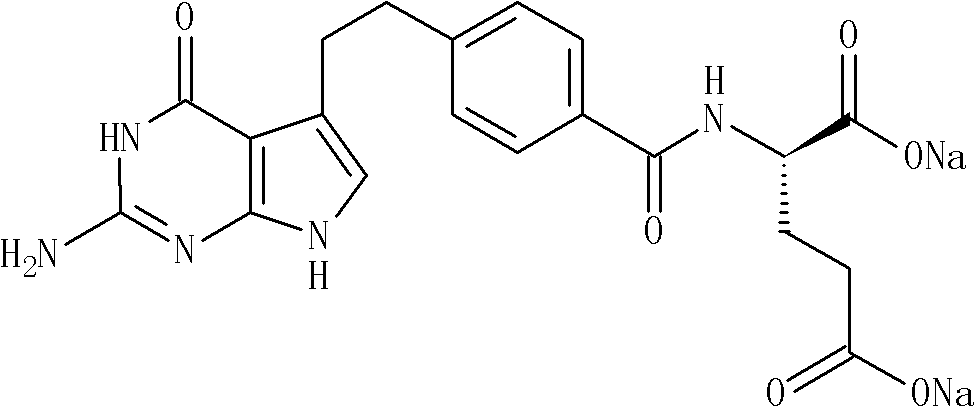
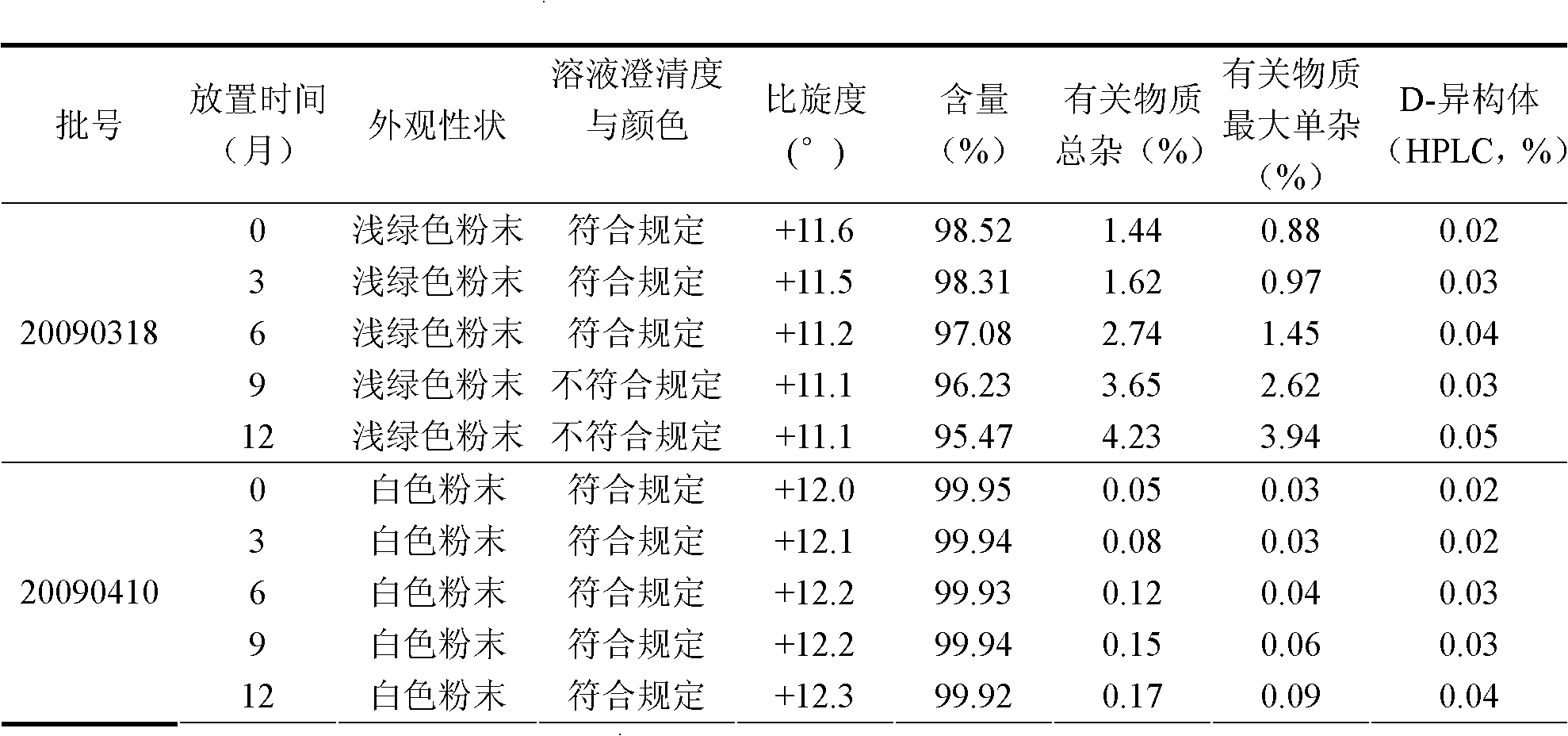
Industrialized production method of high-purity pemetrexed disodium
ActiveCN102086204AReduce Occupational InjuriesEasy to operateOrganic chemistryAcetonitrileSilica gel
The invention provides an industrialized production method of high-purity pemetrexed disodium, comprising the following steps of: (1) adding crude pemetrexed disodium into a reactor, adding water and stirring to dissolve at a temperature of 10-30 DEG C; (2) adding tetrahydrofuran or acetonitrile serving as a dissolvent into the reaction solution of the step (1), dissolving out a part of solids, adding kieselguhr or silica gel and stirring for 5-30 minutes; and (3) filtering the reaction solution of the step (2), adding dissolvent same as the dissolvent added in the step (2) into filtrate, crystallizing for 0.5-10 hours at a temperature of 10-30 DEG C, isolating solids, and drying for 0.5-10 hours at a temperature of 20-40 DEG C to obtain the high-purity pemetrexed disodium. By means of the production method, the shortcomings that in the prior art column chromatography, purification and heating are needed, the product purity is low, the operation is cumbersome and the industrialized production is difficult to realize are overcome; the production method is simple and convenient for operation, is easy to realize the industrialized production and has the advantages of few consumption of dissolvent, energy saving, environmental protection and low labor intensity; and the products have the advantages of white color, high purity, less than 0.05% of impurities in a single product and good stability.
