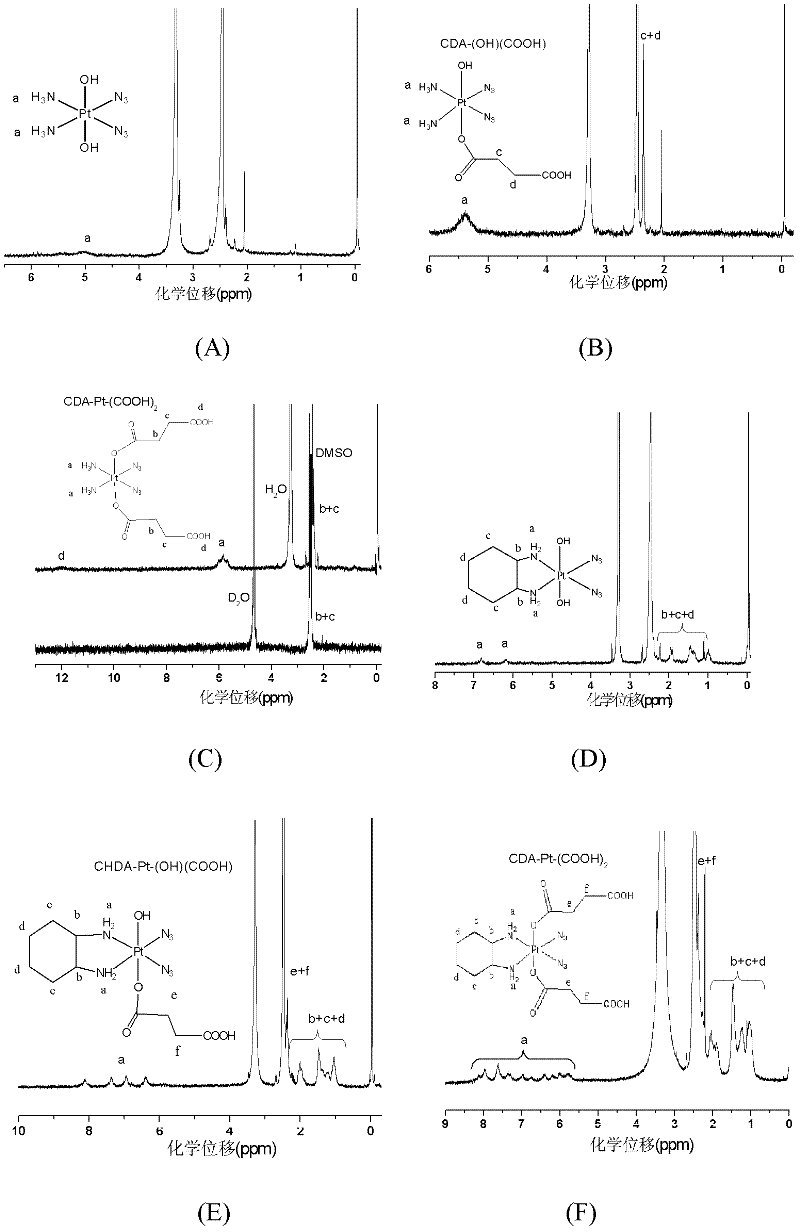
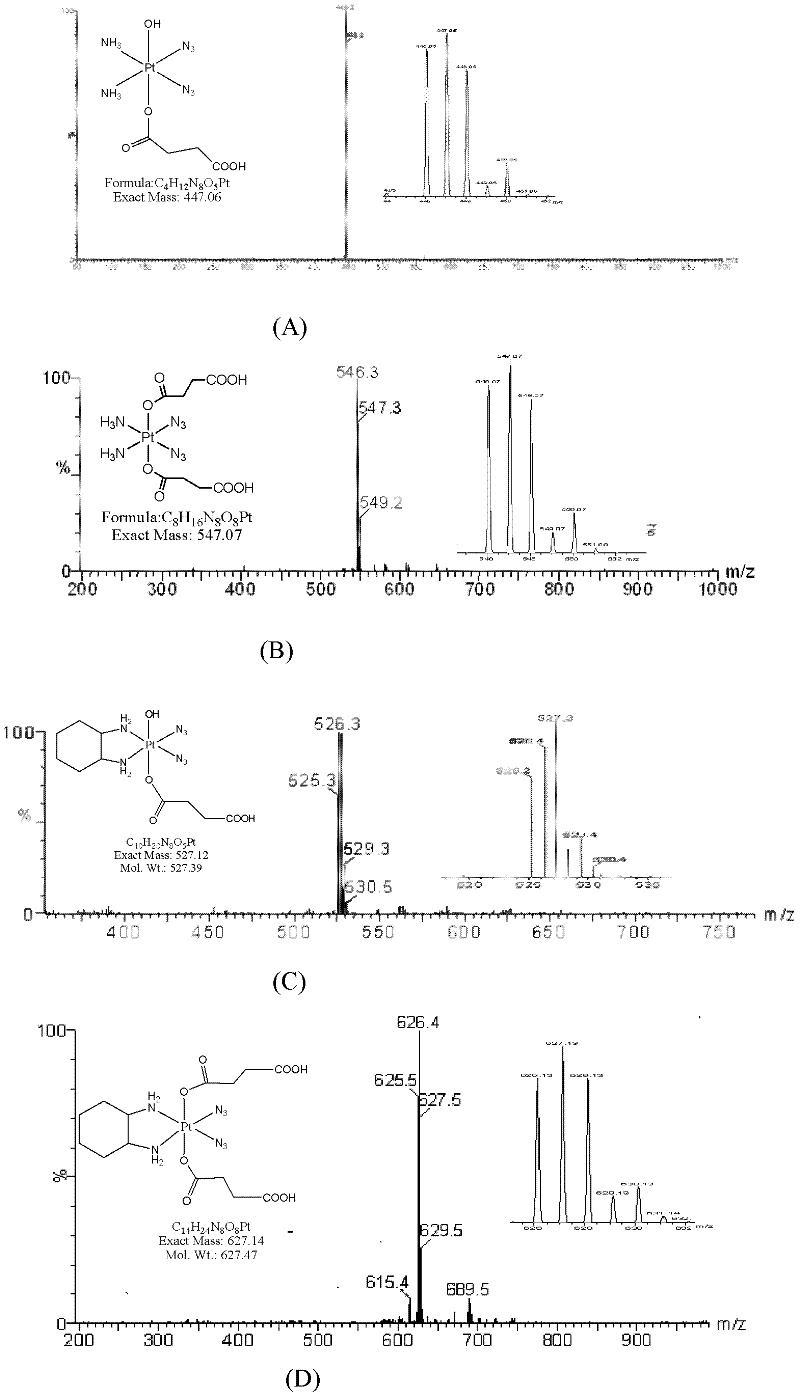
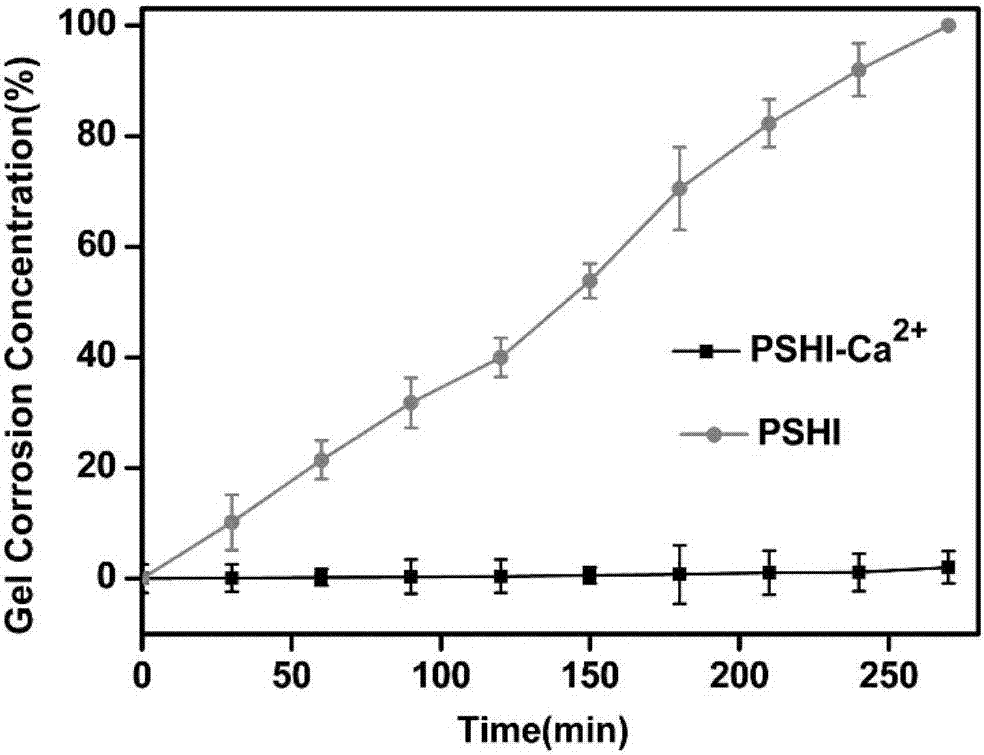
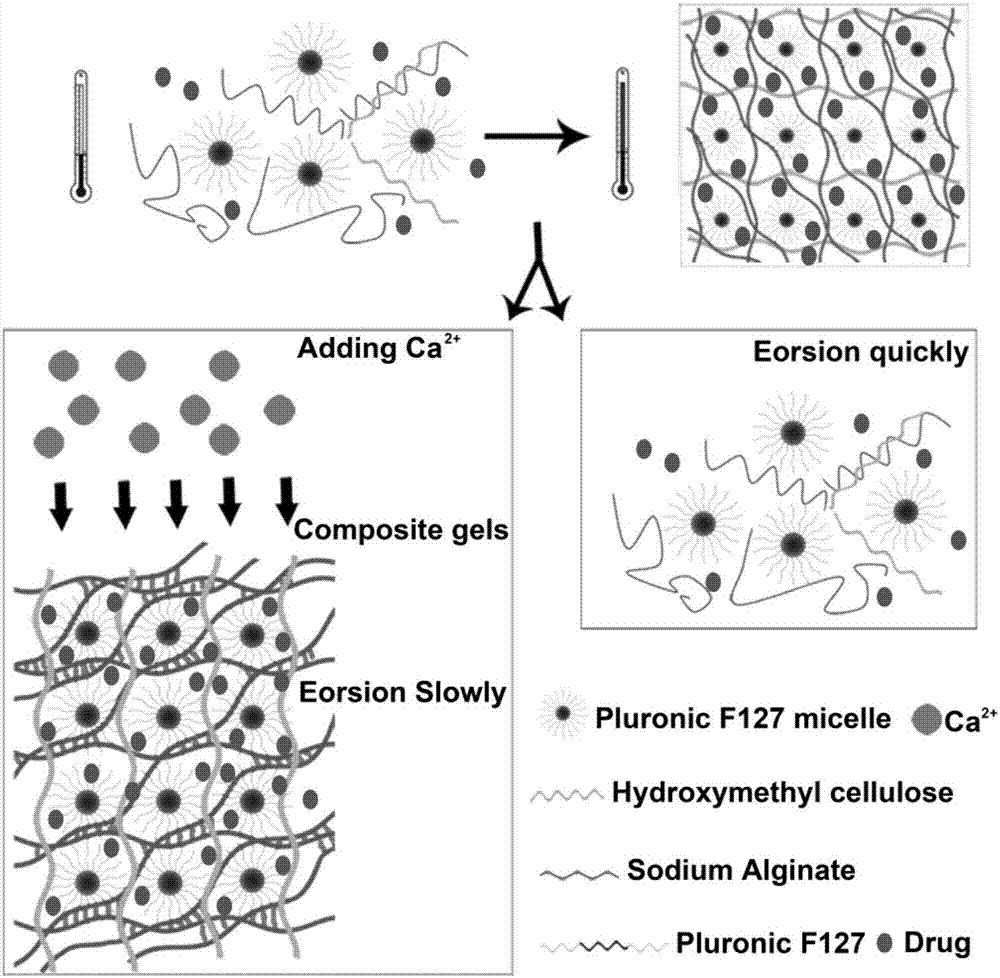
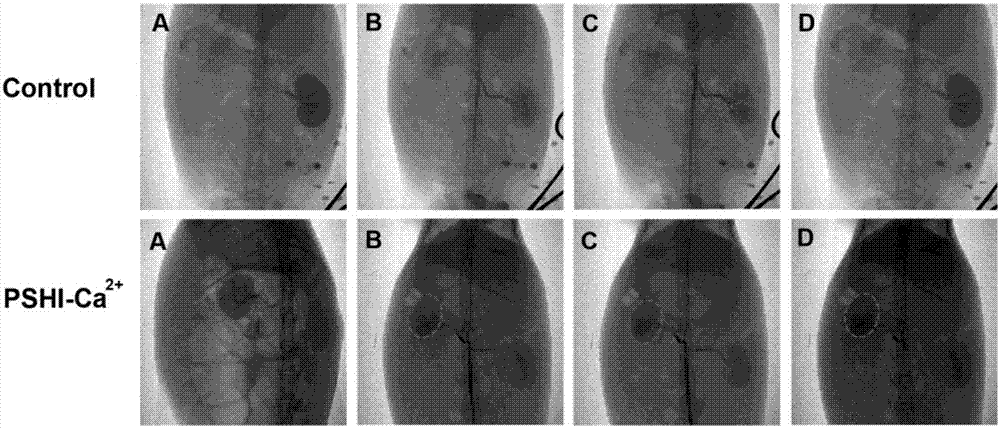
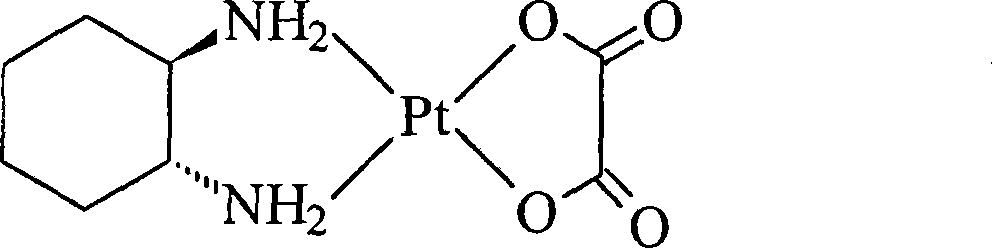
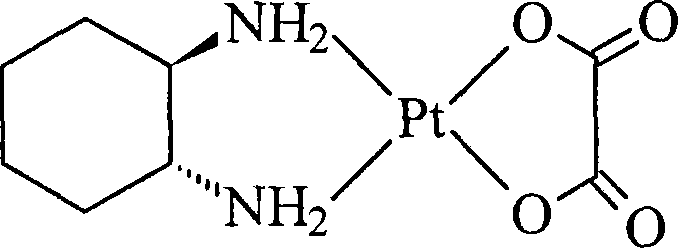
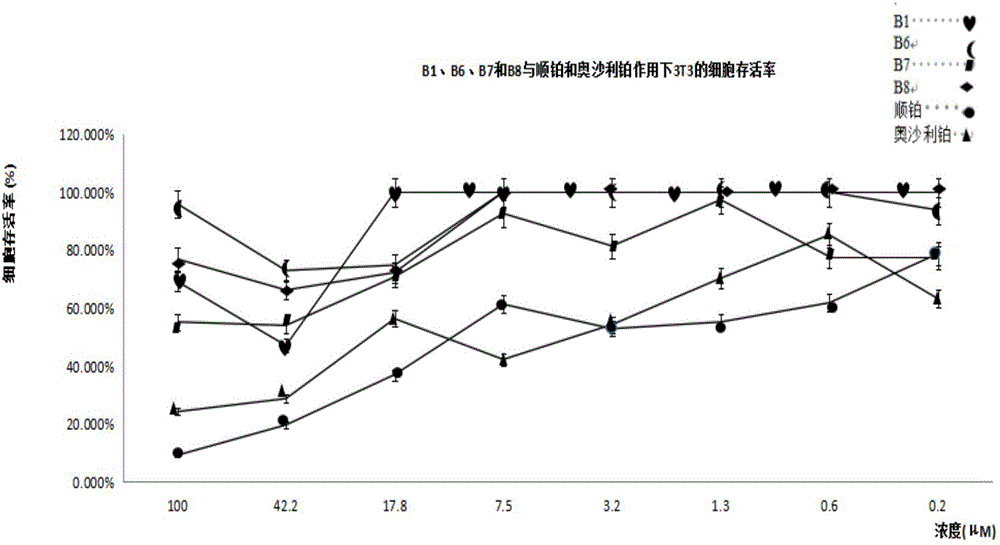
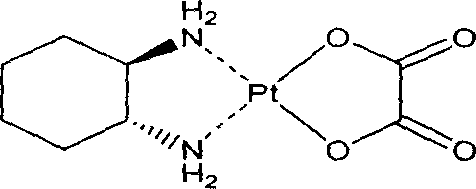
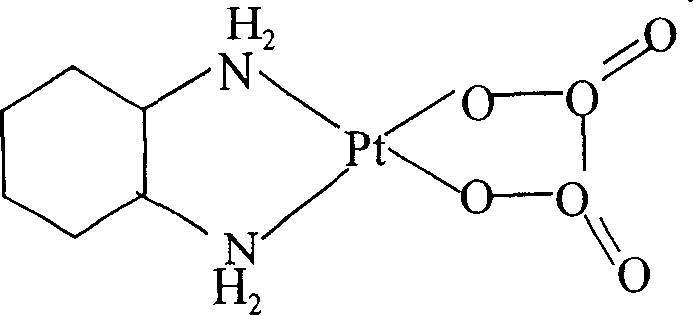
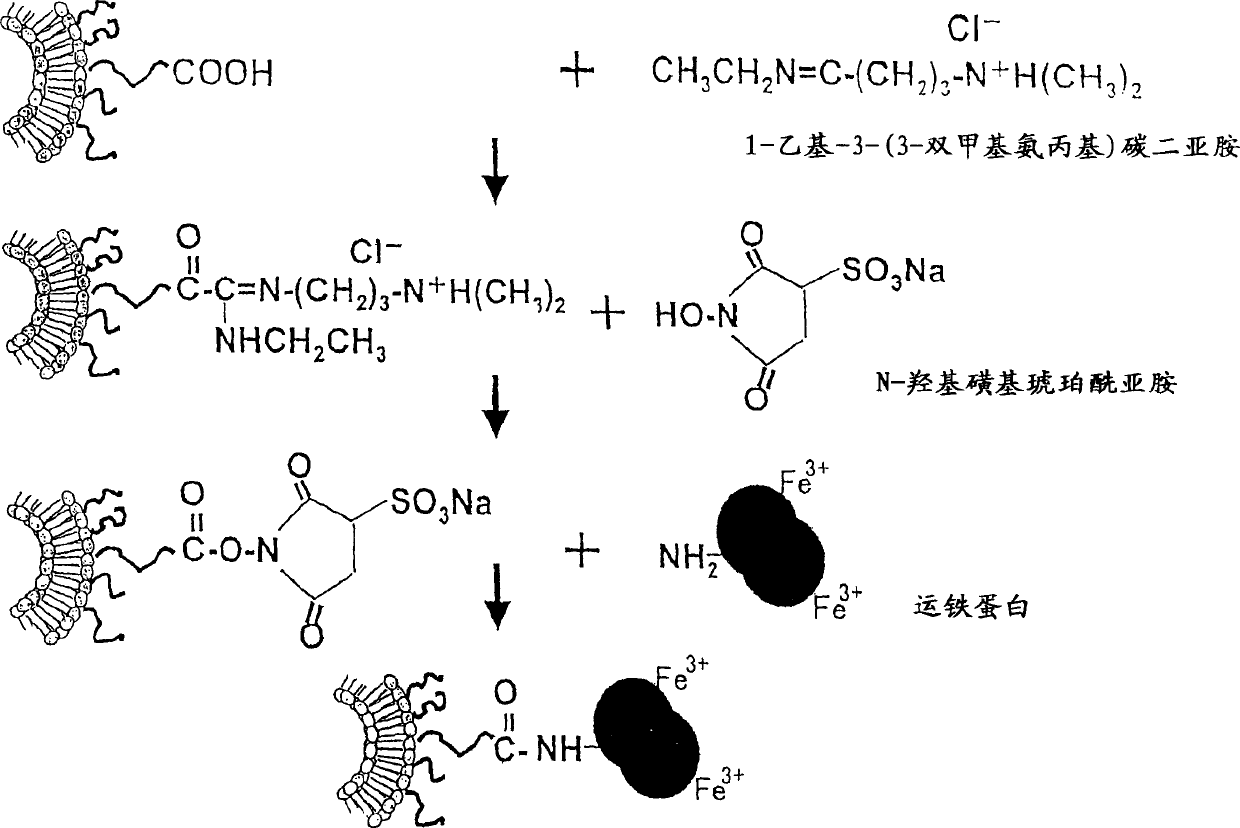
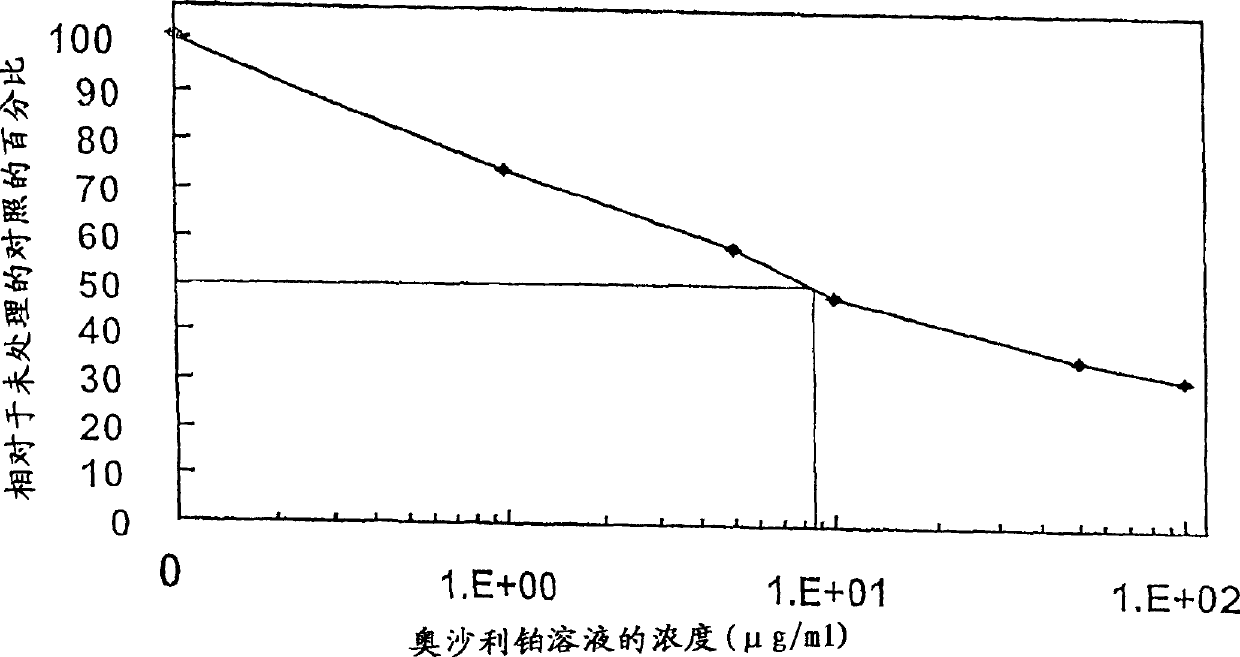
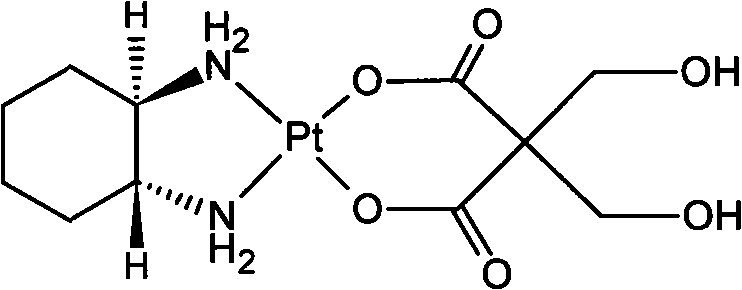
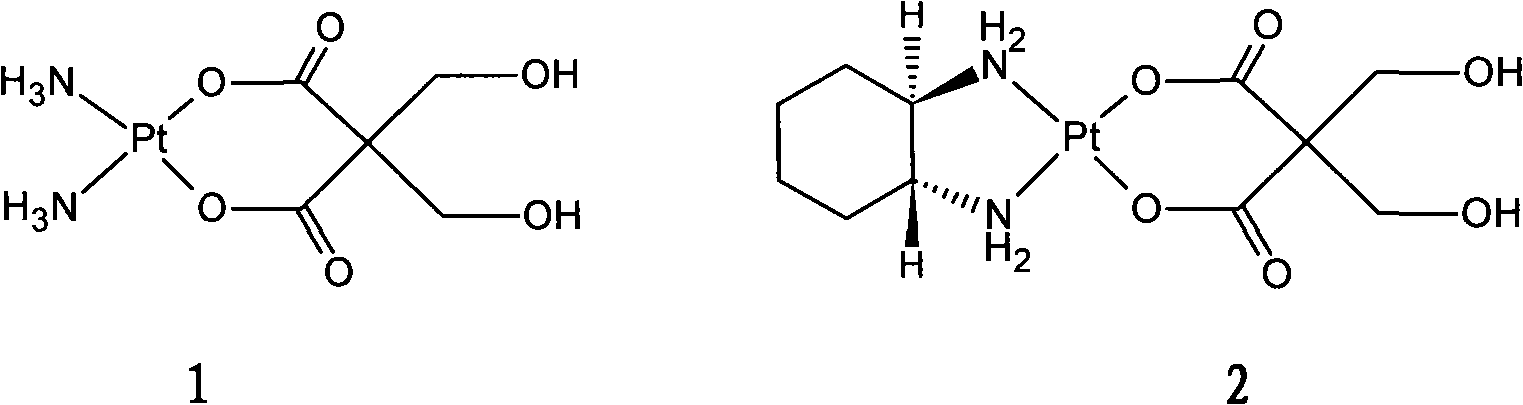
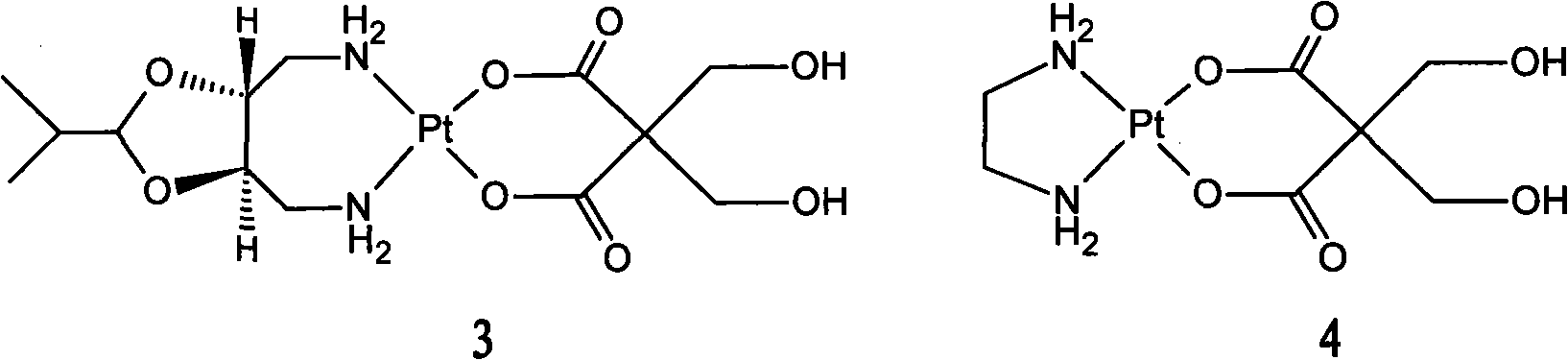
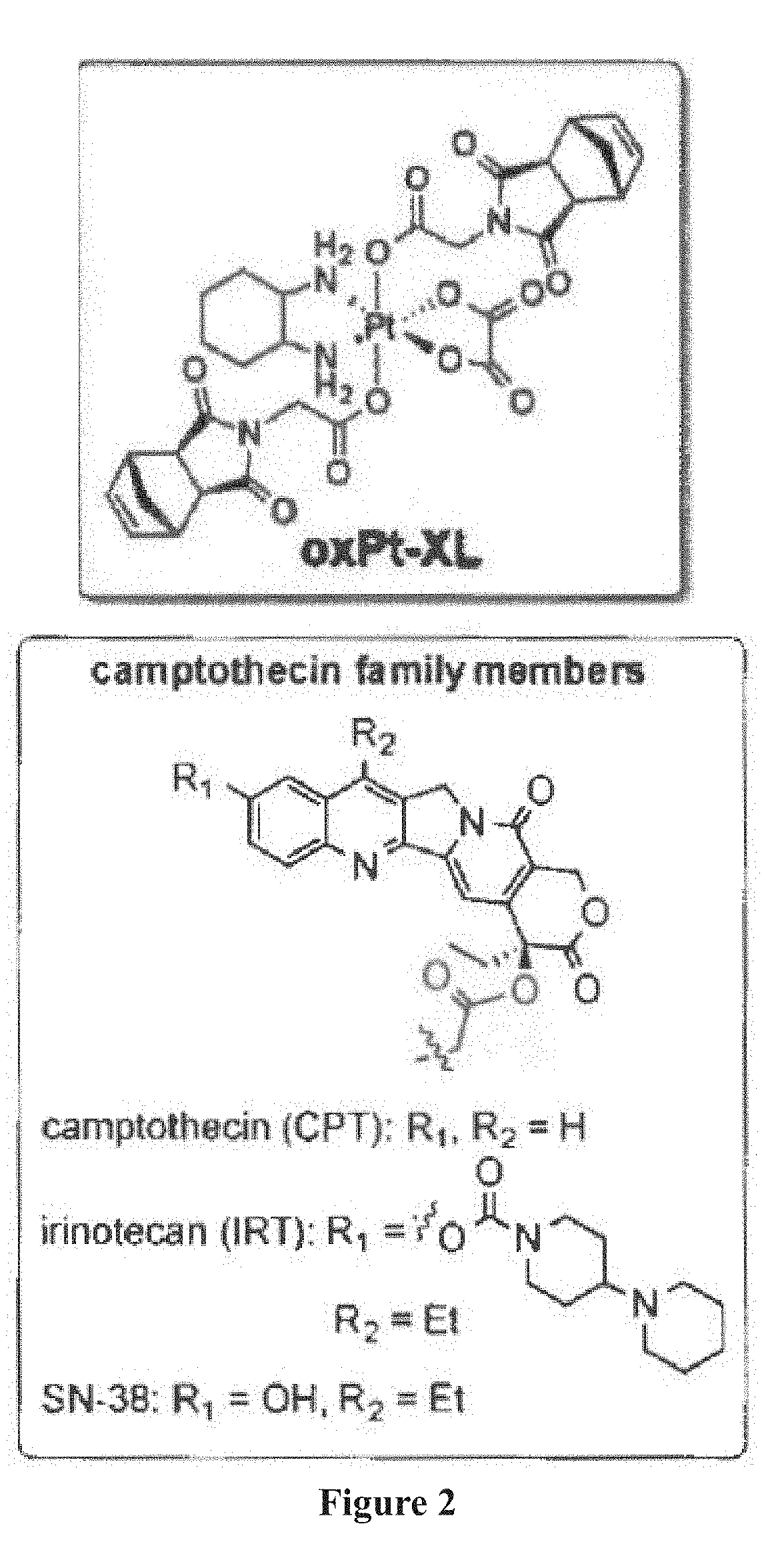
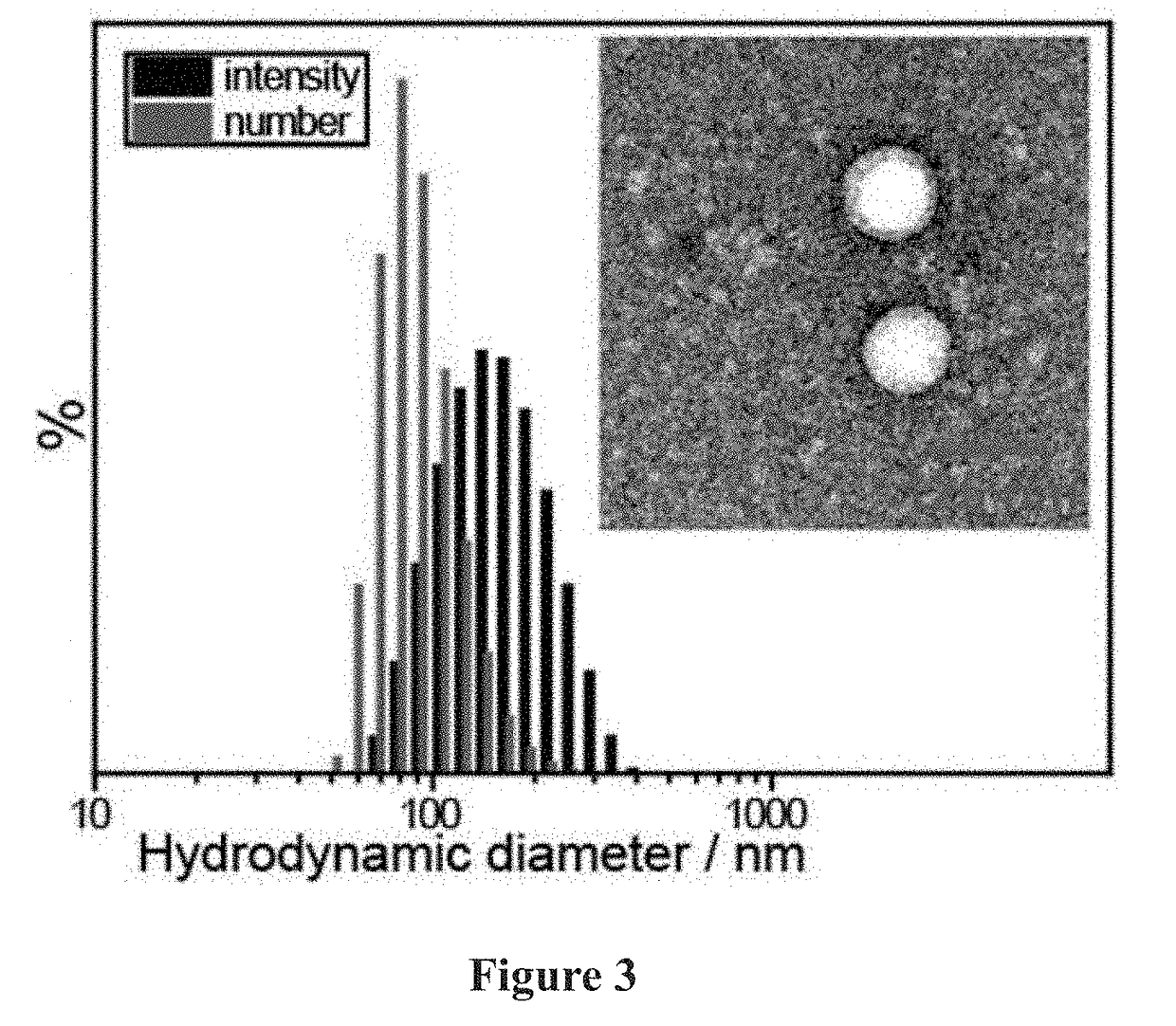
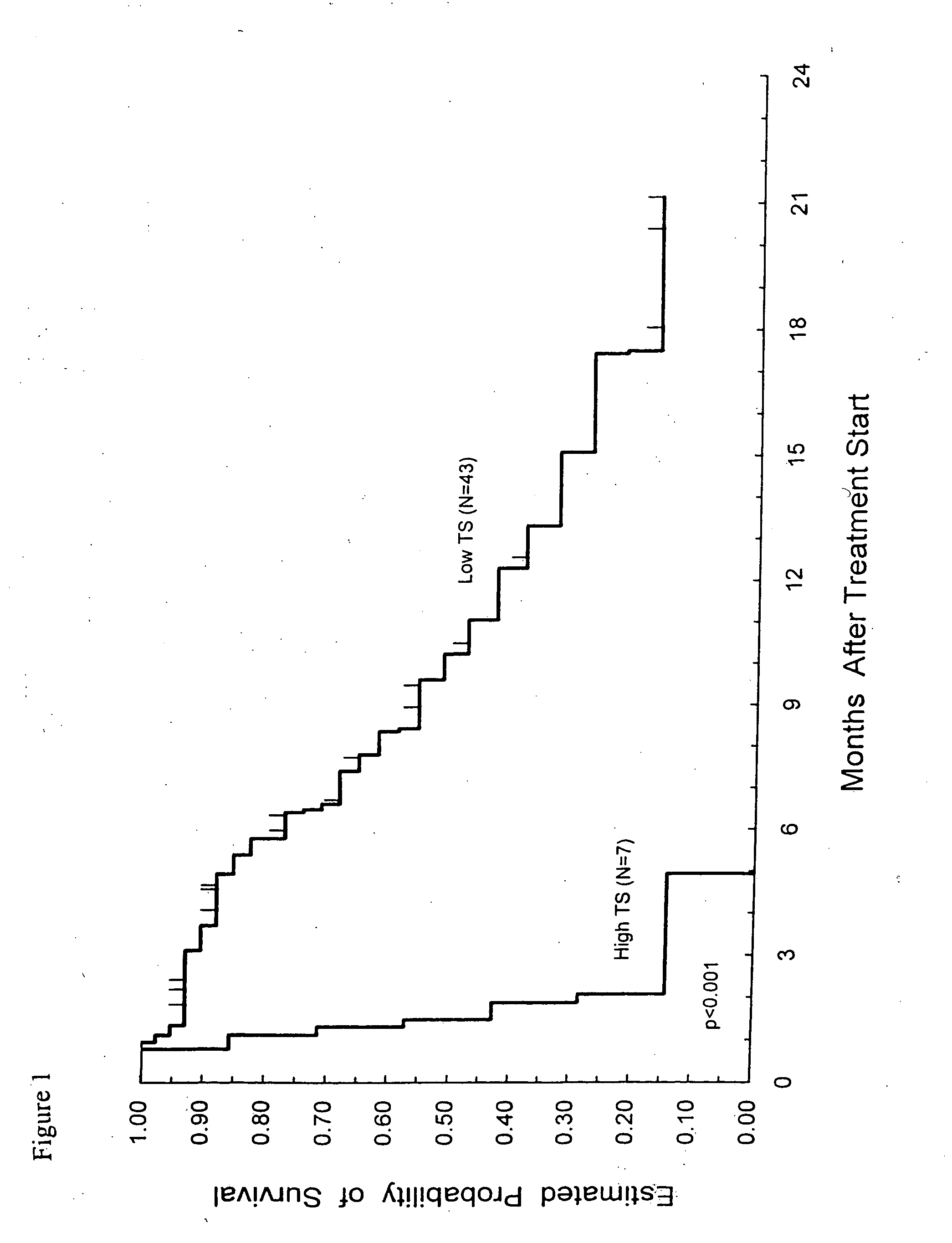
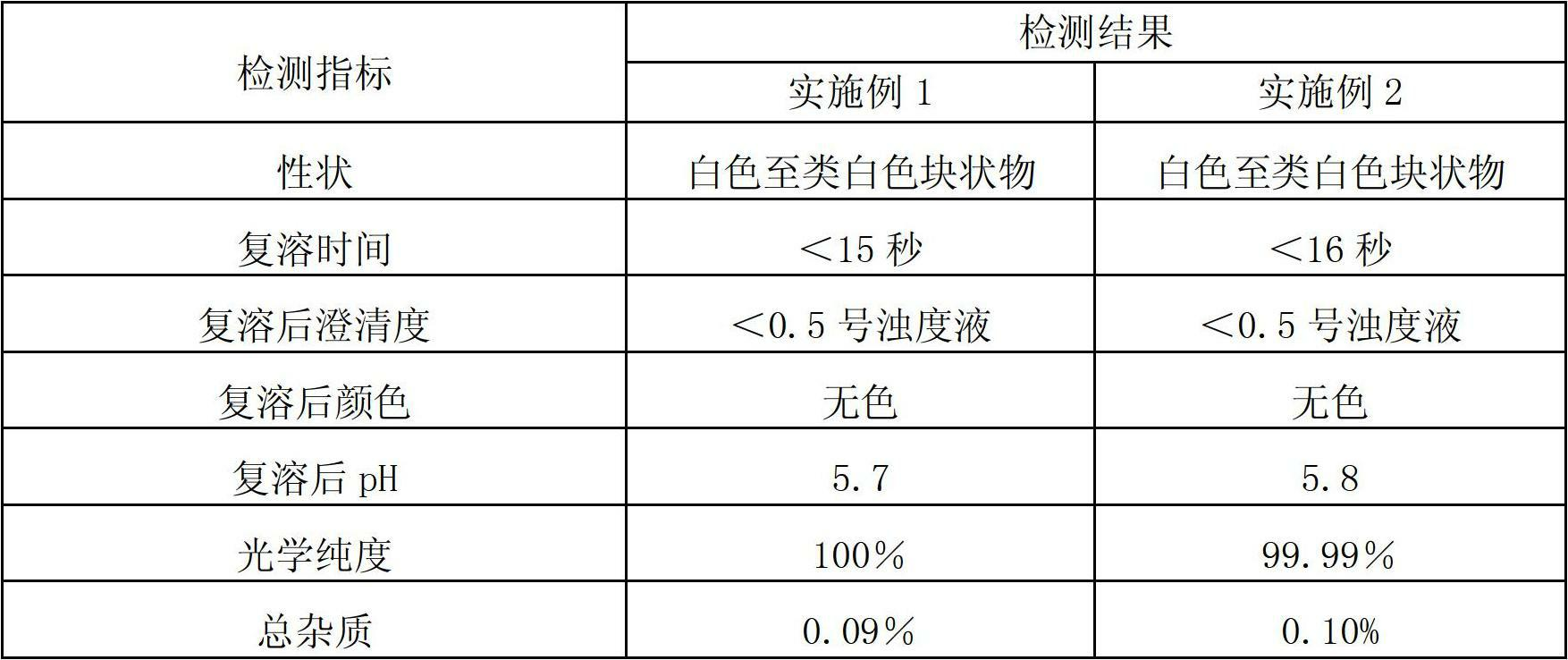
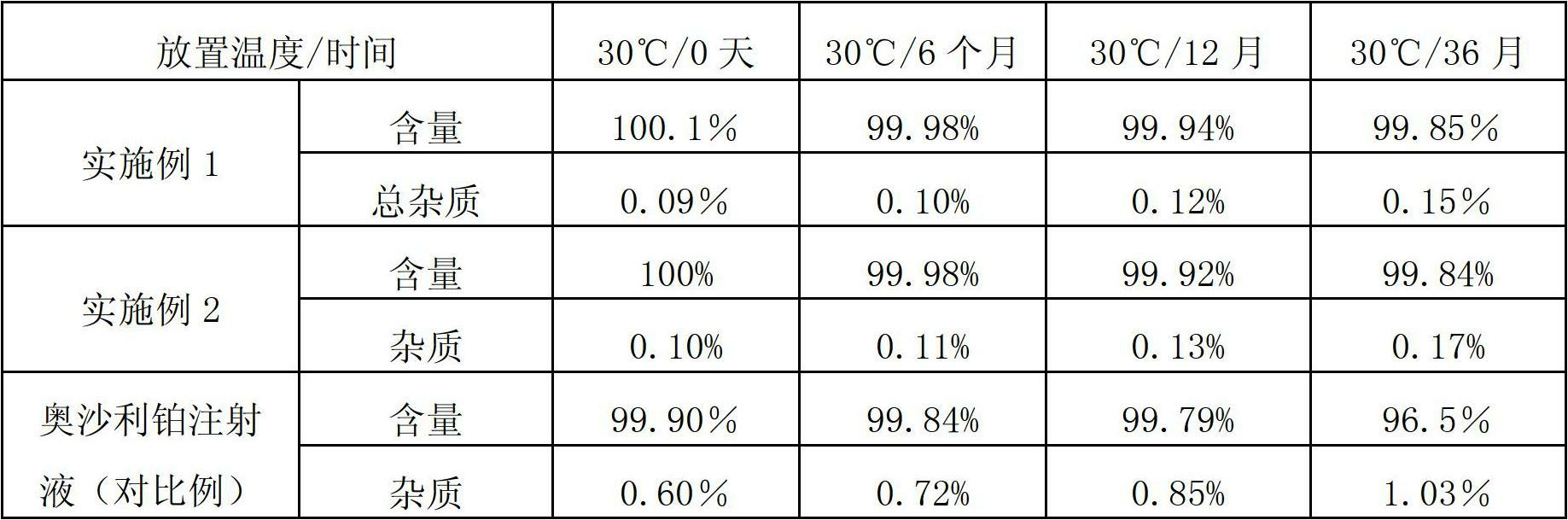
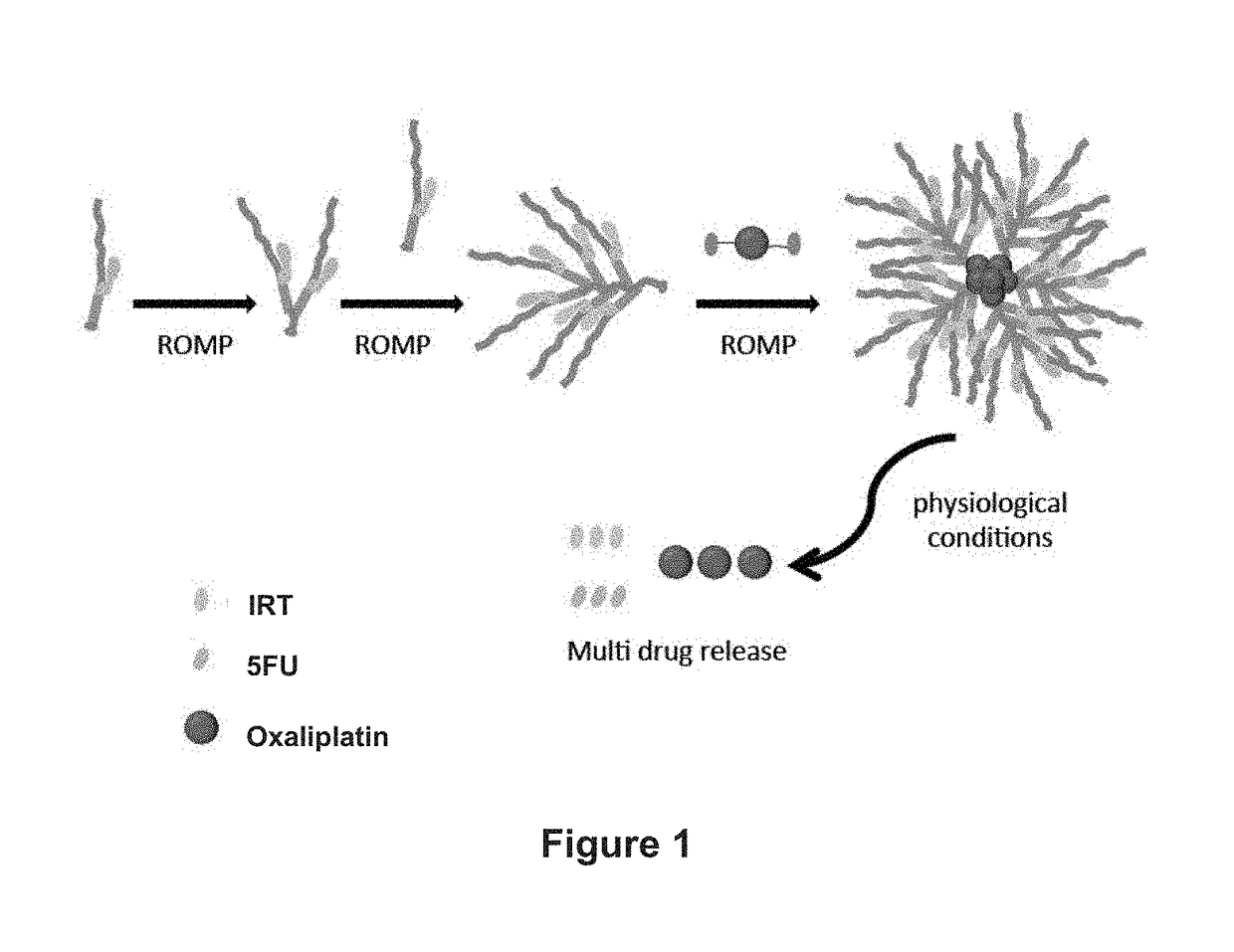
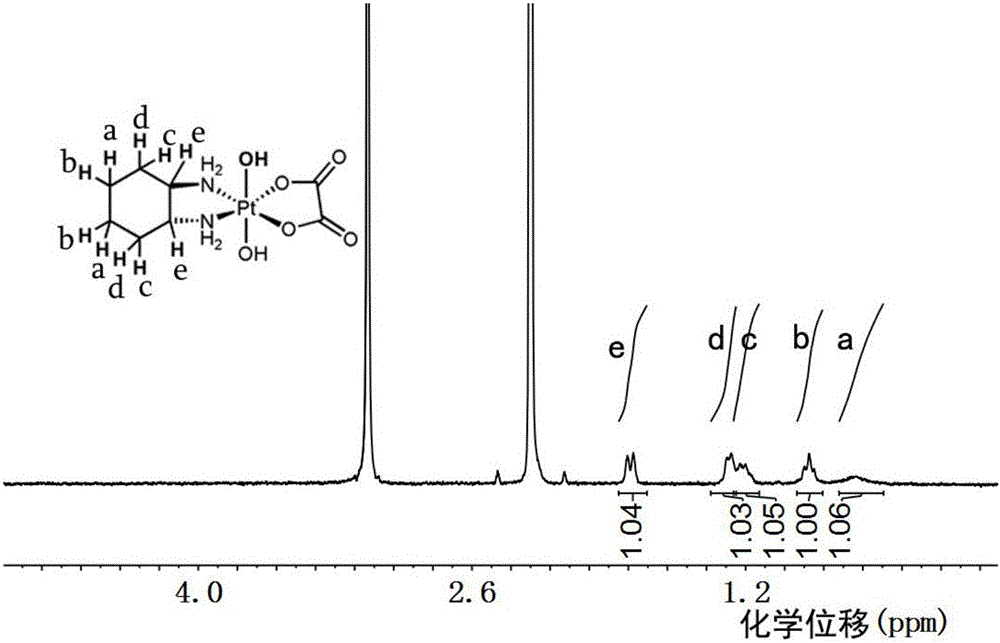
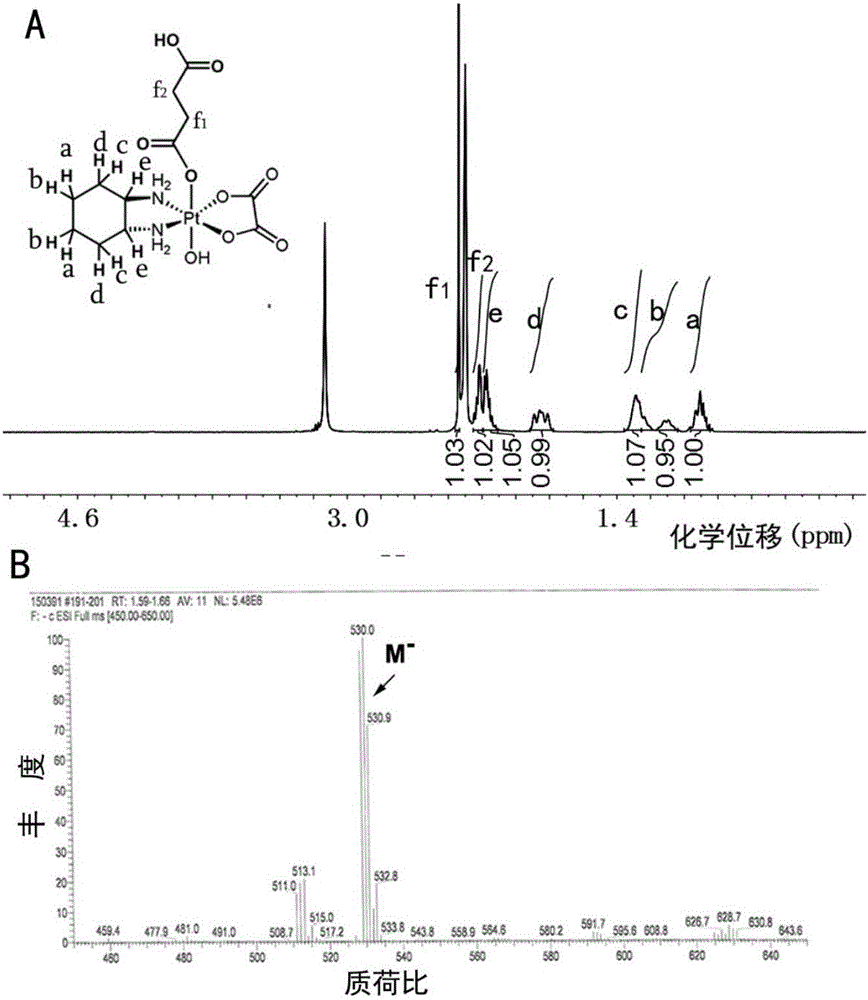
