Method for preparing pimavanserin and tartrate thereof
A technology for pimaserin and hemi-tartrate, which is applied in the field of improved preparation of pimaserin and its tartrate, can solve the problem of 4-(2-methylpropoxy)benzene which endangers the health of production members, is unsuitable for storage, and Problems such as poor stability of methyl isocyanate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 compound 2
[0055] Dissolve (4.7g, 47.5mmol) BTC in 190ml DCM, cool down to 0°C and stir for 30min. (24g, 108mmol) compound 1 and (21.8g, 216mmol) triethylamine were dissolved in 50ml DCM. At 0°C, slowly drop the mixed solution into the above-mentioned triphosgene-dichloromethane solution, stir for 30 minutes after the dropwise addition, warm up to room temperature (30°C), stir and react for 24 hours, and the remaining amount of compound 1 is controlled by HPLC ≤ 1.0% . After the reaction was completed, the pH was adjusted to 5-6, the liquid was separated, extracted with DCM, the organic phases were combined, and the solvent was evaporated under reduced pressure to obtain 27.63 g of compound 2 with a yield of 90% and a purity of 98.2%.
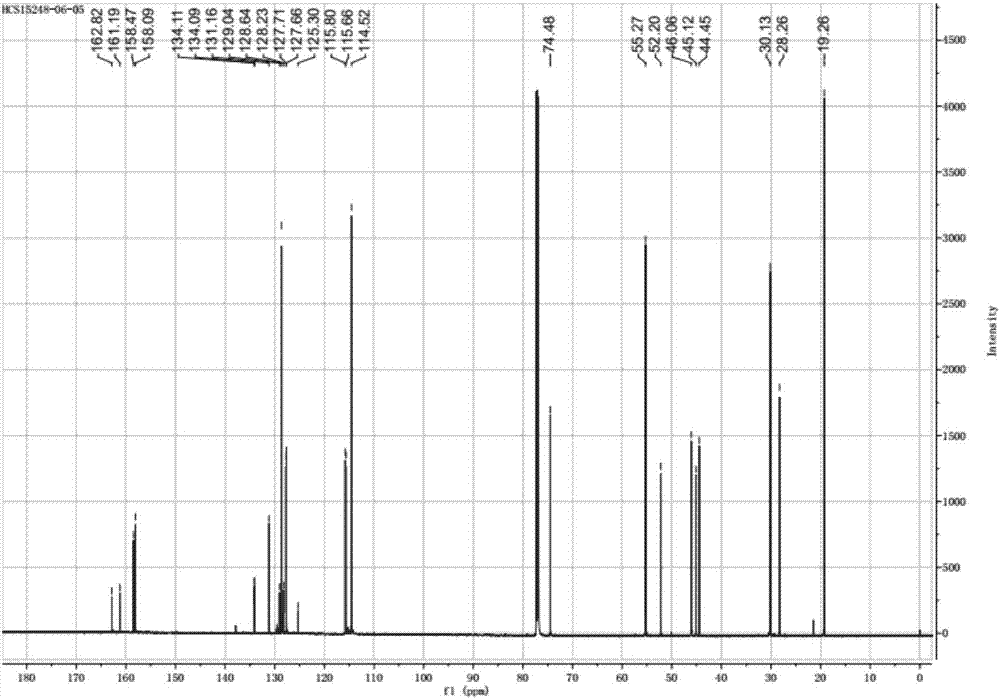
[0056] LCMS-ESI + Characterization: 285.1[M+H + ].
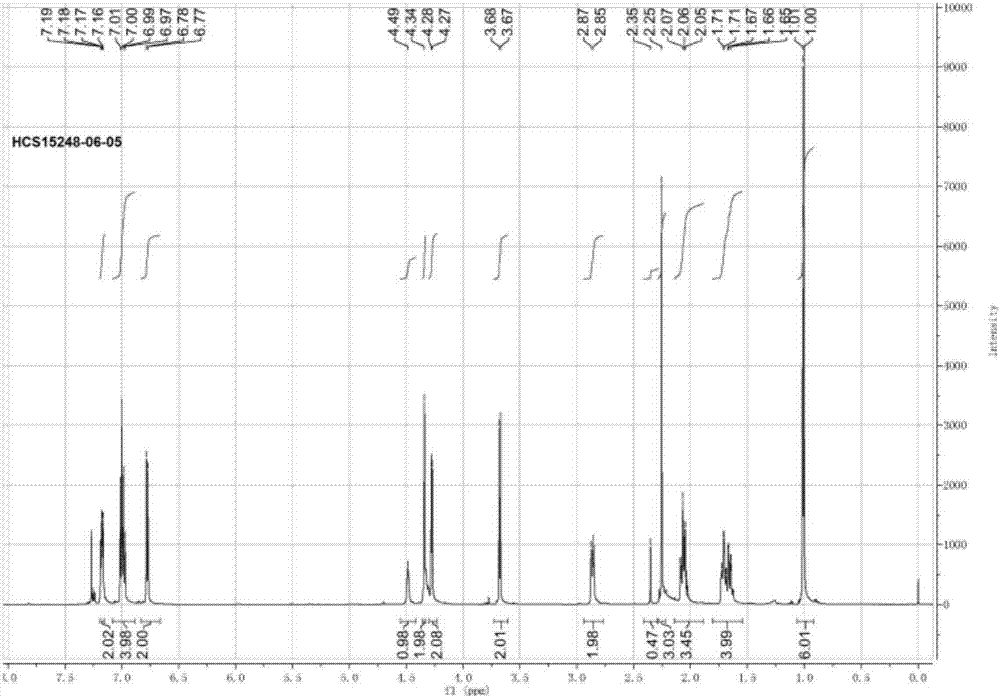
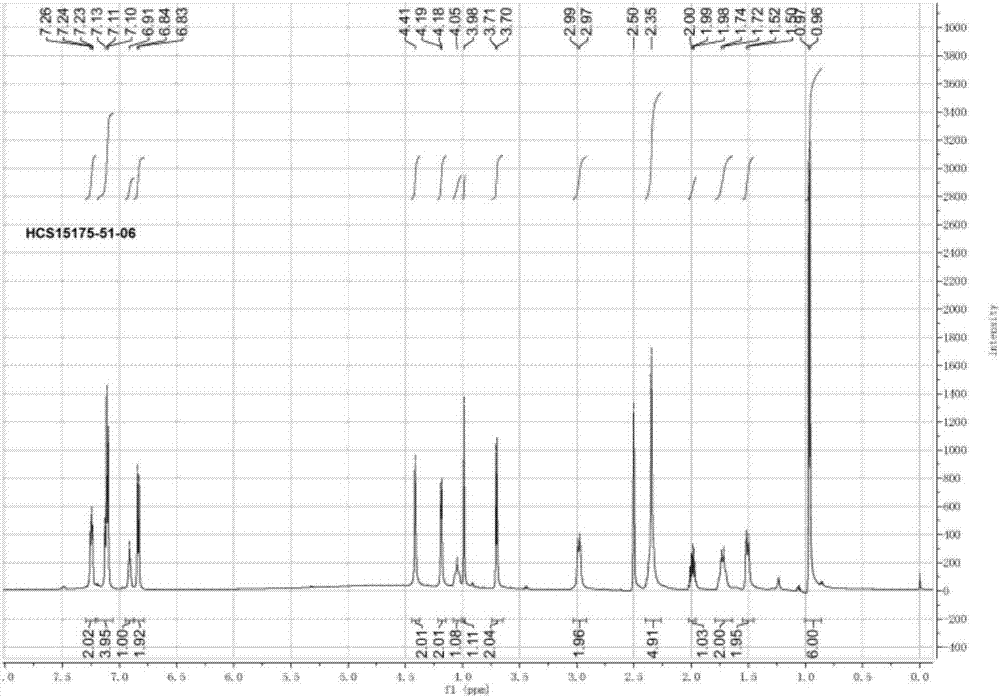
[0057] 1 HNMR characterization: (400MHz, CDCl 3 )7.28(t, J=8.0,2H),6.99(t,J=10.0,2H),5.19(d,J=12.0,1H),3.93(d,J=16.0,1H),3.58(m,3H ), 3.54(m,1...
Embodiment 2
[0059] The preparation of embodiment 2 compound 2
