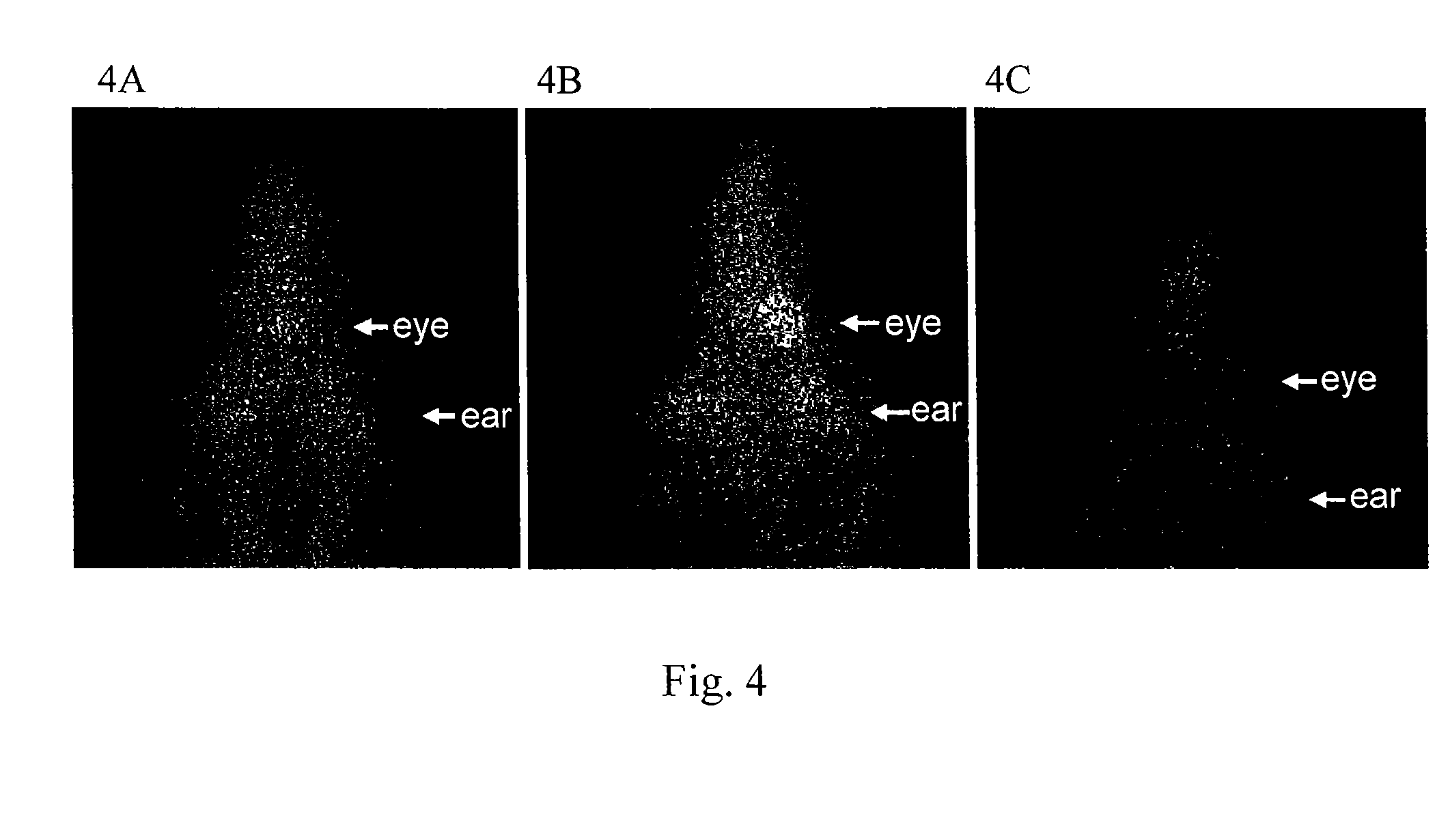
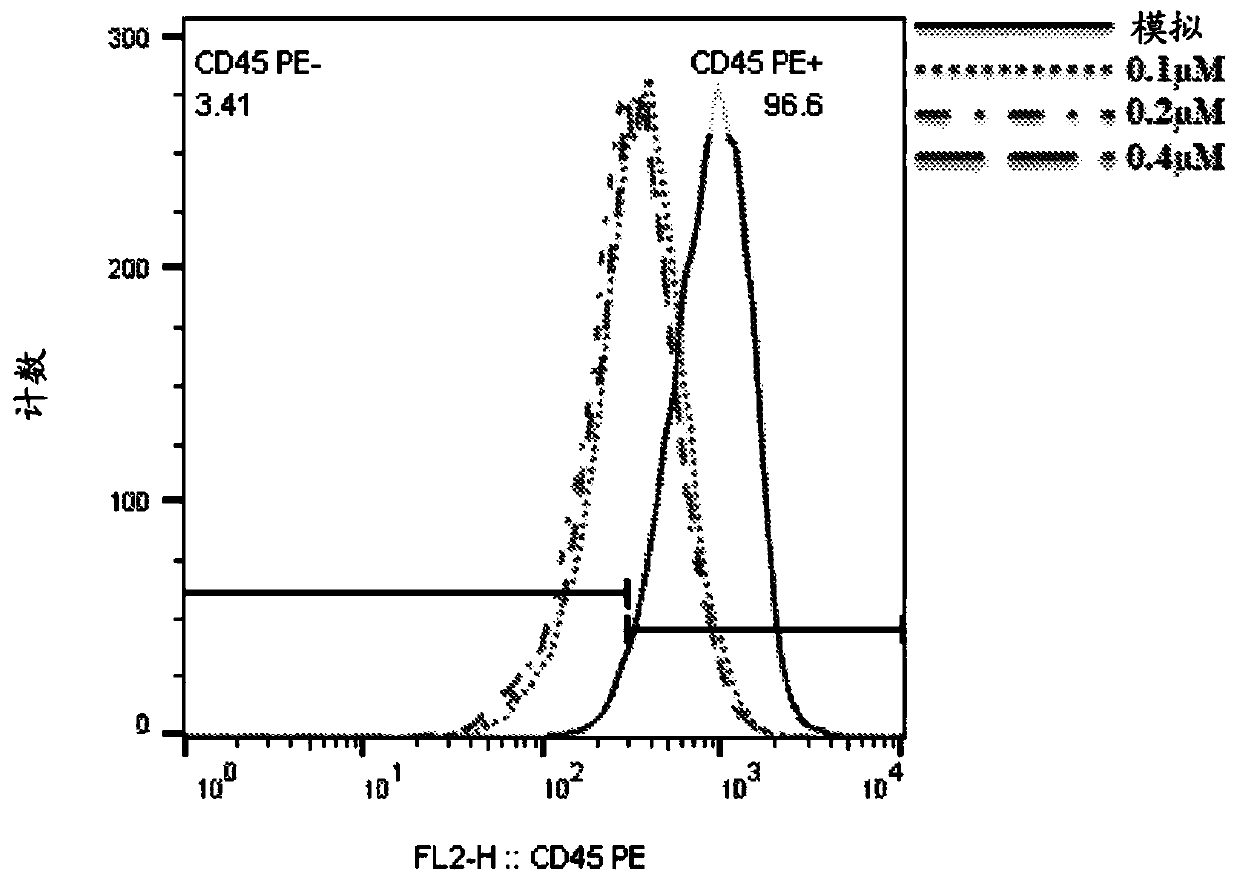
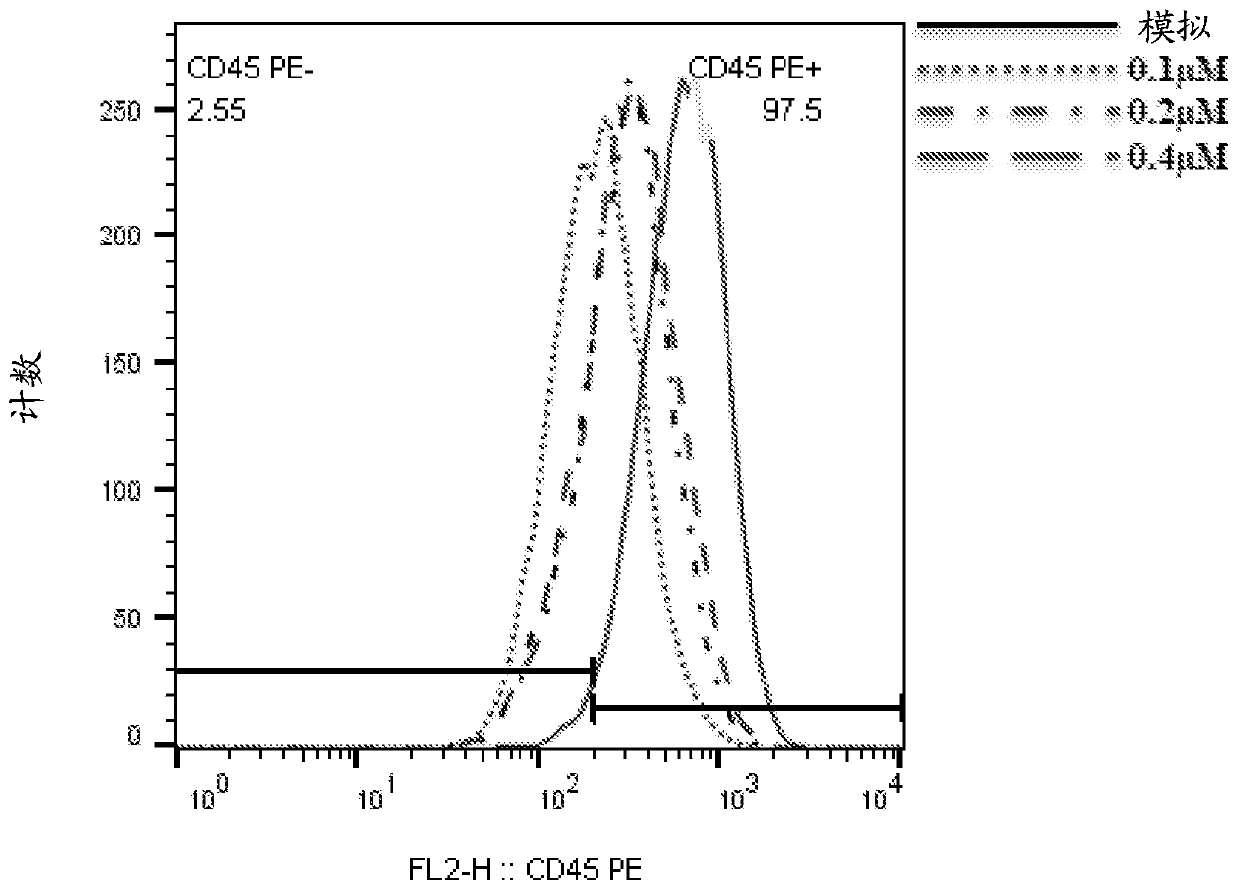
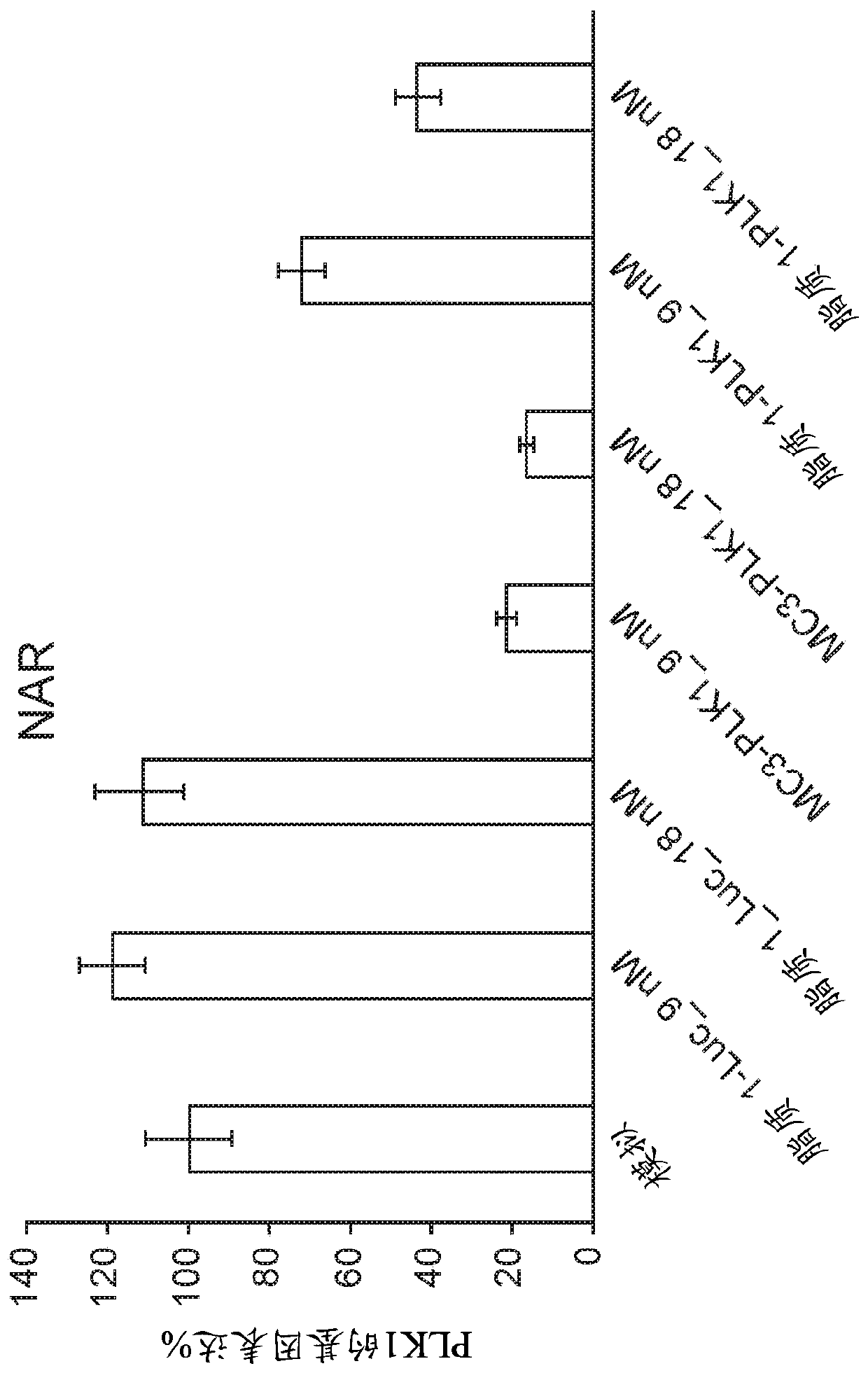
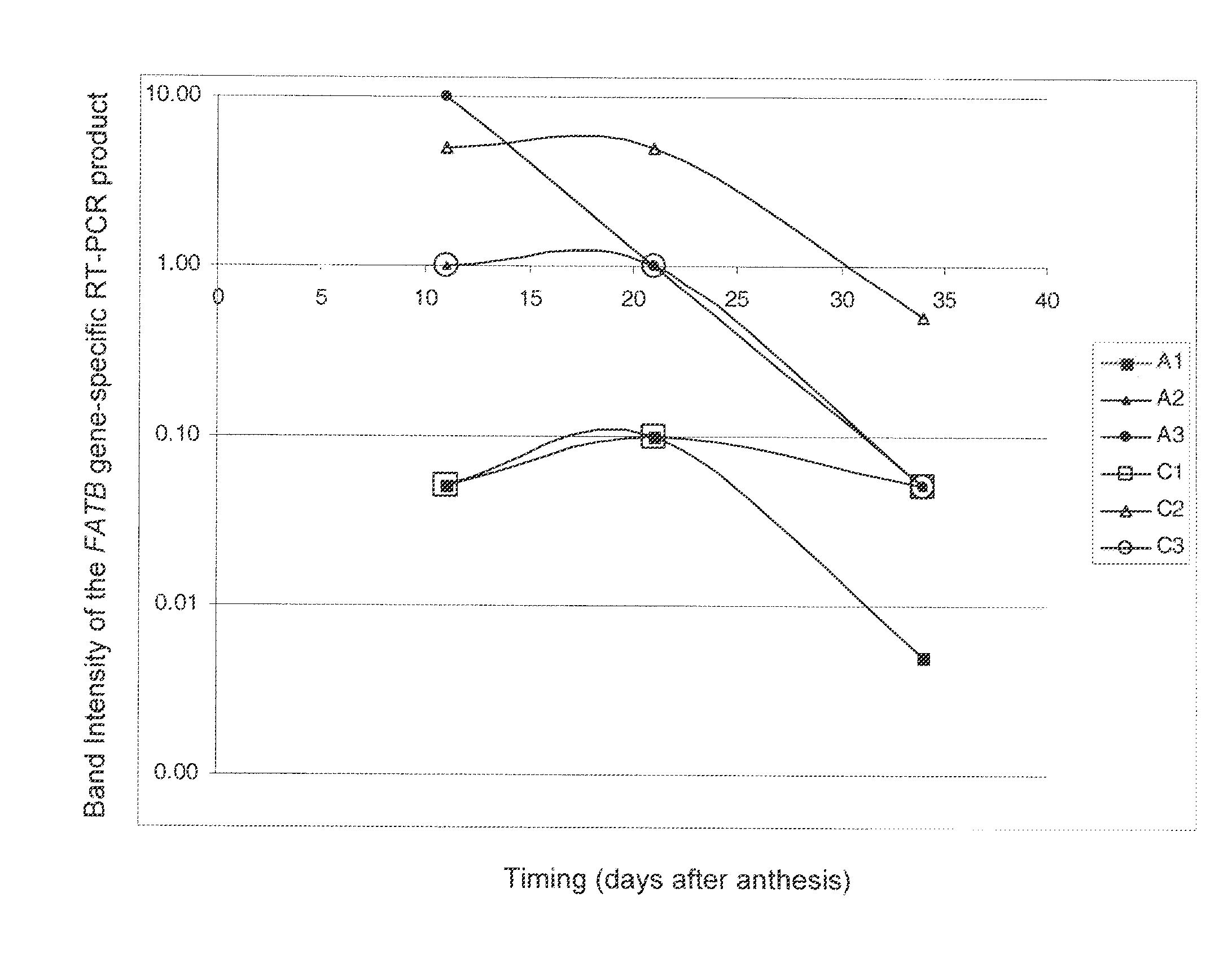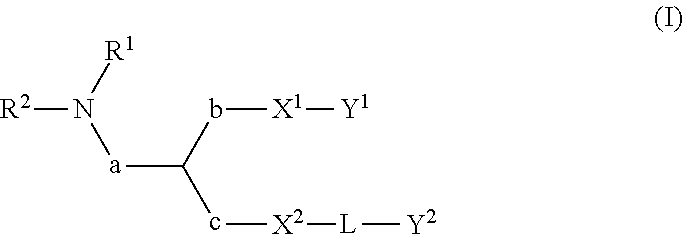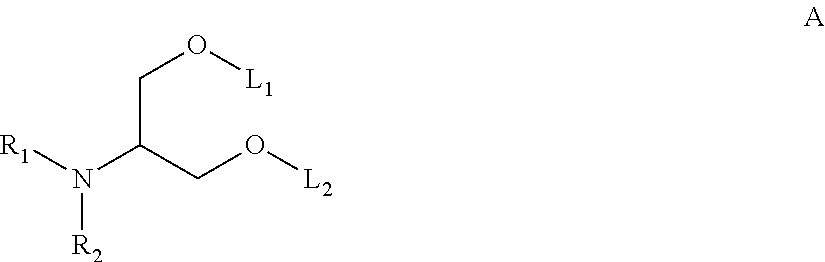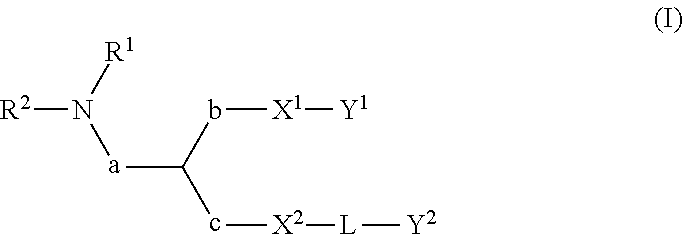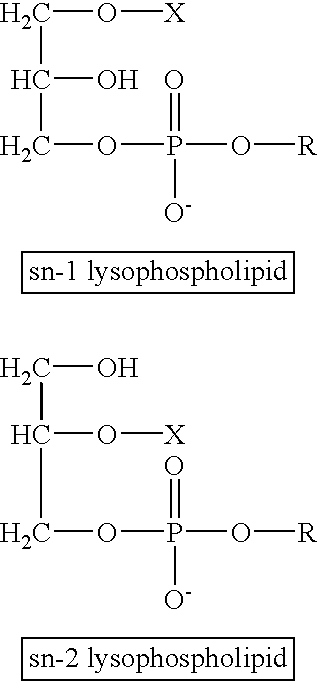Patents
Literature
266 results about "Lipidome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
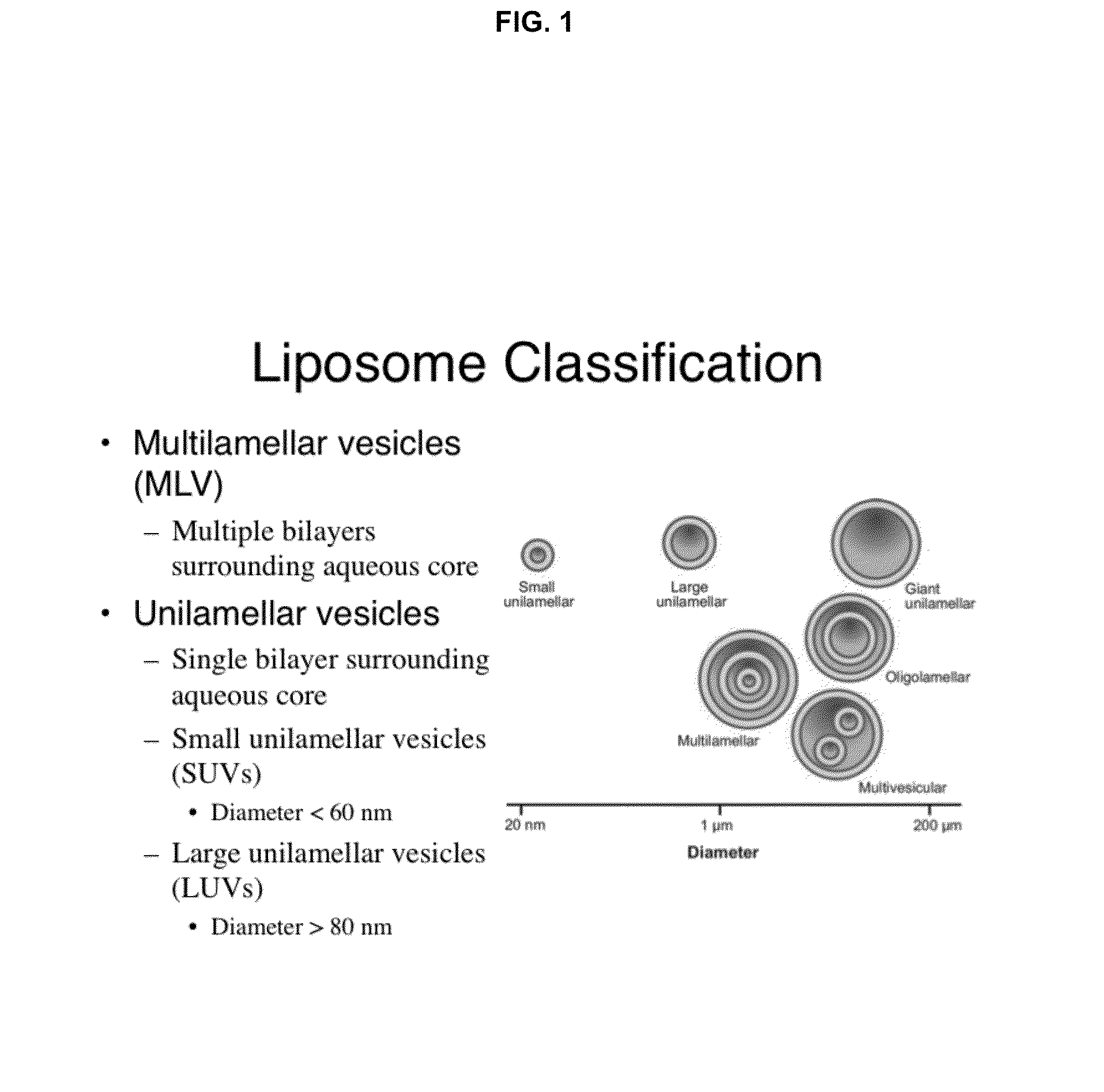
The lipidome refers to the totality of lipids in cells. Lipids are one of the four major molecular components of biological organisms, along with proteins, sugars and nucleic acids. Lipidome is a term coined in the context of omics in modern biology, within the field of lipidomics. It can be studied using mass spectrometry and bioinformatics as well as traditional lab-based methods. The lipidome of a cell can be subdivided into the membrane-lipidome and mediator-lipidome.