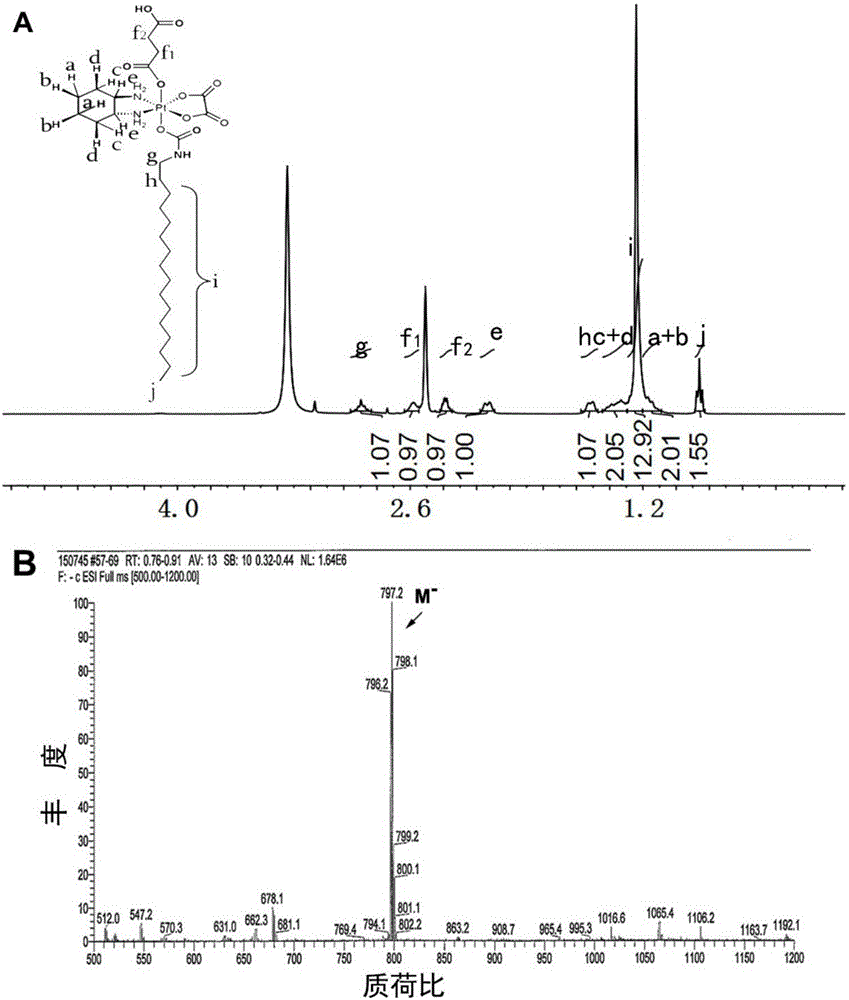
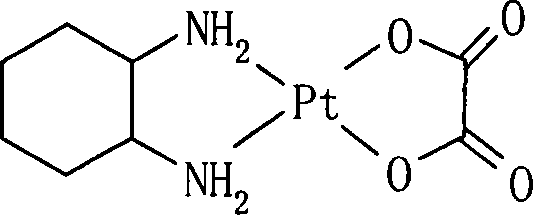
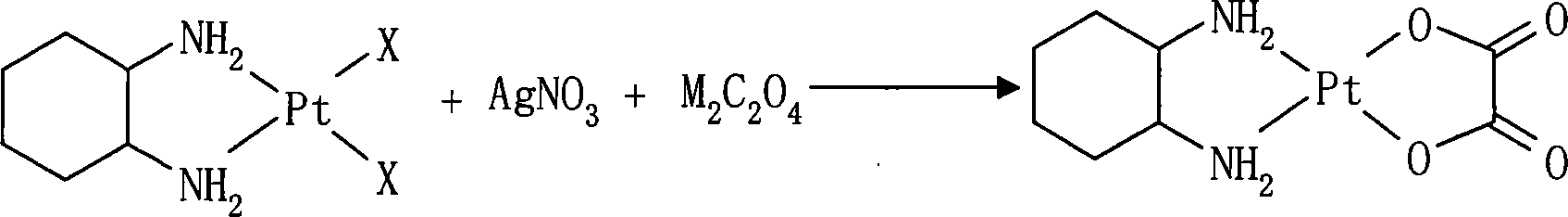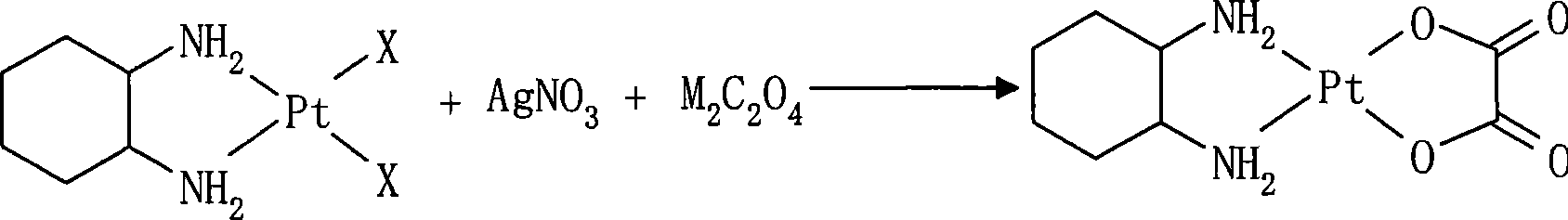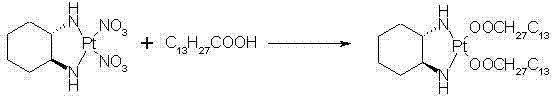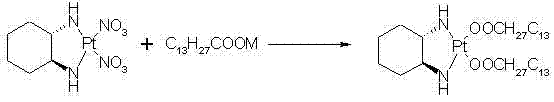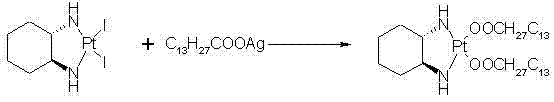Patents
Literature
334 results about "Oxaliplatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
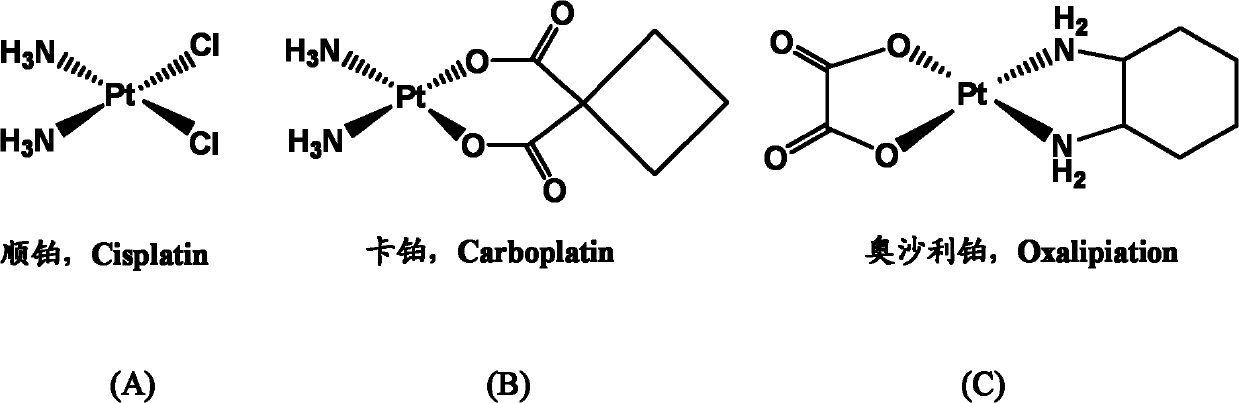
This medication is used to treat advanced cancer of the colon and rectum.
Medicinal compositions for concomitant use as anticancer agent
InactiveUS20030215523A1Good synergyEliminate side effectsHeavy metal active ingredientsBiocideCarboplatinAnticarcinogen
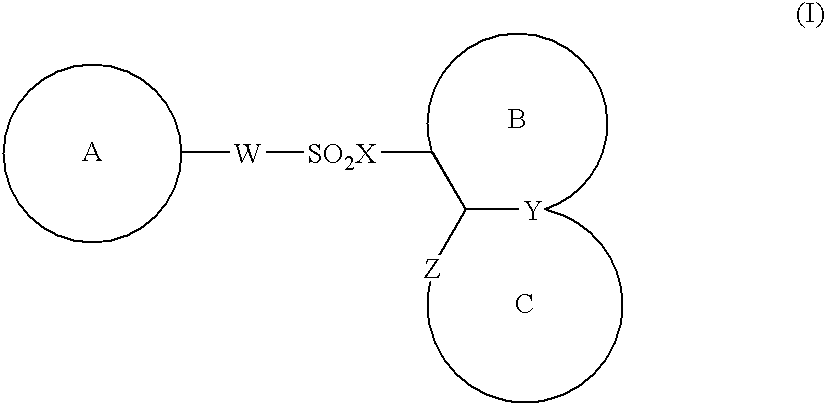
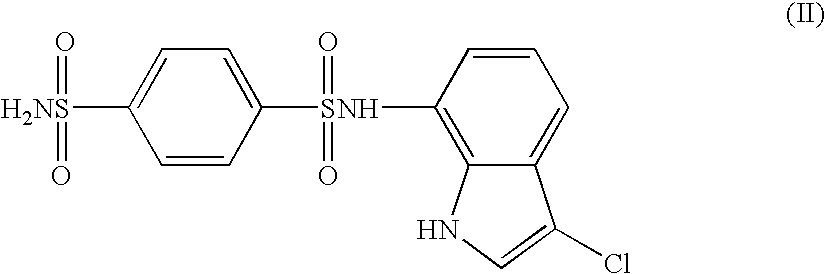
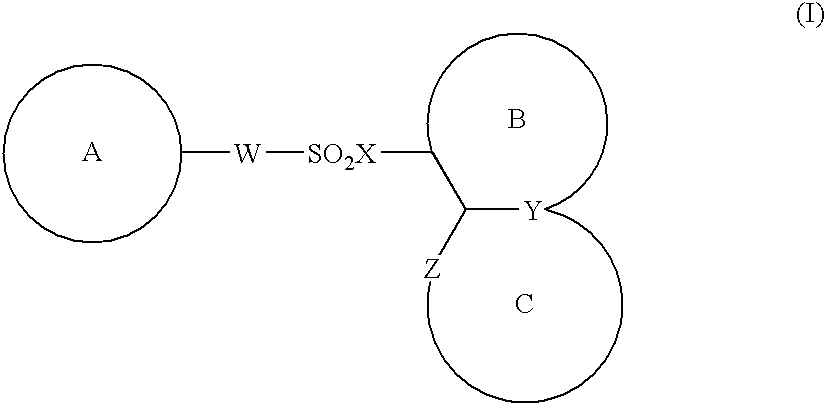
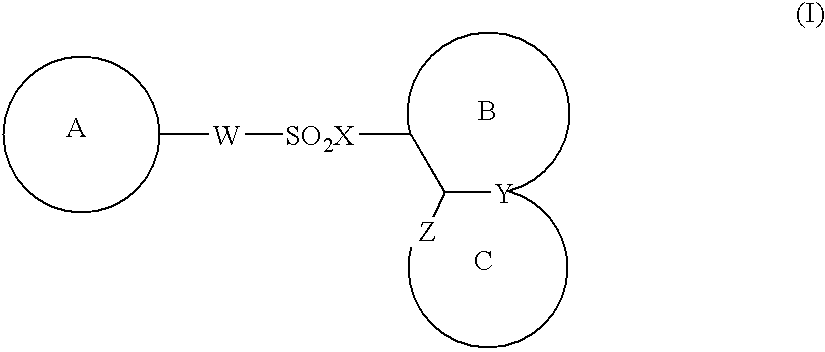
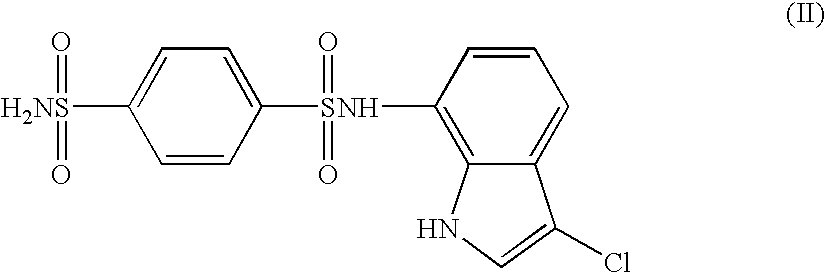
