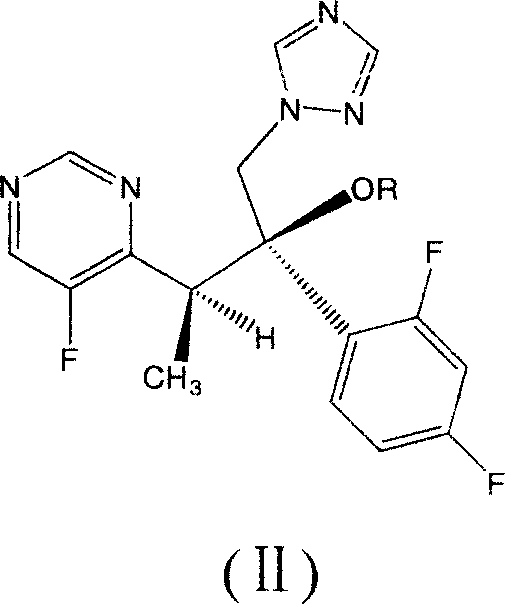
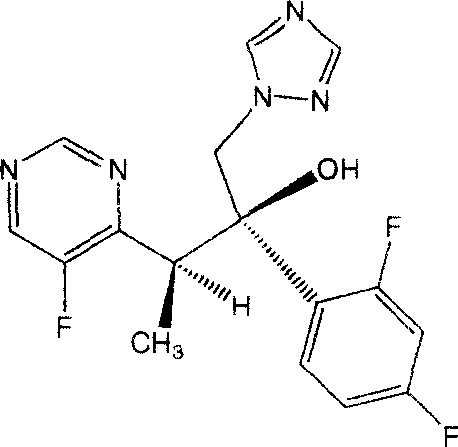
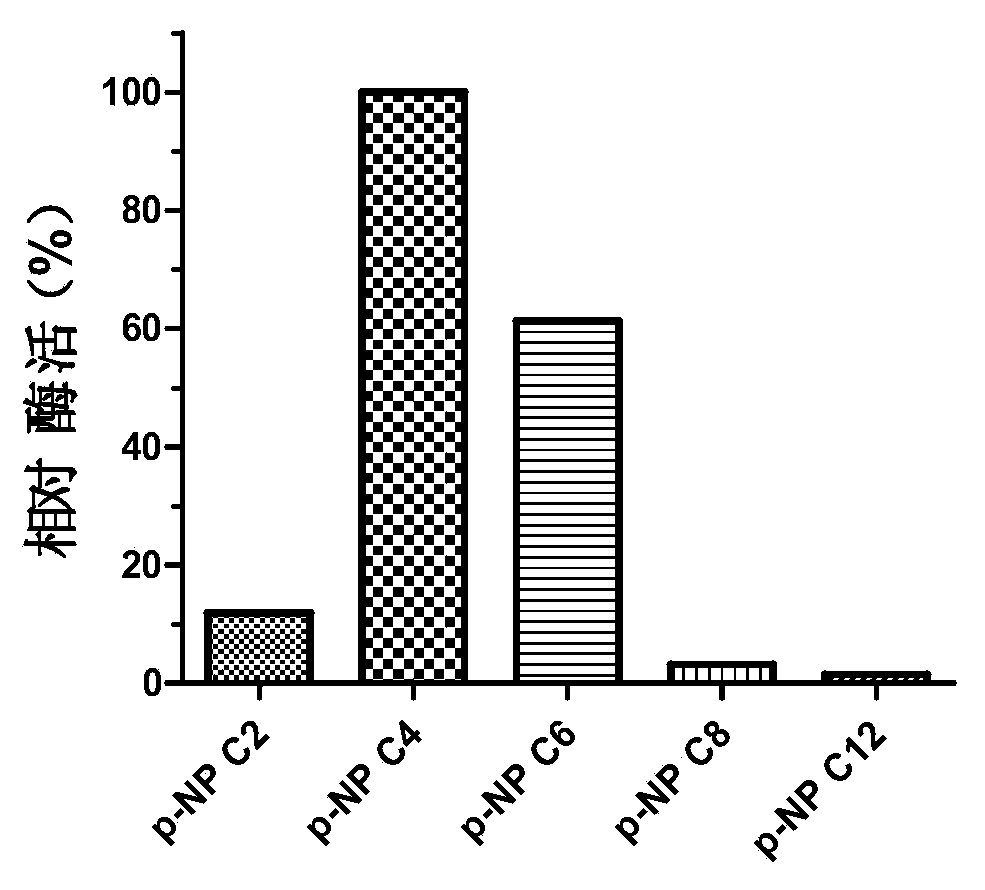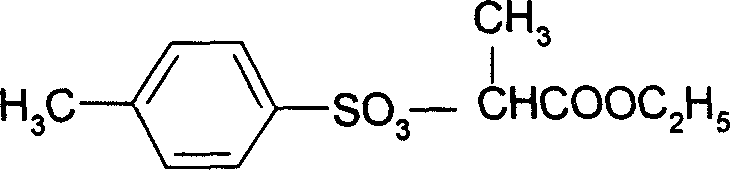Patents
Literature
108 results about "Ethyl propanoate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ethyl propionate is a compound with formula C2H5(C2H5COO). It is the ethyl ester of propionic acid. It has a pineapple-like odor. Some fruits like kiwis and strawberries naturally contain ethyl propionate in small amounts.
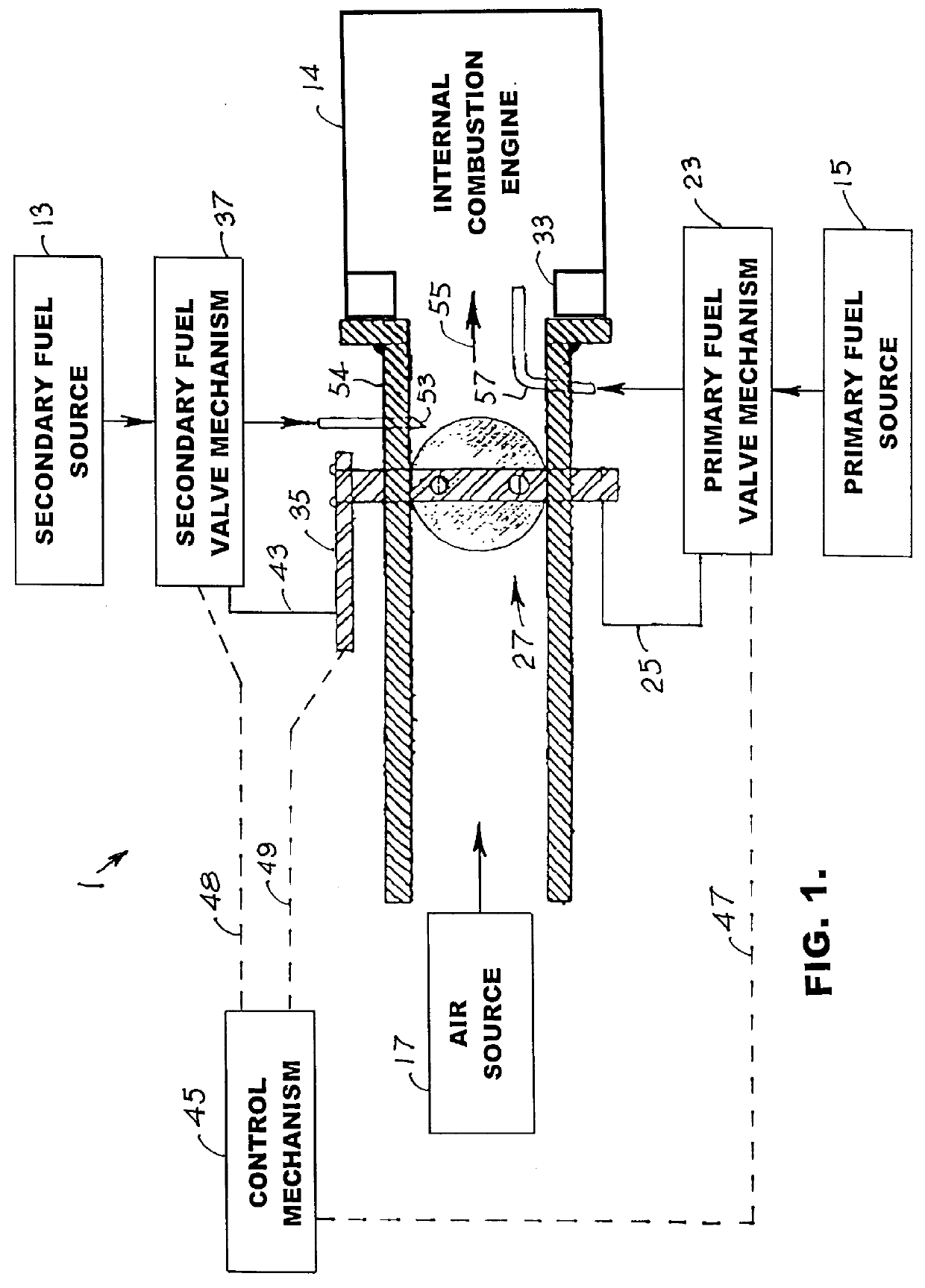
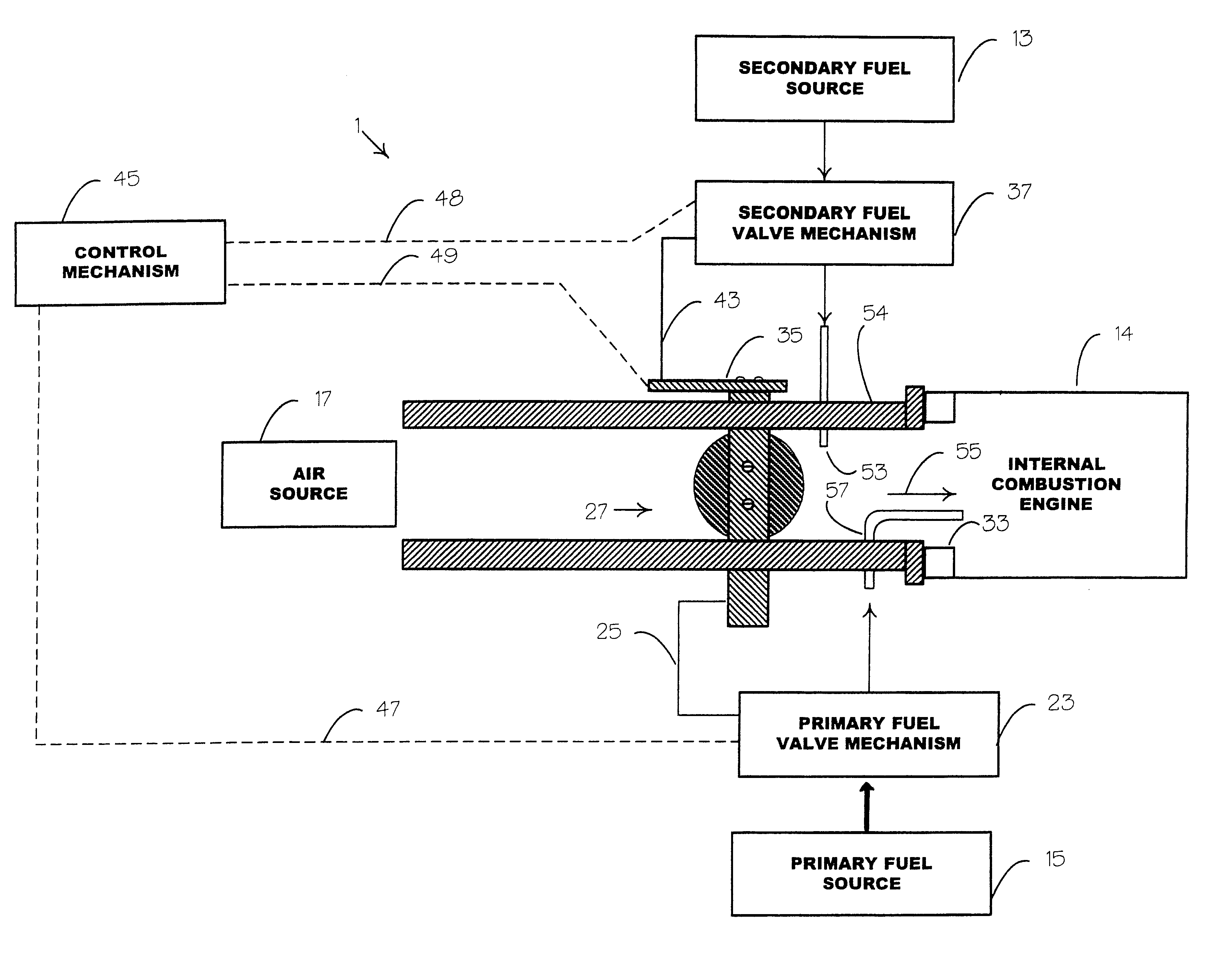
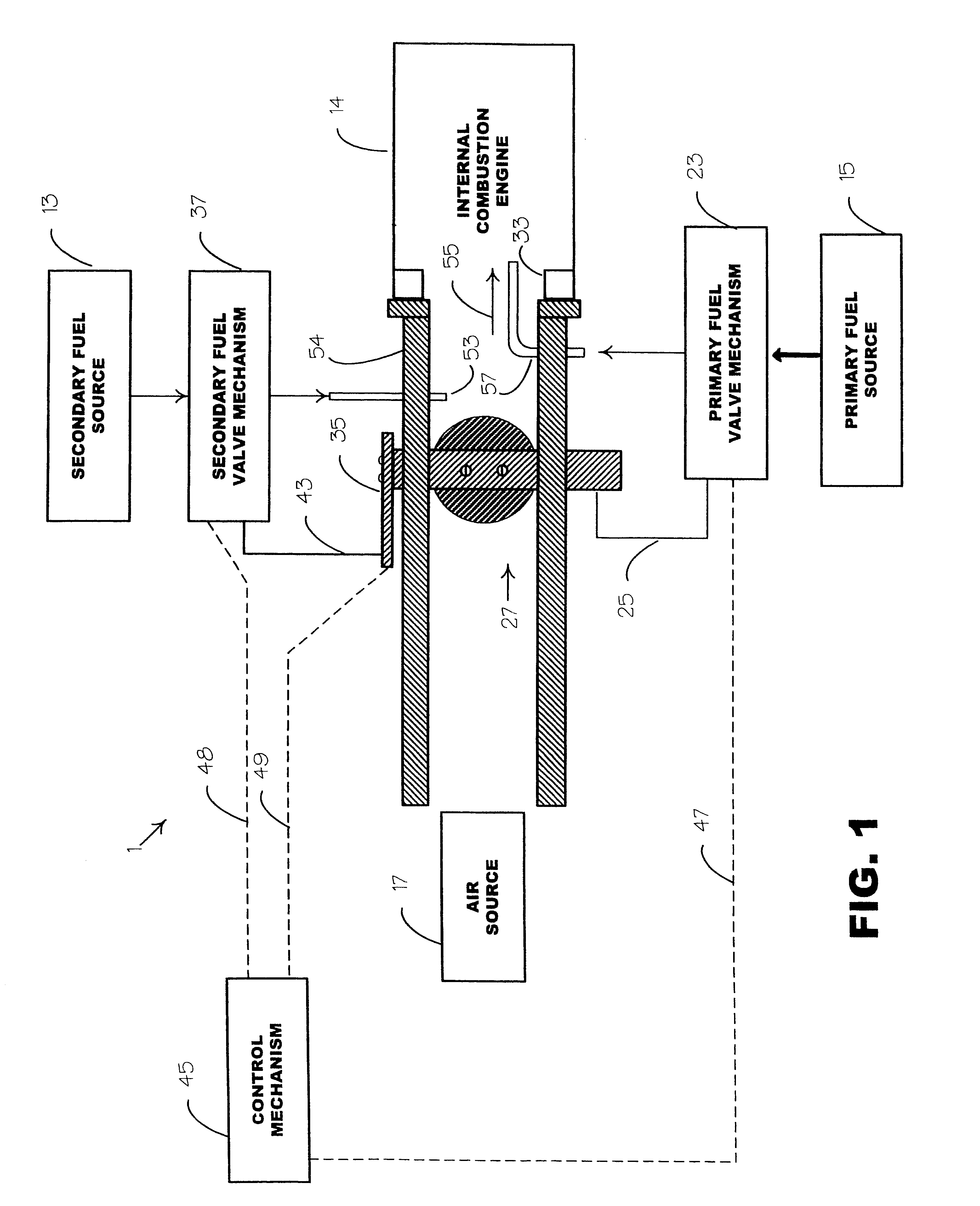
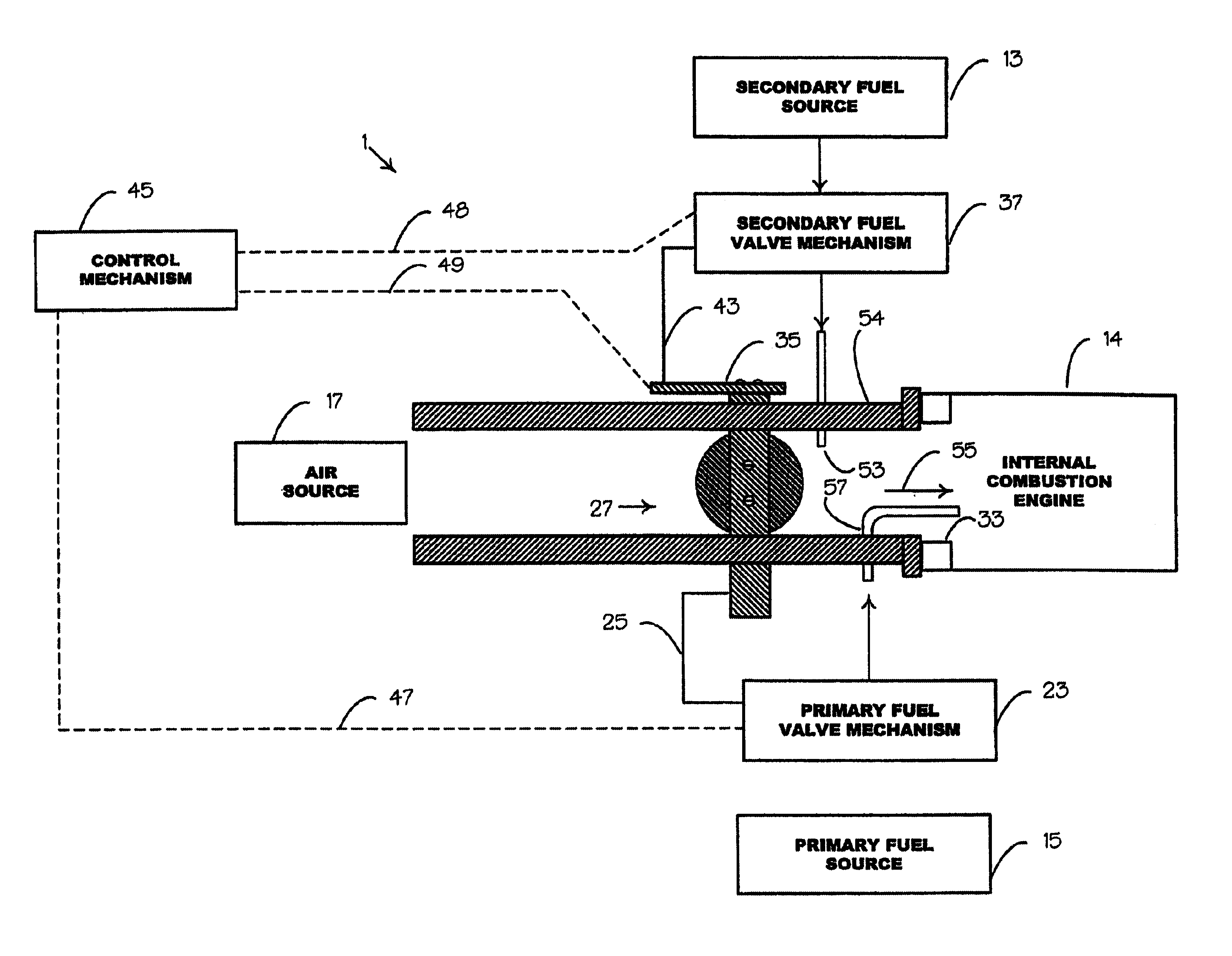
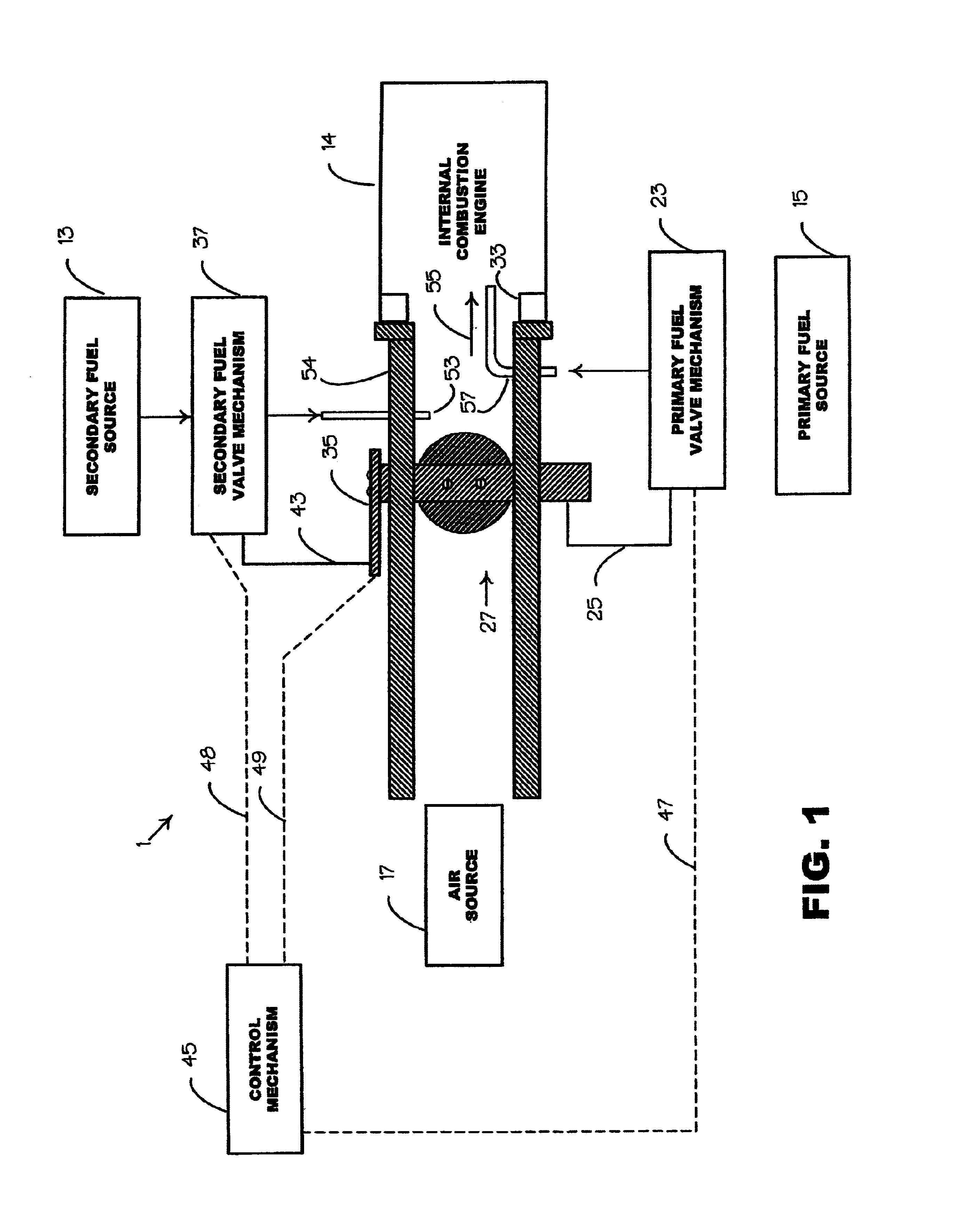
Internal combustion system using acetylene fuel
InactiveUS6076487AInternal combustion piston enginesNon-fuel substance addition to fuelCarbon chainInternal combustion engine
An environmentally clean dual fuel for an internal combustion engine, comprising acetylene as a primary fuel and a combustible fuel, such as one or more fluids selected from an alcohol such as ethanol, methanol or any other alcohol or alcohols from the group comprising C1-C20 carbon chains, ethers such as from the group comprising dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl t-butyl ether, t-amyl methyl ether, di-isopropyl ether and the like, low-molecular-weight esters such as from the group comprising methyl formate, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate and the like, or other suitable combustible fluid such as mineral spirits and the like, as a secondary fuel for operatively preventing early ignition and knock arising from the primary fuel. The dual fuel, internal combustion system, which generally utilizes a two-stage process for start-up and operation and can be operated with air- or liquid-cooling, is environmentally clean with hydrocarbon, CO, NOx, and SOx emissions substantially eliminated.
