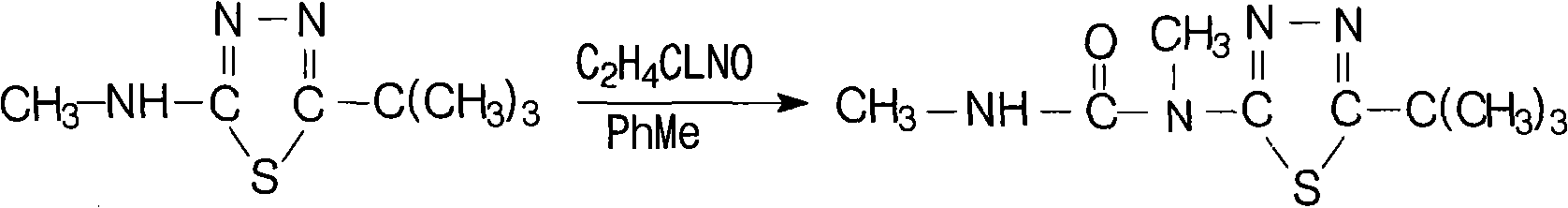
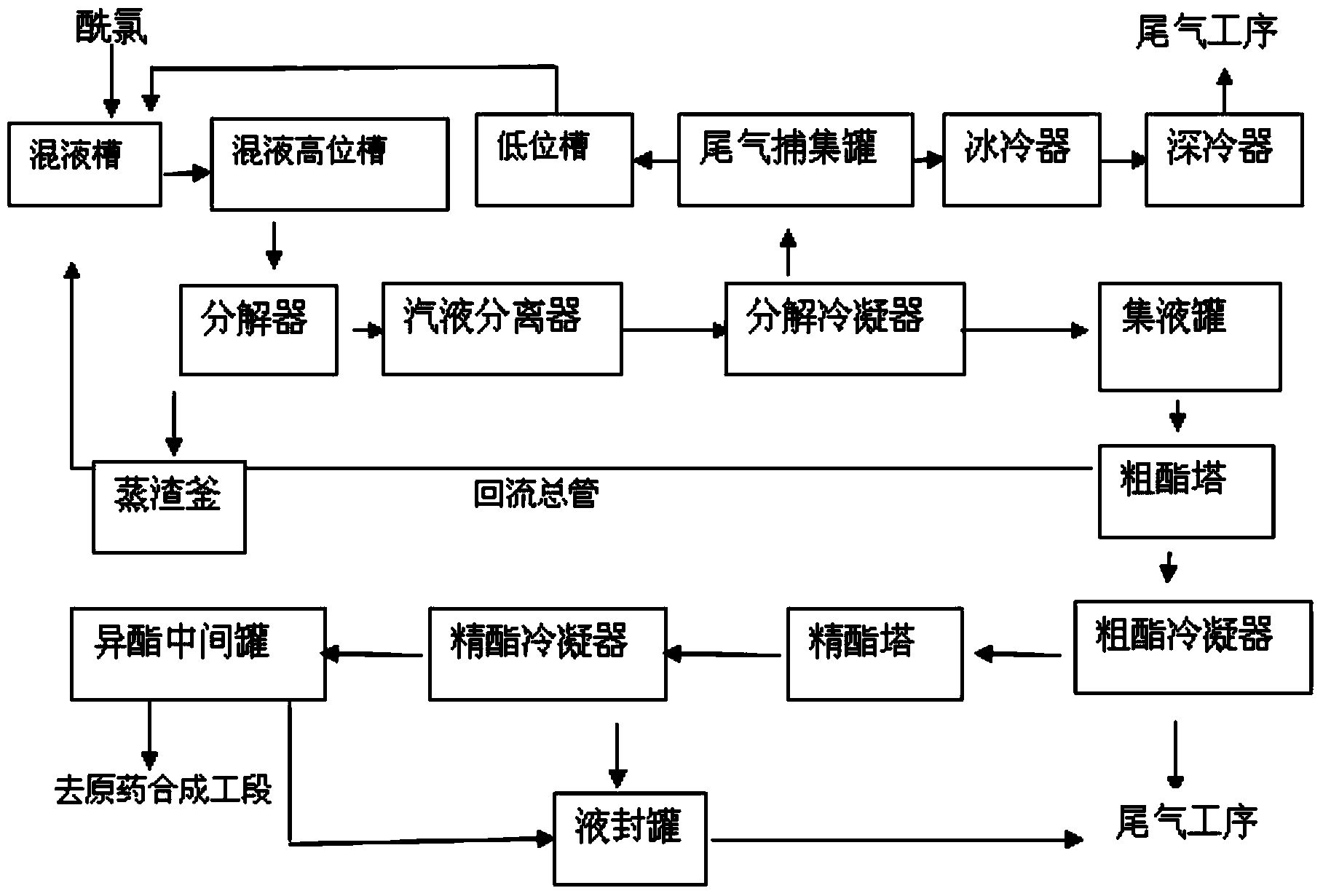
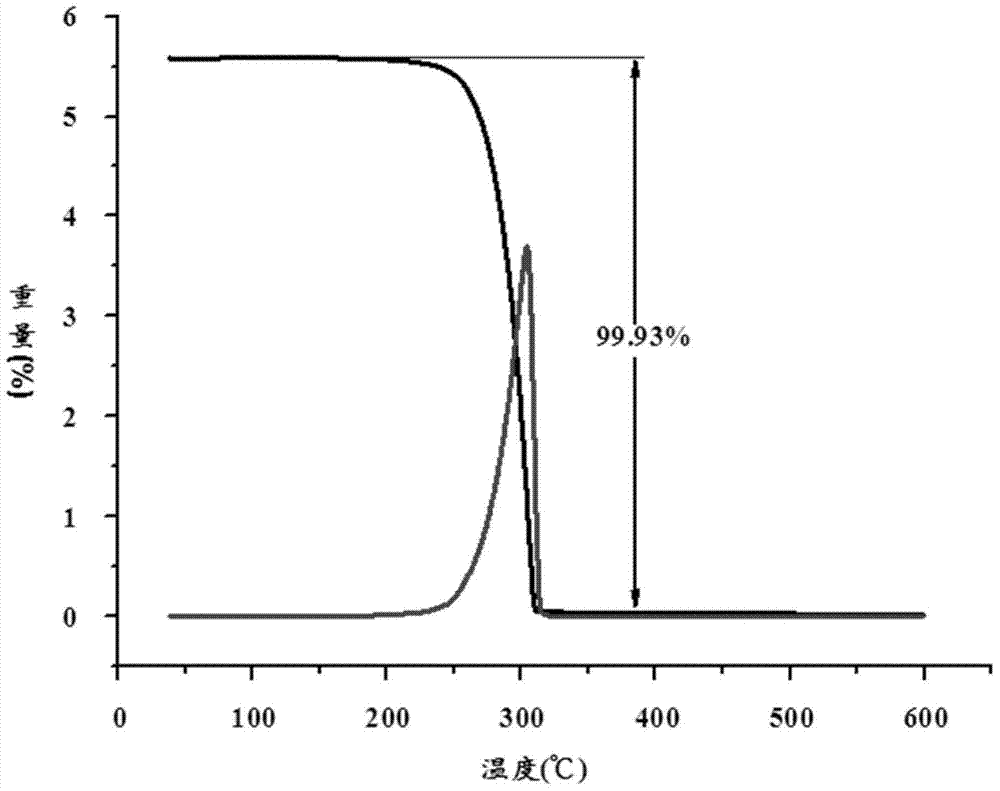
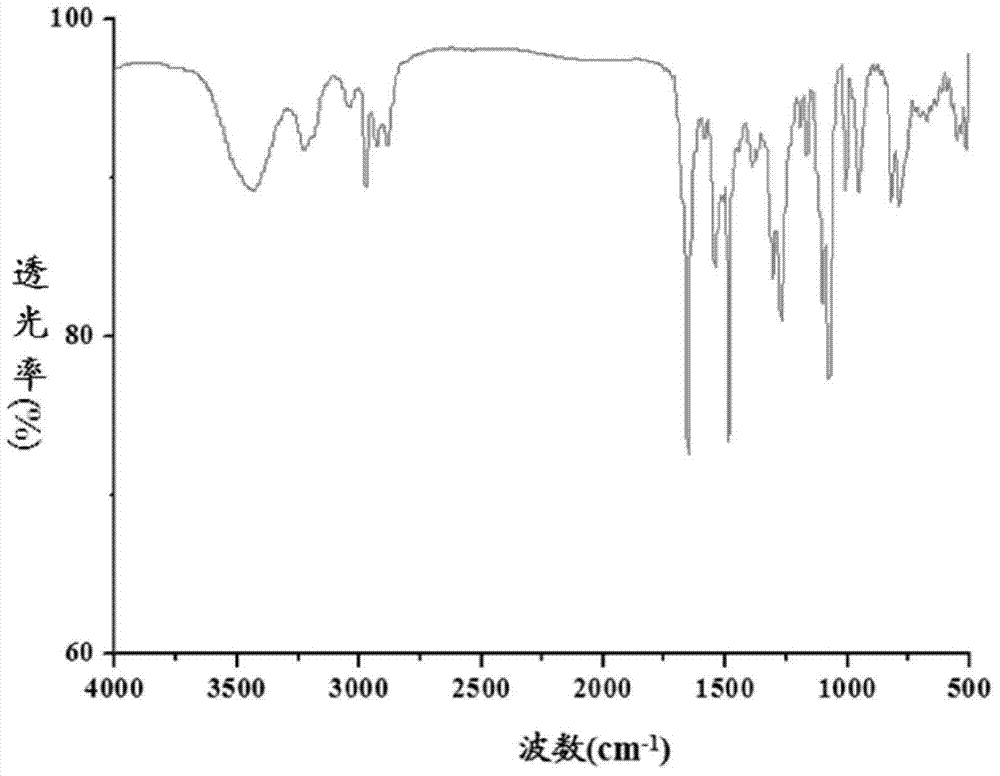
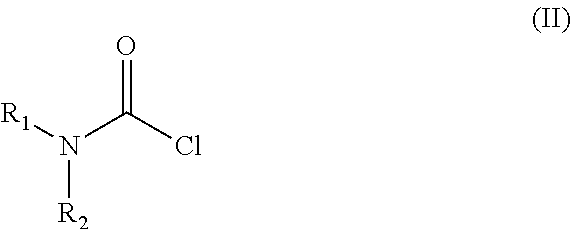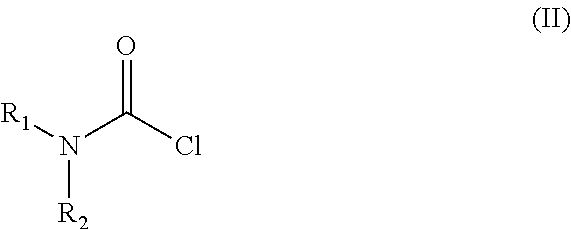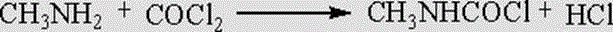Patents
Literature
90 results about "Carbamoyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
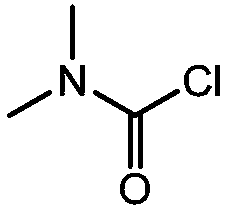
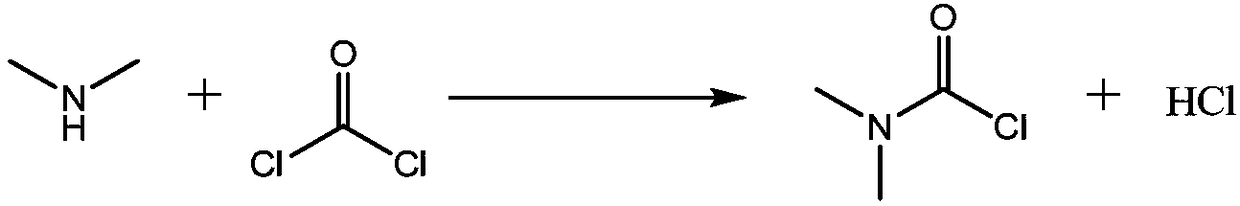
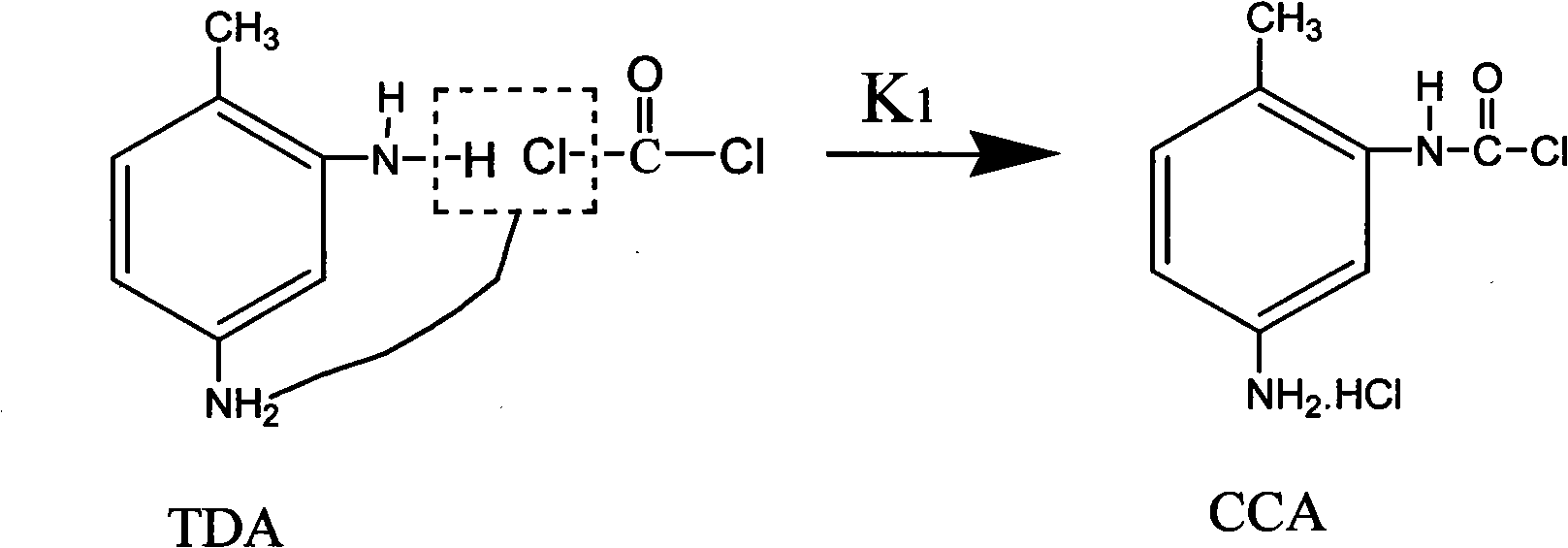
A carbamoyl chloride is the functional group with the formula R₂NC(O)Cl. The parent carbamoyl chloride, H₂NCOCl is unstable, but many N-substituted analogues are known. Most examples are moisture sensitive, colourless, and soluble in nonpolar organic solvents. An example is dimethylcarbamoyl chloride (m.p. −90 °C and b.p. 93 °C). Carbamoyl chlorides are used to prepare a number of pesticides, e.g. carbofuran and aldicarb.
