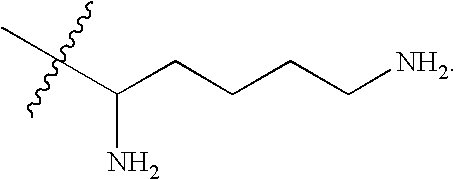
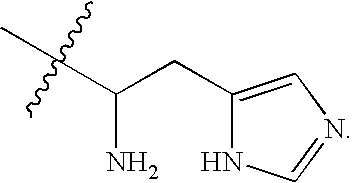
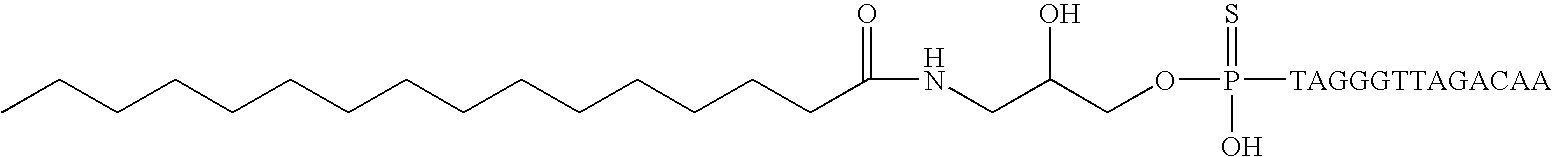
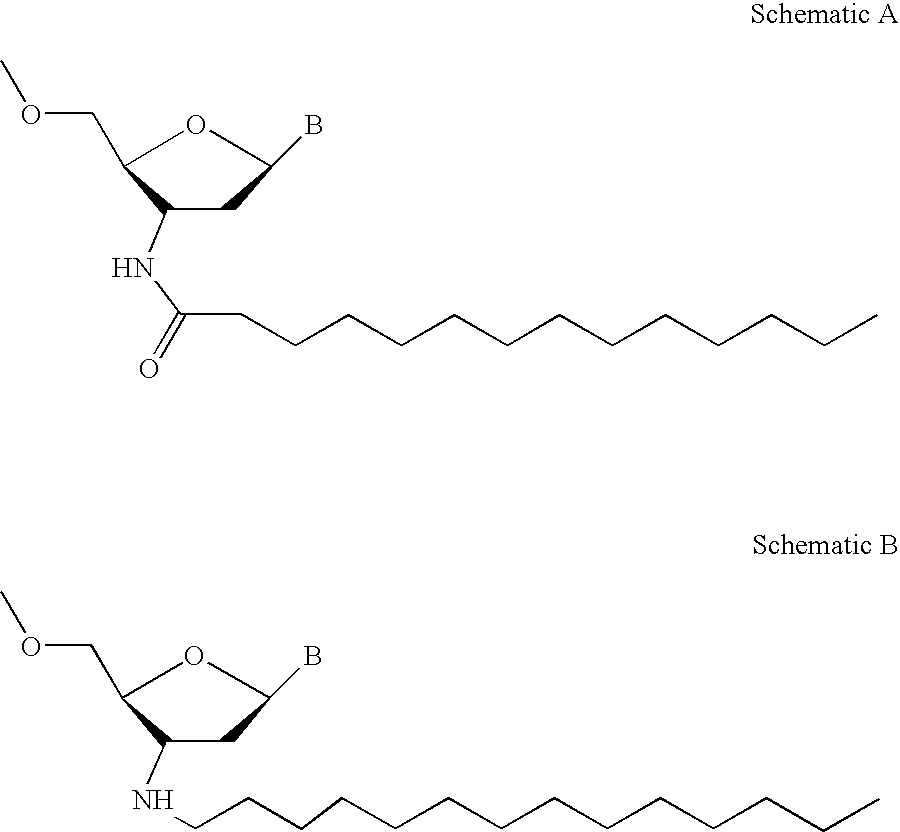
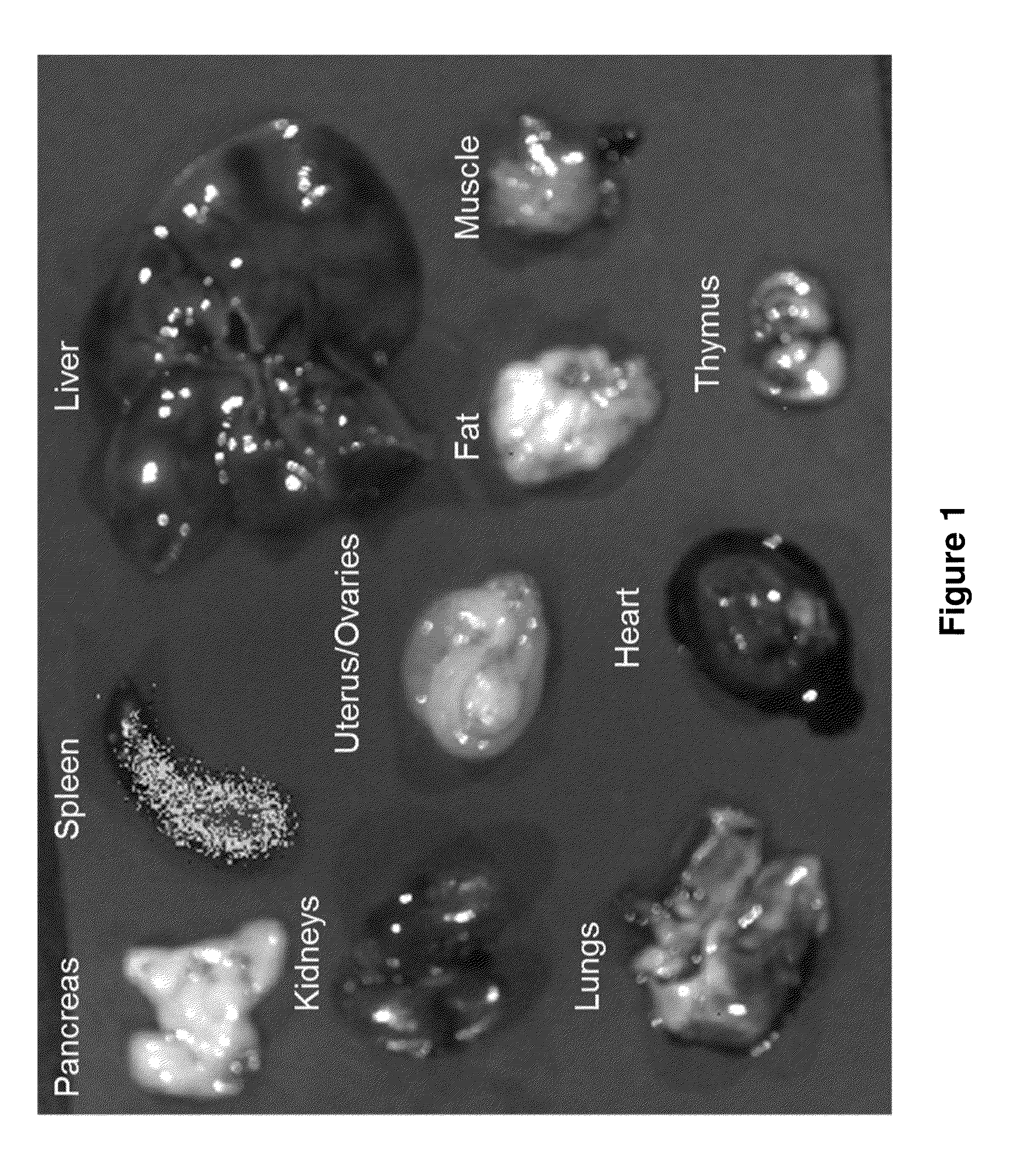
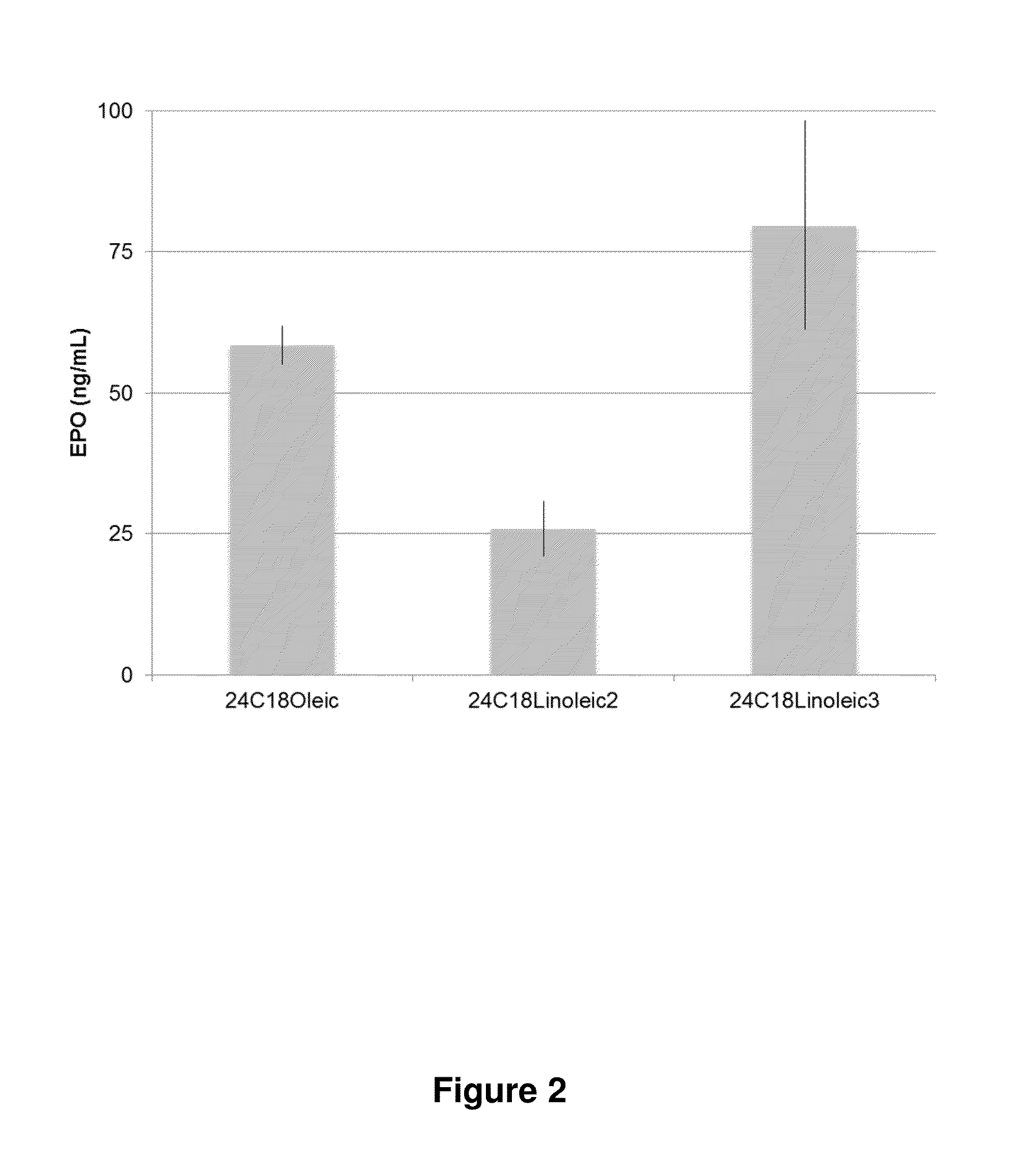
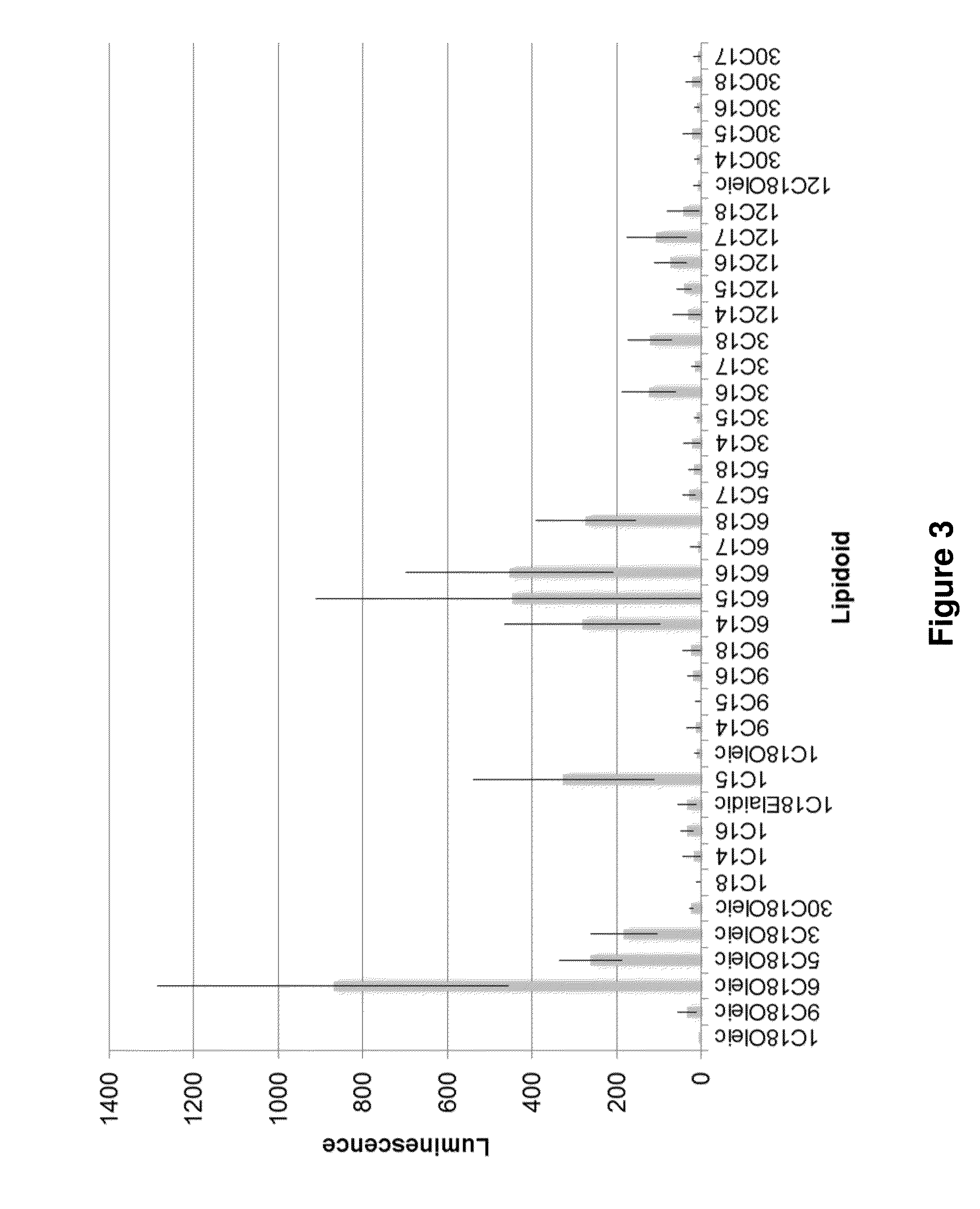
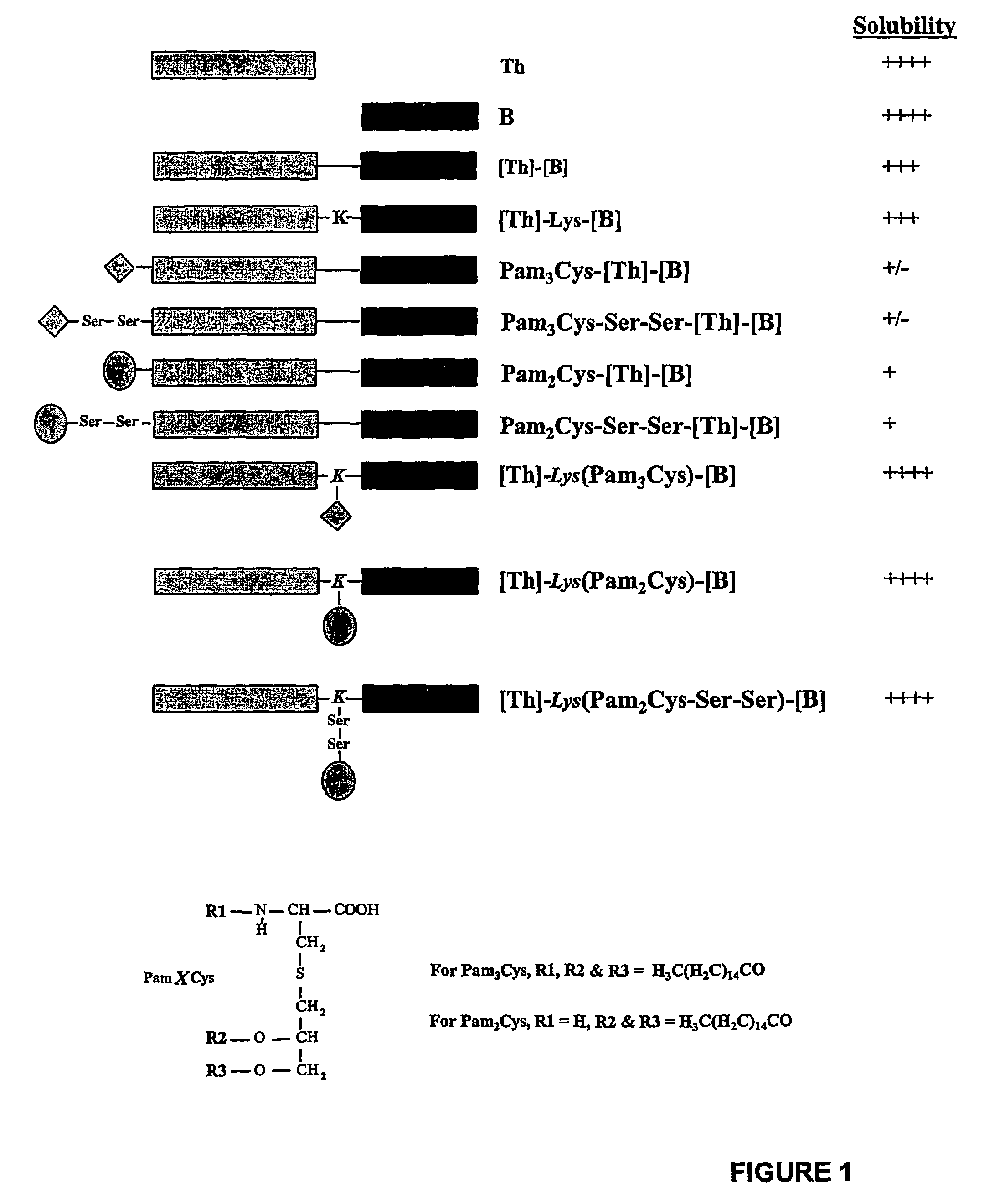
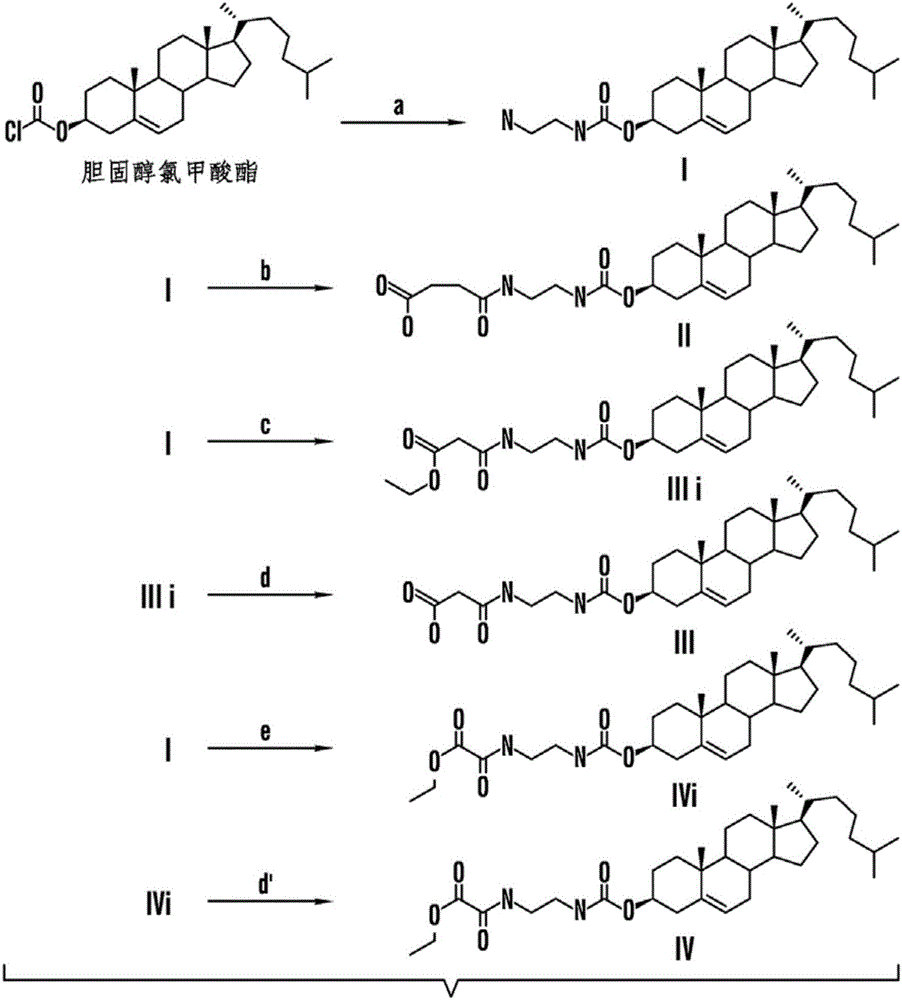
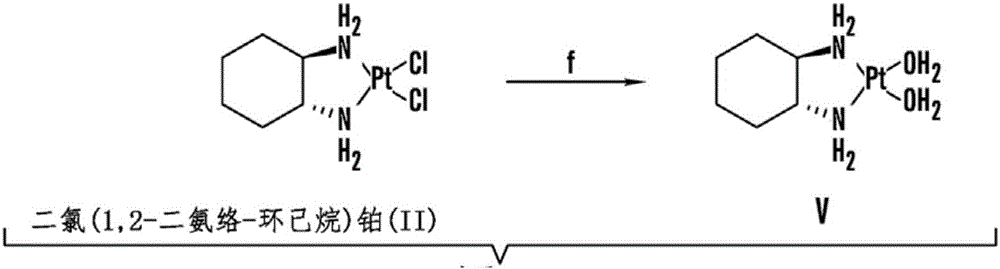
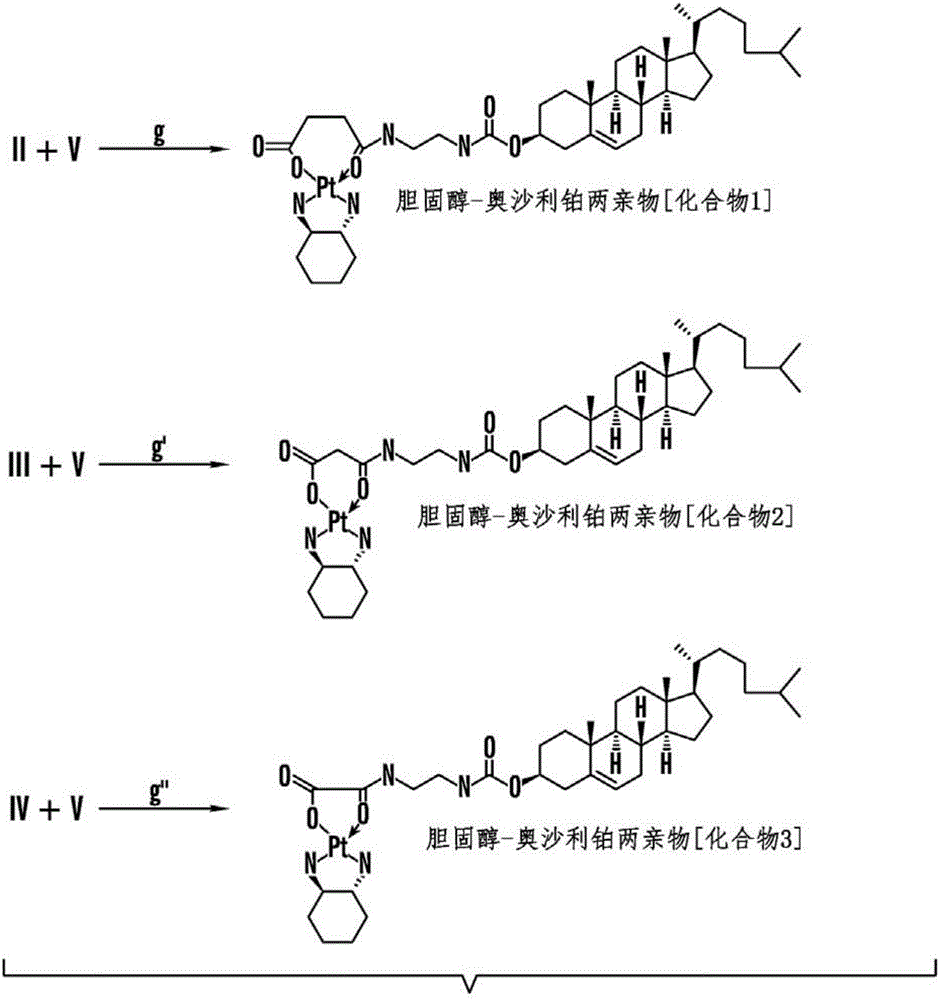
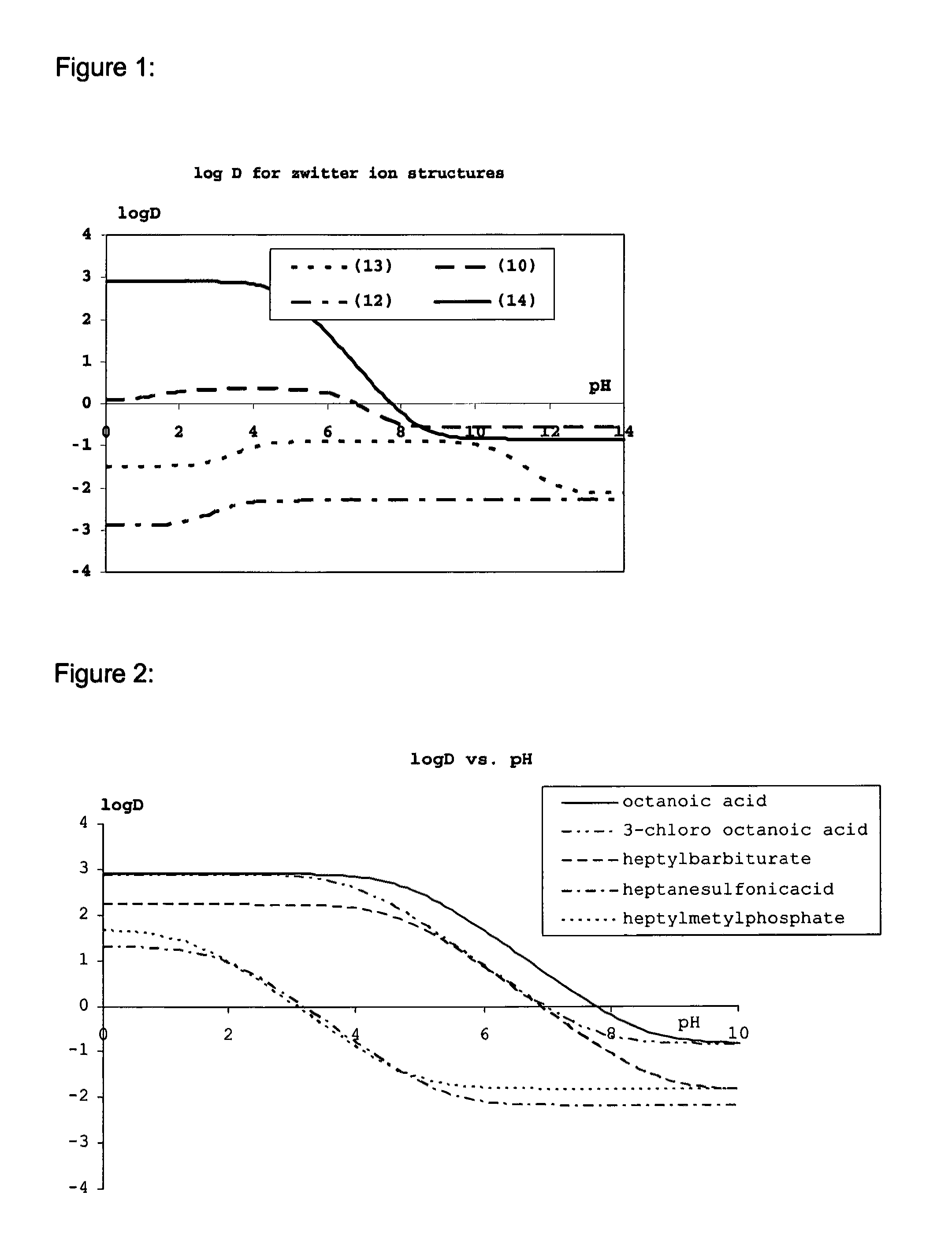
Patents
Literature
66 results about "Lipid moiety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
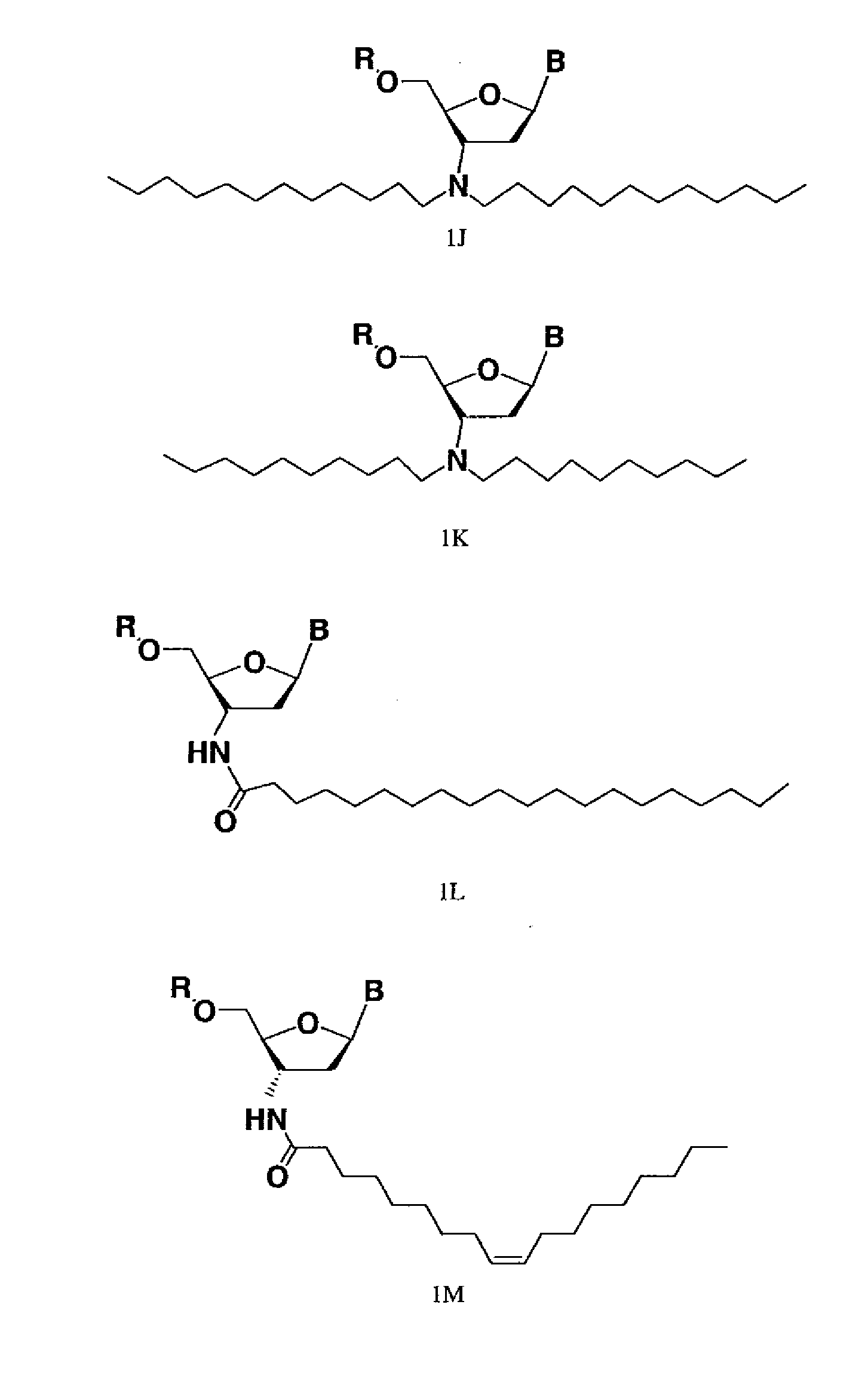
Lipid A (E. coli) is the glycolipid moiety of the lipopolysaccharide produced by E. coli. It has a role as an Escherichia coli metabolite. It is a lipid A, a dodecanoate ester and a tetradecanoate ester. It is a conjugate acid of a lipid A(4-) (E. coli).
Cationic peg-lipids and methods of use
InactiveUS6852334B1High transfection efficiencyMicroencapsulation basedGenetic material ingredientsLipid formationHydrophilic polymers
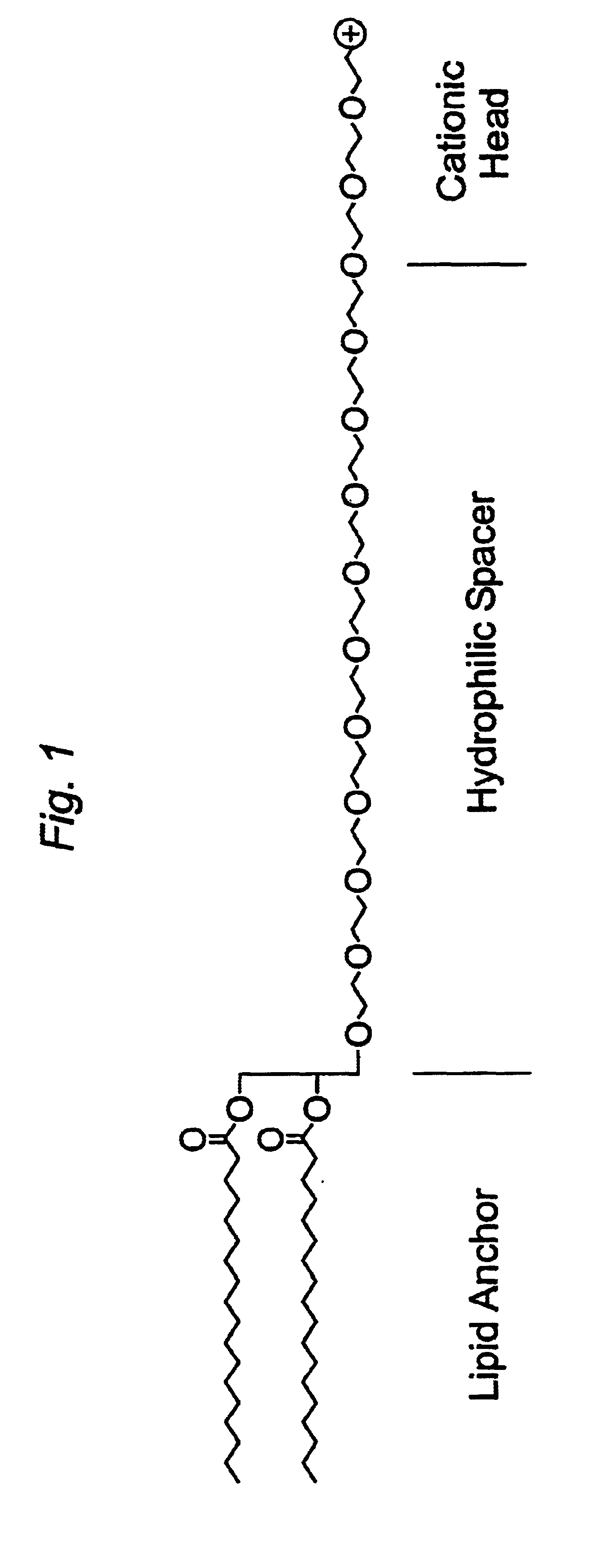
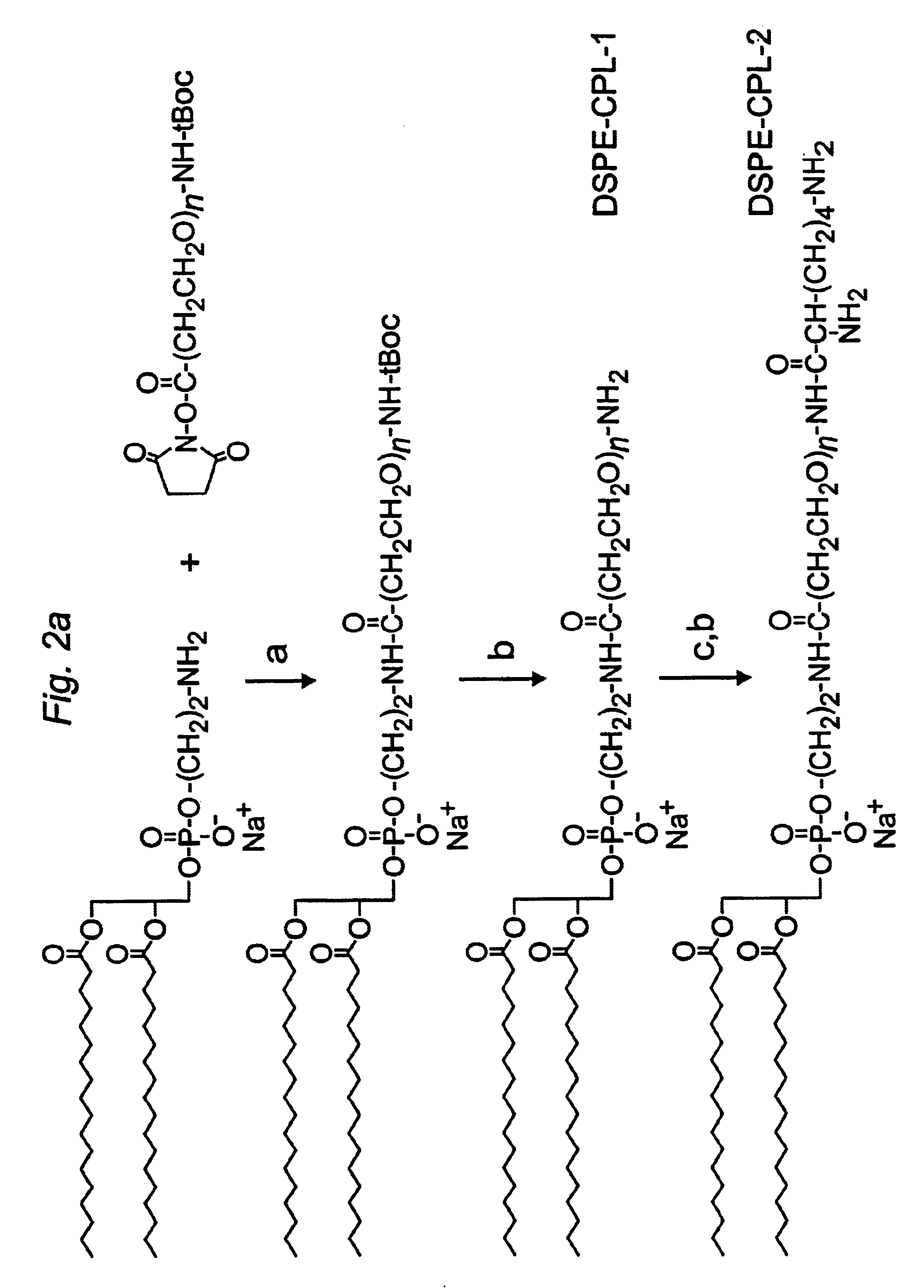