Owner:GOTEC
Internal combustion system adapted for use of a dual fuel composition including acetylene
InactiveUS6575147B2Easy to operateImprove performanceNon-fuel substance addition to fuelInternal combustion piston enginesCarbon chainMineral spirit
An internal combustion engine adapted to use an environmentally clean multi-fuel composition, comprising acetylene as a primary fuel and a combustible fuel, such as one or more fluids selected from an alcohol such as ethanol, methanol or any other alcohol or alcohols from the group comprising C1-C12 carbon chains, ethers such as from the group comprising dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl t-butyl ether, t-amyl methyl ether, di-isopropyl ether and the like, low-molecular-weight esters such as from the group comprising methyl formate, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate, ethyl malate, butyl malate, and the like, or other suitable combustible fluid such as mineral spirits and the like, as a secondary fuel for operatively preventing early ignition and knock arising from the primary fuel.
Owner:GOTEC INC
Functional pit mud for strong-flavor spirit, and manufacturing method thereof
InactiveCN102199505APrevent degradationIncrease contentMicroorganism based processesAlcoholic beverage preparationSodium acetatePhosphate
The invention discloses a functional pit mud for strong-flavor spirit. The pit mud comprises raw materials of, by weight: 70% to 75% of yellow mud, 12% to 14% of lotus pond mud, 1.2% to 1.5% of strong-flavor Daqu powder, 1% to 2% of bean cake powder, 0.05% of potassium dihydrogen phosphate, 0.8% of sodium acetate, 0.01% of magnesium sulfate, 4% to 5% of feints, and 5% to 6% of a functional bacilli liquid. The functional bacilli liquid is a mixed liquid of acetic bacilli, propionic bacilli and saccharomyces cerevisiae. The manufacturing method comprises steps of raw material preparating, mud mixing and in-pit cultivating. The functional pit mud for strong-flavor spirit provided by the present invention fundamentally prevents the pit from retrogression, and provides a good living environment for the microbes in the pit. With the functional pit mud, the flavoring components ethyl caproate and ethyl propanoate of the base spirit of the strong-flavor spirit can be increased, and the quality of the base spirit is improved.
Owner:SITIR LIQUOR
Dual fuel composition including acetylene
InactiveUS7288127B1Easy to operateImprove performanceInternal combustion piston enginesNon-fuel substance addition to fuelCarbon chainDiethyl ether
An environmentally clean dual fuel for use in an internal combustion engine, comprising acetylene as a primary fuel and a combustible fuel, such as one or more fluids selected from an alcohol such as ethanol, methanol or any other alcohol or alcohols from the group comprising C1-C20 carbon chains, ethers such as from the group comprising dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl t-butyl ether, t-amyl methyl ether, di-isopropyl ether and the like, low-molecular-weight esters such as from the group comprising methyl formate, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate, ethyl malate, and butyl malate, and the like, or other suitable combustible fluid such as mineral spirits and the like, as a secondary fuel for operatively preventing early ignition and knock arising from the primary fuel.
Owner:GOTEC
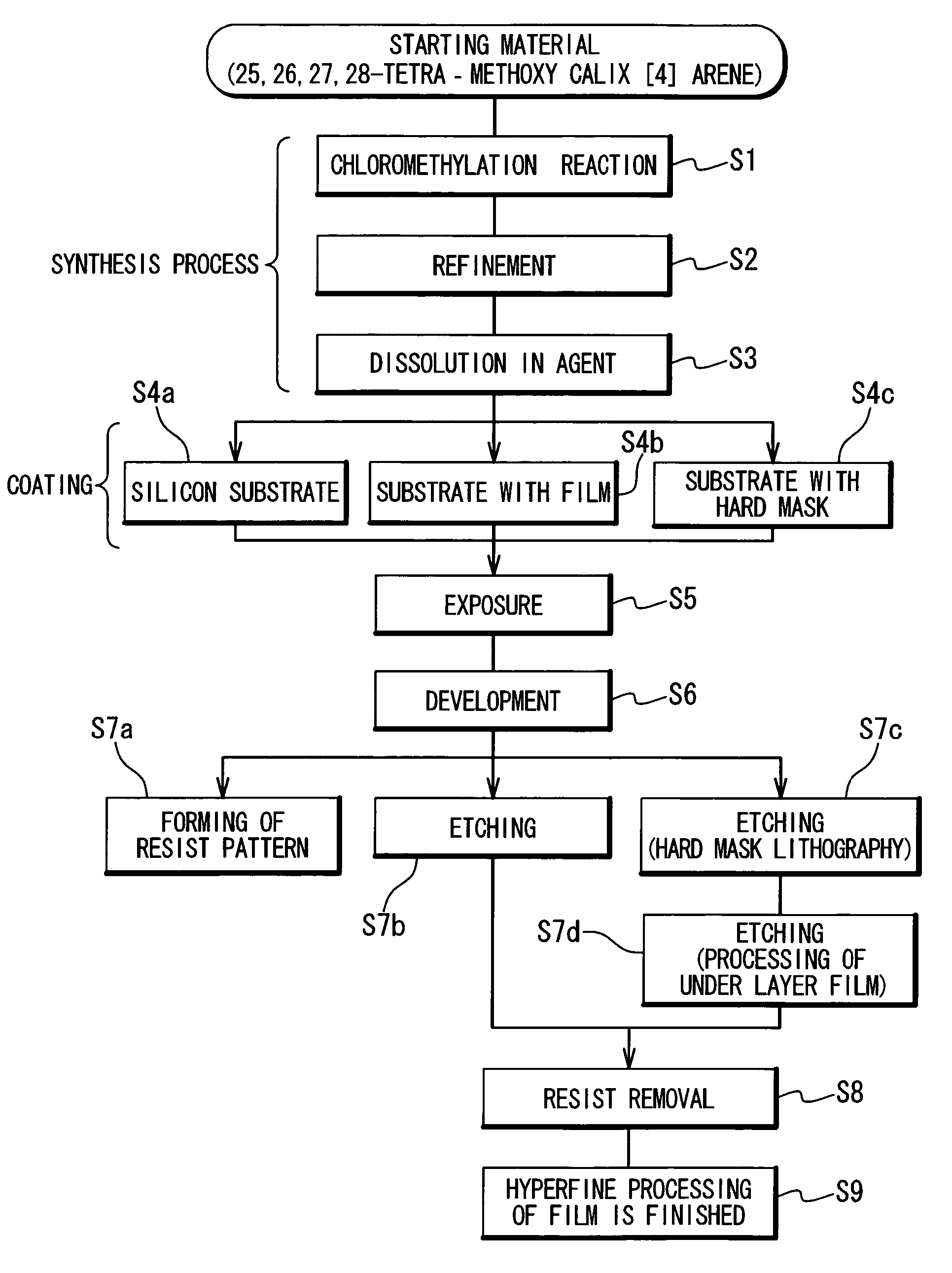
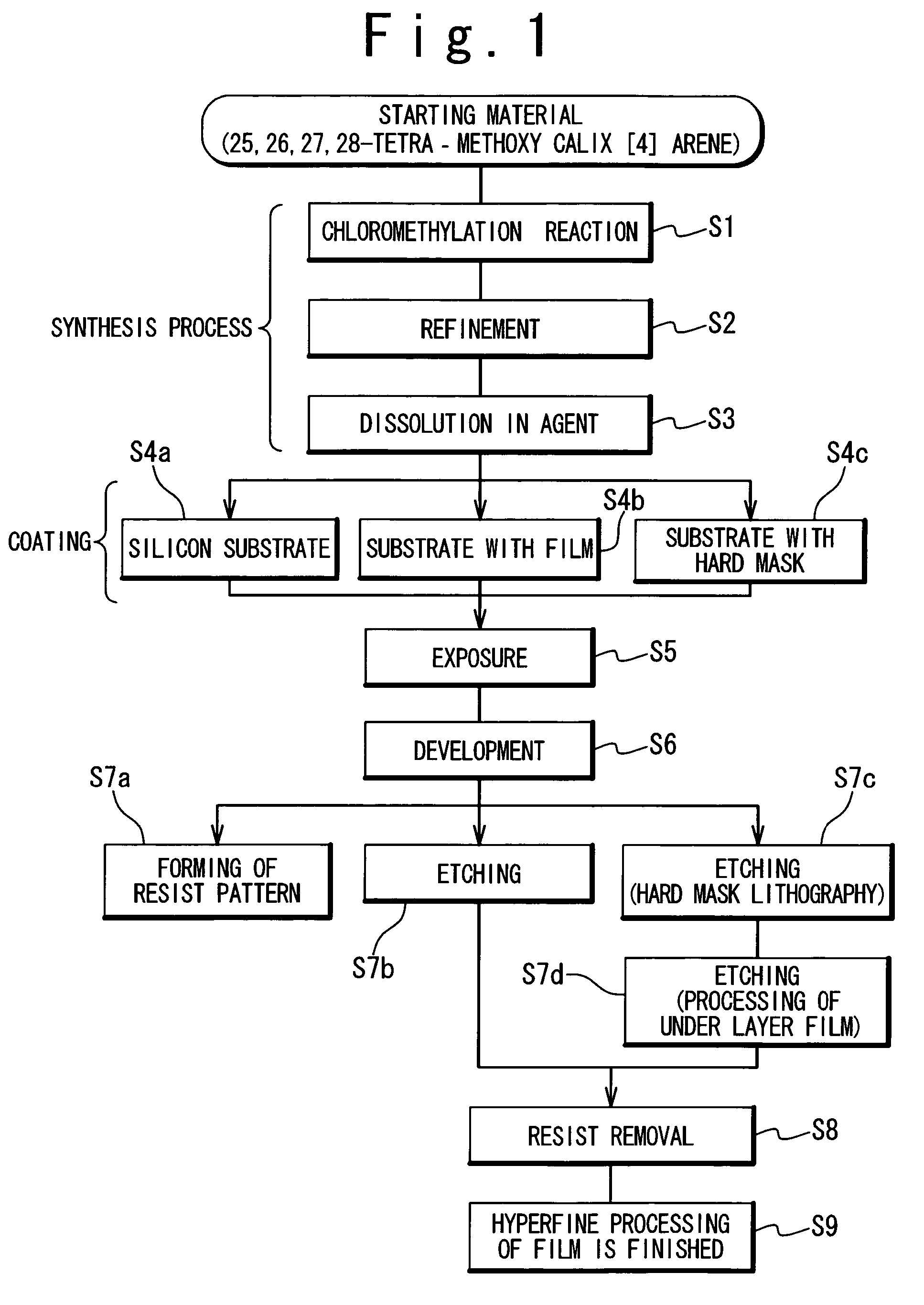
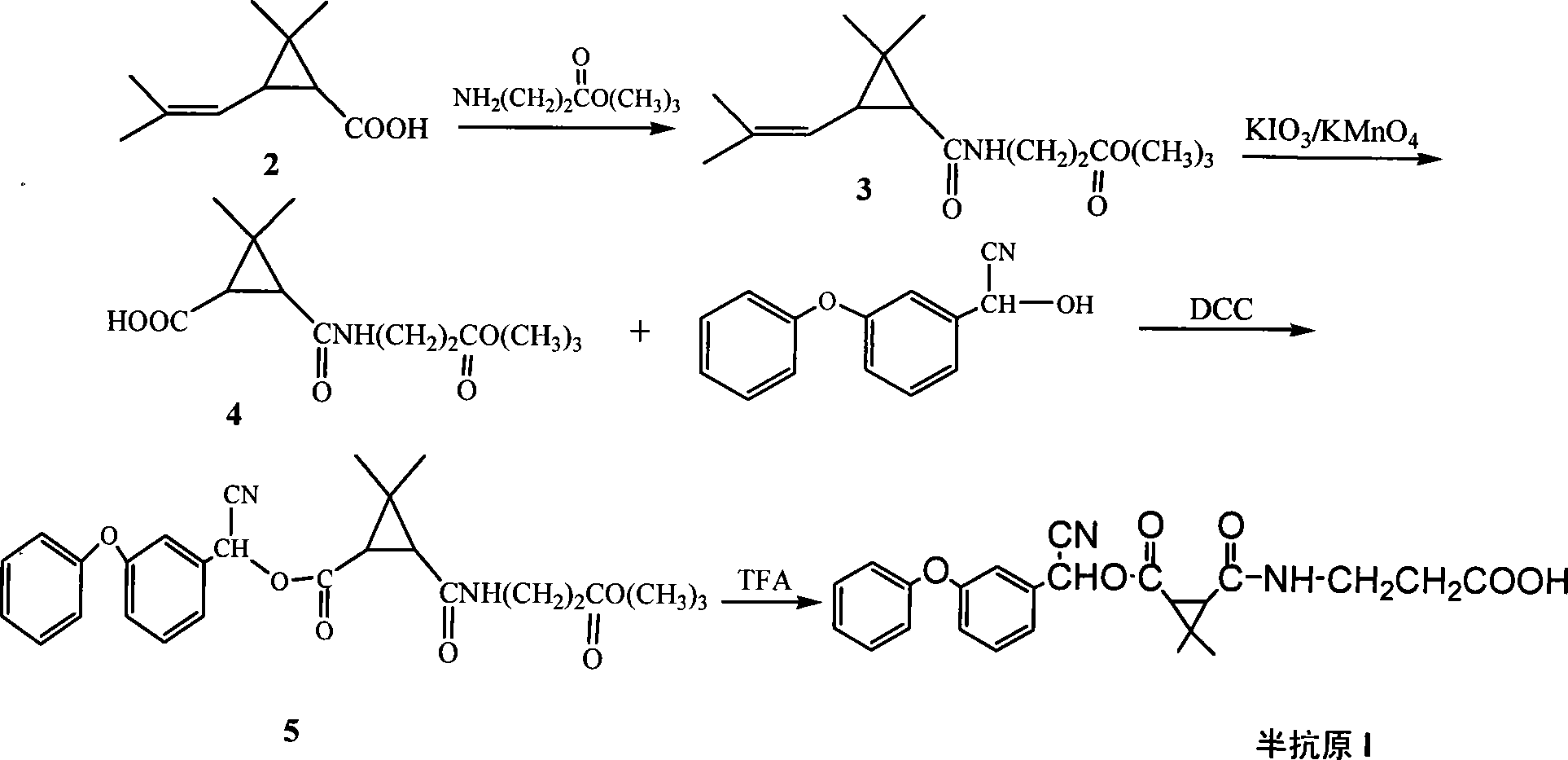
Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine
InactiveCN105622616AIncrease productivityImprove product qualityOrganic chemistryCyanoacetic acidFormamidine acetate
The invention relates to a preparation method of 4-chloropyrrolo[2,3-d]pyrimidine.The method includes the following steps of obtaining 2-cyano-3-(1,3-dioxolan)ethyl propionate after 2-bromomethyl-1,3-dioxolane and ethyl cyanoacetate which are used as the raw materials react with alkaline matter as the catalyst; conducting cyclization on obtained 2-cyano-3-(1,3-dioxolan)ethyl propionate and formamidine acetate with alkaline matter as the catalyst, and adding hydrochloric acid for hydrolysis cyclization to obtain pyrrolo[2,3-d]pyrimidin-4-ol; making obtained pyrrolo[2,3-d]pyrimidin-4-ol react with phosphorus oxychloride to obtain 4-chloropyrrolo[2,3-d]pyrimidine.The method for preparing 4-chloropyrrolo[2,3-d]pyrimidine is simple in technological process, the requirement for production conditions is low, the product is easy to purify and high in yield, and the production efficiency and product quality of 4-chloropyrrolo[2,3-d]pyrimidine are remarkably improved.
