Method for preparing herbicide fenoxaprop-p-ethyl
A technology of fenoxaprop-p-ethyl and herbicides, which is applied in the field of chemical synthesis of fenoxaprop-p-ethyl, can solve problems affecting product quality, increasing raw material costs, and low reaction yield, and achieve high product quality and economical The effect of high raw material and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
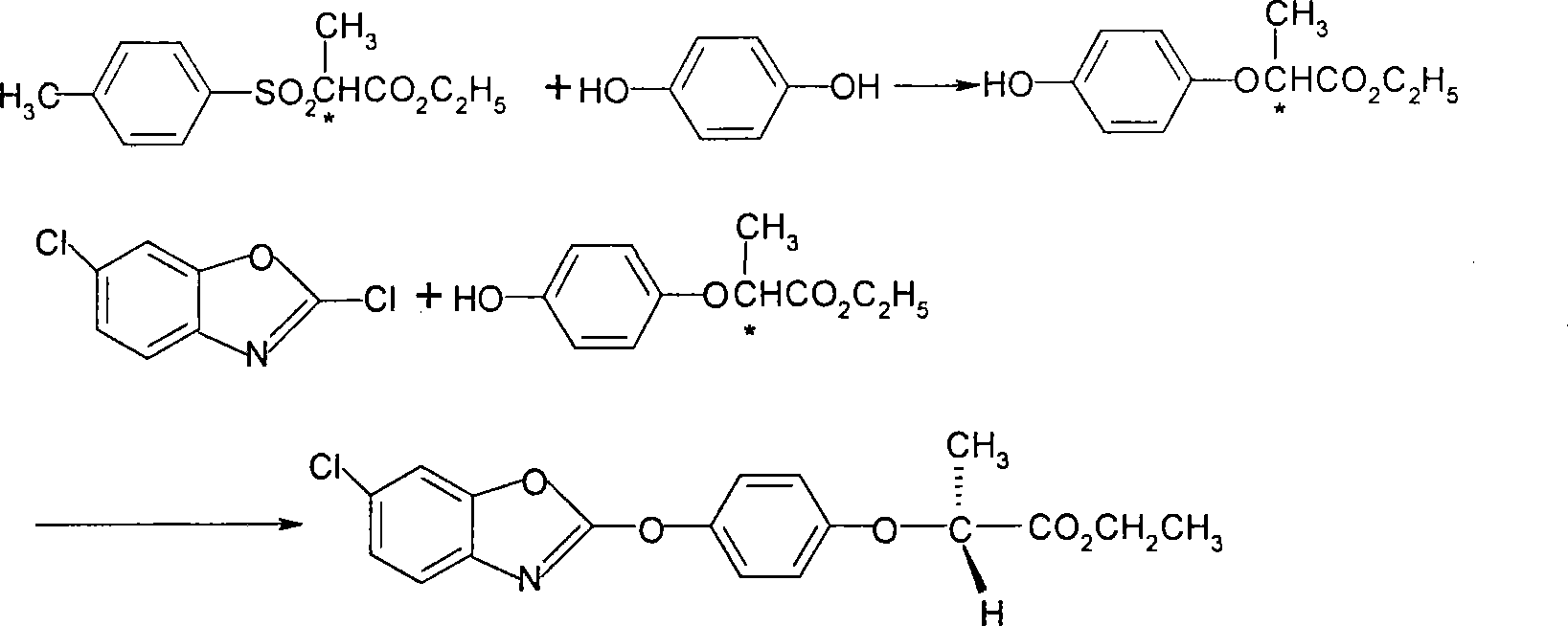
Method used
Image
Examples
Embodiment 1
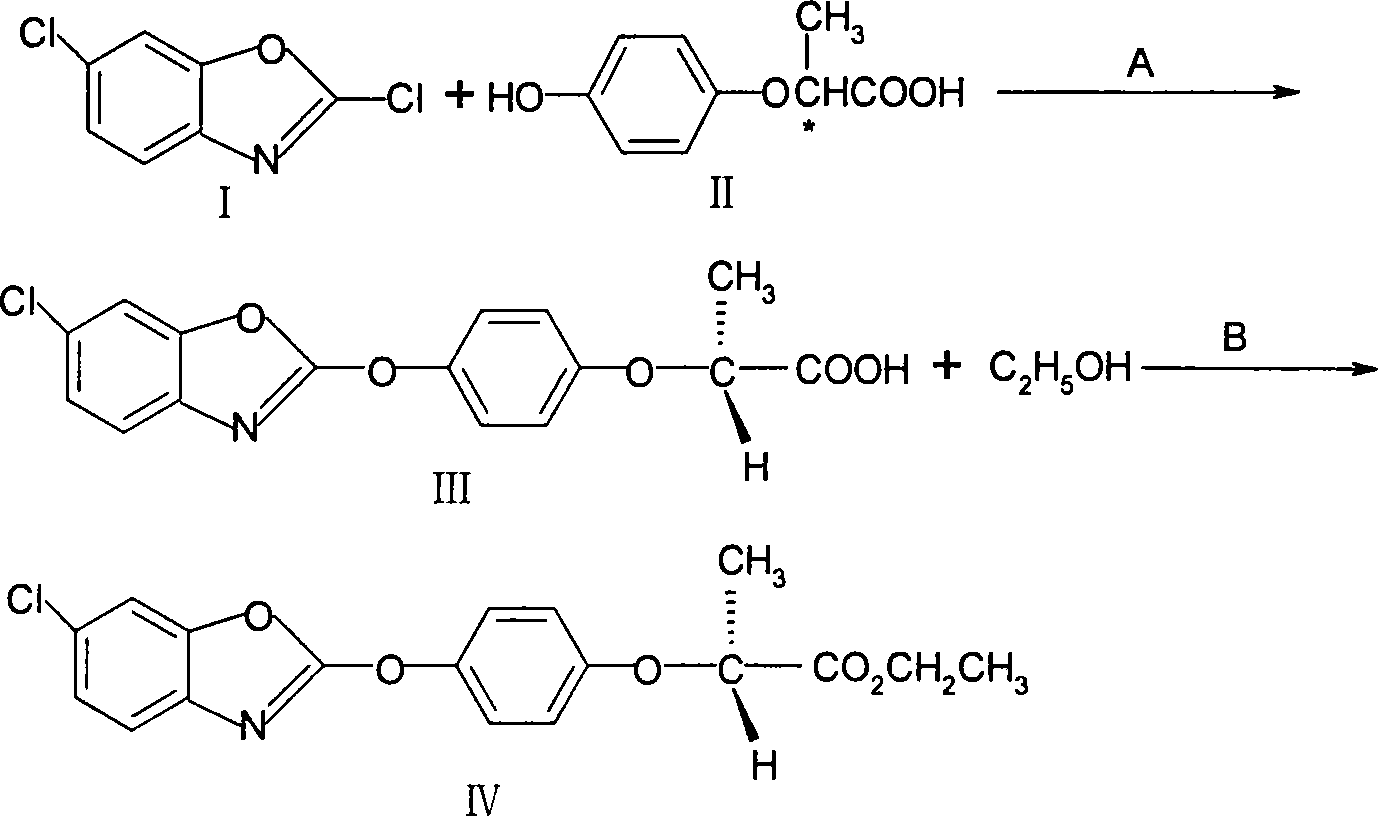
[0025] (1), the preparation of (R)-2-[4-(6-chloro-1,3-benzoxazol-2-yloxy)phenoxy]propionic acid (III)
[0026] Add 150 g of tap water into the reaction flask, cool to 5°C, add 63 g (1.5 mol) of solid sodium hydroxide, add 91 g (0.5 mol) of 2-(4-hydroxyphenoxy)propionic acid (II), stir to form a salt, Then add 1g of phase transfer catalyst, raise the temperature to 50°C, add 197g (0.525mol) of 50% 2,6-dichlorobenzoxazole (I) dropwise, then keep the temperature for 4 hours, then heat up to 80-90°C, keep the temperature After 1 hour, the toluene layer was separated and the pH of the aqueous layer was adjusted to 3-4 with hydrochloric acid. A gray powdery solid was precipitated and dried to obtain 172 g of crude product, with a yield of 95%.
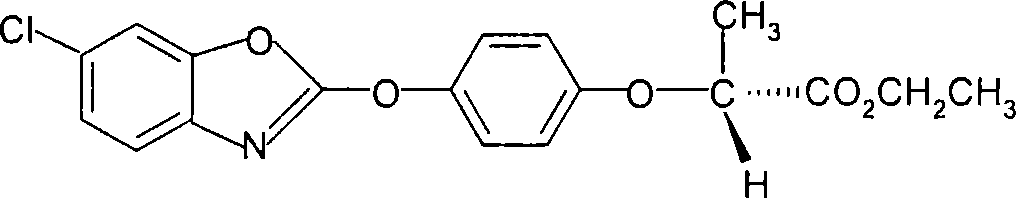
[0027] (2), preparation of (R)-2-[4-(6-chloro-1,3-benzoxazol-2-yloxy)phenoxy]propionate ethyl ester (IV)
[0028] Add (R)-2-[4-(6-chloro-1,3-benzoxazol-2-yloxy)phenoxy]propionic acid (III) 172.5g (0.5mol) in the reaction flask, without 415...