Owner:NANJING HAIRUN PHARM CO LTD
Traditional Chinese medicine compound preparation for treating ovarian cancer and preparation method of traditional Chinese medicine compound preparation
InactiveCN102670944AImprove clinical symptomsImprove the quality of lifeUnknown materialsAntineoplastic agentsTolerabilityWhite blood cell
The invention belongs to the field of traditional Chinese medicine and relates to a traditional Chinese medicine compound preparation for treating ovarian cancer and a preparation method of the traditional Chinese medicine compound preparation. According to the traditional Chinese medicine compound preparation, extracts of bulk medicine such as astragalus mongholicus, codonopsis pilosula, rehmannia root, sculellaria barbata, asparagus cochinchinensis, medlar, cornu cervi, fiveleaf akebia fruit, elecampane and radix paeoniae alba are prepared into solid and liquid preparations. Animal experiment results show that the weight loss and the white cell reduction can be relieved, the T lymphocyte function is improved, the bax expression is increased, the cancer cell apoptosis is promoted, the anti-tumor angiogenesis effect is realized, the oxygen deficiency microenvironment of tumor cells is improved, and the sensitivity of ovarian cancer cells on chemotherapeutics is enhanced. Clinical test results show that the clinical symptoms of later-stage ovarian cancer patients can be relieved, and the survival quality of patients is improved; the chemotherapy toxic and side effect is lightened, and the chemotherapy tolerance and the compliance of the patients are improved; and the uncontrolled and recurrence rate of later-stage ovarian cancer can be reduced, and the five-year survival rate is improved; and no obvious adverse reaction exists, and good safety is realized. The traditional Chinese medicine compound preparation is applicable to the treatment on ovarian cancer and ovarian cancer postoperative chemotherapy patients.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Lipidosome-polymer hybrid nano particle and preparation method and application thereof
ActiveCN103893123AGood curative effectReduce toxic and side effectsPharmaceutical non-active ingredientsAntineoplastic agentsSolubilitySide effect
The invention relates to a lipidosome-polymer hybrid nano particle and a preparation method and an application thereof. The lipidosome-polymer hybrid nano particle comprises an amphiphilic polymer nano particle and a lipid bilayer coating the surface of the amphiphilic polymer nano particle as well as medicines embedded into the amphiphilic polymer nano particle and between the amphiphilic polymer nano particle and the lipid bilayer and having different water solubilities. The nano particle provided by the invention has a multi-layered structure to embed medicines with different water solubilities, thereby realizing a synergistic effect of different medicines. The particle is high in biocompatibility, can circulate in blood for a long time, has the targeted and sustained-release effects, improves the curative effect of the medicines and reduces the toxic and side effects of the medicines, and can be used as a carrier system of an anti-tumor medicine. The preparation method provided by the invention is simple in process, convenient in operating process, and can be produced in a small scale in a laboratory and also can be industrially produced in a large scale.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Industrialized production method for high-purity decitabine
InactiveCN101948493AReduce the impactMeet quality requirementsSugar derivativesSugar derivatives preparationSodium methoxideSolvent
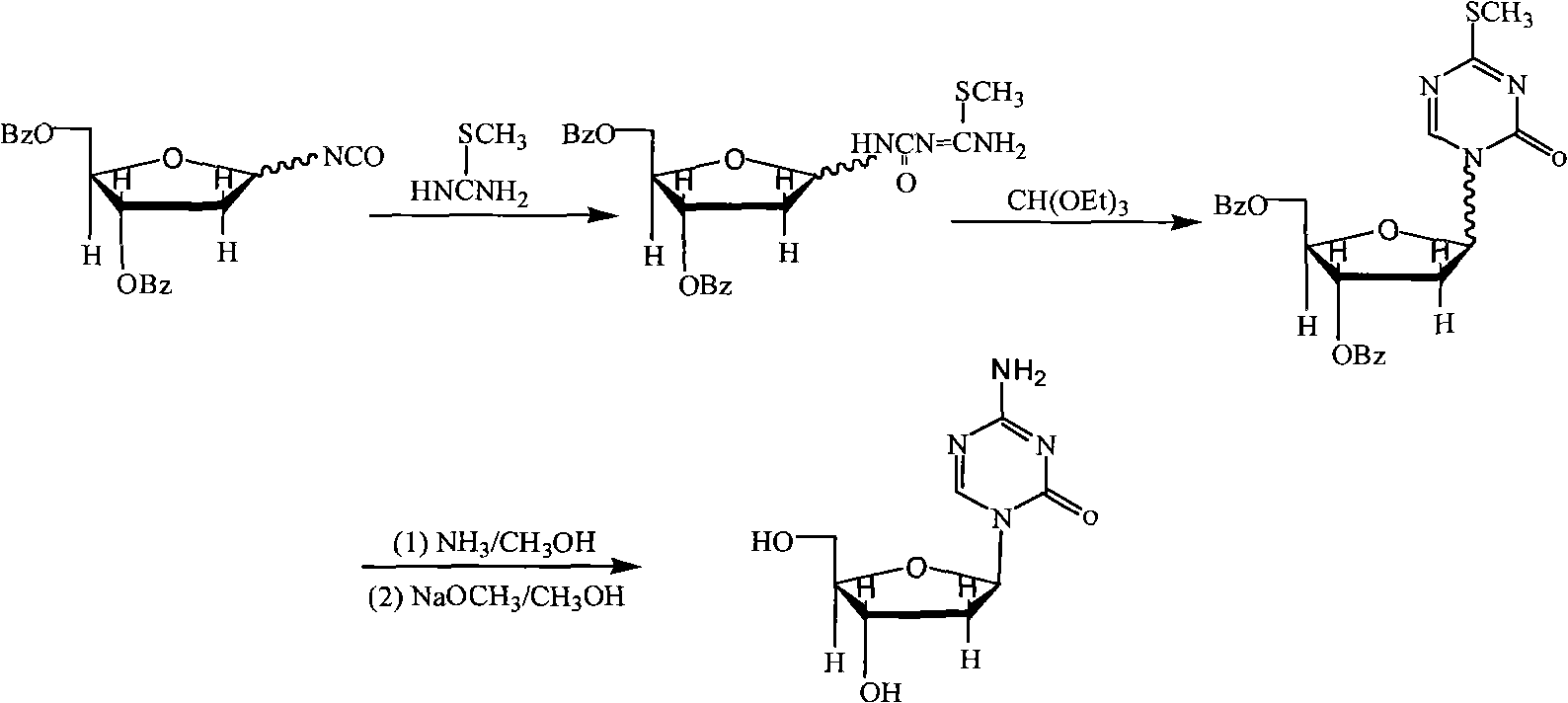
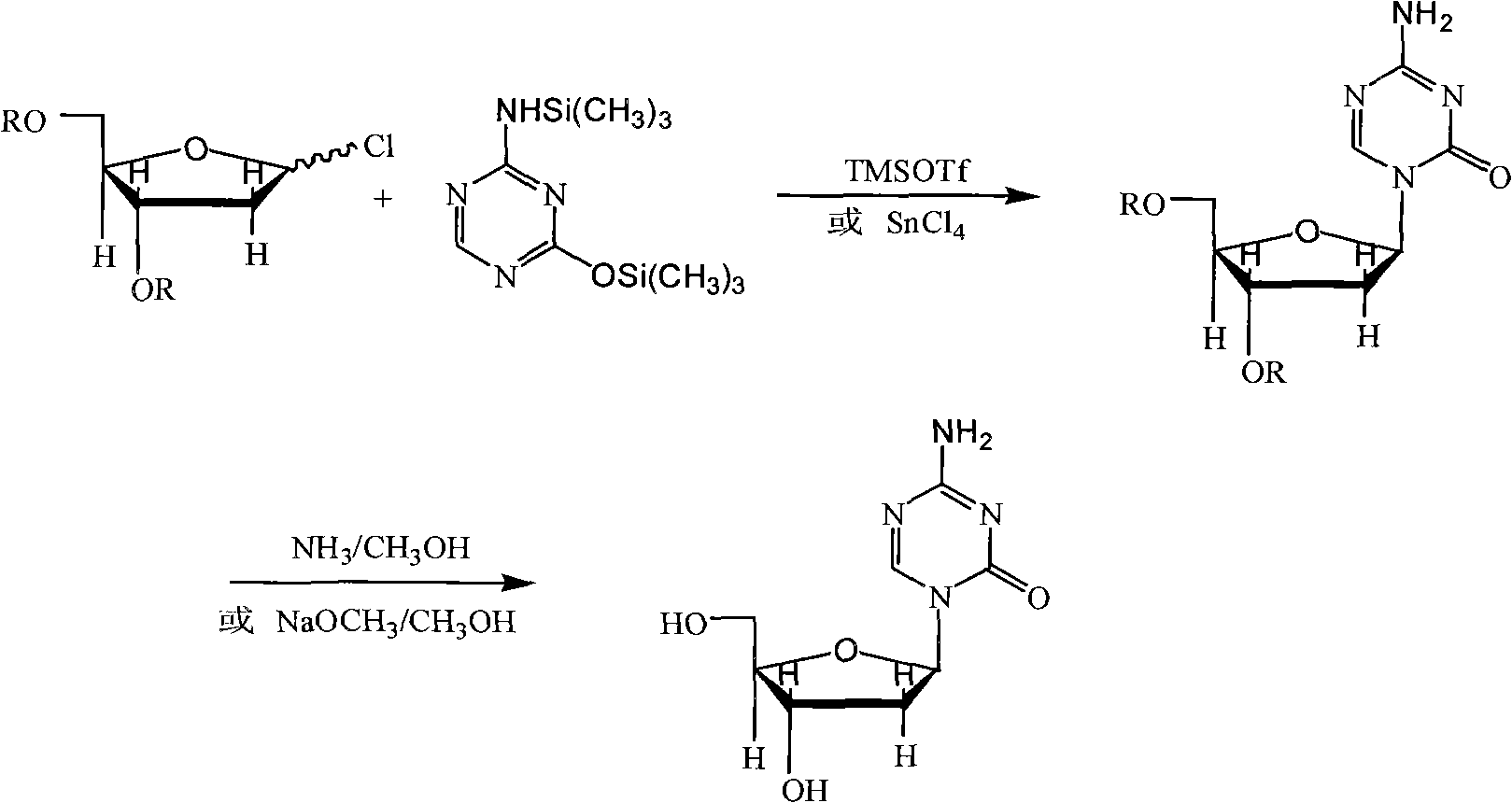
The invention provides an industrialized production method for high-purity decitabine. The method comprises the following steps of: 1, performing silanization reaction of 5-azacytosine, bis(trimethylsilyl)amine and trimethyl chlorosilane, which serve as raw materials, to prepare 2,4-bis(trimethylsilyl)-5-azacytosine; 2, performing reaction of the product obtained by the step 1 and 1-chloro-3,5-bis-(4-chlorobenzoyl)-2-deoxy-D-ribofuranose, which serve as raw materials, to prepare a crude product of 1-(3,5-bis-(4-chlorobenzoyl)-2-deoxy-beta-D-ribofuranose)-5-azacytosine; 3, dissolving the product obtained by the step 2 in a C5 to C7 hydrocarbon, stirring, filtering and drying to obtain a refined product; and 4, producing the high-purity decitabine by using the product obtained by the step 3, methyl alcohol and sodium methoxide as raw materials. The method overcomes the disadvantages of need of column purification, low purity, difficult industrial production in the prior art, and has the advantages of simple and convenient operation, small solvent consumption, small influence on the environment, low labor intensity, short period, high product purity, single impurity and less than 0.1 percent total impurity content.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Compound taxol and its derivative docetaxel fat emulsion and preparation method