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: (wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl)-4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabine hydrochloride; (6) doxorubicin; (7) taxol; (8) carboplatin; (9) oxaliplatin; (10) capecitabine; and (11) a salt of the above-mentioned (1) to (10).
Owner:EISIA R&D MANAGEMENT CO LTD
Cancer treatments
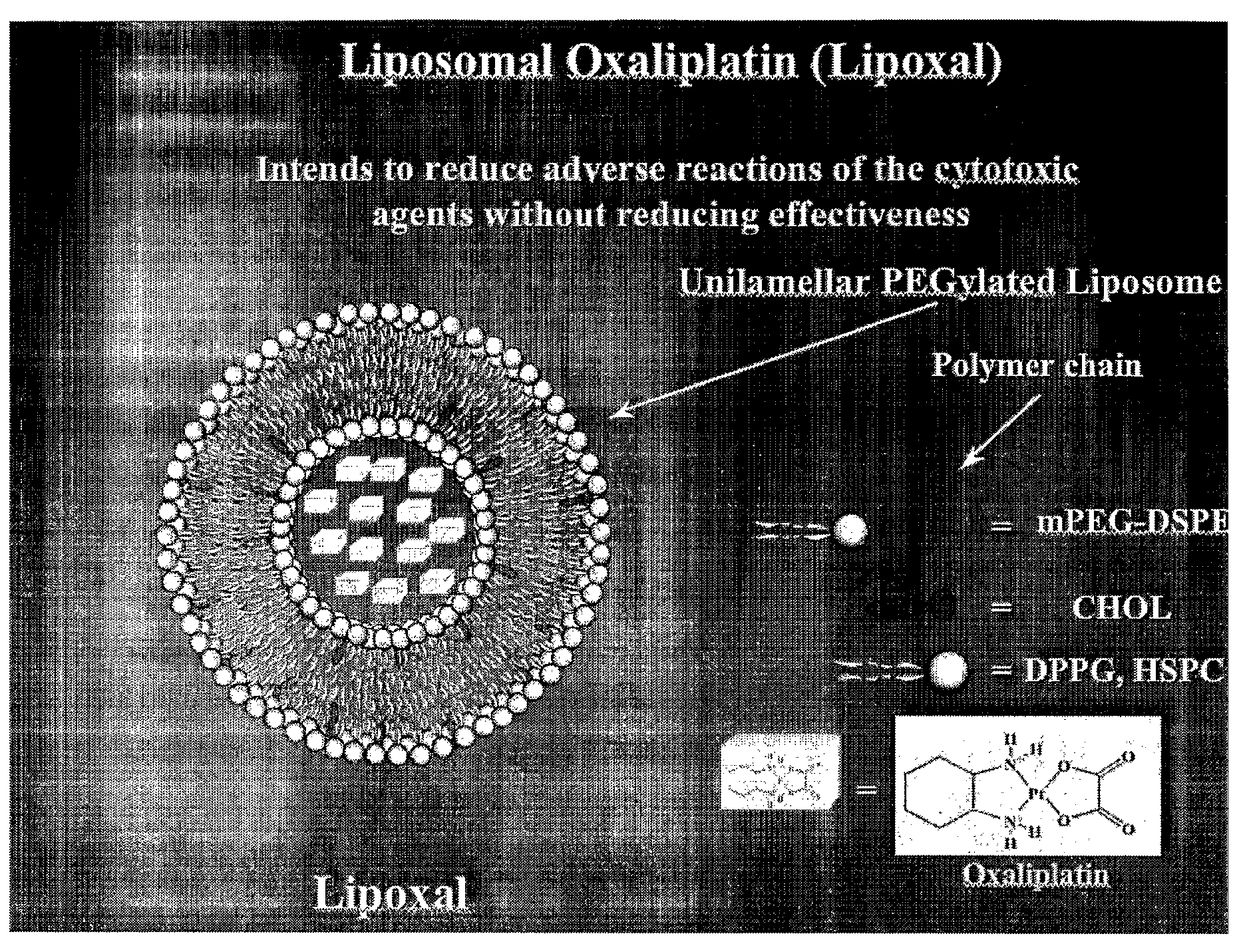
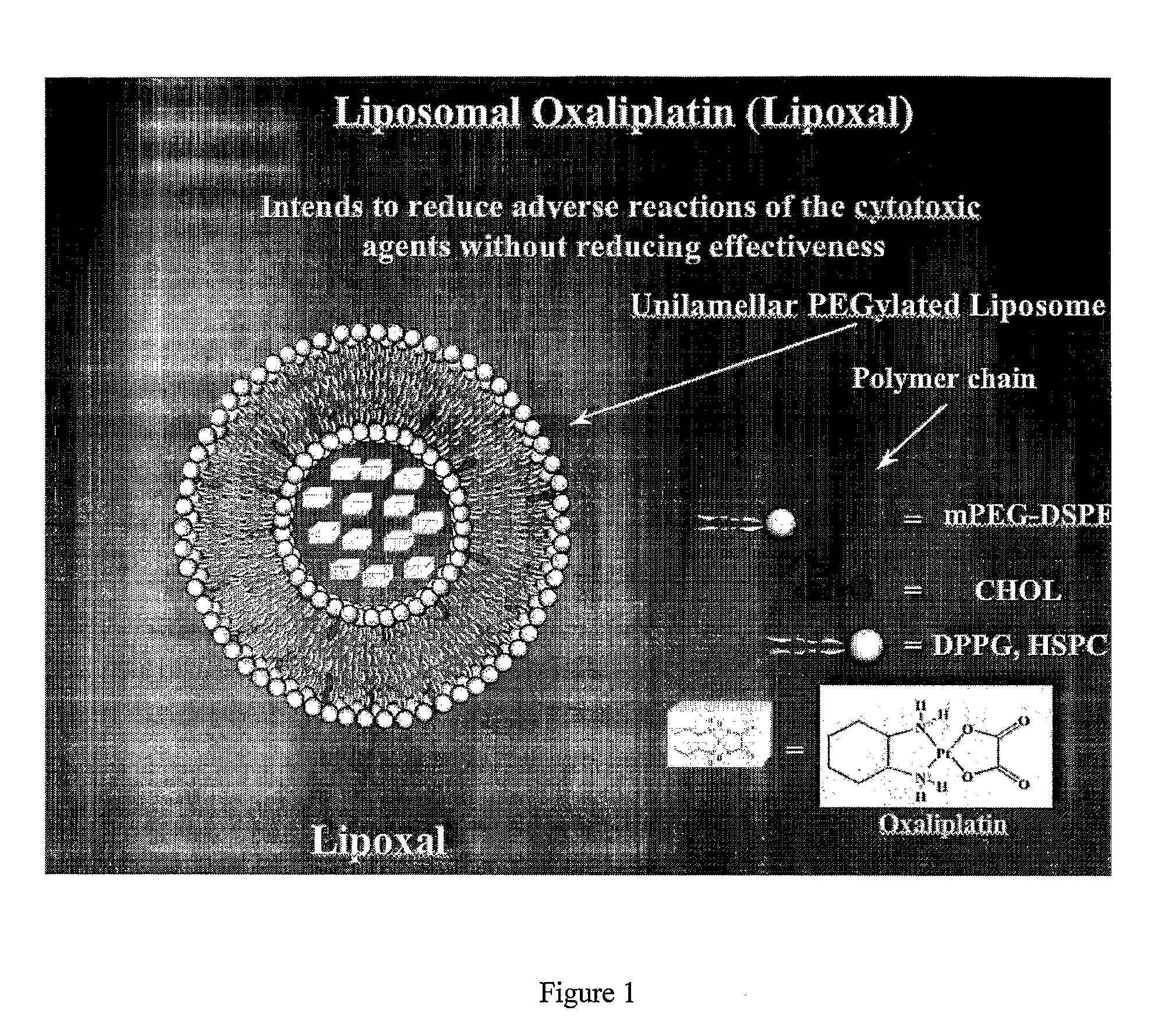
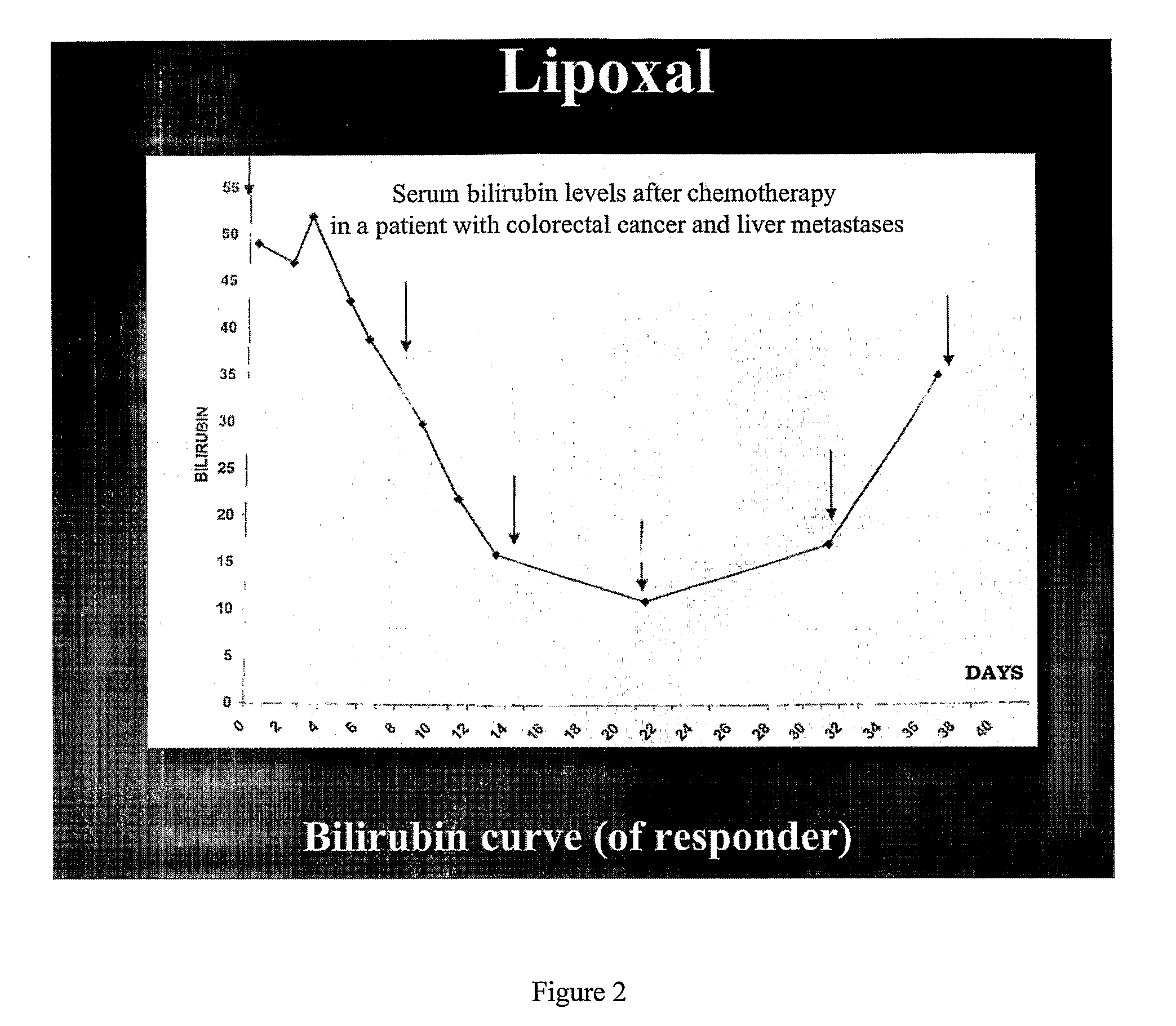
InactiveUS20090053302A1Reduce the adverse reactions of the cytotoxic agents without reducing effectivenessEfficient killingBiocideHeavy metal active ingredientsMedicineCancer therapy
The present invention relates to liposome comprising encapsulated oxaliplatin and methods for making encapsulated oxaliplatin. The invention also relates to liposomes comprising oxaliplatin and another anticancer drug. The liposomes of the invention are useful in cancer treatments.