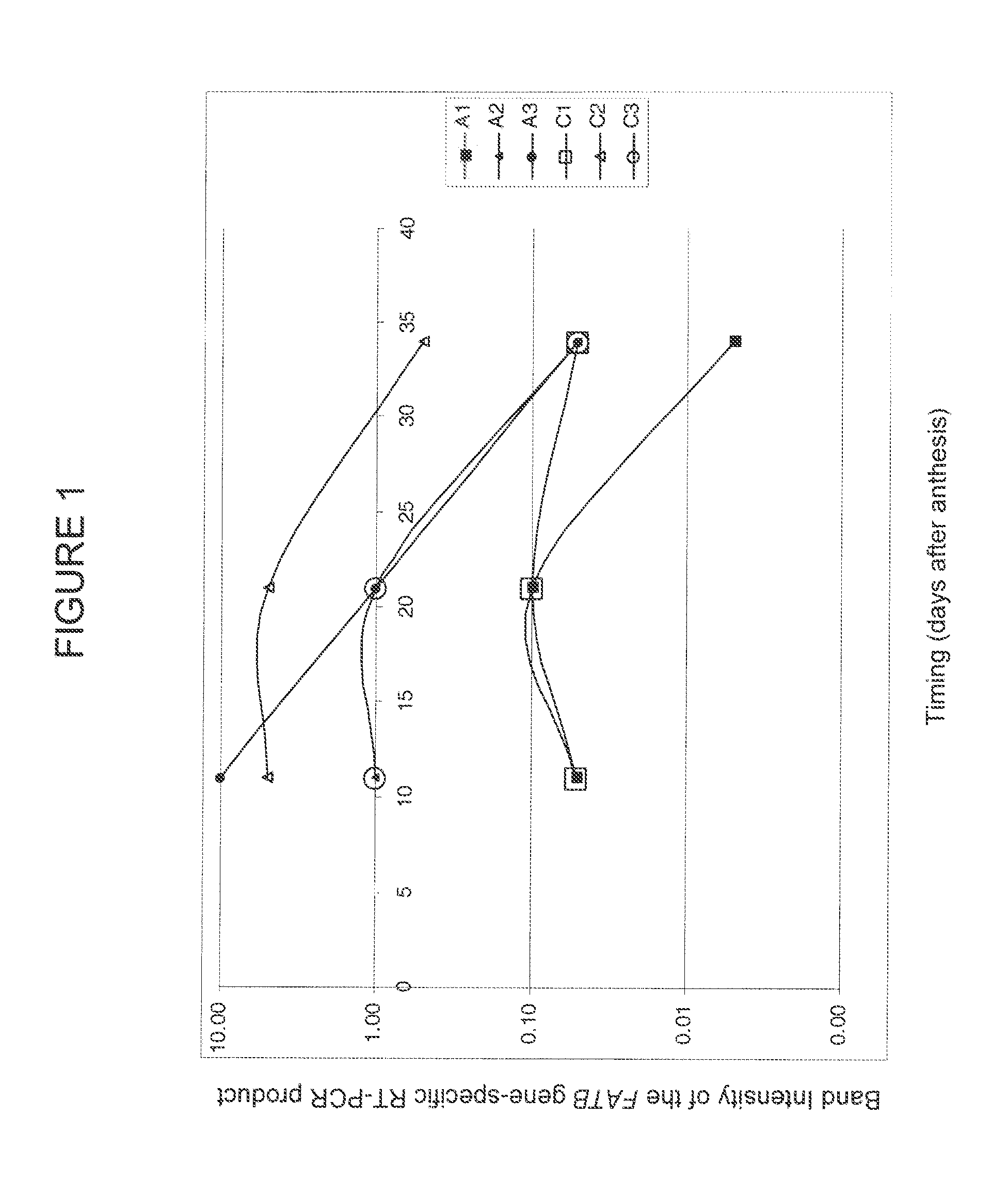
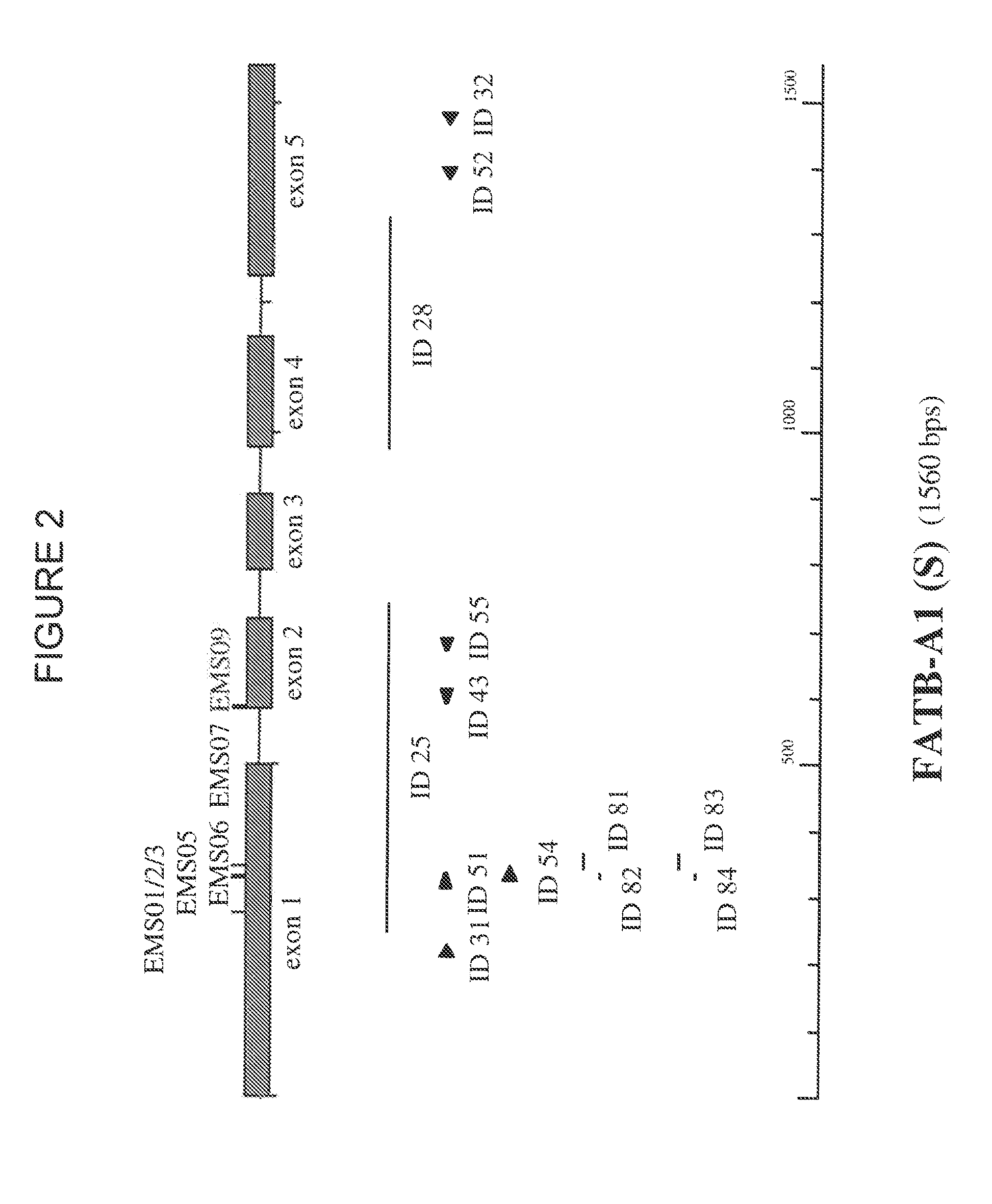
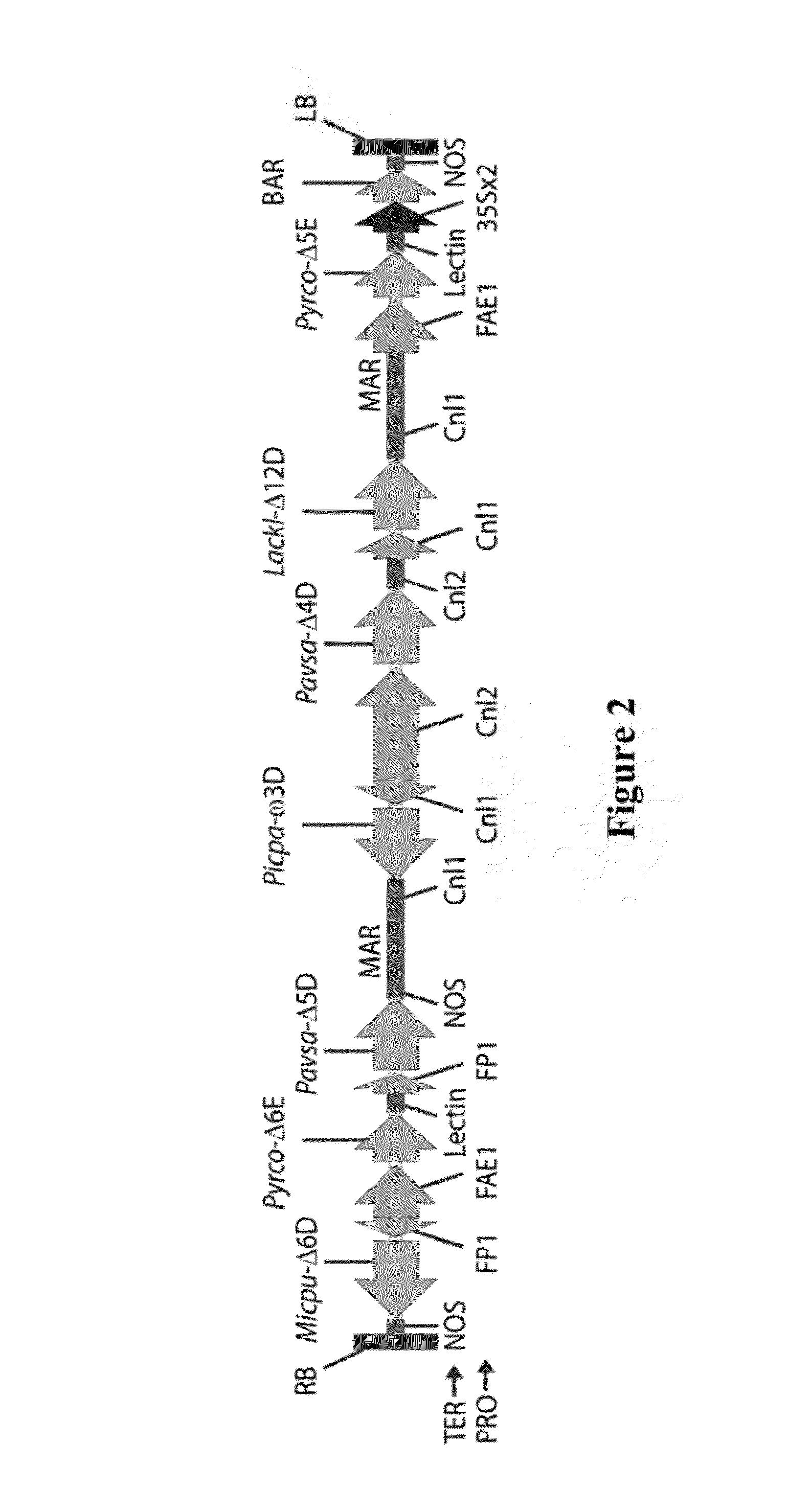
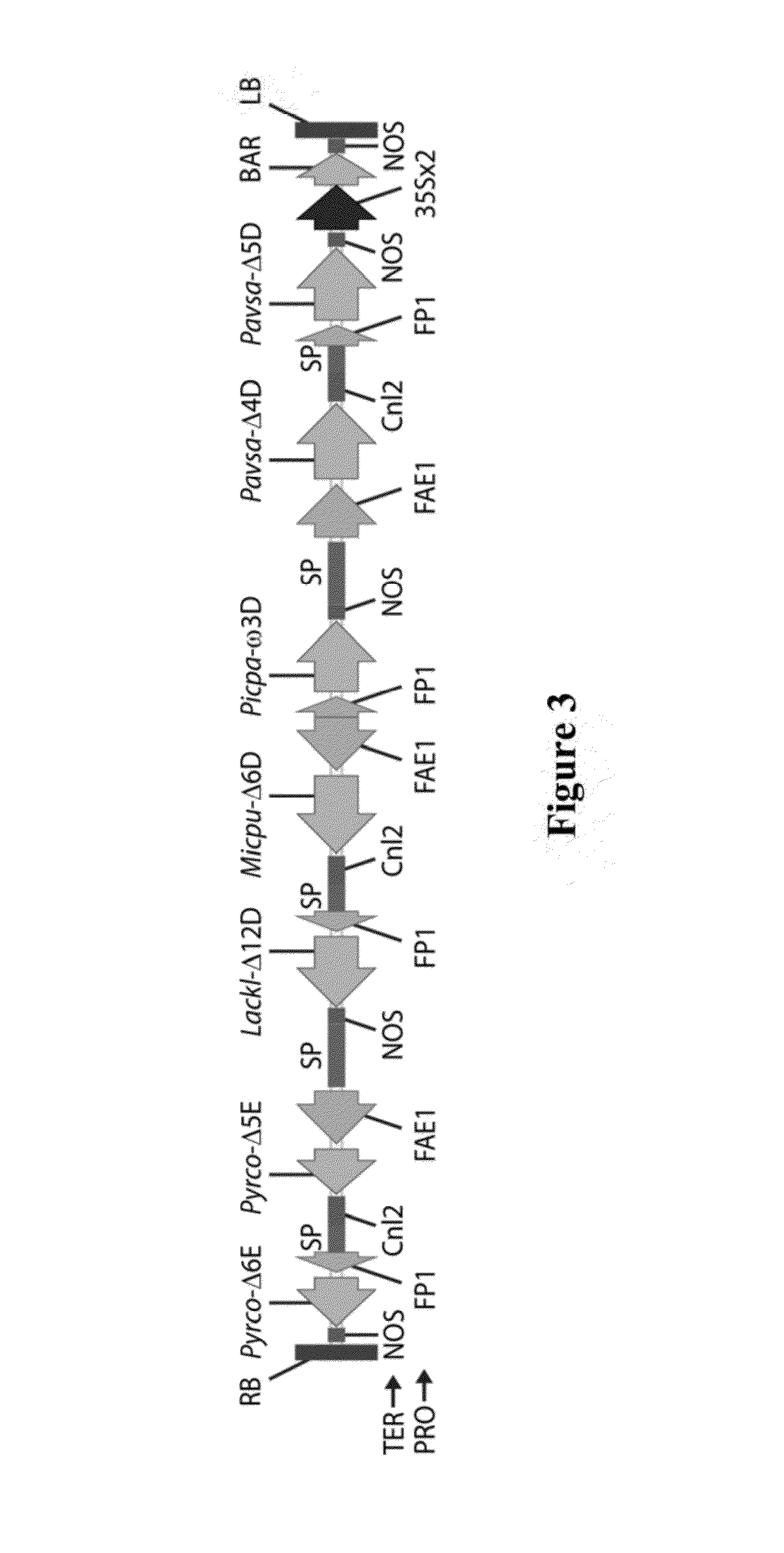
Brassica plant comprising mutant fatty acyl-acp thioesterase alleles
ActiveUS20110145944A1Reduce the amount requiredMinimal level of functional FATB proteinHydrolasesImmunoglobulinsAcyl carrier proteinWild type
The invention relates to crop plants comprising novel seed lipid compositions. Provided are both wild type and mutant nucleic acid molecules encoding Brassica fatty acyl-acyl carrier protein (ACP) thioesterase B proteins (FATB) and the proteins as such. Also provided are Brassica plants, tissue and seeds comprising at least three mutant fatB alleles in their genome, whereby the seed oil fatty acid composition or profile is significantly altered.