InactiveCN101006997APro-apoptosisGood anticancer effectOrganic active ingredientsEmulsion deliverySolubilityVegetable oil
The invention relates to complex paclitaxel and its derivates docetaxel intralipid which includes the following ingredients: paclitaxel or docetaxel, vegetable oil, solubilizing agent, lecithin, glycerine, and water for injection at a ratio of 0.5-10:10-100:10-100:10-20:20-25:700-950. The preparing method includes the following steps: stirring with high speed homogenating machine or ultrasonic oscillating to get the protogala; preparing the complex paclitaxel intralipid with high pressure homogenizer. The preparation is intralipid in O / W type which packages paclitaxel or docetaxel into the compound oil phase. The compound oil phase has good solubility for paclitaxel or docetaxel which has prevented the phenomenon of precipitation after diluting the emulsion; the ingredients of compound oil has the function of coordinated antitumous effect; it can also release the injecting irritative response, haemolysis and hypersensitiveness; it has the function of targeting which has increased the drug action.
Owner:董英杰
Traditional Chinese medicinal composition plaster for curing rheumatism or rheumatoid diseases and preparation method thereof
InactiveCN101167937AReduce toxic and side effectsEasy to useHydroxy compound active ingredientsAntipyreticRegimenLiver and kidney
The invention provides a Chinese traditional medicament composition emplastrum for curing rheumatism or rheumatoid diseases, which has the functions of nourishing liver and kidney, strengthening muscles and bones, dispelling rheumatism and dredging meridian, and is applicable to the patient having the symptoms of arthralgia for a long time and deficient body, arthralgia, local intumescence, rigidity and malformation, unfavorable flexion and extension, and rheumatoid arthritis. The efficacy composition of the invention is that the proportion by weight of the materials is radix rehmanniae 15-25, prepared radix rehmanniae 15-25, black aconite 10-20, japanese teasel 10-20, pubescent angelica 5-15, drynaria fortunei 10-20, cassia twig 5-15, ledebouriella root 5-15, barrenwort 10-20, gleditsia sinensis sting 5-15, sheep bone 15-25, clematis root 10-20, white peony root 10-20, common anemarrhena 10-20, processed cibotium barometz 10-20, safflower 5-15, and lycopodii herb 5-15. The emplastrum of the invention can be directly pasted on the affected part, thereby having convenient utilizing, quick execution and short period of treatment.
Owner:LIAONING HUANREN PHARMA
Externally used medicine for expelling wind and clearing away cold, activating meridians to stop pain and preparation method thereof
InactiveCN101559128ANo allergiesAvoid stimulationAnthropod material medical ingredientsAntipyreticDiseaseIrritation
The invention provides an externally used medicine for expelling wind and clearing away cold, activating meridians to stop pain and a preparation method thereof. The medicine is prepared by 25 types of medicinal materials such as radix aconiti preparata, wild aconite root, nux vomica (processed), epimedium, achyranthes root, notopterygium root, cyrtomium fortunei, phellodendron, zaocys dhumnade, hairy antler, dipsacus root, dark plum, asarum, Chinese ephedra, cassia twig, safflower, acanthopanax, honeysuckle, earth worm, loranthus, licorice, drynaria (scald), anisetree bark, myrrh gum (processed) and red ginseng. The medicine links closely with pathogen and pathogenesis of a disease and a plurality of medicines are compatible reasonably, thus expelling wind and clearing away cold as well as activating meridians to stop pain. As an externally used cataplasm, the medicine is taken through skin, thus avoiding the irritation of an oral preparation to gastrointestinal tract; in addition, relatively slow percutaneous absorption process inevitably greatly mitigates drug toxicity to the whole body. Neither skin sensibility nor skin irritation occurs. Therefore, the transdermal drug delivery of new preparation improves the safety of medicine taking to a certain extent and provides new choices for safe clinical medicine use.
