Technology for templet-free low-temperature preparation of porous boron nitride in one-step method
A template-free, boron nitride technology, applied in inorganic chemistry, nitrogen compounds, chemical instruments and methods, etc., can solve the problems of easy hydrolysis corrosion, complicated process, high reaction temperature, etc., and achieve low price, low reaction temperature, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
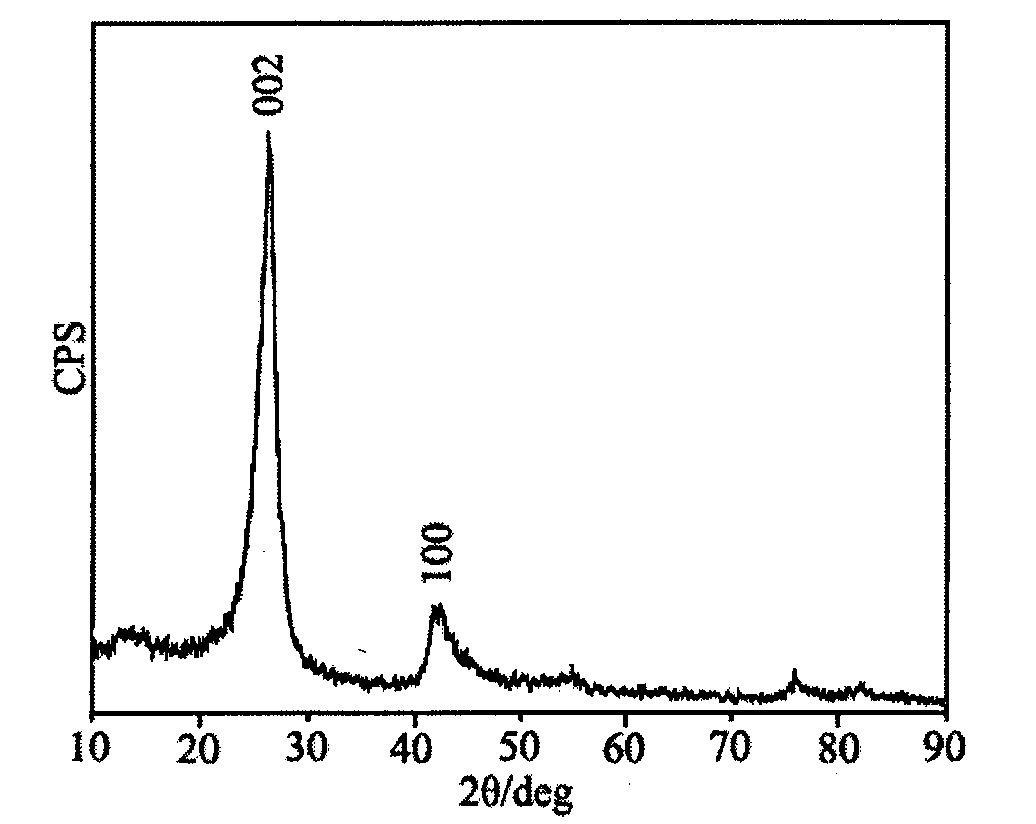
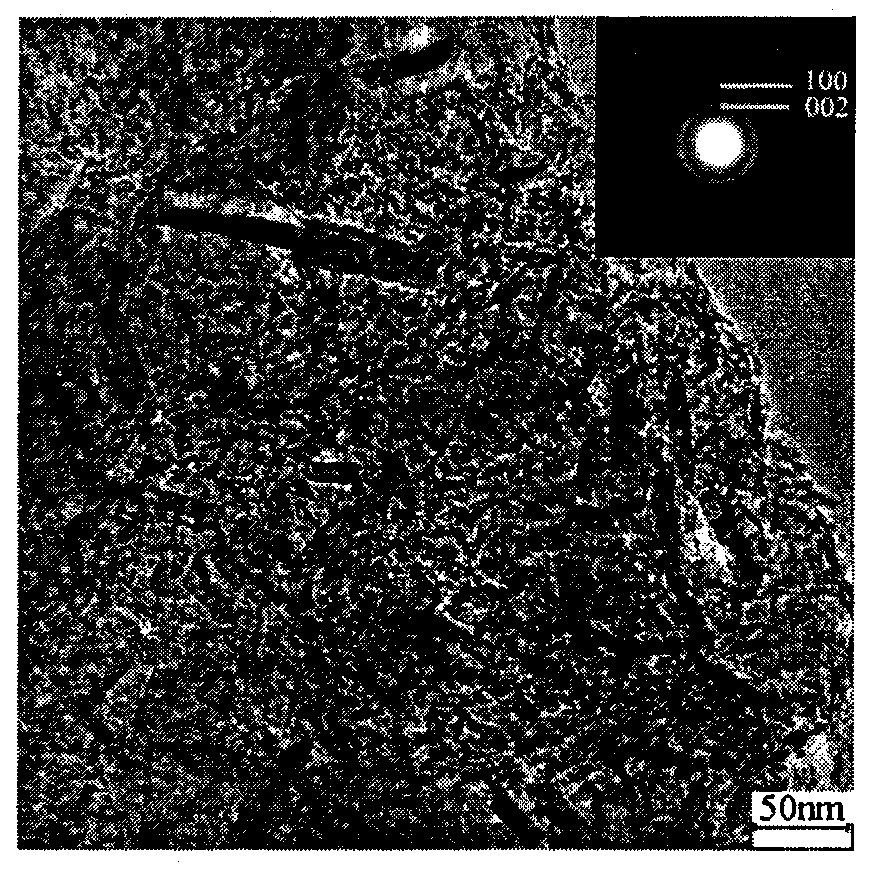
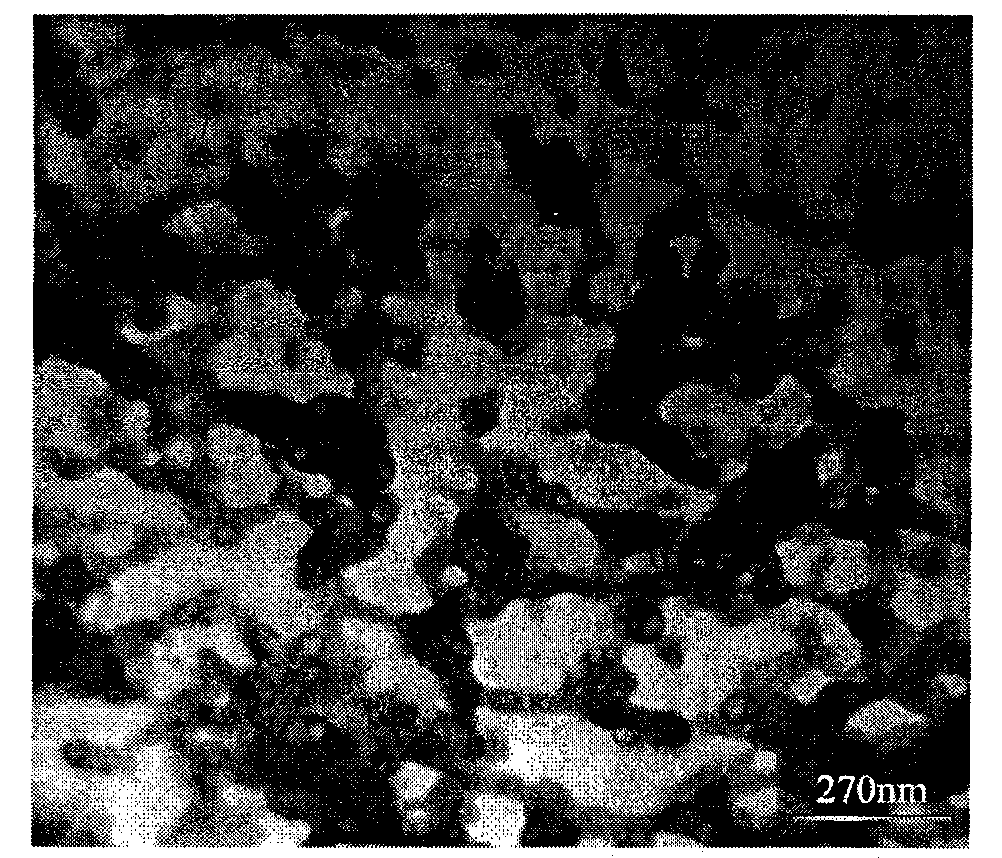
[0023] Preparation of porous boron nitride by reaction of sodium borohydride and urea. Weigh 1.53g of sodium borohydride and 1.2g of urea with a balance, and put them into a stainless steel reactor. After the reactor is sealed, heat it to 600°C in a heating furnace. After keeping the temperature for 10 hours, close the heating furnace, so that the reactor is naturally in the furnace. Let cool to room temperature. The reaction product was washed with absolute ethanol and deionized water, and then dried at 50° C. for 8 hours to obtain an off-white powder.
Embodiment 2
[0025] Preparation of porous boron nitride by reaction of sodium borohydride and urea. Weigh 1.53g of sodium borohydride and 1.2g of urea with a balance and put them into a stainless steel reactor. After the reactor is sealed tightly, heat it to 550°C in a heating furnace. Cool naturally to room temperature. The reaction product was washed with absolute ethanol and deionized water, and then dried at 50° C. for 8 hours to obtain an off-white powder.
Embodiment 3
[0027] Preparation of porous boron nitride by reaction of potassium borohydride and urea. Weigh 2.2g of potassium borohydride and 1.2g of urea with a balance and put them into a stainless steel reactor. After the reactor is sealed tightly, heat it to 550°C in a heating furnace. Cool naturally to room temperature. The reaction product was washed with absolute ethanol and deionized water, and then dried at 50° C. for 8 hours to obtain an off-white powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com