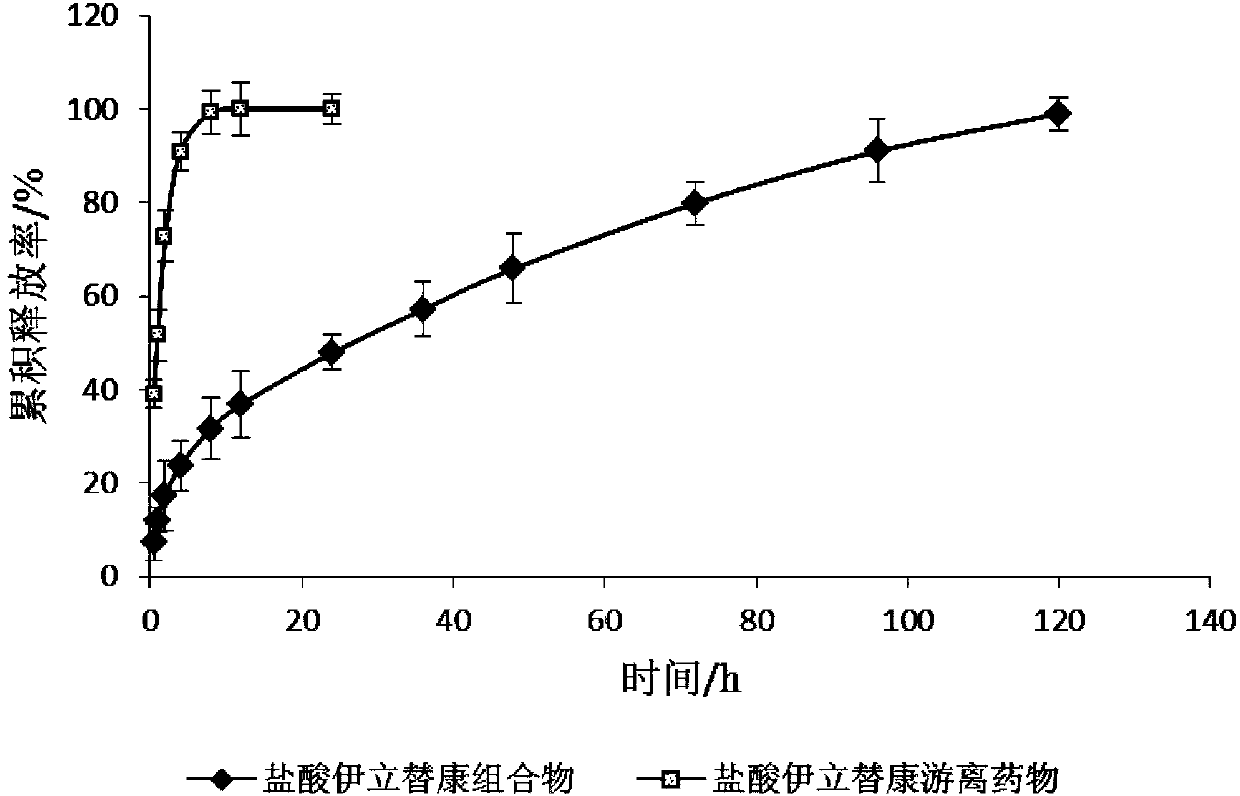
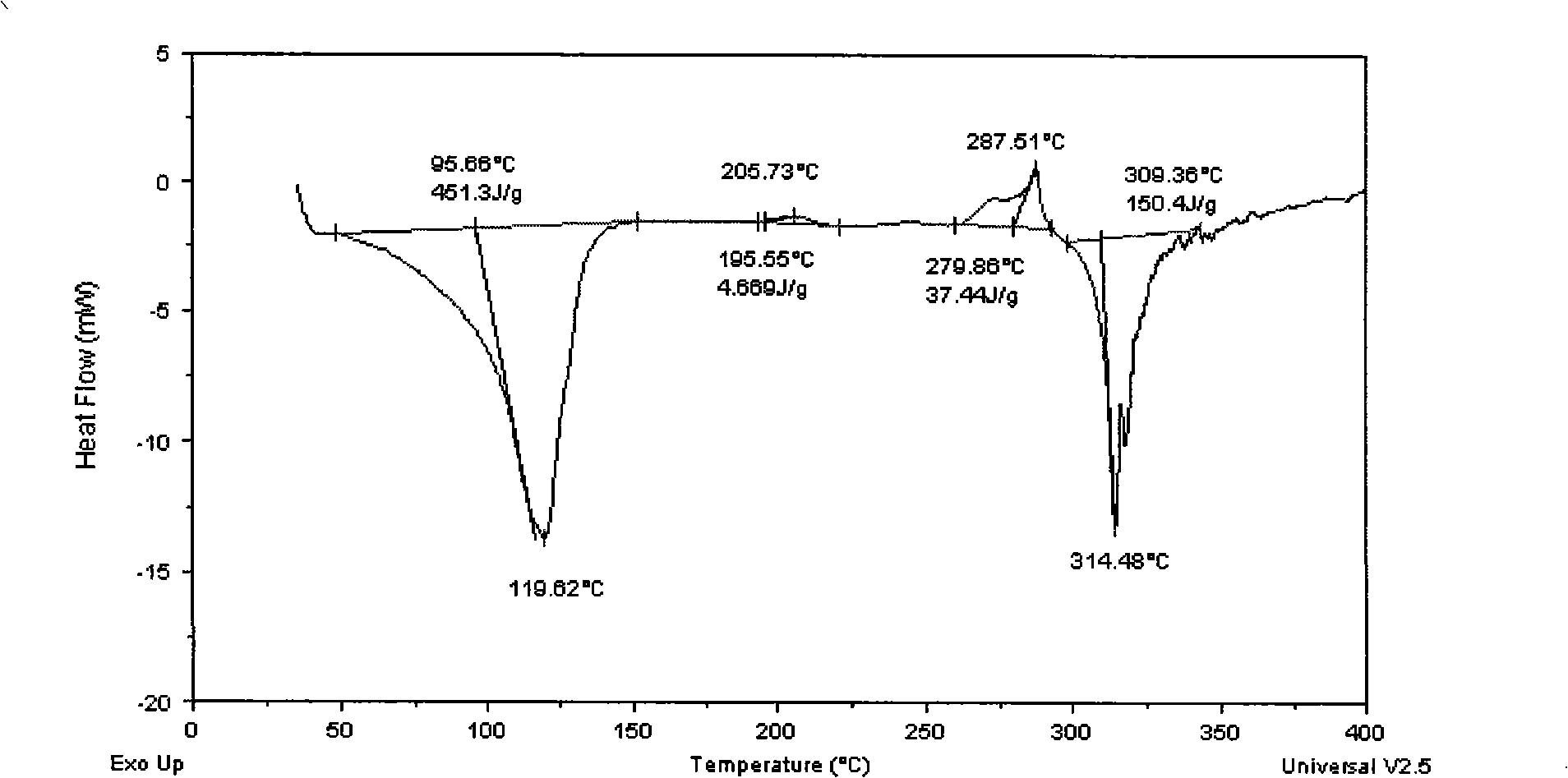
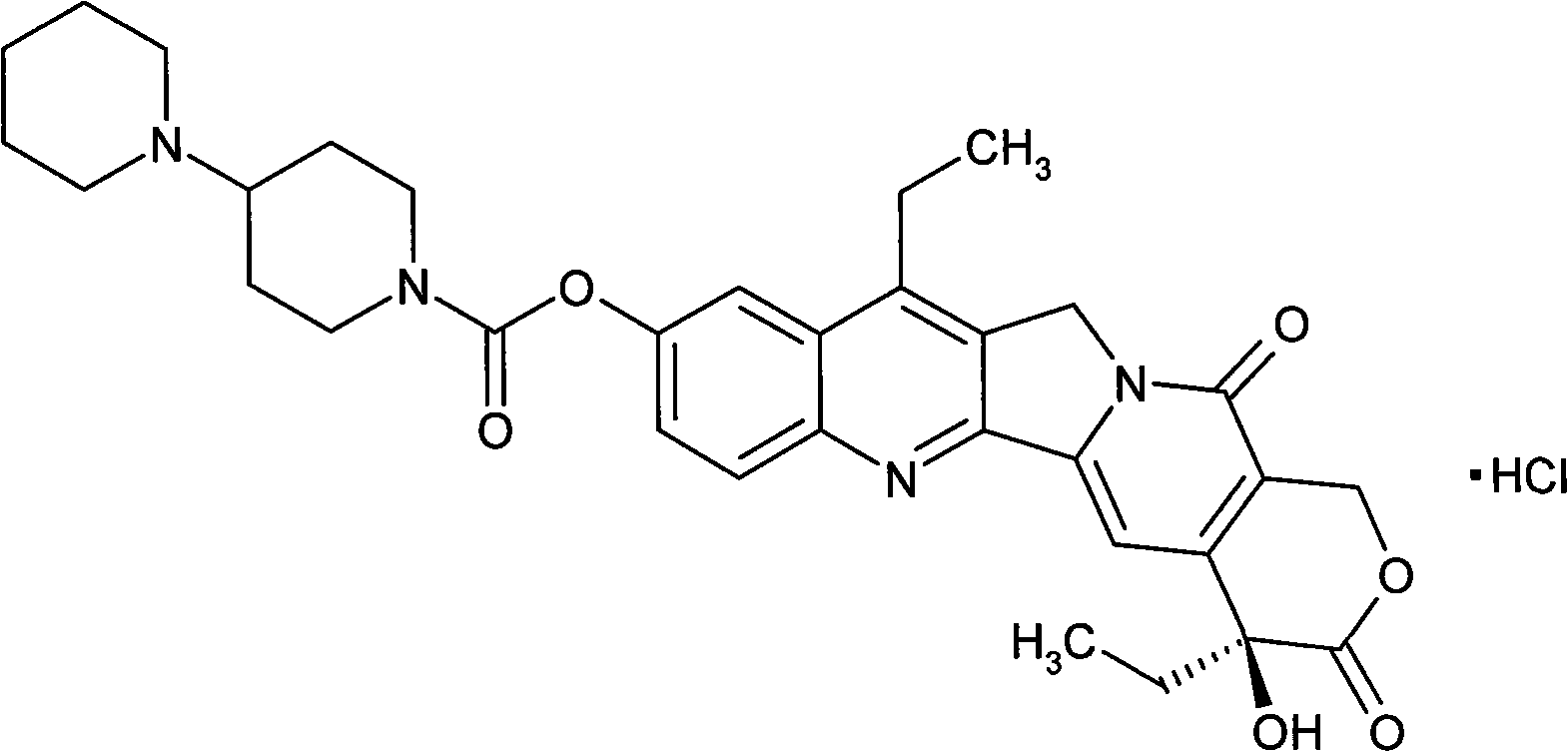
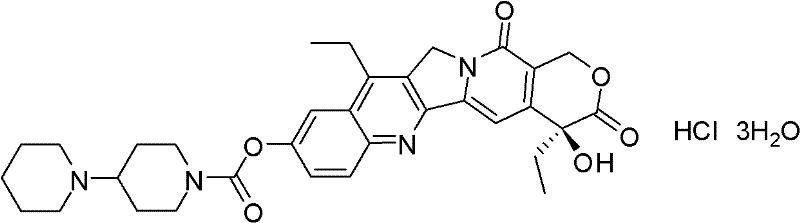
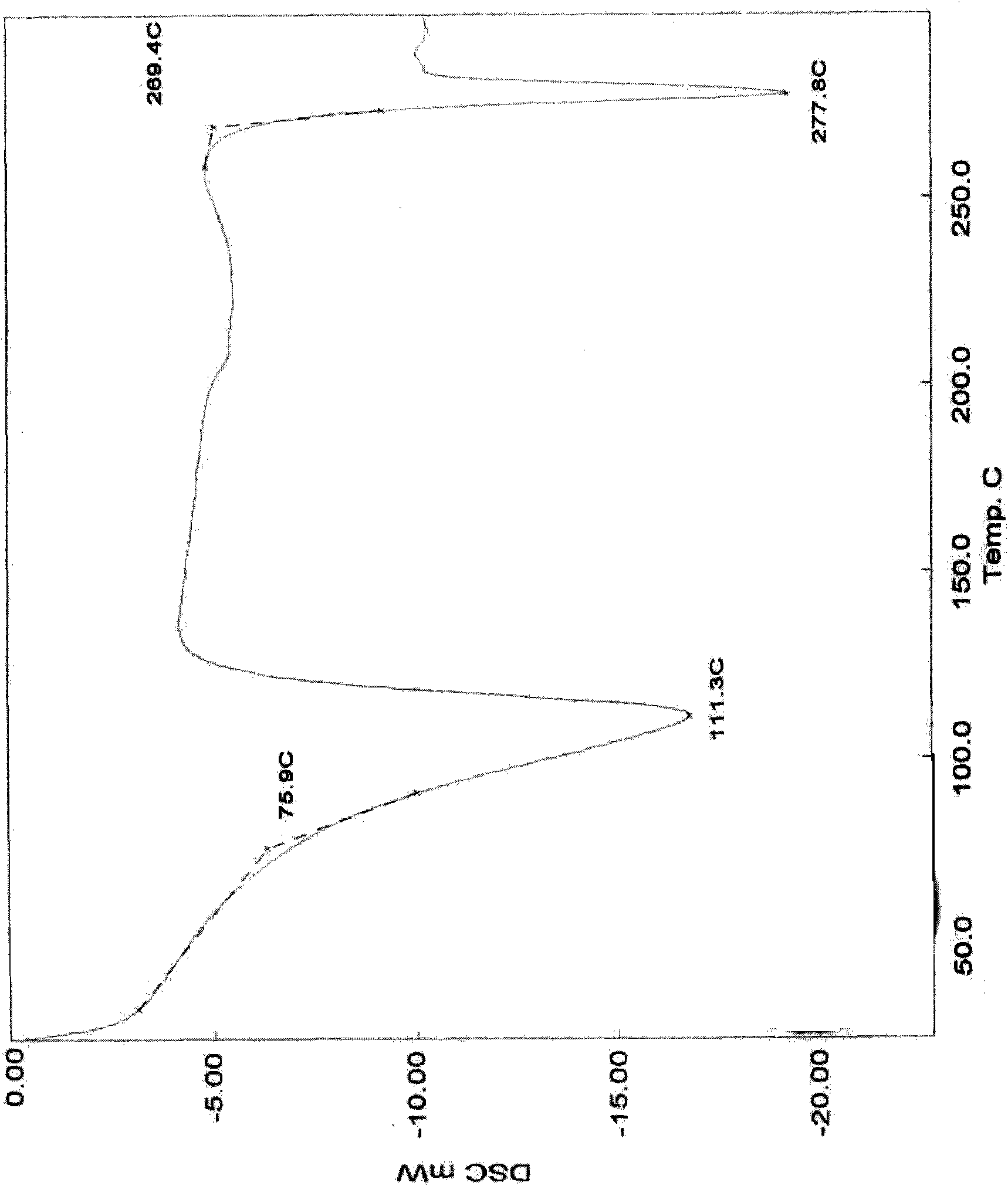
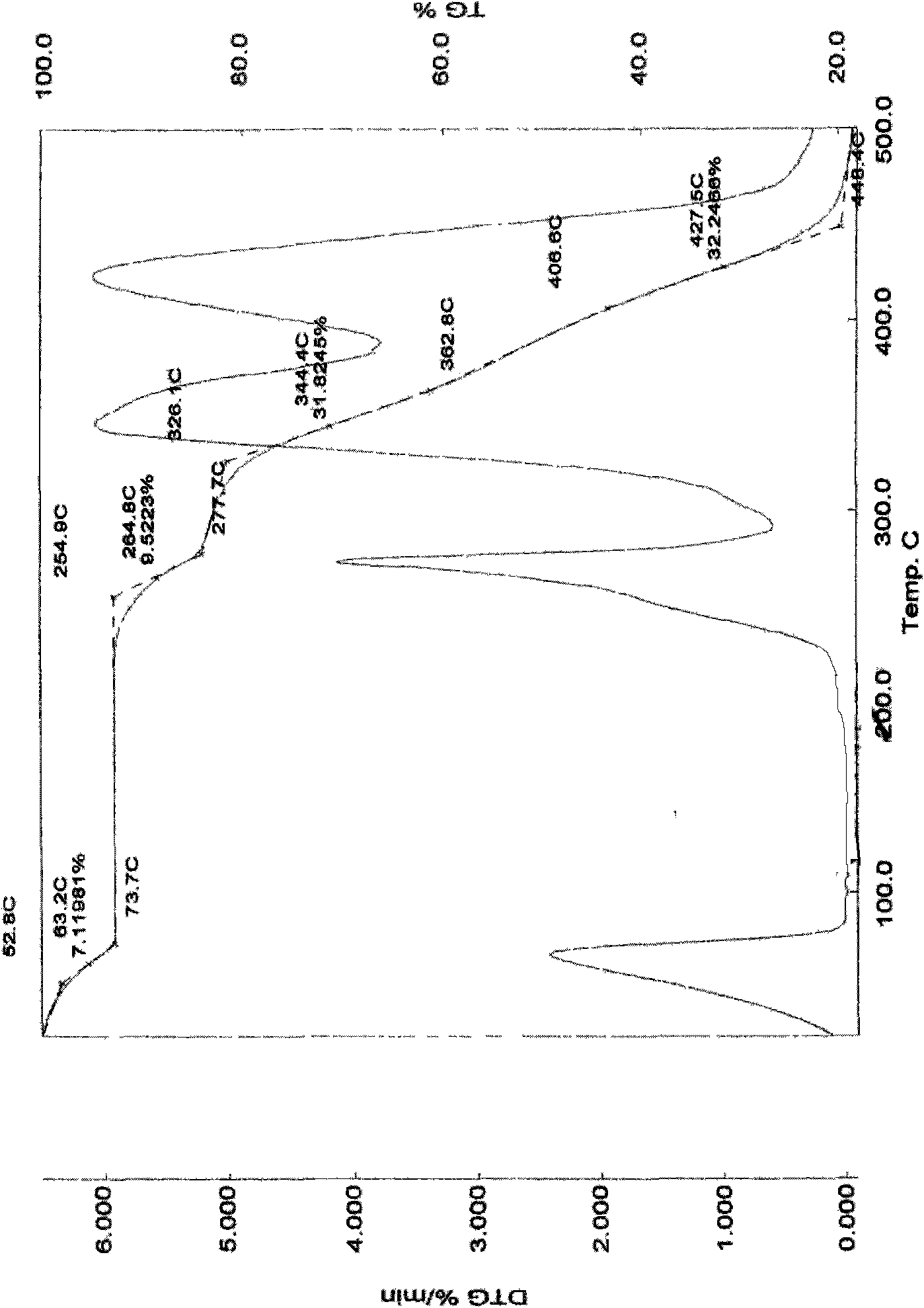
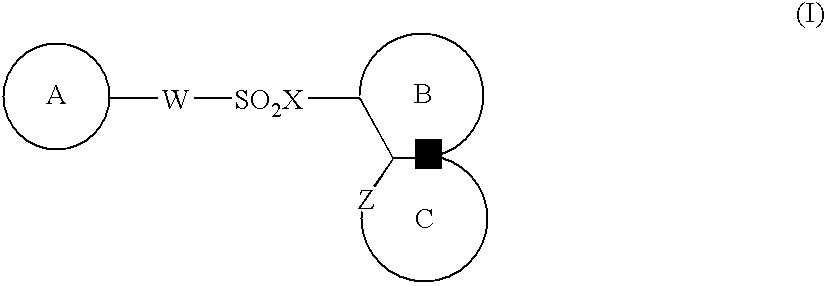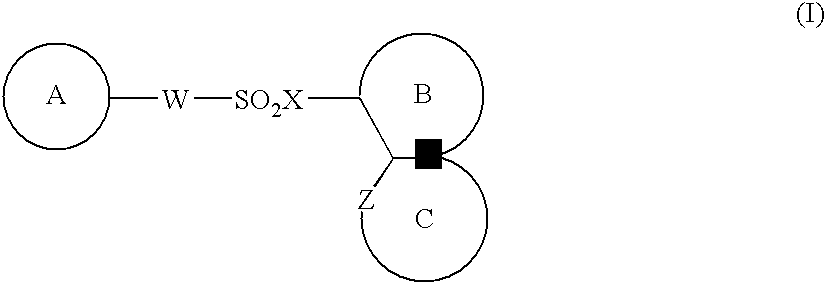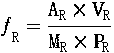Patents
Literature
81 results about "Irinotecan Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
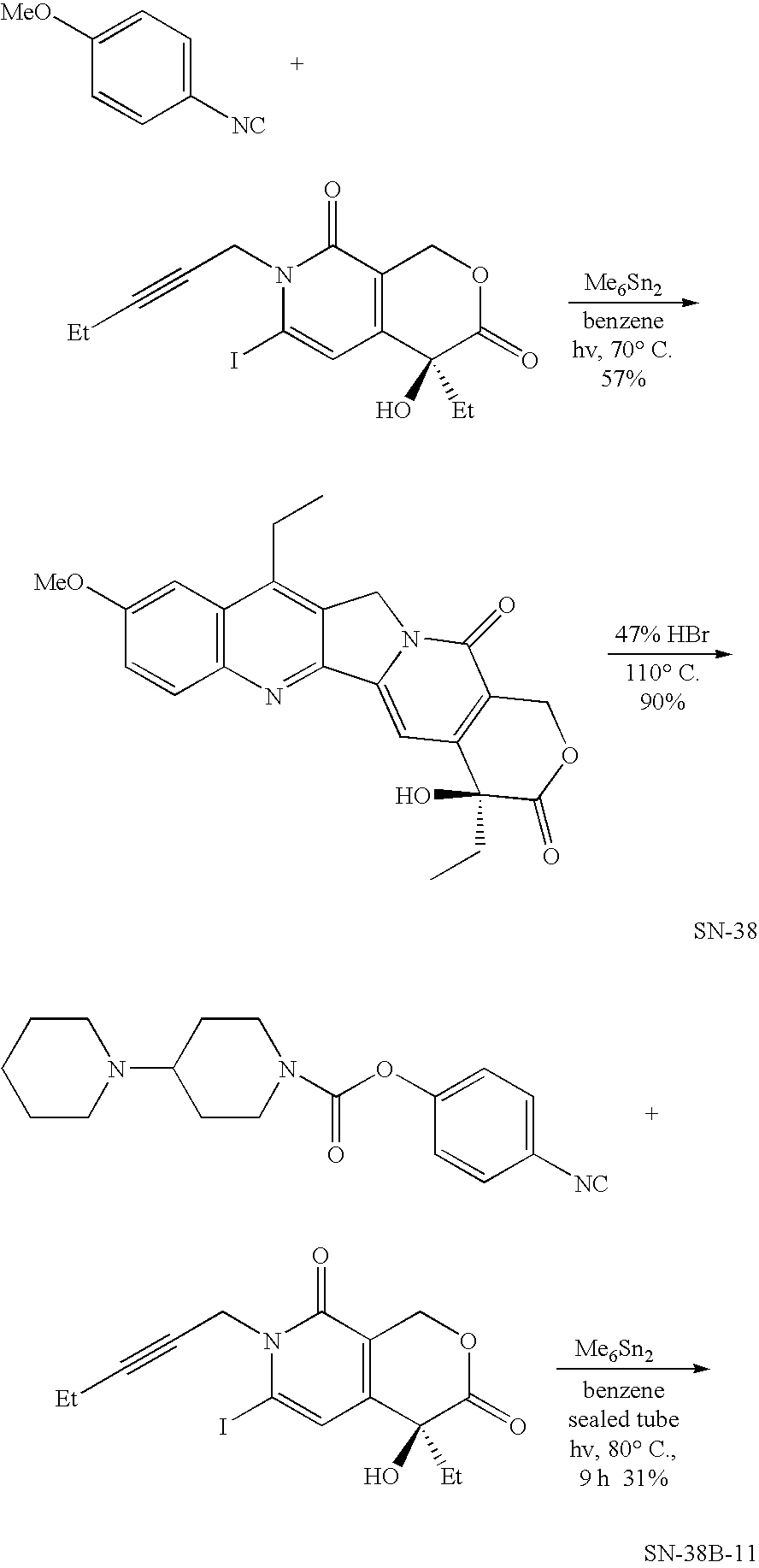
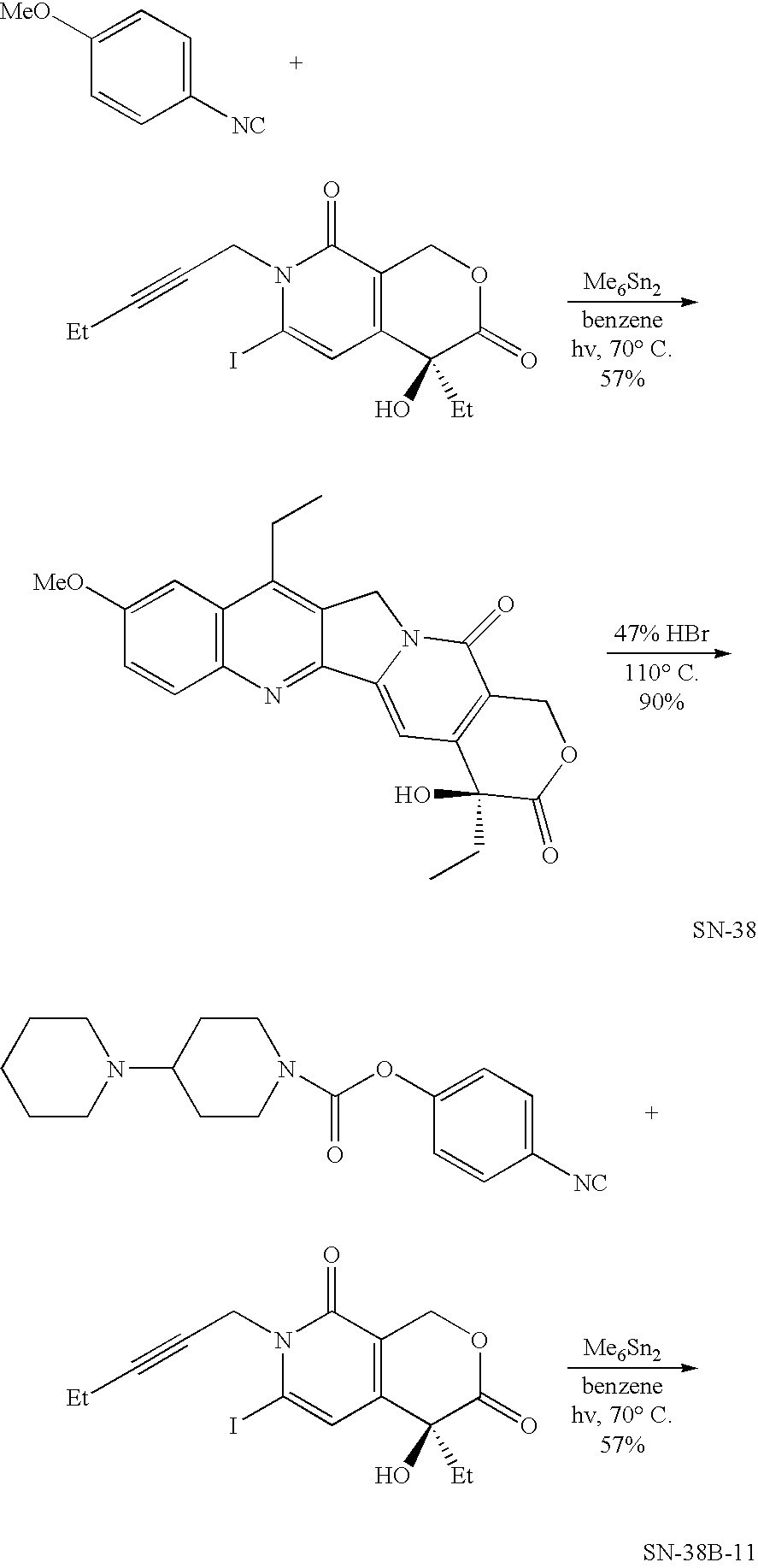
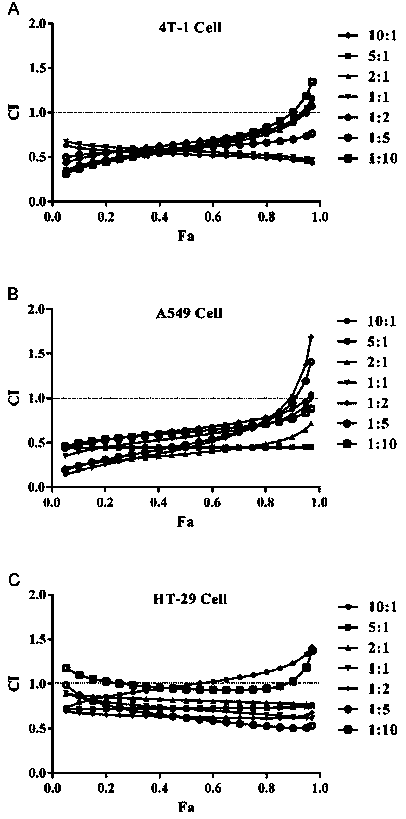
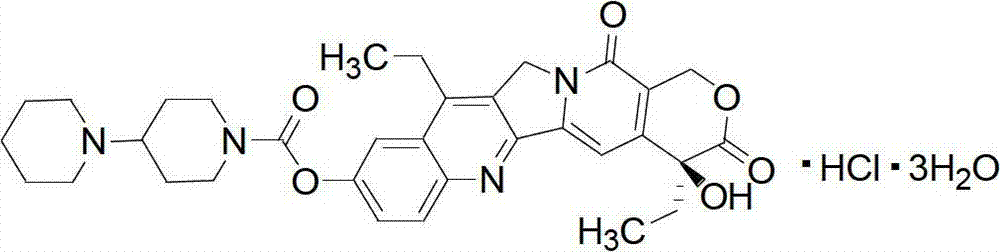
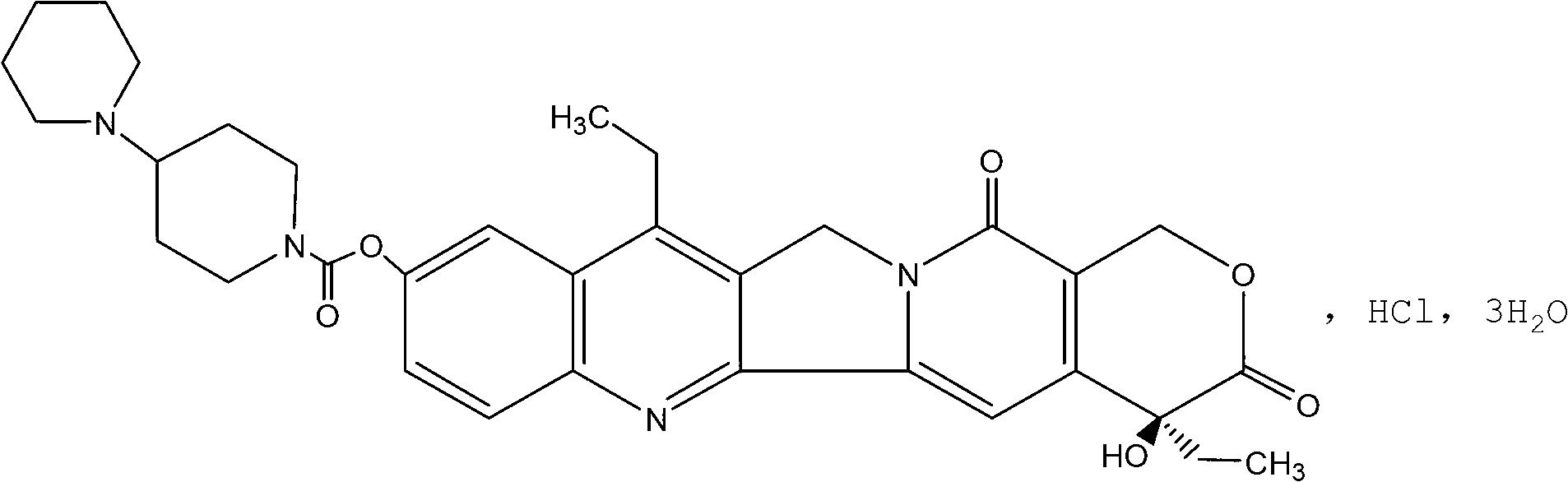
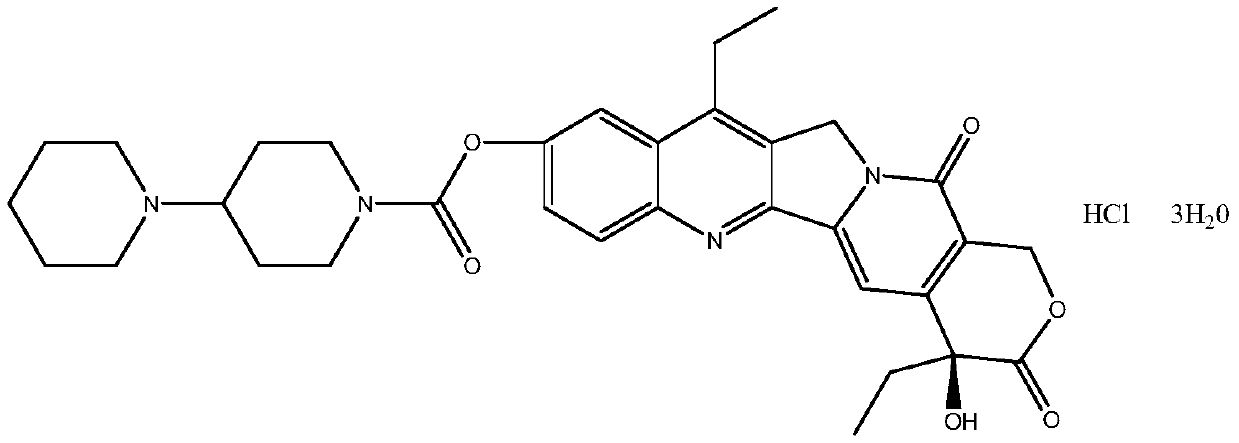
The hydrochloride salt of a semisynthetic derivative of camptothecin, a cytotoxic, quinoline-based alkaloid extracted from the Asian tree Camptotheca acuminata. Irinotecan, a prodrug, is converted to a biologically active metabolite 7-ethyl-10-hydroxy-camptothecin (SN-38) by a carboxylesterase-converting enzyme. One thousand-fold more potent than its parent compound irinotecan, SN-38 inhibits topoisomerase I activity by stabilizing the cleavable complex between topoisomerase I and DNA, resulting in DNA breaks that inhibit DNA replication and trigger apoptotic cell death. Because ongoing DNA synthesis is necessary for irinotecan to exert its cytotoxic effects, it is classified as an S-phase-specific agent.