Owner:潘首德
Chinese traditional medicine composition for treating tumor and preparing method thereof
ActiveCN101612357AWith symptom reliefGrowth inhibitionUnknown materialsAluminium/calcium/magnesium active ingredientsStrychnosToxin
The invention provides a Chinese traditional medicine composition for treating tumor and a preparing method thereof. The Chinese traditional medicine composition is prepared from radix curcumae, agrimony, trogopterus dung, white vitriol, saltpeter, resina toxicodendri, bitter orange and nux vomica powder, wherein the nux vomica poser is prepared from strychnos alkaloid and auxiliary material, and the auxiliary material is one or a mixture of some of dextrine, starch, mannite, microcrystalline cellulose and calcium bicarbonate. The composition has functions of supporting healthy energy, cleaning heat and toxins, and the like, can regulate immune function of an organism, reduce side and toxic effects and combine cancer prevention with cancer treatment, thereby improving the treating effect for tumor.
Owner:XIAN C P PHARMA
Nimodipine lyophilized emulsion for injection and preparing method thereof
InactiveCN101199522AAvoid stimulationAvoid harmOrganic active ingredientsPowder deliveryFreeze-dryingNimodipine
The invention relates to nimodipine lyophilization dry emulsion for injection. Before freeze-dried or reconstituted, according to percentage concentration per 1000 ml of fat emulsion, the lyophilization dry emulsion contains 0.001 percent to 0.2 percent of nimodipine, 0.5 percent to 30 percent of oiliness solvent, 0.1 percent to 5 percent of emulsifier, 5 percent to 40 percent of the freeze-drying protective agent and 0.1 percent to 10 percent of isotonic regulator. The invention also relates to a preparation method of nimodipine lyophilization dry emulsion. The invention has the advantages that ethanol is avoided to decrease irritation; the product can be mixed with any proportion of water for injection, sodium chloride solution, glucose solution, blank fat emulsion or other aqueous solution without phenomena of precipitation or crystallization; in addition, compared with the fat emulsion, the lyophilization dry emulsion is more helpful to improve the stability of nimodipine and excipient of the nimodipine, thereby lowering the requirements of production, transportation and storage conditions and prolonging the period of validity.
Owner:YAOPHARMA CO LTD +1
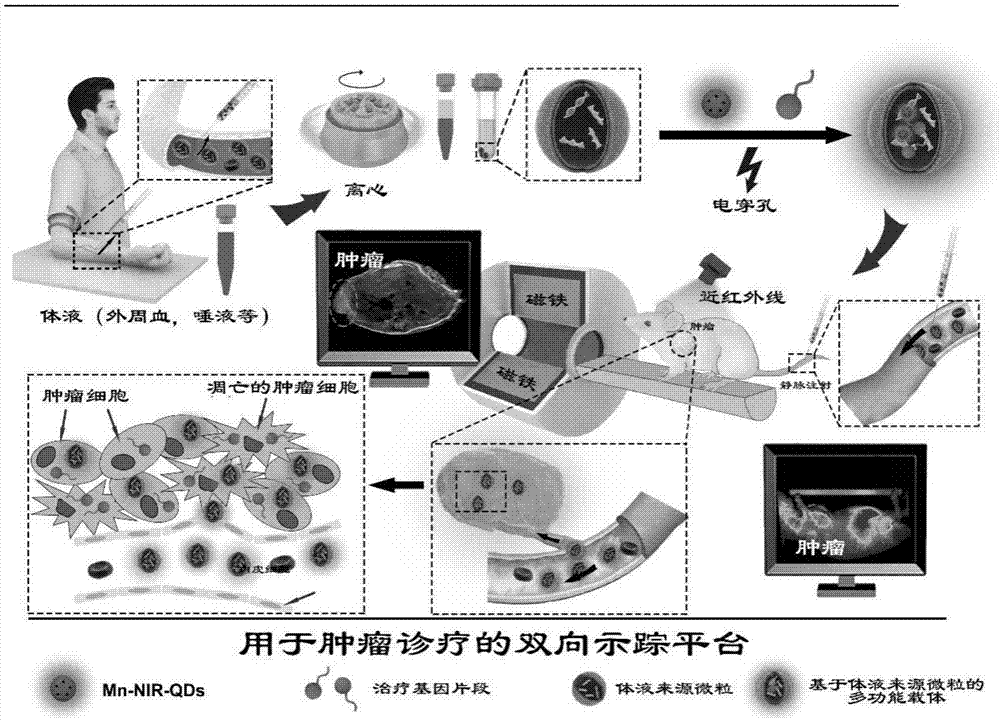
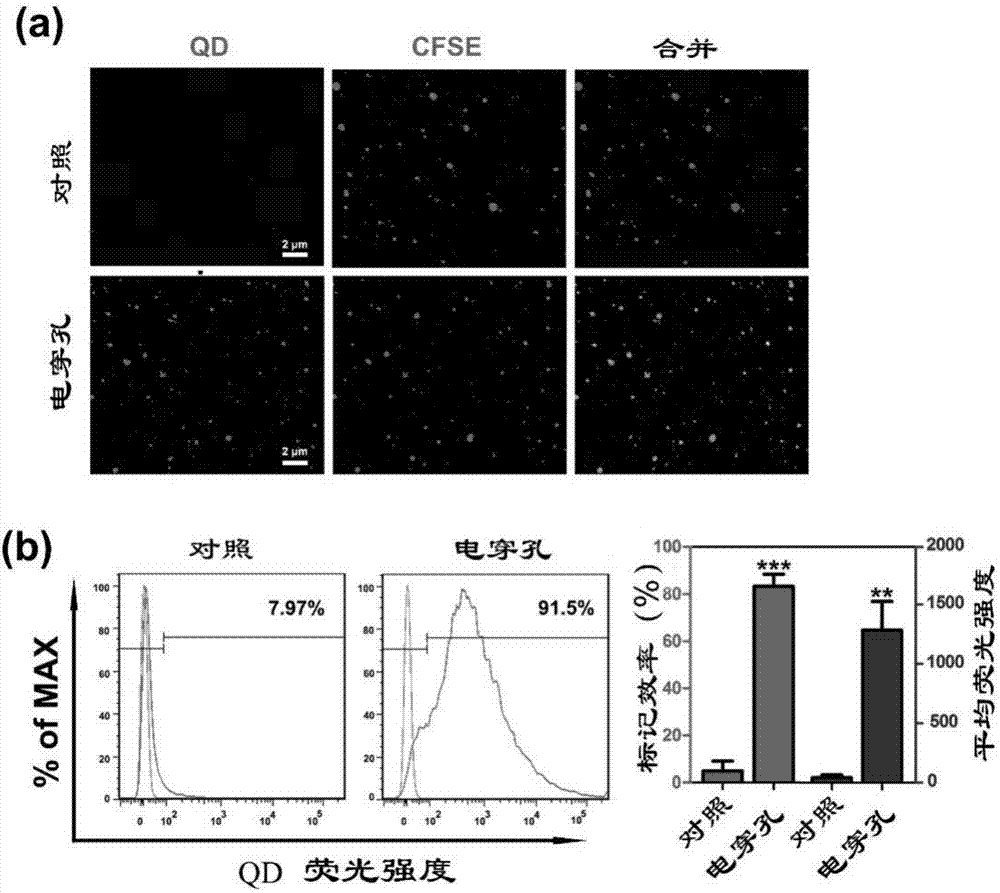
Multifunctional carrier based on cytogenic vesicle in body fluid, preparation method and application
ActiveCN107375234AGood biocompatibilityExtended half-lifeOrganic active ingredientsLuminescence/biological staining preparationChemistry
The invention discloses a multifunctional carrier based on cytogenic vesicle in body fluid, a preparation method and an application. The method comprises the following steps: (1) collecting human body fluid, performing gradient ultra-centrifugation, removing supernatant and using sterile PBS heavy suspension for precipitating and (2) adding the cytogenic membrane vesicle from the body fluid, ultra-small luminescent material and electroporation buffer solution into an electric shock cup, uniformly mixing, placing onto an electroporation instrument and treating, placing under room temperature and repairing after the ending of electroporation, performing ultra-centrifugation and then using sterile PBS heavy suspension for precipitating, thereby acquiring the multifunctional carrier based on the cytogenic membrane vesicle from the body fluid and marked with the ultra-small luminescent material. The carrier is used for preparing a chemotherapeutic drug or gene therapeutic drug for treating or preventing tumor, has high biocompatibility, is convenient to carry various therapeutic drugs, is capable of effectively restraining tumor growth, is suitable for diagnosis and treatment of various innocent and malignant tumors and especially supplies a choice for the diagnosis and treatment of deep tumors in skull, thoracic cavity and abdominal cavity.
