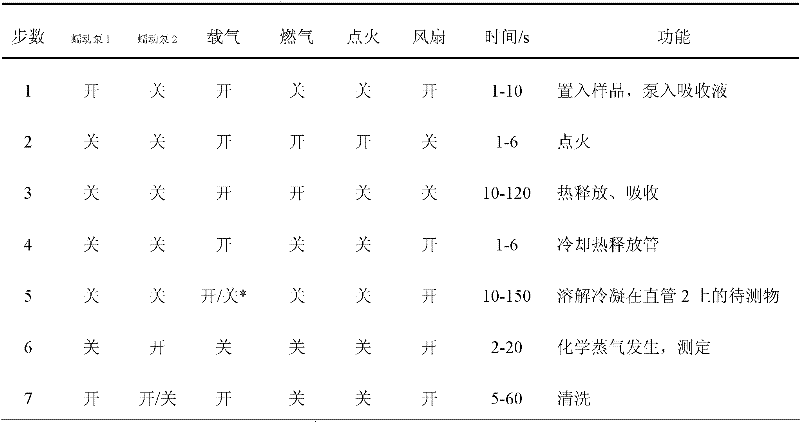
Patents
Literature
665 results about "Potassium borohydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Potassium borohydride. [pə′tas·ē·əm ¦bȯr·ō′hī‚drīd] (inorganic chemistry) KBH4 A white, crystalline powder, soluble in water, alcohol, and ammonia; used as a hydrogen source and a reducing agent for aldehydes and ketones.
Synthesis of several metal selenides and tellurides as semiconductor material
InactiveCN1384047AOvercome the problems of high temperature, highly toxic raw materials, complicated process, etc.Low reaction temperatureSemiconductor/solid-state device manufacturingBinary selenium/tellurium compoundsSemiconductor materialsHydroxylamine
By using the soluble salt of transition metalz Zn, Cd, Pb, Mn, Co, Ni, Cu, Ag, Sb and Bi, selenious acid or its solutl salt, or antimonous acid or its soluble salt as raw material, and hydrazine hydrate, sodium borohydride, potassium borohydride, hydroxylamine or hydrazine sulfate as reductant, and through hydrothermal reduction reaction at 100-200 deg.c in a sealed container for 2 hr to 5 days, selenides or tellurides of the said metals as semiconductor material may be synthesized. Unlike available synthesis process, which needs high temperature, toxic feedstock and complex technological course, the present invention has the advantages of low-cost material, simple apparatus, easy control, good technological reproducibility, stable product quality, etc.
Owner:TSINGHUA UNIV
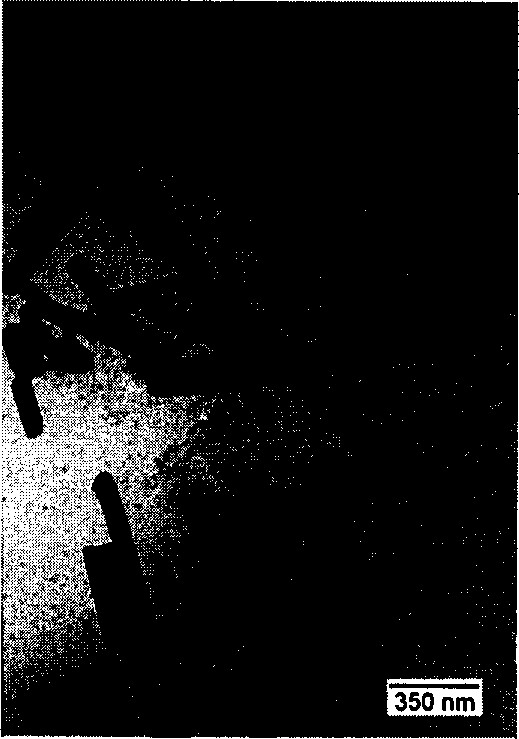
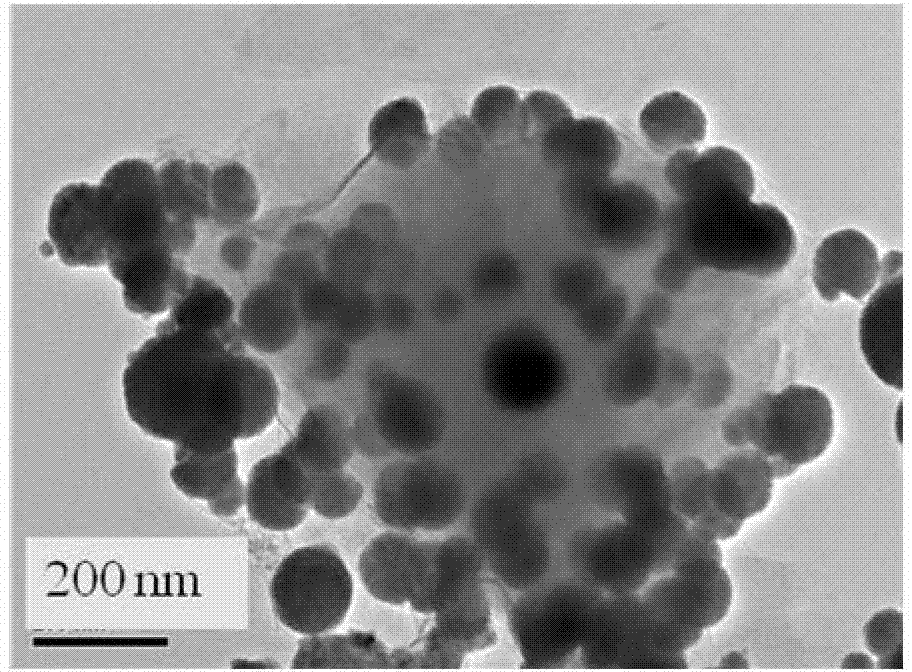
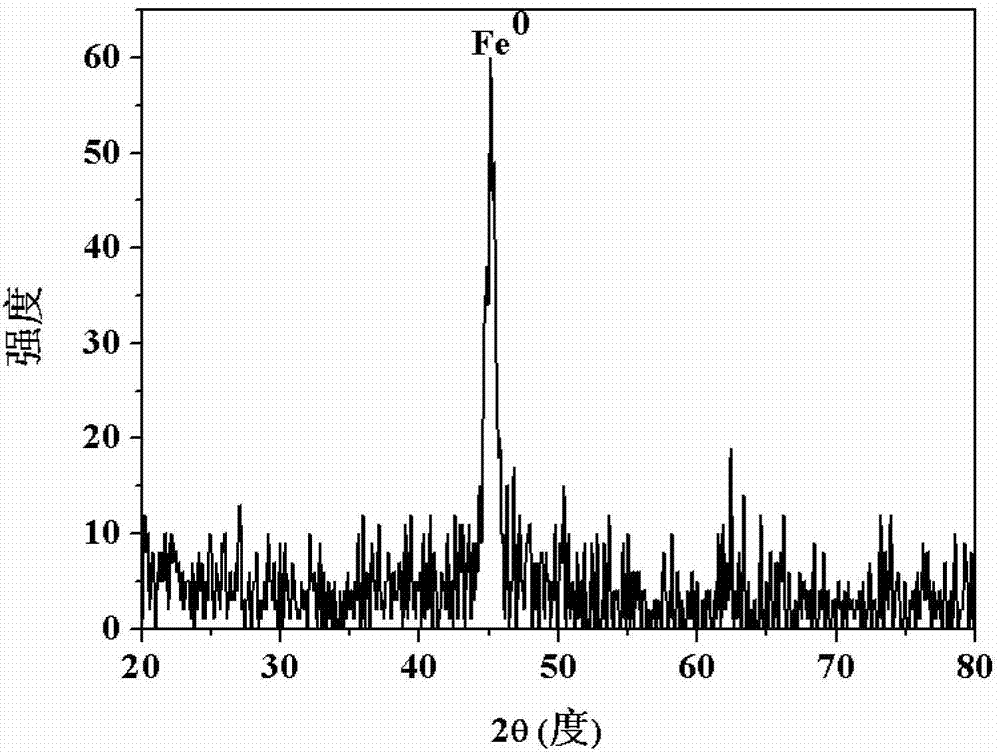
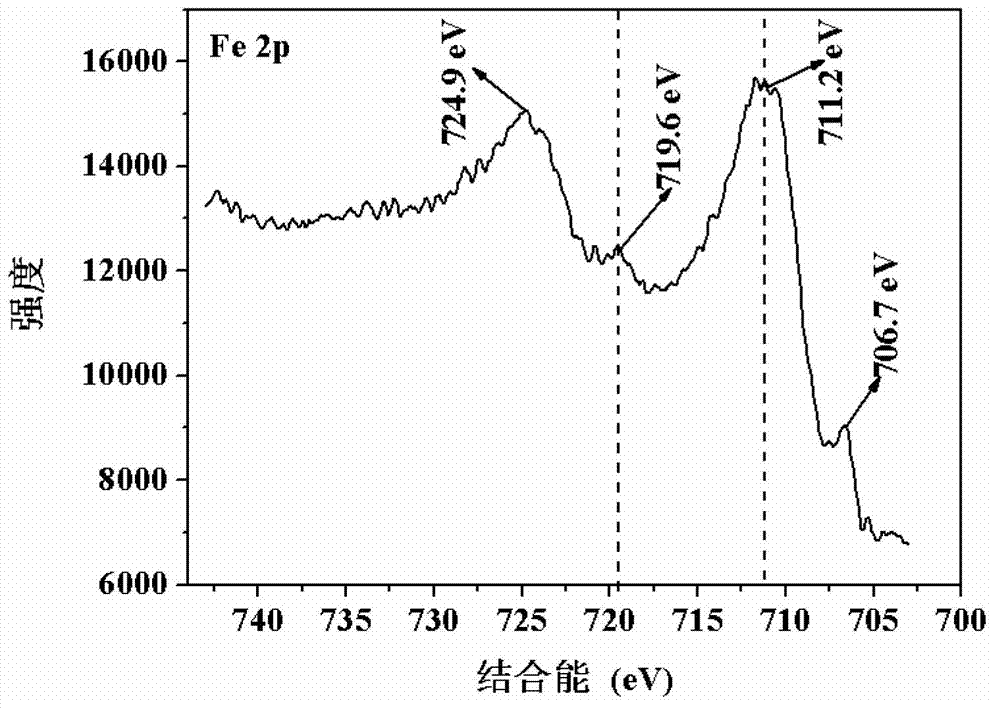
Nano zero-valent iron with montmorillonite serving as carrier, and preparation method and application thereof
InactiveCN102923835AReduce aggregationHigh activityWater/sewage treatment by sorptionWater/sewage treatment by reductionPotassium borohydrideMontmorillonite
The invention provides nano zero-valent iron with montmorillonite serving as a carrier, and a preparation method and application thereof. The method comprises the steps of dissolving ferrous sulfate, adding soluble starch and montmorillonite particles into the ferrous sulfate solution in proportion, and obtaining mixed liquid; stirring after performing ultrasound treatment to the obtained mixed liquid, and obtaining precursor solution; adding the obtained precursor solution into sodium borohydride or potassium borohydride solution in proportion under the conditions of continuous stirring, continuing stirring for a period of time, performing solid-liquid separation, and obtaining the solid which is the nano zero-valent iron with montmorillonite serving as the carrier. According to the prepared nano zero-valent iron with montmorillonite serving as the carrier, interlayer and surface spaces of montmorillonite can be fully utilized, the activity and the stability are high, polymerization of the nano zero-valent iron can be reduced, and the nano zero-valent iron has remarkable effect when used for treating waste water containing hexavalent chromium.
Owner:UNIV OF SCI & TECH OF CHINA
Nano mirror spray coating
InactiveCN1944710AExtended use timeImprove adsorption capacityLiquid/solution decomposition chemical coatingPotassium borohydrideSpray coating
The nanometer mirror spraying process includes the following steps: 1. eliminating surface dust; 2. spraying primer; 3. sensitizing and activating treatment; 4. spraying solution A comprising silver nitrate solution, ammonia water solution and sodium hydroxide solution and solution B comprising sodium borohydride, potassium borohydride or p-methylaminophenol sulfate solution in the volume ratio of 1 to 1-1.7 to produce reduction; 5. shaping and developing; 6. blowing to dry; and 7. drying. The present invention has the advantages of diversified products, high corrosion resistance, suitability to mass production, high hardness, low cost, etc.
Owner:方宏亮
Carbon loaded type noble metal catalyst and preparation method thereof
ActiveCN101450308AHigh activityHigh selectivityCarboxylic preparation by ozone oxidationMetal/metal-oxides/metal-hydroxide catalystsPotassium borohydrideReduction treatment
The invention relates to a carbon loaded noble metal catalyst, which is characterized in that the catalyst consists of a carrier and palladium and ruthenium which are loaded on the carrier, wherein the carrier is powdery fruit shell active carbon and is between 85 and 99.7 percent by weight; the palladium is between 0.2 and 10 percent by weight; and the ruthenium is between 0.1 and 10 percent by weight. The catalyst is obtained by performing acid treatment, ash removal and oxidation treatment on the carrier, namely the active carbon for removing surface reduction functional groups first, using a solution of the palladium and the ruthenium to perform soakage treatment, and using one or more among hydrogen, potassium borohydride or hydrazine hydrate to perform reduction treatment. The catalyst has high activity and selectivity and good stability in hydrogenation reaction of organic compounds containing carbonyl.