Owner:ABA CHEM SHANGHAI
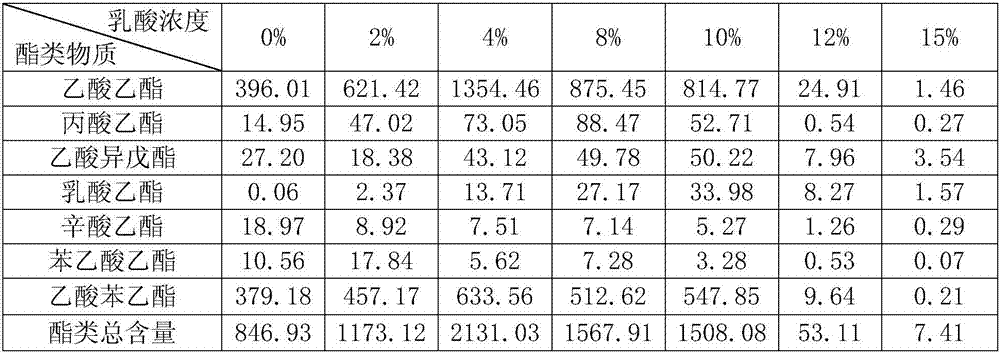
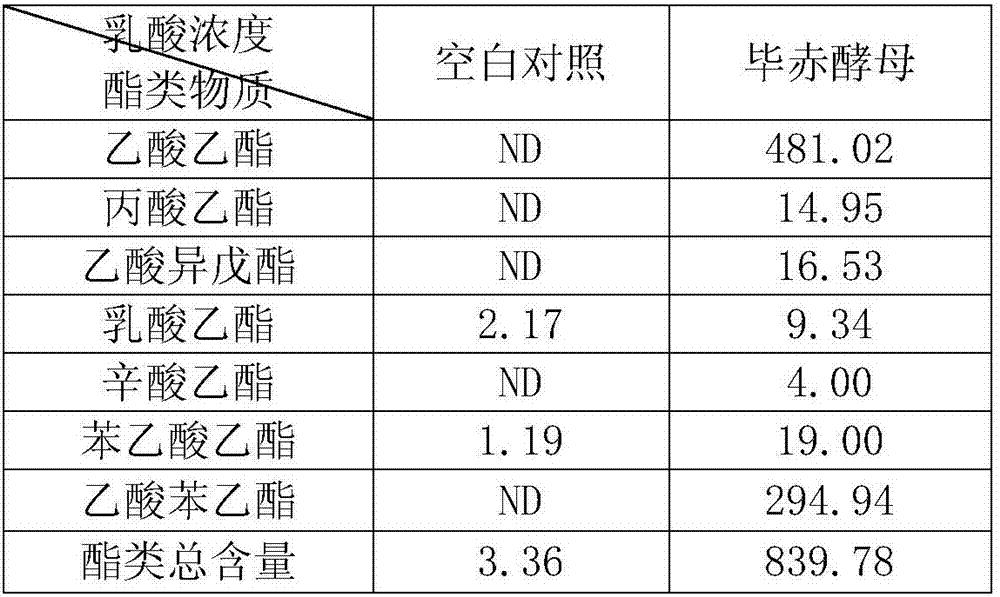
Lactic acid-tolerant ester-producing pichia pastoris
ActiveCN107287127ARapid growthImprove adaptabilityFungiMicroorganism based processesPichia pastorisEthyl phenylacetate
The invention discloses a lactic acid-tolerant ester-producing pichia pastoris, and belongs to the technical field of a bioengineering technology and a brewing biotechnology. The strain is preserved at the General Microbiology Center of the China Committee for Culture Collection of Microorganisms on April 24, 2017, the classification is named pichia kudriavzevii (Pichia kudriavzevii) and the preservation number is CGMCC No.14068. The pichia pastoris is high in environmental tolerance, is capable of tolerating 0-15% lactic acid, is capable of metabolizing to generate multiple ethyl ester volatile flavor substances, such as ethyl acetate, ethyl propionate, ethyl caprylate, phenethyl acetate and ethyl phenylacetate in the environment of 0-12% lactic acid, and is a brewing functional strain with excellent performance.
Owner:KWEICHOW MOUTAI COMPANY
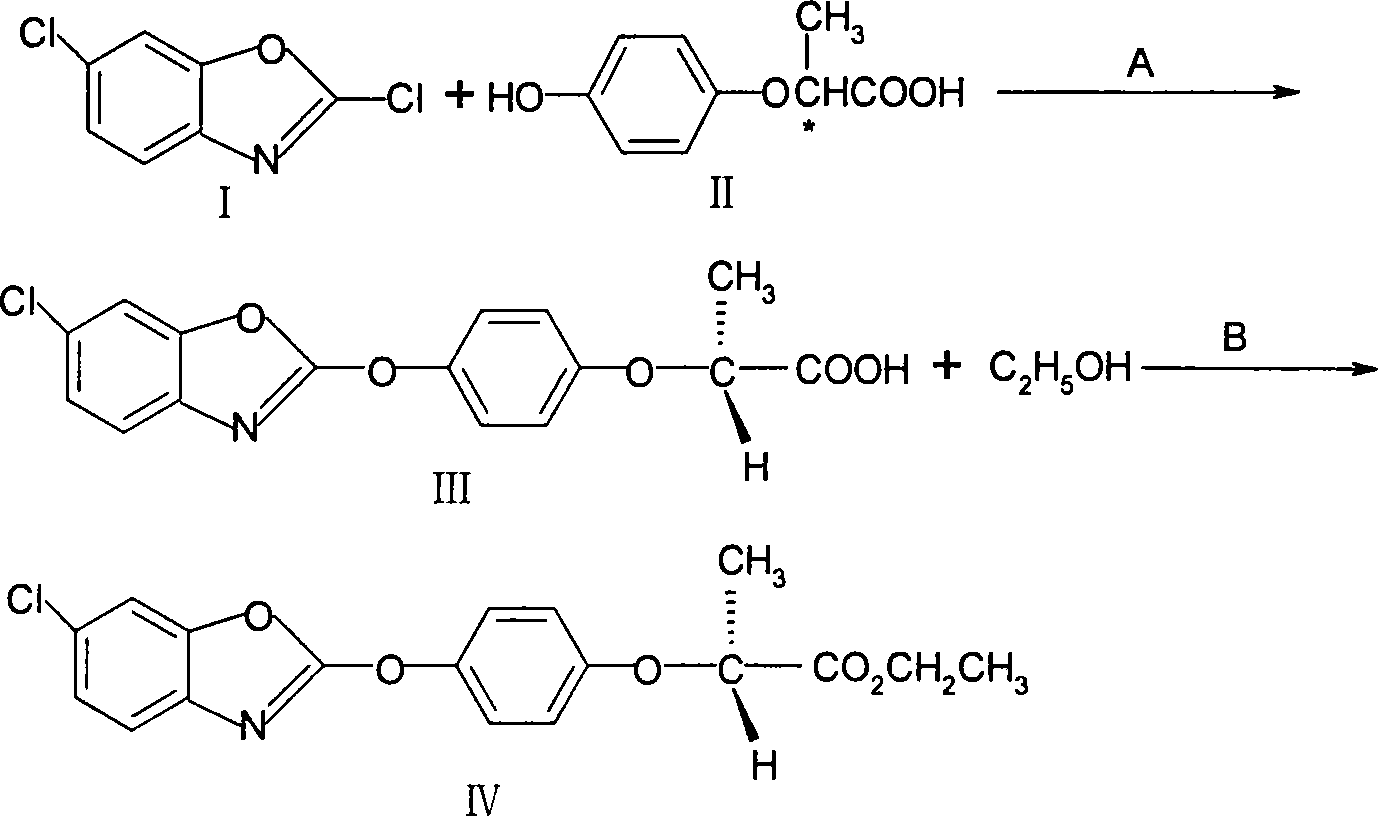
Method for preparing herbicide fenoxaprop-p-ethyl
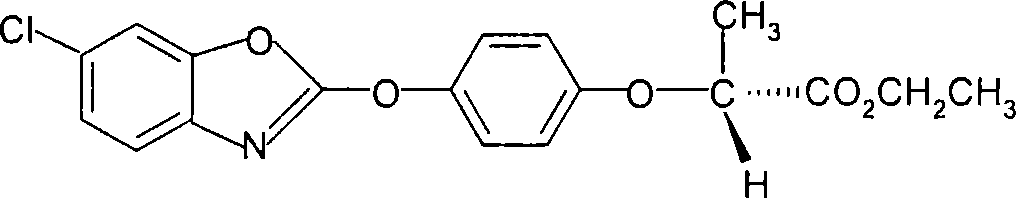
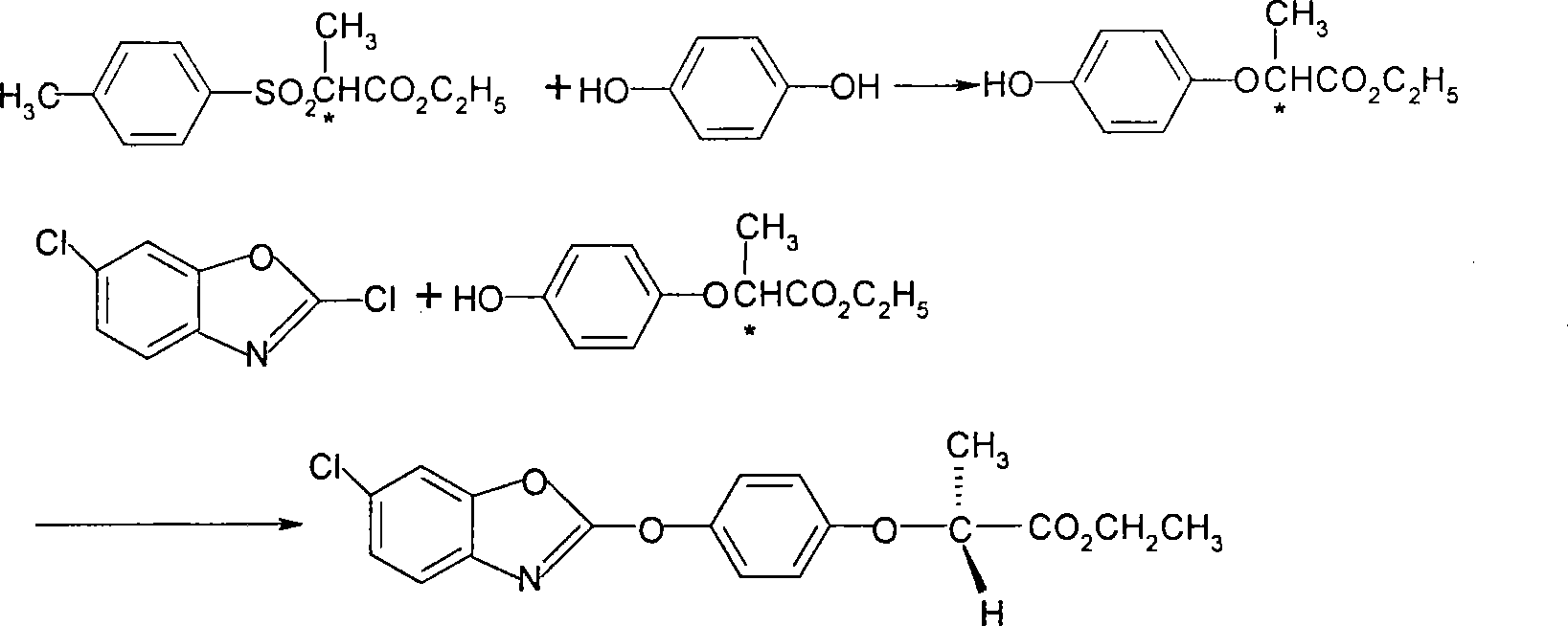
The invention discloses a method for preparing fenoxaprop-p-ethyl of herbicide, which is characterized in that firstly, a compound (I) 2, 6-dichloro benzoxazoles and a compound (II) 2-(4-hydroxyphenoxy) propionic acid are reacted under alkaline condition, thereby getting (III) compound (R) -2-[4-(6-chlorine-1, 3-benzoxazoles-2-based oxygen) phenoxyl] propionic acid, and then reacted with ethanol to get a compound (IV)(R)-2-[4-(6-chlorine-1, 3-benzoxazoles-2-based oxygen) phenoxyl] ethyl propionate. The invention has the advantages of short reaction line, high yield, raw material saving, cost saving, less environmental pollution and high product quality.
Owner:JIANGSU TIANRONG GROUP
Application of modified calcium carbonate in plastics
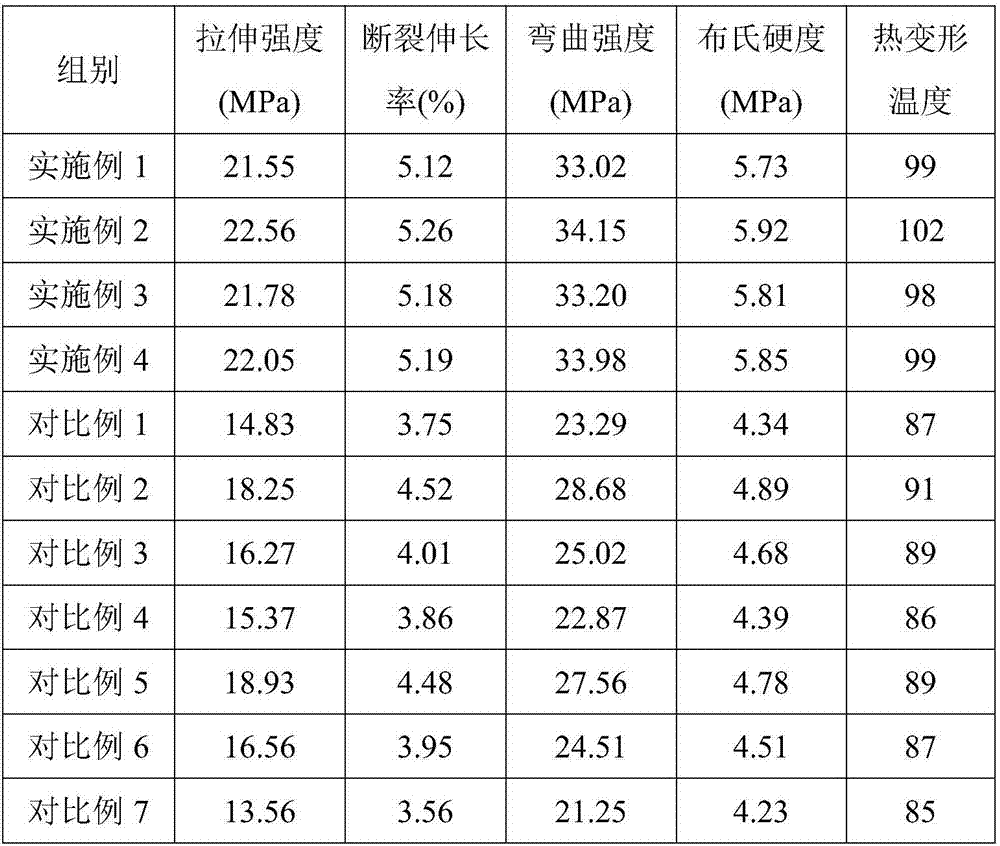
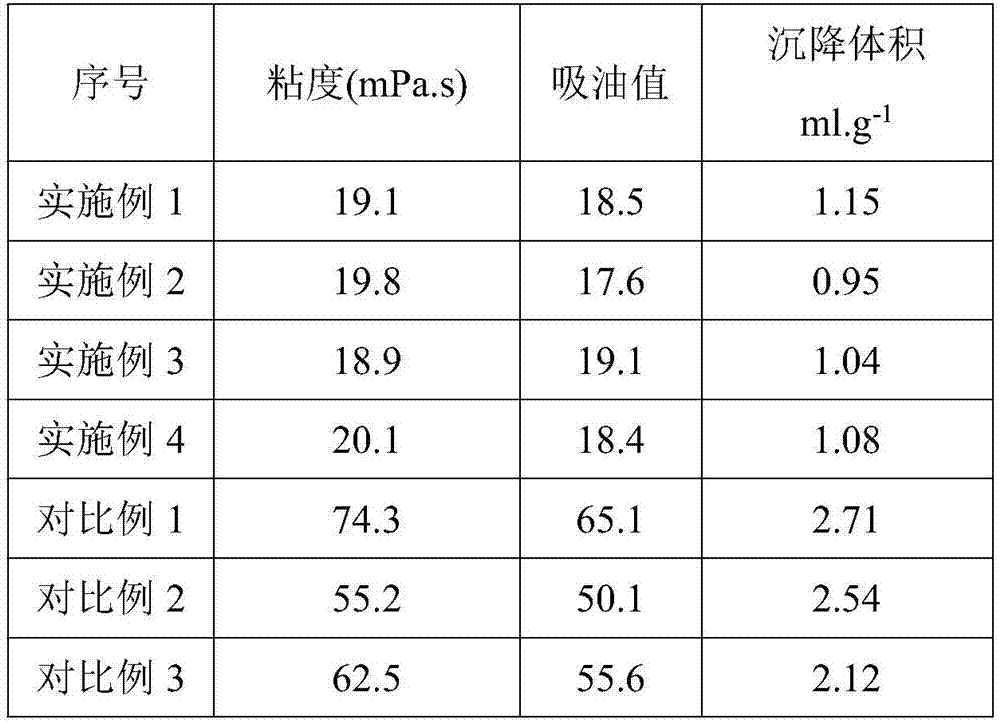
InactiveCN107501891AHigh tensile strengthHigh elongation at breakFlexural strengthThermal deformation
The invention provides application of modified calcium carbonate in plastics. The plastics are prepared from the following raw materials in parts by weight: 3 to 5 parts of modified calcium carbonate, 1 to 3 parts of ethyl propionate, 4 to 6 parts of glass fibers, 40 to 50 parts of polycarbonate, 0.5 to 1 part of alunite, and 0.5 to 1 part of cross-linking agent. The modified calcium carbonate provided by the invention is added in the production process of the plastics, and the prepared plastics have good tensile strength, elongation at break, bending strength and brinell hardness, and high thermal deformation temperature.