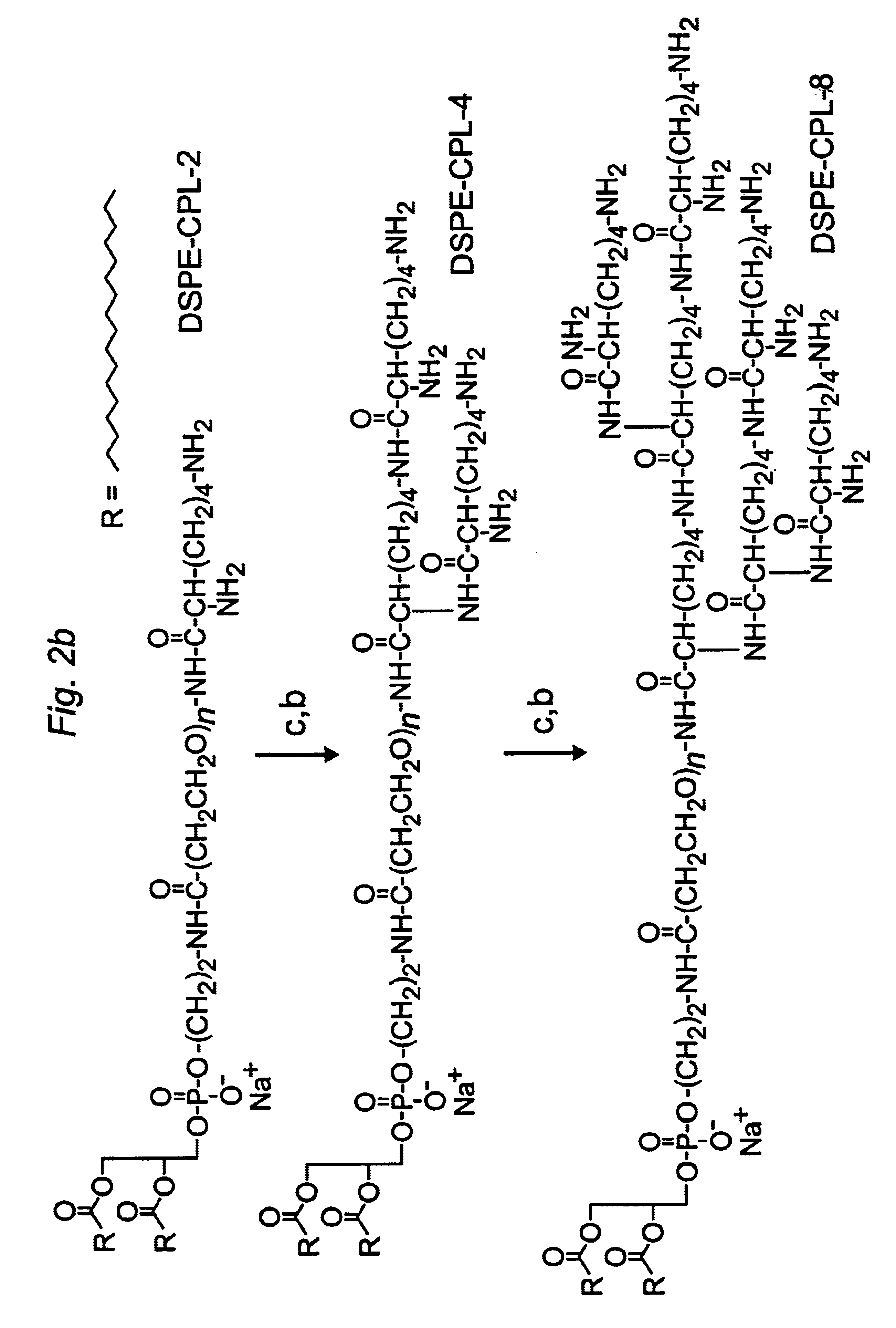
The present invention provides cationic-polymer-lipid conjugates (CPLs) such as distal cationic-poly(ethylene glycol)-lipid conjugates which can be incorporated into conventional and stealth liposomes or other lipid-based formulation for enhancing cellular uptake. The CPLs of the present invention comprise a lipid moiety; a hydrophilic polymer; and a polycationic moiety. Method of increasing intracellular delivery of nucleic acids are also provided.
Owner:THE UNIV OF BRITISH COLUMBIA
Cationic lipids
Owner:ALNYLAM PHARM INC
Modified oligonucleotides for telomerase inhibition
ActiveUS20050113325A1Superior cellular uptake propertyReduce Toxicity RiskBiocideGenetic material ingredientsTelomeraseLipid moiety
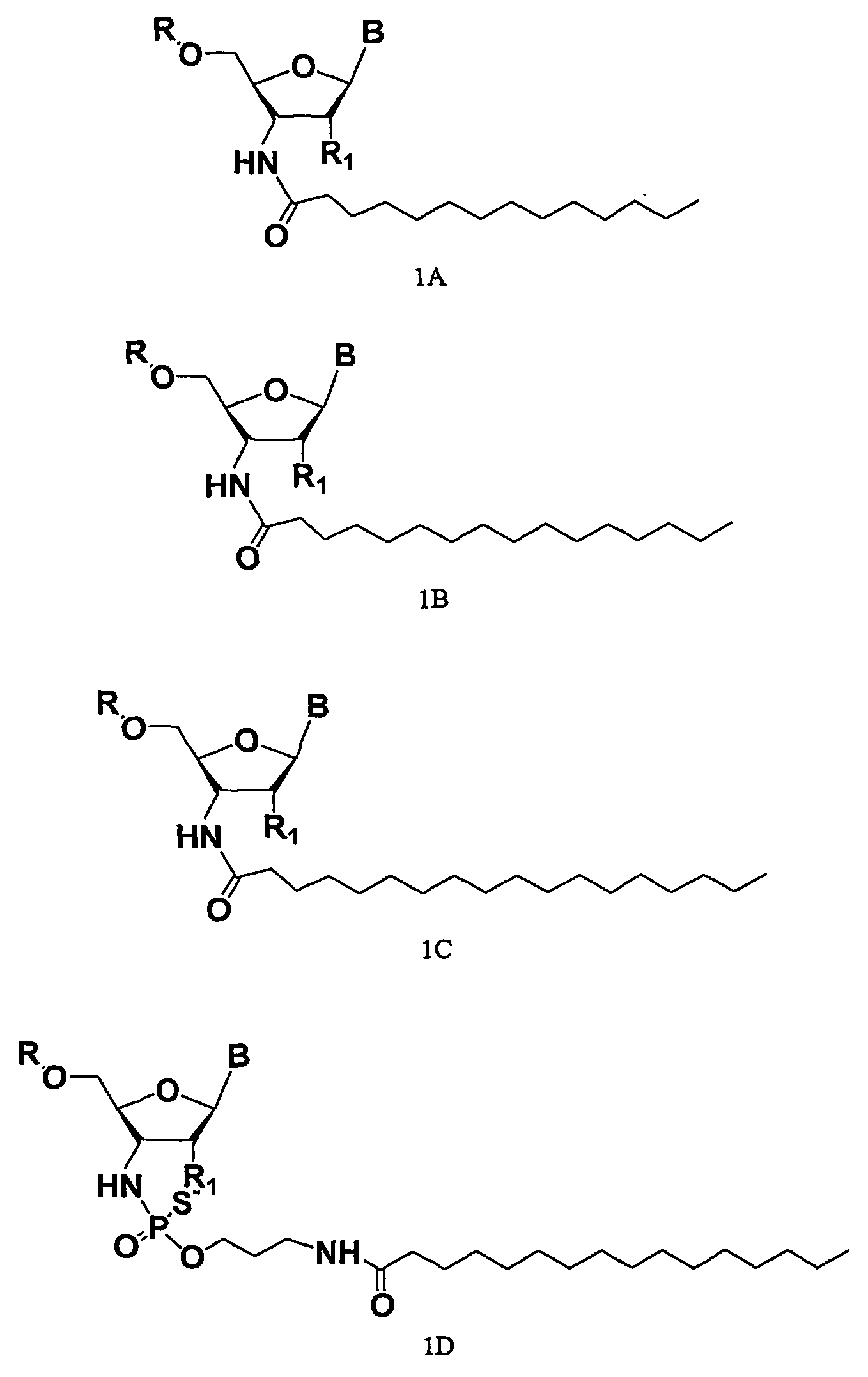
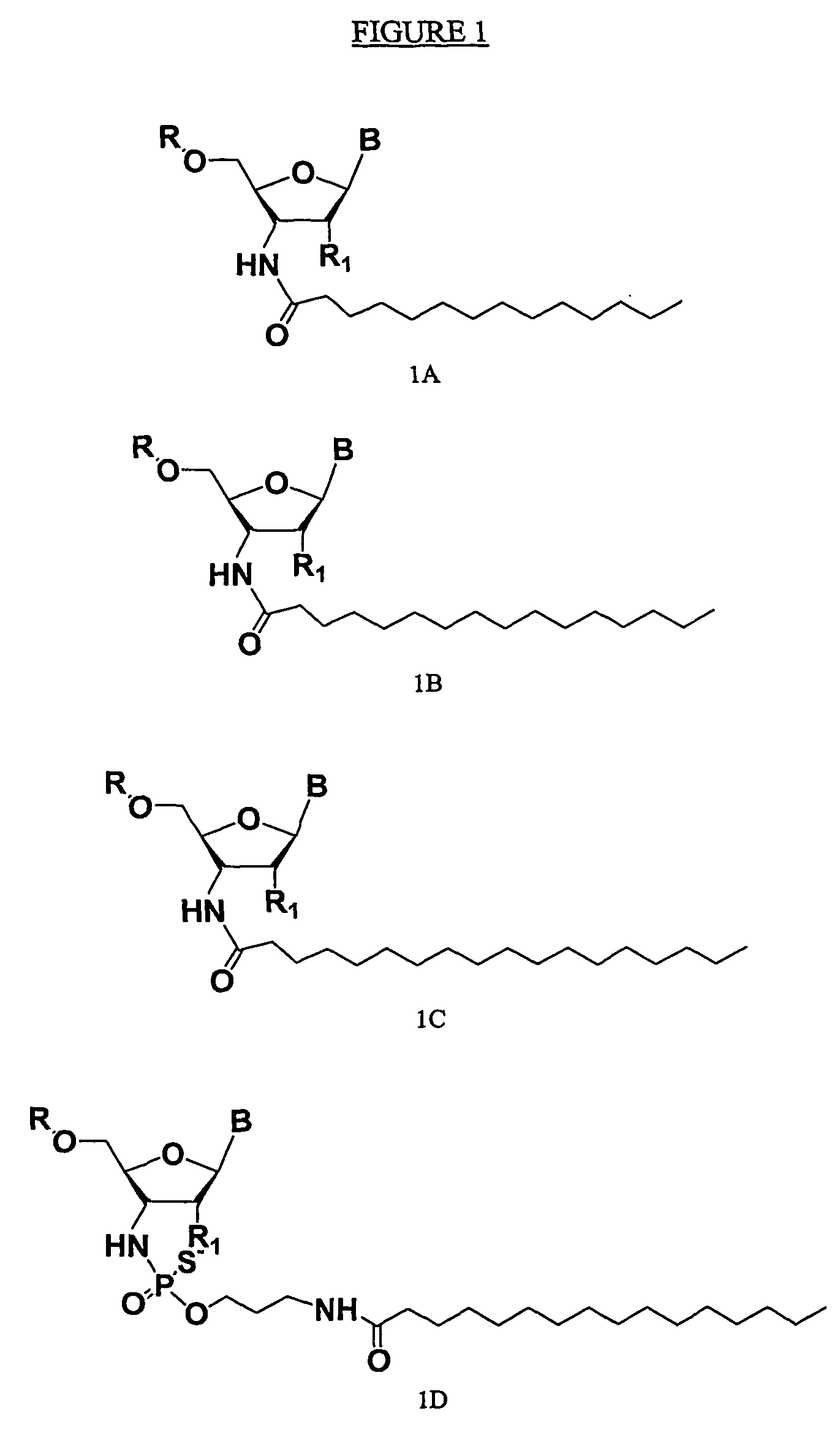
Compounds comprising an oligonucleotide moiety covalently linked to a lipid moiety are disclosed. The oligonucleotide moiety comprises a sequence that is complementary to the RNA component of human telomerase. The compounds inhibit telomerase activity in cells with a high potency and have superior cellular uptake characteristics.
