Proparagyl urea analogue monomer and optical rotation spiral polymer and its preparation method
A technology of propargyl urea and polymer, applied in the field of polymer materials, can solve the problems of low catalytic activity and enantioselectivity, restriction, loss of metal complex catalyst, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]R(+)-N-Benzyl-α-methylbenzylamine (7 mL) was added dropwise to an ice-bathed solution of solid phosgene (Triphosgene 4.64 g) in ethyl acetate. After the dropwise addition, the reaction was heated to reflux, and nitrogen gas was introduced to remove the by-product hydrogen chloride at the same time, and the reaction was stopped after 30 hours. Evaporate the organic solvent to obtain carbamoyl chloride. Carbamoyl chloride was dissolved in dichloromethane and added dropwise to the solution of propargylamine in dichloromethane, and reacted for 5 hours. The resulting solution was washed three times successively with 2N hydrochloric acid and saturated sodium bicarbonate solution, 30 ml each time, and then dried over anhydrous magnesium sulfate. After filtration, the solvent was removed by rotary evaporation to obtain a colorless viscous liquid: propargylcarbamide.
[0042] Take the above monomer (0.1 g) and (nbd) Rh + B - (C 6 h 5 ) 4 Catalysts (the ratio of monomer to ...
Embodiment 2
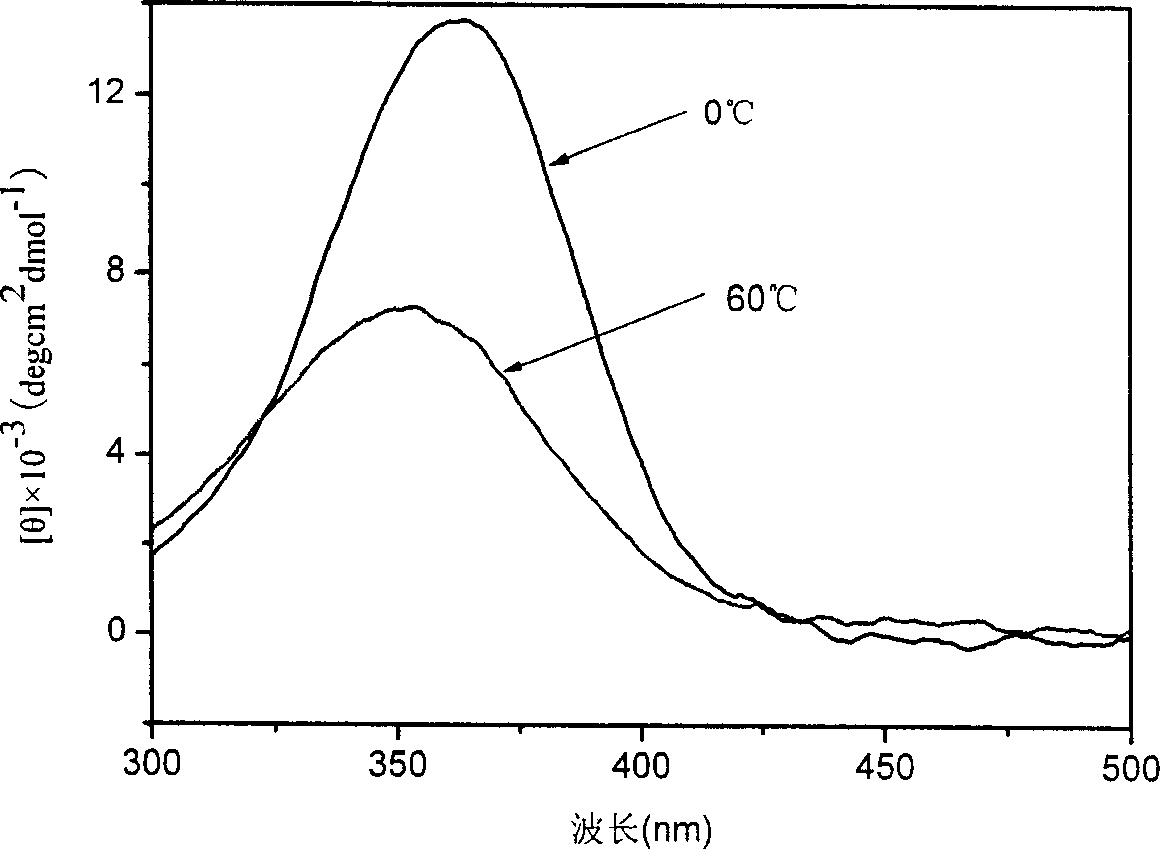
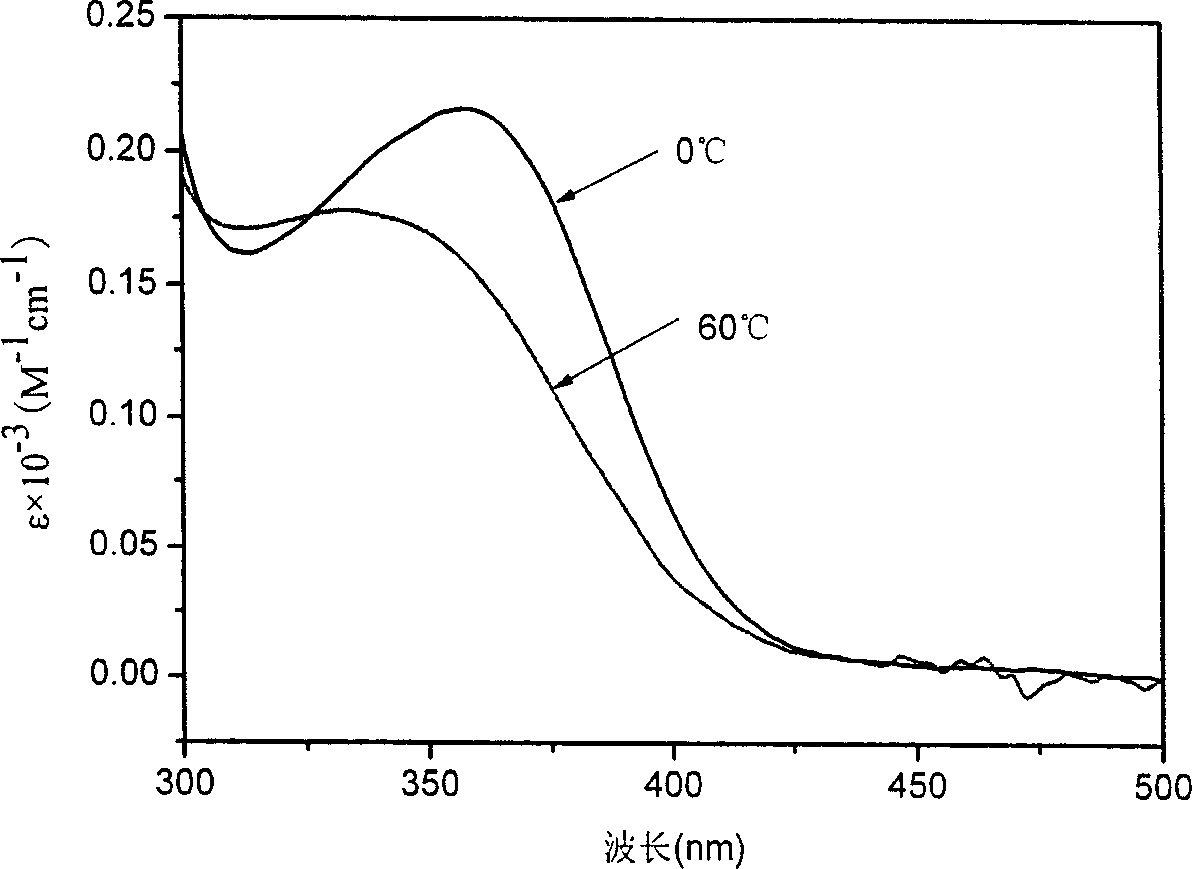
[0045] The difference from Example 1 is that the polymerization temperature is 0°C and the polymerization time is 24h. All the other polymerization steps are the same as in Example 1. The number average molecular weight of the obtained polymer was 7800, and the molecular weight distribution index was 1.81. The specific optical rotation of the polymer is 850°. The peaks of CD and UV-Vis spectra of the obtained polymer reached the maximum at chloroform-40℃, and the minimum at 55℃. The shapes of the two spectra are similar to those of the circular dichroism spectrum and the ultraviolet-visible spectrum in Example 1, respectively, and the peaks are 10% higher than those in Example 1.