Owner:贺州钟山县双文碳酸钙新材料有限公司
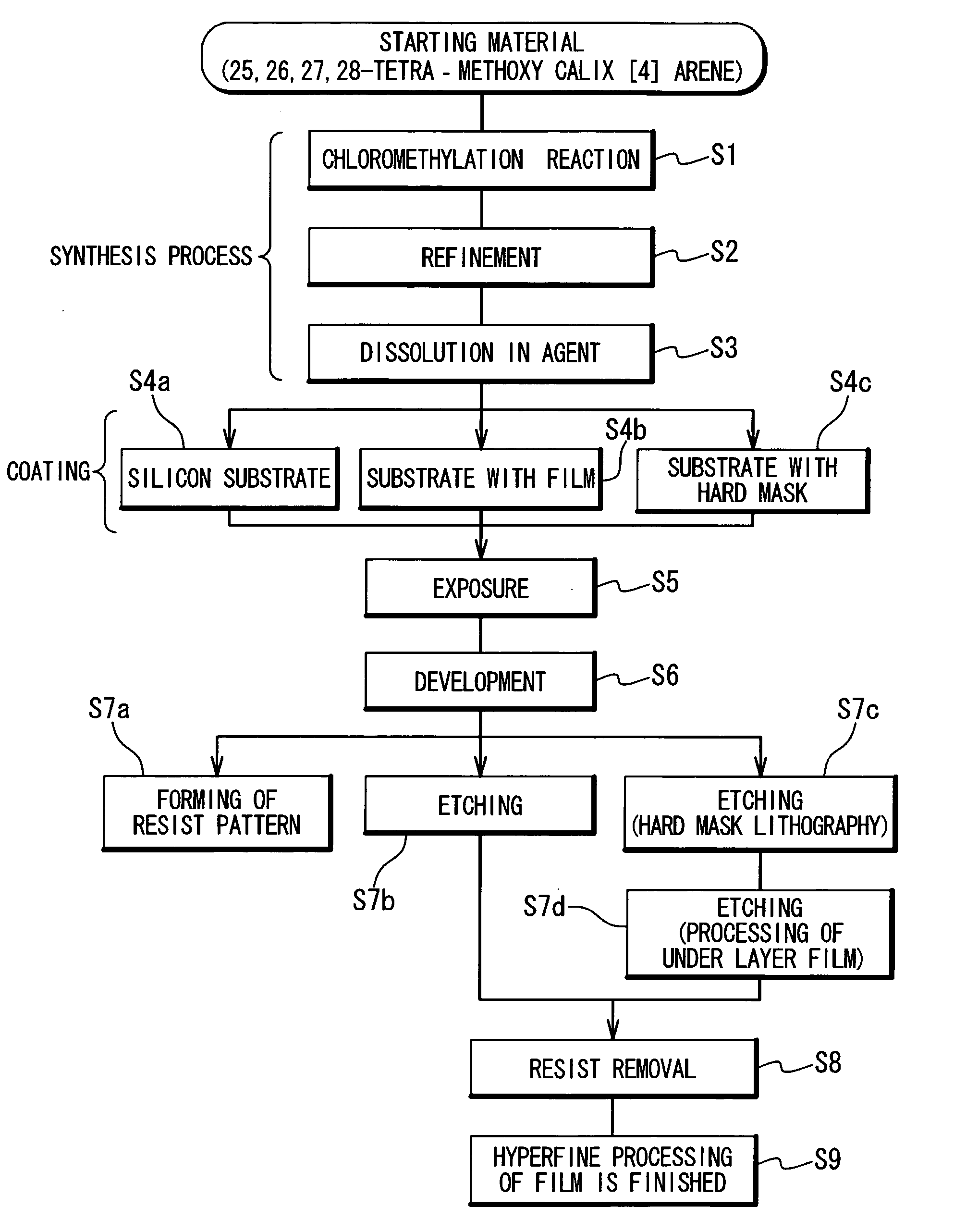
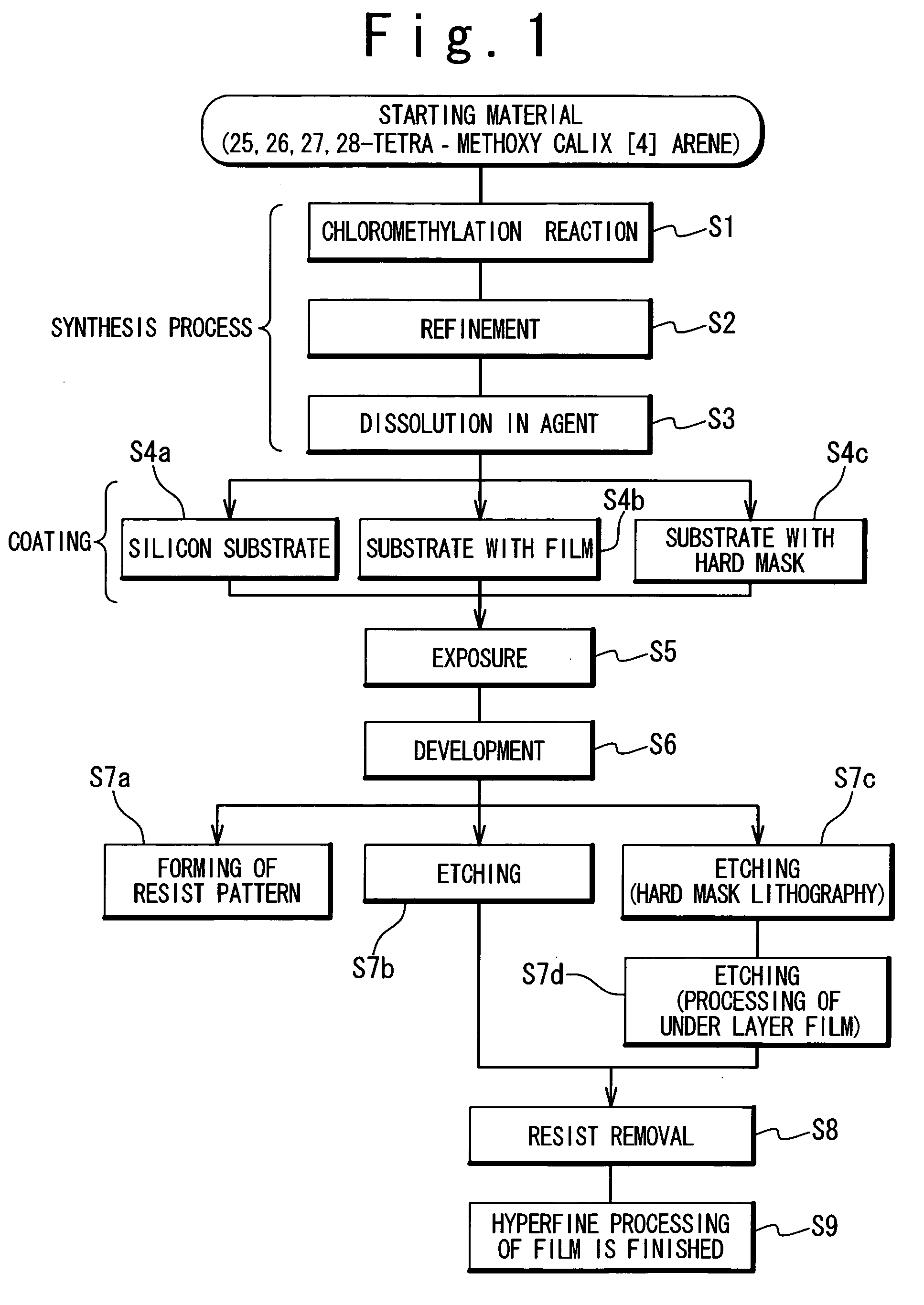
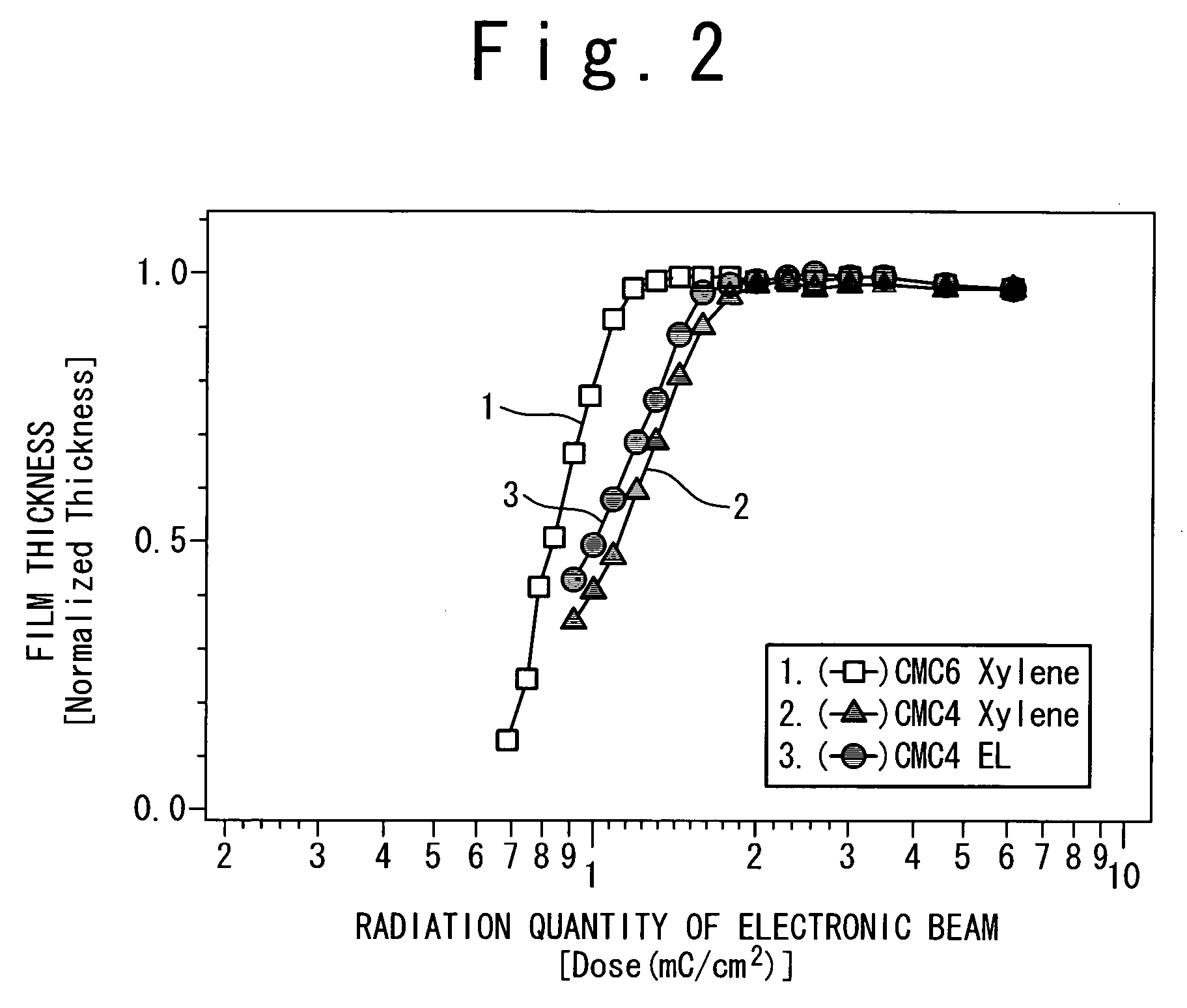
Resist and method of forming resist pattern
InactiveUS20060127798A1High resolutionWorsen working environmentRadiation applicationsPhotomechanical apparatusResistTetramethylammonium hydroxide
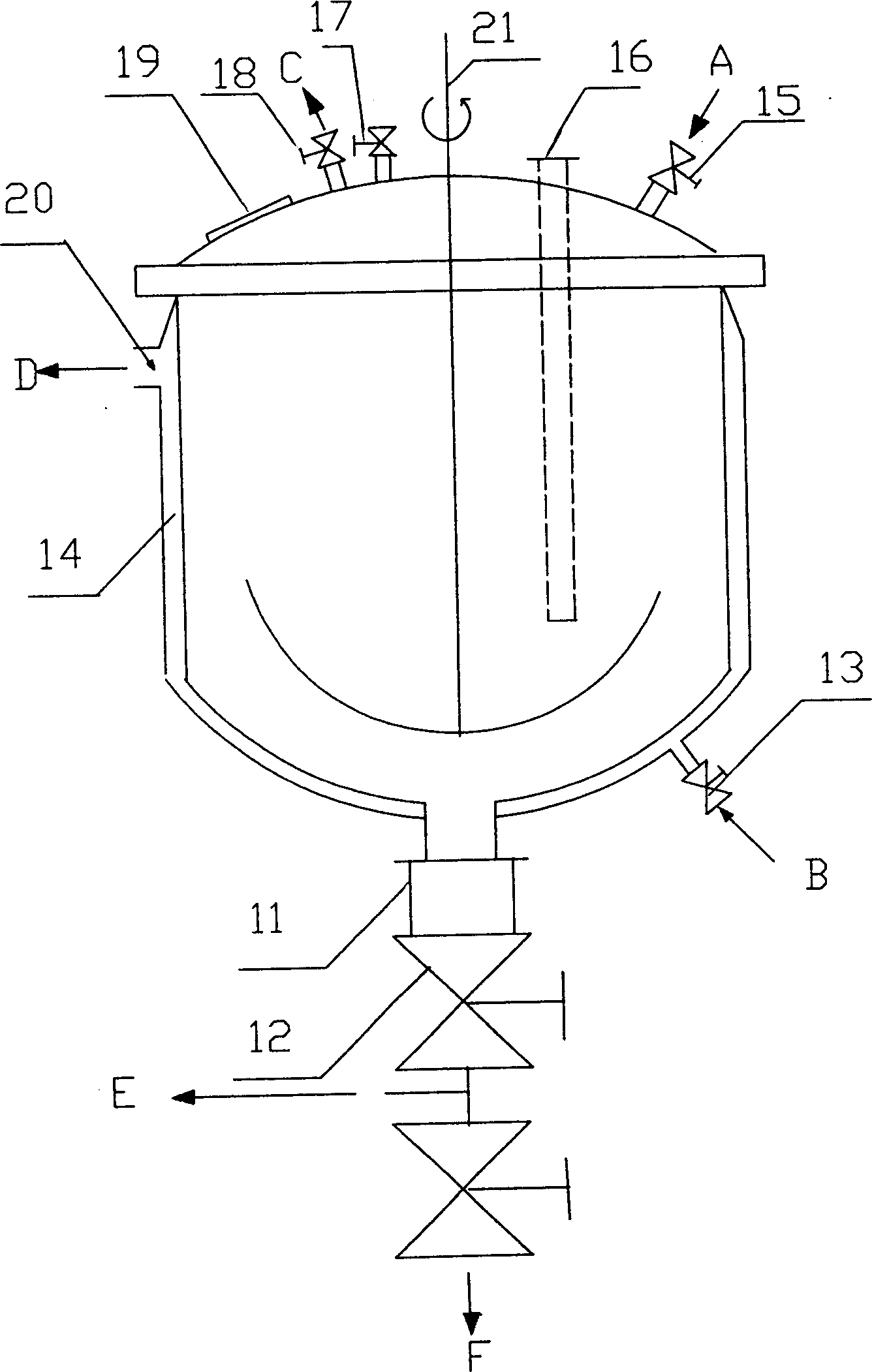
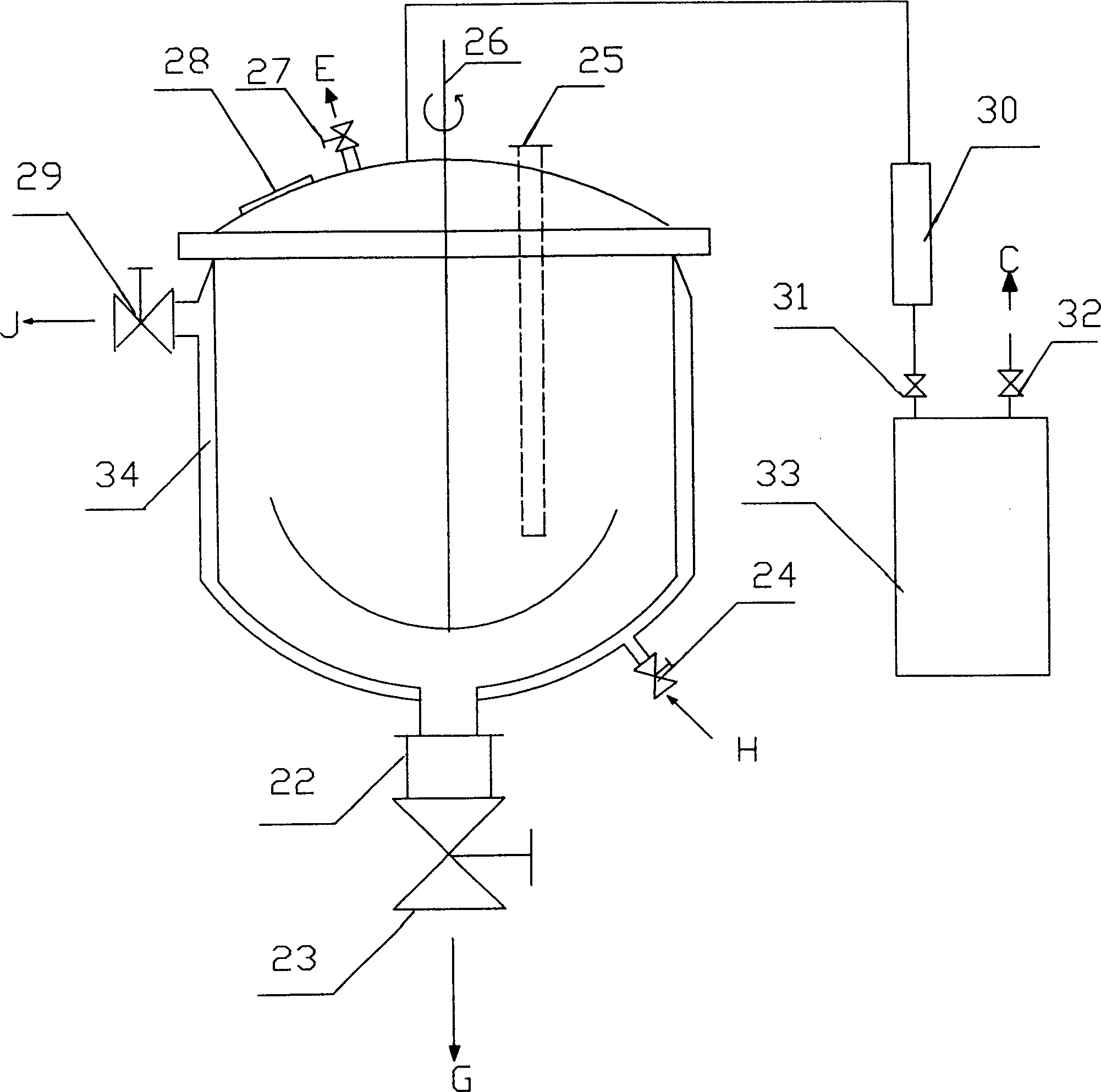
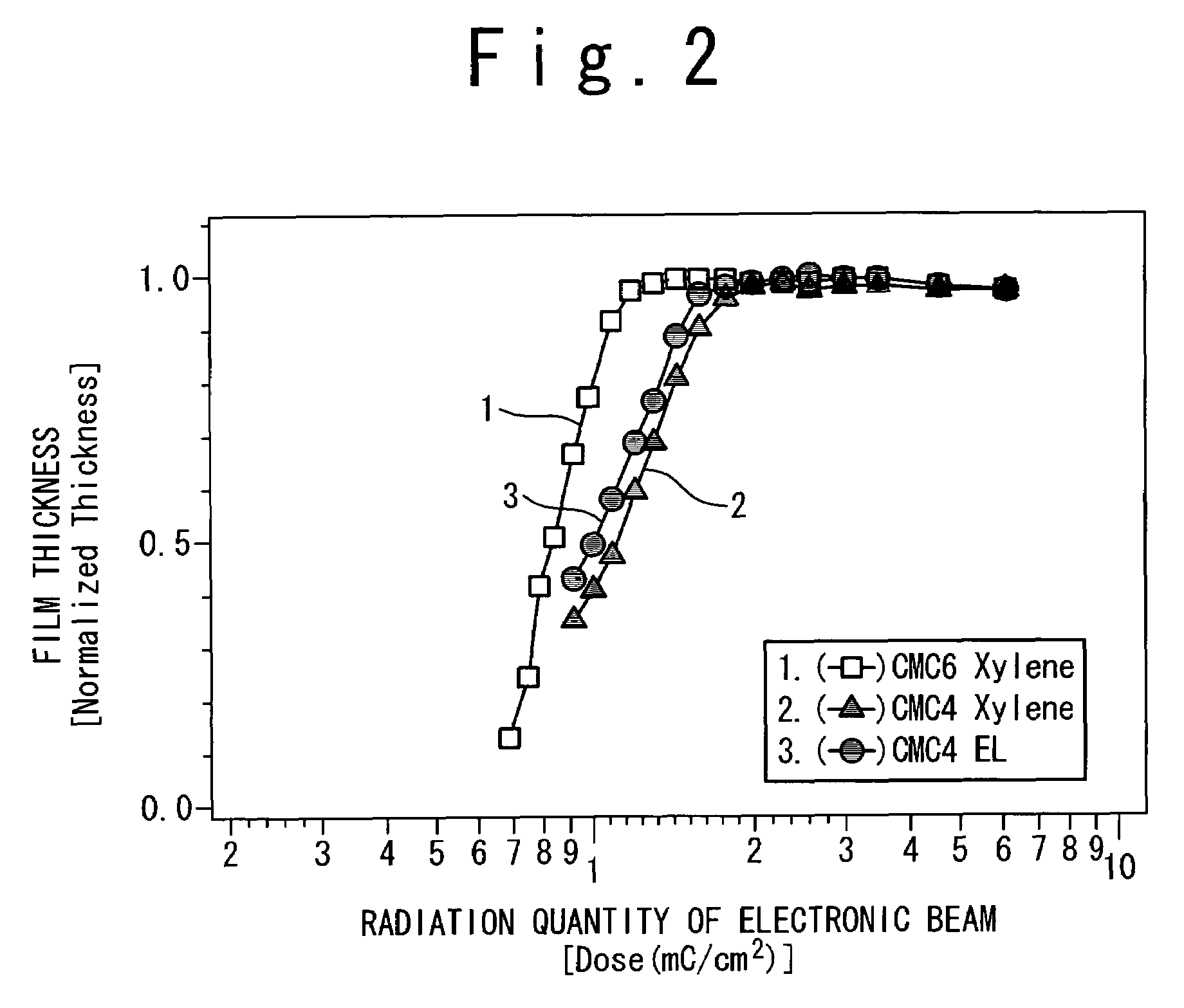
The resist according to the present invention includes any one of tetrachloromethyl tetramethoxycalix [4] arene and trichloromethyl tetramethoxycalix [4] arene. The resist including such kind of components is soluble in the solvent having less effect to worsen a working environment, namely, ethyl lactate (EL), propylene glycol monomethyl ether (PGME), propylene glycol monomethyl ether acetate (PGMEA), ethyl propionate, n-butyl acetate and 2-heptanone. It can be developed by tetra-methyl ammonium hydroxide in addition to the above mentioned solvent. By exposing this resist by electronic ray, high resolution of 8 nm is attained, and by using this resist as a mask, various materials can be formed into a hyperfine shape. According to such kind of resist, a photosensitive resist material which has high resolution and solvable to solvents having less effect to worsen the working environment and can be developed by the solvents, a exposure method using it, and a hyperfine processing method using it are provided.