Owner:GERON CORPORATION
Modified oligonucleotides for telomerase inhibition
ActiveUS7494982B2Inhibit telomeraseMaintain good propertiesBiocideSugar derivativesTelomeraseLipid moiety
Compounds comprising an oligonucleotide moiety covalently linked to a lipid moiety are disclosed. The oligonucleotide moiety comprises a sequence that is complementary to the RNA component of human telomerase. The compounds inhibit telomerase activity in cells with a high potency and have superior cellular uptake characteristics.
Owner:GERON CORPORATION
Lipids and lipid assemblies comprising transfection enhancer elements
ActiveUS20080306153A1Promote absorptionImprove fusion effectAntibacterial agentsBiocideLipid formationLiposome
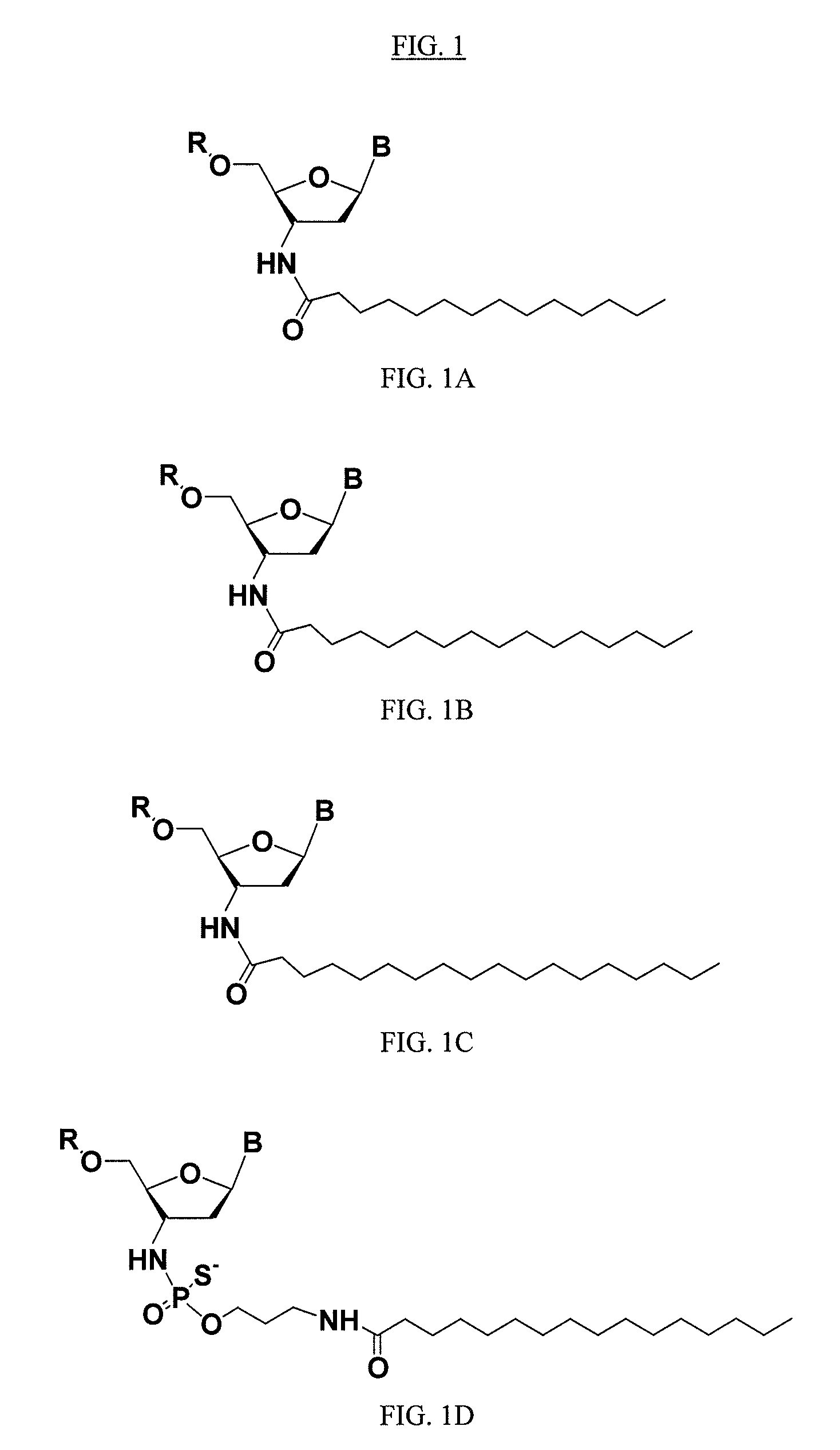
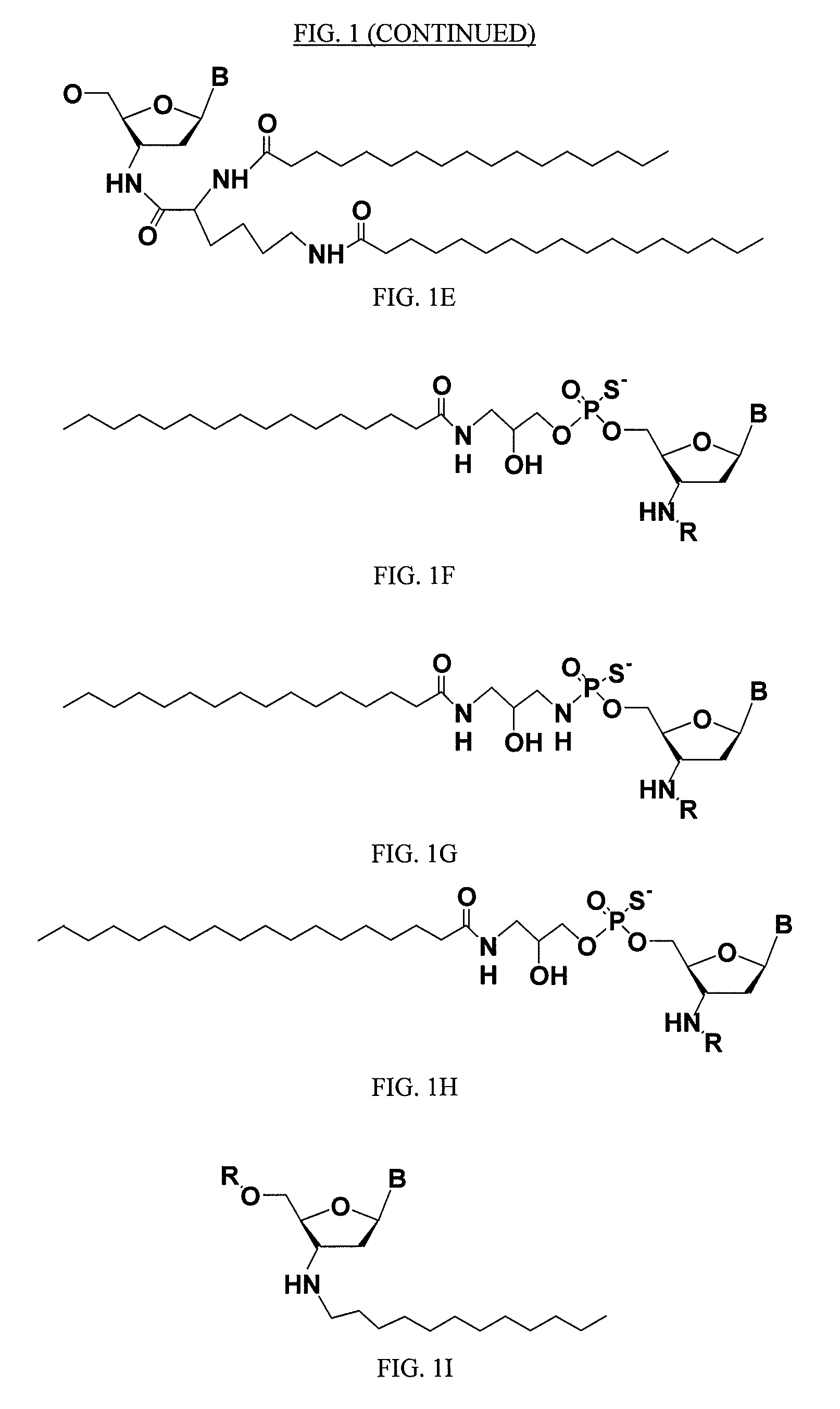
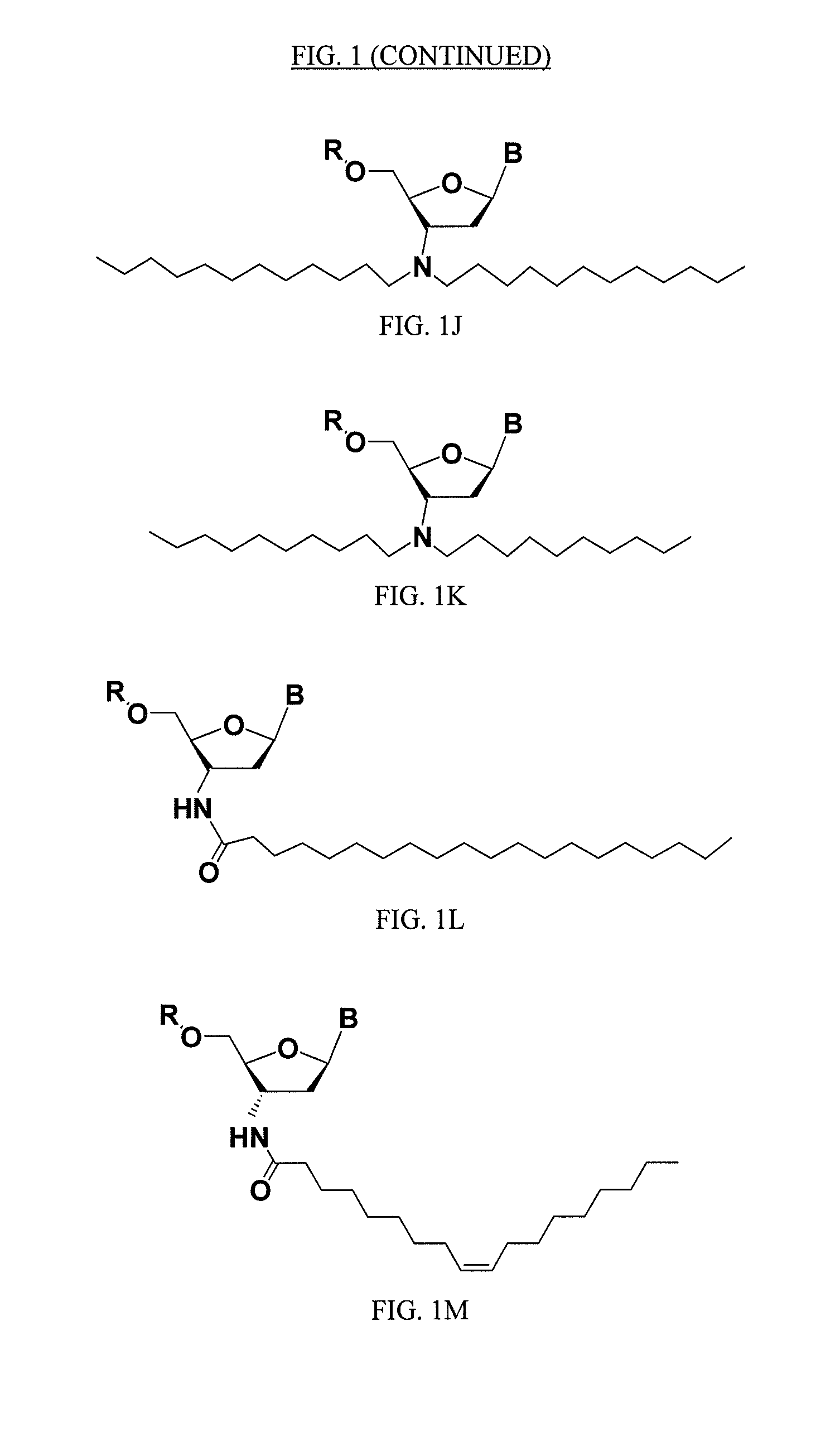
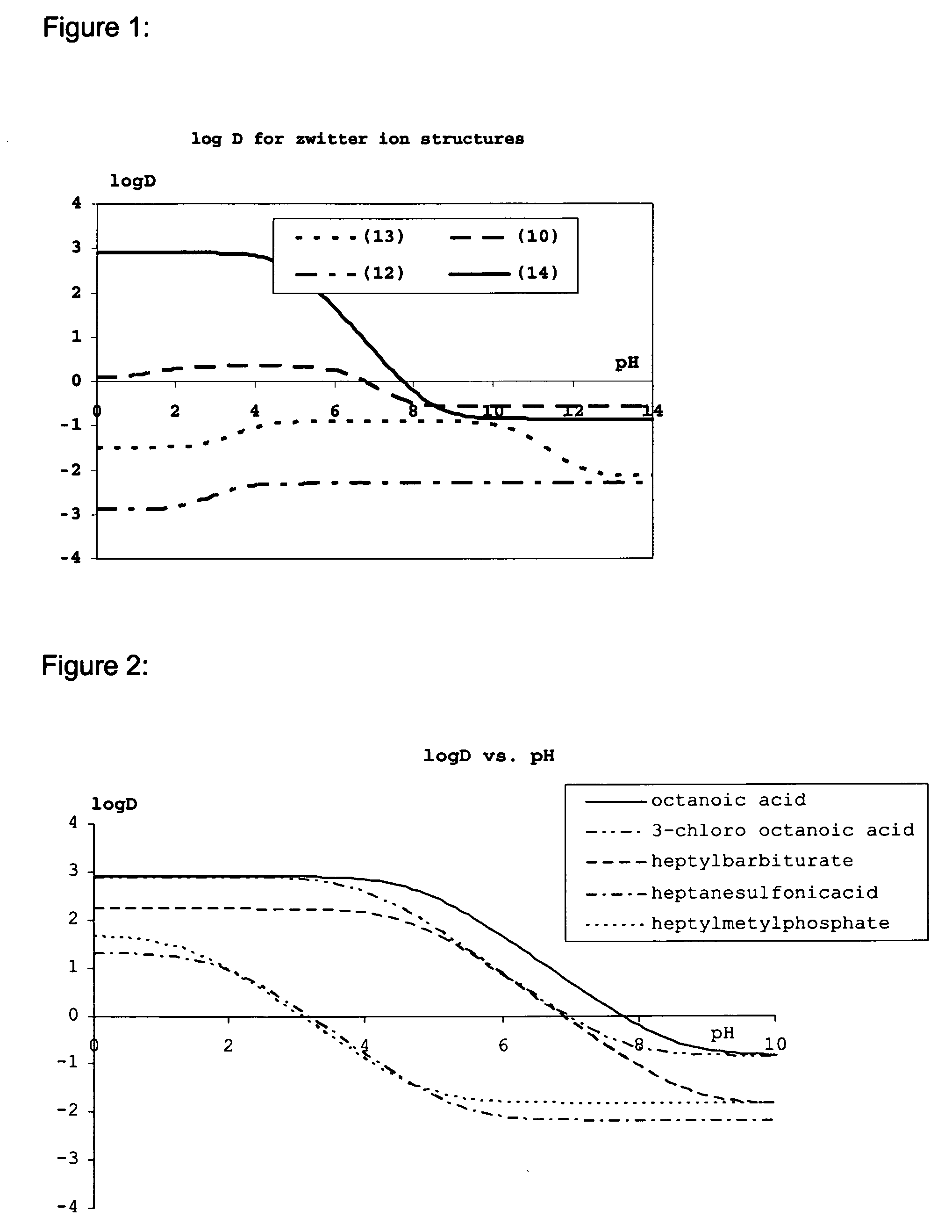
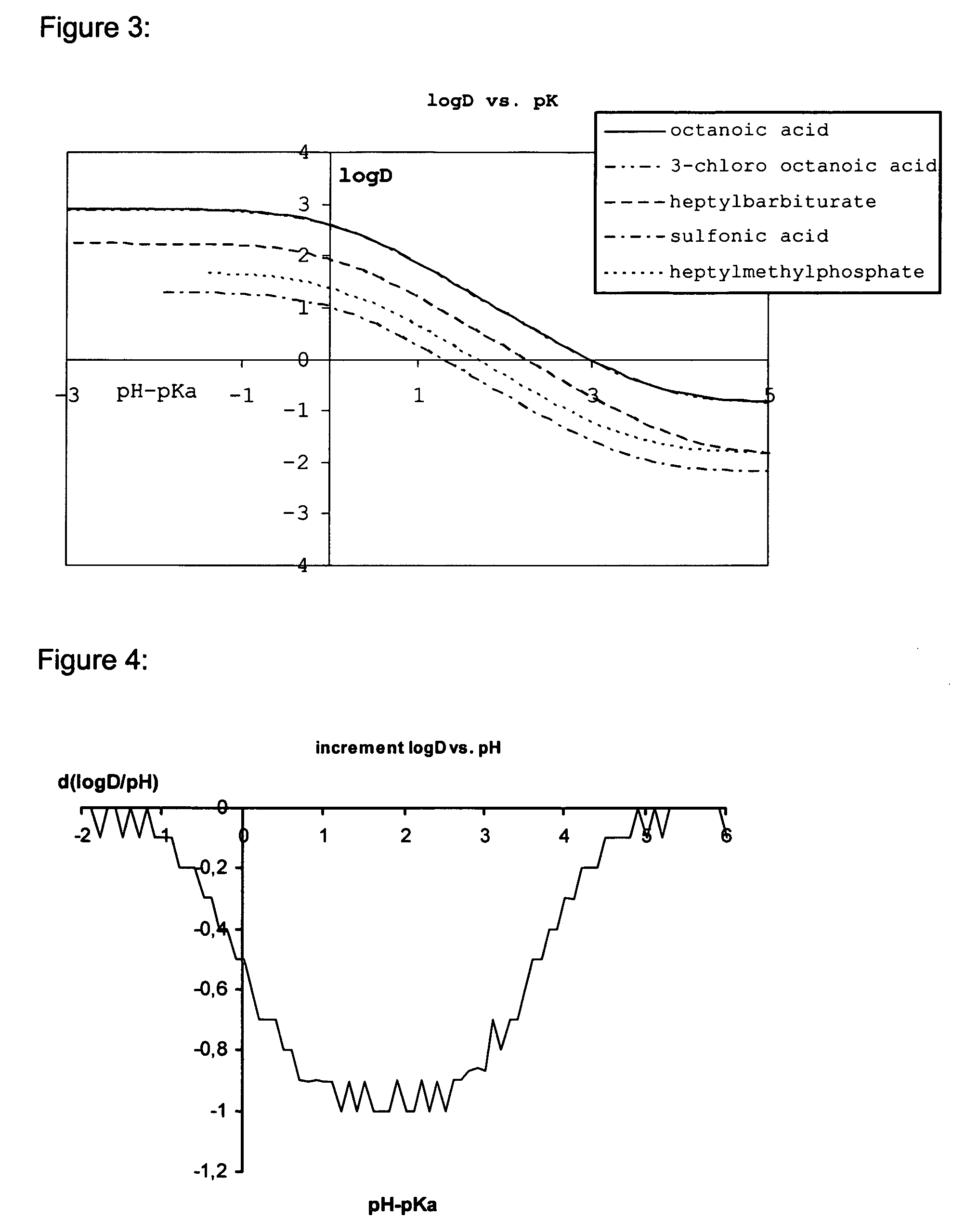
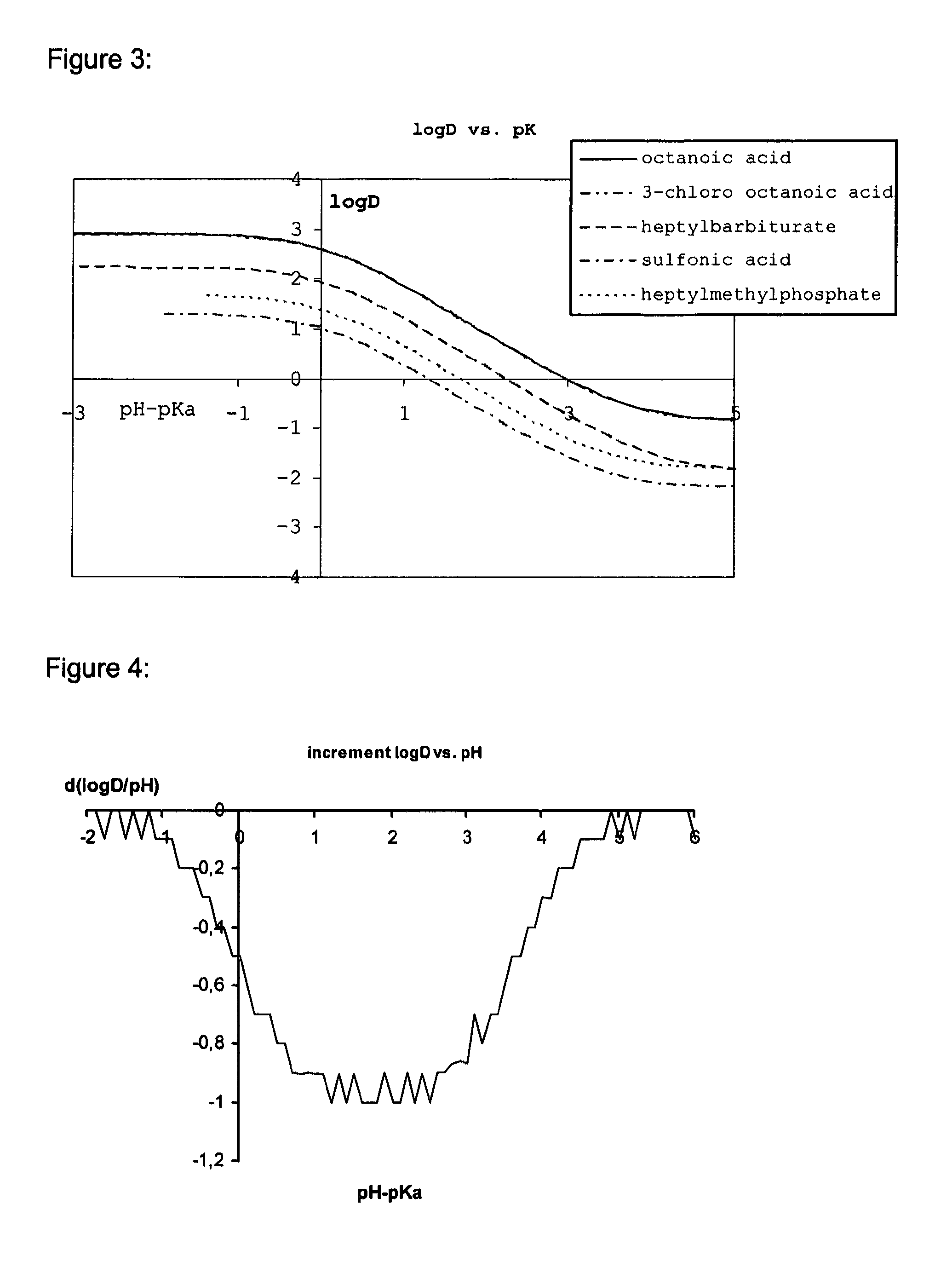
Lipid assemblies, such as liposomes, comprising transfection enhancer elements (TEE's), which are complexed with the lipid assemblies by means of ionic interactions, or lipids incorporating such TEE's are disclosed for enhancing the fusogenicity of the lipid assemblies. The TEE's have the formula:hydrophobic moiety-pH sensitive hydrophilic moiety (II)The pH sensitive hydrophilic moiety of each TEE is a weak acid having a pka of between 2 and 6 or a zwitterionic structure comprising a combination of acidic groups with weak bases having a pKa of between 3 and 8. Lipids incorporating one or more such TEEs have the formula (I):Lipid moiety-[Hydrophobic moiety-pH sensitive hydrophilic moiety] (I)
Owner:BIONTECH DELIVERY TECH GMBH
Modified oligonucleotides for telomerase inhibition
InactiveUS20120329858A1Superior cellular uptake propertyReduce Toxicity RiskOrganic active ingredientsSugar derivativesTelomeraseLipid moiety
Compounds comprising an oligonucleotide moiety covalently linked to a lipid moiety are disclosed. The oligonucleotide moiety comprises a sequence that is complementary to the RNA component of human telomerase. The compounds inhibit telomerase activity in cells with a high potency and have superior cellular uptake characteristics.