Owner:BAYER CROPSCIENCE NV
Lipids, lipid compositions, and methods of using them
InactiveUS20110200582A1Reducing and inhibiting and ameliorating diseaseLower Level RequirementsBiocideOrganic active ingredientsLipidomeActive agent
Owner:NOVARTIS AG
Nutritional supplement containing long-chain polyunsaturated fatty acids
InactiveUS20070166411A1Raise the ratioPrevent health consequenceBiocidePeptide/protein ingredientsNutrition supplementationΩ-3 fatty acids
The present invention relates to a nutritional supplement for administration to children. The supplement comprises a protein component, a carbohydrate component, and a fat or lipid component which further comprises a source of DHA. The supplement has an ω-6:ω3 fatty acid ratio of about 6:1 or less. The present invention also relates to a method of providing nutrition to a pediatric subject comprising administering to the subject a nutritional supplement comprising a protein component; a carbohydrate component; and a fat or lipid component, which further comprises a source of DHA, wherein the composition has an ω-6:ω-3 fatty acid ratio of about 6:1 or less.
Owner:MEAD JOHNSON NUTRITION
Low molecular weight cationic lipids for oligonucleotide delivery
ActiveUS8748667B2Good curative effectReduce liver toxicityOrganic compound preparationOther foreign material introduction processesTolerabilityNanoparticle
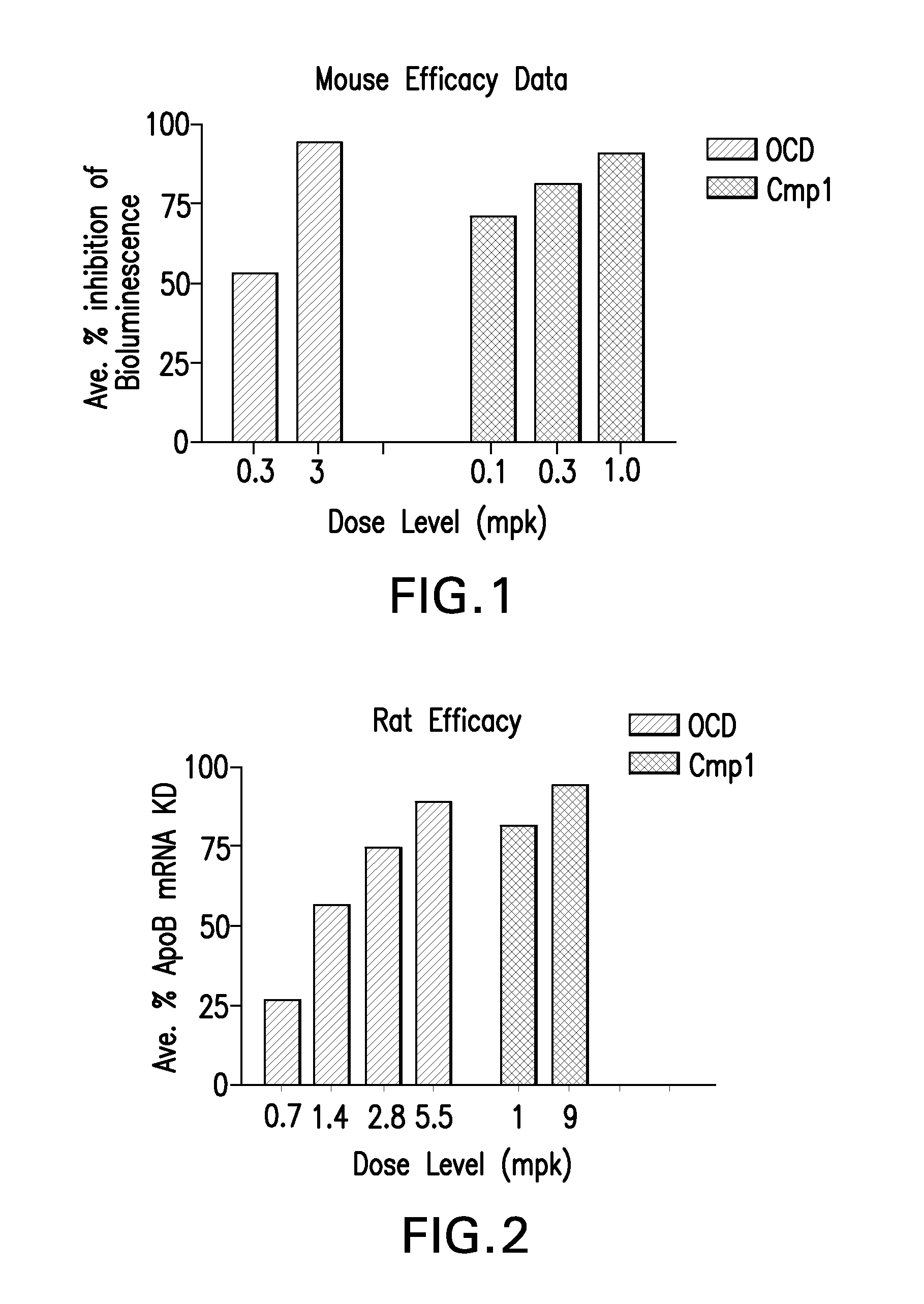
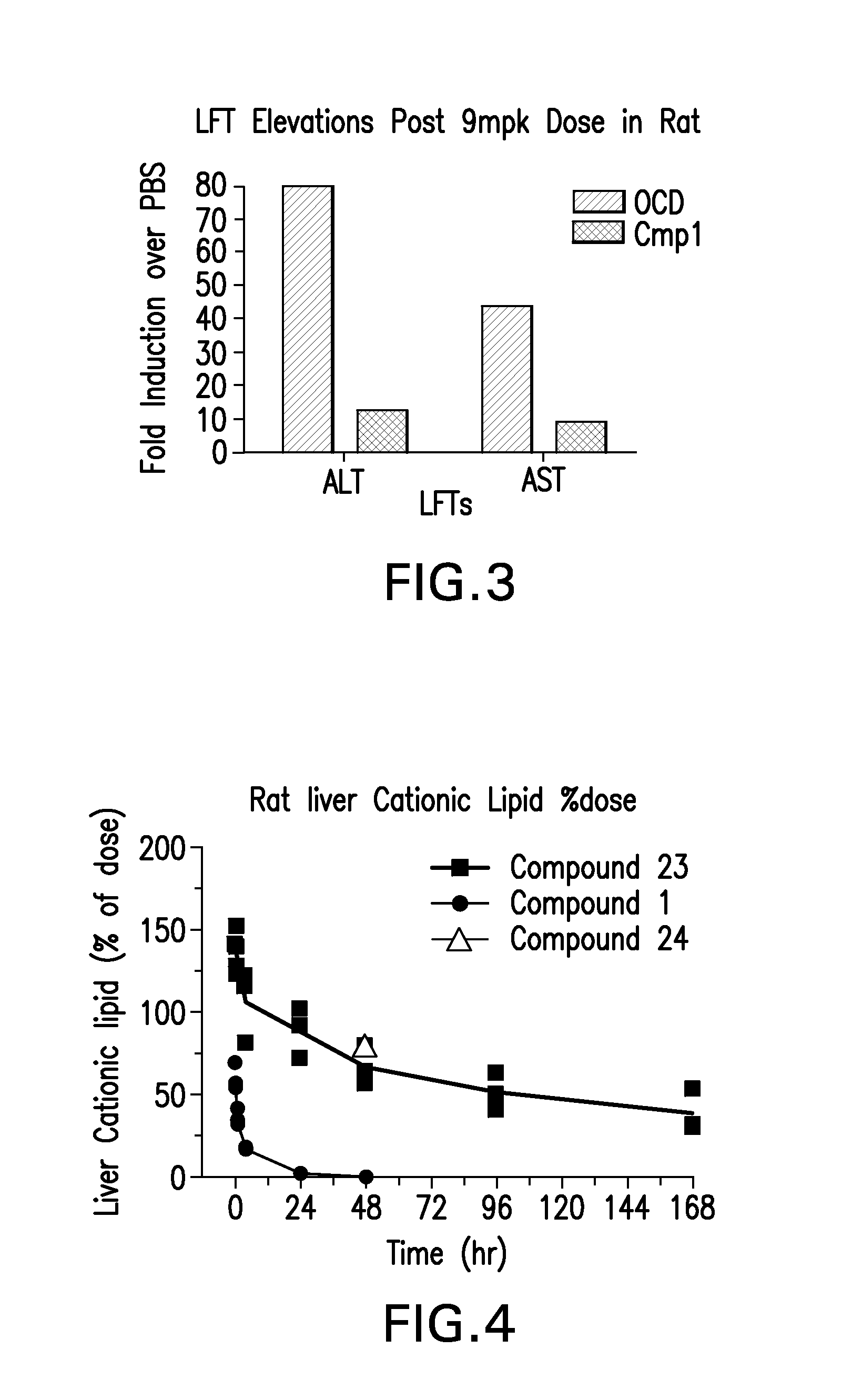
The instant invention provides for novel cationic lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. It is an object of the instant invention to provide a cationic lipid scaffold that demonstrates enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids with one short lipid chain to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
Lipids and Lipid Compositions for the Delivery of Active Agents
ActiveUS20160317458A1Good curative effectLow toxicitySsRNA viruses negative-senseOrganic active ingredientsLipidomeActive agent
Owner:NOVARTIS AG
Systems for treating pulmonary infections
Provided herein are systems for treating a subject with a pulmonary infection, for example, a nontuberculous mycobacterial pulmonary infection, a Burkholderia pulmonary infection, a pulmonary infection associated with bronchiectasis, or a Pseudomonas pulmonary infection. The system includes a pharmaceutical formulation comprising a liposomal aminoglycoside dispersion, and the lipid component of the liposomes consist essentially of electrically neutral lipids. The system also includes a nebulizer which generates an aerosol of the pharmaceutical formulation at a rate greater than about 0.53 gram per minute. The aerosol is delivered to the subject via inhalation for the treatment of the pulmonary infection.
Owner:INSMED INC
Composition for feeding prey organisms in aquaculture
InactiveUS7063855B2Increase concentrationHigh in phospholipidsBiocideAnthropod material medical ingredientsPhospholipidOrganism
A composition is for feeding aquacultural prey organisms such as Artemia and rotifiers, comprising a lipid component comprising at least 25 wt % of phospholipids and providing a DHA content of at least 30 wt %. The lipid component is preferably derived from marine organisms such as fishmeal, phytoplankton or zoo plankton biomass. The composition is used for providing prey organisms having a high content of highly-unsaturated fatty acids (HUFAs) suitable for aquaculture of fish including halibut, turbot, bass and crustaceans and molluses.
Owner:EPAX NORWAY
Lipids, lipid compositions, and methods of using them
ActiveUS20140309277A1Inhibiting and reducingLower Level RequirementsOrganic active ingredientsBiocideLipidomeActive agent
Owner:NOVARTIS AG
Long-wearing cosmetic compositions with improved shine
A cosmetic composition comprising (A) a first structured and adhesive film-forming fatty phase comprising (i) one or more lipidic components that are mutually compatible with one another when liquefied; (ii) at least one adhesive film-forming agent; and (iii) at least one lipophilic structuring agent in an amount effective to retain a second fatty phase entrapped in said cosmetic composition prior to application on mammalian keratinous tissue; and (B) a second fatty phase comprising at least one non-volatile lipidic component wherein said second fatty phase (B) is incompatible with said first structured and adhesive film-forming fatty phase (A) and said second fatty phase (B) is entrapped in said composition where upon application of said composition to mammalian keratinous tissue, said second fatty phase (B) readily separates from said composition to form a barrier layer over said first structured and adhesive film-forming fatty phase (A) and wherein said composition is substantially free of surfactants such that separation of said second fatty phase (B) from said composition is not prevented following application of said composition.
