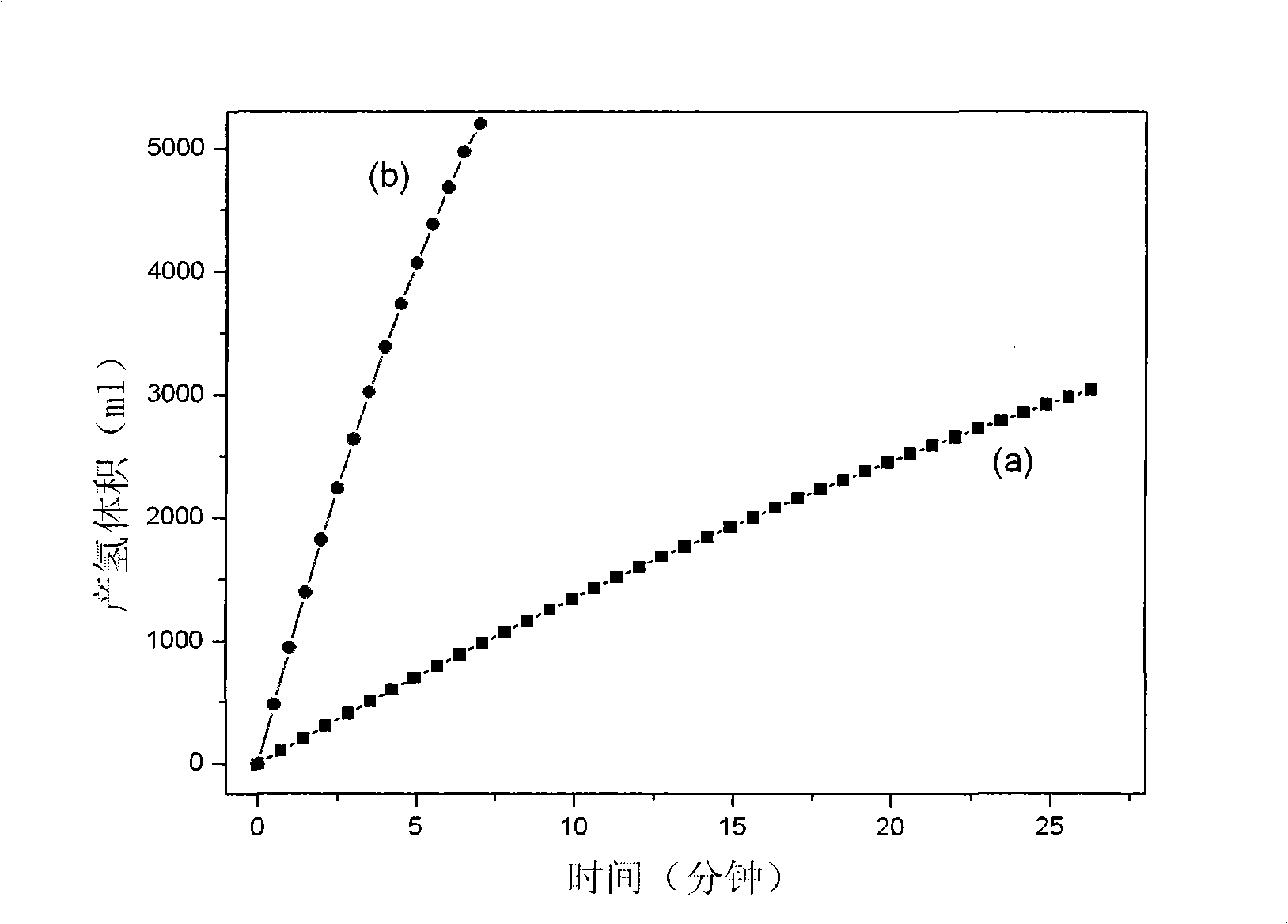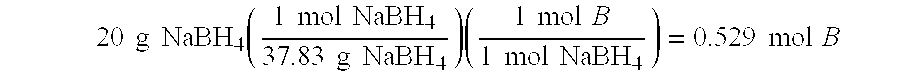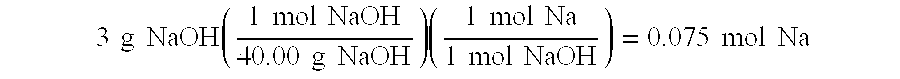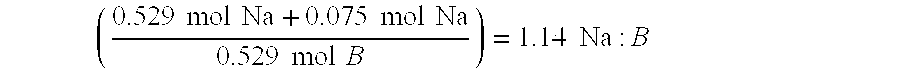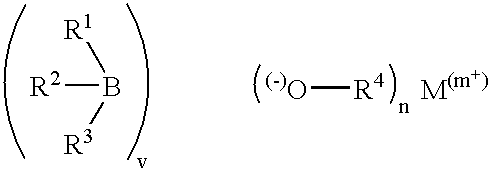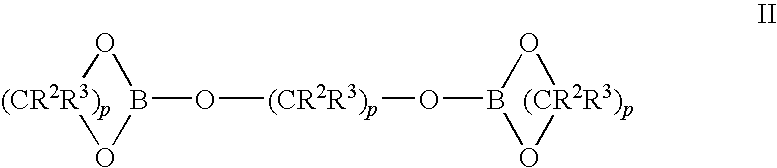Patents
Literature
899 results about "Borohydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Borohydride refers to the anion BH−4 and its salts. Borohydride is also the term used for compounds containing BH₄₋ₙX⁻ₙ, for example cyanoborohydride (B(CN)H⁻₃) and triethylborohydride (B(C₂H₅)₃H⁻). Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known (see Table). Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.
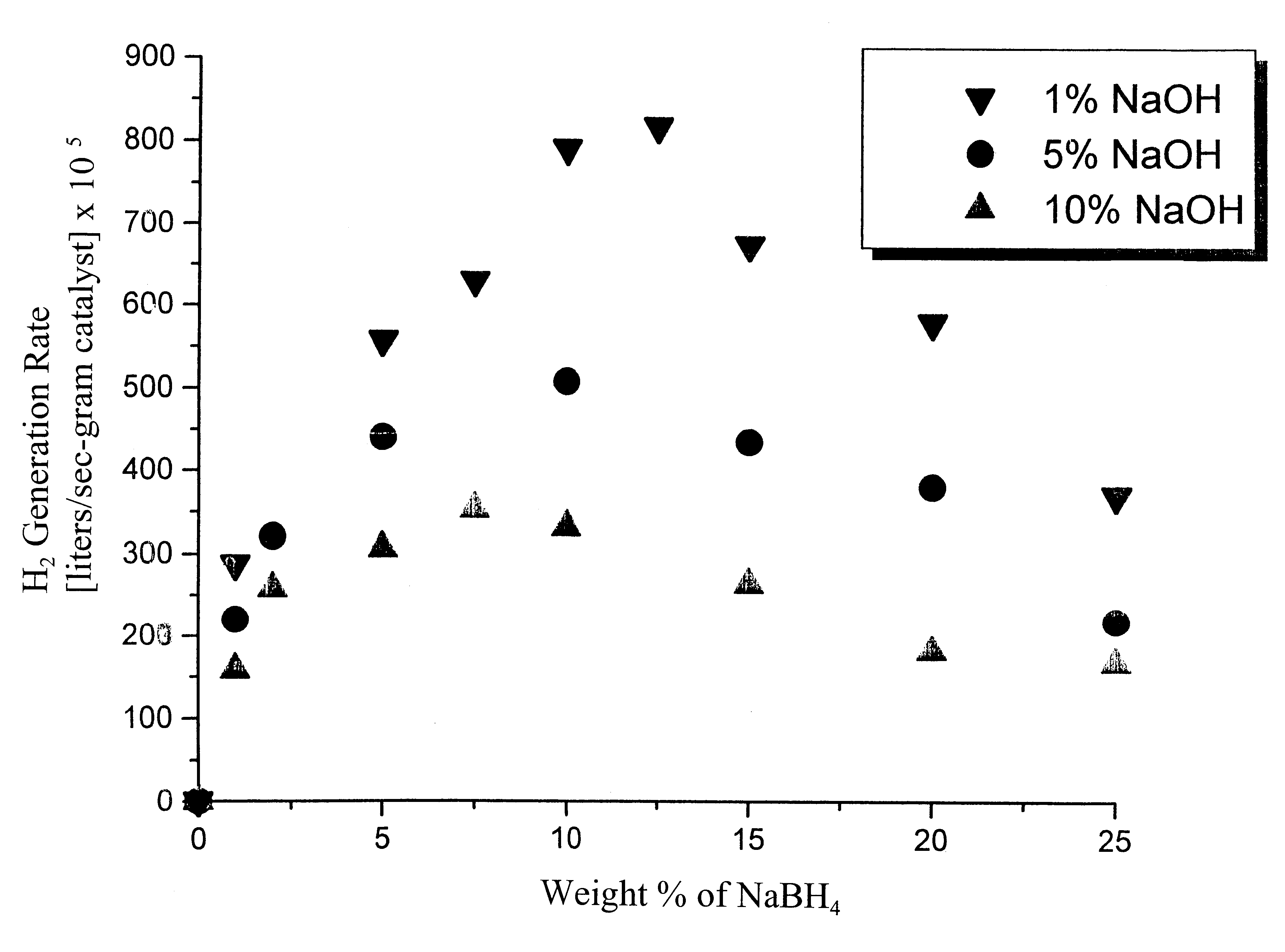
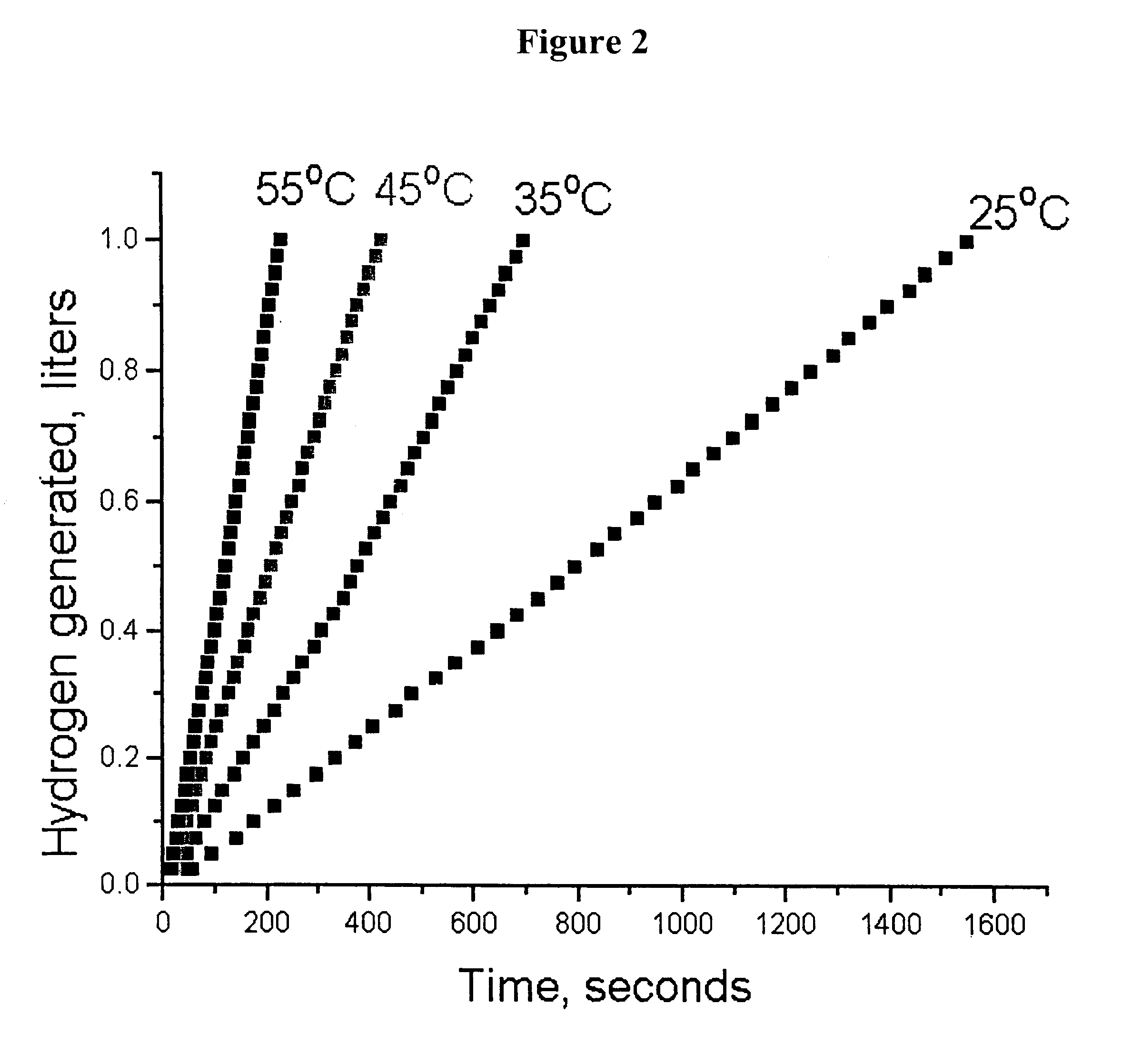
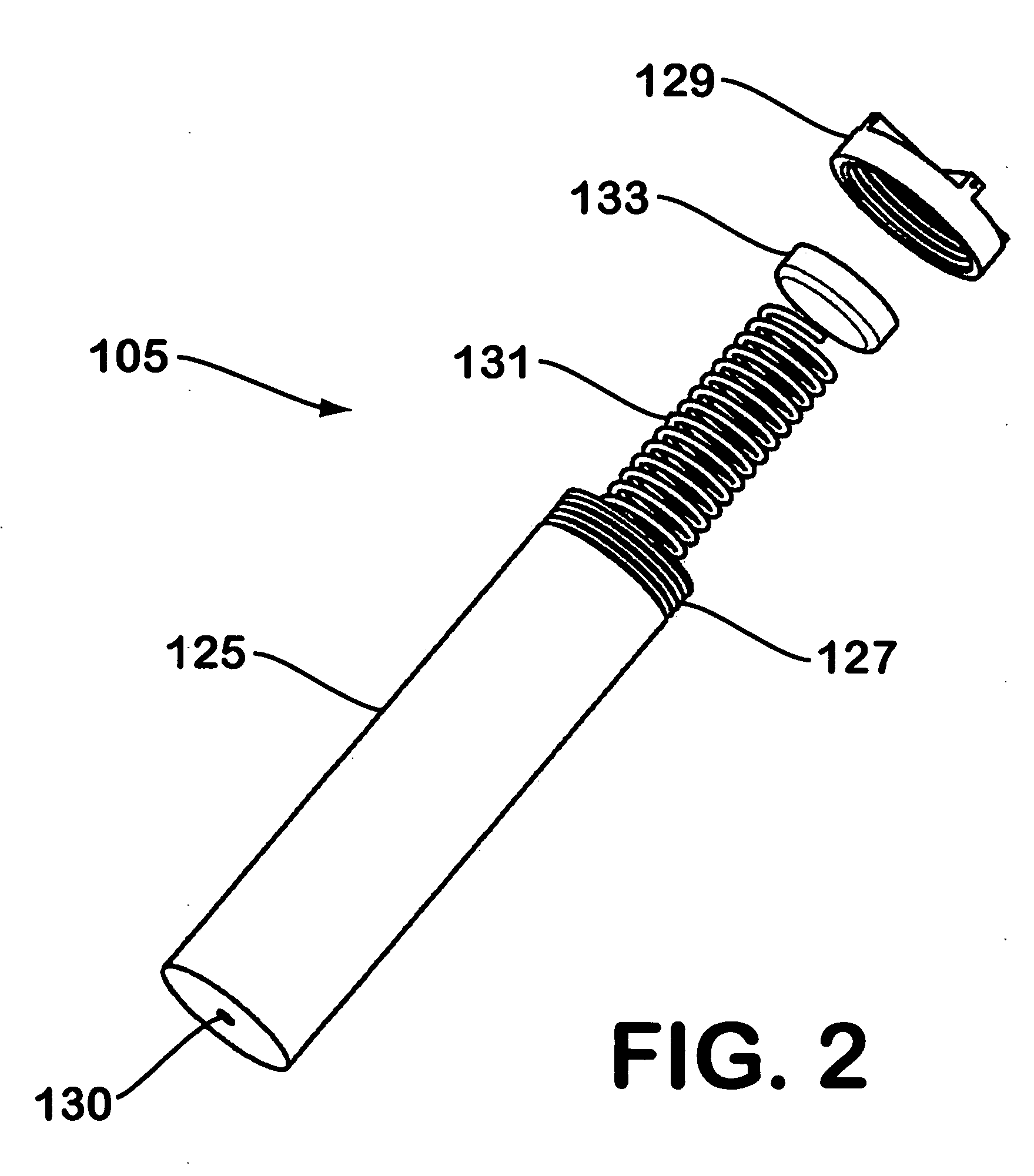
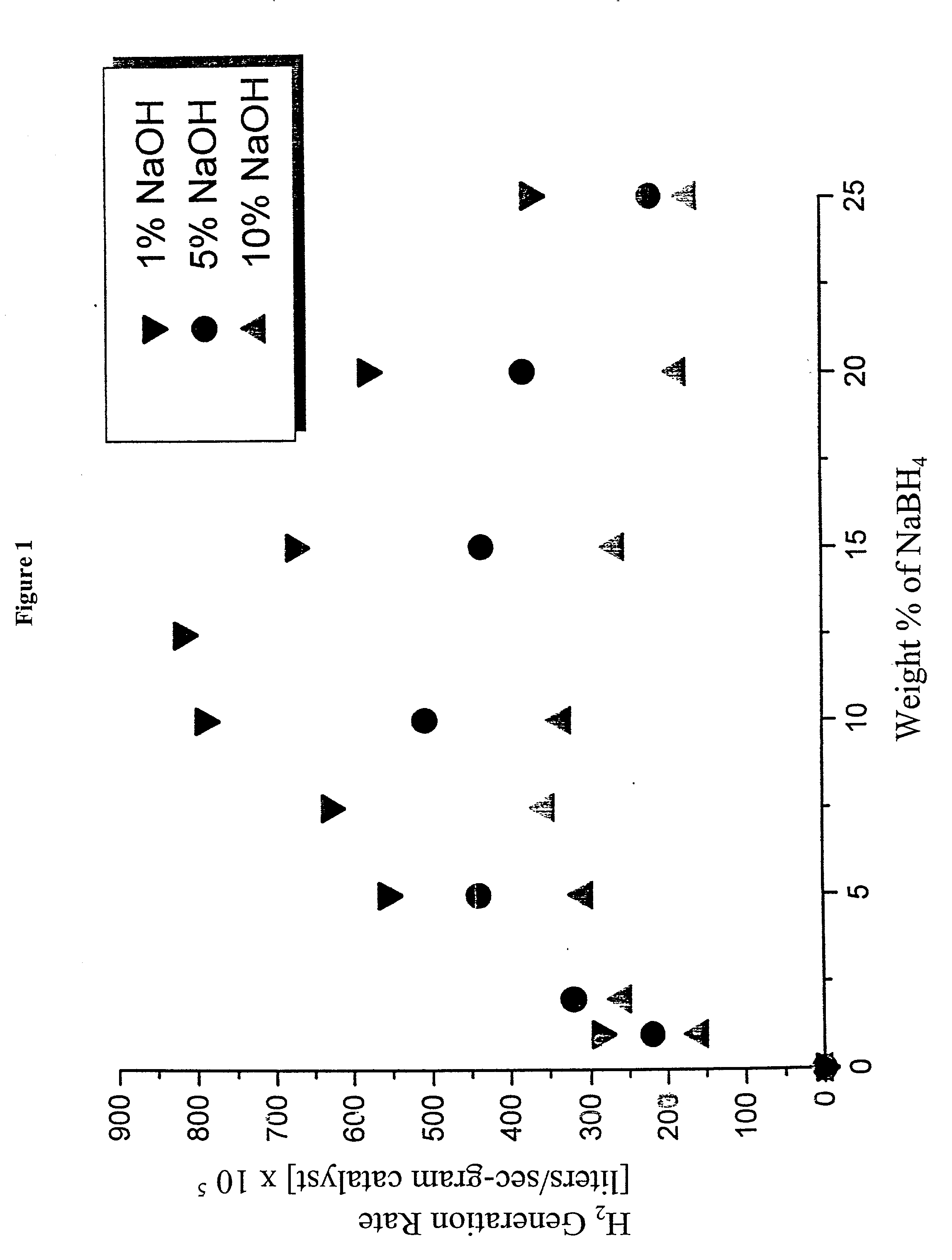
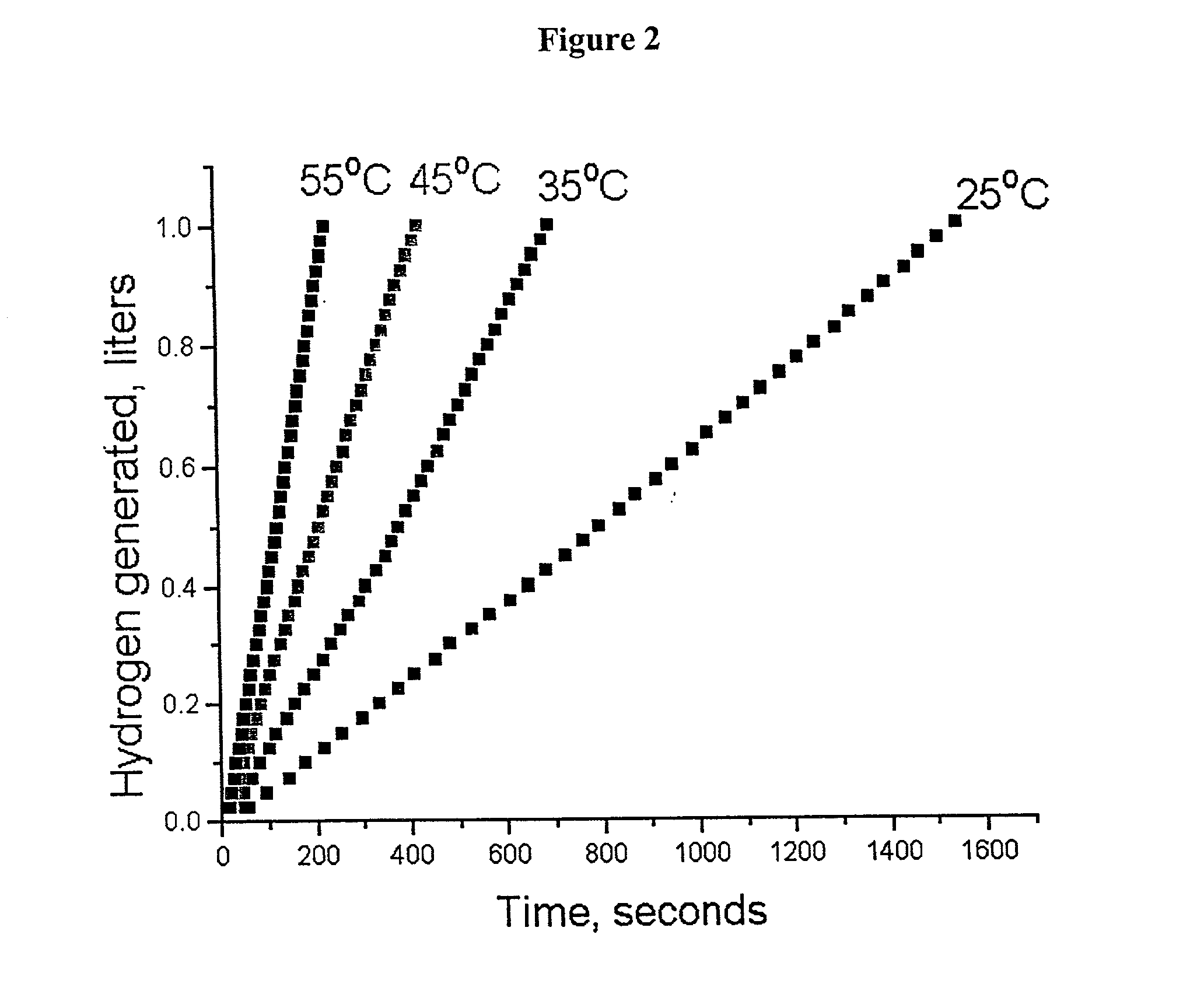
System for hydrogen generation
InactiveUS6534033B1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationControlled releaseBorohydride
The present invention relates to a composition and method for storage and controlled release of hydrogen. In particular, the present invention relates to the use of borohydride based solutions as a hydrogen storage source and a catalyst system to release hydrogen therefrom.
