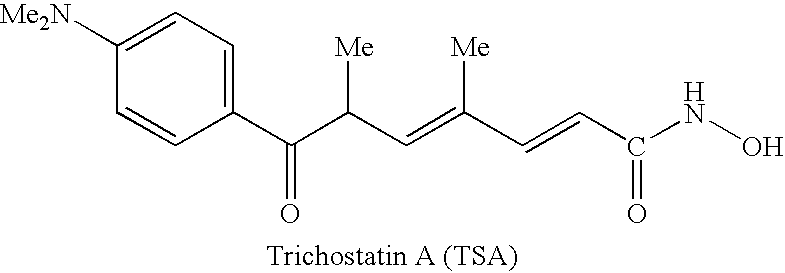
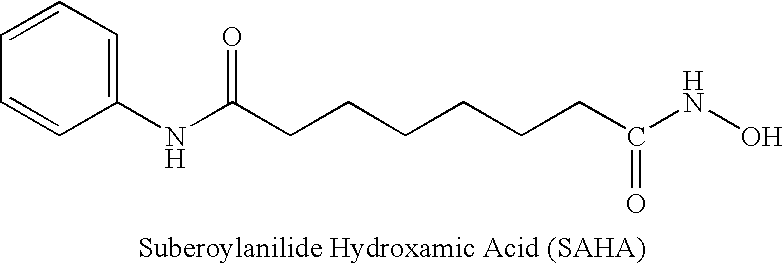
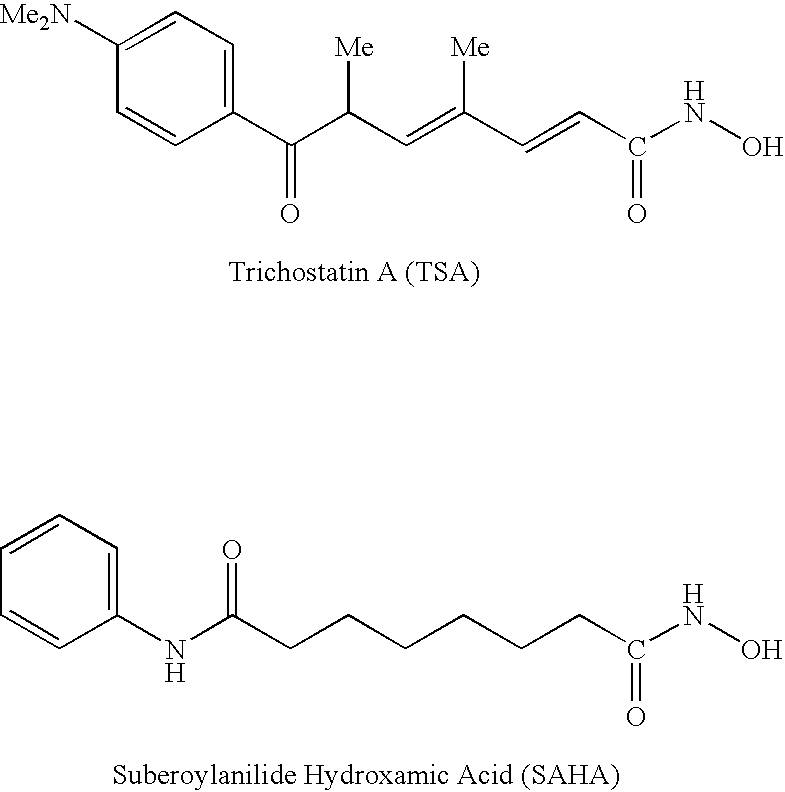
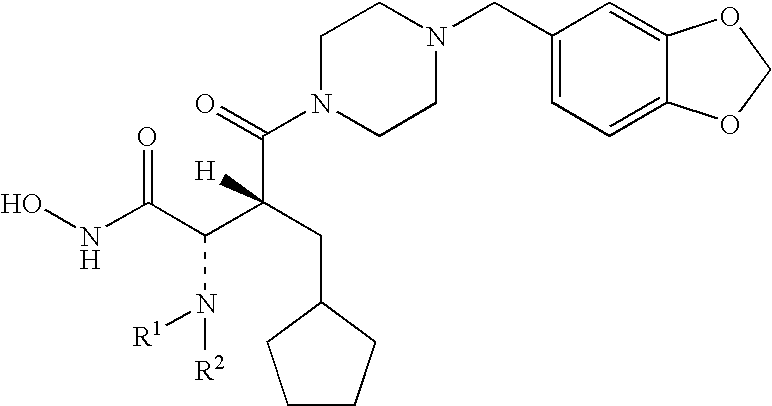
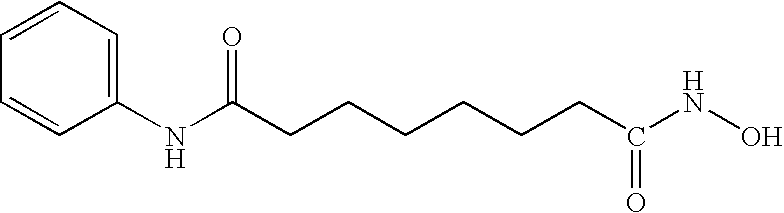
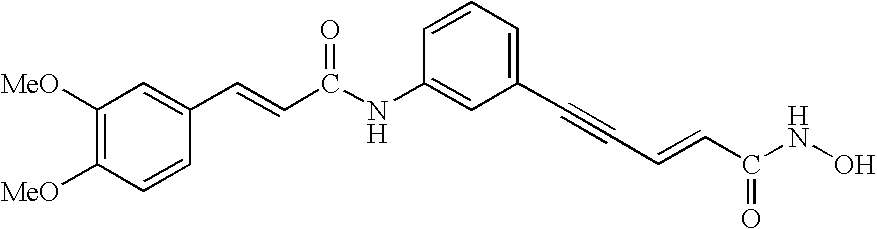
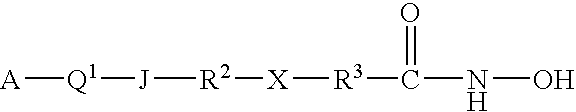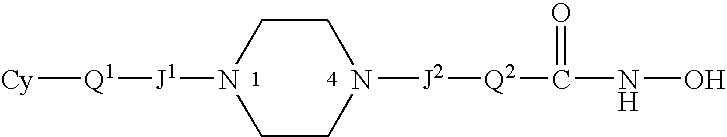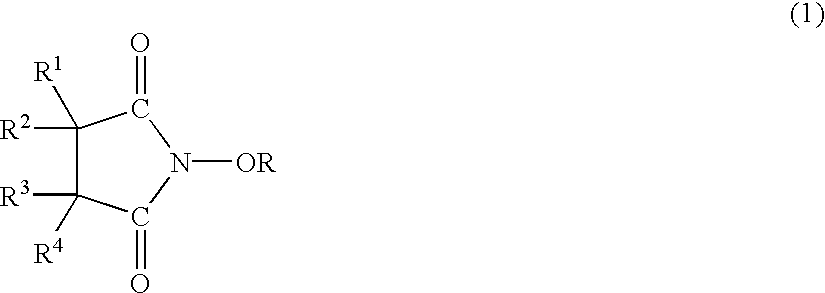Patents
Literature
222 results about "Carbamic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
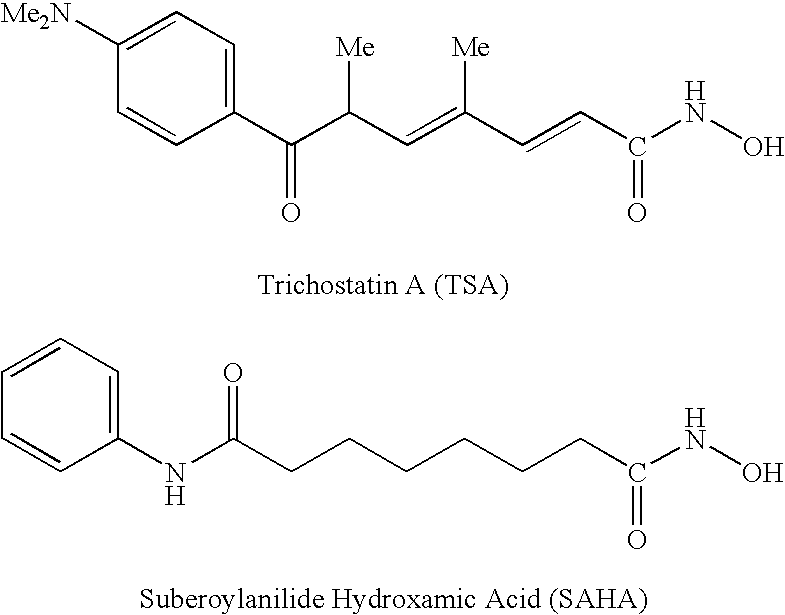
Carbamic acid is the compound with the formula H₂NCOOH. The attachment of the acid group to a nitrogen or amine (instead of carbon) distinguishes it from carboxylic acid and an amide. Many derivatives and analogues of carbamic acid are known. They are generally unstable, reverting to the parent amine and carbon dioxide. The deprotonated anion (or conjugate base) of this functional group is a carbamate. Carbamic acid is a planar molecule.
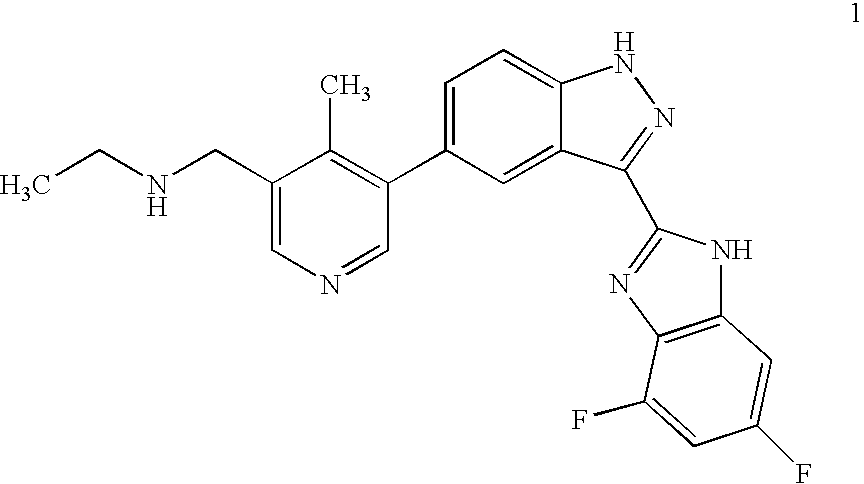
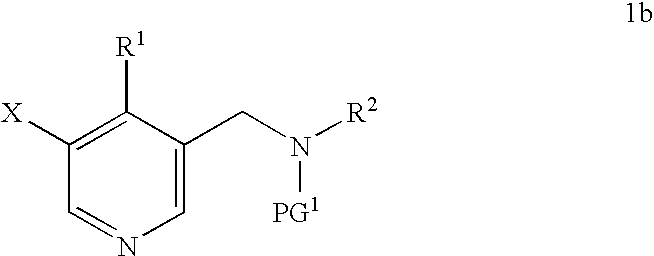
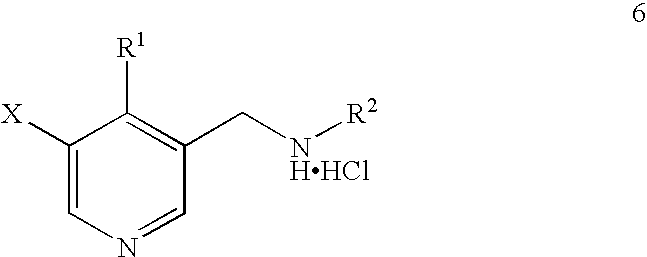
Synthesis of 5-bromo-4-methyl-pyridin-3-ylmethyl)-ethyl-carbamic acid tert-butyl ester
Owner:AGOURON PHARMA INC +1
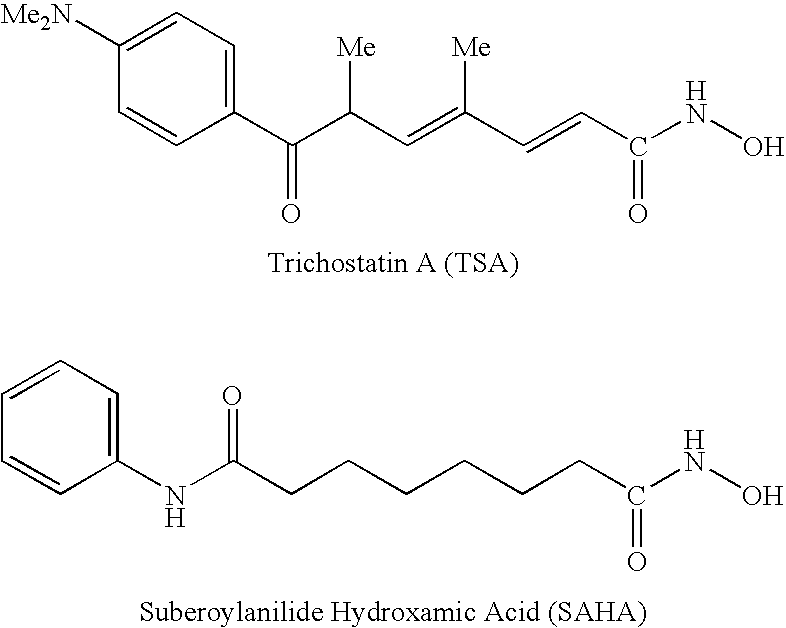
Carbamic acid compounds comprising a sulfonamide linkage as HDAC inhibitors
Owner:TOPOTARGET UK LTD
Carbamic acid compounds comprising an ether linkage as HDAC inhibitors
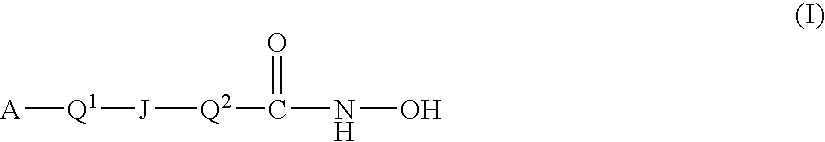
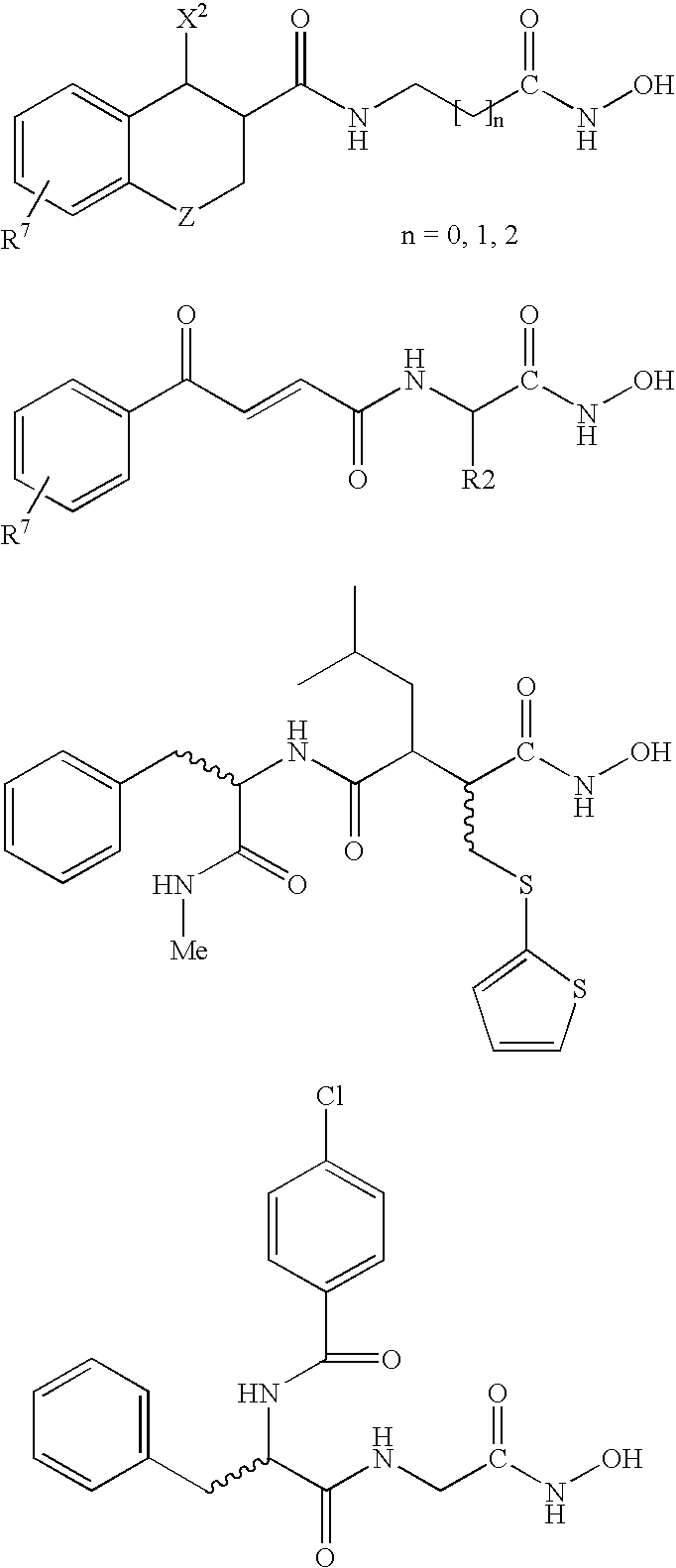
This invention pertains to certain active carbamic acid compounds which inhibit HDAC activity and which have the following formula: wherein: A is an aryl group; Q1 is a covalent bond or an aryl leader group; J is an amide linkage selected from: —NR1C(═O)— and —C(═O)NR1—; R1 is an amido substituent; X is an ether heteroatom, and is —O— or —S—; and, R2 and R3 are each independently an ether group; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and, e.g., to inhibit proliferative conditions, such as cancer and psoriasis.