Owner:BOULIKAS PARTHENIOS
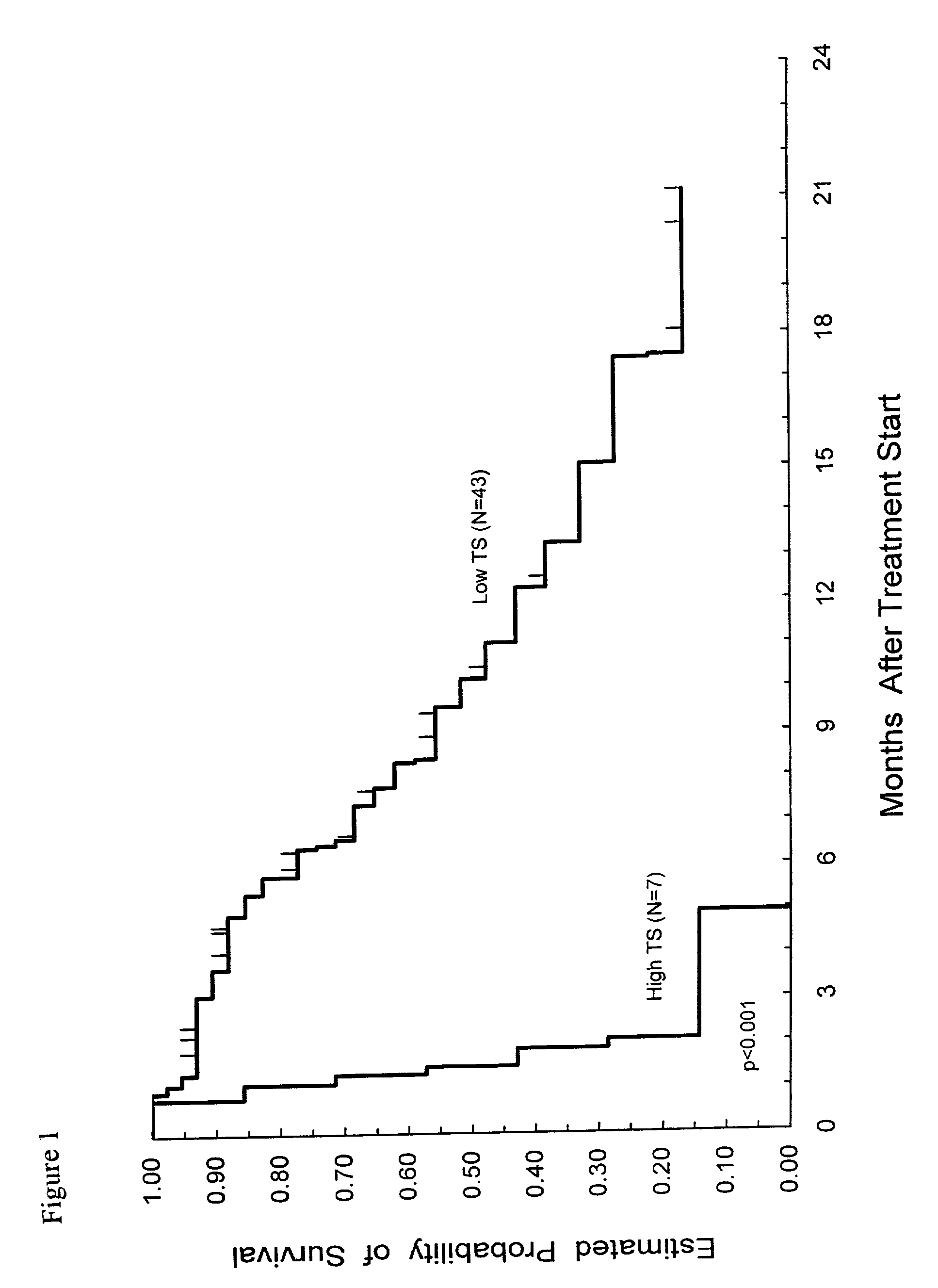
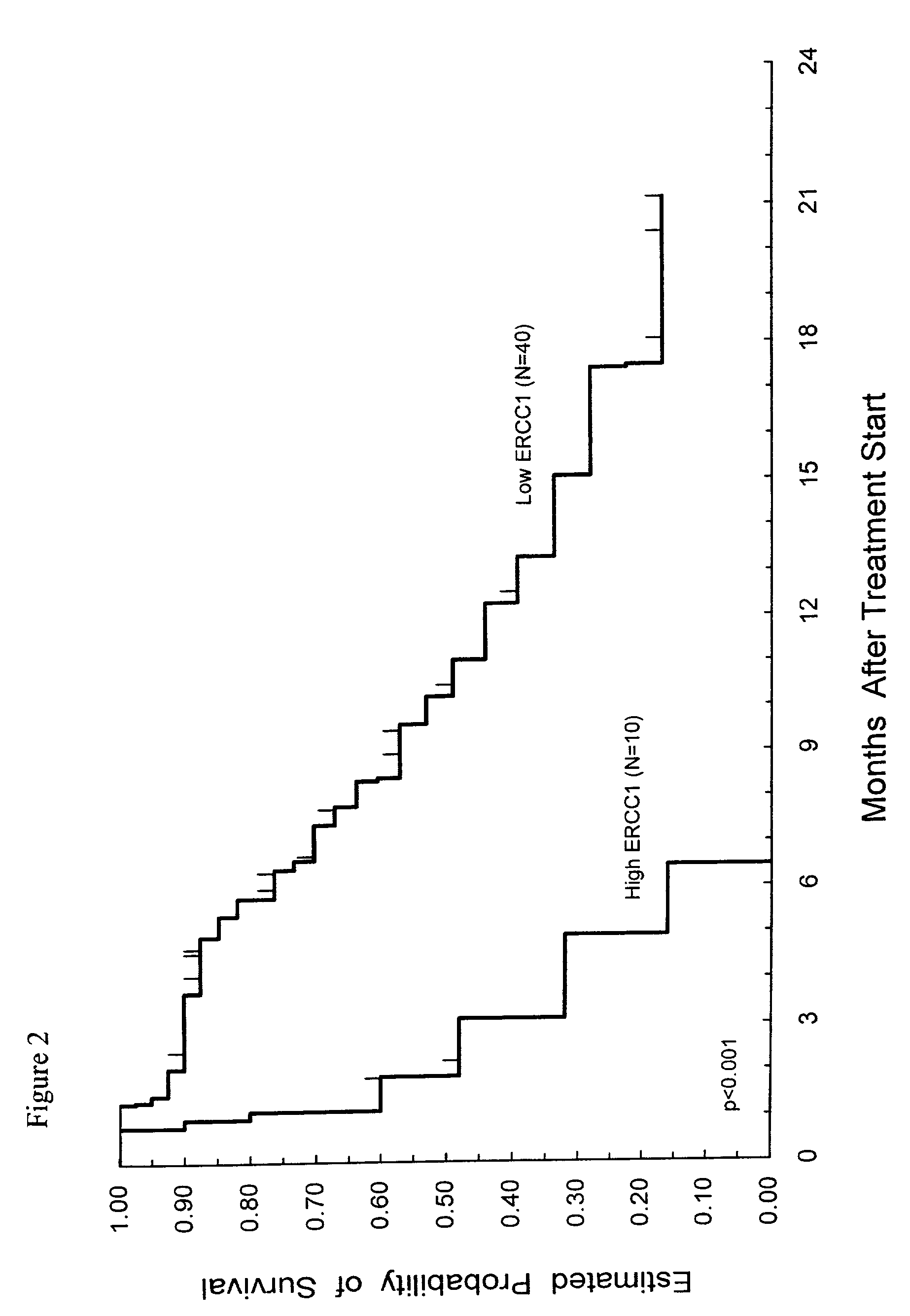
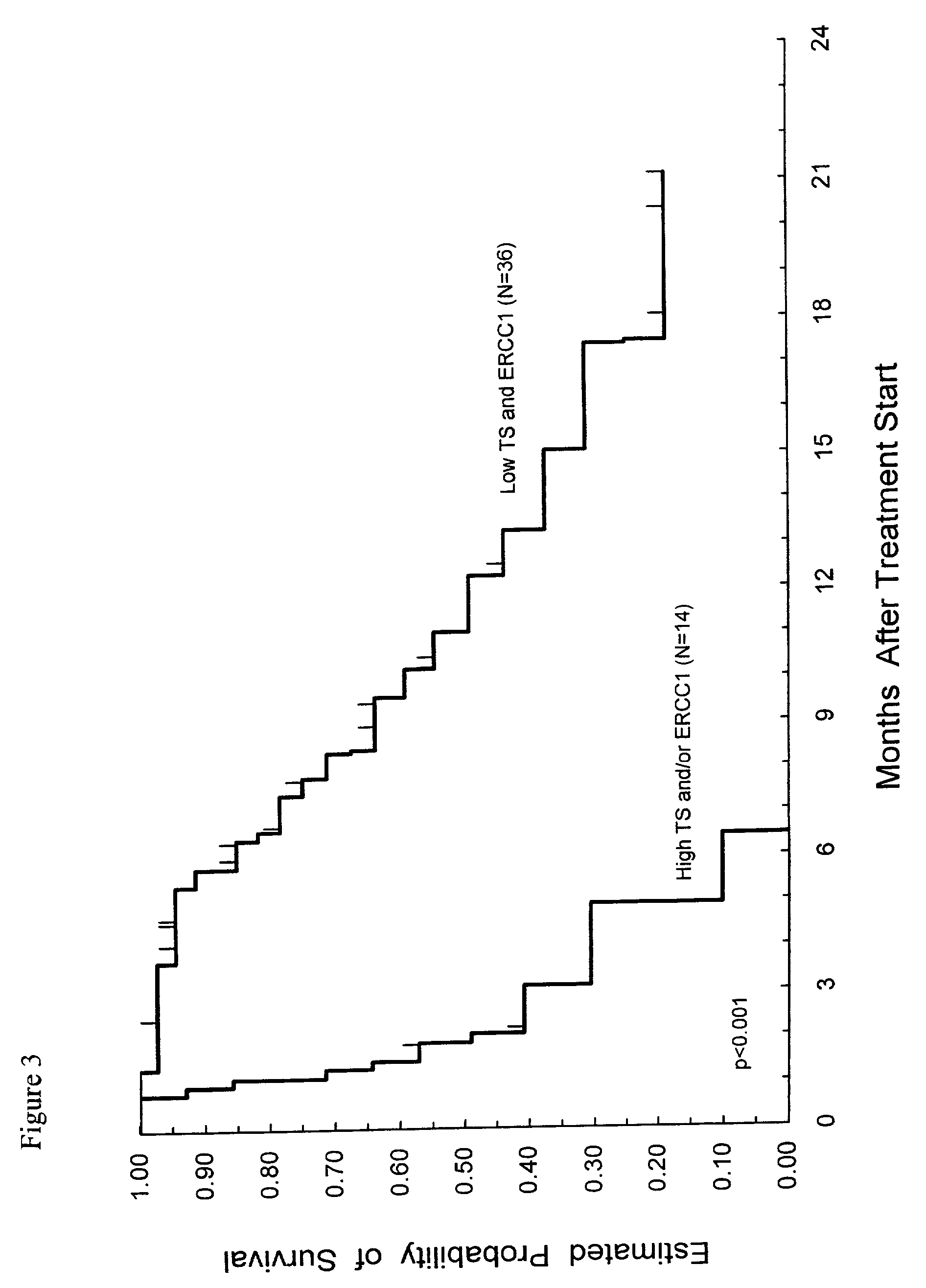
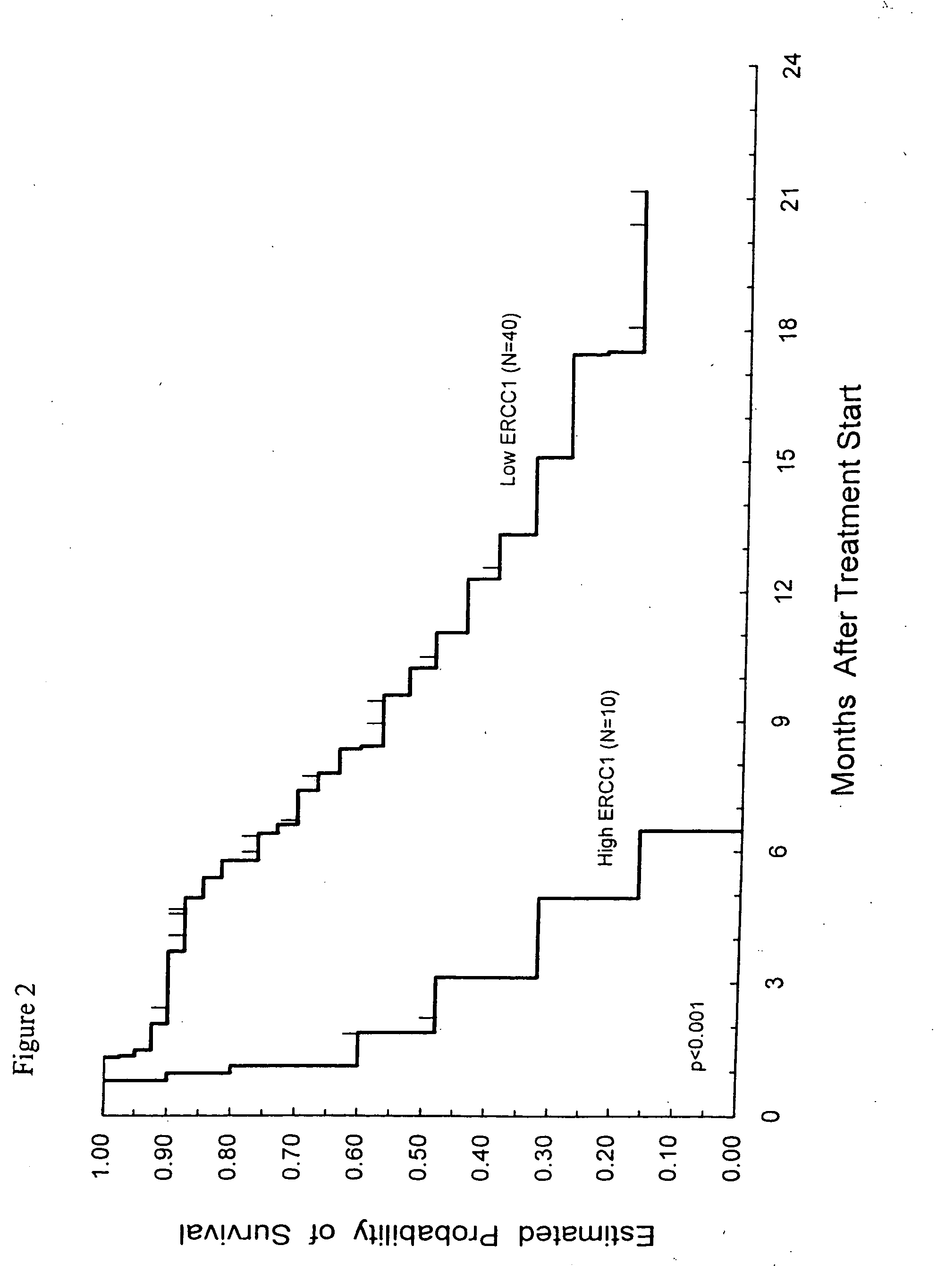
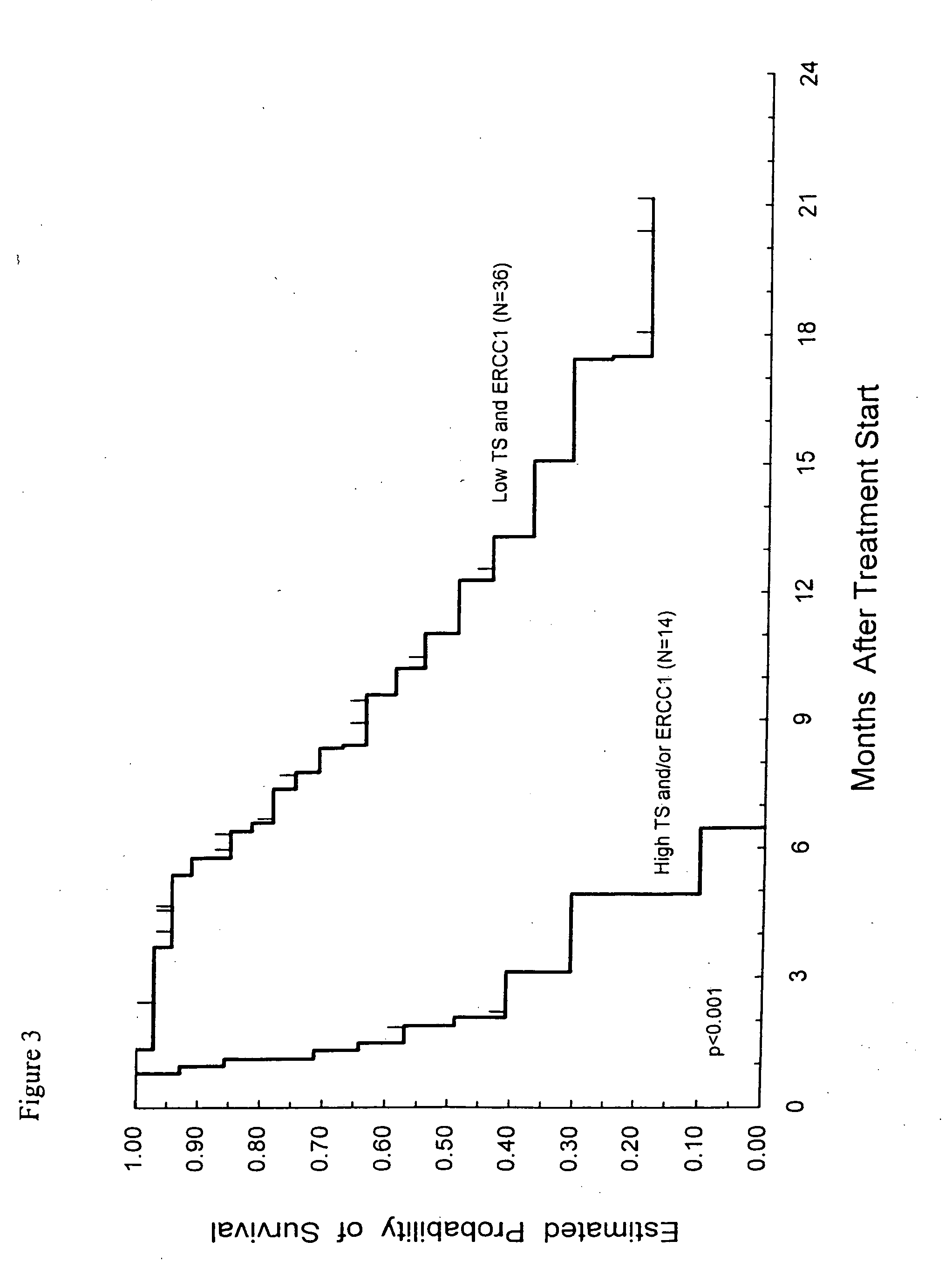
Method of determining a chemotherapeutic regimen based on ERCC1 and TS expression
InactiveUS7049059B2Improve responseMicrobiological testing/measurementRecombinant DNA-technologyAbnormal tissue growthRegimen
The present invention relates to prognostic methods which are useful in medicine, particularly cancer chemotherapy. The object of the invention to provide a method for assessing TS and / or ERCC1 expression levels in fixed or fixed and paraffin embedded tissues and prognosticate the probable resistance of a patient's tumor to treatment with 5-FU and oxaliplatin-based therapies by examination of the amount of TS and / or ERCC1 mRNA in a patient's tumor cells and comparing it to a predetermined threshold expression level for those genes. More specifically, the invention provides to oligonucleotide primer pairs ERCC1 and TS and methods comprising their use for detecting levels of ERCC1 and TS mRNA, respectively.