Medicinal compositions for concomitant use as anticancer agent
InactiveUS20030215523A1Good synergyEliminate side effectsHeavy metal active ingredientsBiocideCarboplatinAnticarcinogen
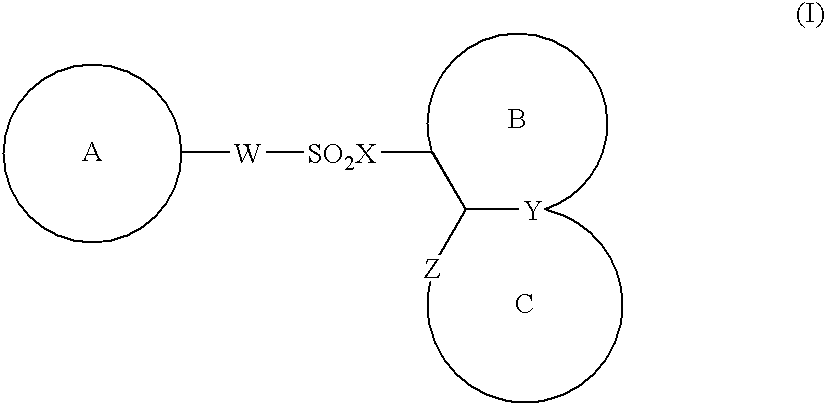
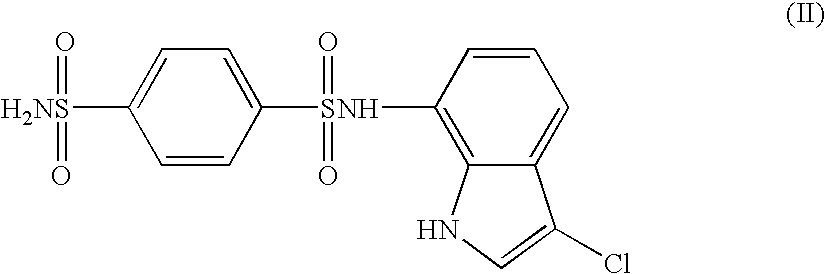
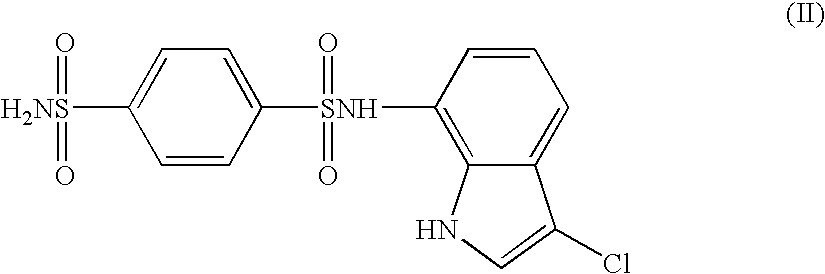
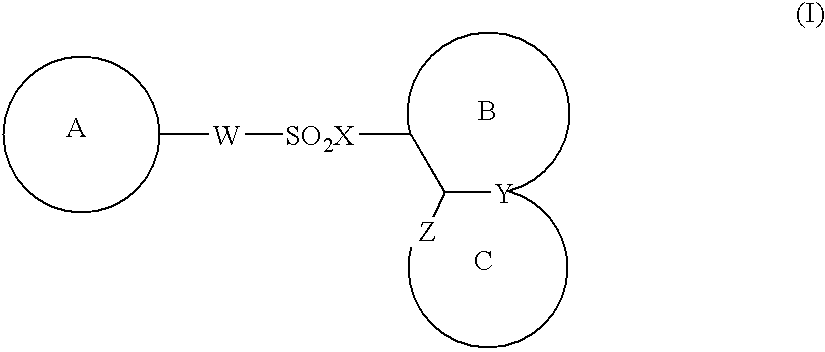
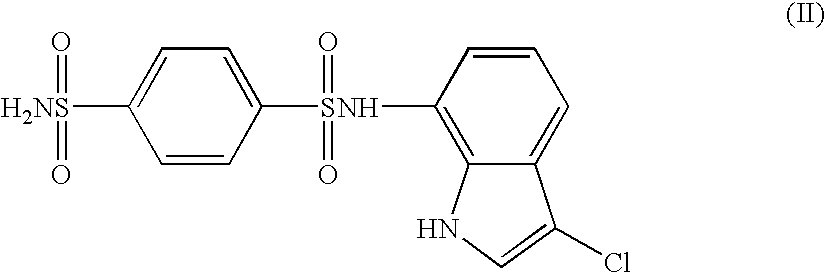
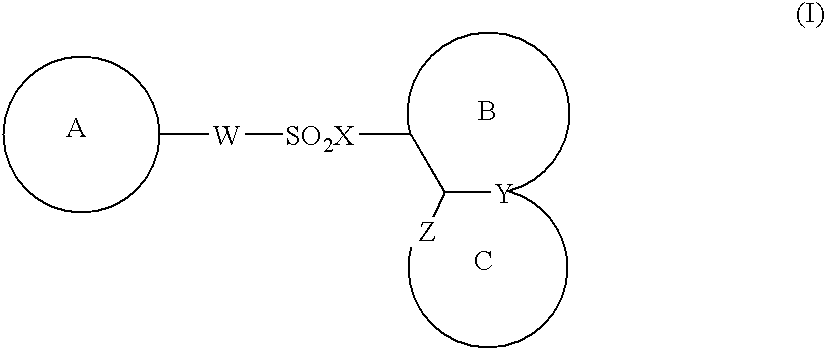
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: (wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl)-4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabine hydrochloride; (6) doxorubicin; (7) taxol; (8) carboplatin; (9) oxaliplatin; (10) capecitabine; and (11) a salt of the above-mentioned (1) to (10).
Owner:EISIA R&D MANAGEMENT CO LTD
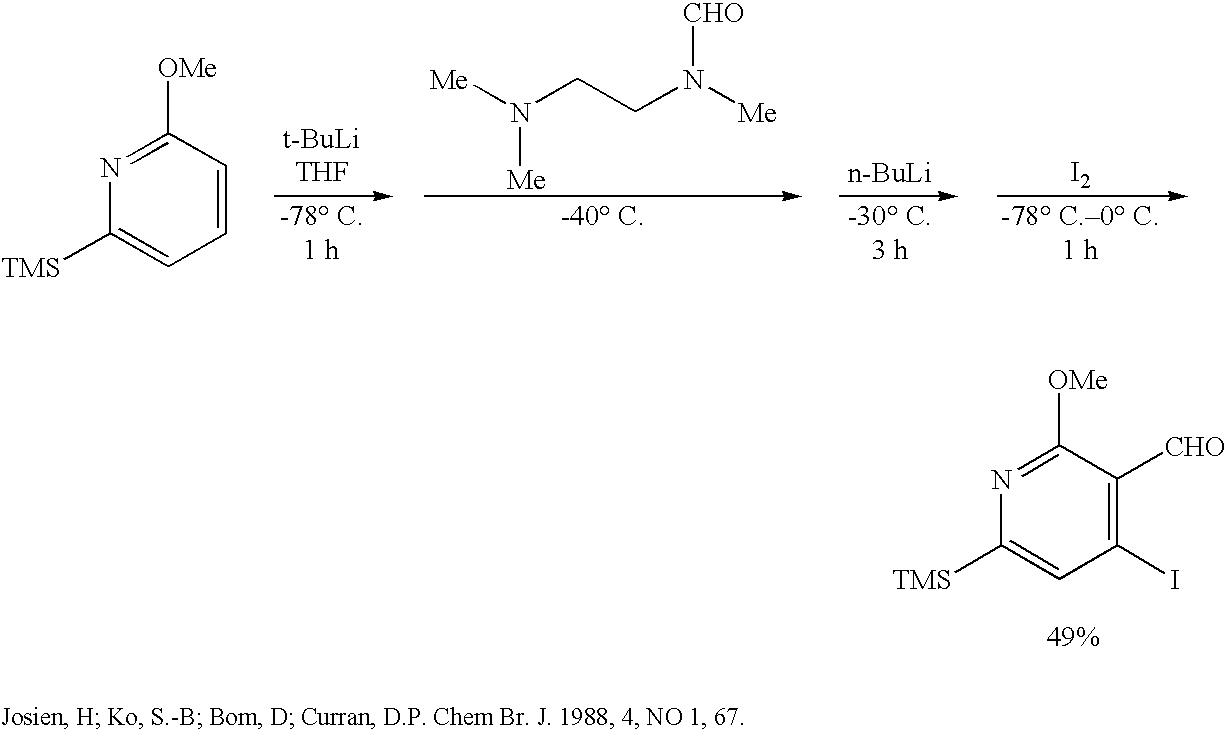
Method of synthesizing camptothecin-relating compounds
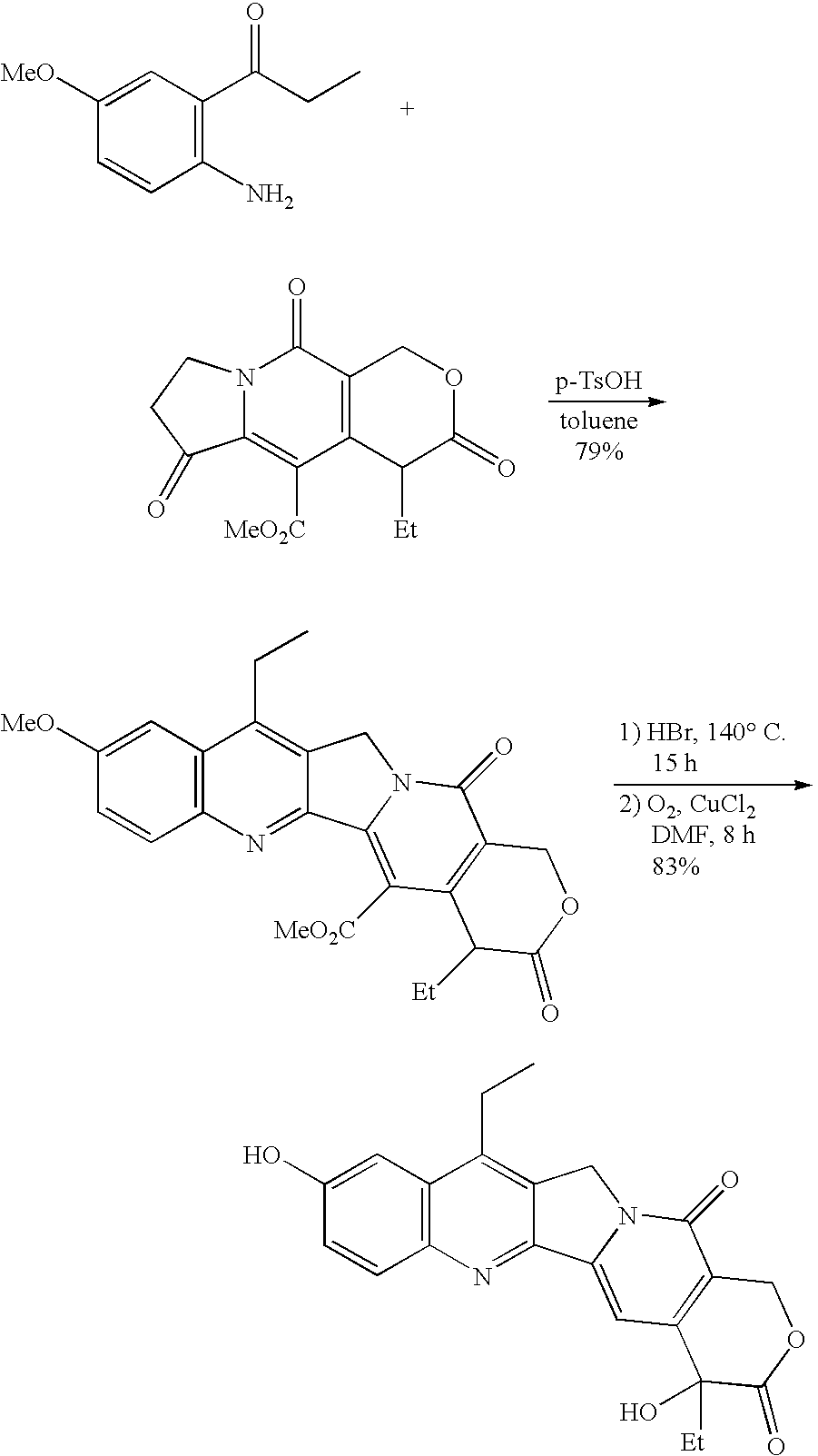
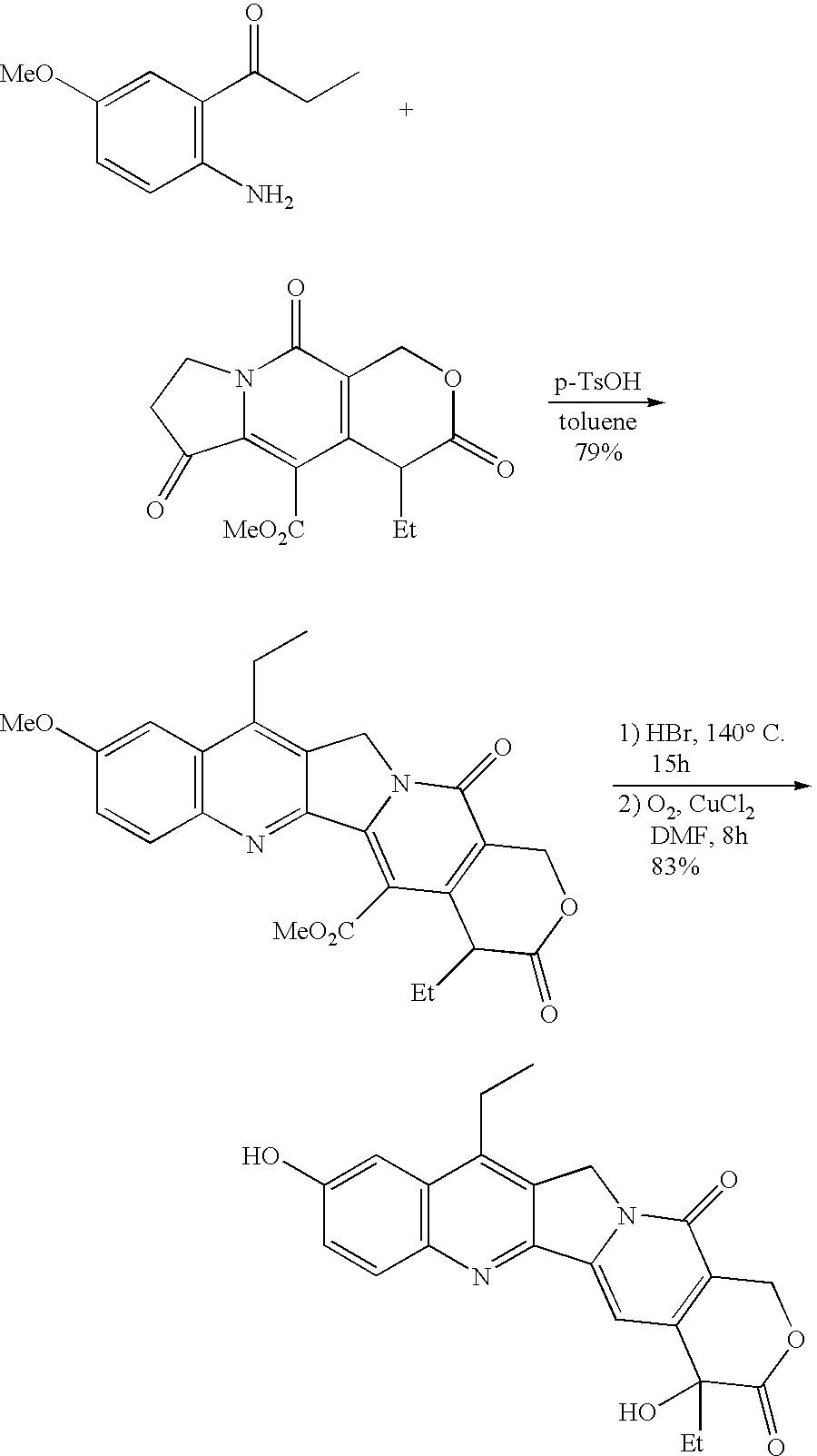
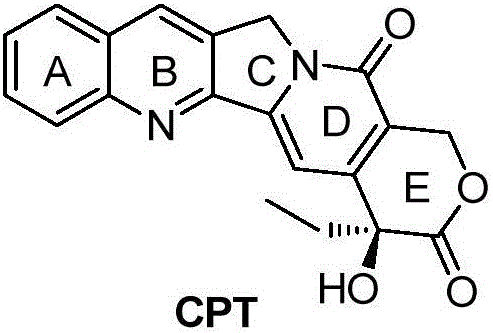
The present invention is to prepare efficiently 2′-amino-5′-hydroxypropiophenone corresponding to the AB-ring part of camptothecin (CPT) skeleton and a tricyclic ketone corresponding to the CDE-ring part in order to provide efficiently CPT by the total synthesis, which is a starting material for irinotecan hydrochloride and various kinds of camptothecin derivatives, and to provide stably CPT and its derivatives.