Owner:TOPOTARGET UK LTD
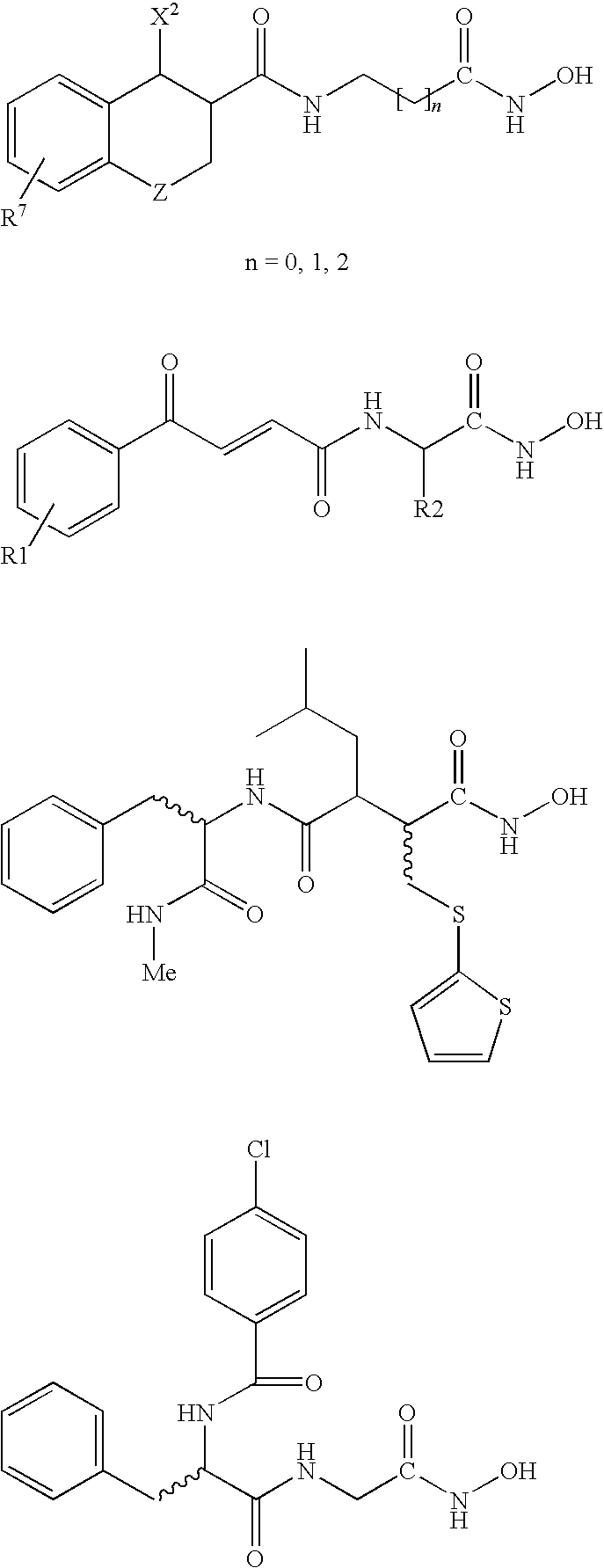
Carbamic acid compounds comprising a bicyclic heteroaryl group as hdac inhibitors
This invention pertains to certain carbamic acid compounds of the following formula, which inhibit HDAC (histone deacetylase) activity wherein: A is independently an unsubstituted or substituted bicyclic C9-10heteroaryl group (e.g., quinolinyl; quinoxalinyl; benzoxazolyl; benzothiazolyl); Q is an acid leader group, and is independently an unsubstituted or substituted, saturated or unsaturated C17alkylene group having a backbone length of 4 or less; with the proviso that if A is unsubstituted benzothiazol-2-yl, then Q is an unsaturated group; and with the proviso that if A is unsubstituted quinolin-6-yl, then Q is unsubstituted at the a-position; and with the proviso that A is not benzimidazol-2-yl; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and in the treatment of conditions mediated by HDAC, cancer, proliferative conditions, psoriasis, etc.
Owner:TOPOTARGET UK LTD
Carbamic acid compounds comprising a sulfonamide linkage as HDAC inhibitors
This invention pertains to certain active carbamic acid compounds which inhibit HDAC activity and which have the following formula: (I) A is an aryl group; Q1 is a covalent bond or an aryl leader group; J is a sulfonamide linkage selected from: —S(═O)2NR1— and —NR1S(═O)2—; R1 is a sulfonamido substituent; and, Q2 is an acid leader group; with the proviso that if J is —S(═O)2NR1—, then Q1 is an aryl leader group; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and, e.g., to inhibit proliferative conditions, such as cancer and psoriasis.
Owner:TOPOTARGET UK LTD
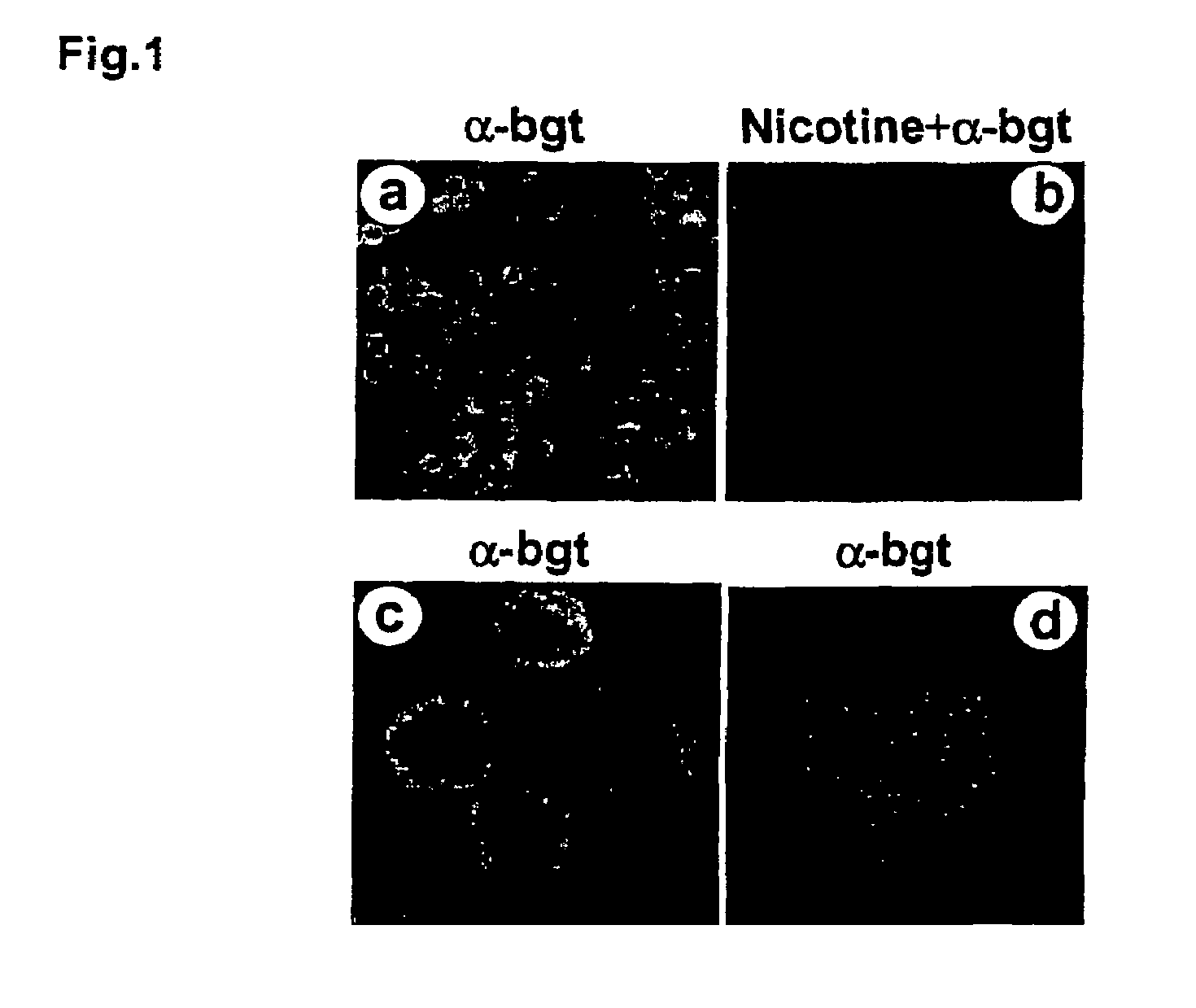
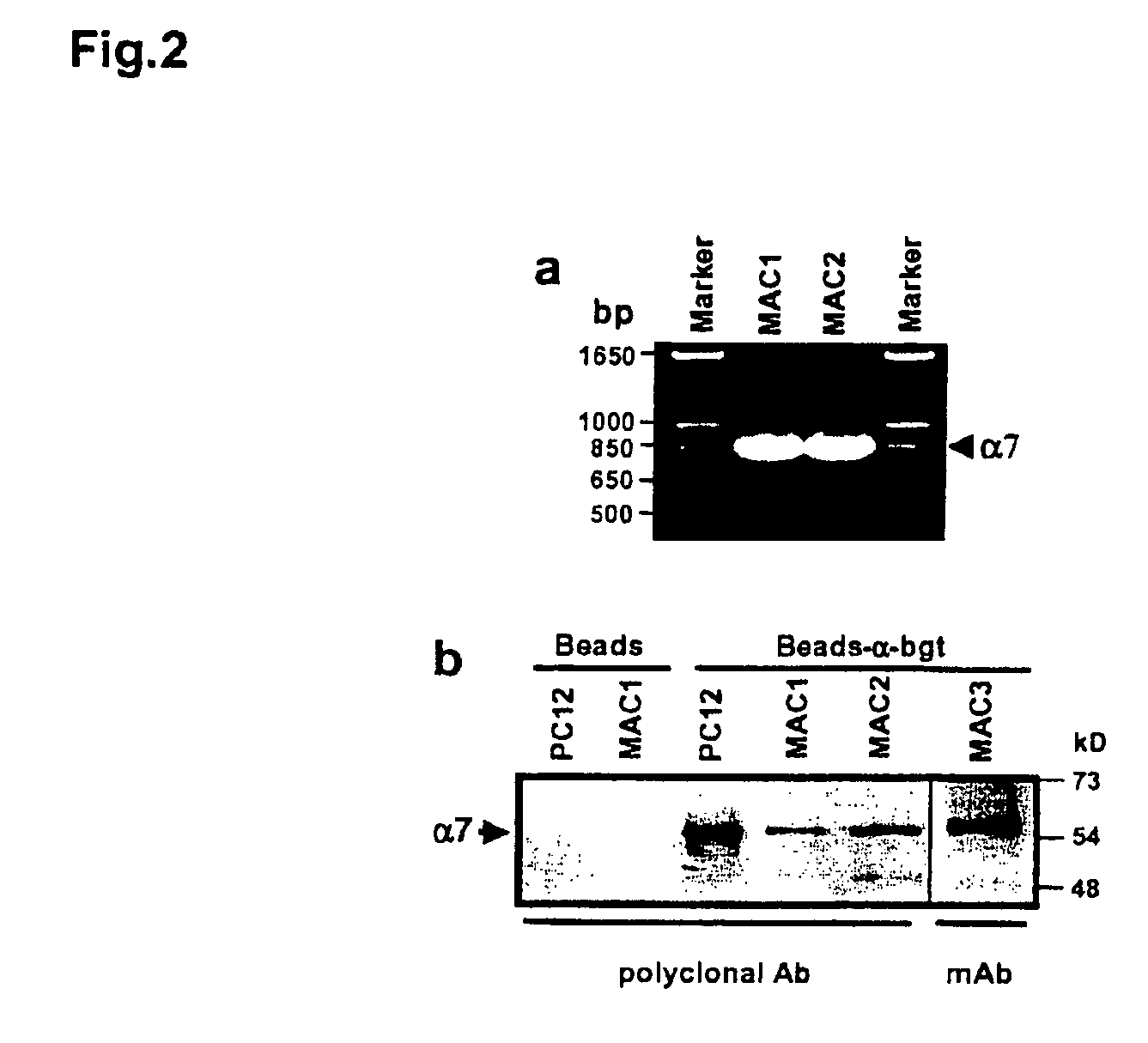
Treatment of pancreatitis using alpha 7 receptor-binding cholinergic agonists
A method of treating a patient suffering from pancreatitis comprising treating said patient with a therapeutically effective amount of a cholinergic agonist selective for an α7 nicotinic receptor in an amount sufficient to decrease the amount of the proinflammatory cytokine that is released from a macrophage wherein said condition is acute pancreatitis. The compounds of the present invention include a quaternary analog of cocaine; (1-aza-bicyclo[2.2.2]oct-3-yl)-carbamic acid 1-(2-fluorophenyl)-ethyl ester; a compound of formula (I), a compound of formula (II), a compound of formula (III), a compound of formula (IV), and an oligonucleotide or mimetic capable of attenuating the symptoms of acute pancreatitis wherein the oligonucleotide or mimetic consists essentially of a sequence greater than 5 nucleotides long that is complementary to an mRNA of an α7 cholinergic receptor. The variables of formulae (I), (II), (III) and (IV) are described herein
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Carbamic acid compounds comprising a piperazine linkage as hdac inhibitors
InactiveUS20050143385A1Promote apoptosisInhibiting cell cycle progressionOrganic active ingredientsNervous disorderAcetylase activityProdrug
This invention pertains to certain carbamic acid compounds which inhibit HDAC (histone deacetylase) activity of the following formula: wherein: Cy is independently a cyclyl group; Q1 is independently a covalent bond or cyclyl leader group; the piperazin-1,4-diyl group is optionally substituted; J1 is independently a covalent bond or —C(═;O)—; J2 is independently —C(═O)— or —S(═O)2—; Q2 is independently an acid leader group; wherein: Cy is independently: C3-20carbocyclyl, C3-20heterocyclyl, or C5-20aryl; and is optionally substituted; Q1 is independently: a covalent bond; C1-7alkylene; or C1-7alkylene-X—C1-7alkylene, —X—C1-7alkylene, or C1-7alkylene-X—, wherein X is —O— or —S—; and is optionally substituted; Q2 is independently: C4-8alkylene; and is optionally substituted; and has a backbone length of at least 4 atoms; or: Q2 is independently: C5-20arylene; C5-20arylene-C1-7alkylene; C1-7alkylene-C5-20arylene; or, C1-7alkylene-C5-20arylene-C1-7alkylene; and is optionally substituted; and has a backbone length of at least 4 atoms; or a pharmaceutically acceptable salt, solvate, amide, ester, ether, chemically protected form, or prodrug thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and in the treatment of conditions mediated by HDAC, cancer, proliferative conditions, psoriasis, etc.
