Lipid-based platinum compounds and nanoparticles
A platinum compound, compound technology, used in nanotechnology and cancer therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
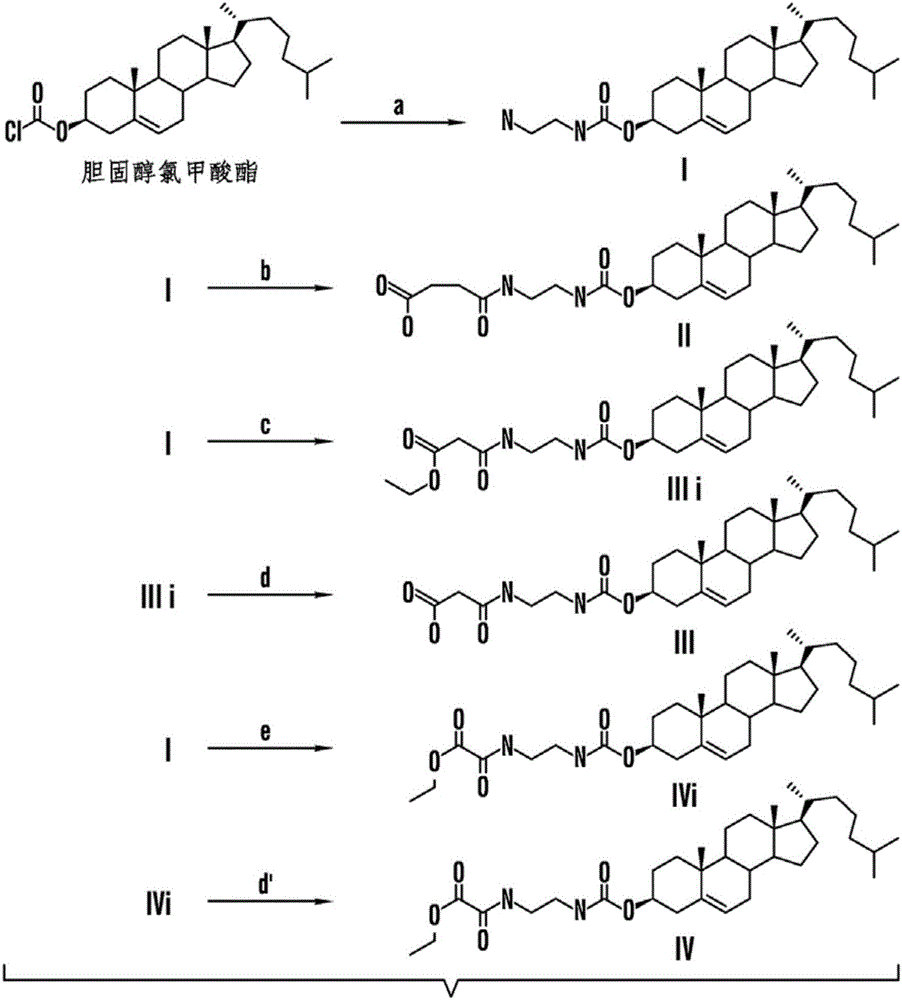
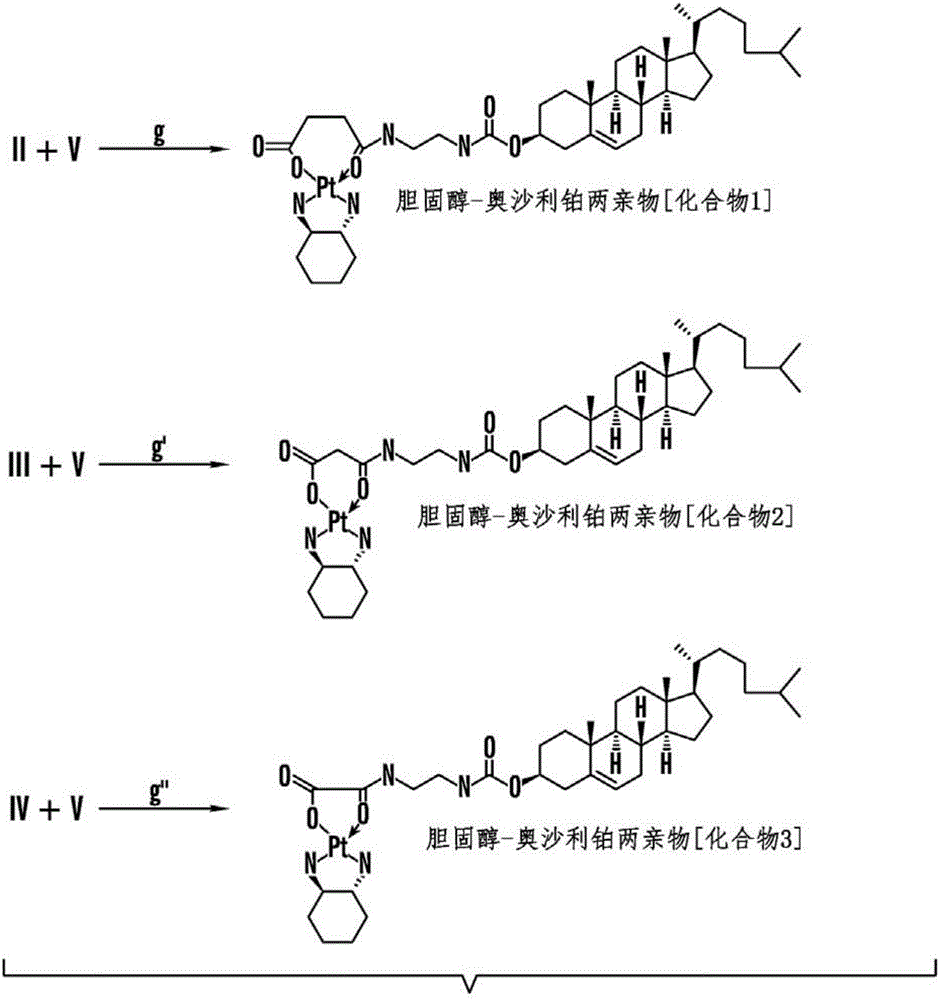
[0351] Example 1: Synthesis of Cholesterol-Oxaliplatin Compounds [Formula I] with Carbamate Linkages
[0352] A cholesterol-oxaliplatin complex containing a carbamate linkage was synthesized as follows (Figure 1):
[0353] Part A ( Figure 1A ), (step a): In a 250 mL round bottom flask, ethylenediamine (about 22.2 mL, 30 eq) was added to about 50 mL of dry DCM (dichloromethane). The reaction flask was cooled to about 0°C under an ice bath. Solid cholesteryl chloroformate (about 5.0 g, 11.14 mmol) dissolved in another 50 mL of dry DCM was added dropwise to the reaction flask with a dropping funnel for about 30 minutes to about 45 minutes with vigorous stirring. The resulting solution was stirred overnight (approximately 8-12 hours) at room temperature (25°C). Then, the solution was taken up in chloroform (about 100 mL), and then washed about 1 to 3 times with water (3×50 mL) and brine (1×50 mL). The resulting organic layer was dried over anhydrous sodium sulfate, filtered, ...
Embodiment 2
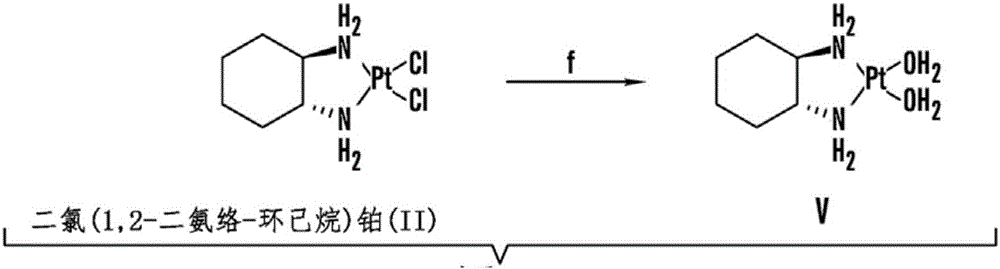
[0370] Example 2: Synthesis of Cholesterol-Oxaliplatin Compounds [Formula I] with Ether Linkages
[0371] A cholesterol-oxaliplatin complex containing an ether linkage was synthesized as follows (Figure 2):
[0372] Part A ( Figure 2A ), (step a-step e), synthesis of amine intermediate (II): step a to step e were carried out as described in Example 3 (step 1 to step 5, synthesis of compound 25).