Owner:WUHAN UNIV
Levetiracetam osmotic pump controlled release tablet and preparation method thereof
InactiveCN101422442ASmooth and sustained releaseReduce toxic and side effectsNervous disorderPharmaceutical delivery mechanismSide effectActive matter
The invention belongs to the technical field of medicament and provides a Levetiracetam osmotic pump controlled-release tablet and a preparation method thereof. The invention consists of the accessory of the Levetiracetam playing the effect of release control and a semi-transparent membrane; in the invention, proper accessory and medicament are mixed to press a tablet core at first; then a layer of semi-transparent membrane is coated outside the tablet core; then at least one small hole is punched on the semi-transparent membrane so as to lead active matters to be released from the semi-transparent membrane, thereby controlling the release of the medicament. Compared with a common preparation, the controlled-release preparation prepared by the invention has the advantages of small wave range of the blood medicine concentration, reducing toxic and side effect, being taken once in one day and improving the compliance of sufferers. The controlled-release preparation is applied on the adjunctive therapy for the partial seizure of epileptics in clinic.
Owner:SHENYANG PHARMA UNIVERSITY
Chinese-medicinal suppository for treating lumbar vertebrae disease
InactiveCN1895600AReduce toxic and side effectsEnhance immune functionAmphibian material medical ingredientsHeavy metal active ingredientsDiseaseLumbar intervertebral disc
A Chinese medicine in the form of suppository for treating lumbar vertebra disease, lumbar intervertebral disc protrusion, tonic rachitis and hyperosteogeny is prepared from 9 Chinese-medicinal materials including astragalus root, capejasmine fruit, musk, cinnamon twig, etc through pulverizing, extracting in alcohol, filtering, concentrating by distilling, to obtain concentrated liquid, immersing catgut suture in physiologic saline, baking, shearing short, putting them in bottle, filling said concentrated liquid, sealing and magnetizing.
Owner:方焕生
Method for preparing hollow fiber with temperature sensitive medicinal hydrogel and application thereof
ActiveCN1961959AAvoid first pass effectAvoid degradationHollow filament manufacturePharmaceutical non-active ingredientsHollow fibreFiber
The invention relates to a method for preparing temperature-sensitive aquagel hollow fiber, wherein it uses hollow fiber as carrier, whose chamber contains temperature-sensitive aquagel; the aquagel will change phase at human temperature to release the drug. And the preparation comprises a, holding the holes of hollow fiber; b, putting temperature-sensitive monomer solution into the chamber of hollow fiber; c, compositing temperature-sensitive aquagel in the chamber; d, using said aquagel to absorb drub. The invention has better temperature-sensitive property and slow-release property.
Owner:LANGSHA KNITTING
Chinese-medicinal suppository for treating cervical spondylosis and its preparation
InactiveCN1895633ANo side effectsSignificant effectAmphibian material medical ingredientsHeavy metal active ingredientsDiseaseCervical spondylosis
A Chinese medicine in the form of suppository for treating lumbar vertebra disease, lumbar intervertebral disc protrusion, tonic rachitis and hyperosteogeny is prepared from 10 Chinese-medicinal materials including astragalus root, capejasmine fruit, musk, cinnamon twig, etc through pulverizing, extracting in alcohol, filtering, concentrating by distilling, to obtain concentrated liquid, immersing catgut suture in physiologic saline, baking, shearing short, putting them in bottle, filling said concentrated liquid, sealing and magnetizing.
Owner:方焕生
Medicine for treating gastroesophageal reflux disease and functional dyspepsia
InactiveCN101143143AImprove toleranceHigh synergistic effectDigestive systemSolution deliveryLansoprazoleRabeprazole
A combination preparation for remedying the gastroesophageal reflux disease (GERD) and the functional dyspepsia is characterized in that the prescription of the combination preparation consists of a proton pump depressor and a gastrointestinal power drug of itopride; the proton pump depressor is selected from one of a Pantoprazole, a Omeprazole, a Esomeprazole, a Lansoprazole, a Rabeprazole, a Tenatoprazole and a Leminorazole, wherein the Pantoprazole is preferential, and at the same time the neutral form of the basic salt of the proton pump depressor is also included, such as Naplus, Mg2plus, Ca2plus, Kplus or Li plus salt and a pure optical stereoisomer of the proton pump depressor or an active metabolite of the proton pump depressor; the gastrointestinal power drug is the itopride and a ramification of the itopride or one of the medicinal salts of the itopride; in the combination preparation, the weight ratio of the Pantoprazole and the itopride is 2 to 5 to 2 to 7. The invention has important affect for remedying the gastroesophageal reflux disease and the functional dyspepsia, and the preparation method of the invention is simple and convenient; the cost is low; the invention is fit for being orally taken by the patient; the invention has good conformance performance, high curative effect, low recrudescence rate and little adverse reaction.
Owner:沈阳东宇药业有限公司
Econazole nitrate microemulsion aerosol and method for preparing same
InactiveCN101518514AGood curative effectHas practical valueOrganic active ingredientsAntimycoticsPolyethylene glycolDissolution
The invention relates to an econazole nitrate microemulsion aerosol and a method for preparing the same. The aerosol is prepared by the materials mainly comprising econazole nitrate, polyethylene glycol 400, polysorbate 80, octane pheno1 polyoxyethylene 10, imidazoline, sodium lauryl sulfate, coconutt diethanol amide, sodium laureth sulfate, dioctyl sulfosuccinate sodium salt, polyoxyethylenated castor oil, purified water, and propane and butane. The method for preparing the aerosol comprises the following steps: dissolving the materials under different conditions; uniformly stirring the solution obtained after dissolution to obtain a primary emulsion; passing the primary emulsion first through a 0.80 mu m of filter membrane, then a nanomachine, and finally a 0.22 mu m of filter membrane; and filling, filling propane-butane projectile and sealing to obtain the finished product of econazole nitrate nanometer microemulsion aerosol.