Owner:TOPOTARGET UK LTD
Process for the recovery of carbon dioxide from a gas stream
ActiveUS7601315B2Reduction in overall mass transfer coefficientHigh viscosityGas treatmentCarbon compoundsDiethylenetriamineTriethylenetetramine
A process for removing acid gas from a gas stream utilizes a low viscosity absorbent comprising a solution of at least one selected amine. The absorbent may include (1) at least one polyamine in the absence of an effective amount of tertiary amine functionalities having a pKa sufficient to neutralize carbamic acid, wherein the polyamine has at least one primary amine functionality having a pKa of <10.0 at 25° C. and wherein the feed gas stream has an SO2 concentration less than 5 ppm vol.; or (2) a polyamine in the absence of an effective amount of tertiary amine functionalities having a pKa sufficient to neutralize carbamic acid, wherein the polyamine has at least one secondary amine functionality having a pKa for each sorbing nitrogen of <10.0 at 25° C. The polyamines may include diethylenetriamine (DETA), triethylenetetramine (TETA) and tetraethylenepentamine (TEPA) or mixtures thereof.
Owner:SHELL OIL CO
Somatostatin analogue compounds
Compounds having somatostatin activity of the following Formula I, wherein,R1 is aryl, substituted-aryl, and aryl-(lower-alkyl)-;R2 is lower alkyl, amino substituted lower alkyl, -carboxy-(lower-alkyl), -carbamic acid-(lower-alkyl) and -carboxy-(lower-alkyl)-aryl; andR3 and R4 are independently, lower-alkyl, aryl, substituted-aryl, (substituted-aryl)-(lower-alkyl)-, heteroaryl, (heteroaryl)-(lower-alkyl)-, substituted-heteroaryl, (substituted heteroaryl)-(lower-alkyl)-, heterocyclic, heterocyclic-(lower-alkyl)-, substituted-heterocyclic, (substituted-heterocylic)-(lower alkyl)-, -carboxy-(lower-alkyl), and -carboxy-(lower-alkyl)-aryl; or a pharmaceutically acceptable, ester, ether, or salt thereof; methods for their use; and preparation.
Owner:CHEMBRIDGE CORP
Hydrophobically-modified polyethyleneimine foaming agent capable of releasing carbon dioxide, and application thereof
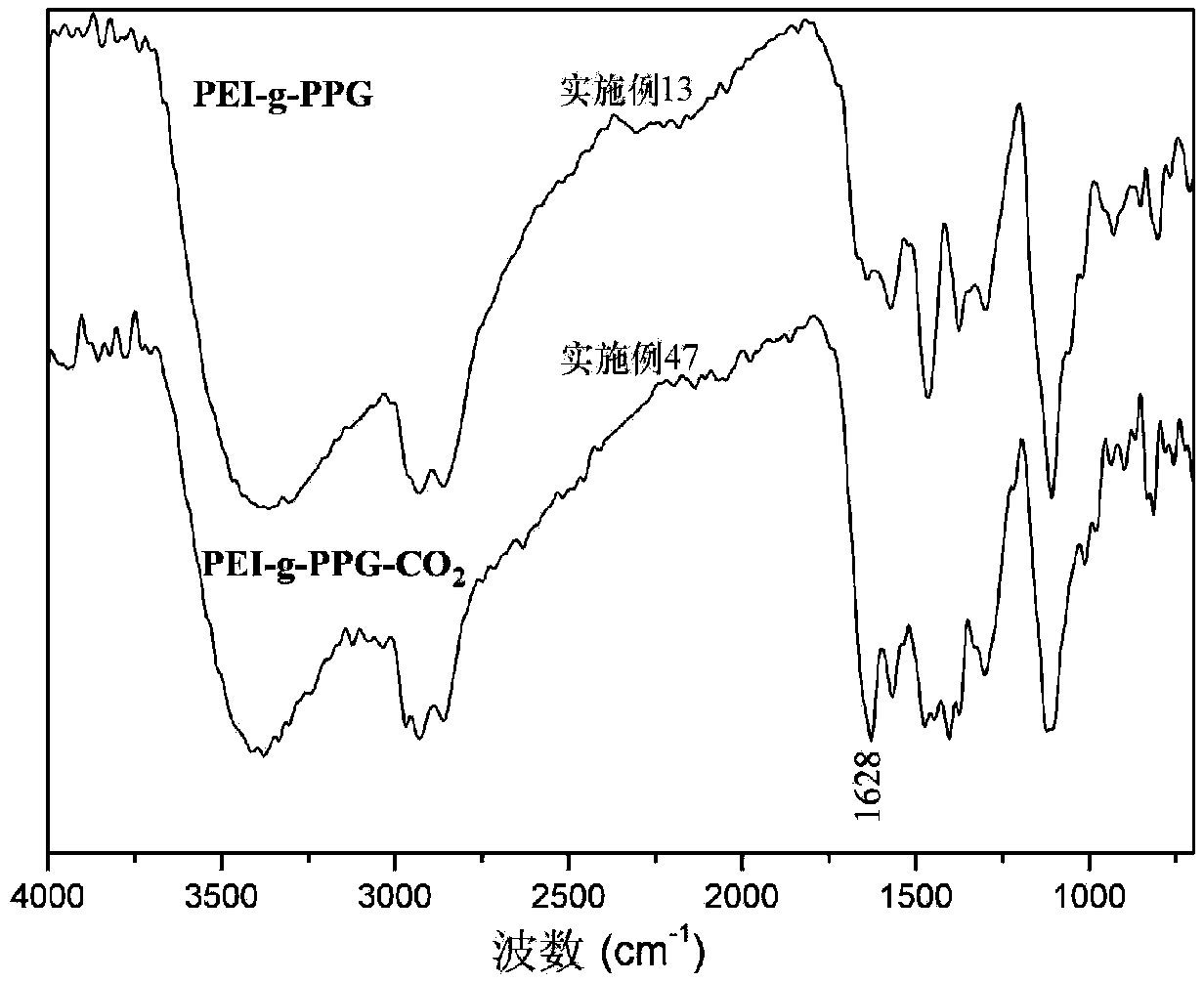
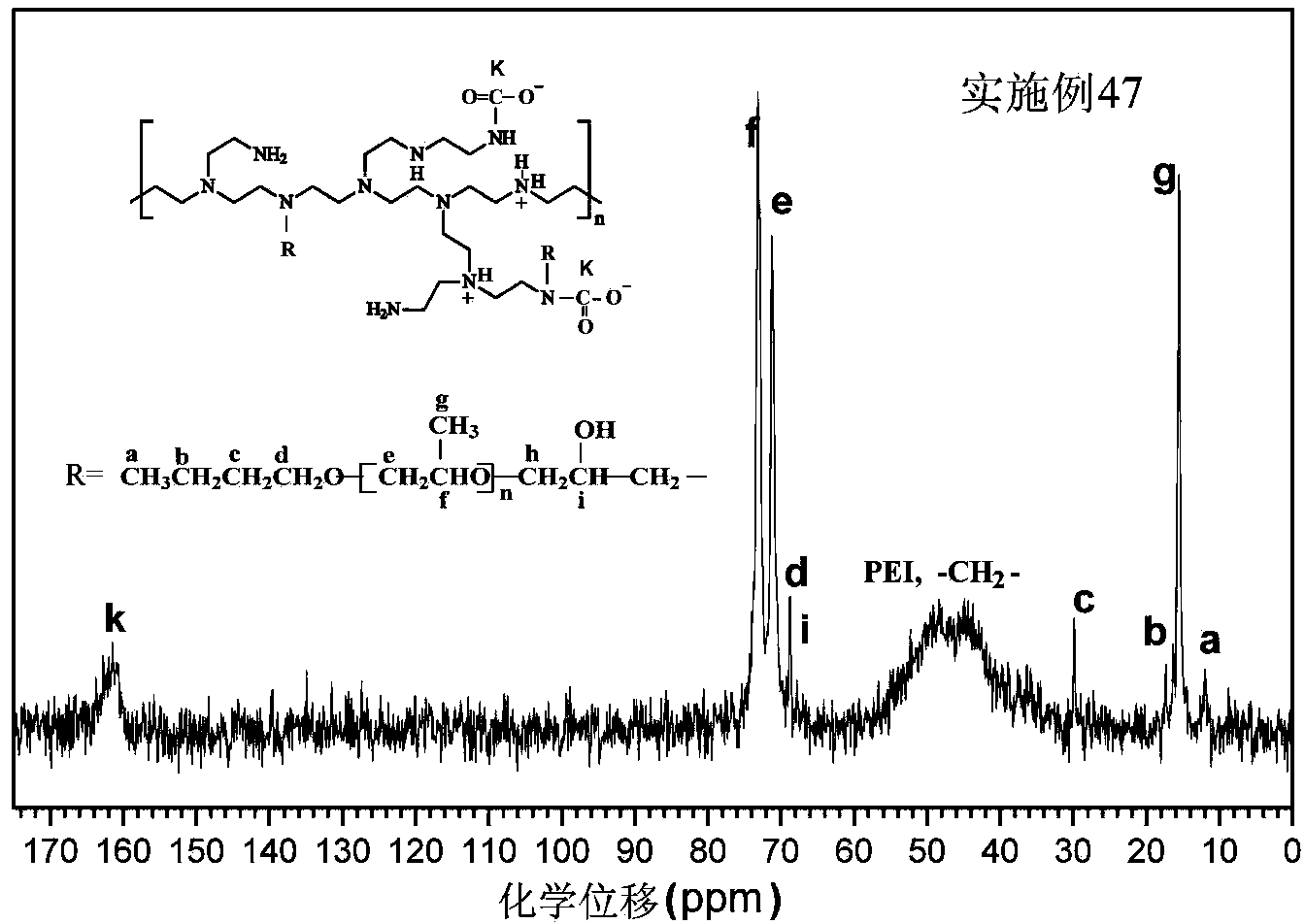
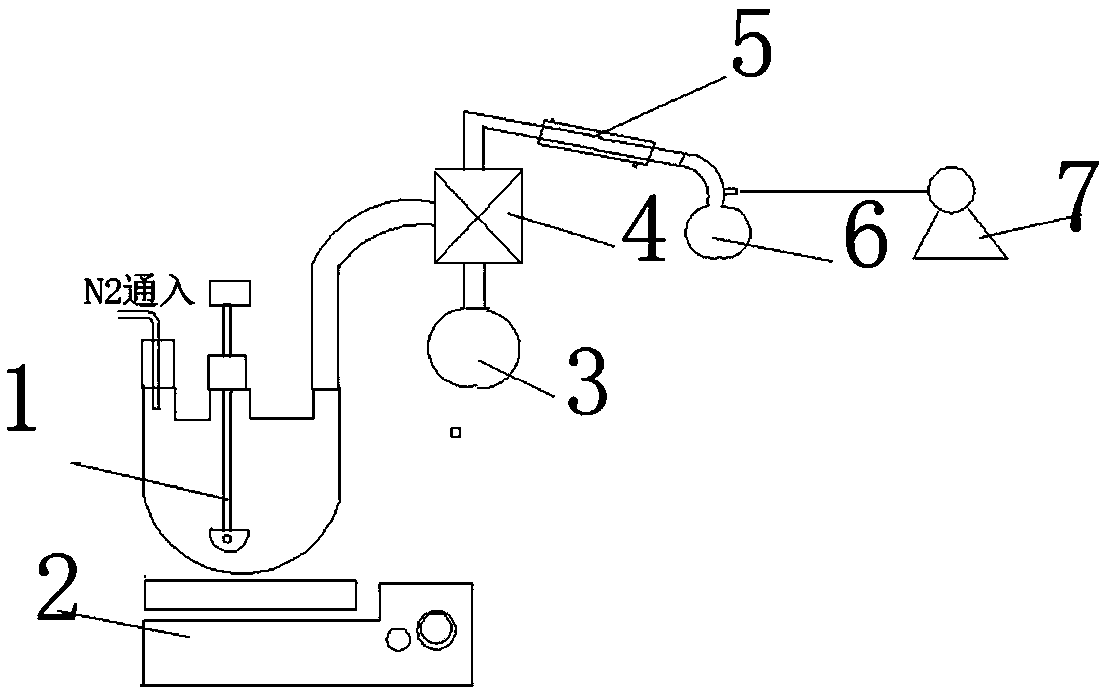
The invention discloses a hydrophobically-modified polyethyleneimine foaming agent capable of releasing carbon dioxide. The hydrophobically-modified polyethyleneimine foaming agent is prepared by reacting polyethyleneimine subjected to hydrophobic modification by a glycidyl ether compound or an alkyl carboxylic compound or a halogenated alkane compound with 0-31% of distilled water at the pressure of 0.1-1 MPa in carbon dioxide at the room temperature for 1-3 days; a 5-40.4 mol% hydrophobic chain is grafted on N atoms in a polyethyleneimine molecular chain of the foaming agent, and a carbamic acid anion (-NCOO-) characteristic absorption peak or a bicarbonate (HCO3-) characteristic absorption peak exists at the range of 159-165 ppm of a C Nuclear magnetic resonance of the foaming agent, and the foaming agent can release carbon dioxide CO2 at the temperature of 50-150 to prepare a polyurethane foam material. The foaming agent provided by the invention can be uniformly dispersed in a preparation raw material of hydrophobic polyurethane foam, can solve a series of problems caused by the fact that non-modified polyethyleneimine is used as a foaming agent, and is climate-friendly.