Owner:SILICON VALLEY BANK +1
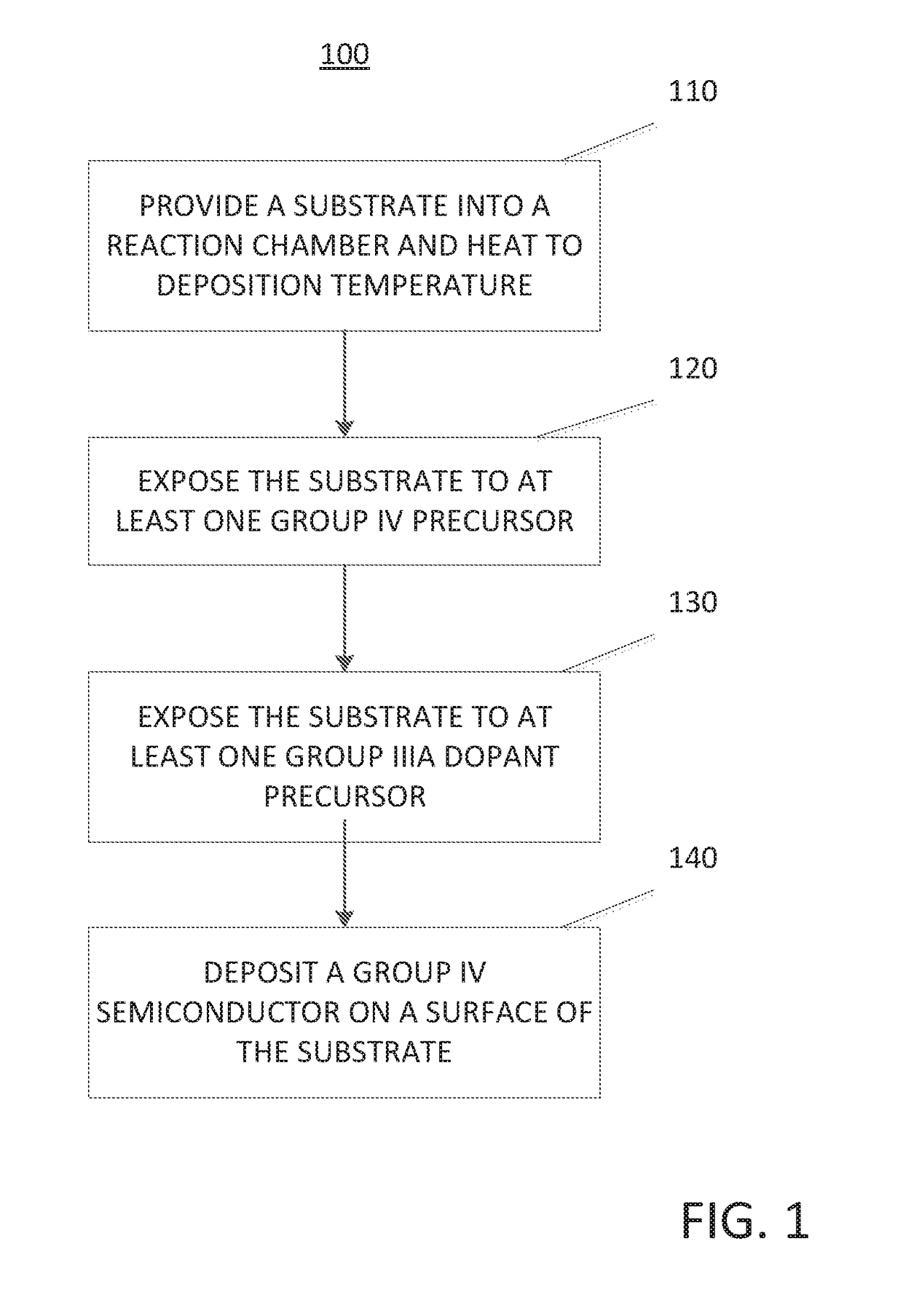
Method for depositing a group iv semiconductor and related semiconductor device structures
ActiveUS20190027605A1TransistorSemiconductor/solid-state device manufacturingDopantDeposition temperature
A method for depositing a Group IV semiconductor on a surface of a substrate is disclosed. The method may include: providing a substrate within a reaction chamber and heating the substrate to a deposition temperature. The methods may further include: exposing the substrate to at least one Group IV precursor and exposing the substrate to at least one Group IIIA dopant precursor; wherein the at least one Group IIIA dopant precursor comprises a borohydride, an organic borohydride, a halide, or an organohalide. Semiconductor device structures including a Group IV semiconductor deposited by the methods of the disclosure are also provided.
Owner:ASM IP HLDG BV
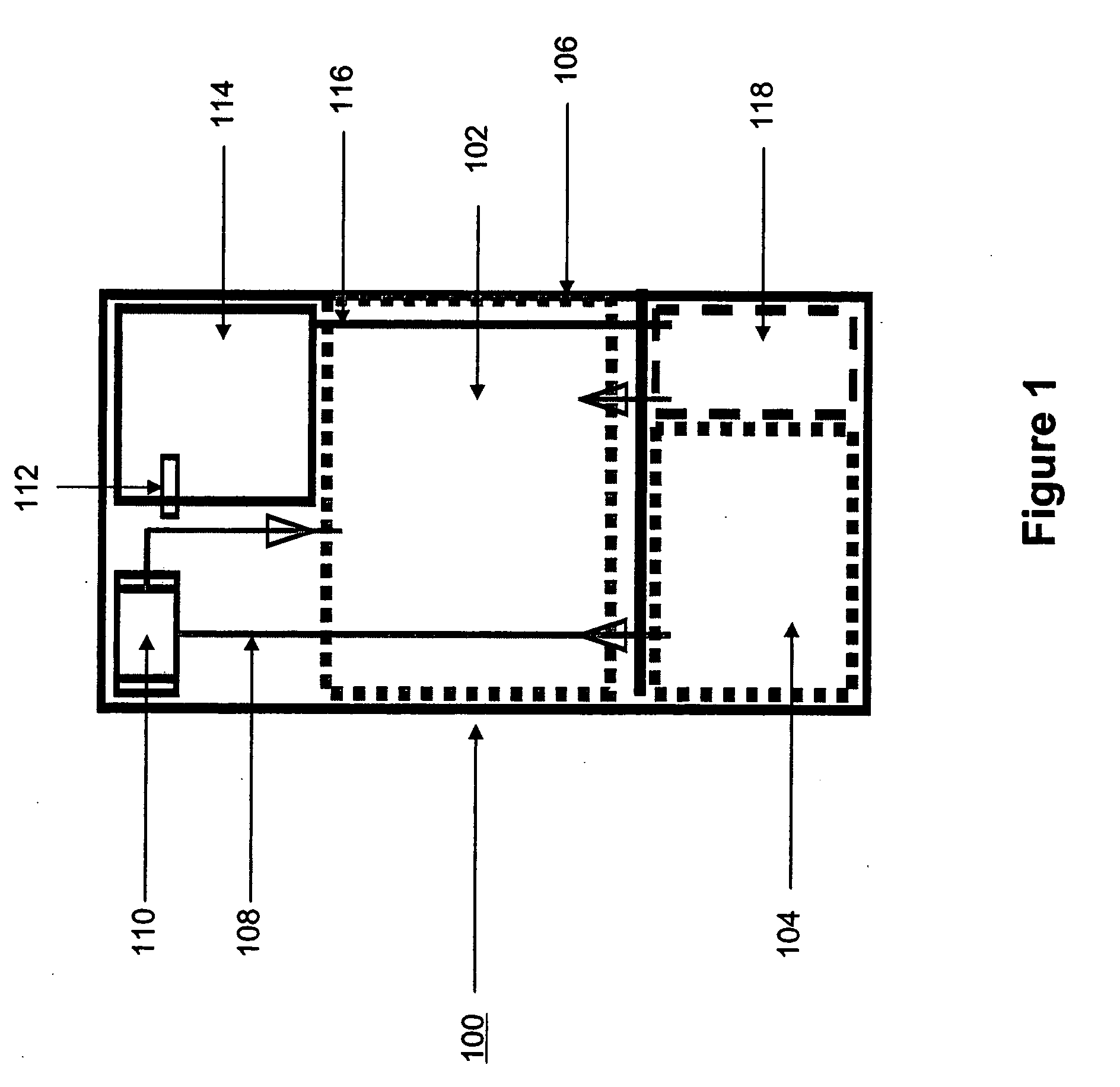
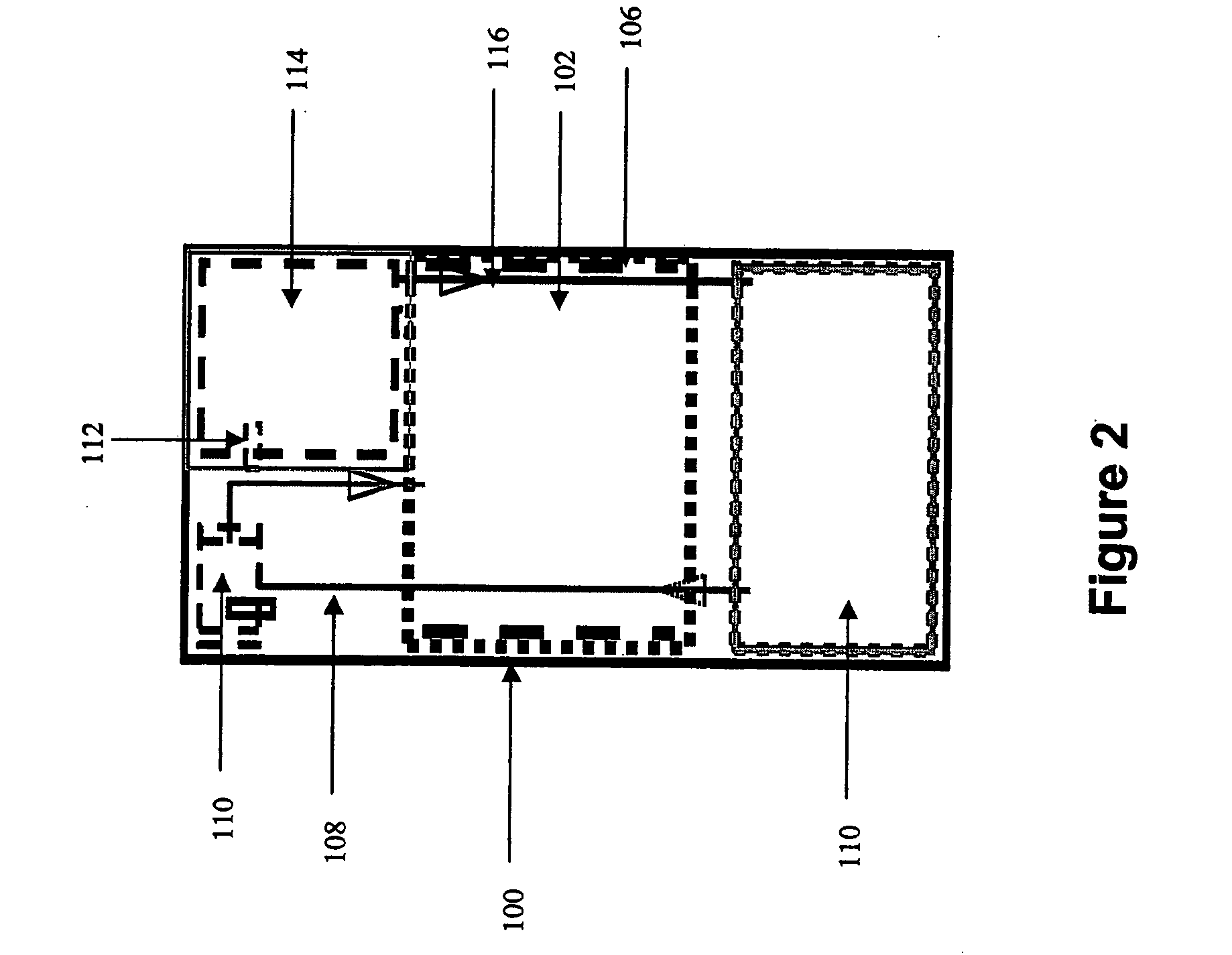
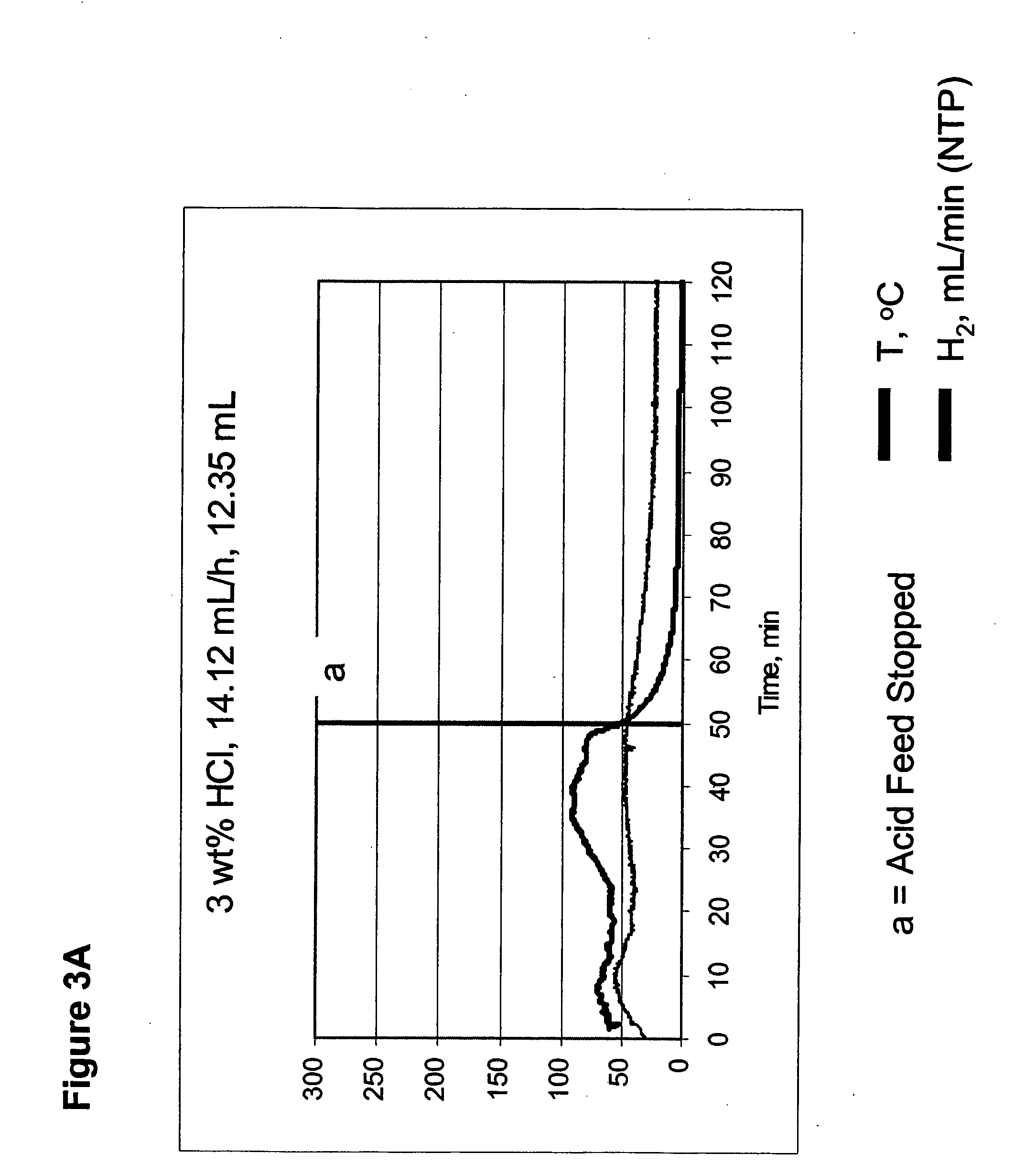
Systems and methods for hydrogen generation from solid hydrides
InactiveUS20050238573A1Regulate rateReactant parameters controlHydrogen productionO-Phosphoric AcidAlkaline earth metal
A system is disclosed for hydrogen generation based on hydrolysis of solid chemical hydrides with the capability of controlled startup and stop characteristics wherein regulation of acid concentration, acid feed rate, or a combination of both control the rate of hydrogen generation. The system comprises a first chamber for storing a solid chemical hydride and a second chamber for storing an acidic reagent. The solid chemical hydride is a solid metal borohydride having the general formula MBH4, where M is selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation. The acidic reagent may comprise inorganic acids such as the mineral acids hydrochloric acid, sulfuric acid, and phosphoric acid, and organic acids such as acetic acid, formic acid, maleic acid, citric acid, and tartaric acid, or mixtures thereof.
Owner:MILLENNIUM CELL
Preparation method for propellant fuel
InactiveCN108910843AShorter ignition delay timeHazard reductionOrganic chemistryHydrazineEvaporationIgnition delay
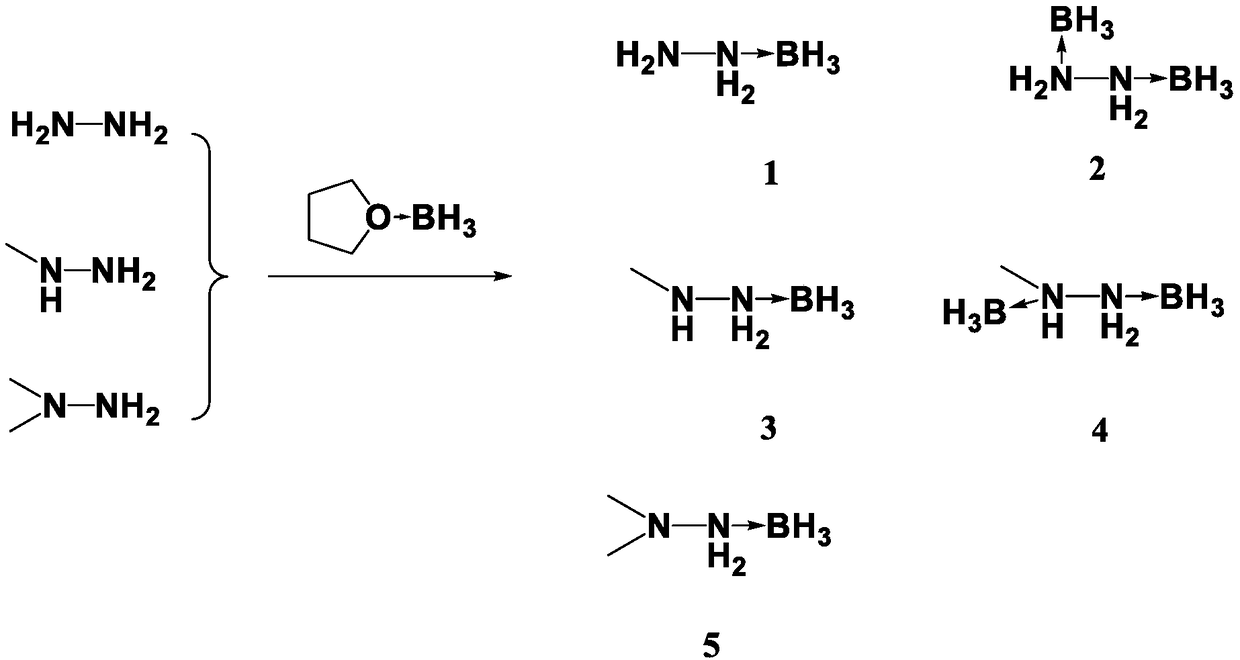
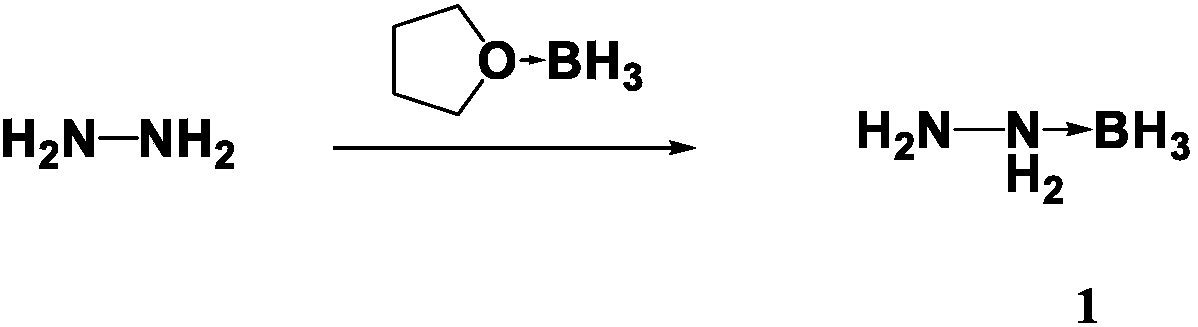
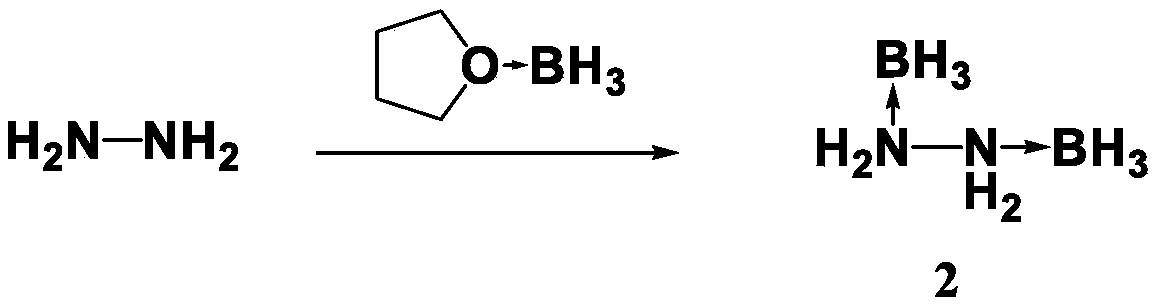