Owner:NOXELL CORP
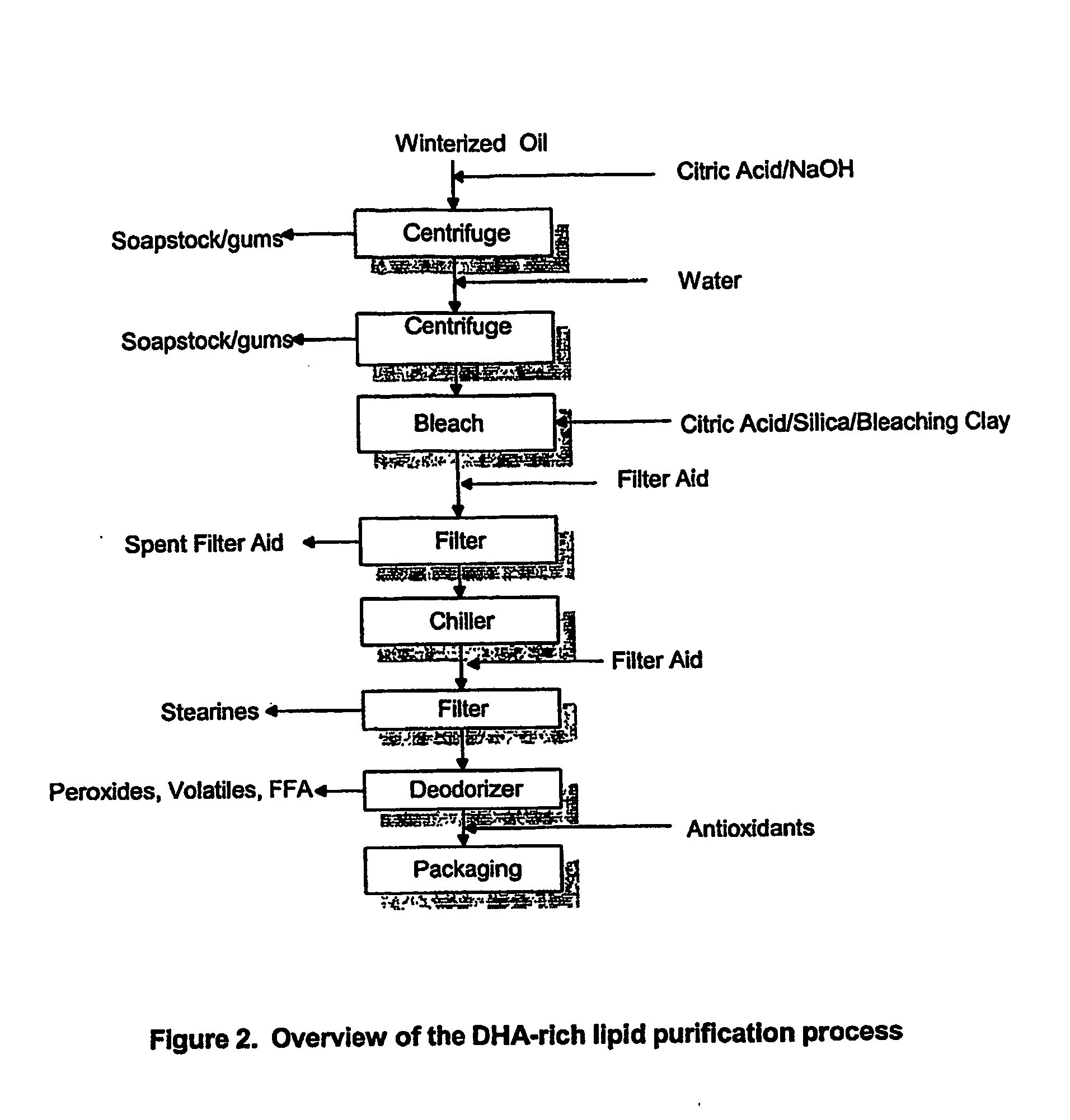
Extraction and winterization of lipids from oilseed and microbial sources
ActiveUS20050115897A1Solve many processesReduce degummingFatty-oils/fats refiningMicroorganism based processesLipid formationMicroorganism
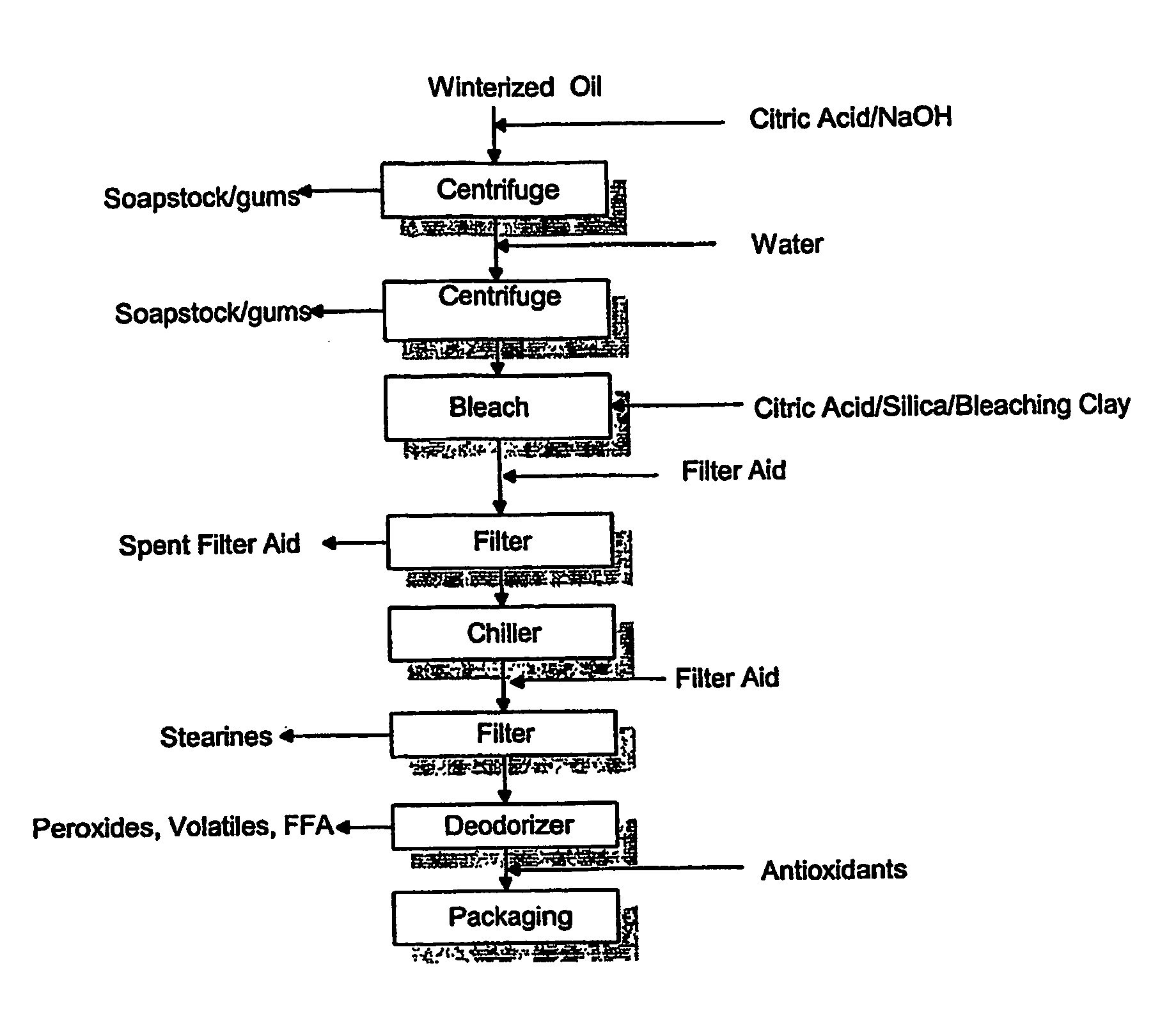
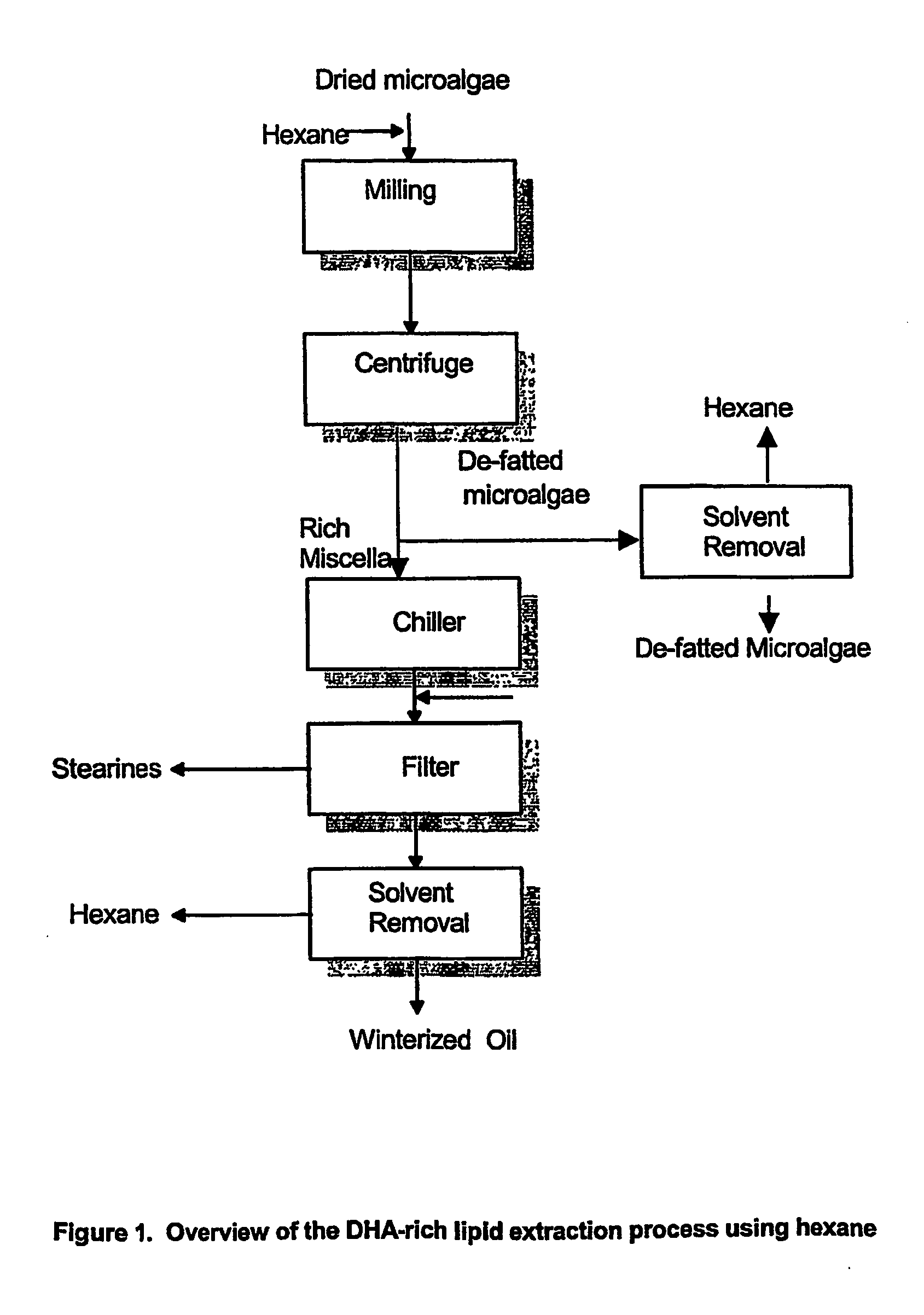
A process for purifying a lipid composition having predominantly neutral lipid components having at least one long chain polyunsaturated fatty acid is disclosed. The process employs contacting the lipid composition with a polar solvent, such as acetone, wherein the solvent is selected such that the contaminants are less soluble in the solvent than in the long chain polyunsaturated fatty acid. The process is typically conducted at cooler temperatures including about 0° C. Upon precipitation of the contaminants from the lipid composition, a separation is conducted to remove the precipitated material from the lipid composition. The long chain polyunsaturated fatty acids can include ARA, DPA, EPA and / or DHA. The process effectively winterizes lipid compositions, thereby reducing the tendency of such compositions to become hazy.
Owner:DSM IP ASSETS BV
Aqueous compositions comprising a lipid and a lanolin-derived surfactant, and their use
InactiveUS6224853B1High viscosityReduce dragCosmetic preparationsHair cosmeticsLipid formationLANOLIN DERIVATIVES
An aqueous composition comprising, in addition to water: (a) one or more surfactant materials selected from polyoxyalkylene condensate derivatives of lanolin or a lanolin derivative; and (b) a lipid component comprising one or more lipid materials, especially lanolin or a lanolin derivative, present as particles emulsified by the said one or more lanolin-derived surfactant materials and having a median particle size of less than about 5 mum, especially from 0.01 to 1 mum. The compositions have very good physical stability and are particularly useful as carriers for transdermal delivery of pharmaceutical actives to the human skin.
Owner:CRODA CHEM INT
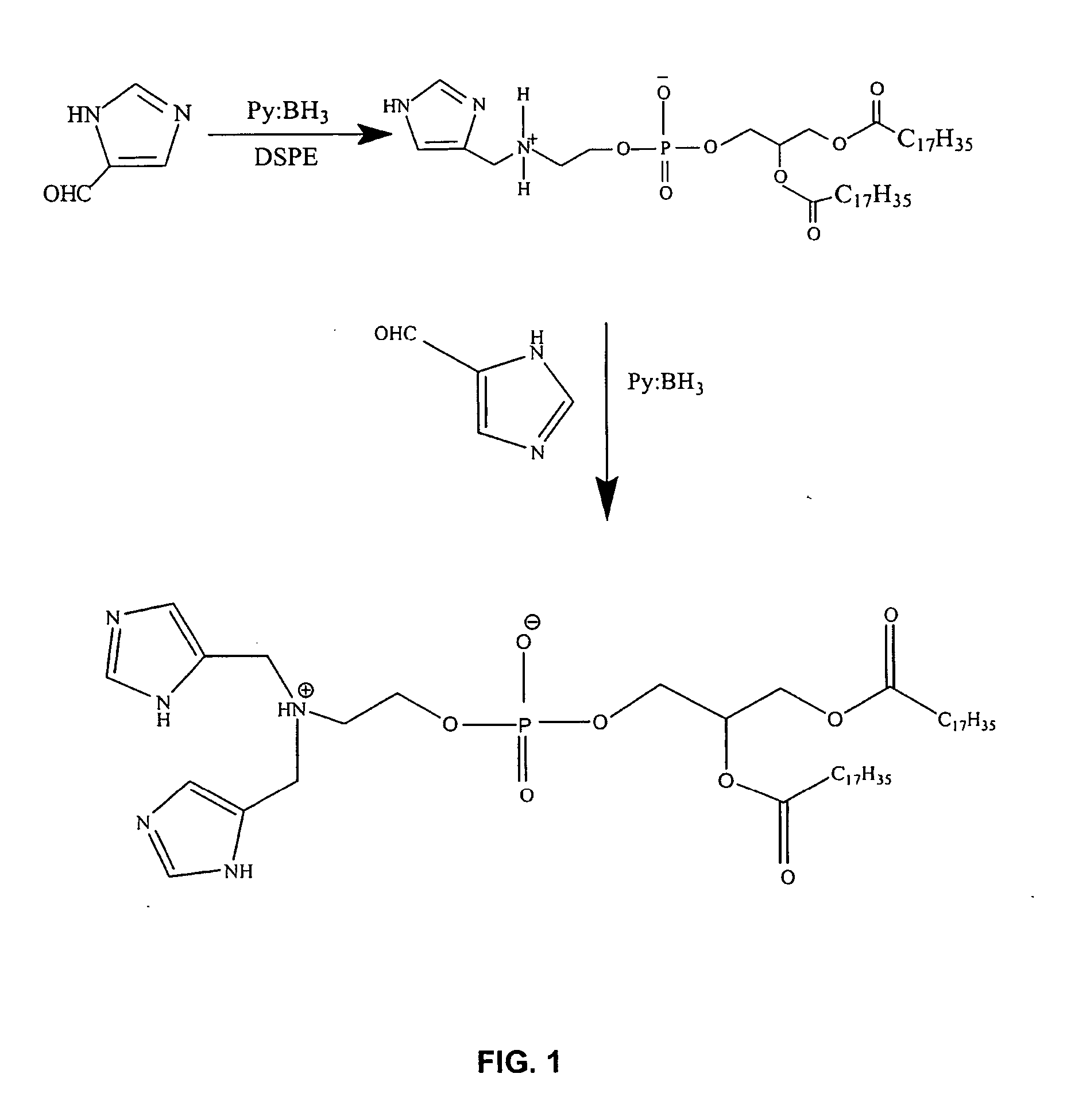
Gene delivery mediated by liposome-DNA complex with cleavable PEG surface modification
A liposome composition and method for delivery of a nucleic acid in vivo or ex vivo is described. The liposomes in the composition are comprised of (i) a cationic lipid and (ii) a lipid joined to a hydrophilic polymer by a releasable linkage. The liposomes are associated with a nucleic acid for delivery to a cell.
Owner:ALZA CORP
Lipids and lipid assemblies comprising transfection enhancer elements
ActiveUS20080306153A1Promote absorptionImprove fusion effectAntibacterial agentsBiocideLipid formationLiposome
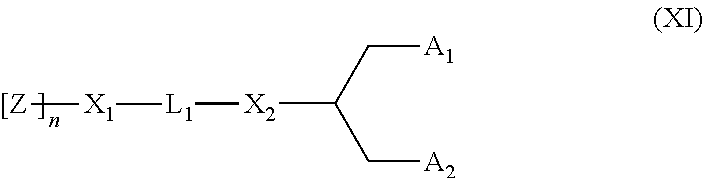
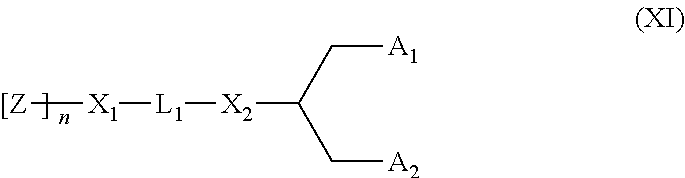
Lipid assemblies, such as liposomes, comprising transfection enhancer elements (TEE's), which are complexed with the lipid assemblies by means of ionic interactions, or lipids incorporating such TEE's are disclosed for enhancing the fusogenicity of the lipid assemblies. The TEE's have the formula:hydrophobic moiety-pH sensitive hydrophilic moiety (II)The pH sensitive hydrophilic moiety of each TEE is a weak acid having a pka of between 2 and 6 or a zwitterionic structure comprising a combination of acidic groups with weak bases having a pKa of between 3 and 8. Lipids incorporating one or more such TEEs have the formula (I):Lipid moiety-[Hydrophobic moiety-pH sensitive hydrophilic moiety] (I)
Owner:BIONTECH DELIVERY TECH GMBH
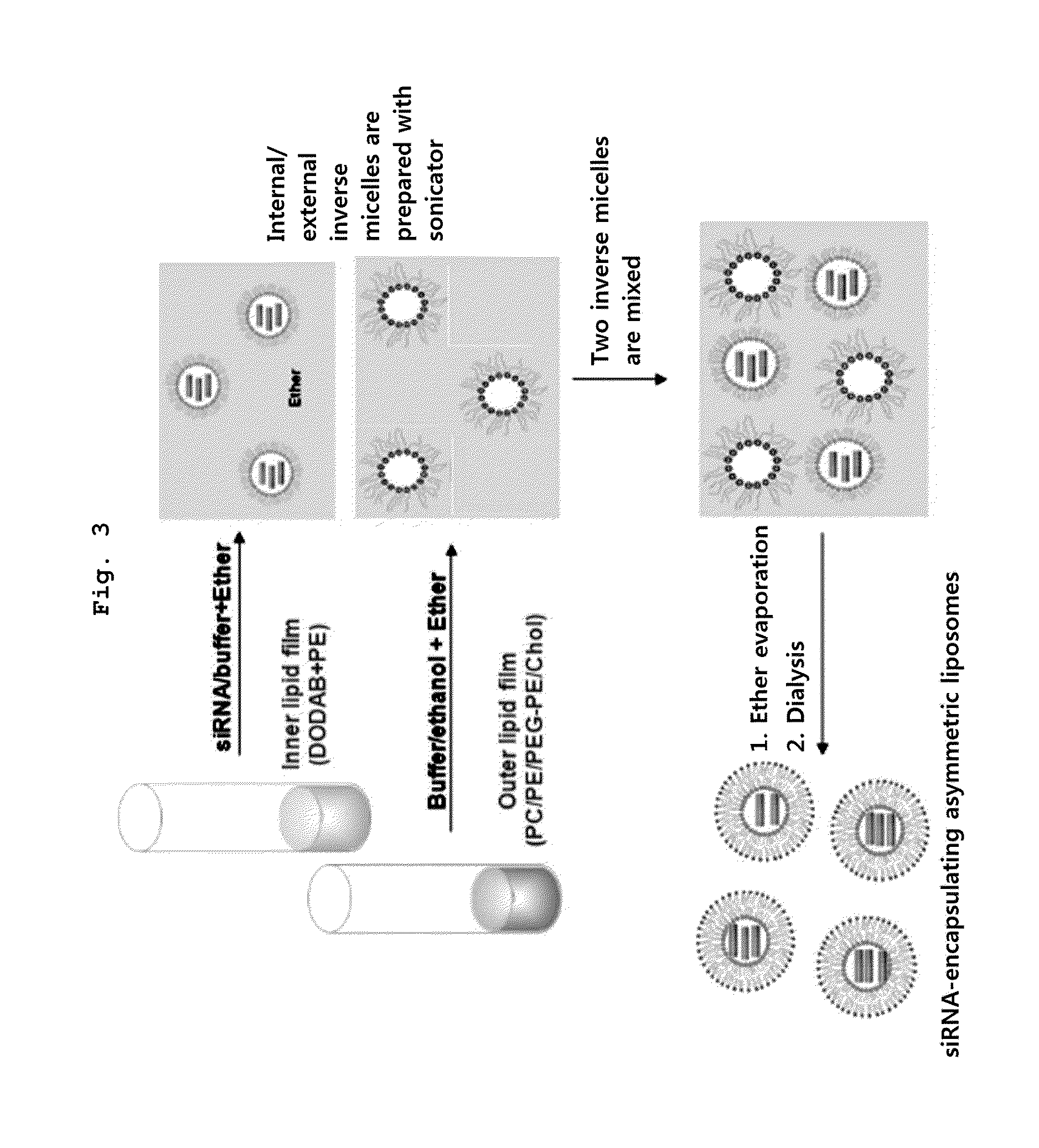
Asymmetric Liposomes for the Highly Efficient Encapsulation of Nucleic Acids and Hydrophilic Anionic Compounds, and Method for Preparing Same
InactiveUS20130149374A1Improve efficiencyCarbohydrate active ingredientsFermentationFluorescenceLiposome