Owner:NEC CORP +1
Novel esterase as well as coding gene and application of esterase
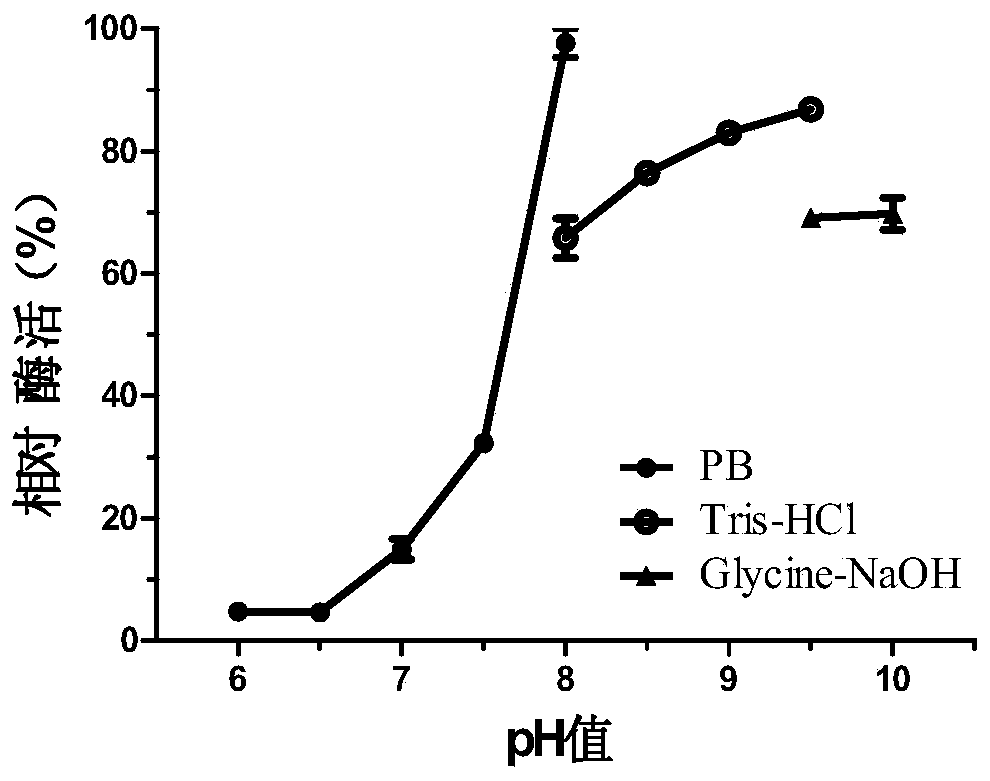
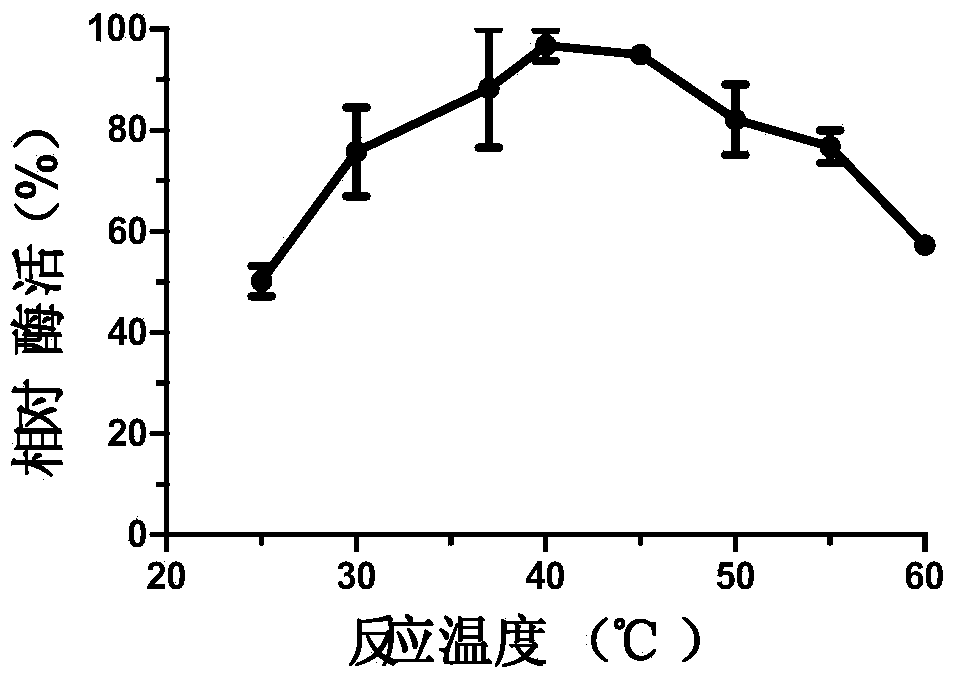
InactiveCN104140959AImprove qualityMild reaction conditionsHydrolasesMicroorganism based processesPropanoic acidNucleotide
The invention discloses a novel esterase as well as a coding gene and an application of esterase. The esterase EST04211 has an amino acid sequence shown in SEQ ID NO. 2. The amino acid sequence of the gene of the esterase EST04211 is shown in SEQ ID NO. 1. The novel esterase disclosed by the invention is obtained by cloning Bacillus SCSIO15121 derived from deep sea of the Indian Ocean. The esterase has the largest characteristic that the esterification rate is 97% in catalytic synthesis of ethyl isobutyrate, and under similar conditions, the esterase can also catalyze propionic acid, butyric acid, valeric acid, caproic acid and ethanol, propanol, butanol, pentanol and hexanol to synthesize corresponding short-chain aromatic ester flavors, such as ethyl propionate, propyl propionate and butyl propionate, the esterification rate mostly reaches 85%-95%. The esterase can be applied in the industrial bio-manufacturing of the short-chain aromatic flavors, such as ethyl isobutyrate and the ester flavors are high in quality, belongs to natural products and can be applied in production industries, such as foods, cigarettes and daily chemical products.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Technology of synthesizing 2,3-dicyano ethyl propionate
InactiveCN1785966AHigh yieldImprove product qualityPreparation by cyanide reactionParaformaldehydeEthyl fumarate
The present invention relates to a process for synthesizing ethyl-2,3-dicyano-propionate which is used for produce pesticide intermediate. The basic method of said process includes the following steps: using the raw materials of ethyl cyanoacetate, paraformaldehyde and sodium cyanide to synthesize ethyl-2,3-dicyano-propionate in the medium dimethyl sulfoxide; after the ethyl-2,3-dicyano-propionate is synthesized, using solvent dichloromethane to extract the synthesized ethyl-2,3-dicyano-propionate from medium dimethyl sulfoxide, reduced pressure desolventizing to obtain crude product, then rectifying said crude product so as to obtain the refined product ethyl-2,3-cyano-propionate. Said invention also provides the concrete requirements of the above-mentioned every step. The purity of the obtained product is greater than 98%.
Owner:栾忠岳
Resist and method of forming resist pattern
InactiveUS7514197B2Difficult to useImprove expectationsPhotosensitive materialsRadiation applicationsTetramethylammonium hydroxideResist
The resist according to the present invention includes any one of tetrachloromethyl tetramethoxycalix [4] arene and trichloromethyl tetramethoxycalix [4] arene. The resist including such kind of components is soluble in the solvent having less effect to worsen a working environment, namely, ethyl lactate (EL), propylene glycol monomethyl ether (PGME), propylene glycol monomethyl ether acetate (PGMEA), ethyl propionate, n-butyl acetate and 2-heptanone. It can be developed by tetra-methyl ammonium hydroxide in addition to the above mentioned solvent. By exposing this resist by electronic ray, high resolution of 8 nm is attained, and by using this resist as a mask, various materials can be formed into a hyperfine shape. According to such kind of resist, a photosensitive resist material which has high resolution and solvable to solvents having less effect to worsen the working environment and can be developed by the solvents, a exposure method using it, and a hyperfine processing method using it are provided.
Owner:NEC CORP +1
Process for crylic acid azeotropism refining and recovering acetic acid
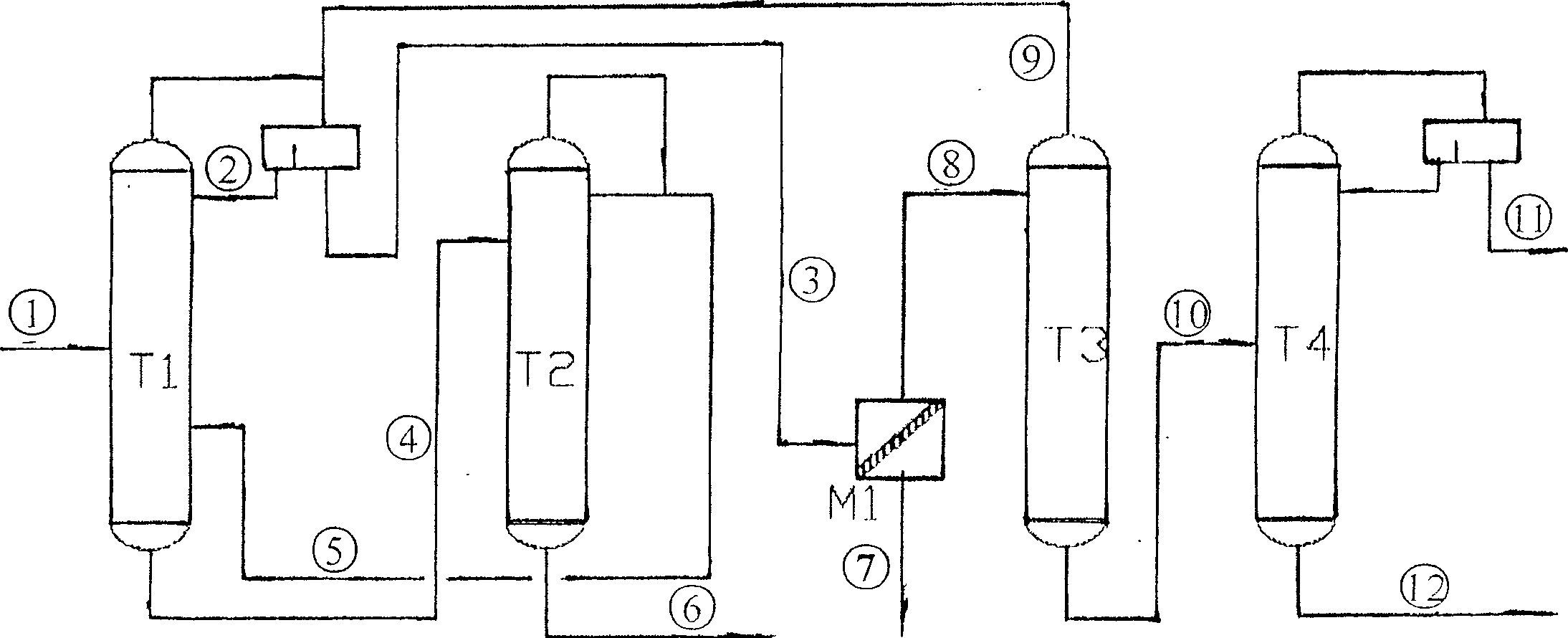
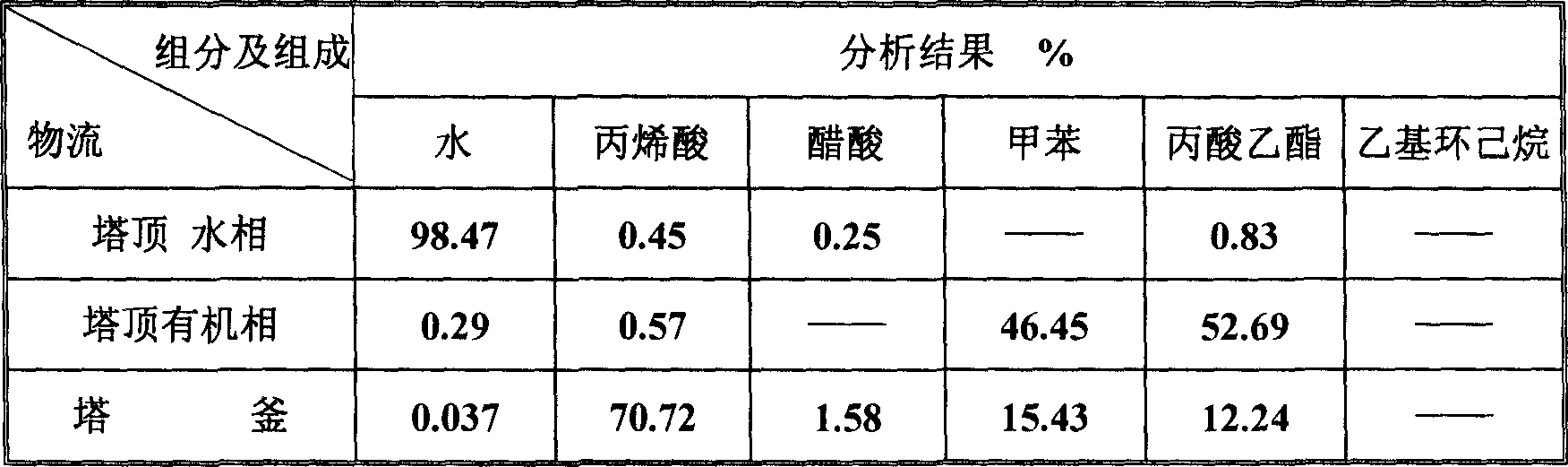
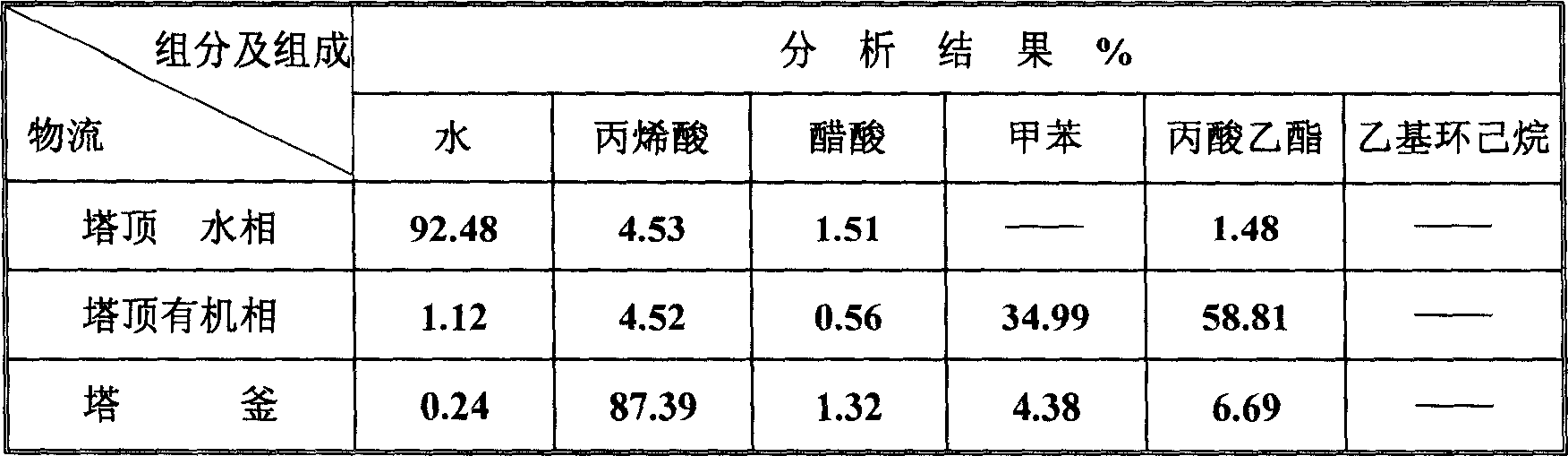
ActiveCN1865216AReduce operating loadAlleviate high temperature self-polymerizationCarboxylic compound separation/purificationOrganic filmAzeotropic distillation
This invention relates to a process for producing acroleic acid and esters thereof, concretely to acroleic acid azeotropic refining and acetic acid recovering process, wherein: apply ethyl cyclohexane, toluene, ethyl propionate and toluene derivative as the entrainer during the acroleic acid azeotropic distillation, set up a acroleic acid azeotropic column and an acetic acid removing column to remove the water and acetic acid in the crude acroleic acid solution, set up an organic film and air extraction column and an acetic acid azeotropic column to purify the 2-8% side produced acetic acid to be acetic acid product with concentration more than 85%. This invention is characterized of high dehydration rate and acetic acid removing rate. The byproduct acetic ester can be produced to be acetic acid product, which saves waste water treatment cost and improves the equipment benefits. By applying ethyl cyclohexane, toluene, ethyl propionate and toluene derivative as the entrainer, it can decrease the bottom temperature of the acroleic acid azeotropic column, which can decrease the polymerization tendency of the acroleic acid, improve the conversion rate of propone, decrease the acroleic acid carry-over loss and acroleic acid consumption, improve the product yield, extend the production cycle and improve the economical benefits of the apparatus.