The invention discloses a preparation method for propellant fuel. The preparation method comprises the following steps: uniformly mixing hydrazine or a methyl derivative thereof with a tetrahydrofuransolution of borane to perform a reaction, and after the reaction, performing rotary evaporation on the tetrahydrofuran solution to obtain a first product; adding a first solvent into the first product, then performing washing, and then performing rotary evaporation to remove the first solvent, so as to obtain a hydrazinoborane derivative. According to the method, hydroboration is carried out on ahighly-volatile hydrazine fuel and a methylated derivative of hydrazine, the obtained borohydride of the hydrazine is a colorless non-volatile liquid or a white solid, has no volatility and greatly reduces hazard of the hydrazine fuel, and is a novel aerospace propellant fuel since the ignition delay time of the hydrazine borofluoride is extremely short.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Monodisperse noble metal nanocrystals
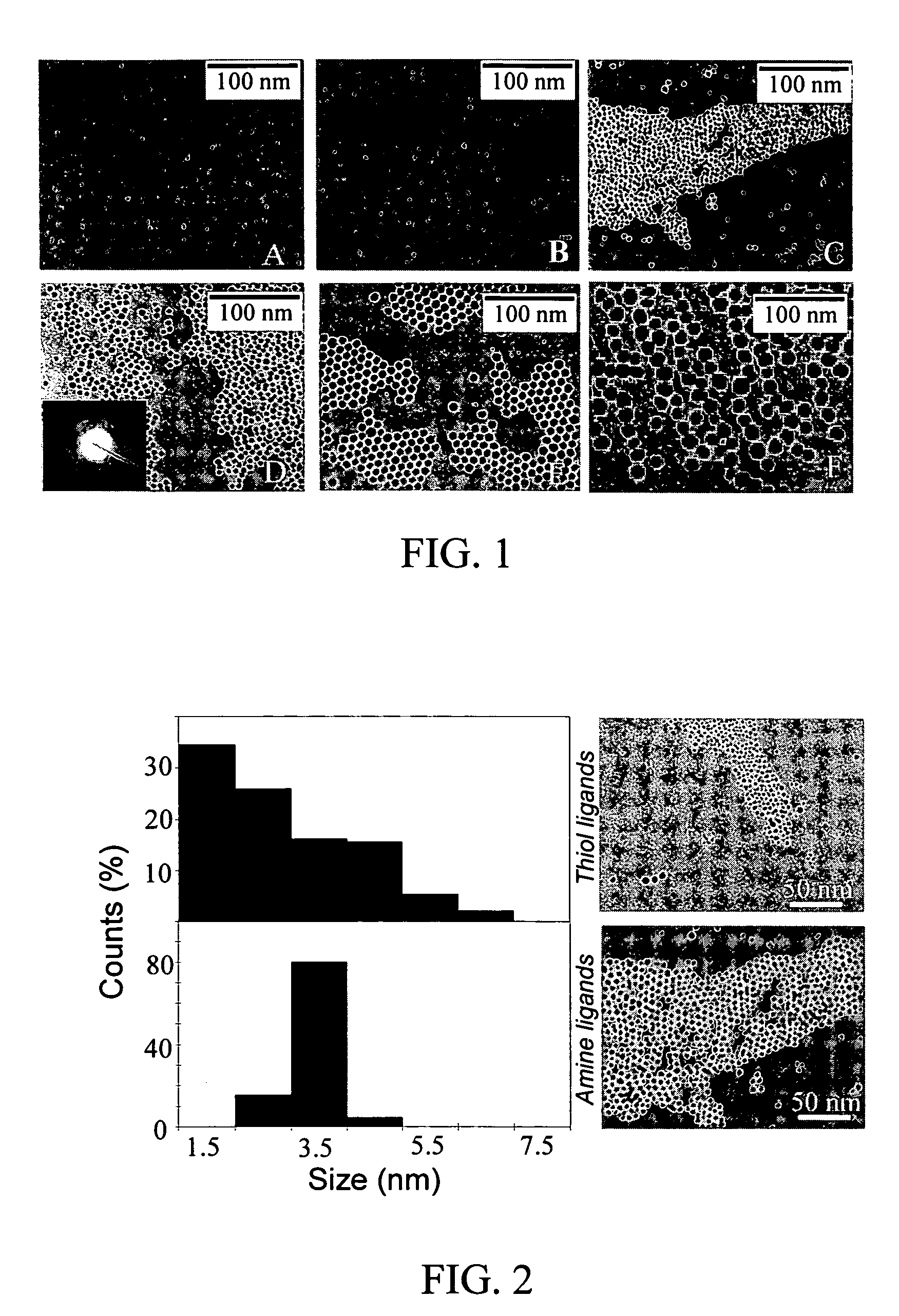
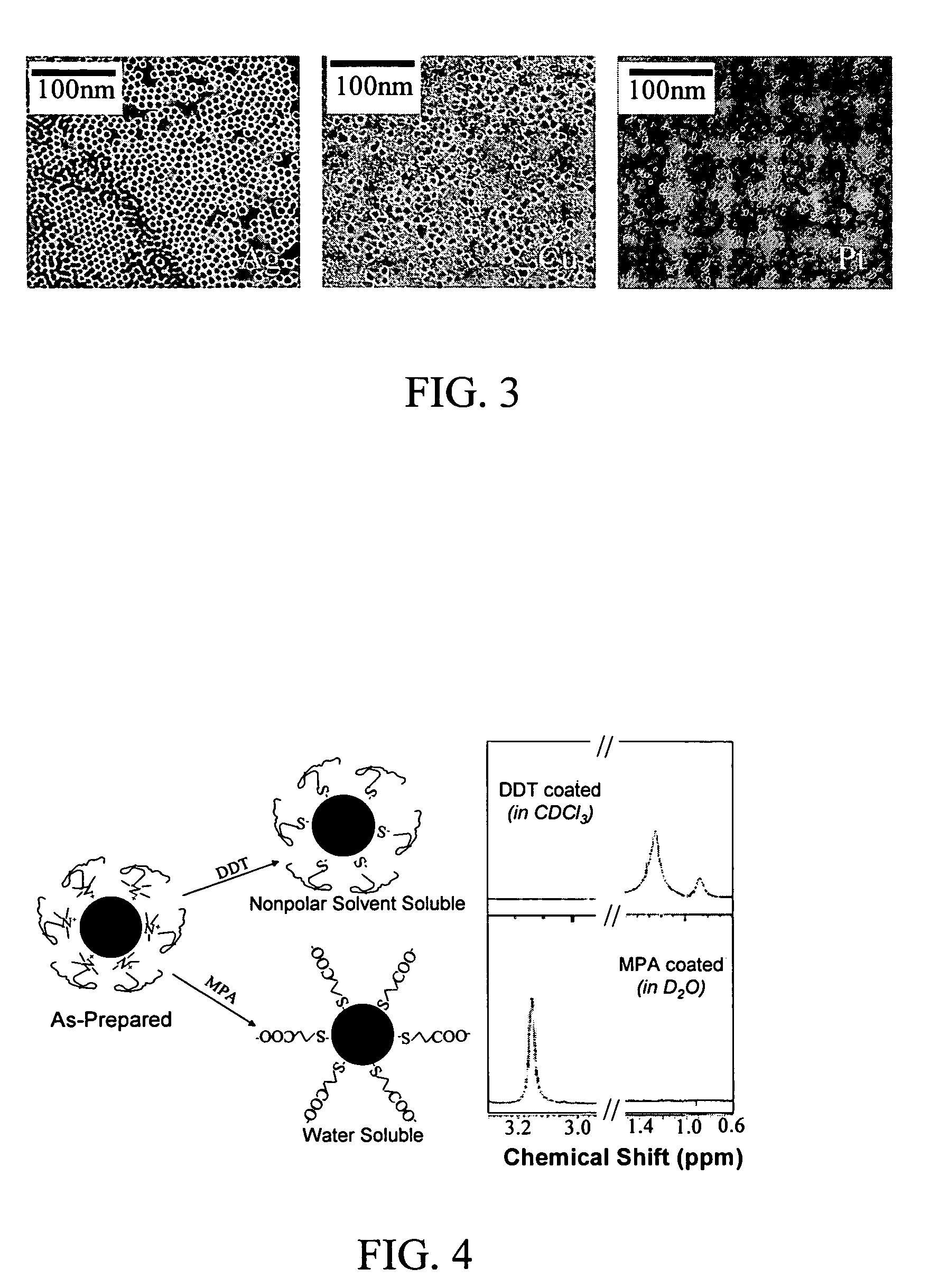
Nanoparticle compositions of noble metals, and methods of making them, are described. The nanoparticle compositions are made by reacting a salt or complex of a noble metal, such as Au, Ag, Cu or Pt, with a weak ligand, and a reducing agent, in a single liquid phase. The noble metal is typically provided as a halide or carboxylate. The ligand is preferably a fatty acid or aliphatic amine. The reducing agent is preferably a borohydride reagent, hydrazine, or a mixture thereof. Nanocrystals in the size range of 1 nm to 20 nm are produced, and can be made in substantially monodisperse form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
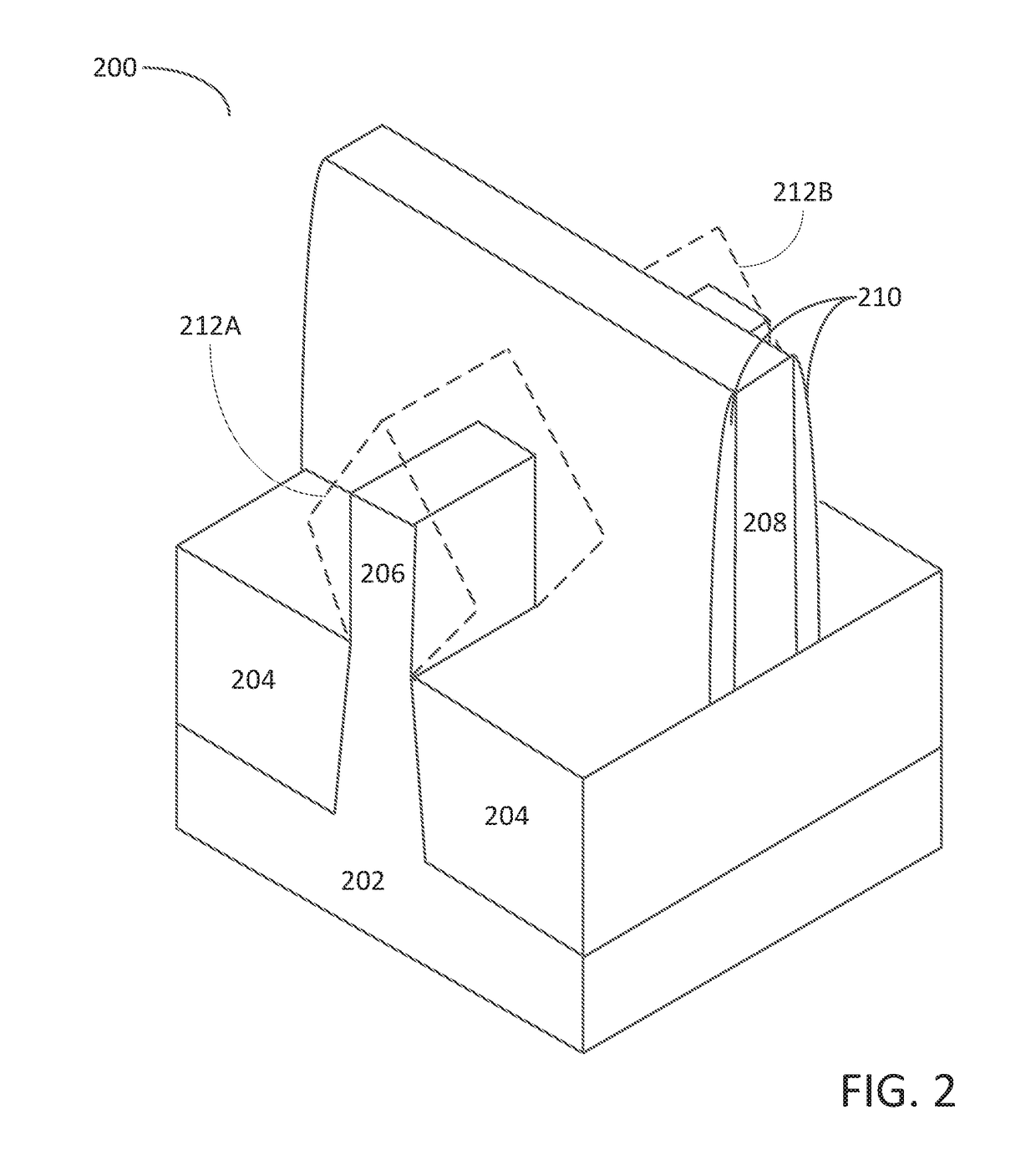
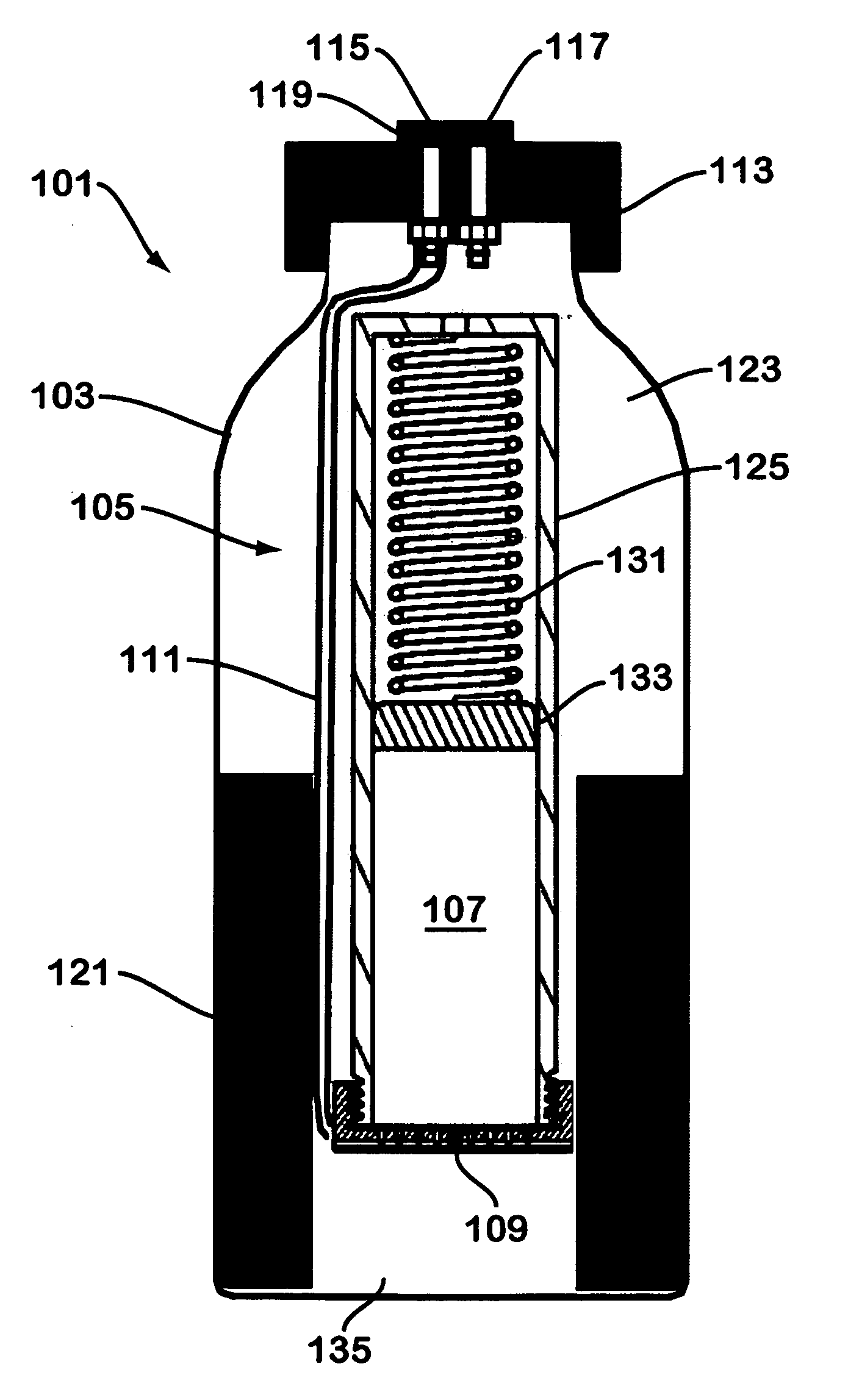
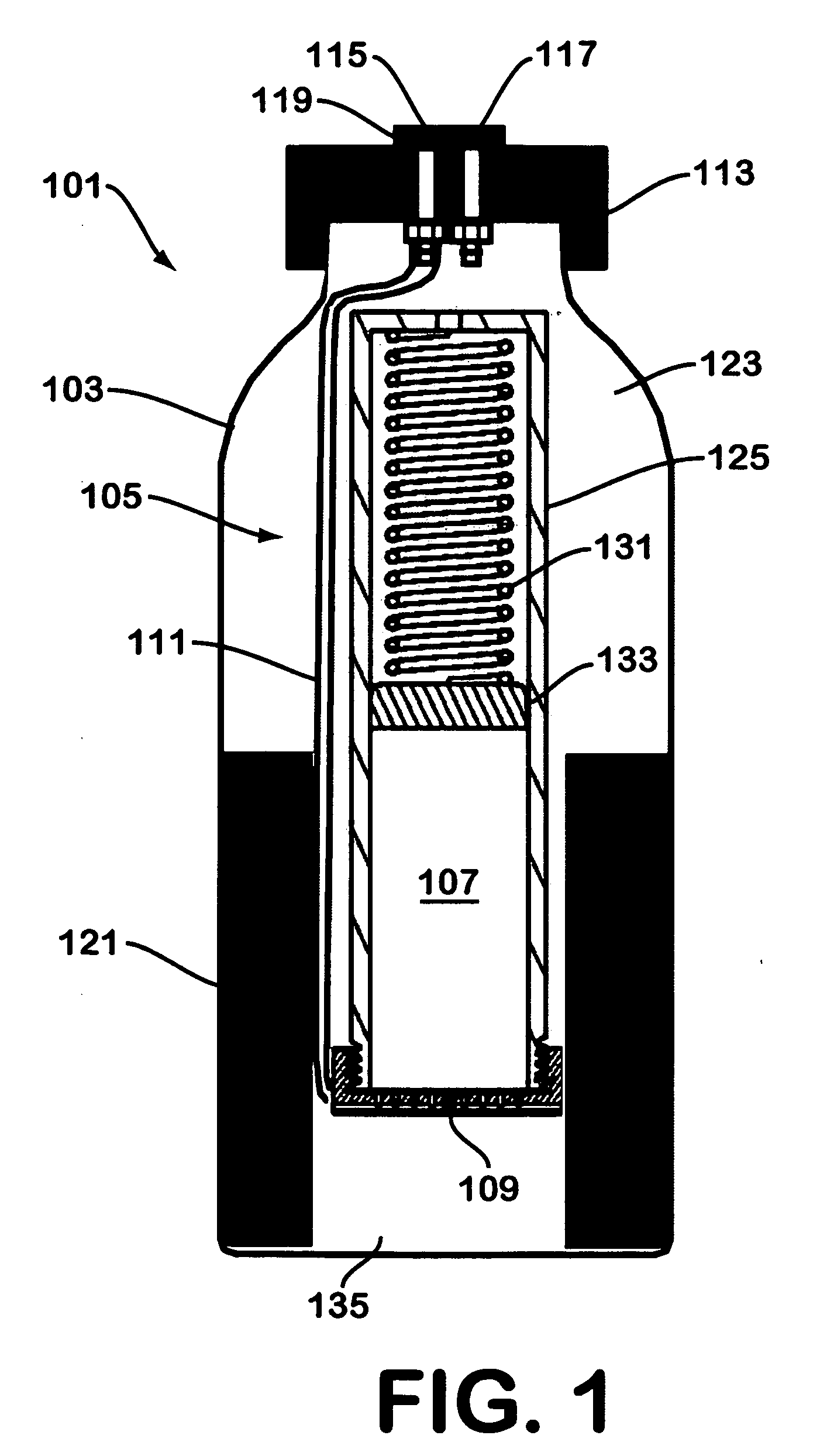
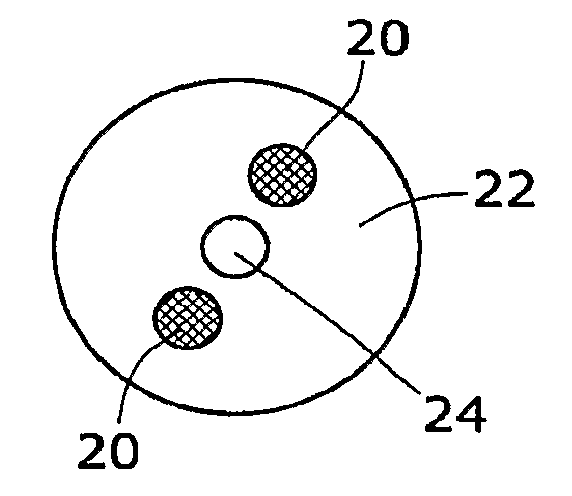
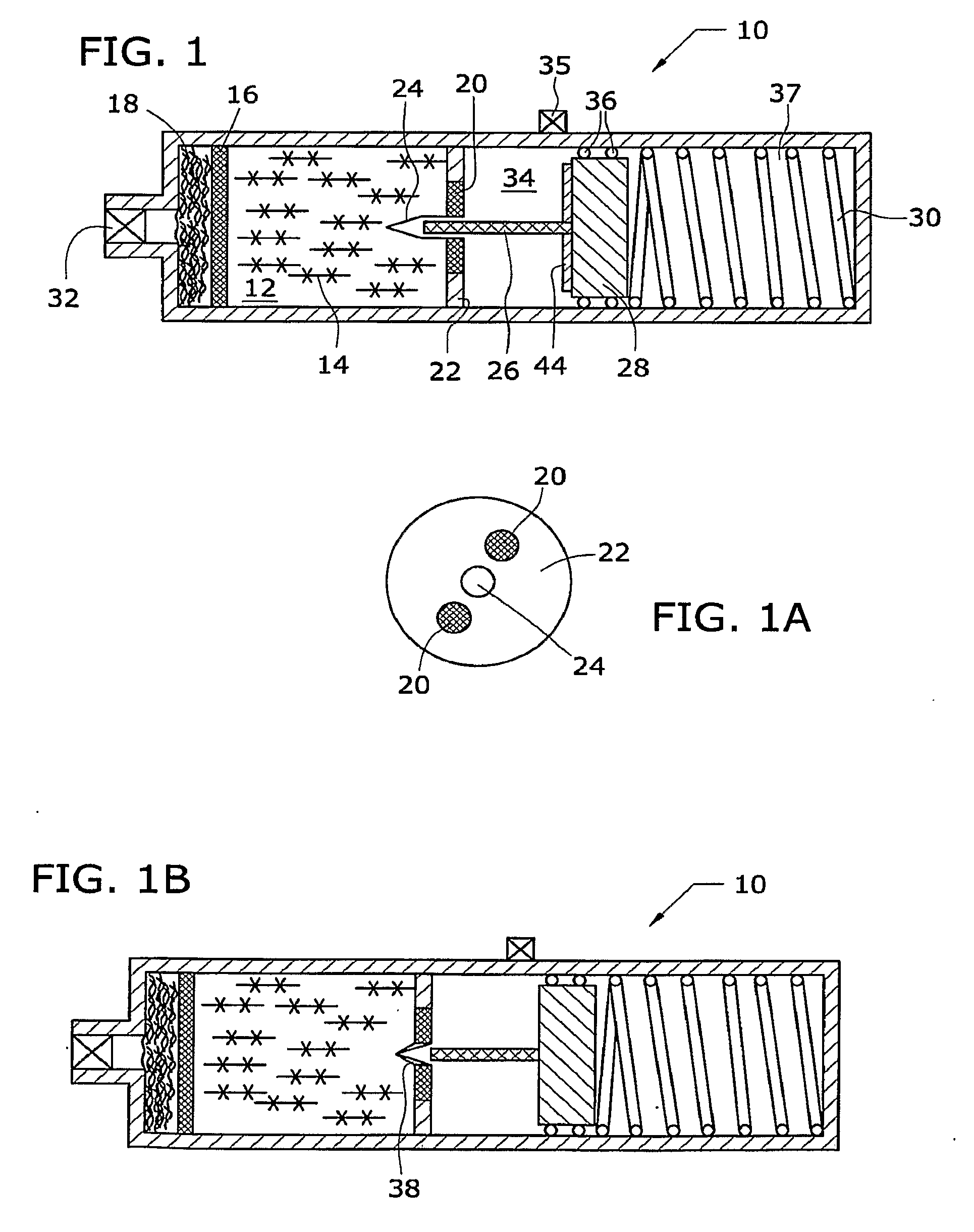
Solid chemical hydride dispenser for generating hydrogen gas
InactiveUS20070020172A1Molybdeum compoundsSynthetic resin layered productsCompound (substance)Hydrogen evolution
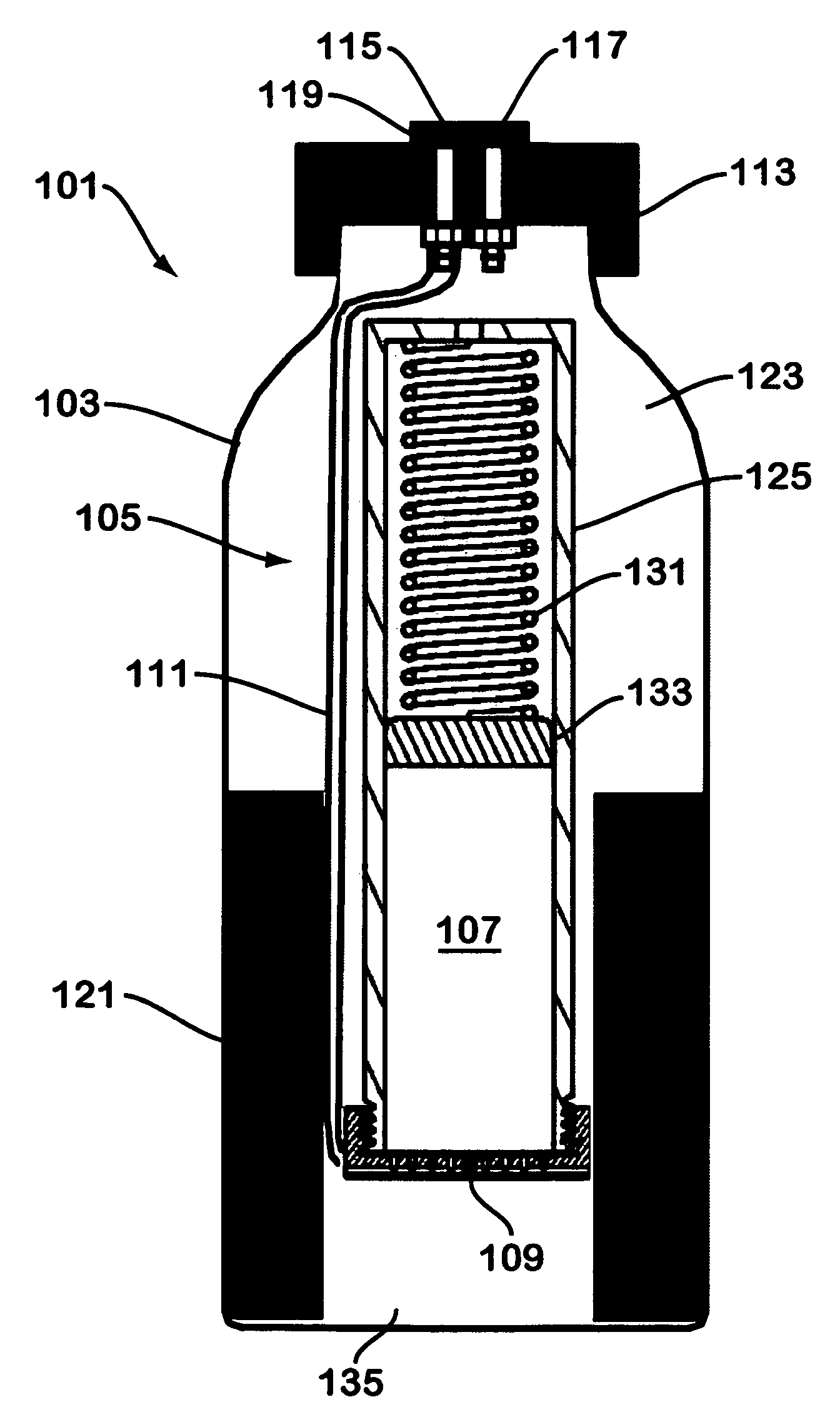
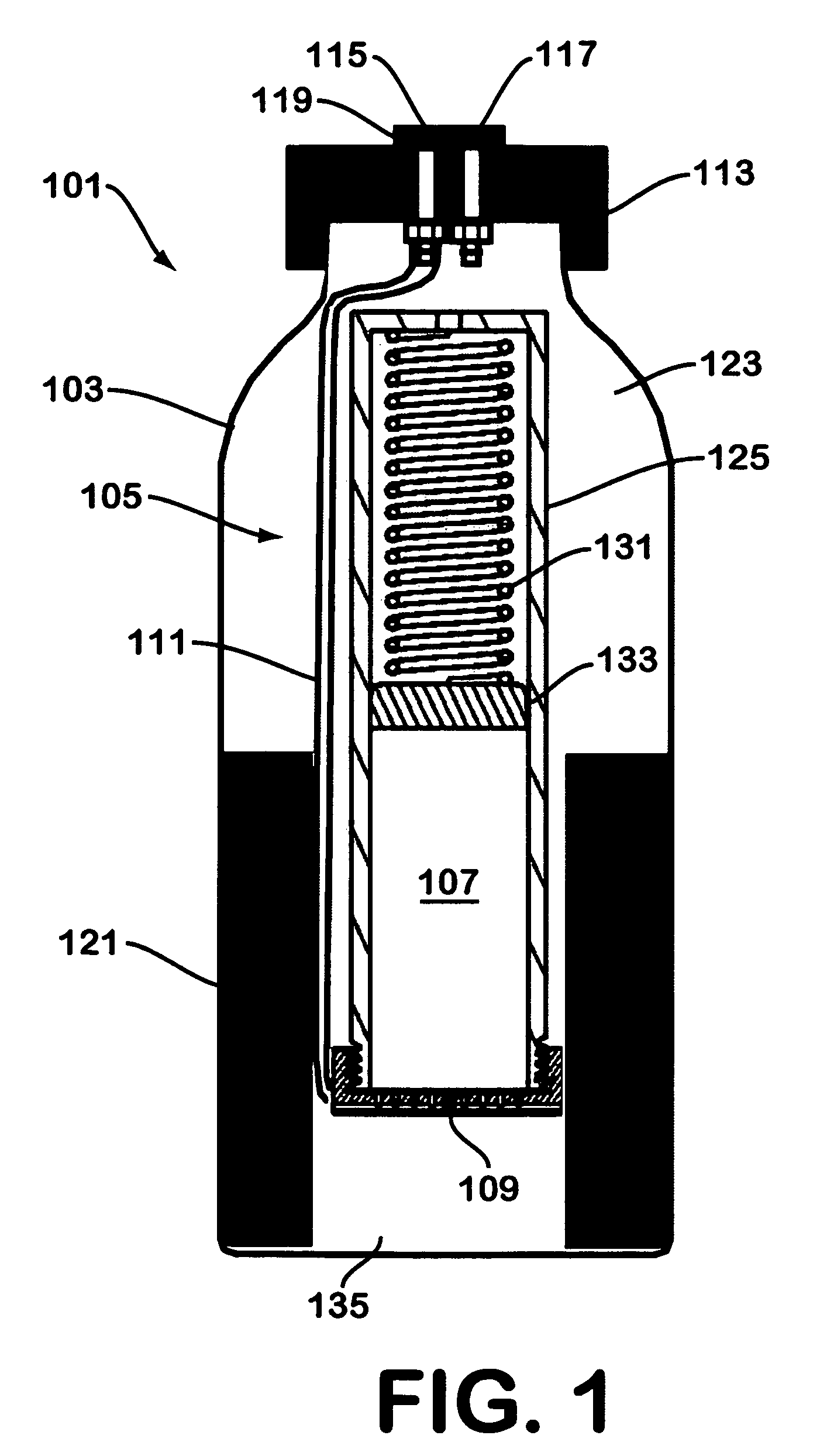
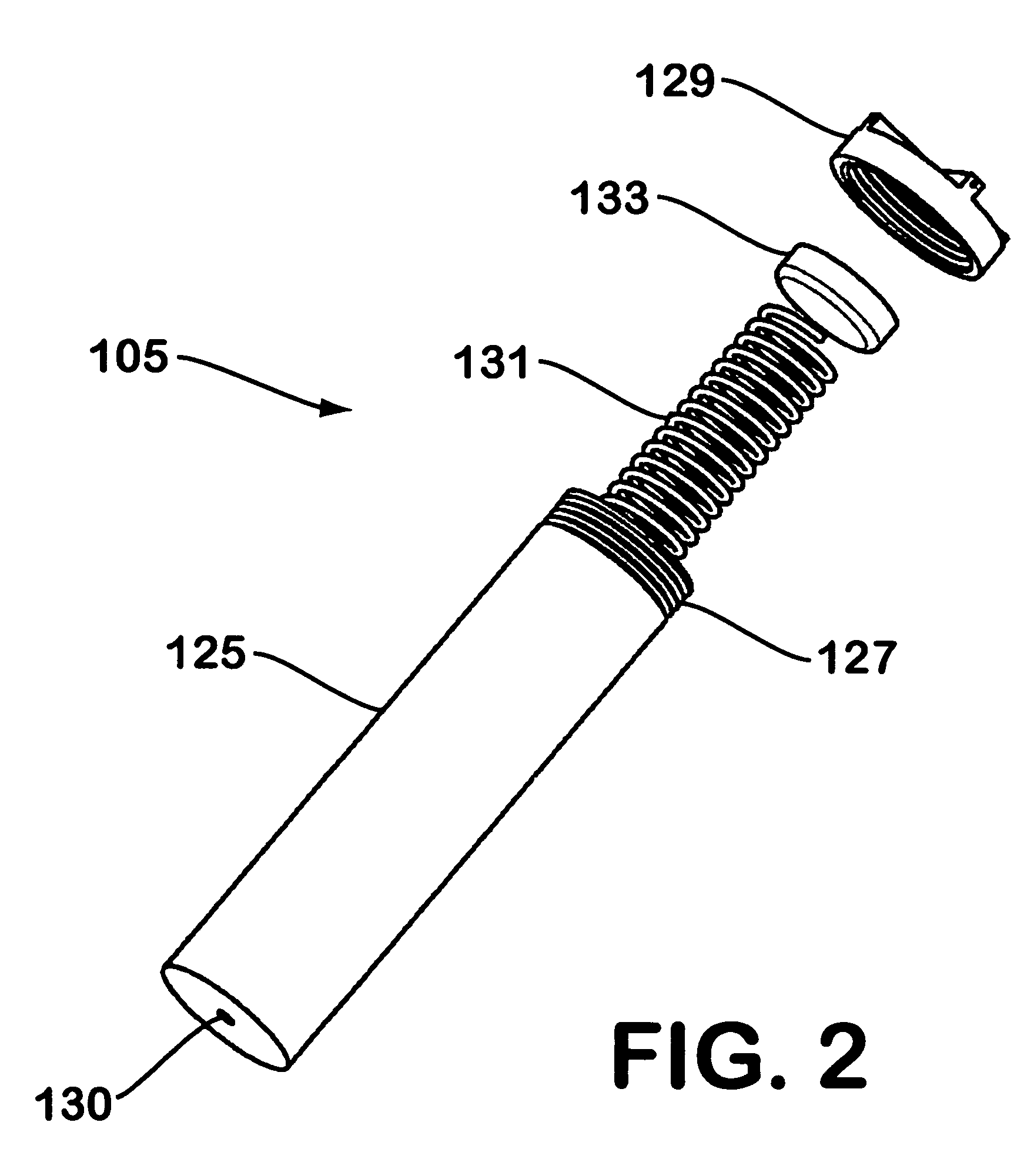
A device for generating hydrogen gas is provided. The device (101) comprises a first hydrogen-containing composition (107) that reacts with a second composition to evolve hydrogen gas; a dispenser (105) adapted to apply the first composition to a first porous member (109); and a conduit (111) adapted to supply the second composition to the first porous member. In a preferred embodiment, the first composition is selected from the group consisting of hydrides, borohydrides and boranes, the second composition is water, and the dispenser is spring-loaded and is charged with the first composition. As the first composition reacts with water at the interface to evolve hydrogen gas, the dispenser forces the reaction product across the interface and out of the dispenser, where it will not interfere with the progress of the hydrogen evolution reaction.
