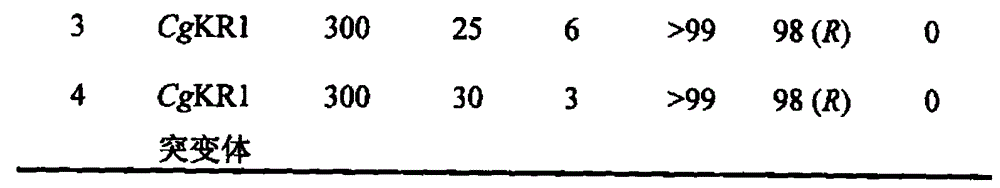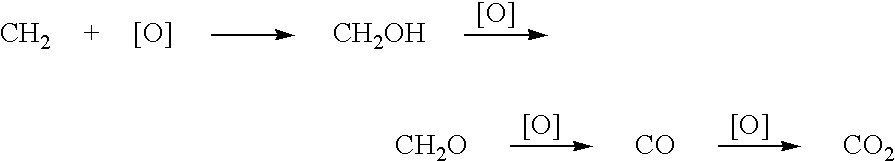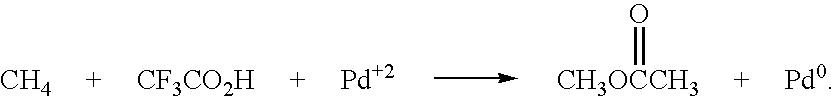Patents
Literature
816 results about "Methyl formate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
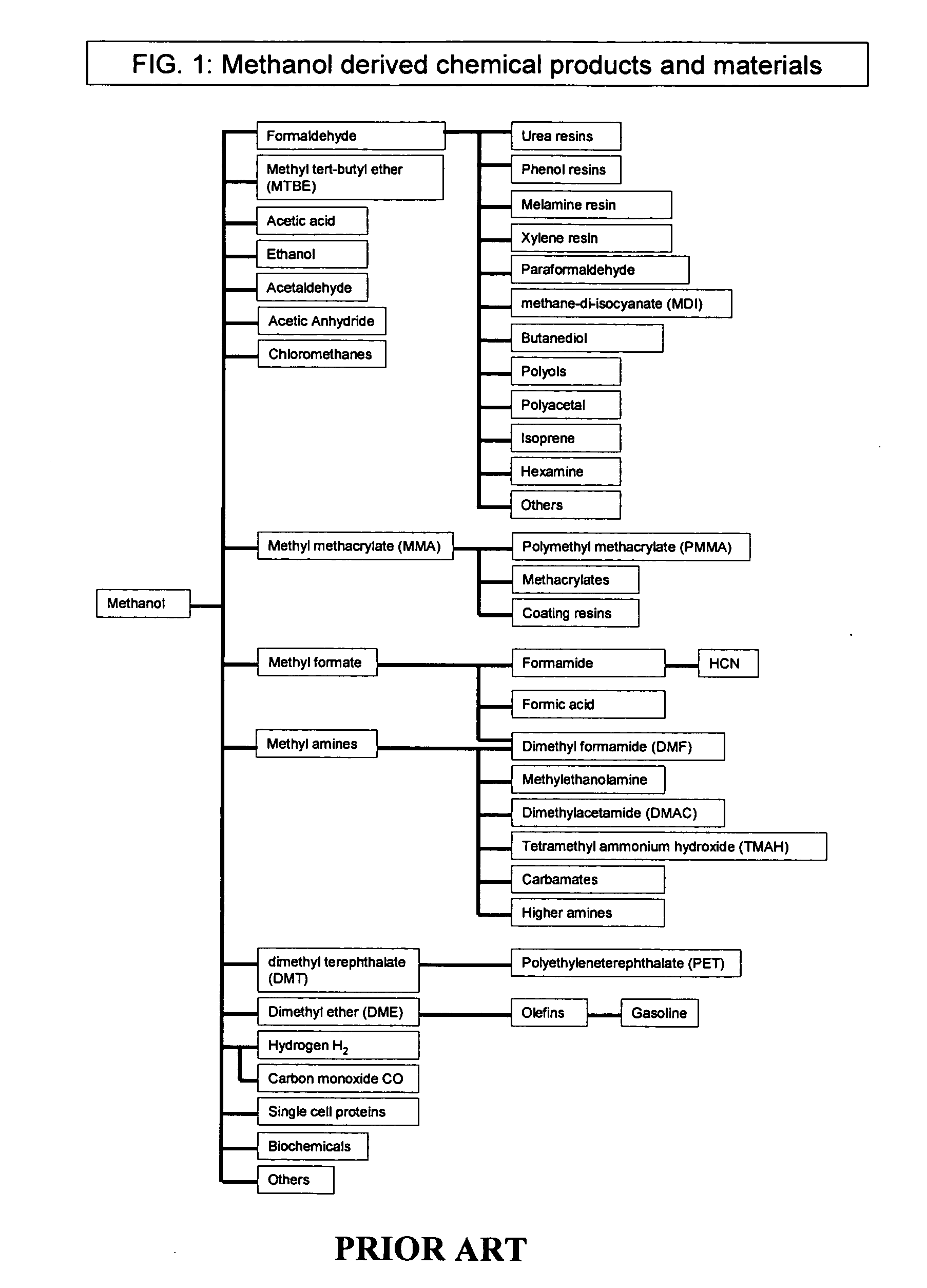
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of an ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other compounds of commercial interest.
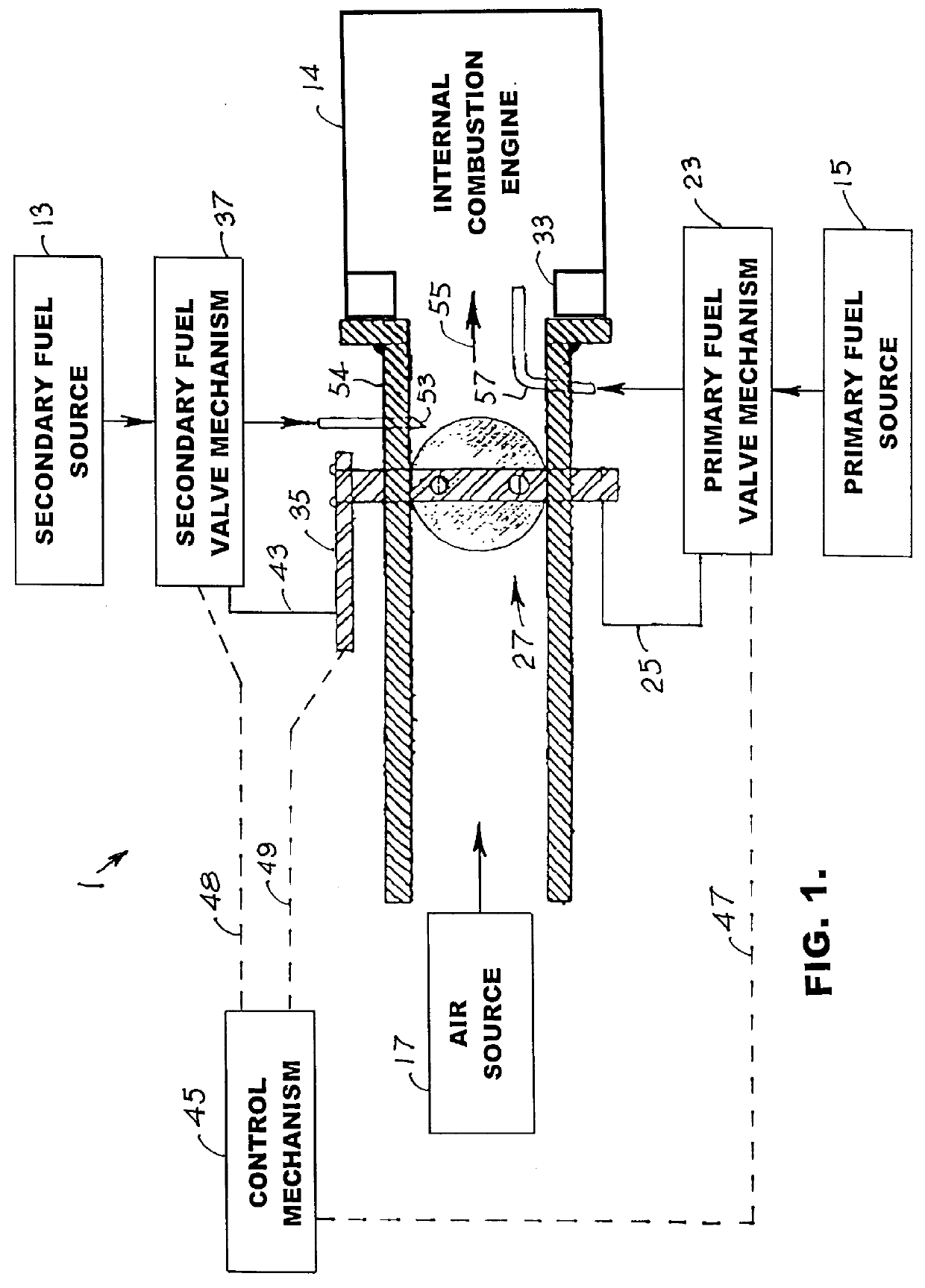
Internal combustion system using acetylene fuel
InactiveUS6076487AInternal combustion piston enginesNon-fuel substance addition to fuelCarbon chainInternal combustion engine
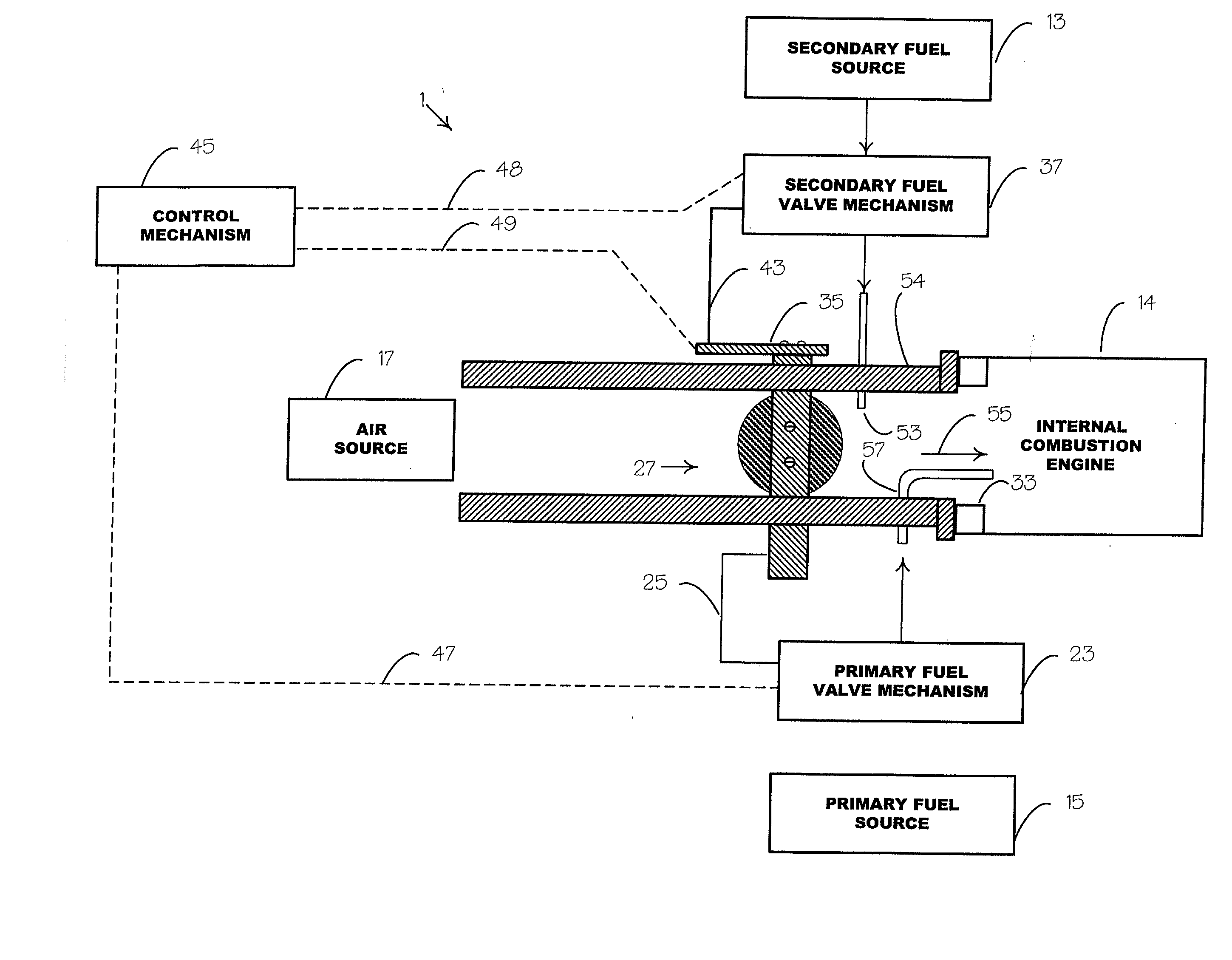
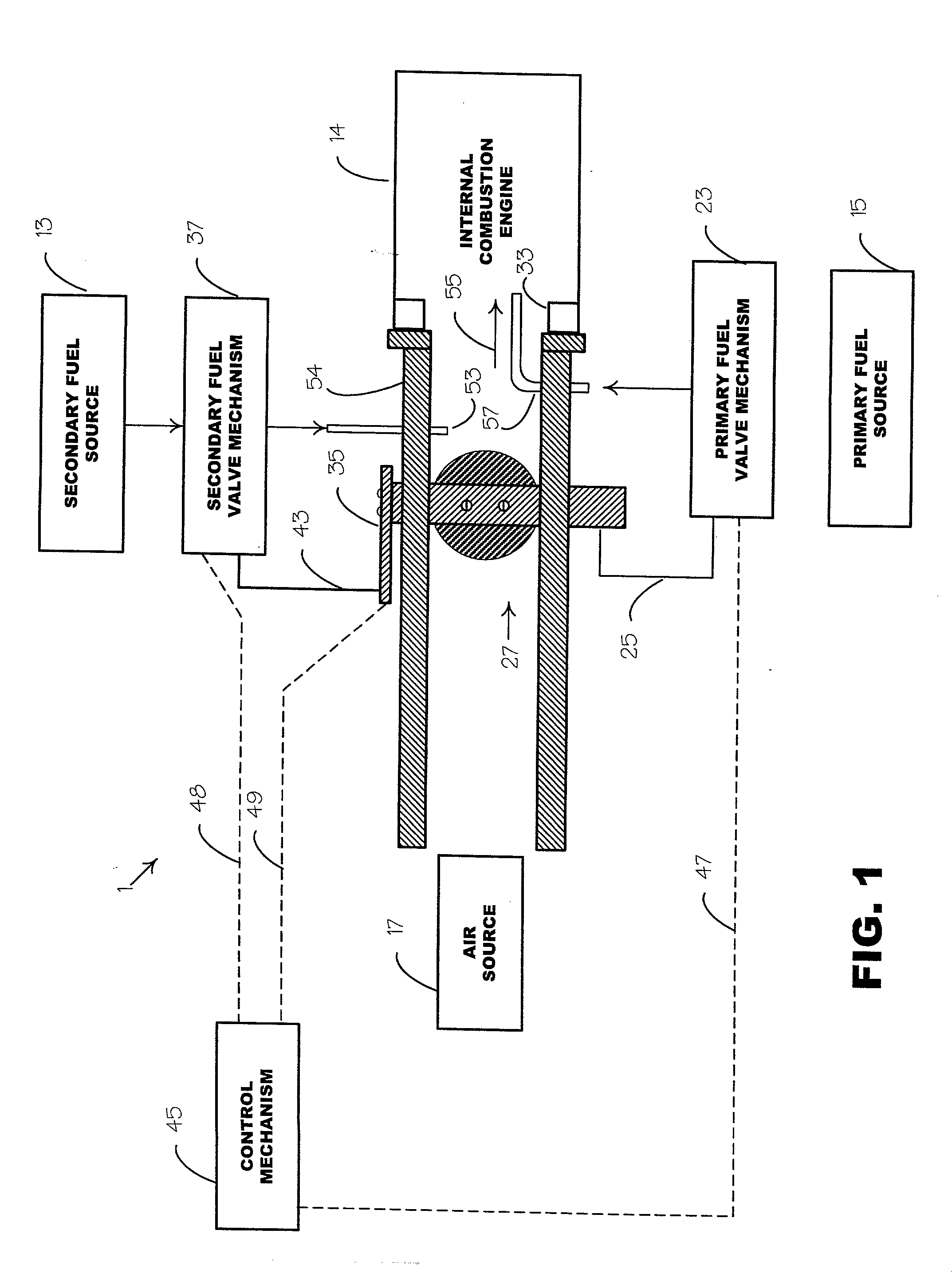
An environmentally clean dual fuel for an internal combustion engine, comprising acetylene as a primary fuel and a combustible fuel, such as one or more fluids selected from an alcohol such as ethanol, methanol or any other alcohol or alcohols from the group comprising C1-C20 carbon chains, ethers such as from the group comprising dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl t-butyl ether, t-amyl methyl ether, di-isopropyl ether and the like, low-molecular-weight esters such as from the group comprising methyl formate, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate and the like, or other suitable combustible fluid such as mineral spirits and the like, as a secondary fuel for operatively preventing early ignition and knock arising from the primary fuel. The dual fuel, internal combustion system, which generally utilizes a two-stage process for start-up and operation and can be operated with air- or liquid-cooling, is environmentally clean with hydrocarbon, CO, NOx, and SOx emissions substantially eliminated.
