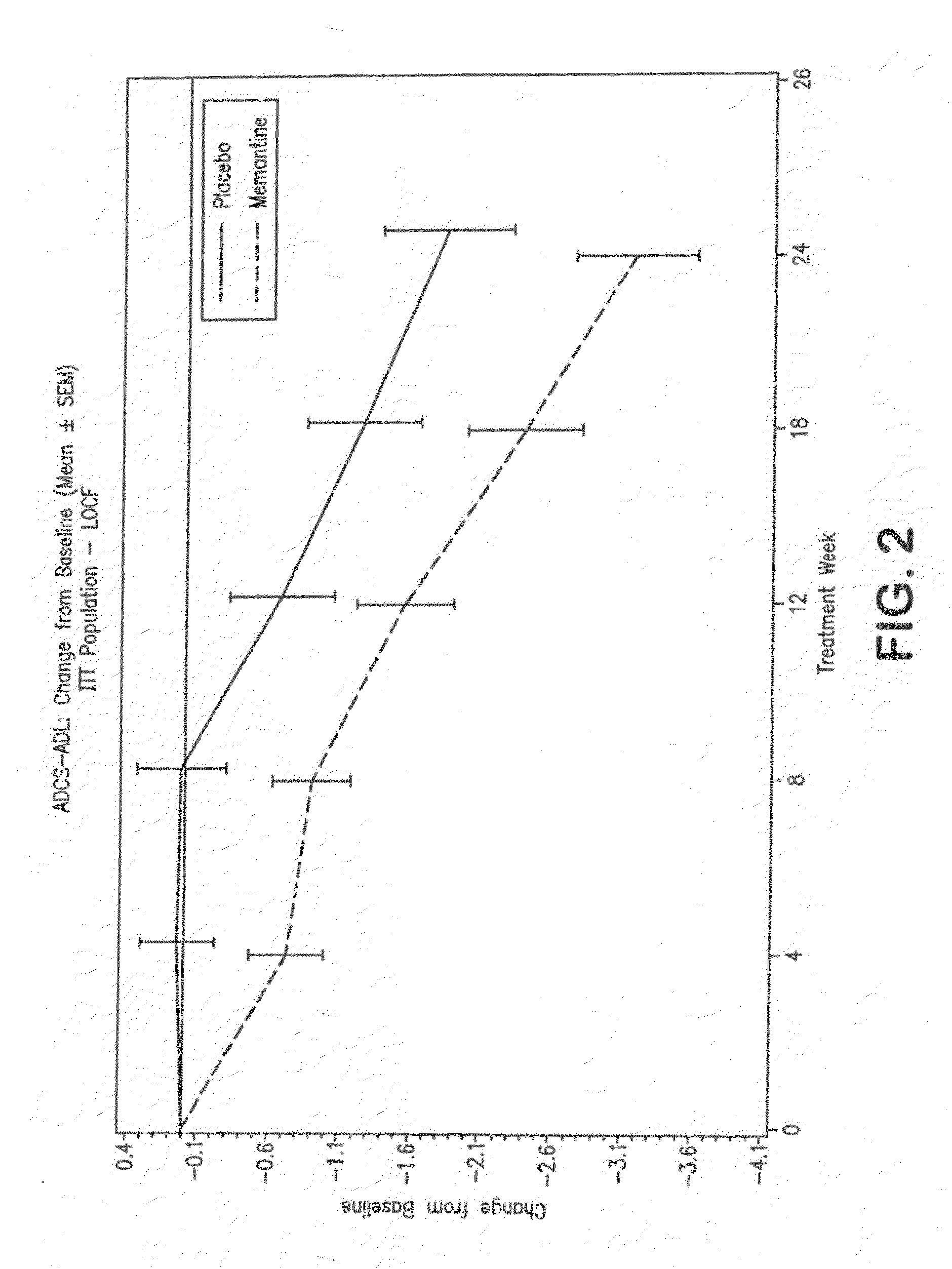
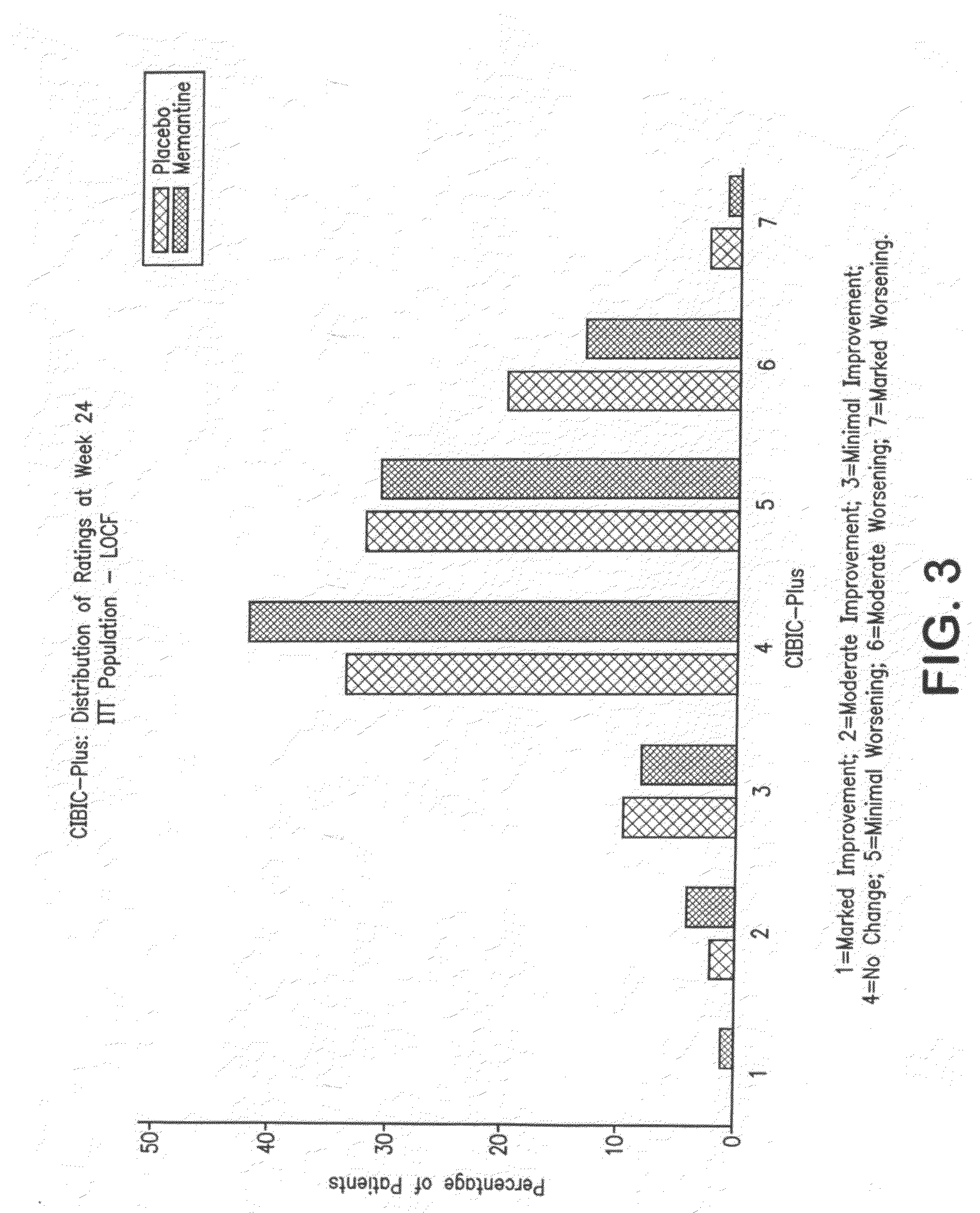
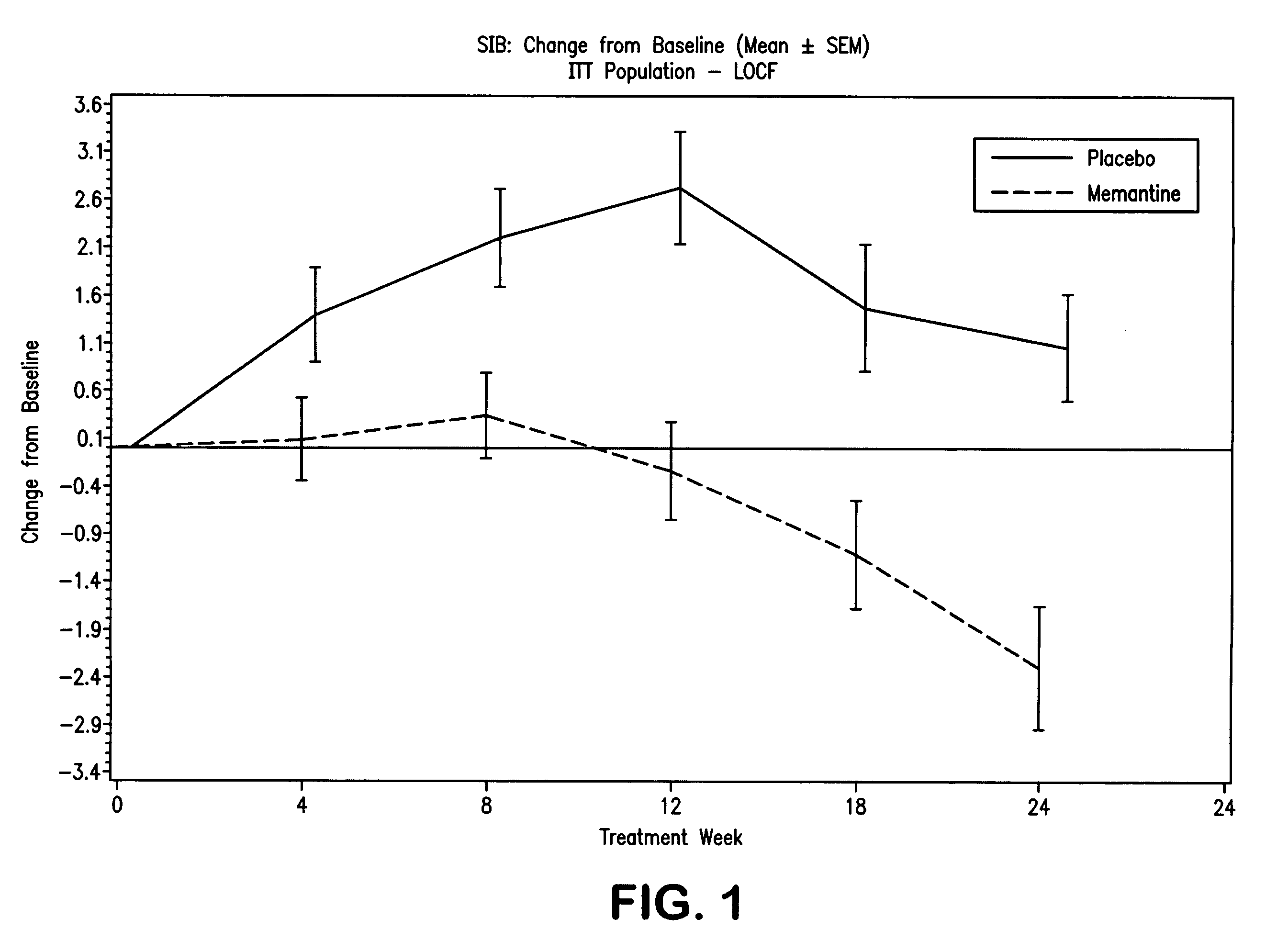
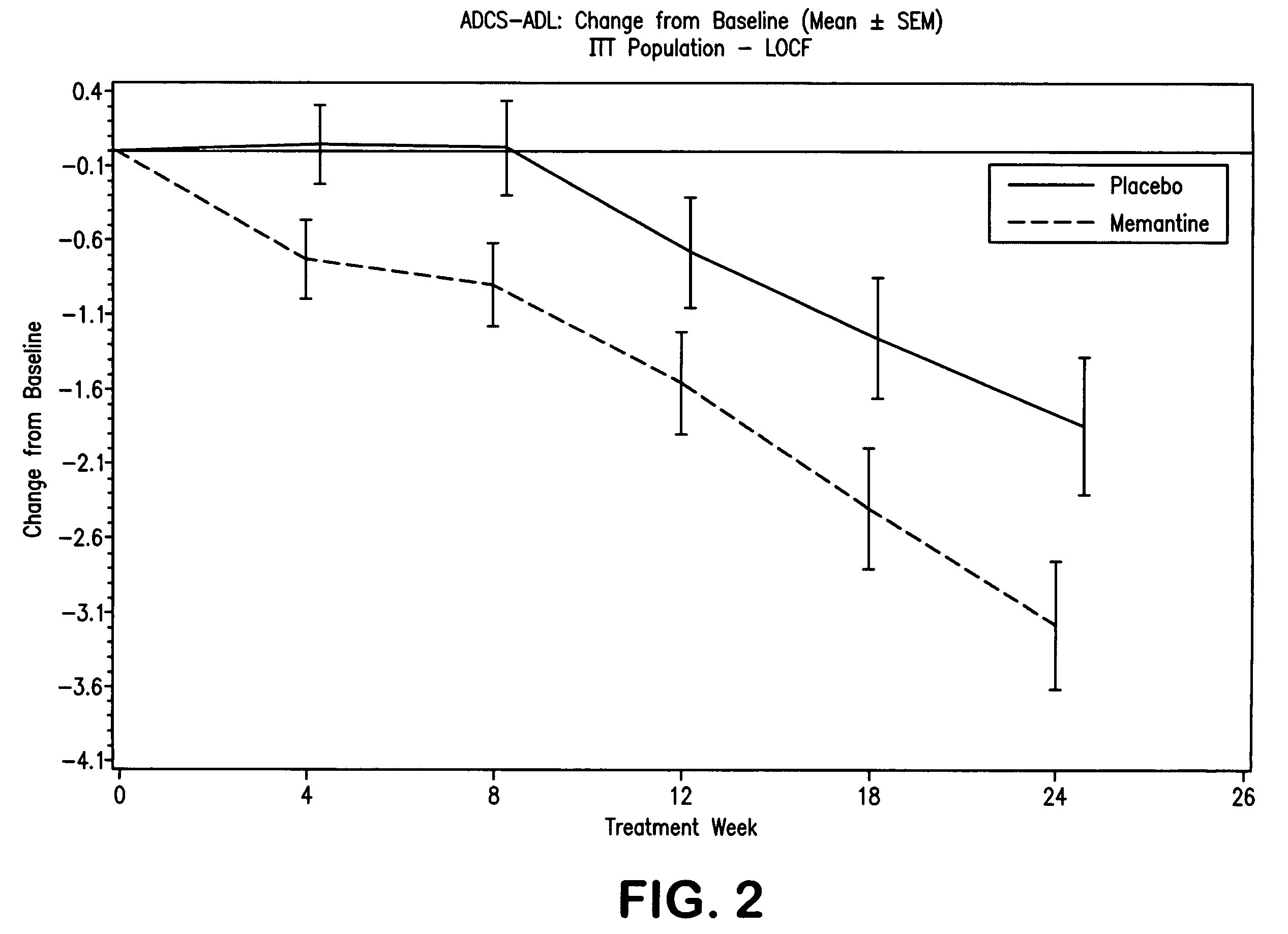
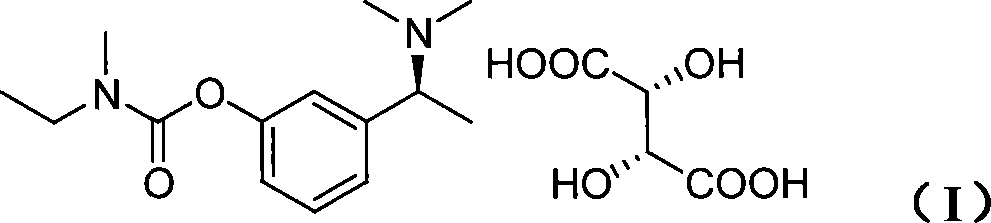
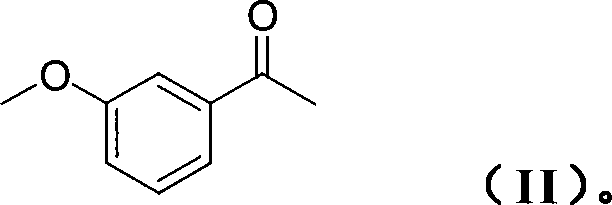
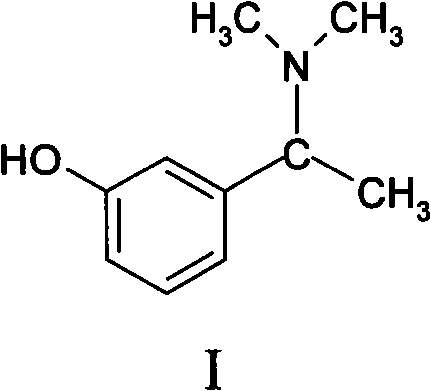
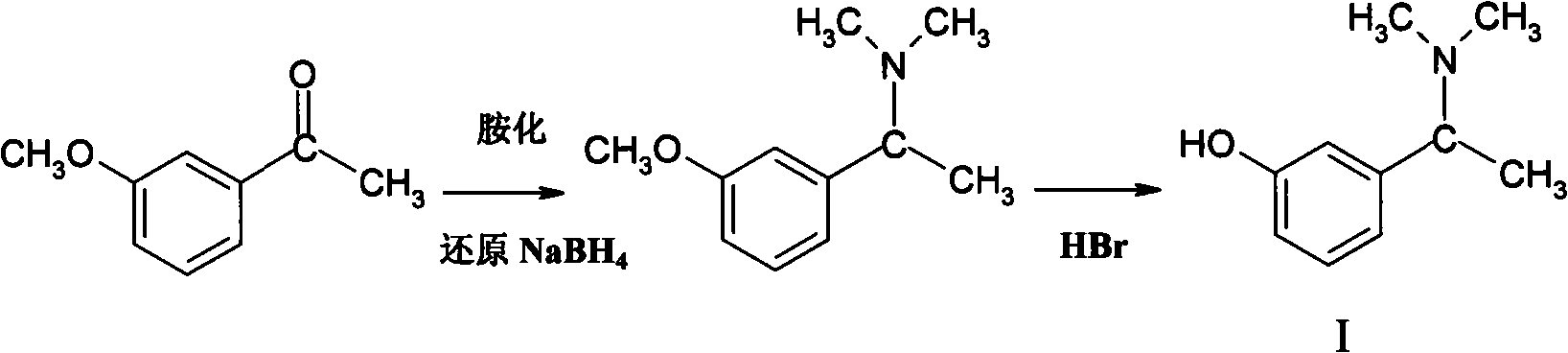
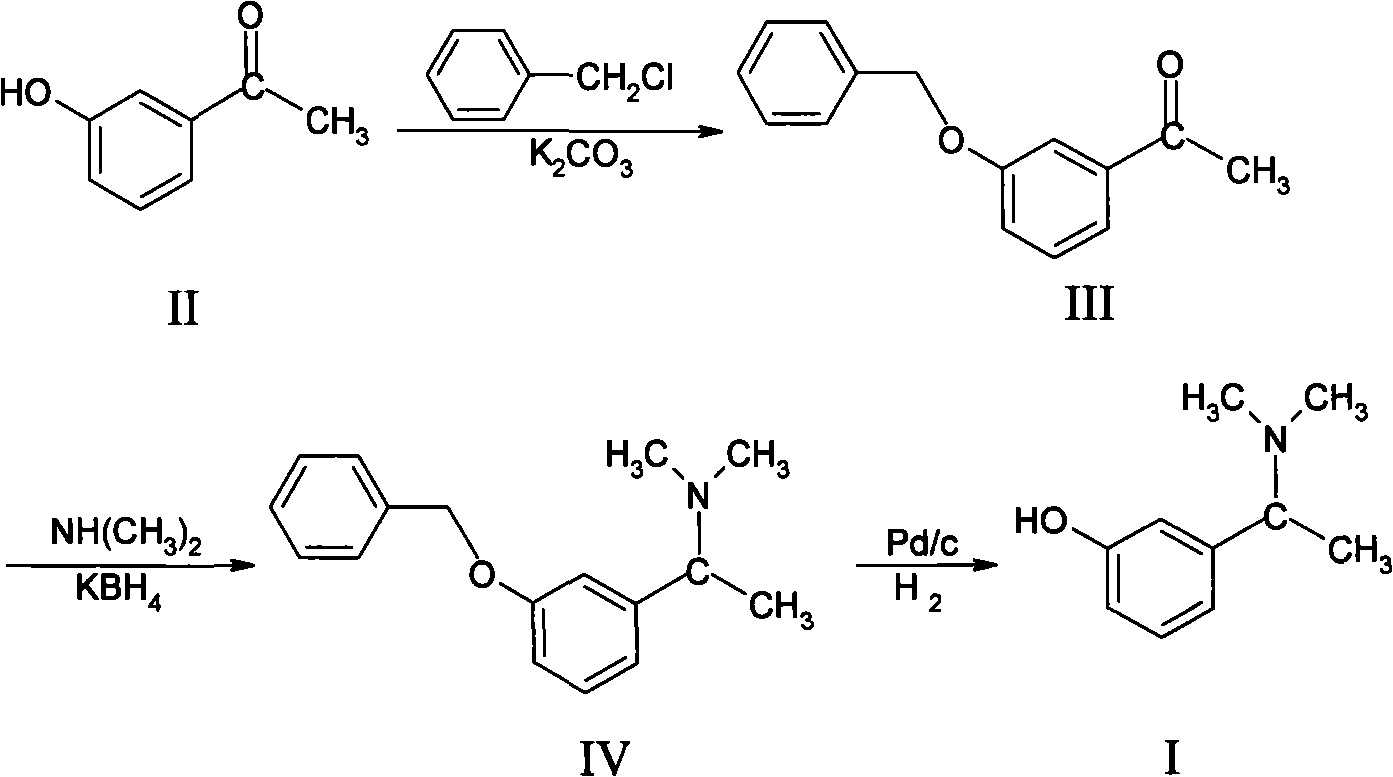
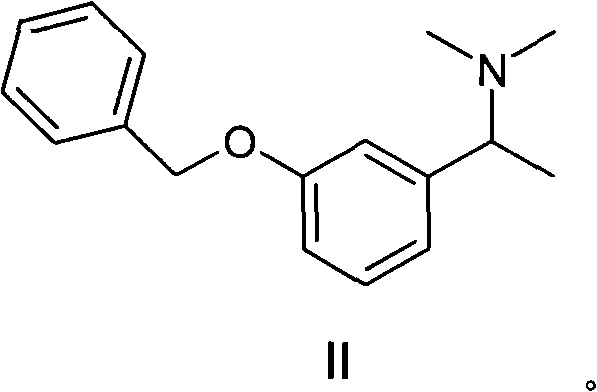
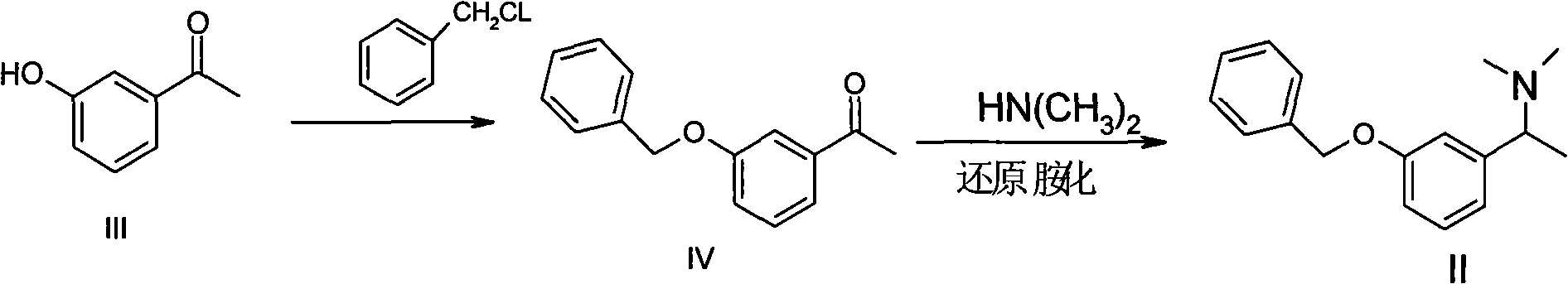