Owner:周小萍
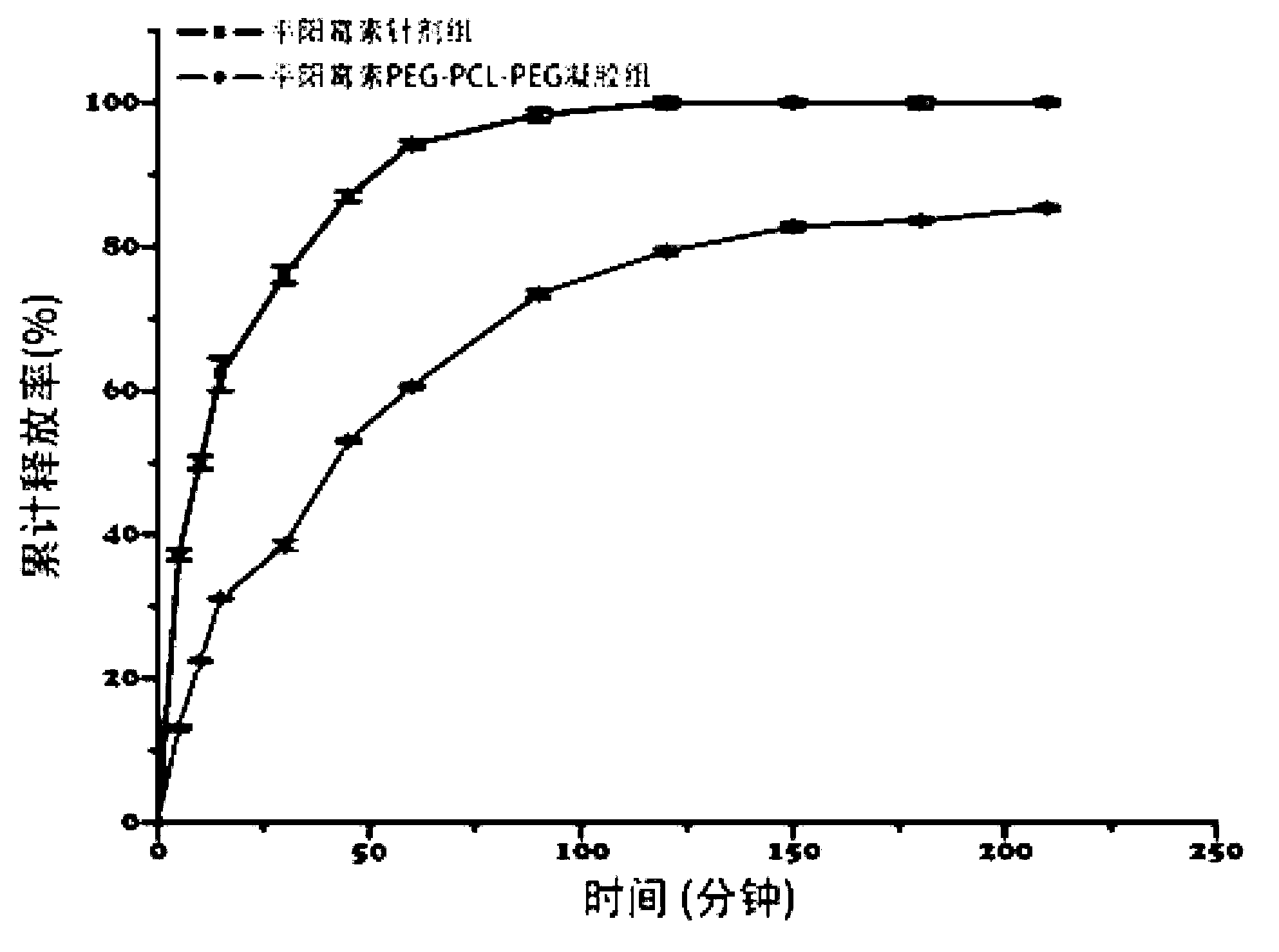
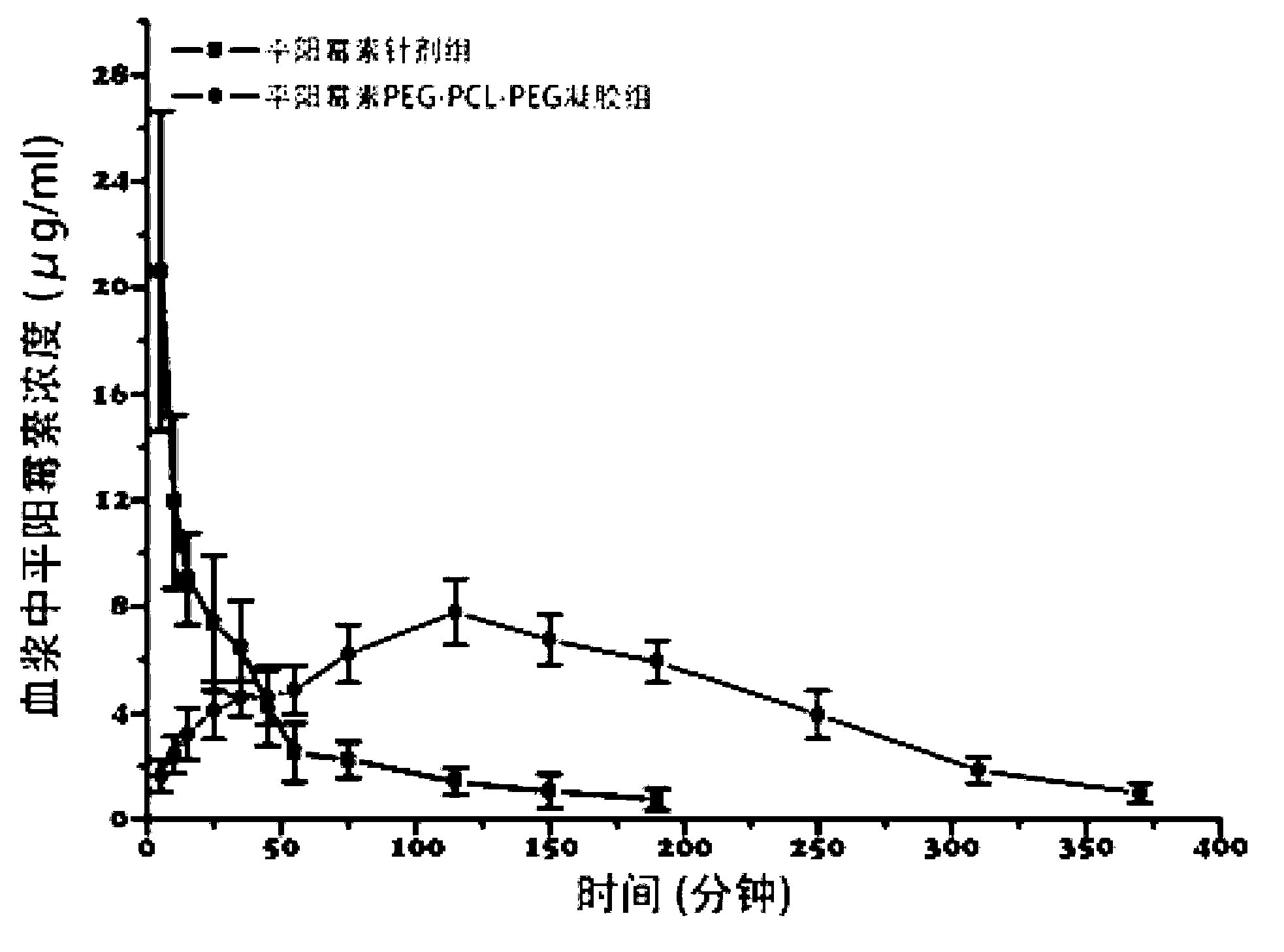
Pingyangmycin polyethylene glycol (PEG)-polycaprolactone (PCL)-polyethylene glycol (PEG) temperature-sensitive slow-release gel, as well as preparation method and application of same
ActiveCN102836418AExtended half-lifeExtension of timeAerosol deliveryOintment deliverySide effectHalf-life
The invention discloses a pingyangmycin polyethylene glycol (PEG)-polycaprolactone (PCL)-polyethylene glycol (PEG) temperature-sensitive slow-release gel, as well as a preparation method and the application of the gel. The Pingyangmycin PEG-PCL-PEG temperature-sensitive slow-release gel mainly consists of two parts comprising PEG (polyethylene glycol)-PCL (polycaprolactone)-PEG (polyethylene glycol) co-polymer and Pingyangmycin, is liquid at room temperature, and is solid gel under the in vivo 37 DEG C condition; the gel system has significant slow-release effect, thereby having functions in prolonging the half-life period and the acting time of the Pingyangmycin, reducing drug concentration in plasma, and reducing systemic toxic and side effects. The gel can be formed in situ after the medicine is injected in a high-flow-speed vessel, and then location embolism is realized, and so as to realize the, and muscularization and closing of muscle is caused, so that the sclerotherapy and the interventional therapy are combined effectively, the gel has excellent biocompatibility and degradability, is beneficial for treating hemangiomas, vascular malformations and cancers, particularly, a new selection is provided to the treatment of the vein malformation and partial malformation of cancers.