[0060] 14.7g of BTC (0.44eq, 47.50mmol) was dissolved in 190ml of dichloromethane (8vol), and the temperature was lowered to 0°C and stirred. 24g (107.96mmol) of compound 1 and 21.8g of triethylamine (2.0eq, 215.92mmol) were miscibly dissolved in 50ml of dichloromethane (2vol), and the mixed solution was slowly Add dropwise the above-mentioned triphosgene in dichloromethane solution. After the dropwise addition was completed and stirred for 30 min, the temperature was raised to room temperature (30° C.) for reaction. The reaction was stirred at room temperature for 24 hours, and the detection of compound 1 by HPLC was below 3%. After the reaction was completed, the pH was adjusted, the liquid was separated, extracted, and the organic phase was rotary evaporated in vacuum to obtain 27.63 g of liquid compound 2 with a yield of 90%.
Embodiment 3
[0061] The preparation of embodiment 3 compound 2
[0062] Dissolve 9.4g of BTC (0.44eq, 31.67mmol) in 130ml of dichloromethane (8vol), and stir at -5°C. Mix 16g of compound 1 (71.97mmol) and 11.36g of pyridine (2.0eq, 143.94mmol) in 30ml of DCM (2vol), and slowly drop the mixed solution into the above-mentioned Sanko gas in dichloromethane solution. After the dropwise addition was completed and stirred for 30 minutes, the temperature was raised to room temperature (30° C.) and the reaction was stirred for 18 hours. Compound 1 was detected to be below 1% by HPLC. After the reaction was completed, the pH was adjusted, the liquid was separated, extracted, and the organic phase was rotary evaporated in vacuum to obtain 19.04 g of liquid compound 2 with a yield of 92.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com