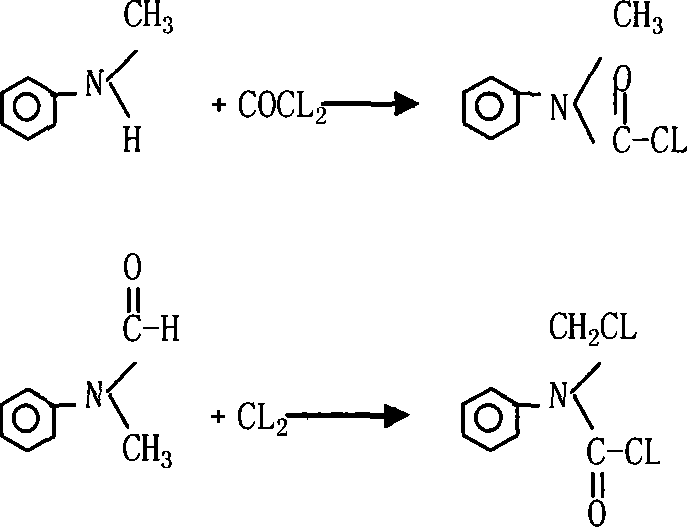
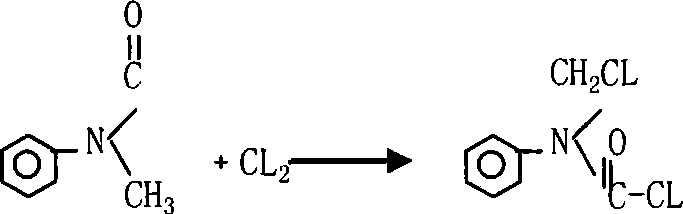
Synthesis process of N-chloromethyl-N-phenyl amino formyl chloride
A technology of phenylcarbamoyl and synthetic method, which is applied in the preparation of carbamic acid derivatives, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as destruction and no related reports, and achieve high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Put 200ml dichloroalkane industrial product into a 500ml four-neck glass flask, add phosgene at 20℃~65℃ under stirring, and add 80gN-methylaniline dropwise at the same time, keep a certain light passing speed and dropping speed, phosgene :N-methylaniline=1.1~2.0:1.0 (molar ratio), after the addition, the temperature is raised to 60℃~70℃, and part of phosgene is added until the N-methylaniline is completely converted, and it will decrease after the end point Pressure desolventization, to 110 ℃, vacuum degree ≥ 0.09mpa after desolventization, directly transferred to a 250ml four-necked flask, and weighed, the product weight 128g, 98% content, 98.5% yield.
[0024] Heat the photochemical obtained above to 80℃~130℃, add 1g of the catalyst azoisobutyronitrile, or keep the temperature 130~60℃ under UV lamp, pass in the chlorine gas 53g, and gradually reduce the chlorination temperature from 80℃~ When the temperature is lowered from 130°C to 60-80°C, the chlorination is over, samp...
Embodiment 2
[0027] The dichloroethane was changed to ethyl acetate, and the feeding amount and method were the same as in Example 1 to obtain 150 g of product, with a content of 93.6% and a yield of 92%.
[0028] The solvent ethyl acetate can also be replaced by methyl acetate or butyl acetate.
Embodiment 3
[0030] In the phosgenation reaction, toluene was used as the solvent to replace the dichloroethane in Example 1, and the treatment was carried out in the same manner as in Example 1, to obtain 153 g of product with a content of 92.5% and a yield of 92.8%.
[0031] The above solvent toluene can also be replaced by benzene, xylene or chlorinated benzene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com