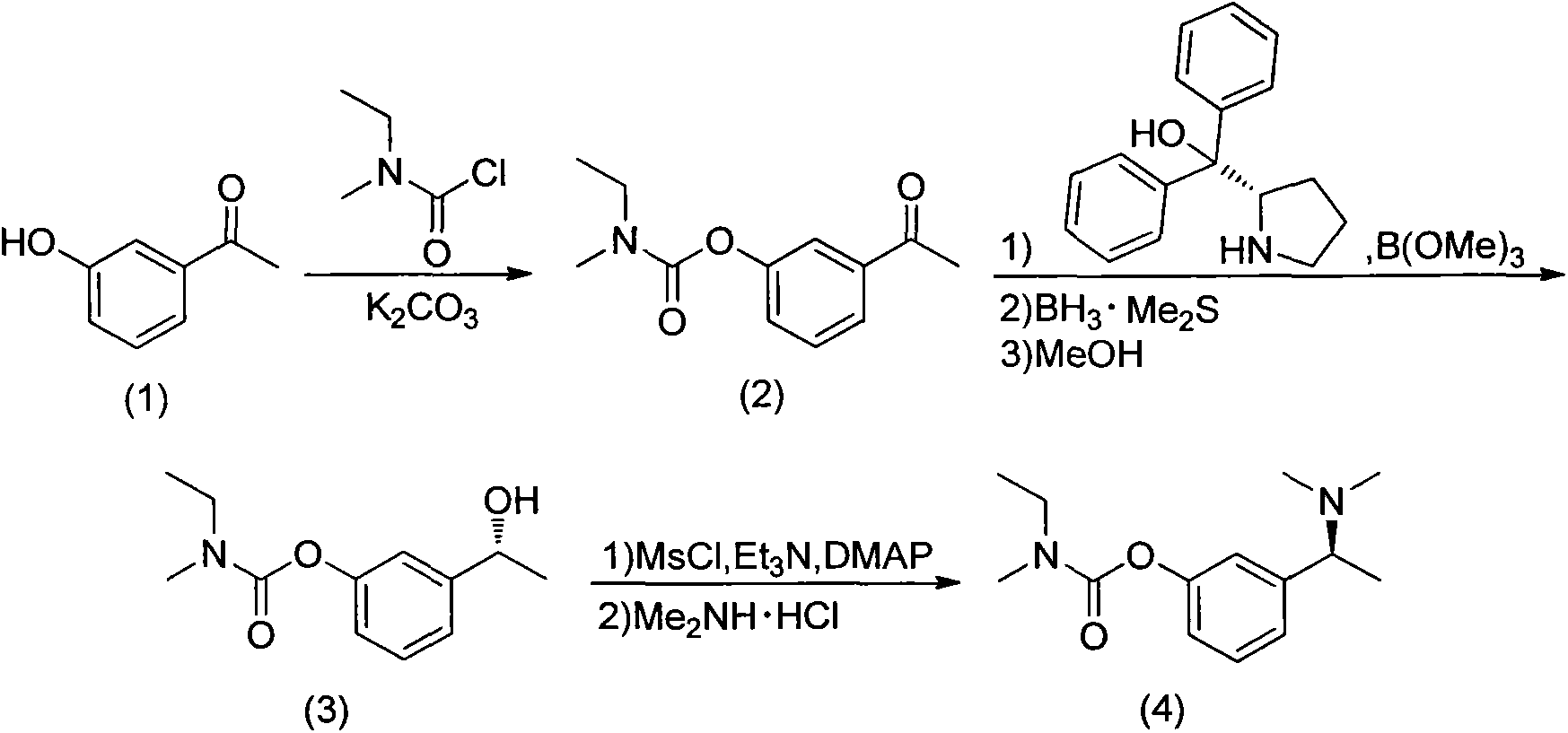
Synthesis process of (S)-rivastigmine
A synthesis method and compound technology, applied in the field of chiral drug synthesis, can solve problems such as the use of toxic and harmful reagents, many steps, complex processes, etc., and achieve the effects of easy industrial production, mild reaction conditions, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of 3-acetylphenyl N-ethyl-N-methyl-carbamate (2)
[0045] Under nitrogen protection at 25°C, m-hydroxyacetophenone (1) (99.0%, 20.0g, 147mmol, 1eq) was dissolved in acetone (AR, 400mL), anhydrous potassium carbonate (AR, 40.6g, 294mmol) was added under stirring , 2eq), slowly added methylethylcarbamoyl chloride (99.4%, 21.4g, 176mmol, 1.2eq) dropwise. 50 ℃ reaction 4h, stop the reaction. The reaction solution was pumped to remove the anhydrous potassium carbonate solid, water (400 mL) was added to the filtrate, extracted with ethyl acetate (250 mL×3), the organic phase was dried with anhydrous magnesium sulfate, and the water pump was spin-dried under reduced pressure to obtain a yellow oil The product N-ethyl-N-methyl-carbamic acid 3-acetylphenyl ester (2) (34.22 g, yield 90%).
[0046] 1HNMR (CDC13) δ1.24(m, 3H, CCH3), 2.61(s, 3H, CCH3), 3.06(d, 3H,, NCH3), 3.47(m, 2H, CH2), 7.35(d, 1H, ArH ), 7.47 (s, 1H, ArH), 7.71 (d, 1H, ArH), 7.80 (t, 1H, ArH); MS, m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com