[0373] (step f): In a 100mL single-neck round bottom flask, under N 2Intermediate II (amine 500 mg, 1.164 mmol) obtained after steps (a-e) [Example 2, part A] was taken in DCM (ca. 10 mL) and stirred at room temperature for about five to about ten minutes under atmosphere . The reaction mixture was cooled to about 0 °C, succinic anhydride (about 570 mg, 5.82 mmol) was added, followed by pyridine (about 1.88 mL, 23.3 mmol), and the reaction mixture was stirred at room temperature for about 24 hours.
[0374] After the stirring process was complete (checked by TLC), the 2 Cl 2 (...
Embodiment 3
[0390] Embodiment 3: the synthesis of the compound of formula II
[0391] Synthesis of compound 25
[0392] Step 1: To the CH 2 Cl 2 To an ice-cold solution of cholesterol 1.01 (about 10 g, 0.026 mol) in (about 45 mL) was added pyridine (about 15 mL) and stirred for about 15 minutes. To this solution was added p-toluenesulfonyl chloride (about 9.8 g, 0.052 mol) and stirred at about 0°C for about 6 hours, after which time it was checked by TLC. Upon completion, the reaction mixture was washed with CHCl 3 (about 20 mL) and washed sequentially with about 1N HCl (3×50 mL) and brine (about 20 mL). The organic layer was washed with anhydrous Na 2 SO 4 Drying and concentration under vacuum provided Intermediate 1.02, which was used directly in the next reaction without further purification.
[0393]
[0394] Step 2: To a solution of tosylated cholesterol 1.02 (about 10 g, 0.018 mol) in dioxane (about 45 mL) was added ethylene glycol (about 15 mL) and refluxed for about 4 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com