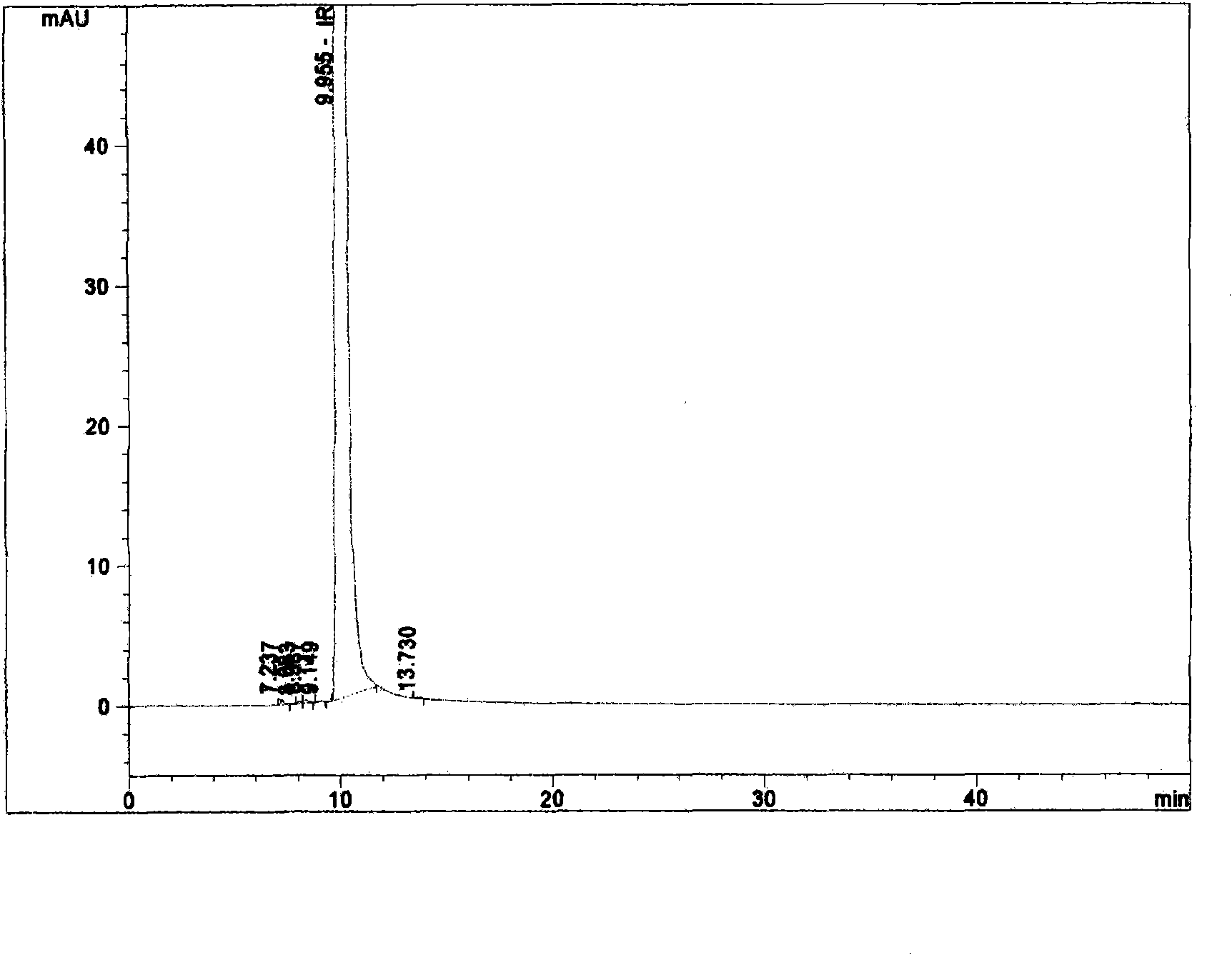
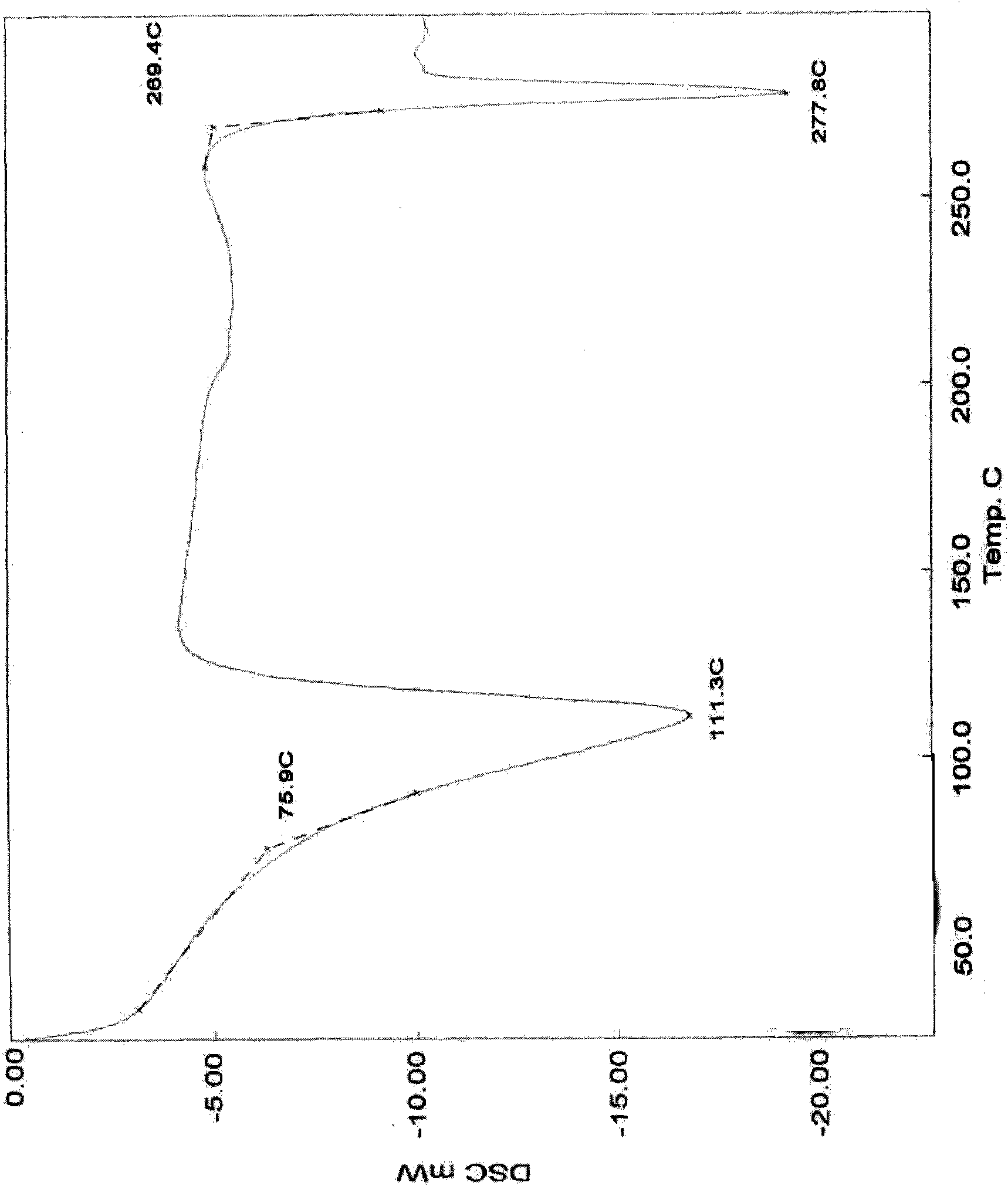
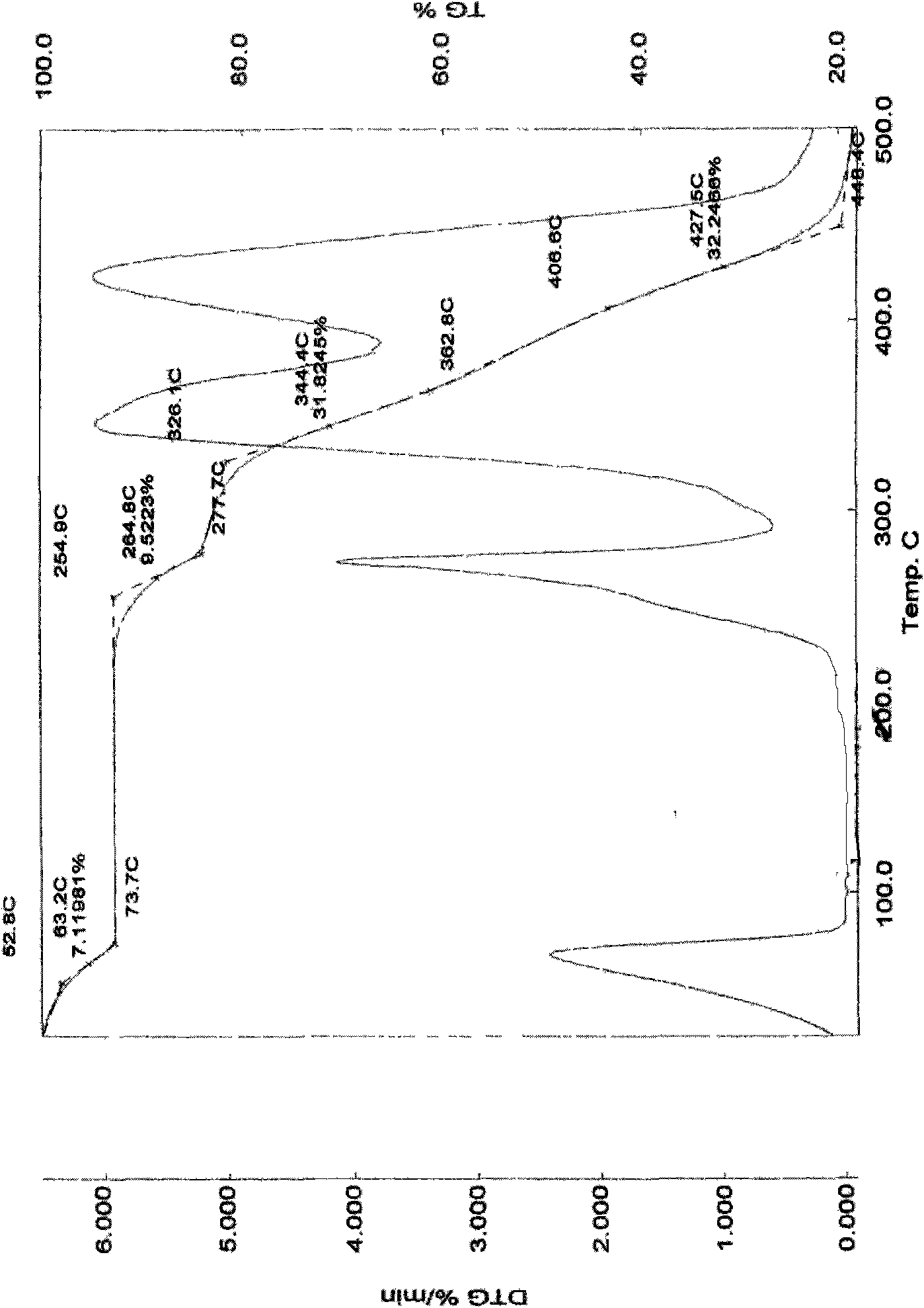
Method for purifying irinotecan hydrochloride
A technology of irinotecan hydrochloride and purification method, which is applied in the field of purification of irinotecan hydrochloride to achieve the effects of simple operation, controllable purification conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Dissolve 30 g of crude irinotecan hydrochloride in 60 ml of methanol, cool to 0°C, add 100 ml of ether, stir well, and then stand at 0°C for crystallization for 48 hours, collect the filter cake after suction filtration, and dry in vacuo to obtain 27.4 g of crude product ;
[0034] 27.4 g of the dried crude product were dissolved in 274 ml of methanol, 137 ml of ethyl acetate and 137 ml of acetonitrile were added, stirred evenly, and the pH value of the feed solution was measured to adjust to pH=2. Stand to crystallize at 0°C for 48 hours, collect the filter cake after suction filtration, and dry in vacuo to obtain 25.2 g of crude product;