Owner:GERON CORPORATION
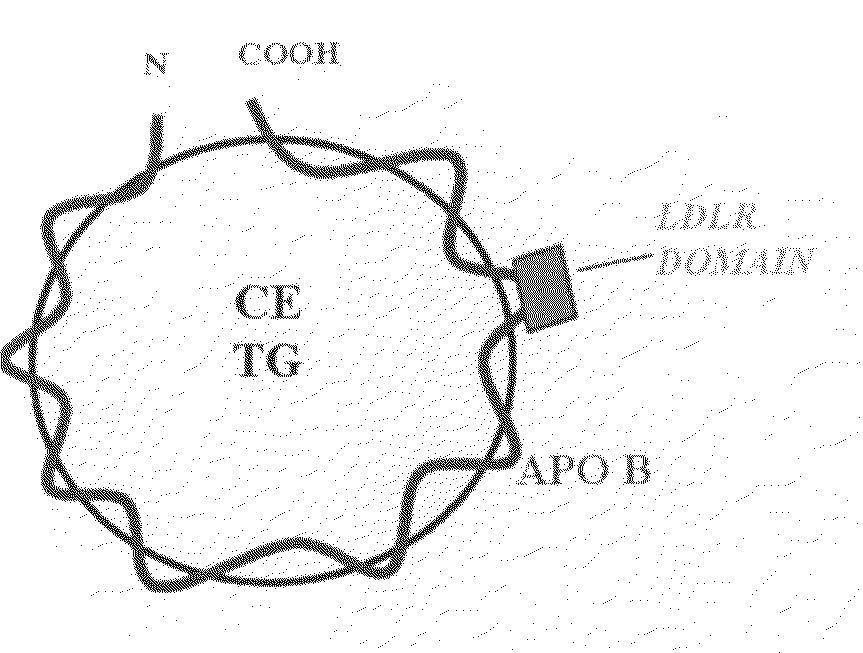
Synthetic LDL as Targeted Drug Delivery Vehicle
The present invention provides a synthetic LDL nanoparticle comprising a lipid moiety and a synthetic chimeric peptide so s to be capable of binding the LDL receptor. The synthetic LDL nanoparticle of the present invention is capable of c incorporating and targeting therapeutics for diseases associated with the expression of the LDL receptor. The invention further provides methods of using such synthetic LDL nanoparticles.
Owner:RGT UNIV OF CALIFORNIA +1
Polyamine-fatty acid derived lipidoids and uses thereof
ActiveUS20160002178A1Increase exposureIncrease concentrationOrganic active ingredientsOrganic chemistryRespiratory diseaseFatty acid
The present disclosure provides polyamine-fatty acid derived lipidoids (e.g., compounds of Formula (I) or (II)) and methods of preparing the lipidoids. A described lipidoid includes R—C(═O)—O— moieties (where R is a lipid moiety), which may be hydrolyzed into non-toxic fatty acids. Also provided are compositions including a described lipidoid and an agent (e.g., polynucleotide, small molecule, peptide, or protein). The present disclosure also provides methods, kits, and uses that involve the lipidoids or compositions for delivering an agent to a subject, tissue, or cell and / or for treating and / or preventing a range of diseases, such as genetic diseases, proliferative diseases, hematological diseases, neurological diseases, immunological diseases, gastrointestinal diseases, respiratory diseases, painful conditions, psychiatric disorders, and metabolic disorders.
Owner:MASSACHUSETTS INST OF TECH
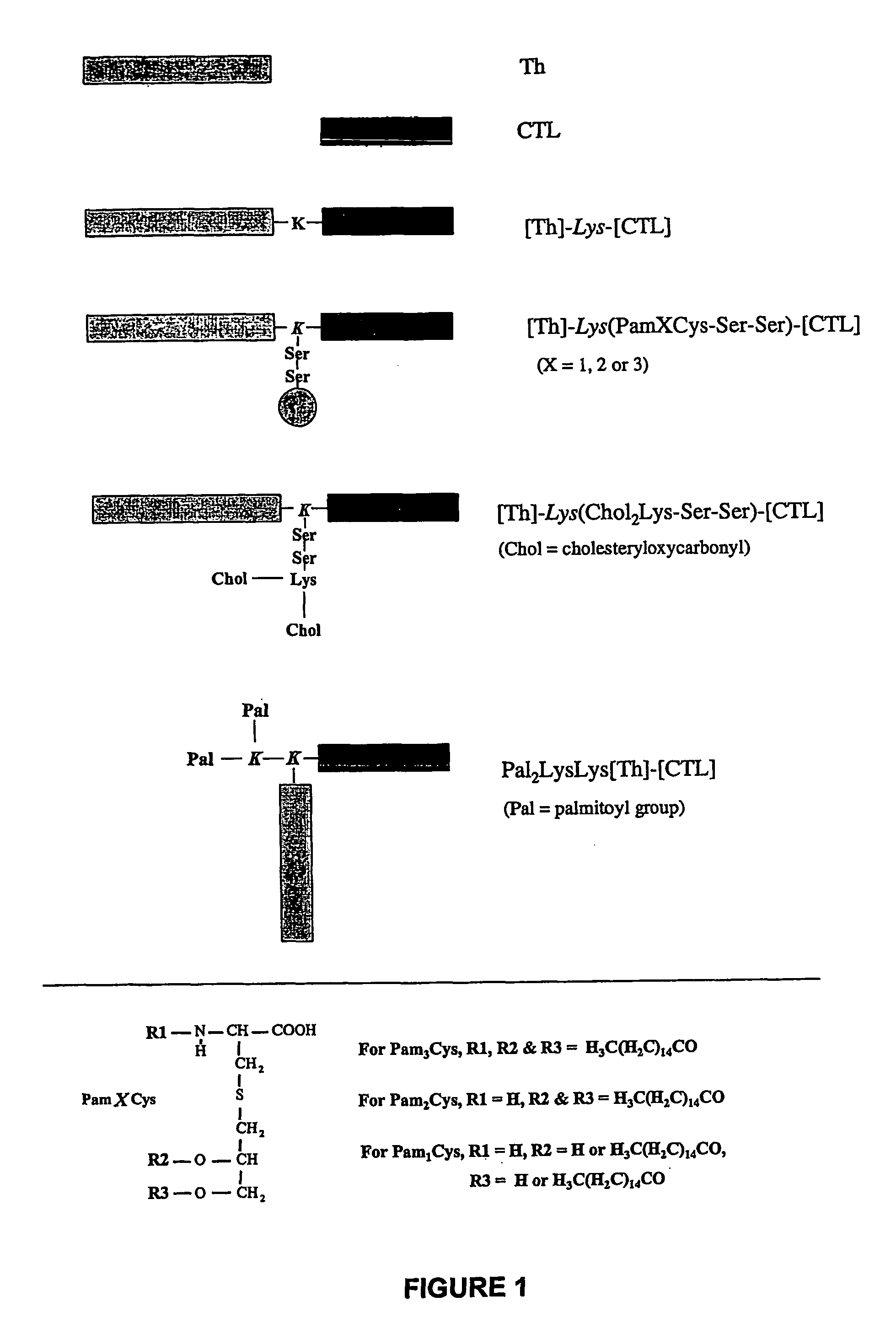
Immunogenic lipopeptides comprising T-helper and B-cell epitopes
InactiveUS7569225B2Increase profitEasy to specifyAntibacterial agentsBiocideSynthetic ImmunogensVaccination
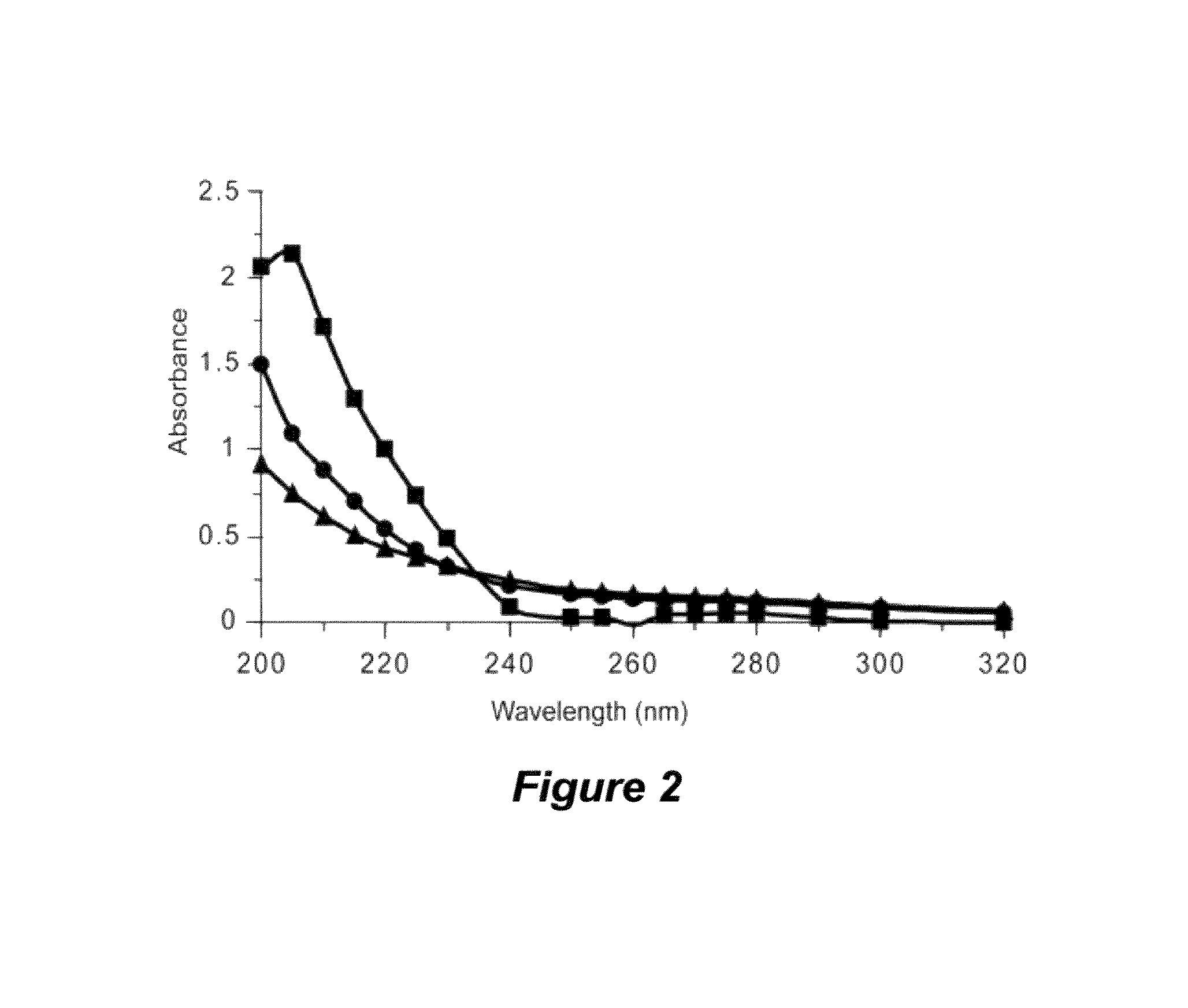
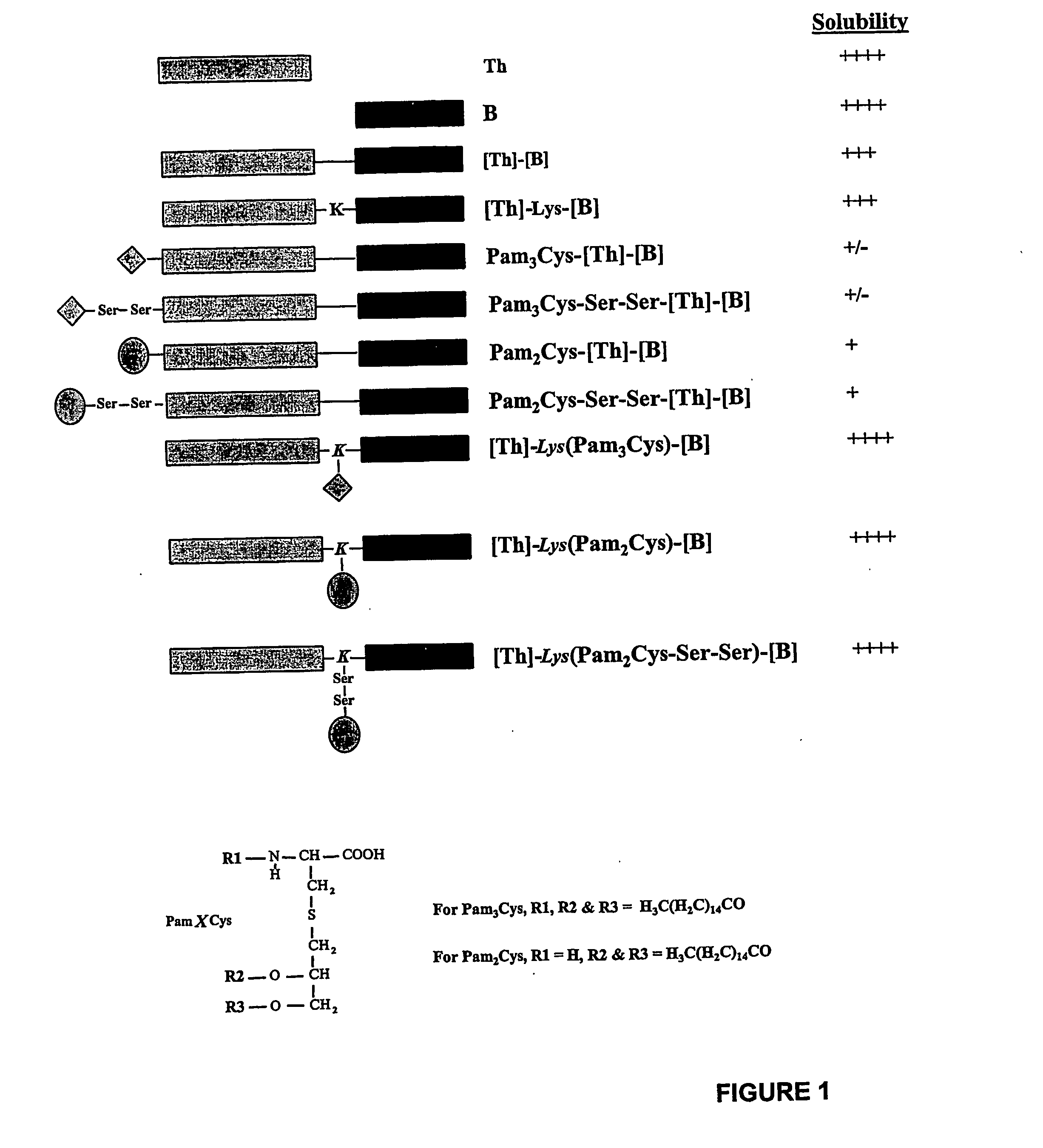
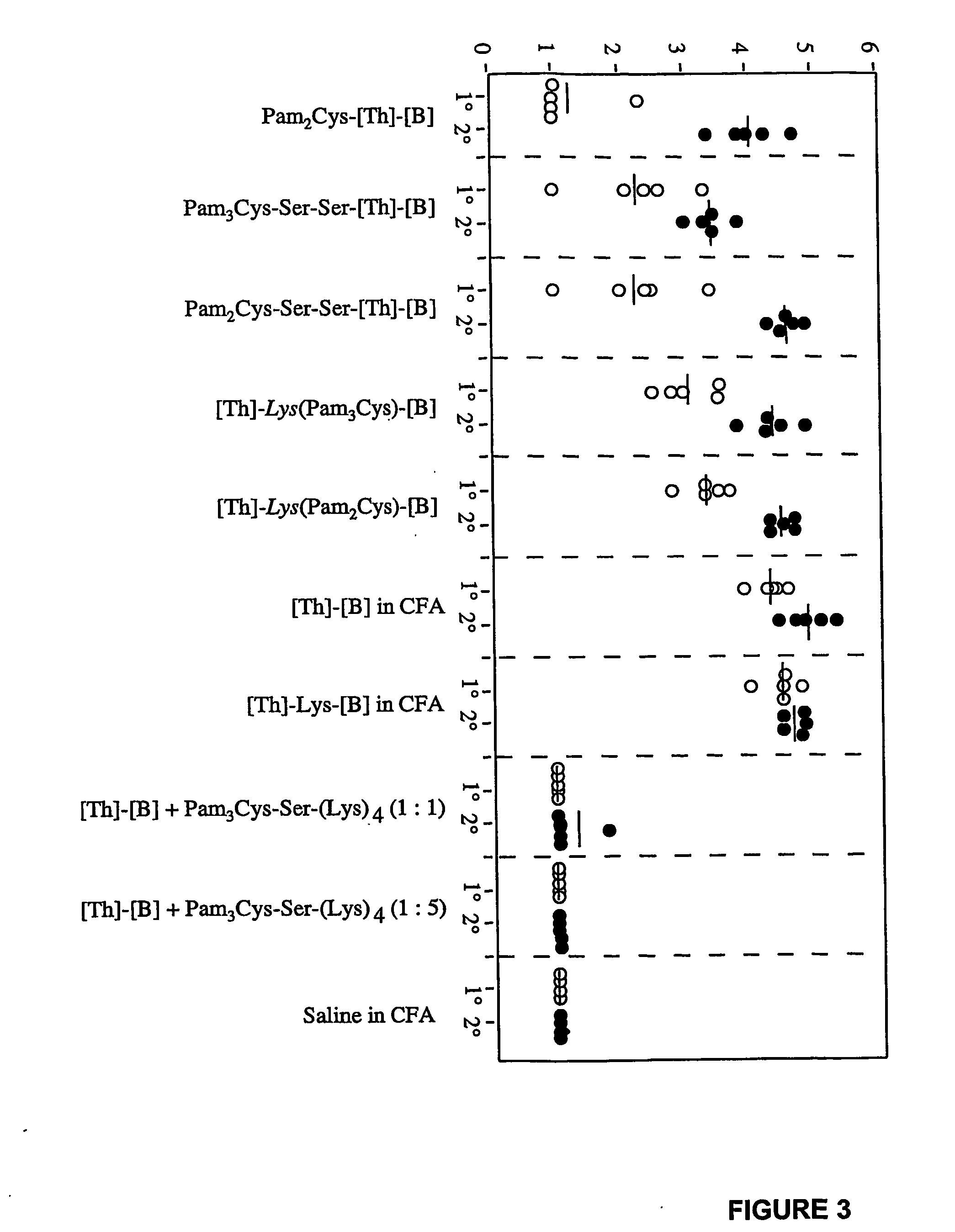
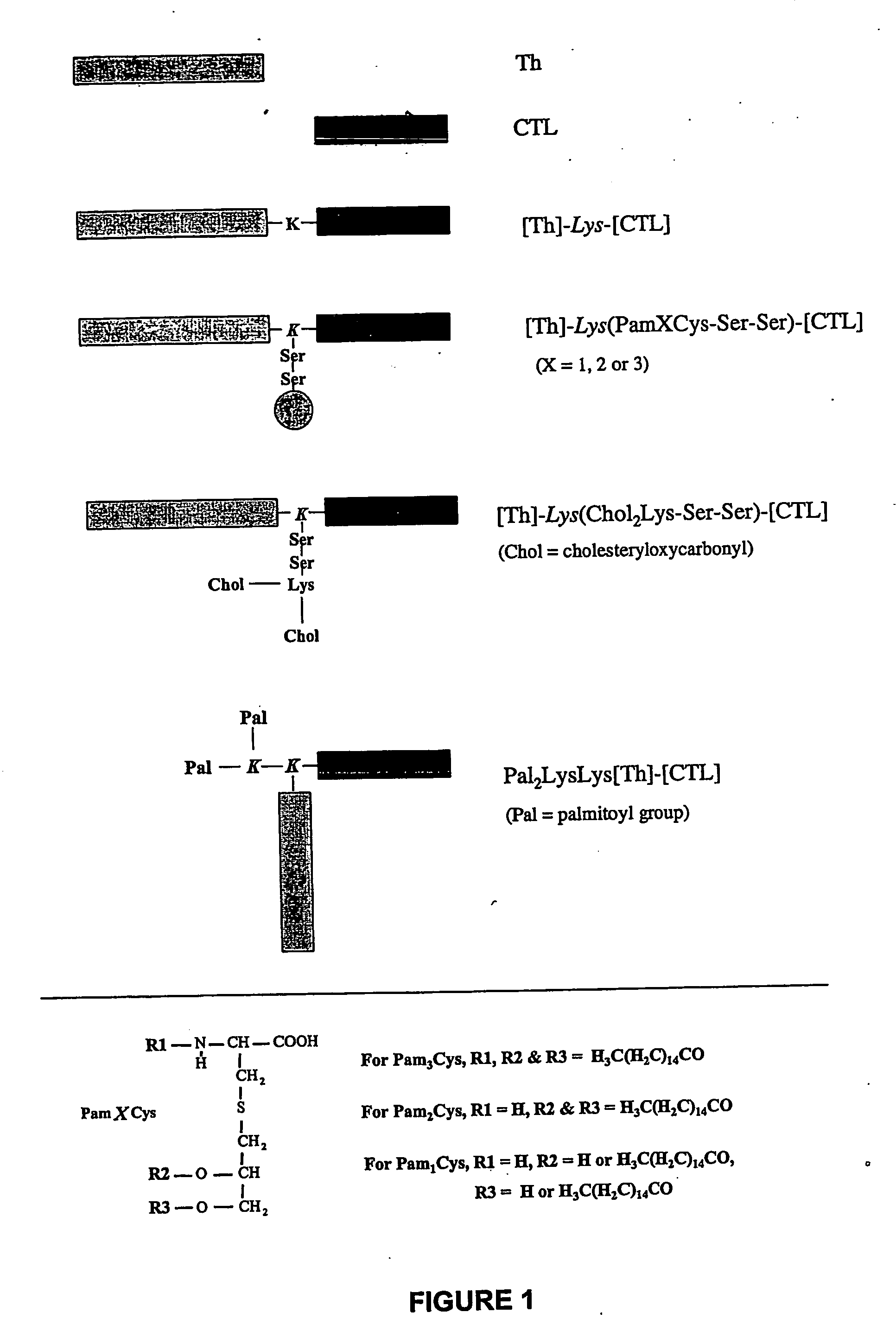
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and B cell epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular antigens. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the B cell epitope or within the T-helper epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Vaccine composition
InactiveUS20060216307A1UpregulationReducing lipid A toxicityAntibacterial agentsSenses disorderProtective antigenBacteroides
The present invention relates to an immuno-protective and non-toxic Gram-negative bleb vaccine suitable for paediatric use. Examples of the Gram-negative strains from which the blebs are made are N. meningitidis, M. catarrhalis and H. influenzae. The blebs of the invention are improved by one or more genetic changes to the chromosome of the bacterium, including up-regulation of protective antigens, down-regulation of immunodominant non-protective antigens, and detoxification of the Lipid A moiety of LPS.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions of active Wnt protein