Owner:YAKULT HONSHA KK
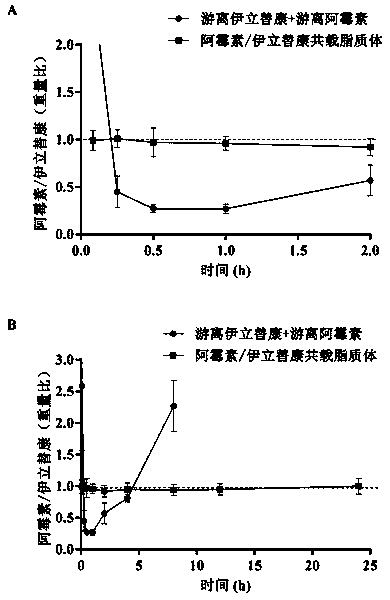
Preparation process of irinotecan hydrochloride liposome for injection
InactiveCN1994279AAvoid adverse reactionsGood curative effectOrganic active ingredientsAntineoplastic agentsOrganic solventIrinotecan hydrochloride liposome
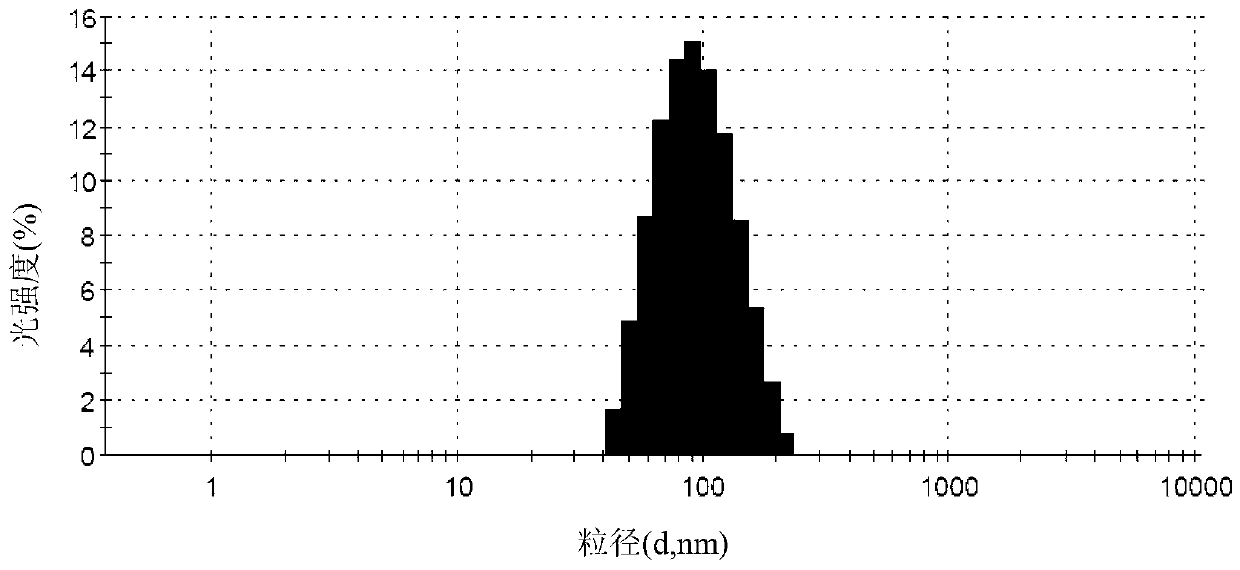
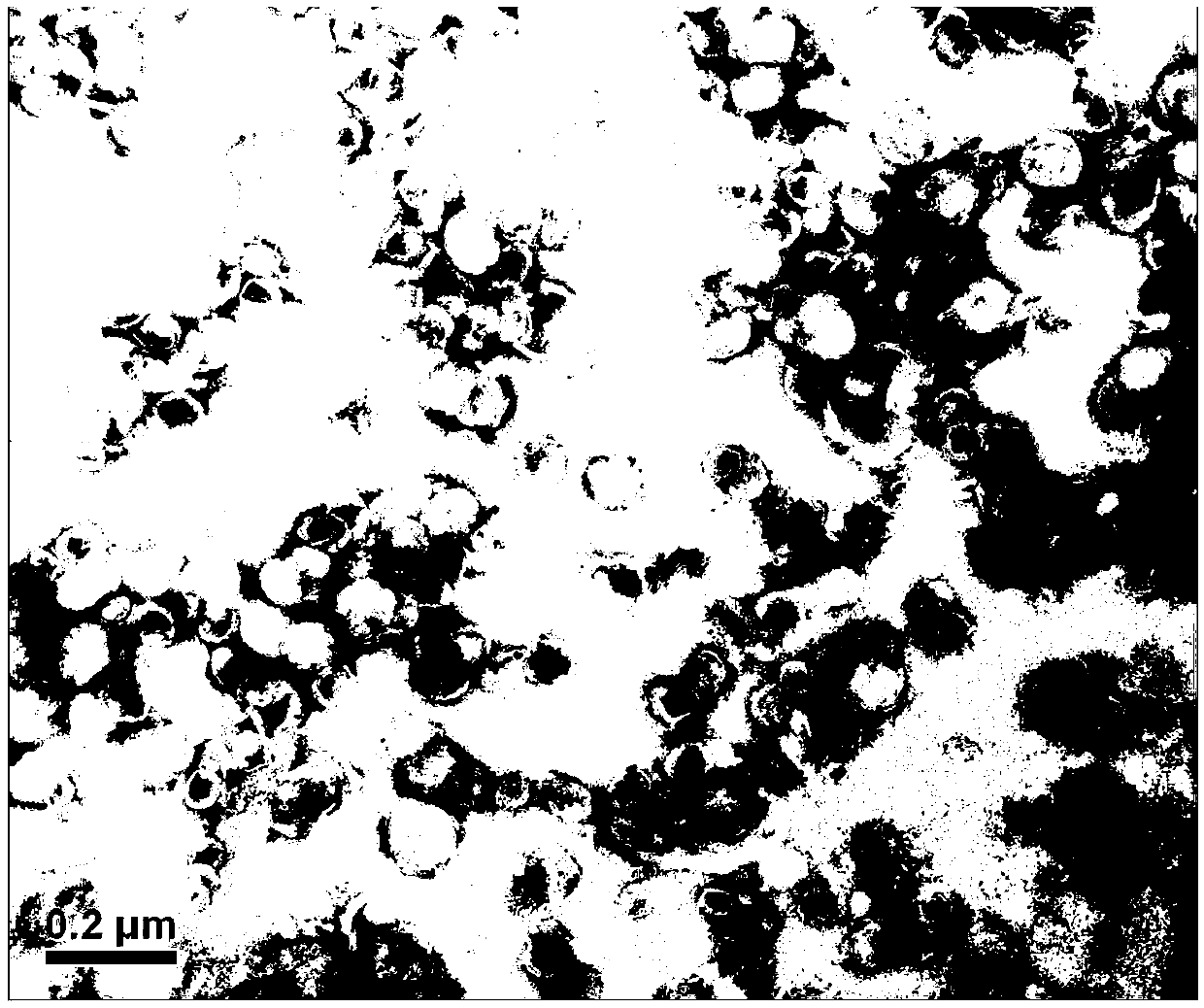
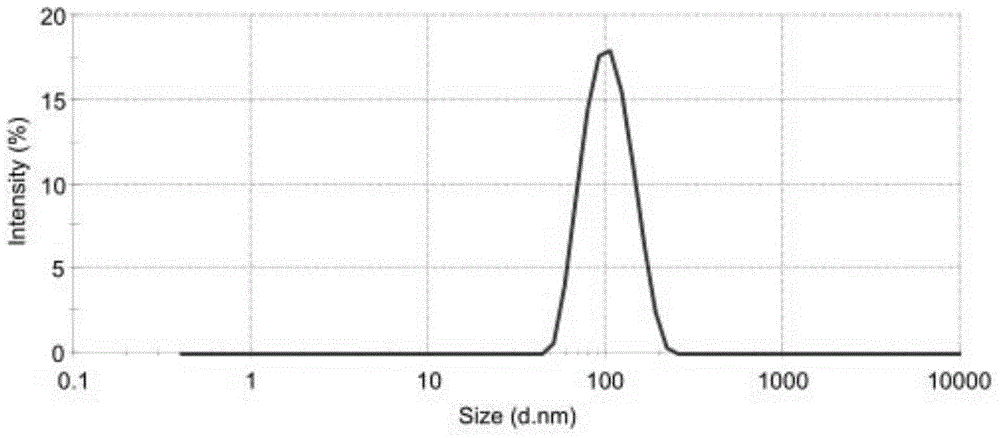
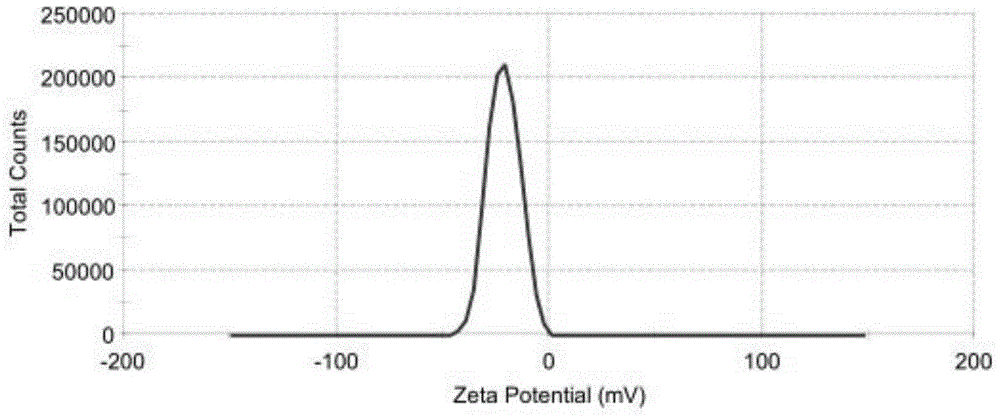
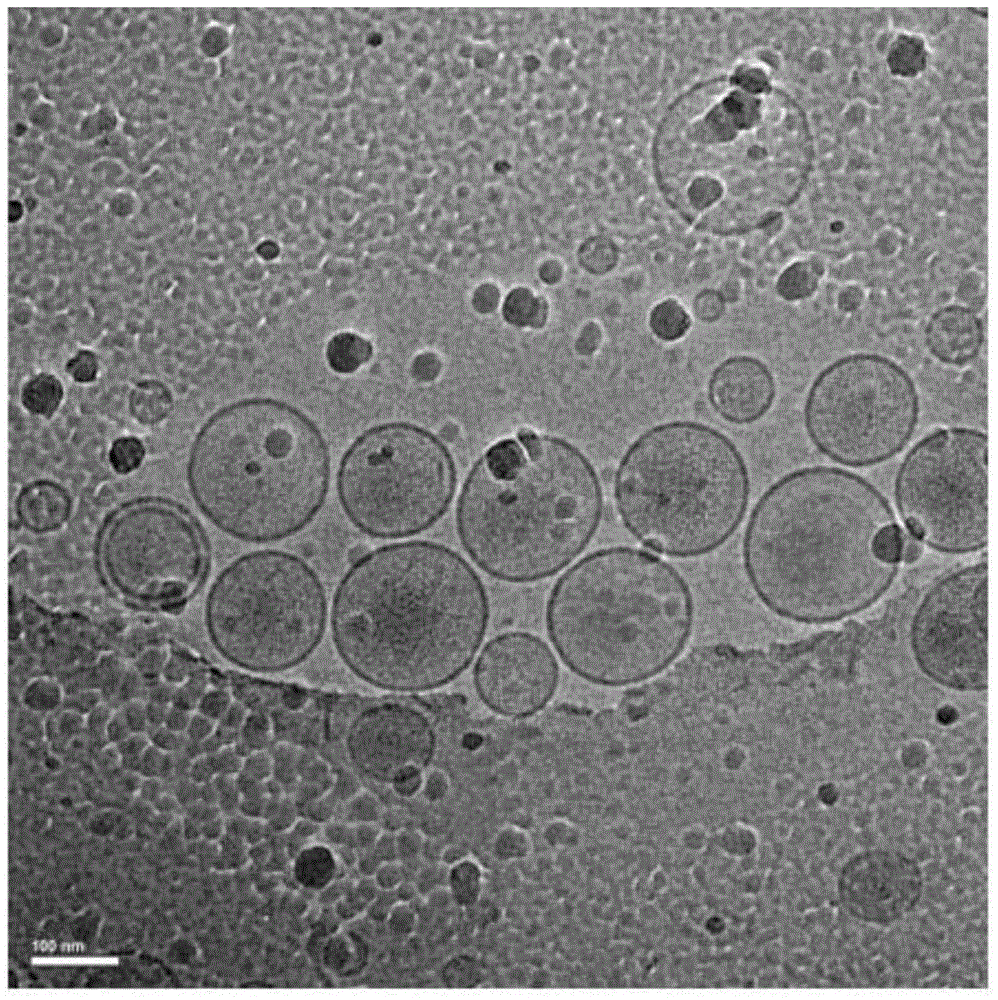
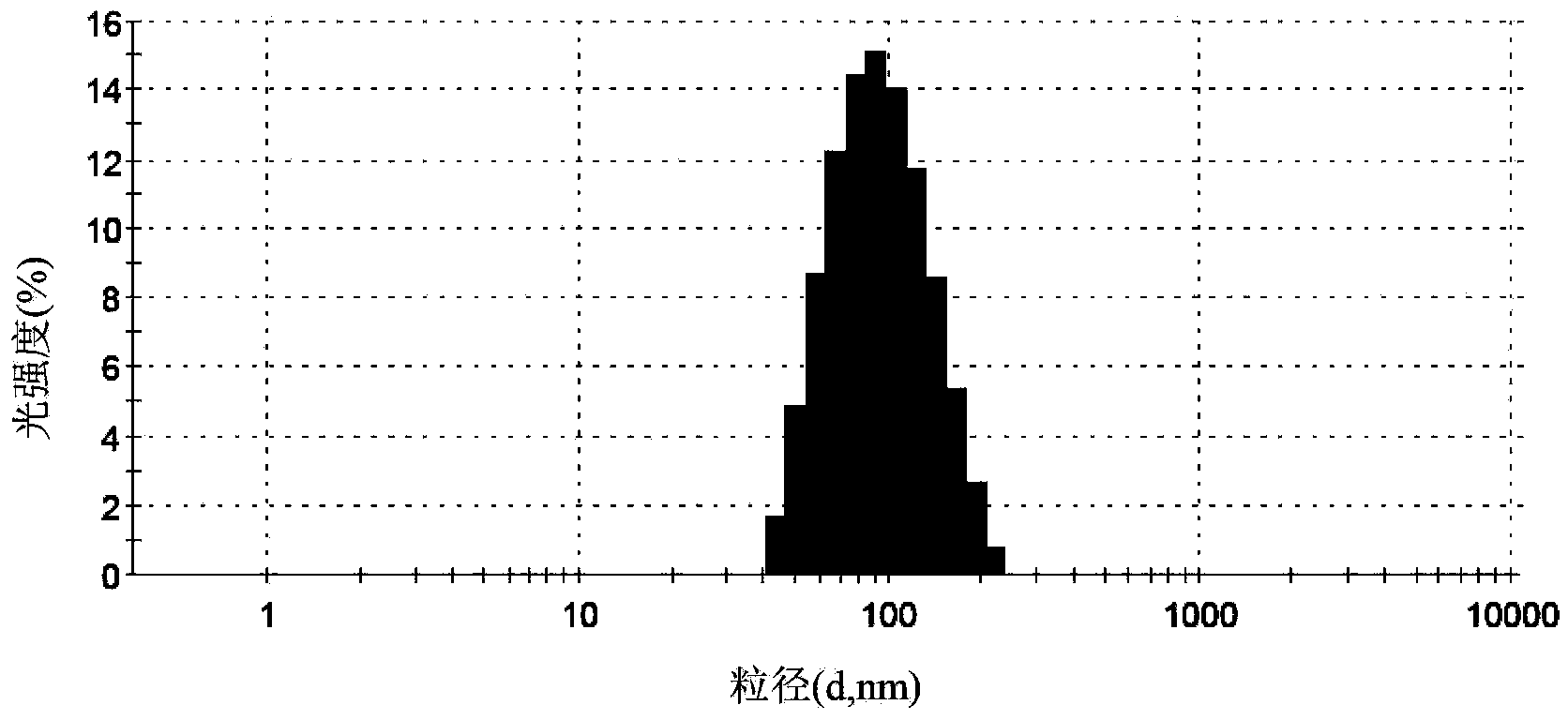
The invention relates to a method for producing injection alcaine liposome, wherein it comprises that: dissolving liposome mixture into organic solvent; mixing liposome solution with buffer solution; compressing it via panlite film into uniform diameter, filtering out the organic solvent, to obtain blank liposome, adding alcaine solution, using pH adjuster to adjust pH value to alkali, to prepare the carrier liposome; or dissolving phosphatide, cholestrin and alcaine into organic solvent; atomizing and drying, vacuum drying or other method to remove organic solvent, to film the liposome; adding hydrating solution with drug to form carrier liposome. The inventive liposome has 50-200nm diameter and more than 95% package rate, with stable property.