The present invention relates to asymmetric liposomes for high encapsulation efficiency of nucleic acids and hydrophilic anionic compounds, and to a method for preparing same, and specifically, to asymmetric liposomes consisting of a cationic lipid having a small head group as an internal lipid and a neutral or PEGylated lipid having a big head group as an external lipid, wherein nucleic acids and / or anionic compounds are encapsulated in the internal lipid. According to the present invention, asymmetric liposomes, in which nucleic acids and hydrophilic anionic compounds are encapsulated with high efficiency, may be prepared, and thus the same may be used for various purposes, such as gene therapy, and the delivery of hydrophilic anionic drugs which are difficult to prepare as prodrugs, and drug delivery, imaging, etc. can be carried out by encapsulating a fluorescent contrast agent in the liposome.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
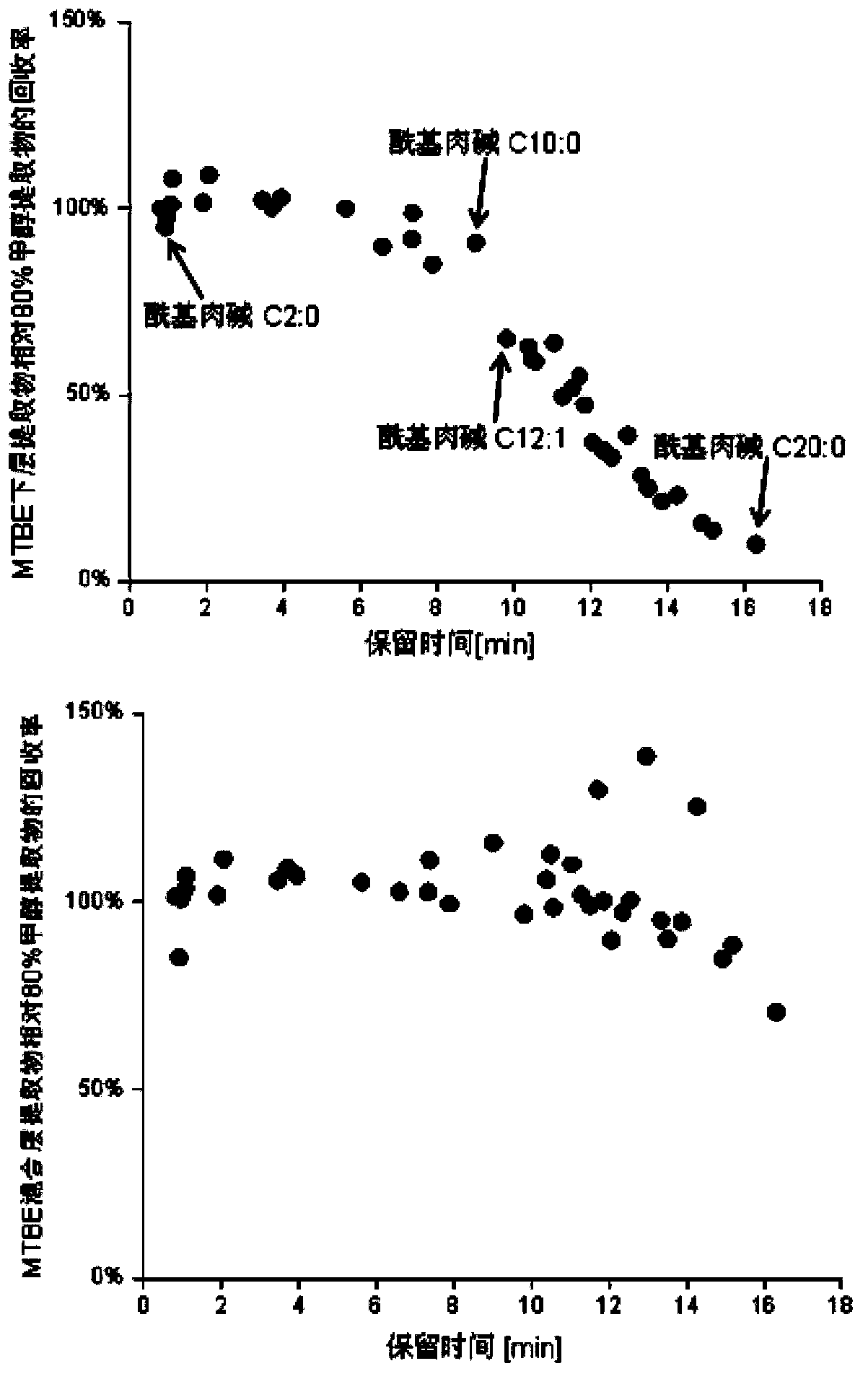
Method for simultaneous extraction and analysis of metabolite group and lipid group in microtissue
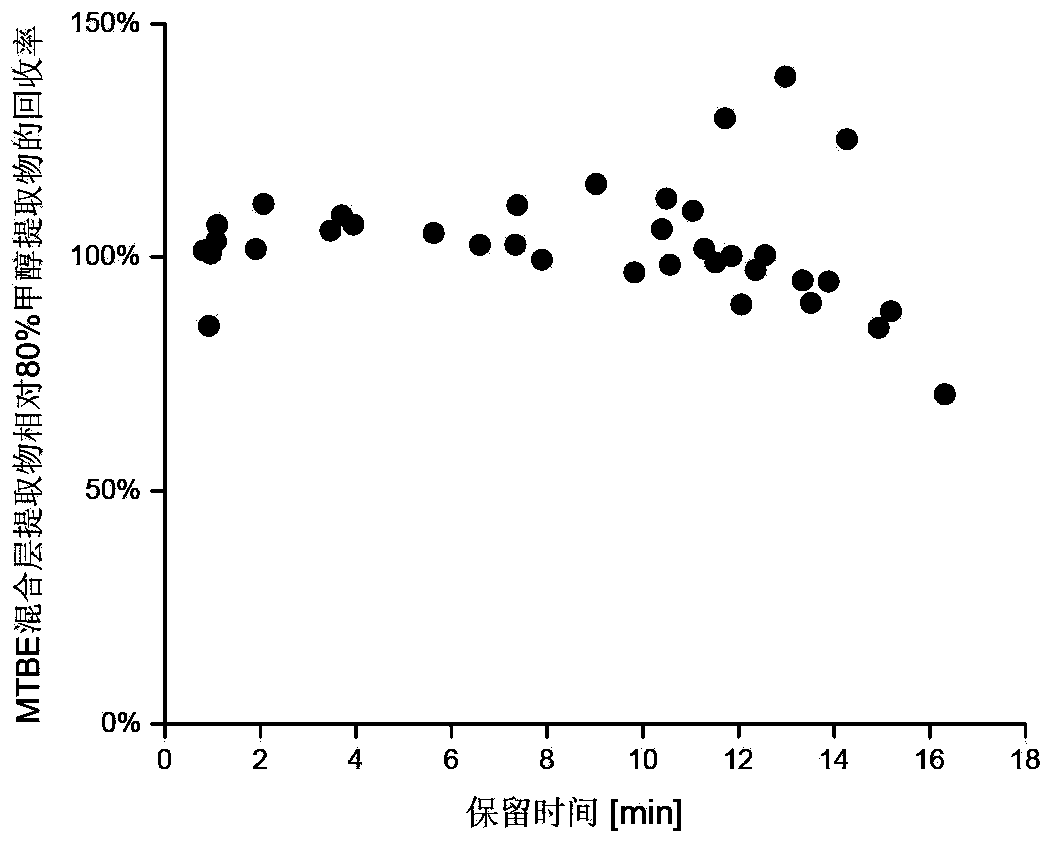
The invention discloses a method for simultaneous extraction and analysis of a metabolite group and a lipid group in microtissue. The method comprises the following steps: freeze drying to-be-analyzed microtissue, accurately weighing 1 to 25 mg of the freeze-dried microtissue and adding solvents like methanol (MeOH), methyl tert butyl ether (MTBE) and water in certain proportion for extraction; allowing a solution obtained after completion of extraction to be divided into two layers, wherein an upper layer mainly contains nonpolar metabolites and lipids, and the lower layer mainly comprises polar and medium-polar metabolites; and subjecting the upper-layer solution and the lower-layer solution to mixing in proportion and freeze-drying, then carrying out redissolving and then metabonomical analysis based on liquid chromatography-mass spectrometry, subjecting the upper-layer solution to freeze-drying and then to redissolving and carrying out lipidomical analysis based on liquid chromatography-mass spectrometry. The method has the following advantages: metabolites and lipids are extracted as many as possible through one extraction of a small amount of tissue, and through metabonomical and lipidomical analysis, the amount of a tissue sample is saved, which benefits other biochemical analysis of the tissue sample.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Liposome composition for delivery of therapeutic agents
InactiveUS20050191344A1Extended circulation timeProlong blood circulation timePharmaceutical non-active ingredientsNon-active genetic ingredientsLipid formationSolubility
A neutral cationic lipid and liposomes prepared from the neutral cationic lipid are described. Liposomes comprised of the lipid are suitable for delivery of a polyanionic compound, such as a nucleic acid. The delivery can be performed in vivo or ex vivo. The neutral cationic lipid, which is neutral in charge at physiologic pH and positively charged at pH values less than physiologic pH, contains a polar head group that imparts solubility of the lipid and permits its packing into a liposomal lipid bilayer.
Owner:ALZA CORP
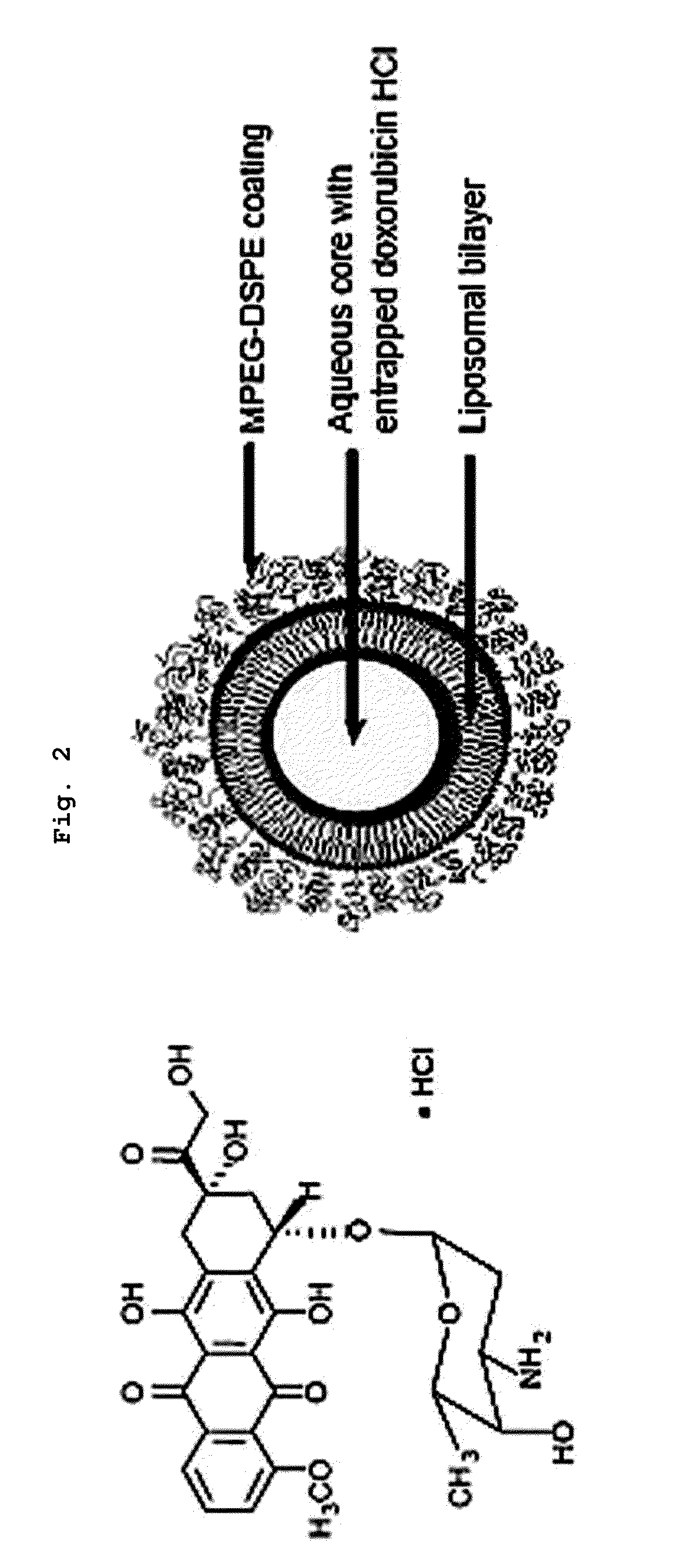
Liposomes with enhanced circulation time and method of treatment
A liposome composition for localizing an anti-tumor compound to a solid tumor via the bloodstream. The liposomes, which contain the agent in entrapped form, are composed of vesicle-forming lipids and between 1-20 mole percent of a vesicle-forming lipid derivatized with hydrophilic biocompatible polymer, and have sizes in a selected size range between 0.07 and 0.12 microns. After intravenous administration, the liposomes are taken up by the tumor within 24-48 hours, for site-specific release of entrapped compound into the tumor. In one composition for use in treating a solid tumor, the compound is an anthracycline antibiotic drug which is entrapped in the liposomes at a concentration of greater than about 50 mug agent / mumole liposome lipid. The method results in regression of solid colon and breast carcinomas which are refractory to anthracycline antibiotic drugs administered in free form or entrapped in conventional liposomes.
Owner:MARTIN FRANCIS J +3
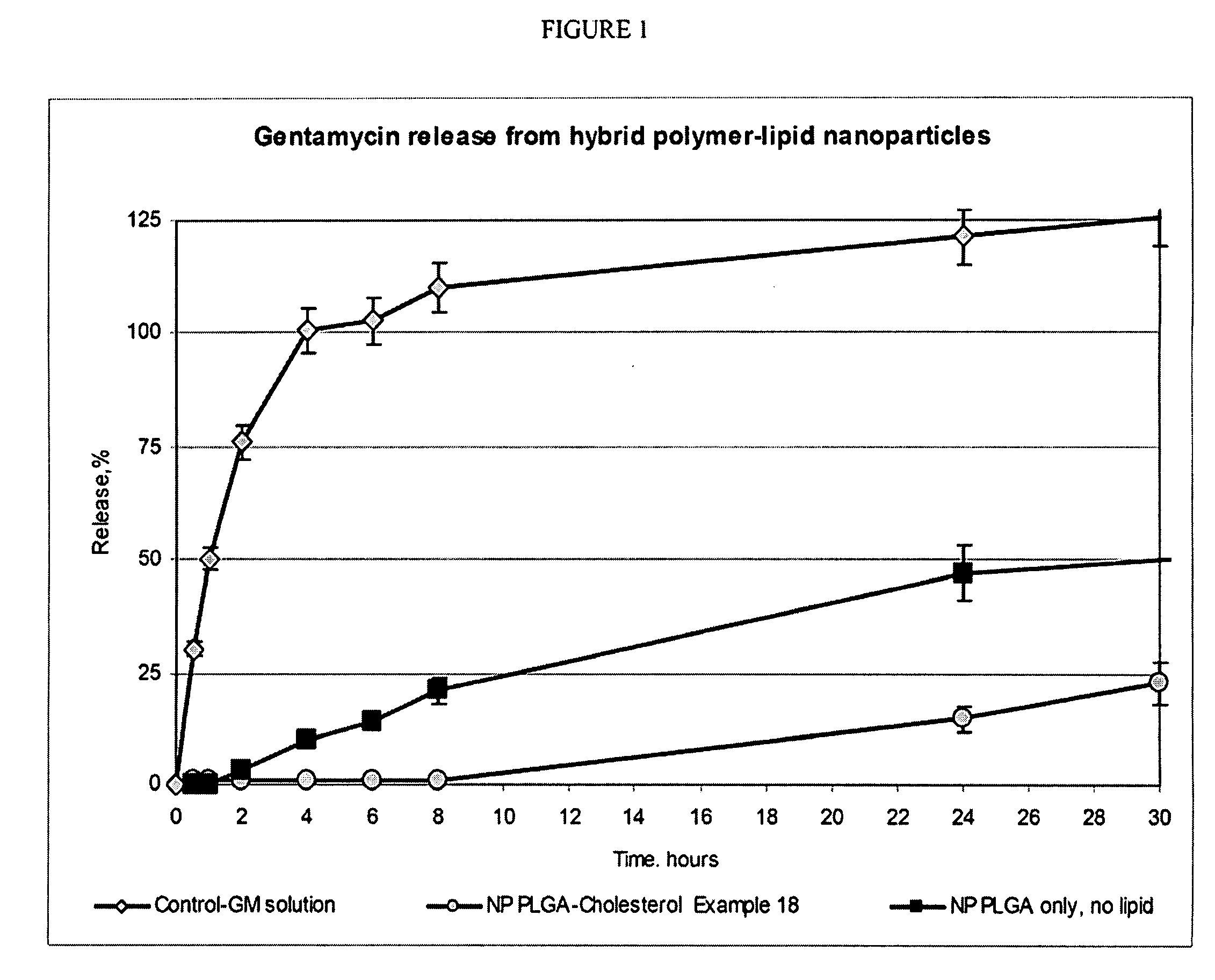
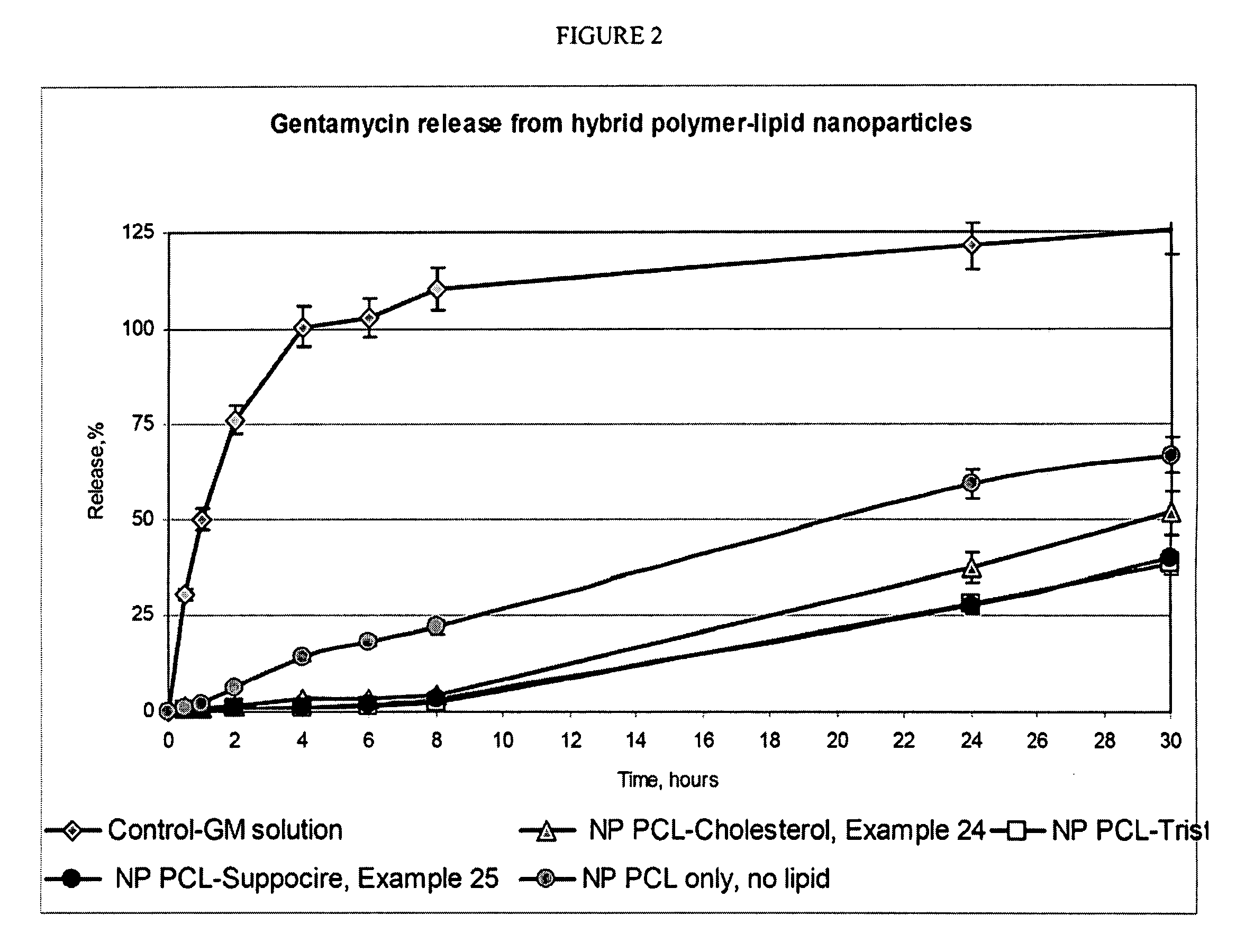
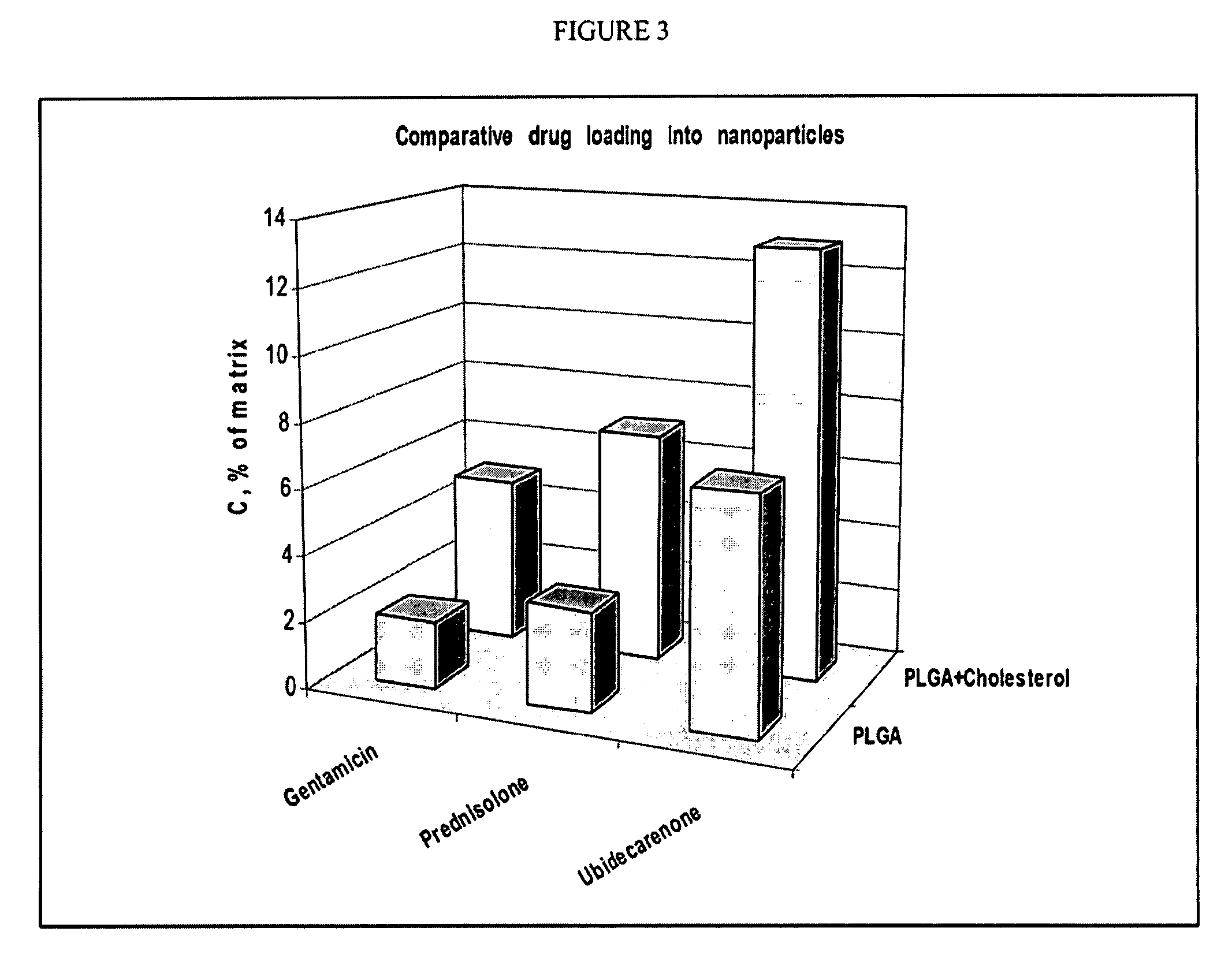
Hybrid lipid-polymer nanoparticulate delivery composition
The invention relates to a nanoparticulate colloidal delivery vehicle comprising a biodegradable polymer in combination with a hydrophobic lipid component. Variation of the lipid and polymer types and variation in the ratio between the polymer and lipid components allows regulation of drug loading and release rate.