Owner:GOTEC
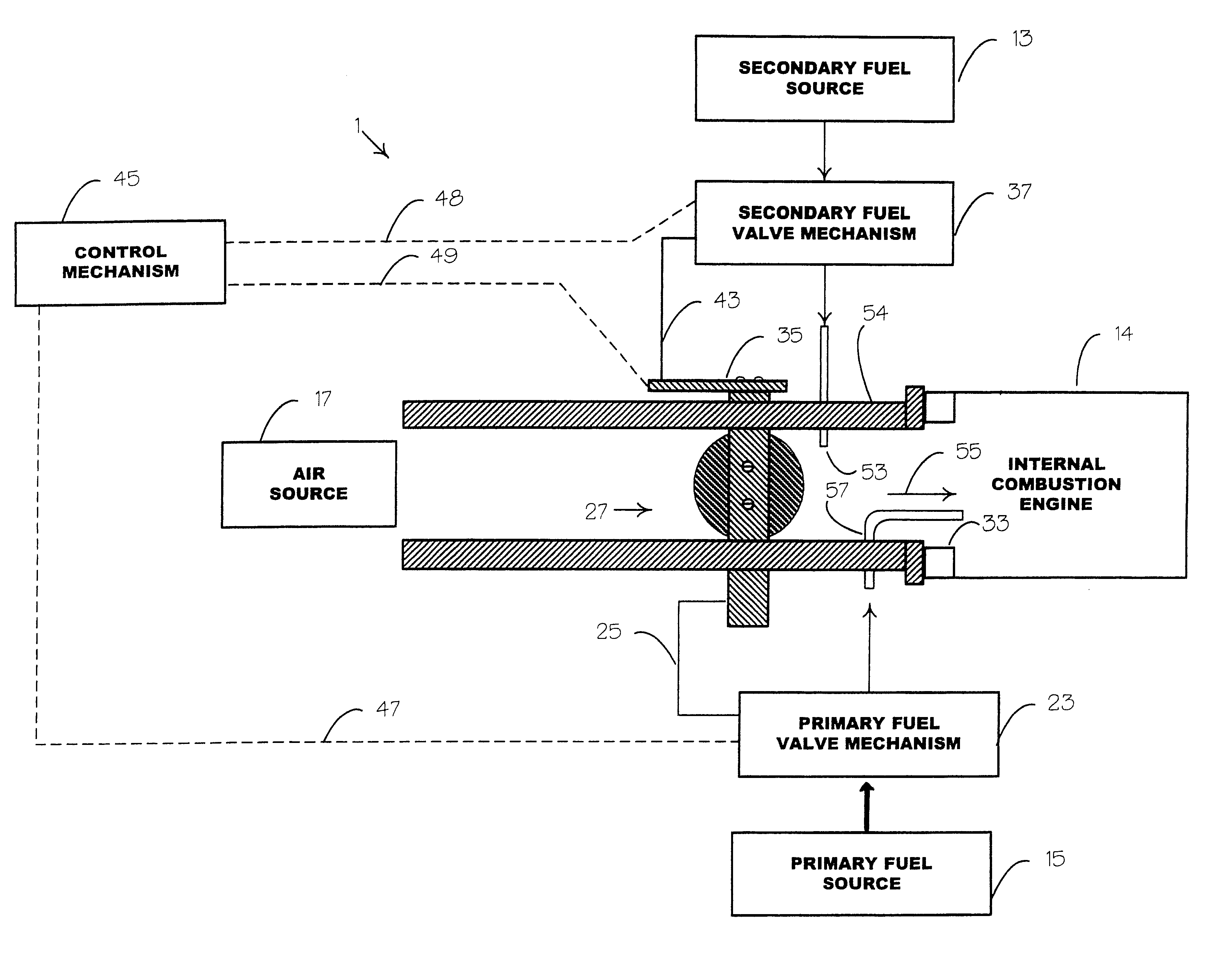
Internal combustion system adapted for use of a dual fuel composition including acetylene
InactiveUS6575147B2Easy to operateImprove performanceNon-fuel substance addition to fuelInternal combustion piston enginesCarbon chainMineral spirit
An internal combustion engine adapted to use an environmentally clean multi-fuel composition, comprising acetylene as a primary fuel and a combustible fuel, such as one or more fluids selected from an alcohol such as ethanol, methanol or any other alcohol or alcohols from the group comprising C1-C12 carbon chains, ethers such as from the group comprising dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl t-butyl ether, t-amyl methyl ether, di-isopropyl ether and the like, low-molecular-weight esters such as from the group comprising methyl formate, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate, ethyl malate, butyl malate, and the like, or other suitable combustible fluid such as mineral spirits and the like, as a secondary fuel for operatively preventing early ignition and knock arising from the primary fuel.
Owner:GOTEC INC
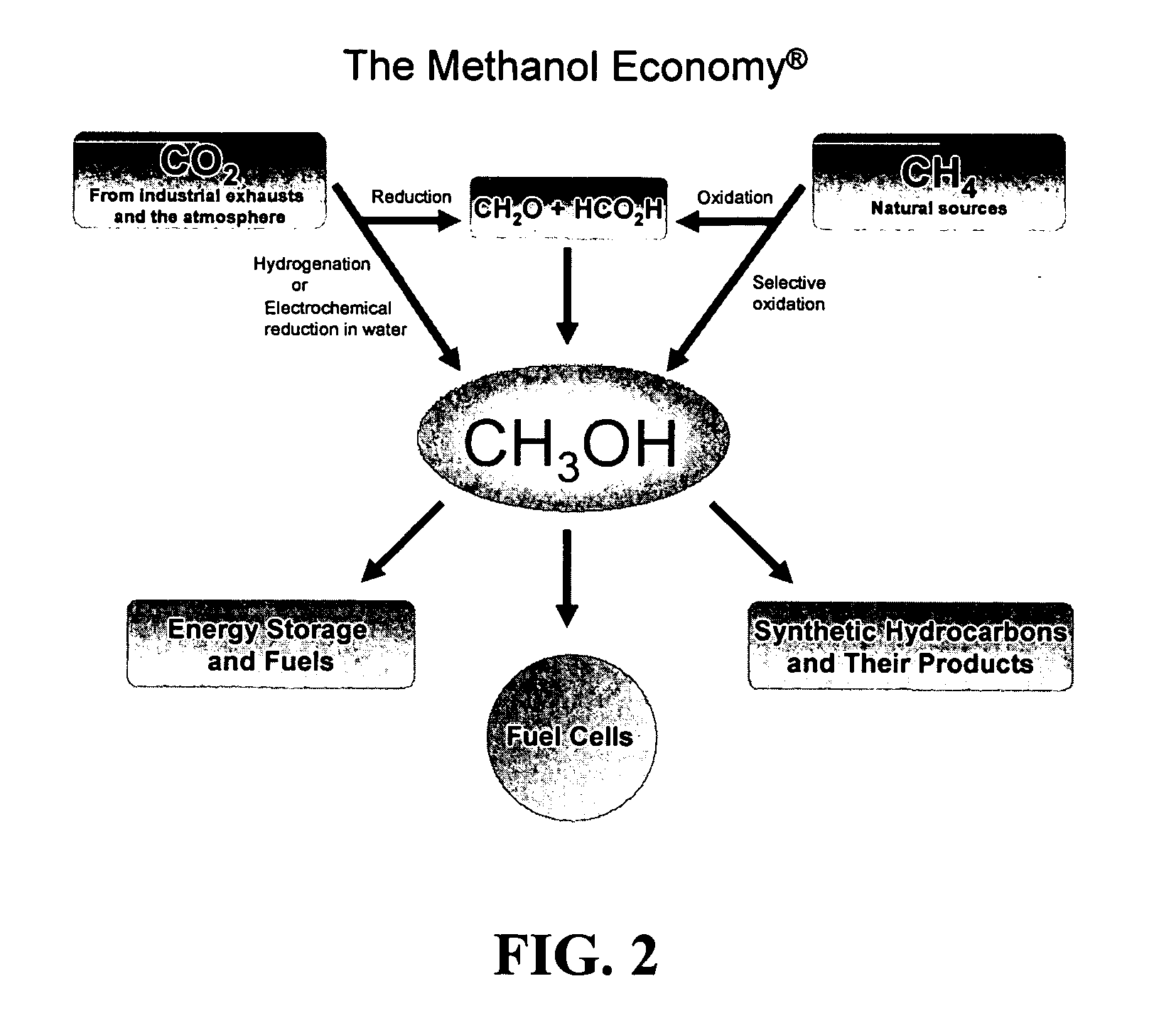
Selective oxidative conversion of methane to methanol, dimethyl ether and derived products
ActiveUS20060235088A1Minimize or eliminate the disadvantages or dangers inherentPreparation by oxidation reactionsElectrolysis componentsFormate EstersDimethyl ether
The present invention relates to a method of producing methanol from a methane source by oxidizing methane under conditions sufficient to a mixture of methanol and formaldehyde while minimizing the formation of formic acid and carbon dioxide. The oxidation step is followed by treatment step in which formaldehyde is converted into methanol and formic acid which itself can further be converted into methanol via catalytic hydrogenation of intermediately formed methyl formate.
Owner:UNIV OF SOUTHERN CALIFORNIA
Novel forms of cddo methyl ester
A triterpenoid compound, methyl 2-cyano-3,12-dioxoleana-1,9(11)-dien-28-oate (CDDO methyl ester), has a non-crystalline, glassy solid form and a non-hydrous crystalline form that can prepared, for example, from a saturated methanol solution. The glassy form displays an enhanced bioavailability over the non-hydrous crystalline form. Each form of CDDO methyl ester is a superior candidate for use, typically in solid dosage form, for treating a variety of disease states, generally associated with inflammation.
Owner:REATA PHARM HLDG LLC
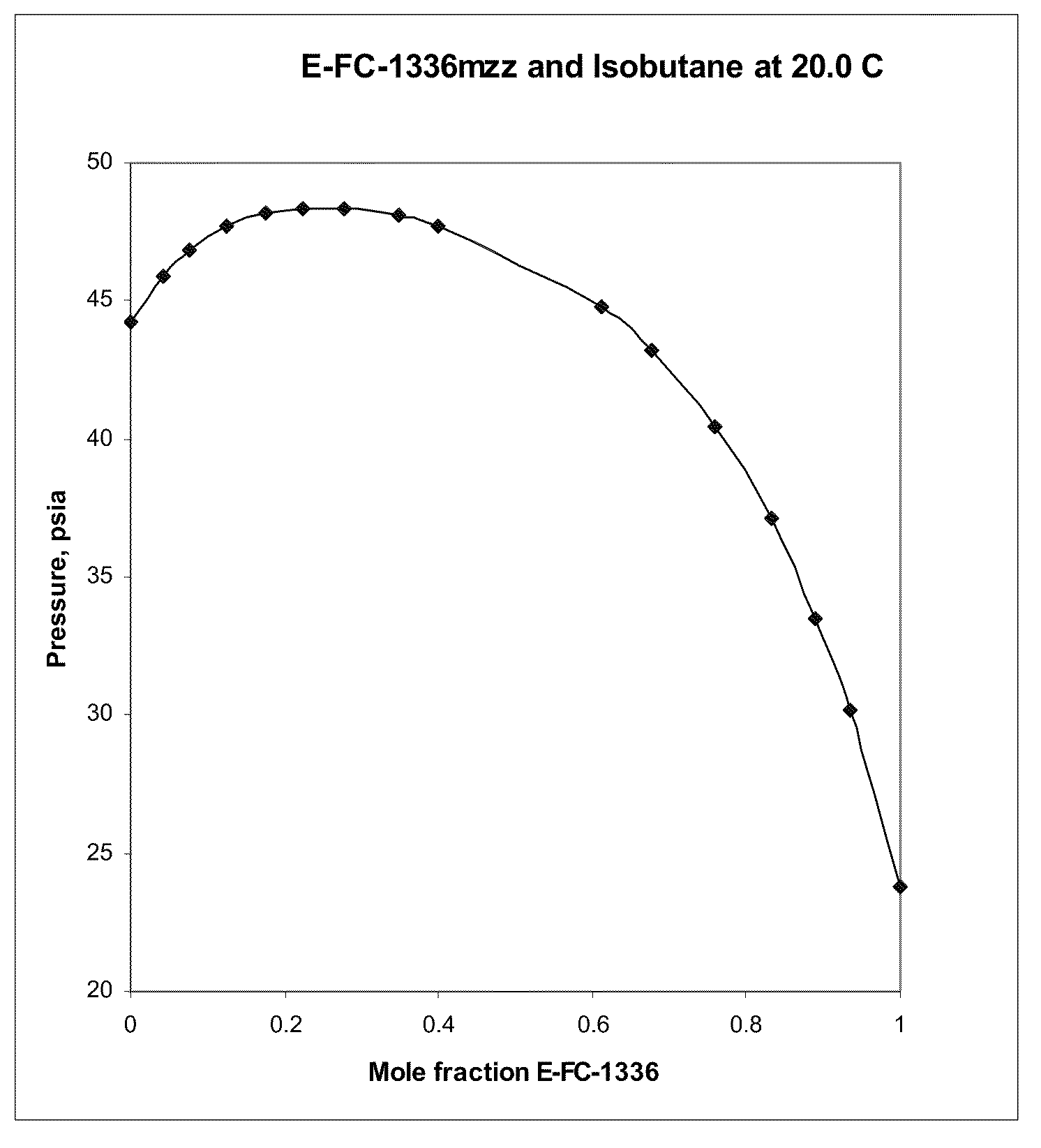
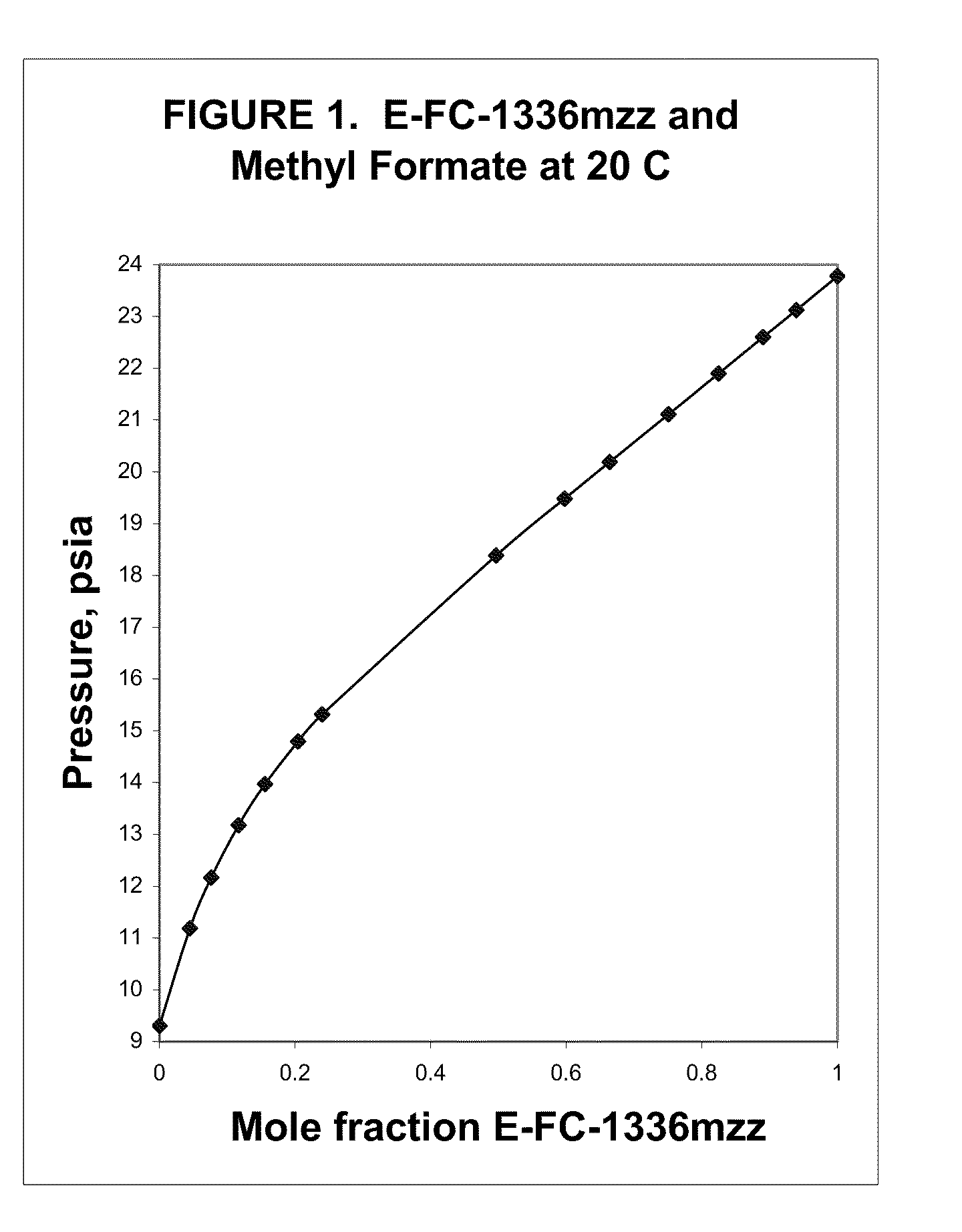
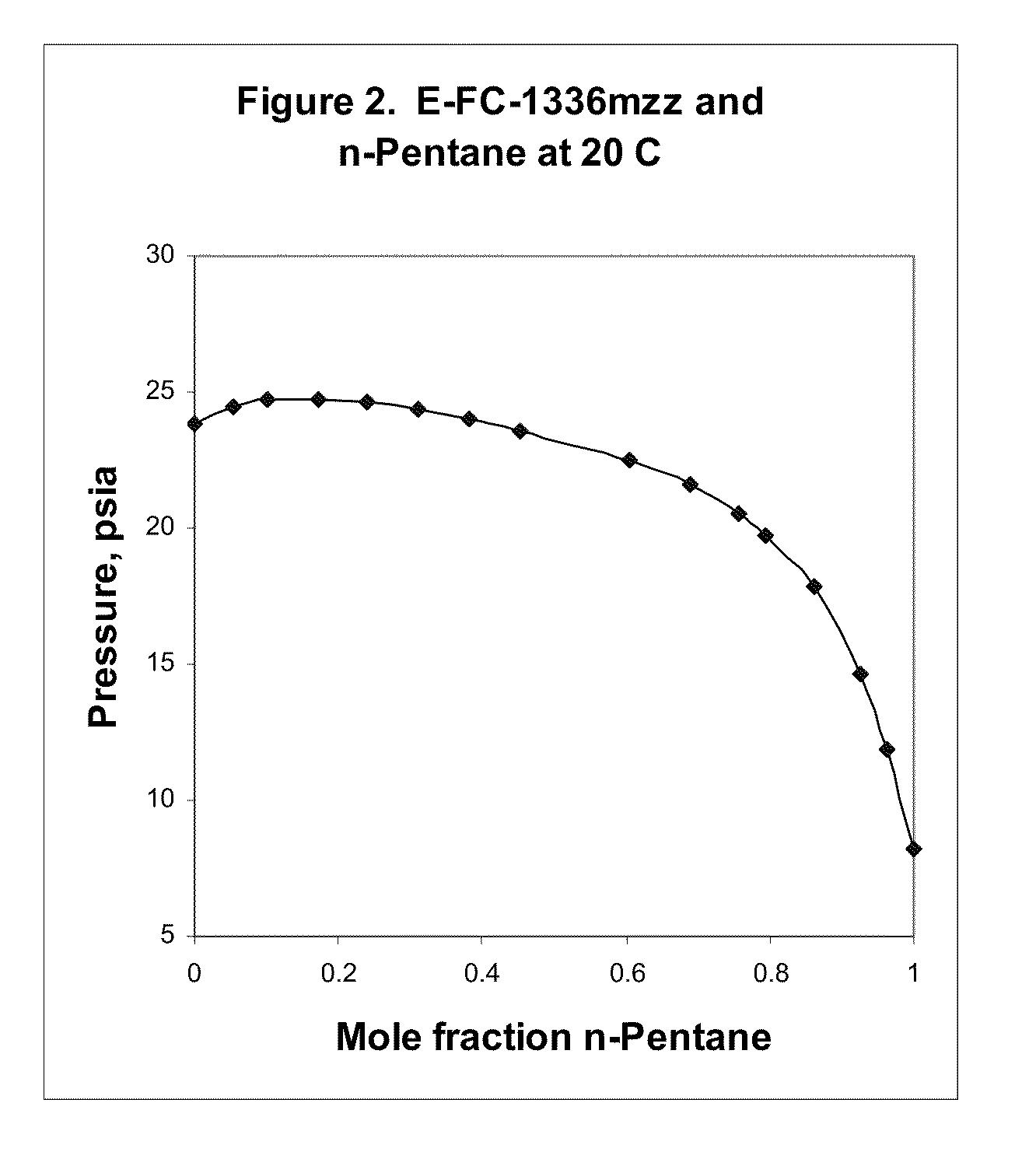
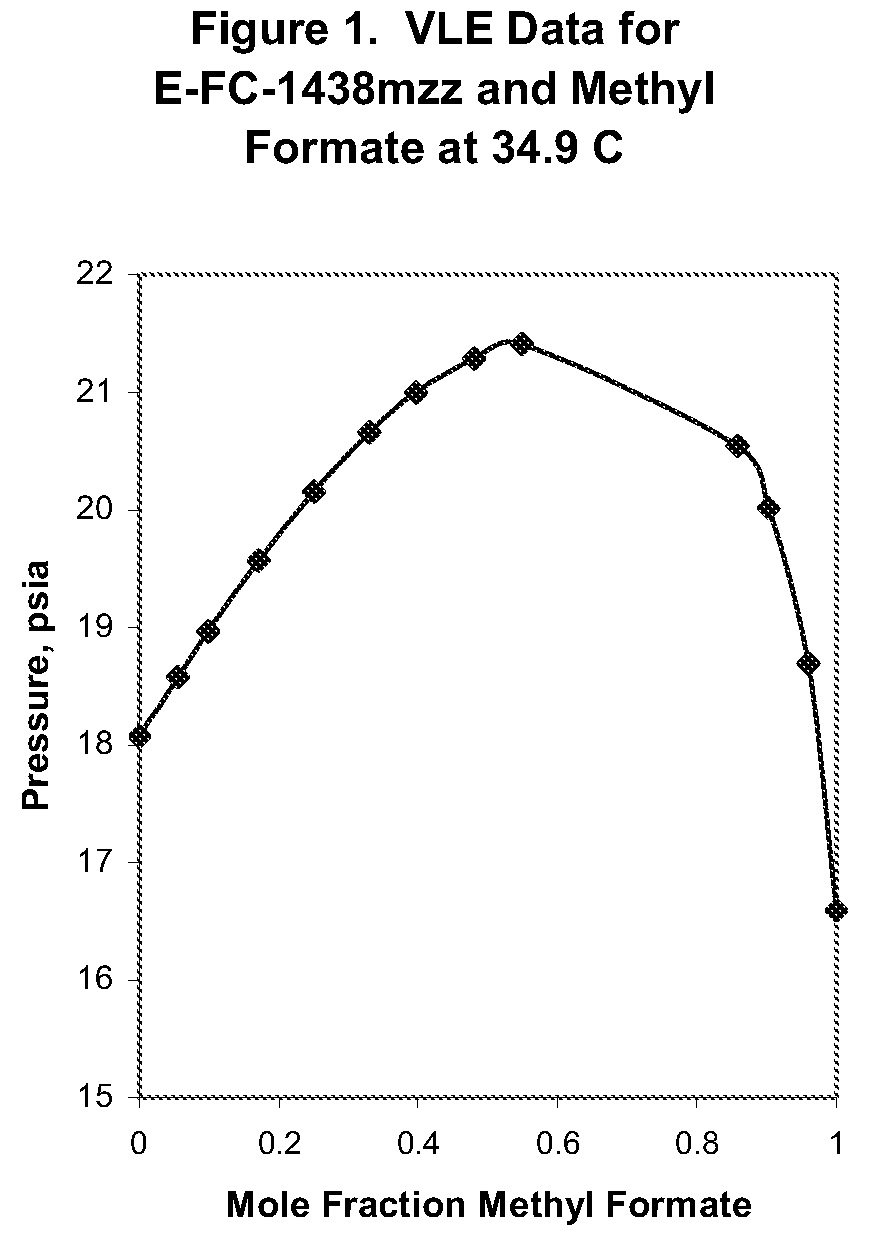
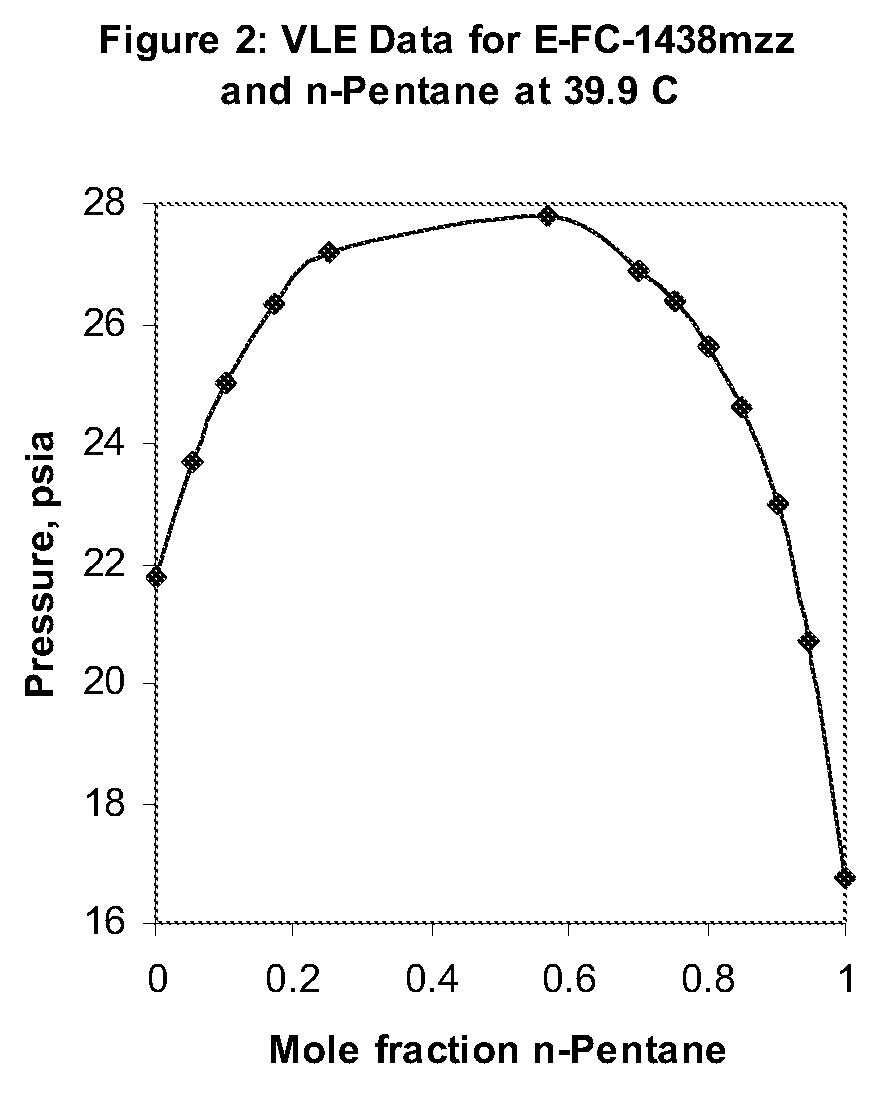
Azeotropic and azeotrope-like compositions of e-1,1,1,4,4,4-hexafluoro-2-butene
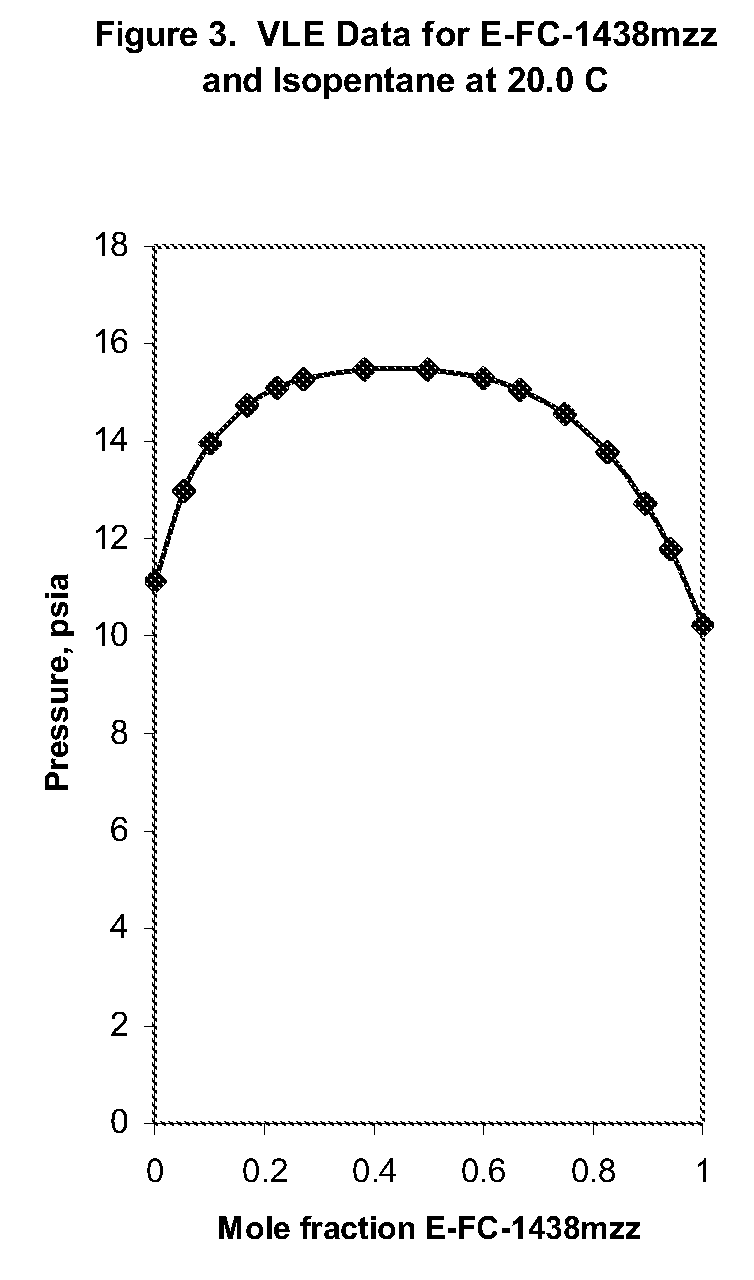