[0035] Dissolve 25.2 g of the dried crude product in 252 ml of acetone-water ~ 3:1 mixed solution, stir and dissolve, then stand for crystallization at 0°C for 48 hours, collect the filter cake after suction filtration, and place the filter cake in a vacuum-dried Under reduced pressure and drying in the box, the vacuum degree was c...
Embodiment 2
[0037] Dissolve 30 grams of irinotecan hydrochloride crude product in 100 ml of isopropanol, cool to 10°C, add 150ml of petroleum ether, stir evenly, stand for crystallization at 10°C for 8 hours, collect the filter cake after suction filtration, and vacuum dry to obtain Crude product 27.9 grams;
[0038] 27.9 g of the dried crude product were dissolved in 420 ml of ethanol, 279 ml of methyl formate and 279 ml of acetonitrile were added, stirred evenly, the pH value of the feed solution was measured, and adjusted to pH=2. Stand to crystallize at 8°C for 24 hours, collect the filter cake after suction filtration, and dry in vacuo to obtain 26.3 g of crude product;
[0039] Dissolve 26.3 grams of the dried crude product in 390 ml of acetonitrile-water=3:1 mixed solution, stir evenly, and then stand for crystallization at 8°C for 24 hours, collect the filter cake after suction filtration, and place the filter cake in a vacuum-dried Under reduced pressure and drying in the box, t...
Embodiment 3
[0041] Dissolve 30 grams of crude irinotecan hydrochloride in 100ml of isobutanol, cool to 15°C, add 60ml of isopropyl ether, stir evenly, stand at 8°C for crystallization for 24 hours, collect the filter cake after suction filtration, and vacuum dry , to get 26.8 grams of crude product;
[0042] Dissolve 26.8 grams of dried crude product in 134ml of isopropanol, add 134ml of propyl propionate and 134ml of acetonitrile in turn, stir evenly, measure the pH value of the feed solution, adjust to pH=2 with methanol:hydrochloric acid=1:1 mixed solution . Stand to crystallize at 10°C for 8 hours, collect the filter cake after suction filtration, and dry in vacuo to obtain 25.9 g of crude product;
[0043]Dissolve 25.9 grams of the dried crude product in 130 ml of tetrahydrofuran-water mixed solution with a volume ratio of 3:1. After stirring and dissolving, stand for crystallization at 10° C. for 12 hours, collect the filter cake after suction filtration, and place the filter cake ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com