Owner:ALPHARX
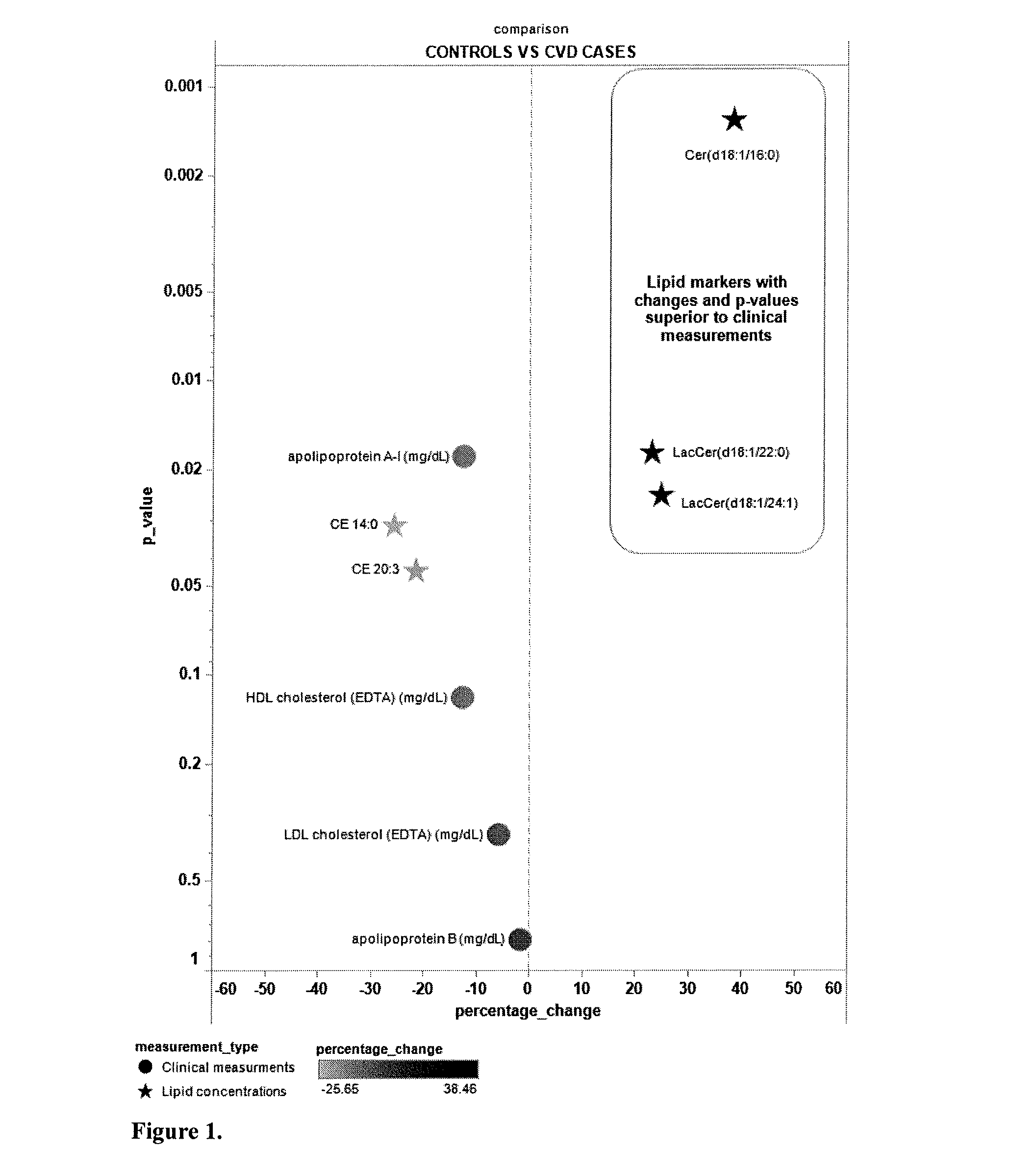
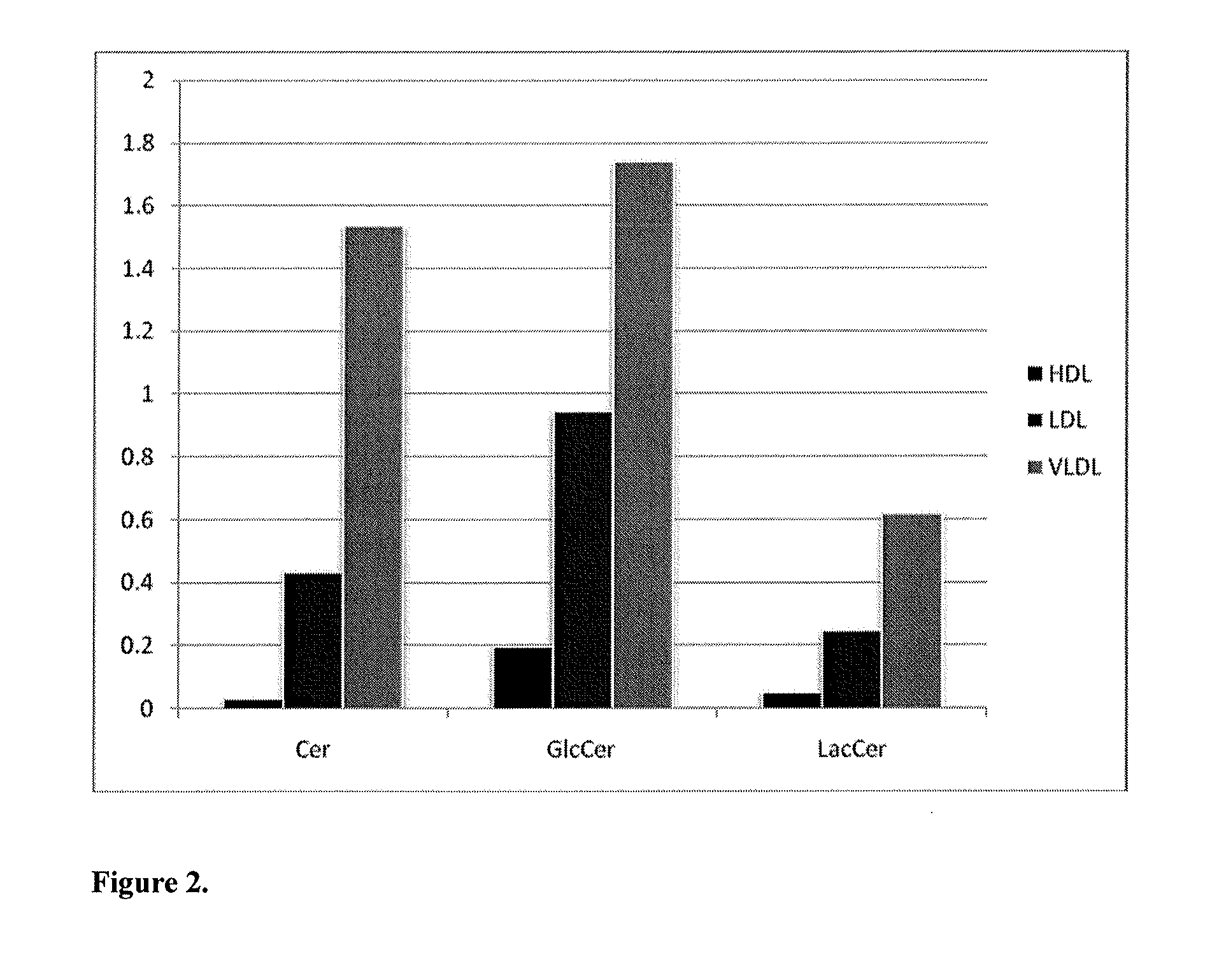
Lipidomic biomarkers for atherosclerosis and cardiovascular disease
ActiveUS20130045217A1Easy diagnosisSuperior prognostic valueBiocideComponent separationLipidomeClinical marker
The present invention inter alia provides a method, and use thereof, of diagnosing and / or predicting atherosclerosis or CVD by detecting the lipid concentrations or lipid ratios of a biological sample and comparing it to a control and has identified specific lipid markers that are more specific and sensitive in detecting and predicting atherosclerosis and CVD than currently utilized clinical markers. Also provided is an antibody towards said lipids, and the use thereof for predicting, diagnosing, preventing and / or treating atherosclerosis or CVD. The invention additionally relates to kits comprising lipids and / or an antibody thereto, for use in the prediction and / or diagnosis of atherosclerosis or CVD.
Owner:ZORA BIOSCIENCES OY
Low molecular weight cationic lipids for oligonucleotide delivery
ActiveUS9029590B2Good curative effectReduce liver toxicityUrea derivatives preparationOrganic active ingredientsLipid formationTolerability
The instant invention provides for novel cationic lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. It is an object of the instant invention to provide a cationic lipid scaffold that demonstrates enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids comprising at least one short lipid chain to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
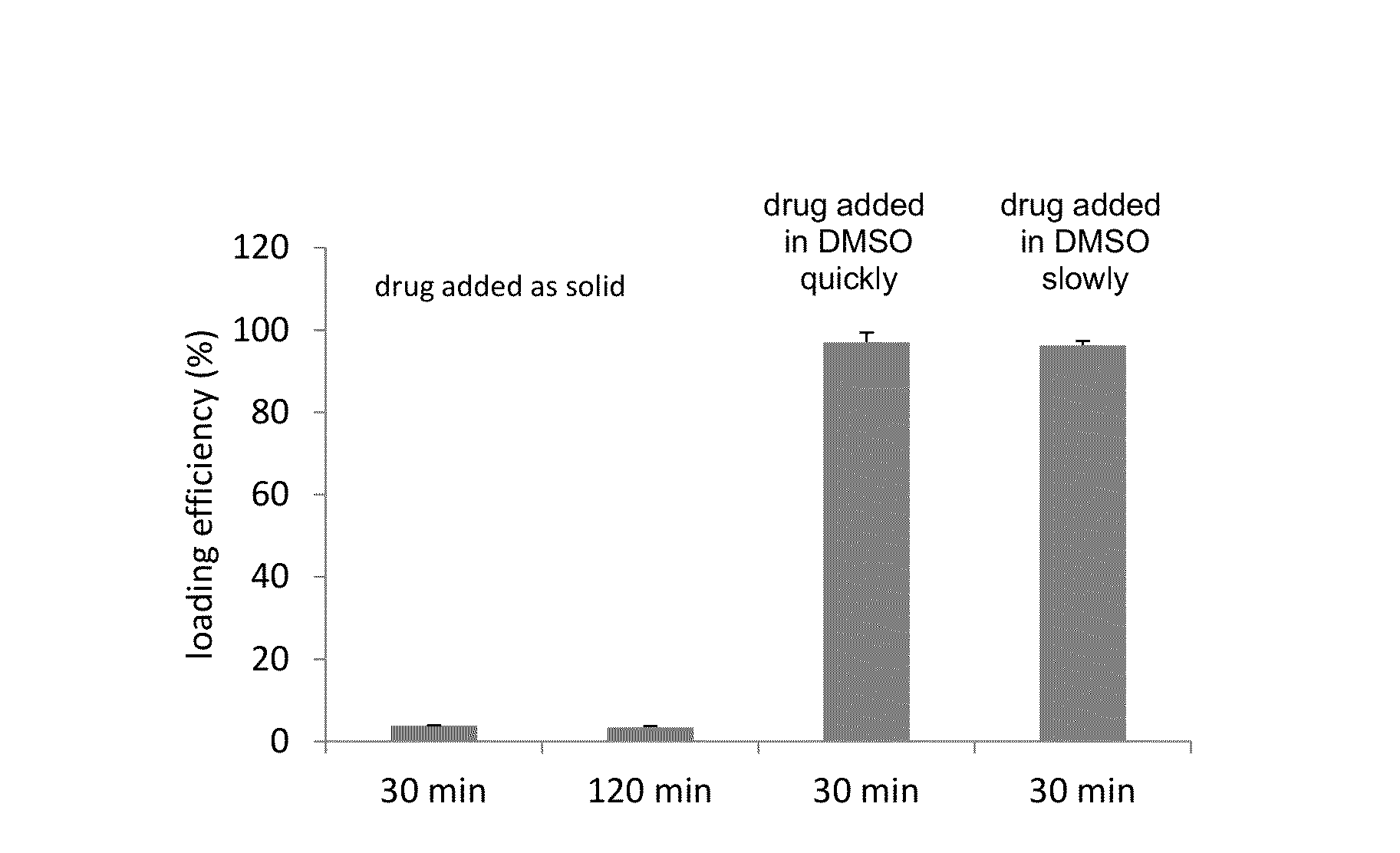
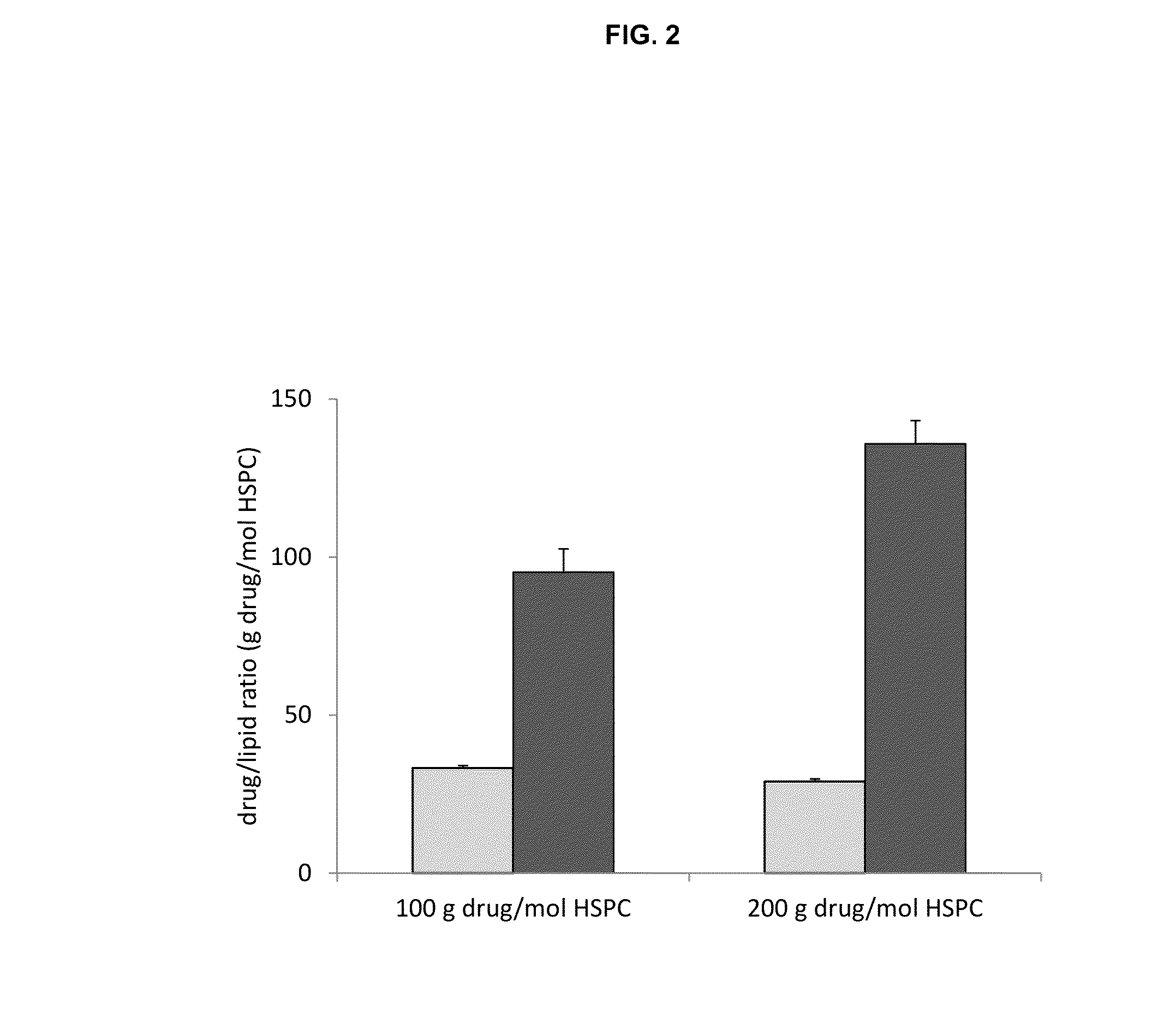
Remote loading of sparingly water-soluble drugs into liposomes
InactiveUS20140220111A1Raise the ratioCost efficiencyOrganic active ingredientsInorganic non-active ingredientsLipid formationDisease
The present invention provides liposome compositions containing sparingly soluble drugs that are used to treat life-threatening diseases. A preferred method of encapsulating a drug inside a liposome is by remote or active loading. Remote loading of a drug into liposomes containing a transmembrane electrochemical gradient is initiated by co-mixing a liposome suspension with a solution of drug, whereby the neutral form of the compound freely enters the liposome and becomes electrostatically charged thereby preventing the reverse transfer out of the liposome. There is a continuous build-up of compound within the liposome interior until the electrochemical gradient is dissipated or all the drug is encapsulated in the liposome. However, this process as described in the literature has been limited to drugs that are freely soluble in aqueous solution or solubilized as a water-soluble complex. This invention describes compositions and methods for remote loading drugs with low water solubility (<2 mg / mL). In the preferred embodiment the drug in the solubilizing agent is mixed with the liposomes in aqueous suspension so that the concentration of solubilizing agent is lowered to below its capacity to completely solubilize the drug. This results in the drug precipitating but remote loading capability is retained. The process is scalable and, in liposomes in which the lipid composition and remote loading agent are optimized, the resulting drug-loaded liposomes are characterized by a high drug-to-lipid ratios and prolonged drug retention when the liposome encapsulated drug is administered to a subject.