Azeotropic or azeotrope-like compositions are disclosed. The azeotropic or azeotrope-like compositions are mixtures of E-1,1,1,4,4,4-hexafluoro-2-butene with methyl formate, n-pentane, 2-methylbutane trans-1,2-dichloroethylene, 1,1,1,3,3-pentafluoropropane, n-butane or isobutane. Also disclosed is a process of preparing a thermoplastic or thermoset foam by using such azeotropic or azeotrope-like compositions as blowing agents. Also disclosed is a process of producing refrigeration by using such azeotropic or azeotrope-like compositions. Also disclosed is a process of using such azeotropic or azeotrope-like compositions as solvents. Also disclosed is a process of producing an aerosol product by using such azeotropic or azeotrope-like compositions. Also disclosed is a process of using such azeotropic or azeotrope-like compositions as heat transfer media. Also disclosed is a process of extinguishing or suppressing a fire by using such azeotropic or azeotrope-like compositions. Also disclosed is a process of using such azeotropic or azeotrope-like compositions as dielectrics.
Owner:THE CHEMOURS CO FC LLC
Fluoropropene compounds and compositions and methods using same
InactiveUS20110037016A1Reduce negative impactImprove environmental desirabilitySolvent extractionOther chemical processesHydrocotyle bowlesioidesKetone
Various uses of fluorinated olefin having an MRI value of less than ethane, in combination with one or more other components, including other fluoroalkenes, hydrocarbons; hydrofluorocarbons (HFCs), ethers, alcohols, aldehydes, ketones, methyl formate, formic acid, water, trans-1,2-dichloroethylene, carbon dioxide and combinations of any two or more of these, in a variety of applications, including as blowing agents, are disclosed.
Owner:HONEYWELL INT INC
Synthetic technology for pyraclostrobin
ActiveCN104211641AFormation reaction is easy to controlSmooth responseOrganic chemistryMethylanilineChlorobenzene
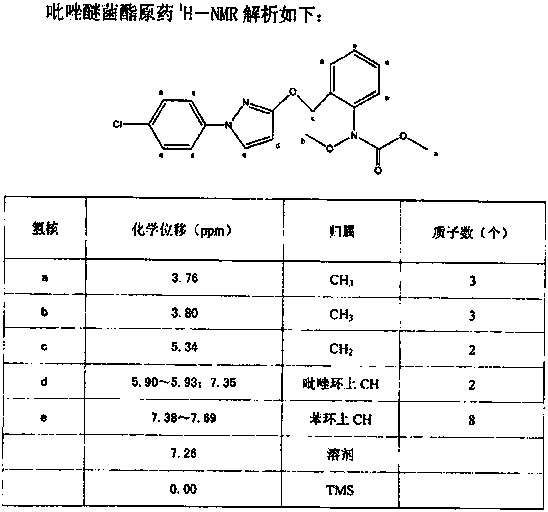
The invention concretely relates to a synthetic technology for pyraclostrobin. The synthetic technology comprises: firstly performing cyclization to obtain 1-(4-chlorophenyl)-pyrazol-3-one, oxidizing the pyrazol ring under the effect of an oxidant to generate 1-(4-chlorophenyl)-3-hydroxypyrazole, then using 2-nitrobenzyl bromide to performing etherification to generate 1-(4-chlorophenyl)-3-[2-(nitrophenyl)methoxy]-1H-pyrazole, then using a reducing agent to perform nitro reducing, so as to generate N-hydroxyl-2-[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]aniline, then using ClCO2CH3 to perform N-acylation reaction to generate methyl N-hydroxyl-N-2-{[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]phenyl}formate, and finally performing hydroxyl methylation under an alkaline condition to generate pyraclostrobin. The technology enables all operations in the pyraclostrobin preparation process to be relatively controllable, helps to improve the stability of the preparation process and improve the product yield, successfully employs low-cost reagents and substantially reduces production cost, and also the employed reagents are relatively small in toxicity, is relatively beneficial for environment protection, and has no corrosivity on plastic pipes, so that the production safety is improved.