Owner:LYNNTECH POWER SYST
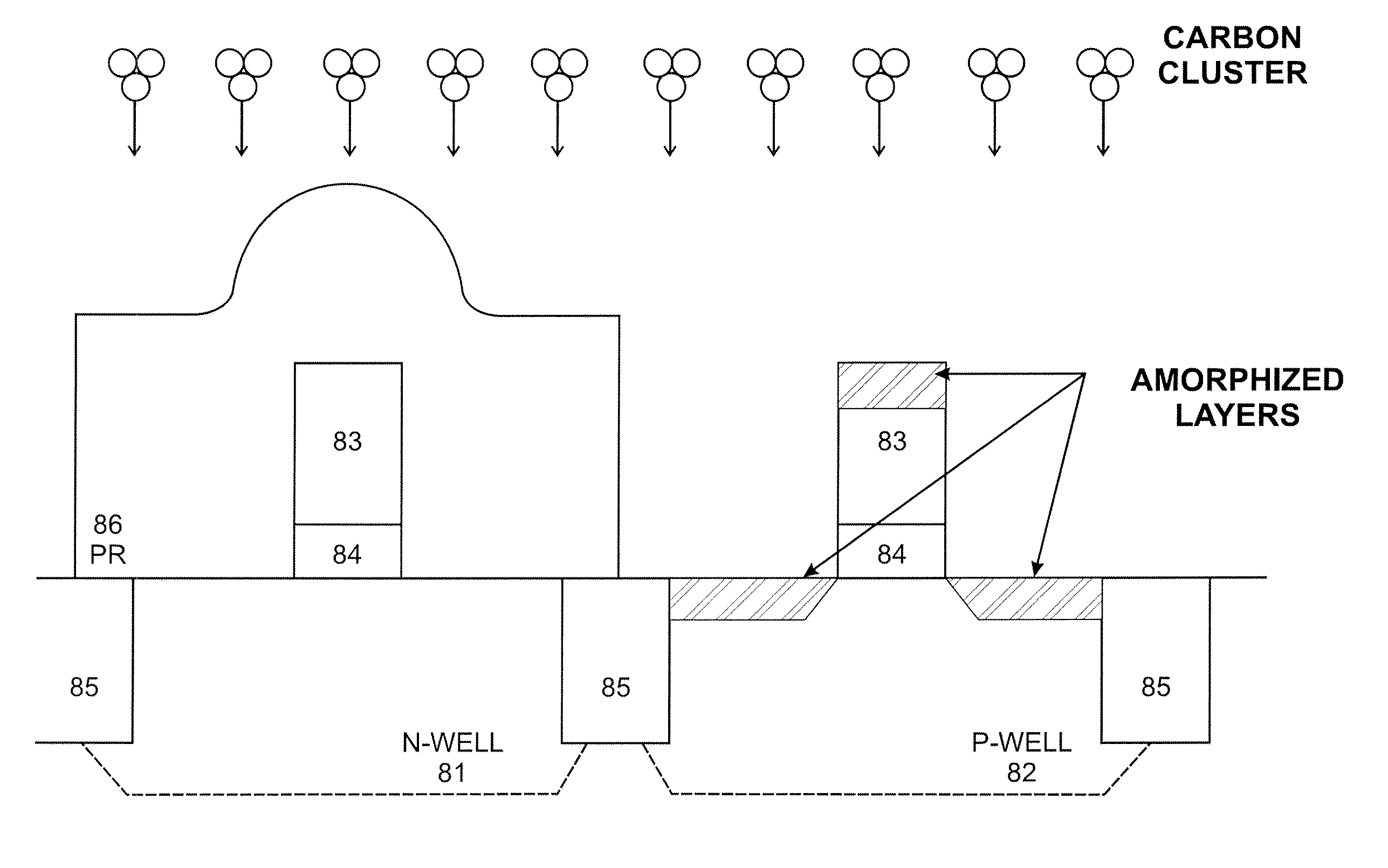
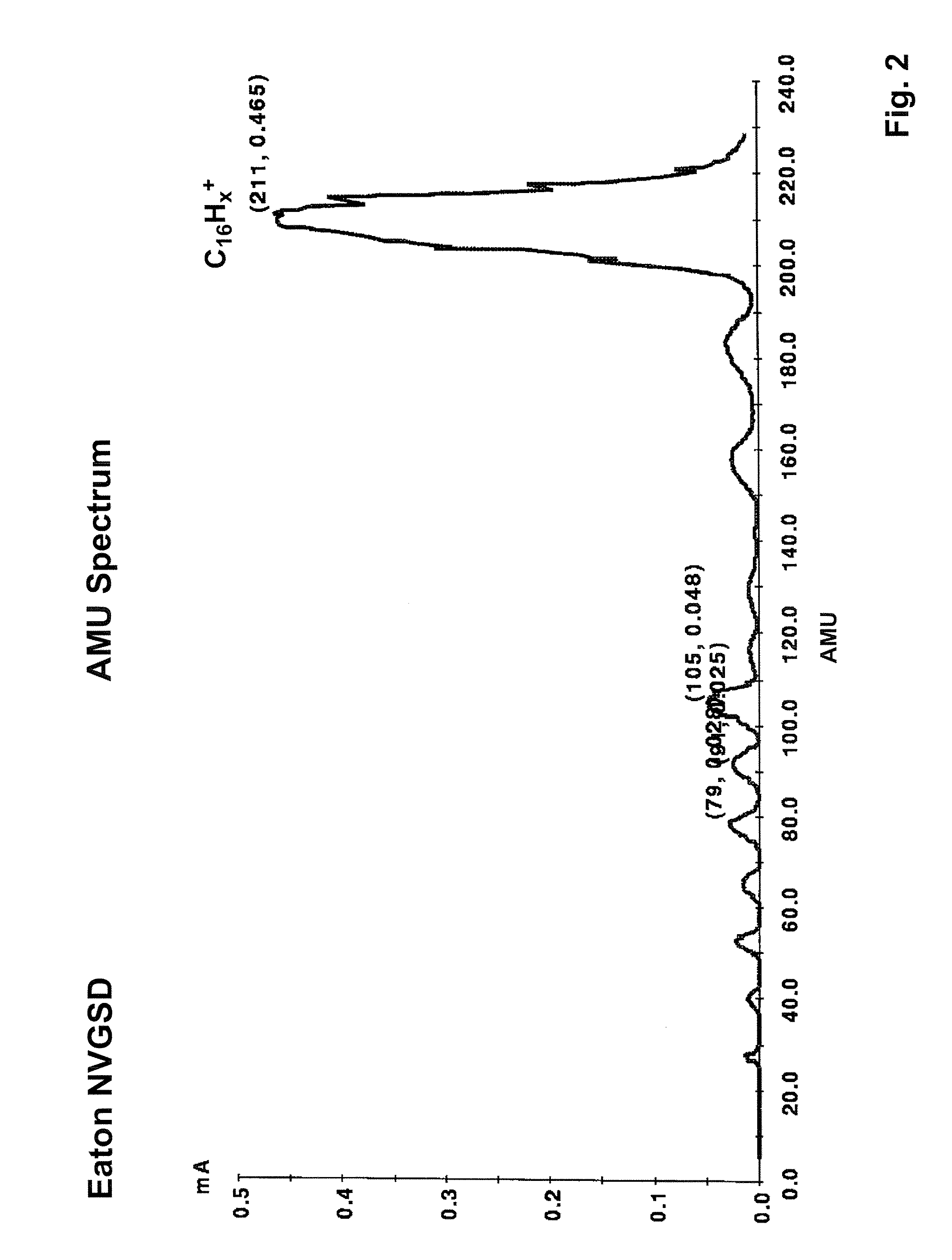
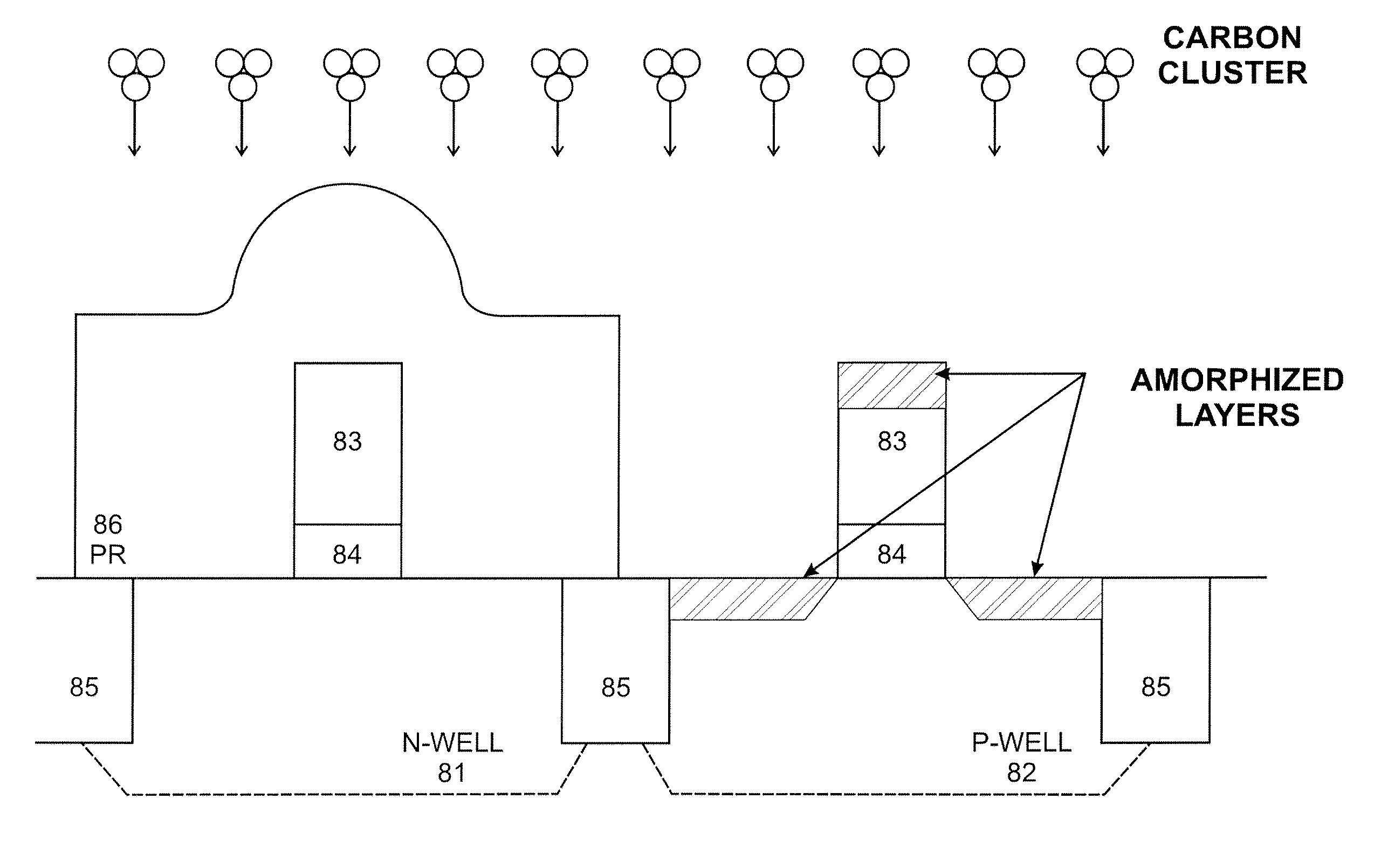
System and method for the manufacture of semiconductor devices by the implantation of carbon clusters
InactiveUS20070148888A1Improve productivityMinimize doseTransistorElectric discharge tubesDevice materialEngineering
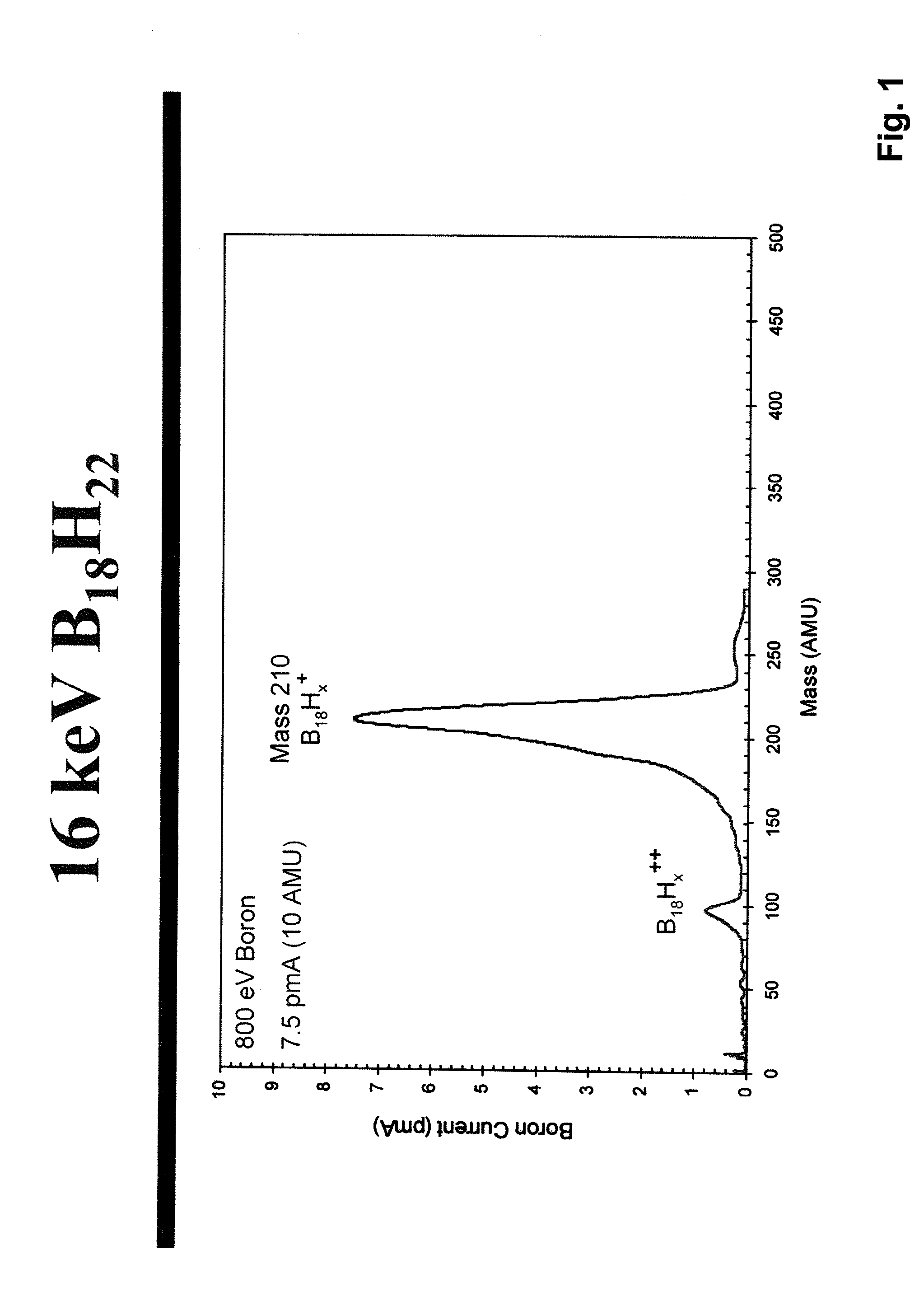
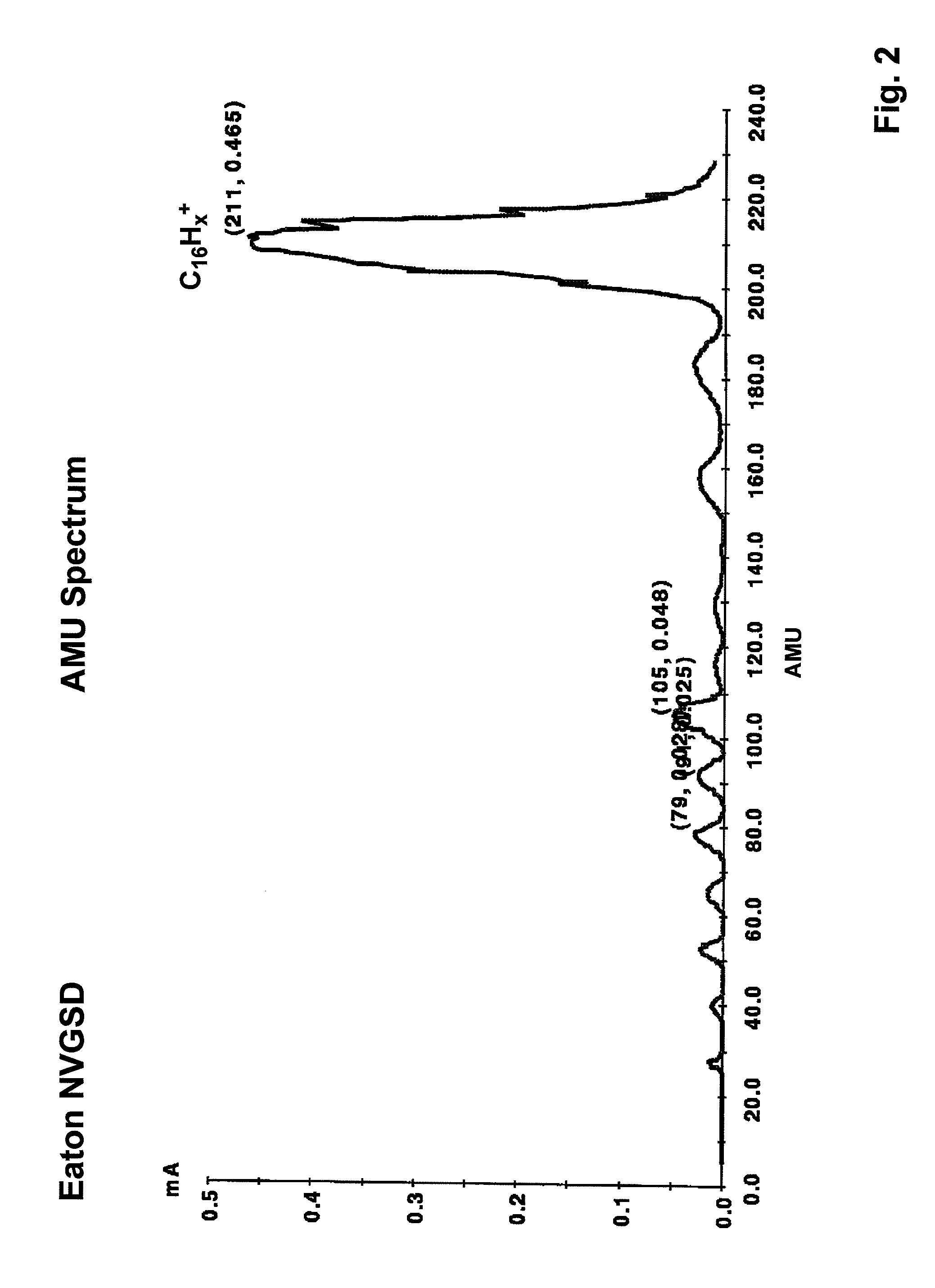
A process is disclosed which incorporates implantation of a carbon cluster into a substrate to improve the characteristics of transistor junctions when the substrates are doped with Boron and Phosphorous in the manufacturing of PMOS transistor structures in integrated circuits. There are two processes which result from this novel approach: (1) diffusion control for USJ formation; and (2) high dose carbon implantation for stress engineering. Diffusion control for USJ formation is demonstrated in conjunction with a boron or shallow boron cluster implant of the source / drain structures in PMOS. More particularly, first, a cluster carbon ion, such as C16Hx+, is implanted into the source / drain region at approximately the same dose as the subsequent boron implant; followed by a shallow boron, boron cluster, phosphorous or phosphorous cluster ion implant to form the source / drain extensions, preferably using a borohydride cluster, such as B18Hx+ or B10Hx+. Upon subsequent annealing and activation, the boron diffusion is reduced, due to the gettering of interstitial defects by the carbon atoms.
Owner:SEMEQUIP
Hydrogen generation catalyst
InactiveUS20020083643A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationControlled releaseBorohydride
The present invention relates to a composition and method for storage and controlled release of hydrogen. In particular, the present invention relates to the use of borohydride based solutions as a hydrogen storage source and a catalyst system to release hydrogen therefrom.