Owner:SICHUAN UNIV
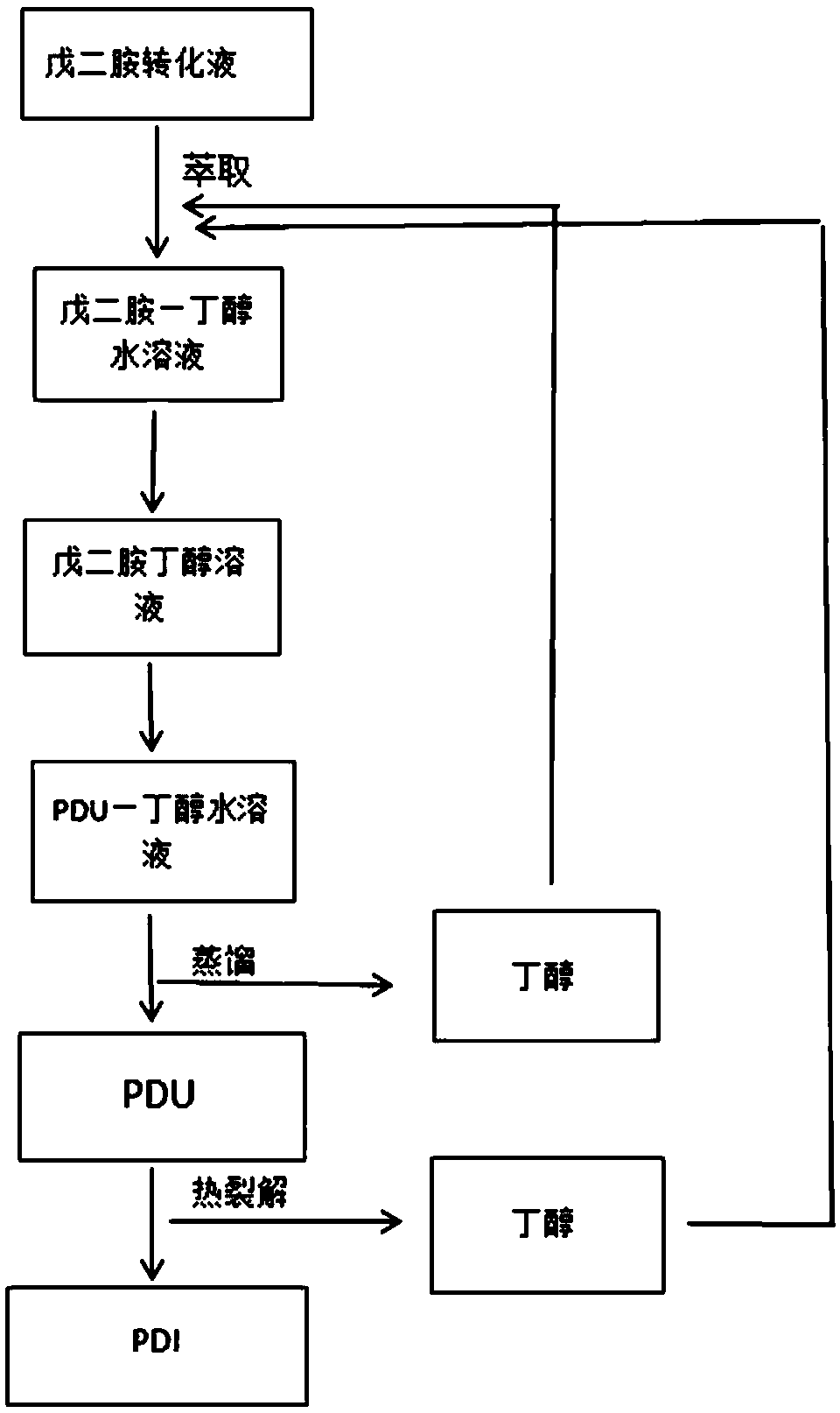
1, 5-pentyl diisocyanate preparation method
InactiveCN108689884AReduce consumptionAchieve recyclingCarbamic acid derivatives preparationOrganic compound preparationHeat carrierDistillation
The invention discloses a 1, 5-pentyl diisocyanate preparation method which includes the steps: (1) mixing 1, 5-pentane diamine transfer solution and extracting solvents, extracting 1, 5-pentane diamine and dewatering extracting solution to obtain mixed solution of the 1, 5-pentane diamine transfer solution and the extracting solvents; (2) adding catalysts and urea into the mixed solution of the 1, 5-pentane diamine transfer solution and the extracting solvents, supplementing an appropriate quantity of extracting solvents to form a reaction system, performing carbamic acid esterification on the 1, 5-pentane diamine and recycling excessive extracting solvents in the reaction system by reduced pressure distillation to obtain PDU; (3) mixing the PDU, heat carriers and the catalysts and performing thermal cracking reaction and separation to obtain the extracting solvents and PDI. According to the method, a large quantity of salts in the transfer solution can