ActiveUS7153832B2Maintain solubilityPeptide/protein ingredientsImmunoglobulinsSolubilityProtein composition
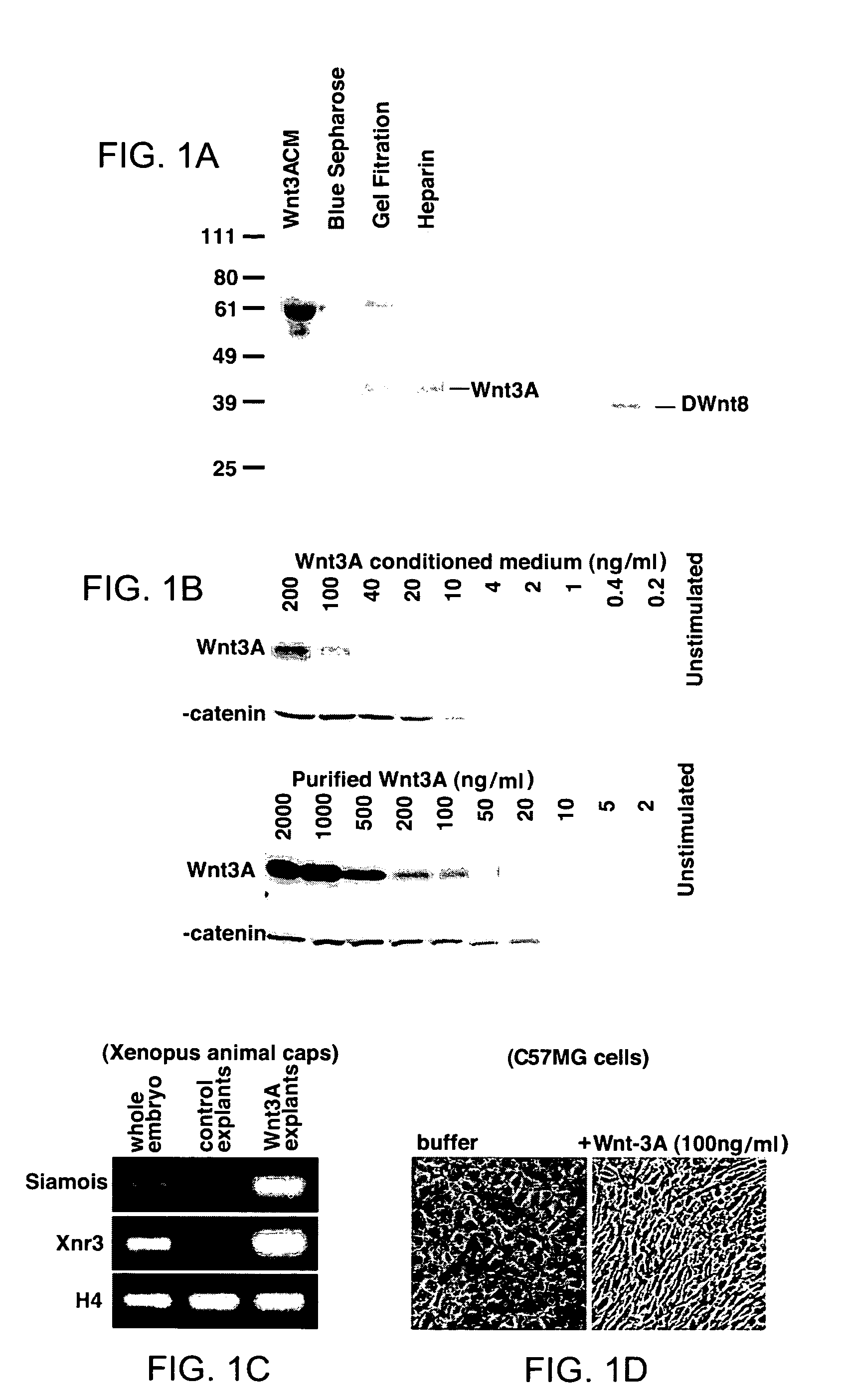
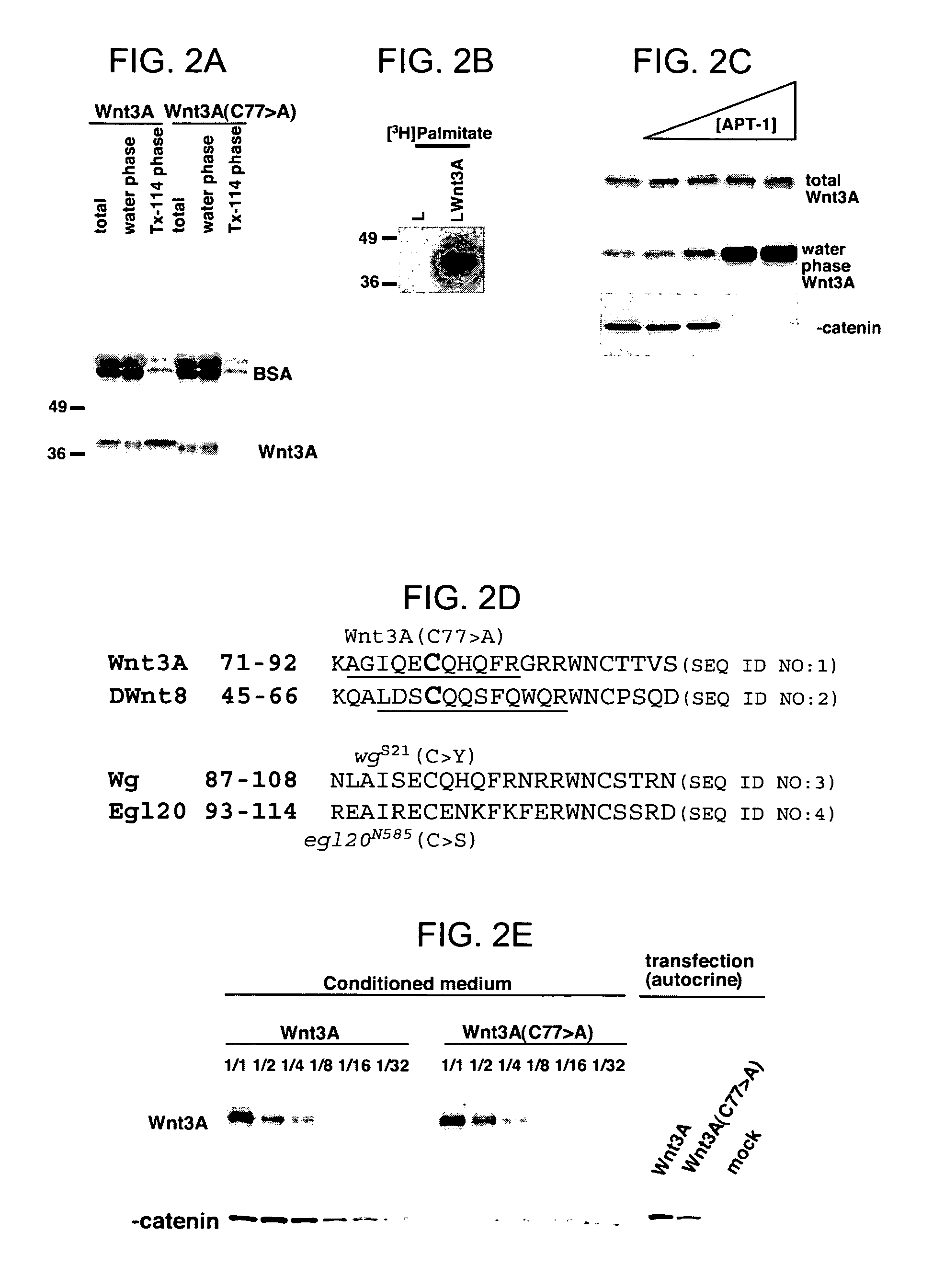
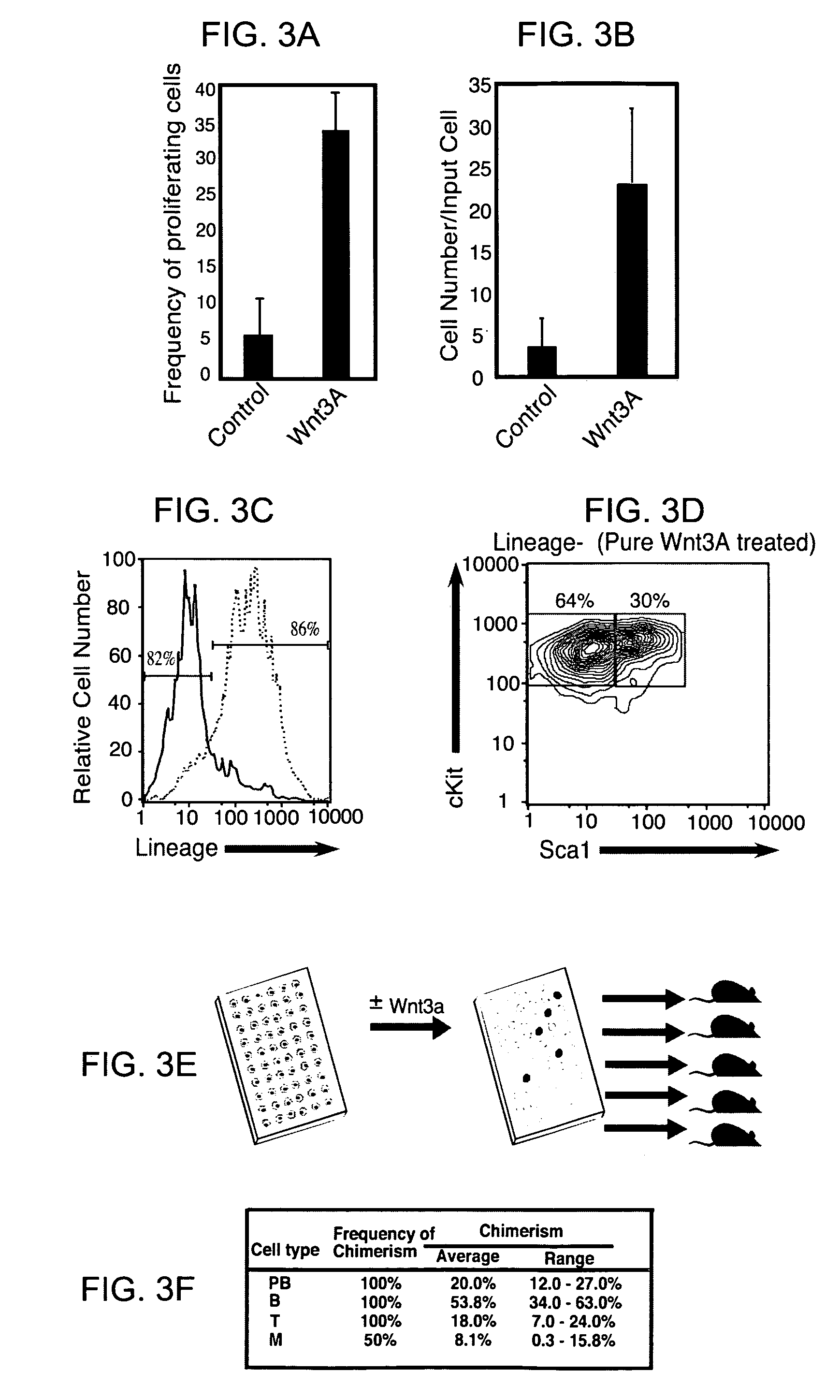
Compositions of purified biologically active Wnt proteins are provided. Wnt proteins are found to be hydrophobic and post-translationally modified by addition of a lipid moiety at a conserved cysteine residue. Methods for isolation of Wnt utilize detergents that maintain the solubility of the modified protein.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Saccharide antifreeze compositions
InactiveUS8604002B1Reducing crystal formationReducing and avoiding frostbiteBiocideOrganic active ingredientsGlycolipidXylose
The invention provides an antifreeze glycolipid compounds and composition comprising a polysaccharide moiety of Formula I:wherein D-Manp represents a D-mannopyranose moiety, D-Xylp represents a D-xylopyranose moiety, and n is about 5 to about 70; and one or more lipid moieties covalently linked to the polysaccharide moiety of Formula I or electrostatically associated with the polysaccharide moiety of Formula I. The antifreeze glycolipid compounds and compositions can be used for a variety of industrial, agricultural, medical, and cosmetic applications where recrystallization-inhibition, cyroprotection, or cryopreservation is desired. The antifreeze glycolipid compounds or compositions can be used as, for example, as cryoprotectants for tissue preservation and transplantation, improving the texture of processed frozen food and frozen meats, frostbite protection, crop protection, and green alternatives for land vehicle antifreeze and aircraft de-icing.
Owner:UNIV OF NOTRE DAME DU LAC
Novel immunogenic lipopeptides comprising t-helper and b-cell epitopes
InactiveUS20070066534A1Promote maturityEnhance antigen presentationAntibacterial agentsBiocideSynthetic ImmunogensDiamino acid
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and B cell epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular antigens. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the B cell epitope or within the T-helper epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
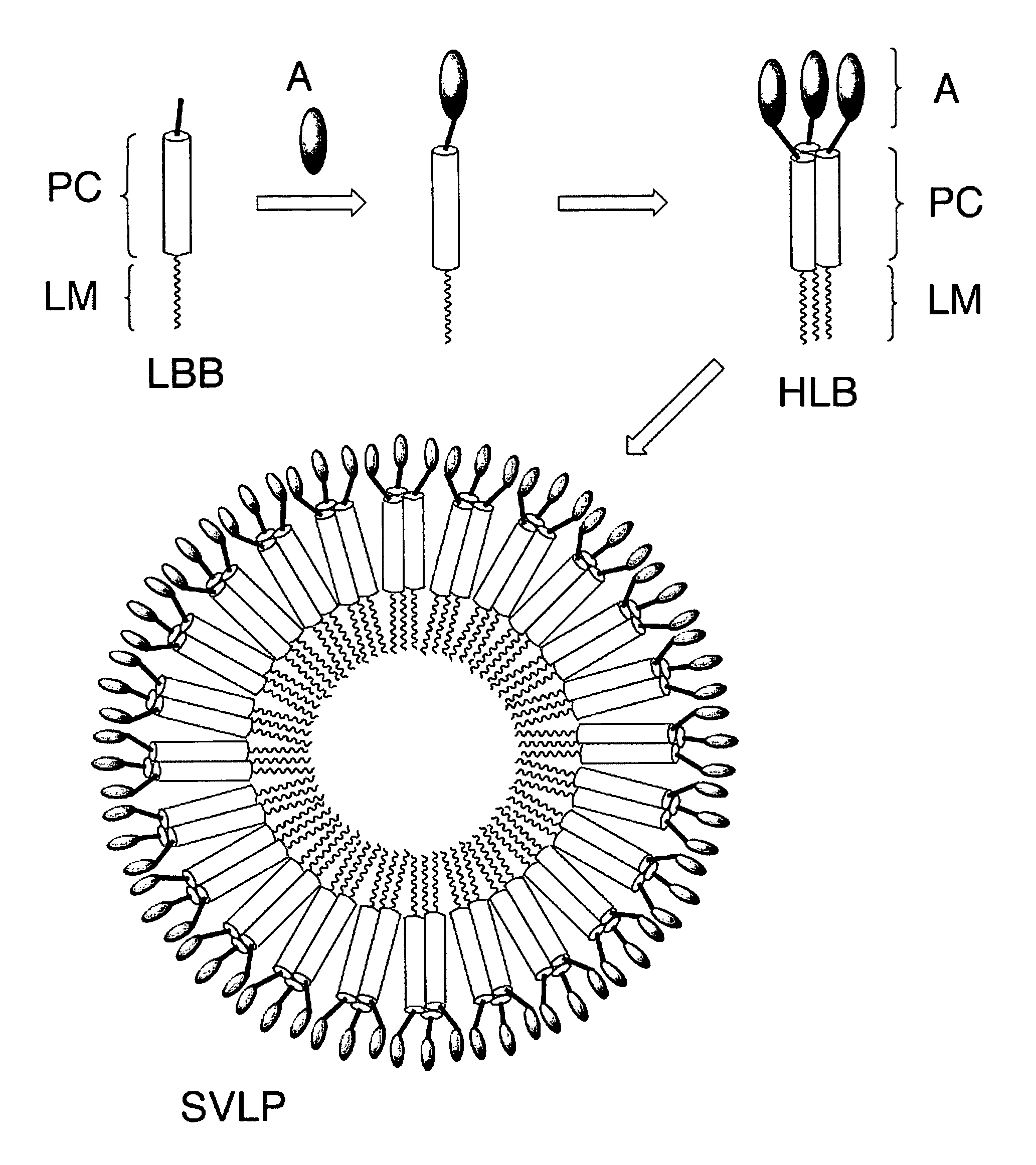
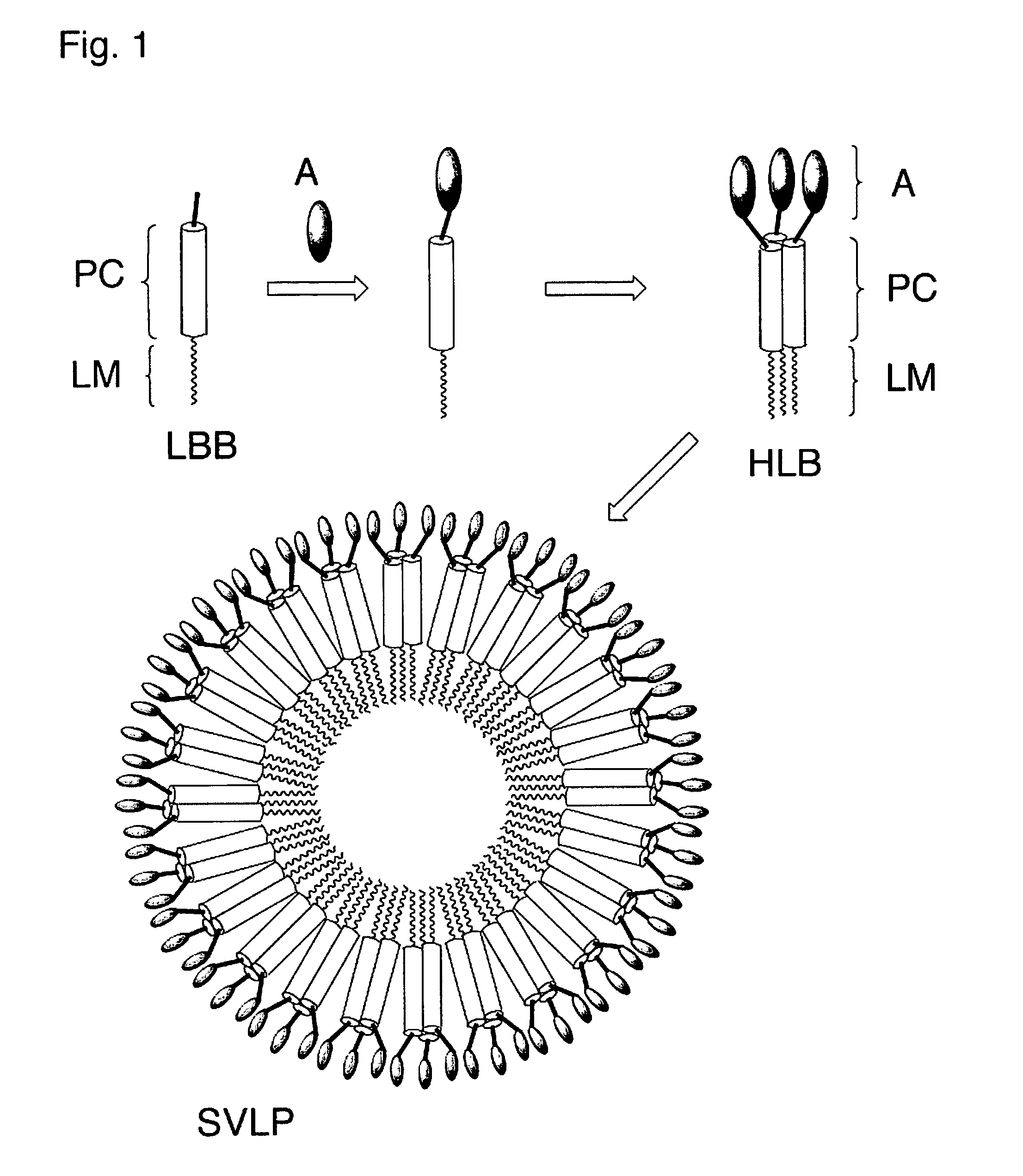
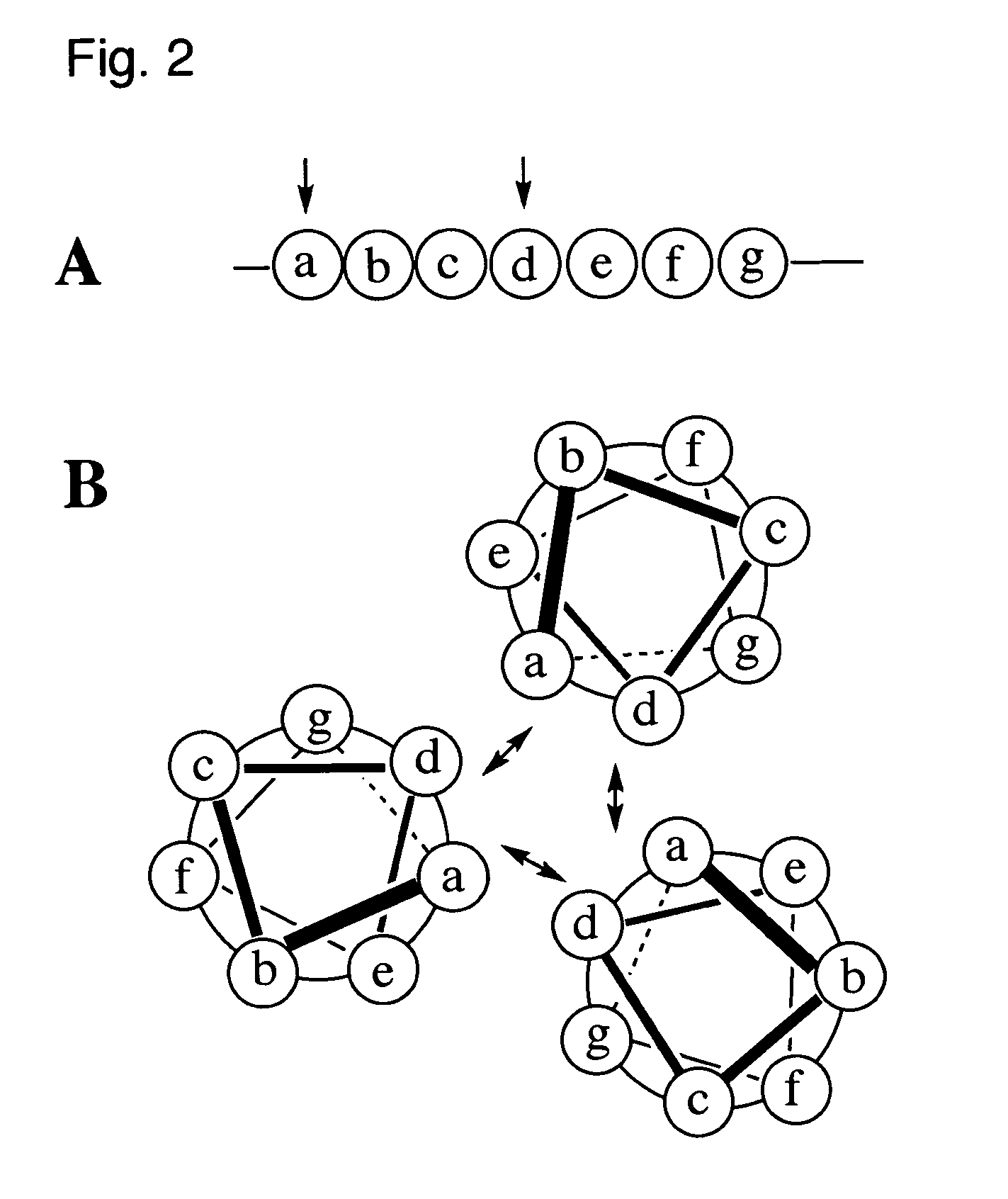
Coiled-coil lipopeptide helical bundles and synthetic virus-like particles
ActiveUS20100015173A1Efficient inductionVirus peptidesSaccharide peptide ingredientsChemical synthesisCoiled coil
The invention relates to lipopeptide building blocks consisting of a peptide chain comprising a coiled-coil domain, linked covalently to a lipid moiety comprising long alkyl or alkenyl chains, and optionally linked to an antigen; and to helical lipopeptide bundles and synthetic virus-like particles formed by aggregation. The nanometer size and shape of these bundles and particles, their stability under aqueous physiological conditions, their chemical composition, the possibility to incorporate B- and T-cell epitopes, and their production by chemical synthesis, make them highly suitable as vaccine delivery vehicles.