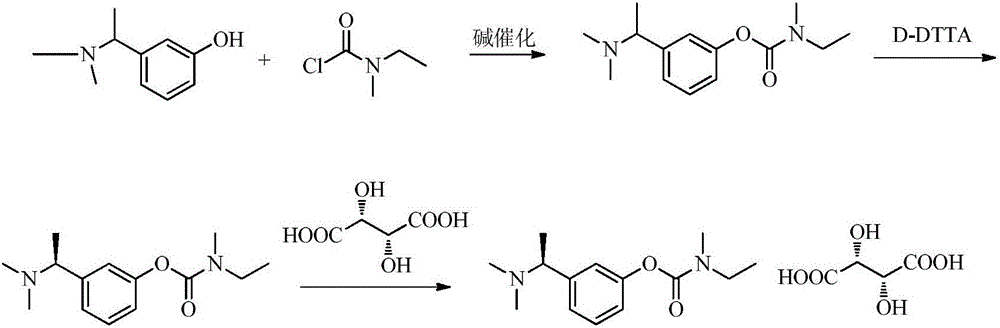
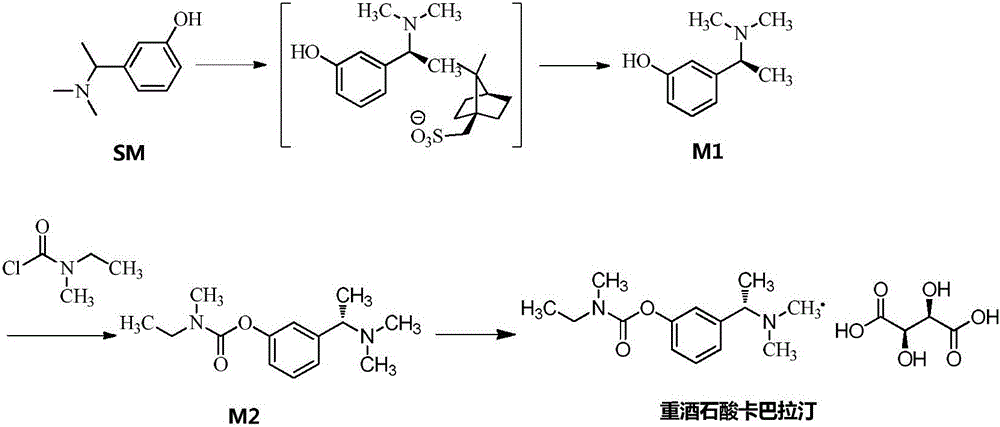
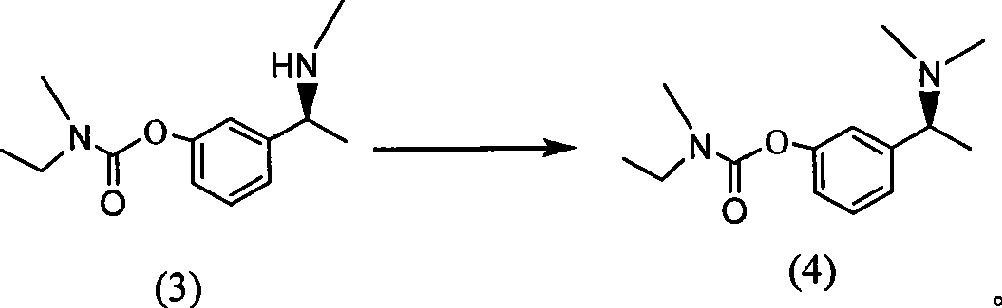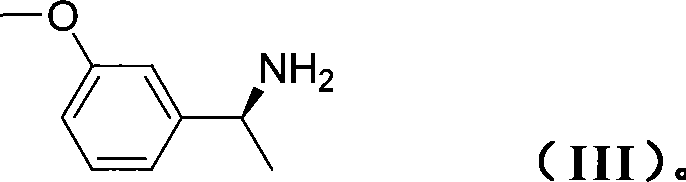Patents
Literature
140 results about "Rivastigmine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
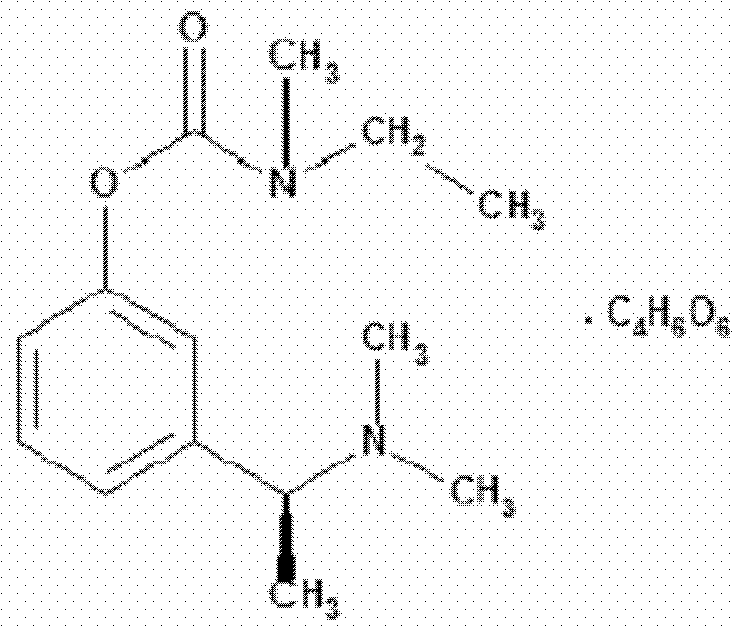
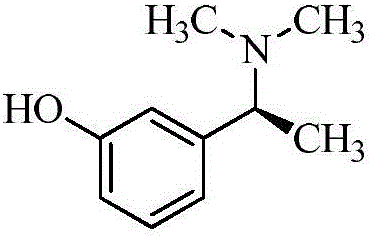
Rivastigmine is used to treat confusion (dementia) related to Alzheimer's disease and to Parkinson's disease.