be removed by extracting solvent extraction, the extracting solvents can serve as raw materials for synthesizing the PDU, the PDI can be prepared by thermal cracking, and the extracting solvents serving as by-products can be recycled.
Owner:NANJING UNIV OF TECH
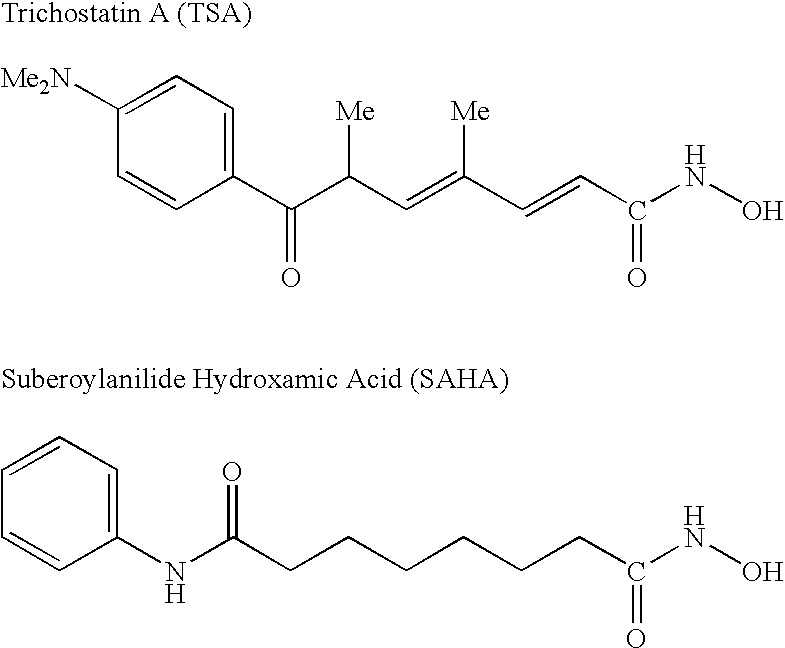
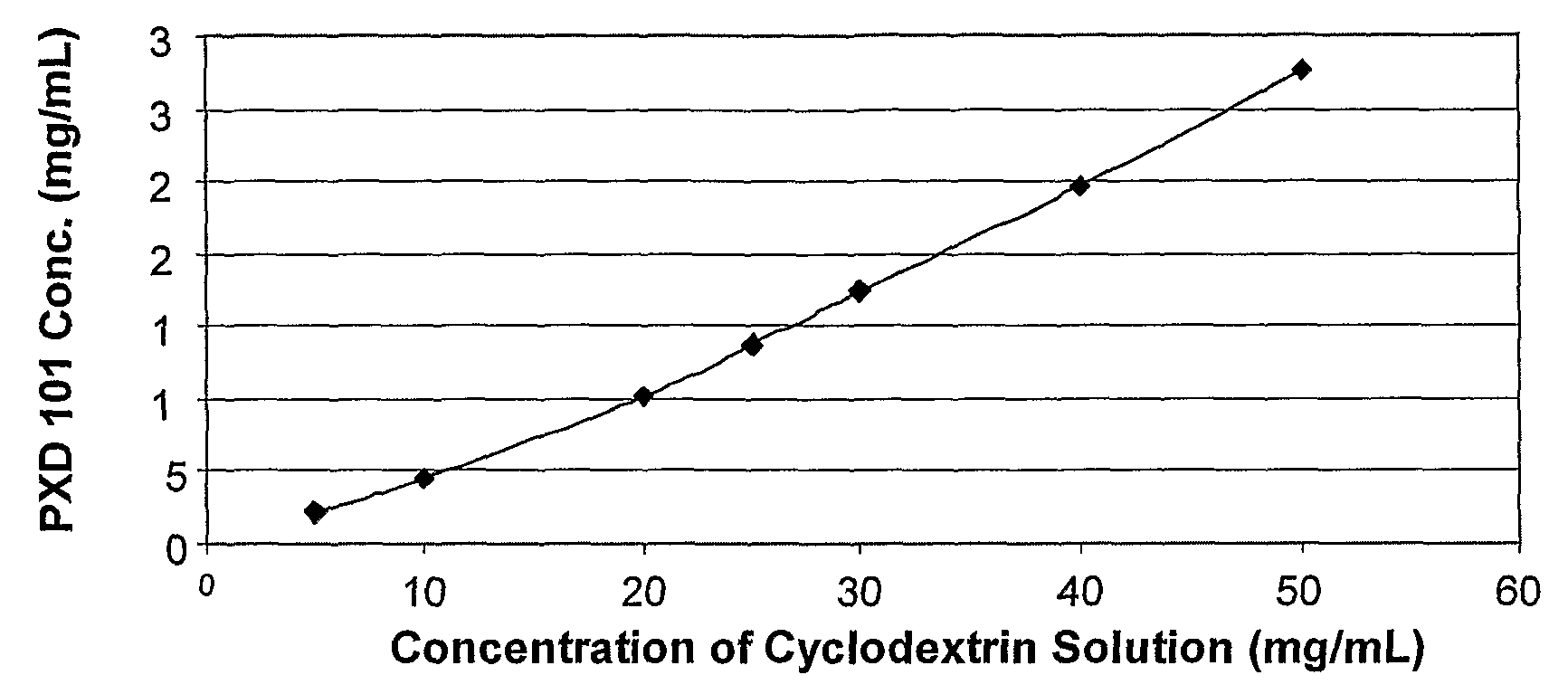
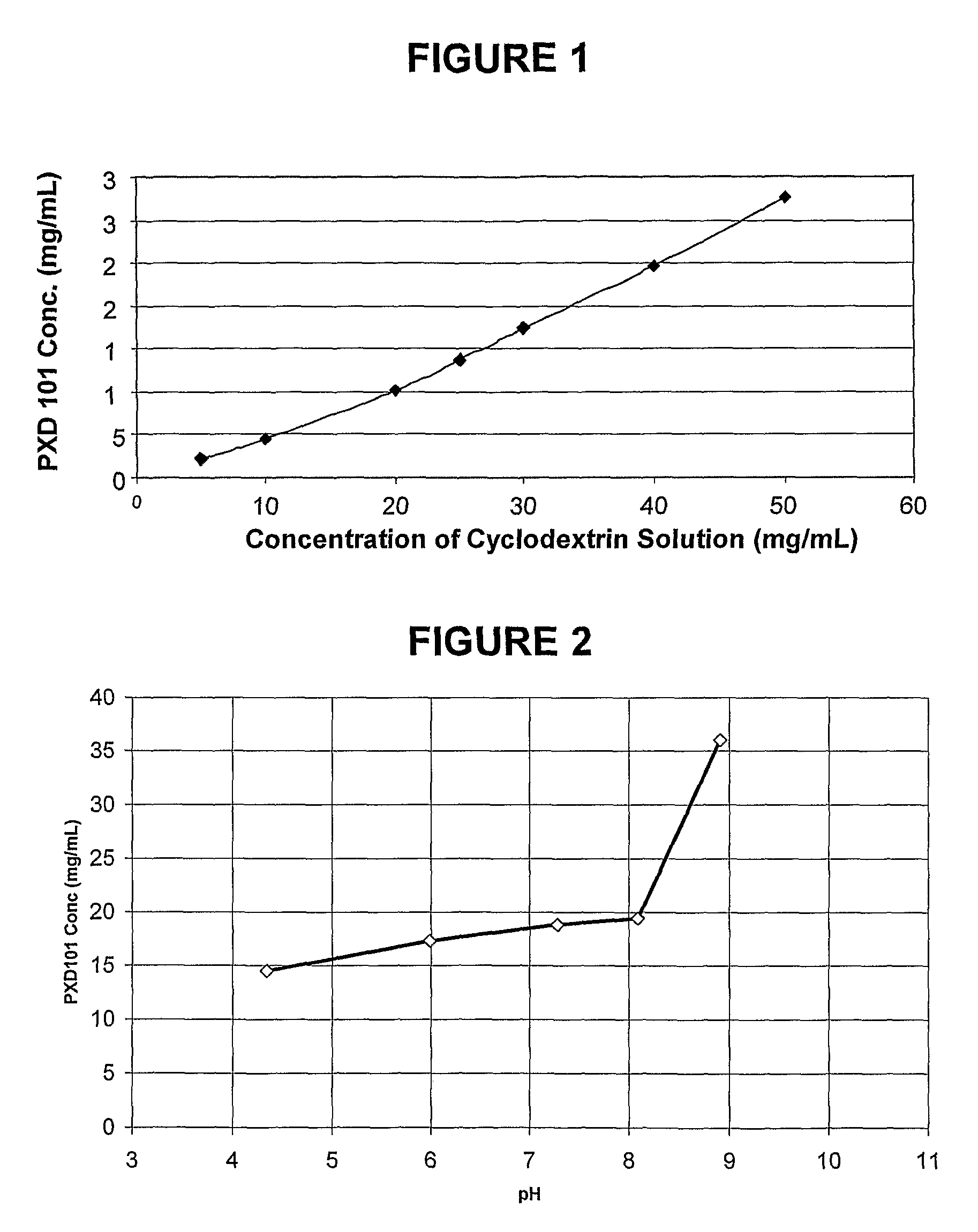
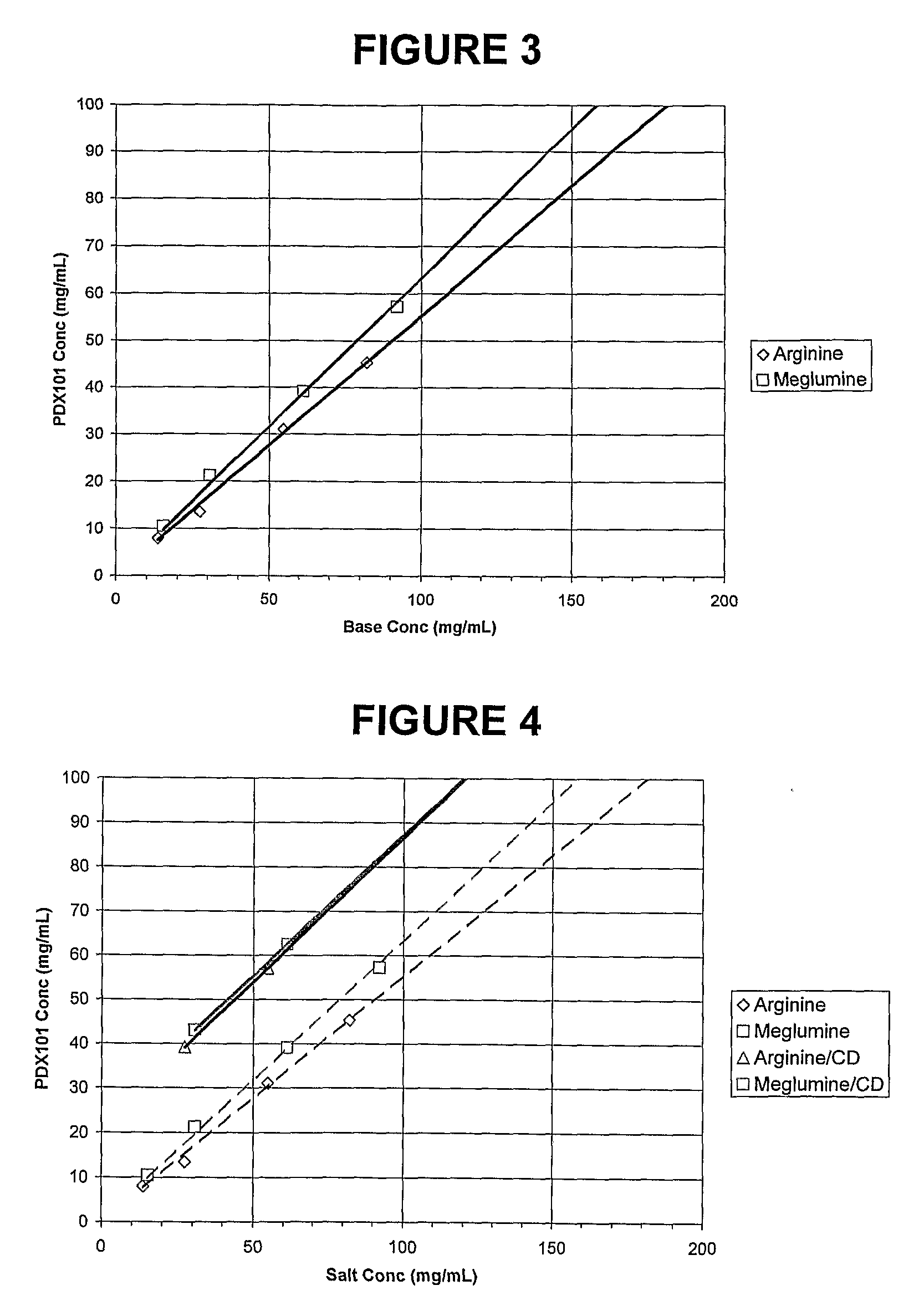
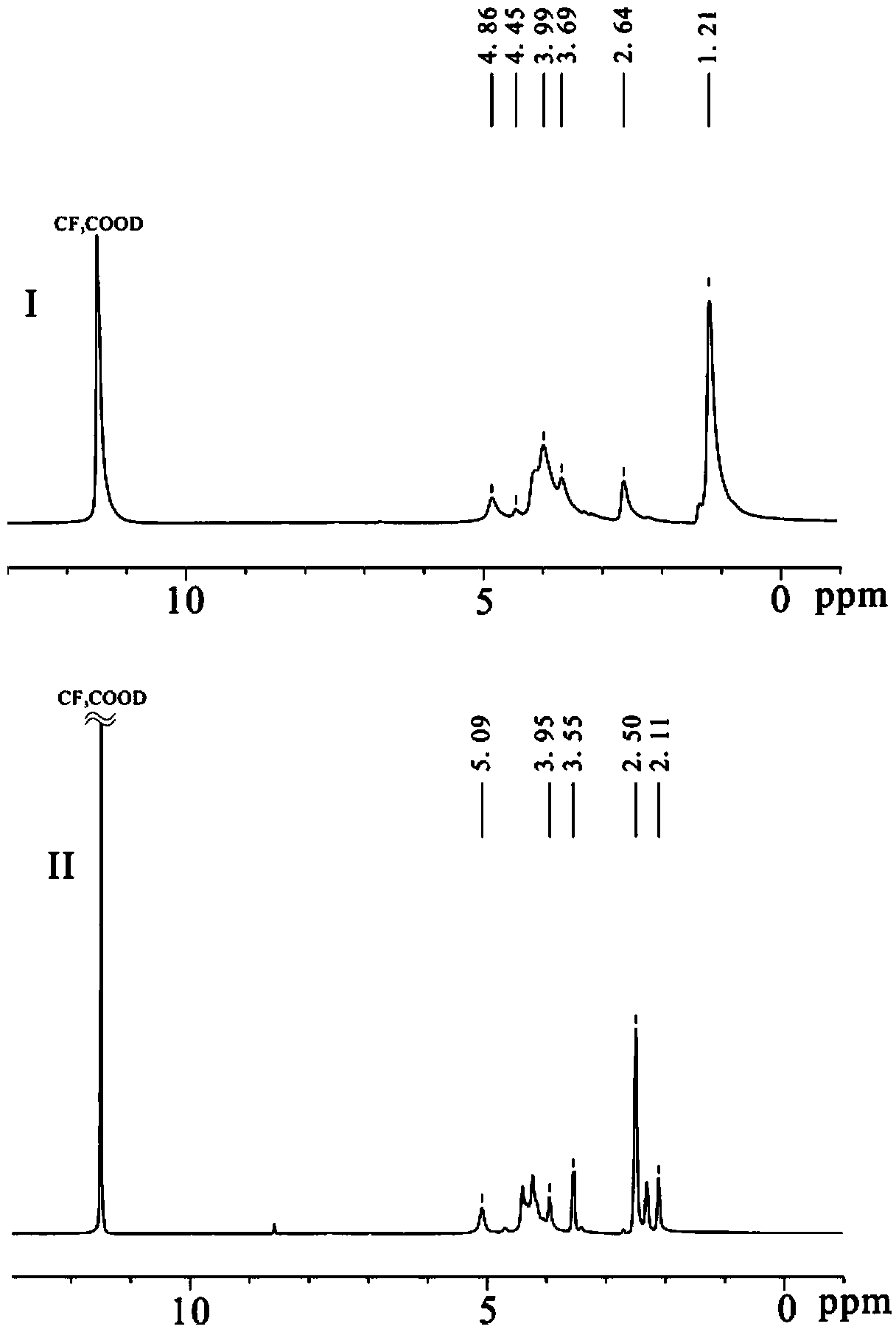
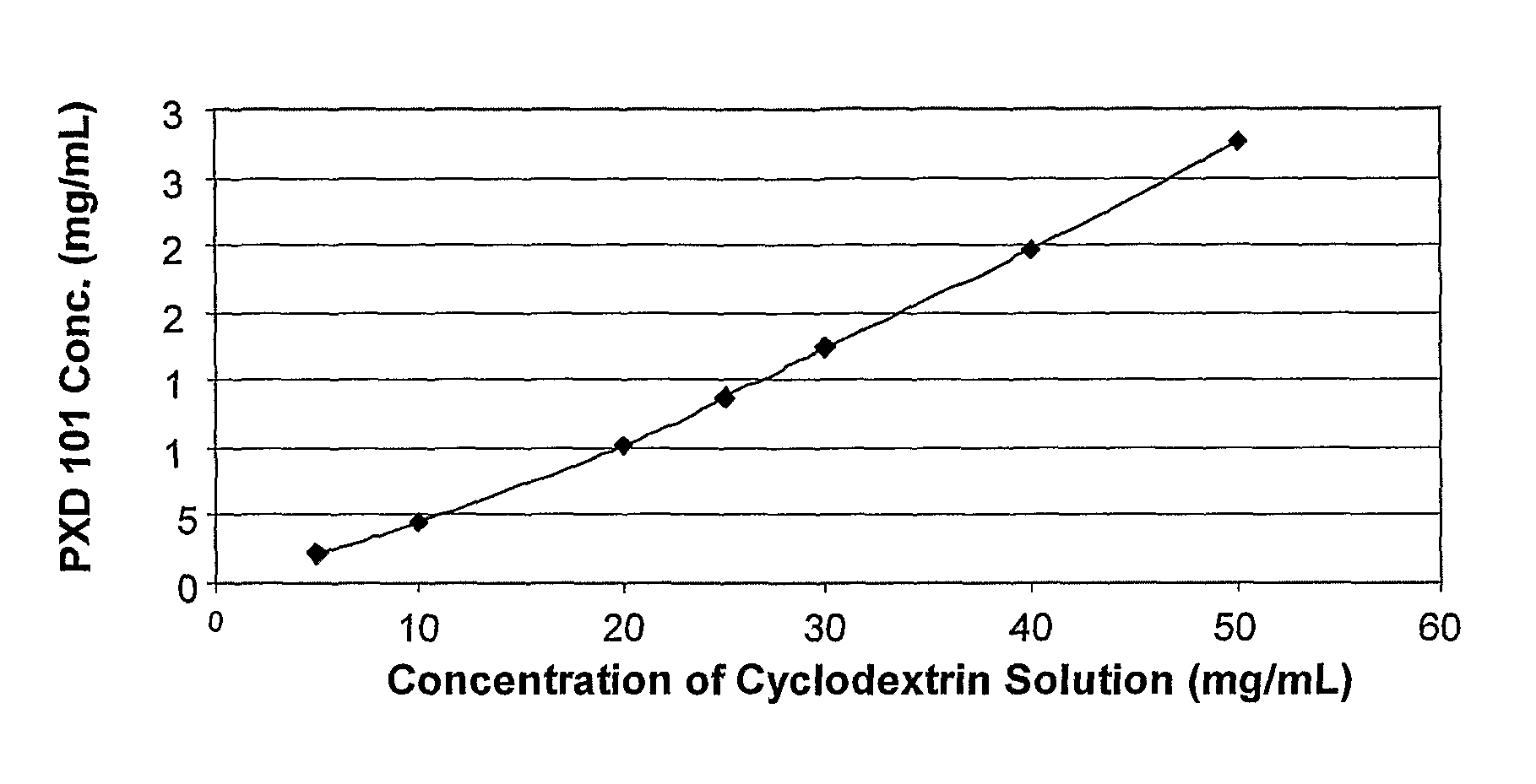
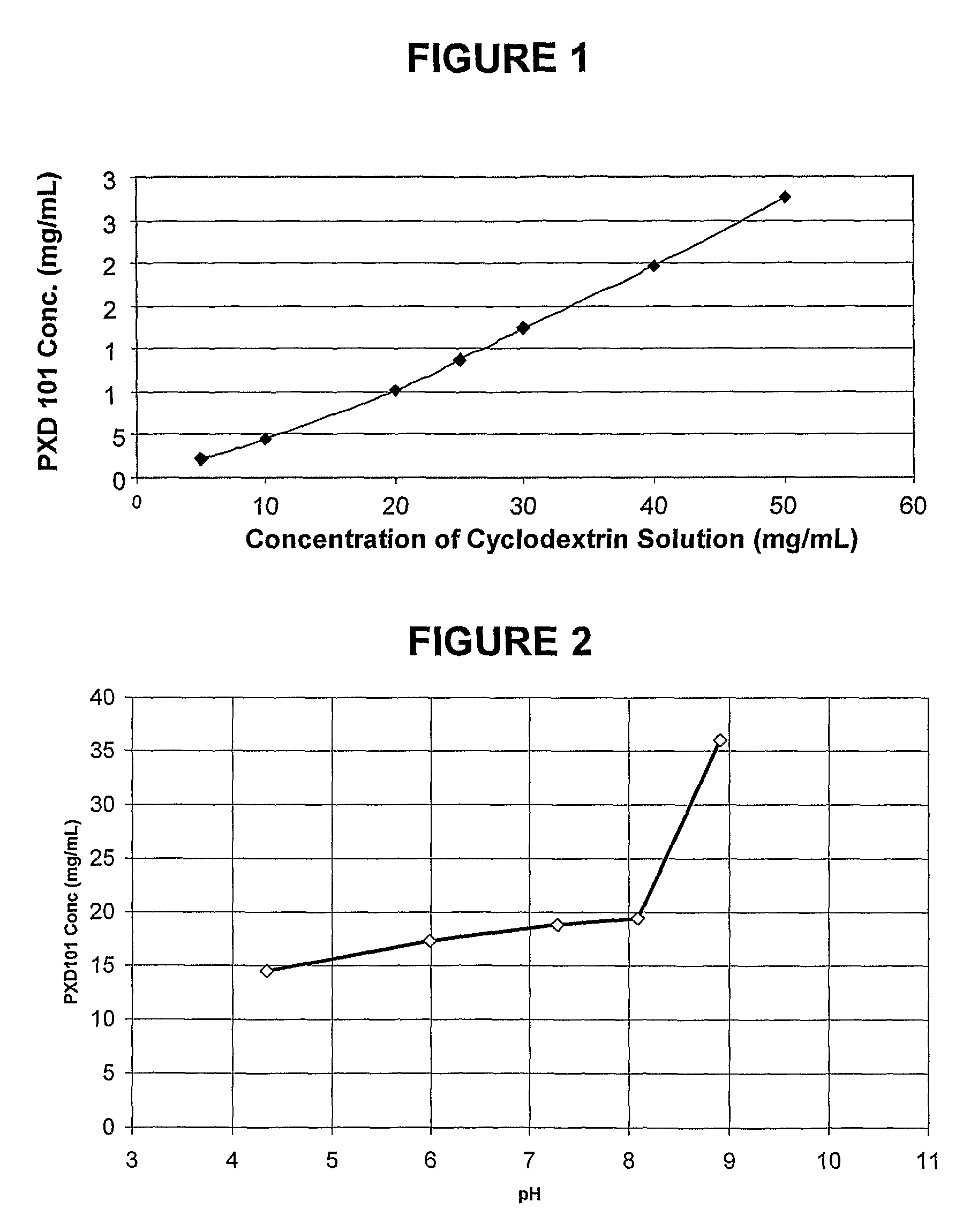
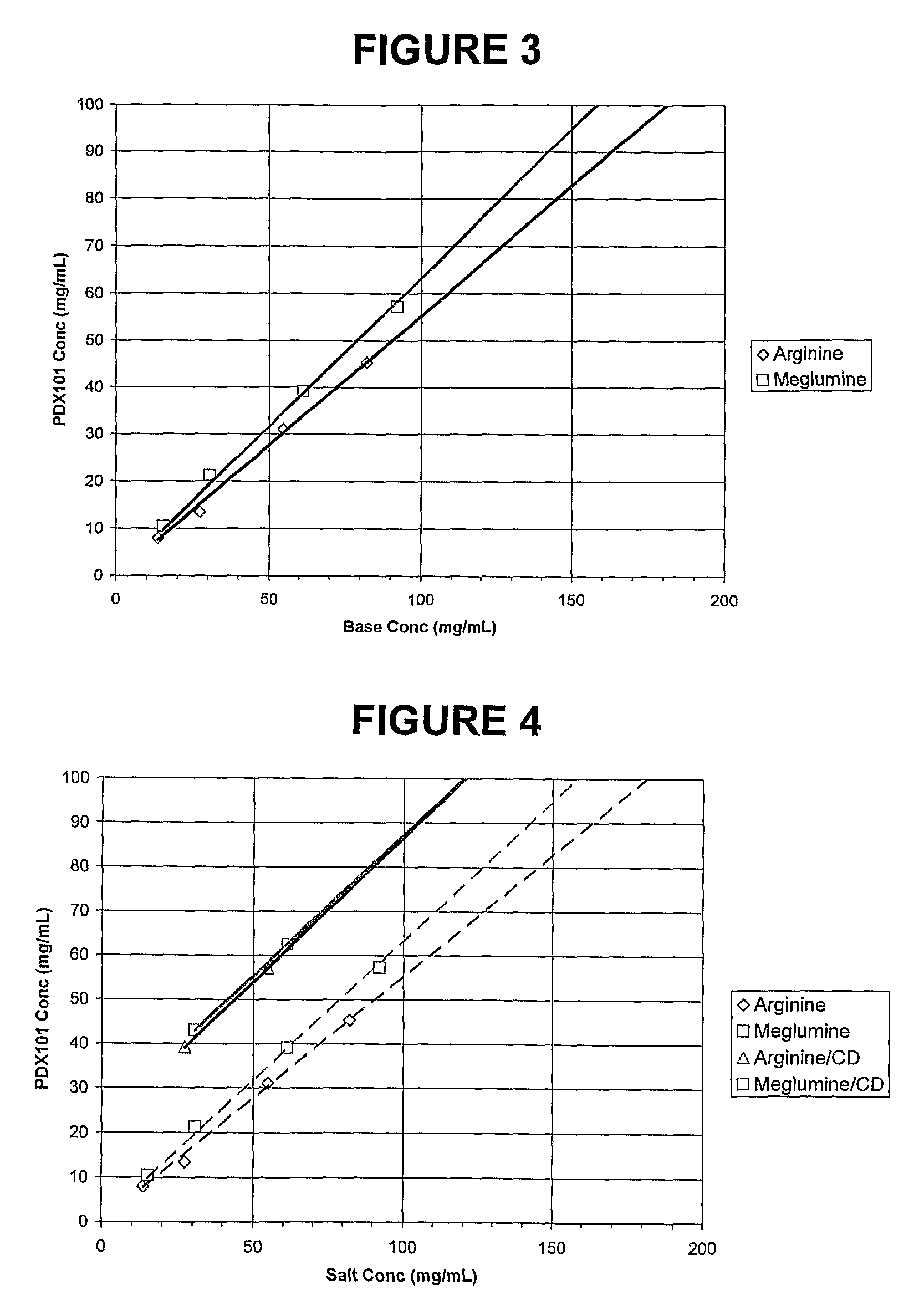
Pharmaceutical Formulations Of Hdac Inhibitors
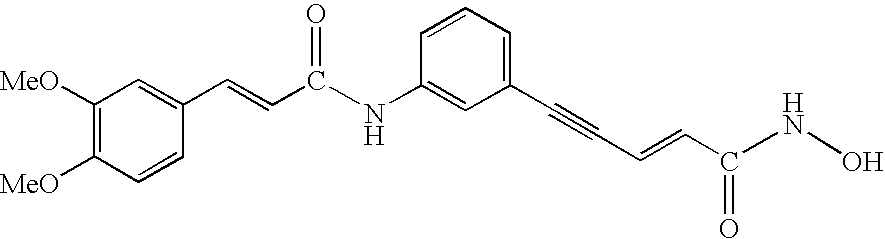
This invention pertains to pharmaceutical compositions comprising certain carbamic acid compounds (e.g., which inhibit HDAC (histone deacetylase) activity) (e.g., PXD-101, N hydroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide)) and one or more additional ingredients selected from cyclodextrin, arginine, and meglumine. The present invention also pertains to the use of such compositions, for example, in the inhibition of HDAC, and in the treatment of conditions mediated by HDAC, cancer, proliferative conditions, psoriasis, etc.