Owner:PROTONEX TECH CORP
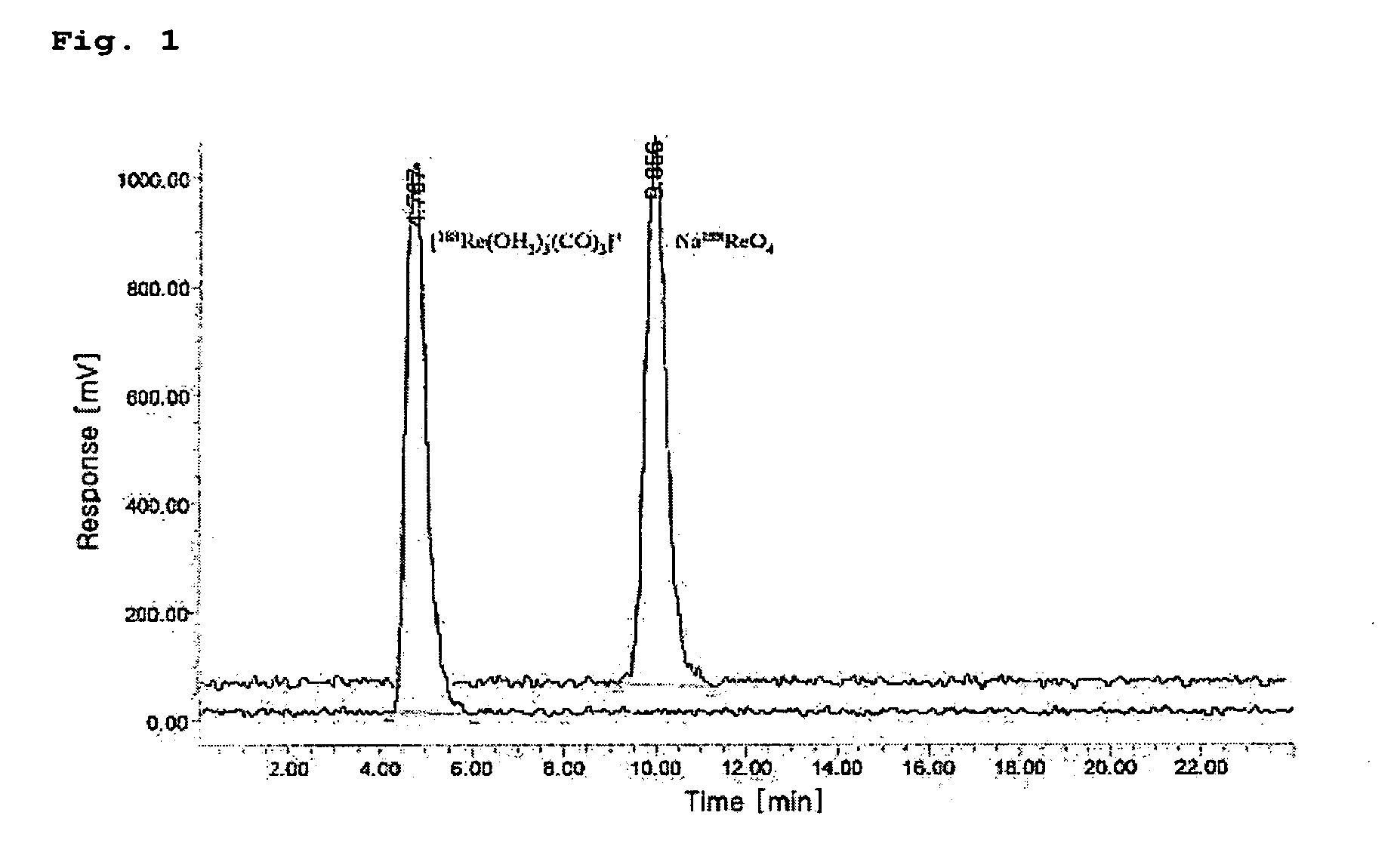
Method of preparing rhenium-tricarbonyl complex and its precursor
InactiveUS20070140959A1High yieldInhibit productionIn-vivo radioactive preparationsRhenium compounds preparationScavengerPhosphate
Disclosed herein is a method of preparing a 188Re-tricarbonyl complex for radiopharmaceutical use and of preparing a precursor thereof, and a contrast agent using the same. Particularly, this invention provides a method of preparing a 188Re-tricarbonyl precursor by reacting perrhenate with borane-ammonia (BH3.NH3), potassium boranocarbonate (K2[H3BCO2]) and phosphate in the presence of borohydride exchange resin as a reducing agent, and a method of preparing a 188Re-tricarbonyl complex by reacting the 188Re-tricarbonyl precursor with a ligand. According to the method of this invention, the borohydride exchange resin is used as a reducing agent and as an anion scavenger, thereby obtaining the 188Re-tricarbonyl precursor and complex having high radiolabeling yield and high purity. In addition, the 188Re-tricarbonyl complex can be used as a contrast agent having excellent plasma stability.
Owner:KOREA ATOMIC ENERGY RES INST
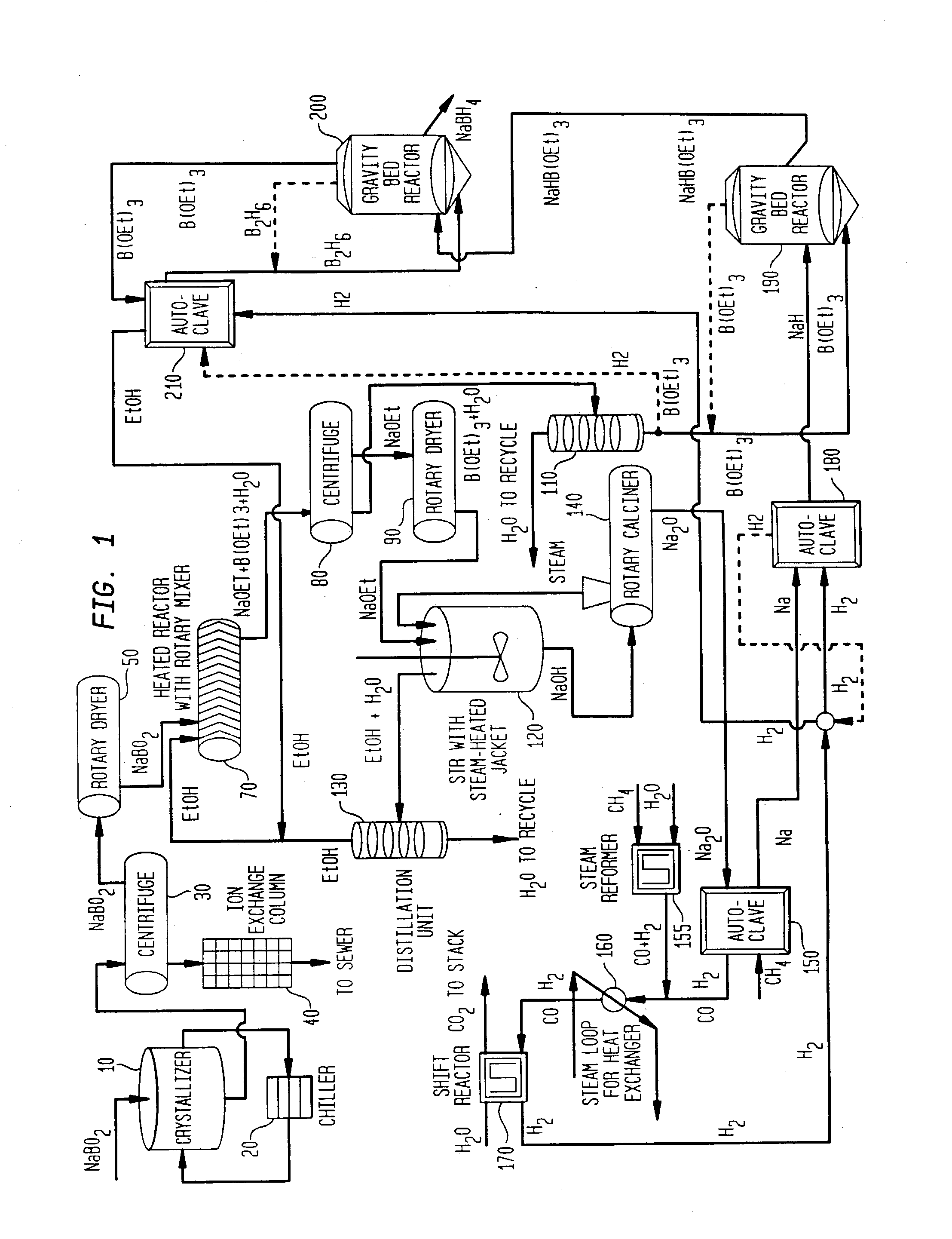
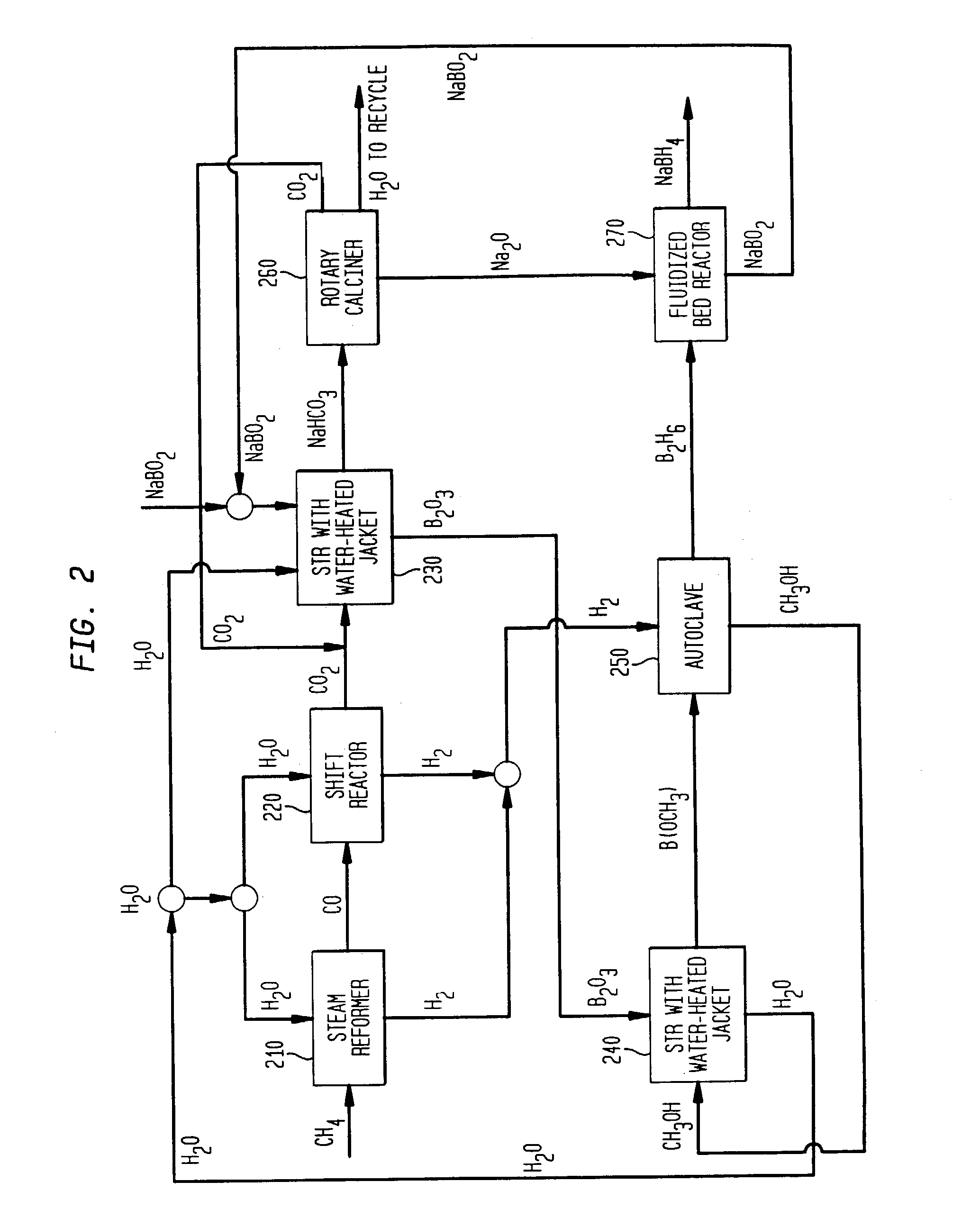
Processes for synthesizing alkali metal borohydride compounds
Processes for synthesizing borohydride compounds with reduced energy requirements and high efficiency are disclosed. The processes include the reaction of a base with a borane complex or diborane to produce a borohydride compound of formula YBH4, where Y is a monovalent cationic moiety.
Owner:MILLENNIUM CELL
Catalyst for hydrogen production by catalyzing and hydrolyzing borohydride and preparation method thereof
InactiveCN101347736AFast deposition rateIncrease concentrationMetal/metal-oxides/metal-hydroxide catalystsMetal hydridesChemical platingRare earth
The invention relates to hydrogen production and hydrogen storage technologies and materials, in particular to a catalyst for catalytic hydrolysis of borane for the hydrogen production and a preparation method thereof, thereby solving the problems that the direct application of powder catalyst in a catalytic hydrolysis solid-liquid reaction system can cause the loss of the catalyst, the catalytic hydrolysis reaction is difficult to control and the hydrolysis by-products are difficult to be recovered, etc. The catalyst is composed of an active component and a carrier; the active component is a binary, ternary or multinary alloy or a single precious metal or the combination thereof which is composed of one or more transition metals, rare earth metals or precious metals and metalloids; the active component is deposited on the carrier through the improved chemical plating technology, the surface thereof is rough and porous, and the structure of the prepared catalyst is the amorphous or the nanocrystalline structure. The preparation method has simple preparation process, high preparation efficiency and convenient large-scale preparation; the sources of the used raw materials are rich; the catalytic activity of the prepared supported catalyst is high, the real-time control of the catalytic hydrolysis reaction of the borane can be realized, the catalytic performance is stable, and the catalyst can be repeatedly used for a plurality of times.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Fuel blends for hydrogen generators
InactiveUS20050132640A1Easy to optimizeEfficient hydrolysisLiquid carbonaceous fuelsHydrogen productionFuel cellsHydrolysis
The present invention relates to improved aqueous fuels for hydrogen generators and a method for using them in the production of hydrogen. The present invention also relates to a system of using the subject aqueous fuels to generate hydrogen gas for use in a fuel cell or other device. The subject fuels contain a mixture of boron hydrides, at least one of which is a metal salt, including metal borohydrides, higher boranes and metal higher boron hydrides. The subject aqueous fuels contain a mixture of boron hydrides having a positive ionic charge (+IC) to boron ratio of between 0.2 and 0.4 or between 0.6 and 0.99. Preferred fuels contain a mixture of boron hydrides having an (+IC) to boron ratio between 0.2 and 0.3 or between 0.7 and 0.8. Mixtures containing a metal borohydride also contain a metal hydroxide to stability it against premature hydrolysis in the aqueous fuel media.
Owner:MILLENNIUM CELL
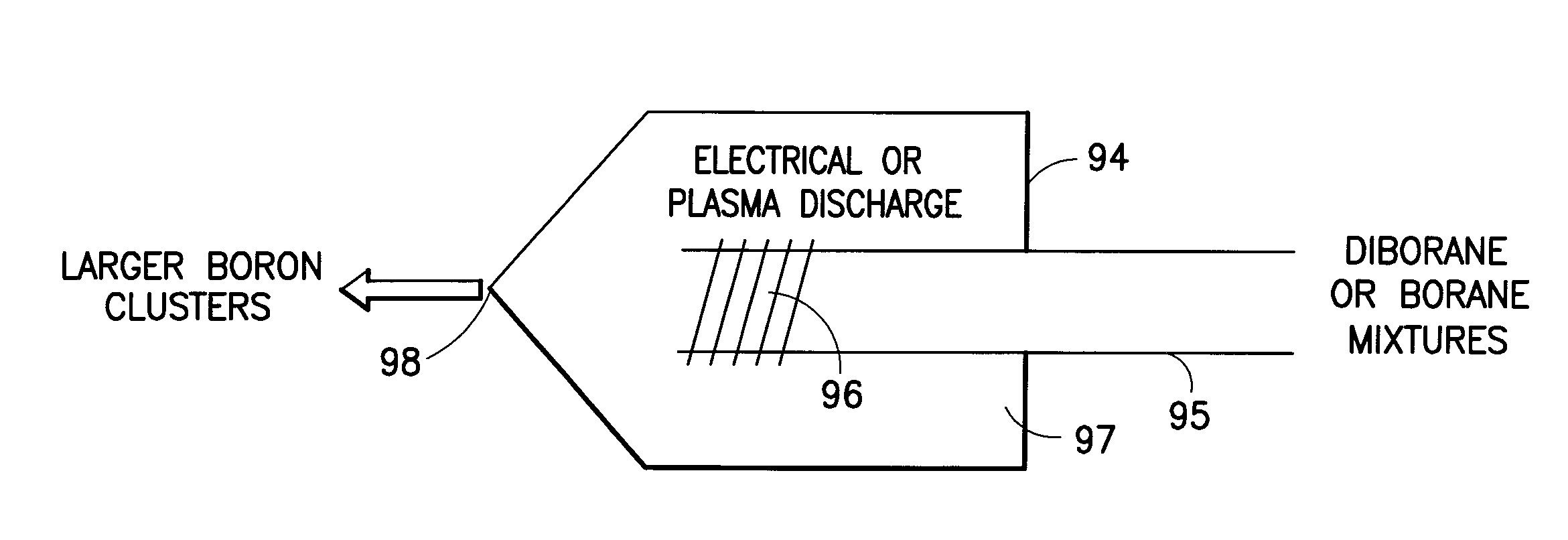
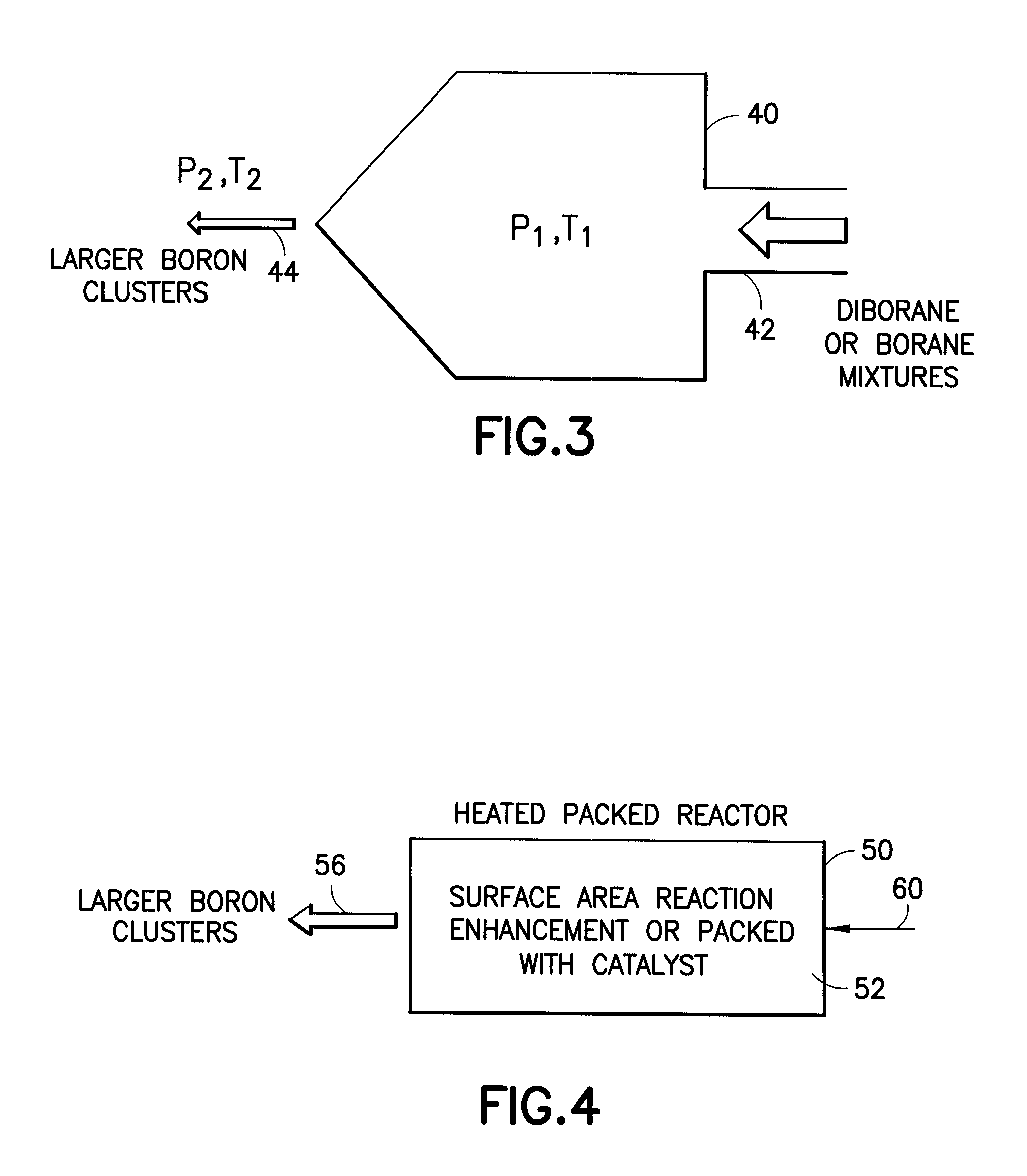
Boron Ion Implantation Using Alternative Fluorinated Boron Precursors, and Formation of Large Boron Hydrides for Implanation
ActiveUS20080248636A1Easy to cutImprove efficiencyOther chemical processesElectric discharge tubesBoron trifluorideIon implantation
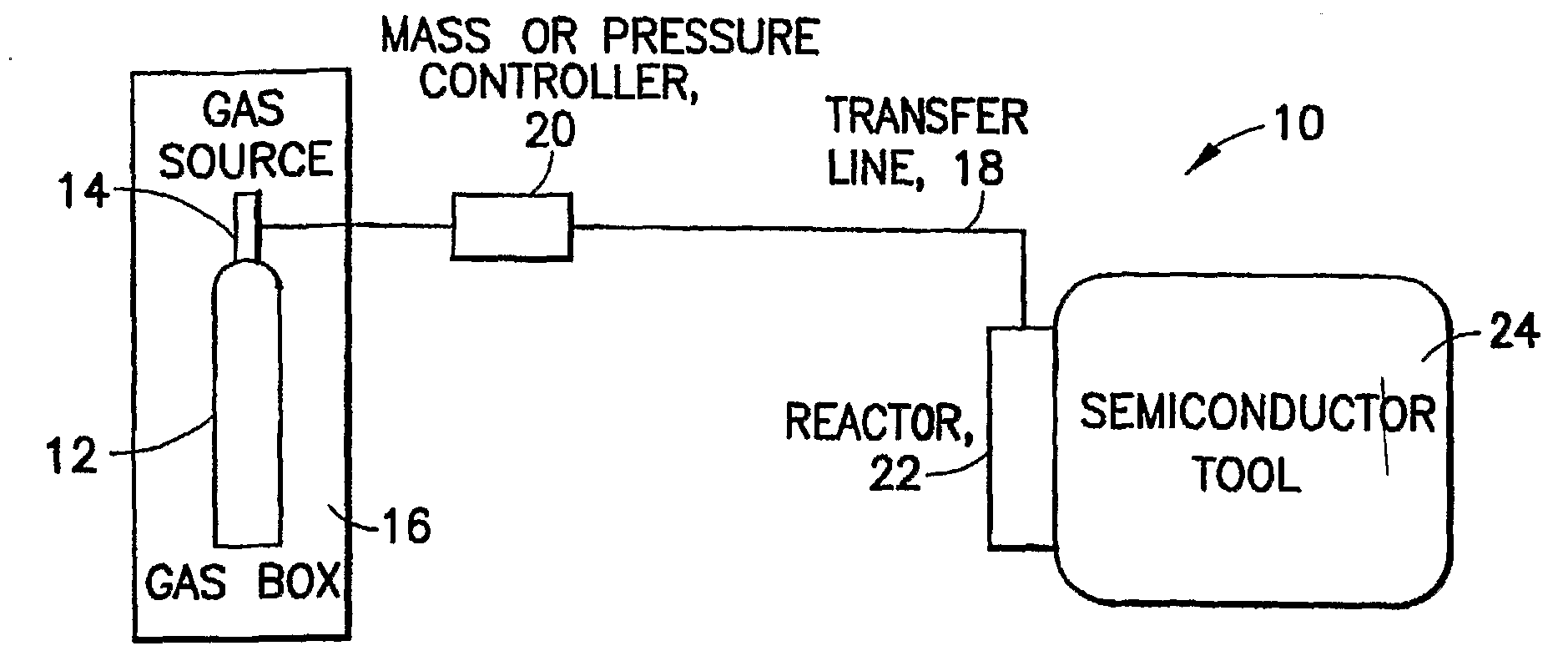
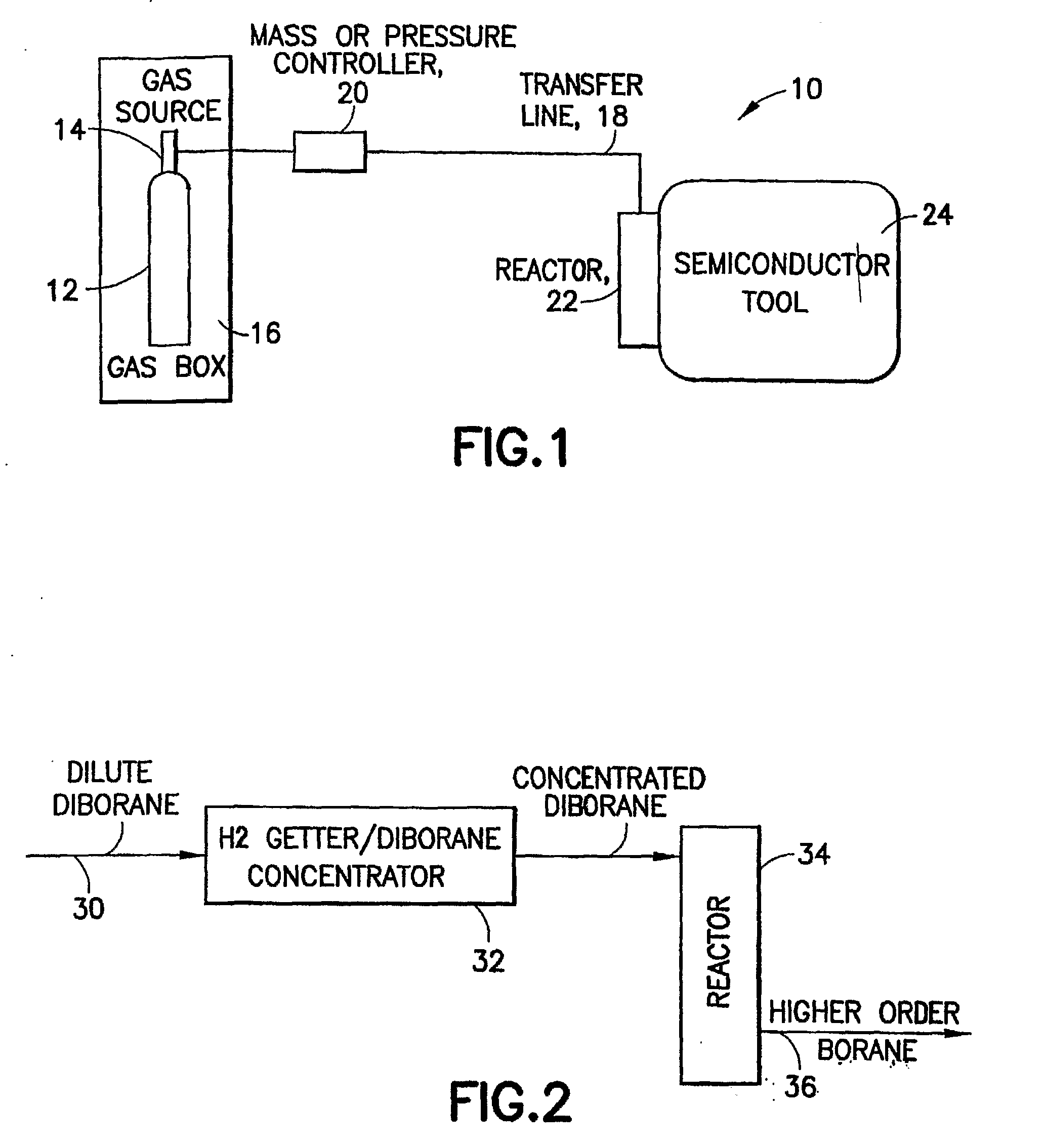
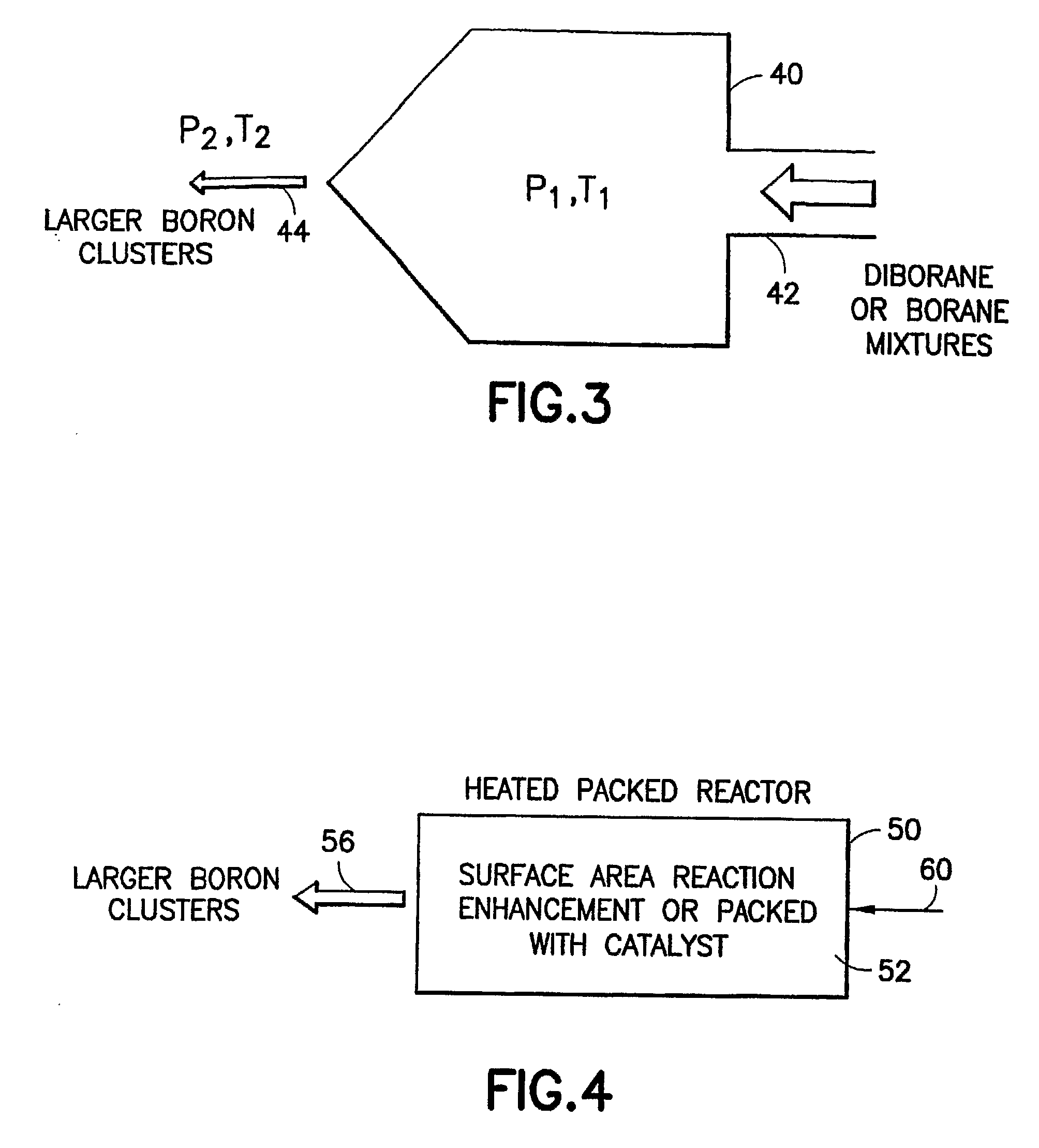
Methods of implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. A method of manufacturing a semiconductor device including implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. Also disclosed are a system for supplying a boron hydride precursor, and methods of forming a boron hydride precursor and methods for supplying a boron hydride precursor. In one implementation of the invention, the boron hydride precursors are generated for cluster boron implantation, for manufacturing semiconductor products such as integrated circuitry.