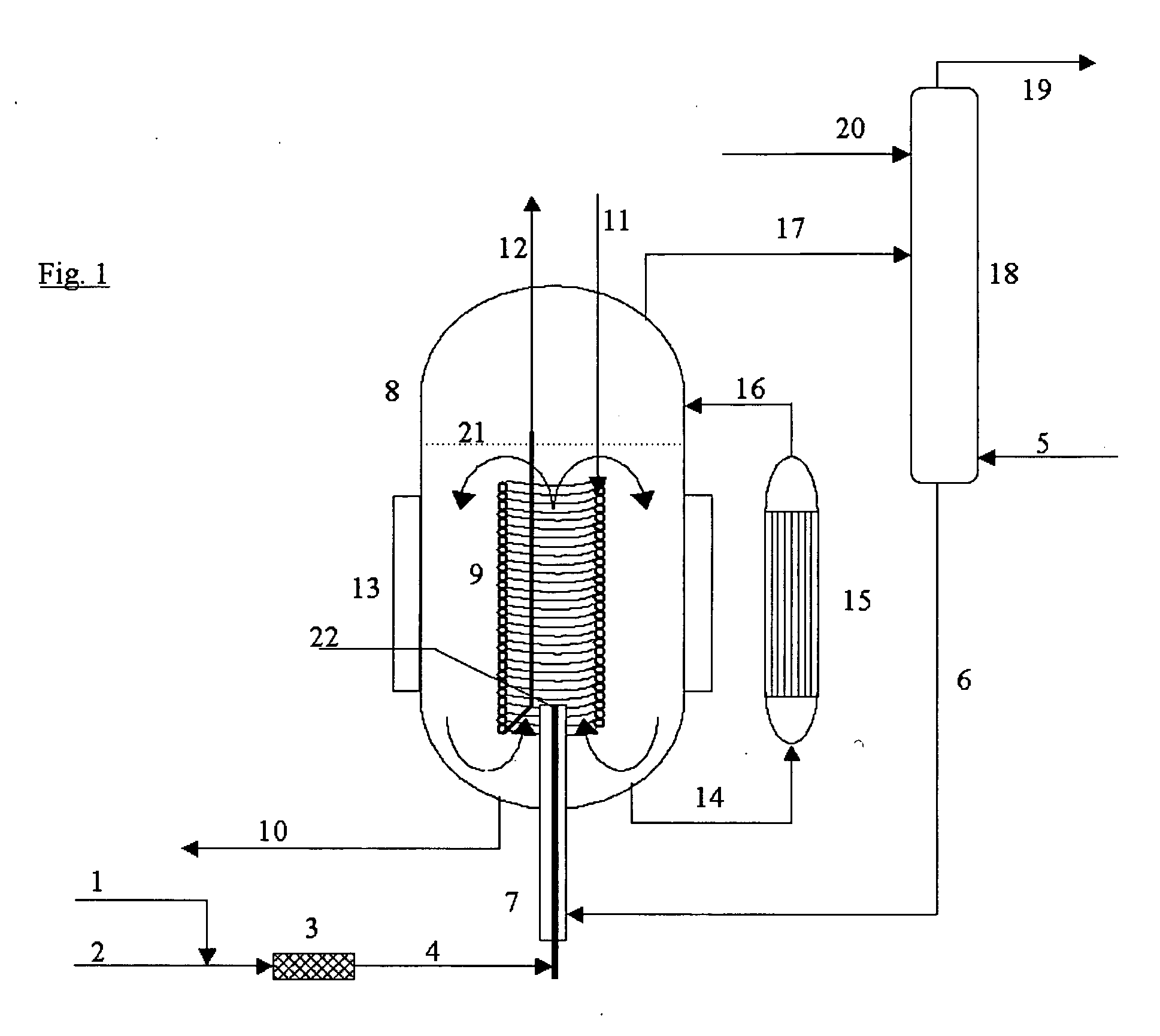
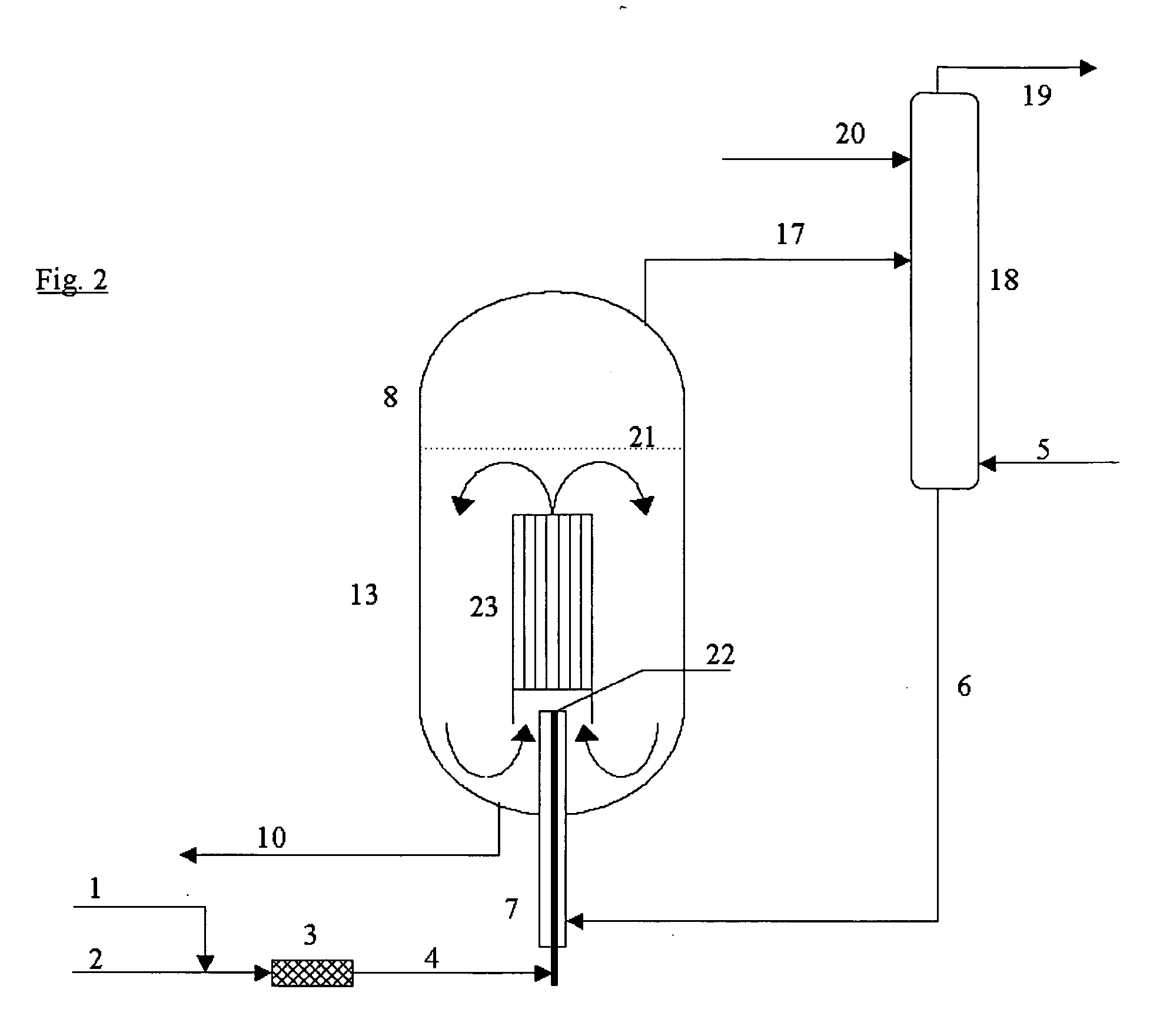
Process for the continuous preparation of organic monoisocyanates and polyisocyanates
InactiveUS20060041166A1Reduce equipment costsMinimize formationIsocyanic acid derivatives preparationOrganic compound preparationOrganic solventDecomposition
The invention relates to a process for the continuous preparation of organic isocyanates through the reaction of organic amines with phosgene in the presence of organic solvents under pressure whereby a concentrated phosgene-containing stream is mixed preferentially with an amine-containing stream in a jet mixer to create a combined jet of reacting amine-phosgene mixture, whereby the combined jet is discharged directly into a reactor vessel and the reactor vessel is operated at a temperature above the decomposition temperature of intermediate carbamoyl chloride products which can be formed upon mixing the aforementioned streams, wherein the combined jet is not pre-mixed with bulk reactor contents, wherein the jet mixer provides sufficiently rapid and thorough mixing and thereby enables an initial reaction temperature lower than the bulk reactor vessel temperature, and the combined jet entering the reactor has sufficient momentum to cause entrainment into it of a sufficient quantity of the bulk reactor contents to be rapidly dispersed and reach the bulk reactor temperature.
Owner:STUART JOSEPH Y
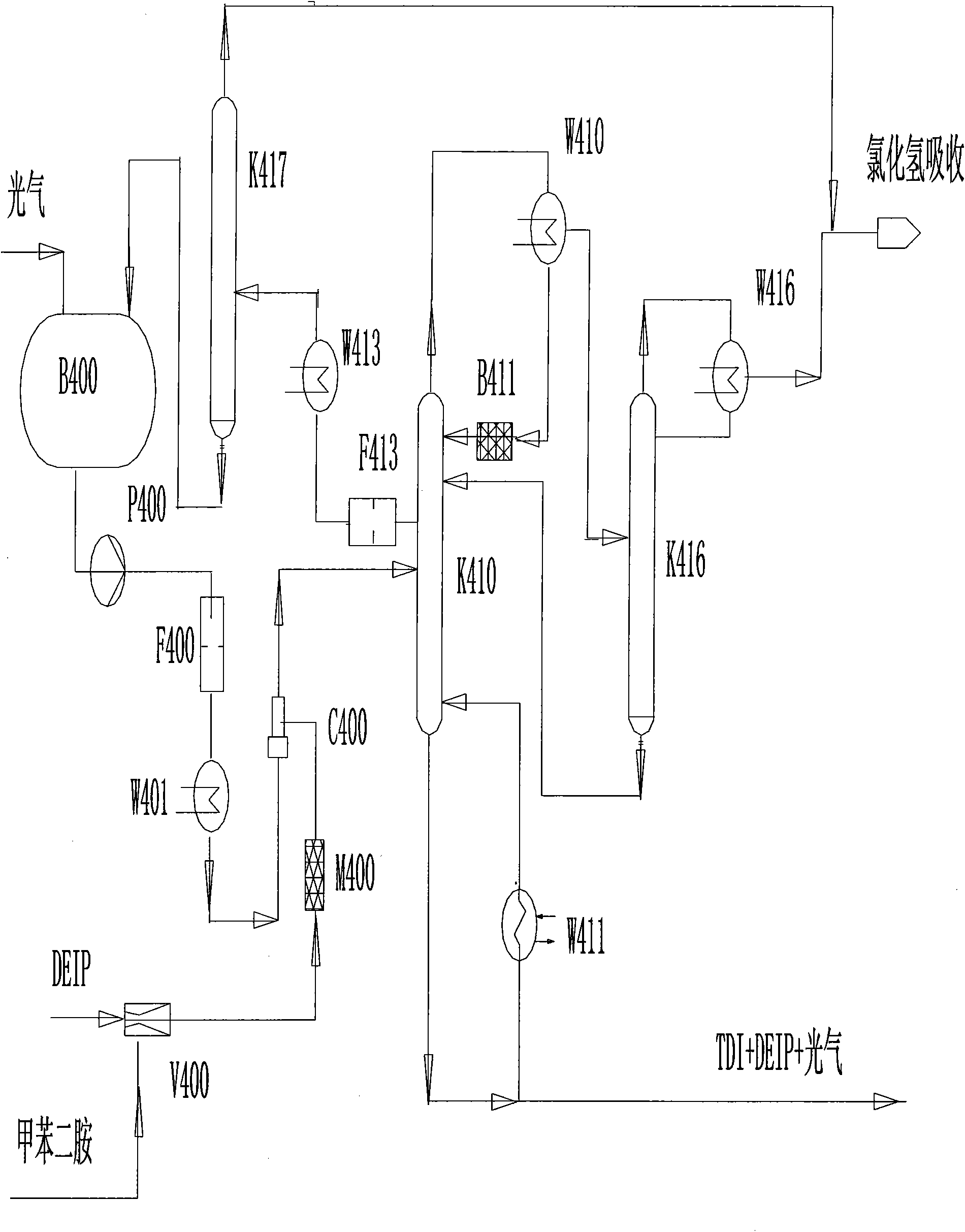
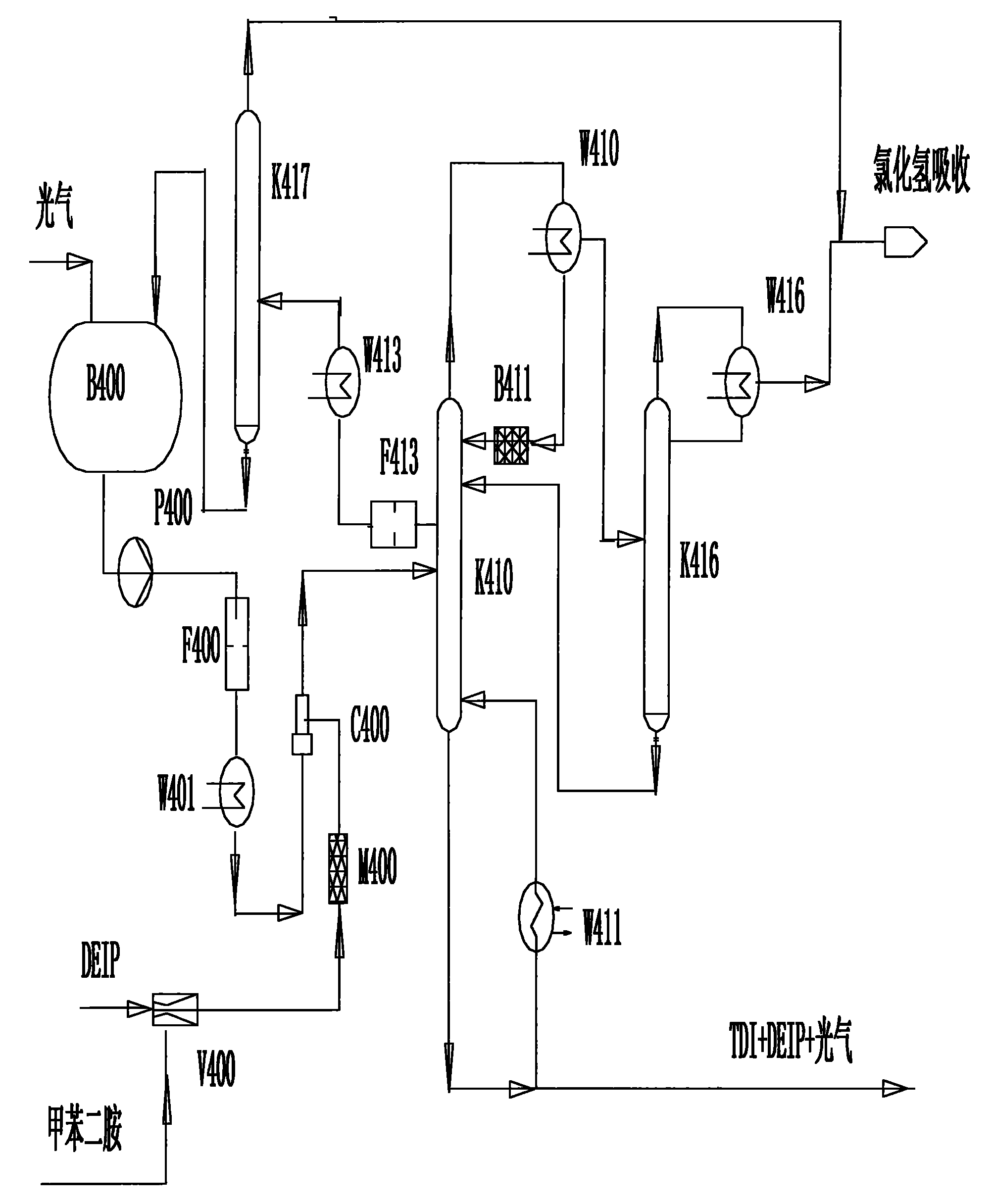
Method for continuously preparing toluene diisocynate
The invention relates to a method for continuously preparing toluene diisocynate, in particular to a method for preparing coarse toluene diisocynate by a phosgene method. The method comprises the following steps: mixing toluene diamine and DEIP solvent, performing low-temperature photochemical reaction on the mixture and excessive liquid phosgene in a jet reactor, heating and cracking a reaction product in a photochemical reaction tower to further decompose toluene dicarbamic chloride serving as an intermediate product to form the toluene diisocynate, recycling the reclaimed phosgene and the DEIP solvent, and delivering the hydrogen chloride gas serving as a side product to a hydrogen chloride absorption system.
