Solid forms of antiviral compound
A compound and crystal form technology, applied in antiviral agents, drug combinations, organic chemistry, etc., can solve unknown problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
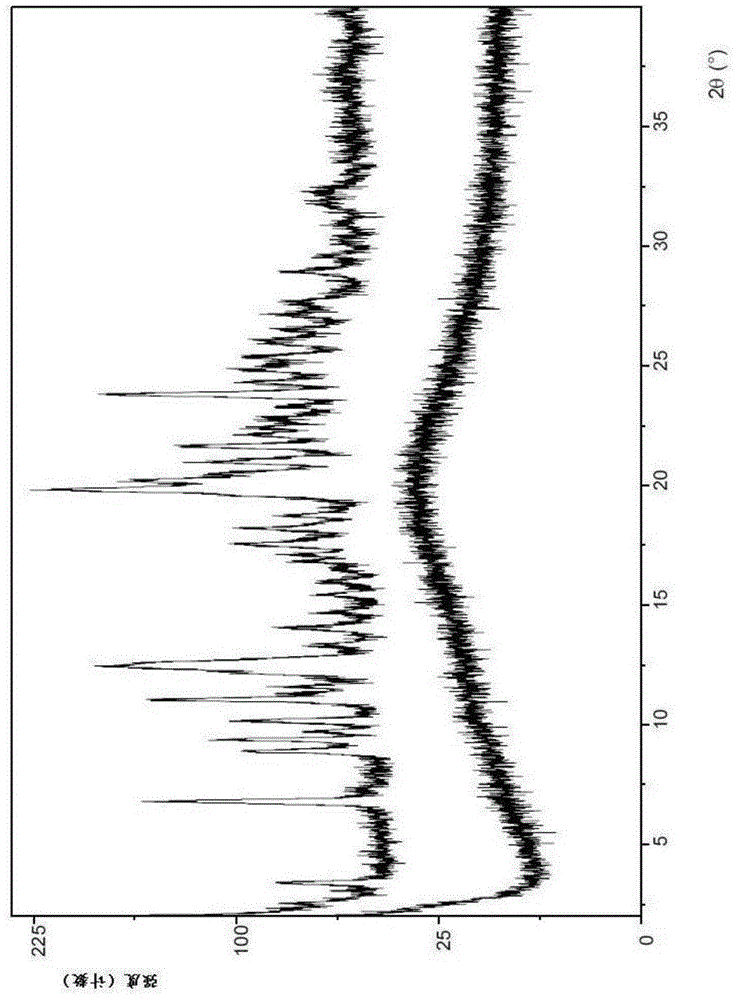
[0180] Example 1: (1-{3-[6-(9,9-difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl)-5-nitrogen Hetero-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzimidazol-2-yl]-2-aza-bicyclo[ 2.2.1] Preparation of heptane-2-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester diacetone solvate (Compound I Form I).
[0181] Weigh approximately 15-60 mg of amorphous (1-{3-[6-(9,9-difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl) )-5-aza-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzimidazol-2-yl]-2- Aza-bicyclo[2.2.1]heptane-2-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester (99.3% HPLC purity) and transferred to a vial equipped with a mini magnetic stir bar . Acetone was added in 200 μL increments, resulting in the formation of a slurry. The slurry was stirred at room temperature (~22°C) for two weeks and checked periodically.
[0182]Two weeks later, a 300 μL sample of the slurry was removed from the vial, transferred to a centrif...
Embodiment 2
[0189] Example 2: (1-{3-[6-(9,9-difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl)-5-nitrogen Hetero-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzimidazol-2-yl]-2-aza-bicyclo[ 2.2.1] Preparation of heptane-2-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester monoacetone solvate (compound I type II).
[0190] Compound I Form II was prepared by partial desolvation of Form I as described more fully below. Methods of desolvation are well known to those skilled in the art. These include, for example, the application of vacuum, prolonged exposure to ambient conditions, subjecting Form I to elevated temperatures, and subjecting Form I to a gas stream, such as air or nitrogen, and any combination thereof. In some embodiments, the preparation of Compound 1 Form I described in Example 1 above results in the formation of a detectable amount of Compound 1 Form II. Thus, in these embodiments, a mixture of Form I and Form II can be prepared.
[0191] As an e...