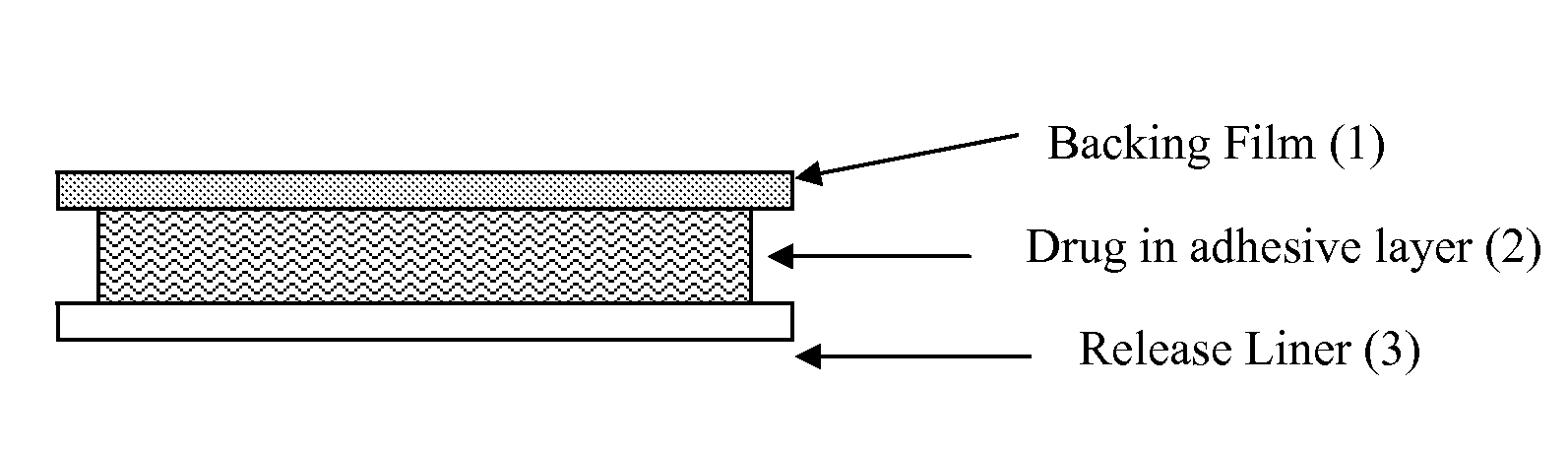
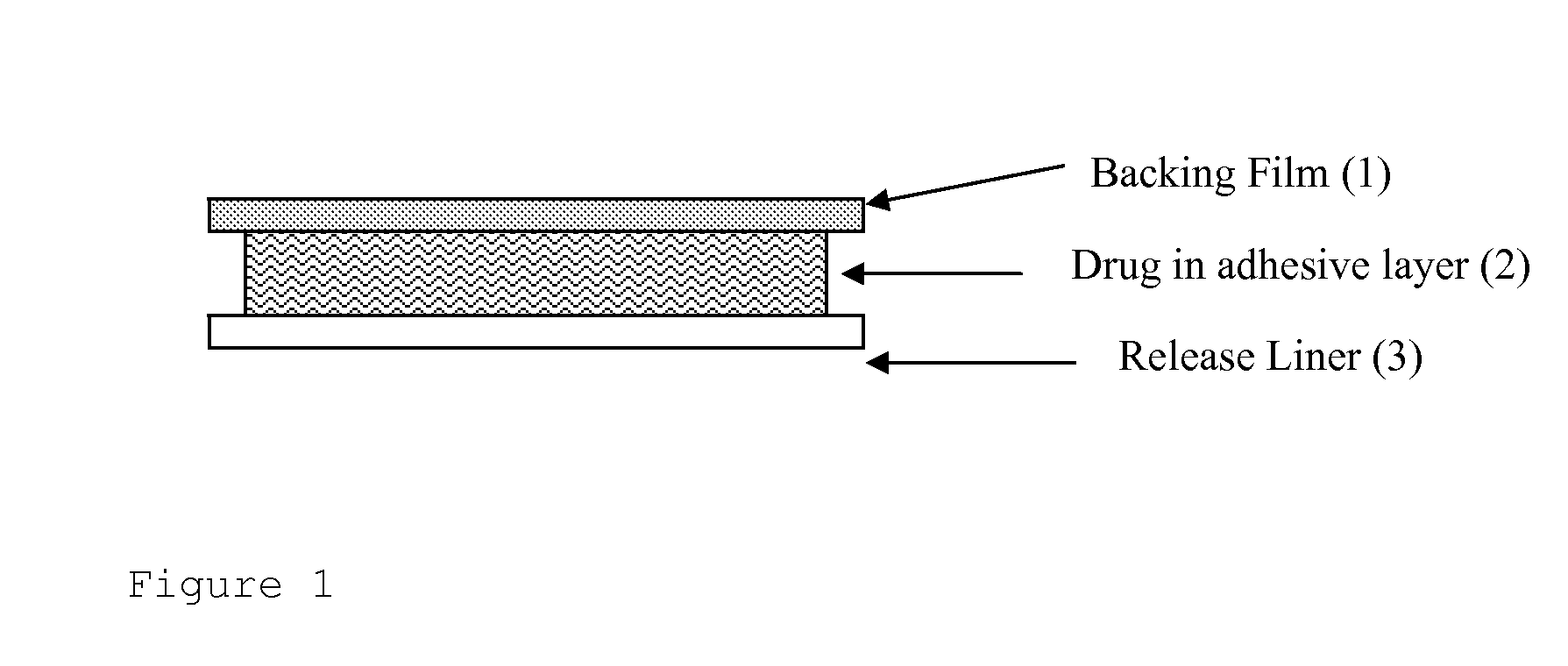
Transdermal drug delivery system for liquid active ingredient
InactiveUS20100087768A1Reduce lossesModerate shearPowder deliveryBiocideAdditive ingredientCross linker
A monolithic device for transdermal administration of an active pharmaceutical ingredient which is selected from propargylamines and rivastigmine and is liquid at 25° C., has an adhesive matrix layer which includes the active ingredient in an acrylic polymer pressure sensitive adhesive without cross-linker agent containing a metal atom, the adhesive having a shear value of between 1.5 and 15 hours, and further includes a non-volatile coadjuvant selected from squalene and triethylcitrate present in the layer in an amount of 1 to 15 wt %. The combination provides good release of the drug in use, reduces loss of the drug during a drying step in manufacture, reduces chemical interaction of the layer with the drug and achieves low level of skin irritation.
Owner:AMARIN TECH
Combination therapy using 1-aminocyclohexane derivatives and acetylcholinesterase inhibitors
InactiveUS20100227852A1Avoid damageReduce riskBiocideNervous disorderCholinesterase inhibitionRivastigmine
Owner:MERZ PHARMA GMBH & CO KGAA
Combination therapy using 1-aminocyclohexane derivatives and acetylcholinesterase and inhibitors
InactiveUS20090124659A1Avoid damageReduce riskBiocideNervous disorderCholinesterase inhibitionDepressant
The invention relates to a novel drug combination therapy useful in the treatment of dementia comprising administering an 1-aminocyclohexane derivative such as memantine or neramexane and an acetylcholinesterase inhibitor (AChEI) such as galantamine, tacrine, donepezil, or rivastigmine.
Owner:MERZ PHARMA GMBH & CO KGAA
New uses for quaternary ammonium anticholinergic muscarinic receptor antagonists in patients being treated for cognitive impairment or acute delirium
ActiveUS20080114014A1Maximizing beneficial effectMaximize the effectBiocideNervous disorderSolubilityFecal incontinence
A method for treating the adverse effects of acetyl-cholinesterase inhibitors used in the treatment of cognitive disorders such as acute delirium and cognitive impairment in elderly human patients. The administration of a clinically effective amount of a quaternary ammonium anti-cholinergic muscarinic receptor antagonist having very low lipid solubility substantially eliminates the adverse effects of urinary and / or fecal incontinence, nausea, bradycardia, bronchorrhea or brochospasm caused by the acetyl-cholinesterase inhibitors, without affecting the beneficial activity of the acetyl-cholinesterase inhibitors. This permits the administration of the optimum effective dosing of acetyl-cholinesterase inhibitors to provide maximum benefit to the patient with the added benefit of reducing or eliminating the unwanted side effects of fecal and urinary incontinence. Further, the combination of rivastigmine and glycopyrrolate has been effective in significantly improving cognitive function in patients suffering from acute dementia or cognitive impairment.
Owner:QAAM PHARMA LLC
Rivastigmine slow-release microspheres and preparation method thereof
InactiveCN101708164AHigh drug loadingHigh encapsulation efficiencyNervous disorderPharmaceutical non-active ingredientsMicrosphereRivastigmine
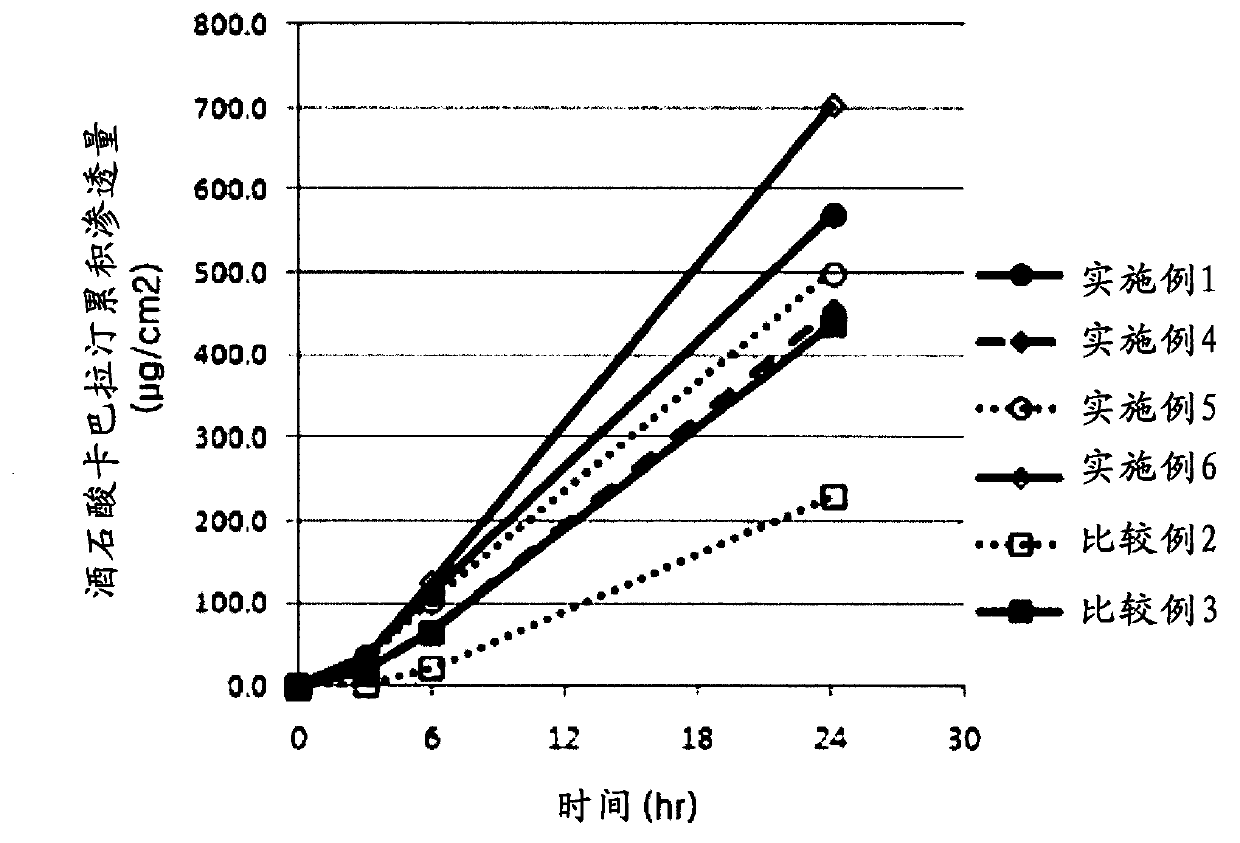
The invention discloses slow-release microspheres coated with rivastigmine tartrate / rivastigmine for PLGA / PLA injection and a preparation method thereof. The slow-release microspheres mainly comprise rivastigmine tartrate / rivastigmine and PLGA and PLA serving as biodegradable polymer medicinal materials, and can be prepared by adopting a W1 / O / W2 composite emulsified solvent volatilization method, an O / W emulsified solvent volatilization method, an O1 / O2 emulsion drying method and a spray drying method. The grain diameter of the microspheres is less than 100 microns. The slow-release microspheres have high medicament loading amount, have no obvious burst release effect, can be continuously released for one week, one mouth or three mouths, are used for treating senile diseases such as Alzheimer's disease, Parkinson disease and the like, and can prolong the acting time of the medicament, reduce the application times and greatly improve the administration compliance of patients.