Owner:ZONEONE PHARMA
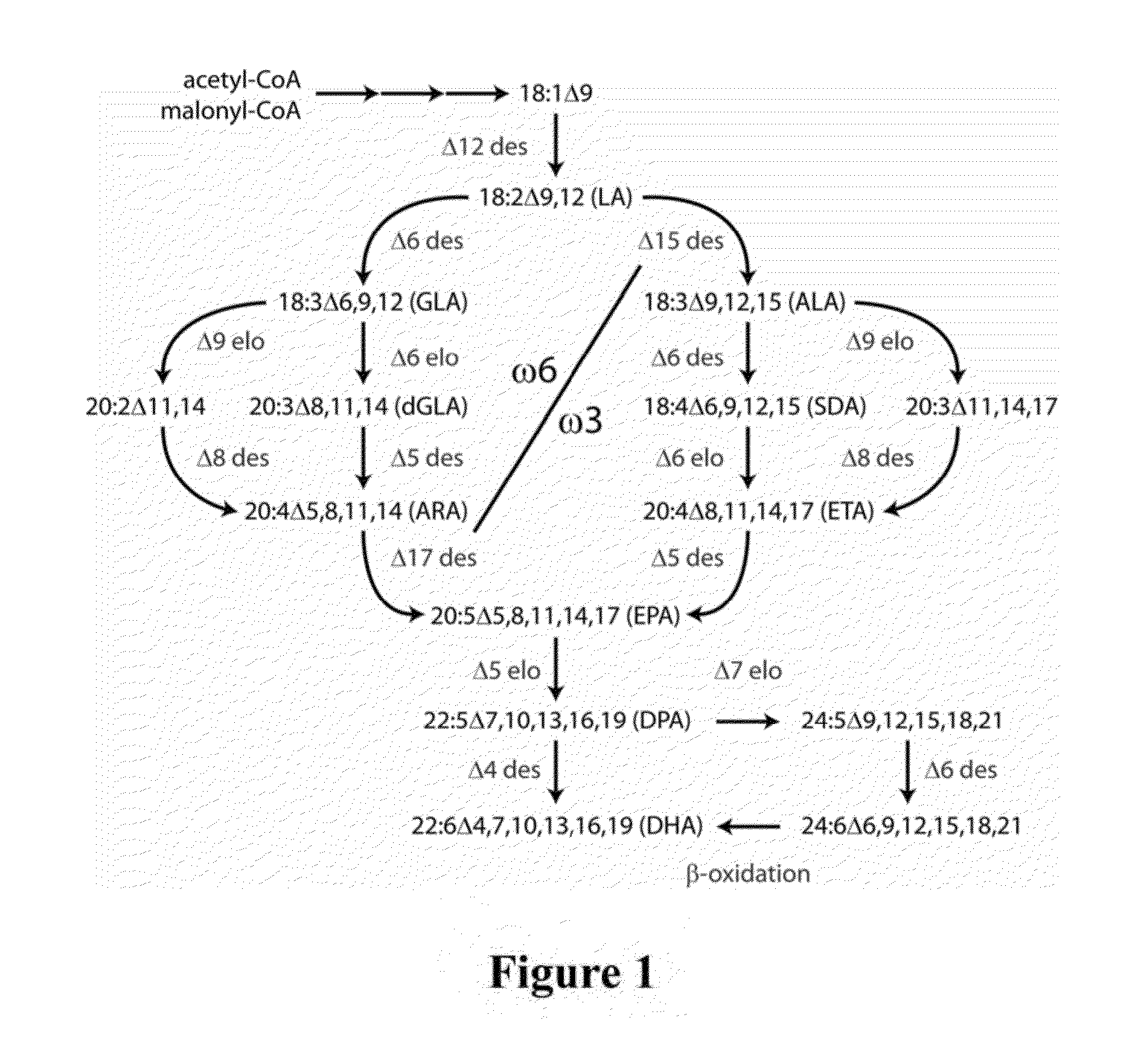
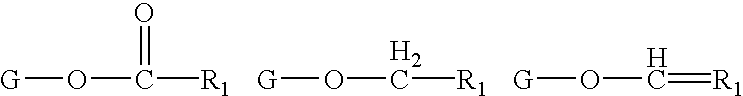
Lipid compositions comprising triacylglycerol with long-chain polyunsaturated fatty acids at the sn-2 position
ActiveUS20160177220A1Efficient productionFatty oils/acids recovery from wasteBiocideLipid compositionUnsaturated fatty acid
The present invention relates to lipid comprising docosapentaenoic acid and / or docosahexaenoic acid preferentially esterified at the sn-2 position of triacylglycerol, and processes for producing and using the lipid.
Owner:NUSEED PTY LTD +3
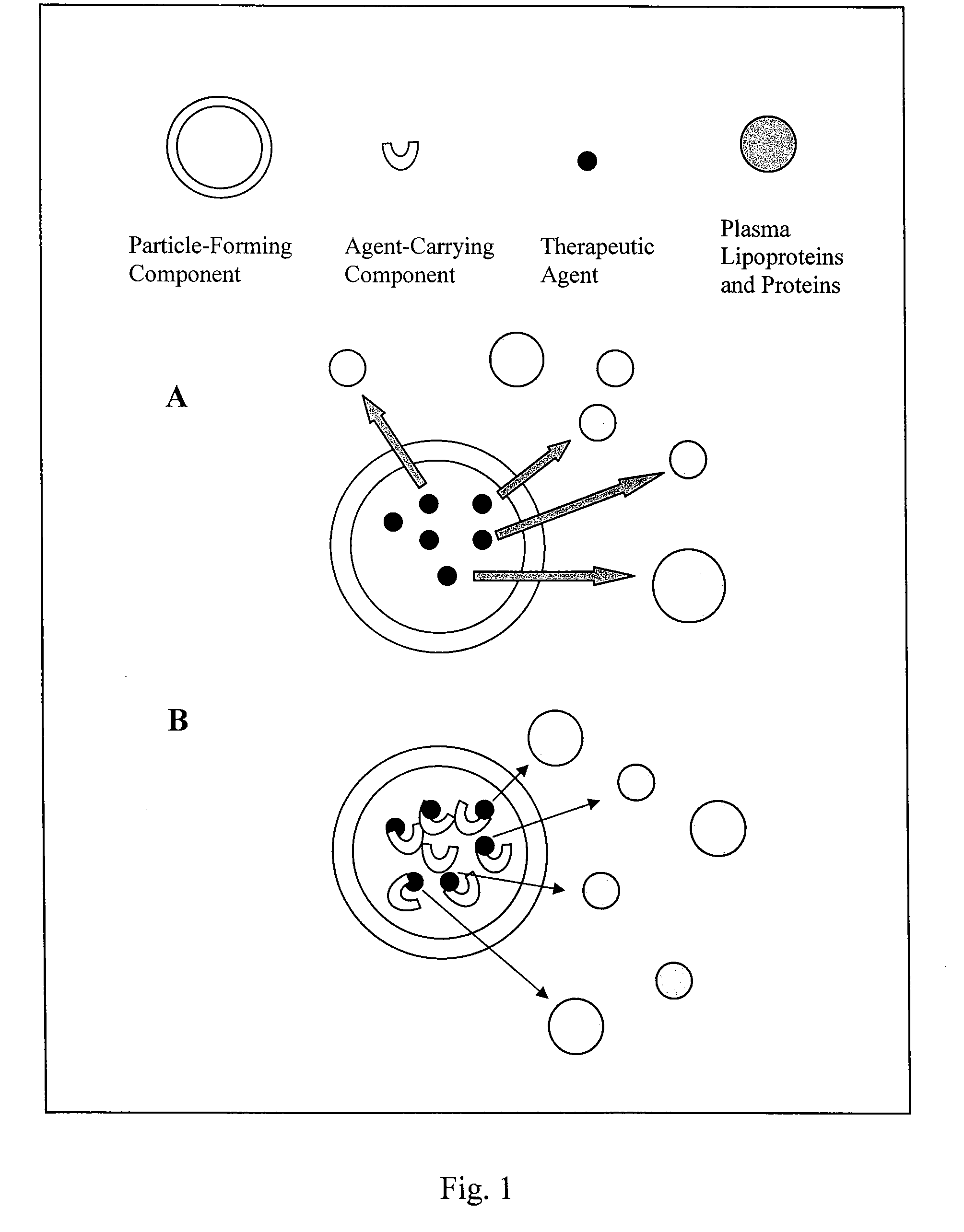
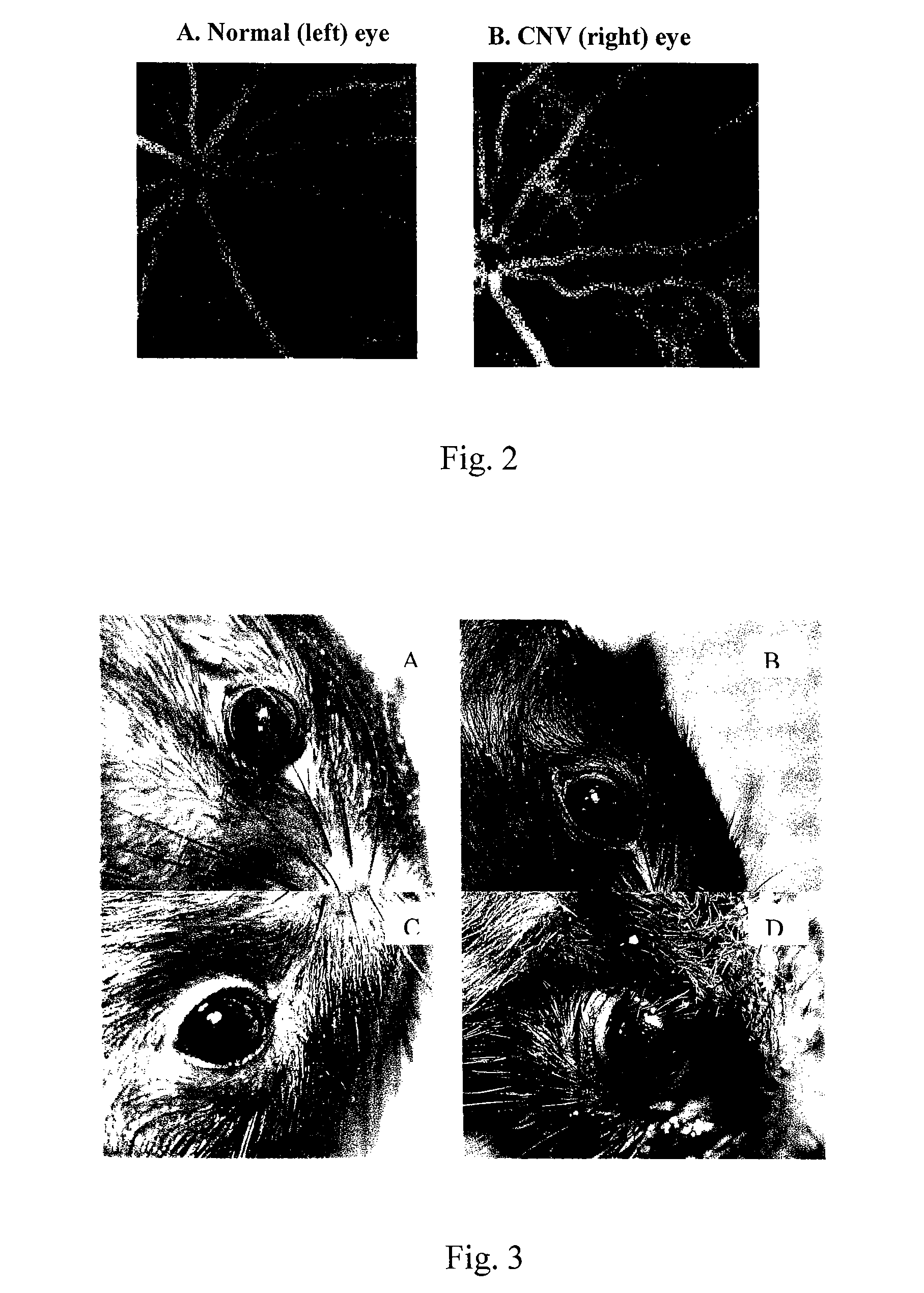
Liposome composition for delivery of a therapeutic agent to eyes
The invention provides a liposome composition for delivering high pay-load of a therapeutic agent to neovascularization sites of the eyes in a patient in need thereof. The liposome composition for entrapping the therapeutic agent comprises a particle forming component composed of a variety of vesicle-forming lipids, and an agent-carrying component able to form a complex with the therapeutic agent via electrostatic charge-charge interaction or hydrophobic-hydrophobic interaction; wherein the liposome composition comprising the therapeutic agent has a mean particle diameter of about 30 to 200 nm and may accumulate at the neovascularization sites of the eyes 24 hours after the intravenous administration of the liposome composition comprising the therapeutic agent to the patient. A method for delivering the therapeutic agent to the eyes in a patient with this liposome composition is also provided
Owner:TAIWAN LIPOSOME CO LTD +1
Marine lipid composition for feeding aquatic organisms
InactiveUS20030124218A1Increase ratingsIncrease chanceBiocideFatty acid esterificationLipidomePlankton biomass
A composition is for feeding aquacultural prey organisms such as Artemia and rotifiers, comprising a lipid component comprising at least 25 wt % of phospholipids and providing a DHA content of at least 30 wt %. The lipid component is preferably derived from marine organisms such as fishineal, phytoplankton or zoo plankton biomass. The composition is used for providing prey organisms having a high content of highly-unsaturated fatty acids (HUFAs) suitable for aquaculture of fish including halibut, turbot, bass and crustaceans and molluses.
Owner:EPAX NORWAY
Cationic lipids for nucleic acid delivery and preparation thereof
The present invention provides cationic lipids and lipid nanoparticle formulations comprising these lipids, alone or in combination with other lipids. These lipid nanoparticles may be formulated withnucleic acids to facilitate their intracellular delivery both in vitro and for therapeutic applications. The present invention also provides methods of chemical synthesis of these lipids, lipid nanoparticle preparation and formulation with nucleic acids.
Owner:RAMOT AT TEL AVIV UNIV LTD
Lipid-containing compositions and methods of use thereof
Lipid compositions comprising nuts, seeds, oils, legumes, fruits, grains, and dairy useful in specified amounts as dietary supplements and diet plans designed around and including the aforementioned for the prophylaxis and treatment of numerous diseases are disclosed. The compositions include omega-6 and omega-3 fatty acids where the ratio of the omega-6 to the omega-3 fatty acids and their amounts are controlled based on one or more factors including age of the subject, sex of the subject, diet of the subject, the body weight of the subject, medical conditions of the subject, and climate of the subject's living area.
Owner:ASHA NUTRITION SCI INC
Method for transdermal or intradermal delivery of molecules
The present invention provides a method for transdermal delivery of molecules. The method comprises the application of electrical pulses concurrently or sequentially with application of the molecules and a lipid composition comprising negatively charged liposomal compositions. The liposomal components are used to enhance permeability of the target site for delivery of the molecule.