Owner:CHINA GASOLINEEUM ENG
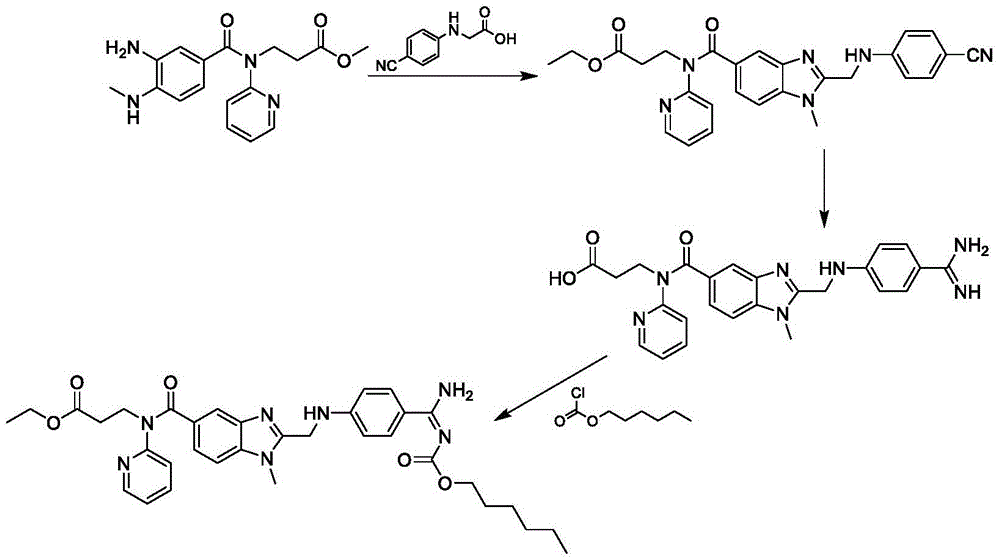
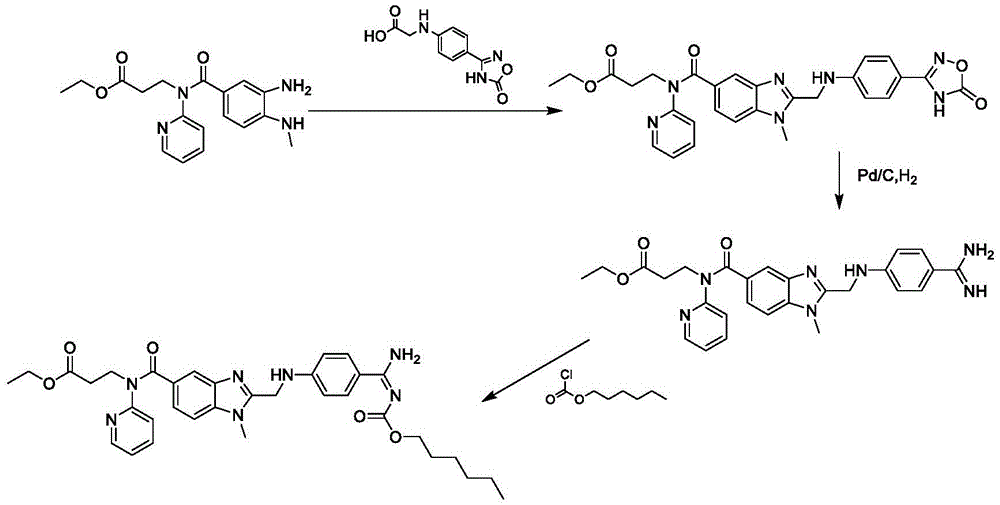
Method for preparing and purifying dabigatran etexilate intermediate
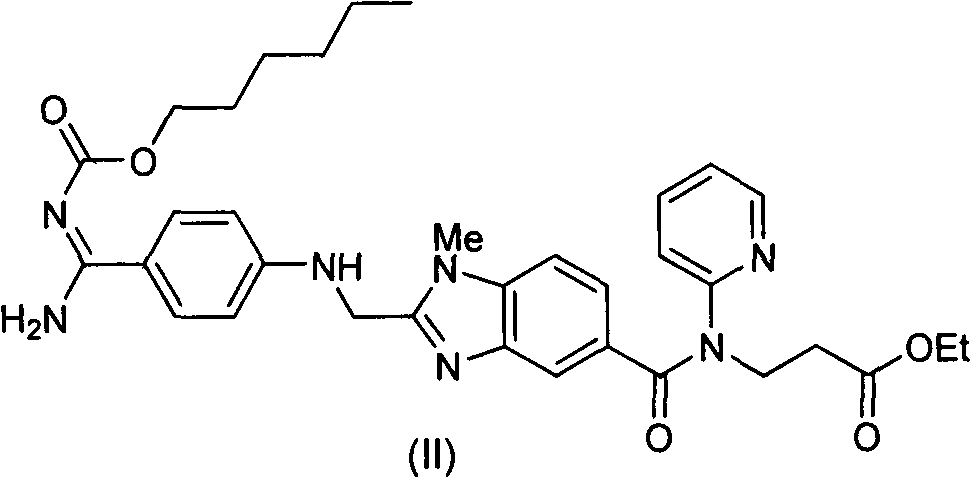
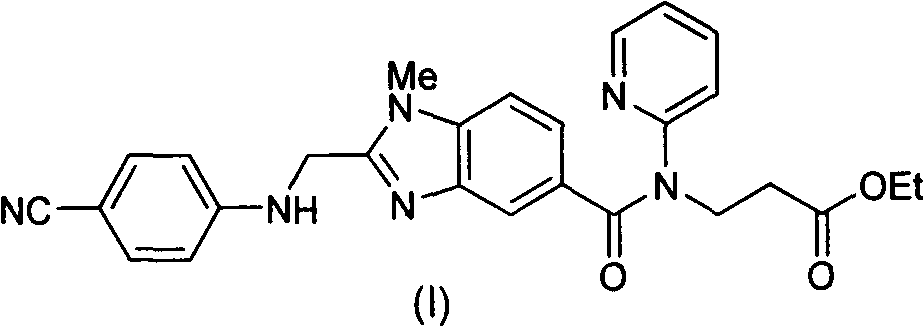
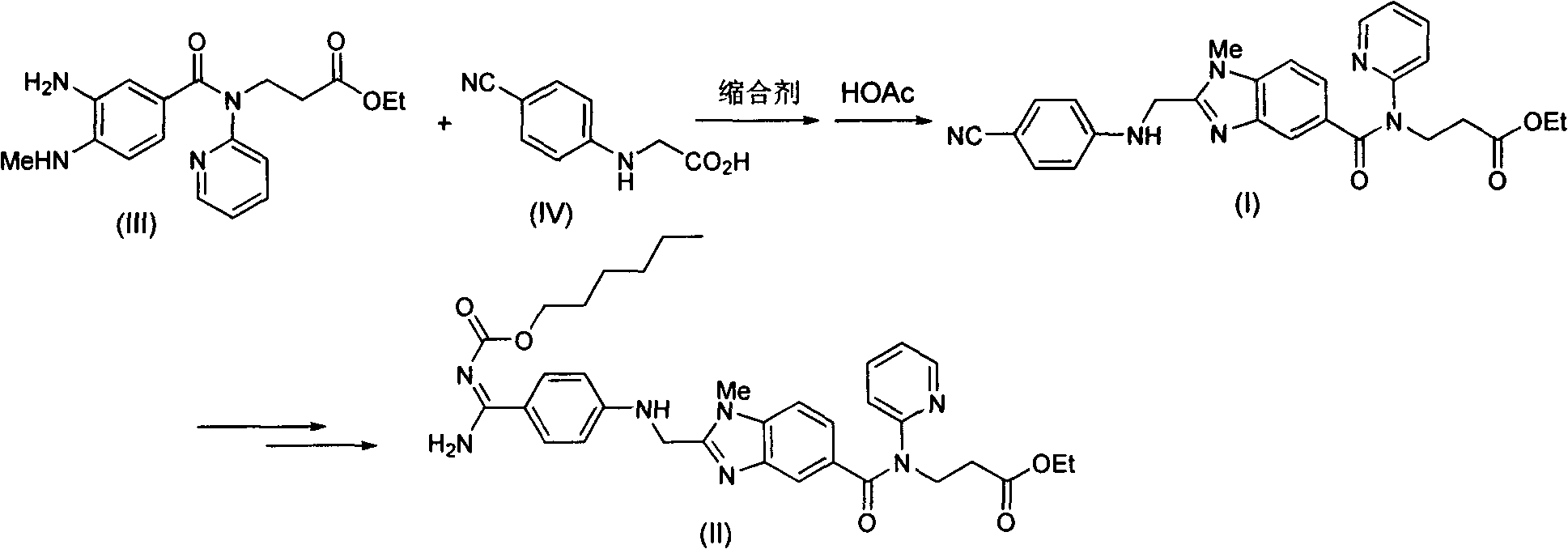
The invention discloses a method for preparing and purifying a dabigatran etexilate key intermediate 3-{2-[(4-cyanoanilino)methyl]-1-methyl-benzimidazolyl-5-[N-(2-pyridyl)formamido]}-ethyl propionate (I). The preparation process comprises the following steps: reacting 2-( 4-cyanoanilino)acetic acid (IV) and 3-[4-methylamino-3-amino-N-(2-pyridyl)-benzamido-]-ethyl acrylate (III) in the presence of a condensing agent to form a condensate; and carrying out cyclization reaction under the catalytic action of acetic acid to prepare the 3-{2-[(4-cyanoanilino)methyl]-1-methyl-benzimidazolyl-5-[N-(2-pyridyl)formamido]}-ethyl propionate (I). The purification process comprises the following steps: reacting the compound (I) and succinic acid to form 3-{2-[(4-cyanoanilino)methyl]-1-methyl-benzimidazolyl-5-[N-(2-pyridyl)formamido]}-ethyl propionate succinate (V), and finally, adding alkali for dissociation to obtain the high-purity compound (I).
Owner:SHANGHAI AOBO PHARMTECH INC LTD
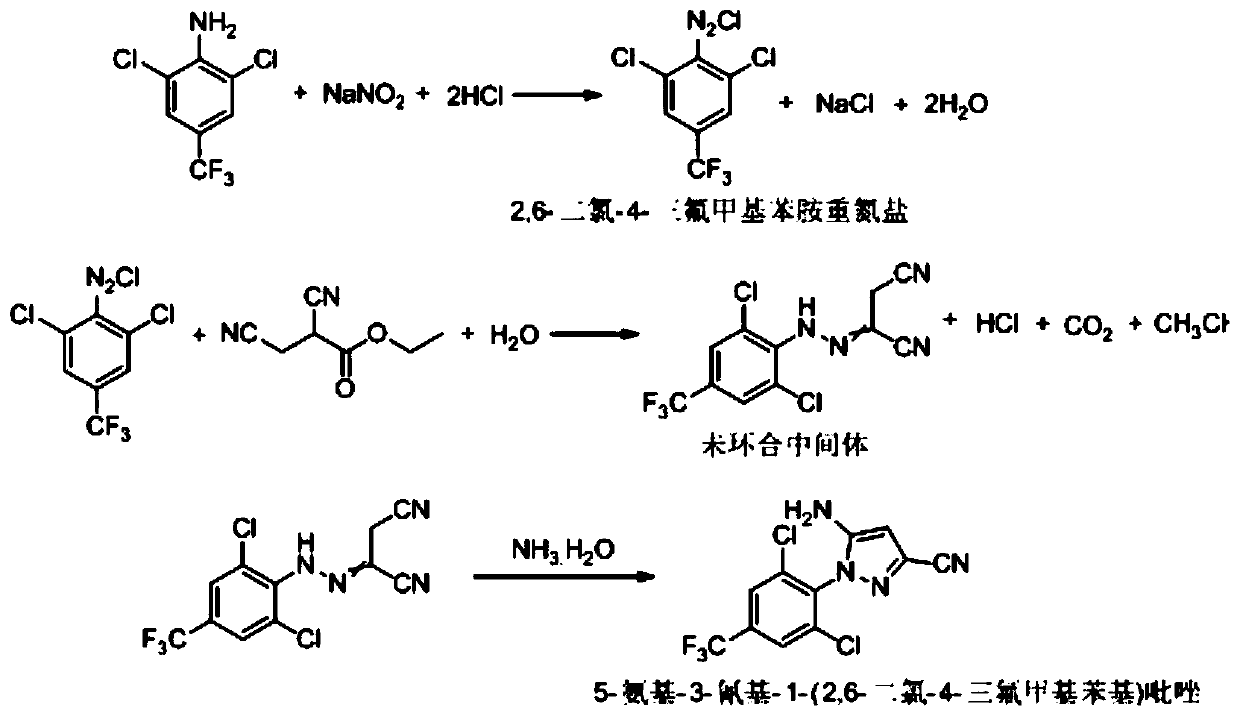
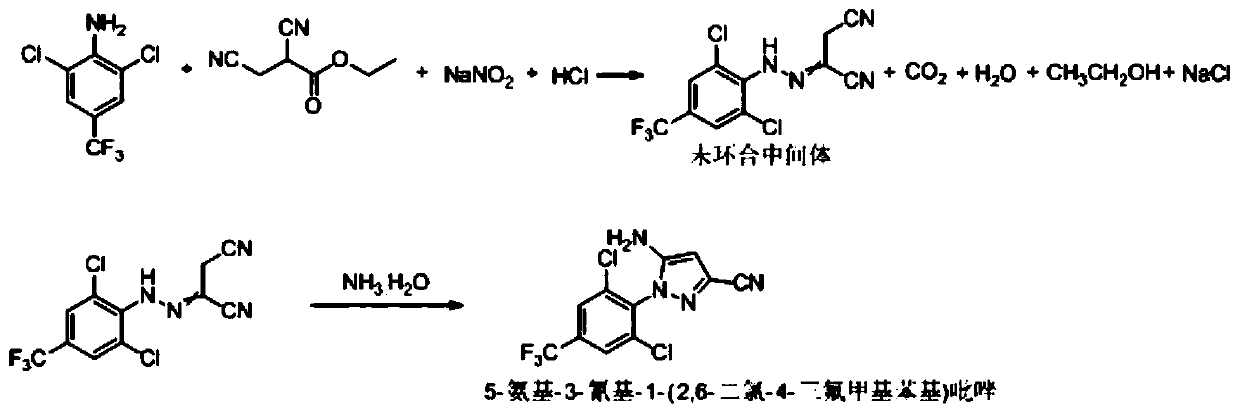
Synthesis method of 5-amidogen-3-cyano-1-(2,6-dichloro-4-trifluoromethyl phenyl) pyrazole
The invention discloses a synthesis method of 5-amidogen-3-cyano-1-(2,6-dichloro-4-trifluoromethyl phenyl) pyrazole. The synthesis method comprises the following steps that at 0 to 5 DEG C, an ethanol solution of 2,6-dichloro-4-trifluoromethyl aniline salt and a sodium nitrite solution with the mass concentration being 40 to 45 percent are simultaneously and dropwise added into a hydrochloric acid ethanol solution of 2,3-dicyan ethyl propionate; reaction liquid is subjected to heat insulation for 2 to 12h at 0 to 5 DEG C until the concentration of 2,6-dichloro-4-trifluoromethyl aniline in the reaction liquid is smaller than or equal to 0.5 percent; reducing reagents are dropwise added into the reaction liquid to remove excessive nitrous acid in the reaction liquid until the nitrous acid in the reaction liquid is completely removed; at 10 to 15 DEG C, ammonium hydroxide and ethanol are added into the reaction liquid, so that the pH of the reaction liquid is greater than or equal to 11; heat insulation is performed for 8 to 24h. The synthesis method has the advantages that the technology prejudice of technicians in the field is broken; the cost is reduced; the generation of three wastes is greatly reduced.
Owner:HAIZHENG CHEM NANTONG CO LTD
Method for loading drugs on drug eluting balloon catheter
ActiveCN104174073ARelease stabilityUniform drug coatingSurgeryCoatingsDrug eluting balloonDrug release
The invention relates to a method for loading drugs on a drug eluting balloon catheter. The method comprises the following steps: (1) swelling a balloon for 0.5-3 hours; (2) spraying a mixed liquid composed of therapeutic drugs, additives and a solvent onto the surface of the swelled balloon by adopting a vacuum spraying technology, and naturally drying by air for 10-30 minutes, wherein the weight ratio of the solvent, the therapeutic drugs and the additives in the mixed liquid is (10-90) to (0.5-30) to (5-60); and the solution for swelling the balloon in the step (1) is any one or more of methyl formate, ethyl acetate, ethyl formate, methyl acetate, propyl formate, methyl propionate, ethyl propionate, phenyl acetate, n-octyl acrylate and methyl benzoate. According to the method for loading drugs on the drug eluting balloon catheter, a drug coating can be uniform and firm, and drugs can be released quickly and stably.
Owner:LIAONING YINYI BIOTECH CO LTD
Low temperature electrolyte for lithium-ion power battery and preparation method thereof
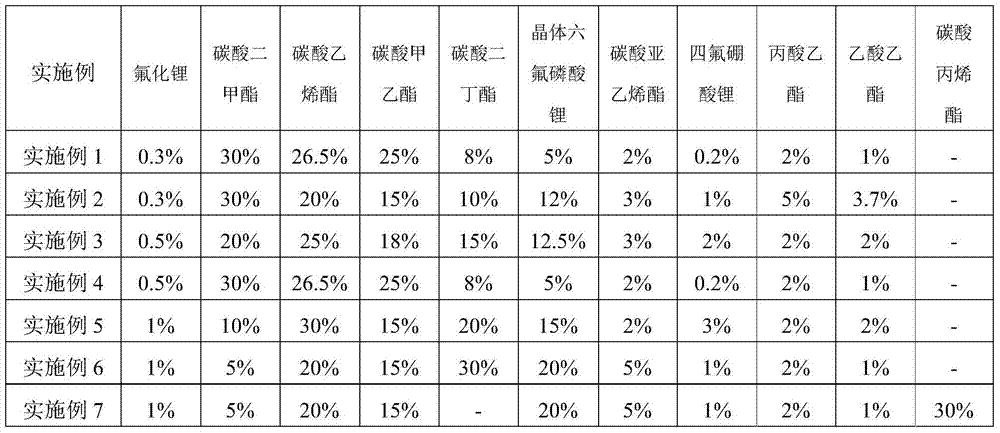
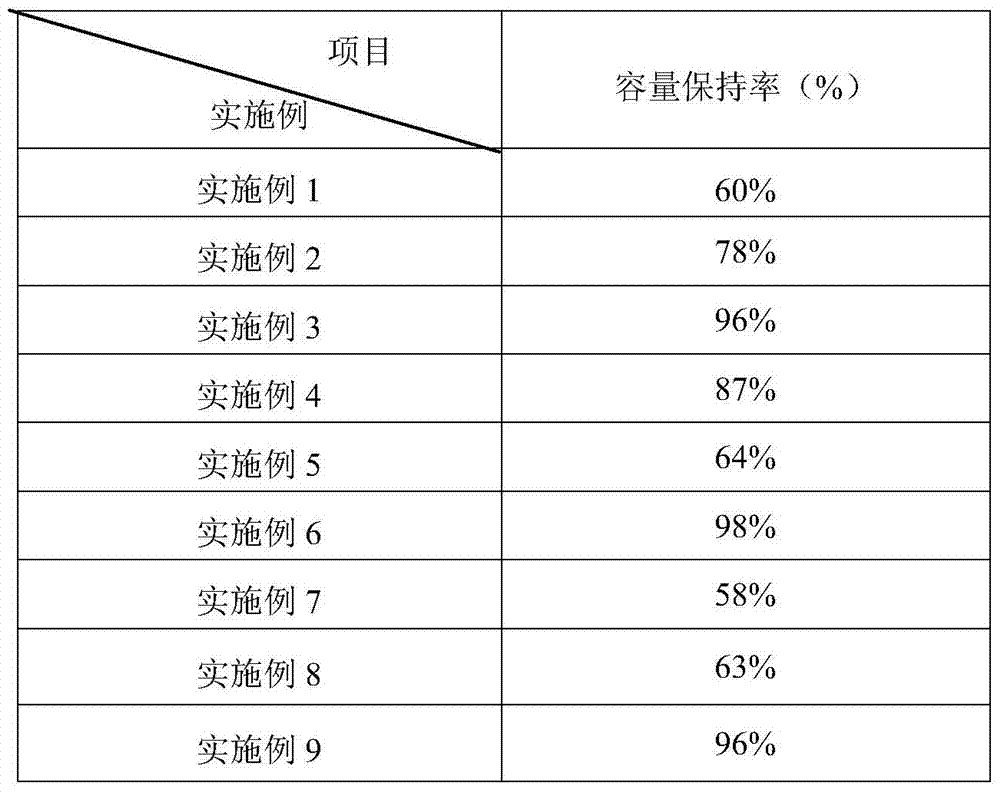
InactiveCN104810554ALow viscosityLower eutectic pointSecondary cellsDischarge efficiencyMethyl carbonate
The invention relates to a low temperature electrolyte for a lithium-ion power battery and a preparation method thereof. Components of the low temperature electrolyte comprise solvents and a solute, wherein the solvents are a mixture of ethylene carbonate, ethyl methyl carbonate, ethyl propionate and an additive. The preparation method comprises steps of uniformly mixing the solvents under a magnetic field and adding the solute. By the use of ethyl propionate, viscosity and eutectic point of the electrolyte system are reduced so as to help migration of lithium ion and reduce content of ethylene carbonate in the electrolyte. Thus, the electrolyte has low melting point and viscosity, and low temperature performance of the electrolyte is improved. By the use of lithium difluoroborate which has good heat stability and oxidation stability, metal dissolution potential of the electrolyte can be improved. The prepared lithium-ion power battery can raise low-temperature discharging efficiency by 15-20% and increase low-temperature mean voltage by 10-15%.