Owner:CANCER GENETICS
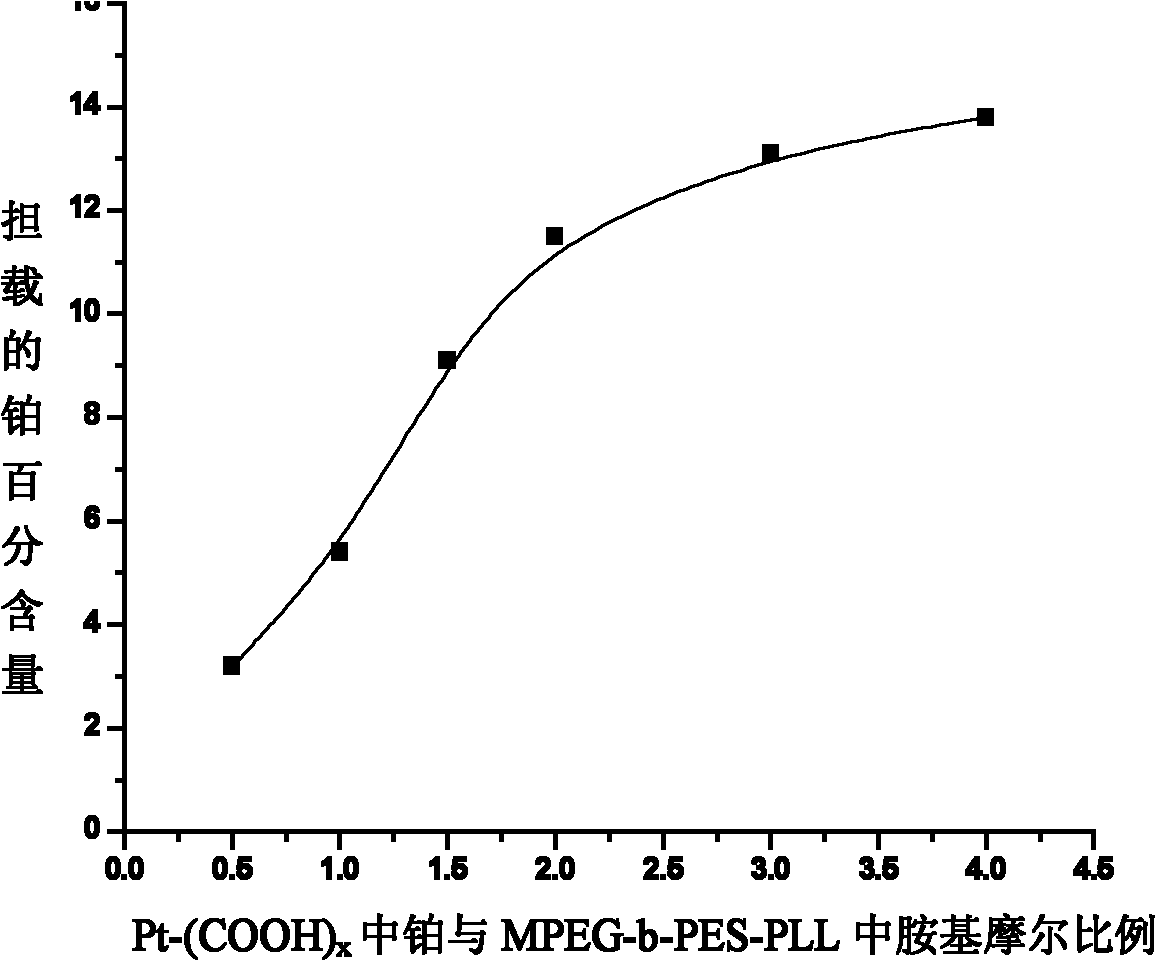
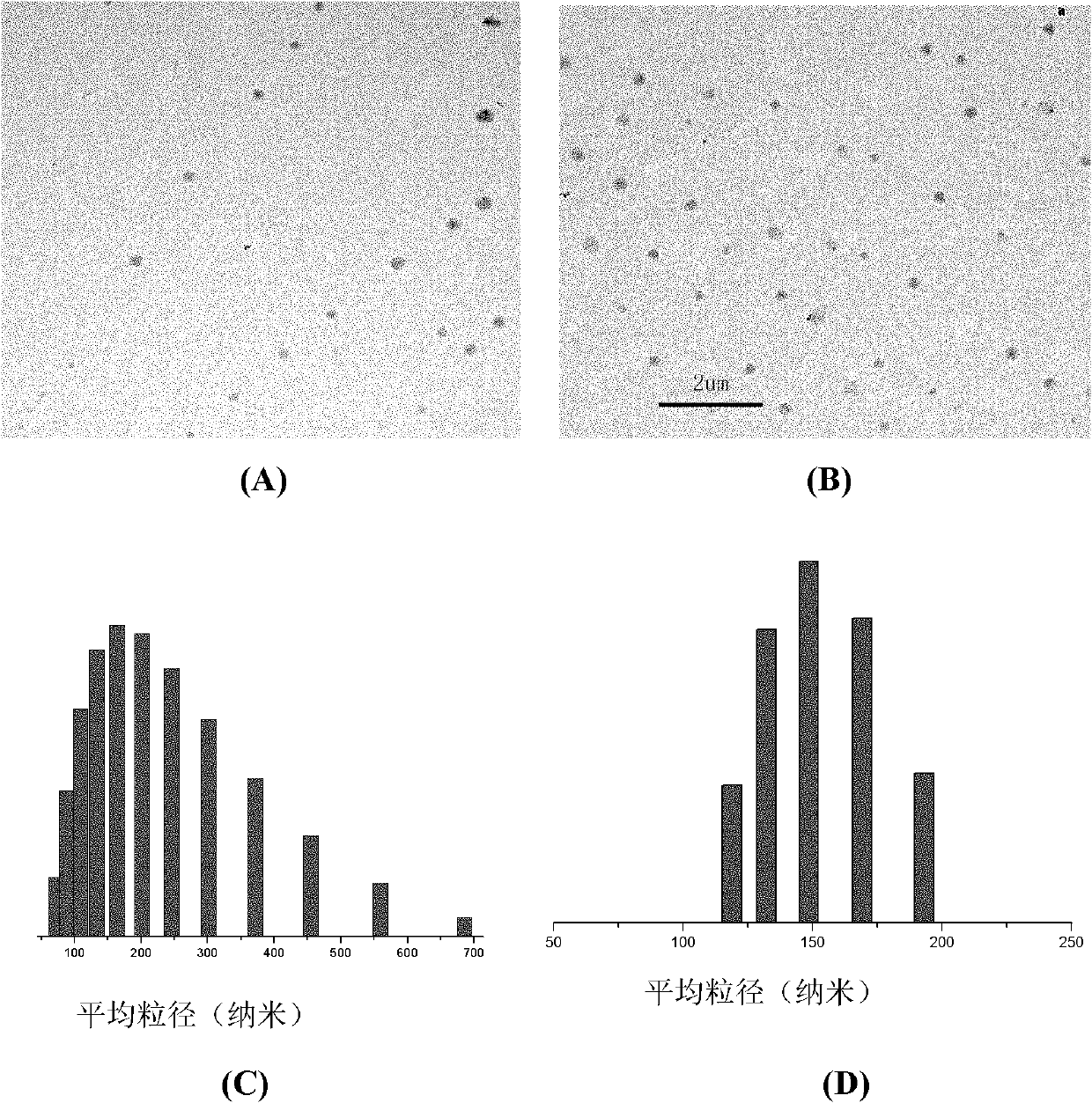
Nano micelle of biodegradable macromolecular-bonding Pt(IV) anti-cancer medicament and preparation method thereof
ActiveCN102120036AReduce toxic and side effectsNo toxicityHeavy metal active ingredientsPharmaceutical non-active ingredientsCarboplatinPolyester
The invention relates to a nano micelle of a biodegradable macromolecular-bonding Pt(IV) anti-cancer medicament and a preparation method thereof. The structural formula of the anti-cancer medicament is defined in the specification, wherein the biodegradable macromolecule is tri-block copolymer, namely polyethylene glycol-b-polyester-b-polylysine; and tetravalency platinum coordination compound Pt(IV) is connected with a side amino on the block of polylysine on a macromolecular carrier through alpha, omega-polyethylene glycol. The carrier is non-toxic and is water-soluble, thus being convenient for reacting with the platinum(IV) coordination compound in water phase; a nano micelle form can be formed through self assembly; a platinum(IV) coordination compound is reduced to platinum(II), namely, cis-platinum, Carboplatin or Oxaliplatin, and the anti-cancer effect is known; the synthesis of the nano micelle is easy; the reduction potential of platinum(IV) is low, so that the platinum(IV) can be rapidly reduced to platinum(II) in cancer cells to take treatment effect; and the platinum(IV) is connected to the side chain of the macromolecule rather than the end of a chain, a macromolecular chain can be connected with multiple platinum(IV)s, and the content of platinum can be as high as 10-20%.
Owner:吉林市博禹祥实工贸有限公司
Medicinal compositions for concomitant use as anticancer agent
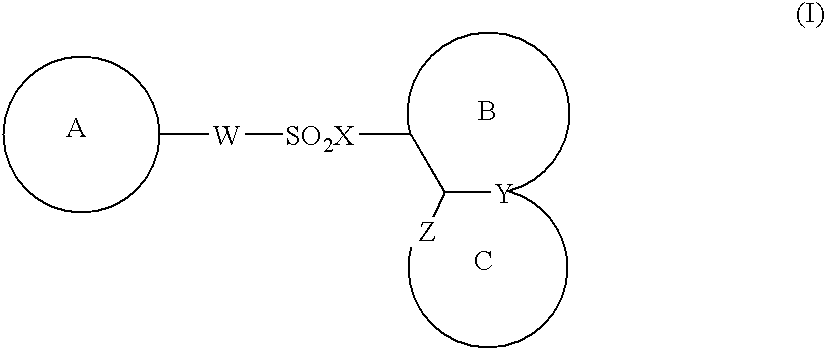
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: (wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl)-4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabine hydrochloride; (6) doxorubicin; (7) taxol; (8) carboplatin; (9) oxaliplatin; (10) capecitabine; and (11) a salt of the above-mentioned (1) to (10).
Owner:EISIA R&D MANAGEMENT CO LTD
Biodegradable high-polymer bonded photoactive Pt (IV) anticancer medicament micelle and preparation method thereof
ActiveCN102416181ASignificant photoactivityFast releasePowder deliveryPharmaceutical non-active ingredientsChemical synthesisPolyester
The invention provides a biodegradable high-polymer bonded photoactive Pt (IV) anticancer medicament micelle and a preparation method thereof, which relate to the field of novel chemical synthesis medicaments and preparations thereof and are used for solving the problem that a high-polymer-platinum (IV) bonded medicament does not have optical activity in the prior art. In an anticancer medicament, ethylene glycol-b-polyester-b-polylysine serving as a biodegradable triblock copolymer or polyethylene glycol-b-poly(ester-co-carbonic ester) serving as a diblock copolymer is taken as a carrier polymer, and Pt (IV) is a photoactive tetravalent platinum composition coordinated with dihydroxyl in the axial direction. The invention further provides a preparation method of the biodegradable high-polymer bonded photoactive Pt (IV) anticancer medicament micelle. The anticancer medicament prepared with the method has remarkable optical activity; under the irradiation of UV (Ultraviolet) of 365 nanometers, Pt (IV) is reduced into Pt (II); a cis-platinum or oxaliplatin antitumor active part is contained; and the mass content of platinum can be up to 10-20 percent.
Owner:吉林市博禹祥实工贸有限公司
Composite developing thermosensitive gel embolizing agent as well as preparation method and application thereof
ActiveCN107281502AIncrease success rateReduce chanceOrganic active ingredientsHeavy metal active ingredientsCarboplatinMiriplatin
The invention relates to a composite developing thermosensitive gel embolizing agent as well as a preparation method and application thereof. The preparation method comprises the following steps: firstly, preparing a mixed aqueous solution of an anticancer active substance, a thermosensitive material and a developing agent into composite developing thermosensitive gel; secondly, forming a composite developing thermosensitive gel embolizing agent by the composite developing thermosensitive gel and a coagulant on the scene, wherein the thermosensitive material is hydroxyl C1-4 alkyl cellulose, Pluronic, alginate or a mixture of the substances; the anticancer active substance is arsenic trioxide, docetaxel, cisplatin, carboplatin, nedaplatin, oxaliplatin, lobaplatin, miriplatin, siRNA or a mixture of the substances; the developing agent is a water-soluble developing agent of iodixanol, ioversol or iohexol and the like. The preparation method disclosed by the invention is simple and convenient, is suitable for industrial large-scale production, is particularly suitable for preparing an embolizing agent which is biodegradable and good in biocompatibility and is used for hemorrhagic diseases, and is especially suitable for preparing the composite developing thermosensitive gel embolizing agent for treating liver cancer, kidney cancer, lung cancer, prostate cancer, uterine myoma or splenic tumor and the like.
Owner:苏州申润医疗科技有限公司 +1
Oxaliplatin lyophilized powder injection and preparing method thereof
ActiveCN101199506AGood reproducibilityLess impuritiesPowder deliveryPharmaceutical non-active ingredientsCITRATE ESTERMANNITOL/SORBITOL
The invention relates to oxaliplatin freeze-dried injection, which is characterized in that the invention is prepared by the method that aqueous solution is freeze-dried. The aqueous solution contains oxaliplatin, mannitol and citrate, wherein, the concentration of the oxaliplatin in the aqueous solution is 2.5 to 6.25 mg / ml; the concentration of the mannitol in the aqueous solution is 25 to 200 mg / ml; and the concentration of the citrate in the aqueous solution is 2 to 20 mg / ml. And sodium citrate can also be added into the aqueous solution so as to adjust the pH of the aqueous solution. The preparation processes are as following: the oxaliplatin is placed inside the container, 80 percent amount of water for injection is added, and then the water for injection is mixed so that the oxaliplatin can be dissolved and mixed evenly in the water for injection; after that, the mannitol and the citrate are added into the water for injection, and then the water for injection is mixed so that the mannitol and the citrate can be dissolved and mixed evenly in the water for injection; the content of the intermediate is measured; if the content of the intermediate is qualified, the volume of the water for injection is fixed to full amount; in the aseptic condition, the water for injection is filtered until to be clear by a microporous membrane of 0.22 microns; the filtered solution is filled into an aseptic silin bottle; part of the aseptic silin bottle is plugged with a butyl rubber closure; and then the aseptic silin bottle is filled in the tray to be sent into the freeze dryer to be freeze-dried; the mouth of the aseptic silin bottle is rolled; the quality of the filtered solution is inspected; and the aseptic silin bottle is packaged.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Drug delivery systems and methods for treatment of bladder cancer
Methods, devices, and medicaments that include oxaliplatin are provided for use in the treatment of bladder cancer by locally administering oxaliplatin into the bladder of a patient to achieve a sustained concentration of oxaliplatin in urine in the bladder sufficient to produce a therapeutic concentration of oxaliplatin in bladder tissue. The oxaliplatin may be delivered into the bladder from an intravesical drug delivery device inserted into the bladder, wherein the device continuously releases the oxaliplatin into the urine in the bladder over an extended period of hours or days.
Owner:TARIS BIOMEDICAL
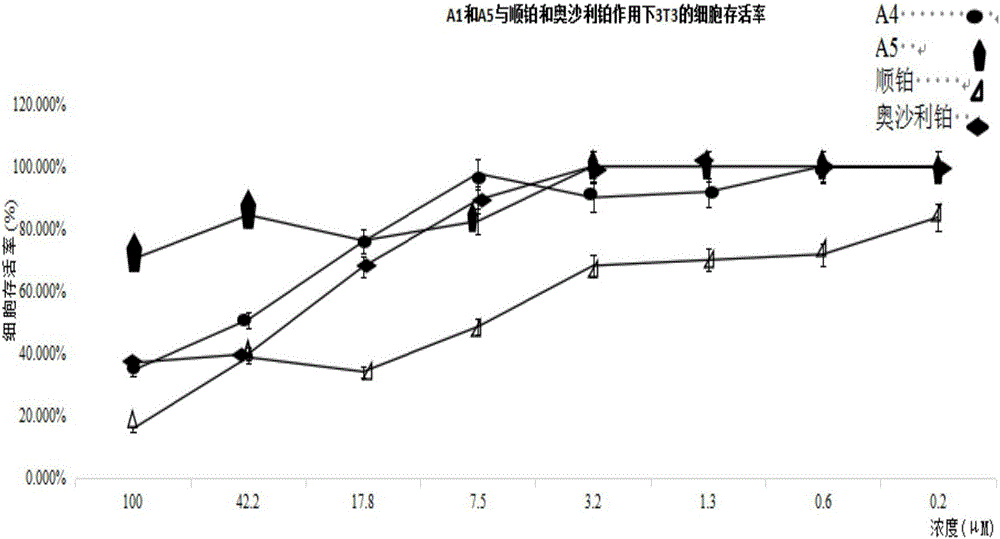
Quadrivalent-platinum glycosyl complex for treating tumors and preparation method thereof
InactiveCN105753922AImprove targetingSmall toxicityOrganic active ingredientsSugar derivativesSolubilityCarboplatin
The invention provides a quadrivalent-platinum glycosyl complex for treating tumors and a preparation method thereof. The synthesized compound has good anti-tumor activity better than that of cisplatin and oxaliplatin, and has better stability than that of bivalent platinums such as cisplatin, carboplatin and oxaliplatin. In addition, since the quadrivalent platinum with galactosylated modification has better targeting for tumor cells, the high selectivity to the tumor cells is improved. Furthermore, the compound provided by the invention has the advantages that the problems of poor solubility and more troublesome clinical compatibility of the original bivalent-platinum anti-tumor medicine are solved, and both fat solubility and water solubility are better. The quadrivalent-platinum glycosyl derivative not only can improve the in-vivo availability and enhance the curative effect, but also can reduce the toxic and side effects of the original bivalent-platinum medicine on the kidney and the like.
Owner:NANKAI UNIV
Method for simultaneously detecting multiple anti-tumor drugs in blood sample
InactiveCN110927297AEasy to handleImprove throughputComponent separationBusulfanTandem mass spectrometry
The invention discloses a method for simultaneously detecting multiple anti-tumor drugs in a blood sample. A pretreated sample to be detected is detected by adopting ultra-high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). The pretreatment process comprises the following steps: adding serum into a mixed solution of methanol and acetonitrile, oscillating and centrifuging,taking out the centrifuged supernatant, drying, dissolving the dried powder into a methanol aqueous solution, and filtering to obtain a sample to be detected. The method can be used for simultaneously detecting 13 kinds of anti-tumor drugs such as methotrexate, 5-fluorouracil, apatinib, busulfan, carboplatin, cyclophosphamide, docetaxel, gemcitabine, imatinib, illinotecan, lenalidomide, oxaliplatin, paclitaxel and the like in blood.
Owner:JINAN YING SHENG BIOTECH
Intravenous nanometer suspension injection contg. oxaliplatin platinum phospholipid compound
InactiveCN1868455AHigh drug loadingImprove stabilityAntineoplastic agentsOil/fats/waxes non-active ingredientsFreeze-dryingPhospholipid complex
A nano-class suspension injection of oxaliplatin-phoshpatide composition for intravenous injection is proportionally prepared from oxaliplatin, surfactant, pH regulator, isotonic agent, antioxidant, water for injection, and optional excipient (for the freeze-dried powder injection).
Owner:SHENYANG PHARMA UNIVERSITY
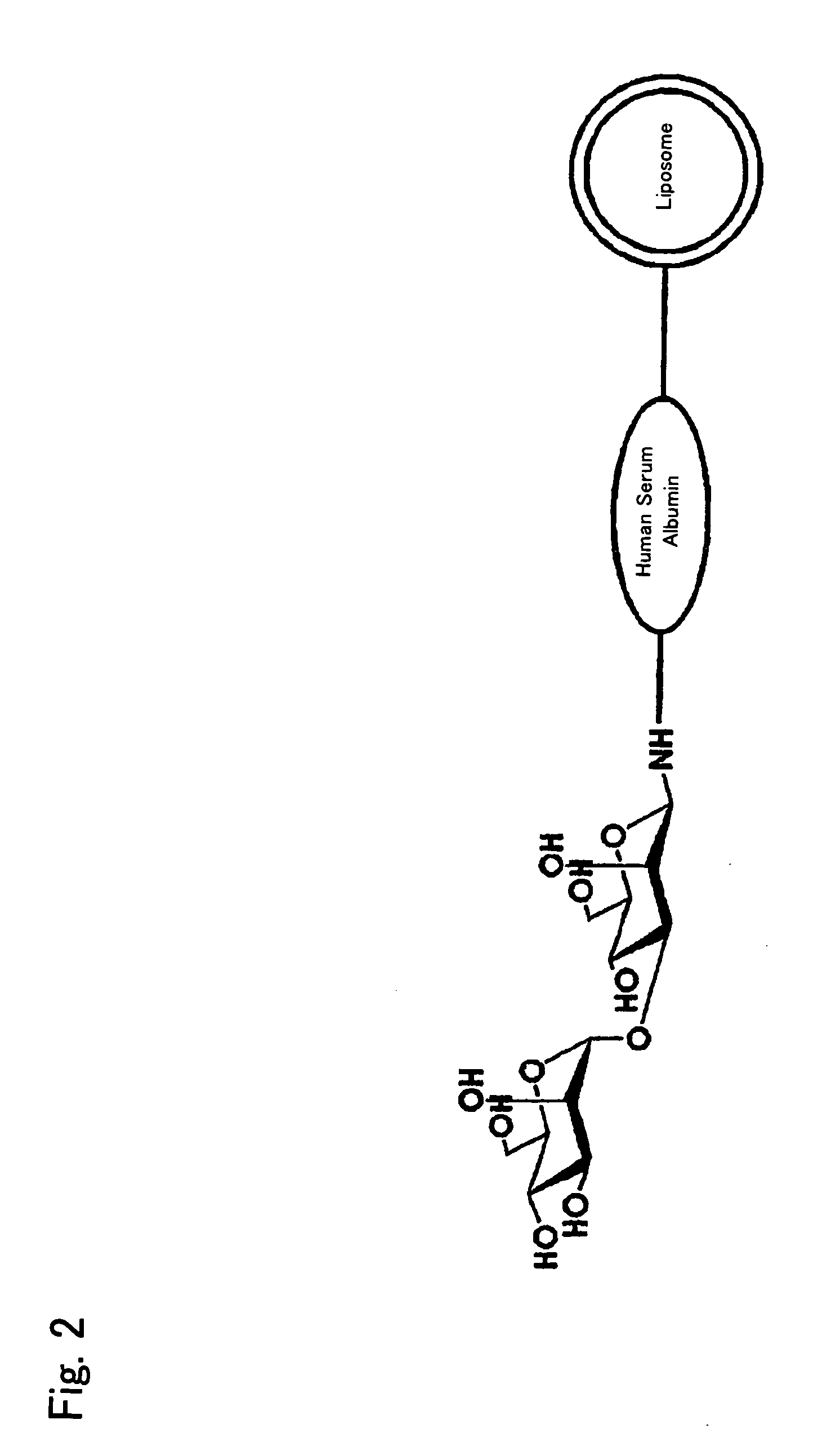
Liposome preparation containing Oxaliplatin
The invention discloses a liposome preparation containing antineoplastic metallic complex which is derived by hydrophilic polymers and aglycones, in one embodiment, the antineoplastic metallic complex includes Oxaliplatin, the hydrophilic polymers include polyethylene glycol, the aglycone is transferrin. In accordance with the invention, the transferring expressed on the surface of the tumor cell can facilitate the absorption of agent in the liposome by the tumor cells. The invention also provides the pharmaceutical composition containing the liposome and method for application.