Owner:甘肃银光聚银化工有限公司
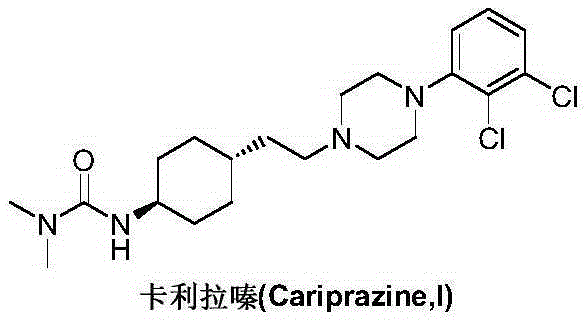
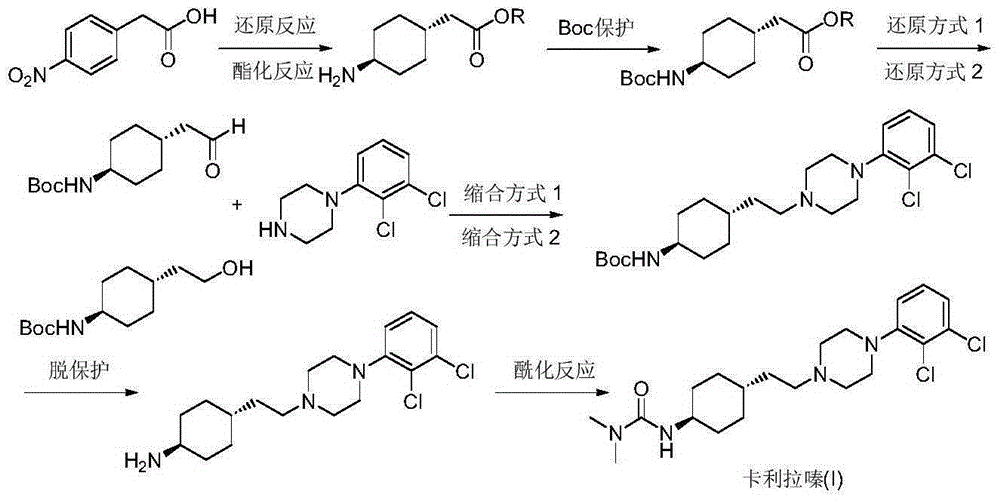
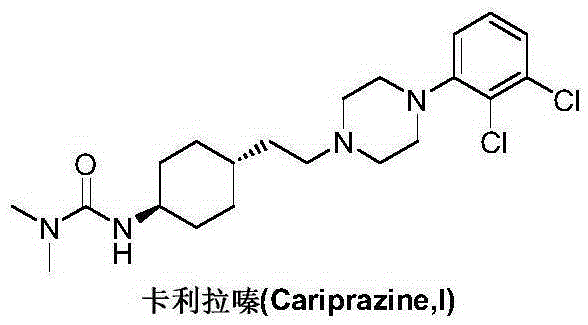
Preparation method of cariprazine
ActiveCN105330616AEase of industrial productionRaw materials are easy to getOrganic active ingredientsNervous disorderCariprazineCyclohexanone
The invention discloses a preparation method of cariprazine (Cariprazine, RGH 188). The method includes the preparation steps of preparing 4-[2-[4-(2,3-dichlorophenyl)piperazine]-1-based]ethyl]cyclohexanone by making 4-(2-hydroxyethyl)cyclohexanone and 1-(2,3-dichlorophenyl)piperazine subjected to a condensation reaction, preparing trans-4-[[2-]4-(2,3-dichlorophenyl)piperazine]-1-based]ethyl]cyclohexylamine by making the obtained intermediate subjected to a reduction ammonolysis reaction, and preparing cariprazine by making the intermediate and N,N-dimethylcarbamyl chloride subjected to an acylation reaction. According to the preparation method, raw materials can be easily obtained, the process is simple, and the method is economical, environmentally friendly and suitable for industrialized production.
Owner:中科恩吉瑞特(烟台)科技发展有限公司
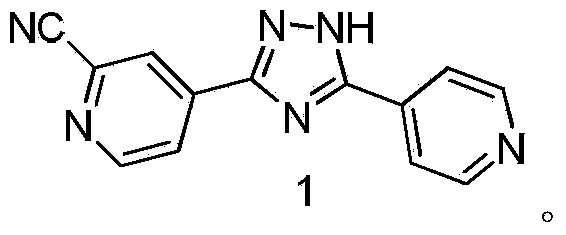
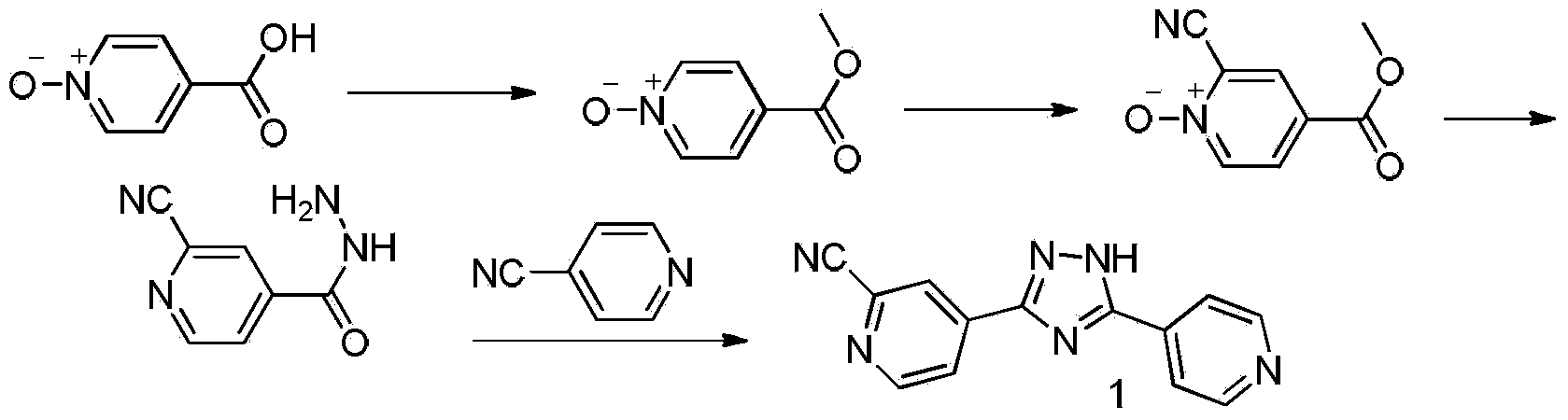
Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile
InactiveCN104151297AEasy to operateSimple processing capacityOrganic chemistryTopiroxostatDimethylcarbamyl chloride
The invention relates to a preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile. The method includes following steps: preparing a compound methyl 2-cyanoisonicotinate represented as the formula (4) with a compound methylisonicotinic-N-oxide (5) being a starting raw material in the presence of a copper catalyst (CuX), a metal cyanide and dimethylcarbamyl chloride; performing hydrazinolysis to the methyl 2-cyanoisonicotinate to obtain a compound 2-cyanoisoniazide represented as the formula (3); and finally performing condensation with a compound 4-cyanopyridine represented as the formula (2) to obtain a compound topiroxostat represented as the formula (1). The method only comprises three steps and is simple in operation and post-treatment. By means of the copper catalyst, a usage amount of the metal cyanide is greatly reduced so that reaction conditions are milder. The method is high in purity of the prepared product and is suitable for industrial production.
Owner:王庆本 +1
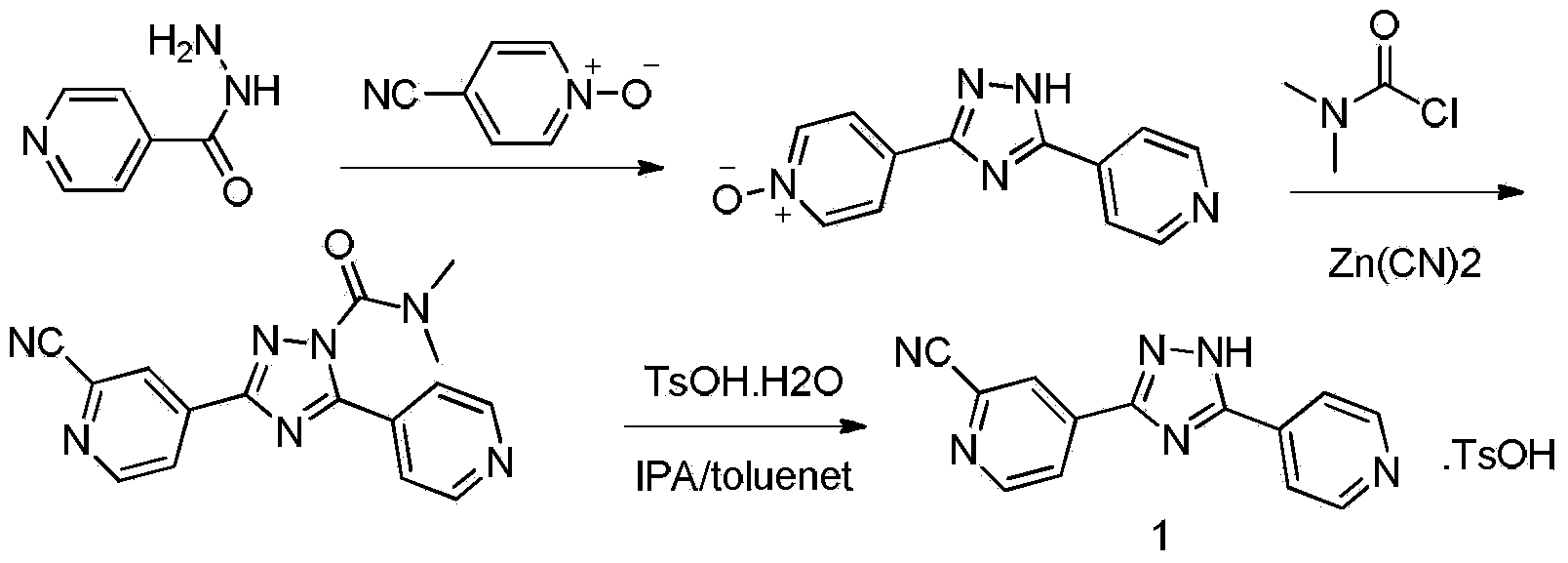
Preparation method of topiroxostat
The invention relates to a preparation method of topiroxostat, which comprises the following steps: reacting an initial raw material compound isoniazide disclosed as Formula (6) with a compound 4-cyano-pyridyl-N-oxide disclosed as Formula (5) to generate a triazole compound disclosed as Formula (4), reacting the triazole compound disclosed as Formula (4) in the presence of a copper catalyst (CuX), zinc cyanide (Zn(CN)2) and dimethylamino formyl chloride to generate a cyanotriazole compound disclosed as Formula (3), reacting the compound disclosed as Formula (3) in the presence of p-toluenesulfonic acid to generate a compound disclosed as Formula (2), and finally, alkalifying with inorganic alkali to obtain the target compound disclosed as Formula (1) (topiroxostat). The method is simple in operation and after-treatment, and greatly lowers the consumption of the zinc cyanide due to the use of the copper catalyst, so that the reaction conditions are milder, and the purity of the prepared product is high; and the method is suitable for industrial production.
