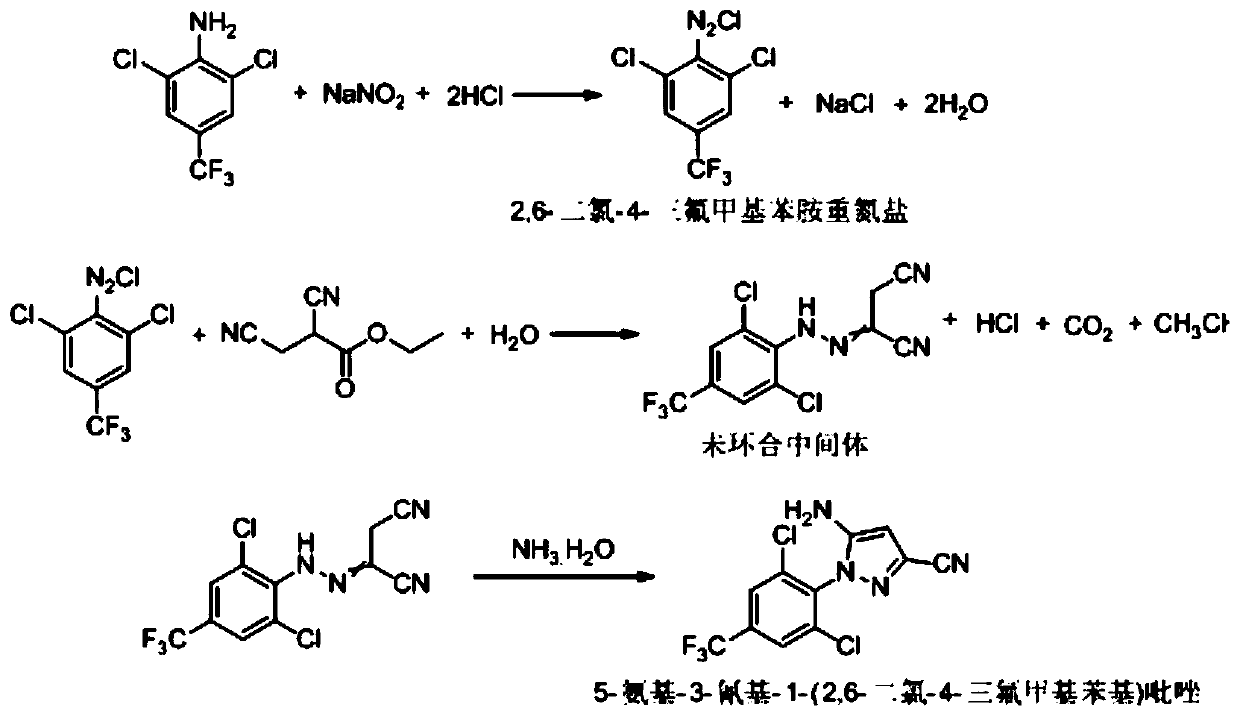
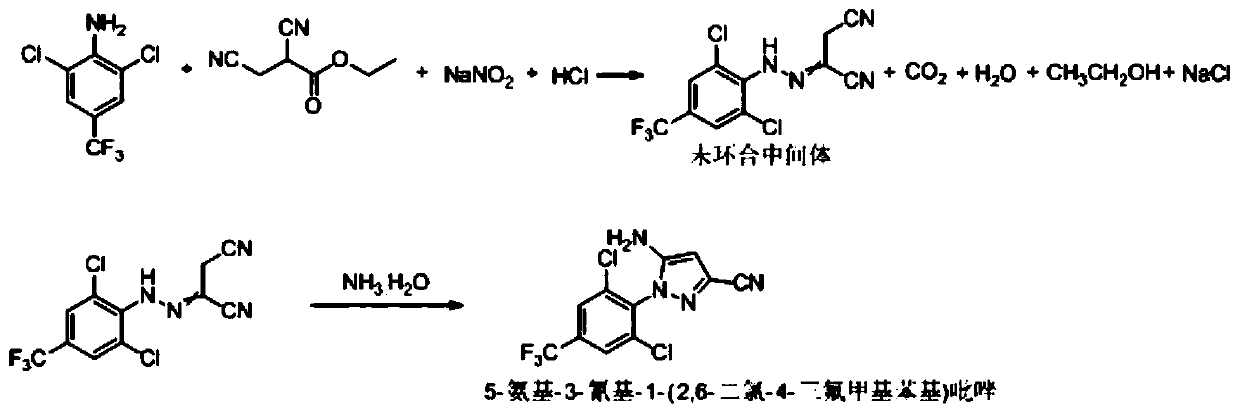
Synthesis method of 5-amidogen-3-cyano-1-(2,6-dichloro-4-trifluoromethyl phenyl) pyrazole
A technology of trifluoromethylphenyl and trifluoromethylaniline, which is applied in the field of synthesis of 5-amino-3-cyano-1-pyrazole, can solve the problem of excessive reaction temperature lag of sodium nitrite and solid material feeding speed Non-uniformity, potential safety hazards and other problems, to achieve the effect of high purity and yield, low production cost and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
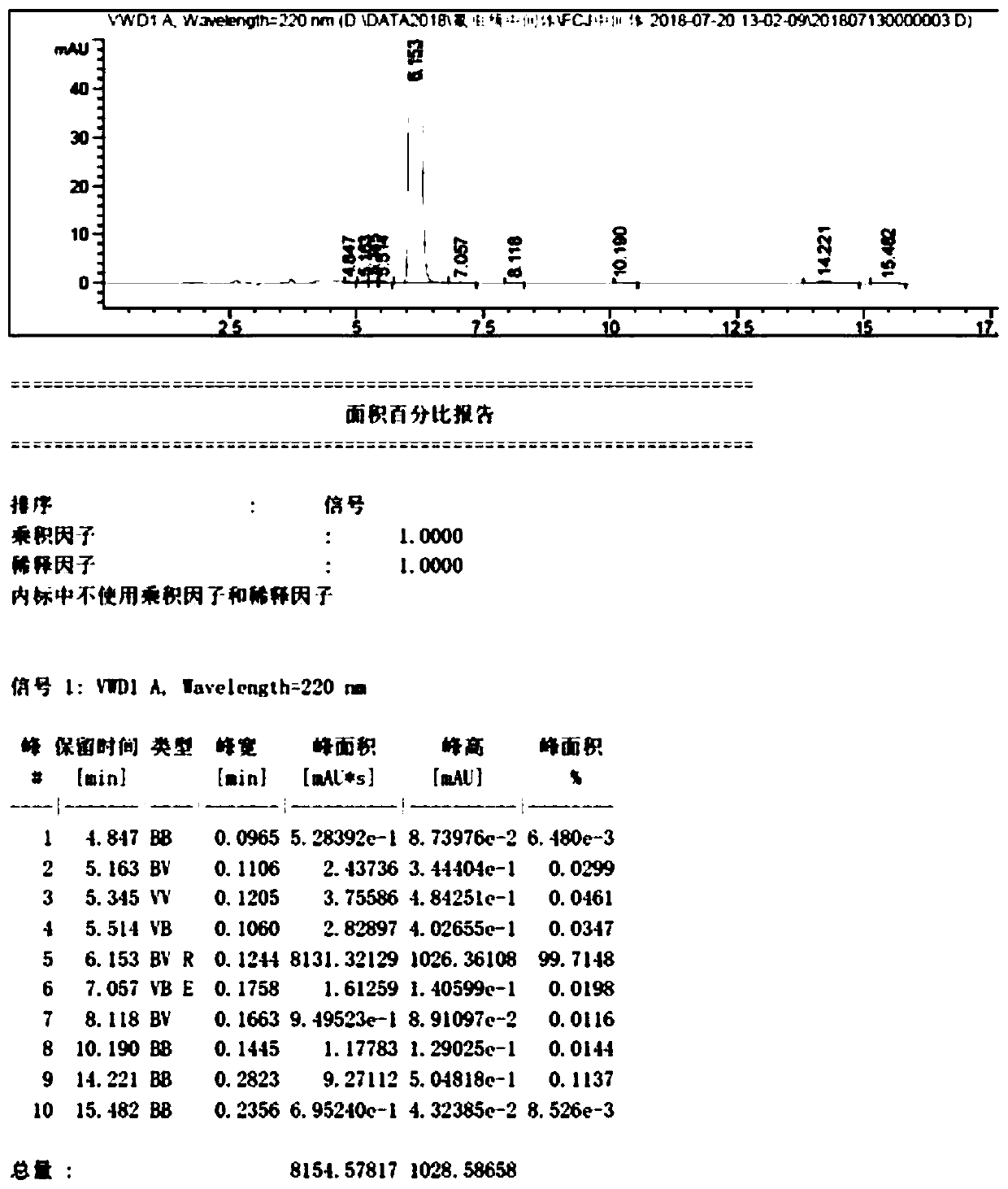
Image
Examples
Embodiment 1
[0051] Add 52g of 2,6-dichloro-4-trifluoromethylaniline, 52g of ethanol, and 19.4g of 30% hydrochloric acid into a 250mL flask, and stir at 30-35°C for 1h to form 2,6-dichloro-4- Ethanol solution of trifluoromethylaniline salt;
[0052] At room temperature, add 34.4 g of ethyl 2,3-dicyanopropionate, 100 g of ethanol, and 20.6 g of 30% hydrochloric acid into a 1000 mL four-necked flask, and stir to obtain ethyl 2,3-dicyanopropionate hydrochloric acid Ethanol solution 155g;