Owner:HEALTH RES INC
Combination Therapy
InactiveUS20080058274A1Beneficial additive effectGood effectBiocideCarbohydrate active ingredientsDoxorubicinLiposome
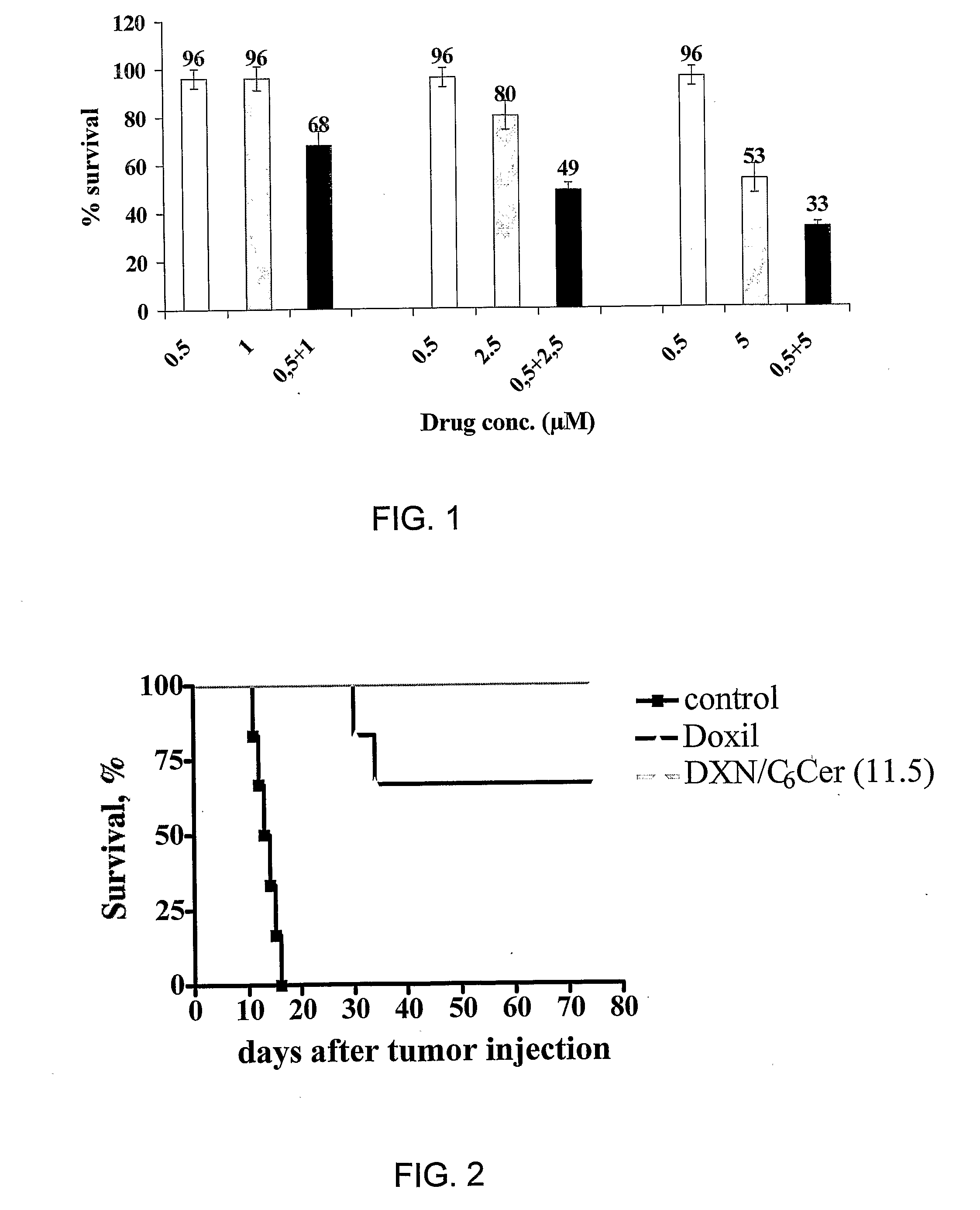
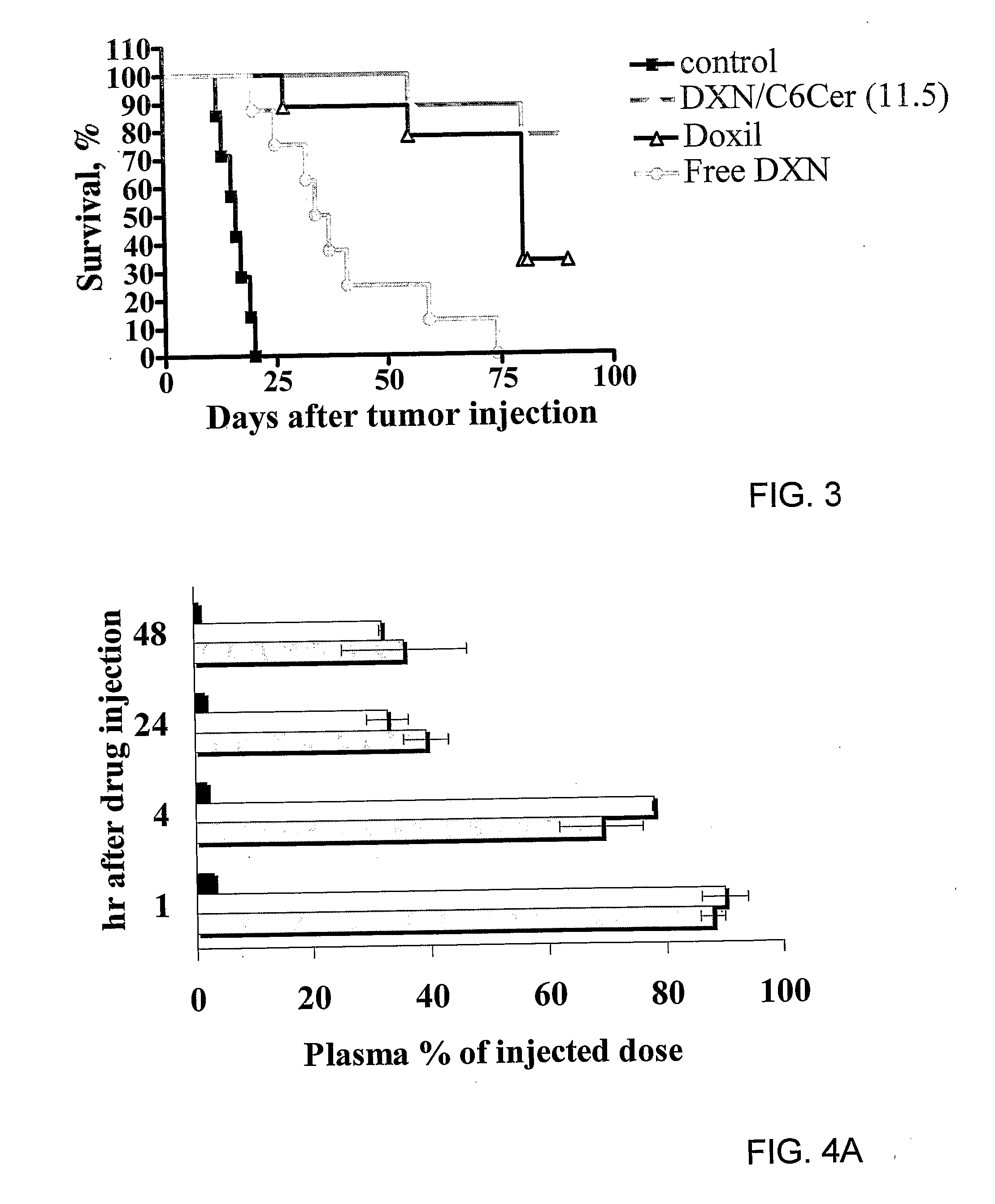
The present invention concerns a new medical treatment involving the combination of two active entities, as well as pharmaceutical compositions comprising the two active entities. Specifically, the invention provides a pharmaceutical composition comprising a stable lipid assembly comprising as a first active entity an apoptosis-affecting lipid which does not self-aggregate in a polar environment to form liposomes and a lipopolymer. The pharmaceutical composition further comprises, as the second active entity, a cytotoxic amphipathic weak base drug carried by the lipid assembly or by a different liposome. According to one embodiment, the apoptotic-affecting lipid is a pro-apoptotic lipid. A preferred pro-apoptotic lipid is ceramide, preferably C6-ceramide. The cytotoxic amphipathic weak base drug is preferably doxorubicin or a biologically active, anthracyline-based doxorubicin analog thereof.
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
Determining existence of complications in pregnancies by measuring levels of bioactive lipids
InactiveUS20030068653A1Microbiological testing/measurementBiological material analysisLipidomePlacental abnormality
The present invention relates generally to methods for detecting complications, or abnormal conditions occurring during pregnancy, including placental abnormalities, eclampsia, or preelcampsia. The present invention comprises the steps of obtaining a sample from a patient, assaying the specimen to determine the level of bioactive lipids and comparing levels in the sample to control values or levels in normal samples, and correlating alterations to disease. The invention includes measuring panels of bioactive lipids to screen patients for disease and to monitor the progress of disease for diagnostic or therapeutic purposes.
Owner:LPL TECH
Lipid compositions and their uses for intratumoral polynucleotide delivery
InactiveUS20190167811A1Easy to chargeHigh retention rateOrganic active ingredientsGenetic material ingredientsNucleotideTherapeutic protein
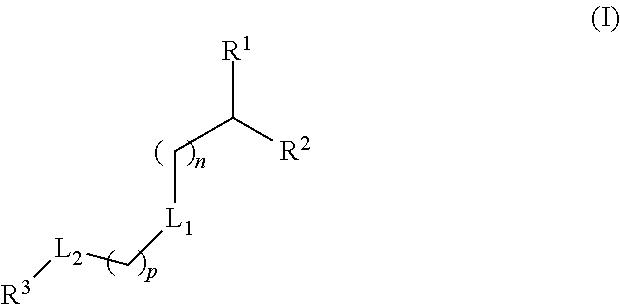
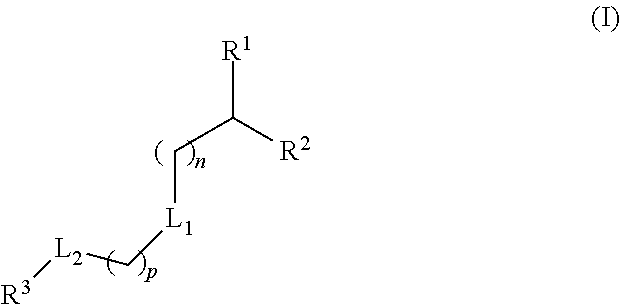
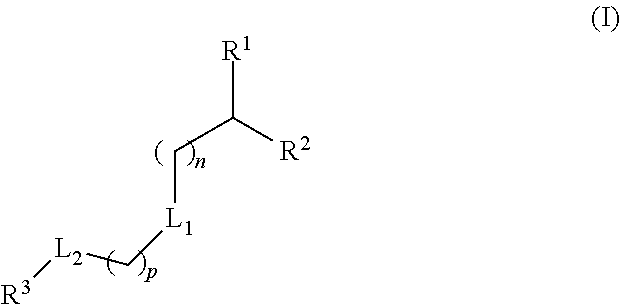
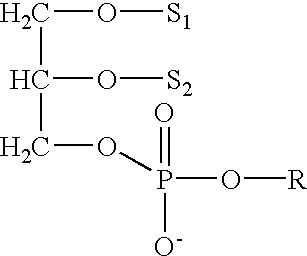
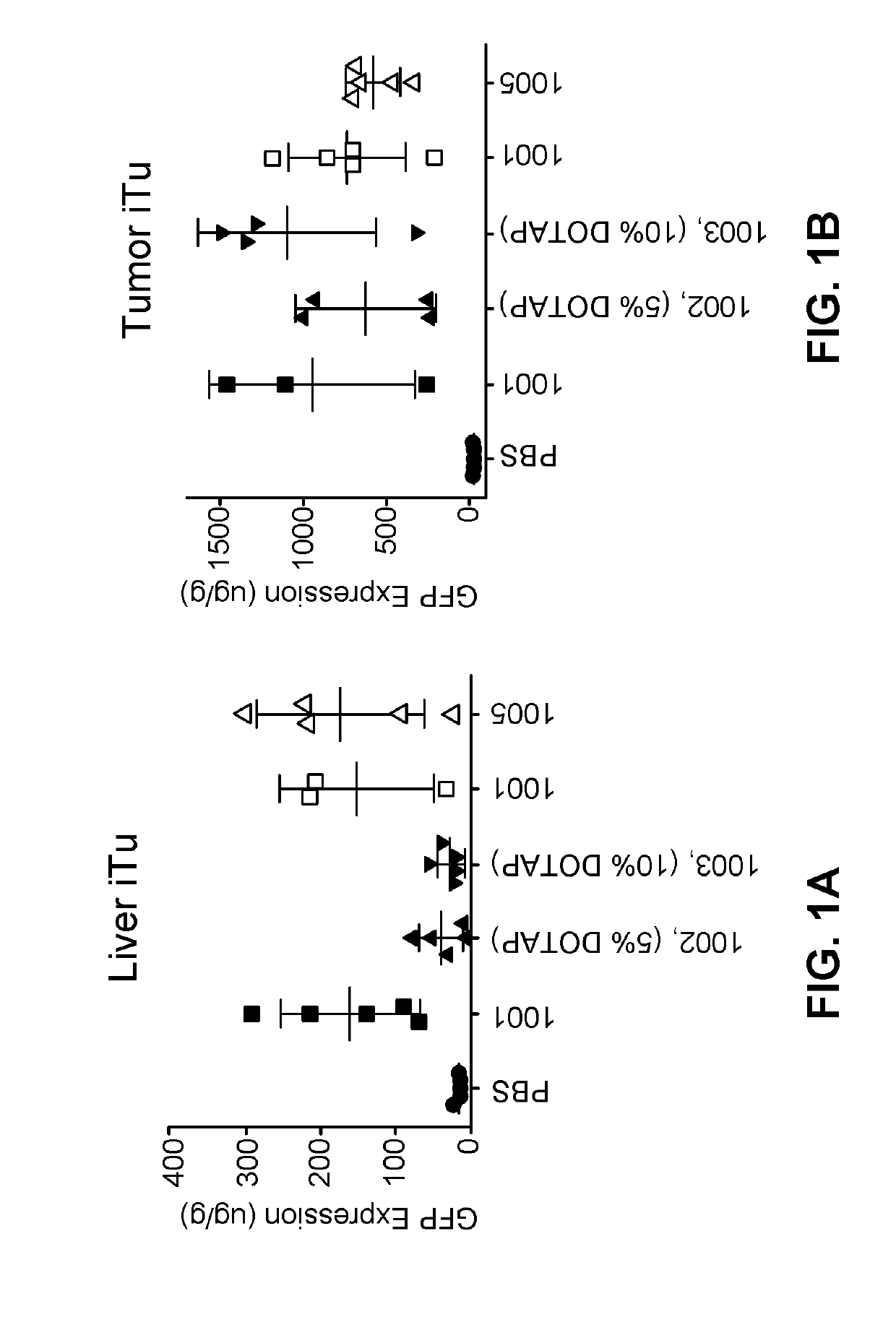
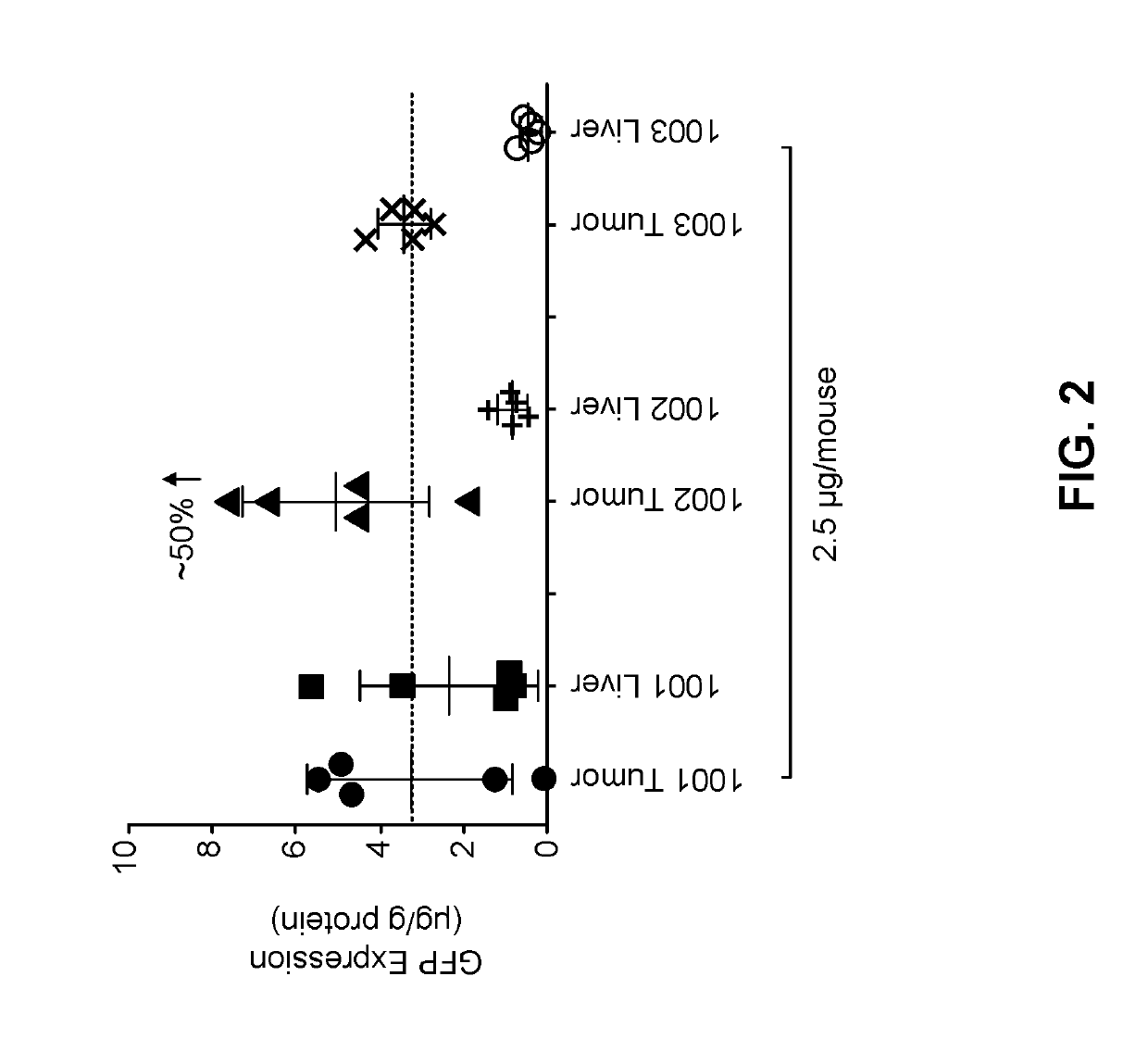
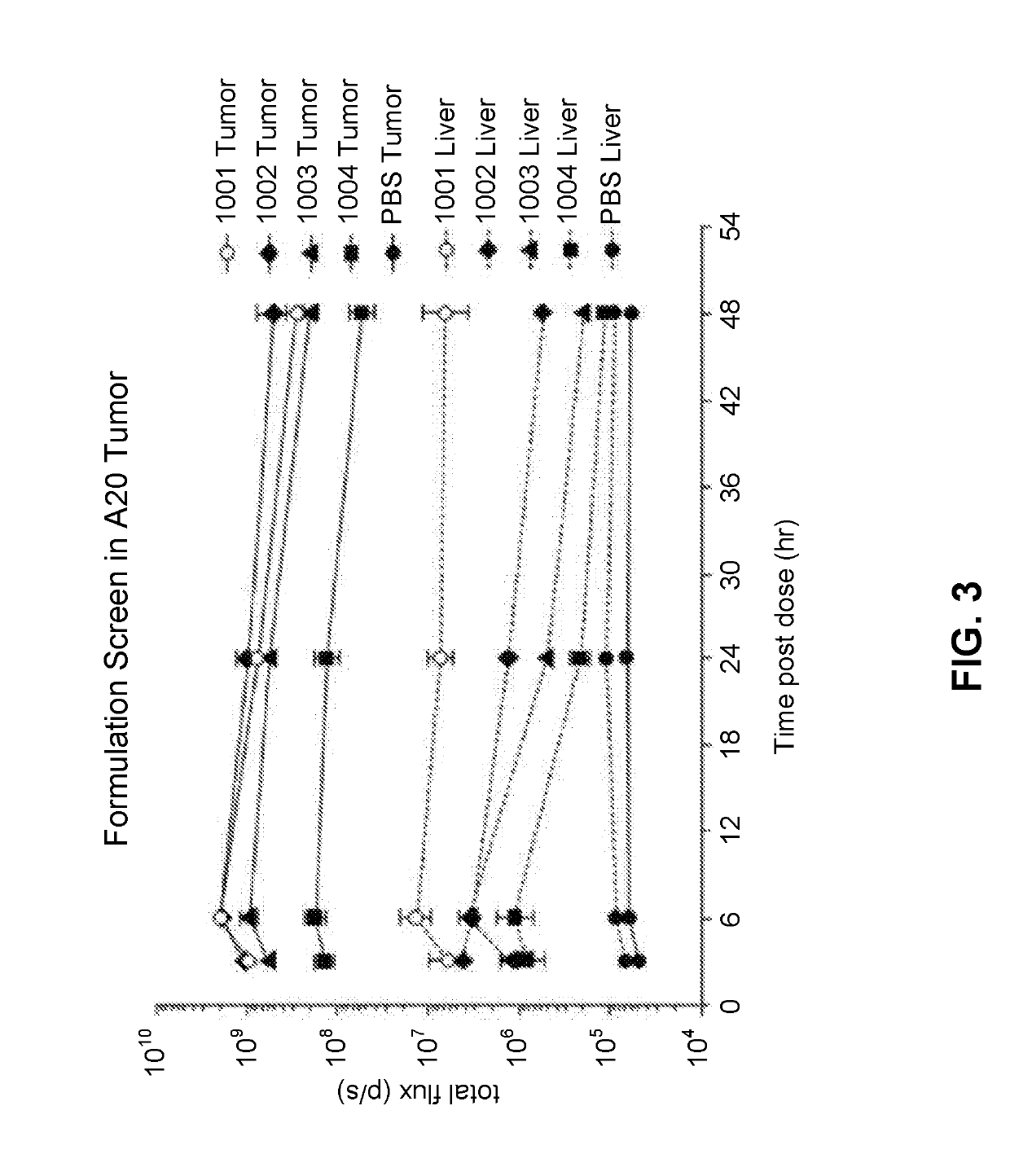
The present application provides a composition comprising (1) a lipid composition comprising an ionizable amino lipid and a quaternary amine compound and (2) a polynucleotide. The present application also provides a composition comprising (1) a lipid composition comprising an asymmetric phospholipid, an ionizable amino lipid, and optionally a quaternary amine compound and (2) a polynucleotide, wherein the composition is formulated for intratumoral delivery of the polynucleotide. The present application further provides pharmaceutical compositions for intratumoral delivery comprising (1) a lipid composition comprising a compound of formula (I) and (2) therapeutic agent or a polynucleotide encoding the therapeutic agent, e.g., an mRNA encoding a therapeutic protein or a fragment thereof. Further provided is a method of increasing retention of a polynucleotide in a tumor tissue by using such a composition.
Owner:MODERNATX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com