Owner:MEBIOPHARM
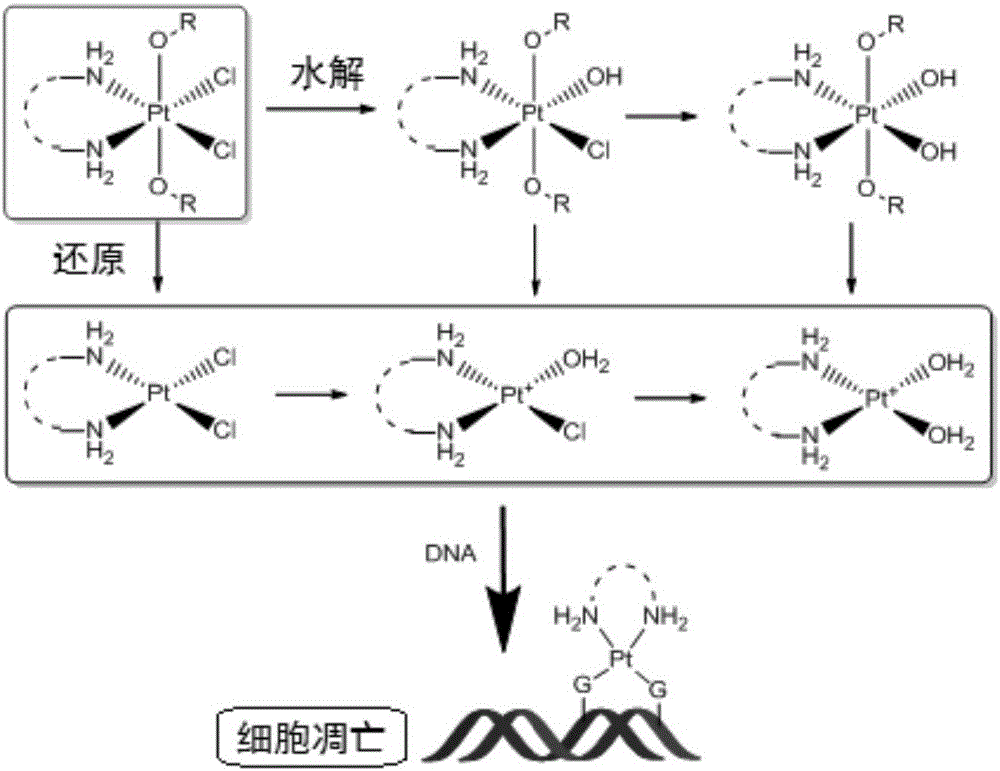
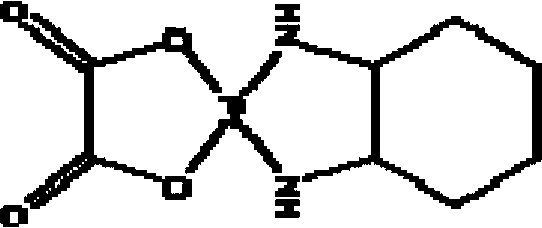
New oxaliplatin derivate
InactiveCN101289468AGood inhibitory effectHigh activityGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSide effectFreeze-drying
The invention relates to a novel oxaliplatin derivative of (1R, 2R-cyclohexanediamine.2, 2-Bis (hydroxymethyl)-1, 3-malonato-platinum (II)) and ((1R, 2R-cyclohexanediamine).2, 2-Bis (hydroxymethyl)malonato-platinum (II)). The derivative is prepared with the steps that the cis-(PtA2I2) (A2 is equal to 1R, 2R-cyclohexanediamine) is adopted as the raw material and quantitatively reacts with the 2, 2-Bis (hydroxymethyl)-1, 3-malonate silver, and a crude product then is obtained through the mother liquor concentration and freeze-drying after the AgI is filtered and separated; a pure product is obtained by recrystallizing the crude product in a system that the ratio of water to ethanol is 1:1. The anti-caner activity of the complex of the derivative is obviously higher than the oxaliplatin and the toxic side effect is obviously less than the oxaliplatin, and can be prepared into freeze-dried powders or injections and used for treating the cancer clinically.
Owner:KUNMING INST OF PRECIOUS METALS +1
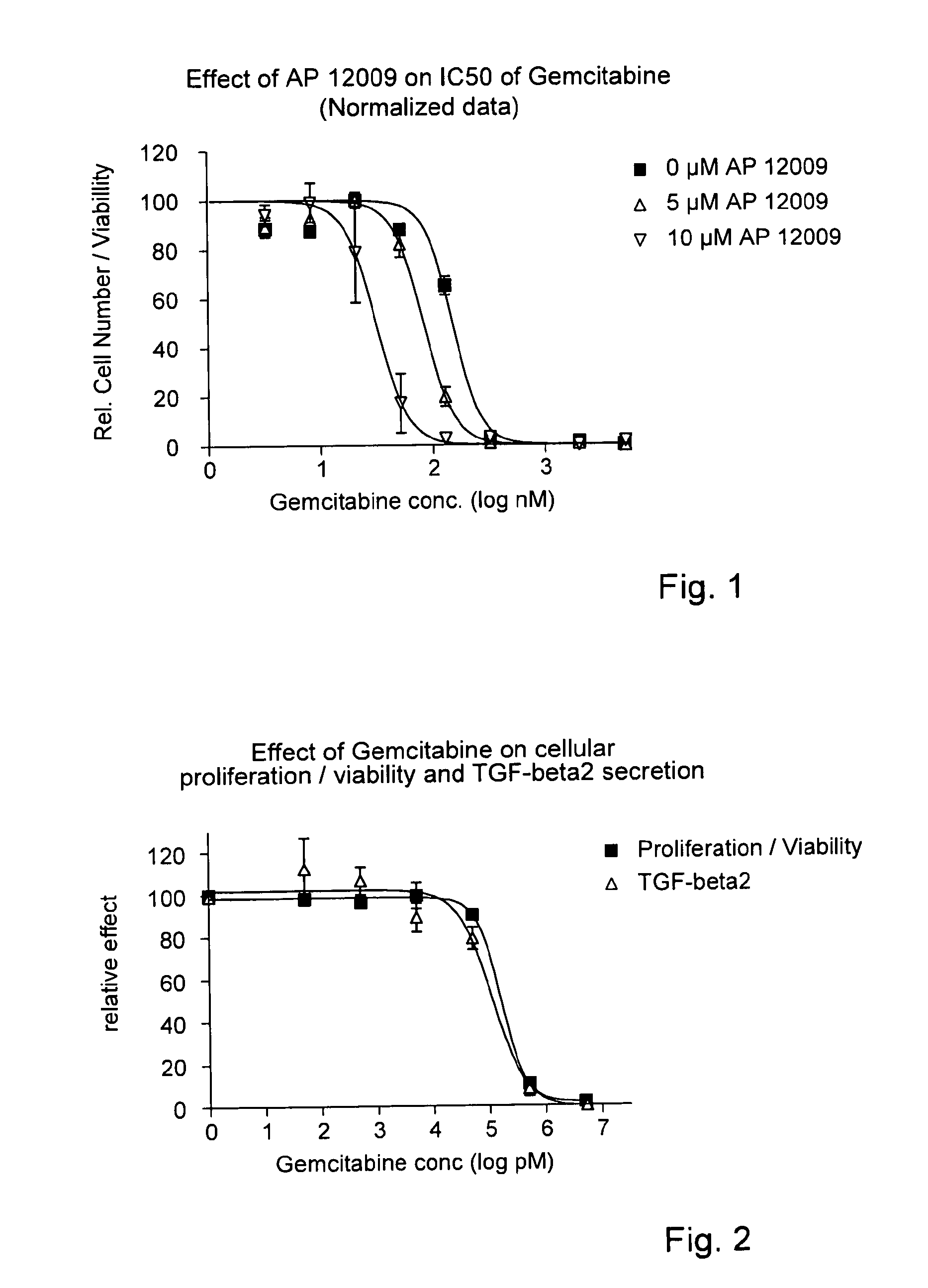
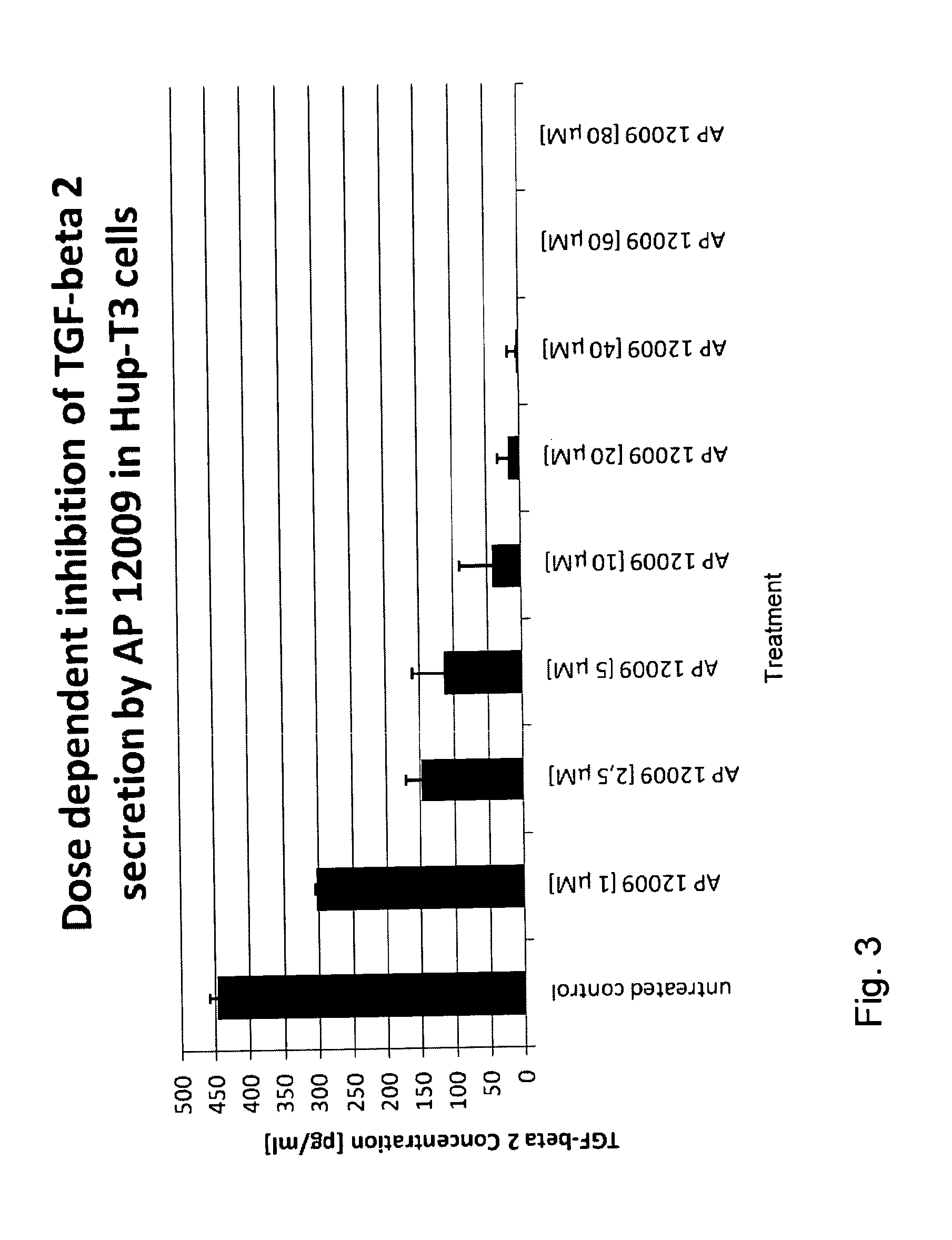
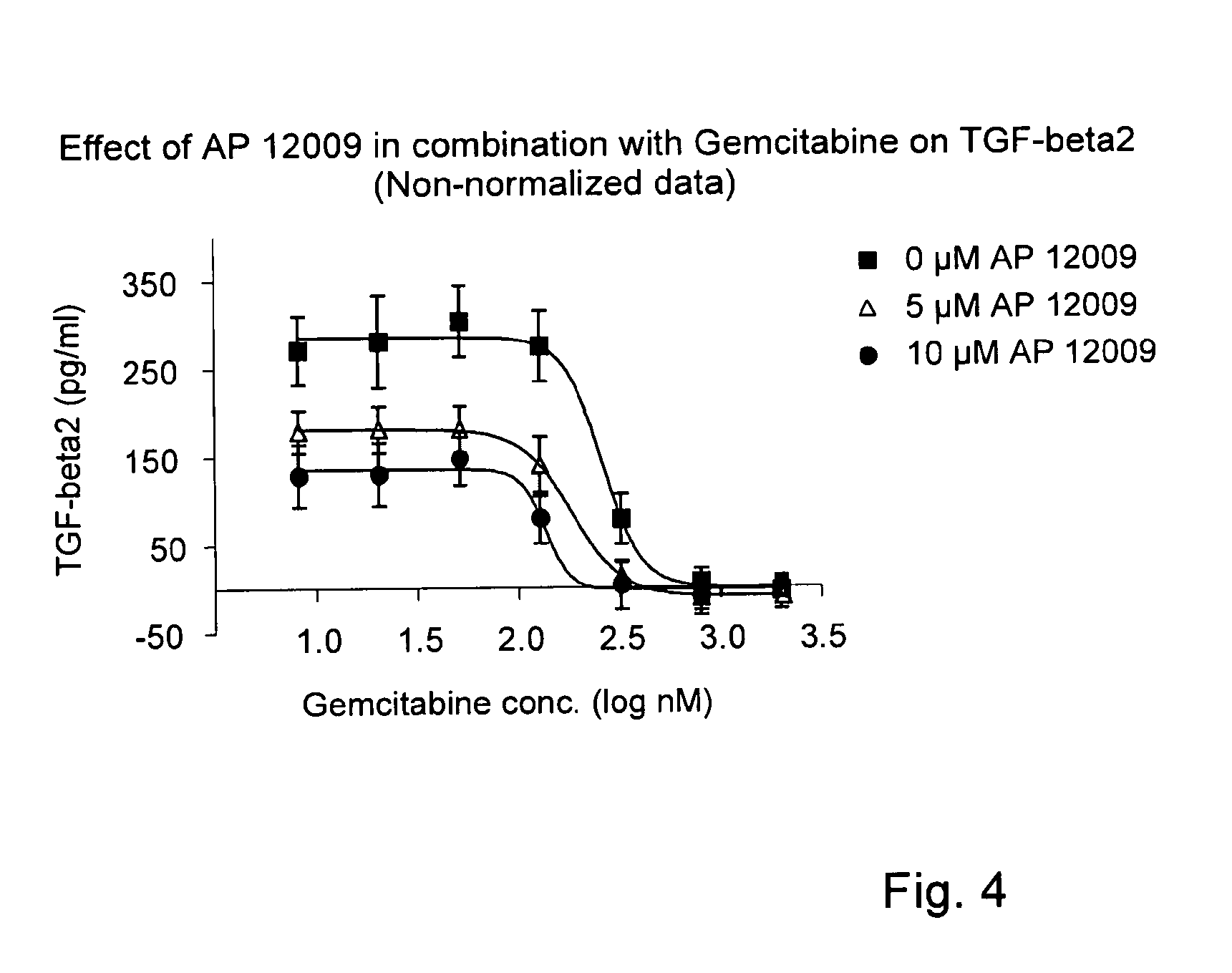
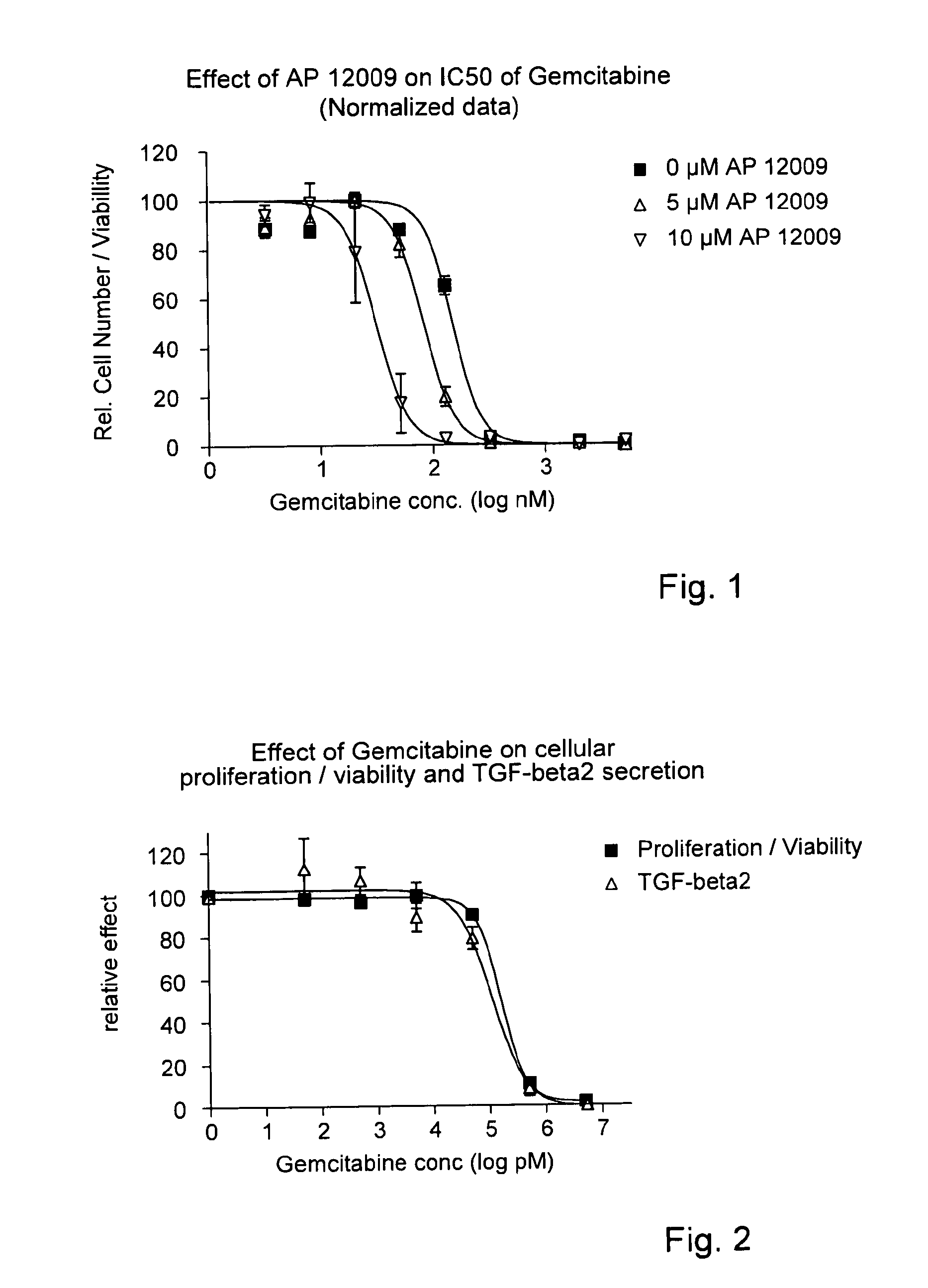
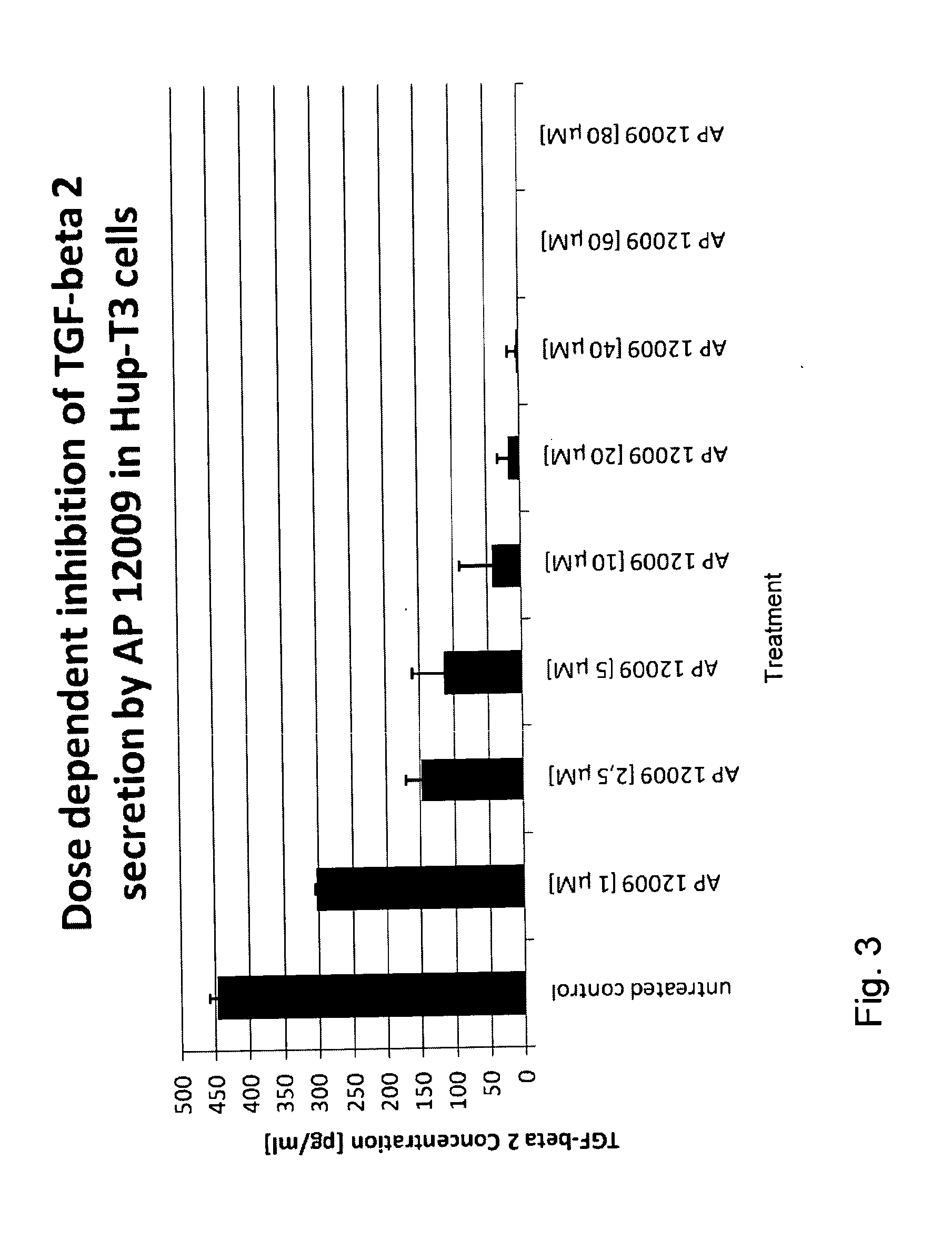
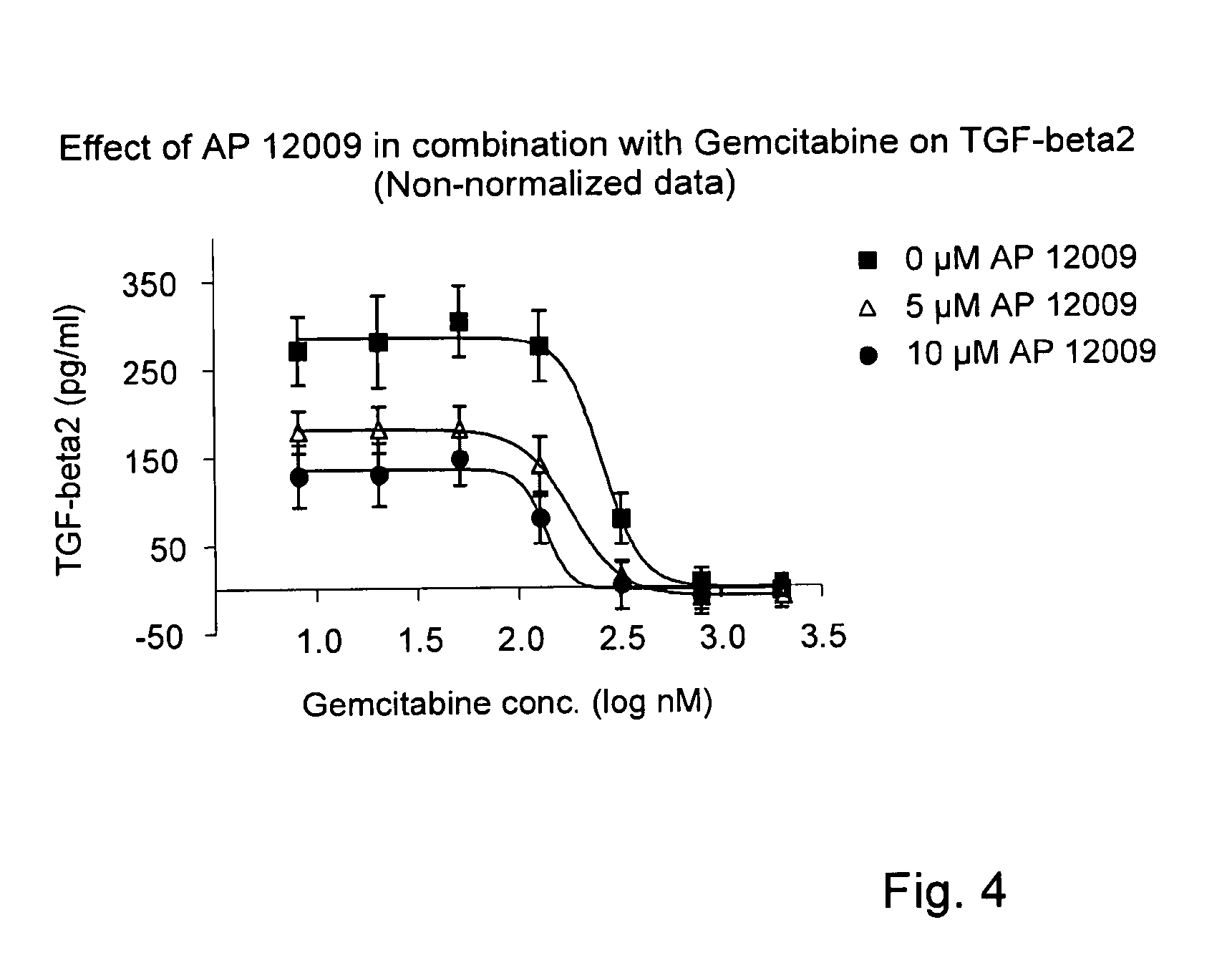
Combination of a chemotherapeutic agent and an inhibitor of the TGF-beta system
ActiveUS8476246B2Reduce IC50Improve efficiencyBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
Pharmaceutical composition comprising a chemotherapeutic agent and a TGF-beta antisense oligonucleotide, wherein the antisense oligonucleotide reduces the sensitivity and IC50, respectively, of the cytotoxicity of the chemotherapeutic agent. Preferably, the antisense oligonucleotide is a TGF-beta 1, 2, and / or 3 antisense oligonucleotide and the chemotherapeutic agent is preferably gemcitabine, 5-fluorouracil, temozolomide, dacarbacine, docetaxel, cisplatin, oxaliplatin, tamoxifen, or irinotecan.