Owner:SHANDONG KANGQIAO BIO TECH CO LTD
Formation method of lithium ion secondary battery
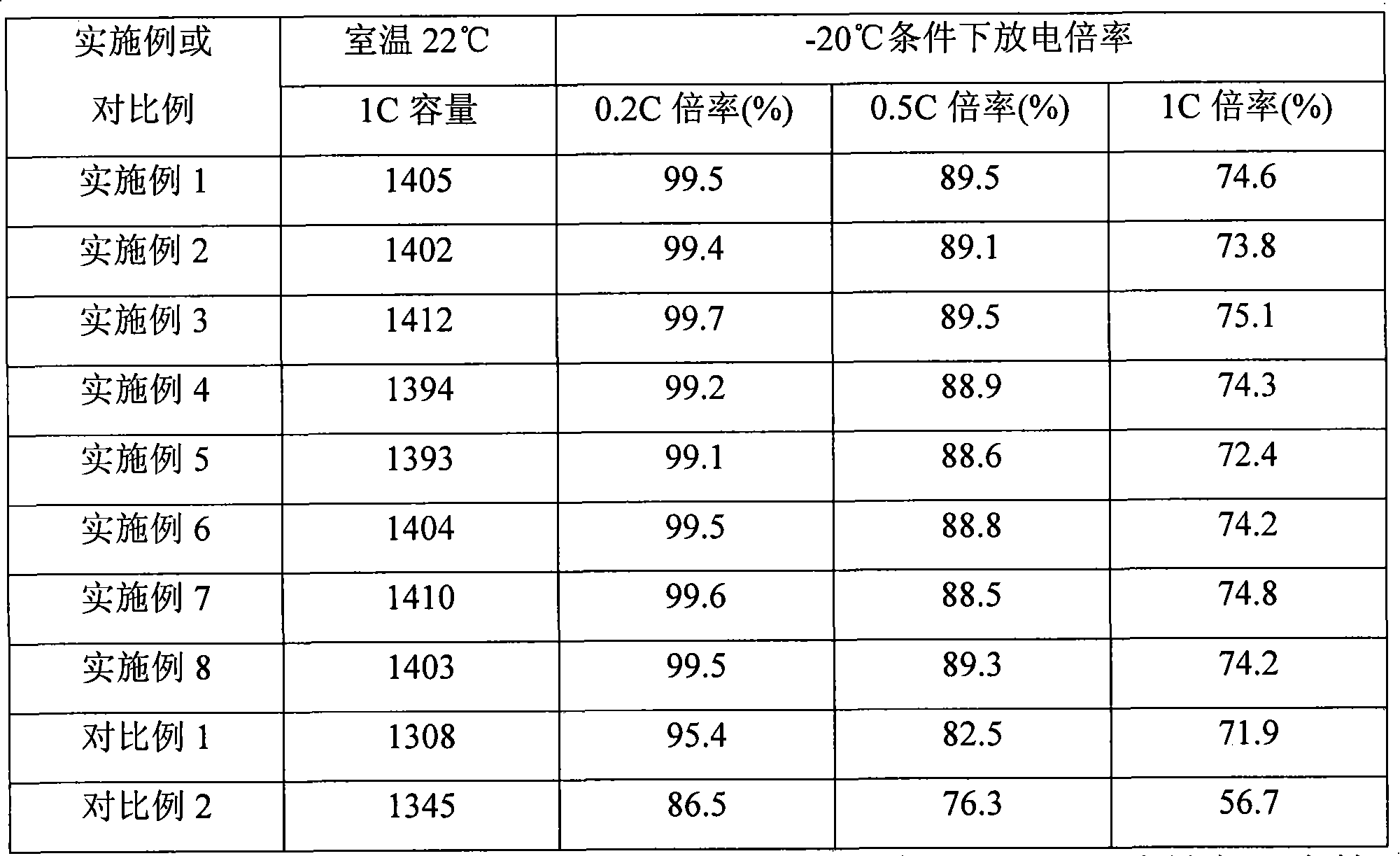
InactiveCN101640285AImprove electrochemical performanceGood electrical conductivitySecondary cells charging/dischargingMethyl formateMethyl acetate
The invention relates to a formation method of a lithium ion secondary battery. The method comprises the following steps: performing a first formation on a battery containing a first electrolytic solution; and then injecting a second electrolytic solution into a battery container after the first formation to perform a second formation, wherein the first electrolytic solution contains an electrolyte and a first solvent which is mixture of a solvent A and a solvent B; the solvent A is ethylene carbonate, and the solvent B is one or more selected from dimethyl carbonate, diethyl carbonate, methylethyl carbonate, dimethyl sulfite and diethyl sulfite; and the second electrolytic solution contains electrolyte and a second solvent, and the second solvent is one or more selected from propylene carbonate, butylene carbonate, dimethoxyethane, gamma-butyrolactone, methyl formate, ethyl formate, methyl acetate, ethyl acetate, ethyl propionate, ethyl butyrate and propyl butyrate. The lithium ion secondary battery obtained by the method has excellent cycle performance and low-temperature multiplying factor discharge performance.
Owner:SHANGHAI BYD