Owner:CHINA PETROLEUM & CHEM CORP +1
Nanogold solution and method for detecting Co<2+> by using same
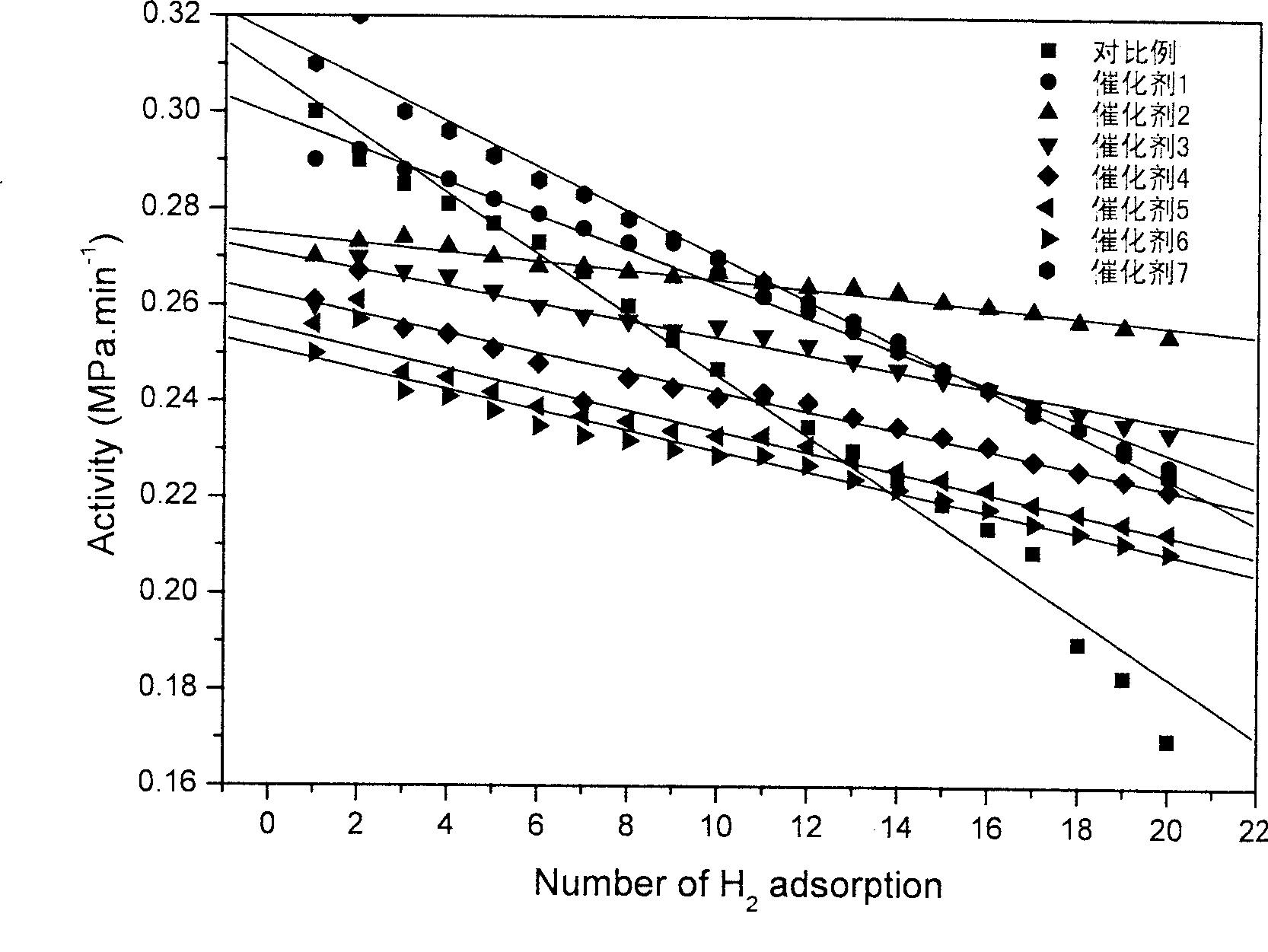
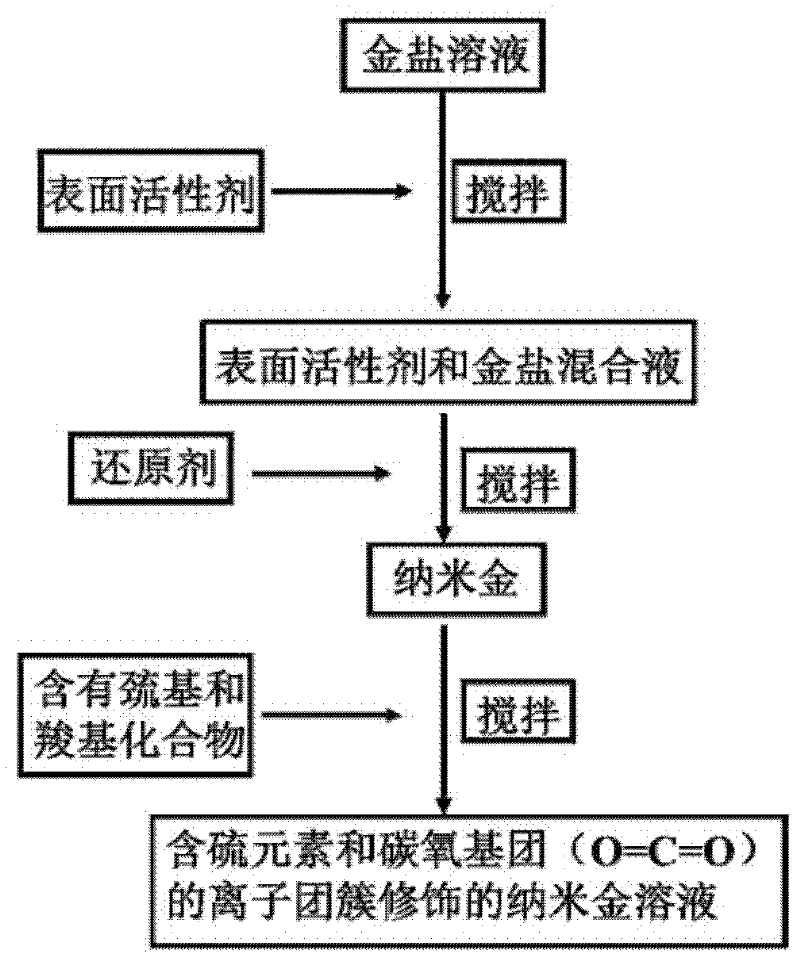
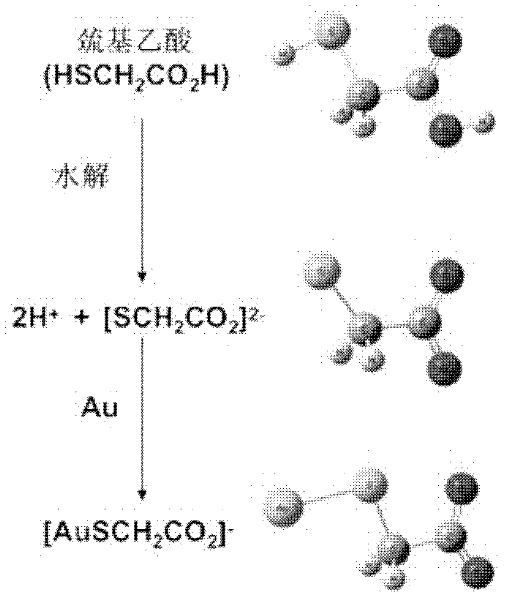
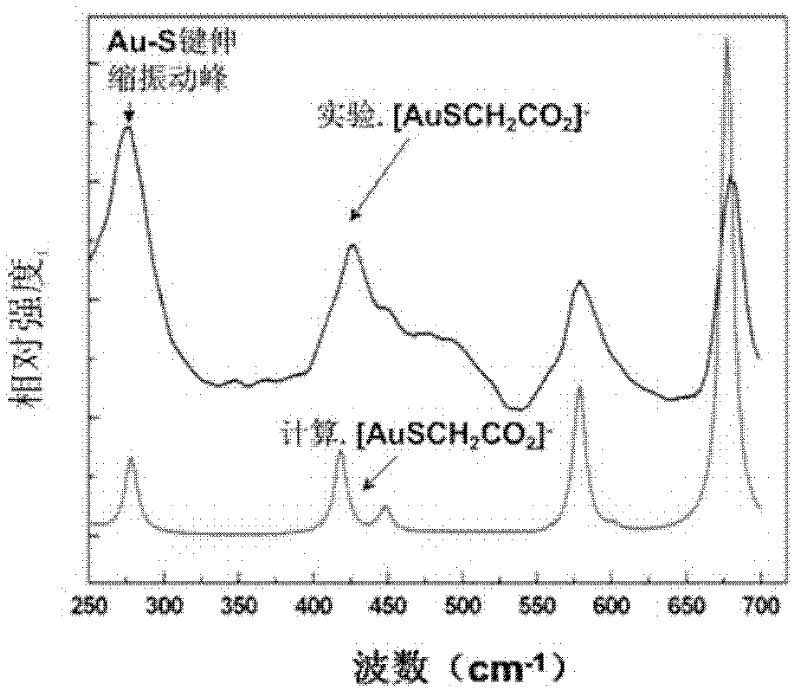
ActiveCN102416482AModification method is simpleGood repeatabilityMaterial nanotechnologyMaterial analysis by observing effect on chemical indicatorIon clustersPotassium borohydride
The invention discloses a nanogold solution and a method for detecting Co<2+> by using the same. The water soluble functional nanogold solution is prepared by using a gold salt as the resource of gold nanoparticles, a quaternary ammonium salt as a surfactant, ion clusters containing sulfur elements and carbon oxygen groups (O=C=O) as ligands, sodium borohydride, potassium borohydride or ascorbic acid as a reducing agent, and deionized water as a synthesis medium and by modifying the surface of the nanogold with the ion clusters containing sulfur elements and carbon oxygen groups (O=C=O) under the action of gold-sulfur bonds (Au-S). The nanogold solution has high speed, selectivity, sensitivity and practicality for detection of trace amount of Co<2+> in the water solution system, and therefore can be used in combination of a colorimetric process for detecting trace amount of Co<2+>; the detection concentration is lower than 8.5*10<-7>M; the detection threshold value may reach 5*10<-10>M; the detection speed is high, the selectivity is high, the cost is low, and the carrying is convenient; and the detection method has a bright application prospect in fields of environmental science, detection chemistry and analysis chemistry, and the like.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Method for preparing load type nano arsenic-removing sorbent for drinking water
InactiveCN101347717ALarge adsorption capacityImprove adsorption capacityOther chemical processesWater/sewage treatment by sorptionPotassium borohydrideSorbent
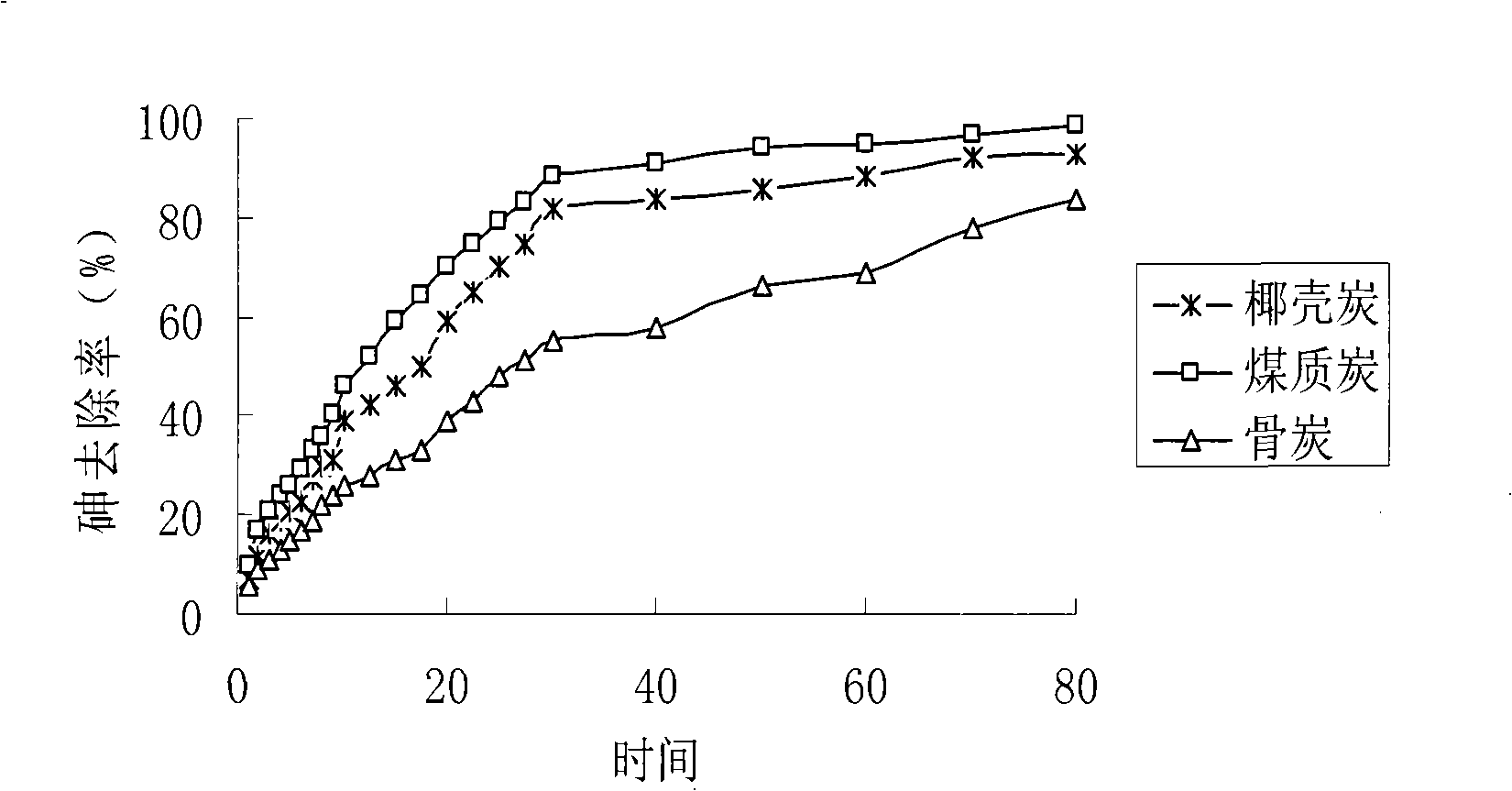
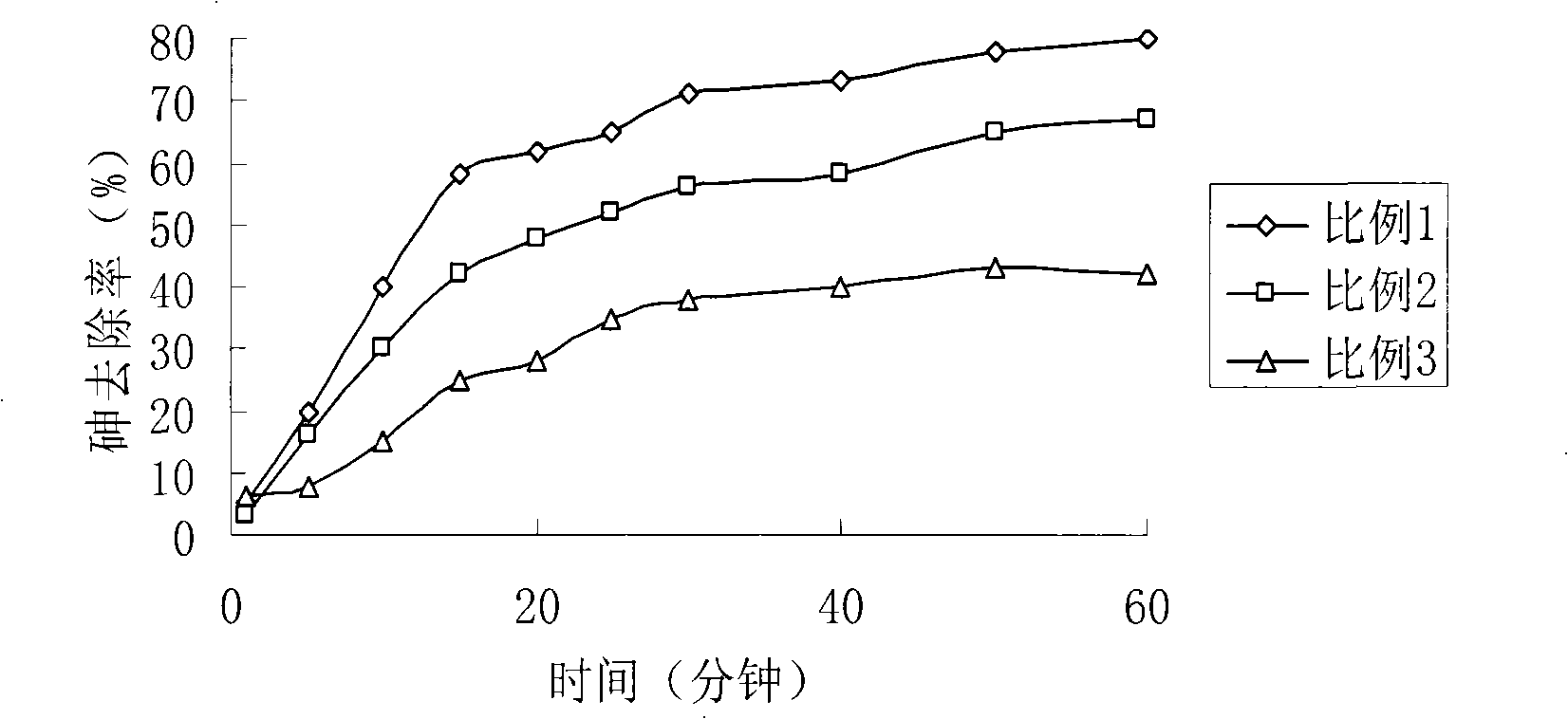
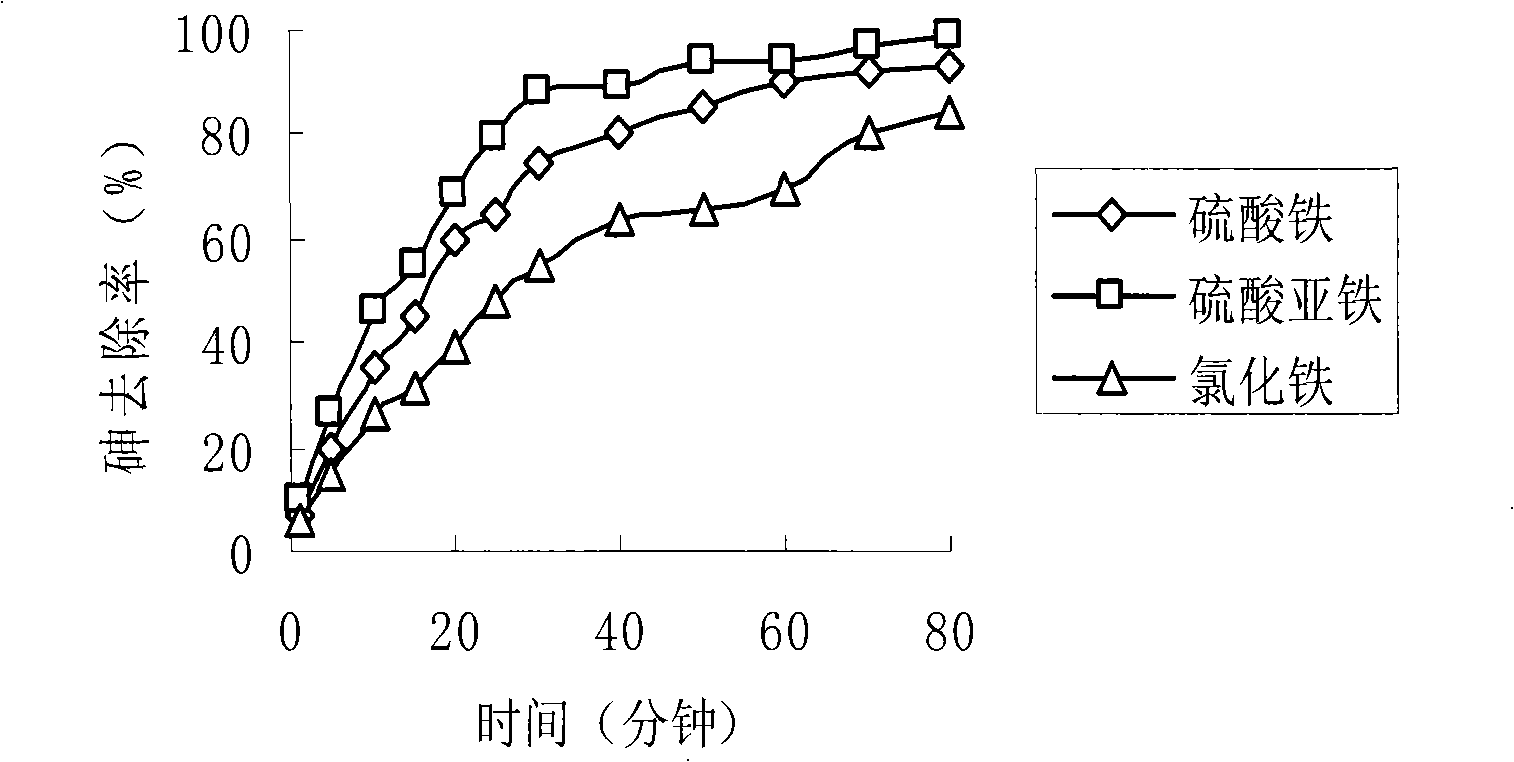
The invention relates to the elimination of the arsenic in drinking water, in particular to a preparation method of a supported type nano-adsorbent for removing arsenic from drinking water; the method includes the following steps: (1) activated carbon material with pore volume of 0.100-0.500cm<3> / g is selected for pretreatment; (2) soluble ferric salt solution is firstly used for soaking the activated carbon for 10-120 min; (3) alcoholic solution is taken as a dispersant to be added into the ferric salt solution; (4) under the protection of inert gases at room temperature, a strong reductant, potassium borohydride or sodium borohydride, is used for titrating the ferric salt, and agitation is carried out under the protection of inert gases; after the titration of potassium borohydride or sodium borohydride solution, agitation lasts for 10-120 min; (5) after agitation, centrifugation is carried out; oxygen-free water is firstly used for washing for 1-3 times, then organic solvent is adopted for washing for 1-3 times, and vacuum drying is done at 40-100 DEG C for 12-48h to obtain the product. The adsorbent of the invention has large adsorption capacity and small volume and is safe, stable and easy to store and transport.
Owner:SHENYANG INST OF APPL ECOLOGY CHINESE ACAD OF SCI
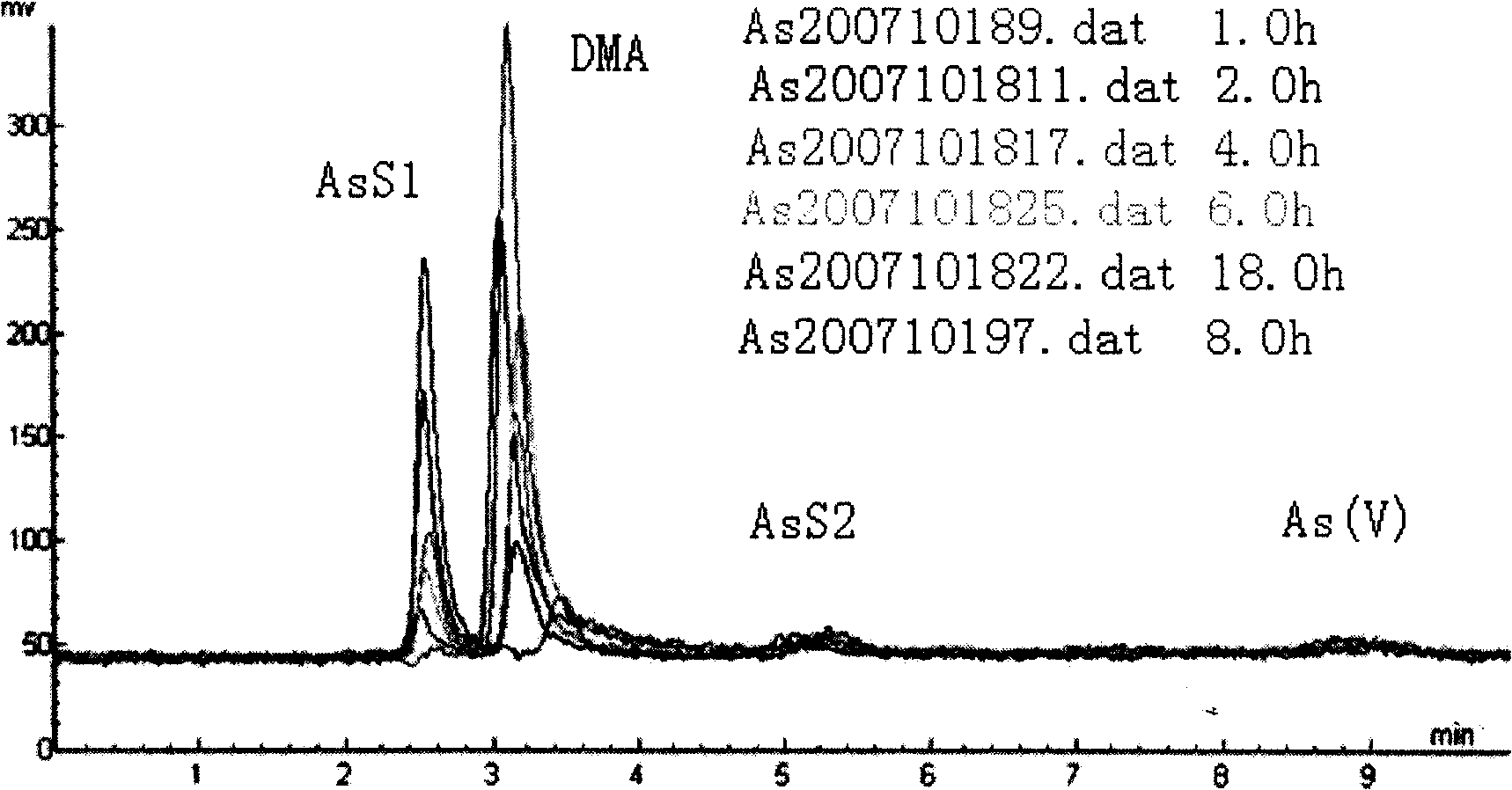
Aquatic product inorganic arsenic determination method
InactiveCN101261258AReasonable selection of experimental conditionsReasonable choiceComponent separationPotassium borohydrideFluorescence
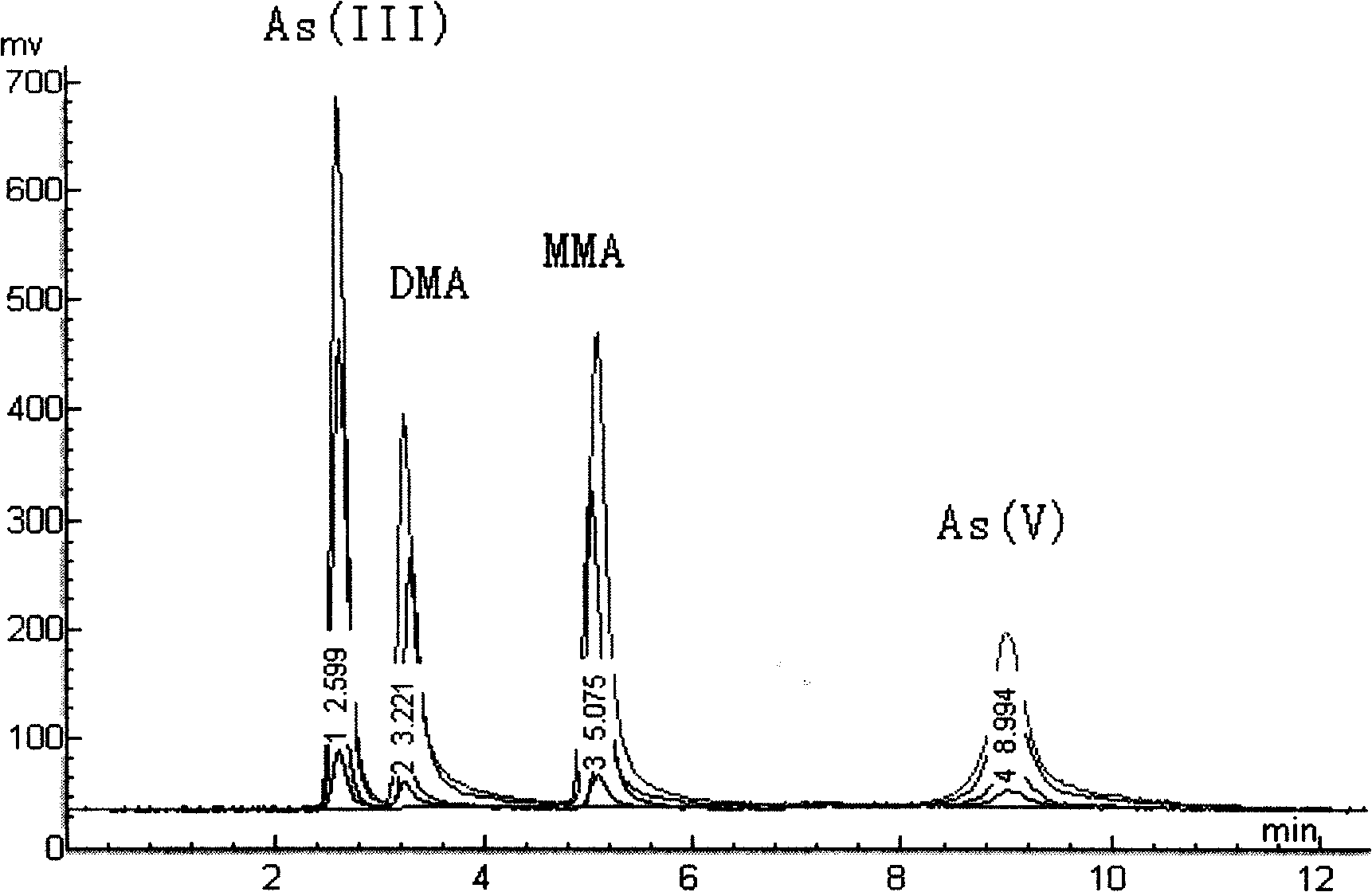