Owner:ANTISENSE PHARMA GMBH
Pharmaceutical composition, method of manufacturing and therapeutic use thereof
InactiveUS20060275331A1Easy to prepareSuitable can be appliedBiocideHydroxy compound active ingredientsActive componentFreeze-drying
The invention relates to a pharmaceutical composition prepared by freeze-drying in vacuo, containing oxaliplatin as the active component and a pharmaceutically acceptable carrier, wherein the carrier is at least one alcoholic sugar of non-animal origin, the weight ratio of oxaliplatin to the alcoholic sugar of non-animal origin or alcoholic sugars of non-animal origin being 1:3 to 1:7. The invention also relates to the method of manufacturing of said composition and to the use of this composition in the treatment of tumors sensitive to oxaliplatin.
Owner:ZALUDEK BOREK +6
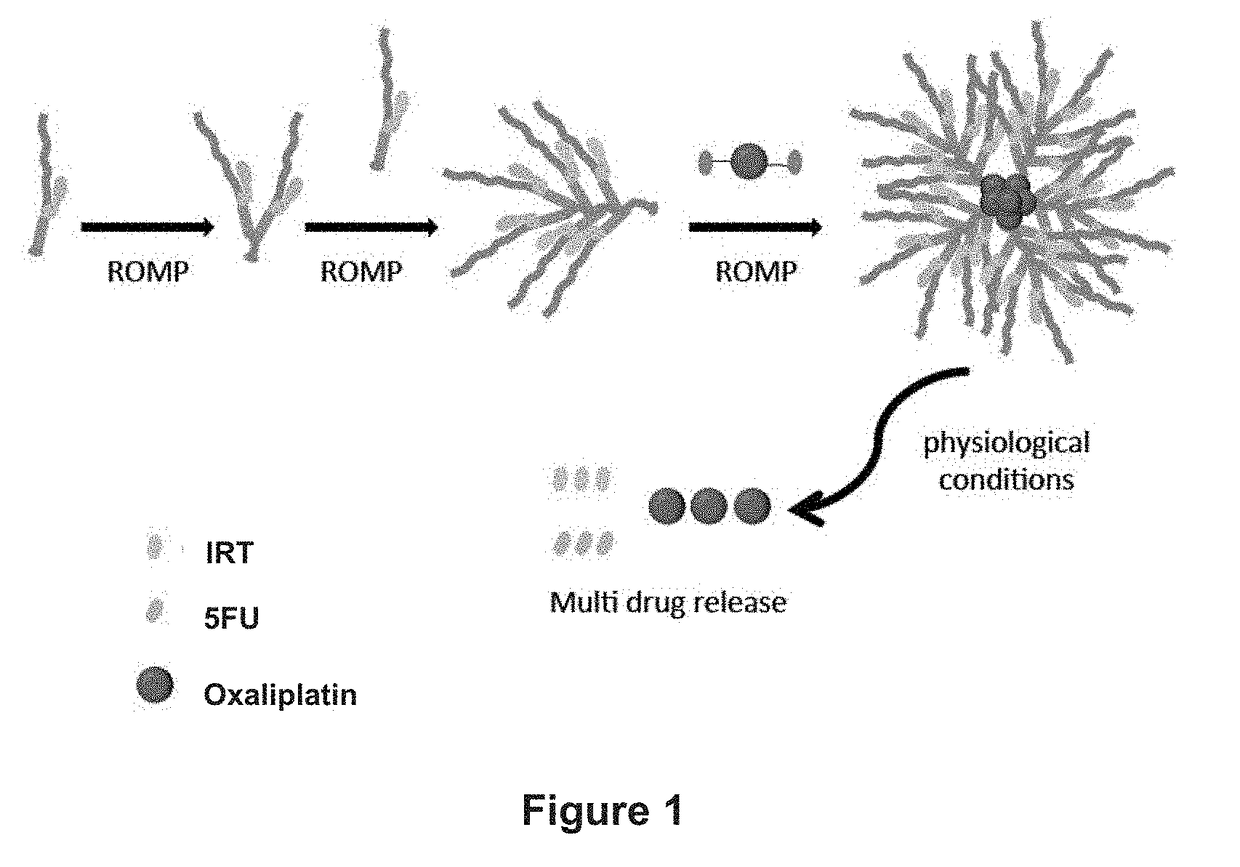
Drug delivery polymers and uses thereof
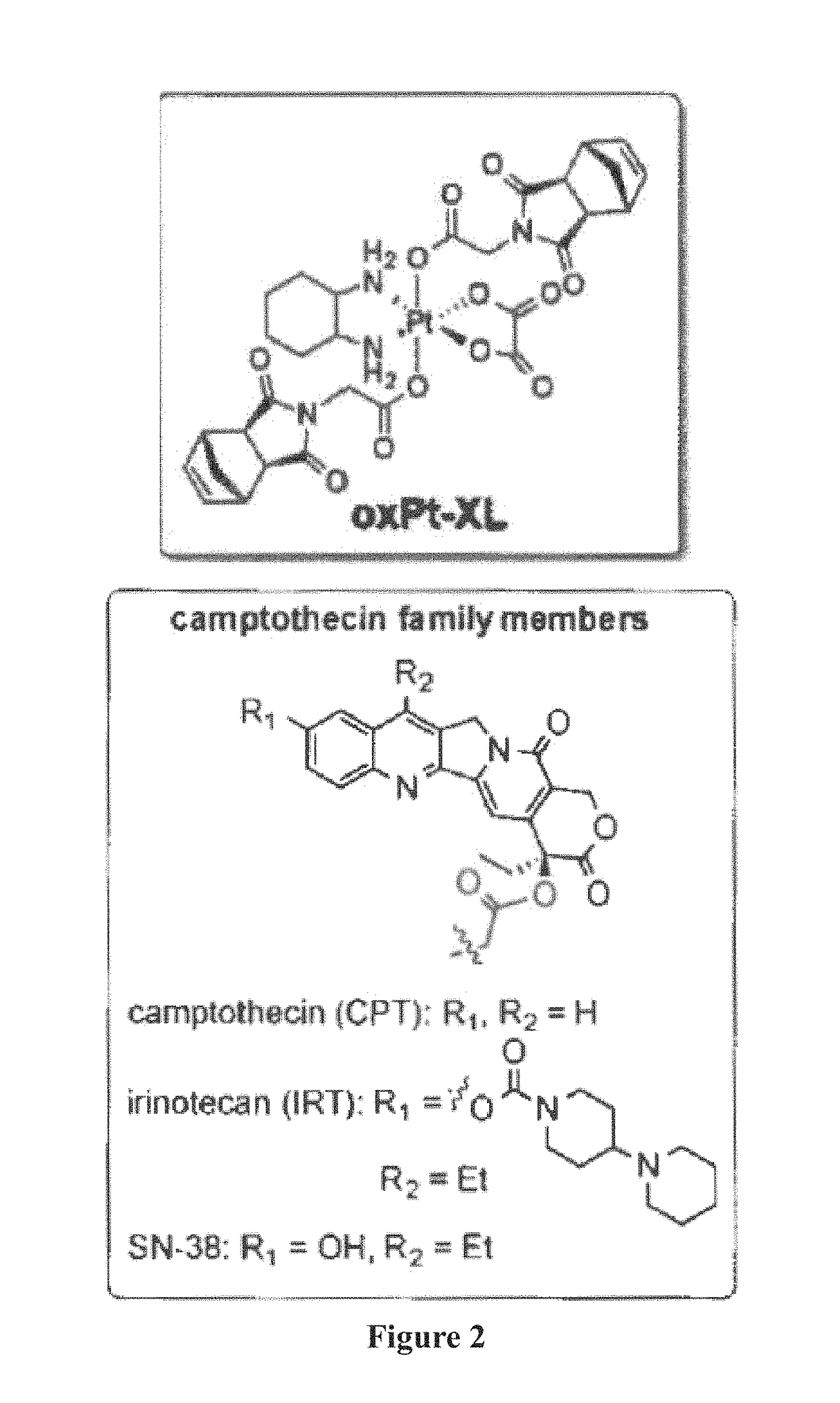
ActiveUS20170348431A1Reduce molecular weightReduces and avoids symptom and causePowder deliveryOrganic active ingredientsPancreas CancersProstate cancer
Described herein are platinum-based brush-arm star polymers (Pt-BASPs), or a pharmaceutical composition thereof, for delivery of platinum-based agents, such as oxaliplatin. Also provided are methods and kits involving the Pt-BASPs, or a pharmaceutical composition thereof, for treating proliferative diseases such as cancers (e.g., lung cancer, head-and-neck cancer, esophagus cancer, stomach cancer, breast cancer, pancreas cancer, colorectal cancer, liver cancer, kidney cancer, or prostate cancer) in a subject.
Owner:MASSACHUSETTS INST OF TECH
Combination therapy for cancer
ActiveUS20180271889A1High plasma concentrationPositive clinical outcomeOrganic active ingredientsPharmaceutical delivery mechanismSatraplatinAnticarcinogen
Disclosed is a combination of gemcitabine-[phenyl-benzoxy-L-alaninyl)]-phosphate (chemical name: 2′-Deoxy-2′,2′-difluoro-D-cytidine-5′-O-[phenyl (benzoxy-L-alaninyl)]phosphate) (NUC-1031) and a platinum-based anticancer agent selected from carboplatin, dicycloplatin, oxaliplatin, satraplatin and nedaplatin. The combinations are useful in the treatment of cancer and particularly ovarian cancer.
Owner:NUCANA PLC
Method of determining a chemotherapeutic regimen based on ERCC1 and TS expression
InactiveUS20060121526A1Sugar derivativesMicrobiological testing/measurementAbnormal tissue growthRegimen
The present invention relates to prognostic methods which are useful in medicine, particularly cancer chemotherapy. The object of the invention to provide a method for assessing TS and / or ERCC1 expression levels in fixed or fixed and paraffin embedded tissues and prognosticate the probable resistance of a patient's tumor to treatment with 5-FU and oxaliplatin-based therapies by examination of the amount of TS and / or ERCC1 mRNA in a patient's tumor cells and comparing it to a predetermined threshold expression level for those genes. More specifically, the invention provides to oligonucleotide primer pairs ERCC1 and TS and methods comprising their use for detecting levels of ERCC1 and TS mRNA, respectively.
Owner:CANCER GENETICS INC
Combination of a chemotherapeutic agent and an inhibitor of the tgf-beta system
ActiveUS20120027873A1Reduce IC50Improve efficiencyHeavy metal active ingredientsBiocideDocetaxel-PNPDocetaxel
Pharmaceutical composition comprising a chemotherapeutic agent and a TGF-beta antisense oligonucleotide, wherein the antisense oligonucleotide reduces the sensitivity and IC50, respectively, of the cytotoxicity of the chemotherapeutic agent. Preferably, the antisense oligonucleotide is a TGF-beta 1, 2, and / or 3 antisense oligonucleotide and the chemotherapeutic agent is preferably gemcitabine, 5-fluorouracil, temozolomide, dacarbacine, docetaxel, cisplatin, oxaliplatin, tamoxifen, or irinotecan.
Owner:ANTISENSE PHARMA GMBH
High optical purity trans-dextro oxaliplatin lyophilized powder injection and preparation method thereof
ActiveCN102657624AHigh optical purityImprove stabilityPowder deliveryLyophilised deliverySucrosePolyethylene glycol
The invention relates to a high optical purity trans-dextro oxaliplatin lyophilized powder injection and a preparation method thereof. The lyophilized powder injection contains high optical purity trans-dextro oxaliplatin with treatment effective dose and pharmaceutically acceptable lyophilized excipient, and does not contain animal-based auxiliary lactose, wherein the optical purity of the trans-dextro oxaliplatin is not lower than 99.95 percent; the pharmaceutically acceptable lyophilized excipient is one or more of cane sugar, trehalose, glycine, histidine, dextran, polyethylene glycol andcyclodextrin. The preparation method of the lyophilized powder injection comprises the following steps: dissolving raw and auxiliary materials in water; subpackaging, pre-freezing, lyophylizing, performing analytic drying; infiltering nitrogen into subpackaged bottles after the lyophilization is completed; and pressing plugs and caps. The high optical purity trans-dextro oxaliplatin lyophilized powder injection is used for preparing an anti-cancer medicament, and can be applied in a parenteral way after being re-dissolved with a compatible solution.
Owner:QILU PHARMA HAINAN
Drug delivery polymers and uses thereof
ActiveUS10105449B2Reduces and avoids symptom and causeGood curative effectHeavy metal active ingredientsPowder deliveryPancreas CancersProstate cancer
Described herein are platinum-based brush-arm star polymers (Pt-BASPs), or a pharmaceutical composition thereof, for delivery of platinum-based agents, such as oxaliplatin. Also provided are methods and kits involving the Pt-BASPs, or a pharmaceutical composition thereof, for treating proliferative diseases such as cancers (e.g., lung cancer, head-and-neck cancer, esophagus cancer, stomach cancer, breast cancer, pancreas cancer, colorectal cancer, liver cancer, kidney cancer, or prostate cancer) in a subject.
Owner:MASSACHUSETTS INST OF TECH
Oxaliplatin crystal compound and freeze-dried powder injection
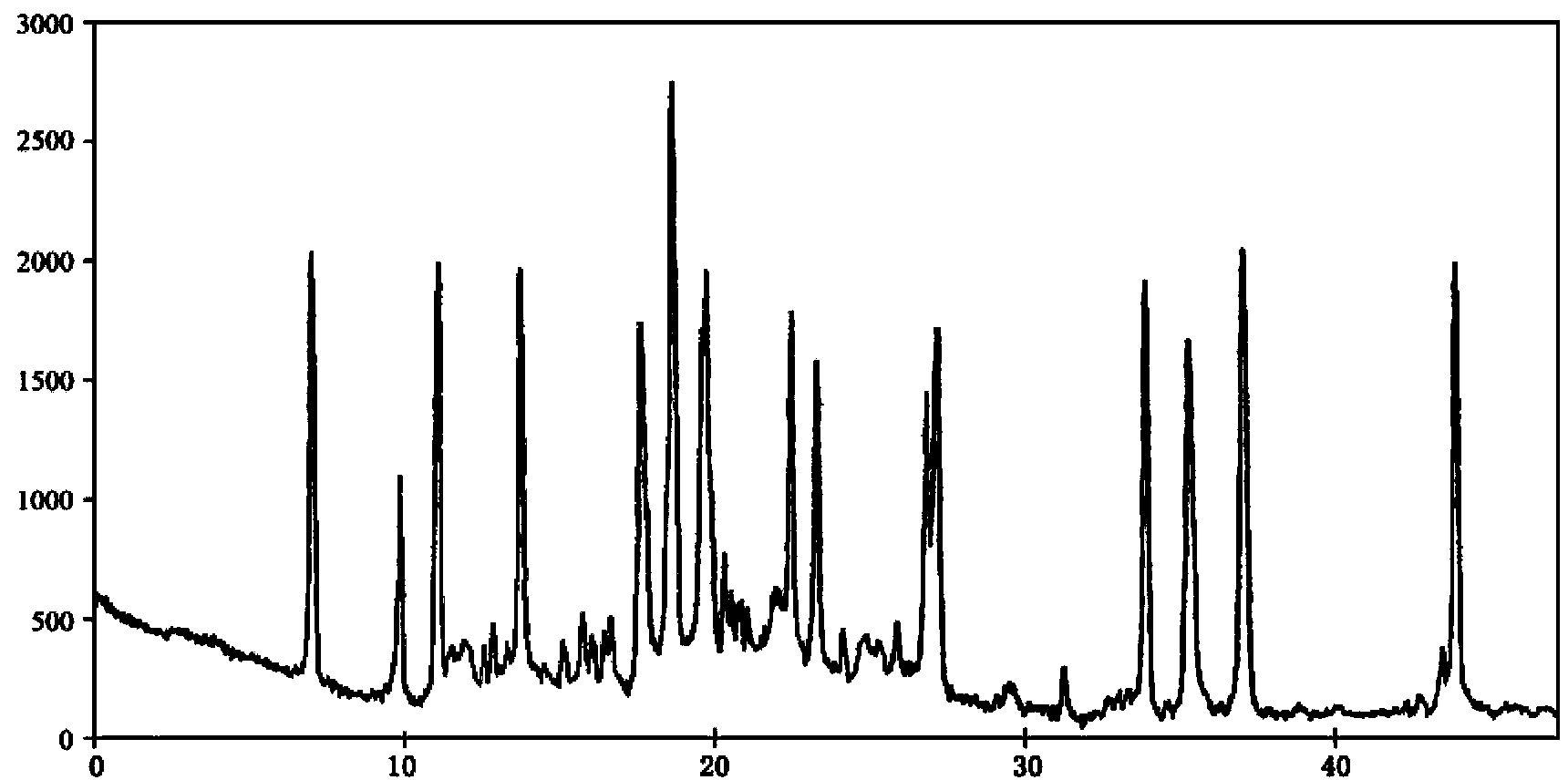
ActiveCN102643308BImprove solubilityNot easy to precipitatePowder deliveryGroup 8/9/10/18 element organic compoundsOxaliplatinPowder diffraction
The invention relates to an oxaliplatin crystal compound. The oxaliplatin crystal compound is measured by using a powder X-ray diffraction measurement method. Characteristic diffraction peaks are shown at positions of 6.5 degrees, 9.8 degrees, 11.2 degrees, 13.7 degrees, 17.5 degrees, 18.5 degrees, 19.7 degrees, 22.4 degrees, 23.2 degrees, 26.8 degrees, 27.1 degrees, 33.8 degrees, 35.2 degrees, 36.9 degrees and 43.8 degrees in an X-ray powder diffraction pattern shown by a diffraction angle of 2theta+ / -0.2 degree. The oxaliplatin crystal compound is good in dissolubility.
Owner:HAINAN JINRUI PHARMA CO LTD
Amphipathic oxaliplatin precursor, preparation method and application thereof
The invention discloses an amphipathic oxaliplatin precursor and a preparation method thereof. The amphipathic oxaliplatin precursor comprises an oxaliplatin unit and hydrophobic and hydrophilic functional groups. The amphipathic oxaliplatin precursor can be self-assembled solely into a micelle in water, the hydrophobic functional group of the amphipathicoxaliplatin precursor can form a hydrophobic core through hydrophobic interaction, the hydrophilic functional group forms a hydrophilic shell layer to maintain colloid stability of the self-assembled micelle. In addition, the invention further discloses application of the amphipathic oxaliplatin precursor. The amphipathicoxaliplatin precursor is mainly used for preparing cancer treatment drugs.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Method of preparing oxaliplatin
ActiveCN101054396AReduce solubilityEasy to remove by precipitationGroup 8/9/10/18 element organic compoundsPlatinumOxalate
The present invention relates to preparation method of anticancer drug oxaliplatin which comprise that material cis-dihalo(trans-(-)-1,2- diamino cyclohexane)platium(II), silver nitrate, univalent metal ion or ammonium oxalate and oxygen-free deionized water are together prepared for oxaliplatin in one step. The invention has a simple process, product is good in color, and material is easy to get which is fit for industrial production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Liposome Preparation
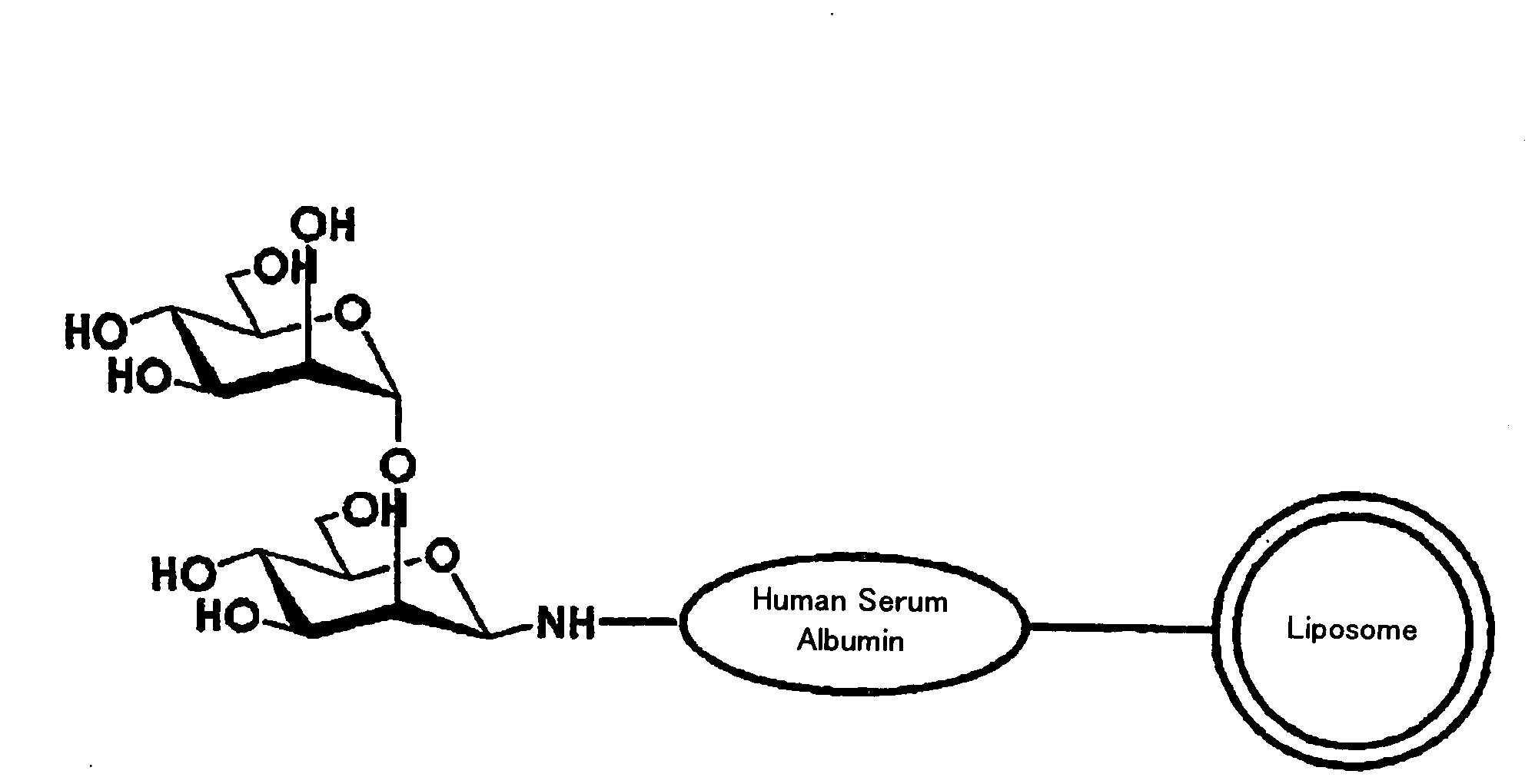
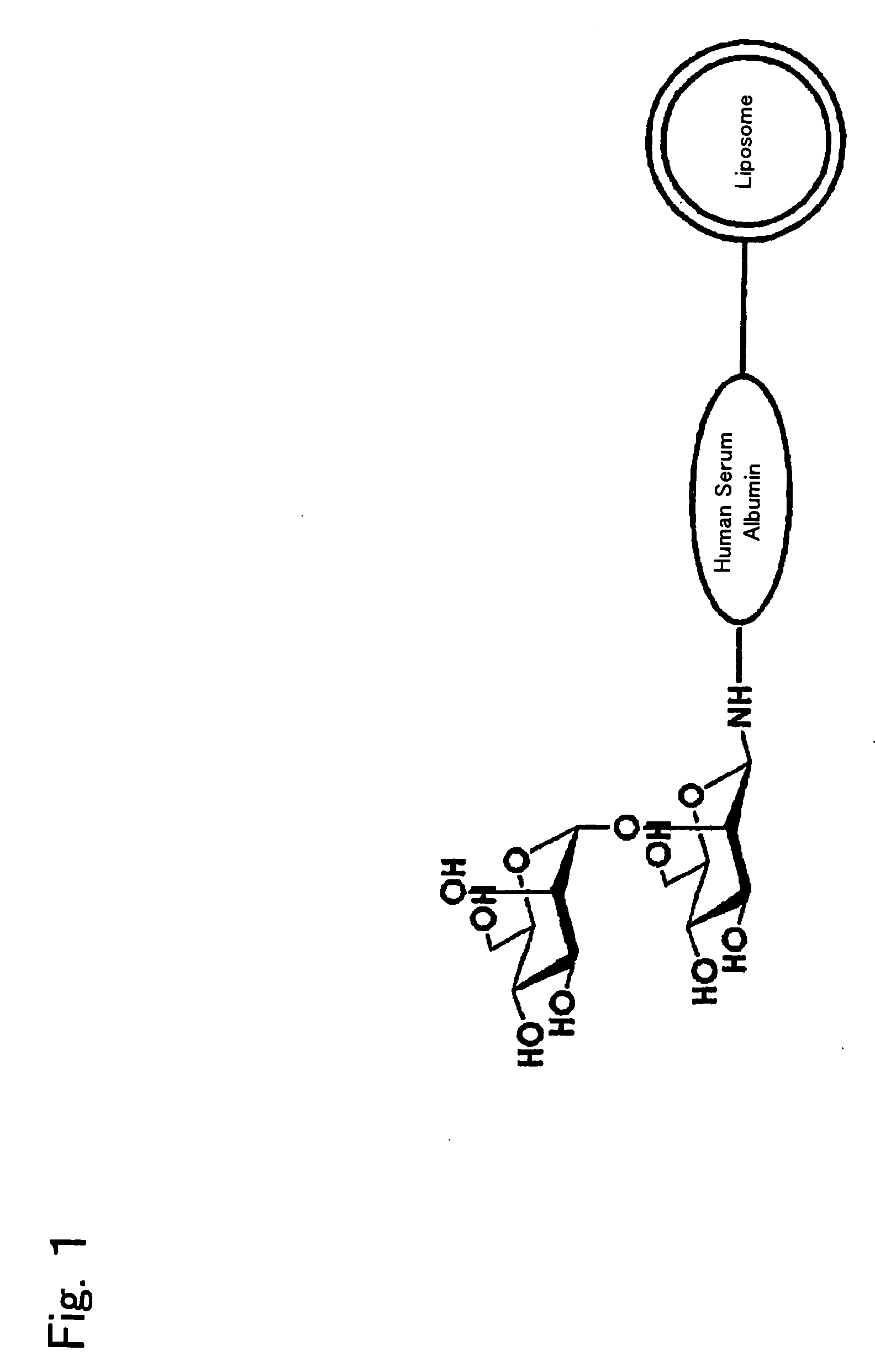
InactiveUS20090169610A1Improve targetingReduce drug doseOrganic active ingredientsAntibody ingredientsGnRH AntagonistLiposome
The present invention provides cancer treatment preparations of excellent targetability. The sugar chain-modified liposomes of the present invention, which contain an aromatase inhibitor, anti-androgenic agent, lyase inhibitor, GnRH agonist, GnRH antagonist, anti-angiogenic agent, tyrosine kinase inhibitor, serine-threonine kinase inhibitor, antibody having an anticancer activity, ansamitocin, capecitabine, celmoleukin, docetaxel hydrate, gemcitabine hydrochloride, oxaliplatin, prednisolone, tegafur-uracil mixtures, zinostatin stimalamer or arsenic trioxide may be used as cancer treatment preparations having an excellent targetability.