Owner:SUZHOU UNIV
Silicone-containing acrylic polymers for transdermal drug delivery compositions
Described herein are silicone-containing acrylic polymers useful, for example, in transdermal drug delivery compositions, to methods of making and using them, to transdermal drug delivery compositions comprising them, and to methods of making and using such transdermal drug delivery compositions. The polymers are particular suitable for formulating amine drugs, such as amphetamine, methylphenidate, rivastigmine, paroxetine and clonidine.
Owner:NOVEN PHARMA
Transdermal plaster of rivastigmine and preparation process thereof
InactiveCN1994290AEasy to useClear curative effectOrganic non-active ingredientsEster active ingredientsTransdermal patchRivastigmine
The invention relates to a method for preparing kabalatin adhere agent for treating senile dementia, wherein said adhere agent can stably hold blood drug density and reduce feeding time, with high safety.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Method for preparing rivastigmine hydrogen tartrate and application thereof
InactiveCN101580482AEasy to operateLow costNervous disorderCarbamic acid derivatives preparationPhosgeneTriphosgene
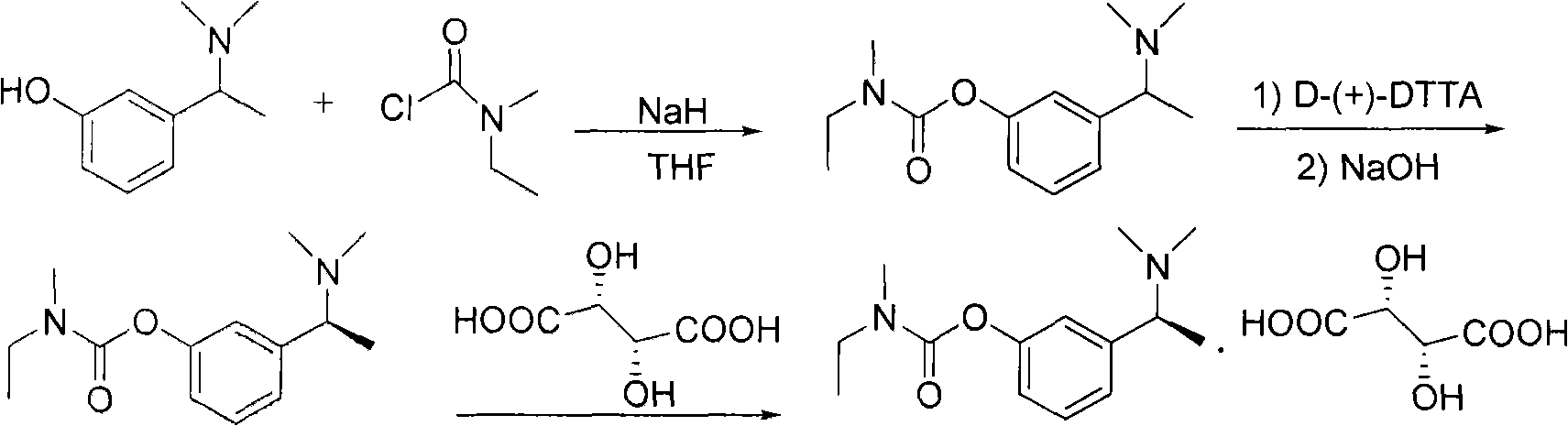
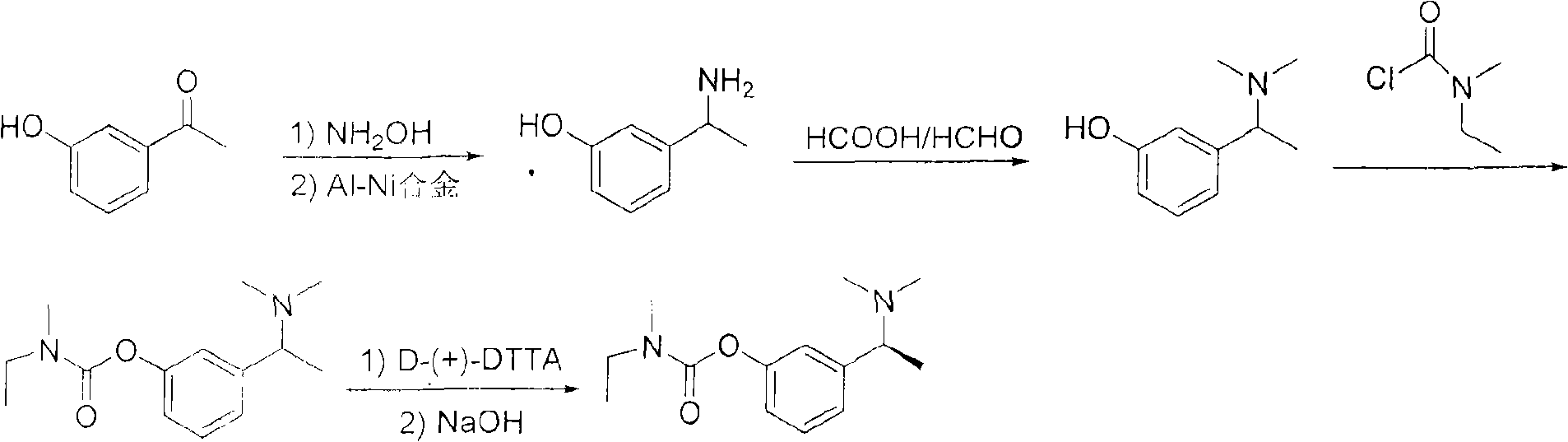
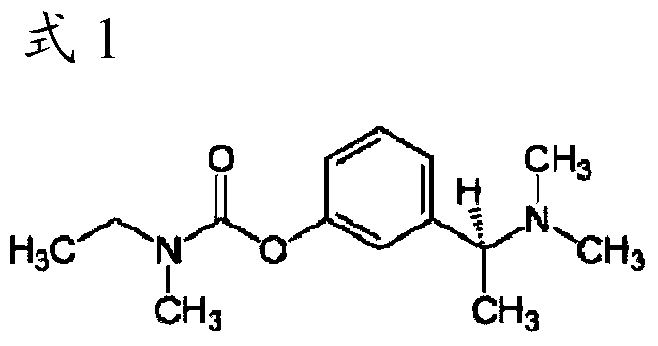
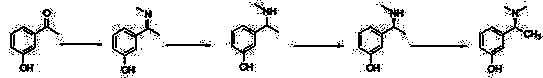
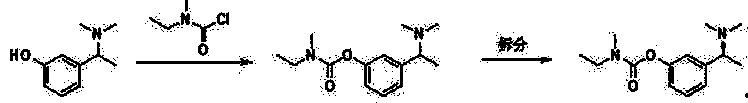
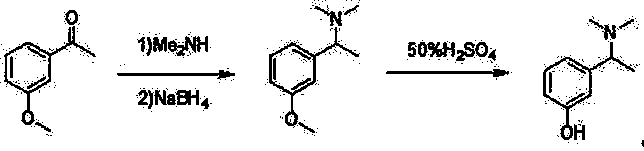
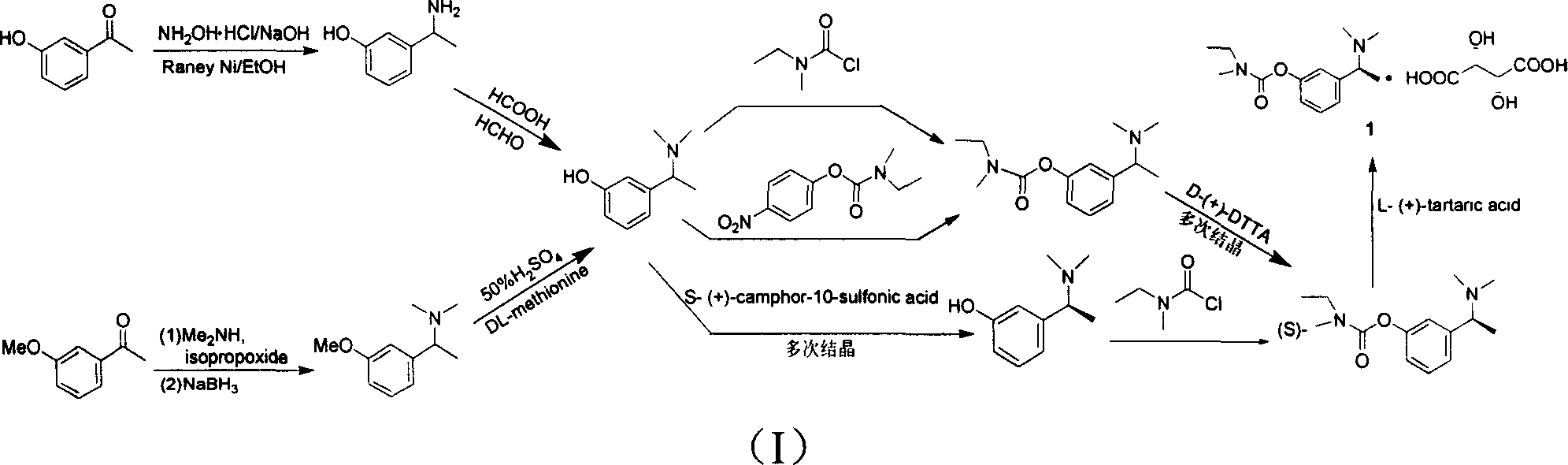
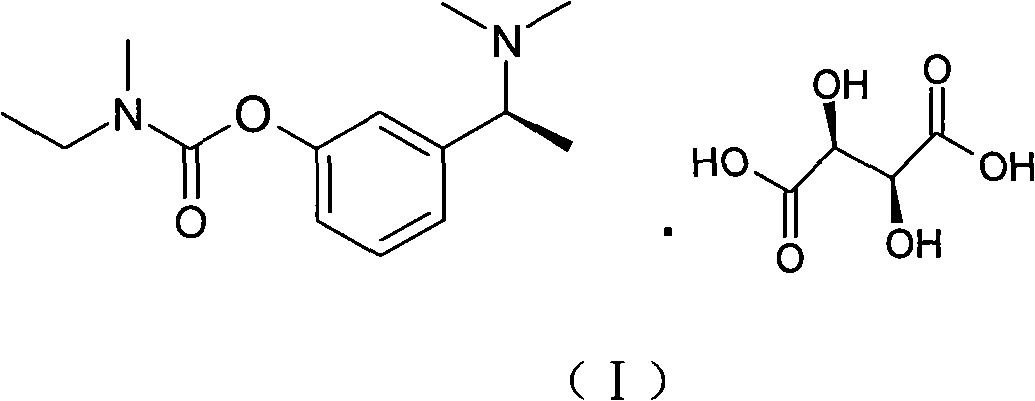
The invention relates to a method for preparing rivastigmine hydrogen and tartrate thereof, which comprises the following steps: taking metamethoxyacetophenone as an initial raw material, and obtaining 1-(3-methoxyphenyl)ethanol by the reduction; then performing the chlorination to obtain 1-(chloroethyl)-3-methoxyphenyl; then reacting the1-(chloroethyl)-3-methoxyphenyl with dimethylamine hydrochloride to obtain 1-(3-methoxyphenyl)-N, N-dimethylethanamine; demethylating the reaction product to obtain 3-[1-(dimethylamino)ethyl]phenol; then performing salt formation resolution with (s)-(+)-camphor-10-sulfonic acid, recrystallizing, and dissociating to obtain (s)-3-[1-(dimethylamino)ethyl]phenol; then taking ethylamine as a raw material to react with ethyl formate to obtain formylethylamine; then reacting the formylethylamine with phosphorus oxychloride to obtain an imine intermediate; reducing the imine intermediate by sodium borohydride to obtain ethyl methyl amine; then reacting the ethyl methyl amine with triphosgene to obtain N-methyl-N-ethylformyl chloride; and finally using (s)-3-[1-dimethylamino)ethyl]phenol to condensate with the N-methyl-N-ethylformyl chloride, and then performing salt formation with levotartaric acid to obtain the rivastigmine hydrogen tartrate. The method has the advantages of easily-obtained raw materials, simple and convenient operation, low cost, high yield and small pollution, and is a brandnew synthesis route at present.
Owner:SHENYANG PHARMA UNIVERSITY
A Device for the Transdermal Delivery of Alkaline Compounds that are Susceptible to Degradation in Their Free Base Form
InactiveUS20140221942A1Sufficient adhesive propertyIncrease polarityNervous disorderInorganic non-active ingredientsRivastigmineFree base
The present invention pertains generally to the field of transdermal drug delivery. More specifically, the invention relates to a device for the transdermal delivery of an alkaline pharmaceutically active compound that is susceptible to degradation in its free base form (e.g., rivastigmine) that comprises an adhesive matrix layer, a backing layer and a release or protective layer, wherein the adhesive matrix layer comprises said pharmaceutically active compound, triethylcitrate and hydrochloric acid. The invention also relates to methods of preparing such devices.
Owner:AMARIN TECH
Cholinesterase Inhibitors In Liposomes And Their Production And Use
InactiveUS20080031935A1Avoid intakeImprove bioavailabilityBiocideNervous disorderEnantiomerRivastigmine
The invention relates to a pharmaceutical composition based on an active ingredient that is enclosed in liposomes for topical, transdermal application. The interior of said liposomes comprises an acidic, aqueous medium containing at least one cholinesterase inhibitor, preferably from the group containing donepezil, rivastigmine, galantamine, physostigmine, heptylphysostigmine, phenserine, tolserine, cymserine, thiatolserine, thiacymserine, neostigmine, huperzine, tacrine, metrifonate and dichlorvos, or an enantiomer or derivative of at least one of said compounds. In addition, the invention relates to a method for producing said composition, optionally in a sterile form and also to the use of the liposomes charged with the active ingredient in various galenic formulations for topical, transdermal application with a depot effect in the epidermis, for the prophylaxis and / or treatment of cutaneous neuropathic pain or the loss of cutaneous sensory function as a result of neuropathy.