Owner:XIAN LIBANG PHARMA TECH
Irinotecan or irinotecan hydrochloride lipidosome and preparation method thereof
ActiveCN103120645ASolve the problem of low drug loadingSmall particle sizePowder deliveryOrganic active ingredientsCholesterolPhospholipid
The invention discloses an irinotecan or irinotecan hydrochloride lipidosome and a preparation method thereof. The lipidosome contains irinotecan or irinotecan hydrochloride, neutral phospholipid and cholesterol, wherein the weight ratio of the cholesterol to the neutral phospholipid is 1:(3-5), and the irinotecan or irinotecan hydrochloride lipidosome is prepared by an ion gradient method.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Medicinal compositions for cocominant use as anticancer agent
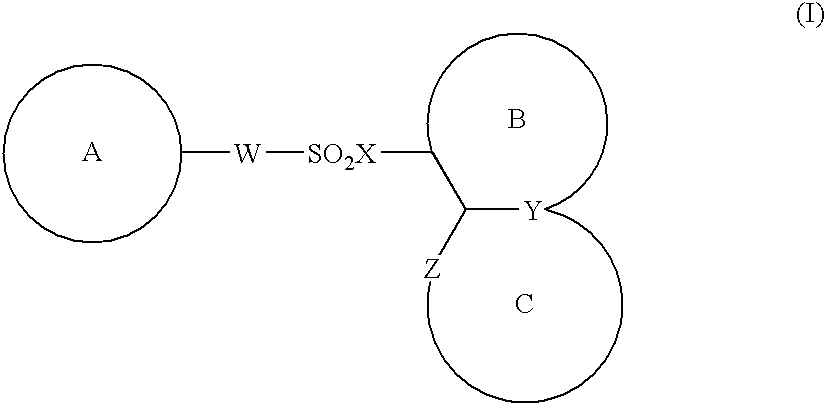
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl) -4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabinehydrochloride; (6) doxorubicin; (7) taxol; and (8) a salt of the above-mentioned (1) to (7).
Owner:EISIA R&D MANAGEMENT CO LTD
Method of synthesizing camptothecin-relating compounds
InactiveUS20040106830A1Efficiently provideOrganic compound preparationAmino-carboxyl compound preparationKetoneCamptothecin derivative
The present invention is to prepare efficiently 2'-amino-5'-hydroxypropiophenone corresponding to the AB-ring part of camptothecin (CPT) skeleton and a tricyclic ketone corresponding to the CDE-ring part in order to provide efficiently CPT by the total synthesis, which is a starting material for irinotecan hydrochloride and various kinds of camptothecin derivatives, and to provide stably CPT and its derivatives.
Owner:YAKULT HONSHA KK
Chemoradiotherapy with TS-1/camptothecins
InactiveUS20070036717A1Improve the level ofLess side effectsBiocideIn-vivo radioactive preparationsAbnormal tissue growthSide effect
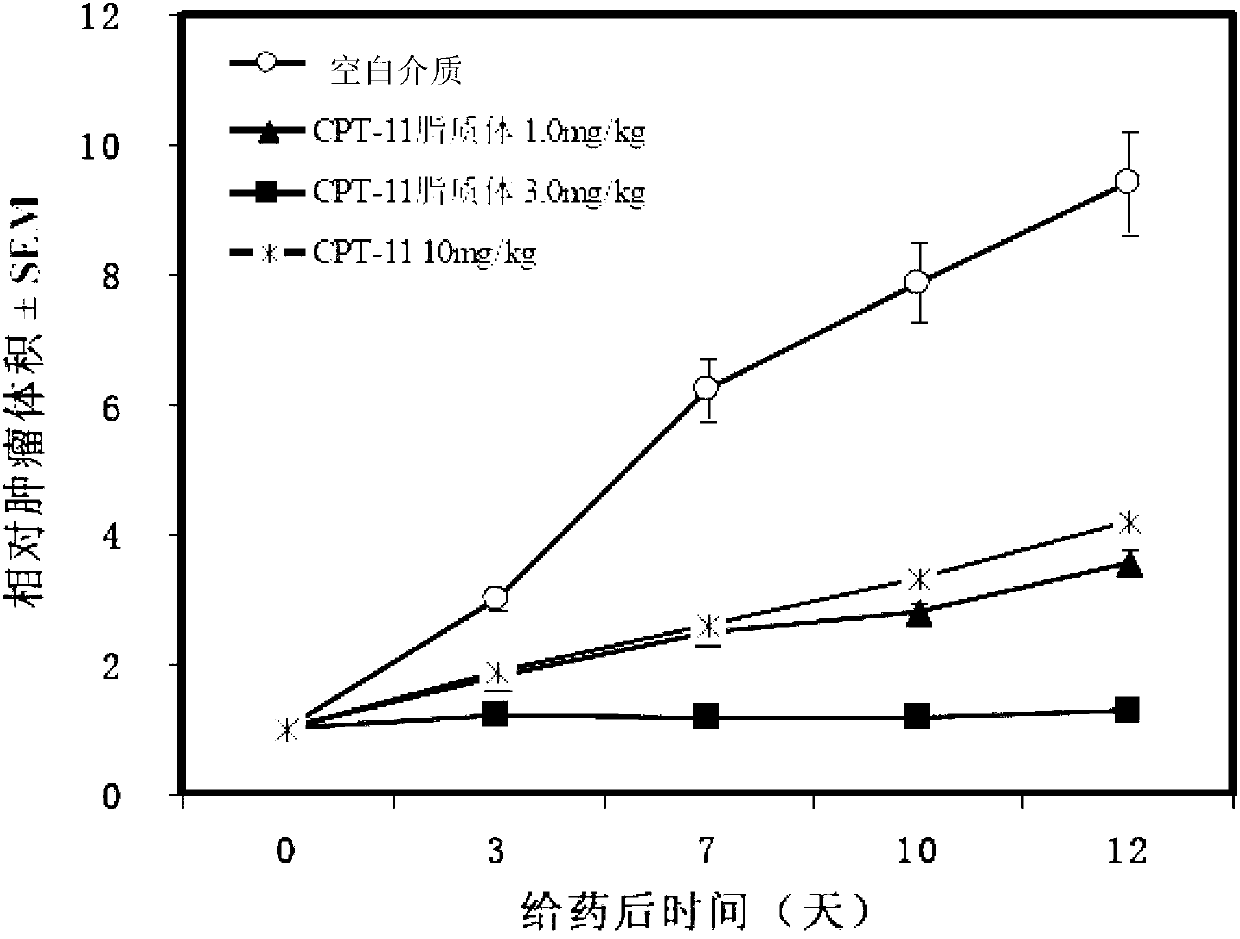
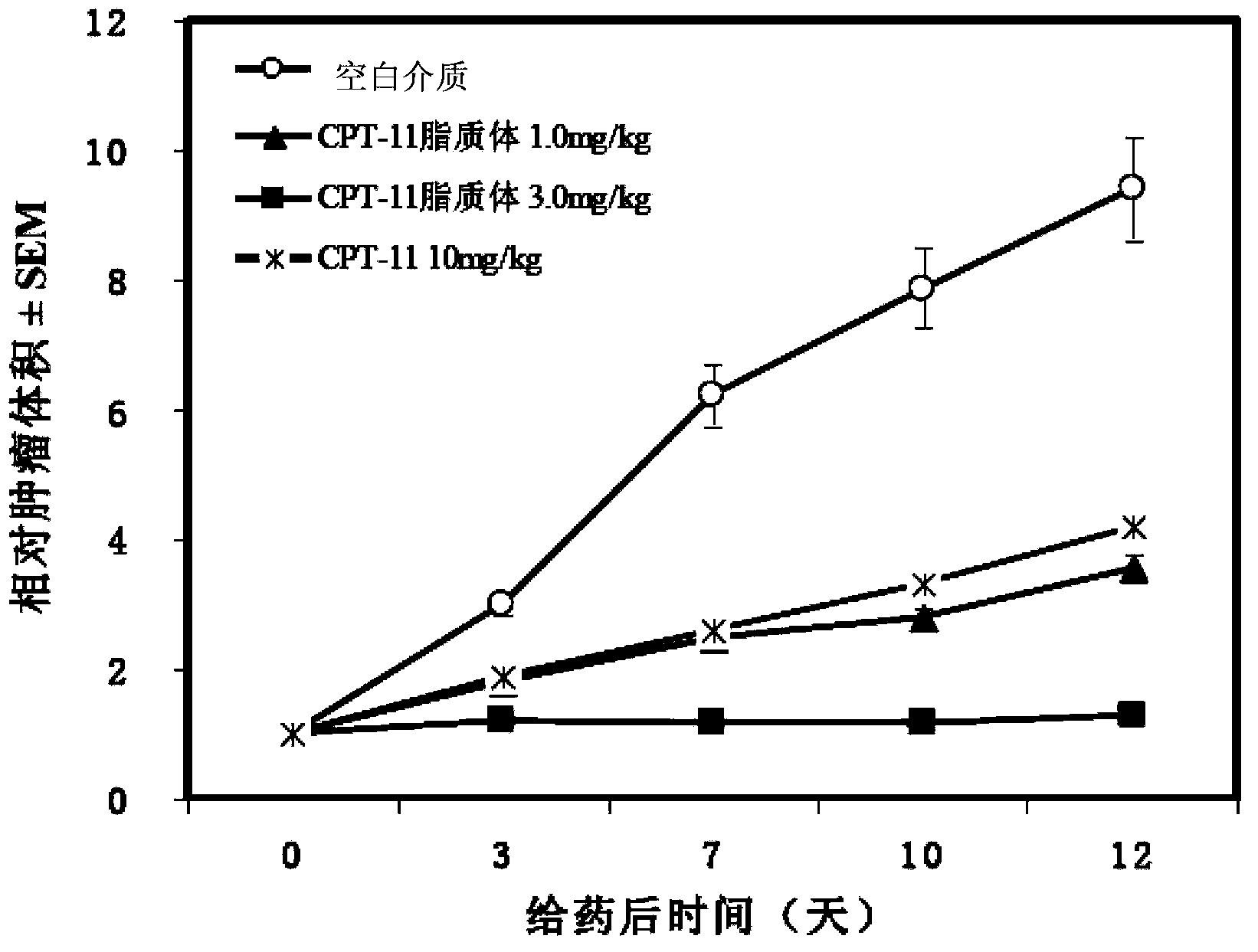
An antitumor agent for chemoradiotherapy of rectal cancer comprising a combination of TS-1 (a combination drug of tegafur, gimeracil, and oteracil potassium at a 1:0.4:1 molar ratio) and CPT-11 (irinotecan hydrochloride). The antitumor agent of the invention can achieve marked tumor volume reduction without causing major side effects, especially by coadministering it with preoperative radiation therapy. The volume of even large tumors that are refractory to surgical resection can be reduced by coadministering the antitumor agent of the invention with preoperative radiation therapy, making the subsequent surgical resection of the tumor easier.
Owner:THE KITASATO INST
Medicinal compositions for concomitant use as anticancer agent
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: (wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl)-4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabine hydrochloride; (6) doxorubicin; (7) taxol; (8) carboplatin; (9) oxaliplatin; (10) capecitabine; and (11) a salt of the above-mentioned (1) to (10).
Owner:EISIA R&D MANAGEMENT CO LTD
Irinotecan hydrochloride liquor type injection and preparation method thereof
ActiveCN101953781AReduce drug riskImprove product qualityOrganic active ingredientsPharmaceutical delivery mechanismStabilizing AgentsChemistry
The invention discloses an irinotecan hydrochloride liquor type injection and a preparation method thereof, which is characterized in that the injection contains buffer salt, no stabilizing agent, i.e. sorbierite. In addition, the invention also discloses a preparation method of the irinotecan hydrochloride injection. The irinotecan hydrochloride injection obtained by utilizing the method has stable quality, pharmacy safety and low production cost.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Irinotecan hydrochloride injection and preparation method thereof
ActiveCN102670500AReduce adverse reactionsPromote drug dependenceOrganic active ingredientsPharmaceutical delivery mechanismO-Phosphoric AcidAnimal testing
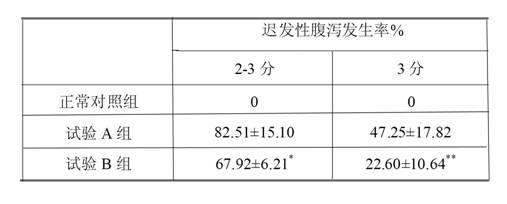
The invention relates to an irinotecan hydrochloride injection consisting of irinotecan hydrochloride (15-25g / L), sorbierite (17.5-22.5g / L), and the balance of phosphoric acid and injection water, wherein the phosphoric acid adjusts the pH of the injection to 3.0-3.8. The invention also relates to a preparation method of the irinotecan hydrochloride injection. Compared with the prior art, the irinotecan hydrochloride injection disclosed by the invention has the following beneficial effects: (1) the untoward effect is reduced; as shown in an animal test, the irinotecan hydrochloride injection disclosed by the invention can greatly reduce the untoward effect of delayed-onset diarrhea caused by irinotecan, which has an active promotion effect on the drug dependency and treatment effect of cancer patients; (2) the stability is high, as shown in an acceleration test, the character, acidity, solution clarity and color, insoluble particles, visible foreign matters, moisture, related substance and content of the irinotecan hydrochloride injection meet the regulations; and (3) the preparation method is simple and is favorable to the industrial mass production.