Owner:WANXIANG 123 CO LTD +2
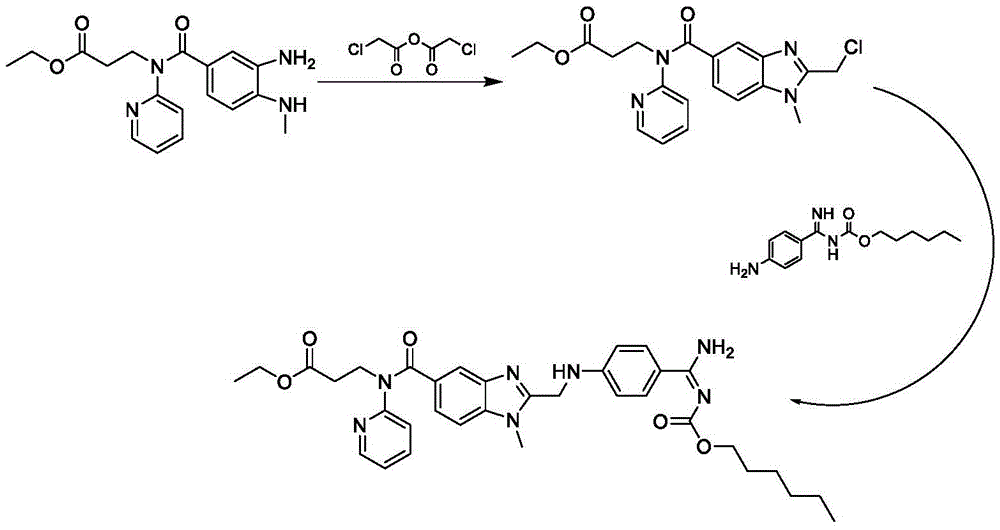
Preparation method of dabigatran etexilate mesylate
InactiveCN105566297ASimple and fast operationHigh selectivityOrganic chemistryPropanoic acidChloroformate
The invention discloses a preparation method of dabigatran etexilate mesylate, and belongs to the technical field of medicine. The preparation method comprises the following steps: taking 3-[(3-amino-4-methylaminobenzoyl)pyridine-2-ylamino]ethyl propanoate and N-(4-cyanphenyl)amino acetic acid as the raw materials to synthesize an intermediate (S3); making the intermediate (S3) carry out ring-closure reactions to generate an intermediate (S4); subjecting the intermediate (S4) to acid splitting in the presence of a hydrogen chloride-ethanol solution at first, then carrying out ammonification in the presence of ammonia water to generate an intermediate (S5); carrying out reactions between the intermediate (S5) and n-hexyl chloroformate under an alkaline condition to generate an intermediate (S6); dissolving the intermediate (S6), and finally carrying out reactions between the intermediate (S6) and methylsulfonic acid to obtain dabigatran etexilate mesylate. The preparation method has the advantages of simpleness, controllable and mild conditions, high yield, high product purity, stable product property, and suitability for industrial production.
Owner:HARBIN PHARMA GROUP TECH CENT
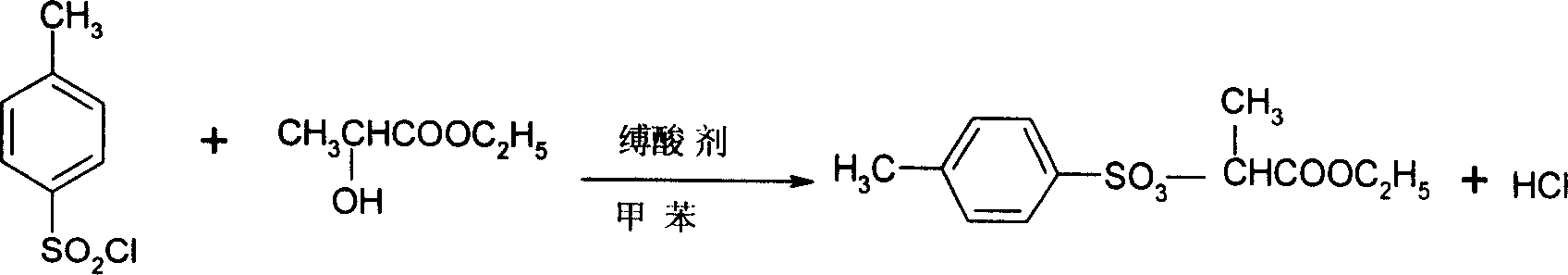
Preparation method of 2-(4'-methyl benzene sulfonyl) ethyl propionate
A process for preparing 2-(4'methylbenzosulfonyl) ethyl propionate includes such steps as adding toluene, P-toluene sulfuryl chloride and sodium hydroxide or potassium carbonate or CaO into reactor, dripping ethyl L-lactate at a temp lower than 10 deg.C, reaction, adding water, stirring, regulating pH=6-7, removing lower-layer water, and negative-pressure distilling to remove toluene and obtain product.
Owner:陈克付
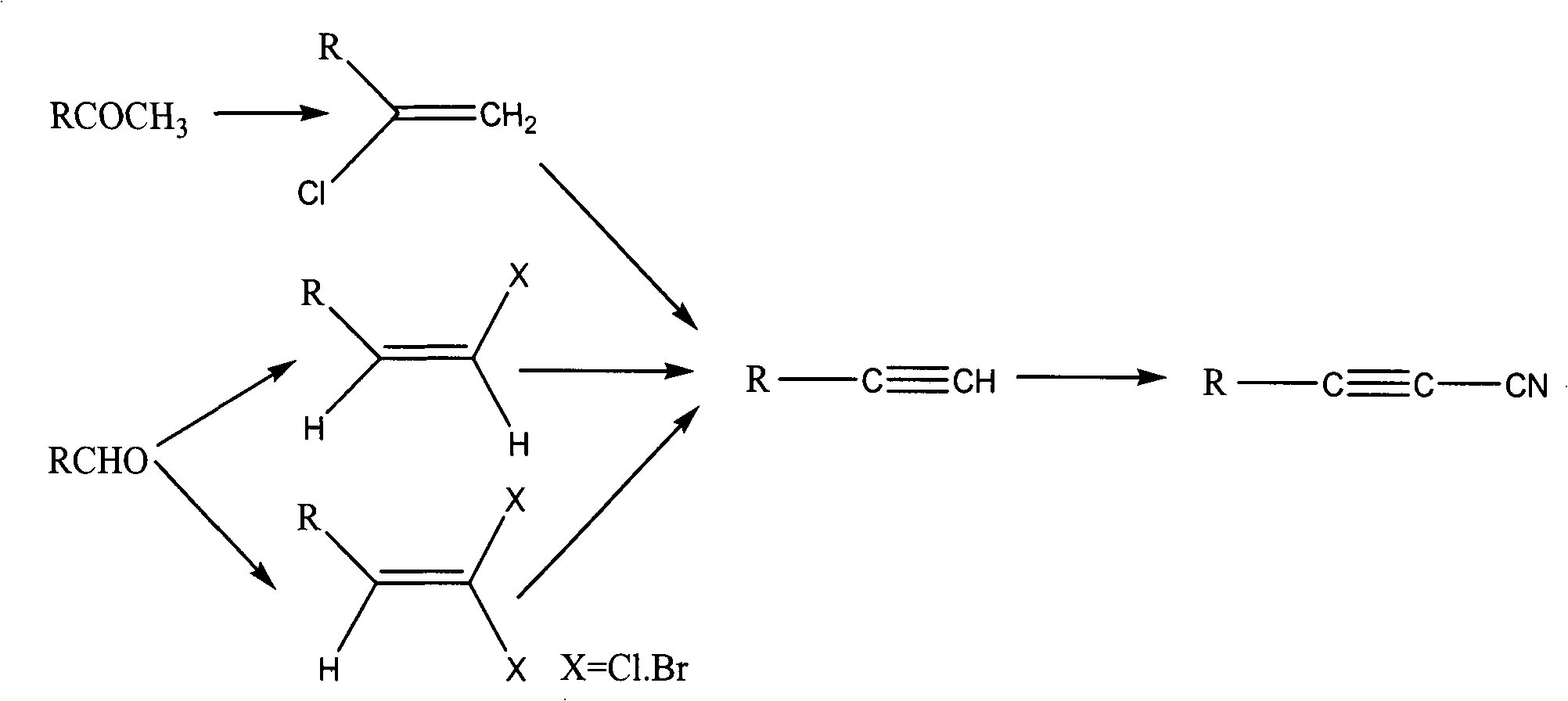
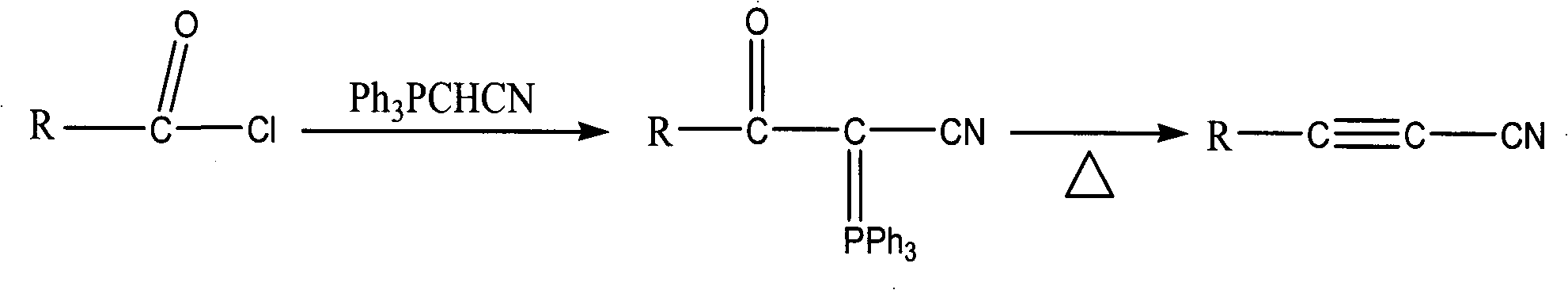
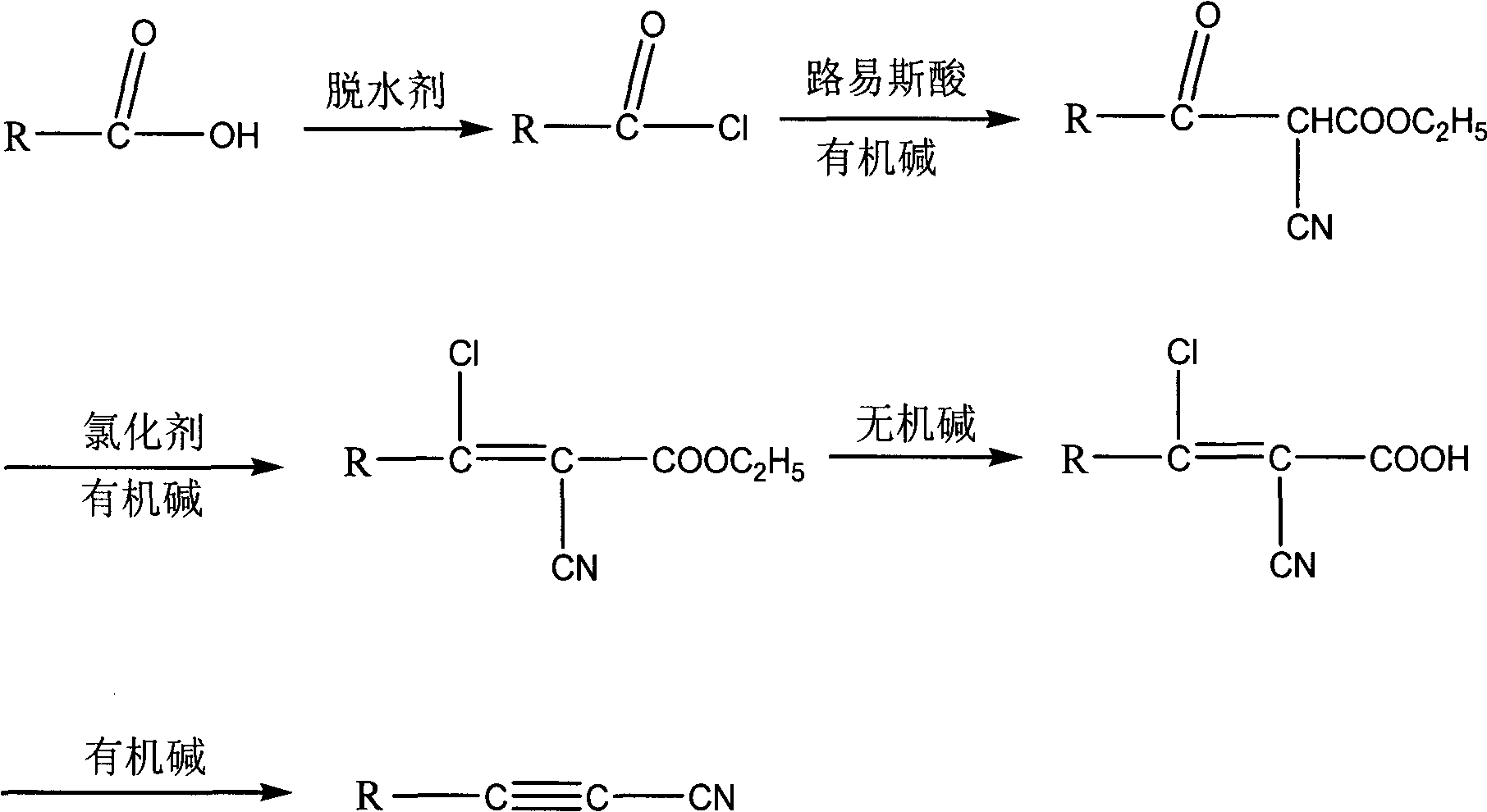
Method for synthesizing cyanoacetylene derivatives
ActiveCN101671270ASimple post-processingReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationEthyl fumarateElimination reaction
The invention discloses a method for synthesizing cyanoacetylene derivatives. The prior preparation method has high production cost and low yield, and is not suitable for industrial production. The method comprises that: in the presence of a dehydrating agent, organic formic acid generates organic formyl chloride; in the presence of Lewis acid and organic alkali, the organic formyl chloride is reacted with ethyl cyanoacetate to generate alpha-cyano-beta-carbonyl ethyl propionate compounds; in the presence of chlorinating agent and organic alkali, the alpha-cyano-beta-carbonyl ethyl propionatecompounds are subjected to chlorination reaction to generate alpha-cyano-beta-chloroacrylate ethyl ester compounds; in the presence of inorganic alkali and lower alcohol solution, the alpha-cyano-beta-chloroacrylate ethyl ester compounds are hydrolyzed to generate alpha-cyano-beta-chloroacrylate compounds; and in the presence of organic alkali, the alpha-cyano-beta-chloroacrylate compounds are subjected to decarboxylation and dehalogenation elimination reaction to generate the cyanoacetylene derivatives. The whole process operation is simple and convenient, the post treatment is simple and the yield is high, so the method is quite suitable for industrialized production.
Owner:HANGZHOU ALLSINO CHEM
Essence with natural pear extraction components
ActiveCN104172090AImprove heat resistanceImprove stabilityAlcoholic food ingredientsFood ingredient as flavour affecting agentFood additiveAdditive ingredient
The invention discloses essence with natural pear extraction components. The essence mainly comprises the components such as propylene glycol, alcohol, ethyl acetate, butanol, ethyl propionate, butyl acetate, 2-methyl ethyl butyrate, leaf alcohol, hexyl alcohol, isoamyl acetate, ethyl hexanoate, hexyl acetate, anti-2-hexene aldehyde and natural pear extracted liquid, wherein the natural pear extracted liquid is taken as the main component. Compared with other pear essence, the essence with natural pear extraction components is more tender in mouthfeel, is more vivid and natural in fragrance and aroma, and has no unpleasant chemical smell of other essences, has fresh flavor of natural pear, is used for enhancing flavor of final products of food, beverages and the like as a food additive, and endows the mouthfeel of natural and fresh pears on these products.