Owner:于宗光
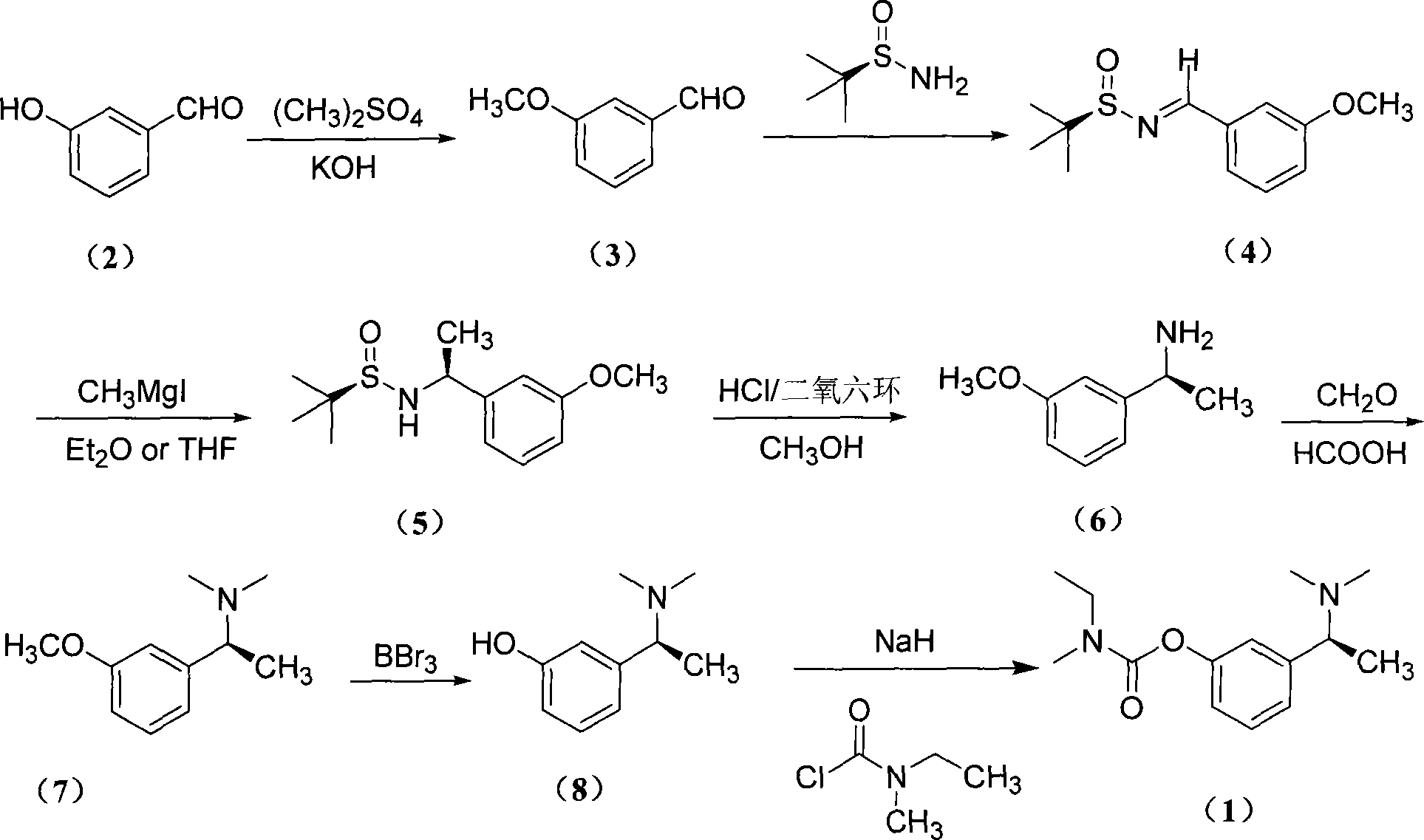
Asymmetric synthesis method of (S)-rivastigmine
InactiveCN101134738AFew reaction stepsHigh reaction yieldCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsGrignard reagent
The present invention is asymmetrical (S)-carbalatine synthesizing process including the following steps: protecting the phenolic hydroxyl group of m-hydroxy benzaldehyde, reacting with chiral tert-butyl sulfonamide to produce (R, E)-3-methoxyl-phenyl methylene tert-butyl sulfonamide, addition reacting with methyl Grignard reagent, hydrolyzing, Eschweiler-Clarke methylation reaction, demethylating BBr3 to synthesize important intermediate (S)-1-(3-hydroxy phenyl)-N, N-dimethyl ethyl amine, and esterification with methyl ethyl carbamyl chloride to obtain (S)-carbalatine. The present invention has less reaction steps, high yield, total yield up to 21.85 %, and low cost.
Owner:JINAN UNIVERSITY
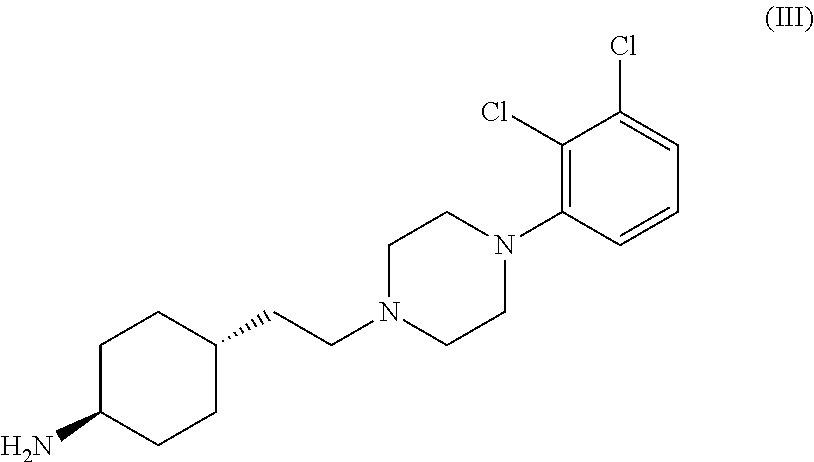
Process for the preparation of piperazine derivatives
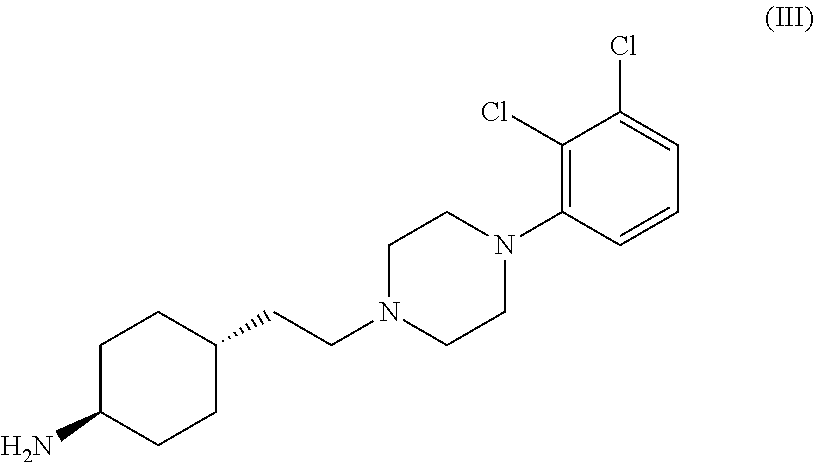
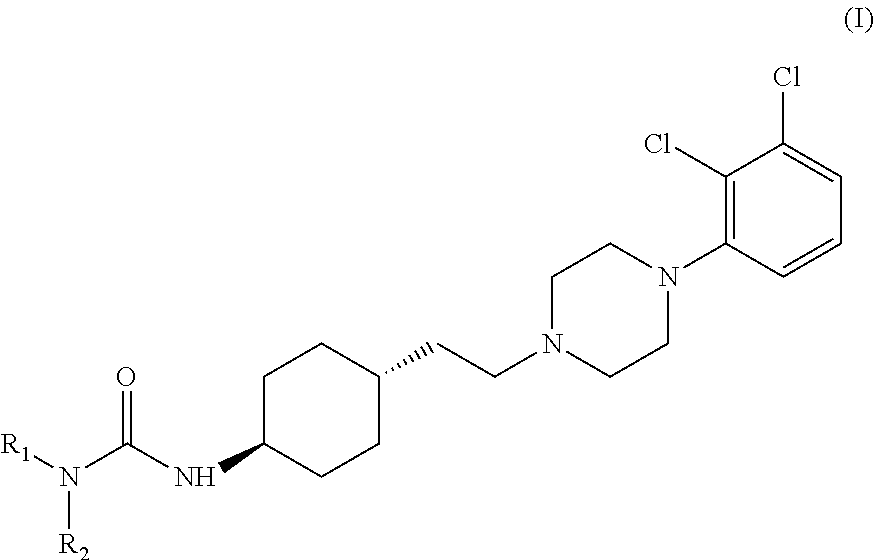
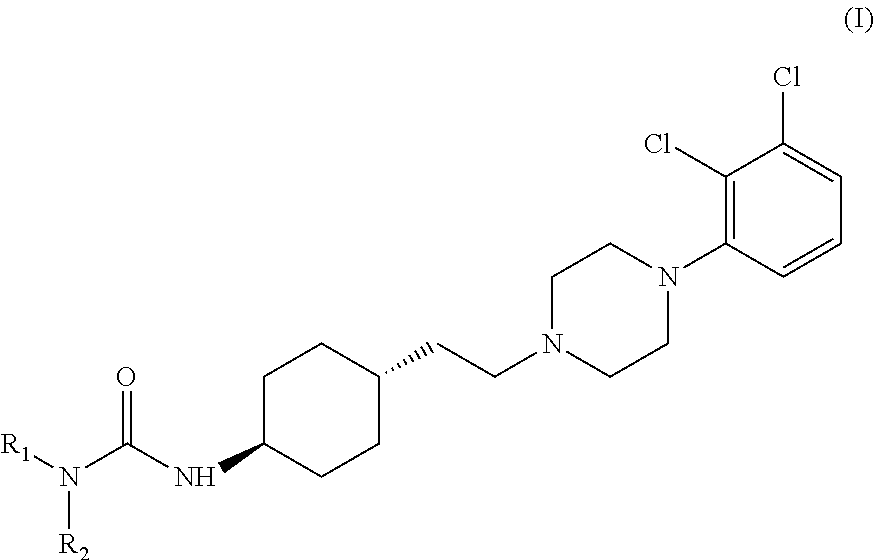
The invention relates to a process for the preparation of the trans N-{4-{2-[4-(2,3-dichlorophenyl)-piperazine-1-yl]-ethyl}-cyclohexyl}-carbamide derivatives of general formula (I) by reacting the compound of formula (III) with a carbamoylchloride of general formula (II) which comprises carrying out the reaction in a mixture of a solvent and concentrated aqueous solution of an alkali hydroxide at a temperature between 40-100° C. in the presence of a phase transfer catalyst, separating the phases and washing the organic layer then removing the solvent and drying the compound of formula (I) obtained until its weight is constant.
Owner:GEDEON RICHTER NYRT
Proparagyl urea analogue monomer and optical rotation spiral polymer and its preparation method
The invention discloses a propargyl urea monomer in the macromolecular material domain, which is characterized by the following: group R1 and R2 in the structure formula (1) of propargyl urea monomer are the same or different, which are aromatic radical or alkyl; the number average molecular weight of structure formula (2) of propargyl urea monomer is 6800-136600 and the molecular weight distribution index is 1.34-2.91 with 100 percent sym-form structure. The making method comprises the following steps: adding secondary amine drip at 3:1 rate in the organic solution of solid phosgene; heating to reflux for 10-30h; cooling; removing organic solvent through boiling to produce carbamyl chloride; reacting carbamyl chloride and propargyl amine at 1:1 rate for 2-5 h; neutralizing through alkali after acid cleaning; dewater; boiling out carrene to produce propargyl urea monomer; solving propargyl urea monomer and transition metal accelerant at 50:1-100:1 rate in the organic solvent protected by nitrogen; blending two solutions to polymerize for 2-24 h at -15-30 deg.c; adding skellysolve B; settling; filtering; drying in the vacuum. The invention can produce polymerization with more ideal molecular weight and molecular weight distribution, which can be used in optical, biological and medicine domain.
Owner:BEIJING UNIV OF CHEM TECH
Method for synthesizing pentamethylene diisocyanate
InactiveCN106045882AAccurate measurementAvoid reactionCarbamic acid derivatives preparationOrganic compound preparationHigh pressureMethylene diisocyanate
The invention discloses a method for synthesizing pentamethylene diisocyanate. 1,5-pentanediamine or salt produced by 1,5-pentanediamine and dry carbon dioxide or 1,5-pentanediamine and dry hydrogen chloride reacts with solid-state phosgene, a middle product is produced, carbon dioxide or hydrogen chloride is exhausted, and the middle product is pentamethylene dimethylcarbamoyl chloride. The middle product is decomposed at the high temperature to produce pentamethylene diisocyanate and hydrogen chloride. The synthesis method adopts a solid-state phosgene method for synthesis, and solid-state phosgene is in a solid form at the normal temperature, has a higher melting point and a stable property and can be accurately metered during a reaction; high temperature and high pressure are not required in the technological process, the operation is simple and the cost is low.
Owner:山东崇舜新材料科技有限公司
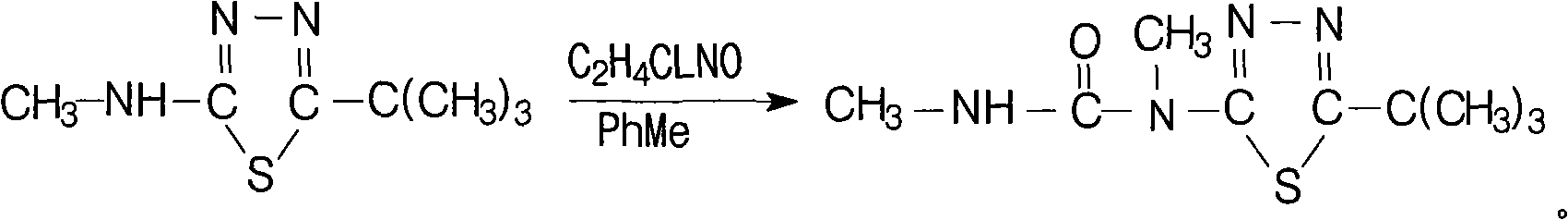
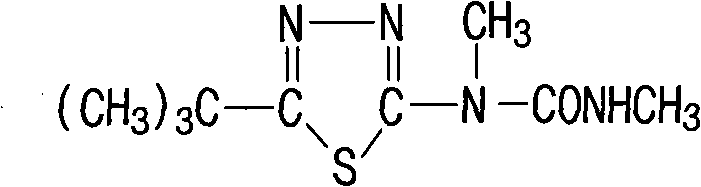
Method for synthesizing tebuthiuron technical
The invention relates to a method for synthesizing tebuthiuron technical. The method adopts methylaminoformyl chloride and 5-tert-butyl-2-methylamino-1,3,4 thiadiazole to directly react to generate the tebuthiuron with the reaction formula as the above. The new method avoids using inflammable and explosive dangerous chemical methyl isocyanate with great toxicity, and is safer for production. The method reduces the requirement on equipment materials, and reduces equipment investment.
Owner:YIFAN AGRI CHEM PLANT ZHEJIANG PROV
Production method and equipment of methyl isocyanate
InactiveCN103694190AAvoid downtimeEnsure continuous productionIsocyanic acid derivatives purification/separationPreparation from carbamatesChlorideMethyl isocyanate
The invention discloses a production method of methyl isocyanate. The method comprises the following steps: producing CO from CO2, O2 and coke, producing phosgene from CO and chlorine gas, synthesizing methylaminoformyl chloride from the phosgene and the monomethylamine, decomposing and purifying to prepare the methyl isocyanate. The invention further discloses production equipment of methyl isocyanate. According to the production method and equipment, the energy consumption can be effectively reduced, byproducts are reduced, the production cost is lowered, the product yield is increased, the labor intensity of workers is reduced, the production field environment is improved, the reaction safety is further enhanced, and the three-waste emission is reduced.