Embodiment 3
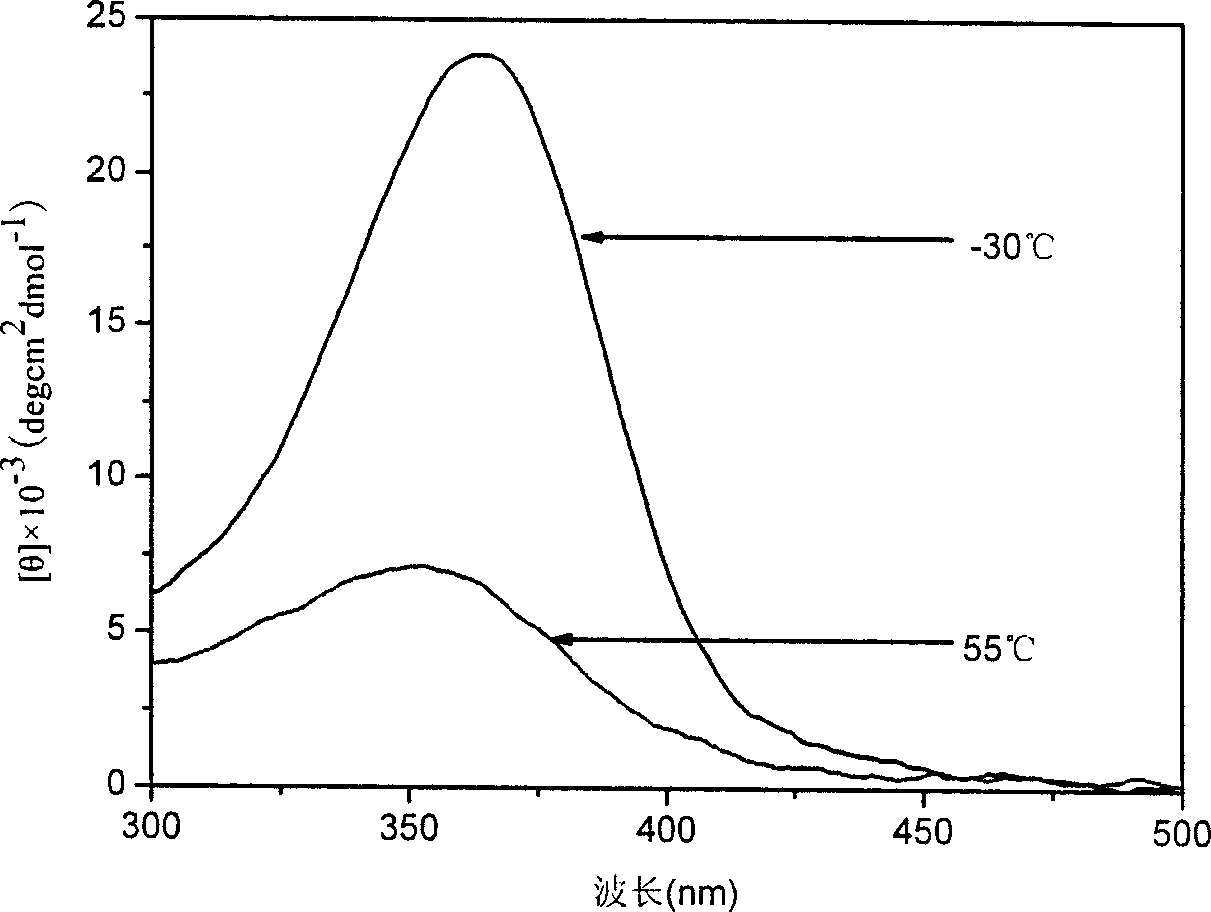
[0047] The difference from Example 1 is that the polymerization temperature is -15°C and the polymerization time is 24h. The remaining polymerization steps were the same as in Example 1, and the number average molecular weight of the obtained polymer was 6800, and the molecular weight distribution index was 1.62. The specific rotation of the polymer is 880°. The peaks of CD and UV spectra of the obtained polymer reach the maximum at chloroform -30°C and the minimum at 55°C. The shapes of the two spectrograms are similar to the circular dichroic spectrum and the ultraviolet-visible spectrum in Example 1, respectively, and the peaks are 15% higher than those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com