Method for synthesis of rivastigmine
A synthetic method, ethyl technology, which is applied in the field of anti-senile dementia drug rivastigmine, can solve the problems of high cost, low yield, and long synthetic route, and achieve low cost, good recovery yield, and few reaction by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
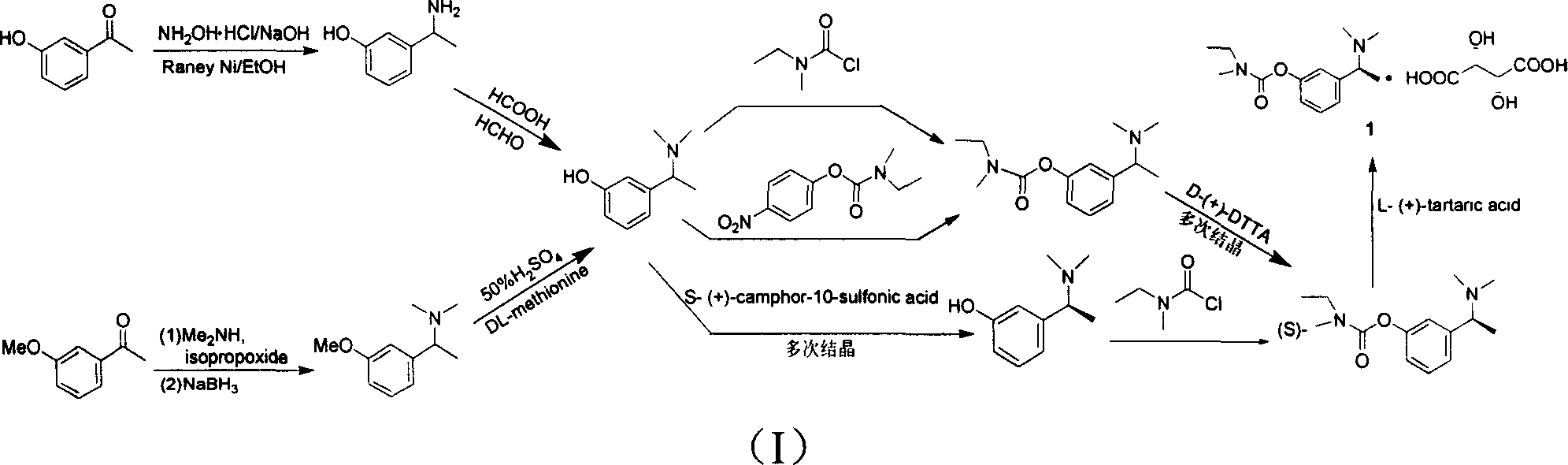
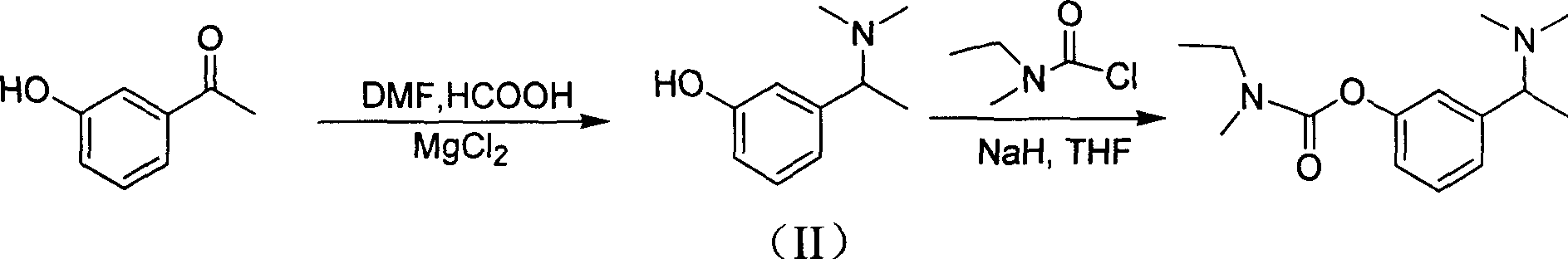
[0024] (1) Under ice bath conditions, slowly add 33% dimethylamine aqueous solution (54.5g, 0.4mol) to a 250ml three-necked flask containing 88% formic acid (20.9g, 0.4mol). After the addition is complete, heat for distillation , Distill about 46ml of water to prepare new ecological N,N-dimethylformamide (DMF). When the temperature drops below 100°C, add m-hydroxyacetophenone (13.6g, 0.1mol), formic acid (88% concentration) (4.6g, 0.1mol), MgCl to the reaction flask 2 ·6H 2 O (3g, 0.015mol). Raise the temperature to 170℃ directly, heat the reaction for 5h, after the reaction is completed, pour the reaction solution into 80ml water, wash the reaction flask with about 5ml water and combine them together, adjust the pH value to about 1-2 with concentrated hydrochloric acid, filter and extract with ether , The ether phase is dried with anhydrous magnesium sulfate, rotary evaporated to dryness, the solid raw material m-hydroxyacetophenone 6.47g is recovered and reused. Adjust the pH va...
Embodiment 2
[0027] (1) In a 100ml three-necked flask, add m-hydroxyacetophenone (4g, 0.029mol), 88% formic acid (4ml, 0.093mol), N,N-dimethylformamide (DMF) (20ml, 0.26mol) . Heat to 155°C and reflux for 24h, adjust the pH to about 1-2 with concentrated hydrochloric acid, filter, extract with ether, dry the ether phase with anhydrous magnesium sulfate, first distill off the ether by ordinary distillation, and then distill under reduced pressure to remove excess DMF to obtain a solid raw material 2.55g of m-hydroxyacetophenone. Adjust the pH value of the aqueous phase to 8.5 with sodium bicarbonate, extract with ethyl acetate, anhydrous MgSO 4 Drying, rotary evaporation and concentration, the remaining residual liquid is cooled, the solid is separated out, filtered, and vacuum dried to obtain the product 3-(1-(dimethylamino)ethyl)phenol (0.65g, yield: 13.4%; recovery yield) : 36.9%).
[0028] (2) Same as Example 1.
Embodiment 3
[0030] (1) Same as in Example 1, to a 2000ml three-necked flask containing 88% formic acid (418g, 8mol), slowly add 33% dimethylamine aqueous solution (1090g, 8mol), after the addition is complete, heat and distill to remove water. The new ecological N,N-dimethylformamide (DMF) is prepared. After cooling to about 100°C, add m-hydroxyacetophenone (1.36g, 0.01mol), formic acid (88% concentration) (4.6g, 0.1mol), MgCl to the reaction flask 2 ·6H 2 O (2g, 0.01mol). The temperature was directly raised to 170°C, and the reaction was heated for 5 hours. After the reaction was completed, the excess DMF was distilled off under reduced pressure, and the residue was poured into 10 ml of water. Other operations are the same as in Example 1. The 3-(1-(dimethylamino)ethyl)phenol (0.7 g, yield: 42.4%; recovery yield: 79.0%) was obtained.
[0031] (2) Same as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com