Owner:荆州沙隆达控股有限公司
Functional organosiloxane containing asymmetrical substituted urea and preparation method thereof
InactiveCN103755733AImprove conversion rateImprove thermal stabilityGroup 4/14 element organic compoundsOther chemical processesPolymer scienceUltraviolet
The invention discloses functional organosiloxane containing asymmetrical substituted urea and a preparation method thereof. The preparation method comprises the following steps: carrying out reaction between 4, 4'-dibromodiphenylamine and triphosgene to obtain (i) N, N( / i)-di(4-bromo phenyl) carbamyl chloride; and then, carrying out reaction with 3-aminopropyl triethoxysilane to obtain functional organosiloxane, wherein the organosiloxane is the functional organosiloxane containing asymmetrical substituted urea through the verification of infrared spectrum, ultraviolet spectrum, 1H-NMR (1Hydrogen-Nuclear Magnetic Resonance) and 13C-NMR. The structure of the compound contains a plurality of functional groups such as siloxane, amide, urea groups and bromoaryl. The functional organosiloxane has a potential application value on the respects of molecular imprinting recognition materials, structural flame retardant materials, rare earth light emitting materials, white carbon black and the like. The functional organosiloxane disclosed by the invention is reasonable in synthetic line, mild in condition, easy to operate, low in cost of raw materials and high in product yield.
Owner:FUJIAN NORMAL UNIV
Method for synthesizing tebuthiuron technical material
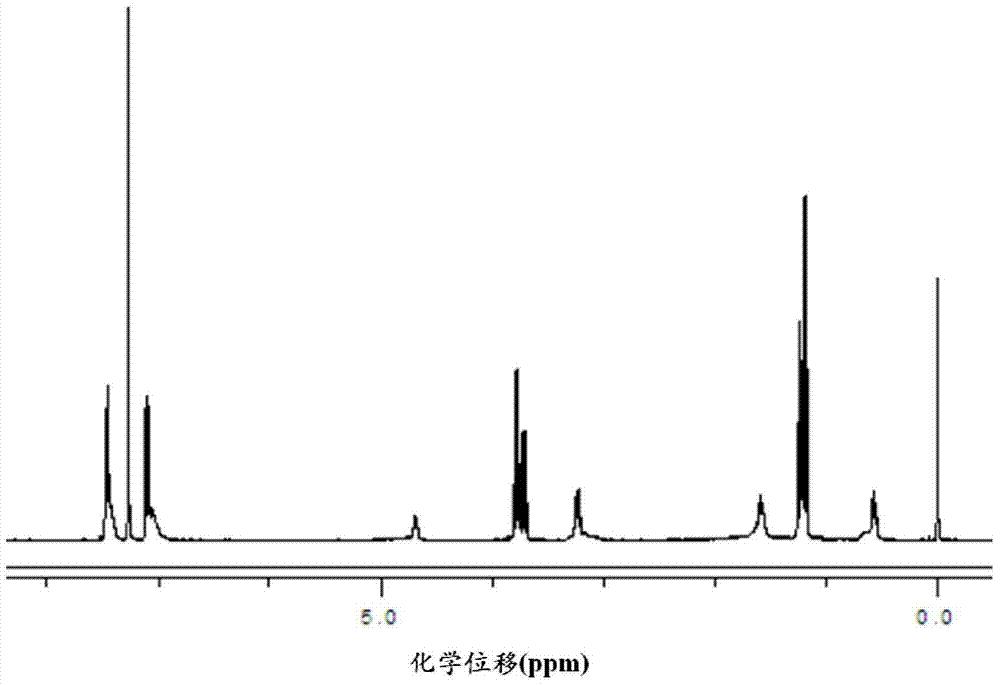
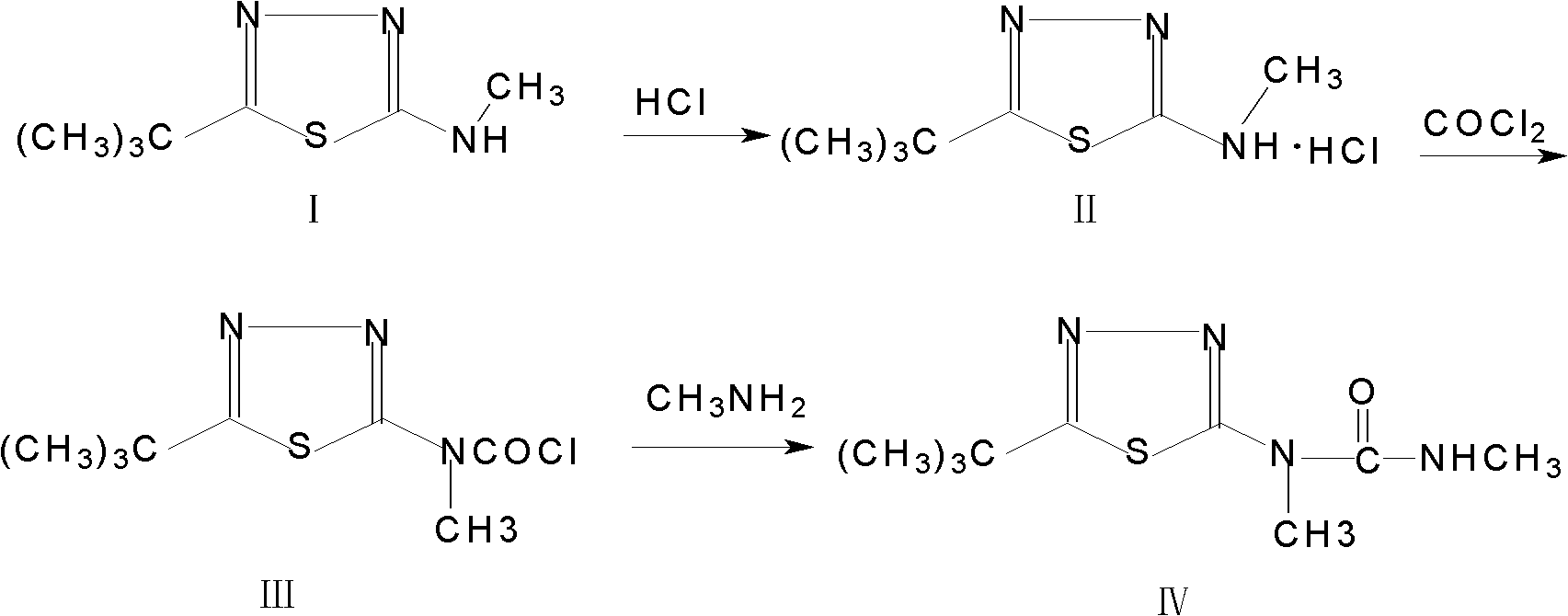
The invention discloses a method for synthesizing a tebuthiuron technical material. The method comprises the following steps: taking 5-tert-butyl-2-methylamino-1,3,4-thiadiazole as a starting material; carrying out salt forming reaction and photochemical reaction in an organic solvent to prepare N-methyl-N-(5-tert-butyl-1,3,4-thiadiazole) carbamyl chloride; and introducing monomethylamine gas into the N-methyl-N-(5-tert-butyl-1,3,4-thiadiazole) carbamyl chloride to generate the tebuthiuron. The method has the advantages of simplicity in process, low production cost, high product purity, high yield, high production safety and the like.
Owner:JIANGSU KUAIDA AGROCHEM
Method for synthesis of rivastigmine
InactiveCN1962624ALess investmentSimple and safe operationCarbamic acid derivatives preparationOrganic compound preparationMethylcarbamic acidRivastigmine
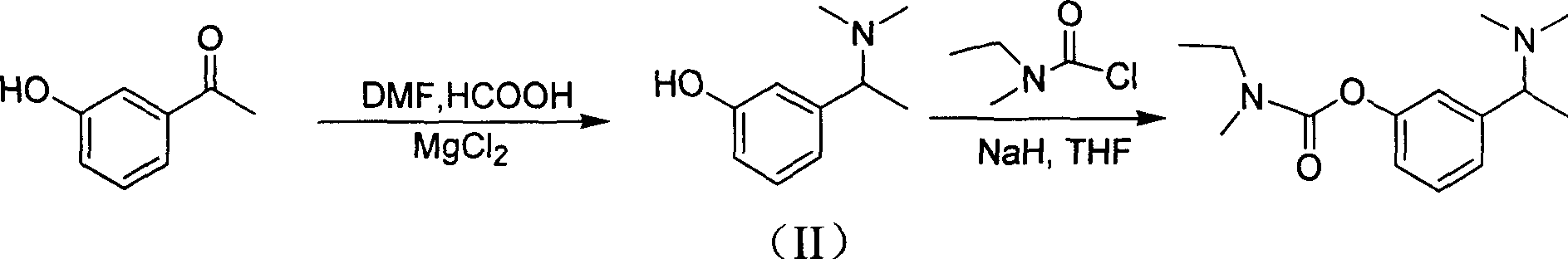
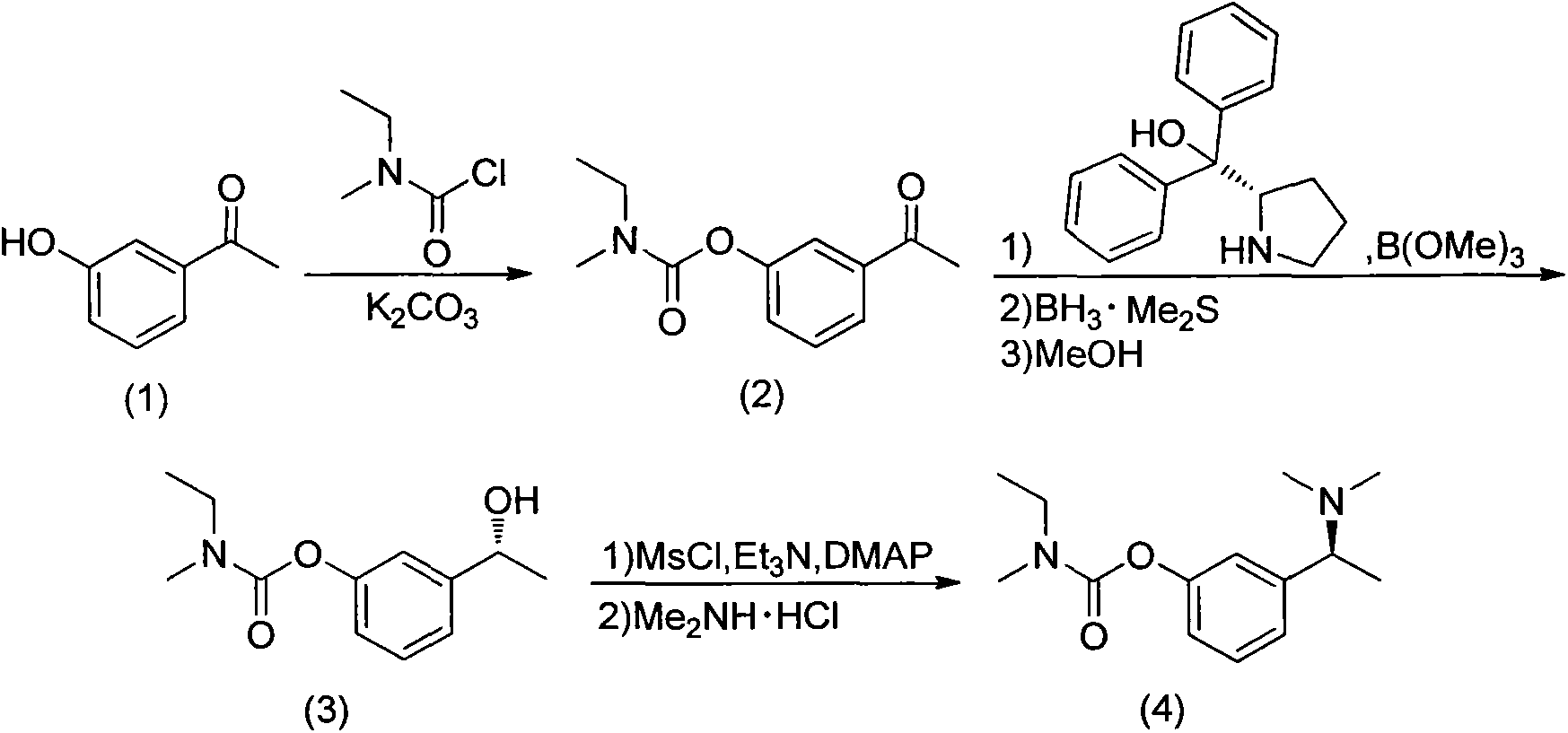
The invention discloses a synthesizing method of (S)-N-ethyl-3-[(1-dimethylamino) acetyl]-N-methyl amido phenyl formate, which is characterized by the following: adopting m-hydroxy acetophenone as raw material; synthesizing intermediate 3-(1-(dimethylamino) ethyl) phenol; condensing 3-(1-(dimethylamino) ethyl) phenol and formyl chloride to produce the product with high receiving rate.