Owner:SANOCHEMIA PHARMA AG
Transepidermal drug delivery system containing rivastigmine
InactiveUS20120197221A1Promote dissolutionTransdermal absorptivity of increaseBiocideNervous disorderRivastigmineRosin
Disclosed is a pharmaceutical composition containing rivastigmine. Specifically, disclosed is a transepidermal drug delivery system including a rivastigmine-containing drug layer and a supporter adhered to one surface of the drug layer to support the drug layer, wherein the drug layer contains 10 to 40 parts by weight of a rubber, 20 to 80 parts by weight of a rosin ester resin and 0.1 to 10 parts by weight of an acrylic adhesive and the drug layer has a thickness of 40 μm to 100 μm.
Owner:SINSIN PHARMA
Patch
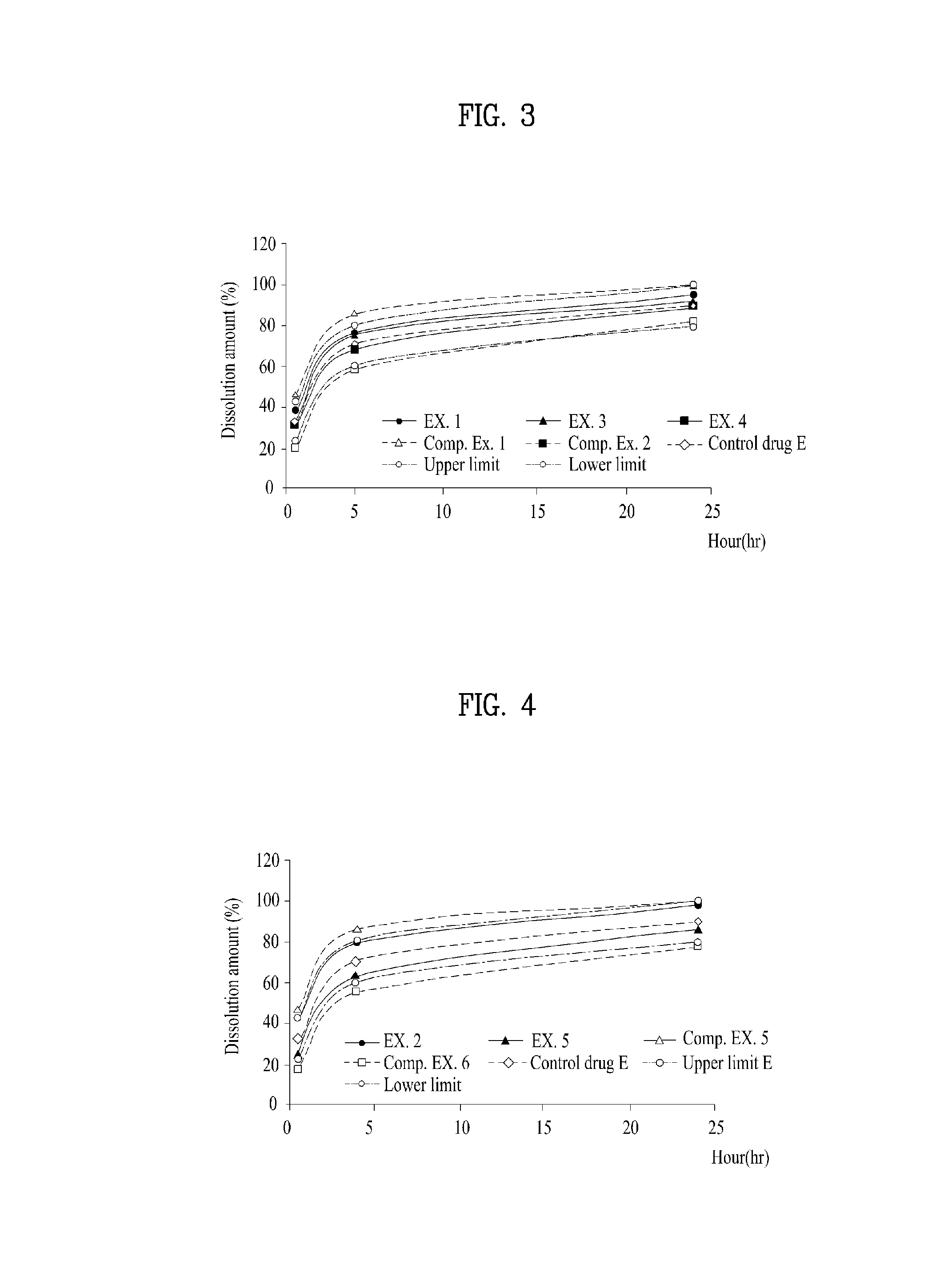
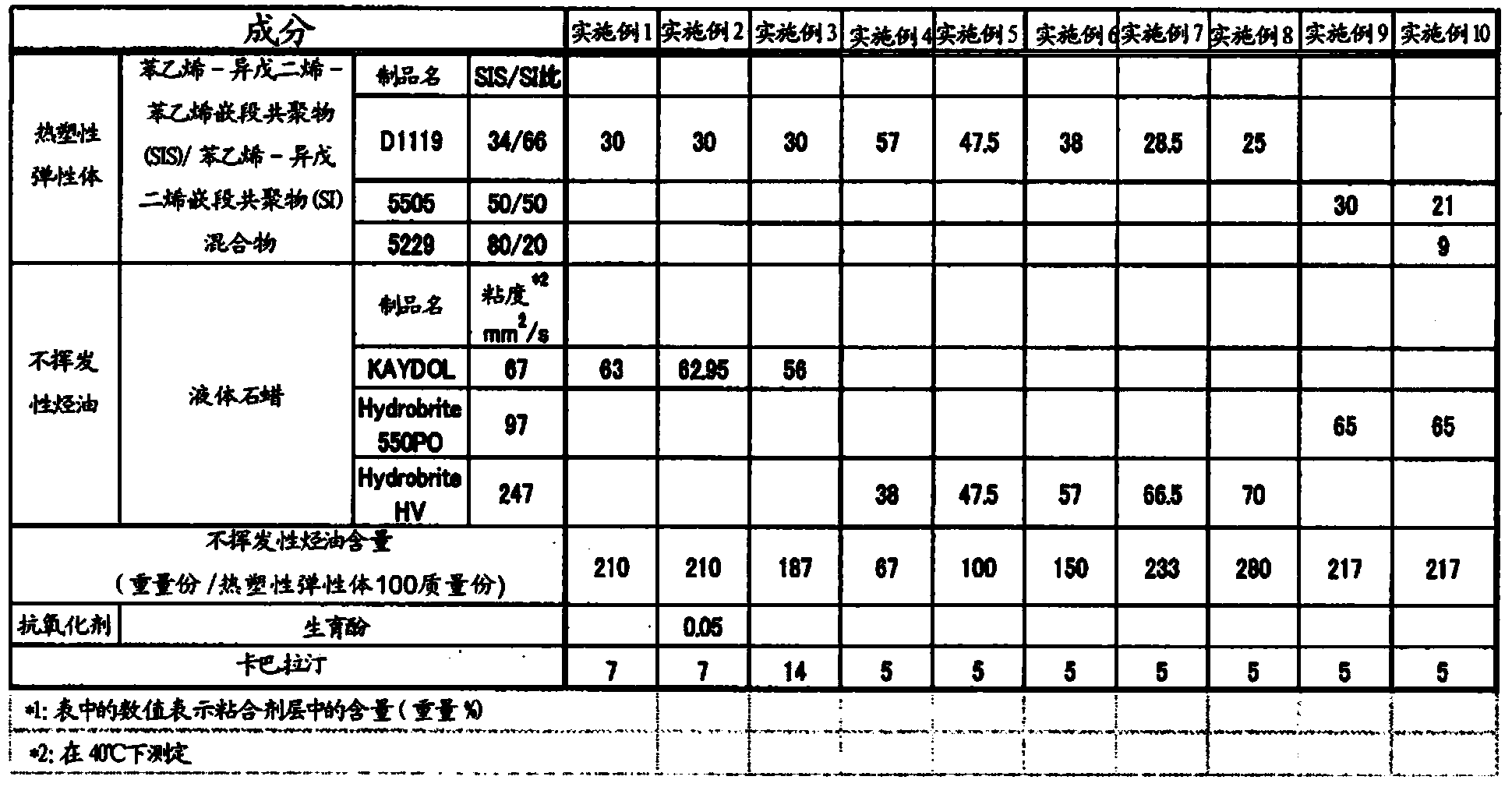
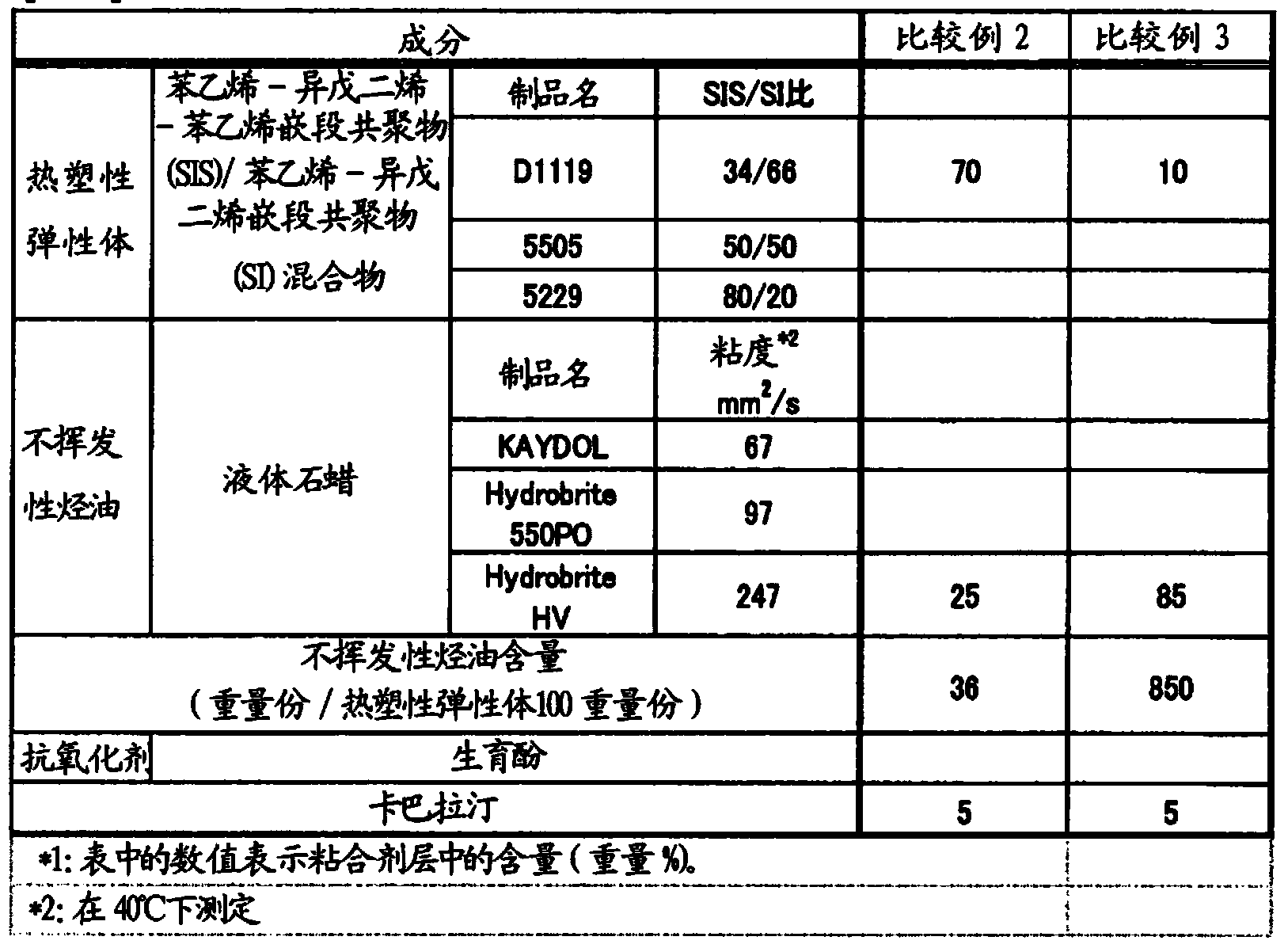
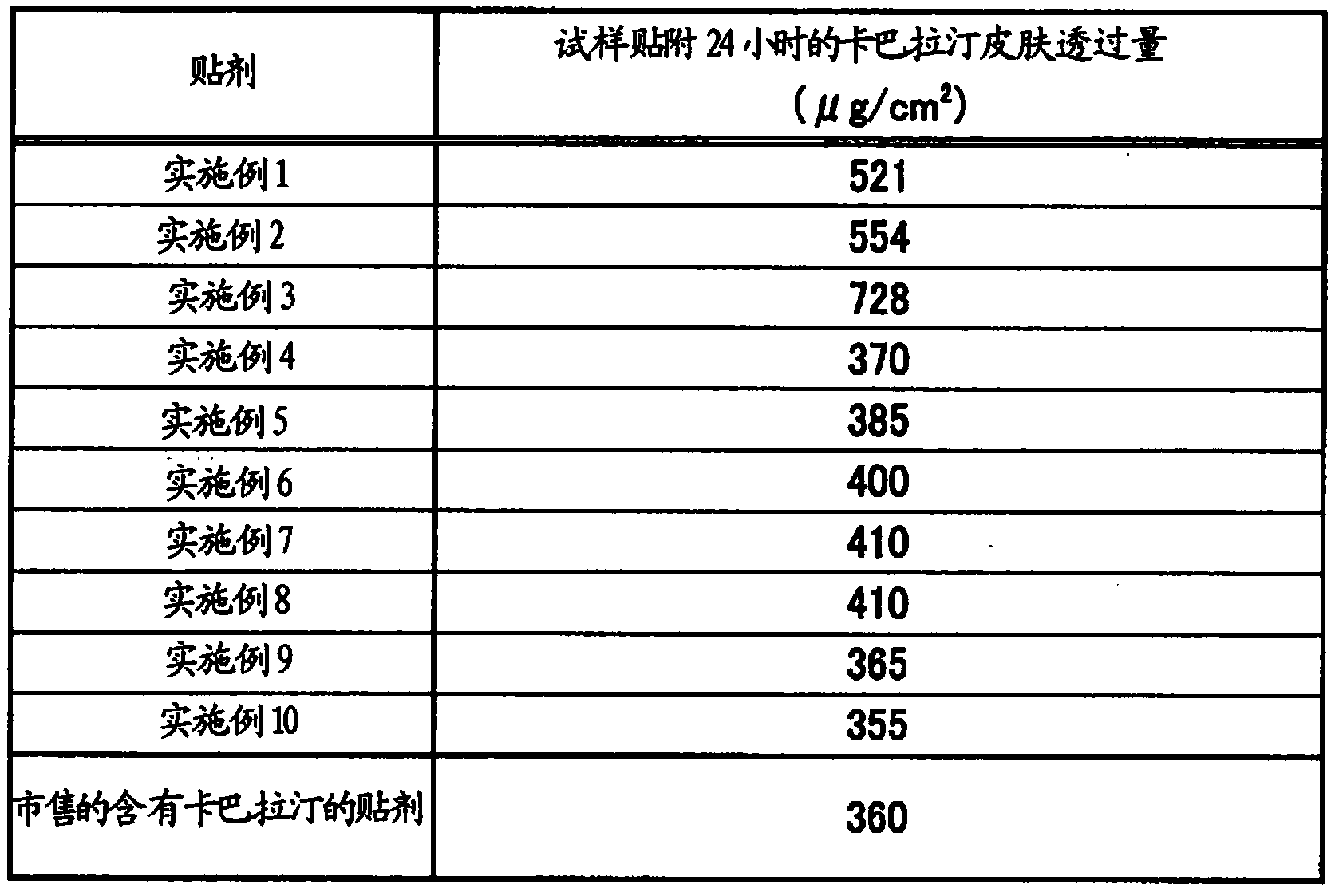
ActiveCN104114167APromote transdermal absorptionFull adhesionNervous disorderOrganic non-active ingredientsElastomerThermoplastic elastomer
Provided is a patch which is formed with an adhesive layer that retains a medicine on a support, wherein the adhesive layer contains a thermoplastic elastomer, a non-volatile hydrocarbon oil in an amount of over 50 parts by weight and 800 parts by weight or less with respect to 100 parts by weight of the elastomer, and rivastigmine or a salt thereof, and may further contain a tackifier in an amount of 10 parts by weight or less of the adhesive layer.