The invention relates to a determination method for inorganic arsenic in aquatic products. After being extracted by hydrochloric acid solution, the inorganic arsenic (1plus1) in the aquatic products passes through an anion-exchange column, and arsenite As (III), dimethyl arsenic compound DMA, methyl arsenic compound MMA and arsenate As (V) are eluted sequentially by mobile phase; owing to different adsorption capacities of the anion-exchange column on the arsenate As (V), the arsenite As (III), the methyl arsenic compound MMA, the dimethyl arsenic compound DMA and arsenic sugar AsS, the eluted solution is hydrogenated by borohydride potassium reducing agent and hydrochloride, and hydride is generated, enters an atomizer, and is subject to analytic determination by being combined with atomic fluorescence. The experimental condition is reasonably selected, and the detecting data is accurate and reliable and can not be influenced by extraction time and temperature. The determination method of inorganic arsenic in aquatic products of the invention not only can detect the inorganic arsenic iAs accurately, but also can determine the methyl arsenic compound MMA and the dimethyl arsenic compound DMA of the organic arsenic, and can be used for the morphometry of the arsenic in the aquatic products.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
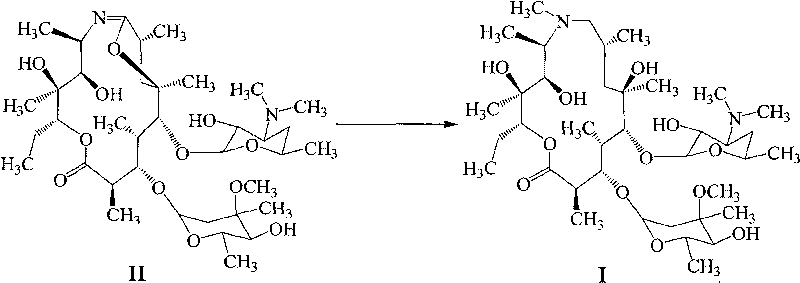
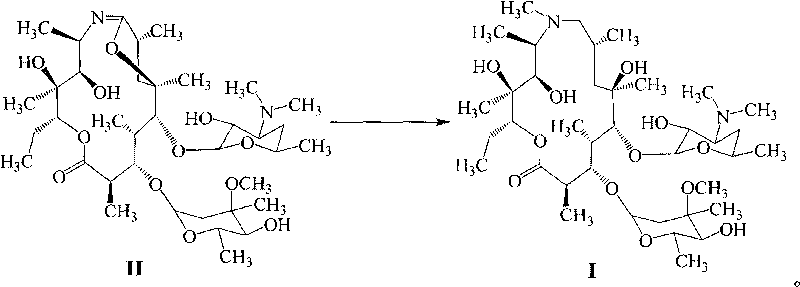
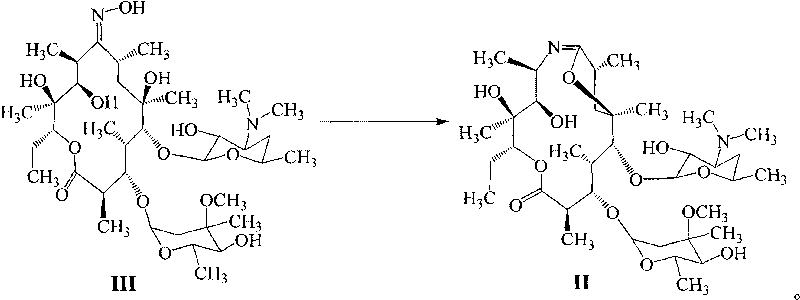
Method for preparing azithromycin and method for preparing intermediate of azithromycin
ActiveCN101712703ALow costModerate dosageSugar derivativesSugar derivatives preparationPotassium borohydrideSolvent
The invention discloses a method for preparing azithromycin. The method comprises the following steps of: in acid aqueous solution and under an action of potassium borohydride, performing the reduction reaction of erythromycinA6, 9-imine ether represented by a formula II, then performing methylation reaction, and putting the reaction products in a mixed solvent of water and halogenated hydrocarbons for hydrolysis reaction so as to obtain the azithromycin represented by a formula I. In the method, the reducer is properly consumed, the yield is high, and the cost of the preparation of the azithromycin is obviously reduced. The invention also relates to a method for preparing the erythromycinA6, 9-imine ether and erythromycinA9-oxime.
Owner:BEIJING RED SUN PHARMA
Test method of micro amount of arsenic or antimony in steel
InactiveCN101650302AQuality assuranceEnsure performancePreparing sample for investigationColor/spectral properties measurementsPotassium borohydrideHigh energy
The invention relates to a test method of steel, in particular to a test method of a micro amount of arsenic or antimony in steel. The test method is based on a principle of a flow injection-hydride generation-atomic absorption spectroscopic methodology, and experiments are determined in a flow-injection sample-injection mode. The method comprises the following steps: a test solution is reduced bya thiourea-ascorbic acid mixed solution and a hydrochloric acid solution to convert arsenic (V) into arsenic (III), and convert antimony (V) into antimony (III); the test solution reacts with a potassium borohydride solution under a carrier band of a carrier solution to generate a great amount of nascent oxygen which reacts with the arsenic (III) or the antimony (III) to generate gaseous AsH3 orSbH3; the AsH3 or the SbH3 is led into a specially-designed quartz tube by high-purity argon gas as carrier gas and is atomized into ground-state atomic vapor; ground-state electrons which are on theoutermost layer of the atom are excited by the light energy of a light source of a hollow cathode lamp to transit to a high energy level; and the amount of abortion light intensity is directly proportional to the concentration of the atom. Accordingly, the arsenic or antimony content in the test solution can be quantitatively analyzed.
Owner:CHINA YITUO GROUP
Nanometer bismuth/carbon composite material and preparation method thereof