Owner:JINAN UNIVERSITY
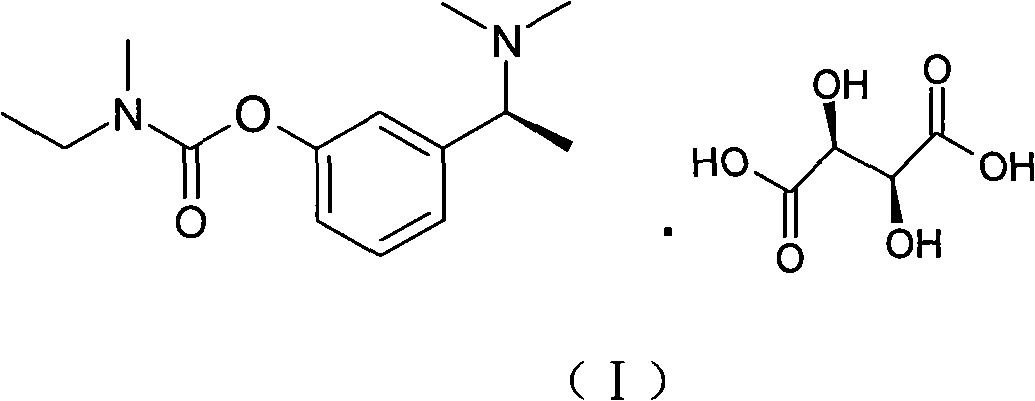
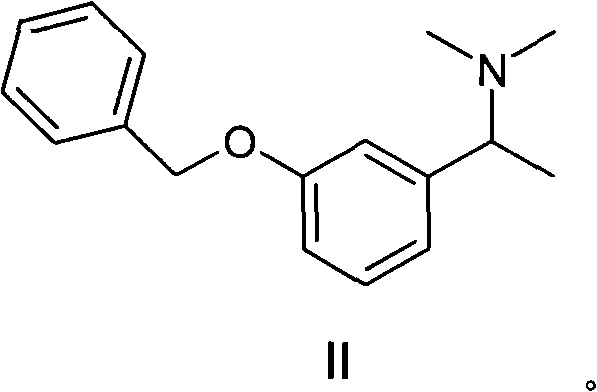
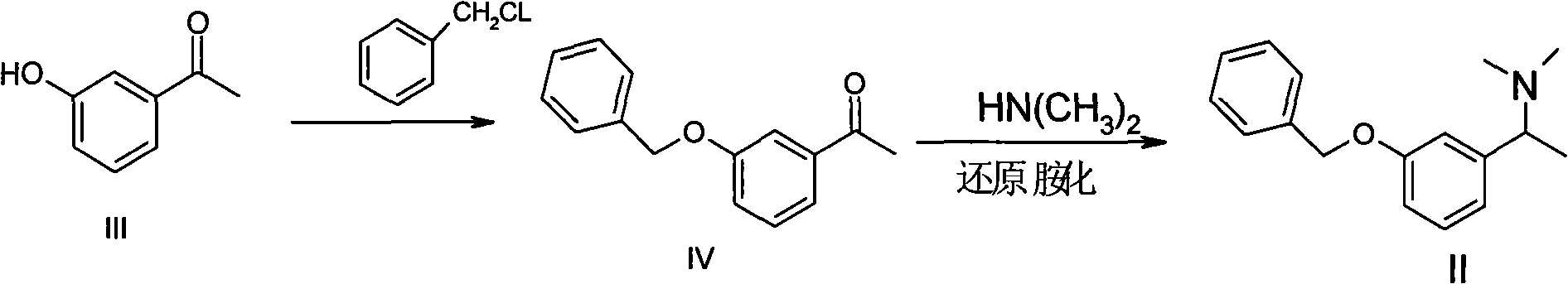
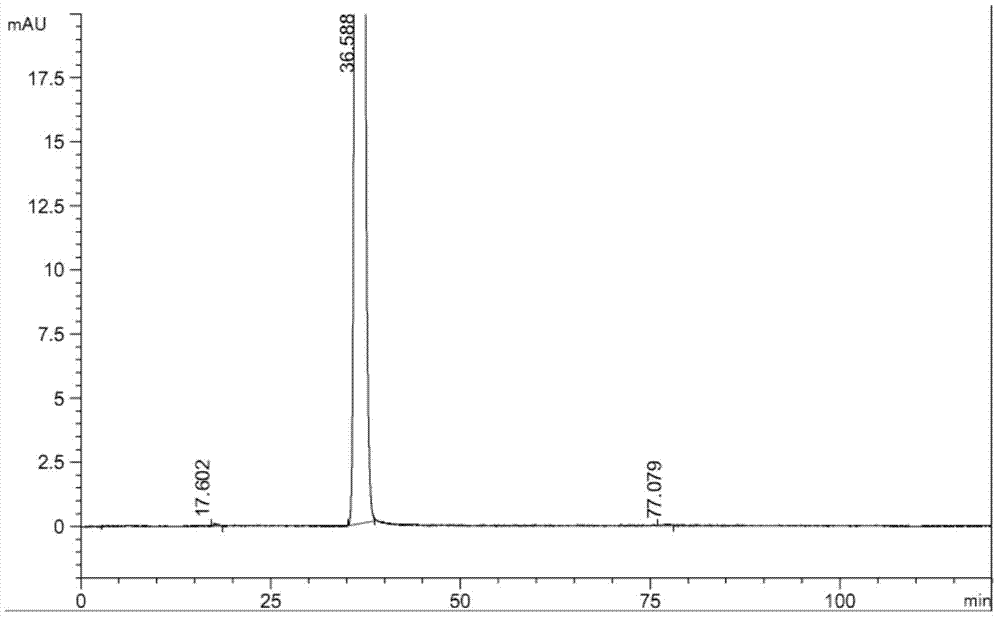
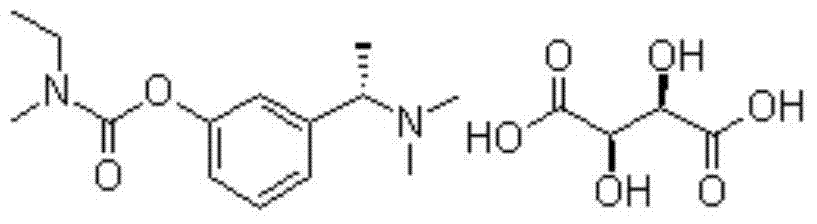
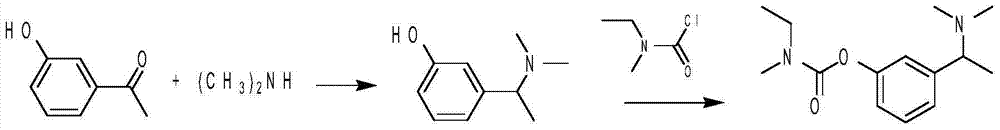
Synthetic method of rivastigmine and intermediates thereof
ActiveCN101823970ASolve the problems of difficult operation and low production efficiencyEasy to separate and purifyNervous disorderCarbamic acid derivatives preparationRivastigminePhenol
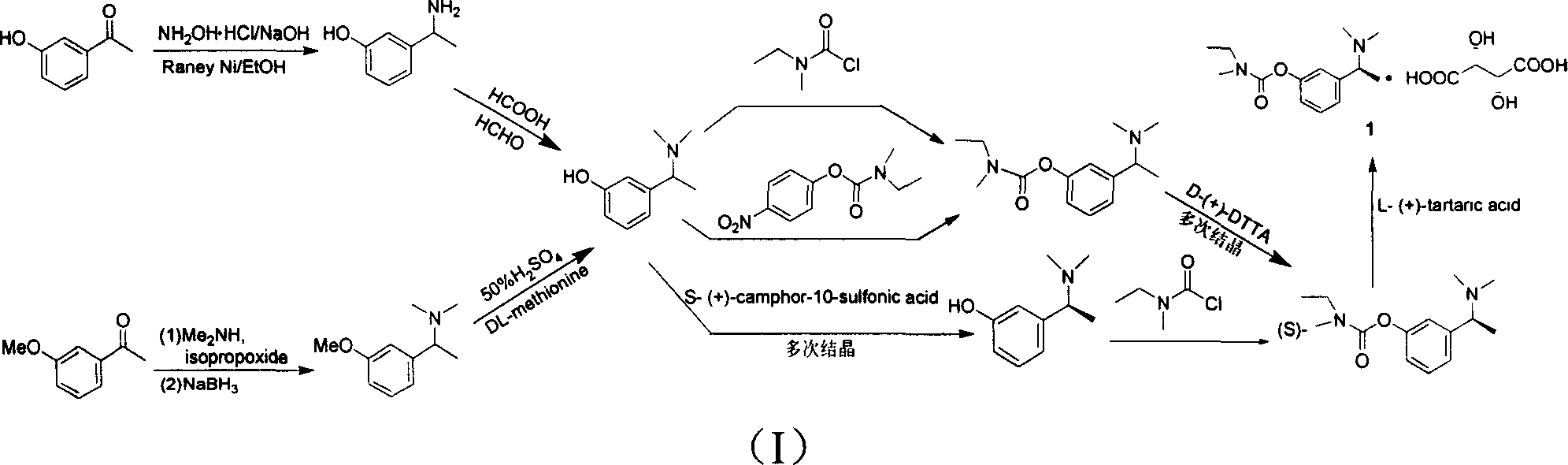
The invention relates to a preparation method of rivastigmine tartrate and intermediates thereof, which comprises the steps of carrying out reductive amination on 3-benzyloxy acetophenone and dimethyl for obtaining a (1-(3-benzyloxy-phenyl)-ethyl)-dimethylamine (II) compound, removing benzyl with hydrogenated amine, obtaining 3-(1-(dimethylamino) ethyl) phenol (V), further carrying out condensation reaction with N-methyl-N-ethyl carbamoyl chloride, resolving a rivastigmine racemate with D-(+)-p-methyl dibenzoyl tartaric acid, and further salifying with L-(+)-tartaric acid for obtaining the rivastigmine tartrate (I). The method has the advantages of cheap price and easy obtainment of raw materials, short synthetic route, few reaction steps, mild reaction conditions and easy separation and purification of all the intermediates, thereby being applicable to industrial production.
Owner:NHWA PHARMA CORPORATION
Preparation method of dimethylcarbamoyl chloride
InactiveCN109369461AReduce wasteIn line with the production concept of green environmental protectionCarbamic acid derivatives preparationOrganic compound preparationPhosgeneCarbamoyl chloride
The invention discloses a preparation method of dimethylcarbamoyl chloride. The preparation method comprises the following steps: simultaneously introducing dimethylamine and phosgene into a micro-reactor to perform the reaction to obtain the dimethylcarbamoyl chloride. The dimethylcarbamoyl chloride prepared in the preparation method disclosed by the invention is high in content, the content reaches 98.5 to 99 percent, and the yield is greatly increased and can reach 95.0 to 96.2 percent (referring to dimethylamine). The preparation method disclosed by the invention has the advantages of simple operation, high yield, less waste, safety, environmental protection and the like, is suitable for industrialized production and good in application value and application prospect.
Owner:湖南海利常德农药化工有限公司
Method for synthesizing diisocyanate containing ether linkage through direct phosgenation method
InactiveCN110305041AGood yellowing resistanceHigh hardnessOrganic compound preparationIsocyanic acid derivatives purification/separationEtherChloride
The invention provides a method for synthesizing diisocyanate containing ether linkage through a direct phosgenation method. The method comprises the specific steps that (1) phosgene is introduced to-10-40 DEG C inert liquid to be dissolved until phosgene saturates; (2) the phosgene is introduced continuously, meanwhile an ether linkage containing diamine solution is dropwise added at -10-40 DEGC, and cold photochemical reaction is conducted to generate carbamic chloride; (3) the temperature is risen to 80-200 DEG C, hydrogen chloride of the carbamic chloride is removed to generate a diisocyanate containing the ether linkage actinic solution; and (4) phosgene removing, solvent removing, and product purification are conducted on the actinic solution to obtain diisocyanate containing the ether linkage. The diisocyanate containing the ether linkage belongs to the field of fat diisocyanate, oxygen is introduced in the main chain except a NCO perssad in the molecular structure; and meanwhile, downstream products further have excellent high temperature resistant performance, and the special requirements of performance of the downstream products are met. The operating process is simpleand easy to control, and the diisocyanate containing the ether linkage is suitable for commercial process.
Owner:甘肃银光聚银化工有限公司
Dehydration method during production process of toluene diisocynate
ActiveCN103724229AEfficient removalReduce corrosionOrganic compound preparationPreparation from carbamatesPhosgeneCarbamoyl chloride
The invention belongs to the field of toluene diisocynate production technology and relates to a dehydration method during the production process of toluene diisocynate. Toluenediamine and phosgene have cold light gasification reaction to obtain a mixture flow of carbamyl chloride, amidogen hydrochloride and hydrogen chloride; the mixture flow flows into a phosgenation reaction tower; carbamyl chloride and amidogen hydrochloride react to produce toluene diisocynate and the by-product hydrogen chloride; at the bottom of a hydrogen chloride rectifying tower, the mixture flow of amidogen and hydrogen chloride is divided into two parts, most of the mixture flow returns to the top of the phosgenation reaction tower and serves as the backflow, and the rest of the mixture flow enters into a drain sump containing activated carbon as the catalyst; water and phosgene in the mixture flow react to produce hydrogen chloride and carbon dioxide to remove water in the mixture flow under the function of the activated carbon catalyst; the dehydrated mixture flow enters into the bottom of the phosgenation reaction tower for further rectification and is discharged from the bottom of the phosgenation reaction tower for next step. The dehydration technology is simple and environmentally friendly, the cost is low, and the dehydration effect is excellent.
Owner:QINGDAO UNIV OF SCI & TECH +1
Synthesis process of (S)-rivastigmine
ActiveCN103304447AFew stepsHigh reaction yieldCarbamic acid derivatives preparationOrganic compound preparationMethyl carbamateFormate
The invention discloses an unsymmetrical synthesis method of (S)-rivastigmine. The method comprises the following steps of: reacting hydroxyacetophenone with methylethylcarbamoyl chloride to generate N-ethyl-N-methyl-carbamate 3-acetylphenyl formate, carrying out chiral reduction on the N-ethyl-N-methyl-carbamate 3-acetylphenyl formate under the catalysis effect of a complex of (S)-(-)-alpha, alpha-diphenyl prolinol and trimethyl borate, carrying out methanol dissociation to generate N-ethyl-N-methyl- carbamate 3-[(R)-1-hydroxyethyl]phenyl formate, and reacting the N-ethyl-N-methyl- carbamate 3-[(R)-1-hydroxyethyl]phenyl formate with methylsufonyl chloride and dimethylamine hydrochloride to obtain the (S)-rivastigmine. According to the method disclosed by the invention, a target product can be prepared from conventional reagents only through a three-step reaction, the reaction yield reaches 40% and is far higher than that by a racemic resolution method and that by other chiral synthesis methods, the application of virulent reagents and highly corrosive reagents are avoided in the process, and the unsymmetrical synthesis method is easy to operate, environment-friendly, low in production cost, and suitable for industrialized production.
Owner:YABAO PHARMA GRP CO LTD
Synthesis process of N-chloromethyl-N-phenyl amino formyl chloride
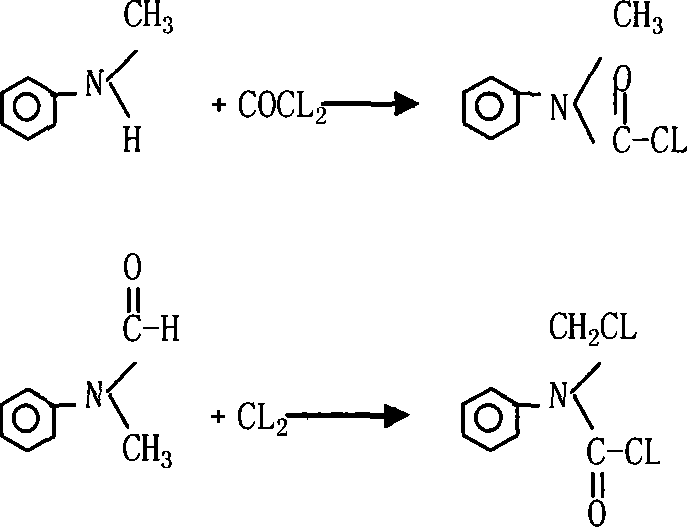
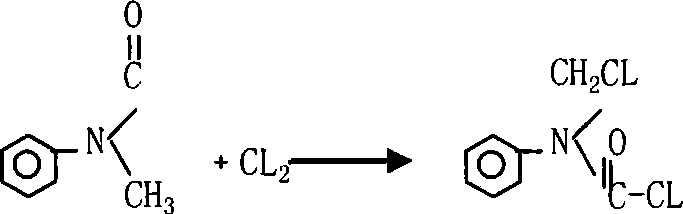
InactiveCN101066937ASolve substitution problemsImprove conversion rateCarbamic acid derivatives preparationOrganic compound preparationChlorideFormamide
The present invention relates to synthesis process of N-chloromethyl-N-phenyl amino formyl chloride. The technological process includes the chloroformylating reaction of N-methyl aniline and phosgene in solvent to obtain N-methyl-N-phenyl amino formyl chloride, and the subsequent chlorination reaction of the resultant solution directly or after eliminating solvent with introduced chlorine at 60-130 deg.c under the action of catalyst or ultraviolet ray to obtain the product. Or, N-chloromethyl-N-phenyl amino formyl chloride is prepared with N-methyl-N-phenyl formamide as the initial material and through chlorination reaction with introduced chlorine at 60-130 deg.c under the action of catalyst or ultraviolet ray to obtain the product. The present invention realizes no solvent synthesis, and has high conversion rate and high product yield.