Owner:KM TRANSDERM LTD
Rivastigmine-hydrogentartrate-containing pharmaceutical composition and preparation method
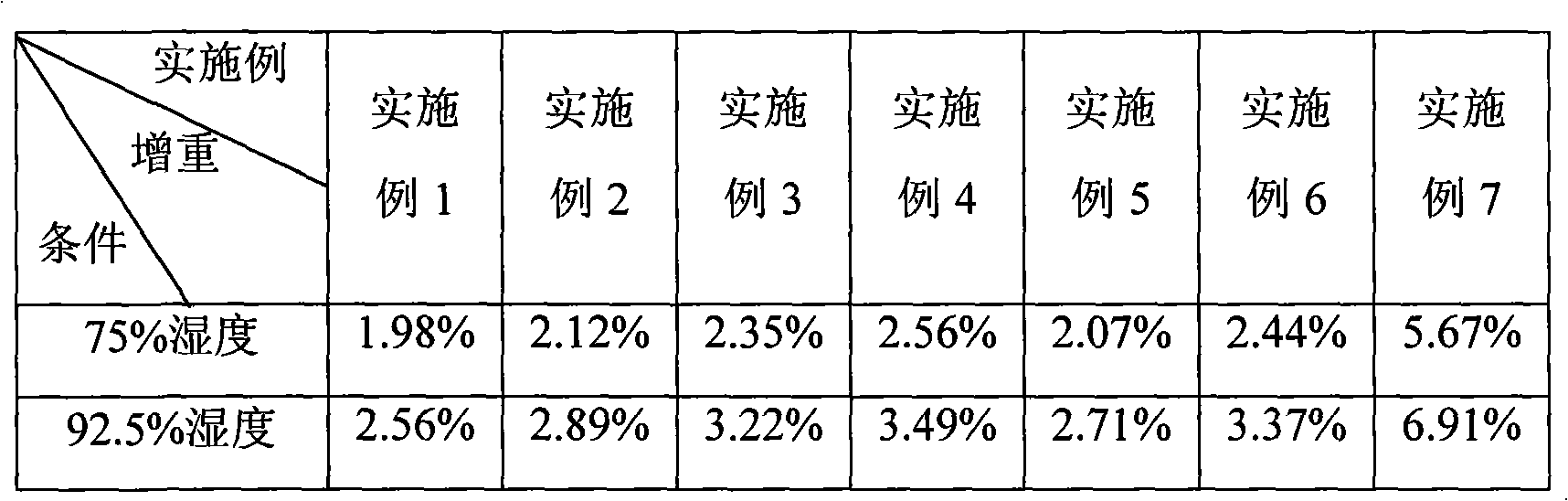
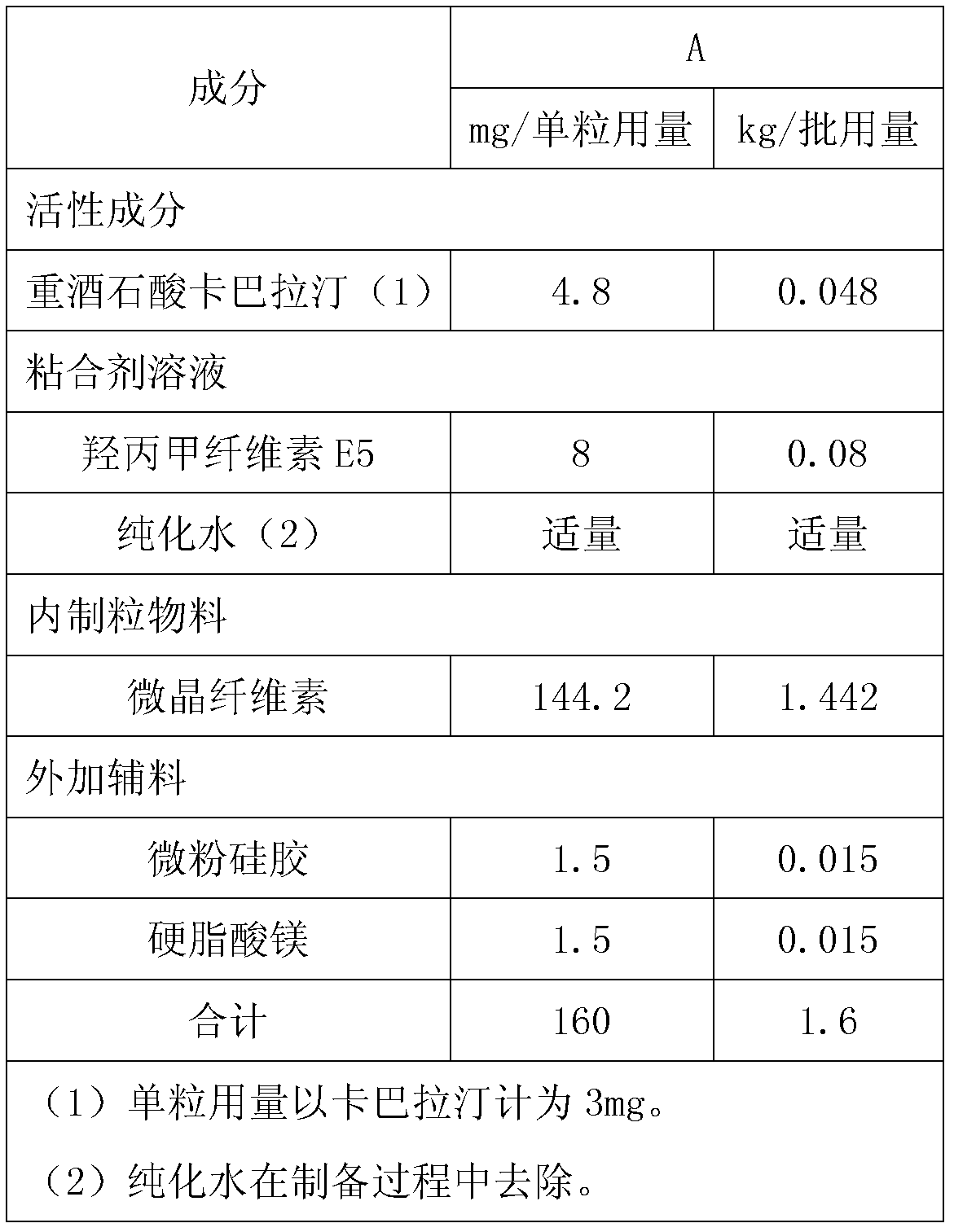
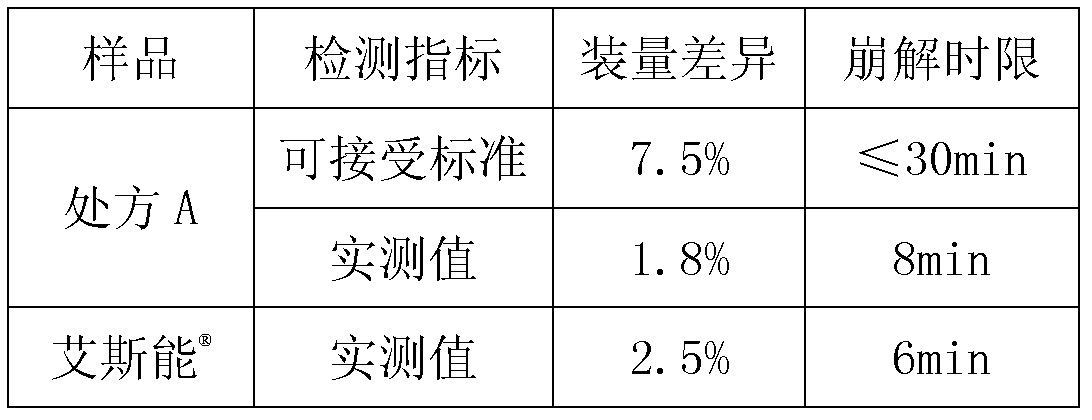
InactiveCN101836974AIncrease humidityQuality assuranceNervous disorderPharmaceutical non-active ingredientsRivastigmineBULK ACTIVE INGREDIENT
The invention discloses a pharmaceutical composition containing an active ingredient rivastigmine hydrogentartrate. The pharmaceutical composition comprises the rivastigmine hydrogentartrate and microcrystalline cellulose with weight percent of more than 50 percent, and then other excipients are added to be made into a corresponding formulation. The invention effectively solves the problem of hygroscopicity.