InactiveCN108134090AAvoid the case of bulky bismuth metalEvenly distributedMaterial nanotechnologyAlkaline accumulatorsCarbon compositesPotassium borohydride
The invention relates to a nanometer composite material, in particular to a nanometer bismuth / carbon composite material and a preparation method thereof. A nanometer bismuth and carbon compound is obtained by taking various carbon materials as a substrate, bismuth nitrate, bismuth chloride, bismuth sulfate, bismuth acetate, bismuth citrate and the like as a bismuth source, water containing an organic complexing agent, ethylene glycol, propylene glycol or other mixture as a solvent and sodium borohydride, potassium borohydride, hydrazine hydrate and the like as a reducing agent and by an absorption-thermal decomposition-reduction method. According to the method, a solution containing bismuth ions is absorbed to a surface of a carbon material, remaining solution is filtered, the bismuth oxide / bismuth and carbon compound is obtained after drying and thermal treatment, and the nanometer bismuth / carbon composite material is finally obtained by reduction reaction. In the composite material obtained by the method, metal bismuth particles are uniformly distributed in surfaces of carbon particles in nanometer size, and a phenomenon that a large amount of bismuth can be agglomerated by a traditional bismuth reduction method is prevented.
Owner:CENT SOUTH UNIV
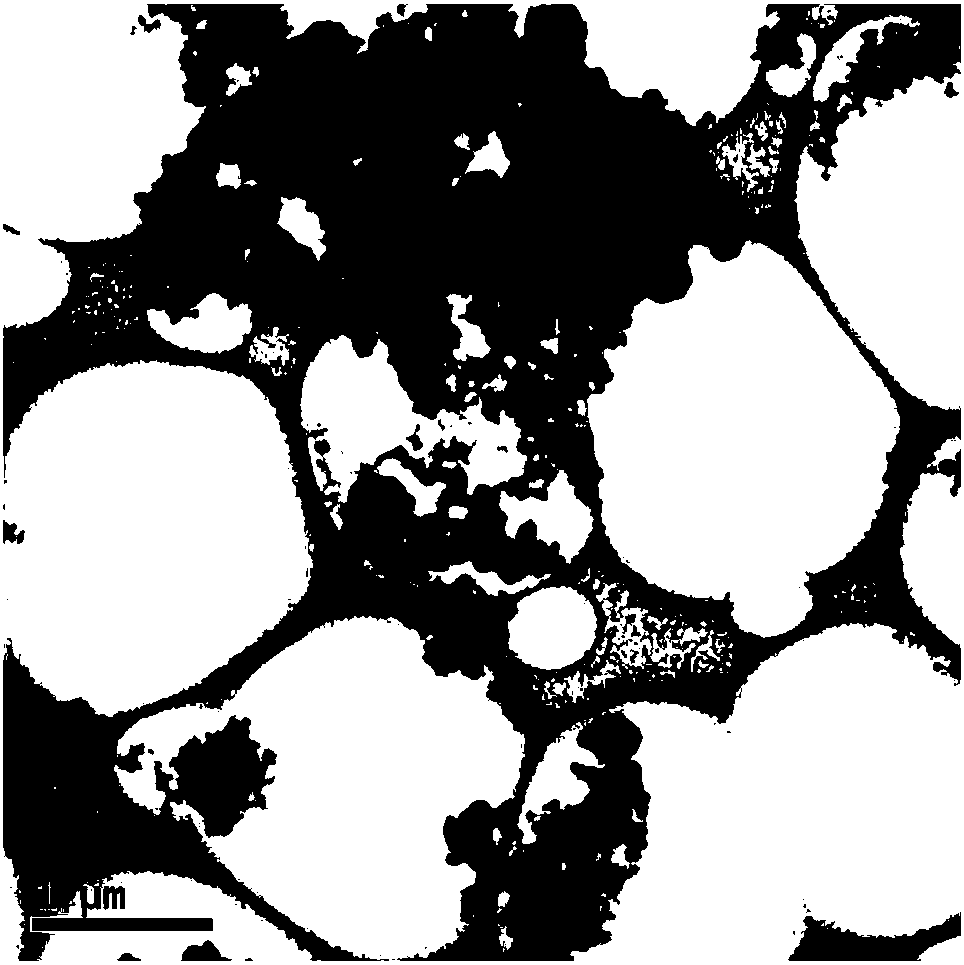
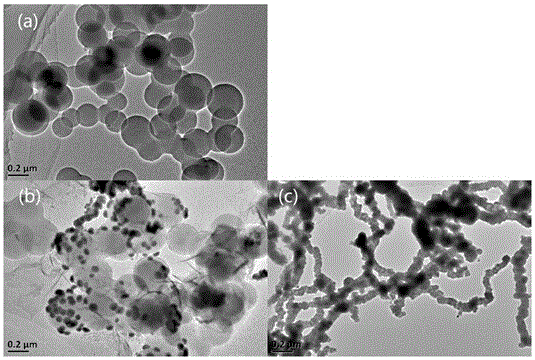
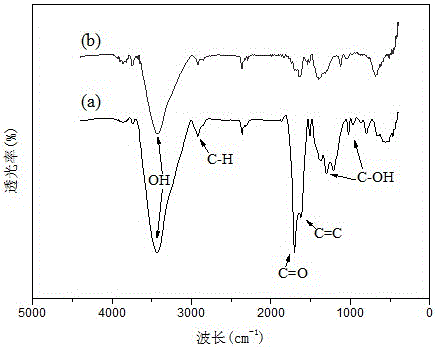
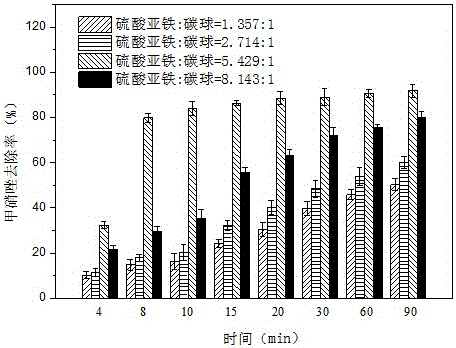
Preparation method and application of carbon sphere loaded nanoscale zero valent iron composite material
ActiveCN106044921AWide variety of sourcesLow priceWater contaminantsWater/sewage treatment by electrochemical methodsDispersityPotassium borohydride
The invention provides a preparation method of a carbon sphere loaded nanoscale zero valent iron composite material. The method comprises: (1) preparing carbon spheres containing hydrophilic functional groups (-OH, -COOH) by a hydrothermal method; (2) chelating iron ions and the functional groups by soaking; (3) finally, dropping potassium borohydride or sodium borohydride solution into carbon sphere mixed solution with the iron ions, and forming the carbon sphere loaded nanoscale zero valent iron composite material by a strong reducing effect. The material prepared by the preparation method provided by the invention not only simultaneously has an adsorption effect of the carbon spheres and the strong reducing effect of nanoscale zero valent iron, but also can form a micro primary cell between the iron and carbon; not only are the problems of clustering and the like of nanoscale zero valent iron particles solved, but also electron transfer is reinforced and a degrading effect on polluted waste water is promoted; the method provided by the invention is low in cost and simple to operate, and the nano particles have high dispersity and stability.
Owner:KUNMING UNIV OF SCI & TECH
Microwave assistant method for quickly synthesizing quanta point of zinc selenide fluorescence
InactiveCN1687303AGood water solubilityImprove stabilityLuminescent compositionsZinc selenidePotassium borohydride
The present invention relates to a method for microwave-aided quickly synthesizing zinc selenide fluorescence quantum point. Said method uses water as solvent, and adopts the following steps: mixing zinc salt or zinc oxide and water-soluble mercaptocompound, injecting soduim hydrogen selenide or potassium hydrogen selenide produced by adopting sodium borohydride or potassium borohydride and zinc powder and making them produce reaction to obtain zinc solenide ZnSe precursor solution, then placing said solution in a closed teflon tank and making reaction in a temperature-controllable and pressure-controllable microwave reactor so as to quickly synthesize ZnSe fluorescence quantum point.