Owner:UNIV ZURICH
Biodegradation Process and Composition
Owner:AMERICAN CHEM CORP
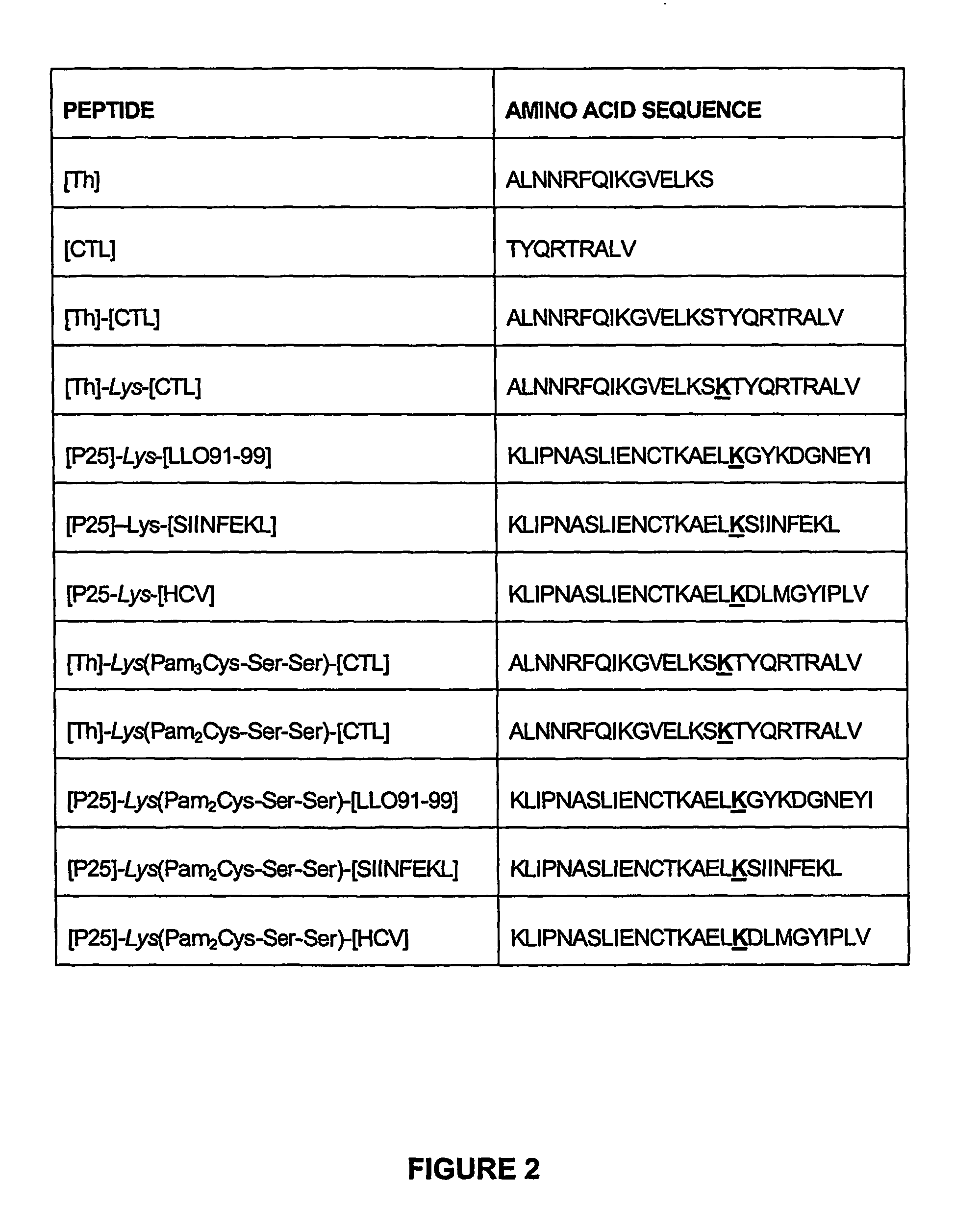
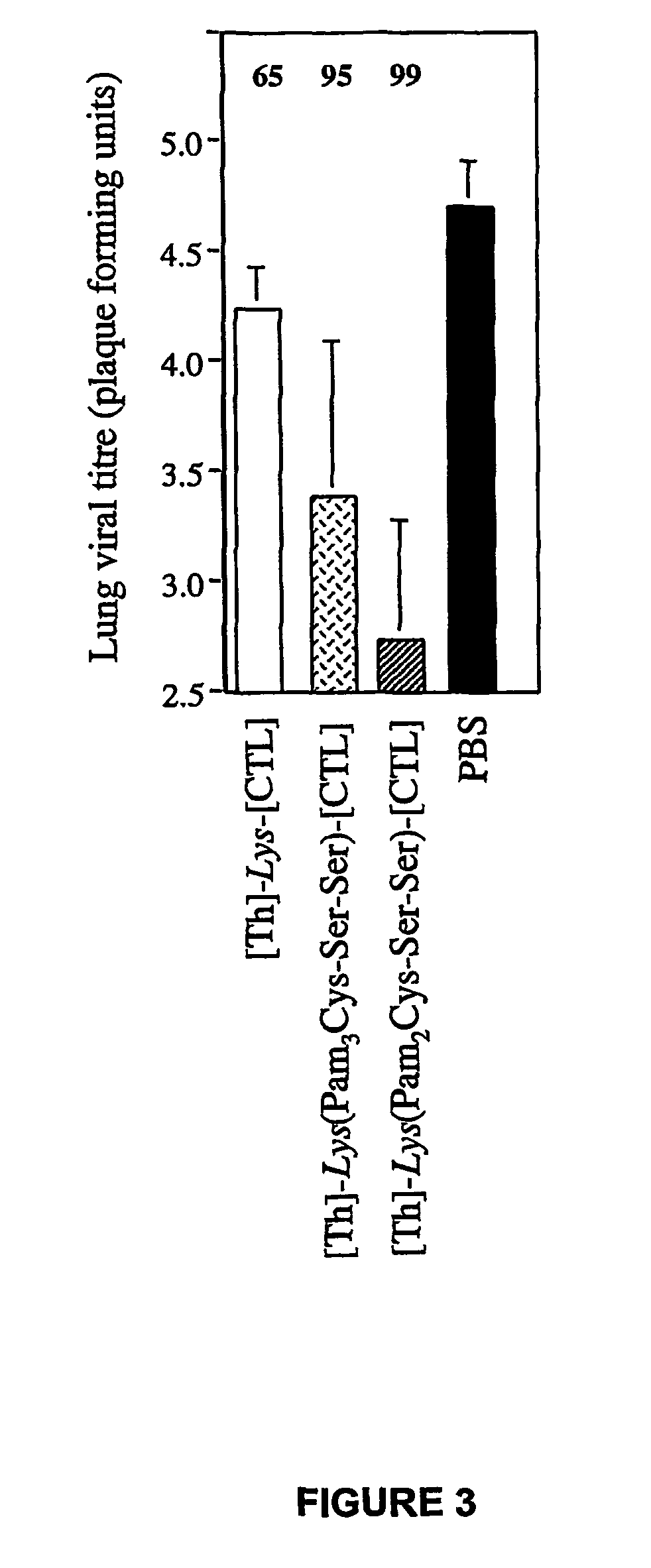
Immunogenic lipopeptides comprising T-helper and cytotoxic T lymphocyte (CTL) epitopes
InactiveUS7833532B2Easy to synthesizeIncrease profitSsRNA viruses negative-senseBiocideSynthetic ImmunogensCtl epitope
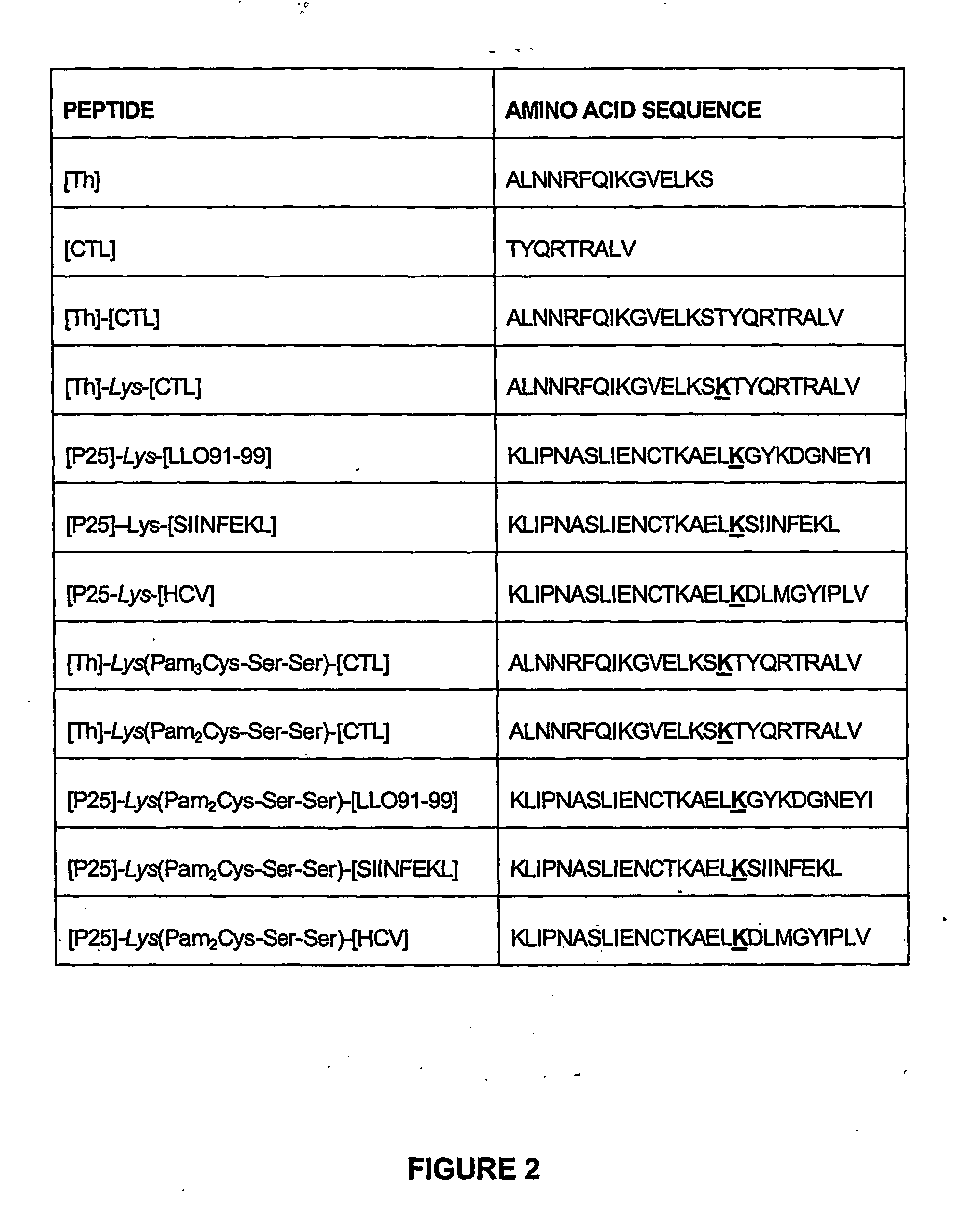
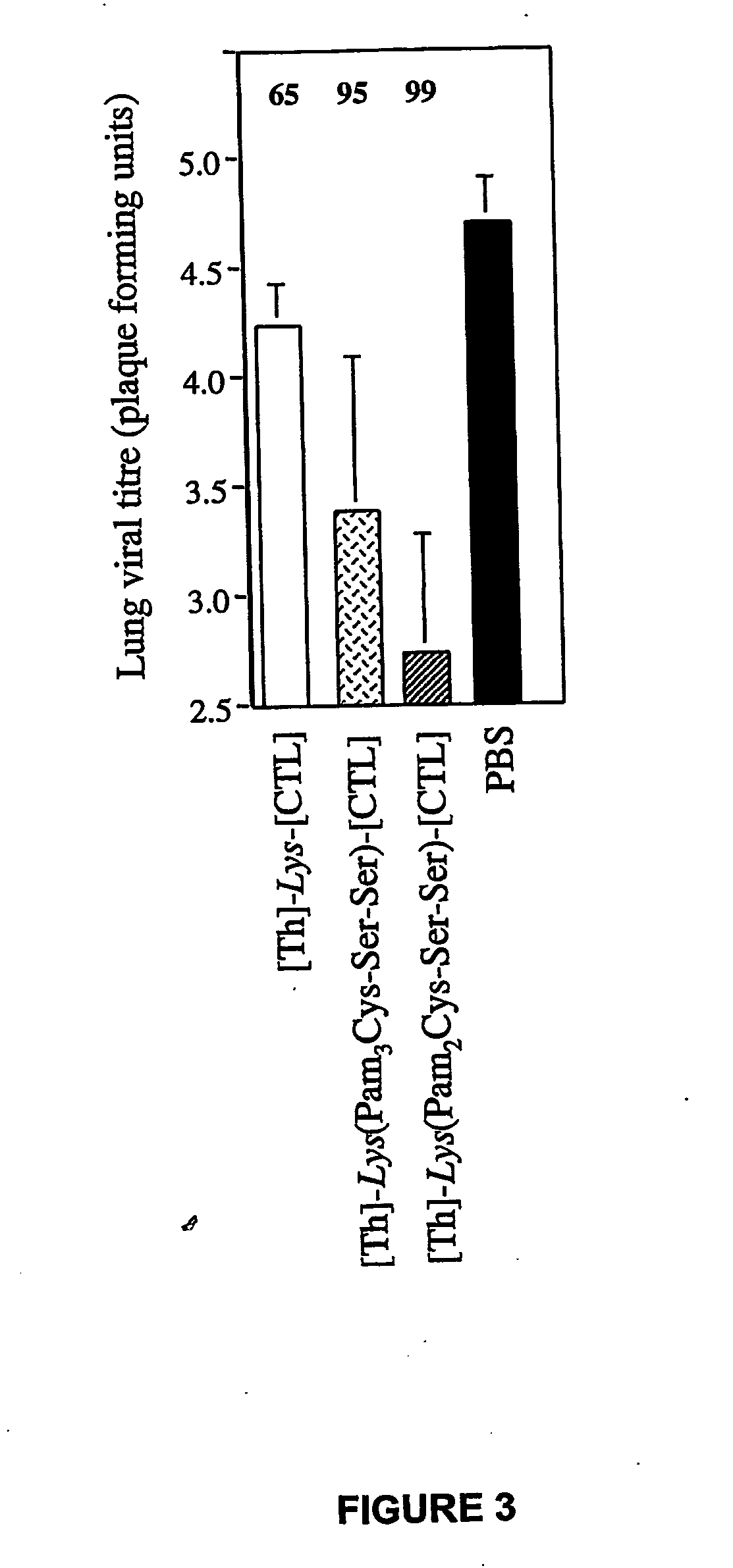
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and CTL epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular CTL epitopes. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the CTL epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Compound
ActiveUS20110002983A1Reduce capacityEnhance antibody responseOrganic active ingredientsAntimycoticsAntigenAdjuvant
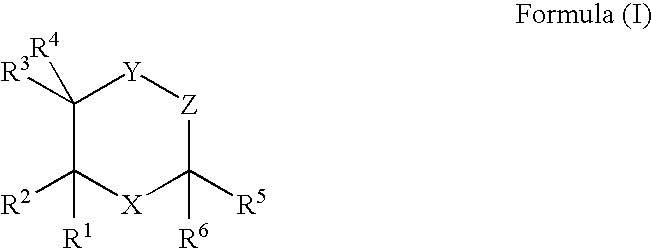
The invention provides a compound (which can act as an adjuvant) of Formula I or Formula (II), wherein R1 R4 R5 R6 and R7 are each independently selected from hydrogen, acetyl, hydrocarbyl, a lipid moiety and a lipid acyl moiety; R2 is a hydroxyl, a hydrocarbyl, a lipid moiety, a lipid acyl moiety; or an amino hydrocarbyl group optionally substituted with a hydrocarbyl, a lipid moiety or a lipid acyl moiety; R3 and R8 are each independently selected from acetyl, a hydrocarbyl, a lipid moiety and a lipid acyl moiety; X is a peptide chain; The above normuramylglycopeptide compounds can be located in liposomes and micelles and can function as immunomodulators, along with a desired antigen (or DNA encloding the antigen), in (e.g. DNA) vaccines.
Owner:IMUTHES LTD +2
Short antimicrobial lipopeptides
ActiveUS20150080291A1Enhanced anti-microbial activityPromotes skin healthAntibacterial agentsCosmetic preparationsArginineTyrosine
Disclosed are peptides having biological and therapeutic activity. Particularly disclosed are lipidated di- or tri-peptides analogs of KPV or KdPT that exhibit antimicrobial activity. In particular, the peptides of this invention provide enhanced anti-microbial activity over the base tri-peptides, lysine-proline-valine and lysine-d-proline-tyrosine. The disclosed peptides have the general formula of C12-18 lipid-KXZ-NH2i wherein K is lysine; X is proline, d-proline, histidine or arginine; Z is optional and if present Z is valine, threonine, alanine or leucine; and the terminal COOH is NH2 amidated. The C12-18 lipid is preferably the lipid moiety of lauric acid (C12), myristic acid (C14), pentadecanoic acid (C15), palmitic acid (C16), or stearic acid (C18). The invention is further related to methods of using of these peptides to treat various insults, inflammations or bacterial infections affecting the skin and other related mucosal body surfaces such as the oral cavity.
Owner:HELIX BIOMEDIX INC
Method and apparatus for preparing novel liposome
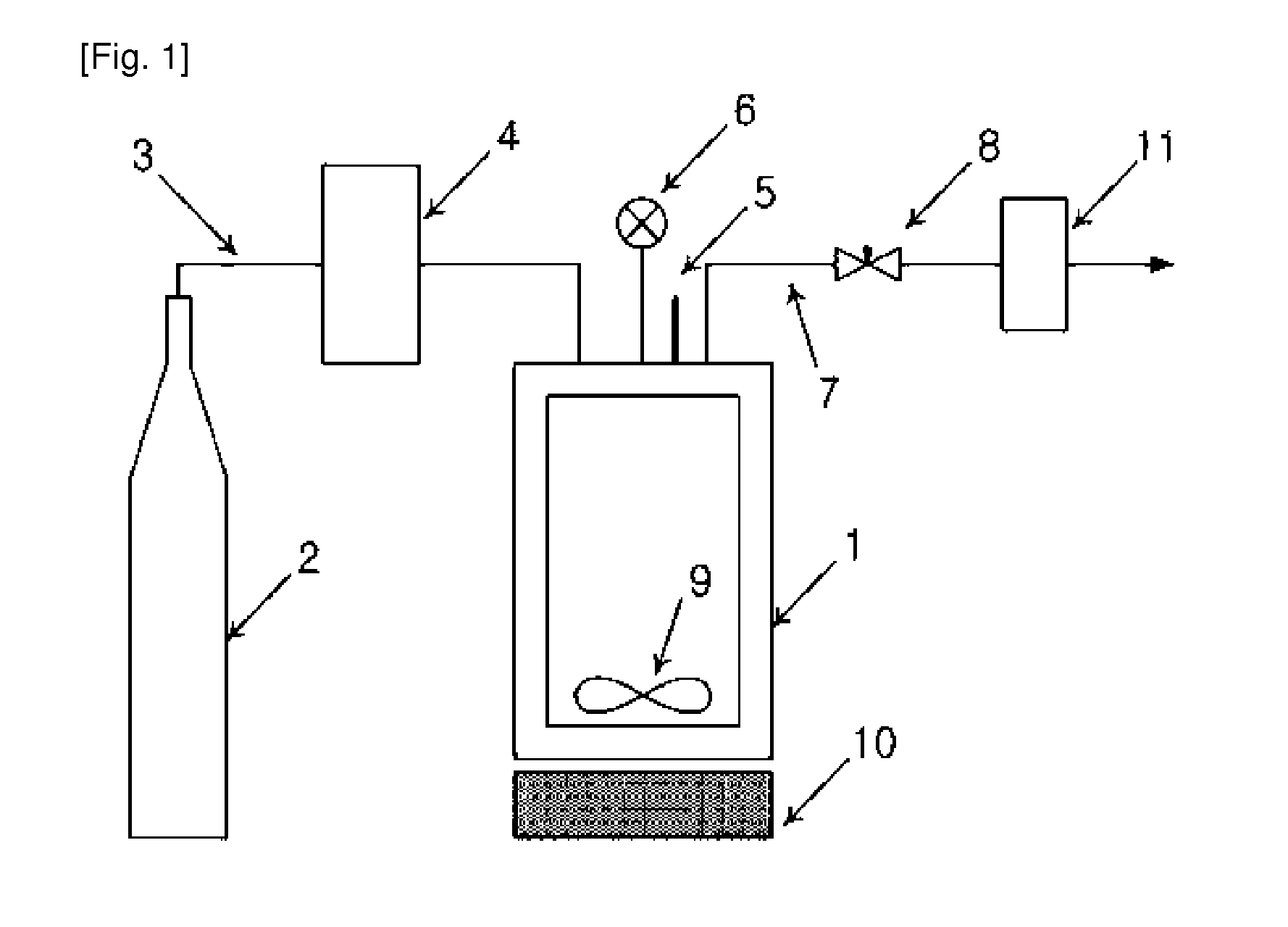
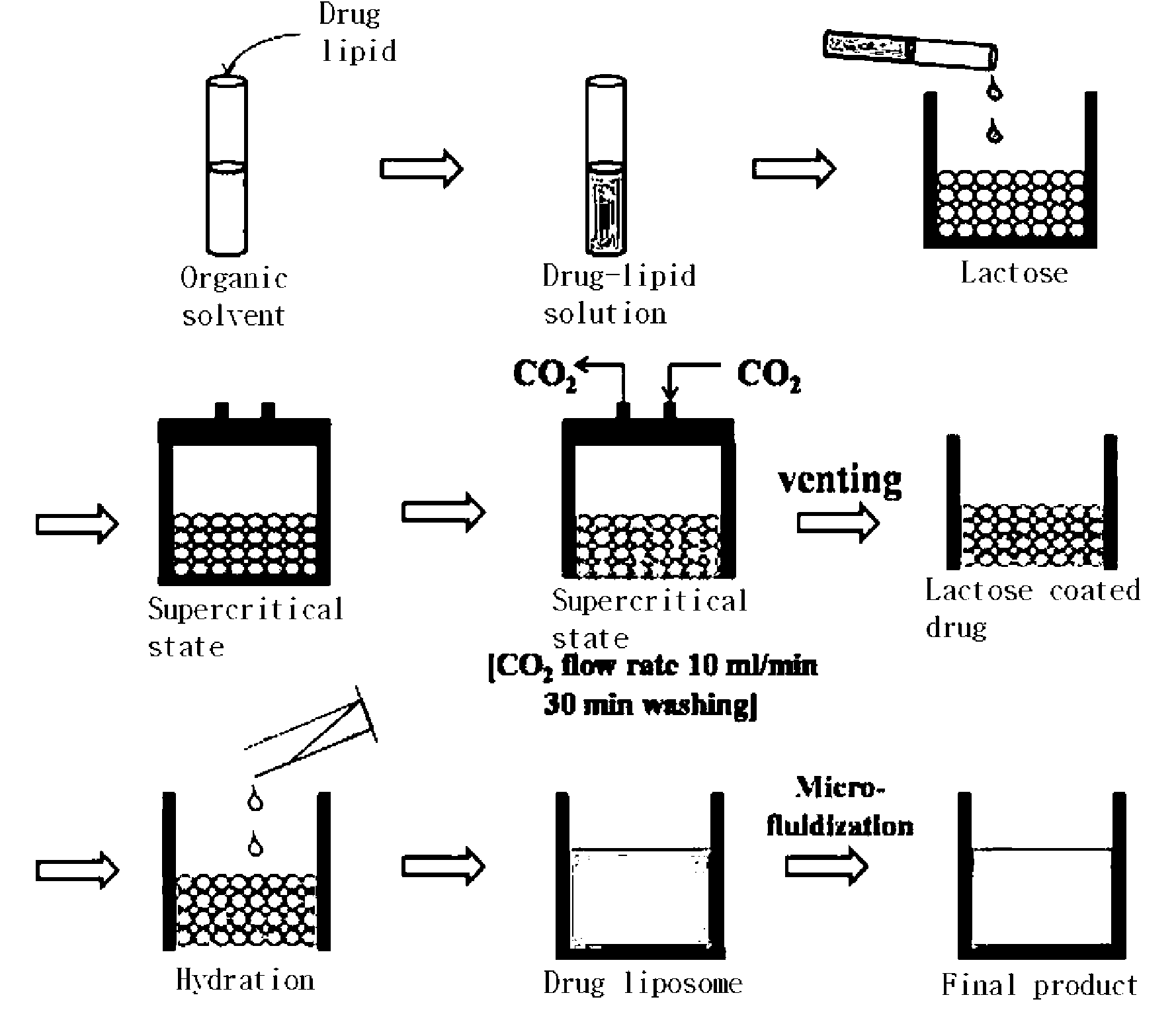
ActiveUS20130069261A1Increase contentImprove loading efficiencyAntimycoticsSolvent extractionLaboratory scaleFreeze-drying
Disclosed is a method for preparing a liposome formulation. In the disclosed method, a lipid fraction is dissolved in an organic solvent. The solution including a bioactive component and the lipid fraction, together with a carrier, is put in a reaction vessel, and a supercritical fluid is introduced thereto, so as to prepare particles coated with the bioactive component-lipid. The supercritical fluid is discharged by compression to obtain proliposome particles, and then the proliposome particles are hydrated by an aqueous solution including water so as to form a liposome solution. Preferably, the formulation may include one or more bioactive components. As required, the liposome formulation may be further processed by methods such as particle size reduction, removal of organic solvent, and freeze-drying. The preparation method can be easily carried out at a laboratory scale. Furthermore, the same method can be employed in liposome formulation preparation in mass production, or at a commercial scale.