Owner:蒋亦清
Process for the preparation of piperazine derivatives
The invention relates to a process for the preparation of the trans N-{4-{2-[4-(2,3-dichlorophenyl)-piperazine-1-yl]-ethyl}-cyclohexyl}-carbamide derivatives of general formula (I) by reacting the compound of formula (III) with a carbamoylchloride of general formula (II) which comprises carrying out the reaction in a mixture of a solvent and concentrated aqueous solution of an alkali hydroxide at a temperature between 40-100° C. in the presence of a phase transfer catalyst, separating the phases and washing the organic layer then removing the solvent and drying the compound of formula (I) obtained until its weight is constant.
Owner:GEDEON RICHTER NYRT
Method for preparing pimavanserin and tartrate thereof
The invention provides an improved method for preparing pimavanserin and tartrate thereof. The method comprises the following steps: taking a compound 4-(4-fluorobenzylamino)-1-methylpiperidine as a starting material and carrying out acylation reaction on the starting material and triphosgene in the presence of triethylamine to prepare a compound (4-fluorobenzyl)-(1-methylpiperidine-4-yl)carbamyl chloride; then taking the (4-fluorobenzyl)-(1-methylpiperidine-4-yl)carbamyl chloride and 4-isobutoxybenzylamine to react in the presence of triethylamine, so as to prepare the pimavanserin and the tartrate thereof. The method provided by the invention is simple, efficient and economical and the quality of an intermediate is controllable, so that the method is suitable for industrial preparation.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of pirimicarb
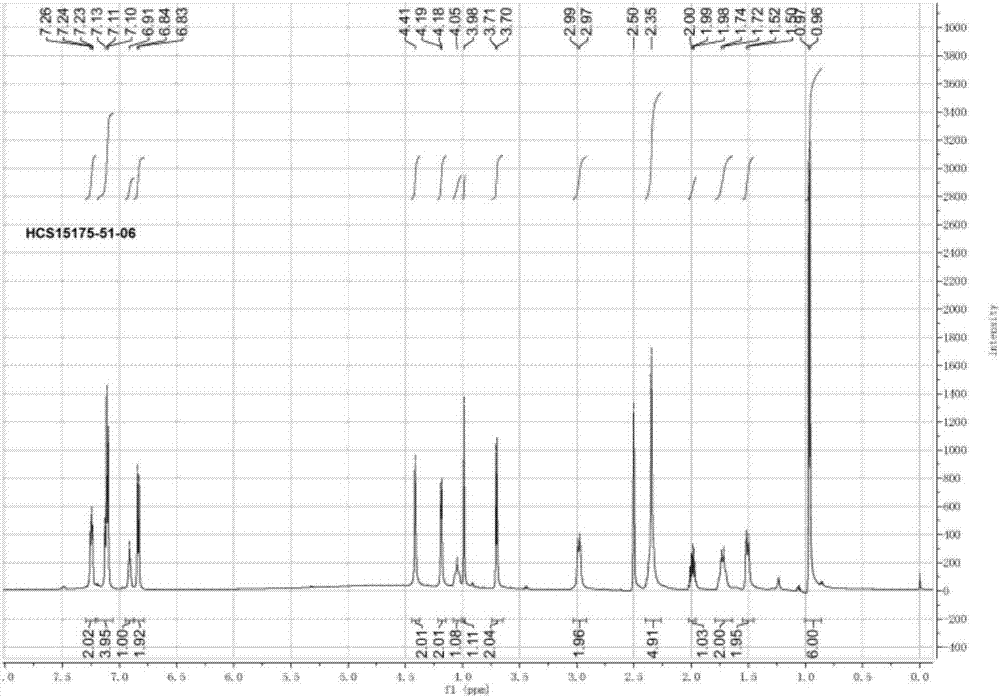
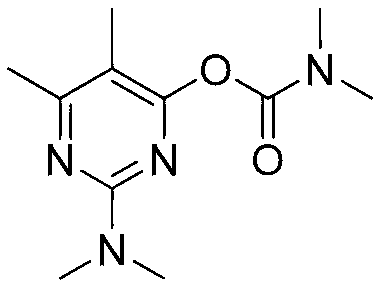
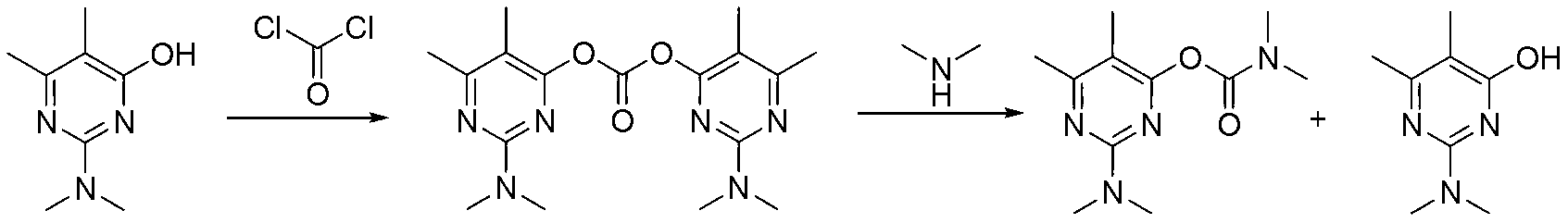
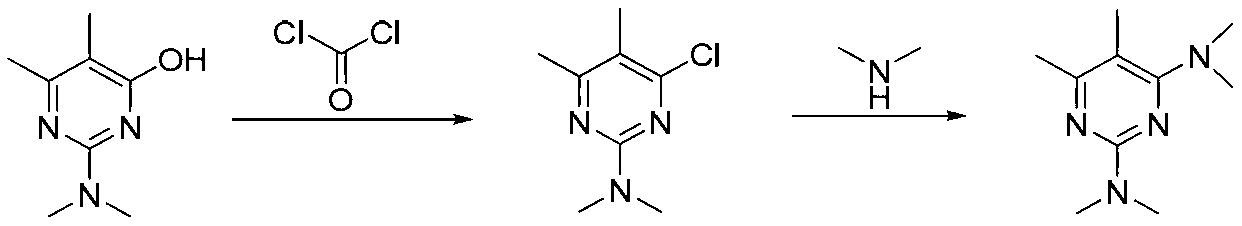
ActiveCN103193720AHigh content of pirimicarbHigh reaction yieldBiocideOrganic chemistryPirimicarbChloride
The invention discloses a preparation method of pirimicarb. The method is used for preparing the pirimicarb by reaction in which 5,6-dimentyl-2-dimethylamino-4-hydroxypyrimidine and dimethylamino carbamyl chloride are taken as raw materials, sodium hydroxide is taken as an acid-binding agent, an inert solvent, namely toluene or dimethylbenzene, is taken as a reaction solvent, and 4-dimethylamino pyridine is taken as a catalyst. The reaction formula is shown in the description, the pirimicarb content is as high as 98.1% to 99.2%, the reaction yield is as high as 98.1%-99.4% metered by 5,6-dimentyl-3-dimethylamino-4-hydroxypyrimidine, the reaction time is as short as 1h-3h, the acid-binding agent is unnecessary to recycle, the operation and subsequent processing are simple, and the production cost is low.
Owner:HUNAN HAILI CHEM IND
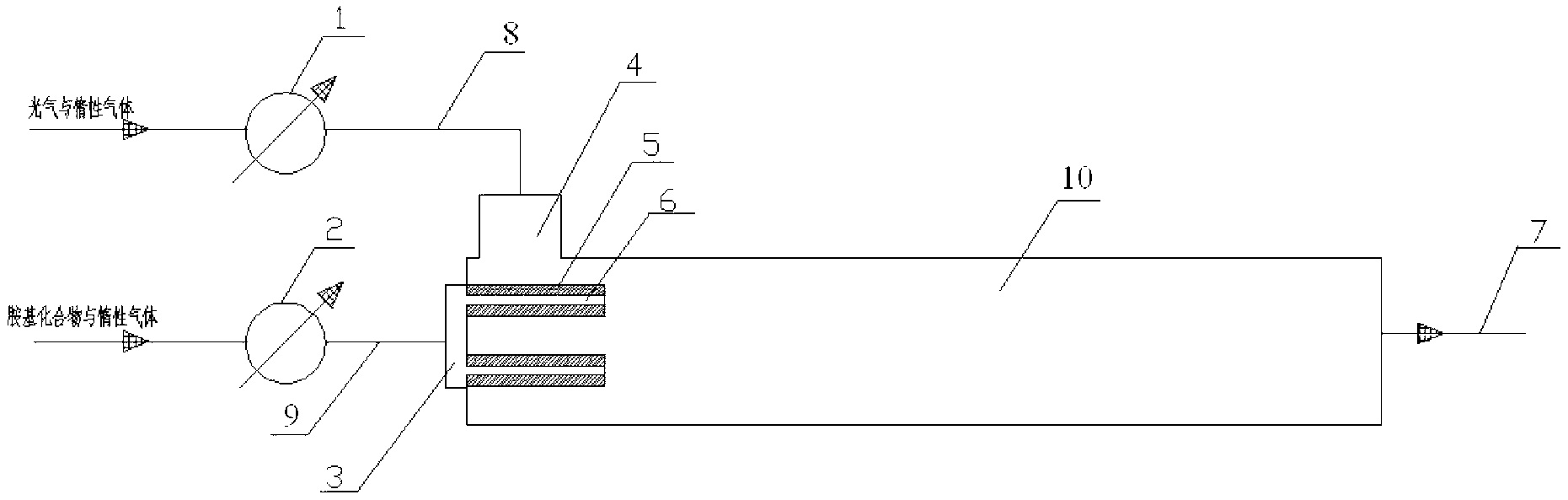
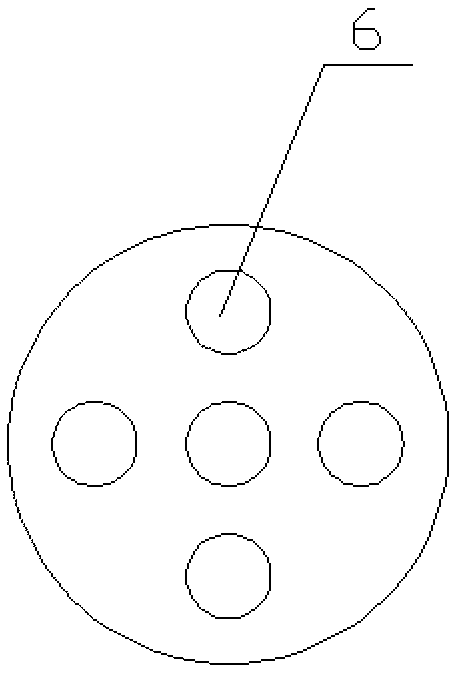
Method and device for continuous preparation of carbamyl chloride
InactiveCN103224457AHigh yieldLess side effectsCarbamic acid derivatives preparationOrganic compound preparationInert gas dilutionChloride
The invention discloses a method and a device for continuous preparation of carbamyl chloride. The method includes: diluting an amino compound by an inert gas and preheating it, letting the amino compound continuously enter a ceramic membrane microporous gas distributor at one end inside a tubular reactor and diffuse into the tubular reactor through pores on membrane tube walls of the ceramic membrane microporous gas distributor; preheating inert gas diluted phosgene, and making it continuously enter the tubular reactor through a phosgene inlet at the above end inside the tubular reactor along the membrane tube wall tangential direction of the gas distributor and mix with the diffused amino compound to undergo a reaction, and then carrying out separation so as to obtain carbamyl chloride. The device mainly comprises the tubular reactor and the membrane distributor. The method can effectively improve the yield of the isocyanate intermediate carbamyl chloride, is simple and has rapid reaction, thus being able to realize continuous industrial production. The device disclosed in the invention can well disperse the reaction raw materials and achieve a good mixing effect, thus being beneficial to improve the reaction yield and product quality and reducing side reaction in the reaction process.
Owner:XIANGTAN UNIV
Synthesis method of 1,3-dimethyl isocyanate cyclohexane
InactiveCN108752240AIncrease profitReduce the concentration of amine saltsIsocyanic acid derivatives preparationOrganic compound preparationReaction temperatureSlurry
The invention discloses a synthesis method of 1,3-dimethyl isocyanate cyclohexane. The method comprises the steps of pumping a metered inert solvent into reactor capable of performing stirring and with phosgene ingress pipe for bedding, performing cooling, beginning to continuously supply metered phosgene, dropwise adding a metered mixed solution of 1,3-hexamethylene dimethylamine and the inert solvent into a reaction system to form a mixture of 1,3-hexanaphthene dimethylamine hydrochloride and 1,3-hexanaphthene dimethylcarbamoyl chloride (a material in the reactor is slurry and the process isa cold photochemical reaction), after dropwise adding, heating to a reaction temperature (the process is a heat photochemical reaction), after the reaction, removing excessive phosgene and hydrogen chloride through nitrogen, and performing high vacuum rectification to form high-purity 1,3-dimethyl isocyanate cyclohexane. The method has the advantages that raw materials are easy to obtain, the solvent can be recycled, and the method is simple in technology, easy and simple to operate, higher in yield, mild in reaction condition and suitable for industrial production.