Embodiment 2
[0030] (1), the preparation of (R)-2-[4-(6-chloro-1,3-benzoxazol-2-yloxy)phenoxy]propionic acid (III)
[0031] Add 150 g of tap water in the reaction flask, cool to 5° C., add 84 g (2 mol) of solid sodium hydroxide, add 91 g (0.5 mol) of 2-(4-hydroxyphenoxy) propionic acid (II), stir to form a salt, and then Add 1 g of phase transfer catalyst, raise the temperature to 50° C., add 206 g (0.55 mol) of 50% 2,6-dichlorobenzoxazole (I) dropwise, then keep the temperature for 4 hours, then raise the temperature to 70-80° C., and keep the temperature for 1 After 1 hour, the toluene layer was separated, and the pH of the water layer was adjusted to 3-4 with hydrochloric acid. A gray powdery solid was precipitated, and the crude product was dried to obtain 173.4 g, with a yield of 96%.
[0032] (2), preparation of (R)-2-[4-(6-chloro-1,3-benzoxazol-2-yloxy)phenoxy]propionate ethyl ester (IV)
[0033] Add (R)-2-[4-(6-chloro-1,3-benzoxazol-2-yloxy)phenoxy]propionic acid (III) 172.5g (0.5...
Embodiment 3
[0035] Take by weighing 8.76 grams of fenoxaprop-methyl, 2.9 grams of oxapropen, and 12 grams of 20 grams of nonylphenol polyoxyethylene ether as a solvent, 4 grams of ethylene glycol, 0.2 grams of xanthan gum, and 0.15 grams of benzoic acid, and hydrolyze to 100 g to obtain the product. The obtained product is measured with a particle size distribution tester to have an average particle diameter of 1.05 microns.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com