Owner:BCWORLD PHARMA
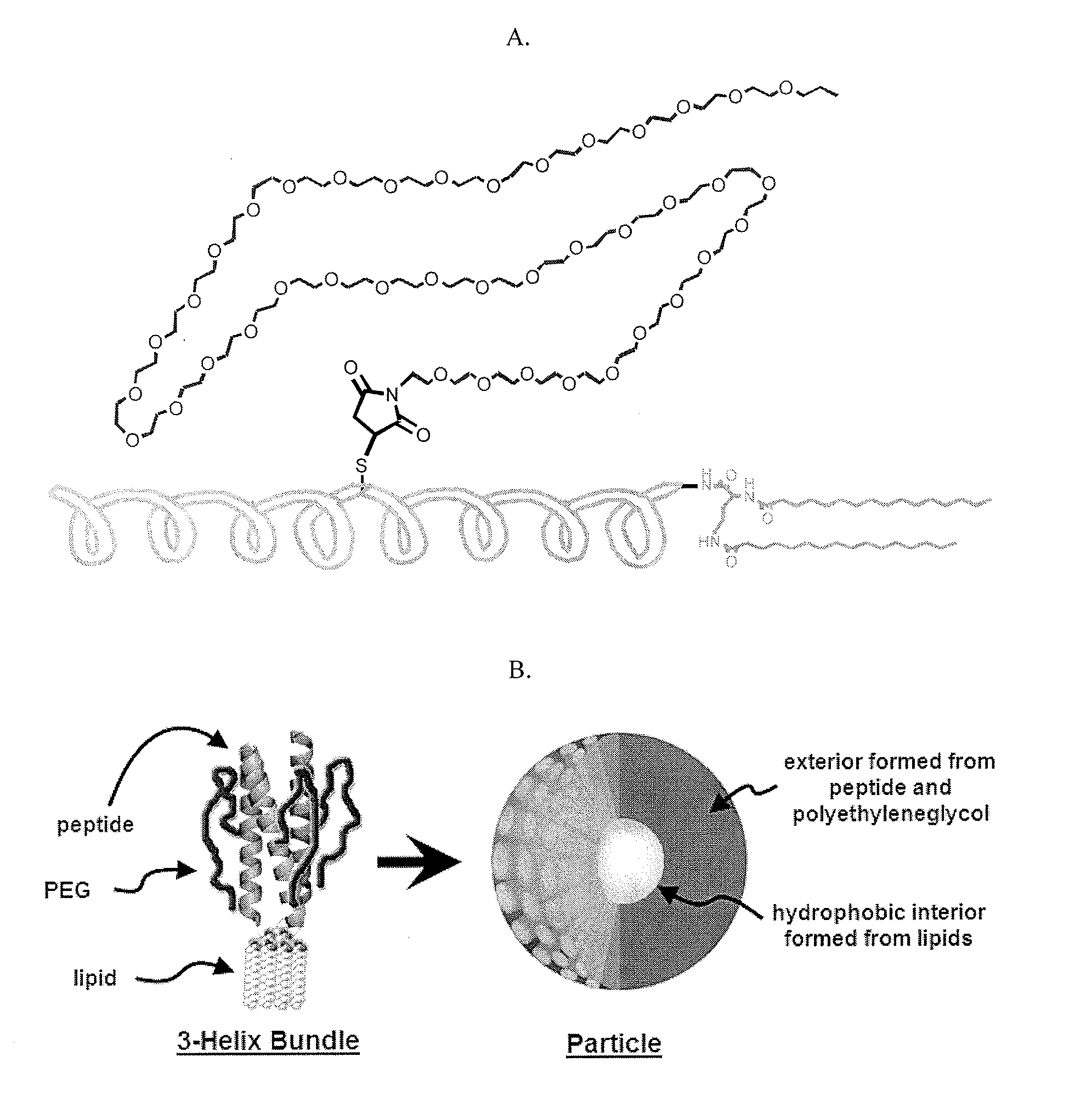
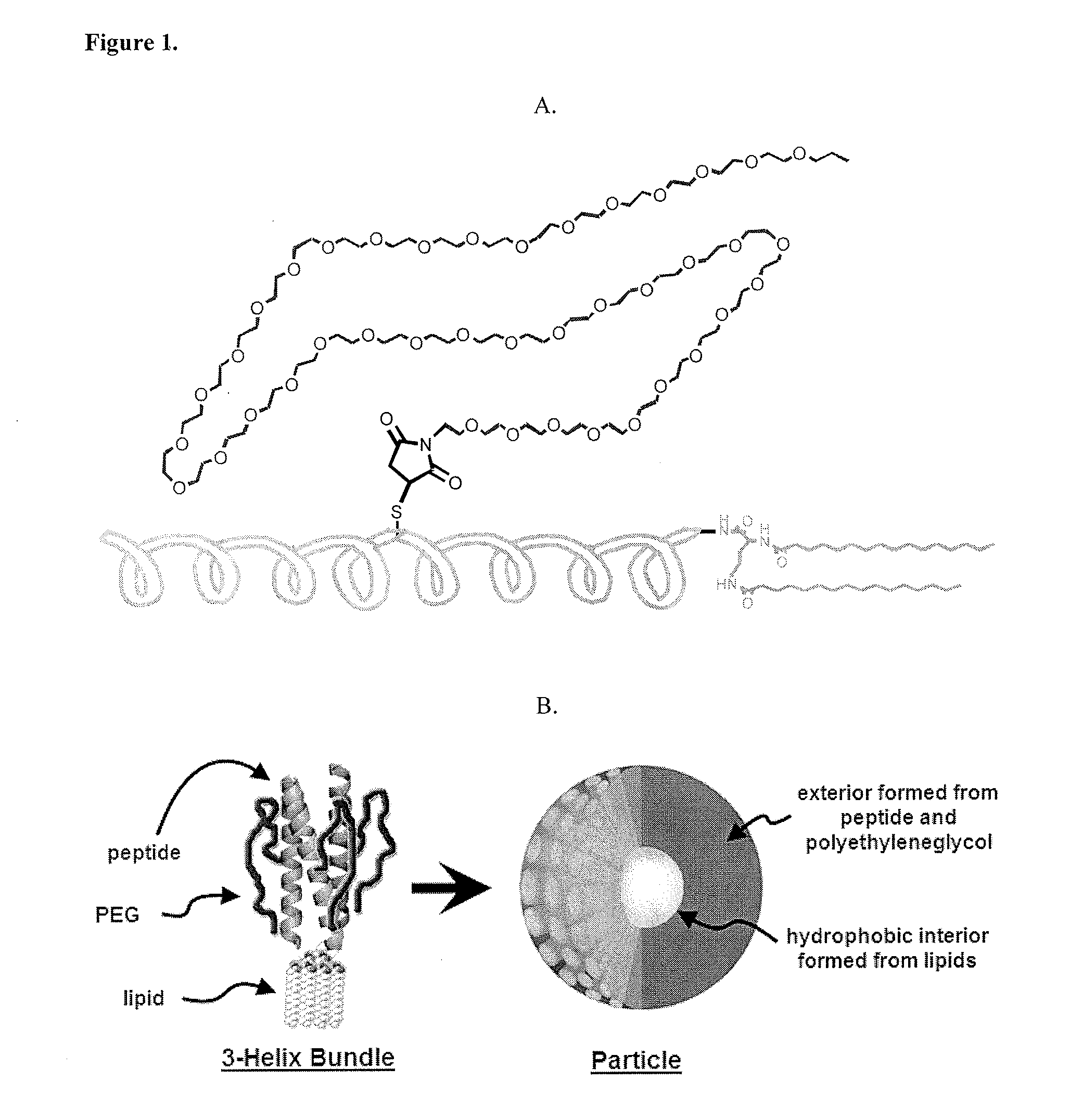
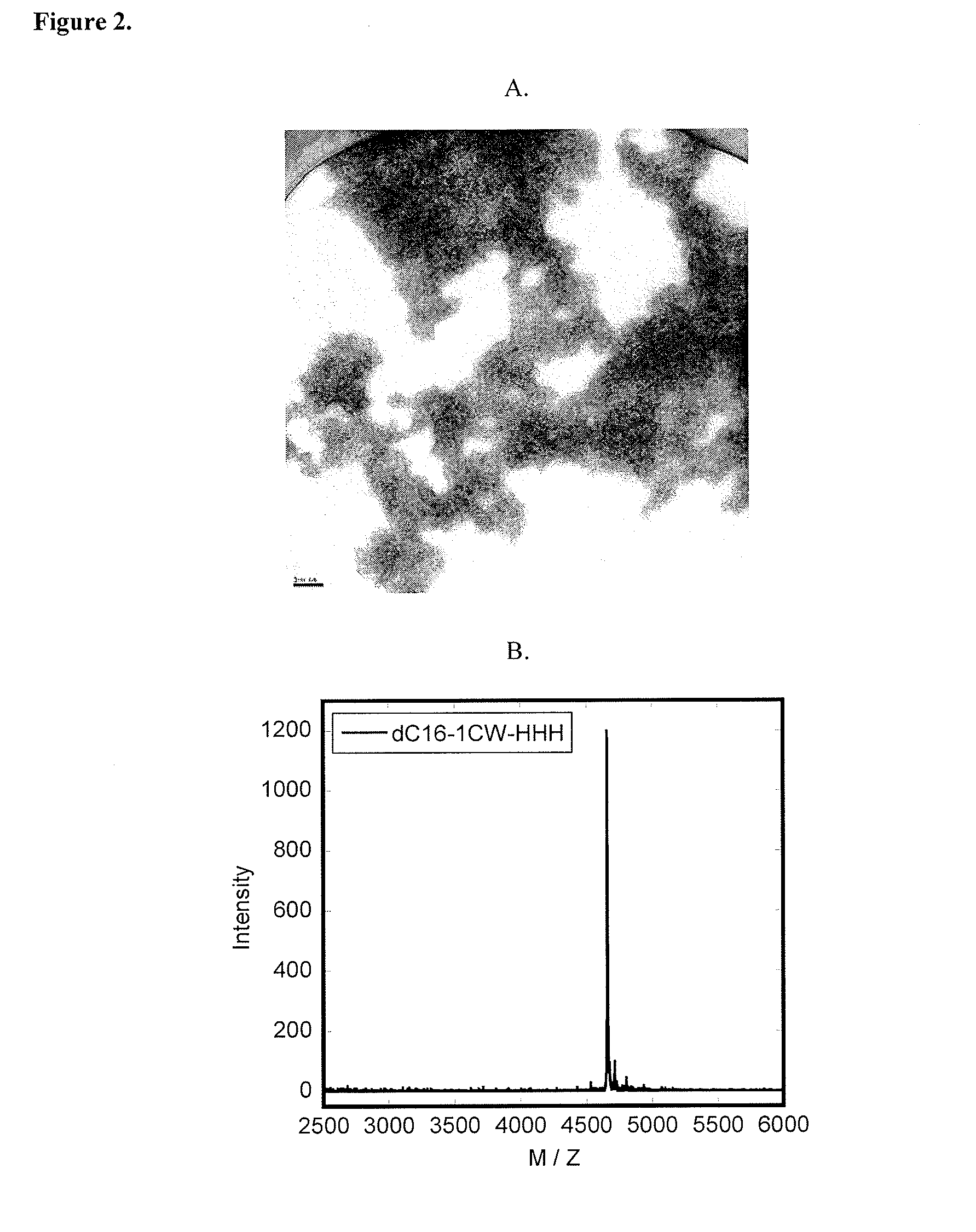
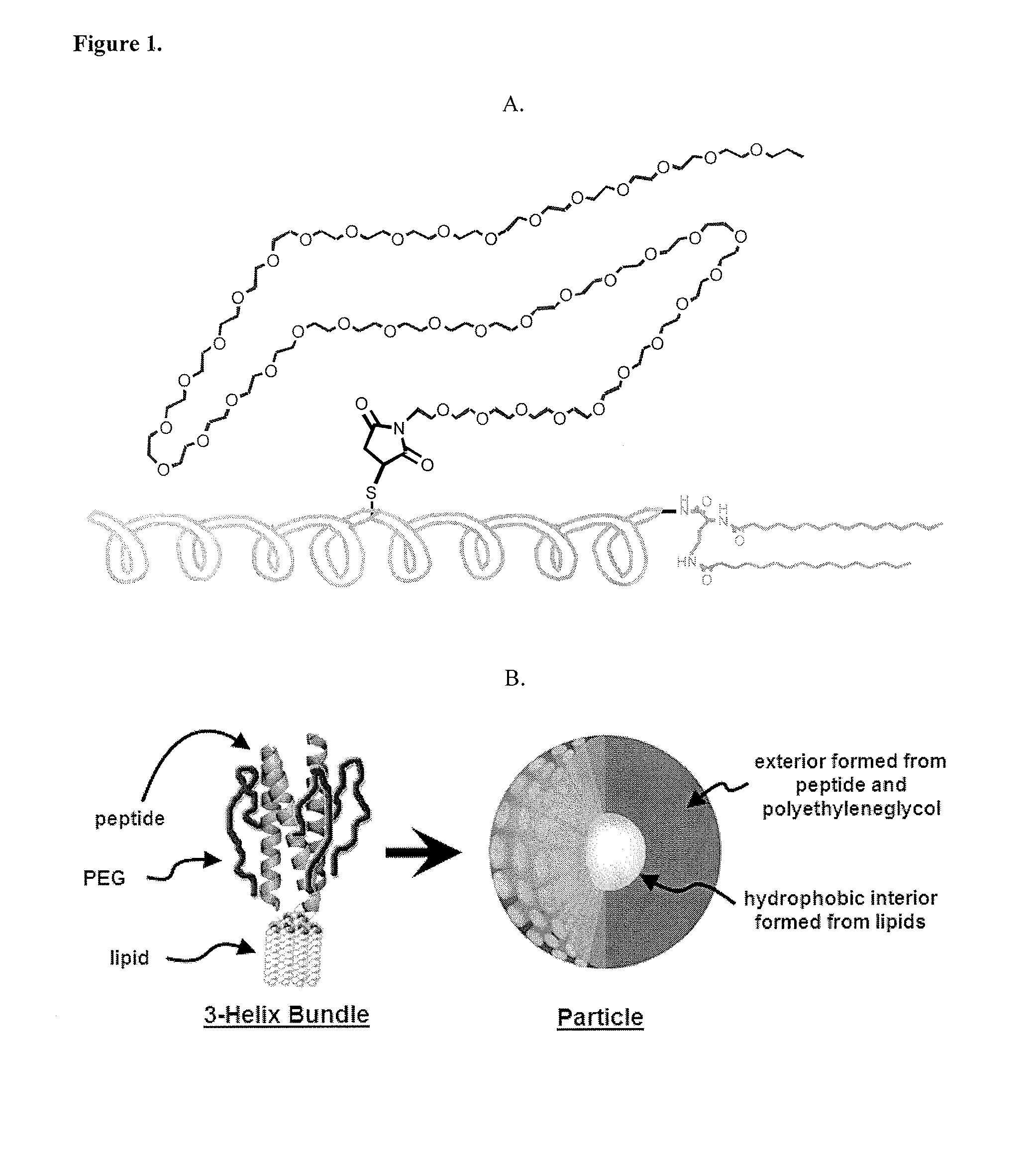
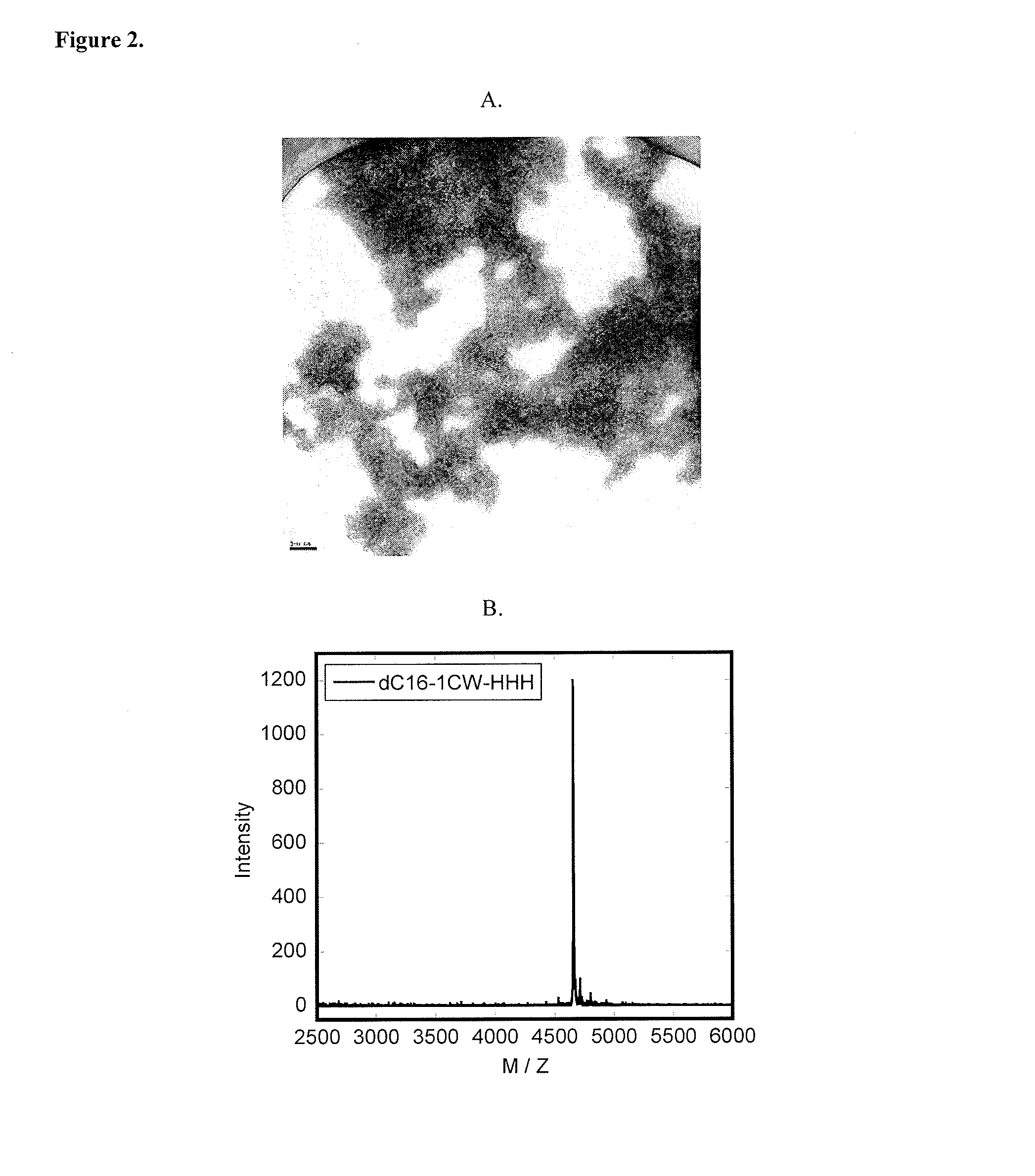
Lipid-Peptide-Polymer Conjugates and Nanoparticles Thereof
The present invention provides a conjugate having a peptide with from about 10 to about 100 amino acids, wherein the peptide adopts a helical structure. The conjugate also includes a first polymer covalently linked to the peptide, and a hydrophobic moiety covalently linked to the N-terminus of the peptide, wherein the hydrophobic moiety comprises a second polymer or a lipid moiety. The present invention also provides helix bundles form by self-assembling the conjugates, and particles formed by self-assembling the helix bundles. Methods of preparing the helix bundles and particles are also provided.
Owner:LAWRENCE BERKELEY NAT LAB
Lipid-peptide-polymer conjugates and nanoparticles thereof
Owner:LAWRENCE BERKELEY NAT LAB
Rna Amidates and Thioamidates for Rnai
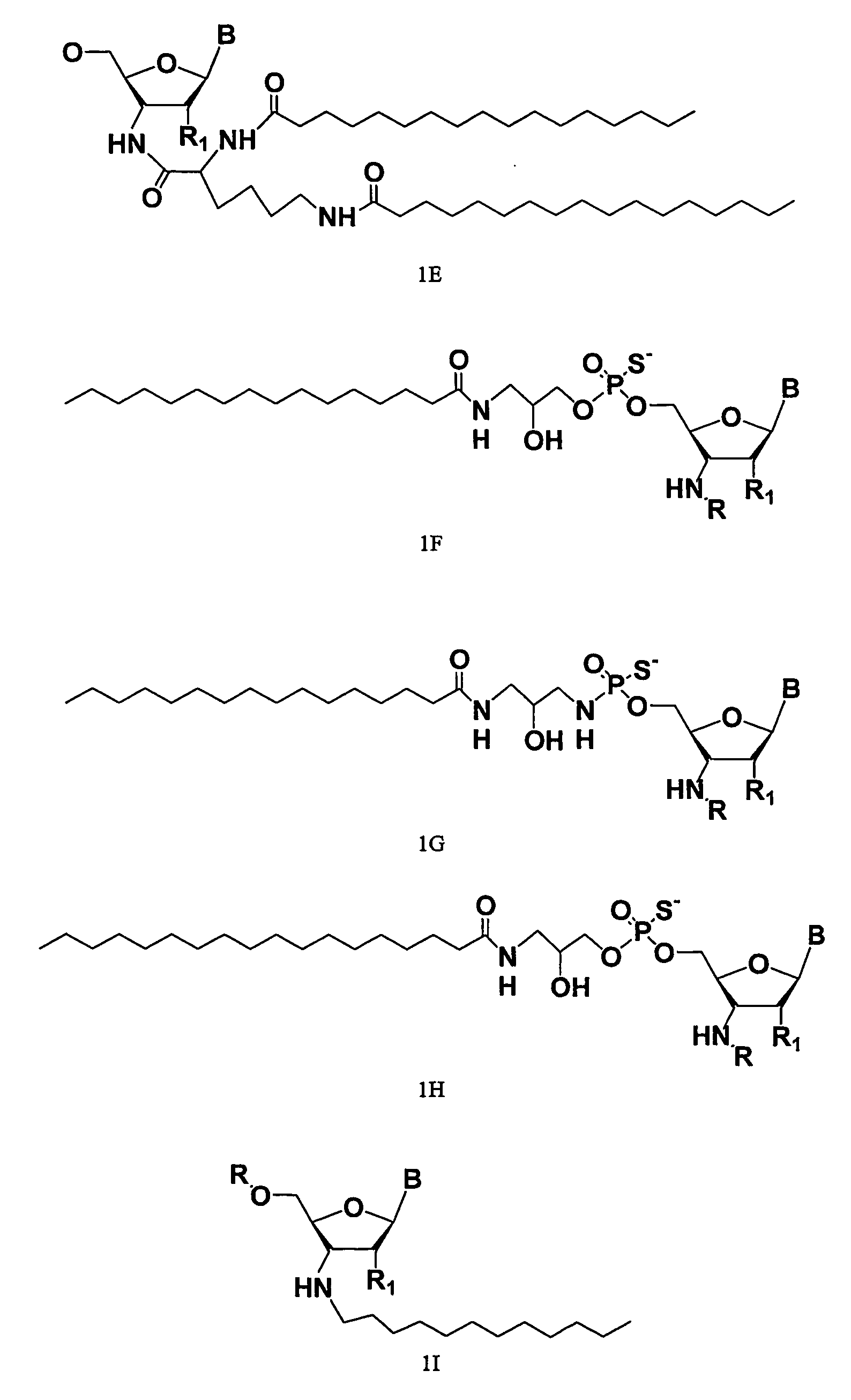
ActiveUS20070275919A1Improve the immunityLess criticalOrganic active ingredientsSugar derivativesRegulator geneBiology
The present disclosure relates to RNA amidates and thioamidates useful for RNA interference applications. The RNA amidates and thioamidates contain at least one internucleoside linkage chosen from ribo-N3′→P5′ phosphoramidate (NP) and ribo-N3′→P5′ thiophosphoramidate (NPS) linkages, and optionally further containing at least one covalently conjugated lipid moiety. Compositions comprising the amidates and thioamidates are disclosed, as are methods for their use in modulating gene expression.
Owner:GERON CORPORATION
Enteral composition for the prevention and/or treatment of sepis
InactiveUS20070190064A1Improve stabilityComposition is stableAntibacterial agentsOrganic active ingredientsPhospholipidEndocrinology
Enteral compositions and use thereof, which compositions contain proteins and lipids, for the prevention and / or treatment of sepsis; the lipid fraction being particularly rich in phospholipids.
Owner:NUTRICIA
Enteral composition for the prevention and/or treatment of sepsis
InactiveCN1802102AImprove stabilityAntibacterial agentsOrganic active ingredientsPhysiologyPhospholipid
Enteral compositions and use thereof, which compositions contain proteins and lipids, for the prevention and / or treatment of sepsis; the lipid fraction being particularly rich in phospholipids.