Owner:TOPOTARGET UK LTD
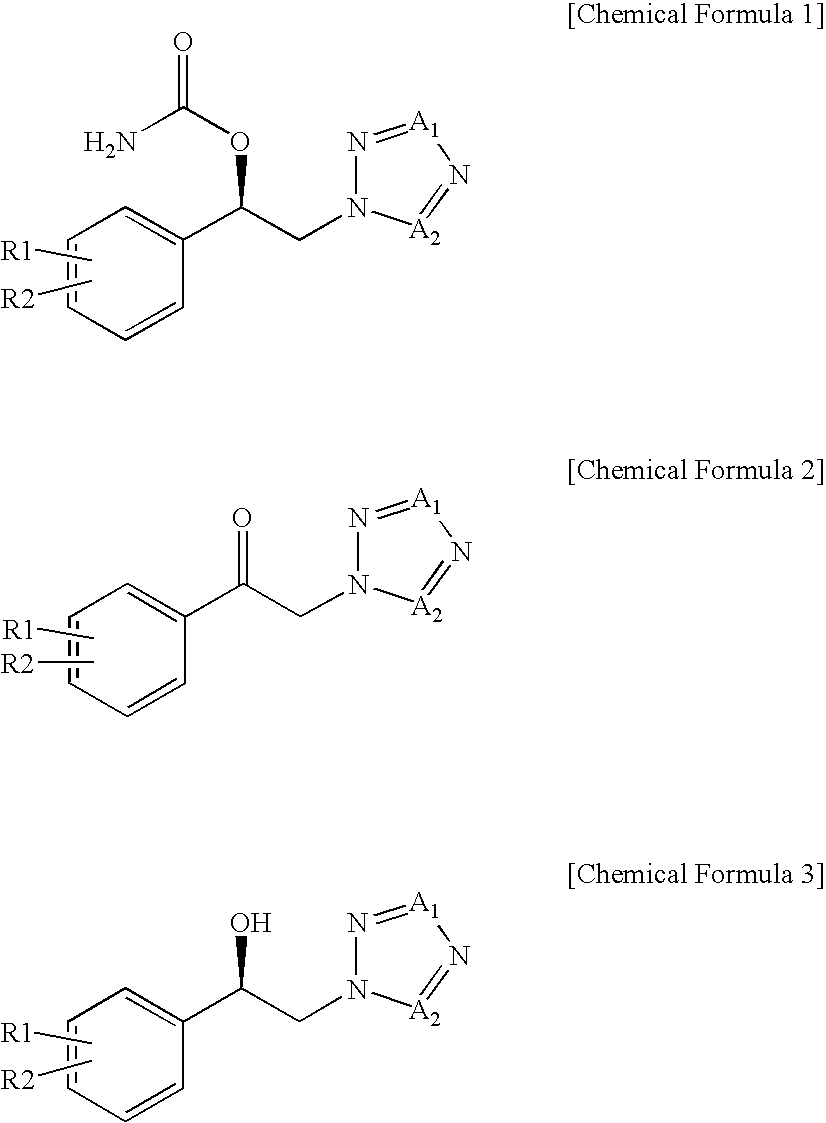
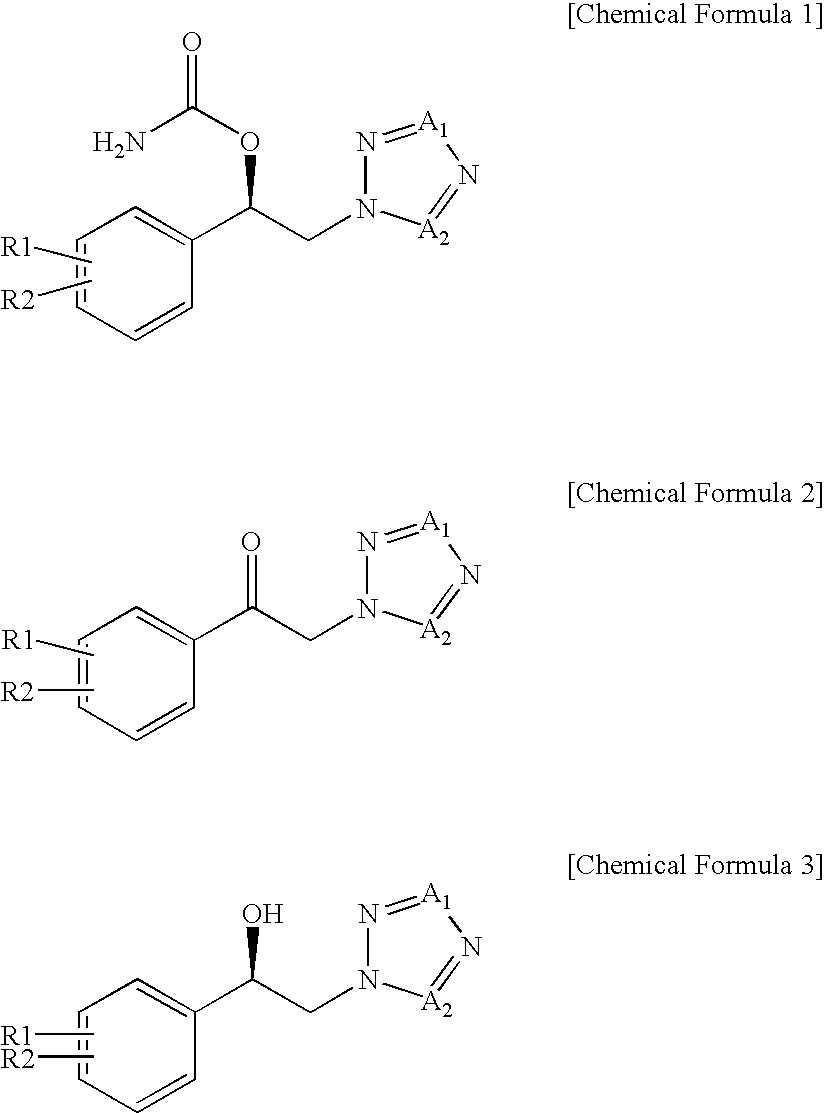
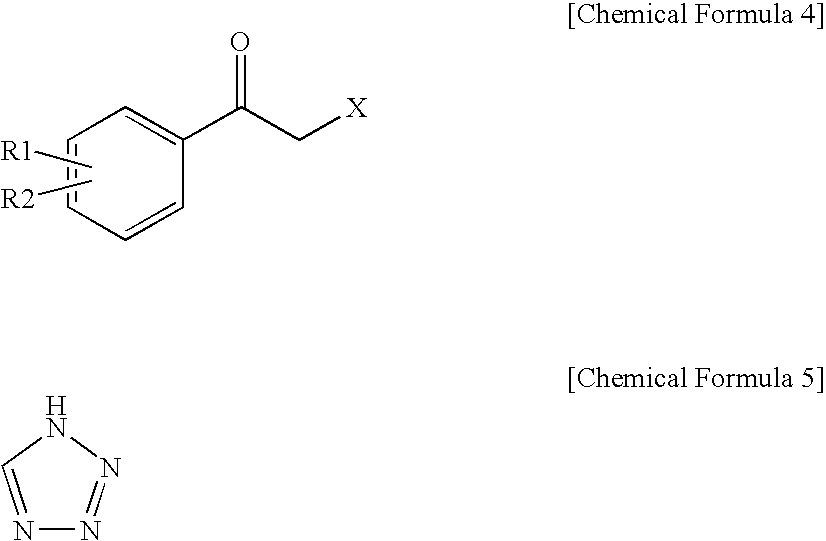
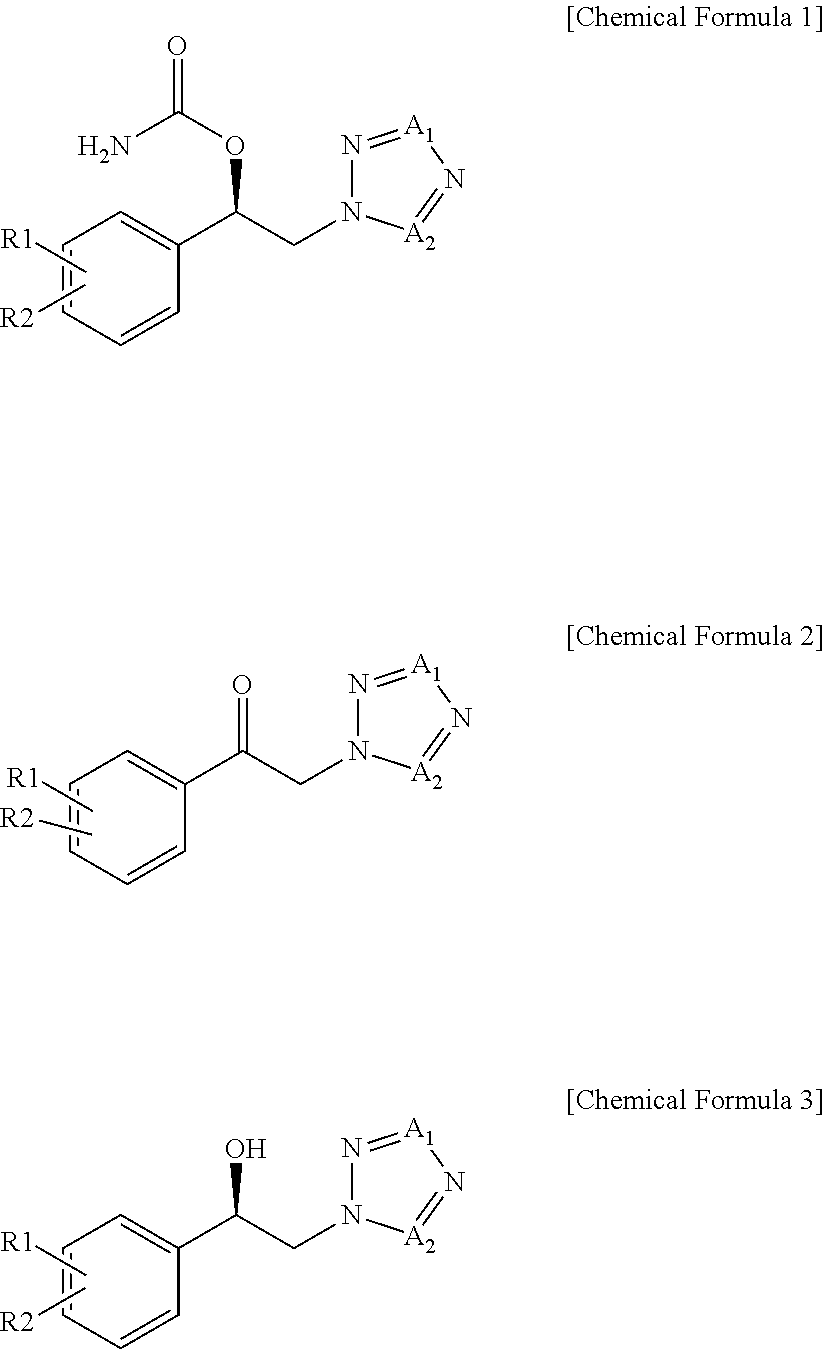
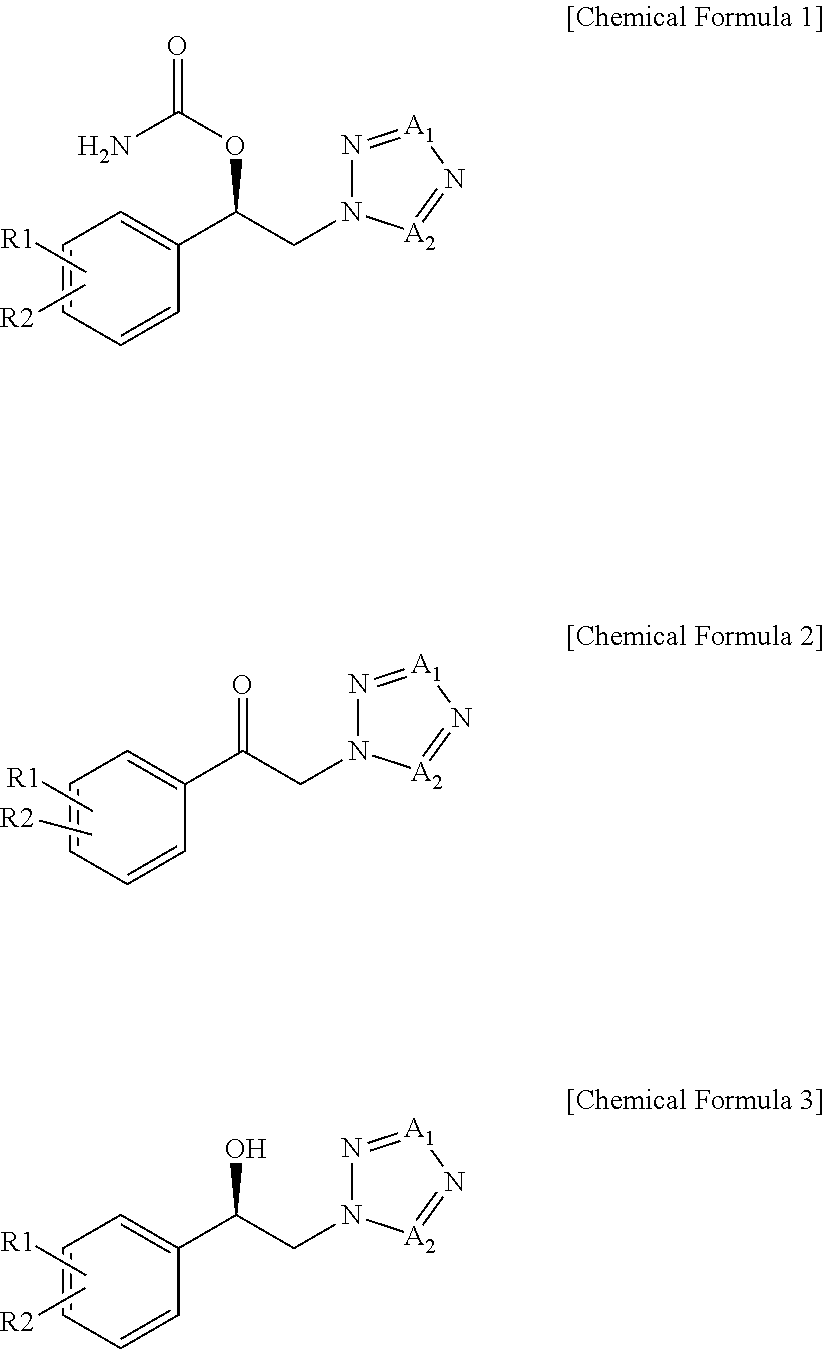
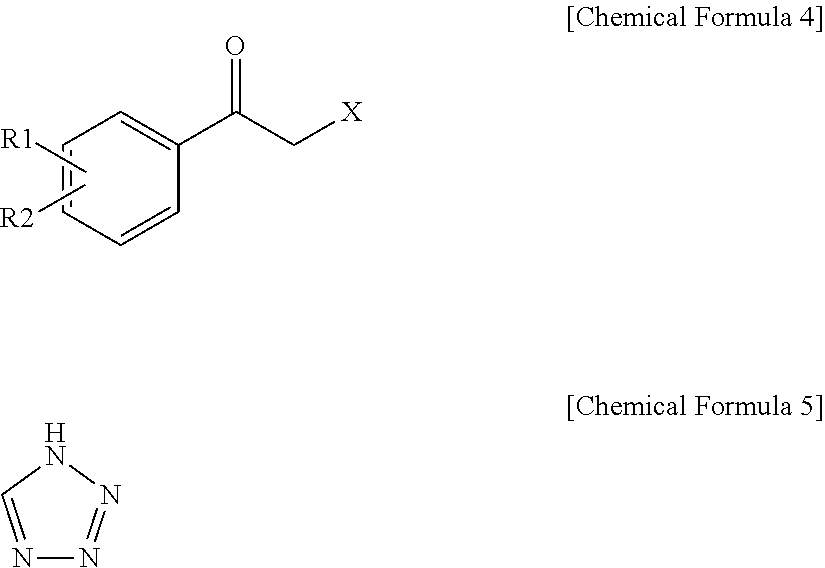
Method for preparation of carbamic acid (r)-1-aryl-2-tetrazolyl-ethyl ester
ActiveUS20100323410A1High product yieldImprove purification effectOrganic chemistryMicrobiological testing/measurementArylAlcohol
Disclosed is a method for the preparation of carbamic acid (R)-1-aryl-2-tetrazolyl-ethyl ester, comprising the asymmetric reduction of arylketone and the carbamation of alcohol.