Owner:万全万特制药江苏有限公司
Novel rivastigmine preparation
InactiveCN101481333AAvoid deprotection problemsAvoid huge lossesCarbamic acid derivatives preparationOrganic compound preparationCarbamateRivastigmine
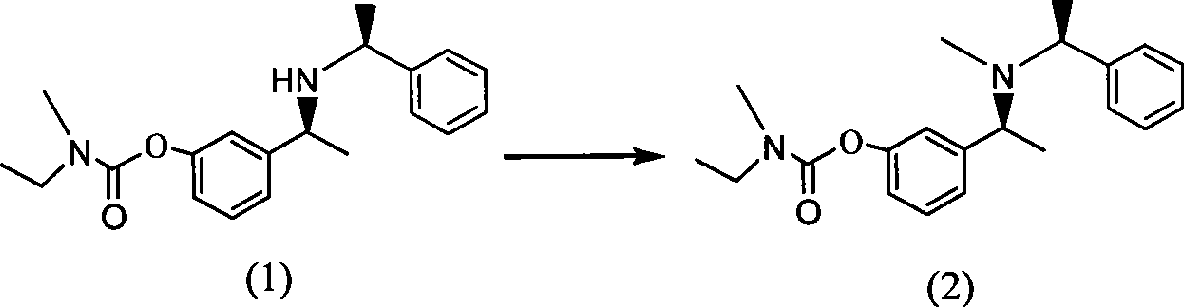
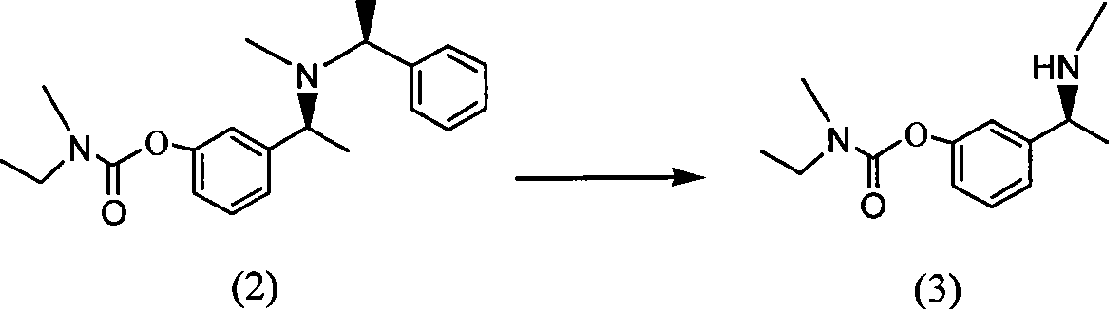
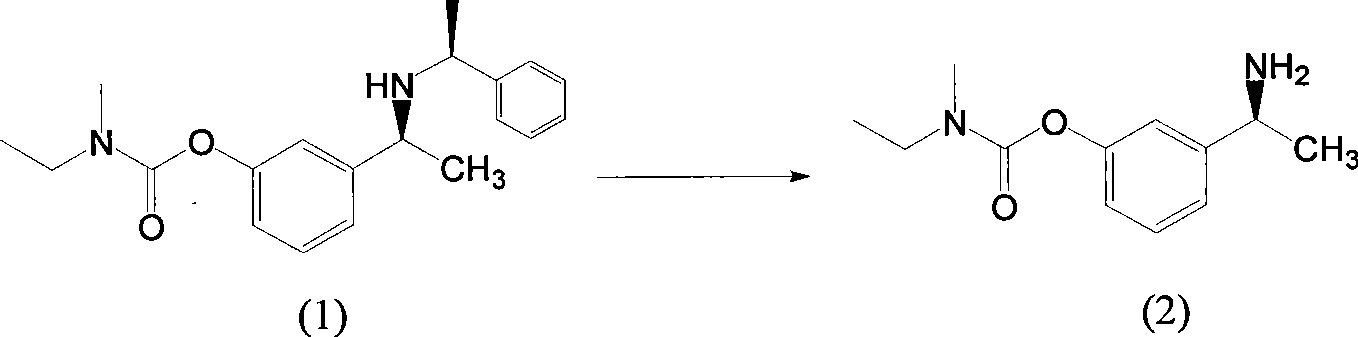
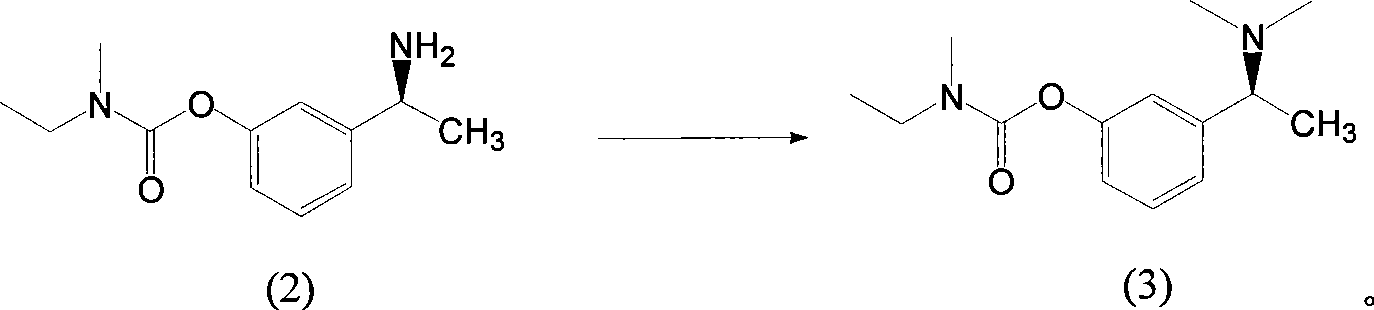
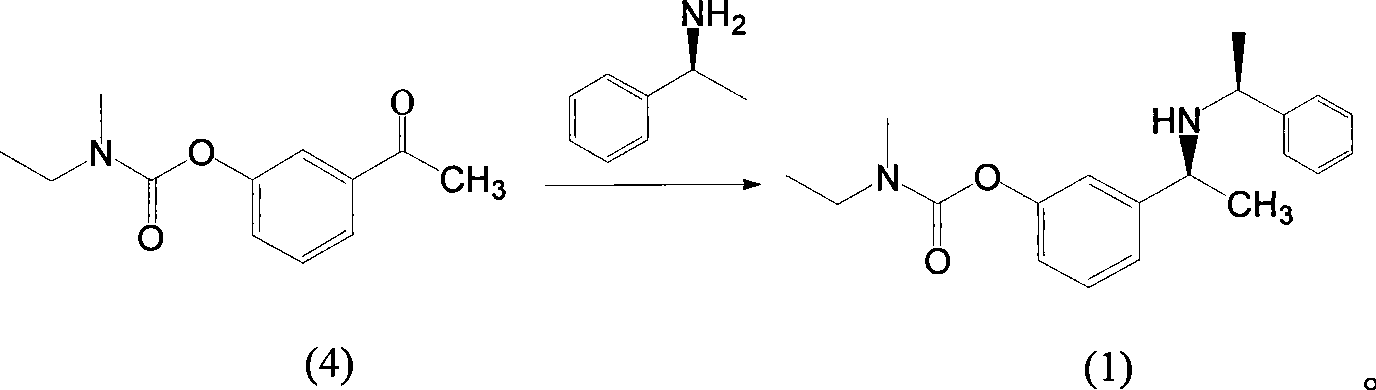
The invention belongs to the technical field of a method for preparing Rivastigmine, comprising the following steps: methylation reaction is carried out on 3-((S)-1-((S)-1-phenethylamine group) ethide) phenyl-ethyl (methyl) carbamate, then catalytic hydrogenation is carried out to remove phenylethane group and the methylation is carried out again to obtain the Rivastigmine. By adopting the synthetic route in the method, huge losses caused by disconnecting route later can be avoided, the problem of deprotection of hydroxide radical caused by first disconnecting route and then forming ester is avoided by forming ester first and pollution is lessened. The obtained final product has high purity and simple post-treatment. In every step of the method, reaction yield is relatively high, raw material is accessible, no special equipment is needed, operation is simple and convenient, pollution is little and integrated cost is low, therefore, the method is suitable for domestic industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Technique for producing rivastigmine hydrogen tartrate
InactiveCN101239934AAvoid huge lossesLow production costCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsRivastigmine
The invention discloses a production process of rivastigmine, comprising using m-methoxy hypnone as raw material, adding chiral auxiliary, reducing and aminating through an asymmetry synthesis method to obtain chiral amine, then demethylatig and acylating to prepare rivastigmine, and finally reacting rivastigmine with tartaric acid to obtain rivastigmine. The invention has advantages of simple operation, low cost, high yield, small pollution, and is suitable for industrialisation production.
Owner:苏州凯达生物医药技术有限公司
Preparation method for Rivastigmine midbody 3-hydroxyl-alpha-N, N-dimethyl phenylethylamine
InactiveCN101343237ARaw materials are easy to getSimple methodOrganic compound preparationBulk chemical productionRivastigmineAcetophenone
The invention relates to a preparation method of an intermediate (R, S)3-oxhydryl-Alpha-N, N-phenpromethamine of rivastigmine. The method takes 3-hydroxyl acetophenone as a raw material, and prepares the target product under the protection of oxhydryl, amination and reduction of carbonyl, and after the dehydroxylation protecting group reaction. The method has advantages of easy taking of materials, simple operation and high product purity.
Owner:SHANGHAI ZHONGXI SUNVE PHARMA
Transdermal drug delivery system containing rivastigmine
InactiveUS20140271866A1High skin penetration rateInhibition of recrystallizationBiocidePowder deliveryRivastigmineTransdermal medication
The present invention provides a transdermal drug delivery system comprising rivastigmine or its pharmaceutically acceptable salt and method of making the same.
Owner:NAL PHARM LTD
Pharmaceutical compositions and methods for treating Alzheimer's disease
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and donepezil or rivastigmine or galantamine or a pharmaceutically acceptable salt of thereof is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMATECH INC
Rivastigmine intermediate preparation
InactiveCN101481335AEasy to operateHigh purityCarbamic acid derivatives preparationOrganic compound preparationTetraisopropyl titanateCarbamate
The invention belongs to the technical field of a method for preparing Rivastigmine intermediate. As is shown above, the Rivastigmine intermediate is obtained by causing the following formula to react with chiral amine under catalysis of tetraisopropyl titanate and then carrying out catalytic hydrogenation. In the method, 3-((S)-1- ((S)-1-phenethylamine group) ethide) phenylethyl (methyl) carbamate is obtained under the catalysis of the tetraisopropyl titanate. As the price of the tetraisopropyl titanate is 10% of the price of the currently used ytterbium acetate, the cost advantage is obvious. In addition, the method has the advantages of simple operation, low cost and high purity and yield of the obtained isomer. By adopting the intermediate to synthesize the Rivastigmine, the reaction route is short and the yield is high; the intermediate is the key intermediate for synthesizing the Rivastigmine.
Owner:SHANGHAI INST OF PHARMA IND +1
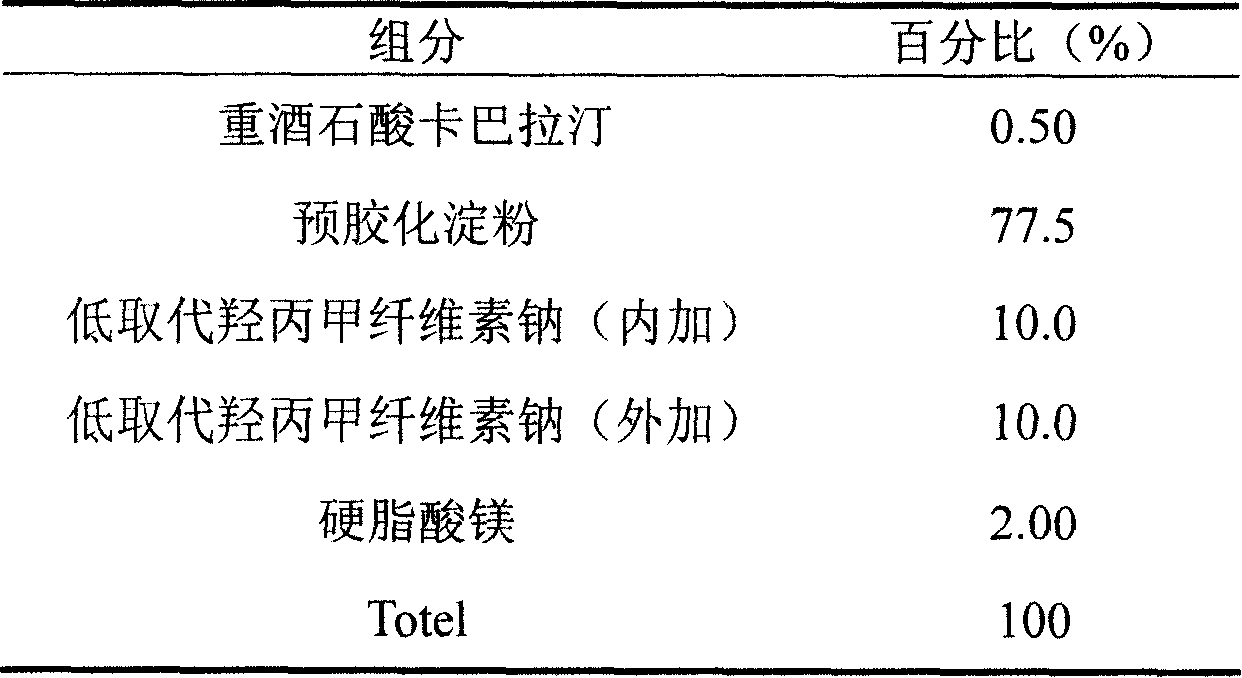
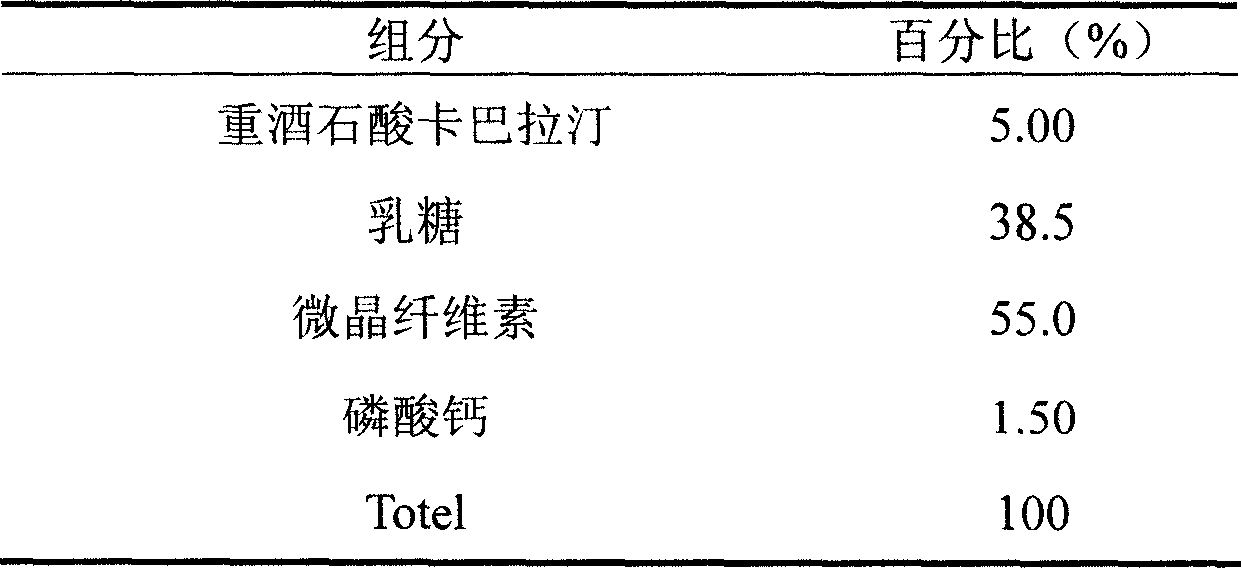
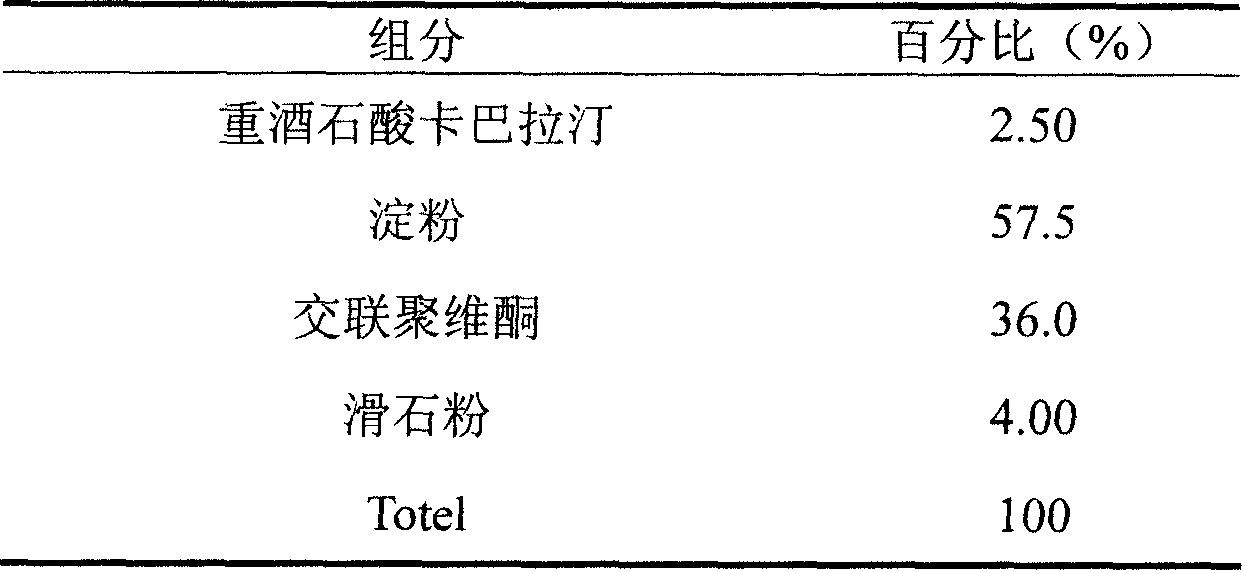
Iatric composite containing Rivastigmine and preparation method thereof
InactiveCN101204389ASimple preparation processGood process reproducibilityNervous disorderPharmaceutical non-active ingredientsDiseaseAdditive ingredient
The invention discloses a drug composition containing rivastigmine, which comprises the active ingredient and the excipient of the rivastigmine and exists in the form of a troche.The drug composition is mainly used for curing senile dementia caused by alzheimer disease and paralysis agitans.