Owner:HANGZHOU EVER MAPLE FLAVOR & FRAGRANCE
Batch preparation method of 3,5-heptandiol dibenzoate
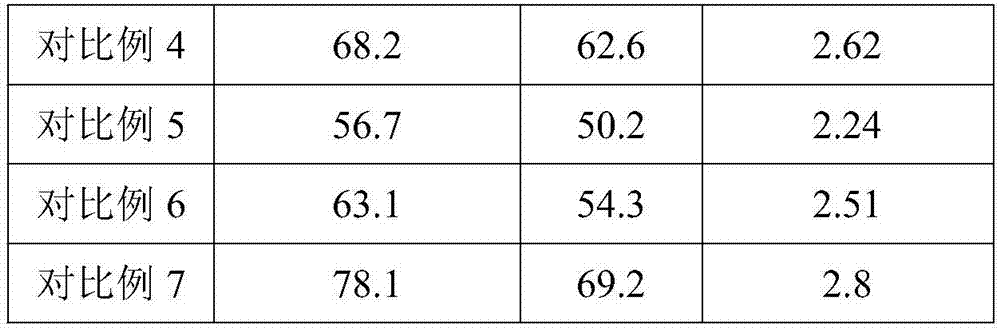
InactiveCN102417455APreparation from carboxylic acid halidesPreparation by ester-hydroxy reactionPurification methodsEthyl propanoate
The invention discloses a batch preparation method of 3,5-heptandiol dibenzoate. The 3,5-heptandiol dibenzoate is 4-substituted 3,5-heptandiol dibenzoate or unsubstituted 3,5-heptandiol dibenzoate. The batch preparation method of the 3,5-heptandiol dibenzoate comprises the following steps that 1, in the presence of a solvent and a basic catalyst, ethyl propanoate and butanone undergo an acylation reaction to produce 3,5-heptanedione, and specially, after the acylation reaction is finished, the 3,5-heptanedione is processed into a 3,5-heptanedione product by a chelating purification method, 2, in the presence of a solvent and a basic catalyst, the 3,5-heptanedione product contacts and undergoes a 4-substitution reaction with a reagent to produce 4-substituted 3,5-heptanedione, and 3, in the presence of a solvent and a basic catalyst, the 4-substituted 3,5-heptanedione or unsubstituted 3,5-heptanedione is reduced into 3,5-heptandiol by a reducing agent under alkaline conditions, and 4, in the presence of a solvent, the reduced 3,5-heptandiol reacts with a carbonyl-containing compound under certain conditions to produce the 3,5-heptandiol dibenzoate. Through the batch preparation method, cheap raw materials as initiators can be prepared into needed heptandiol dibenzoate products at a high yield. Therefore, the batch preparation method can be utilized for mass preparation of the 3,5-heptandiol dibenzoate.
Owner:CHINA PETROLEUM & CHEM CORP +1
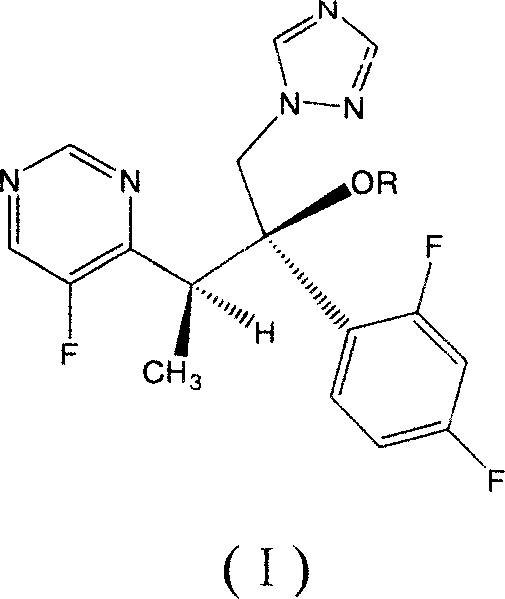
Voriconazole derivate and preparation process thereof
InactiveCN100999518ATo achieve asymmetric synthesisHigh yieldOrganic active ingredientsAntimycoticsPropanoic acidEnantiomer
This invention involves Voriconazole derivatives. The invention also involves Voriconazole derivatives preparation methods, including the following steps : 2 - (5-fluoro-4-yl) acetate and ethanol for esterification, generate 2 -(5-fluoro-4-yl) ethyl acetate, in alkaline conditions takes reaction with methylation agent, generate 2 - (5-fluoro-4-yl) ethyl propionate; hydrolysis of 2 - (5-fluoro-4-yl) propionic acid, through split gain S-2 - (5-fluoro-4-yl) propionic acid; for chlorination to gain S-2 - (5-fluoro-4-yl) propionyl chloride; occurred Friedel-Crafts reaction, generating S-1 - 2, 4-difluoro-2 - (5-fluoro-4-yl) acetone; with 1-methyl - 1-H-1 ,2,4 - triazol under alkali conditions to take reaction to gain voriconazole and the series of voriconazole derivatives. The methods described in this invention has short routes, only use of a pair of enantiomers separation, the overall yield has been greatly improved, is a simple and easy method for synthesis of voriconazole and its derivatives.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Production method for preparing epsilon-caprolactone solution by using novel dehydrating agent
InactiveCN105906603AHigh yieldOrganic compound preparationPeroxy compound preparationCyclohexanoneCaprolactone
The invention relates to a production method for preparing an epsilon-caprolactone solution by using a novel dehydrating agent; the production method comprises the specific steps: taking one of organic solvents butyl acetate, cyclohexane and ethyl propionate as a dehydrating agent, taking a Mg / Sn / W composite oxide as a catalyst, and oxidizing acetic acid by using hydrogen peroxide to obtain a peracetic acid solution; then adding cyclohexanone to the peracetic acid solution; and after completion of the reaction, cooling the reaction to room temperature, and thus obtaining the epsilon-caprolactone solution. The method provided by the invention can reduce water as far as possible before caprolactone is produced, so as to avoid the polymerization of caprolactone and improve the yield.
Owner:WUHAN UNIV OF TECH
Method for synthesizing pyrethroid hapten compounds
InactiveCN101215247AQuick checkCarboxylic acid nitrile preparationOrganic compound preparationGamma-Aminobutyric acidAcetonitrile
The invention discloses a synthesis process of pyrethroids hapten compound by using dimethyl trichloromethyl chloroformate, gamma-aminobutyric acid and as main material, which comprises following steps that firstly generating 3-(2, 2-dimethyl-3-(2'-methacryloyl) cyclopropyl carbonyl amino) ethyl propionate, secondly generating 3-(3-ethyoxyl-3-oxopropanecarbonic acyl radical)-2, 2-dimethyl cyclopropane aminic acid, thirdly generating 2-hydroxy-2-(3-pheonexyphenyl) acetonitrile, fourthly generating cyano-(3-phenoxy phenyl) methyl N-2-ethyoxylcarbonylethyl-2, 2-dimethylcypromethyl carbonate, fifthly generating 3-(3-(( cyano(3-phenoxyphenyl) methoxyl) carbonic acyl radical)-2, 2-phenoxy phenylcypro carbamoyl) ethylformic acid. Hapten which is prepared by the invention comprises three similarity structures of most pyrethroids pesticides, ester with-CN and three-membered ring structure.
Owner:ZHEJIANG UNIV
Synthesis method of chiral fenoxanil
ActiveCN106366020AEasy to getLower synthesis costCarboxylic acid nitrile preparationOrganic compound preparationPropanoic acidSynthesis methods
The invention relates to a synthesis method of chiral fenoxanil. The synthesis method of the chiral fenoxanil is characterized in that chiral ethyl lactate is subjected to sulfonylation reaction to obtain sulfonic acid ester; the sulfonic acid ester reacts with 2,4-dichlorophenol to generate 2-(2,4-dichlorophenoxy) ethyl propionate; the 2-(2,4-dichlorophenoxy) ethyl propionate and 2,-amino-2,3-dimethyl nitrile are subjected to ammonia ester exchange reaction to generate corresponding chiral fenoxanil, wherein the molar ratio of the 2-(2,4-dichlorophenoxy) ethyl propionate to the 2,-amino-2,3-dimethyl nitrile is 1:1 and the reaction temperature is 60 to 85 DEG C. The method is simple in synthesis route, hydrolysis and chlorination are not needed, little waste water and waste gas are generated, a high environmentally-friendly benefit is achieved, the production cost is greatly reduced, the condition is mild, the fenoxanil with optical chirality can be synthesized in an oriented mode by selecting different configuration of ethyl lactate, and important significance of further exploring the optical property of the fenoxanil is achieved.
Owner:JINGBO AGROCHEM TECH CO LTD
A kind of lithium-ion battery low-temperature electrolyte and lithium-ion battery
ActiveCN105047996BImprove low temperature performanceSecondary cellsOrganic electrolytesPhysical chemistryCharge discharge
The present invention relates to a lithium ion battery low temperature electrolyte and a lithium ion battery, and belongs to the technical field of lithium ion batteries. The lithium ion battery low temperature electrolyte comprises, by weight, 5-20% of lithium hexafluorophosphate, 0.1-3% of lithium fluoride, 0.2-5% of lithium tetrafluoroborate, 48-94.7% of an electrolyte solvent, 2-5% of vinylene carbonate, 2-10% of ethyl propionate, and 1-5% of ethyl acetate. With the lithium ion battery low temperature electrolyte of the present invention, the charge-discharge performance and the cycle performance of the electrolyte at the low temperature can be improved.
Owner:HENAN FAENLAITE NEW ENERGY SCI & TECH CO LTD
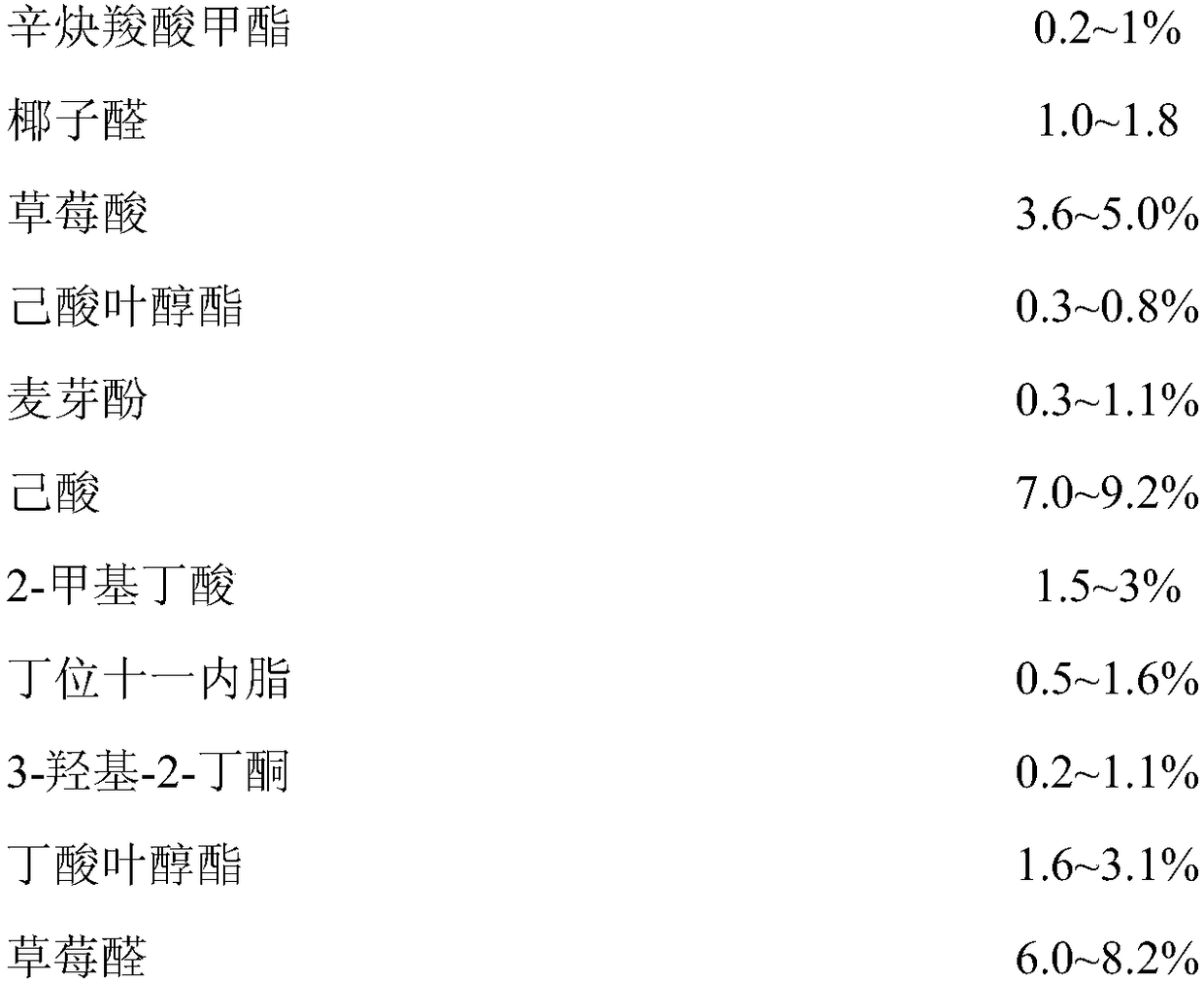
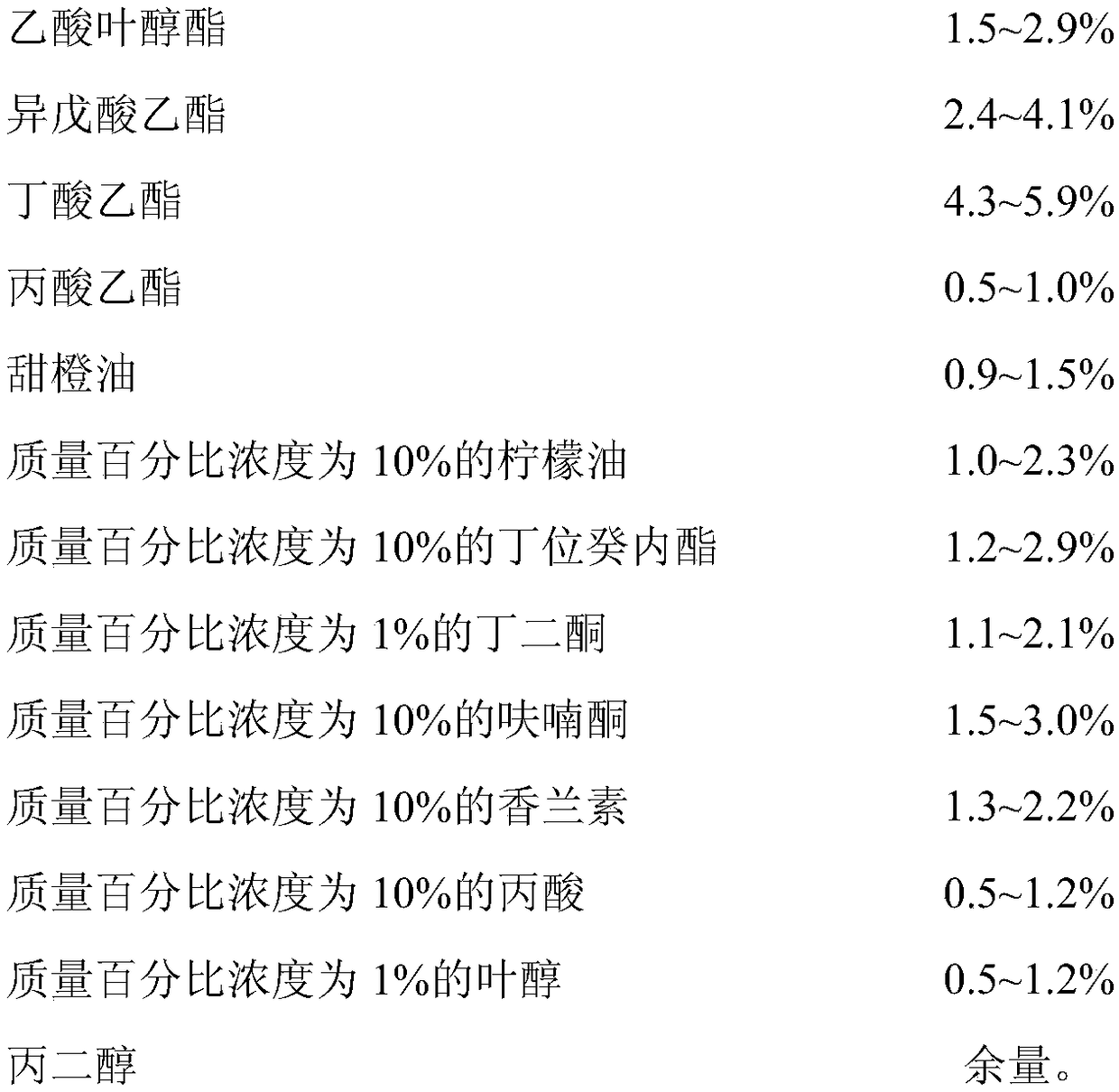
Waxberry essence and preparation method thereof
InactiveCN108576763AImprove fidelitySignificant technological progressFood scienceFood additivePropanoic acid
The invention discloses waxberry essence. The essence consists of the following raw materials: methyl 2-nonynoate, coconut aldehyde, strawberry acid, cis-3-hexenyl hexanoate, maltol, caproic acid, 2-methylbutyric acid, undecanolactone, 3-hydroxy-2-butanone, cis-3-hexenyl butyrate, strawberry aldehyde, cis-3-hexenyl acetate, ethyl isovalerate, ethyl butyrate, ethyl propionate, sweet orange oil, 10%of lemon oil, 10% of 5-decanolide, 1% of butanedione, 10% of furanone, 10% of vanillin, 10% of propionic acid, 1% of leaf alcohol, and propylene glycol. The invention also provides a preparation method of the waxberry essence. The waxberry essence is prepared by mixing and uniformly stirring the raw materials weighed in percentage by mass. The fragrant raw materials used for the waxberry essenceare convenient in source, and the characteristic waxberry aroma is prominent, and the natural feeling is strong. The essence is added to foods such as cans, fruit wine and beverages as a food additiveso as to endow the product with a natural waxberry aroma and taste.
Owner:SHANGHAI INST OF TECH
Preparation method of 2-(4-oxethyl phenyl)-2-methyl propanol
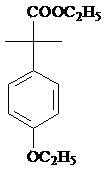
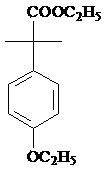
InactiveCN103467254AReduce riskReasonable workmanshipOrganic chemistryOrganic compound preparationPropanolPotassium borohydride
The invention provides a preparation method of 2-(4-oxethyl phenyl)-2-methyl propanol. The preparation method comprises the following steps of (1) dissolving 2-(4-oxethyl phenyl)-2-methyl ethyl propanoate in an ethyl alcohol solvent, and adding potassium borohydrate and lithium chloride; after ending reaction, dropwise adding hydrochloric acid, adding water, recovering the solvent under reduced pressure, extracting by using dichloromethane, drying an organic phase by using sodium sulphate anhydrous, and carrying out vacuum concentration, thus obtaining the 2-(4-oxethyl phenyl)-2-methyl propanol. The preparation method has the advantages that the dangerousness is reduced, and the process is relatively reasonable, simple and convenient. The 2-(4-oxethyl phenyl)-2-methyl propanol is low in cost, high in quality, environment-friendly and is suitable for industrial production. The reaction total yield can reach above 80% by the 2-(4-oxethyl phenyl)-2-methyl ethyl propanoate, and the content of the product reaches 98%.
Owner:LIANYUNGANG GUOSHENG CHEM
Method for preparing N-benzyl-3-pyrrolidone
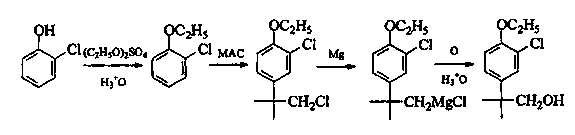
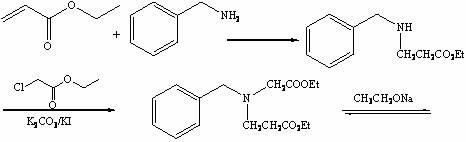
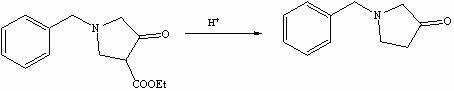
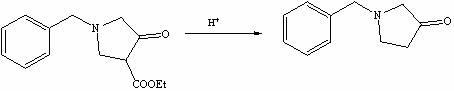
The invention discloses a method for preparing N-benzyl-3-pyrrolidone. The method comprises the following steps of: (a) synthesizing 3-benzylamine ethyl propionate; (b) synthesizing 3-(N-carbethoxy methylene) benzylamine ethyl propionate; (c) synthesizing N-benzyl-4-carbethoxy-3-pyrrolidone; and (d) synthesizing N-benzyl-3-pyrrolidone. The preparation method of the N-benzyl-3-pyrrolidone has the advantages of fewer synthesis steps and high yield.
Owner:ZHANGJIAGANG HICOMER CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000011.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000012.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000021.PNG)