Owner:SHANGHAI JIAO TONG UNIV
Method for synthesizing noble metal superfine nanowire water phase and establishing noble metal nanopore membrane by self-precipitation thereof
InactiveCN101935017AWide applicabilityGood biocompatibilityPolycrystalline material growthNanostructure manufacturePlatinumNanowire
The invention provides a method for synthesizing a noble metal (gold, palladium, platinum) nanowire and establishing a noble metal nanopore membrane by self-precipitation thereof. The method comprises the following steps of: adding a 0.05 percent (W / V) nonionic surfactant into 1 mmol.L<-1> solution of noble metal precursor (HAuCl4, H2PtCl6, Pd(NO3)2) and mixing the solution and the surfactant by stirring; stirring the mixture in ice bath for 5 to 10 minutes and adding potassium borohydride (or sodium borohydride) in an amount which is 6 times that of the HAuCl4 of the metal precursor, 4 timesthat of the H2PtCl6 and twice that of the Pd(NO3)2; stirring violently to fully reduce the metal precursor in the mixture so as to synthesize the metal superfine (less than or equal to 3 nanometers) netlike nanowire; adding the 0.05 percent (W / V) nonionic surfactant into synthesized nanowire dispersion liquid, mixing uniformly and centrifuging at the temperature of 60 DEG C for 10 minutes so as to separate and purify a nanomaterial; and adding 5mM of NaCl (additional NaCl does not need to be added into an Au nanowire) into the synthesized nanowire dispersion liquid and standing for 12 hours so as to obtain a corresponding noble metal nanopore membrane.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
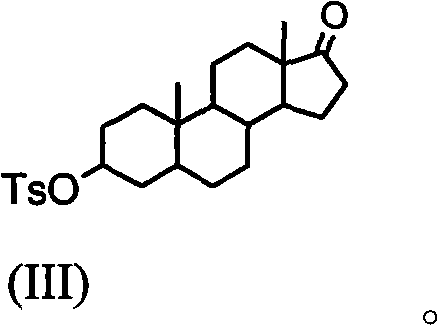
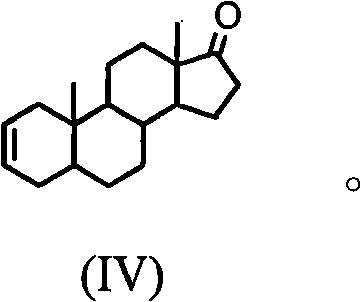
Synthesis process of vecuronium bromide
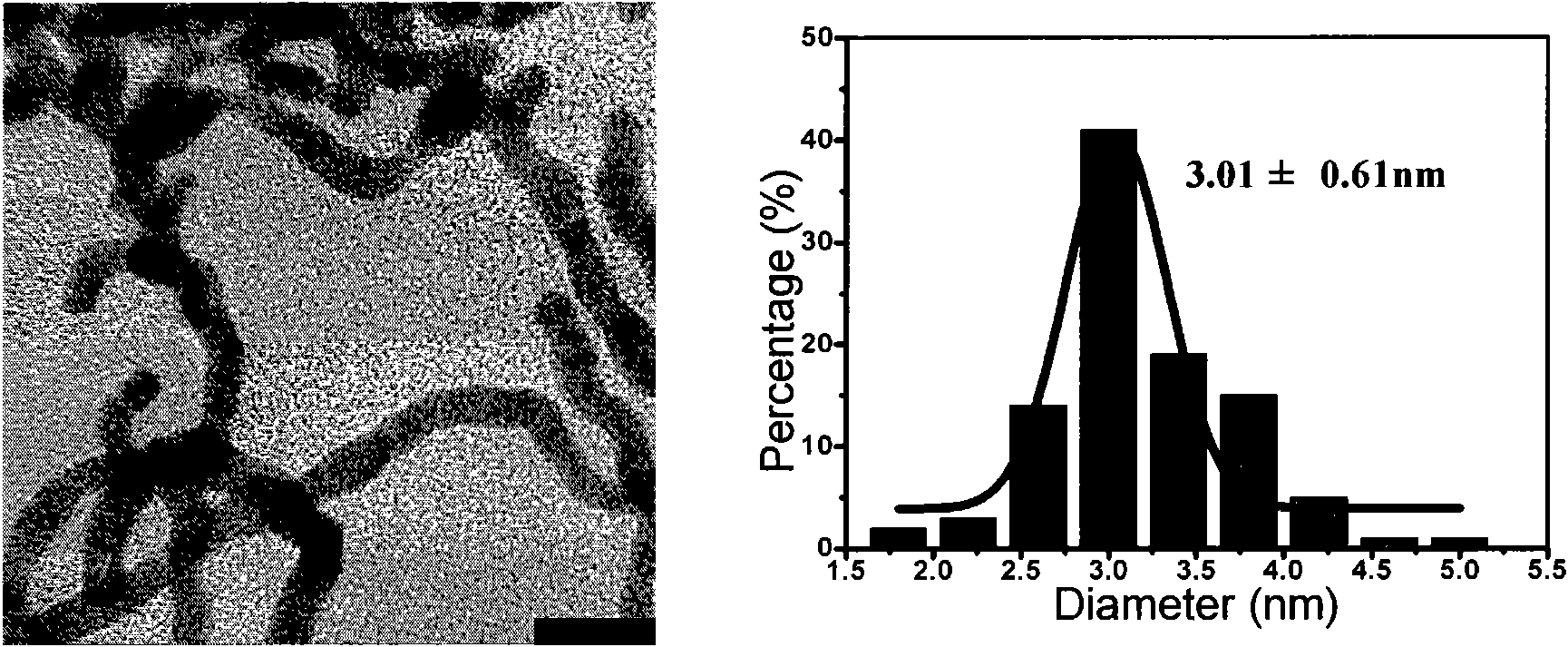
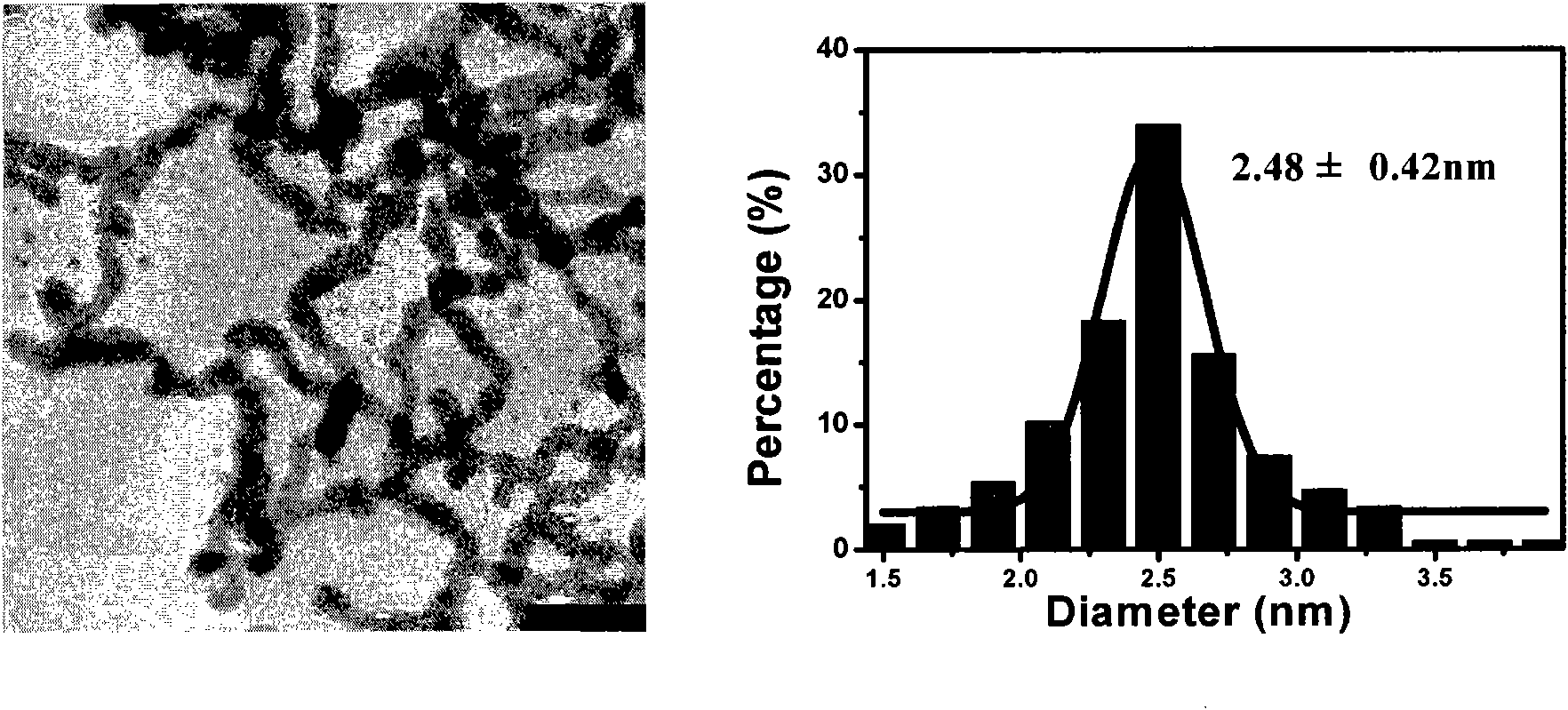
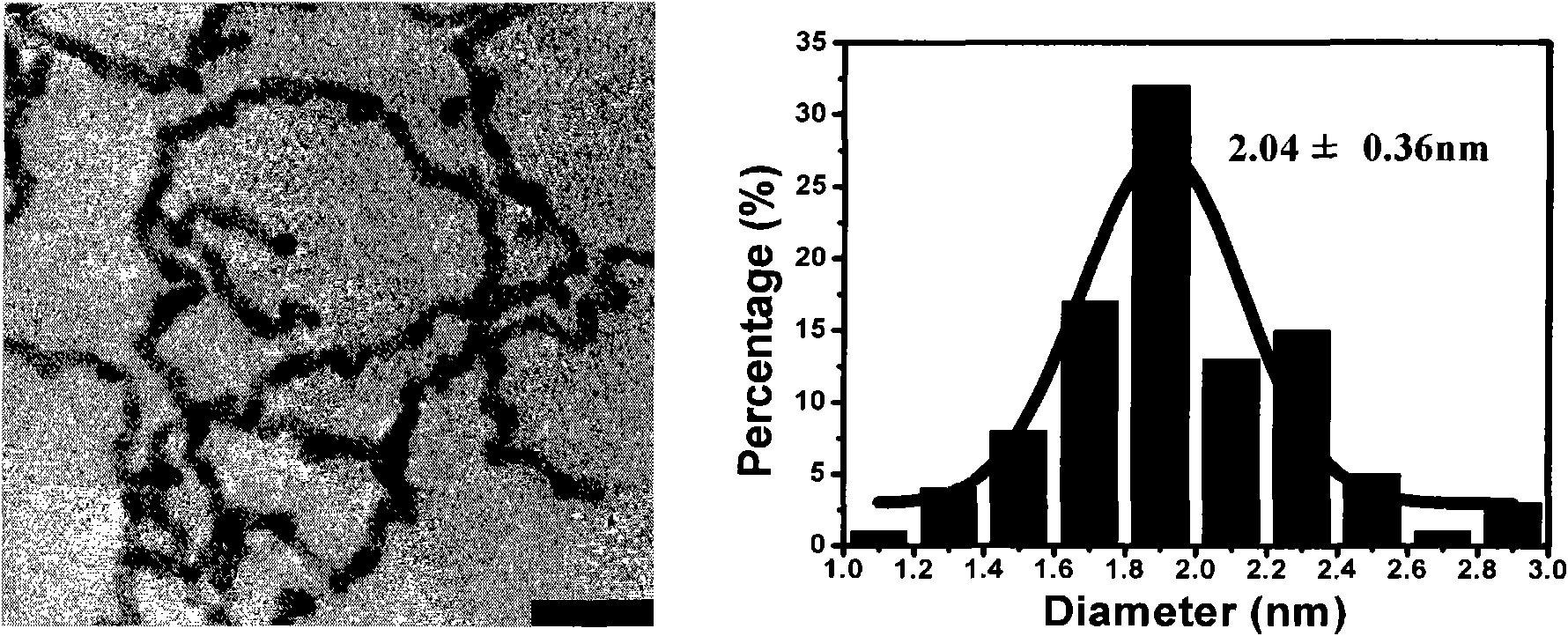
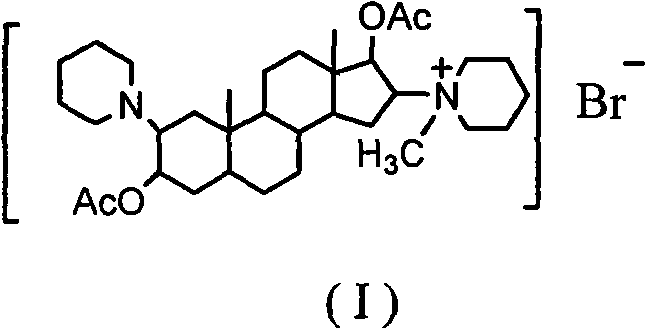
The invention discloses a synthesis process of vecuronium bromide. The synthesis process comprises the following steps: generating epiandrosterone sulfonyl ester (III) by carrying out esterification reaction between epiandrosterone (II) and paratoluensulfonylchloride; generating 5Alpha-androst-2-alkene-17-ketone (IV) by carrying out elimination and dehydration reaction between the (III) and 2,6-lutidines; generating 17-acetoxyl-5Alpha-androstane-2,16-diene (V) by carrying out enolization and esterification reaction between the (IV) and isopropenyl acetate; generating (2Alpha, 3Alpha, 16Alpha,17Alpha)-diepoxy-17Beta-acetyl-5Alpha-androstane (VI) by epoxy reaction of the (V) under the effect of hydrogen peroxide; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha-hydroxyl-17-ketone (VII) by ring-opening and addition reaction of the (VI) under the effect of hexahydropyridine; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha,17Beta-diol (VIII) by the (VII)under the reduction of potassium borohydride; generating 2Beta, 16Beta-di(1-piperidyl)-3Alpha, 17Beta- acetoxyl-5Alpha-androstane (IX) by carrying out esterification reaction of the (VIII) under the acetylation of acetic anhydride; and generating vecuronium bromide (I) by carrying out quaternary ammonium salt reaction between the (IX) and bromomethane. The invention has the advantages of low cost,less pollution and high yield.