Owner:BEIJING D VENTUREPHARM TECH DEV
Rivastigmine capsules and preparation method thereof
InactiveCN102805740AEasy to storeReduce liquidityNervous disorderPharmaceutical non-active ingredientsFluidized bedMethyl cellulose
The present invention relates to rivastigmine capsules and a preparation method thereof. The capsule contains rivastigmine adopted as an active ingredient, hydroxy propyl methyl cellulose adopted as a binder and a film forming material, and other medicinal auxiliary materials. The preparation method comprises: adopting a fluidized bed process to prepare particles, and further adopting hydroxy propyl methyl cellulose to carry out coating on the particles, wherein the prepared product has characteristics of low hygroscopicity and good storage stability so as to easily store the product, and the particles prepared according to the method of the present invention have characteristics of good fluidity and uniform particle size distribution. In addition, the preparation process is stable, quality is controllable, and various detection indexes of the finished product meet requirements, such that low production cost is ensured, and the method is suitable for industrial production.
Owner:ZHEJIANG JINGXIN PHARMA
Percutaneous absorption preparation containing rivastigmine
InactiveCN103313705AReduce stimulationEffective in treating dementiaNervous disorderPlastersAdditive ingredientPlasticizer
Disclosed is a percutaneous absorption preparation for treatment of dementia that comprises rivastigmine or its pharmaceutically acceptable salt as an active ingredient, a styrene-isoprene-styrene block copolymer, a tackifier resin, and a plasticizer. The preparation contains a small amount of rivastigmine but allows a sufficient amount of the active ingredient penetrated into the skin to achieve an effective treatment of dementia.
Owner:SAMYANG BIOPHARMLS CORP
Chiral intermediate of rivastigmine, and preparation method thereof
ActiveCN104151176AReduce pollutionHigh yieldOrganic compound preparationAmino-hyroxy compound preparationEthyl groupRivastigmine
The invention discloses a chiral intermediate of rivastigmine, and a preparation method thereof. The preparation method comprises the steps of preparing 3-[1-(methyllamino)ethyl]phenol through reacting a raw material of 3-hydroxyacetophenone with an methylamine water solution to form an imine and reducing the imine; carrying out resolution by using a chiral reagent to obtain a novel intermediate of (s)-3-[1-(methyllamino)ethyl]phenol of rivastigmine; and further loading methyl on basis of the intermediate to obtain an important intermediate of (S)-3-[1-(dimethyllamino)ethyl]phenol. Compared with a conventional synthetic method of (s)-rivastigmine, the preparation method provided by the invention has high resolution rate, low cost and small load for treatment of three wastes, has little pollution to an environment, and is beneficial to large-scale production.
Owner:DALIAN WONDERSUN BIOCHEM TECH
Method for synthesis of rivastigmine
InactiveCN1962624ALess investmentSimple and safe operationCarbamic acid derivatives preparationOrganic compound preparationMethylcarbamic acidRivastigmine
The invention discloses a synthesizing method of (S)-N-ethyl-3-[(1-dimethylamino) acetyl]-N-methyl amido phenyl formate, which is characterized by the following: adopting m-hydroxy acetophenone as raw material; synthesizing intermediate 3-(1-(dimethylamino) ethyl) phenol; condensing 3-(1-(dimethylamino) ethyl) phenol and formyl chloride to produce the product with high receiving rate.
Owner:JINAN UNIVERSITY
Method for preparing intermediate compound of Rivastigmine and intermediate compound
ActiveCN102381988AHigh yieldHigh chemical purityCarbamic acid derivatives preparationPreparation from carboxylic acid halidesRivastigmineCombinatorial chemistry
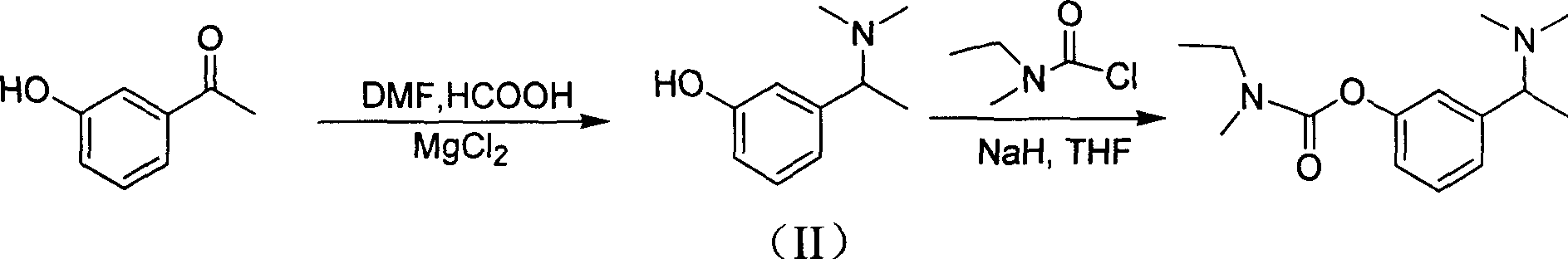
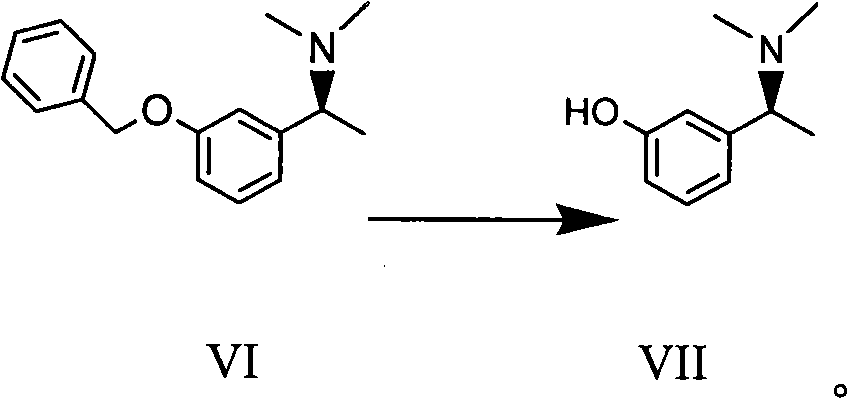
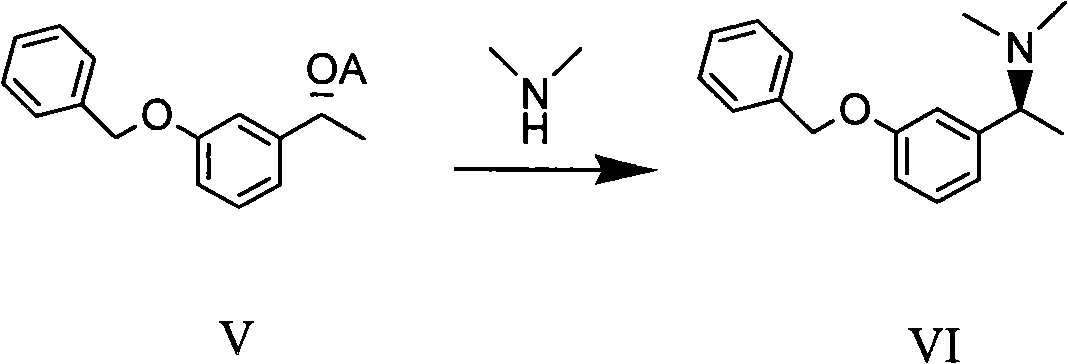
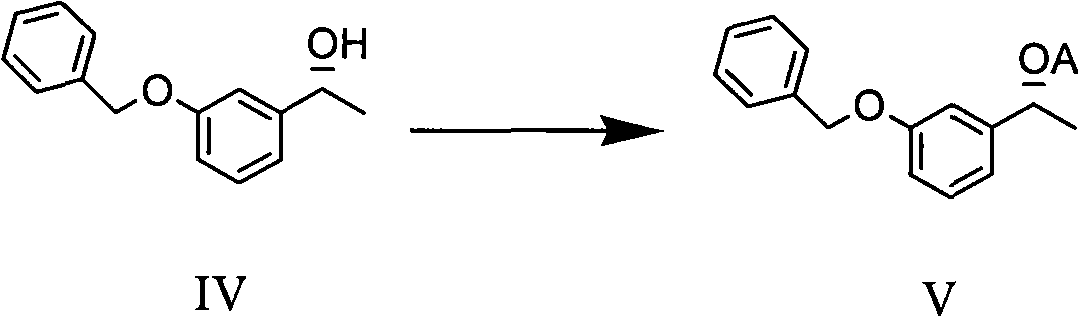
The invention discloses a method for preparing an intermediate compound VII of Rivastigmine, which is characterized by comprising the step of conducting the reaction of removing a benzyl protection group of hydroxy on a compound VI. The invention also discloses intermediate compounds V and VI of Rivastigmine and hydrochloride of the compound VI. By adopting the preparation method, the product productivity is high, the chemical impurity and optical purity are very high, the cost is low, the postprocessing is simple, and the method can be applicable to industrialization production.
Owner:CHIRAL QUEST (SUZHOU) CO LTD +1
Rivastigmine preparation suitable for industrial production
InactiveCN101481334AShort stepsAvoid deprotection problemsCarbamic acid derivatives preparationOrganic compound preparationEnantioselective synthesisRivastigmine
The invention belongs to the technical field of a method for preparing Rivastigmine. In the preparation method for the Rivastigmine which is suitable for industrial production, catalytic hydrogenation is carried out on 3-((S)-1-((S)-1-phenethylamine group) ethide) phenylethyl (methyl) carbamate to remove phenylethane group and methylation reaction is carried out to obtain the Rivastigmine. The synthetic route in the invention is the shortest among the current routes of asymmetric synthesis of Rivastigmine, so that huge losses caused by disconnecting route later can be avoided, the problem of deprotection of hydroxide radical caused by first disconnecting route later and then forming ester can be avoided by forming ester first and pollution is lessened. In every step of the method, reaction yield is relatively high, raw material is accessible, no special equipment is needed, operation is simple and convenient, pollution is little and integrated cost is low, therefore, the method is suitable for domestic industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Synthetic method of rivastigmine and intermediates thereof
ActiveCN101823970ASolve the problems of difficult operation and low production efficiencyEasy to separate and purifyNervous disorderCarbamic acid derivatives preparationRivastigminePhenol
The invention relates to a preparation method of rivastigmine tartrate and intermediates thereof, which comprises the steps of carrying out reductive amination on 3-benzyloxy acetophenone and dimethyl for obtaining a (1-(3-benzyloxy-phenyl)-ethyl)-dimethylamine (II) compound, removing benzyl with hydrogenated amine, obtaining 3-(1-(dimethylamino) ethyl) phenol (V), further carrying out condensation reaction with N-methyl-N-ethyl carbamoyl chloride, resolving a rivastigmine racemate with D-(+)-p-methyl dibenzoyl tartaric acid, and further salifying with L-(+)-tartaric acid for obtaining the rivastigmine tartrate (I). The method has the advantages of cheap price and easy obtainment of raw materials, short synthetic route, few reaction steps, mild reaction conditions and easy separation and purification of all the intermediates, thereby being applicable to industrial production.
Owner:NHWA PHARMA CORPORATION
Transdermal patch
InactiveUS20130220846A1Improve practicalityGood time stabilityNervous disorderAdhesive articlesTransdermal patchMedicine
A transdermal patch for the treatment of Alzheimer's disease includes: a backing, a rivastigmine-containing layer, a pressure-sensitive adhesive layer, and a release liner. In the transdermal patch, the rivastigmine-containing layer contains rivastigmine and an alkyl (meth)acrylate resin, the pressure-sensitive adhesive layer is composed of an acrylic pressure-sensitive adhesive containing a (meth)acrylic acid ester having a hydroxy group, and neither the rivastigmine-containing layer nor the pressure-sensitive adhesive layer contains an anti-oxidizing agent.
Owner:NICHIBAN CO LTD
Preparation method of rivastigmine tartrate
ActiveCN106565543AEffective controlHigh yieldCarbamic acid derivatives preparationOrganic compound preparationRivastigminePhenol
The invention discloses a preparation method of rivastigmine tartrate. The preparation method comprises the following steps: using (R, S)-3-(1-(dimethylamino)ethyl)phenol as a raw material, and carrying out resolution on the raw material by the use of S-(+)-camphorsulfonic acid so as to obtain enantiomerically pure (S)-3-(1-(dimethylamino)ethyl)phenol; letting enantiomerically pure (S)-3-(1-(dimethylamino)ethyl)phenol be in butt-joint with N-ethyl-N-methylcarbamyl chloride to obtain rivastigmine free alkali; and finally letting rivastigmine free alkali react with L-(+)-tartaric acid for salifying so as to obtain rivastigmine tartrate. Through process control such as treatment during the synthetic process of raw materials, acid-base alternative impurity removal during refining of an intermediate and acetone pulping for purification during later period, the preparation method of rivastigmine tartrate is obtained. By the method, purity and yield of the product are both raised, the isomer impurity can be more effectively controlled, and cost is greatly reduced and process is simplified. The preparation method is more suitable for industrial production.
Owner:KUNMING YUANRUI PHARMA
Uses for quaternary ammonium anticholinergic muscarinic receptor antagonists in patients being treated for cognitive impairment or acute delirium
A method for treating the adverse effects of acetyl-cholinesterase inhibitors used in the treatment of cognitive disorders such as acute delirium and cognitive impairment in elderly human patients. The administration of a clinically effective amount of a quaternary ammonium anti-cholinergic muscarinic receptor antagonist having very low lipid solubility substantially eliminates the adverse effects of urinary and / or fecal incontinence, nausea, bradycardia, bronchorrhea or brochospasm caused by the acetyl-cholinesterase inhibitors, without affecting the beneficial activity of the acetyl-cholinesterase inhibitors. This permits the administration of the optimum effective dosing of acetyl-cholinesterase inhibitors to provide maximum benefit to the patient with the added benefit of reducing or eliminating the unwanted side effects of fecal and urinary incontinence. Further, the combination of rivastigmine and glycopyrrolate has been effective in significantly improving cognitive function in patients suffering from acute dementia or cognitive impairment.
Owner:QAAM PHARMA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com