Owner:BIOPHARM
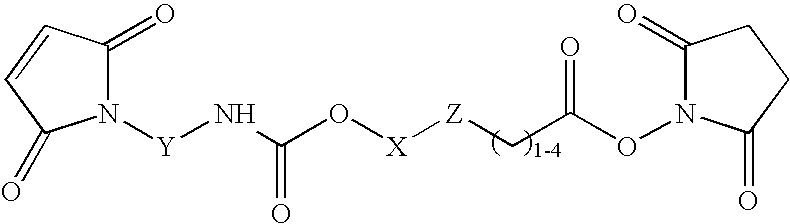
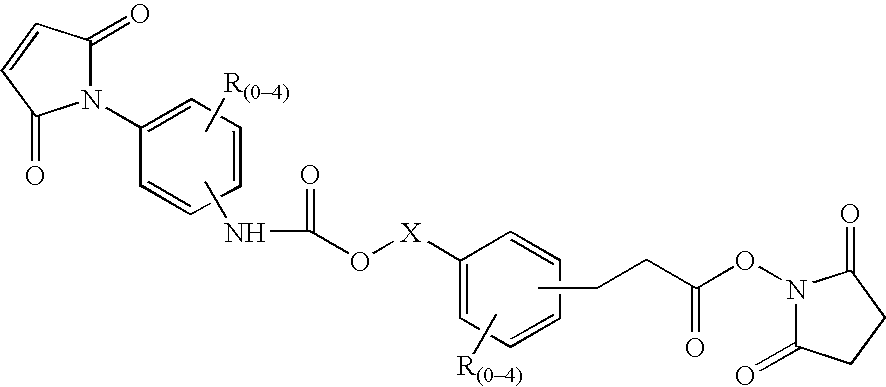
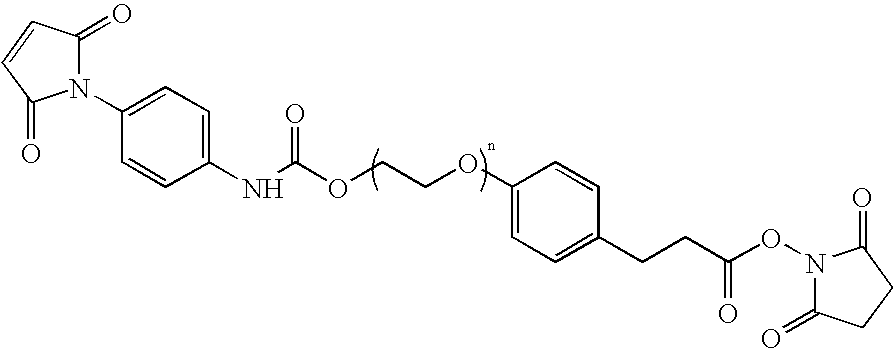
N-aryl-carbamic acid ester-derived and valeric acid ester-derived cross-linkers and conjugates, and methods for their synthesis and use
ActiveUS6887952B1Simplified synthetic routeAbility to monitor synthesisOrganic active ingredientsOrganic chemistrySolubilityAryl
The present invention describes carbamic acid ester-derived and valeric acid ester-derived polyfunctional cross-linker molecules, and methods for their synthesis and use. The inclusion of polymeric moieties such as poly(alkylene oxide) in the cross-linkers of the present invention can provide advantageous solubility properties in aqueous environments. Such cross-linkers may be used to form conjugates for use in a variety of assay formats.
Owner:BIOSITE DIAGNOSTICS
Catalyst comprising n-substituted cyclic imides and processes for preparing organic compounds with the catalyst
InactiveUS7115541B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhosphoric acidCarboxylic acid
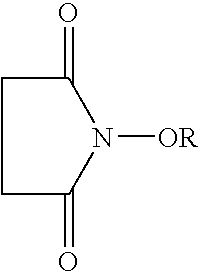
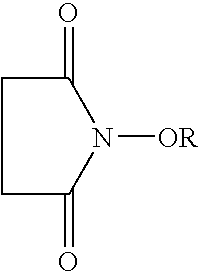
A catalyst of the invention includes an imide compound having a N-substituted cyclic imide skeleton represented by following Formula (I):wherein R is a hydroxyl-protecting group. Preferred R is a hydrolyzable protecting group. R may be a group obtained from an acid by eliminating an OH group therefrom. Such acids include, for example, carboxylic acids, sulfonic acids, carbonic acid, carbamic acid, sulfuric acid, nitric acid, phosphoric acids and boric acids. The catalyst may include the imide compound and a metallic compound in combination. In the presence of the catalyst, (A) a compound capable of forming a radical is allowed to react with (B) a radical scavenging compound and thereby yields an addition or substitution reaction product of the compound (A) and the compound (B) or a derivative thereof. This catalyst can produce an organic compound with a high selectivity in a high yield as a result of, for example, an addition or substitution reaction under mild conditions.
Owner:DAICEL CHEM IND LTD
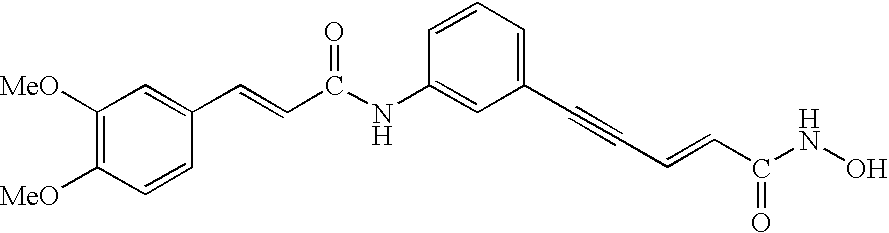
Carbamic acid compounds comprising an amide linkage as HDAC inhibitors
This invention pertains to certain active carbamic acid compounds which inhibit HDAC activity and which have the formula (1) wherein: A is an aryl group; Q1 is an aryl leader group having a backbone of at least 2 carbon atoms; J is an amide linkage selected from: —NR1C(═O)— and —C(═O)NR1—; R1 is an amido substituent; and, Q2 is an acid leader group; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and, e.g., to inhibit proliferative conditions, such as cancer and psorias
Owner:TOPOTARGET UK LTD
Catalyst comprised of n-substituted cyclic imides and processes for preparing organic compounds with the catalyst
InactiveUS7084090B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhosphoric acidCarboxylic acid
A catalyst of the invention includes an imide compound having a N-substituted cyclic imide skeleton represented by following Formula (I):wherein R is a hydroxyl-protecting group. Preferred R is a hydrolyzable protecting group. R may be a group obtained from an acid by eliminating an OH group therefrom. Such acids include, for example, carboxylic acids, sulfonic acids, carbonic acid, carbamic acid, sulfuric acid, nitric acid, phosphoric acids and boric acids. The catalyst may include the imide compound and a metallic compound in combination. In the presence of the catalyst, (A) a compound capable of forming a radical is allowed to react with (B) a radical scavenging compound and thereby yields an addition or substitution reaction product of the compound (A) and the compound (B) or a derivative thereof. This catalyst can produce an organic compound with a high selectivity in a high yield as a result of, for example, an addition or substitution reaction under mild conditions.
Owner:DAICEL CHEM IND LTD
Carbamic acid compounds comprising an amide linkage as HDAC inhibitors
This invention pertains to certain active carbamic acid compounds which inhibit HDAC activity and which have the formula (1) wherein: A is an aryl group; Q1 is an aryl leader group having a backbone of at least 2 carbon atoms; J is an amide linkage selected from: —NR1C(═O)— and —C(═O)NR1-; R1 is an amido substituent; and, Q2 is an acid leader group; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and, e.g., to inhibit proliferative conditions, such as cancer and psoriasis.
Owner:TOPOTARGET UK LTD
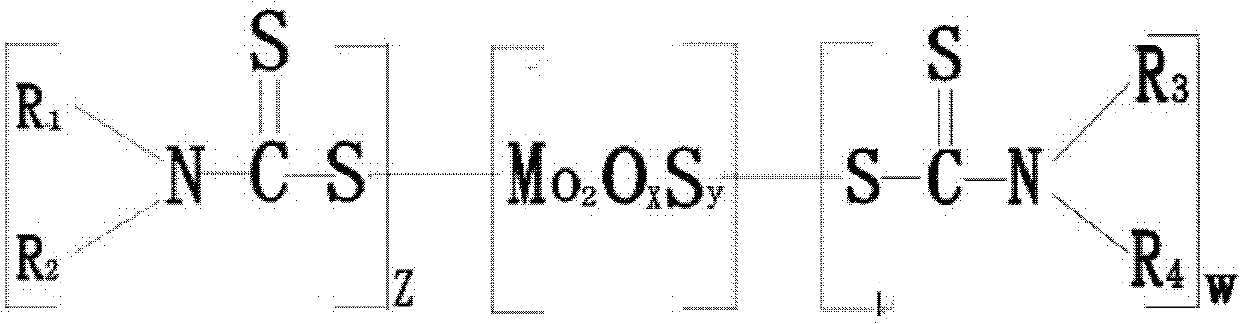
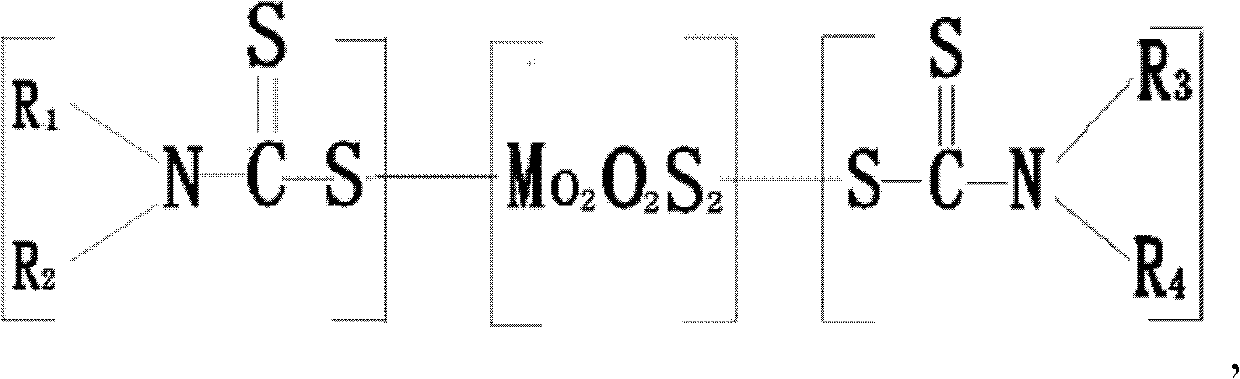
Carbamic acid molybdenum lubricating grease additive, its preparation method and application
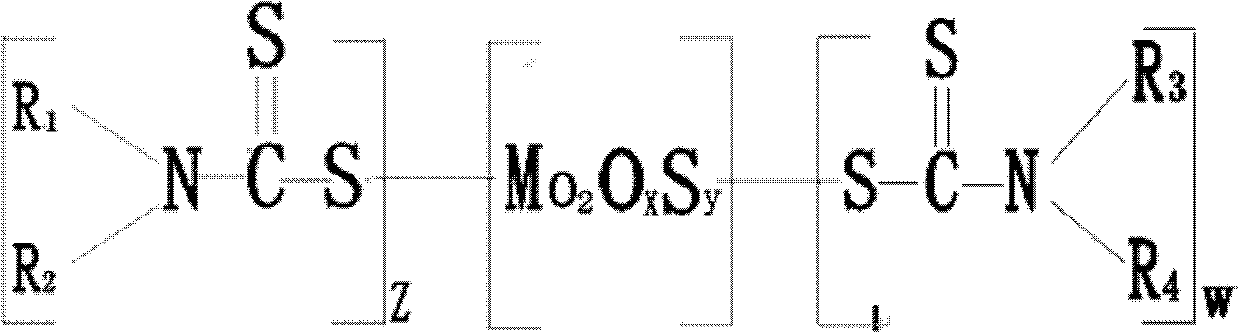
ActiveCN102311841AImprove anti-friction and anti-wear propertiesShorten the timeAdditivesGroup 6/16 element organic compoundsBase oilAlkyl amine
The invention relates to a lubricating grease additive, which has a structure as shown in a general formula, wherein R1, R2, R3 and R4 are selected from C4-C15 straight chain and branched chain alkyl group; R1 is different from R2; R3 is the same with R4; R1 and R2 can be the same with or different from R3 and R4; Z equals to 1-3, W equals to 1-3; and x+y=3-7. The invention also provides a methodfor preparing the above additive by a reaction of a dialkylamine mixture, comprising the following steps of: mixing water, the dialkylamine mixture, cycloalkyl base oil and molybdenum resource with uniformly stirring, cooling to 15-20DEG C, pressurizing to 830-900mmHg, adding dropwisely carbon disulfide, heating up to 60-80DEG C, followed by reflux reaction for 3-5 hours, reducing pressure to 760-800mmHg for water evaporation, completely vacuumizing at low speed, heating to 120-140DEG C, continuously reacting for 1-3 hours, cooling, and filtering. The additive provided by the invention contains no phosphorus, and has not only excellent antifriction and anti-wear performance but also improved oil dissolving performance and antioxidation performance.