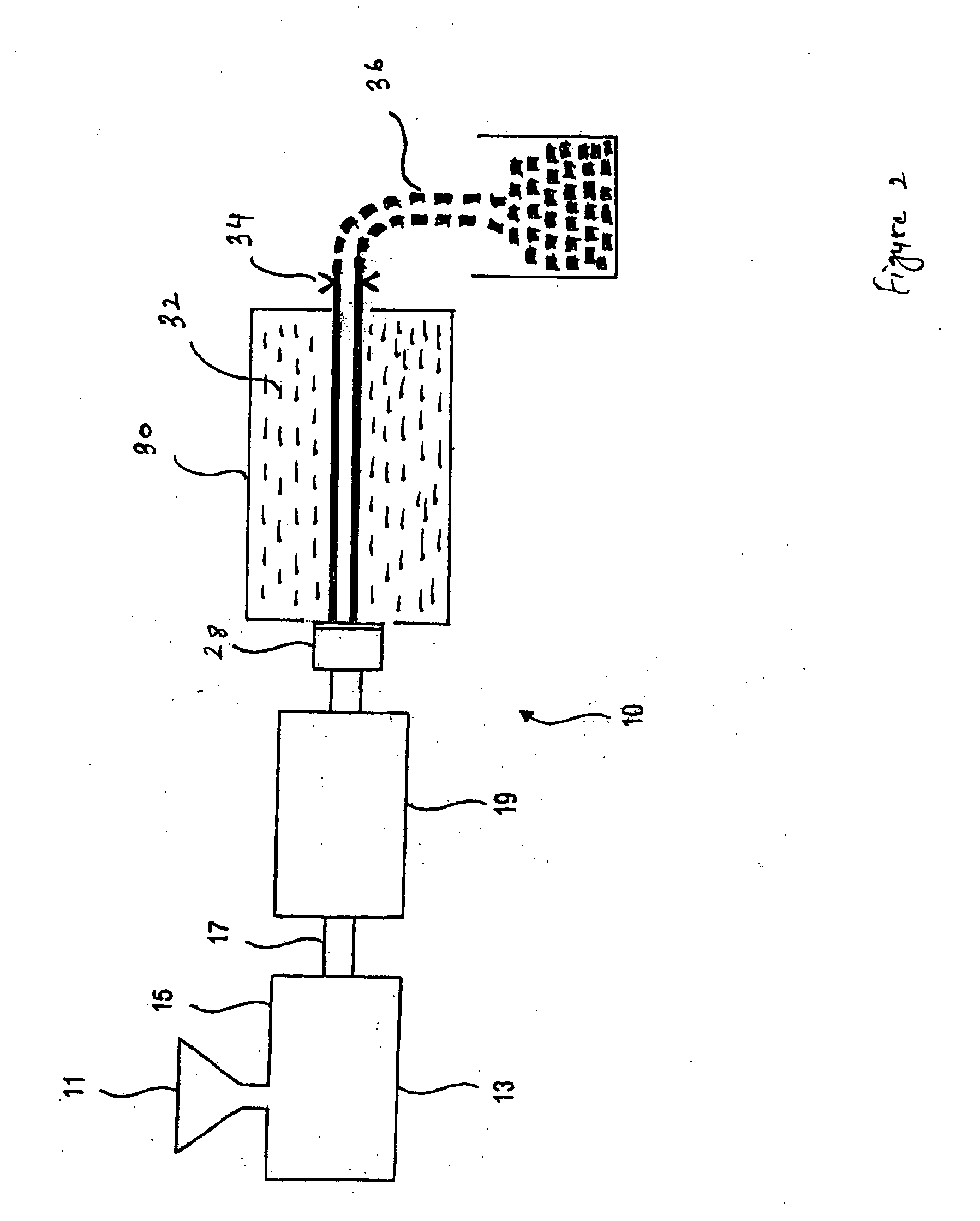
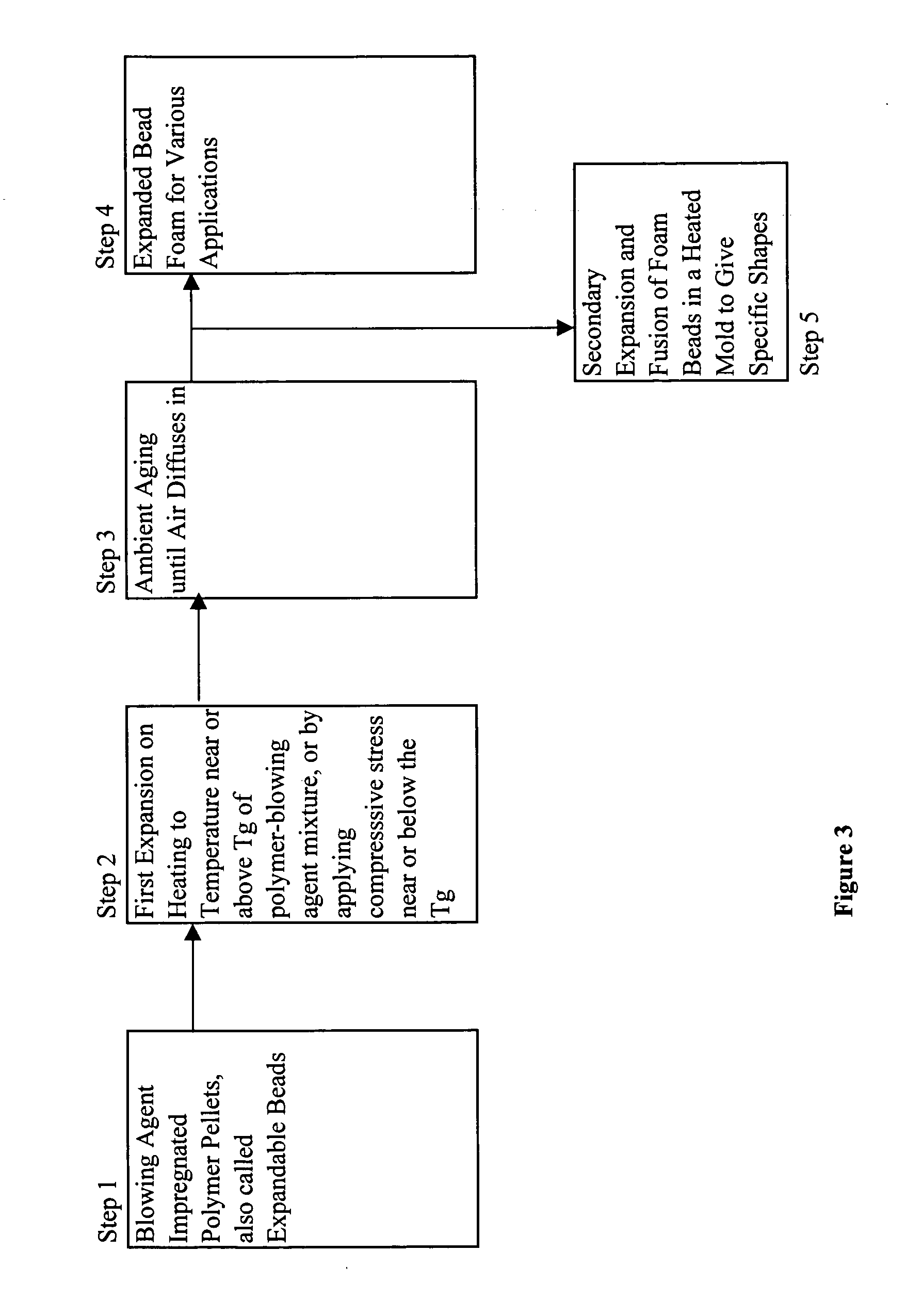
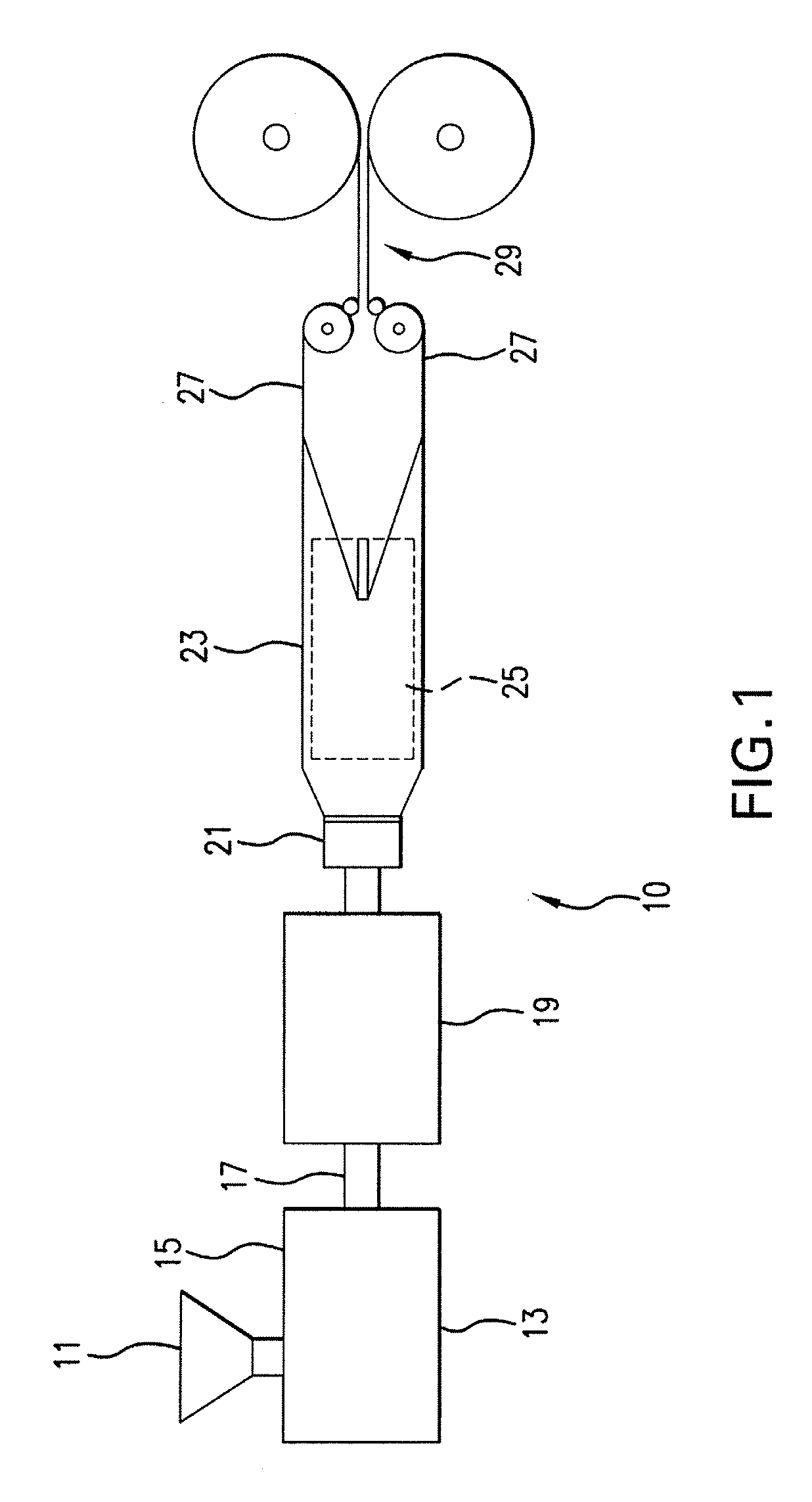
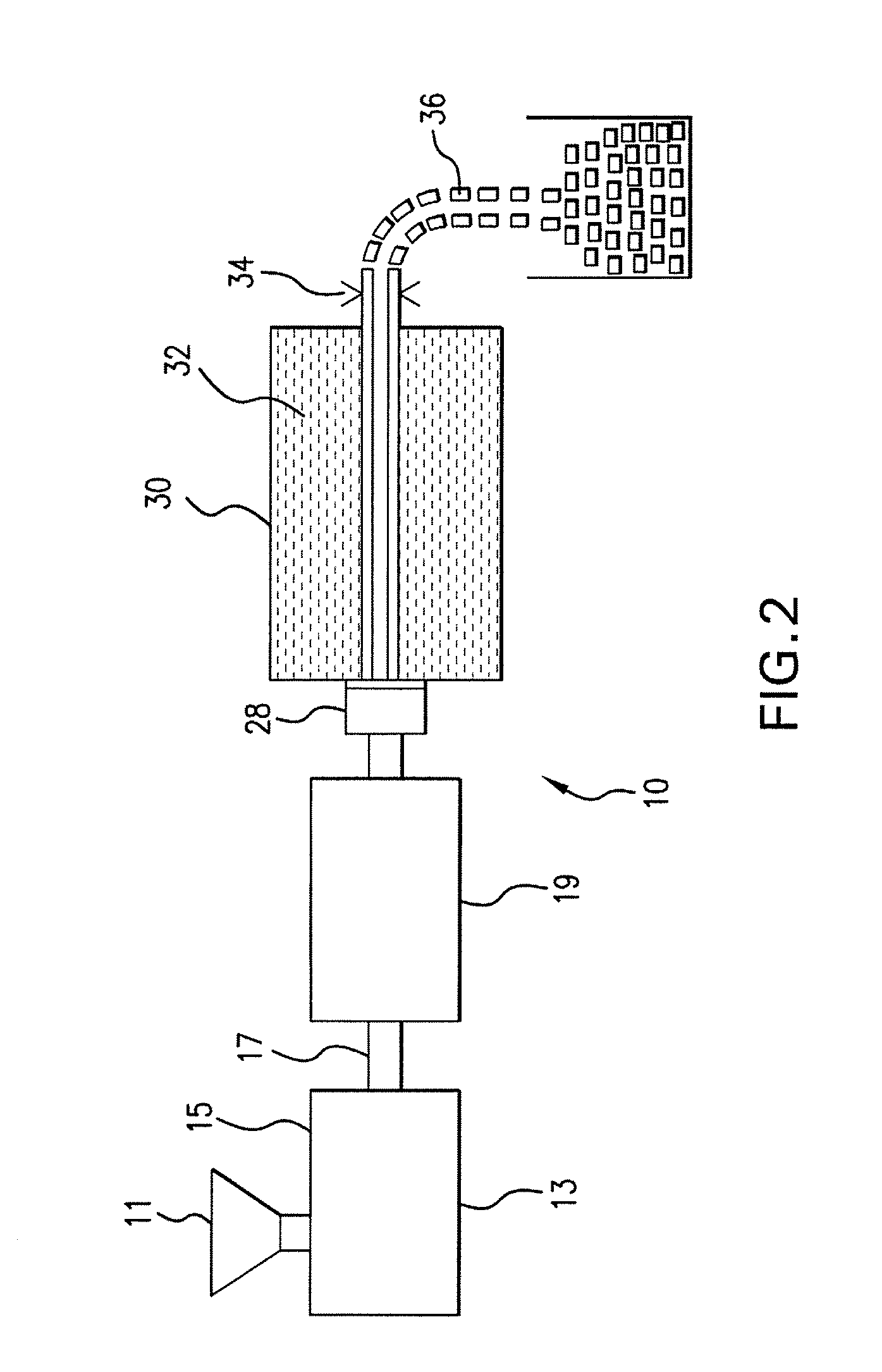
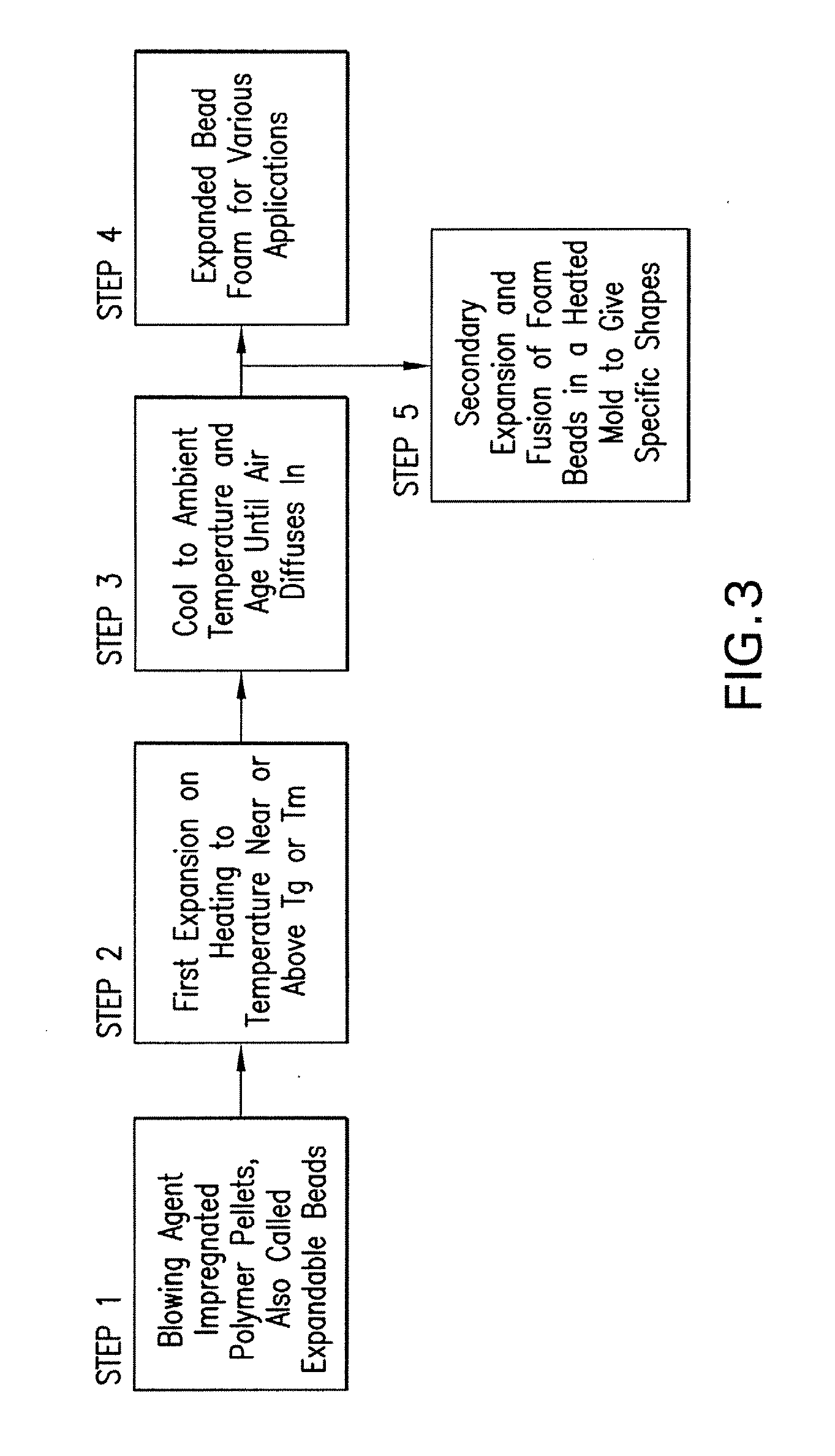
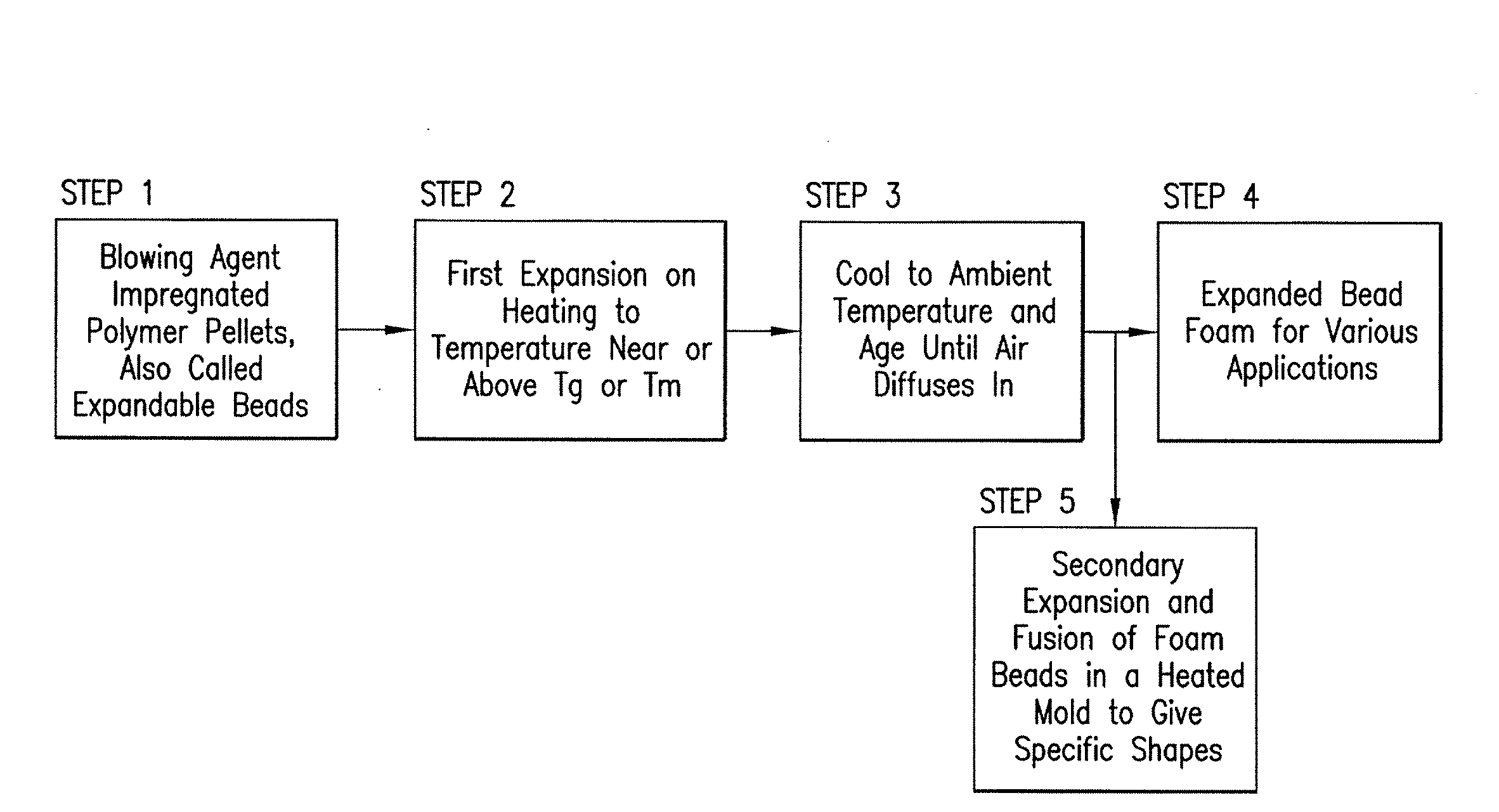
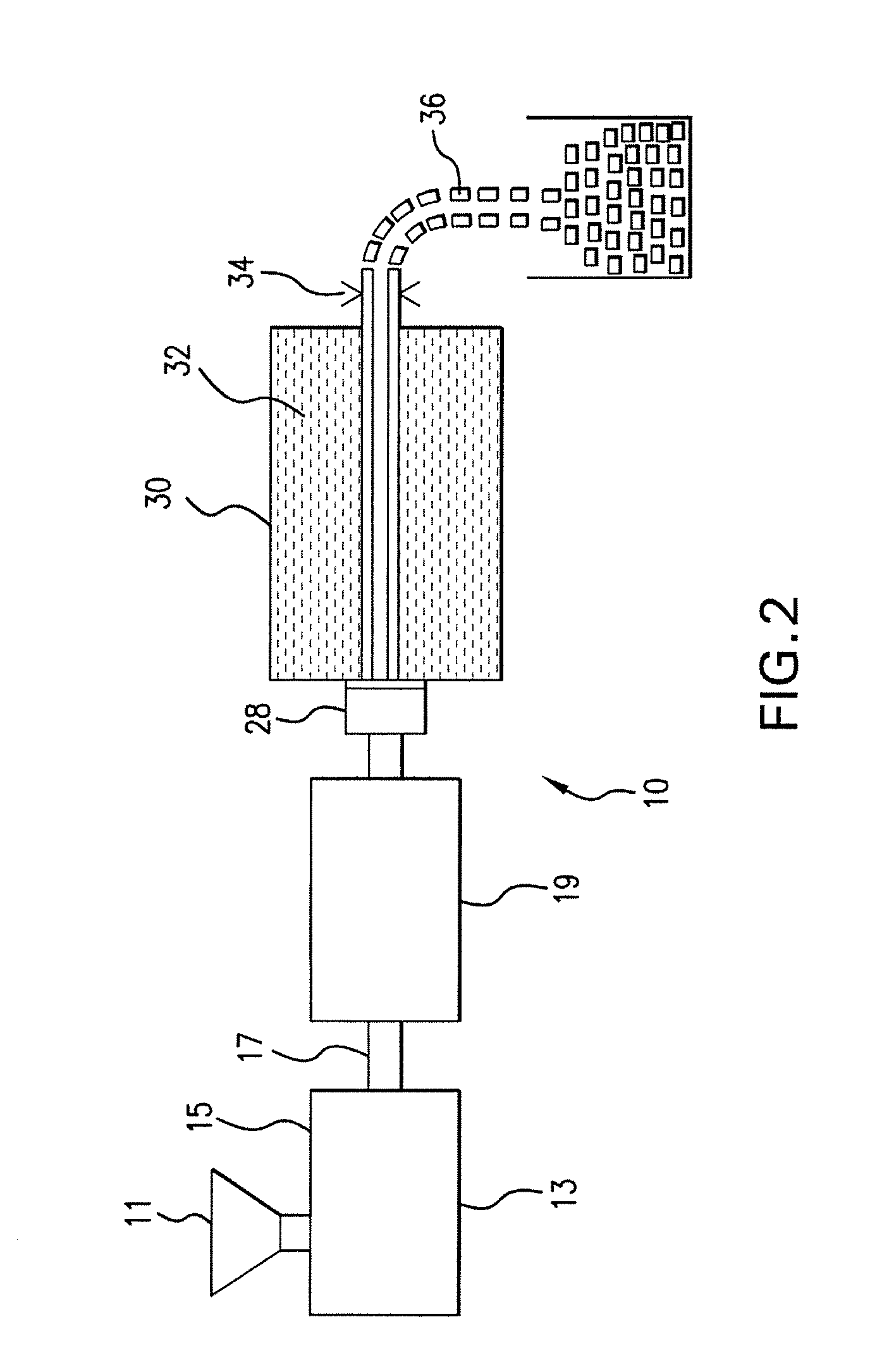
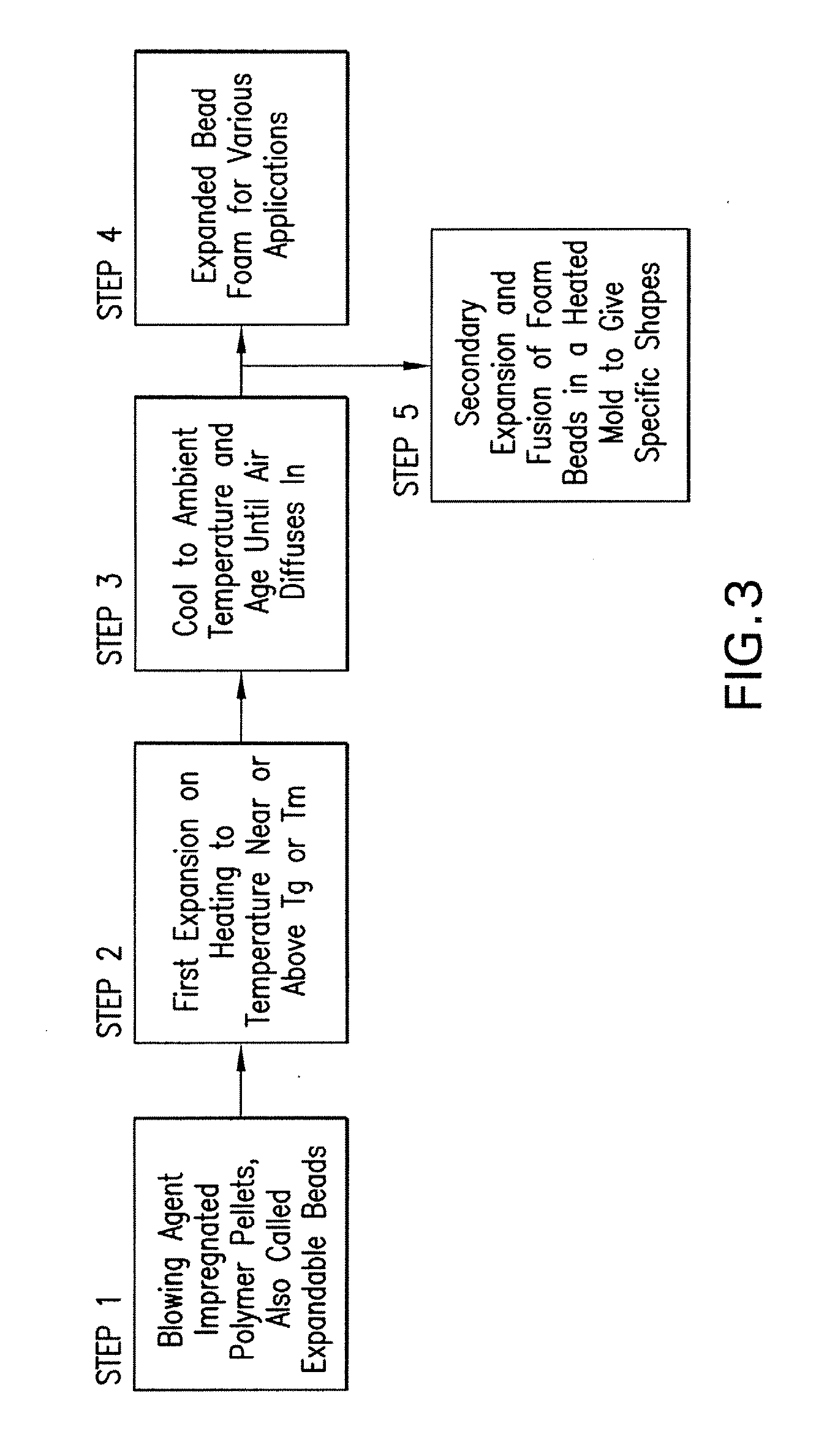
Expanded and extruded thermoplastic foams made with methyl formate-based blowing agents
Low density expanded and extruded thermoplastic polymer foams are obtained using an environmentally benign non-VOC and non-HAP methyl formate as a blowing agent. The blowing agent can be a blend further including at least one co-blowing agent, preferably an environmentally friendly species (e.g., non-VOC and / or non-HAP). The co-blowing agent is either a physical co-blowing agent (e.g. an inorganic agent, a hydrocarbon, a halogenated hydrocarbon, a hydrocarbon with polar, functional group(s), water or any combination thereof), or a chemical co-blowing agent, or combinations thereof The thermoplastic polymer foam can be an alkenyl aromatic polymer foam, e.g. a polystyrene foam. The blowing agent blend can include any combination of methyl formate and one or more co-blowing agents. The methyl formate-based blowing agent blends produce stable foams for various applications, including containers, packaging systems, as well as insulation boards and building materials. Processes for the preparation of such foams are also provided.