Owner:XUZHOU NORMAL UNIVERSITY
High-strength and high-toughness composite silver solder ring for in-situ synthesis of soldering flux
ActiveCN105127618AHigh activityStrong ability to remove filmWelding/cutting media/materialsSoldering mediaPotassium borohydridePotassium fluoride
The invention discloses a high-strength and high-toughness composite silver solder ring for in-situ synthesis of soldering flux. The high-strength and high-toughness composite silver solder ring comprises a silver solder ring body of a hollow structure. The silver solder ring body is formed by winding composite silver solder formed by a silver solder pipe and a flux core with which the silver solder pipe is filled. The flux core is prepared from boron micro-powder, sodium borohydride or potassium borohydride, potassium fluoborate, boric anhydride or boric acid, potassium fluoride or sodium fluoride or lithium fluoride, potassium bifluoride and potassium fluoroaluminate according to a certain proportion. The purpose of in-situ synthesis of the soldering flux through the boron micro-powder in the flux core and metal elements in the silver solder pipe is achieved, so that the content of the flux core is reduced, and when the content of the flux core is low, the solder still achieves good brazing manufacturability; meanwhile, due to the fact that the wall thickness of the solder pipe is increased, good toughness and high stiffness are achieved, the processing performance of the solder is greatly improved, the minimum diameter can be reduced to 0.8 mm, the solder can be easily wound into the solder ring with the intermediate diameter below 6 mm, and application and popularization of the automatic brazing process are facilitated.
Owner:ZHENGZHOU RES INST OF MECHANICAL ENG CO LTD
Method for removing nitrate nitrogen in water body
InactiveCN103964550AHigh removal rateLarge specific surface areaWater/sewage treatment using germicide/oligodynamic-processPotassium borohydrideNitrate nitrogen
The invention provides a method for removing nitrate nitrogen in a water body, which comprises the following steps: 1, adding ferrous sulfate heptahydrate, polyethyleneglycol and graphene into deoxidized distilled water, mixing to prepare a suspension, then adding a potassium borohydride solution into the suspension, stirring, filtering, and washing to obtain graphene loaded nano iron; and 2, uniformly mixing the graphene loaded nano iron and a water body to be treated, and then performing constant-temperature oscillation treatment, thus ensuring that the removal rate of nitrate nitrogen in the water body is above 85%. According to the invention, the nitrate nitrogen in the water body is removed by using the graphene loaded nano iron, so that the method is simple in technical process, low in production cost and easy to realize popularization and application, maximally keeps the favorable characteristics of graphene and nano iron, and can efficiently and quickly remove the nitrate nitrogen in the water body, thereby obviously improving the nitrate nitrogen removal effect and having wide application value.
Owner:CHANGAN UNIV
Solid sampling-non-dispersion atomic fluorescence photometer collocating device and analyzing method
ActiveCN102305779AReduce preprocessing timeSolve pollutionPreparing sample for investigationFluorescence/phosphorescenceEnvironmental resistancePeristaltic pump
The invention relates to a solid sampling-non-dispersion atomic fluorescence photometer collocating device and an analyzing method. The device comprises a solid sampler, an integrated interface, a sample pipe, a peristaltic pump, a tee joint, a gas-liquid separator, a detector and a computer. The method comprises the following steps of: volatilizing an analyzing element in a volatile halide way and absorbing by absorption liquid by adding an auxiliary heat release agent or an ashing auxiliary agent in the solid sampler. The absorption liquid is introduced into a chemical steam generating system for reacting with potassium borohydride (sodium); and the generated volatile hydride or atomic steam is carried into an instrument atomization area for being detected by atomic fluorescence. The invention directly adopts solid sampling, rapidly realizes the detection of toxic elements, such as arsenic, stibium, selenium, mercury, and the like in the samples, such as geology, environment, food, biology, and the like and has the advantages of higher analyzing speed, low analyzing cost, high detecting sensitivity, high device portability, environmental protection, and the like.
Owner:西安西北有色地质研究院有限公司
Technology for templet-free low-temperature preparation of porous boron nitride in one-step method
InactiveCN101602497AUniform pore size distributionHigh yieldNitrogen compoundsPotassium borohydrideThiourea
The invention discloses a technology for the templet-free low-temperature preparation of porous boron nitride in a one-step method. In the technology, sodium borohydride or potassium borohydride is used as a boron source, urea or thiourea or aminothiourea is used as a nitrogen source, the boron source and the nitrogen source are proportionally weighed, are heated to 500 to 600 DEG C in a reaction kettle after being mixed evenly, and react, the mixture is naturally cooled to room temperature after heat preservation for 5 to 10 hours, and finally, the porous boron nitride is prepared by impurity removal and drying. The preparation technology has low price of a reactant, low reaction temperature, short reaction time, energy saving, simple operation, good repeatability and no pollution and is beneficial to the mass production of the porous boron nitride, a templet is not used in the reaction process, a residual reactant and a reaction byproduct are easy to remove, and the porous boron nitride has high yield and higher thermal stability.
Owner:SHANDONG UNIV
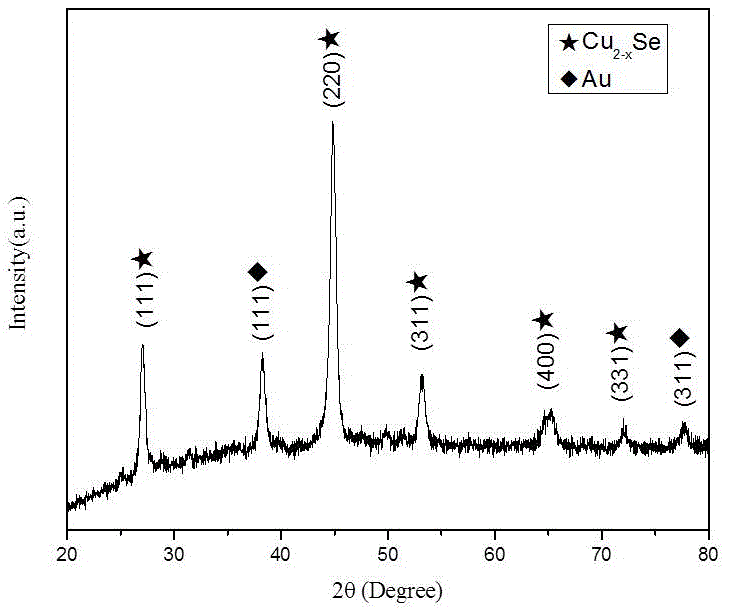
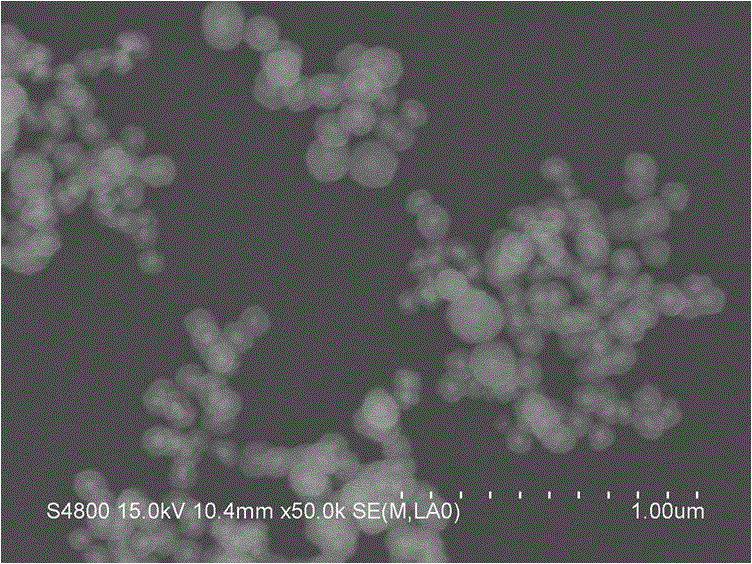
Preparation method of Au@Cu2-xSe cage-like core-shell nanostructures
The invention discloses a preparation method of Au@Cu2-xSe cage-like core-shell nanostructures. The preparation method comprises: 1, using sodium citrate to reduce chloroauric acid to obtain spherical gold nanoparticles; 2, by using the spherical gold nanoparticles as a template, using ascorbic acid to reduce a copper salt to obtain gold / cuprous oxide nano core-shell structures; 3, using potassium borohydride to reduce selenium powder in a strong base environment to obtain a selenium source, and mixing and reacting the obtained selenium source with the gold / cuprous oxide core-shell nanoparticles; and 4, carrying out centrifugal washing treatment, and vacuum drying treatment, and finally obtaining the gold / cuprous oxide cage-like core-shell nanostructures. The method is easy in operation, good in repeatability, and high in yield, and the prepared product is good in stability.
Owner:EAST CHINA UNIV OF SCI & TECH
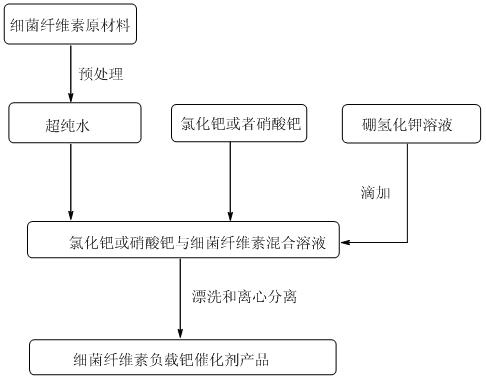
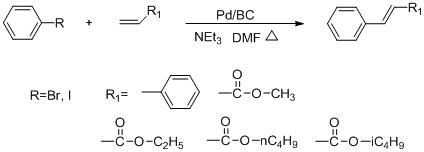
A kind of preparation method of bacterial cellulose supported nanometer palladium catalyst
ActiveCN102274753AEasy to makeImprove thermal stabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPotassium borohydridePtru catalyst
The invention discloses a method for preparing a bacterial cellulose loaded nanometer palladium catalyst. The method comprises the following steps of: pretreating bacterial cellulose, stirring the pretreated bacterial cellulose in ultrapure water, and dispersing; adding palladium chloride or palladium nitrate, dispersing fully, removing oxygen, and heating; adding potassium borohydride solution; and after reaction is finished, blanching and centrifugal separation repeatedly to obtain the bacterial cellulose loaded nanometer palladium catalyst. In the method, divalent potassium ions are reduced by using the potassium borohydride solution under the hydrothermal condition to form the zero-valent palladium catalyst with small grain diameters and uniform distribution; and the preparation process is simple, the prepared catalyst is high in catalytic efficiency, and the method can be applied in the fields of organic synthesis and the like.
Owner:NANJING UNIV OF SCI & TECH
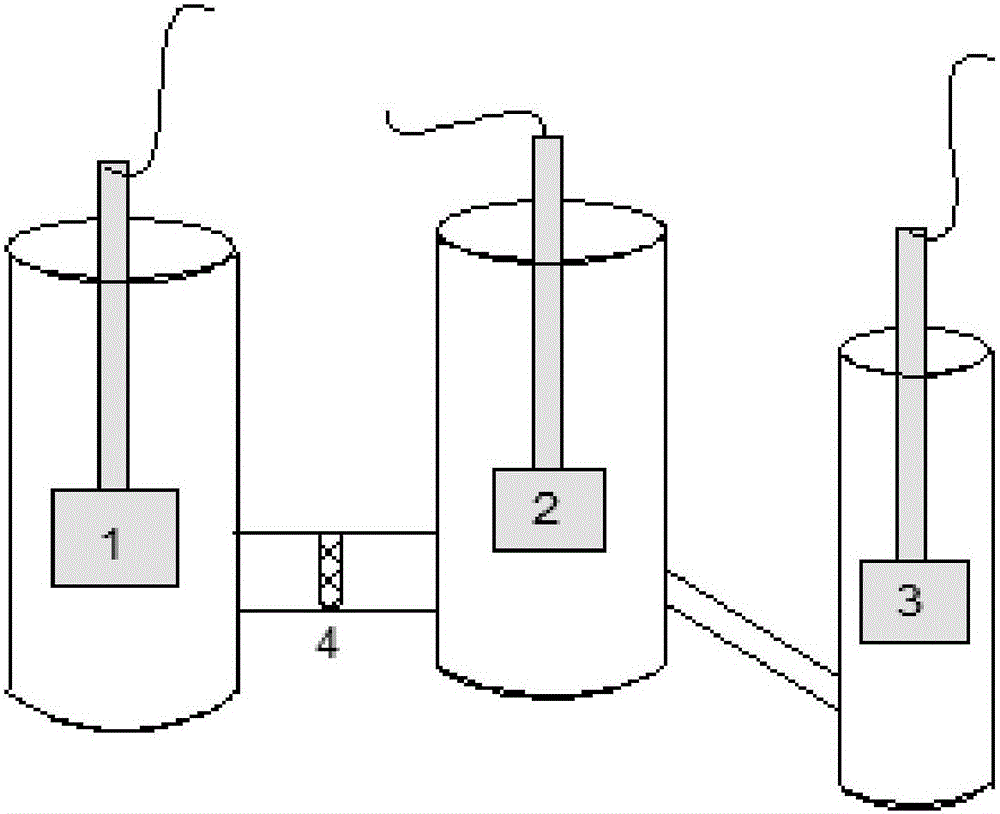
Method for recycling iron waste for fenton technology and water treating device of method
InactiveCN105174413AIncrease reaction rateRealize resource utilizationWater/sewage treatment by oxidationPotassium borohydrideSulfite salt
The invention discloses a method for recycling iron waste for the fenton technology. The method comprises the following steps that hydrogen peroxide, the iron waste and an activating agent are added to waste water to be treated, stirring is conducted so that the iron waste can be dispersed in a reaction system, a reaction is conducted, and then solid and liquid separation is conducted, wherein the activating agent is any one or a mixture of sodium sulfite, potassium sulfite, magnesium sulfite, calcium sulfite, sodium thiosulfate, potassium thiosulfate, calcium thiosulfate, magnesium thiosulfate, sodium borohydride, potassium borohydride, hydroxylamine hydrochloride and ascorbic acid. By means of the method, various kinds of iron waste are recycled, meanwhile, zero iron mud emission is achieved, and thus pollution to the environment is effectively reduced. The invention further provides a water treating device capable of achieving the method. By means of the method and the water treating device, the reaction speed of the iron waste in the fenton system is greatly increased, the effective pH range of the fenton system is expanded, meanwhile zero iron mud emission is achieved, and thus pollution to the environment can be easily reduced.