Owner:NANJING CHENGONG PHARM CO LTD
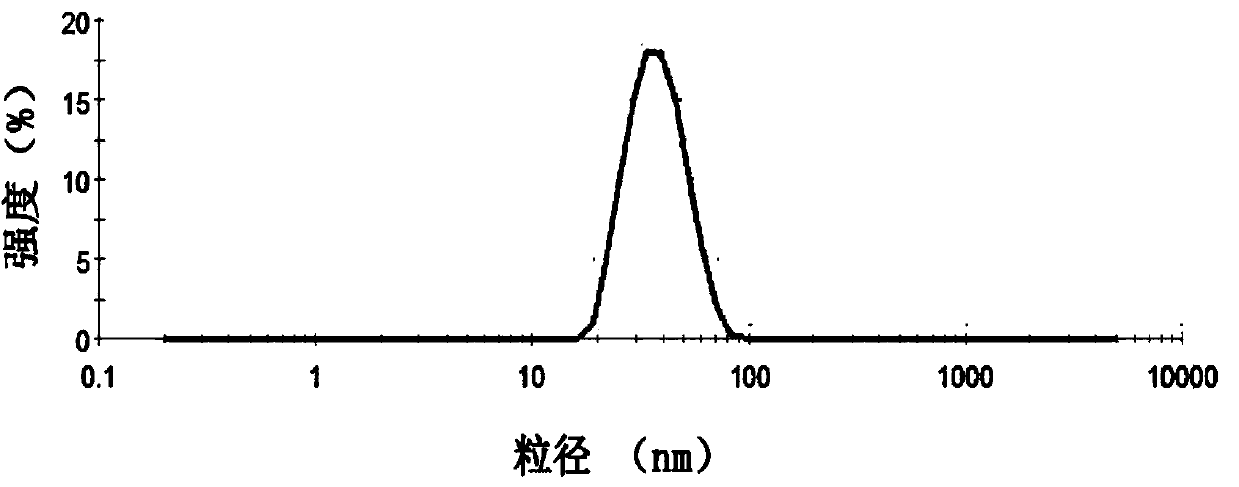
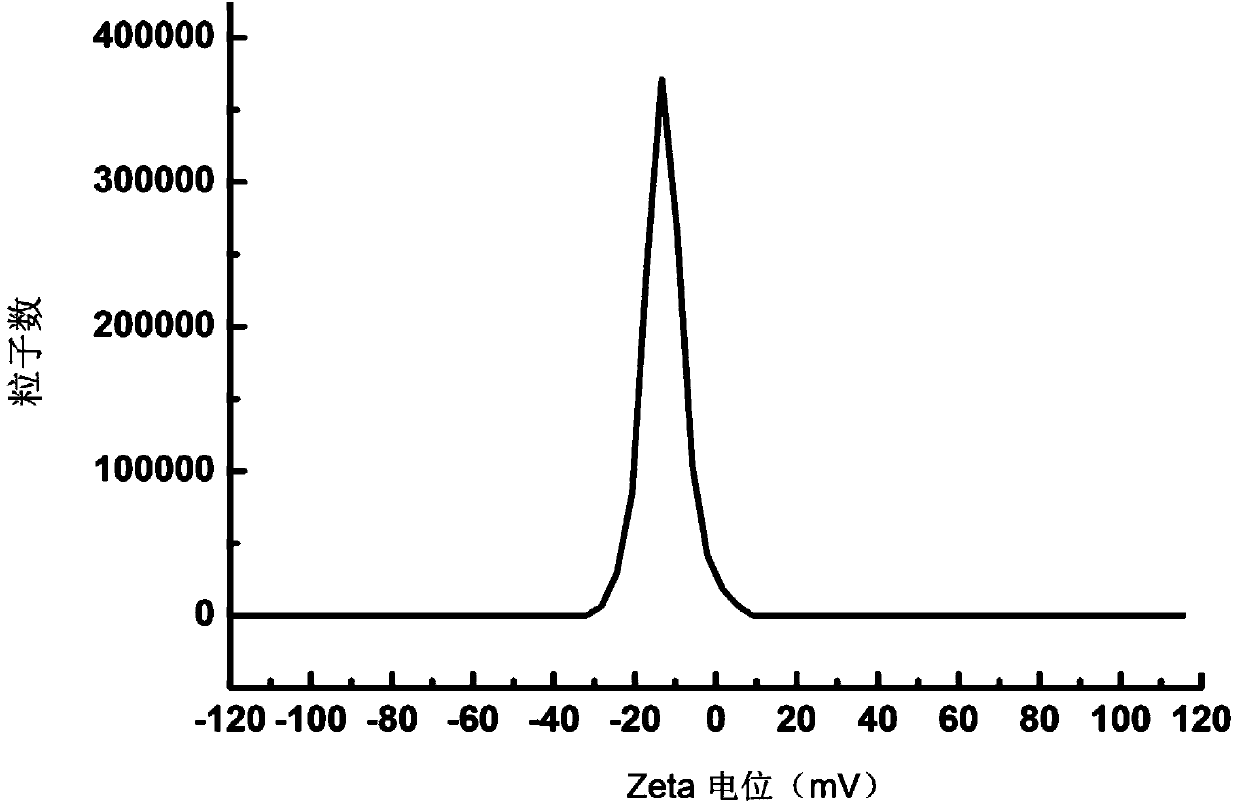
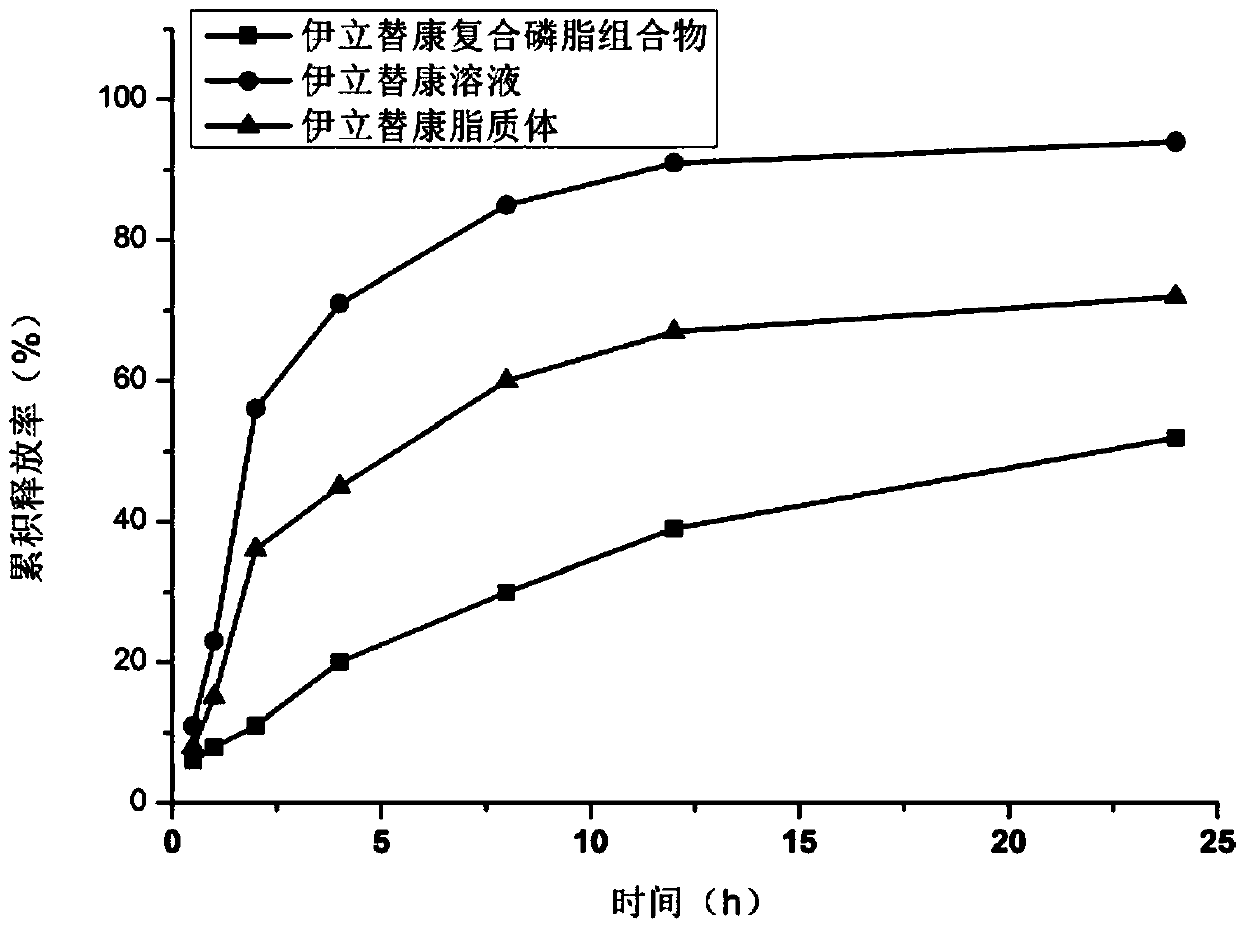
Irinotecan hydrochloride composite phospholipid composition, preparation method and applications thereof
InactiveCN104906586AImprove rigidityClosely arrangedOrganic active ingredientsInorganic non-active ingredientsCholesterolActive agent
An irinotecan hydrochloride composite phospholipid composition, preparation method and uses thereof in the preparation of drugs for treating tumors or drug resistant tumors. The composite phospholipid composition comprises irinotecan hydrochloride, composite phospholipid, cholesterol, long-circulating membrane material, surfactant and a buffer medium. The composition improves stability of lipid formulation and the anti-tumor effect of irinotecan hydrochloride, and can overcome multidrug resistance of a tumor.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Irinotecan hydrochloride liposome pharmaceutical composition and preparation method thereof
ActiveCN105796495AHigh encapsulation efficiencyHigh drug loadingOrganic active ingredientsPharmaceutical non-active ingredientsIrinotecan hydrochloride liposomeCholesterol
The invention provides an irinotecan hydrochloride liposome pharmaceutical composition and a preparation method thereof. The irinotecan hydrochloride liposome pharmaceutical composition comprises irinotecan hydrochloride, hydrogenated soy phosphatidylcholine (HSPC), distearoyl phosphatidylcholine (DSPC), cholesterol (CHol) and Phosphatidylethanolamine Pegol. The irinotecan hydrochloride liposome pharmaceutical composition obtained through the preparation method has a high encapsulation rate, a high drug loading amount and good stability.
Owner:NANJING LUYE PHARMA
Irinotecan hydrochloride nanometer fat beam preparation and preparation method thereof
ActiveCN104083325AImprove solubilityHigh encapsulation efficiencyOrganic active ingredientsEmulsion deliveryWater basedSide effect
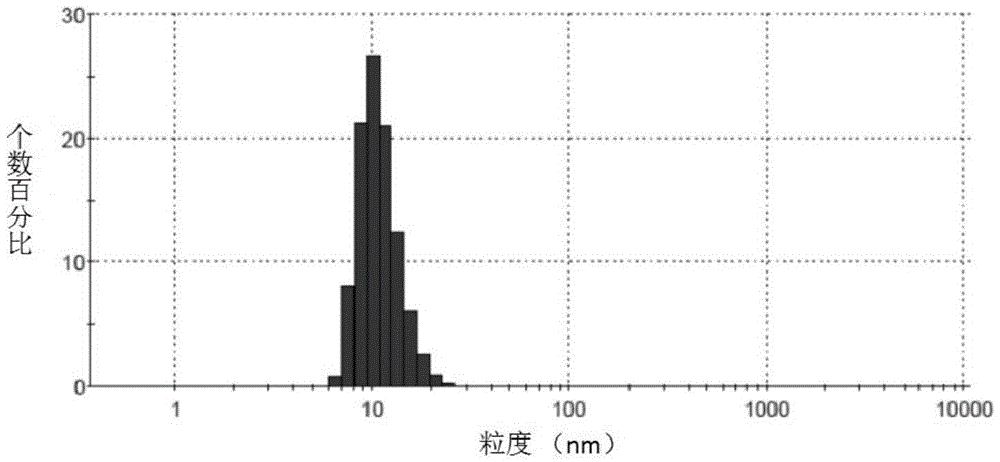
The invention discloses an irinotecan hydrochloride nanometer fat beam preparation, which comprises an entrapment material, and irinotecan hydrochloride and a water-based solvent for injection, wherein the entrapment material is one or at least two of PLGA-PEG, PGA-PEG, PCL-PEG, PEG-NH2, PEG-COOH, DSPE-PEG, Solutol HS15, and phospholipid. The invention also discloses a preparation method for the irinotecan hydrochloride nanometer fat beam preparation and irinotecan hydrochloride nanometer fat beam powder preparation prepared by the method. The encapsulation efficiency of the irinotecan hydrochloride nanometer fat beam preparation reaches up to 85%, the grain size of the irinotecan hydrochloride nanometer fat beam preparation is about 10nm, and the irinotecan hydrochloride nanometer fat beam preparation can realize passive target to tumors and can increase pharmacological function and reduce the toxic and side effects of the system. The irinotecan hydrochloride nanometer fat beam powder preparation improves the safety and compliance of the irinotecan hydrochloride preparation, lowers the toxicity of the irinotecan hydrochloride, and prolongs the circulation time in the body. The preparation method is simple and feasible, has good repeatability, and is applicable to industrial production.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Irinotecan hydrochloride composition and preparation method thereof
ActiveCN103989624AImprove stabilityPrevent leakageOrganic active ingredientsPharmaceutical delivery mechanismDrugIrinotecan Hydrochloride
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
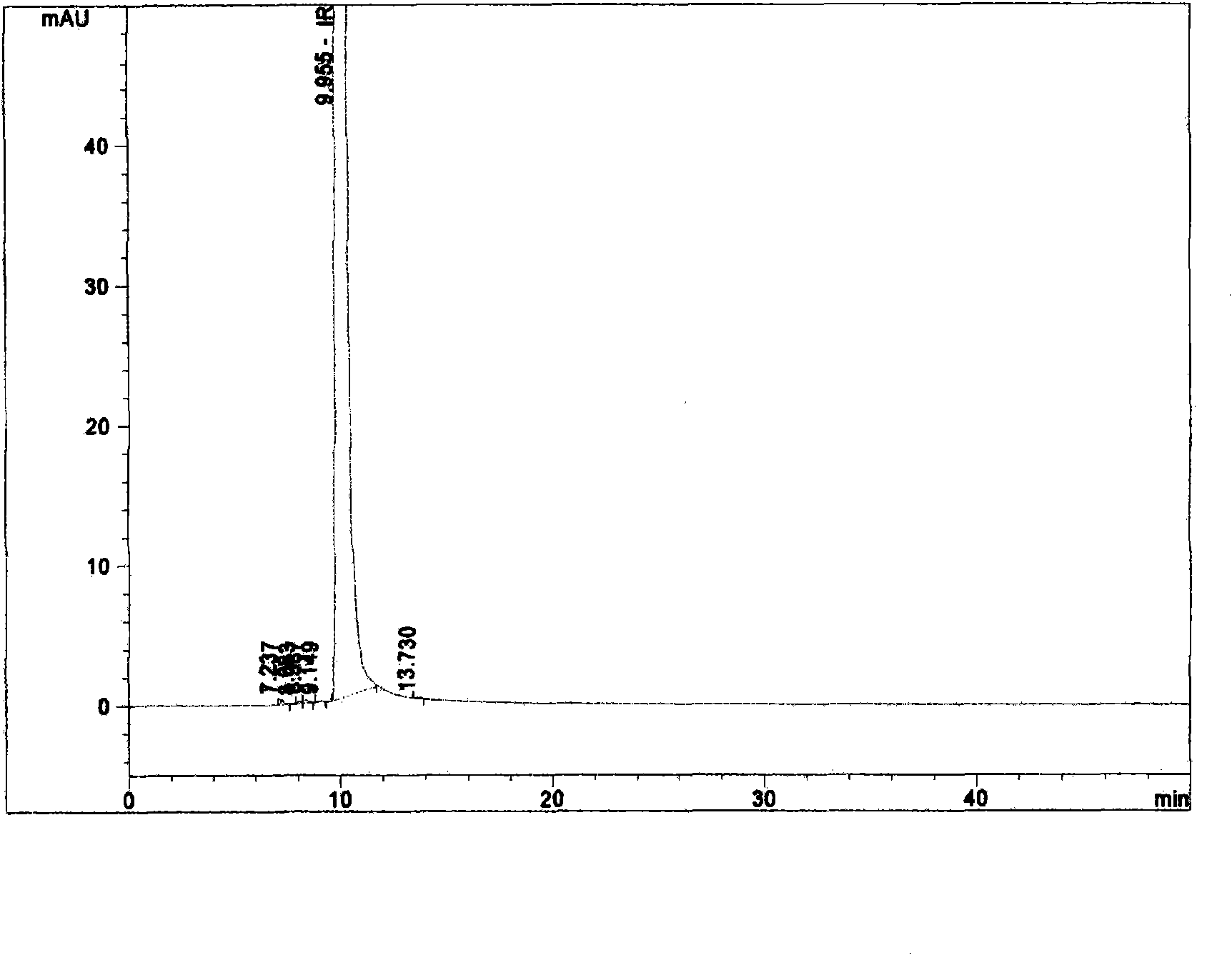
Novel crystal system of irinotecan hydrochloride and preparation method thereof
ActiveCN101318964AImprove solubilityGood storage stabilityOrganic chemistryAntineoplastic agentsSolubilityCrystal system
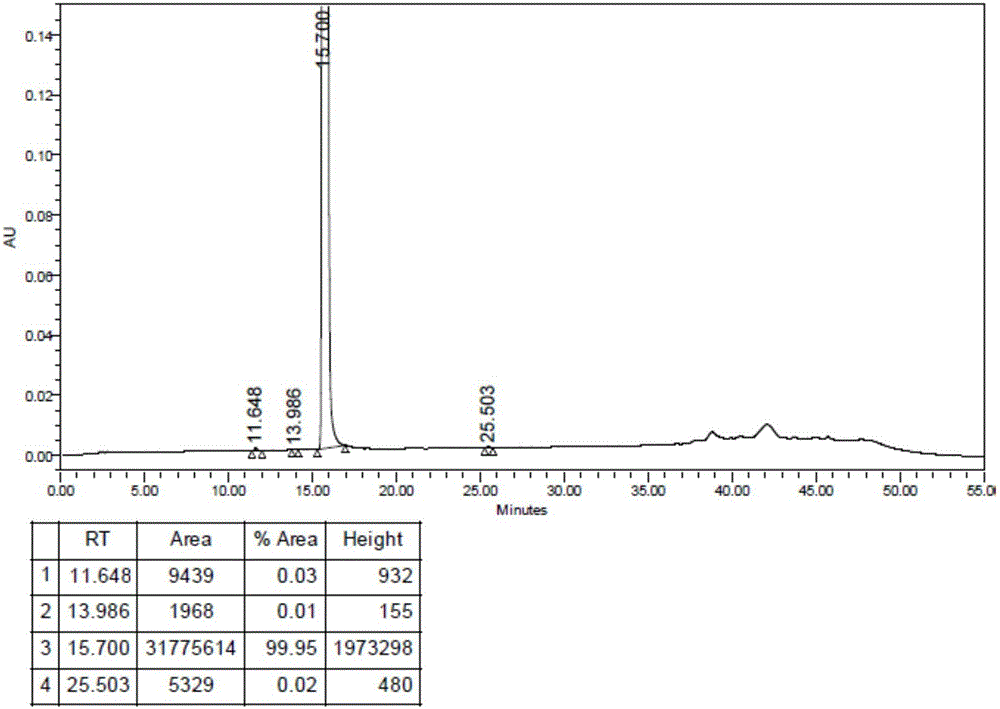
The invention discloses a new crystal of irinotecan hydrochloride. The crystal has characteristic peaks under powder X-ray diffraction at the following angles (2 theta): 7.80 degrees, 9.96 degrees, 13.28 degrees, 15.62 degrees, 19.98 degrees, 20.36 degrees, 22.34 degrees, 22.66 degrees, 26.60 degrees and 30.18 degrees. In addition, the invention also discloses a method for preparing a new crystal form of the irinotecan hydrochloride. The irinotecan hydrochloride prepared by the method has good water-solubility, good storage stability, high content and purity and less impurity.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
A kind of method for preparing irinotecan hydrochloride
ActiveCN102260272AAvoid foul smellHigh activityOrganic chemistrySide effect7-ethyl-10-hydroxycamptothecin
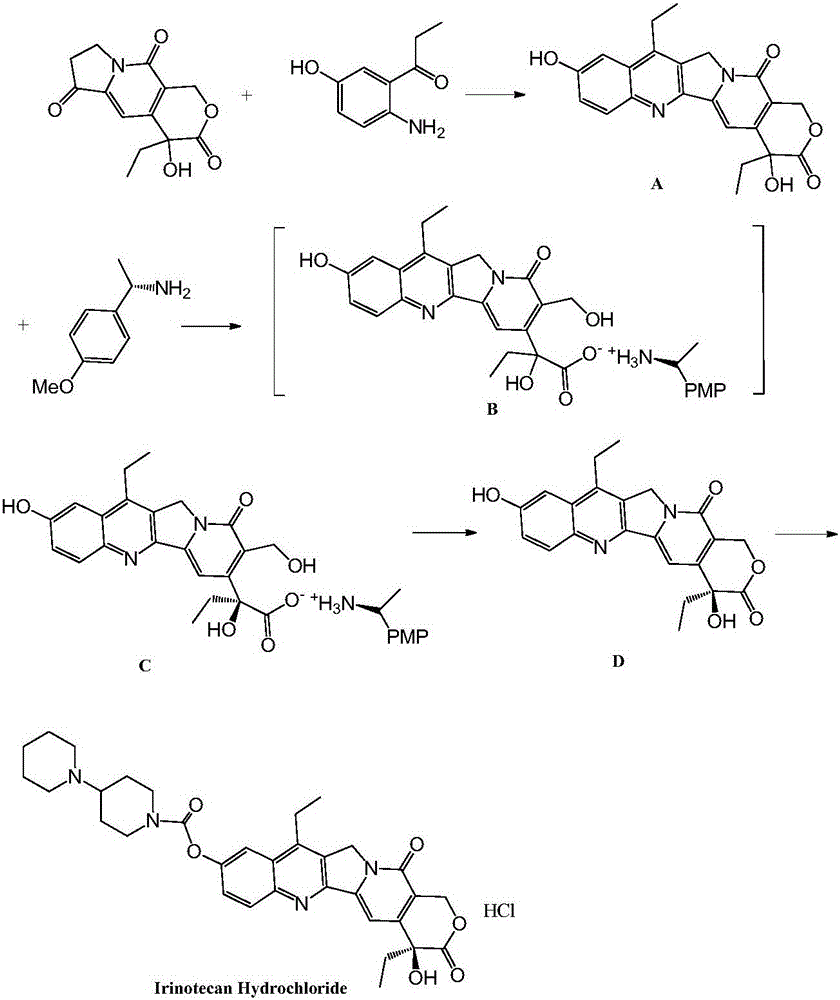
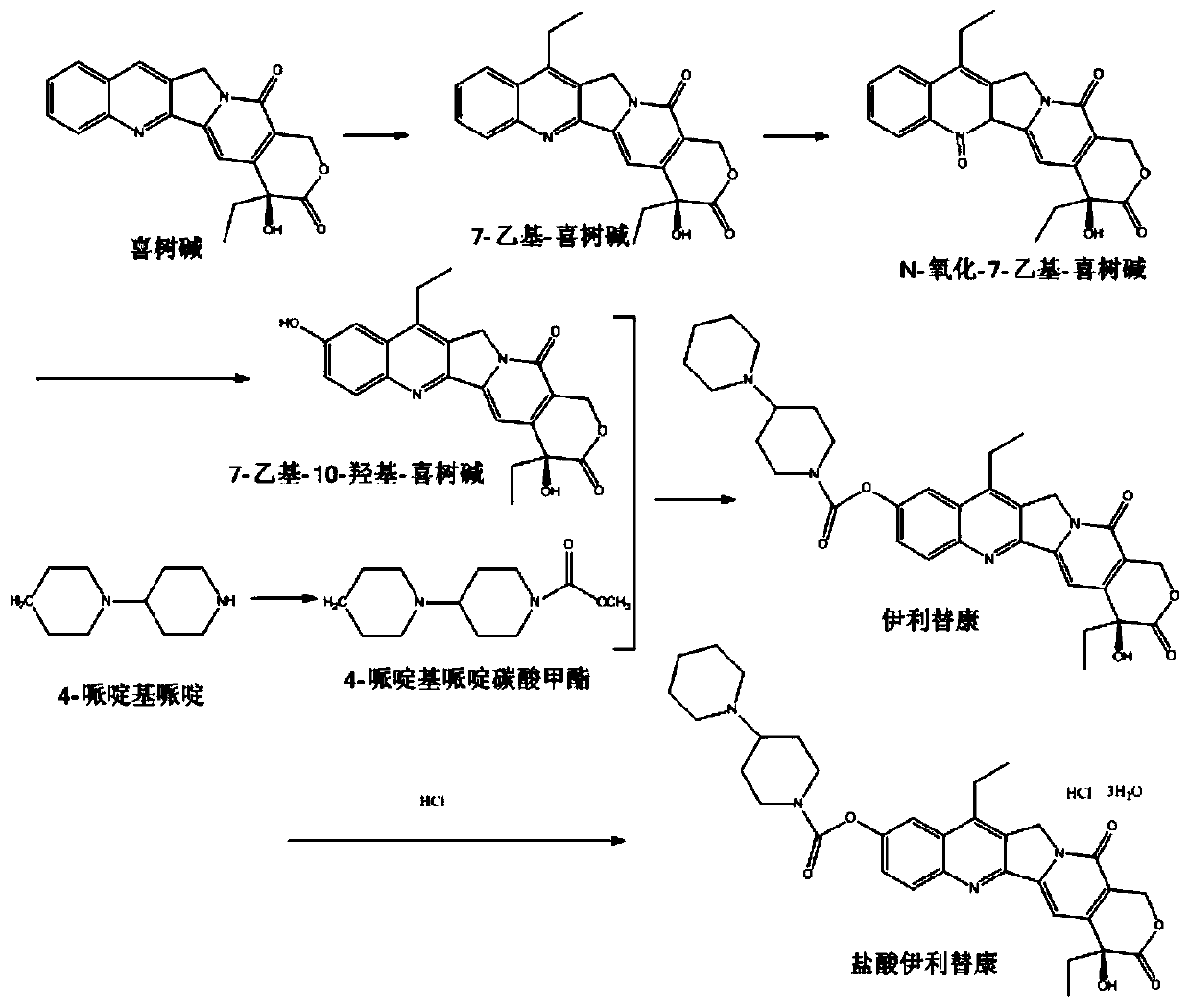
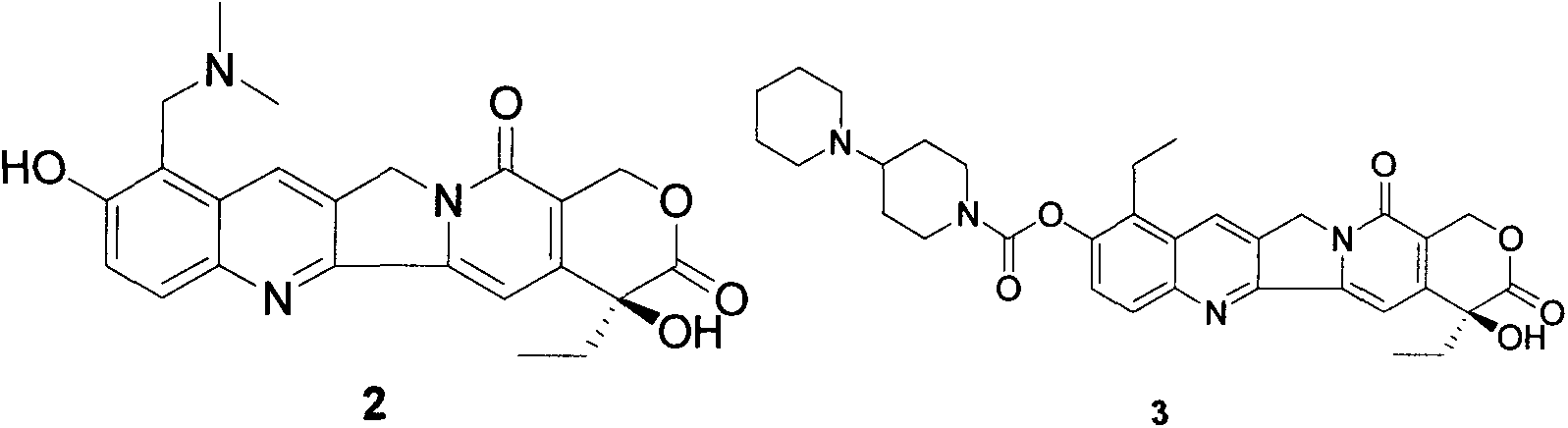
The invention provides a method for preparing irinotecan hydrochloride. The method reacts the intermediate 7-ethyl-10-hydroxycamptothecin with 4-piperidinylpiperidine carboxylic acid chloride or its salt in the presence of 4-dimethylaminopyridine or its salt, or its analogue, The final product is obtained through steps such as concentration, washing, and salt formation. The method of the invention avoids the use of malodorous and easily discolored pyridine to participate in the reaction, reduces side reactions, improves the purity and yield of the product, improves the color of the product at the same time, and is easy for large-scale production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Irinotecan or irinotecan hydrochloride lipidosome and preparation method thereof
ActiveCN103120645BGood curative effectImprove stabilityOrganic active ingredientsPowder deliveryCholesterolPhospholipid