Owner:PACTIV CORP
Process and catalysts for the oxidation of methanol and/or ethanol
InactiveUS20050059839A1Good choiceIncrease surface densityOrganic compound preparationCarboxylic acid esters preparationOxygenProcess conditions
A process for oxidation of methanol, ethanol, or a mixture of methanol and ethanol comprising contacting the methanol and / or ethanol with an oxygen-containing gas and a supported catalyst comprising one or more platinum group oxides. The process conditions and / or catalyst may be selected to as to selectively produce methyl formate from methanol or diethoxyethane from ethanol. The invention also includes certain novel supported platinum group metal oxide catalysts. Preferred catalysts include one or more ruthenium oxides.
Owner:RGT UNIV OF CALIFORNIA
Metal carrier loaded catalyst for purifying formaldehyde at room temperature
InactiveCN102941111AReduce wind resistanceHigh bonding strengthGas treatmentDispersed particle separationAlkaline earth metalPt element
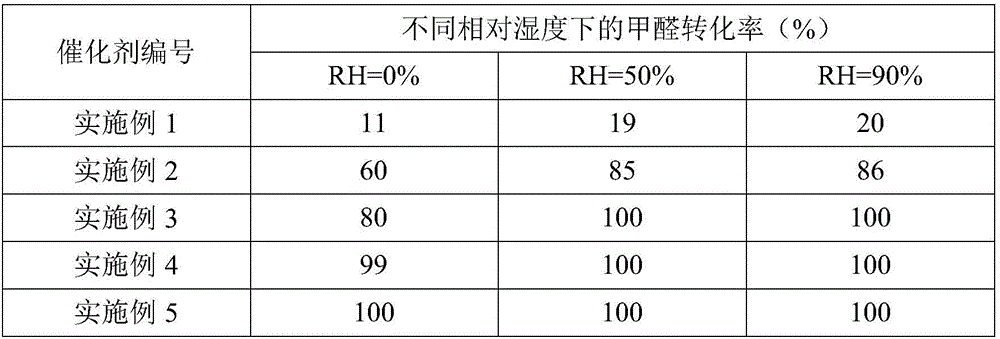
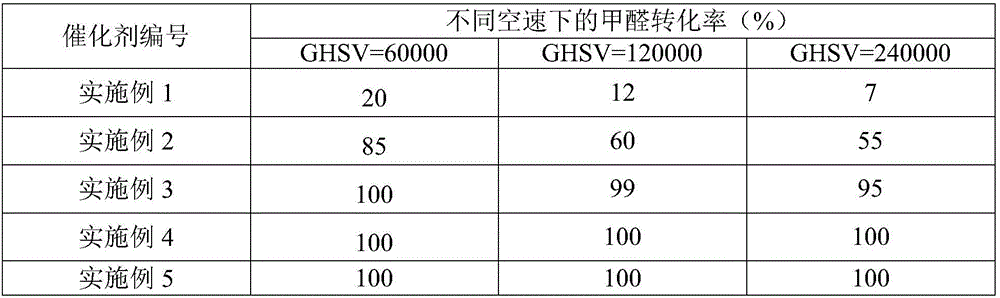
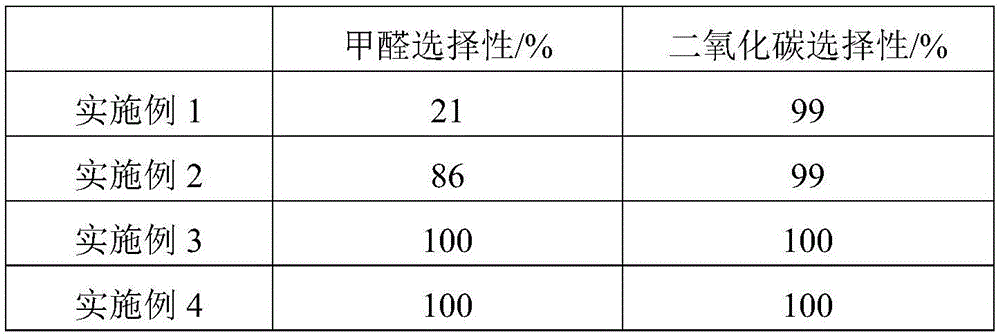
The invention relates to a metal carrier loaded catalyst for purifying formaldehyde at a room temperature. The catalyst comprises a metal carrier, porous inorganic material loaded on the metal carrier, noble metal active components loaded on the porous inorganic material and an additive, wherein the metal carrier is aludirome, the noble metal is any one or a mixture of at least two of platinum, rhodium, palladium, gold or silver and the additive is any one or a mixture of at least two of alkali metal simple substance, alkali metal compound, alkaline earth metal simple substance or alkaline earth metal compound. By the metal carrier load catalyst for purifying formaldehyde at the room temperature, most or all formaldehyde can be converted into carbon dioxide and water; by-products such as formic acid, carbon monoxide and methyl formate are not generated; and the formaldehyde conversion rate can be up to 100%.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Monochlorotrifluoropropene compounds and compositions and mehtods using same
ActiveUS20120161063A1Reduce flammabilityHigh degree of miscibilityMolecular sieve catalystsSolvent extractionAlcoholEther
Various uses of monochlorotrifluoropropenes, in combination with one or more other components, including other fluoroalkenes, hydrocarbons; hydrofluorocarbons (HFCs), ethers, alcohols, aldehydes, ketones, methyl formate, formic acid, water, trans-1,2-dichloroethylene, carbon dioxide and combinations of any two or more of these, in a variety of applications, including as blowing agents, are disclosed.
Owner:HONEYWELL INT INC
Expanded and extruded polyolefin foams made with methyl formate-based blowing agents
Expanded and extruded polyolefin foams are obtained using environmentally benign non-VOC methyl formate as a blowing agent. The blowing agent can be a blend further including at least one co-blowing agent, preferably an environmentally friendly species (e.g., non-VOC), which is either a physical co-blowing agent (e.g. an inorganic agent, a hydrocarbon, a halogenated hydrocarbon, a hydrocarbon with polar, functional group(s) or any combination thereof), or a chemical co-blowing agent, or combinations thereof. The blowing agent blend can include any combination of methyl formate and one or more co-blowing agents. The polymer foam can include polyethylene, polypropylene or a combination thereof. The methyl formate-based blowing agent blends produce stable foams for various applications, including containers, packaging systems, as well as for insulation and protective cushioning. Processes for the preparation of such foams are also provided.
Owner:PACTIV CORP
Metallic catalyst for synthesizing dimethoxym ethane and methyl formate and production method thereof and use
InactiveCN101327444ALarge specific surface areaSmall particlesMolecular sieve catalystsOrganic compound preparationFormate EstersTitanium
The present invention relates to a metal catalyst synthesizing methylal and methyl formate. In weight percentage of the metal catalyst, the content of vanadium is calculated by the weight of V2O5; the content of titanium is calculated by the weight of TiO2; the weight proportion of the V2O5 and the TiO2 in the catalyst is 5 to 70 to 30 to 95; the V2O5 and the TiO2 take 20 percent to 100 percent of the catalyst in weight percentage; the content of accessory ingredient is calculated by the content of the oxide of the accessory ingredient and takes 0wt percent to 2.0wt percent of the catalyst in weight percentage; support takes 0wt percent to 78wt percent of catalyst in weight percentage. The catalyst has the advantages of high methanol conversion rate, the high selectivity of the methylal and the methyl formate, the good stability of the catalyst and long life.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Expanded and extruded biodegradable and reduced emission foams made with methyl formate-based blowing agents
Expanded and extruded biodegradable polymer foams are obtained using biodegradable polymers and environmentally benign non-VOC methyl formate as a blowing agent. The blowing agent can be a blend further including at least one co-blowing agent, preferably an environmentally friendly species (e.g., non-VOC), which is either a physical co-blowing agent (e.g. an inorganic agent, a hydrocarbon, a halogenated hydrocarbon, a hydrocarbon with polar, functional group(s), water or any combination thereof), or a chemical co-blowing agent, or combinations thereof. The blowing agent blend can include any combination of methyl formate and one or more co-blowing agents. The polymer foam can include a biodegradable polymer or its blends with other biodegradable polymers or conventional (non-biodegradable) polymers. The methyl formate-based blowing agent blends produce stable foams for various applications, including containers, packaging systems, as well as for insulation and protective cushioning. Processes for the preparation of such foams are also provided.
Owner:PACTIV CORP
Azeotropic and azeotrope-like compositions of e-1,1,1,4,4,5,5,5-octafluoro-2-pentene
InactiveUS20100243943A1Organic compounds purification/separation/stabilisationOther chemical processesDielectricThermoplastic
Azeotropic or azeotrope-like compositions are disclosed. The azeotropic or azeotrope-like compositions are mixtures of E-1,1,1,4,4,5,5,5-Octafluoro-2-pentene with methyl formate, n-pentane, 2-methylbutane, 1,1,1,3,3-pentafluorobutane, trans-1,2-dichloroethylene, 1,1,1,3,3-pentafluoropropane, dimethoxymethane, cyclopentane or Z-1,1,1,4,4,4-hexafluoro-2-butene. Also disclosed is a process of preparing a thermoplastic or thermoset foam by using such azeotropic or azeotrope-like compositions as blowing agents. Also disclosed is a process of producing refrigeration by using such azeotropic or azeotrope-like compositions. Also disclosed is a process of using such azeotropic or azeotrope-like compositions as solvents. Also disclosed is a process of producing an aerosol product by using such azeotropic or azeotrope-like compositions as propellants. Also disclosed is a process of using such azeotropic or azeotrope-like compositions as heat transfer media. Also disclosed is a process of extinguishing or suppressing a fire by using such azeotropic or azeotrope-like compositions. Also disclosed is a process of using such azeotropic or azeotrope-like compositions as dielectrics. Also disclosed is a process for the separation of a chemical compound from a mixture of two or more chemical compounds using such azeotropic or azeotrope-like compositions.
Owner:THE CHEMOURS CO FC LLC
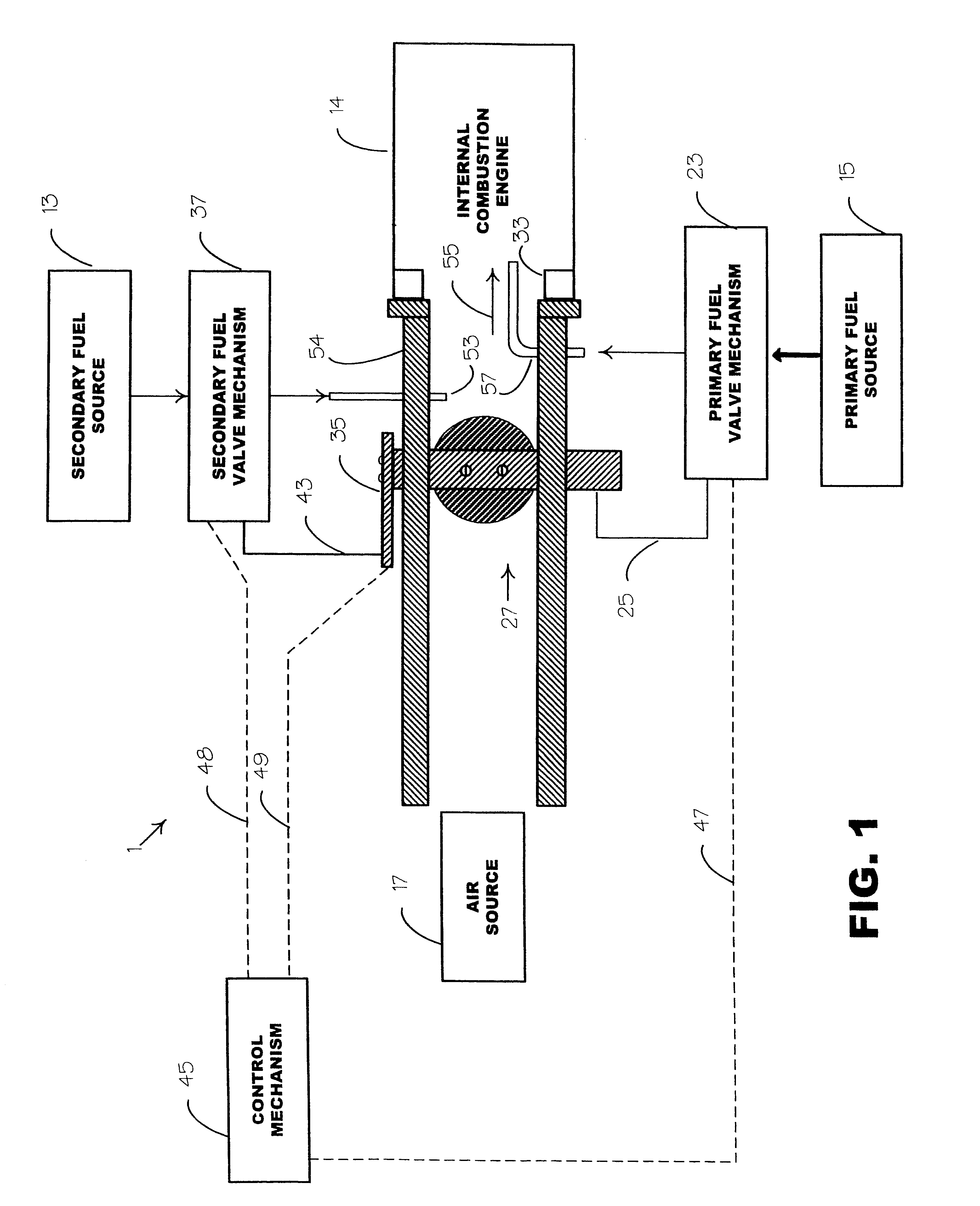
Internal combustion system adapted for use of a dual fuel composition including acetylene
InactiveUS20020014226A1Early ignition be preventEfficient in operationInternal combustion piston enginesNon-fuel substance addition to fuelChemistryFormate Esters
An internal combustion engine adapted to use an environmentally clean multi-fuel composition, comprising acetylene as a primary fuel and a combustible fuel, such as one or more fluids selected from an alcohol such as ethanol, methanol or any other alcohol or alcohols from the group comprising C1-C12 carbon chains, ethers such as from the group comprising dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl t-butyl ether, t-amyl methyl ether, di-isopropyl ether and the like, low-molecular-weight esters such as from the group comprising methyl formate, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate, ethyl malate, butyl malate, and the like, or other suitable combustible fluid such as mineral spirits and the like, as a secondary fuel for operatively preventing early ignition and knock arising from the primary fuel.
Owner:GOTEC
Expanded and extruded polyolefin foams made with methyl formate-based blowing agents
Expanded and extruded polyolefin foams are obtained using environmentally benign non-VOC methyl formate as a blowing agent. The blowing agent can be a blend further including at least one co-blowing agent, preferably an environmentally friendly species (e.g., non-VOC), which is either a physical co-blowing agent (e.g. an inorganic agent, a hydrocarbon, a halogenated hydrocarbon, a hydrocarbon with polar, functional group(s) or any combination thereof), or a chemical co-blowing agent, or combinations thereof. The blowing agent blend can include any combination of methyl formate and one or more co-blowing agents. The polymer foam can include polyethylene, polypropylene or a combination thereof. The methyl formate-based blowing agent blends produce stable foams for various applications, including containers, packaging systems, as well as for insulation and protective cushioning. Processes for the preparation of such foams are also provided.
Owner:PACTIV CORP
Azeotropic compositions comprising methyl perfluoropentene ethers for cleaning applications
The present disclosure provides azeotropic and azeotrope-like compositions comprised of methylperfluoropentene ethers and at least one of methanol, ethanol, 2-propanol, hexane, heptane, trans-1,2-dichloroethylene, ethyl formate, methyl formate, HFE-7100, HFE-7200 and 1-bromopropane or combinations thereof. The present disclosure also provides for methods of use for the azeotropic and azeotrope-like compositions.
Owner:EI DU PONT DE NEMOURS & CO
Gas self-production system used for plug removal and energy increase of oil-water well and application method thereof
ActiveCN103333670ALess equipment required for preparationSimple processDrilling compositionHexamethylenetetraminePotassium nitrite
The invention relates to a gas self-production system used for plug removal and energy increase of an oil-water well and an application method thereof. The gas self-production system comprises, by weight, 3-10% of urea, 5-25% of a nitrite, 5-25% of an activator and 40-85% of water. The nitrite is one of sodium nitrite, ammonium nitrite and potassium nitrite. The activator is one of methyl formate, ammonium amidosulfonate, ammonium-monochloracetat, hexamethylenetetramine and formaldehyde. When the gas self-production system is used, the above materials are mixed at ambient temperature under ambient pressure, and injected into oil-bearing strata having a temperature higher than 50 DEG C, so as to remove plug, increase energy and increase enhance rate. The gas self-production system can be applied for plug removal and energy increasing of low-pressure low-energy wells in an oil field scattered reserve unit.