Owner:NV NUTRICIA
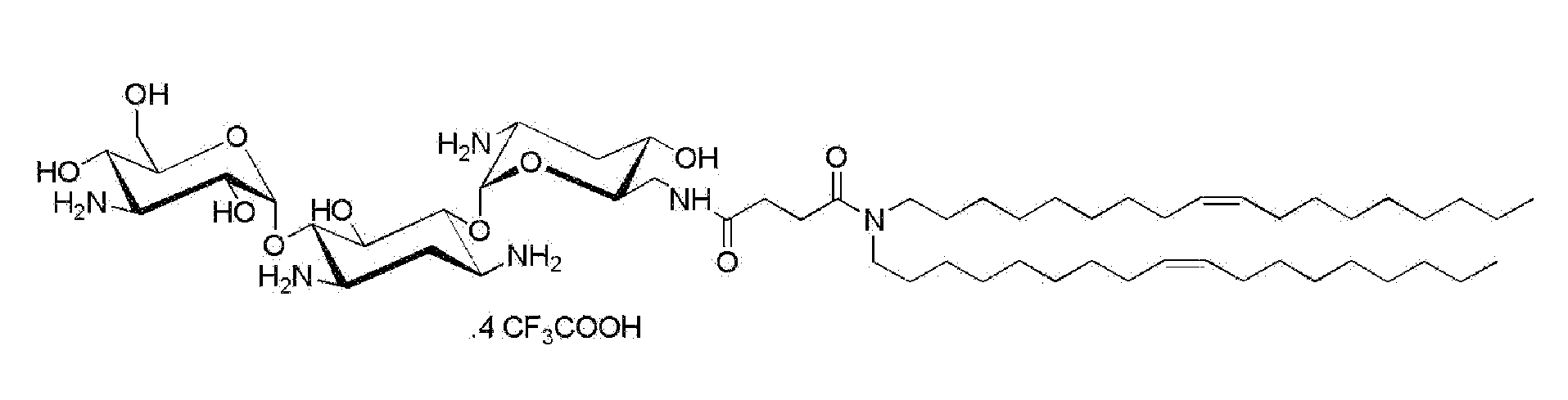
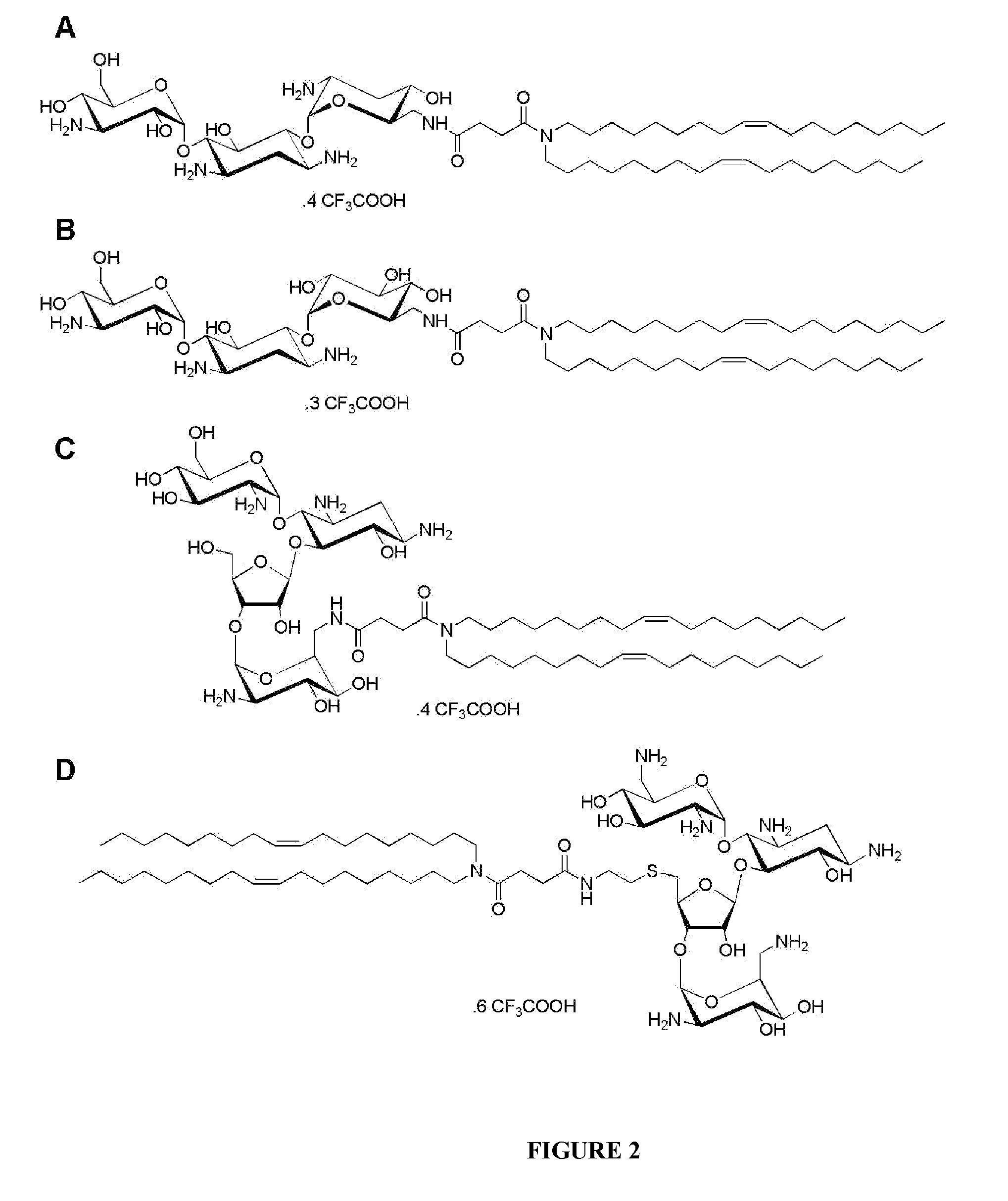
Compositions comprising a sirna and lipidic 4,5-disubstituted 2-deoxystreptamine ring aminoglycoside derivatives and uses thereof
InactiveUS20100035974A1Increase powerImprove transfection efficiencyOrganic active ingredientsSpecial deliveryGlycoside formationIn vivo
The present invention relates to a composition comprising a siRNA and a transfecting compound consisting of an aminoglycoside of the class of 4,5-disubstituted 2-deoxystreptamine ring linked via a spacer molecule to a lipid moiety of formula —(R1)R2 wherein R1 and R2 represent, independently of one another, a hydrogen atom or a fatty aliphatic chain or R1 or R2 is absent, with the proviso that at least one of R1 and R2 represents a fatty aliphatic chain; or —OR3 or —NR3 wherein R3 represents a steroidal derivative. The invention also concerns in vitro and in vivo applications of these compositions.
Owner:CENT NAT DE LA RECHERCHE SCI +1
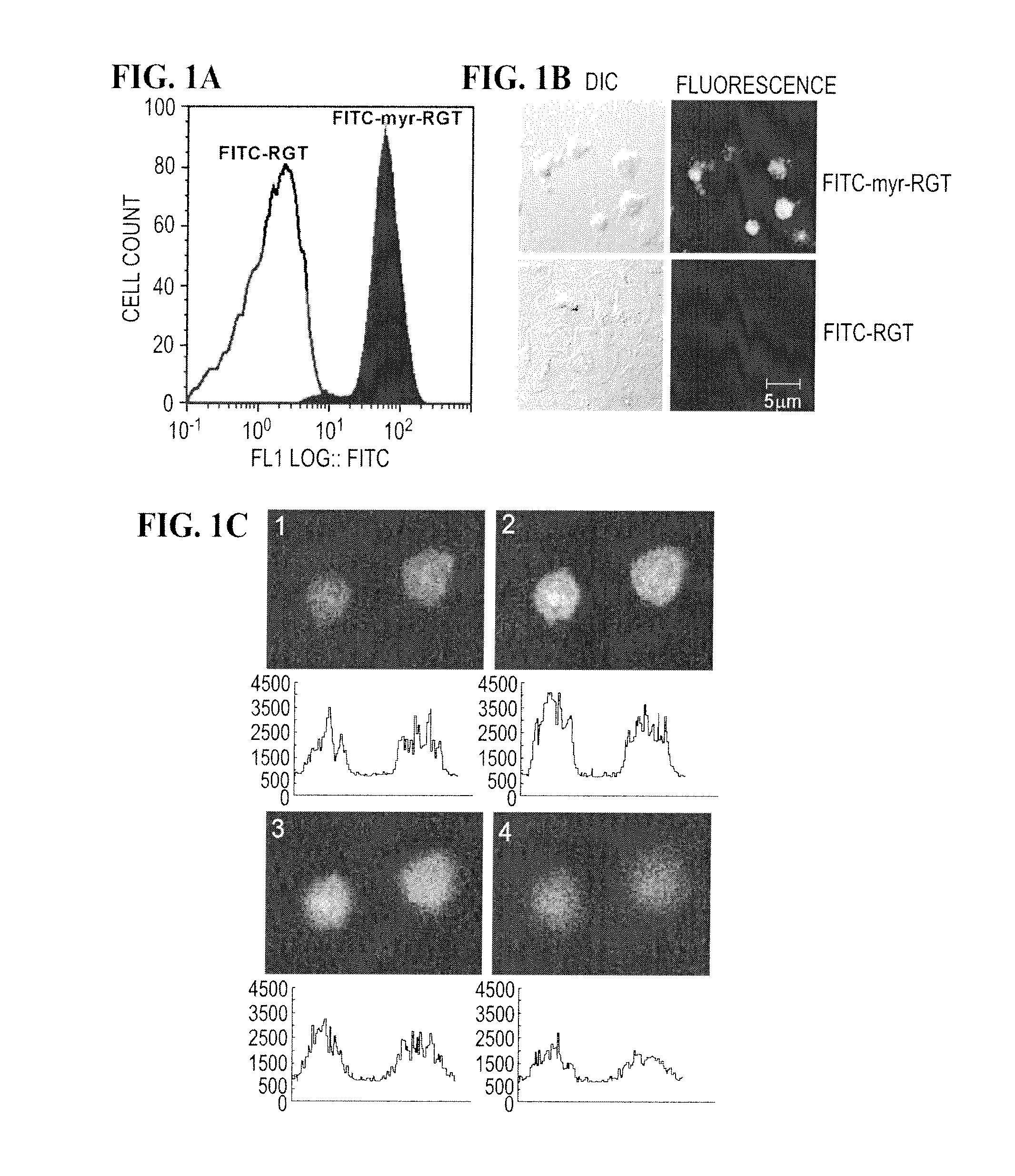
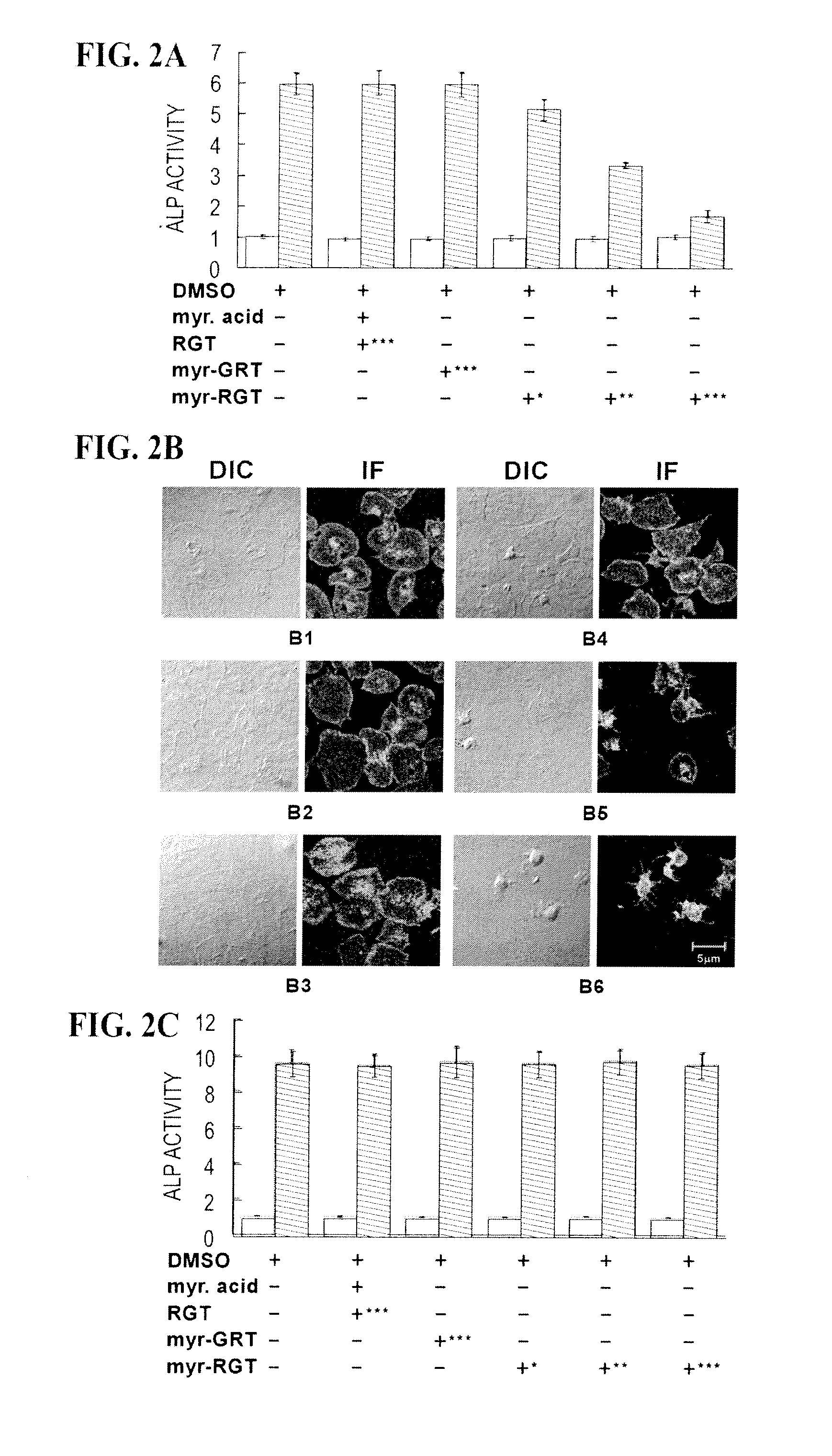
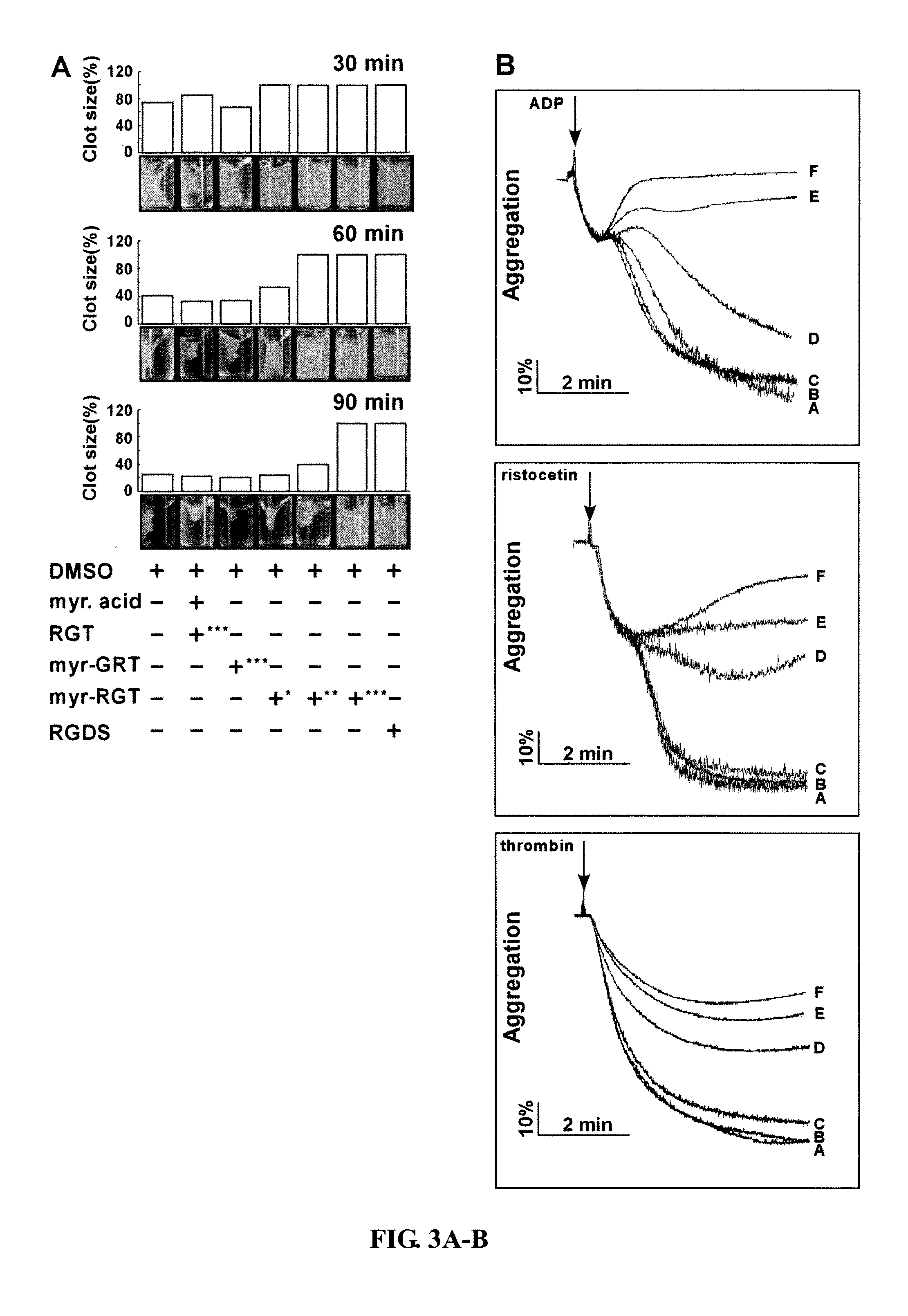
Modulation of Platelet Aggregation
Methods and compositions for inhibition of platelet cell aggregation are described. In particular, compositions comprising cell permeant RGT peptides, such as RGT bound to a lipid moiety are provided. Compositions may be used in the treatment and prevention of clot related diseases such as stroke and myocardial infarction.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
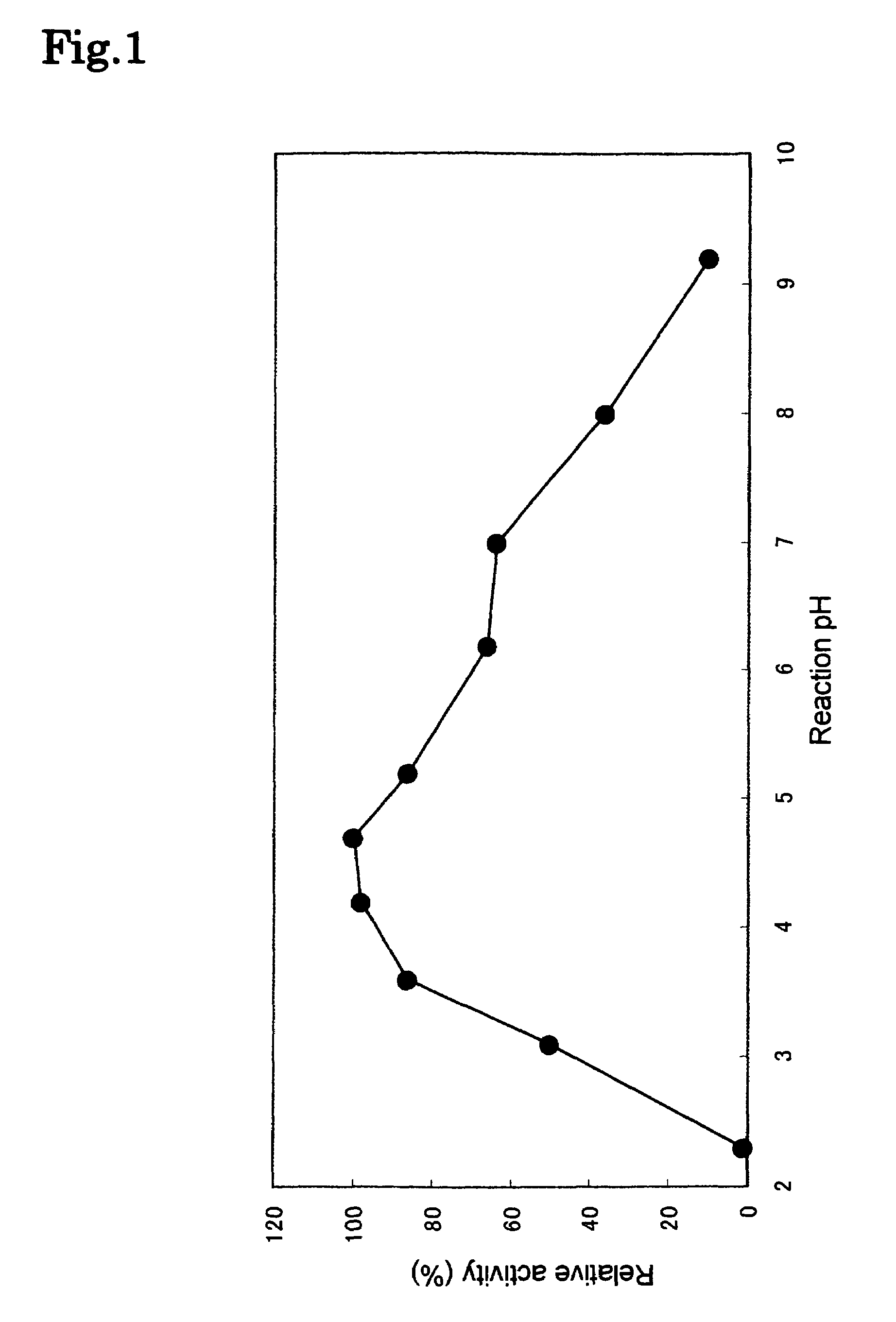
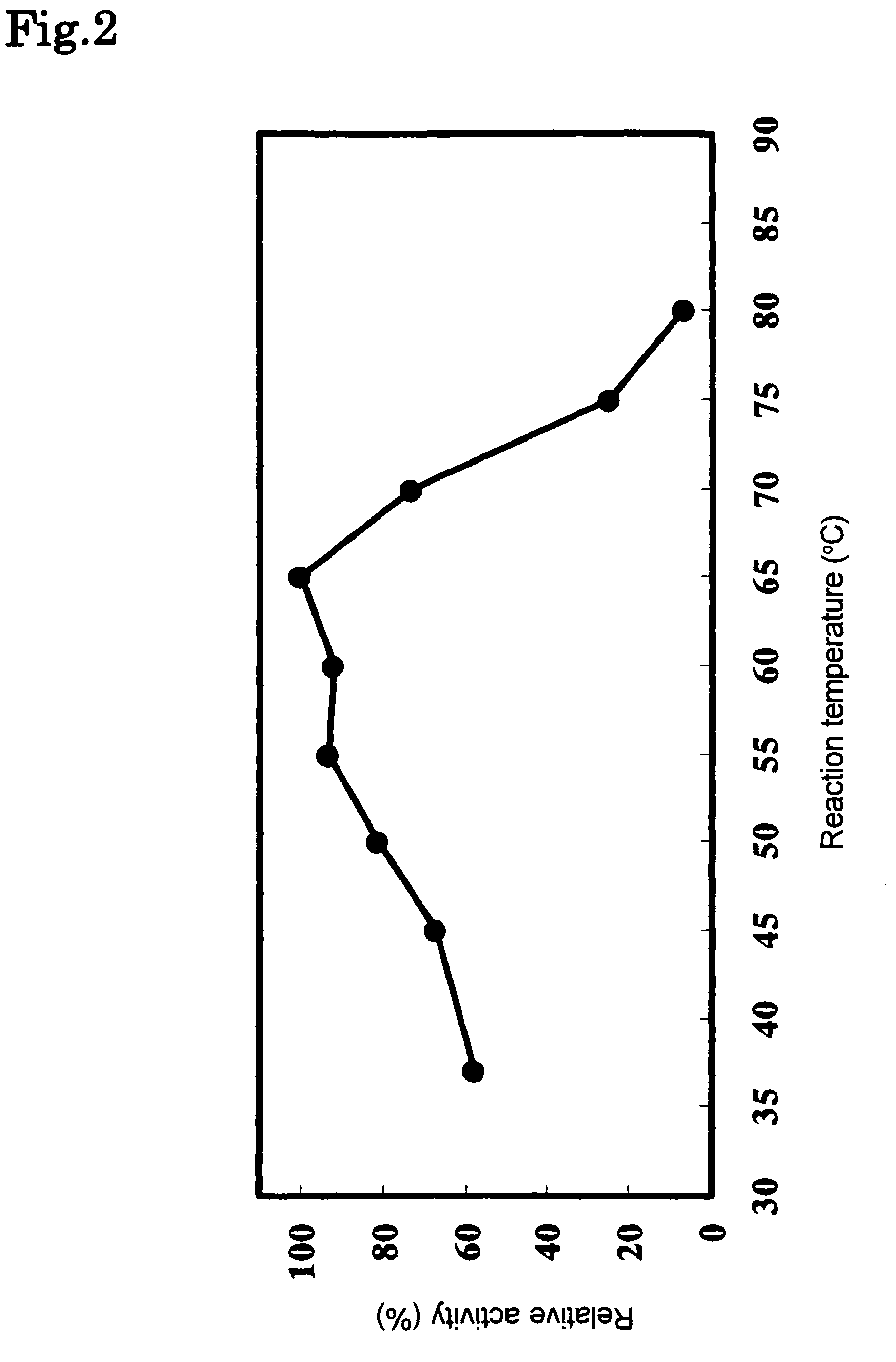
Phospholipase C enzyme(s)
The present invention provides a phospholipase C enzyme(s) having ability to hydrolyze phospholipid in both acidic and around neutral ranges and the activity in a citrate buffer solution as well as having some degree of heat stability, and having a property not to hydrolyze phosphate esters not containing lipid moieties. The phospholipase C enzyme(s) shows the activity at from acidic to neutral pH and does not substantially hydrolyze any phosphate esters except for phospholipids.
Owner:MITSUBISHI CHEM CORP
Novel immunogenic lipopeptides comprising t-helper and cytotoxic t lymphocyte (ctl) epitopes
InactiveUS20070160631A1Conveniently synthesizedEasy to synthesizeSsRNA viruses negative-senseBiocideSynthetic ImmunogensCtl epitope
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and CTL epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular CTL epitopes. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the CTL epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Lipid-based platinum compounds and nanoparticles
ActiveCN105705152AHeavy metal active ingredientsOrganic active ingredientsLipid formationNanoparticle
The present disclosure is in relation to the field of nanotechnology and cancer therapeutics. In particular, the present disclosure relates to platinum based compounds comprising platinum moiety, linker moiety and lipid moiety and corresponding nanoparticles thereof. The disclosure further relates to synthesis of said platinum based compounds, nanoparticles and compositions comprising said platinum based compounds / nanoparticles. The disclosure also relates to methods of managing cancer by employing aforesaid carbene compounds, platinum based compounds, nanoparticles and compositions thereof.
Owner:AKAMARA THERAPEUTICS INC
Lipids and lipid assemblies comprising transfection enhancer elements
Lipid assemblies, such as liposomes, comprising transfection enhancer elements (TEE's), which are complexed with the lipid assemblies by means of ionic interactions, or lipids incorporating such TEE's are disclosed for enhancing the fusogenicity of the lipid assemblies. The TEE's have the formula:hydrophobic moiety-pH sensitive hydrophilic moiety (II)The pH sensitive hydrophilic moiety of each TEE is a weak acid having a pka of between 2 and 6 or a zwitterionic structure comprising a combination of acidic groups with weak bases having a pKa of between 3 and 8. Lipids incorporating one or more such TEEs have the formula (I):Lipid moiety-[Hydrophobic moiety-pH sensitive hydrophilic moiety] (I).
Owner:BIONTECH DELIVERY TECH GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com