The invention discloses an irinotecan or irinotecan hydrochloride lipidosome and a preparation method thereof. The lipidosome contains irinotecan or irinotecan hydrochloride, neutral phospholipid and cholesterol, wherein the weight ratio of the cholesterol to the neutral phospholipid is 1:(3-5), and the irinotecan or irinotecan hydrochloride lipidosome is prepared by an ion gradient method.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Irinotecan hydrochloride and doxorubicin hydrochloride co-loaded liposome and preparation method thereof
ActiveCN109528654AHigh encapsulation efficiencyIncrease loading capacityOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolPhospholipid
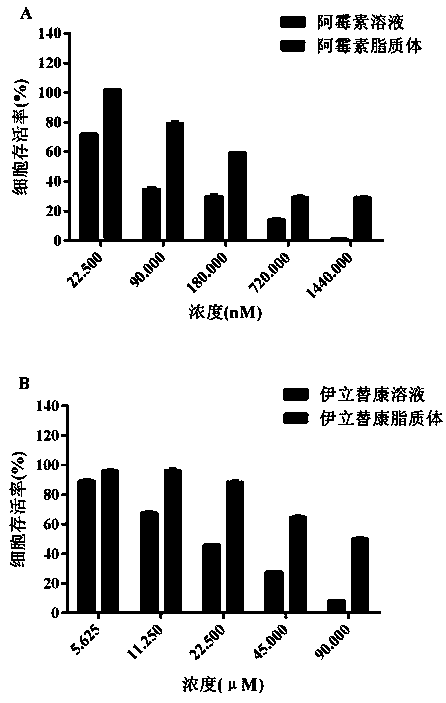
The invention belongs to the technical field of medicines, and relates to an irinotecan hydrochloride and doxorubicin hydrochloride co-loaded liposome and a preparation method thereof. The liposome comprises the following components of medicines, phosphatide, cholesterol, triethylammonium sucrose octasulfate salt and a buffering agent, wherein the medicines include irinotecan hydrochloride and doxorubicin hydrochloride, the weight ratio of the irinotecan hydrochloride to the doxorubicin hydrochloride is (1-10) to (1-10), the weight ratio of the irinotecan hydrochloride to the phosphatide is (0.1-1) to 1, and the weight ratio of the phosphatide to the cholesterol is (2-9) to 1. The liposome is high in encapsulation efficiency and high in medicine loading quantity and can co-deliver two kinds of medicines, the situation that the medicines can maintain cooperating proportion when reaching tumor positions is guaranteed, and the purposes of improving effects and reducing toxin can be achieved.
Owner:SHENYANG PHARMA UNIVERSITY
Irinotecan hydrochloride composition and preparation method thereof
InactiveCN102824345AStable contentAccurate contentOrganic active ingredientsPharmaceutical delivery mechanismActivated carbonAlcohol
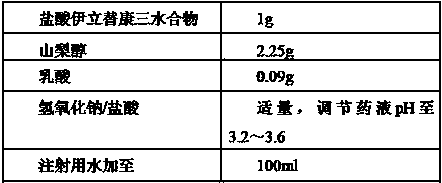
The invention relates to an irinotecan hydrochloride composition, which is a composition by using irinotecan hydrochloride as a main drug and an auxiliary solution consisting of sorbic alcohol, lactic acid, alkaline regulation solution and injection water as dissolving solution. The preparation method comprises the following steps: weighing sorbic alcohol and lactic acid in a container according to the prescription, adding the injection water, stirring to dissolve, and regulating the pH to be between 3.0 and 4.0 with the alkaline regulation solution; adding the irinotecan hydrochloride with the amount of the prescription, dissolving, adding 0.1 percent activated carbon, stirring for 20 minutes, and decarburizing; filtering with a 0.22mu m microporous filter membrane until the mixture is clear; detecting the intermediate substance; filling in a penicillin bottle, plugging a butyl rubber plug, and rolling a cap; and performing leakage inspection and light inspection to the sample after high-pressure sterilization, and packaging after the sample is qualified in quality inspection.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for purifying irinotecan hydrochloride
The invention discloses a method for purifying irinotecan hydrochloride. The method performs crystallization and purification through three different crystallization systems, the irinotecan hydrochloride is purified by a low-temperature drying method, the purity of the irinotecan hydrochloride in the purified product is up to 99.9 percent, the total impurity content is not more than 0.1 percent, and adsorbed water is not contained. The purifying method has the advantages of controllable conditions, simple operation, low solvent consumption, recycle of irinotecan hydrochloride in the mother liquor and feasible industrial production.
Owner:CHONGQING TAIHAO PHARM CO LTD
Irinotecan hydrochloride-containing drug composition and preparation method thereof
ActiveCN108992400AOrganic active ingredientsPharmaceutical delivery mechanismAqueous solutionImpurity
The invention provides an irinotecan hydrochloride-containing drug composition and a preparation method thereof. The preparation method comprises the following steps: before irinotecan hydrochloride is added into a lactic acid aqueous solution, adjusting the lactic acid aqueous solution to a specific pH range at first, and then after the irinotecan hydrochloride, sorbitol and injection water are added, adjusting medicinal liquid to the specific pH range. Through twice pH value adjustment, the long-term stability of the injection can be obviously improved, and impurities can be reduced; the drug use risk of a patient is greatly lowered; meanwhile, the preparation method is simple; no special equipment requirement is needed; the production cost is obviously reduced.
Owner:SICHUAN HUIYU PHARMA
Method for encapsulating hydrophobic antitumor drug by amphiphilic medicament and preparation
ActiveCN103638027AImprove solubilityPreserve the characteristics of high anti-tumor activityOrganic active ingredientsPowder deliverySolubilityWater insoluble
The invention discloses a method for encapsulating a hydrophobic antitumor drug by an amphiphilic medicament, comprising: (1) adding the amphiphilic medicament and the hydrophobic antitumor drug into an organic solvent, and uniformly mixing; (2) adding distilled water into an obtained mixture, and uniformly mixing to obtain a preparation solution; and (3) removing the organic solvent in the preparation solution, adding a freeze-drying protective agent, and carrying out freezing and drying treatment, to obtain an antitumor drug pulvis encapsulated with the hydrophobic antitumor drug. The invention further discloses a medicinal preparation prepared by the method. The amphiphilic medicament irinotecan hydrochloride or topotecan hydrochloride is used as an emulsifier to encapsulate a water insoluble anticancer medicament, which greatly increases solubility of the hydrophobic medicament in water and reserves high antineoplastic activity characteristics of the medicament itself, has advantages of simple preparation technology, strong applicability and convenience for large scale production, and develops a new approach for in vivo conveying of the hydrophobic medicament.