[0053] Controlled at 0-5°C, add dropwise the ethanol solution of 2,6-dichloro-4-trifluoromethylaniline salt and 43g of 40% sodium nitrite aqueous solution to a 1000mL four-necked flask at the same time, drop as much as possible at the same time After the addition is completed, continue to incubate at 0-5°C for 4 hours, and after sampling and detecting 2,6-dichloro-4-trifluoromethylaniline ≤ 0.5% by HPLC, add 40% aqueous urea solution to the reaction solution dropwise until the starch Potassium iodide test...
Embodiment 2
[0074] Add 78g of 2,6-dichloro-4-trifluoromethylaniline, 78g of ethanol, and 20g of 35% hydrochloric acid into a 250mL flask, and stir at 30-35°C for 1.5h to form 2,6-dichloro-4- Ethanol solution of trifluoromethylaniline salt;
[0075] At room temperature, add 51.6 g of ethyl 2,3-dicyanopropionate, 150 g of ethanol, and 22.4 g of 35% hydrochloric acid into a 1000 mL four-necked flask, and stir to obtain ethyl 2,3-dicyanopropionate hydrochloric acid Ethanol solution 224g;
[0076] Controlled at 0-5°C, add dropwise the ethanol solution of 2,6-dichloro-4-trifluoromethylaniline salt and 62.4g of 45% sodium nitrite aqueous solution to a 1000mL four-necked flask at the same time. After the dropwise addition, continue to incubate at 0-5°C for 2 hours, take a sample and detect 2,6-dichloro-4-trifluoromethylaniline ≤ 0.5% by HPLC, then add ammonium sulfite with a concentration of 35% to the reaction solution Water solution to starch potassium iodide test paper test does not change c...
Embodiment 3
[0082] Add 156g of 2,6-dichloro-4-trifluoromethylaniline, 104g of ethanol, and 38.8g of 32% hydrochloric acid into a 500mL flask, and stir at 30-35°C for 1h to form 2,6-dichloro-4- Ethanol solution of trifluoromethylaniline salt;
[0083] At room temperature, add 103.2 g of ethyl 2,3-dicyanopropionate, 200 g of ethanol, and 38.8 g of 32% hydrochloric acid into a 2000 mL four-necked flask, and stir to obtain ethyl 2,3-dicyanopropionate hydrochloric acid Ethanol solution 342g;
[0084] Controlled at 0-5°C, add dropwise the ethanol solution of 2,6-dichloro-4-trifluoromethylaniline salt and 122.6g of 42% sodium nitrite aqueous solution to a 2000mL four-necked flask at the same time. After the dropwise addition, continue to keep warm at 0-5°C for 12 hours, and after sampling and detecting 2,6-dichloro-4-trifluoromethylaniline ≤ 0.5% by HPLC, add hydrogen sulfite with a concentration of 38% to the reaction solution Sodium aqueous solution to starch potassium iodide test paper test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com