Embodiment 3
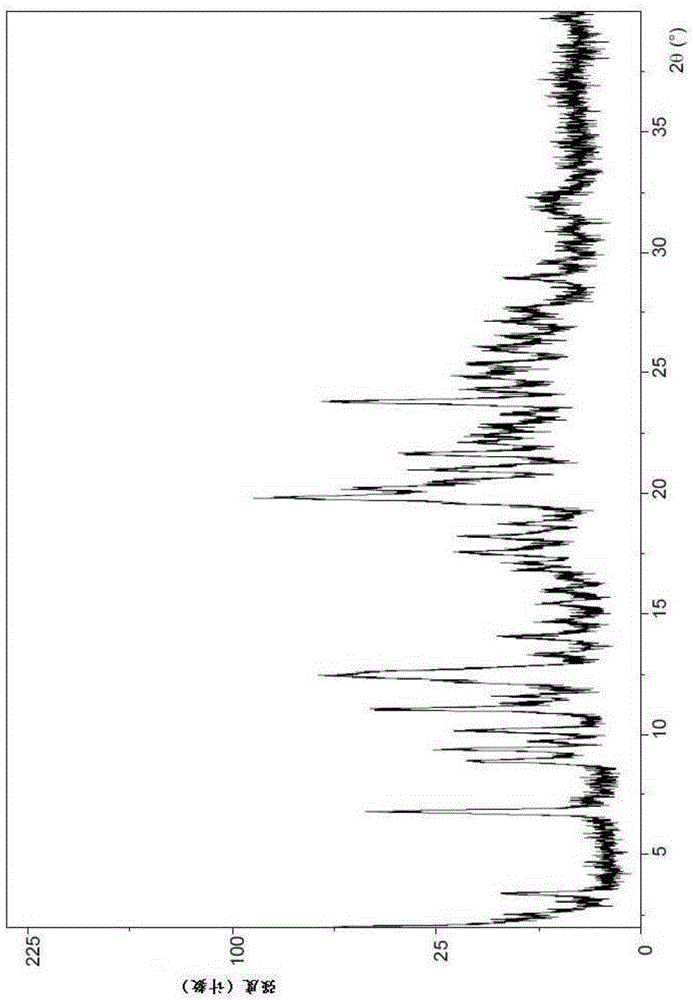
[0197] Example 3: (1-{3-[6-(9,9-difluoro-7-{2-[5-(2-methoxycarbonylamino-3-methyl-butyryl)-5-nitrogen Hetero-spiro[2.4]hept-6-yl]-3H-imidazol-4-yl}-9H-fluoren-2-yl)-1H-benzimidazol-2-yl]-2-aza-bicyclo[ 2.2.1] Preparation of heptane-2-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester (compound I type III).
[0198] Although Form II confers surprising advantages over amorphous compound I, it still has a significant content of tightly bound acetone in its structure. The purpose of this example was to break the solvate and produce the anhydrous crystalline form.
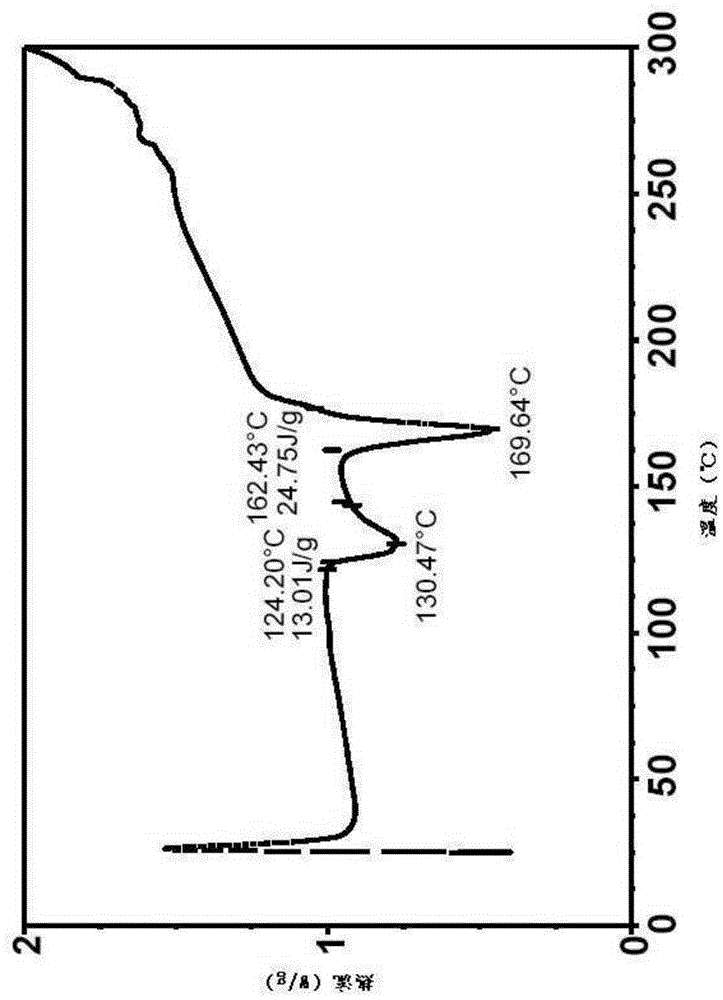
[0199] Therefore, the Form II crystals were heated to complete dryness or desolvated at -115°C, which led to the discovery of a third crystal form, Form III.
[0200] Form III was revealed by variable temperature XRPD experiments. In this experiment, samples of Form II were heated at 10°C / min starting at 25°C. After each 10°C temperature increase, the samples were held at the elevated temperature for 20 min to al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com