Owner:WUHAN UNIV
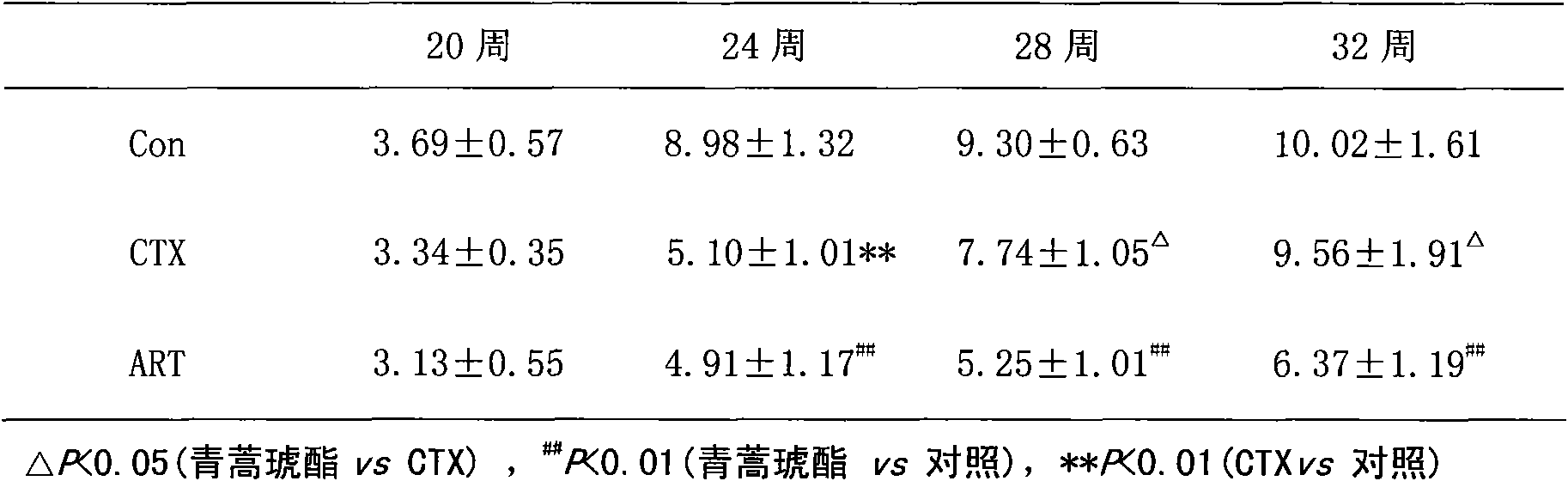
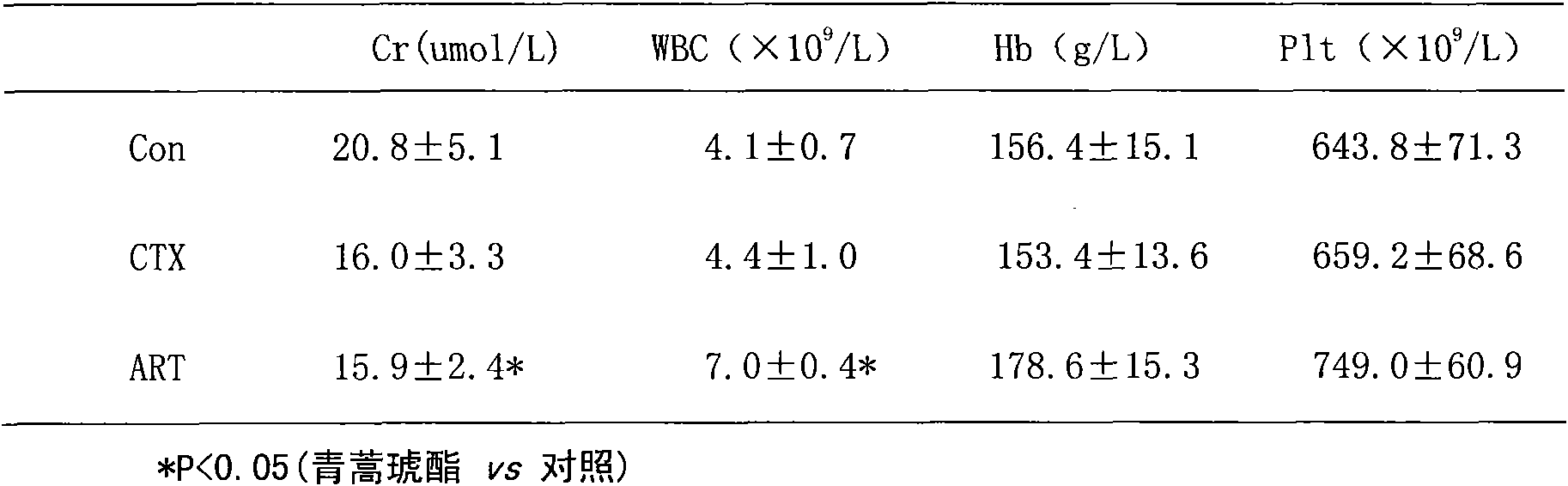
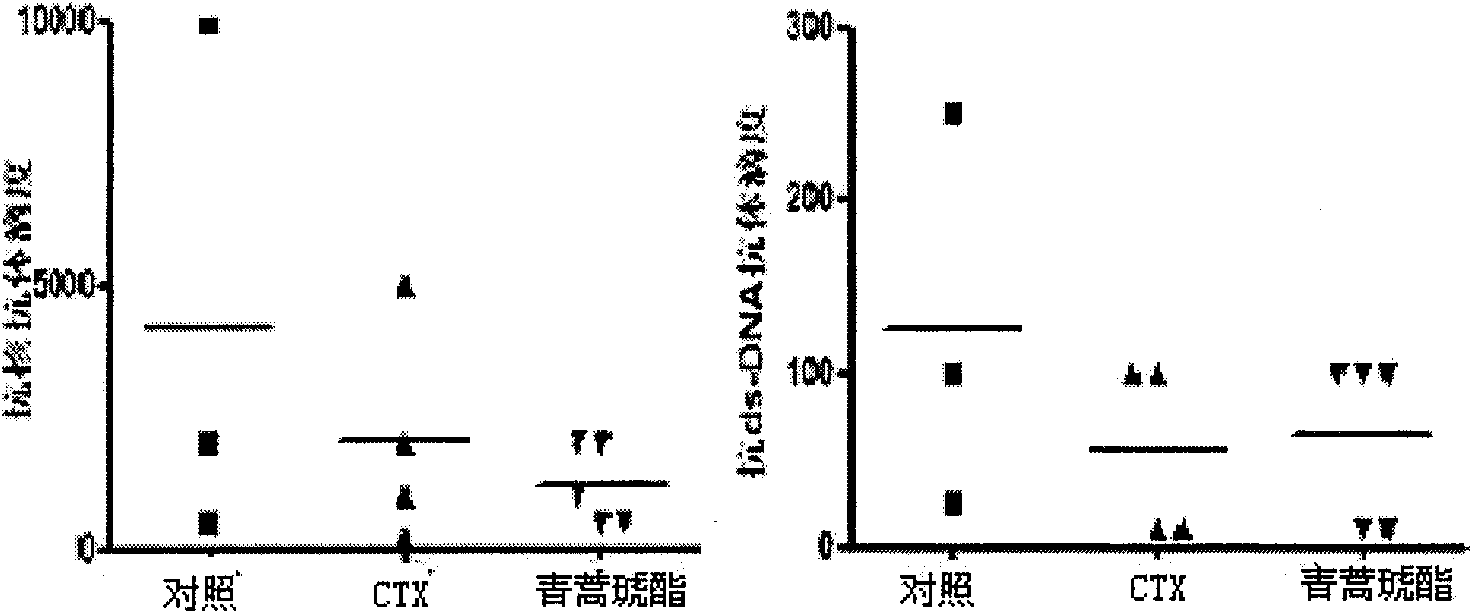
Application of artesunate as drug for treating systemic lupus erythematosus
InactiveCN101632657AMature production processQuality improvementOrganic active ingredientsSkeletal disorderLupus pernioSide effect
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
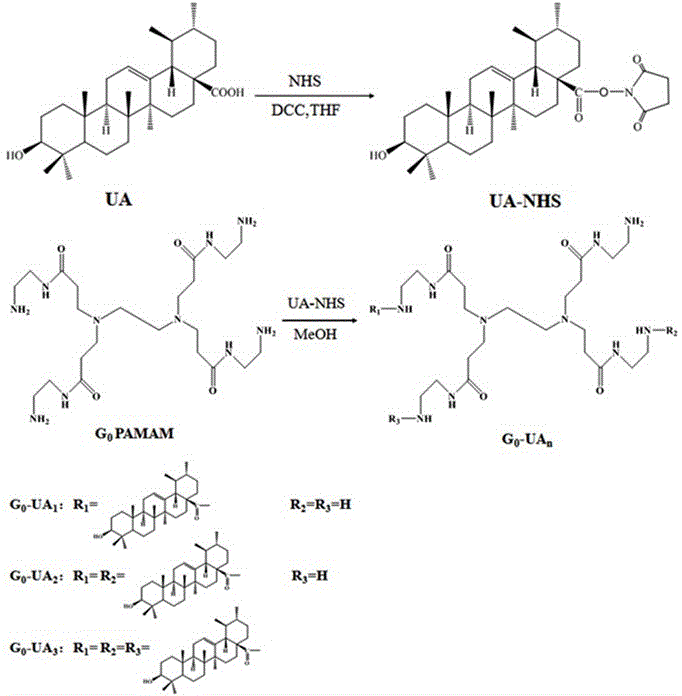
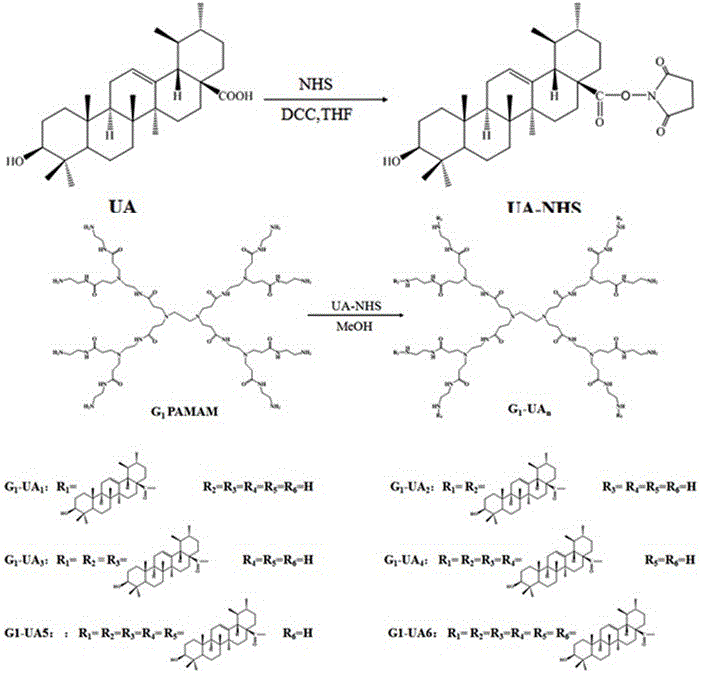
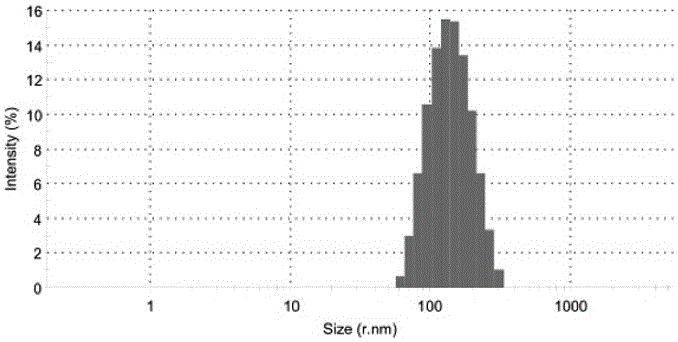
Amphiphilic self-assembled nanomicelle based on nano Low-generation PAMAM (polyamidoamine) dendrimer and application thereof
InactiveCN105854027AThe synthesis steps are simpleLow costOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityNanocarriers
The invention relates to an amphiphilic self-assembled nanomicelle based on nano Low-generation PAMAM (polyamidoamine) dendrimer and application thereof, particularly obtained conjugating low-generation PAMAM dendrimer and a hydrophobic agent and self-assembling in water via ultrasonic vibration. The nanomicelle has active molecules or a prodrug with drug loading capacity; the chemical conjugation of the hydrophobic agent and the low-generation PAMAM dendrimer helps improve the defects of poor solubility of the hydrophobic drug and low bioavailability, and a new idea is provided to solve the problem that traditional clinical chemotherapeutics are high in toxicity and low in safety coefficient; the nanomicelle can also function as a nanocarrier to carry other hydrophobic drugs, enabling joint use of drugs and enhancing therapeutic effect.
Owner:FUZHOU UNIV
Preparation of Chinese traditional medicine for treating trmor in alimentary canal
InactiveCN1695731AEnhance immune functionPromotes Health and LongevityUnknown materialsGranular delivery
A Chinese medicine for treating the tumor in digestive tract is proportionally prepared from 8 Chinese-medicinal materials including pseudostellaria root, Chinese angelica root, ganoderma, yam, etc.
Owner:SHANGHAI FANGXIN HEALTH TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com