Owner:太平洋联合(北京)石油化工有限公司
Medicinal composition combining paclitaxel with novel phthalazinone compound
InactiveCN109288830AGood anticancer effectImprove biological activityOrganic active ingredientsOrganic chemistry methodsCrystalCarbamic acid
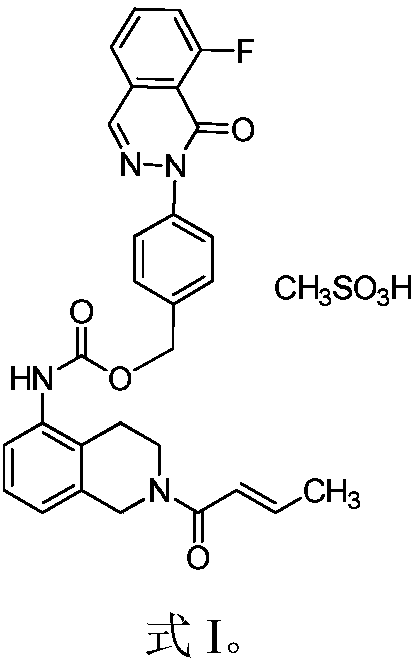
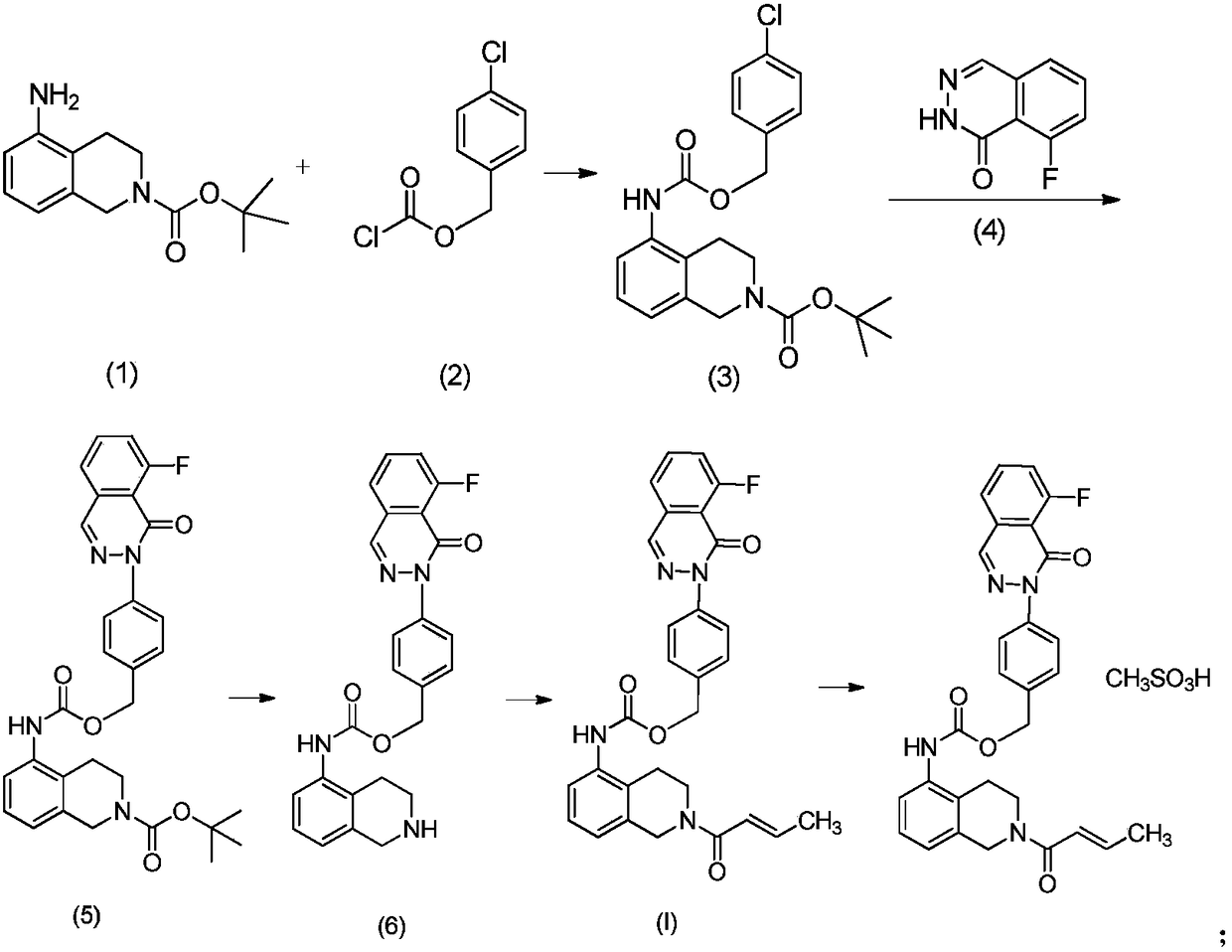
A medicinal composition combining paclitaxel with a novel phthalazinone compound, provided by the invention, comprises an active component and a pharmaceutically-acceptable auxiliary material, the active component is composed of paclitaxel and the phthalazinone compound with an N crystal form, represented by formula I, and a mass ratio of the paclitaxel to the phthalazinone compound of the formulaI in the active component is (0.1-0.3):1, wherein the chemical name of the phthalazinone compound of the formula I is [2(1H)-butyl-2-enoyl-3,4-dihydroisoquinolin-5-yl]-carbamic acid-4-[8-fluoro-(2H)-phthalazin-1-one]benzyl ester mesylate. The medicinal composition has a good anticancer effect. It is found that the paclitaxel and the phthalazinone compound with an N crystal form, represented by formula I, are combined to greatly enhance the antitumor growth bioactivity and achieve antitumor proliferation synergistic effects, so the medicinal composition is expected to be developed into clinically anti-pancreatic cancer first-line drugs.
Owner:NANJING ADVANCED BIOLOGICAL MATERIALS & PROCESS EQUIP INST CO LTD
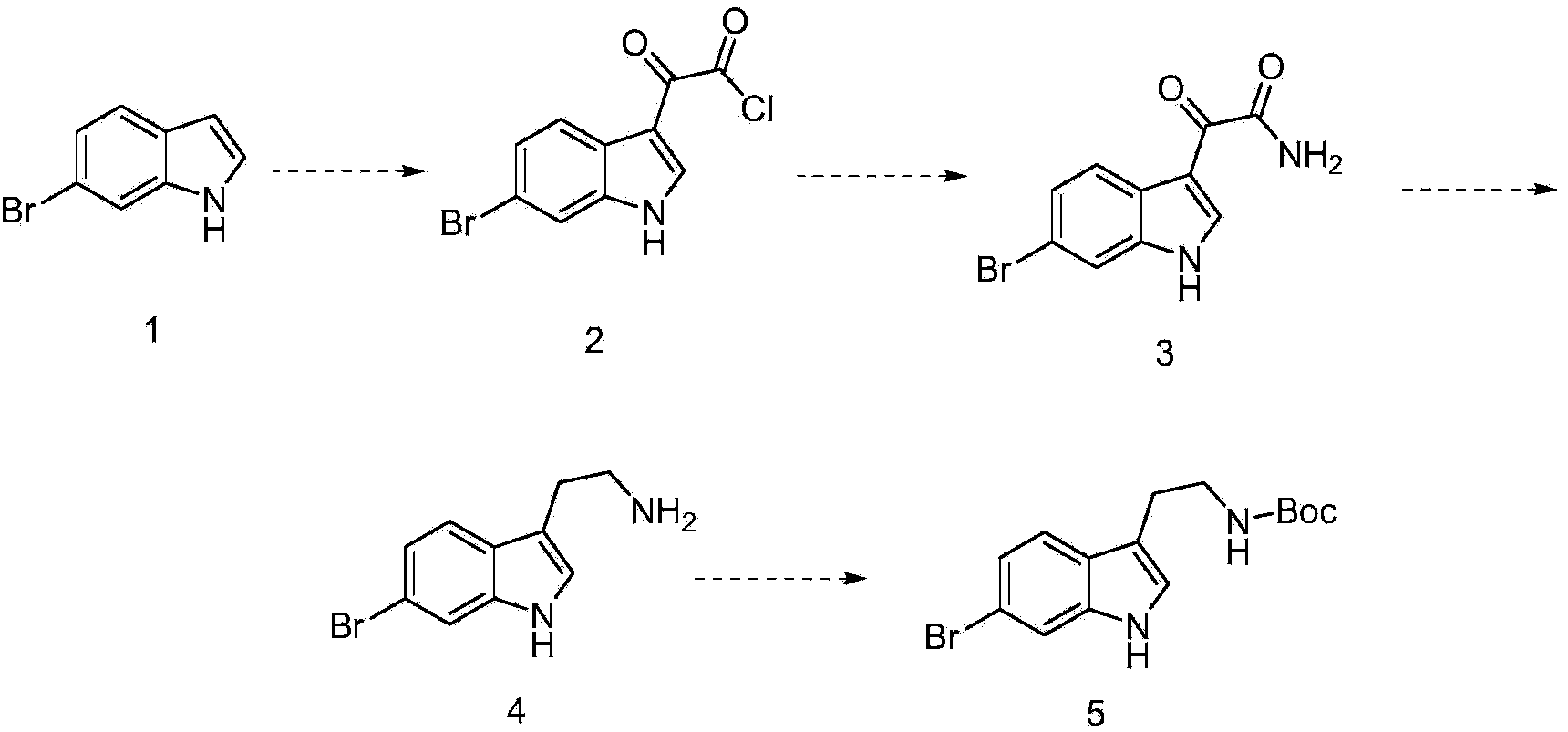
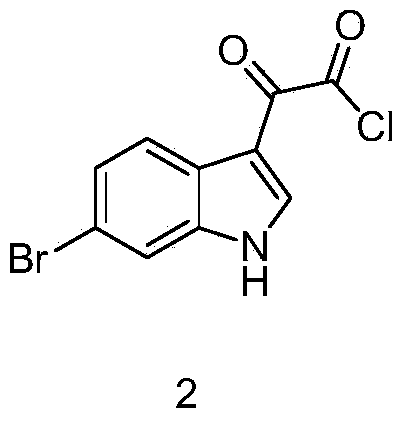
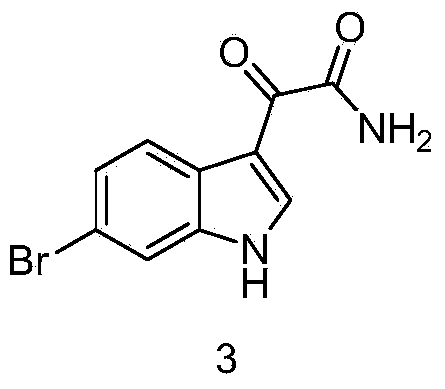
Preparation method of 6-bromoindole derivative
The invention discloses a preparation method of a 6-bromoindole derivative: tertiary butyl 2-(6-bromo-1H-indole-3-yl) ethyl carbamic acid. The preparation method comprises the following steps: by using 6-bromoindole as an initial raw material, carrying out Friedel-Craft reaction; carrying out amidation; reducing; and carrying out t-butyloxycarboryl protective reaction to obtain the target product. The compound is an important medical intermediate.
Owner:湖南华腾制药有限公司
Solid forms of antiviral compound
Amorphous and crystalline solid forms of the anti-HCV compound (1-{3-[6-(9,9-difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl)-5-aza-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzoimidazol-2-yl]-2-aza-bicyclo[2.2.1]heptane-2-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester (Compound I) were prepared and characterized in the solid state: Also provided are processes of manufacture and methods of using the amorphous and crystalline forms.
Owner:GILEAD PHARMASSET LLC
Piperazine salt and a process for the preparation thereof
ActiveUS20110275816A1High purityHigh yieldOrganic chemistryBulk chemical productionEthyl methanesulfonate2,3-Dichlorophenylpiperazine
The invention relates to novel trans N-{4-{2-[4-(2, 3-dichlorophenyl)-piperazine-1-il]-ethyl}-cyclohexylamine dihydrochloride monohydrate and a process for the preparation of the trans N-{4-{2-[4-(2,3-dichlorophenyl)-piperazine-1-il]-ethyl}-cyclohexylamine dihydrochloride monohydrate, said process comprising the stepsa) reacting trans 2-{1-[4-(N-tert-butoxycarbonyl) amino]-cyclohexyl}-acetic acid ester with sodium borohydride and aluminium trichloride to give trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-ethanol;b) reacting trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]cyclohexyl}-ethanol obtained with methanesulfonic acid chloride in the presence of an acid binding agent to give trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-ethyl methanesulfonate;c) reacting trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-ethyl methanesulfonate obtained with 2,3-dichlorophenyl-piperazine in the presence of an acid binding agent to give trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-carbamic acid tert-butylester;d) heating trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-carbamic acid tert-butylester obtained to a temperature between 40-100° C. in a mixture of aqueous hydrochloric acid / methanol to give trans N-{4-{2-[4-(2,3-dichlorophenyl) piperazine-1-il]-ethyl}-cyclohexylamine dihydrochloride monohydrate.
Owner:RICHTER GEDEON NYRT
Chitosan-bi(aryl-carbamate)-(amide) and preparation method thereof
The invention relates to a material chitosan-bi(aryl-carbamate)-(amide) for preparing a chiral stationary phase and a preparation method of the chitosan-bi(aryl-carbamate)-(amide). The preparation method comprises the following steps: 1) acylating chitosan amino, namely enabling chitosan with the deacetylation degree of over 98 percent to be reacted with excessive anhydride, thereby obtaining acylated N-chitosan; and 2) performing carbamic acid esterification on acylated N-chitosan, namely dissolving the acylated N-chitosan in N,N-dimethylacetamide solution of lithium chloride, adding excessive isocyanate containing different substituent groups on the benzene ring, and reacting at the temperature of 80-95 DEG C for 24-36 hours, thereby generating a chitosan derivative, namely the chitosan-bi(aryl-carbamate)-(amide). The structural formula is as shown in the specification.
Owner:WUHAN INSTITUTE OF TECHNOLOGY +1
Polyurethane coating cure enhancement using zinc carbonate initiators
InactiveUS20050154111A1Low costWell mixedPretreated surfacesPolyurea/polyurethane coatingsCarbamatePolyurethane coating
Zinc carbonates such as carbonic acid zinc salt, zinc carbonate hydroxide monohydrate, zinc bicarbonate, zinc tetraamine carbonate, zinc ammonium carbonate, carbamic acid zinc salt and zinc carbamate can initiate hardening of polyurethane coatings and decrease the coating tack-free time. The decreased tack-free times facilitate earlier application of additional polyurethane layers and earlier return of a coated article to service, and can provide improved floor finishes.
Owner:ECOLAB USA INC
Pharmaceutical formulations of HDAC inhibitors
This invention pertains to pharmaceutical compositions comprising certain carbamic acid compounds (e.g., which inhibit HDAC (histone deacetylase) activity) (e.g., PXD-101, N hydroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide)) and one or more additional ingredients selected from cyclodextrin, arginine, and meglumine. The present invention also pertains to the use of such compositions, for example, in the inhibition of HDAC, and in the treatment of conditions mediated by HDAC, cancer, proliferative conditions, psoriasis, etc.
Owner:TOPOTARGET UK LTD
Novel high-strength foam concrete and preparation method for same
InactiveCN106186954ASimple production processProduction process safetyCeramicwareFoam concreteUltimate tensile strength
The invention discloses novel high-strength foam concrete and a preparation method for the same. The novel high-strength foam concrete is characterized in that the novel high-strength foam concrete is prepared from the following raw materials in parts by weight: 300 to 800 parts of a gel material, 250 to 850 parts of a fine aggregate, 375 to 1,250 parts of a coarse aggregate, 4 to 8 parts of a water reducing agent, 250 to 850 parts of water, 20 to 40 parts of a foaming agent and 10 to 20 parts of a foam stabilizer, wherein the foaming agent is an isocyanate derivative. According to the novel high-strength foam concrete and the preparation method for the same, the following principle is utilized: isocyanate adopted as the foaming agent is reacted with the water to generate unstable carbamic acid at first, the unstable carbamic acid is rapidly decomposed into amine and carbon dioxide, and the discharged carbon dioxide acts for foaming to generate a foam body; a prepared foam concrete material is favorable for making a heat insulation, soundproof and high-strength foam concrete building block on one hand, and on the other hand, the construction engineering cost is reduced, and the high-strength foam concrete can be widely applied to construction engineering.
Owner:JIANGSU CHINA RAILWAY ARIT NEW MATEIRALS CO LTD
Composition for forming a coating film, method of preparing the composition, tantalum oxide film and method of forming the tantalum oxide film
InactiveUS20050003085A1High dielectric constantSolve the large leakage currentTransistorPlastic/resin/waxes insulatorsCarboxylic acidSolvent
A composition for forming a coating film, comprising a reaction product of a tantalum alkoxide and at least one compound selected from carbamic acid, carboxylic acid and carboxylic anhydride and a solvent. A tantalum oxide film is obtained by forming a coating film of this composition and thermally and / or optically treating the coating film. This tantalum oxide film has a large dielectric constant and a small leak current.
Owner:JSR CORPORATIOON
Gypsum board having mold resistance
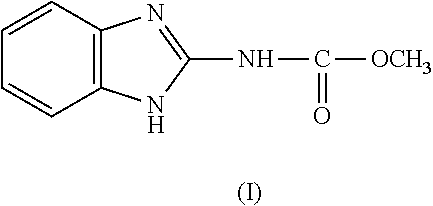
A gypsum board having mold resistance includes a gypsum core and gypsum board paper covering two surfaces of the gypsum core. The gypsum core contains first and second anti-mold agents, each having a water solubility of 200 ppm or less, and a waterproofing agent. A mixture consisting of the first and second anti-mold agents and starch is applied on a surface of the gypsum board paper, which surface is out of contact with the gypsum core. The first anti-mold agent is 2-(4-thiazolyl)-benzimidazole (TBZ) or 2-benzimidazol carbamic acid methyl ester (BCM), and the second anti-mold agent is 3-iodo-2-propyl butyl carbamate (IPBC). The total amount of the first and second anti-mold agents contained in the gypsum core is 0.03% to 0.2% of gypsum forming the gypsum core on an active ingredient basis, and the additive amount of the waterproofing agent is 0.3% to 1.5% of the gypsum forming the gypsum core.
Owner:YOSHINO GYPSUM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com