Owner:CHINA PETROCHEM & CHEM JIANGSU OILFIELD BRANCH +2
Carbonyl reductase mutant as well as gene and application thereof
InactiveCN104099305AImprove thermal stabilityIncreased reductase activityBacteriaOxidoreductasesMethyl o-chloromandelateMandelic acid
The invention relates to a carbonyl reductase CgKR1 mutant, a coding gene of the mutant, a recombinant expression vector containing the gene of the carbonyl reductase mutant, a recombinant expression transformant, a recombinase, a preparation method of the recombinase, and an application of the carbonyl reductase mutant to asymmetric reduction of ketonic ester for preparation of optically pure chiral hydroxyl ester, such as catalysis of o-cyano methyl phenylglyoxylate for asymmetric reduction to prepare (R)-o-chloro mandelic acid methyl ester. Compared with wild enzymes, the carbonyl reductase mutant has the advantages that the thermal stability is substantially improved, and the catalytic activity of part of the mutant to the o-chlorobenzoic acid formyl methyl ester is also obviously improved. The multiple mutants can be applied to catalysis of the ketonic ester for asymmetric reduction to prepare the optical purely-chiral hydroxyl ester, such as catalysis of the o-cyano methyl phenylglyoxylate for asymmetric reduction to prepare the optically pure (R)-o-chloro mandelic acid methyl ester. The carbonyl reductase mutants have the very good industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
Catalyst for elimination of formaldehyde at room temperature and preparation method thereof
ActiveCN106540741ASimple conditions of useEasy to operateGas treatmentMolecular sieve catalystsCatalytic oxidationMoisture resistance
The invention relates to the field of environmental catalysis and provides a catalyst for elimination of formaldehyde at a room temperature and a preparation method thereof. The catalyst comprises a Beta molecular sieve obtained by an organic template-free seed crystal method and a noble metal active component supported on a Beta molecular sieve. The noble metal active component comprises one or at least two of noble metals such as platinum, ruthenium, gold, silver and palladium. The catalyst has the advantages of simple use condition and convenient operation, can be effectively used for catalytic oxidation of the main pollutant formaldehyde in the room at the room temperature, does not produce by-products such as formic acid, carbon monoxide and methyl formate, has a formaldehyde conversion rate of 100%, a less catalyst use amount and excellent resistance to a high speed, does not need a specific light source, does not consume power and heat, saves energy and has excellent stability and moisture resistance.
Owner:苏州美吉科环保科技有限公司
Low temperature double-layer capacitors
ActiveUS20080304207A1Low melting pointHybrid capacitor electrolytesClosuresTetrafluoroborateDevice form
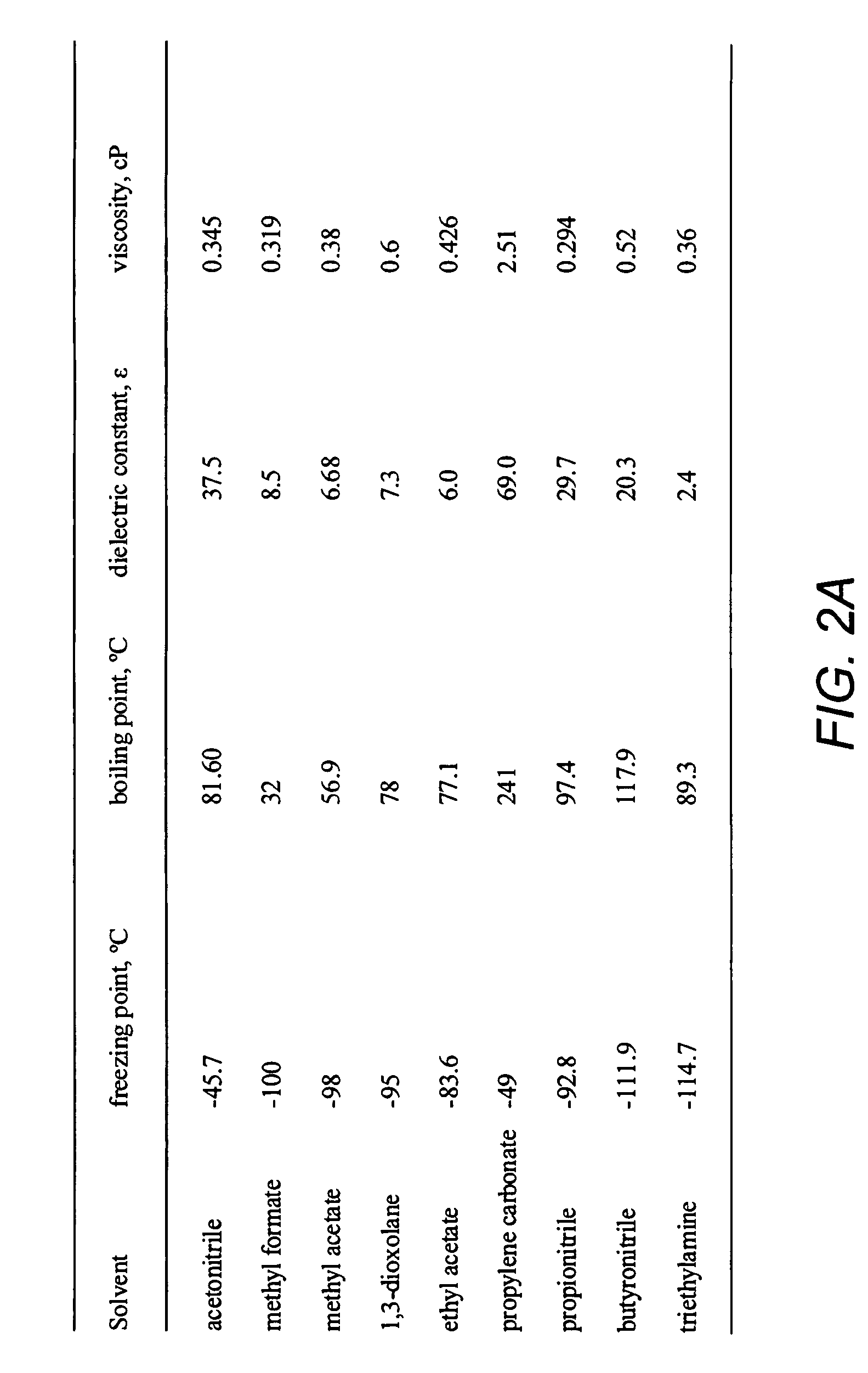
Double-layer capacitors capable of operating at extremely low temperatures (e.g., as low as −75° C.) are disclosed. Electrolyte solutions combining a base solvent (e.g., acetonitrile) and a cosolvent are employed to lower the melting point of the base electrolyte. Example cosolvents include methyl formate, ethyl acetate, methyl acetate, propionitrile, butyronitrile, and 1,3-dioxolane. An optimized concentration (e.g., 0.10 M to 0.75 M) of salt, such as tetraethylammonium tetrafluoroborate, is disolved into the electrolyte solution. In some cases (e.g., 1,3-dioxolane cosolvent) additives, such as 2% by volume triethylamine, may be included in the solvent mixture to prevent polymerization of the solution. Conventional device form factors and structural elements (e.g., porous carbon electrodes and a polyethylene separator) may be employed.
Owner:CALIFORNIA INST OF TECH
Process for Producing Acetic Acid
ActiveUS20050197506A1Easy to separateFacilitate phase separationOrganic compound preparationCarboxylic preparation from carbon monoxide reactionMethyl acetateFormate Esters
An improved process is disclosed for producing acetic acid, including the following steps: reacting a carbonylatable reactant such as methanol, methyl acetate, methyl formate or dimethyl ether with carbon monoxide in a reaction medium containing water, methyl iodide, and a catalyst to produce a reaction product that contains acetic acid; separating the reaction product to provide a volatile phase containing acetic acid, water, and methyl iodide and a less volatile phase; distilling the volatile phase to produce a purified acetic acid product and a first overhead containing water, methyl acetate, and methyl iodide; phase separating the first overhead to provide a first liquid phase containing water and a second liquid phase containing methyl iodide; and adding dimethyl ether to the process in an amount effective to enhance separation of the first overhead to form the first and second liquid phases.
Owner:CELANESE INT CORP
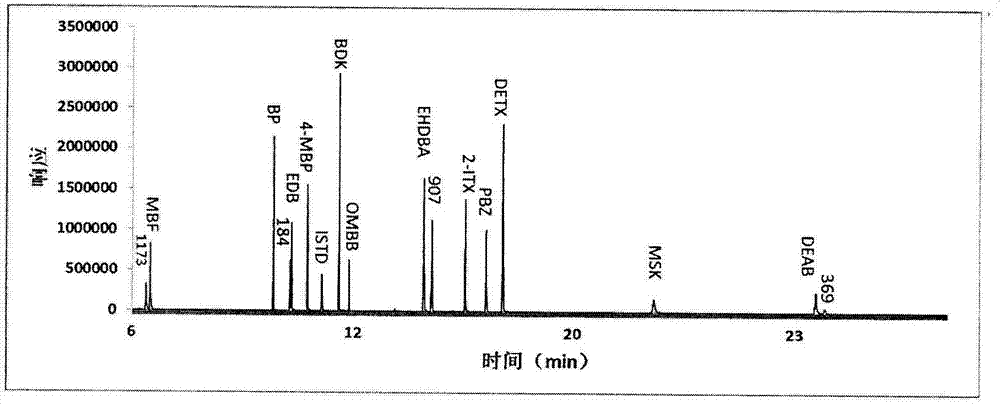
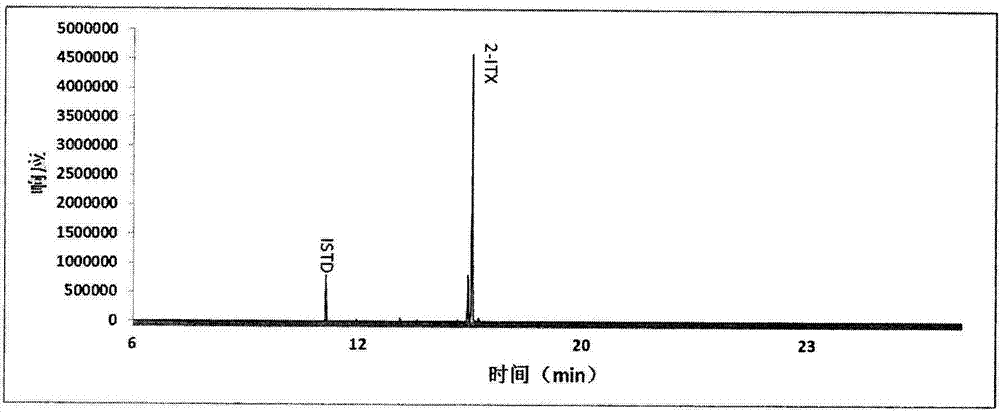
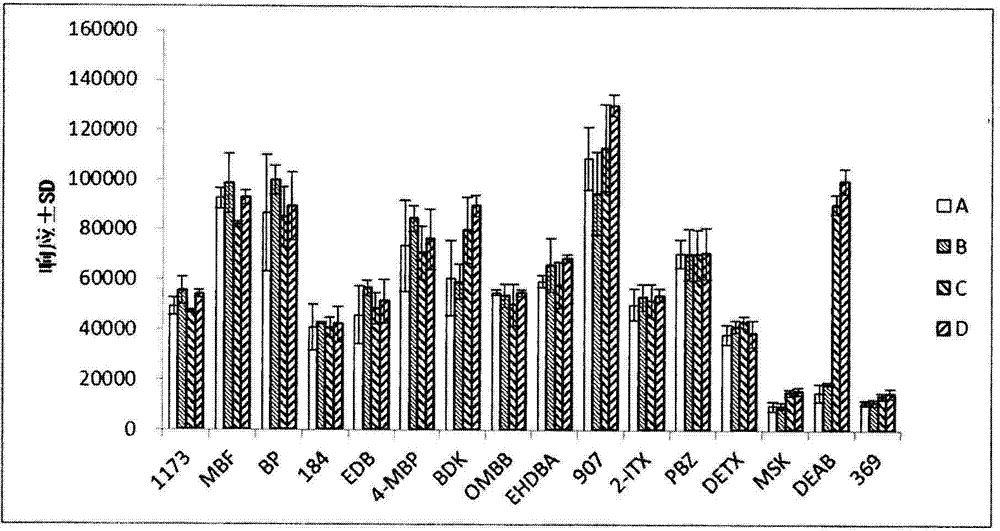
Detection method of determining 16 types of photoinitiators in paper printing and packaging material
ActiveCN102818874AEnable simultaneous analysisRealize determinationComponent separationLiquid liquid partitionPrinting ink
The invention relates to a detection method of determining 16 types of photoinitiators in a paper printing and packaging material, which is used for determining residual amounts of the 16 types of photoinitiators of 2-hodroxy-2-methyl-1-phenylacetone, mehtyl phenylglyoxylate, benzophenone and the like and is characterized by comprising the following concrete steps of: a, sample pretreatment; b, preparation of a standard work solution; c, instrumental analysis; and d, result calculation. Compared with the prior art, through sample pretreatment and optimization of instrumental analysis conditions, the detection method has the advantages that efficiency is high, the 16 kinds of photoinitiators can be simultaneously analyzed and determined within 25m; and printing ink impurities in a sample extraction solution can be better removed in a liquid-liquid distribution impurity removal mode adopted in the sample pretreatment process, so that cost is low, consumption is little, efficiency is high, and sensitivity is high; and in addition, the detection method also has the advantages of high accuracy and good repeatability.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Method for refining and purifying propylene oxide
ActiveCN103172594AGuaranteed separation effectHigh yieldOrganic chemistryBulk chemical productionIon exchangeKetone
The invention provides a method for refining and purifying propylene oxide. The method is characterized by comprising the steps that an epoxidation reaction product enters a propylene separating column to be separated into column top materials and column bottom materials, wherein the column top materials contain propylene, propylene oxide and methanol and contain or do not contain propane and water, and the column bottom materials contain methanol, water, hydrogen peroxide and high-boiling-point byproducts; column top materials from the propylene separating column enter a propylene stripping column, and the column top gases contain propylene and little propylene oxide and contain or do not contain propane and circulate to a reaction system after compression; and column bottom materials contain methanol, propylene oxide and few aldehyde ketone impurities and contain or do not contain water, enter a reactor filled with alkaline ion exchange resins to react to remove methyl formate and aldehyde out of the column bottom materials and are further refined through rectification to obtain the high-purity propylene oxide product. The invention also provides a separation method for preparing propylene oxide and the product through direct epoxidation. The method provided by the invention has the beneficial effects that the low temperature and low pressure process is adopted, thus increasing the yield of propylene oxide, reducing the losses of propylene oxide and reducing the operating costs of the devices.