Owner:JIANGSU KUAIDA AGROCHEM
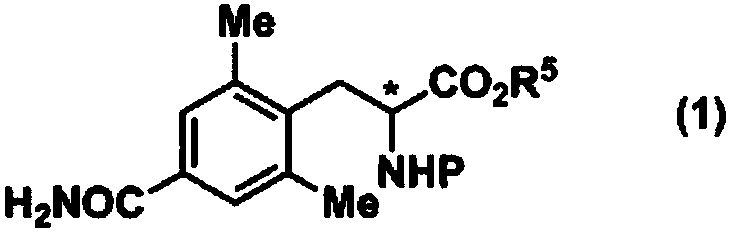
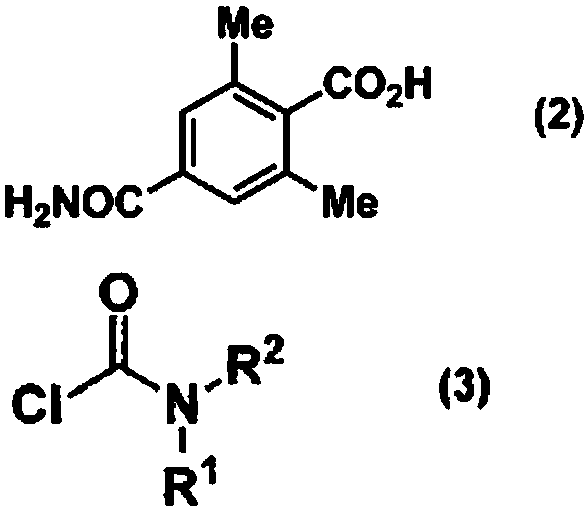
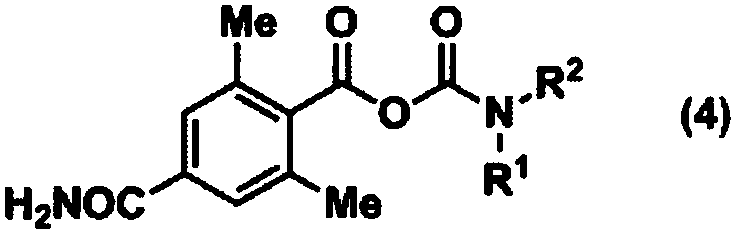
Method for producing optically active 4-carbamoyl-2,6-dimethylphenylalanine derivative
ActiveCN108026032AEasy and Efficient ManufactureCarbamic acid derivatives preparationOrganic compound preparationAlcoholMedicinal chemistry
Provided is a method for producing an optically active 4-carbamoyl-2,6-dimethylphenylalanine derivative, which is a compound useful as an intermediate for a medicine, in a simple manner and with highefficiency. 4-carbamoyl-2,6-dimethylbenzoic acid, a carbamoyl chloride and a base are reacted together to produce a corresponding acid anhydride mixture, and then the acid anhydride mixture is reducedto produce 4-carbamoyl-2,6-dimethylbenzyl alcohol. The use of this compound enables an optically active 4-carbamoyl-2,6-dimethylphenylalanine derivative to be produced in a simple manner and with high efficiency.
Owner:KANEKA CORP
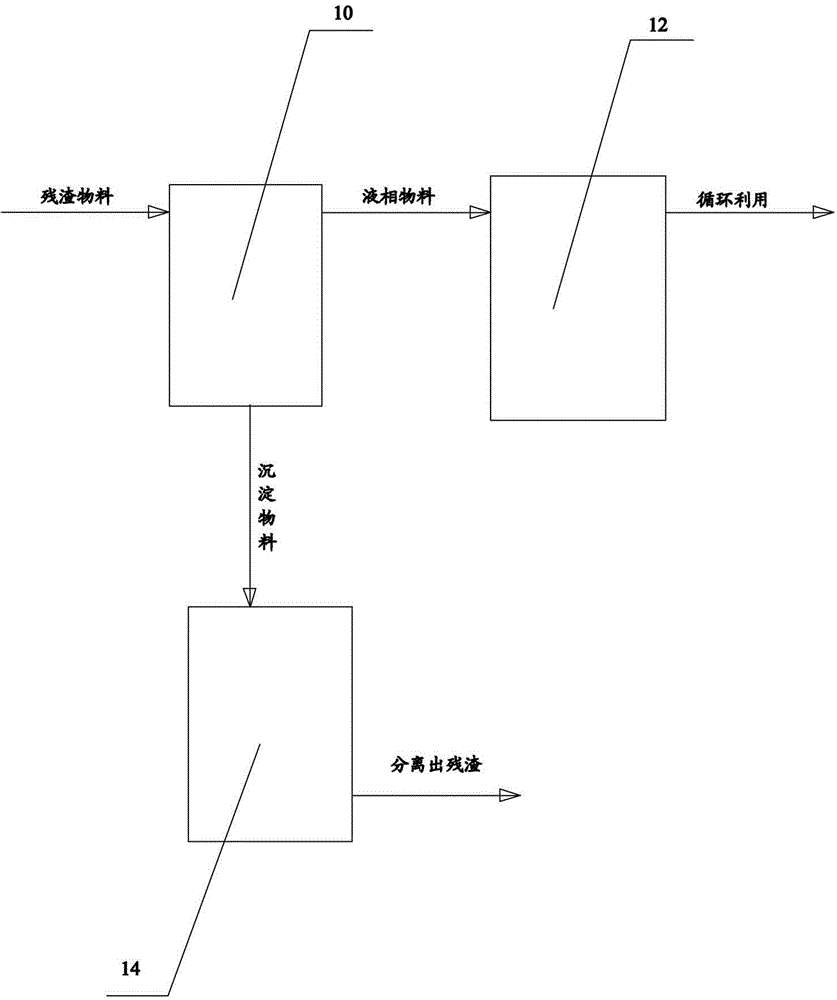
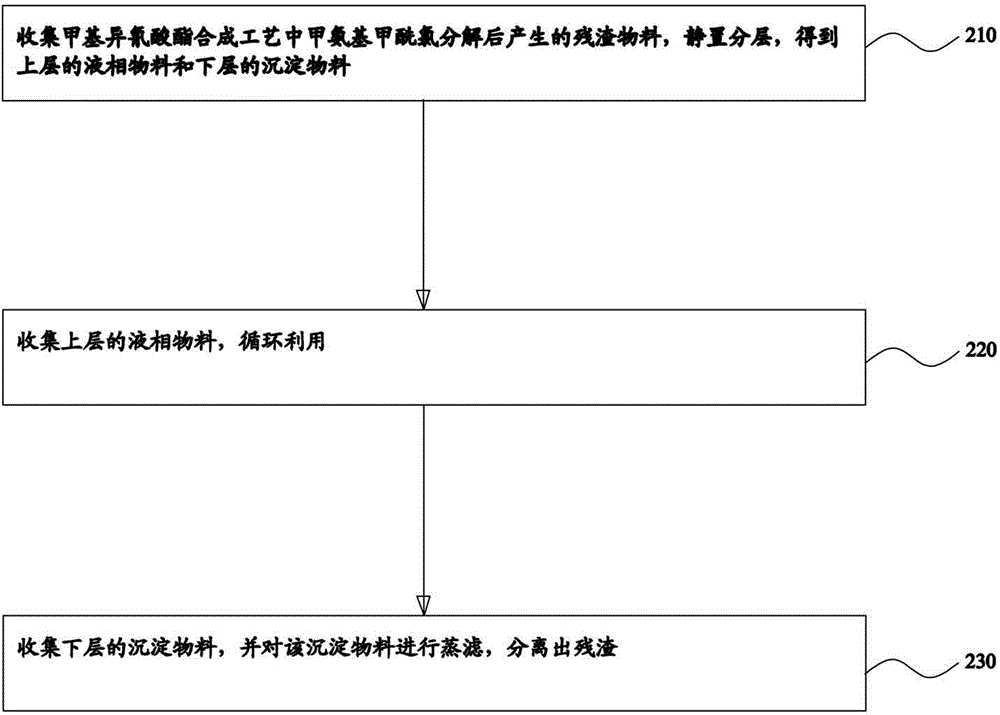
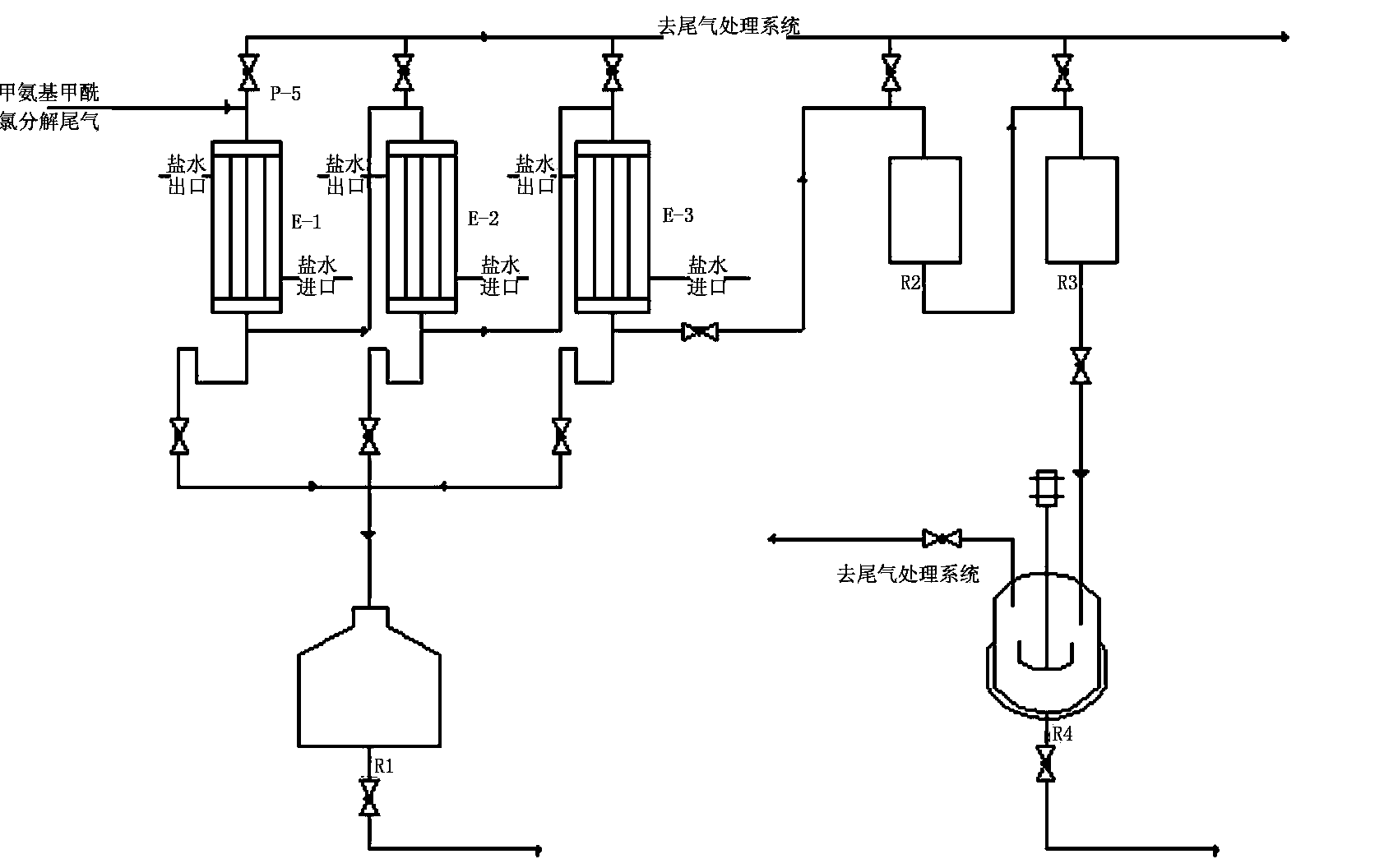
Treatment system and treatment method of residues in methyl isocyanate synthetic process
InactiveCN104447410AEfficient separationAddress effectivenessIsocyanic acid derivatives purification/separationDecompositionChloride
The invention provides a treatment system of residues in a methyl isocyanate synthetic process. The treatment system comprises a residue isolation tank, a blending groove and a residue steaming tank, wherein the residue isolation tank is used for collecting a residue material generated after decomposition of methylaminoformyl chloride in the methyl isocyanate synthetic process, standing and layering to obtain a liquid-phase material on the upper layer and a sediment material on the lower layer; the blending groove is connected with the top of the residue isolation tank and is used for collecting the liquid-phase material on the upper layer for cyclic utilization; the residue steaming tank is connected with the bottom of the residue isolation tank, and is used for collecting the sediment material on the lower layer as well as steaming and filtering the sediment material to separate the residues. The invention further provides a treatment method of the residues in the methyl isocyanate synthetic process. According to the system and the method, the generated residues can be separated effectively, and the liquid-phase material can be continuously and cyclically utilized, so that the problem that the residues in the methyl isocyanate synthetic process are difficult to effectively clear in time, and the production efficiency is improved.
Owner:HUNAN GOFAR FINE CHEM IND TECH CO LDT
Product for absorption purposes
Owner:WADSTROM TORKEL +4
Production process of rivastigmine
InactiveCN103664702AEasy to produceLow costCarbamic acid derivatives preparationOrganic compound preparationChlorideRivastigmine
The invention discloses a production process of rivastigmine. The production process comprises the steps as follows: 1), m-hydroxyacetophenone and dimethylamine are taken as raw materials, and 3-(1-(dimethylamino) ethyl) phenol is synthesized through one-step Leucart reaction; and 2), 3-(1-(dimethylamino) ethyl) phenol and ethylmethyl-carbamic chloride result in condensation reaction to produce rivastigmine. With the adoption of the production process, the production yield and the purity of rivastigmine can be improved remarkably, the process steps are reduced, and the production cost is reduced.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Application method of methylamino formyl chloride decomposition tail gas hydrogen chloride in cartap synthesis
ActiveCN103848768AFew preparation stepsIncrease added valueIsocyanic acid derivatives purification/separationCyanidePhysical chemistry
The invention relates to an application method of a methylamino formyl chloride decomposition tail gas hydrogen chloride in cartap synthesis. After a tail gas mixture of hydrogen chloride, trichloromethane and methyl isocyanate produced by decomposing methylamino formyl chloride is condensed by a condenser, the condensed condensate is transferred into a methylamino formyl chloride mixing slot, and then enters a circulating system of methyl isocyanate production for being collected and recycled; uncondensed gas hydrogen chloride is directly added into thiocyanide liquor of cartap synthesis after pressure is stabilized by a secondary buffer tank, so that the cartap is synthesized by catalytic hydrolysis. According to the invention, tertiary step cooling and condensing is adopted to separate a hydrogen chloride gas in the tail gas from a trichloromethane gas and a methyl isocyanate gas. The whole separating and recycling process is less in equipment investment and less in amount of byproduct hydrochloric acid; moreover, the separated hydrogen chloride gas is used for a catalytic hydrolysis process of the cartap.
Owner:HUNAN GOFAR FINE CHEM IND TECH CO LDT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile](https://images-eureka.patsnap.com/patent_img/55e48882-21a1-424c-b066-a4def80b3d6e/BDA0000560558790000021.PNG)
![Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile](https://images-eureka.patsnap.com/patent_img/55e48882-21a1-424c-b066-a4def80b3d6e/BDA0000560558790000022.PNG)
![Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile Preparation of 4-[5-(pyridine-4-yl)-1H-[1,2,4]triazole-3-yl]pyridine-2-formonitrile](https://images-eureka.patsnap.com/patent_img/55e48882-21a1-424c-b066-a4def80b3d6e/BDA0000560558790000023.PNG)