Owner:SIEMENS AG +1
Pegylated oxaliplatin prodrug as well as preparation method and application thereof
ActiveCN106074379AHeavy metal active ingredientsPharmaceutical non-active ingredientsCancer treatmentOxaliplatin
The invention discloses a pegylated oxaliplatin prodrug as well as a preparation method and application thereof. The pegylated oxaliplatin prodrug has a structure as shown in the formula 1 or 2, wherein the linkage unit L is as shown in the description. The pegylated oxaliplatin prodrug is mainly used for preparing medicaments for cancer treatment and can be independently self-assembled into micelles in water. In addition, the invention also discloses pegylated oshari. The formulae 1 and 2 are as shown in the description.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
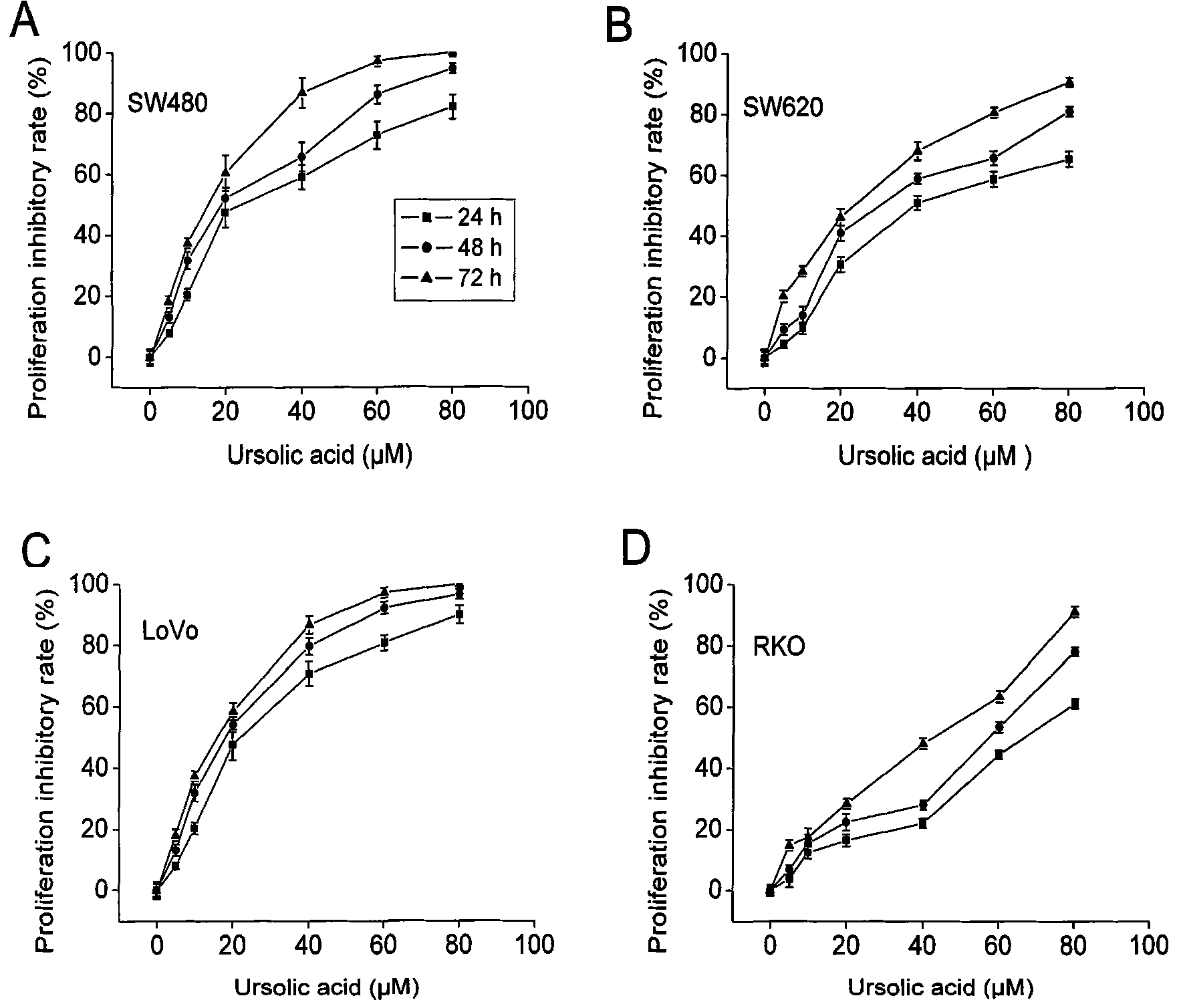
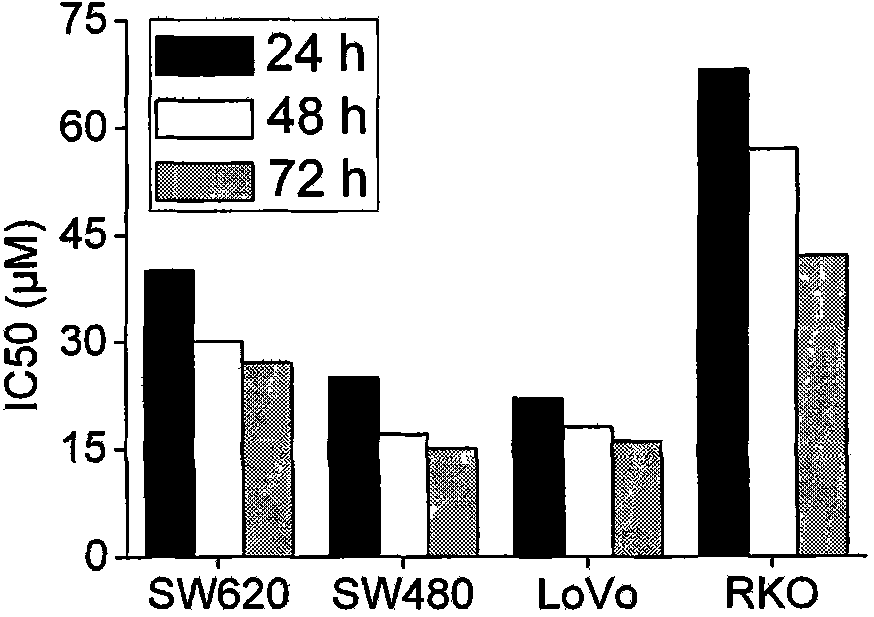
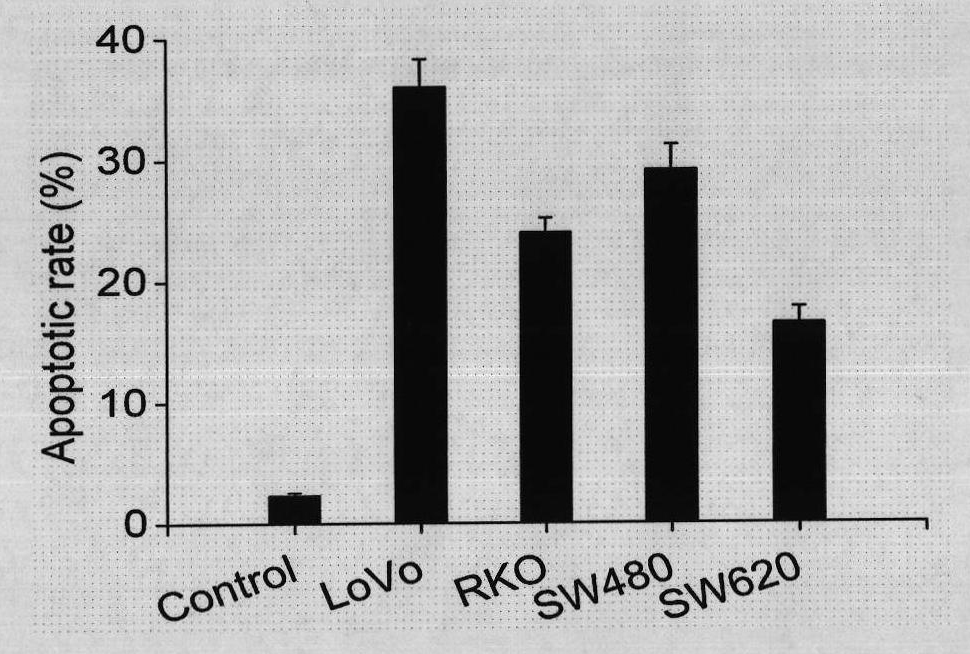
Use of ursolic acid as colon tumor resistant medicament
InactiveCN101991579ABlock EGFR signaling pathwayOrganic active ingredientsAntineoplastic agentsSide effectApoptosis
The invention relates to use of ursolic acid as a colon tumor resistant medicament. The ursolic acid has the effects of inhibiting colon cancer / tumor cell proliferation and inducing apoptosis. In naked mouse in vivo experiments, the ursolic acid can effectively inhibit the proliferation of colon cancer transplanted tumor and inhibit the generation of tumor blood vessels, and has no obvious toxic or side effect on mice. The ursolic acid has the effect of inhibiting EGFR / MAPK signal channel phosphorylation of colon cancer cells, and the key target of the ursolic acid is to inhibit ERK1 / 2 protein phosphorylation. The ursolic acid which is synergetic with oxaliplatin serving as a clinical first-line chemotherapy medicament of the colon cancer has better curative effect. The ursolic acid has better proliferation inhibiting effect on the colon cancer cells of K-RAS gene mutation. The use of the ursolic acid as the colon tumor resistant medicament provides a new thought for treating the colon tumor.
Owner:ZHEJIANG UNIV
Screen method of sensitization tumour cell pharmaceutical product and use thereof
InactiveCN101457251AToxicAlleviate or even reverse drug resistancePeptide/protein ingredientsMicrobiological testing/measurementCoralyne sulfoacetateCancer cell
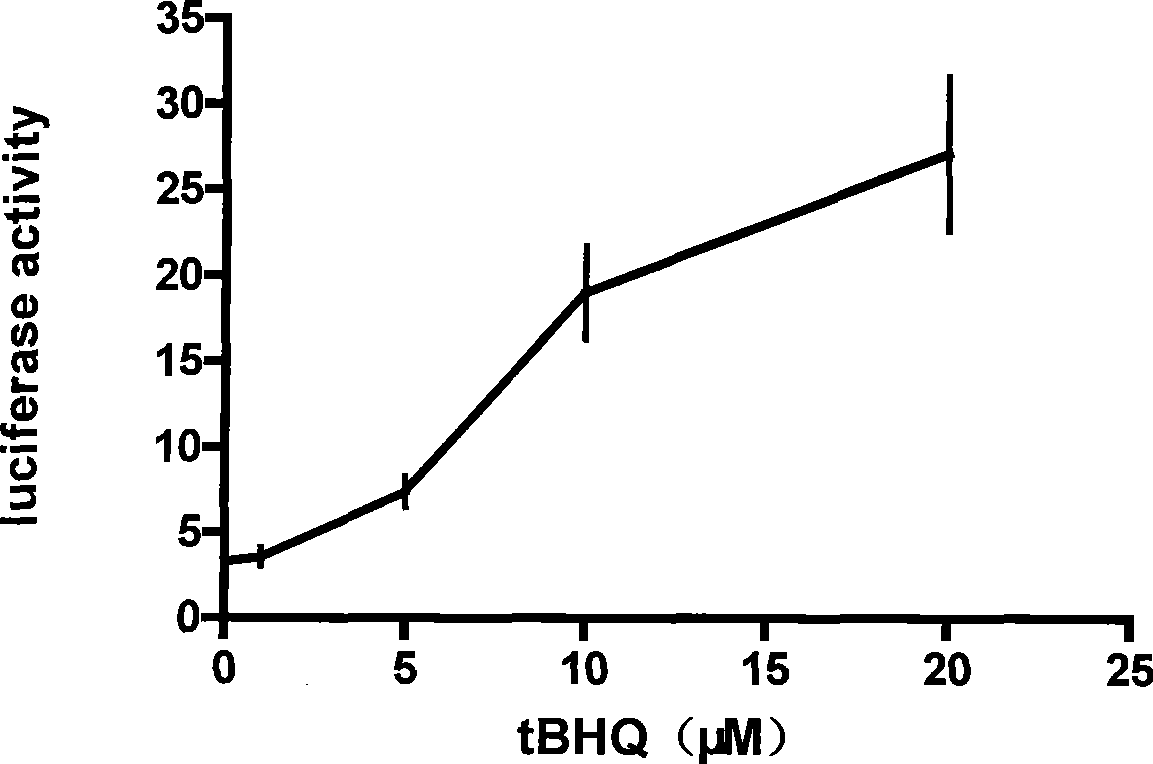
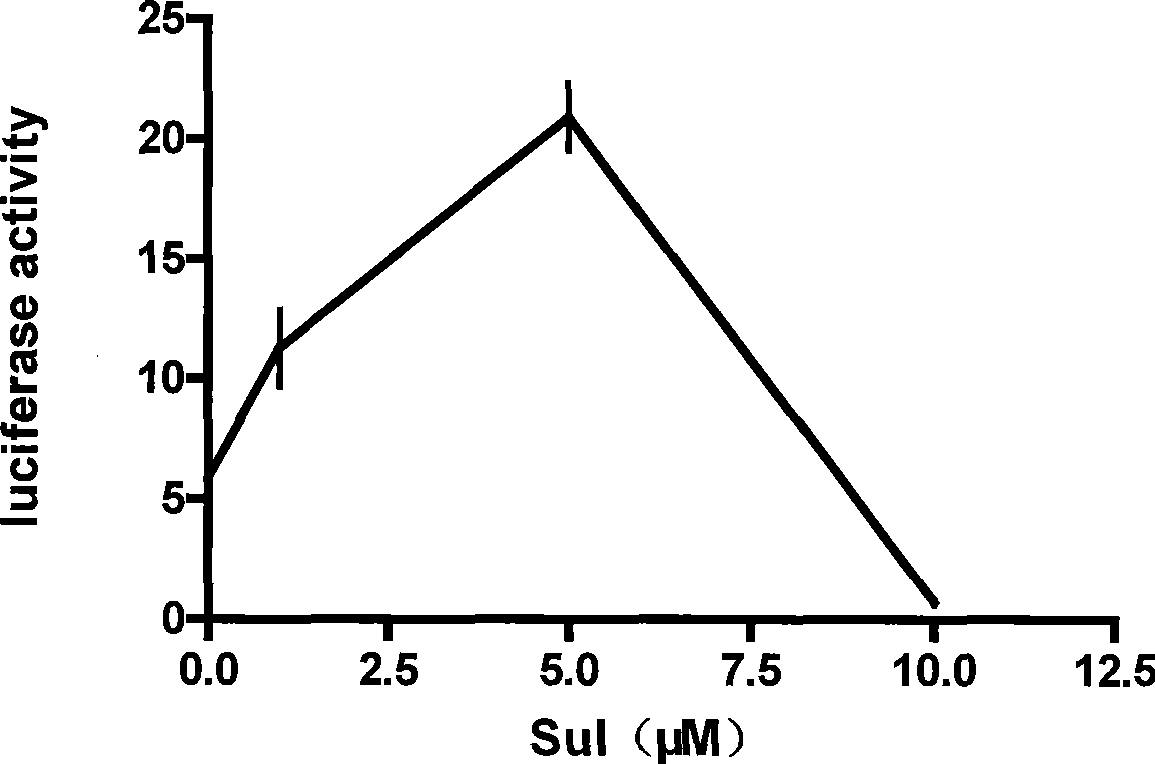
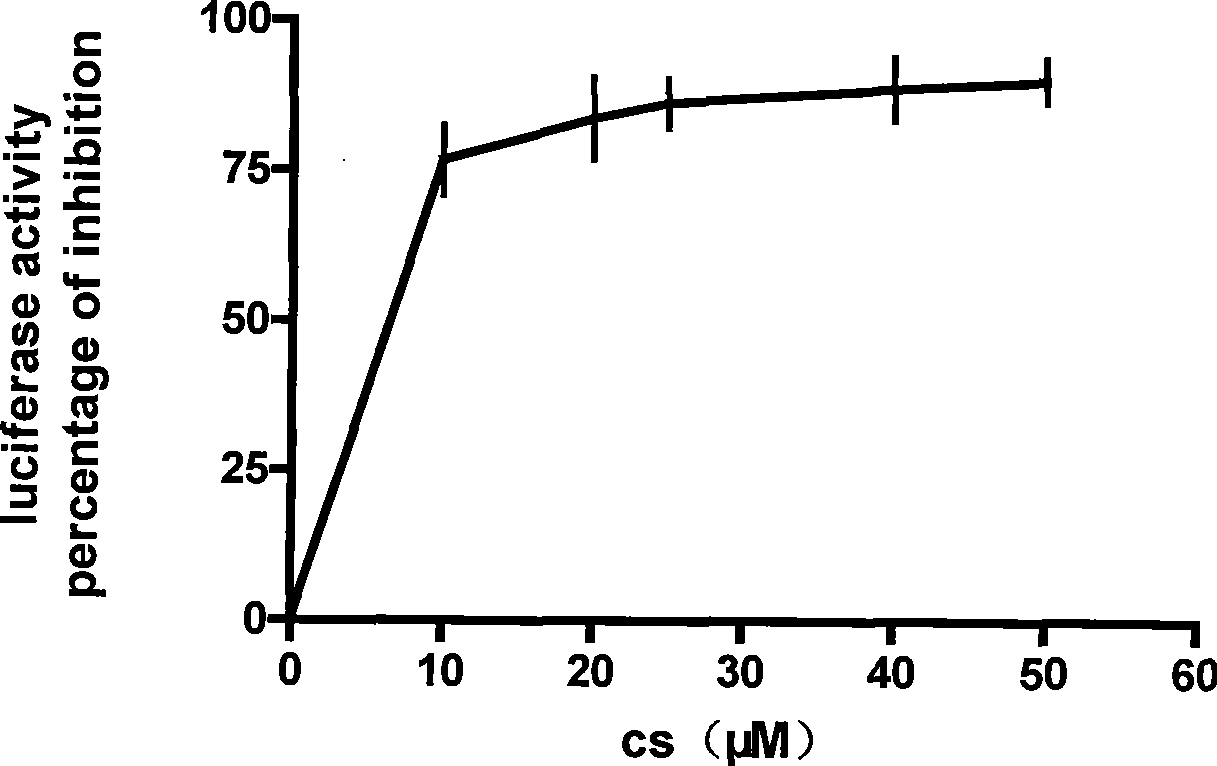
The invention discloses an enhancement tumor cell medicament screening method and its application mainly for effectively overcoming the multidrug resistance to improve the anti-cancer drug treatment effect in a tumor chemotherapy process. The invention screens an inhibitor which can effectively inhibiting the induction action through luciferase activity detection by using an Nrf2 as a cell drug screen platform of a molecular target and a tBHQ as an Nrf2 inducer, and the inhibitor is a medicament of the enhancement tumor cell. The medicament obtained by the screen is a small molecular compound ZDAK02 (coralyne sulfoacetate coralyne thioacetate). The combined use of the medicament of the invention with the anti-cancer drug Chlorambucil or anti-cancer drug oxaliplatin can enhance the toxicity of the anti-cancer drug to the cancer cells and be helpful for easing even reversing the multidrug resistance to the anti-cancer drugs.
Owner:ZHEJIANG UNIV
Preparation method of miriplatin hydrate
ActiveCN102516311AQuality improvementReduced responseGroup 8/9/10/18 element organic compoundsMiriplatinPotassium
The invention discloses a preparation method of miriplatin hydrate, which comprises the following steps: controlling the temperature to 40-90 DEG C, adding Oxaliplatin and C13H27COOM in a dissolved reaction solvent, and reacting for 1-6d to prepare the miriplatin hydrate, wherein the molar ratio of the Oxaliplatin to the C13H27COOM is 1:0.5-5, and M is sodium, potassium, ammonia, calcium or nitrogenous organic alkali. Compared with the existing preparation method of miriplatin hydrate, the preparation method disclosed by the invention has the outstanding advantages of environment-friendly synthesis lines, no silver ion residues, no toxic solvents, short lines and convenience in operation, the obtained product has good quality (chemical purity of 99.5% and optical purity more than 99.9%), high yield (more than 92%) which is 10% higher than that of other lines and low cost, is public-benefit and environment-friendly, is easy for industrial production, has good practicability and can produce better economic and social benefits. In addition, the miriplatin hydrate used for injection prepared according to the preparation method of the miriplatin hydrate disclosed by the invention has low production cost and high quality, and can reduce adverse reactions and treatment cost for patients to a great extent.
Owner:NANJING YOKO PHARMA GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com