Owner:ENTEGRIS INC
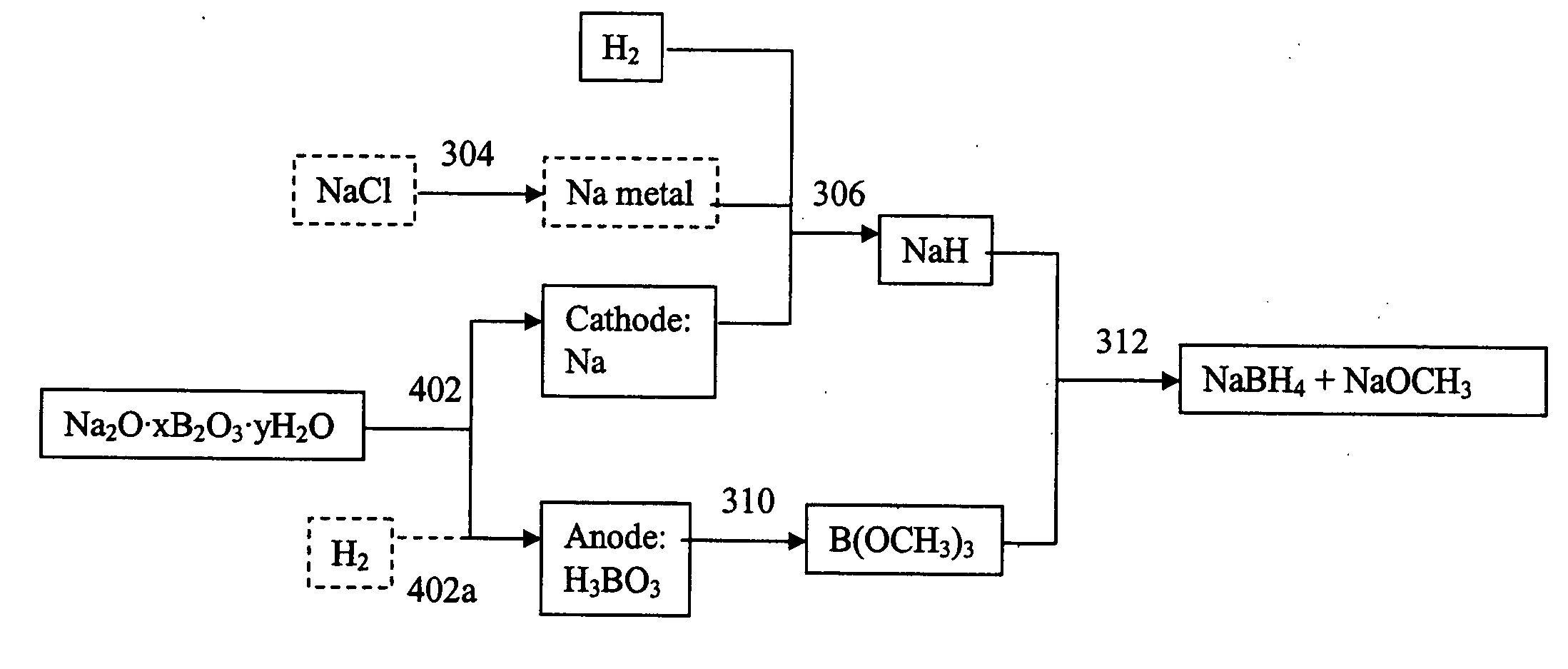
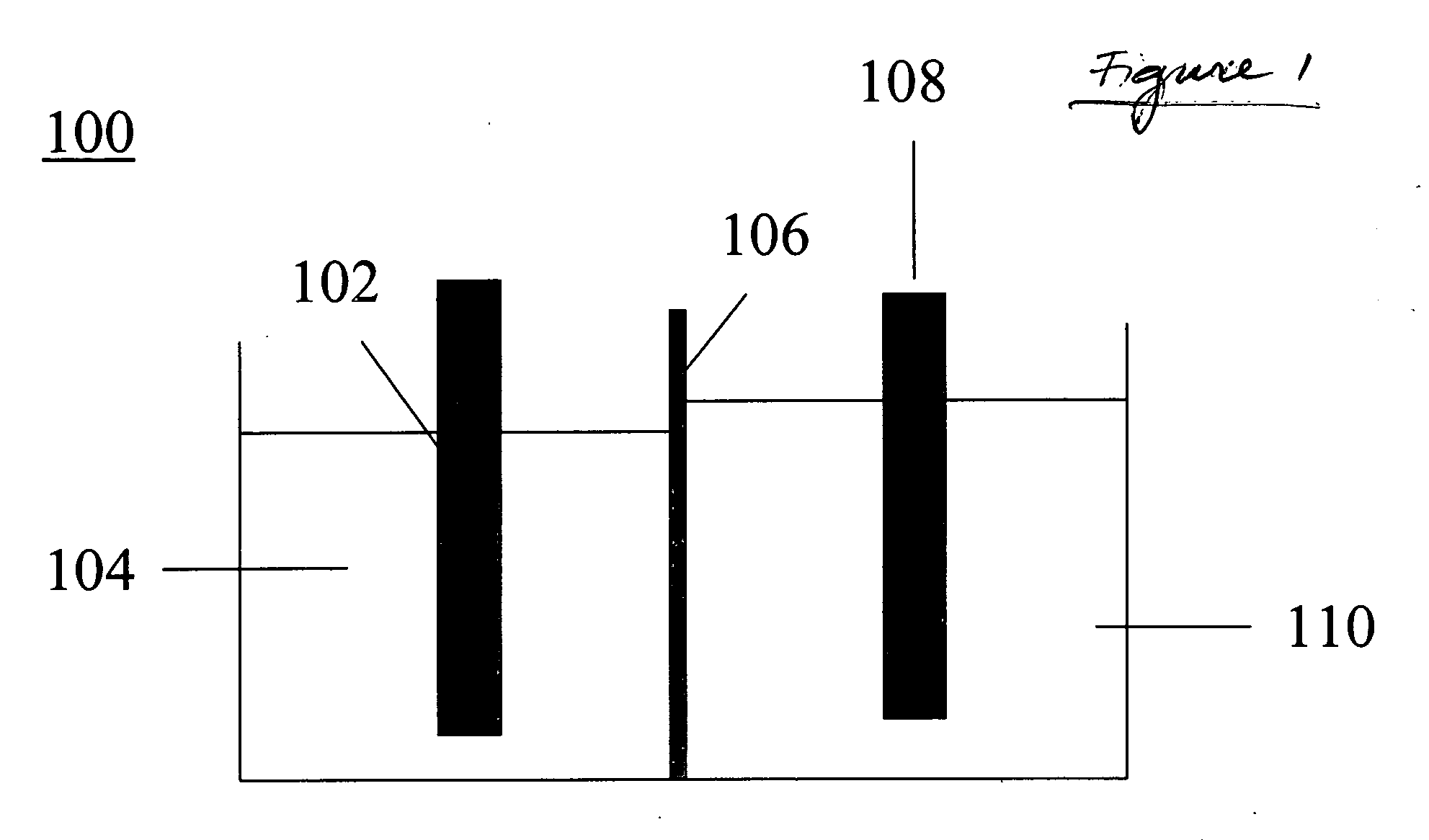
Methods and apparatus for synthesis of metal hydrides
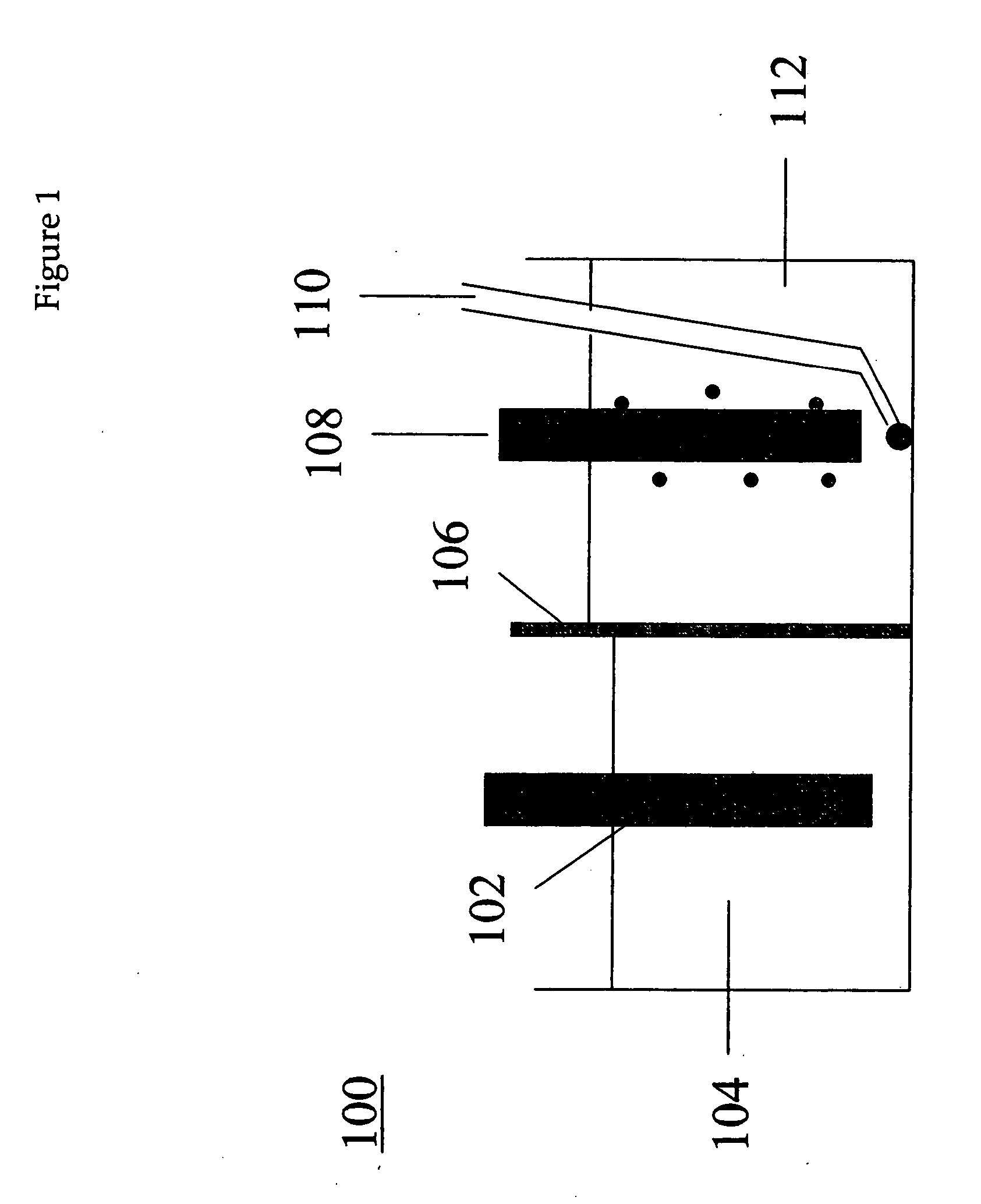
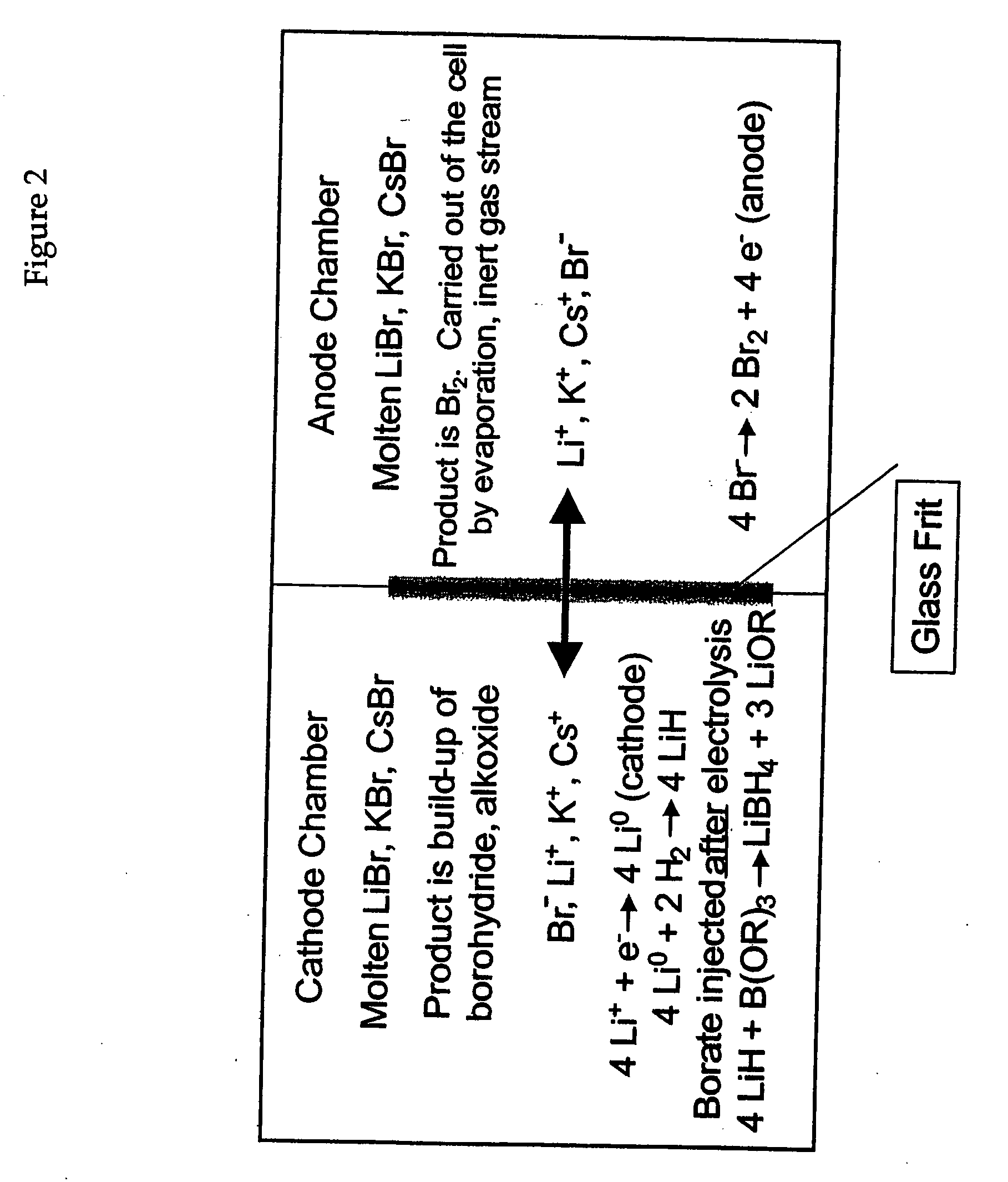
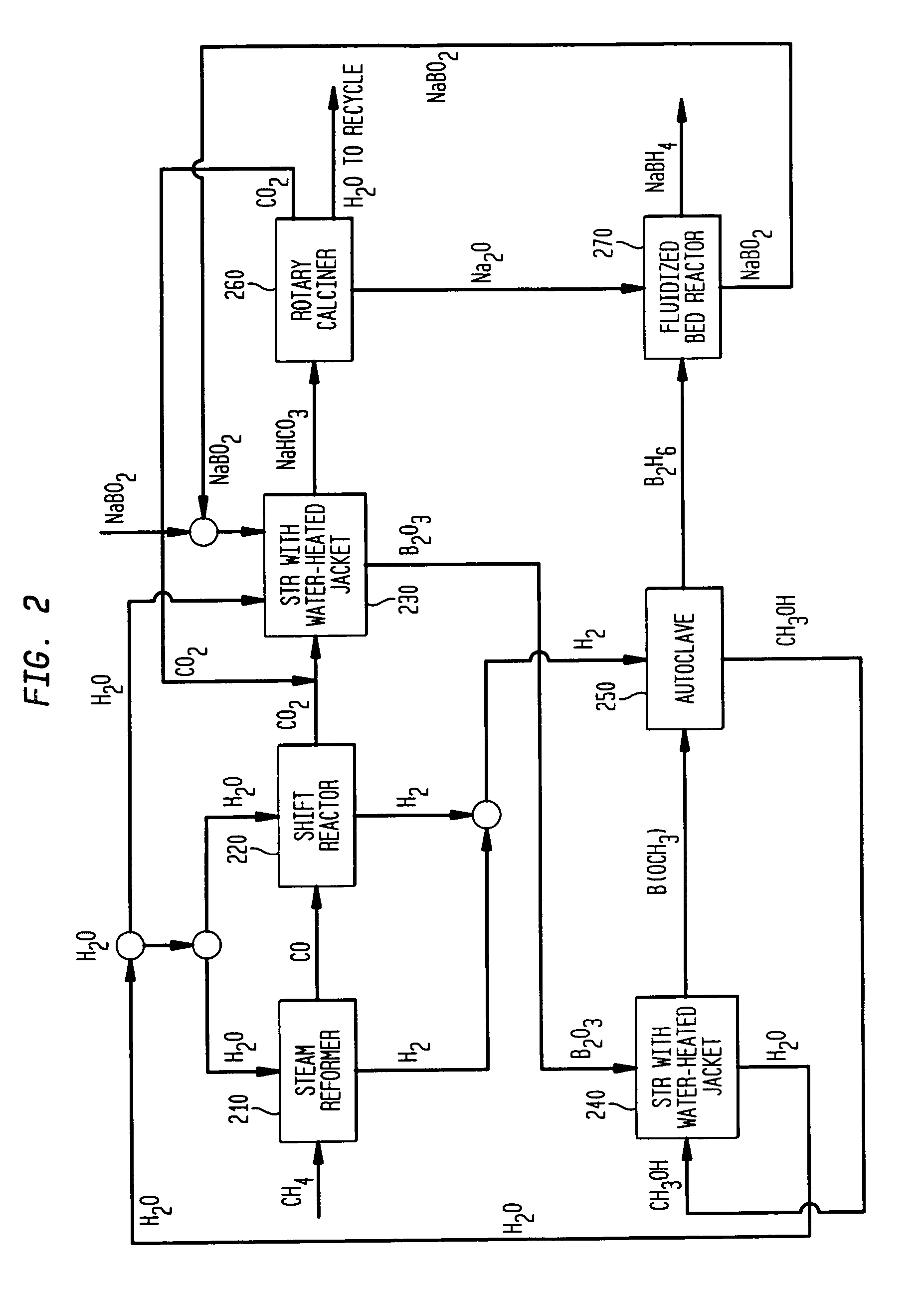
An electrochemical process and apparatus for preparing metal hydride compounds from metal salts under a hydrogen atmosphere are disclosed. The electrochemical process may be integrated with chemical reaction of a boron compound to produce borohydride compounds. A metal salt and a borate are charged to the cathode of an electrolytic cell wherein the borate reacts with the hydride, to produce the borohydride compound.
Owner:MILLENNIUM CELL
Accelerated hydrogen generation through reactive mixing of two or more fluids
InactiveUS6818334B2Reactivity issueStability issueCell electrodesFuel cell auxillariesPtru catalystProton exchange membrane fuel cell
Owner:INTELLIGENT ENERGY LTD
Borohydride fuel compositions and methods
Solid self-stabilized borohydride fuel compositions, fuel cartridges, related methods of preparation and hydrogen generation are provided. The fuel compositions comprise mixtures of at least one borohydride salt with a cation selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation, preferably sodium borohydride, and at least one hydroxide salt with a cation selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation, preferably sodium hydroxide.