Owner:杭州磐田生物技术有限公司
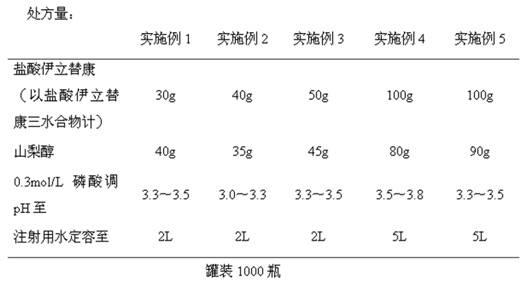
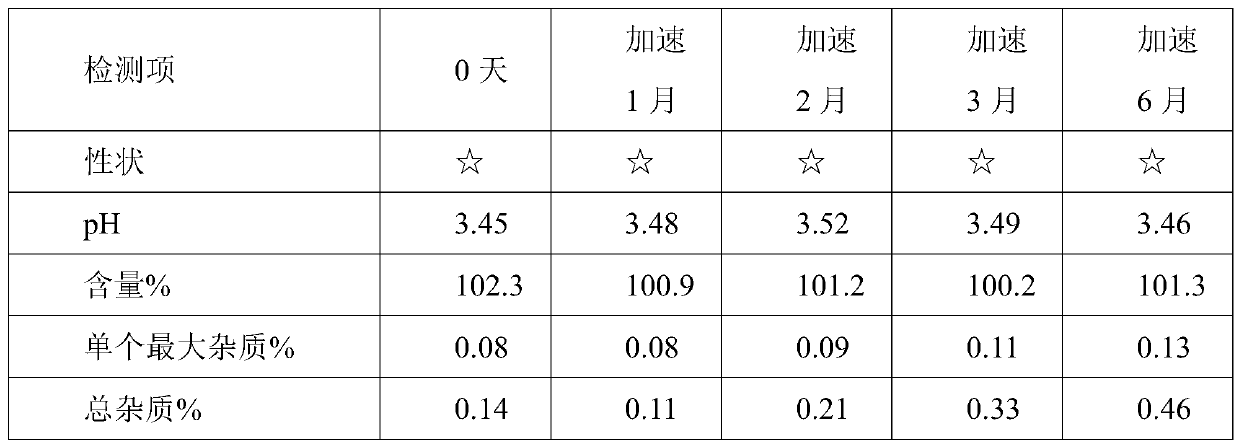
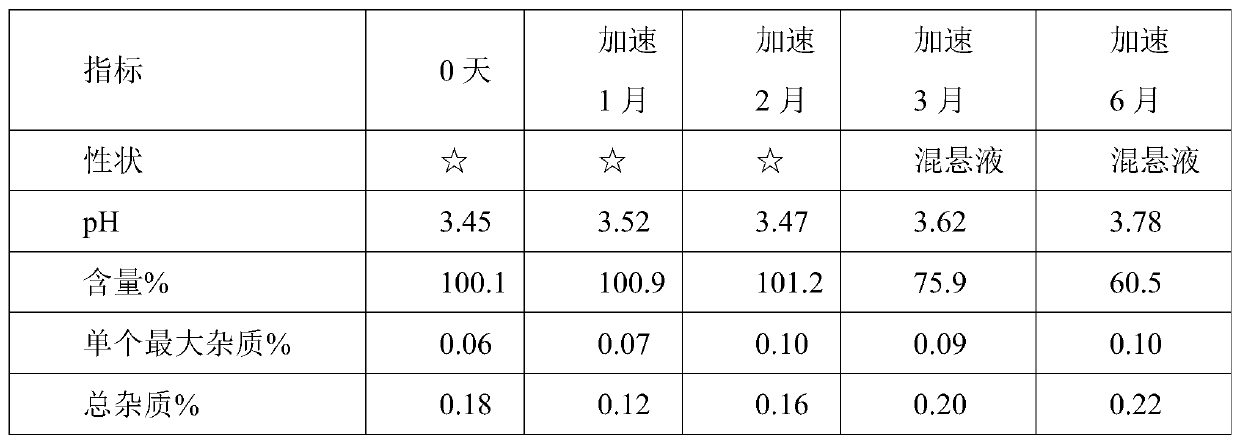
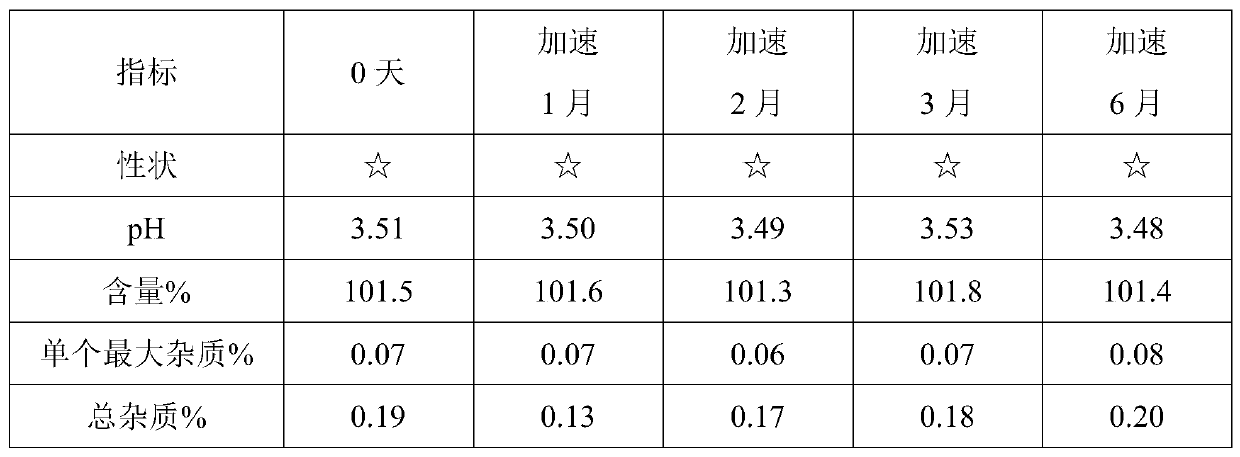
Prescription and preparation process for irinotecan hydrochloride injection
InactiveCN103099779AImprove sterilization effectEffective controlOrganic active ingredientsPharmaceutical delivery mechanismWater bathsFiltration
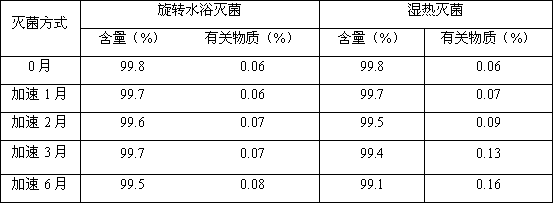
The invention provides a prescription and a preparation process for an irinotecan hydrochloride injection. The preparation process comprises the following steps: weighing and re-checking; preparation of a soup; removal of bacteria through filtration; sterile filling; and terminal sterilization. The invention is characterized in that since the process employs rotating water-bath for terminal sterilization, fast production is realized, the amount of related substances can be effectively controlled, the period of validity is prolonged and irinotecan hydrochloride in the injection is allowed to be more stable.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG +1
Preparation method of irinotecan hydrochloride injection
PendingCN109908077ASolve the precipitation problemImprove product qualityOrganic active ingredientsDigestive systemMedicineRoom temperature
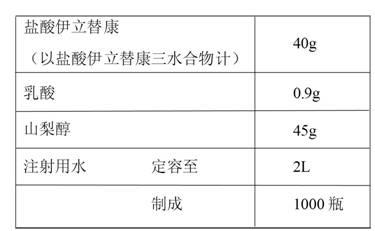
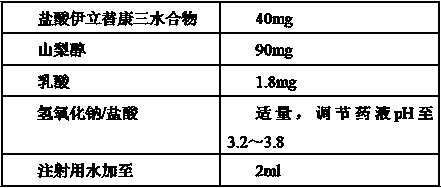
The invention provides a preparation method of an irinotecan hydrochloride injection. Each ml of the injection contains 20mg of irinotecan hydrochloride, 45mg of sorbitol, 0.9mg of lactic acid and thebalance of pH regulator and injection water. The preparation method includes: a), taking 50-70% of total volume of the injection water at room temperature, sequentially adding lactic acid, sorbitol and irinotecan hydrochloride of prescription volume sequentially, stirring, heating to 70-80 DEG C, and cooling to the room temperature after irinotecan hydrochloride is fully stirred and dissolved; b), using the pH regulator to adjust pH of medicine liquid to 3.0-4.0 (3.5 preferably), supplementing the injection water, and fixing volume to the total volume; c), filtering, filling, plugging and capping, sterilizing, and packaging to obtain the injection. Compared with the prior art, the preparation method has the advantages that the problem of separation-out of irinotecan hydrochloride in a solution is solved effectively, the injection is reliable in quality, and the preparation method is simple, convenient and suitable for industrial mass production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Irinotecan hydrochloride lipidosome composition and preparation method thereof
ActiveCN105982857ADoes not affect encapsulation rateNo additional high temperature processing timeOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolAdditive ingredient
The invention provides an irinotecan hydrochloride lipidosome composition, which consists of the following ingredients in parts by weight: 1 part of irinotecan hydrochloride, 2-6 parts of hydrogenated soybean phosphatide, 1.0-2.25 -parts of cholesterol and 0.04-0.20 parts of distearoyl phosphoethanolamine-polyethylene glycol 2000. The invention also provides a preparation method of the lipidosome composition. The lipidosome disclosed by the invention significantly reduces cost, and the lipidosome is good in stability,low in content of impurities, high in safety and easy for industrial mass production.
Owner:HUNAN KELUN PHARM RES CO LTD +1
Irinotecan hydrochloride lipid nanoparticles injection
InactiveCN102697720AProtection formProtection stabilityOrganic active ingredientsPowder deliveryPolyethylene glycolOrganosolv
The invention discloses an irinotecan hydrochloride lipid nanoparticles injection and a preparation method thereof. Mixed phase is formed by dissolving irinotecan hydrochloride and stearic acid in organic solvent and dissolving lecithin in buffer salt solution; aqueous solution of PEG (polyethylene glycol) 400, glycerol and trehalose is adopted as aqueous phase; and then irinotecan hydrochloride is loaded in lipid nanoparticles by combining stirring emulsification and high-pressure emulsion homogenizing to obtain the irinotecan hydrochloride lipid nanoparticles injection. The inventive lipid nanoparticles preparation has the advantages of high drug loading rate, uniform particle size, long drug retention time in blood circulation, better sustained-release and controlled-release effect, simple preparation method and device, easy operation, improved product quality, reduced toxic and side effects, and suitability for industrialized production.
Owner:灵康药业集团股份有限公司
Preparation method of irinotecan hydrochloride
ActiveCN106632368AGood split effectLow isomer contentOptically-active compound separationOrganic racemisationOrganic acidChiral amine
The invention discloses a preparation method of irinotecan hydrochloride. The preparation method comprises following steps: 1, chemical raw materials of racemic indolizine derivatives are taken as initial raw materials, ring closing reaction and alkali ring-opening reaction are carried out so as to obtain an organic acid intermediate; 2, a chiral amine and the organic acid intermediate are subjected to salt forming resolution so as to obtain a chiral intermediate; 3, the chiral intermediate is subjected to acid ring-opening reaction so as to remove the chiral amine; 4, esterification with a piperidine derivative, and salt forming are carried out so as to obtain the target product irinotecan hydrochloride. The preparation method is used for total synthesis of irinotecan hydrochloride, large scale production is realized, reaction conditions are mild, the preparation method is easy to control, product purity is 98% or higher, and the largest individual impurity content is lower than 0.1%.
Owner:SHANGHAI JINHE BIO TECH
Preparation method of irinotecan hydrochloride
InactiveCN109796462AReduce manufacturing costAvoid the disadvantages of separation and purificationOrganic chemistryMethyl carbonateSynthesis methods
The invention relates to a preparation method of irinotecan hydrochloride. The method comprises the steps that a mother ring reacts with propionaldehyde by taking camptothecin as a raw material to generate 7-ethylcamptothecin, and then hydrogen peroxide is used for oxidization to generate N-oxide-7-ethylcamptothecin; illumination rearrangement is carried out to generate 7-ethyl-10-hydroxycamptothecine; 4-piperidinopiperidine reacts with dimethyl carbonate to generate 4-piperidinopiperidine methyl carbonate; 7-ethyl-10-hydroxycamptothecine reacts with 4-piperidinopiperidine methyl carbonate togenerate irinotecan monomer; the irinotecan monomer and hydrochloric acid are salified to obtain an irinotecan hydrochloride finished product. Compared with the prior art, in the reaction process, phosgene, chloroform and other toxic substances are prevented from being used, and the irinotecan hydrochloride has the advantages of being safe, convenient to use, little in pollution and the like during production. Besides, the synthesis method avoids the defect that a chromatographic column is needed for separation and purification in the prior art, the production cost of irinotecan is reduced, and huge economic benefits are achieved.
Owner:CISEN PHARMA
Method for synthesizing camptothecin serving as active intermediate of anti-tumor medicament
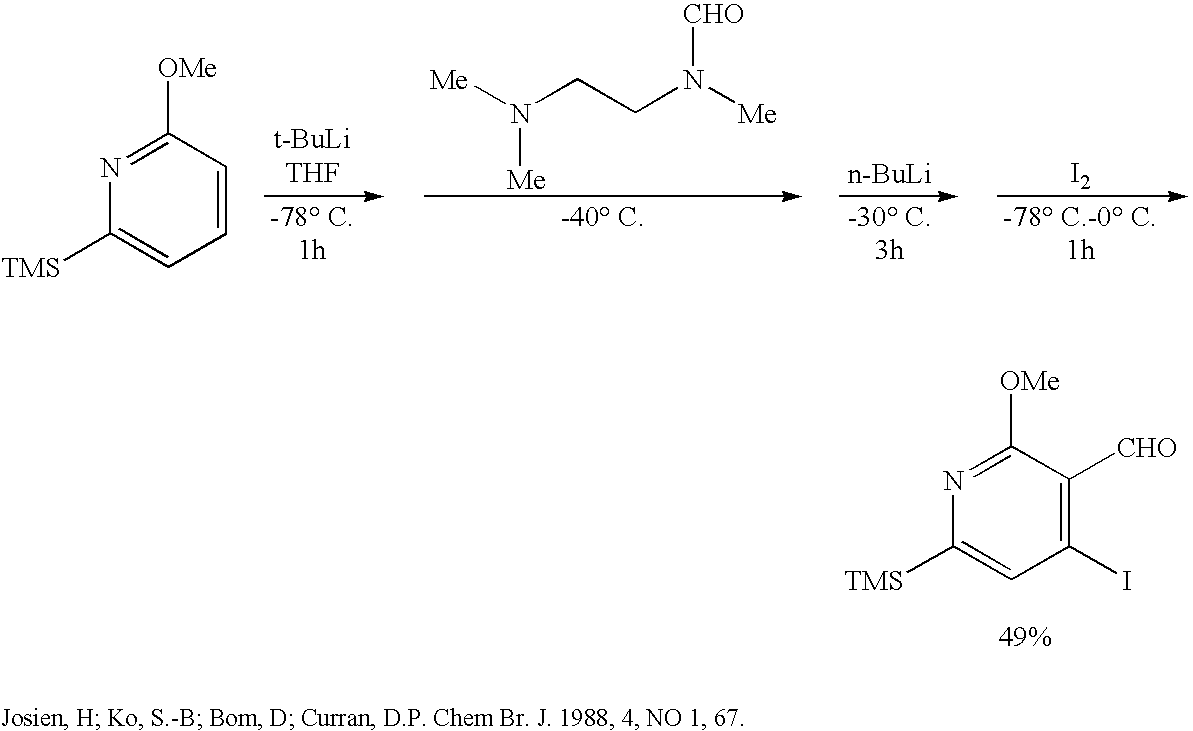
InactiveCN103265551ASimple and fast operationImprove efficiencyOrganic chemistryAbnormal tissue growthCamptothecin derivative
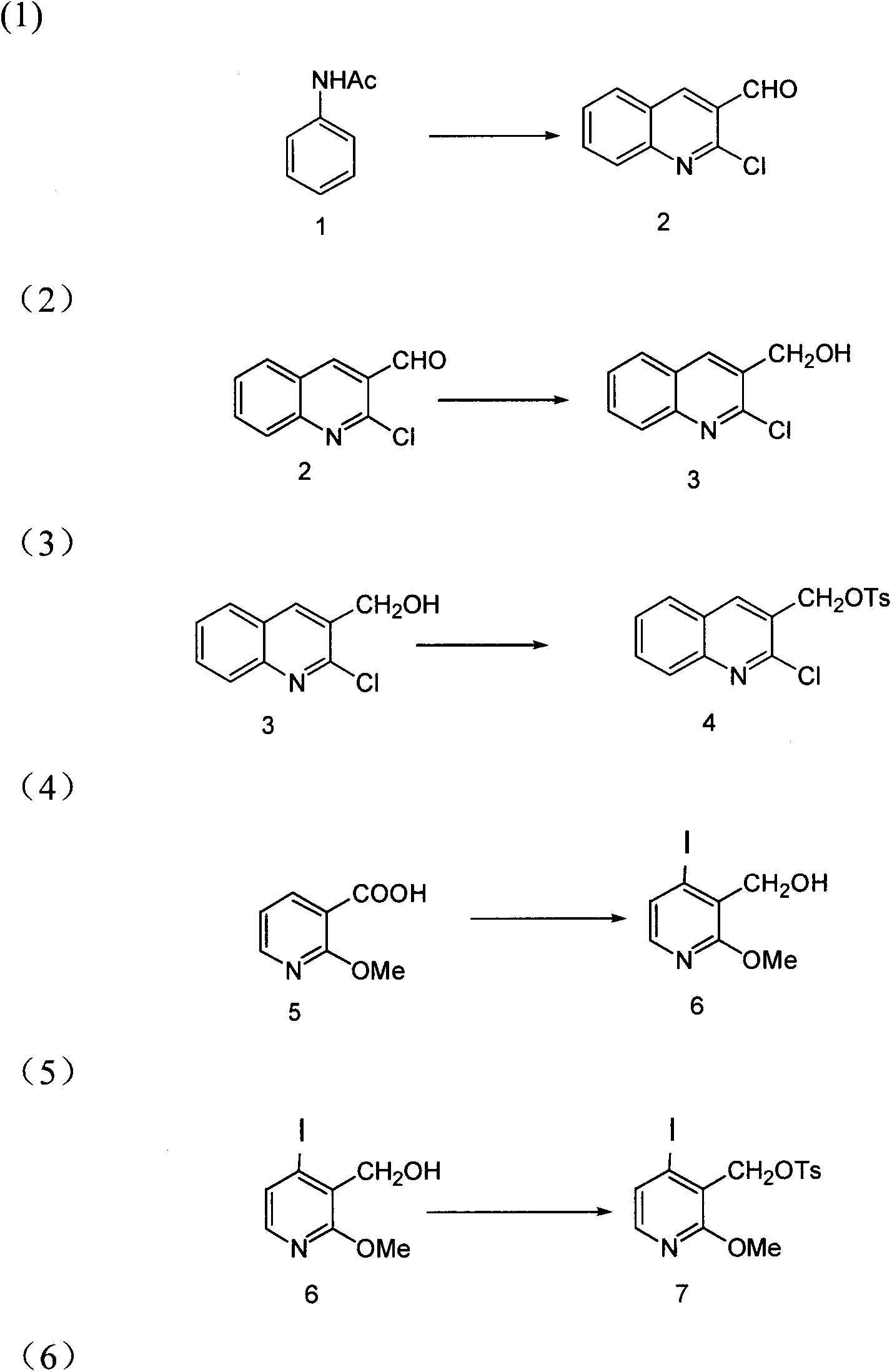
The invention discloses a novel synthetic method for camptothecin serving as an intermediate of anti-tumor medicament irinotecan hydrochloride and various functional medicament camptothecin derivatives. According to the technical scheme, the method mainly comprises the following steps of: (1) obtaining a compound 2 from a compound 1; (2) obtaining a compound 3 from the compound 2; (3) obtaining a compound 4 from the compound 3; (4) obtaining a compound 6 from a compound 5; (5) obtaining a compound 7 from the compound 6; (6) obtaining a compound 8 from the compound 7; (7) obtaining a compound 9 from the compound 8; (8) obtaining a compound 10 from the compounds 4 and 9; and (9) obtaining a compound 11 from the compound 10, namely (20S)-camptothecin. By adopting the technical scheme, the method can be used for efficiently completing full synthesis of the camptothecin with short route based on simple commercial raw materials by using common reagents and simple operation.
Owner:NANJING YIBEIJIA TECH
Method for preparing irinotecan hydrochloride trihydrate pure product
The invention relates to a method for preparing an irinotecan hydrochloride trihydrate pure product, which belongs to the field of medicinal chemistry. The method comprises the steps of first preparing dissociative 4-piperidyl piperidine formyl chloride, performing condensation reaction of the 4-piperidyl piperidine formyl chloride and 7-ethyl-10-hydroxycamptothecine with the presence of 4-dimethylamino piperidine or 4-dimethylamino pyridinium or analogues, and finally performing salifying to obtain an end-product. By means of the method, fetid and easily-discoloring piperidine participated condensation reaction is avoided, side reaction is decreased, the defects including increased period, large solvent dosage and the like caused by the fact that the product is purified through column chromatography are overcome, the purity and the yield of the product are improved, simultaneously color and luster of the product are improved, and the scale production is easy to achieve.
Owner:JIANGSU HONGDOUSHAN BIOLOGICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com