Owner:CHINA PETROLEUM & CHEM CORP +1
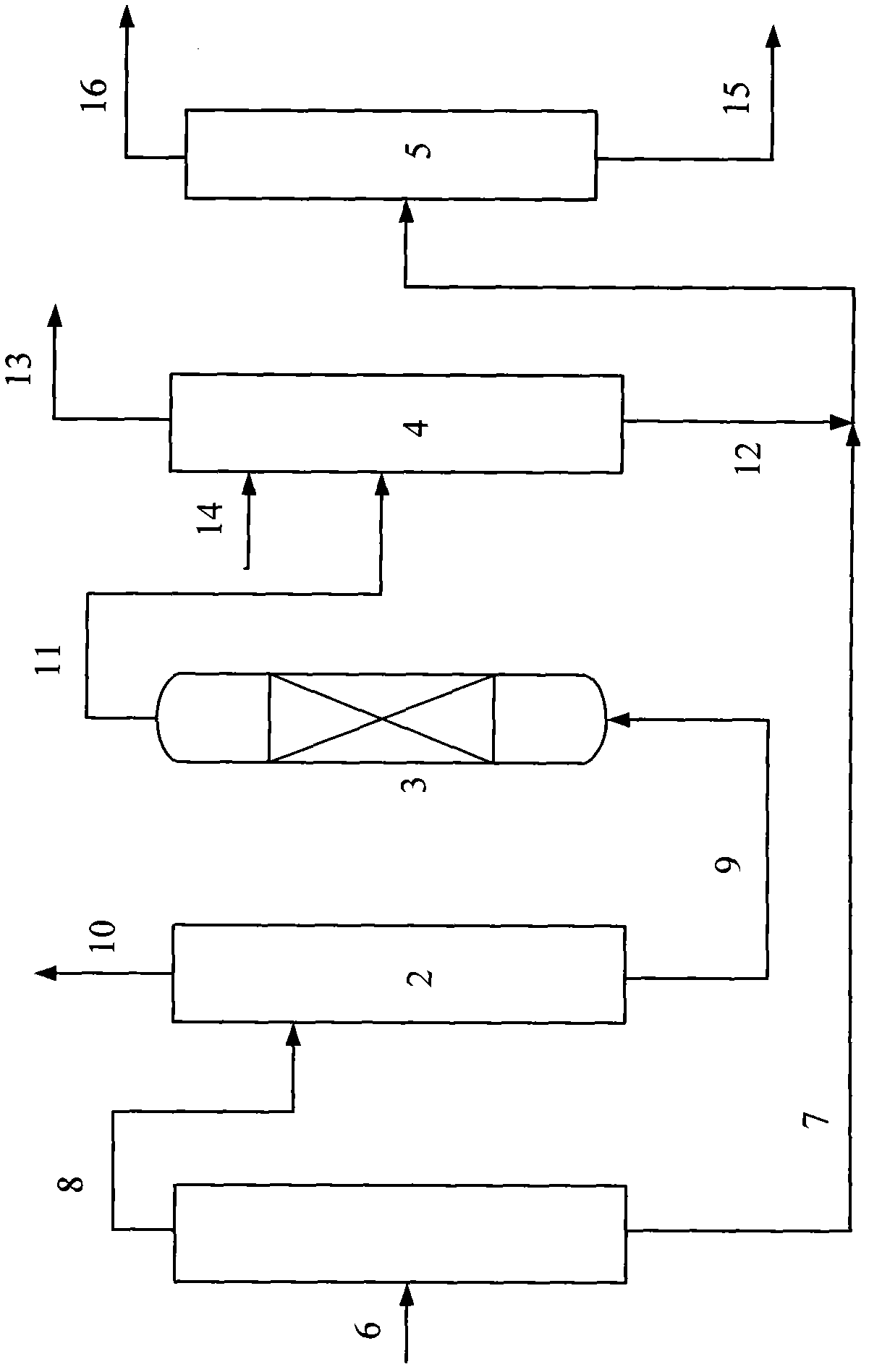
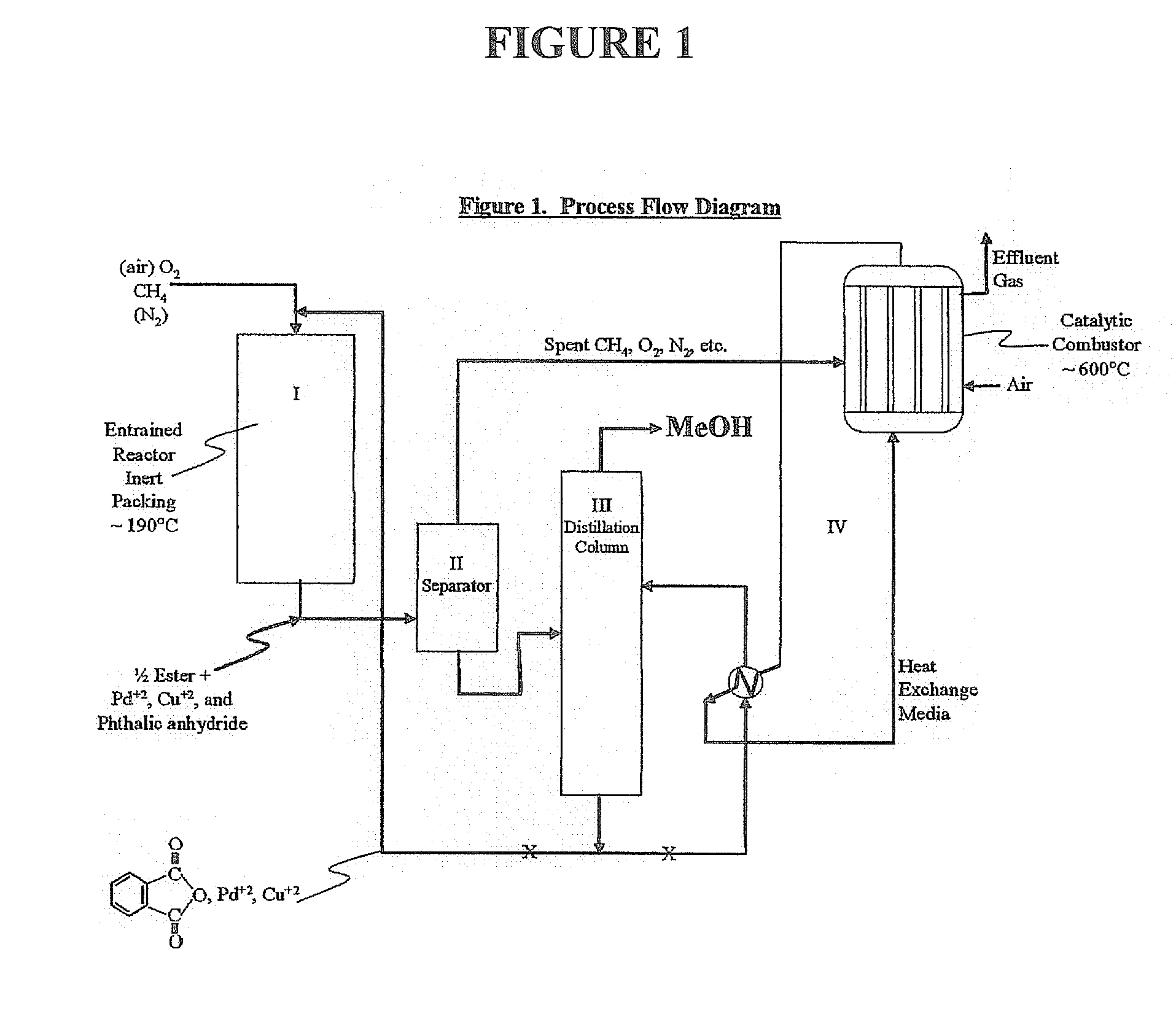
Method for deriving methanol from waste generated methane and structured product formulated therefrom
InactiveUS20060264683A1Less capitolInexpensively separatedPreparation by oxidation reactionsOrganic compound preparationPtru catalystReactive distillation
Methanol is produced from bacterially oxidized waste methane by reaction with Pd+2, Cu+2 air, and molten phthalic anhydride in an entrained oxidizer generating half ester of methyl phthalate which is reaction distilled to prodtice methanol and recycle phthalic anliydride containing the Pd+2,Cu+2 phthalate catalyst.
Owner:RINNOVI
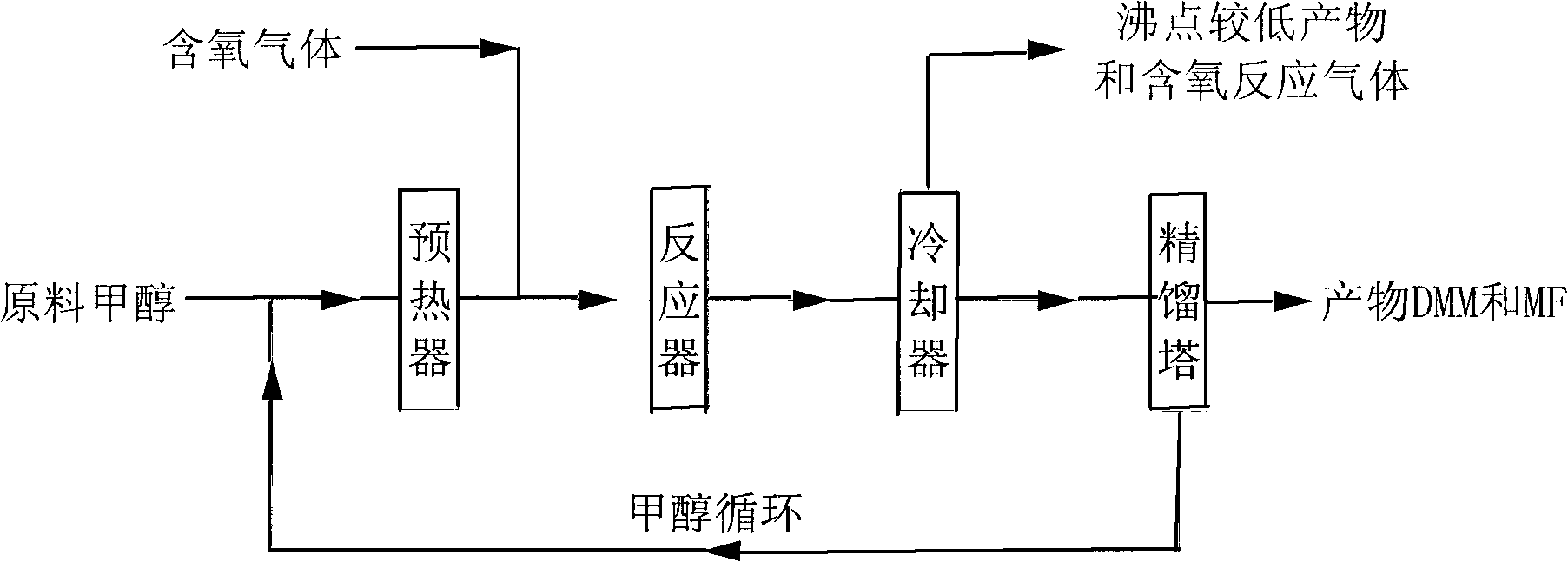
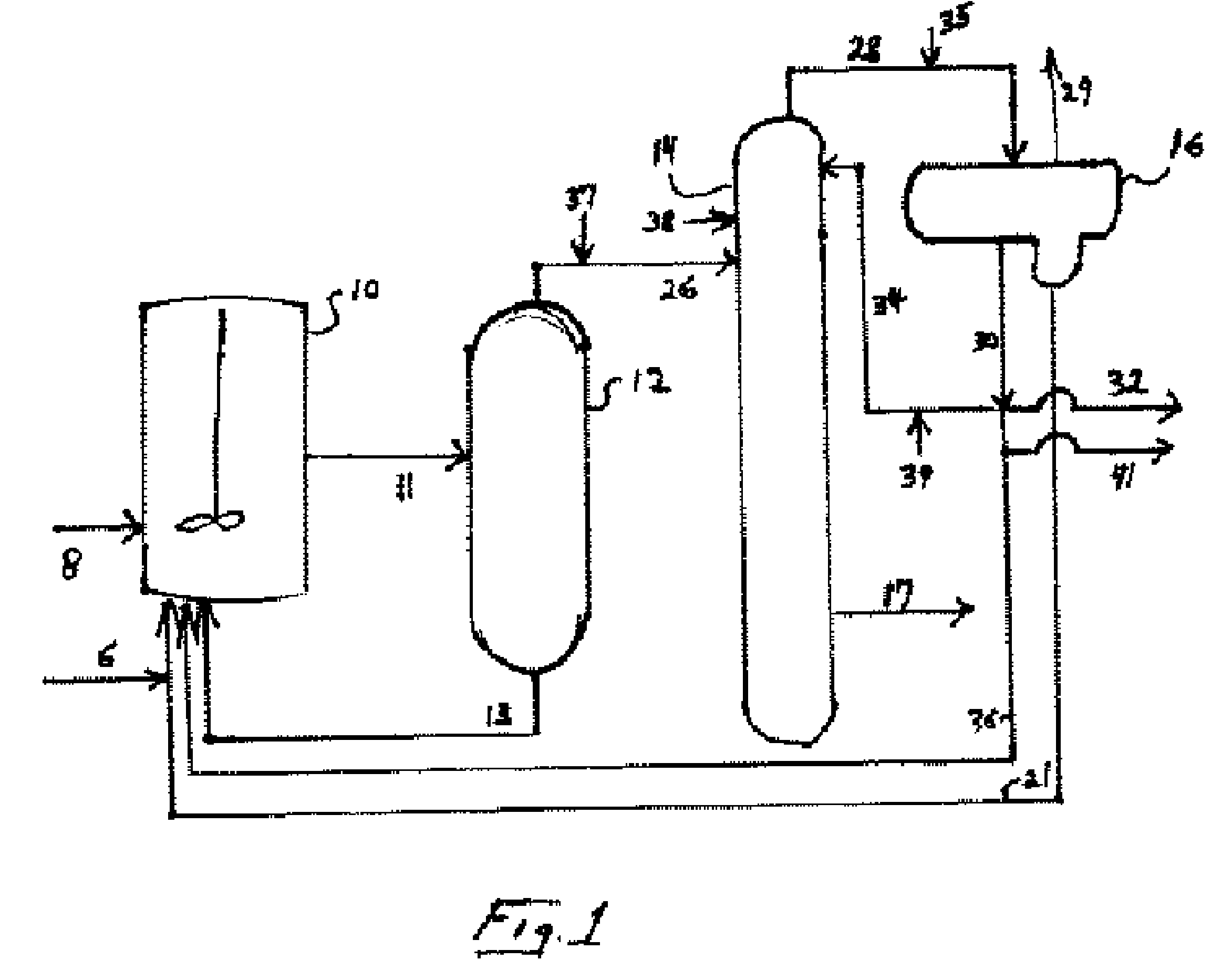
Method for synthesizing methyl formate by gas-phase carbonylation of methyl alcohol
ActiveCN103694116AAvoid high purity requirementsEasy to separatePreparation by carbon monoxide or formate reactionMetal/metal-oxides/metal-hydroxide catalystsGas phaseFixed bed
The invention discloses a method for synthesizing methyl formate by gas-phase carbonylation of methyl alcohol. A fixed bed reaction technology is used, and raw materials including methyl alcohol, carbon monoxide, hydrogen and oxygen generate gas-phase carbonylation reaction under the action of supported nano platinum family metal heterogeneous catalyst so as to generate methyl formate. The reaction raw materials include 10%-50% of methyl alcohol, 10%-50% of carbon monoxide, 10%-30% of hydrogen and 5%-20% of oxygen by volume ratio, and react under the conditions that air speed is 500-5000h<-1>, reaction temperature is 323K-423K and reaction pressure is 0.01Mpa-2Mpa.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Method for preparing catalyst for methanol dehydrogenation to methyl formate through step coprecipitation-spray process
ActiveCN102755897AImprove catalytic performanceSmall particle sizeOrganic compound preparationCarboxylic acid esters preparationPtru catalystDehydrogenation
The invention discloses a method for preparing catalyst for methanol dehydrogenation to methyl formate through a step coprecipitation-spray drying process. The method is that step coprecipitation is performed to copper, zinc and aluminum salt solution and alkaline solution, after the copper, zinc and aluminum salt solution and alkaline solution are pulped and filtered with hot desalted water, a qualified filter cake is obtained and is subjected to colloid mill processing, spray drying is performed to size, and dried catalyst precursor is roasted and formed so as to obtain the catalyst for methanol dehydrogenation. The catalyst prepared by the method has the characteristics of high catalytic activity and selectivity and strong stability.
Owner:HAO HUA CHENGDU TECH
A process for vapor-phase methanol carbonylation to methyl formate, a catalyst used in the process and a method for preparing the catalyst
ActiveUS20160332953A1Low purityReduce investmentHeterogenous catalyst chemical elementsCatalyst activation/preparationGas phaseOxygen
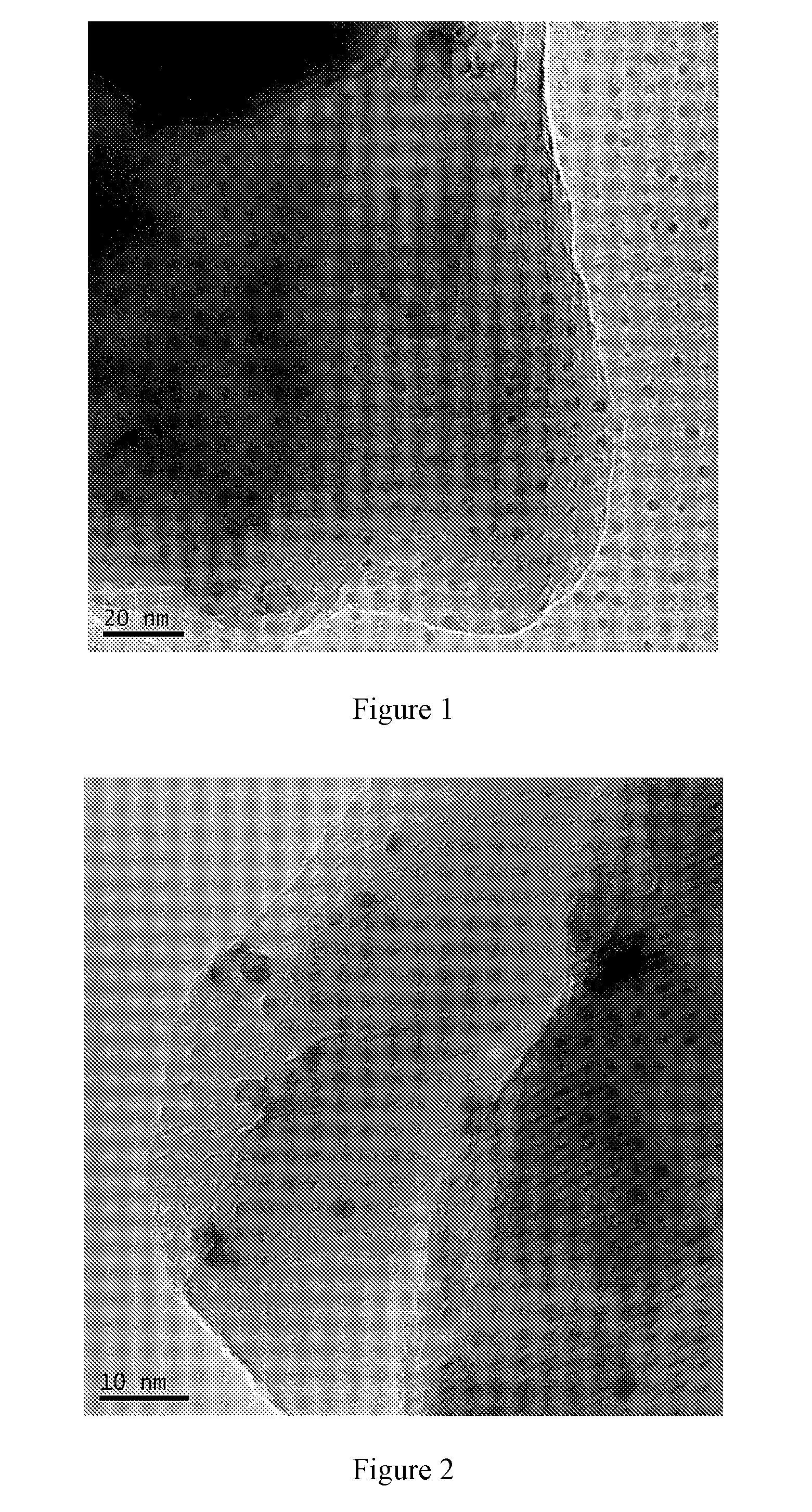
A process for vapor-phase methanol carbonylation to methyl formate, a supported nano-scaled platinum group metal heterogeneous catalyst used in said process and a method for preparing said catalyst are disclosed. A feed gas containing methanol, carbon monoxide, hydrogen and oxygen is passed through a reactor loaded with supported nano-scaled platinum group metal heterogeneous catalyst to produce methyl formate by vapor-phase carbonylation reaction, in the reaction conditions with a space velocity of 500-5000 h−1, a temperature of 50-150° C. and a pressure of 0.01-2 MPa. The active component of the supported nano-scaled platinum group metal heterogeneous catalyst is platinum group metal with a particle size of 0.5-10 nm. The process and catalyst solve the problems in present industrial technology, such as high requirement for the purity of raw materials, severe corrosion of the equipment by catalyst, difficult separation of catalyst from product, high reaction pressure, difficult operation and the like, and provide a novel technical way to produce methyl formate.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com