Owner:MILLENNIUM CELL
Compositions and processes for synthesizing borohydride compounds
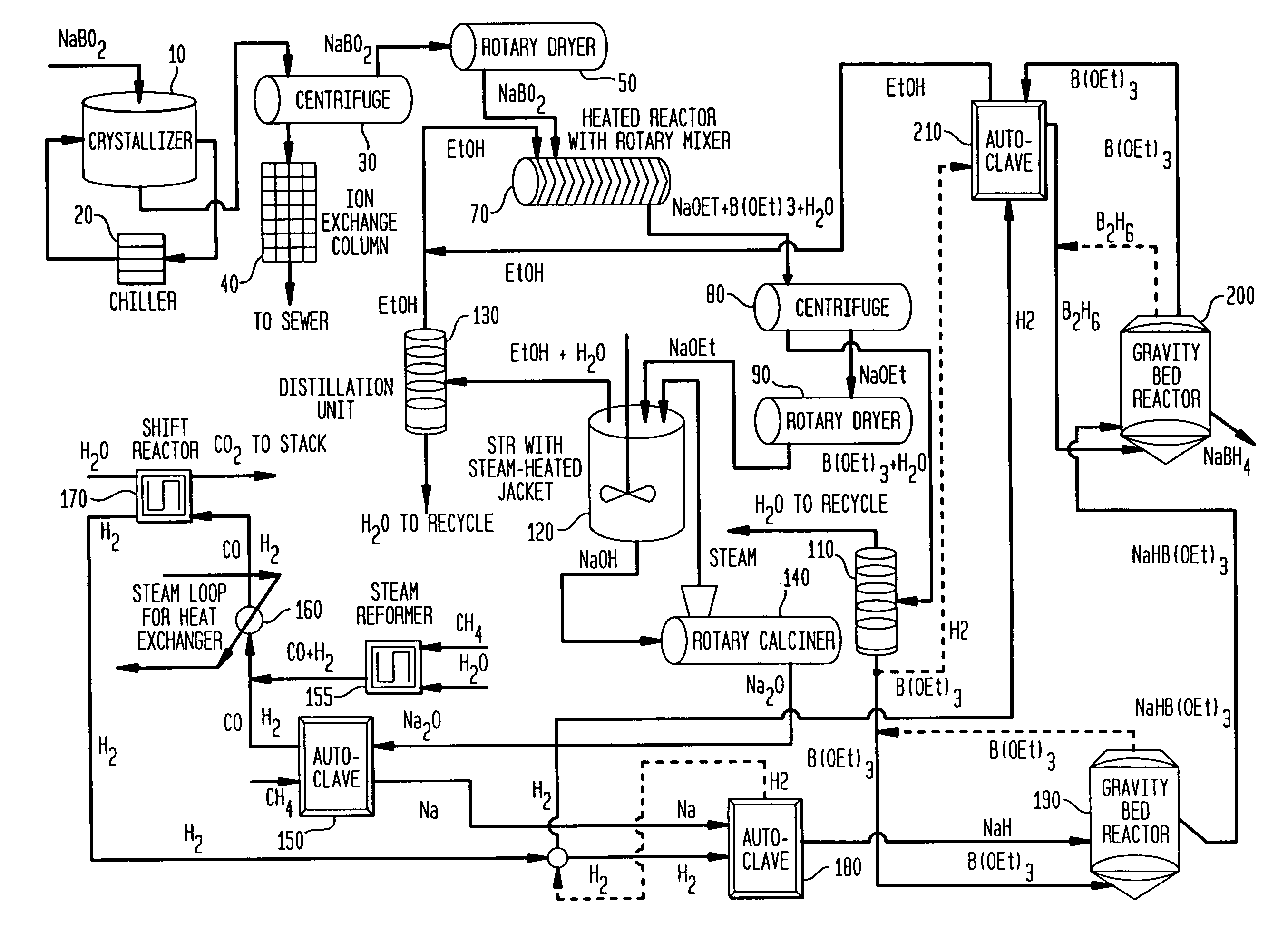
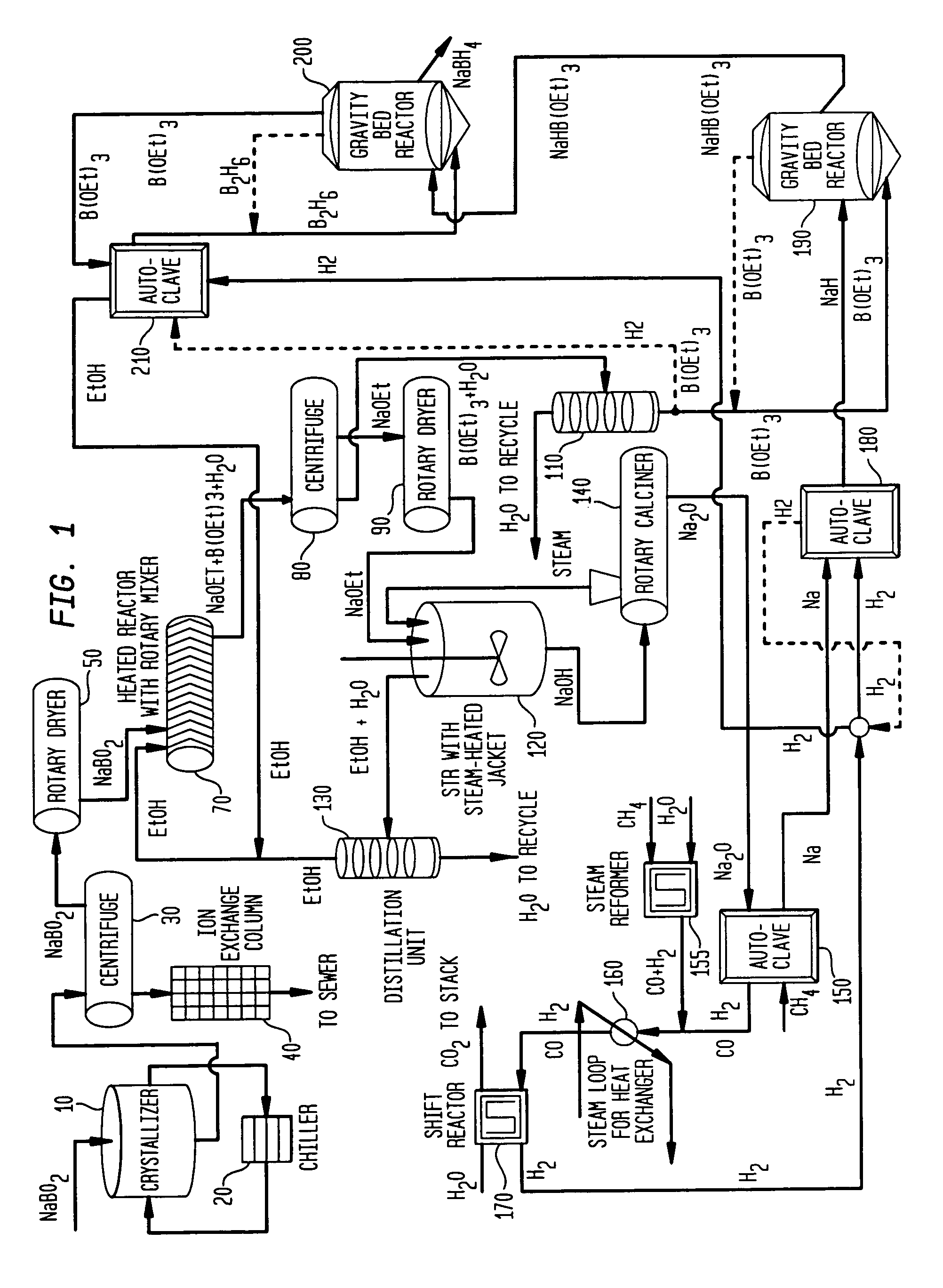
InactiveUS7019105B2Improve processing efficiencyOvercome deficienciesHydrogenMonoborane/diborane hydridesOrganic chemistryBorohydride
The present invention relates to compositions and processes for producing borohydride compounds. In particular, the present invention provides efficient processes and compositions for the large-scale production of borohydride compounds.
Owner:MILLENNIUM CELL
Metal alkyl borphydride polymerisation initiators, polymerisable compositions, and uses thereof
InactiveUS6844080B2Wide rangeImprove impact resistanceSynthetic resin layered productsOrganic non-macromolecular adhesiveLithiumPolyolefin
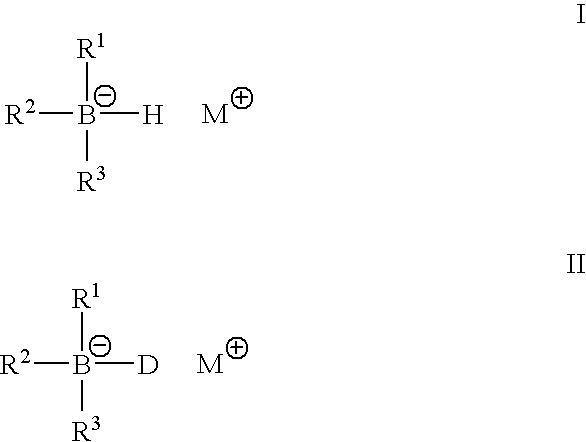
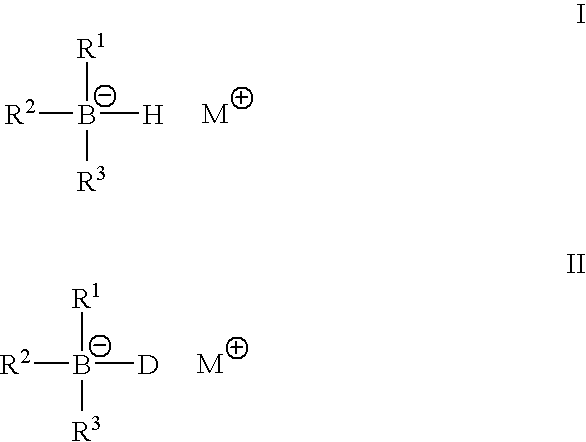
Metal alkyl borohydrides are used as initiators of polymerisation, particularly in adhesive compositions for bonding a wide range of substrates including low surface energy substrates such as polyolefins. As described, the metal alkyl borohydrides are of the formula I or II. whereinR1 is C1-C10 alkyl,R2 and R3, which may be the same or different, are H, D, C1-C10 alkyl or C3-C10 cycloalkyl, phenyl, or phenyl-substituted C1-C10 alkyl or C3-C10 cycloalkyl, provided that any two of R1-R3 may optionally be part of a carbocyclic ring, andM+ is a metal ion.In particular, alkali metal trialkyl borohydrides are used, the alkali metal salt being selected from: Lithium triethylborohydride, Sodium triethylborohydride, Potassium triethylborohydride, Lithium tri-sec-butylborohydride, Sodium tri-sec-butylborohydride, Potassium tri-sec-butylborohydride, and Lithium triethylborodeuteride. Other exemplified compounds which are less effective on low surface energy substrates include Lithium 9-borabicyclo [3.3.1]-nonane (9BBN) hydride, Lithium thexylborohydride, Lithium trisiamylborohydride and Potassium trisiamylborohydride.
Owner:HENKEL IP & HOLDING GMBH
Isotopically-enriched boranes and methods of preparing them
InactiveUS20050163693A1High synthetic yieldHigh isolation yieldSemiconductor/solid-state device manufacturingChemical recyclingIsotopeConjugate acid
Owner:SEMEQUIP
Destabilized and catalyzed borohydrided for reversible hydrogen storage
InactiveUS20060194695A1Reduce the temperatureImproved hydrogen binding/release kineticsHydrogenPhysical/chemical process catalystsIndiumCobalt
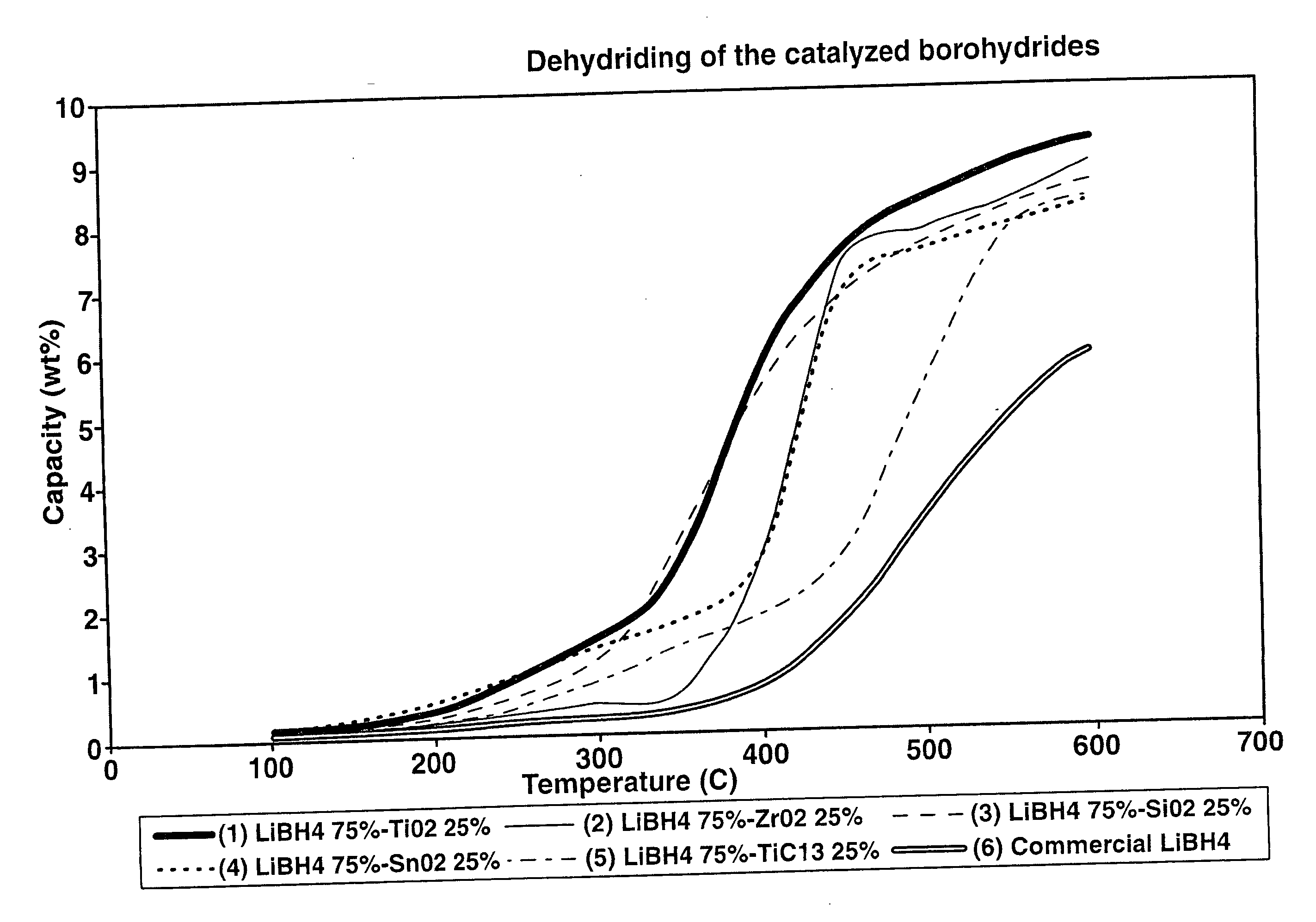
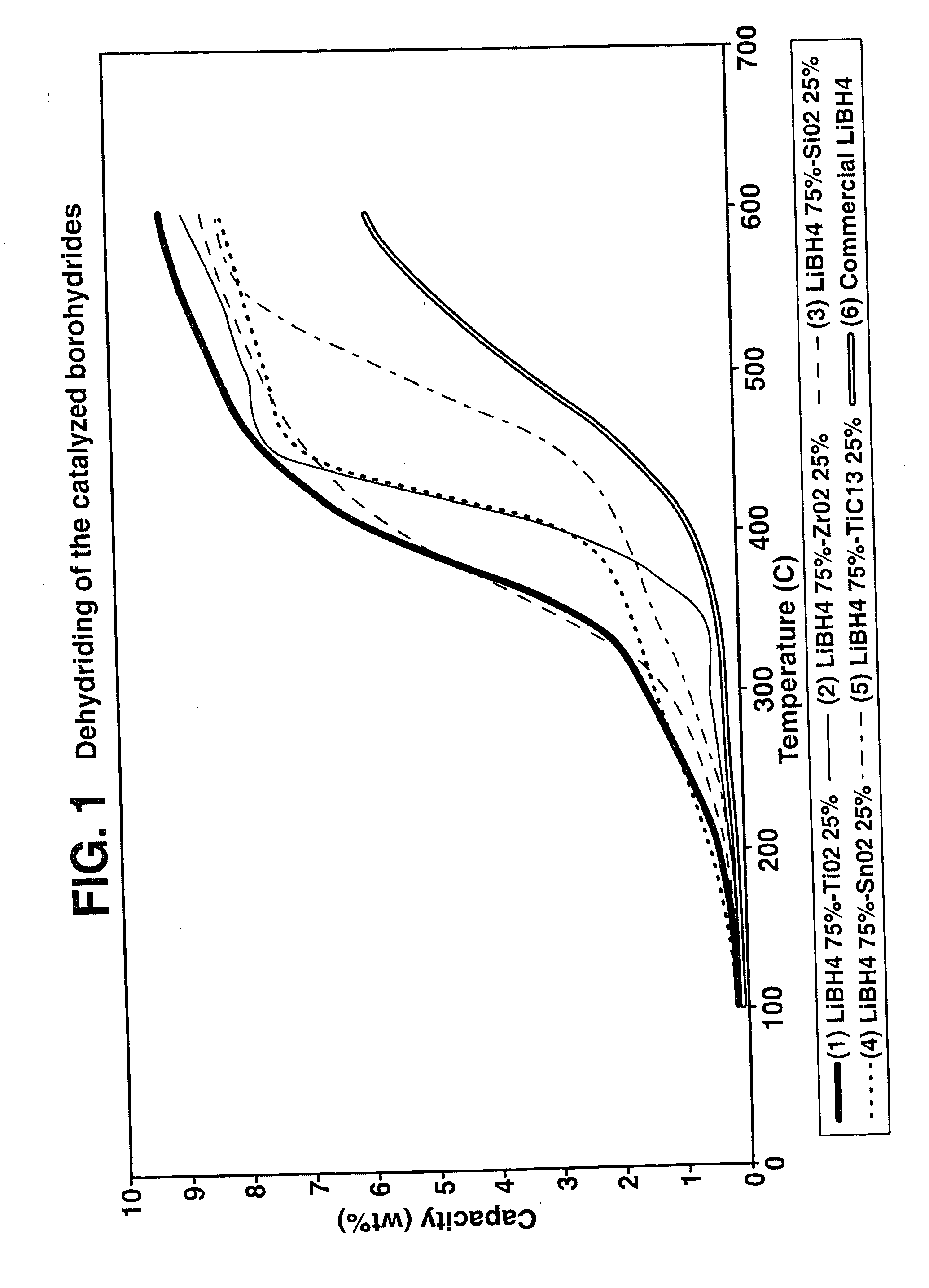
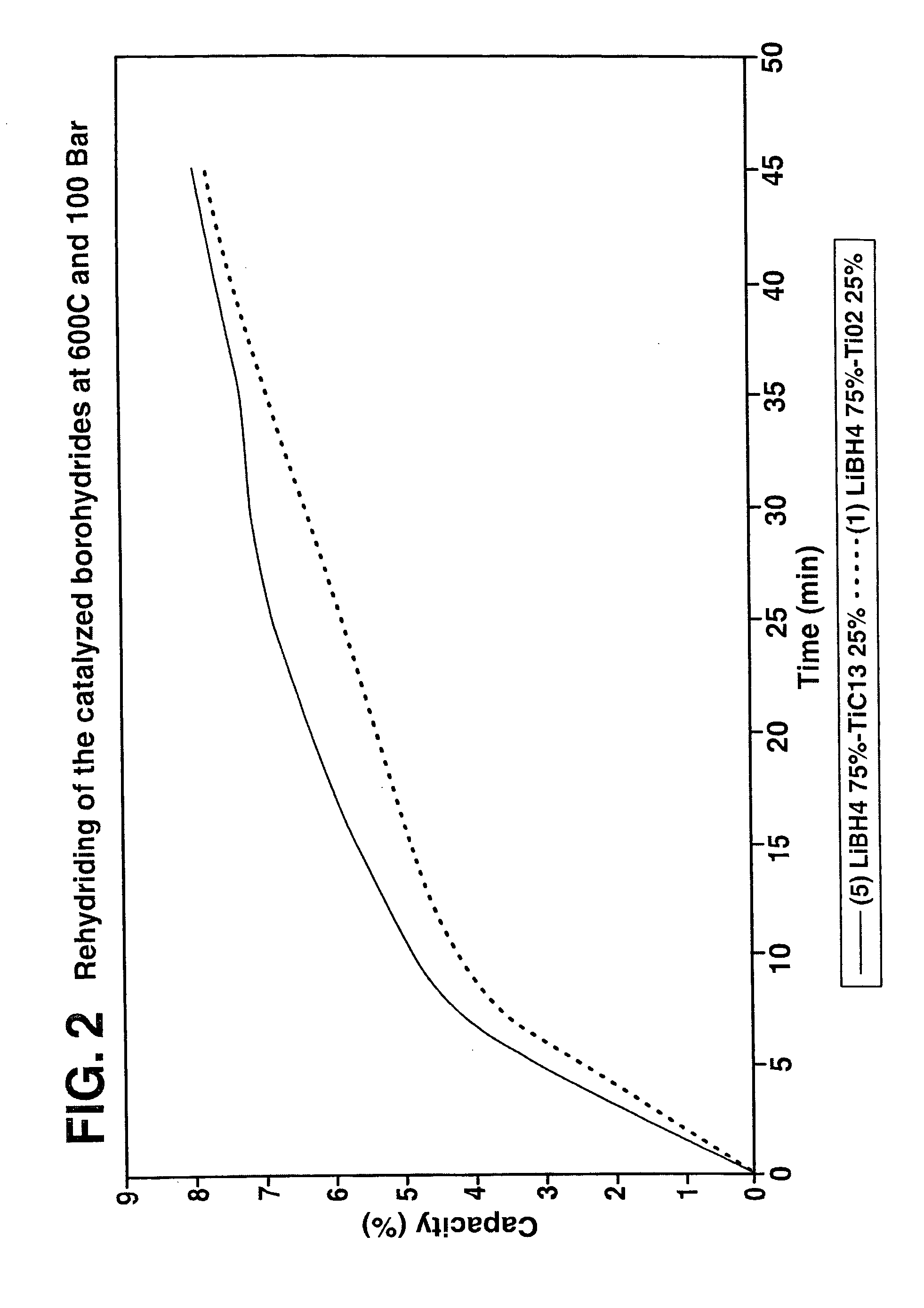
A hydrogen storage material and process is provided in which catalyzed alkali borohydride materials and partially substituted borohydride materials are created and which may contain effective amounts of catalyst(s) which include transition metal oxides, halides, and chlorides of titanium, zirconium, tin, vanadium, iron, cobalt and combinations of the various catalysts and the destabilization agents which include metals, metal hydrides, metal chlorides and complex hydrides of magnesium, calcium, strontium, barium, aluminum, gallium, indium, thallium and combinations of the various destabilization agents. When the catalysts and destabilization agents are added to an alkali borodydride such as a lithium borohydride, the initial hydrogen release point of the resulting mixture is substantially lowered. Additionally, the hydrogen storage material may be rehydrided with weight percent values of hydrogen of at least about nine percent.