Owner:HOHAI UNIV
Carbon nitrogen nano fiber loaded platinum ruthenium nano particle electrode catalyst and preparation method
InactiveCN101288849AInhibitory activityAvoid retouchingCell electrodesMetal/metal-oxides/metal-hydroxide catalystsFiberPotassium borohydride
The invention provides an electrode catalyst with carbon-nitrogen nanometer fiber loading platinum-ruthenium nanometer particle. The carbon-nitrogen nanometer fiber is dispersed in solution containing platinum salt and ruthenium salt; a reducing agent is adopted for reduction; the electrode catalyst with carbon-nitrogen nanometer fiber loading platinum-ruthenium nanometer particle is gained after purification; the diameter of the carbon-nitrogen nanometer fiber ranges from 5nm to 300nm; the N / C atomic ratio is 0.01-0.25 and marked as CNx; wherein, x ranges from 0.01 to 0.25; the particle size of the platinum-ruthenium nanometer particle is 0.1-15nm; the proportion of the content (wt%) of the platinum or / and ruthenium nanometer particle and the carbon-nitrogen nanometer fiber mass is 1%-100%:1; the molar ratio of the platinum salt and the ruthenium salt is m:n; wherein, m ranges from 0.5 to1 and n ranges from 0 to1; the used reducing agent is ethylene glycol, sodium borohydride, potassium borohydride or nitrogen.
Owner:NANJING UNIV
Novel high performance alkaline fuel cell
InactiveCN1901261ALow costAvoid hydrogen purificationCell electrodesAqueous electrolyte fuel cellsPotassium borohydrideCatalytic oxidation
This invention relates to a new high performance alkali fuel battery, in which, MnO is used as the catalyst of the positive, various kinds of stored alloys are used as the catalyst of th negative and an alkali solution (such as NaOH or KOH) containing a certain volume of hydrides (such as BNaH and BKH) as the electrolyte, during the work of a battery, a catalytic and reductive reaction happens to oxygen at the positive and hydrogen in the hydride is catalyzed and oxidated at the negative.
Owner:FUDAN UNIV
Process for production of sodium borohydride from sodium aluminum hydride with recycle of byproducts
A process for production of sodium borohydride. The process comprises the steps of: (a) combining a boric acid ester, B(OR)3 and sodium aluminum hydride to produce sodium borohydride and Al(OR)3; and (b) combining Al(OR)3 and sulfuric acid to produce alum and ROH.
Owner:ROHM & HAAS CO
Composite metal oxide loaded Pt-based nano metal catalyst and preparation method therefor
InactiveCN105854878ALarge specific surface areaImprove structural stabilityOrganic compound preparationHydroxy compound preparationDispersityPotassium borohydride
The invention relates to a composite metal oxide loaded Pt-based nano metal catalyst and a preparation method therefor and belongs to the technical field of preparation of catalysts. A composite metal oxide of the composite metal oxide loaded Pt-based nano metal catalyst is one of CeO2-ZrO2, TiO2-ZrO2 and ZrO2-TiO2, the specific surface area of the catalyst is 250m<2> / g to 400m<2> / g, the average particle size of Pt particles is 2nm to 5nm, and the mass percentage of Pt is 1.0% to 5.5%. According to the method, a metal salt solution, a chloroplatinic acid solution and a potassium borohydride solution are mixed in a colloid mill and then are subjected to high-speed coring, then, a hydrothermal reaction is controlled by using kinetics, then, the compositing of metal oxides and the reduction of noble metals are achieved in one step, and finally, the composite metal oxide loaded Pt-based nano metal catalyst is prepared. By applying the catalyst to a reaction for preparing cinnamyl alcohol through selective hydrogenation of cinnamyl aldehyde, the conversion ratio of the cinnamyl aldehyde and the selectivity to the cinnamyl alcohol separately can reach 90% to 100% and 90% to 100%. The catalyst has the advantages that the dispersity of metal nanoparticles is high, the size is small, the carrier action is high, and the preparation method is simple.
Owner:BEIJING UNIV OF CHEM TECH
Chemical method for preparing spherical porous hollow nanometer cobalt powder
InactiveCN104607651ASmall particle sizeParticle size control and adjustmentNanotechnologyPotassium borohydrideCobalt
The invention provides a chemical method for preparing spherical porous hollow nanometer cobalt powder and relates to the chemical method for preparing nanometer cobalt powder materials. According to the chemical method, hydrazine hydrate and potassium borohydride are used as reducing agents to restore metallic cobalt and iron ions to metallic cobalt and iron atoms in specific solvent, metal atoms re-associate and grow up, and ferrocobaltnano-alloy particles are obtained finally. Then de-alloying for the prepared spherical ferrocobaltnano-alloy particles is conducted in acid solutions or acid mist with certain concentration to remove iron elements, and the spherical porous hollow nanometer cobalt powder particles are prepared out. The chemical method for preparing the spherical porous hollow nanometer cobalt powder is simple in technology, low in raw material cost and convenient to operate; in addition, by means of the chemical method, the spherical porous hollow nanometer particle powder can be prepared out at normal temperature and pressure and conditions are provided for practical application of the nanometer cobalt powder.
Owner:UNIV OF SCI & TECH BEIJING
Method for purifying butadiene hexafluoride
ActiveCN106349008AImprove adsorption capacityGood flexibilityOther chemical processesHalogenated hydrocarbon separation/purificationMolecular sieveHexafluoride
The invention provides a method for purifying butadiene hexafluoride. The method comprises the following steps: pumping pure water, adsorption resin, a graphene oxide aqueous solution and potassium borohydride into a reaction kettle, heating to reaction temperature and stirring, performing filtering, drying and heating roasting at the end of the reaction, and cooling to room temperature to obtain an efficient adsorbent; performing an impurity removal rectification technology which is publically known in the industry, including performing an extraction technology, on a coarse butadiene hexafluoride product to separate out a solvent, then adsorbing by a molecular sieve to remove water, putting into an adsorption tower with the efficient adsorbent, adjusting to proper reaction temperature, pressure and flowing rate for adsorption, performing the impurity removal rectification technology which is publically known in the industry again, including decompression rectification, on adsorbed fluorine-containing organic gas, and removing impurities to obtain a high-purity butadiene hexafluoride product.
Owner:ZHEJIANG BRITECH CO LTD
A nano-metal coated sulfur composite material and applications thereof
InactiveCN103066255AWide variety of sourcesLow priceCell electrodesPotassium borohydrideLithium sulfur
The present invention discloses a nano-metal coated sulfur composite material and applications thereof. The preparation method for the nano-metal coated sulfur composite material comprises the following steps of: (1) dissolving the metal compound in deionized water to prepare a precursor aqueous solution; (2) adding powdered sulfur into the precursor aqueous solution, and fully stirring for uniformly mixing to form a suspension; (3) by using sodium borohydride or potassium borohydride as a r reducing agent, dissolving the reducing agent in deionized water to prepare a reducing agent aqueous solution; (4) under the condition of stirring at room temperature, adding the reducing agent aqueous solution dropwise to the suspension to make the metal ions fully reduced, and coated on the powdered sulfur surface, and isolating to obtain the nano-metal coated sulfur composite material. The composite material is used as the cathode material for lithium sulfur batteries, has the characteristics of high specific capacity and excellent cycling stability; and the material of the present invention is simple in the preparation method, inexpensive in the cost, excellent in performance, and easy to realize industrialization.
Owner:ZHEJIANG UNIV OF TECH
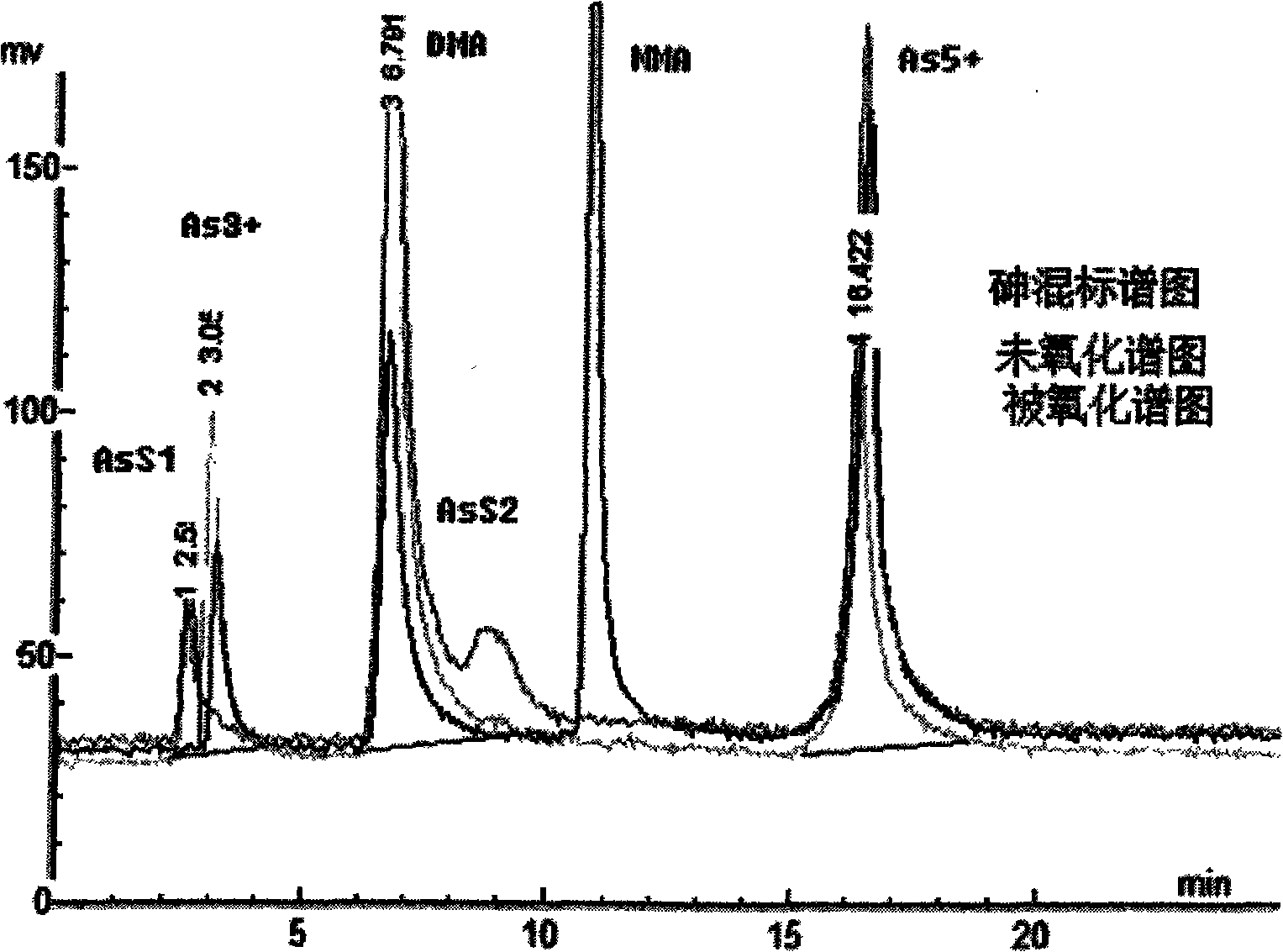
Detection method of selenium form in aquatic product
InactiveCN103399117AReasonable selection of experimental conditionsReasonable choiceComponent separationPotassium borohydrideFluorescence
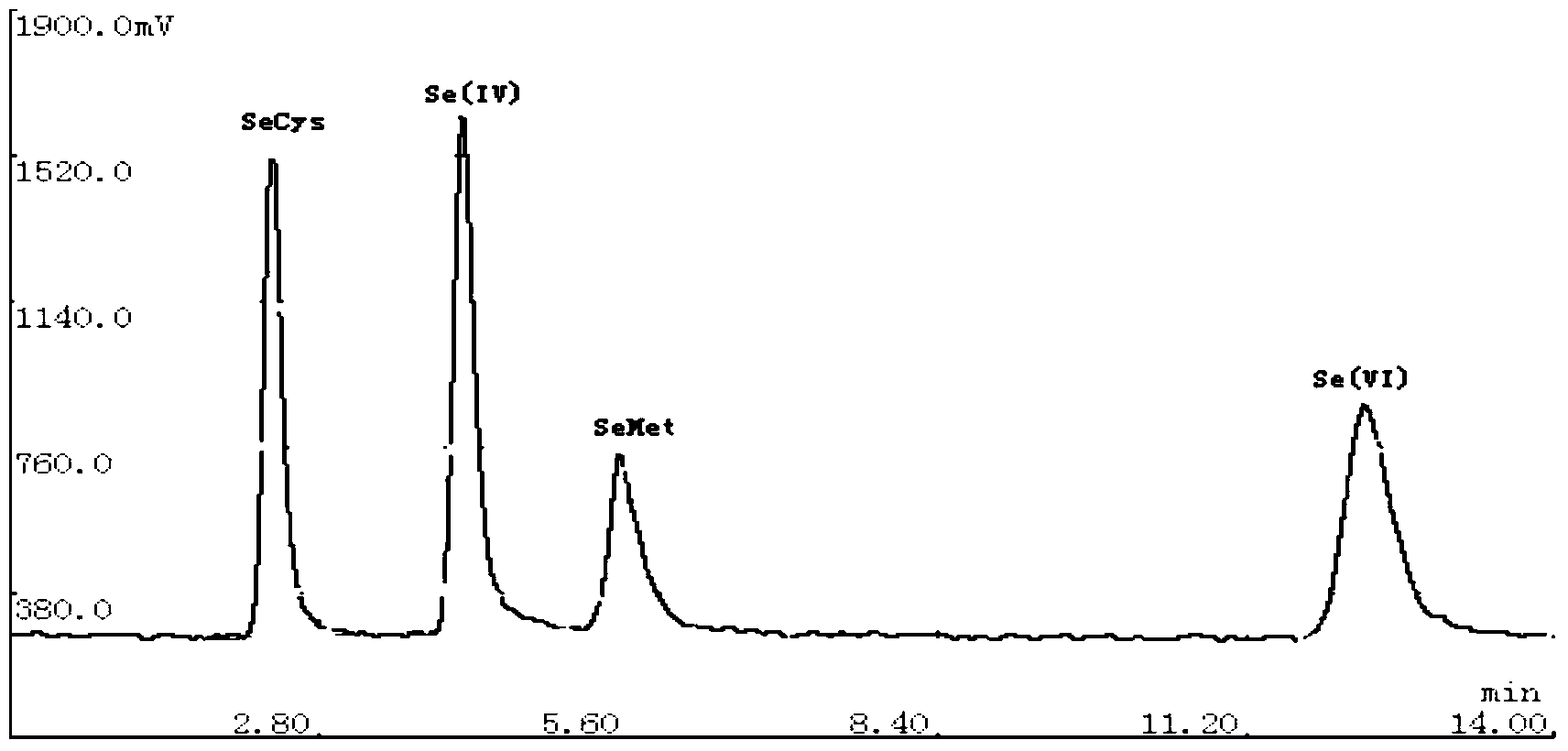
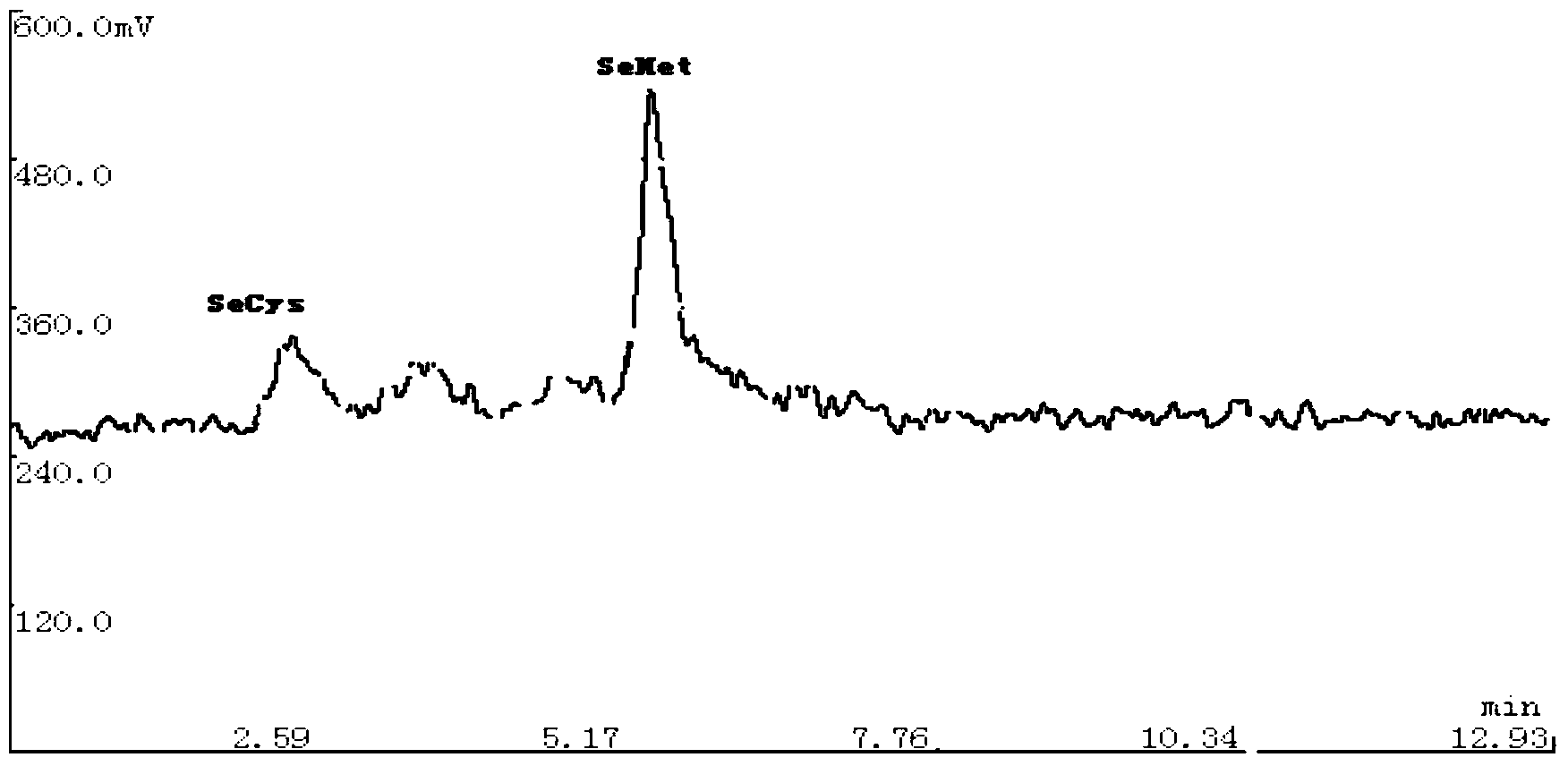
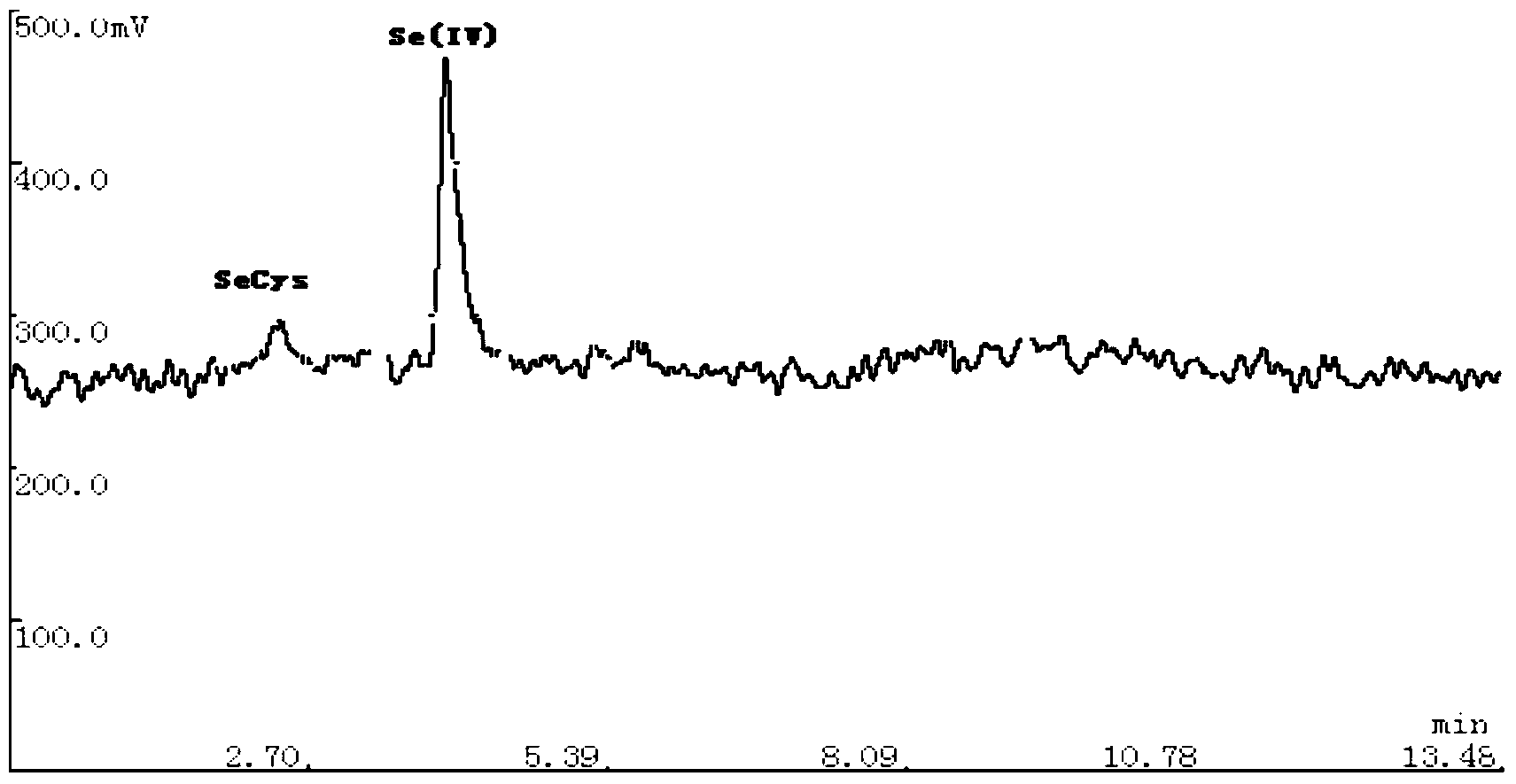
The invention relates to a detection method of selenium form in an aquatic product, and belongs to the technical field of aquatic product detection. The selenium form in the aquatic product is extracted by oscillating with a super constant-temperature mixer and measured by a high performance liquid chromatography-hydride generation atomic fluorescence spectroscopy combined morphological analysis technology, the extracting solution is separated by an anion chromatographic column, different forms of selenium are eluted successively by a moving phase, hydrogenation is carried out on the elution solution through a potassium borohydride reducing agent and hydrochloric acid to generate a hydride which enters an atomizer and is used in combination with atomic fluorescence for analysis and measurement, so that a more complete biological property of the selenium is provided. The experiment condition is reasonable, the detection data are accurate and reliable, the sample recovery rate is 88.79-94.88% and the degree of precision is less than 5%. The method is high in sensitivity, low in interference and simple in sample pretreatment, can be used for accurately measuring inorganic selenium, selenite and selenate as well as organic selenium, selenocysteine and selenomethionine and detecting selenium form in aquatic product.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Preparation method of boron nitrogen doped graphene supported palladium catalyst
ActiveCN105833893AImprove electrocatalytic performancePhysical/chemical process catalystsCell electrodesDoped graphenePotassium borohydride
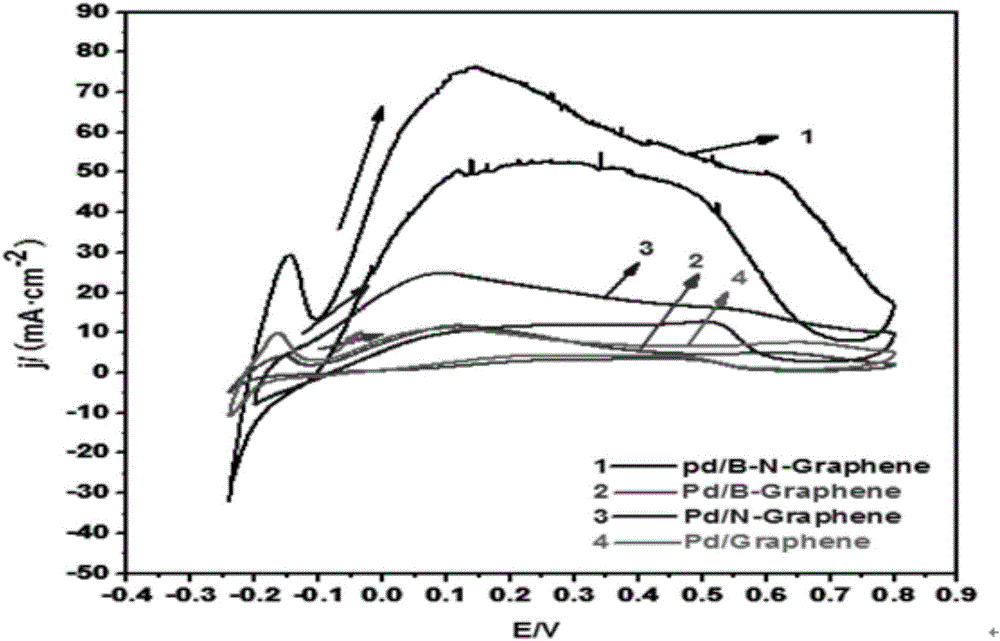
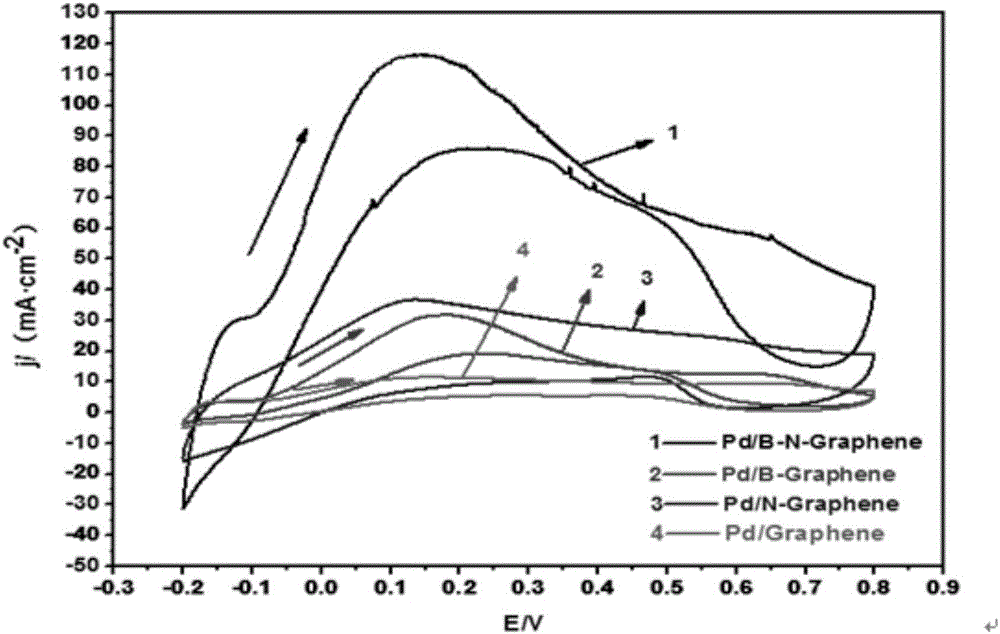
The invention relates to a preparation method of a graphene catalyst, in particular to a preparation method of a boron nitrogen doped graphene supported palladium catalyst. The method includes the steps of preparing graphene oxide through a Hummers synthesis method, heating graphene oxide and boric acid together to prepare boron doped graphene oxide, dipping boron doped graphene oxide in ammonium hydroxide, doping boron doped graphene oxide with nitrogen to be subjected to reduction, conducting suction filtration, conducting roasting at 400-450 DEG C under the protection of nitrogen, and loading boron doped graphene oxide with Pd metal through a potassium borohydride method to obtain the boron nitrogen doped graphene supported palladium catalyst. By means of the method, graphene oxide is prepared through the Hummers synthesis method, graphene oxide and boric acid are heated together to prepare boron doped graphene oxide, boron doped graphene oxide is dipped in ammonium hydroxide, boron doped graphene oxide is doped with nitrogen to be subjected to reduction, suction filtration is conducted, then roasting is conducted under the protection of nitrogen, boron doped graphene oxide is loaded with Pd metal through the potassium borohydride method, and then the boron nitrogen doped graphene supported palladium catalyst is obtained.
Owner:TAIZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com