Owner:SAVANNAH RIVER NUCLEAR SOLUTIONS
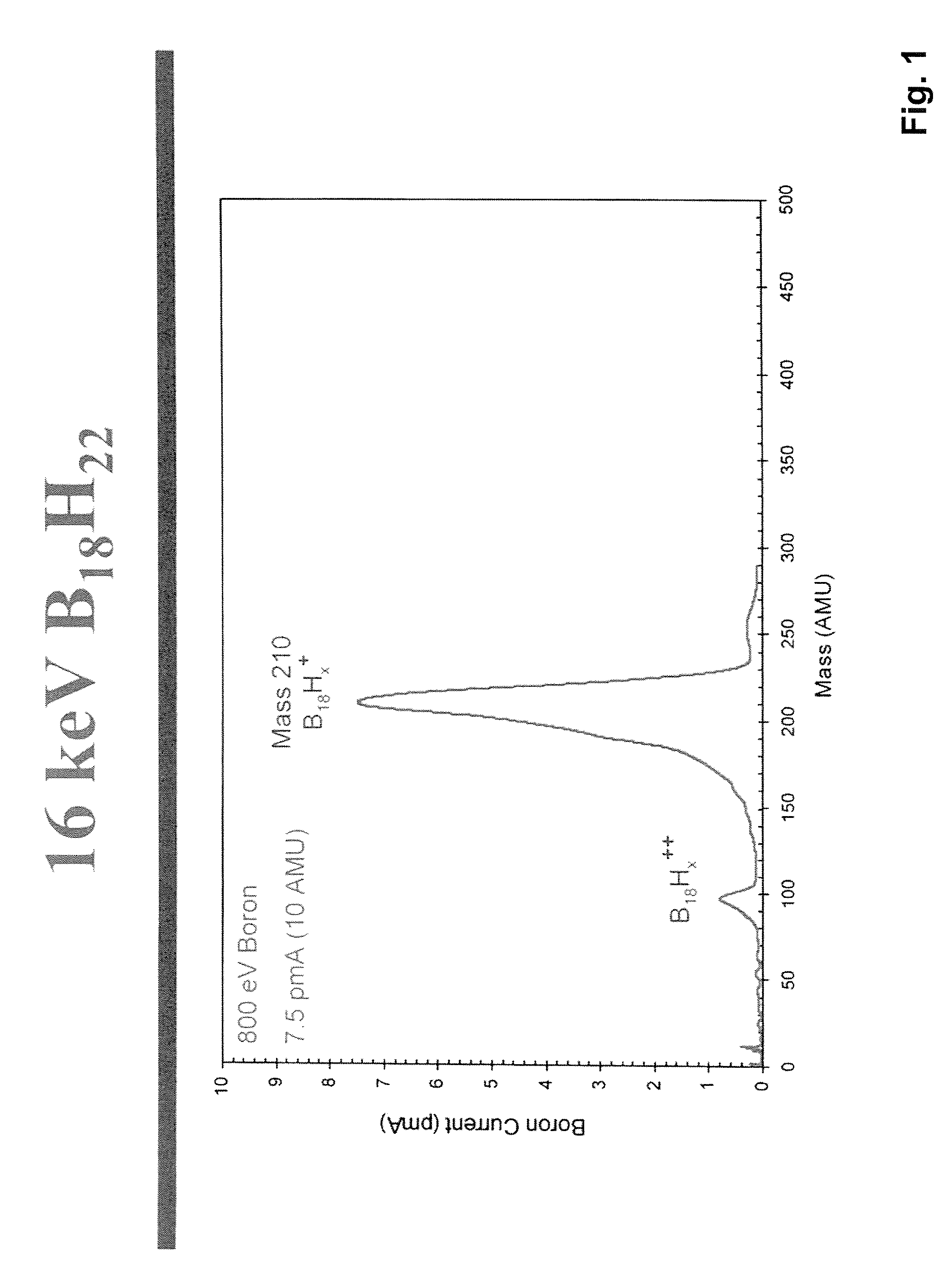
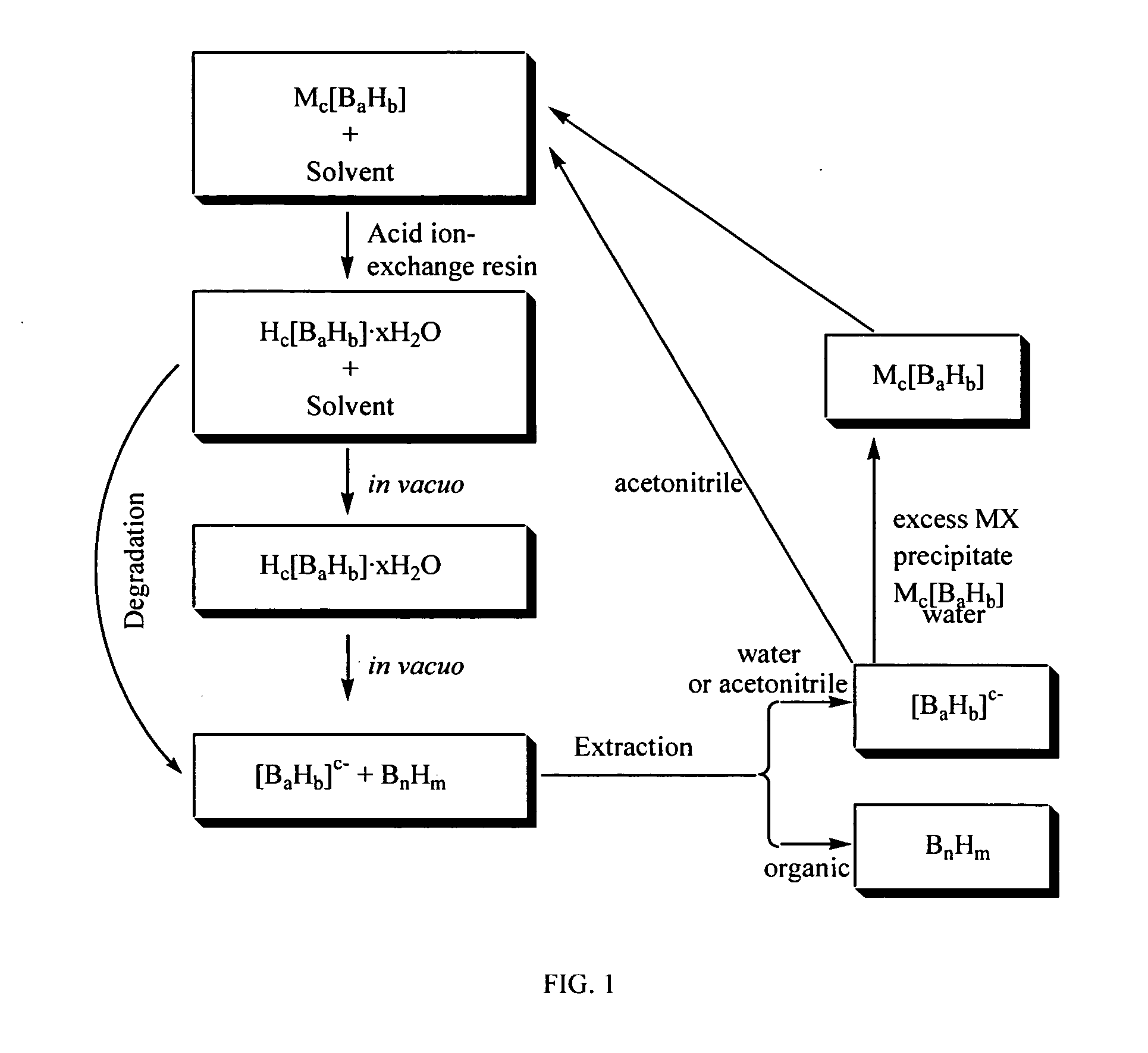
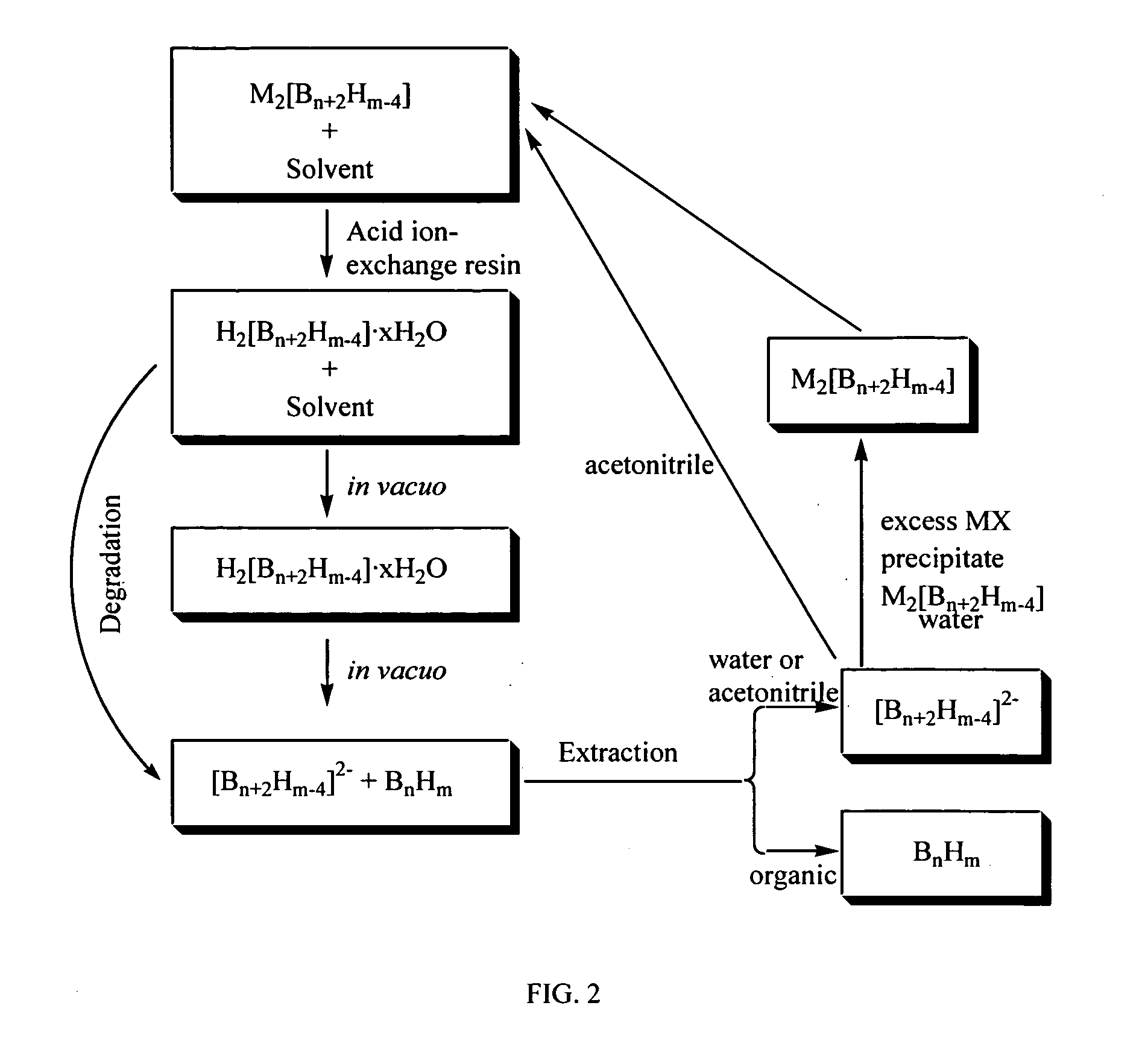
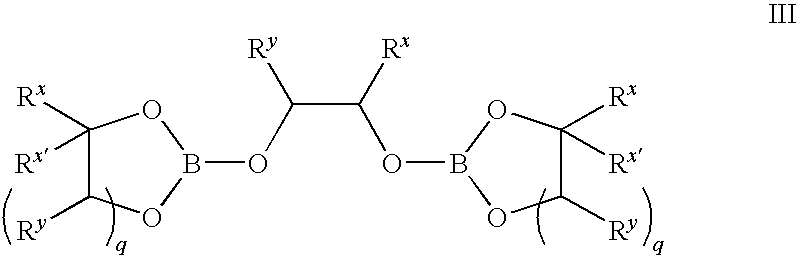
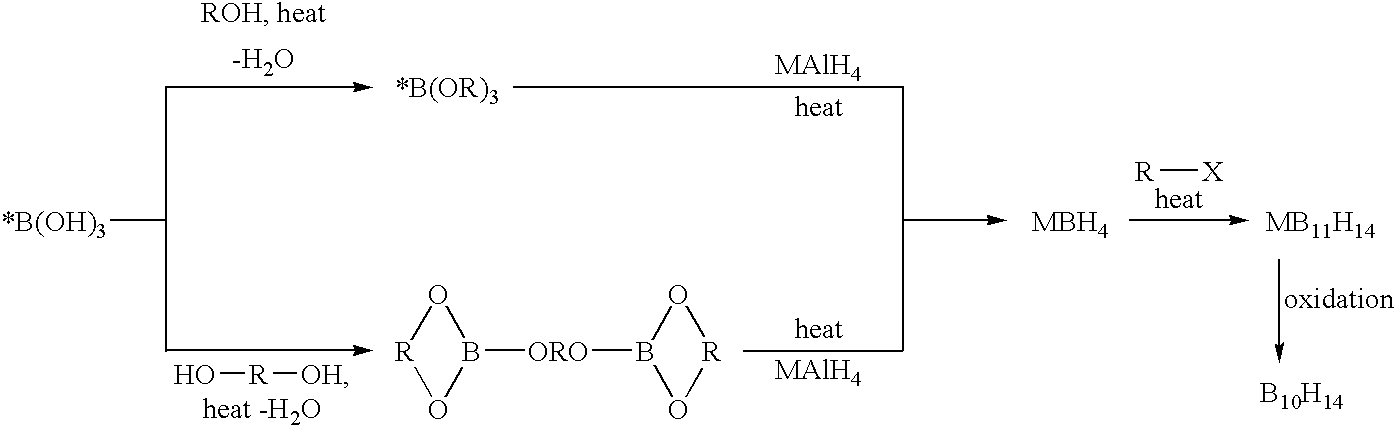
Method of production of B10H102-ammonium salts and methods of production of B18H22
InactiveUS20050169828A1High yieldSemiconductor/solid-state device manufacturingGroup 3/13 element organic compoundsMethods of productionOctadecaborane
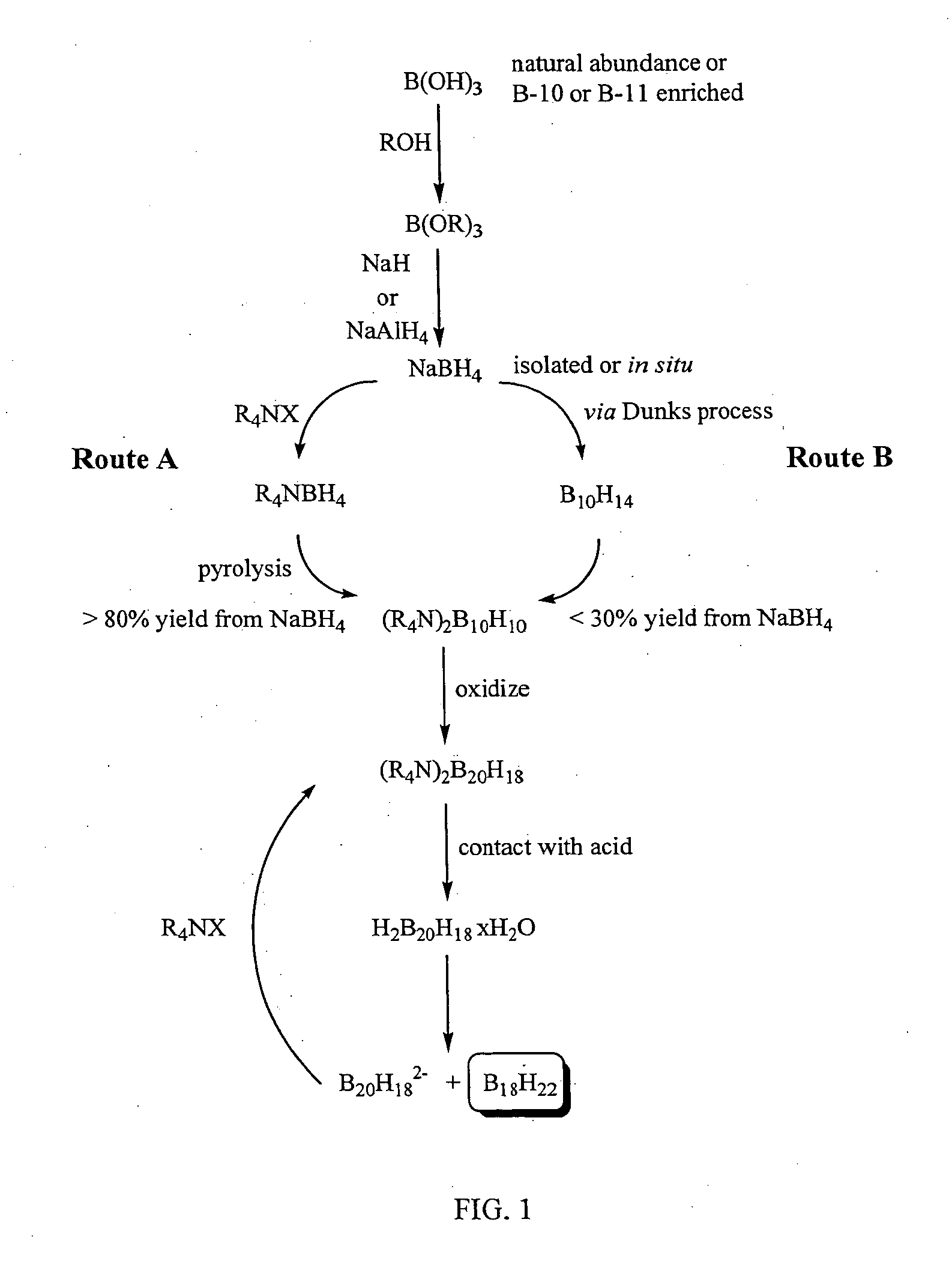
The invention provides new methods for synthesis of B9H9−, B10H102−, B11H14−, and B12H122− salts, particularly alkylammonium salts of B9H9−, B10H102−, B11H14−, and B12H122−. More particularly, the invention provides methods of preparing tetraalkylamronium salts of B9H9−, B10H102−, B11H14−, and B12H122− by pyrolysis of tetraalkylammonium borohydrides under controlled conditions. The invention additionally provides methods of preparing, in an atom efficient process, octadecaborane from the tetraalkylammonium salts of the invention. Preferred methods of the invention are suitable for preparation of isotopically enriched boranes, particularly isotopically enriched 10B18H22 and 11B18H22.
Owner:SEMEQUIP
Triborohydride salts as hydrogen storage materials and preparation thereof
InactiveUS20050135996A1Physical/chemical process catalystsAlkali/alkaline-earth/beryllium/magnesium hydridesFuel cellsSlurry
The present invention relates to the use of triborohydride salts as hydrogen storage materials. The present invention also relates to a system of using triborohydride salts to generate hydrogen gas for use in a fuel cell or other hydrogen-consuming device. A novel method of preparing triborohydride salts is also disclosed, wherein gaseous diborane is reacted with a carbonate suspended in a non-aqueous solvent in a suitable vessel with agitation. The process is typically carried out utilizing sodium carbonate to form sodium triborohydride. Other triborohydride salts can then be formed by cationic exchange. Hydrogen generating fuels according to the present invention include aqueous or hydroalcoholic solutions or slurries of a triborohydride salt, which may additionally contain a borohydride salt to provide operation over a broader temperature range.
Owner:MILLENNIUM CELL
Solid chemical hydride dispenser for generating hydrogen gas
InactiveUS7666386B2Molybdeum compoundsSynthetic resin layered productsCompound (substance)Hydrogen evolution
A device for generating hydrogen gas is provided. The device (101) comprises a first hydrogen-containing composition (107) that reacts with a second composition to evolve hydrogen gas; a dispenser (105) adapted to apply the first composition to a first porous member (109); and a conduit (111) adapted to supply the second composition to the first porous member. In a preferred embodiment, the first composition is selected from the group consisting of hydrides, borohydrides and boranes, the second composition is water, and the dispenser is spring-loaded and is charged with the first composition. As the first composition reacts with water at the interface to evolve hydrogen gas, the dispenser forces the reaction product across the interface and out of the dispenser, where it will not interfere with the progress of the hydrogen evolution reaction.
Owner:LYNNTECH POWER SYST
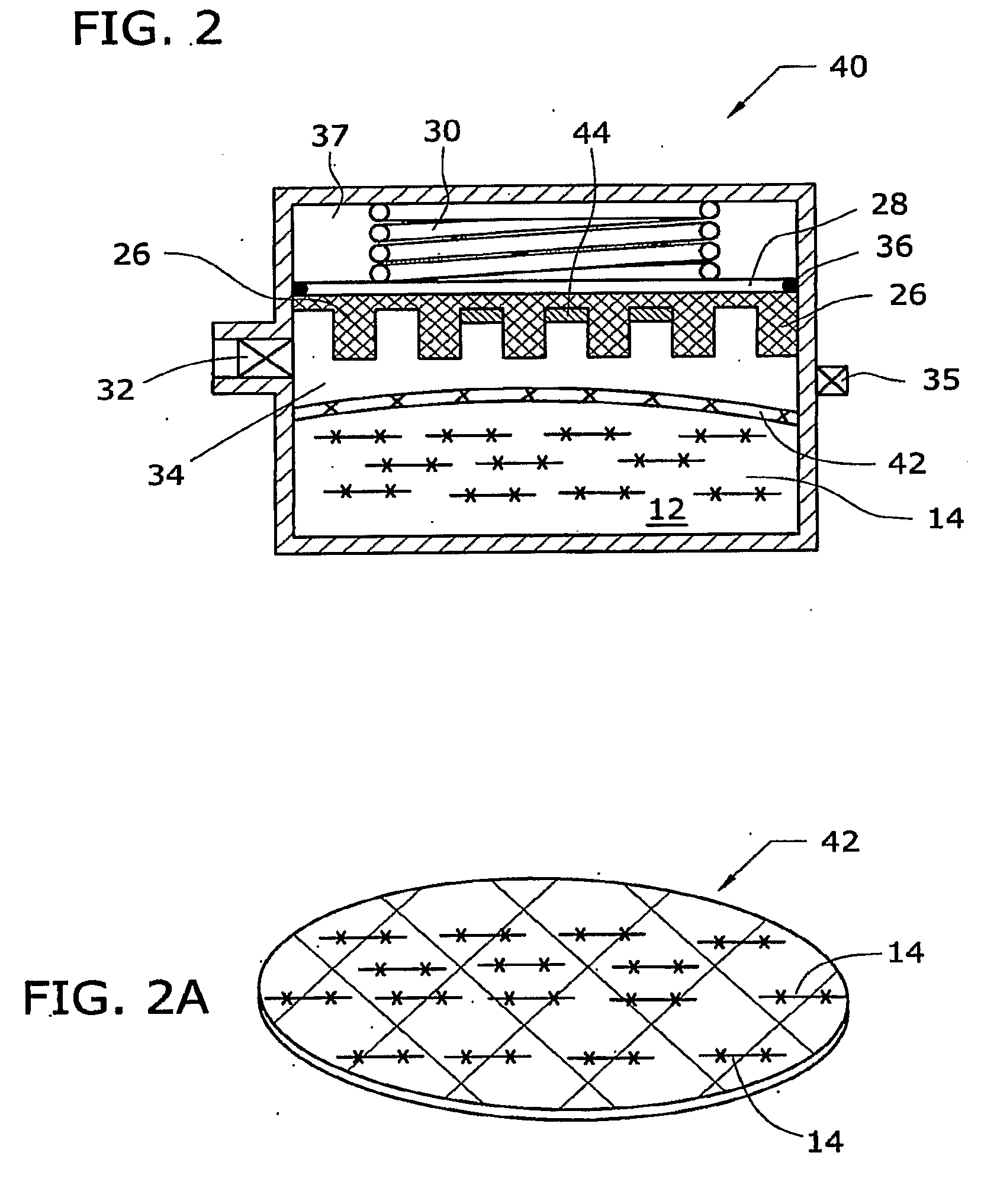
Fuel Compositions for Fuel Cells and Gas Generators Utilizing Same
InactiveUS20090060833A1Gaseous chemical processesReactant parameters controlAutomatic controlReaction rate
In a reaction of water or other reactable liquids with solid borohydride fuels, the liquid reactant and / or additives are converted to a gel form (14). The solid metal hydride and catalyst are formed into a single solid member (26). The single metal hydride / catalyst member is inserted into the gel (14) to initiate the reaction to produce hydrogen and is withdrawn from the gel to stop or slow the reaction. A self-regulating gas generator (10, 40) using such a fuel-production formulation automatically controls the reaction rate thereof to control the internal pressure of the gas generator.
Owner:SOC DITE SOC BIC
System and method for the manufacture of semiconductor devices by the implantation of carbon clusters
InactiveUS7666771B2Increase junction depthReduce leakage currentTransistorElectric discharge tubesDevice materialEngineering
A process is disclosed which incorporates implantation of a carbon cluster into a substrate to improve the characteristics of transistor junctions when the substrates are doped with Boron and Phosphorous in the manufacturing of PMOS transistor structures in integrated circuits. There are two processes which result from this novel approach: (1) diffusion control for USJ formation; and (2) high dose carbon implantation for stress engineering. Diffusion control for USJ formation is demonstrated in conjunction with a boron or shallow boron cluster implant of the source / drain structures in PMOS. More particularly, first, a cluster carbon ion, such as C16Hx+, is implanted into the source / drain region at approximately the same dose as the subsequent boron implant; followed by a shallow boron, boron cluster, phosphorous or phosphorous cluster ion implant to form the source / drain extensions, preferably using a borohydride cluster, such as B18Hx+ or B10Hx+. Upon subsequent annealing and activation, the boron diffusion is reduced, due to the gettering of interstitial defects by the carbon atoms.
Owner:SEMEQUIP
Processes for synthesizing borohydride compounds
InactiveUS20030092877A1HydrogenMonoborane/diborane hydridesAlkaline earth metalCombinatorial chemistry
The present invention relates to processes for producing borohydride compounds. In particular, the present invention provides efficient processes and compositions for the large-scale production of borohydride compounds of the formula YBH.sub.4 by the reaction of a boron-containing compound represented by the formula BX.sub.3 with hydrogen or an aldehyde to obtain diborane and HX, and reacting the diborane with a Y-containing base selected from those represented by the formula Y.sub.2O, YOH and Y.sub.2CO.sub.3 to obtain YBH.sub.4 and YBO.sub.2. Y is selected from the group consisting of the alkali metals, pseudo-alkali metals, alkaline earth metals, an ammonium ion, and quaternary amines of the formula NR.sub.4.sup.+, wherein each R is independently selected from hydrogen and a straight- or branched-chain C.sub.1-4 alkyl group, and X is selected from the group consisting of halide ions, --OH, --R' and --OR' groups, chalcogens, and chalcogenides, wherein R' is a straight- or branched-chain C.sub.1-4 alkyl group.
Owner:MILLENNIUM CELL
Processes for separating metals from metal salts
InactiveUS20060102491A1Electrolysis componentsGroup 3/13 element organic compoundsBorate ionSodium borohydride
Electrochemical processes and apparatus for obtaining metals from metal salts, particularly separating alkali metal and borate ions from alkali metal borate compounds, are disclosed. Aqueous solutions of metal borates or metal carbonates are converted to metals by preferred electrochemical processes. These electrochemical processes also may be integrated into processes for the production of borohydrides, such as sodium borohydride.
Owner:MILLENNIUM CELL
Methods of synthesis of isotopically enriched borohydride and methods of synthesis of isotopically enriched boranes
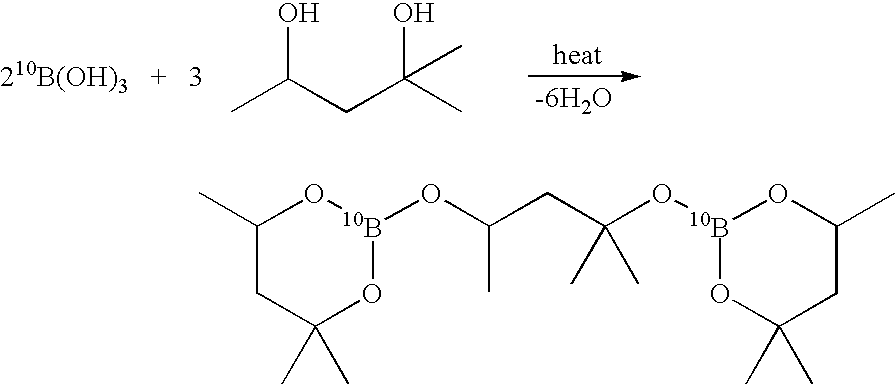
InactiveUS7641879B2High yieldPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesMonoborane/diborane hydridesIsotopeBoric acid
The invention provides new methods for the synthesis of isotopically enriched metal borohydrides, metal tetrahydroundecaborate salts, and decaborane from isotopically enriched 10B-boric acid or 11B-boric acid. The invention is particularly useful for synthesis of isotopically enriched sodium or lithium borohydride, MB11H14 (where M is Li, Na, K, or alkylammonium), and decaborane.
Owner:SEMEQUIP
Aqueous borohydride compositions
InactiveUS6866689B2Use minimizedHigh catalytic efficiencyOther chemical processesMonoborane/diborane hydridesHydrogenSodium borohydride
An aqueous fuel for generating hydrogen includes alkaline aqueous composition of about 17 to 37 mole percent of a sodium borohydride, and from about 0.001 to 1 mole percent of sodium hydroxide.
Owner:MONTGOMERY CHEM
Boron ion implantation using alternative fluorinated boron precursors, and formation of large boron hydrides for implantation
ActiveUS20110065268A1Easy to cutImprove efficiencyElectric discharge tubesVacuum evaporation coatingBoron trifluorideBoron containing
Methods of implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. A method of manufacturing a semiconductor device including implanting boron-containing ions using fluorinated boron-containing dopant species that are more readily cleaved than boron trifluoride. Also disclosed are a system for supplying a boron hydride precursor, and methods of forming a boron hydride precursor and methods for supplying a boron hydride precursor. In one implementation of the invention, the boron hydride precursors are generated for cluster boron implantation, for manufacturing semiconductor products such as integrated circuitry.
Owner:ENTEGRIS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com